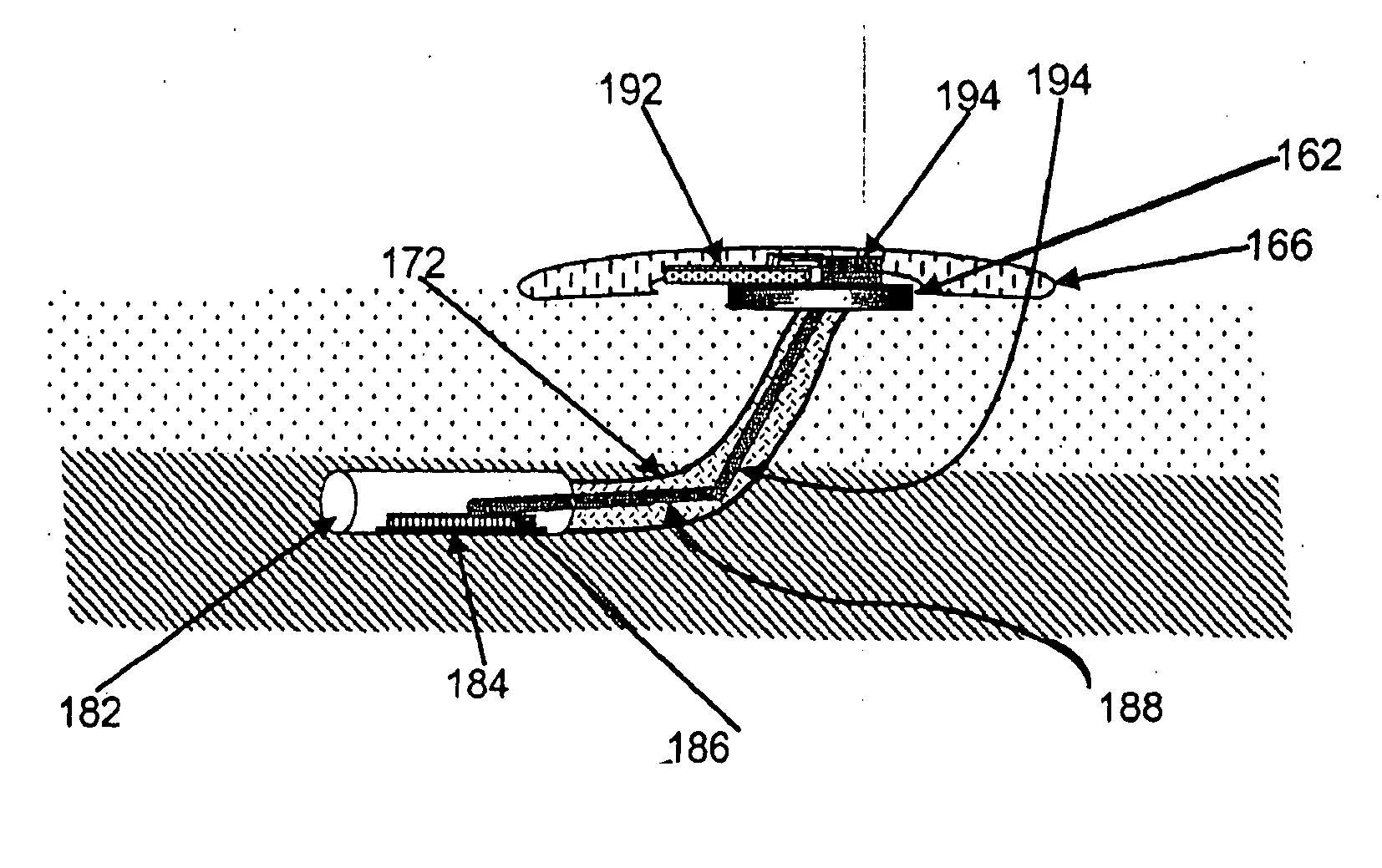
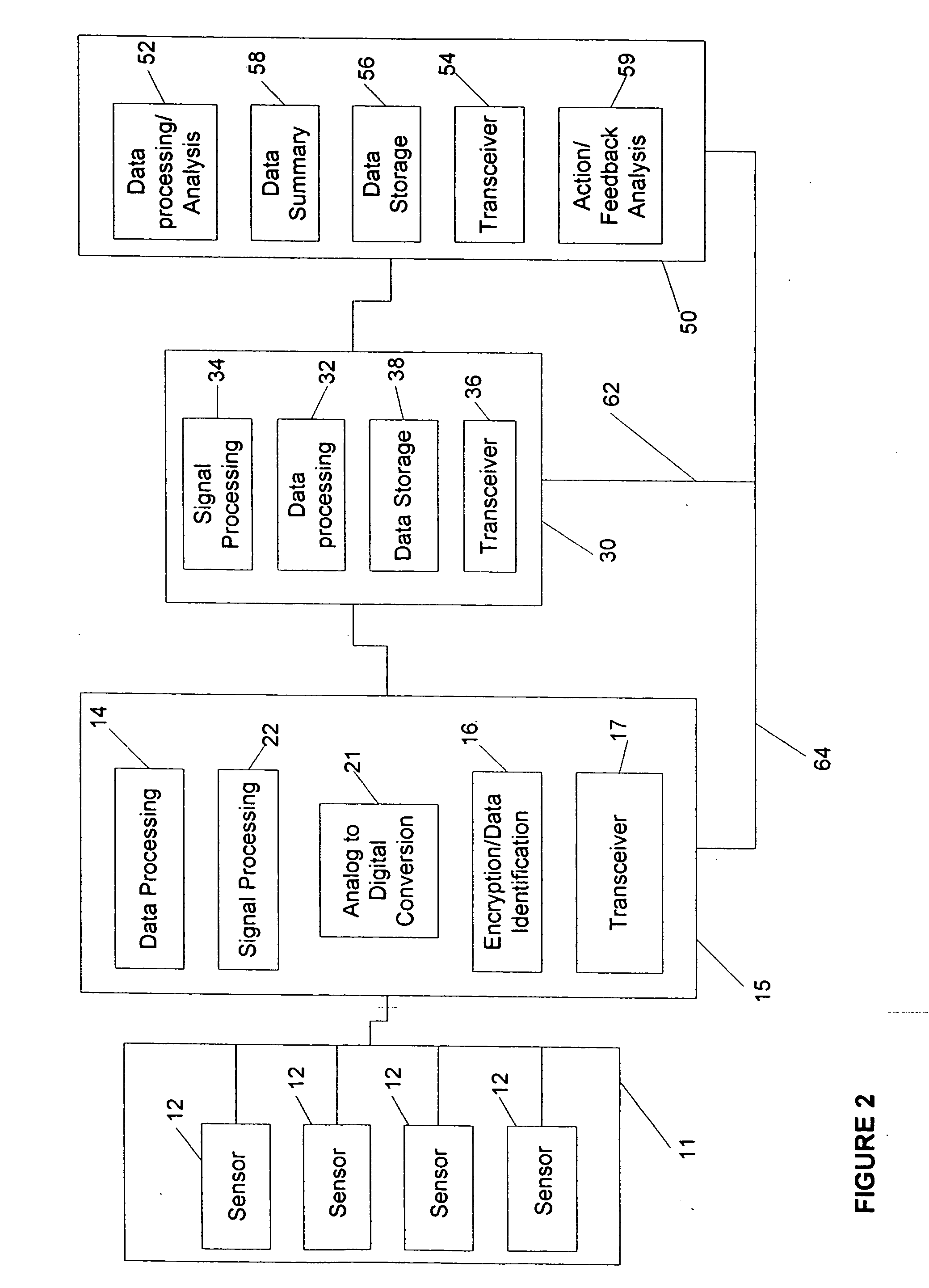
[0011] One aspect of this invention is a device which automatically and continuously or periodically monitors physiological conditions in vivo using surface or sub-surface implanted sensors linked to CCM's and DCU's. By continuously monitoring physiological parameters remotely or in a distributed environment, baseline or reference data can be obtained, allowing detection of deviations in measured subjects. The device particularly distinguishes itself from long-term monitoring devices currently available by: 1) improving measured data quality by diminishing data variation caused by the user, technique or compliance issues; 2) converting, encrypting and identifying data for further transmission and processing of data; 3) incorporating a wireless transmission signal system (e.g. radio frequency, acoustic or optical) or other remote communication method to allow automatic transmission of data collected from the CCM / BIH assembly to either adjacent or remote CCM's or DCU's; 4) reducing biocompatibility issues associated with implantable sensors with the use of novel biomaterials and devices to decrease the adhesion or encapsulation of the biofluid access port by biological processes; and 5) coupling the wireless signal system to enable a two-way wireless-based control system to allow controlled or automatic delivery of compounds or devices from the CCM / BIH assembly.
[0012] In one aspect, the BIH assembly may comprise various types of sensing mechanisms, including thermal sensors (thermoresistors, thermocouples), electrical sensors (EKG, ECG, impedance, frequency or capacitance), optical sensors (photonic wavelength, calorimetric, turbidity), chemical sensors (pH, biomolecules, gases such as CO2, and other chemical sensors), enzyme-linked sensors (glucose oxidase, phosphatase, coupled substrates (e.g. horseradish peroxidase or alkaline phosphatase and other enzyme-linked sensors)), radiation sensors (gamma, beta and other radiation detectors), magnetic sensors (micro NMR circuitry and magnetic spin state) and physical sensors, such as flow meters and pressure sensors. Alternatively, the sensor may also comprise a MEMS (Micro Electrical Mechanical Systems) or a MOEMS (Micro Optical Electrical Mechanical Systems) sensing device, comprising at least one cantilever beam coated with polymeric compounds for detection of various physiological substances or conditions. The microcantilever beams allow increases in sensitivity and specificity, as compared to currently available technologies, and simplifies detection by coupling the beam to transducers which measure changes in capacitance, resonant frequency, or other techniques used in detecting mass changes in the spring element of the cantilever beam. In still other embodiments, nanotechnology devices may be incorporated into the sensor head or other components of the device for more accurate detection, cellular manipulation and measurement of physiological parameters. In one embodiment the BIH assembly, as well as other components of the system, may contain components micron, submicron or nanoscale in dimension, further lessening the obtrusiveness of the device to wearer.
[0013] In another aspect, the BIH assembly of the sensor element comprises materials that permit interaction of the sensor with the host environment. This includes microchannels, gel, fine mesh, screen, membrane, filters or a microporous frit, which permit interaction of sensors to the host environment while maintaining a segregated and sterile environment within the sensing element itself. This tends to extend the life of the sensor by preventing fouling of the biological sensor with macromolecules and other substances that can adhere onto the sensor mechanism.
[0014] In accordance with another aspect of the invention, the use of specialized biomedia can be incorporated into the sensing head device and may decrease the exposure of the sensor element to the external environment. This biomedia system may also decrease the adherence of the sensor element onto the host tissue or layer, a large component of the rejection mechanism of biological sensors. Moreover, the use of a biomedia system may lower trauma to the surrounding tissue or layer by providing medium that is physiologically compatible with the host, mimicking the tissue environment in which the sensor is implanted. In yet another aspect of the invention, growth factors, cell signaling and cell adhesion molecules will be integrated into the biomedia system, mimicking the tissue and further improving biocompatibility issues of the sensor implantation into the host species.
[0015] In other aspects, the biomedia may have gel-like properties at ambient room temperature, whereupon exposure to higher body temperatures changes the material to a fluid-like state and becomes less viscous. One utility of this gel-like material may be its use as part of a calibration process for the sensor elements. When the sensor is implanted on or into the host, the sensor itself is shielded from the host environment by the gel-like material. As the temperature around the sensor increases, the gel-like material changes viscosity, freeing calibration molecules from the matrix that then enter into the sensor. The sensor can then be accurately calibrated before being equilibrated into the host environment. The bio-media may also be used as a process or method during manufacturing. The bio-media may also provide increased product shelf-life storage by insulating the sensors on the BIH from degradation caused by ambient conditions such as temperature, humidity or other degenerative storage issues.
[0016] In another aspect of the invention, the BIH assembly, located on top or within the dermal layer, interacts with the CCM (Control and Communication Module) that is also located on top or within the dermal layer. The CCM interacts with the sensor unit either directly through a physical means (e.g. conductive wire, optical, acoustic or other means) or indirectly using a remote wireless-based signal and control system. The CCM also contains a power supply consisting of either a removable or responder power source. The CCM and the BIH assembly, if located on top of the dermal layer, are attached to the host patient through a bioadherence system, which allows minimal irritation of the outer body surface, thereby tending to decrease rejection and increase the longevity of the BIH and CCM assemblies.
 Login to View More
Login to View More  Login to View More
Login to View More