Patents
Literature
141 results about "Implantation procedure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
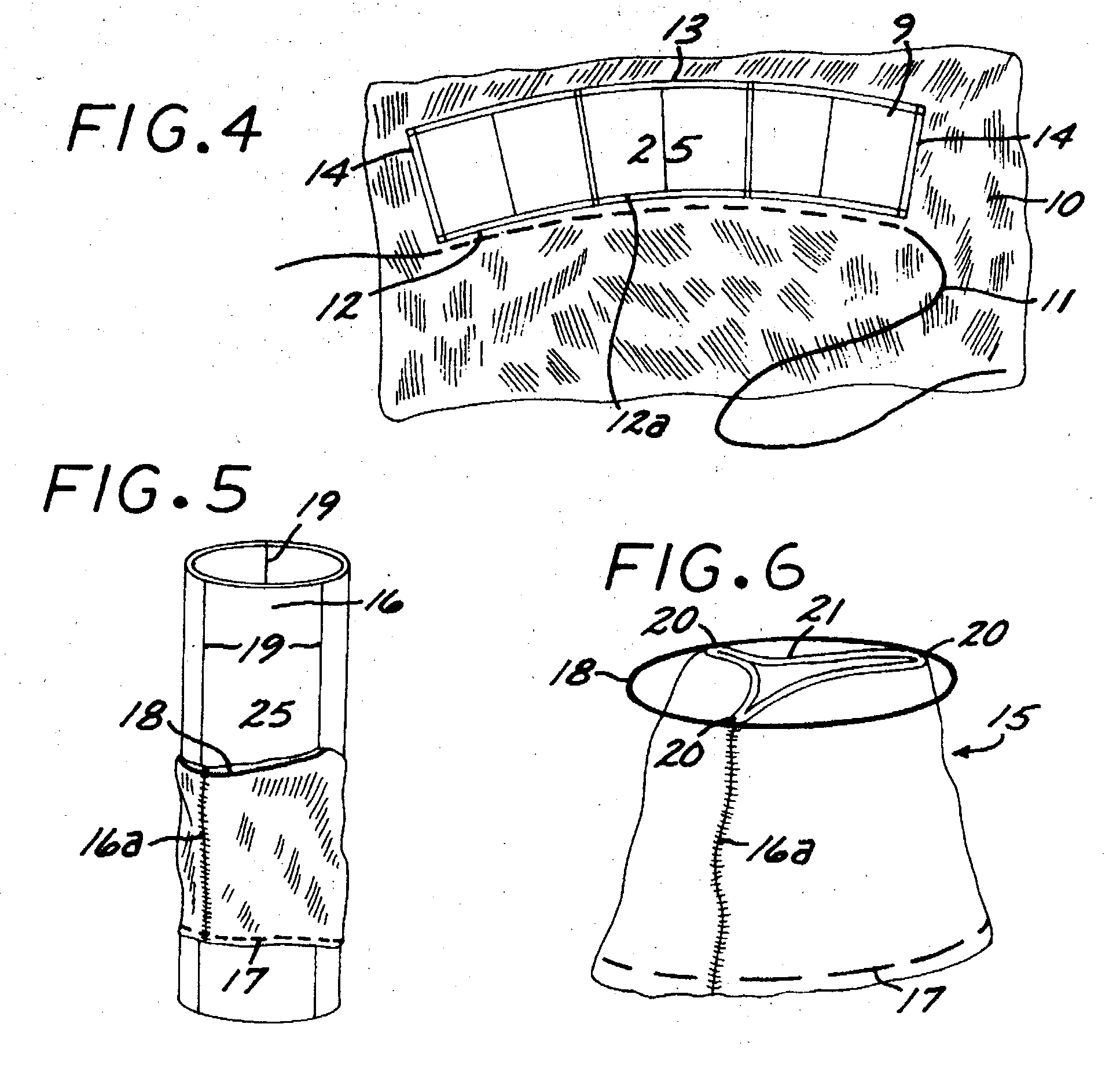
Prosthetic Valve for Transluminal Delivery
InactiveUS20100004740A1Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesVenous accessImplantation Site
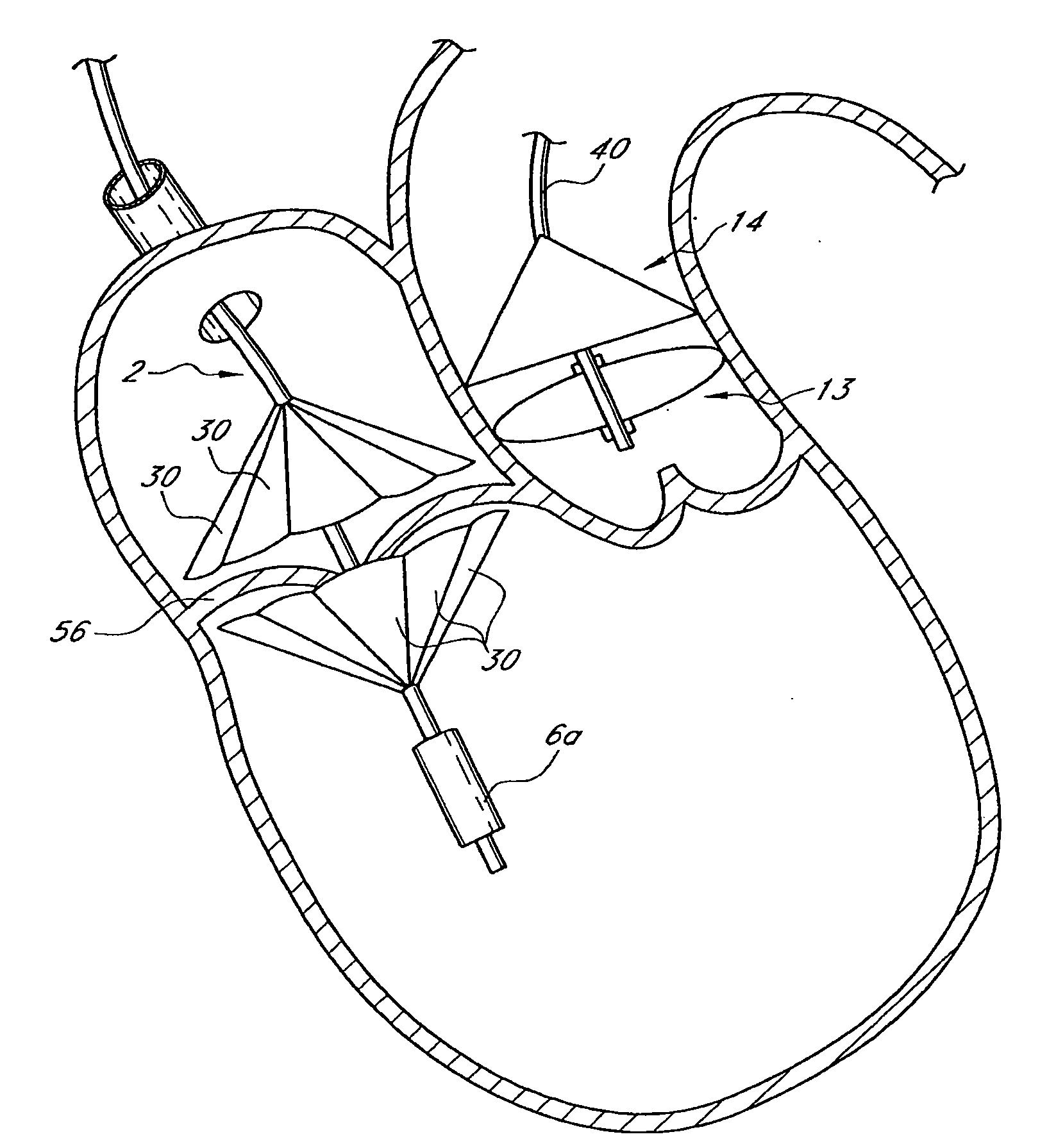
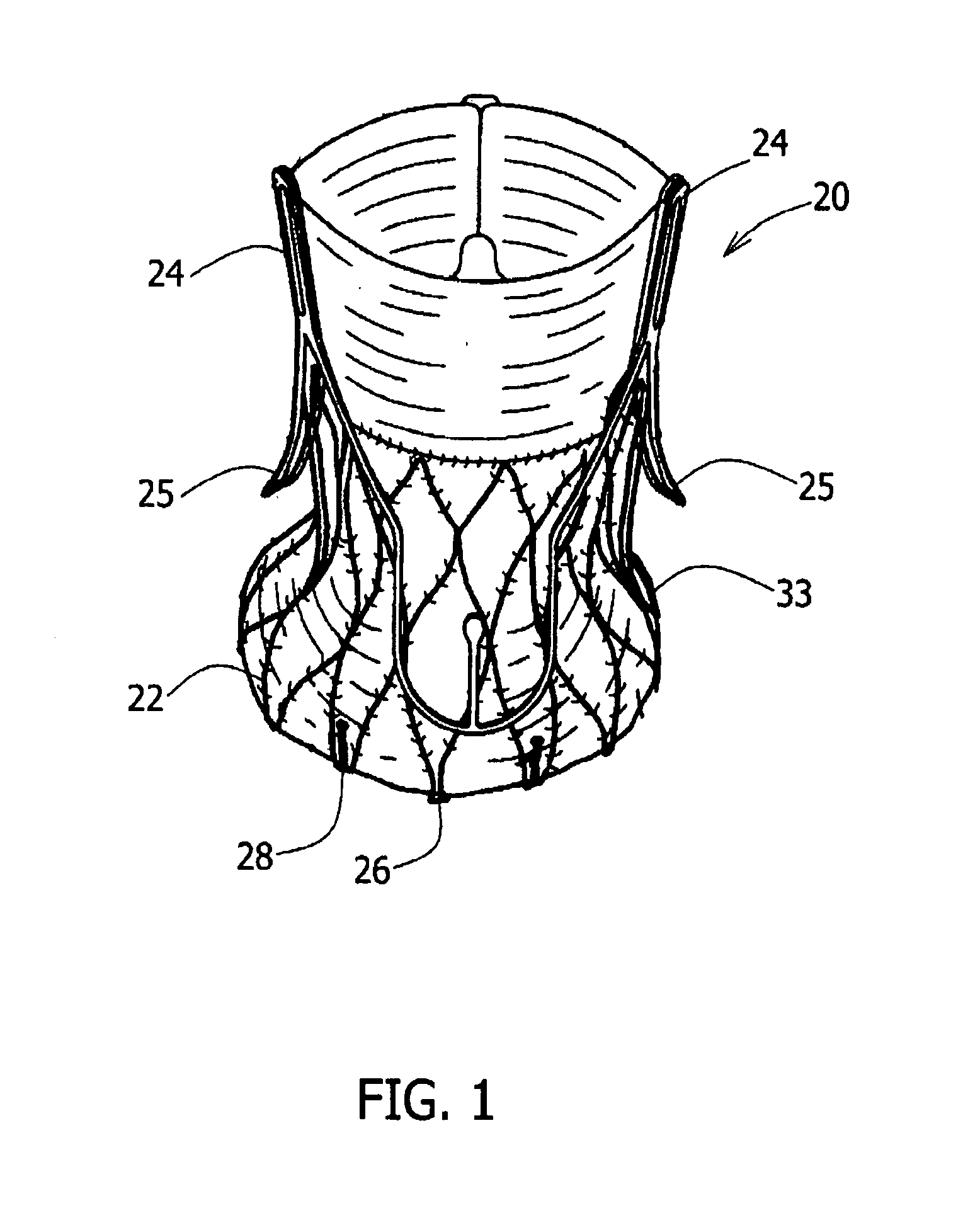
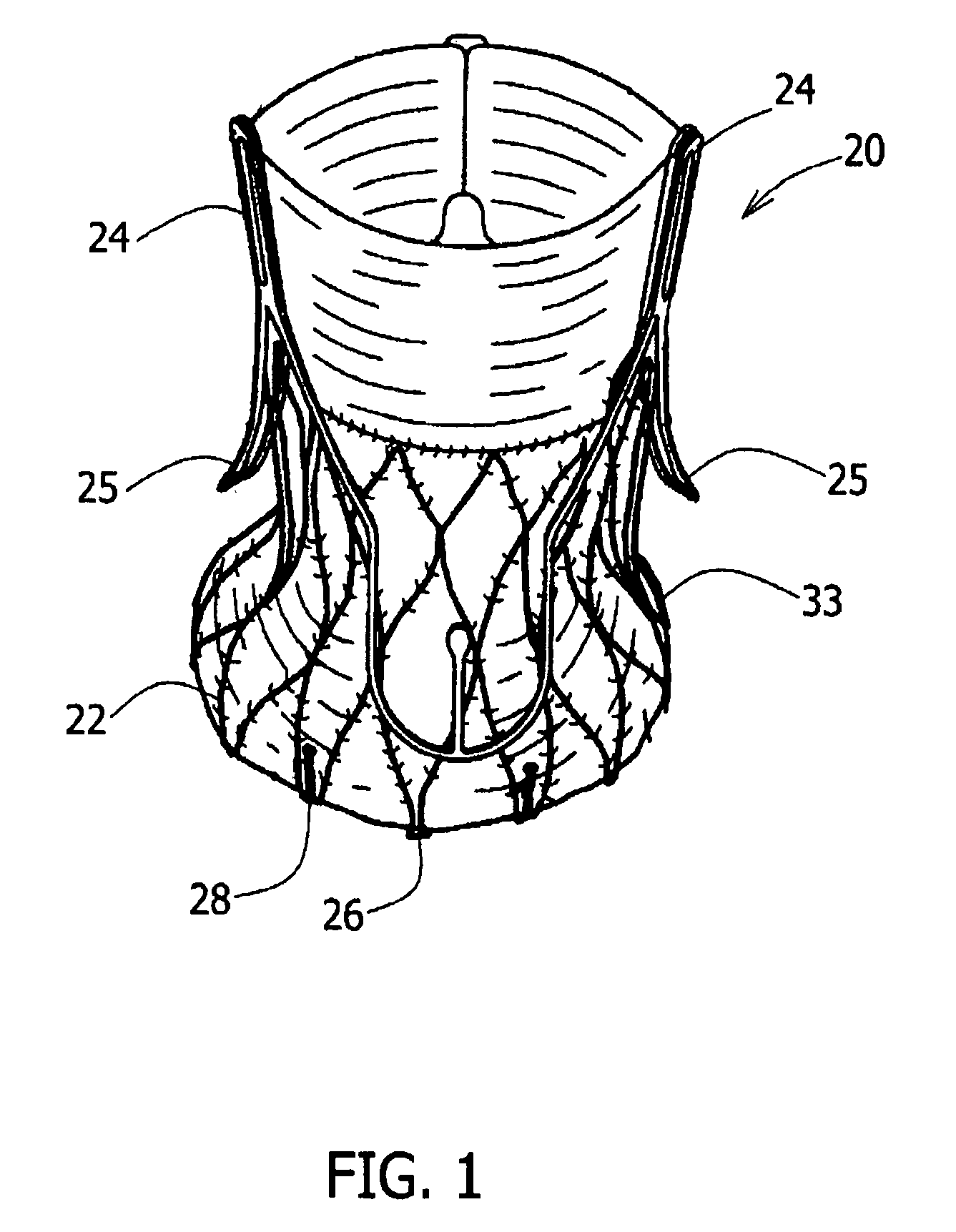
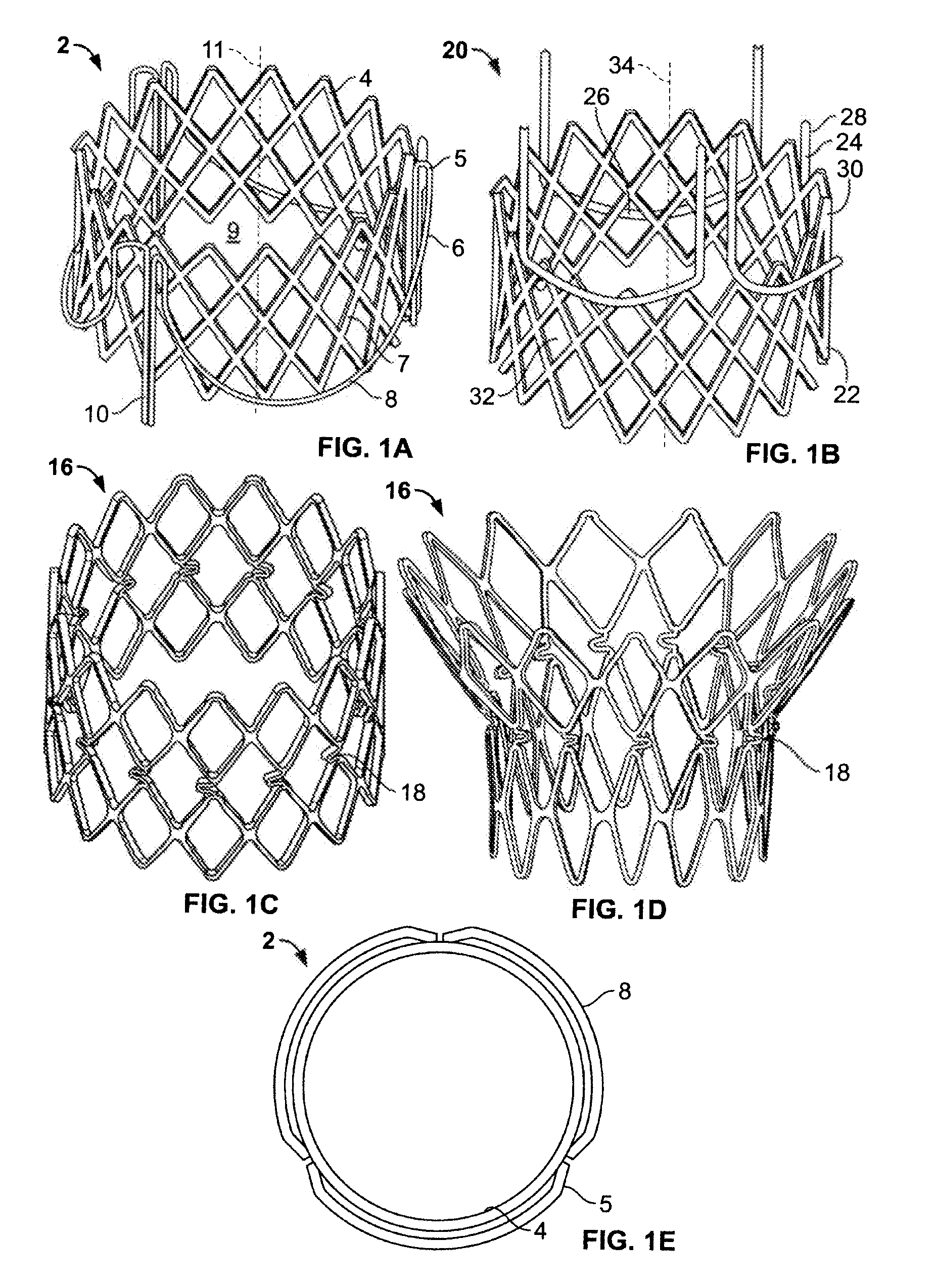
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable valve support. If desired, one or more anchors may be used. The valve support, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. Portions of the valve support may expand to a preset diameter to maintain coaptivity of the replacement valve and to prevent occlusion of the coronary ostia. A radial restraint, comprising a wire, thread or cuff, may be used to ensure expansion does not exceed the preset diameter. The valve support may optionally comprise a drug elution component. The anchor engages the lumen wall when expanded and prevents substantial migration of the valve assembly when positioned in place. The prosthetic valve assembly is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart. A blood pump may be inserted into the catheter to ensure continued blood flow across the implantation site during implantation procedure. When the outer sheath is retracted, the prosthetic valve assembly expands to an expanded position such that the valve and valve support expand at the implantation site and the anchor engages the lumen wall. Insertion of the catheter may optionally be performed over a transseptally delivered guidewire that has been externalized through the arterial vasculature. Such a guidewire provide dual venous and arterial access to the implantation site and allows additional manipulation of the implantation site after arterial implantation of the prosthetic valve. Additional expansion stents may be delivered by venous access to the valve.
Owner:MEDTRONIC COREVALVE
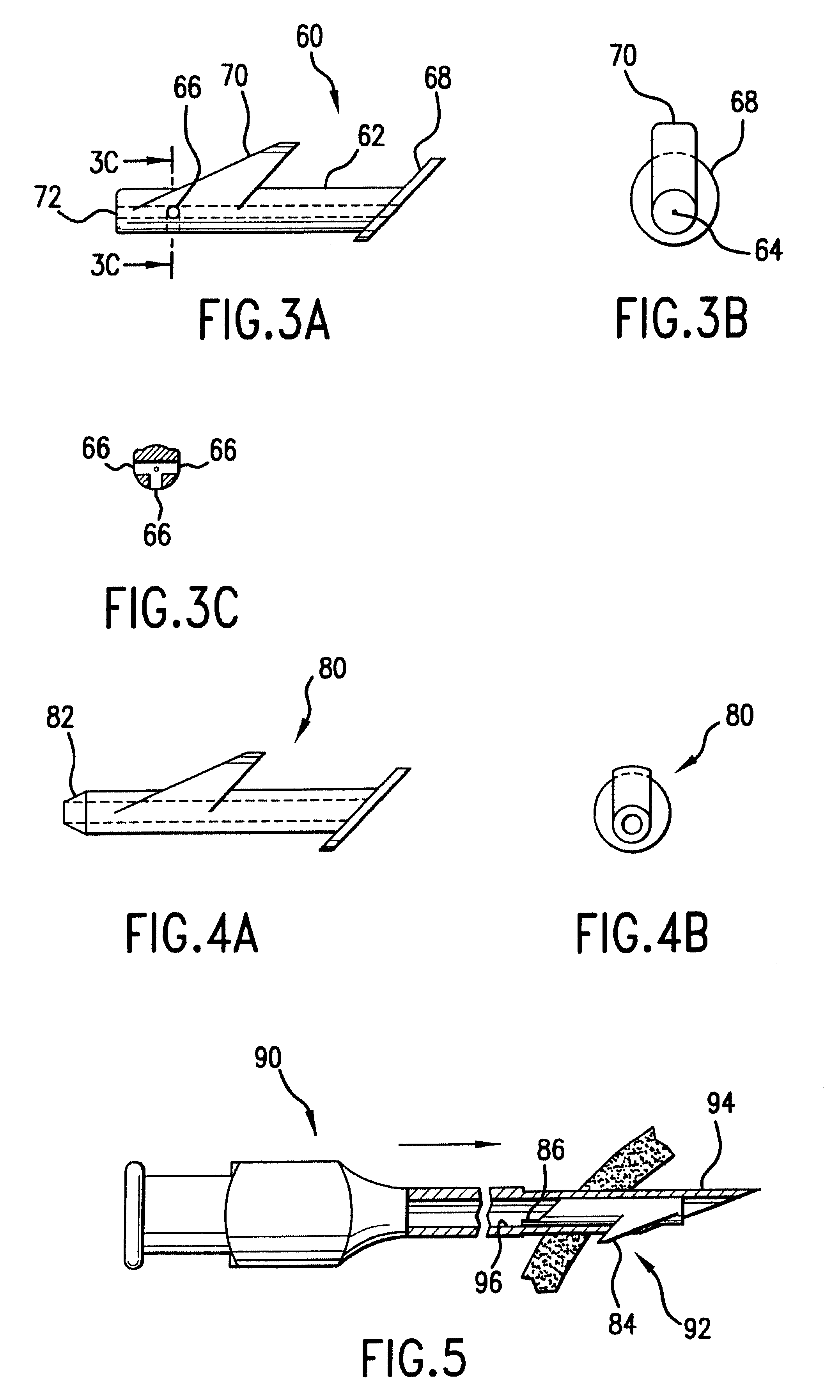
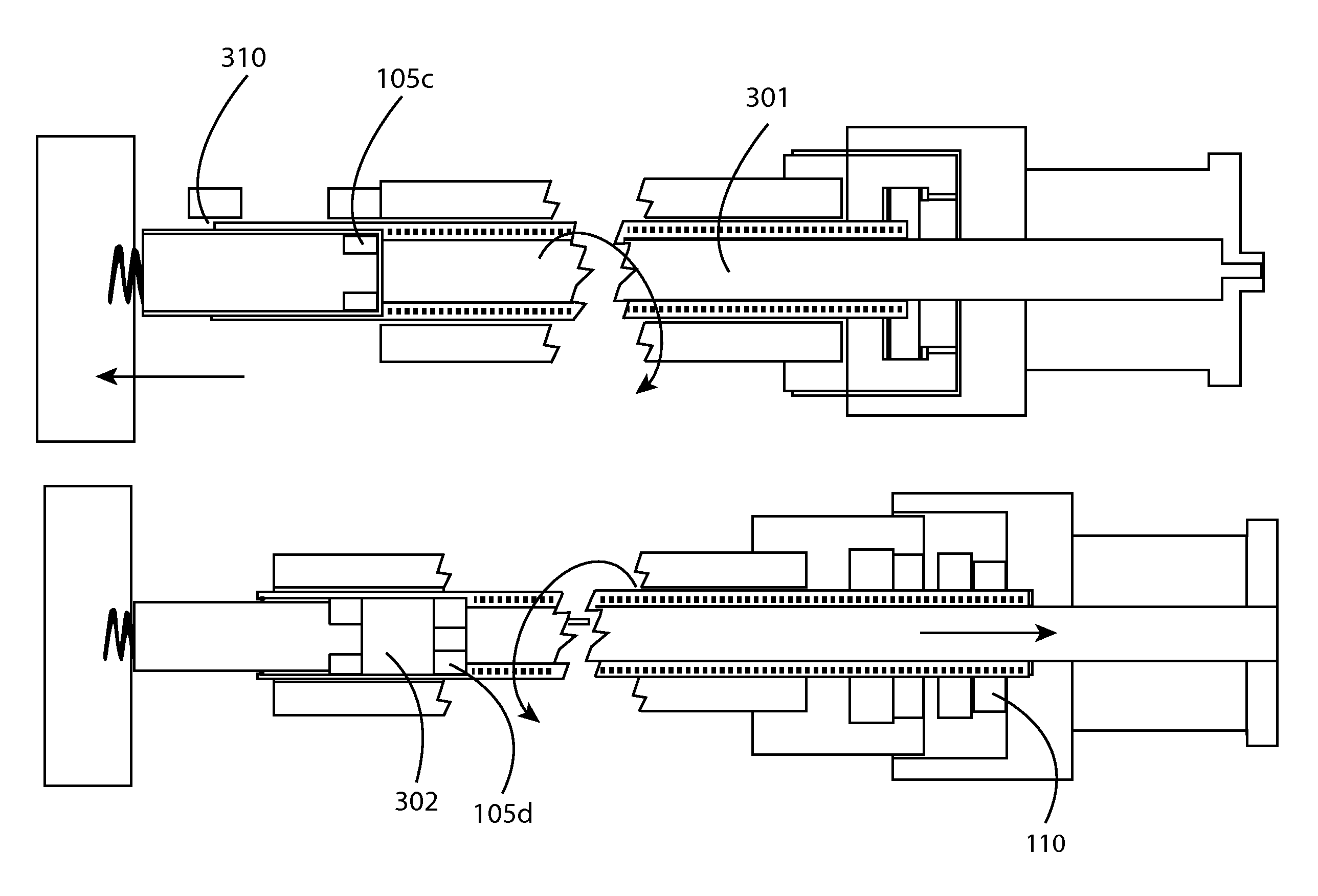
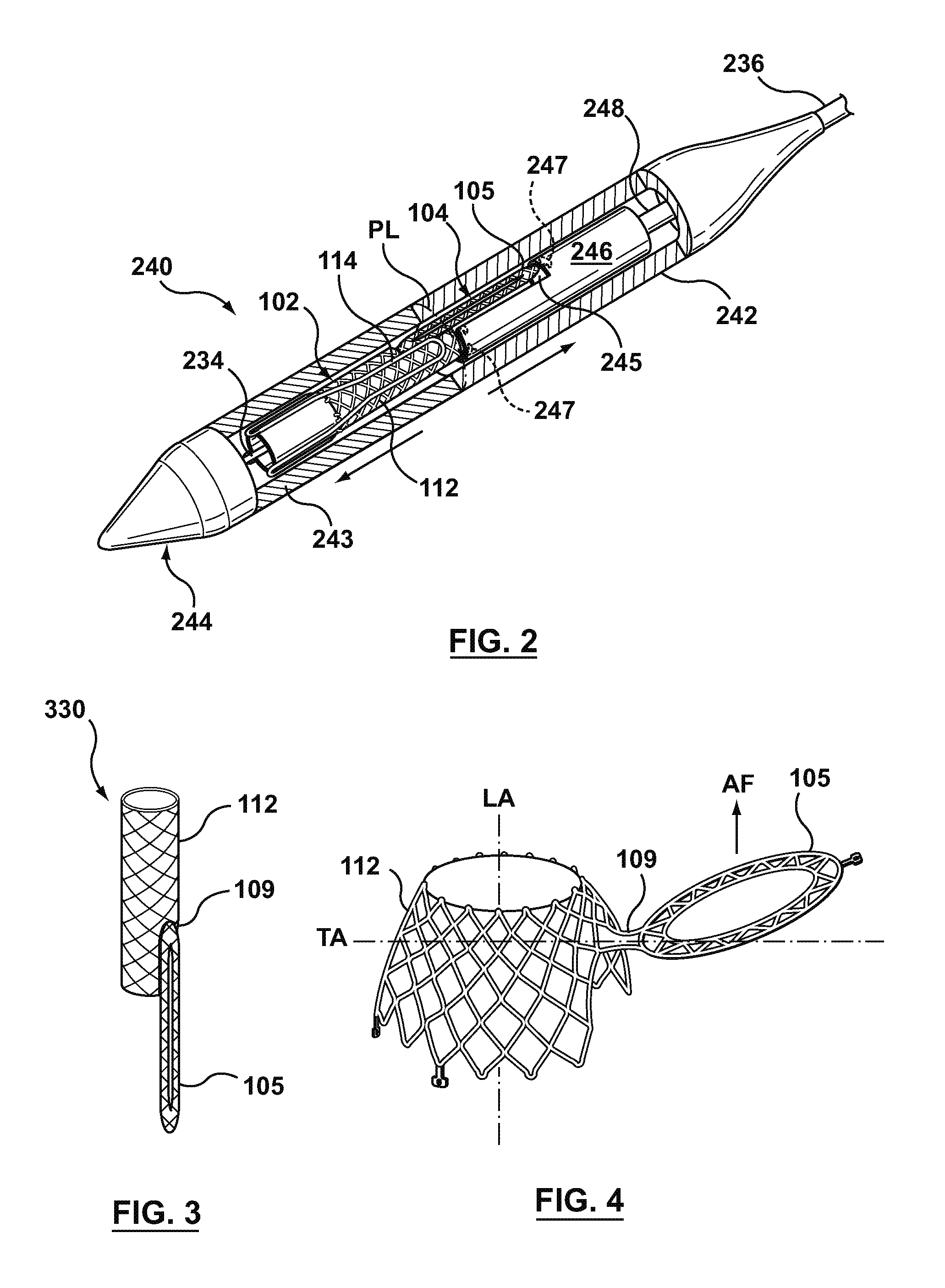
Stent loading tool and method for use thereof
ActiveUS20090054976A1The method is simple and reliableEasy to pushStentsHeart valvesVALVE PORTCatheter device
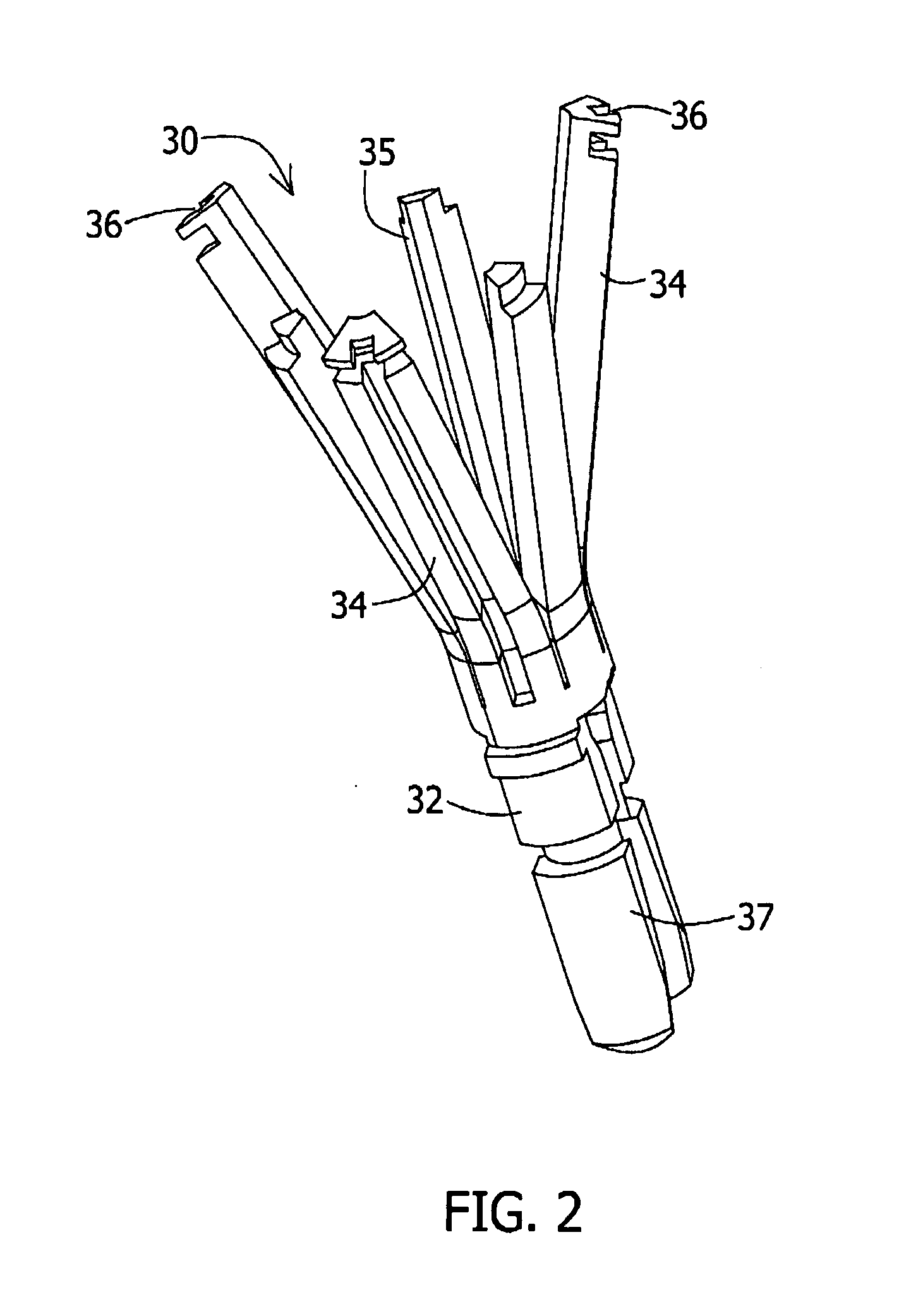
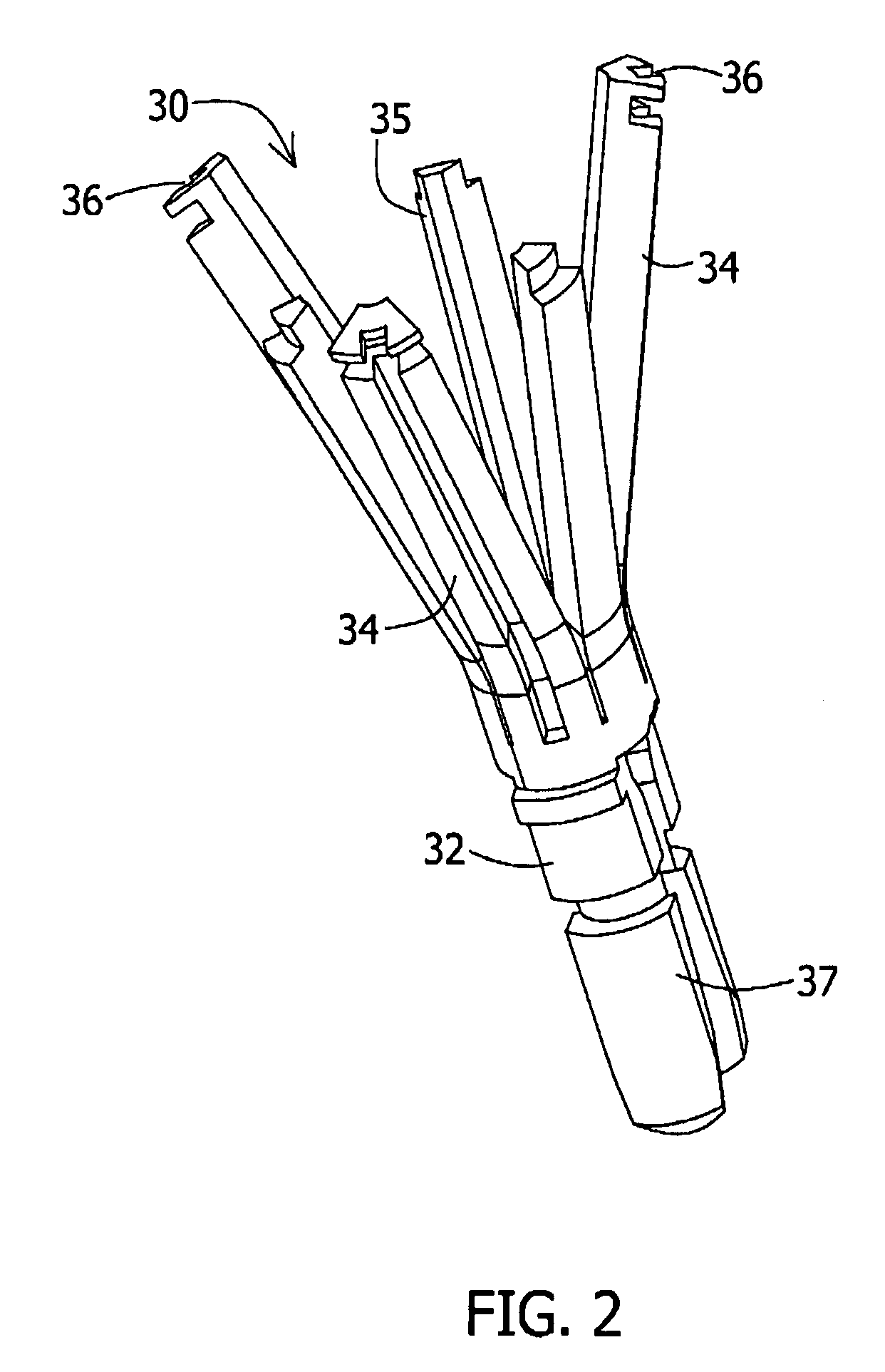
A loading tool for withdrawing, crimping, and loading a stent-mounted valve into a delivery catheter, and for pushing the stent-mounted valve from the delivery catheter into a native heart valve orifice. The loading tool comprises at least one connector adapted for being removably connected to the stent of the stent-mounted valve. A crimping tool having a generally converging shape is adapted for use with the loading tool. Following connection of the loading tool to the stent-mounted valve, the loading tool operates to allow the stent-mounted valve to be drawn through the crimping tool, and loaded, in a crimped state, into a delivery catheter. Also disclosed is a kit of the of the various components for effecting the delivery of the stent-mounted valve and a method for withdrawing, crimping, and loading a stent-mounted valve from a storage container into a delivery catheter for the performance of a transcatheter valve implantation procedure.
Owner:MEDTRONIC VENTOR TECH
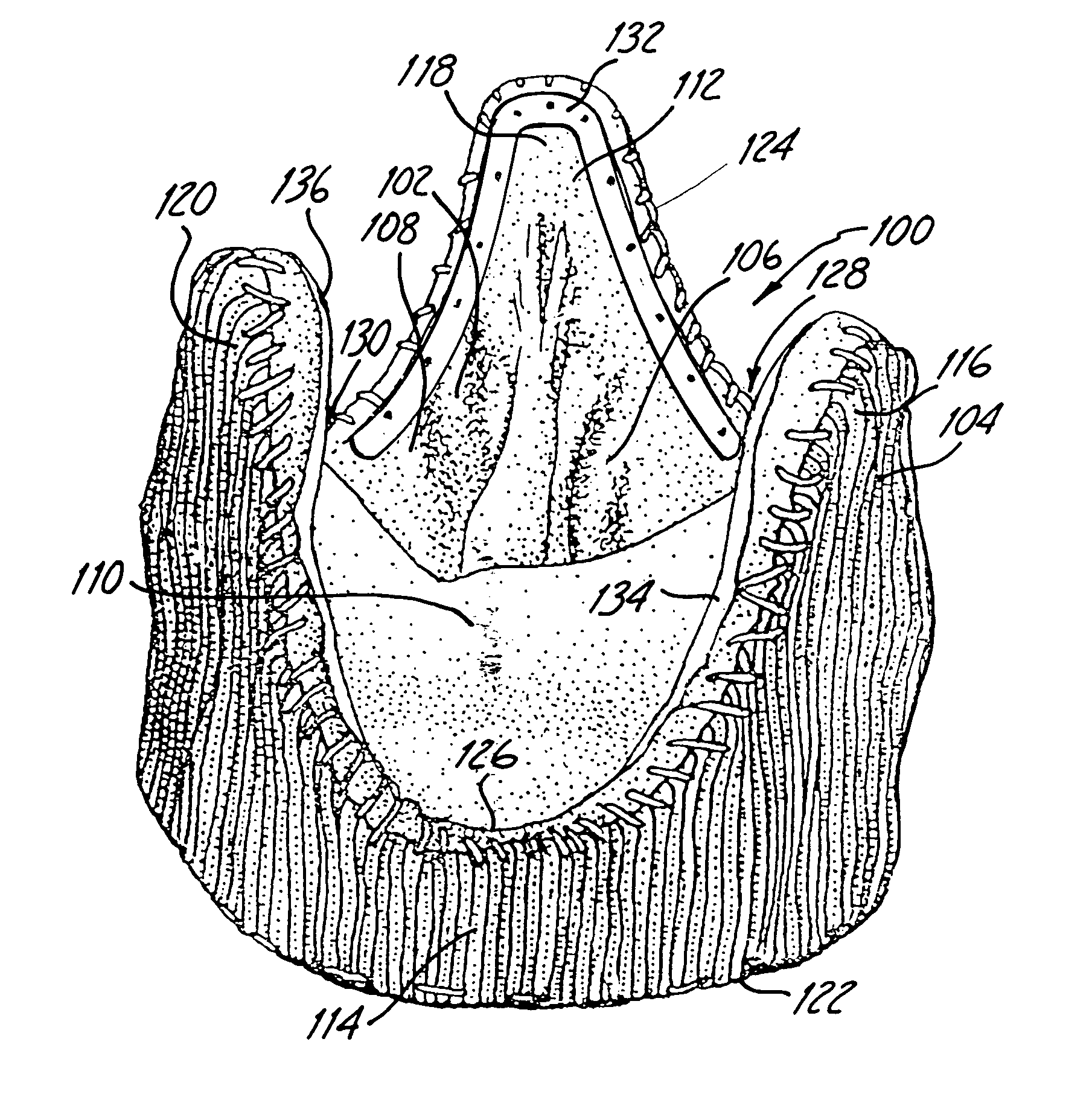
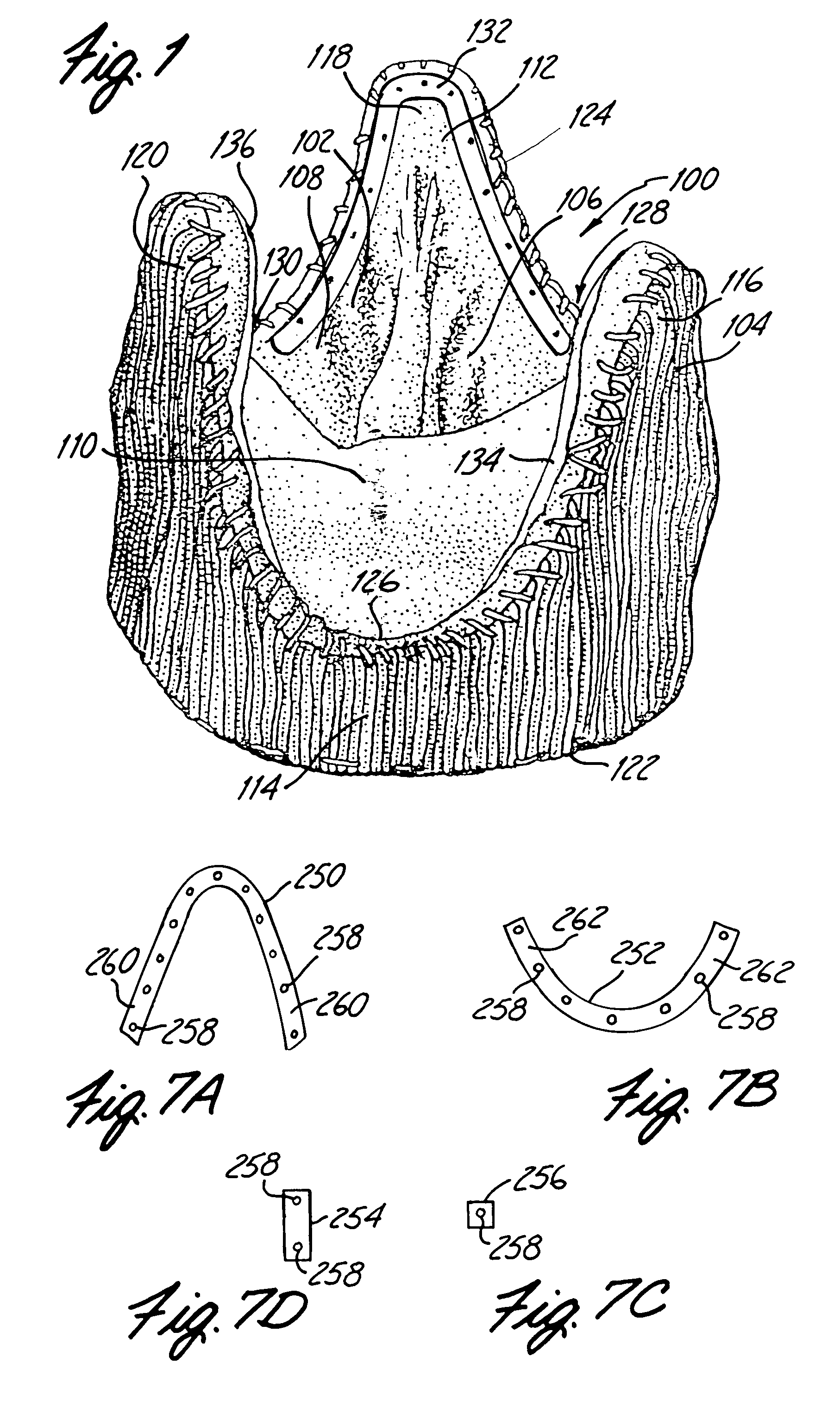
Sigmoid valve and method for its percutaneous implantation
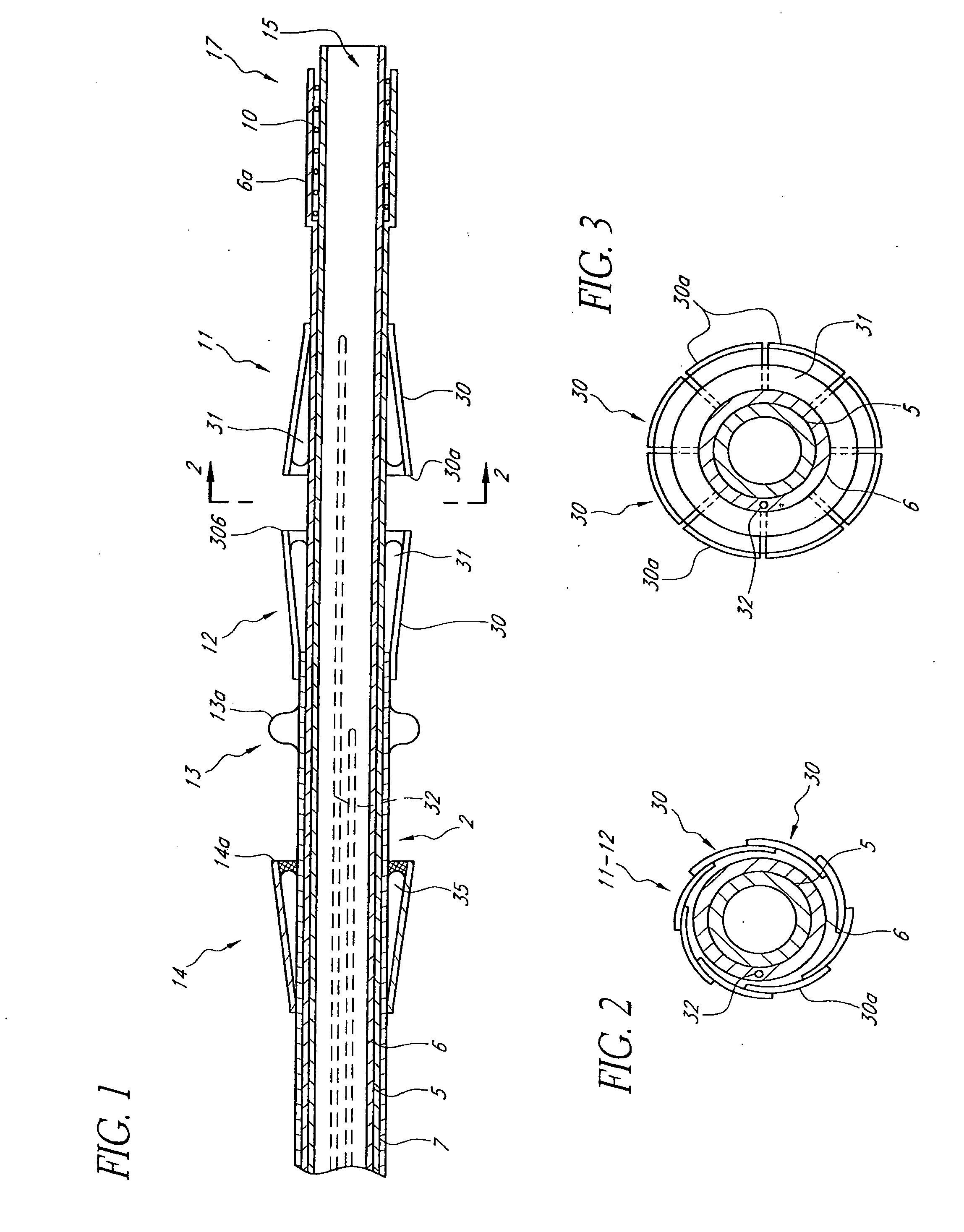
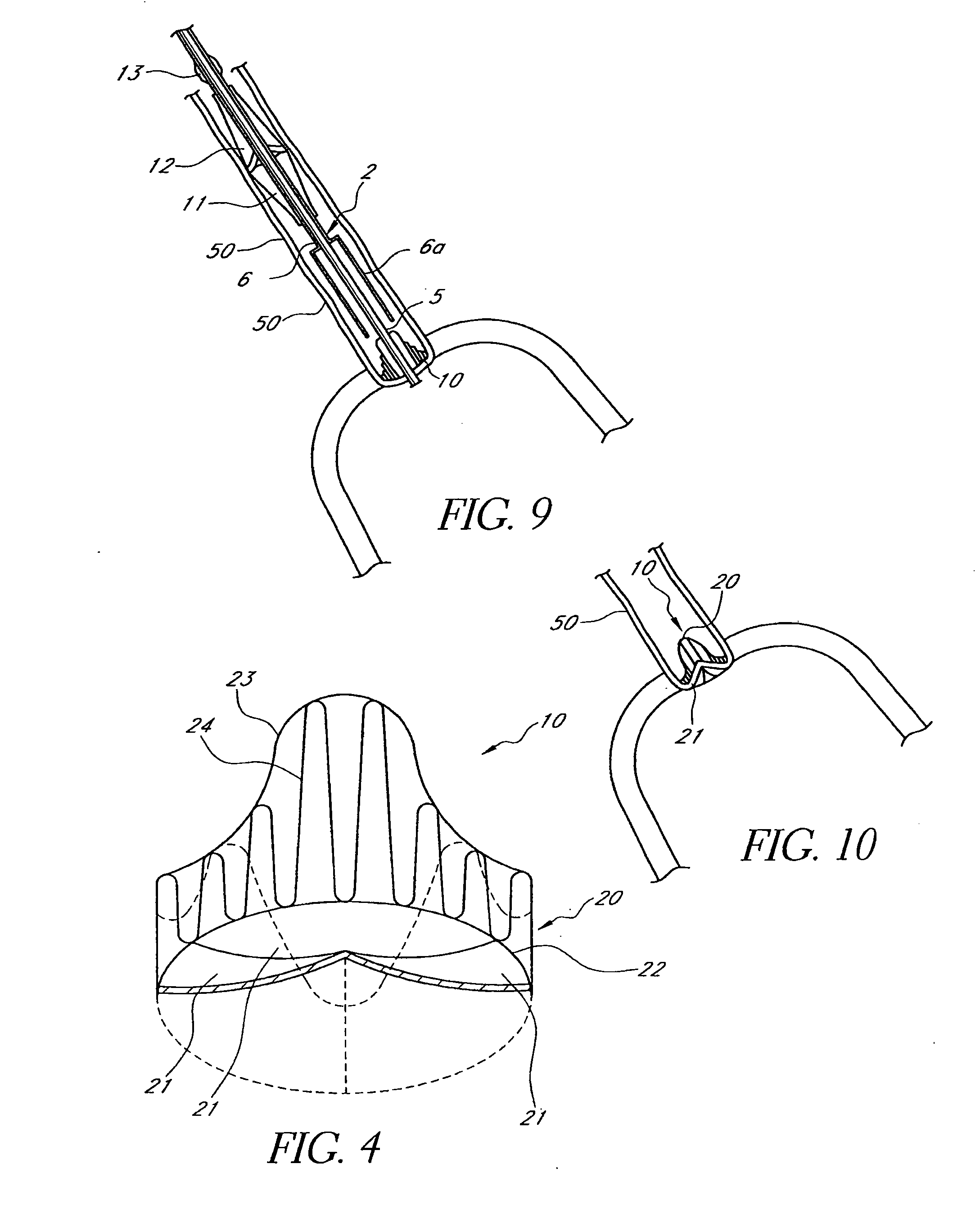
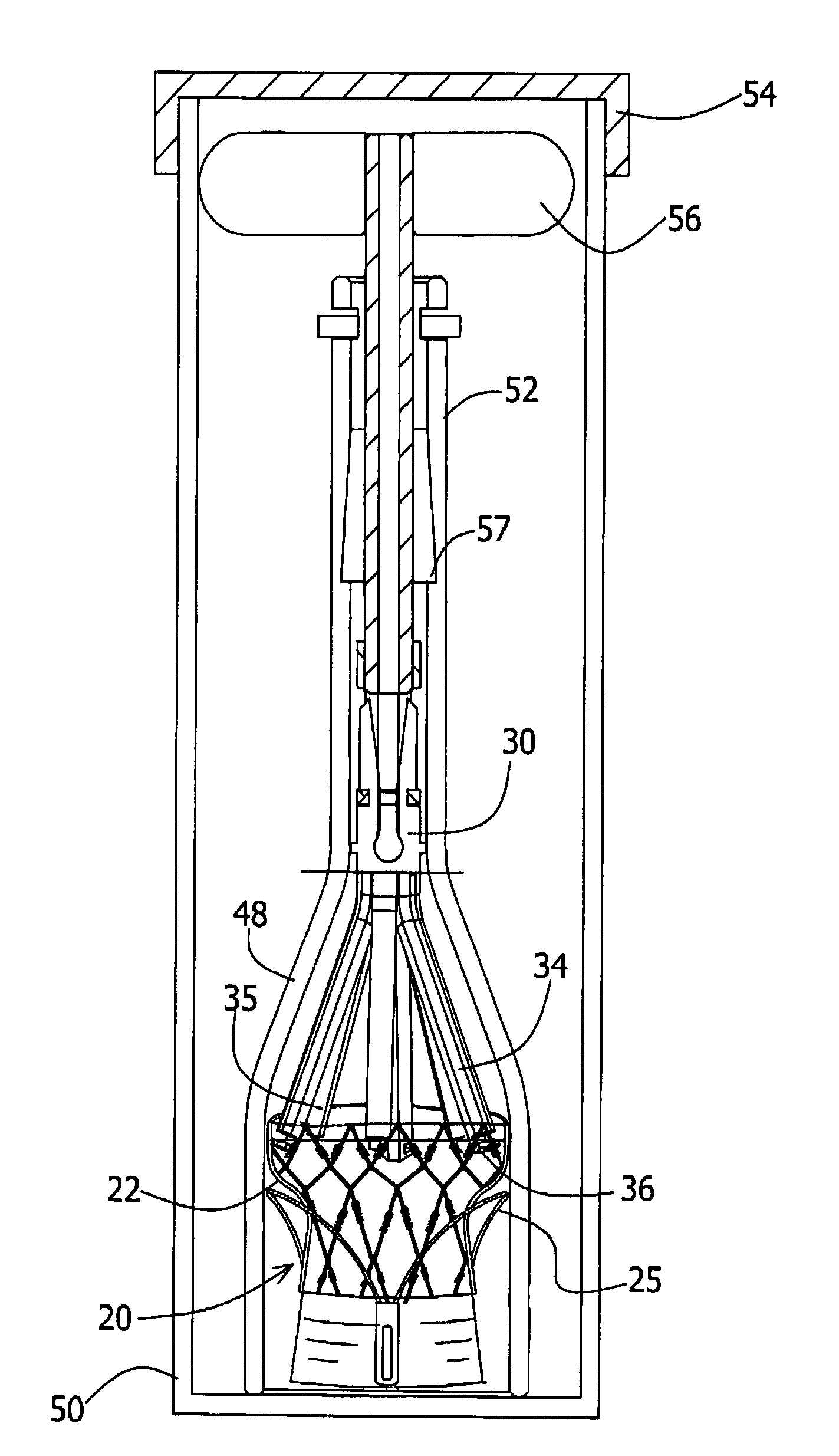
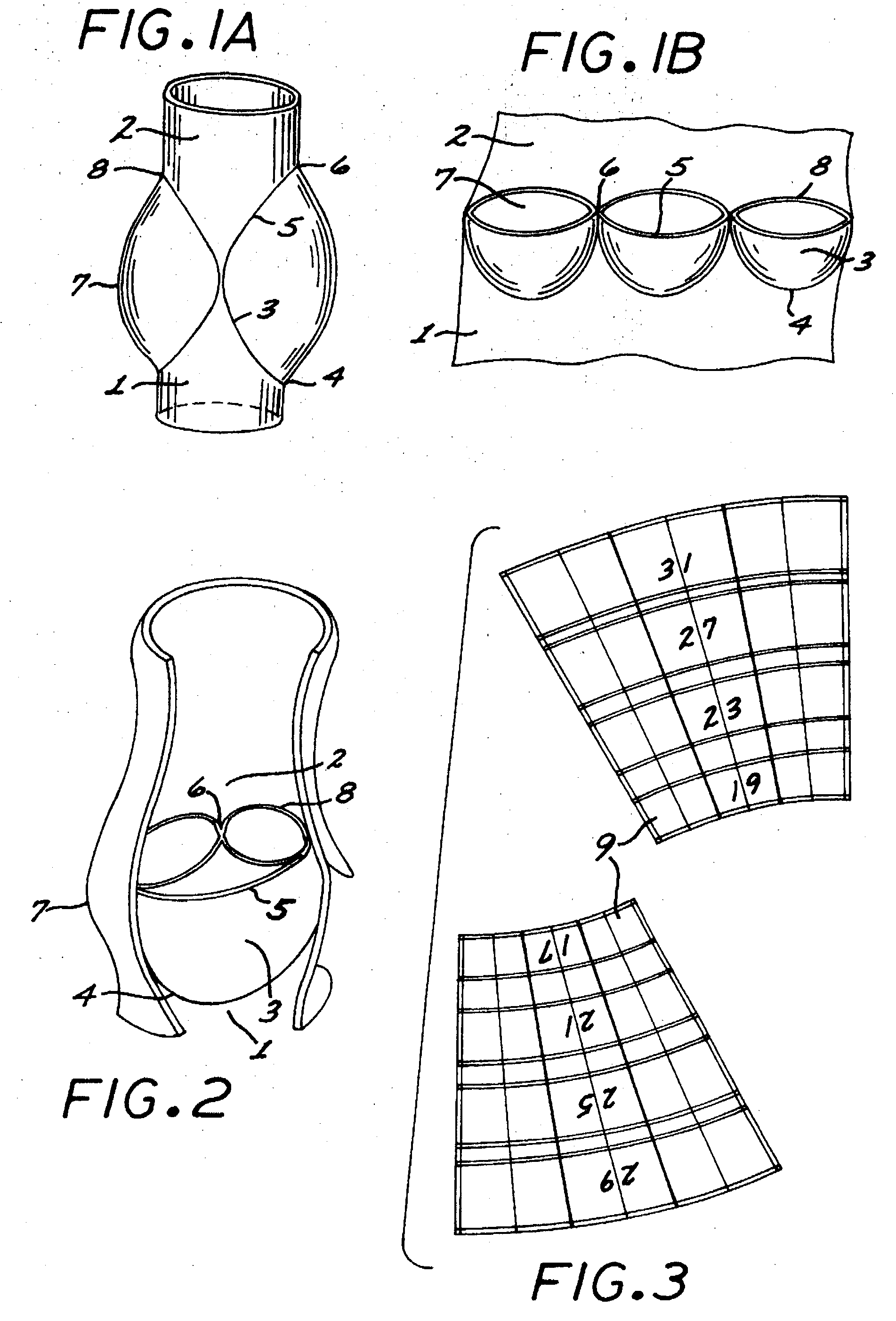
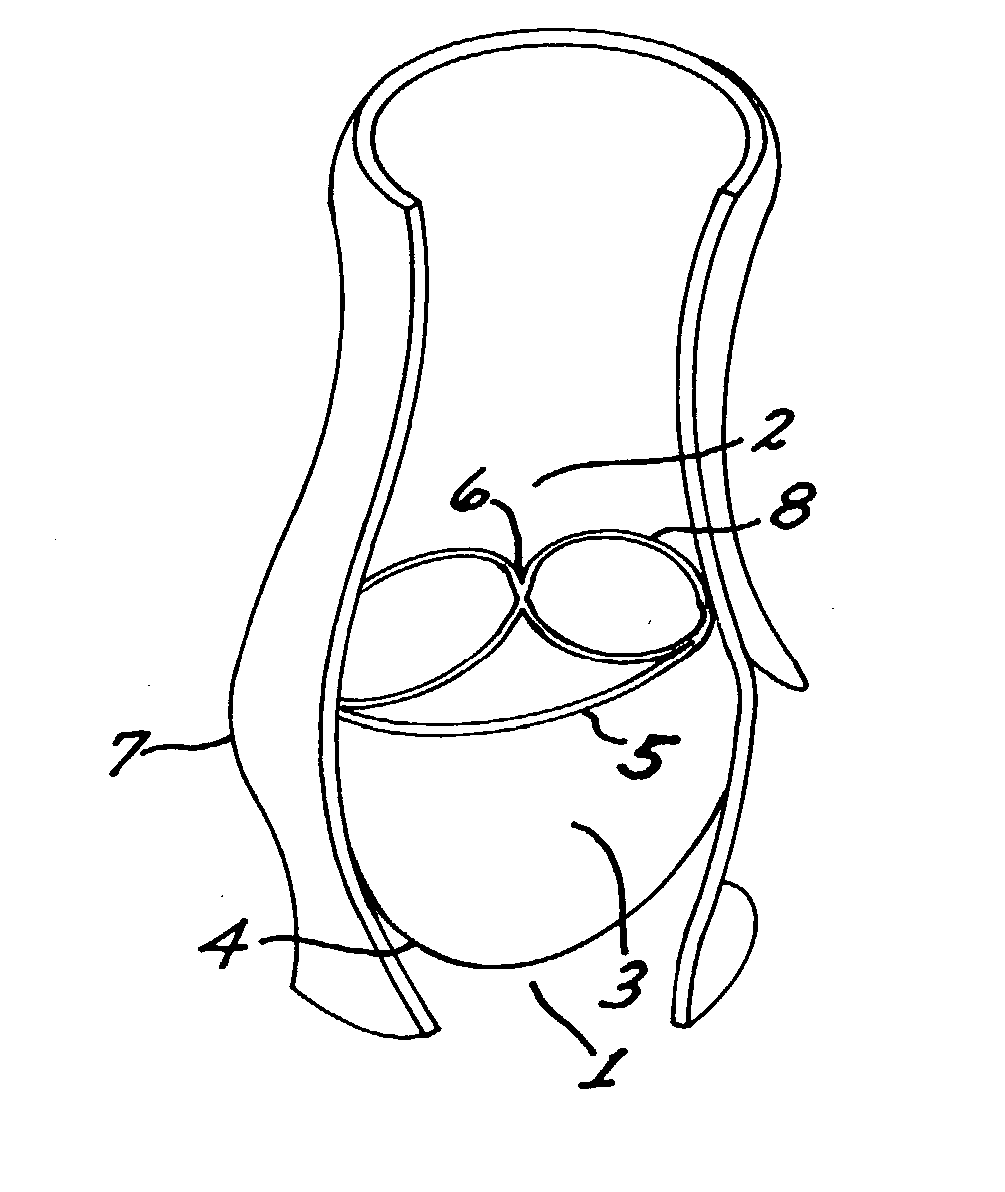
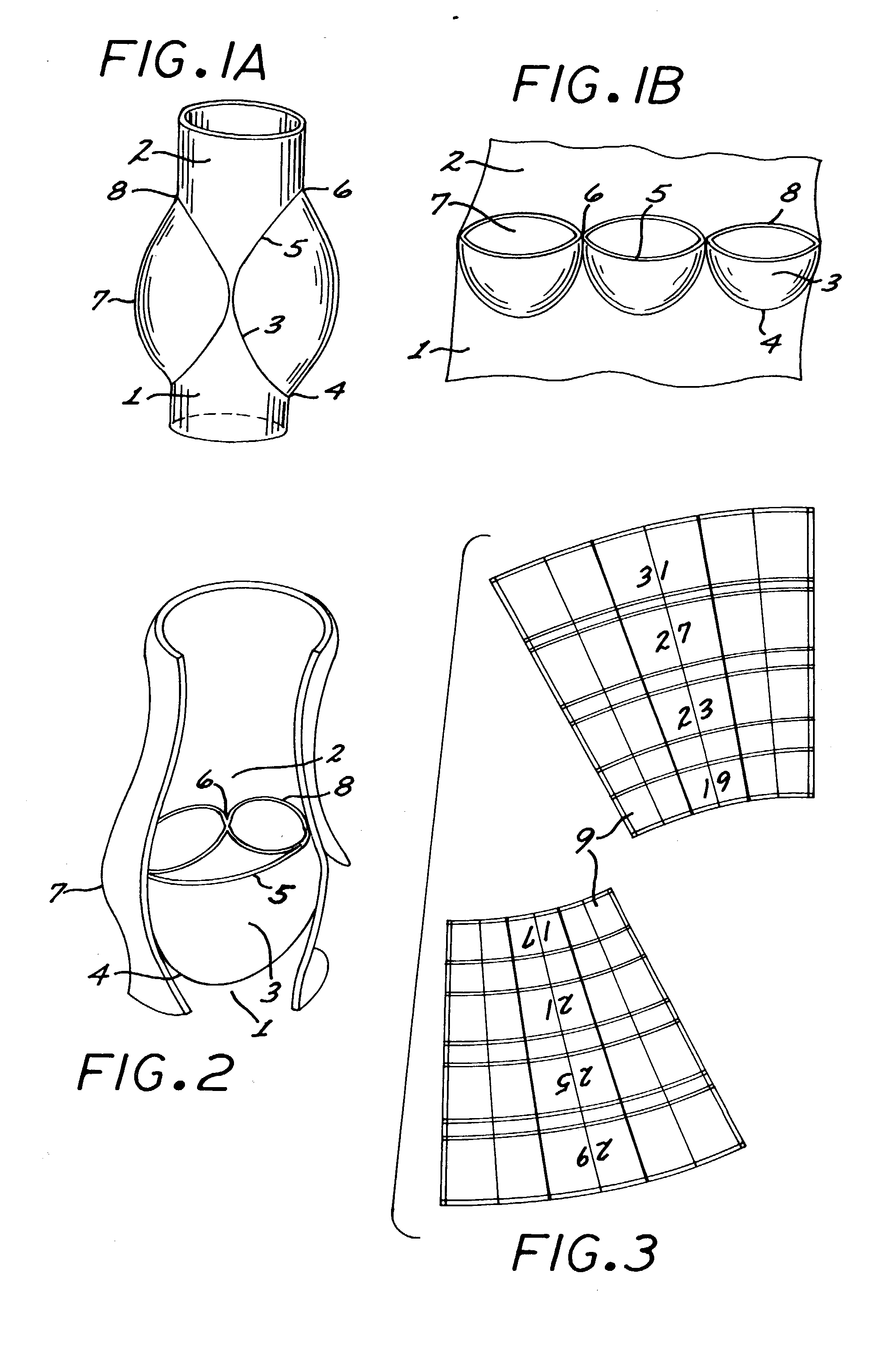
A multi-leaflet valve adapted to serve as a prosthesis for diseased native valve of a mammal is incorporated in self-expandable or inflatable endovascular stents or stents to form a combination which is introduced on a catheter with a guide wire into the circulatory system of the mammal to replace the diseased native valve. Once the combination is at the desired location the stent is caused to expand and affix itself to the patient's vessel wall. The prosthetic valve has the shape of a truncated cone that has an inflow and an outflow orifice with leaflets forming the outflow orifice and forming a plurality of commissures. A first flexible circular support is affixed in a substantially circular fashion around the truncated cone in proximity of the inflow orifice, and a second flexible circular support is affixed at the location of the commissures to form a circle around the truncated cone in proximity of the outflow orifice. The circular supports maintain the shape of the valve during the surgical implantation procedure and thereafter.
Owner:THE INT HEART INST OF MONTANA FOUND
Valve prosthesis
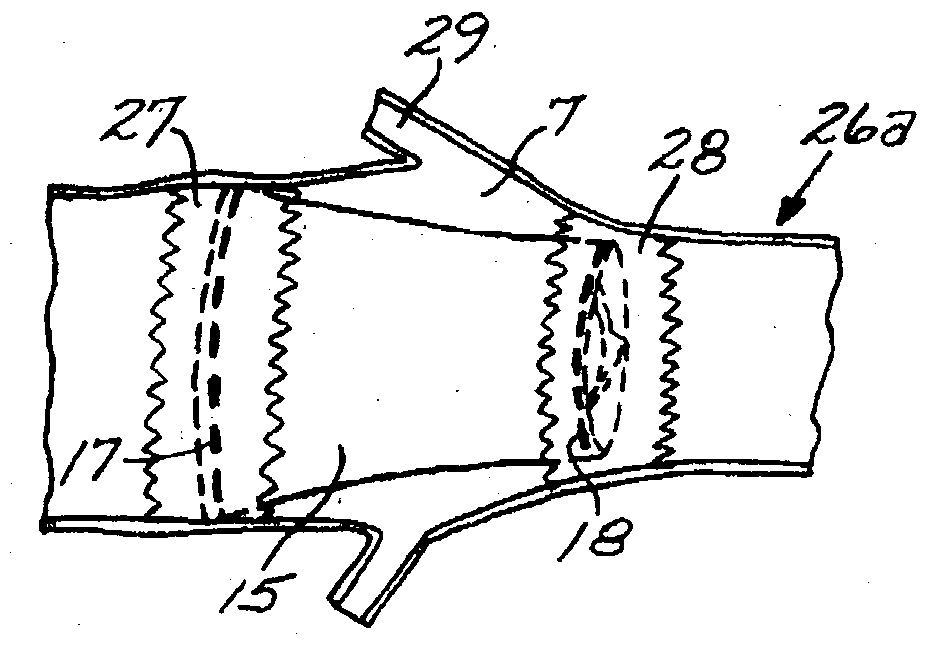
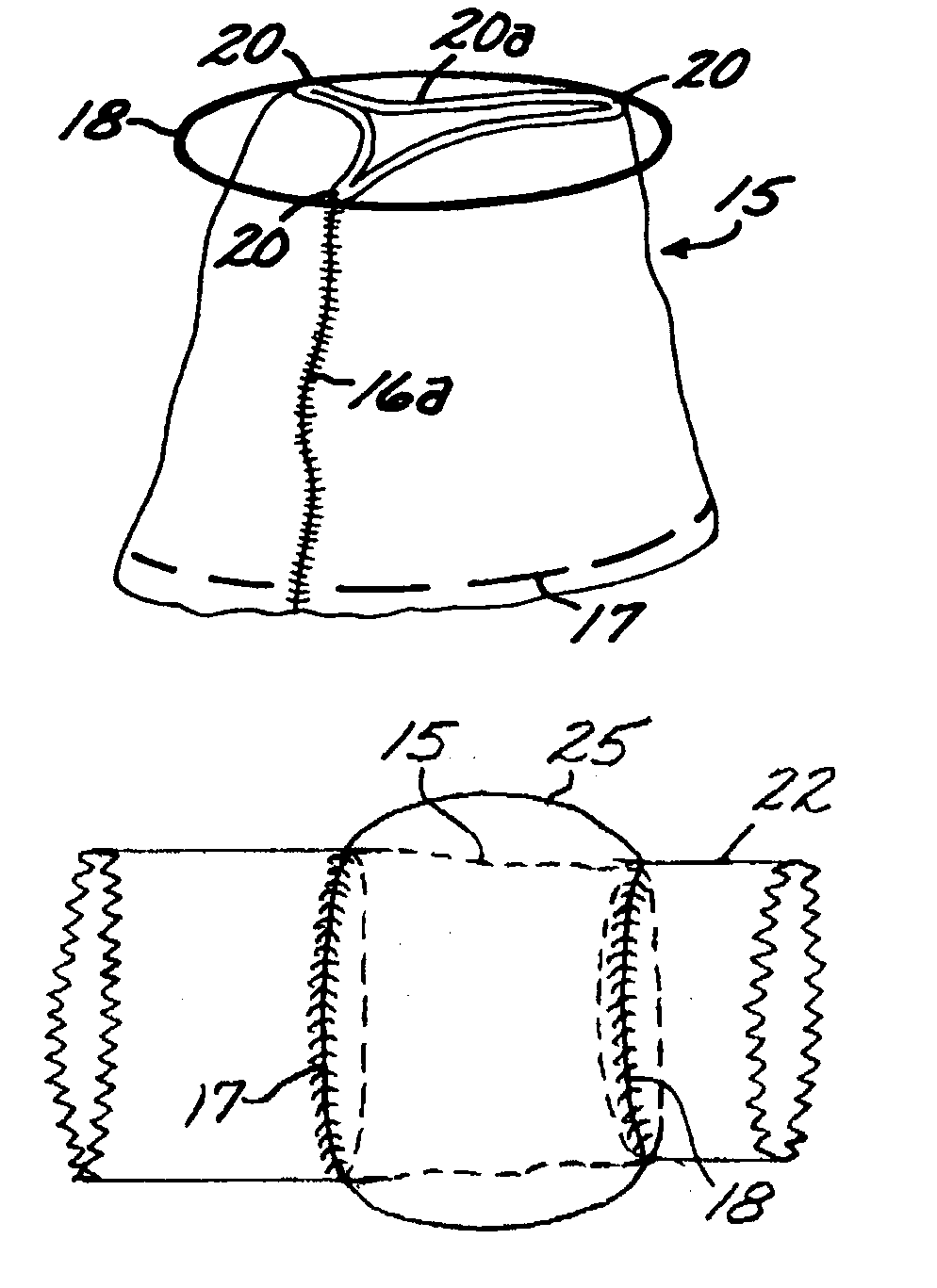
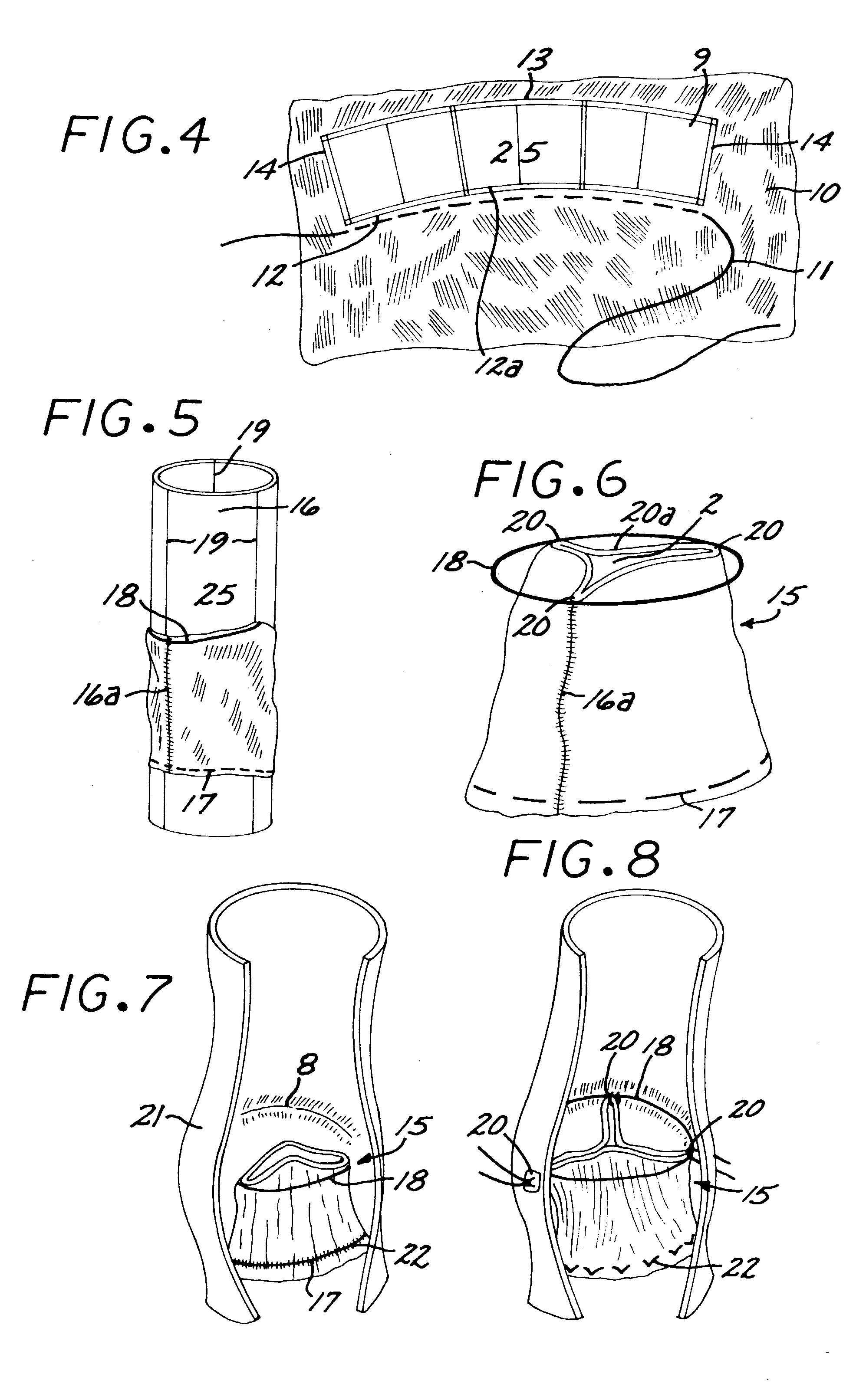
The present disclosure relates to valve replacement devices that are foldable for catheter-based deployment to the site of implantation, as well as systems for the delivery of valve prostheses, including prostheses having the special characteristics of the disclosed valve replacement devices. The devices include highly effective adhering mechanisms for secure and enduring precision implantation. The adhering mechanisms may employ a unique sealing mechanism that includes a cuff that expands slowly whereby the device is not secured in place until the completion of the implantation procedure. The implanted device, optionally together with the cuff, prevents perivalvular leaks and incorporate an appropriate leaflet system for reliable functioning in situ.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
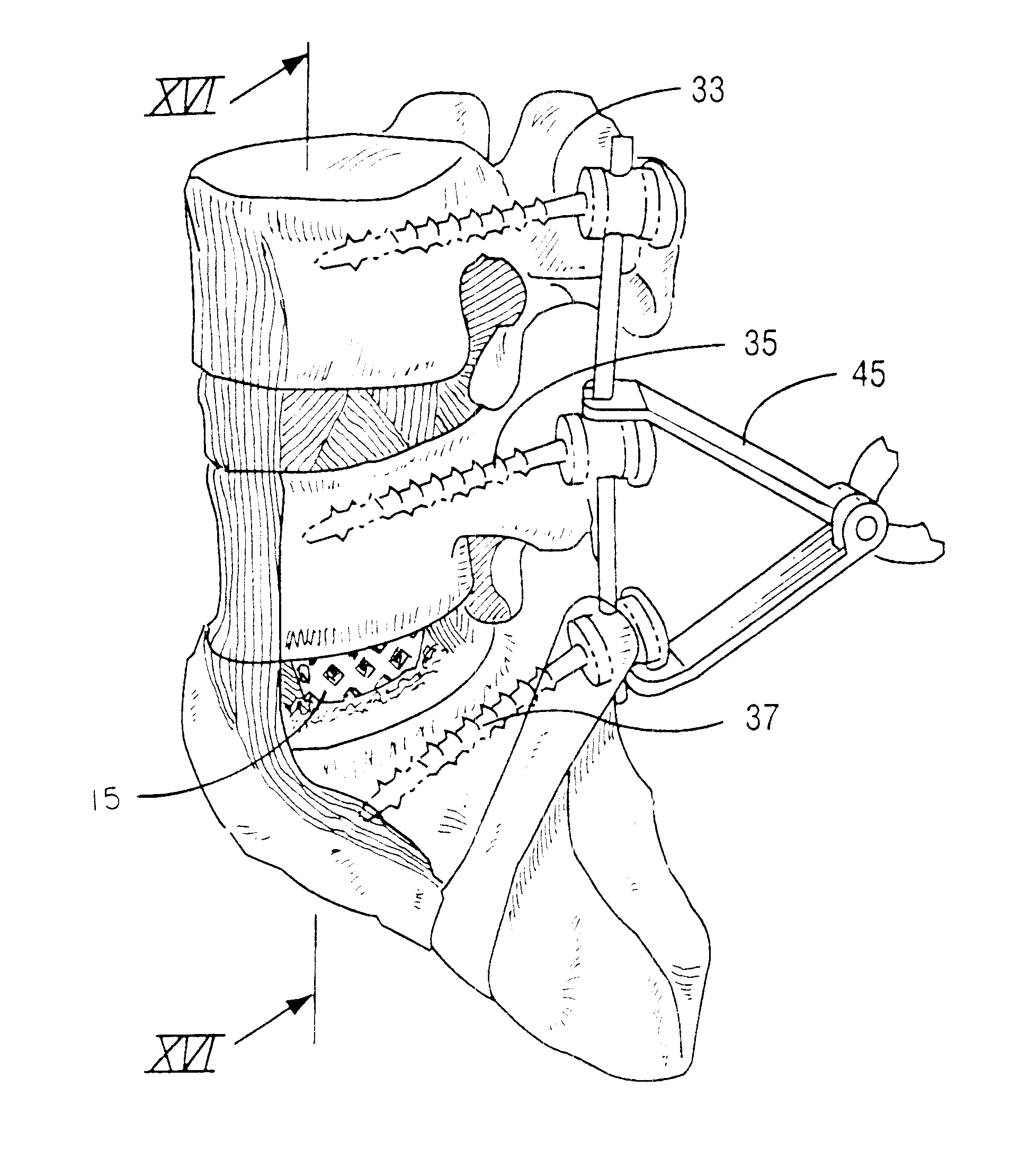
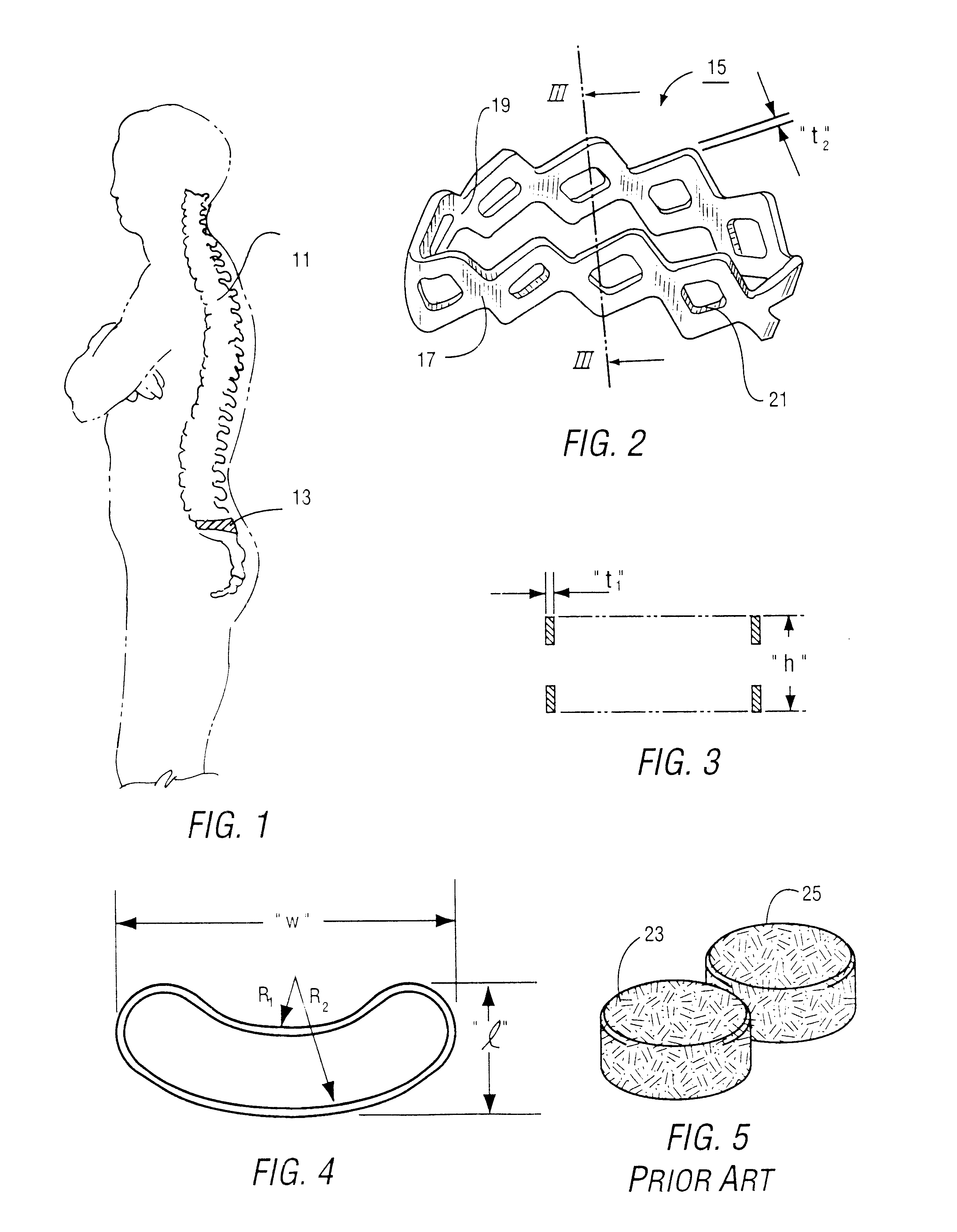
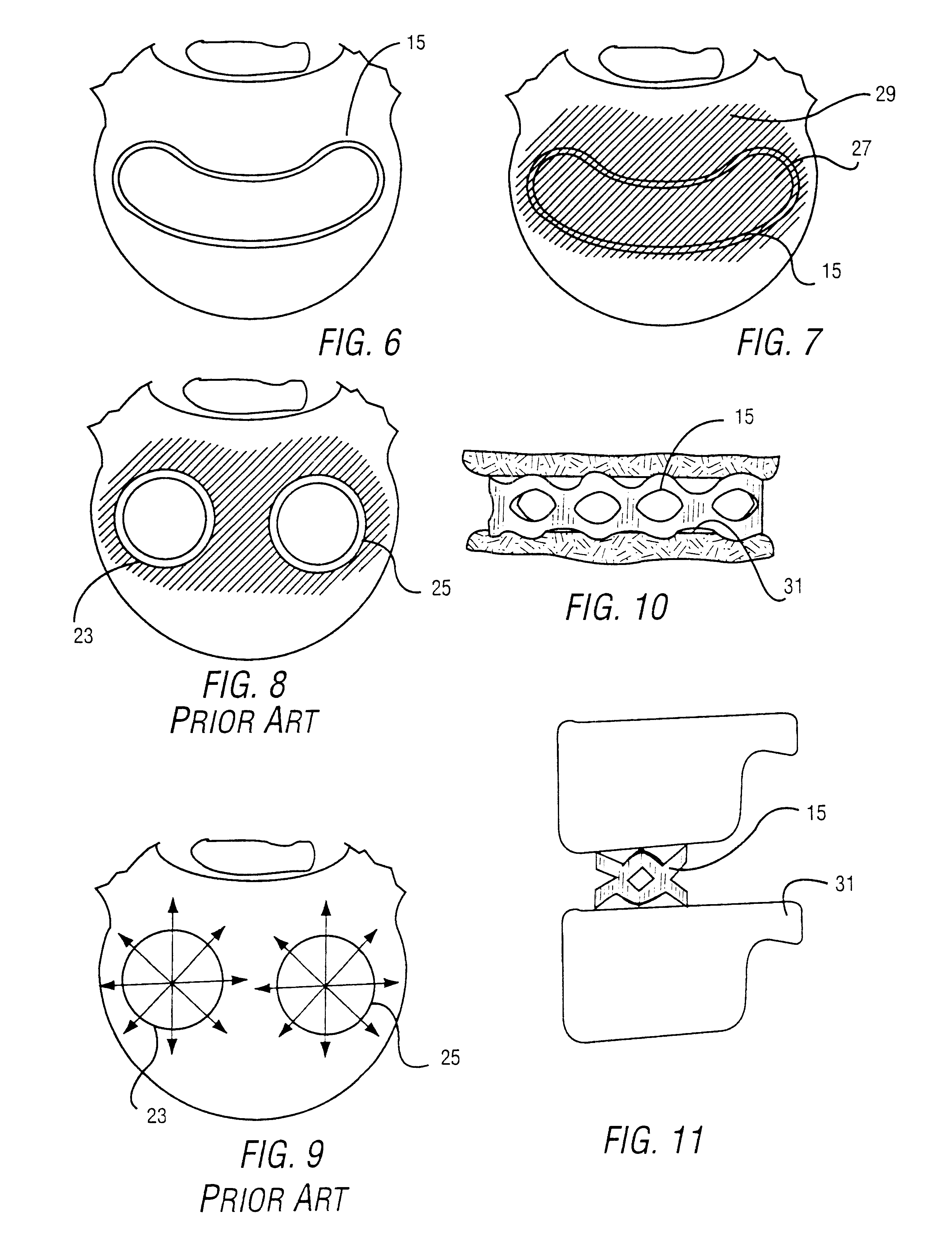
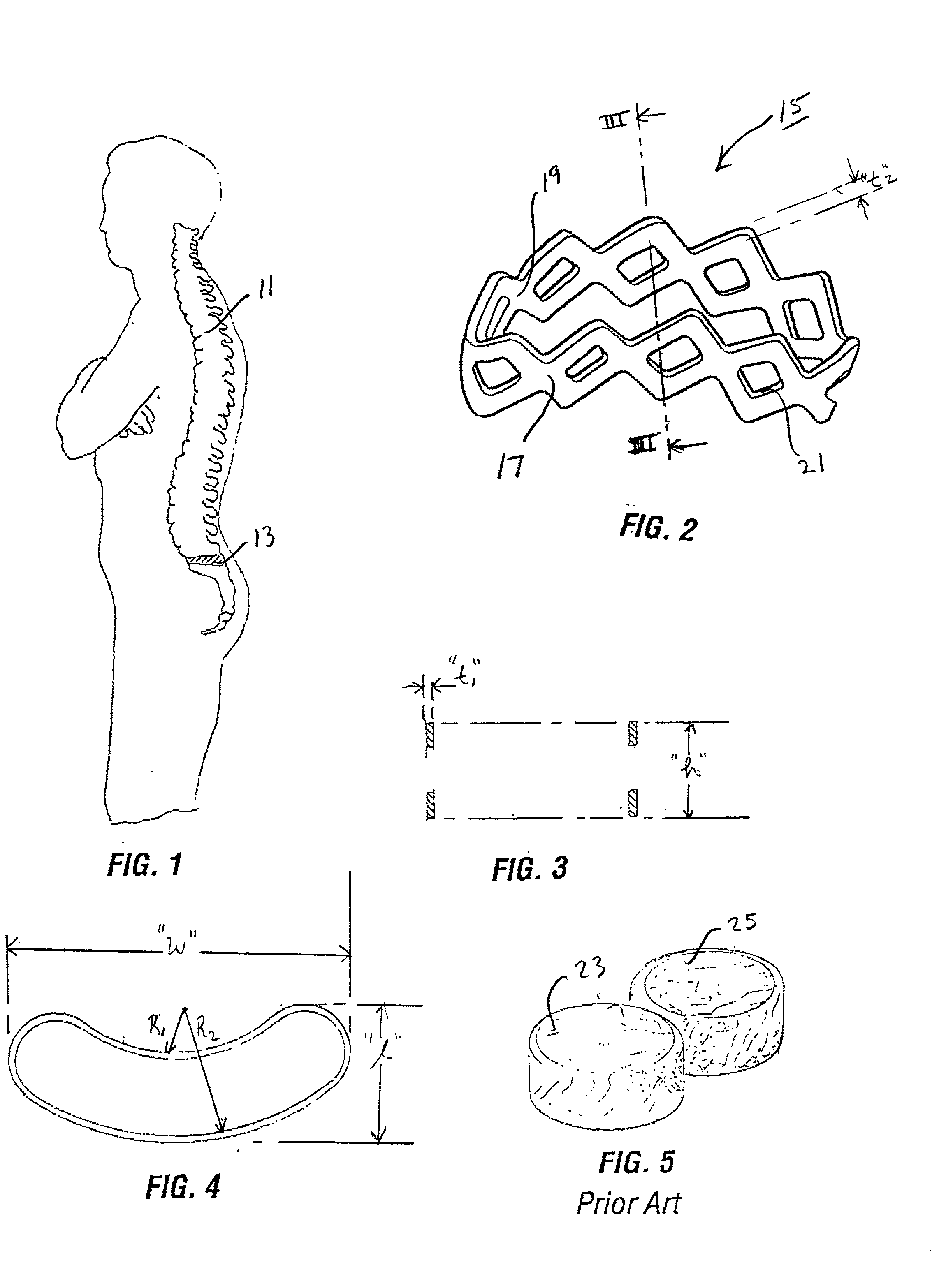
Intervertebral cage and method of use
InactiveUS6648915B2Restore tensionStable mechanical propertiesInternal osteosythesisBone implantSpinal columnHuman body
An intervertebral prosthesis for implantation between adjacent vertebrae of the human spine is shown. The prosthesis is formed as a unitary cage body configured and sized to be inserted between adjacent vertebrae in a single step implantation procedure. The cage body is banana shaped as viewed from above, the body having an exterior surface and an interior surface, the interior surface defining an internal recess for receiving cancellous bone material during an implantation procedure. The cage body can be formed as an interlinked mesh.
Owner:DEPUY ACROMED INC
Devices, systems and methods for delivering a prosthetic mitral valve and anchoring device
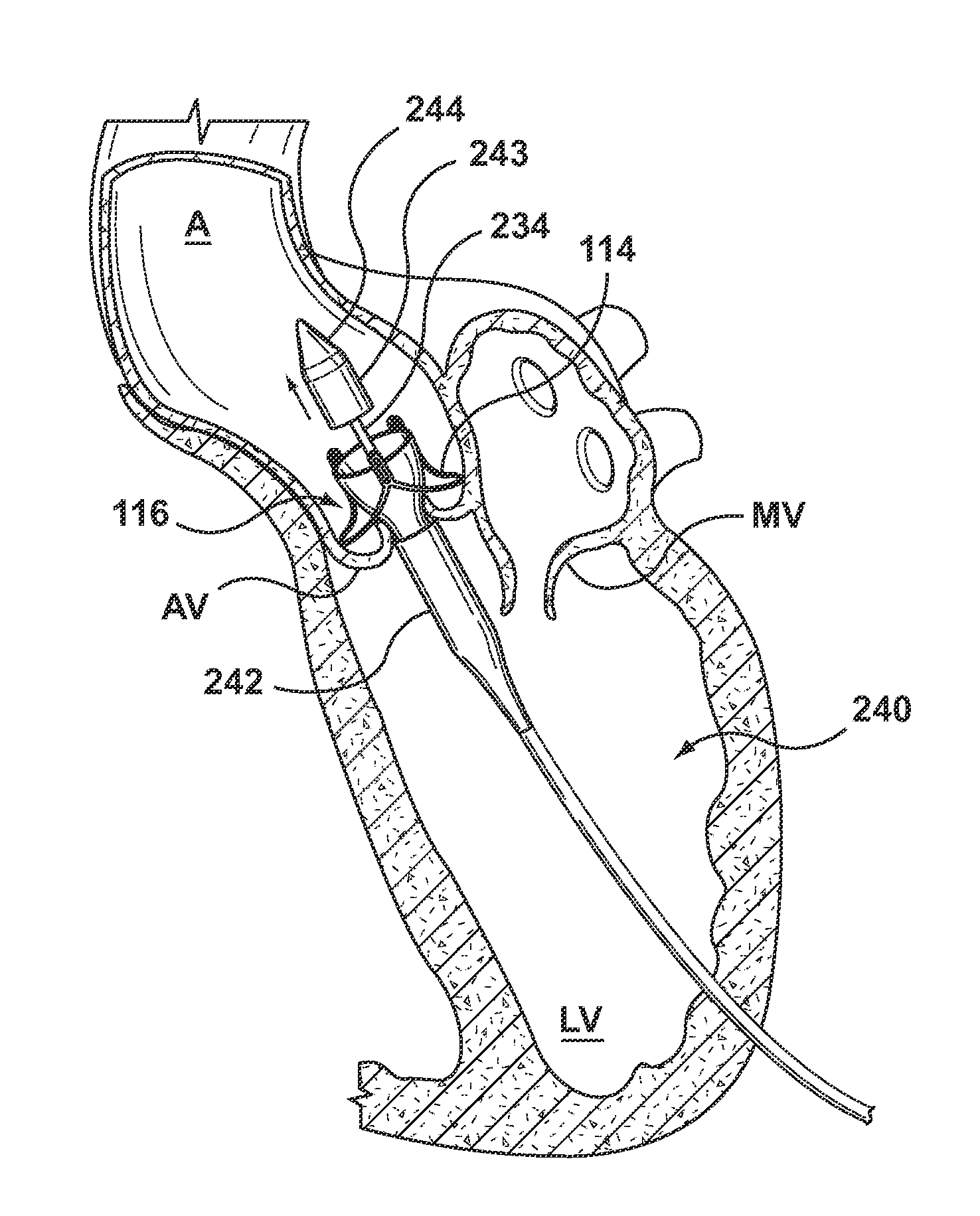
Prosthetic mitral heart valves and anchors for use with such valves are provided that allow for an improved implantation procedure. In various embodiments, a helical anchoring device is formed as a coiled or twisted anchor that includes one or more turns that twist or curve around a central axis. Curved arms attached to the frame of the valve guide the helical anchoring device into position beneath the valve leaflets and around the mitral valve annulus as it exits the delivery catheter, and the expandable prosthetic mitral valve is held within the coil of the anchoring device. The anchoring device and the valve can be delivered together, simplifying the valve replacement procedure.
Owner:MITRAL VALVE TECHNOLOGIES SARL
Flow control device, introducer and method of implanting
InactiveUS6558342B1Simple and efficient procedureAvoid tearingEye surgeryCatheterTemporary occlusionBiomedical engineering
An implant having a tube for permitting fluid flow has an outer flange at the outlet end and a retention projection near the inlet end. A delivery device for implanting the implant has a central bore for accommodating the implant during the implantation procedure. When the implant is loaded in the delivery device, the retention projection of the implant protrudes beyond the outside surface of the delivery device. After the delivery device and implant have penetrated the tissue through which drainage is desired, and the retention projection has fully penetrated through the tissue, the delivery device is withdrawn. The retention projection acts as a hook engaging the inside surface of the tissue, causing the implant to stay implanted in the tissue. An implant may also be provided with a mechanism for temporary occlusion, in whole or in part, of the flow passage. Thus, the tube passage may be filled, partially or wholly, with absorbable material and / or a plurality of withdrawable or advanceable flow controlling strands.
Owner:MARY C WERNER
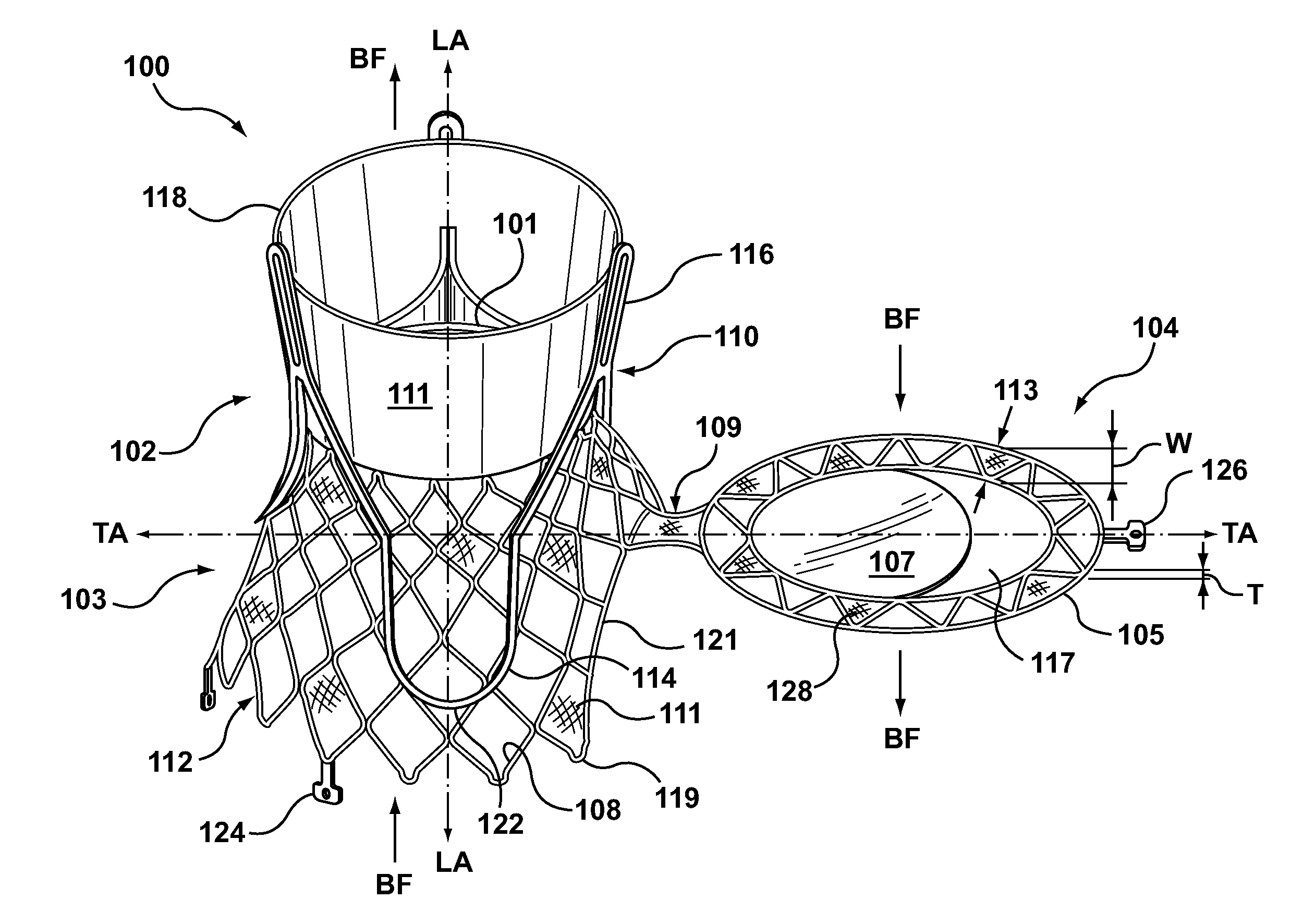
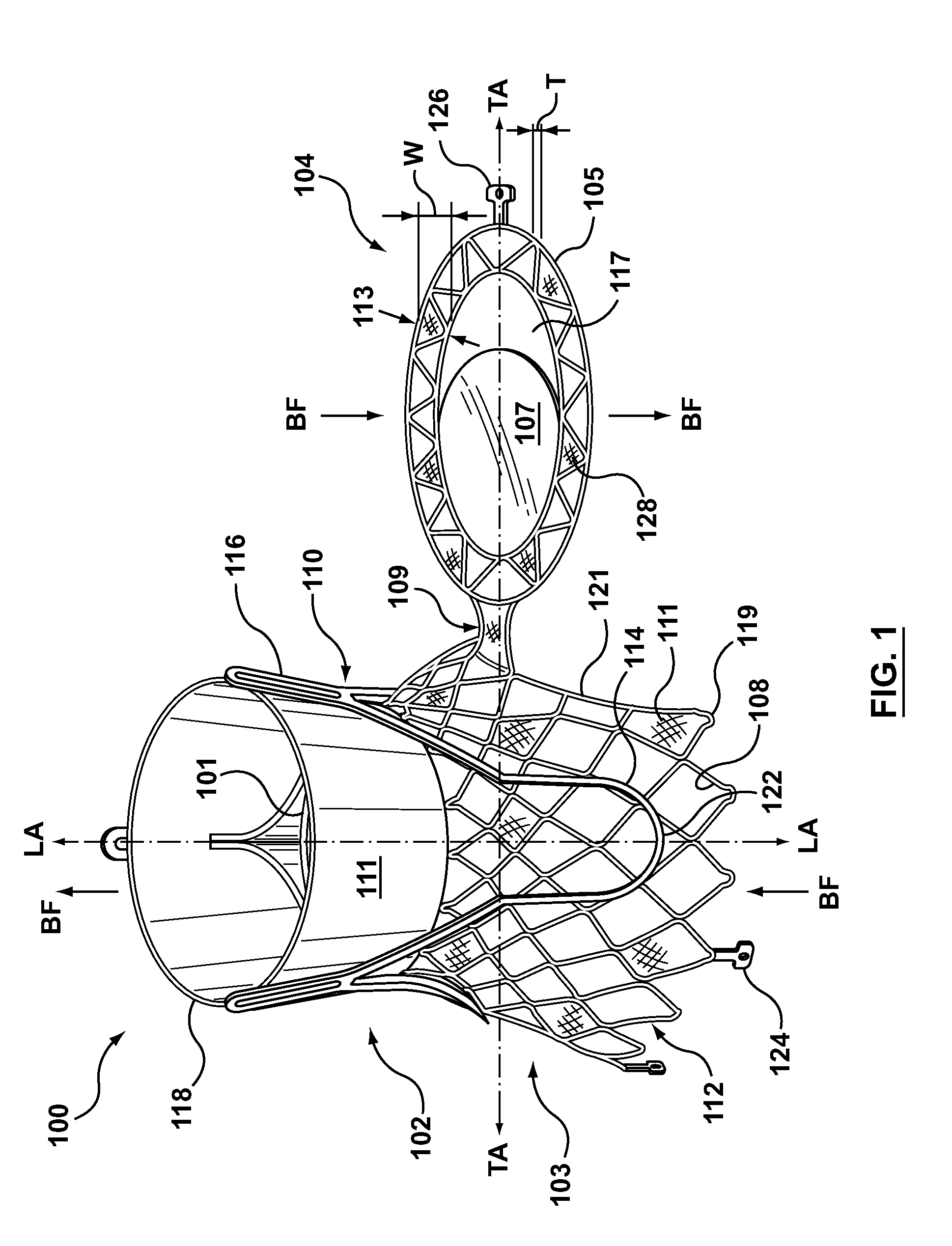
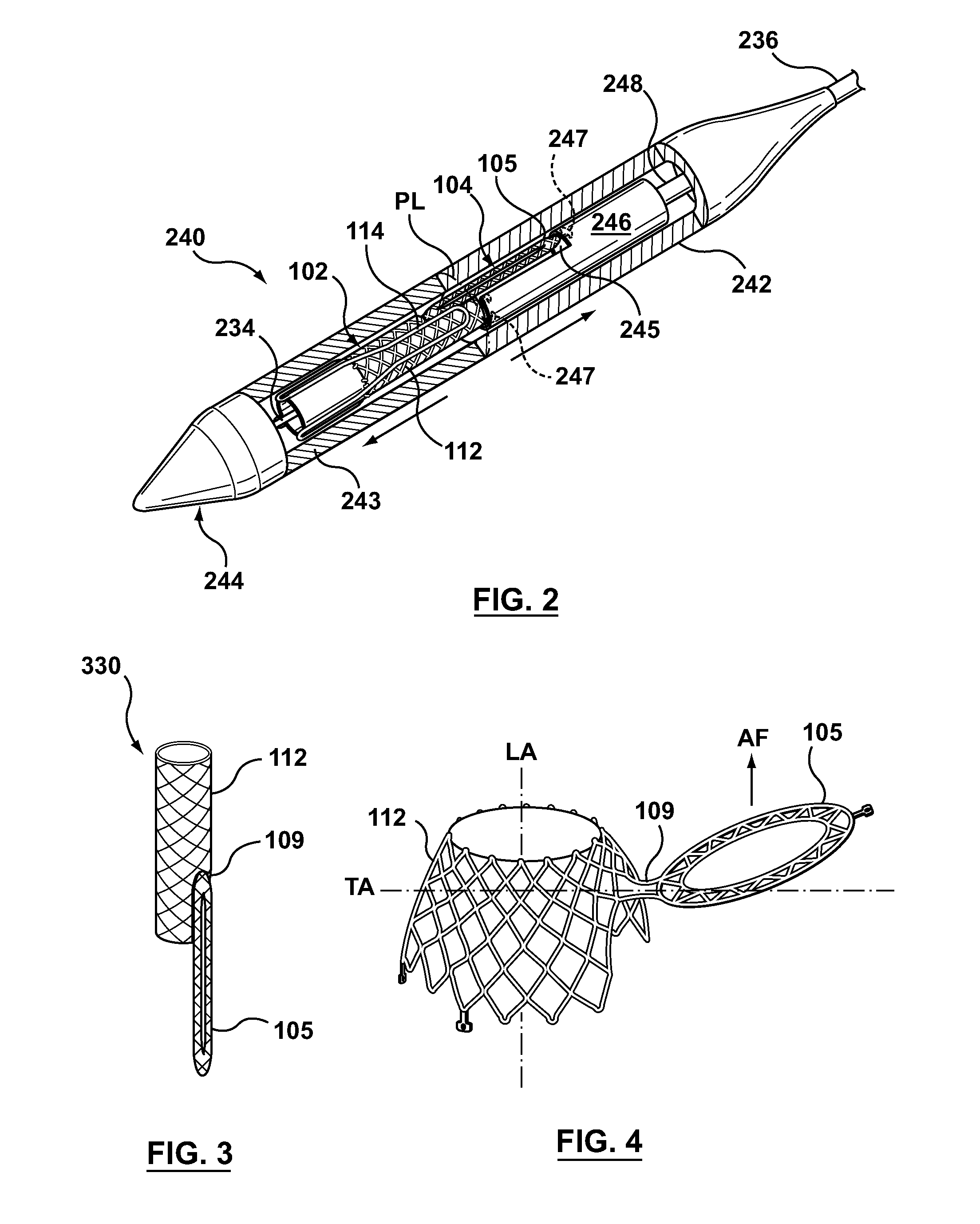
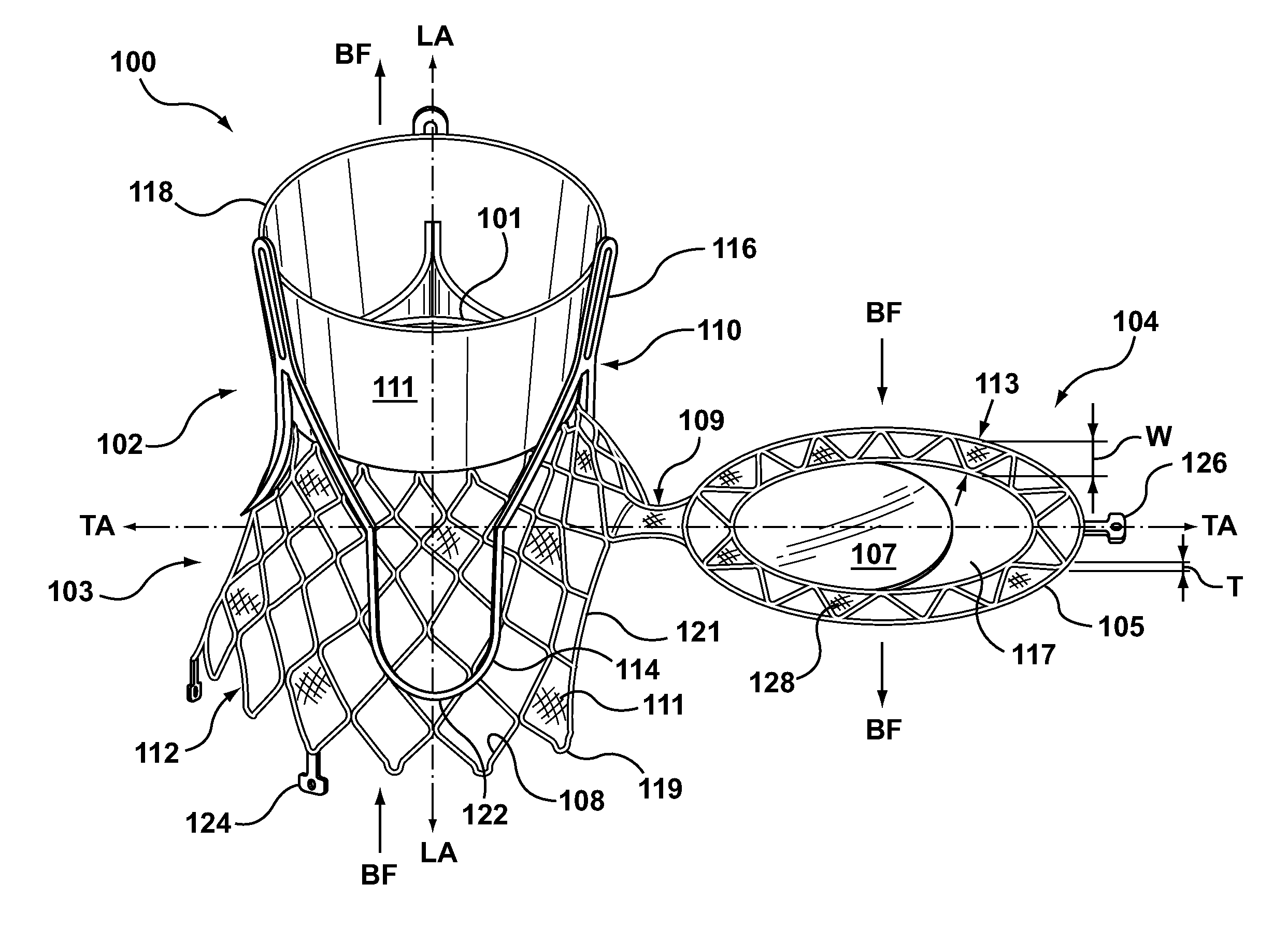
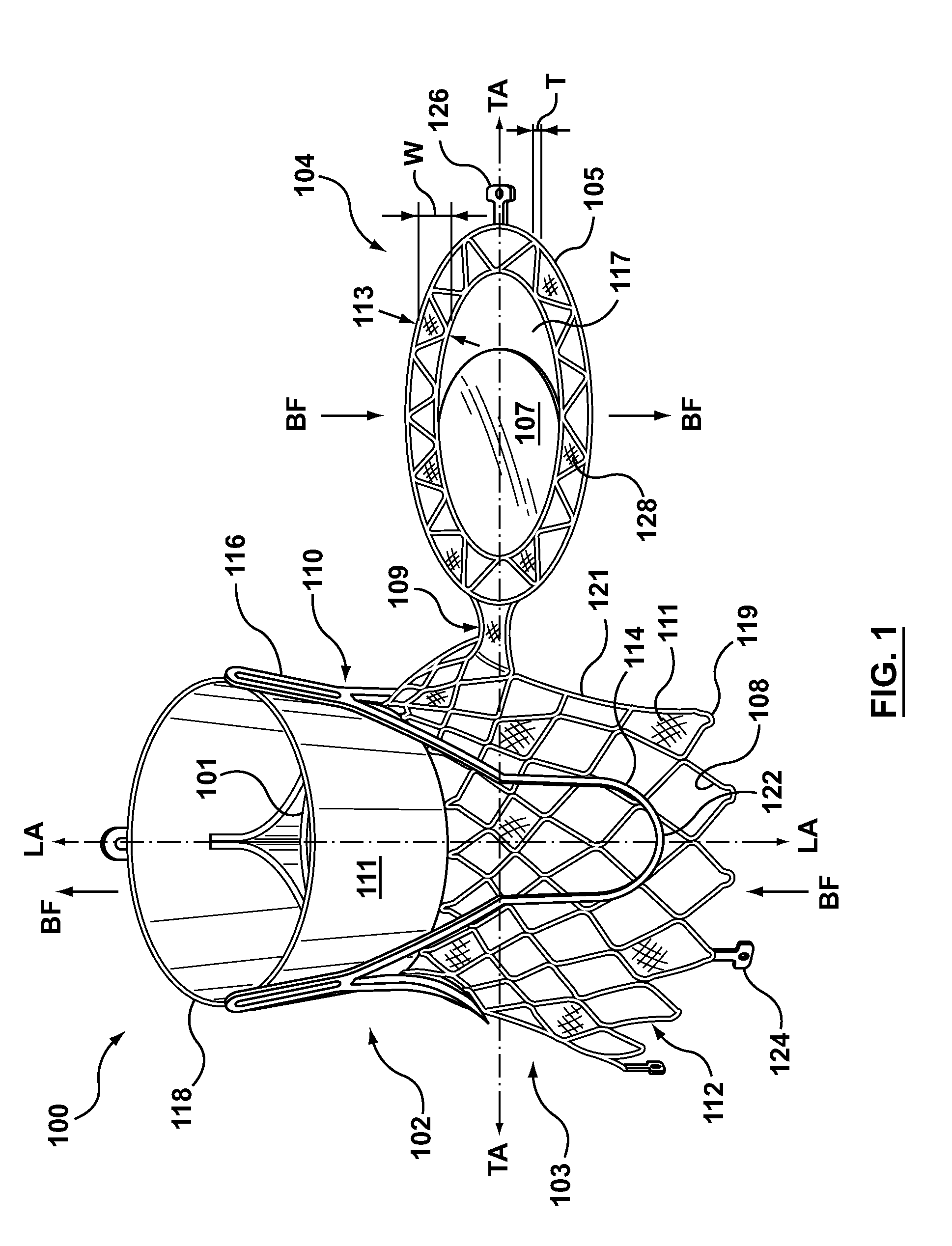
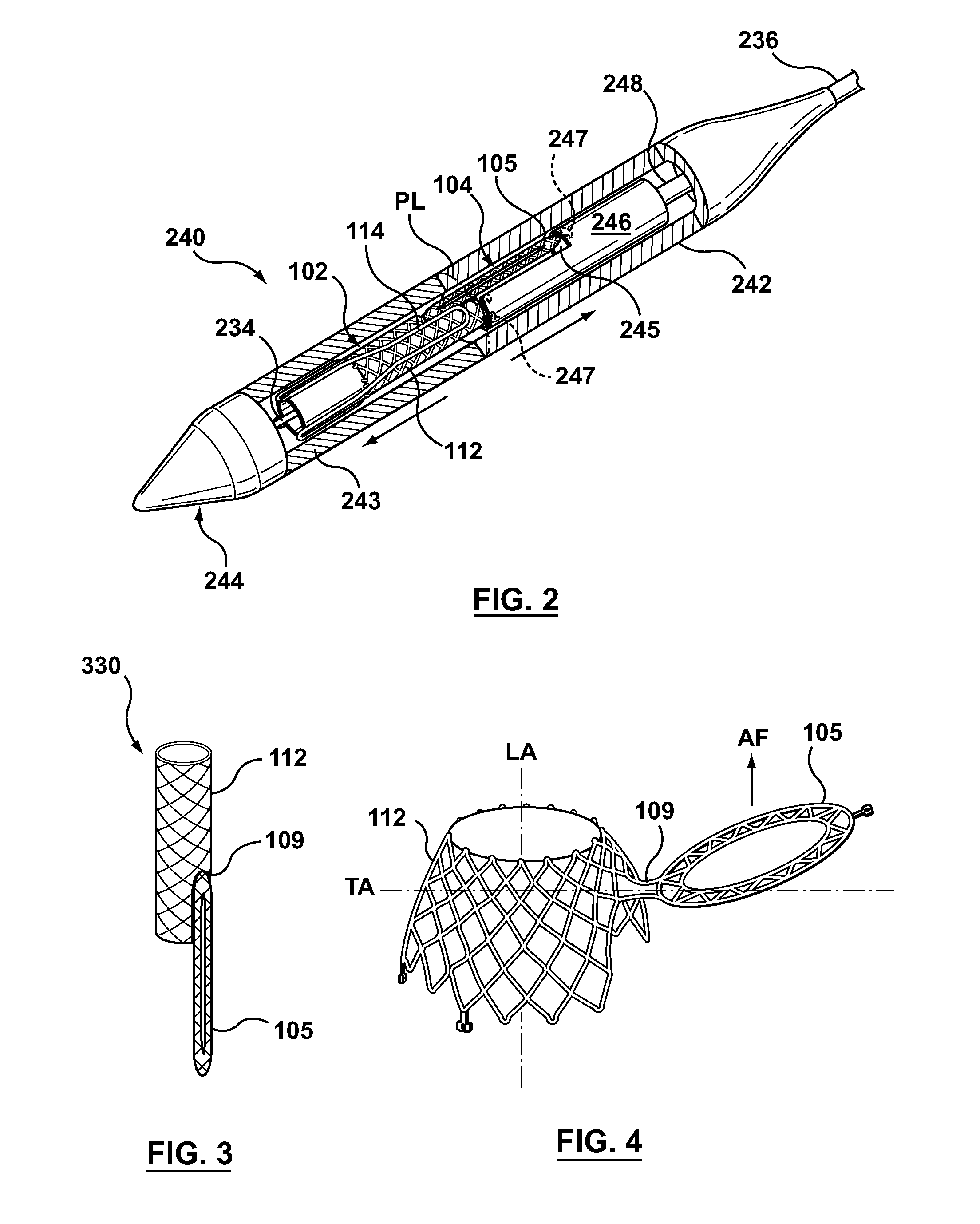
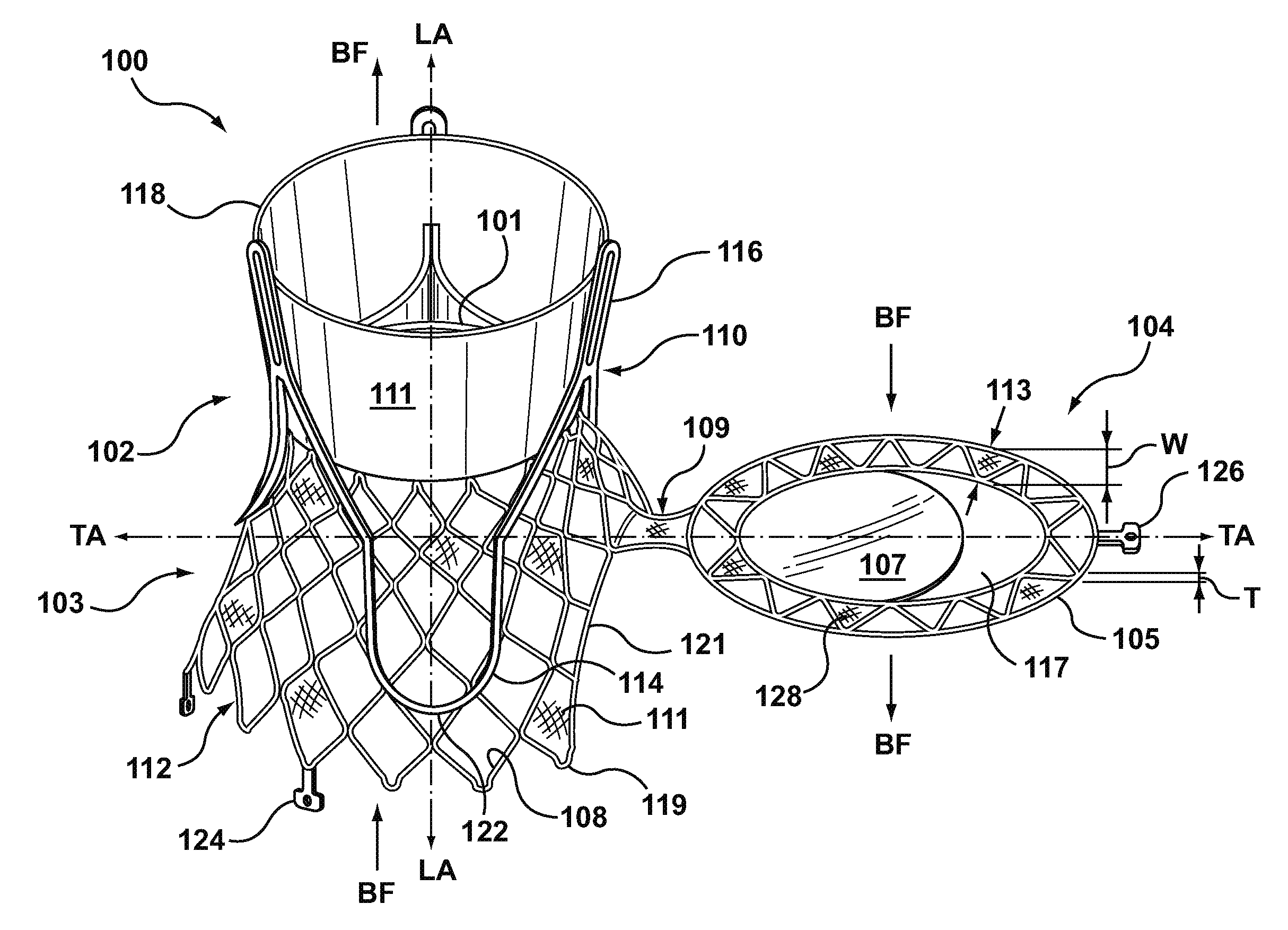
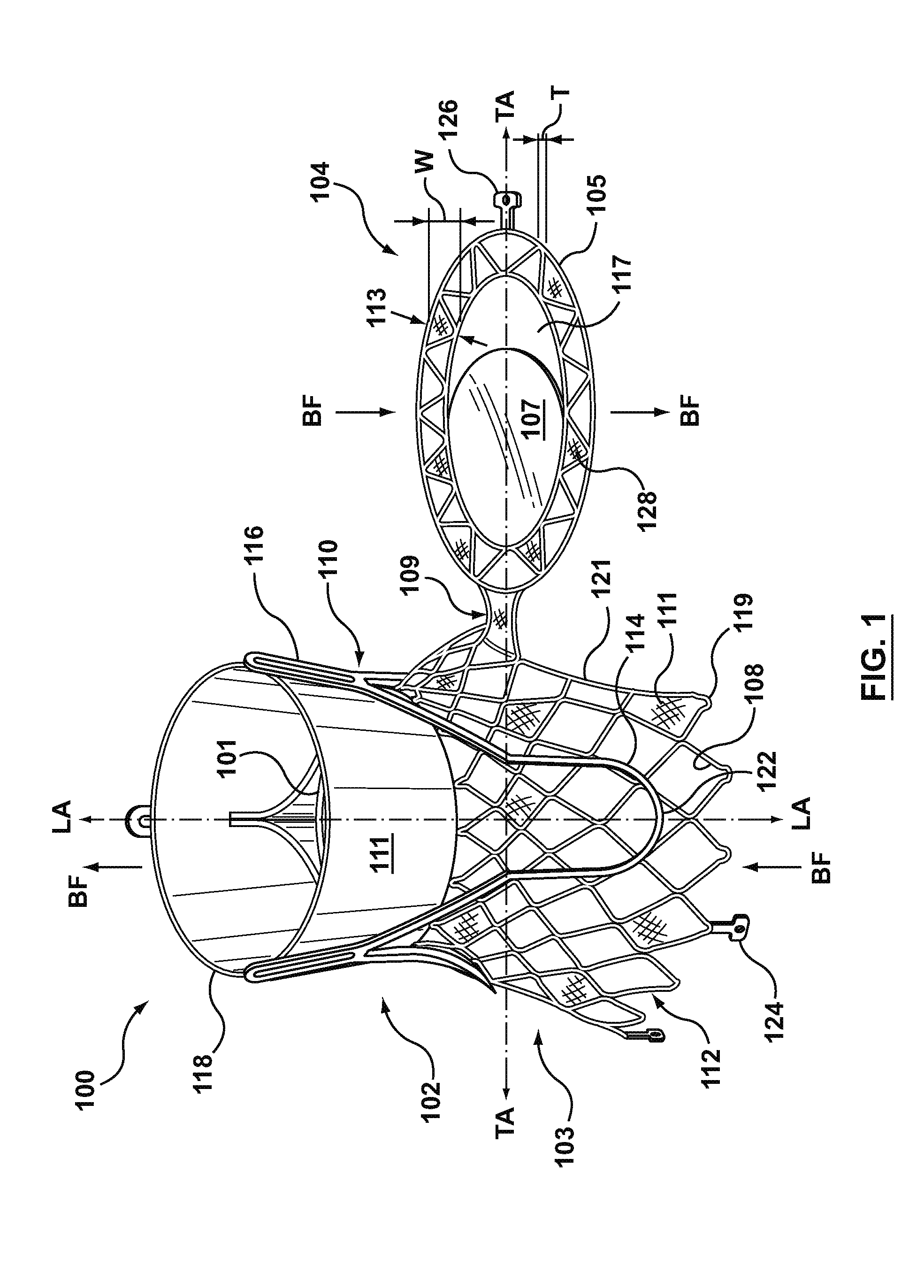
Dual Valve Prosthesis for Transcatheter Valve Implantation
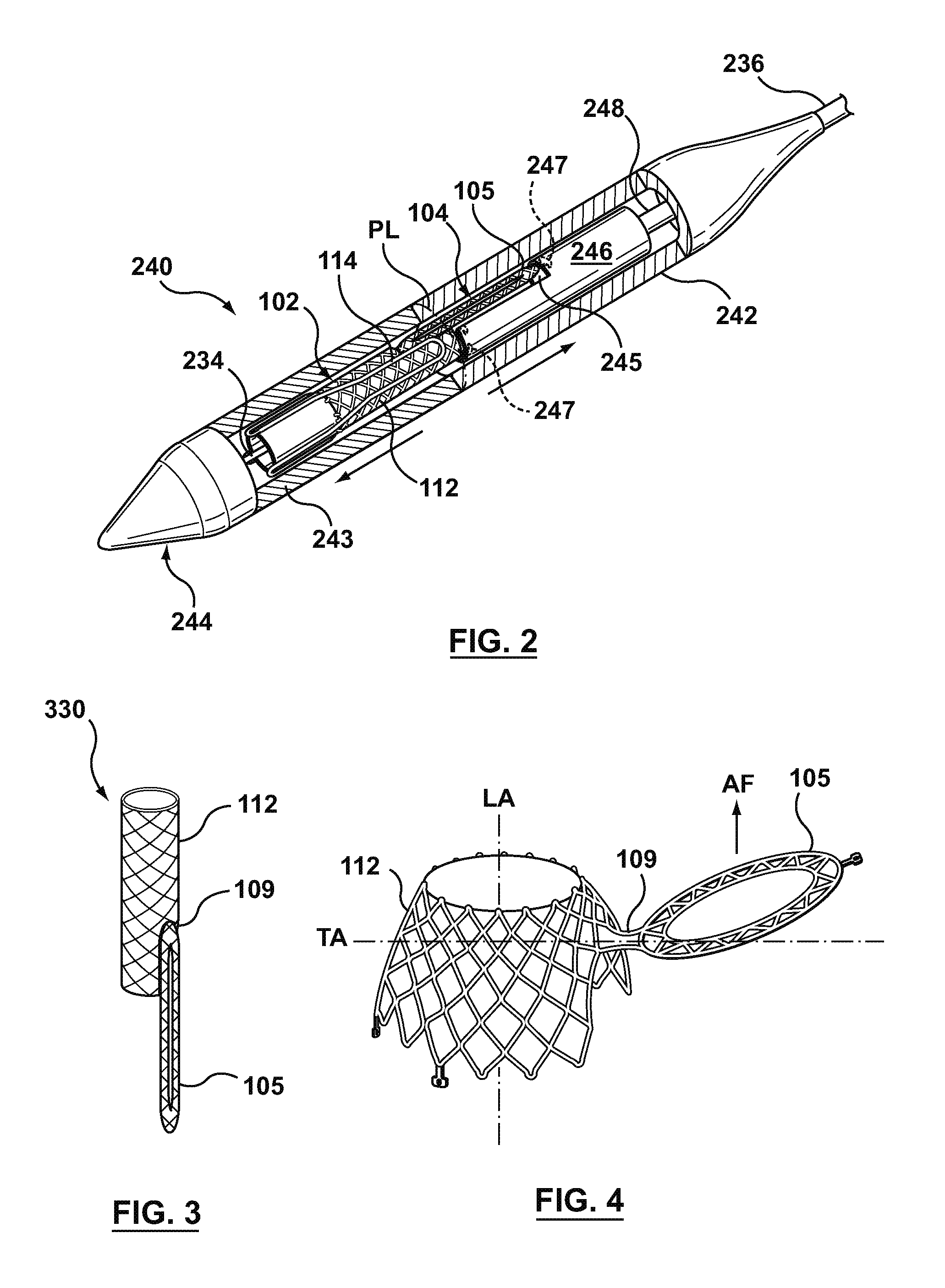
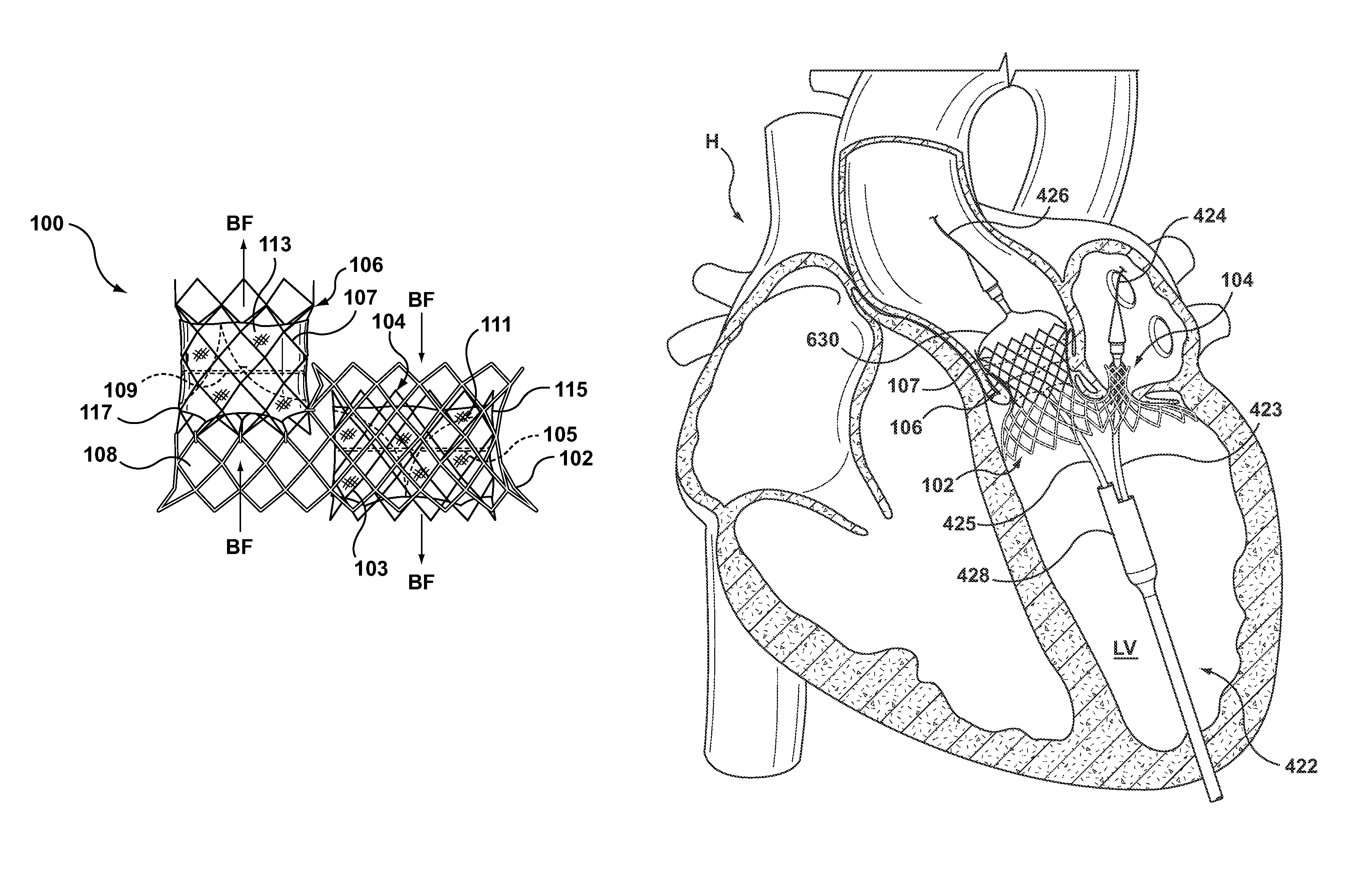
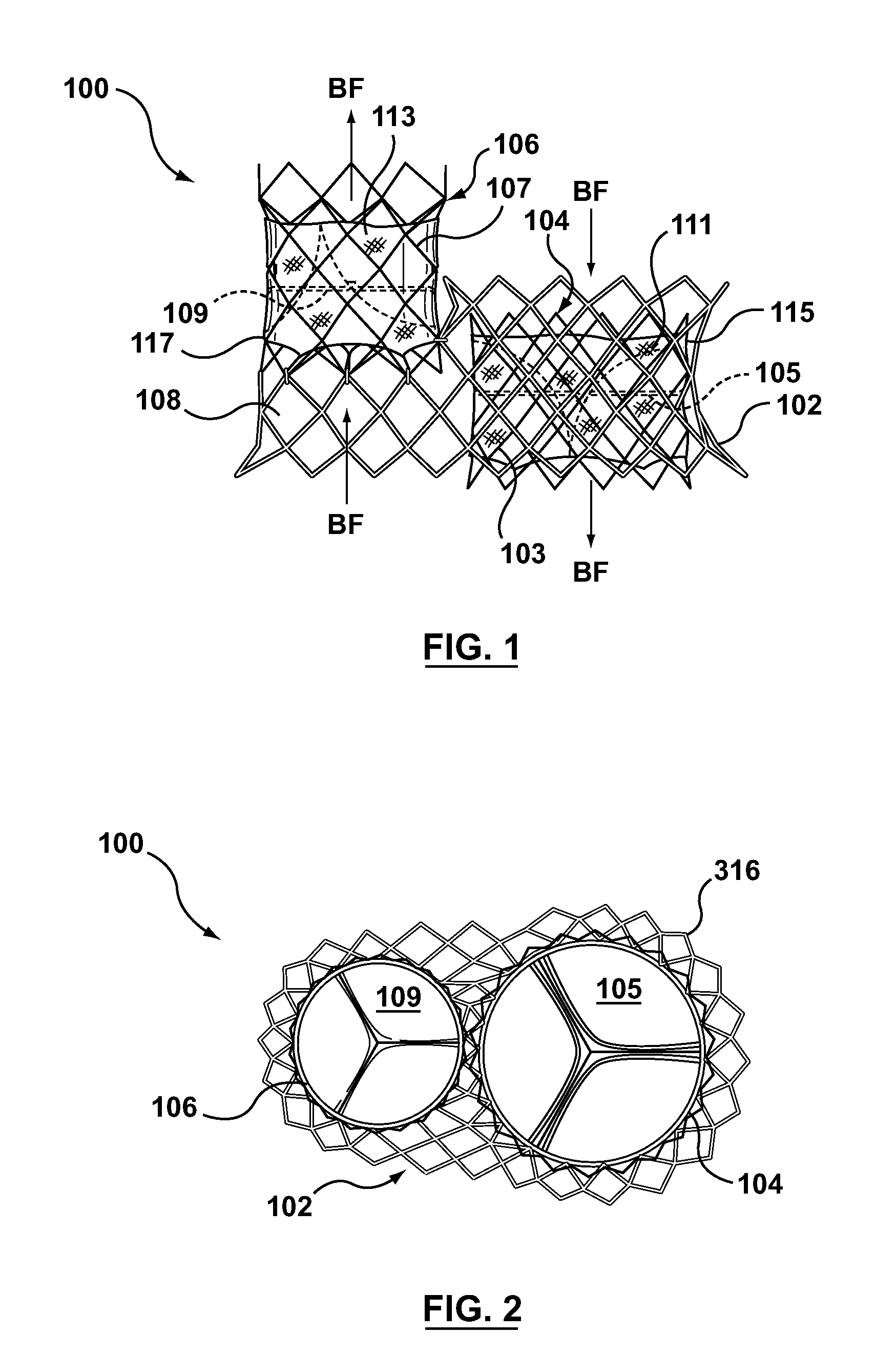
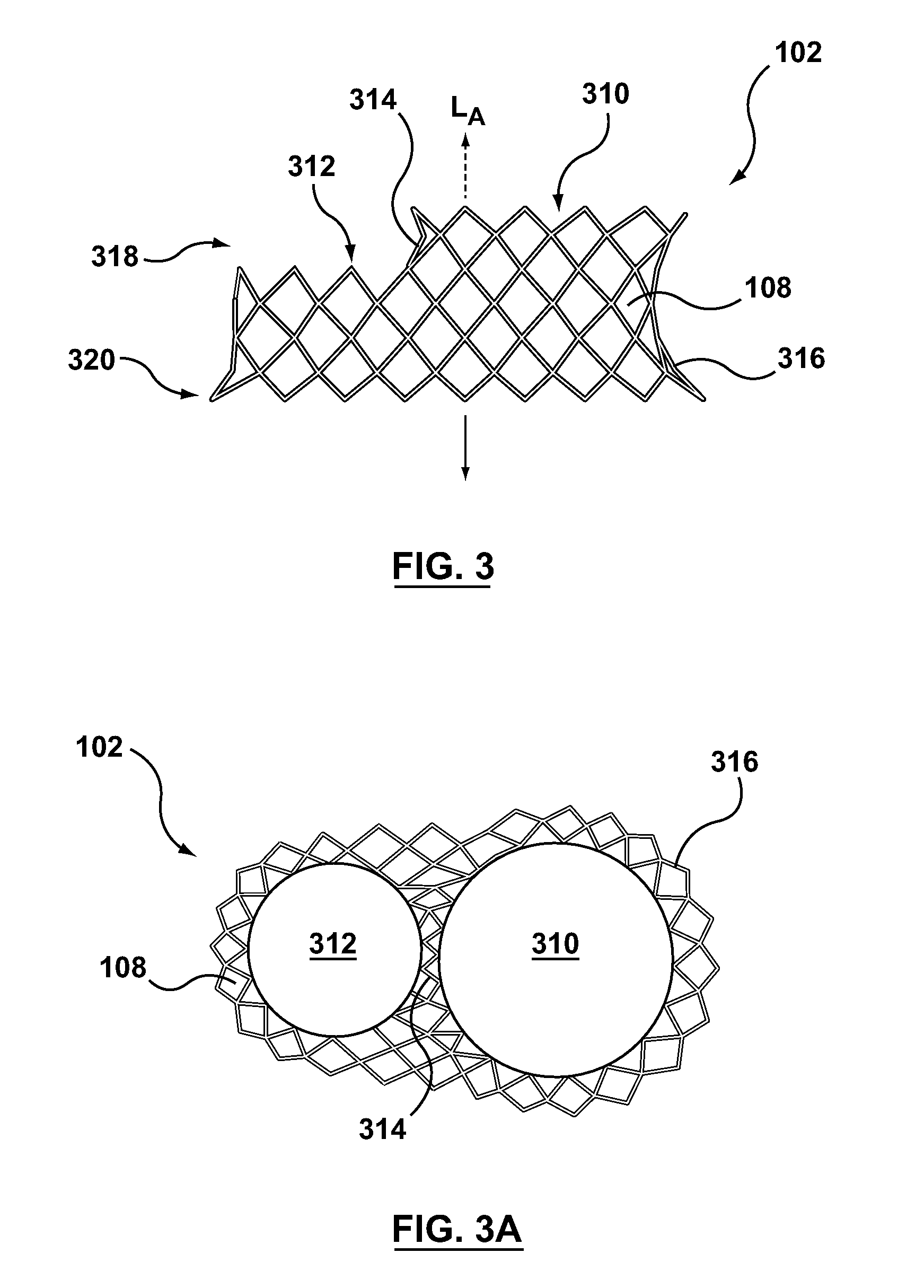
A dual valve prosthesis having first and second prosthetic valve sections is disclosed. The first prosthetic valve section includes a stent structure with a first prosthetic valve secured therein and the second prosthetic valve section includes an annular frame with a second prosthetic valve secured therein. When the dual valve prosthesis is in an expanded configuration, the annular frame extends from the stent structure such that the first and second prosthetic valves are laterally offset from each other. In a method in accordance herewith, the first and second prosthetic valve sections include prosthetic aortic and mitral valves, respectively, and the dual heart valve prosthesis is configured to replace both the native aortic and mitral valves of the heart in a single transcatheter heart valve implantation procedure.
Owner:MEDTRONIC VSACULAR GALWAY
Sigmoid valve and method for its percutaneous implantation
Owner:THE INT HEART INST OF MONTANA FOUND
Methods, compositions and systems for local delivery of drugs
ActiveUS20110195123A1Reduce degradationImprove permeabilityBiocidePowder deliveryCell-Extracellular MatrixBrachytherapy
Implantable medical device eluting drug locally and in prolonged period is provided, including several types of such a device, the treatment modes of implementation and methods of implantation. The device comprising of polymeric substrate, such as a matrix for example, that is used as the device body, and drugs, and in some cases additional scaffolding materials, such as metals or additional polymers, and materials to enhance visibility and imaging. The selection of drug is based on the advantageous of releasing drug locally and in prolonged period, where drug is released directly to the extracellular matrix (ECM) of the diseased area such as tumor, inflammation, degeneration or for symptomatic objectives, or to injured smooth muscle cells, or for prevention. One kind of drug is the gene silencing drugs based on RNA interference (RNAi), including but not limited to si RNA, sh RNA, or antisense RNA / DNA, ribozyme and nucleoside analogs. The modes of implantation in some embodiments are existing implantation procedures that are developed and used today for other treatments, including brachytherapy and needle biopsy. In such cases the dimensions of the new implant described in this invention are similar to the original implant. Typically a few devices are implanted during the same treatment procedure.
Owner:SILENSEED LTD
Dual Valve Prosthesis for Transcatheter Valve Implantation
A dual valve prosthesis having first and second prosthetic valve components with a linkage that connects the first and second prosthetic valve components together is disclosed. Each of the first and second prosthetic valve components includes a stent structure with a prosthetic valve secured therein. In a disclosed method, the first and second prosthetic valve components include prosthetic mitral and aortic valves, respectively, and the dual heart valve prosthesis is configured to replace both the native mitral and aortic valves of the heart in a single transcatheter heart valve implantation procedure. The linkage between the first and second prosthetic valve components is configured to secure the anterior mitral valve leaflet against a wall of the left ventricle when the dual valve prosthesis is implanted within the heart.
Owner:MEDTRONIC INC
Modular total ankle prosthesis apparatuses, systems and methods, and systems and methods for bone resection and prosthetic implantation
Ankle prosthesis apparatuses, systems and methods are provided as disclosed herein. Additionally, systems and methods for bone resection and implantation of prosthetics are provided, including surgical techniques and related instrumentation. An ankle prosthesis apparatus can include a talar component having a lower surface with a bone fixation portion for fixation to a talus bone and an upper surface designed for articulation with a bearing component. The bearing component can have a lower surface for articulation with the talar component and an upper surface for articulation with a tibial component. The tibial component can have a lower surface for articulation with the bearing component and an upper surface with a bone fixation portion for fixation to a tibia bone and / or a fibula bone. The bearing component can have a protrusion on its upper surface adapted for engagement with a recess on the tibial component to allow desired rotational and translational movement. Methods and systems can be used to prepare a bone surface for implantation of a prosthesis including determining a location for a curved cut line on the bone surface and drilling a series of holes tangent to the curved cut line to create a curved bone resection surface. Methods and systems can be used for the implantation of an ankle joint prosthesis including the use of an alignment guide, tibia and talus drill guides, tibia and talus saw guides, and tibia and talus broach guides, all components of which can be placed on and removed from a plurality of alignment anchor pins throughout the implantation procedure. A method for medially to laterally implanting an ankle joint prosthesis can include exposing tibia and talus bones from the medial side, resection of the tibia and talus bones, broaching the tibia and talus bones, and positioning and affixing the ankle joint prosthesis components.
Owner:INTEGRA LIFESCI
Method for contouring bone reconstruction plates
ActiveUS6978188B1Medical simulationAdditive manufacturing apparatusAnatomical structuresBiomedical engineering
Systems and methods are provided for designing and producing custom-made templates for implantation or for pre-contouring metallic or polymer implantable plates prior to surgery. According to one embodiment, medical image data representing surrounding portions of a patient's anatomy to be repaired by surgical implantation of a bone reconstruction plate is received. Next, three-dimensional surface reconstruction is preformed based on the medical image data. Virtual removal of a bone or portion thereof to be reconstructed is performed with reference to the medical image data by simulating the contemplated surgical implantation procedure. Then, a representation of a template is created that is countered to fit the patient's anatomy to be repaired. Finally, a replica of the template is produced by using Solid Freeform Fabrication manufacturing techniques.
Owner:3D SYST INC
Intervertebral cage and method of use
InactiveUS20020055781A1Facilitates inter-body fusionInternal osteosythesisBone implantProsthesisCancellous bone
An intervertebral prosthesis for implantation between adjacent vertebrae of the human spine is shown. The prosthesis is formed as a unitary cage body configured and sized to be inserted between adjacent vertebrae in a single step implantation procedure. The cage body is banana shaped as viewed from above, the body having an exterior surface and an interior surface, the interior surface defining an internal recess for receiving cancellous bone material during an implantation procedure. The cage body can be formed as an interlinked mesh.
Owner:DEPUY ACROMED INC
Dual valve prosthesis for transcatheter valve implantation
A dual valve prosthesis having first and second prosthetic valve sections is disclosed. The first prosthetic valve section includes a stent structure with a first prosthetic valve secured therein and the second prosthetic valve section includes an annular frame with a second prosthetic valve secured therein. When the dual valve prosthesis is in an expanded configuration, the annular frame extends from the stent structure such that the first and second prosthetic valves are laterally offset from each other. In a method in accordance herewith, the first and second prosthetic valve sections include prosthetic aortic and mitral valves, respectively, and the dual heart valve prosthesis is configured to replace both the native aortic and mitral valves of the heart in a single transcatheter heart valve implantation procedure.
Owner:MEDTRONIC VSACULAR GALWAY
Non-sheath based medical device delivery system
InactiveUS7092765B2Reduce the overall diameterEasy to disassembleEpicardial electrodesCannulasMedical deviceDelivery system
Owner:MEDTRONIC INC
Leadless implantable device delivery apparatus
A leadless implantable device delivery apparatus that enables testing of an implantation site before permanent implantation and enables secure attachment at the site while minimizing effects of the implantation procedure. Embodiments include a delivery sheath configured to accommodate a leadless implantable device, the leadless implantable device having an anchor that includes at least one projection configured to physically attach the anchor to tissue, such as heart tissue. In addition, embodiments include an adapter that resides within the delivery sheath and is configured to impart rotational force at the distal end of the adapter that is applied to the proximal end of the adapter to rotate the anchor associated with the implantable device.
Owner:BIOTRONIK SE & CO KG
Stent loading tool and method for use thereof
ActiveUS8747458B2The method is simple and reliableEasy to pushStentsHeart valvesInsertion stentEngineering
Owner:MEDTRONIC VENTOR TECH
Reed valve for implantation into mammalian blood vessels and heart with optional temporary or permanent support
A multi-leaflet valve adapted to serve as a prosthesis for diseased native valve of a mammal is constructed of biologic membrane or of biocompatible synthetic membrane. The valve has the shape of a truncated cone that has an inflow and an outflow orifice with leaflets forming the outflow orifice and forming a plurality of commissures. A first flexible stent is removably affixed in a substantially circular fashion around the truncated cone in proximity of the inflow orifice, and a second flexible stent is removably affixed at the location of the commissures to form a circle around the truncated cone in proximity of the outflow orifice. The stents maintain the shape of the valve during the surgical implantation procedure. Each stent independently can be left in the valve or can be removed during the implantation procedure based upon the judgement of the cardiac surgeon performing the implantation procedure. A holder designed to maintain the geometry of the valve during implantation to a mammal is also disclosed.
Owner:THE INT HEART INST OF MONTANA FOUND
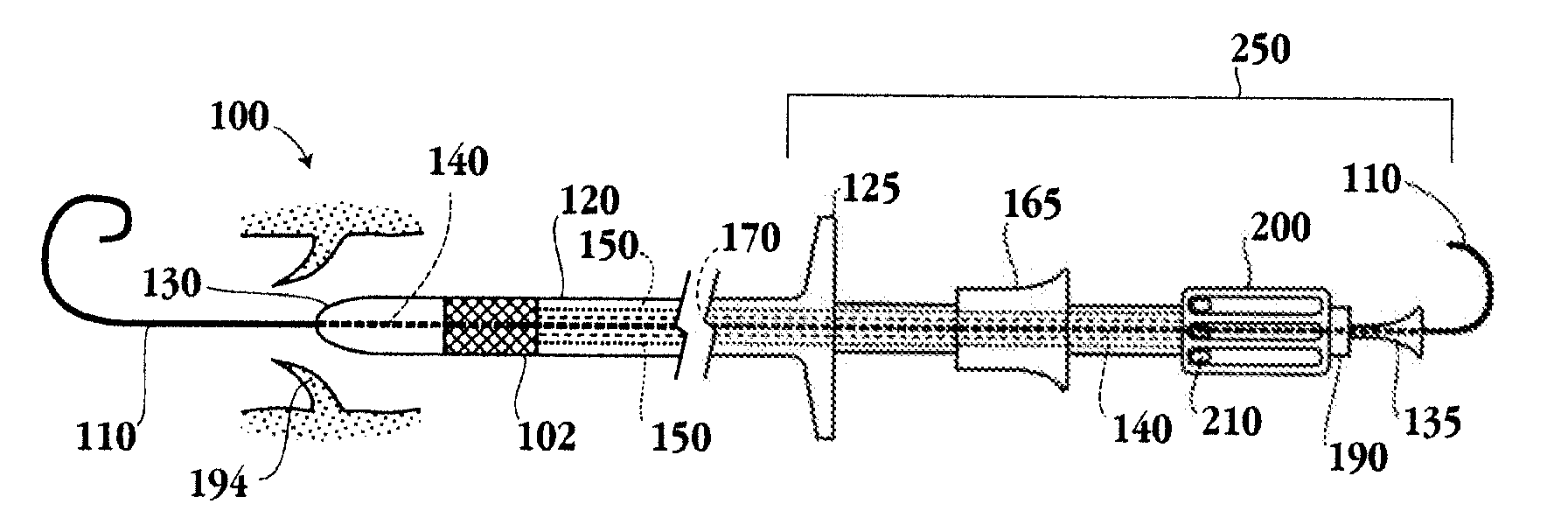
Devices and methods for delivery of aortic and mitral valve prostheses
A valve prosthesis and implantation device, and methods for use are provided. The devices described herein may be used for transcatheter delivery of an aortic valve prosthesis or transapical delivery of a mitral valve prosthesis. The implantation device utilizes movable claspers for both positioning and anchoring the valve prosthesis, reducing the extent of imaging needed during the implantation procedure.
Owner:JC MEDICAL INC
Leadless implantable device delivery apparatus
Owner:BIOTRONIK SE & CO KG
Systems and methods for transcatheter aortic valve treatment
ActiveUS20110213459A1Shorter and straight access pathEasy to placeSuture equipmentsStentsTRANSCATHETER AORTIC VALVE IMPLANTCatheter
Devices and methods are configured to allow transcervical or subclavian access via the common carotid artery to the native aortic valve, and implantation of a prosthetic aortic valve into the heart. The devices and methods also provide means for embolic protection during such an endovascular aortic valve implantation procedure.
Owner:SILK ROAD MEDICAL
Efficient implantation of heart valve prostheses
Tools can be used to assist with the implantation of heart valve prostheses, especially stentless aortic heart valve prostheses. In some embodiments, a heart valve prosthesis includes a plurality of flexible leaflets, a commissure support and a plurality of fasteners inserted into the inner surface of the commissure support and protruding from the outer surface of the commissure support. The fasteners comprise an elongated portion and a tip at an end of the elongated portion. In other embodiments, a heart valve prosthesis includes a plurality of flexible leaflets and a reinforcement secured to an inner surface of a valve commissure support. The reinforcement has an aperture. The heart valve prosthesis and fastener components can be placed into kits. The fasteners can be used to perform improved implantation procedures.
Owner:ST JUDE MEDICAL
Prosthesis for transcatheter valve implantation
A prosthesis having first and second prosthetic sections is disclosed. The first prosthetic section includes a stent structure that may contain a first prosthetic valve secured therein and the second prosthetic section includes an annular frame that may contain a second prosthetic valve secured therein. When the prosthesis is in an expanded configuration, the annular frame extends from the stent structure such that the first and second prosthetic sections are laterally offset from each other. In a method in accordance herewith, the first and second prosthetic sections may include prosthetic aortic and mitral valves, respectively, and the heart valve prosthesis is configured to replace one or both of the native aortic and mitral valves of the heart in a single transcatheter heart valve implantation procedure.
Owner:MEDTRONIC VSACULAR GALWAY
Prosthesis for Transcatheter Valve Implantation
A prosthesis having first and second prosthetic sections is disclosed. The first prosthetic section includes a stent structure that may contain a first prosthetic valve secured therein and the second prosthetic section includes an annular frame that may contain a second prosthetic valve secured therein. When the prosthesis is in an expanded configuration, the annular frame extends from the stent structure such that the first and second prosthetic sections are laterally offset from each other. In a method in accordance herewith, the first and second prosthetic sections may include prosthetic aortic and mitral valves, respectively, and the heart valve prosthesis is configured to replace one or both of the native aortic and mitral valves of the heart in a single transcatheter heart valve implantation procedure.
Owner:MEDTRONIC VSACULAR GALWAY
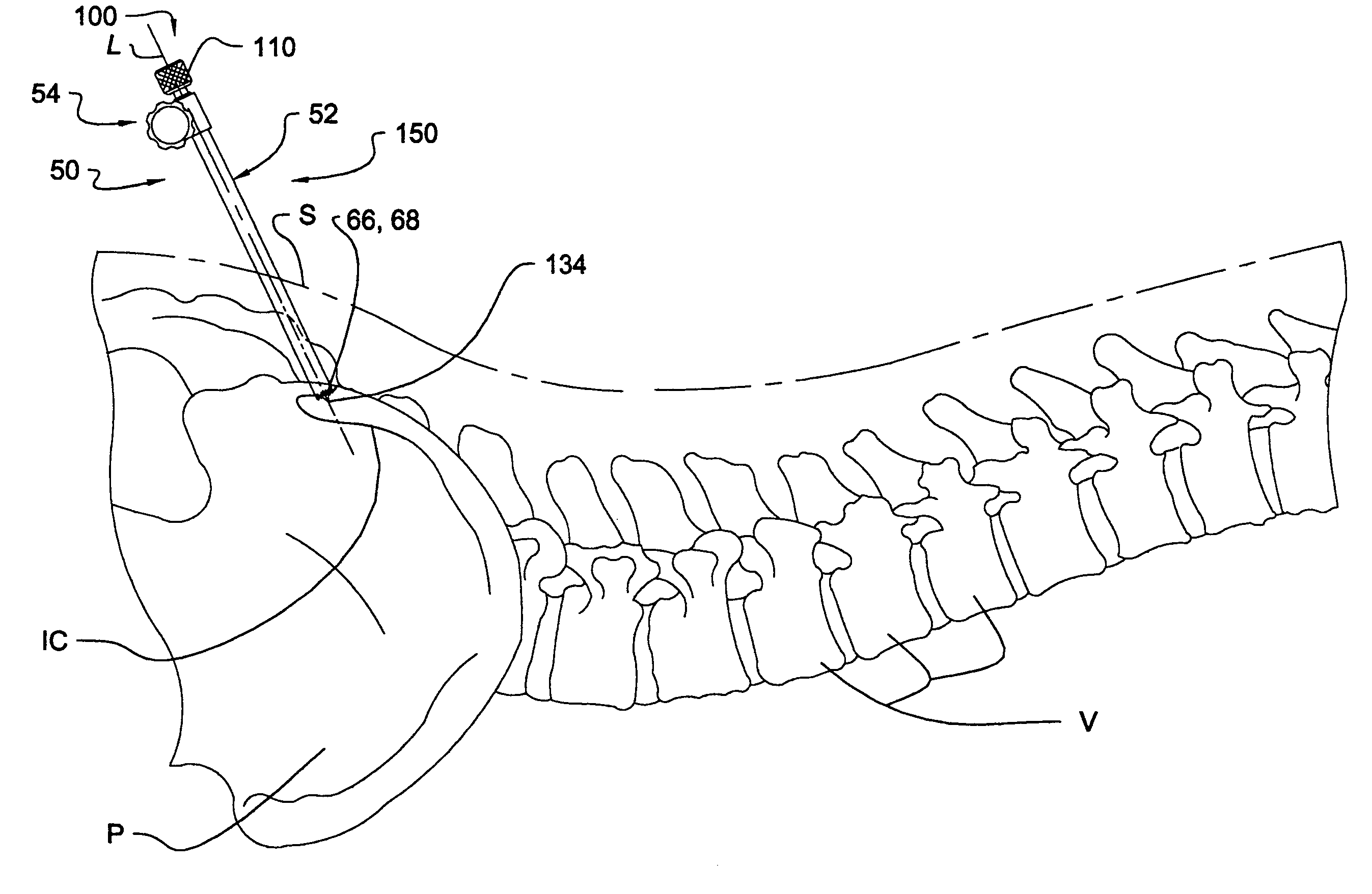
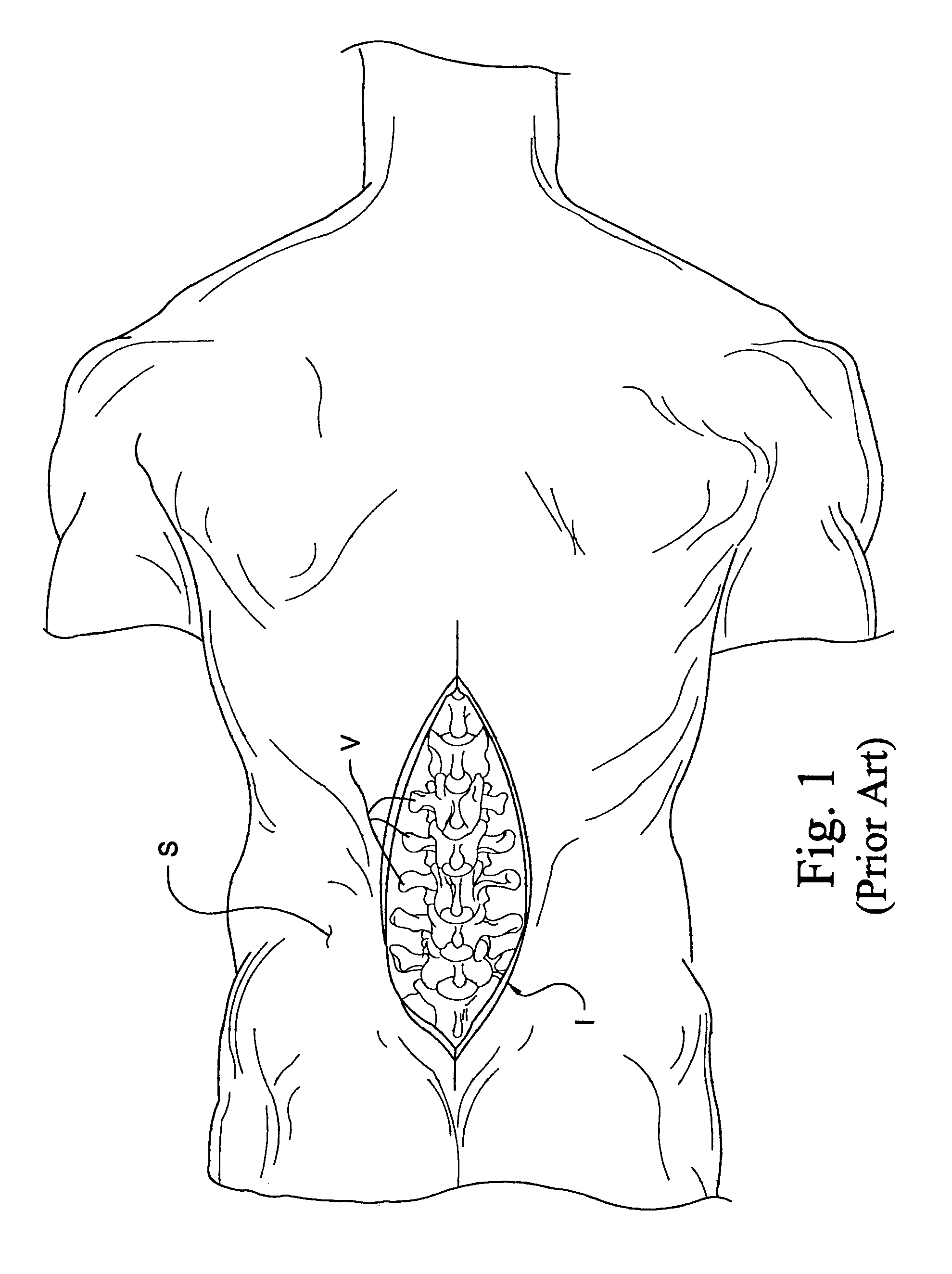
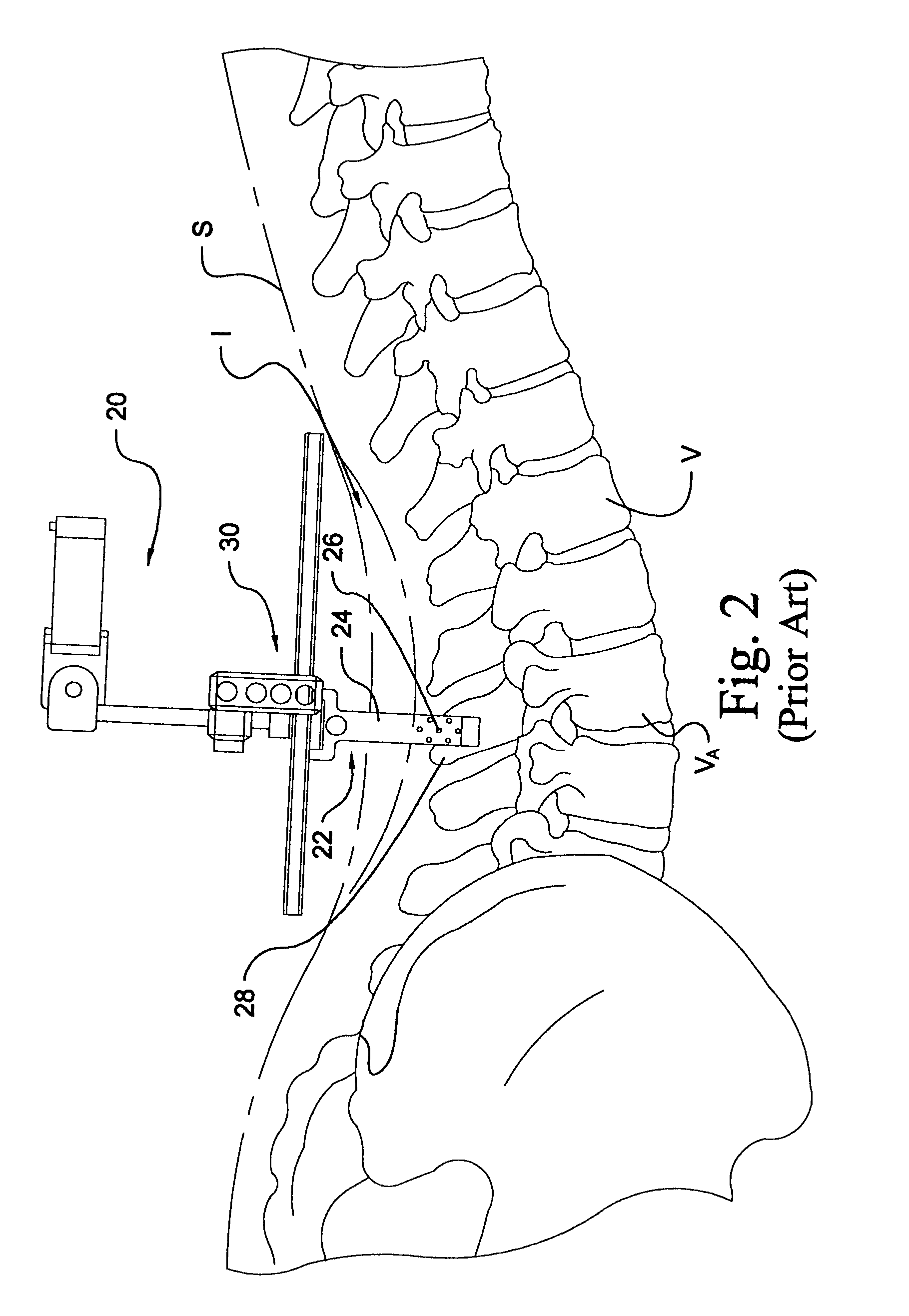
Instrumentation and method for performing image-guided spinal surgery using an anterior surgical approach
InactiveUS6980849B2Improved instrumentationImprove methodSurgical instrument detailsDiagnostic markersSpinal columnSurgical approach
Instrumentation and methods are provided for performing image-guided spinal surgery using an anterior surgical approach. In one embodiment, the method comprises providing a surgical navigation reference device, mounting the reference device to bone at a location remote from the spinal column and in a substantially fixed position relative thereto, accessing a portion of the spinal column from an anterior direction, and performing an image-guided surgical procedure on the spinal column using an anterior surgical approach. In another embodiment, the mounting of the reference device comprises anchoring the reference device to a portion of the patient's pelvic bone, and more specifically the anterior region of the iliac crest. In a further embodiment, the image-guided surgical procedure comprises a spinal implantation procedure wherein a spinal implant is inserted into an intervertebral opening formed along the lumbar region of the spinal column using an anterior surgical approach.
Owner:WARSAW ORTHOPEDIC INC
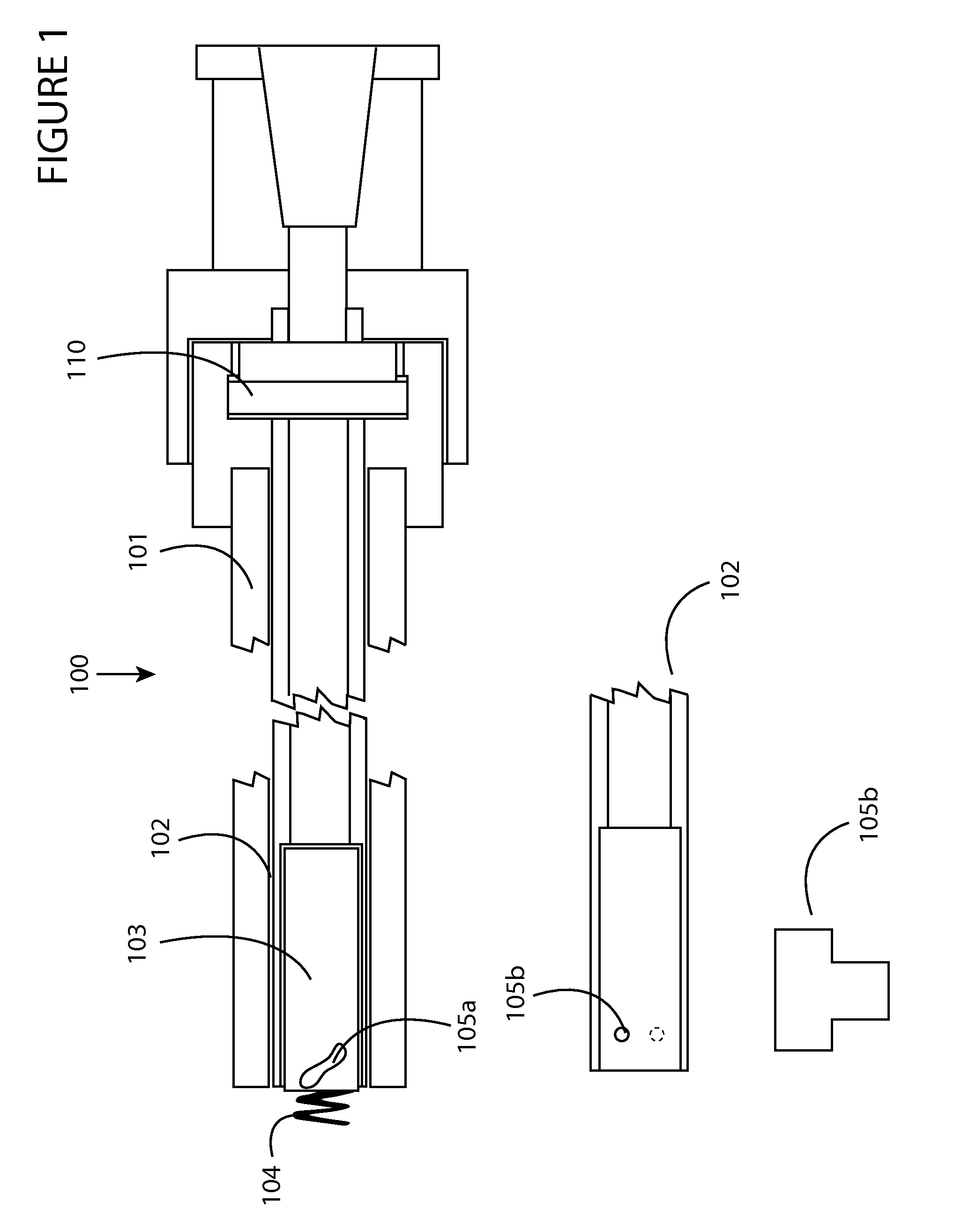
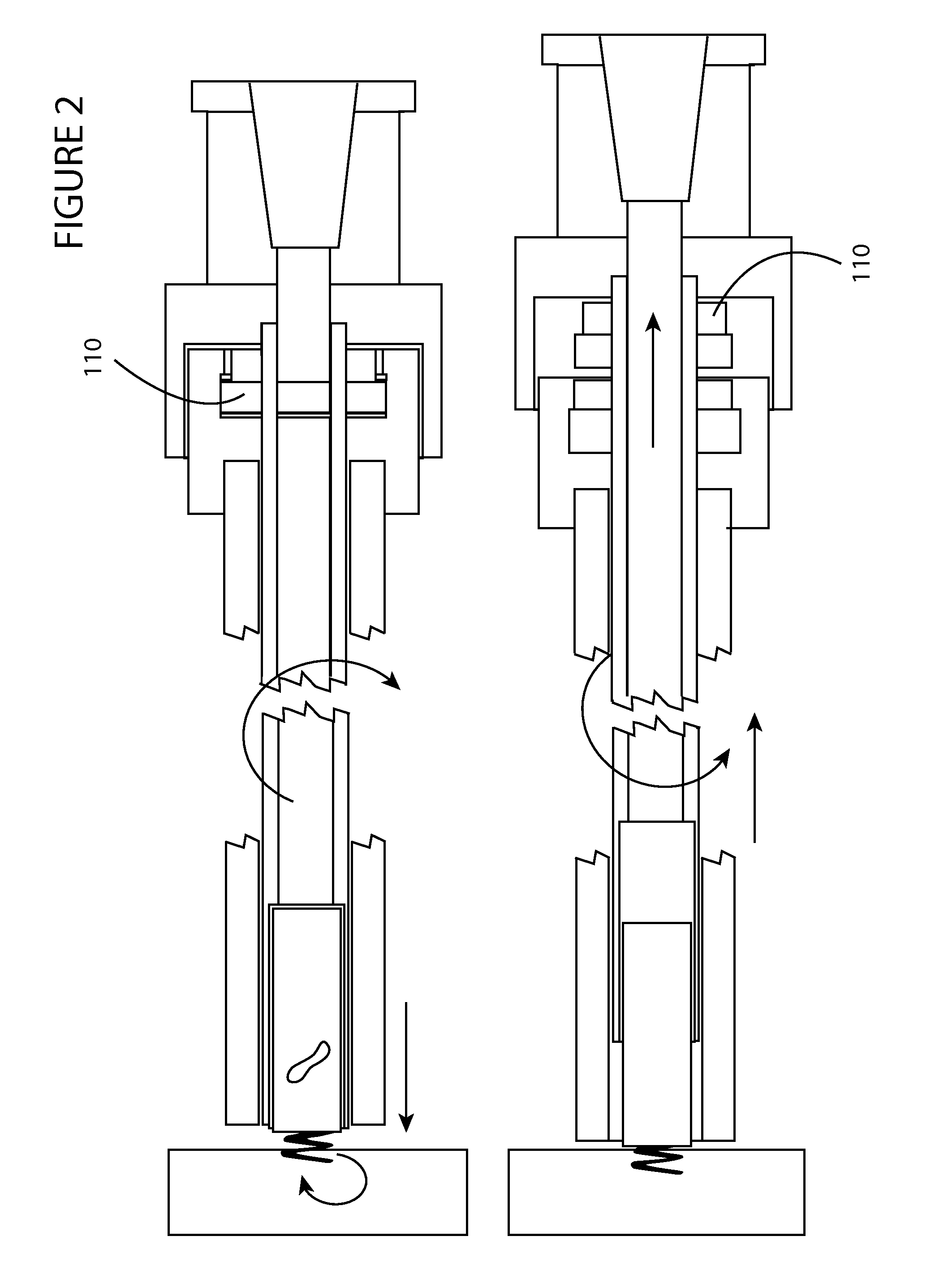
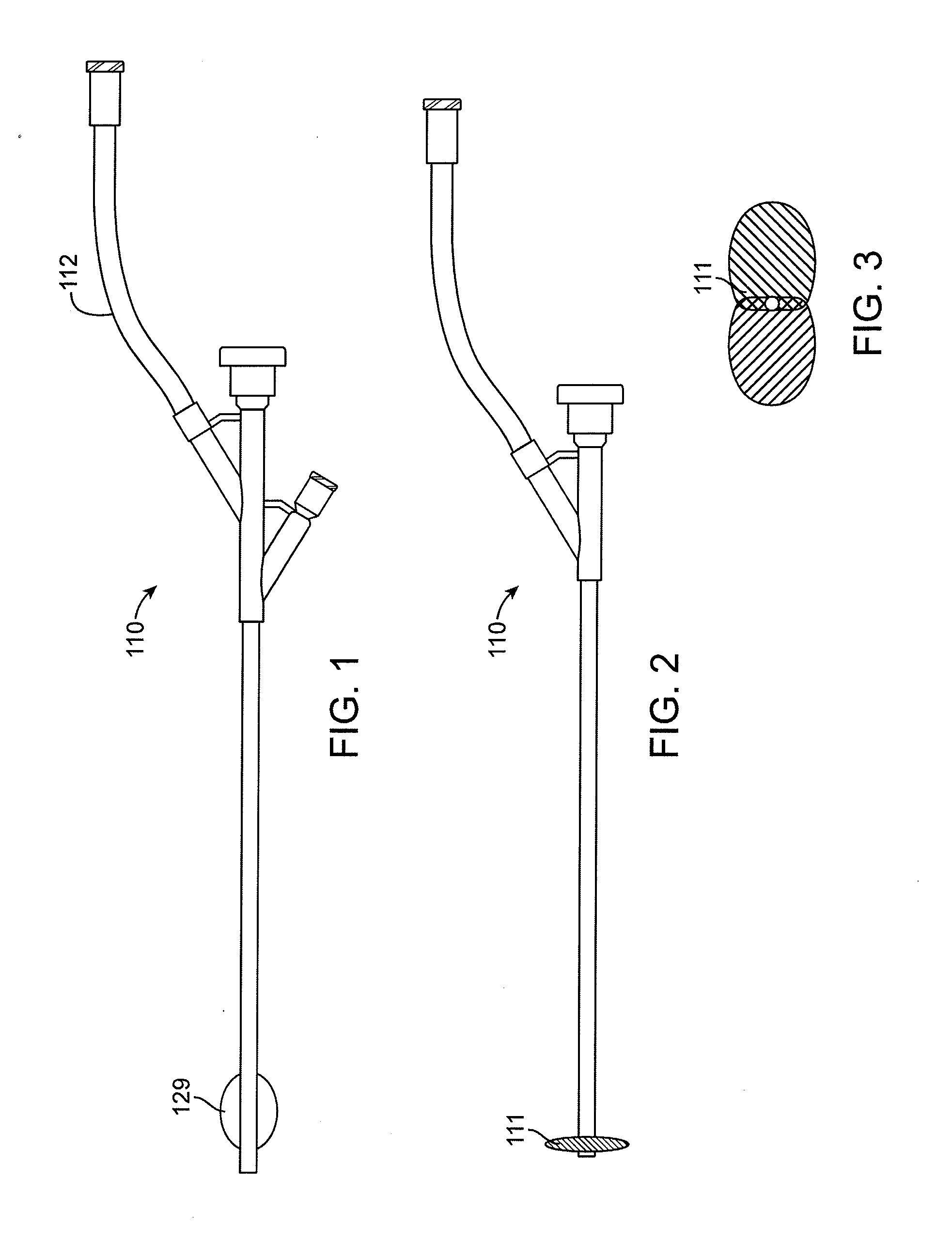
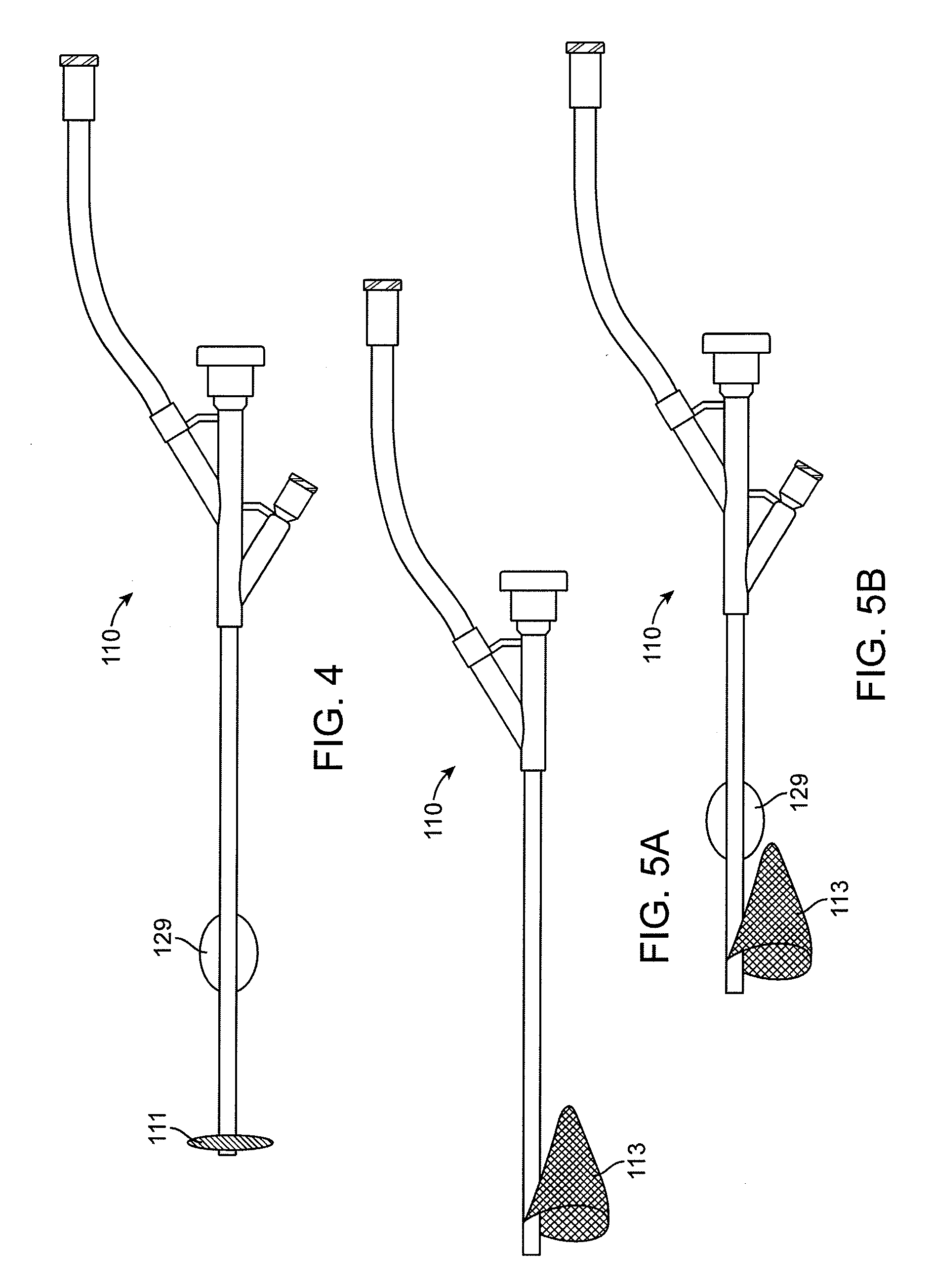
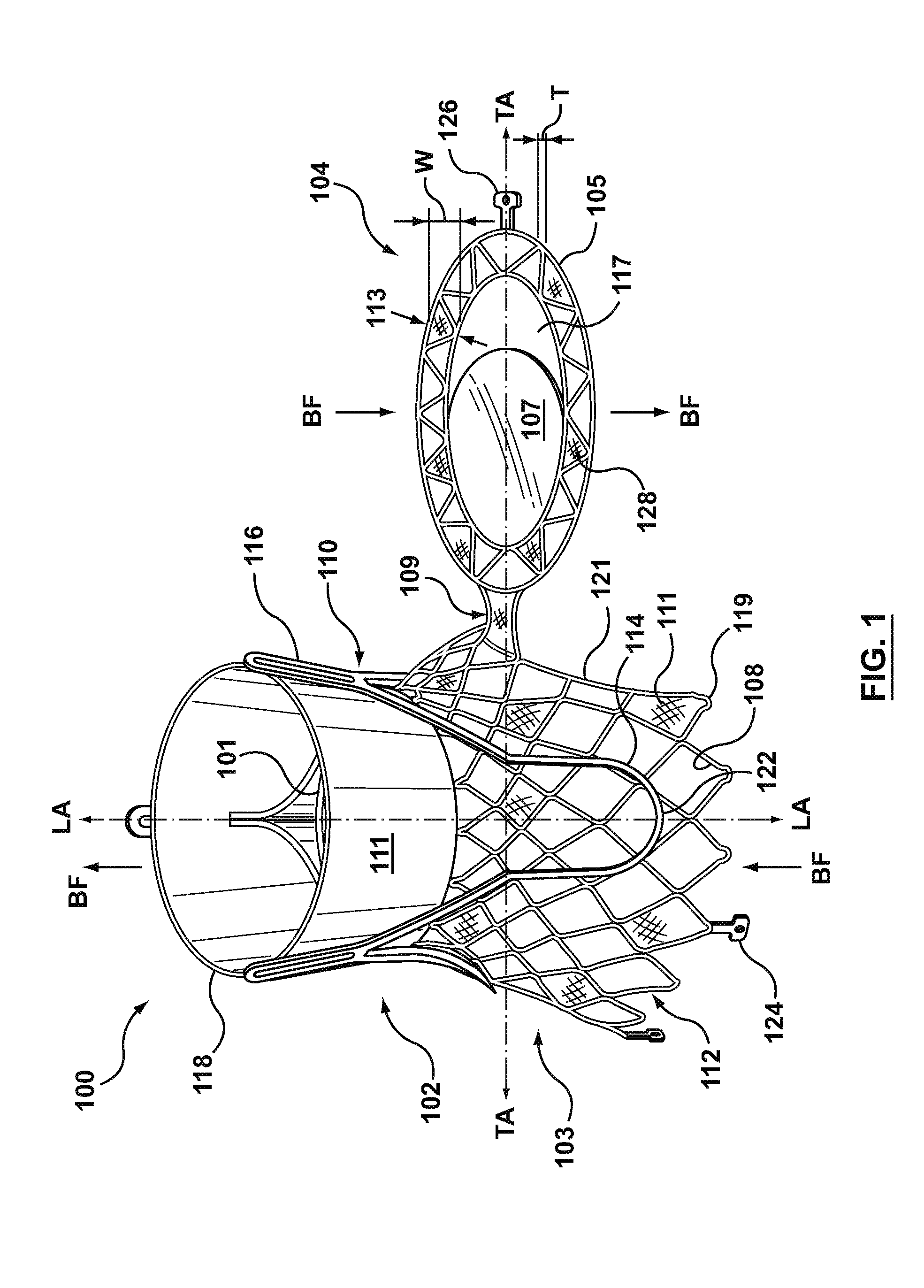
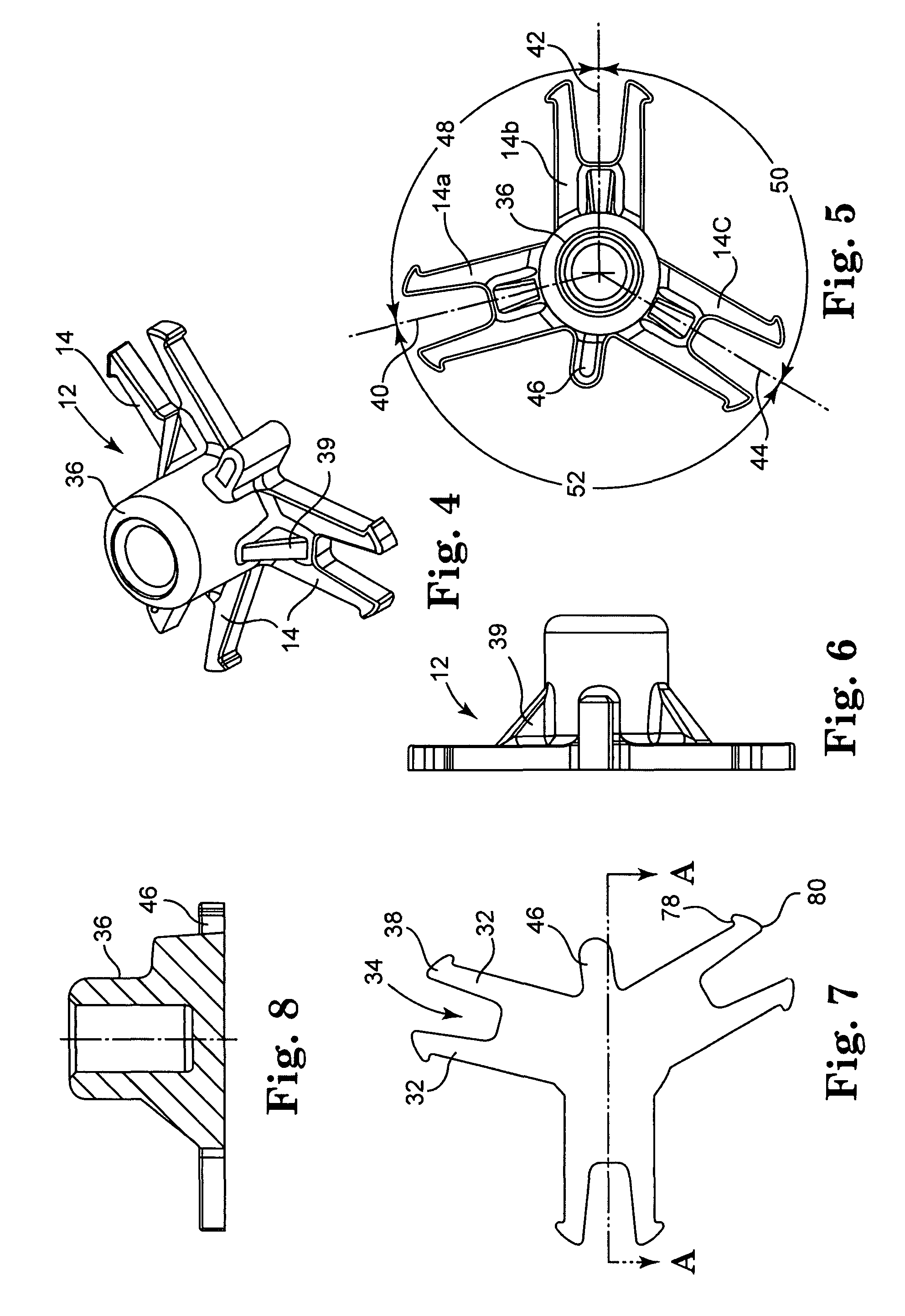
Heart valve holder for use in valve implantation procedures
A valve holder for a prosthetic valve having a stent with a stent base and multiple commissure posts projecting from the stent base, the valve holder including a central base portion, a plurality of legs radially extending from the central base portion, each leg comprising a first prong portion spaced from a second prong portion by a gap distance, a plurality of commissure post engaging members, wherein one commissure post engaging member is slideably engaged with each of the plurality of legs, and a handle extending from the central base portion.
Owner:MEDTRONIC INC
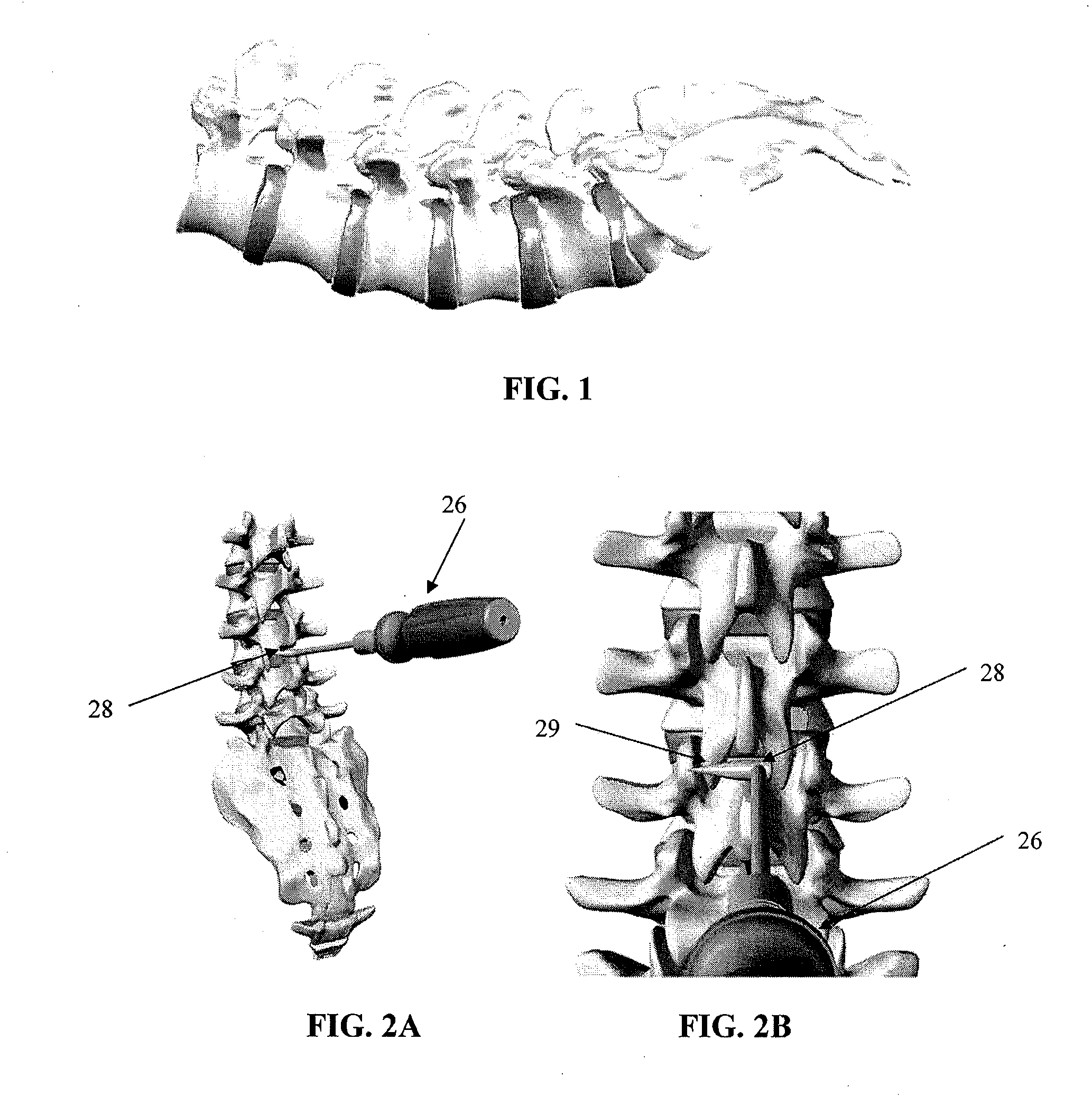
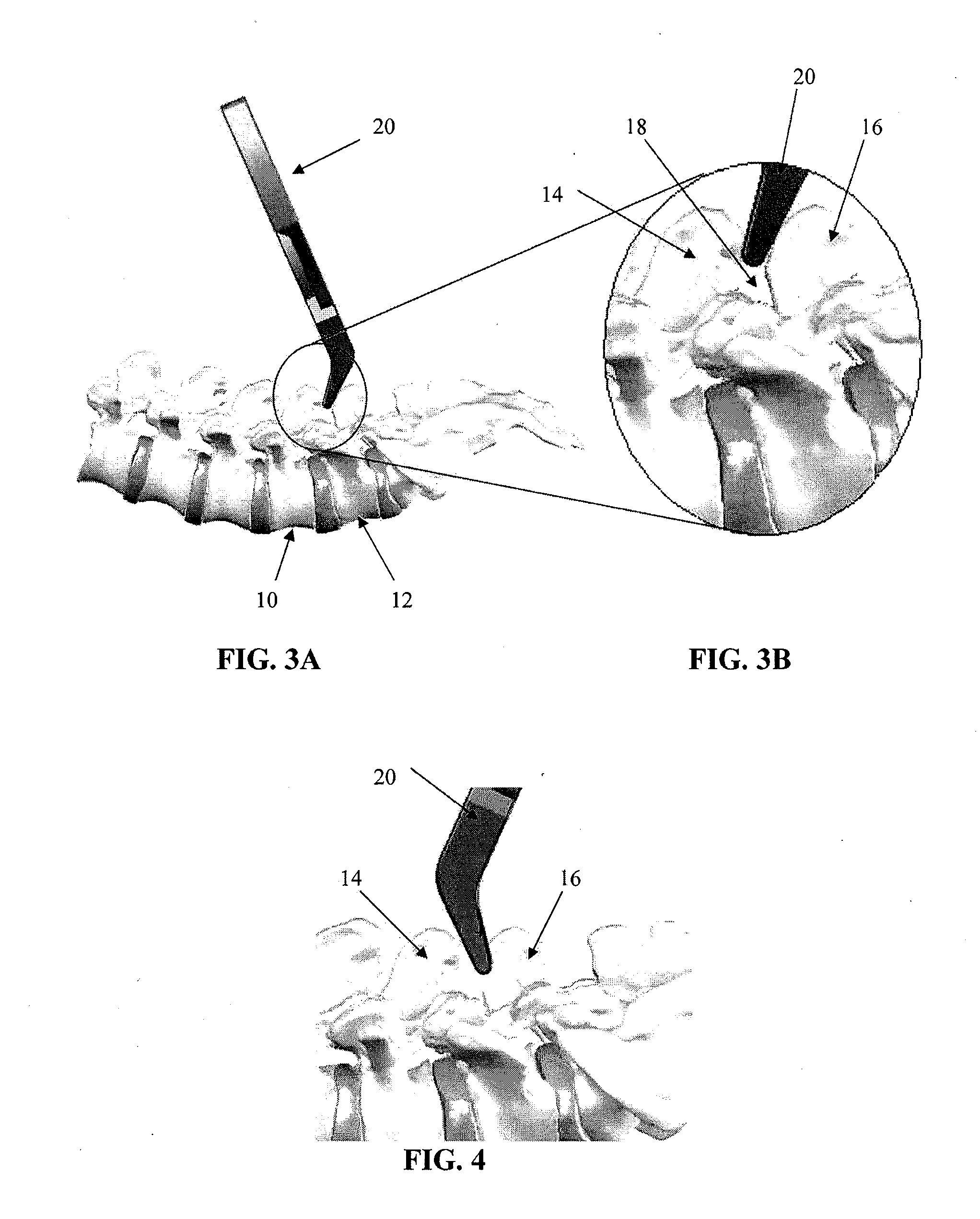
Spinous process spacer and implantation procedure
InactiveUS20120215262A1Improve joint qualityIncrease ratingsInternal osteosythesisJoint implantsBiomedical engineeringBone screws
A spinal fixation procedure and system are provided for fixing the spacing of an inferior vertebra relative to a superior vertebra. The procedure for implanting a spinous process spacer can comprise decorticating and / or forming a notch in adjacent spinous processes, measuring the distance between the notches formed in the spinous processes, and inserting an interspinous process implant such that the implant is fitted into the notches of the spinous processes. Other fixation devices, such as bone screws, can also be used for fixing the position of the vertebrae and to create facet fusion.
Owner:INTERVENTIONAL SPINE
Dual valve prosthesis for transcatheter valve implantation
A dual valve prosthesis having a self-expanding anchoring frame with first and second prosthetic valve assemblies attached to the anchoring frame is disclosed. Each of the first and second prosthetic valve assemblies includes a balloon-expandable stent structure with a prosthetic valve secured therein. In a disclosed method, the first and second prosthetic valve assemblies include prosthetic mitral and aortic valves, respectively, and the dual heart valve prosthesis is configured to replace both the native mitral and aortic valves of the heart in a single transcatheter heart valve implantation procedure.
Owner:MEDTRONIC INC
Spinous process spacer and implantation procedure
InactiveUS20110040332A1Improve joint qualityIncrease ratingsInternal osteosythesisJoint implantsIliac screwBiomedical engineering
A spinal fixation procedure and system are provided for fixing the spacing of an inferior vertebra relative to a superior vertebra. The procedure for implanting a spinous process spacer can comprise decorticating and / or forming a notch in adjacent spinous processes, measuring the distance between the notches formed in the spinous processes, and inserting an interspinous process implant such that the implant is fitted into the notches of the spinous processes. Other fixation devices, such as bone screws, can also be used for fixing the position of the vertebrae and to create facet fusion.
Owner:INTERVENTIONAL SPINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com