Patents
Literature
445results about "Gastrointestinal cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
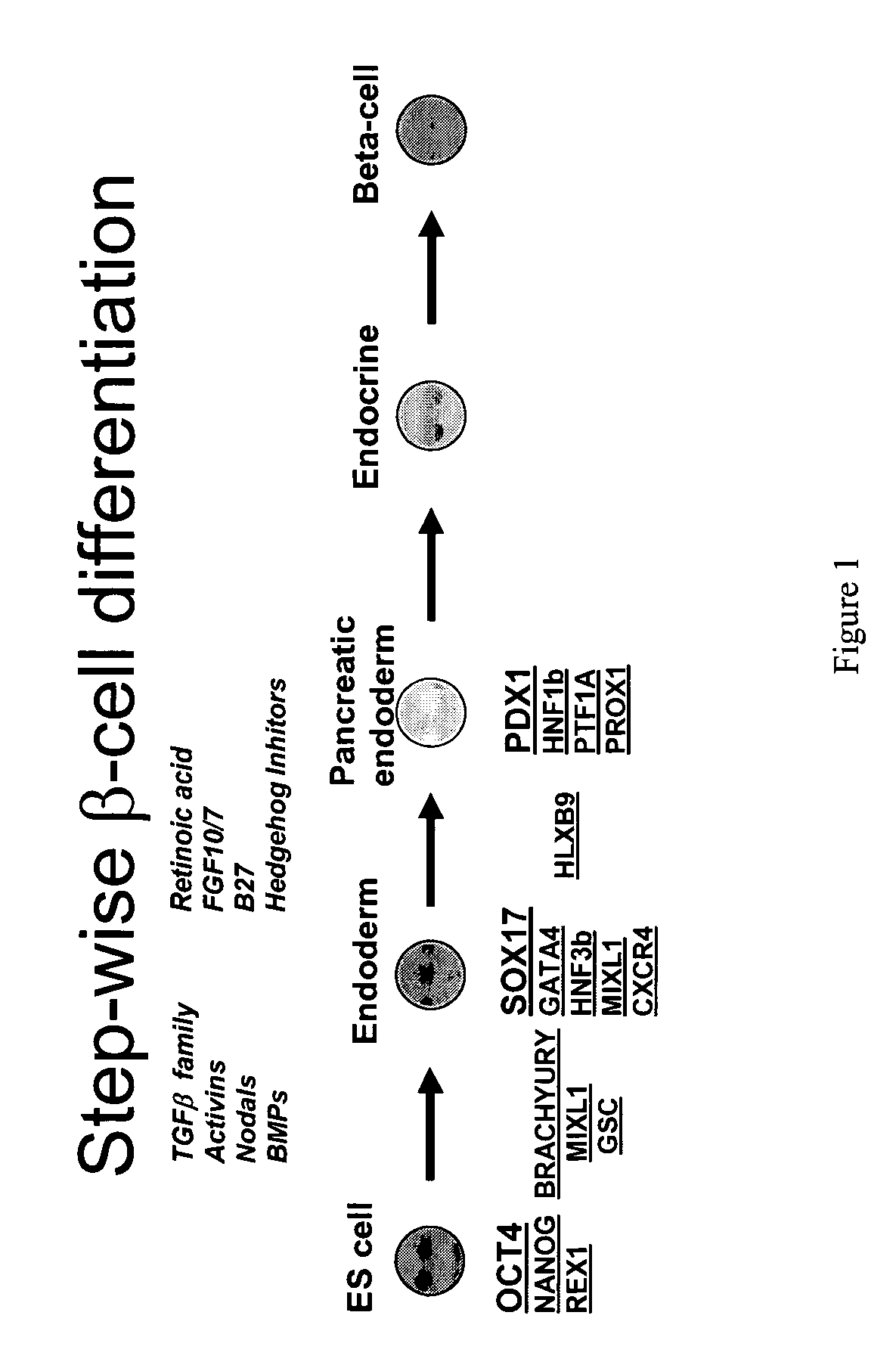
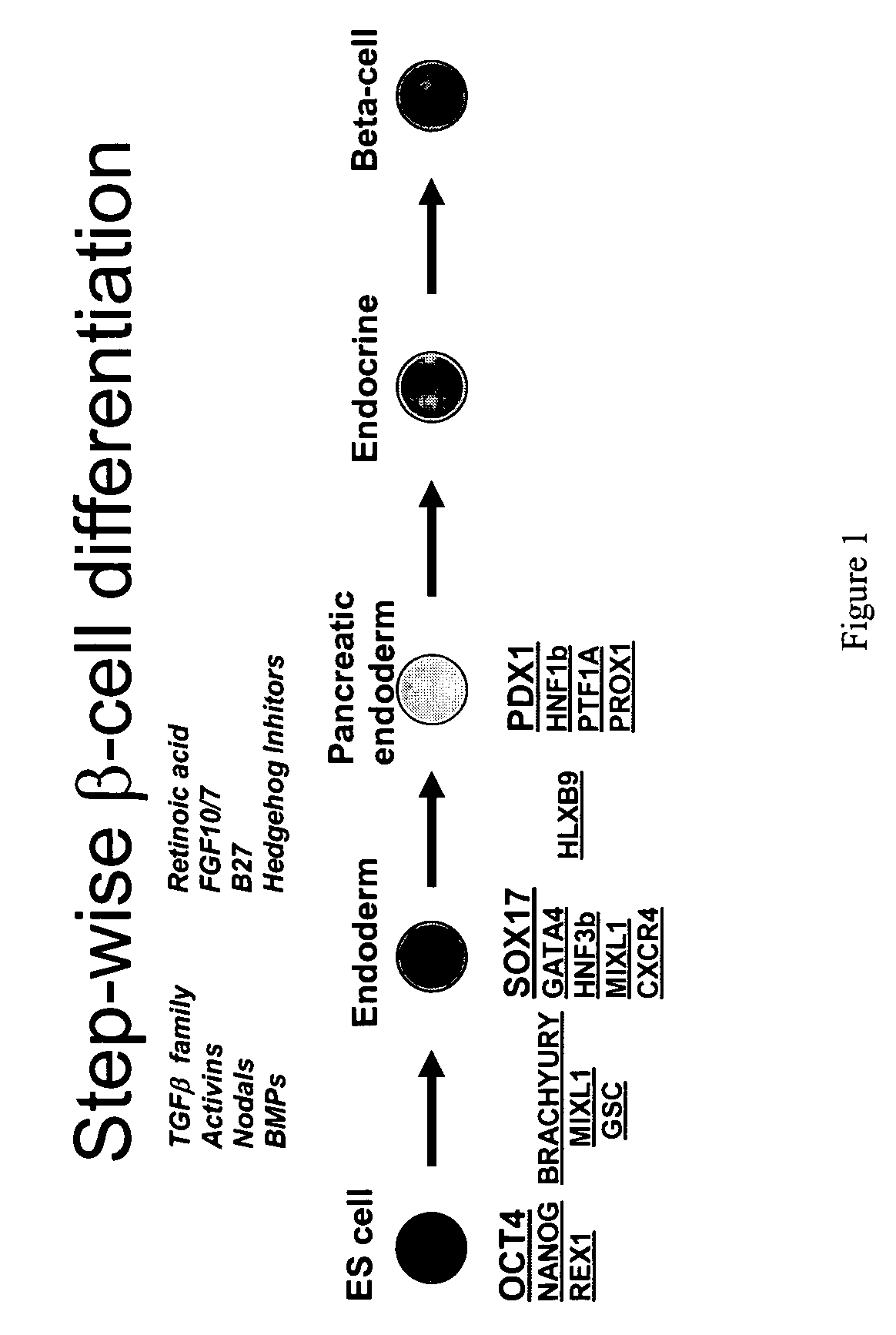
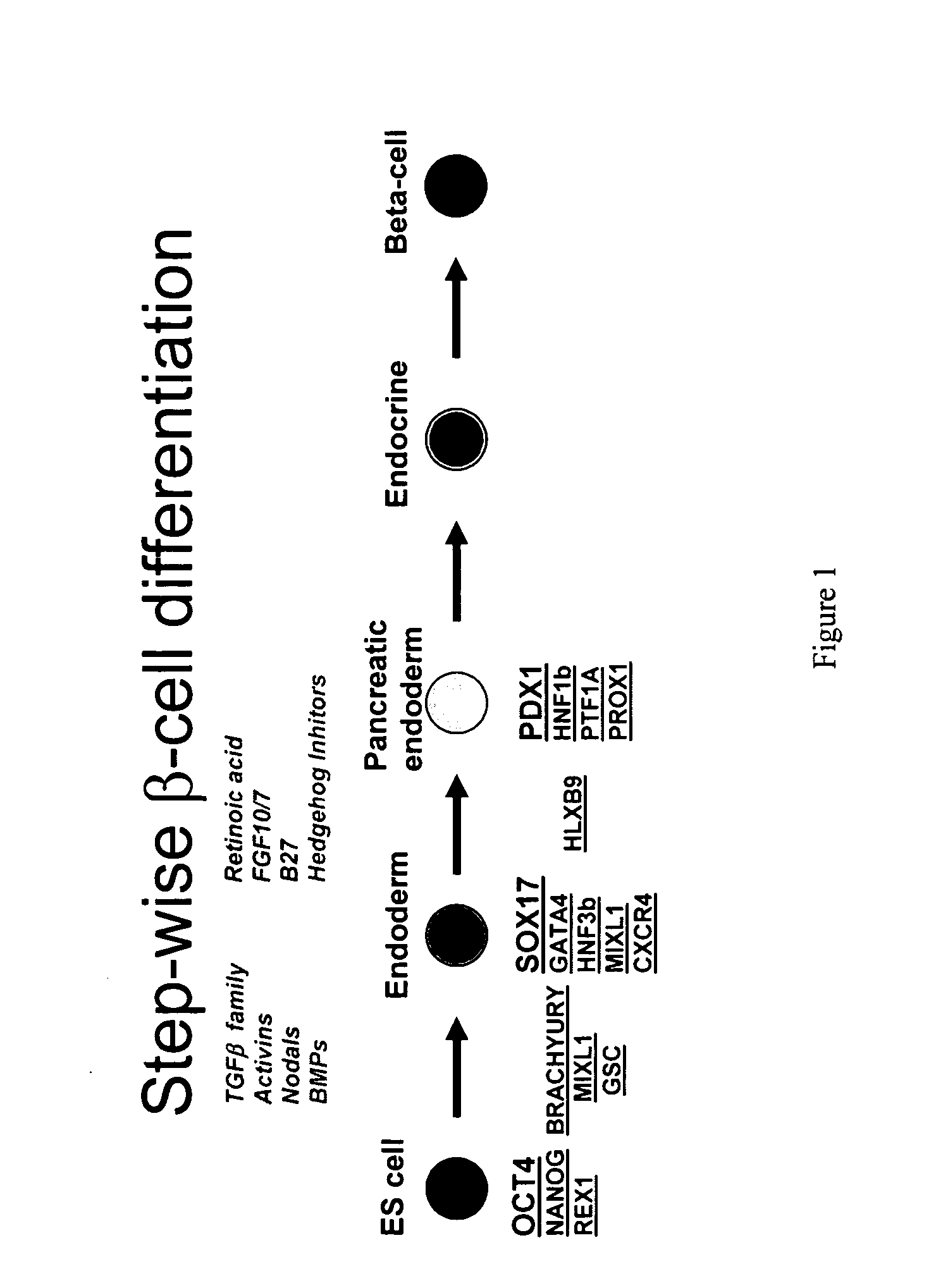
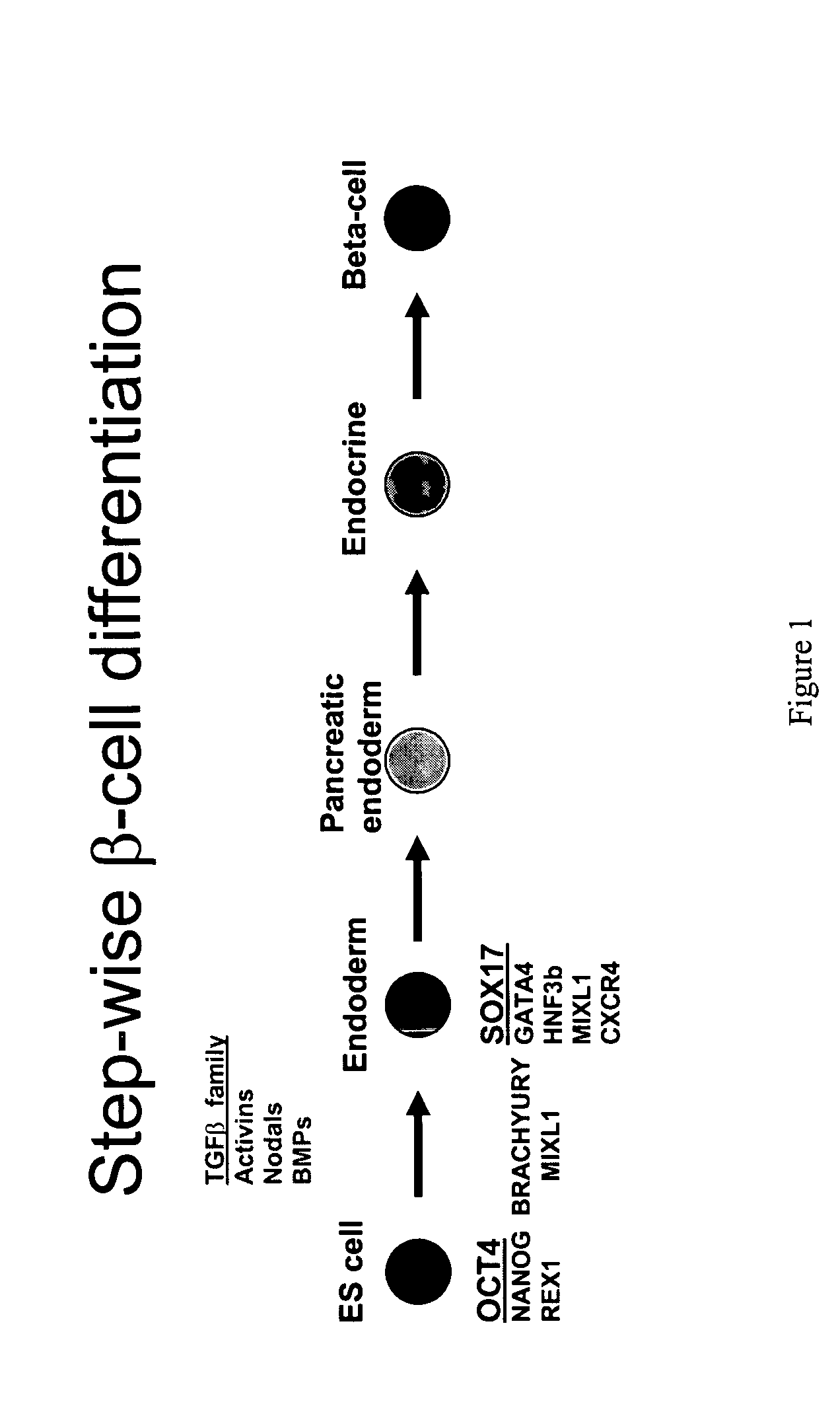
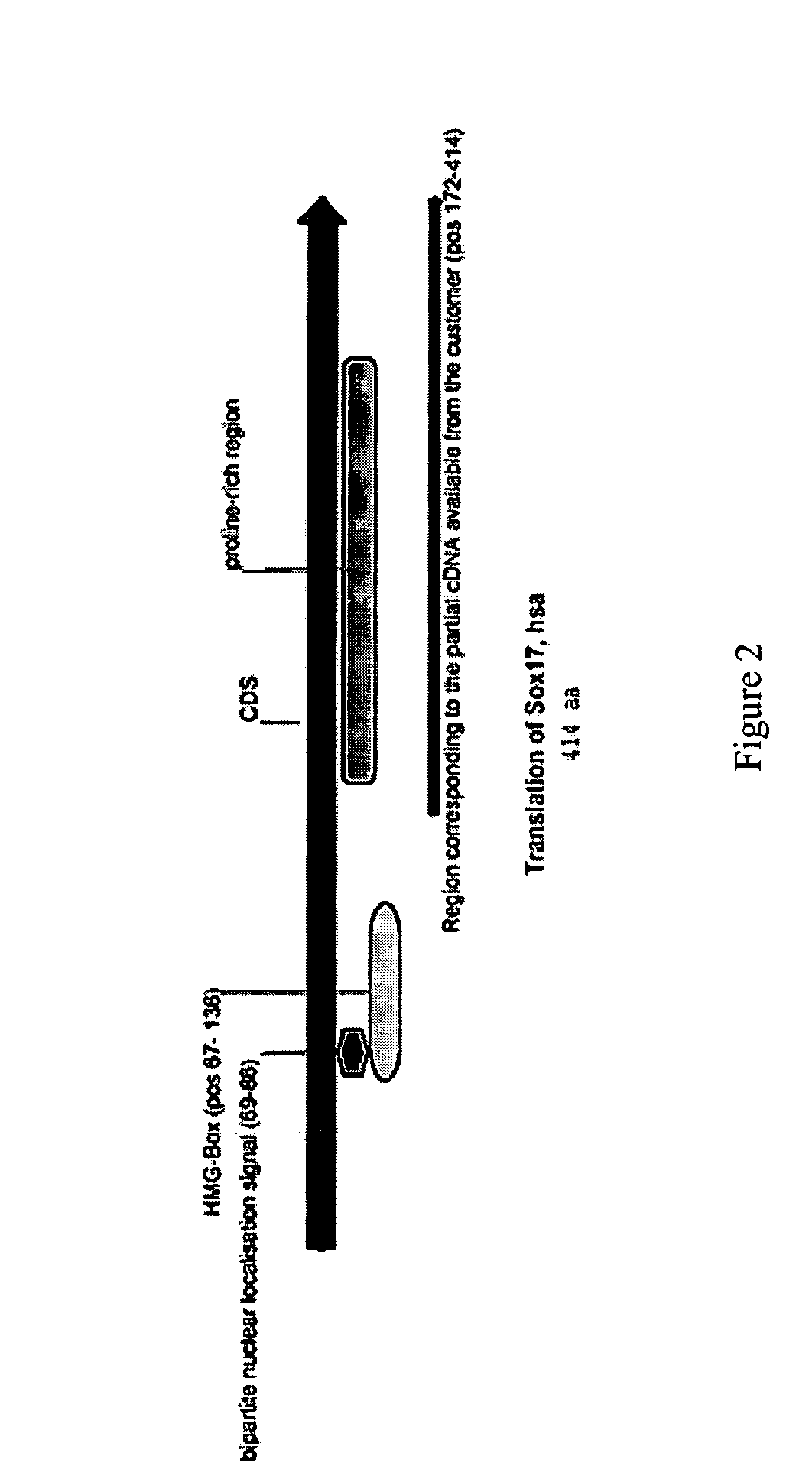
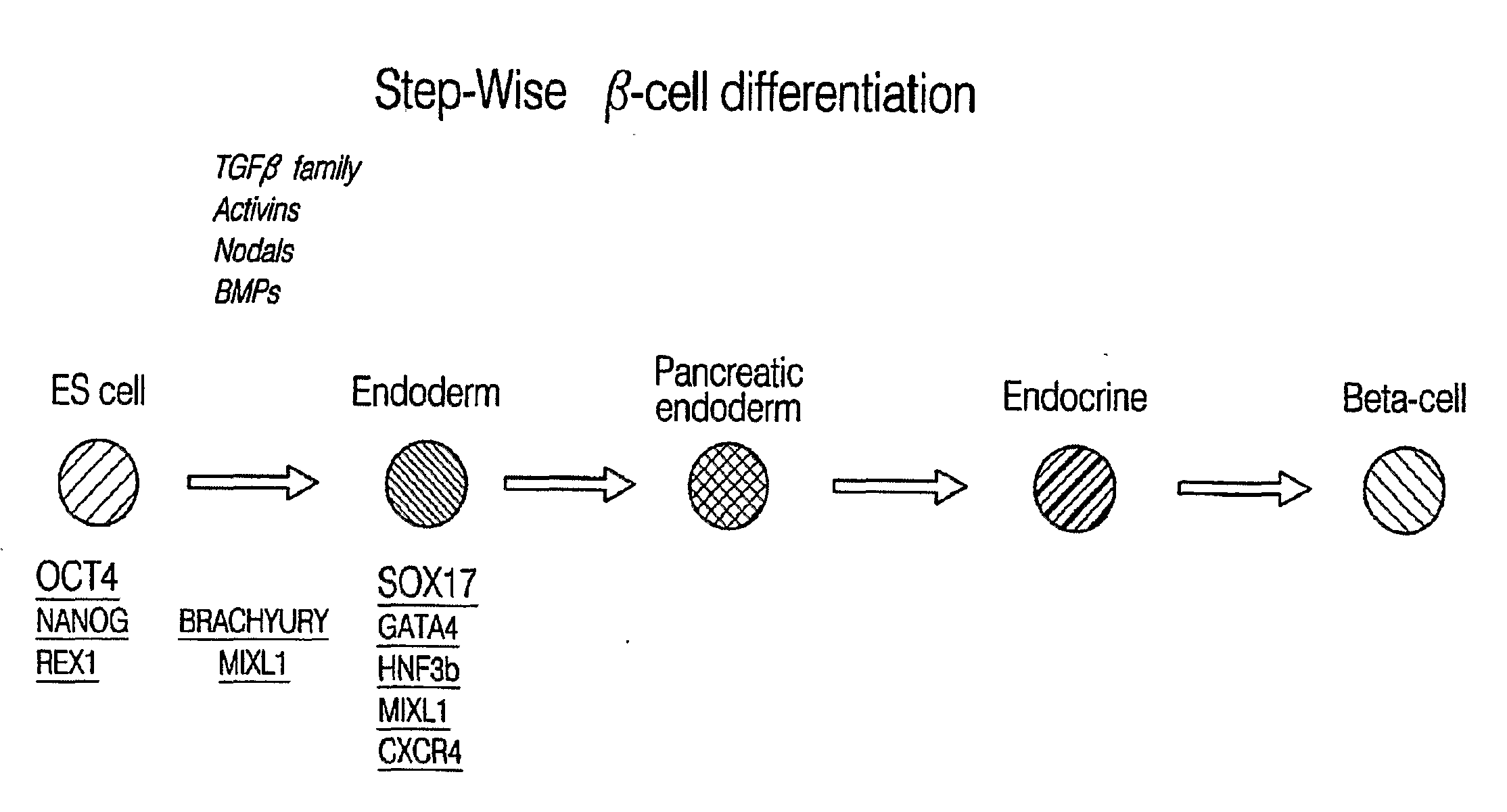
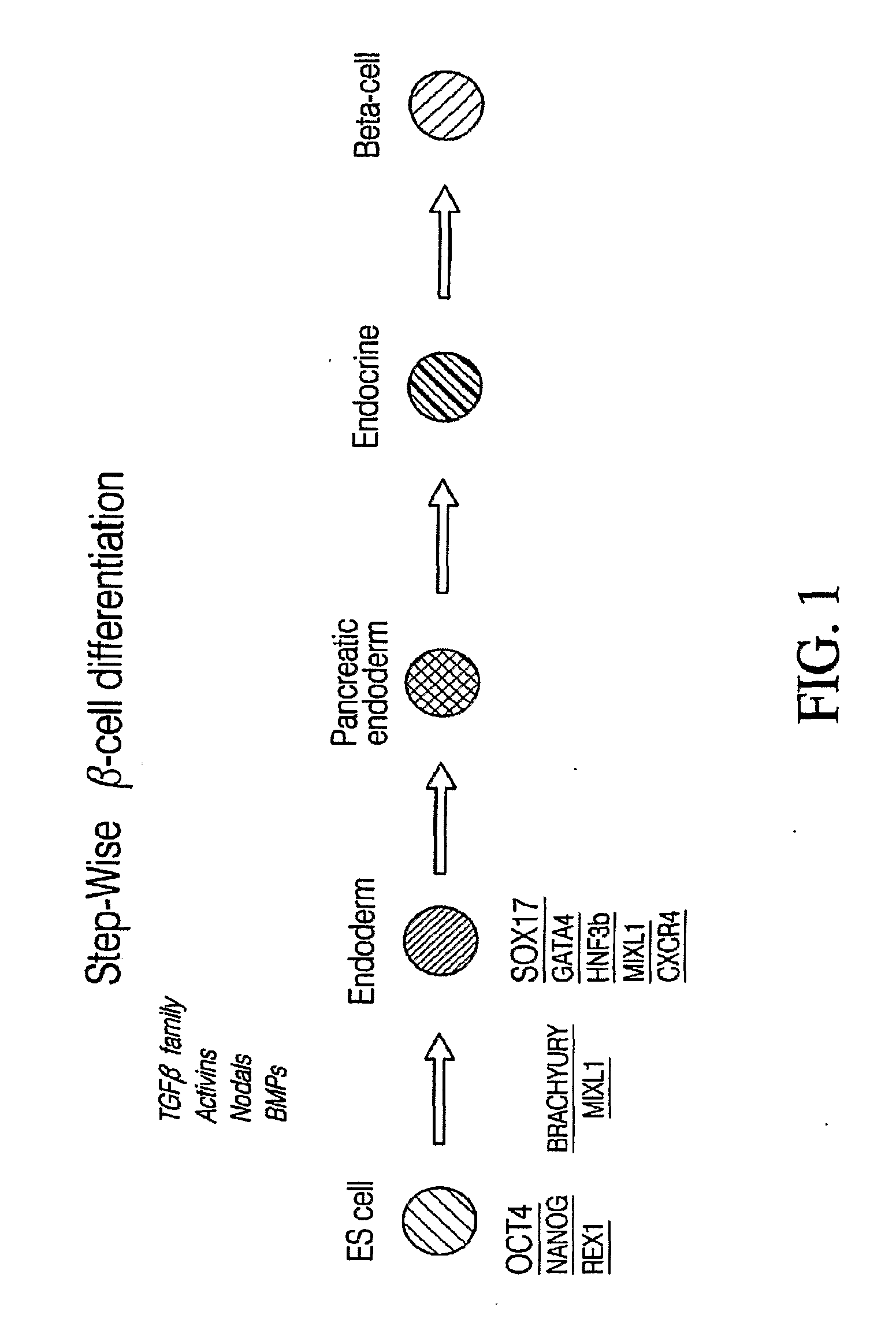
PDX1 expressing endoderm
InactiveUS20050266554A1Increase differentiationIncrease productionGastrointestinal cellsDiagnosticsGerm layerCell type
Disclosed herein are cell cultures comprising PDX1-positive endoderm cells and methods of producing the same. Also disclosed herein are cell populations comprising substantially purified PDX1-positive endoderm cells as well as methods for enriching, isolating and purifying PDX1-positive endoderm cells from other cell types. Methods of identifying differentiation factors capable of promoting the differentiation of endoderm cells, such as PDX1-positive foregut endoderm cells and PDX1-negative definitive endoderm cells, are also disclosed.
Owner:CYTHERA
Decellularized extracellular matrix of conditioned body tissues and uses thereof
InactiveUS20050013870A1Increase in sizeHigh strengthGastrointestinal cellsGenetically modified cellsCell-Extracellular MatrixECM Protein
The present invention relates generally to decellularized extracellular matrix of conditioned body tissues. The decellularized extracellular matrix contains a biological material, preferably vascular endothelial growth factor (VEGF), produced by the conditioned body tissue that is in an amount different than the amount of the biological material that the body tissue would produce absent the conditioning. The invention also relates to methods of making and methods of using said decellularized extracellular matrix. Specifically, the invention relates to treating defective, diseased, damaged or ischemic cells, tissues or organs in a subject by administering, injecting or implanting the decellularized extracellular matrix of the invention into a subject in need thereof. The invention is further directed to a tissue regeneration scaffold for implantation into a subject inflicted with a disease or condition that requires tissue or organ repair, regeneration and / or strengthening. Additionally, the invention is directed to a medical device, preferably a stent or an artificial heart, having a surface coated or covered with the decellularized extracellular matrix of the invention or having a component comprising the decellularized extracellular matrix of the invention for implantation into a subject, preferably a human. Methods for making the tissue regeneration scaffold and methods for manufacturing a coated or covered medical device having a component comprising decellularized extracellular matrix of conditioned body tissues are also provided.
Owner:BOSTON SCI SCIMED INC
Methods for identifying factors for differentiating definitive endoderm
Disclosed herein are methods of identifying one or more differentiation factors that are useful for differentiating cells in a cell population comprising definitive endoderm cells into cells which are capable of forming tissues and / or organs that are derived from the gut tube.
Owner:VIACYTE INC
PDX1-expressing dorsal and ventral foregut endoderm
Disclosed herein are cell cultures comprising dorsal and / or ventral PDX1-positive foregut endoderm cells and methods of producing the same. Also disclosed herein are cell populations comprising substantially purified dorsal and / or ventral PDX1-positive foregut endoderm cells as well as methods for enriching, isolating and purifying dorsal and / or ventral PDX1-positive foregut endoderm cells from other cell types. Methods of identifying differentiation factors capable of promoting the differentiation of dorsal and / or ventral PDX1-positive foregut endoderm cells, are also disclosed.
Owner:CYTHERA
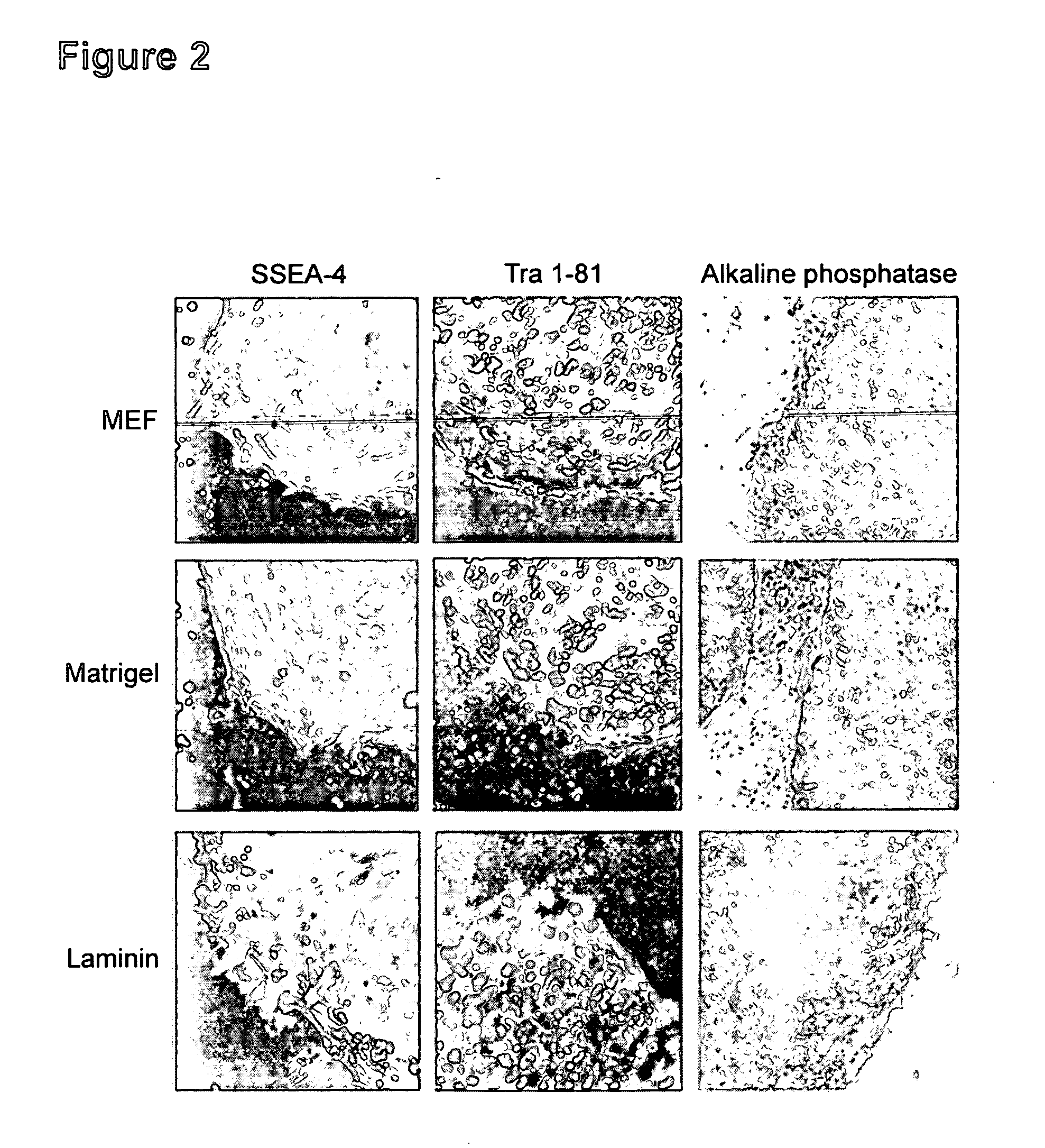
Medium for growing human embryonic stem cells
InactiveUS7297539B2Rapid productionExpanding primate pluripotent stem (pPS) cellsHepatocytesGastrointestinal cellsGerm layerFiber
This disclosure provides an improved system for culturing human pluripotent stem cells. Traditionally, pluripotent stem cells are cultured on a layer of feeder cells (such as mouse embryonic fibroblasts) to prevent them from differentiating. In the system described here, the role of feeder cells is replaced by components added to the culture environment that support rapid proliferation without differentiation. Effective features are a suitable support structure for the cells, and an effective medium that can be added fresh to the culture without being preconditioned by another cell type. Culturing human embryonic stem cells in fresh medium according to this invention causes the cells to expand surprisingly rapidly, while retaining the ability to differentiate into cells representing all three embryonic germ layers. This new culture system allows for bulk proliferation of pPS cells for commercial production of important products for use in drug screening and human therapy.
Owner:ASTERIAS BIOTHERAPEUTICS INC
Decullularized extracellular matrix of conditioned body tissues and uses thereof
InactiveUS20050181016A1Good curative effectMinimize delayGastrointestinal cellsGenetically modified cellsAbnormal tissue growthCell-Extracellular Matrix
The invention is directed to an apparatus, such as a medical device, having a surface coated or covered with a decellularized extracellular matrix or having a component comprising the decellularized extracellular matrix for implantation into a subject, preferably a human. In one embodiment of the invention, a decellularized extracellular matrix is used to form a bodily implant such as a vein, an artery, an esophagus, or a ventricular restraining device. In some embodiments of the invention, the decellularized extracellular matrix is configured to be a time released therapeutic. In another embodiment of the invention, a decellularized extracellular matrix forms an aneurysm treatment device, such as an aneurysm coil, a seal, a pouch, or a filler. In a further embodiment of the invention, decellularized extracellular matrix is used to embolize lesions, tumors, or vessels. Methods for making the tissue regeneration scaffold and methods for manufacturing a coated or covered medical device having a component comprising decellularized extracellular matrix of body tissues are also provided.
Owner:BOSTON SCI SCIMED INC
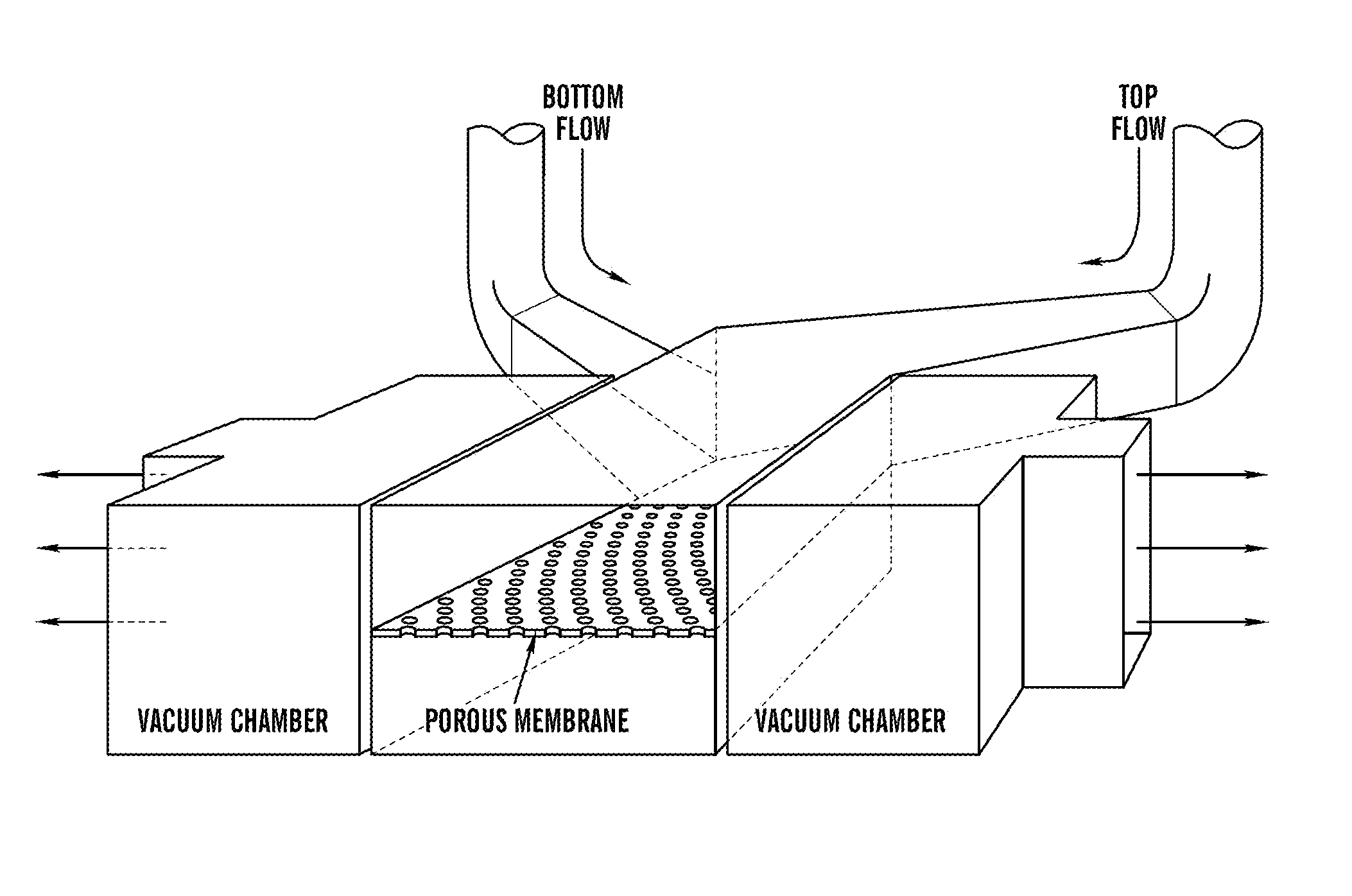
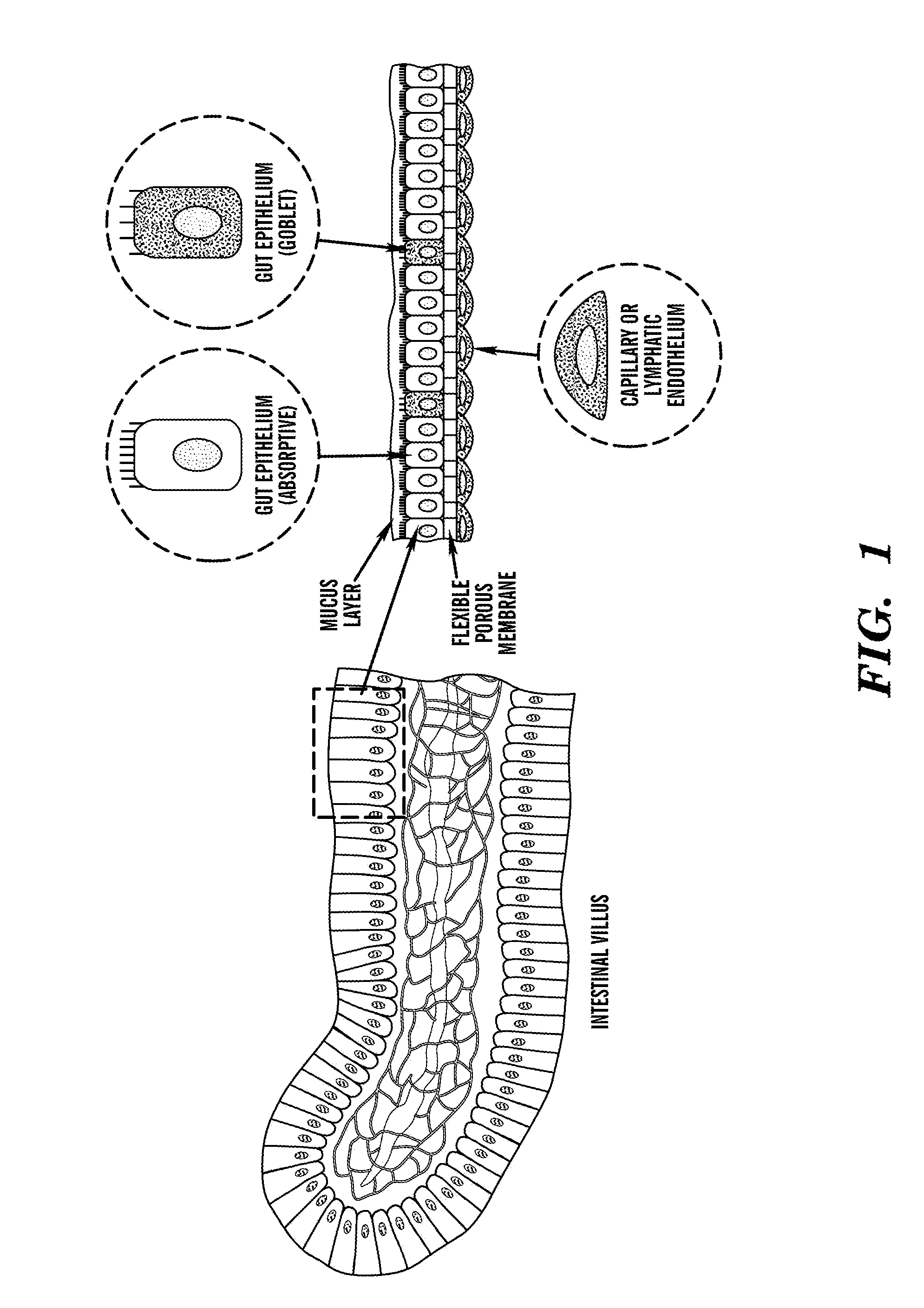
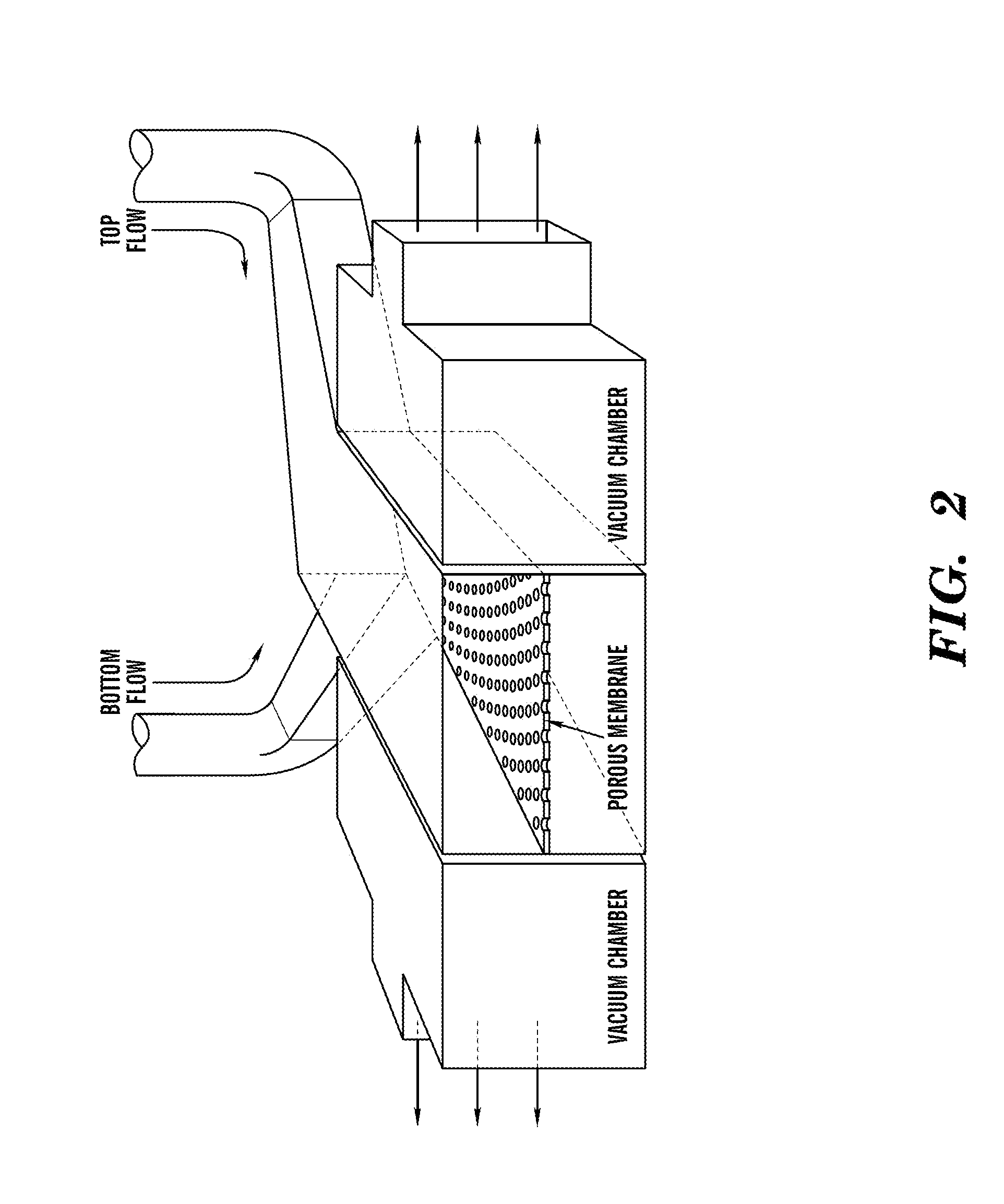
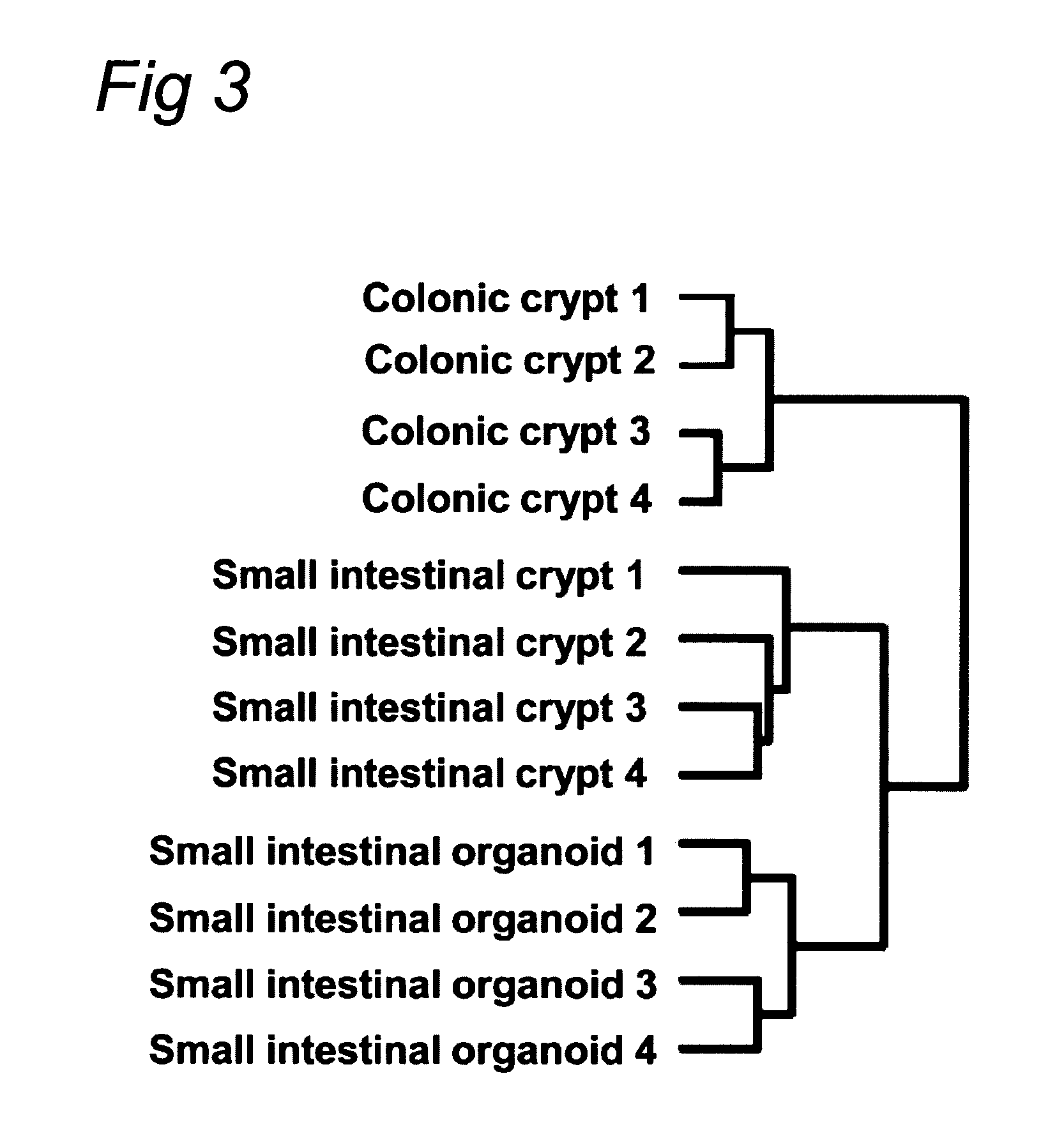
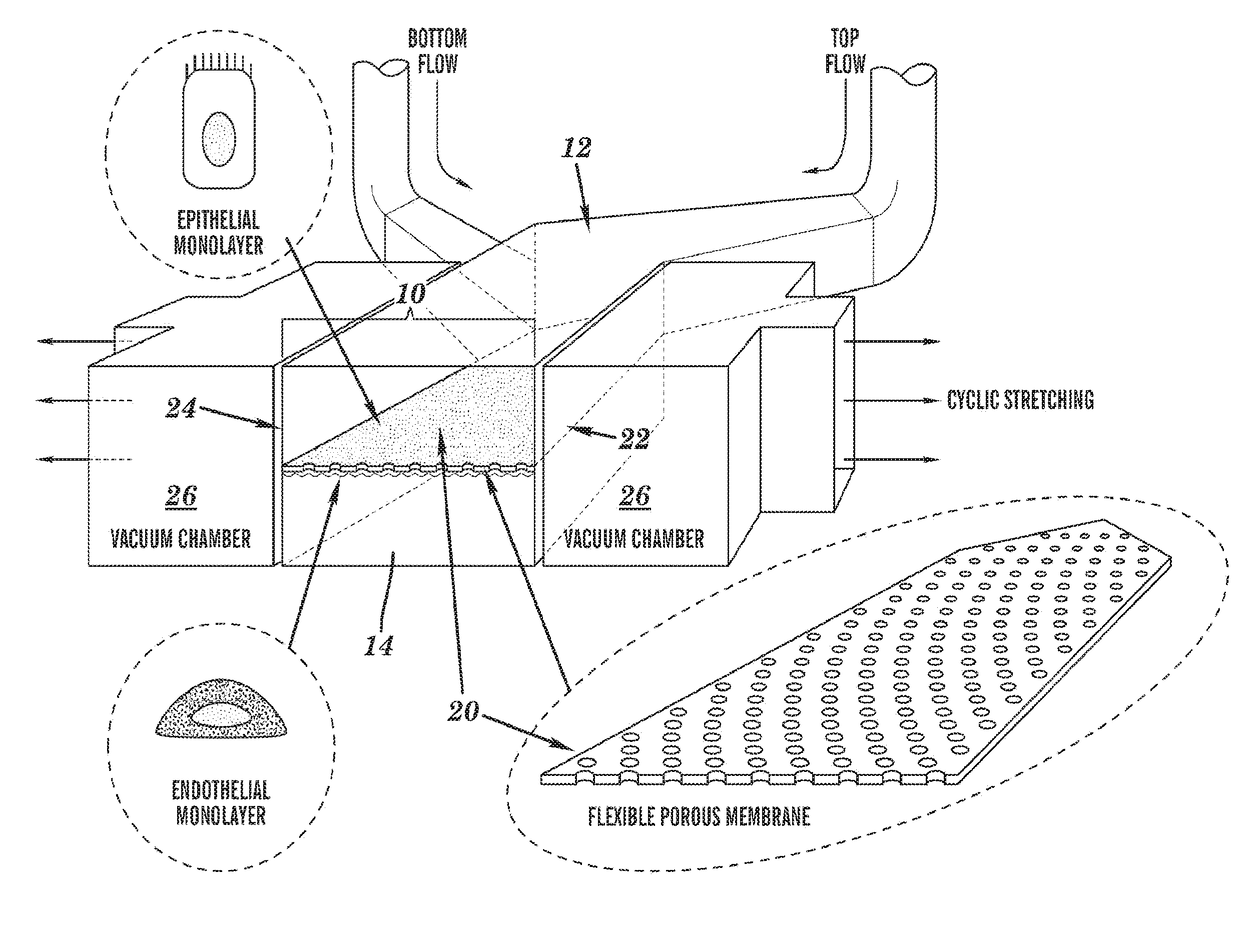
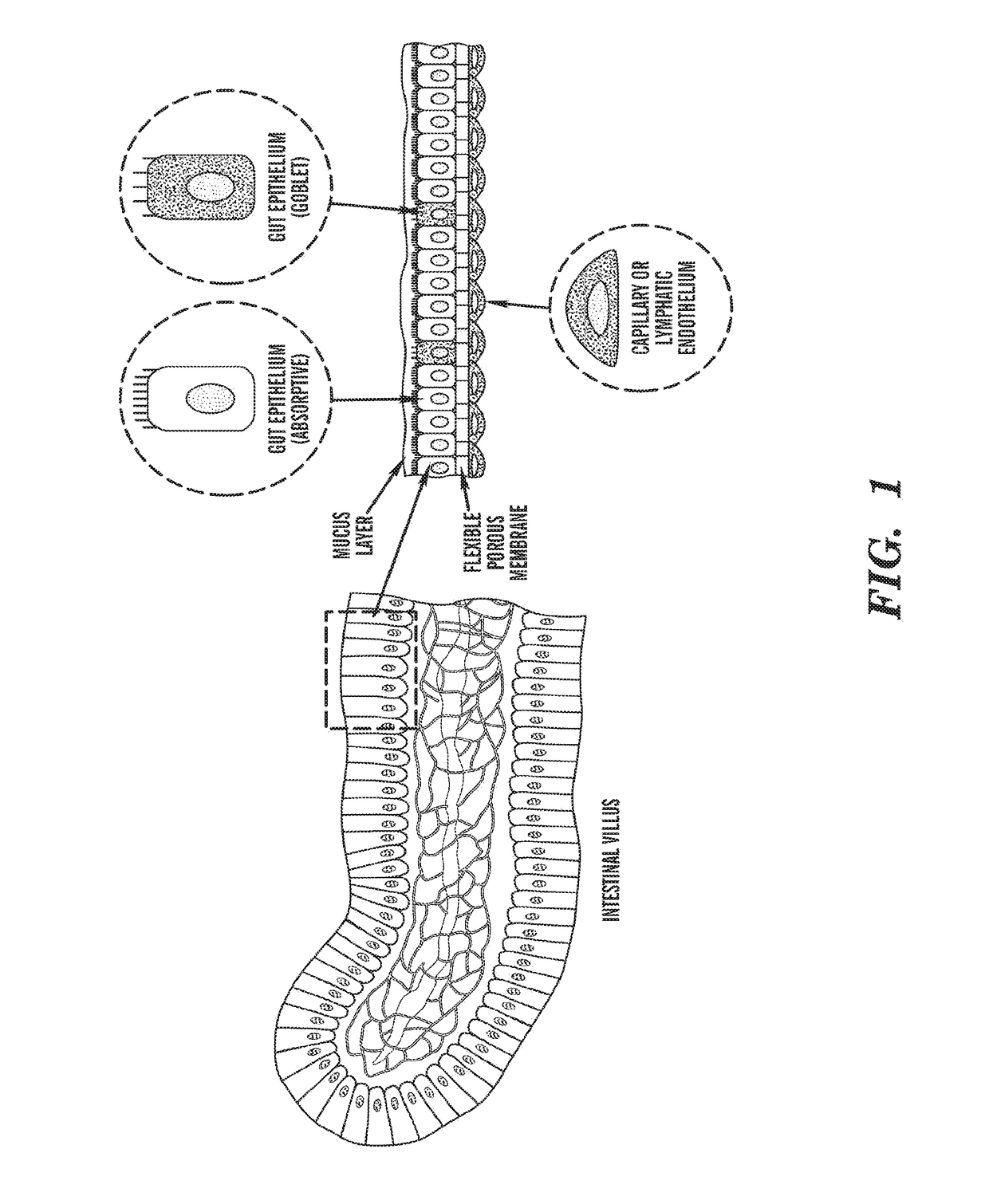
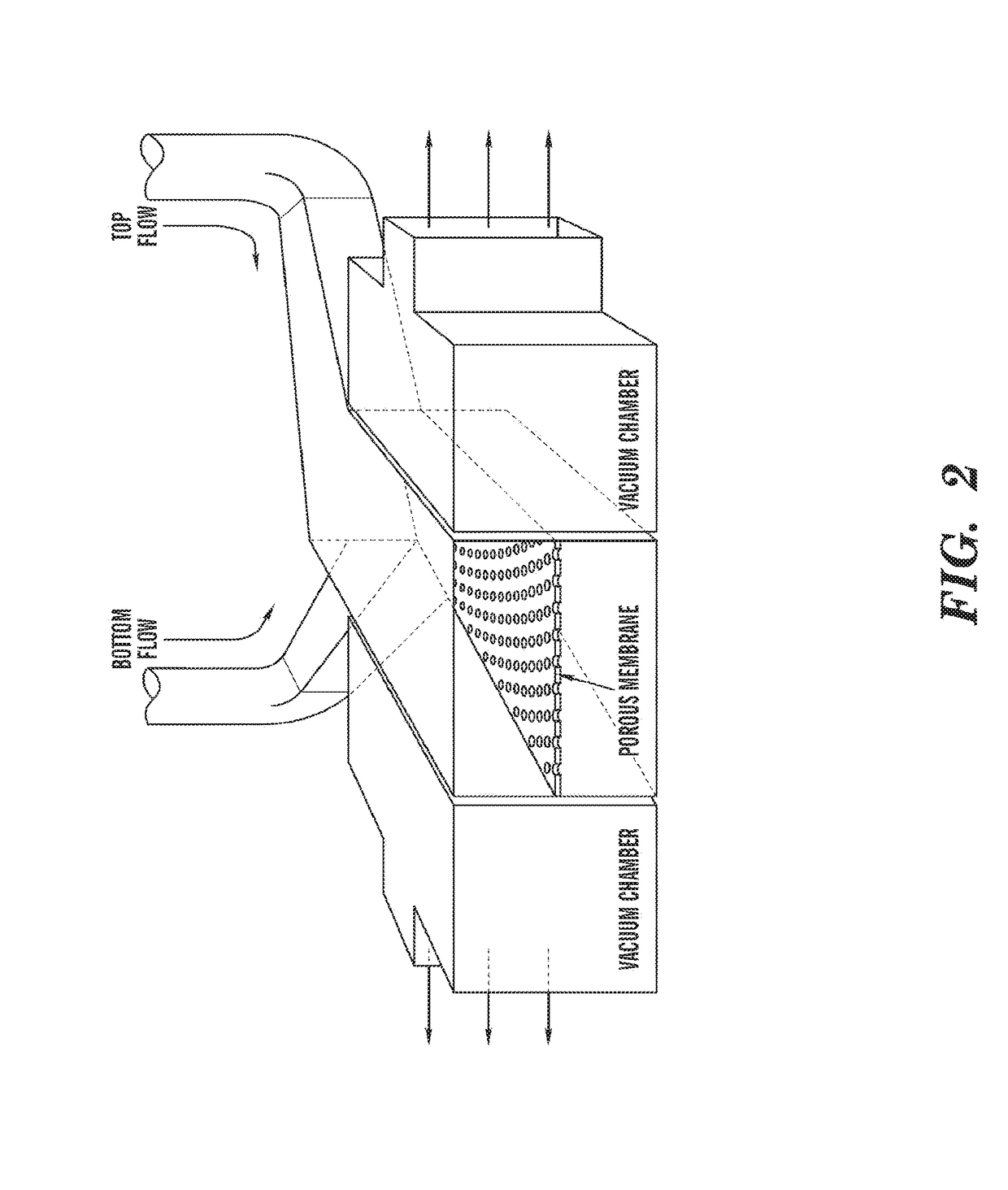
Cell culture system
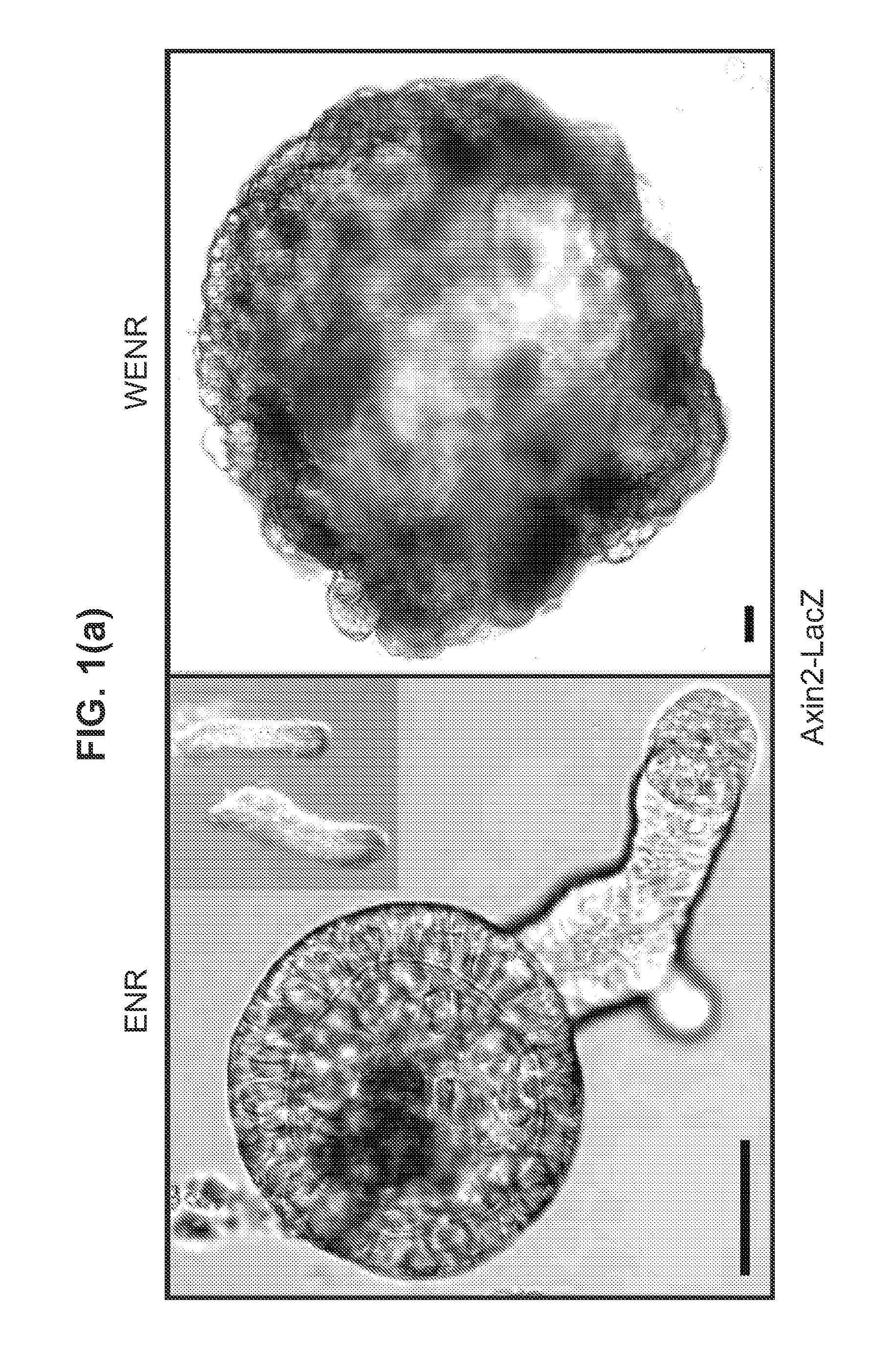
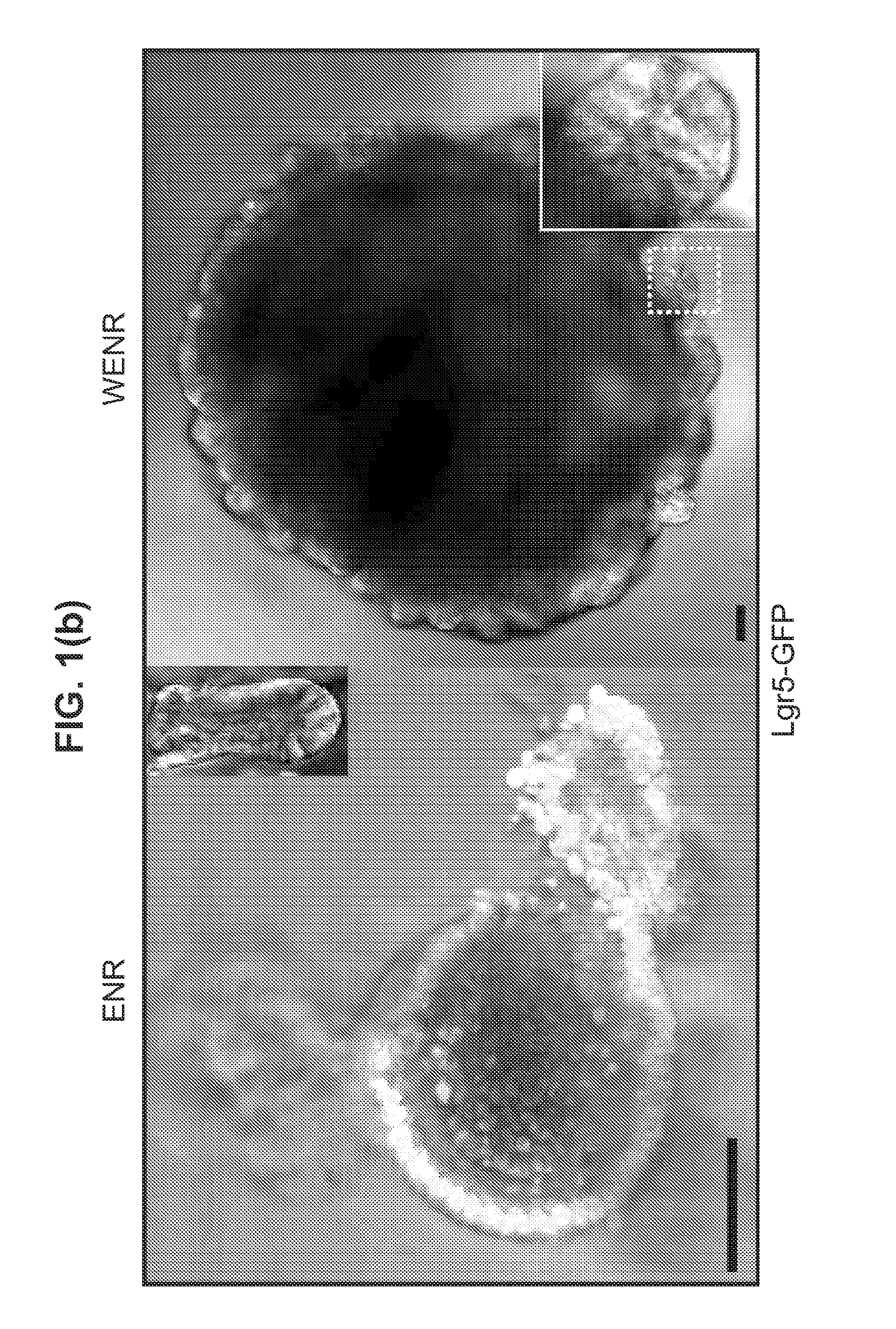
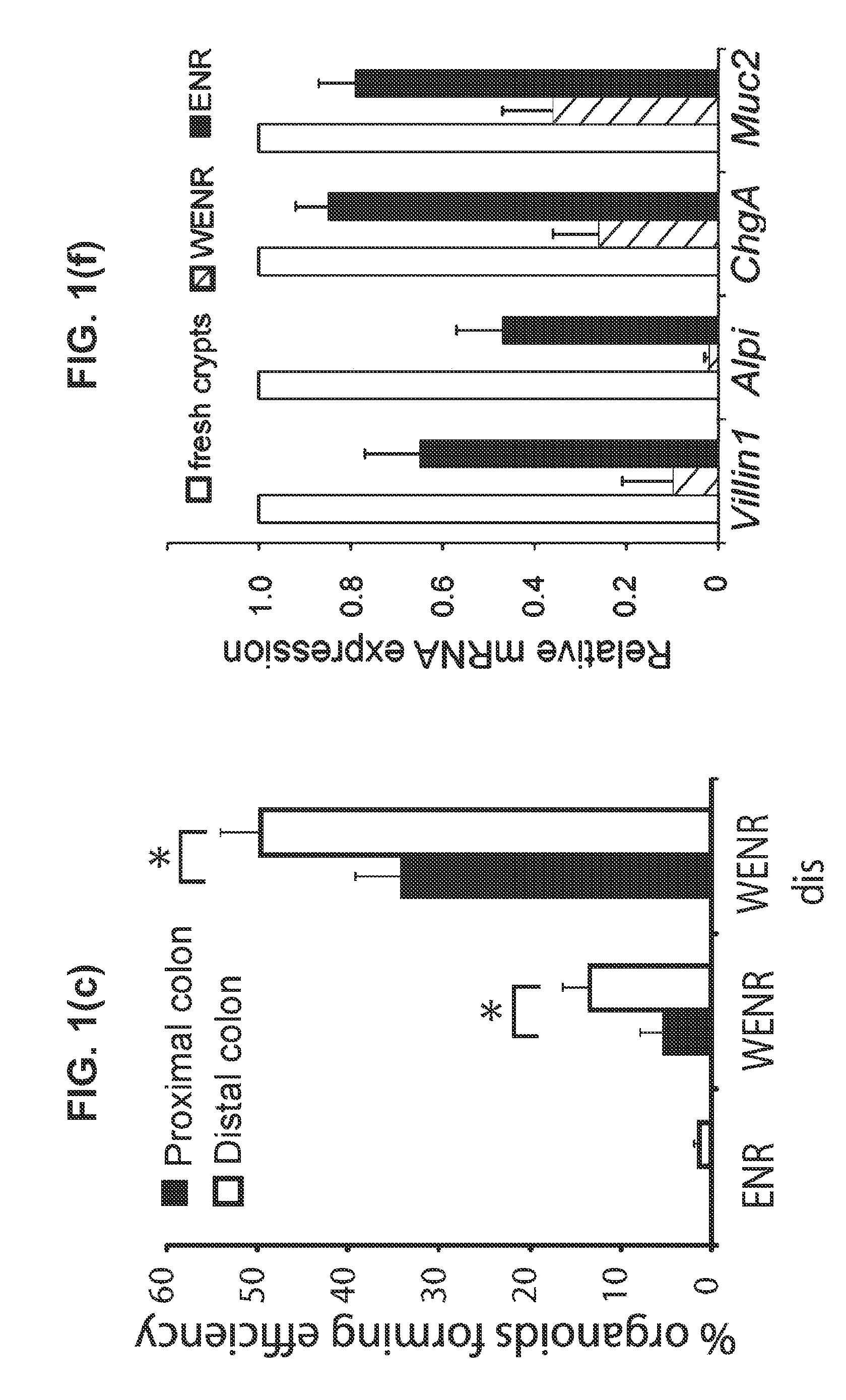
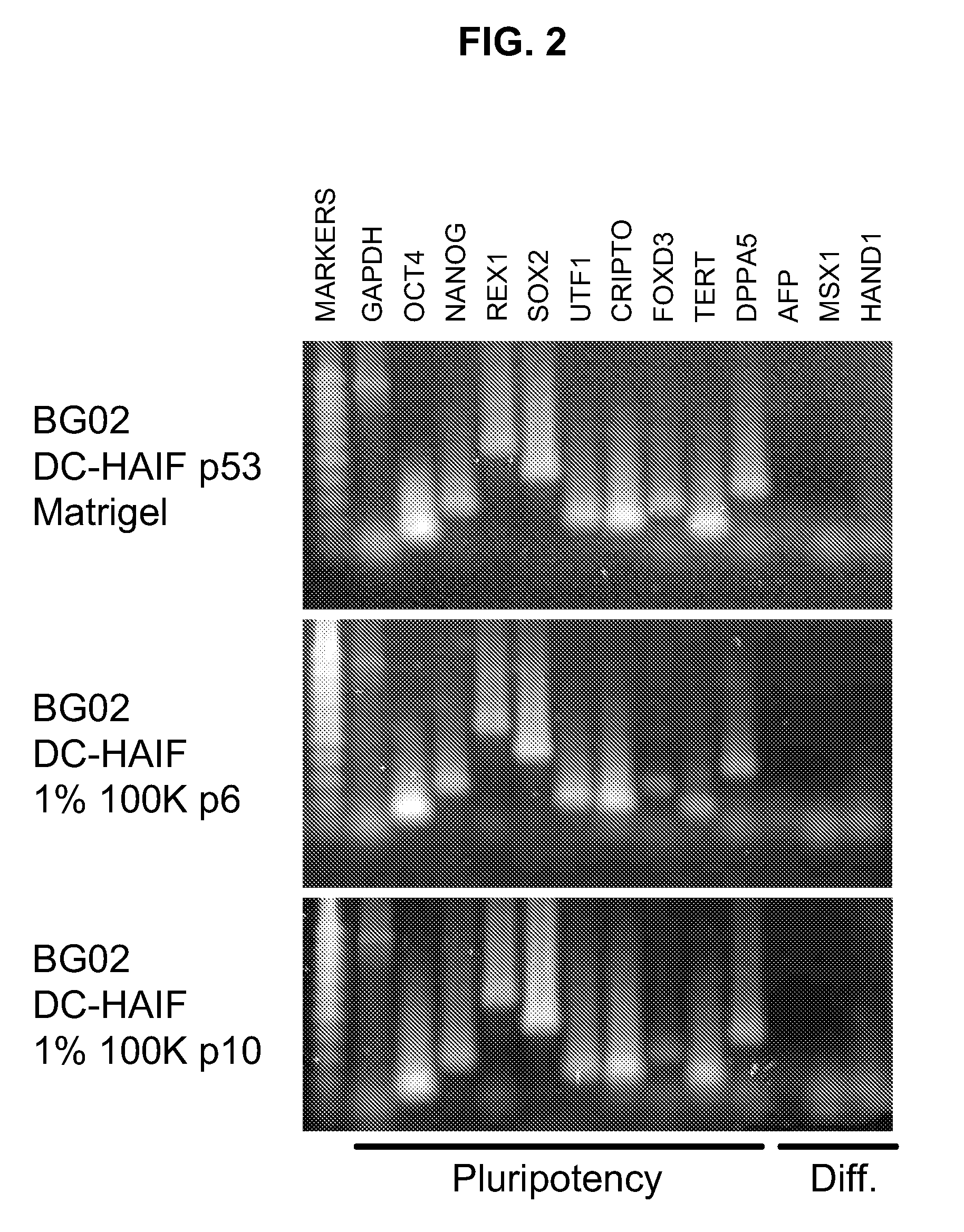
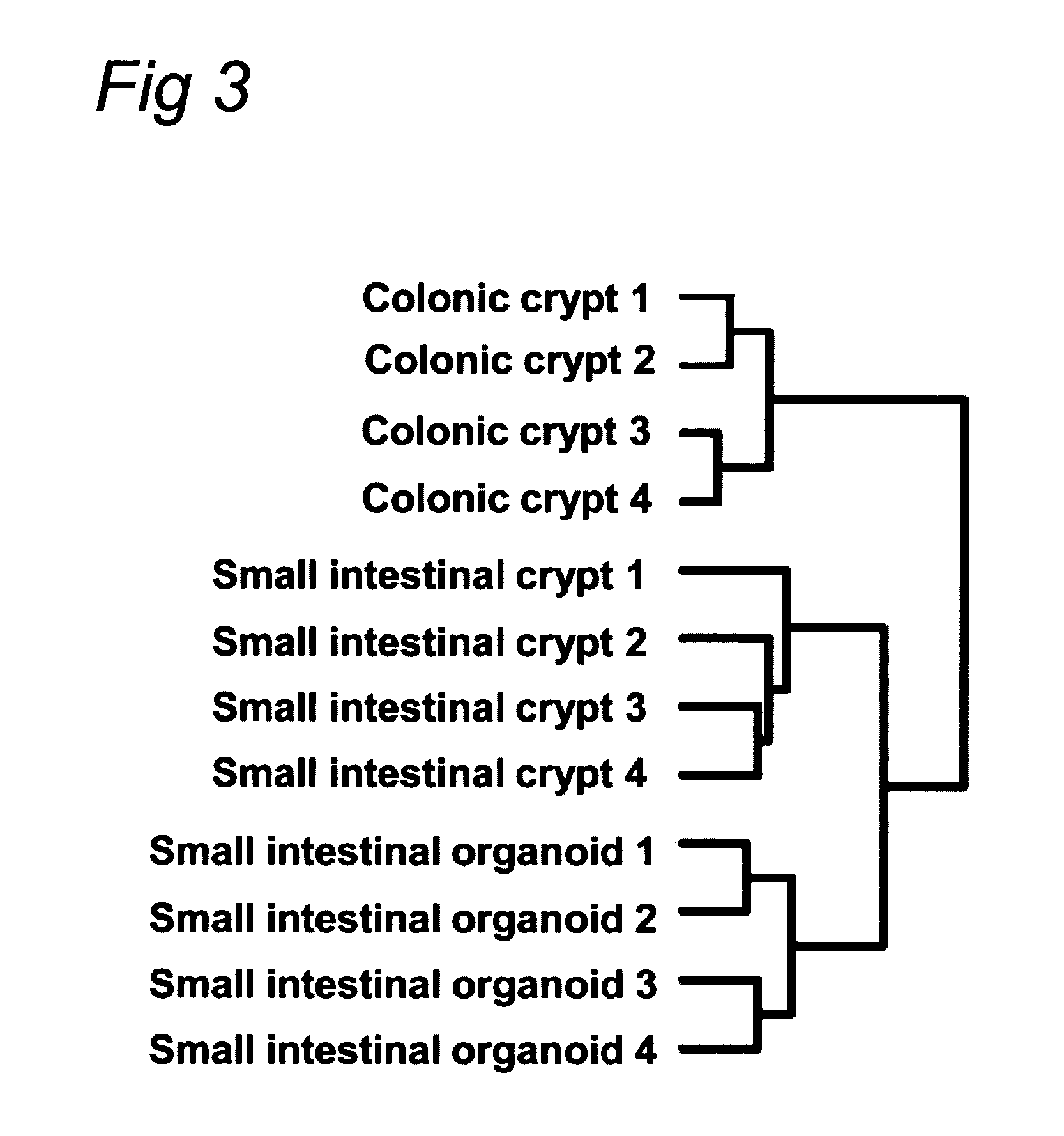
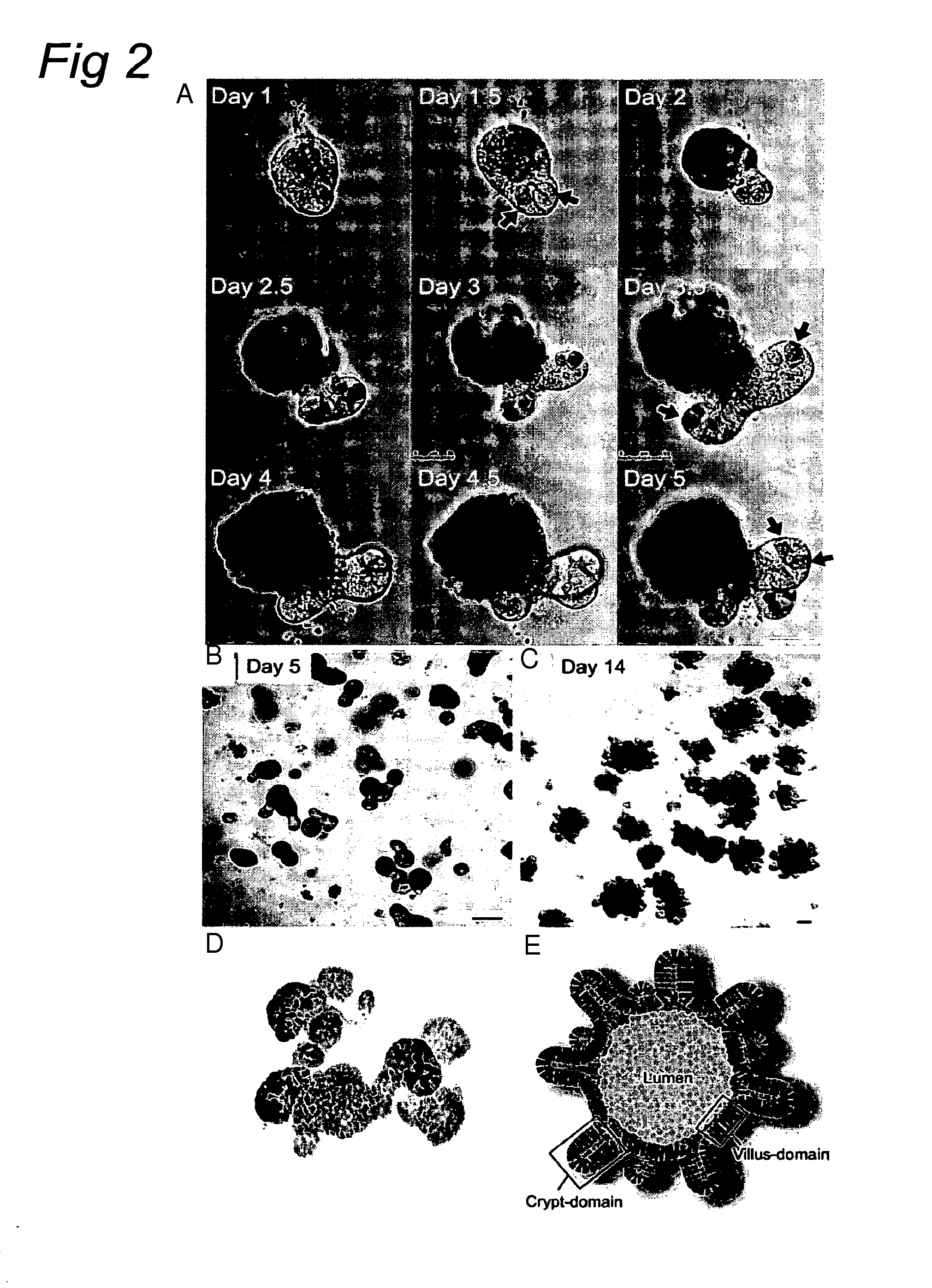
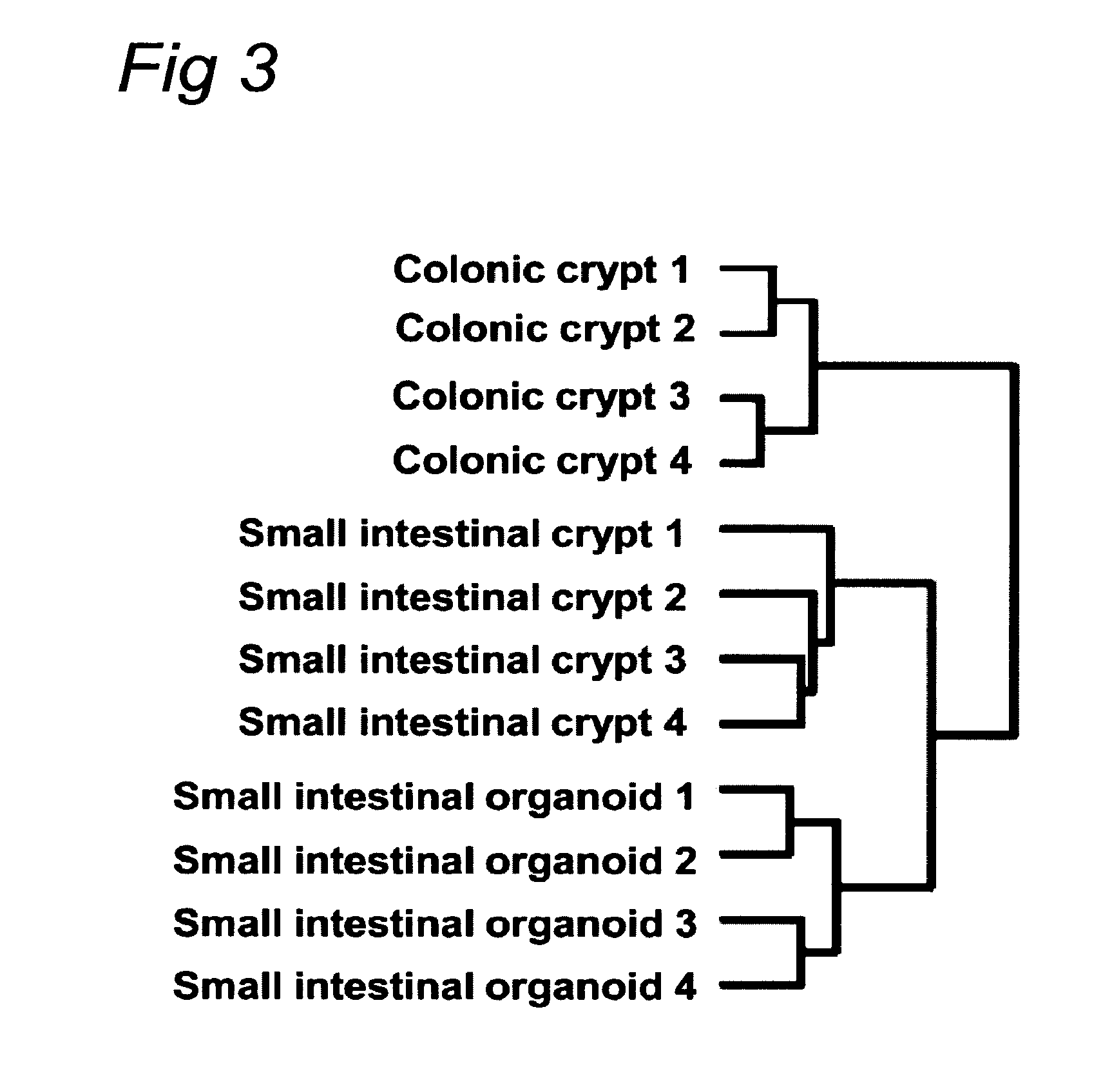
The embodiments of the invention described herein relate to systems and methods for culturing and / or maintaining intestinal cells, tissues and / or organoids in vitro. The cells, tissues and / or organoids cultured according to the methods and systems described herein can mimic or reproduce natural intestinal epithelial structures and behavior as well as support co-culture of intestinal microflora.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
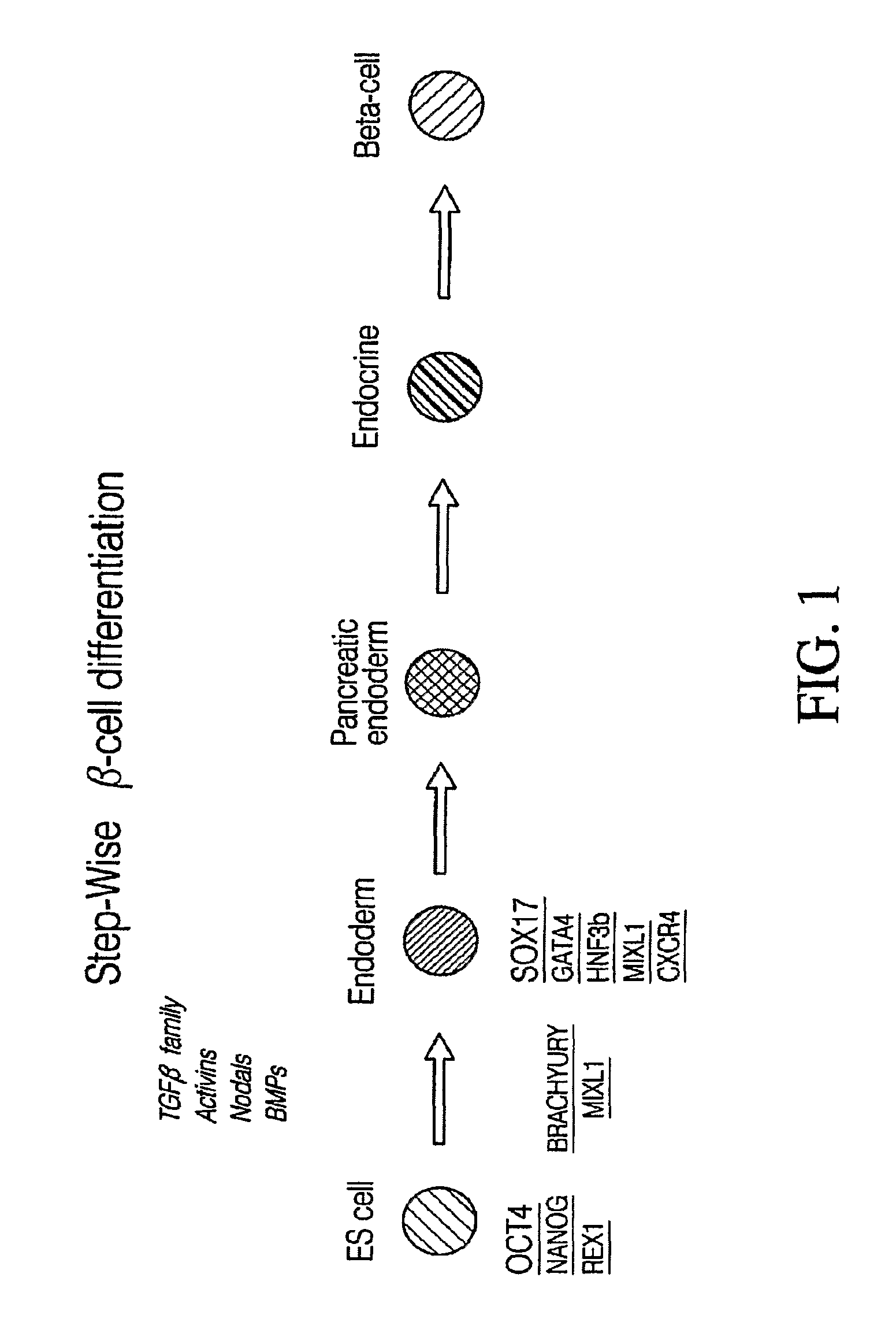
Definitive endoderm
ActiveUS20050158853A1Enough timeGastrointestinal cellsMicrobiological testing/measurementGerm layerCell type
Disclosed herein are cell cultures comprising definitive endoderm cells and methods of producing the same. Also disclosed herein are cell populations comprising substantially purified definitive endoderm cells as well as methods for enriching, isolating and purifying definitive endoderm cells from other cell types.
Owner:VIACYTE INC
Methods for identifying factors for differentiating definitive endoderm
Disclosed herein are methods of identifying one or more differentiation factors that are useful for differentiating cells in a cell population comprising definitive endoderm cells into cells which are capable of forming tissues and / or organs that are derived from the gut tube.
Owner:VIACYTE INC
Definitive endoderm
Disclosed herein are cell cultures comprising definitive endoderm cells and methods of producing the same. Also disclosed herein are cell populations comprising substantially purified definitive endoderm cells as well as methods for enriching, isolating and purifying definitive endoderm cells from other cell types.
Owner:VIACYTE INC
Culture media for stem cells
PendingUS20140243227A1Slow proliferationIncrease surface areaBioreactor/fermenter combinationsBiological substance pretreatmentsStem cell cultureBiology
Culture media and methods for expanding and differentiating populations of stem cells and for obtaining organoids. Expanded cell populations and organoids obtainable by methods of the invention and their use in drug screening, toxicity assays and regenerative medicine.
Owner:KONINK NEDERLANDSE AKADE VAN WETENSCHAPPEN
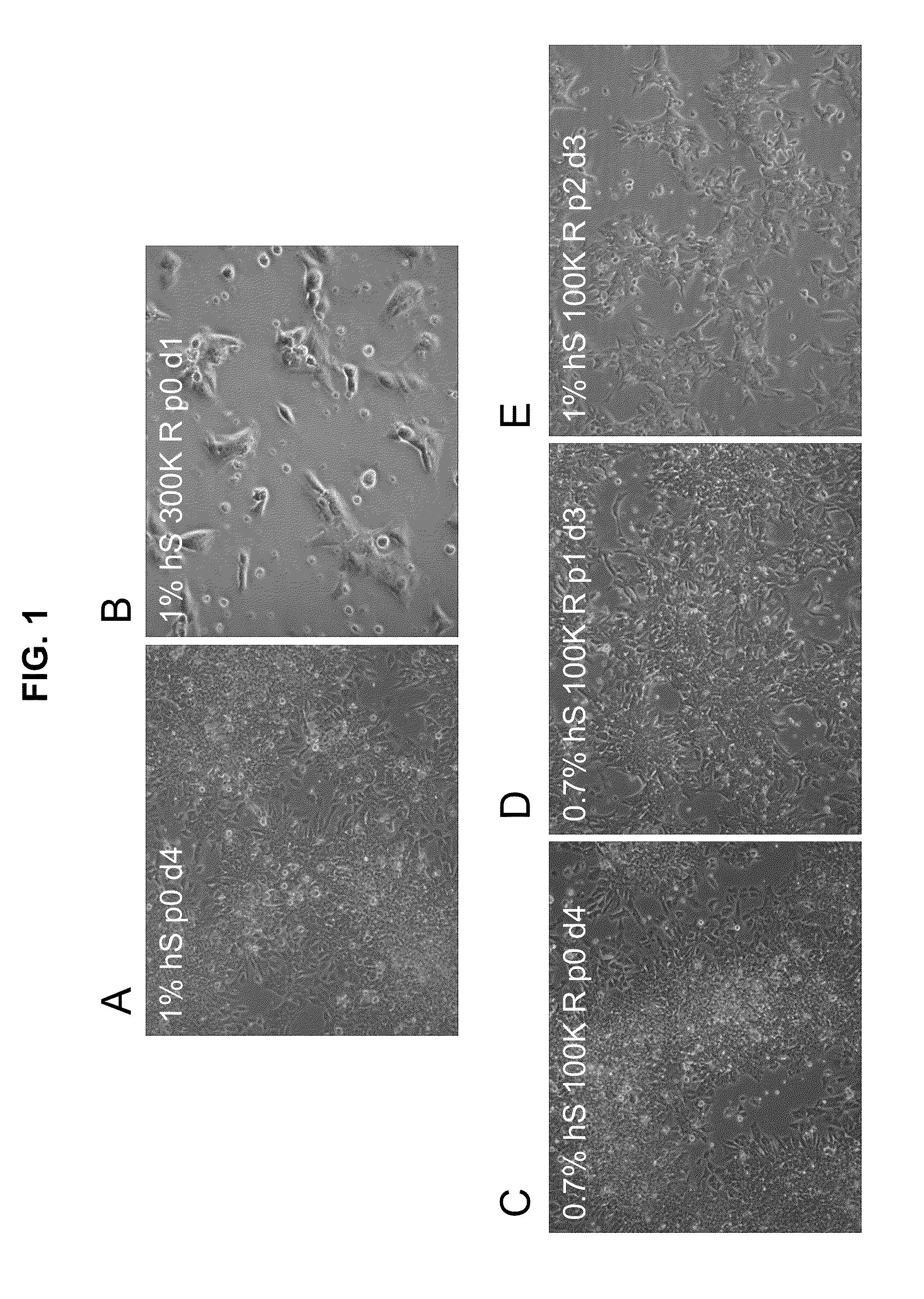
Methods and compositions for feeder-free pluripotent stem cell media containing human serum
The present invention provides compositions and methods for the culture and maintenance of pluripotent stem cells. More particularly, the present invention provides for compositions and methods for culturing, maintaining, growing and stabilizing primate pluripotent stem cells in a feeder-free defined media further comprising human serum, or a soluble attachment component of the human serum, for promoting cell attachment.
Owner:VIACYTE INC
Methods and Compositions for Feeder-Free Pluripotent Stem Cell Media Containing Human Serum
The present invention provides compositions and methods for the culture and maintenance of pluripotent stem cells. More particularly, the present invention provides for compositions and methods for culturing, maintaining, growing and stabilizing primate pluripotent stem cells in a feeder-free defined media further comprising human serum, or a soluble attachment component of the human serum, for promoting cell attachment.
Owner:VIACYTE INC
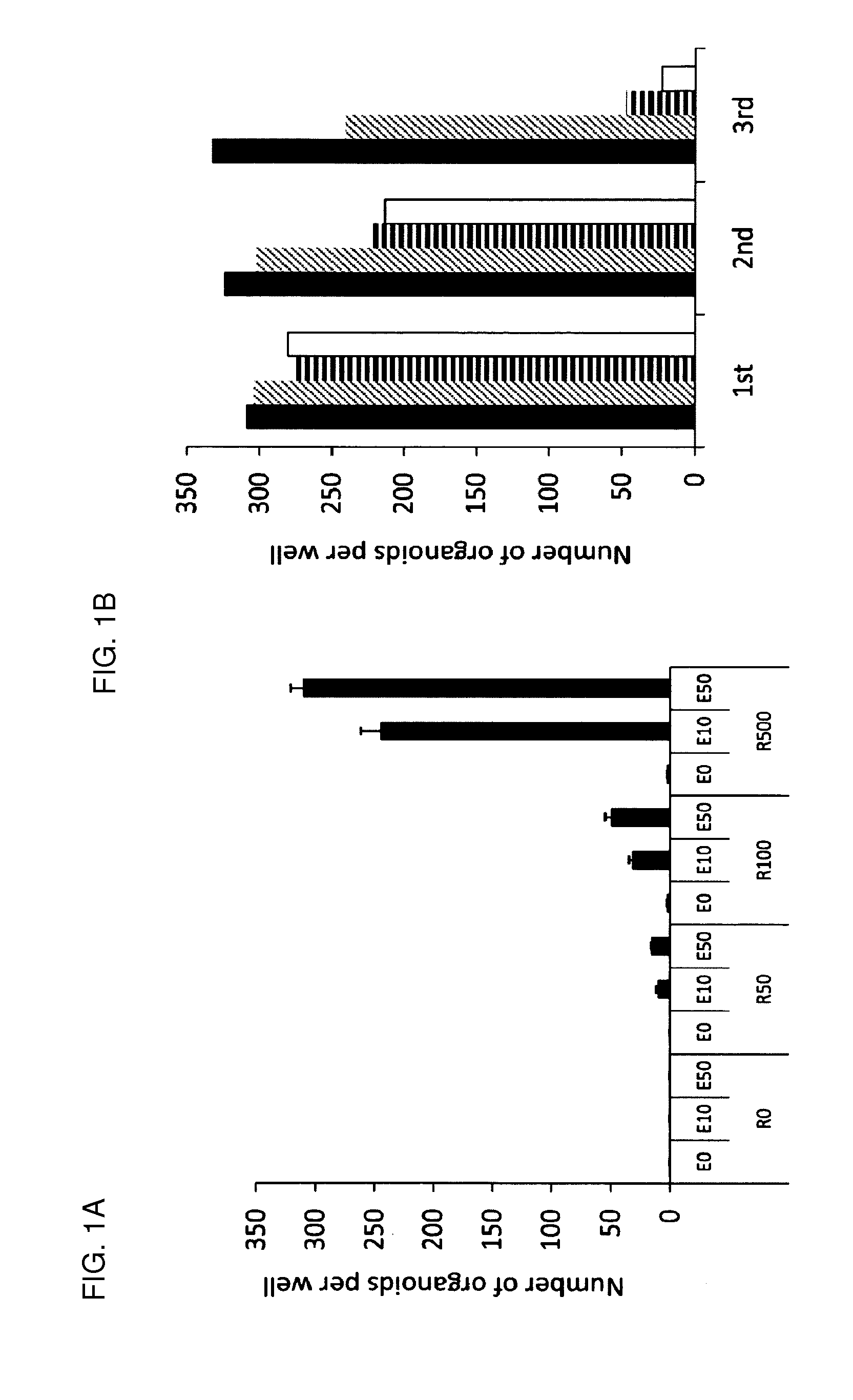
Culture medium for epithelial stem cells and organoids comprising said stem cells
ActiveUS20120028355A1Profound effectGastrointestinal cellsMetabolism disorderCell culture mediaAdenoma
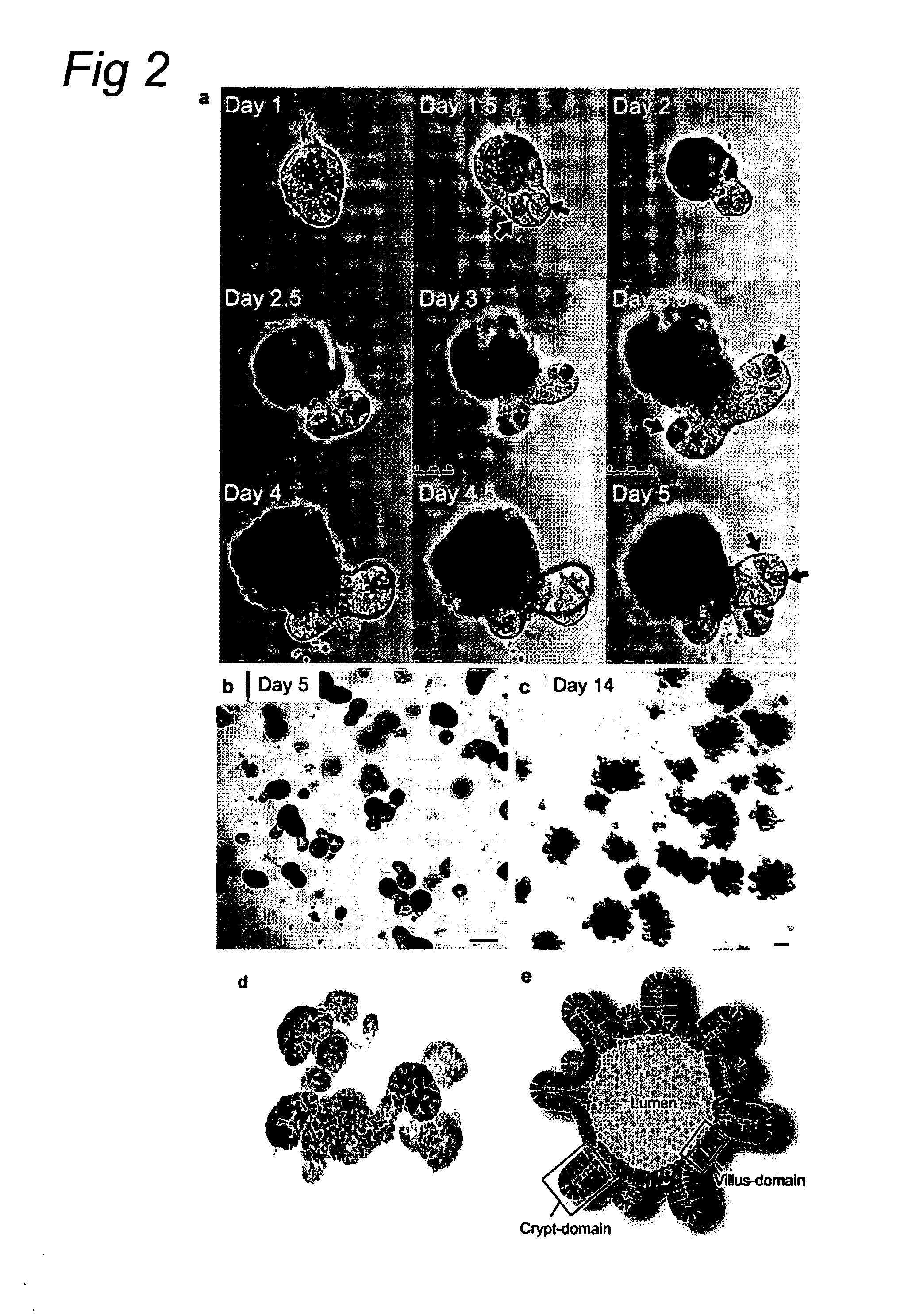
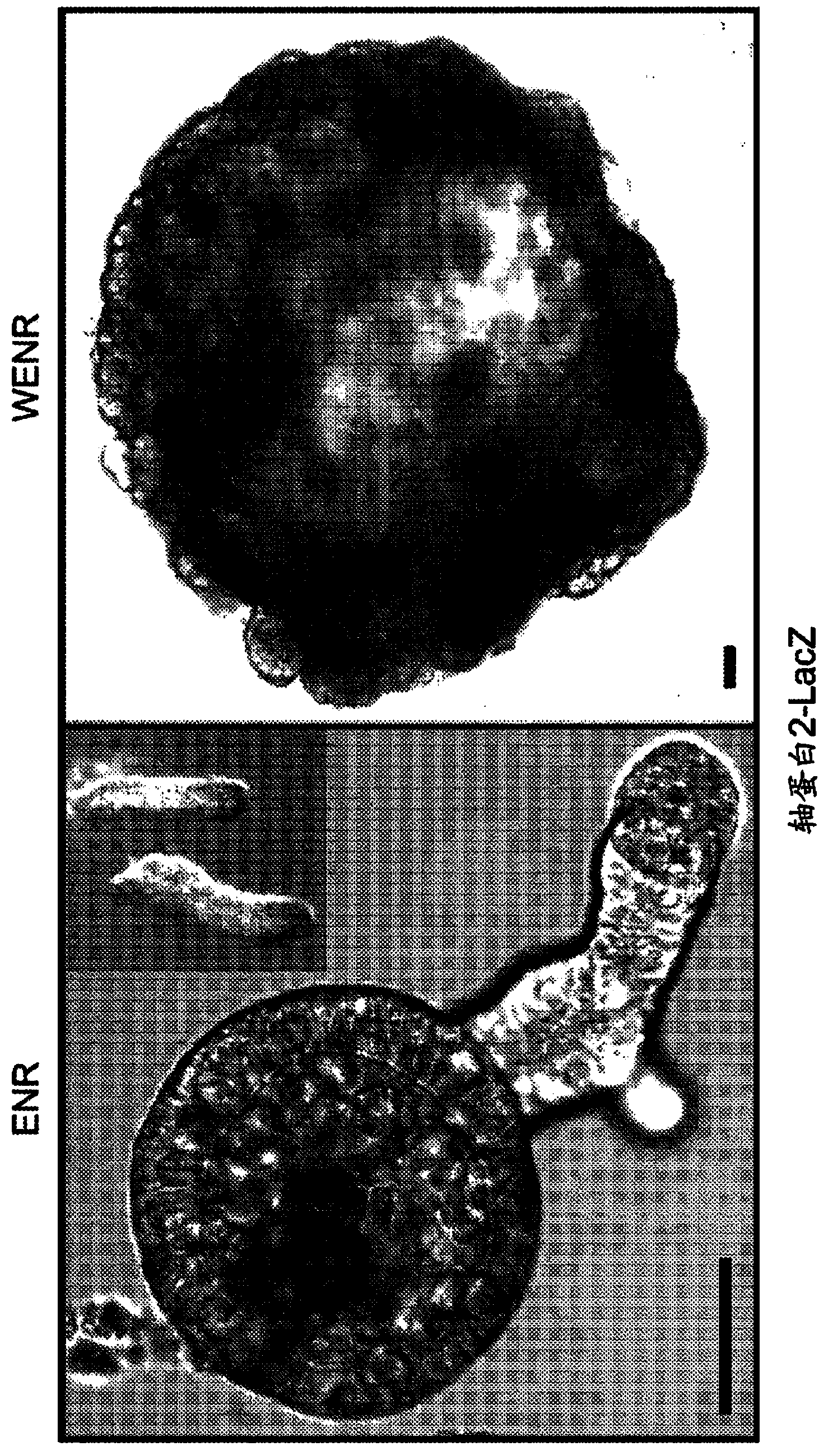
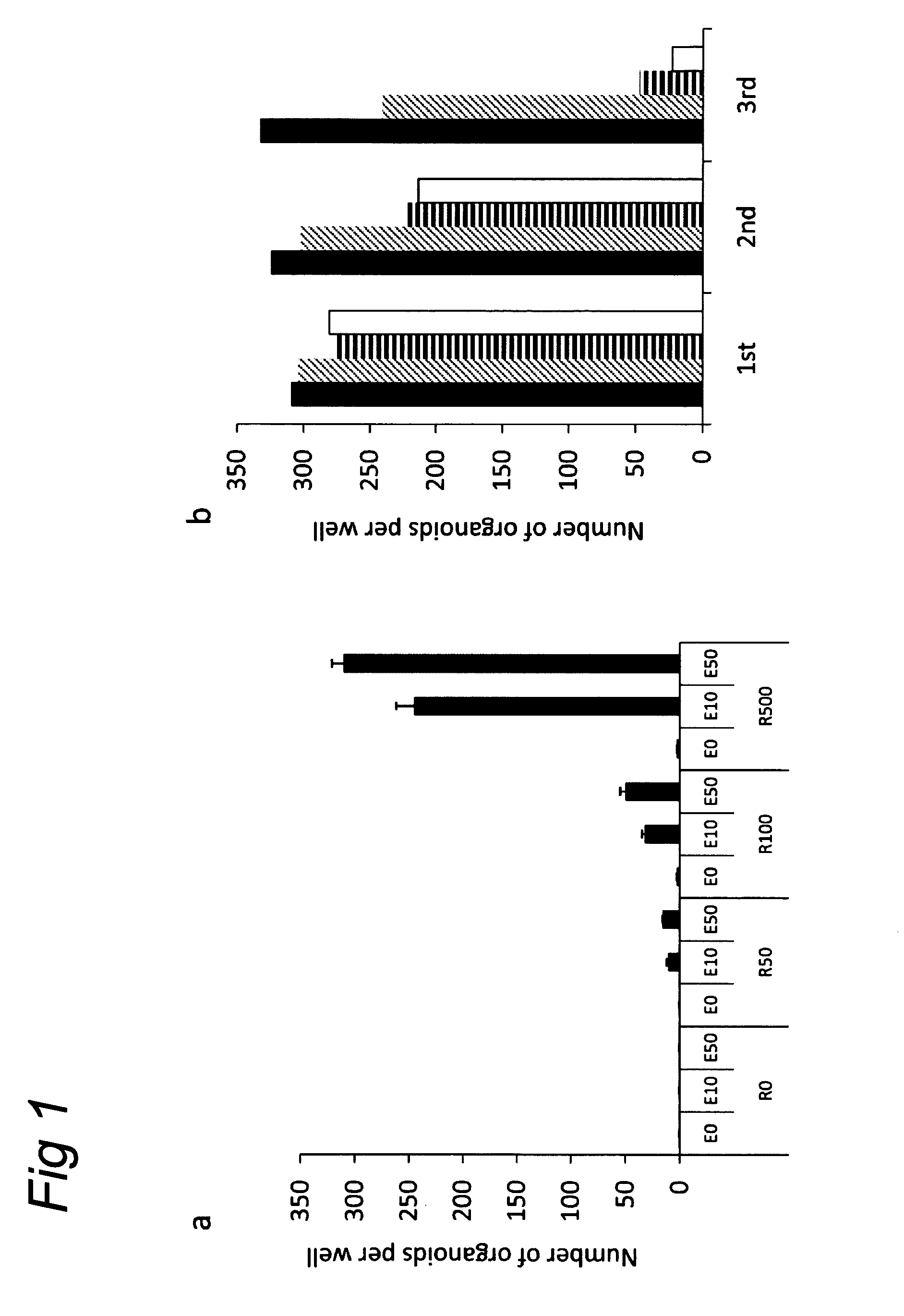
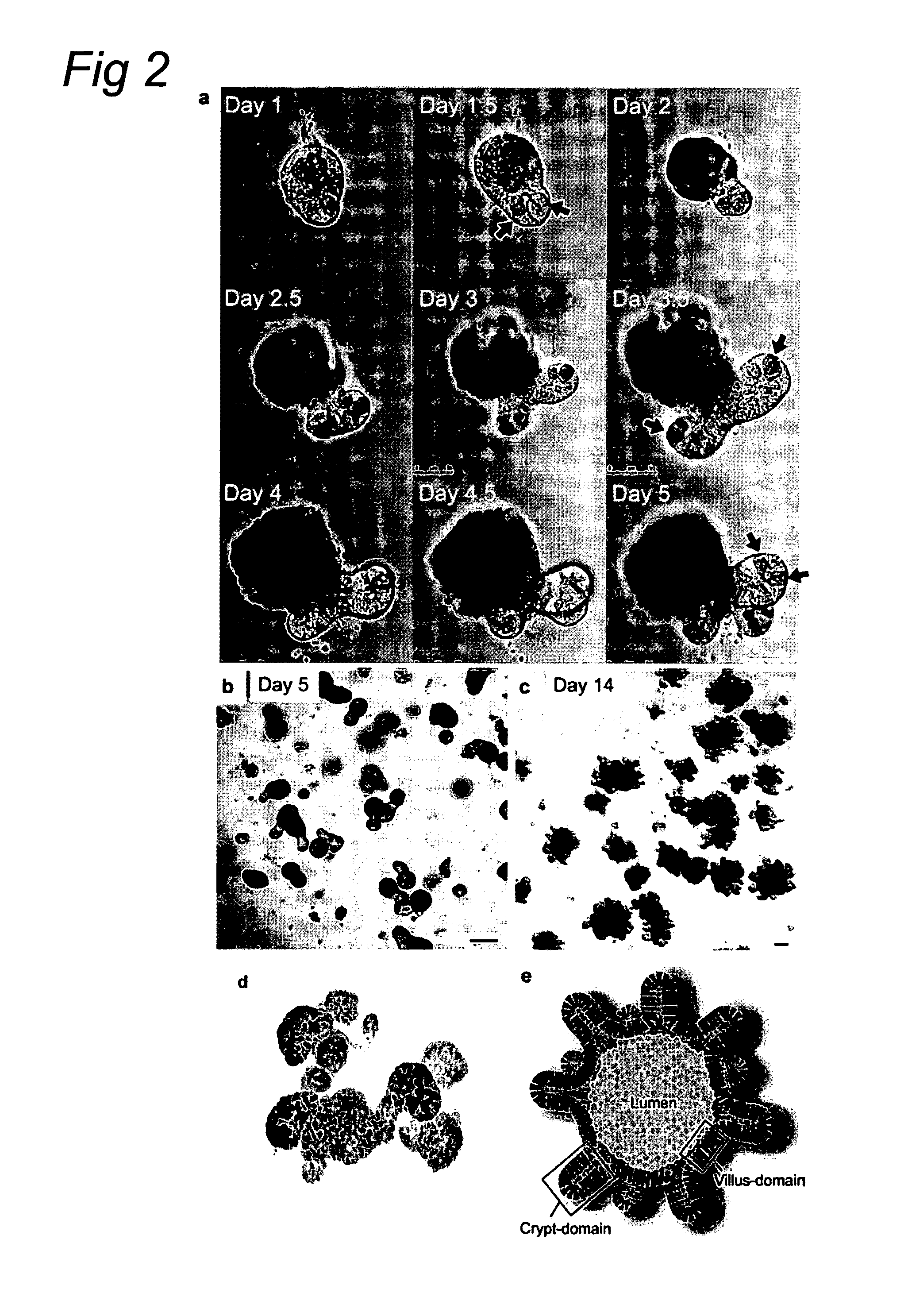
The invention relates to a method for culturing epithelial stem cells, isolated tissue fragments comprising the epithelial stem cells, or adenoma cells, and culturing the cells or fragments in the presence of a Bone Morphogenetic Protein (BMP) inhibitor, a mitogenic growth factor, and a Wnt agonist when culturing epithelial stem cells and isolated tissue fragments. The invention further relates to a cell culture medium comprising a BMP inhibitor, a mitogenic growth factor, and a Wnt agonist, to the use of the culture medium, and to crypt-villus organoids, gastric organoids and pancreatic organoids that are formed in the culture medium.
Owner:KONINK NEDERLANDSE AKADE VAN WETENSCHAPPEN
Culture media for stem cells
Culture media and methods for expanding and differentiating populations of stem cells and for obtaining organoids. Expanded cell populations and organoids obtainable by methods of the invention and their use in drug screening, toxicity assays and regenerative medicine.
Owner:KONINK NEDERLANDSE AKADE VAN WETENSCHAPPEN
Cell culture system
The embodiments of the invention described herein relate to systems and methods for culturing and / or maintaining intestinal cells, tissues and / or organoids in vitro. The cells, tissues and / or organoids cultured according to the methods and systems described herein can mimic or reproduce natural intestinal epithelial structures and behavior as well as support co-culture of intestinal microflora.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Markers of definitive endoderm
ActiveUS20090220959A1Promote differentiationEnough timeHepatocytesGastrointestinal cellsGerm layerEndoderm cell
Disclosed herein are reagent-cell complexes comprising one or more definitive endoderm cells. Also described herein are compositions for detecting definitive endoderm. Method of enriching, isolating and / or purifying definitive endoderm cells are also disclosed.
Owner:VIACYTE INC
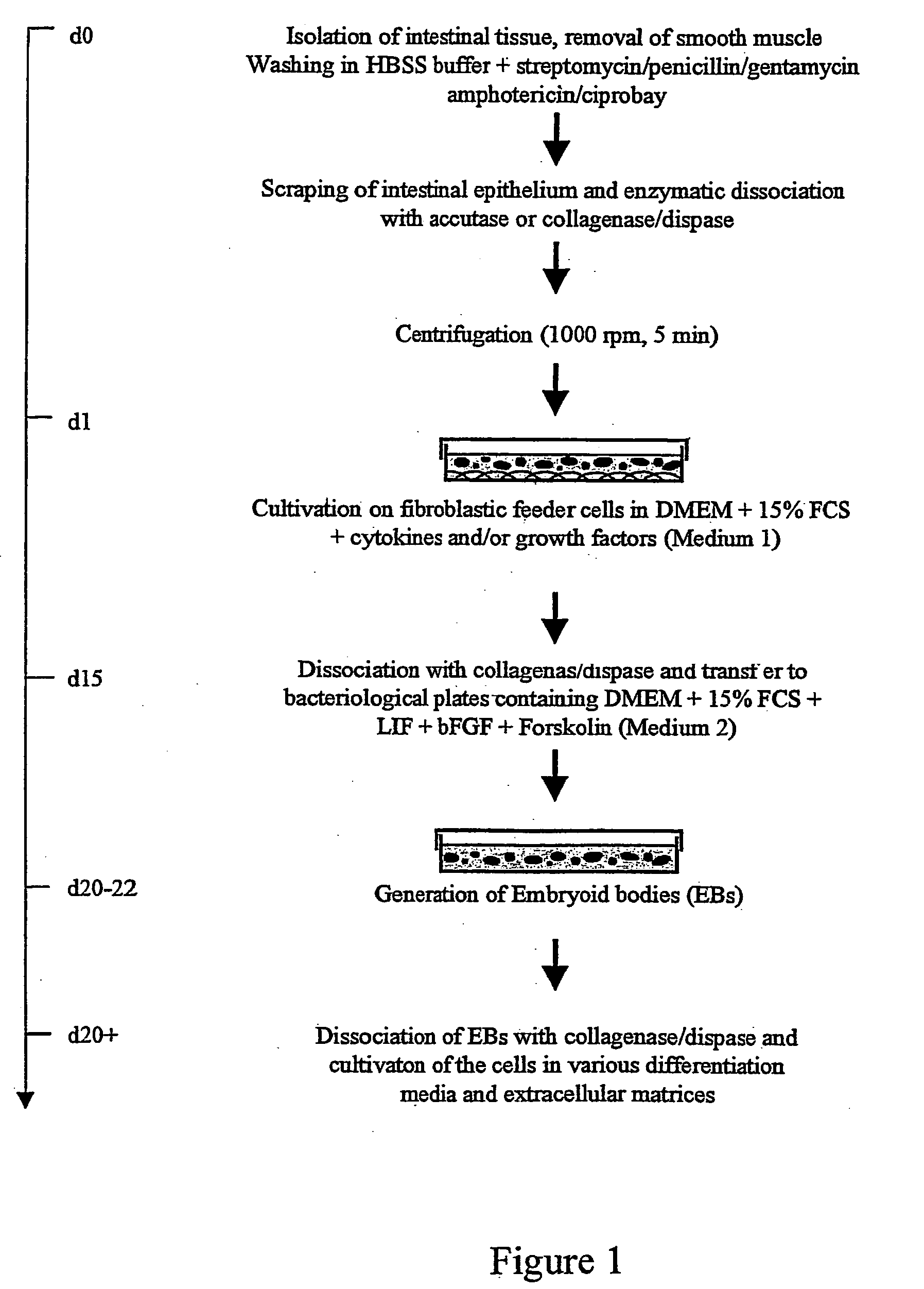
Method for isolating, culturing and differentiating intestinal stem cells for therapeutic use
InactiveUS20050032207A1Promote differentiationEfficient proliferation and differentiationNervous disorderGastrointestinal cellsProgenitor
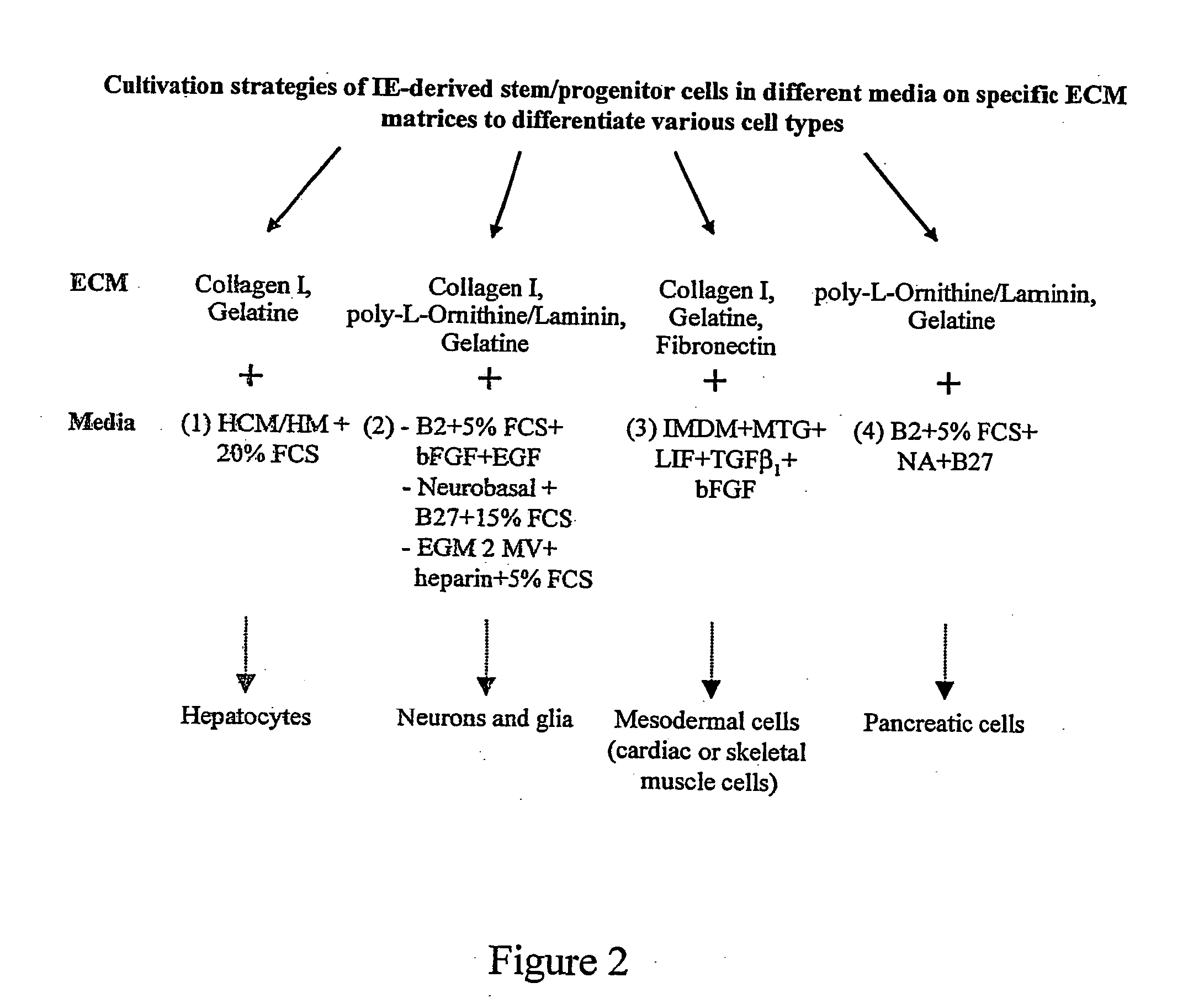
The present invention relates to methods for the isolation, culture, and production of undifferentiated somatic intestinal stem / progenitor cells of mammalian, preferably human origins. The resulting stem / progenitor cells resemble properties of embryonic stem (ES) and multipotent progenitor cells with respect to morphology, biochemical property, and in pluripotency.
Owner:DEVELOGEN AKTIENGES FUR ENTWICKLUNGSBIOLOGISCHE FORSCHUNG +1
Culture medium for epithelial stem cells and organoids comprising the stem cells
The invention relates to a method for culturing epithelial stem cells, isolated tissue fragments comprising the epithelial stem cells, or adenoma cells, and culturing the cells or fragments in the presence of a Bone Morphogenetic Protein (BMP) inhibitor, a mitogenic growth factor, and a Wnt agonist when culturing epithelial stem cells and isolated tissue fragments. The invention further relates to a cell culture medium comprising a BMP inhibitor, a mitogenic growth factor, and a Wnt agonist, to the use of the culture medium, and to crypt-villus organoids, gastric organoids and pancreatic organoids that are formed in the culture medium.
Owner:KONINK NEDERLANDSE AKADE VAN WETENSCHAPPEN
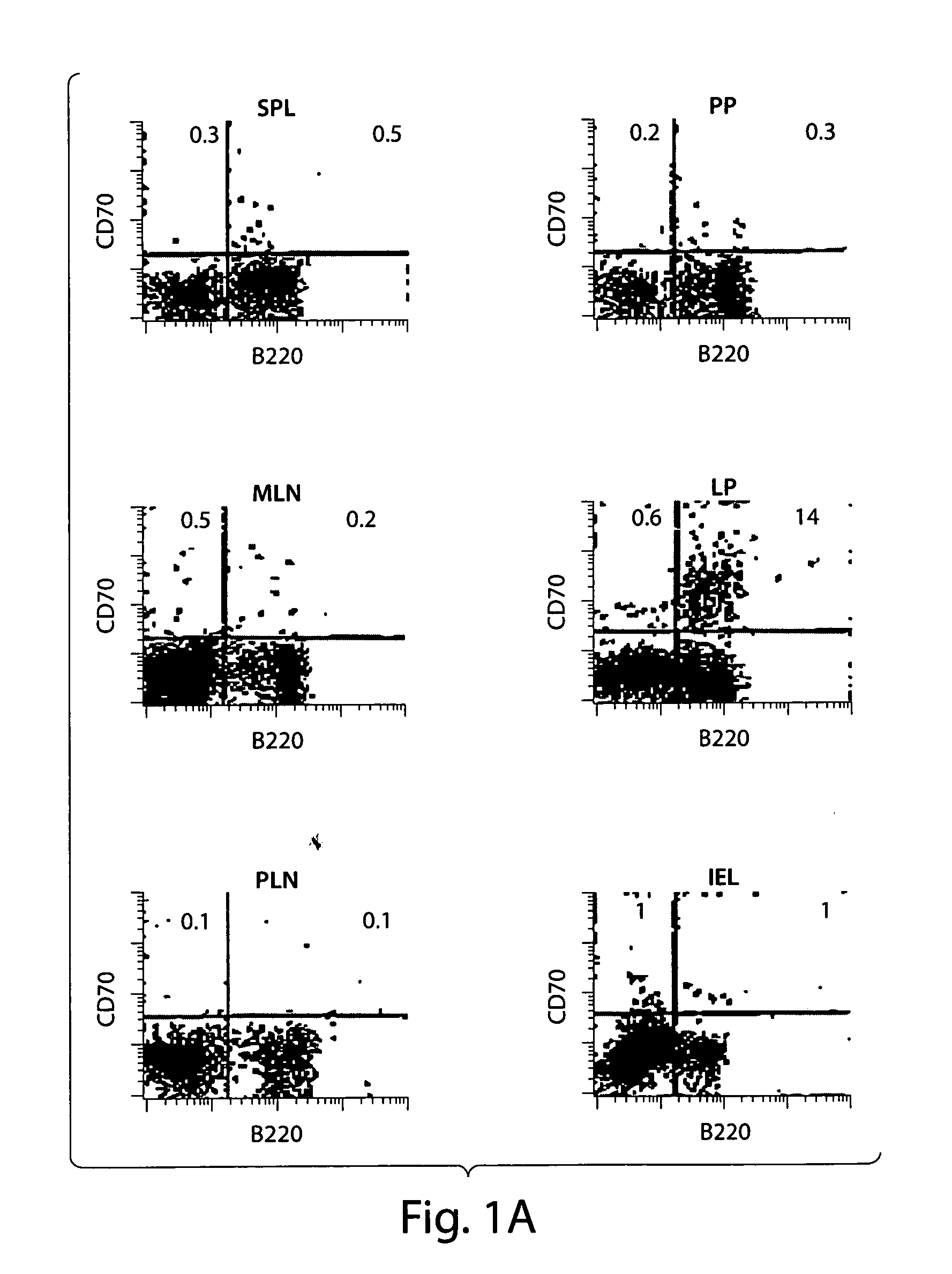
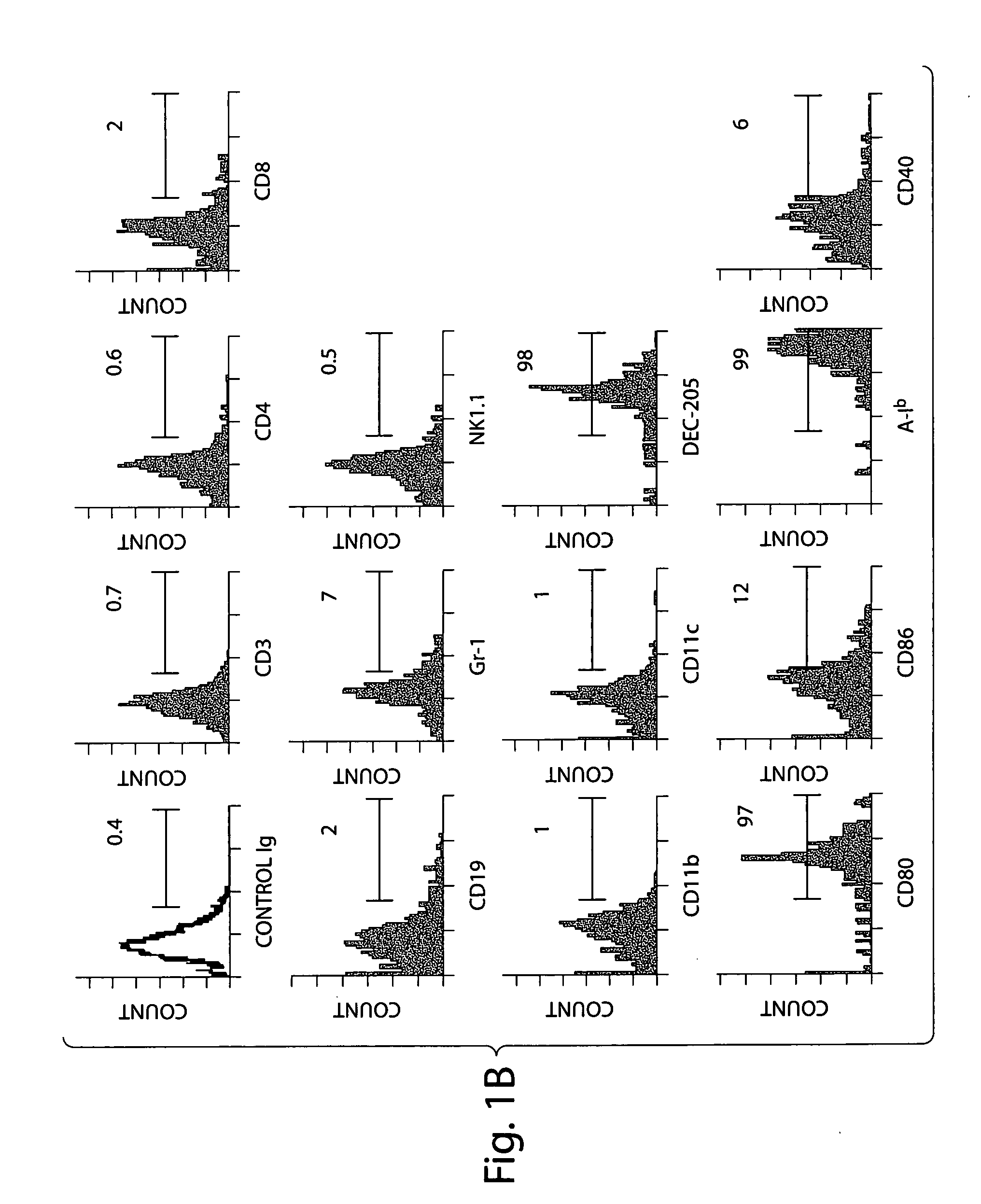
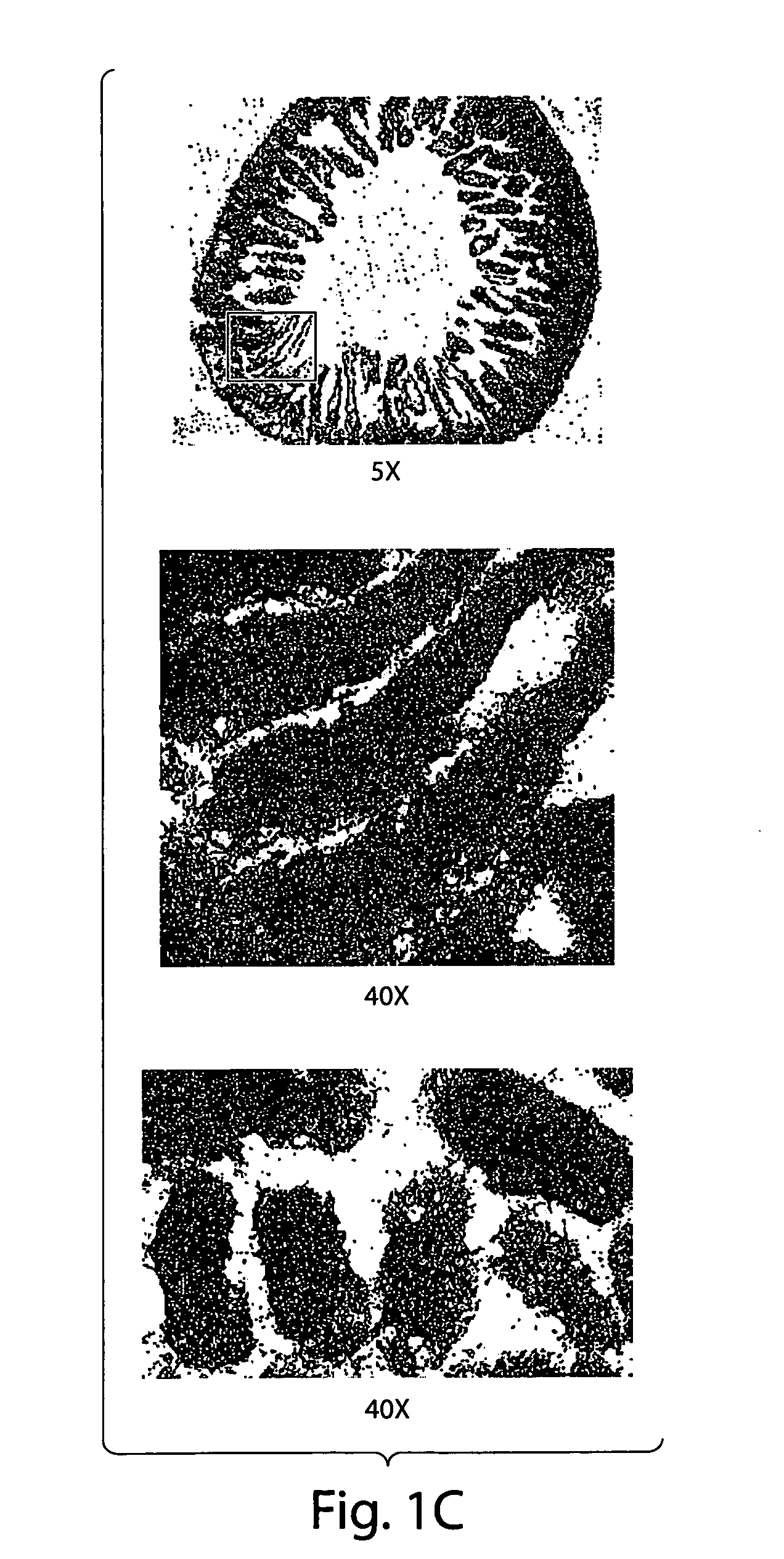
CD70 inhibition for the treatment and prevention of inflammatory bowel disease
ActiveUS20050191299A1Reduce and eliminate inflammatory conditionGastrointestinal cellsDigestive systemInflammatory bowel diseaseCD70
Substantially purified populations of APCLP cells capable of expressing CD70 are described. Also described are methods for the treatment of certain diseases and medical conditions of the gastrointestinal tract, such as inflammatory bowel disease, by utilizing inhibitors of CD70 activity.
Owner:CENT FOR BLOOD RES
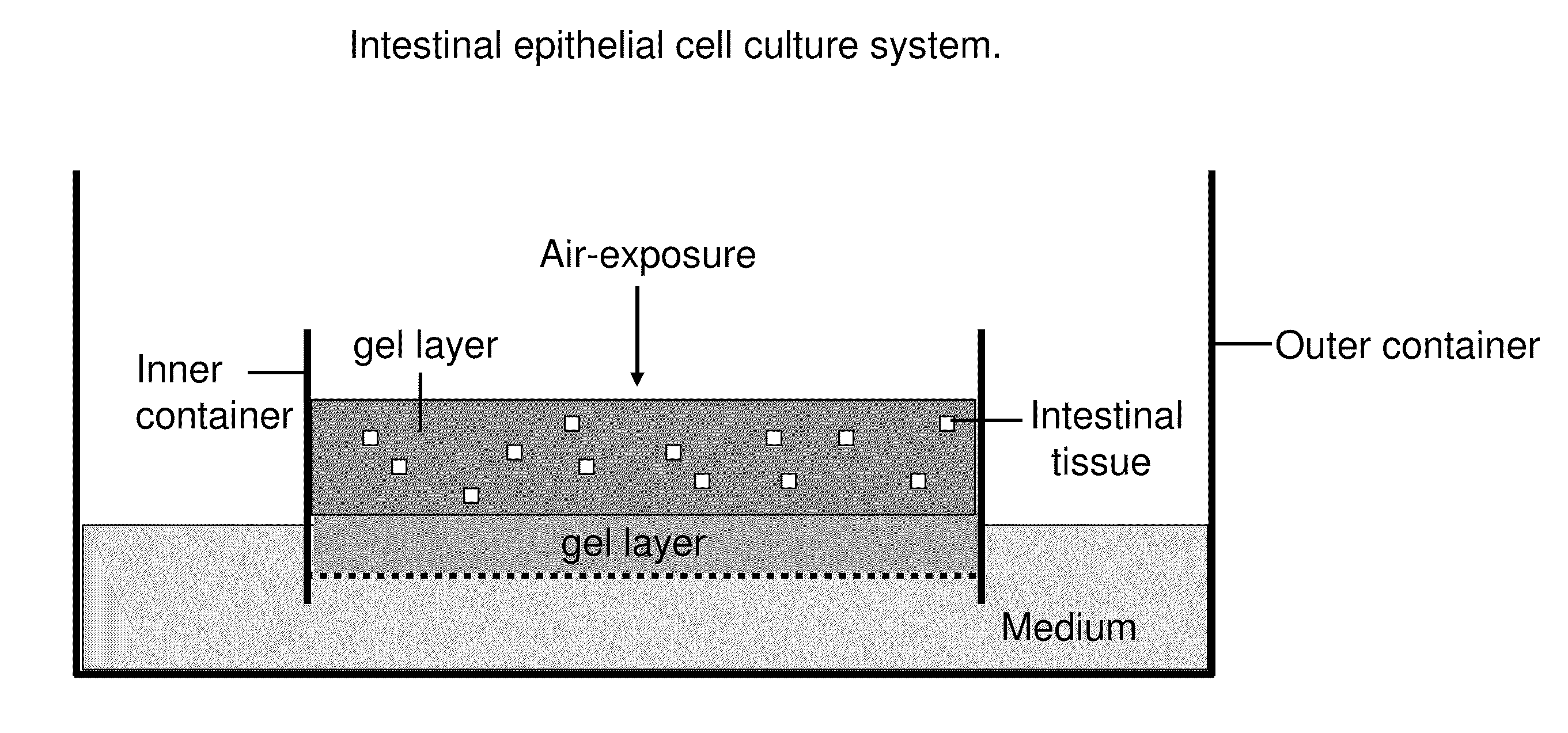
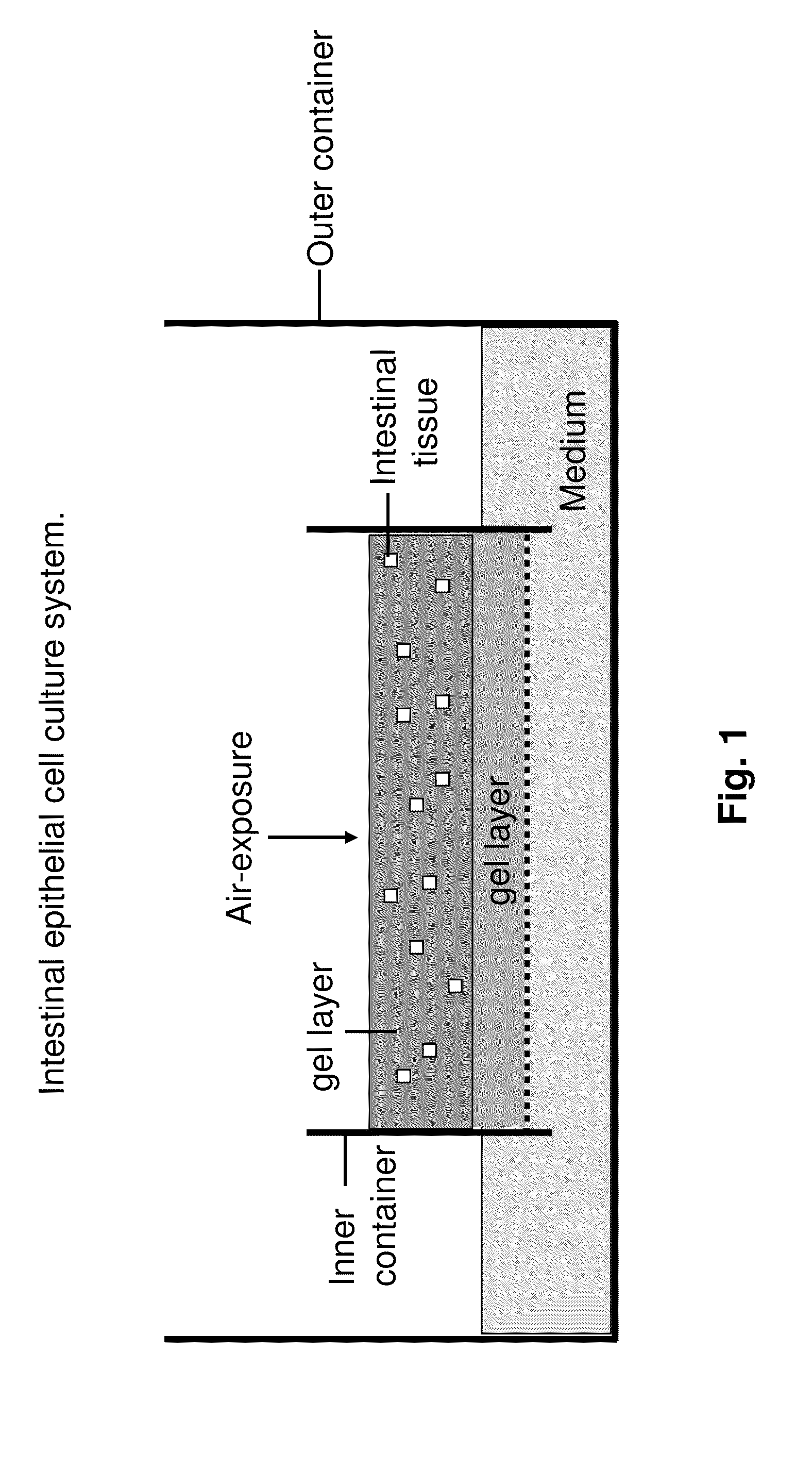
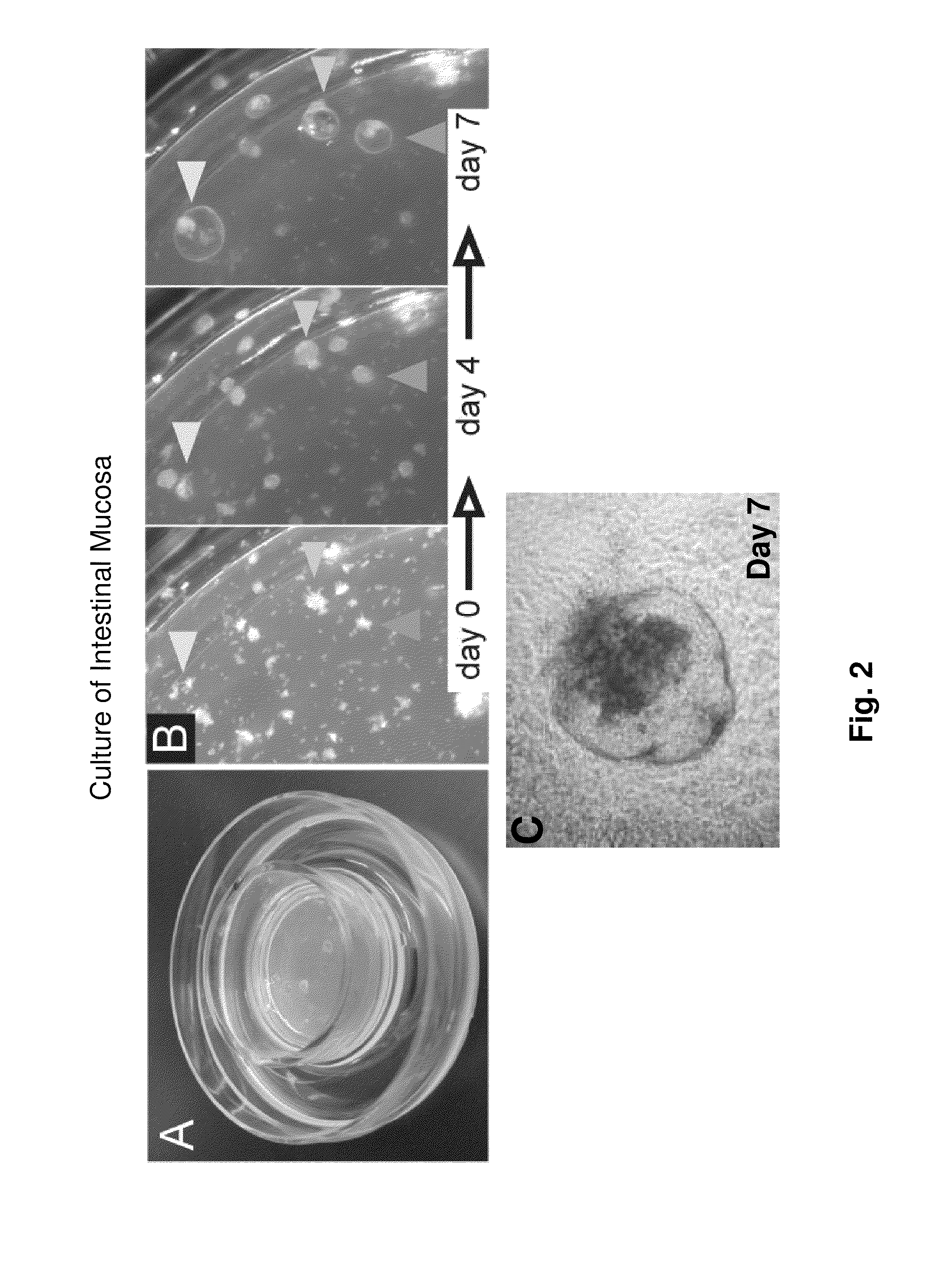
Ex Vivo Culture, Proliferation and Expansion of Intestinal Epithelium
ActiveUS20100047853A1Induce pluripotencyAlter differentiationGastrointestinal cellsMicrobiological testing/measurementAir liquid interfaceMammal
Methods are provided for long term culture of mammalian intestinal cells. Cultures are initiated with fragments of mammalian intestinal tissue, which are then maintained embedded in a gel substrate that provides an air-liquid interface. Intestinal epithelium in cultures of the invention can be continuously grown for extended periods of time. Mammalian intestinal cells cultured by the methods of the invention recapitulate features of intestinal growth in vivo.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Cell culture system
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Markers of definitive endoderm
ActiveUS8586357B2Promote differentiationEnough timeHepatocytesGastrointestinal cellsGerm layerEndoderm cell
Disclosed herein are reagent-cell complexes comprising one or more definitive endoderm cells. Also described herein are compositions for detecting definitive endoderm. Method of enriching, isolating and / or purifying definitive endoderm cells are also disclosed.
Owner:VIACYTE INC
Composition and method for culturing potentially regenerative cells and functional tissue-organs in vitro
Compositions and methods are provided for culturing in vitro potentially regenerative cells (PRCs) from which functional tissue-organs are regenerated. In one aspect of the invention, a tissue culture medium is provided which comprises at least 50% of water and a sterol compound that is dissolved in a fatty acid-containing oil at a concentration at least 0.1% by weight based on the weight of the oil and added to the water. The culture medium can be used to culture PRCs that are isolated from the body of a mammal to generate functional tissue-organs in vitro with substantially the same physiological structure and function as the corresponding ones existing in vivo and in situ. The cultured PRCs, tissues, and tissue-organs can serve as valuable models for scientific investigation in life sciences, nutraceutical discovery, drug screening, pharmacokinetic studies, medical devices and tissue / organ transplantation.
Owner:RONGXIANG XU
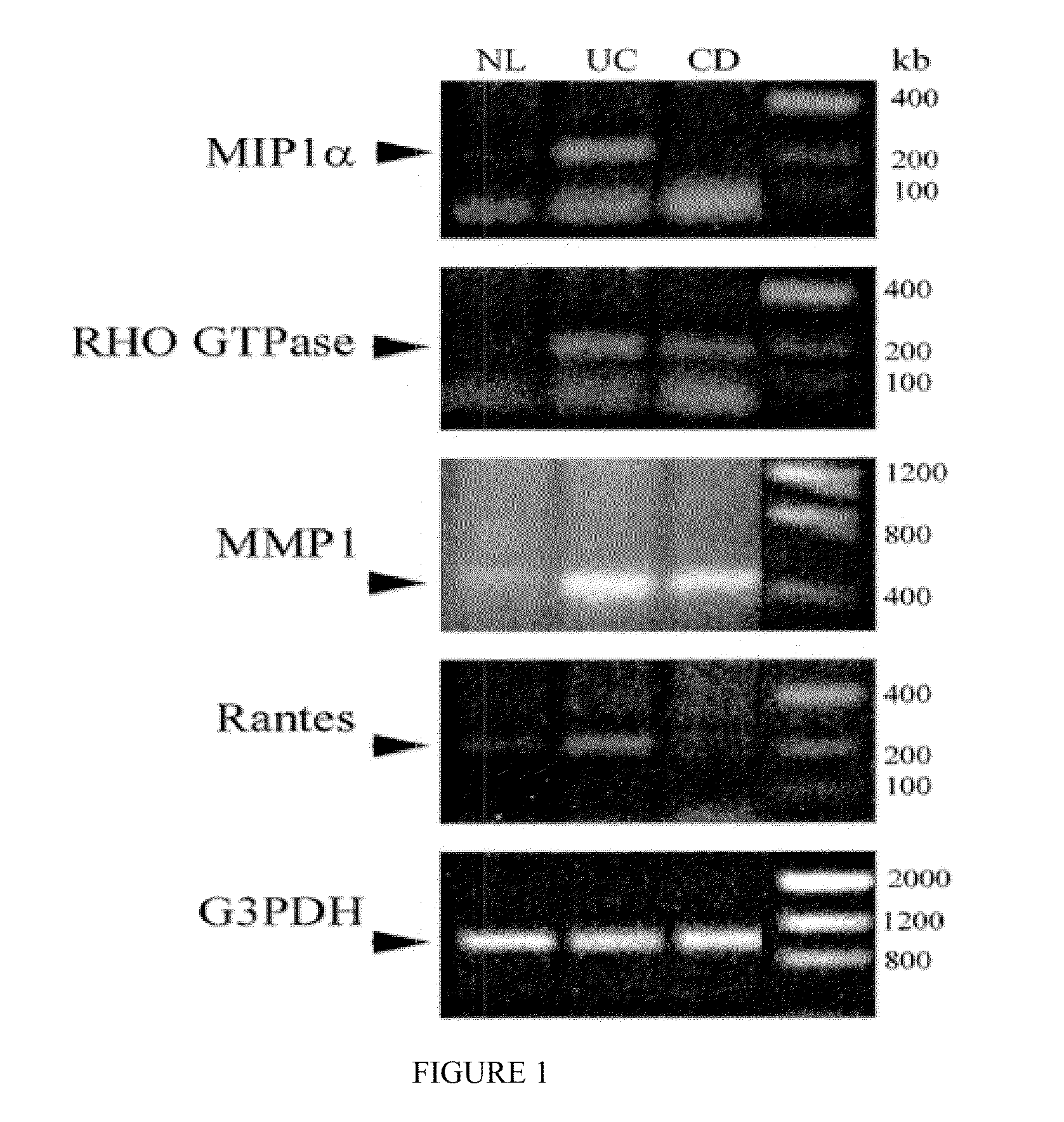
Use and identification of biomarkers for gastrointestinal diseases
InactiveUS20100093552A1Compound screeningApoptosis detectionUlcerative colitisManagement of Crohn's disease
The described invention relates to the identification of biomarkers for gastrointestinal diseases and provides methods utilizing the biomarkers for in drug discovery, monitoring of treatment efficacy, and diagnostics. The invention further provides methods for identifying a therapeutic target to treat ulcerative colitis, colorectal cancer, and Crohn's disease.
Owner:ALFAGENE BIOSCI
Culture medium for epithelial stem cells and organoids comprising the stem cells
The invention relates to a method for culturing epithelial stem cells, isolated tissue fragments comprising said epithelial stem cells, or adenoma cells, and culturing the cells or fragments in the presence of a Bone Morphogenetic Protein (BMP) inhibitor, a mitogenic growth factor, and a Wnt agonist when culturing epithelial stem cells and isolated tissue fragments. The invention further relates to a cell culture medium comprising a BMP inhibitor, a mitogenic growth factor, and a Wnt agonist, to the use of said culture medium, and to crypt-villus organoids, gastric organoids and pancreatic organoids that are formed in said culture medium.
Owner:KONINK NEDERLANDSE AKADE VAN WETENSCHAPPEN
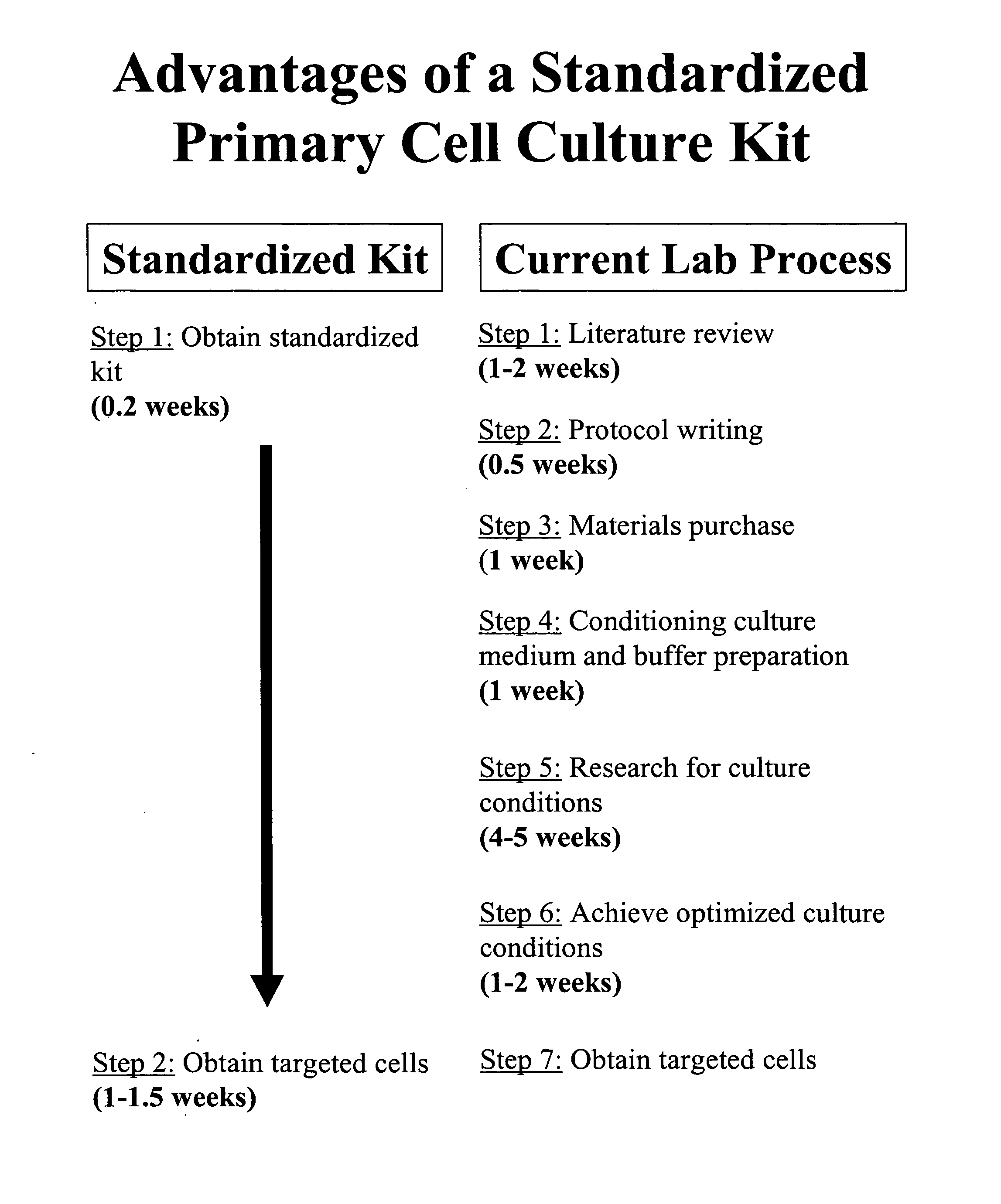
Standardization of processes for culturing primary cells
InactiveUS20070238175A1Promotes target cell enrichmentPromote growthCell dissociation methodsGastrointestinal cellsCell specificPrimary cell
The present invention provides a standardized tissue-specific and cell-specific kit and methods for promoting the enrichment and expansion of primary cells in culture while reducing the contamination of unwanted cell types. The present invention further provides the compositions for optimized tissue-specific and cell-type specific dissociation of tissues and inhibition of contaminating cell-types in primary cultures.
Owner:CHI SCI
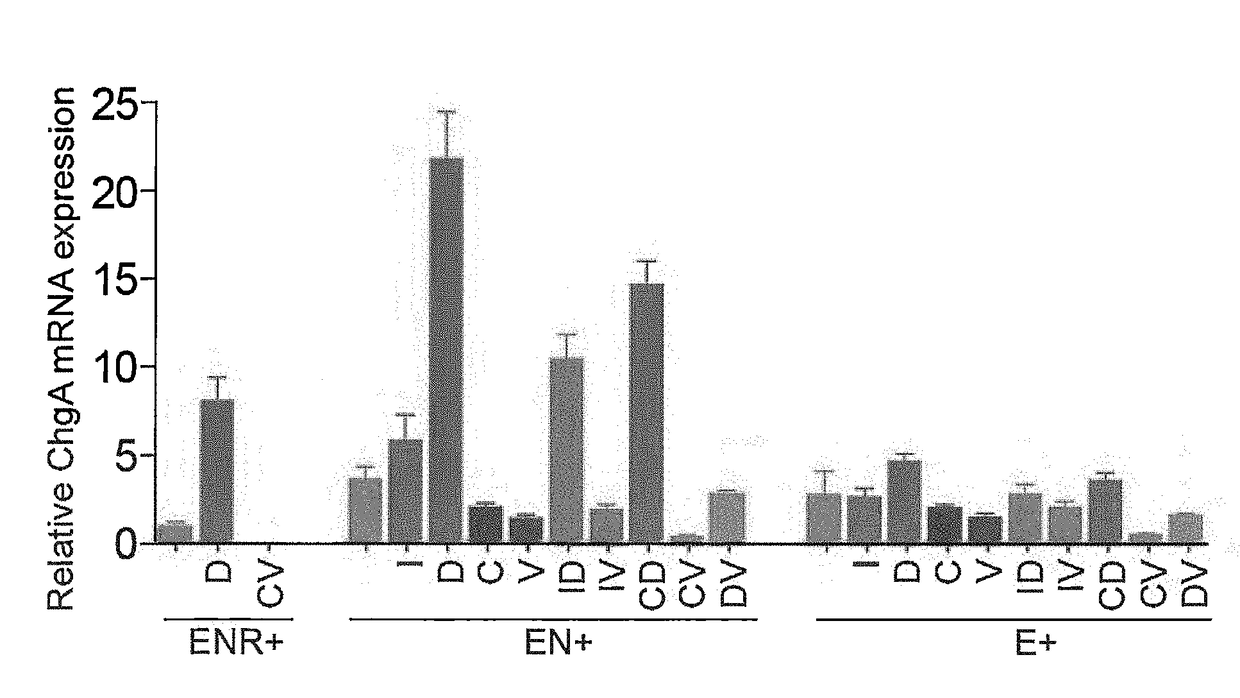
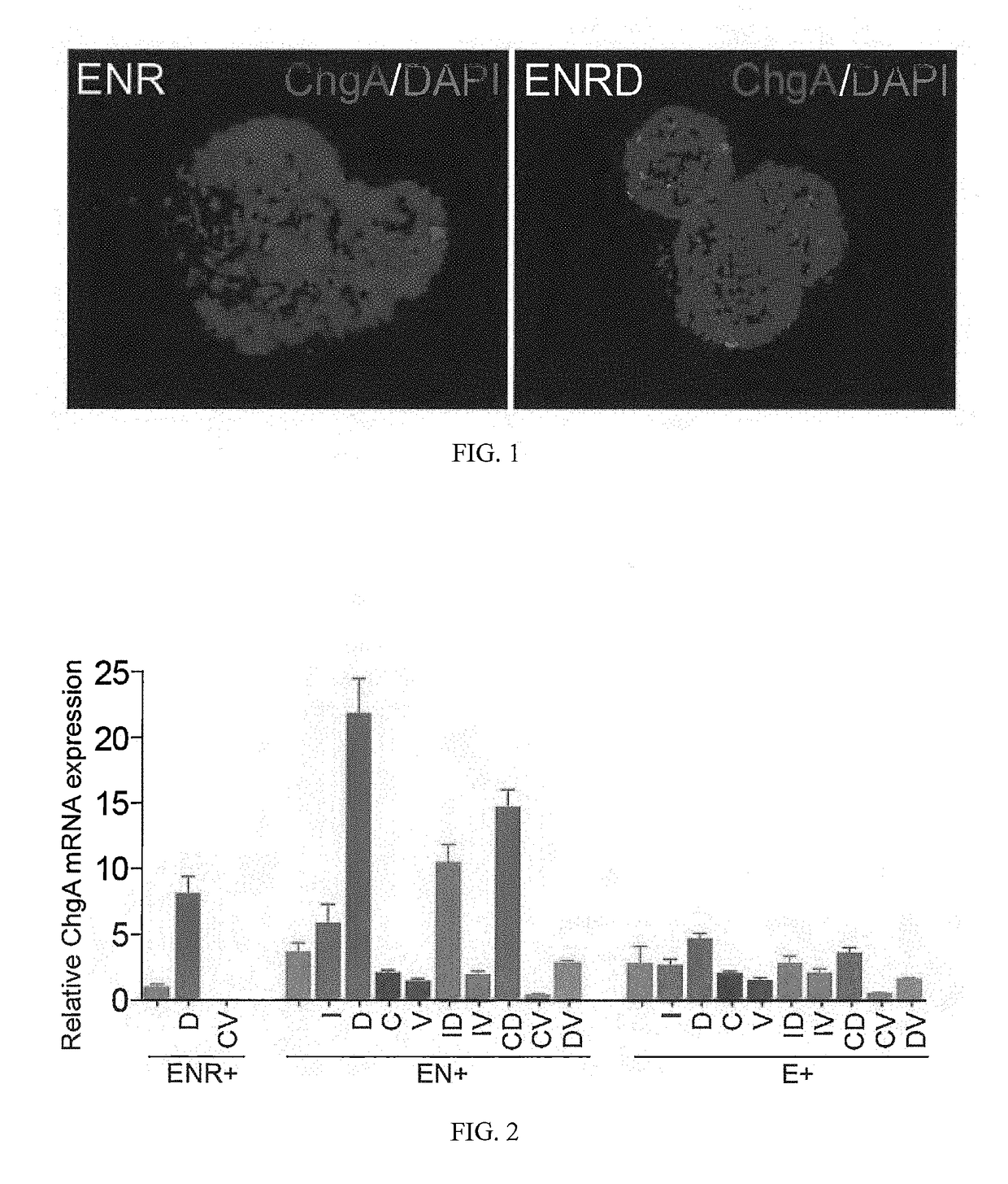
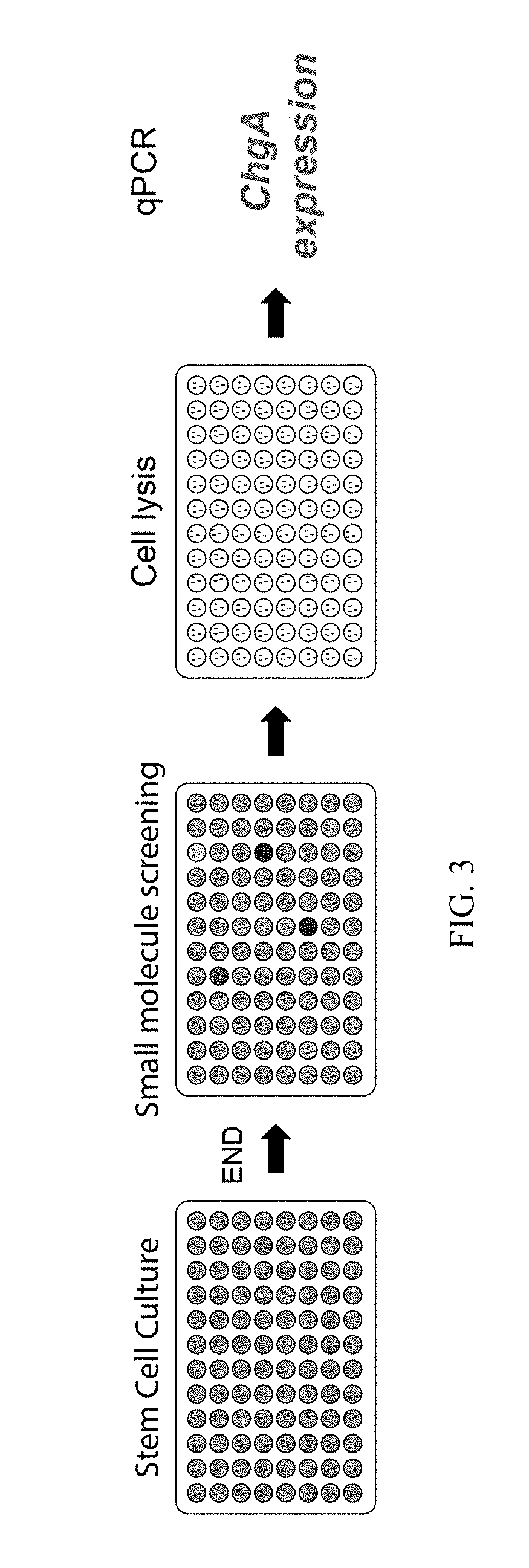
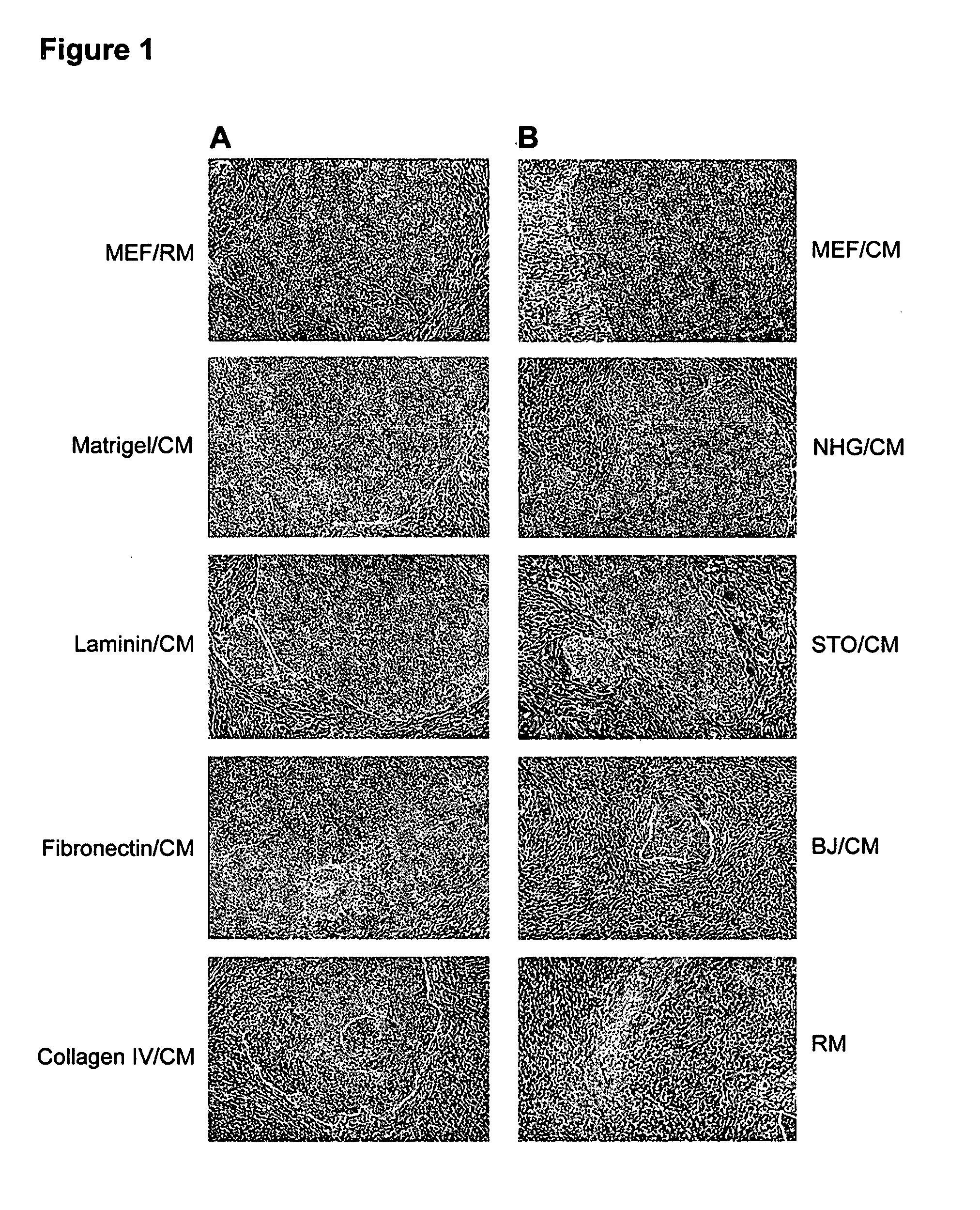
Production of Differentiated Enteroendocrine Cells and Insulin Producing Cells
ActiveUS20170349884A1Promote cell differentiationHigh expressionGastrointestinal cellsAntipyreticHistone methylationWnt inhibitor
A population of enteroendocrine cells (EEC) is obtained from a mammalian post-natal cell population, such as a population including post-natal stem cells, by treating the population with a plurality of small molecules that upregulate ChgA and promote differentiation of the cells to form the enteroendocrine cells. The upregulation of ChgA is such that the fraction of cells expressing CGA in the obtained cell population, as measured by a ChgA Immunostaining Assay, is at least about 1.5%. Small molecules that can be used to differentiate the post-natal cells into the enteroendocrine cells can include at least one of a Wnt activator, a Notch inhibitor, a Wnt inhibitor, a MEK / ERK inhibitor, a growth factor, a HDAC inhibitor, a Histone Methylation Inhibitor, a Tgf-β inhibitor, and a NeuroD1 activator. Also, the insulin expression of a population of mammalian cells is increased by treating the population with a plurality of small molecules that increase the insulin expression.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
System for expanding and differentiating human embryonic stem cells
InactiveUS20050153445A1Rapid productionExpanding primate pluripotent stem (pPS) cellsHepatocytesGastrointestinal cellsGerm layerPluripotential stem cell
This disclosure provides an improved system for culturing human pluripotent stem cells. Traditionally, pluripotent stem cells are cultured on a layer of feeder cells (such as mouse embryonic fibroblasts) to prevent them from differentiating. In the system described here, the role of feeder cells is replaced by components added to the culture environment that support rapid proliferation without differentiation. Effective features are a suitable support structure for the cells, and an effective medium that can be added fresh to the culture without being preconditioned by another cell type. Culturing human embryonic stem cells in fresh medium according to this invention causes the cells to expand surprisingly rapidly, while retaining the ability to differentiate into cells representing all three embryonic germ layers. This new culture system allows for bulk proliferation of pPS cells for commercial production of important products for use in drug screening and human therapy.
Owner:ASTERIAS BIOTHERAPEUTICS INC
Using fibroblast growth factor to establish a line of embryonic stem cells
InactiveUS20050153444A1Rapid productionSimple systemHepatocytesGastrointestinal cellsGerm layerPluripotential stem cell
This disclosure provides an improved system for culturing human pluripotent stem cells. Traditionally, pluripotent stem cells are cultured on a layer of feeder cells (such as mouse embryonic fibroblasts) to prevent them from differentiating. In the system described here, the role of feeder cells is replaced by components added to the culture environment that support rapid proliferation without differentiation. Effective features are a suitable support structure for the cells, and an effective medium that can be added fresh to the culture without being preconditioned by another cell type. Culturing human embryonic stem cells in fresh medium according to this invention causes the cells to expand surprisingly rapidly, while retaining the ability to differentiate into cells representing all three embryonic germ layers. This new culture system allows for bulk proliferation of pPS cells for commercial production of important products for use in drug screening and human therapy.
Owner:ASTERIAS BIOTHERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com