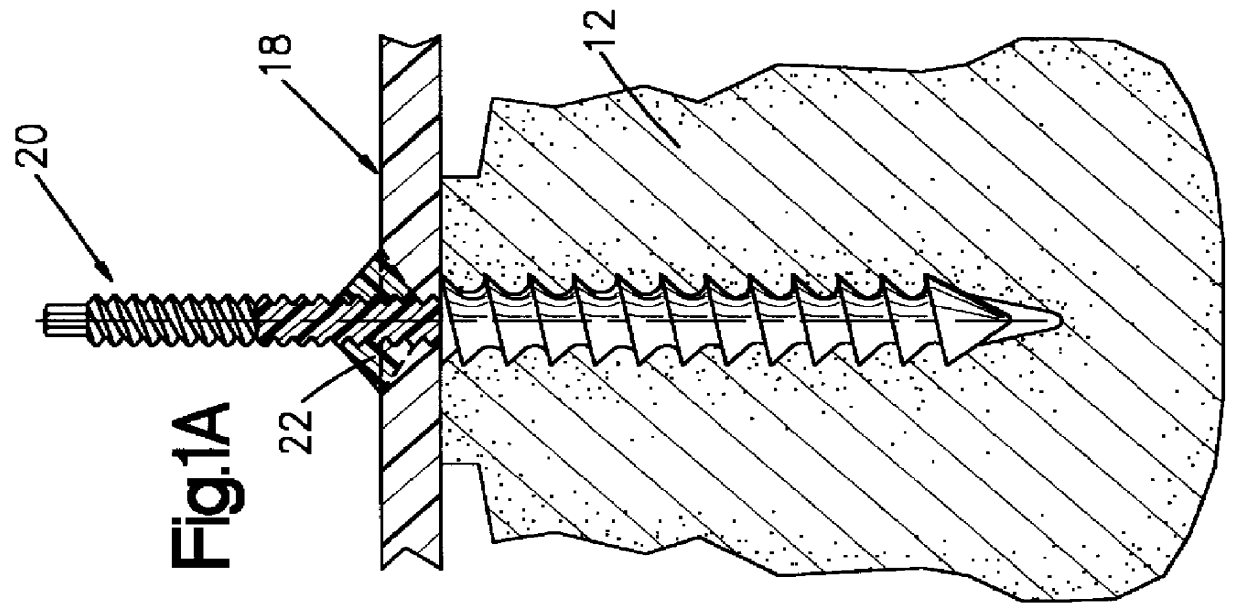
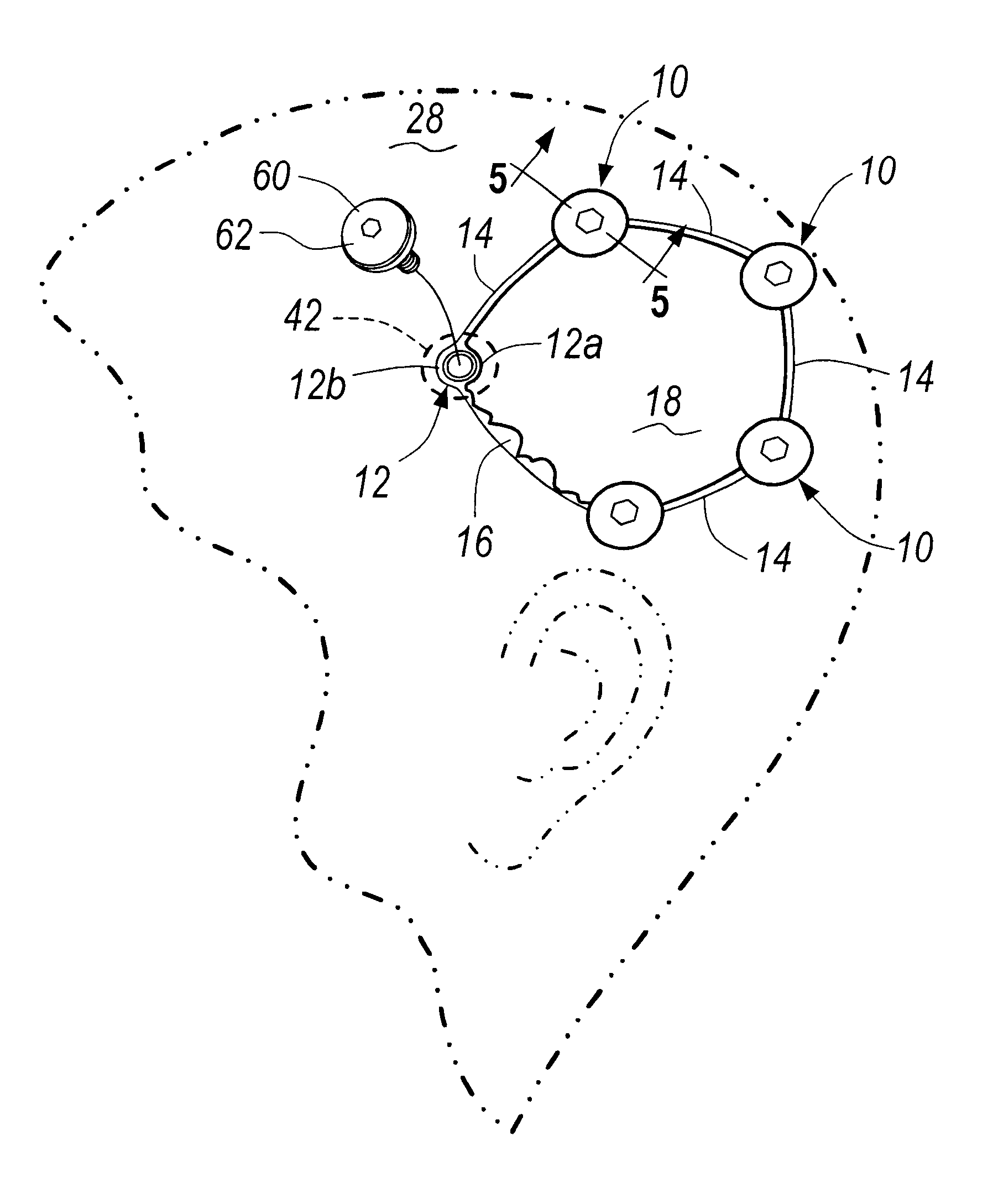
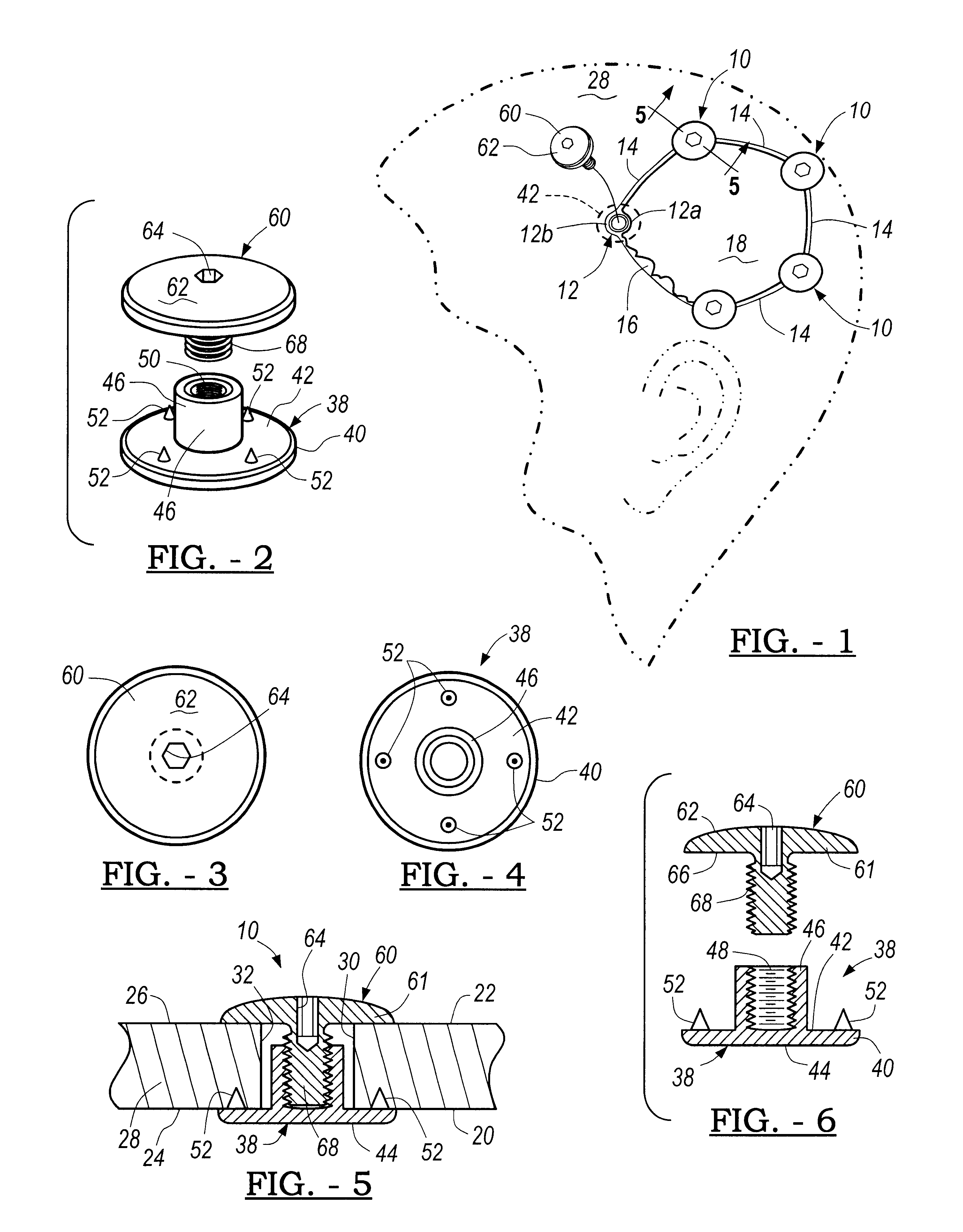
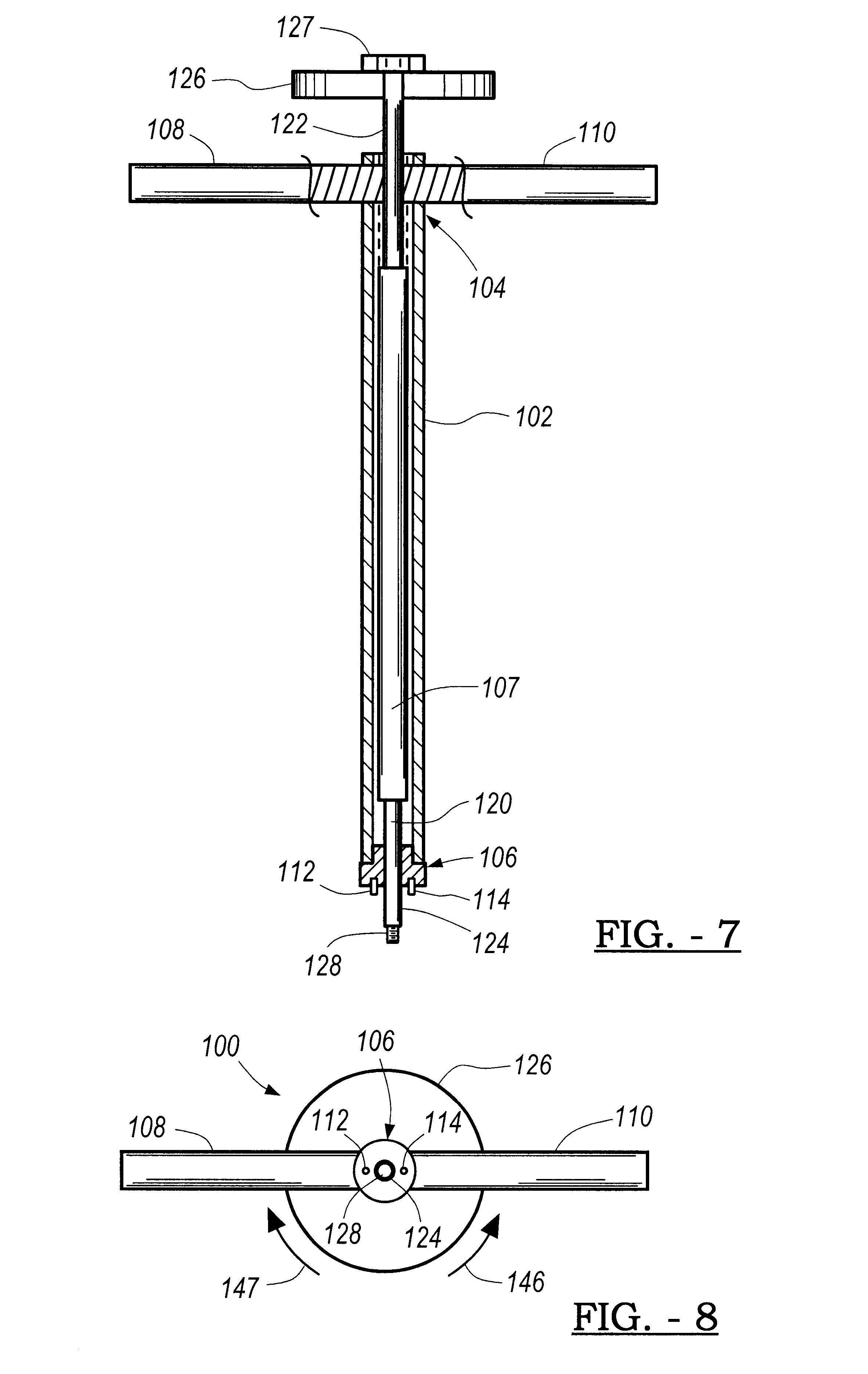
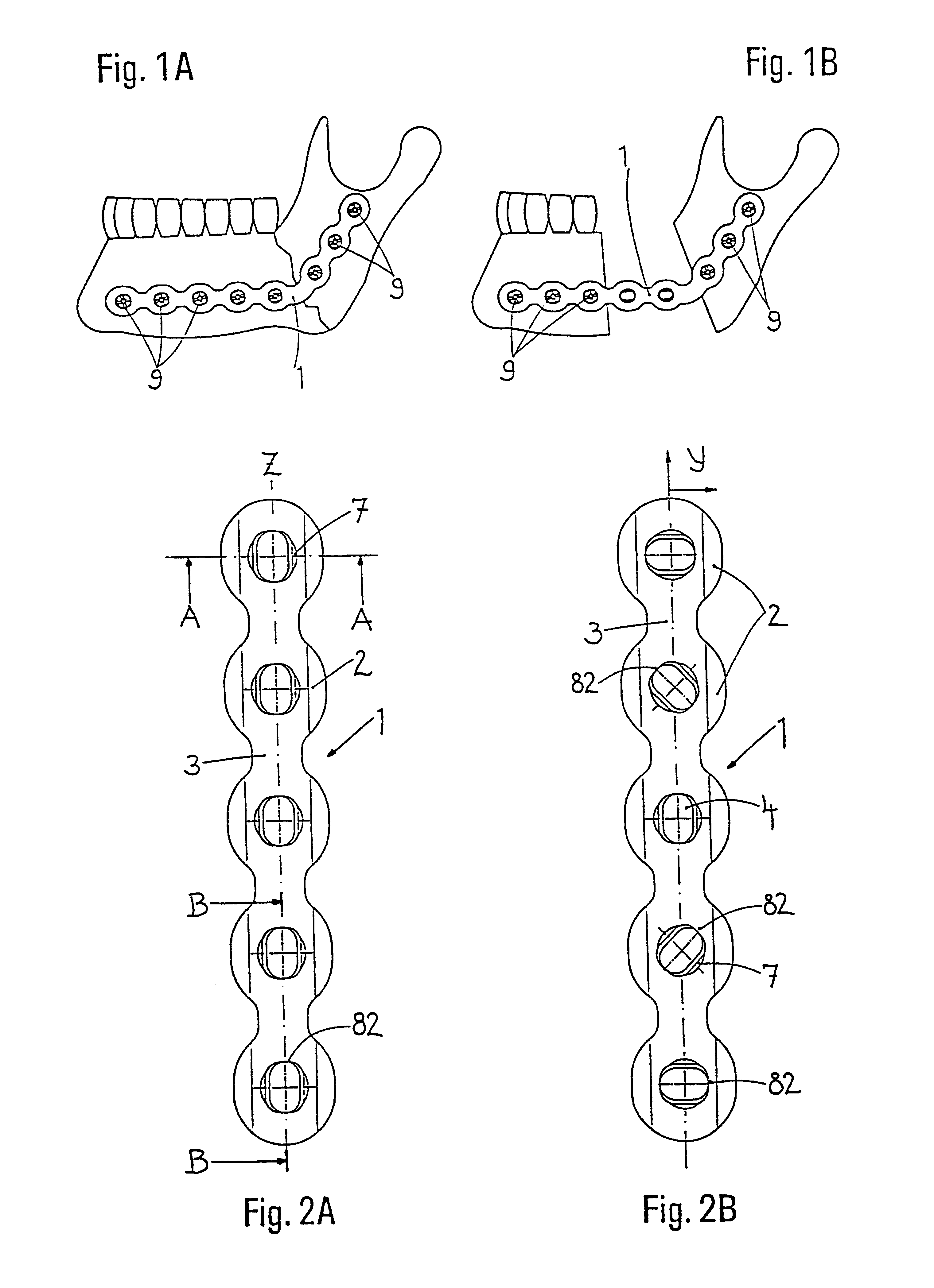Patents
Literature
1580 results about "Bone plate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Non-metallic implant devices and intra-operative methods for assembly and fixation
This invention relates to orthopedic implants and to methods of treating bone defects. More specifically, but not exclusively, the present invention is directed to non-metallic implants and to methods for intra-operative assembly and fixation of orthopedic implants to facilitate medical treatment. The non-metallic implant assembly can be secured to underlying tissue by a fastener, such as a bone screw, that is capable of swelling on contact with fluid in the underlying tissue. Alternatively, the non-metallic implant assembly can be assembled intra-operatively using a fastener that is adhesively bonded to a bone plate or the bone plate can be deformed using heat, force, or solvents to inhibit withdrawal of the fastener. In preferred embodiments, both the fastener and the bone plate are formed of biodegradable material.
Owner:WARSAW ORTHOPEDIC INC
Surgical devices assembled using heat bondable materials
Surgical devices such as implants or suture fastenings are assembled from a plurality of discrete components, one of which components includes a heat bondable plastic material for bonding the components together. At least two components are bonded to each other by the applying heat to the heat bondable plastic material of one component. The heat bondable plastic material is preferably a polymeric or composite material suitable for surgical applications and implantation in humans, and may be a biodegradable material. A laser may be used as the heat source. The present invention is advantageously embodied in heat bonded fastenings for sutures or K-wires, in which a variety of different suture anchors are usable, including expandable distal suture anchors. Other embodiments include a metal bone plate which is held to bone by a metal bone screw and a nut of bondable material bonded to the plate to secure the connection; a piece of bondable material bonded to a metal prosthesis to custom fit the prosthesis; and a surgical implant custom formed by bonding together a plurality of discrete elements one or more of which is bondable.
Owner:P TECH +1
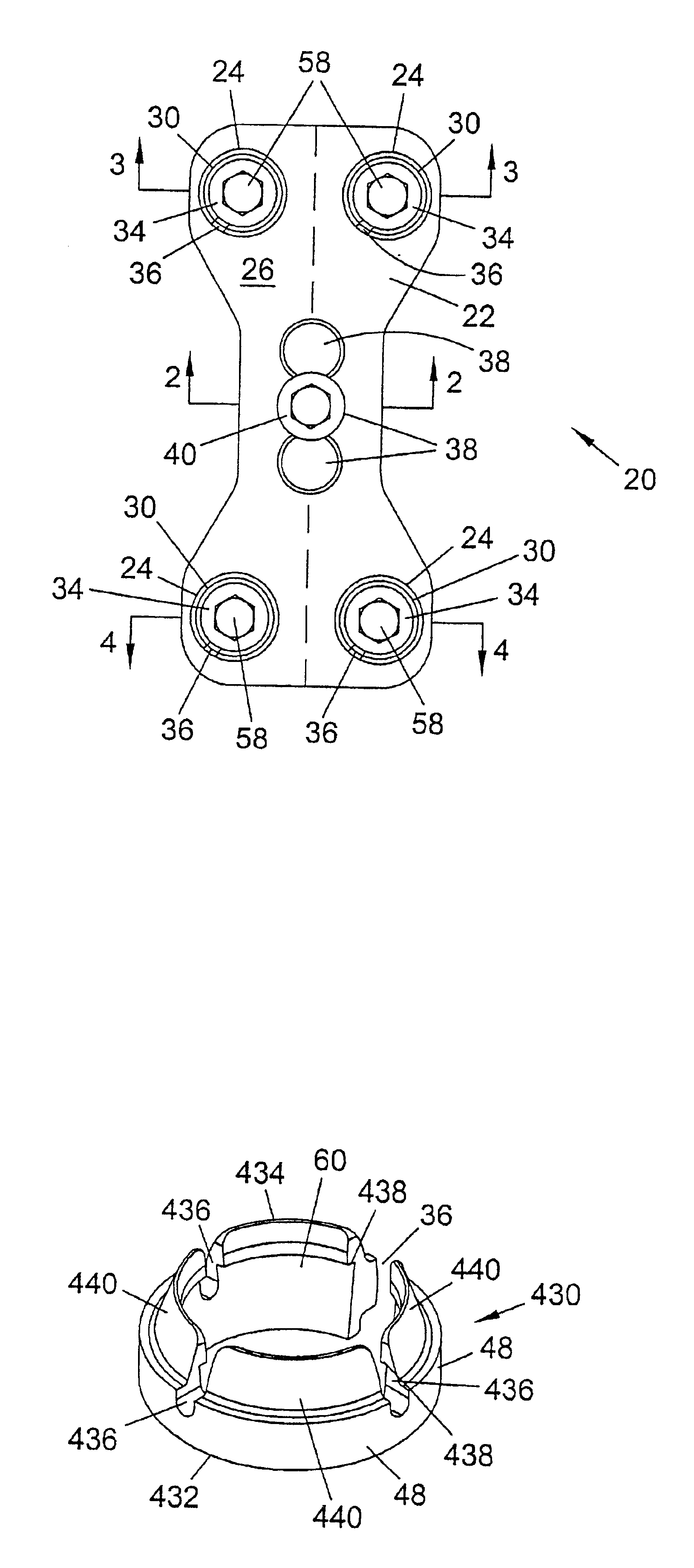
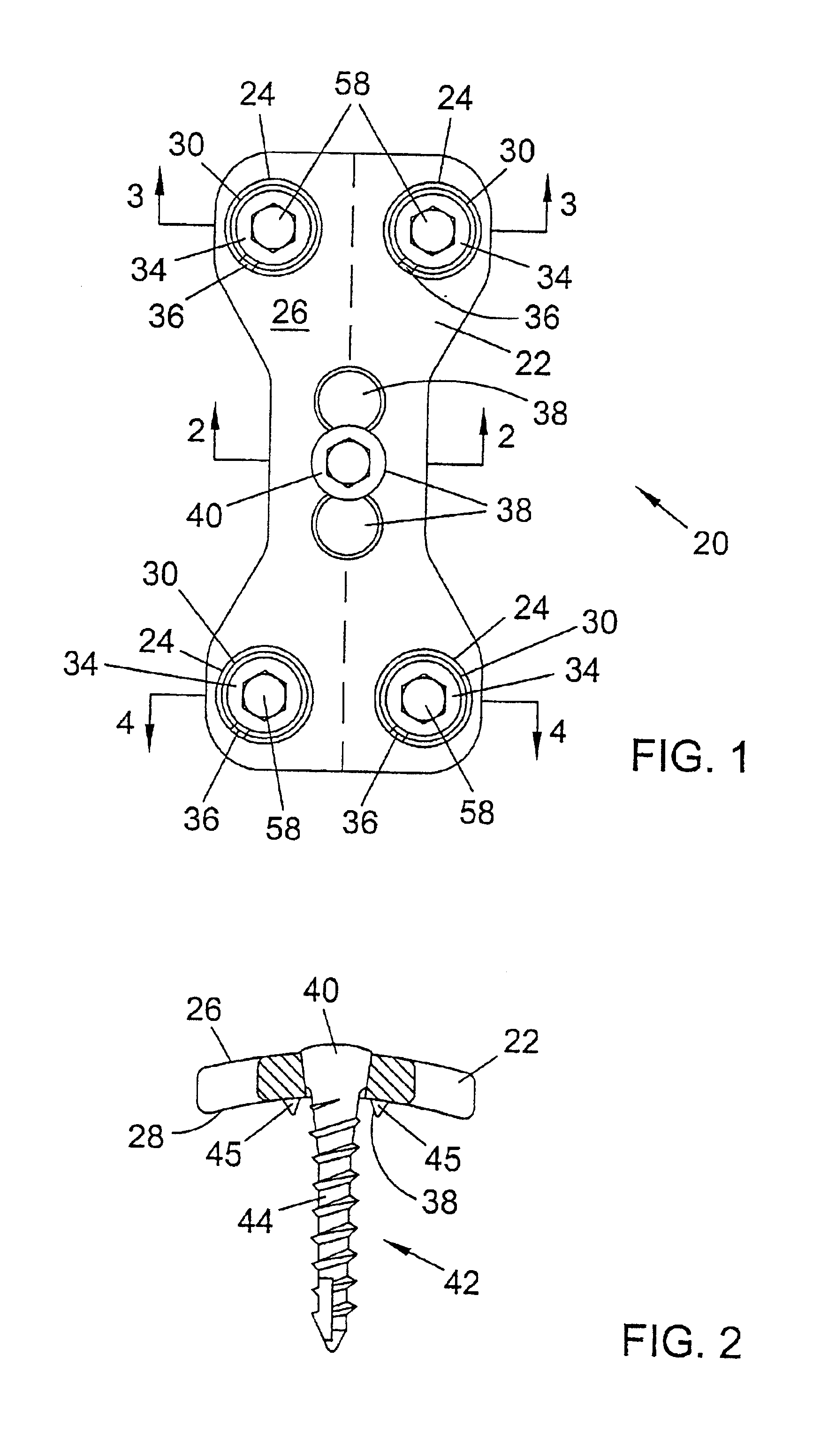
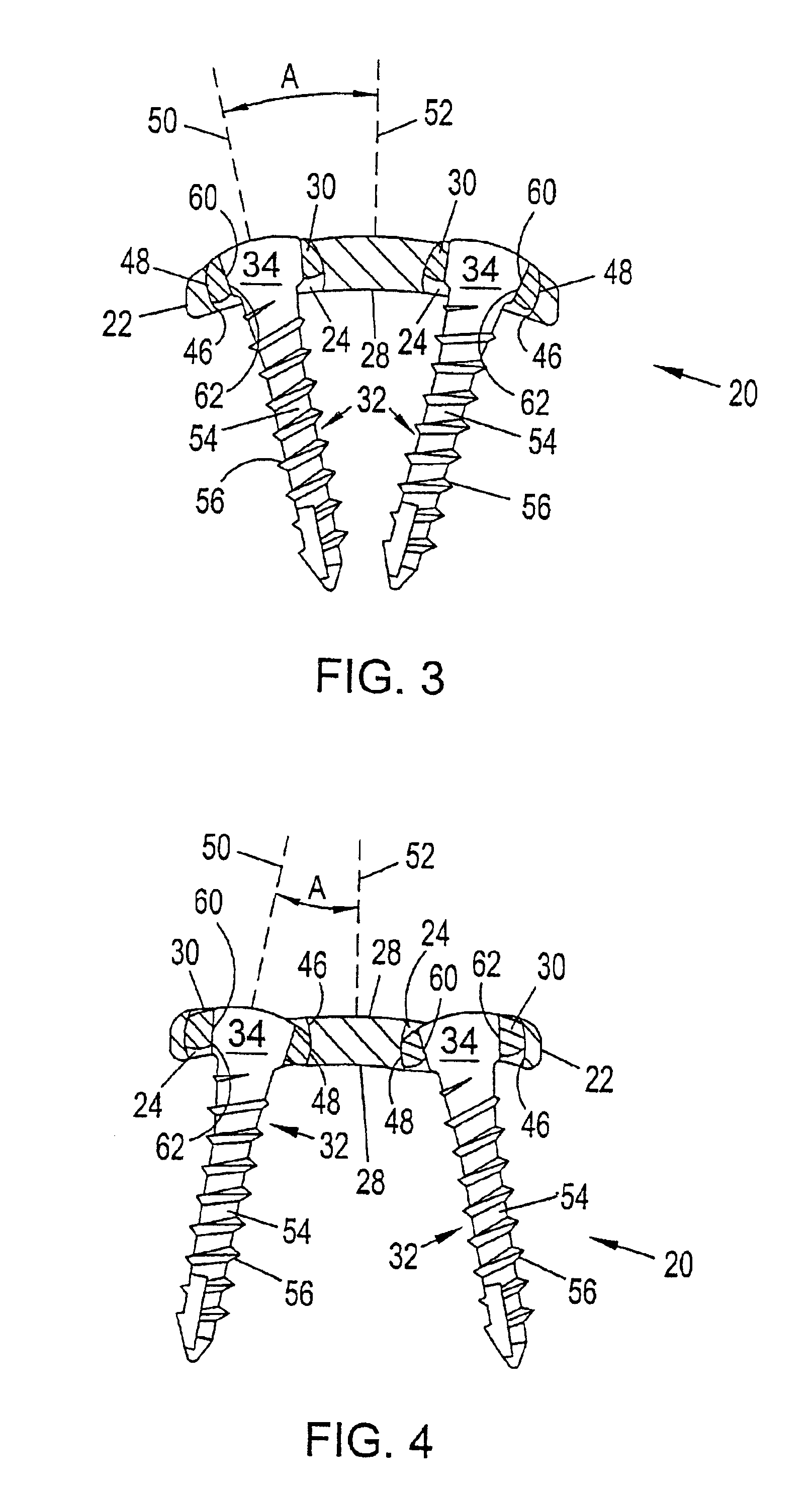
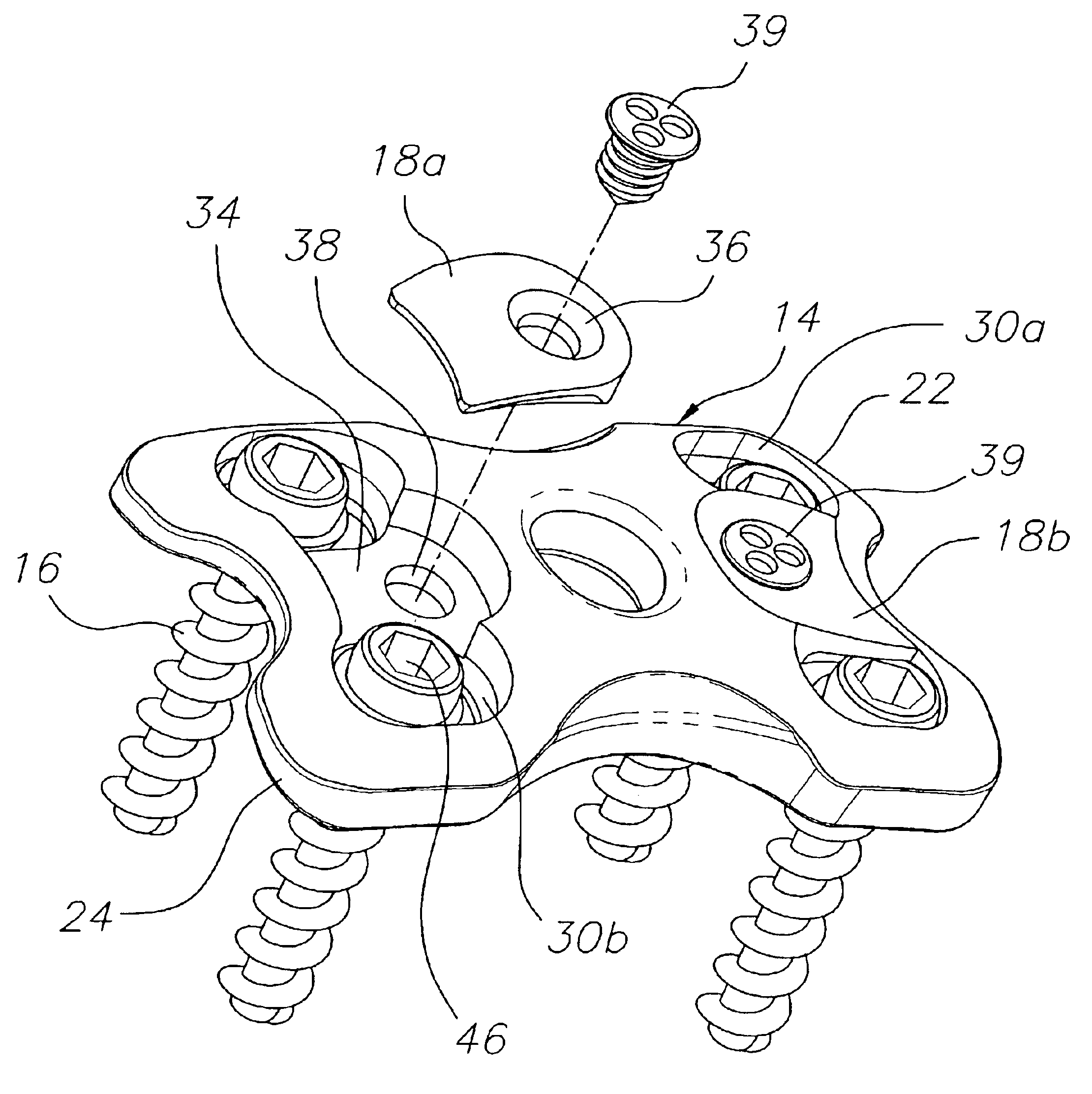
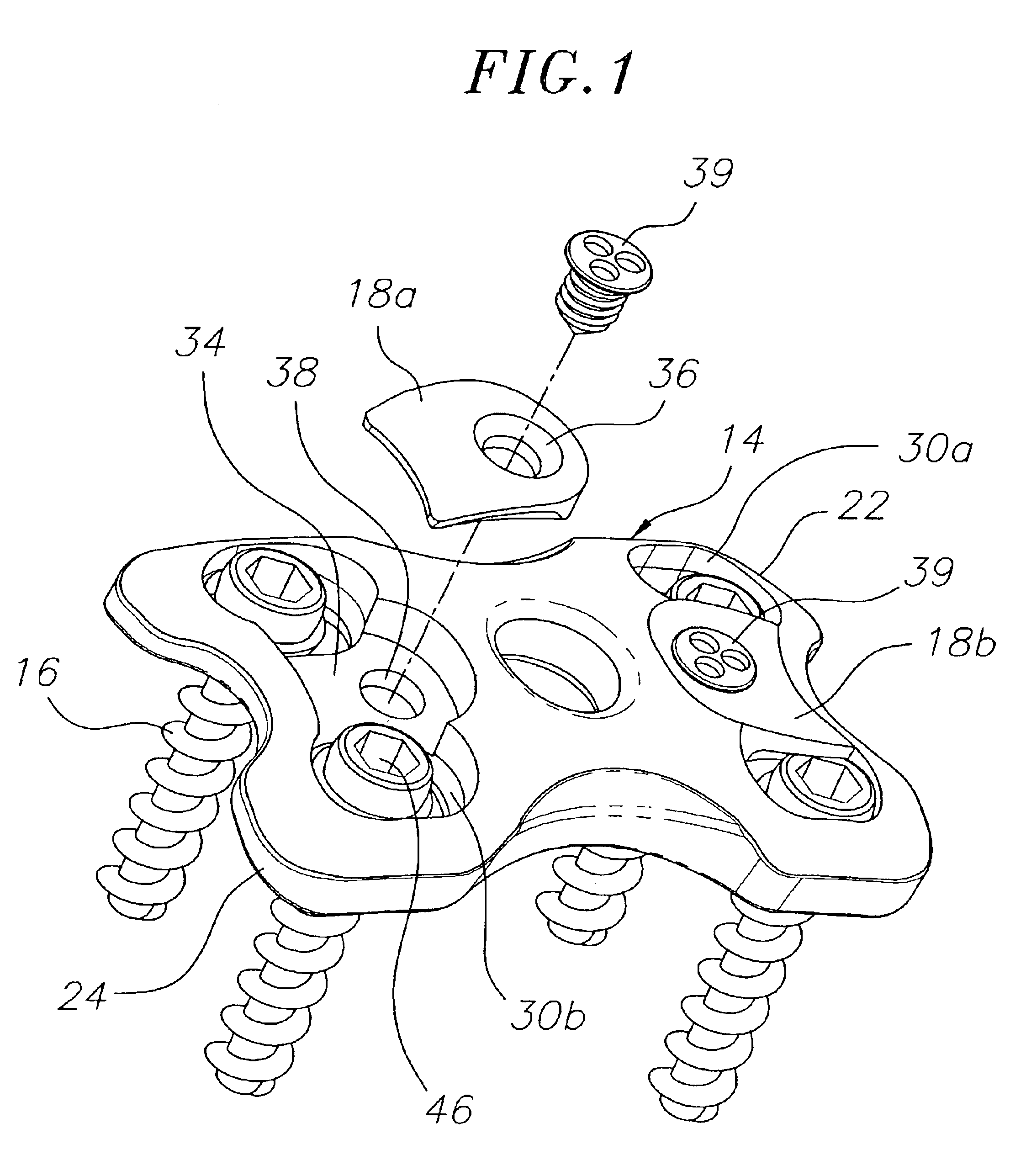
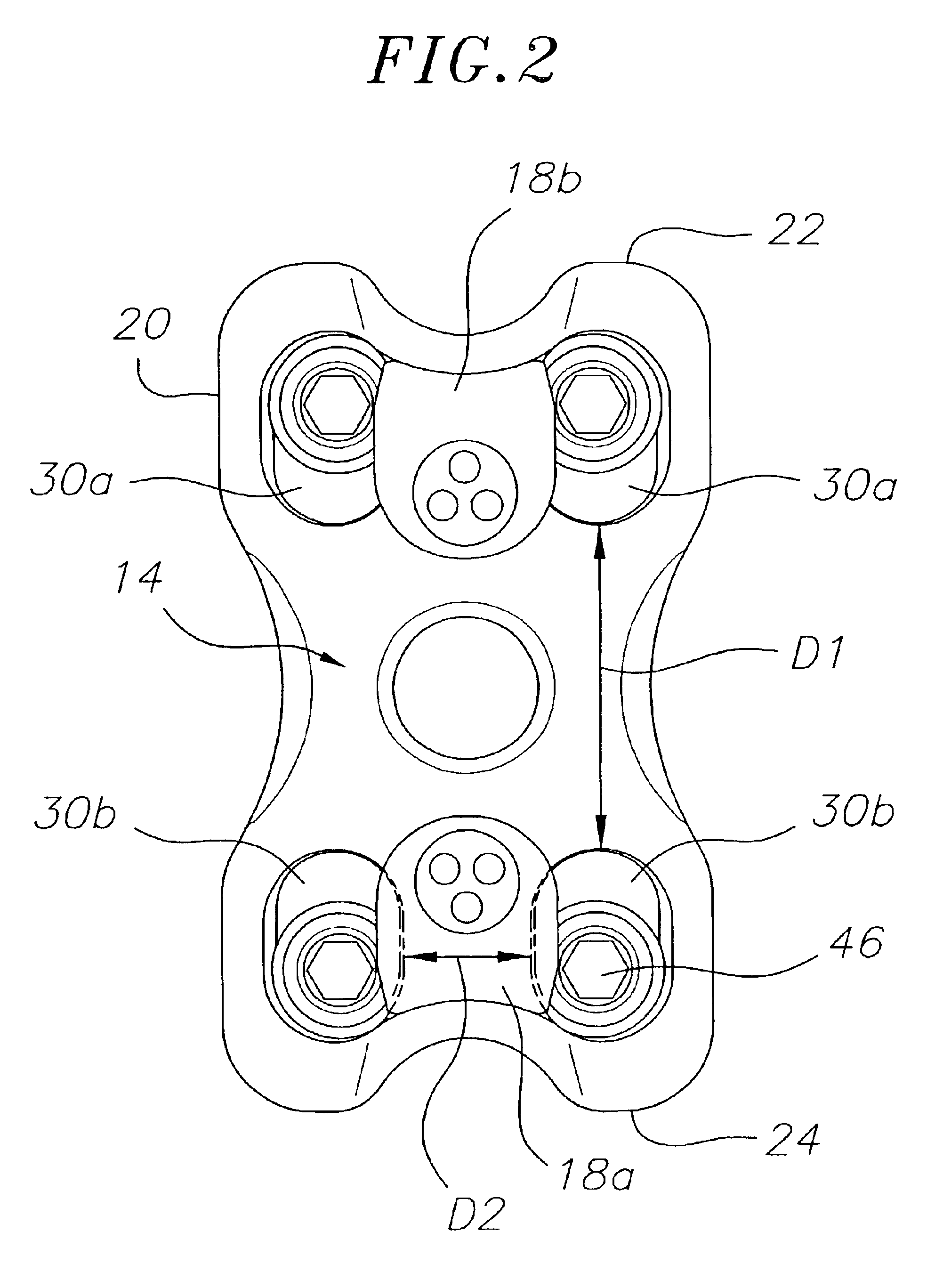
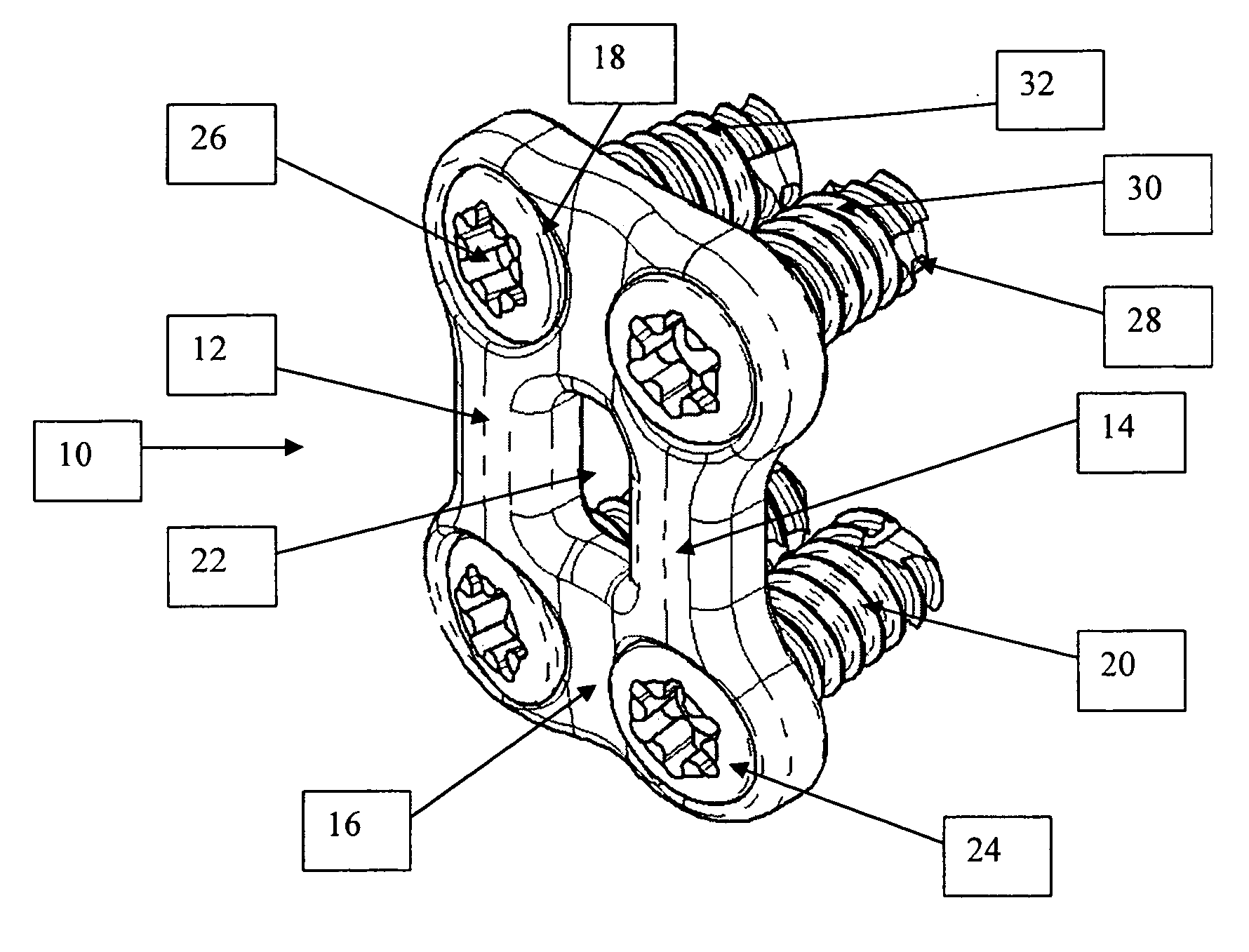
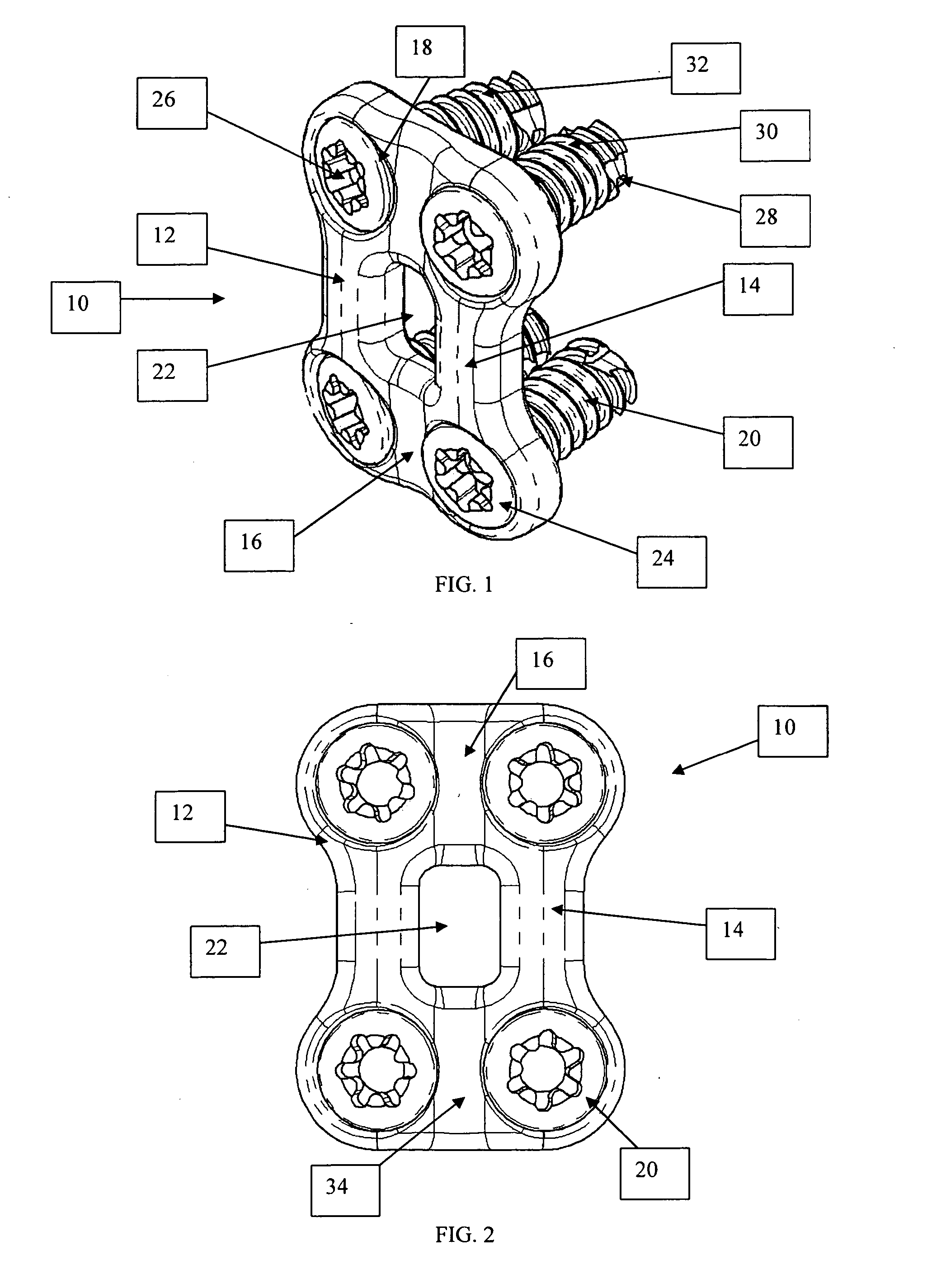
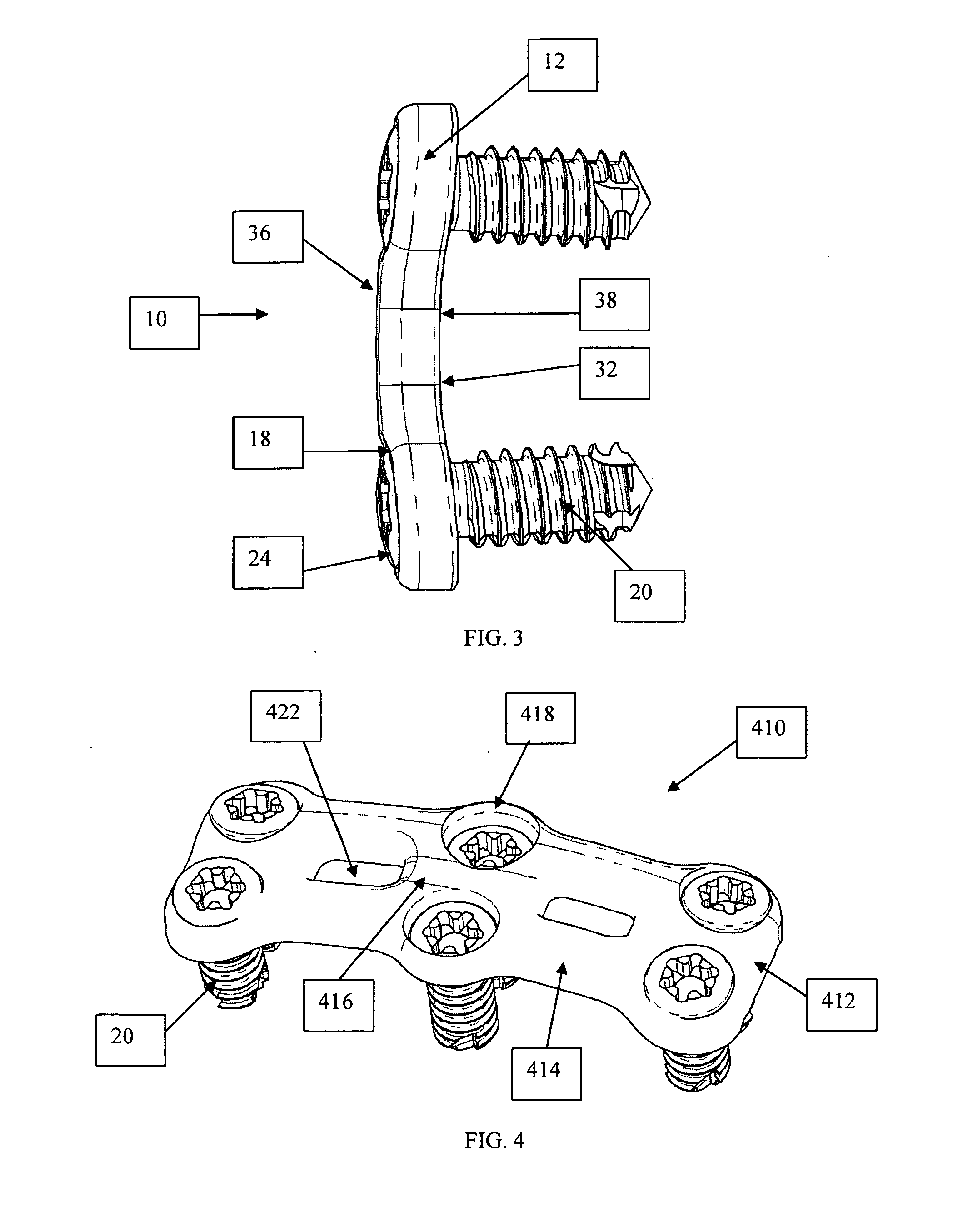
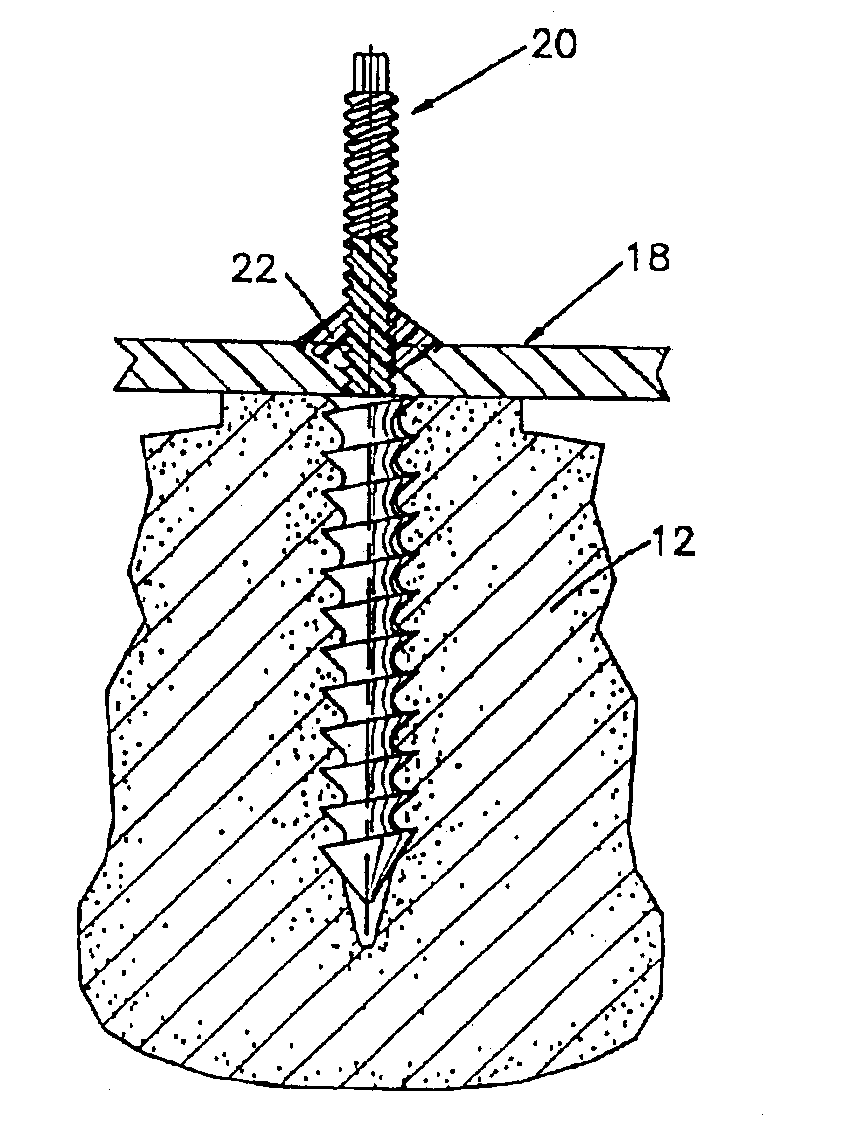
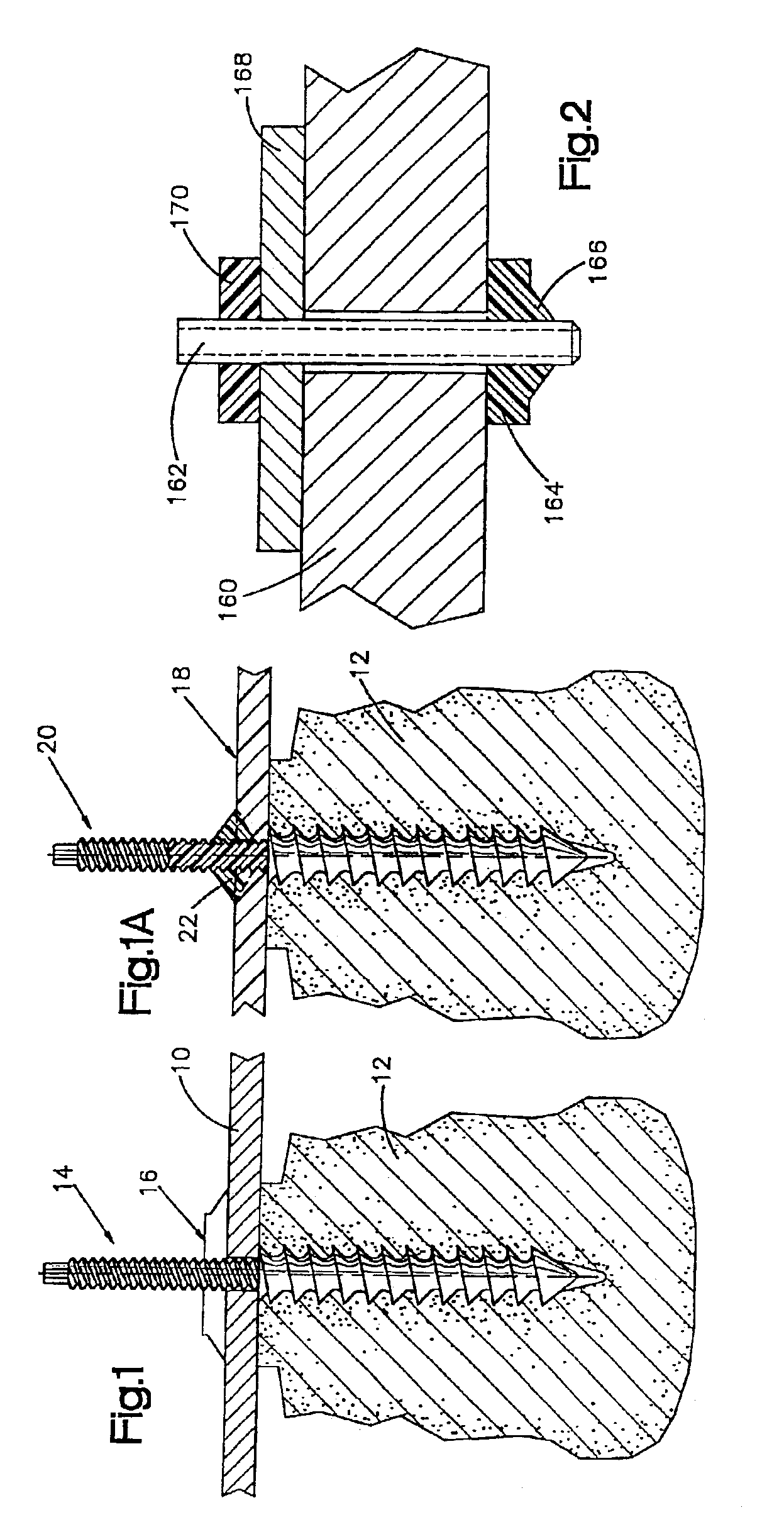
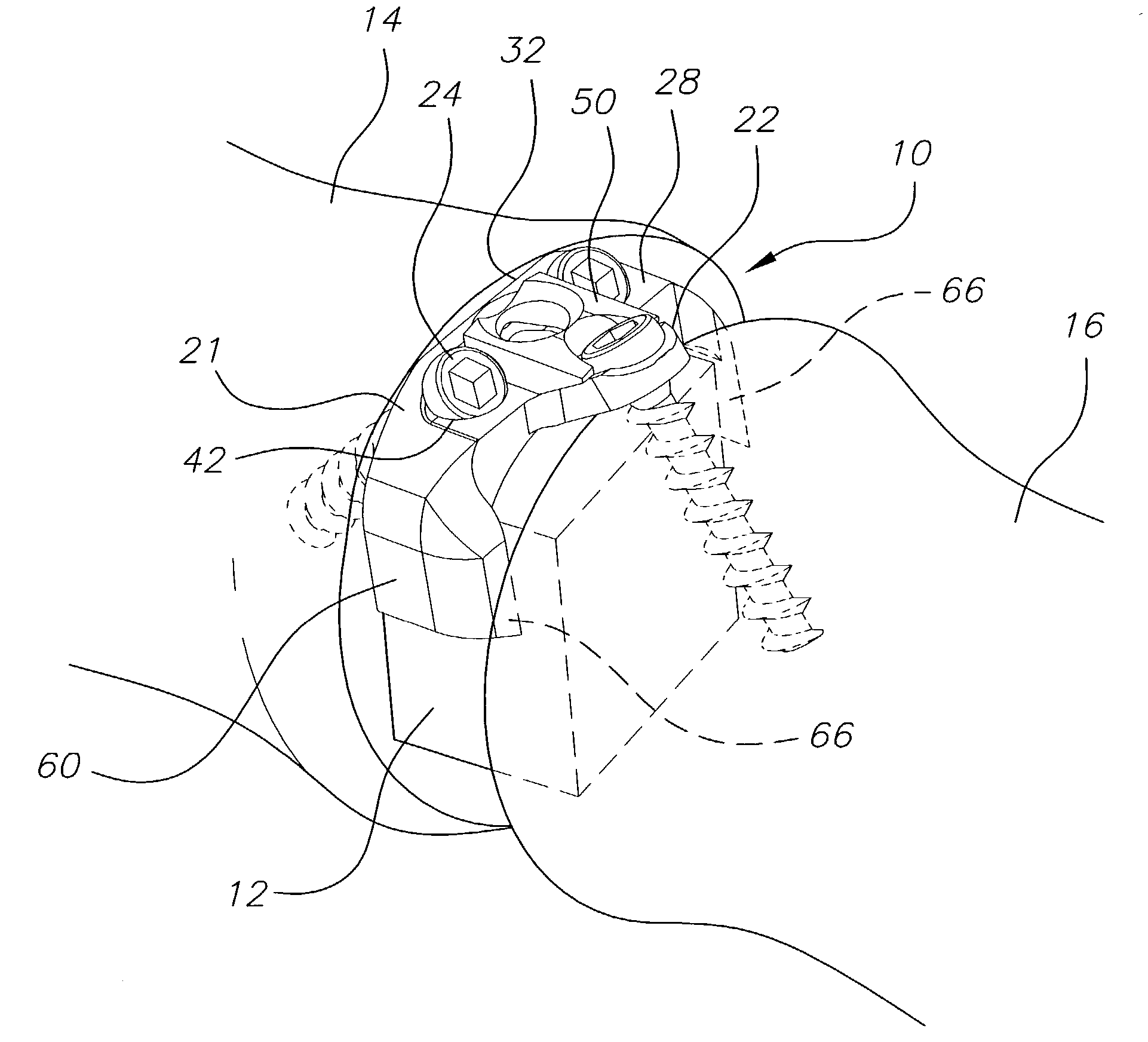
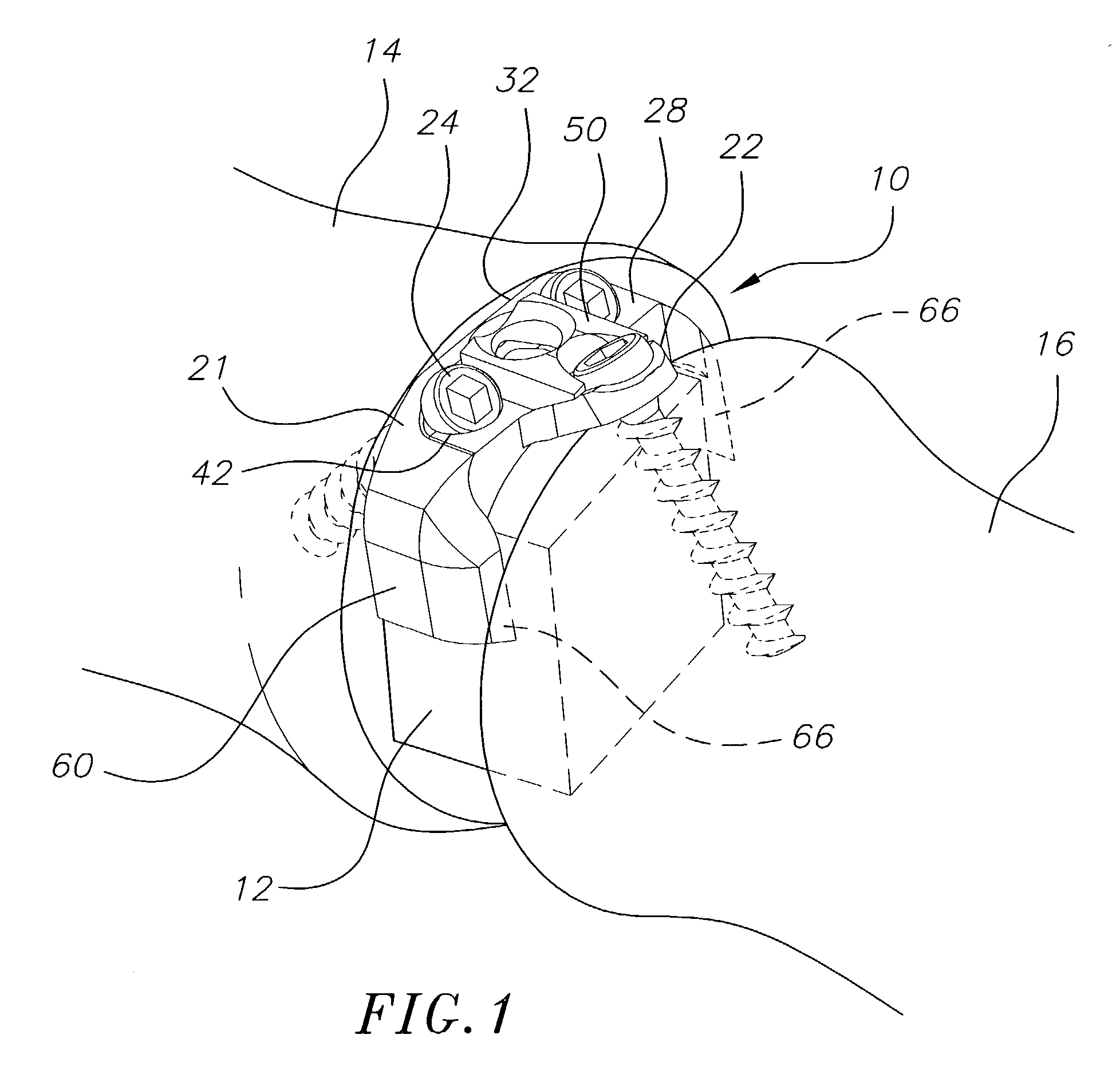
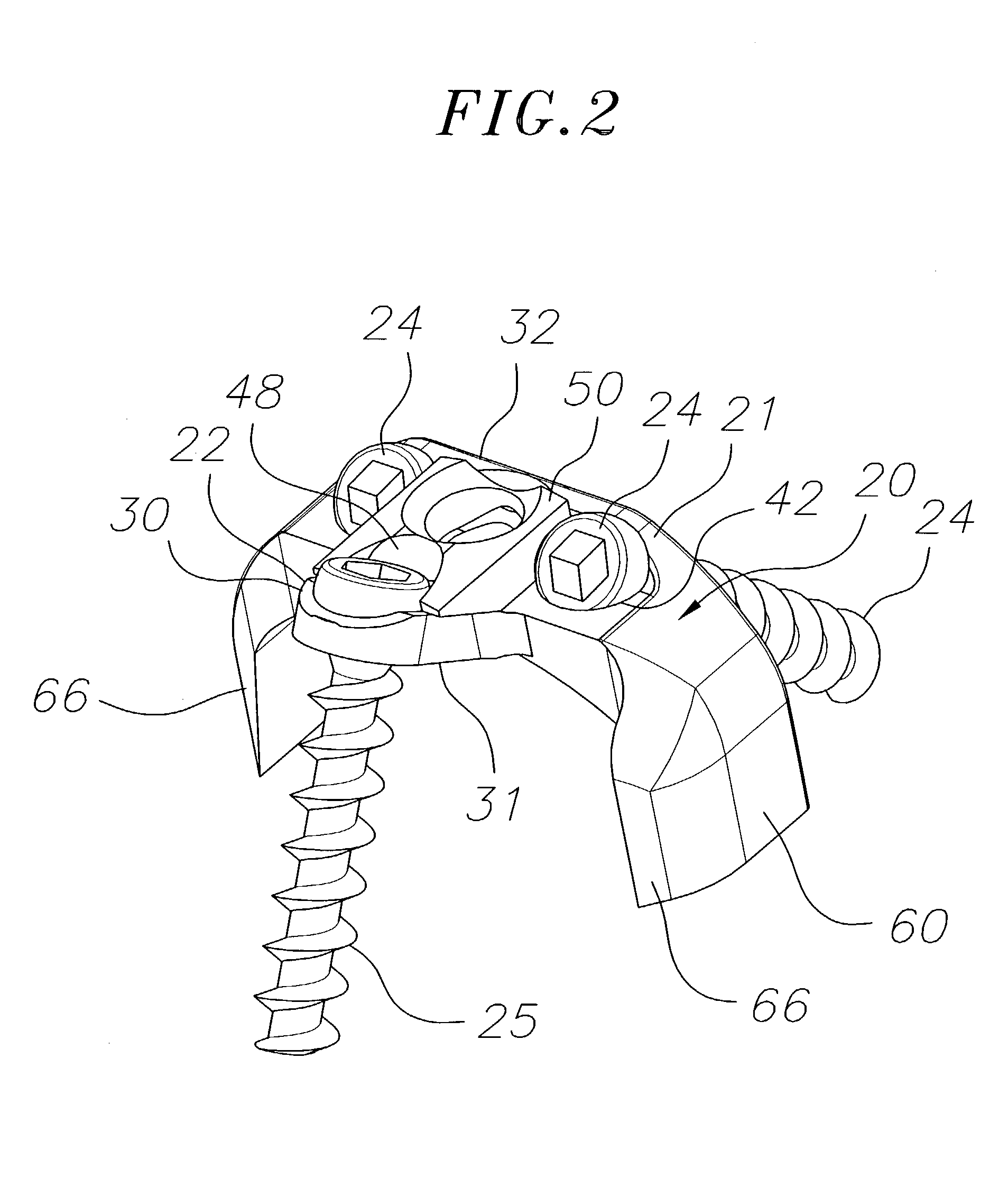
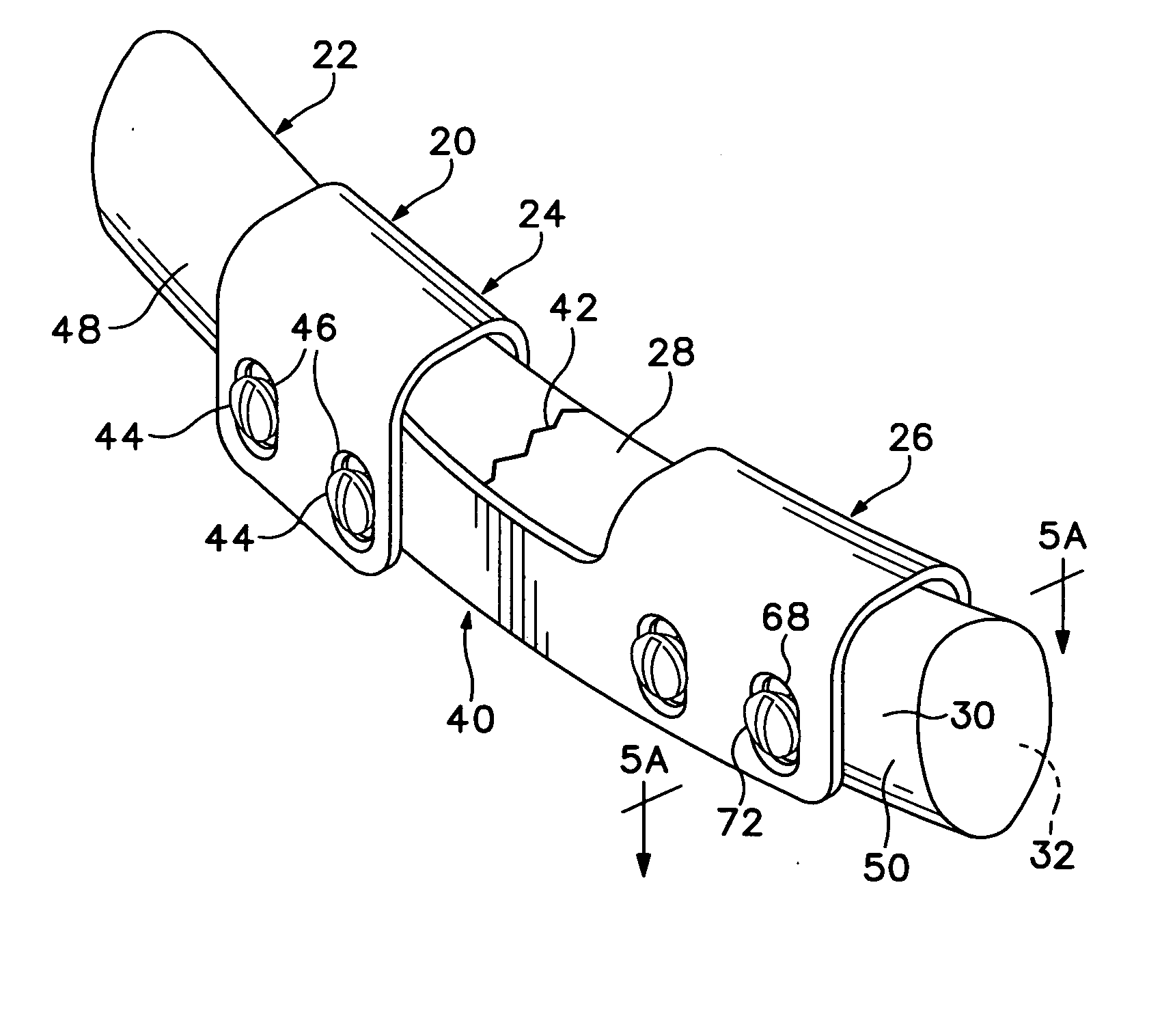
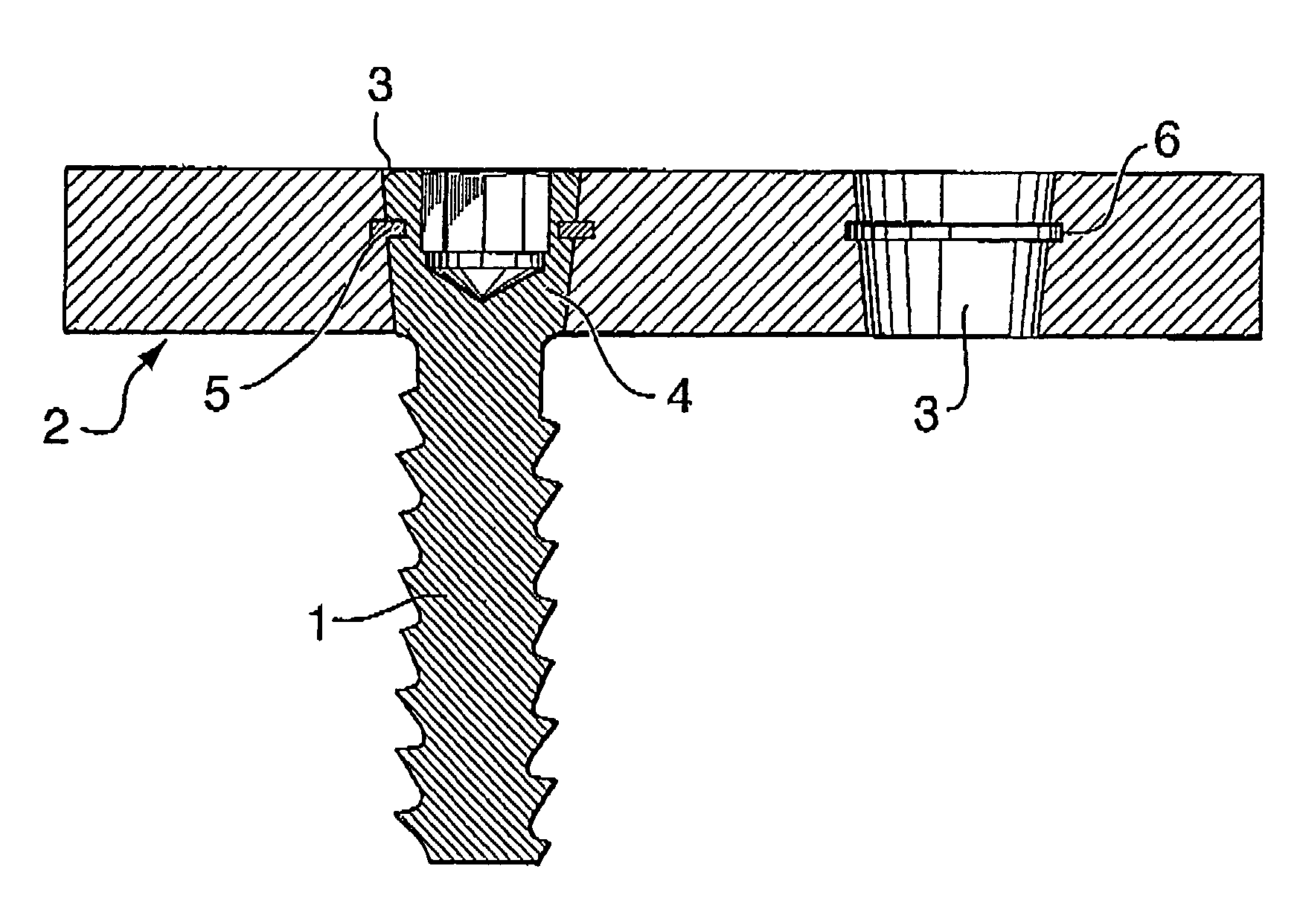
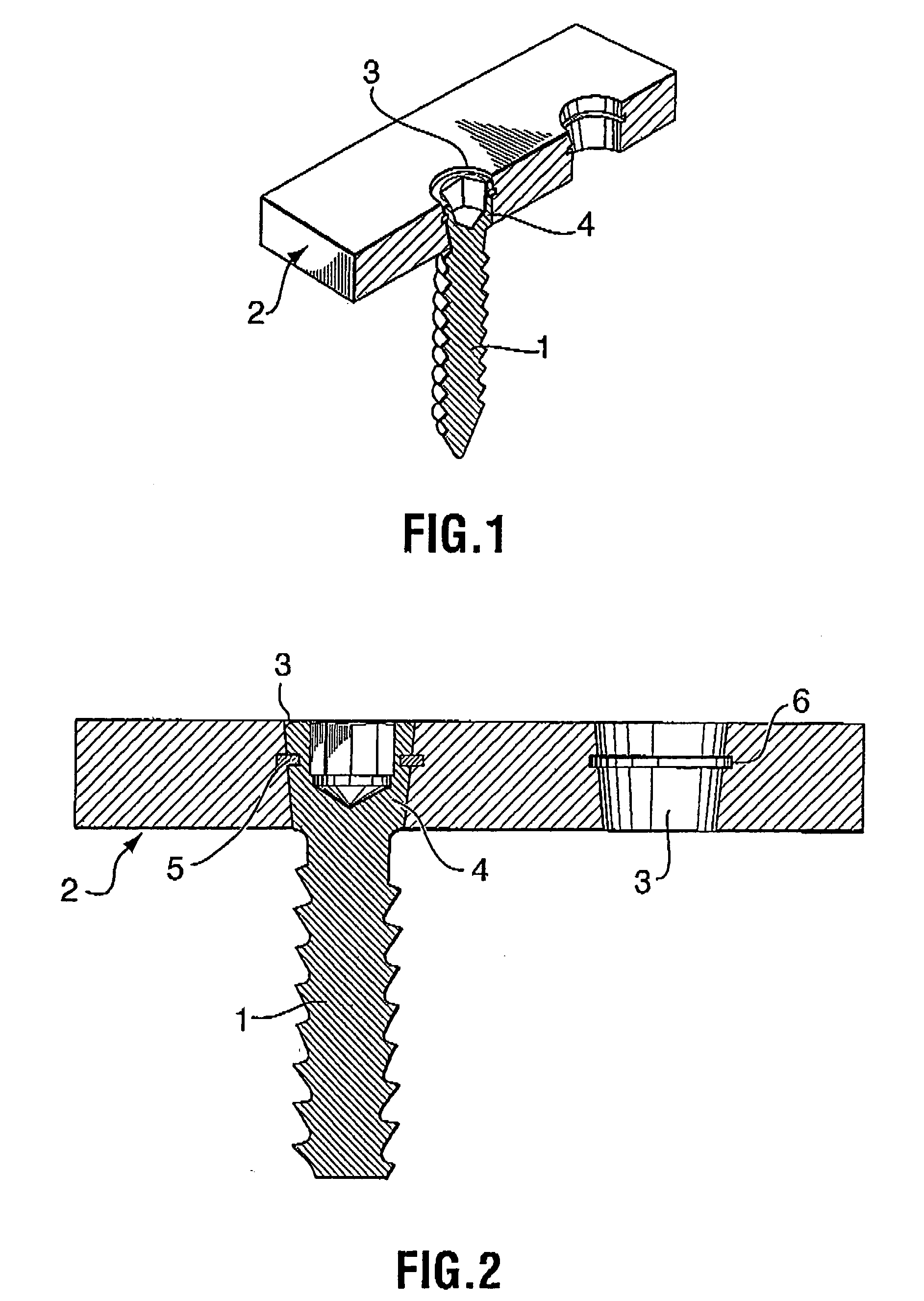
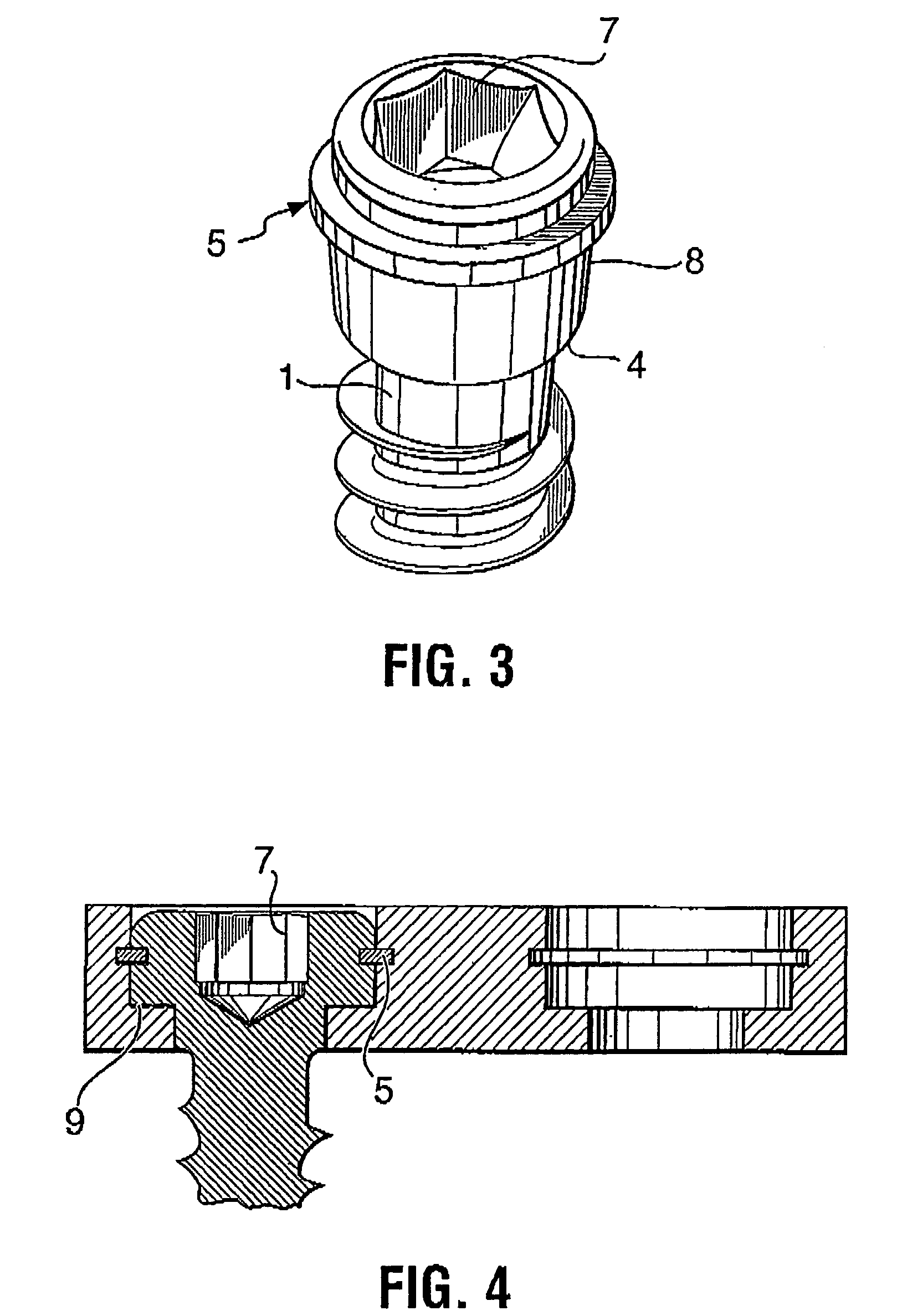
Bone plate with interference fit screw
InactiveUS20050131413A1Relieve painMinimally invasiveSuture equipmentsLigamentsInterference fitScrew system
Systems, including apparatus and methods, for internal fixation of a fractured or otherwise compromised bone. These systems may include and / or make use of bone plates, locking screws, and / or kits, among others.
Owner:ODRISCOLL SHAWN W +1
Implants formed of shape memory polymeric material for spinal fixation
ActiveUS20050033295A1Absorb energySuture equipmentsInternal osteosythesisJoint arthrodesisImplanted device
This invention relates to a orthopedic implant that comprises a shape memory polymeric material. The orthopedic implant can be fabricated or molded in a desired configuration selected to provide support or tension to bony structures. Examples of implantable devices include spinal rods, bone plates, and bone fixation cords. The orthopedic implant can be deformed to a second configuration different from the first configuration either prior to implantation or after implantation. When desired, the shape memory polymeric material can be induced to revert to it original molded configuration. This can compress the attached bony structure and / or promote arthrodesis.
Owner:WARSAW ORTHOPEDIC INC
Variable angle locked bone fixation system
Owner:SYNTHES USA
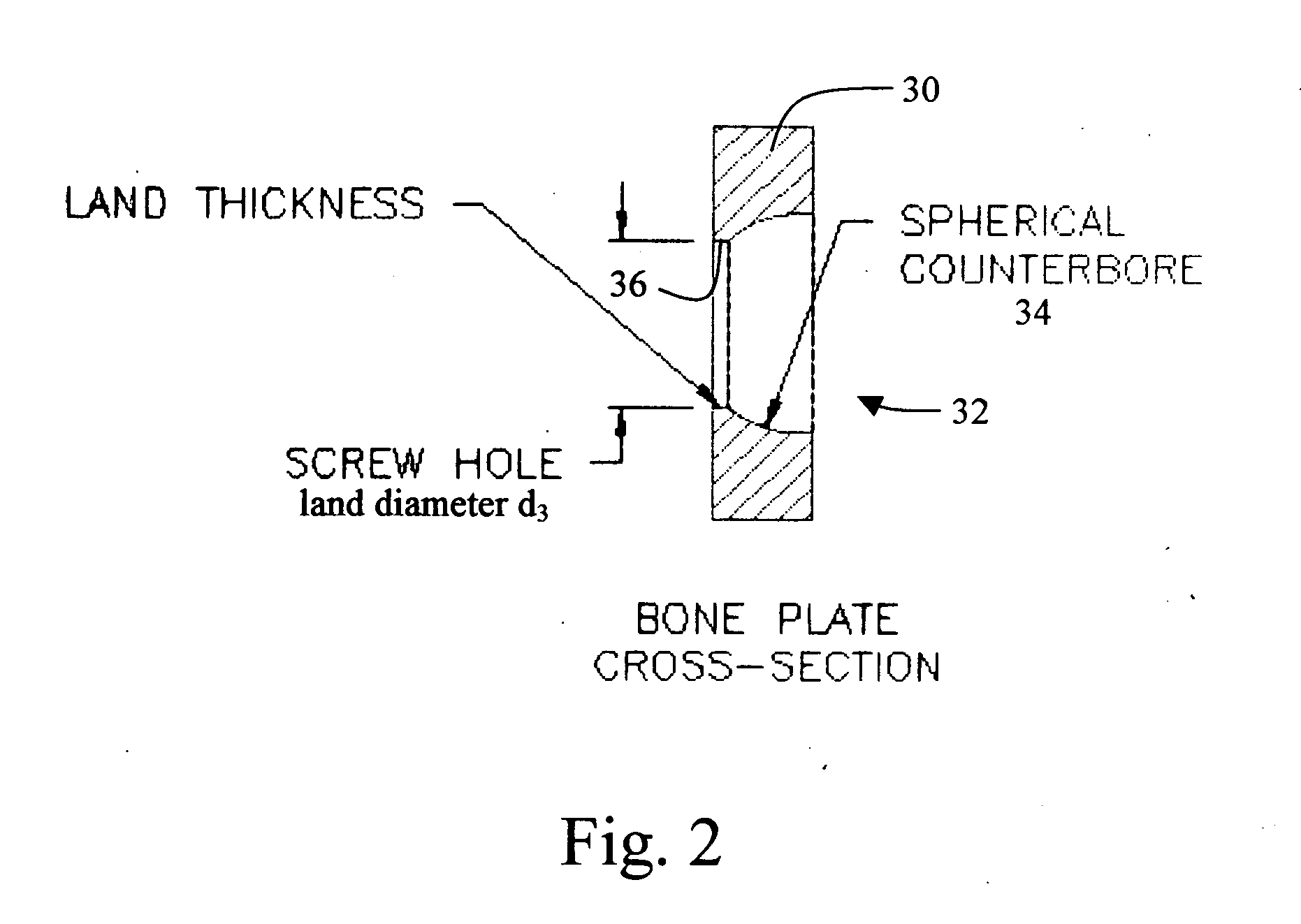
Blockable bone plate
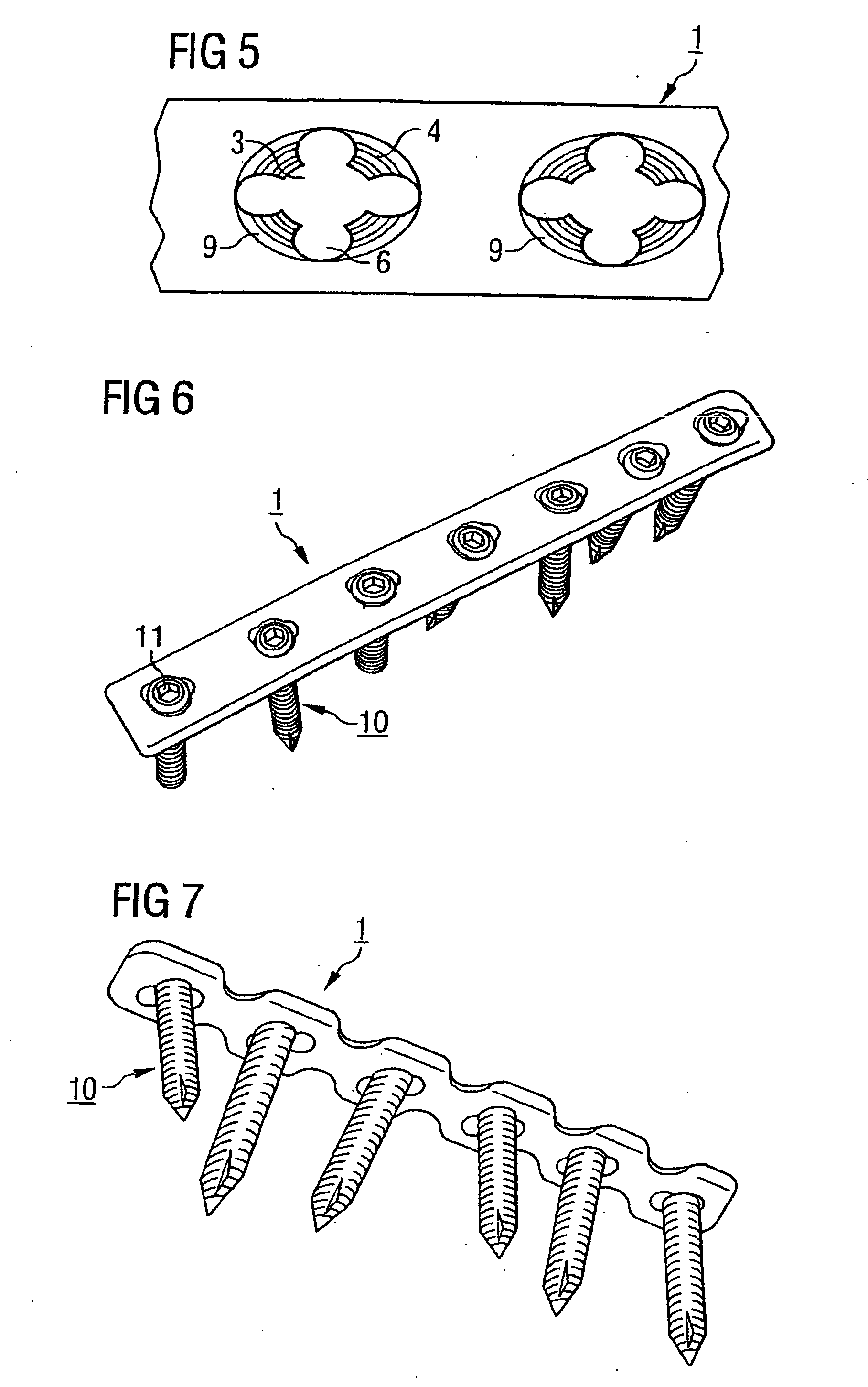
InactiveUS6730091B1Cost effective productionLow costSuture equipmentsInternal osteosythesisEngineeringIliac screw
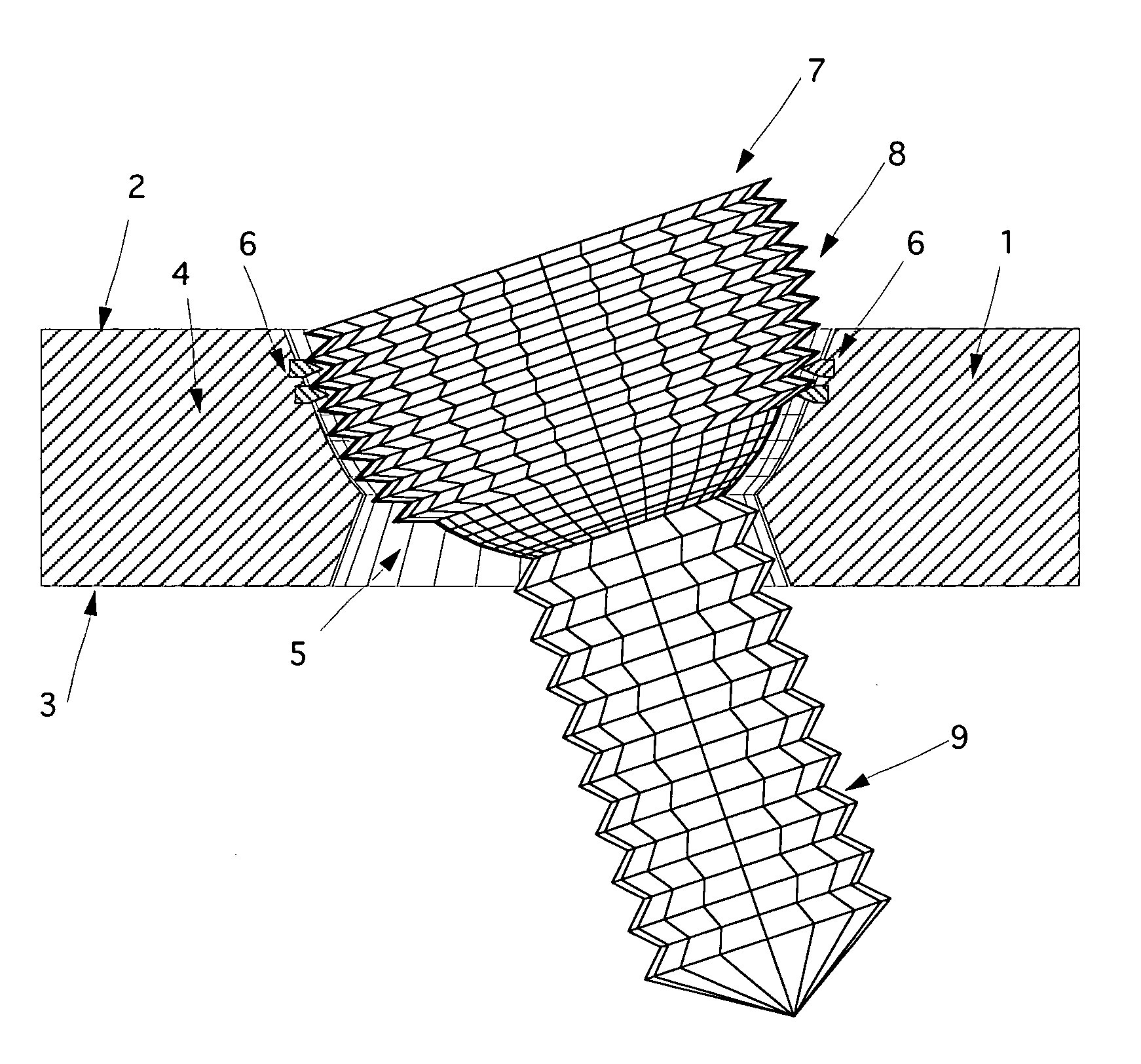
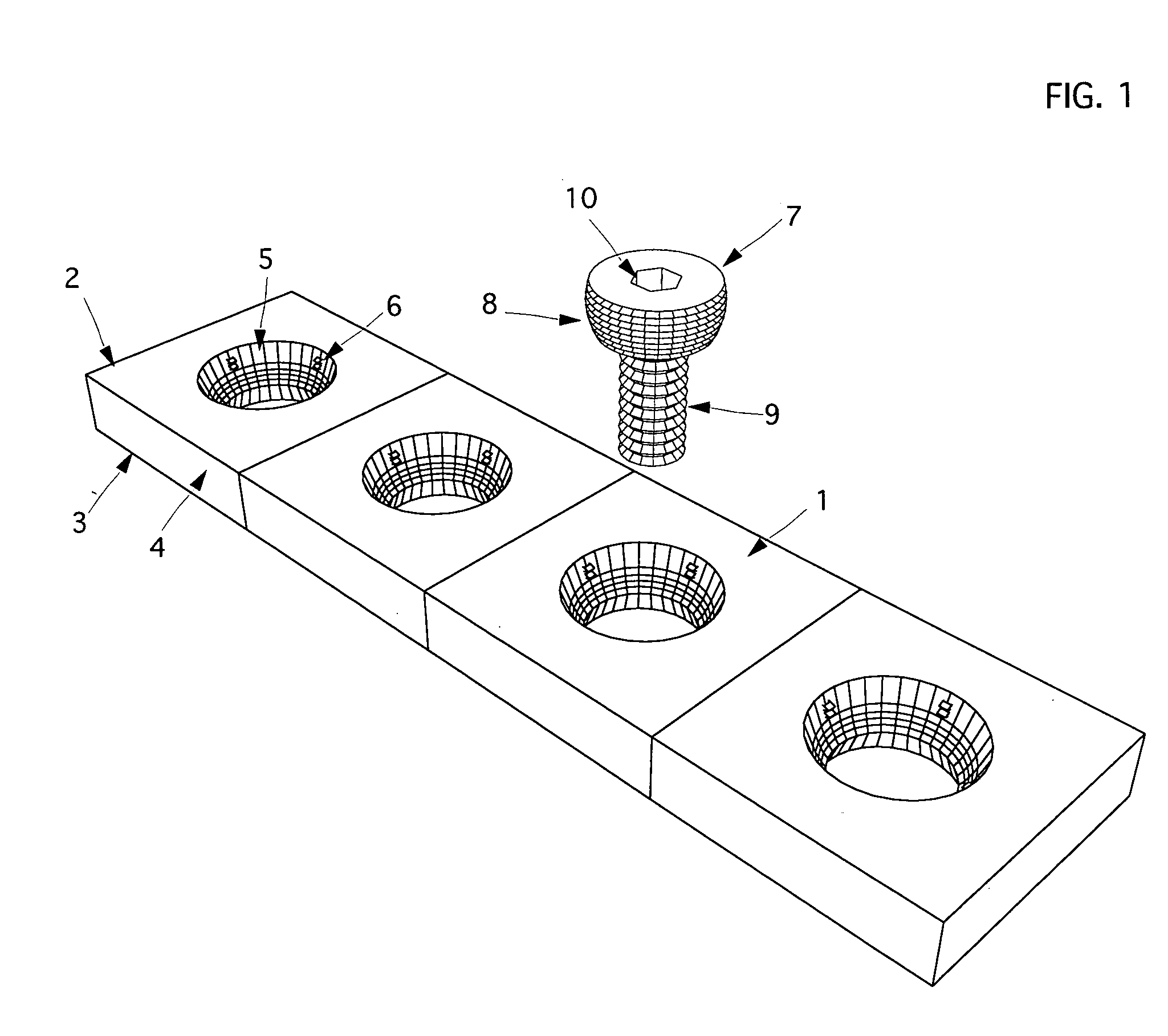
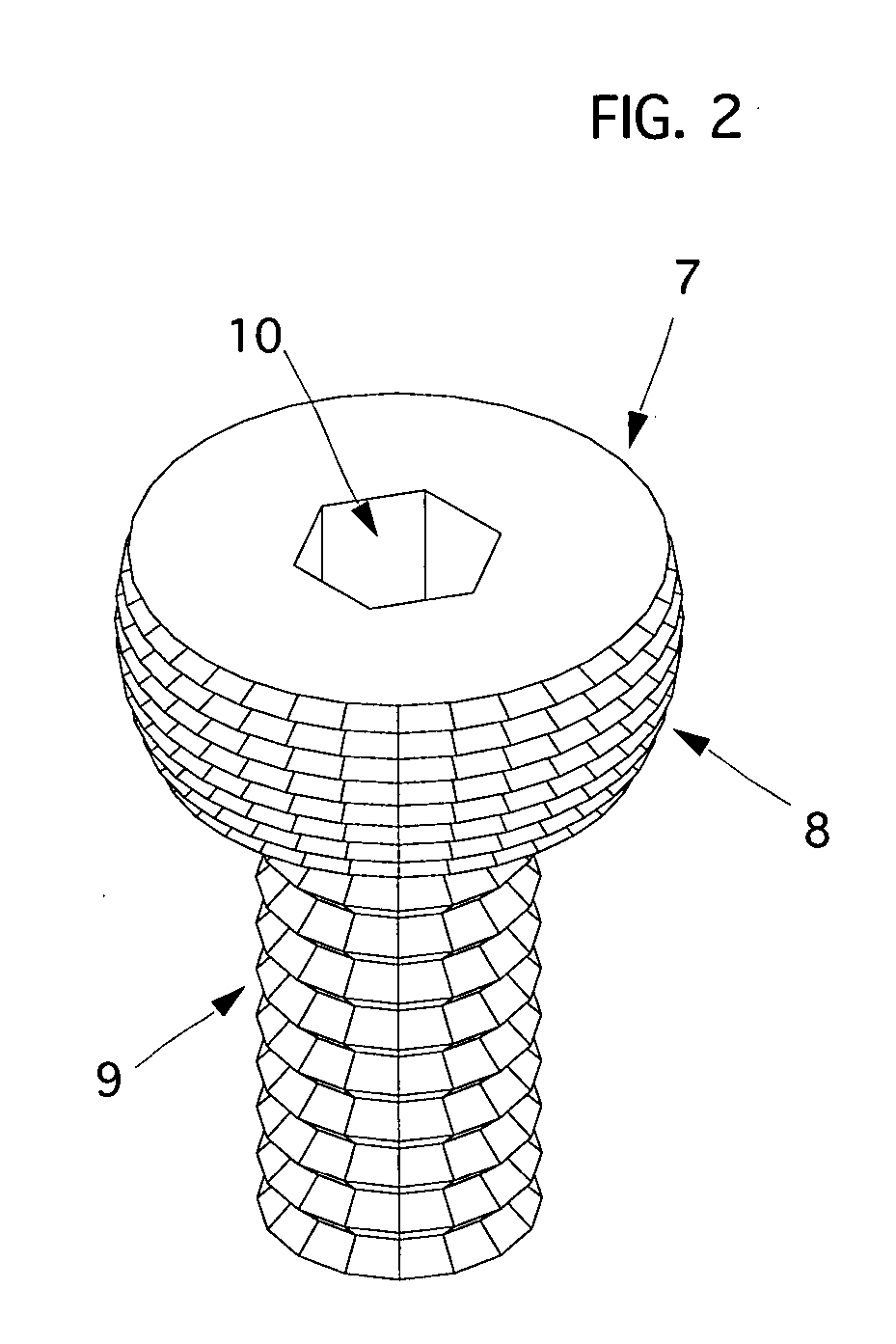
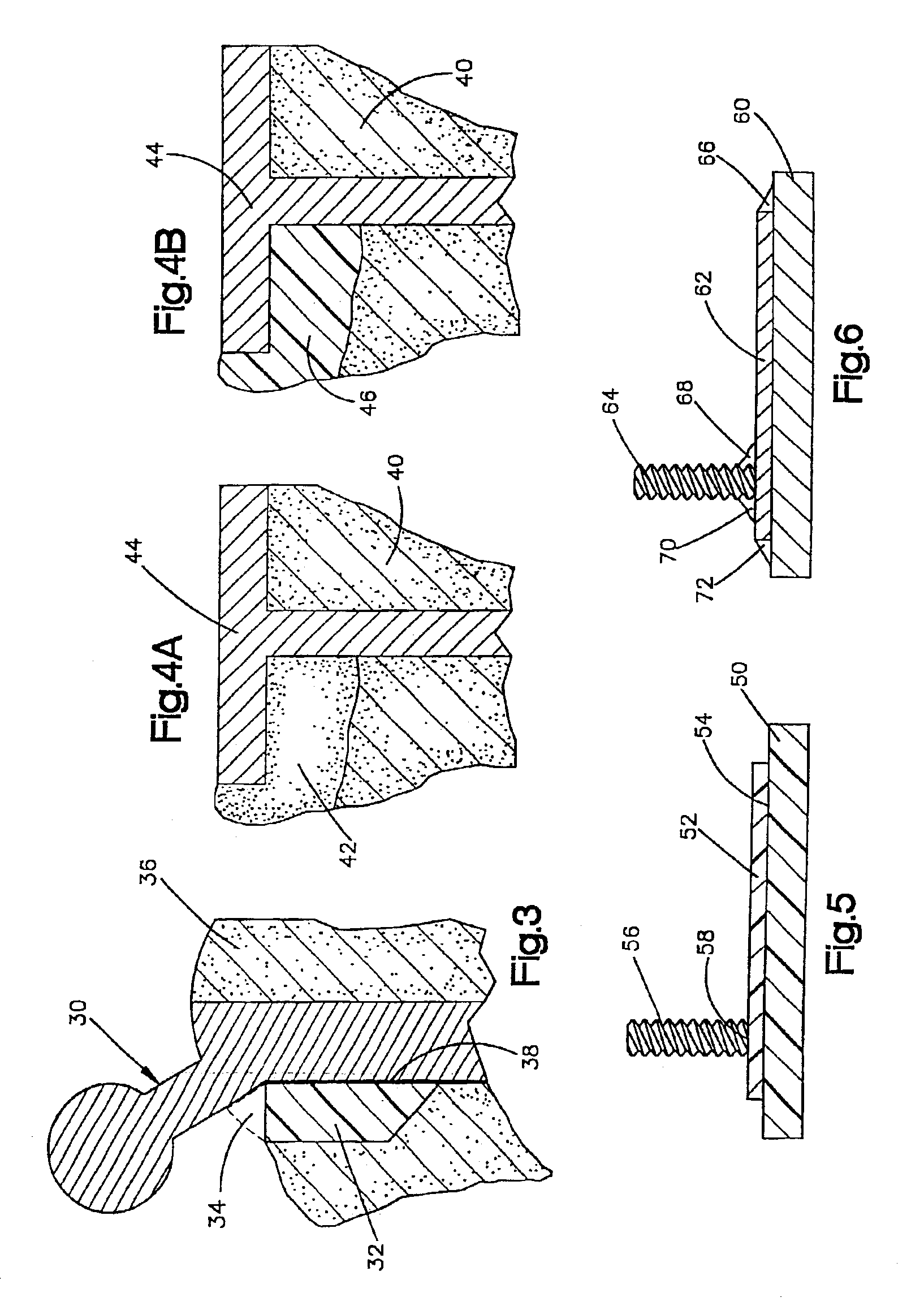
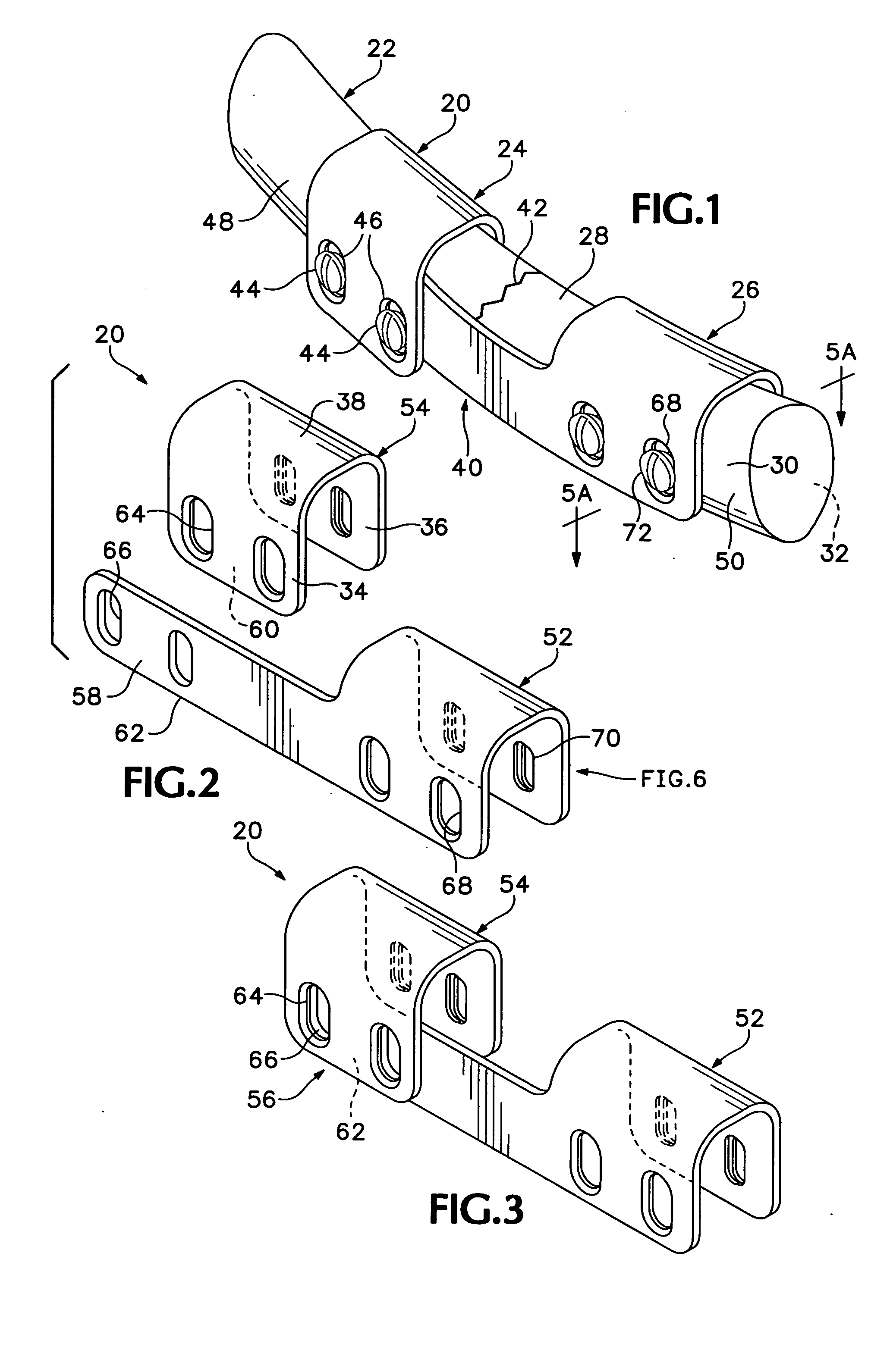
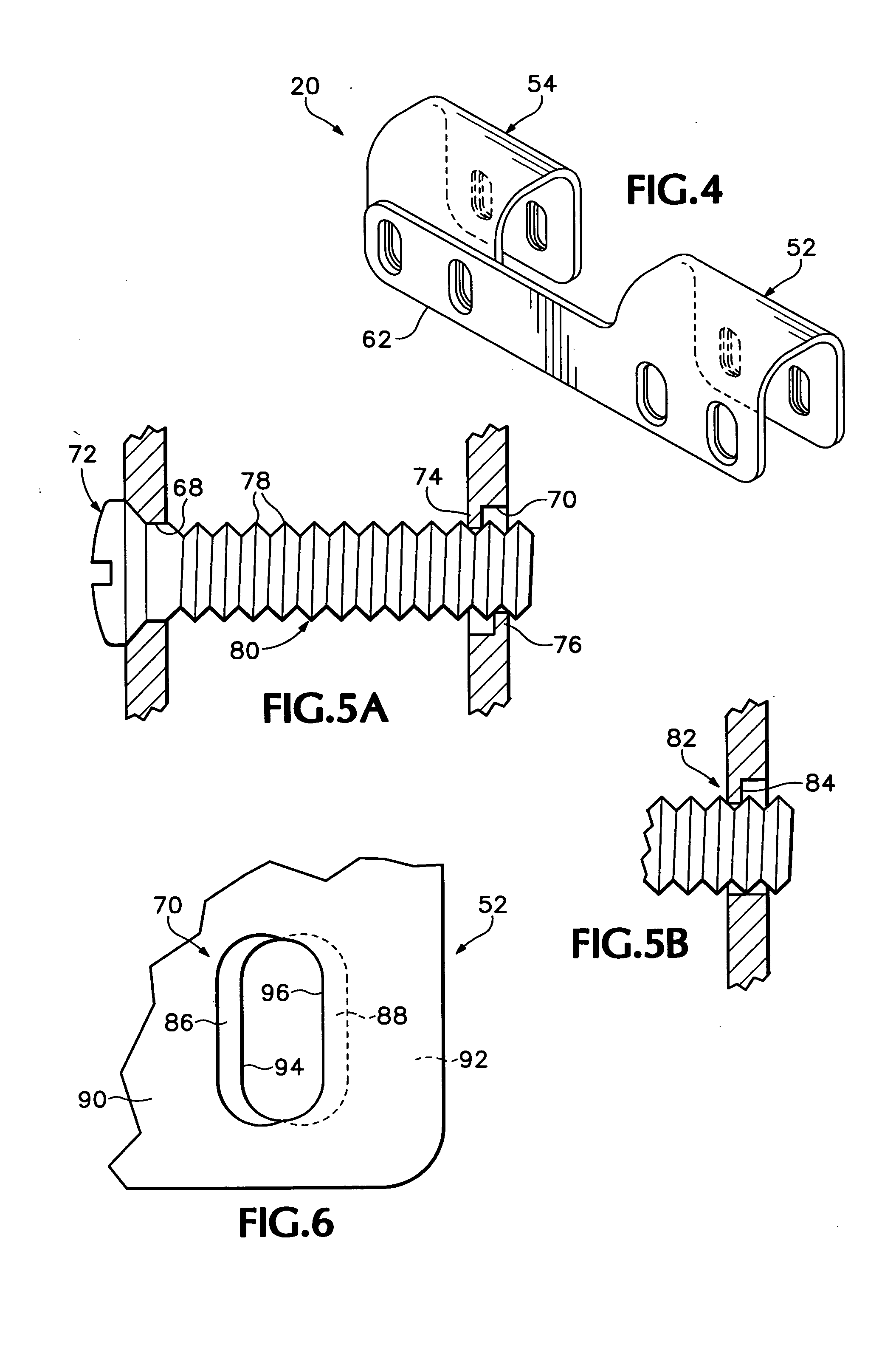
The blockable bone plate consists of a plurality of plate members which are connected to each other via webs. A screw hole is provided in at least some plate members, preferably in each plate member. The screw hole is surrounded by a spherical countersink on the upper surface of the plate. Provided internally in the screw hole there is an engagement contour which consists of contour valleys and contour peaks partially running in a horizontal and radial peripheral direction on the wall of the screw hole. The engagement contour is preferably produced by milling and has for example a pointed, round, trapezoidal or serrated configuration. A blocking thread is provided under the screw head of the screw intended for blocking. As the screw is screwed in, the blocking thread engages in the engagement contour. The particular advantages lie in increased security against the screw coming loose and in the possibility of also inserting the screw through the plate at an inclination. Furthermore, a bone plate is proposed which has the shape of an arc of a circle in the longitudinal axis of the plate and which requires less bending, particularly on the human lower jaw, and adapts to the bone in a more ideal manner.
Owner:MEDARTIS AG
Surgical devices assembled using heat bondable materials
Surgical devices such as implants or suture fastenings are assembled from a plurality of discrete components, one of which components includes a heat bondable plastic material for bonding the components together. At least two components are bonded to each other by the applying heat to the heat bondable plastic material of one component. The heat bondable plastic material is preferably a polymeric or composite material suitable for surgical applications and implantation in humans, and may be a biodegradable material. A laser may be used as the heat source. The present invention is advantageously embodied in heat bonded fastenings for sutures or K-wires, in which a variety of different suture anchors are usable, including expandable distal suture anchors. Other embodiments include a metal bone plate which is held to bone by a metal bone screw and a nut of bondable material bonded to the plate to secure the connection; a piece of bondable material bonded to a metal prosthesis to custom fit the prosthesis; and a surgical implant custom formed by bonding together a plurality of discrete elements one or more of which is bondable.
Owner:P TECH +1
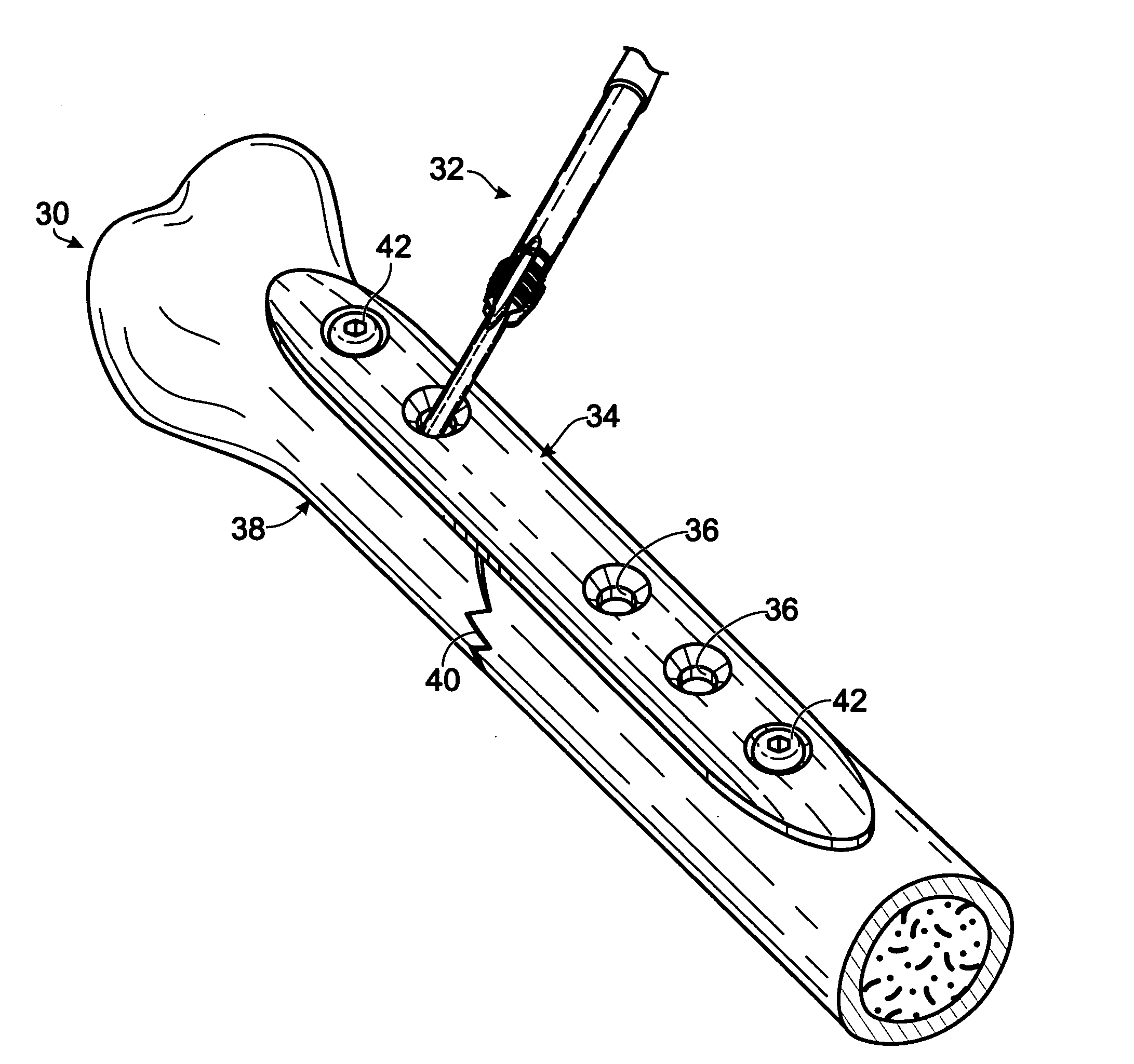
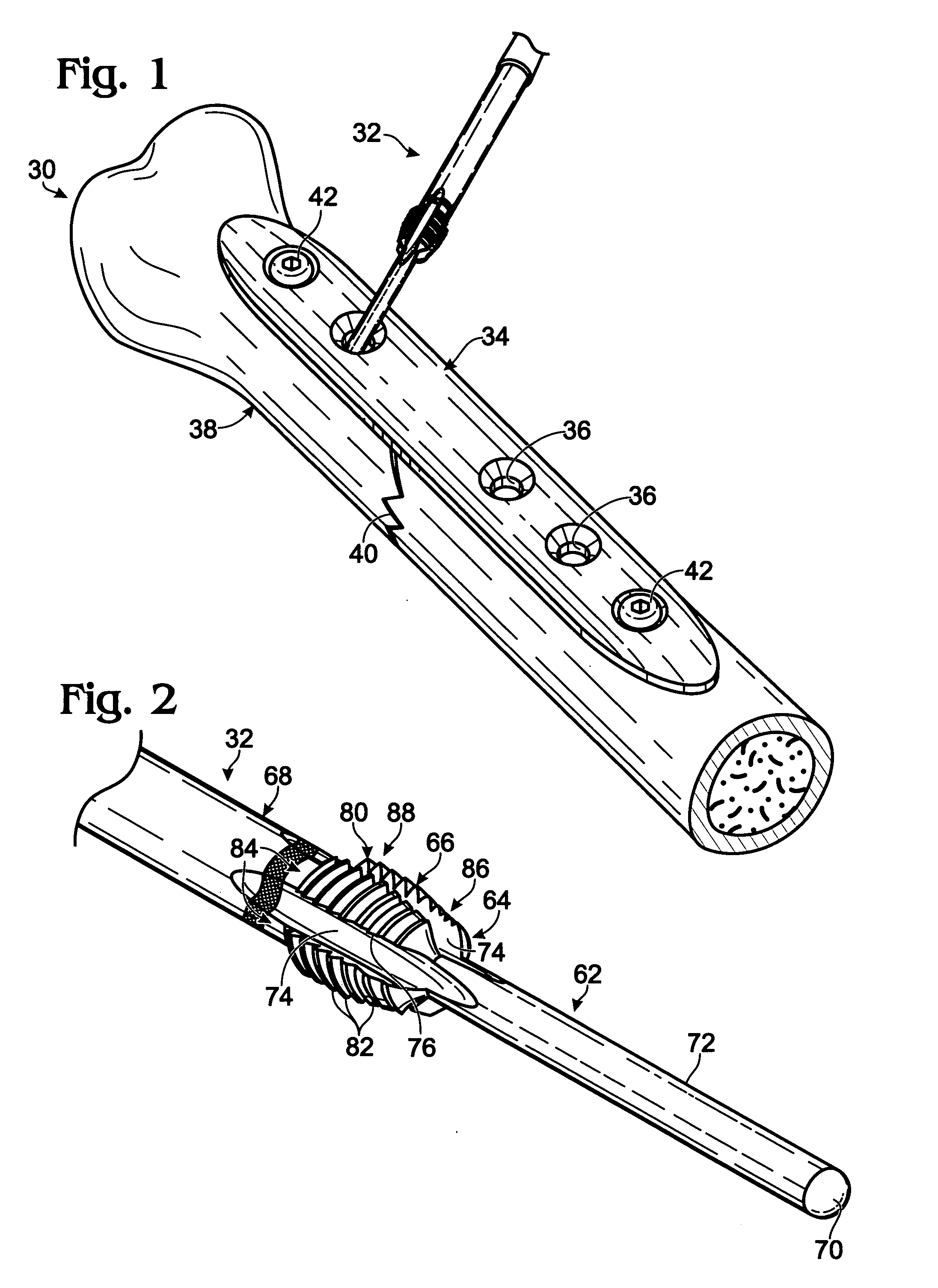
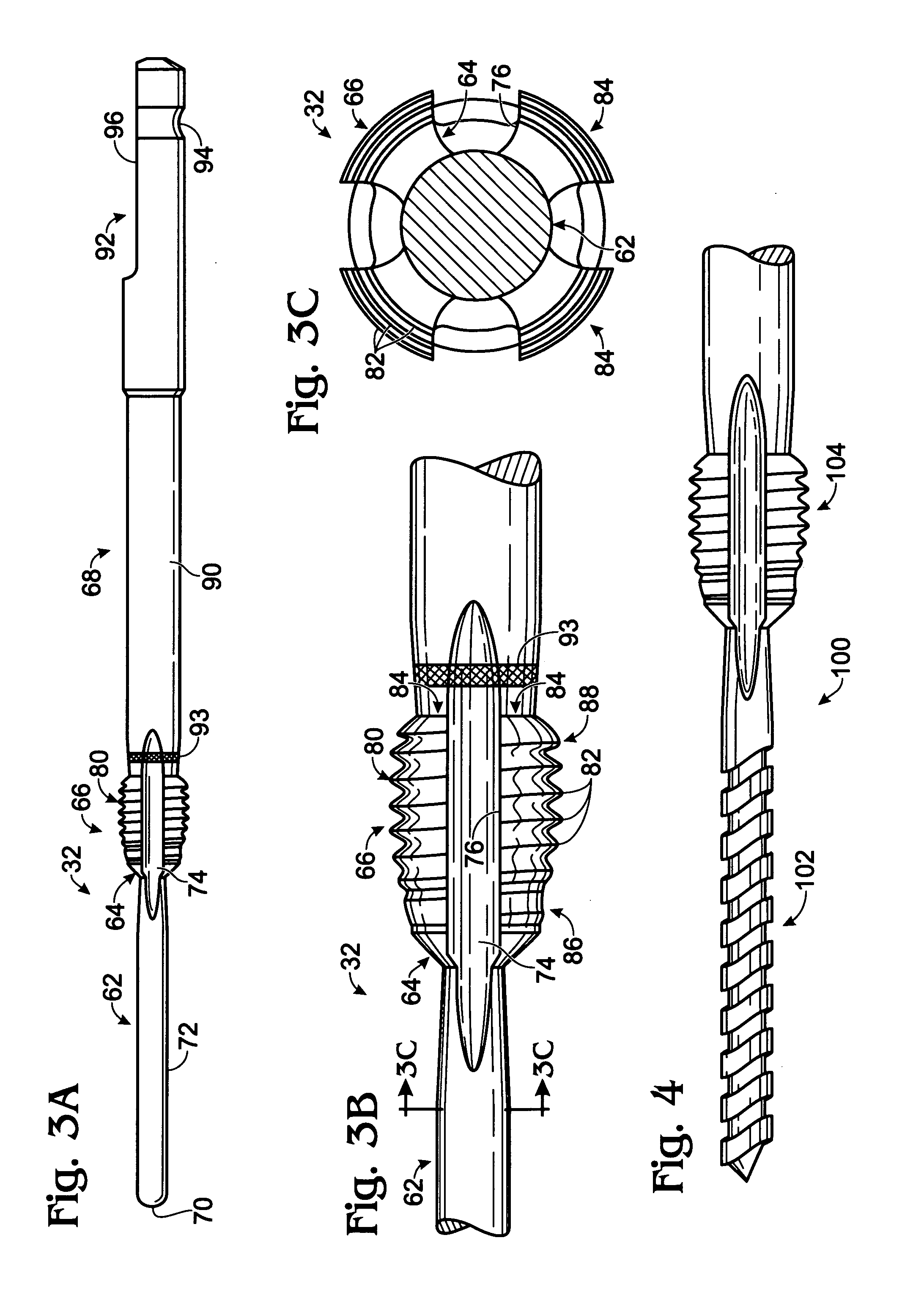
Bone fastener and instrument for insertion thereof
InactiveUS6258091B1Quickly and efficientlyEasy to disassembleSuture equipmentsInternal osteosythesisArcuate shapeScrew thread
A bone member fastener for closing a craniotomy includes a cap and a base interconnected by a narrow cylindrical collar. The cap has an externally threaded stud that screws into an internally threaded bore of the collar, thereby allowing the cap and base to be brought into clamping engagement against the internal and external faces of a bone plate and surrounding bone. In a particularly disclosed embodiment, the base of the fastener is placed below a craniotomy hole with the collar projecting into the hole, and the stud of the cap is screwed into the bore of the base from above the hole to clamp a bone flap against the surrounding cranium. This device provides a method of quickly and securely replacing a bone cover into a craniotomy. The distance between the cap and base can be selected by how far the threaded stud of the cap is advanced into the internally threaded collar. The fastener is therefore adaptable for use in several regions of the skull having various thicknesses. An insertion tool with a long handle permits safe and convenient placement of the base between the brain and the internal face of the bone plate. Some disclosed embodiments of the fastener have a cap and base that conform to the curved surface of the skull, for example by having an arcuate shape or flexible members that conform to the curvature of the bone plate and surrounding cranial bone as the fastener is tightened.
Owner:ZIMMER BIOMET CMF & THORACIC
System and method for stabilizing the human spine with a bone plate
InactiveUS6964664B2Reduce risk of backoutFirmly connectedInternal osteosythesisFastenersEngineeringCoupling system
A spinal plate system and method for fixation of the human spine is provided. In an embodiment, the spinal fixation system includes a plate, a coupling member, a locking system for substantially locking the coupling member in a desired position, and an anchoring system to secure the coupling member in the locking system. The plate may have a hole that allows the coupling member to couple the plate with a bone. At least a portion of the coupling member may swivel in the hole so that a bottom end of the member may extend at a plurality of angles substantially oblique to the plate. The locking system may lock the coupling member in desired positions relative to the plate. The anchoring system may secure the coupling member in the locking system to inhibit the coupling system from detaching from the locking system when stressed. An assembly tool may be used to engage and disengage the anchoring system during the installation or removal of the spinal fixation system.
Owner:ZIMMER SPINE INC
Degradable implantable medical devices
InactiveUS20060229711A1Reduce probabilityLower resistanceStentsBlood vesselsVascular implantBlood vessel
Devices and methods are provided for an implantable medical device which is degradable over a clinically relevant period of time. The medical devices may have the form of implants, graft implants, vascular implants, non vascular implants, wound closure implants, sutures, drug delivery implants, biologic delivery implants, urinary tract implants, inter-uterine implants, organ implants, bone implants including bone plates, bone screws, dental implants, spinal disks, or the like. In preferred embodiments, the implantable medical device comprises an implantable luminal prosthesis, such as vascular and non-vascular stents and stents grafts.
Owner:ELIXIR MEDICAL CORP
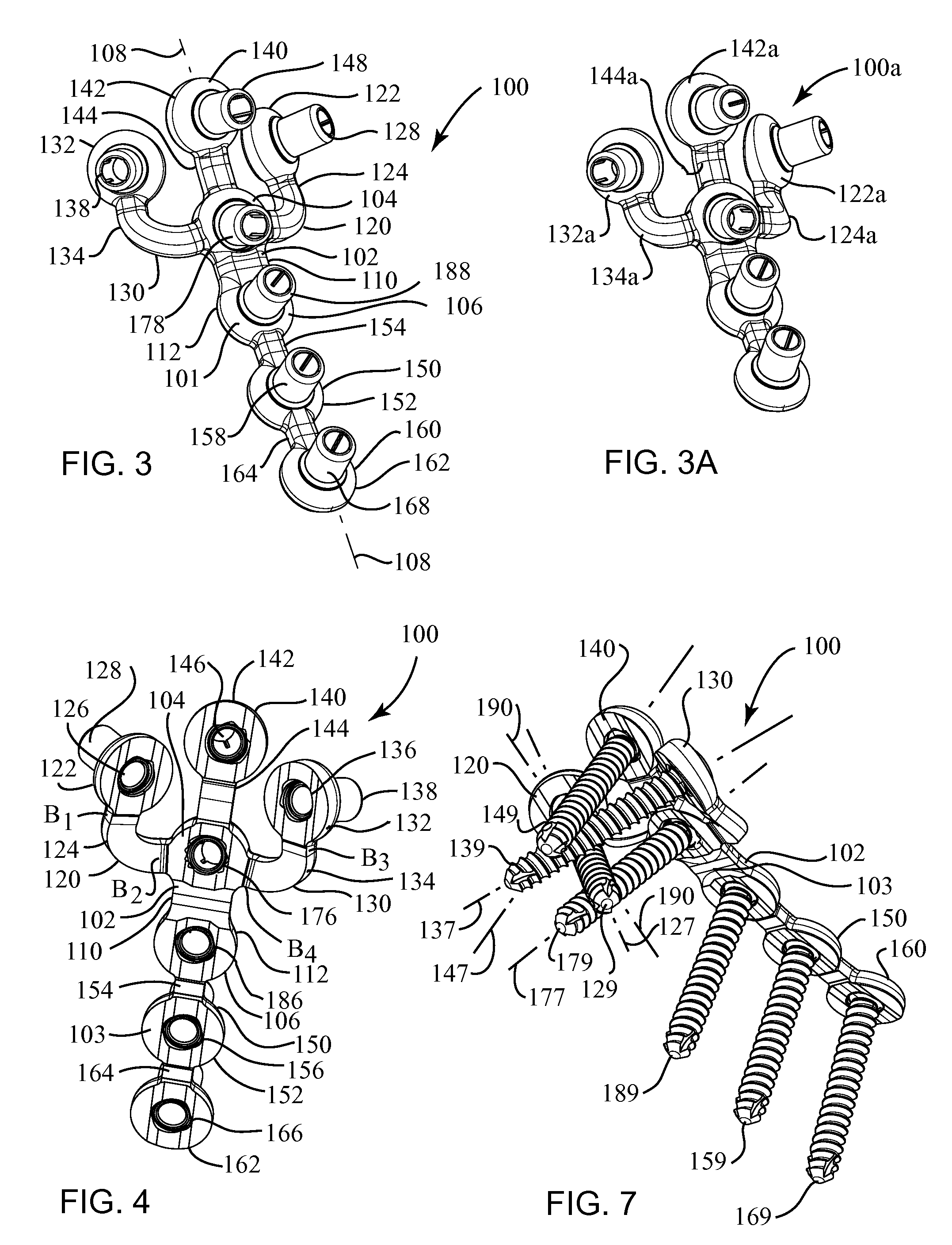
Highly-versatile variable-angle bone plate system
ActiveUS20080140130A1Improve versatilitySuture equipmentsThread cutting machinesEngineeringInternal fixation
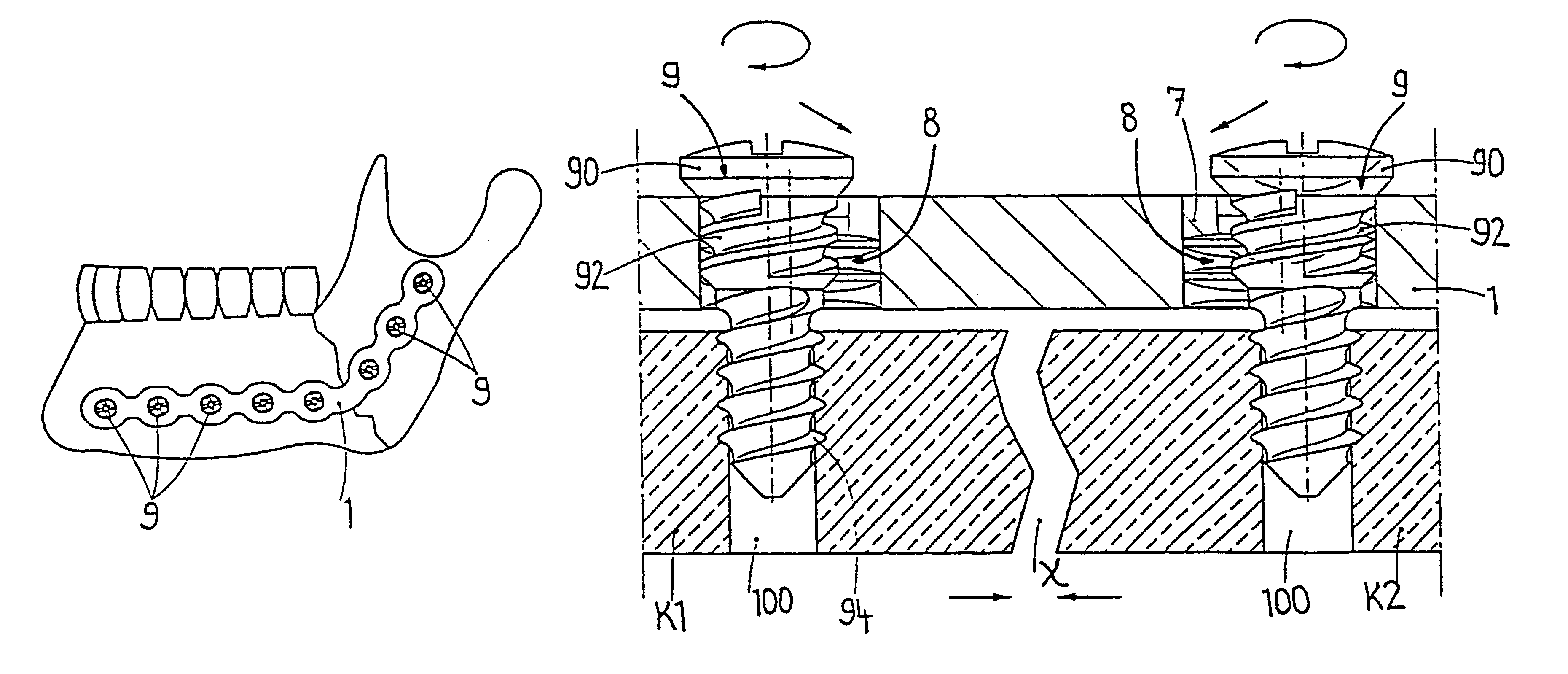
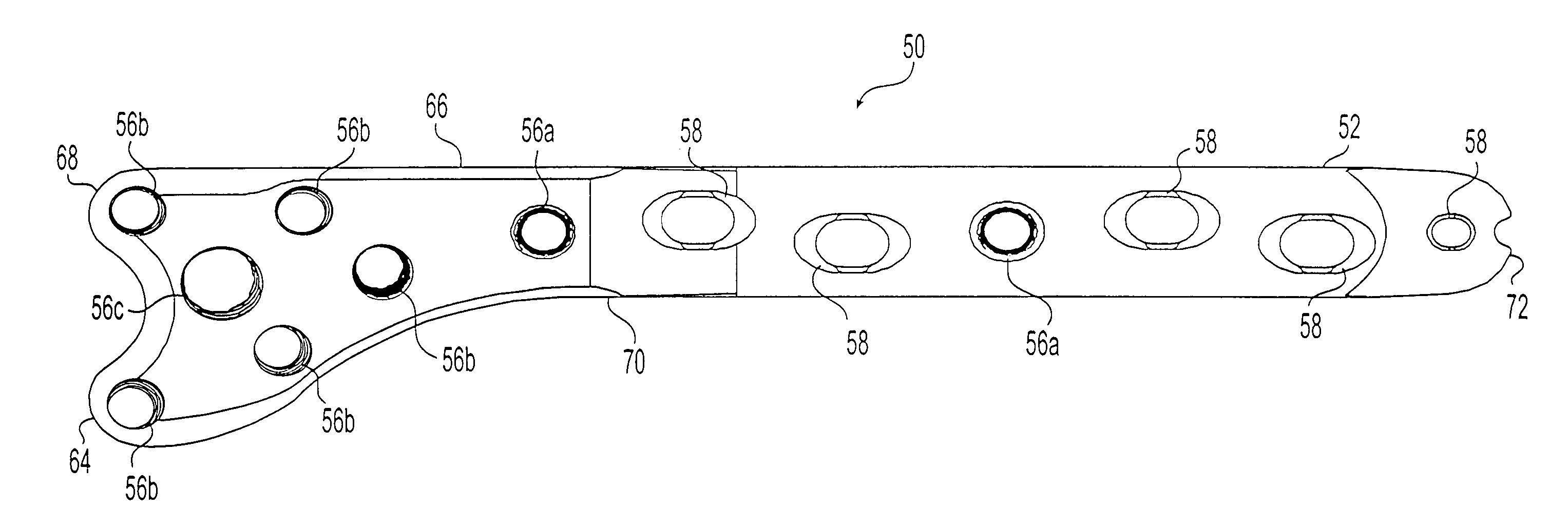
A bone plate system for internal fixation of bone fractures includes a bone plate having a plurality of bone plate holes. The holes are constructed to receive either a non-locking, locking, or variable-angle locking bone screw. The holes have discrete columns of teeth or thread segments arranged around the inner surface of the hole for engaging threads on the heads of locking and variable-angle locking bone screws. Conventional locking bone screws engage the bone plate coaxially with the central axis of the bone plate hole. Variable-angle locking bone screws can engage the bone plate at a selectable angle within a range of selectable angles relative to the central axis of the bone plate hole. The head of the variable-angle locking screw is at least partially spherical, and the thread thereon has a profile that follows the arc-shaped radius of curvature of the spherical portion of the screwhead.
Owner:DEPUY SYNTHES PROD INC
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6867247B2Reduce probabilityHigh porositySuture equipmentsStentsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Slidable bone plate system
The invention is directed to a bone plate system. The bone plate system comprises a base plate having two generally parallel elongated screw slots extending therethrough. Two bone screws are provided that are capable of securing the base plate to a bone by insertion through the screw slots into the bone. Each bone screw has a screw head and a threaded portion extending therefrom. An interference device is attached to the base plate and retains the bone screws while permitting the bone screws to toggle and to controllably slide in the screw slots of the base plate. This design is particularly useful for joining adjacent vertebral bodies, as it permits controlled settling of the vertebral bodies, thereby enhancing the healing process.
Owner:NUVASIVE
Bone fixation plate with self-locking screws
InactiveUS20060276793A1Solve the lack of spaceHigh strengthSuture equipmentsLigamentsEngineeringSelf locking
A dynamic bone fixation plate assembly includes a bone plate with at least one fastener-receiving aperture, and at least one self-locking fastener. Each fastener includes a threaded shaft or shank for secure engagement with patient bone, and a head for engaging the bone plate in a manner providing a low profile orthopedic device. The fastener shank includes features lock the fastener to the bone plate to prevent the fastener from backing out of the bone plate while still allowing rotational movement between the fastener and the plate. Utilizing the features of the present invention, the bone plate controllably subsides and settles into a position of stability.
Owner:AMEDICA A DELAWARE
Surgical devices having a biodegradable material with a therapeutic agent
Surgical devices such as implants or suture fastenings are assembled from a plurality of discrete components, one of which components includes a heat bondable plastic material for bonding the components together. At least two components are bonded to each other by the applying heat to the heat bondable plastic material of one component. The heat bondable plastic material is preferably a polymeric or composite material suitable for surgical applications and implantation in humans, and may be a biodegradable material. A laser may be used as the heat source. The present invention is advantageously embodied in heat bonded fastenings for sutures or K-wires, in which a variety of different suture anchors are usable, including expandable distal suture anchors. Other embodiments include a metal bone plate which is held to bone by a metal bone screw and a nut of bondable material bonded to the plate to secure the connection; a piece of bondable material bonded to a metal prosthesis to custom fit the prosthesis; and a surgical implant custom formed by bonding together a plurality of discrete elements one or more of which is bondable.
Owner:P TECH +1
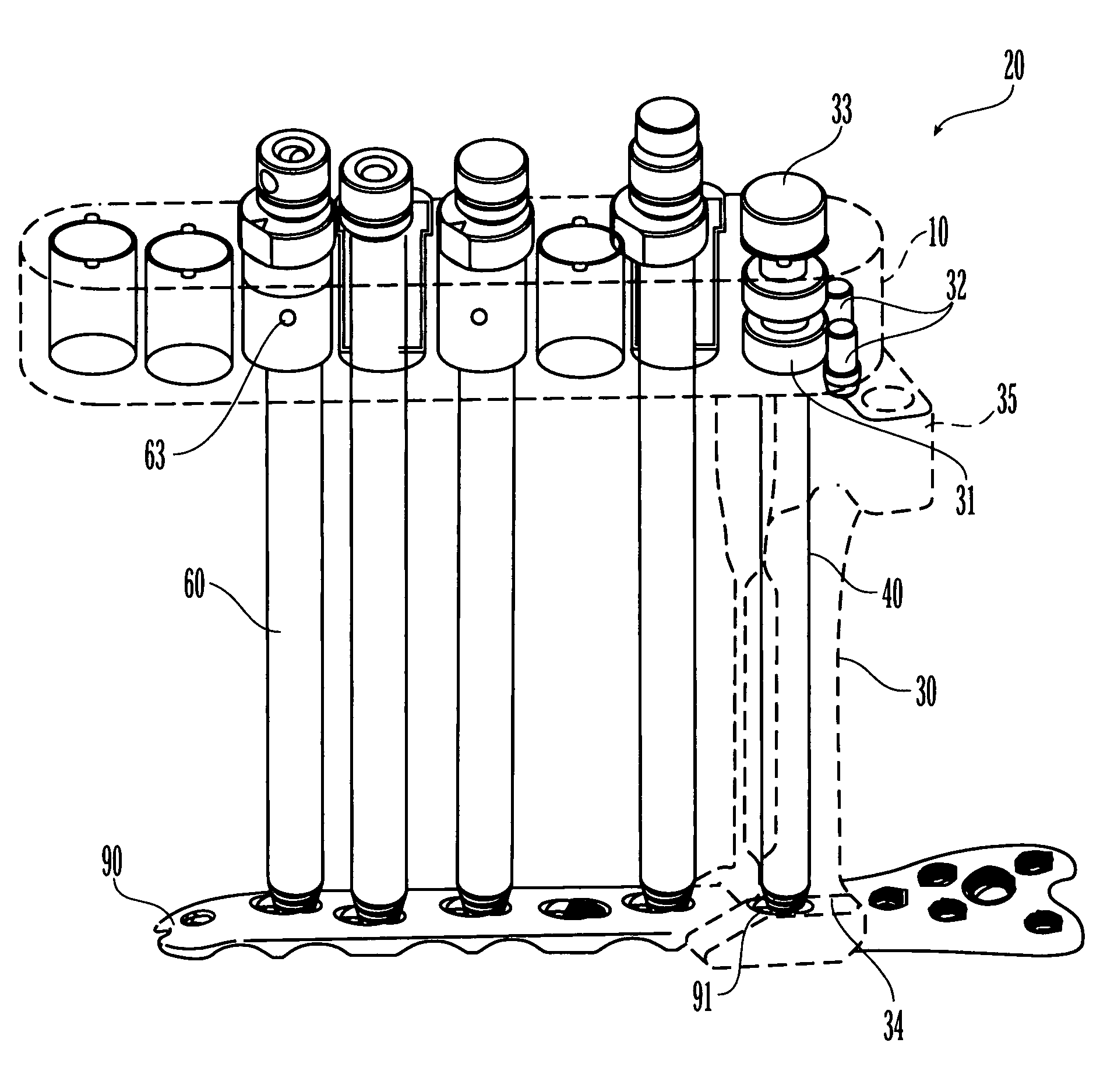
Bone plating system
The present invention relates to a bone plating system and method for fracture fixation of bone. The bone plating system includes a bone plate, at least one locking screw, and at least one non-locking screw. The bone plate has locking holes with threads and non-locking holes. The locking screws have a shaft with a thread for engaging bone and a head with a thread configured and dimensioned to mate with the thread of the locking holes. The non-locking screws have a thread for engaging bone and a non-threaded head. Both the locking and non-locking screws remain seated in their respective holes for substantially as long as the bone plate is implanted. The non-locking screws compress the bone plate against the bone and hold fracture reduction while the locking screws are secured to the plate at a fixed angular relationship. The mixed fixation achieved by this bone plating system and method is particularly useful for treatment of per-articular fractures.
Owner:DEPUY SYNTHES PROD INC
Bone plate stabilization system and method for its use
A method for joining first and second bones is provided. The method comprises inserting between the side surfaces of the bones a base plate having a first end nearer the first bone and a second end nearer the second bone. The base plate has a first screw hole extending through the first end and a second screw hole extending through the second end. A first bone screw is introduced through the first screw hole and into the first bone. The first bone screw is introduced at an angle relative to the top surface of the bone ranging from about 20° to about 60°. A second bone screw is introduced through the second screw hole and into the second bone. The second bone screw is introduced at an angle relative to the top surface of the bone ranging from about 20° to about 70°. At least a part of the first bone screw and at least a part of the second bone screw are covered to prevent the first and second bone screws from backing out of the first and second bones, respectively.
Owner:RSB SPINE
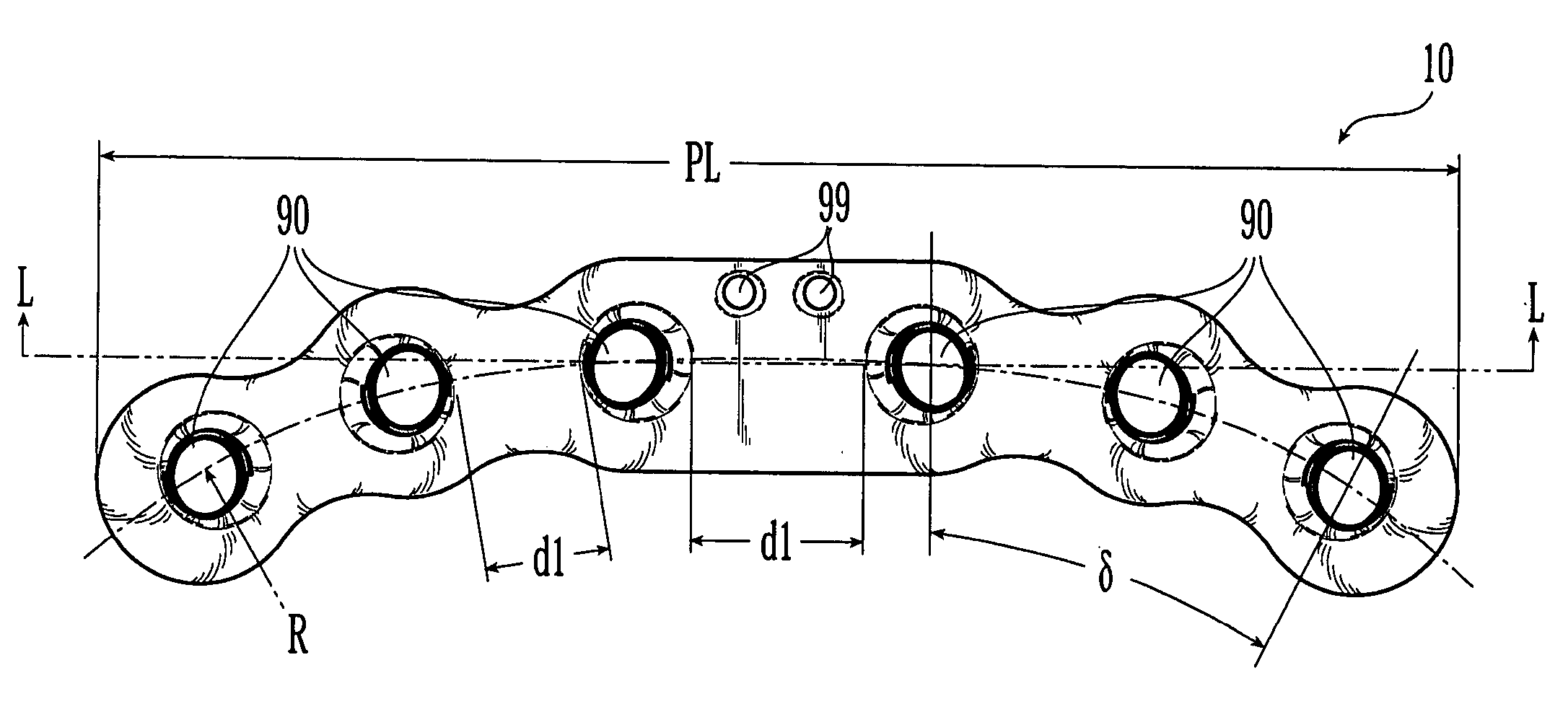
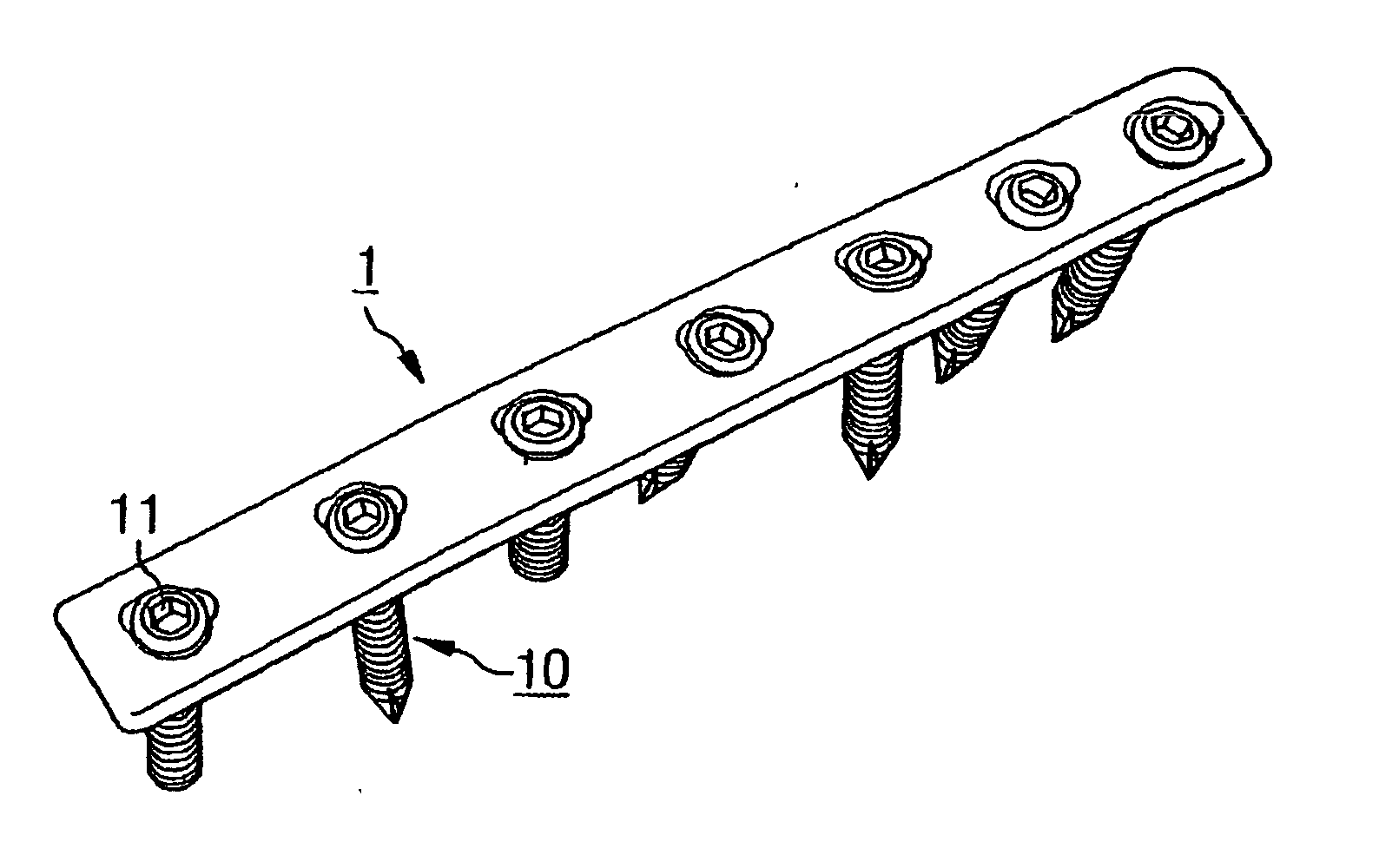
Bone plates
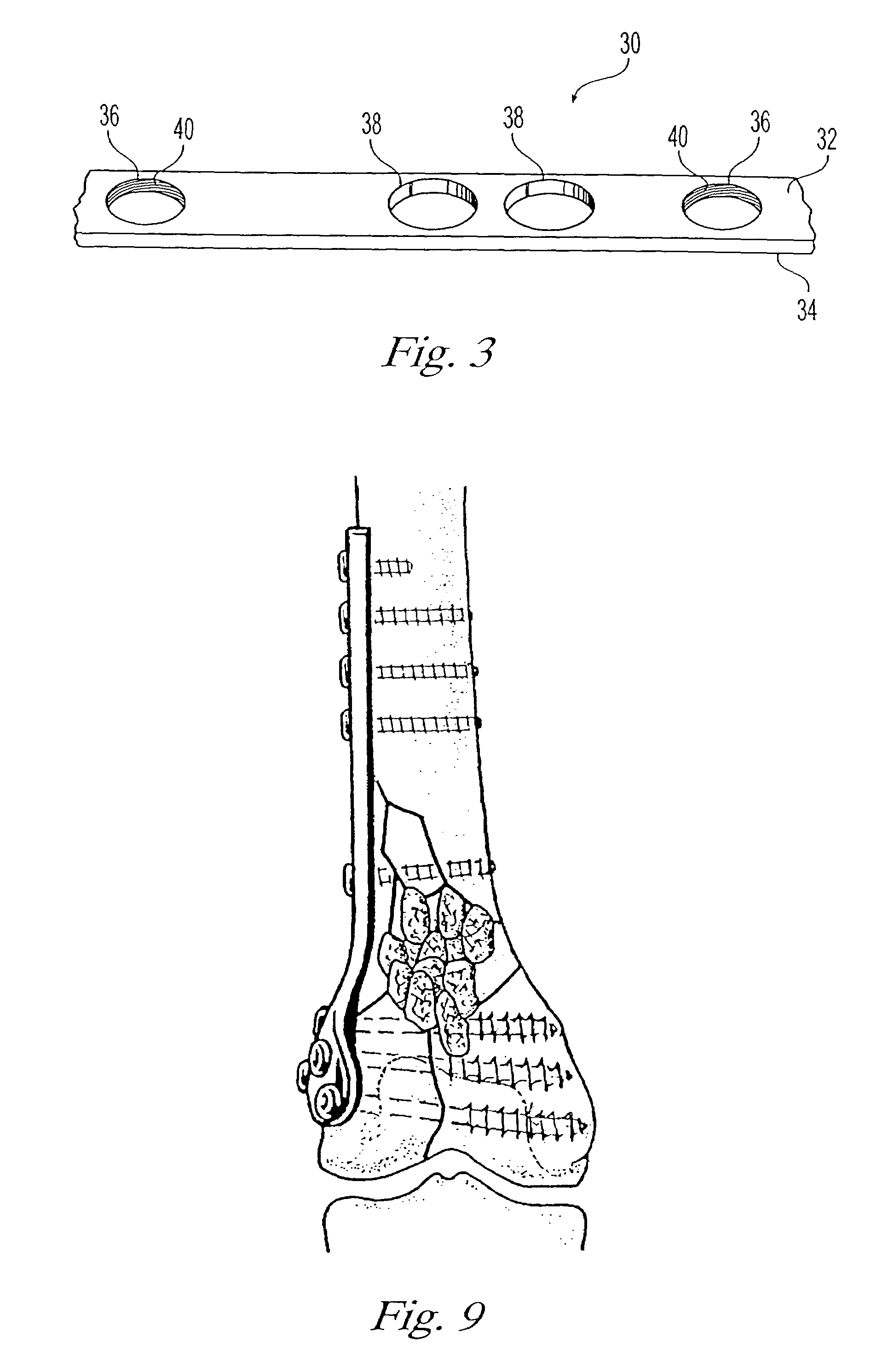
Systems, including methods, apparatus, and kits, for fixing bones, such as rib bones, with bone plates.
Owner:ELLIS THOMAS J +2
Spinal Plate
ActiveUS20130060337A1Improve securityImprove stabilityInternal osteosythesisJoint implantsSet screwIliac screw
Spinal plates with additional features to improve the stability of the interface between the plate and the underlying bone. A bone plate may include one or more sharp ridges along the periphery of its underside. When attached to bone, the ridge digs into the bone and increases stability. A bone plate may alternatively or additionally include one or more holes for optional spikes, which may be inserted once the plate is attached to the bone. By separating the spikes and including them as an optional component, the plate may enhance stability while reducing or eliminating the chance of the spike injuring the patient. Furthermore, bone screws may incorporate alternating notches and ridges into the head of the screw. The notches and ridges may interface with a set screw, thereby preventing rotation and loosening of the screw.
Owner:GLOBUS MEDICAL INC
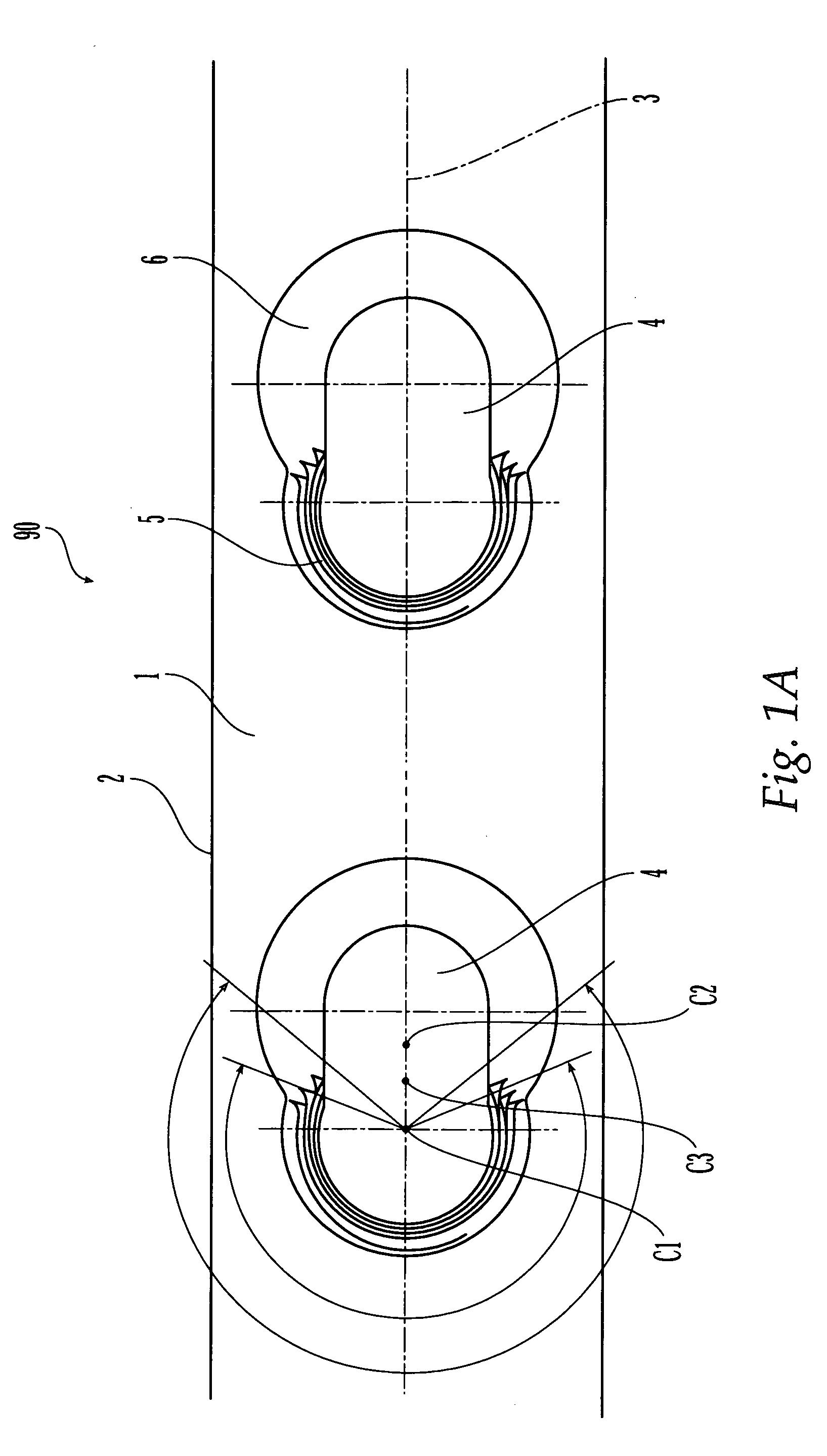
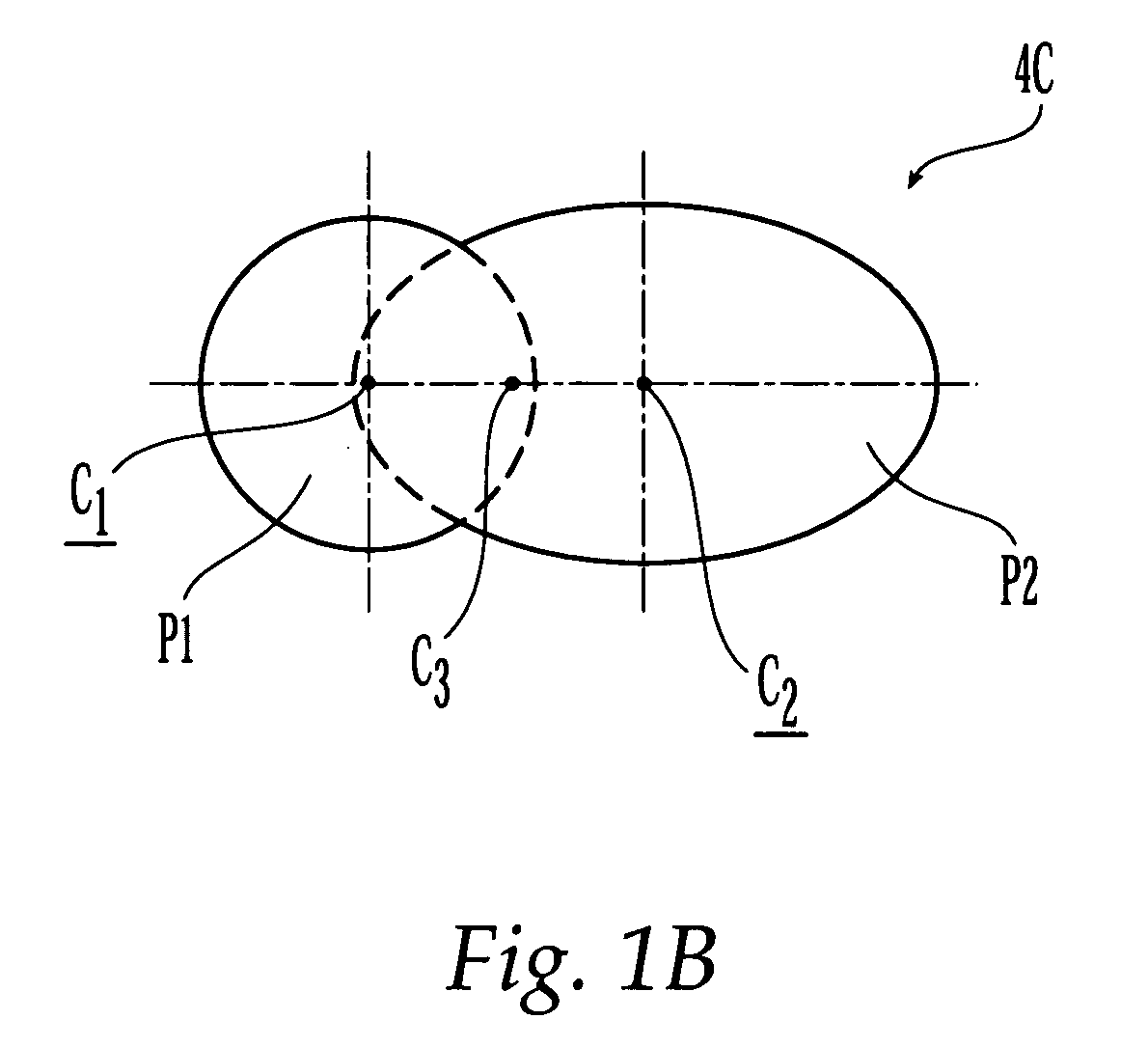
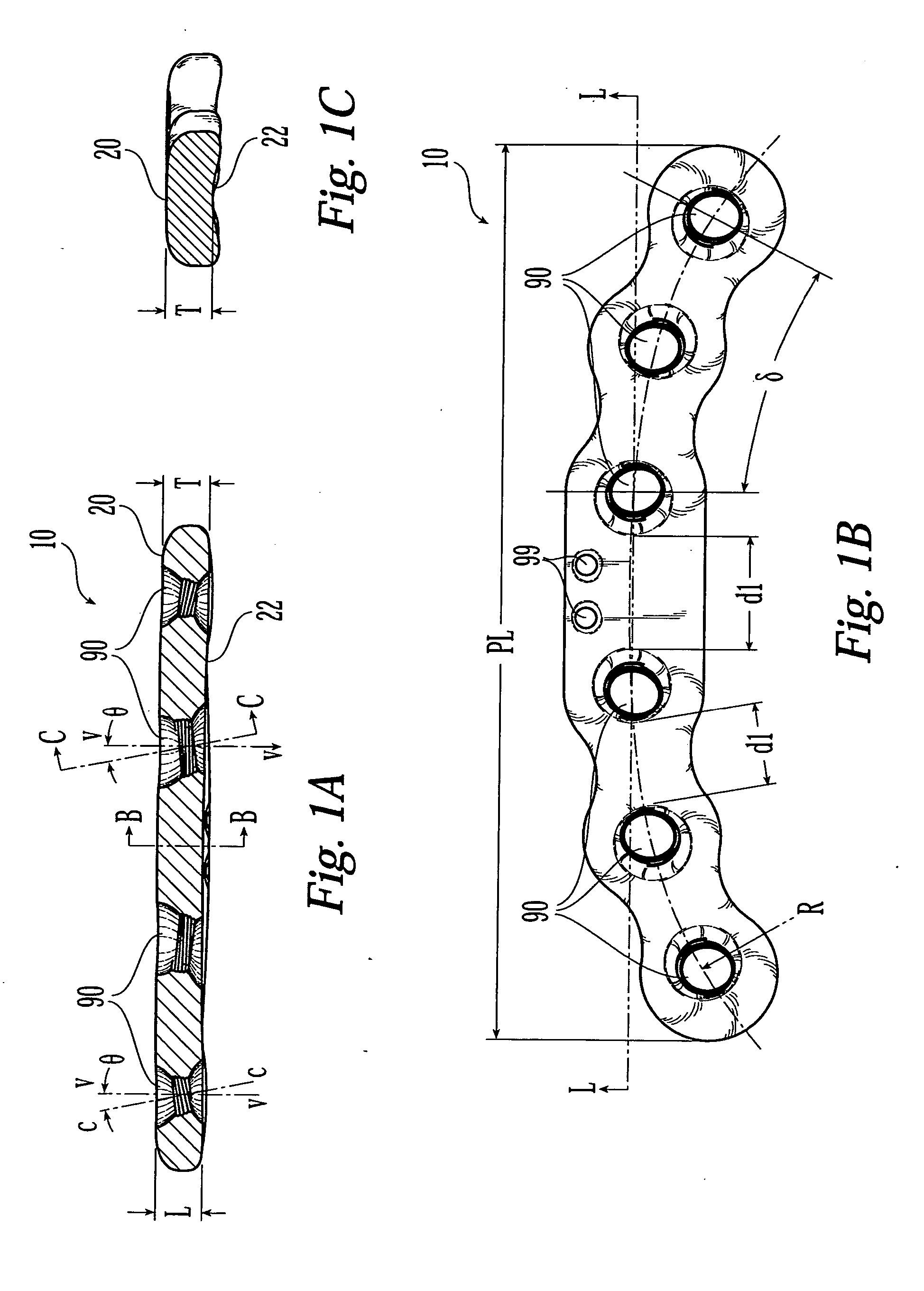
Bone plate
ActiveUS20050010226A1Reduce fracturesReduce gapInternal osteosythesisFastenersFracture reductionBone anchor
The present invention is directed to a bone plate and its method of use for reducing a bone fracture, the bone plate having a longitudinal axis, the bone plate comprising an upper surface, a lower surface, a first hole for engaging a head of a first bone anchor. The first hole is configured and adapted to fix a shaft of the first bone anchor along a first axis. A second hole is spaced apart from the first hole along the longitudinal axis. The second hole is for engaging a head of a second bone anchor, and is configured and adapted to fix a shaft of the second bone anchor along a second axis. The first hole and the second hole are configured such that the first axis and the second axis define a single plane and intersect at a point below the lower surface. The shafts of the first and second bone anchors may touch or nearly touch at the point of intersection, such that the first and second bone anchors form a truss.
Owner:SYNTHES USA
Bone plate and bone screw system
Owner:SPEIRS ANDREW +1
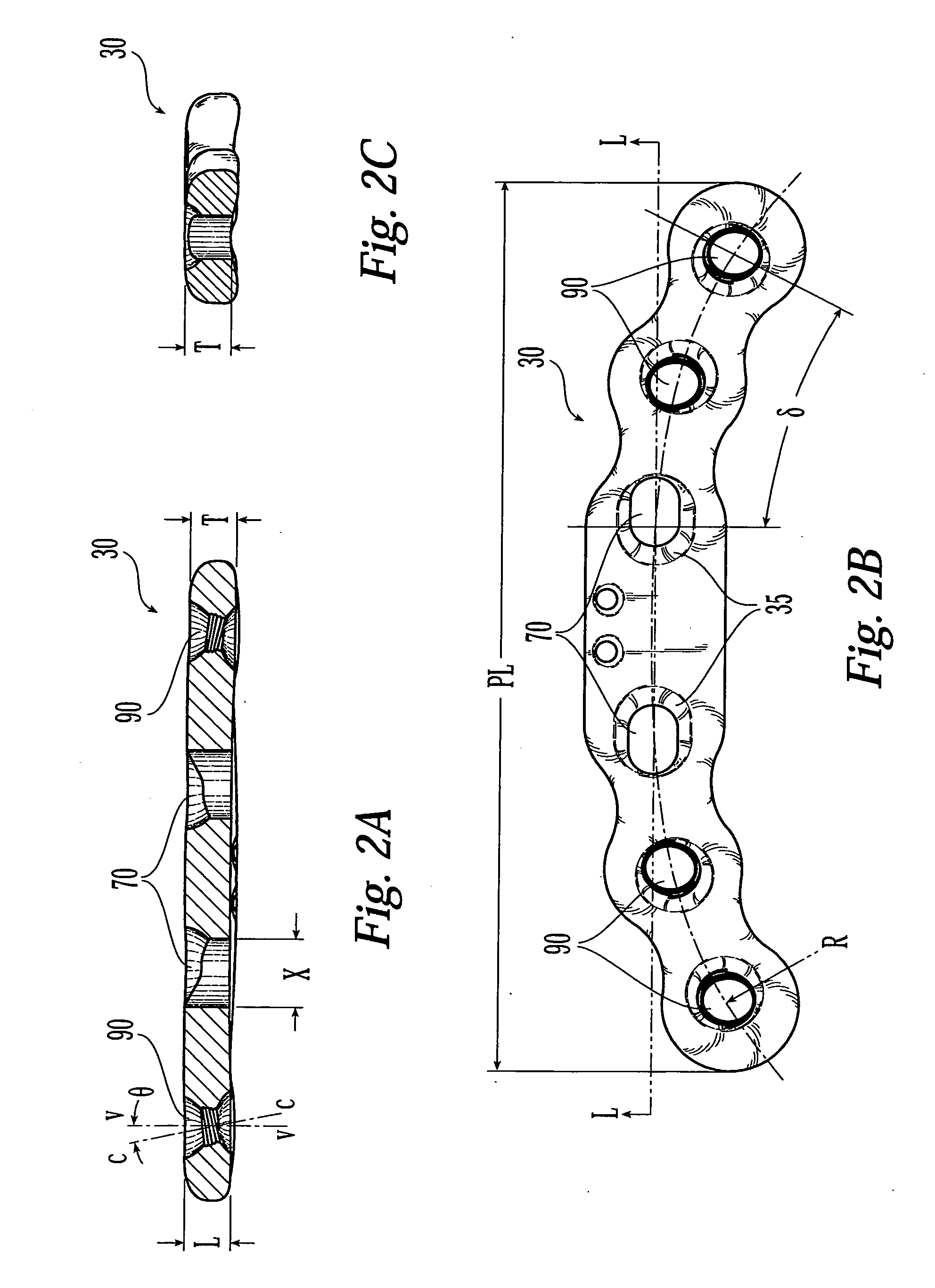
Bone plates with movable locking elements
ActiveUS20070055251A1Reduce discontinuityReduce chanceJoint implantsBone platesEngineeringMechanical engineering
System, including methods, apparatus, and kits, for fixing bones with bone plates having movable locking elements.
Owner:ACUMED
Orthopaedic implant with sensors
The present invention relates to an orthopaedic implant, such as a bone plate, for the fixation of bone where the implant also has at least one microchip and at least one sensor connected to the microchip. The sensor or sensors are configured to receive physical stimulus from a portion of the implant or the patient's tissue such as temperature, pressure, and strain. The information received from the sensor or sensors is gathered by the microchip and transmitted to a receiver, such as a personal computer, outside the patient. This information enables doctors to diagnose the useful life of the implant, the load sharing of the bone plate, and possible complications typically associated with orthopaedic implants such as infection, fracture non-union, and fatigue. The implant may also have one or more electrodes located on its surface which emit an electric current to stimulate healing of the broken or fractured bone.
Owner:SYNTHES USA
Orthopedic systems
Orthopedic systems, including apparatus, methods, and kits. In some embodiments, these systems may be used for selectively tapping apertures of bone plates, to form threaded apertures during installation of the bone plates (i.e., intraoperatively).
Owner:ACUMED
Aiming arm for bone plates
An aiming guide aligns a surgical tool with a hole in a bone plate. The aiming guide includes an arm portion having a longitudinal axis, top and bottom surfaces, and first and second ends. The aiming guide further includes a handle portion having upper and lower ends. The handle portion is, at its lower end, connected to the bone plate. The handle portion is, at its upper end, connected to the arm portion. Bores extend from the top surface to the lower surface of the arm portion. Each bore is configured and dimensioned to receive a tool guide in at least two different preset positions which locates the channel in at least two different hole positions. The bore includes two diametrically opposed slots extending along at least a portion of a length of the bore, the slots configured and dimensioned to mate with diametrically opposed knobs extending radially outward from a tool guide. The tool guide has a head and a sleeve portion, which has a centered channel. The sleeve portion and channel are eccentric with respect to the head portion. The tool guide is inserted in a first preset position in order to align a surgical tool with one portion of a two-portion bone plate hole, or in a second preset position rotated 180° from the first preset position, in order to align a surgical tool with the second portion of a two-portion bone plate hole.
Owner:SYNTHES USA
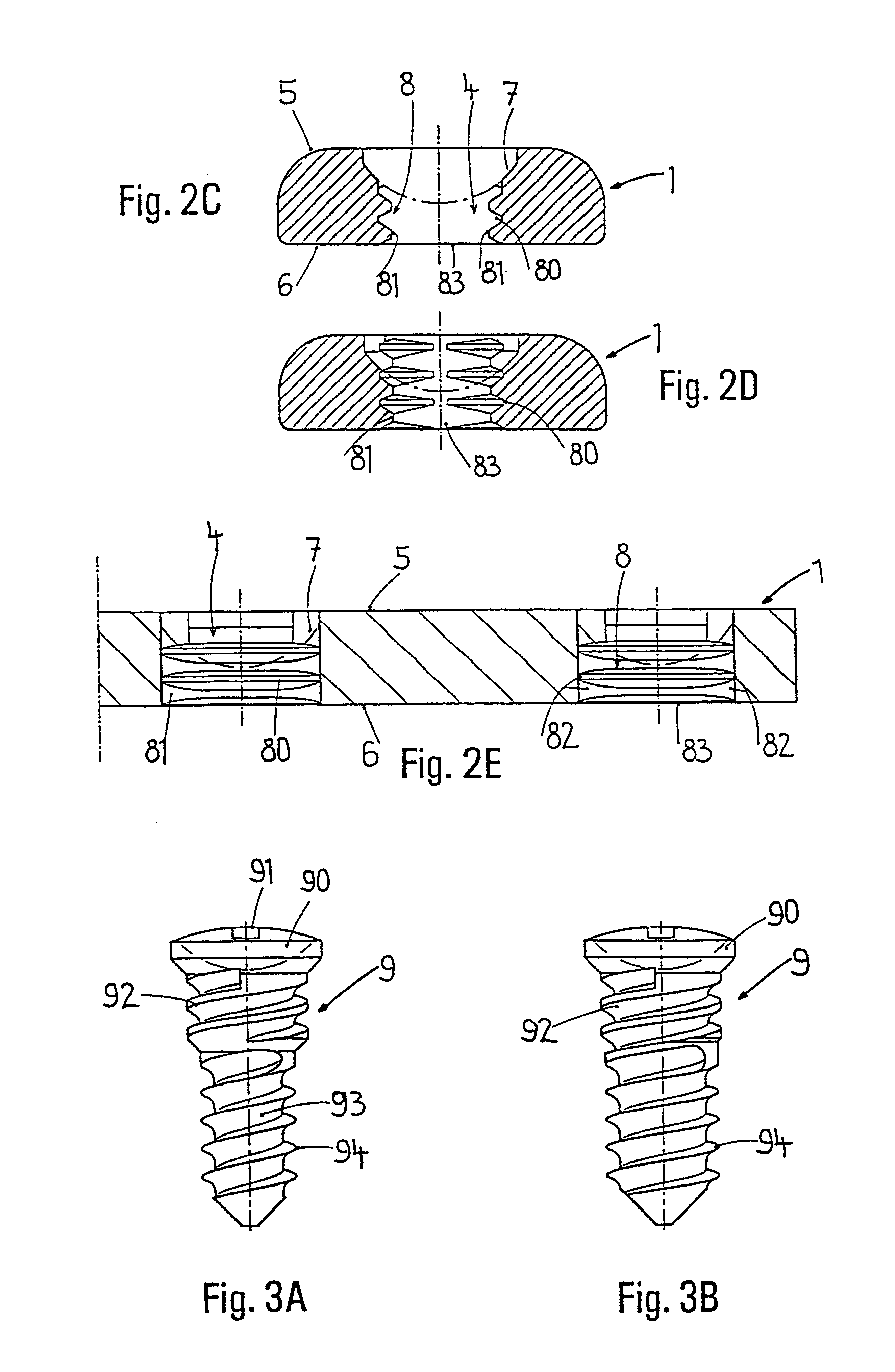
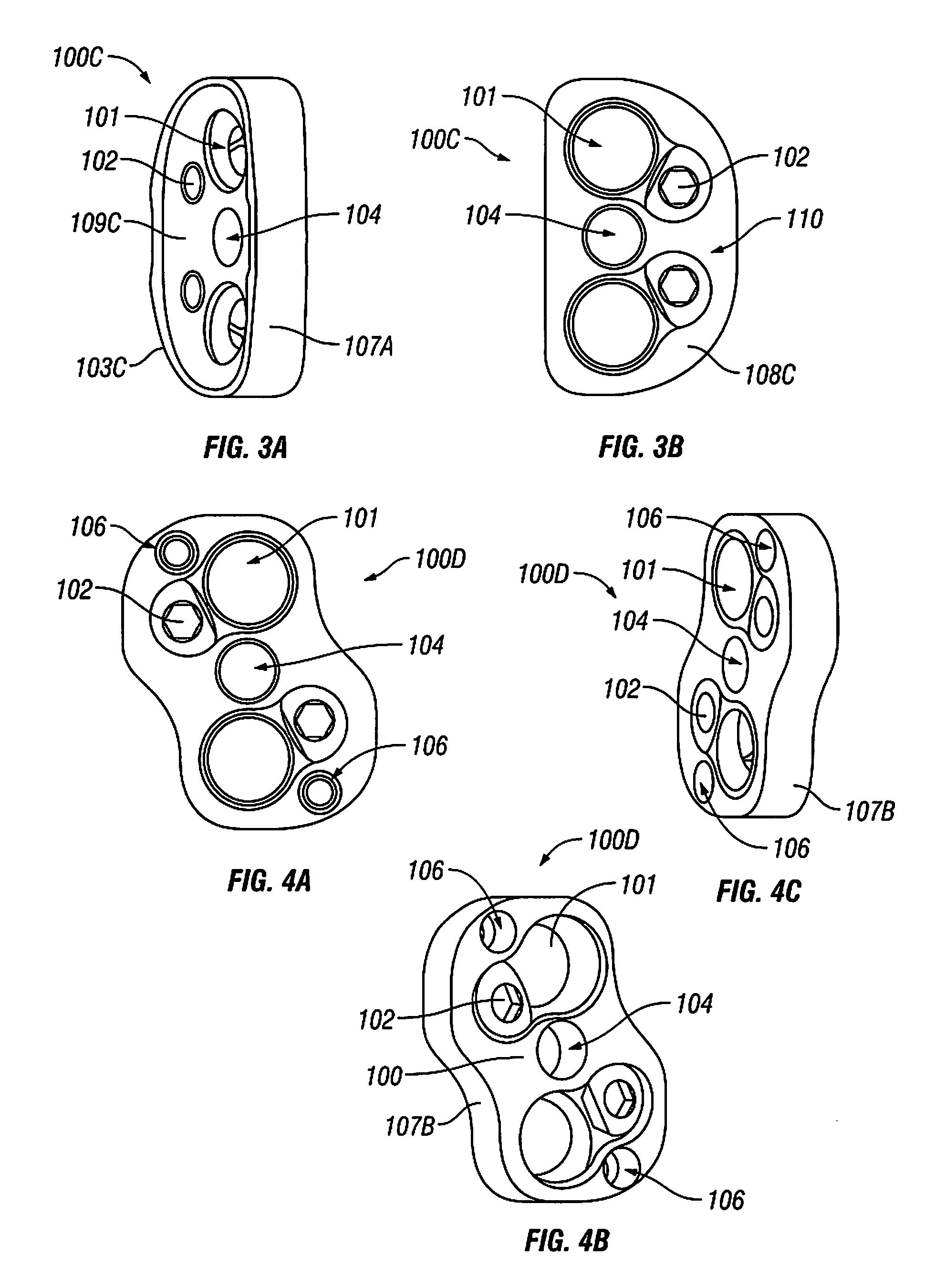
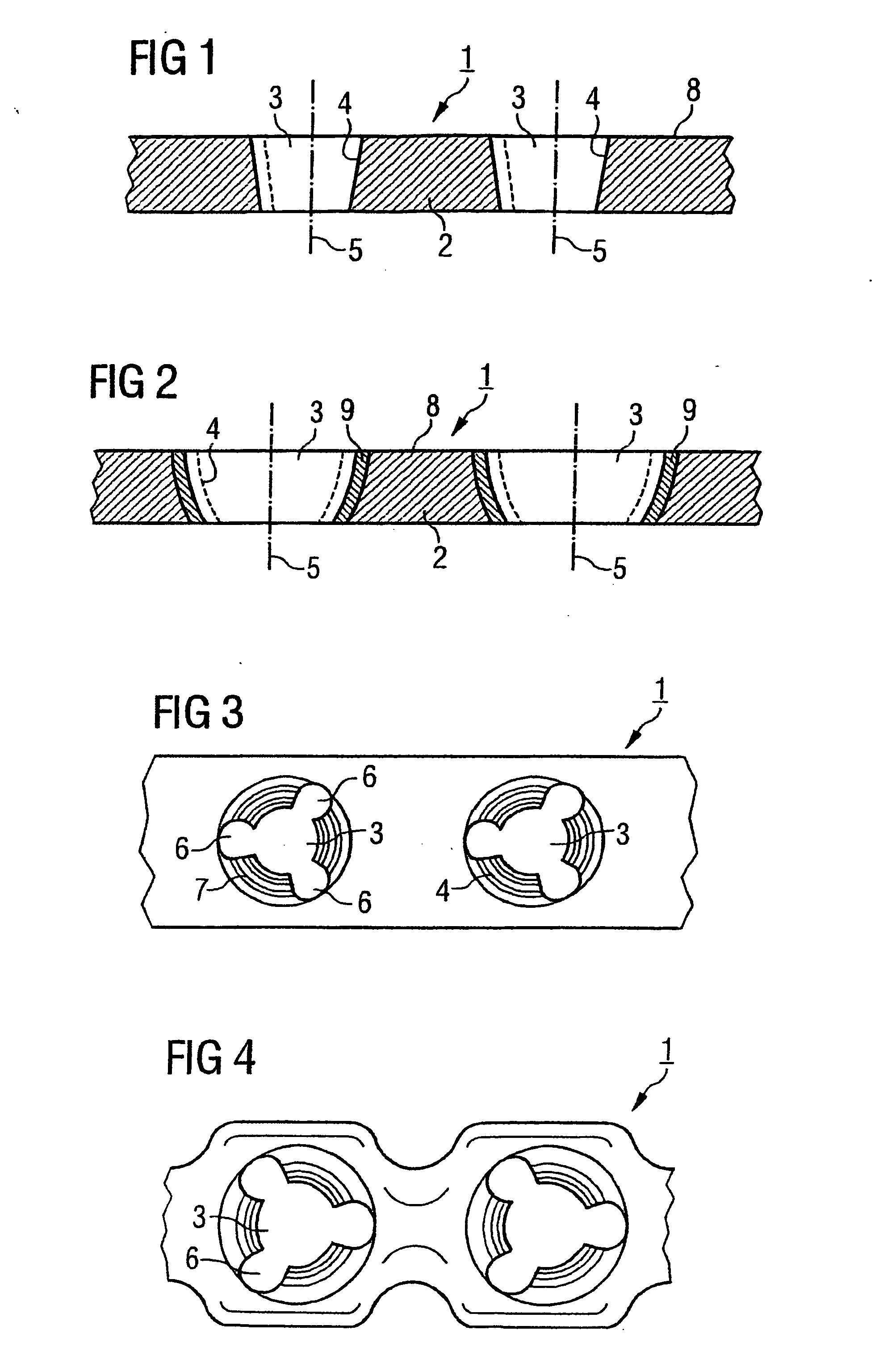
Bone plate
ActiveUS20050261688A1Integrity compromisedSize compromisedSuture equipmentsInternal osteosythesisEngineeringBone plate
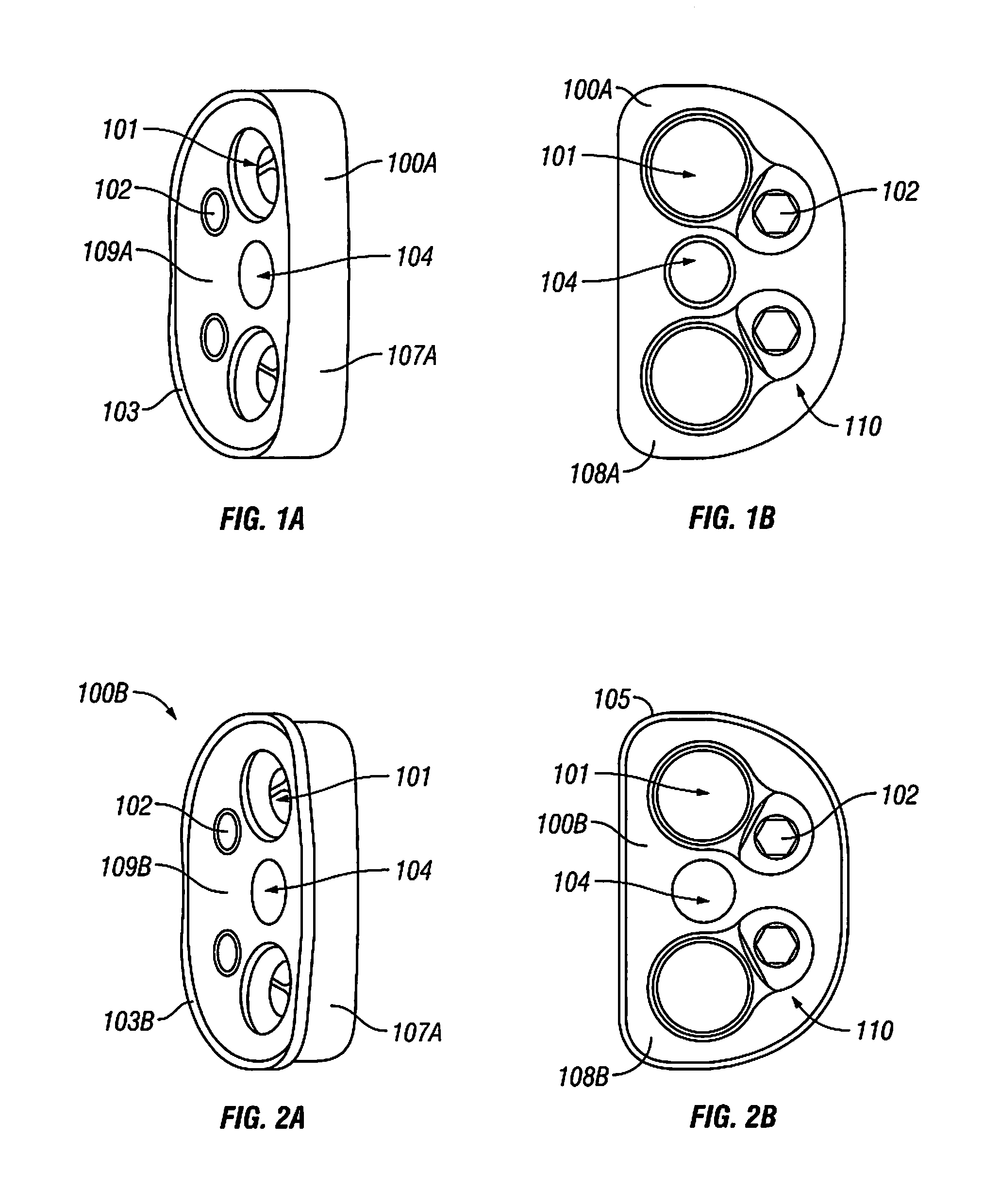
A bone plate has an upper surface, a lower surface, and at least one first hole extending through the upper and lower surfaces. The first hole has two or three vertically separate regions, each region communicating with or abutting the adjacent region. The first hole has a first upper region, which is unthreaded and which, from the plate's upper surface to the plate's lower surface, has a curved inward taper. The first hole has a second middle region, which is threaded and which, from the plate's upper surface to the plate's lower surface, has a conical inward taper. The first hole has a third lower region, which is unthreaded and which, from the plate's upper surface to the plate's lower surface, has a conical outward taper. The bone plate is straight, curved, or both straight and curved. The bone plate may have at least one second hole, different from the first hole. The second hole is an elongated hole, which has a compression ramp or which has a threaded portion through part of its perimeter and a non-threaded portion through the other part of its perimeter.
Owner:SYNTHES USA
Bone plate
A bone plate has an underside on the side of the bone, an upper side and a plurality of holes in the plate connecting the underside with the upper side, with a central hole axis. At least one of these holes in the plate has an internal jacket surface that tapers towards the underside, while the internal jacket surface has N≧3 recesses which extend radially away from the axis of the hole.
Owner:DEPUY SYNTHES PROD INC
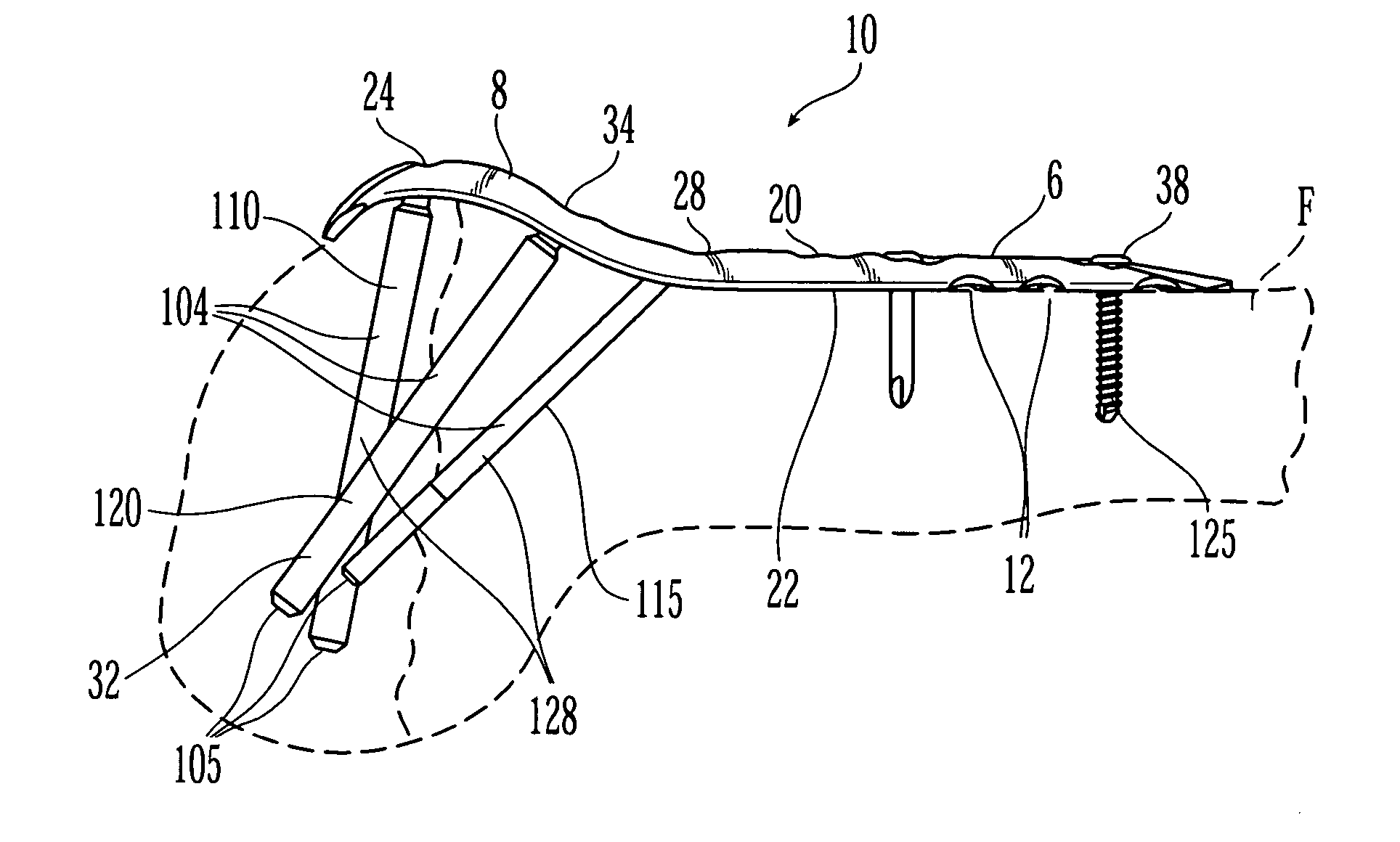
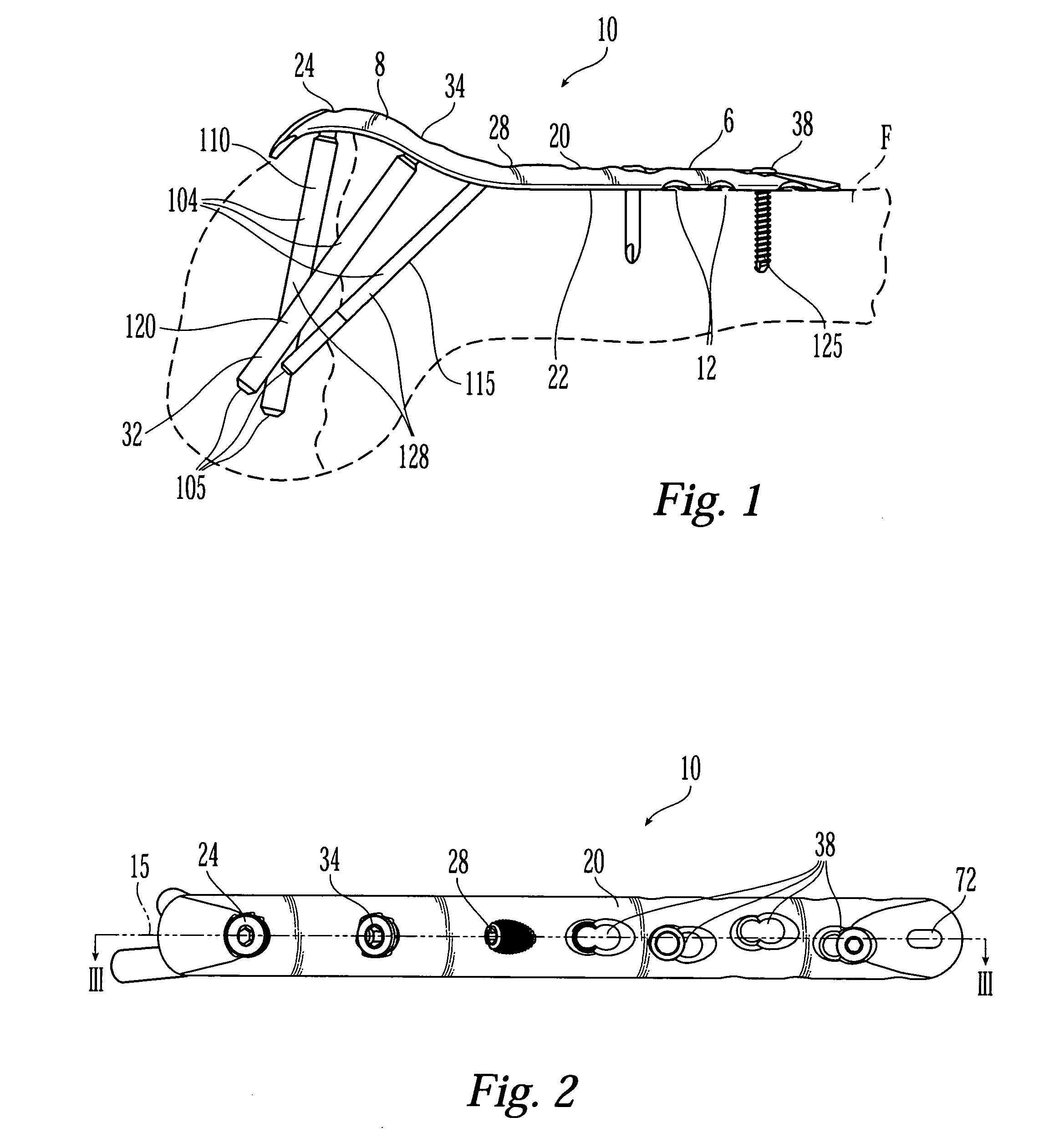
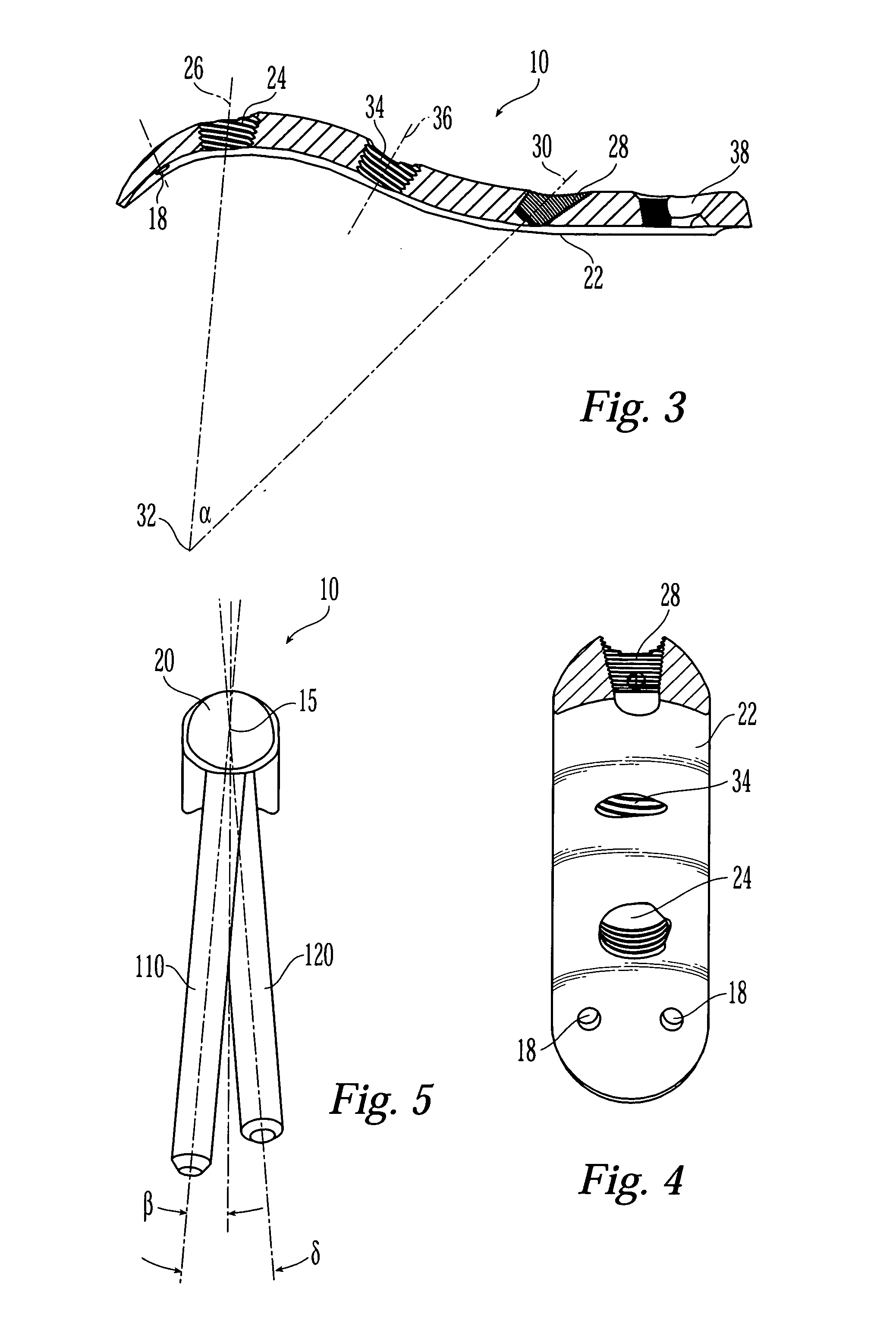
Bone fixation assembly and method of securement
A bone plate is provided for fixation of spaced vertebra. The bone plate has at least one through passage for securing the plate to bone with a bone fixation screw. The threaded shaft of a bone fixation screw is inserted through a bushing located in the through passage of the bone plate and the screw is thereby threadably secured to the underlying bone and the bushing is then compressed inward against the head of the screw with cams that are actuated by rotating the bushing in the through passage whereby the screw is locked relative to the bone plate. The bushing is not only compressed inwardly against the head of the screw but is also compressed downwardly by the cams into a seat to clamp separate elements of the bone plate together.
Owner:AESCULAP INC
Fracture Fixation Plate for the Proximal Radius
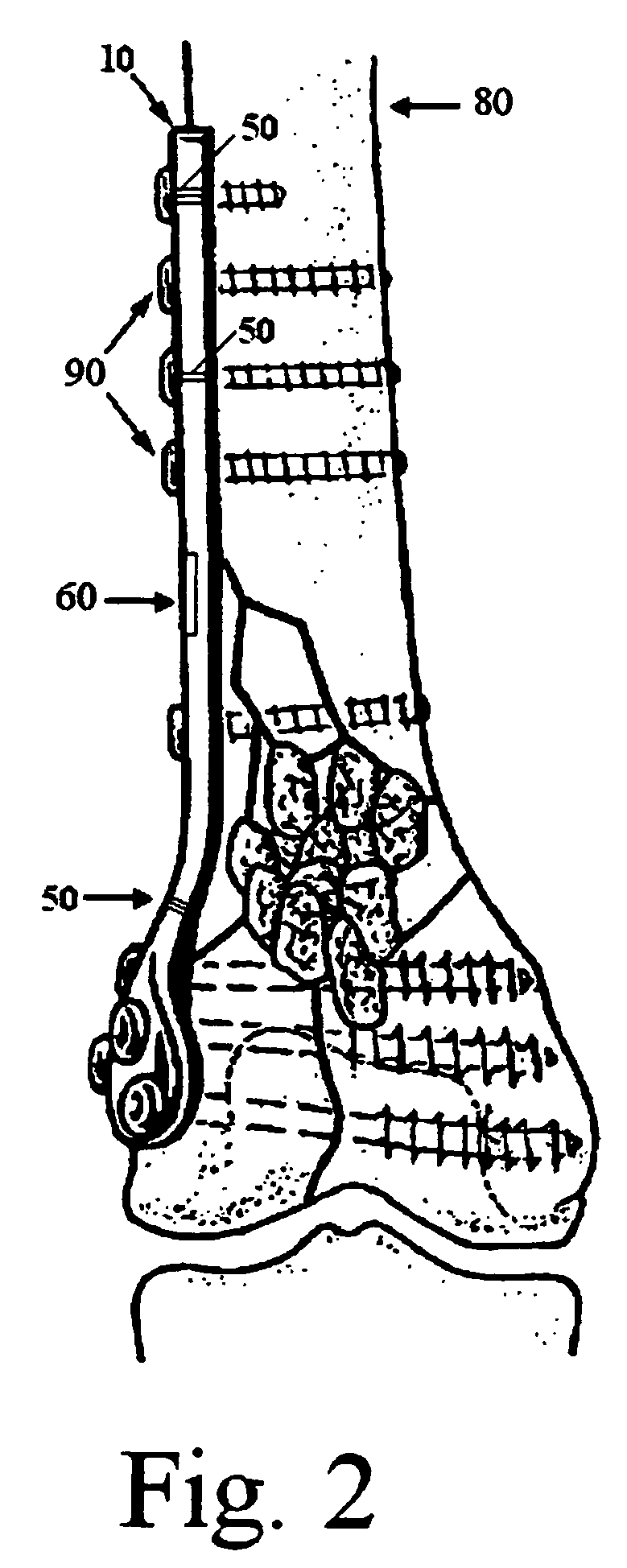
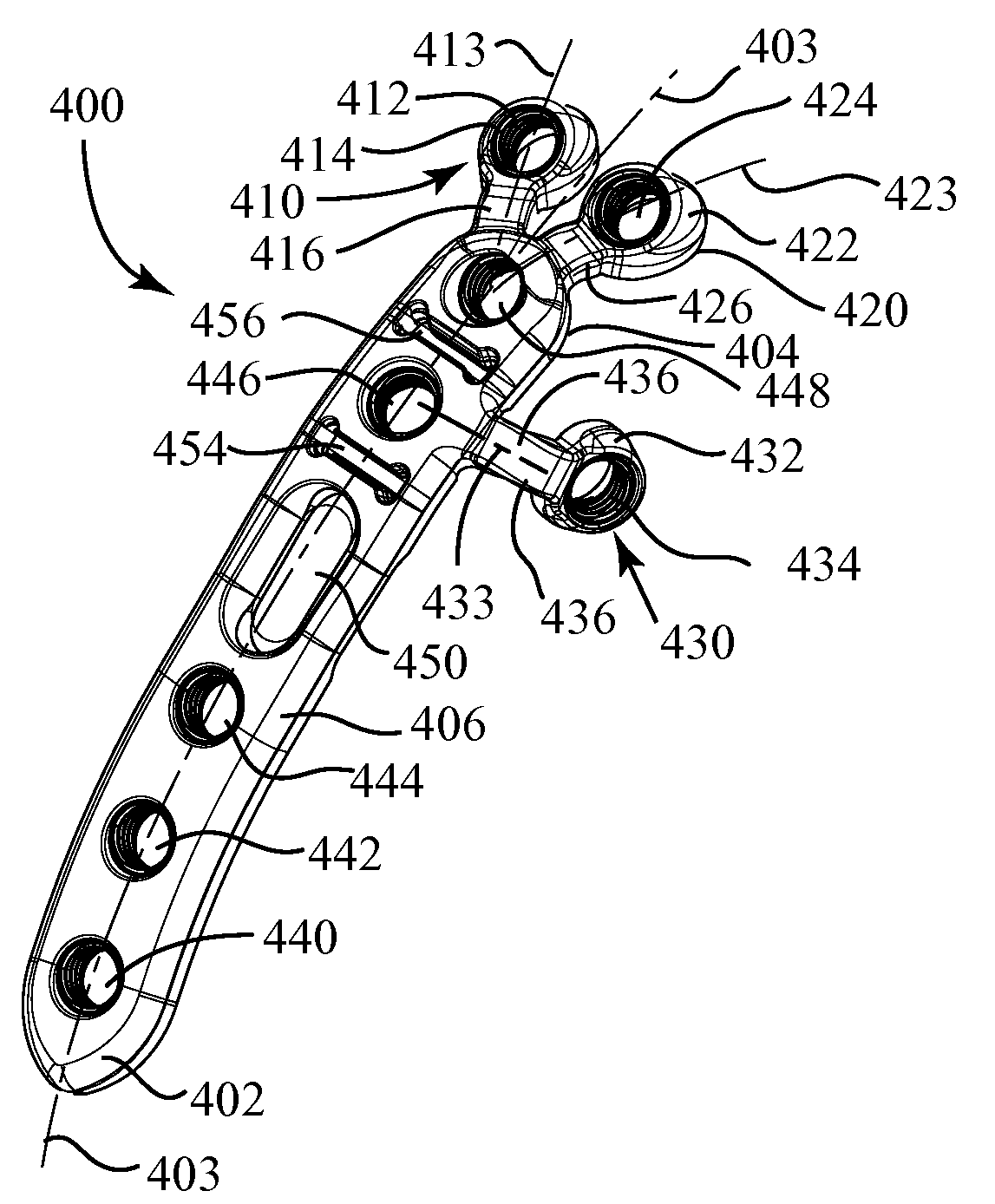
ActiveUS20090118769A1Simple and safe processEasily and safely reconfiguredInternal osteosythesisMetal-working hand toolsProximal radiusInternal fixation
A system for the internal fixation of a fractured bone of an elbow joint of a patient includes at least one bone plate, each bone plate having a plurality of holes and generally configured to fit an anatomical surface of the fractured bone. The at least one plate is adapted to be customized to the shape of a patient's bone. The system also includes a plurality of fasteners including at least one locking fastener for attaching the bone plate to the bone. At least one of the holes is a threaded hole. Guides for plate benders, drills, and / or K-wires can be pre-assembled to the threaded holes, and the locking fastener can lock into any of the threaded holes after the guides are removed.
Owner:BIOMET CV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com