Patents
Literature
72 results about "Resorbable polymers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Resorbable polymers are polymers, which degrade completely without presenting any threat to human body. This gives medical practitioners scope to incorporate resorbable polymers as implants in surgery, thereby, reducing the need for second surgery to remove temporary implants and reducing post-surgery risk to the patients.
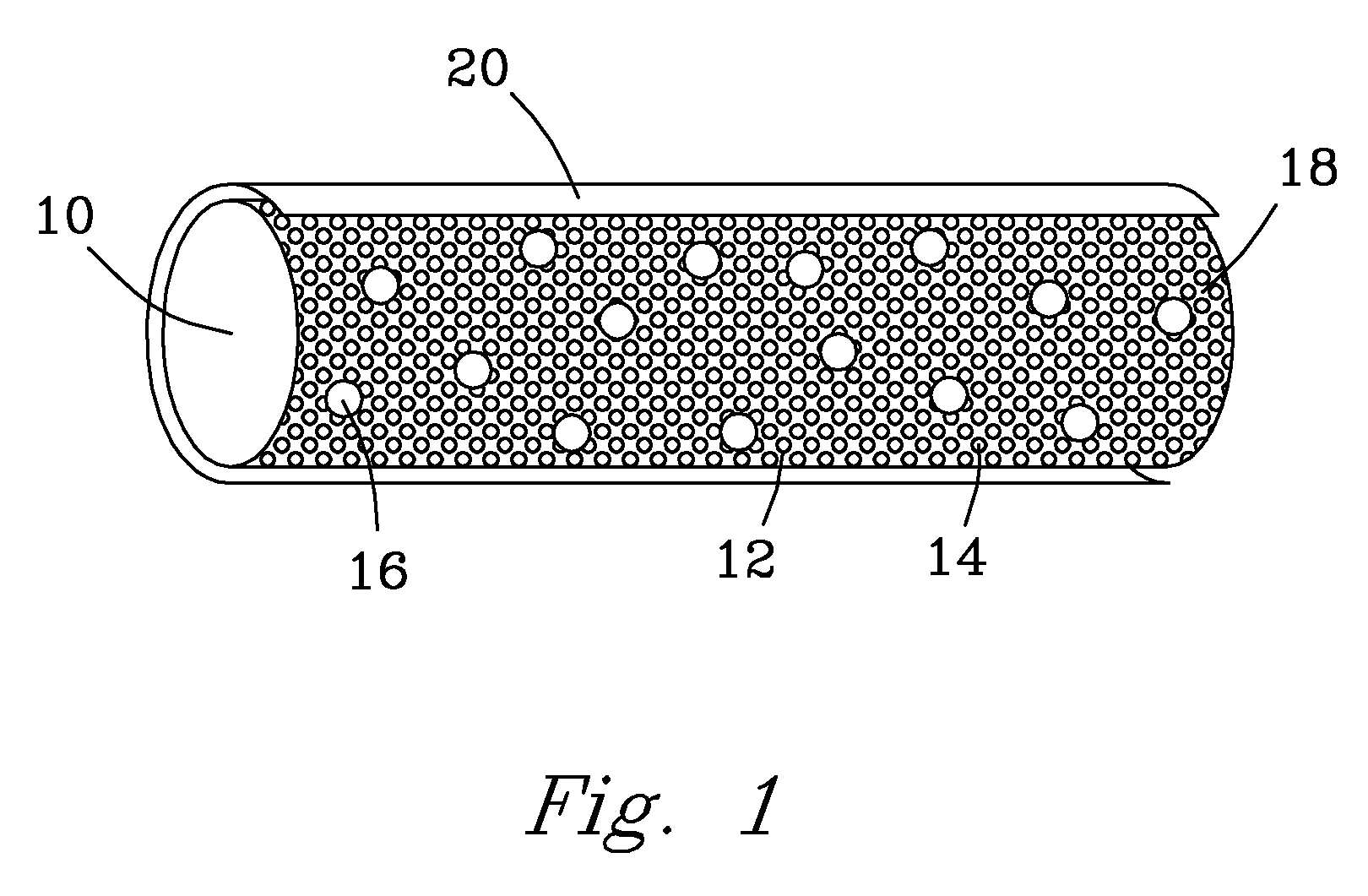
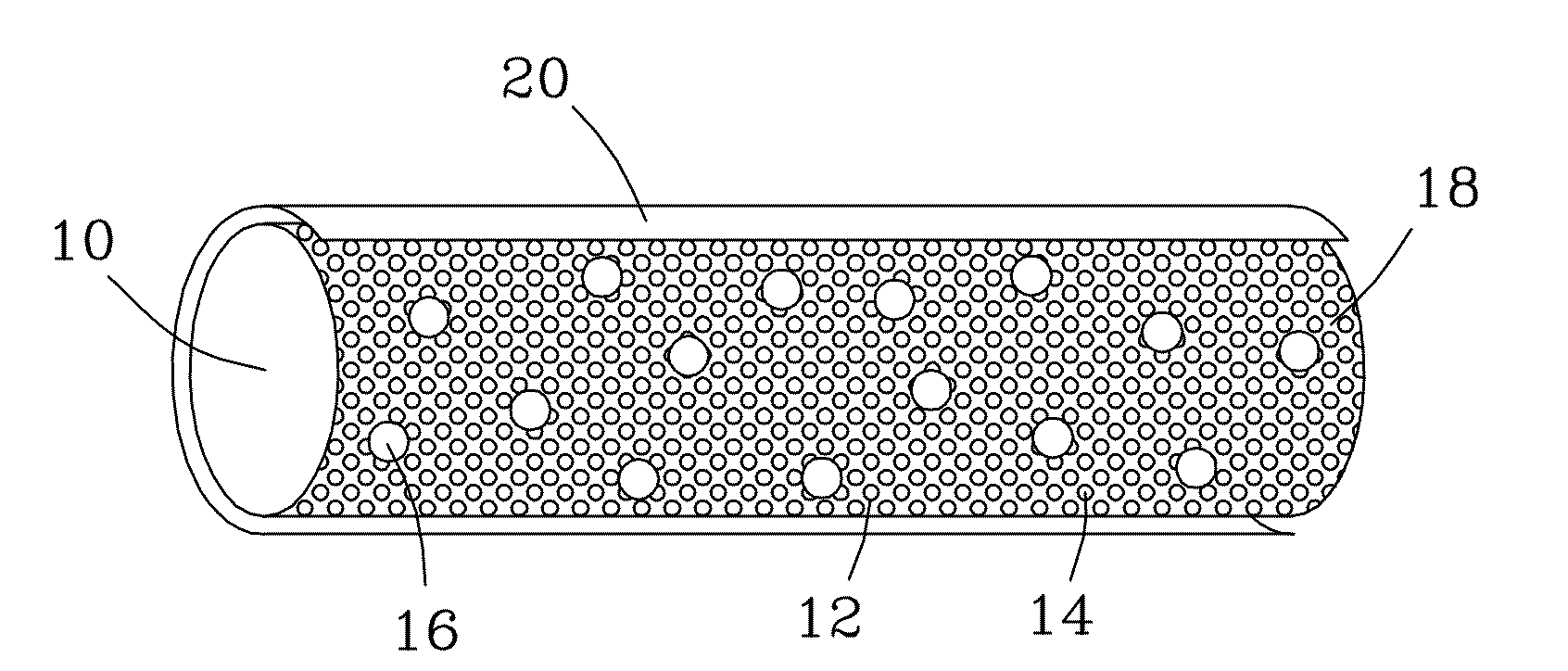
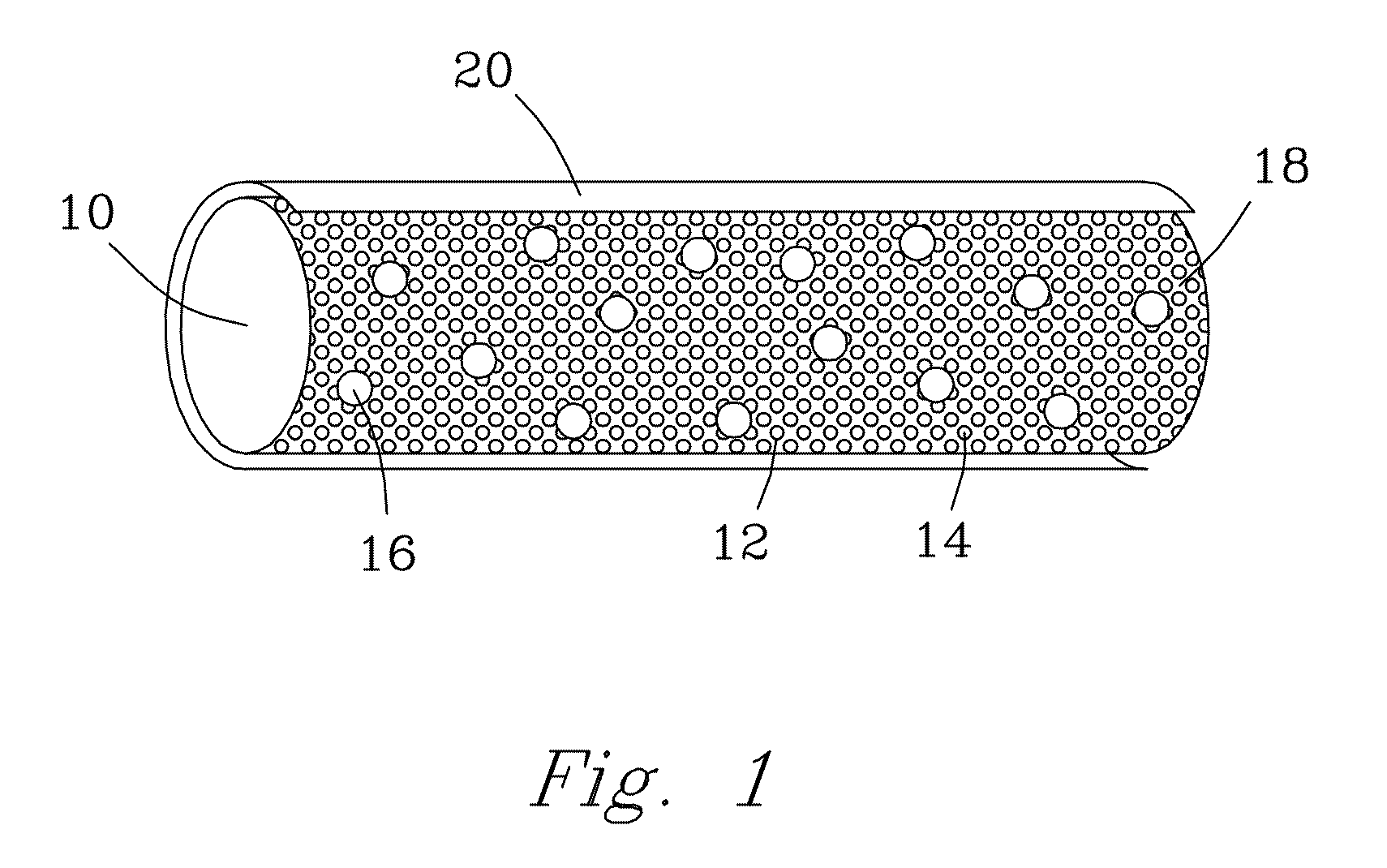
Bioactive, resorbable scaffolds for tissue engineering
InactiveUS20050118236A1High porosityImprove manufacturabilityBiocideSynthetic resin layered productsPorosityFiber
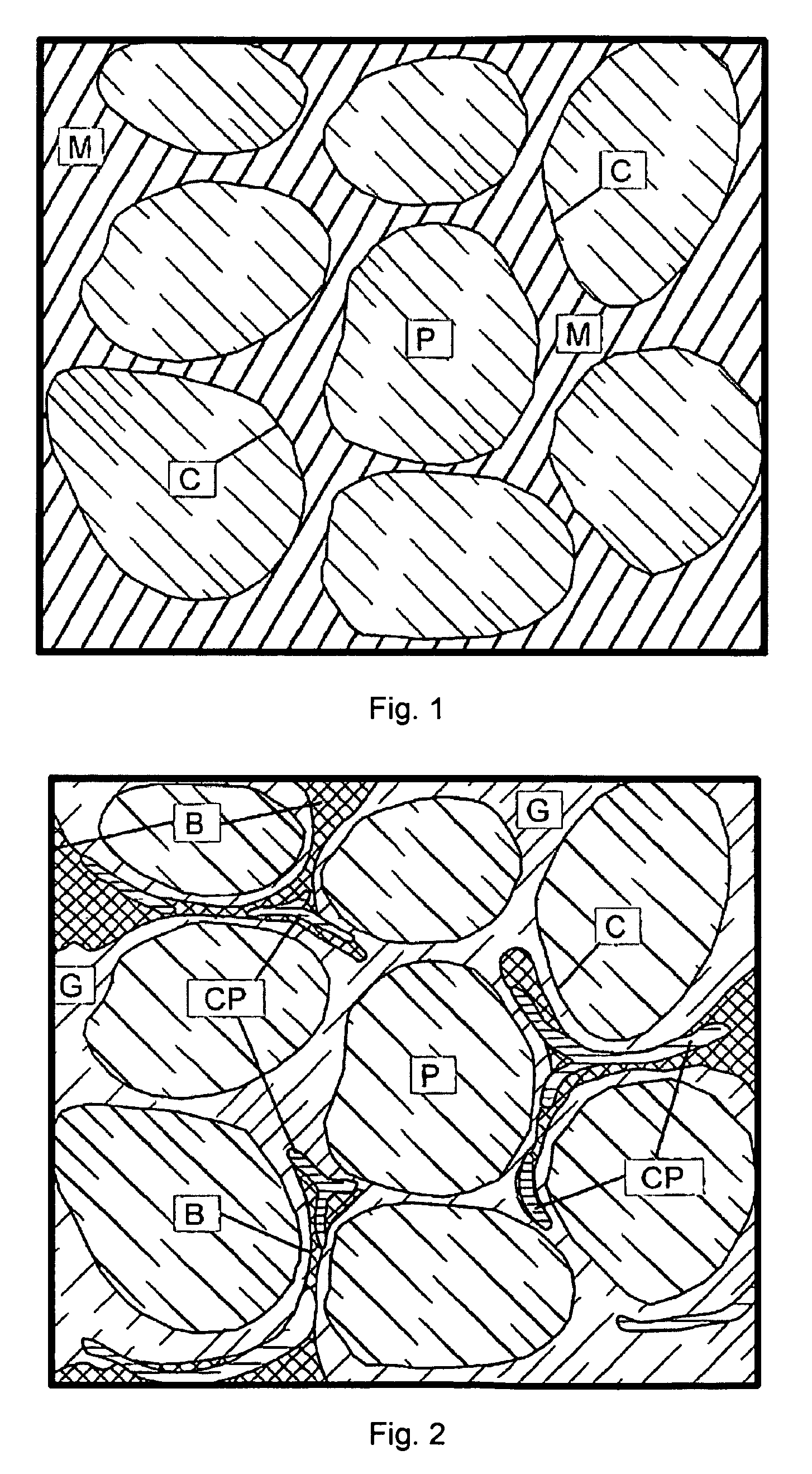
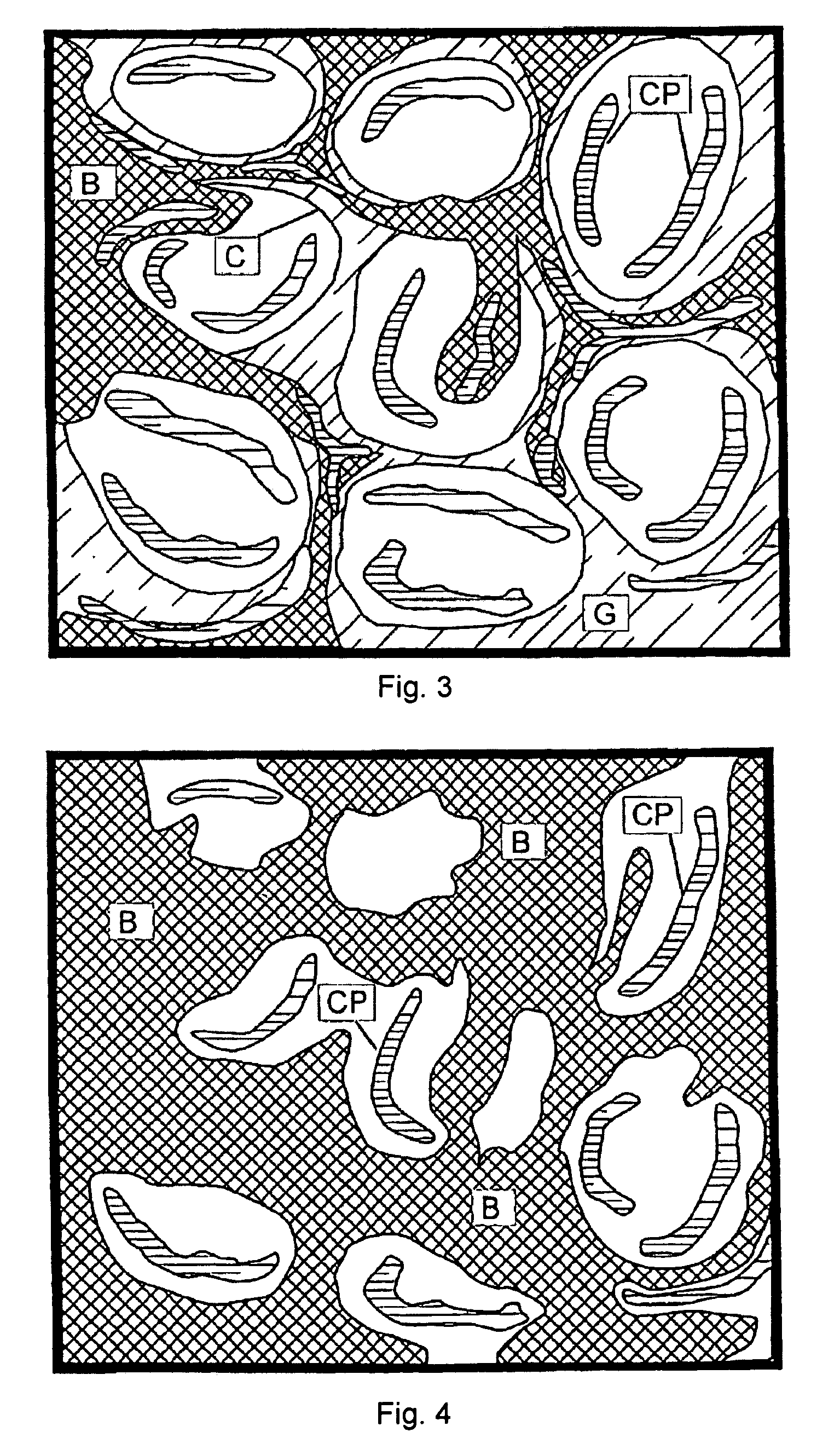
Flexible, bioactive glass meshes and scaffolds made therefrom are provided. The meshes comprise interwoven bioactive glass fibers that can be coated with resorbable polymers. Meshes can also be woven from glass fibers and resorbable polymers. Scaffolds can be constructed by a plurality of meshes, which can have varying porosities to create porosity gradients in the scaffold. Methods of making scaffolds are provided which can comprise pulling bioactive glass fibers, winding the fibers, forming the fibers into bundles, coating the fibers with a resorbable polymer, and creating a biaxial weave with the bundles. Soft tissue engineering methods are also provided for creating scaffolds for incubating cells such as fibroblasts and chondroblasts. Meshes and scaffolds are suitable for tissue engineering, such as bone tissue engineering and cartilage tissue engineering.
Owner:GENTIS
Resorbable Pouches for Implantable Medical Devices
ActiveUS20080128315A1Reduces and prevents implantReduces and prevents and surgery-related complicationBiocideElectrotherapyResorbable polymersSide effect
Biodegradable and resorbable polymer pouches are described for use with cardiac rhythm mamagent devices (CRMs) and other implantable medical devices (IMDs), i.e., a pouch, covering, or other receptacle capable of encasing, surrounding and / or holding the CRM or other IMD for the purpose of securing it in position, inhibiting or reducing bacterial growth, providing pain relief and / or inhibiting scarring or fibrosis on or around the CRM or other IMD. Optionally, the biodegradable and resorbable pouches of the invention include one or more drugs in the polymer matrix to provide prophylactic effects and alleviate side effects or complications associated with the surgery or implantation of the CRM or other IMD.
Owner:MEDTRONIC INC
Fracture fixation systems
ActiveUS20120029102A1Firmly connectedImprove permeabilityImpression capsSurgical adhesivesResorbable polymersPolymer resin
Systems for bone fracture repair are disclosed. One system includes a biocompatible putty that may be packed about a bone fracture to provide full loadbearing capabilities within days. The disclosed putties create an osteoconductive scaffold for bone regeneration and degrade over time to harmless 5 resorbable byproducts. Fixation devices for contacting an endosteal wall of an intramedullary (IM) canal of a fractured bone are also disclosed. One such fixation device includes a woven elongated structure fabricated from resorbable polymer filaments. The woven elongated structure has resilient properties that allow the woven 10 structure to be radially compressed and delivered to the IM canal using an insertion tube. When the insertion tube is removed, the woven structure expands towards its relaxed cross-sectional width to engage the endosteal wall. The woven elongated structure is impregnated with a resorbable polymer resin that cures in situ, or in the IM canal.
Owner:SMITH & NEPHEW INC
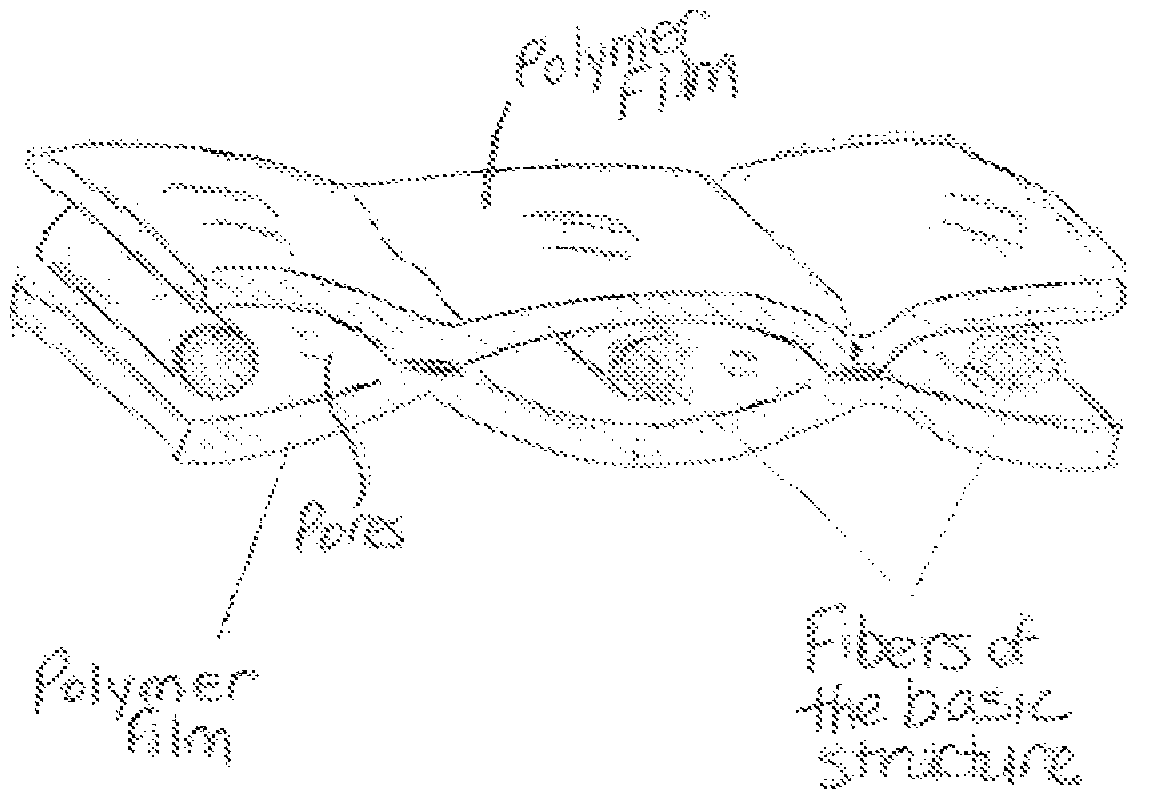
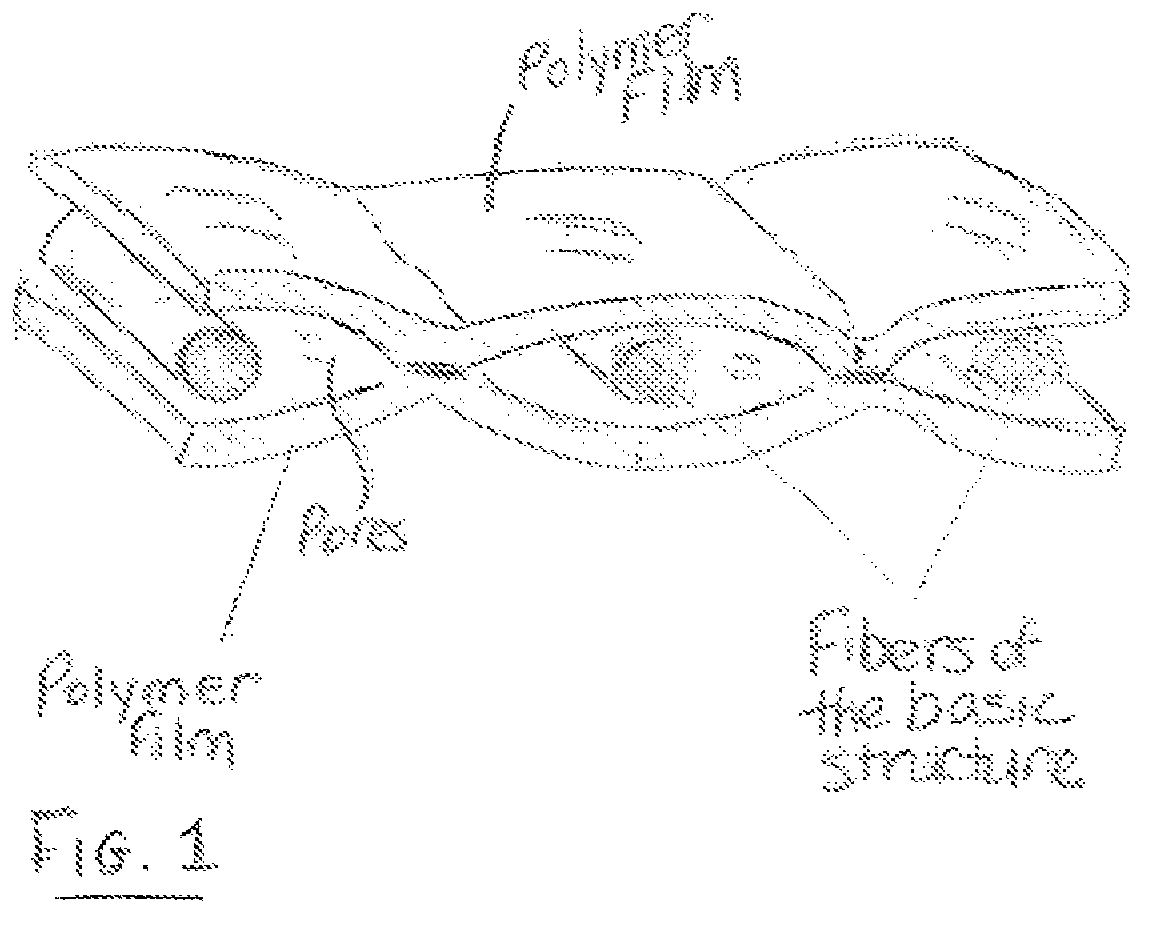
Areal implant
ActiveUS7615065B2Reduces formation of fusion (adhesions) of internal structurePromote growthWarp knittingProsthesisResorbable polymersPolymer thin films
Owner:ETHICON GMBH
Areal implant
ActiveUS20050010306A1Improve toleranceReduces formation of fusion (adhesions)Warp knittingProsthesisResorbable polymersPolymer thin films
An areal implant has a long-term-stable, mesh-like basic structure which has pores of a size in the range from 1.5 mm to 8 mm and is provided, at least in a part area, on both sides with a synthetic, resorbable polymer film. The two polymer films are glued or welded together in pores of the basic structure.
Owner:ETHICON GMBH
Pliable conformable bone restorative
Owner:ORTHOVITA INC
Methods for governing bone growth
Resorbable polymer barrier membranes and methods of their applications are disclosed. In a broad embodiment, methods of governing bone growth, or preventing bone growth into a certain spatial area, includes the step of forming a spatial barrier with the present resorbable barrier membrane. The barrier membrane separates a bone-growth area and a non-bone-growth area, and prevents bone from growing into the non-growth area.
Owner:CYTORI THERAPEUTICS INC
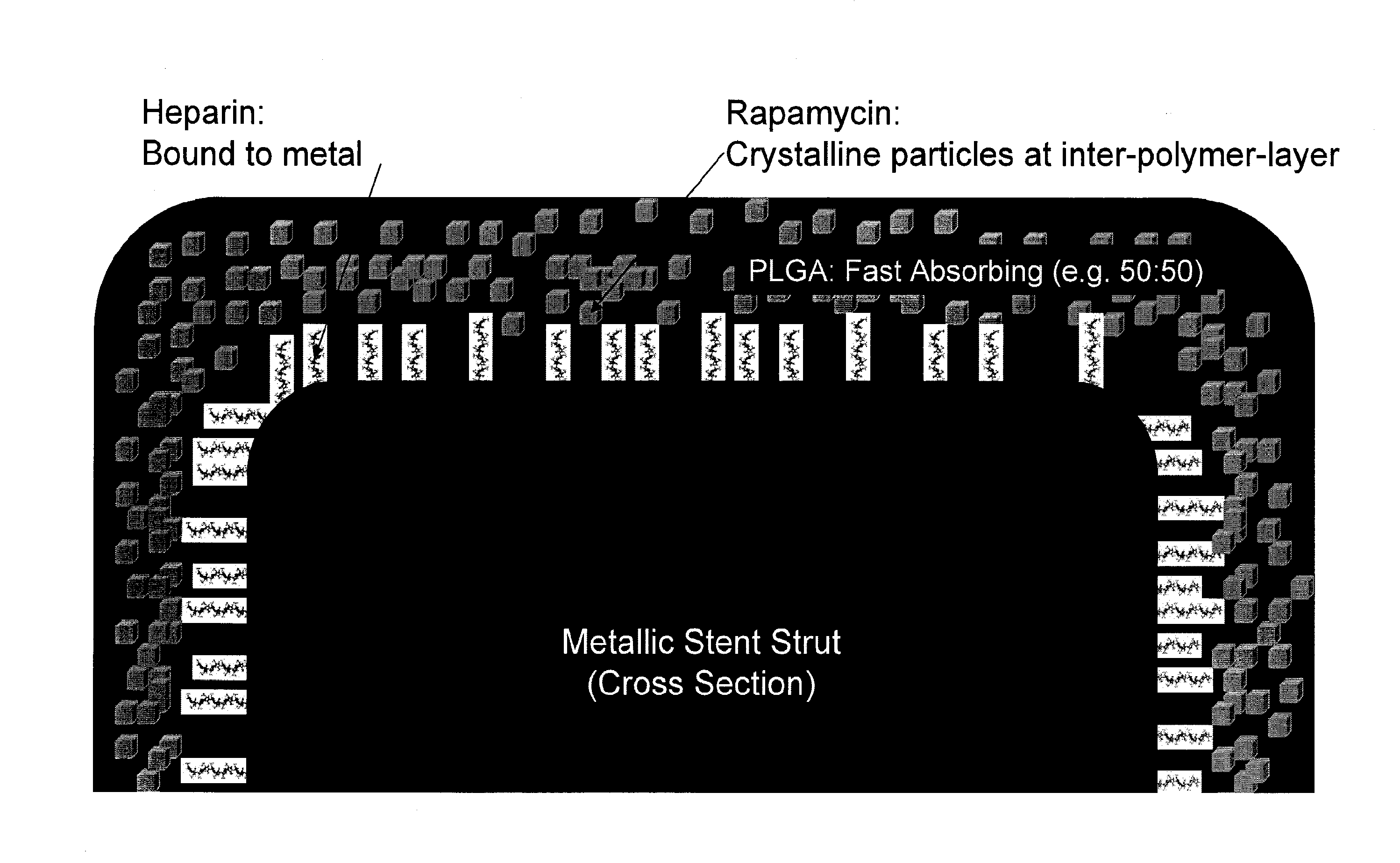
Drug Coated Stents
Provided herein is a coated coronary stent, comprising: a stent framework; heparin molecules attached to the stent framework; and a rapamycin-polymer coating wherein at least part of rapamycin is in crystalline form. In one embodiment, the rapamycin-polymer coating comprises one or more resorbable polymers.
Owner:MICELL TECH INC
Minimal injury resorbable stent
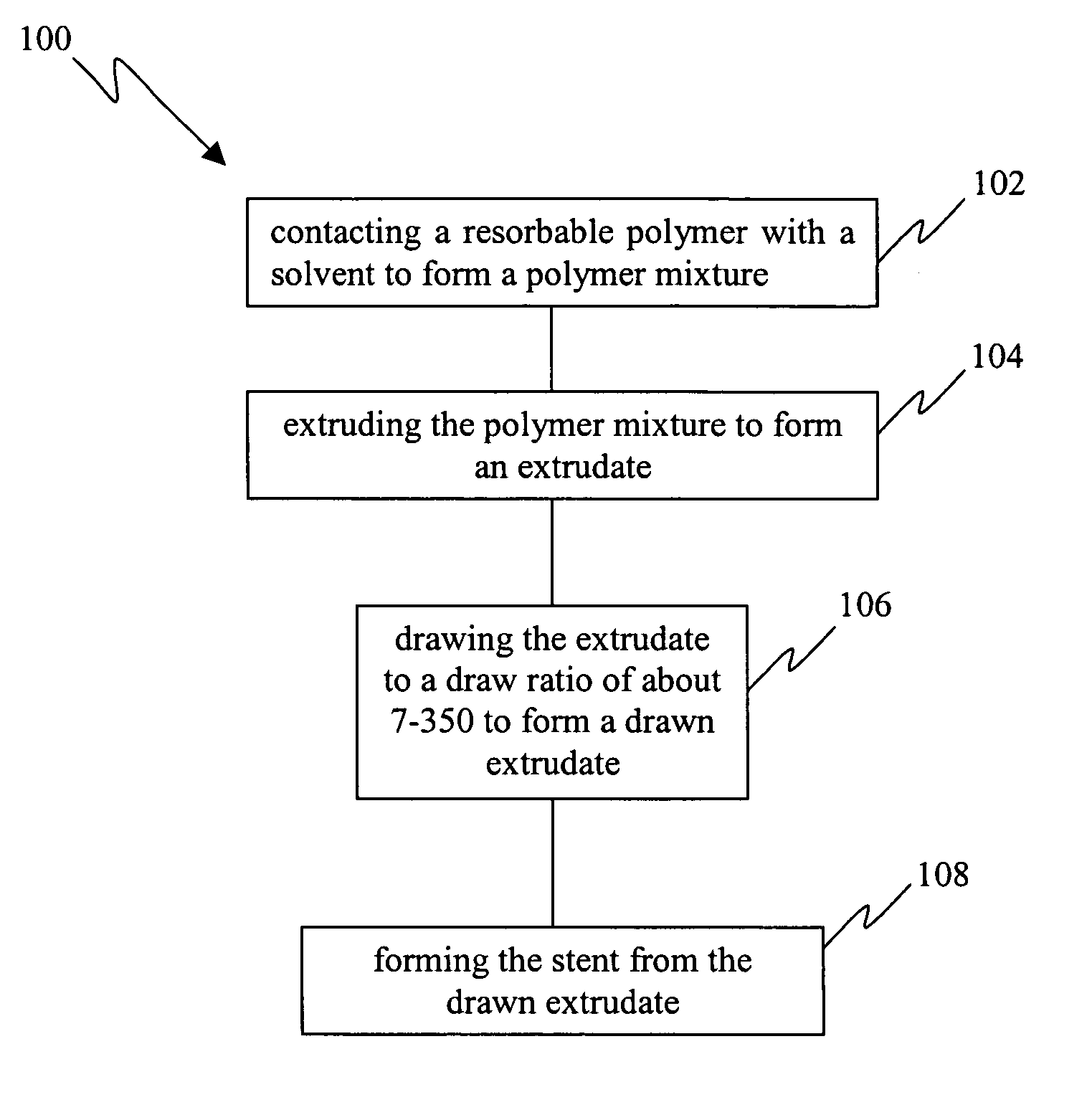
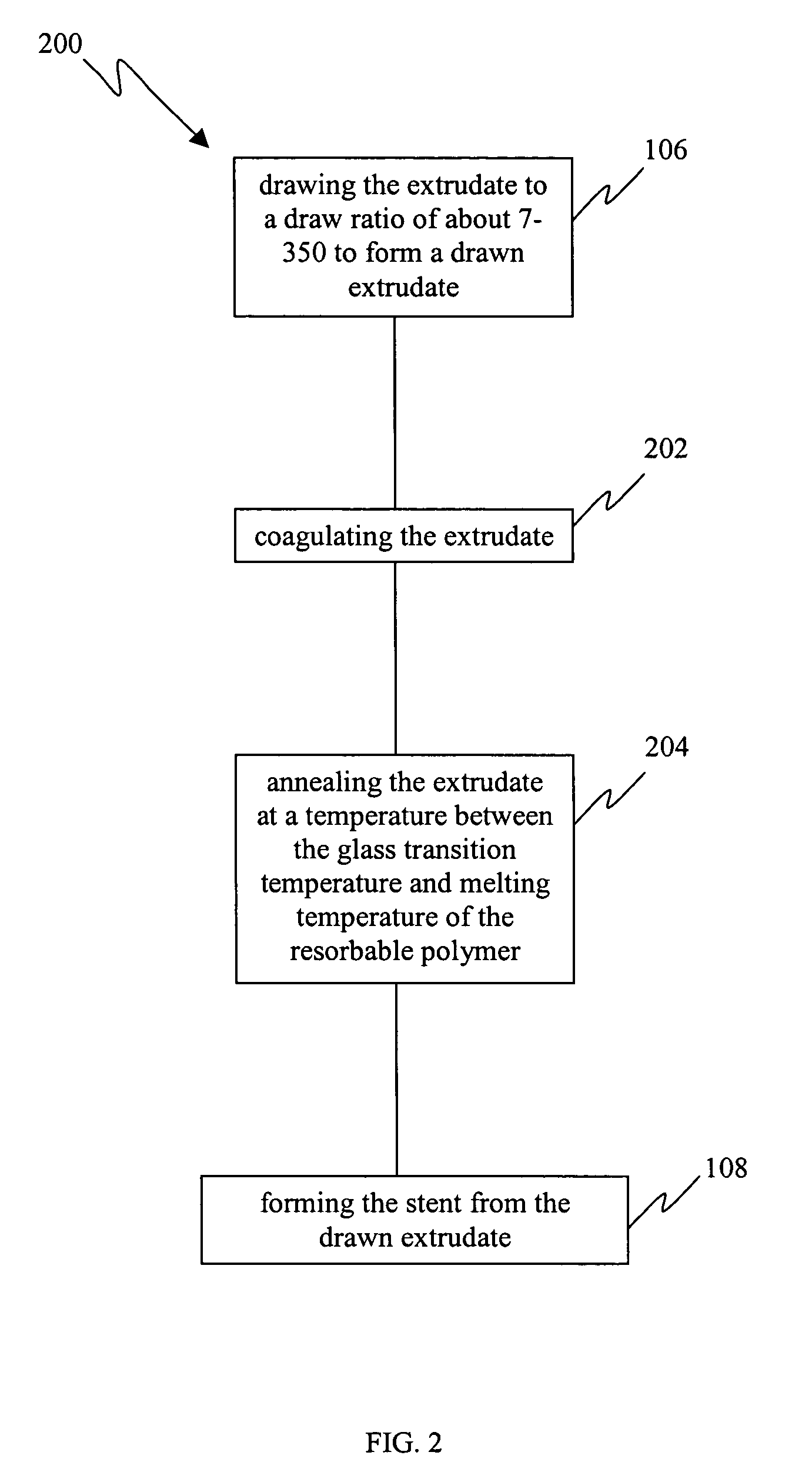
InactiveUS20050149172A1Improved propertyMinimal injuryMonocomponent protein artificial filamentSurgeryResorbable polymersPlasticizer
A minimal injury resorbable stent comprising an oriented resorbable material drawn to a ratio about 7-350 is disclosed. The drawn materials of the present invention have tensile strength of about 50-500 MPa and Young's modulus of about 2-300 GPa. The bioresorbable stents can have cylindrical shape and optionally further comprise one or more of a solvent, plasticizer, biologically active agent and modifier. Also disclosed is a method for manufacturing a minimal injury resorbable stent. The process comprises contacting a resorbable polymer with a solvent to form a polymer mixture, extruding said polymer mixture to form an extrudate, drawing said extrudate to a draw ratio in the range of about 7-350 to form a drawn extrudate and forming said stent from said drawn extrudate. The process optionally further comprises coagulating the extrudate and annealing the extrudate and / or stent.
Owner:MEDTRONIC VASCULAR INC
Composition and Method For Treating Tissue Defects
InactiveUS20100080791A1Peptide/protein ingredientsMetabolism disorderResorbable polymersTissue defect
Owner:ETHICON INC
Fracture fixation systems
InactiveUS20120095463A1Firmly connectedImprove permeabilityInternal osteosythesisSurgical adhesivesResorbable polymersPolymer resin
Systems for bone fracture repair are disclosed. One system includes a biocompatible putty that may be packed about a bone fracture to provide full loadbearing capabilities within days. The disclosed putties create an osteoconductive scaffold for bone regeneration and degrade over time to harmless resorbable byproducts. Fixation devices for contacting an endosteal wall of an intramedullary (IM) canal of a fractured bone are also disclosed. One such fixation device includes a woven elongated structure fabricated from resorbable polymer filaments. The woven elongated structure has resilient properties that allow the woven structure to be radially compressed and delivered to the IM canal using an insertion tube. When the insertion tube is removed, the woven structure expands towards its relaxed cross-sectional width to engage the endosteal wall. The woven elongated structure is impregnated with a resorbable polymer resin that cures in situ, or in the IM canal.
Owner:SMITH & NEPHEW INC
Resorbable polymer compositions for use in medicine, dentistry, and surgery
ActiveUS20090286886A1Overcomes lack of resorbabilityLittle tendency to absorb waterCosmetic preparationsPowder deliveryResorbable polymersMedicine
Owner:SYNCERA
Stent Formed from Crosslinked Bioabsorbable Polymer and Methods of Making the Stent
A stent having a stent body made from a crosslinked bioabsorbable polymer is disclosed. A method of making the stent including exposing a tube formed from a bioabsorbable polymer to radiation to crosslink the bioabsorbable polymer and forming a stent body from the exposed tube is disclosed. The tube can include a crosslinking agent which induces crosslinking upon radiation exposure. Additionally or alternatively, the bioabsorbable polymer can be a copolymer that crosslinks upon exposure to radiation in the absence of a crosslinking agent.
Owner:ABBOTT CARDIOVASCULAR
Methods for governing bone growth
Owner:CYTORI THERAPEUTICS INC
Resorbable Phenolic Polymers
ActiveUS20090088548A1Inhibition formationRobust mechanical propertyOrganic chemistryResorbable polymersMedical device
The invention provides biocompatible resorbable polymers, comprising monomer units having formula (I), formula (II), formula (III) or formula (IV). The polymers degrade over time when implanted in the body, and are useful as components of implantable medical devices.
Owner:MEDTRONIC INC
Tendon and Ligament Repair Sheet and Methods of Use
InactiveUS20090157193A1High suture pull-out strengthHigh strengthDiagnosticsSurgeryResorbable polymersPorous layer
Methods and device for treating or healing an injured tendon or ligament is disclosed. The device, a tendon and ligament repair sheet, has a porous layer and a denser layer, and optionally a therapeutic agent in the porous layer, the denser layer or both. The repair sheet is made from a resorbable or non-resorbable polymer. The repair sheet is securely attached to the injured tendon, ligament, muscle, or bone and has a suture pull out strength of at least 3N. If the injury involve severing of a ligament or tendon, one should place the severed ends in close proximity to each other and securely attach the repair sheet to both sides of the severed tendon or ligament at a distance from the injury so that the repair sheet remains securely attached to the tendon, ligament, muscle, or bone while the tissue is healing.
Owner:WARSAW ORTHOPEDIC INC
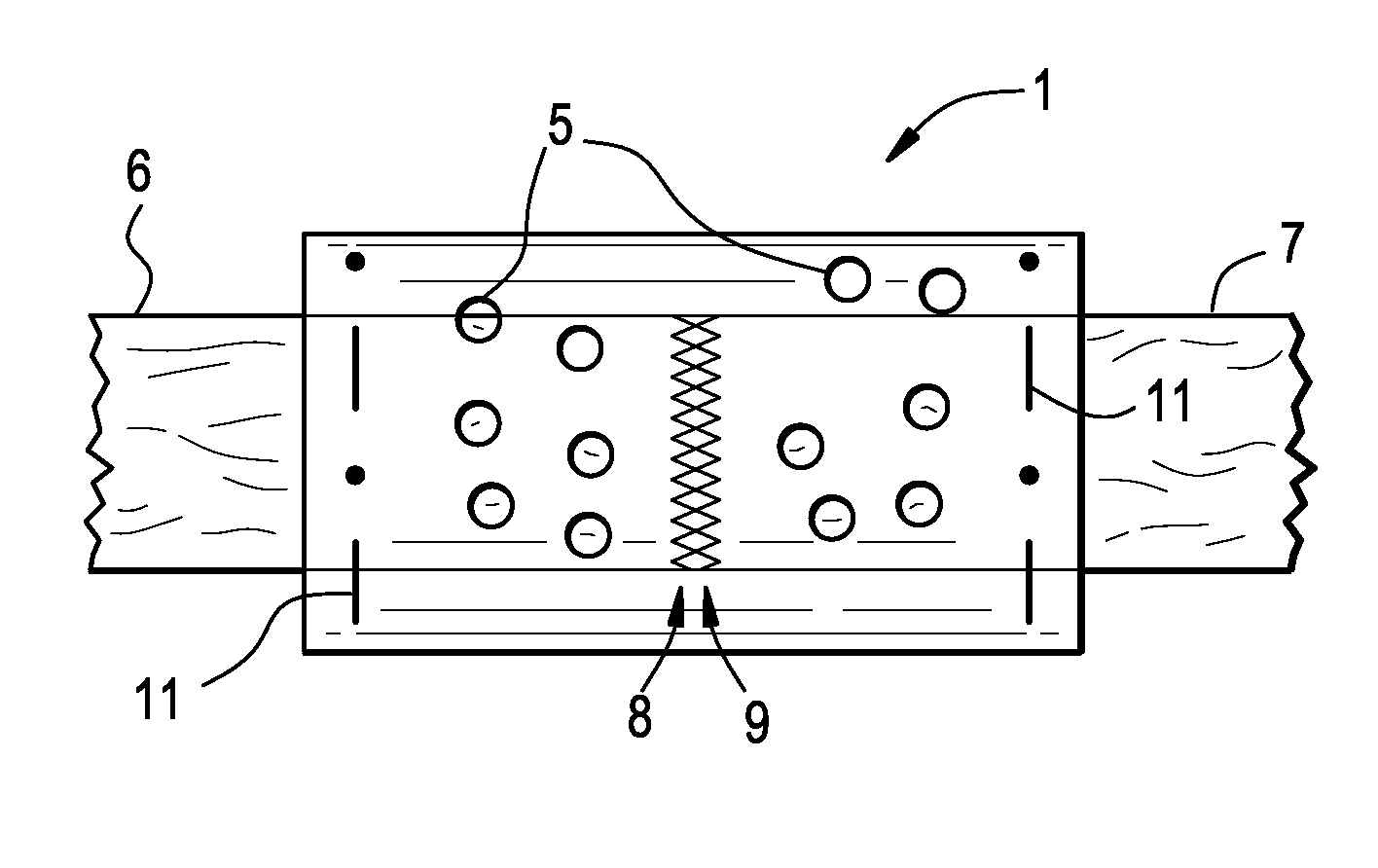
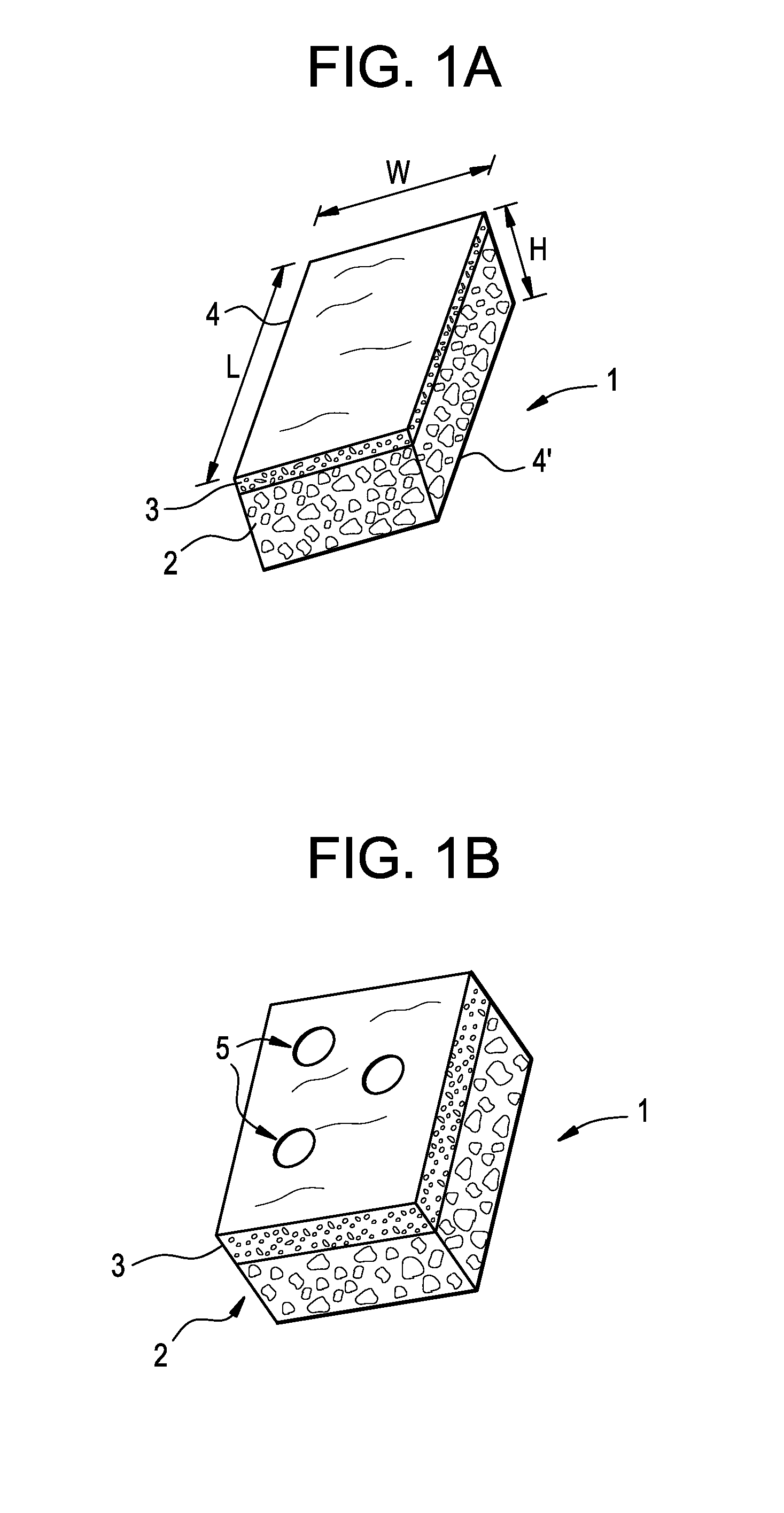
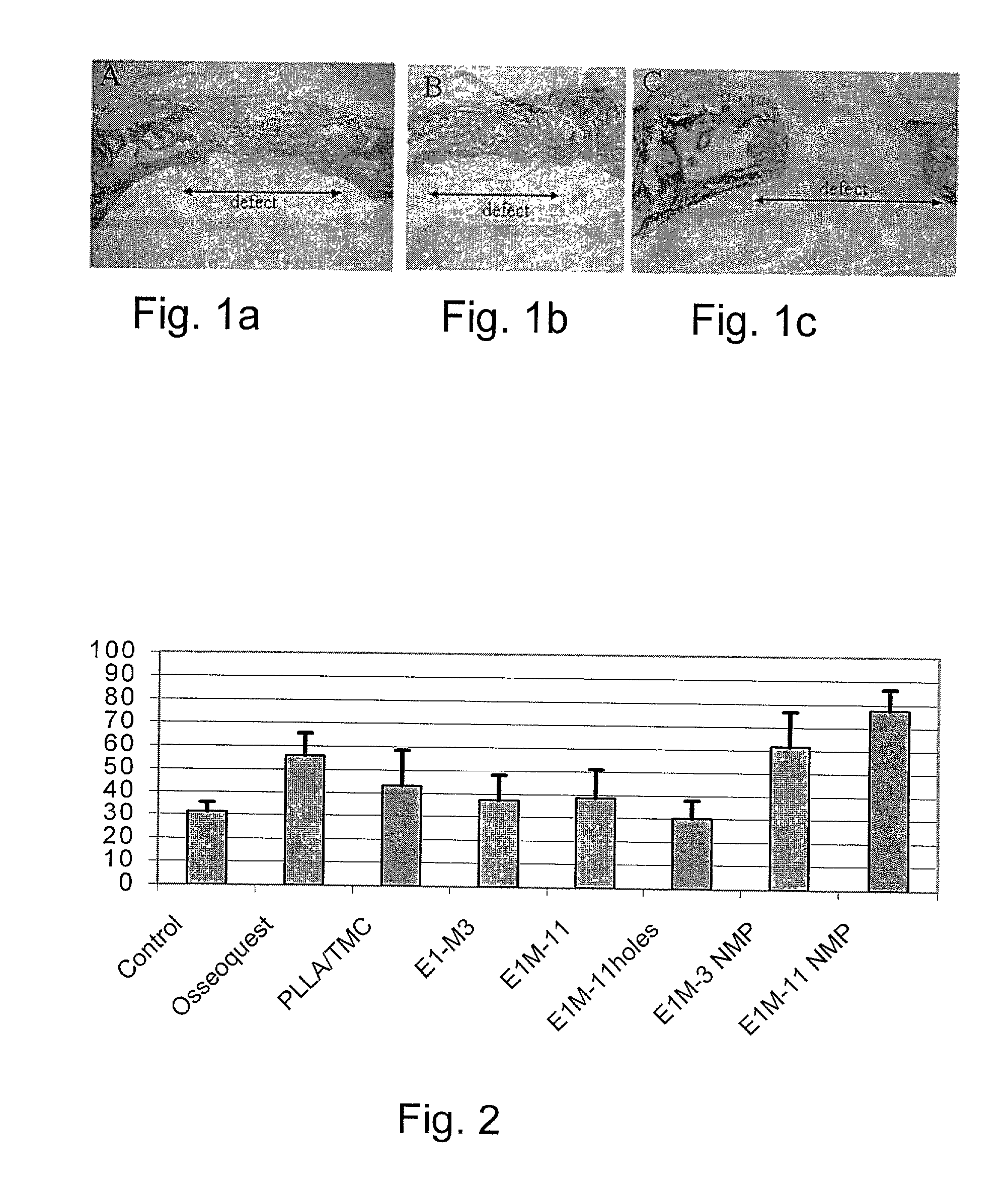
Resorbable polymer composition, implant and method of making implant
InactiveUS20030104029A1Induce bone growthEnhances bone healingBiocideImpression capsResorbable polymers2-Pyrrolidone
Novel polymer compositions that are useful in the manufacture of medical implants, implants having osteogenic properties and methods of making said implants are disclosed. Polymer compositions comprise a base material including a polymer matrix of resorbable polymer(s) or copolymer(s), and N-methyl-2-pyrrolidone (NMP), wherein NMP is present in an amount imparting osteogenic properties for the composition.
Owner:INION
Resorbable pouches for implantable medical devices
ActiveUS9023114B2Reduces and prevents implant and surgery-related complicationReduce swellingBiocideElectrotherapyResorbable polymersSide effect
Biodegradable and resorbable polymer pouches are described for use with cardiac rhythm mamagent devices (CRMs) and other implantable medical devices (IMDs), i.e., a pouch, covering, or other receptacle capable of encasing, surrounding and / or holding the CRM or other IMD for the purpose of securing it in position, inhibiting or reducing bacterial growth, providing pain relief and / or inhibiting scarring or fibrosis on or around the CRM or other IMD. Optionally, the biodegradable and resorbable pouches of the invention include one or more drugs in the polymer matrix to provide prophylactic effects and alleviate side effects or complications associated with the surgery or implantation of the CRM or other IMD.
Owner:MEDTRONIC INC
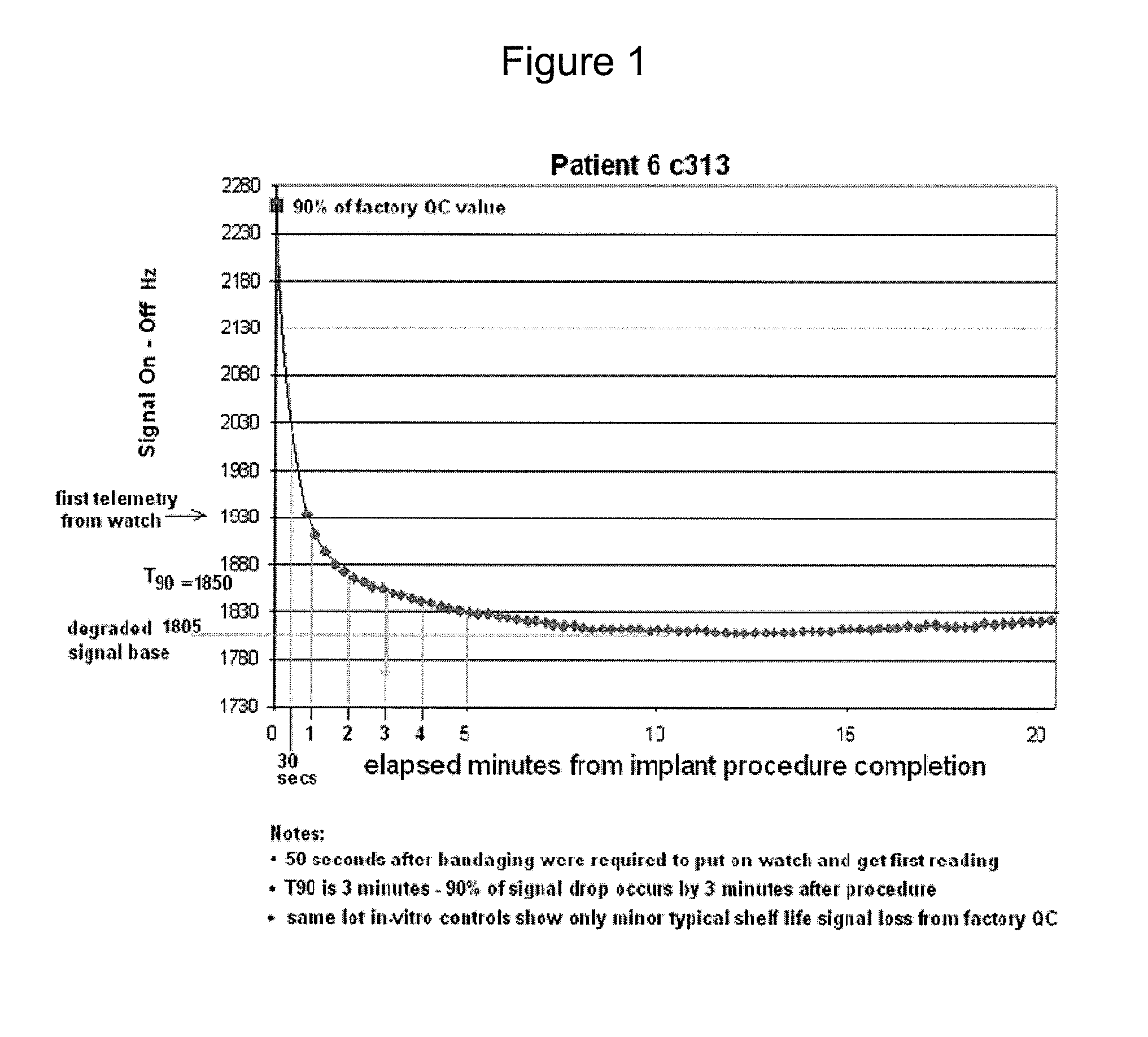
Protective shell for an in vivo sensor made from resorbable polymer
InactiveUS20100298674A1Prevents and reduces degradation and interferenceCatheterSensorsInjury causeResorbable polymers
An implantable device with in vivo functionality, where the functionality of the device is negatively affected by the inflammation reaction generally associated with tissue injury, encapsulated by a protective coating that prevents damage to the device from any inflammation reactions. The protective coating is designed to persist for a set period of time, generally until after the inflammation reaction of the surrounding in vivo environment in response to the injury caused by the implantation procedure has concluded. The protective coating is further designed to “resorb” (i.e. to dissociate from the device, dissolve, and be absorbed into the surrounding environment) after a set period of time, allowing the device to perform its in vivo functionality unhindered without loss of performance.
Owner:SENSORS FOR MEDICINE & SCI INC
Resorbable barrier micro-membranes for attenuation of scar tissue during healing
InactiveUS20090060978A1Reduce scarsTo offer comfortPeptide/protein ingredientsSurgeryUltrasound attenuationResorbable polymers
Resorbable lactide polymer micro-membranes are disclosed. The micro-membranes are constructed of polylactide resorbable polymers, which are engineered to be absorbed into the body relatively slowly over time in order to reduce potential negative side effects. The membranes are formed to have very thin thicknesses, for example, thicknesses between about 0.010 mm and about O.300 mm. The membranes can be extruded from polylactide polymers having a relatively high viscosity property, can be preshaped with relatively thick portions, and can be stored in sterile packages.
Owner:MAST BIOSURGERY
Resorbable polymer composition, implant and method of making implant
Novel polymer compositions that are useful in the manufacture of medical implants, implants having osteogenic properties and methods of making said implants are disclosed. Polymer compositions comprise a base material including a polymer matrix of resorbable polymer(s) or copolymer(s), and N-methyl-2-pyrrolidone (NMP), wherein NMP is present in an amount imparting osteogenic properties for the composition.
Owner:INION
Time release calcium sulfate and growth factor matrix for bone augmentation
A bone-growth stimulating composition for forming a resorbable implant, methods for making such a composition and a corresponding putty / paste material. In some embodiments of the invention, such a material includes a plurality of particles having a predetermined size and comprising a first calcium sulfate compound, a resorbable polymer in a predetermined weight ratio and a growth factor.
Owner:ORTHOGEN LLC
Implants for Soft and Hard Tissue Regeneration
ActiveUS20140200667A1Promote osseointegrationPromote formationWeft knittingBone implantFiberCalcium biphosphate
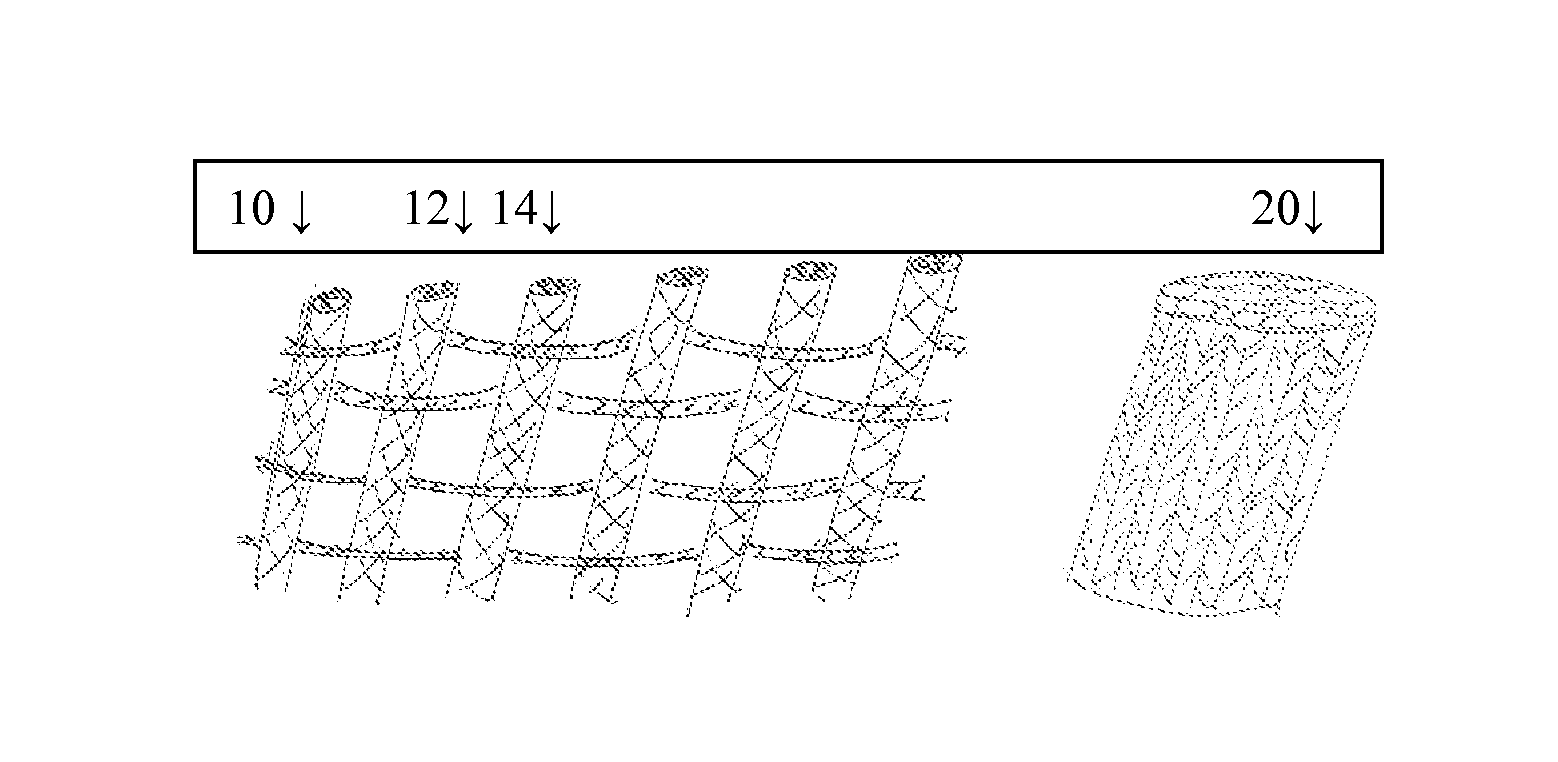
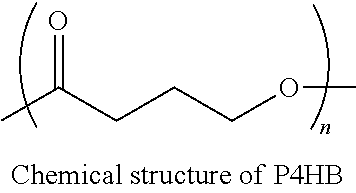
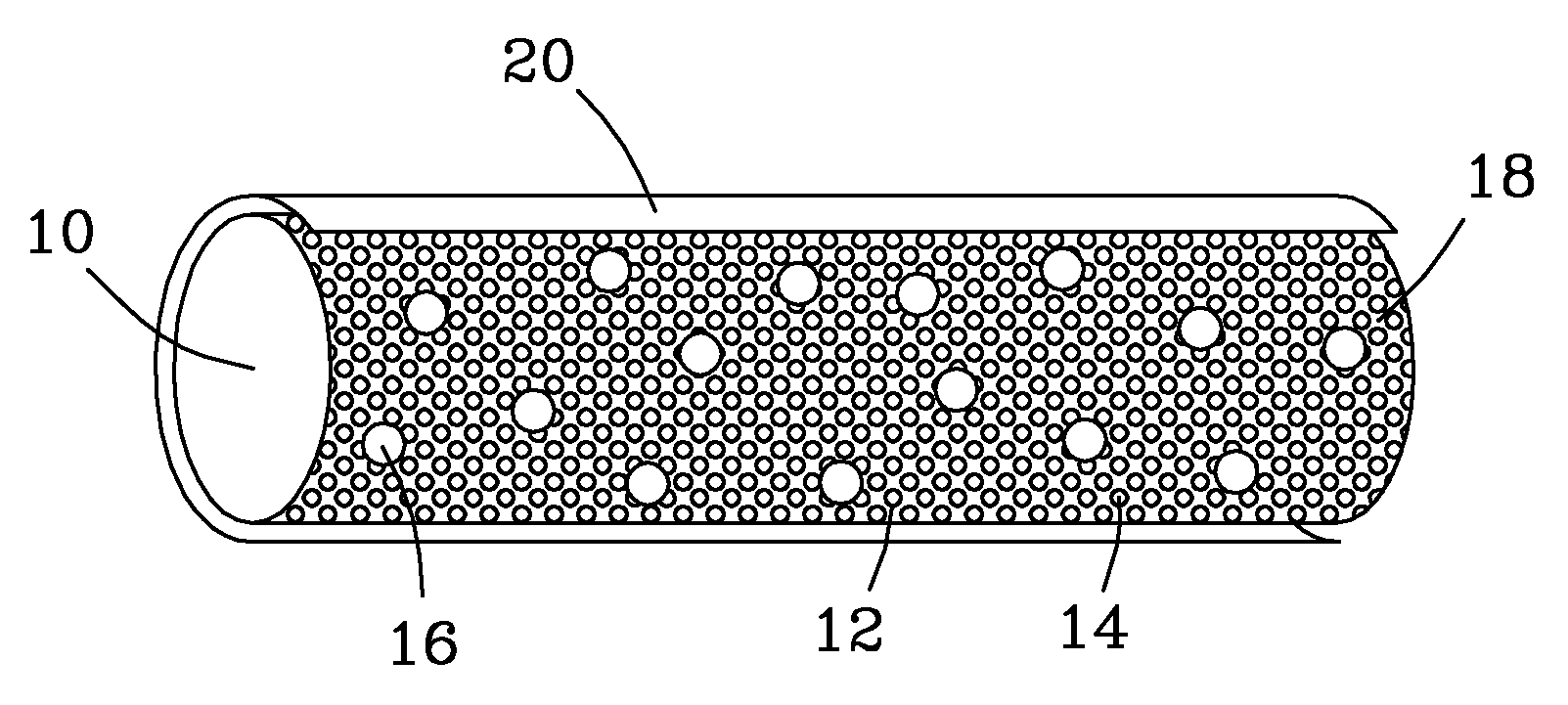
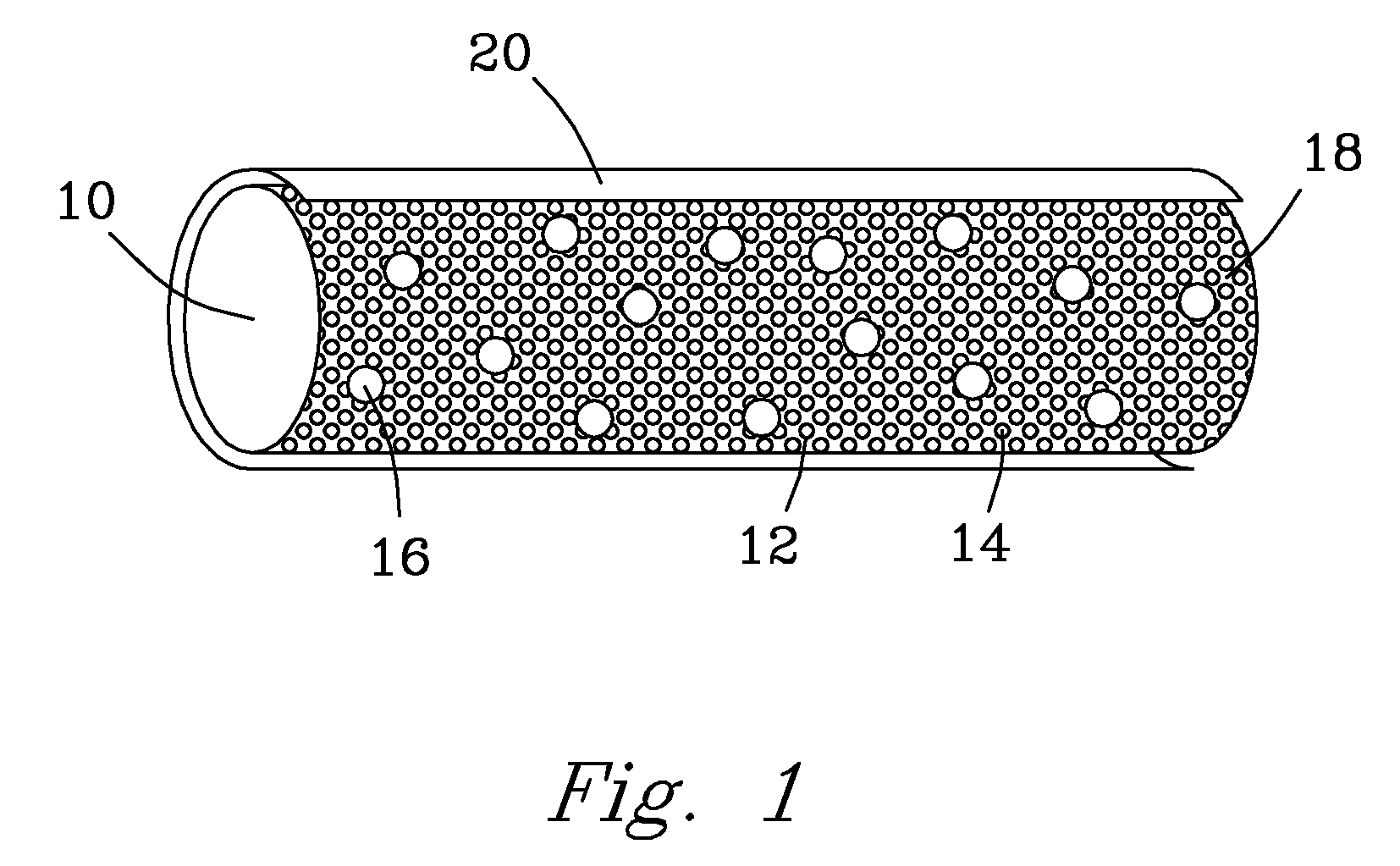
Implants for osteo and osteochondral repair have been developed. These implants include a series of channels between the upper and lower surfaces of the implants, such that when implanted the lower surfaces are situated in an area rich in bone marrow and the channels provide a means for the bone marrow to migrate through the implant. Preferably the implants are made from resorbable polymer fibers, preferably arranged in braids that are knitted or woven together such that the braids are substantially parallel with each other. The implants may be rolled into a bundle of braids with the axis of the braids substantially parallel to the axis of the bundle, to provide channels along the axis of the bundle. A preferred embodiment includes P4HB fibers braided and knitted into a structure that is coated with a ceramic, preferably physiologic calcium phosphate.
Owner:TEPHA INC
Brachytherapy seed with fast dissolving matrix for optimal delivery of radionuclides to cancer tissue
A system, method and device for treating tumor cells utilizing a resorbable therapy seed made up of microspheres containing a beta- or alpha-particle-emitting radiation source and a resorbable polymer matrix. These seeds are implanted within the tumor and then rapidly dissolved so as to release the microspheres from the polymer matrix. These microspheres then spread within a preselected target area and provide radiation therapy in a predetermined amount and at a preselected rate according the specific needs and necessities of the users. The configuration of the microspheres, the types of radiation provided and the location and use of these microspheres provides desired localized treatment to target cells while preferentially avoiding or minimizing undesired damage to surrounding tissue. The present invention provides a method for making the seeds, as well as a method for utilizing the seeds as a part of the treatment method.
Owner:BATTELLE MEMORIAL INST +1
Resorbable thin membranes
InactiveUS20070116739A1Simple chemical reaction and formulationReduce scarsOrganic active ingredientsSurgeryResorbable polymersSide effect
Resorbable lactide polymer thin membranes are disclosed. The thin membranes are constructed of polylactide resorbable polymers, which are engineered to be absorbed into the body relatively slowly over time in order to reduce potential negative side effects. The membranes are formed to have very thin thicknesses, for example, thicknesses between about 0.010 mm and about 0.300 mm. The membranes can be extruded from polylactide polymers having a relatively high viscosity property, can be preshaped with relatively thick portions, and can be stored in sterile packages.
Owner:MAST BIOSURGERY
Brachytherapy Seed With Fast Dissolving Matrix for Optimal Delivery of Radionuclides To Cancer Tissue
InactiveUS20100056843A1Improve radiation qualityLow costX-ray/gamma-ray/particle-irradiation therapyAbnormal tissue growthResorbable polymers
A system, method and device for treating tumor cells utilizing a resorbable therapy seed made up of microspheres containing a beta-particle-emitting radiation source and a resorbable polymer matrix. These seeds are implanted within the tumor and then rapidly dissolved or broken so as to release the microspheres. These microspheres then spread within a preselected target area and provide radiation therapy in a predetermined amount and at a preselected rate according the specific needs and necessities of the users. The configuration of the microspheres, the types of radiation provided and the location and use of these microspheres provides desired localized treatment to target cells while preferentially avoiding undesired damage to surrounding tissue. The present invention provides a method for making the seeds, as well as a method for utilizing the seeds as a part of the treatment method.
Owner:BATTELLE MEMORIAL INST +1
Brachytherapy seed with fast dissolving matrix for optimal delivery of radionuclides to cancer tissue
InactiveUS20100056844A1Improve radiation qualityLow costPowder deliverySurgeryAbnormal tissue growthResorbable polymers
A system, method and device for treating tumor cells utilizing a resorbable therapy seed made up of microspheres containing a beta- or alpha-particle-emitting radiation source and a resorbable polymer matrix. These seeds are implanted within the tumor and then rapidly dissolved so as to release the microspheres from the polymer matrix. These microspheres then spread within a preselected target area and provide radiation therapy in a predetermined amount and at a preselected rate according the specific needs and necessities of the users. The configuration of the microspheres, the types of radiation provided and the location and use of these microspheres provides desired localized treatment to target cells while preferentially avoiding or minimizing undesired damage to surrounding tissue. The present invention provides a method for making the seeds, as well as a method for utilizing the seeds as a part of the treatment method.
Owner:BATTELLE MEMORIAL INST +1
Pre-shaped user-formable micro-membrane implants
InactiveUS20100310628A1Shorten the timeThin thicknessSurgeryProsthesisResorbable polymersPolymer science
Precut, user-shapeable, resorbable polymer micro-membranes are disclosed. The micro-membranes are constructed of resorbable polymers, which are engineered to attenuate adhesions and to be absorbed into the body relatively slowly over time. The membranes can formed to have very thin thicknesses, for example, thicknesses between about 0.010 mm and about 0.300 mm, while maintaining adequate strength. The membranes can be extruded from polylactide polymers having a relatively high viscosity property, can be stored in sterile packages, and can be preshaped with relatively high reproducibility during implantation procedures.
Owner:MAST BIOSURGERY
Resorbable polymer composition, implant and method of making implant
InactiveUS20060257448A1Impart propertySurgeryPharmaceutical non-active ingredientsResorbable polymersGlycerol
Novel polymer compositions that are useful in the manufacture of medical implants, implants having osteogenic properties and methods of making the implants are disclosed. Polymer compositions include a base material having a polymer matrix of resorbable polymer(s) or copolymer(s), and a glycerol mono-, di-, or triester derivative, wherein the glycerol mono-, di-, or triester derivative is present in an amount imparting osteogenic properties for the composition.
Owner:UNIV ZURICH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com