Patents
Literature
50 results about "Arteriovenous grafts" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
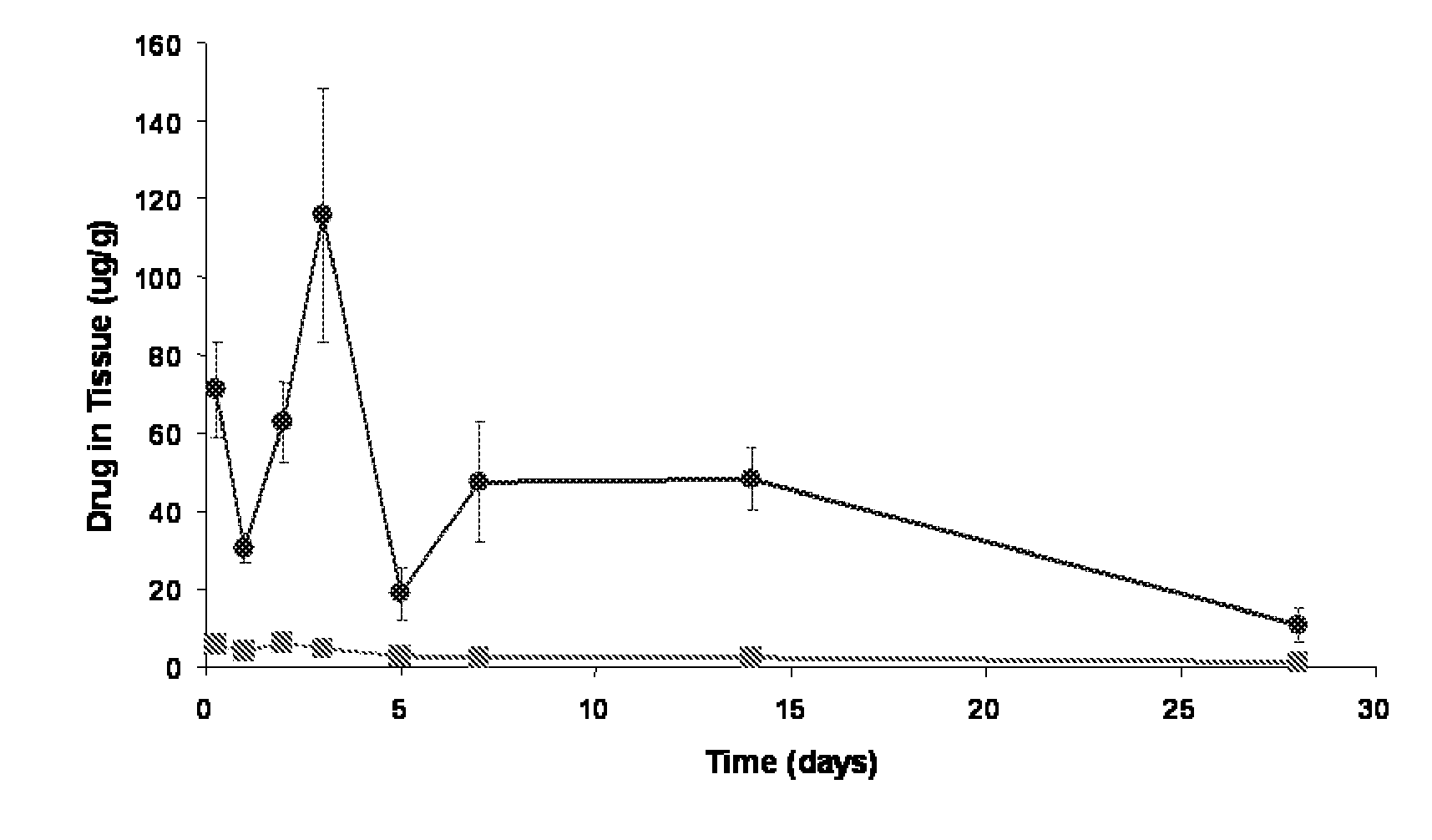
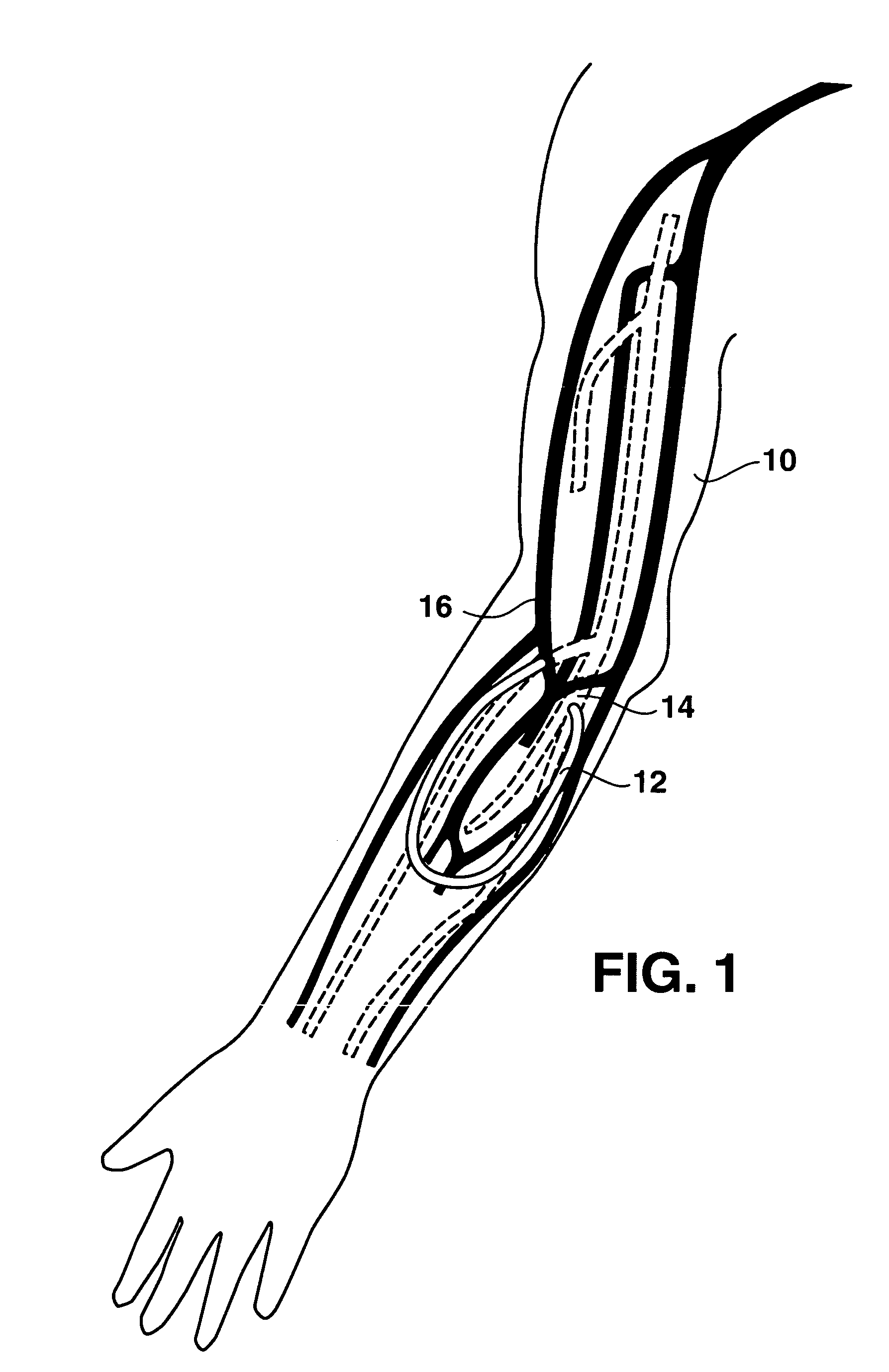
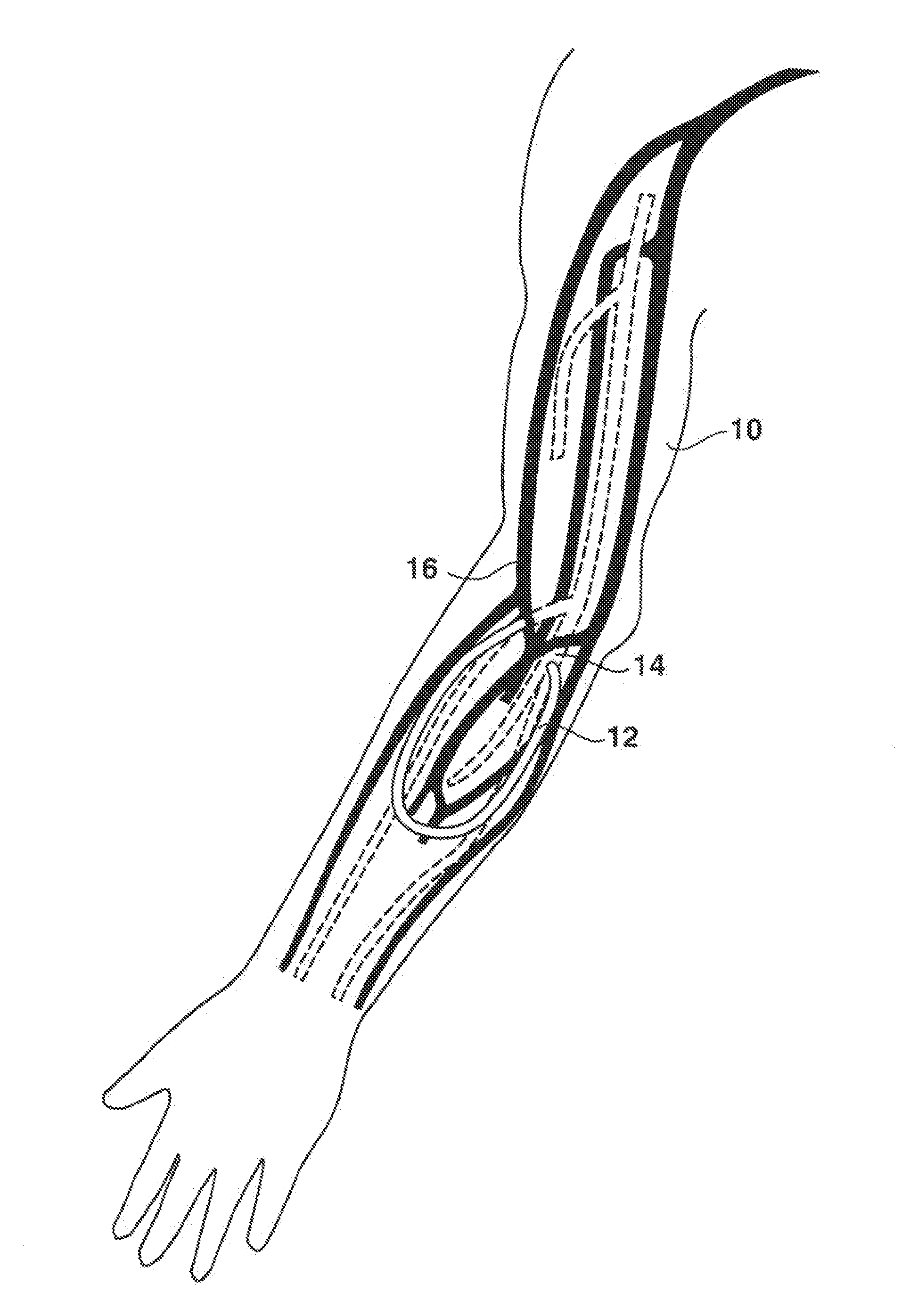
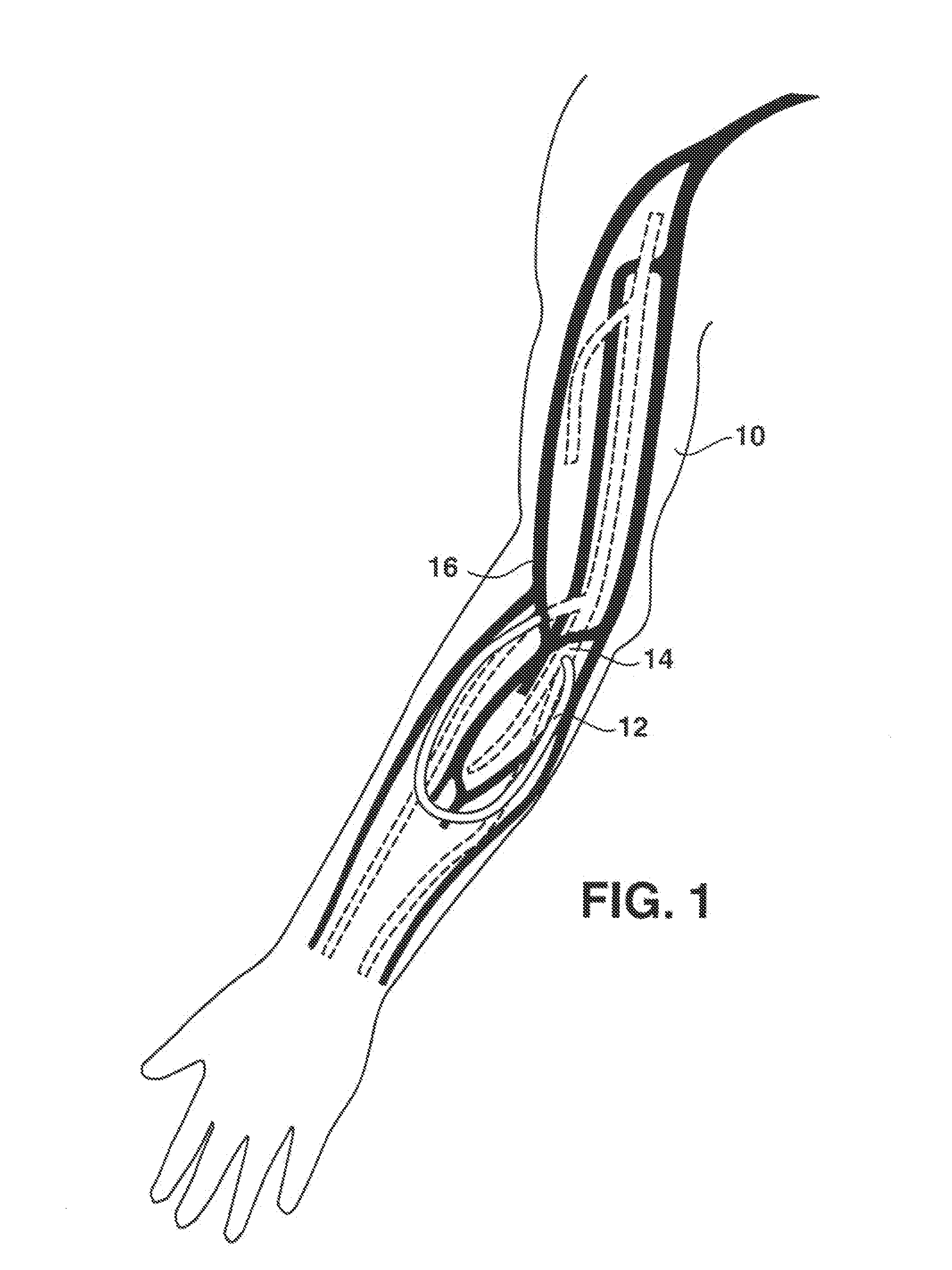
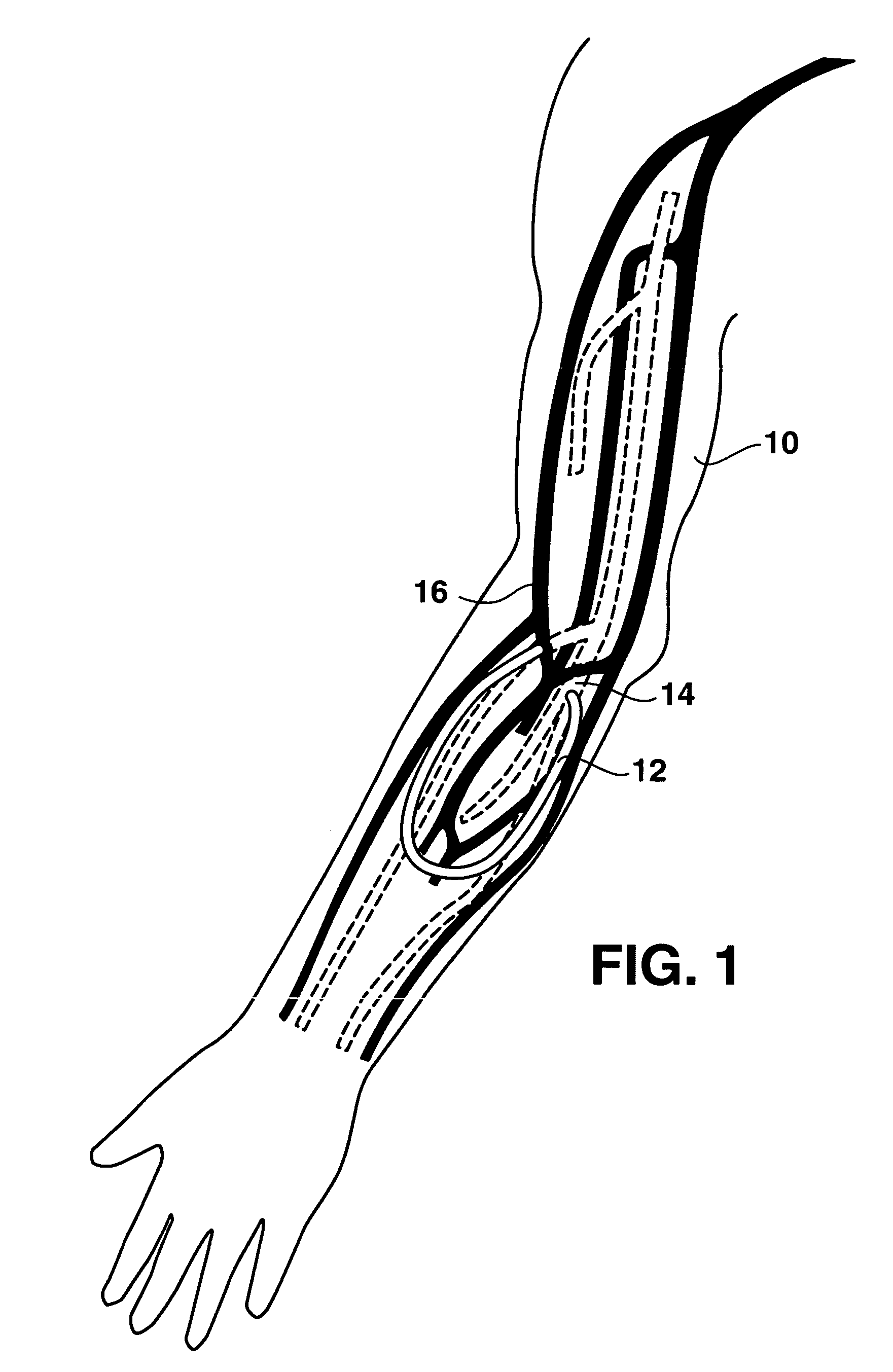
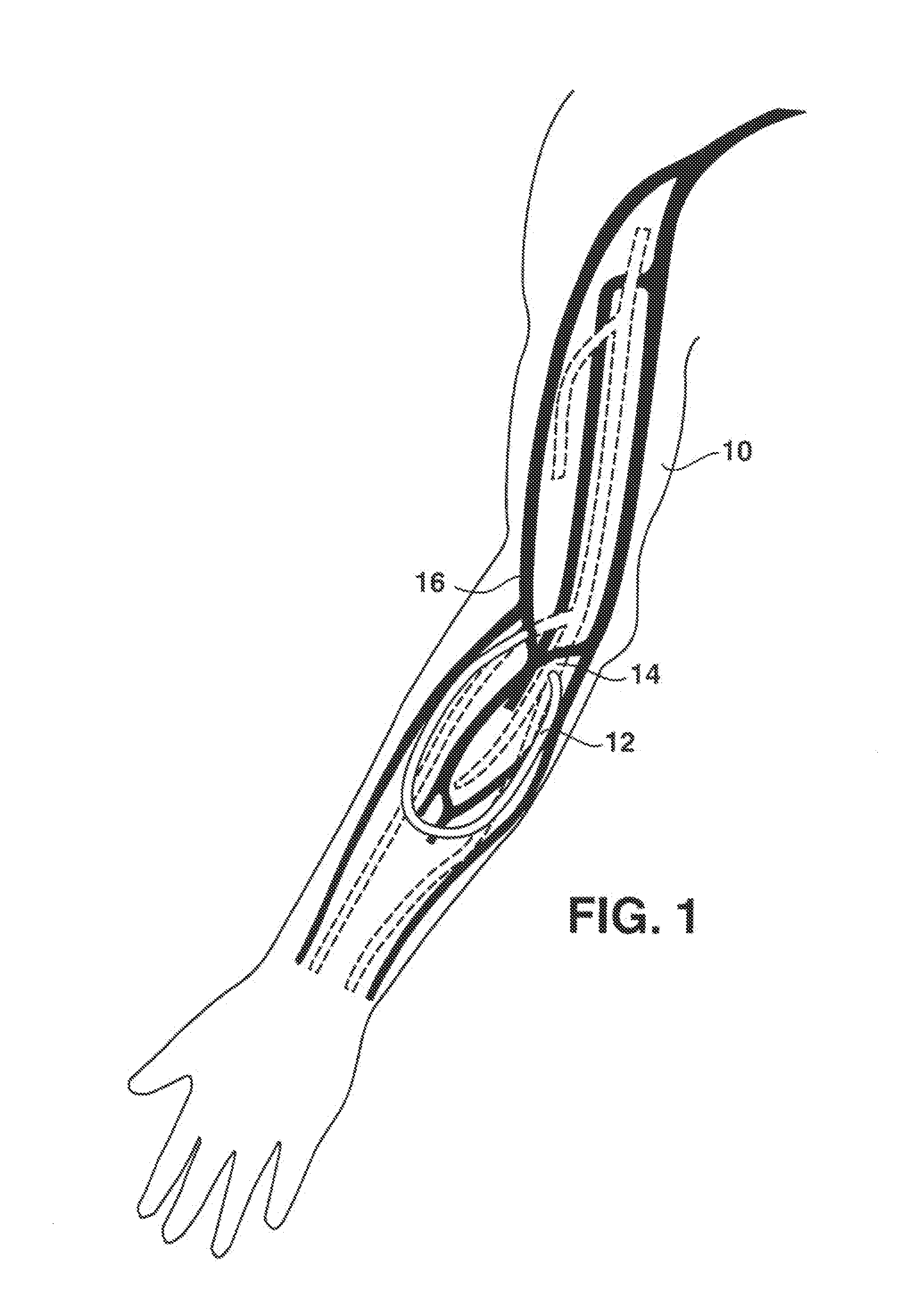
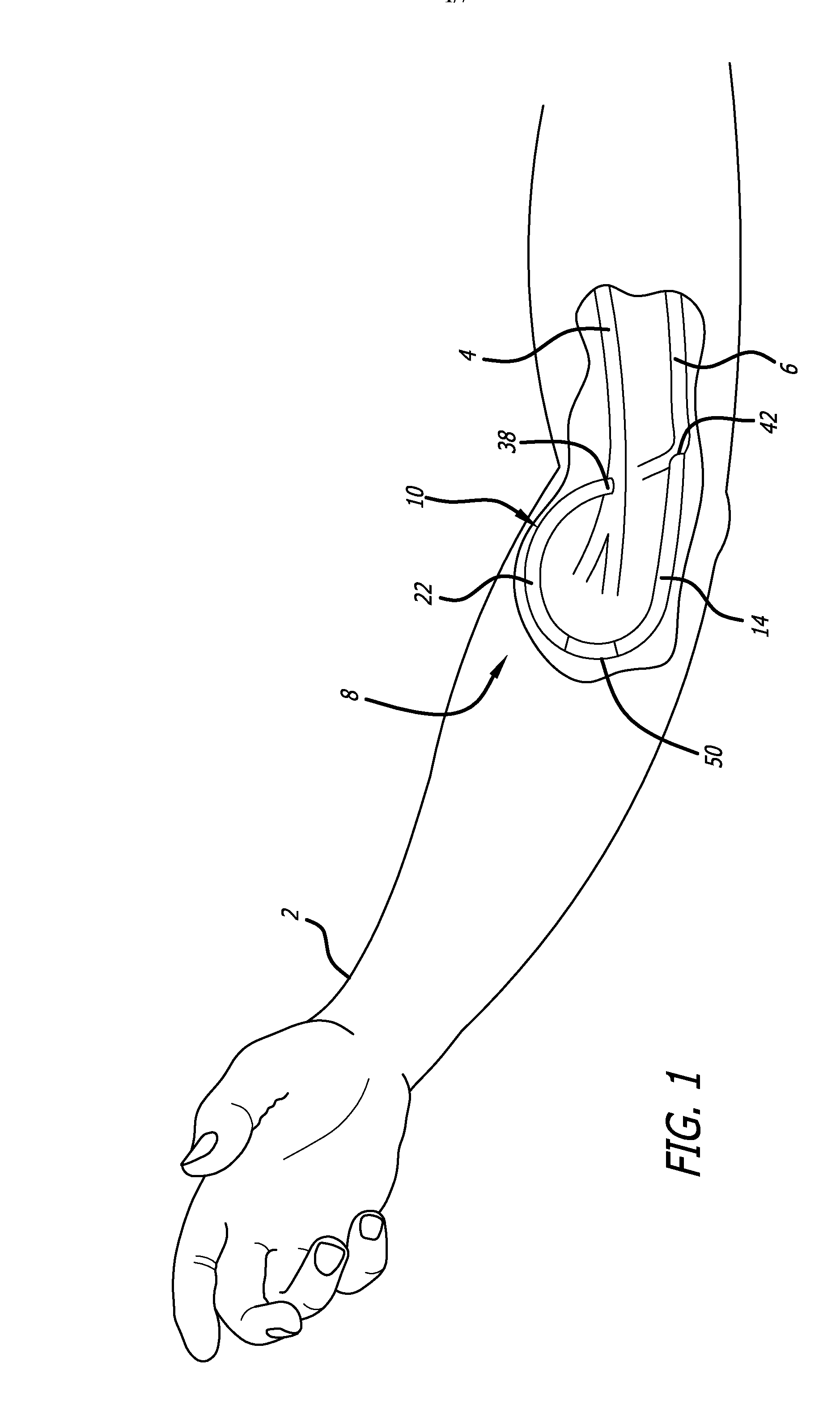
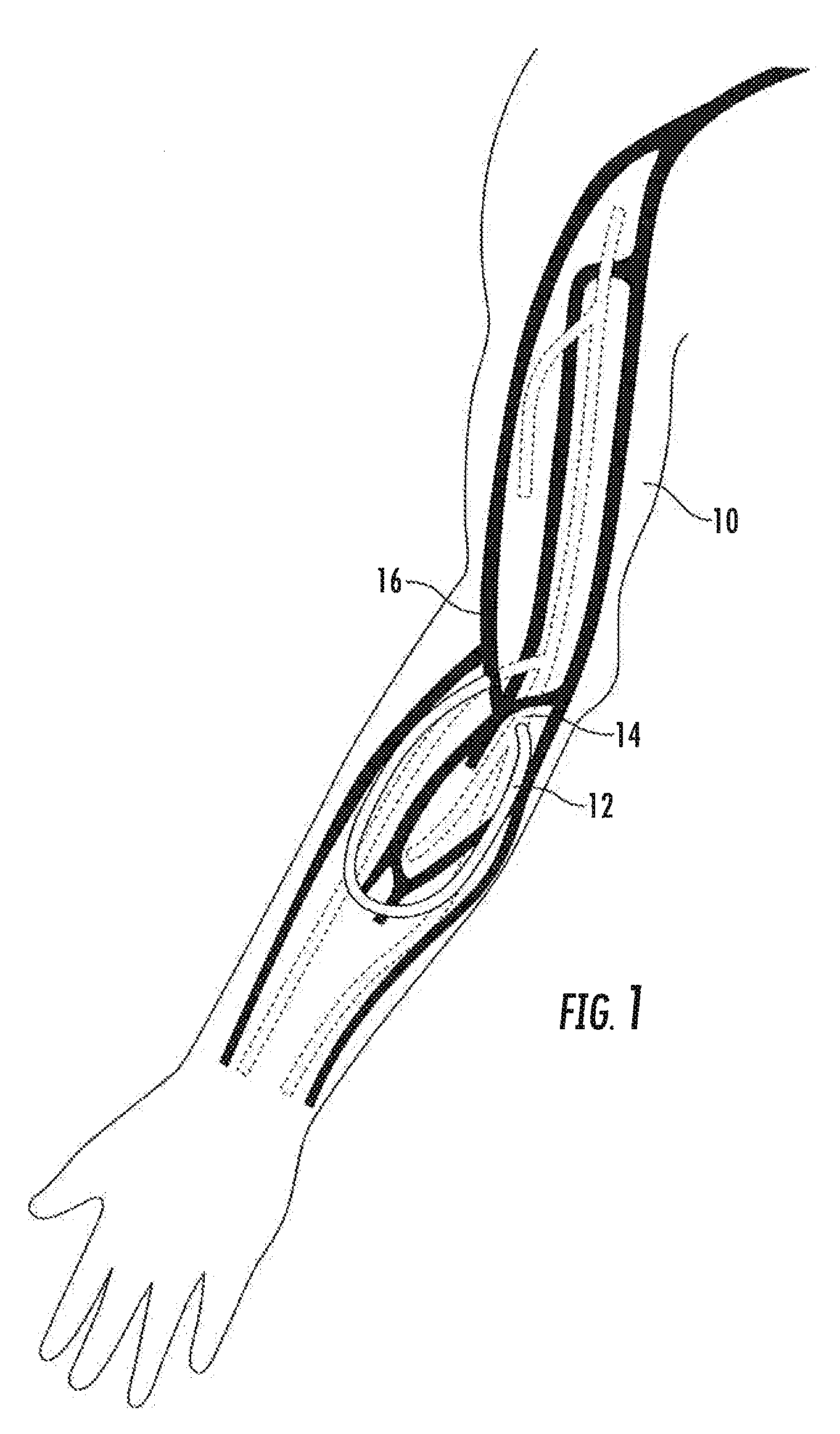
An arteriovenous (AV) graft is a type of access used for hemodialysis. The graft is usually placed in the arm, but may be placed in the leg if necessary.
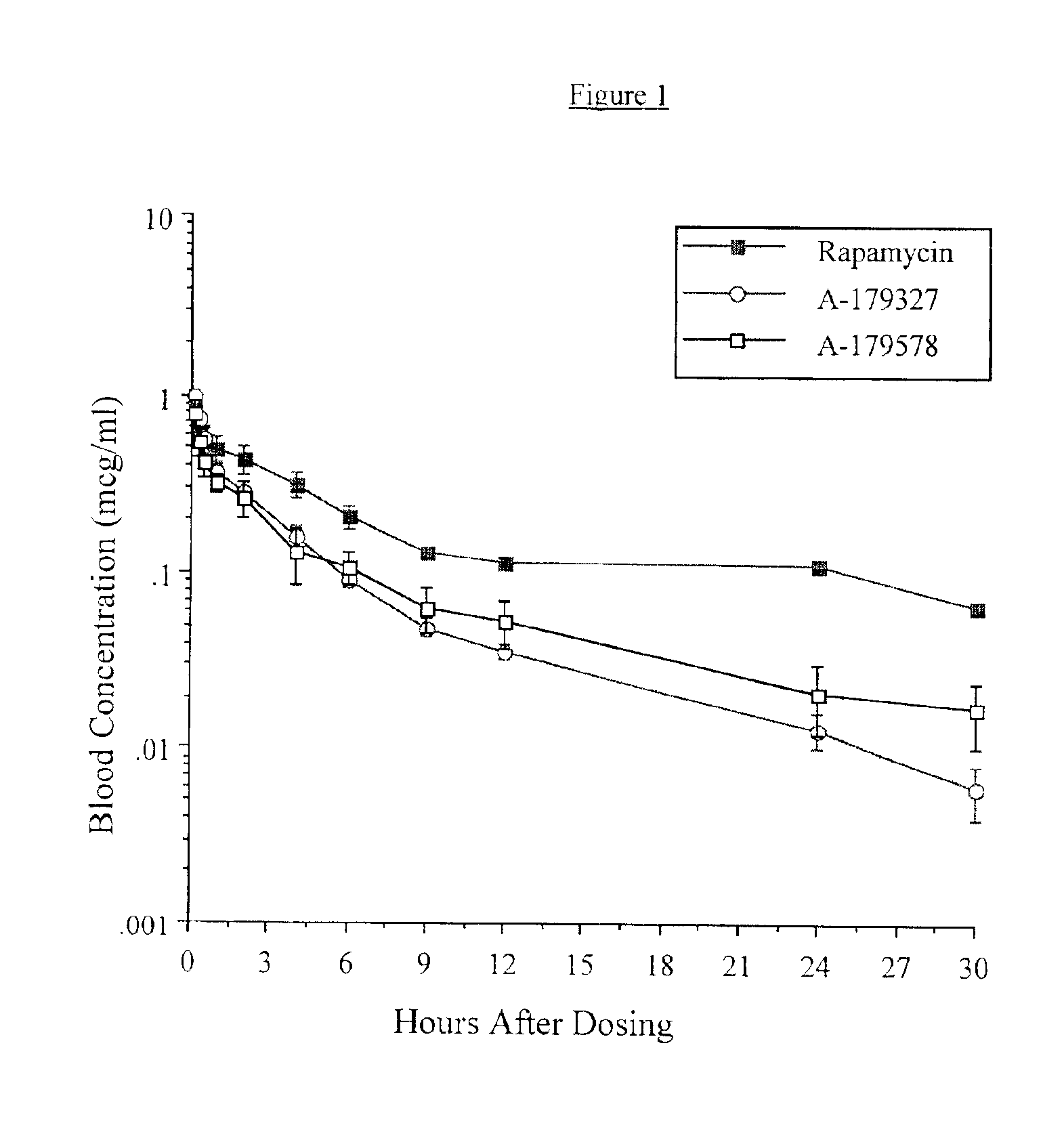
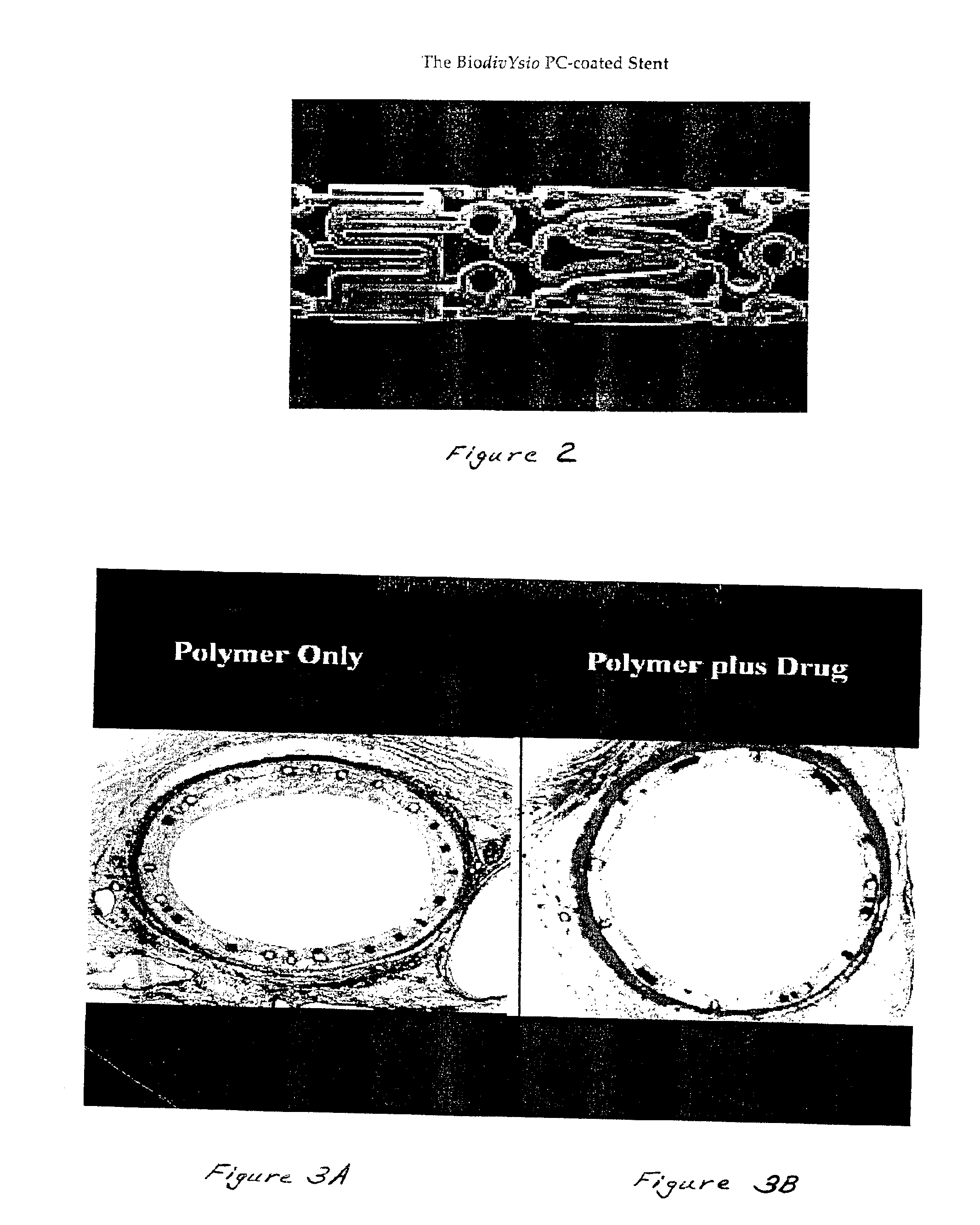
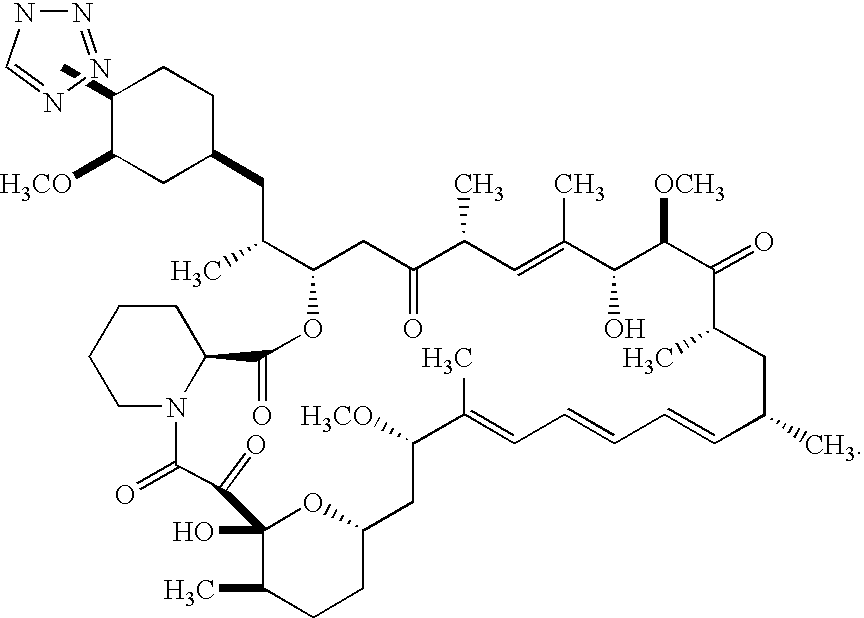
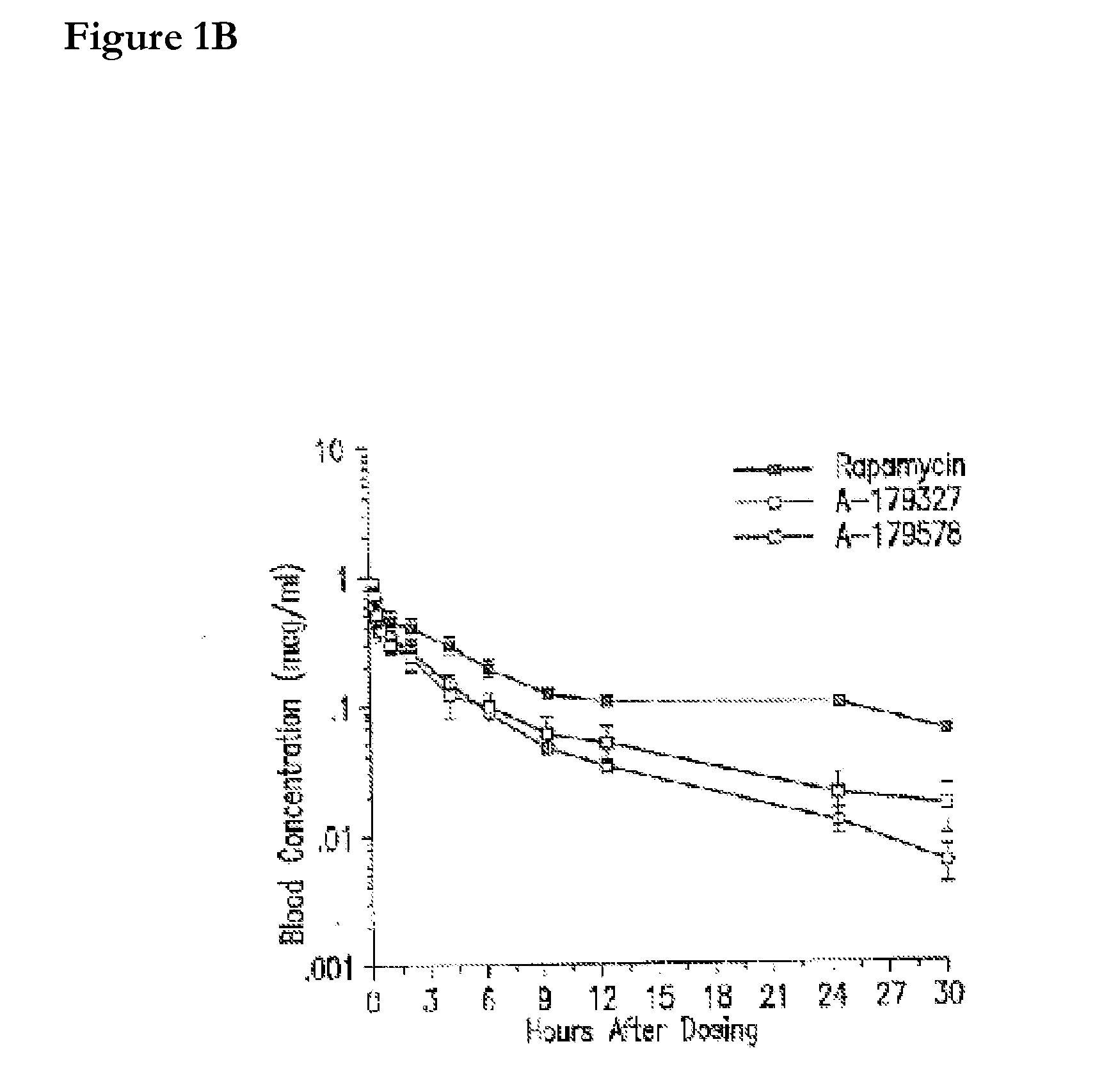
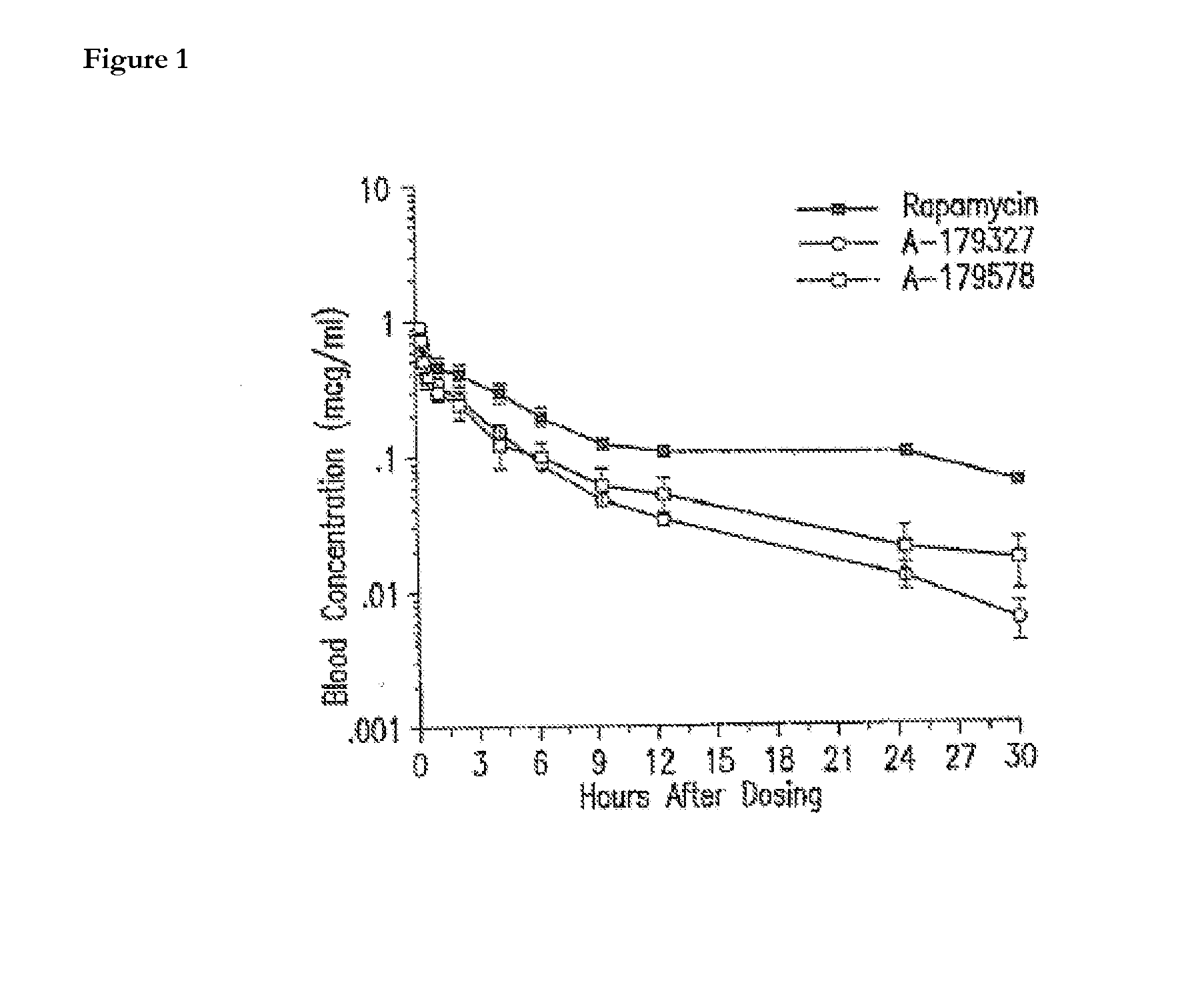
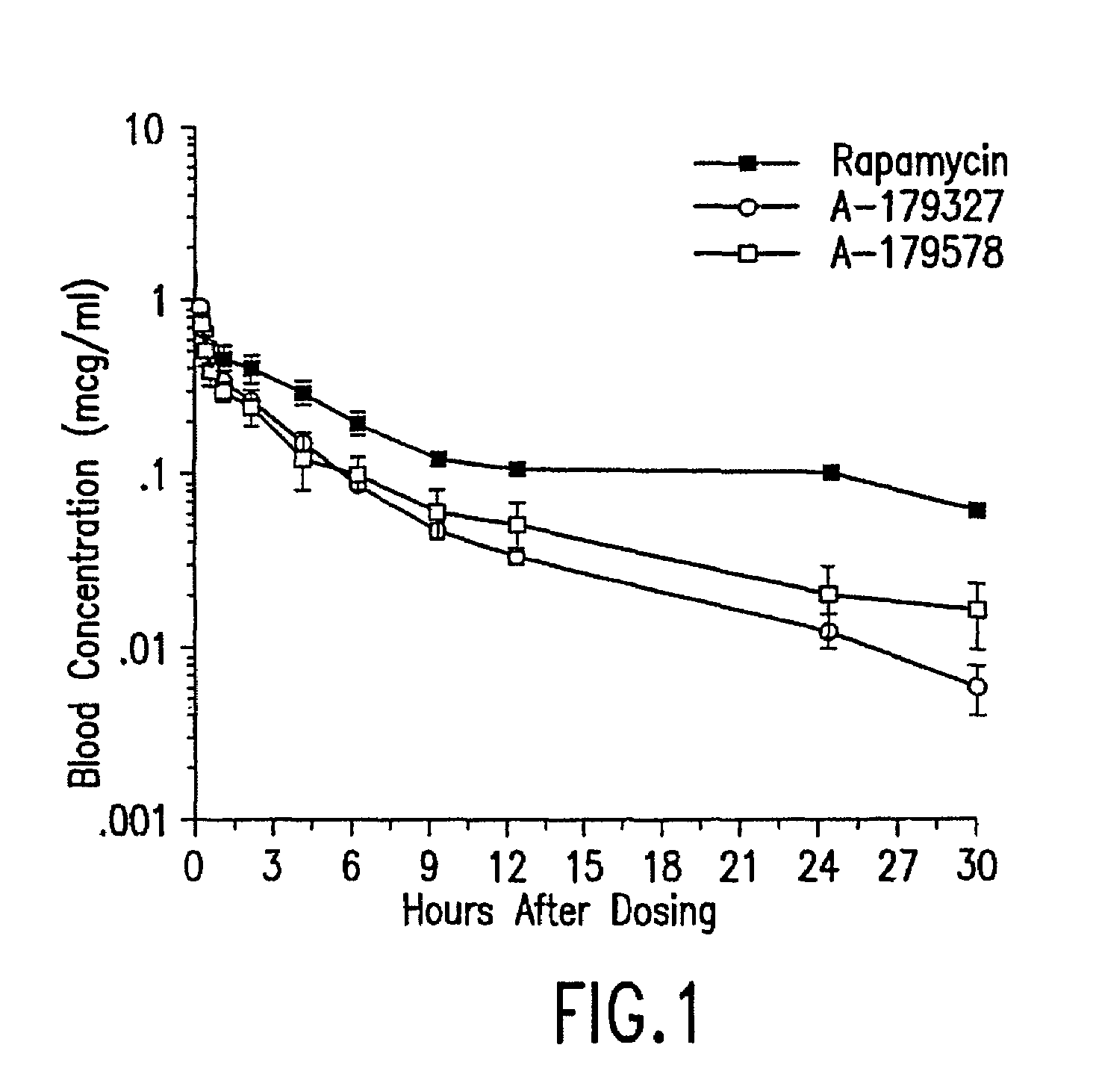
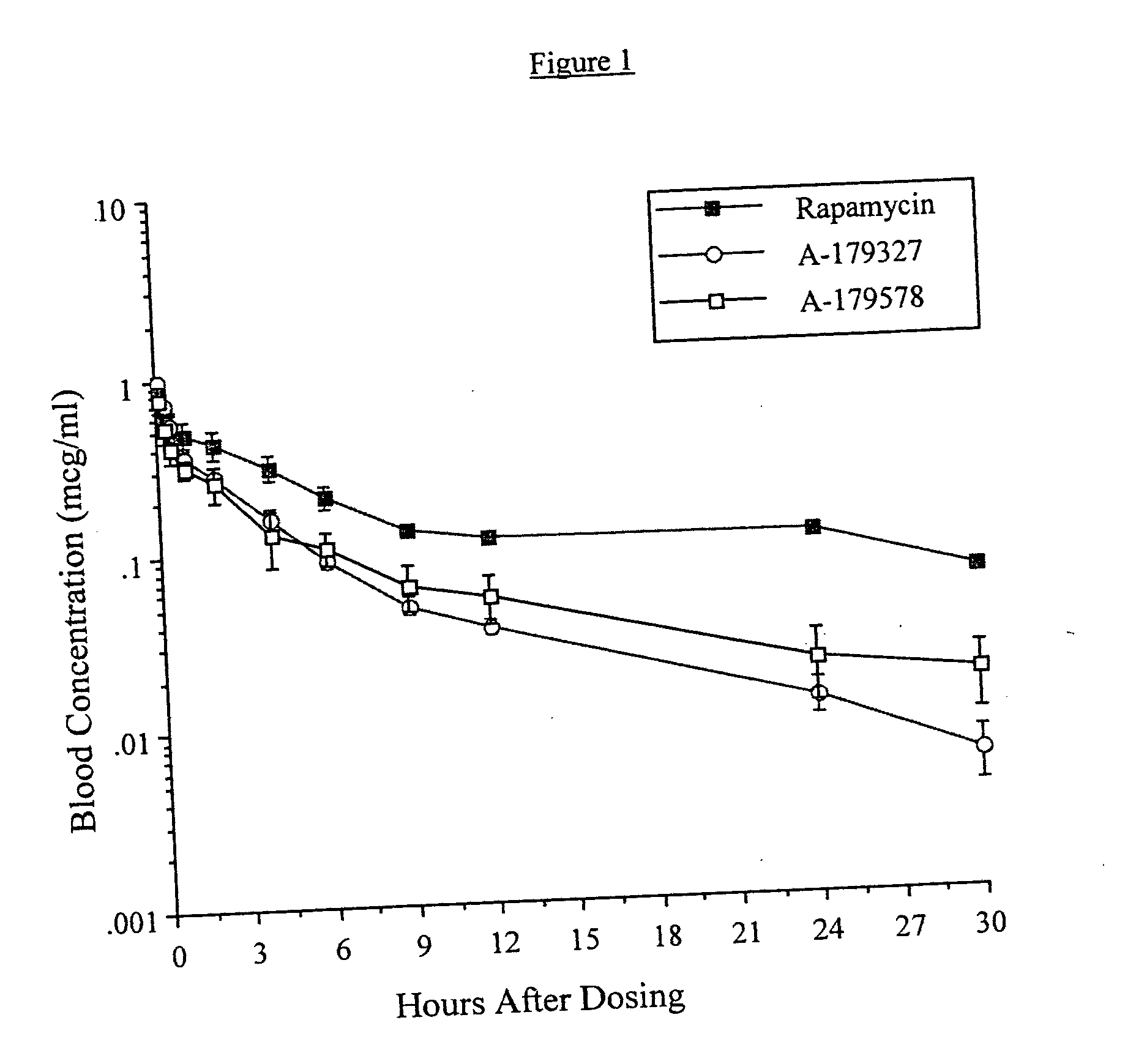
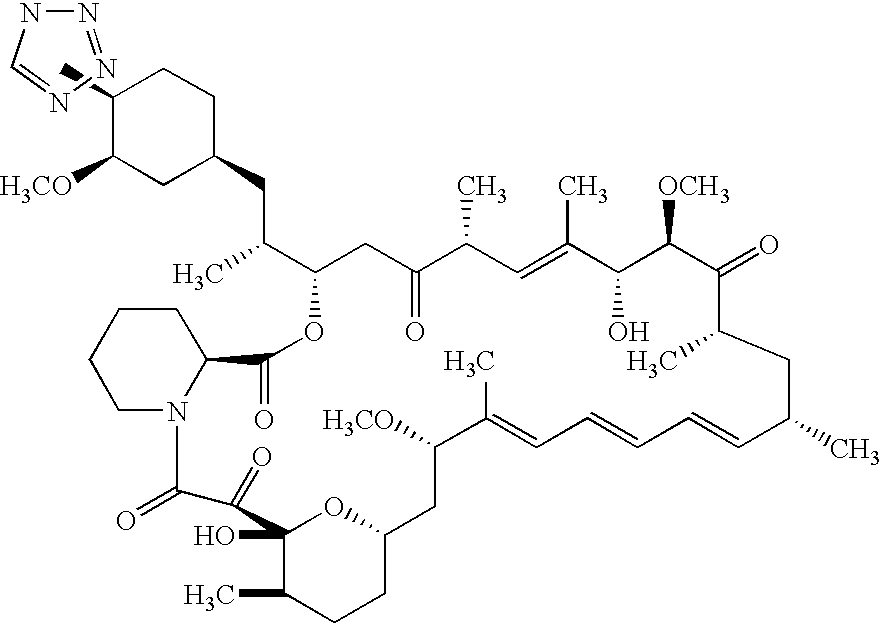
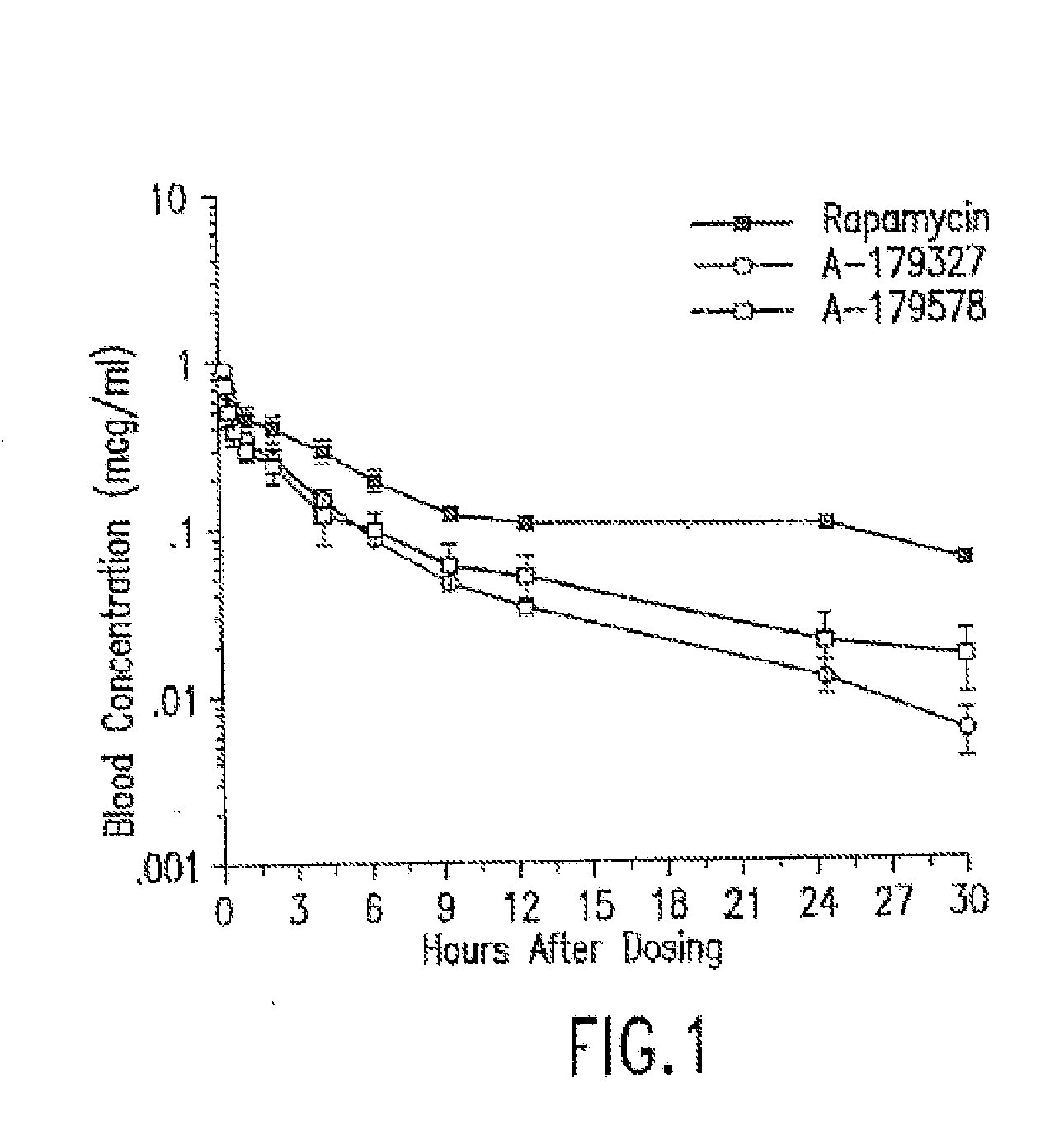
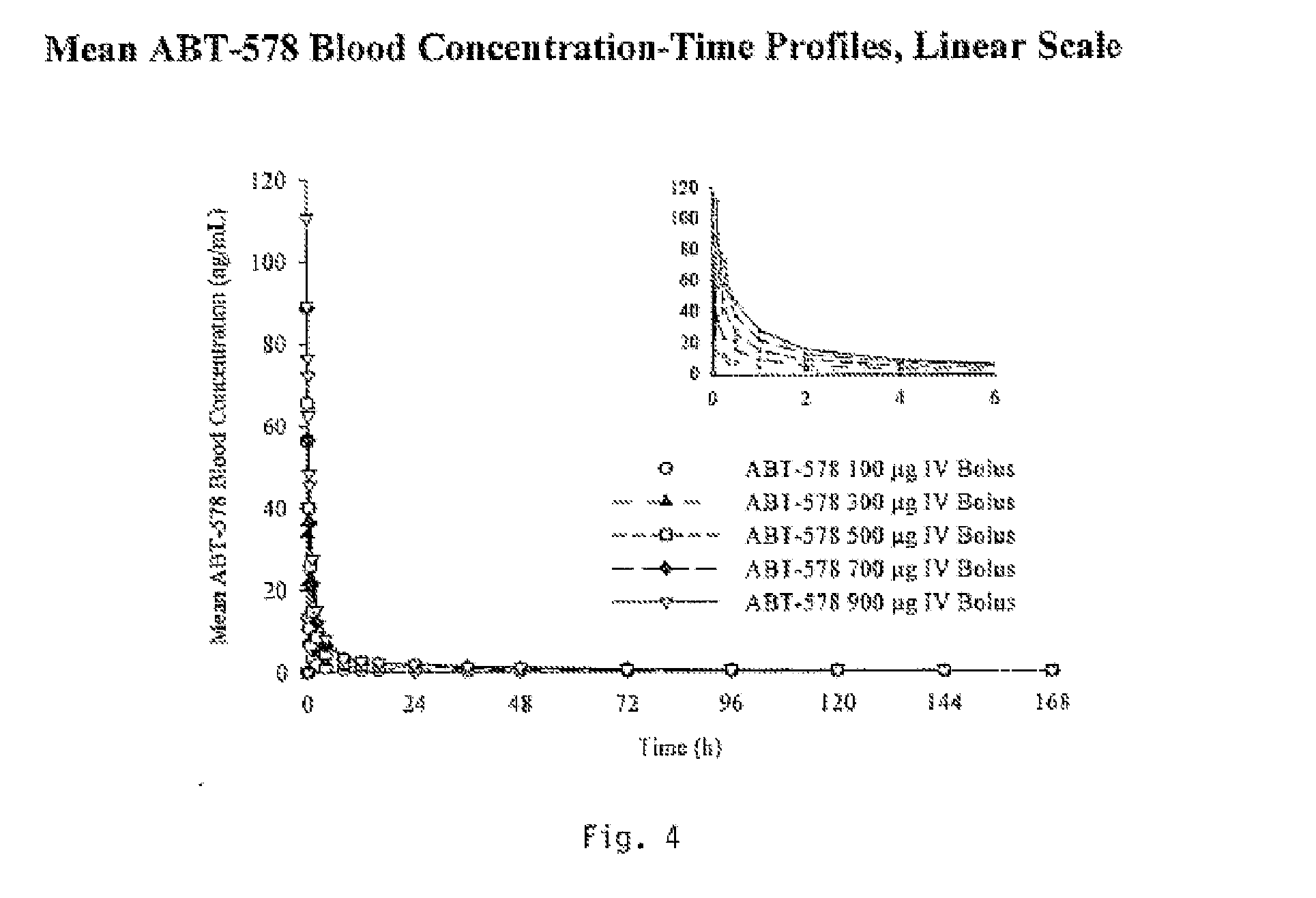
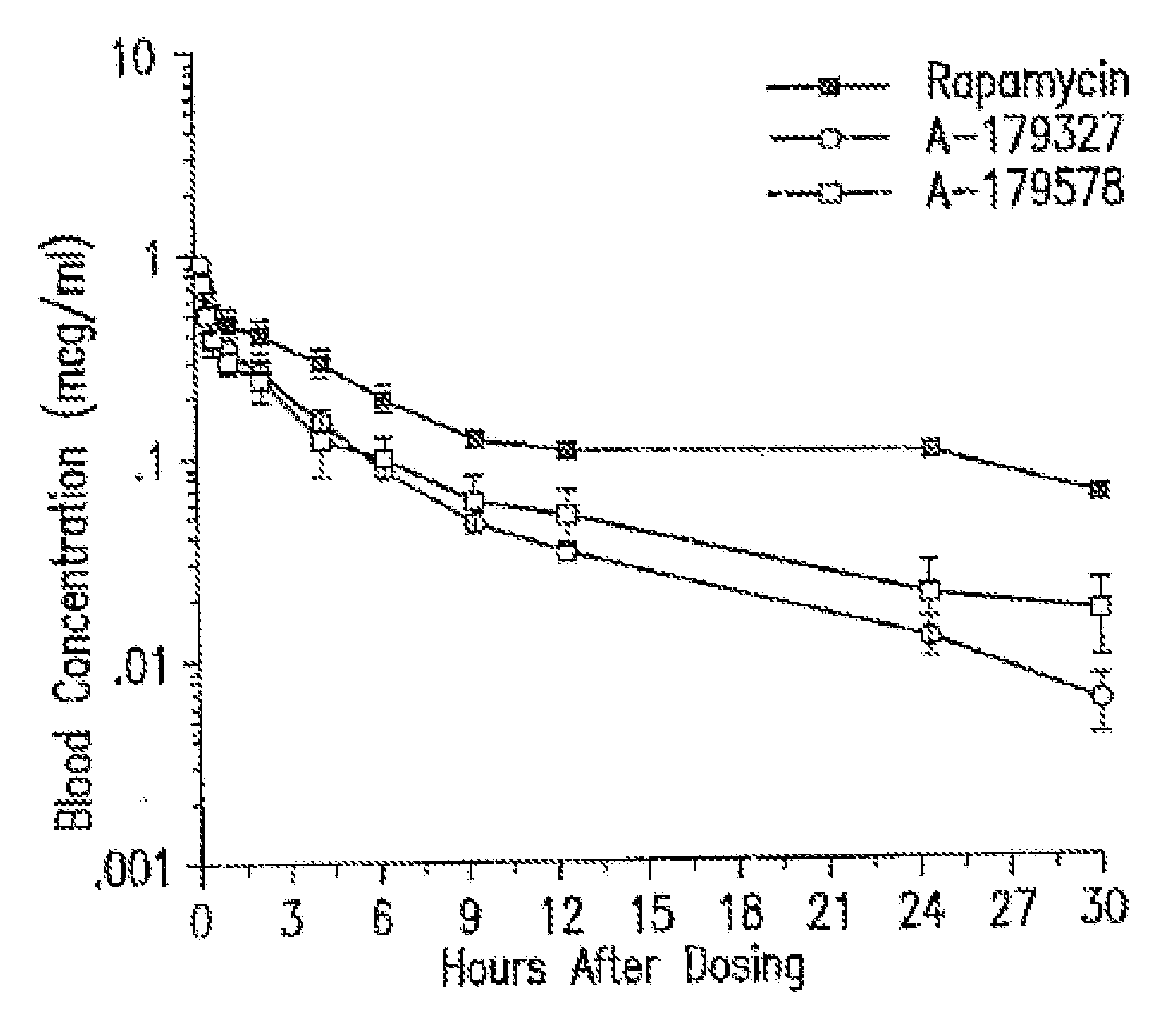
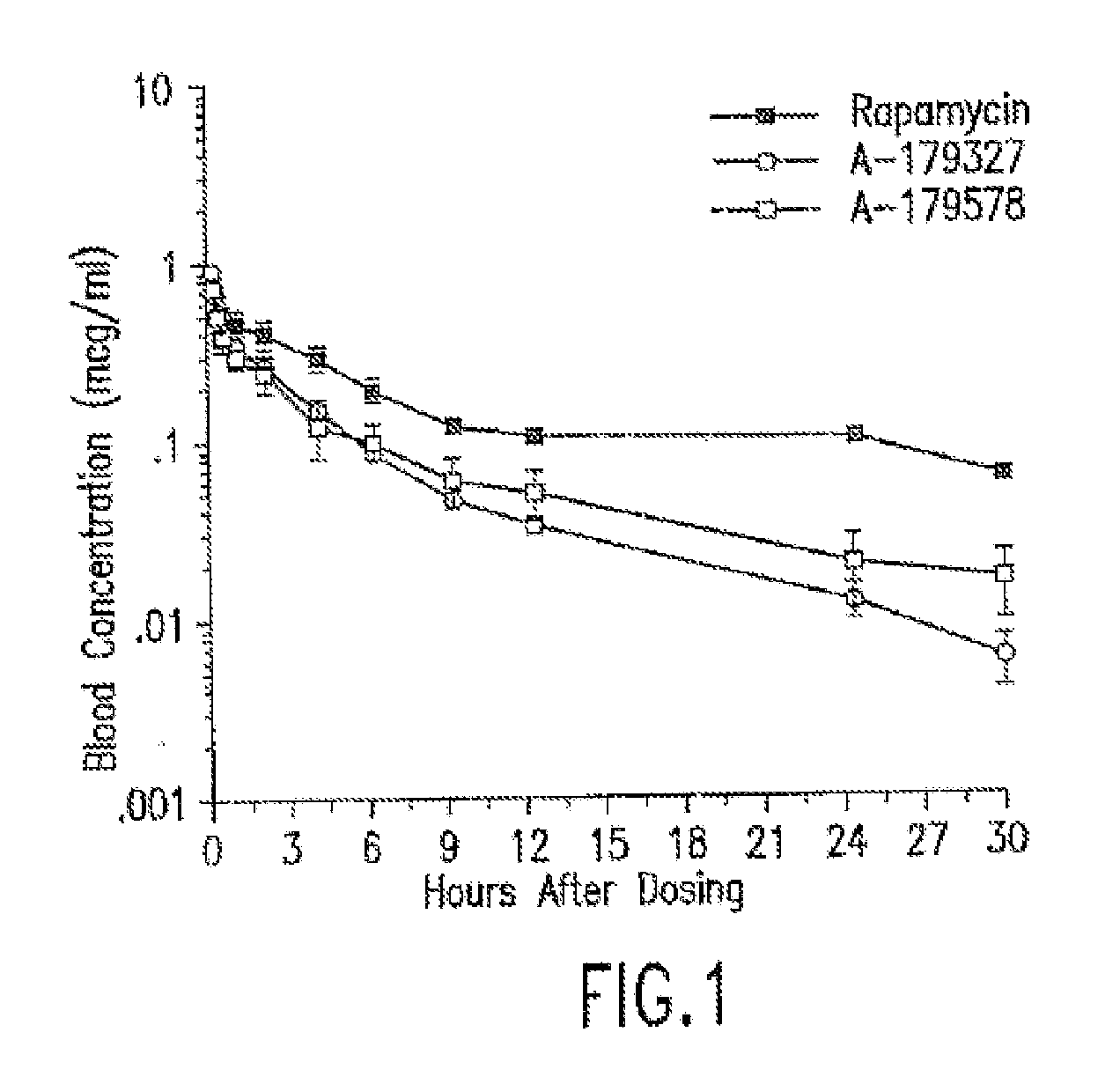
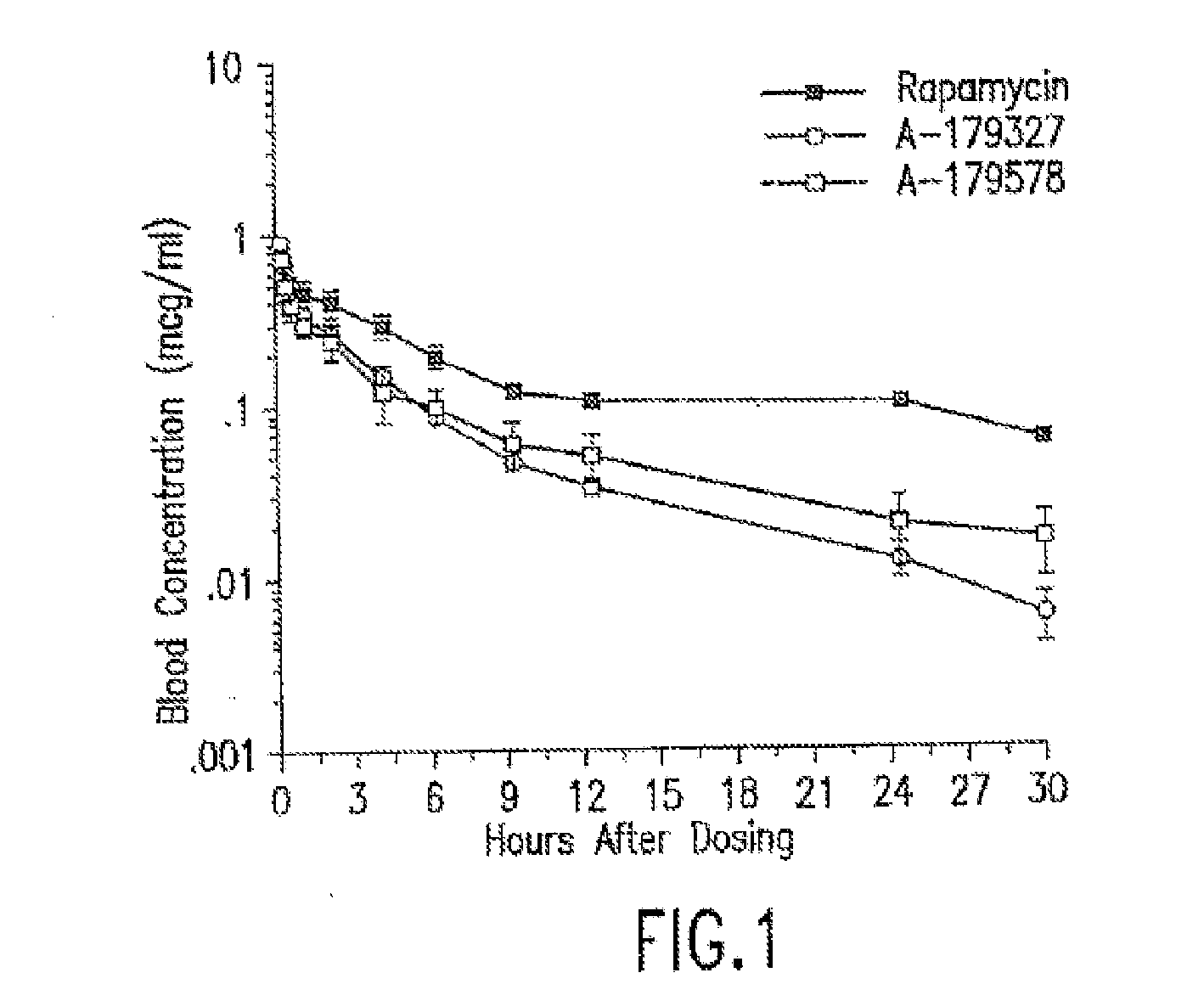
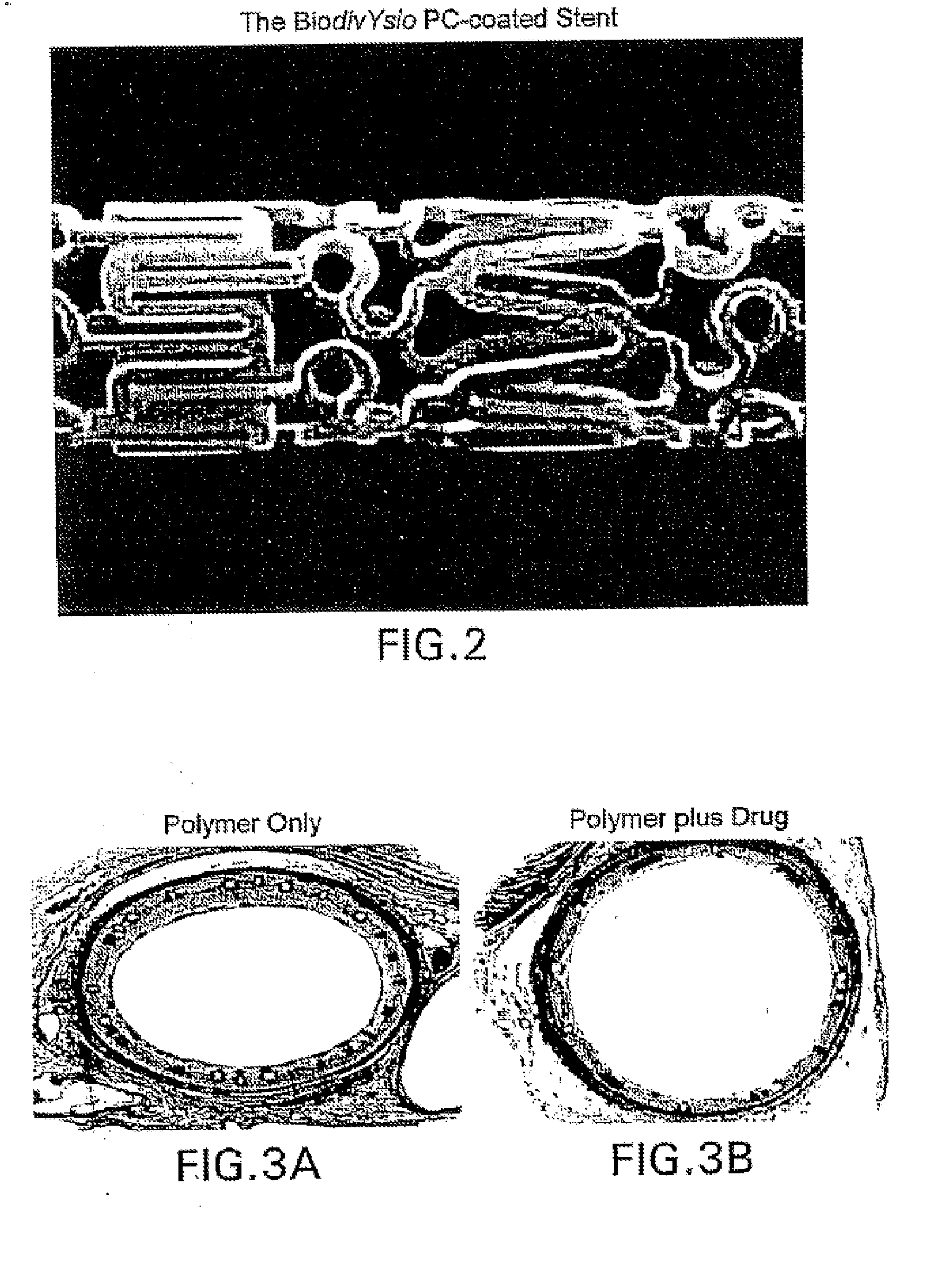
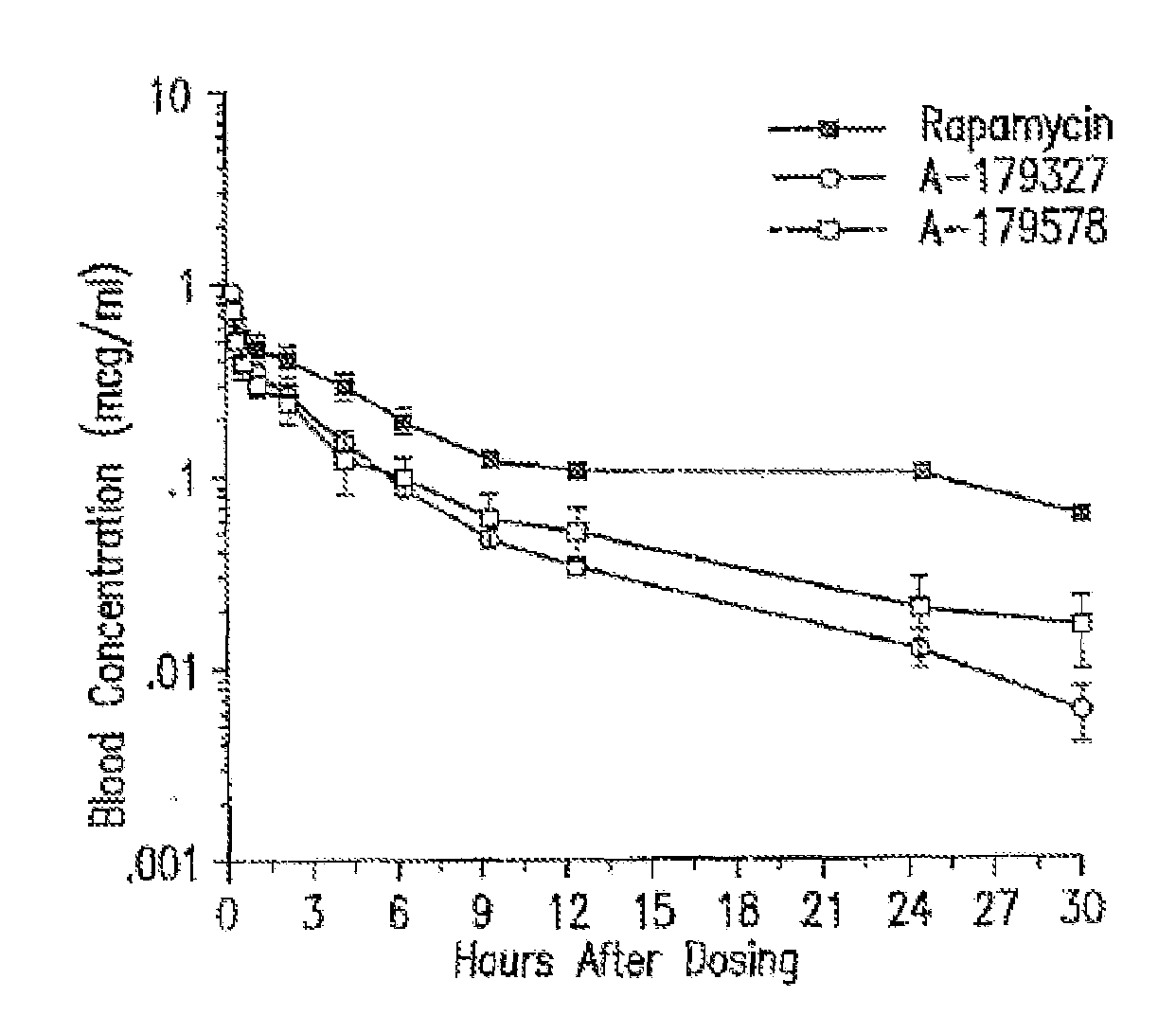
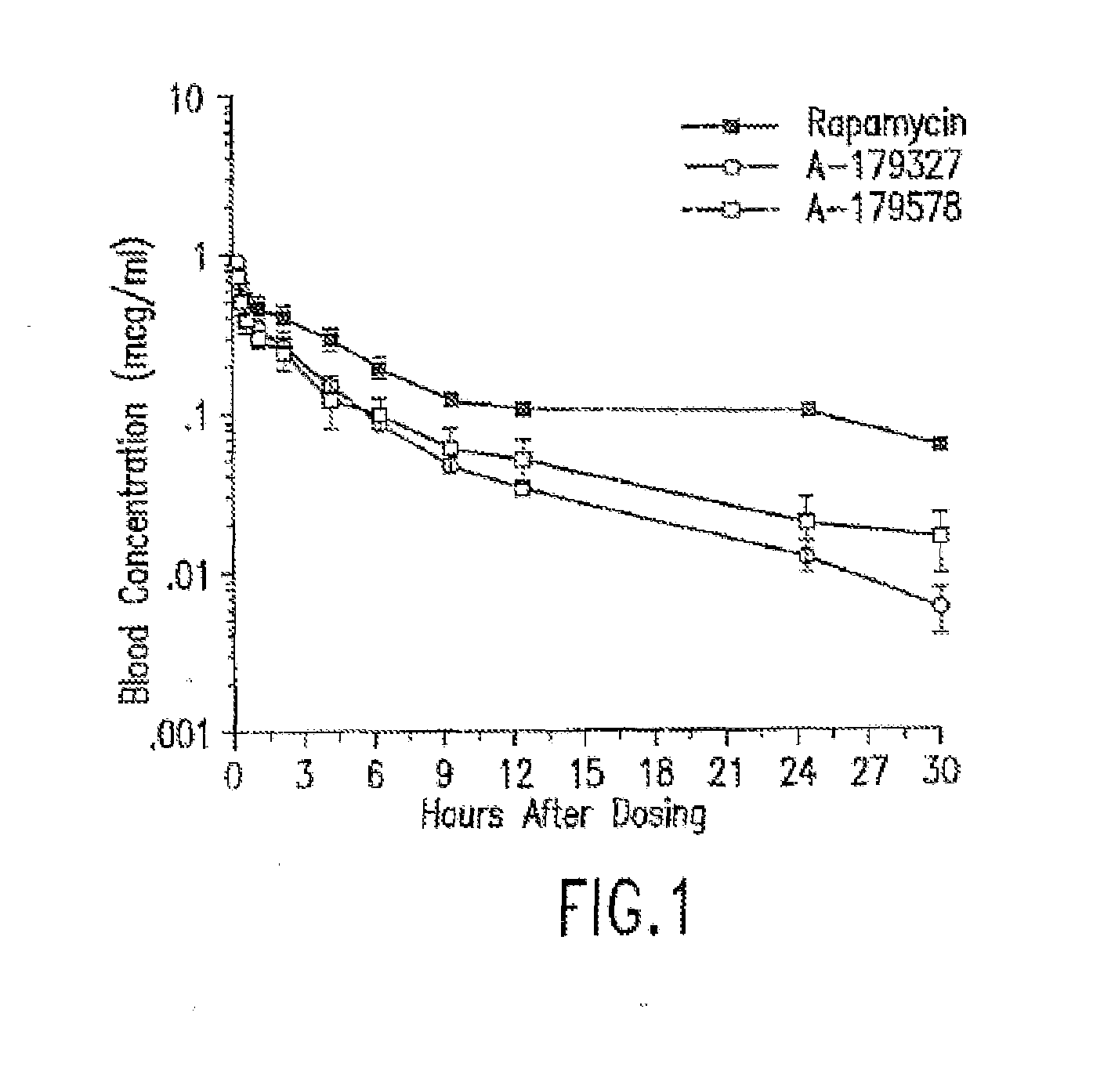
Medical devices containing rapamycin analogs
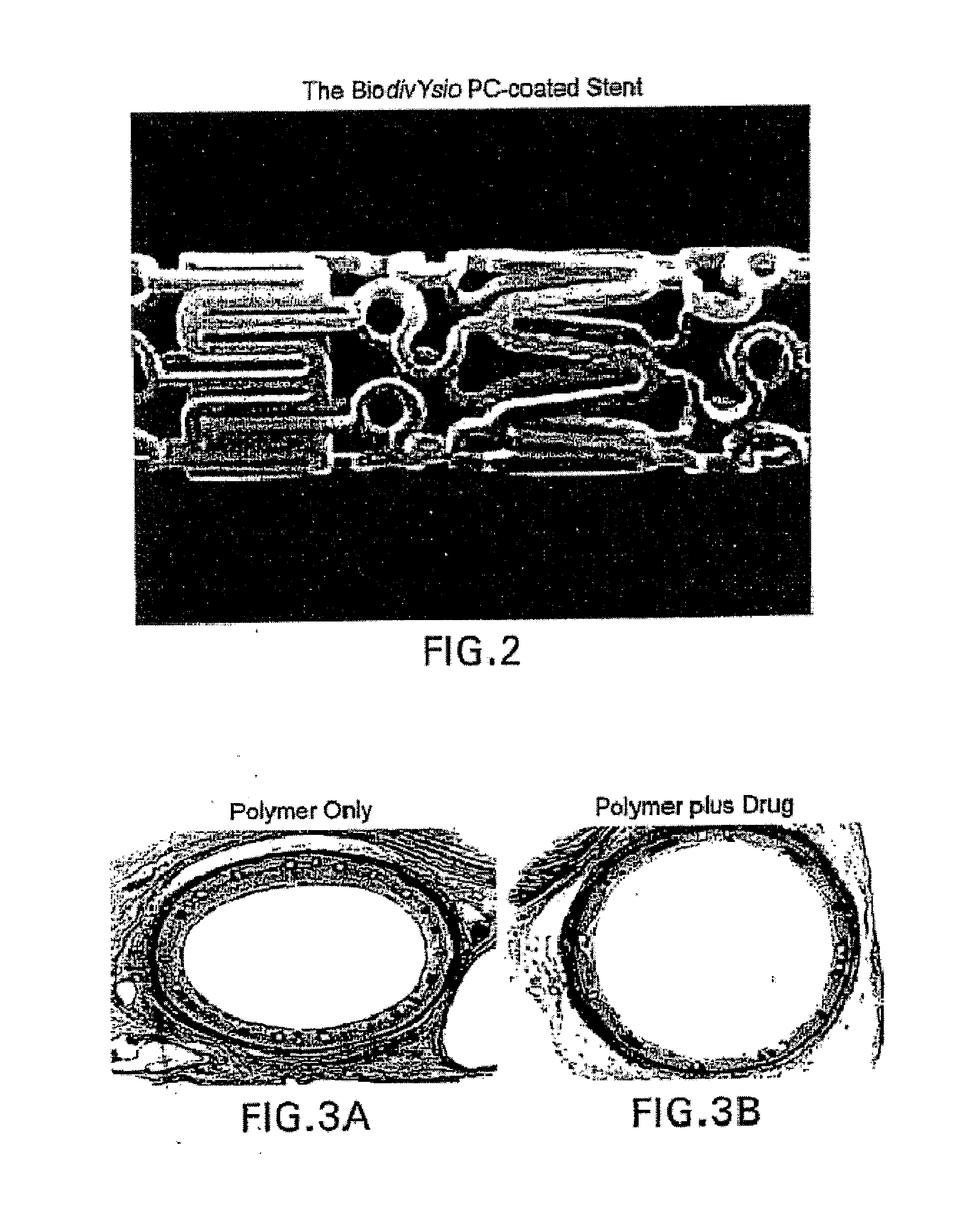
InactiveUS6890546B2Reduce probabilityReduces restenosis in vasculatureBiocideAntimycoticsArteriovenous graftsMedical treatment
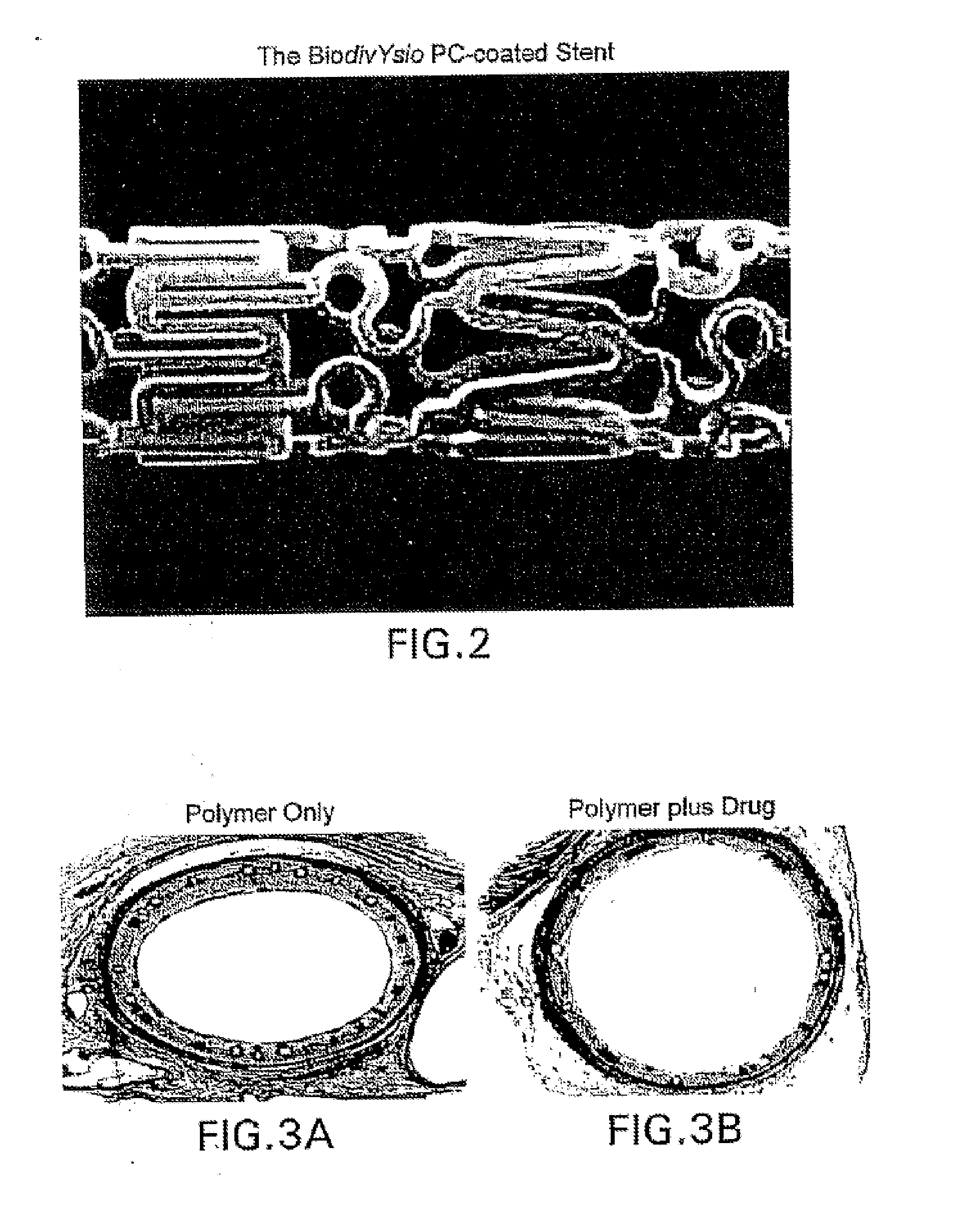
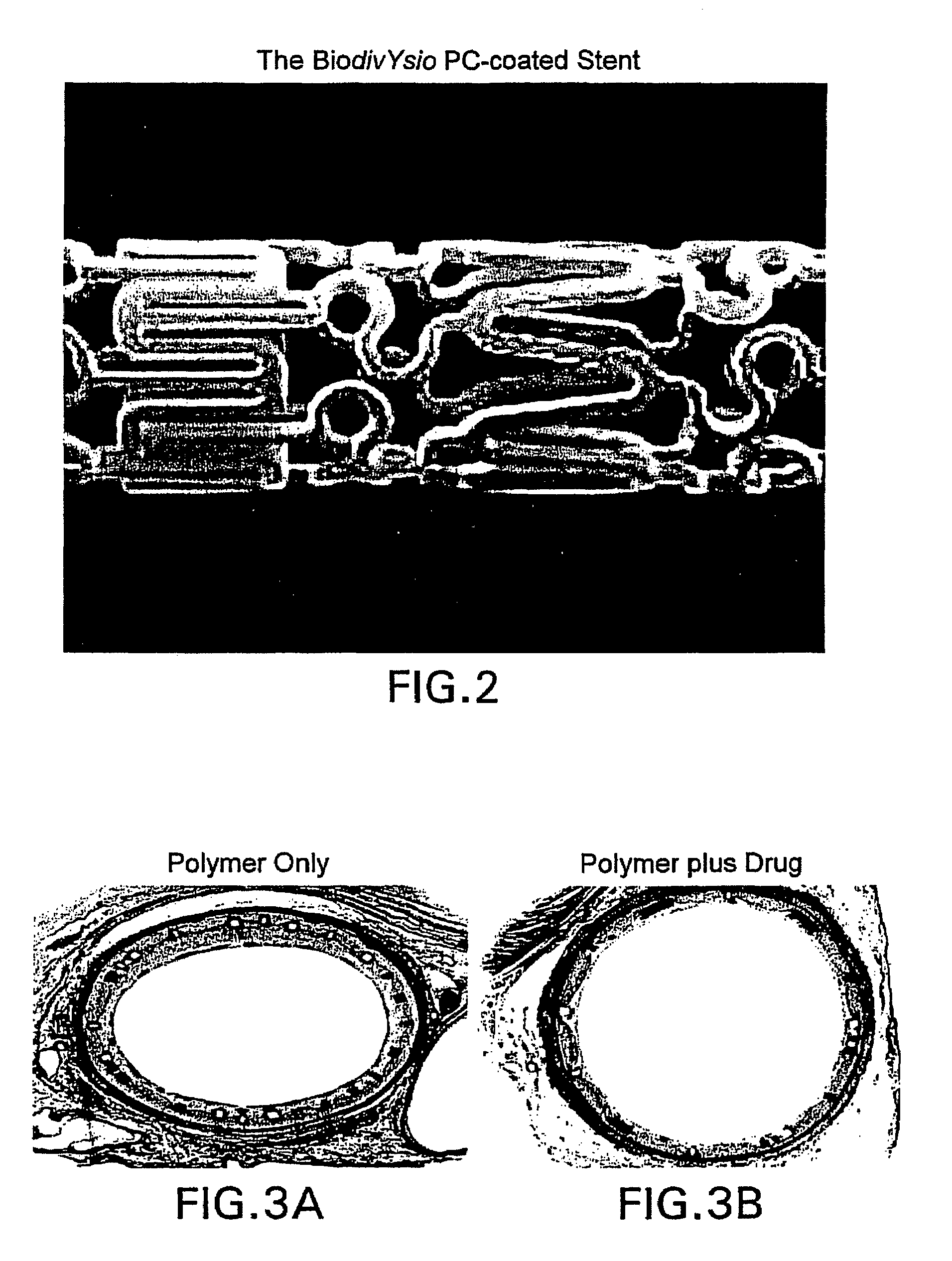
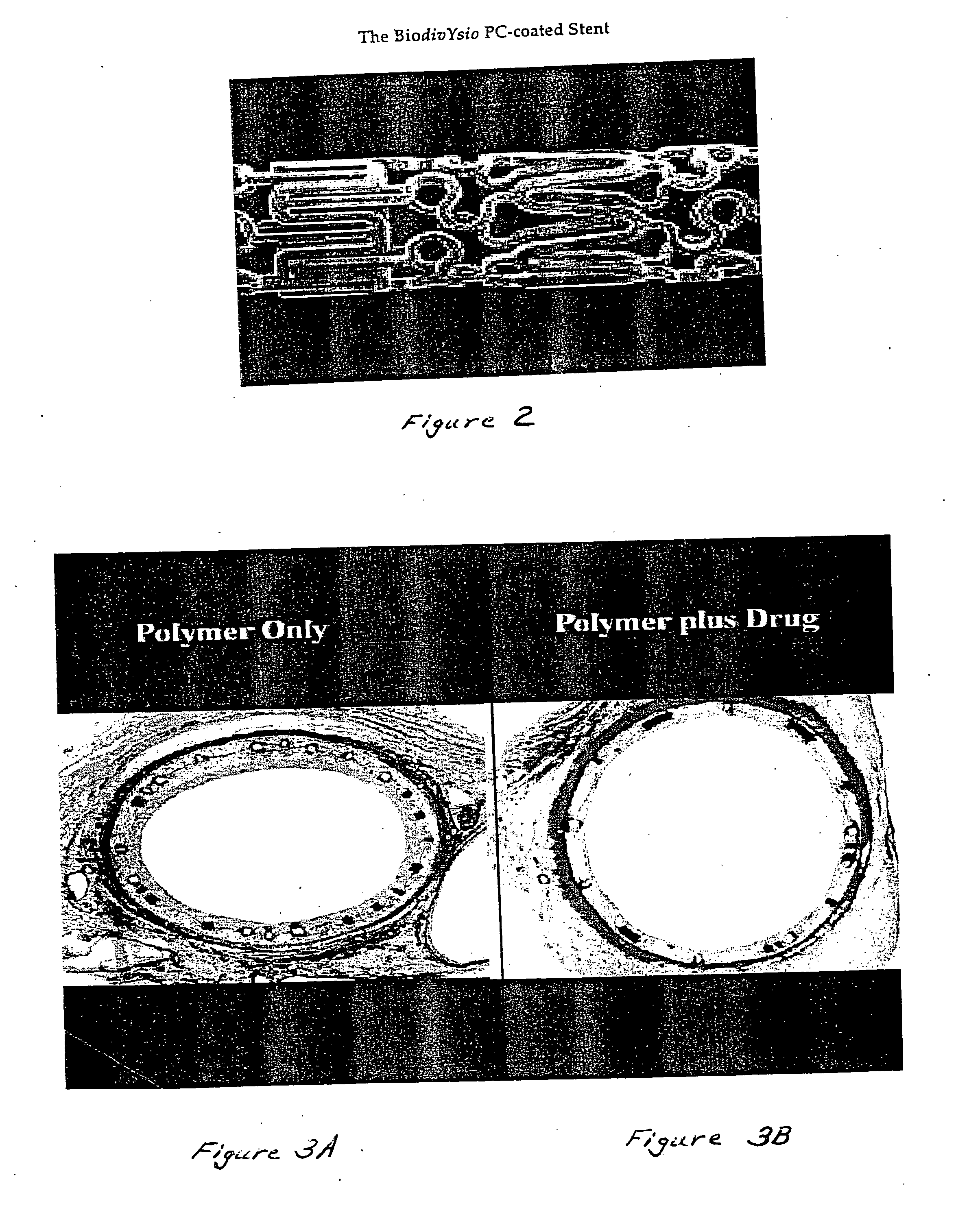
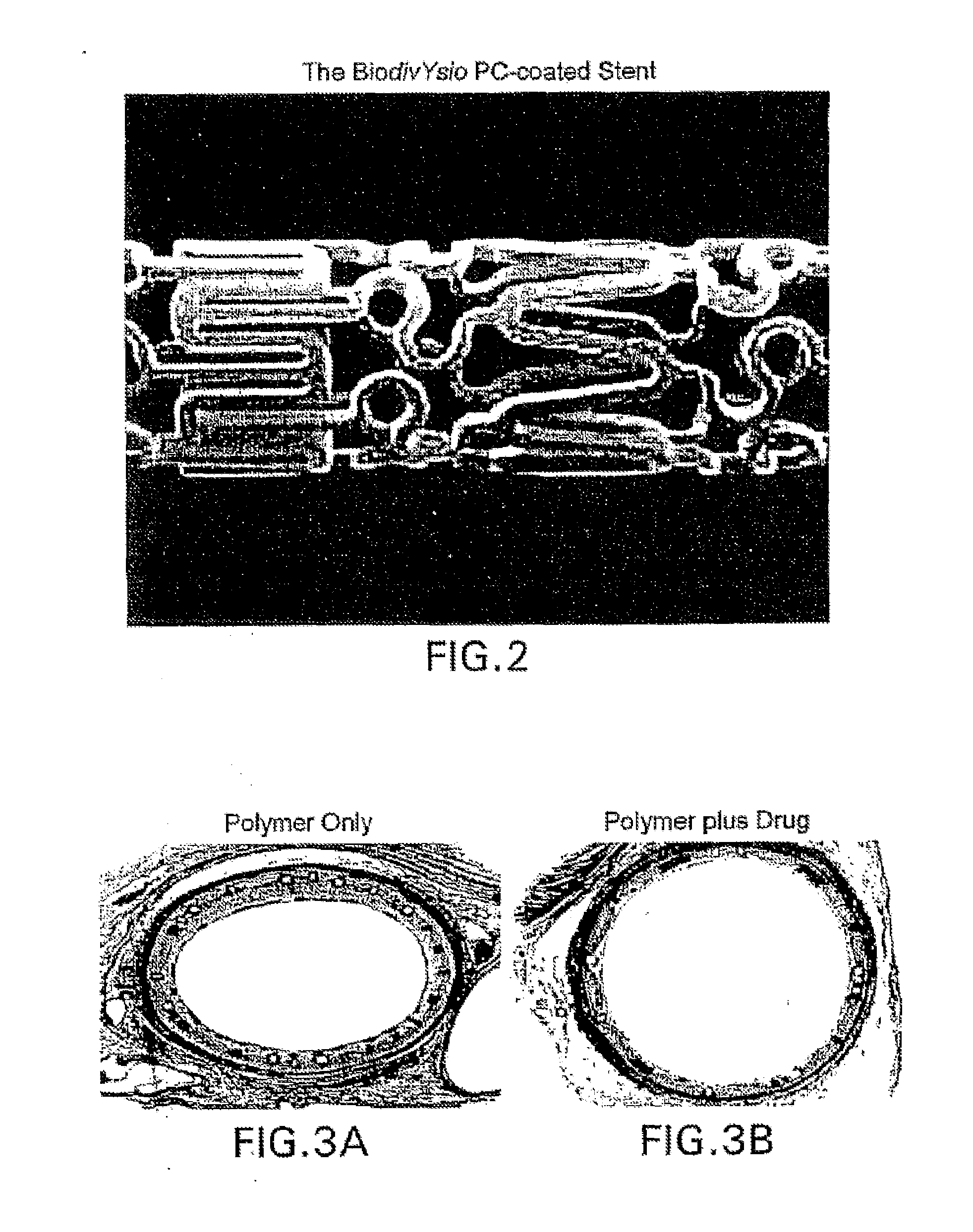
A medical device comprising a supporting structure having a coating on the surface thereof, the coating containing a therapeutic substance, such as, for example, a drug. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to,
Owner:ABBOTT LAB INC
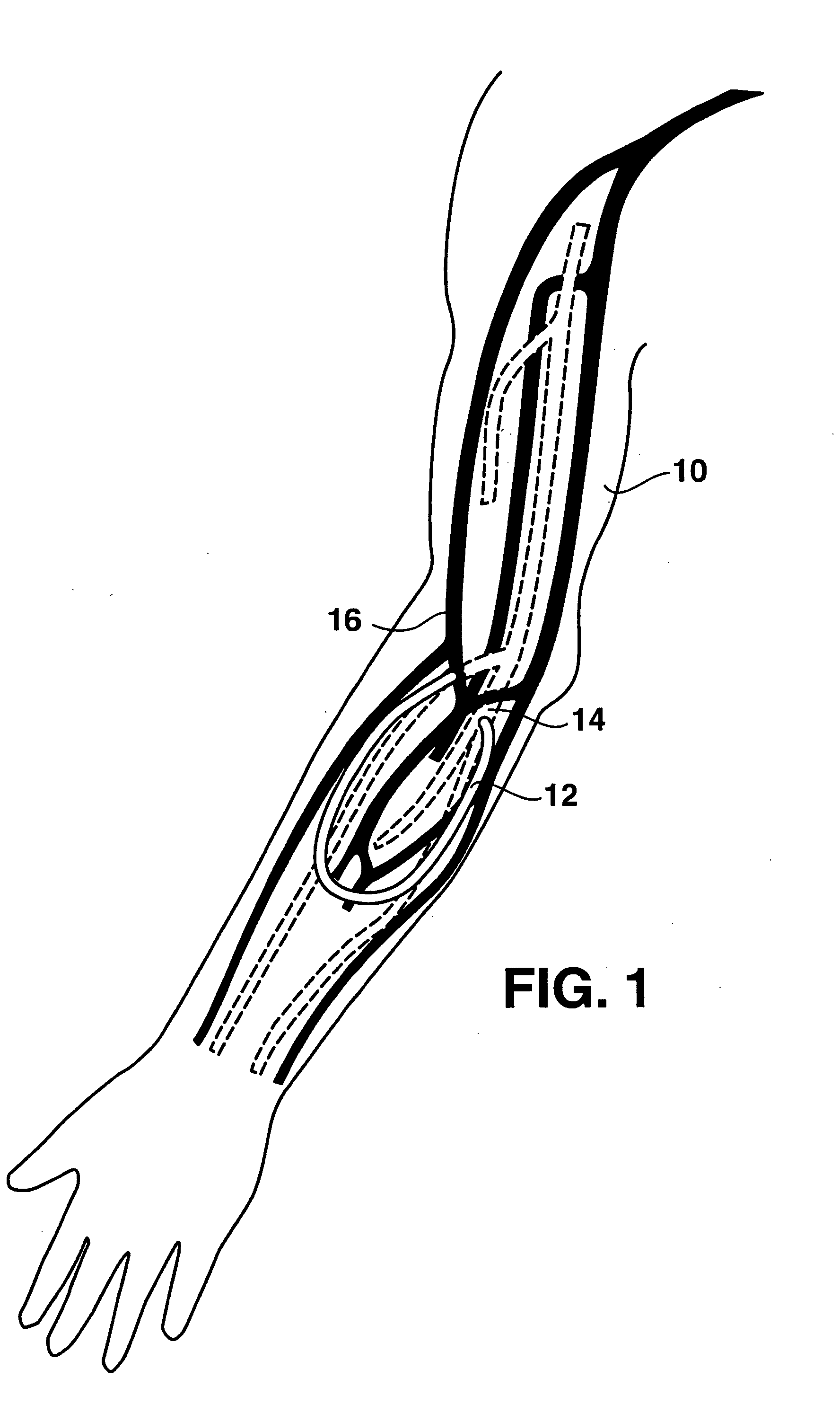
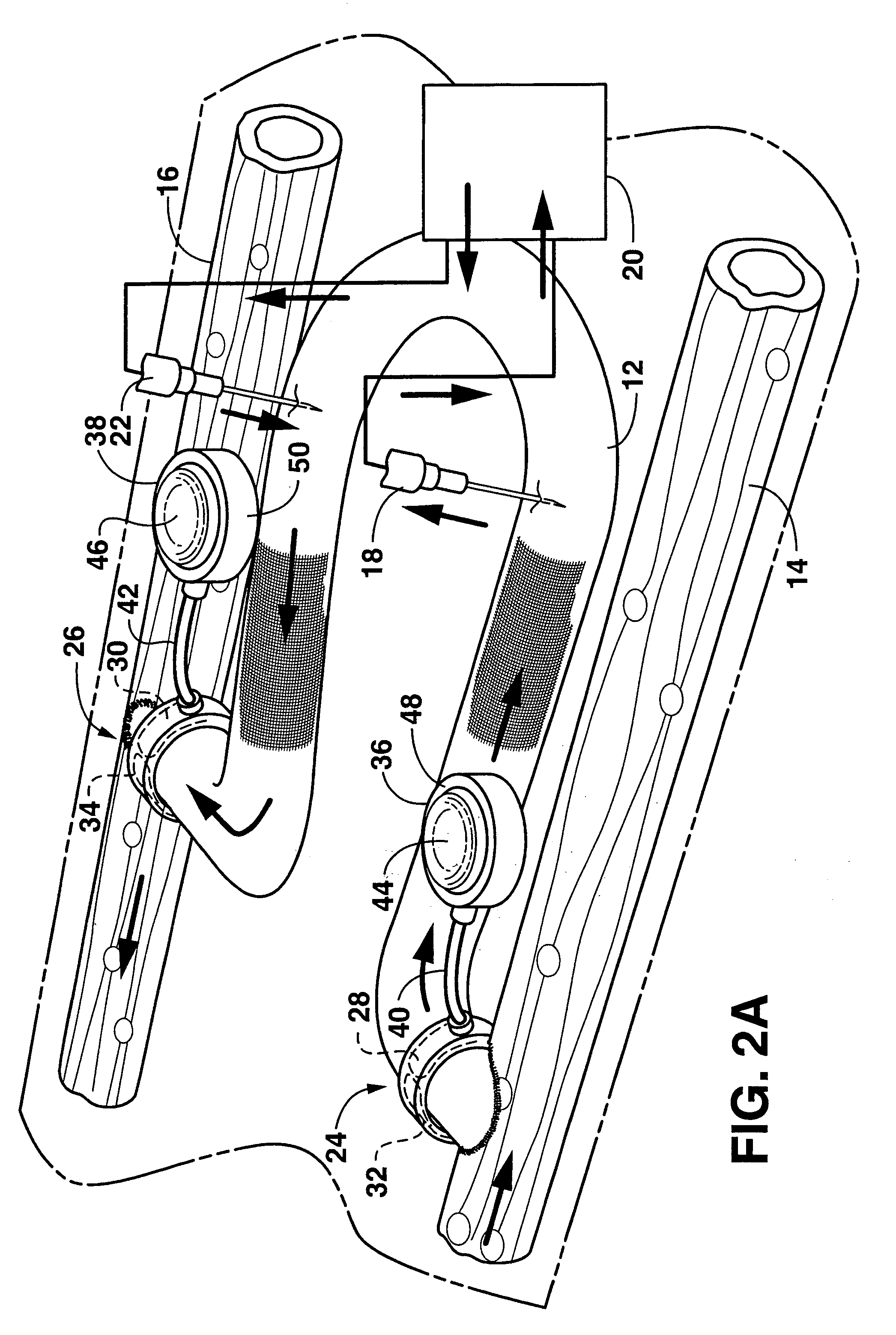
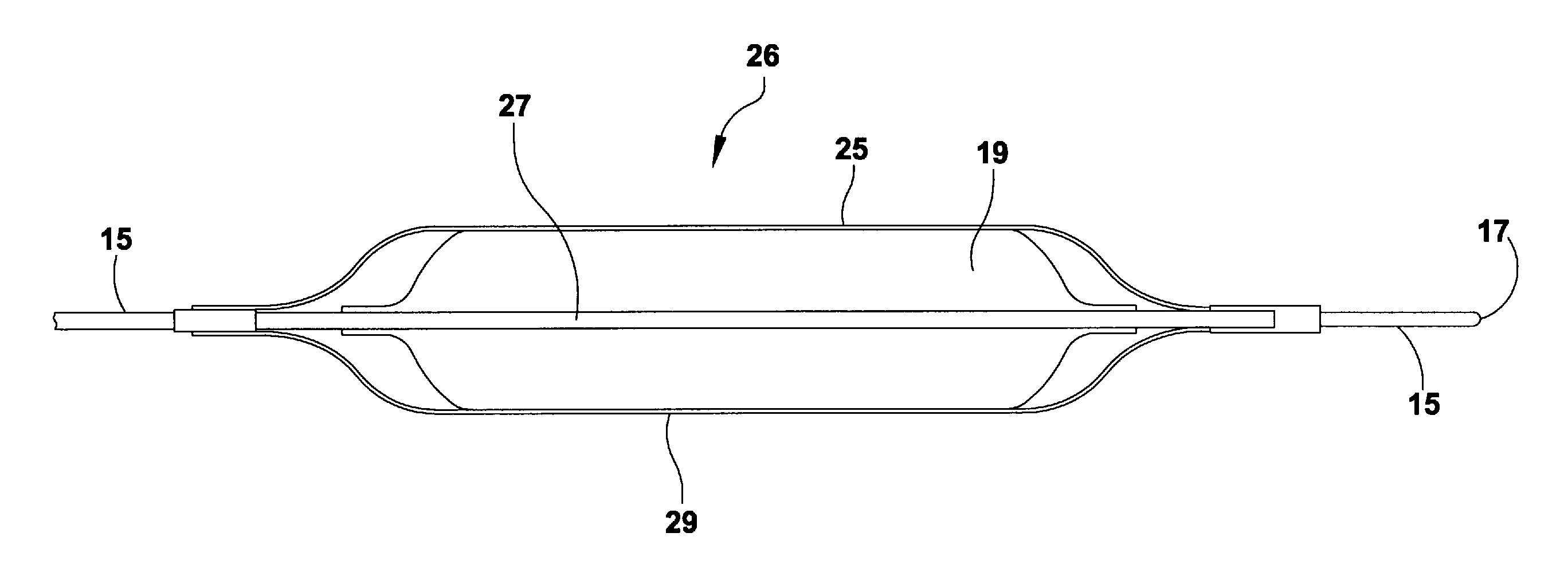
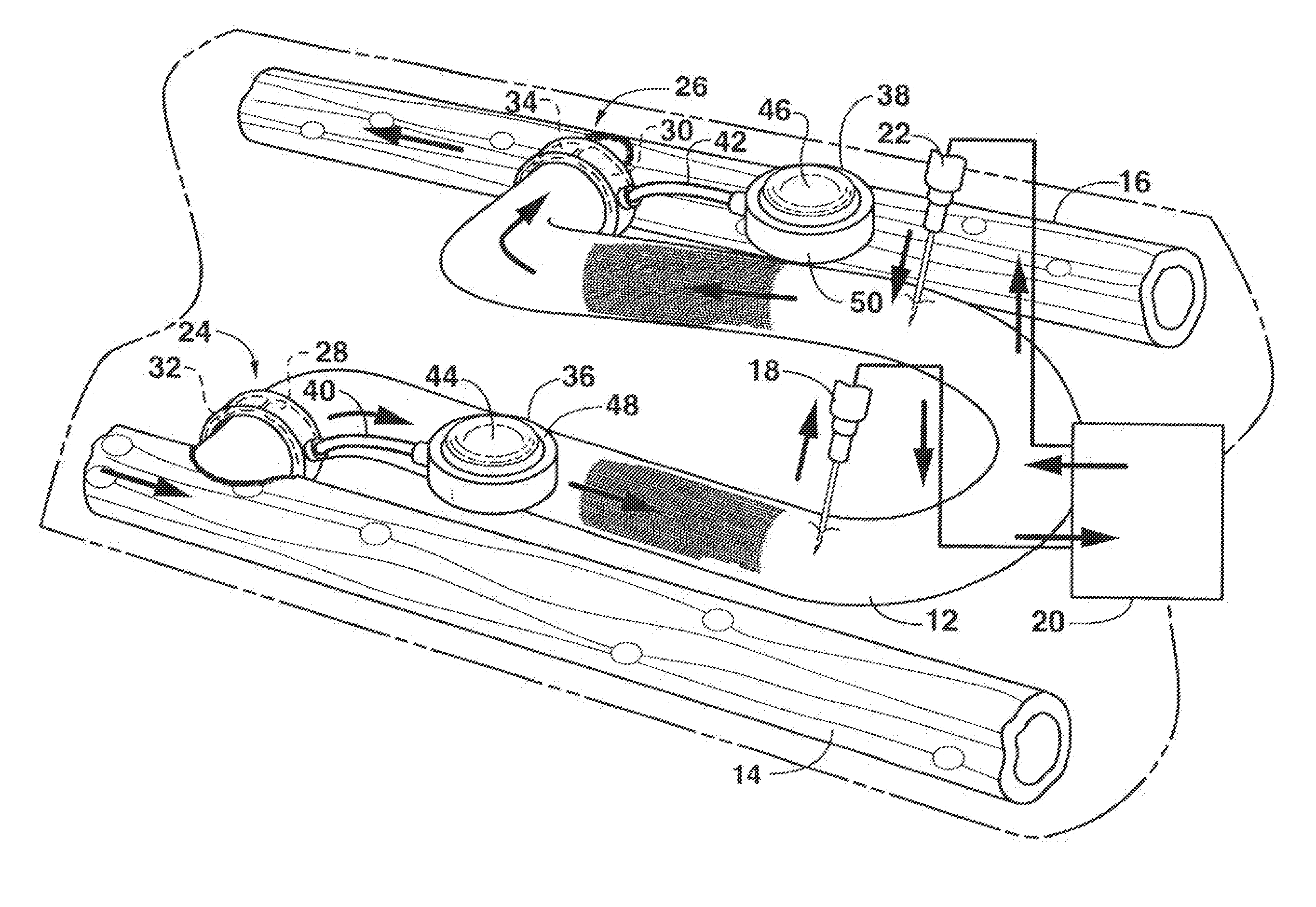
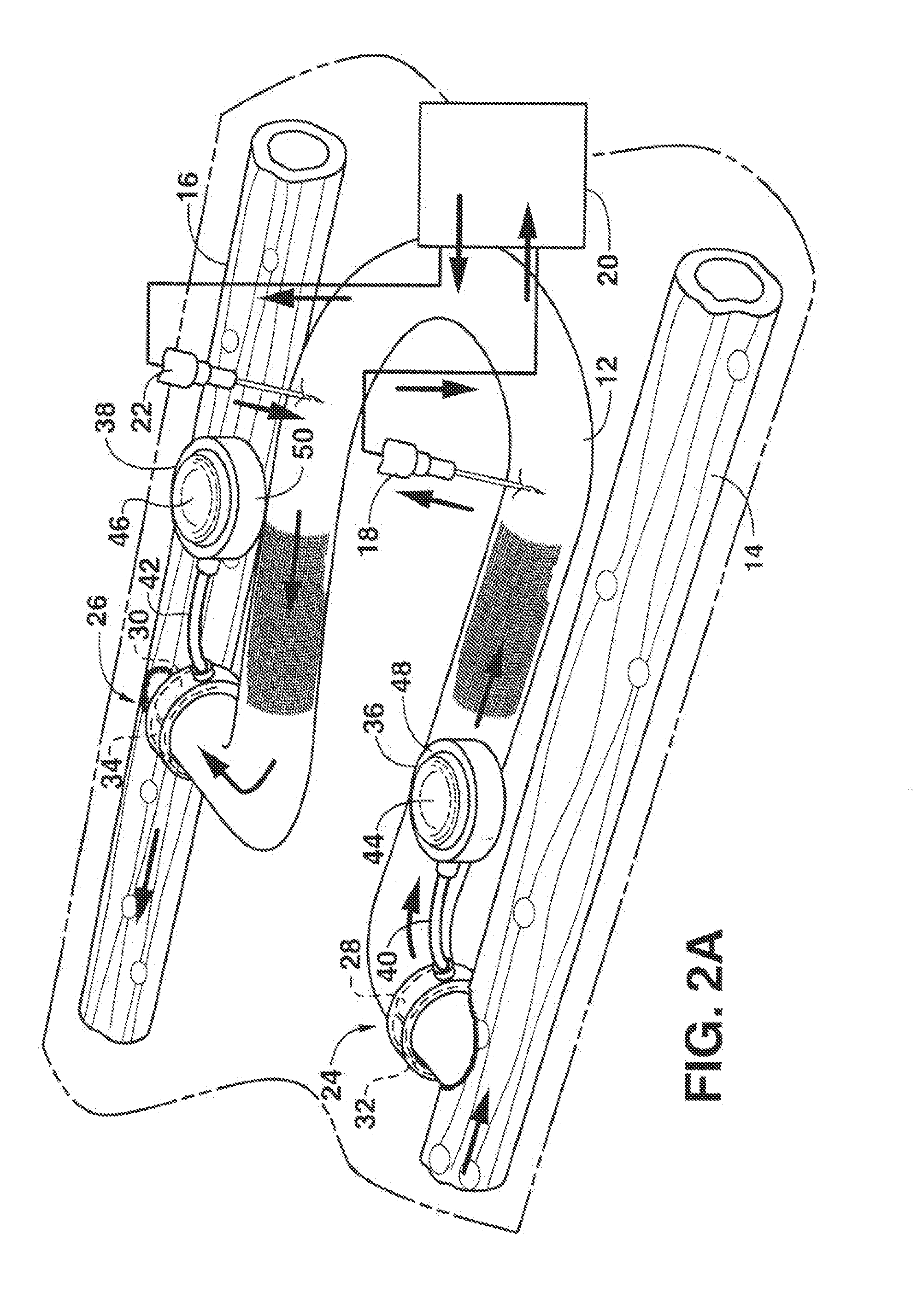
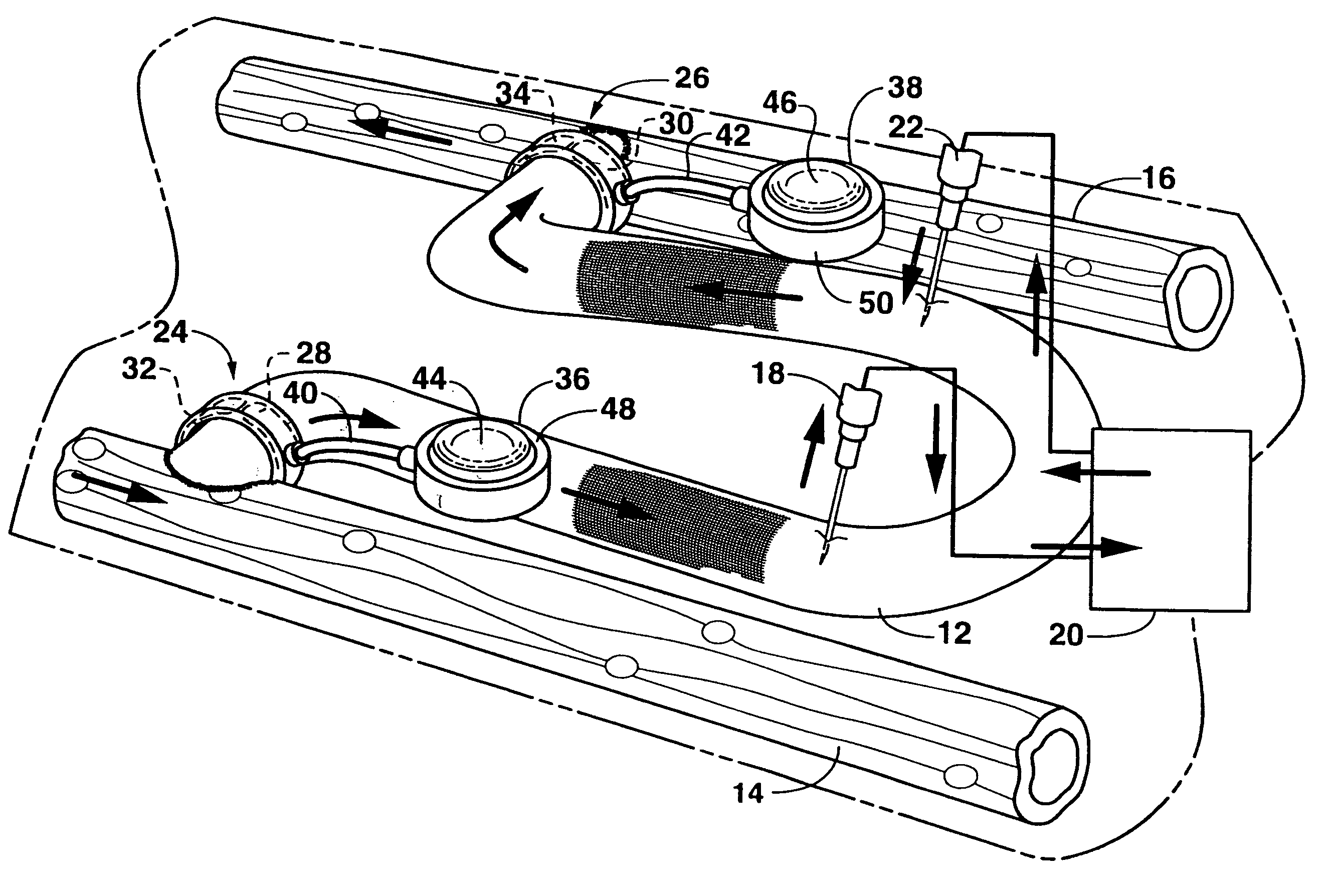
Arteriovenous access valve system and process
InactiveUS7025741B2Eliminates orReduces arterial steal and graft thrombosisOther blood circulation devicesDilatorsHaemodialysis machineVein graft
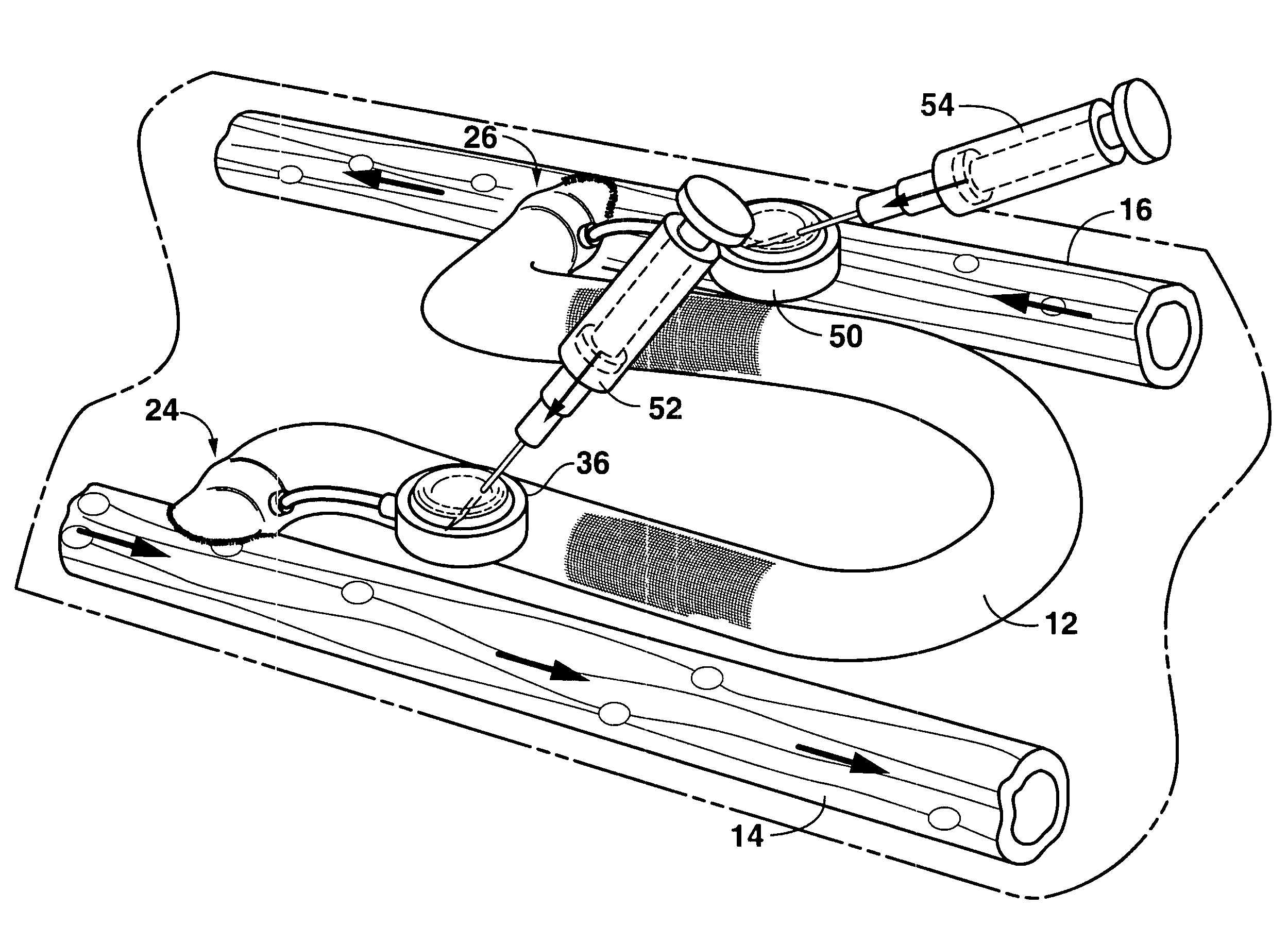
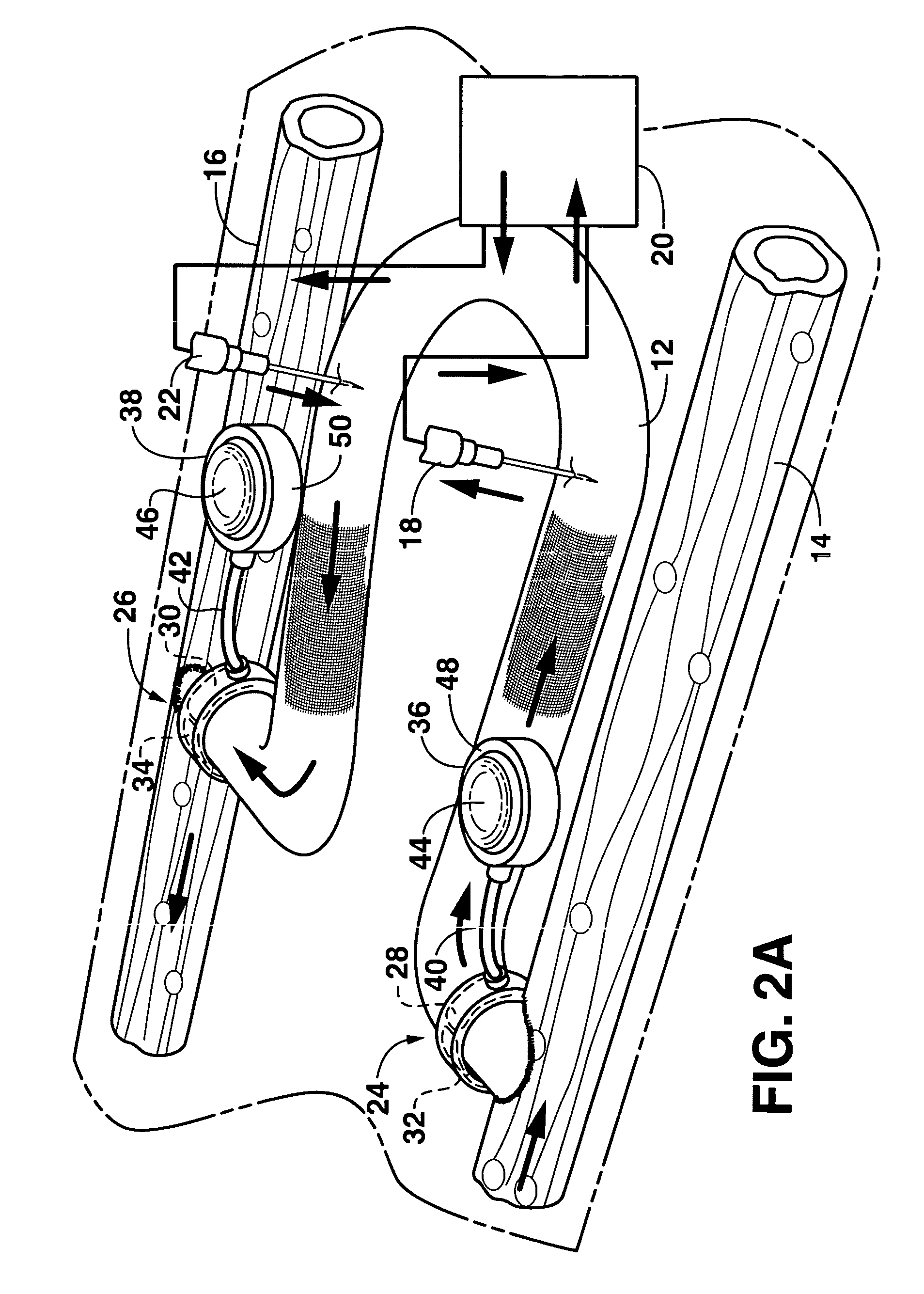
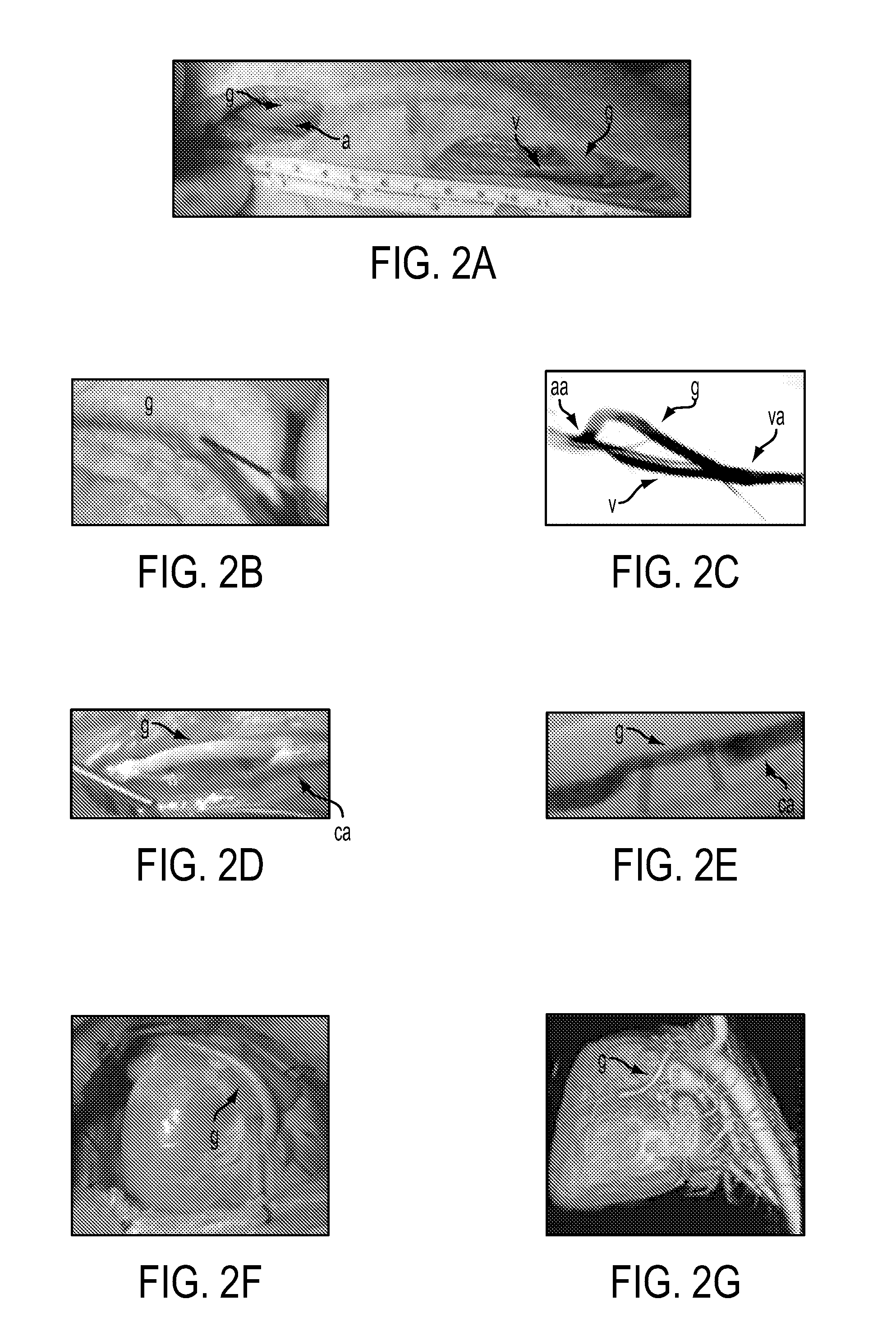
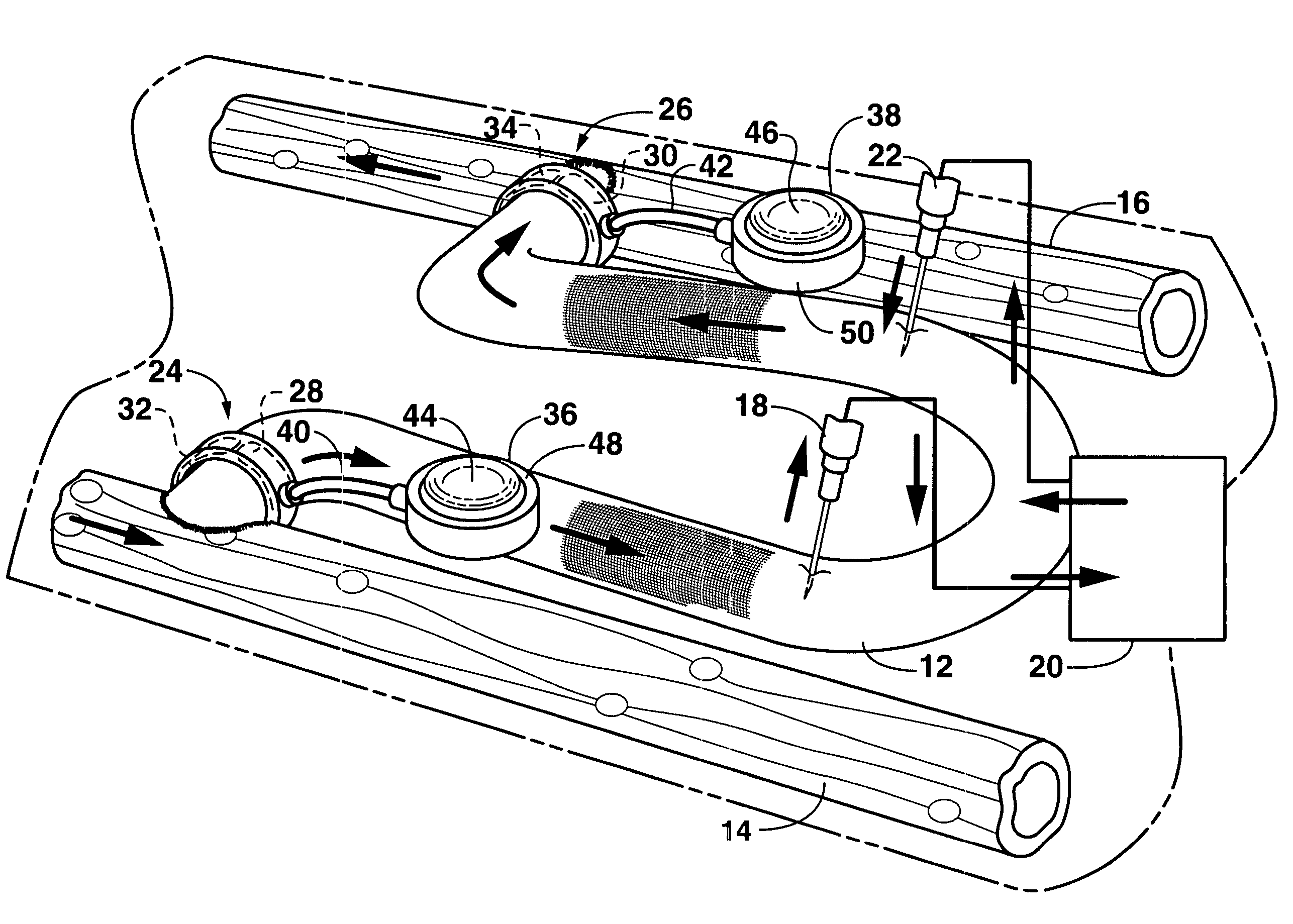
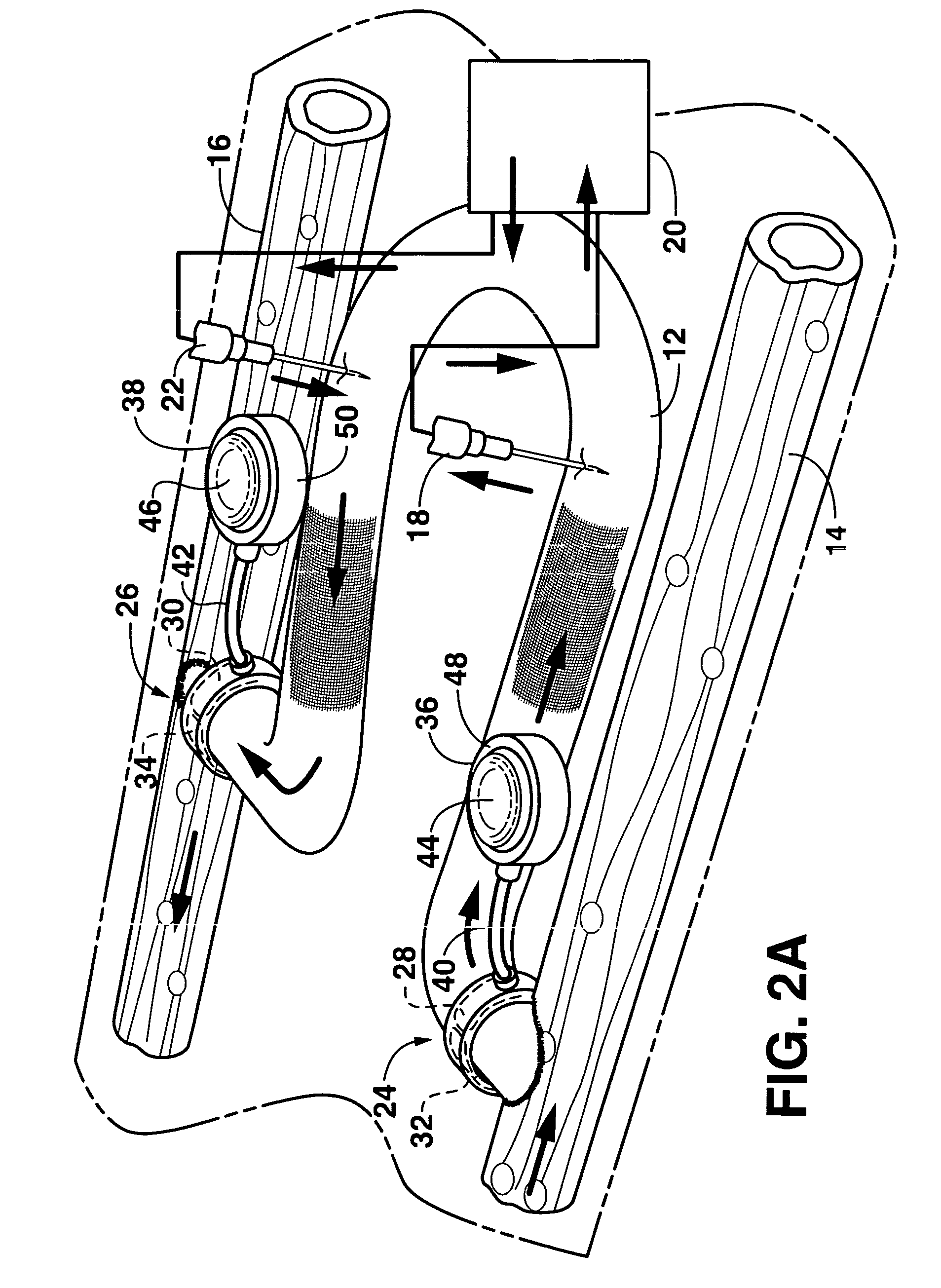
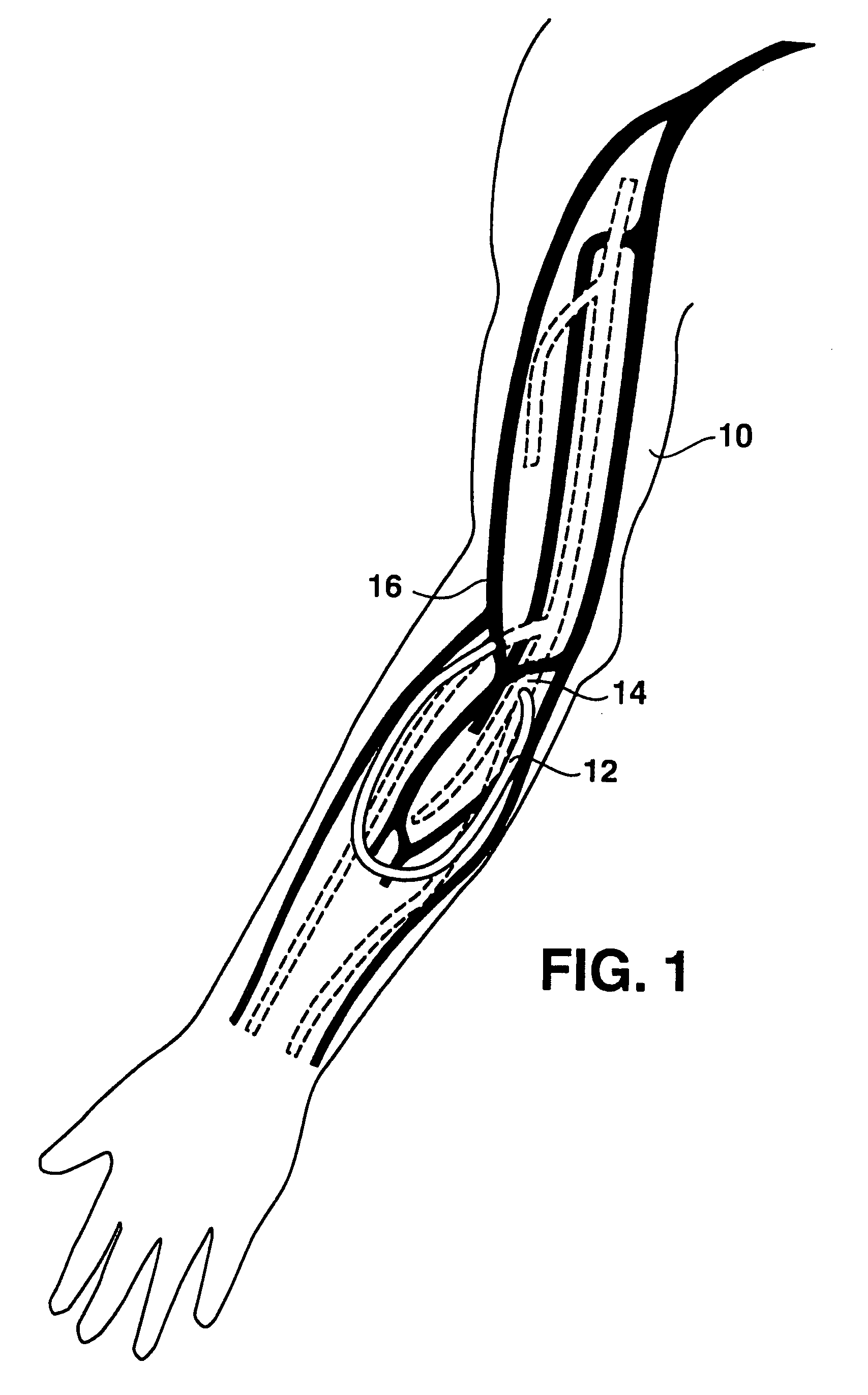
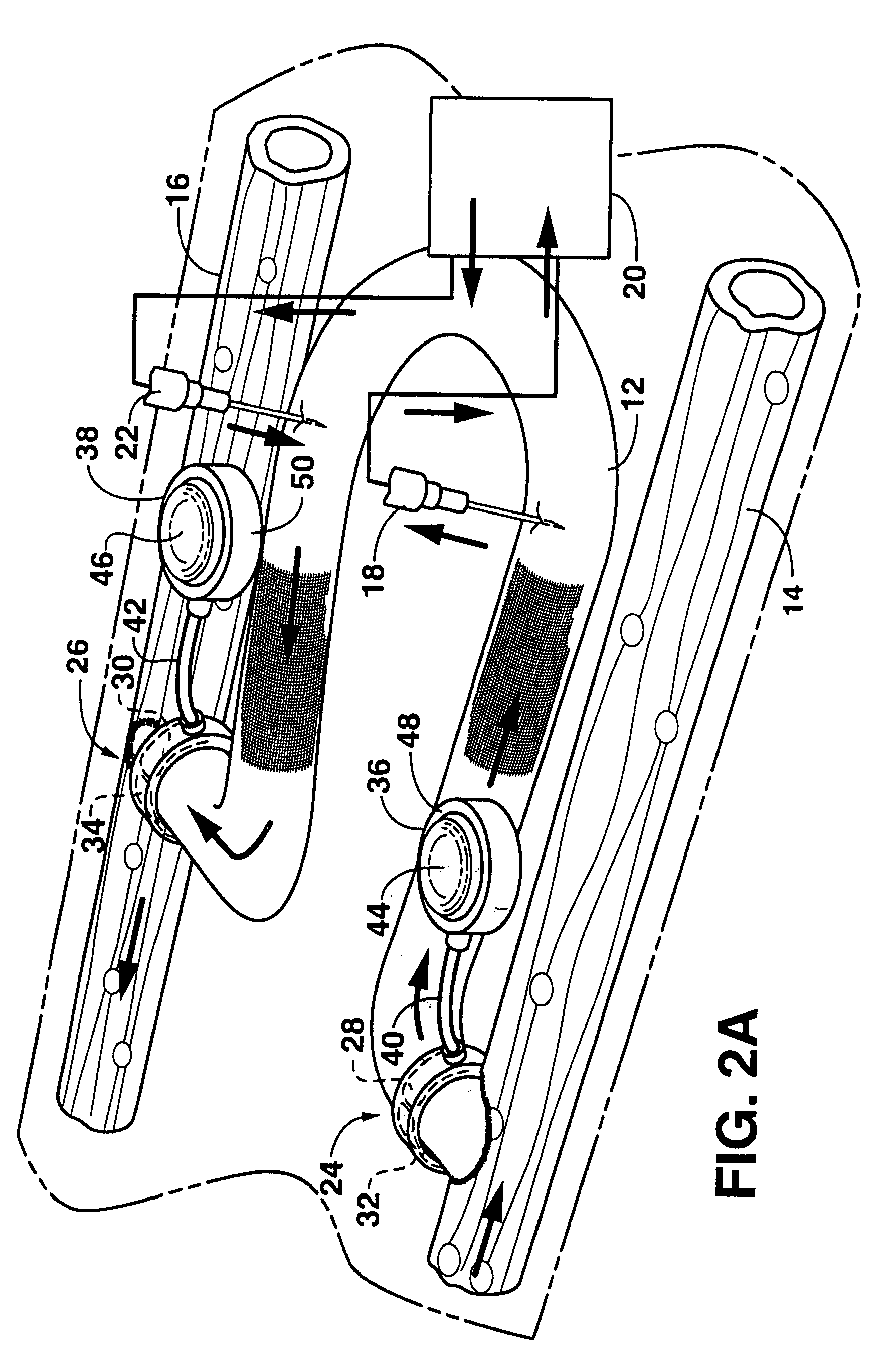
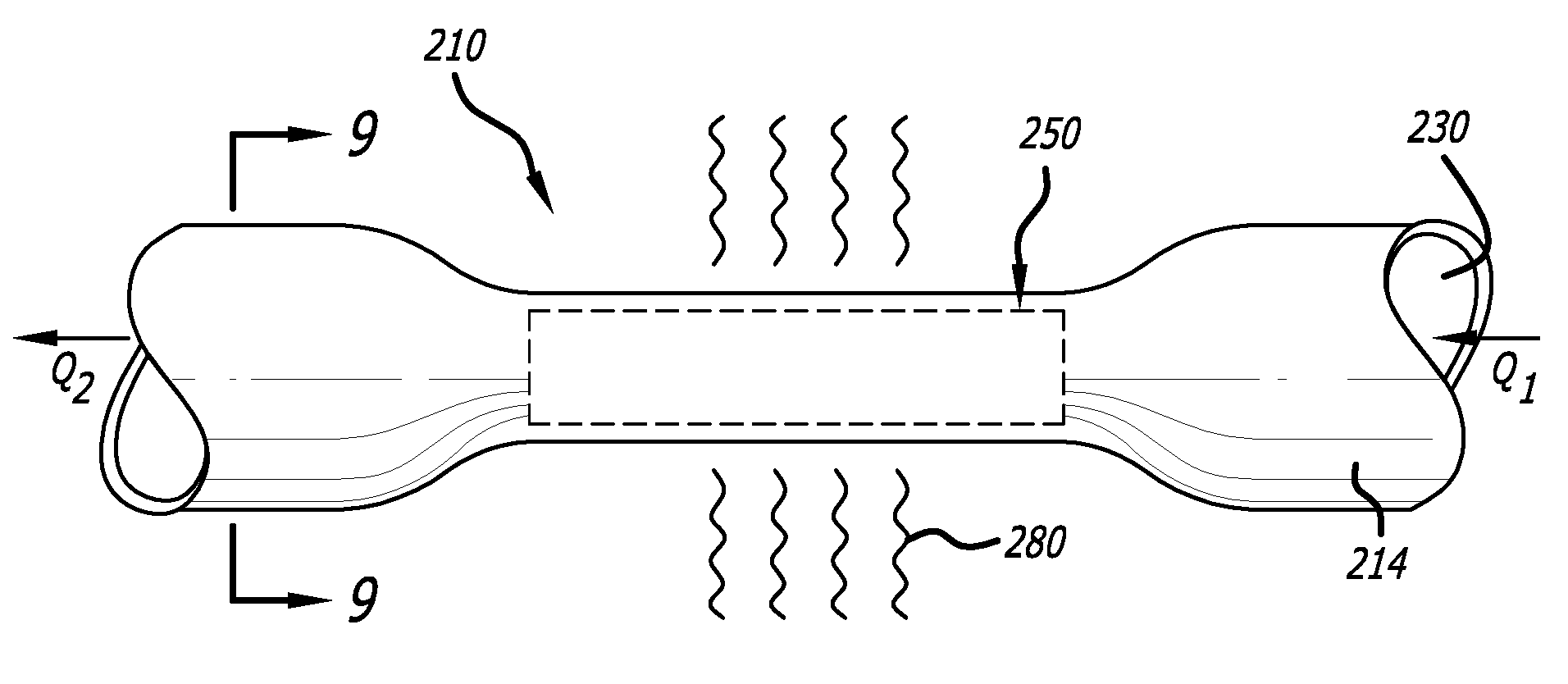
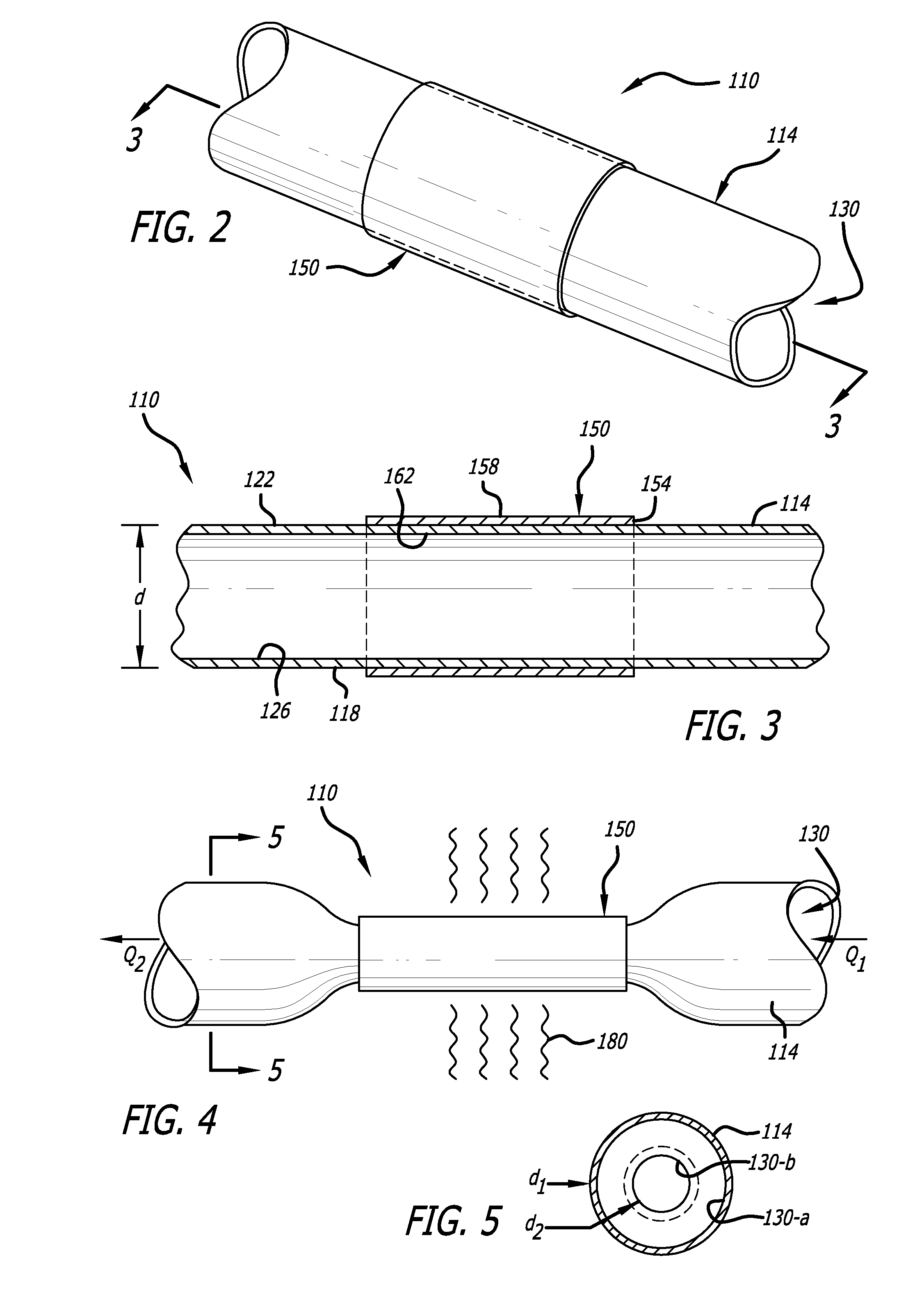
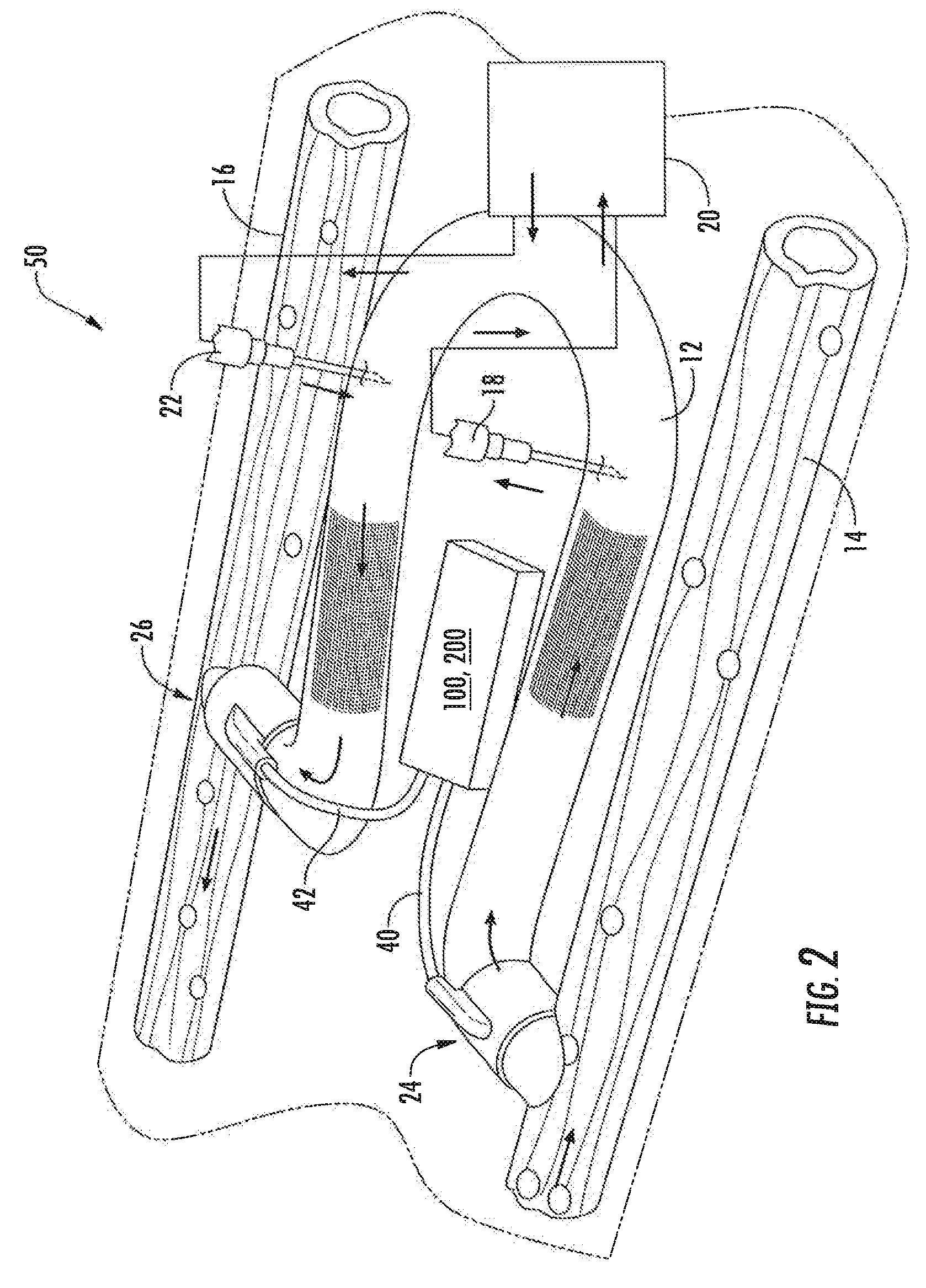
An arteriovenous graft system is described. The arteriovenous graft system includes an arteriovenous graft that is well suited for use during hemodialysis. In order to minimize or prevent arterial steal, at least one valve device is positioned at the arterial end of the arteriovenous graft. In one embodiment, for instance, the arteriovenous graft system includes a first valve device positioned at the arterial end and a second valve device positioned at the venous end. In one embodiment, the valve devices may include an inflatable balloon that, when inflated, constricts and closes off the arteriovenous graft. By minimizing or preventing arterial steal, other complications associated with arteriovenous grafts are also avoided. For instance, the present invention is also well suited to preventing arteriovenous graft thrombosis, eliminating dialysis needle hole bleeding, and eliminating or minimizing arteriovenous graft pseudoaneurism formation.
Owner:DIAXAMED LLC
Drug delivery systems, kits, and methods for administering zotarolimus and paclitaxel to blood vessel lumens
A system and compositions including zotarolimus and paclitaxel are disclosed, as well as methods of delivery, wherein the drugs have effects that complement each other. Medical devices are disclosed which include supporting structures that include at least one pharmaceutically acceptable carrier or excipient, which carrier or excipient can include one or more therapeutic agents or substances, with the carrier including at least one coating on the surface thereof, and the coating associated with the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
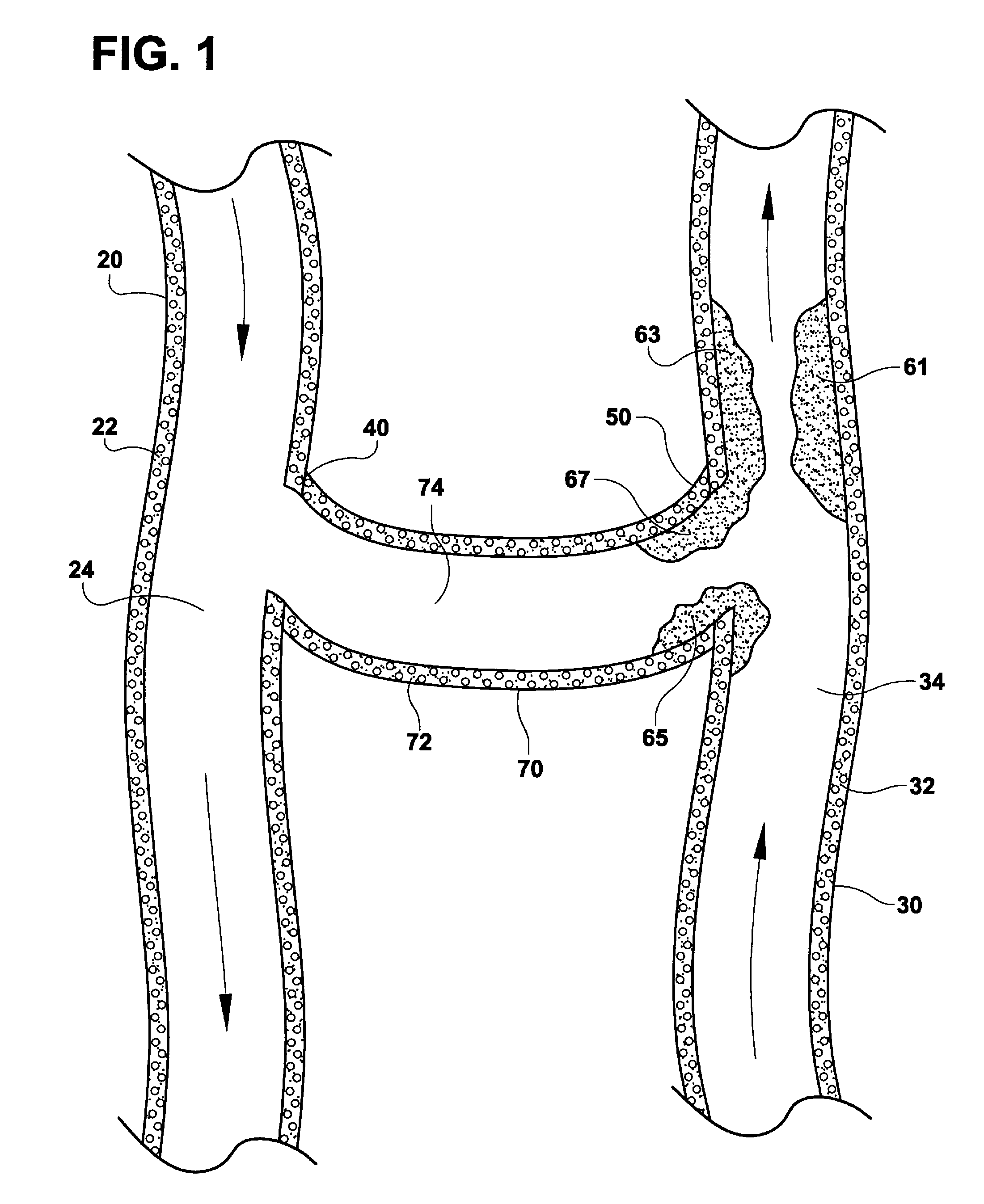
Method and apparatus for detecting vulnerable atherosclerotic plaque
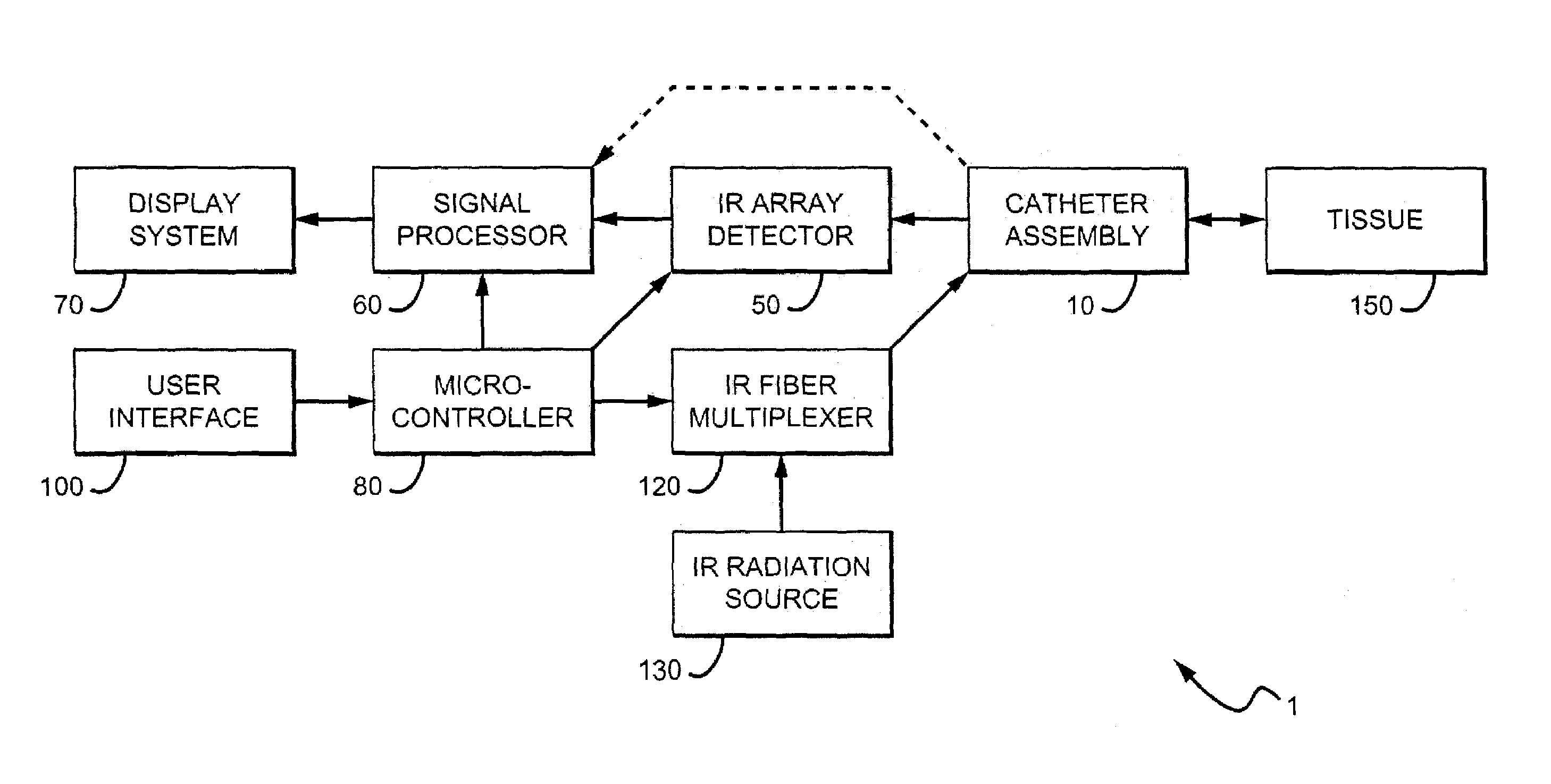
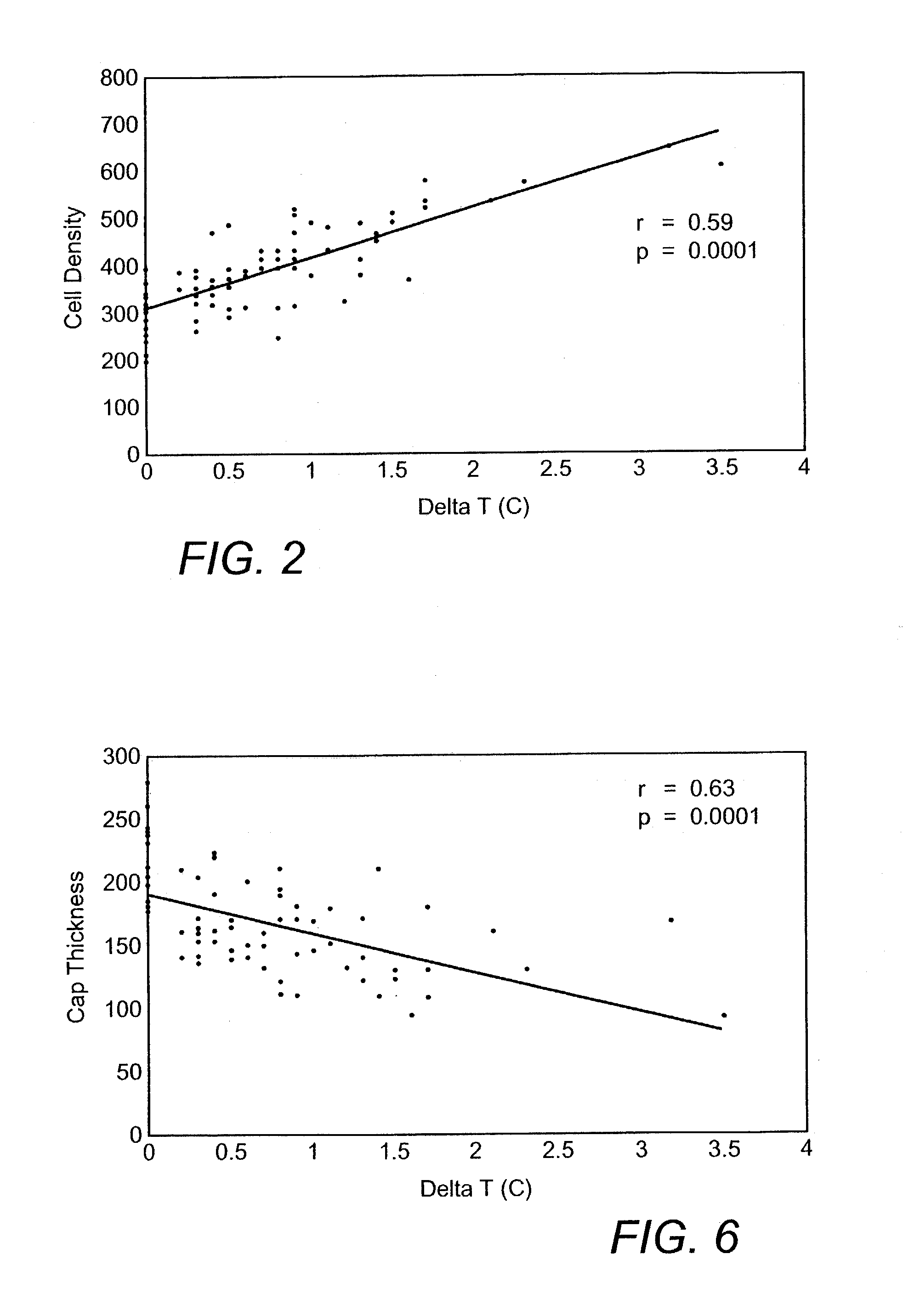
Methods and devices are disclosed for detecting vulnerable atherosclerotic plaque, or plaque at risk of reducing blood flow in a vessel, by identifying a region of elevated temperature along a living vessel wall. The disclosure that human atherosclerotic plaque with measurable temperature heterogeneity has the morphological characteristics of plaque that is likely to ulcerate provides a new and sensitive technique for detecting and treating these dangerous plaques before myocardial infarction and its consequences occur. The disclosed methods are advantageous over conventional plaque detection techniques because they are capable of differentiating between those plaques that are at great risk of rupture, fissure, or ulceration, and consequent thrombosis and occlusion of the artery, and those that are not presently at risk. Infrared heat-sensing catheters useful for identifying potentially fatal arterial plaques in patients with disease of the coronary or other arteries are also described. In some embodiments a coherent infrared fiber optic bundle is employed to radially and longitudinally explore a luminal wall to identify inflamed, heat-producing, atherosclerotic plaque. Certain other methods and devices are disclosed which are particularly suited for non-invasively identifying and then monitoring the progression or amelioration of an inflamed plaque in a patient, and for monitoring for onset of inflammation in an implanted arteriovenous graft. Also disclosed are thermocouple basket catheters and thermistor basket catheters which are also capable of detecting temperature heterogeneity along a vessel wall.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for Treatment of Complications Associated with Arteriovenous Grafts and Fistulas Using Electroporation
Electroporation devices and methods for use in the treatment of complications, such as thrombosis, stenotic segments, or infections, associated with an arteriovenous graft or fistula are provided. The devices include at least two electrodes. The electrodes are adapted to be positioned near the target zone of complication for applying electrical pulses and thereby causing electroporation. In a preferred embodiment, the electroporation pulses are sufficient to subject substantially all cells within the target zone to irreversible electroporation without creating a thermally damaging effect.
Owner:ANGIODYNAMICS INC
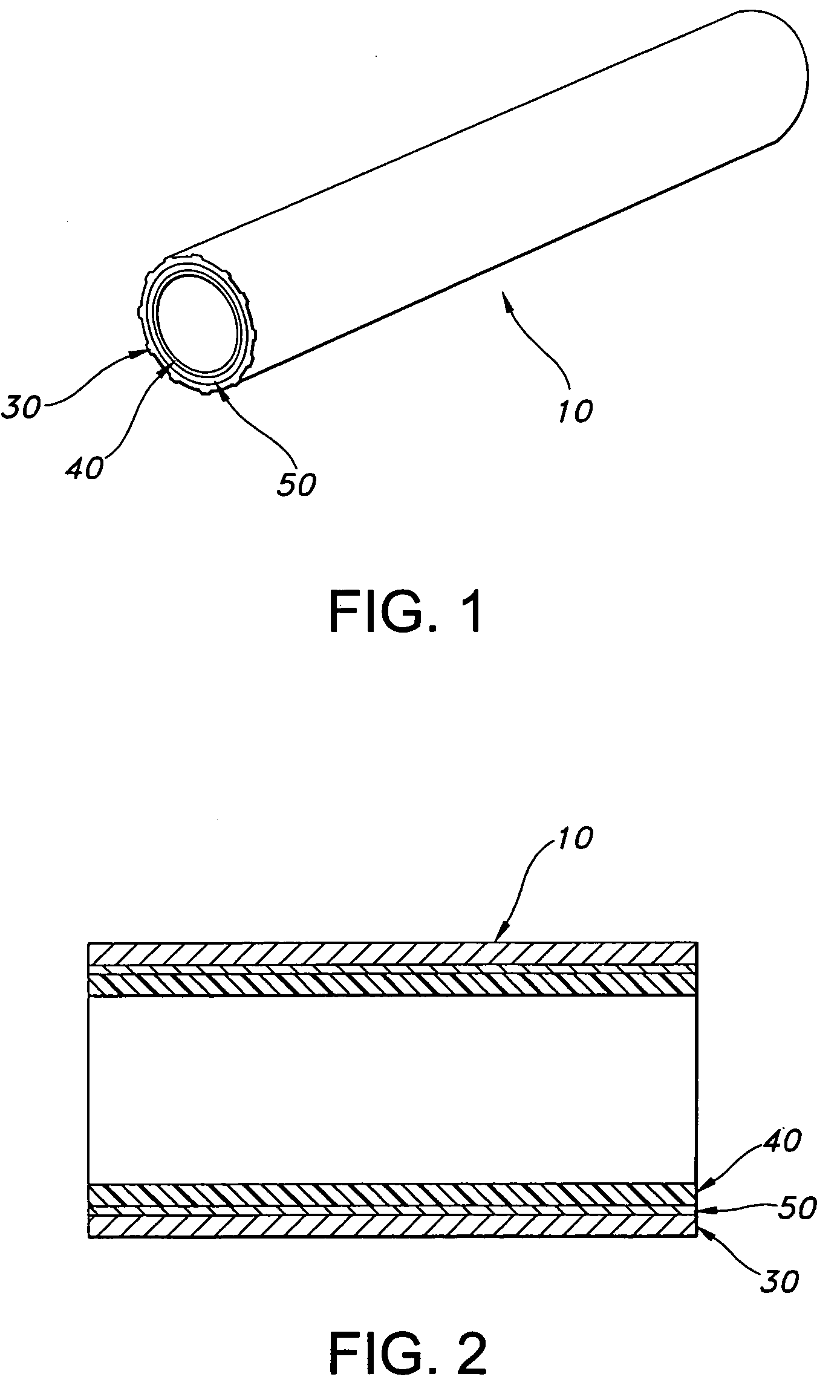
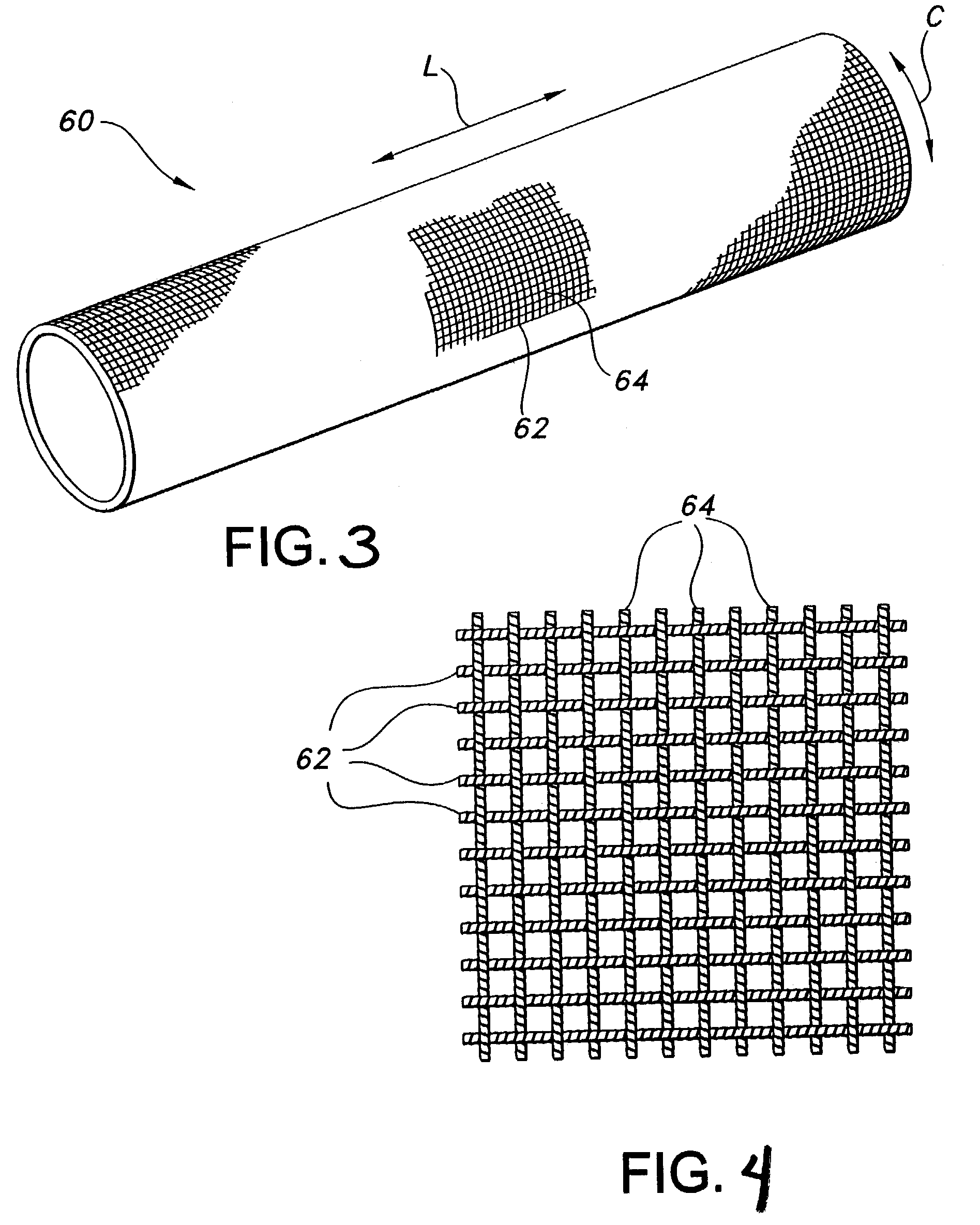
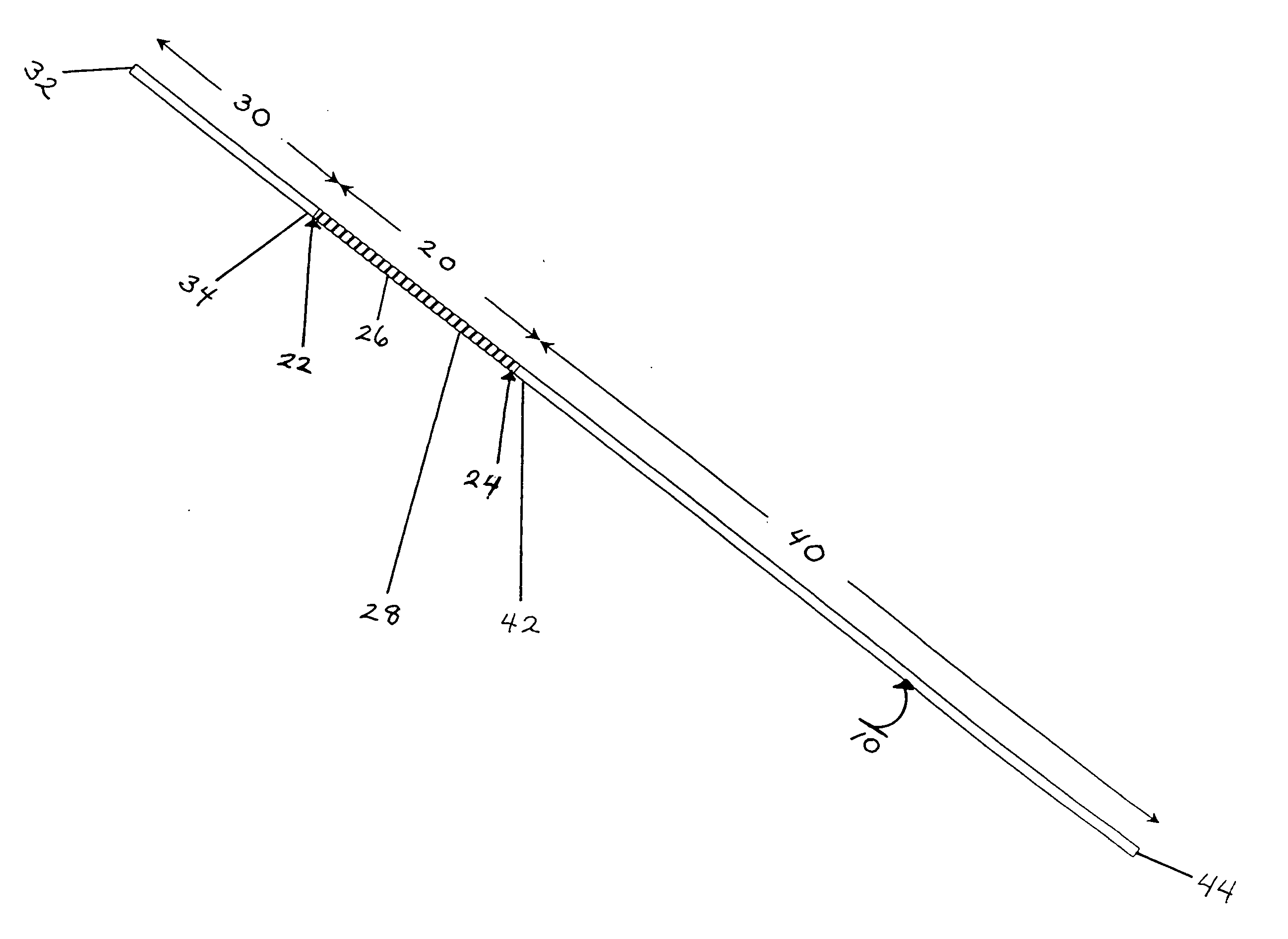
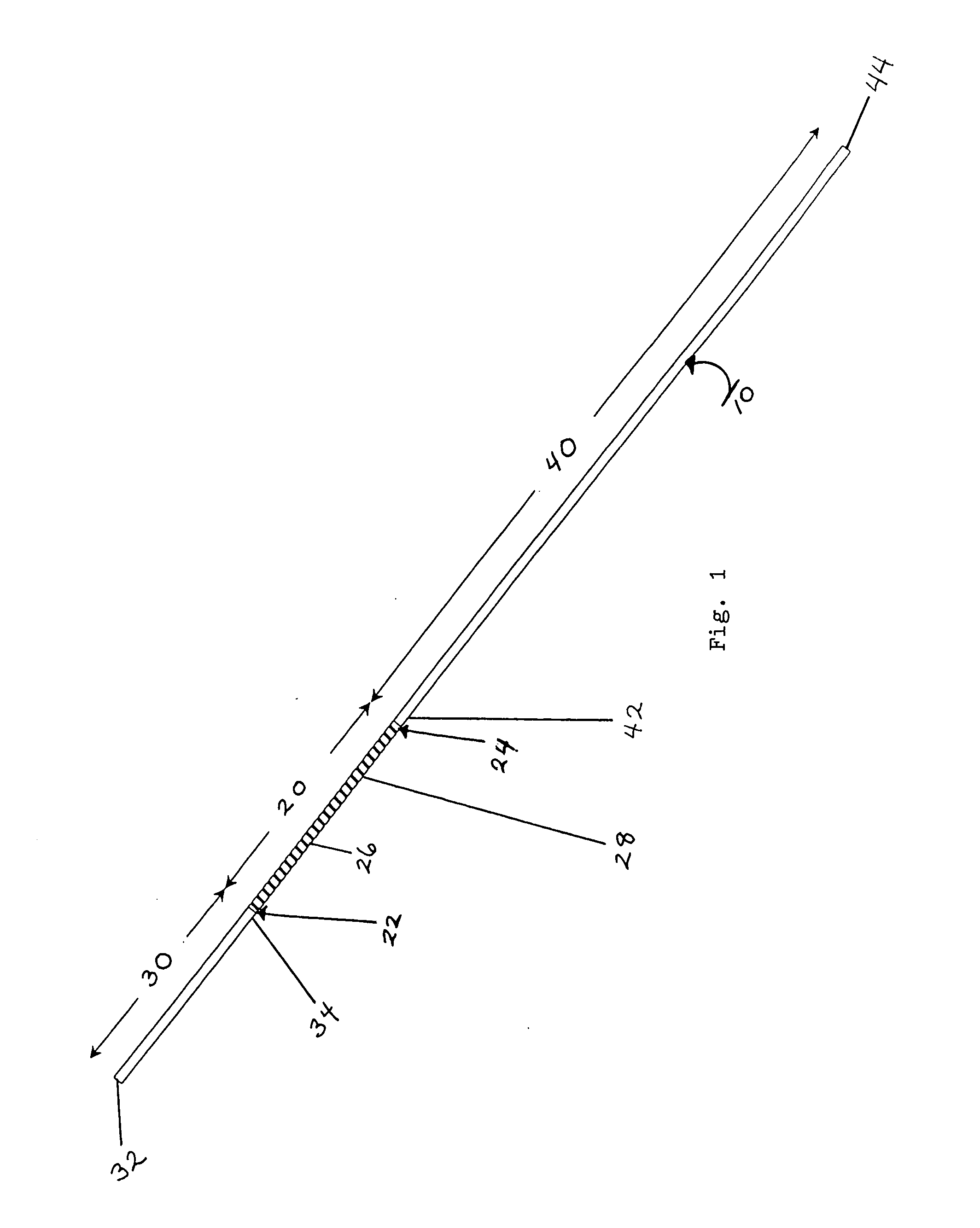
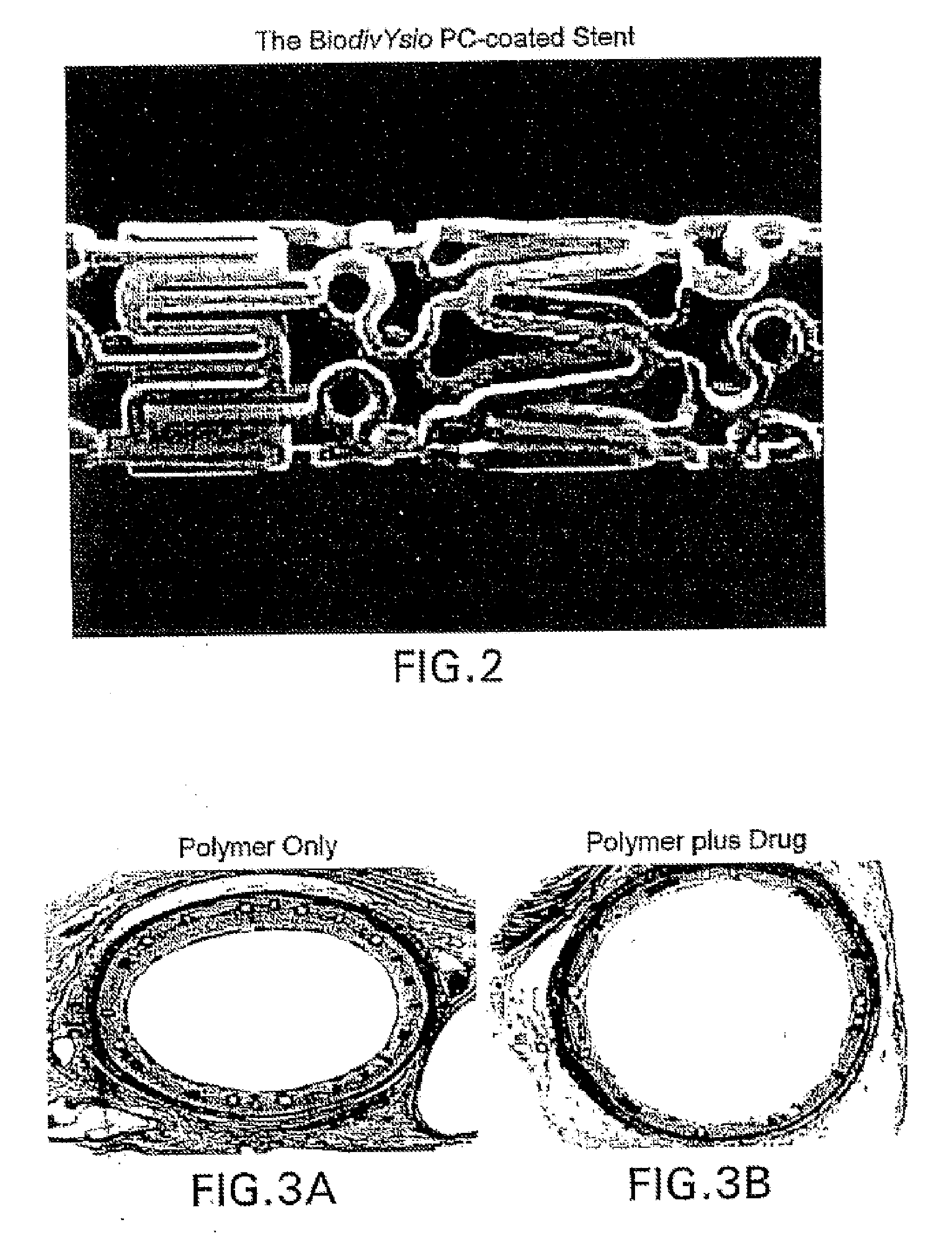
AV grafts with rapid post-operative self-sealing capabilities
The invention provides a self-sealing arteriovenous graft including a tube having a polymeric inner layer defining a lumen through which blood may flow and an outer textile layer. The outer textile layer is concentrically disposed about the inner layer. Furthermore, an intermediate self-sealing layer is concentrically positioned between the inner and outer layers. The self-sealing layer includes a biocompatible polymer.
Owner:MAQUET CARDIOVASCULAR LLC
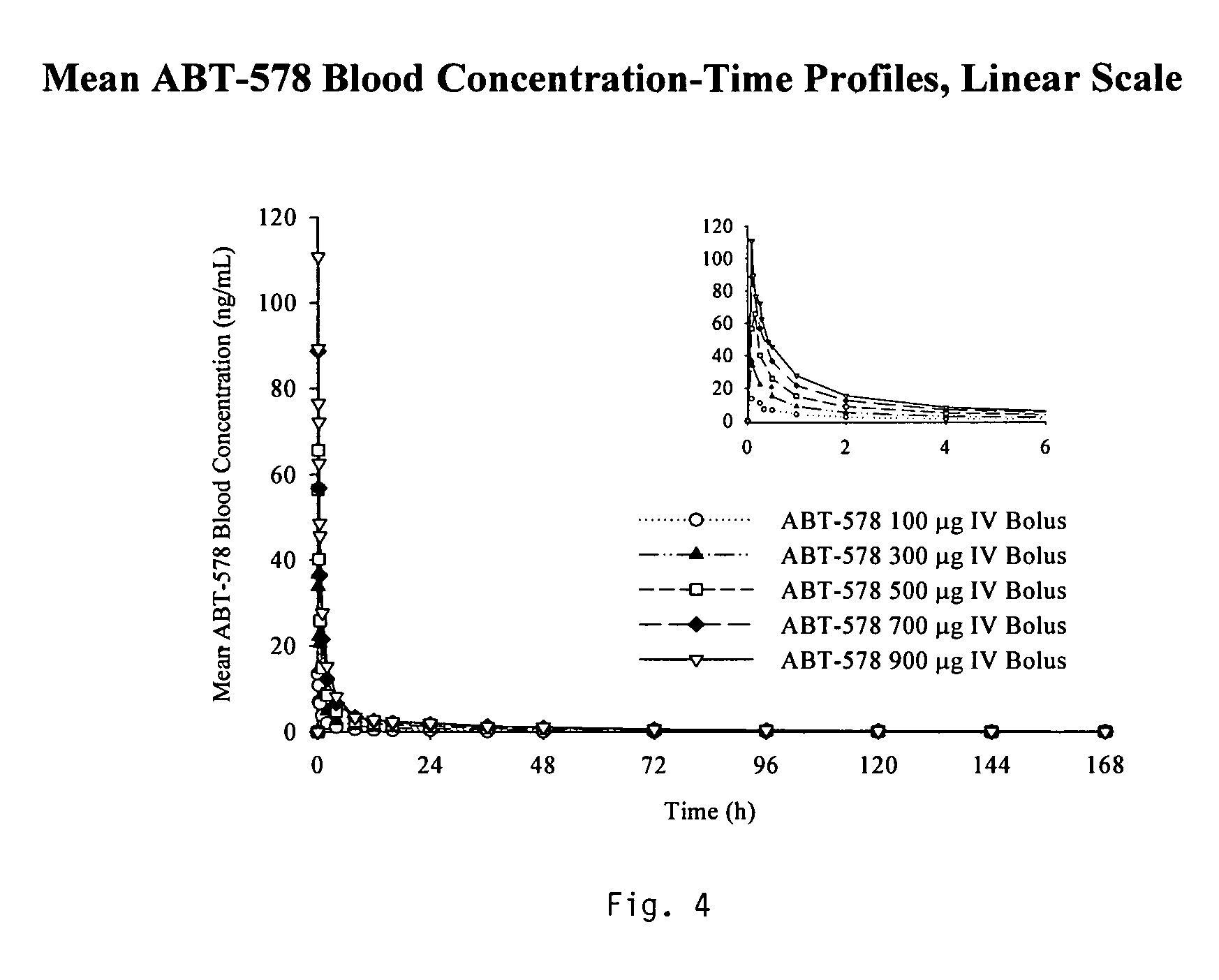
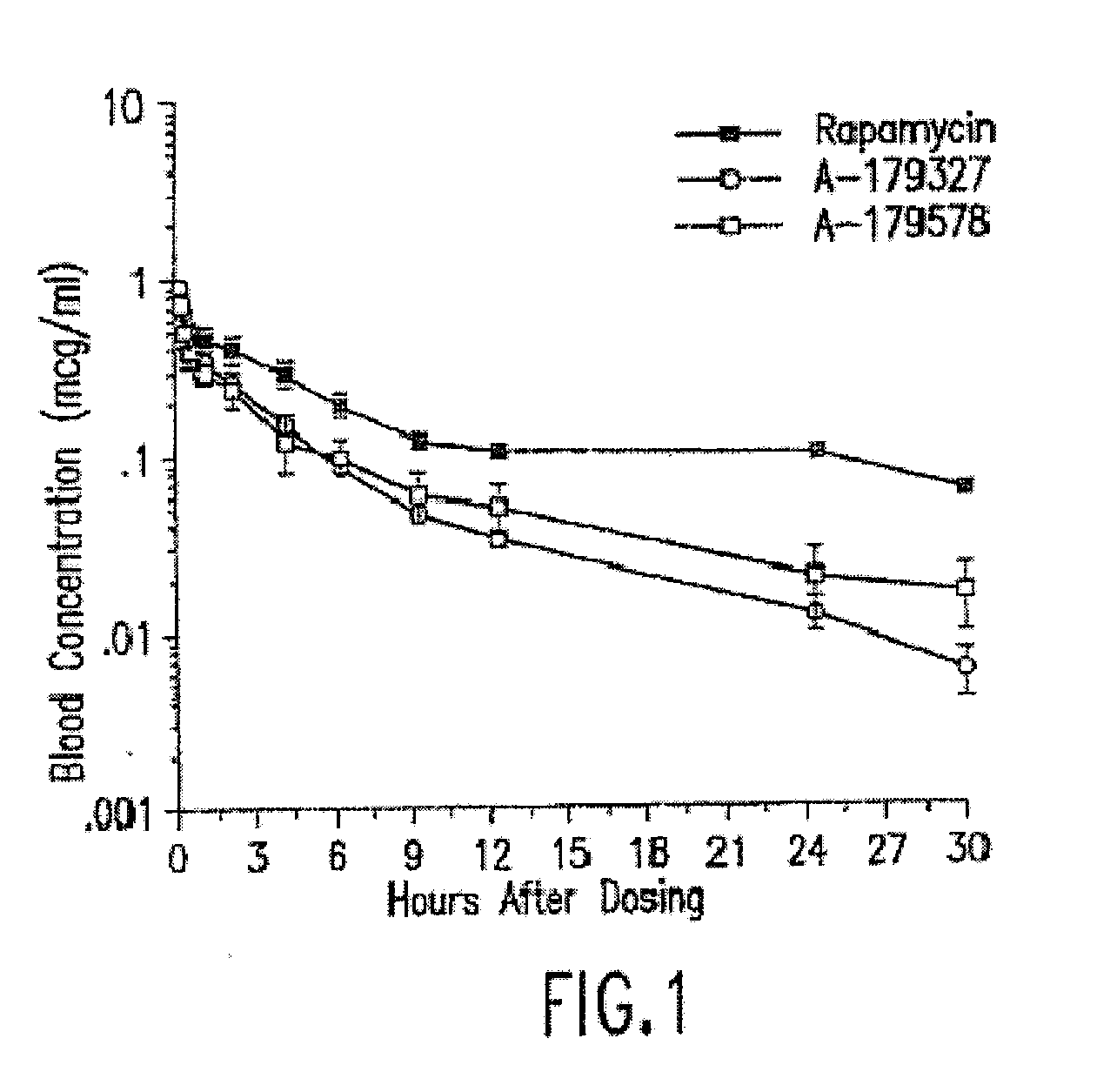
Compositions and methods of administering rapamycin analogs using medical devices for long-term efficacy
Systems and compositions comprising zotarolimus that are safer, more effective and produce less inflammation than rapamycin and paclitaxel systems are disclosed. Medical devices comprising supporting structures capable of containing or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient can contain one or more therapeutic agents or substances, with the carrier including a coating on the surface thereof, and the coating having the therapeutic compounds, including, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
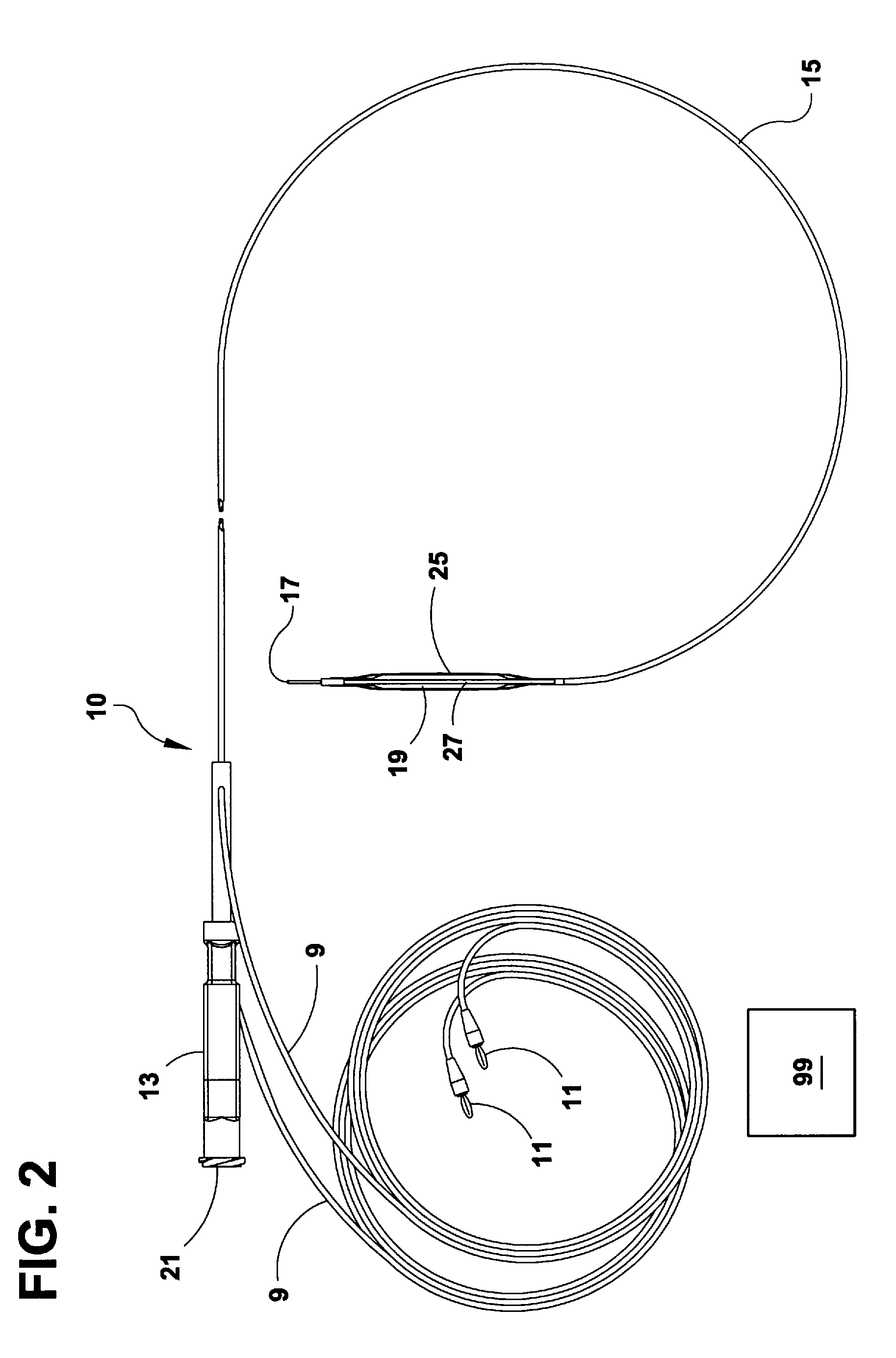
Apparatus and method for creating an arterio-venous connection in hemodialysis maintenance
The present invention provides a kit apparatus and a methodology to prevent the primary causes of arterio-venous graft thrombosis; and provides a durable vascular access for successful long term use in hemodialysis. The invention employs a patient-customized prosthetic endograft as an subcutaneously implanted vascular access; and utilizes a surgical method for endovascular insertion of the prosthetic endograft into a pre-chosen vein, which does not require a distal anastomosis, and thus allows the distal outflow end of the implanted vascular access to remain unattached and freely floating at a precisely located anatomic position within the internal lumen the pre-chosen vein.
Owner:PERMAGRAFT +1
Compositions, systems, and kits for administering zotarolimus and paclitaxel to blood vessel lumens
A system and compositions including zotarolimus and paclitaxel are disclosed, as well as methods of delivery, wherein the drugs have effects that complement each other. Medical devices are disclosed which include supporting structures that include at least one pharmaceutically acceptable carrier or excipient, which carrier or excipient can include one or more therapeutic agents or substances, with the carrier including at least one coating on the surface thereof, and the coating associated with the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
Methods of administering rapamycin analogs with anti-inflammatories using medical devices
A medical device comprising a supporting structure capable of including or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient may include one or more therapeutic agents or substances, with the carrier including a coating on the surface thereof, and the coating including the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to, This drug can be used in combination with another drug including those selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
Arteriovenous access for hemodialysis employing a vascular balloon catheter and an improved hybrid endovascular technique
The present invention provides a kit apparatus and a methodology to prevent the primary causes of arteriovenous graft thrombosis; and provides a durable vascular access for successful long term use in hemodialysis. The invention employs a patient-customized prosthetic endograft as an subcutaneously implanted vascular access; and utilizes a surgical method for endovascular insertion of the prosthetic endograft into a pre-chosen vein, which does not require a distal anastomosis, and thus allows the distal outflow end of the implanted vascular access to remain unattached and freely floating at a precisely located anatomic position within the internal lumen the pre-chosen vein.
Owner:SMEGO DOUGLAS R
Medical devices containing rapamycin analogs
InactiveUS20050175660A1Reduce restenosisReduce restenosis rateBiocideOrganic chemistryTherapeutic effectAnti platelet
A medical device comprising a supporting structure capable of containing or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient may contain one or more therapeutic agents or substances, with the carrier preferably including a coating on the surface thereof, and the coating containing the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to, This drug can be used in combination with another drug including those selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
Arteriovenous access valve system and process
ActiveUS20060229548A1Eliminates orReduces arterial steal and graft thrombosisOther blood circulation devicesMedical devicesVein graftHaemodialysis machine
An arteriovenous graft system is described. The arteriovenous graft system includes an arteriovenous graft that is well suited for use during hemodialysis. In order to minimize or prevent arterial steal, at least one valve device is positioned at the arterial end of the arteriovenous graft. In one embodiment, for instance, the arteriovenous graft system includes a first valve device positioned at the arterial end and a second valve device positioned at the venous end. In one embodiment, the valve devices may include an inflatable balloon that, when inflated, constricts and closes off the arteriovenous graft. By minimizing or preventing arterial steal, other complications associated with arteriovenous grafts are also avoided. For instance, the present invention is also well suited to preventing arteriovenous graft thrombosis, eliminating dialysis needle hole bleeding, and eliminating or minimizing arteriovenous graft pseudoaneurism formation.
Owner:DIAXAMED LLC
Methods of administering tetrazole-containing rapamycin analogs with other therapeutic substances using medical devices
InactiveUS7399480B2Reduce probabilityReduces restenosis in vasculatureBiocideOrganic chemistryAnti plateletCytostatic drugs
A medical device comprising a supporting structure capable of containing or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient may contain one or more therapeutic agents or substances, with the carrier preferably including a coating on the surface thereof, and the coating containing the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to,This drug can be used in combination with another drug including those selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
Medical devices containing rapamycin analogs
InactiveUS20050032826A1Reduce probabilityReduces restenosis in vasculatureBiocideAntimycoticsArteriovenous graftsMedical treatment
A medical device comprising a supporting structure having a coating on the surface thereof, the coating containing a therapeutic substance, such as, for example, a drug. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to,
Owner:MOLLISON KARL W +4
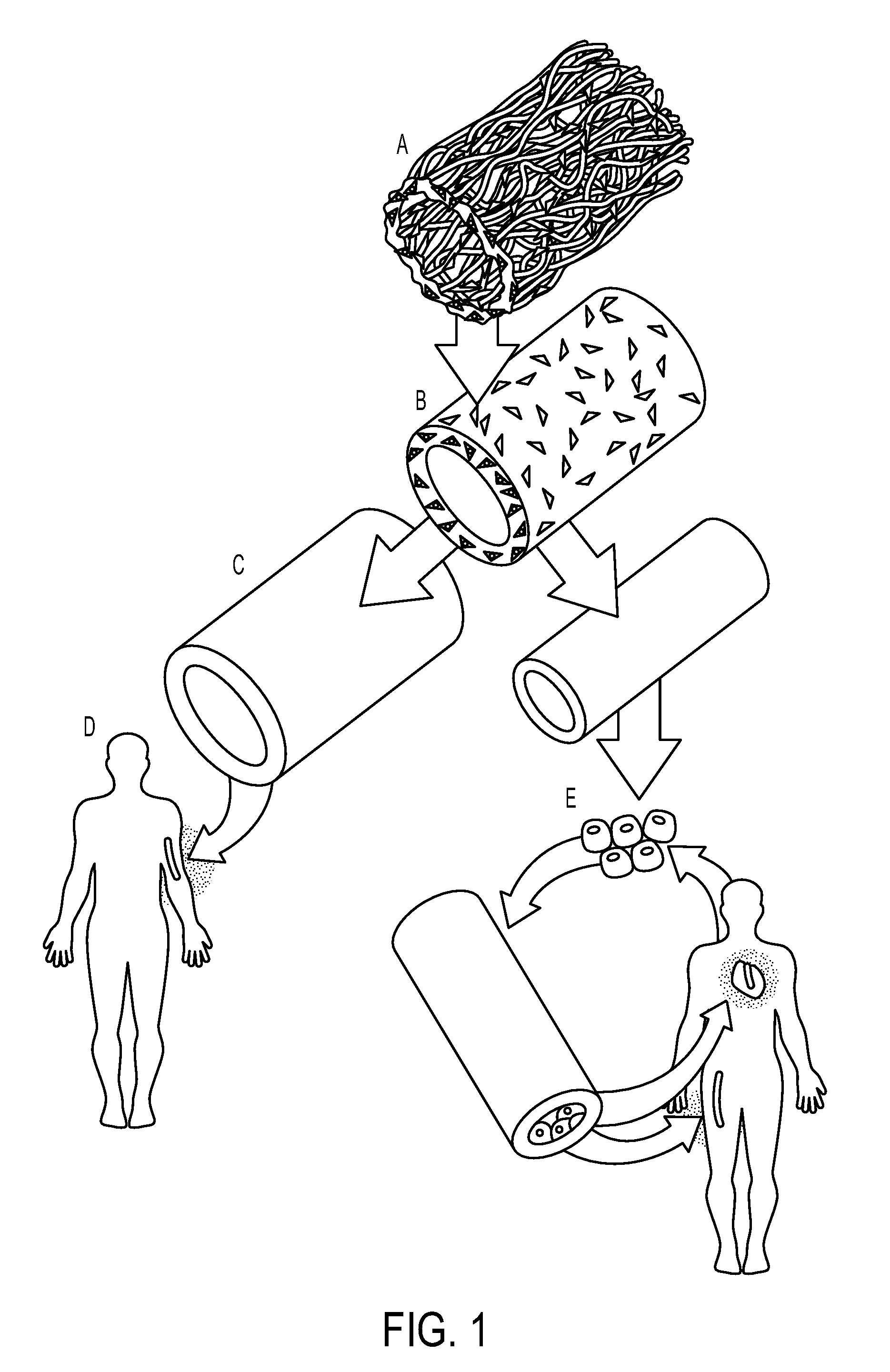
Tissue-Engineered Constructs
InactiveUS20130013083A1Improve degradation rateImprove wettabilityUrethraeCell culture supports/coatingCell-Extracellular MatrixVein graft
Constructs including a tubular biodegradable polyglycolic acid scaffold may be coated with extracellular matrix proteins and are substantially acellular. The constructs can be utilized as an arteriovenous graft, a coronary graft, an arterial graft, a venous graft, a duct graft, a skin graft, or a urinary graft or conduit.
Owner:HUMACYTE INC
Methods And Compositions For Enhacing Vascular Access
Disclosed is an implantable material comprising a biocompatible matrix and cells which, when provided to a vascular access structure, can promote functionality generally. For example, implantable material of the present invention can enhance maturation of an arteriovenous native fistula as well as prolong the fistula in a mature, functional state suitable for dialysis. Additionally, the present invention can promote formation of a functional arteriovenous graft suitable for dialysis as well as promote formation of a functional peripheral bypass graft. Implantable material can be configured as a flexible planar form or a flowable composition with shape retaining properties suitable for implantation at, adjacent or in the vicinity of an anastomoses or arteriovenous graft. According to the methods disclosed herein, the implantable material is provided to an exterior surface of a blood vessel. Certain embodiments of the flexible planar form define a slot. The materials and methods of the present invention comprise cells, preferably endothelial cells or cells having an endothelial-like phenotype.
Owner:SHIRE REGENERATIVE MEDICINE INC
Compositions and methods of administering paclitaxel with other drugs using medical devices
Systems and compositions comprising paclitaxel and a second drug, such as rapamycin, analogs, derivatives, salts and esters thereof are disclosed, as well as methods of delivery wherein the drugs have effects that complement each other. Medical devices comprising supporting structures capable of including or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient can contain one or more therapeutic agents or substances, with the carrier preferably including a coating on the surface thereof, and the coating including the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
Arteriovenous Access Valve System and Process
ActiveUS20130303959A1Eliminates orReduces arterial steal and graft thrombosisDialysis systemsMedical devicesHemodialysisVein graft
An arteriovenous graft system is described. The arteriovenous graft system includes an arteriovenous graft that is well suited for use during hemodialysis. In order to minimize or prevent arterial steal, at least one valve device is positioned at the arterial end of the arteriovenous graft. In one embodiment, a subcutaneous arteriovenous graft system is described. The system includes an arteriovenous graft having an arterial end and an opposite venous end with a first valve device positioned at the arterial end of the arteriovenous graft and a second valve device positioned at the venous end of the arteriovenous graft. The system also includes an actuator having an accumulator. The actuator is in communication with both the first valve device and the second valve device and is configured to cause each valve device to open or close simultaneously. The accumulator assists in maintaining a generally constant pressure when the actuator causes each valve device to close.
Owner:DIAXAMED LLC
Medical devices containing rapamycin analogs
InactiveUS20060198870A1Reduce probabilityReduces restenosis in vasculatureBiocideAntimycoticsAnti plateletAntiproliferative Agents
A medical device comprising a supporting structure having a coating on the surface thereof, the coating containing a therapeutic substance, such as, for example, a drug. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to, This drug can be used in combination with a drug selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
Compositions, systems, kits, and methods of administering rapamycin analogs with paclitaxel using medical devices
A system and compositions including zotarolimus and paclitaxel are disclosed, as well as methods of delivery, wherein the drugs have effects that complement each other. Medical devices are disclosed which include supporting structures that include at least one pharmaceutically acceptable carrier or excipient, which carrier or excipient can include one or more therapeutic agents or substances, with the carrier including at least one coating on the surface thereof, and the coating associated with the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
Medical Devices Containing Rapamycin Analogs
InactiveUS20080145402A1Reduce probabilityReduces restenosis in vasculatureBiocideSurgeryAnti plateletAntiproliferative Agents
A medical device comprises a supporting structure capable of containing or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient may contain one or more therapeutic agents or substances, with the carrier preferably including a coating on the surface thereof, and the coating containing the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to drugs of Formula (I). The drugs of Formula (I) can be used in combination with another drug including those selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
Tissued-engineered constructs
ActiveUS20130345824A1Improve degradation rateImprove wettabilityUrethraeCell culture supports/coatingCell-Extracellular MatrixMedicine
The present invention provides constructs including a tubular biodegradable polyglycolic acid scaffold, wherein the scaffold may be coated with extracellular matrix proteins and substantially acellular. The constructs can be utilized as an arteriovenous graft, a coronary graft, a peripheral artery bypass conduit, or a urinary conduit. The present invention also provides methods of producing such constructs.
Owner:HUMACYTE INC
Arteriovenous access valve system and process
ActiveUS8114044B2Eliminates orReduces arterial steal and graft thrombosisOther blood circulation devicesMedical devicesVein graftHaemodialysis machine
An arteriovenous graft system is described. The arteriovenous graft system includes an arteriovenous graft that is well suited for use during hemodialysis. In order to minimize or prevent arterial steal, at least one valve device is positioned at the arterial end of the arteriovenous graft. In one embodiment, for instance, the arteriovenous graft system includes a first valve device positioned at the arterial end and a second valve device positioned at the venous end. In one embodiment, the valve devices may include an inflatable balloon that, when inflated, constricts and closes off the arteriovenous graft. By minimizing or preventing arterial steal, other complications associated with arteriovenous grafts are also avoided. For instance, the present invention is also well suited to preventing arteriovenous graft thrombosis, eliminating dialysis needle hole bleeding, and eliminating or minimizing arteriovenous graft pseudoaneurism formation.
Owner:DIAXAMED LLC
Arteriovenous Access Valve System and Process
InactiveUS20090030498A1Eliminates and least reduces arterial stealEliminates and least reduces and graft thrombosisDialysis systemsMedical devicesArteriovenous graftsHemodialysis
An arteriovenous graft system is described. The arteriovenous graft system includes an arteriovenous graft that is well suited for use during hemodialysis. In order to minimize or prevent arterial steal, at least one valve device is positioned at the arterial end of the arteriovenous graft. In one embodiment, a subcutaneous arteriovenous graft system is described. The system includes an arteriovenous graft having an arterial end and an opposite venous end with a first valve device positioned at the arterial end of the arteriovenous graft and a second valve device positioned at the venous end of the arteriovenous graft. The system also includes an actuator having an accumulator. The actuator is in communication with both the first valve device and the second valve device and is configured to cause each valve device to open or close simultaneously. The accumulator assists in maintaining a generally constant pressure when the actuator causes each valve device to close.
Owner:CREATIVASC MEDICAL
Arteriovenous access valve system and process
InactiveUS20080300528A1Eliminates and least reduces arterial stealEliminates and least reduces and graft thrombosisStentsMedical devicesVeinHemodialysis
An arteriovenous graft system is described. The arteriovenous graft system includes an arteriovenous graft that is well suited for use during hemodialysis. In order to minimize or prevent arterial steal, at least one valve device is positioned at the arterial end of the arteriovenous graft. In one embodiment, for instance, the arteriovenous graft system includes a first valve device positioned at the arterial end and a second valve device positioned at the venous end. In one embodiment, the valve devices may include an inflatable balloon that, when inflated, constricts and closes off the arteriovenous graft. If desired, a single actuator can be used to open and close both valve devices.
Owner:DIAXAMED LLC
Compositions, systems, kits, and methods of administering rapamycin analogs with paclitaxel using medical devices
A system and compositions including zotarolimus and paclitaxel are disclosed, as well as methods of delivery, wherein the drugs have effects that complement each other. Medical devices are disclosed which include supporting structures that include at least one pharmaceutically acceptable carrier or excipient, which carrier or excipient can include one or more therapeutic agents or substances, with the carrier including at least one coating on the surface thereof, and the coating associated with the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
Vascular graft prosthesis with selective flow reduction
InactiveUS20100318175A1Reducing inner luminal diameterReduce flow rateStentsBlood vesselsStealing syndromeProsthesis
A vascular graft prosthesis comprising a tubular member having a luminal wall defining a lumen and an inner luminal diameter; and means for non-invasively constricting at least a portion of the luminal wall to reduce the inner luminal diameter and reduce a volumetric flow rate through the tubular member at the site of implantation of the vascular graft prosthesis, and during normal operating conditions, the means for constricting being operable to therefore facilitate an increase in a volumetric flow rate through the target limb to treat various symptoms manifesting themselves as a result of the implanted graft prosthesis, such as those resulting from an arteriovenous graft access and indicative of Steal syndrome.
Owner:CR BARD INC
Medical Devices Containing Rapamycin Analogs
InactiveUS20080153790A1Reduce probabilityReduces restenosis in vasculatureBiocideAntimycoticsAnti plateletCytostatic drugs
A medical device comprises a supporting structure capable of containing or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient may contain one or more therapeutic agents or substances, with the carrier preferably including a coating on the surface thereof, and the coating containing the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to drugs of Formula (I). The drugs of Formula (I) can be used in combination with another drug including those selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
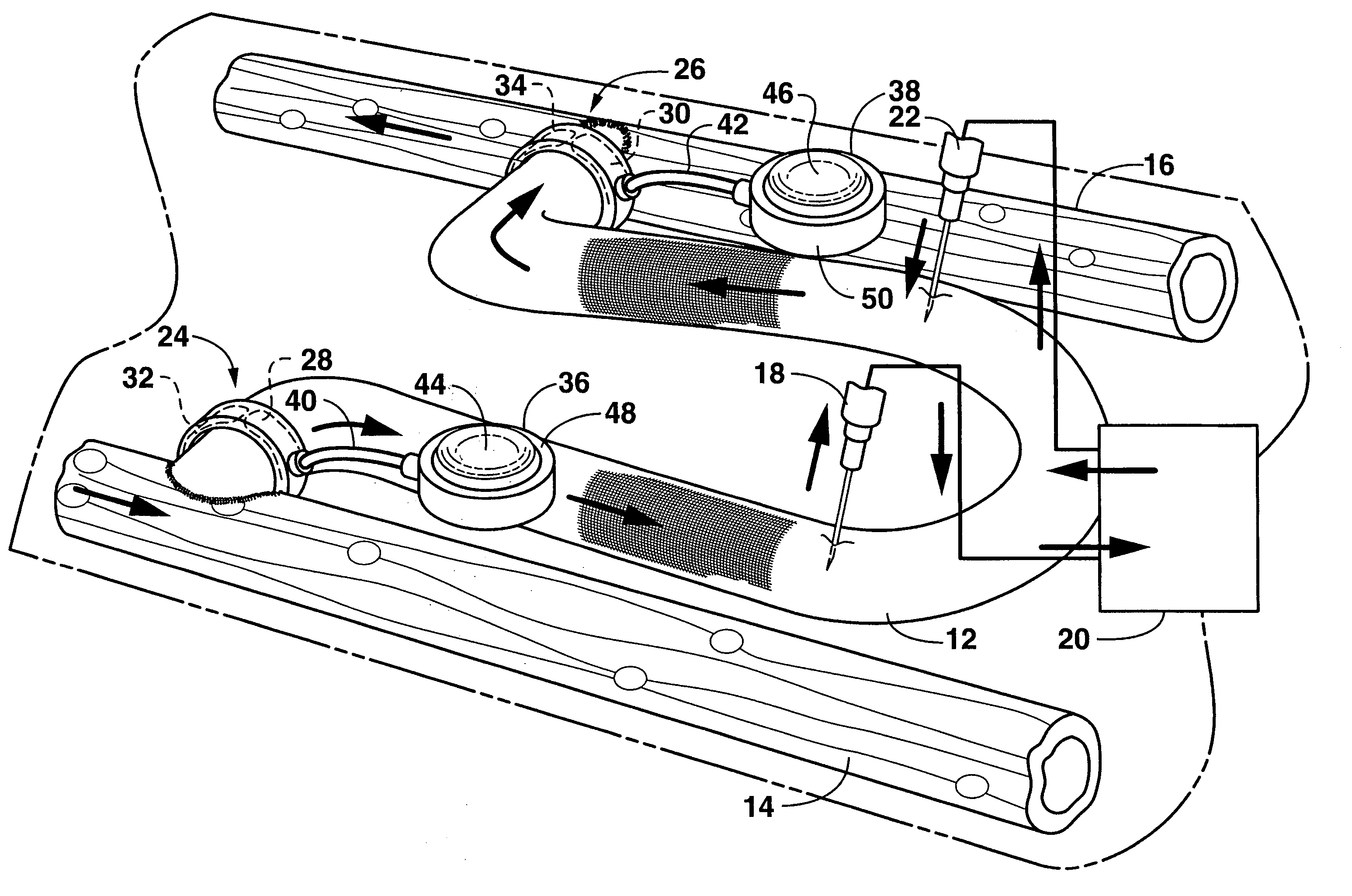
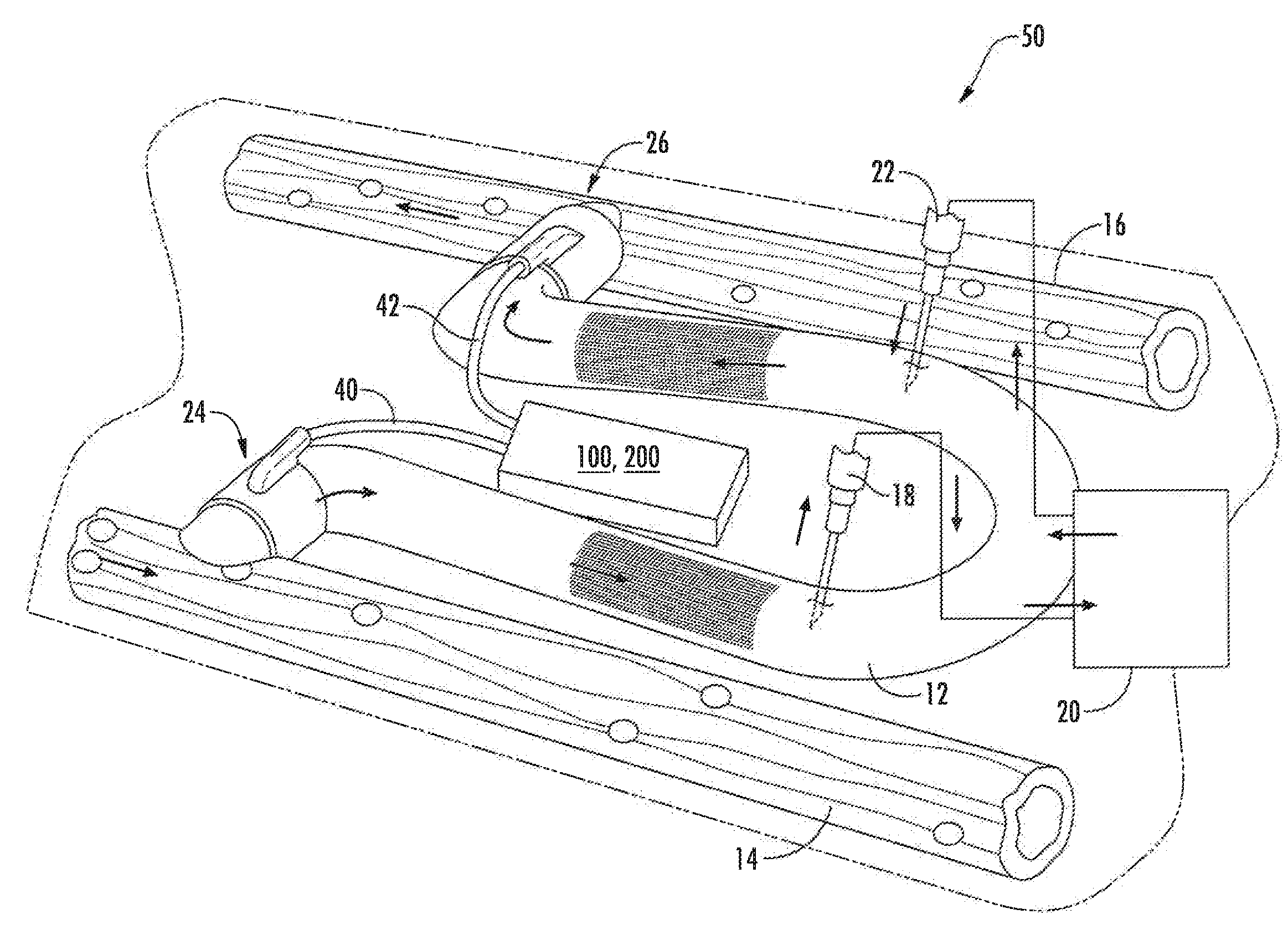
Magnetically activated arteriovenous access valve system and related methods
In one aspect, an arteriovenous access valve system may generally include a first valve configured to be positioned at or adjacent to an end of an arteriovenous graft and a second valve configured to be positioned at or adjacent to an opposite end of the arteriovenous graft. In addition, the system may include an actuator assembly in fluid communication with the first and second valves. The actuator assembly may include a housing, a driver assembly positioned within the housing and a drive magnet positioned within the housing. The drive magnet may be rotatably coupled to the driver assembly such that, when the drive magnet is rotated, the driver assembly is configured to be rotatably driven so as to supply fluid to the first and second valves or to draw fluid out of the first and second valves depending on a rotational direction of the driver assembly.
Owner:DIAXAMED LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com