Patents
Literature
120 results about "Coronary stent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
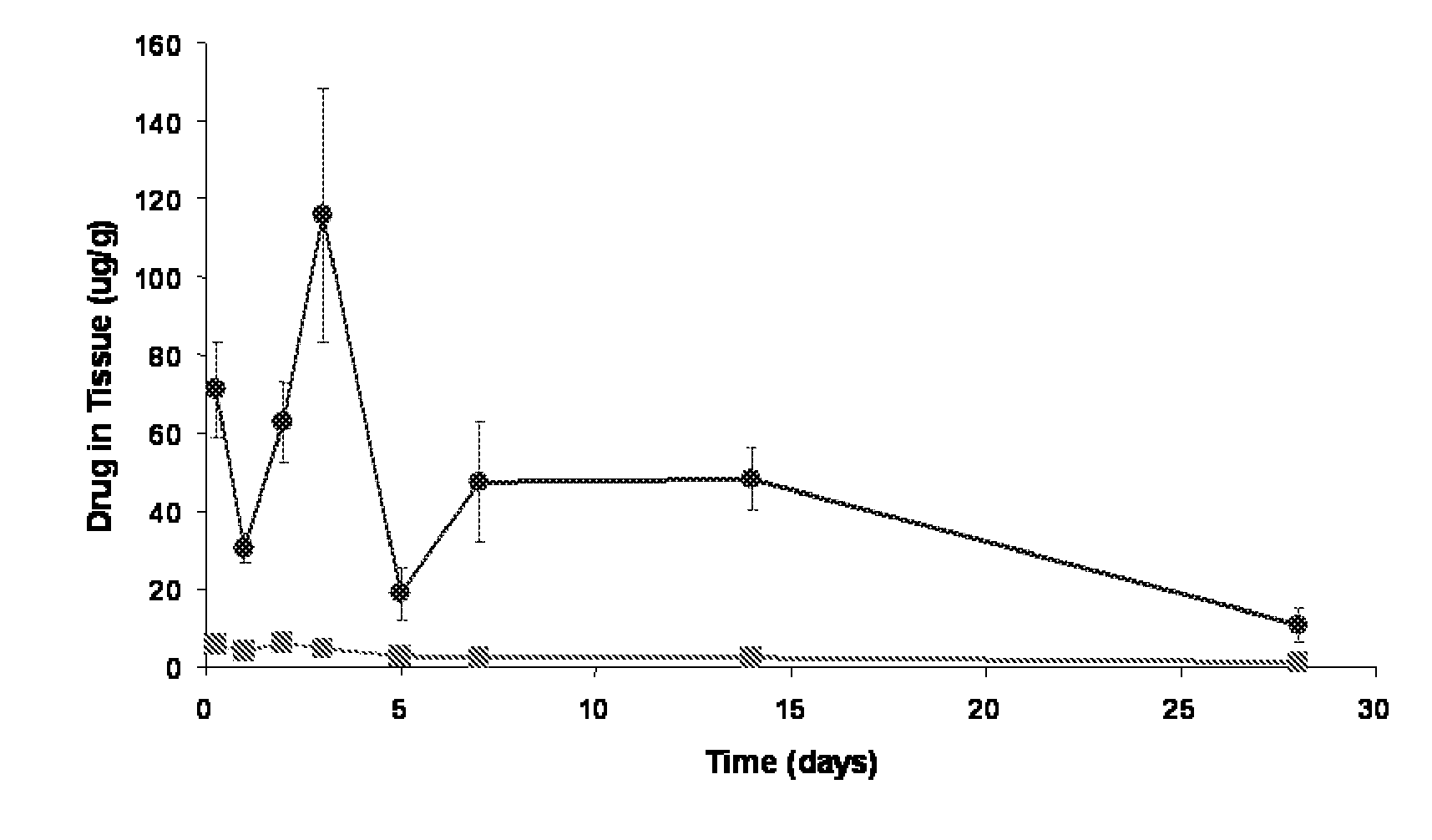
A coronary stent is a tube-shaped device placed in the coronary arteries that supply blood to the heart, to keep the arteries open in the treatment of coronary heart disease. It is used in a procedure called percutaneous coronary intervention (PCI). Coronary stents are now used in more than 90% of PCI procedures. Stents reduce angina (chest pain) and have been shown to improve survivability and decrease adverse events in an acute myocardial infarction.
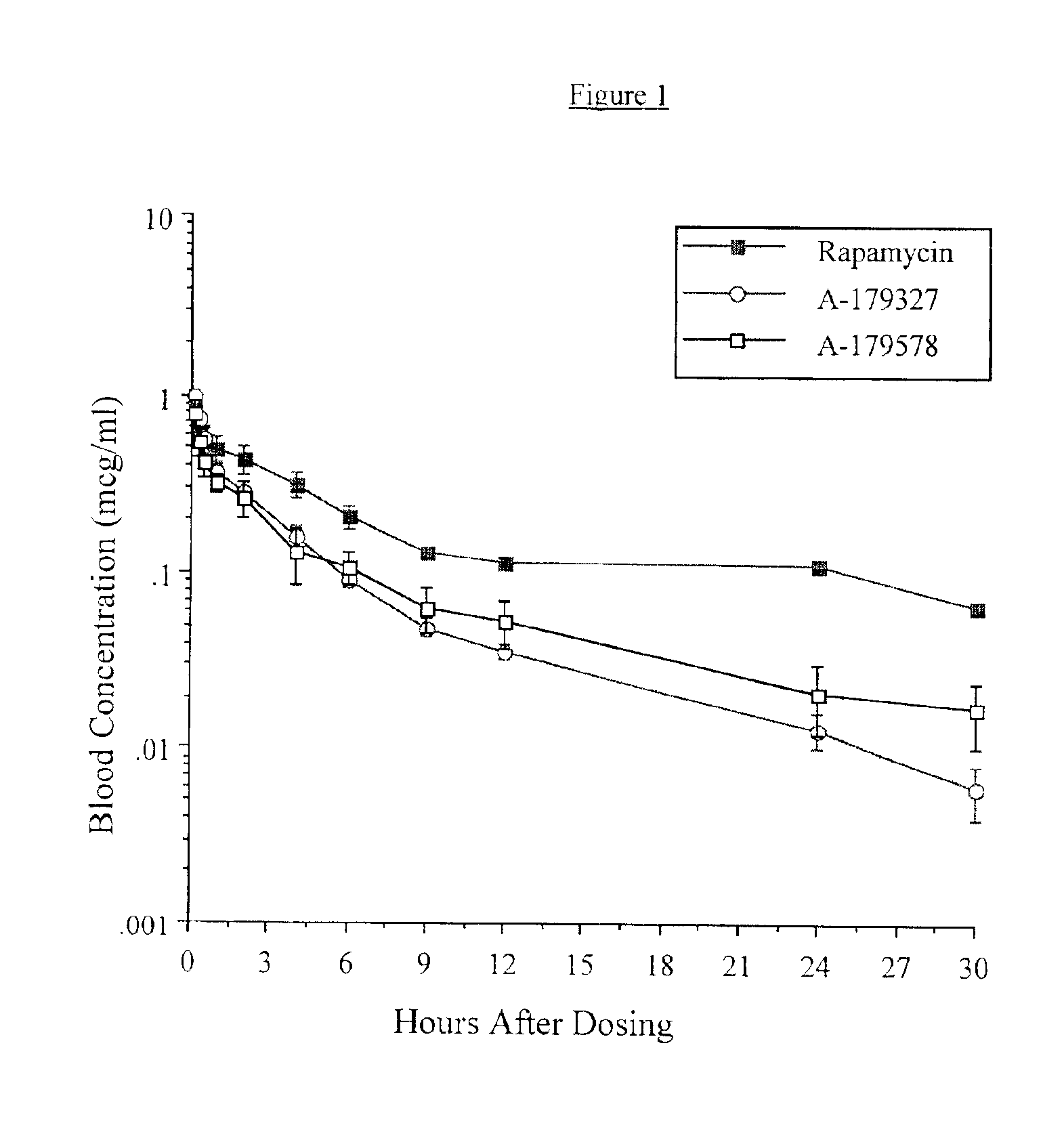
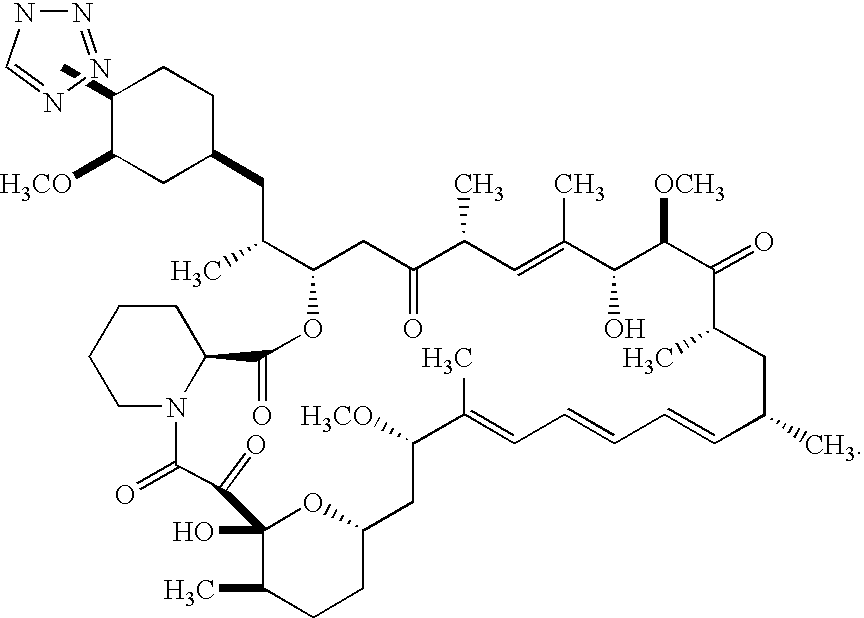
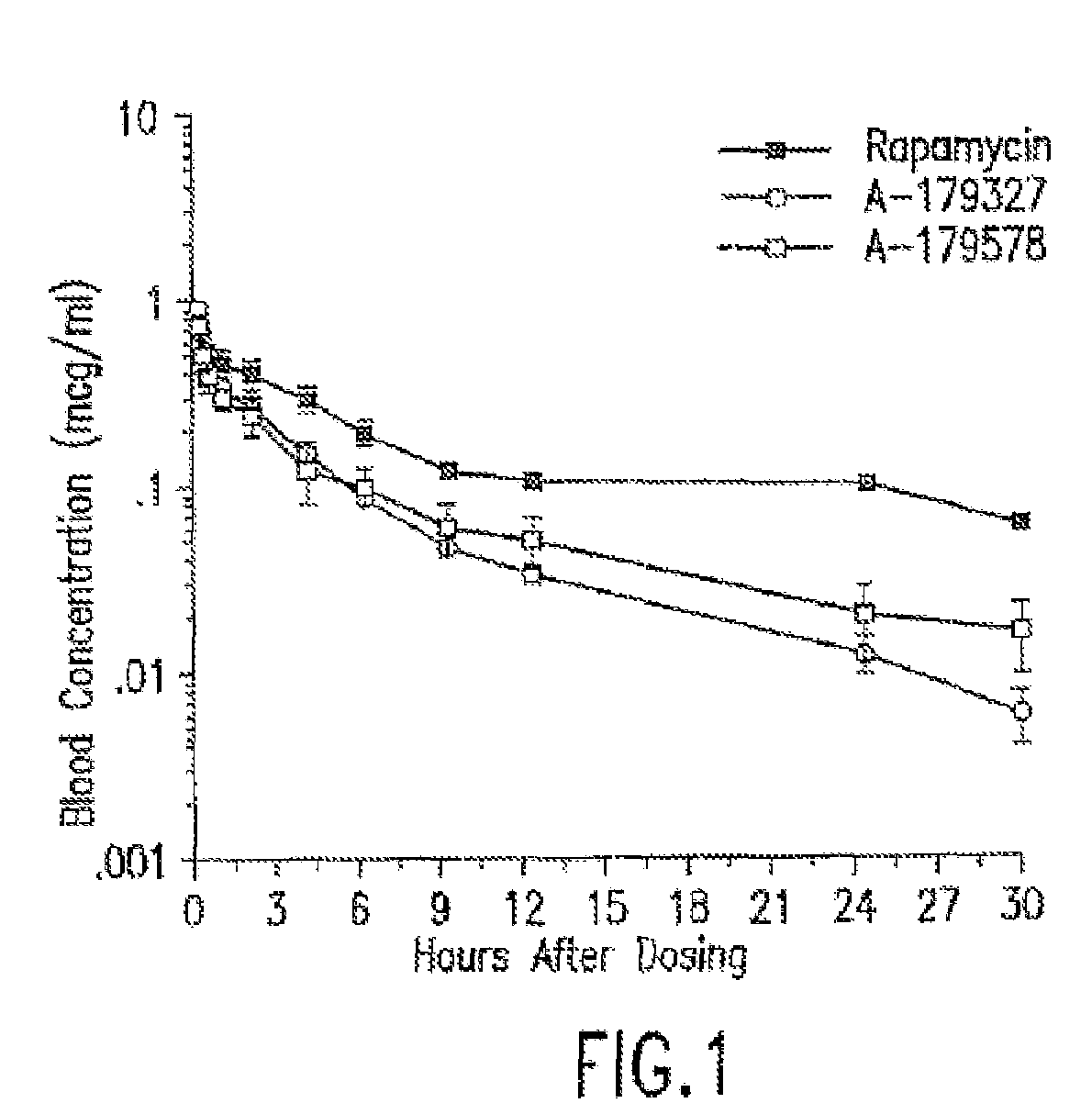
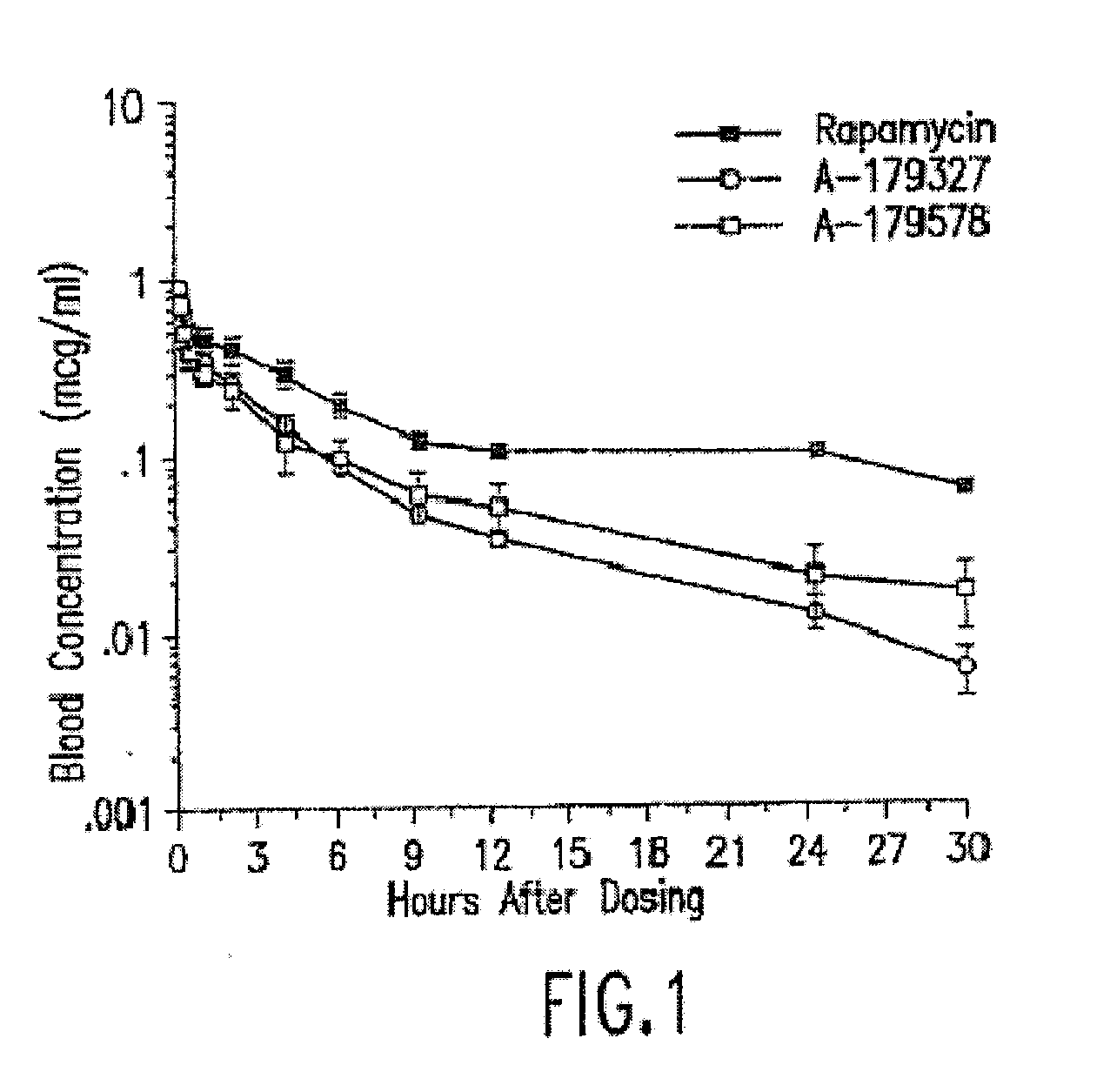
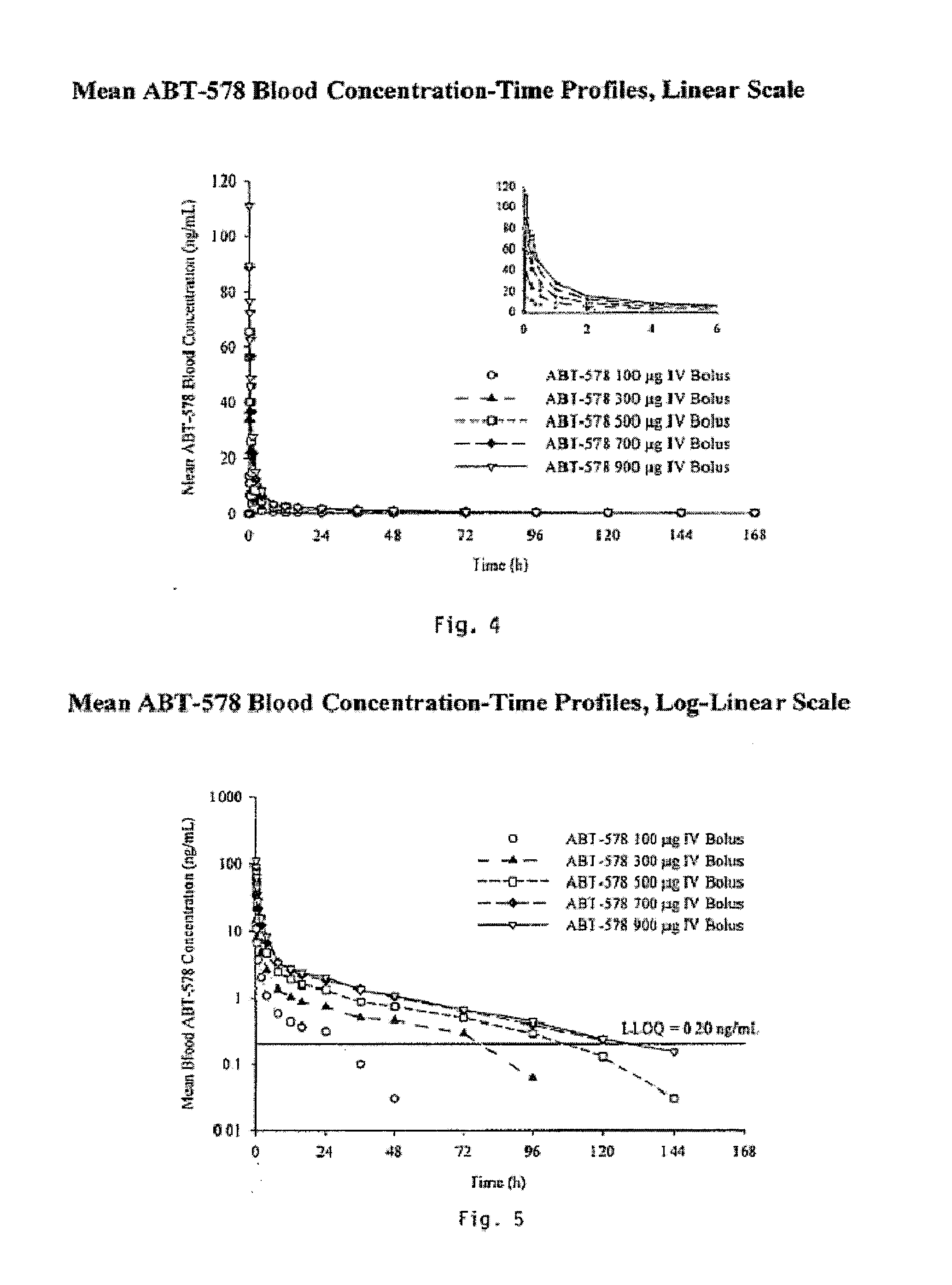
Medical devices containing rapamycin analogs
InactiveUS6890546B2Reduce probabilityReduces restenosis in vasculatureBiocideAntimycoticsArteriovenous graftsMedical treatment
A medical device comprising a supporting structure having a coating on the surface thereof, the coating containing a therapeutic substance, such as, for example, a drug. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to,
Owner:ABBOTT LAB INC
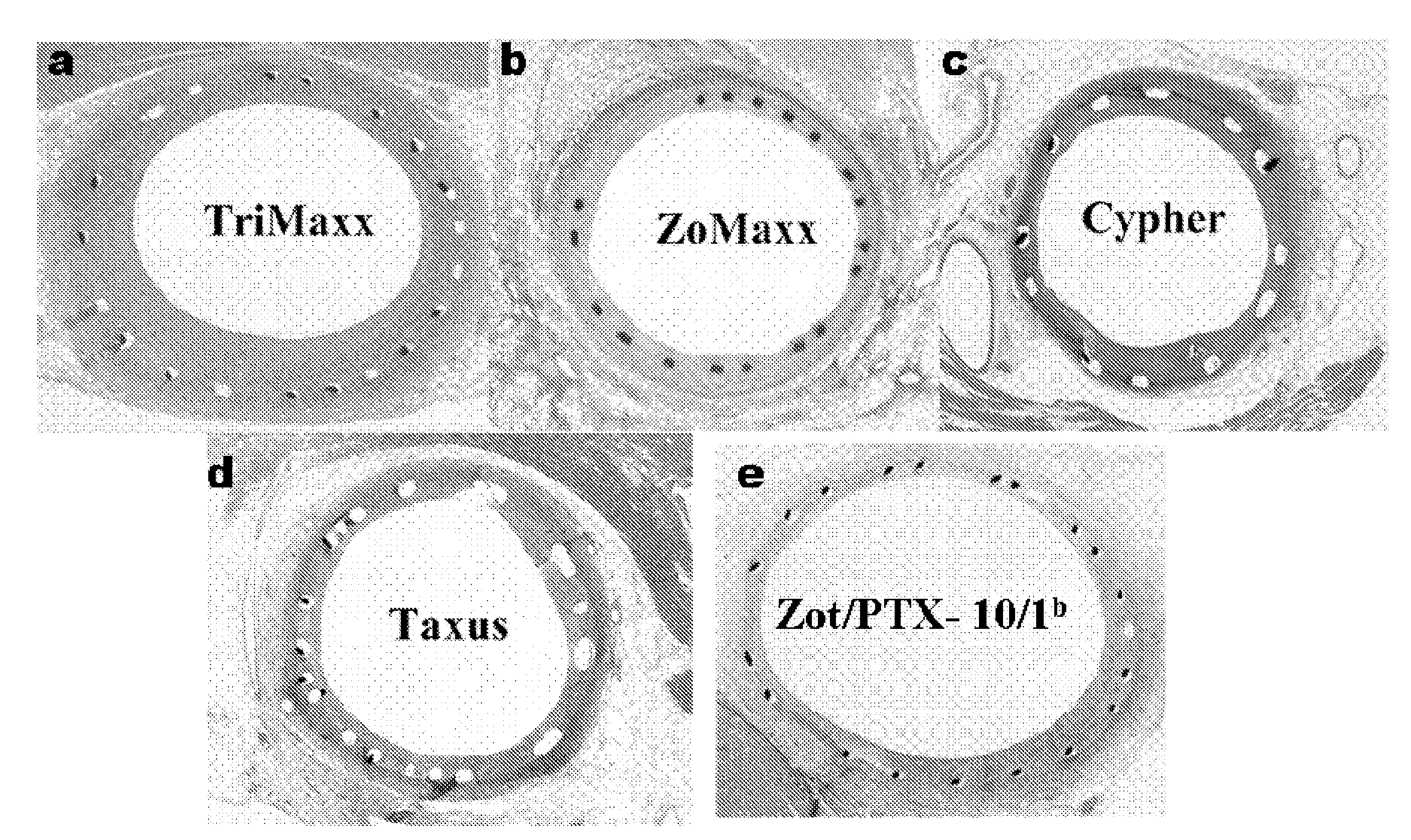
Drug delivery systems, kits, and methods for administering zotarolimus and paclitaxel to blood vessel lumens
A system and compositions including zotarolimus and paclitaxel are disclosed, as well as methods of delivery, wherein the drugs have effects that complement each other. Medical devices are disclosed which include supporting structures that include at least one pharmaceutically acceptable carrier or excipient, which carrier or excipient can include one or more therapeutic agents or substances, with the carrier including at least one coating on the surface thereof, and the coating associated with the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
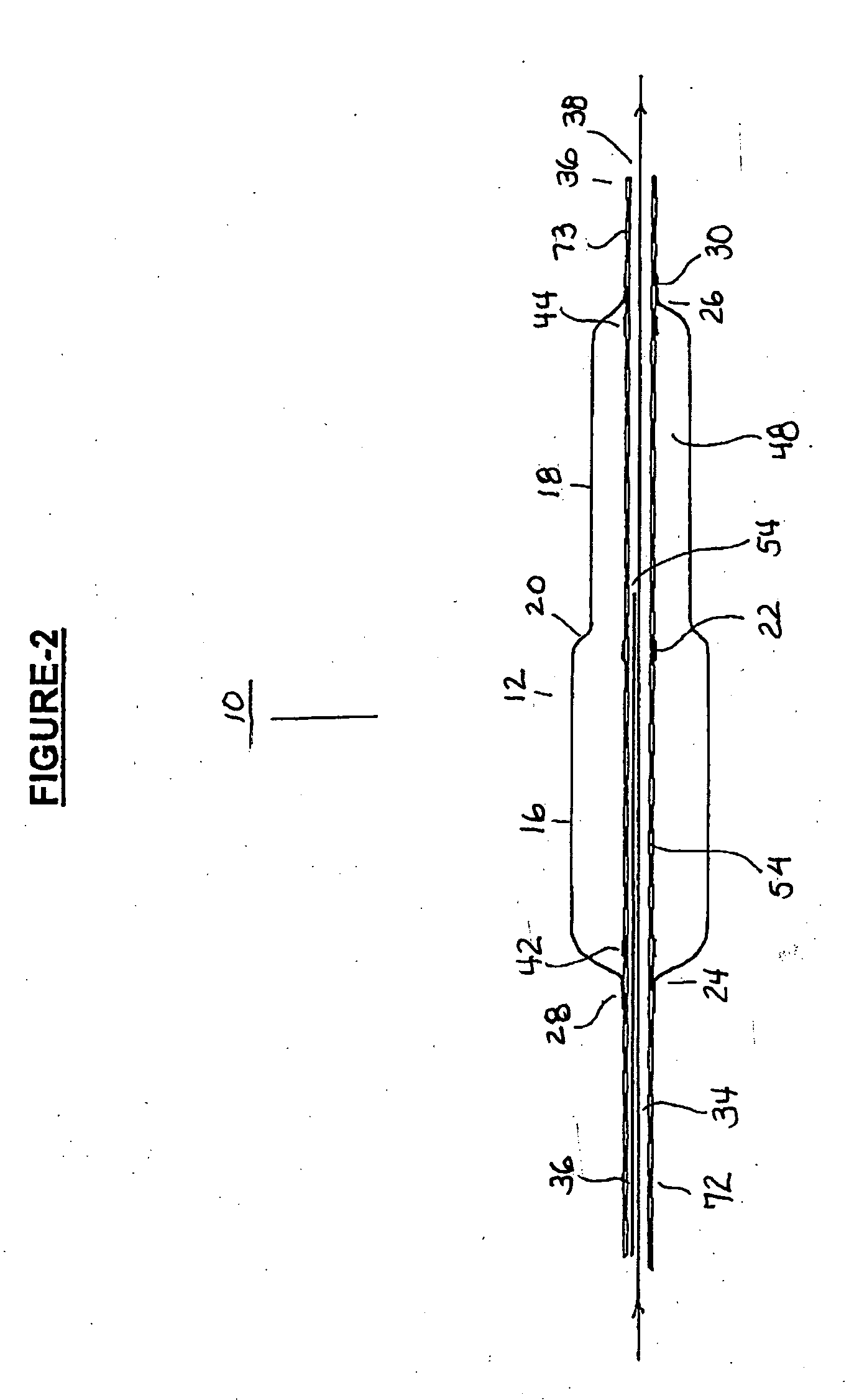
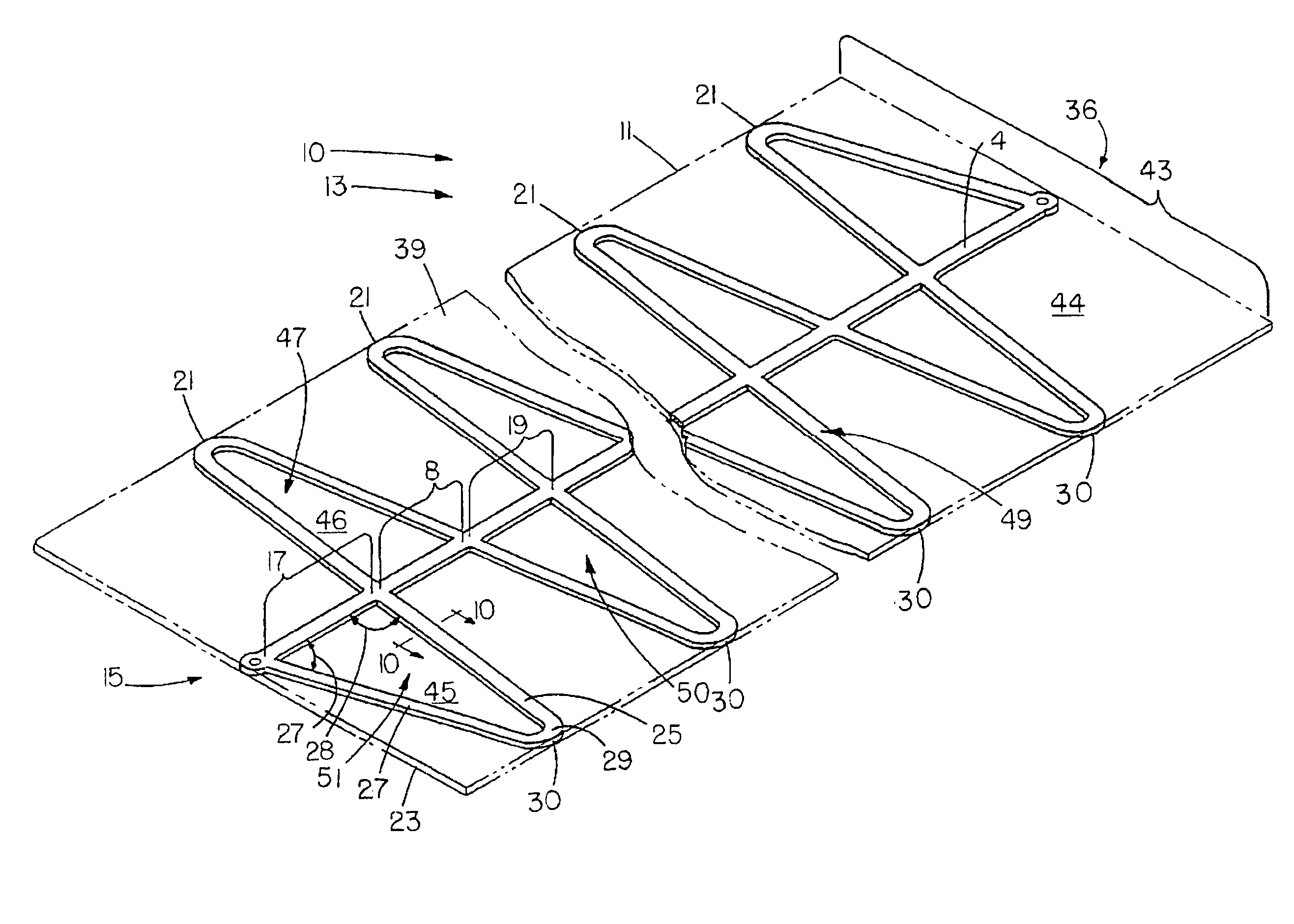
Coronary stent
In an embodiment, a stent is provided for use in blood vessels with blockage near or in a bifurcation. The stent includes a side aperture. The stent is inserted into one of the daughter branches of the bifurcation and positioned with the use of markers. The stent is then expanded so as to support the wall of the blood vessel while allowing the blood to continue to flow to both daughter branches.
Owner:RBKPARK
Degradable coronary stent and manufacturing method thereof
InactiveCN102228721AImprove hydrogen evolution corrosion rateIncreased corrosion/degradation rateStentsSurgeryMedicineBiocompatibility
The invention provides a degradable coronary stent, which is characterized in that an iron-based material is taken as a matrix, and the surface of the matrix is covered with a degradable macromolecule coating; and components in the degradable macromolecule coating contain ester bonds (-COO-). The manufacturing method comprises the following concrete steps: a macromolecule material containing the ester bonds is dissolved in an organic solvent; and a dip-coating or spray-coating method is utilized to coat the mixture on the surface of an iron-based alloyed matrix, wherein the coating thickness is 1-40mu m. The degradable coronary stent is used for enhancing the degradation / corrosion speed of the iron-based coronary stent and improving the biocompatibility of the iron-based alloy, and is beneficial to rapid endothelialisation of endothelial cells on the surface of the stent. The method is simple and is convention to operate.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
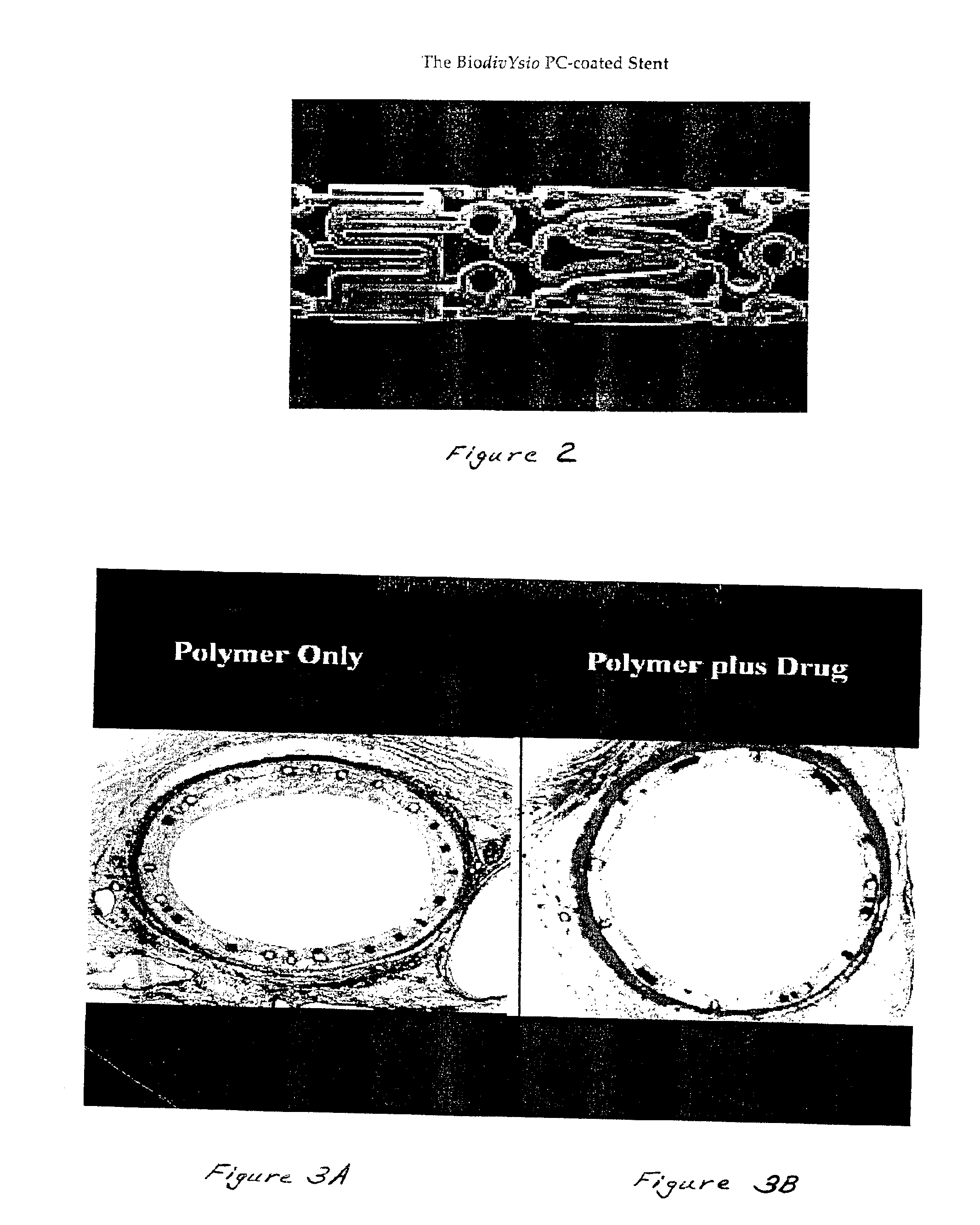
Coated implantable medical device
InactiveUS20070050010A1Prevent degradationIncrease release rateStentsIn-vivo radioactive preparationsParyleneGas phase
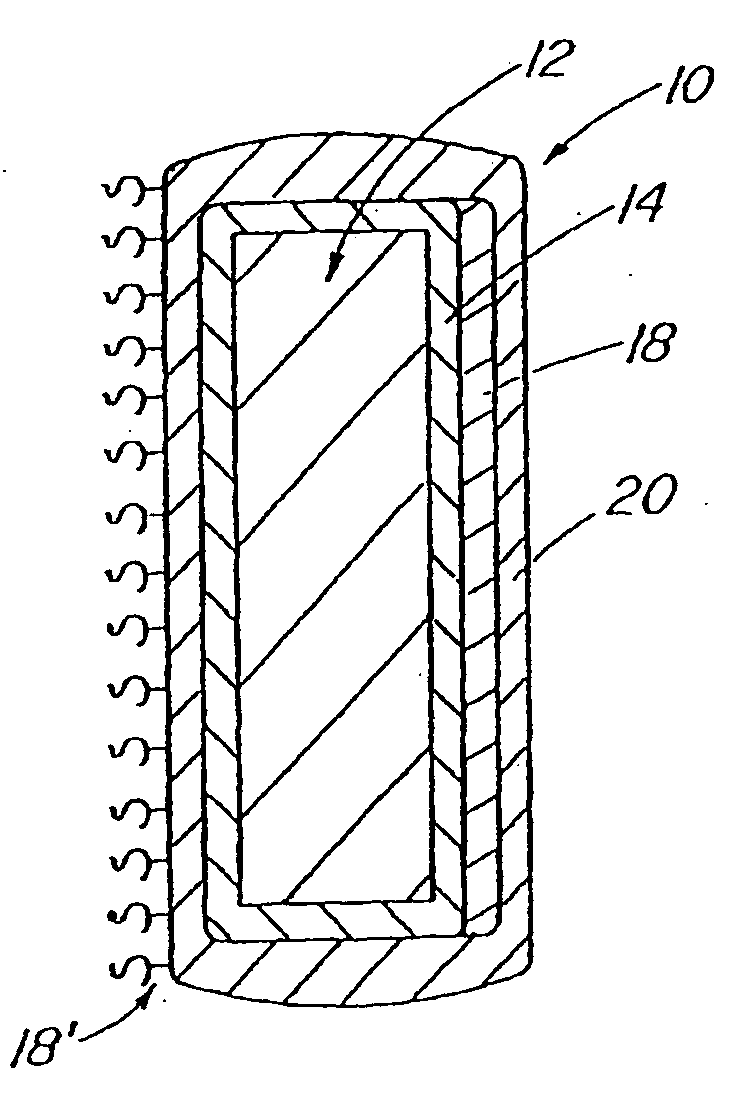
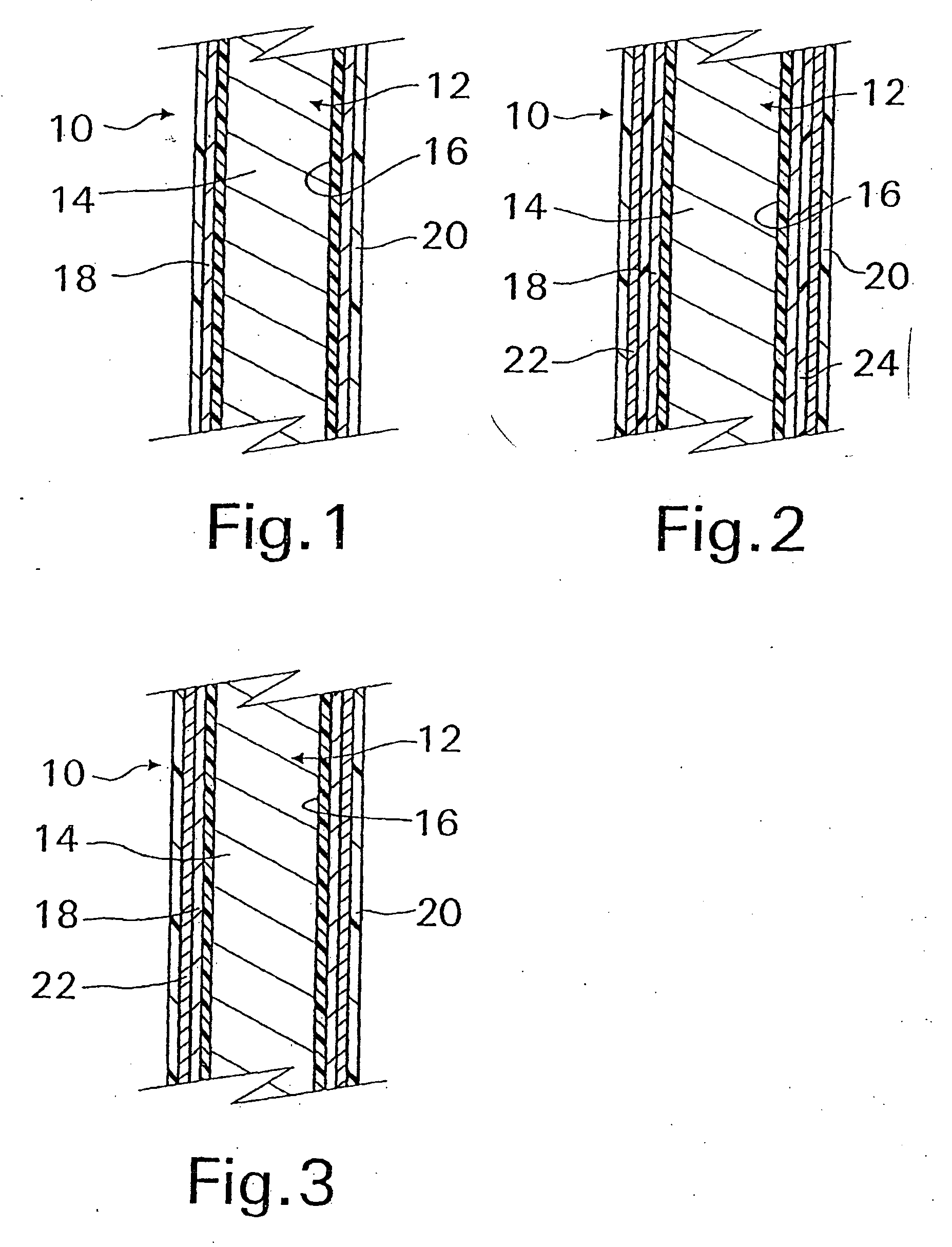
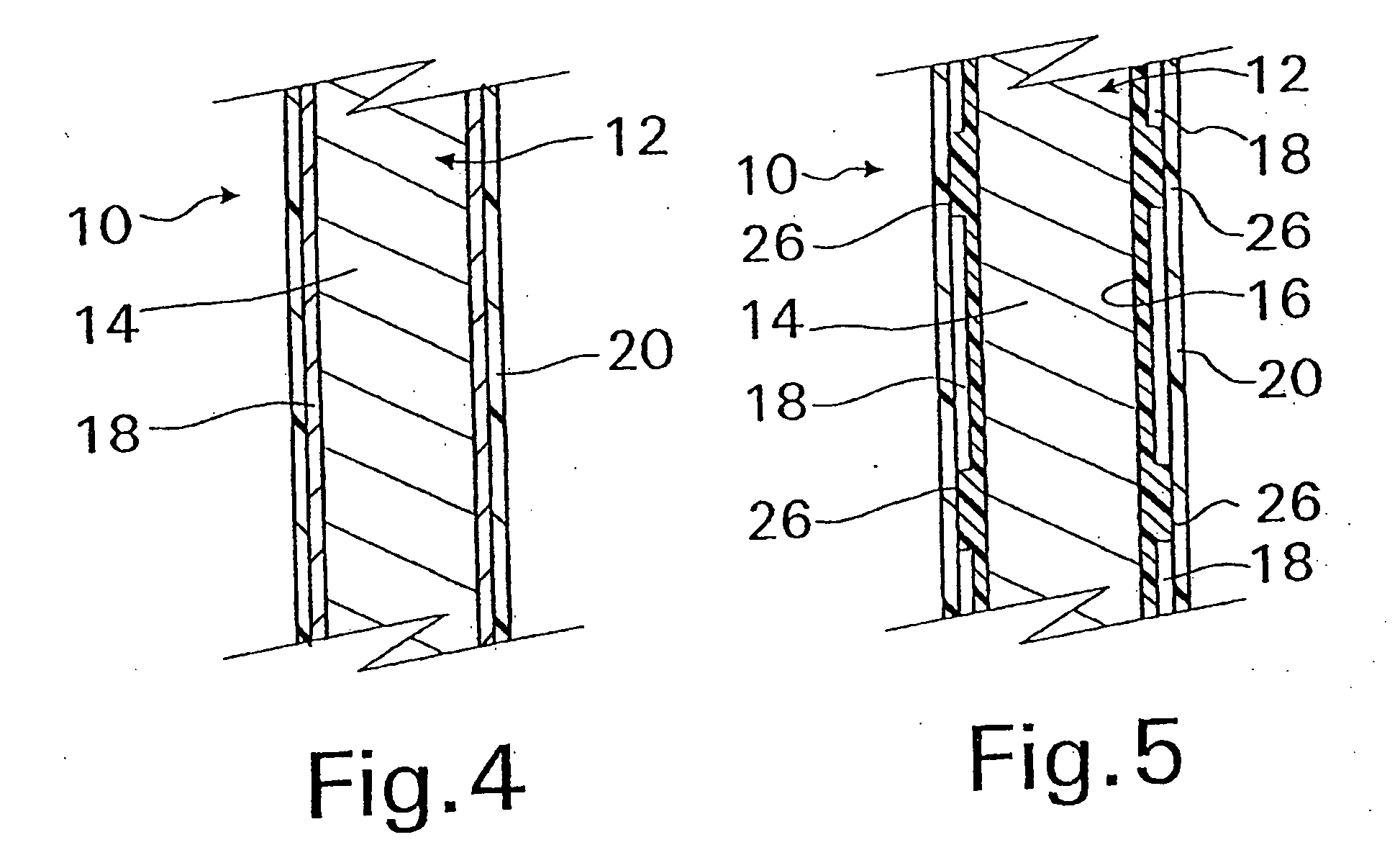
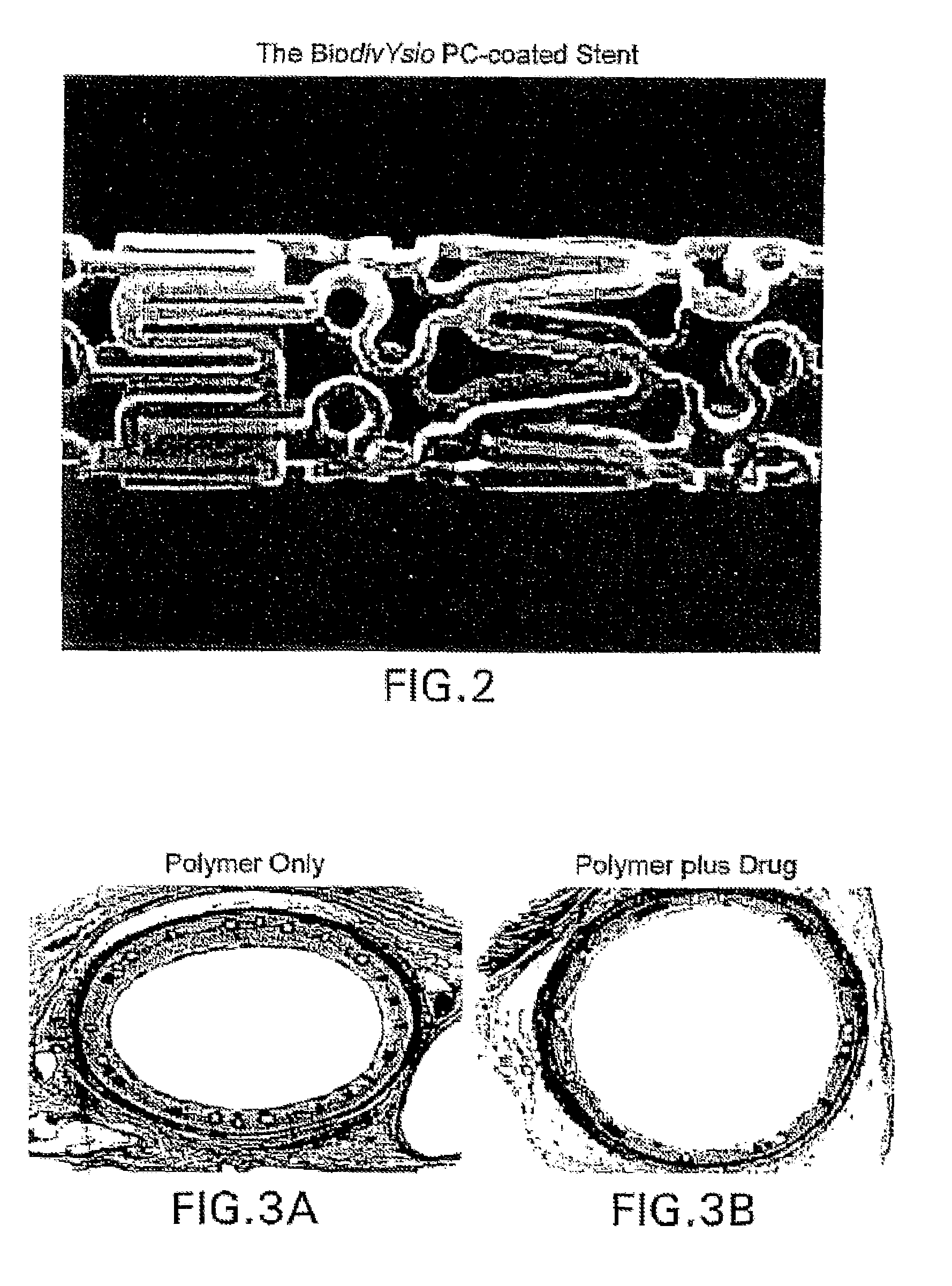
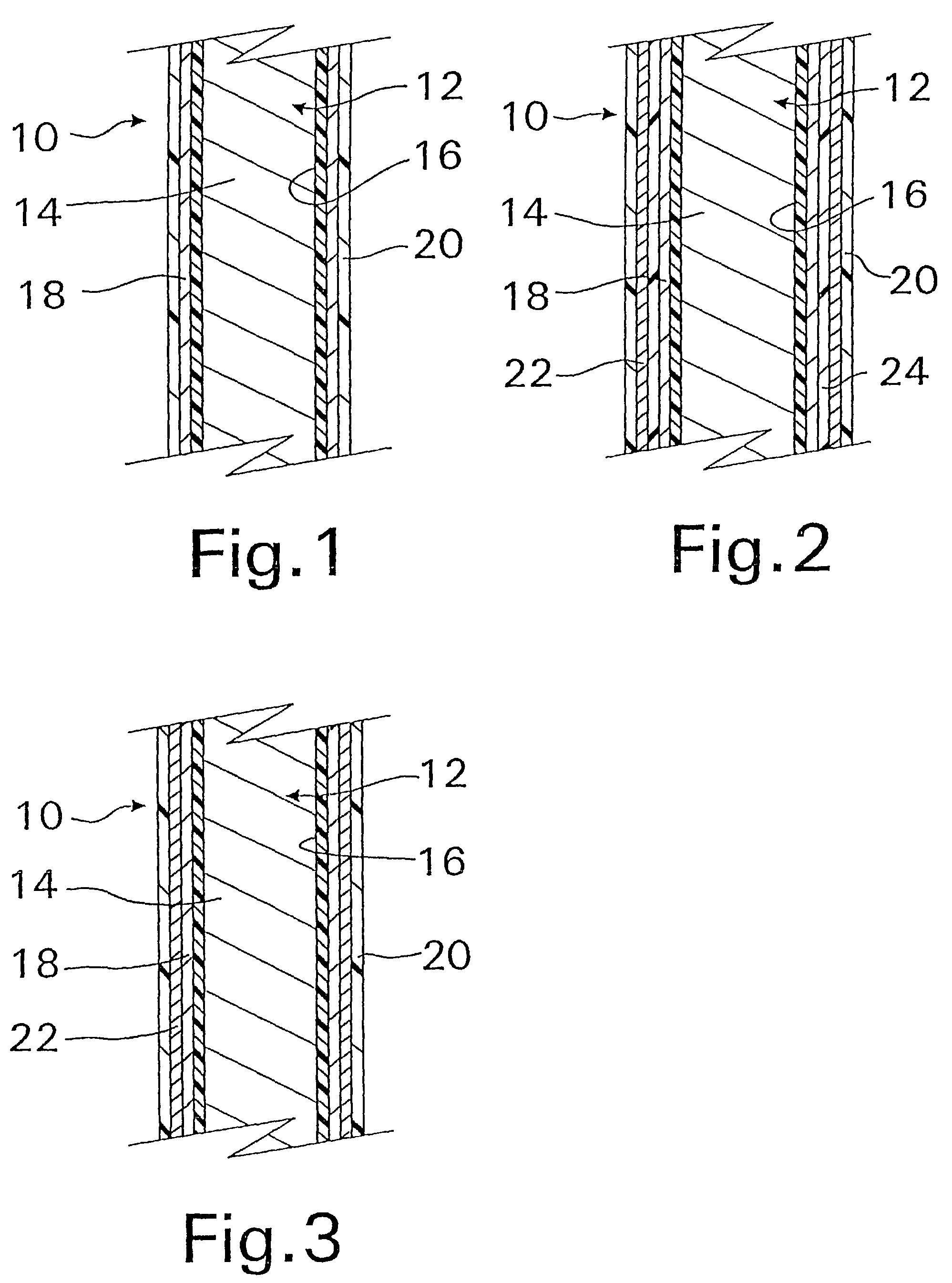
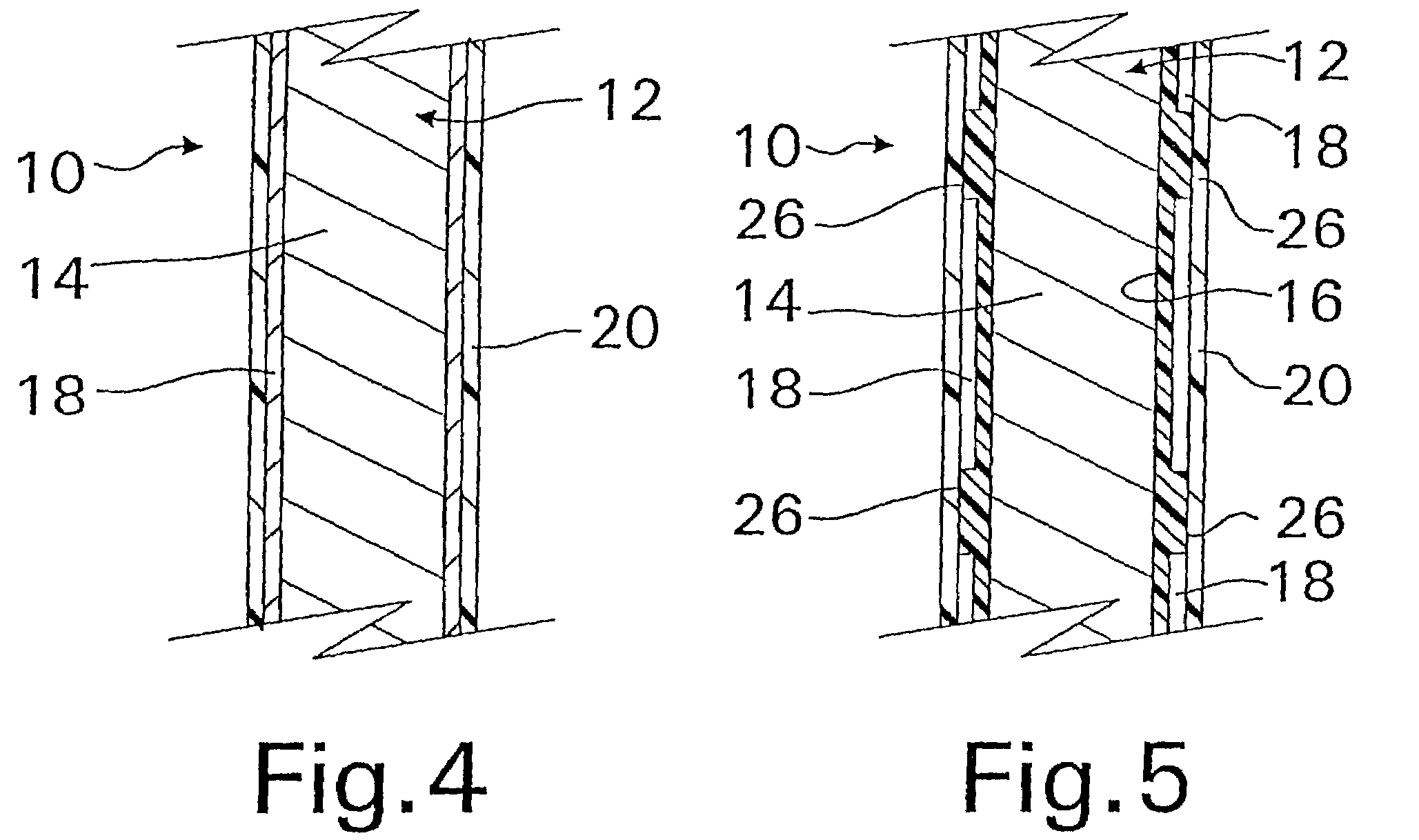
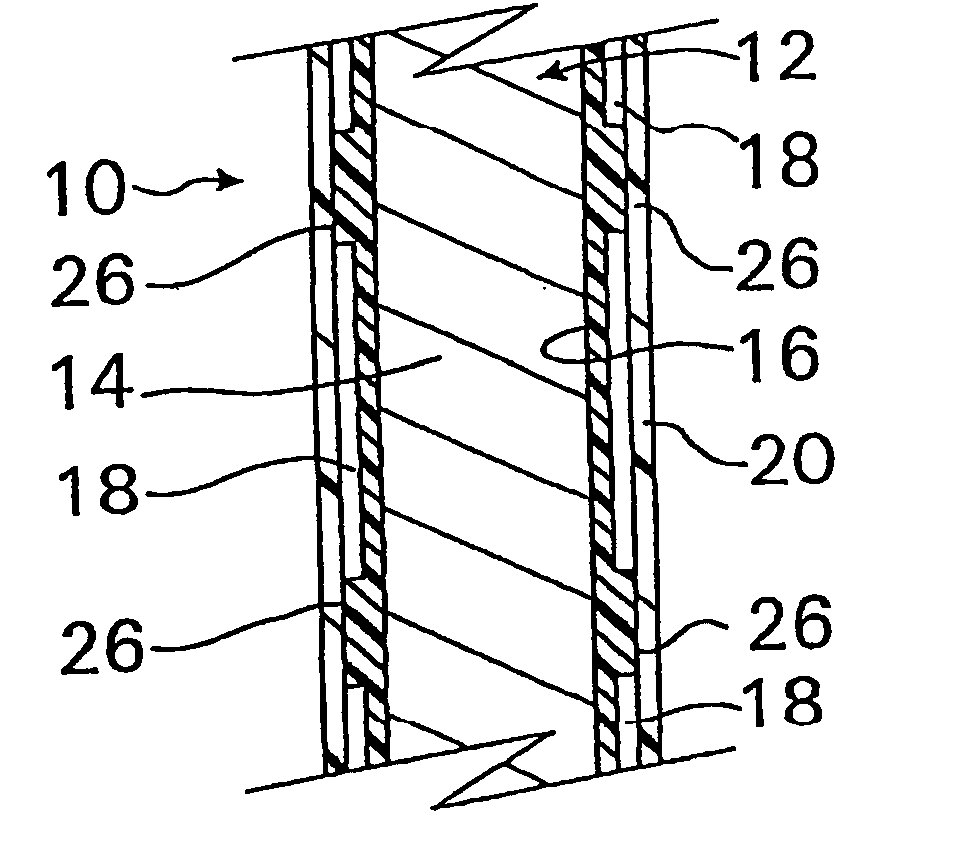
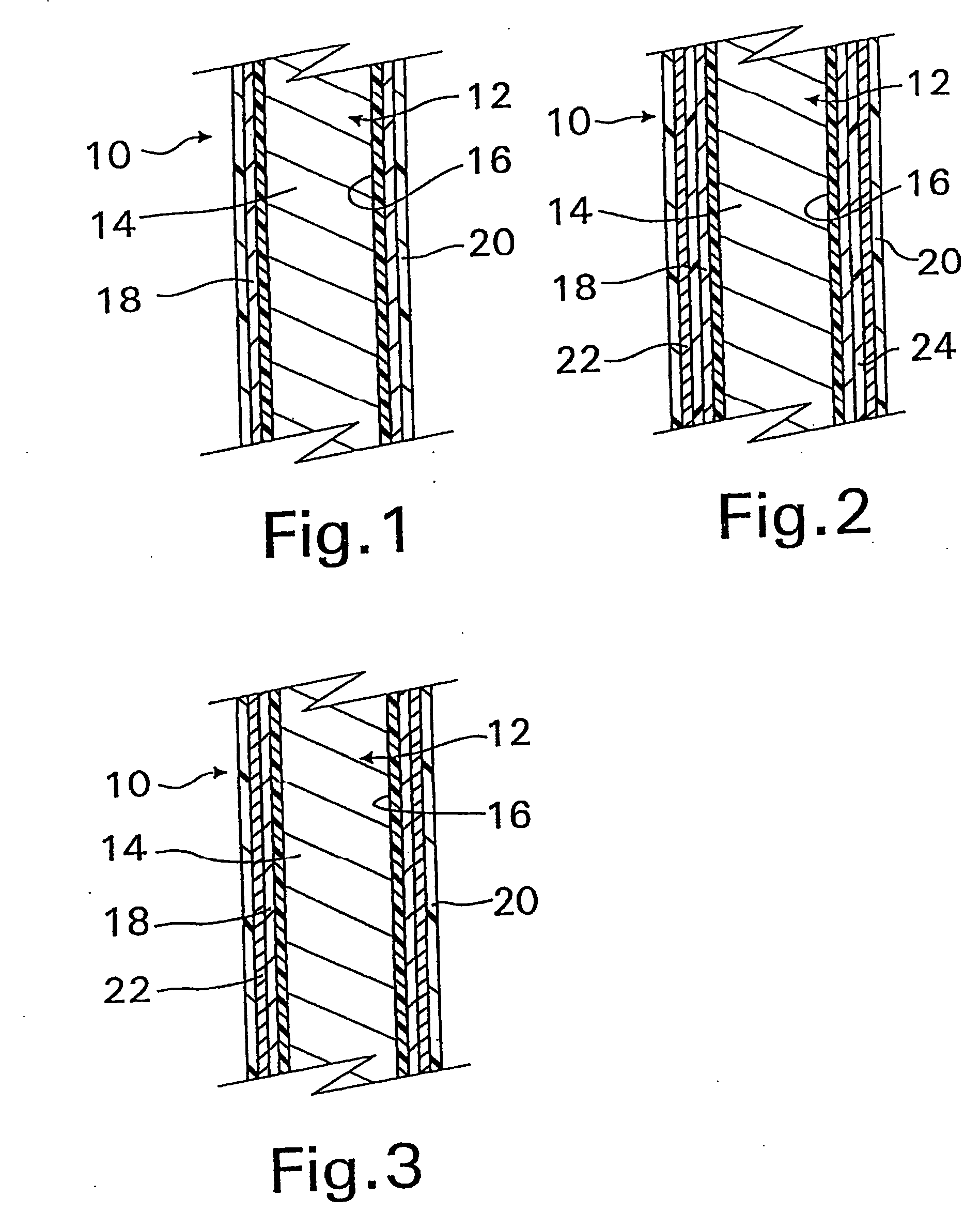
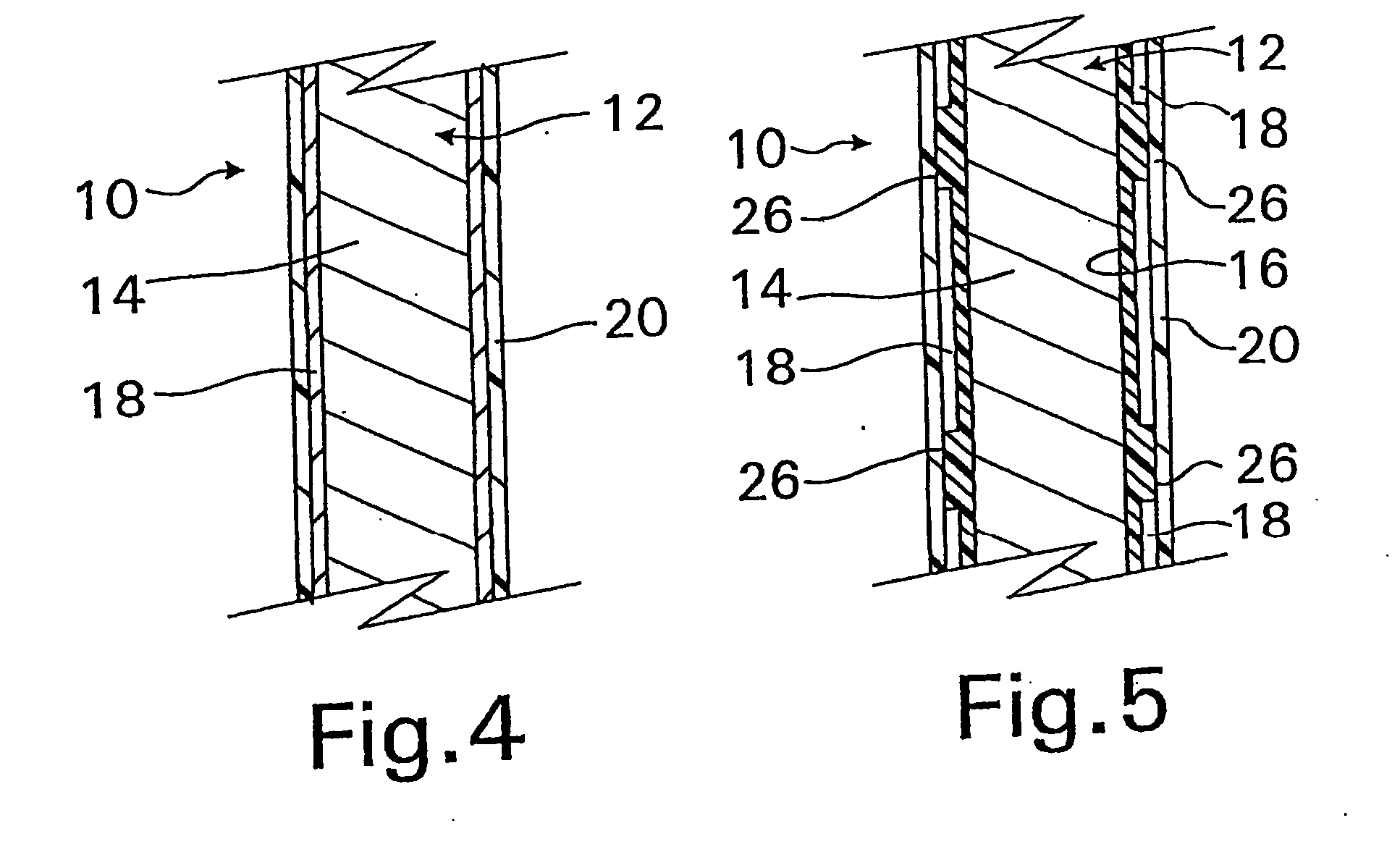
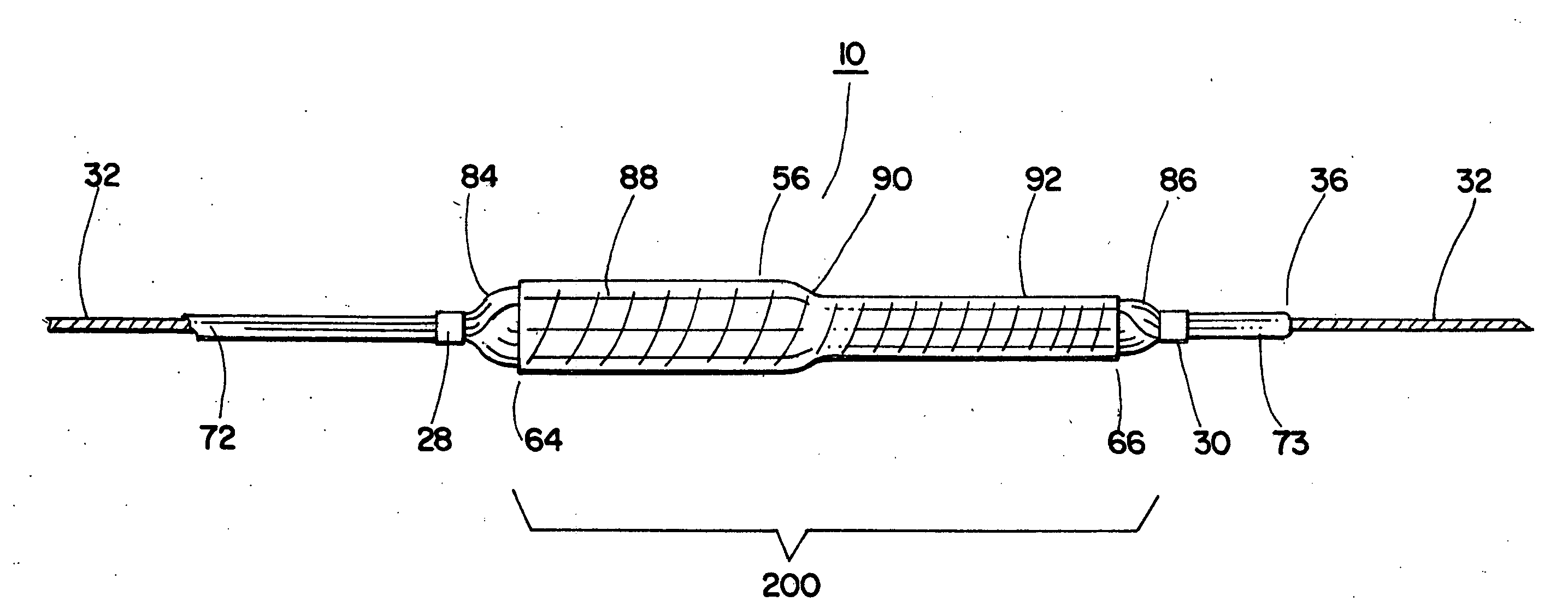
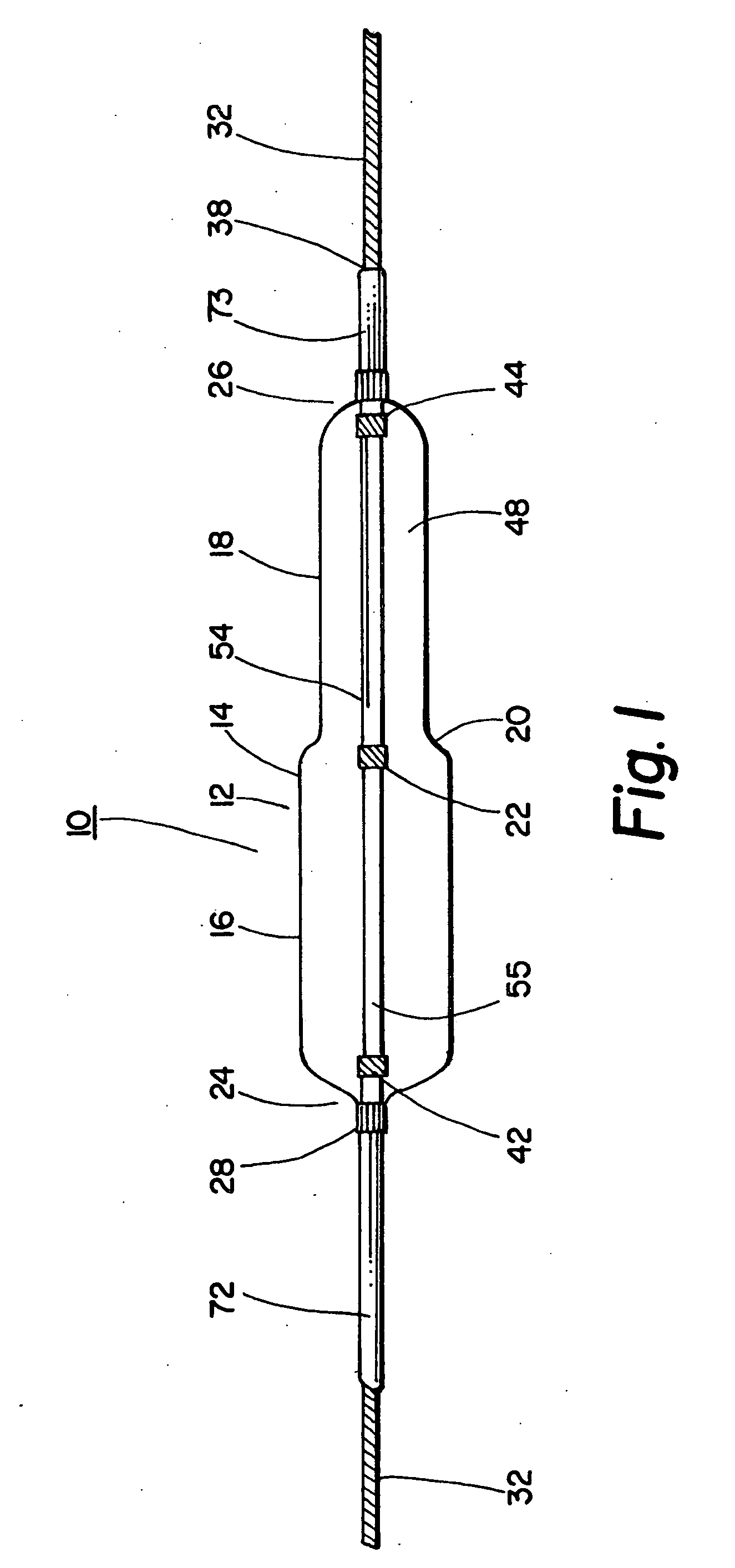
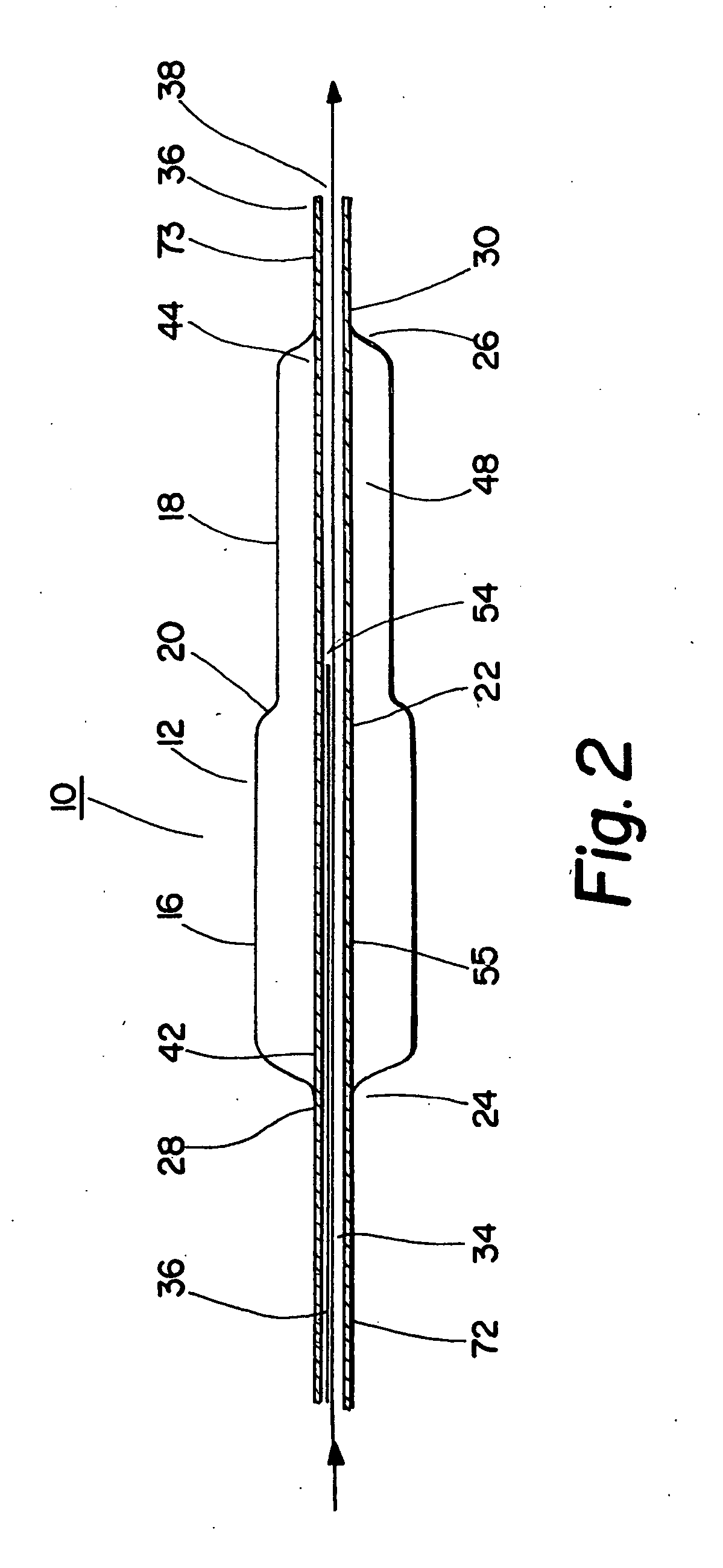
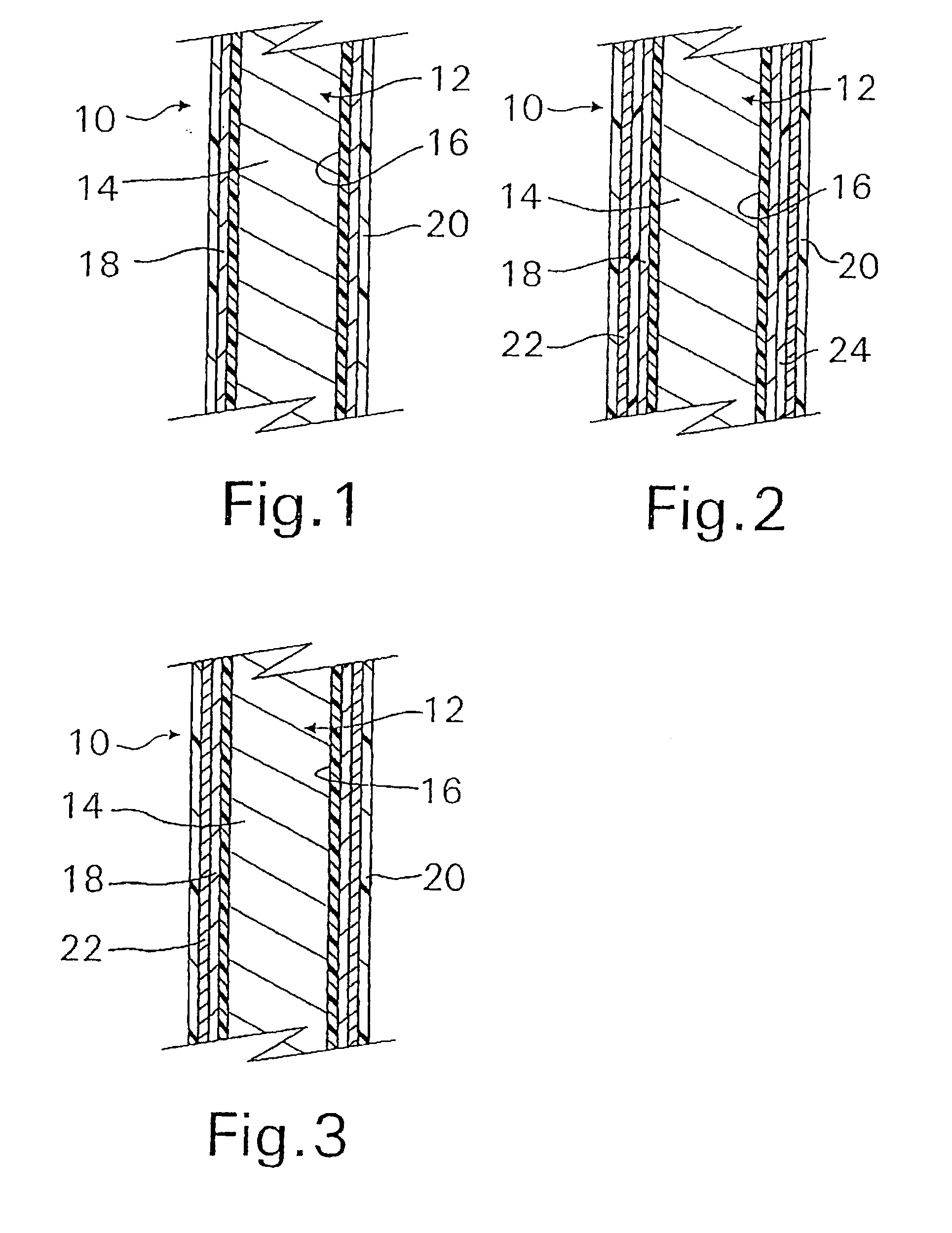
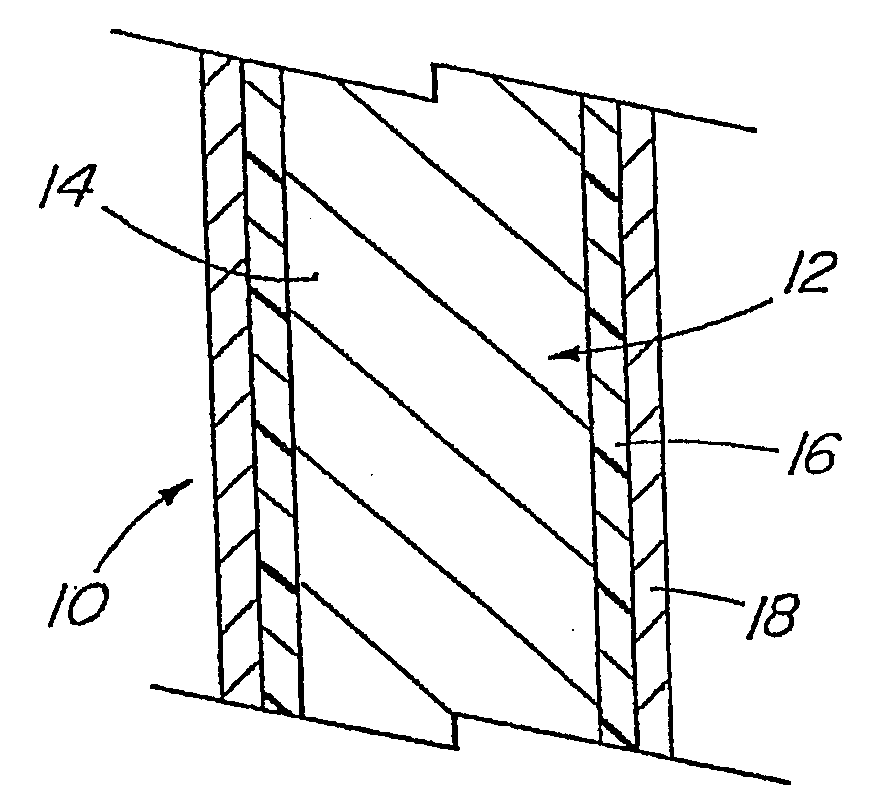
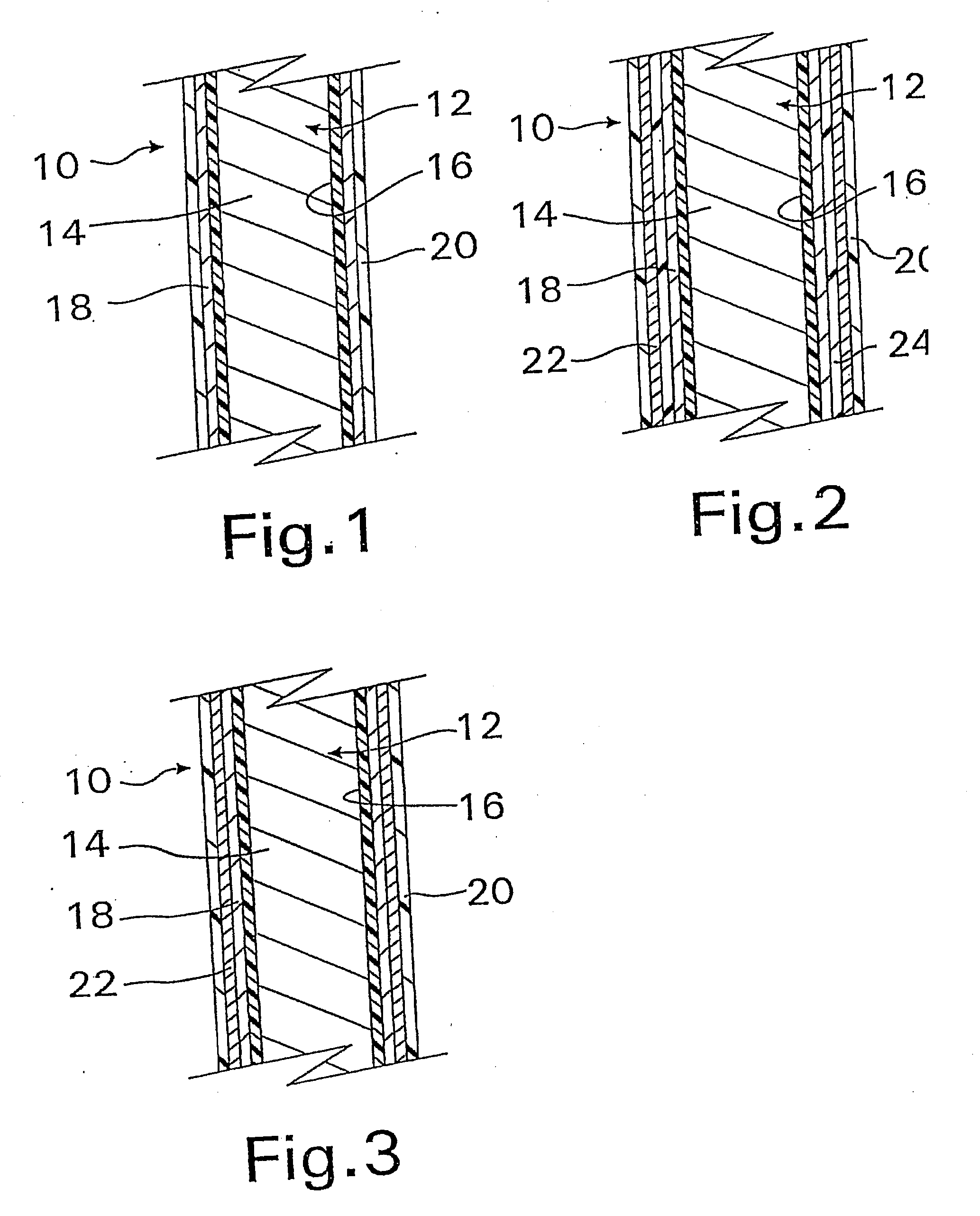
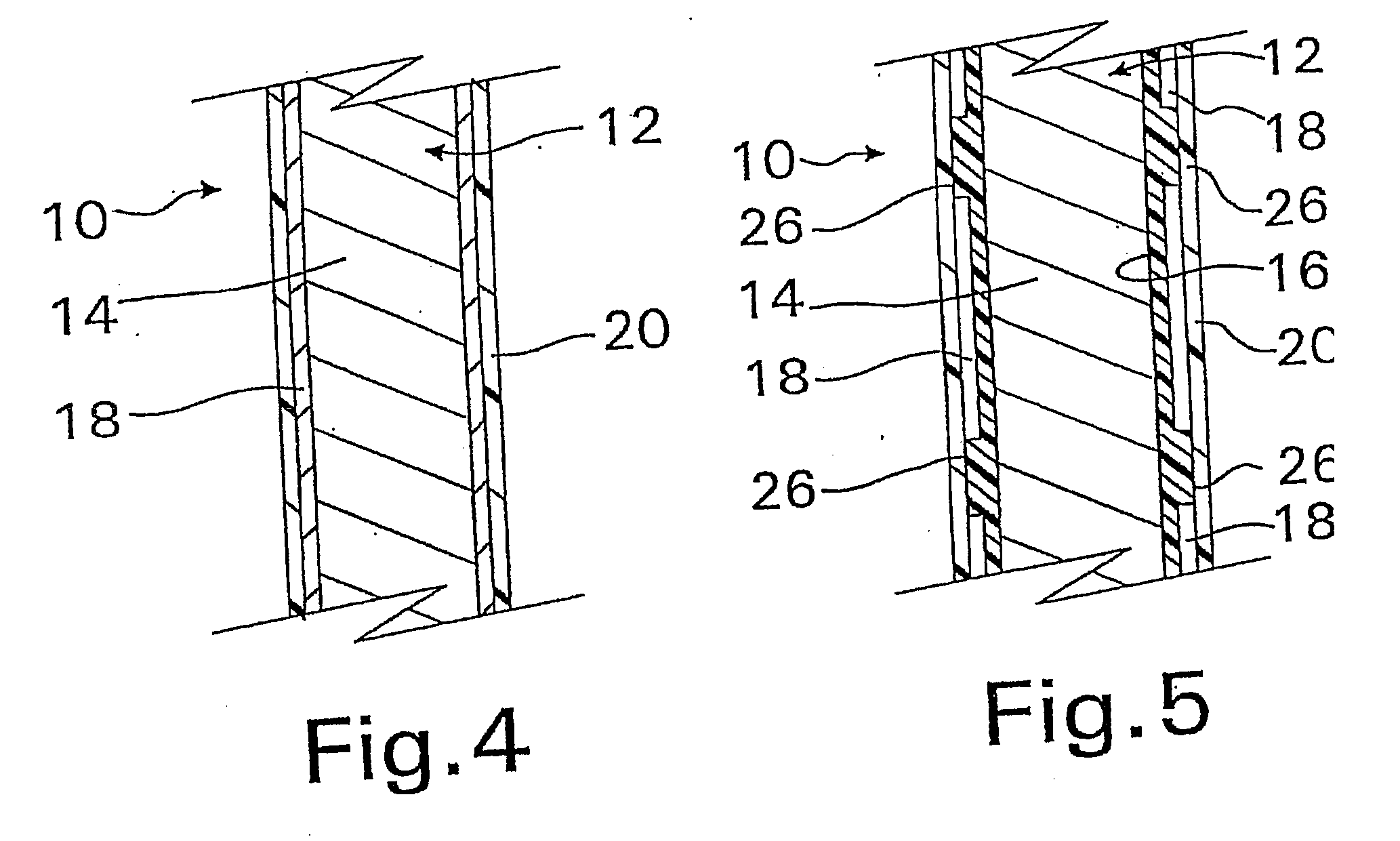
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract; at least one coating layer 16 posited on one surface of the structure; and at least one layer 18 of a bioactive material posited on at least a portion of the coating layer 16, wherein the coating layer 16 provides for the controlled release of the bioactive material from the coating layer. In addition, at least one porous layer 20 can be posited over the bioactive material layer 18, wherein the porous layer includes a polymer and provides for the controlled release of the bioactive material therethrough. Preferably, the structure 12 is a coronary stent. The porous layer 20 includes a polymer applied preferably by vapor or plasma deposition and provides for a controlled release of the bioactive material. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative, which is deposited without solvents, heat or catalysts, and merely by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
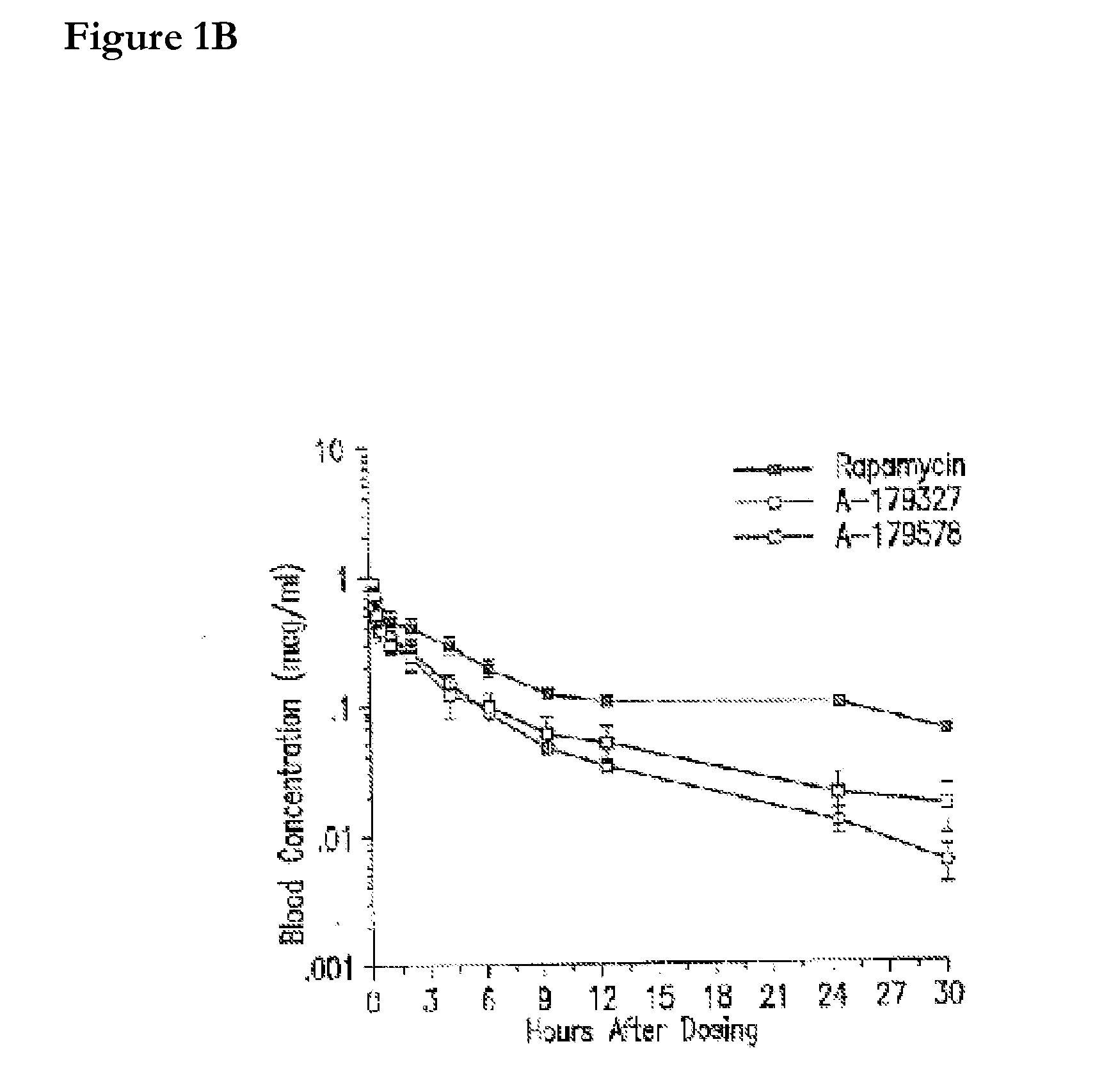
Compositions and methods of administering rapamycin analogs using medical devices for long-term efficacy
Systems and compositions comprising zotarolimus that are safer, more effective and produce less inflammation than rapamycin and paclitaxel systems are disclosed. Medical devices comprising supporting structures capable of containing or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient can contain one or more therapeutic agents or substances, with the carrier including a coating on the surface thereof, and the coating having the therapeutic compounds, including, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
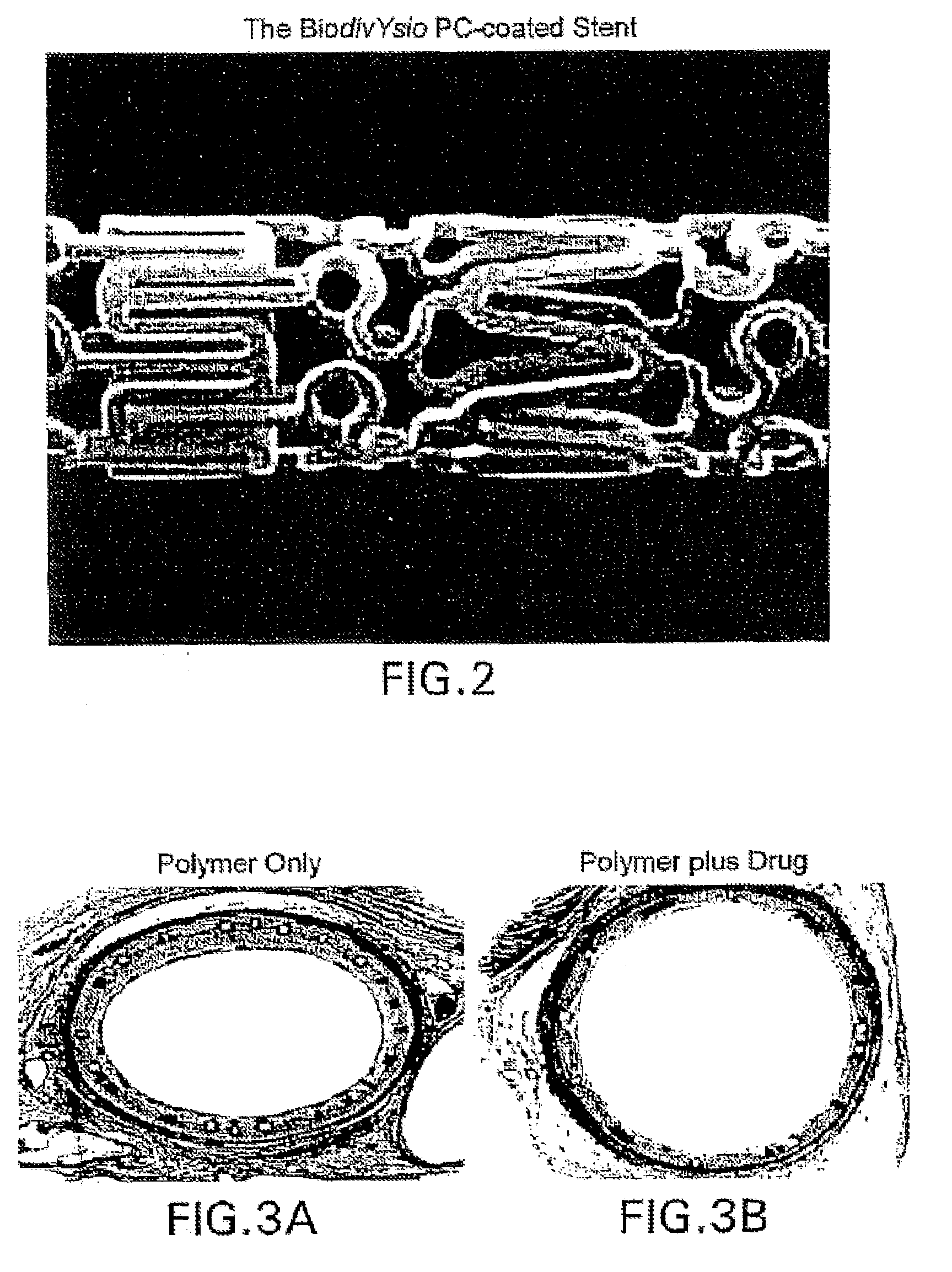
Stent with polymer coating containing amorphous rapamycin
InactiveUS20090062909A1Extensive adhesionBulk propertiesStentsSurgeryPolymer coatingsPolybutyl methacrylate
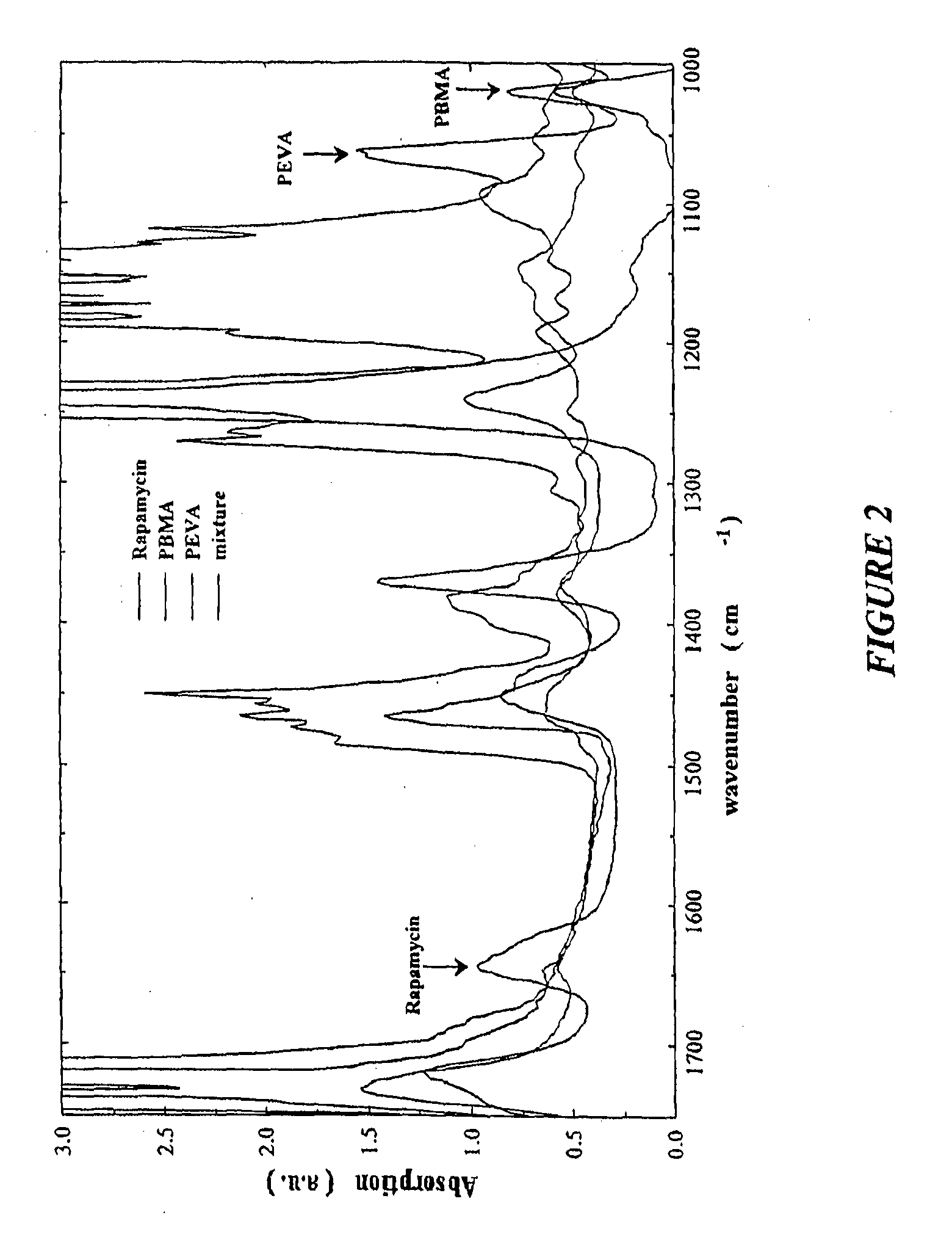
A coated coronary stent, comprising: a stainless steel sent framework coated with a primer layer of Parylene C; and a rapamycin-polymer coating having substantially uniform thickness disposed on the stent framework, wherein the rapamycin-polymer coating comprises polybutyl methacrylate (PBMA), polyethylene-co-vinyl acetate (PEVA) and rapamycin, wherein substantially all of the rapamycin in the coating is in amorphous form and substantially uniformly dispersed within the rapamycin-polymer coating.
Owner:MICELL TECH INC
Compositions, systems, and kits for administering zotarolimus and paclitaxel to blood vessel lumens
A system and compositions including zotarolimus and paclitaxel are disclosed, as well as methods of delivery, wherein the drugs have effects that complement each other. Medical devices are disclosed which include supporting structures that include at least one pharmaceutically acceptable carrier or excipient, which carrier or excipient can include one or more therapeutic agents or substances, with the carrier including at least one coating on the surface thereof, and the coating associated with the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
Coated implantable medical device
InactiveUS7550005B2Prevent degradationIncrease release rateStentsHeart valvesParylenePlasma deposition
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract; at least one coating layer 16 posited on one surface of the structure; and at least one layer 18 of a bioactive material posited on at least a portion of the coating layer 16, wherein the coating layer 16 provides for the controlled release of the bioactive material from the coating layer. In addition, at least one porous layer 20 can be posited over the bioactive material layer 18, wherein the porous layer includes a polymer and provides for the controlled release of the bioactive material therethrough. Preferably, the structure 12 is a coronary stent. The porous layer 20 includes a polymer applied preferably by vapor or plasma deposition and provides for a controlled release of the bioactive material. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative, which is deposited without solvents, heat or catalysts, and merely by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
Methods of administering rapamycin analogs with anti-inflammatories using medical devices
A medical device comprising a supporting structure capable of including or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient may include one or more therapeutic agents or substances, with the carrier including a coating on the surface thereof, and the coating including the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to, This drug can be used in combination with another drug including those selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
Matrix Coated Stent
InactiveUS20110172763A1Promote endothelializationSimplifying device manufacture and loadingStentsSurgeryBiocompatibility TestingDisease progression
The present invention relates generally to a drug eluting stent containing metallic surfaces modified in microsphere metallic matrix structure and methods for making same. More specifically, the invention relates to an expandable and implantable vascular stent having at least one matrix layer that promotes improved cellular adhesion properties for healing promotion healing and long term biocompatibility. In the case of coronary stents, the metallic matrix layer promotes re-endothelialization at sites of stent implantation, improves overall healing, and reduces inflammation and intimal disease progression. The microsphere metallic matrix layer may be optionally loaded with one or more therapeutic agent to further improve the function of the implanted stent and further augment clinical efficacy and safety. The active compounds are selected primarily for their anti-proliferative, immunosuppressive, and anti-inflammatory activities, among other properties, which prevent, in part, smooth muscle cell proliferation and promote endothelial cell growth.
Owner:STERLING VASCULAR SYST
Drug Coated Stents
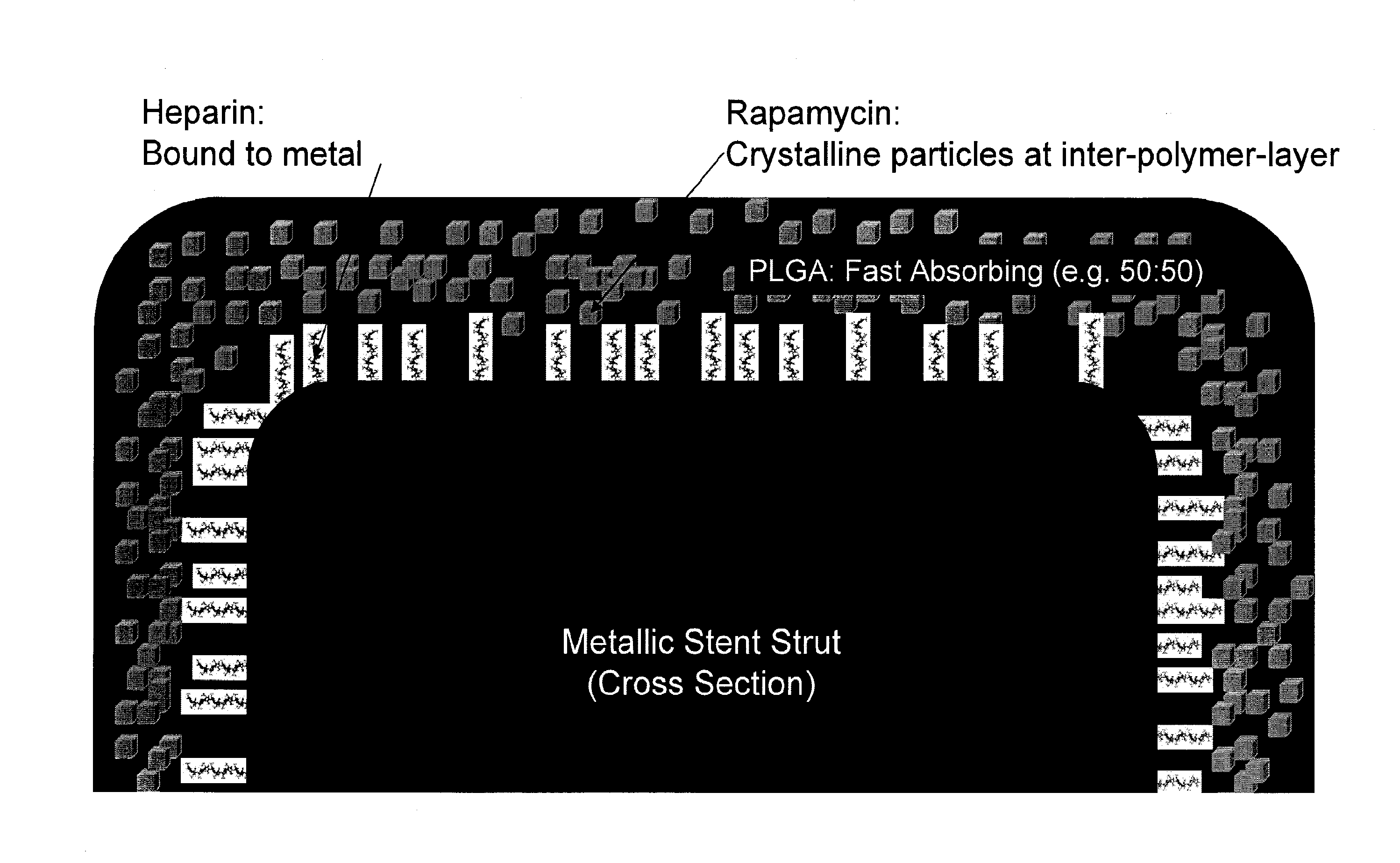
Provided herein is a coated coronary stent, comprising: a stent framework; heparin molecules attached to the stent framework; and a rapamycin-polymer coating wherein at least part of rapamycin is in crystalline form. In one embodiment, the rapamycin-polymer coating comprises one or more resorbable polymers.
Owner:MICELL TECH INC
Medical devices containing rapamycin analogs
InactiveUS20050175660A1Reduce restenosisReduce restenosis rateBiocideOrganic chemistryTherapeutic effectAnti platelet
A medical device comprising a supporting structure capable of containing or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient may contain one or more therapeutic agents or substances, with the carrier preferably including a coating on the surface thereof, and the coating containing the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to, This drug can be used in combination with another drug including those selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
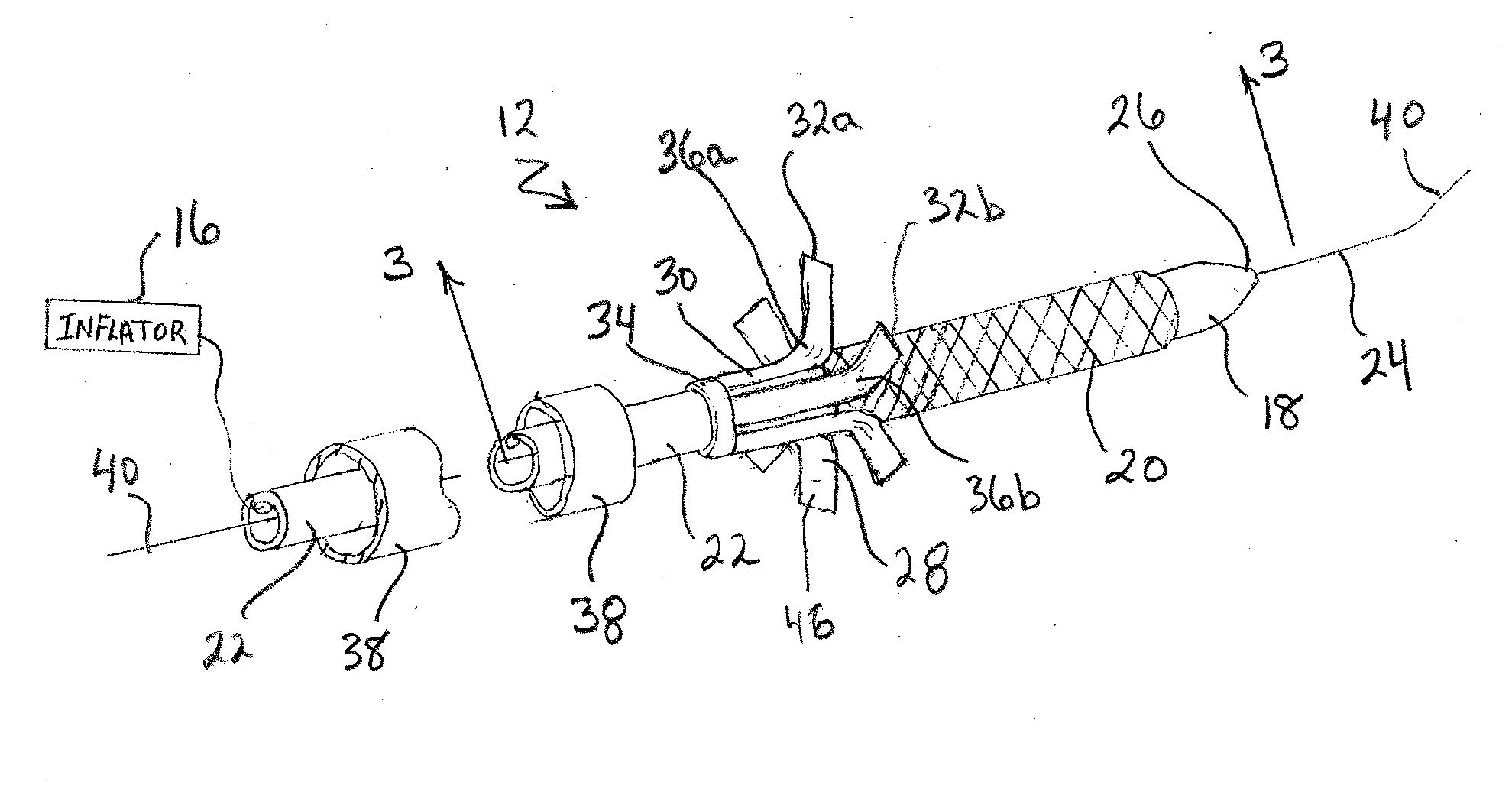
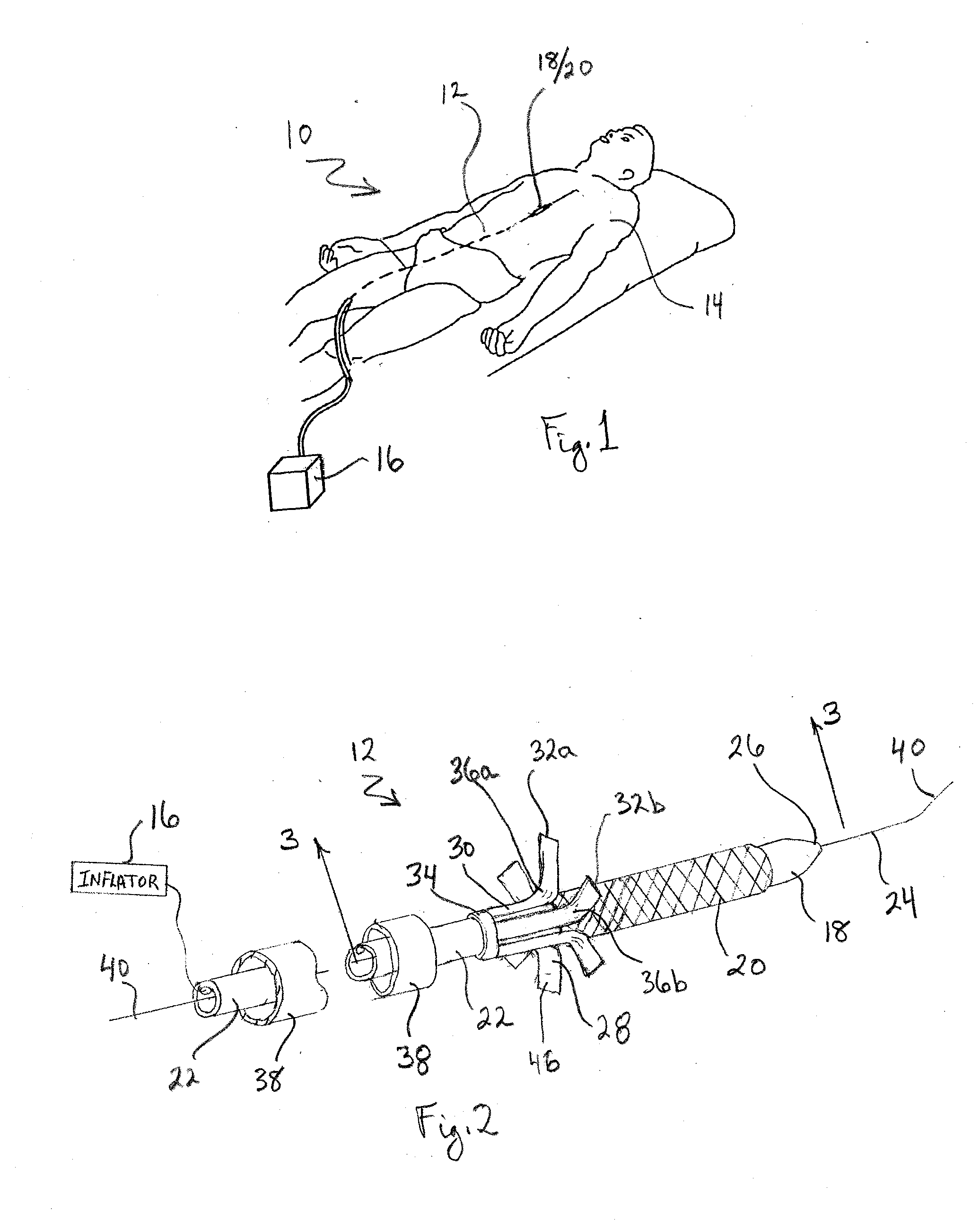
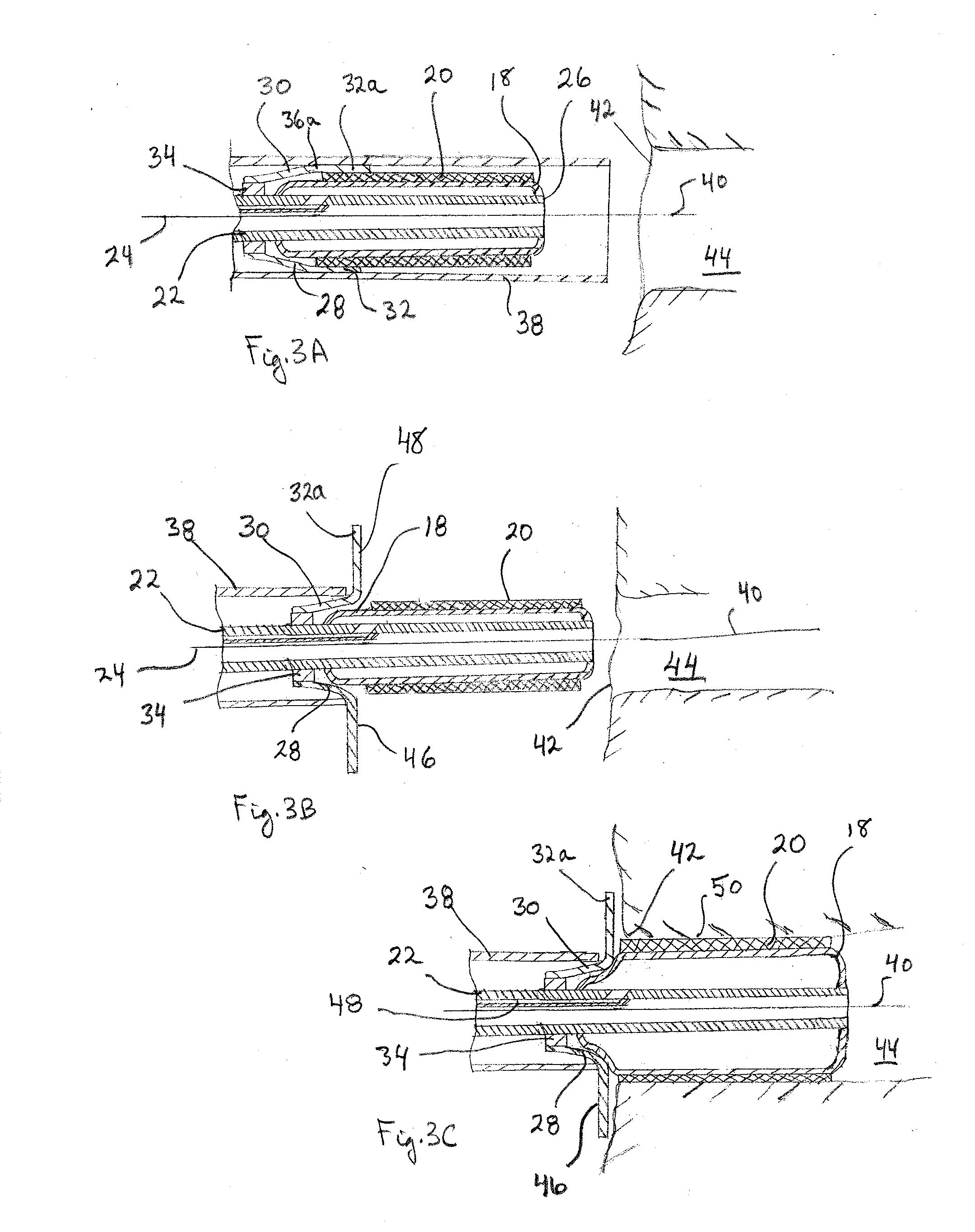
Two-step/dual-diameter balloon angioplasty catheter for bifurcation and side-branch vascular anatomy
The present invention tackles the challenging anatomic characteristics of the coronary artery disease in the bifurcation point and the origin of side-branch. The invention has a specifically designed angioplasty balloon catheter, particularly the balloon shape and profile, to be used in the diseased vessels at these difficult anatomic locations. In stent implanting into a coronary artery, a balloon catheter application is an inseparable requirement. A stent is a passive device that cannot be deployed in a diseased or stenosed artery without a pre-stent, with-stent and / or post-stent balloon dilatation. In majority (more than 95%) of available coronary stents, a stent is deployed by balloon expandable mode, meaning that the stent is delivered and expanded inside a vessel lumen by expanding a delivery balloon. This is done by crimping a stent over a folded balloon for delivery into a coronary artery. When expanded by balloon inflation, a stent is expanded and shaped passively by the inflated balloon shape and profile. The balloon catheter is designed to do angioplasty in the bifurcation and side-branch anatomy of coronary arteries, while minimizing the side effect. This specially designed balloon catheter is not only for balloon angioplasty dilatation of the bifurcation and side-branch anatomy, but is also for delivering and deploying specially designed bifurcation or side-branch stents into these difficult anatomic locations, as a stent delivery system.
Owner:JANG G DAVID
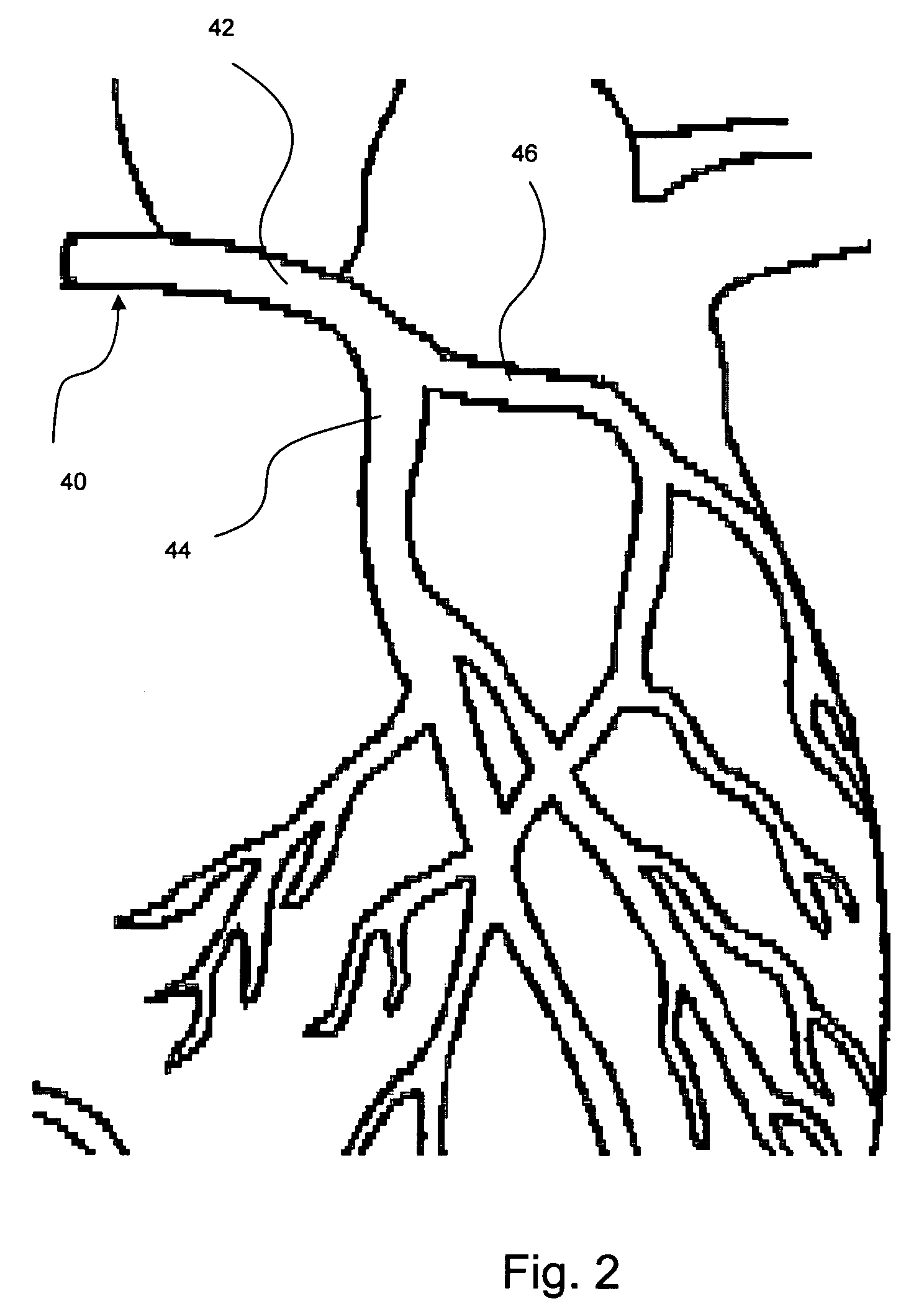
System and Method for Placing a Coronary Stent at the Ostium of a Blood Vessel
A system and method are provided that use a balloon / stent catheter for placing a stent at the ostium of a blood vessel. Included is a barrier member that is attached to the catheter at a location proximal the balloon. In use, the barrier member is reconfigured, prior to insertion of the balloon / stent portion of the catheter through the ostium. With this reconfiguration, a barrier (array) is established that limits advancement of the catheter into the blood vessel to ensure proper placement of the stent at the ostium.
Owner:SCHATZ RICHARD A
Coated implantable medical device
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract; at least one coating layer 16 posited on one surface of the structure; and at least one layer 18 of a bioactive material posited on at least a portion of the coating layer 16, wherein the coating layer 16 provides for the controlled release of the bioactive material from the coating layer. In addition, at least one porous layer 20 can be posited over the bioactive material layer 18, wherein the porous layer includes a polymer and provides for the controlled release of the bioactive material therethrough. Preferably, the structure 12 is a coronary stent. The porous layer 20 includes a polymer applied preferably by vapor or plasma deposition and provides for a controlled release of the bioactive material. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative, which is deposited without solvents, heat or catalysts, and merely by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
Compositions, systems, kits, and methods of administering rapamycin analogs with paclitaxel using medical devices
A system and compositions including zotarolimus and paclitaxel are disclosed, as well as methods of delivery, wherein the drugs have effects that complement each other. Medical devices are disclosed which include supporting structures that include at least one pharmaceutically acceptable carrier or excipient, which carrier or excipient can include one or more therapeutic agents or substances, with the carrier including at least one coating on the surface thereof, and the coating associated with the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
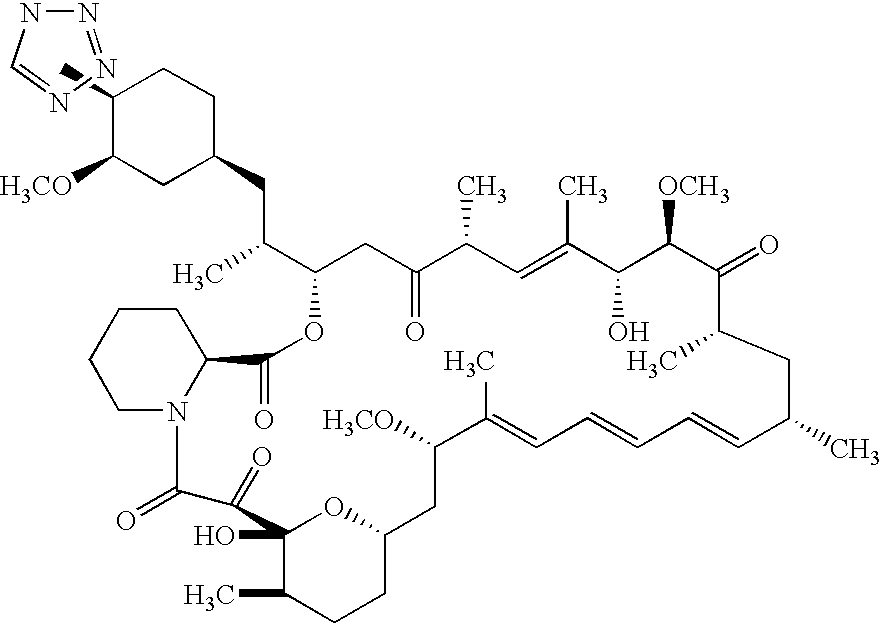
Methods of administering tetrazole-containing rapamycin analogs with other therapeutic substances using medical devices
InactiveUS7399480B2Reduce probabilityReduces restenosis in vasculatureBiocideOrganic chemistryAnti plateletCytostatic drugs
A medical device comprising a supporting structure capable of containing or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient may contain one or more therapeutic agents or substances, with the carrier preferably including a coating on the surface thereof, and the coating containing the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to,This drug can be used in combination with another drug including those selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
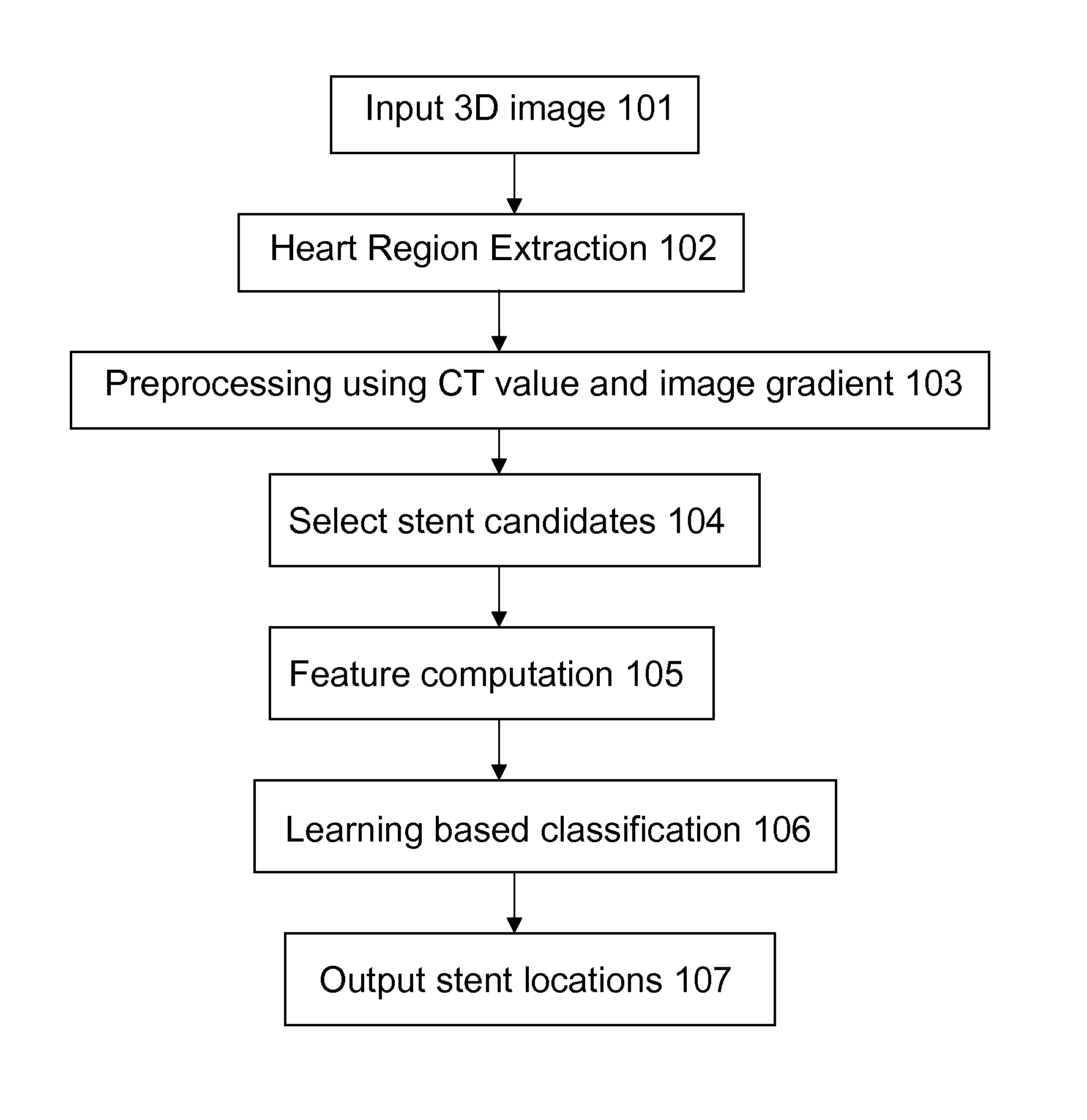
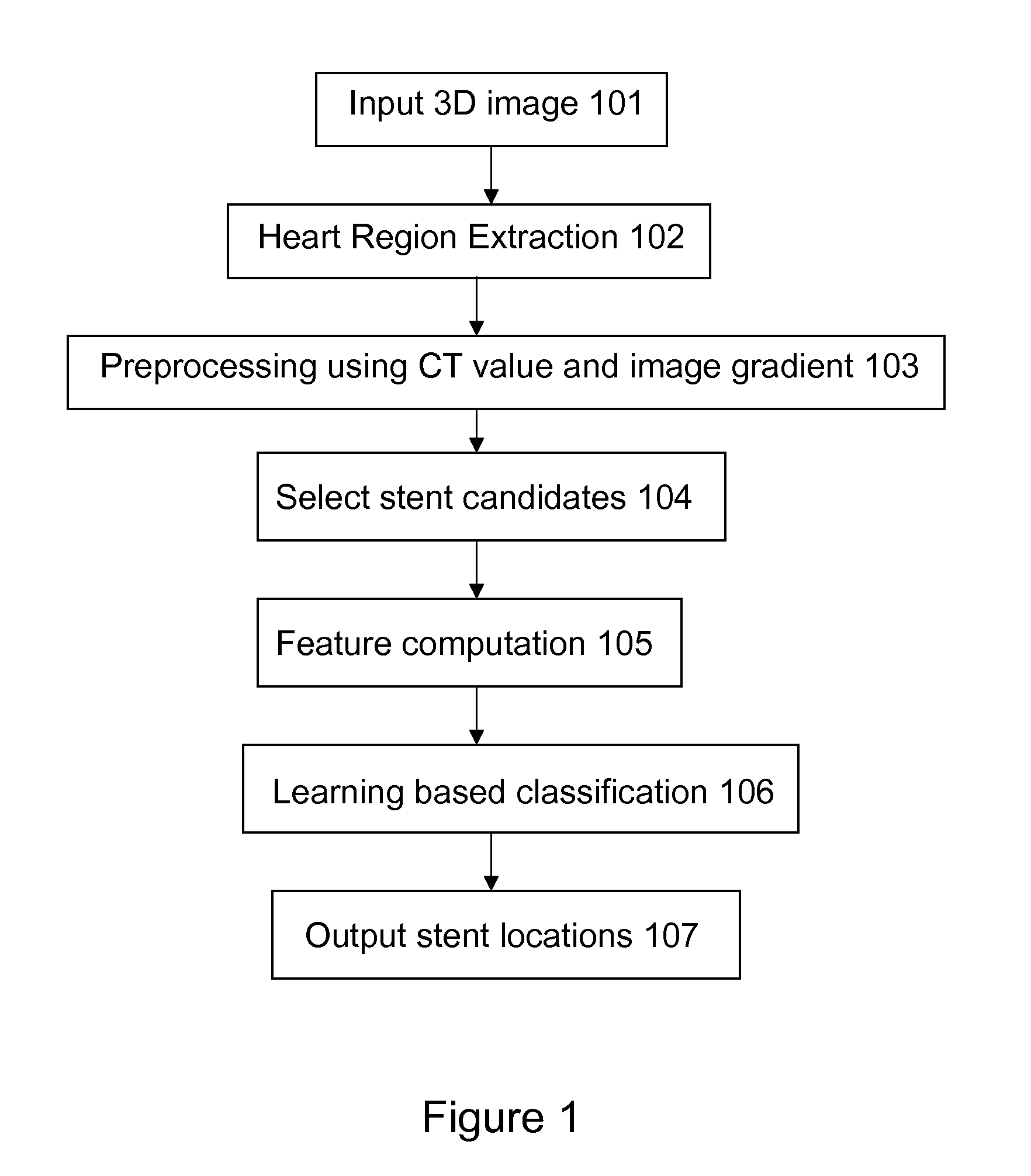
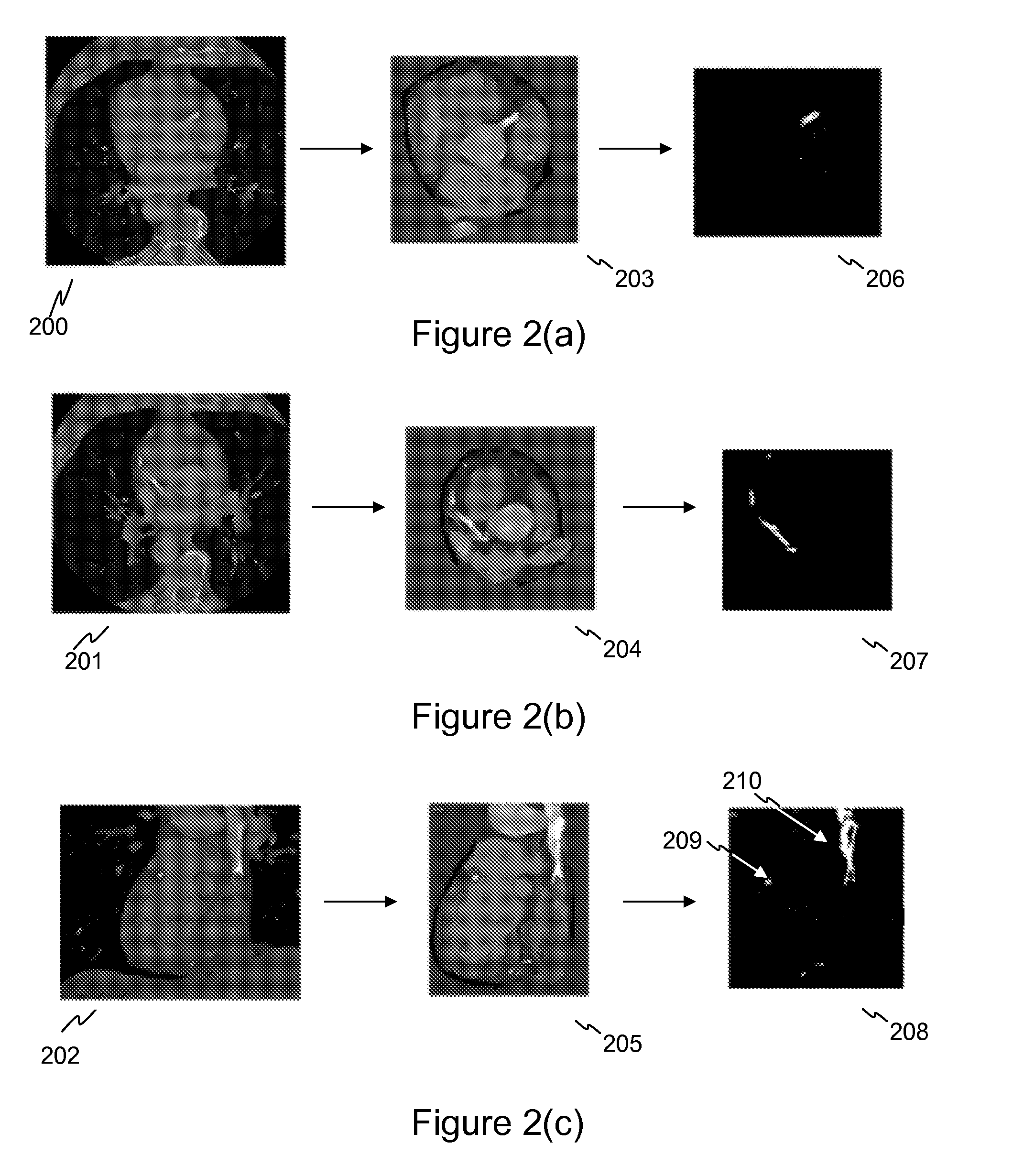
Stent localization in 3D cardiac images
Systems and methods are described for automatically identifying coronary stents within a 3D cardiac image. Based on feature analysis on a cardiac image, coronary stents can be detected by filtering the cardiac images for stent candidates, applying a score based on factors related to coronary stents and applying a threshold. Once coronary stents are identified, in-stent restenosis and stent fractures can be further detected.
Owner:FUJIFILM CORP
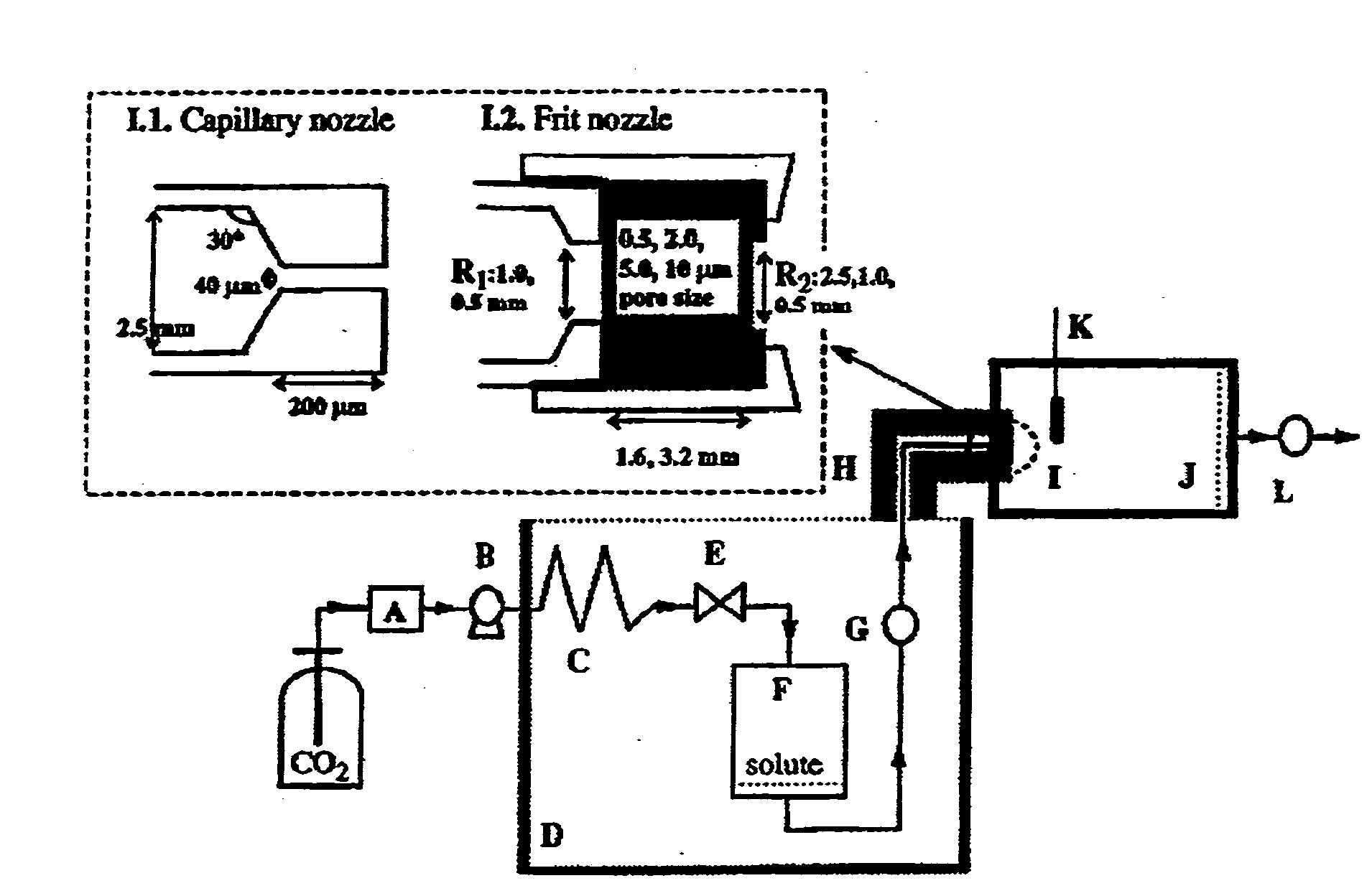
Method of producing personalized biomimetic drug-eluting coronary stents by 3d-printing
ActiveUS20160256610A1Avoid influenceReduce complicationsStentsAdditive manufacturing apparatusThrombusPercent Diameter Stenosis
A method for using 3D printing technology produces personalized biomimetic drug-eluting coronary stent and the product thereof. The process of manufacturing stent, based on coronary angiography imaging data, measures the diameter of diseased coronary and conducts 3D reconstruction. A personalized stent for each patient according to diameter, length, and morphological characteristics of target vessel that suited to the lesion is produced. The coronary stent is formed from biodegradable poly-L-lactic acid (PLLA) or other materials. The stent is modeled by 3D printing and then coated with polymers carrying antiproliferative drug to reduce restenosis (the polymers is a mixture of antiproliferative drug and PDLLA at a ratio of 1:1). The biomimetic drug-eluting coronary stent produced by 3D printing technology is personalized stent for each patient according to different characteristics of diseased coronary, reduces the incidence of vascular injury, thrombosis, dissection and other complications caused by stent and vessel diameter mismatch.
Owner:ZHOU YUJIE +3
Medical devices containing rapamycin analogs
InactiveUS20050032826A1Reduce probabilityReduces restenosis in vasculatureBiocideAntimycoticsArteriovenous graftsMedical treatment
A medical device comprising a supporting structure having a coating on the surface thereof, the coating containing a therapeutic substance, such as, for example, a drug. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to,
Owner:MOLLISON KARL W +4
Compositions and methods of administering paclitaxel with other drugs using medical devices
Systems and compositions comprising paclitaxel and a second drug, such as rapamycin, analogs, derivatives, salts and esters thereof are disclosed, as well as methods of delivery wherein the drugs have effects that complement each other. Medical devices comprising supporting structures capable of including or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient can contain one or more therapeutic agents or substances, with the carrier preferably including a coating on the surface thereof, and the coating including the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
Method for preparing personalized bionic drug eluting coronary stent by using 3D printing technology
InactiveCN105877881AAvoid influenceImprove the quality of lifeStentsAdditive manufacturing apparatusThrombusCoronary angiogram
The invention discloses a method for preparing a personalized bionic drug eluting coronary stent by using a 3D printing technology and a product. The method for preparing the personalized bionic drug eluting coronary stent comprises the step that according to image data of coronary angiogram, adopting a QCA technique for measuring the diameter of a diseased coronary artery and reconstructing in a three-dimensional manner. According to indexes such as lesion vascular diameter, lesion length and lesion vascular pattern, a personalized coronary stent can be made for each patient in a customized manner and a stent most suitable for the lesion state of a patient can be prepared. The personalized bionic drug eluting coronary stent can be made of degradable poly L-lactic acid (PLLA) or other materials, after 3D printing molding, the surface of the stent can be coated with a polymer with anti-proliferation medicines by using a conventional process, and in-stent restenosis can be reduced (the polymer is prepared by mixing a medicine with PDLLA in a ratio of the medicine to PDLLA being 1:1). The most suitable personalized bionic drug eluting coronary stent can be customized for the patient according to diseased coronary arteries of different states, requirements of different lesions can be met, customization of coronary stents can be achieved, and complications such as vascular injury, thrombus and coronary arterial dissection caused by mismatching of the diameter of the stent and that of the blood vessel can be reduced.
Owner:周玉杰 +3
Anchoring ball sac in coronary stent
InactiveCN103356251ASolve technical problems with poor passabilityIncrease frictionSurgeryCalcificationEngineering
The invention discloses an anchoring ball sac in a coronary stent, which comprises a hollow conduit; the outer wall of the front end of the conduit is provided with a first elastic ball sac; a liquid-injecting channel communicated with the first elastic bass sac is arranged in the conduit wall along the axial direction; and a plurality of bosses are arranged at the outer face of the first elastic ball sac. The using method comprises the steps of placing a guide wire into the far end of a coronary blood vessel by an already placed stent, and positioning the elastic ball sac at the front end of the conduit in the stent under the guide of the guide wire, wherein the outer end of the conduit is connected with a booster pump, and a developing agent is injected by the liquid-injecting channel to expand the elastic ball sac; and the bosses at the face of the bal sac can increase anchoring force, thus enhancing the ability of the coronary stent to pass through lesions such as torsion and calcification, better solving the technical problem that as the traditional coronary stent is poor in passing complicated lesions, the ball sac and the stent difficultly reach in place.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
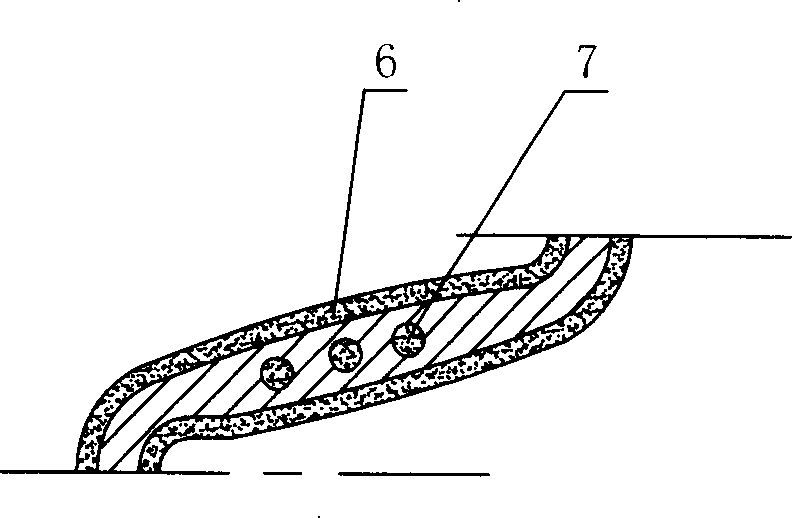
Double medicament controlled-release coating micropore ACS bracket
The invention relates to a millipore coronary stent with double-drug-slow-released coatings The coronary stent is structured by a plurality of metal main-structural members and connecting rods, wherein, the metal main-structural member is a regular snake-shaped ring which is formed by connecting an arc segment and a snake segment; neighboring main-structural members are connected by the connecting rod to form a ring support of a cylinder-wall; a plurality of millipores are opened on the snake-shaped segment; an outer drug-slow-released coating is coated on all metal main-structural members and connecting rods; an inner drug-slow-released coating is filled in the millipores on the snake-shaped segment of the metal-main-structural members. The double-drug-slow-released coatings of the coronary stent, through the slow-released function of the polymer coatings, can guarantee that different drugs can release at the given drug-releasing speeds, so as to guarantee that the drugs can adopts different releasing orders and durative acting times according to different pathogenesis.
Owner:天津市凯迪亚医疗器械有限公司
Two-step/dual-diameter balloon angioplasty catheter for bifurcation and side-branch vascular anatomy
The present invention tackles the challenging anatomic characteristics of the coronary artery disease in the bifurcation point and the origin of side-branch. The invention has a specifically designed angioplasty balloon catheter, particularly the balloon shape and profile, to be used in the diseased vessels at these difficult anatomic locations. In stent implanting into a coronary artery, a balloon catheter application is an inseparable requirement. A stent is a passive device that cannot be deployed in a diseased or stenosed artery without a pre-stent, with-stent and / or post-stent balloon dilatation. In majority (more than 95%) of available coronary stents, a stent is deployed by balloon expandable mode, meaning that the stent is delivered and expanded inside a vessel lumen by expanding a delivery balloon. This is done by crimping a stent over a folded balloon for delivery into a coronary artery. When expanded by balloon inflation, a stent is expanded and shaped passively by the inflated balloon shape and profile. The balloon catheter is designed to do angioplasty in the bifurcation and side-branch anatomy of coronary arteries, while minimizing the side effect. This specially designed balloon catheter is not only for balloon angioplasty dilatation of the bifurcation and side-branch anatomy, but is also for delivering and deploying specially designed bifurcation or side-branch stents into these difficult anatomic locations, as a stent delivery system.
Owner:JANG G DAVID
Coated implantable medical device
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract; at least one coating layer 16 posited on one surface of the structure; and at least one layer 18 of a bioactive material posited on at least a portion of the coating layer 16, where the coating layer 16 provides for the controlled release of the bioactive material from the coating layer. In addition, at least one porous layer 20 may be posited over the bioactive material layer 18, where the porous layer includes a polymer and provides for the controlled release of the bioactive material therethrough. Preferably, the structure 12 is a coronary stent. The porous layer 20 includes a polymer applied preferably by vapor or plasma deposition and provides for a controlled release of the bioactive material. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative, which is deposited without solvents, heat or catalysts, and merely by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
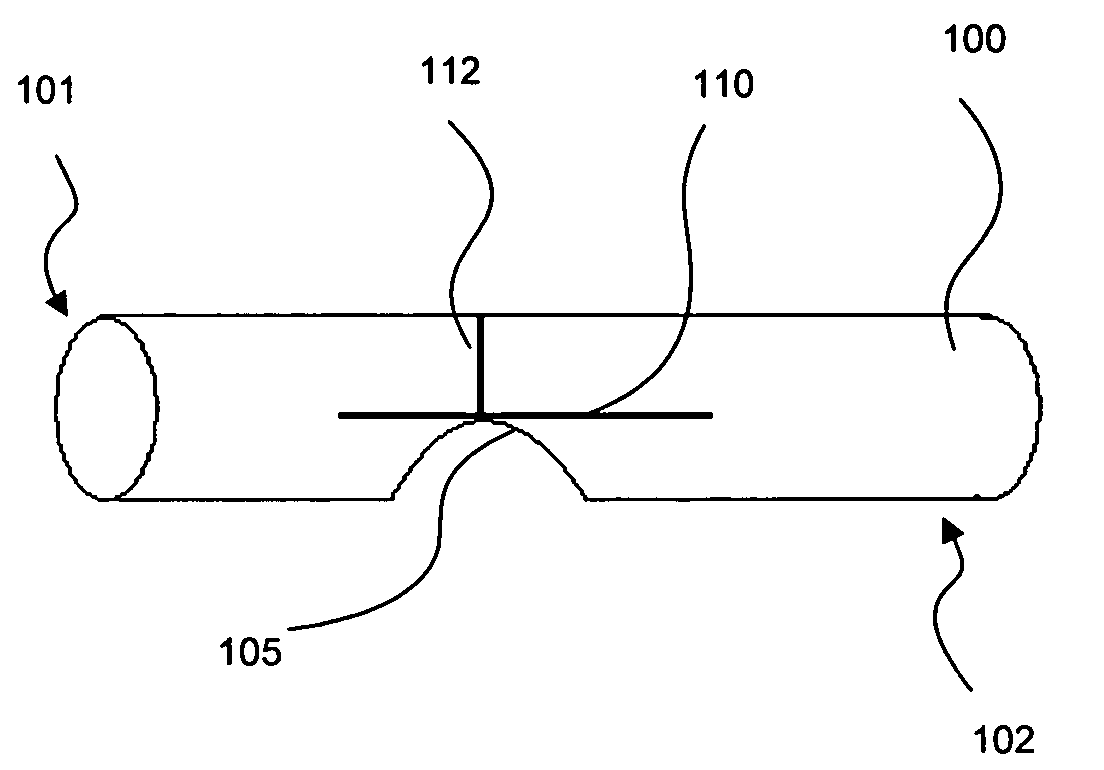
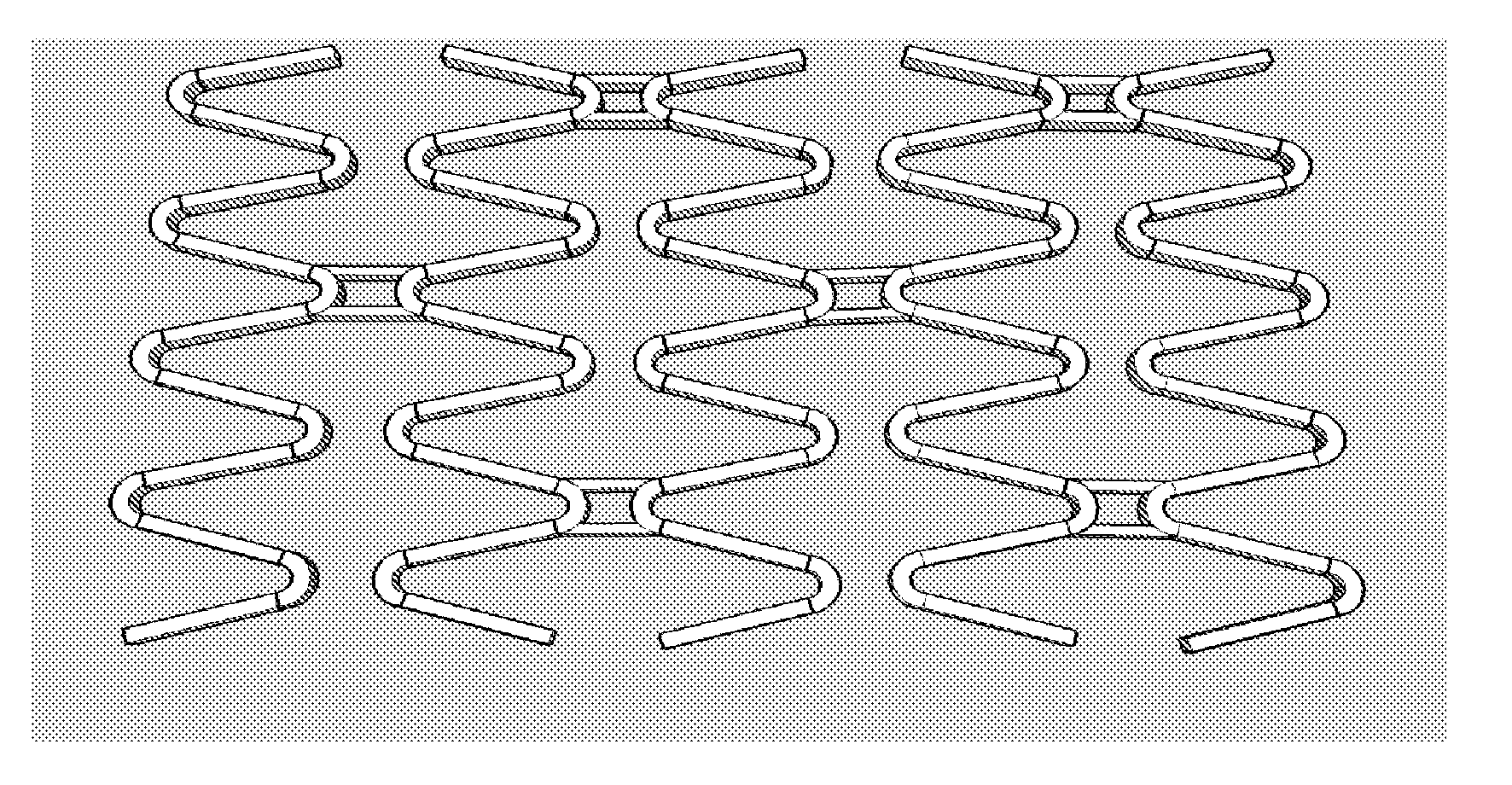
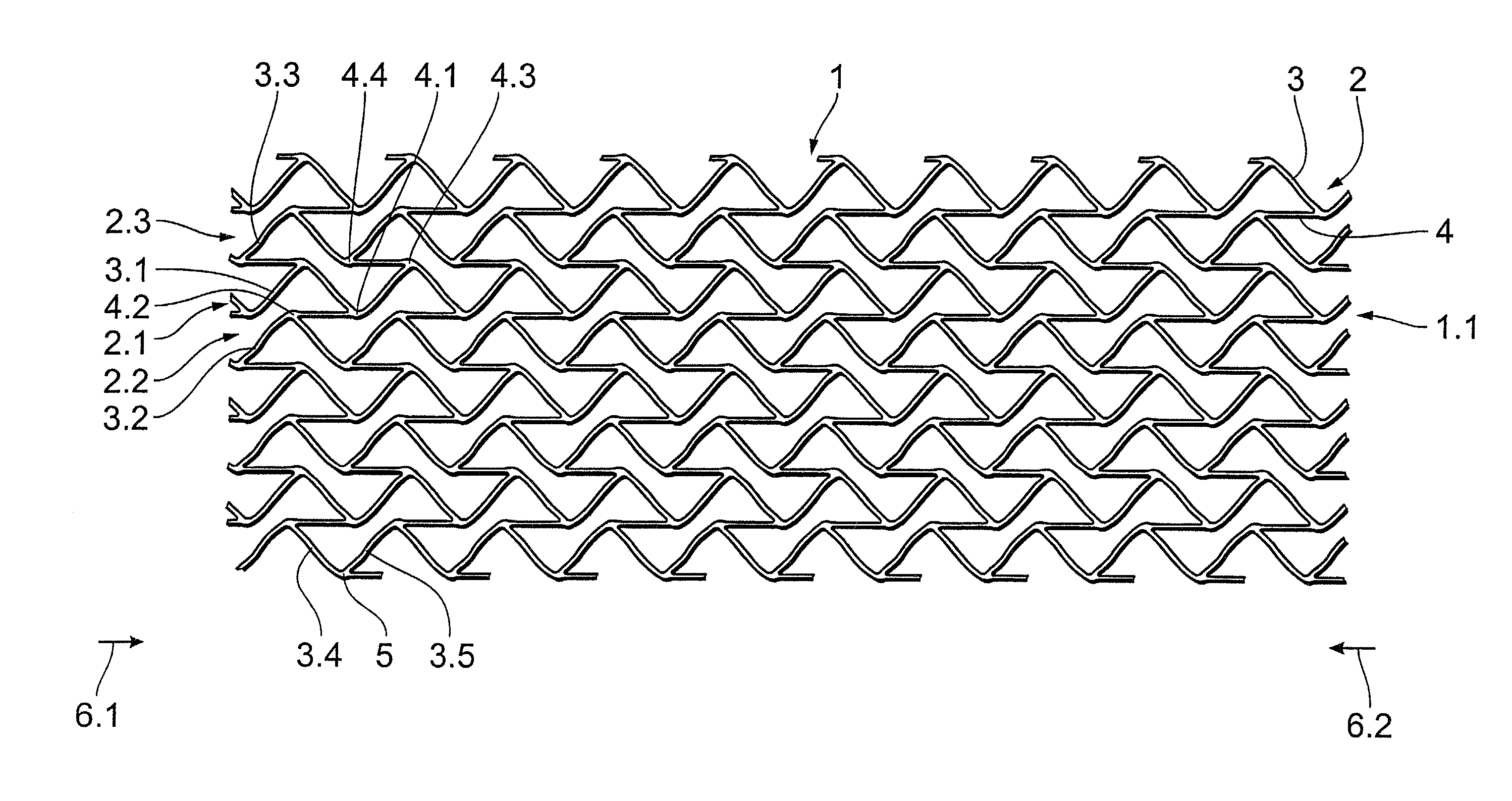
Stent
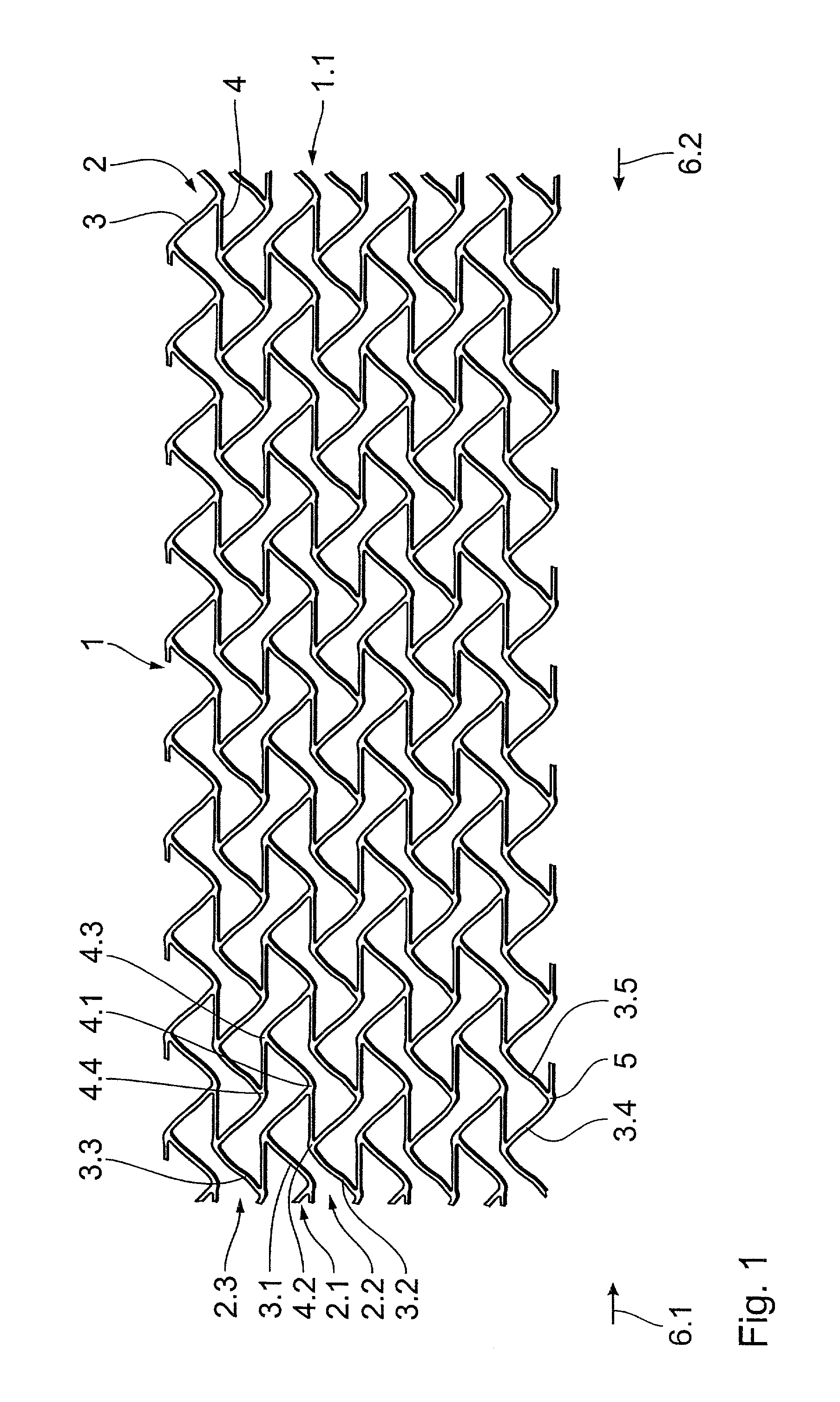
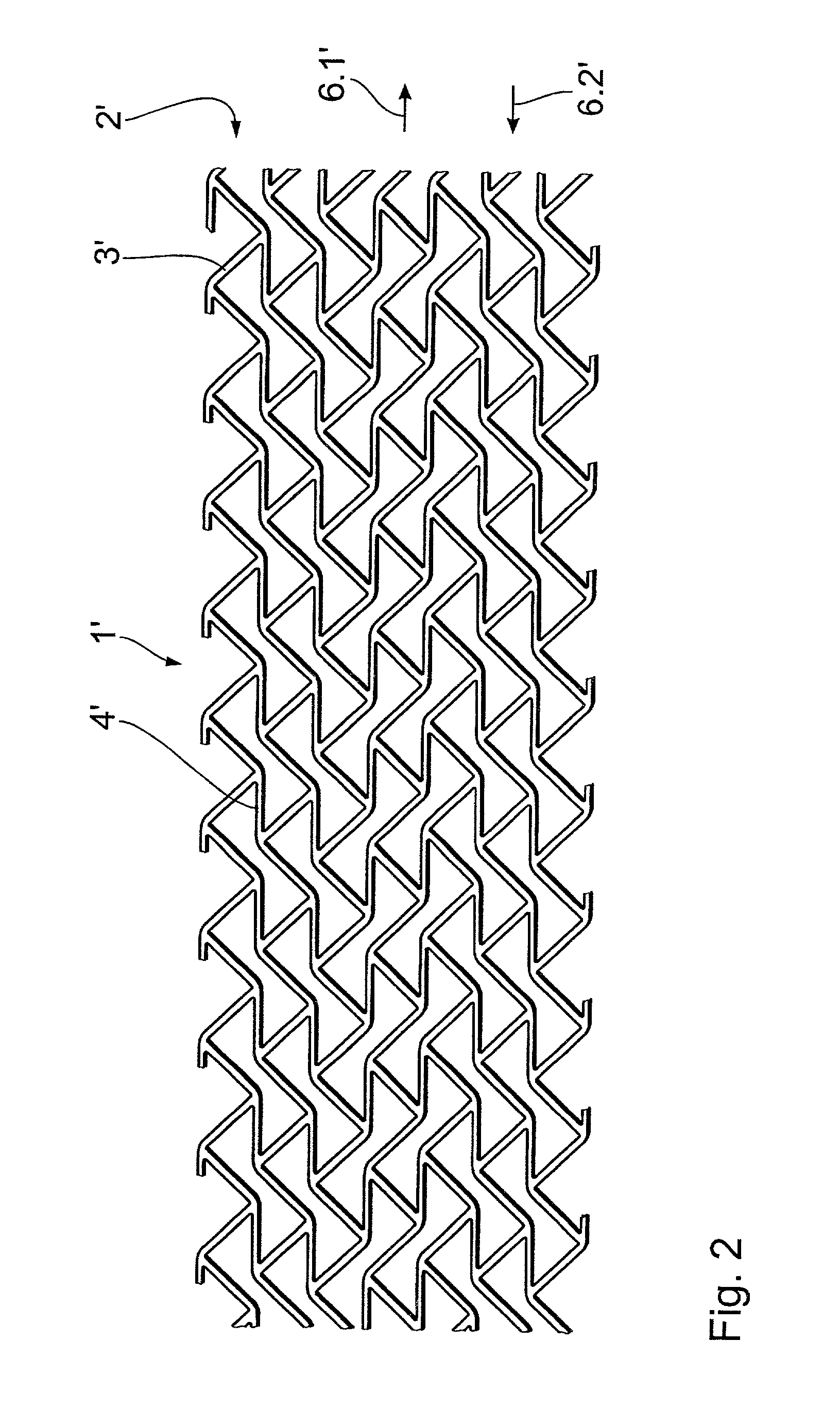
A stent, in particular a coronary stent, for expansion from a first condition into an expanded second condition in which it holds a vessel in an expanded state, comprising a tubular body whose peripheral surface (1) is formed by a number of support portions (2) which extend in the longitudinal direction of the stent and which comprise bar elements (3) which are connected by way of connecting bars (4), wherein there is provided a number of support portion groups (1.1) with at least a first support portion (2.1) and a second support portion (2.2, 2.3) in adjacent relationship in the peripheral direction of the stent, whose bar elements (3.1, 3.2, 3.3) extend in a meander configuration in the longitudinal direction of the stent, and wherein the first engagement points (4.1, 4.2) of first connecting bars (4) engage the first support portion (2.1) and the second engagement points (4.2, 4.4) of the first connecting bars (4) engage the second support portion (2.2, 2.3), wherein the first and second engagement points (4.1, 4.3, 4.2, 4.4) of the first connecting bars (4) are spaced from each other in the longitudinal direction of the stent and the first connecting bars (4) are of such a configuration and arrangement that the spacing between the first and second engagement points (4.1, 4.3, 4.2, 4.4) of the first connecting bars (4) in the longitudinal direction of the stent changes upon expansion of the stent to compensate for the reduction in length of the support portions.
Owner:BIOTRONIK MESS UND THERAPIEGERAETE GMBH & CO
Manufacturing method of cobalt/iron composite tube for coronary stent and auxiliary equipment
ActiveCN103286155AHigh dimensional accuracySmooth inner and outer surfacesMetal rolling arrangementsMetallurgyEngineering
The invention relates to a processing method of a novel cobalt / iron composite tube for a coronary stent and auxiliary equipment. The problem that the existing stent is thick in tube wall and capable of easily causing repeat narrow of focus blood vessel is solved. The process flow comprises the following steps of: smelting, forging, hot rolling, hot rolling piercing, boring and polishing inner surface and outer surface, assembling tube blank, primarily cold rolling and compounding, annealing, cold rolling / drawing, annealing, repeatedly cold rolling / drawing, and obtaining the finished product. In the repeated cold rolling process, the composite tube deformation is controlled at each time, and meanwhile, the matching relation of the reducing deformation and wall-reducing deformation is adjusted so that the stress of the composite interface is less than 0.2 Sigmab cobalt (breaking strength) to prevent the composite interface from cracking. The rough blank of the tube is obtained at high temperature, the cobalt / iron composite tube is obtained by rolling at room temperature, and the common tube rolling process is used for processing; the composite tube is high in size precision, excellent in dynamic performance, simple in process, good in controllability, and high in economic benefit.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
Coated implantable medical device
InactiveUS20080145398A1Prevent degradationIncrease release rateHeart valvesSurgeryParyleneBiliary tract
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract, and at least one layer 18 of an immunosuppressive agent posited over at least one surface of the structure 12. Optionally, the device 10 can include at least one porous, preferably polymeric layer 20 posited over the layer 18 of immunosuppressive agent, and can alternatively or additionally include at least one coating layer 16 posited on one surface of the structure 12, the at least one layer 18 of immunosuppessive agent being posited in turn on at least a portion of the coating layer 16. The porous layer 20 and the coating layer 16 each provide for the controlled release of the bioactive material from the device 10. The structure 12 is preferably configured as a coronary stent. The polymer of the porous layer 20 is preferably applied by vapor or plasma deposition. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative which is deposited without solvents, heat or catalysts, but rather by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com