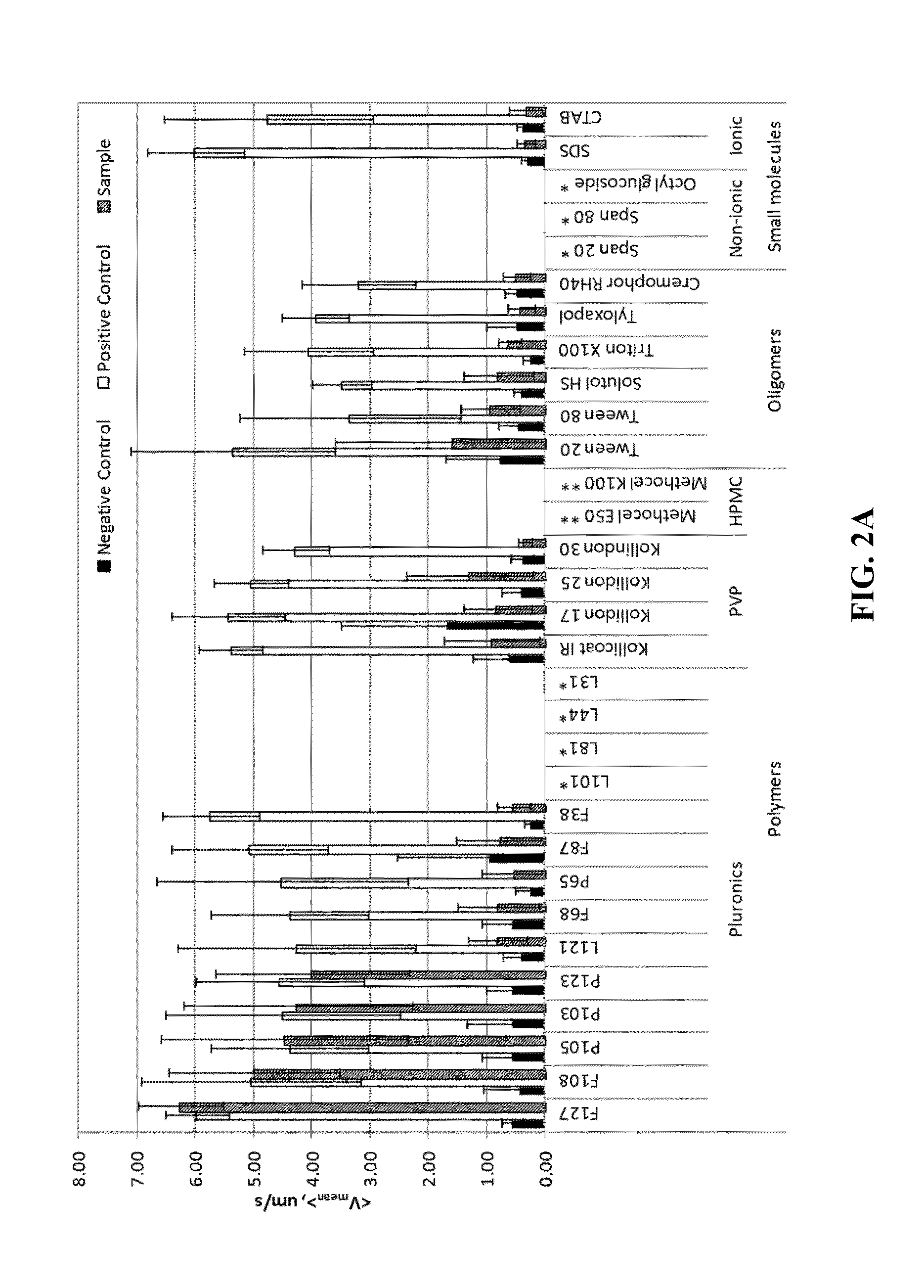
Patents
Literature
8050results about How to "Reduce adhesion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
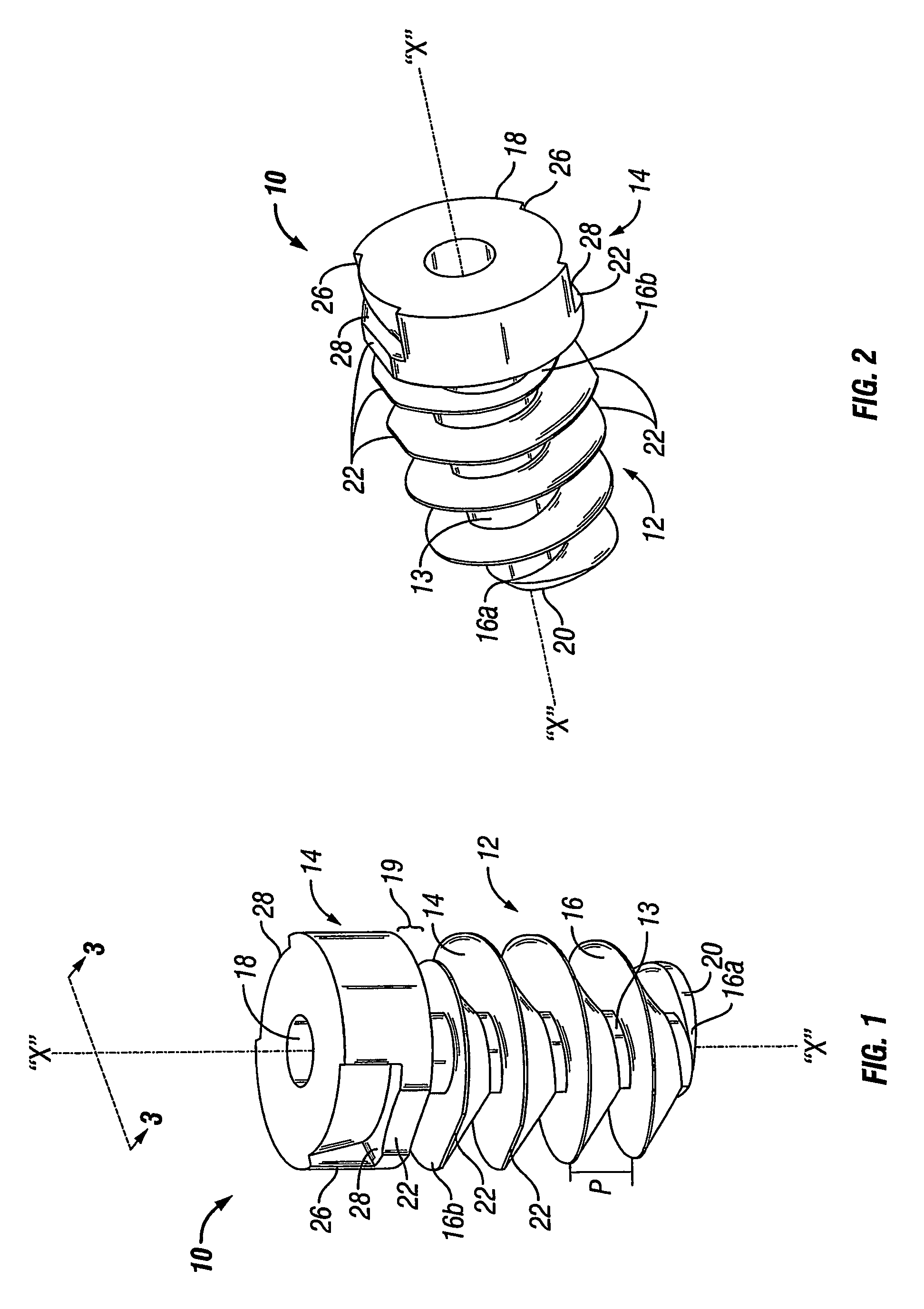
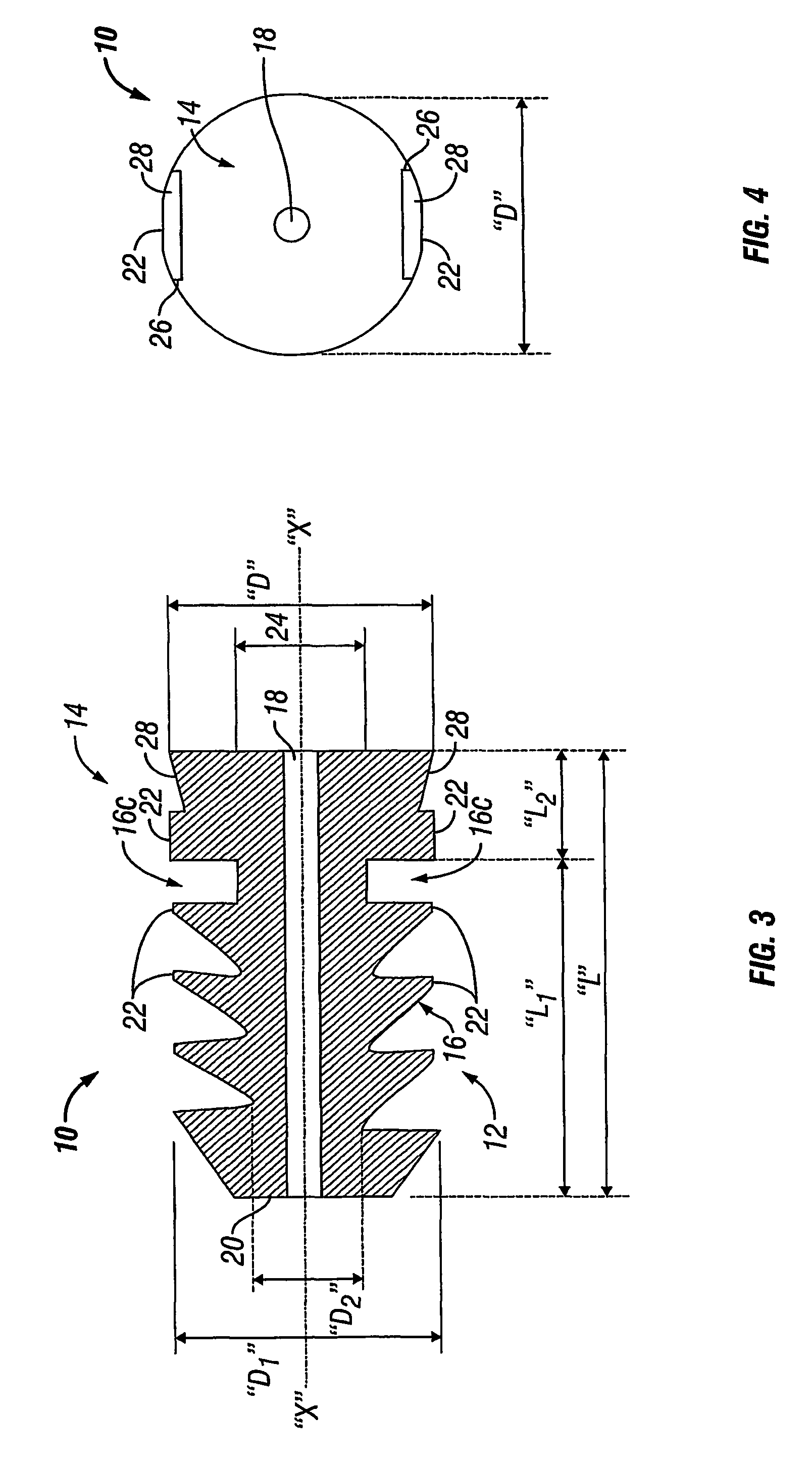
Method and device for handling bone adhesives
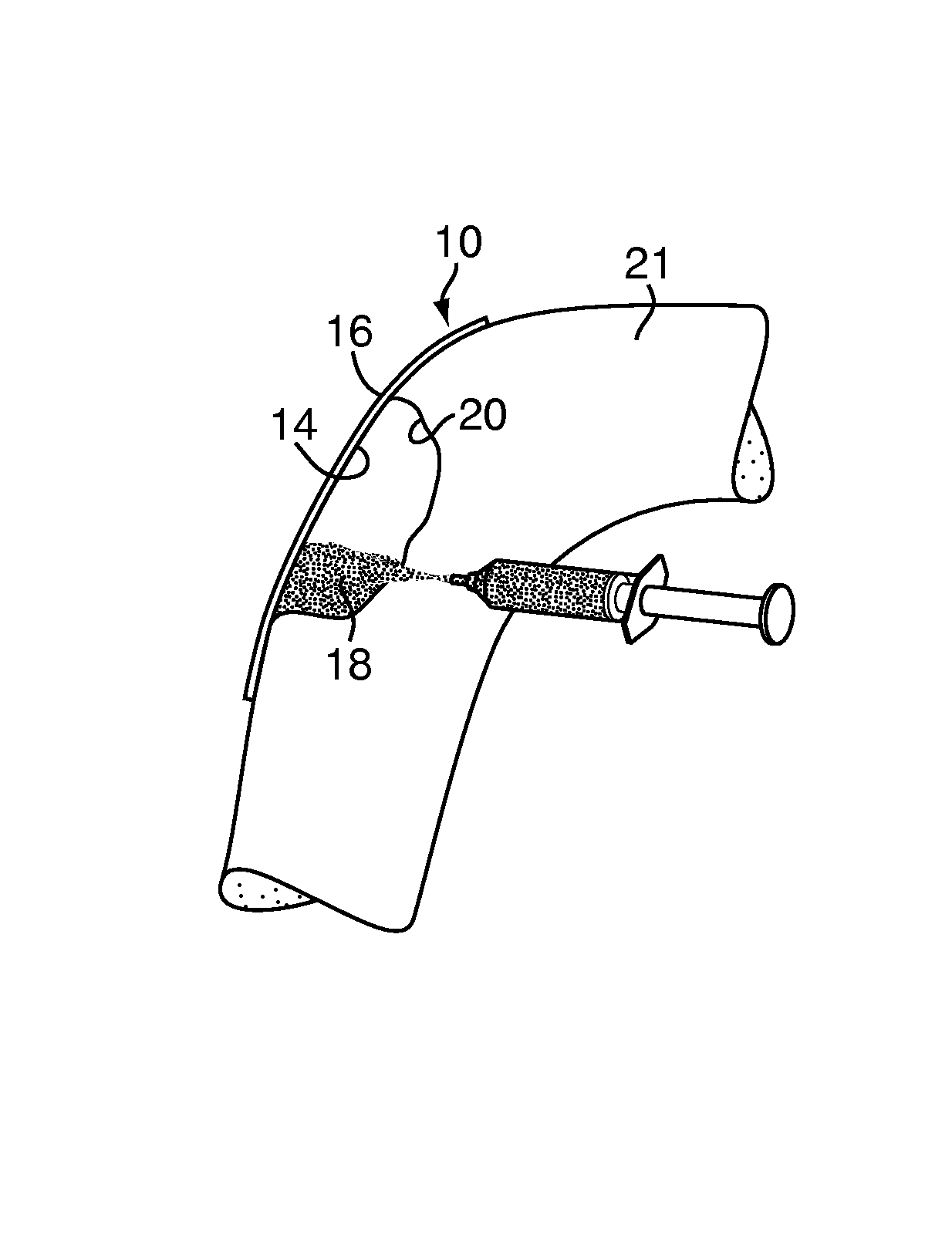
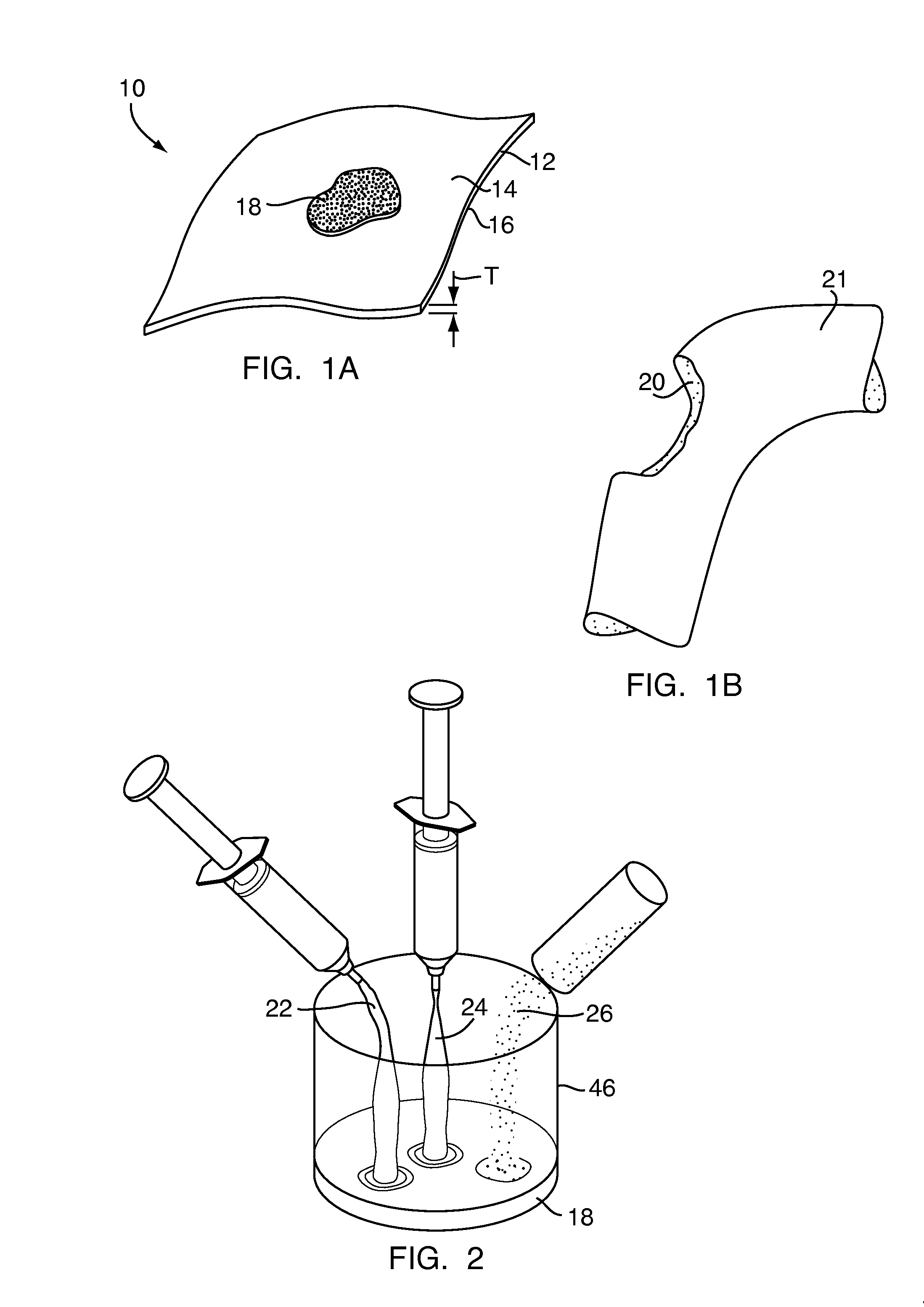
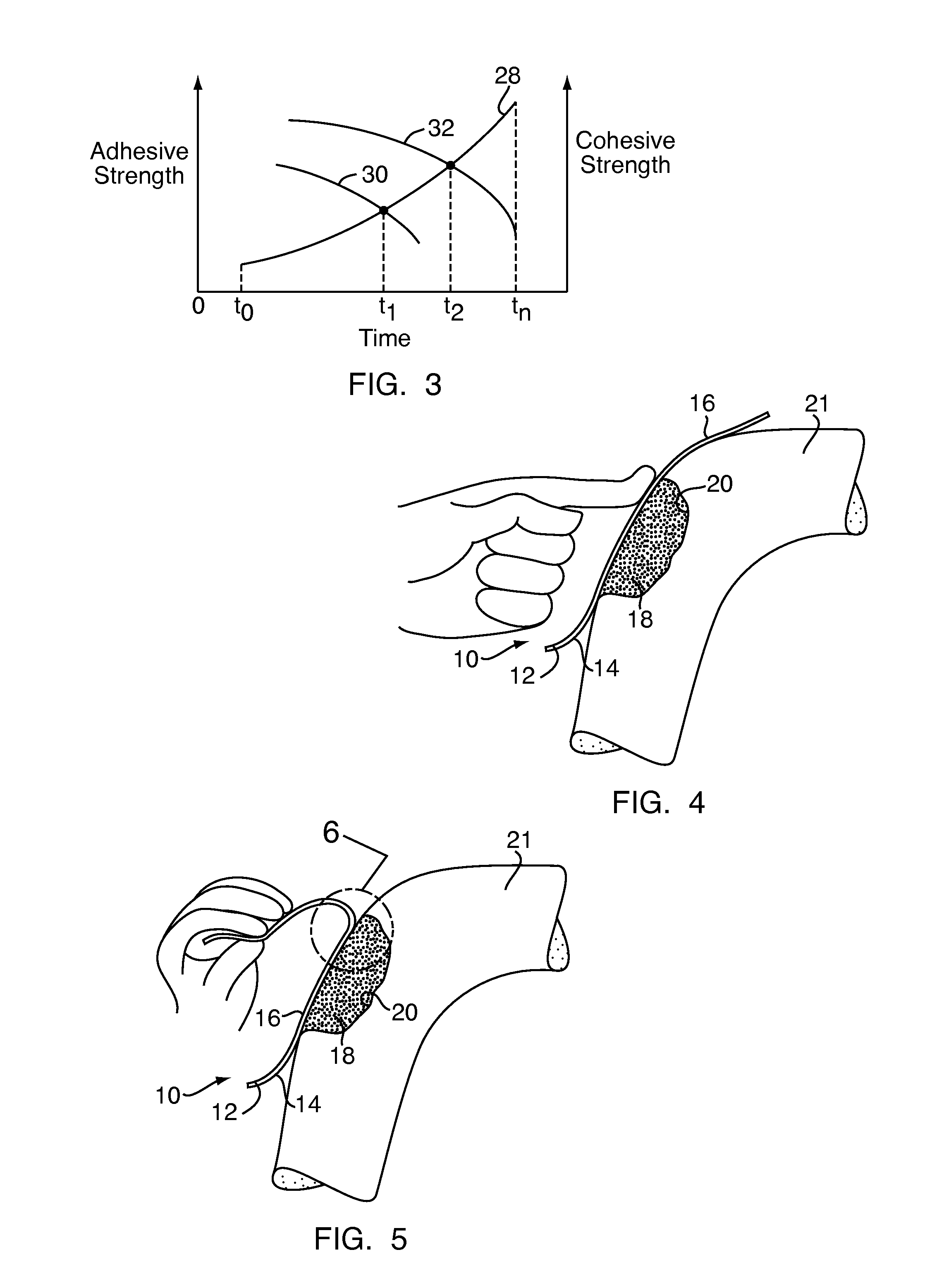
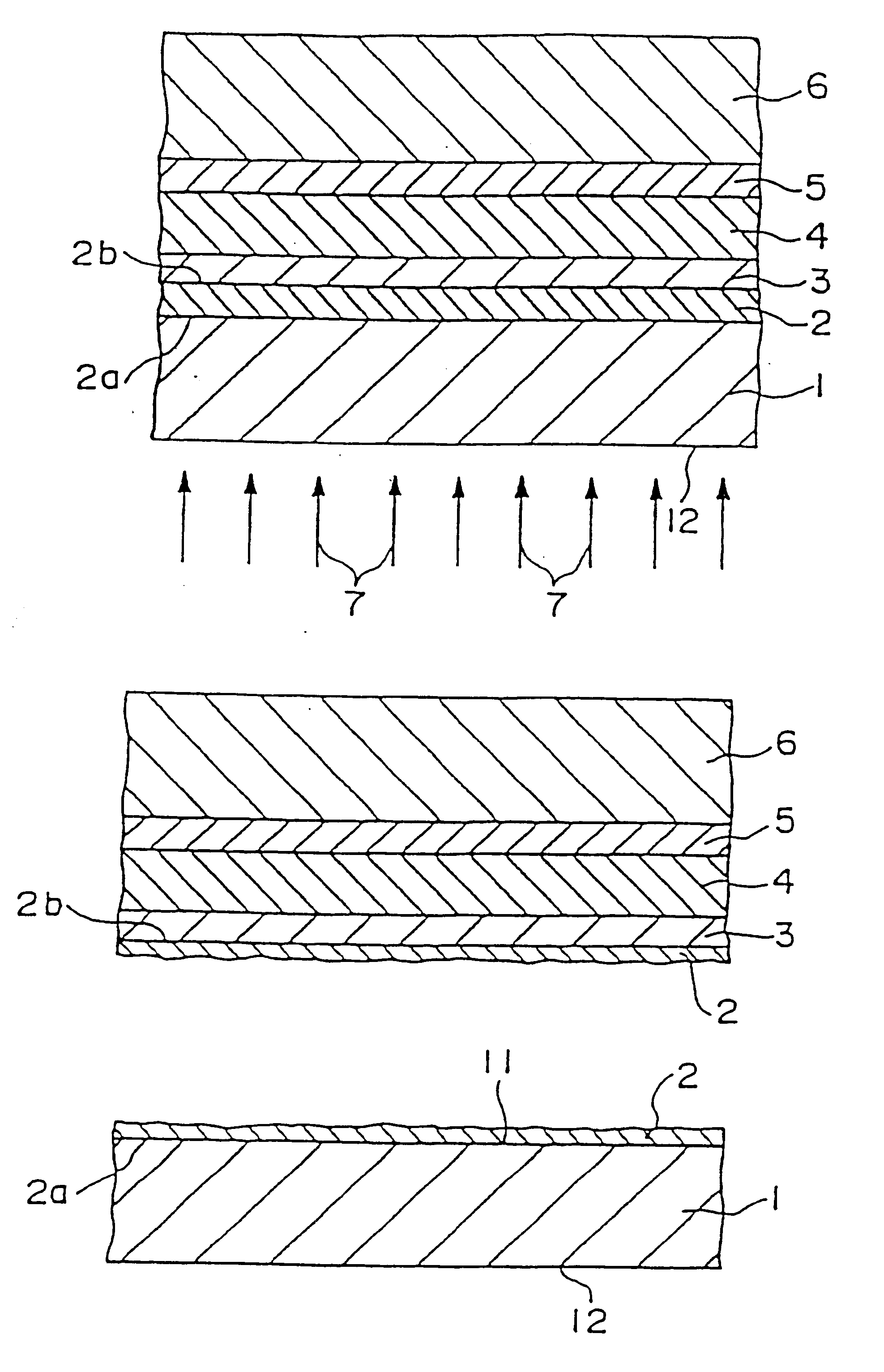
A bone adhesive application device has a pliable structure with an application surface upon which a bone adhesive may be applied. The application surface has a surface energy substantially equal to or less than a surface energy of the bone adhesive to reduce adhesion between the bone adhesive and the bone adhesive application device. The bone adhesive application device may be included in a kit for repairing bone defects having a bone adhesive formed from a reactive biocompatible polyurethane material. The bone adhesive may be applied to a bone defect by positioning the pliable structure over at least a portion of the bone defect, delivering the bone adhesive to the bone defect and removing the pliable structure from the bone adhesive.
Owner:ABYRX
Exfoliating method, transferring method of thin film device, and thin film device, thin film integrated circuit device and liquid crystal display device produced by the same
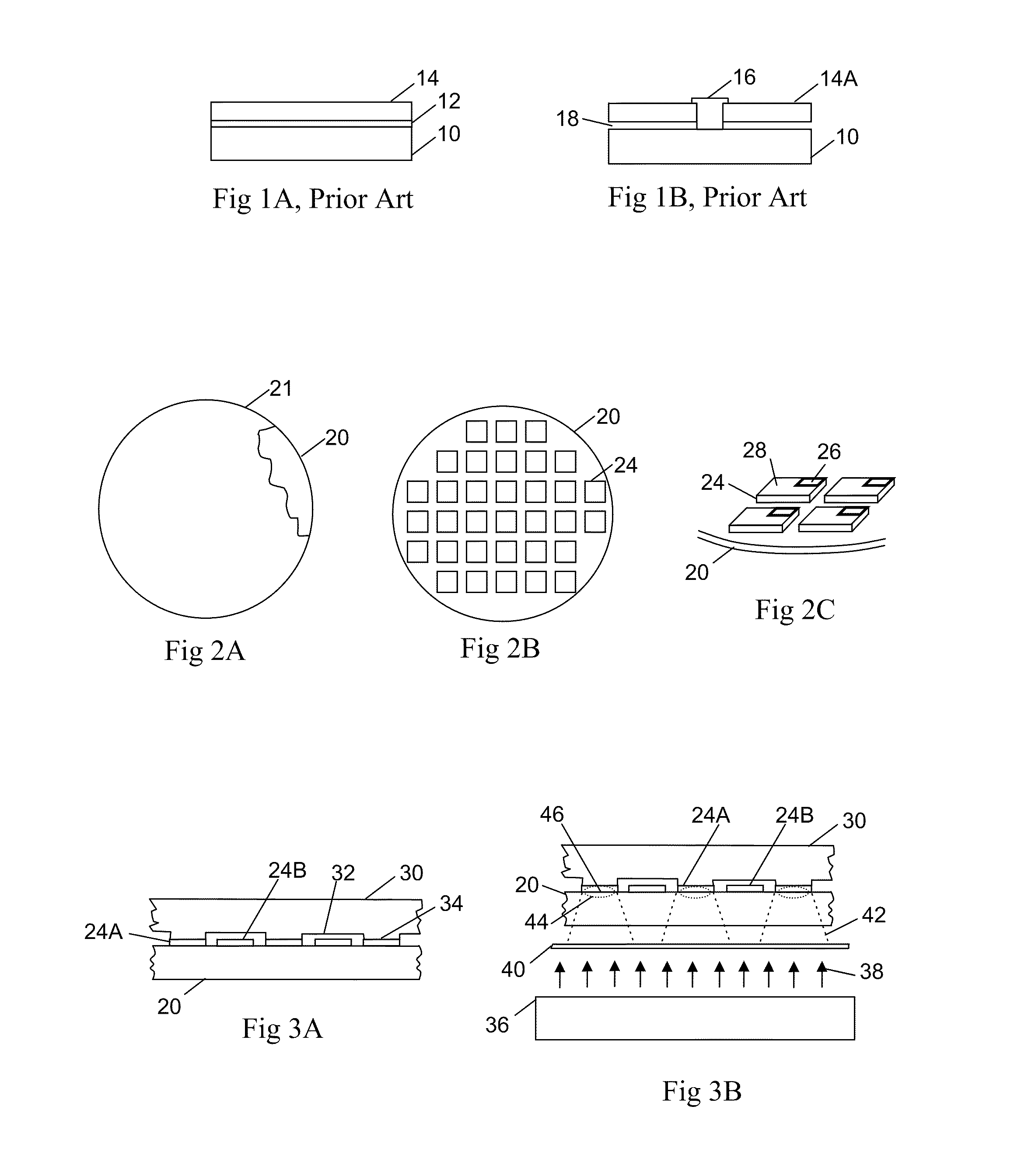
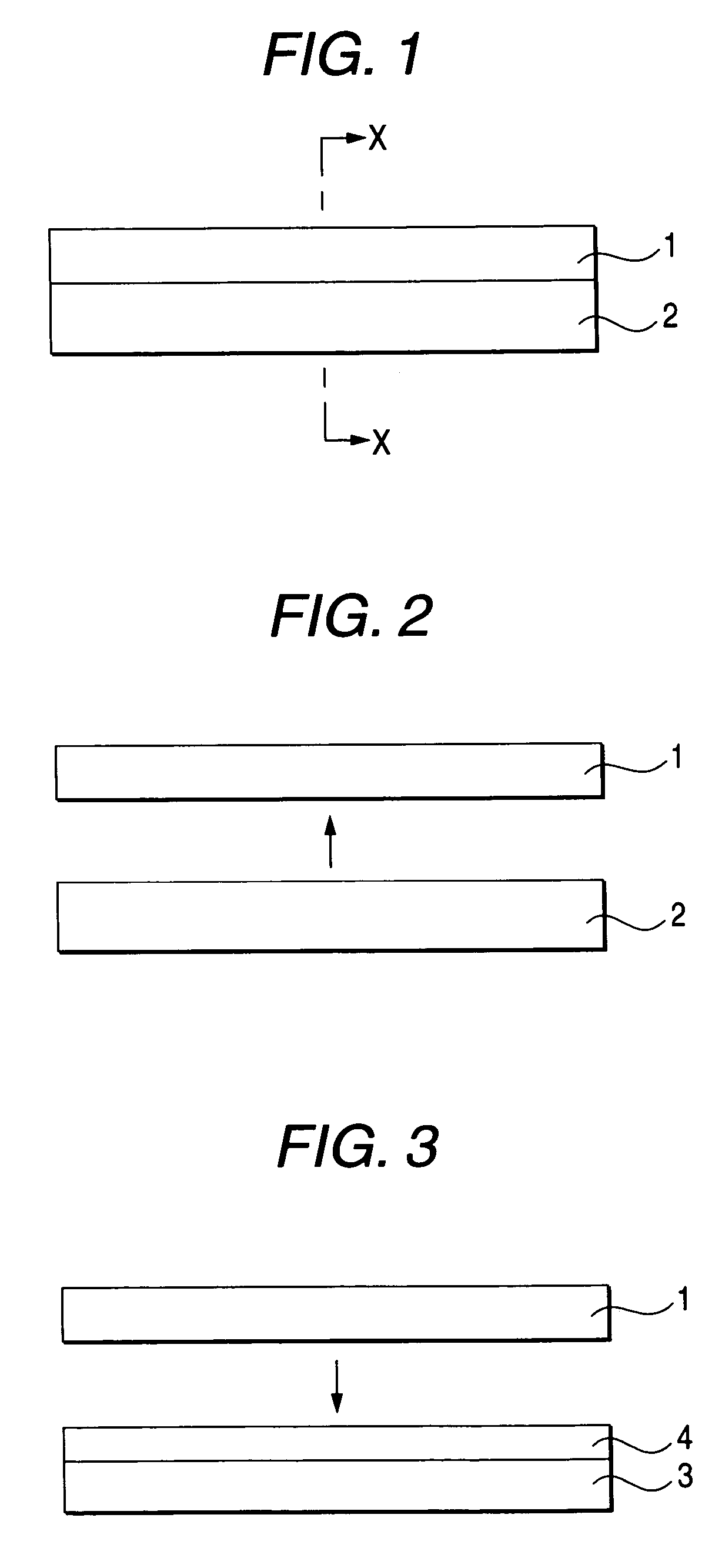
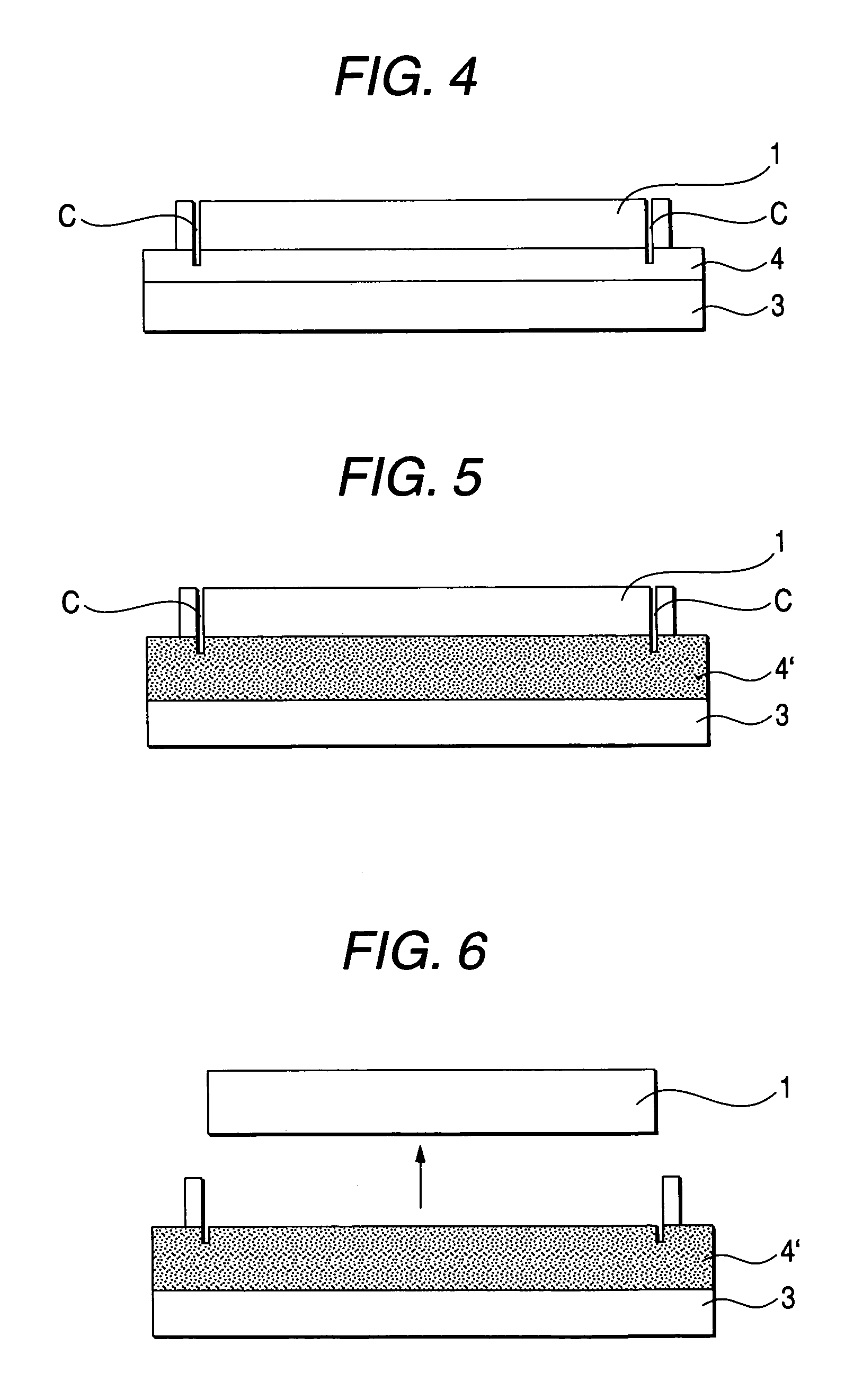
InactiveUS6645830B2Improve reliabilityReduce adhesionSolid-state devicesSemiconductor/solid-state device manufacturingLiquid-crystal displayPellicle membrane
A method for transferring a thin film device on a substrate onto a transfer member, includes a step for forming a separation layer on the substrate, a step for forming a transferred layer including the thin film device on the separation layer, a step for adhering the transferred layer including the thin film device to the transfer member with an adhesive layer therebetween, a step for irradiating the separation layer with light so as to form internal and / or interfacial exfoliation of the separation layer, and a step for detaching the substrate from the separation layer.
Owner:SAMSUNG ELECTRONICS CO LTD
Article having a lotioned topsheet
InactiveUS6861571B1Reduce adhesionImprove usabilityCosmetic preparationsToilet preparationsMedicineLotion
An article containing a liquid pervious topsheet coated with a lotion composition is disclosed. The lotion composition provides a skin benefit and / or reduces the adherence of BM to the skin of the wearer, thereby improving the case of BM clean up. The lotion composition applied to the article in a nonuniform manner, preferably such there are regions on the article's topsheet that are not coated with lotion.
Owner:THE PROCTER & GAMBLE COMPANY
Semiconductor processing apparatus comprising chamber partitioned into reaction and transfer sections
InactiveUS6899507B2Reduce adhesionImprove efficiencySemiconductor/solid-state device manufacturingCharge manipulationEngineeringSemiconductor
Semiconductor processing equipment that has increased efficiency, throughput, and stability, as well as reduced operating cost, footprint, and faceprint is provided. Other than during deposition, the atmosphere of both the reaction chamber and the transfer chamber are evacuated using the transfer chamber exhaust port, which is located below the surface of the semiconductor wafer. This configuration prevents particles generated during wafer transfer or during deposition from adhering to the surface of the semiconductor wafer. Additionally, by introducing a purge gas into the transfer chamber during deposition, and by using an insulation separating plate 34, the atmospheres of the transfer and reaction chambers can be effectively isolated from each other, thereby preventing deposition on the walls and components of the transfer chamber. Finally, the configuration described herein permits a wafer buffer mechanism to be used with the semiconductor processing equipment, thereby further increasing throughput and efficiency.
Owner:ASM JAPAN
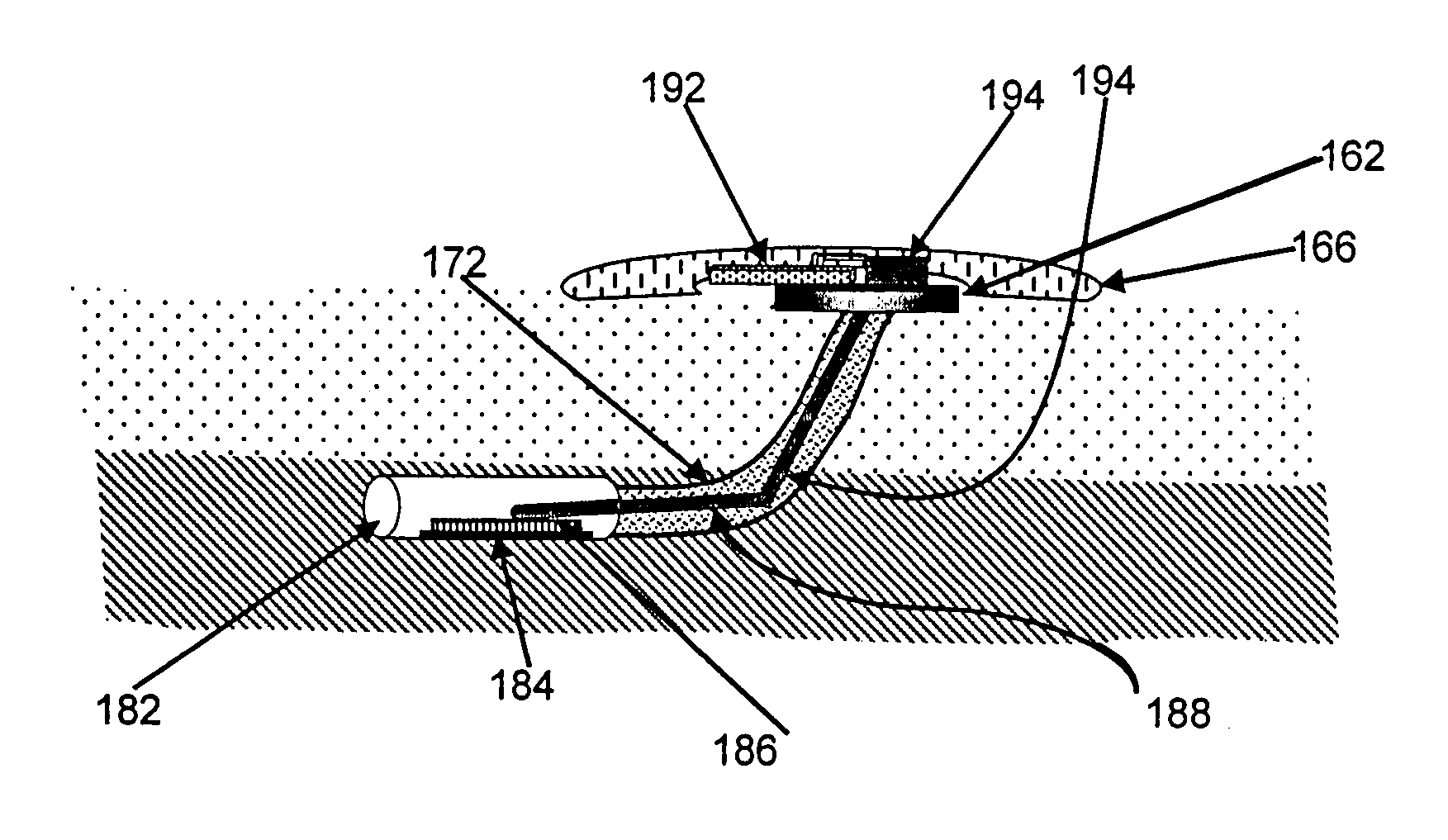
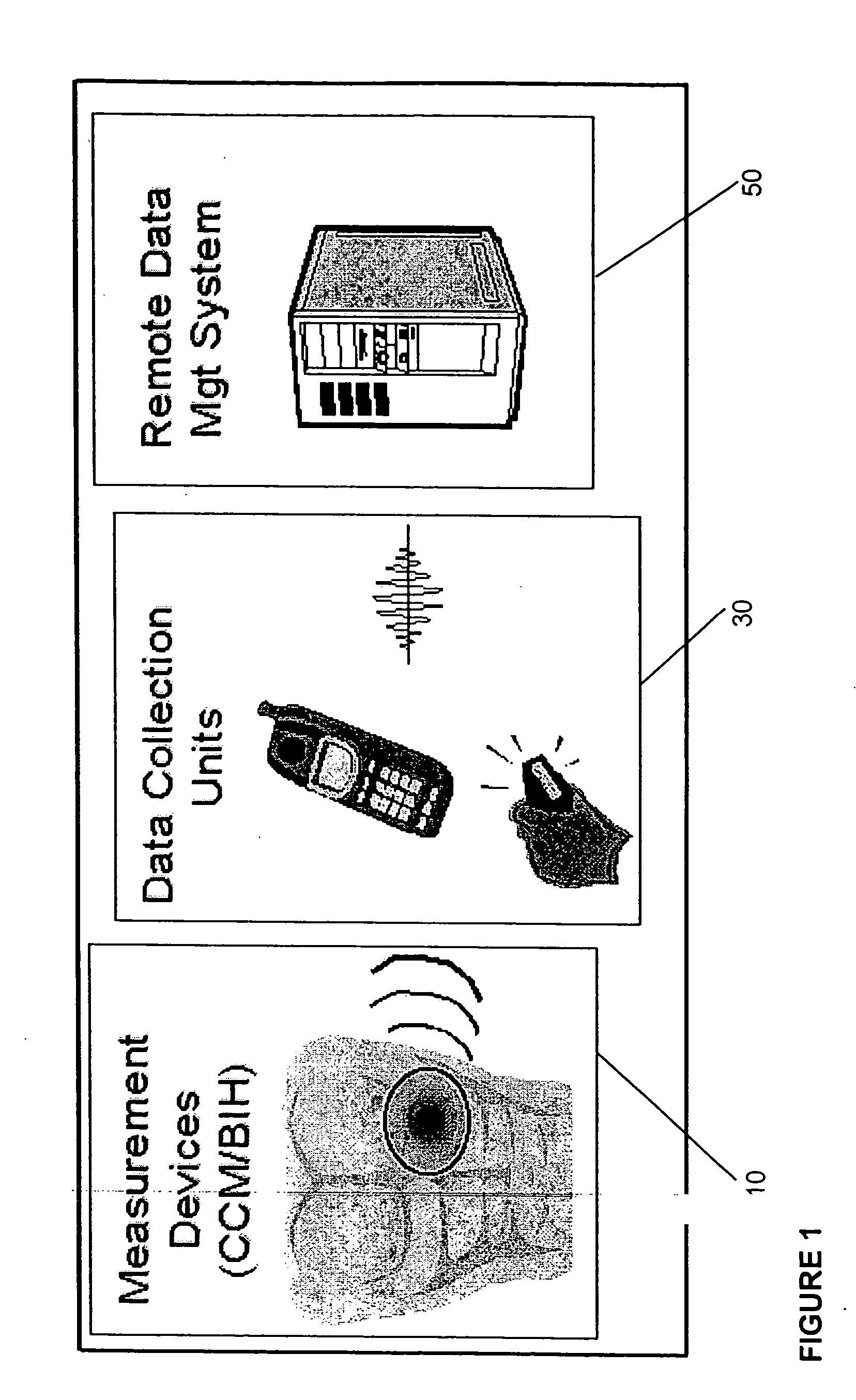
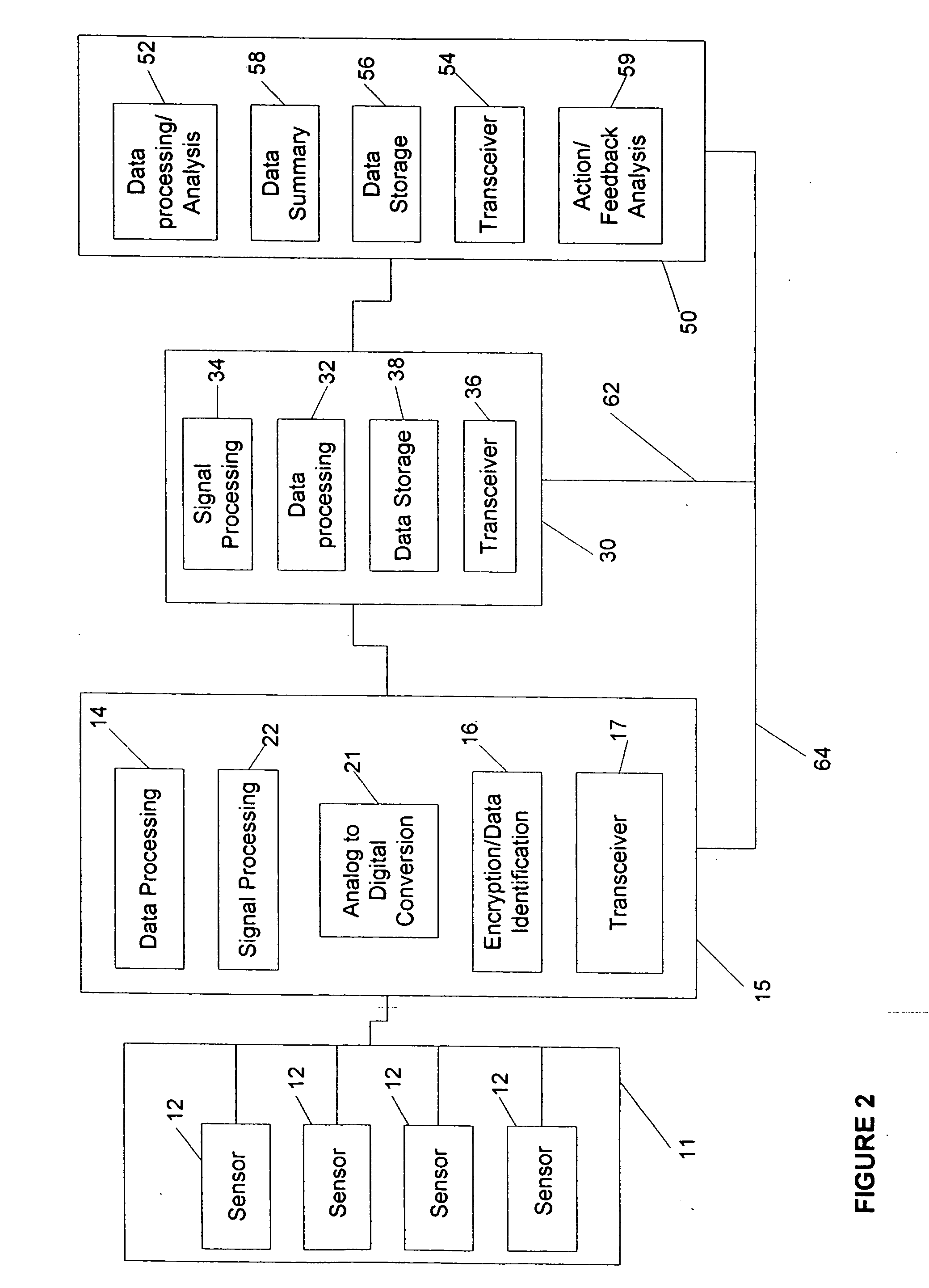
Gateway platform for biological monitoring and delivery of therapeutic compounds
InactiveUS20070060800A1Decrease adhesion and encapsulationImprove data qualityBioelectric signal measurementDrug and medicationsBiomonitoringComputer science
The invention relates to methods and devices for remote or distributed continuous monitoring of physiologically relevant states. The invention provides for methods to automatically detect deviations or other states in physiological parameters and automatically alert a measured subject, user or other authorized party. The device provides for a universal platform for sensors, and further provides for the automatic compensation or distribution of devices or bioactive agents at appropriate levels and / or intervals in response to deviations or other states sensed in various physiological parameters.
Owner:PHILOMETRON
Method of manufacturing transferable elements incorporating radiation enabled lift off for allowing transfer from host substrate
ActiveUS20110151602A1Reduce usageReduces cost and complexitySolid-state devicesSemiconductor/solid-state device manufacturingOptical transparencySemiconductor materials
Semiconductor material is formed on a host substrate of a material exhibiting optical transparency with an intervening radiation lift off layer. A transfer device, intermediate substrate or target substrate is brought into adhesive contact with the semiconductor material and the radiation lift off layer is irradiated to weaken it, allowing the semiconductor material to be transferred off the host substrate. Electronic devices may be formed in the semiconductor layer while it is attached to the host substrate or the intermediate substrate.
Owner:EPISTAR CORP
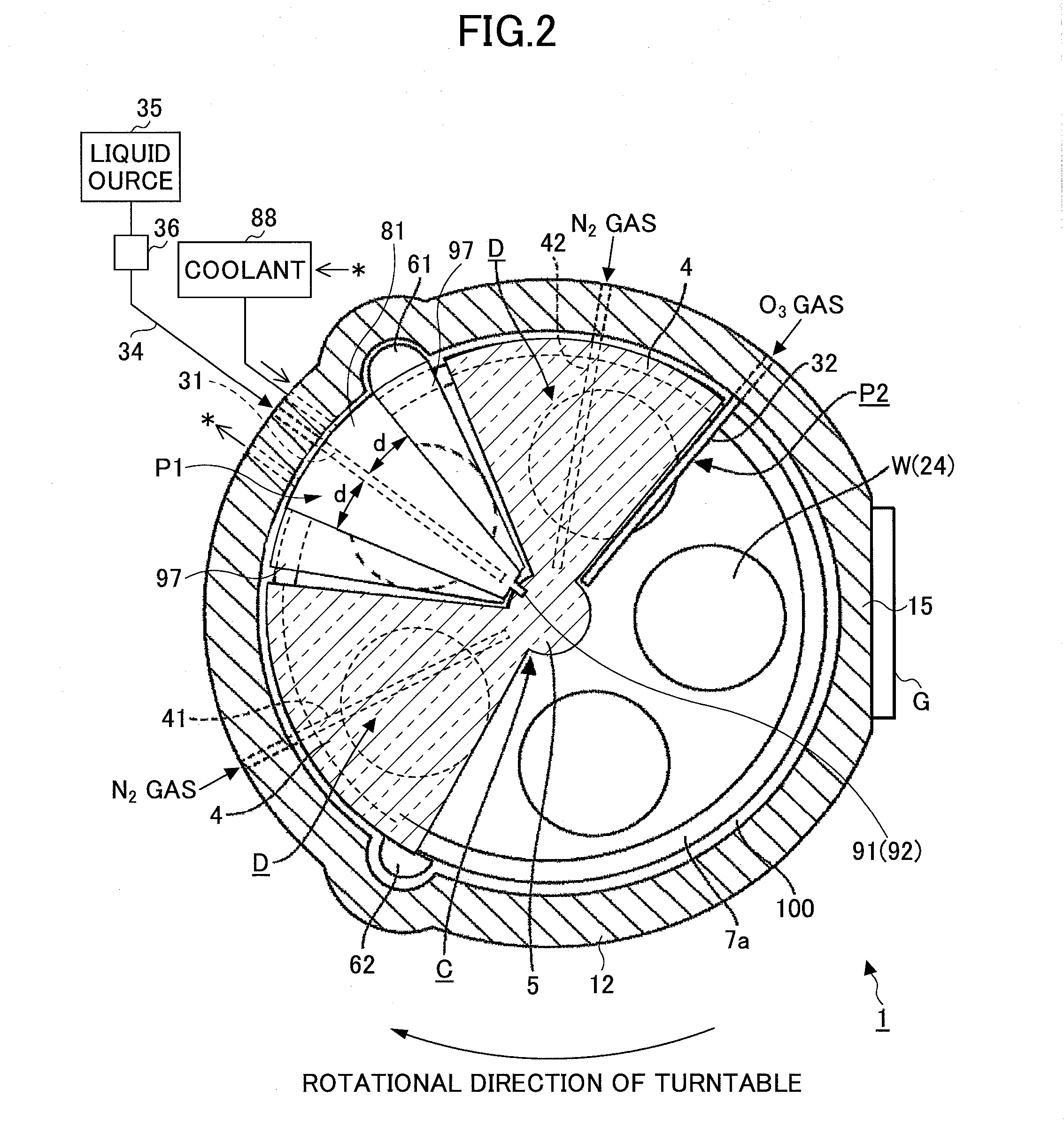
Film deposition apparatus
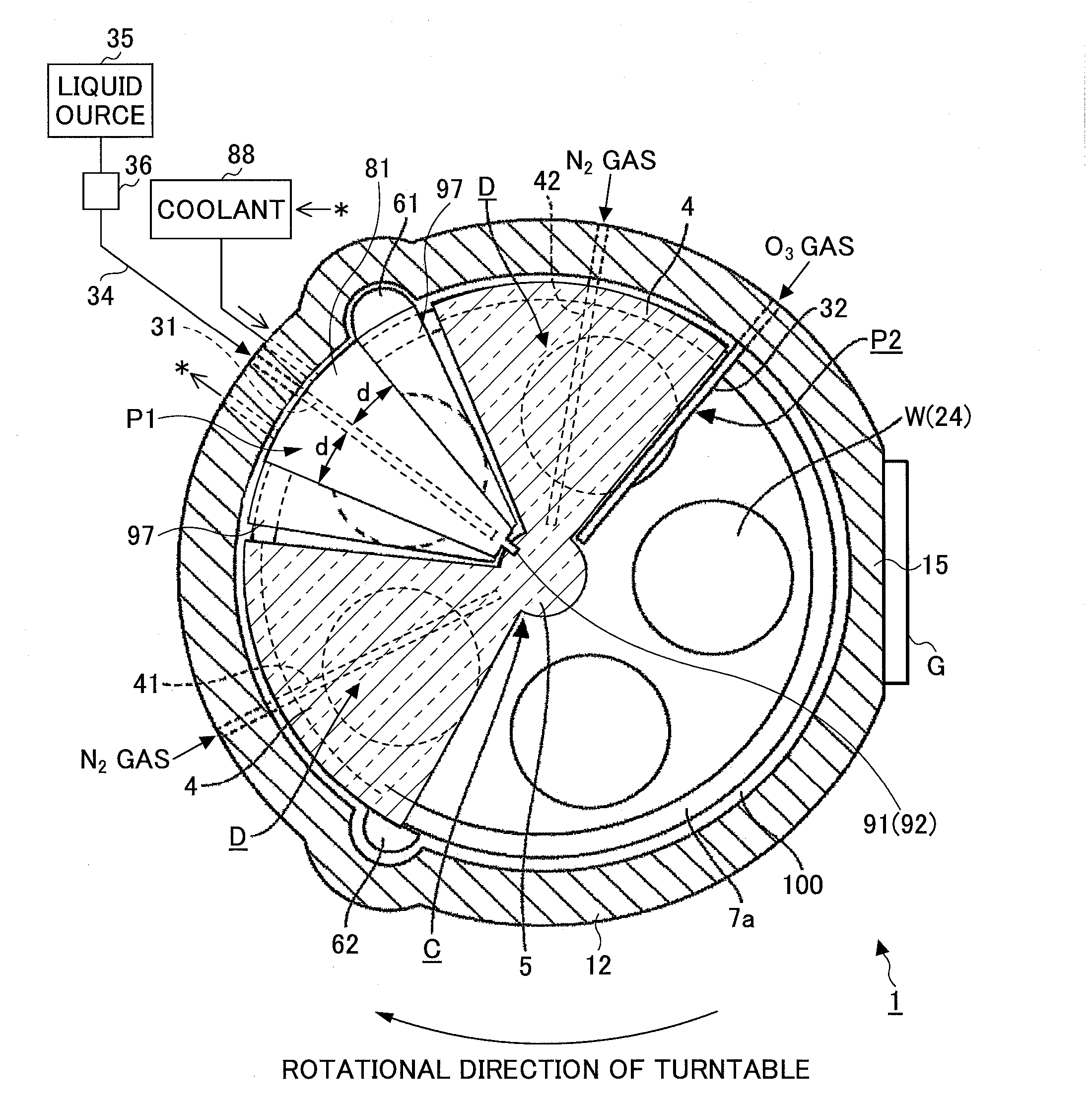
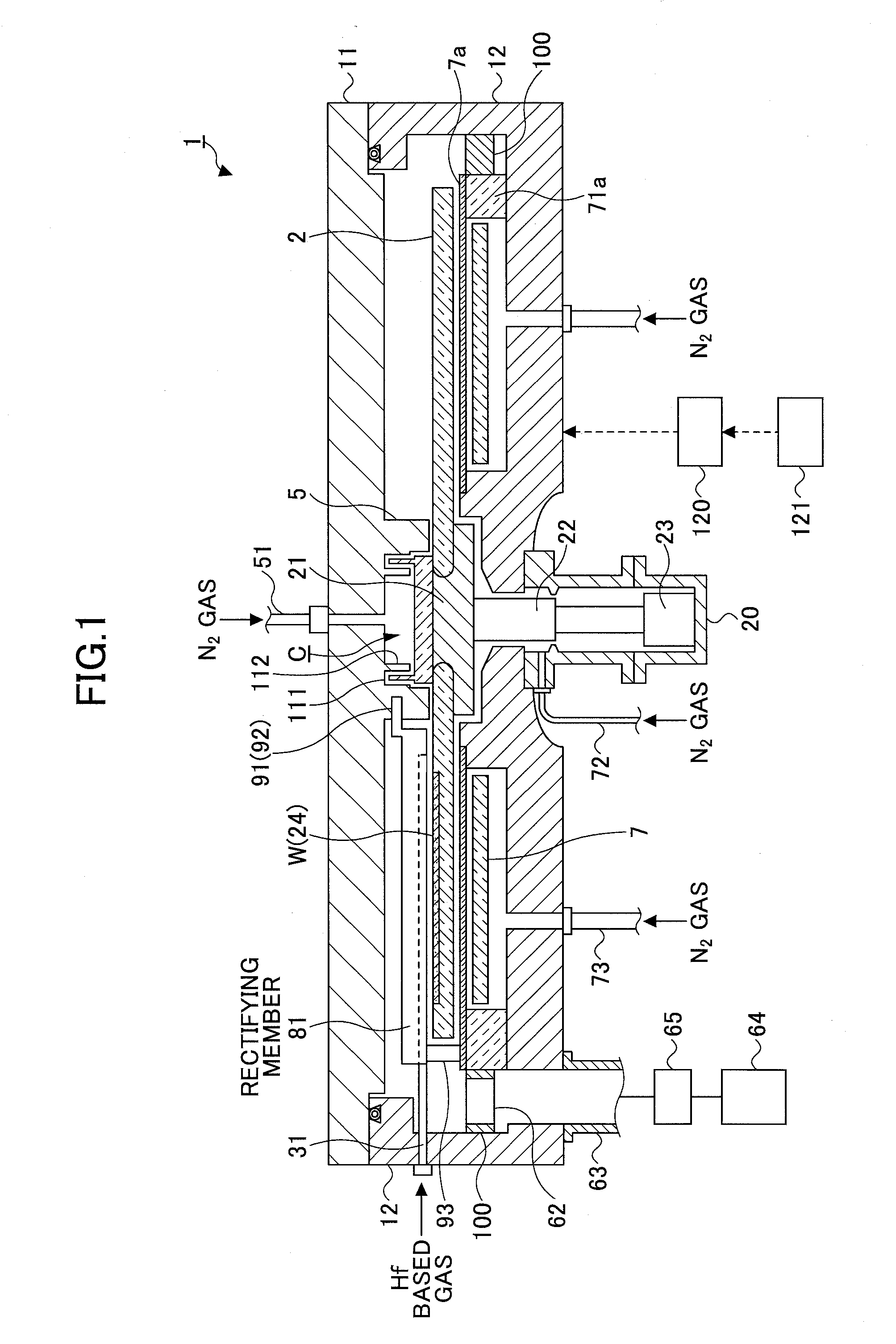
In discharging a source gas from a first process gas nozzle, rectifying members including a coolant flow passage provided in a concertinaing manner therein are arranged both sides of the first process gas nozzle. Then, a coolant at a temperature higher than a liquefaction temperature of the source gas and lower than a thermal decomposition temperature of the source gas is flown through the coolant flow passage, by which the first process gas nozzle is cooled through the rectifying member.
Owner:TOKYO ELECTRON LTD
Exfoliating method, transferring method of thin film device, and thin film device, thin film integrated circuit device, and liquid crystal display device produced by the same
InactiveUS20020146893A1Improve reliabilityReduce adhesionSolid-state devicesSemiconductor/solid-state device manufacturingLiquid-crystal displayEngineering
A method for transferring a thin film device on a substrate onto a transfer member, includes a step for forming a separation layer on the substrate, a step for forming a transferred layer including the thin film device on the separation layer, a step for adhering the transferred layer including the thin film device to the transfer member with an adhesive layer therebetween, a step for irradiating the separation layer,with light so as to form internal and / or interfacial exfoliation of the separation layer, and a step for detaching the substrate from the separation layer.
Owner:SAMSUNG ELECTRONICS CO LTD
Process for producing flexible optical waveguide
InactiveUS7120345B2Peeling can be safelyReduce adhesionCoupling light guidesOptical waveguide light guideFoaming agentWaveguide
Owner:NITTO DENKO CORP
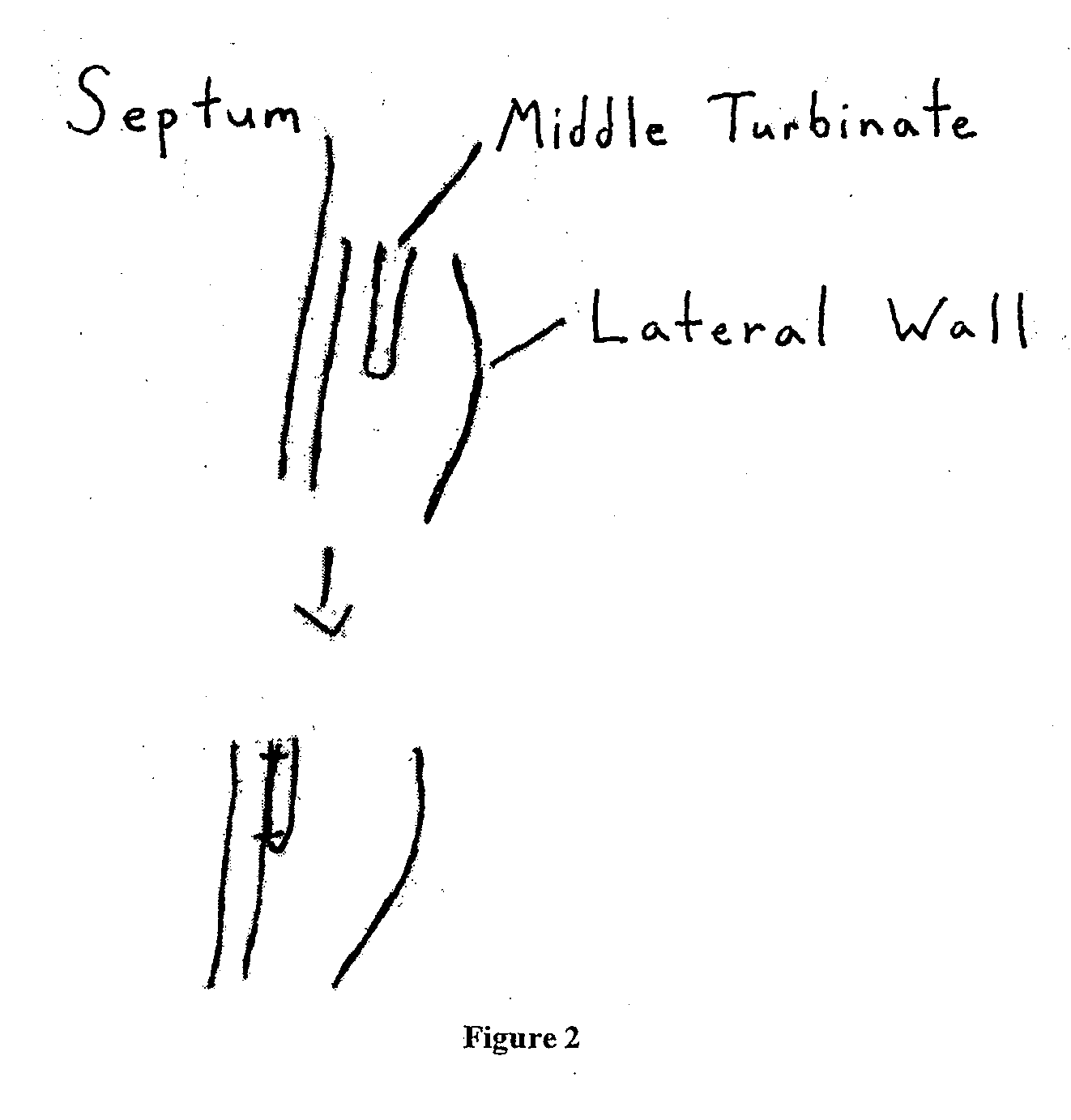
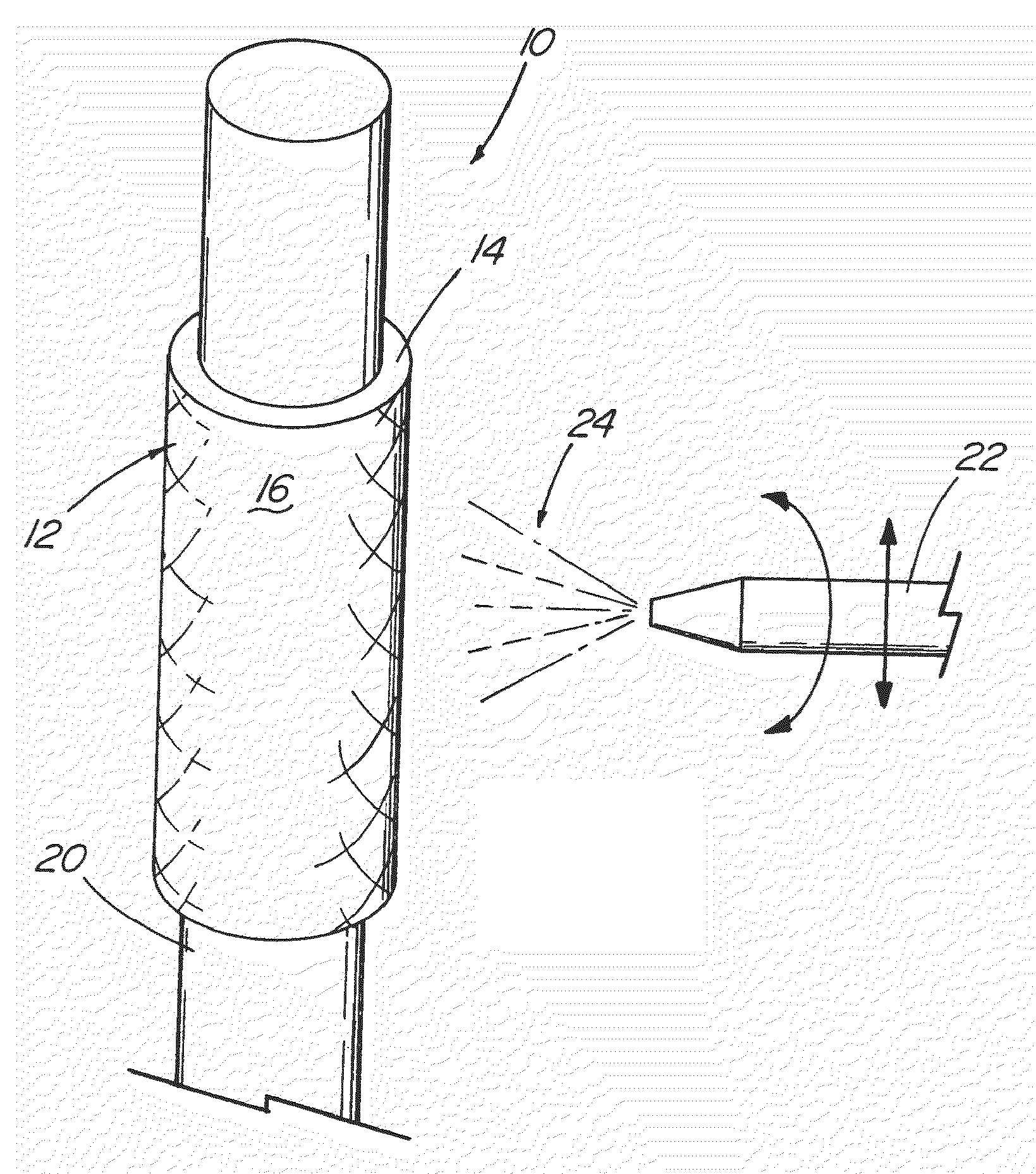
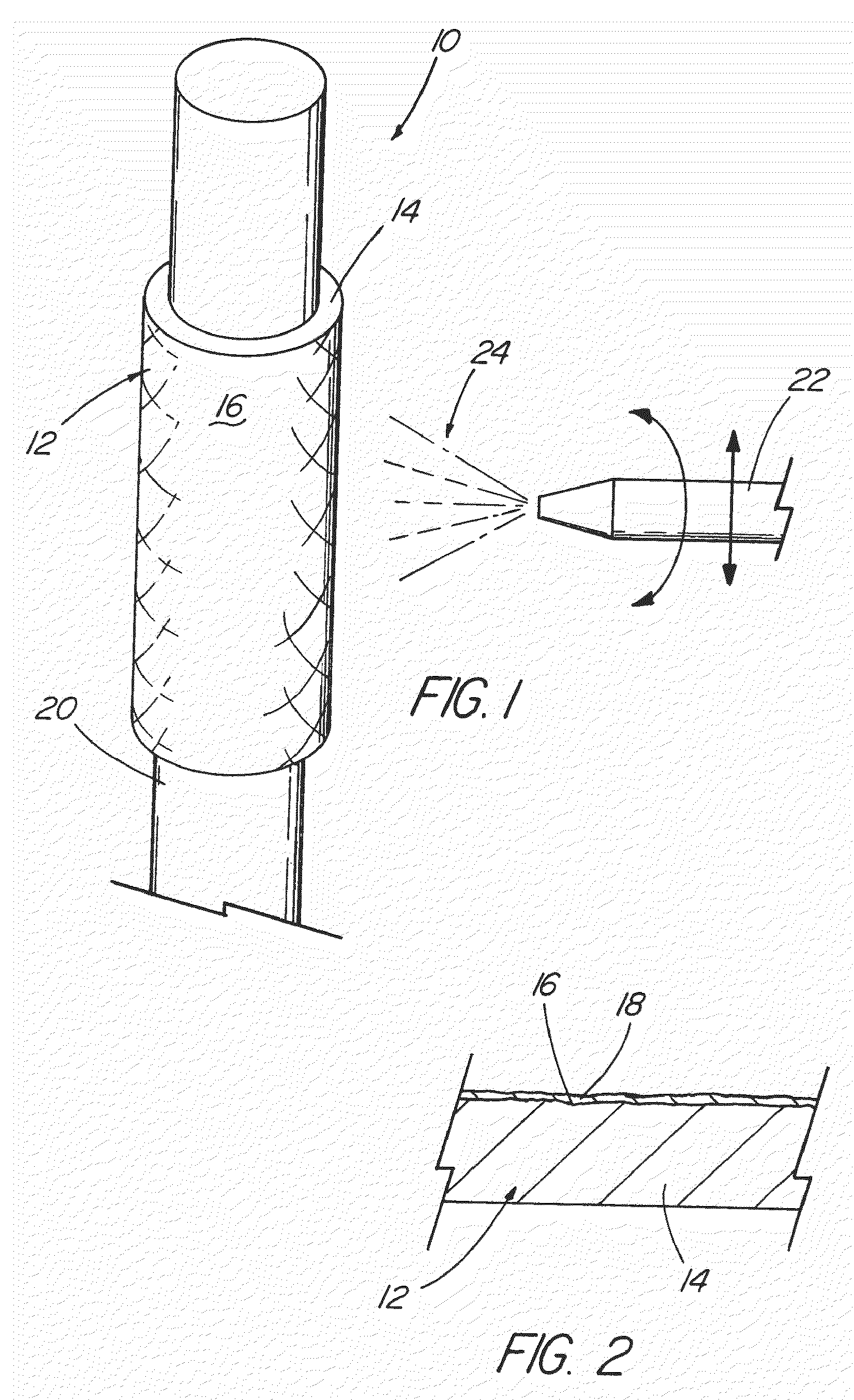
Middle Turbinate Medializer
InactiveUS20070293946A1Avoid stickingRestoring natural anatomySuture equipmentsDiagnosticsPalate muscleMiddle turbinates
Medializing the middle turbinate in the nose has been realized as a solution to the common complication of adhesions following nasal and sinus surgery. The invention provides a system for medializing the middle turbinate by attaching the middle turbinate temporarily to the nasal septum. The attachment is performed using a wafer with means on both sides for attaching the wafer to a mucosal surface. The attachment may also be performed using a tissue adhesive, pins, or other medical devices described herein. The invention also provides a system for attaching the uvula to the nasopharyngeal side of the soft palate. The invention provides a medical device for use in the inventive procedures as well as methods for the procedures and kits for use by a physician.
Owner:ARTHROCARE
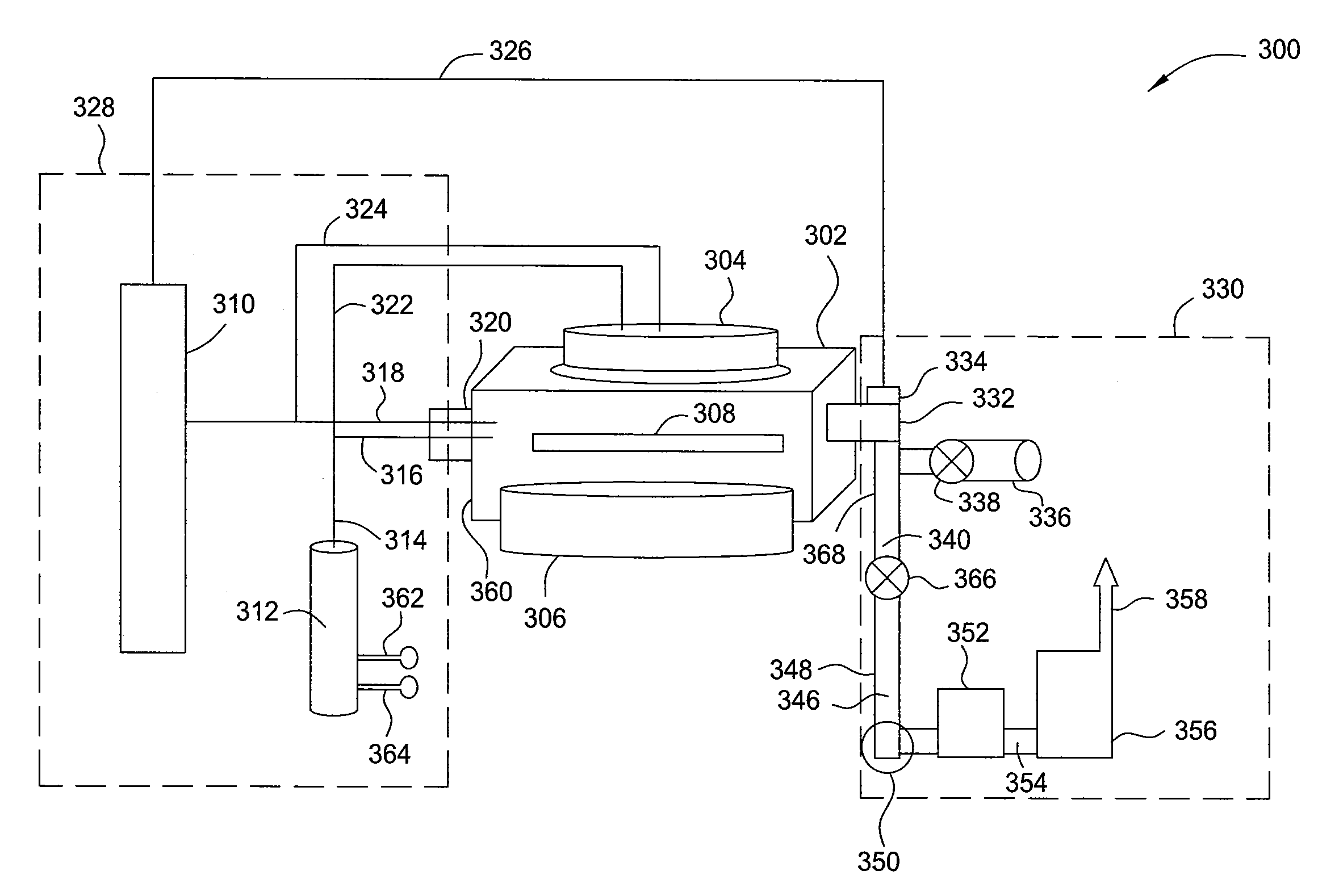
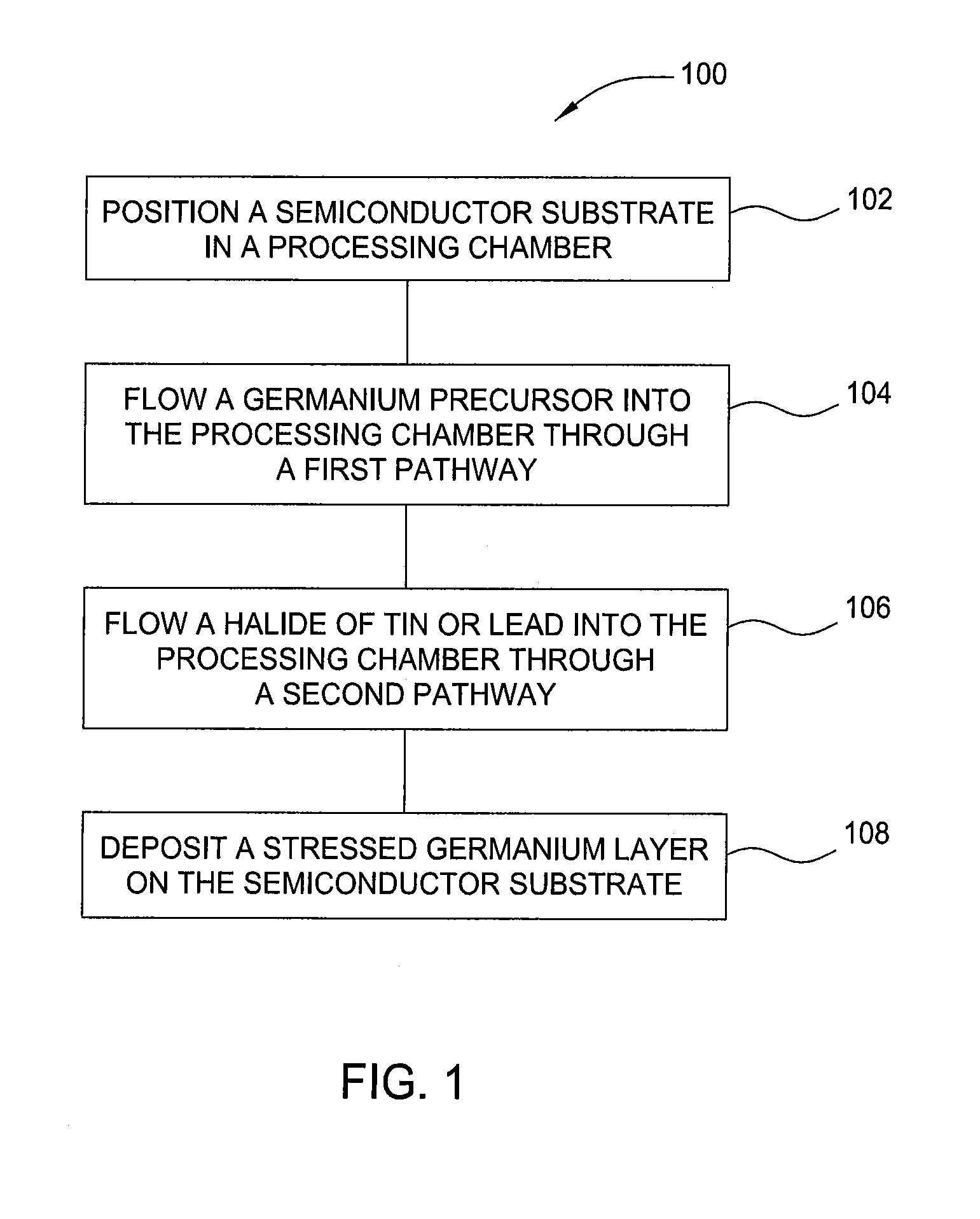
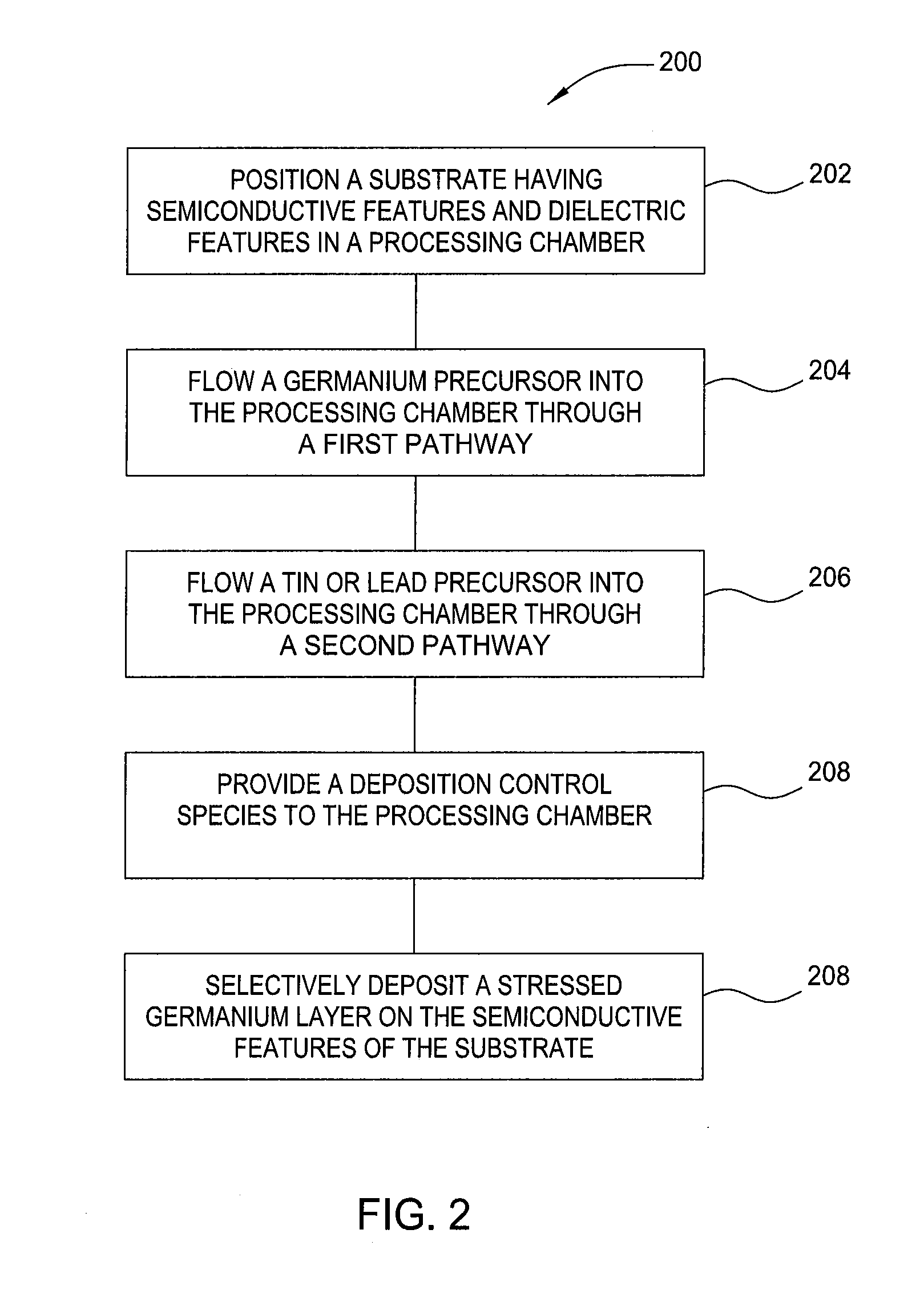
Method and apparatus for the selective deposition of epitaxial germanium stressor alloys
ActiveUS20120247386A1Reduce adhesionPolycrystalline material growthSemiconductor/solid-state device manufacturingHeterojunctionHalogen
A method and apparatus for forming heterojunction stressor layers is described. A germanium precursor and a metal precursor are provided to a chamber, and an epitaxial layer of germanium-metal alloy formed on the substrate. The metal precursor is typically a metal halide, which may be provided by subliming a solid metal halide or by contacting a pure metal with a halogen gas. The precursors may be provided through a showerhead or through a side entry point, and an exhaust system coupled to the chamber may be separately heated to manage condensation of exhaust components.
Owner:APPLIED MATERIALS INC
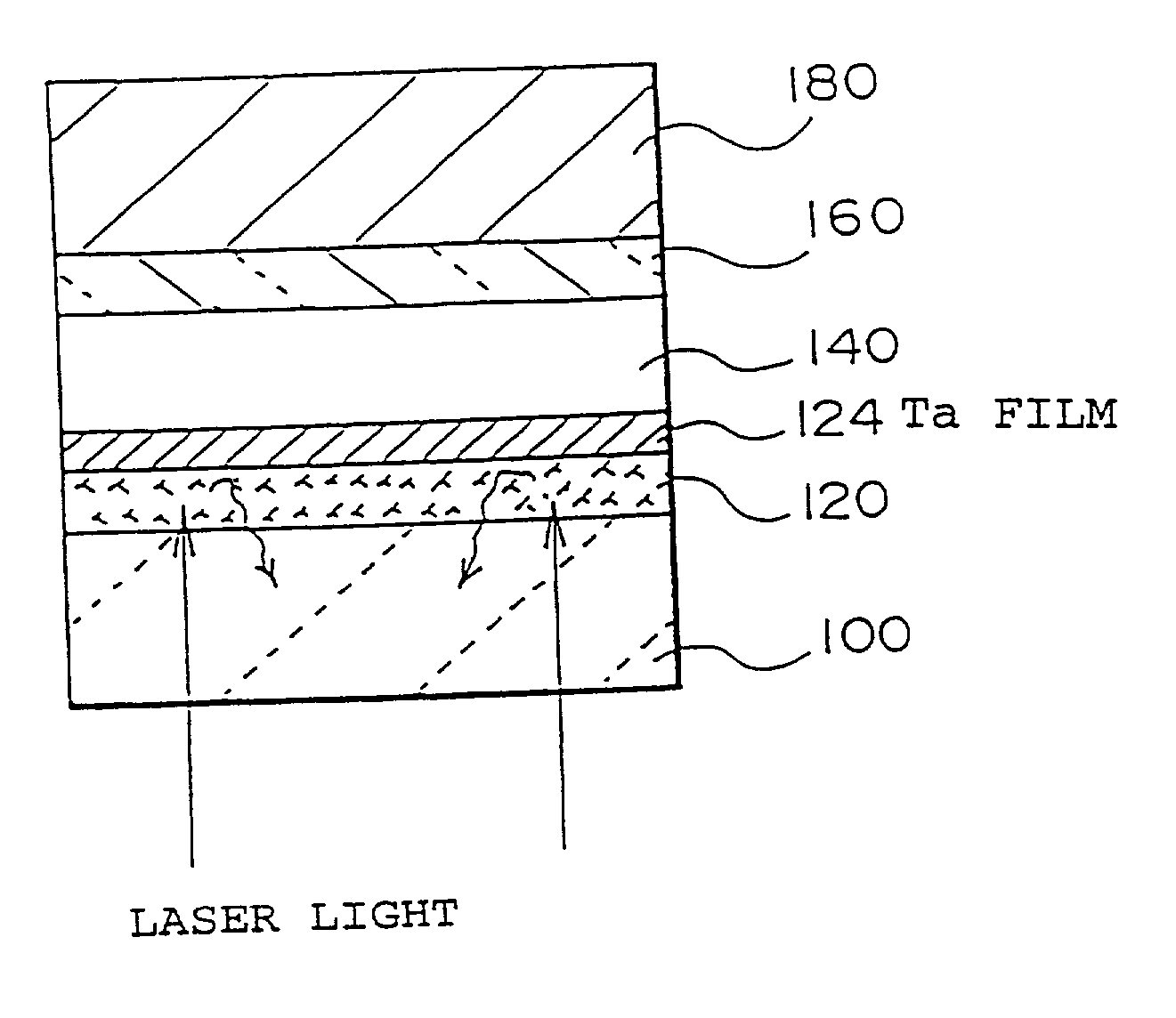
Imaged article on polymeric substrate
InactiveUS6203952B1Increasing the thicknessSmooth peelingAntenna supports/mountingsLoop antennasResistPolymer substrate
Patterned articles, such as RFID antenna, are made by subablation, a process comprising the steps of:A. providing a substrate having a coating, such as a metal or metal oxide, and an interface comprising the thin region where the coating and the substrate are closest to each other;B. exposing at least one part of the total area of the coating to a flux of electromagnetic energy, such as a focused excimer laser beam, sufficient to disrupt the interface but insufficient to ablate the coating; andC. removing the parts of the coating in registry with the portion of the interface area that was disrupted, by means such as ultrasonic agitation.The process has advantages over photo-resist processes in that there is no residual chemical resist left on the product and no undercutting of the pattern or image. It has advantages over laser ablation processes in that higher throughput is possible at the same energy level and there is no microscopic debris left on the product surface.
Owner:3M INNOVATIVE PROPERTIES CO
Gateway platform for biological monitoring and delivery of therapeutic compounds
InactiveUS20060253005A1Decrease adhesion and encapsulationImprove data qualityBioelectric signal measurementDrug and medicationsBiomonitoringComputer science
Owner:PHILOMETRON
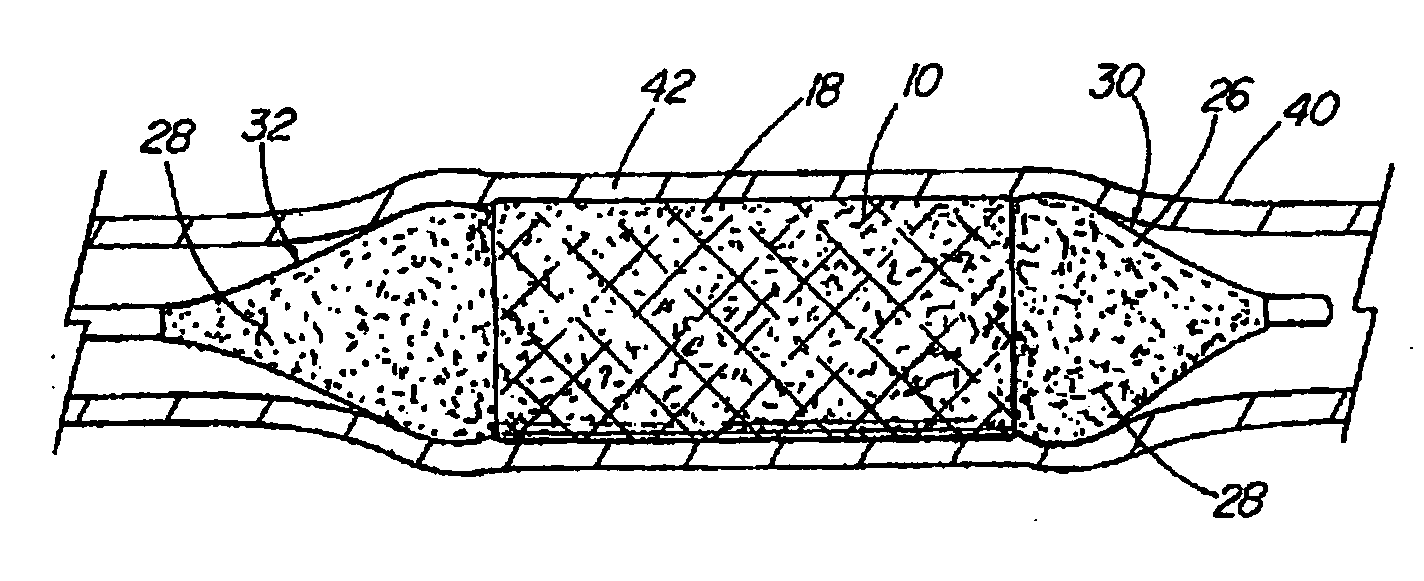
Coated medical device
A coated medical device (10) including a structure (12) adapted for introduction into a passage or vessel of a patient. The structure is formed of preferably a non-porous base material (14) having a bioactive material layer (18) disposed thereon. The medical device is preferably an implantable stent or balloon (26) of which the bioactive material layer is deposited thereon. The stent can be positioned around the balloon and another layer of the bioactive material posited over the entire structure and extending beyond the ends of the positioned stent. The ends of the balloon extend beyond the ends of the stent and include the bioactive material thereon for delivering the bioactive material to the cells of a vessel wall coming in contact therewith. The balloon further includes a layer of hydrophilic material (58) positioned between the base and bioactive material layers of the balloon.
Owner:COOK MEDICAL TECH LLC
Collagen binding protein compositions and methods of use
InactiveUS6288214B1Prevent and lessen adhesionReduce adhesionAntibacterial agentsPeptide/protein ingredientsPassive ImmunizationsCarrier protein
Disclosed are the cna gene and cna-derived nucleic acid segments from Staphylococcus aureus, and DNA segments encoding cna from related bacteria. Also disclosed are Col binding protein (CBP) compositions and methods of use. The CBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological infections, and in particular, for use in the prevention of bacterial adhesion to Col. DNA segments encoding these proteins and anti-(Col binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of bacterial colonization in an animal such as a human. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of S. aureus infection.
Owner:TEXAS A&M UNIVERSITY
Coated medical device
InactiveUS20090136560A1Convenient treatmentReduce adhesionStentsBalloon catheterMedical deviceBlood vessel
A coated medical device (10) including a structure (12) adapted for introduction into a passage or vessel of a patient. The structure is formed of preferably a non-porous base material (14) having a bioactive material layer (18) disposed thereon. The medical device is preferably an implantable stent or balloon (26) of which the bioactive material layer is deposited thereon. The stent can be positioned around the balloon and another layer of the bioactive material posited over the entire structure and extending beyond the ends of the positioned stent. The ends of the balloon extend beyond the ends of the stent and include the bioactive material thereon for delivering the bioactive material to the cells of a vessel wall coming in contact therewith. The balloon further includes a layer of hydrophilic material (58) positioned between the base and bioactive material layers of the balloon.
Owner:COOK MEDICAL TECH LLC
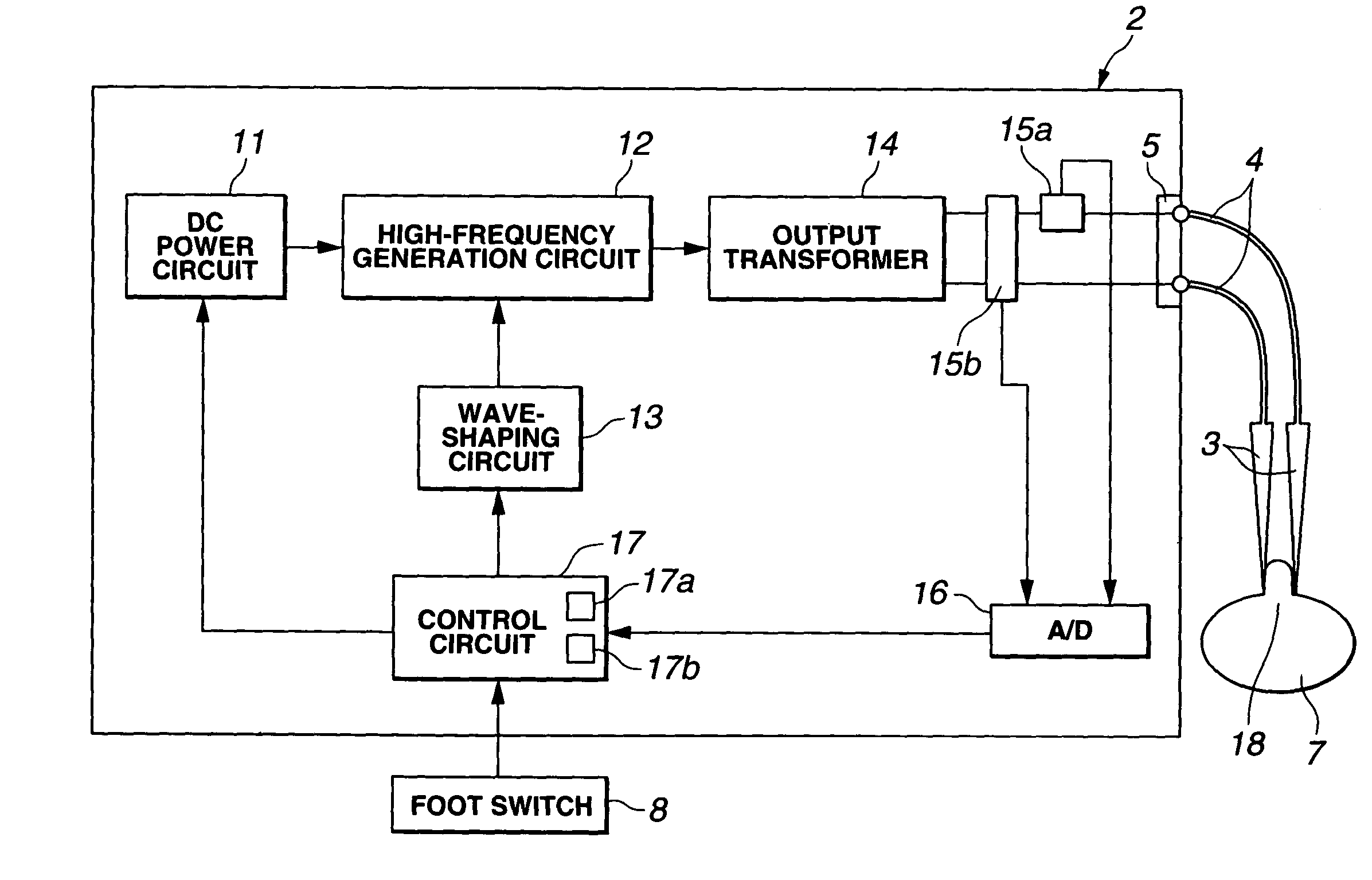
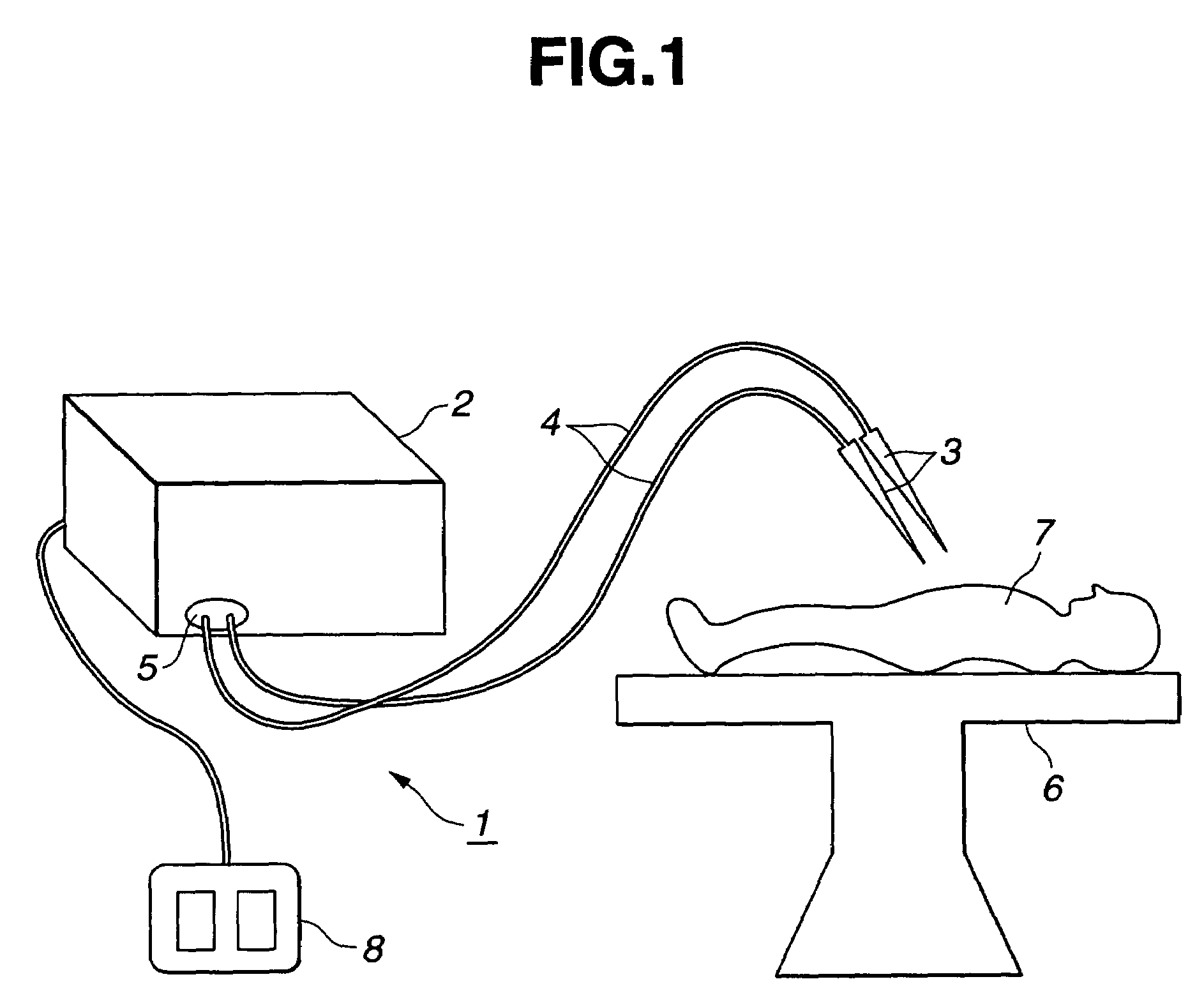
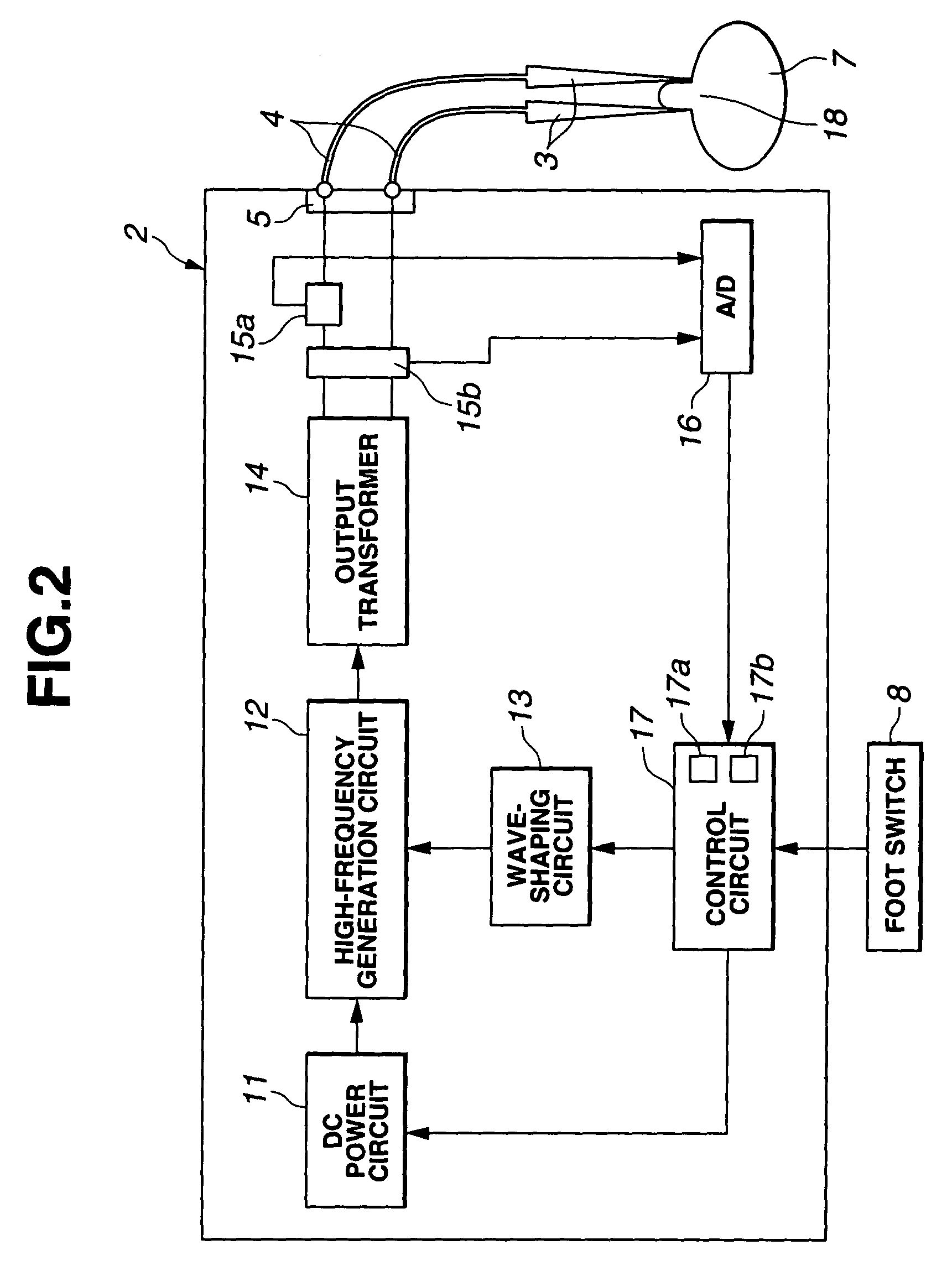
Electric operation apparatus
InactiveUS7172591B2Satisfactory coagulation abilityAvoid carbonizationControlling energy of instrumentSurgical instruments for heatingEngineeringControl circuit
When a user steps on a footswitch, high-frequency output power is delivered. A control circuit included in a diathermic power supply calculates the impedance ZSn that is offered by a living tissue immediately after delivery of high-frequency output power is started during the n-th delivery period. The control circuit also calculates the impedance ZEn that is offered thereby immediately before delivery of high-frequency output power is discontinued with elapse of predetermined time. The control circuit then discontinues delivery of high-frequency output power for the predetermined time, and calculates a difference ΔZn between the impedances. When the difference meets a predetermined condition that implies coagulation or when the number of times of delivery reaches a predetermined value, the control circuit discontinues delivery of high-frequency output power.
Owner:OLYMPUS CORP
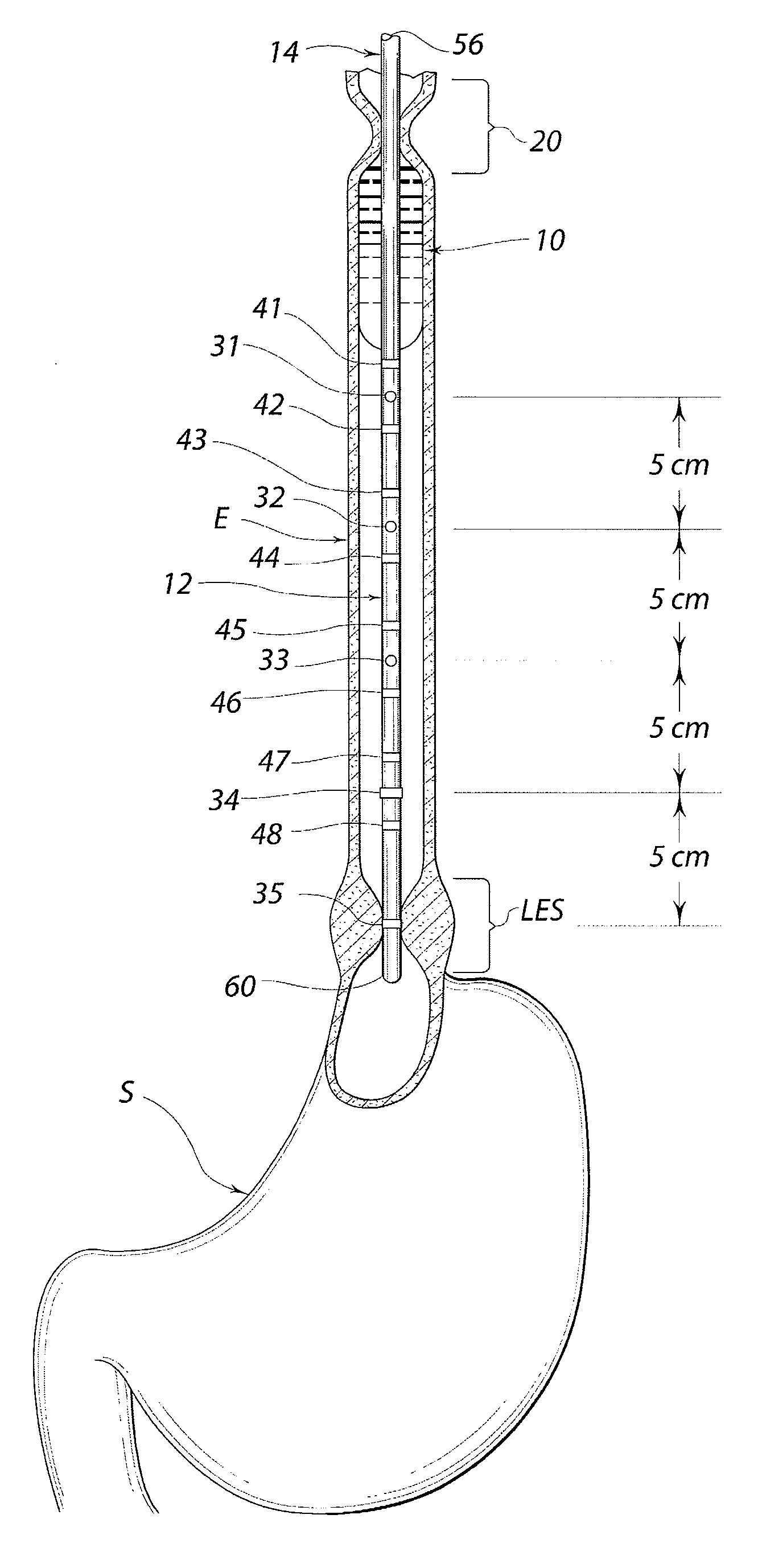
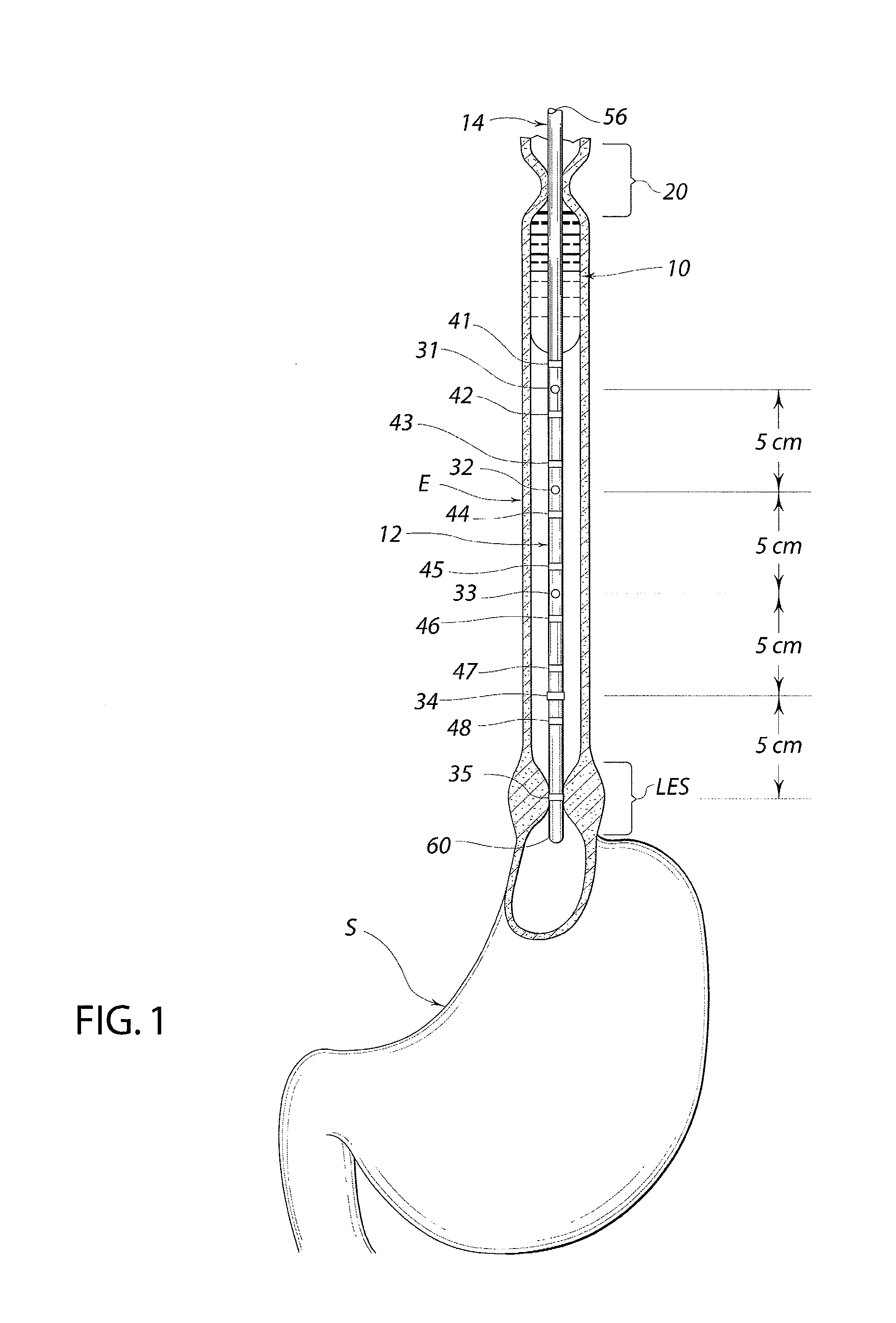
Standardized swallow challenge medium and method of use for esophageal function testing
ActiveUS7236820B2Long enough shelf lifeMeaningful resultDispersion deliverySurgeryMedicineEsophageal function
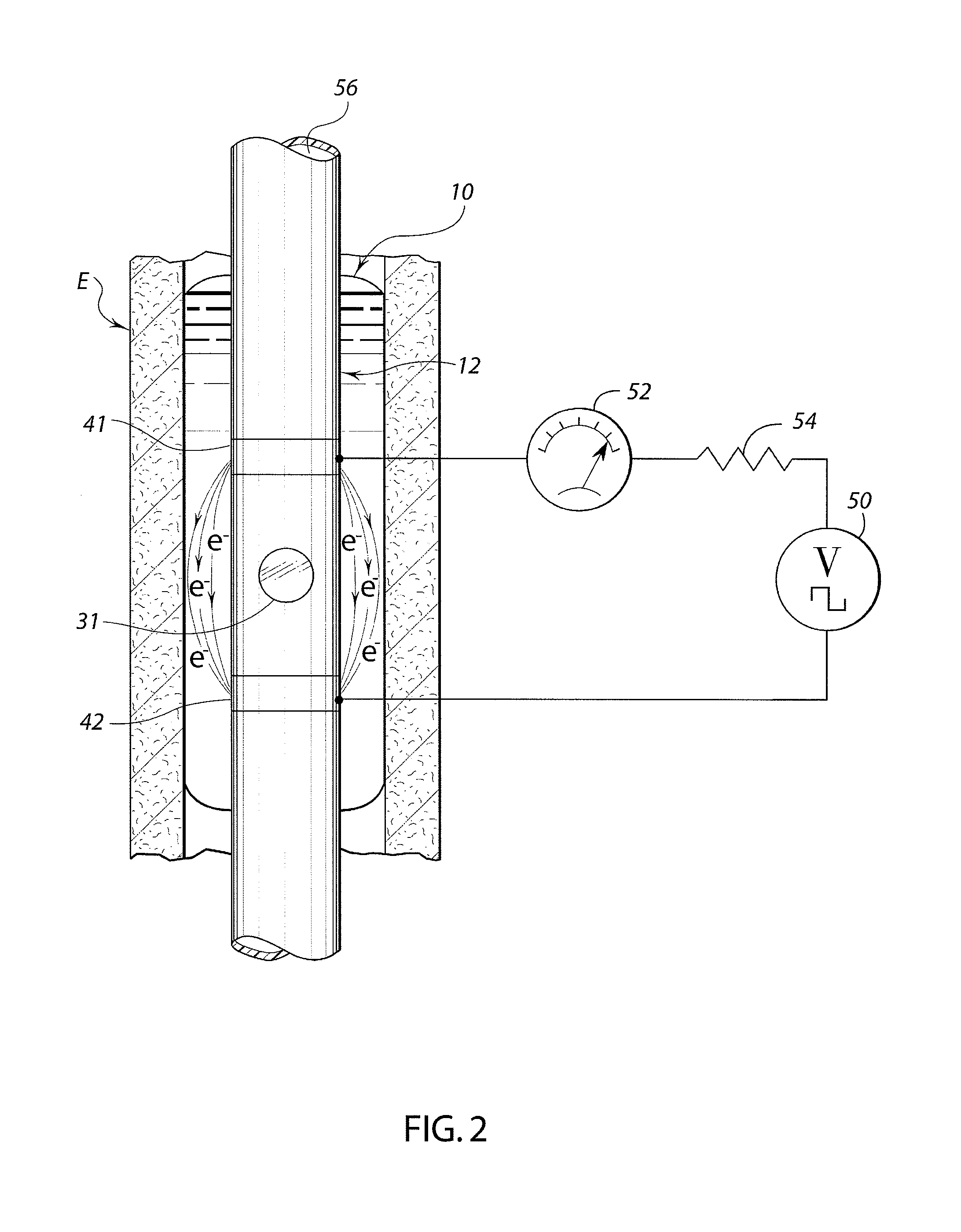
A swallow challenge medium (10) is thixotropic for easy swallowing and to provide enough viscosity for effective challenge to peristalsis (20) and has high ionic density for effective impedance measurements by contact with electrodes (41–48) positioned in a person's esophagus (E) or oropharynx during swallow testing. The medium (10) also has a high surface tension so as not to adhere to or coat the electrodes (41–48) or probe (12) surfaces. These physical characteristics are stabilized and consistent enough to provide standard for esophageal and / or oropharyngeal function testing and diagnostics.
Owner:DIVERSATEK HEALTHCARE INC
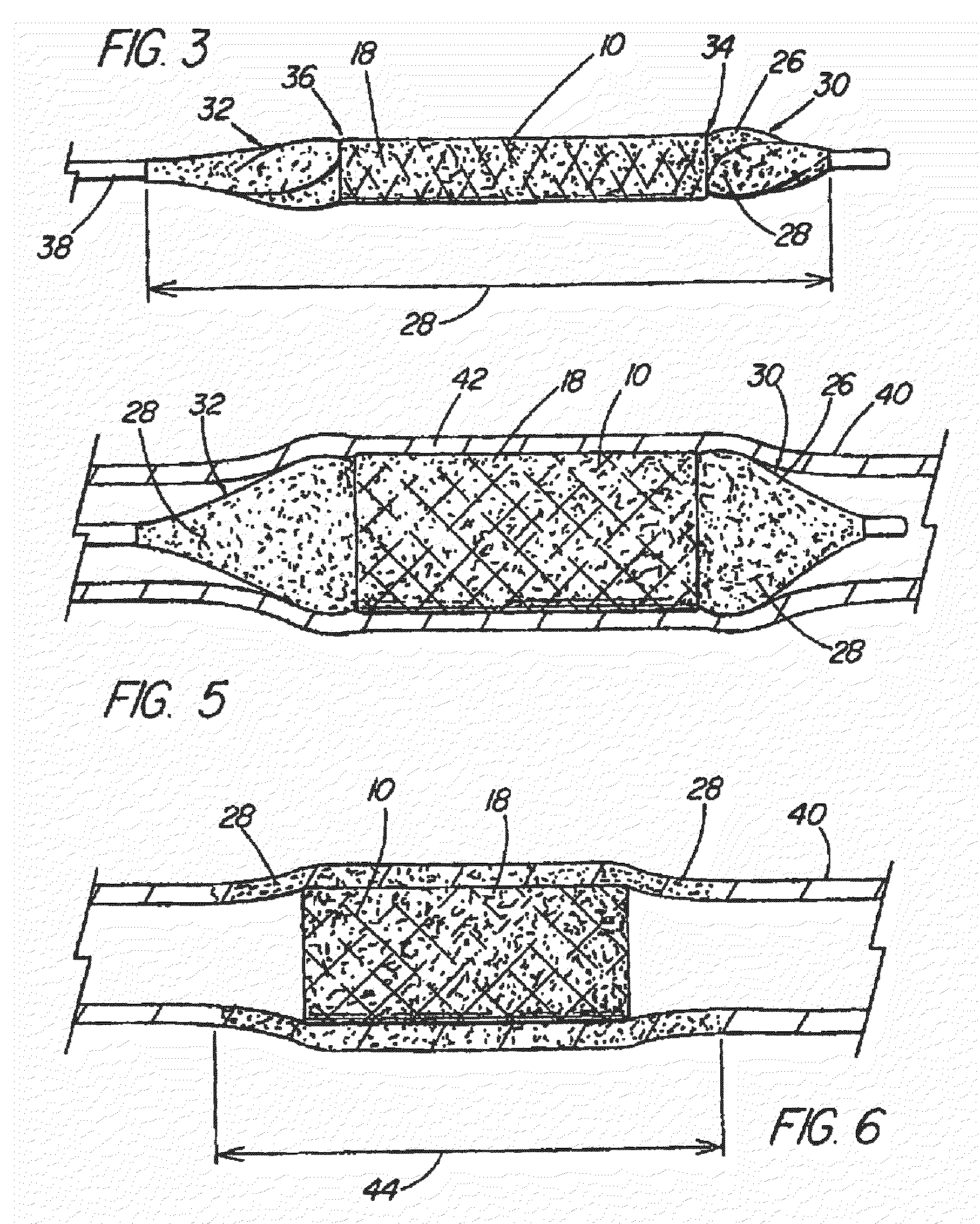
Multiple member interconnect for surgical instrument and absorbable screw fastener
ActiveUS20060241622A1Improve stabilityAvoid displacementSuture equipmentsLigamentsSurgical departmentSurgical Fasteners
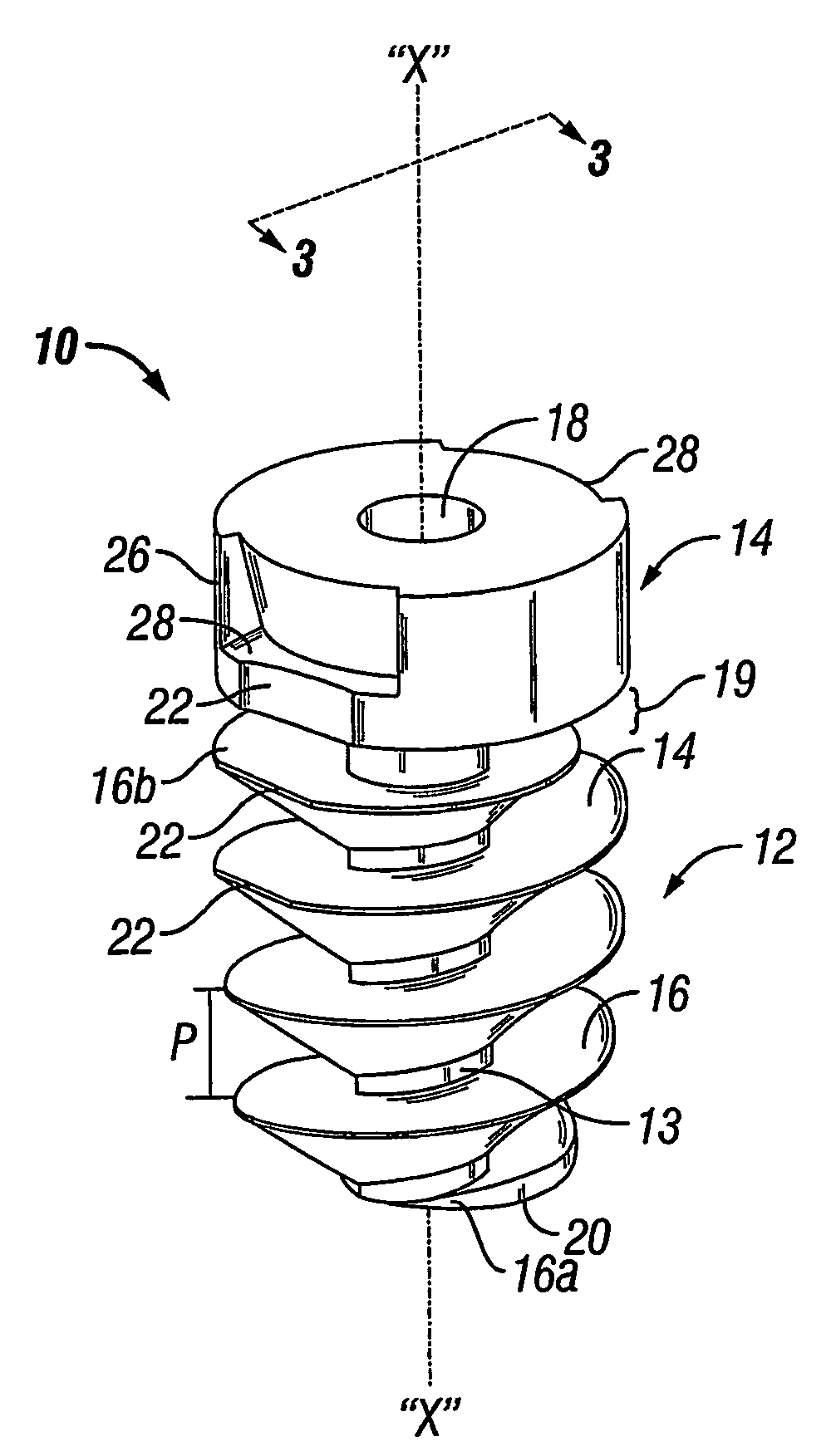
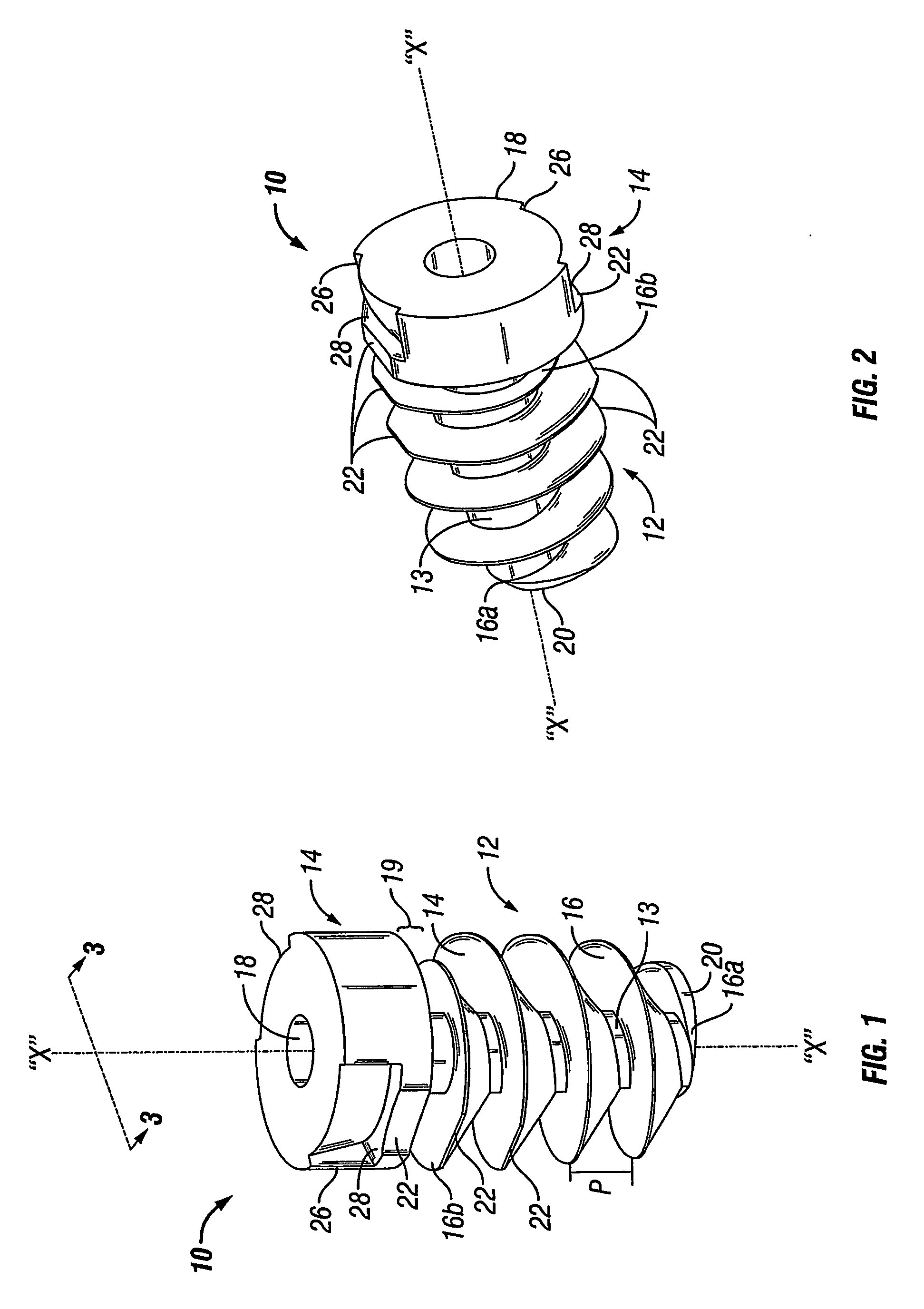
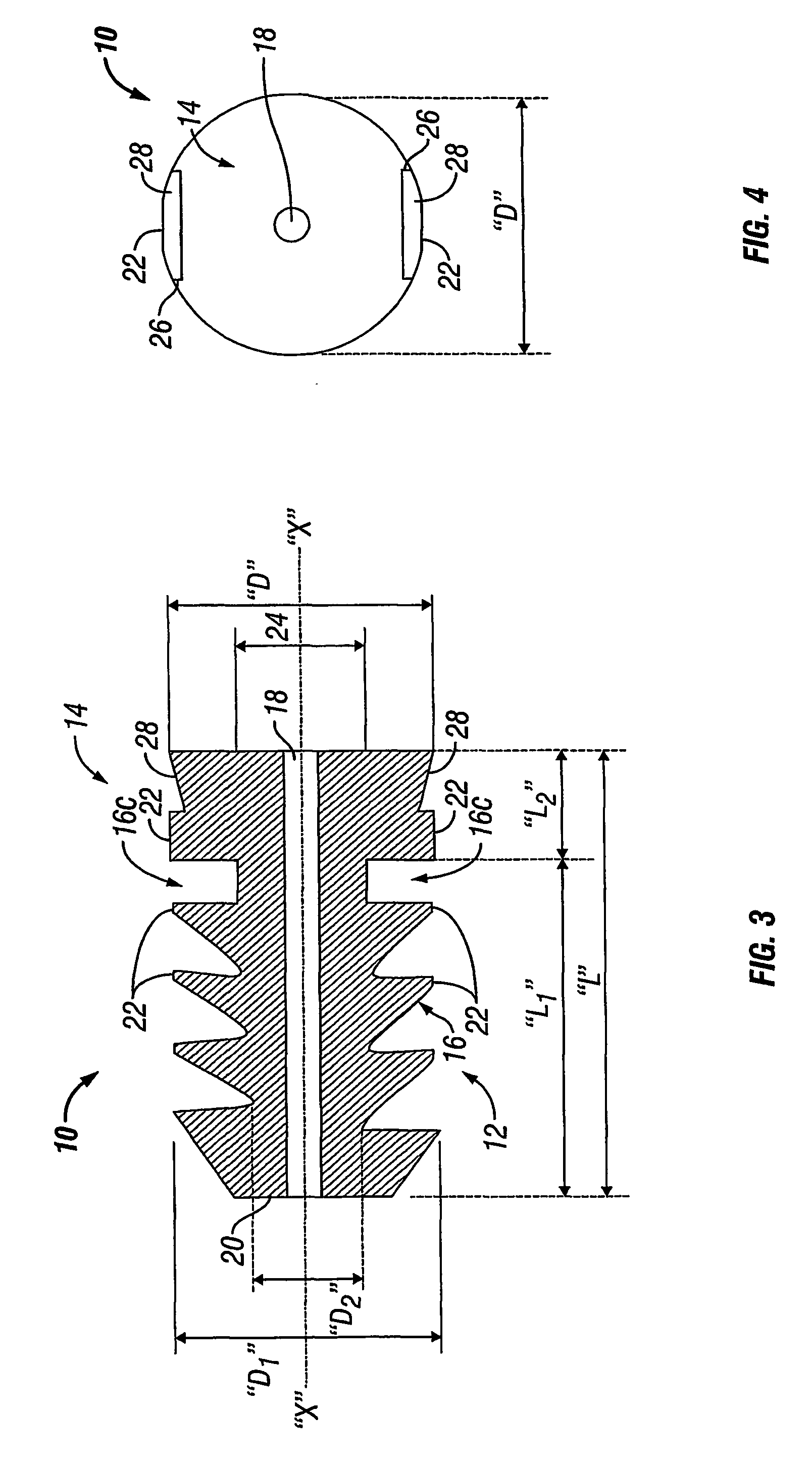
An absorbable screw fastener and a method of firing with an applicator capable of applying a surgical fastener to tissue in order to form tissue connection to secure objects to tissue, the fastener including a body portion having a helical thread, a head portion disposed at the proximal end of the body portion. The head portion includes a driver receiving configuration on its outer surface. The screw fastener further includes a cannulated center lumen with an opening extending from the head portion through the longitudinal length of the body portion.
Owner:TYCO HEALTHCARE GRP LP
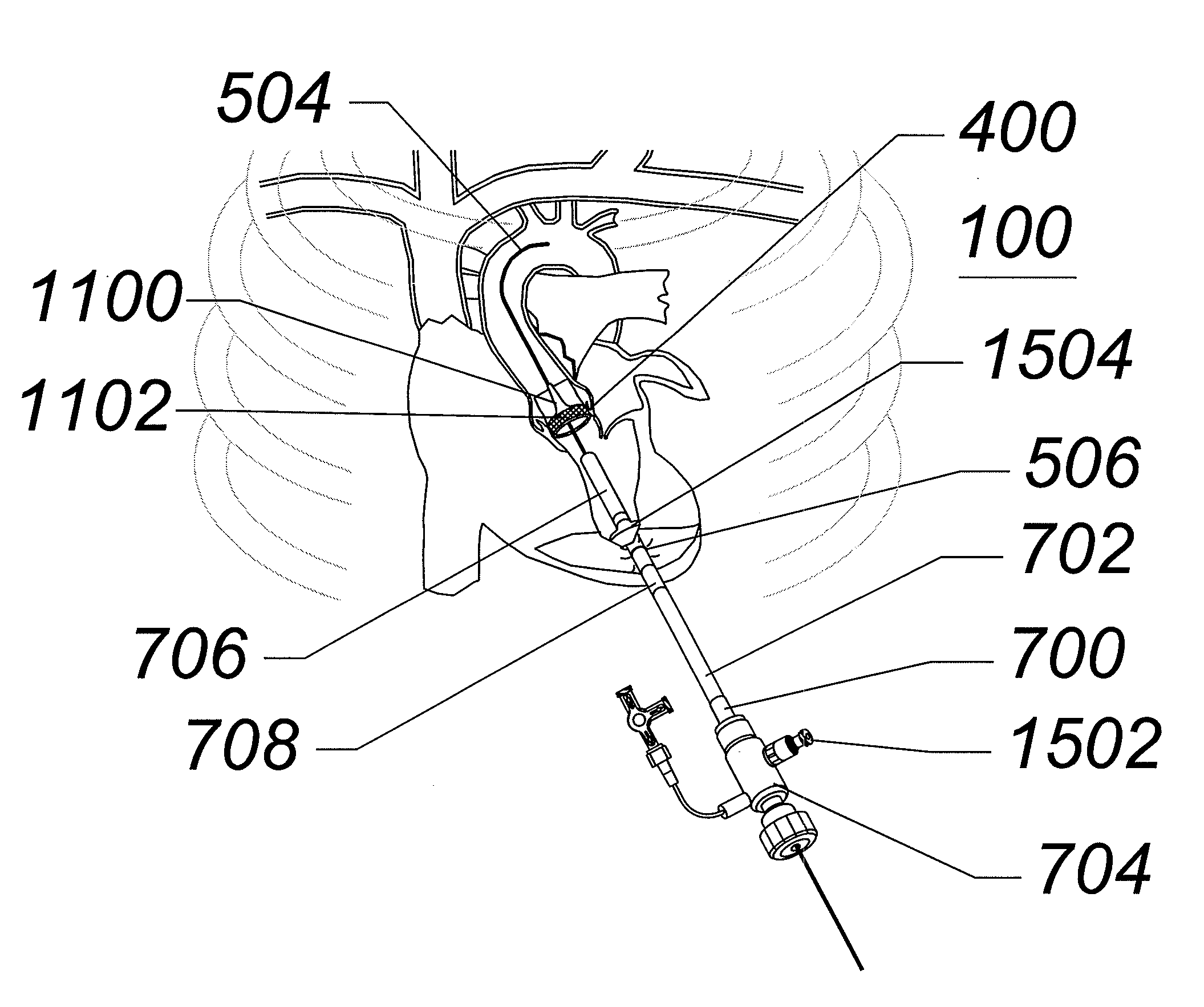
Expandable transapical sheath and method of use
Disclosed is an expandable transluminal sheath, for introduction into the body while in a first, small cross-sectional area configuration, and subsequent expansion of at least a part of the distal end of the sheath to a second, enlarged cross-sectional configuration. The sheath is configured for use in the vascular system and has utility in the introduction and removal of implant delivery catheters. The access route is through the ventricular myocardium, more specifically at the left ventricular apex, into the aortic root. The distal end of the sheath is maintained in the first, low cross-sectional configuration during advancement to the arteries into the aorta. The distal end of the sheath is subsequently expanded using a radial dilatation device, which is removed prior to the introduction of implant delivery catheters. In an exemplary application, the sheath includes a supported proximal end, a supported distal end, and a collapsible center section. Certain configurations of the sheath are capable of being inserted in a first, small cross-sectional configuration, being expanded diametrically to a second, larger cross-sectional configuration, and then being reduced to a diametrically small size for removal.
Owner:ONSET MEDICAL CORP
Multiple member interconnect for surgical instrument and absorbable screw fastener
ActiveUS7670362B2Improve stabilityAvoid displacementSuture equipmentsLigamentsEngineeringSurgical department
Owner:COVIDIEN LP
Particles, compositions and methods for ophthalmic and/or other applications
Particles, compositions, and methods that aid particle transport in mucus are provided. The particles, compositions, and methods may be used, in some instances, for ophthalmic and / or other applications. In some embodiments, the compositions and methods may involve modifying the surface coatings of particles, such as particles of pharmaceutical agents that have a low aqueous solubility. Such compositions and methods can be used to achieve efficient transport of particles of pharmaceutical agents though mucus barriers in the body for a wide spectrum of applications, including drug delivery, imaging, and diagnostic applications. In certain embodiments, a pharmaceutical composition including such particles is well-suited for ophthalmic applications, and may be used for delivering pharmaceutical agents to the front of the eye and / or the back of the eye.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Compositions for prevention of adhesions and other barrier applications
ActiveUS20080032934A1Minimizing contaminationMinimizing infectionBiocideNervous disorderNucleotideMedicinal chemistry
A method has been developed of preventing or limiting formation of adhesions by administering to a site in need thereof, in the absence of or after bleeding or leakage of fluid has been substantially stopped, a self-assembling material which forms a barrier to formation of adhesions. In one embodiment, the self-assembling material comprises peptides having a sequence of amino acid residues conforming to one or more of Formulas I-IV: ((Xaa°′−Xaa+)x(Xaane1_Xaa−)y)n (I); ((Xaa′_Xaa)x(Xaa°e°−Xaa+)y)n (II); ((Xaa+−Xaa″e1)x(Xaa−Xaa″)y)n (III); and ((Xaa−XaaQe7)x(Xaa+Xaa1e″)y)n (IV), where Xaan′ represents an amino acid residue having a neutral charge; Xaa+ represents an amino acid residue having a positive charge; Xaa represents an amino acid residue having a negative charge; x and y are integers having a value of 1, 2 or 4, independently; and n is an integer having a value of 1-5. In another embodiment, the self assembling materials are peptidomimetics, nucleotidomimetics, di- and triblock copolymers, N-alkylacrylamides, or dendimers. These materials are also useful in a method for regeneration or repair of tissue or cells forming tissue.
Owner:ARCH BIOSURGERY
Hemostatic compositions, assemblies, systems, and methods employing particulate hemostatic agents formed from hydrophilic polymer foam such as chitosan
ActiveUS20070021703A1Safe and effective deliveryIncrease ratingsPharmaceutical delivery mechanismAbsorbent padsParticulatesPolymer
Improved hemostatic agents take the form of granules or particles that can be used to stanch, seal, or stabilize a site of hemorrhage, including a noncompressible hemorrhage.
Owner:TRICOL BIOMEDICAL INC
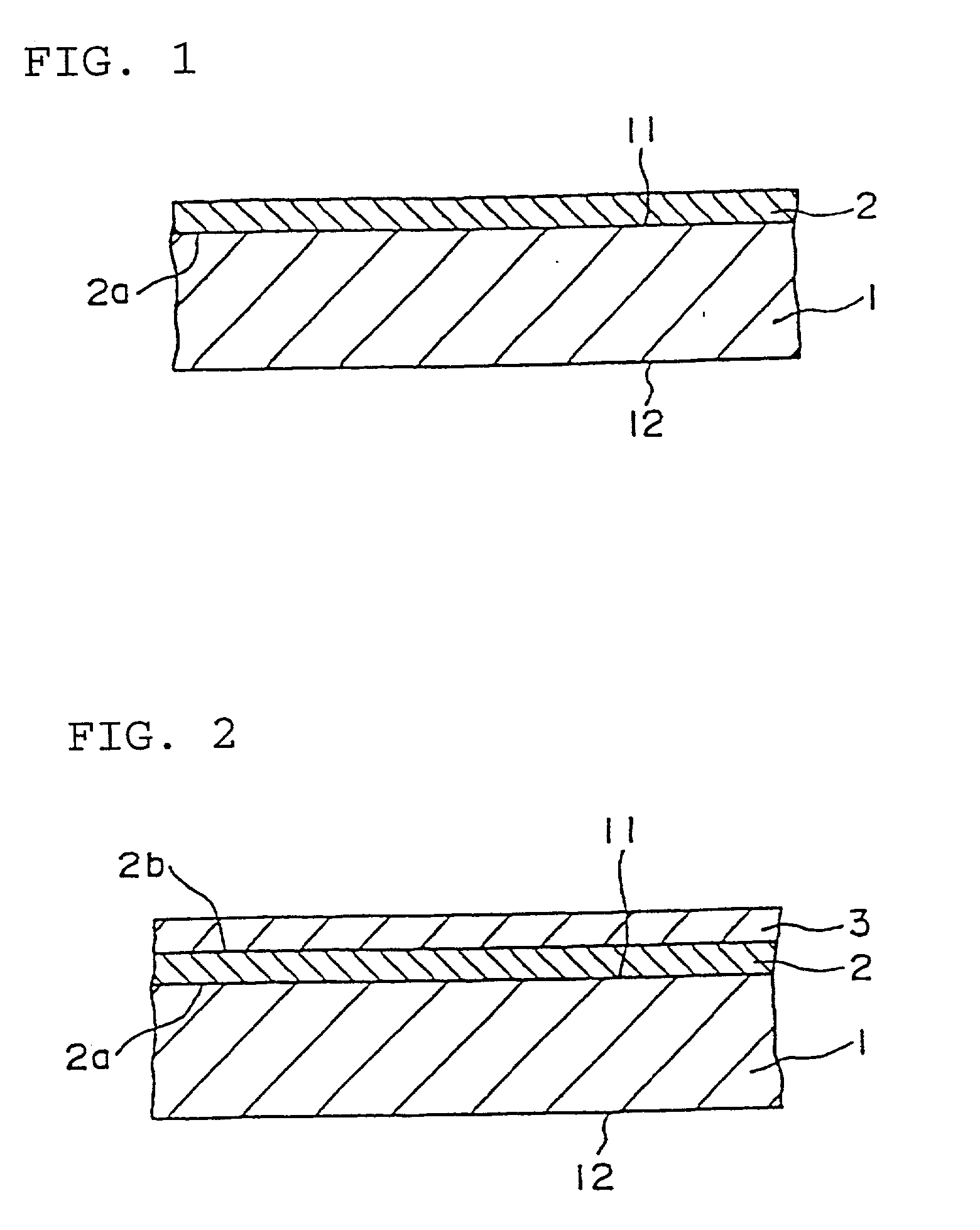
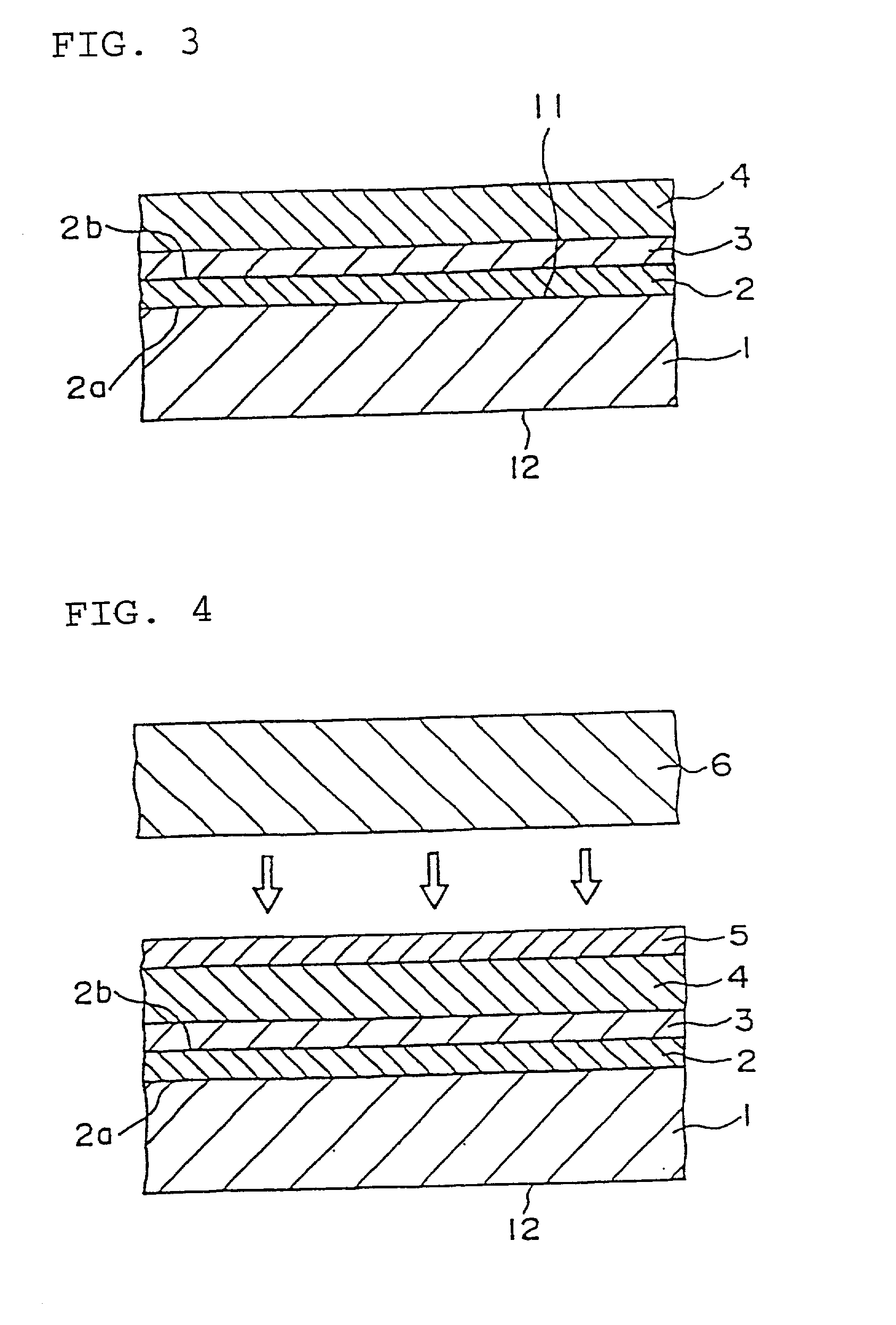
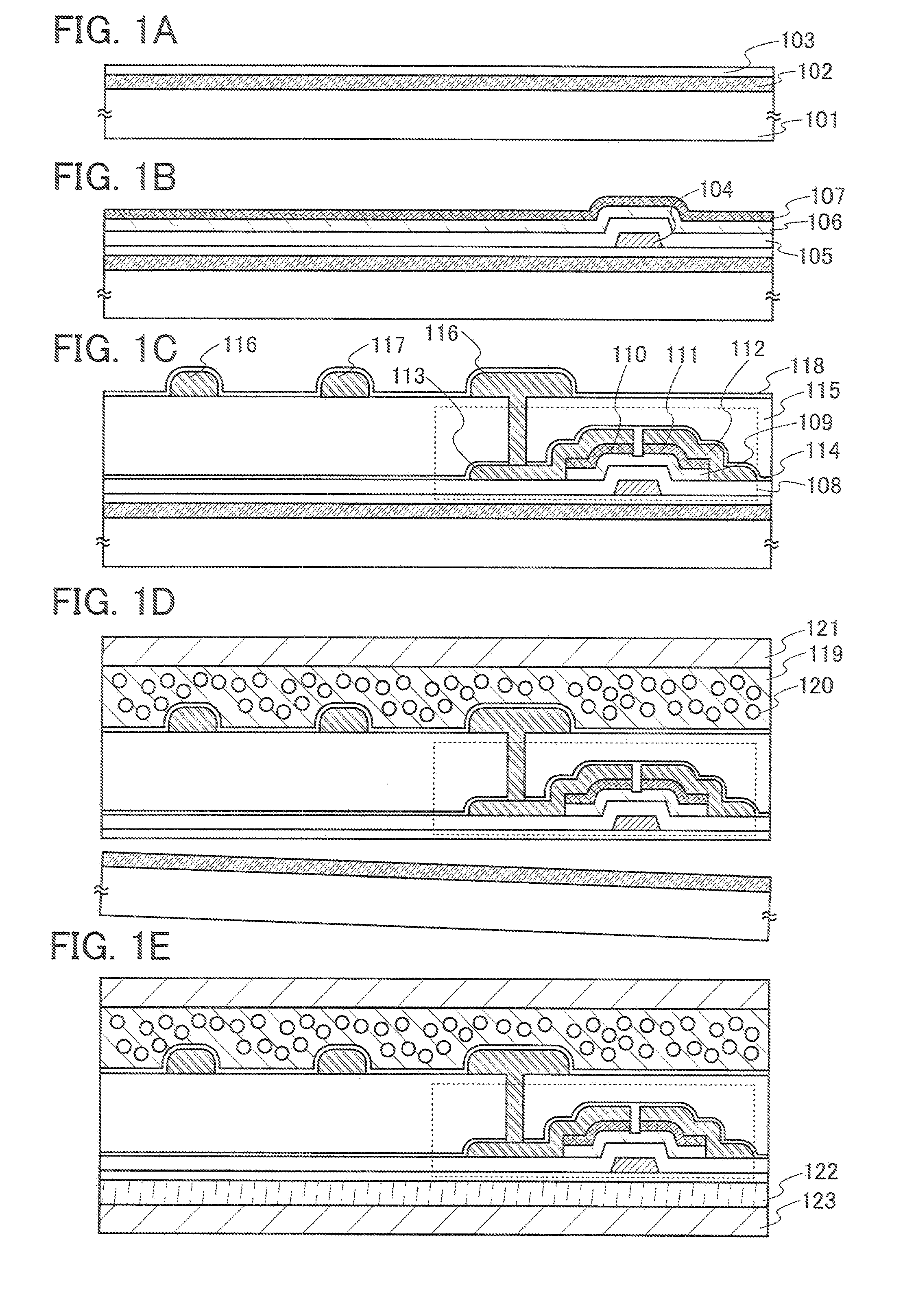
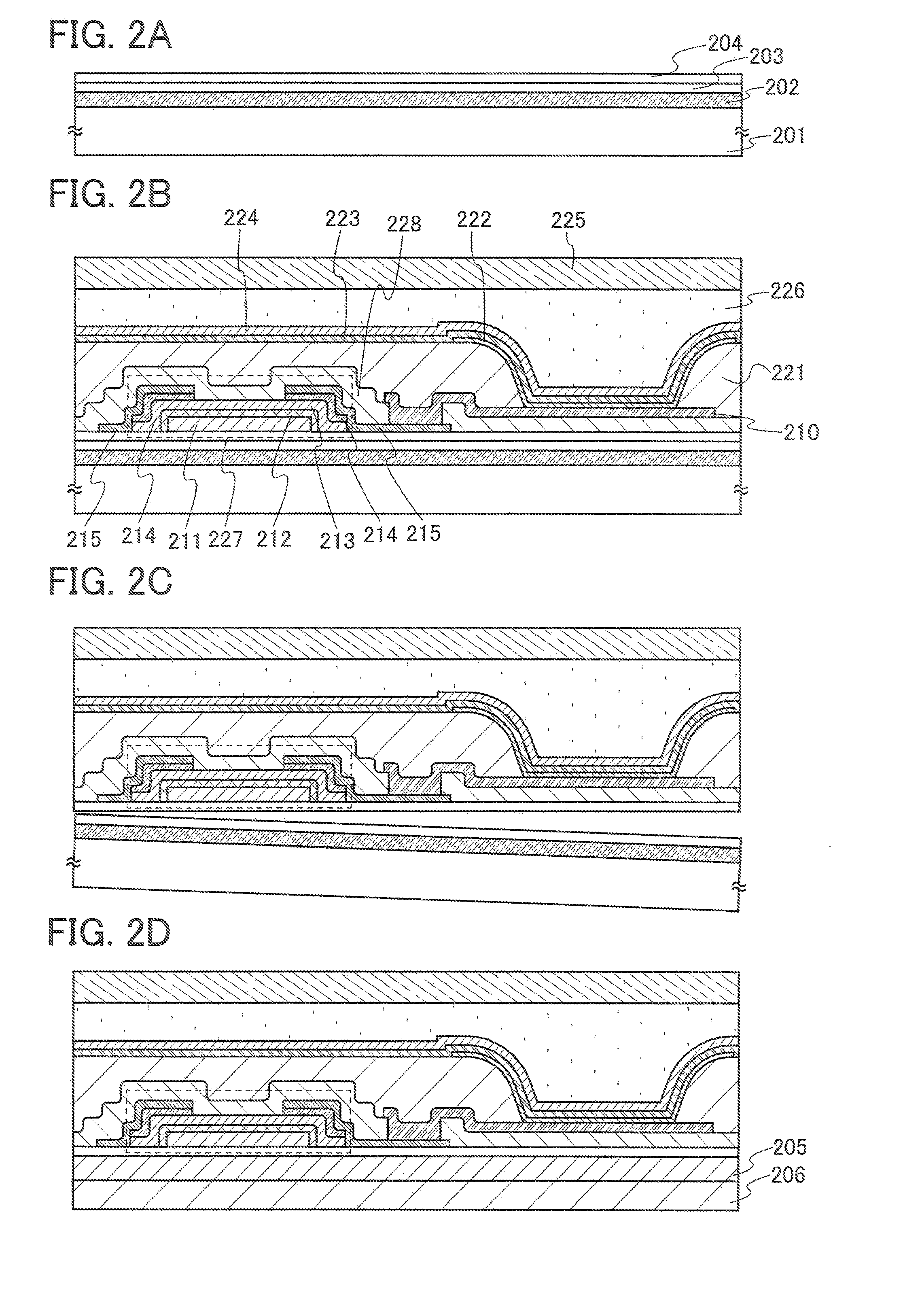
Semiconductor device and method of manufacturing the same
InactiveUS7091070B2Enhanced adhesivenessPrevent peelingTransistorSolid-state devicesIntegrated circuitEngineering
To provide a method for manufacturing a semiconductor device including a transfer step that is capable of controlling the adhesiveness of a substrate and an element-formed layer in the case of separating the element-formed layer including a semiconductor element or an integrated circuit formed over the substrate from the substrate and bonding it to another substrate. An adhesive agent made of a good adhesiveness material is formed between the semiconductor element or the integrated circuit comprising plural semiconductor elements formed over the substrate (a first substrate) and the substrate, and thus it is possible to prevent a semiconductor element from peeling off a substrate in manufacturing the semiconductor element, and further, to make it easier to separate the semiconductor element from the substrate by removing the adhesive agent after forming the semiconductor element.
Owner:SEMICON ENERGY LAB CO LTD
Method of using water-in-oil emulsion to remove oil base or synthetic oil base filter cake
ActiveUS7481273B2Improve breathabilityHighly efficient in breakingCleaning apparatusScale removal and water softeningParticulatesParaffin wax
Fluid producing or injecting wells may be treated with a water-in-oil emulsion for the removal or inhibition of unwanted particulates, including pipe dope, asphaltenes and paraffins. In addition, such emulsions are effective in the displacement of oil base drilling muds and / or residues from such muds from wells. The emulsion may also be used to break the interfacial and / or rheological properties of oil base mud and synthetic oil base mud filter cakes, and act as a demulsifier to break the water-in-oil emulsion present in such oil base and synthetic oil base muds. The water-in-oil emulsions may optionally contain a dispersing agent as well as a surfactant.
Owner:BAKER HUGHES INC
Low peel adhesive
InactiveUS20130084459A1Sufficient shear propertyLow peel adhesionLamination ancillary operationsSynthetic resin layered productsOligomerAdhesive
A curable adhesive composition is disclosed comprising: at least one free radically polymerizable oligomer component; optionally at least one diluent monomer; at least one perfluorinated ether monomer; and a photoinitiator. The curable liquid adhesive is easily removable with low force and low adhesive transfer.
Owner:3M INNOVATIVE PROPERTIES CO
Particles, compositions and methods for ophthalmic and/or other applications
InactiveUS20130316001A1Improves ocular bioavailabilityImprove concentrationNanotechSenses disorderDrug deliveryOphthalmology
Particles, compositions, and methods that aid particle transport in mucus are provided. The particles, compositions, and methods may be used, in some instances, for ophthalmic and / or other applications. In some embodiments, the compositions and methods may involve modifying the surface coatings of particles, such as particles of pharmaceutical agents that have a low aqueous solubility. Such compositions and methods can be used to achieve efficient transport of particles of pharmaceutical agents though mucus barriers in the body for a wide spectrum of applications, including drug delivery, imaging, and diagnostic applications. In certain embodiments, a pharmaceutical composition including such particles is well-suited for ophthalmic applications, and may be used for delivering pharmaceutical agents to the front of the eye and / or the back of the eye.
Owner:ALCON INC
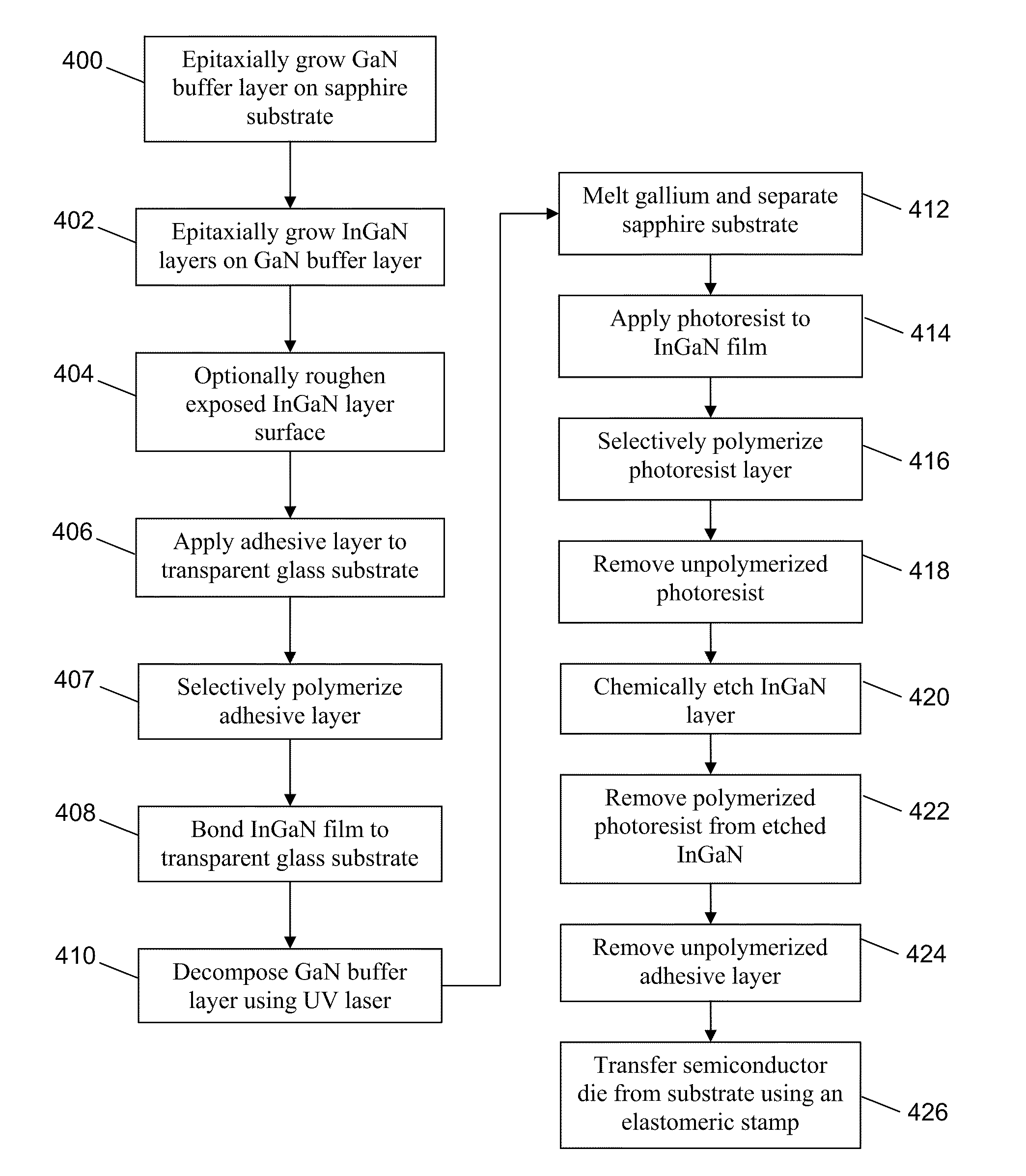
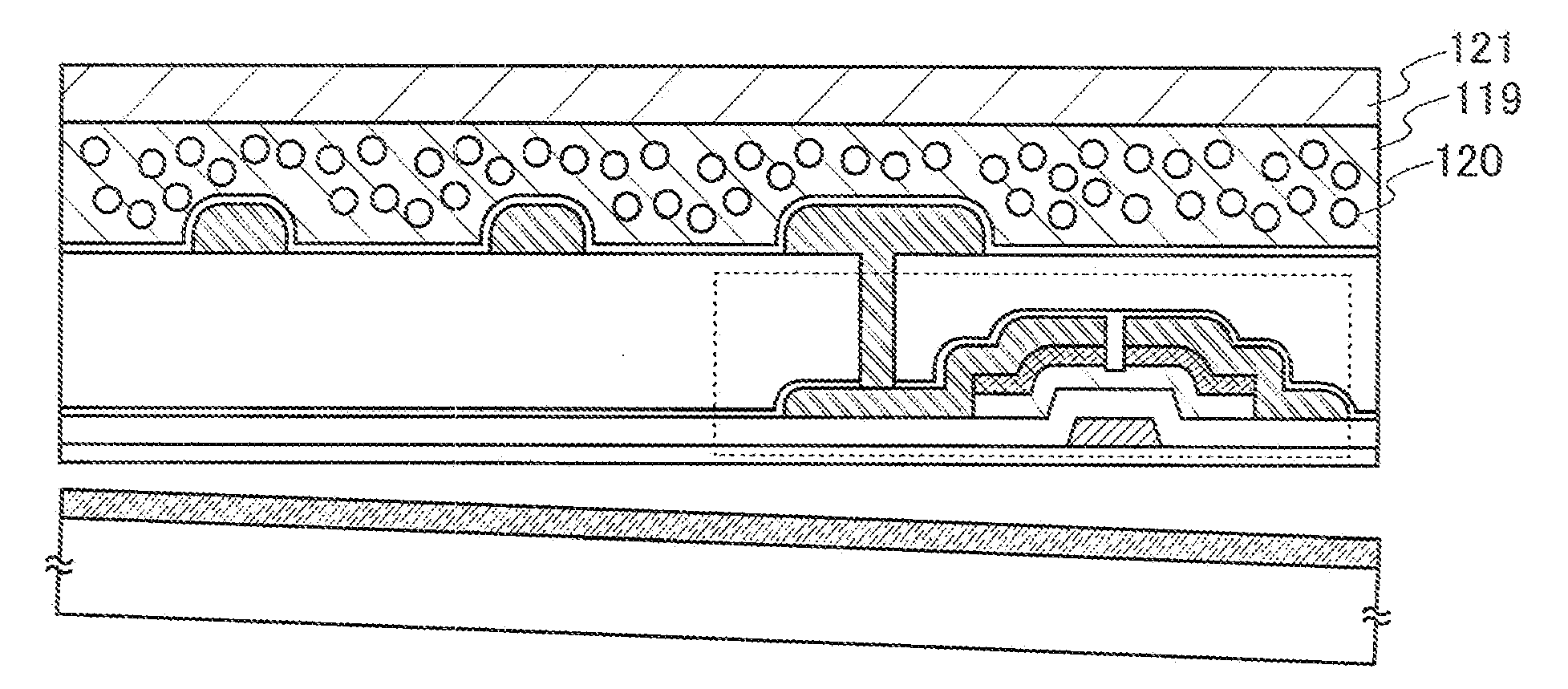
Method for manufacturing semiconductor device
ActiveUS20070254456A1Process stabilityPoor heat resistanceFinal product manufactureSemiconductor/solid-state device detailsEngineeringPlastic film
A technique for peeling an element manufactured through a process at relatively low temperature (lower than 500° C.) from a substrate and transferring the element to a flexible substrate (typically, a plastic film). With the use of an existing manufacturing device for a large glass substrate, a molybdenum film (Mo film) is formed over a glass substrate, an oxide film is formed over the molybdenum film, and an element is formed over the oxide film through a process at relatively low temperature (lower than 500° C.). Then, the element is peeled from the glass substrate and transferred to a flexible substrate.
Owner:SEMICON ENERGY LAB CO LTD
Method for finishing bacterium fixation by utilizing polyvinyl alcohol-borate secondary crosslinking
The invention relates to a method for finishing bacterium fixation by utilizing polyvinyl alcohol-borate secondary crosslinking, belonging to the field of water treatment. The method comprises the steps of: after mixing polyvinyl alcohol and bacteria in proportion, putting the mixture into saturated borate solution and carrying out first crosslinking fixation, after the fixation, taking out supernate and regulating pH of the supernate, carrying out second crosslinking fixation on the polyvinyl alcohol by utilizing the supernate in which the pH is regulated, and cleaning the polyvinyl alcohol after the fixation, so as to finish the whole fixation process. The method has the advantages that the damage to the bacteria in the fixation process is greatly reduced, and the phenomena of adhesion, water solubility and expansion are effectively reduced as well, thus providing conditions for large-scale industrial application of biologic active fillers and biologic active filter materials based on the polyvinyl alcohol.
Owner:BEIJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com