Resorbable pouches for implantable medical devices
a medical device and pouch technology, applied in the direction of prosthesis, rigid containers, drug compositions, etc., can solve the problems of undue complexity of replacement surgery and little that can be done, and achieve the effects of reducing or preventing implant- or surgery-related complications, reducing swelling and inflammation associated with implantation, and reducing the swelling and inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Resorbable Pouch
[0085]To prepare a resorbable pouch, 18.0 g of polymer, 1.0 g minocycline and 1.0 g rifampin are dissolved in 75 mL of a solution of tetrahydrofuran-methanol. This solution is poured over a level non-stick Teflon surface. A calibrated stainless steel gardner knife is used to spread the solution to the desired thickness, typically to a range of from about 2 and about 400 microns.
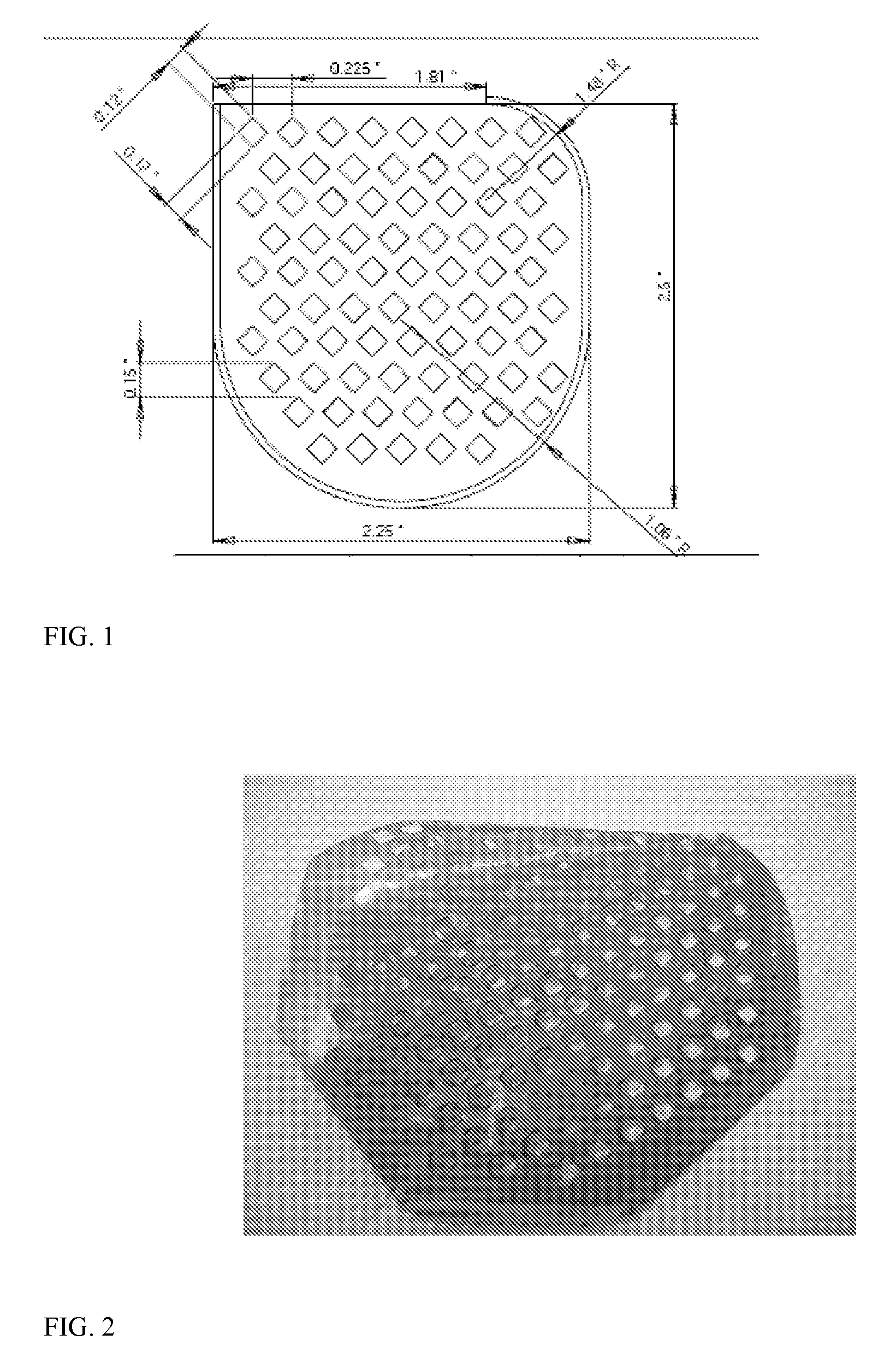
[0086]The film is dried at ambient temperature overnight. Thereafter, the solvent cast film is dried in a convection oven at 50° C. for 1 day, the temperature of the oven is increased to 80° C. and the film is dried further for 2 days. At this point, the dried film is ready for laser cutting and further processing to create the pouch, i.e., cutting and sealing three of the sides. The pouch is cut for overall shape to match the desired CRM and to create a mesh like covering. A CRM pouch made using a DT-DTE succinate polymer is shown in FIG. 4.
example 2
Resorbable Clamshell Pouch
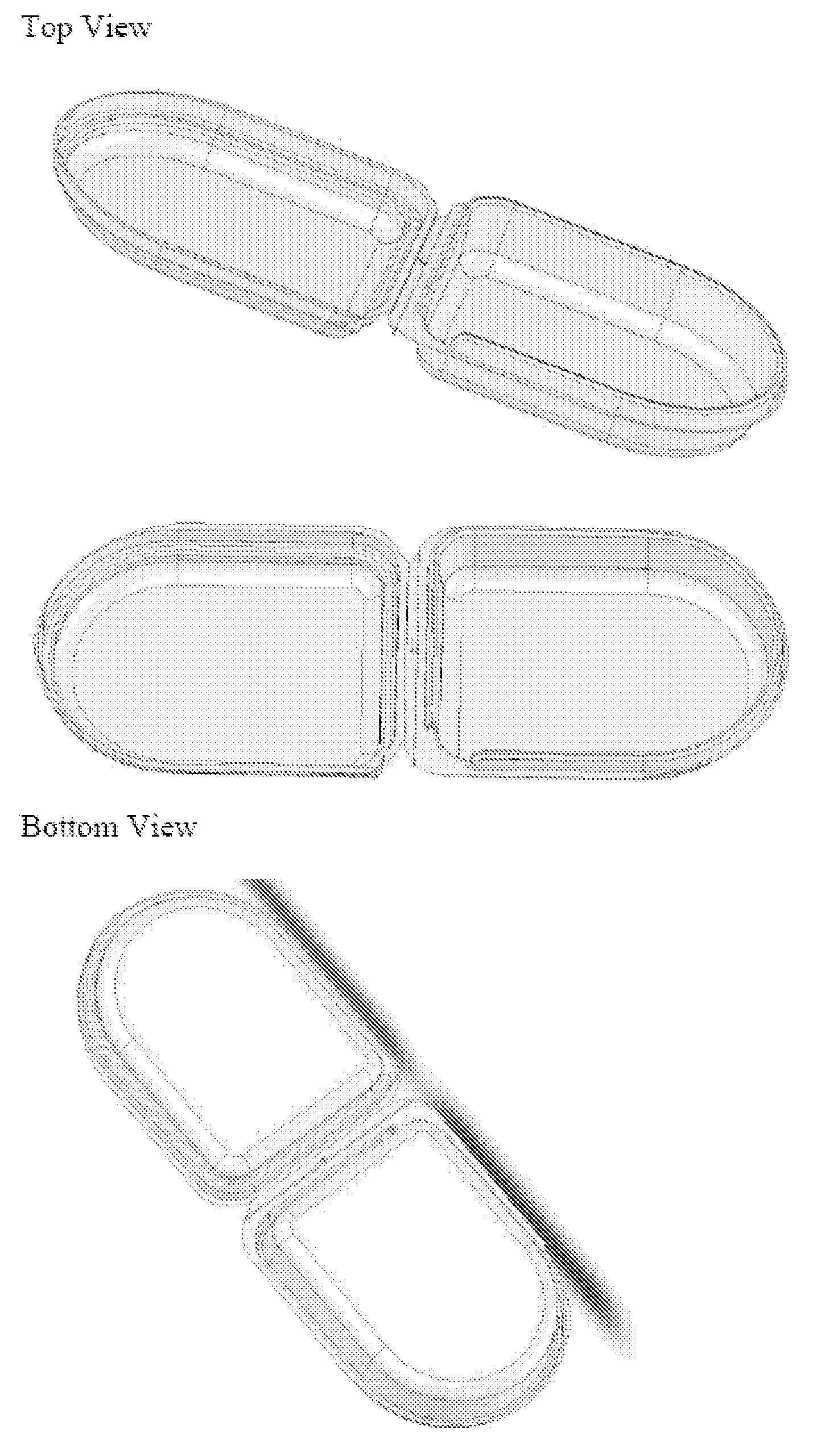
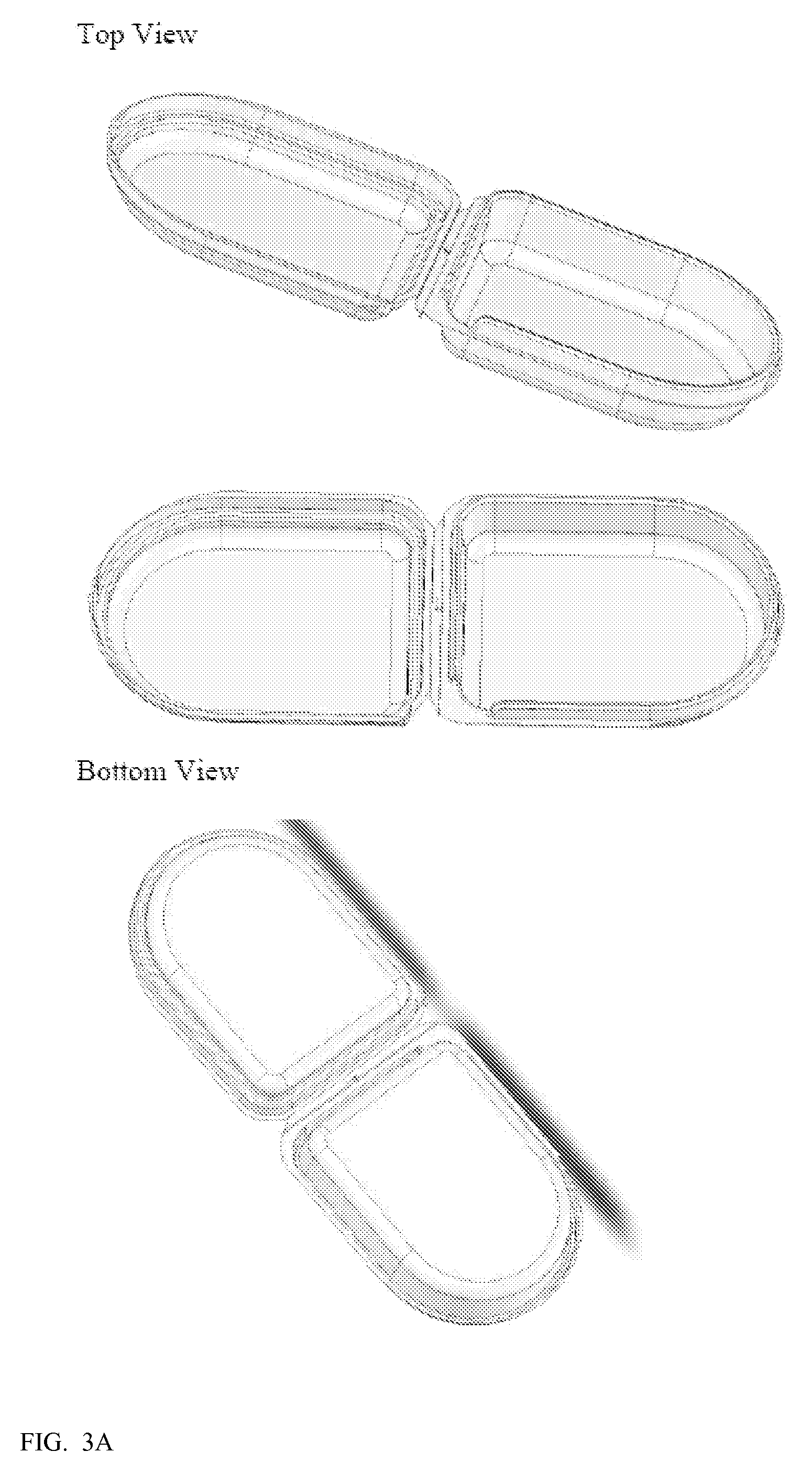
[0087]To prepare a resorbable clamshell pouch, a film is prepared as described above in Example 1. After the film is dried, it is thermoformed into the clamshell shape by placing a film sheet into a frame and heating the film to about 90-100° C. and lowering the frame over a clamshell-shaped mold as shown in FIG. 7 whereby the film takes the shape of the clamshell mold. The molded clamshell is cooled, freed from the mold, and laser cut from the film sheet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com