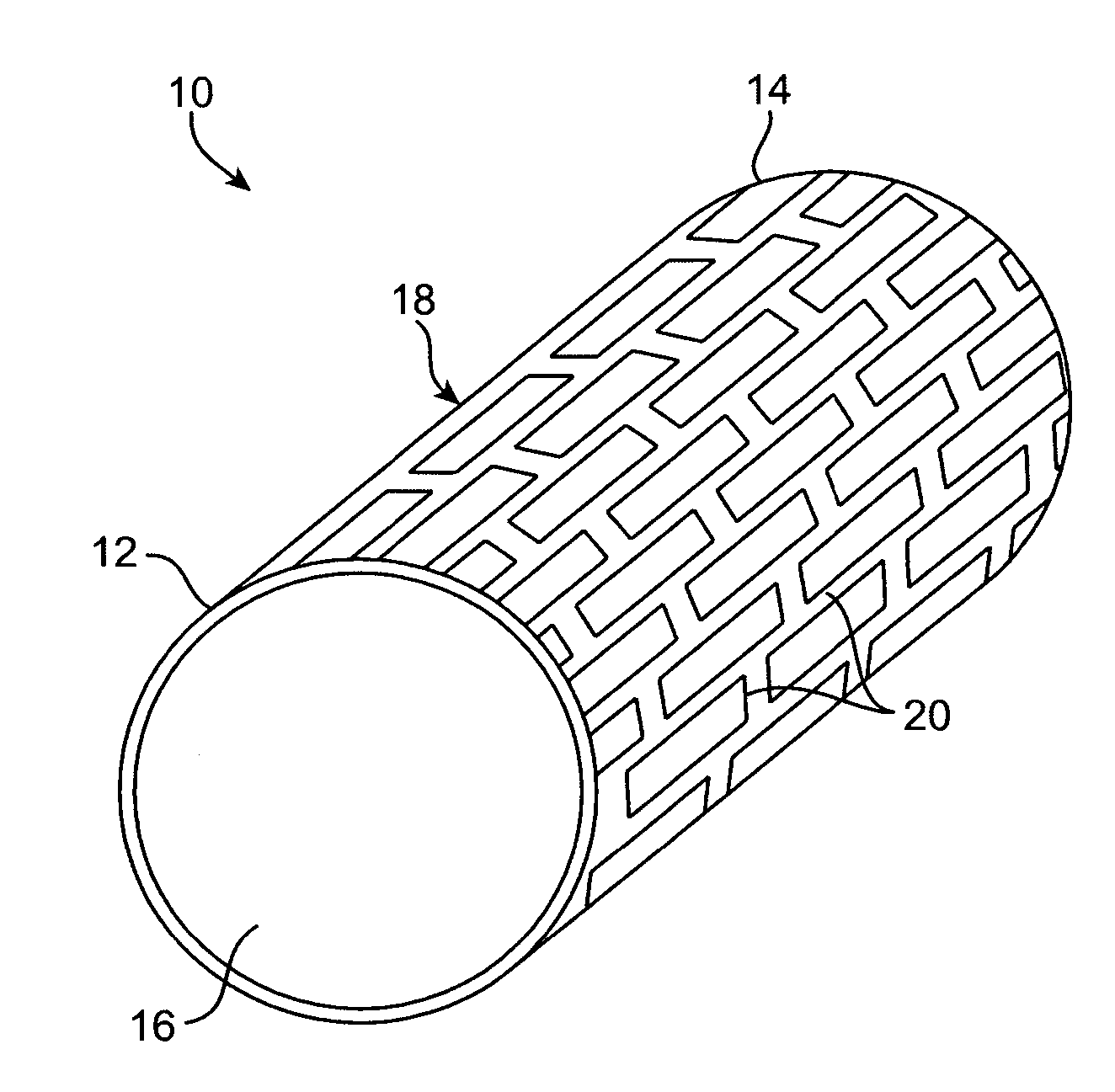
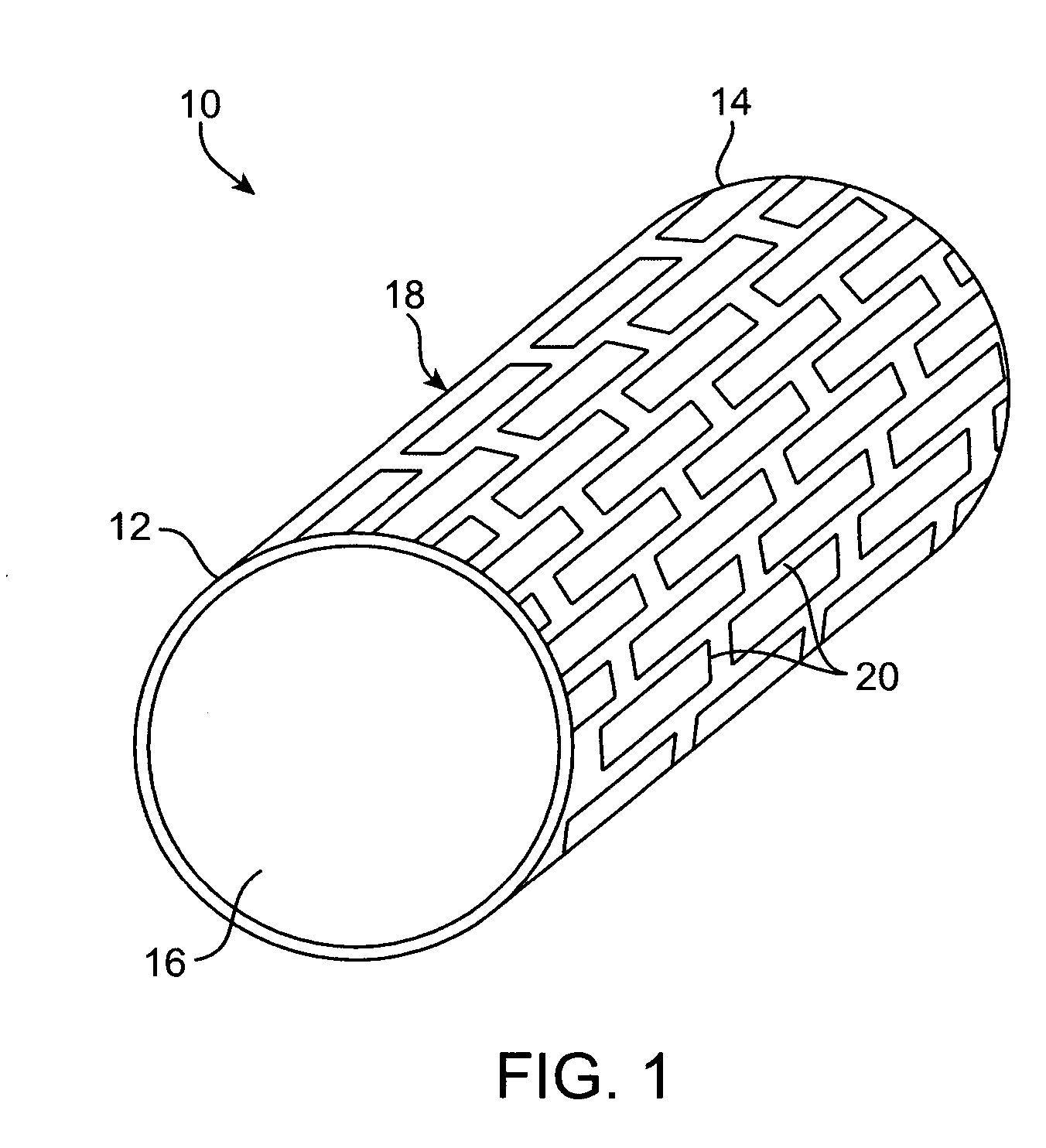
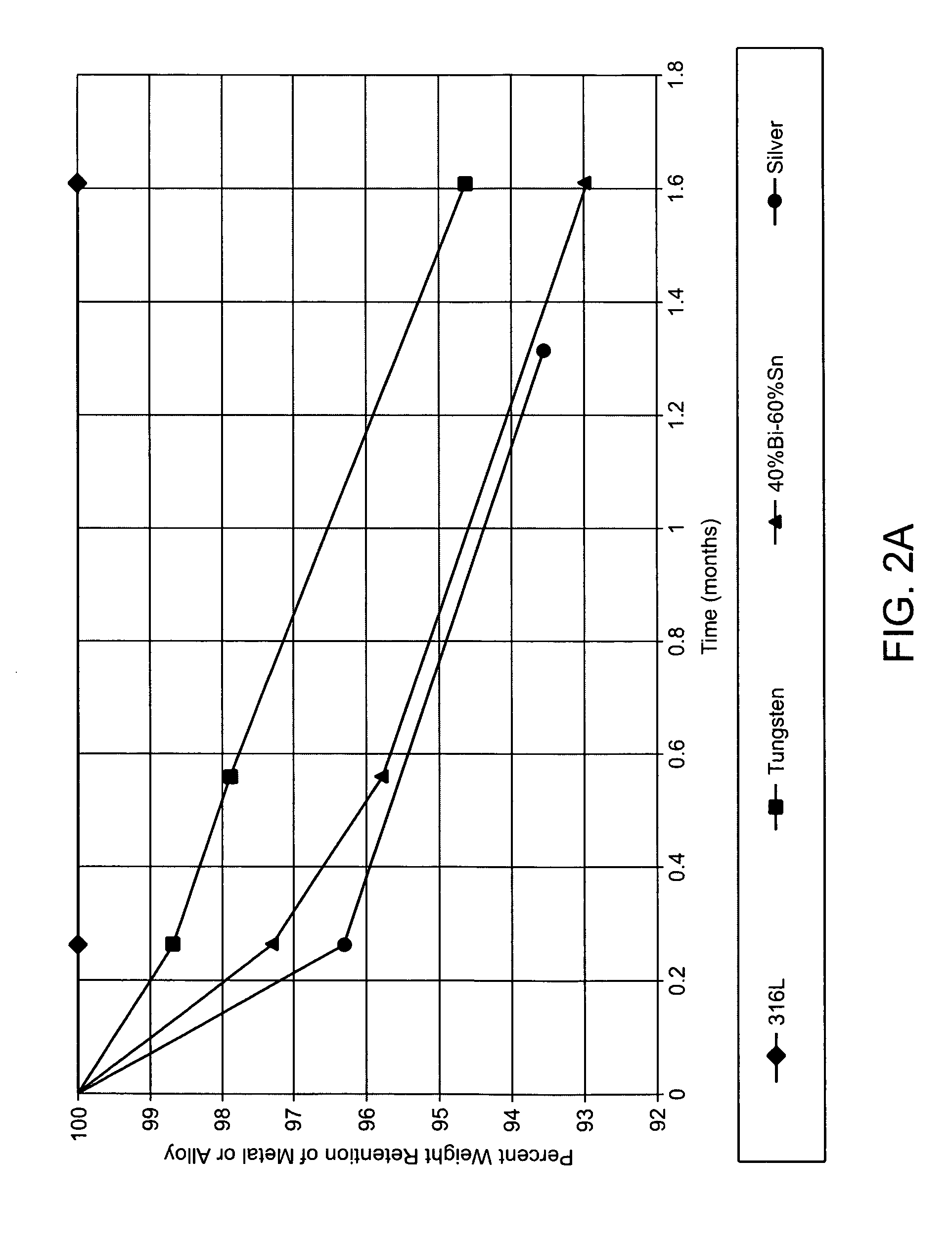
Degradable implantable medical devices
a medical device and implantable technology, applied in the field of medical devices and methods, can solve the problems of reocclusion of the treated lesion, thrombosis or other adverse medical effects, affecting the treatment effect, and increasing the amount of intimal hyperplasia, etc., and achieve the effect of reducing the rate of the implantable structur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0134] A 1.52 mm diameter hole is drilled in the center of a 5 mm diameter cobalt rod by EDM drill or Swiss screw. The rod is then centerless grind to 1.63 mm outer diameter. The resulting cobalt tube is annealed at 850 degrees C. for 1 hr in a vacuum oven. The tube is laser cut into an 18 mm long coronary stent. After the slag and scale are removed by chemical treatment, the stent is placed on a 0.020″ diameter metal mandrel and rotated. The air nozzle of a sandblaster (Econoline of Grand Haven, Mich.) with 360 mesh aluminum oxide abrasive blasting media is directed at the stent and air activated for 10 seconds to create a textured surface on all surfaces of the cobalt stent. The stent is cleaned by dipping in hot water beakers several times followed by dipping in 100% isopropanol beaker. The stent is then placed inside a beaker filled with 100% isopropanol. This beaker is placed in an ultrasonic bath (Branson Ultrasonic Corporation of Danbury Conn.) and the stent is cleaned for 5 ...
example 2
[0136] Before crimping onto a delivery catheter, the coronary stent made in Example 1 is coated with a matrix comprising of 33% Rapamycin and 66% Polyethylene-vinyl alcohol copolymer. After drying, the stent is crimped onto the balloon of a stent delivery catheter. The stent delivery system is sealed inside a pouch and sterilized by EtO.
[0137] The stent delivery system is inserted over a guidewire until the stent is within a lesion in the left circumflex artery. The stent is deployed onto the lesion and the cobalt metal starts to dissolve its mass over a period of time while the drug is released.
example 3
[0138] A coronary stent is made from 1.6 mm OD and 0.6 mm ID tungsten rod. The hole is enlarged to 1 mm by an EDM drill. The rod is then centerless grind to 1.11 mm outer diameter. The resulting tungsten tube is annealed at 1400 degrees C. in a vacuum oven for 1 hour. Slots are cut into the tube with a stamp EDM such that a 25 mm long stent pattern is formed. The non EDM ends are removed. After the slag and scale are removed by chemical treatment, the stent is etched for 6 hours in 45 degree Celsius heated 2N Hydrochloric Acid to induce corrosive pit features on its surface.
[0139] The stent is cleaned by dipping in hot water beakers several times followed by dipping in 100% ethanol beaker. The stent is then placed inside a beaker filled with 100% ethanol. This beaker is placed in an ultrasonic bath (Branson Ultrasonic Corporation of Danbury Conn.) and the stent is cleaned for 5 minutes in the bath. After drying, the stent is crimped onto the balloon of a 3.5×27 mm stent delivery ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface roughness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com