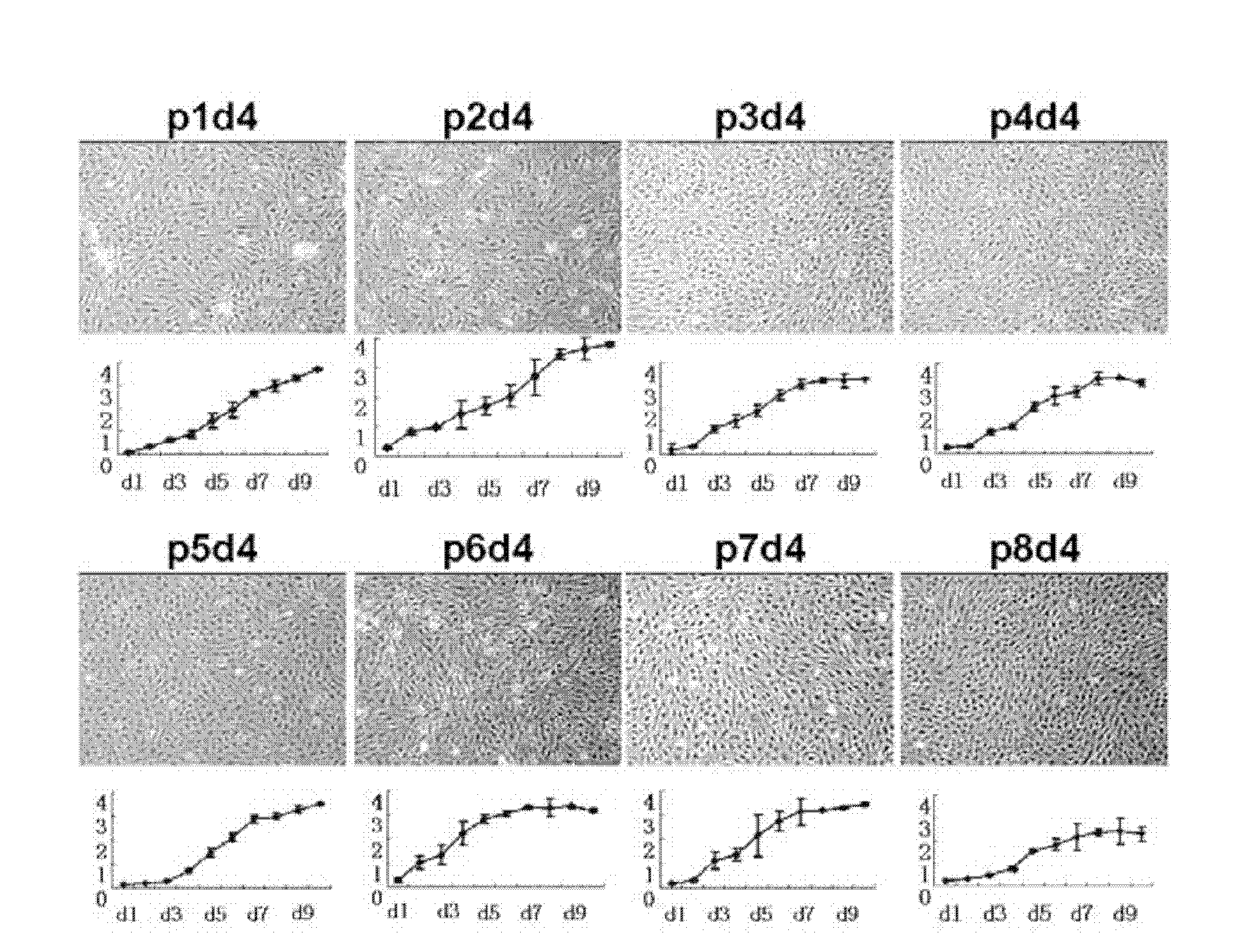Patents
Literature
31 results about "Cartilage graft" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
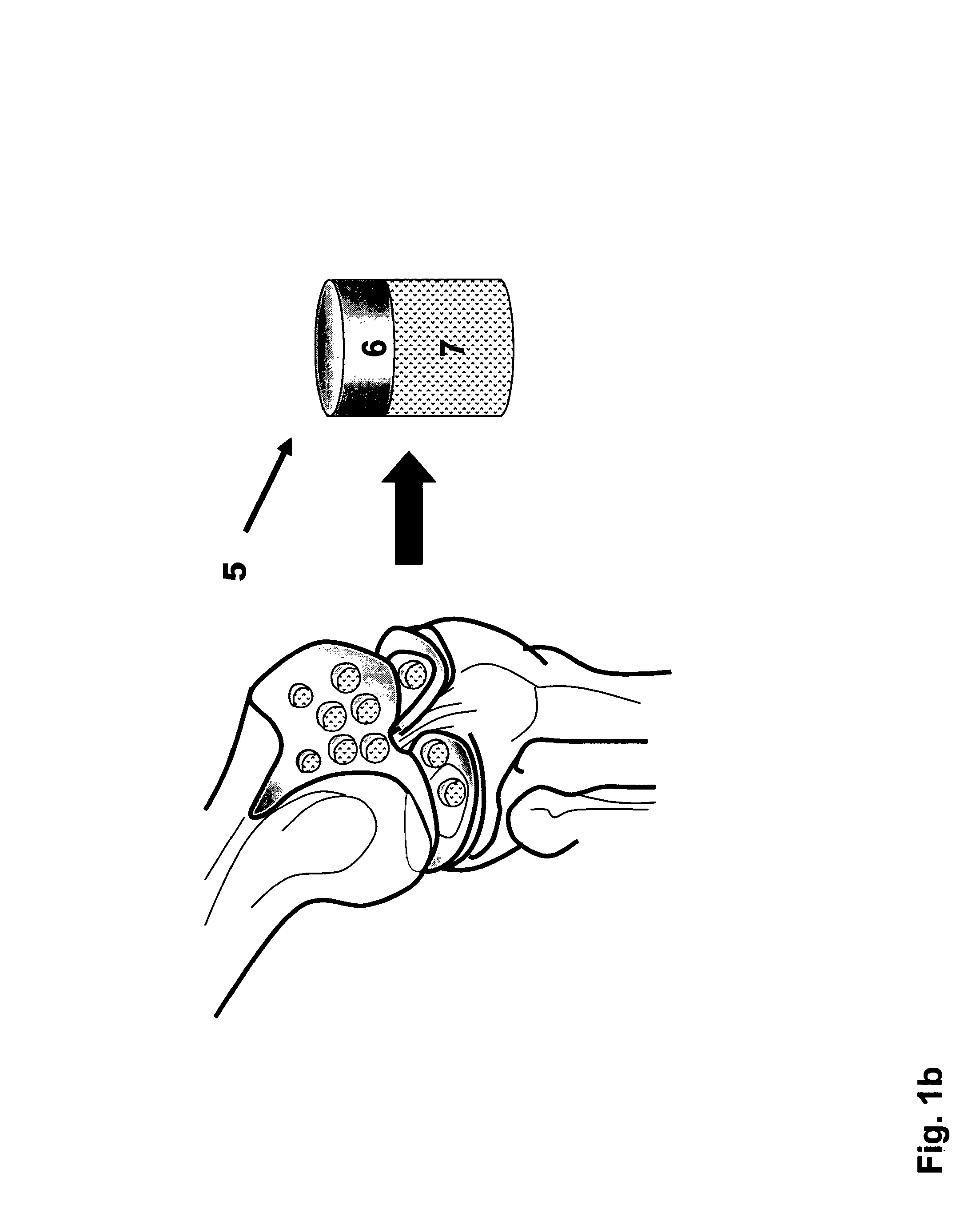
Cartilage Grafts. Cartilage grafting is a surgical procedure that replaces damaged cartilage with healthy cartilage from a non-weight bearing joint. Cartilage grafting is performed to correct joint deformities and restore the weight-bearing capability of the affected joint.
Cleaning and devitalization of cartilage
InactiveUS20080077251A1Improve recellularizationBone implantDead animal preservationCellular DebrisMedicine
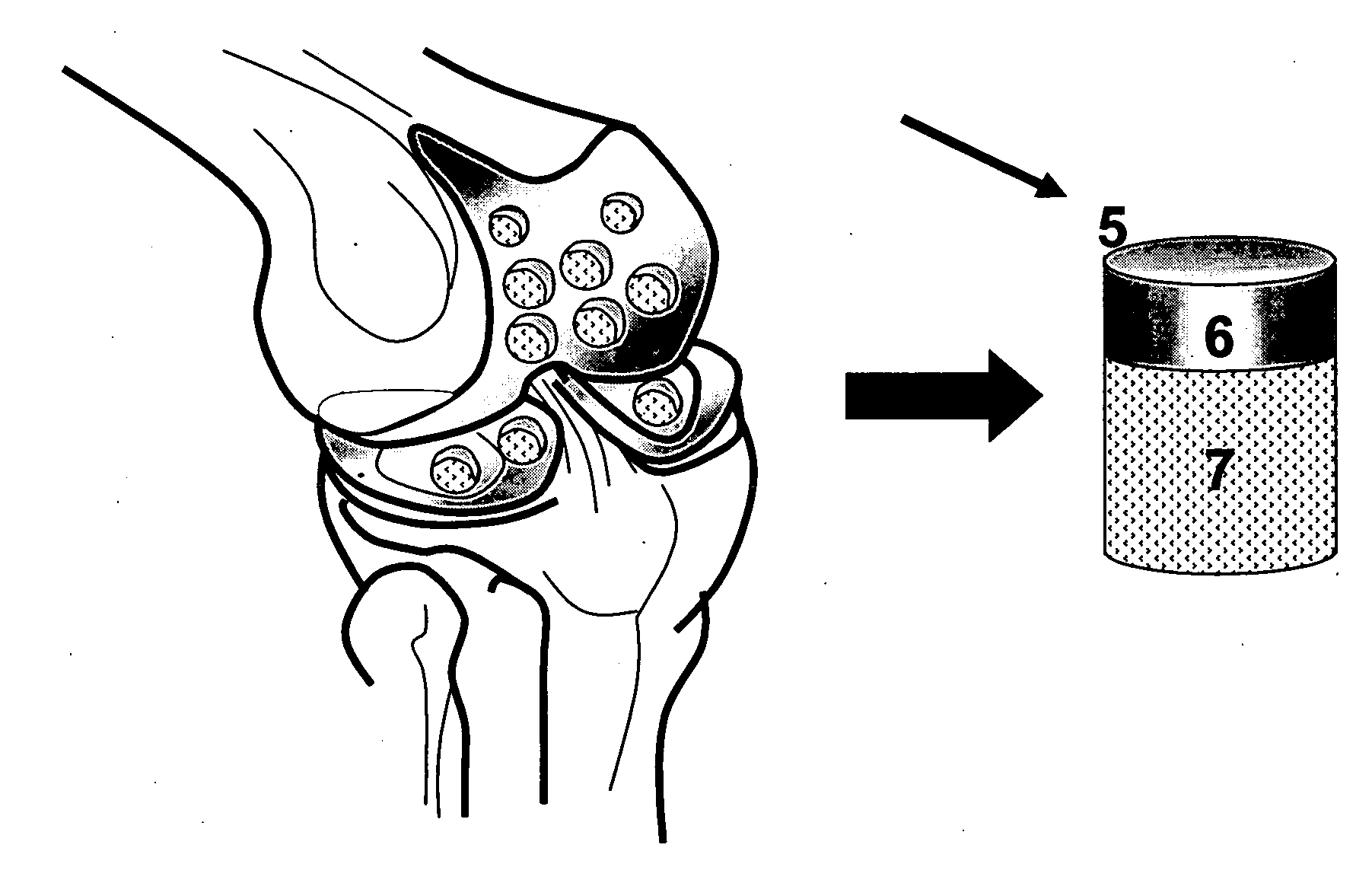
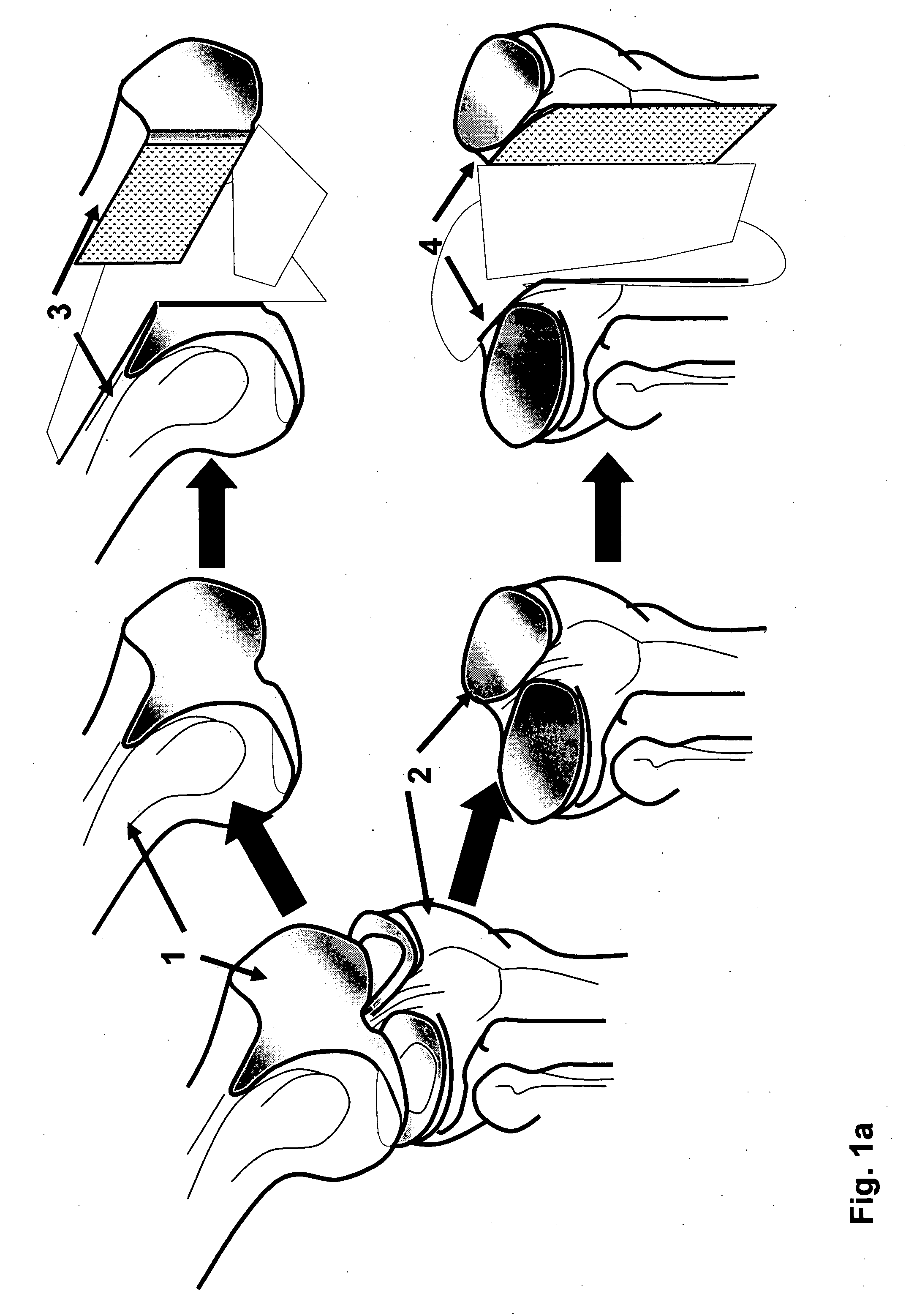
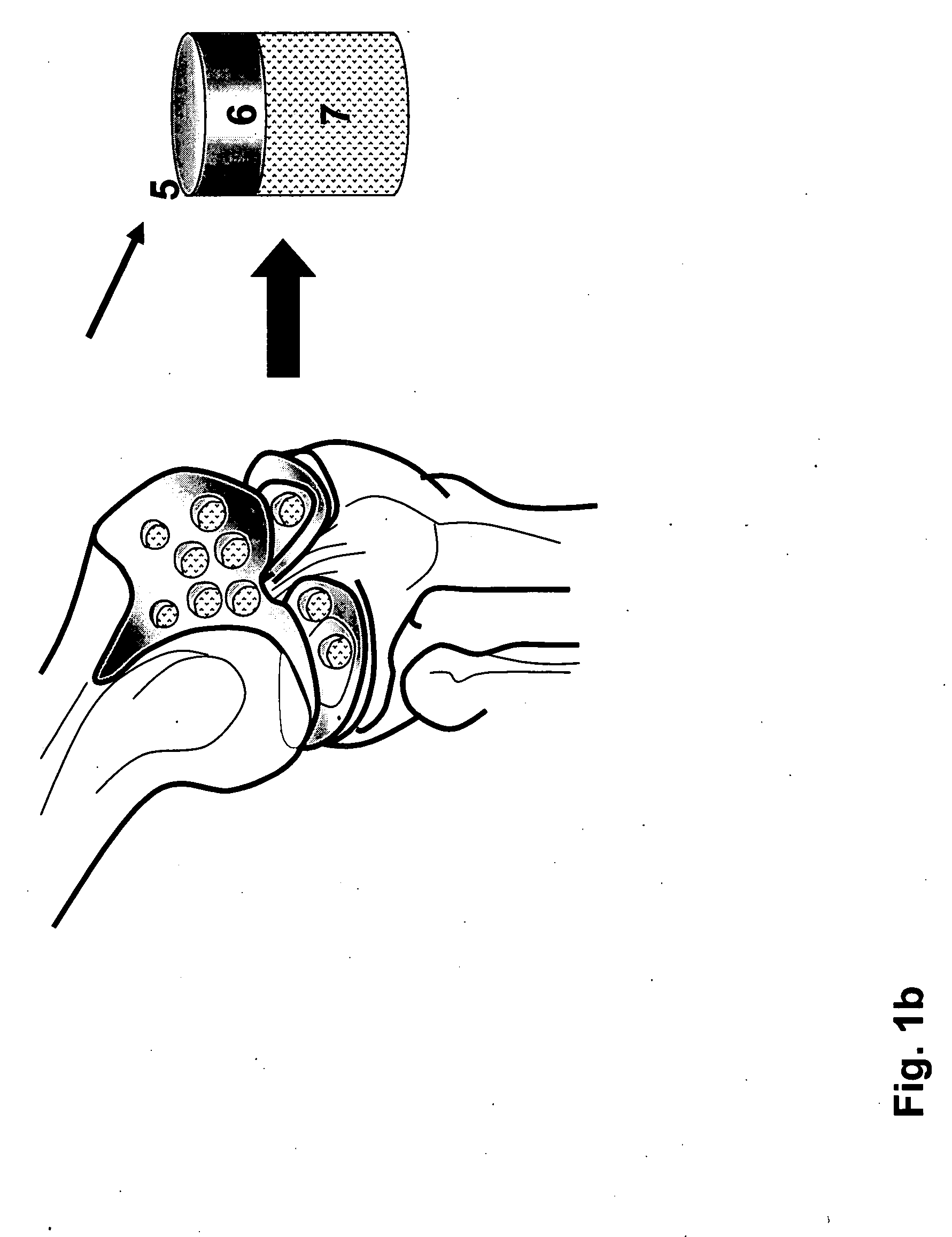
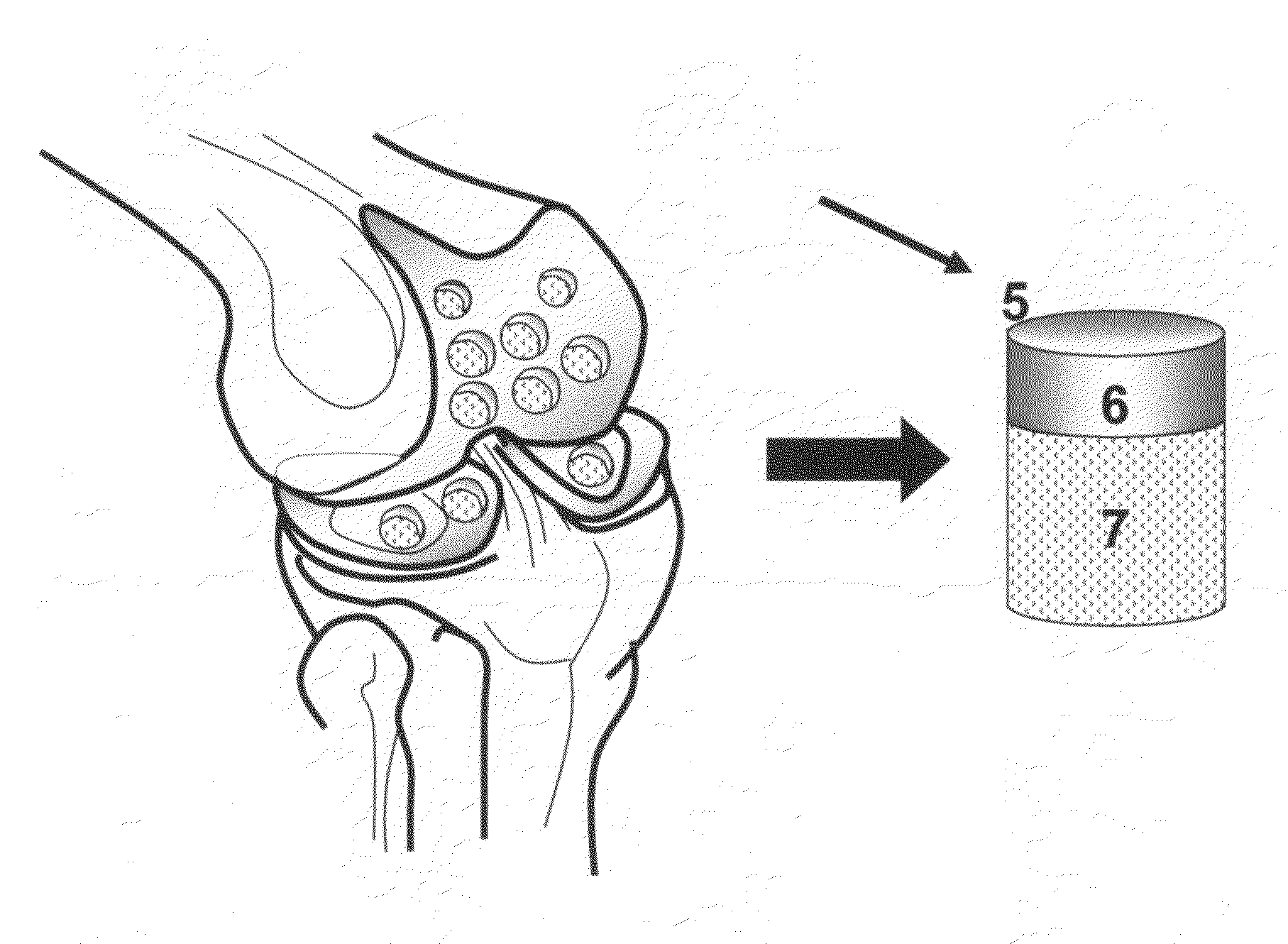
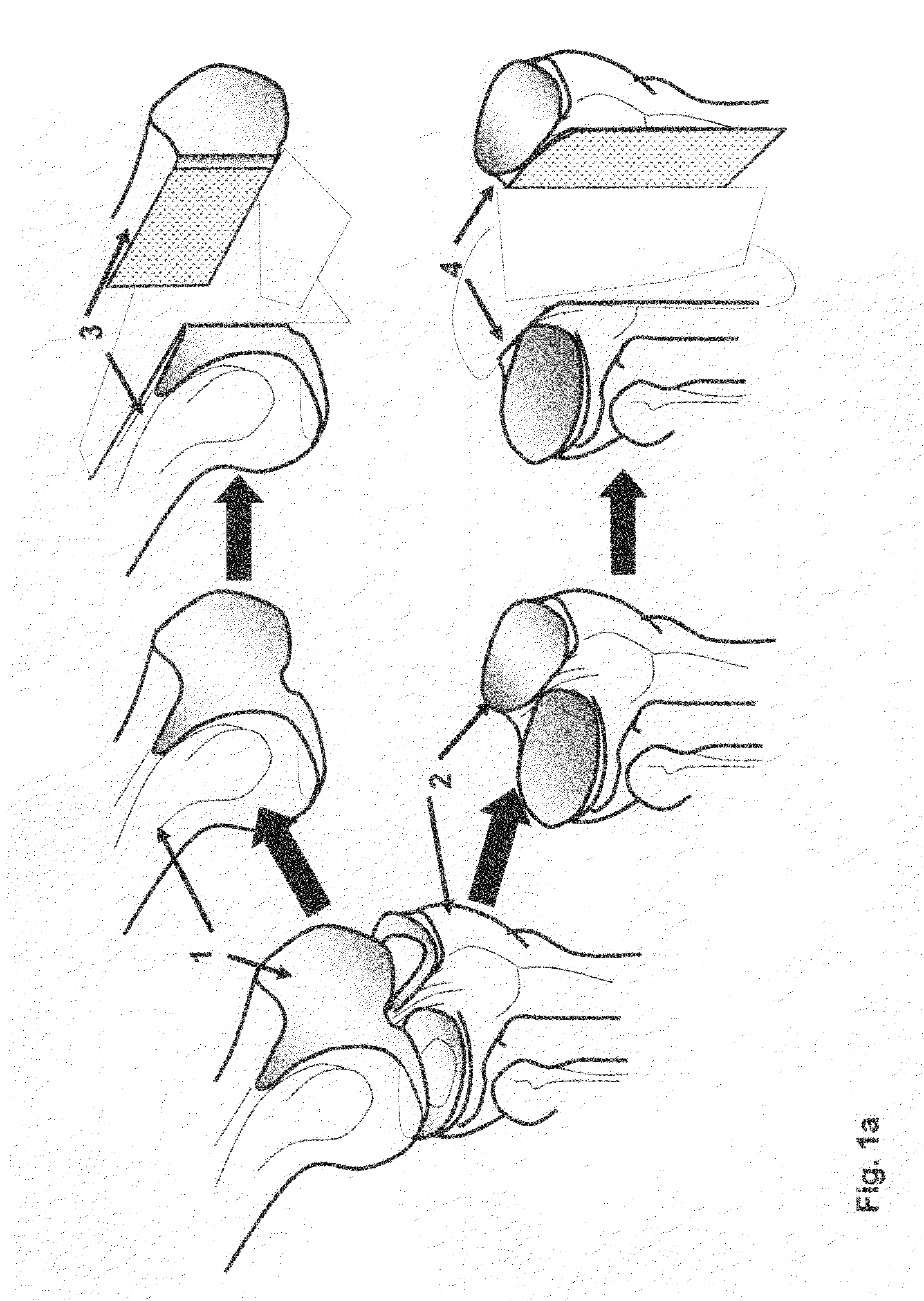
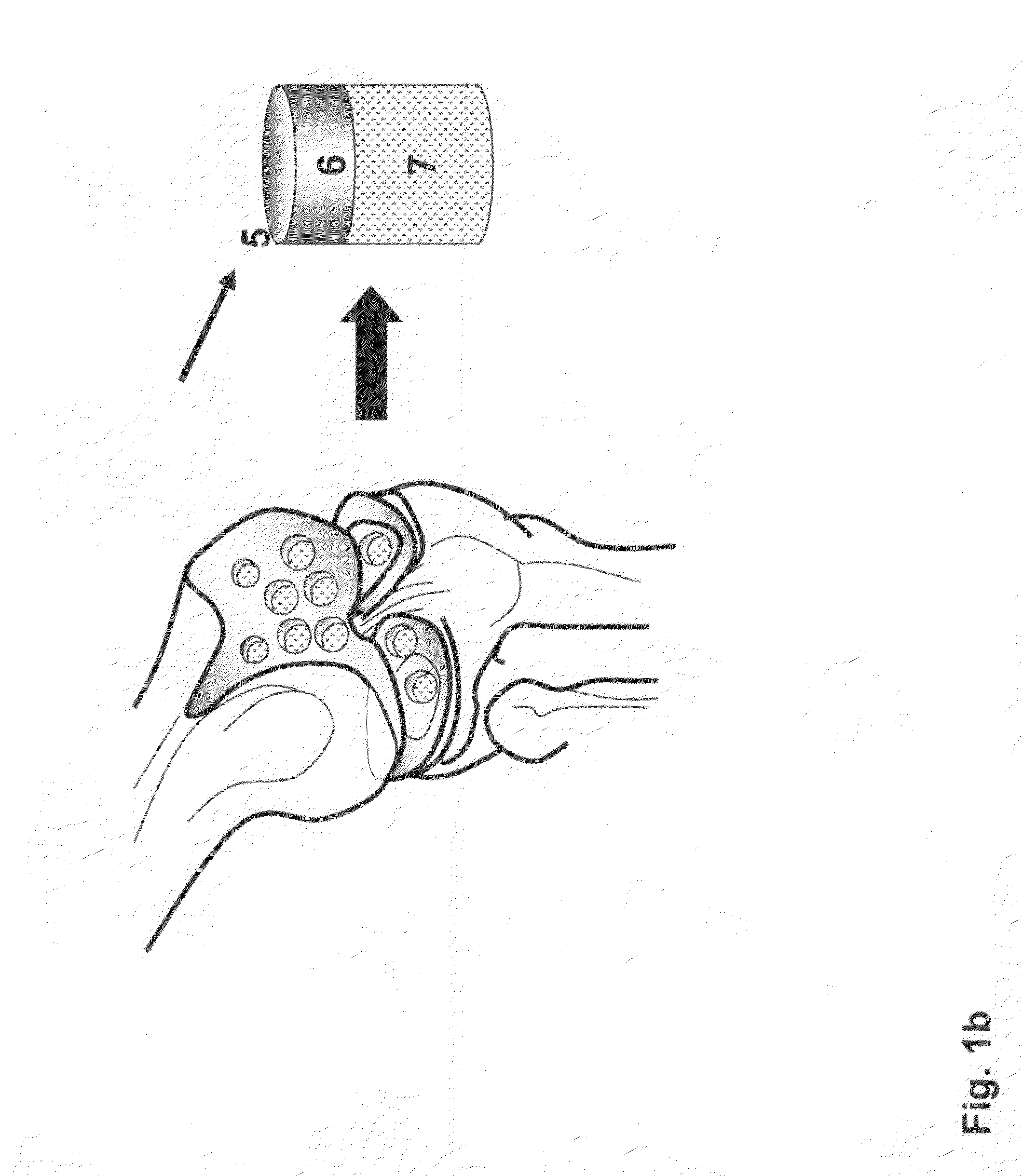
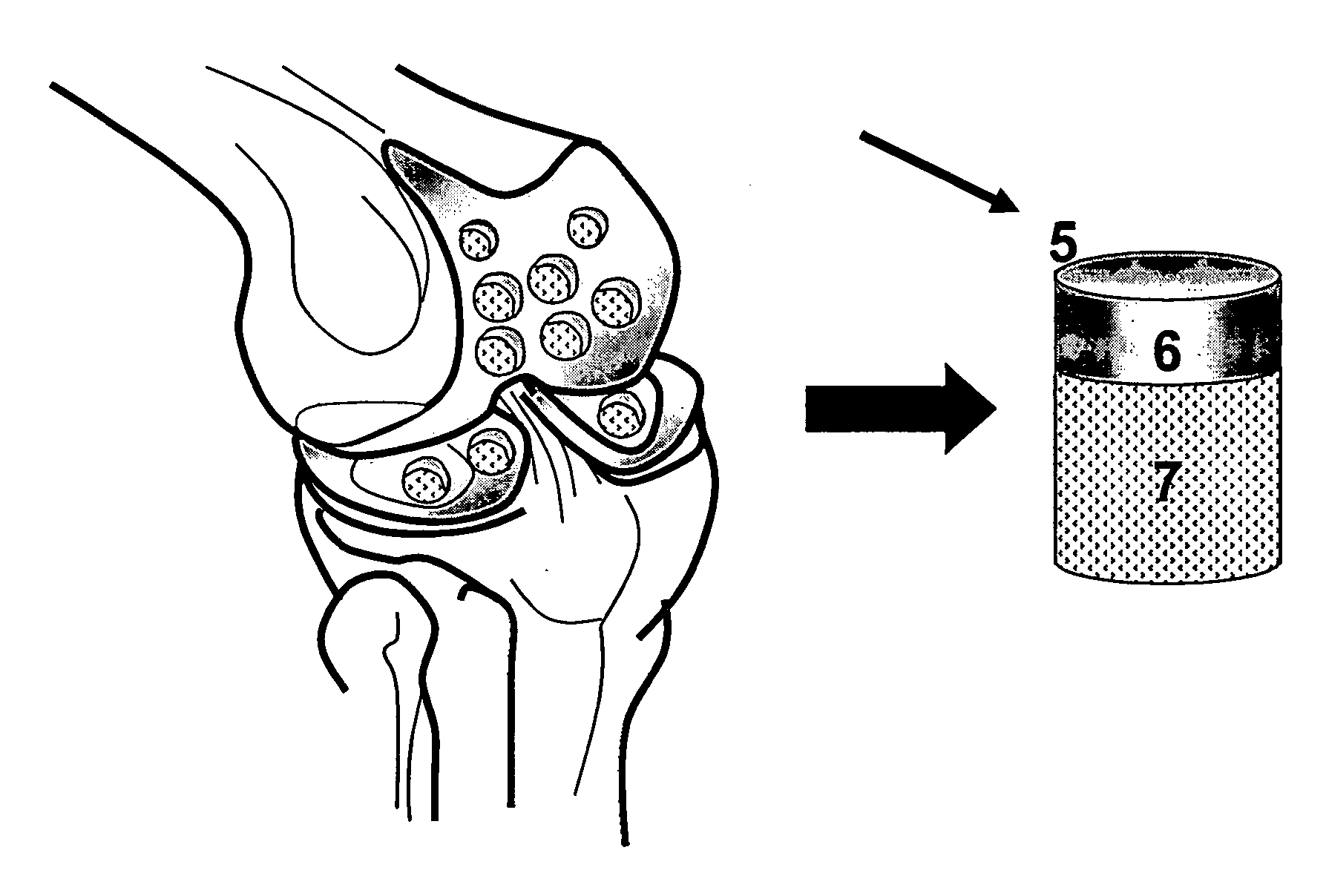
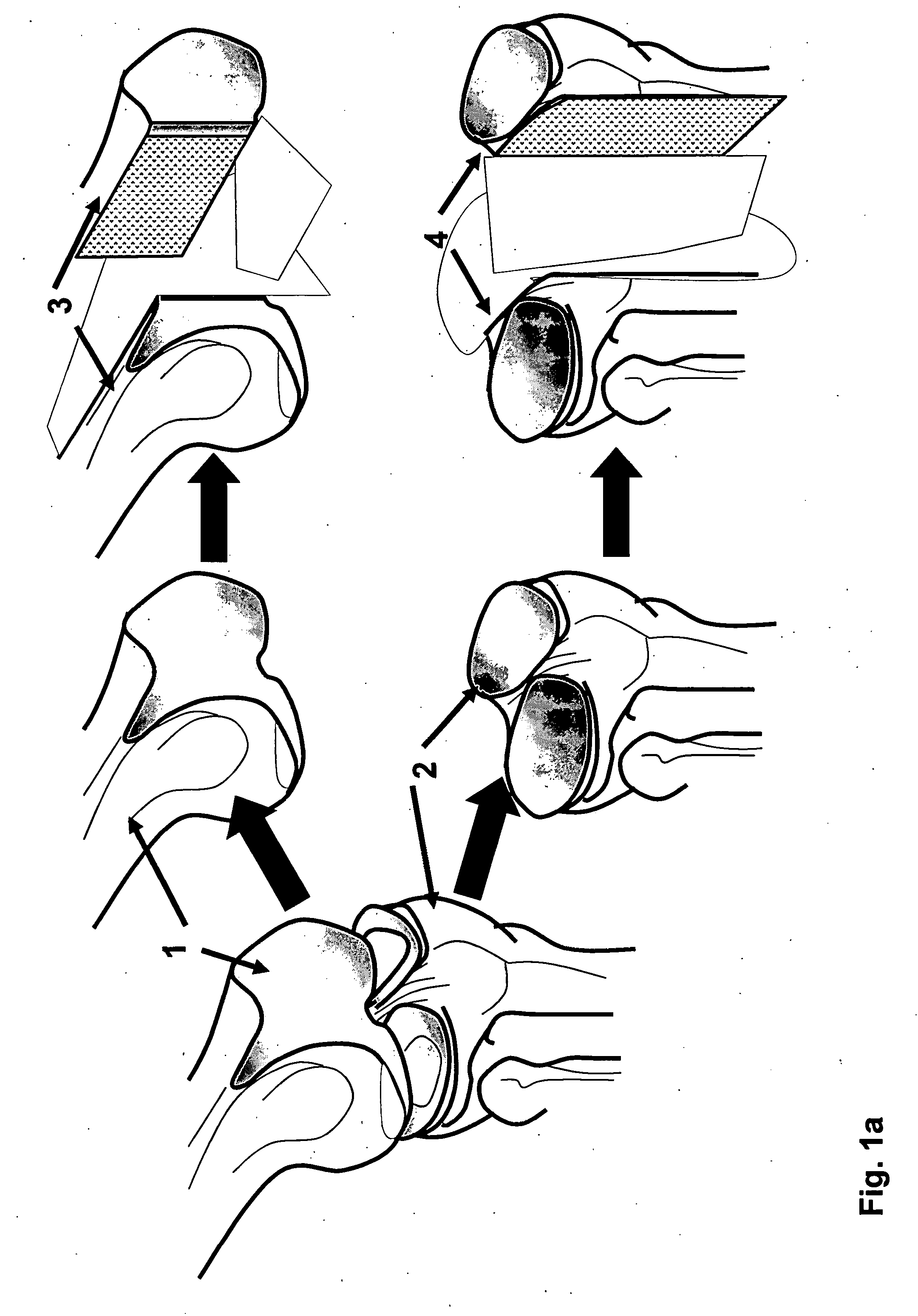
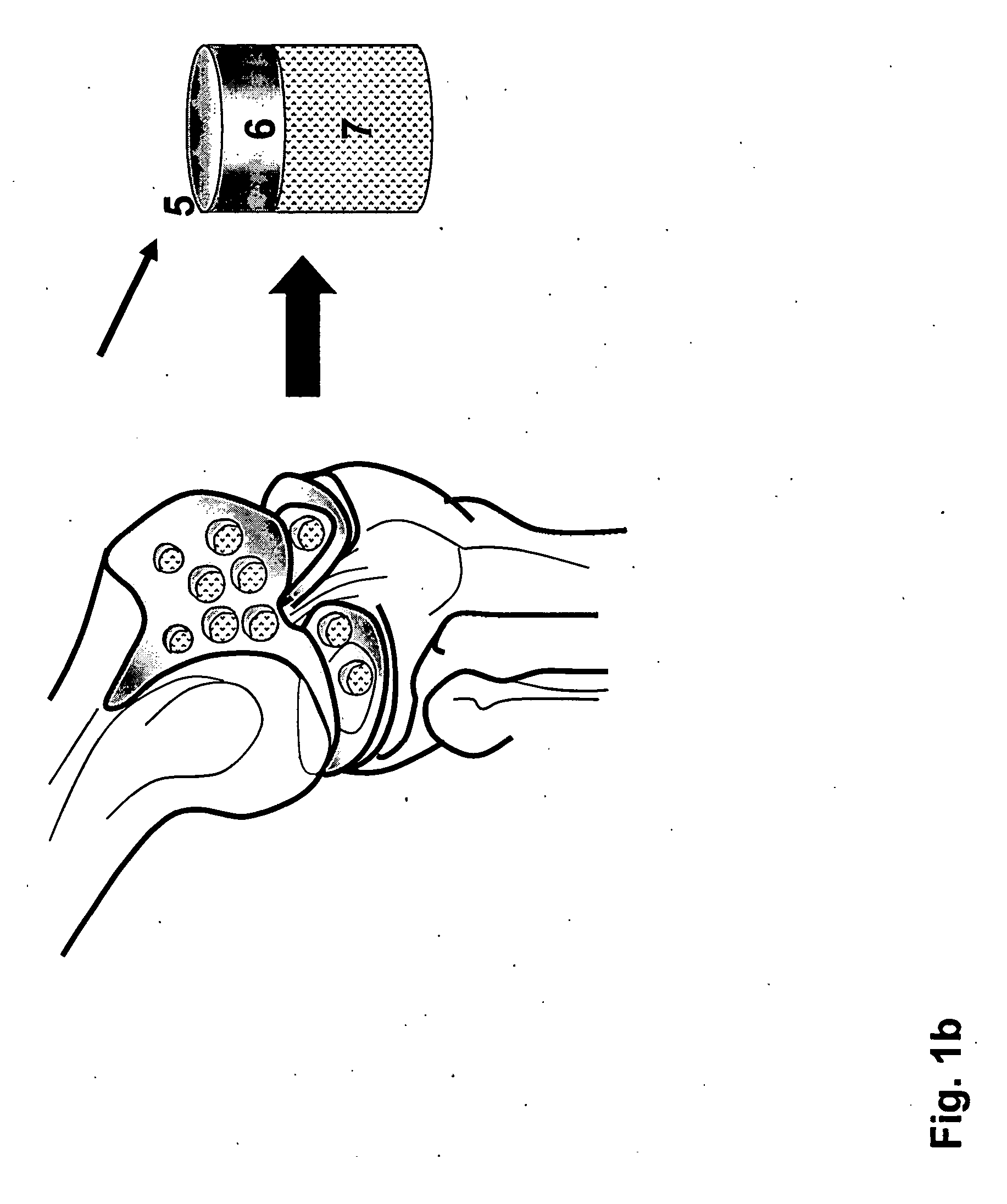
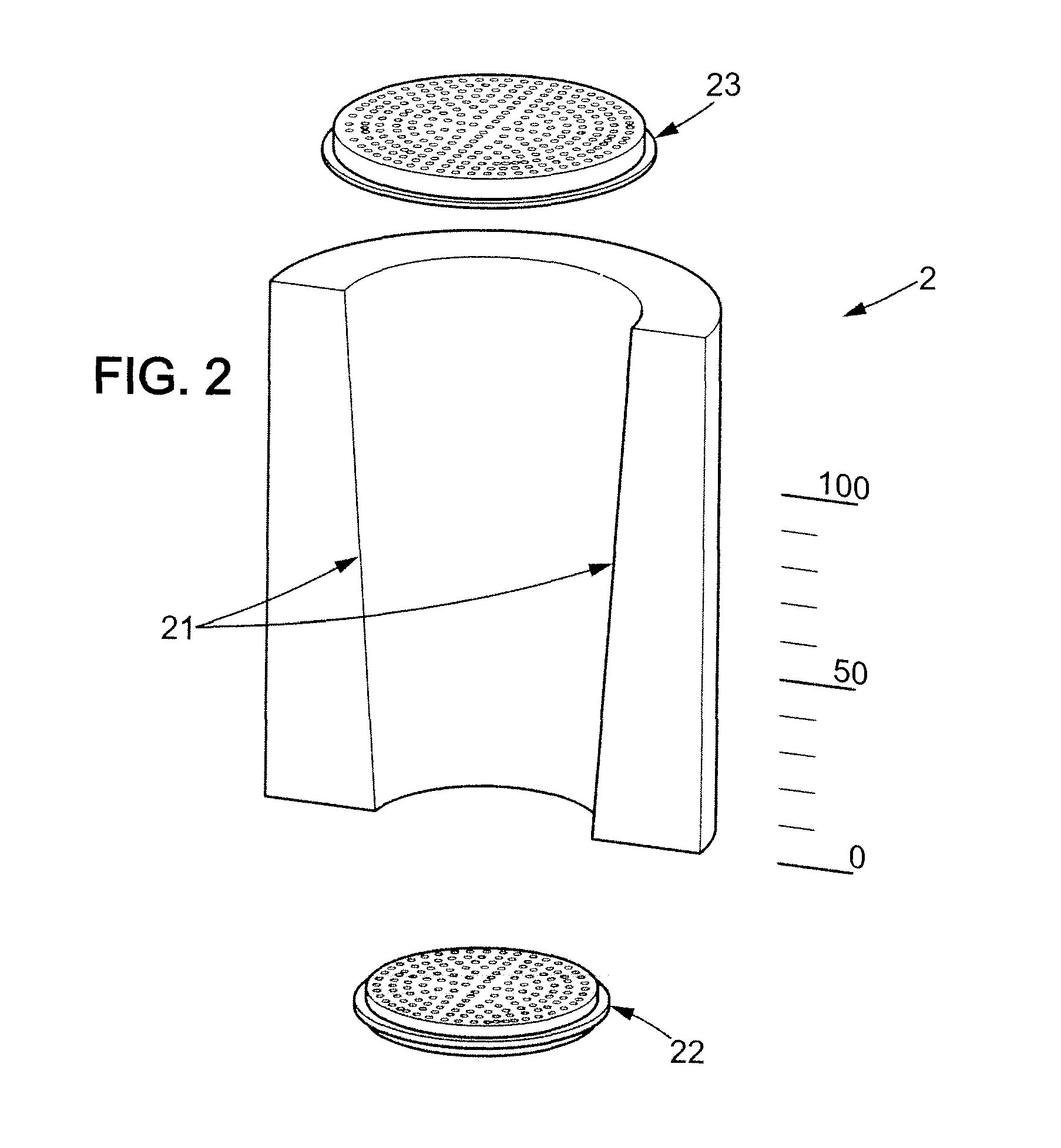
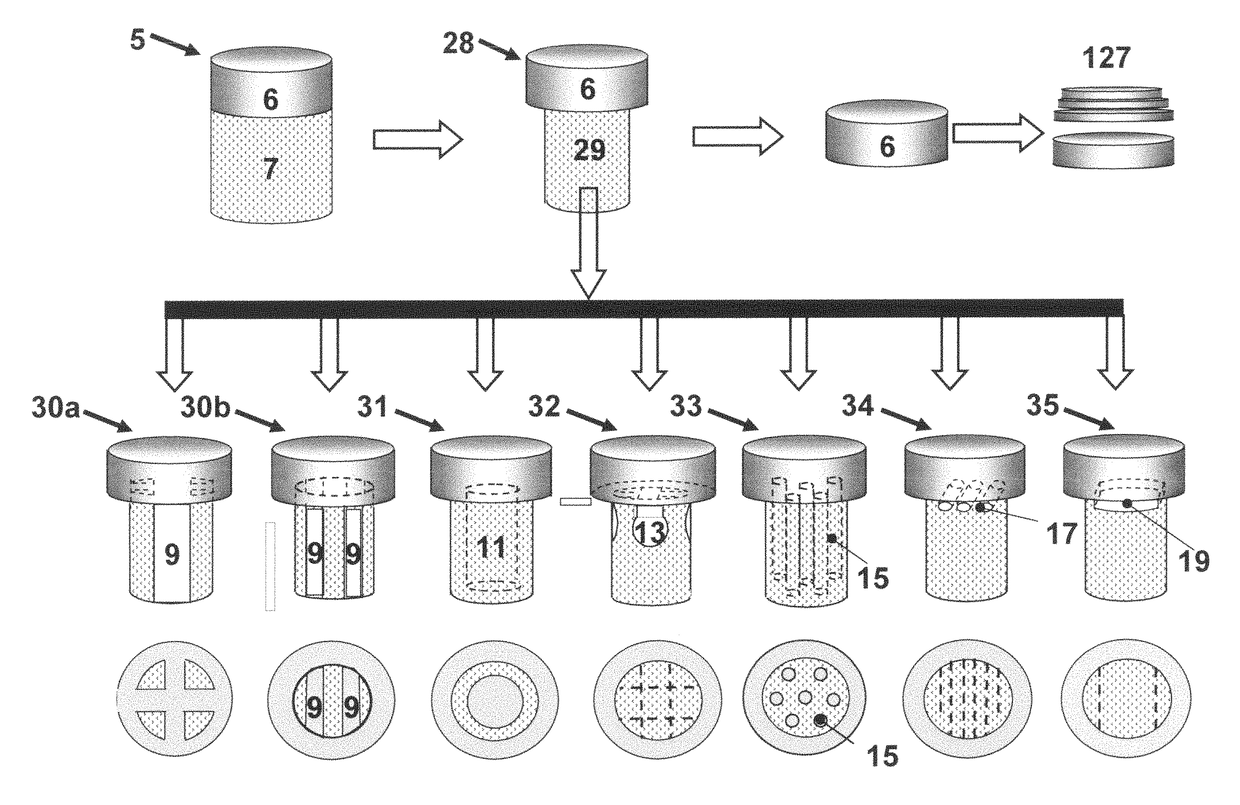
The invention is further directed to producing a cleaned, disinfected, and devitalized cartilage graft by optionally cleaning and disinfecting the cartilage graft; treating the cartilage graft in a pretreatment solution; treating the cartilage graft in an extracting solution; washing the extracted cartilage graft with a rinsing solution; and subsequently soaking the devitalized cartilage graft in a storage solution. The devitalized cartilage graft is essentially free from metabolically viable and / or reproductively viable cells and the rinsing solution is hypotonic solution or isotonic solution. The present invention is further directed to a cleaned, disinfected, and devitalized cartilage graft and a process for cleaning, disinfecting, and devitalizing cartilage grafts. The invention also relates to a process for repairing a cartilage defect and implantation of a cartilage graft into a human or animal by crafting the cartilage matrix into individual grafts, disinfecting and cleaning the cartilage graft, applying a pretreatment solution to the cartilage graft, removing cellular debris using an extracting solution to produce a devitalized cartilage graft, implanting the cartilage graft into the cartilage defect with or without an insertion device, and sealing the implanted cartilage graft with recipient tissue. The devitalized cartilage graft is optionally recellularized in vitro, in vivo, or in situ with viable cells to render the tissue vital before or after the implantation. The devitalized cartilage graft is also optionally stored between the removing cellular debris and the recellularizing steps.
Owner:LIFENET HEALTH
Crafting of cartilage
The invention is directed to producing a shaped cartilage matrix isolated from a human or animal where the cartilage has been crafted to facilitate disinfection, cleaning, devitalization, recellularization, and / or integration after implantation. The invention relates to a process for repairing a cartilage defect and implantation of a cartilage graft into a human or animal by crafting the cartilage matrix into individual grafts, disinfecting and cleaning the cartilage graft, applying a pretreatment solution to the cartilage graft, removing cellular debris using an extracting solution to produce a devitalized cartilage graft, implanting the cartilage graft into the cartilage defect with or without an insertion device, and sealing the implanted cartilage graft with recipient tissue. The devitalized cartilage graft is optionally recellularized in vitro, in vivo, or in situ with viable cells to render the tissue vital before or after the implantation. The devitalized cartilage graft is also optionally stored between the removing cellular debris and the recellularizing steps.
Owner:LIFENET HEALTH
Methods and instruments for forming non-circular cartilage grafts
Techniques and instruments for knee replacement surgery that allow for the removal of an oval oblong-shaped allograft bone and cartilage plug from a donor distal femur. The instruments include (i) sizing guides to match the recipient's femoral size and curvature to that of a donor femur (the sizing guides also acting as a wide pin placement template for the donor distal femur); (ii) osteotomes that cut the curved and straight portions of the implant shape (these may be disposable or reusable); and (iii) templates that fit over the guide pins and have openings to allow the osteotomes to cut the donor femur plug to the correct size, shape and depth. The instruments allow for a non-circular shape to be extracted from a donor femur for use in a bone-saving osteoarthritis distal femur resurfacing procedure.
Owner:ARTHREX
Methods and instruments for forming non-circular cartilage grafts
Techniques and instruments for knee replacement surgery that allow for the removal of an oval oblong-shaped allograft bone and cartilage plug from a donor distal femur. The instruments include (i) sizing guides to match the recipient's femoral size and curvature to that of a donor femur (the sizing guides also acting as a wide pin placement template for the donor distal femur); (ii) osteotomes that cut the curved and straight portions of the implant shape (these may be disposable or reusable); and (iii) templates that fit over the guide pins and have openings to allow the osteotomes to cut the donor femur plug to the correct size, shape and depth. The instruments allow for a non-circular shape to be extracted from a donor femur for use in a bone-saving osteoarthritis distal femur resurfacing procedure.
Owner:ARTHREX
Methods of promoting healing of cartilage defects and method of causing stem cells to differentiate by the articular chondrocyte pathway
InactiveUS20060111778A1Promote healingBone implantSkeletal/connective tissue cellsCell-Extracellular MatrixArticular chondrocyte
Methods of promoting healing of a cartilage defect in a region of cartilage, which comprises the defect and which may further comprise stem cells, and methods of promoting healing of a cartilage defect in a region of cartilage, which comprises the defect and an implant comprising cartilage scaffold or a cartilage graft, which methods comprise contacting the region with various combinations of cartilage fragments, a growth factor, a partially synthesized extracellular matrix, a scaffold, an implant comprising cartilage scaffold, an implant comprising a cartilage graft, stem cells, chondrocytes, a proteoglycan, an anti-oxidant, a collagen precursor, a vitamin, a mineral, and / or a cartilage-degrading enzyme; and a method of causing stem cells to differentiate by the articular chondrocyte pathway comprising contacting the stem cells with a compound comprising an active alcohol moiety.
Owner:MICHALOW ALEXANDER E
Implantation of Cartilage
ActiveUS20130030528A1Restrict subsequent apoptosisMaintain compatibilityBone implantSurgeryChondral defectBiomedical engineering
The invention is directed towards a process for implanting a cartilage graft into a cartilage defect and sealing the implanted cartilage graft with recipient tissue. The invention is also directed towards a process for repairing a cartilage defect and implanting a cartilage graft into a human or animal. The invention is further directed toward a repaired cartilage defect.
Owner:LIFENET HEALTH
Bioreactor for cell culture on a three-dimensional substrate
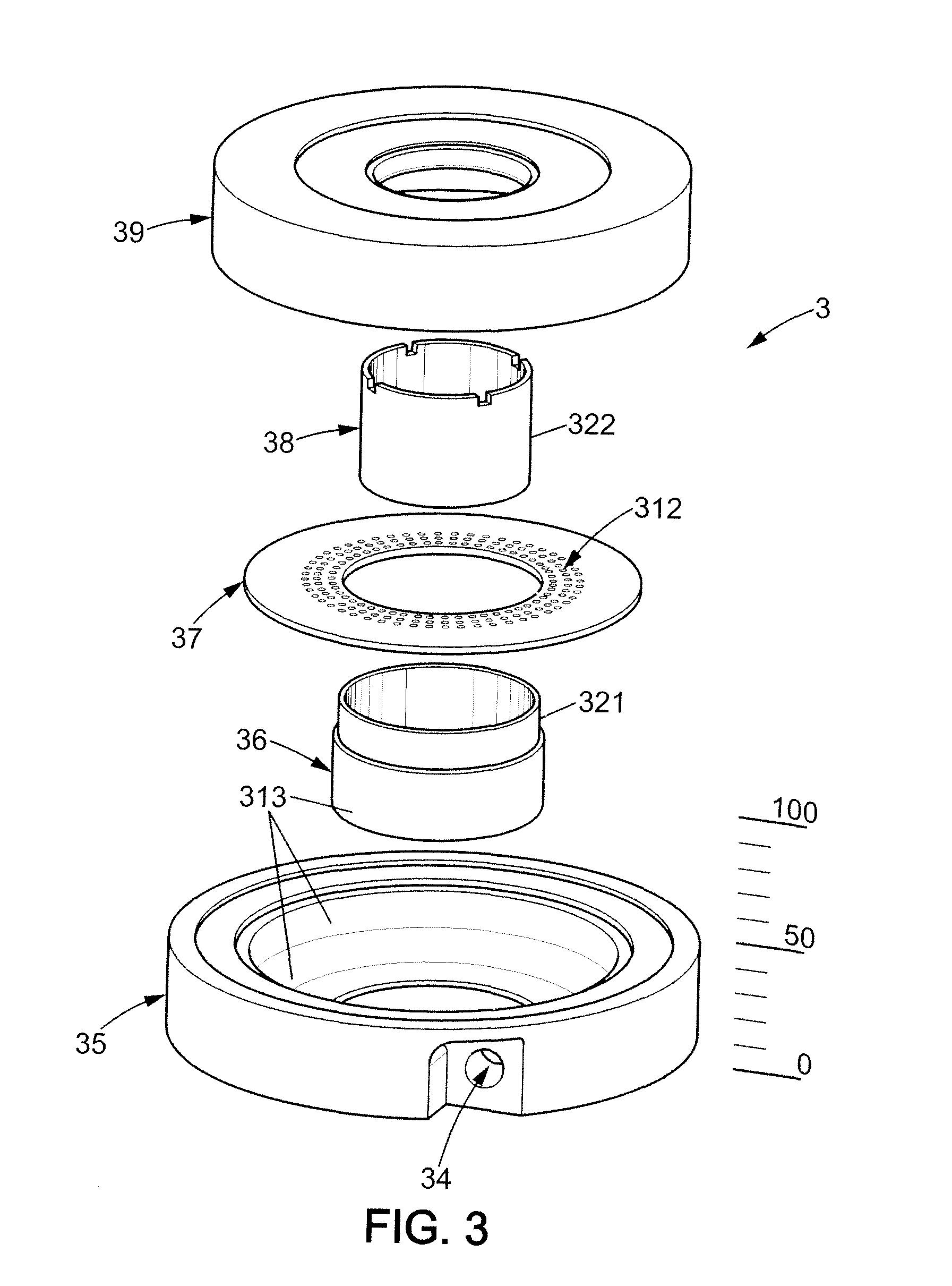
InactiveUS20140030762A1Reduce defectsInteraction is limitedBioreactor/fermenter combinationsBiological substance pretreatments3D cell cultureTissue Graft
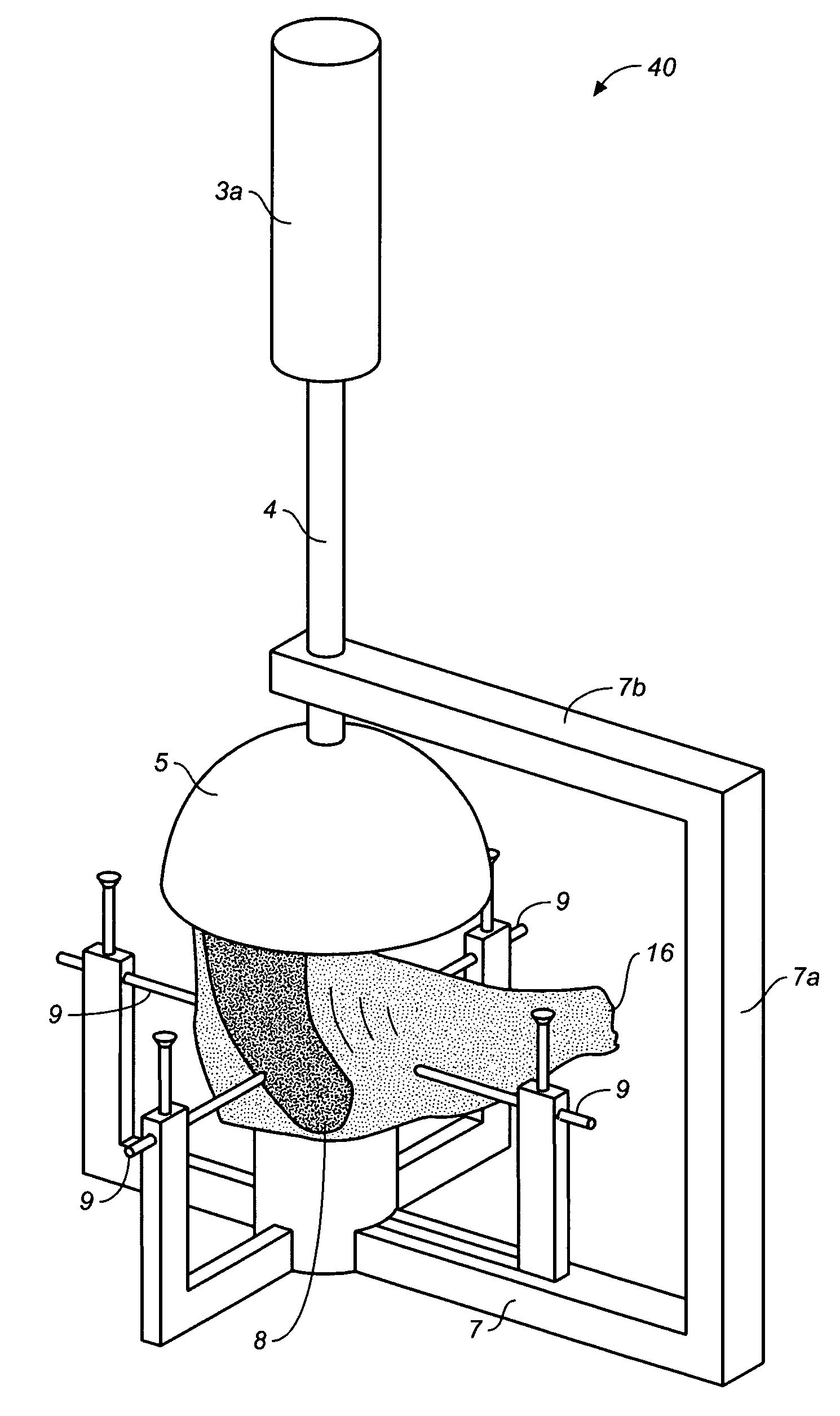
The invention relates to a bioreactor (1) for cell culture on a three-dimensional substrate, comprisinga culture chamber (2), the inner walls of which form a vertical duct, preferably, tapered, with a diameter that widens regularly form the duct inlet to the duct outlet, means (3, 4) enabling the culture medium to flow in said vertical duct.The invention also relates to the advantageous use of these bioreactors in tissue engineering, for the production of tissue grafts, notably a bone or cartilage graft.
Owner:UNIV DE PROVENCE D AIX MARSEILLE I +2
Articular cartilage graft and preparation method thereof
ActiveCN103920190AAvoid allergiesLower immune responseJoint implantsCell-Extracellular MatrixCartilage lesion
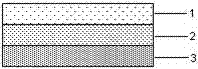
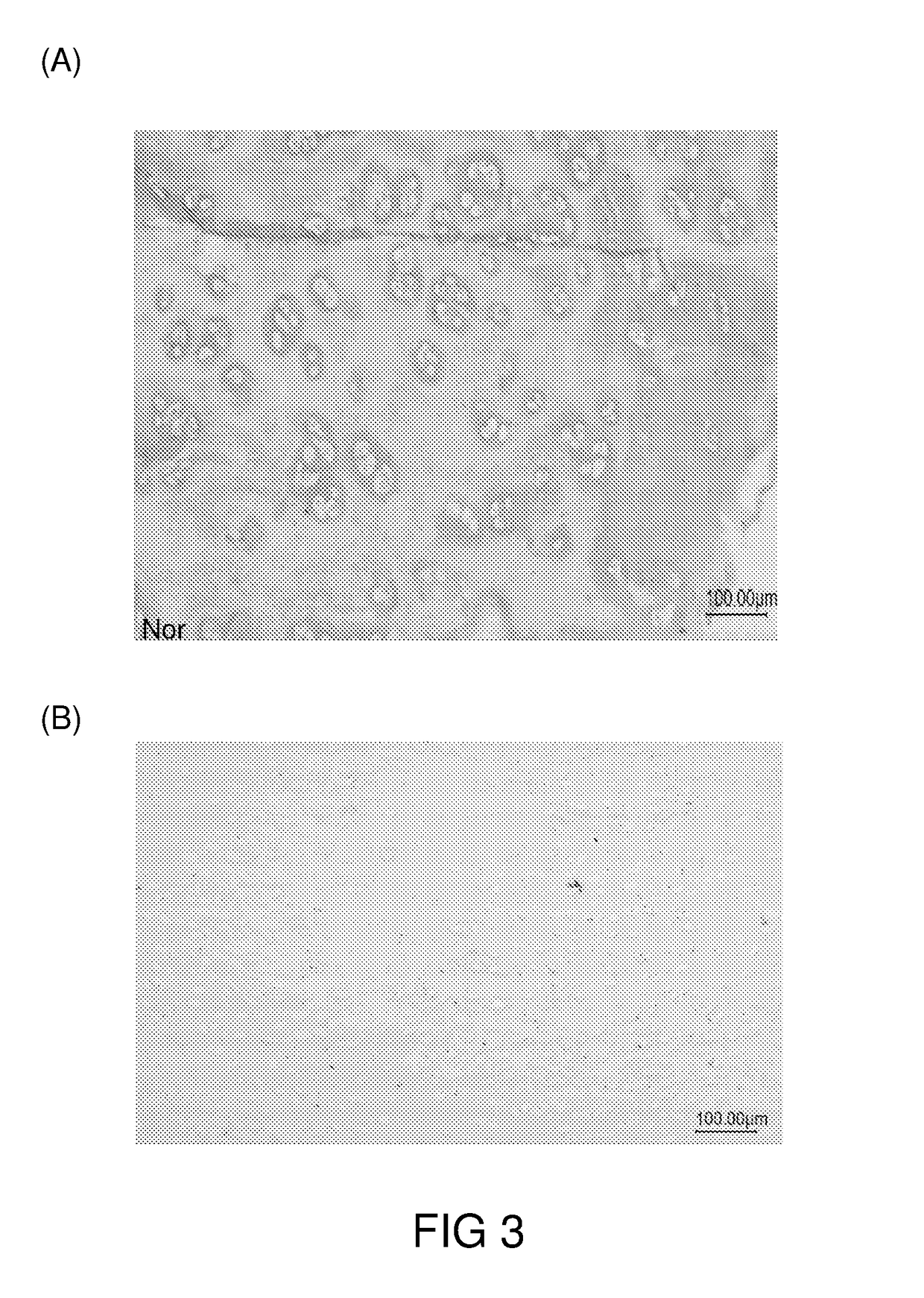
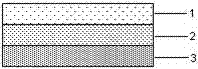
An articular cartilage graft and a preparation method thereof are provided. The prepared articular cartilage graft is composed of a superficial layer, a middle layer and a deep layer from outside to inside; the thickness, shape and size of each layer are all matched with a cartilage injury part, and thus preoperative shaping is not required; after grafting, the articular cartilage graft has layer distribution corresponding to distribution of each layer of surrounding normal cartilages, is conducive to intercellular signal transmission, transduction and regulation, and can be better integrated with surrounding normal cartilage tissues; the articular cartilage graft has structure characteristics consistent with those of the natural cartilages, the collagen type II content is gradually decreased from the superficial layer to the deep layer, the GAG content is increased gradually from the superficial layer to the deep layer, and compression resistance and wear resistance are good; and chondrocytes in the cartilage graft are wrapped with an extracellular matrix, have low immunogenicity, allow generation of immunologic rejection to be avoided after grafting, can survive for a long term and exert functions, and improve cartilage repair long-term curative effects.
Owner:西安博鸿生物技术有限公司
Autologous fat stem cell constructing tissue engineering cartilage
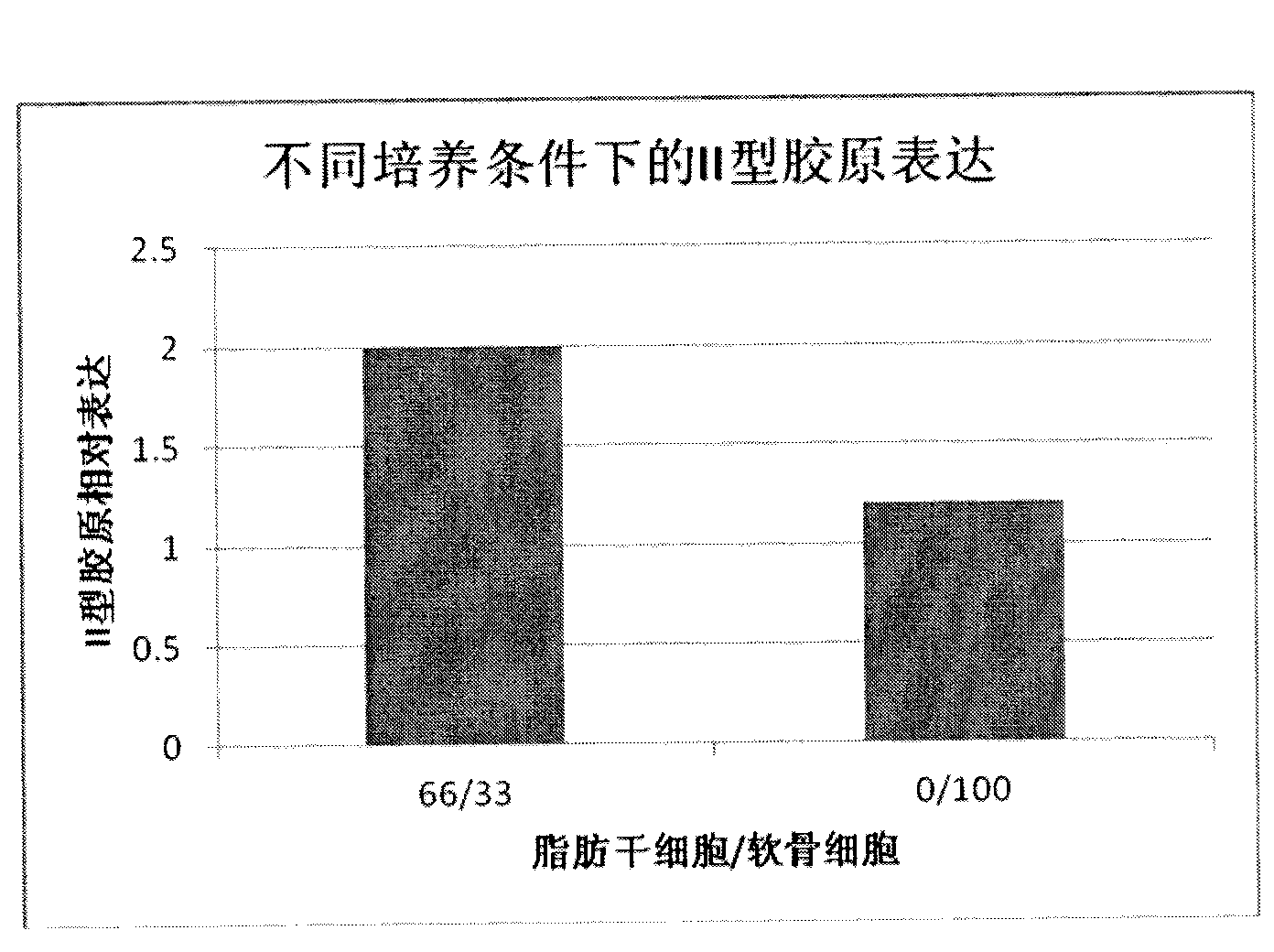
The present invention discloses one kind of cartilage graft, which includes pharmaceutically acceptable biodegradable material and fat stem cell and is in the shape matching the cartilage defect part to be repaired. The tissue engineering cartilage constructed with the autologous fat stem cell may be used in treating cartilage impairment effectively. The present invention also provides the preparation process and use of the tissue engineering cartilage.
Owner:SHANGHAI TISSUE ENG LIFE SCI
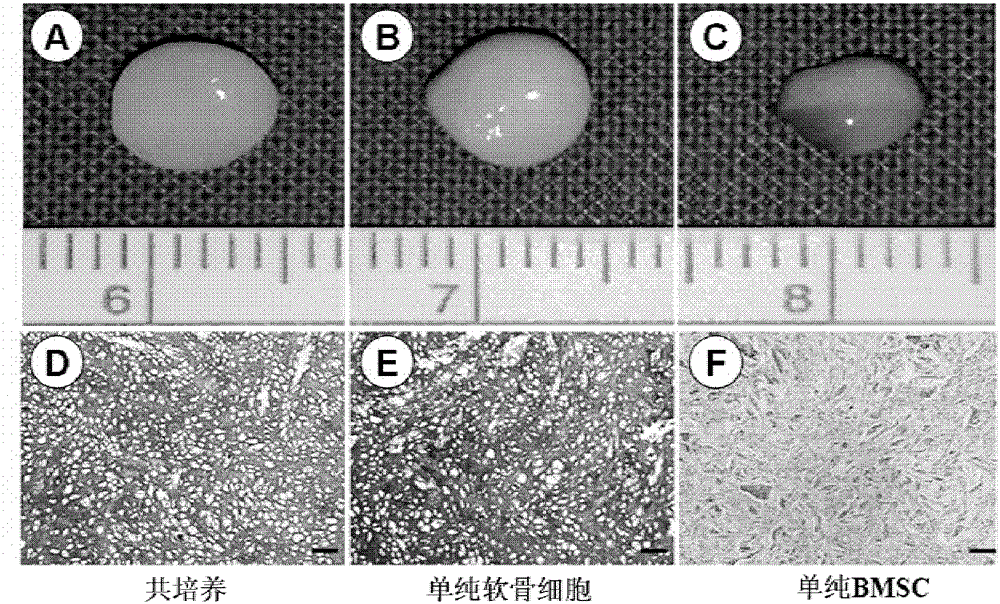
Cartilage graft for cartilage injury repair and preparation method thereof
InactiveCN103223194AImprove proliferative abilityHigh activityProsthesisCartilage cellsCartilage injury
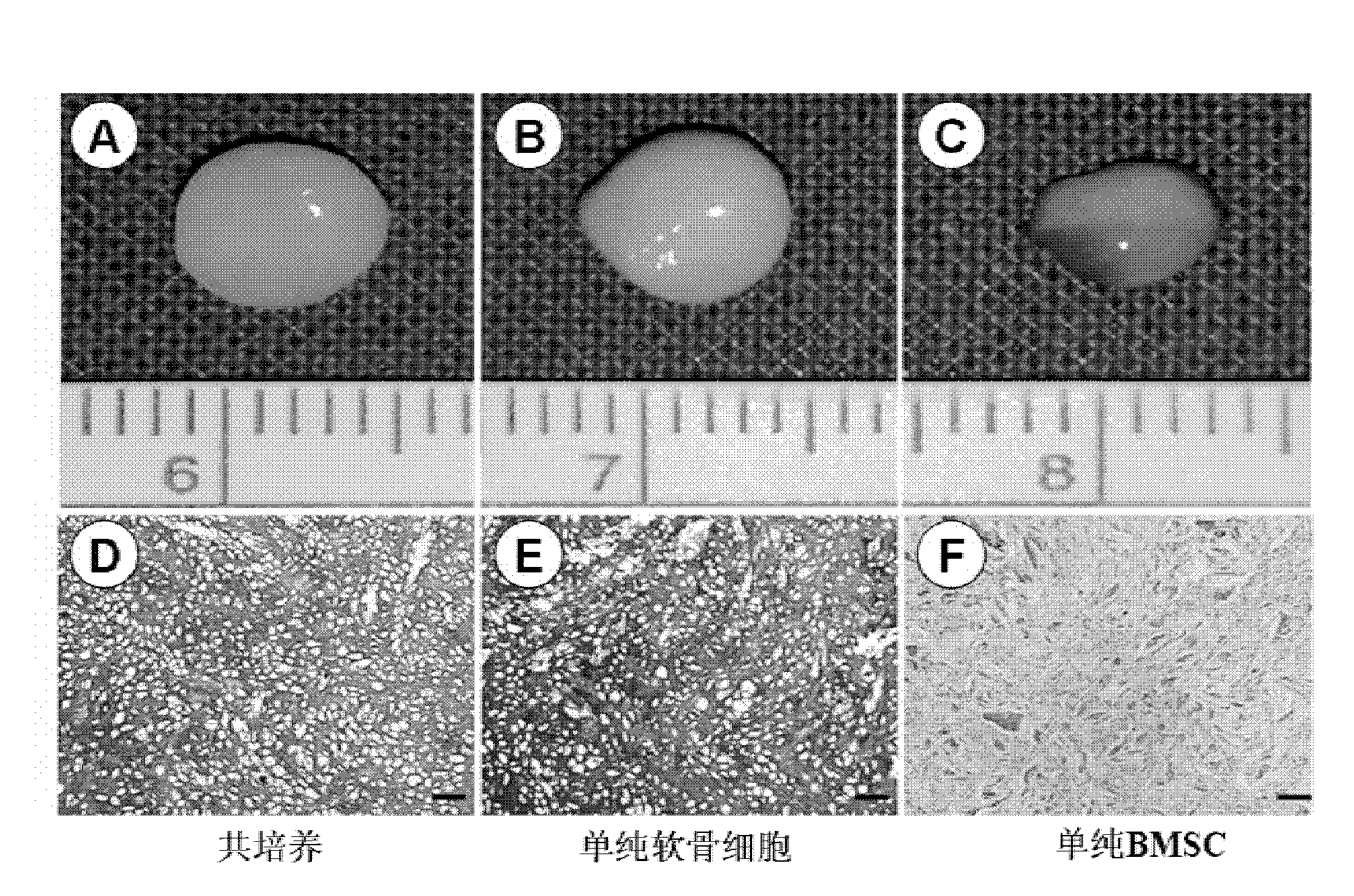
The invention discloses a cartilage graft for cartilage injury repair and a preparation method thereof. The cartilage graft is prepared based on an adipose-derived stem cell-cartilage cell co-culture technology. The preparation method is effective and safe, can prepare enough cartilage cells for transplant in a short time, can efficiently transform cartilage tissue and can improve cartilage tissue transplant treatment effects. The preparation method utilizes nutrition functions of adipose-derived stem cells and carries out co-culture of adipose-derived stem cells and cartilage cells in a three-dimensional culture system. Through interaction of the adipose-derived stem cells and the cartilage cells, proliferation and activity of the cartilage cells are promoted. The adipose-derived stem cells are influenced by the environment and are partly differentiated into cartilage cells and thus under the condition of undersampling or activity lack of the cartilage cells, a high-quality cartilage graft can be obtained and graft viability is promoted and shallow-layer cartilage coloboma can be repaired well.
Owner:GUANGZHOU LAND BIOLOGY TECH
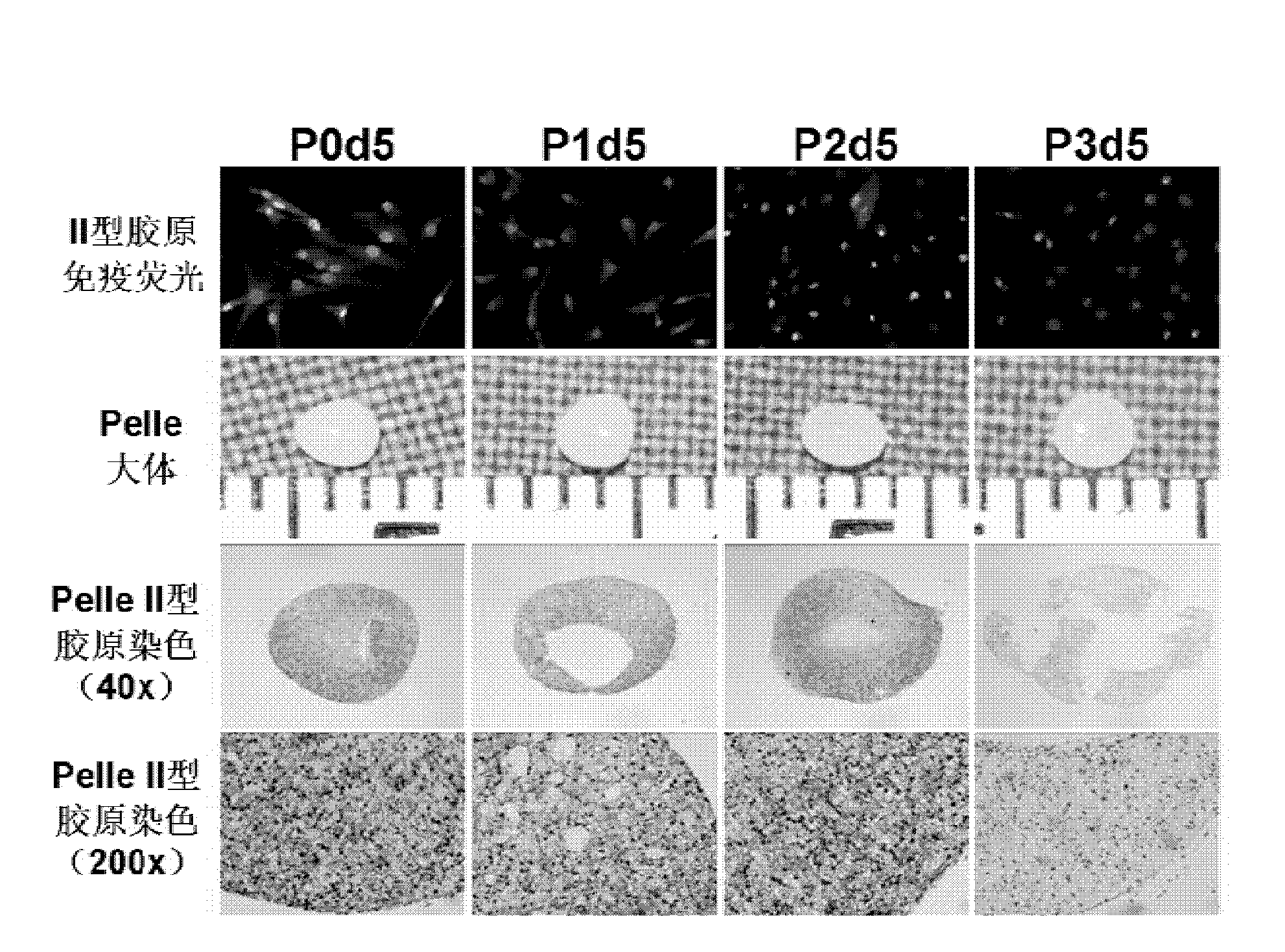
Human residual ear cartilage stem cells, and method for constructing tissue engineering cartilages
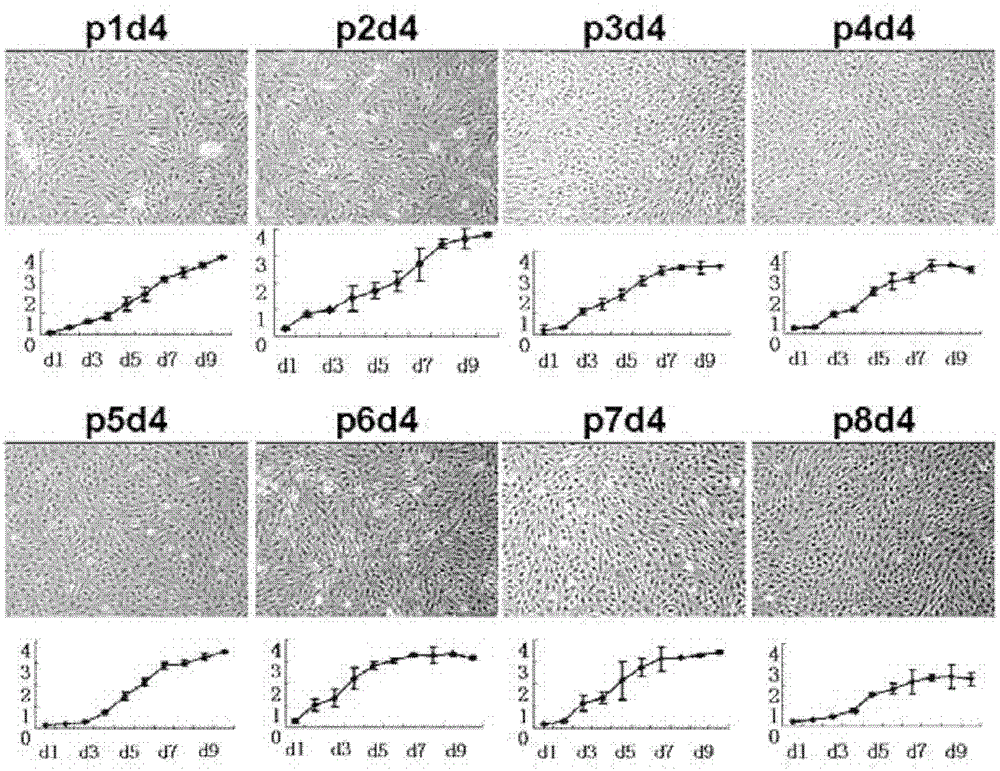
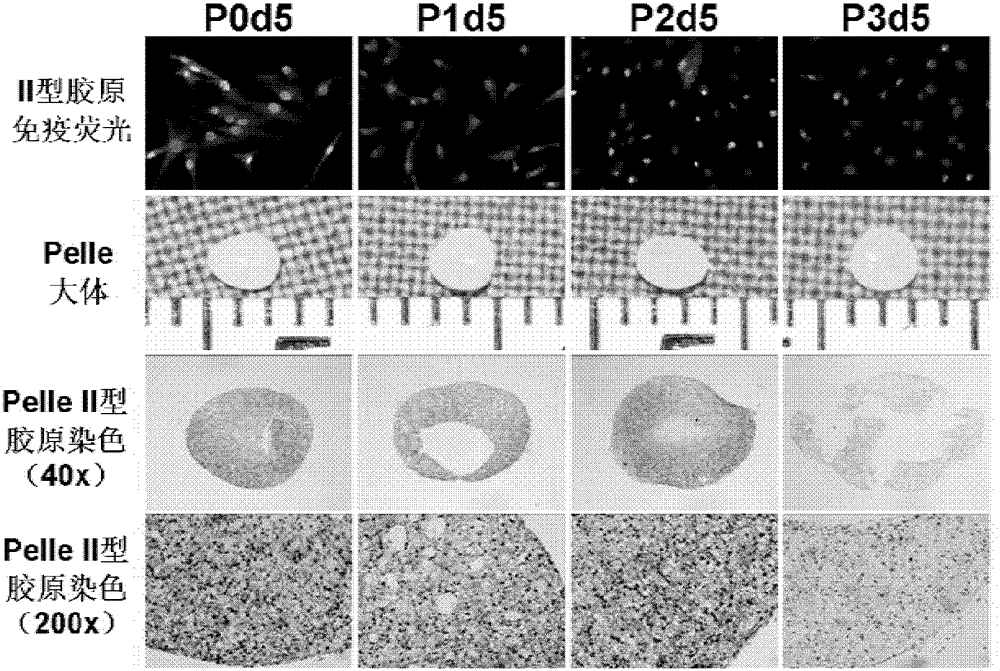
The invention discloses a tissue engineering cartilage graft. The tissue engineering cartilage graft contains human residual ear cartilage cells growing under a three-dimensional structure, and the human residual ear cartilage cells are passaged at the first, second, third, fourth, fifth, sixth, seventh or eighth passage. The invention also discloses an application of the human residual ear cartilage cells in the in-vitro construction of the cartilage graft.
Owner:SHANGHAI TISSUE ENG LIFE SCI
Crafting of cartilage
The invention is directed to producing a shaped cartilage matrix isolated from a human or animal where the cartilage has been crafted to facilitate disinfection, cleaning, devitalization, recellularization, and / or integration after implantation. The invention relates to a process for repairing a cartilage defect and implantation of a cartilage graft into a human or animal by crafting the cartilage matrix into individual grafts, disinfecting and cleaning the cartilage graft, applying a pretreatment solution to the cartilage graft, removing cellular debris using an extracting solution to produce a devitalized cartilage graft, implanting the cartilage graft into the cartilage defect with or without an insertion device, and sealing the implanted cartilage graft with recipient tissue. The devitalized cartilage graft is optionally recellularized in vitro, in vivo, or in situ with viable cells to render the tissue vital before or after the implantation. The devitalized cartilage graft is also optionally stored between the removing cellular debris and the recellularizing steps.
Owner:LIFENET HEALTH
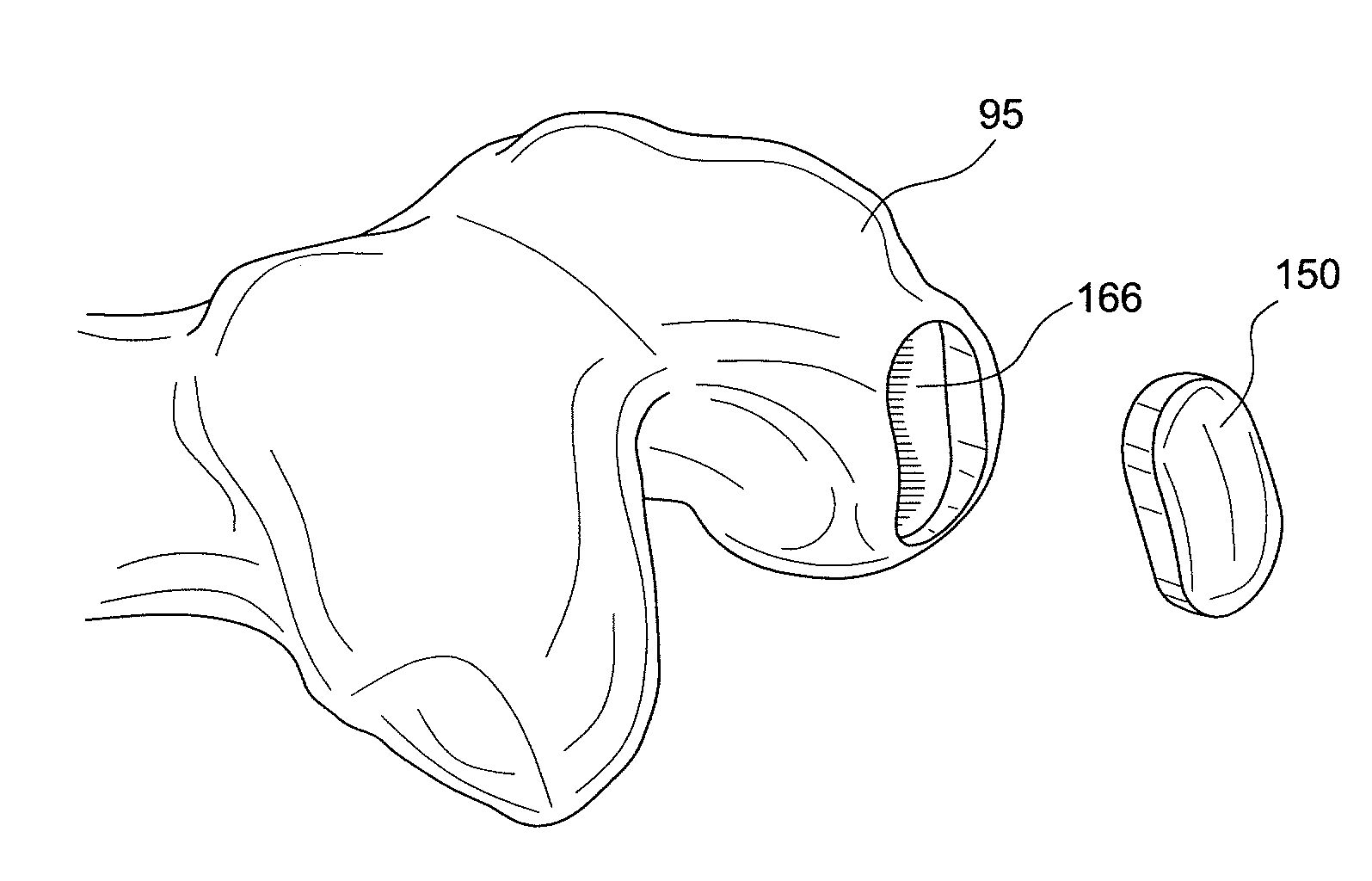
Device and method for allograft total hip arthroplasty
ActiveUS8439921B2Precise preparationBone implantJoint implantsTotal hip arthroplastyTissue engineered bone
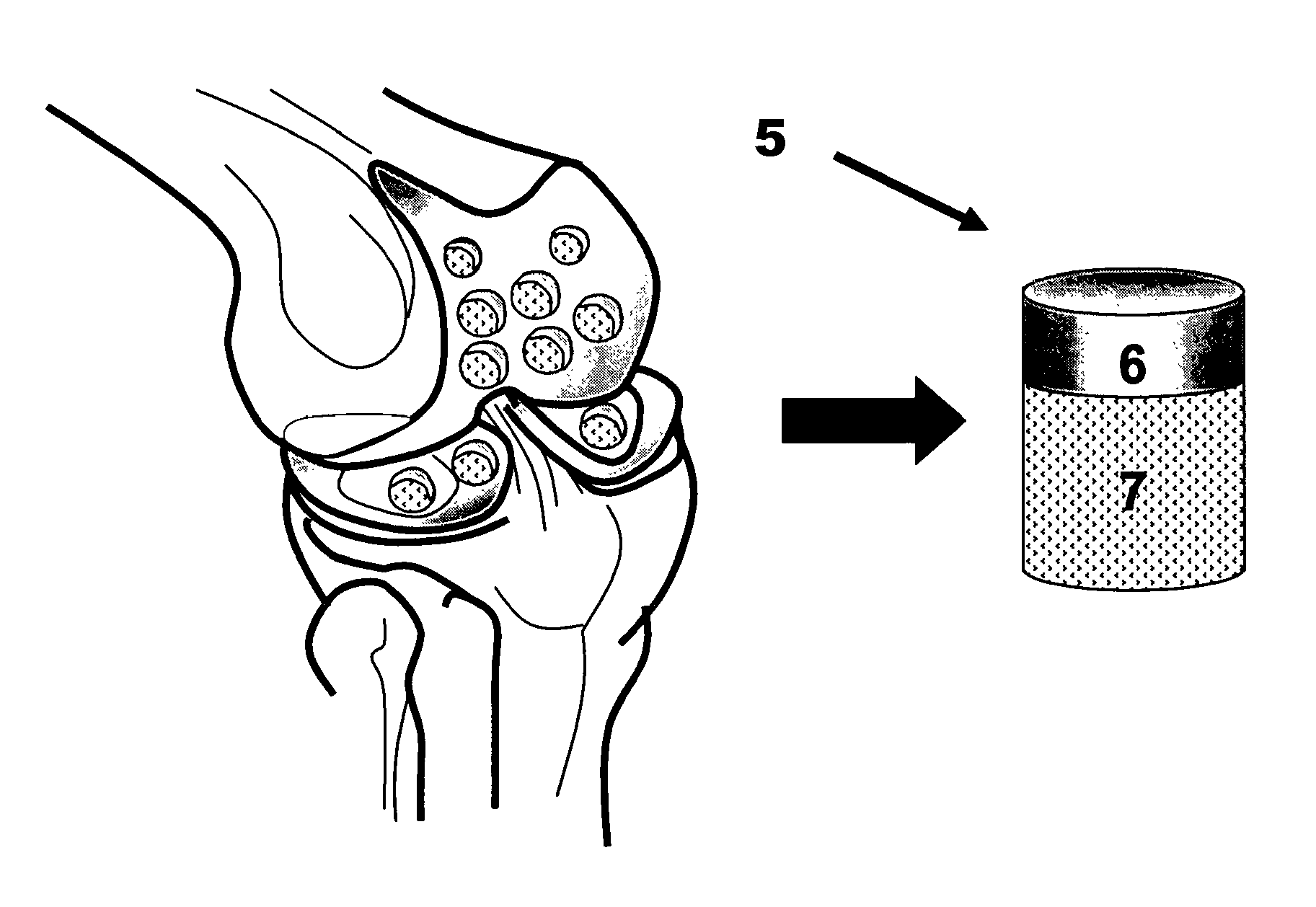
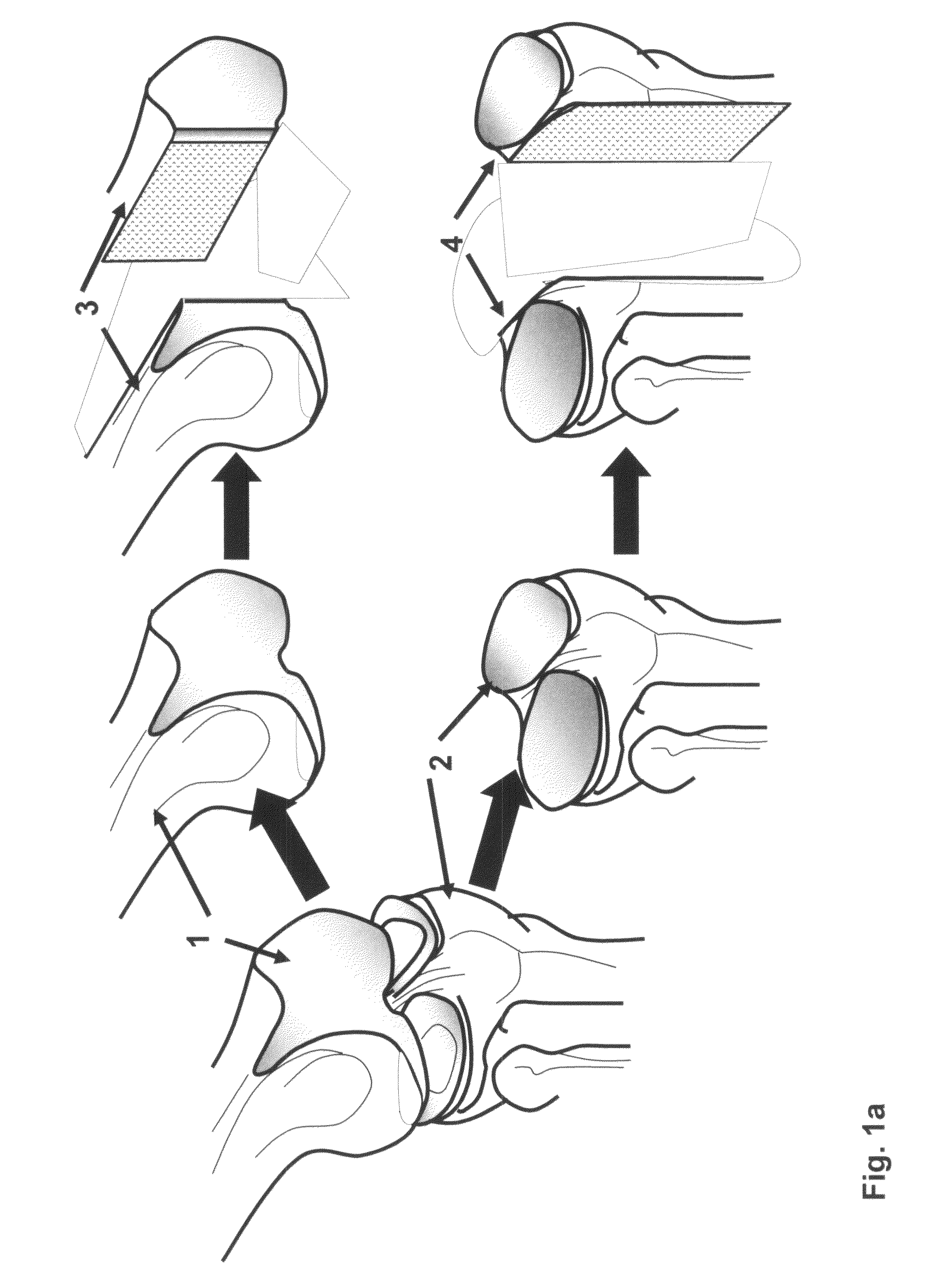
Method and apparatus for preparing bone and cartilage transplants for the reconstruction of the acetabulum, femoral head, or both with tissue engineered osteochondral constructs or and osteochondral allograft transplant.
Owner:JAMALI AMIR
Incus replacement prosthesis
ActiveUS7025785B1Effective positioningImproving lateral relationshipEar implantsIncusMiddle ear pressure
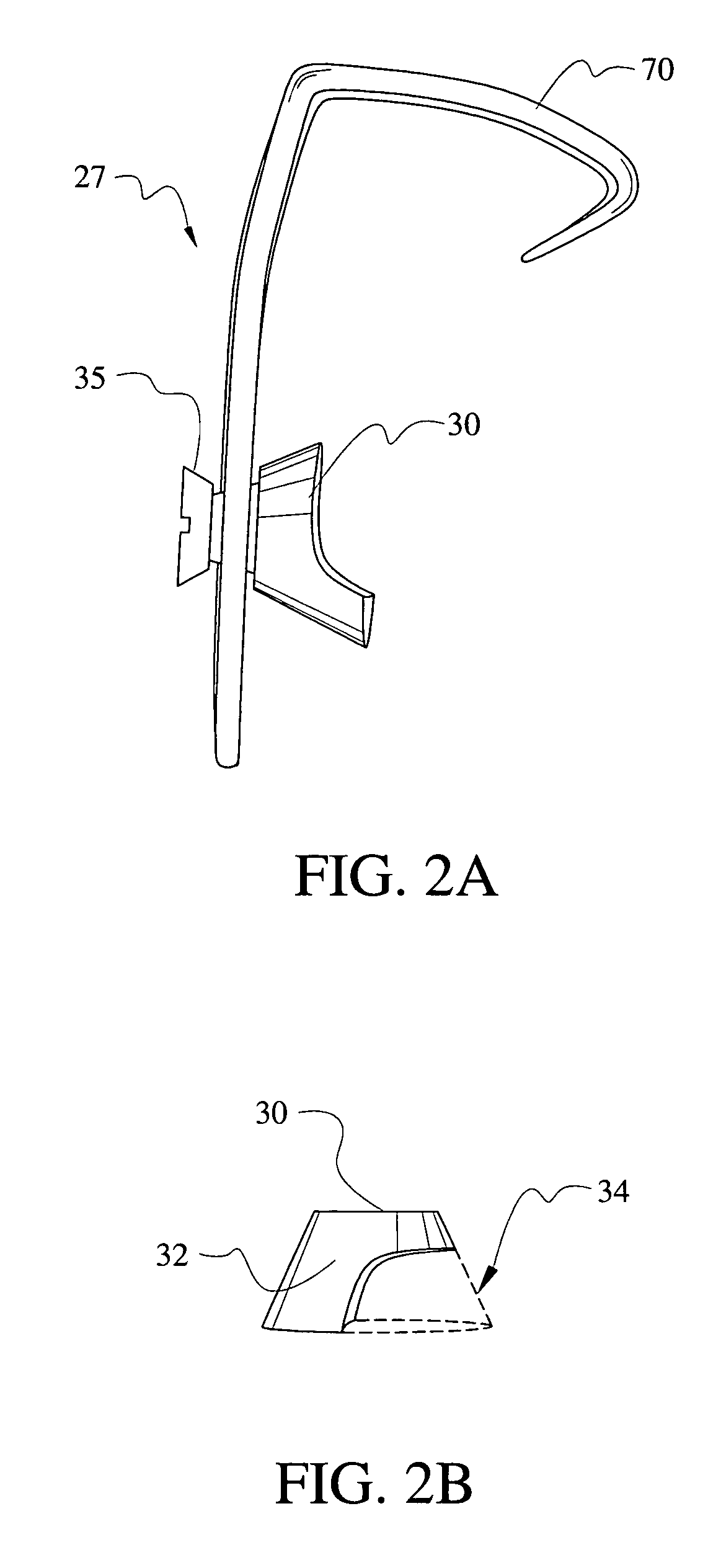
The present invention provides a device to restore hearing to individuals who have a discontinuity in the middle ear sound conductive mechanism. The device in accordance with the present invention addresses a specific problem arising often in middle ear surgery. Currently available middle ear prostheses are inadequate to remedy the specific problem of a lateral relationship of the stapes capitulum to the malleus, thereby necessitating a cartilage graft resulting in poor sound conductive properties. The present invention provides a middle ear prosthesis that solves the problems associated with the lateral relationship of the stapes capitulum to the malleus.
Owner:UNIV OF SOUTH FLORIDA
Cartilage graft and construction method thereof
PendingCN108342356AReverse dedifferentiationLess trauma to the donor siteBone implantSkeletal/connective tissue cellsCartilage cellsMedicine
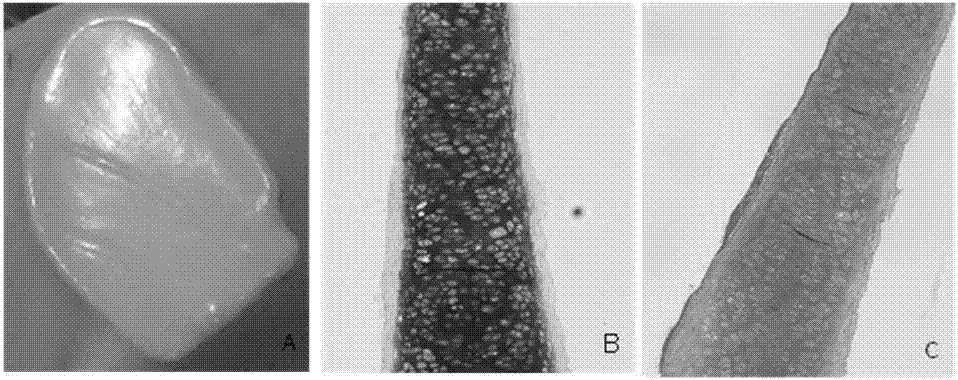
The invention discloses a cartilage graft and a construction method thereof. The cartilage graft is a cartilage membrane or injectable cartilage graft and contains a cartilage membrane or cartilage micro-tissue formed by in-vitro culture of cartilage cells.
Owner:SHANGHAI RESTHETIC BIO CO LTD
Devitalization and recellularization of cartilage
Owner:LIFENET HEALTH
Processing method of tissue engineered implant
ActiveCN101332312ANo damageBest physiological performanceProsthesisShort termsBiomedical engineering
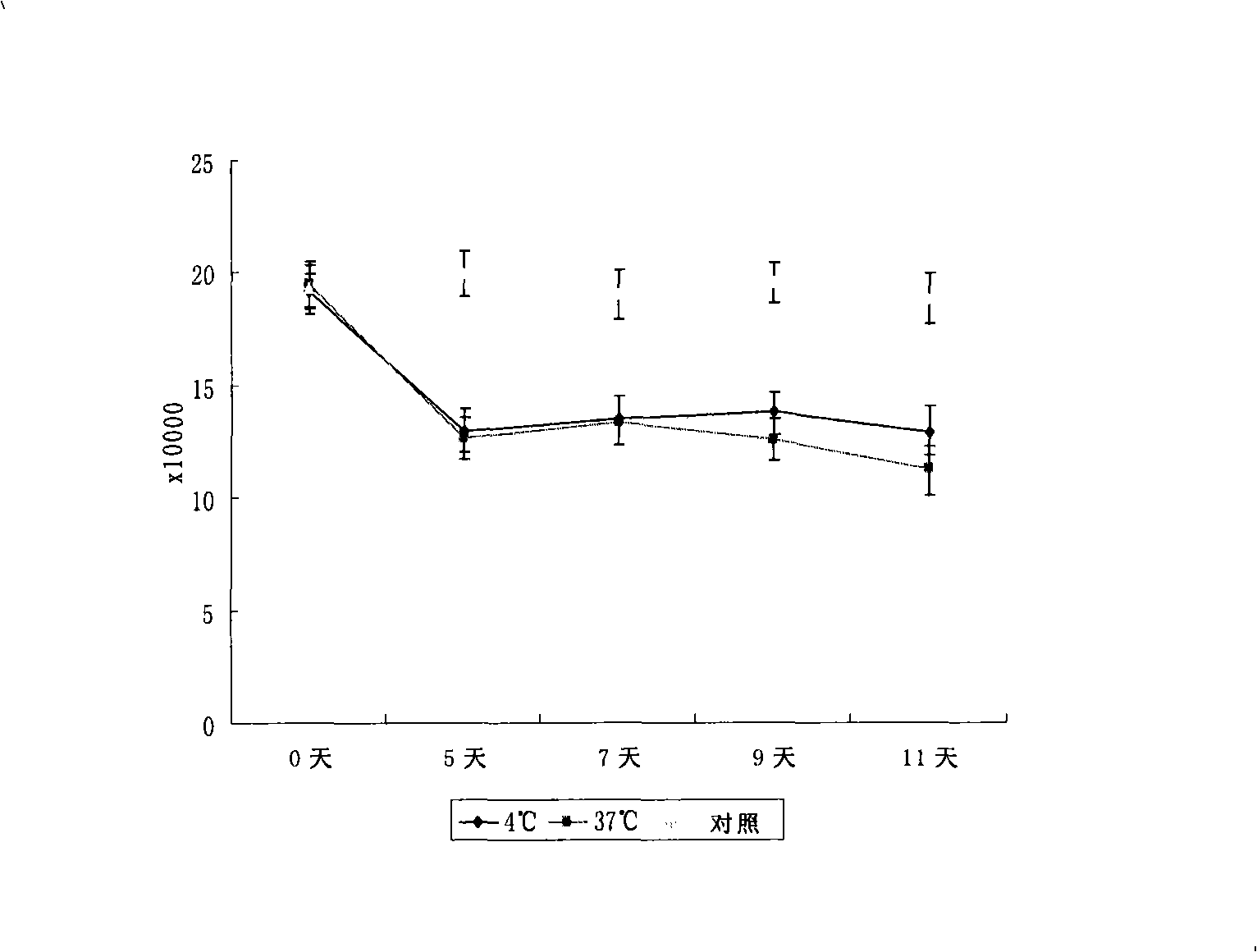
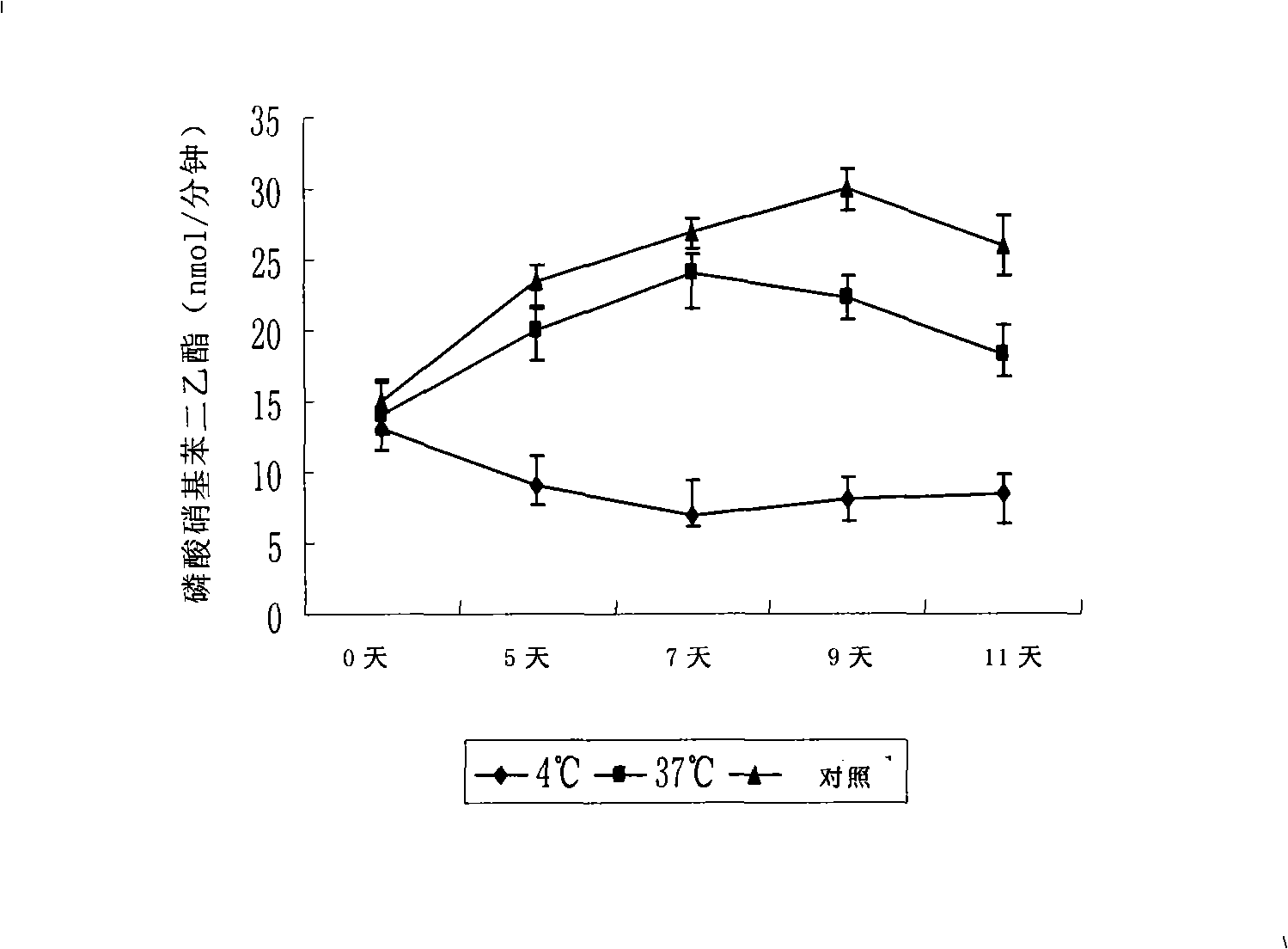
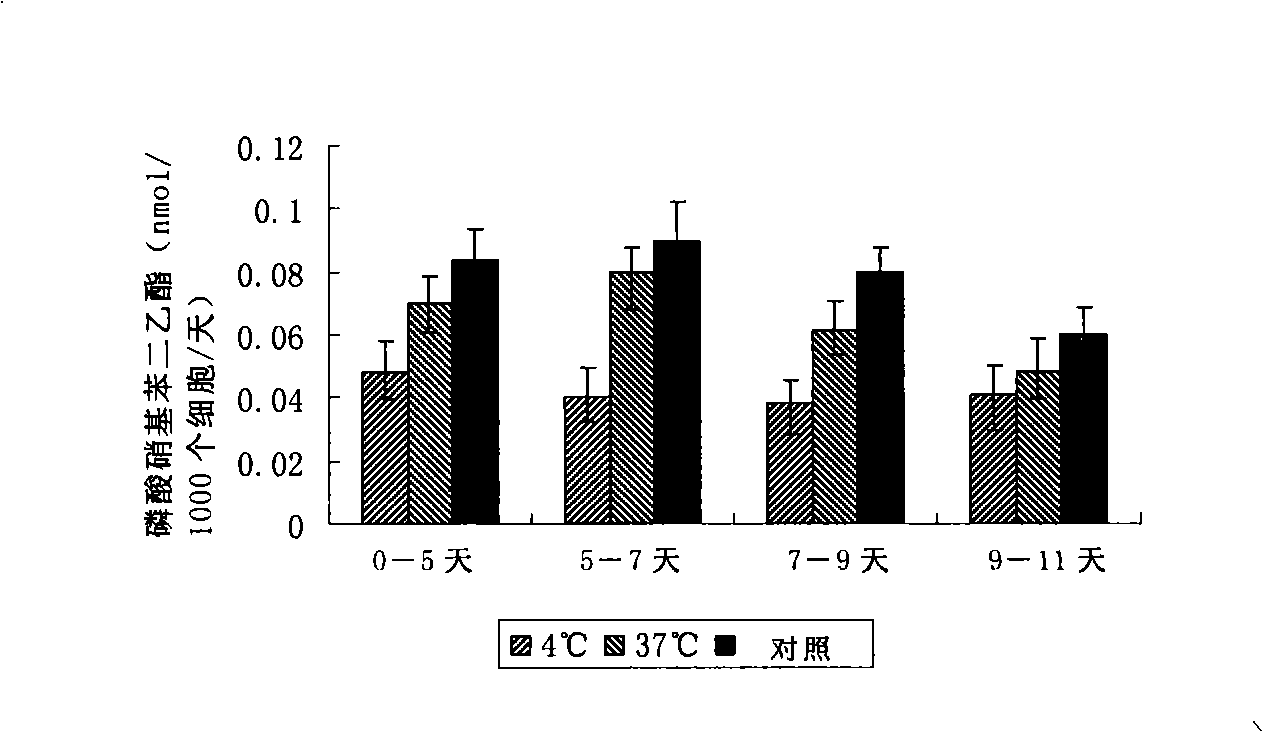
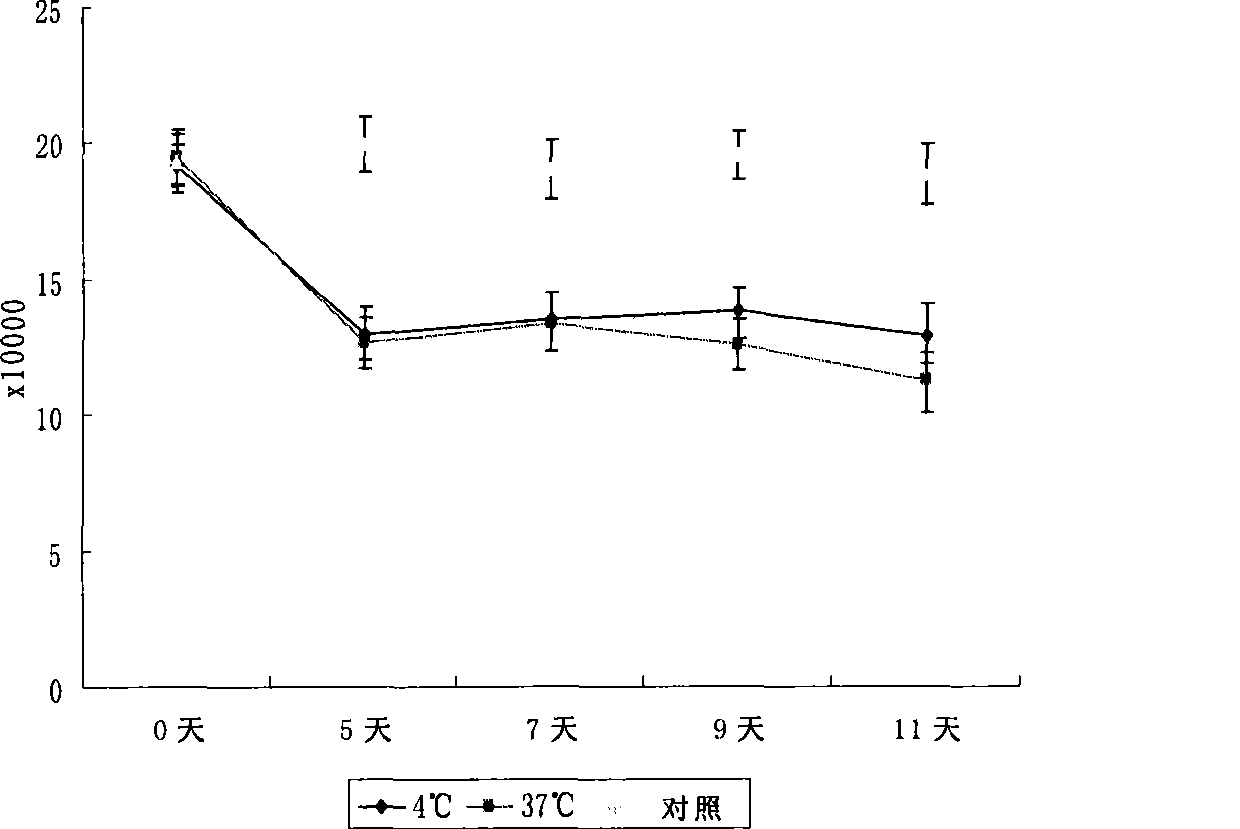
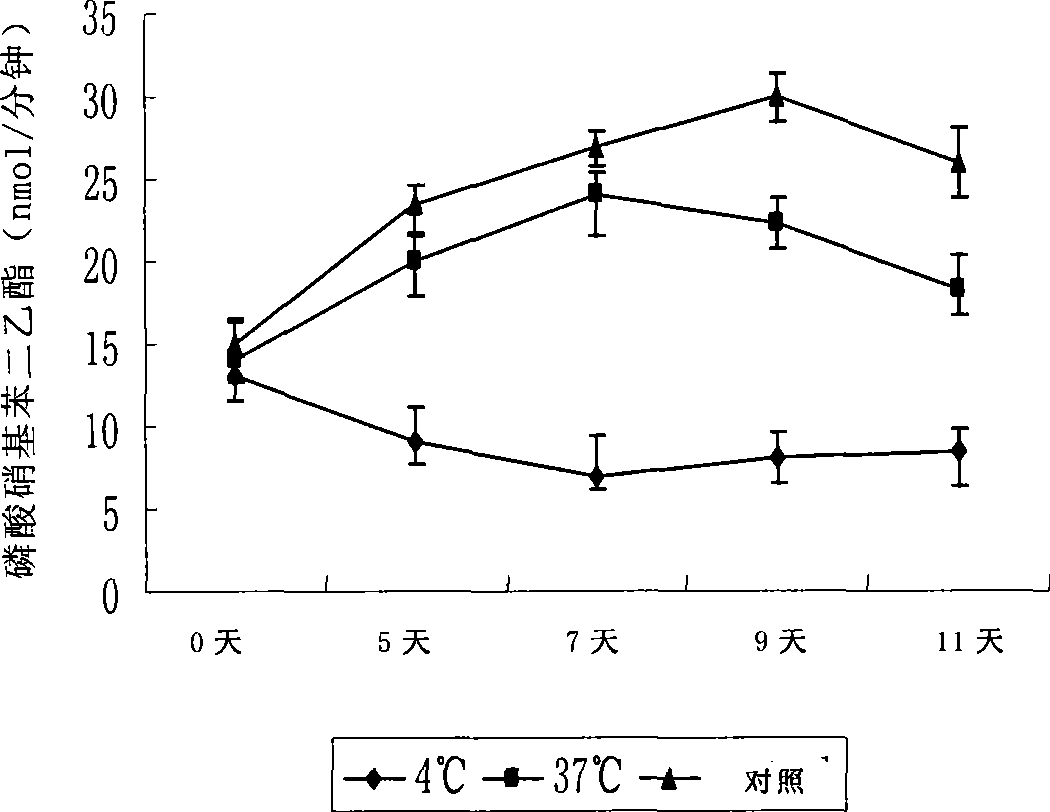
The invention discloses a disposal method for a tissue-engineered graft, comprising the following steps: (a) placing a bone graft or a cartilage graft and osteogenesis induced liquid or chondroblast induced liquid in a container for 2 hours to 14 days under 37 plus or minus 2 DEG C; (b) taking out of the bone graft or the cartilage graft from the container as the preparation material for transplant operation. The method can preserve the graft in a short term but brings no damage to the graft and keep good physical performance while the cost is low and the operation is easy.
Owner:SHANGHAI TISSUE ENG LIFE SCI
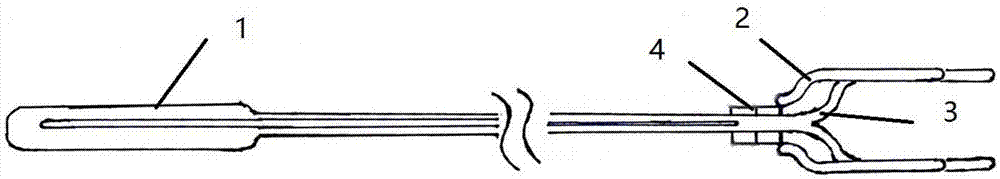
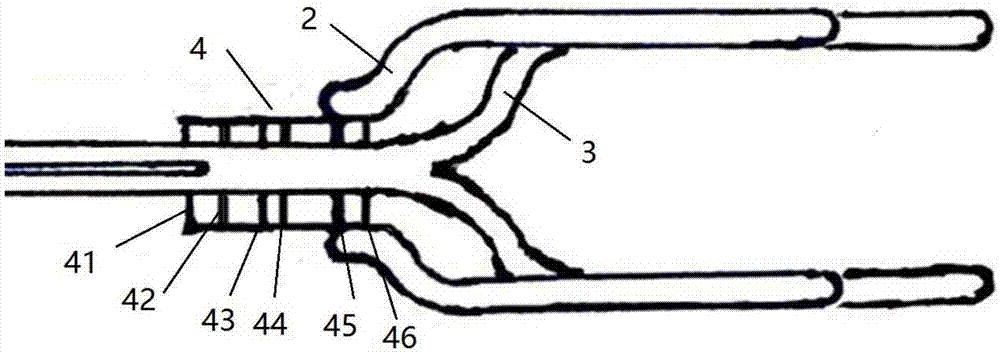
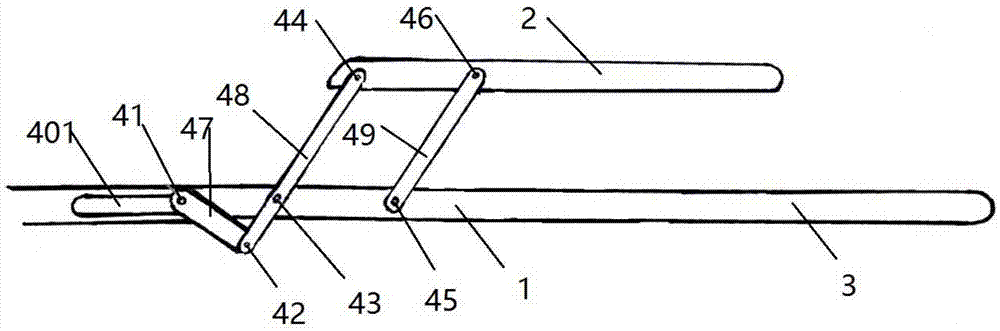
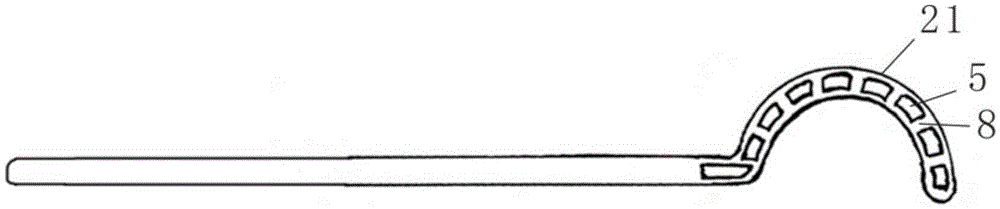
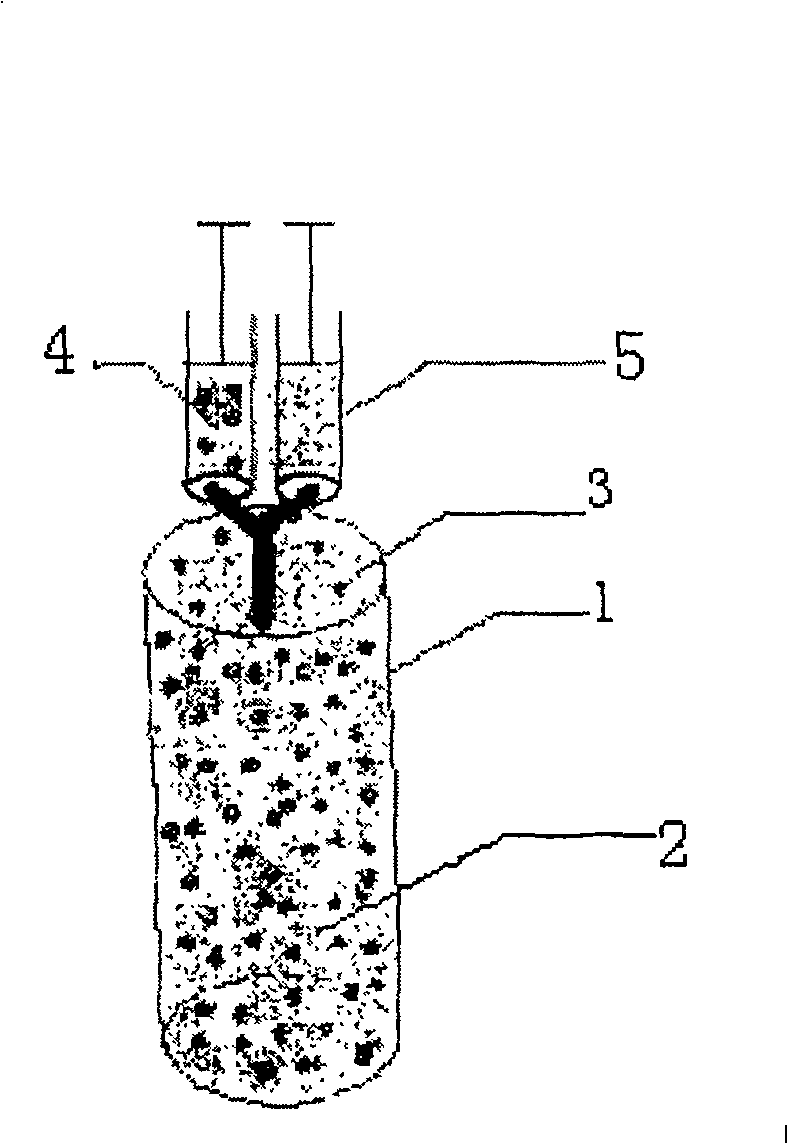
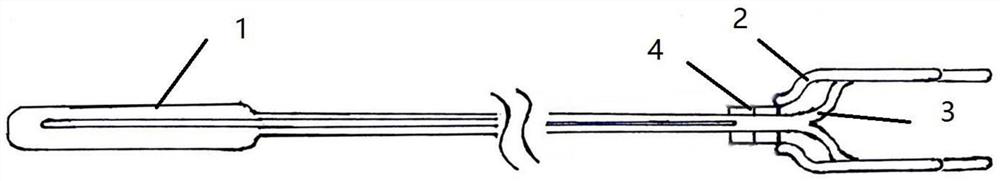
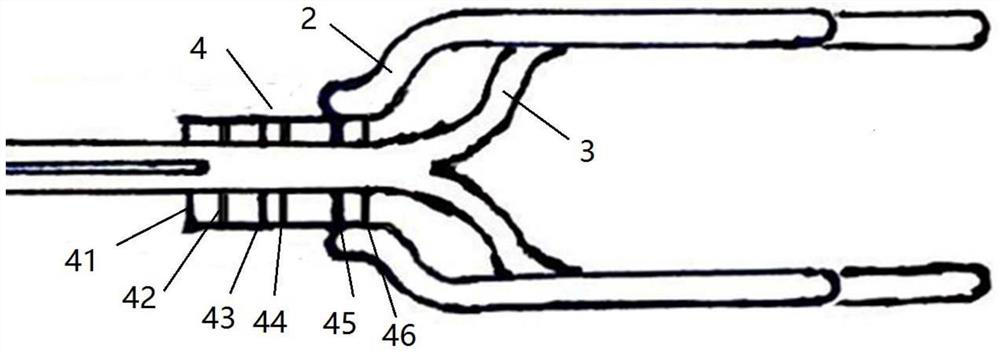
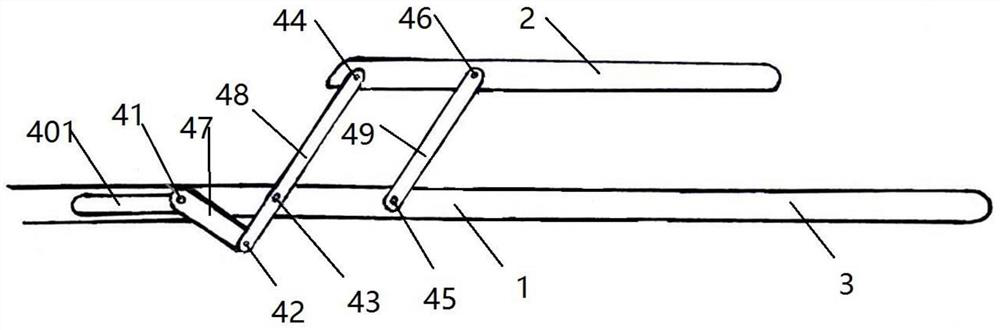
Minimally invasive collagen membrane retainer used for tissue engineering cartilage grafting
ActiveCN106901803ASmall expansion areaNo enlargement of the surgical woundSurgical forcepsForcepsEngineering
A minimally invasive collagen membrane retainer used for tissue engineering cartilage grafting comprises a body, a first clamping arm set, a second clamping arm set and a control mechanism for driving the first clamping arm set and the second clamping arm set to close. The two clamping arms are located at the front end of the body. The control mechanism comprises a sliding assembly and a connection rod assembly which are in driving connection, and the connection rod assembly is connected with the first and second clamping arm sets and used for driving the first clamping arm set to overlap in the extending direction. According to the retainer, the two clamping arm sets are overlapped under the drive of the sliding assembly and the connection rod assembly, a collagen membrane is clamped, the sliding assembly moves to the body and drives the connection mechanism, the connection rod mechanism makes the clamping arm sets extend forward to the body and overlap, and clamping is achieved. Compared with operating forceps and retainers in the prior art, the expanded area is small, surgical wounds are not expanded, and operation is convenient.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
Method for forming non-circular cartilage grafts
A technique for knee replacement surgery that allow for the removal of an oval oblong-shaped allograft bone and cartilage plug from a donor distal femur. The technique uses instruments including (i) sizing guides to match the recipient's femoral size and curvature to that of a donor femur (the sizing guides also acting as a wide pin placement template for the donor distal femur); (ii) osteotomes that cut the curved and straight portions of the implant shape (these may be disposable or reusable); and (iii) templates that fit over the guide pins and have openings to allow the osteotomes to cut the donor femur plug to the correct size, shape and depth. The instruments allow for a non-circular shape to be extracted from a donor femur for use in a bone-saving osteoarthritis distal femur resurfacing procedure.
Owner:ARTHREX INC
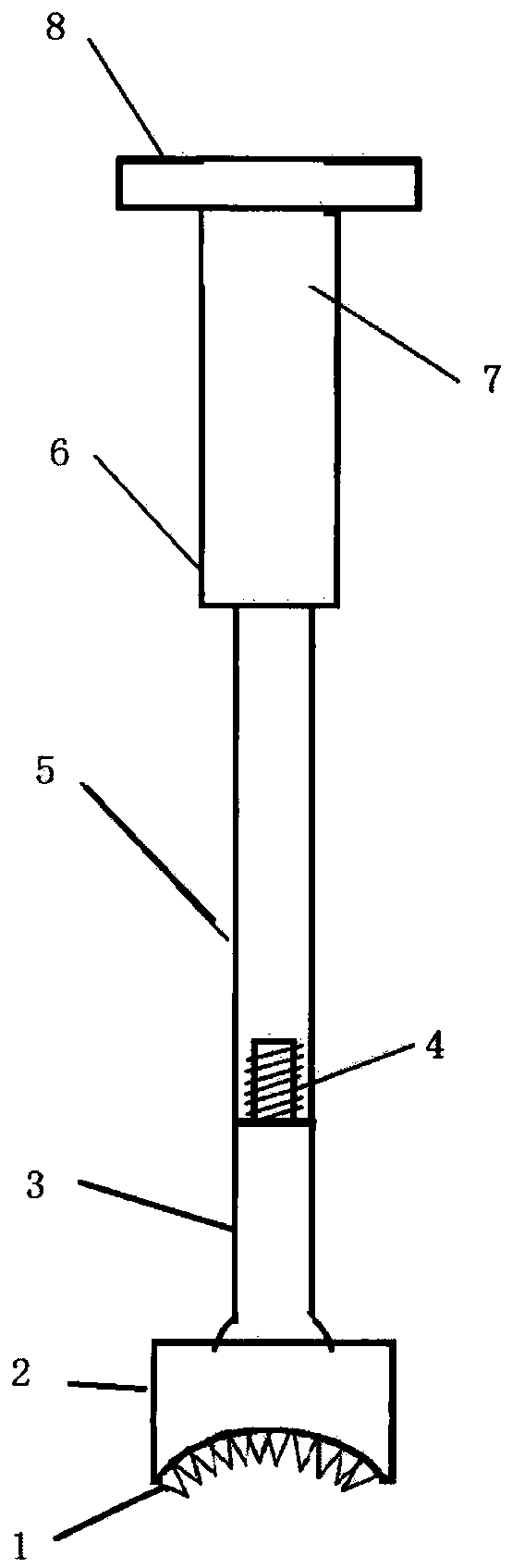
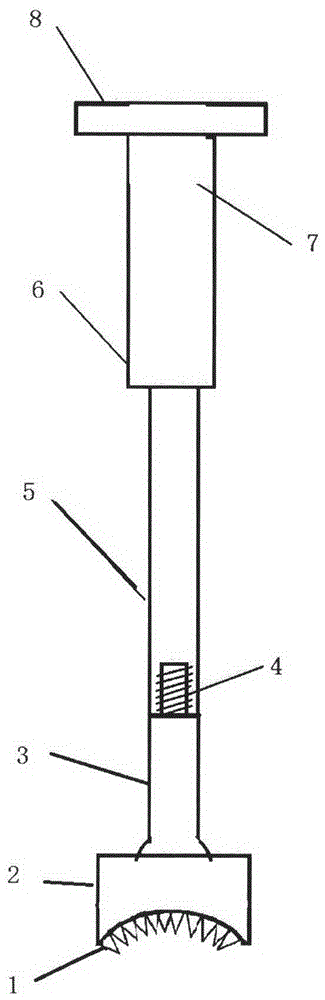
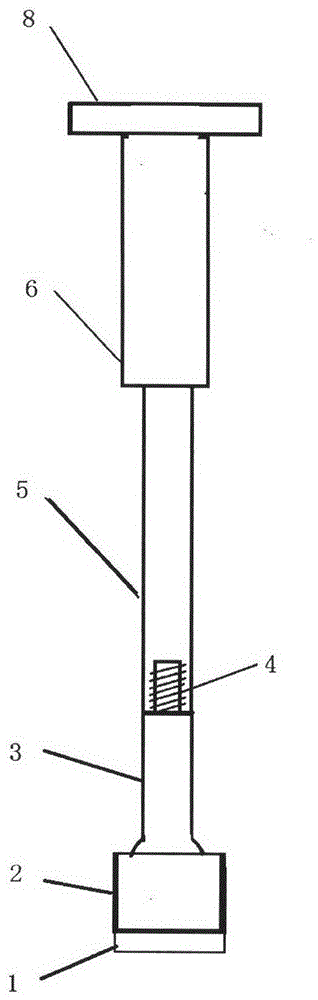
Medical forming device for gristle transplanting receptor spongy bone bed
The invention relates to a medical forming device for a gristle transplanting receptor spongy bone bed. Multiple knife edges are connected to a knife head base connected with a knife head connecting rod through a universal ball joint. The knife head connecting rod is connected with a handle connecting rod and a handle, the handle is connected with a handle stopping block, and the handle stopping block is connected with a manual sliding impacting device or an electric impacting device. The defects that in the prior art, because a bone knife, a bone chisel and other simple tools are used for manually trimming, the bone cutting face is not flat, randomness is high, and the bone cutting face of a receptor and the bone cutting face of a donor are prone to being mismatched, which is adverse to bone healing are overcome. The receptor spongy bone bed can be formed through one-off impacting, so that forming efficiency is improved, operation time is shortened, and the form of the receptor spongy bone bed is kept uniform. The manual sliding impacting device and the electric impacting device can be selected for use, so that the form of the spongy bone, after cutting, below the transplanted donor gristle is completely matched with that of the receptor sponge bone bed, a transplanted donor and a receptor are completely matched, and bone healing is facilitated.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
Composite structured tissue engineering cartilage graft and its preparation method
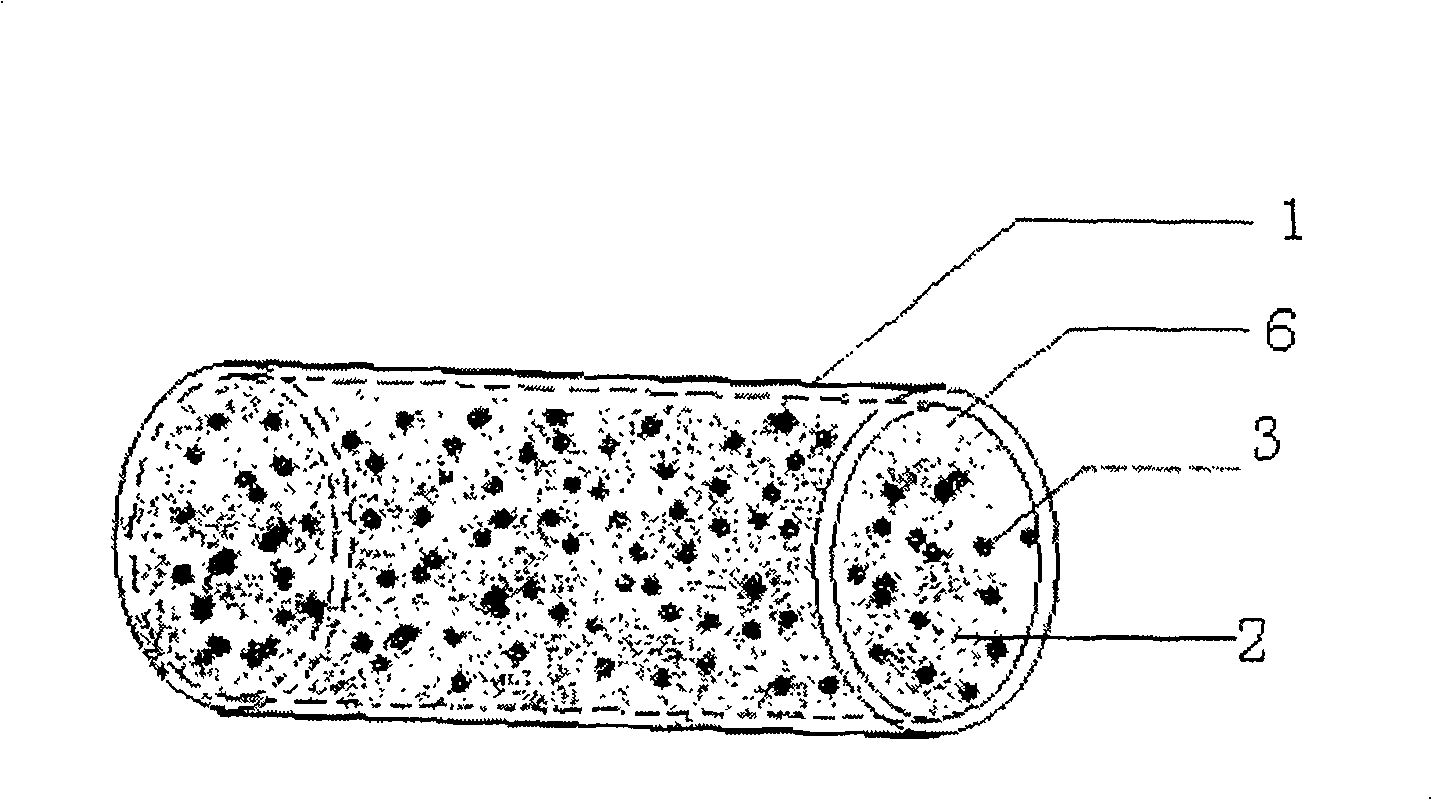
A tissue-engineered cartilage transplant with composite structure for treating the cartilage defect and surficial depressed defect is composed of the medically acceptable solid carrier with internal cavity, and the mixture of medically acceptable colloid and normal cartilage cells through filling said mixture in said cavity of biodegradable solid carrier.
Owner:北京市创伤骨科研究所
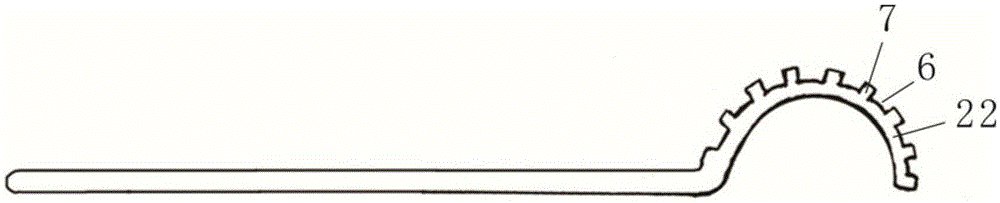
Minimally invasive surgery instrument for tissue-engineered cartilage transplantation
ActiveCN105232113ASolve the problem of clamping and fixingTraumaSurgical pincettesLess invasive surgeryForceps
The invention discloses a minimally invasive surgery instrument for tissue-engineered cartilage transplantation. According to the minimally invasive surgery instrument, the technical problems of clamping and fixation of a collagen membrane under an arthroscope during sewing are solved. The minimally invasive surgery instrument comprises forceps, wherein two forceps heads are arc-shaped bent heads, through holes are uniformly formed along the surface of the forceps head at the upper end and penetrate through the upper and lower end surfaces, and a first separation part is arranged between every two adjacent through holes; grooves corresponding to the positions of the through holes are formed at intervals along the peripheral wall of the outer diameter of the forceps head at the lower end, a second separation part is arranged between every two adjacent grooves, and a rectangular tooth-shaped structure is formed between the grooves and the second separation parts. Compared with the prior art, the minimally invasive surgery instrument has the advantages that a clamper for the arc-shaped bent heads is arranged, and holes and teeth are respectively formed in and arranged on the two forceps heads and form sewing holes after being folded, so that the clamping and fixation problems of the collagen membrane under the arthroscope during the sewing are solved.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
Preparation of acellular cartilage graft and uses thereof
ActiveUS20180369449A1Improve mechanical propertiesConnective tissue peptidesPharmaceutical delivery mechanismFiberDisease
Disclosed herein is a method of producing acellular cartilage grafts. The method includes steps of, subjecting a cartilage matrix derived from an animal to alkaline, disinfection and decelluarization treatments. The thus produced cartilage graft is devoid of any cellular matters, while maintaining the porosity and integrity of collagen fibers therein, thus is suitable as a xenograft for host cells to grown thereon. Also disclosed herein is a method for treating osteochondral disease of a subject, in which the present acellular cartilage graft is applied to a lesion site of the subject.
Owner:ACRO BIOMEDICAL CO LTD
A kind of articular cartilage graft and preparation method thereof
ActiveCN103920190BImprove stress resistanceImprove wear resistanceJoint implantsCell-Extracellular MatrixECM Protein
An articular cartilage graft and a preparation method thereof. The articular cartilage graft prepared by the present invention consists of three layers from the outside to the inside: superficial layer, middle layer and deep layer, and the thickness, shape and size of each layer are matched with the cartilage damage site, No need for preoperative shaping, after implantation, it corresponds to the distribution of the surrounding normal cartilage layers, which is conducive to the signal transmission, transduction and regulation between cells, and can better integrate with the surrounding normal cartilage tissue; it has a structure consistent with natural cartilage Features, from the superficial layer to the deep layer, the content of type II collagen gradually decreases, the content of GAG gradually increases, and the compression resistance and abrasion resistance are good; the chondrocytes in the cartilage graft are wrapped by extracellular matrix, and the immunogenicity is low, avoiding implantation After immune rejection occurs, it can survive and function for a long time, and improve the long-term curative effect of cartilage repair.
Owner:西安博鸿生物技术有限公司
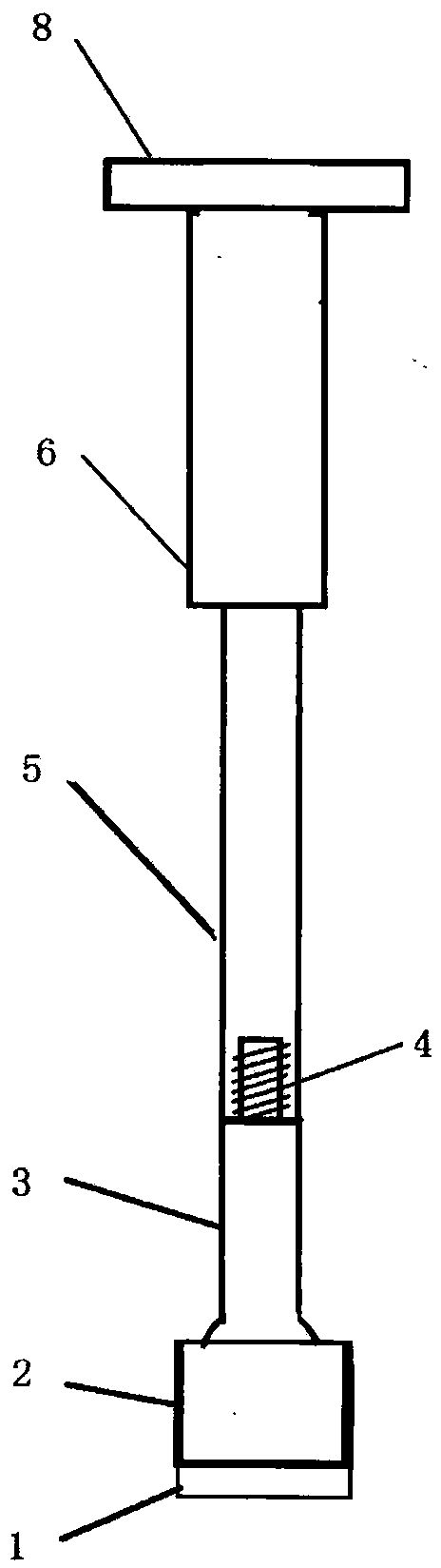
Cancellous bone bed shaper for medical cartilage graft recipients
The invention relates to a medical forming device for a gristle transplanting receptor spongy bone bed. Multiple knife edges are connected to a knife head base connected with a knife head connecting rod through a universal ball joint. The knife head connecting rod is connected with a handle connecting rod and a handle, the handle is connected with a handle stopping block, and the handle stopping block is connected with a manual sliding impacting device or an electric impacting device. The defects that in the prior art, because a bone knife, a bone chisel and other simple tools are used for manually trimming, the bone cutting face is not flat, randomness is high, and the bone cutting face of a receptor and the bone cutting face of a donor are prone to being mismatched, which is adverse to bone healing are overcome. The receptor spongy bone bed can be formed through one-off impacting, so that forming efficiency is improved, operation time is shortened, and the form of the receptor spongy bone bed is kept uniform. The manual sliding impacting device and the electric impacting device can be selected for use, so that the form of the spongy bone, after cutting, below the transplanted donor gristle is completely matched with that of the receptor sponge bone bed, a transplanted donor and a receptor are completely matched, and bone healing is facilitated.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
Human residual ear cartilage stem cells and method for constructing tissue engineered cartilage
Owner:SHANGHAI TISSUE ENG LIFE SCI
Processing method of tissue engineered implant
ActiveCN101332312BNo damageBest physiological performanceProsthesisEngineeringBiomedical engineering
The invention discloses a disposal method for a tissue-engineered graft, comprising the following steps: (a) placing a bone graft or a cartilage graft and osteogenesis induced liquid or chondroblast induced liquid in a container for 2 hours to 14 days under 37 plus or minus 2 DEG C; (b) taking out of the bone graft or the cartilage graft from the container as the preparation material for transplant operation. The method can preserve the graft in a short term but brings no damage to the graft and keep good physical performance while the cost is low and the operation is easy.
Owner:SHANGHAI TISSUE ENG LIFE SCI
Cartilage graft scaffolds
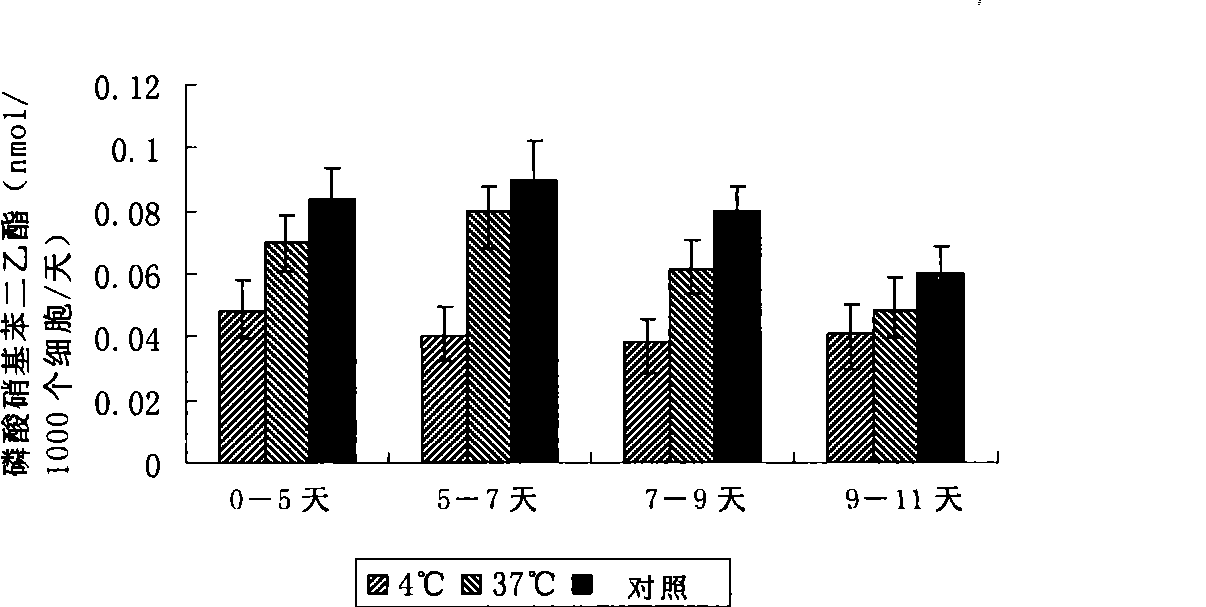
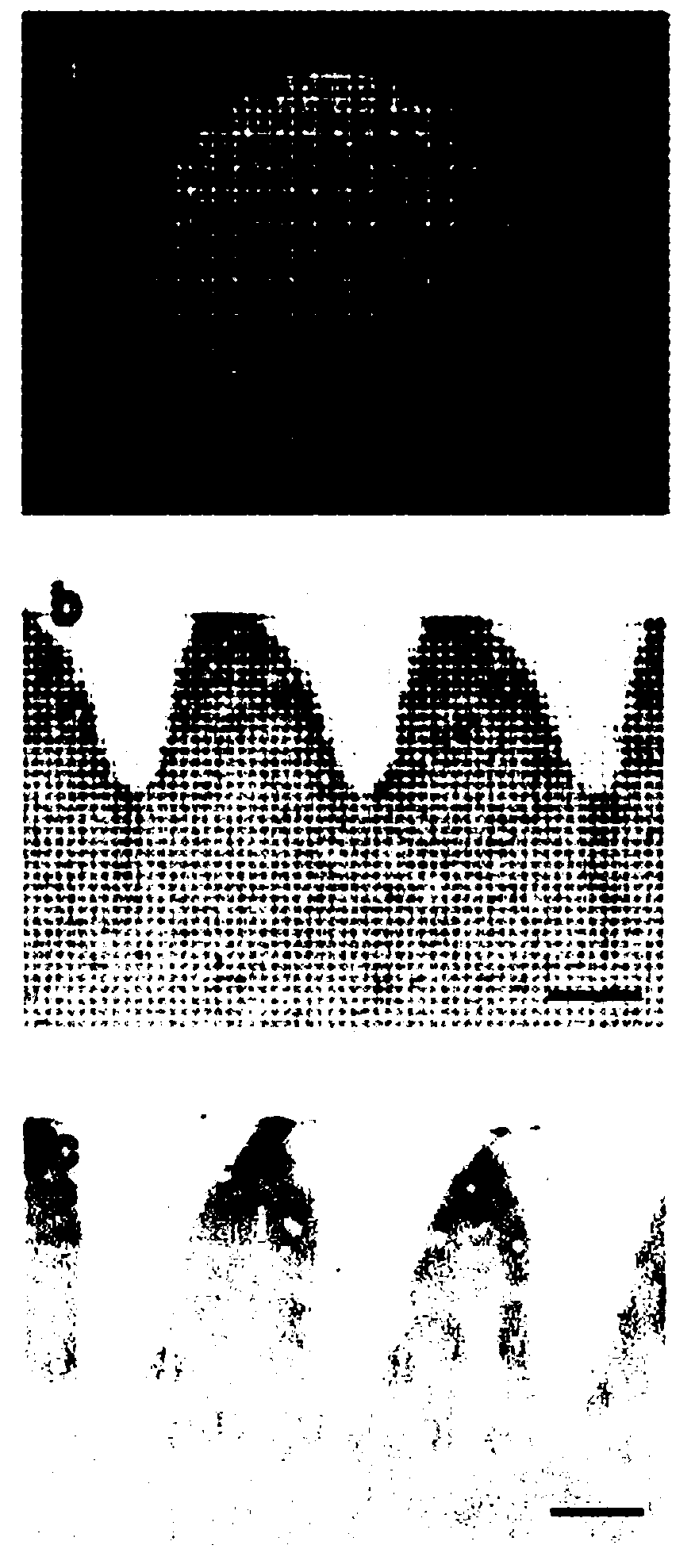
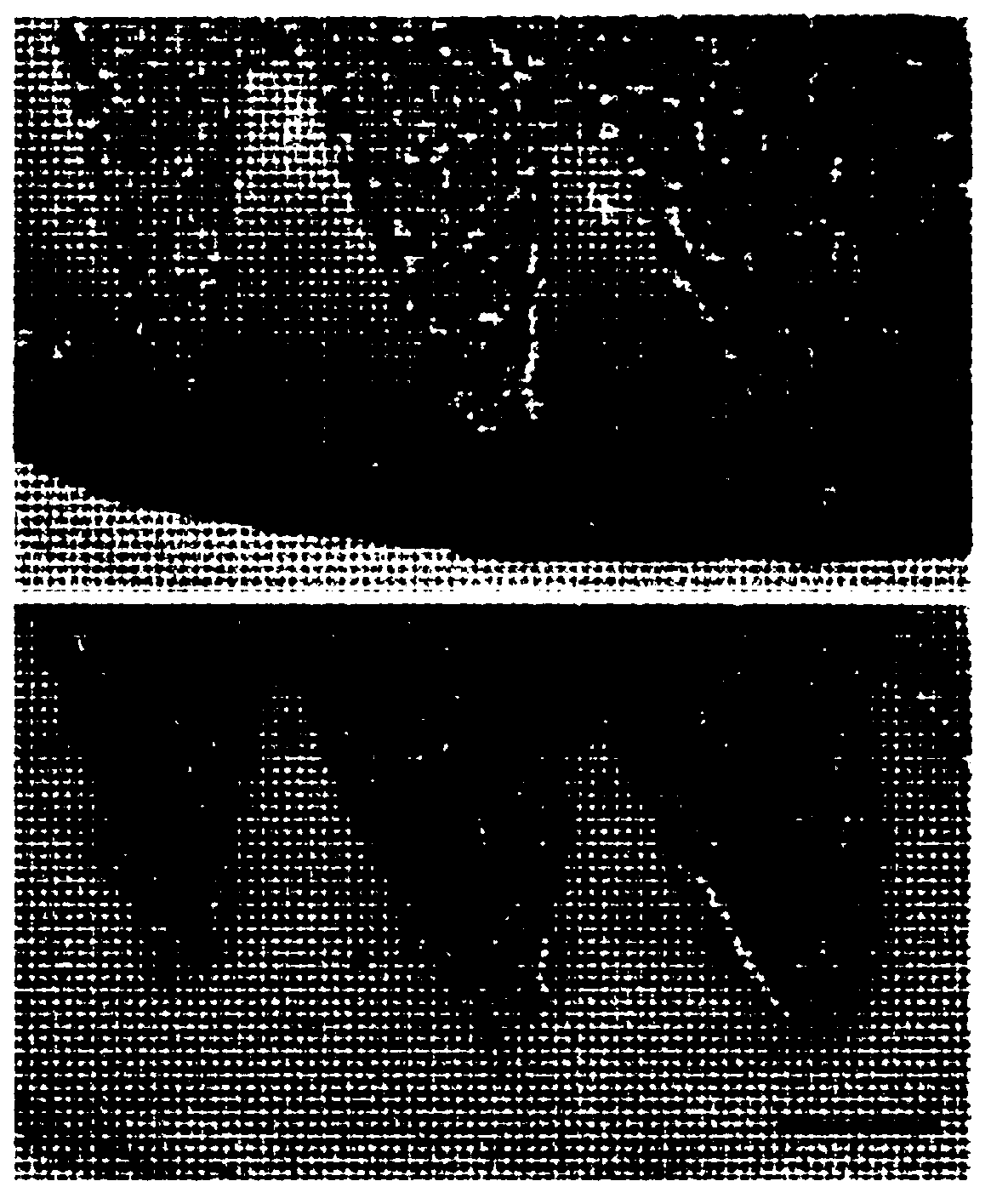
The present invention relates to a biomaterial comprising a cartilage graft scaffold substantially free of viable cells, wherein the cartilage graft scaffold exhibits a plural of notches in form of lamellae or grids.
Owner:TRAUMA CARE CONSULT
Composite structured tissue engineering cartilage graft and preparation method thereof
A tissue-engineered cartilage transplant with composite structure for treating the cartilage defect and surficial depressed defect is composed of the medically acceptable solid carrier with internal cavity, and the mixture of medically acceptable colloid and normal cartilage cells through filling said mixture in said cavity of biodegradable solid carrier.
Owner:北京市创伤骨科研究所
A Minimally Invasive Collagen Membrane Holder for Tissue-Engineered Cartilage Transplantation
ActiveCN106901803BSmall expansion areaNo enlargement of the surgical woundSurgical forcepsRetainerForceps
A minimally invasive collagen membrane retainer used for tissue engineering cartilage grafting comprises a body, a first clamping arm set, a second clamping arm set and a control mechanism for driving the first clamping arm set and the second clamping arm set to close. The two clamping arms are located at the front end of the body. The control mechanism comprises a sliding assembly and a connection rod assembly which are in driving connection, and the connection rod assembly is connected with the first and second clamping arm sets and used for driving the first clamping arm set to overlap in the extending direction. According to the retainer, the two clamping arm sets are overlapped under the drive of the sliding assembly and the connection rod assembly, a collagen membrane is clamped, the sliding assembly moves to the body and drives the connection mechanism, the connection rod mechanism makes the clamping arm sets extend forward to the body and overlap, and clamping is achieved. Compared with operating forceps and retainers in the prior art, the expanded area is small, surgical wounds are not expanded, and operation is convenient.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com