System for expanding and differentiating human embryonic stem cells
a human embryonic stem cell and system technology, applied in the field of regenerative medicine, can solve the problems that the technology for culturing and differentiating human pluripotent stem cells is considerably less advanced, and achieve the effects of rapid production of high-quality pps cells, improving the quality and expansion rate of culture, and expanding primate pluripotent stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Growing hES cells without feeder cells in conditioned medium
[0106] In this example, undifferentiated hES cells that had been maintained on primary mouse embryonic feeder cells were maintained in the absence of feeders. The culture wells were coated with Matrigel®, and the cells were cultured in the presence of conditioned nutrient medium obtained from a culture of irradiated primary fibroblasts.
[0107] Conditioned medium (CM) was prepared as follows. The fibroblasts were harvested from T150 flasks by washing once with Ca++ / Mg++ free PBS and incubating in trypsin / EDTA (Gibco). After the fibroblasts detached from the flask, they were collected in mEF medium (DMEM+10% FBS). The cells were irradiated at 4000 rad, counted and seeded at about 55,000 cells cm−2 in mEF medium. After at least 4 hours, the medium was exchanged with SR containing ES medium. Conditioned medium was collected daily for feeding of hES cultures. Alternatively, medium was prepared using mEF plated in culture flasks...
example 2
Phenotypic Markers of hES Cells in Feeder-Free Culture
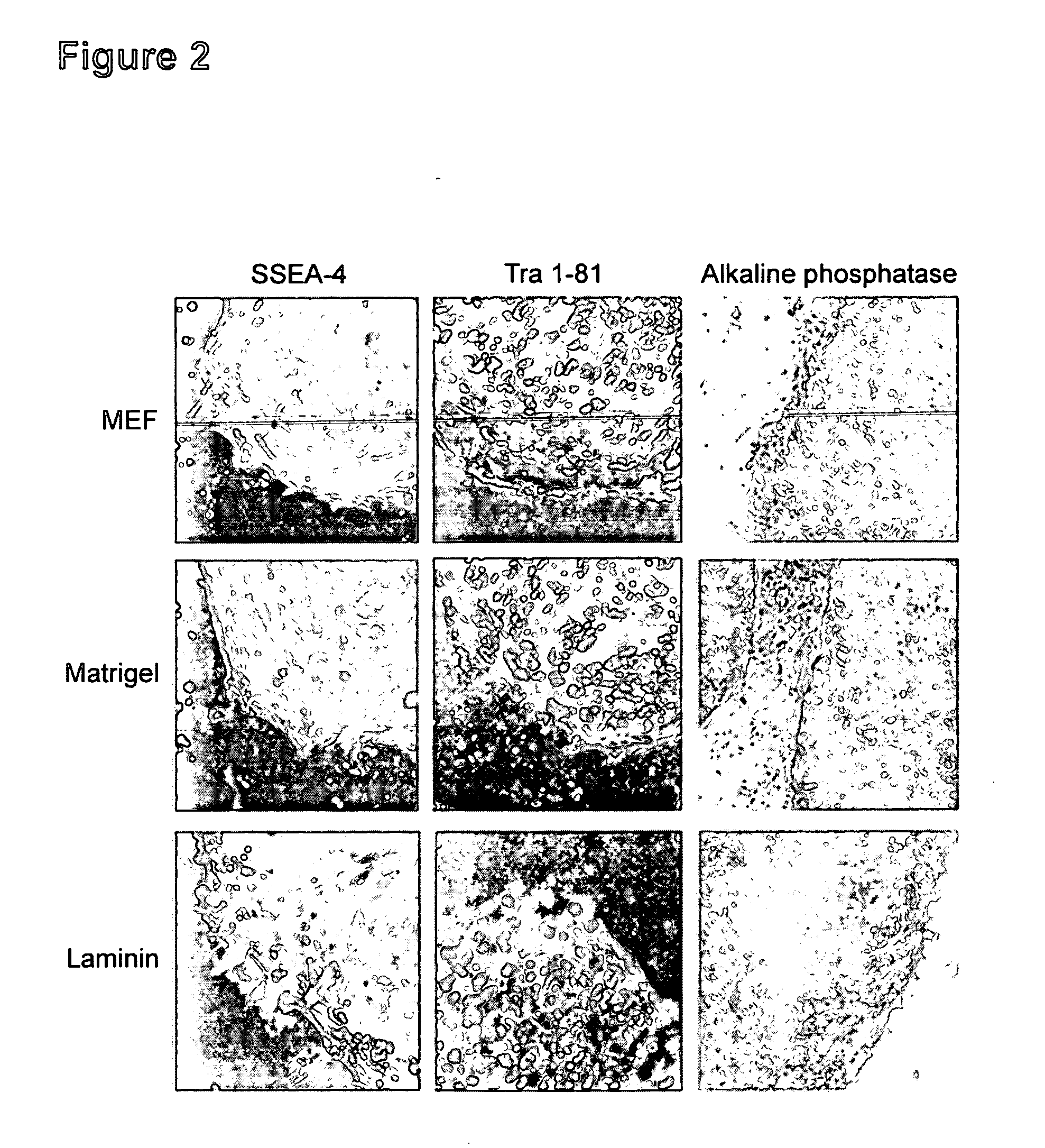
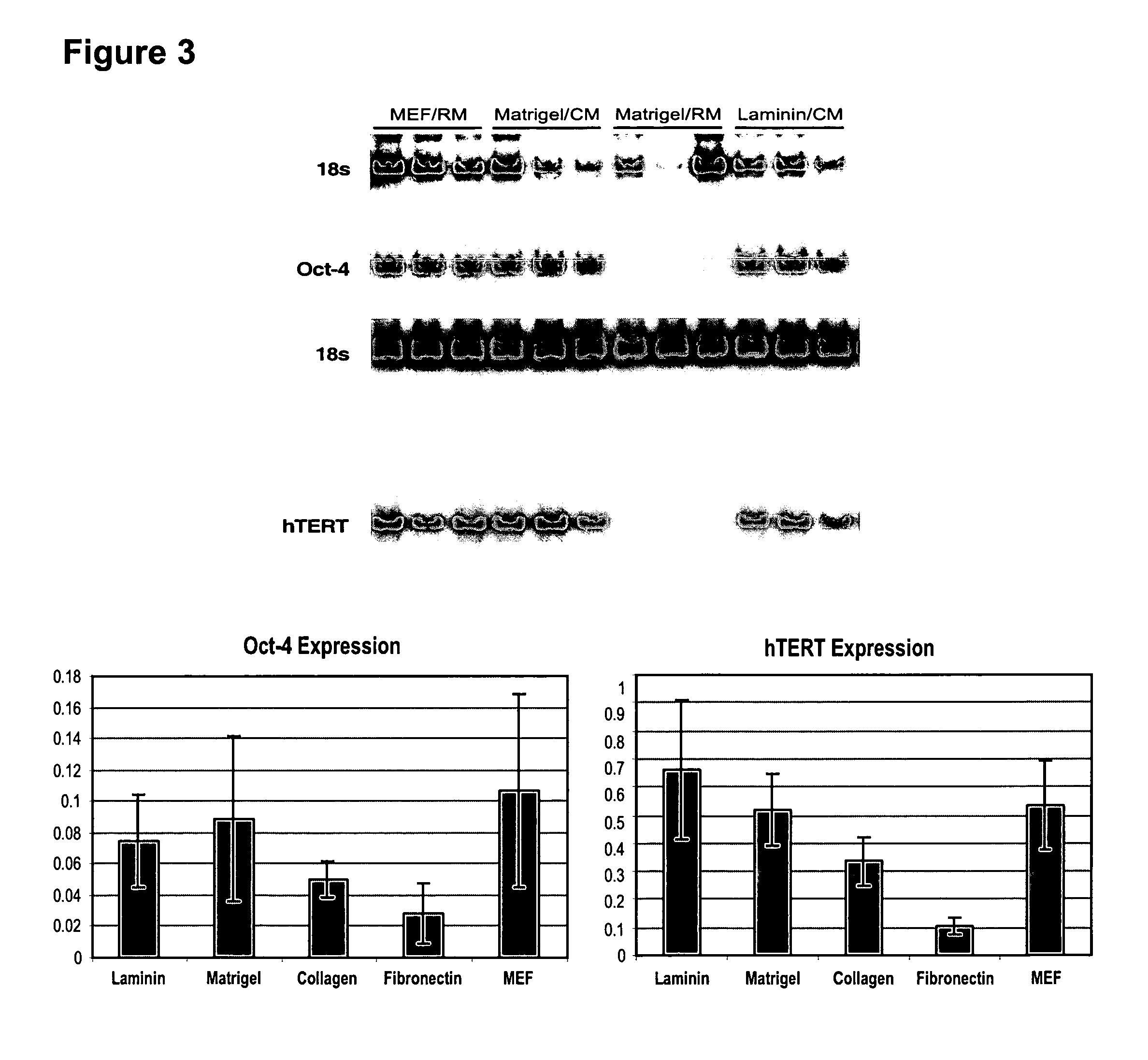
[0115] Undifferentiated hES cells express SSEA-4, Tra-1-60, Tra-1-81, OCT-4, and hTERT. In order to assess whether the cells maintained in feeder-free conditions retained these markers, cells were evaluated by immunostaining, reverse transcriptase PCR amplification, and assay for telomerase activity.
[0116] For analysis by fluorescence-activated cell sorting (FACS), the hES cells were dissociated in 0.5 mM EDTA in PBS and resuspended to about 5×105 cells in 50 μL diluent containing 0.1% BSA in PBS. They were labeled with specific primary antibody and then fluorescent second antibody, and analyzed on a Flow Cytometer.
[0117] Similar to the hES cells on feeders, cells on Matrigel®, laminin, fibronectin or collagen IV expressed SSEA-4, Tra-1-60 and Tra-1-81. There was very little expression of SSEA-1, a glycolipid that is not expressed by undifferentiated hES cells.
[0118]FIG. 2 shows marker expression detected by immunocytochemist...
example 3
Pluripotency of hES Cells in Feeder-Free Culture
[0123] In vitro differentiation was induced in H1 hES cells maintained in conditioned medium on Matrigel®, laminin, fibronectin or collagen IV for 26 days. The hES cells were dissociated into small clumps by incubating in ˜200 U / mL collagenase IV at 37° C. for 10 min, and cultured in suspension to form embryoid bodies (EBs) in medium containing DMEM, 20% FBS (Hyclone), 1 mM glutamine, 0.1 mM β-mercaptoethanol, and 1% non-essential amino acids (Gibco). After 4 days in suspension, the aggregates were transferred onto poly-ornithine-coated plates, and cultured for additional 7 days. The cultures were then examined for the presence of beating cells, and processed for immunocytochemistry.
[0124] The staining patterns were consistent with cells of the neuron and cardiomyocyte lineages (β-tubulin III and cardiac troponin 1, respectively). About 8 days after differentiation, beating regions were identified in all cultures. There were also cel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| doubling time | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com