Patents
Literature
1437 results about "Blood pump" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The blood pump was patented in 1855 by Porter and Bradley and was hand operated. A modification first named surgical pump, designed and manufactured by E. E. Allen in 1887, was intended for direct blood transfusion. Truax, who also distributed and promoted the Allen pump with one roller, developed the first double roller pump in 1899. In the following decades, several researchers, including Beck, Van Allen, Bayliss and Müller as well as Henry and Jouvelet, refined the apparatus and recommended the use of roller pumps for blood transfusion and other applications. After further modifications made by DeBakey in 1934, and application of this pump in one of the first heart-lung machines constructed by Gibbon, DeBakey's name became inseparably attached to this type of pump. For perfusion experiments, an electrically powered roller pump was first used by Fleisch in 1935. Today, the roller pump is the most frequently used blood pump for cardiopulmonary bypass worldwide, having prevailed against the early pulsatile tube compression pumps and ventricular pumps. In recent years, centrifugal pumps have increasingly competed with roller pumps as systemic blood pumps for cardiopulmonary bypass and have become the preferred arterial pump in a variety of centers. Application of mechanical cardiac assistance has evolved from nonpulsatile roller pump support, followed by an era of pulsatile ventricular pumps to the rediscovery of the nonpulsatile flow mode with modern axial flow pumps.
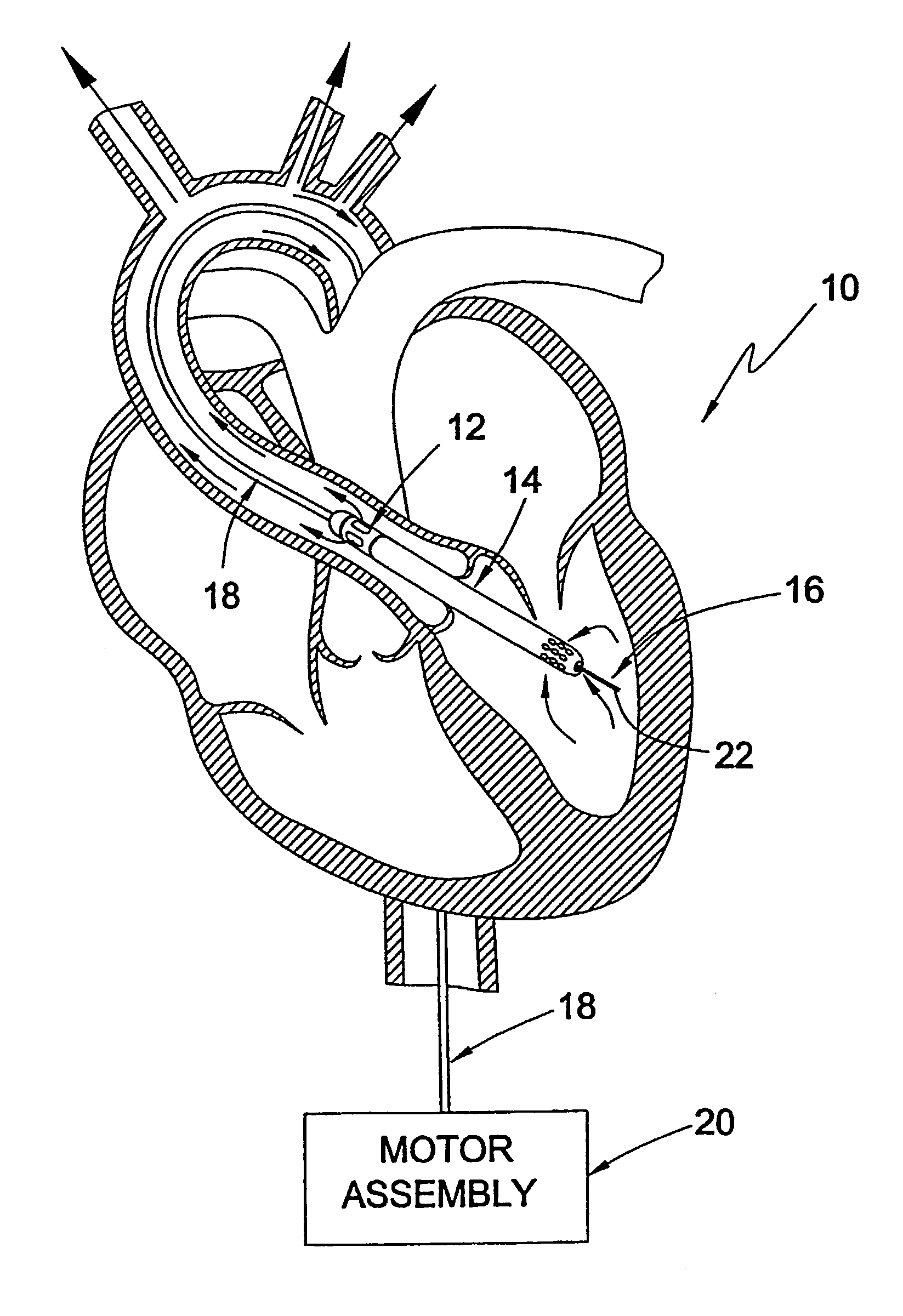
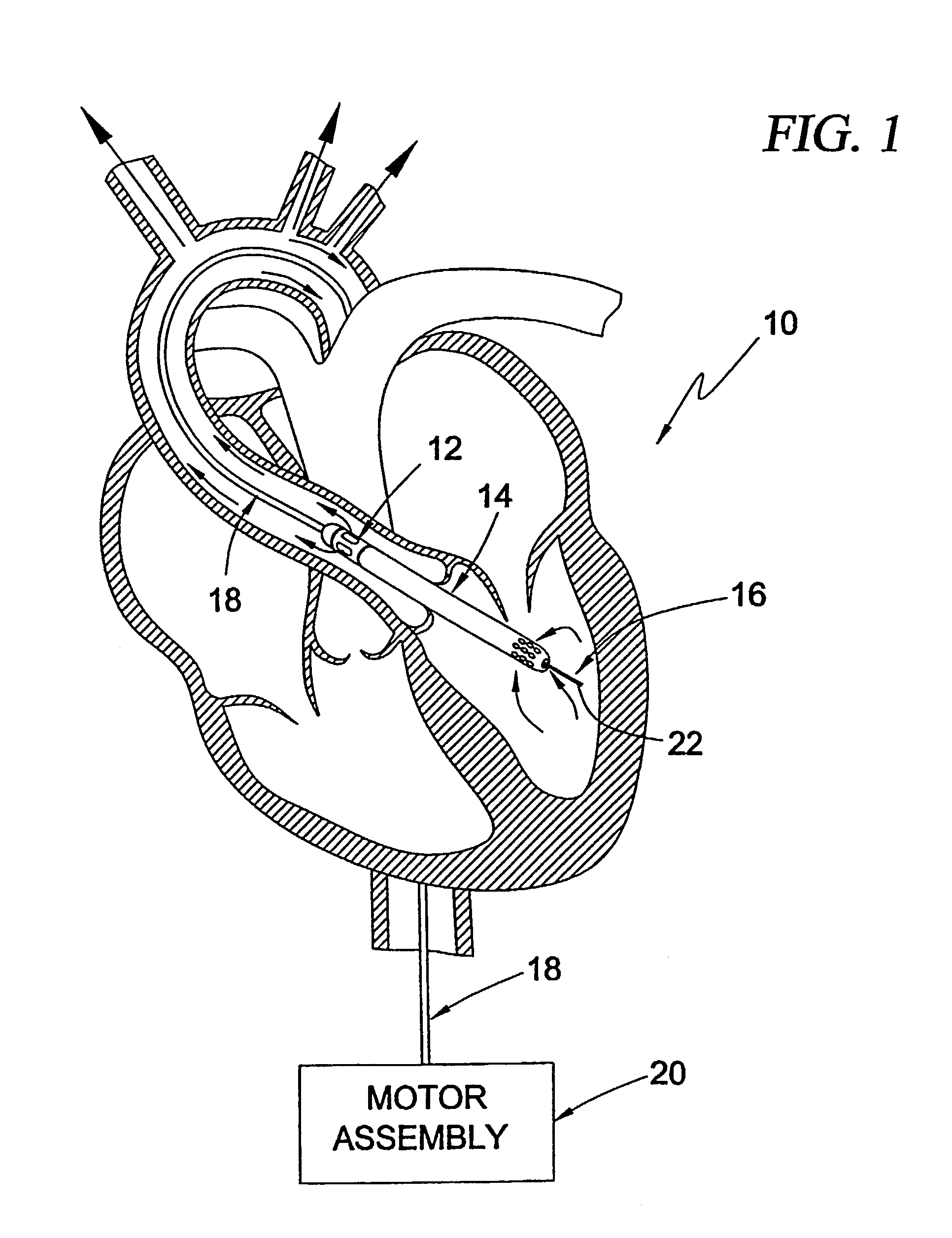
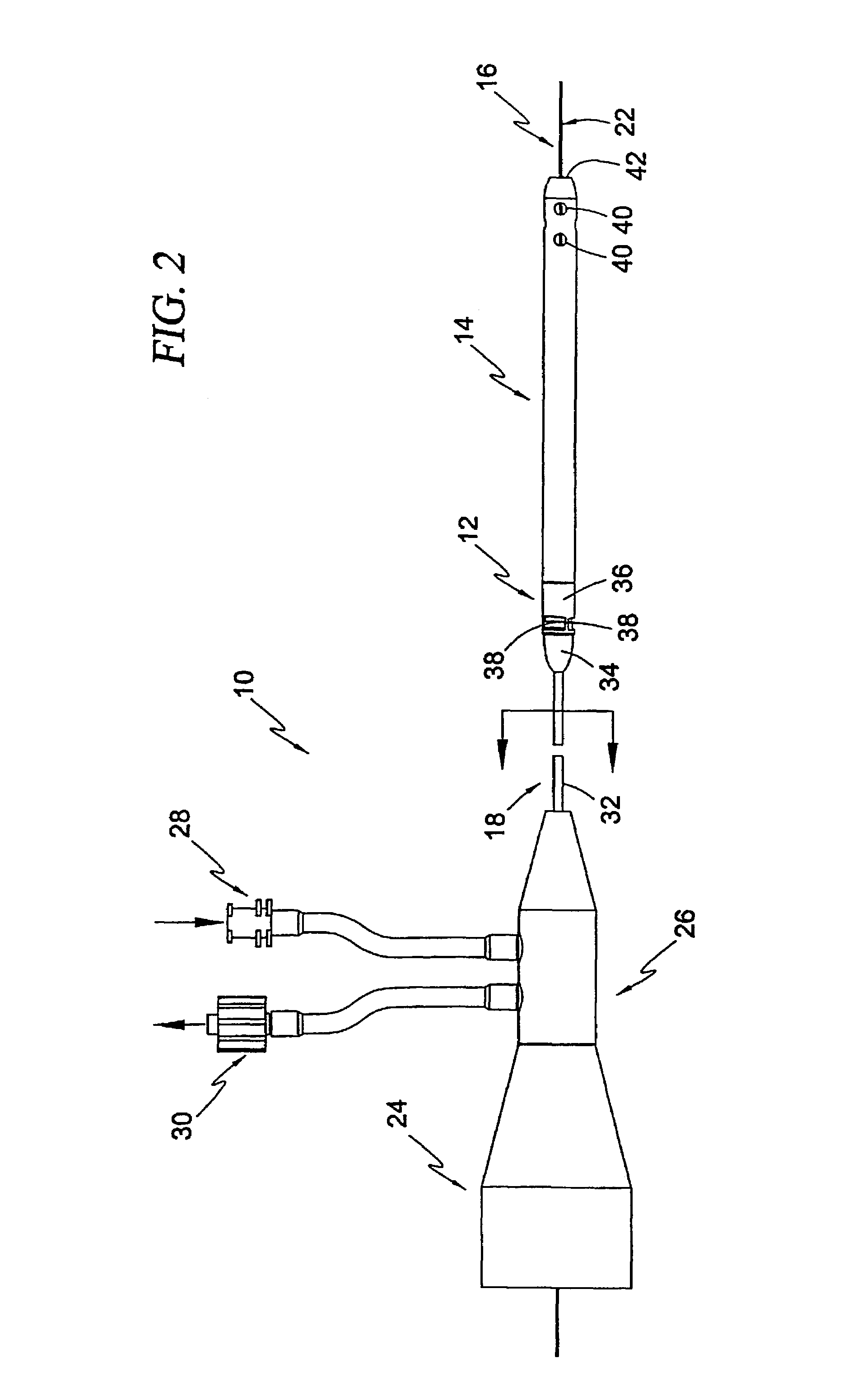
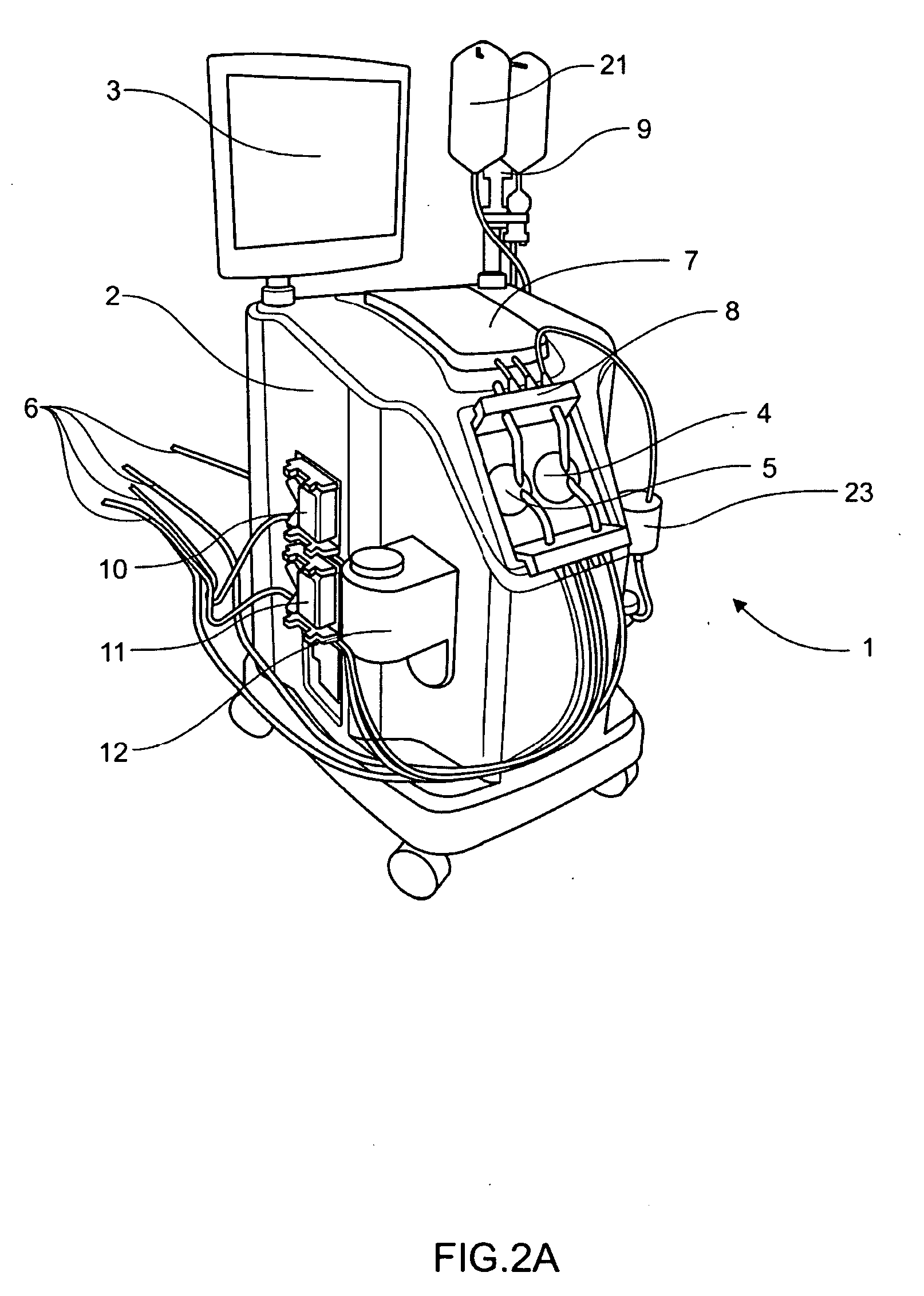
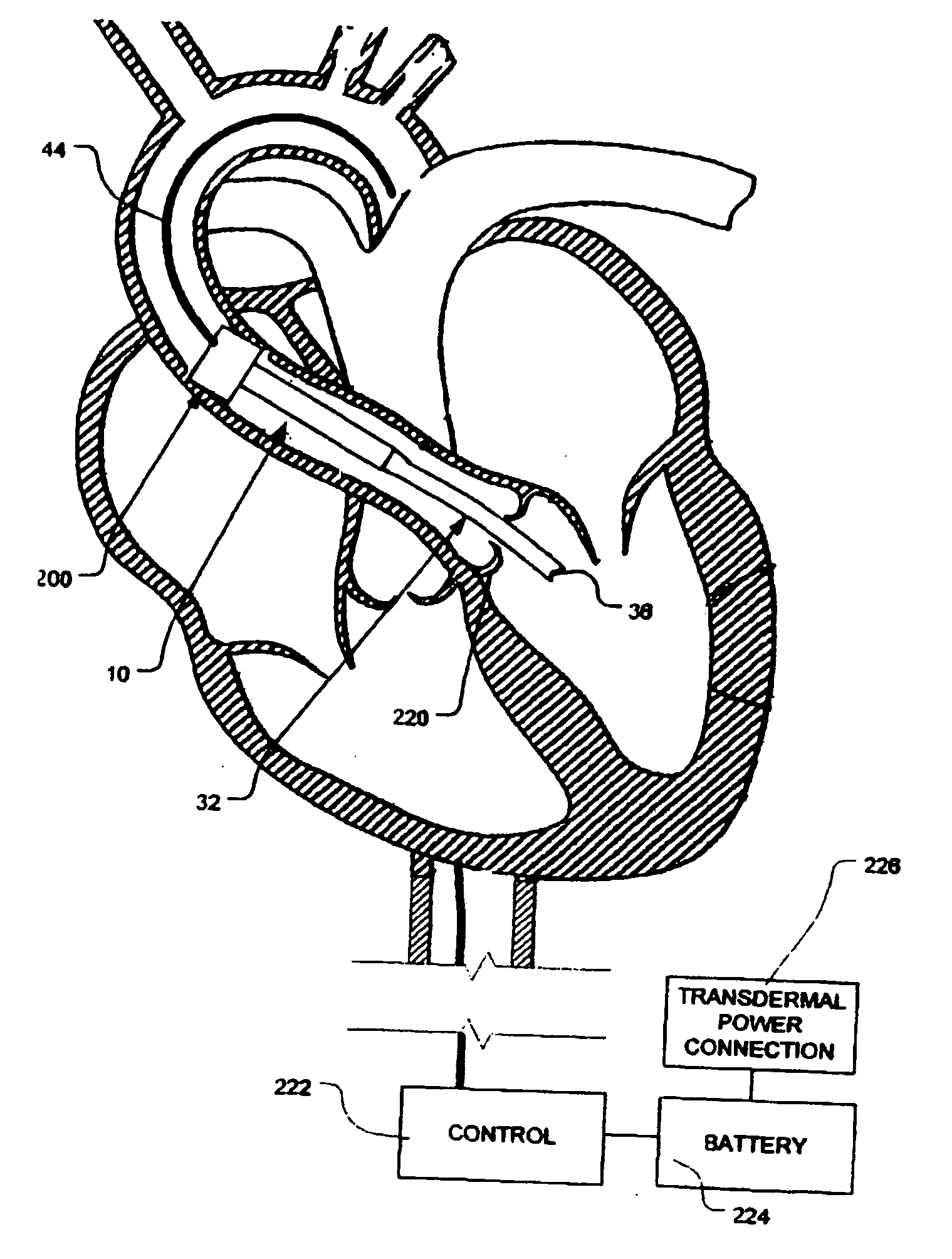
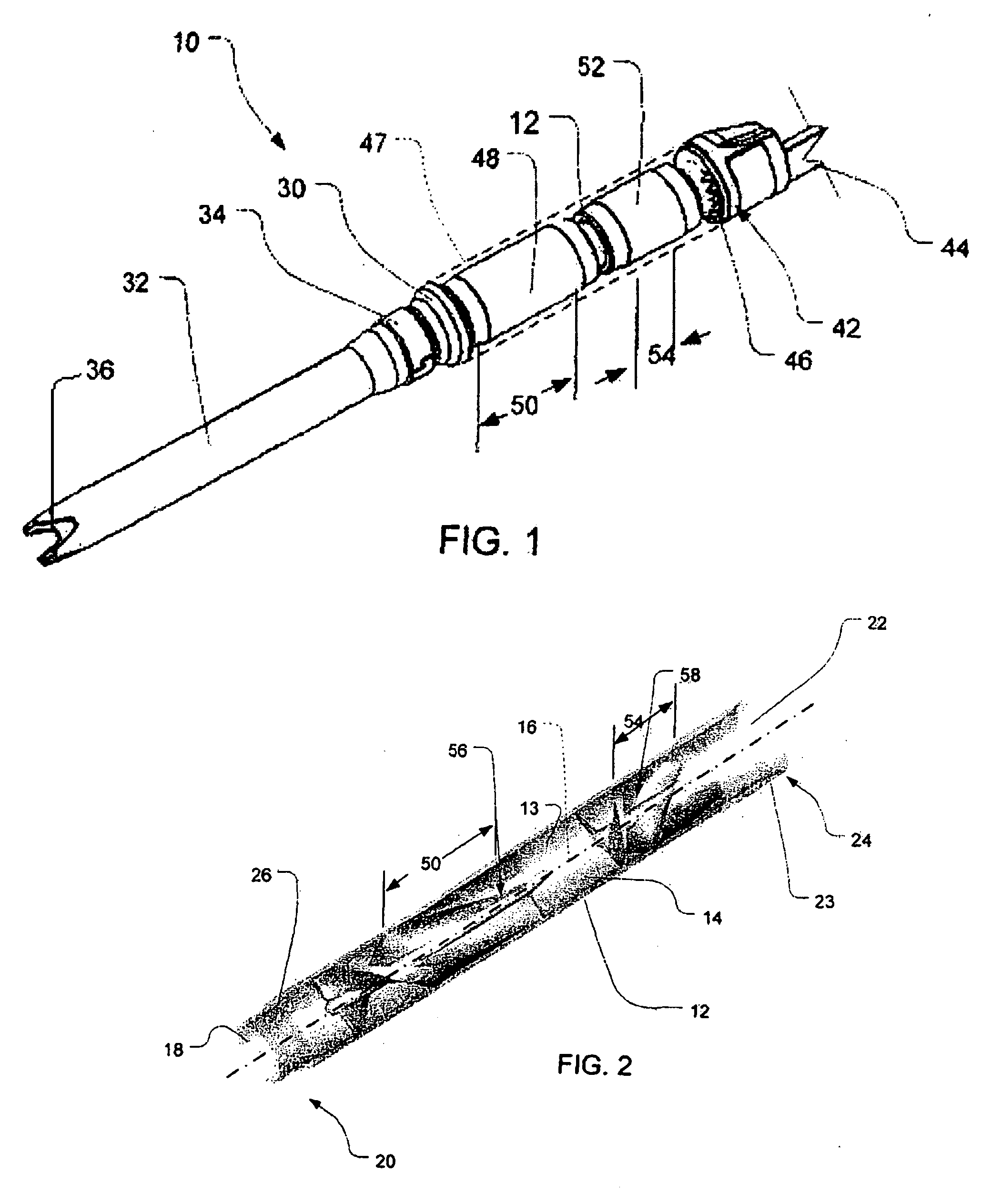
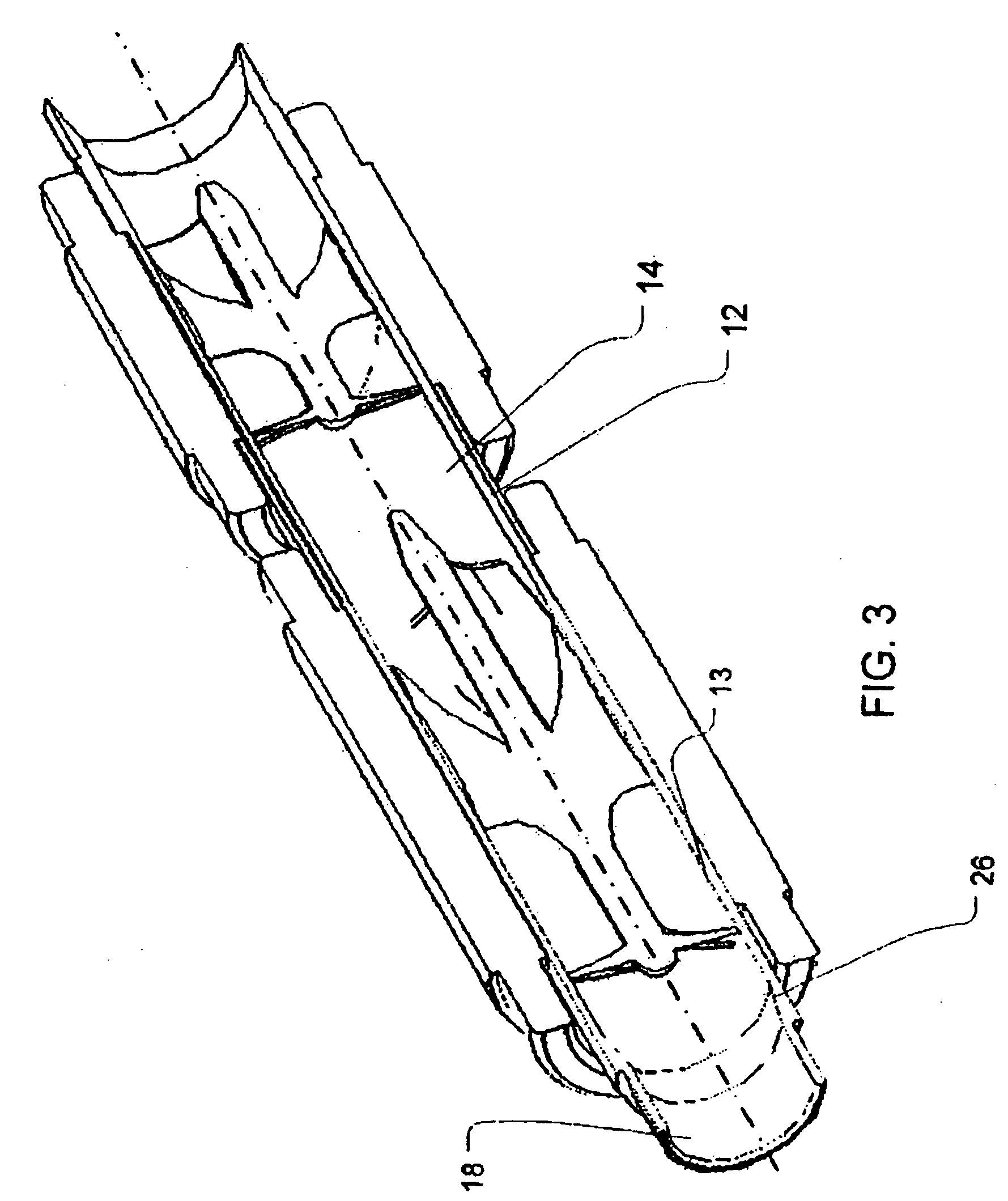
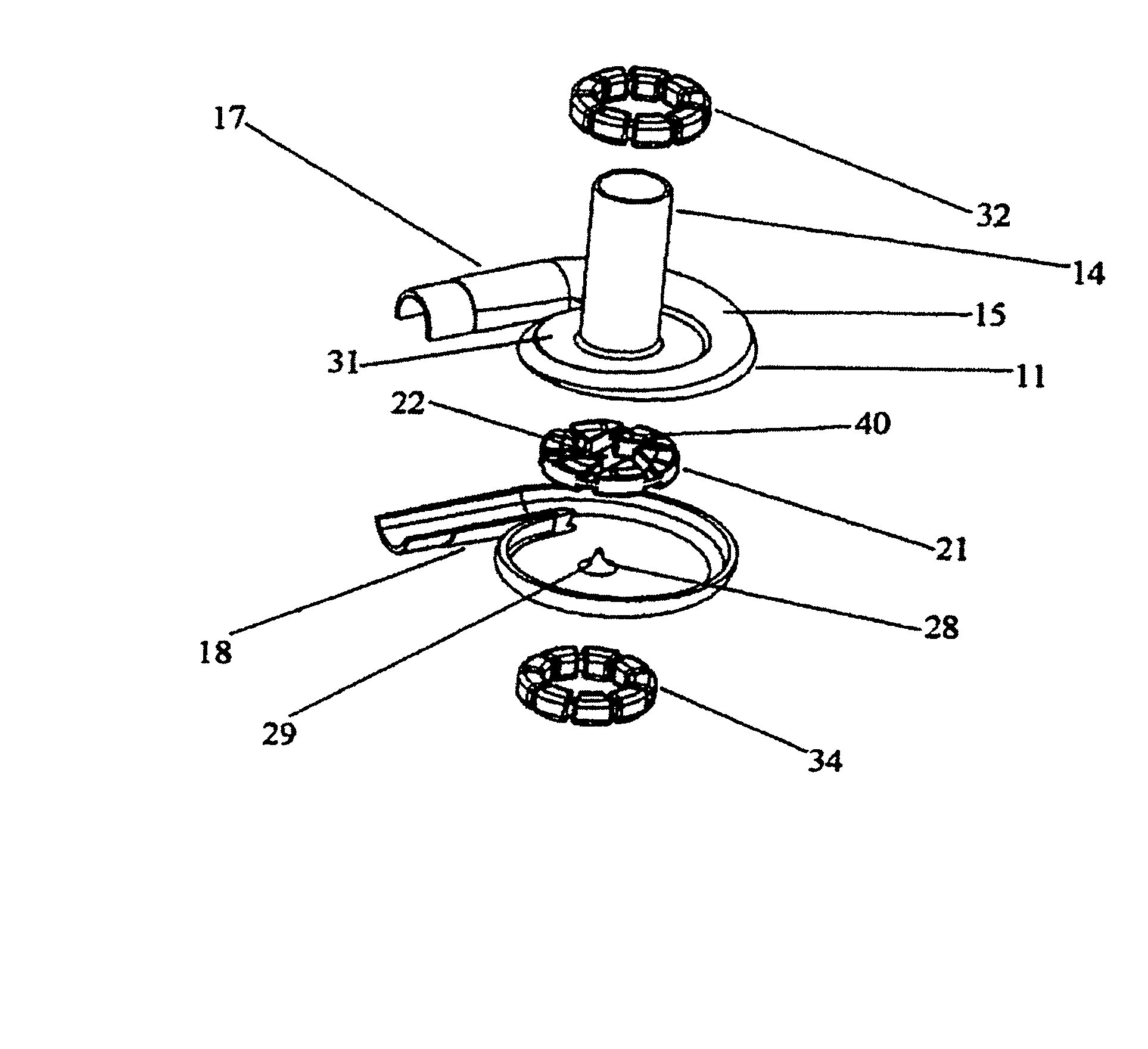
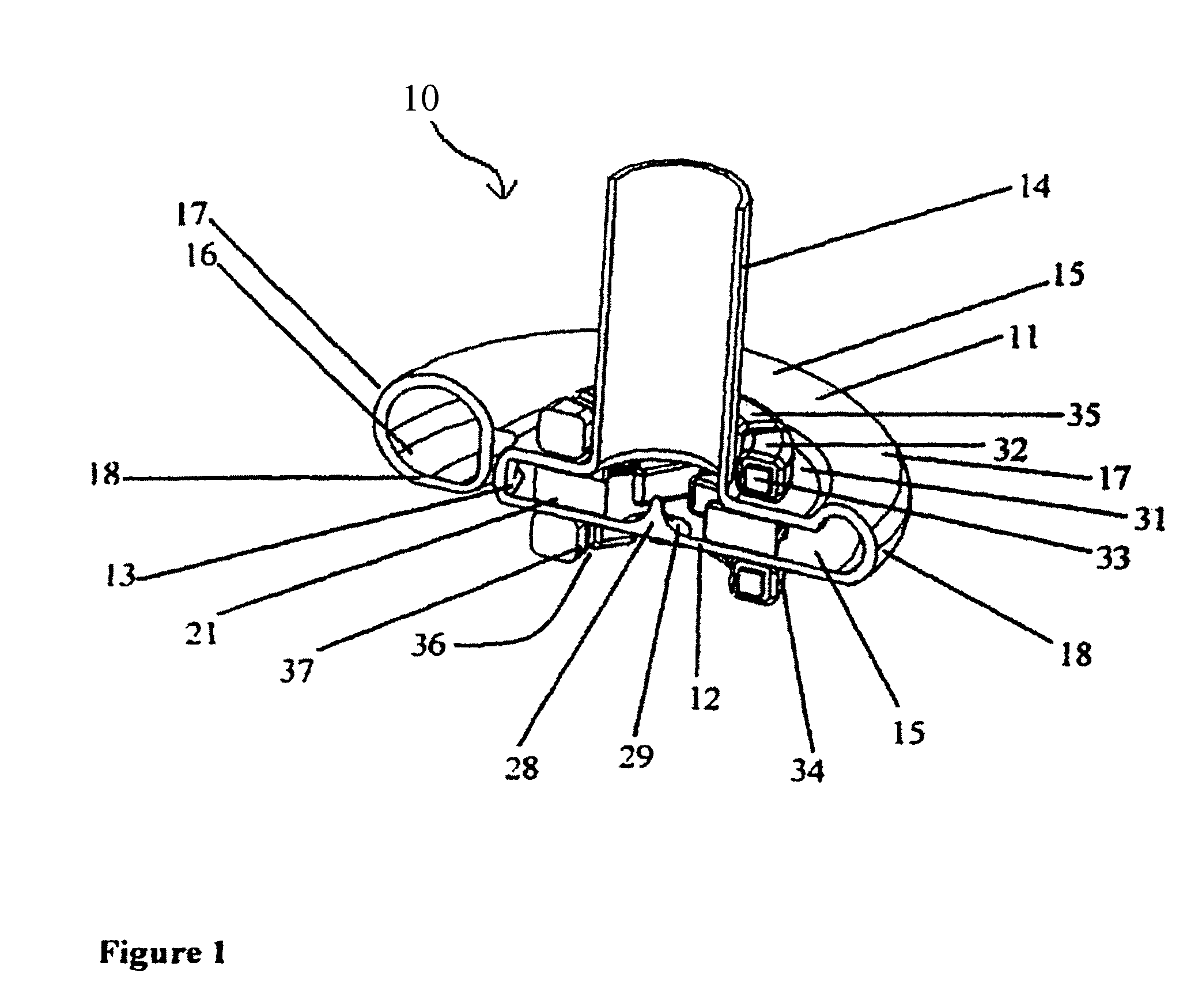
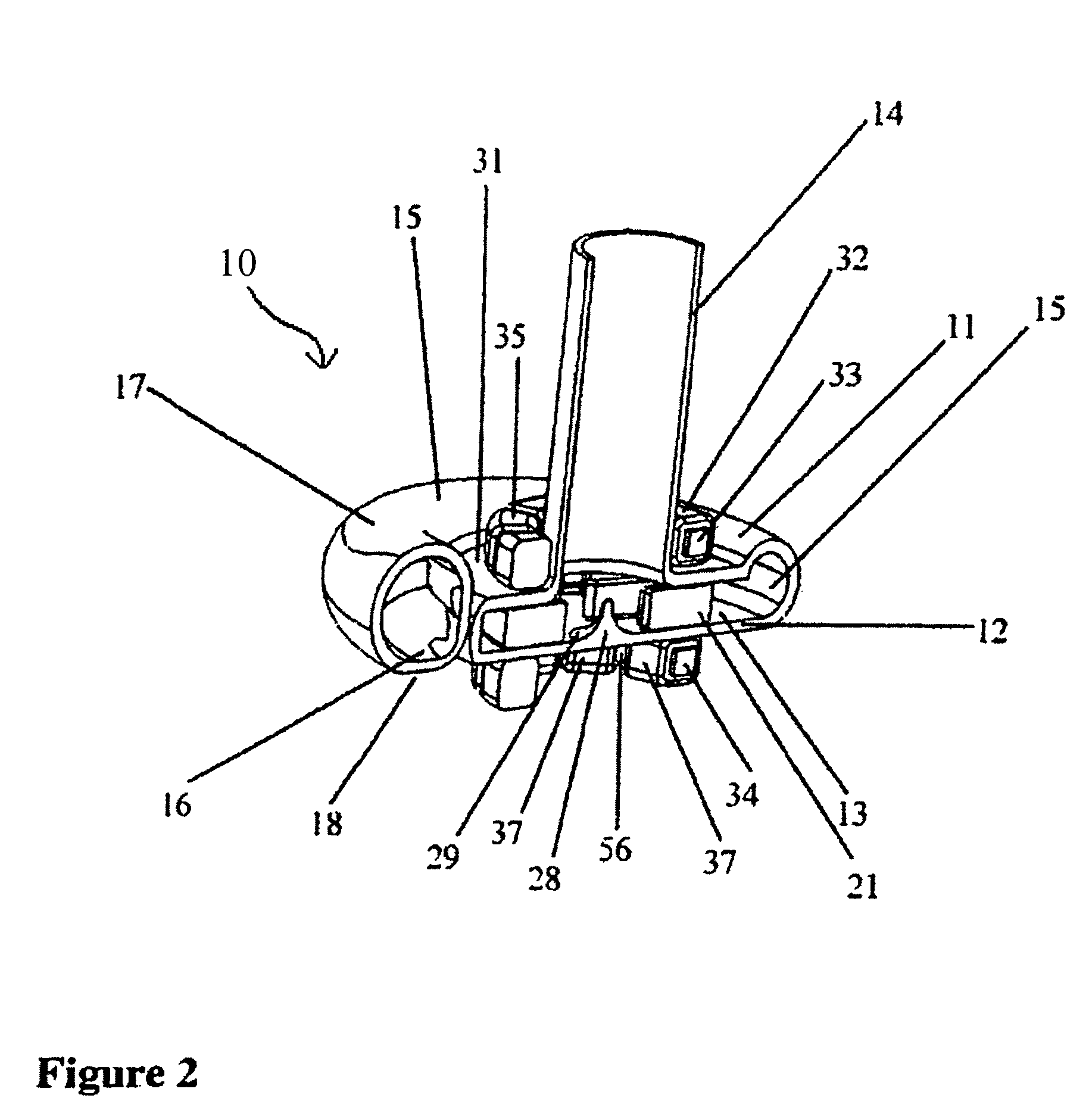
Guidable intravascular blood pump and related methods
InactiveUS7022100B1Integration of featureEliminate needGuide wiresControl devicesBlood pumpBlood vessel
An improved intravascular blood pump system (10) and related methods involving the broad inventive concept of equipping the intravascular blood pump (12) with guiding features such that the intravascular blood pump can be selectively positioned at a predetermined location within the circulatory system of a patient.
Owner:MAQUET CARDIOVASCULAR LLC
Prosthetic Valve for Transluminal Delivery
InactiveUS20100004740A1Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesVenous accessImplantation Site
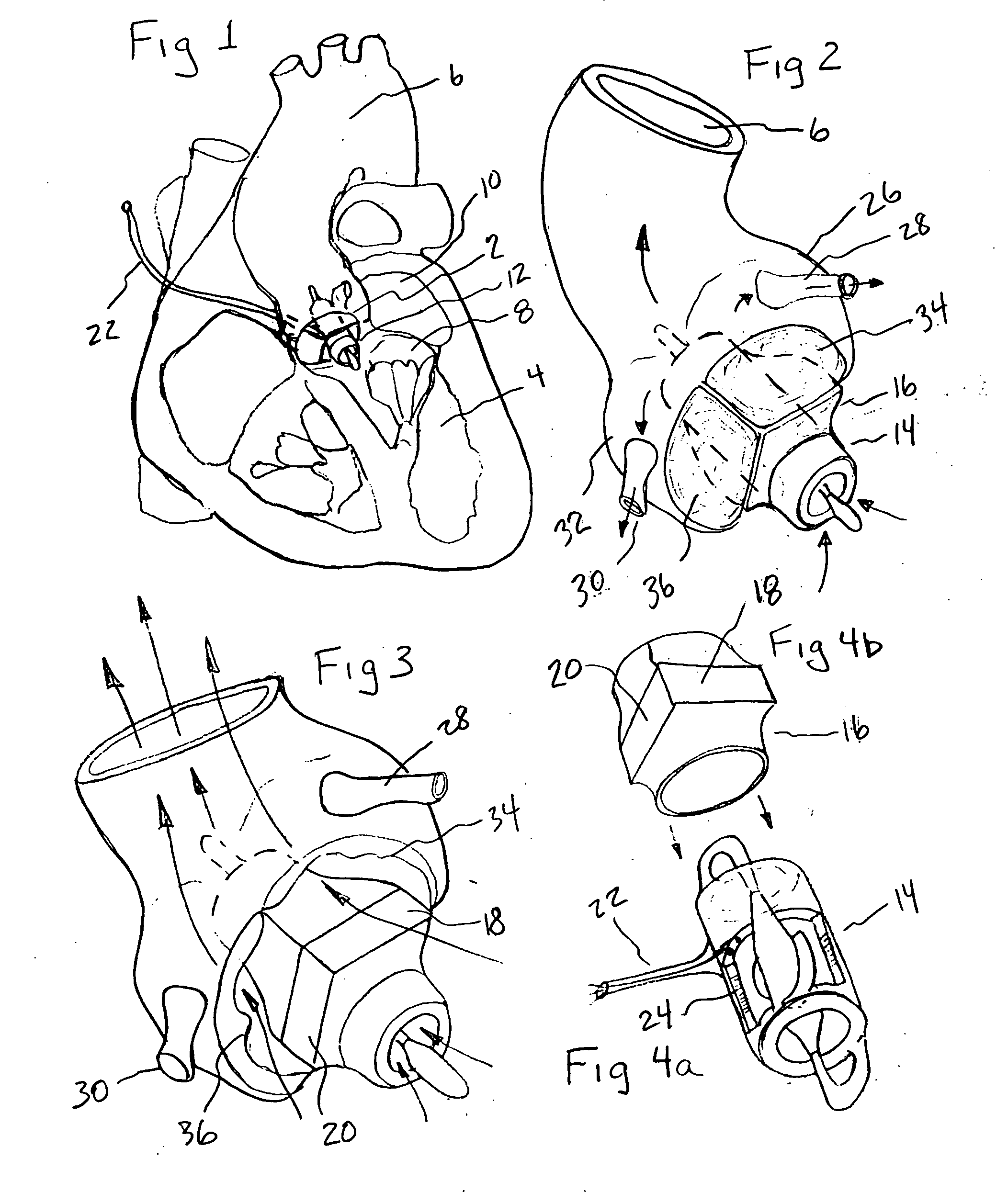
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable valve support. If desired, one or more anchors may be used. The valve support, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. Portions of the valve support may expand to a preset diameter to maintain coaptivity of the replacement valve and to prevent occlusion of the coronary ostia. A radial restraint, comprising a wire, thread or cuff, may be used to ensure expansion does not exceed the preset diameter. The valve support may optionally comprise a drug elution component. The anchor engages the lumen wall when expanded and prevents substantial migration of the valve assembly when positioned in place. The prosthetic valve assembly is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart. A blood pump may be inserted into the catheter to ensure continued blood flow across the implantation site during implantation procedure. When the outer sheath is retracted, the prosthetic valve assembly expands to an expanded position such that the valve and valve support expand at the implantation site and the anchor engages the lumen wall. Insertion of the catheter may optionally be performed over a transseptally delivered guidewire that has been externalized through the arterial vasculature. Such a guidewire provide dual venous and arterial access to the implantation site and allows additional manipulation of the implantation site after arterial implantation of the prosthetic valve. Additional expansion stents may be delivered by venous access to the valve.
Owner:MEDTRONIC COREVALVE
Heart assist device with expandable impeller pump
An impeller includes a hub and at least one blade supported by the hub. The impeller has a stored configuration in which the blade is compressed so that its distal end moves towards the hub, and a deployed configuration in which the blade extends away from the hub. The impeller may be part of a pump for pumping fluids, such as pumping blood within a patient. A blood pump may include a cannula having a proximal portion with a fixed diameter, and a distal portion with an expandable diameter. The impeller may reside in the expandable portion of the cannula. The cannula may have a compressed diameter which allows it to be inserted percutaneously into a patient. Once at a desired location, the expandable portion of the cannula may be expanded and the impeller expanded to the deployed configuration. A flexible drive shaft may extend through the cannula for rotationally driving the impeller within the patient's body.
Owner:PENN STATE RES FOUND +2
Wide blade, axial flow pump
ActiveUS7699586B2Increase the magnetic fluxReduce air gapControl devicesBlood pumpsAxial-flow pumpImpeller
A blood pump comprises a pump housing; a rotor positioned in the housing and comprising an impeller having a hydrodynamic surface for pumping blood; and a motor including a plurality of magnets carried by the impeller, plus a rotor stator, including an electrically conductive coil located adjacent to or within the housing. The impeller comprises radially outwardly extending, bladelike projections that define generally longitudinally extending spaces between the projections. The shape of the projections and the spaces therebetween tend to drive blood in the spaces in an axial direction as the impeller is rotated. The spaces collectively have a total width along most of their lengths at the radial periphery of the rotor, that is substantially equal to or less than the collective width of the projections along most of their lengths at the radial periphery. Thus, the bladelike projections are thicker, achieving significant advantages.
Owner:HEARTWARE INC
Blood pump with expandable cannula
ActiveUS20110004046A1Minimizing potential poolingPromote crashBlood pumpsIntravenous devicesImpellerDistal portion
A blood pump includes an impeller having a plurality of foldable blades and a cannula having a proximal portion with a fixed diameter, and a distal portion with an expandable diameter. The impeller can reside in the expandable portion of the cannula. The cannula has a collapsed condition for percutaneous delivery to a desired location within the body, and an expanded condition in which the impeller can rotate to pump blood. A flexible drive shaft can extend through the cannula for rotationally driving the impeller within the patient's body.
Owner:TC1 LLC +1
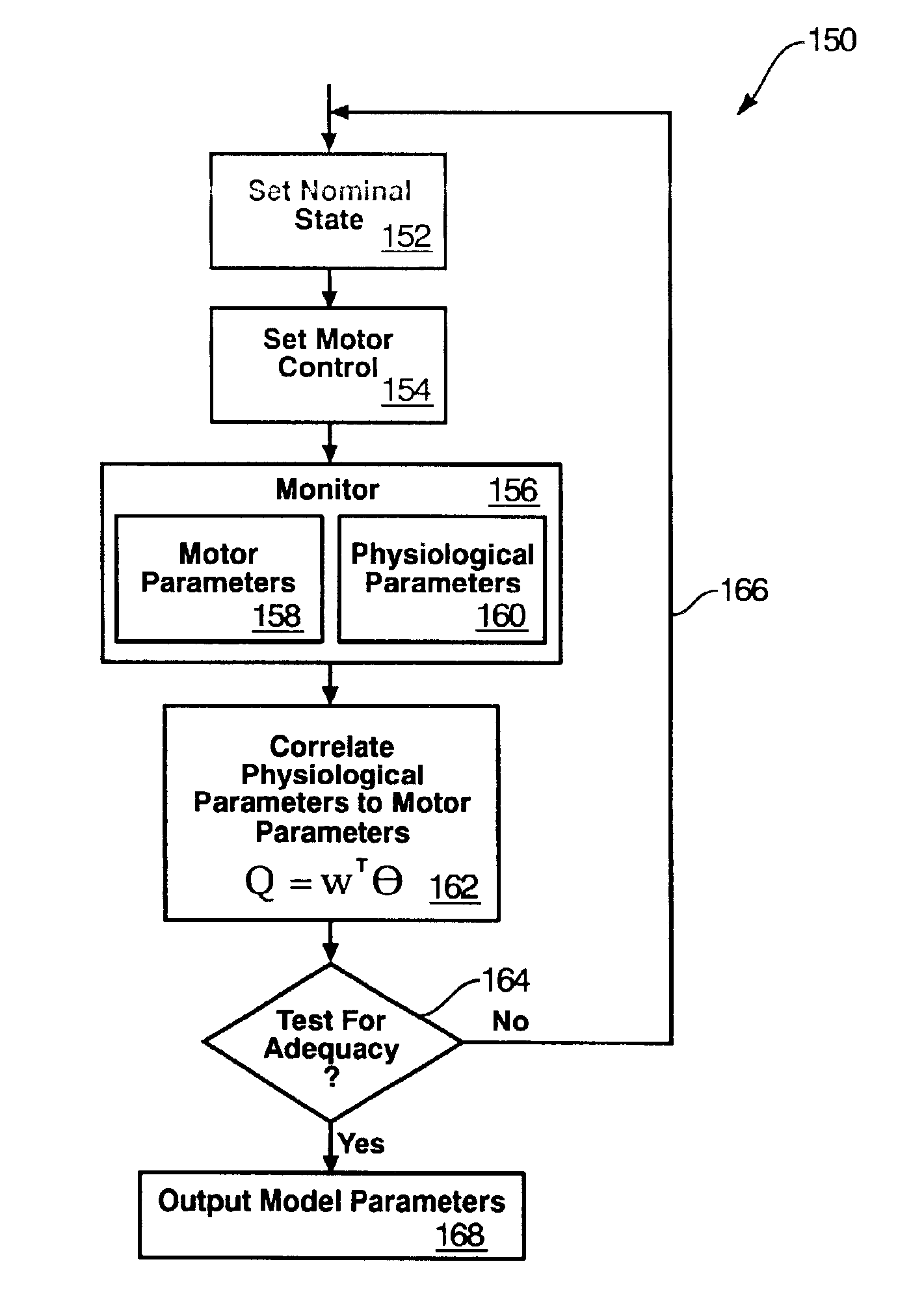
Rotary blood pump diagnostics and cardiac output controller
A method and apparatus for controlling a ventricular assist device are disclosed. The method includes the step of providing a ventricular assist device which can be defined in terms of operational parameters such as pump speed or current. Measuring at least one physiological parameter reflecting a physiological state corresponding to a patient. Correlating at least one physiological parameter measured from the patient to at least one operational parameter using an estimation method. Selecting a physiological state definable by desired values of the physiological parameters. Monitoring at least one operational parameter. Controlling input values of the operational parameter based on output from the monitoring step. The apparatus includes a pump driven by a motive drive and having an impeller. A sensor detects the value of an operational parameter of the pump. A processor provides a statistical correlation between patients physiological parameter and the operational parameter of the pump and adjusts the operational parameter to affect a predetermined optimal physiological state.
Owner:WORLD HEART
Heart assist device with expandable impeller pump
An impeller includes a hub and at least one blade supported by the hub. The impeller has a stored configuration in which the blade is compressed so that its distal end moves towards the hub, and a deployed configuration in which the blade extends away from the hub. The impeller may be part of a pump for pumping fluids, such as pumping blood within a patient. A blood pump may include a cannula having a proximal portion with a fixed diameter, and a distal portion with an expandable diameter. The impeller may reside in the expandable portion of the cannula. The cannula may have a compressed diameter which allows it to be inserted percutaneously into a patient. Once at a desired location, the expandable portion of the cannula may be expanded and the impeller expanded to the deployed configuration. A flexible drive shaft may extend through the cannula for rotationally driving the impeller within the patient's body.
Owner:PENN STATE RES FOUND +2
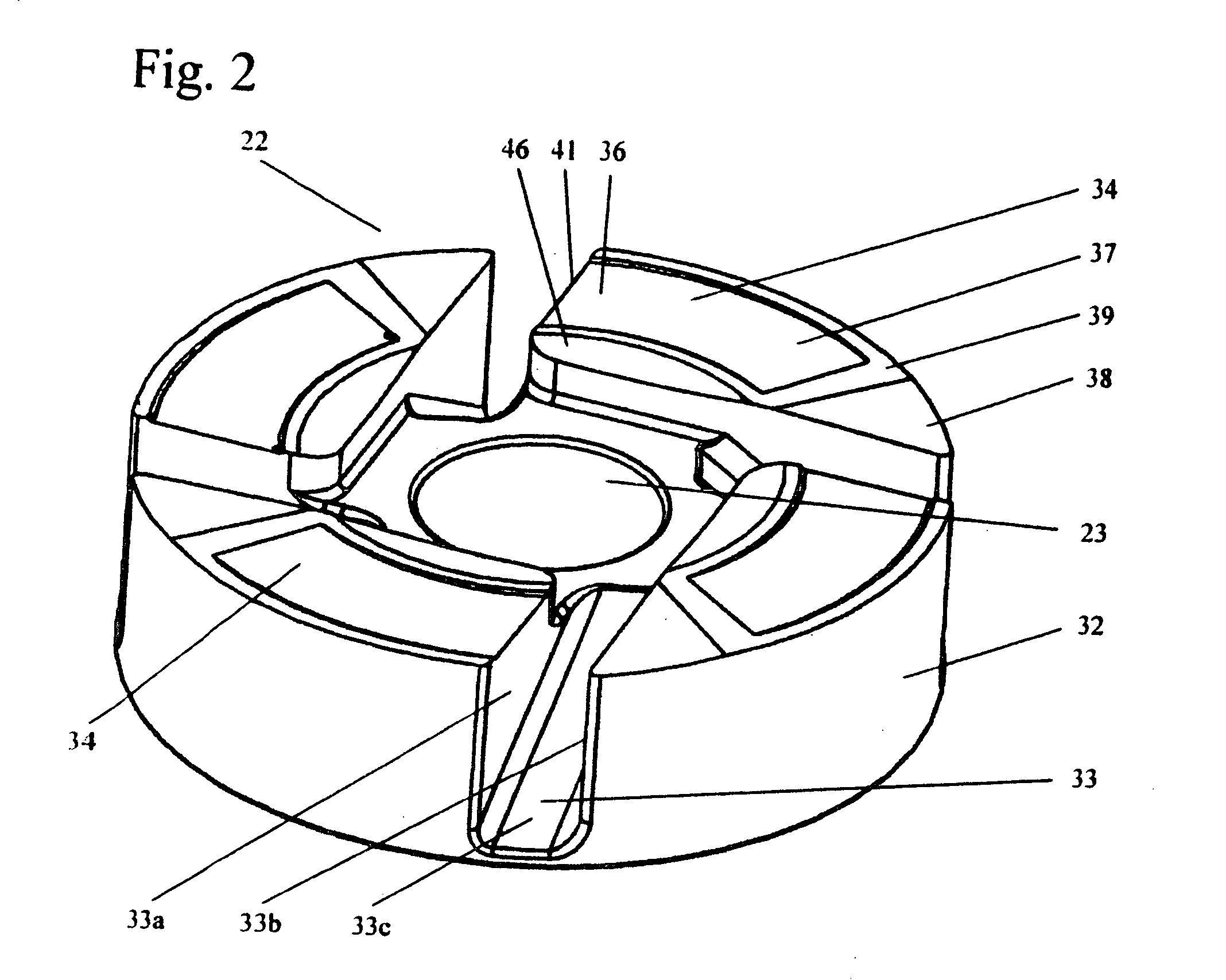
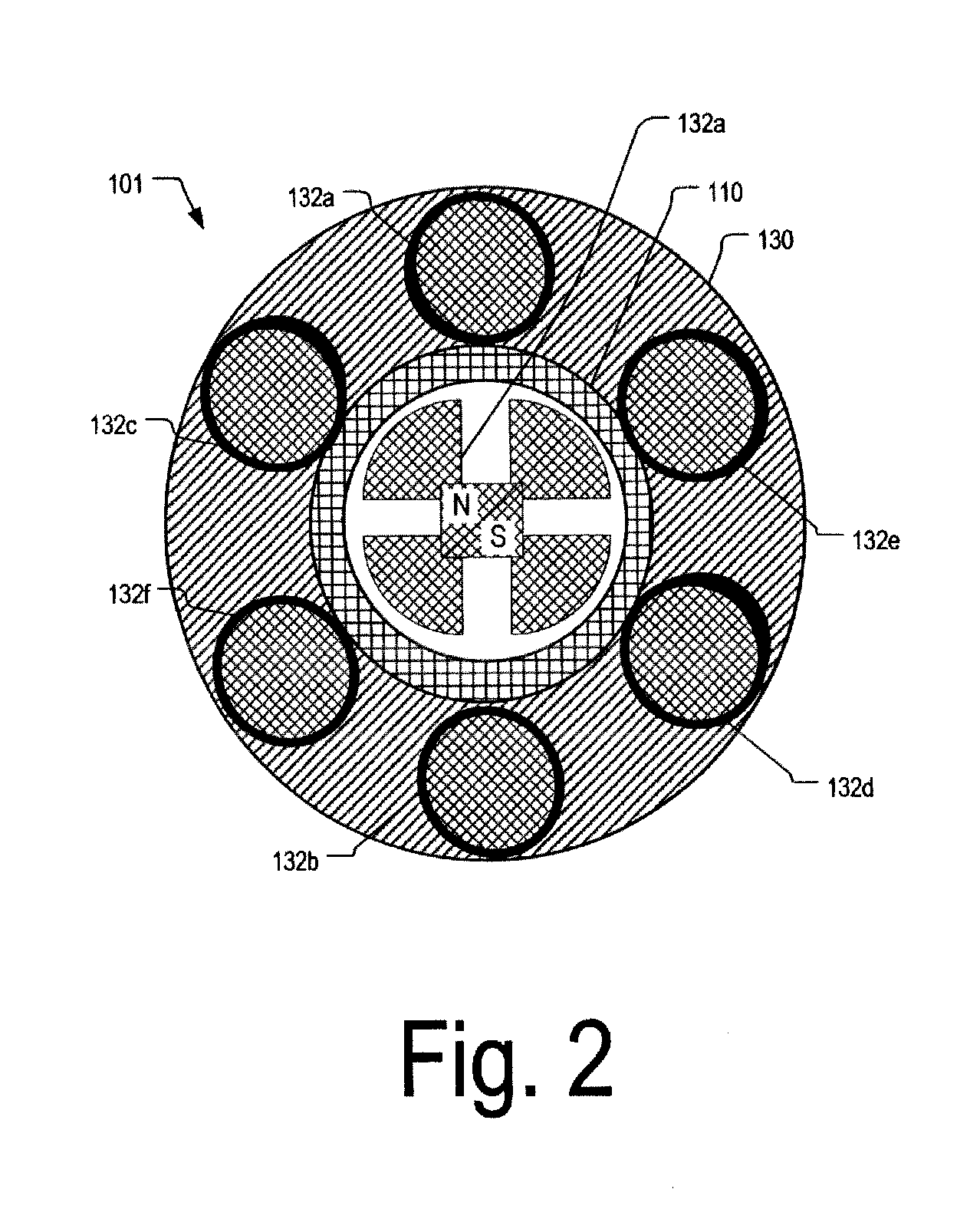
Magnetically driven axial-flow pump
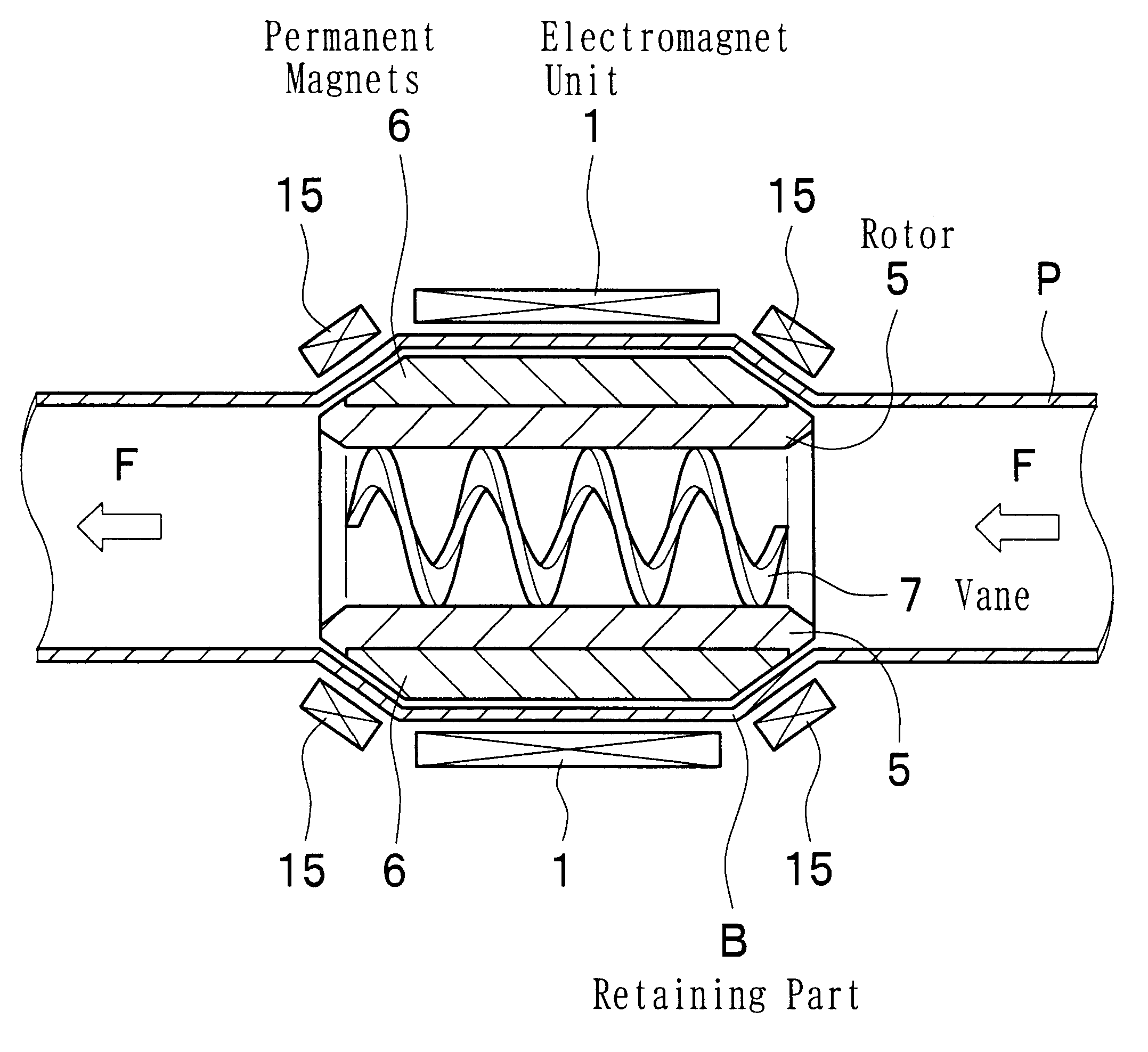
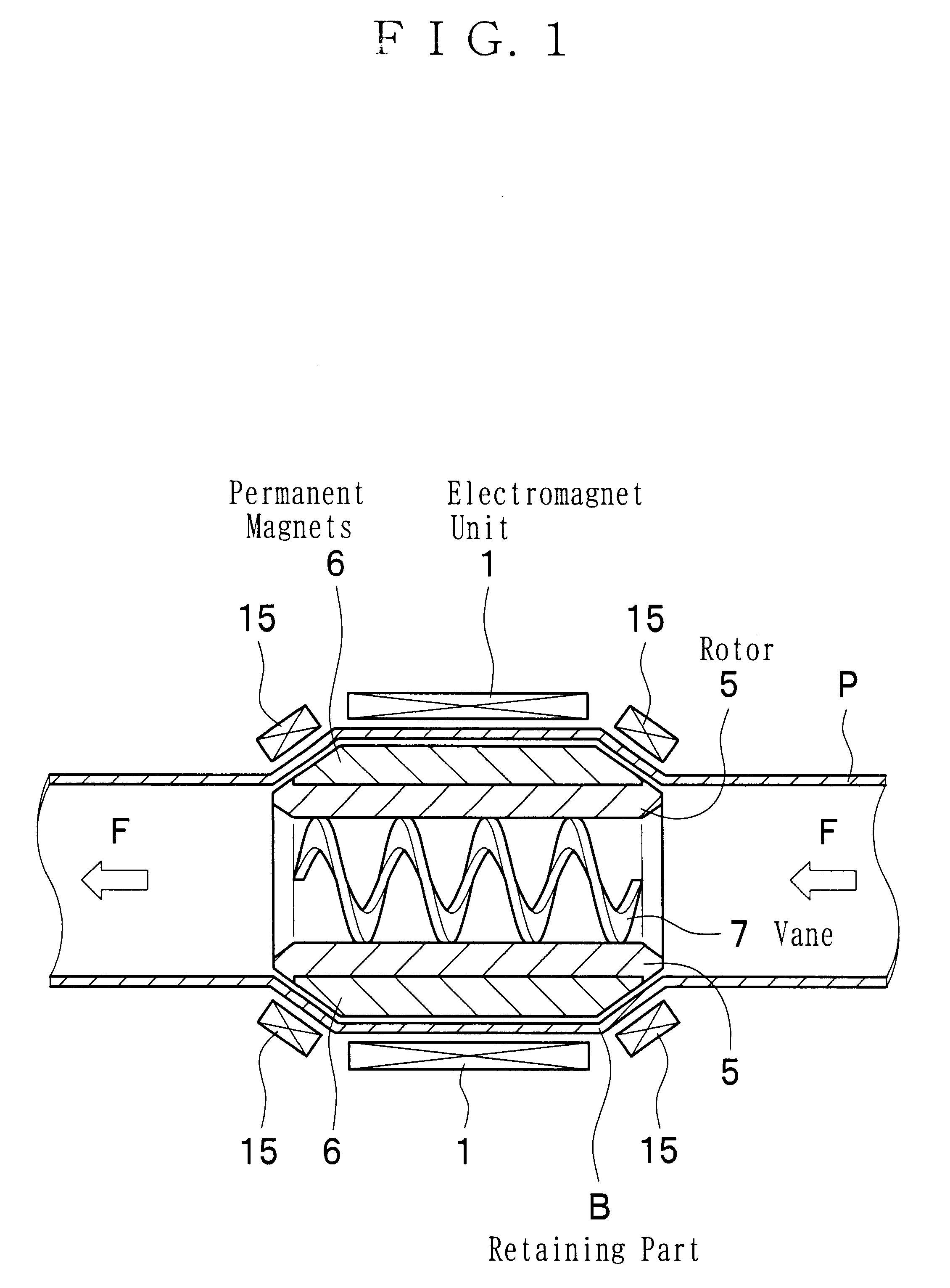
A magnetically driven, axial flow pump comprises an electromagnetic unit arranged about the periphery of a pipe. A cylindrical rotor is accommodated within the pipe and is equipped with permanent magnets mounted on the periphery. A spiral, hollow vane is formed on an inner surface of the rotor by either casting or by cutting, such as with a NC machine, thus precluding the occurrence of gaps between the rotor and the vane. The lack of gaps within the pump makes the pump suitable for use as a blood pump.
Owner:NIPRO CORP
Axial force null position magnetic bearing and rotary blood pumps which use them
InactiveUS6227820B1Improve radial stiffnessSimple designPump componentsSurgeryAxial-flow pumpMagnetic tension force
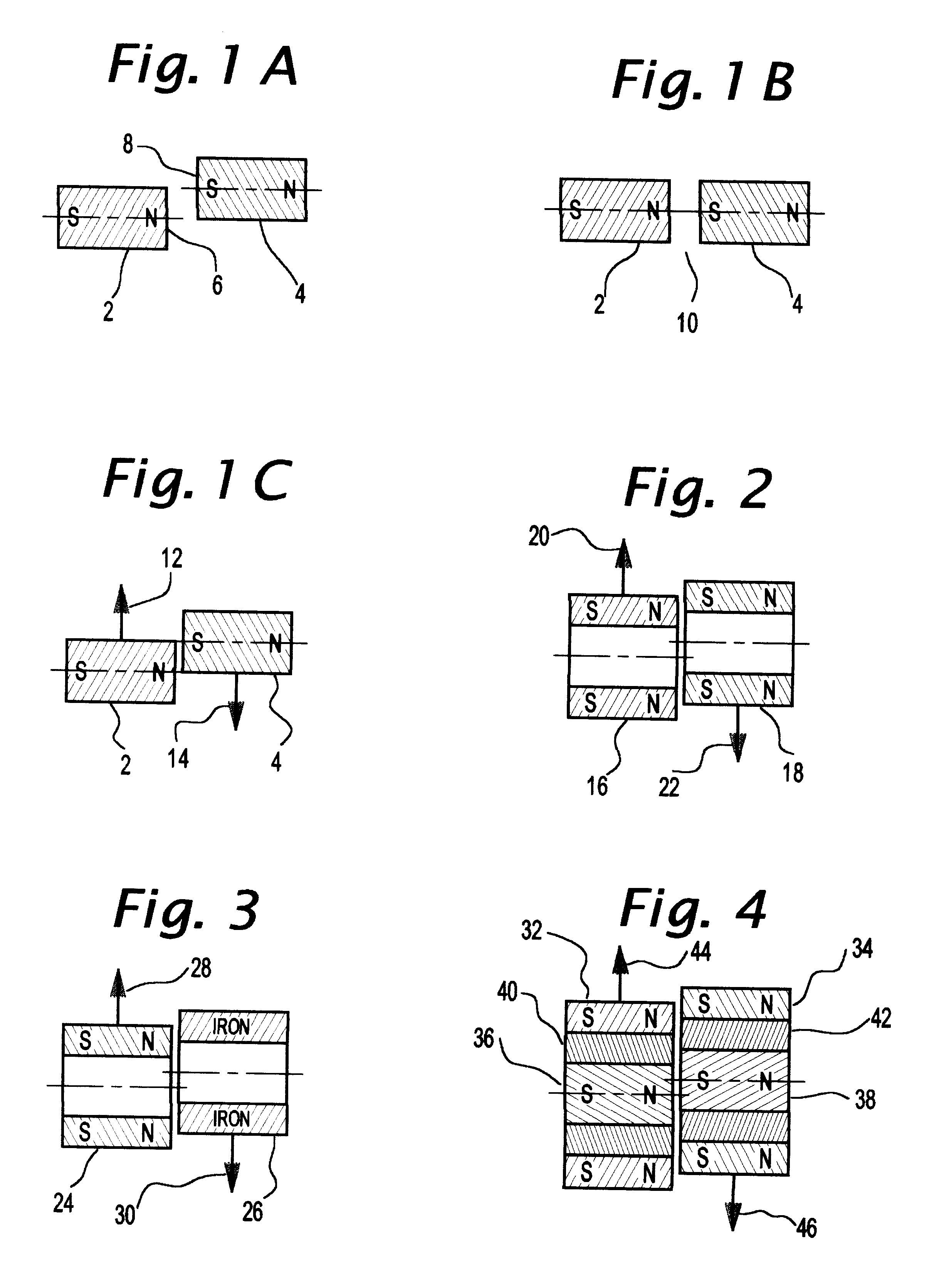
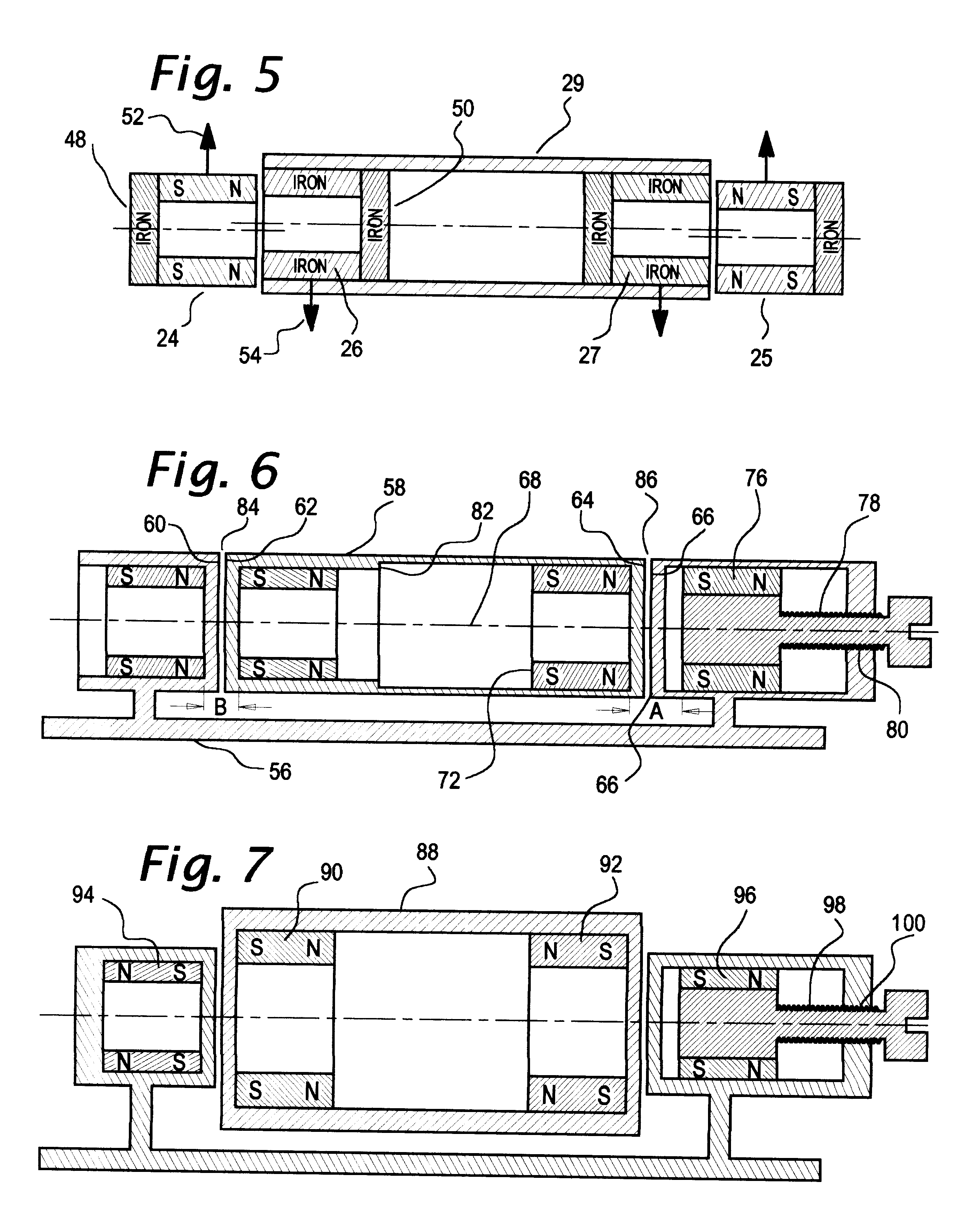
A generally cylindrical rotor very closely confined between two rigid thrust bearing surfaces is radially suspended by an array of attracting or repelling magnets or by a combination of permanent magnets and ring shaped members composed of ferromagnetic material. The geometry permits very small spacing between magnetic components to achieve high radial stiffness. High magnetic axial forces exerted between the rotor and stationary component on one end of the rotor are counter-balanced by equal and opposite forces at the other end of the rotor. Precise positioning of the rotor in the location where the opposing axial magnetic forces counterballance each other yields a net magnetic axial force on the rotor of near zero, hence the reference to this as the null position. Wear resistant mechanical thrust bearings confine the rotor axially to maintain this position during rotatioin. Precisely balance the magnetic axial forces in the proper geometry with relation to the mechanical thrust bearings. Blood pumps utilizing this type of bearing are disclosed, including both axial flow pump and centrifugal flow pump configurations with high flow washing of the junction of the rotating and stationary parts to prevent thrombus accumulation.
Owner:JARVIK ROBERT
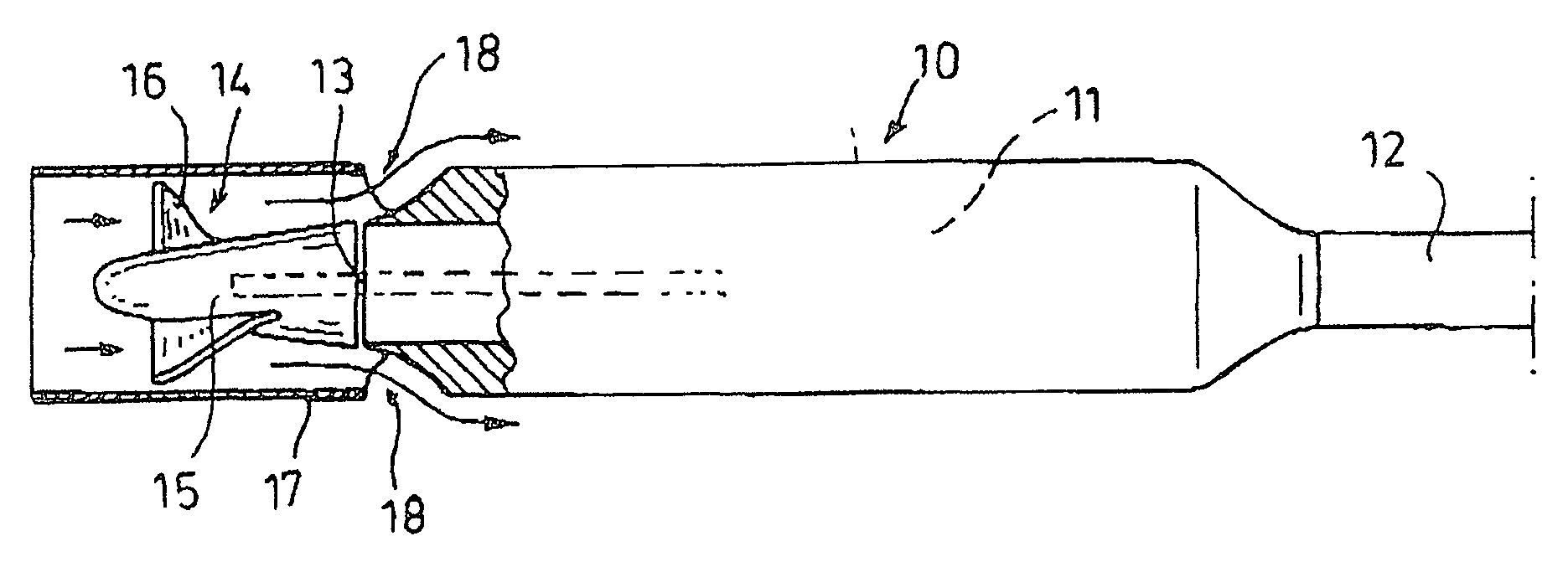
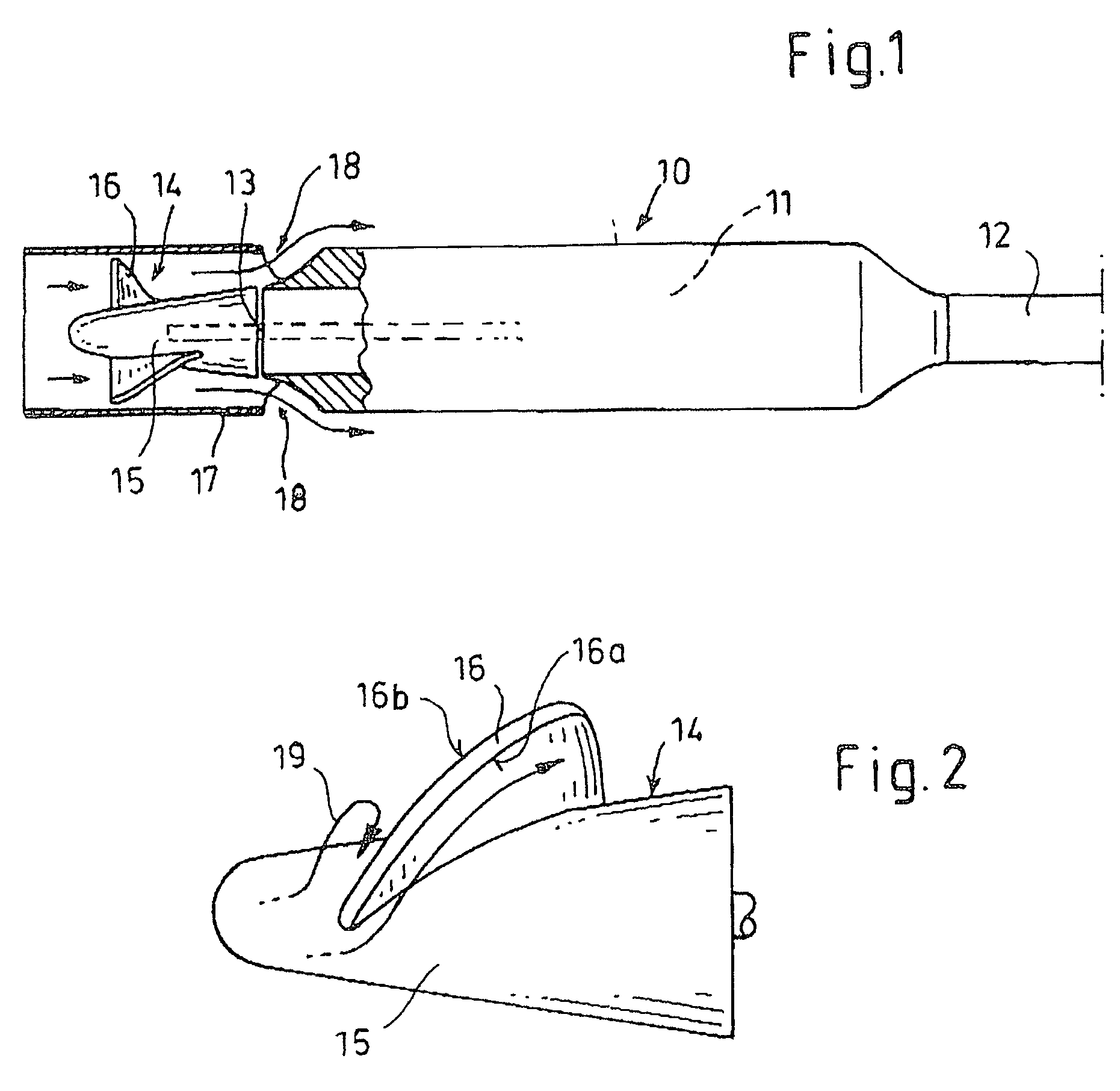
Foldable Intravascularly Inserted Blood Pump
ActiveUS20080103591A1Simple configurationReduced susceptibility to failureBlood pumpsIntravenous devicesRelative displacementImpeller
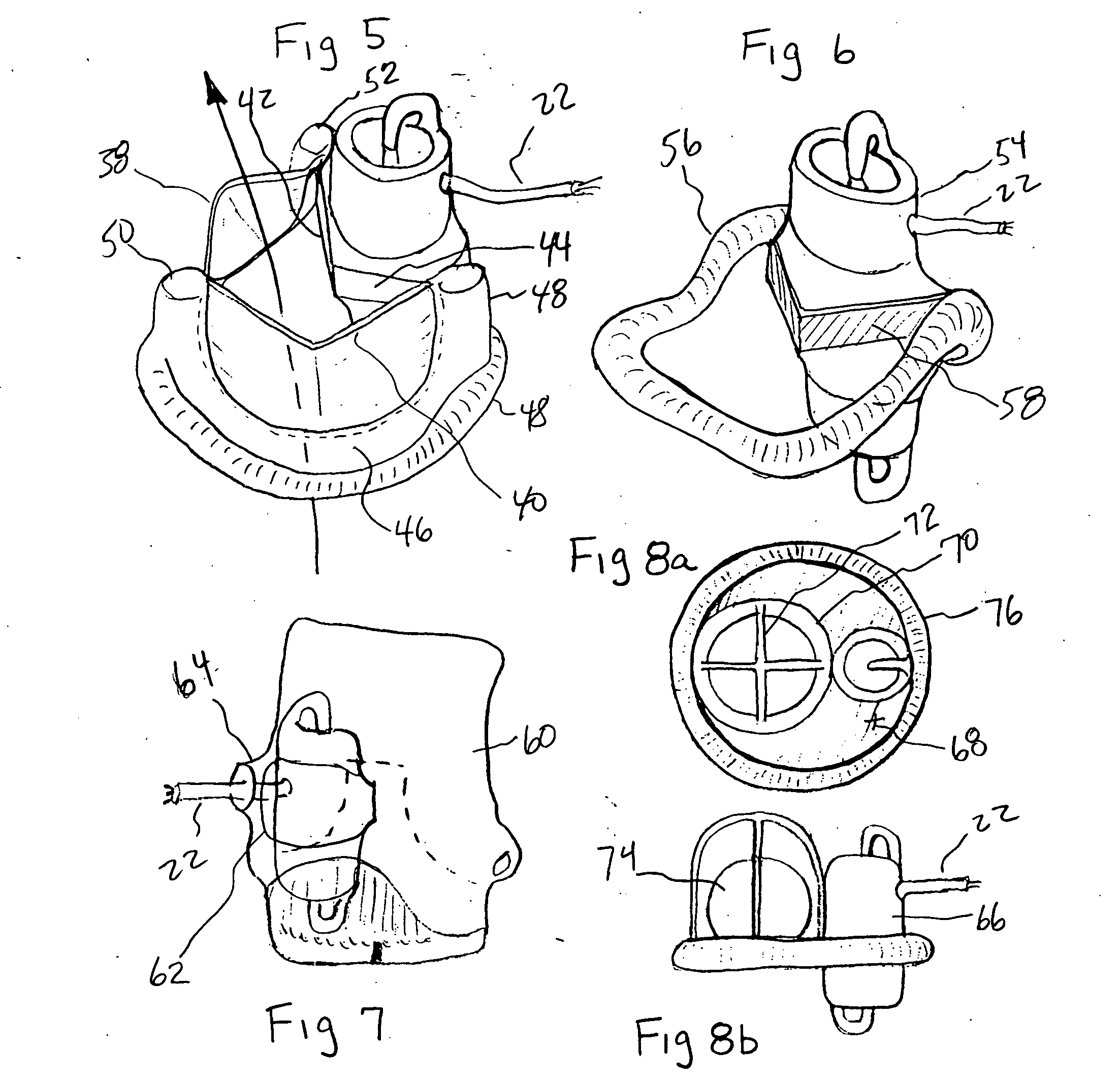
A foldable intravascularly insertable blood pump comprises a rotatable impeller provided with vanes, a flexible shaft extending through a catheter and adapted to drive said impeller, and an envelope enclosing said impeller. For simplifying the technical setup of the blood pump and enhancing its robustness, the impeller comprises radially delivering vanes. The envelope comprises an annular bulge in the region of the impeller, wherein between the radially outer ends of the vanes and the envelope an annular deflection channel is defined. The impeller and the envelope are adapted to be folded by relative displacement of the shaft and the catheter.
Owner:ABIOMED EURO
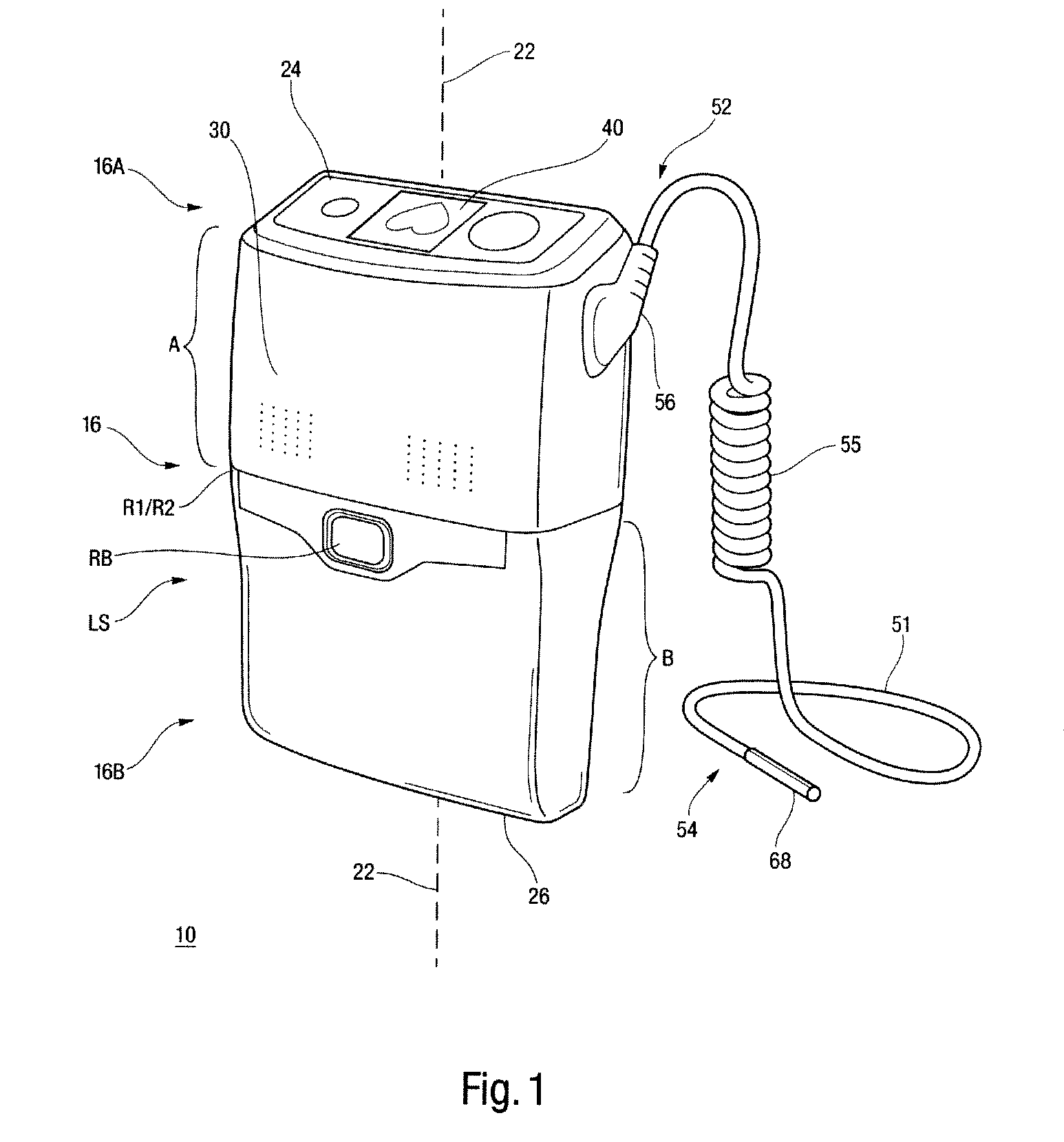
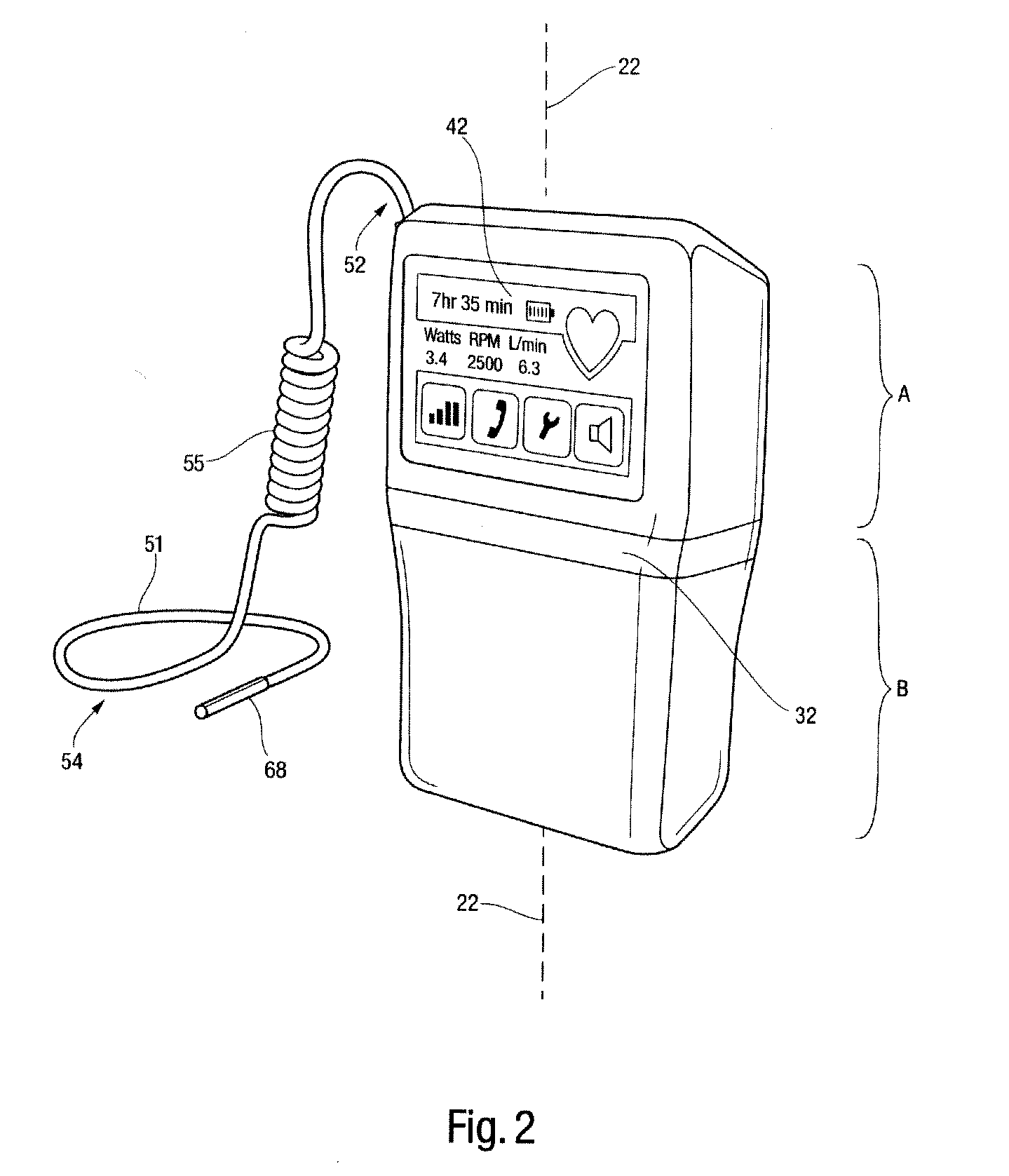
Systems, Devices and Methods for Cardiopulmonary Treatment and Procedures
ActiveUS20090099498A1Reduces hard snappingReduce shear forceVolume measurement apparatus/methodsControl devicesBlood pumpControl room
A cardiopulmonary bypass system utilizing membrane-based reciprocating positive displacement blood pumps (“pod pumps”). In one aspect, the pod pumps are constructed to provide reduced shear forces on the blood being pumped. In another aspect blood flow through the pod pumps can be controlled by a controller using information from pressure sensors in the control chamber of the pod pumps. In another aspect, the pod pumps are included on a disposable unit that can be received and held by a receptacle means on a base unit, the base unit also providing pressurized control fluid to the pod pumps on the disposable unit through the receptacle means.
Owner:DEKA PROD LLP
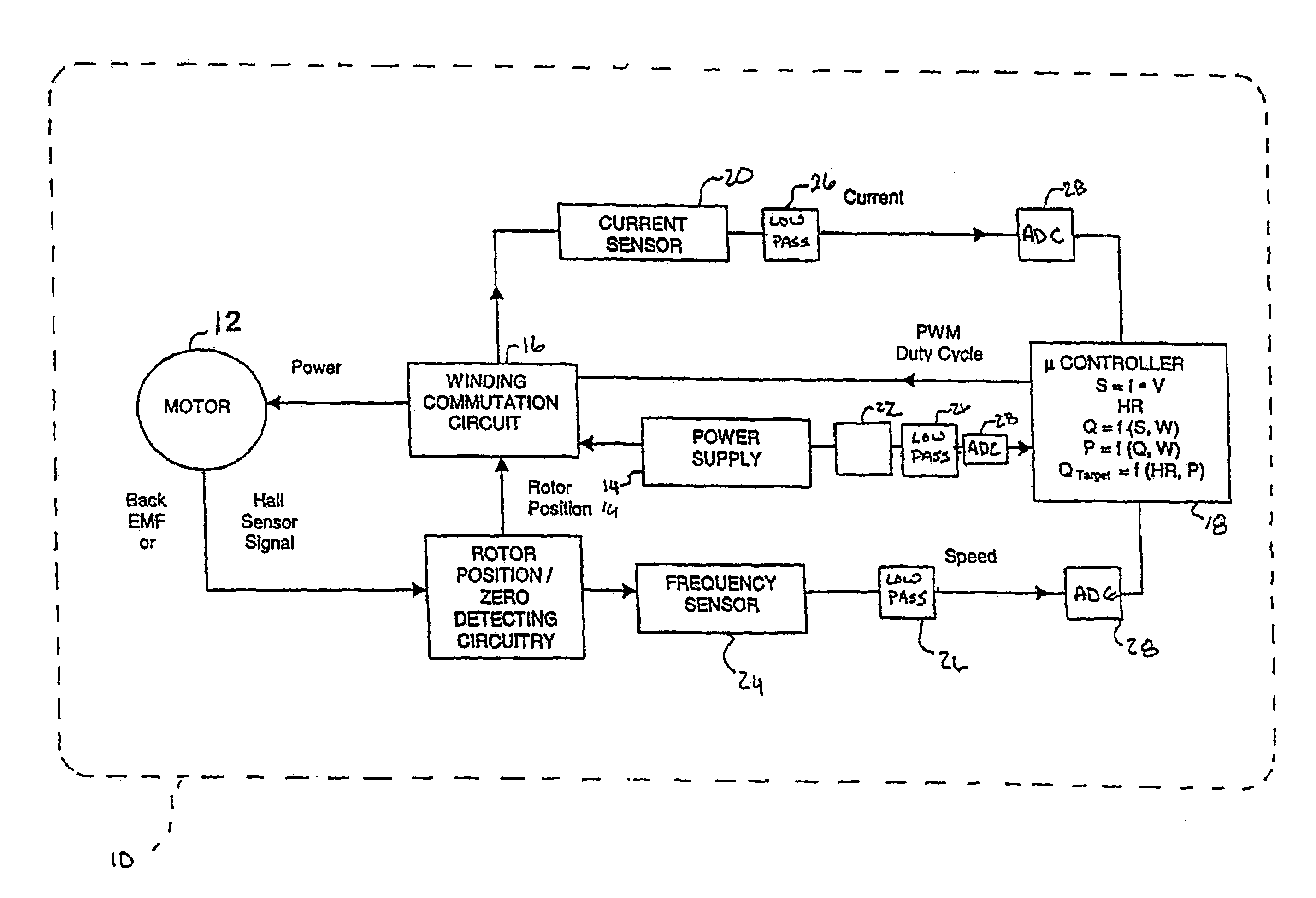
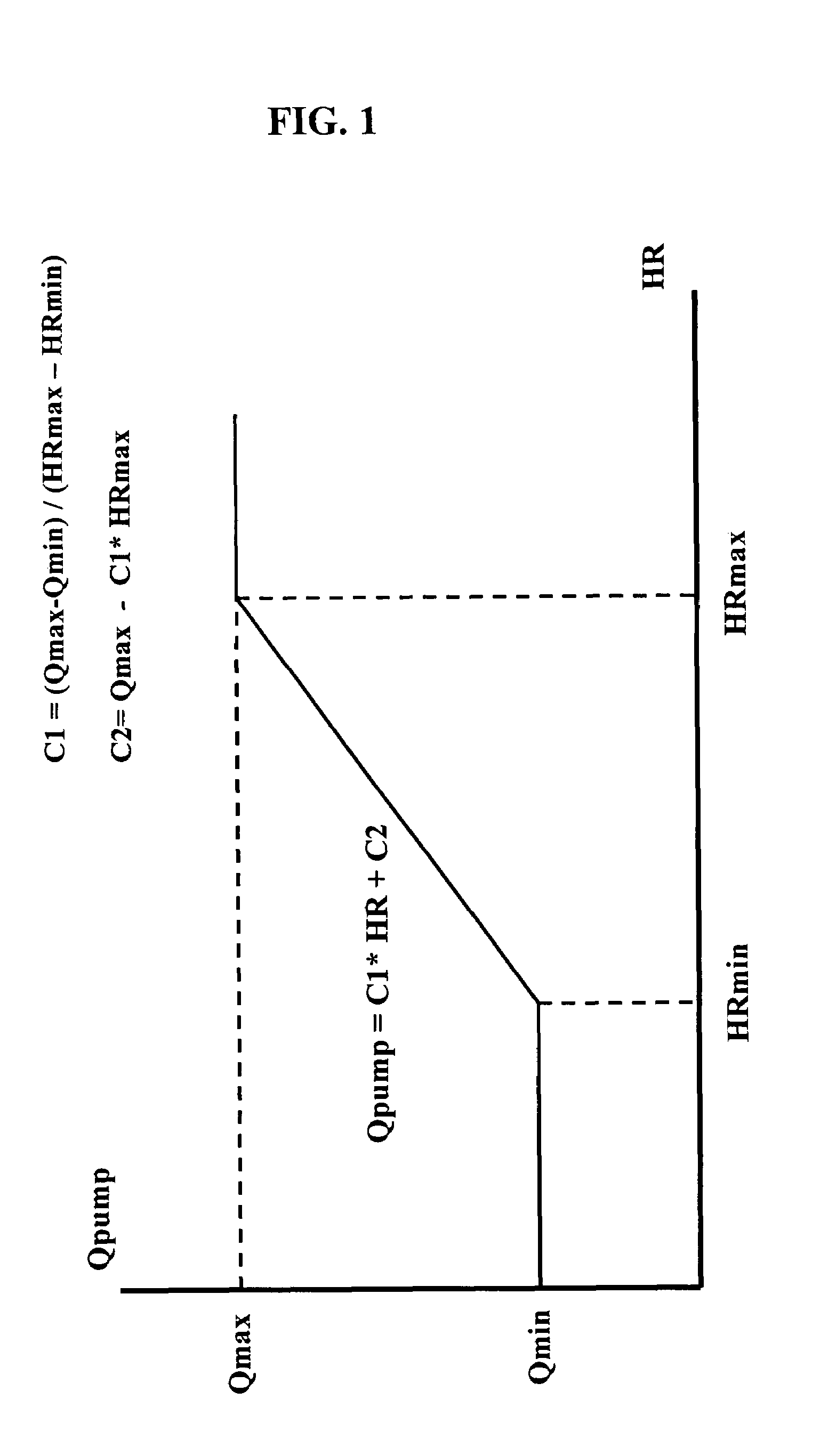
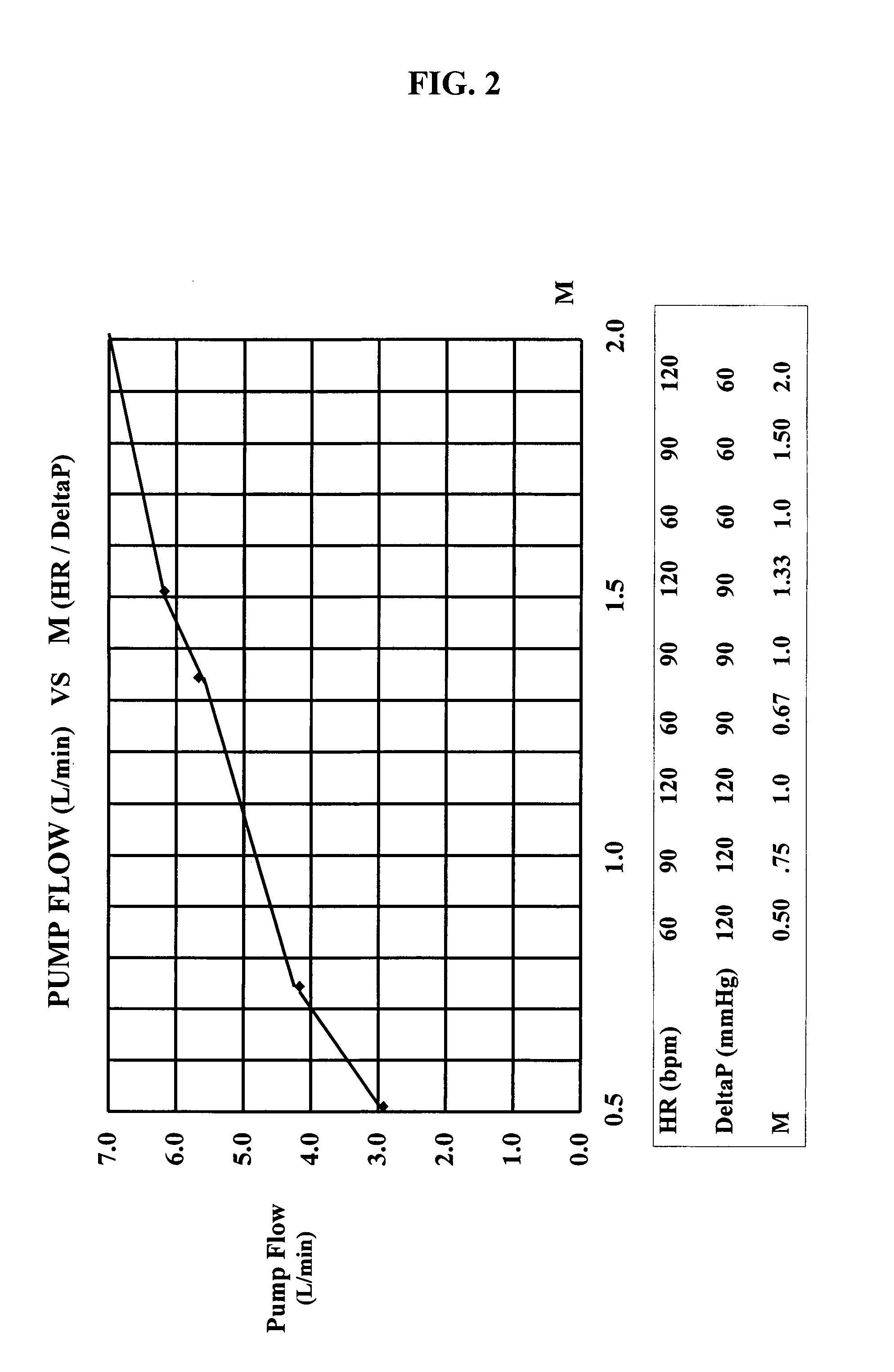
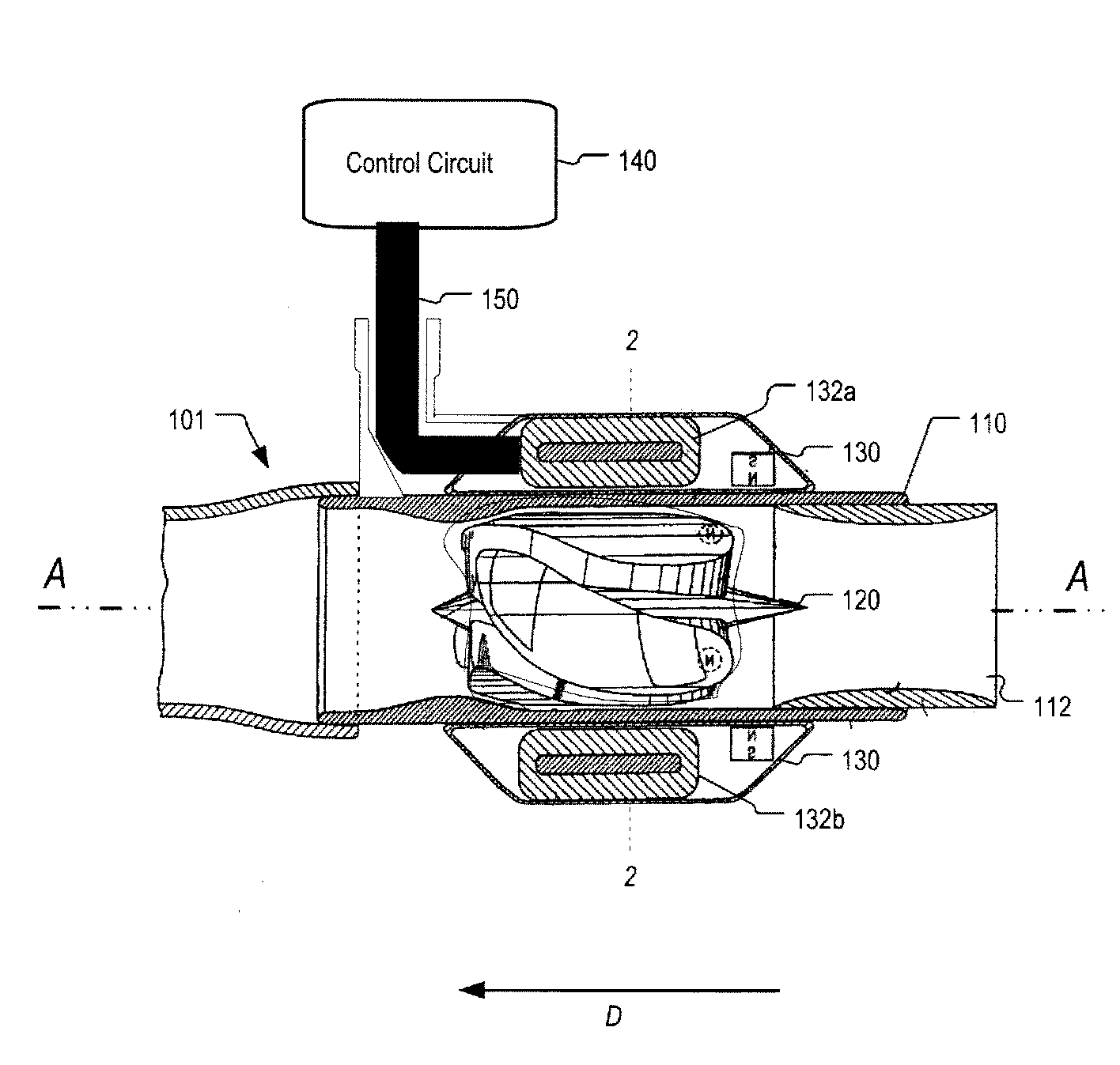
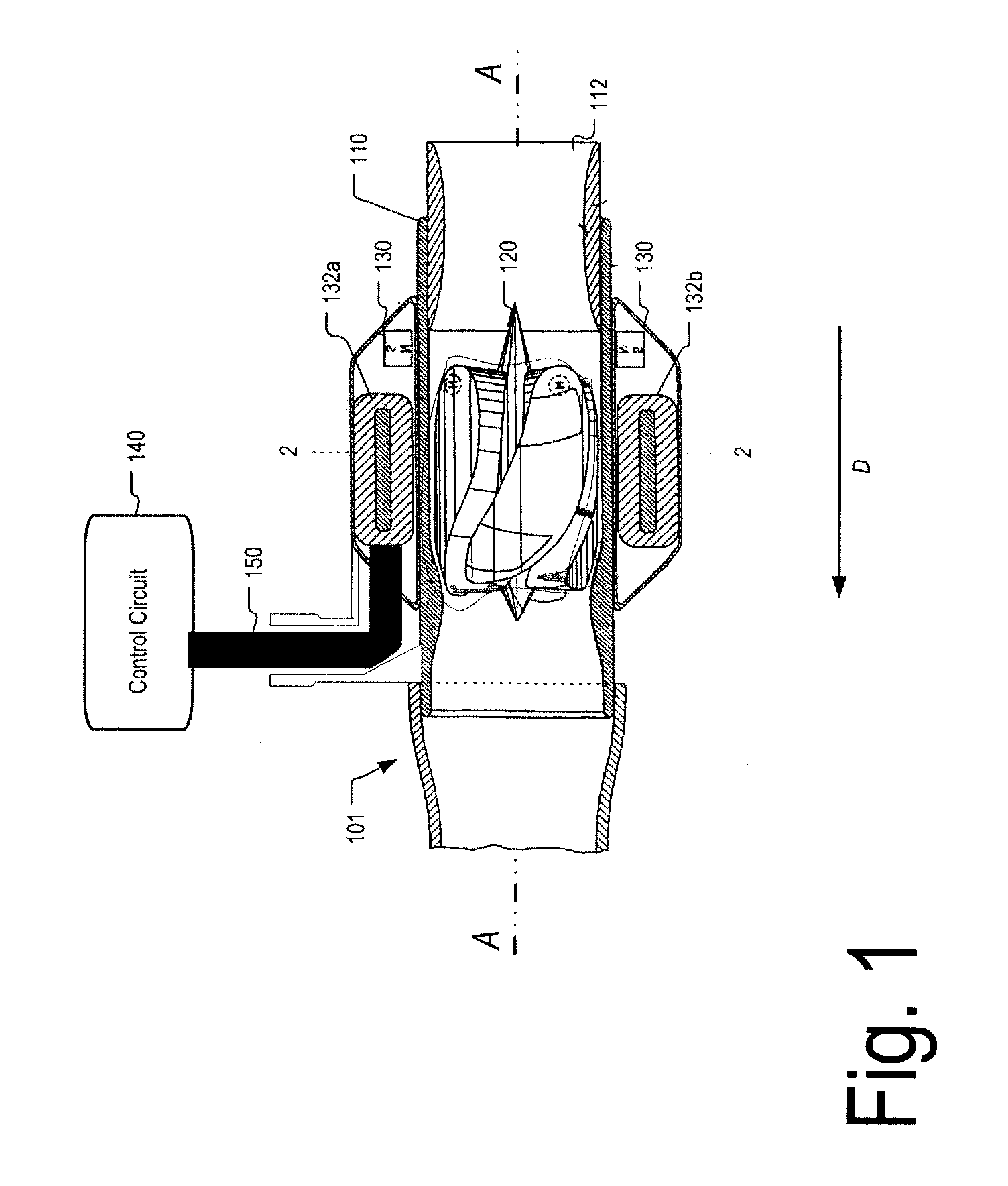
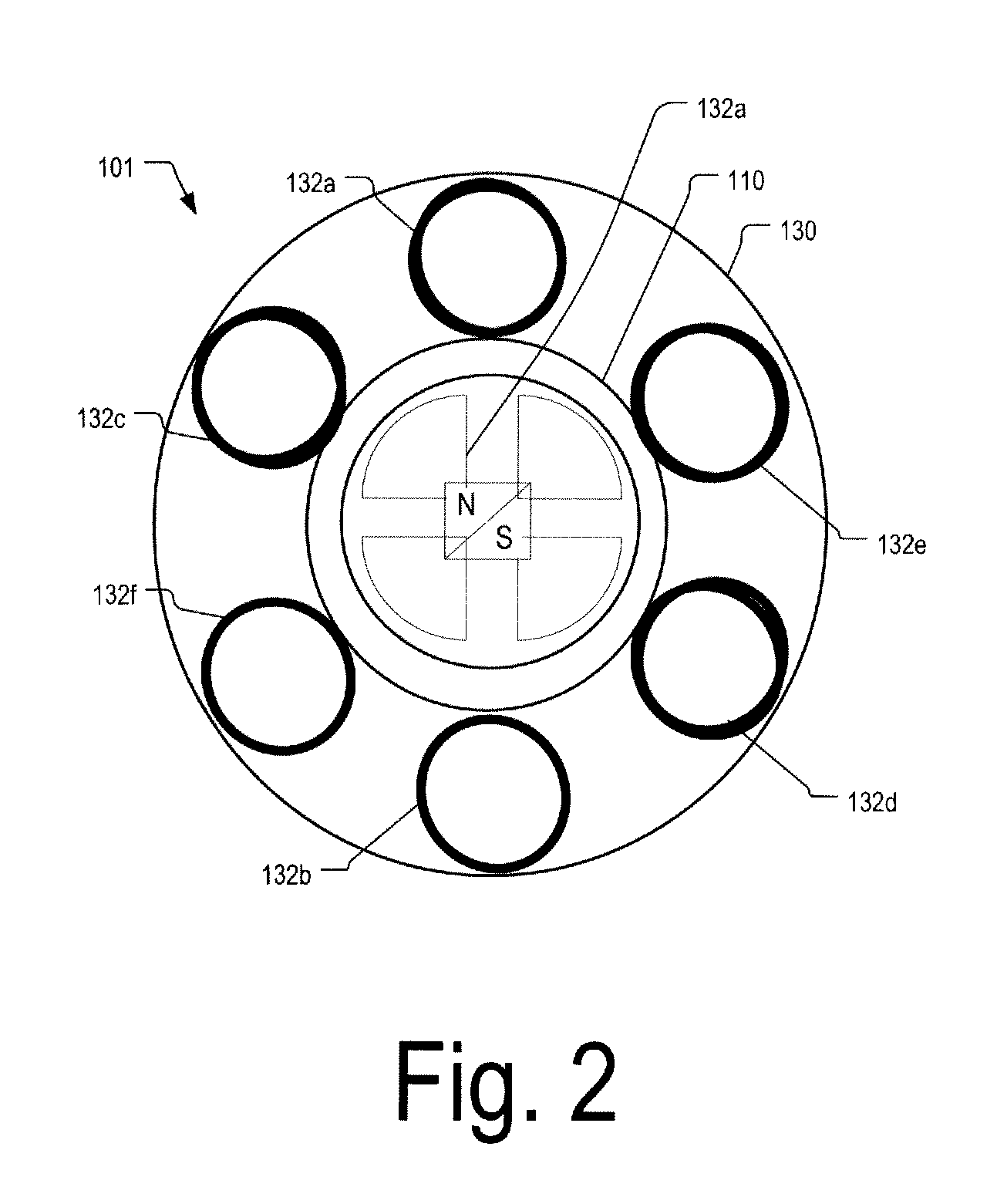
Chronic performance control system for rotodynamic blood pumps
InactiveUS7645225B2Simple control circuitIncrease control flexibilityDC motor speed/torque controlAC motor controlMicrocontrollerMotor speed
In a left ventricular assist device (LVAD) a rotodynamic blood pump (10) is powered by a brushless DC motor (12). A power supply (14) supplies power to the motor (12). Three feedback channels, one for each of voltage, current, and motor speed lead to a microcontroller or microprocessor (18). The three feedback waveforms are analyzed, and from these waveforms, motor input power, patient heart rate, current pump flow rate, and systemic pressure are determined. The microprocessor (18) then calculates a desired flow rate proportional to the patient heart rate. The microprocessor communicates a new power output to a commutation circuit (16), which regulates power to the motor (12). The pump (10) also includes safety checks that are prioritized over desired pump flow. These include prevention of ventricular suction, low pulsatility, minimum and maximum pump speed, minimum speed-relative pump flow, minimum absolute pump flow, minimum and maximum motor input power.
Owner:MEDVEDEV ALEXANDER +2
Intravascular ventricular assist device
One aspect of an intravascular ventricular assist device is an implantable blood pump where the pump includes a housing defining a bore having an axis, one or more rotors disposed within the bore, each rotor including a plurality of magnetic poles, and one or more stators surrounding the bore for providing a magnetic field within the bore to induce rotation of each of the one or more rotors. Another aspect of the invention includes methods of providing cardiac assistance to a mammalian subject as, for example, a human. Further aspects of the invention include rotor bodies having helical channels formed longitudinally along the length of the body of the rotor where each helical channel is formed between peripheral support surface areas facing radially outwardly and extending generally in circumferential directions around the rotational axis of the rotor.
Owner:HEARTWARE INC
Rotary blood pump and control system therefor
Owner:TC1 LLC +1
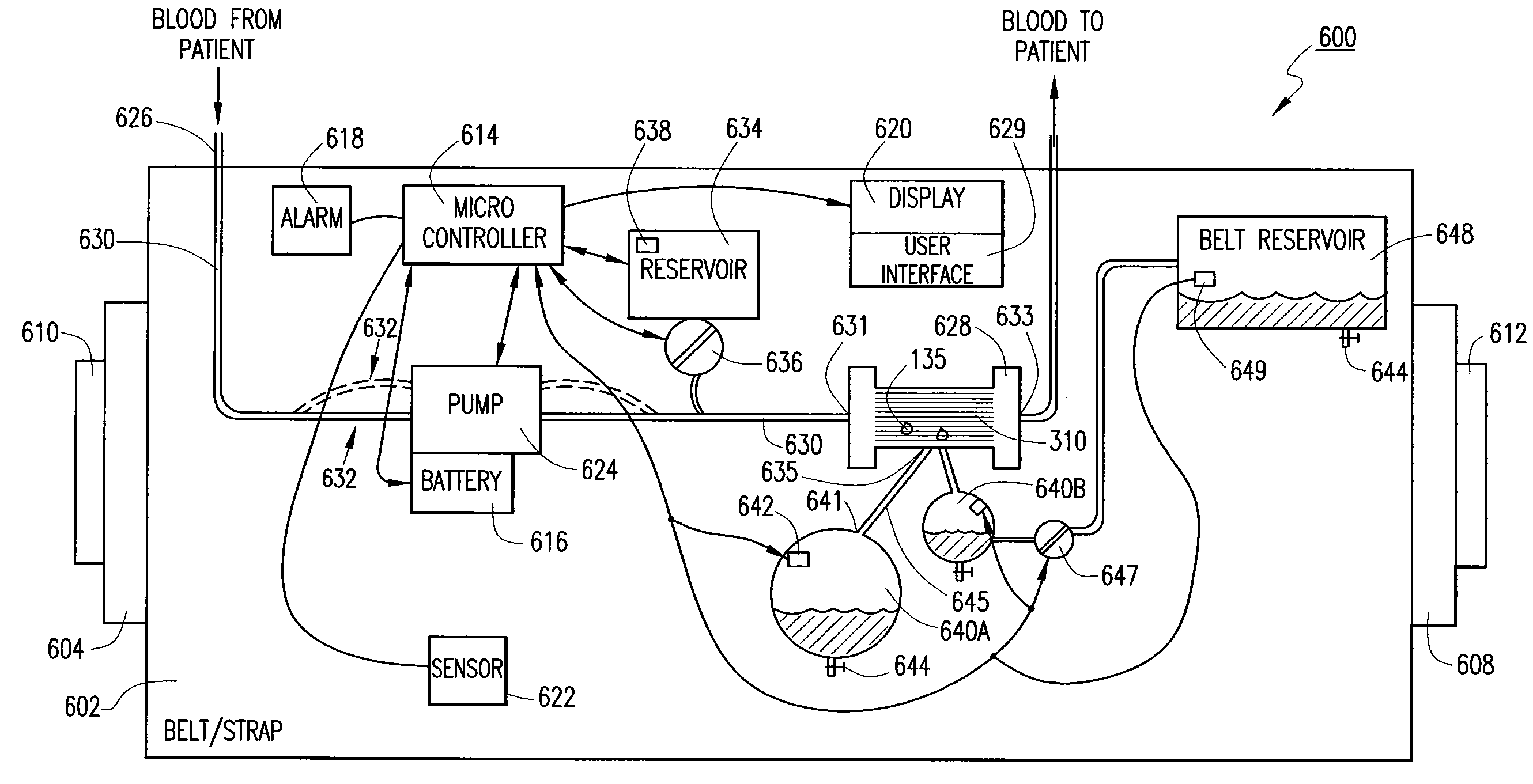
Wearable ultrafiltration device
InactiveUS7645253B2Steady and smooth removalPreventing the shortness of breath and swellingMembranesSemi-permeable membranesUltrafiltrationBlood pump
An ultrafiltration device adapted to be worn on a portion of the body of a patient includes a blood inlet tube leading from a first blood vessel, a blood pump, an anticoagulant reservoir for infusing anticoagulants into the blood, a blood filter including a substrate through which the blood is circulated and filtered, a fluid bag for storing the excess fluid and a blood outlet tube leading to a second blood vessel.
Owner:FRESENIUS MEDICAL CARE HLDG INC
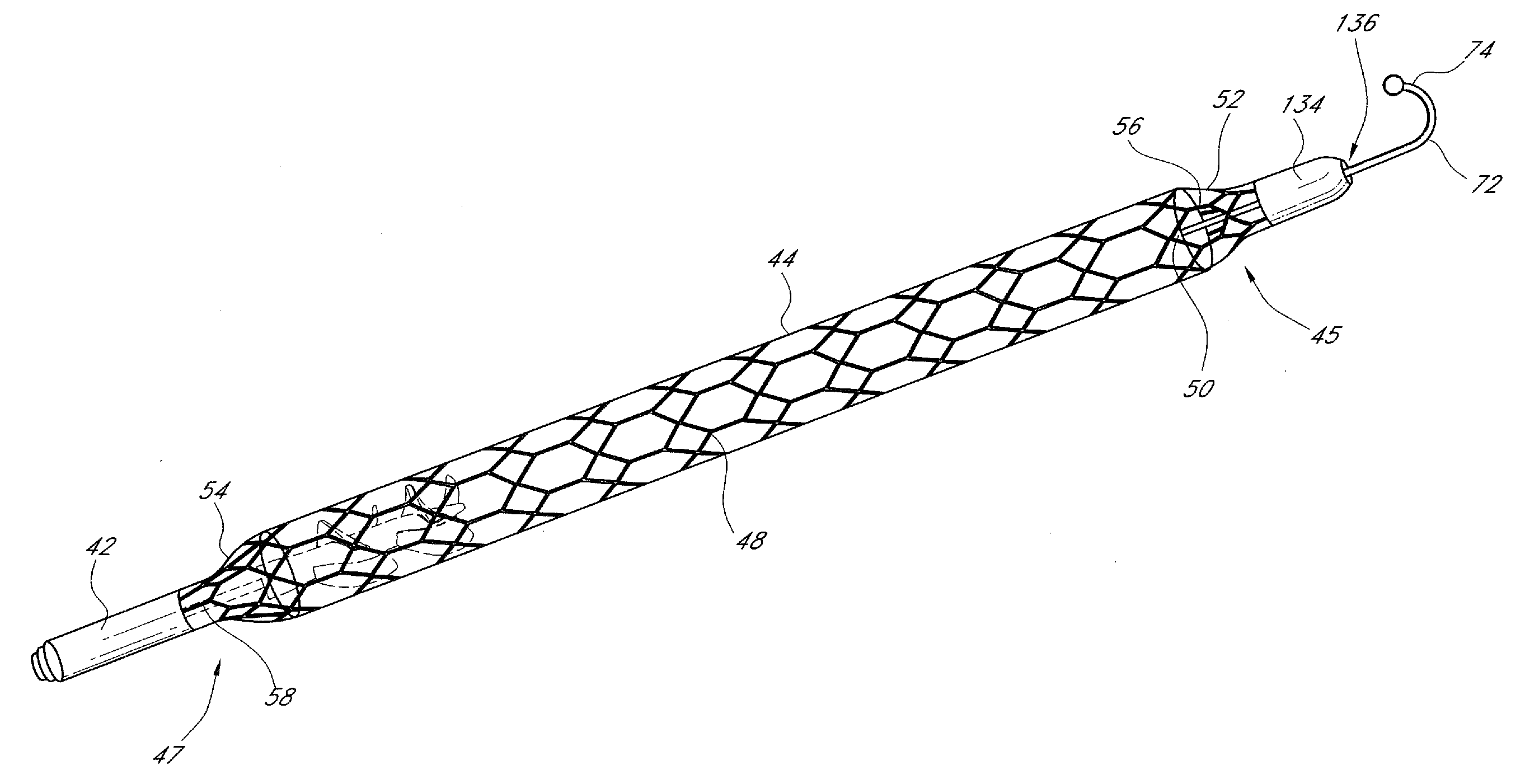
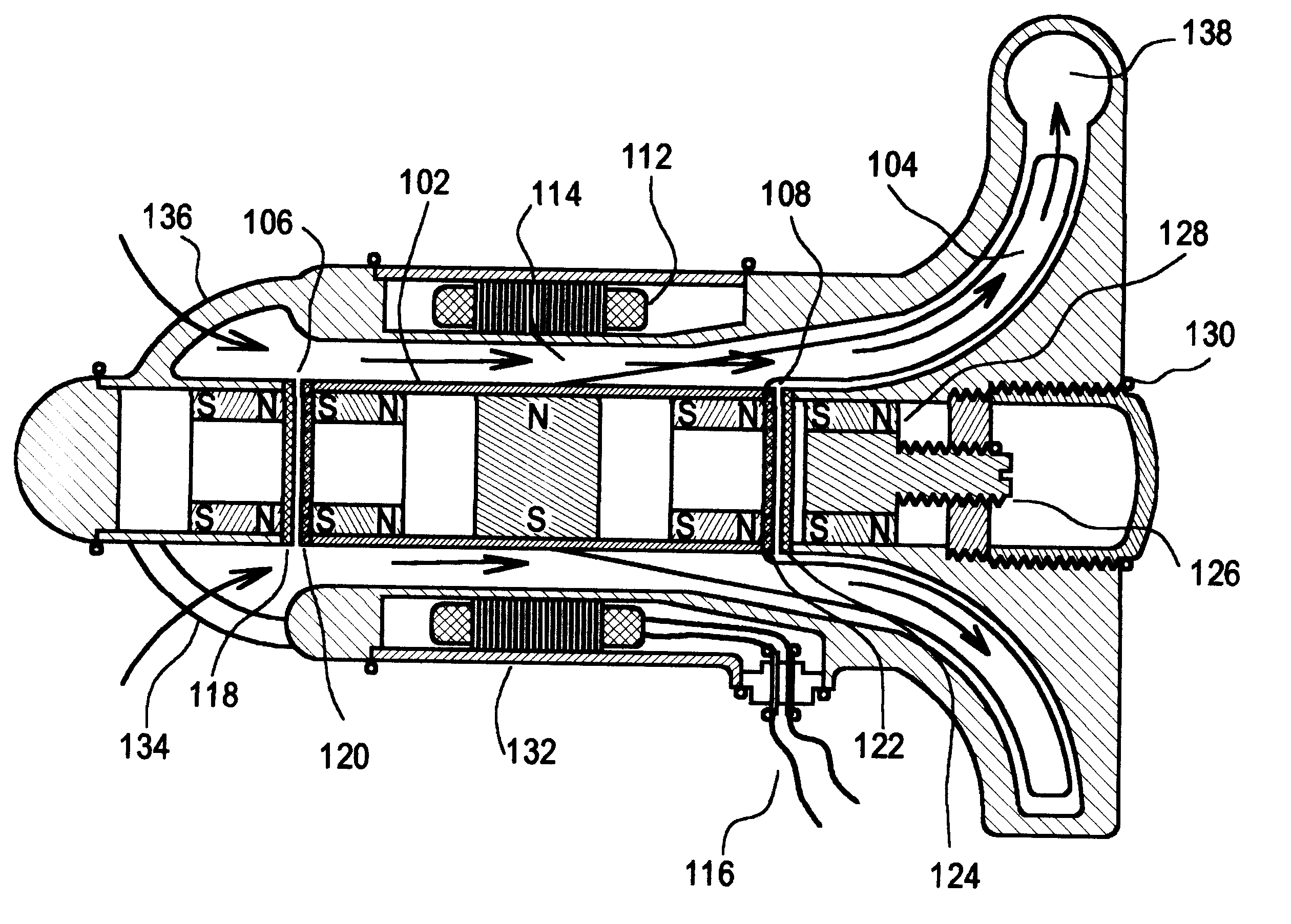
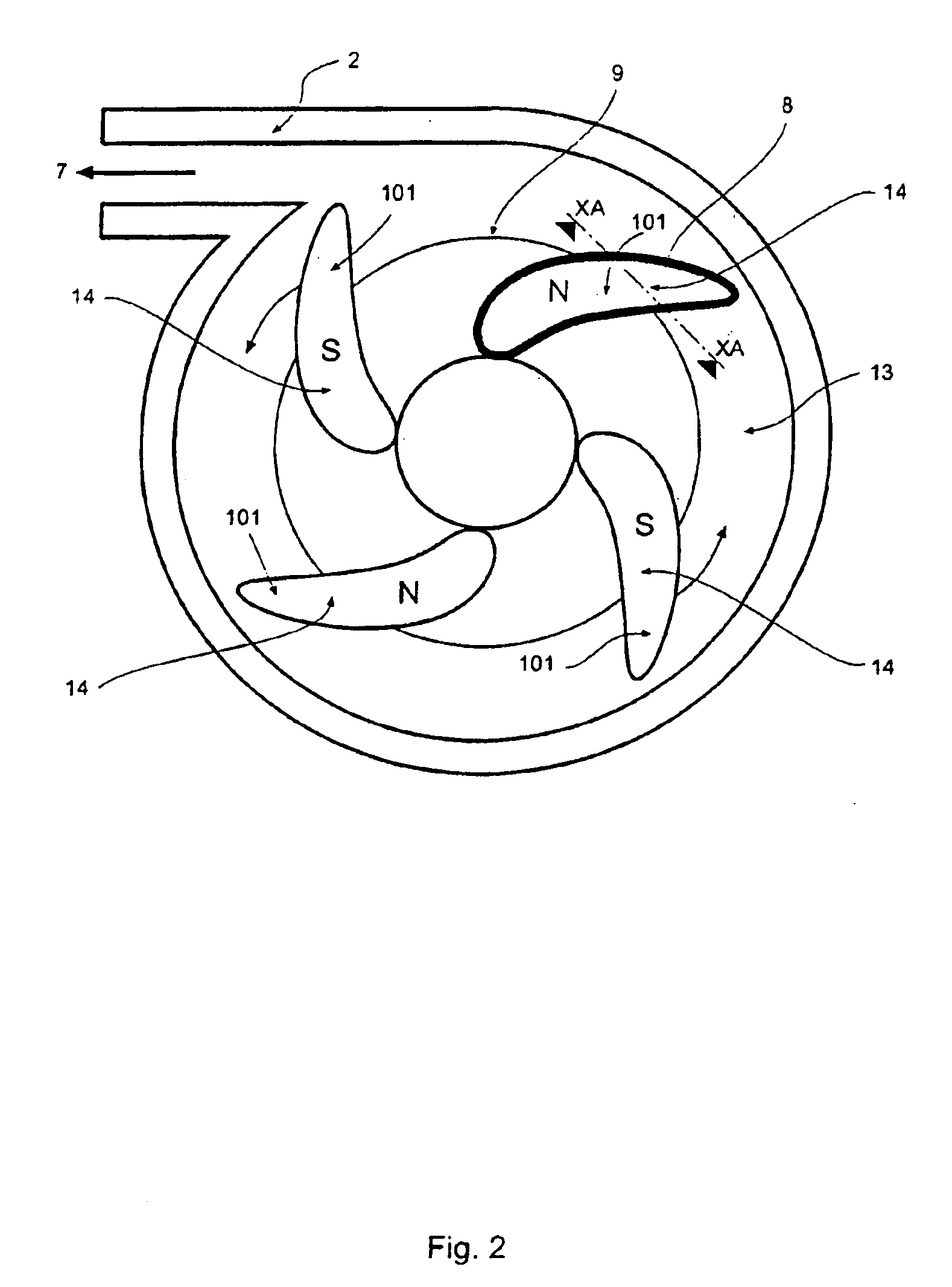
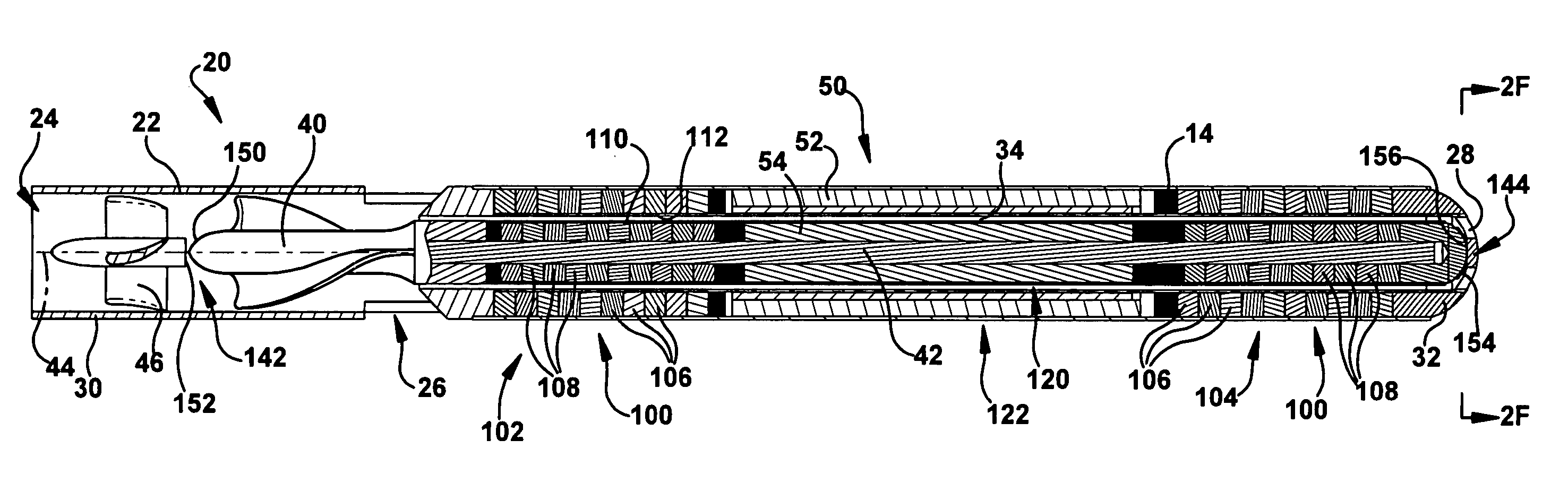
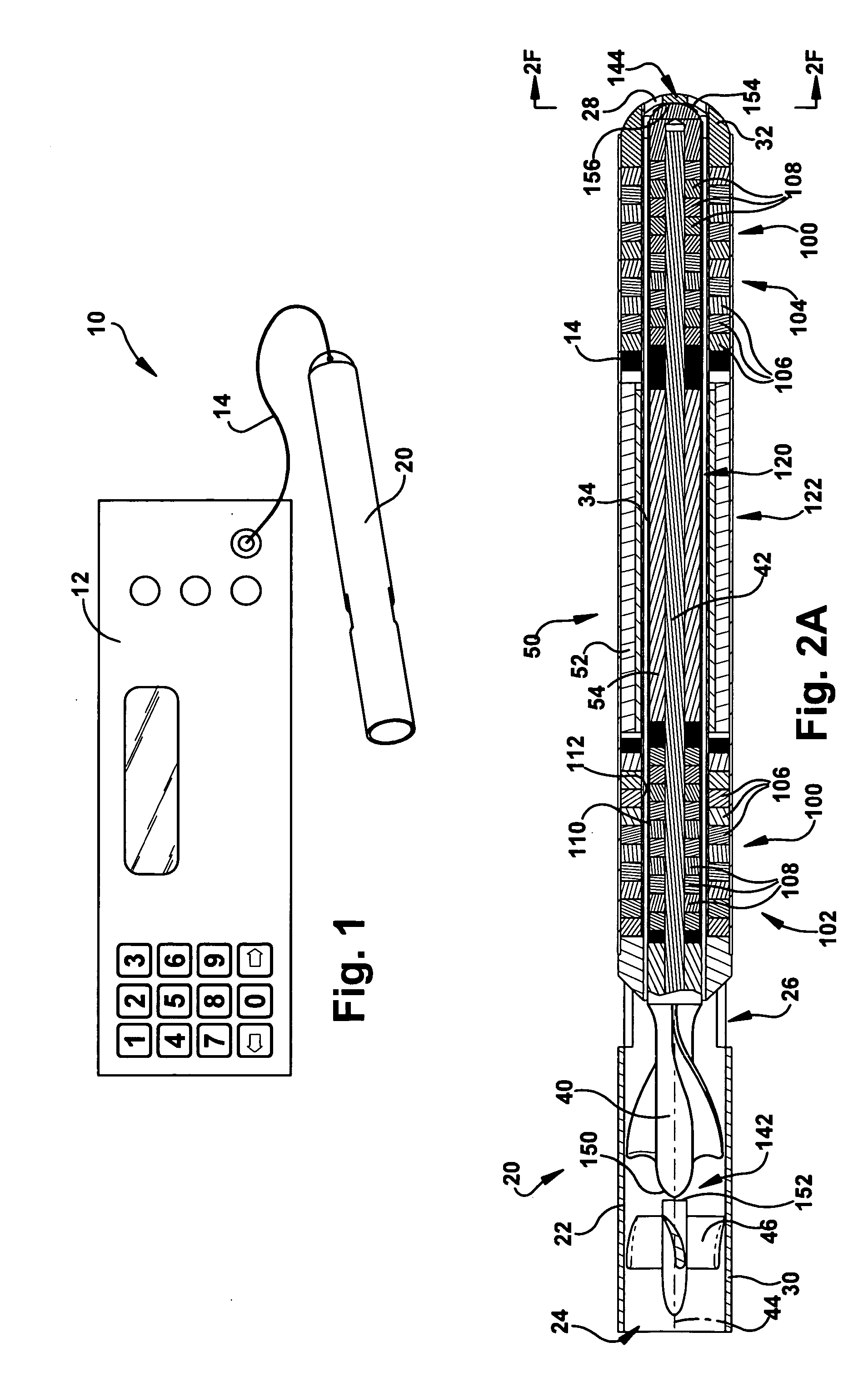
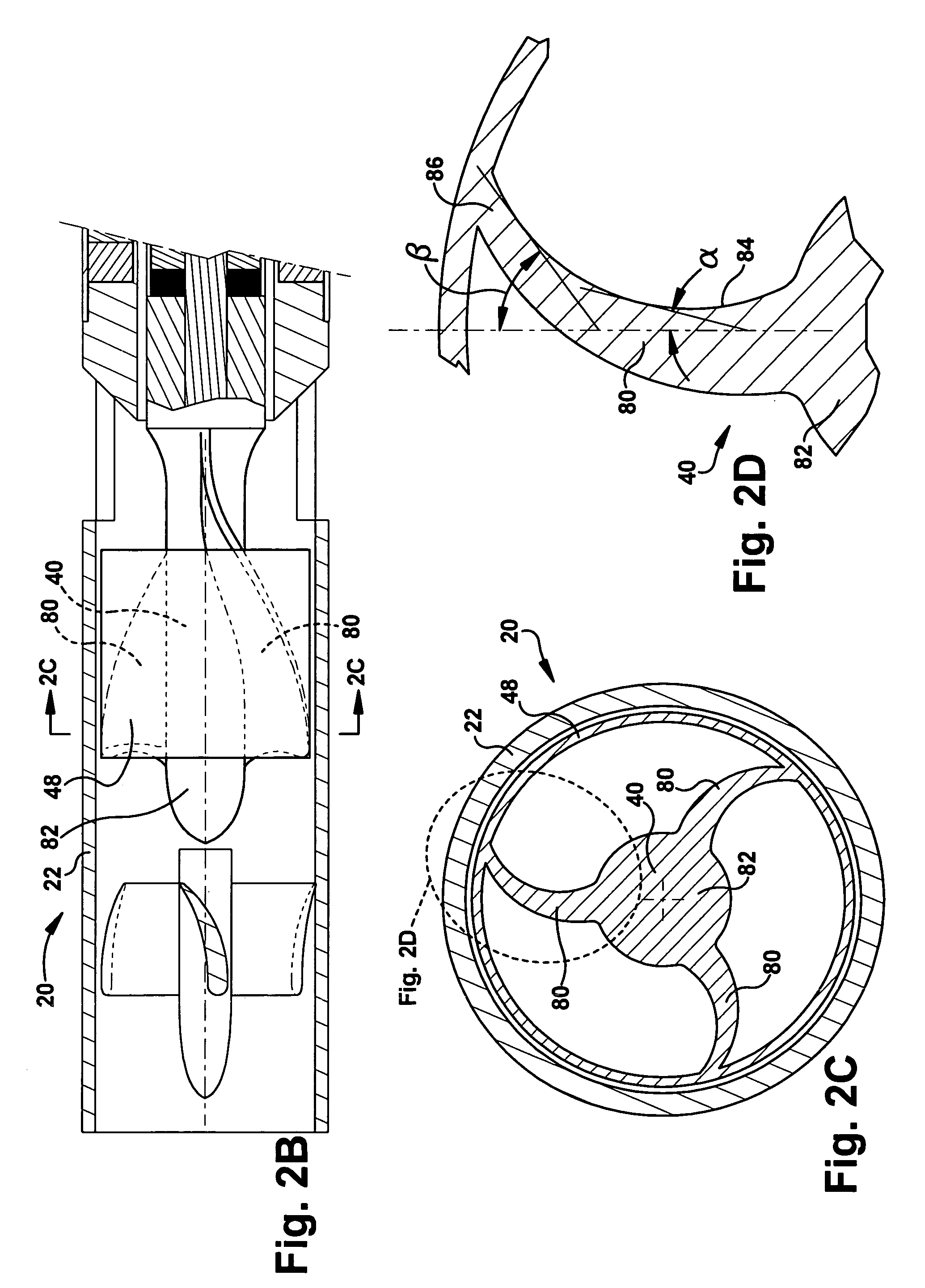
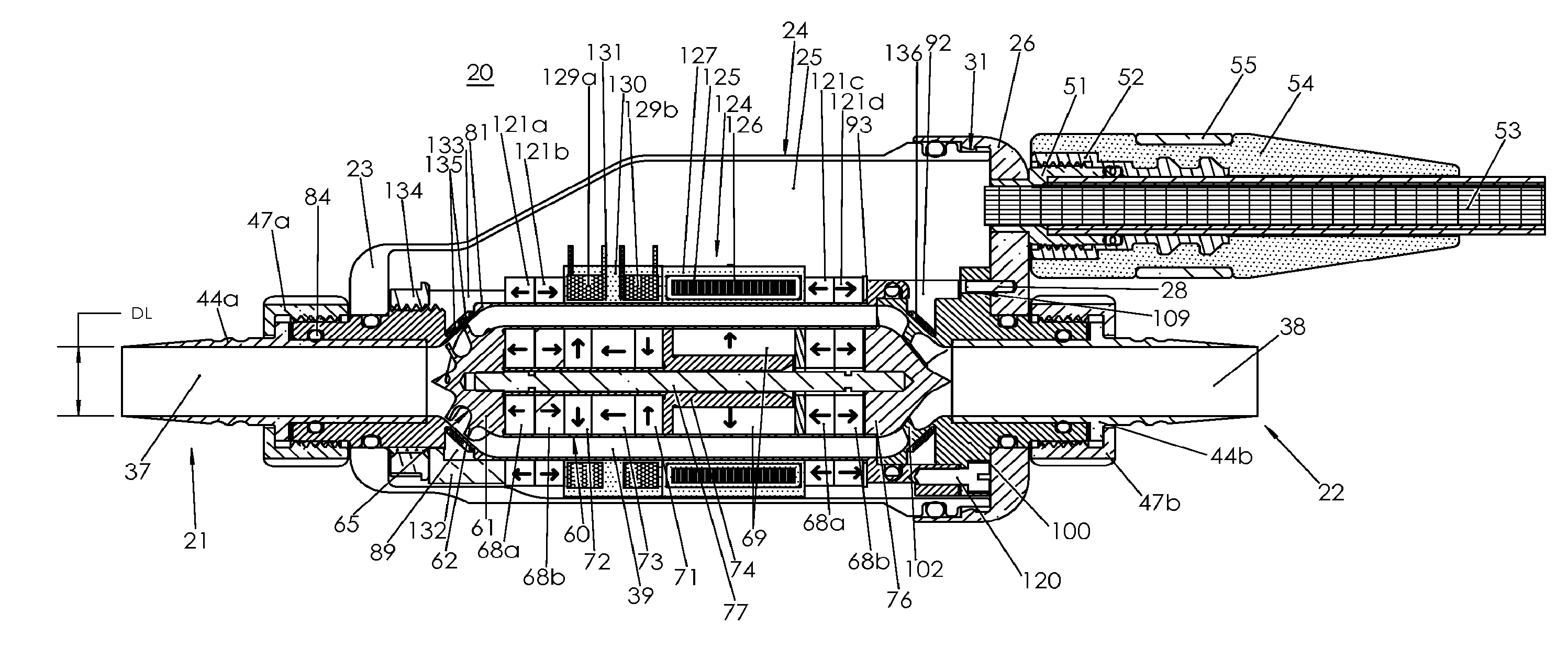
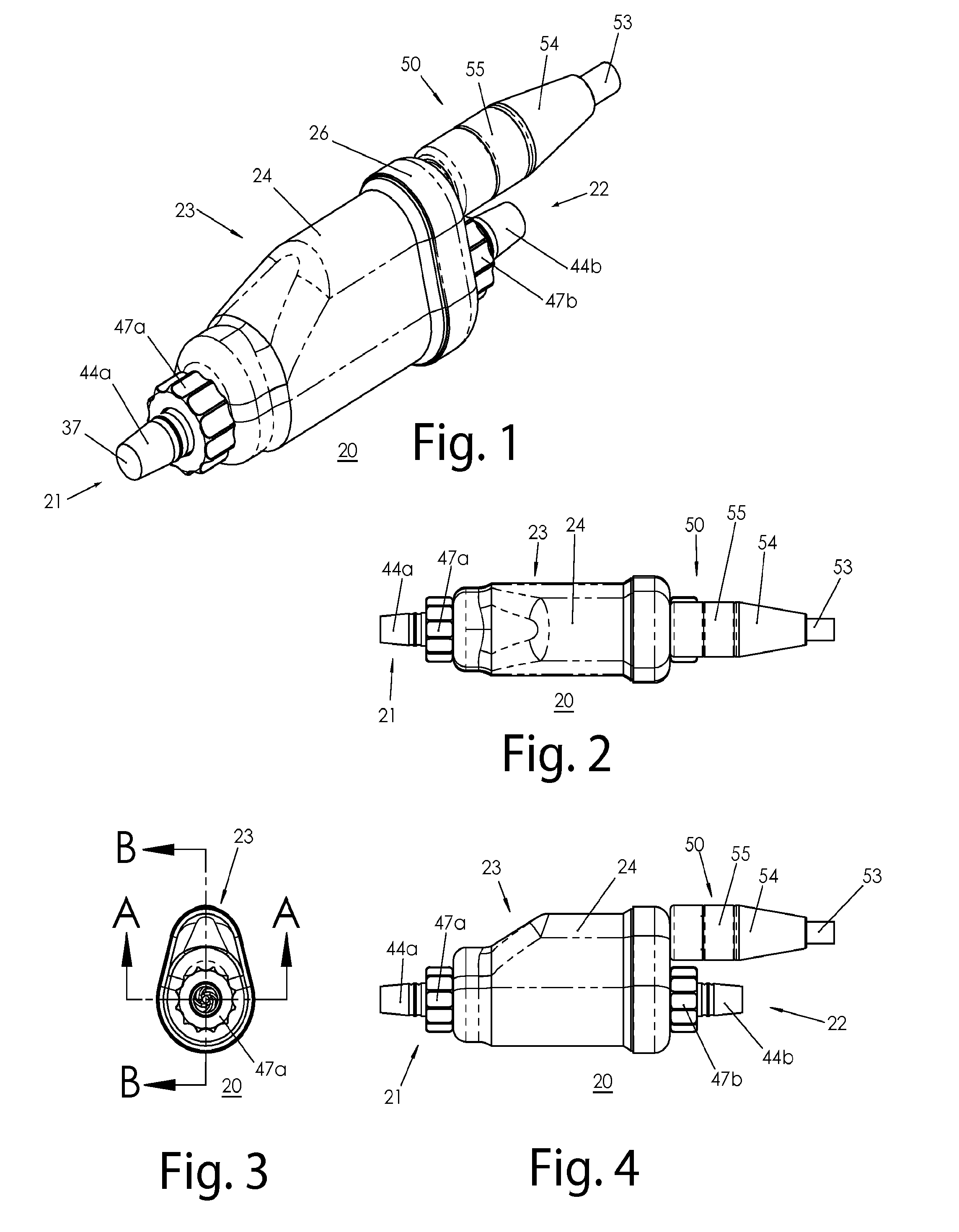
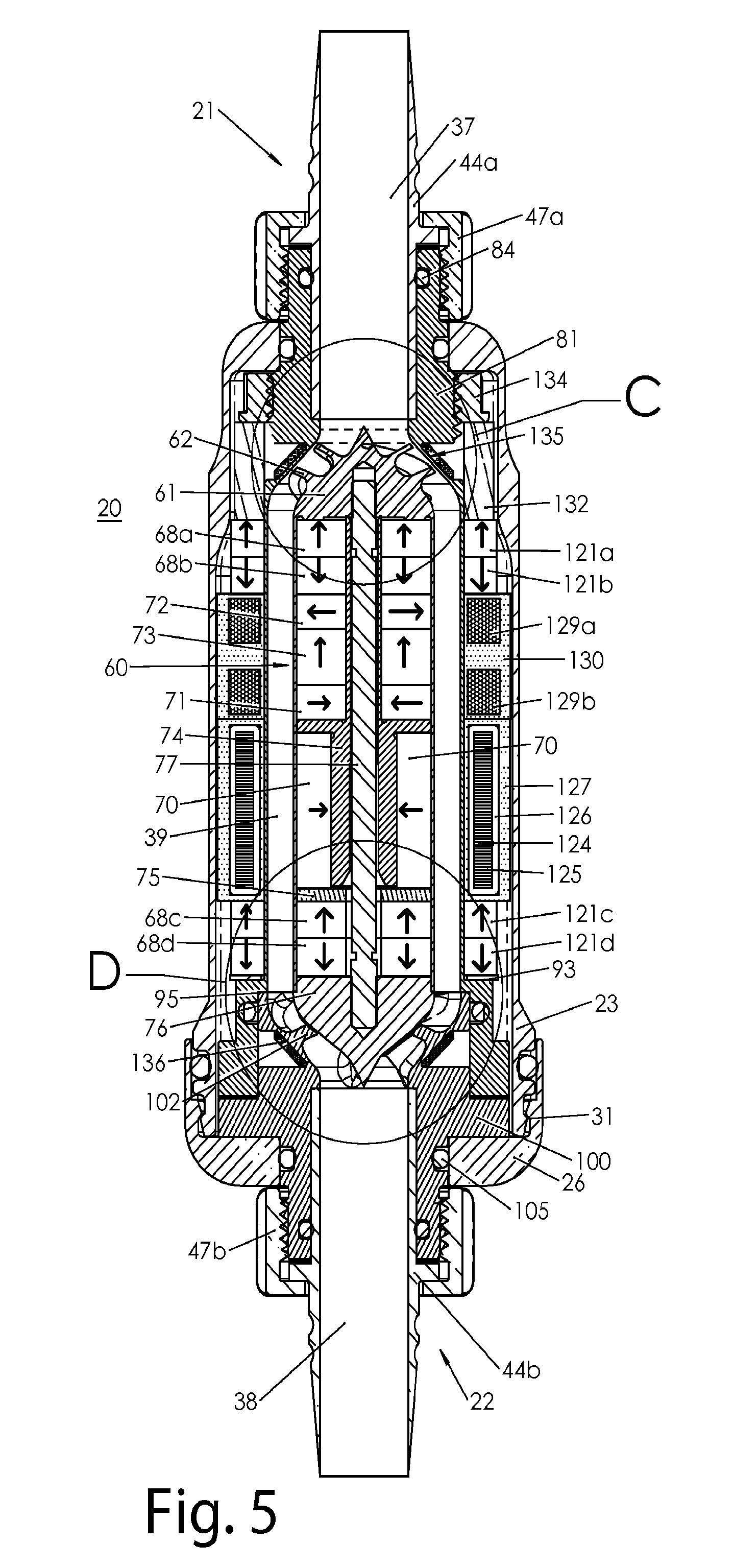
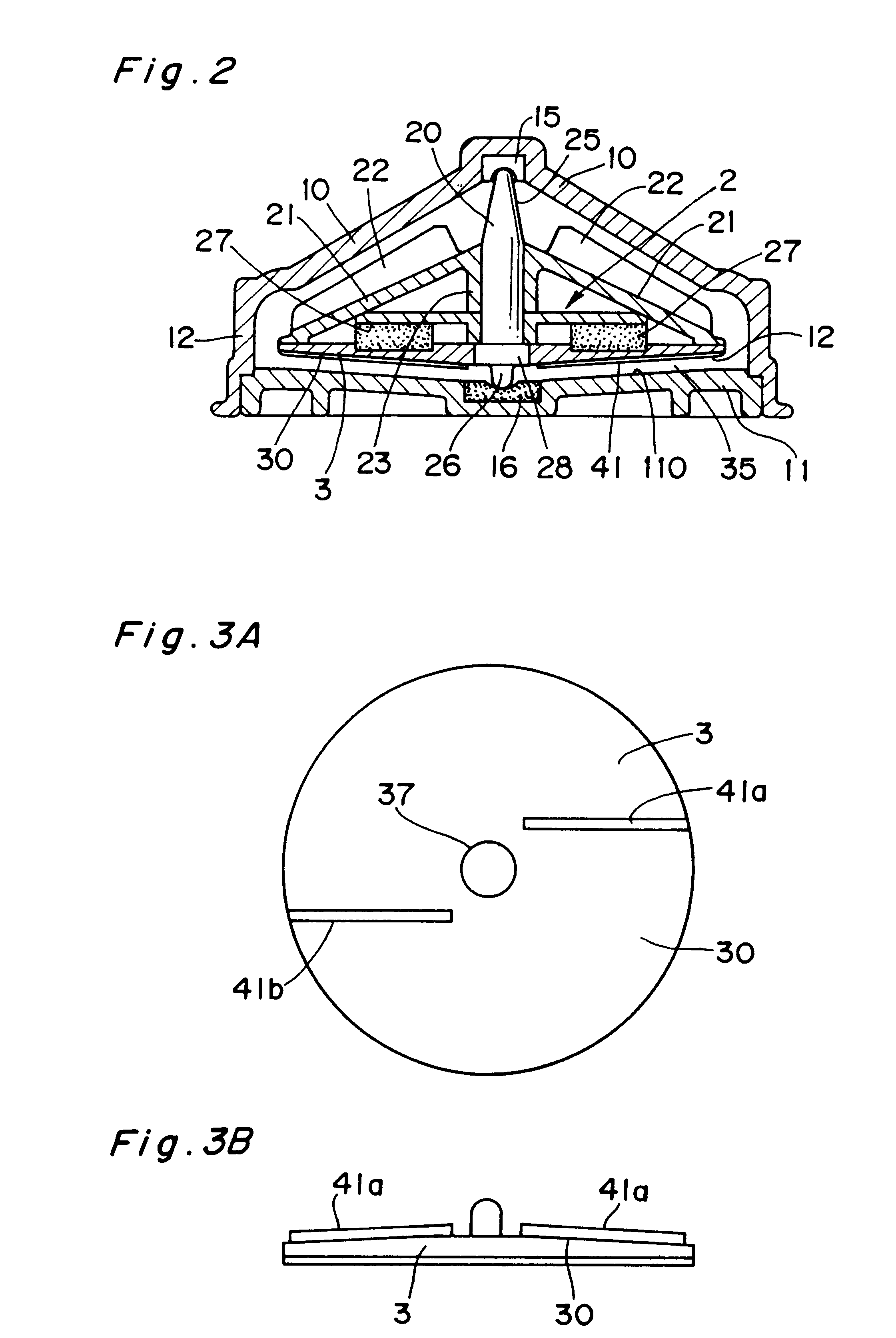
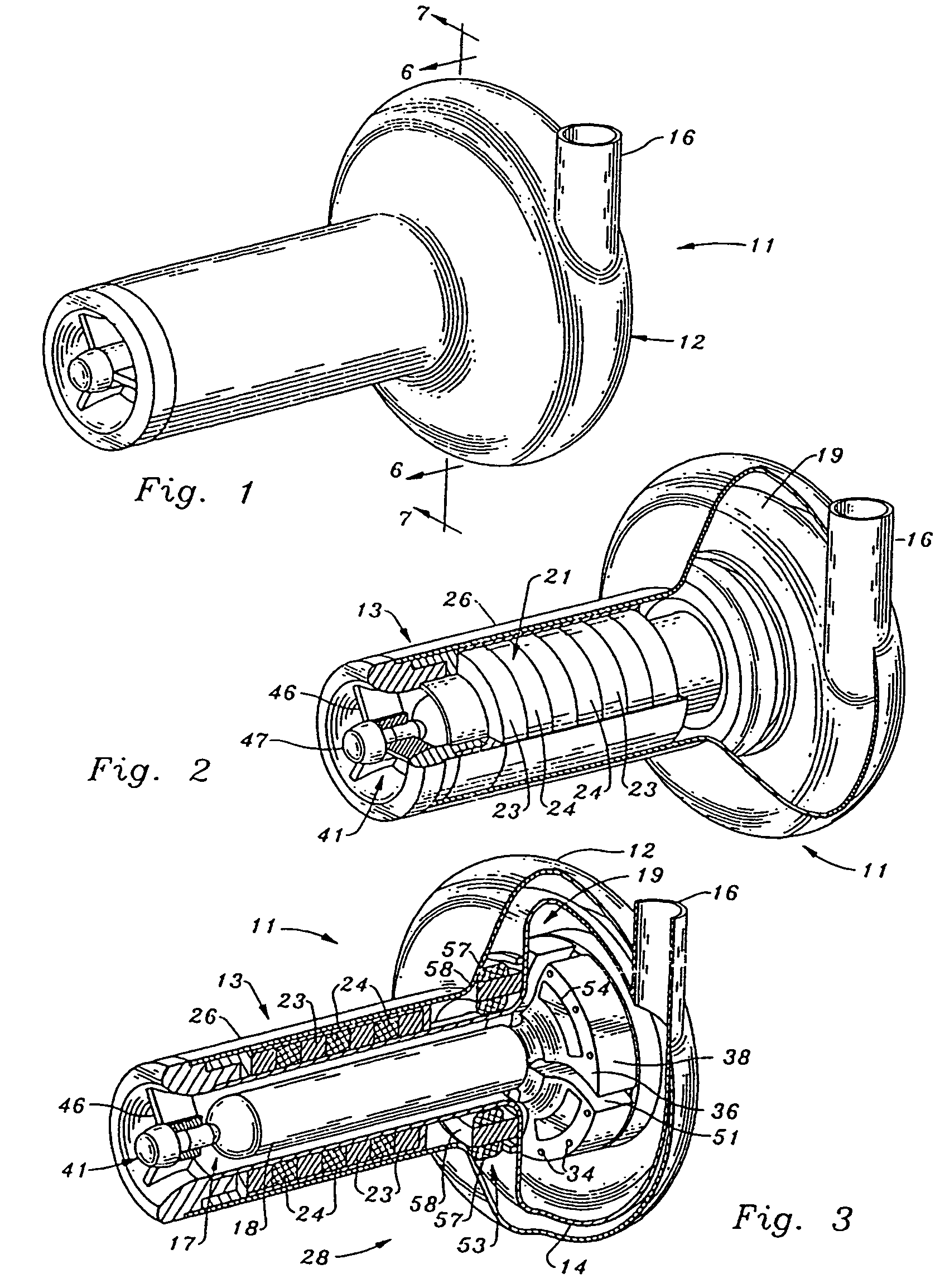
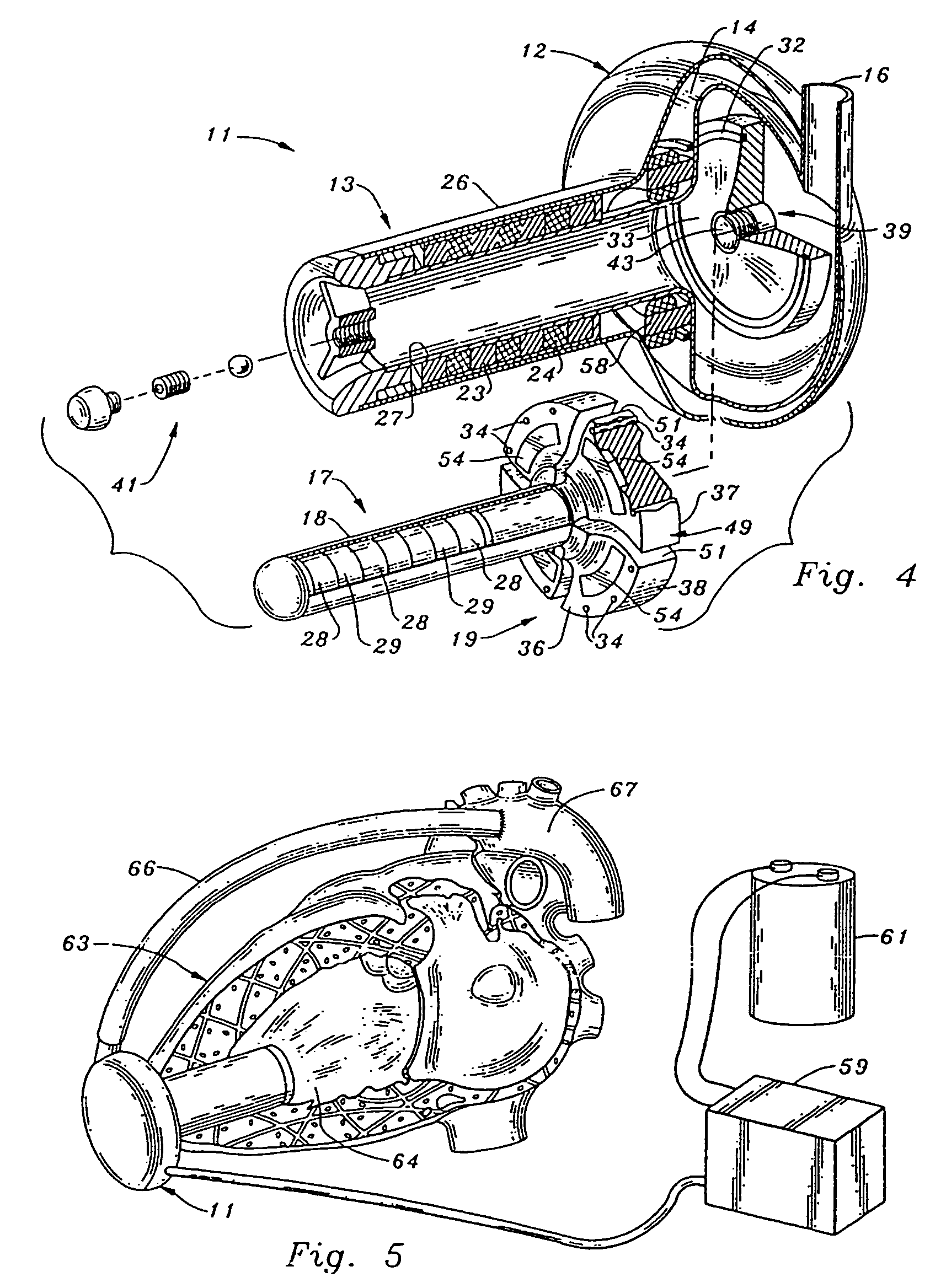
Blood pump
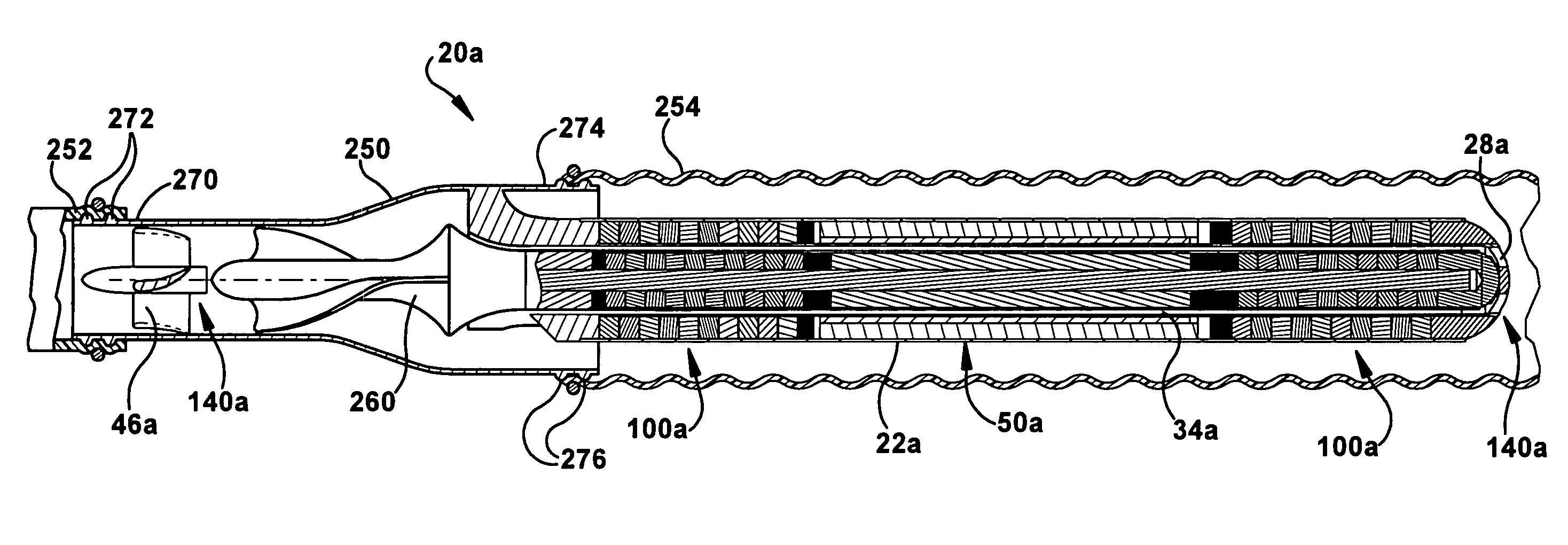
A blood pump (26) includes a stator assembly including a fluid inlet (24) and a fluid outlet (26). A rotor assembly (120) includes an impeller (40) rotatable about an axis (44) to move fluid from the inlet (24) to the outlet (26). A motor (50) imparts rotation of the impeller (40) about the axis (44). The motor (50) includes a motor stator (52) fixed to the stator assembly (122), a motor rotor (54) fixed to the rotor assembly (120), and a radial motor gap (34) between the stator (52) and the rotor (54). The pump (20) is configured to direct a mixed blood flow from the fluid inlet (24) to the fluid outlet (26) and a wash flow through the motor gap (34).
Owner:THE CLEVELAND CLINIC FOUND +1
Magnetically-levitated blood pump with optimization method enabling miniaturization
ActiveUS20110237863A1Quality improvementReduce stiffnessControl devicesBlood pumpsBlood pumpMiniaturization
A magnetically-levitated blood pump with an optimization method that enables miniaturization and supercritical operation. The blood pump includes an optimized annular blood gap that increases blood flow and also provides a reduction in bearing stiffness among the permanent magnet bearings. Sensors are configured and placed optimally to provide space savings for the motor and magnet sections of the blood pump. Rotor mass is increased by providing permanent magnet placement deep within the rotor enabled by a draw rod configuration.
Owner:WORLD HEART +1
Stabilizing drive for contactless rotary blood pump impeller
ActiveUS20080021394A1Good for healthSpeed up the flowSpecific fluid pumpsPump componentsImpellerCoupling
A rotary blood pump includes a casing defining a pumping chamber. The pumping chamber has a blood inlet and a tangential blood outlet. One or more motor stators are provided outside of the pumping chamber. A rotatable impeller is within the pumping chamber and is adapted to cause blood entering the pumping chamber to move to the blood outlet. The impeller has one or more magnetic regions. The impeller is radially constrained in rotation by magnetic coupling to one or more motor stators and is axially constrained in rotation by one or more hydrodynamic thrust bearing surfaces on the impeller.
Owner:HEARTWARE INC
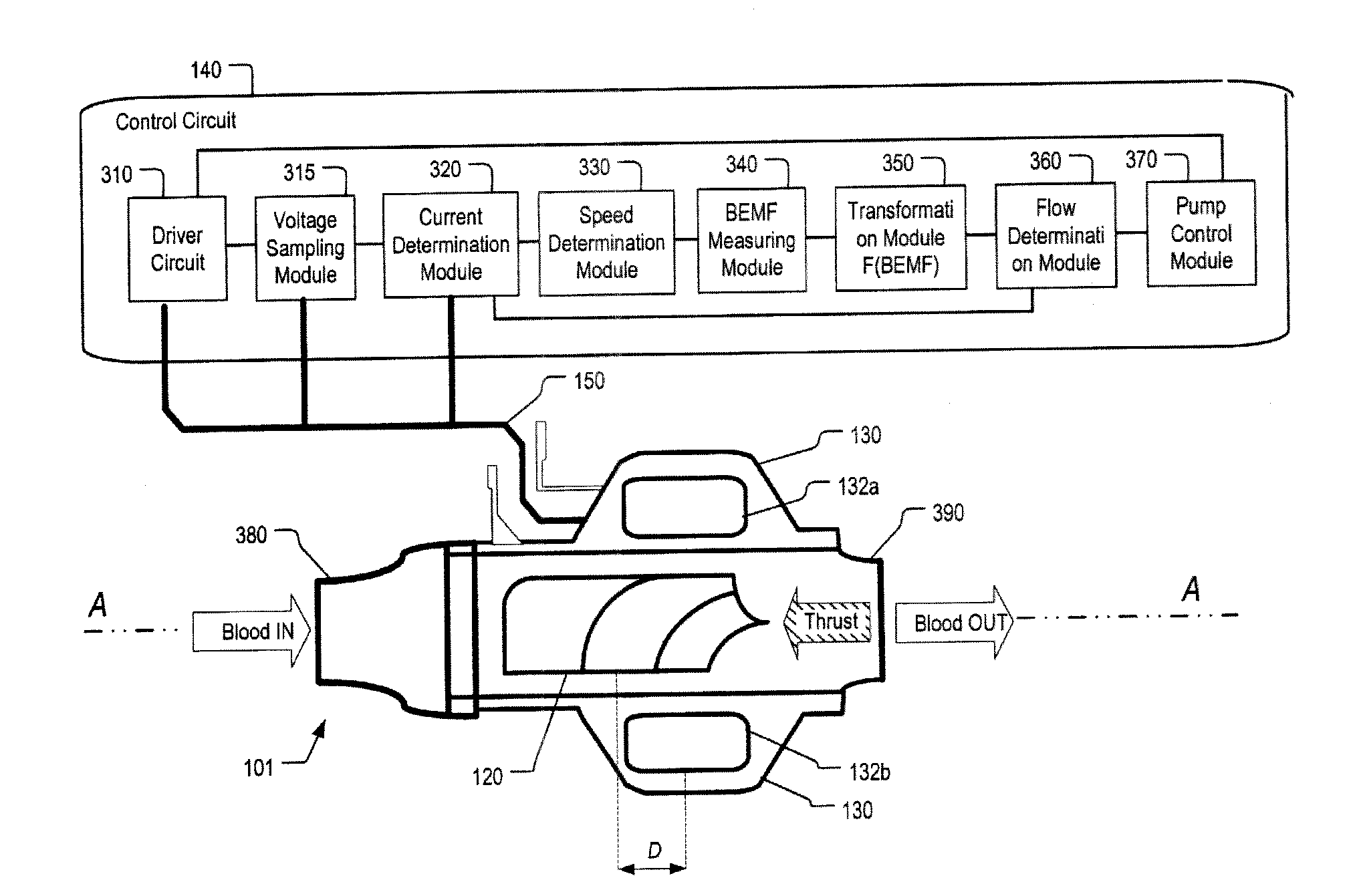
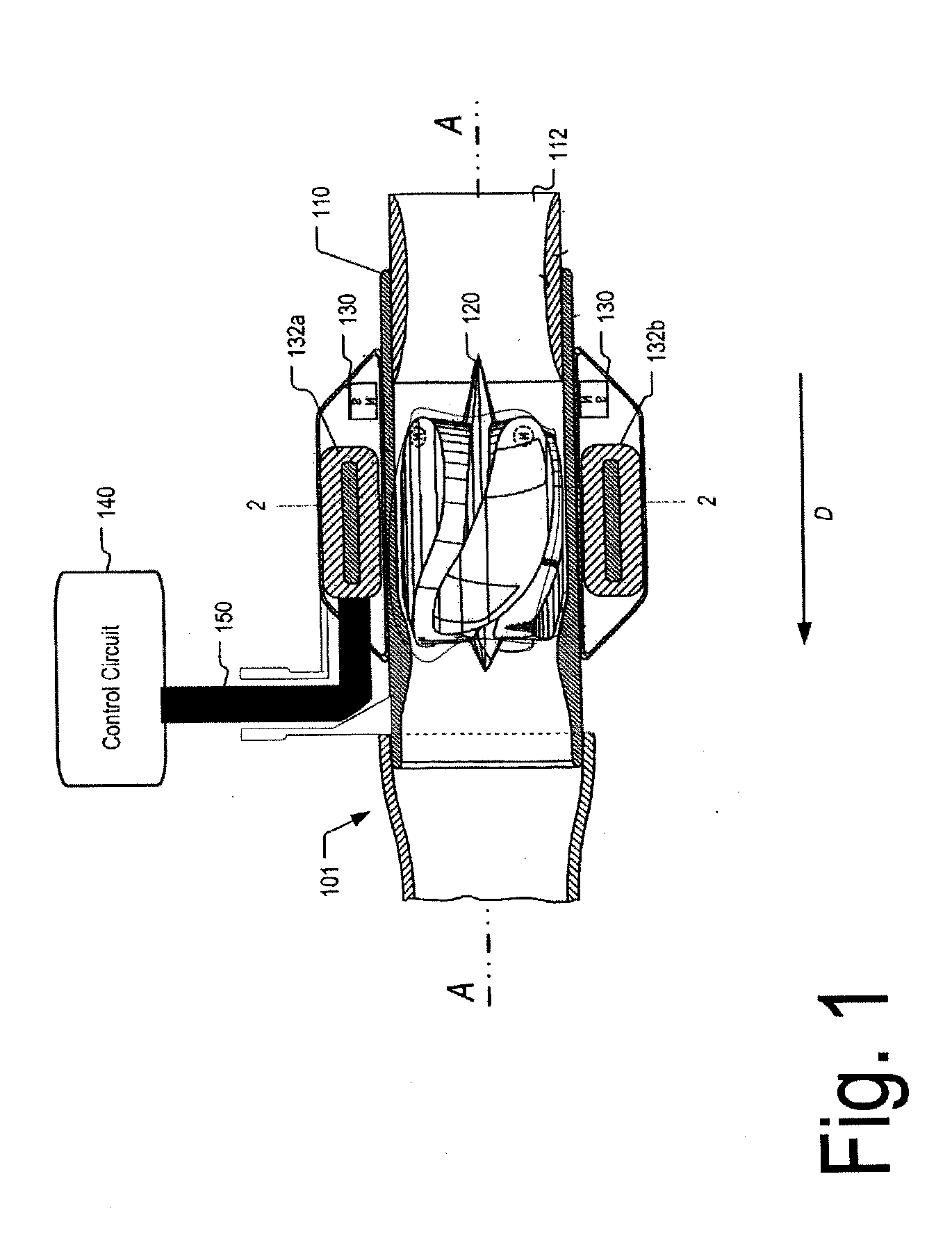
Flow estimation in a blood pump
The flow rate of blood in an implantable blood pump is determined at least in part based on a parameter related to thrust on the rotor of the pump. The parameter may be a parameter related to displacement of the rotor along its axis, such as a function of the back electromotive force generated in one or more coils of the stator. The back electromotive force may be measured during open-phase periods of a particular coil or set of coils, during which no power is applied to the coil or set of coils by the motor drive circuit. The parameter related to thrust may be used in conjunction with the speed of rotation of the rotor, the magnitude of current supplied to the rotor, or both to determine the flow rate. The pump may be controlled responsive to the determined flow rate.
Owner:HEARTWARE INC
Single port cardiac support apparatus
Owner:MAQUET CARDIOVASCULAR LLC
Controller and power source for implantable blood pump
Methods and apparatus for controlling the operation of, and providing power for and to, implantable ventricular assist devices which includes a pump employing a brushless DC motor-driven blood pump, are disclosed. In one embodiment, a control system for driving an implantable blood pump is provided. The digital processor is responsive to data associated with the operation of the pump received at the data transfer pump, and from program data stored in the memory, (i) to determine therefrom, the identity of the pump, (ii) to determine therefrom, electrical characteristics and features of the identified pump, and (iii) to adaptively generate and apply to the data port, control signals for driving the identified pump.
Owner:HEARTWARE INC
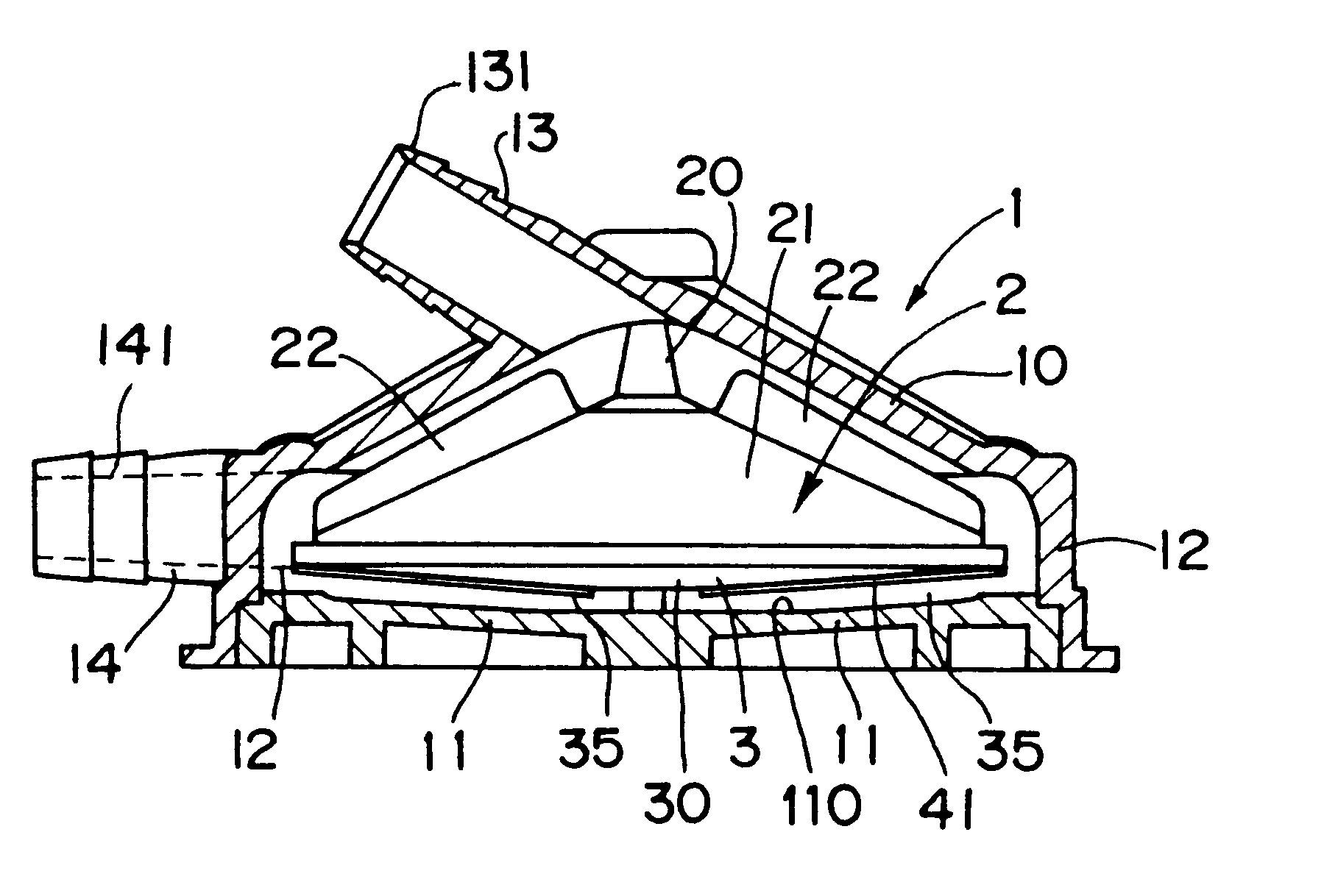
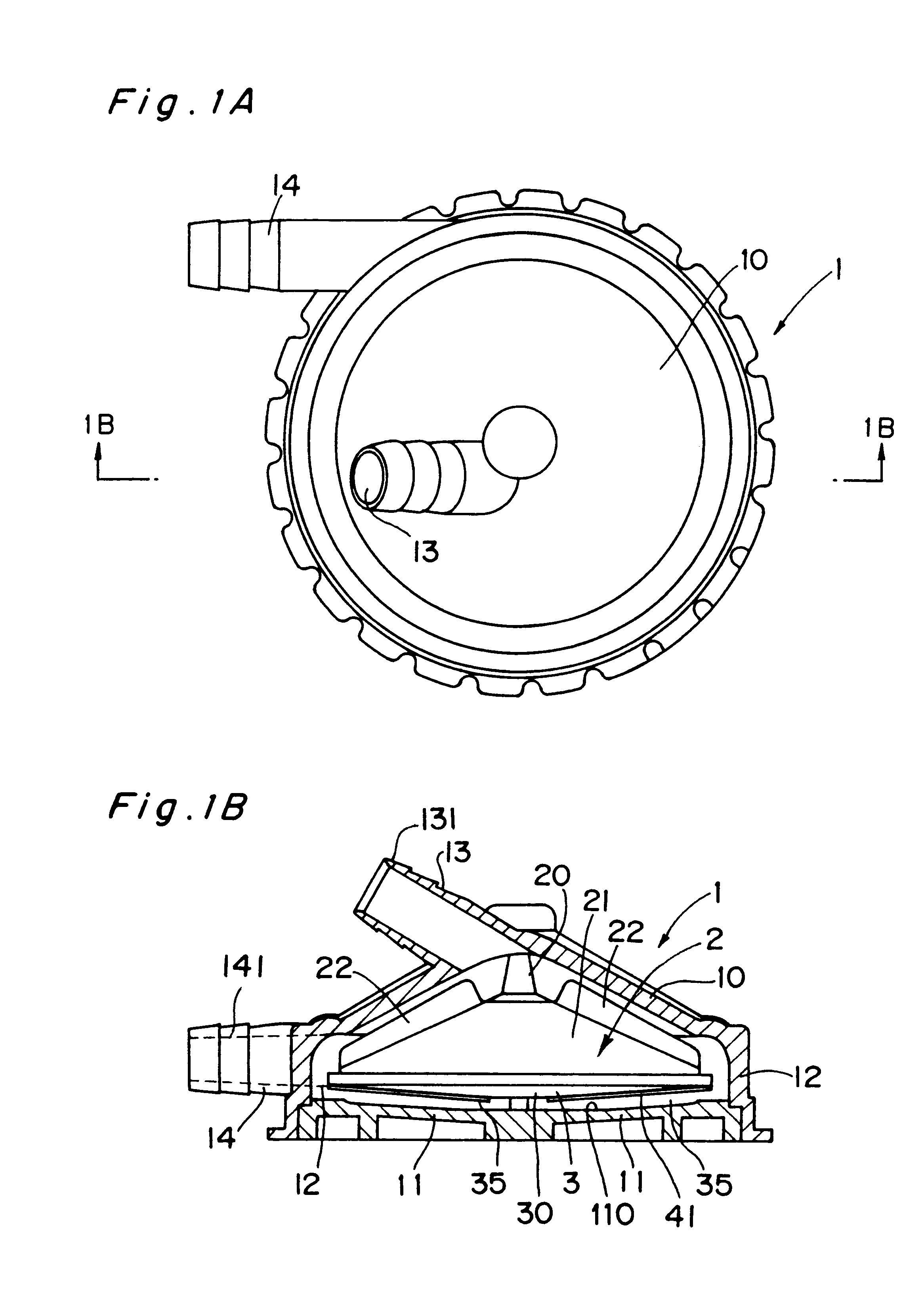
Centrifugal blood pump
The present invention provides a blood pump capable of substantially completely preventing thrombus from attaching to the inner bottom portion of the casing without lowering the anti-hemolytic characteristic of blood. A centrifugal blood pump in accordance with the present invention comprises a pump casing, a suction inlet disposed at the central portion on the upper side of the casing, a delivery outlet disposed at the bottom peripheral portion of the casing, a main impeller (D in diameter) for forming a centrifugal flow of blood supplied from the suction inlet in the range from the central portion to the peripheral portion to feed the blood to the delivery outlet, wherein the main impeller is provided with an stirring impeller, the surface of which is provided with one or more stirring elements (L in entire length) having the shape of a fin or a groove, and the dimensions of the stirring elements are determined to satisfy inequality (1): 0.43<LD<1.30 and inequality (2) 0.03<S / A<0.21, where S is the surface area of the blood contact surfaces of the stirring elements.
Owner:KYOCERA CORP +1
Centrifugal rotary blood pump with impeller having a hydrodynamic thrust bearing surface
ActiveUS8152493B2Facilitate the fit of the blood pumpSpeed up the flowPump componentsControl devicesImpellerMagnetic tension force
A rotary blood pump may include one or more motor stators overlying exterior surfaces of a wall defining a pumping chamber. A rotatable impeller within the pumping chamber may have a hydrodynamic thrust bearing surface adapted to constrain a position of the impeller along an axis of rotation relative to the wall when the impeller is rotating about the axis of rotation. The impeller position may then be constrained without requiring a constant polarity magnetic force to be applied in the axial direction from a fixed position of the housing.
Owner:HEARTWARE INC
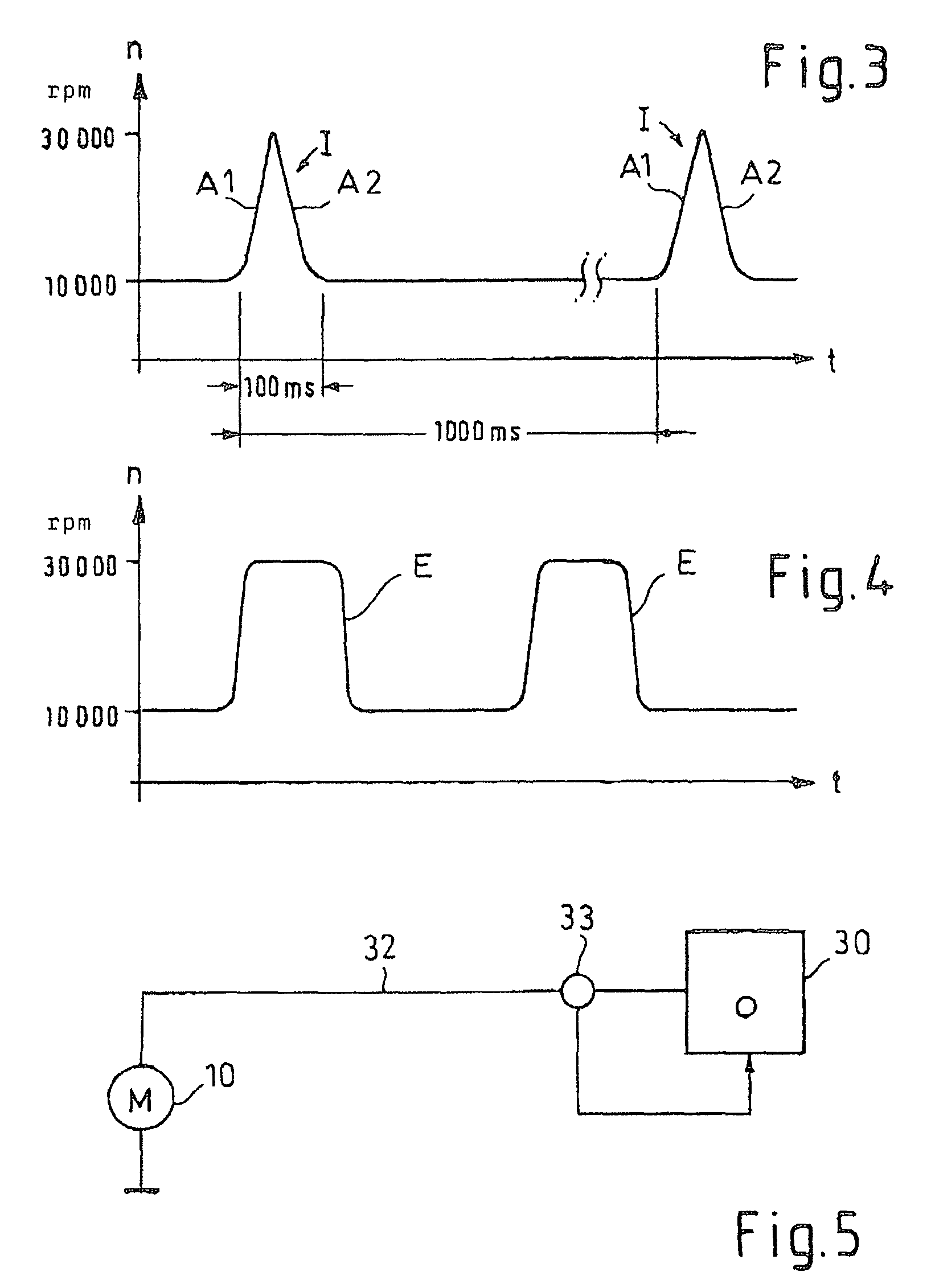
Method for controlling a blood pump
A blood pump is temporarily operated at a low rotational speed lying below a design rotational speed. This involves a risk of thrombogenesis since flow detachments may occur at blades of an impeller of the rotary blood pump. For eliminating deposits at said impeller, the rotational speed of said pump is temporarily increased to the design rotational speed. Alternatively, said pump alternately operates at said design rotational speed and a low rotational speed, and this pulsed operation is synchronized with the heart rate.
Owner:ABIOMED EURO
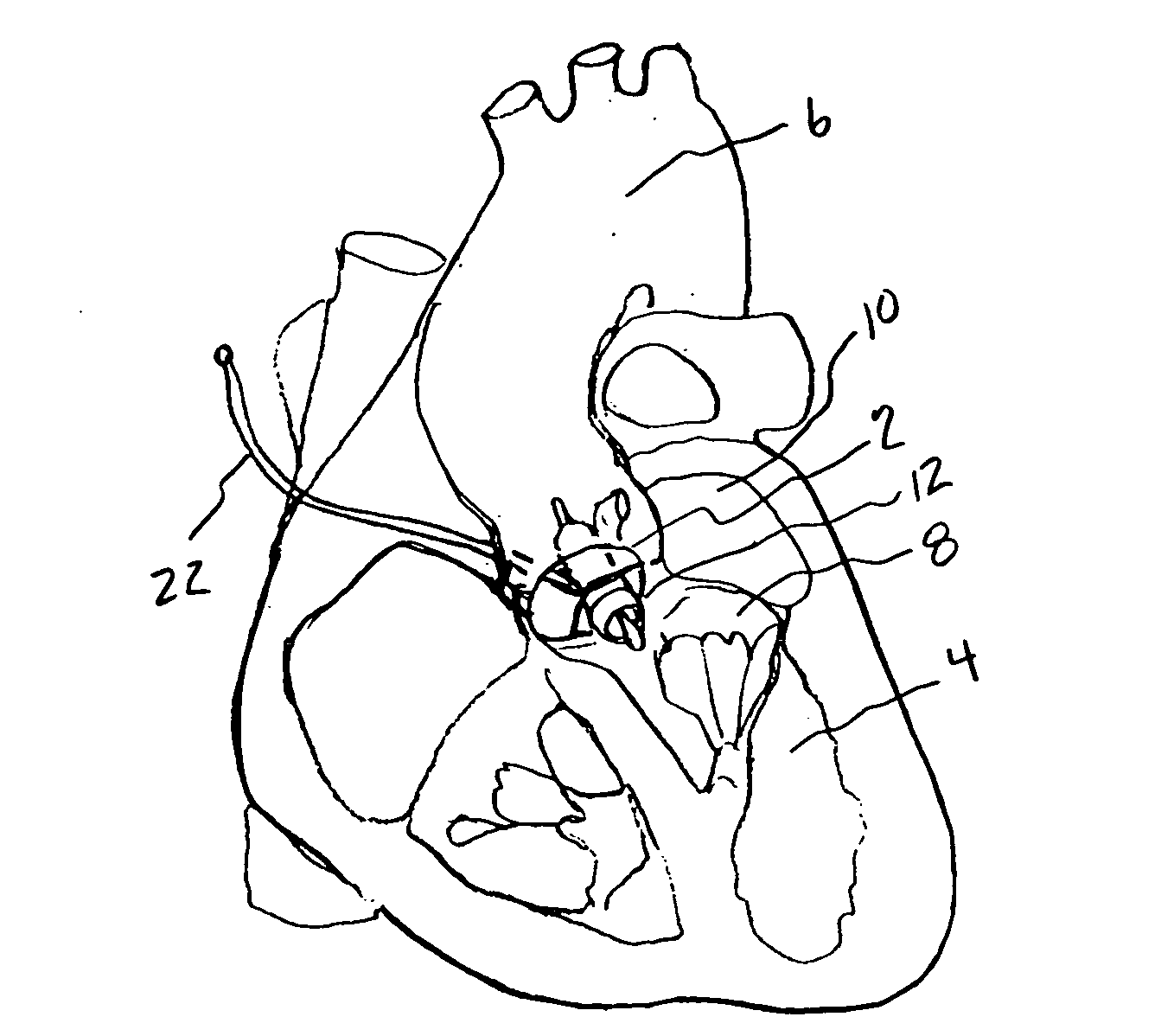
Minimally invasive transvalvular ventricular assist device
ActiveUS20060195004A1Highly effectiveHighly miniaturizedAdditive manufacturing apparatusBlood pumpsThree dimensional ctVentricular cavity
A tiny electrically powered hydrodynamic blood pump is disclosed which occupies one third of the aortic or pulmonary valve position, and pumps directly from the left ventricle to the aorta or from the right ventricle to the pulmonary artery. The device is configured to exactly match or approximate the space of one leaflet and sinus of valsalva, with part of the device supported in the outflow tract of the ventricular cavity adjacent to the valve. In the configuration used, two leaflets of the natural tri-leaflet valve remain functional and the pump resides where the third leaflet had been. When implanted, the outer surface of the device includes two faces against which the two valve leaflets seal when closed. To obtain the best valve function, the shape of these faces may be custom fabricated to match the individual patient's valve geometry based on high resolution three dimensional CT or MRI images. Another embodiment of the invention discloses a combined two leaflet tissue valve with the miniature blood pump supported in the position usually occupied by the third leaflet. Either stented or un-stented tissue valves may be used. This structure preserves two thirds of the valve annulus area for ejection of blood by the natural ventricle, with excellent washing of the aortic root and interface of the blood pump to the heart. In the aortic position, the blood pump is positioned in the non-coronary cusp. A major advantage of the transvalvular VAD is the elimination of both the inflow and outflow cannulae usually required with heart assist devices.
Owner:JARVIK ROBERT
Blood pump
A blood pump (26) includes a stator assembly including a fluid inlet (24) and a fluid outlet (26). A rotor assembly (120) includes an impeller (40) rotatable about an axis (44) to move fluid from the inlet (24) to the outlet (26). A motor (50) imparts rotation of the impeller (40) about the axis (44). The motor (50) includes a motor stator (52) fixed to the stator assembly (122), a motor rotor (54) fixed to the rotor assembly (120), and a radial motor gap (34) between the stator (52) and the rotor (54). The pump (20) is configured to direct a mixed blood flow from the fluid inlet (24) to the fluid outlet (26) and a wash flow through the motor gap (34).
Owner:THE CLEVELAND CLINIC FOUND +1
Streamlined unobstructed one-pass axial-flow pump
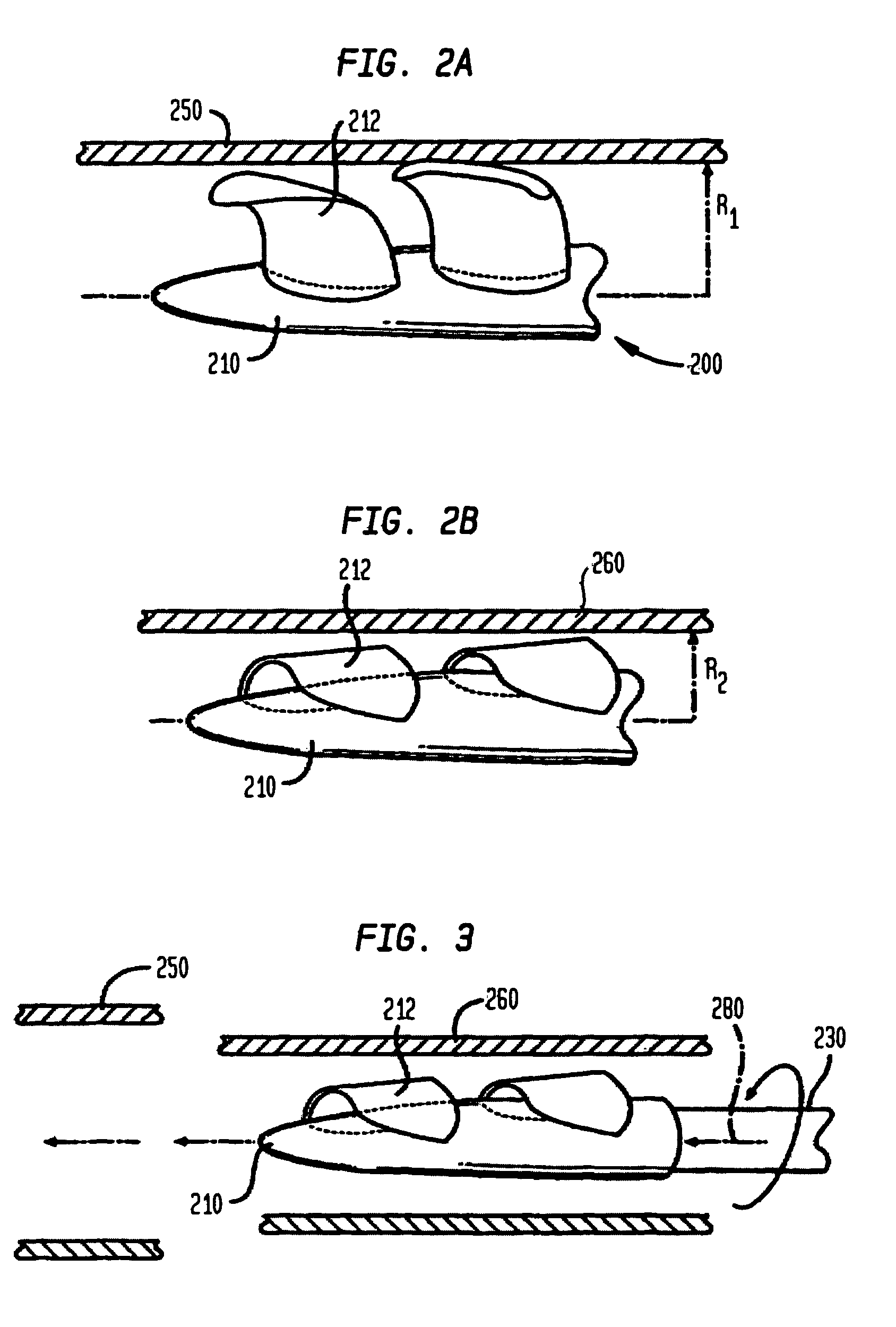
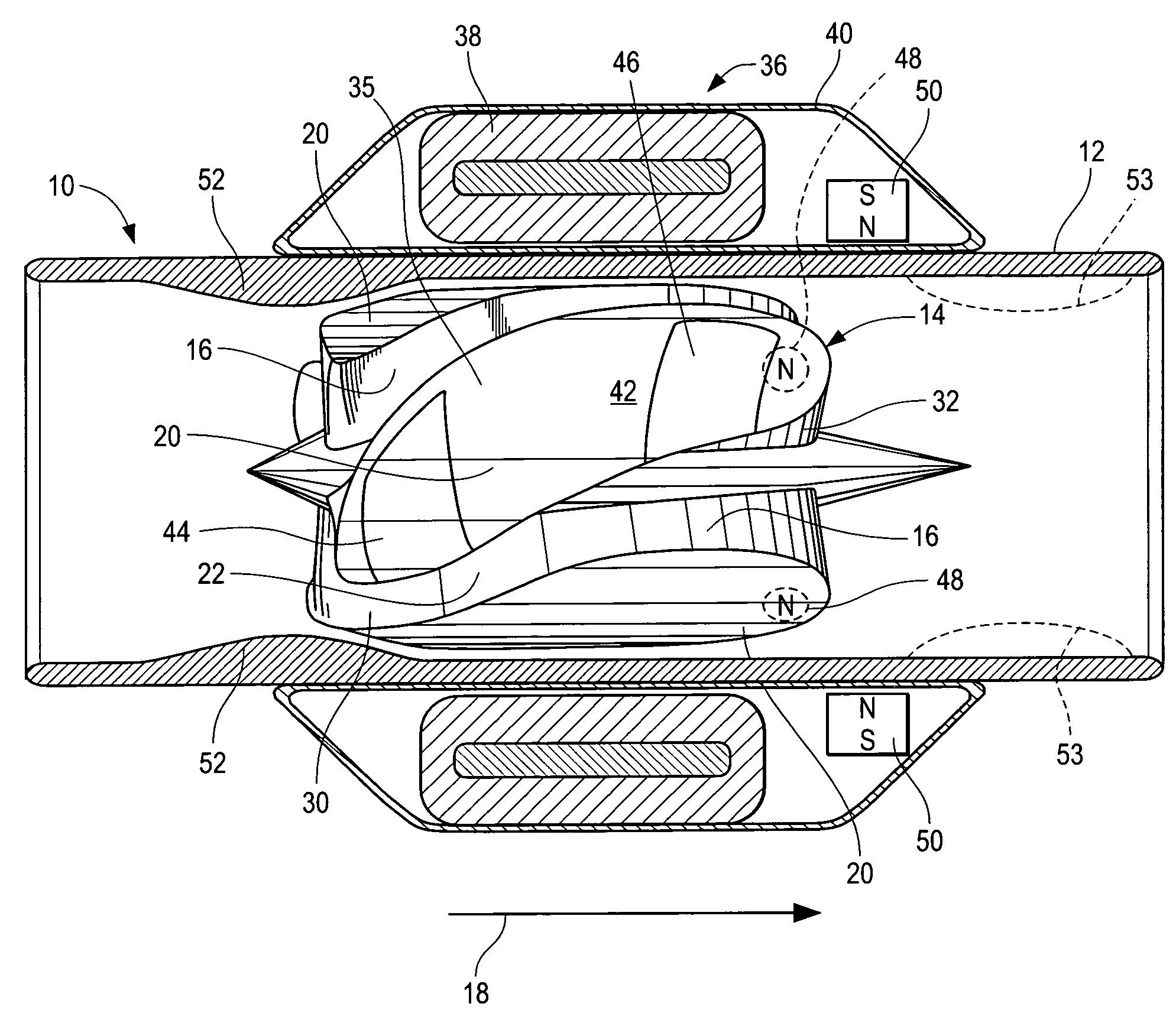
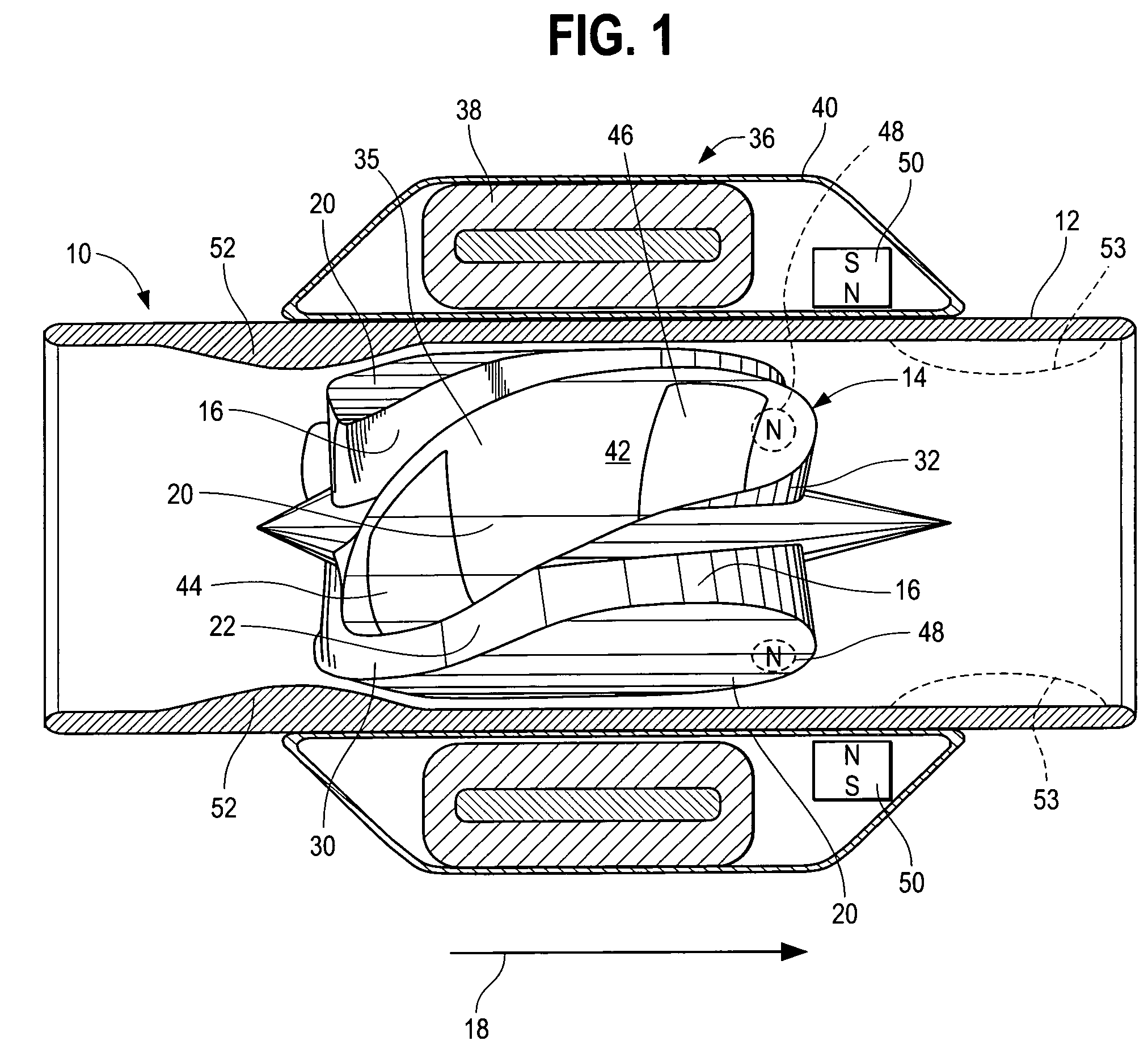
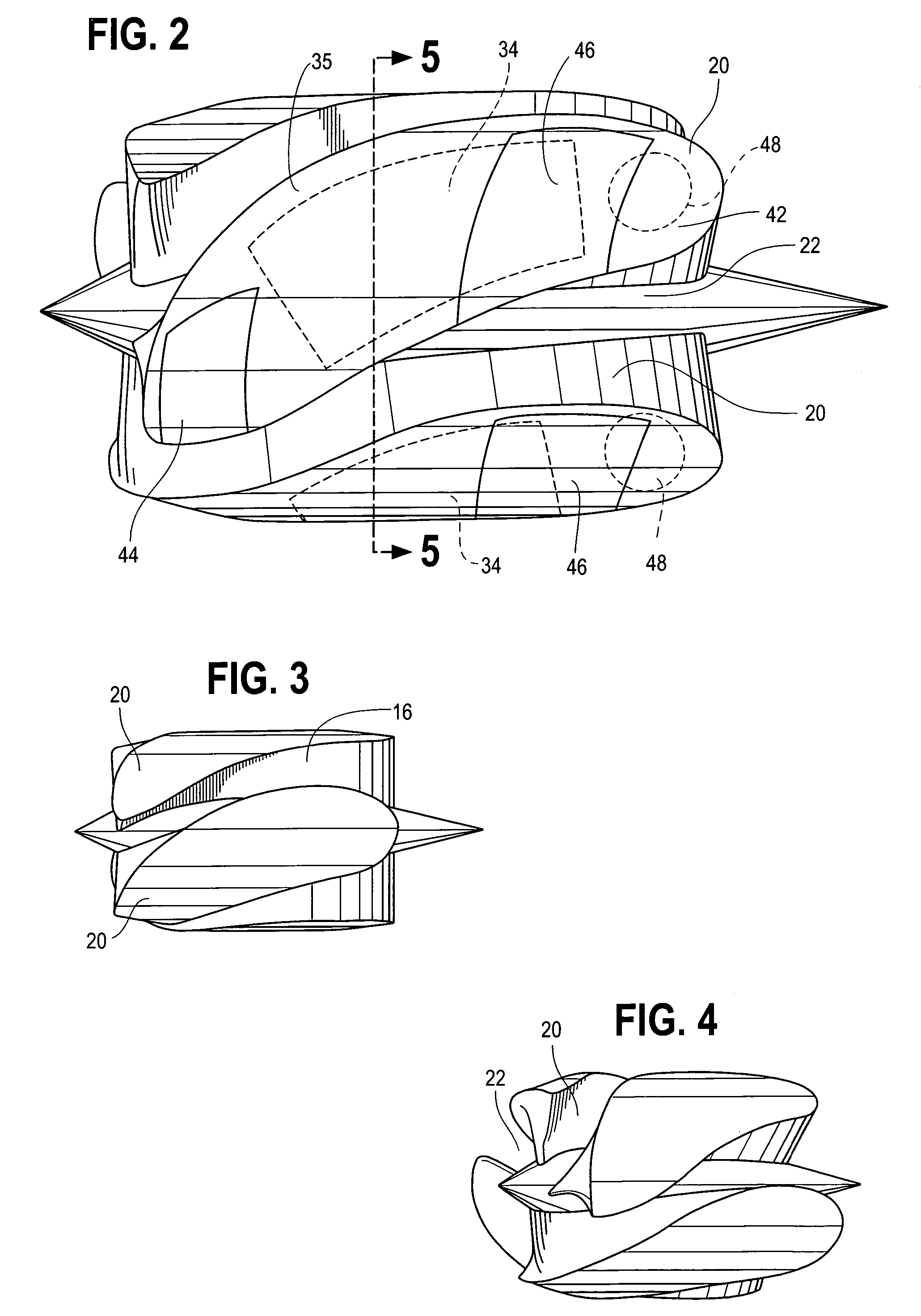
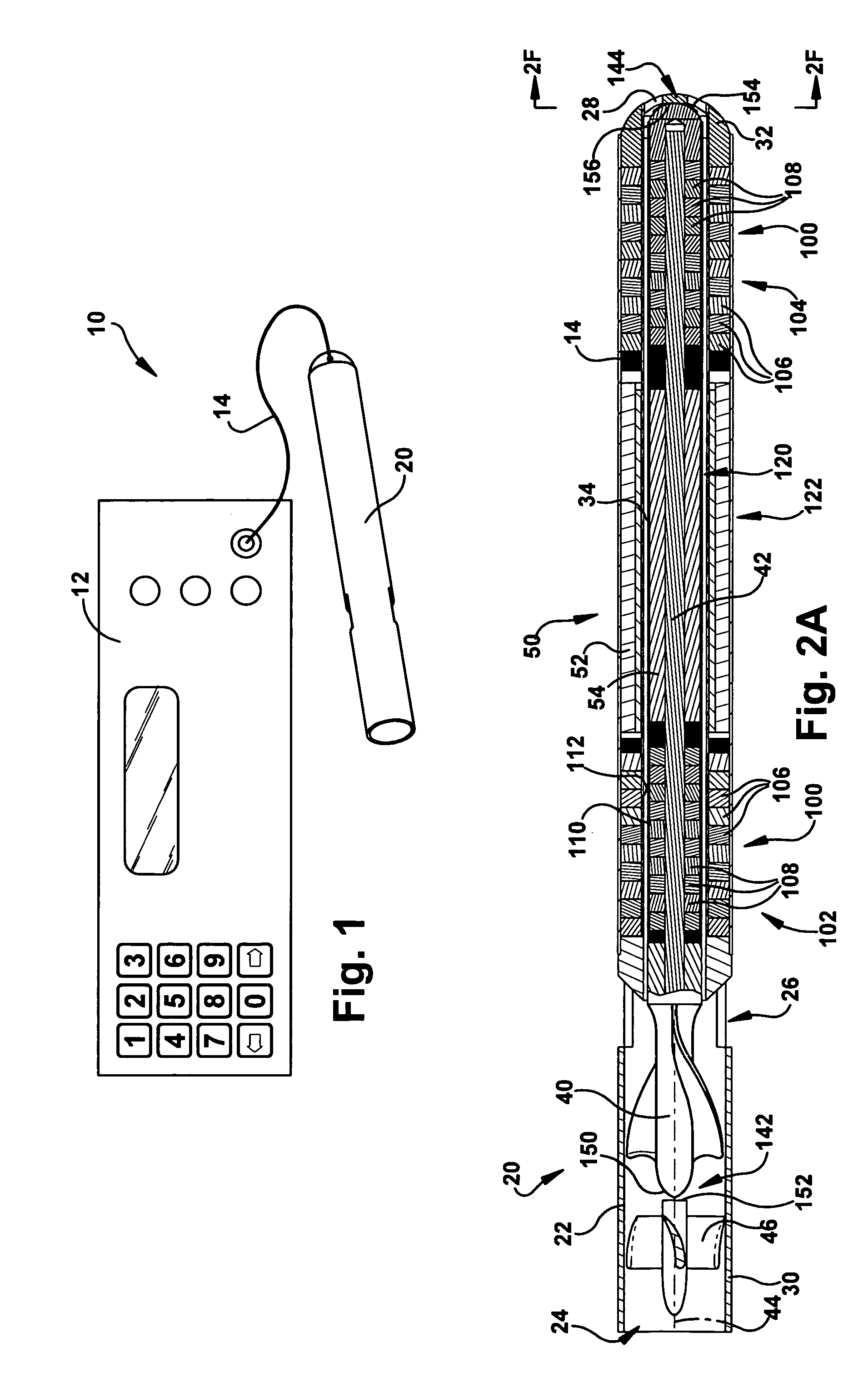
InactiveUS7229258B2Small sizeIncreased operating lifeBlood pumpsIntravenous devicesAxial-flow pumpOne pass
A blood pump has an impeller rotatably disposed and magnetically suspended within a cavity of a stator by a plurality of magnetic bearings (passive permanent and active electromagnetic) having impeller magnets on the impeller and stator magnets or coils / poles on the stator. A motor includes impeller magnets on the impeller and coils / poles associated with the stator. A single, annular blood flow path extends axially through the cavity between the impeller and the stator, and between the impeller magnets on the impeller and the stator magnets or the coils / poles on the stator.
Owner:MEDFORTE RES FOUND +1
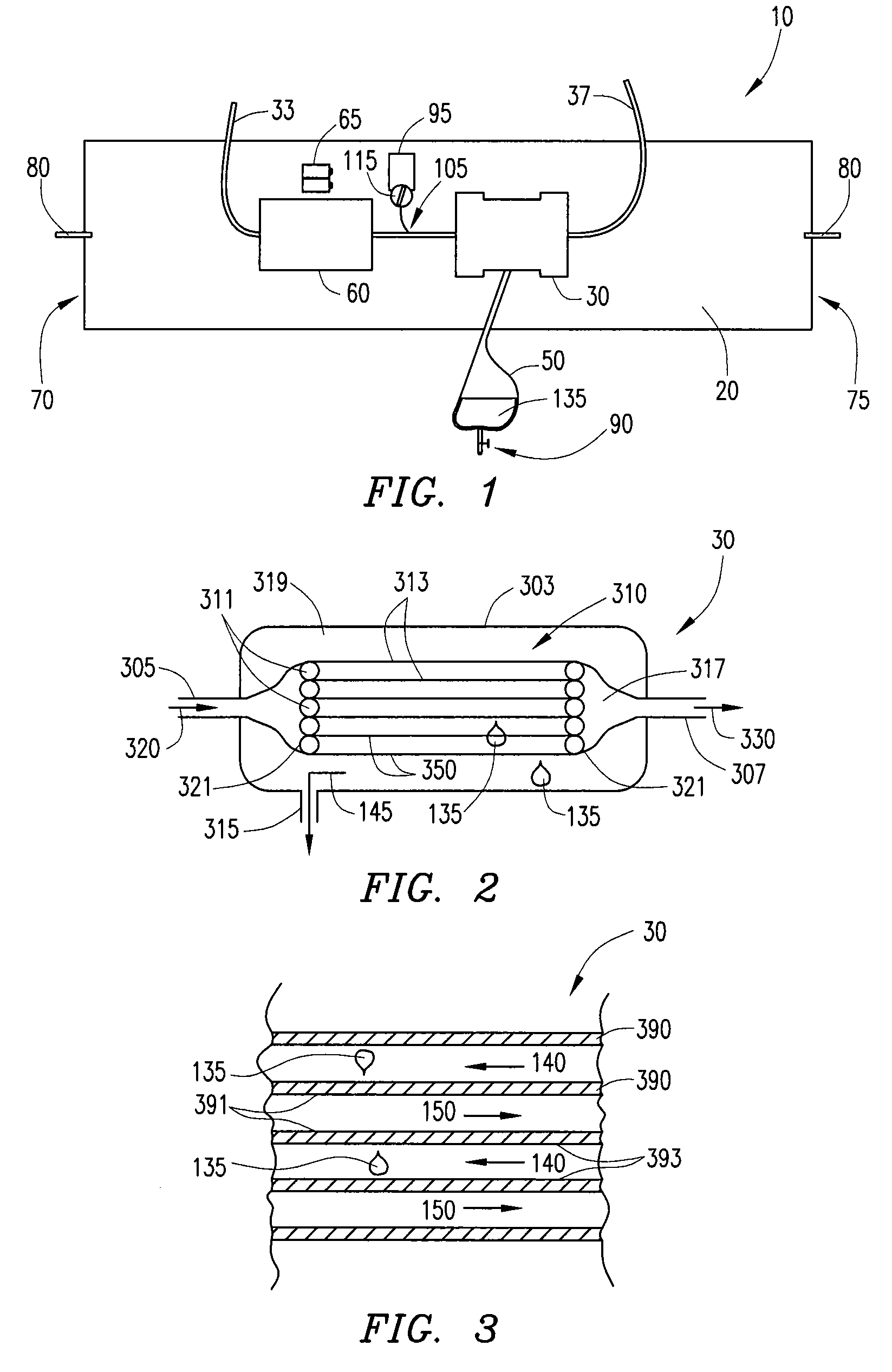
Blood pump having a disposable blood passage cartridge with integrated pressure sensors
InactiveUS6887214B1Significant limitationNo loss of accuracySemi-permeable membranesOther blood circulation devicesMedicineUltrafiltration
An integrated disposable cartridge for dialysis or ultrafiltration treatment of blood is disclosed that includes integral miniature pressure sensors. Sensors are embedded in the tubing of the cartridge to measure pressure of blood or other fluids. Cartridge elements form a continuous smooth bore passage for blood that reduces probability of clotting.
Owner:GAMBRO LUNDIA AB
Sealless rotary blood pump
InactiveUS7575423B2Minimize the possibilityShortening its residence timePump componentsMedical devicesImpellerThrust bearing
A rotary blood pump is provided which includes a pump housing and a rotor mounted for rotation with the housing. The rotor has an impeller. A rotor motor is provided including a plurality of permanent magnets carried by the impeller. A first motor stator is positioned on one side of the impeller and a second motor stator is positioned on an opposite side of the impeller. The motor stators each include a plurality of electrically conductive coils and pole pieces located within the housing. A plurality of wedge-shaped hydrodynamic thrust bearings are located outside of the axis of the rotor. During rotation of the impeller, the hydrodynamic bearings are separated from the housing by a fluid film and are not in direct mechanical contact with the housing.
Owner:THORATEC CORPORTION
Suction detection on an axial blood pump using bemf data
ActiveUS20140100413A1Increase pumping speedShorten speedControl devicesBlood pumpsPR intervalBlood pump
The presence or absence of a suction condition in an implantable blood pump is determined at least in part based on a parameter related to flow, such as a parameter related to thrust on the rotor of the pump. A local extreme of the parameter representing the minimum flow during ventricular diastole in an earlier interval is used to establish a threshold value. A value of the parameter representing the minimum flow during ventricular diastole in a later interval is compared to this threshold. If the comparison indicates a substantial decline in the minimum flow between the earlier and later intervals is associated with a suction condition. During the absence of a suction condition, the threshold is continually updated, so that the system does not indicate presence of a suction condition if the flow decreases gradually.
Owner:HEARTWARE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com