Composite structured tissue engineering cartilage graft and its preparation method
A composite structure and tissue engineering technology, which is applied in the fields of medicine and biomedical engineering, can solve the problems of biological glue not reaching the effective concentration, causing great damage to patients, and decreasing viscosity, and achieve the effects of saving chondrocytes, reducing pain, and not easy to fall off
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation and molding of allogeneic demineralized bone:
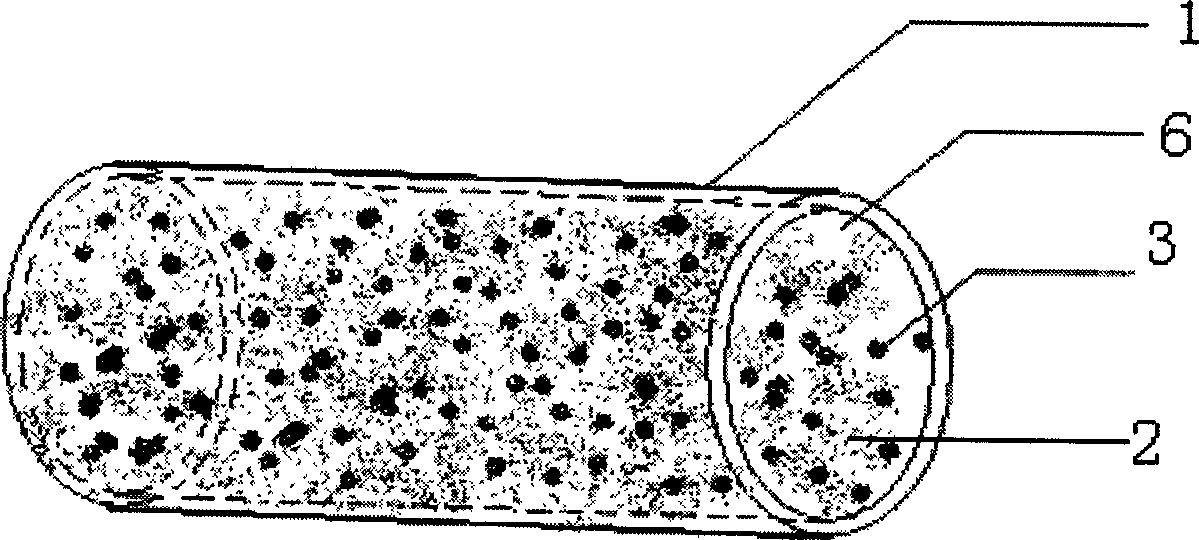
[0031] 1. The cancellous bone part of the allograft bone was made into a cylinder with a diameter of 4 mm and a length of 6 mm.
[0032] 2. Defat and protein the prepared cylindrical allogeneic bone to prepare antigen-free bone: use surfactant to defat and decellularize. For the specific method, refer to the patented method for the preparation of biologically derived artificial bone without antigen.
[0033] 3. Demineralize the allogeneic bone of the cylinder with hydrochloric acid at a concentration of 0.1-2.0 mol / L at a weight ratio of 50:1, and the demineralization time is 0.5-3 hours. In this embodiment, the concentration of hydrochloric acid is 0.5 mol / L, the weight ratio of hydrochloric acid to artificial bone is 50:1, and the demineralization time is 1 hour.
[0034] 4. After decellularization, degreasing and demineralization, the allograft bone forms an internally connected grid structure.
...
Embodiment 2
[0037] The acquisition of embodiment 2 chondrocytes:
[0038]We use dogs as experimental subjects. The knee joint of the dog's left hind leg was exposed, some cartilage in the non-load-bearing area of the lower end of the femur was taken, and the joint cavity was sutured. Soak the removed cartilage tissue in sterile PBS (concentration 0.01mol / L, pH7.4±0.1) containing gentamicin (40 units / ml) at a ratio of 200mg cartilage / 50ml PBS for 45-60 minutes, Afterwards, the cartilage tissue was manually cut into small pieces of 1mm×1mm×1mm size; the crushed cartilage tissue pieces were placed in 0.2% mg / ml type II collagenase solution, and digested in a constant temperature oscillator at 37°C±0.5°C for 8- After 12 hours, filter and centrifuge; the precipitated cells were washed twice with sterile PBS (concentration 0.01 mol / L, pH 7.4±0.1), stained with trypan blue to detect cell viability, and the viability was > 60%, and set aside.
Embodiment 3
[0039] The preparation of embodiment 3 cartilage tissue engineering graft: (following steps all require aseptic operation)
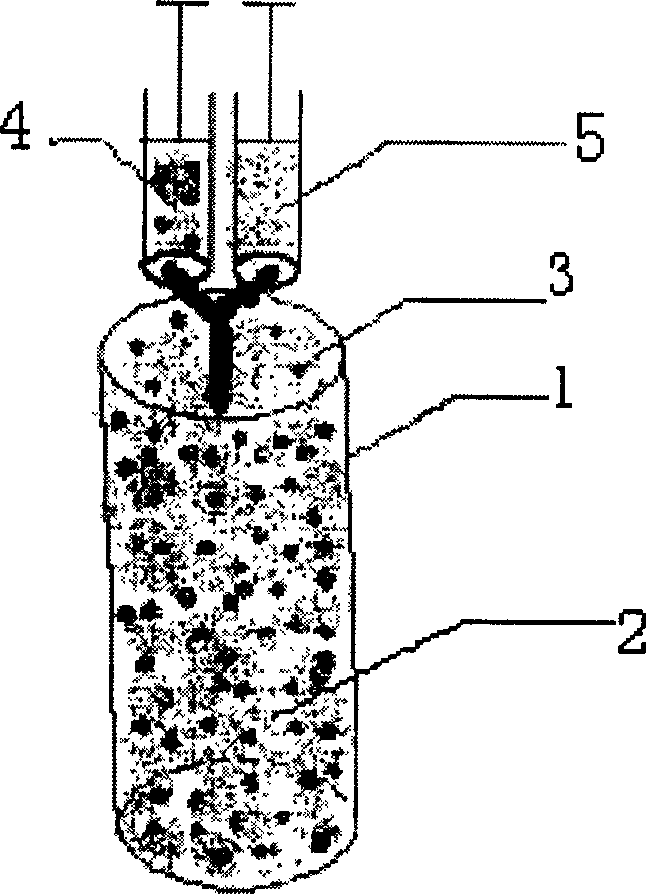
[0040] 1. Put 2.5×10 5 chondrocytes with 25 μl containing thrombin and Ca 2+ The solution (sterilized) was mixed evenly, and prepared into solution 4 for later use.
[0041] 2. Take the solution whose main components are fibrinogen and factor XIII as solution 5, inhale solution 5 into another needle syringe, and the inhalation volume is equal to solution 4.
[0042] 3. Place the cylindrical solid carrier with a diameter of 4 mm and a length of 6 mm upright, and slowly inject solution 4 and solution 5 into the cylindrical solid carrier after the needle of the syringe containing solution 4 and solution 5 is pressed against the top of the cylindrical solid carrier.
[0043] 4. Let it stand for 5 minutes until the mixture of solution 4 and solution 5 coagulates. At this time, the colloidal carrier formed by the agglutination of the mixture of solution 4 a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com