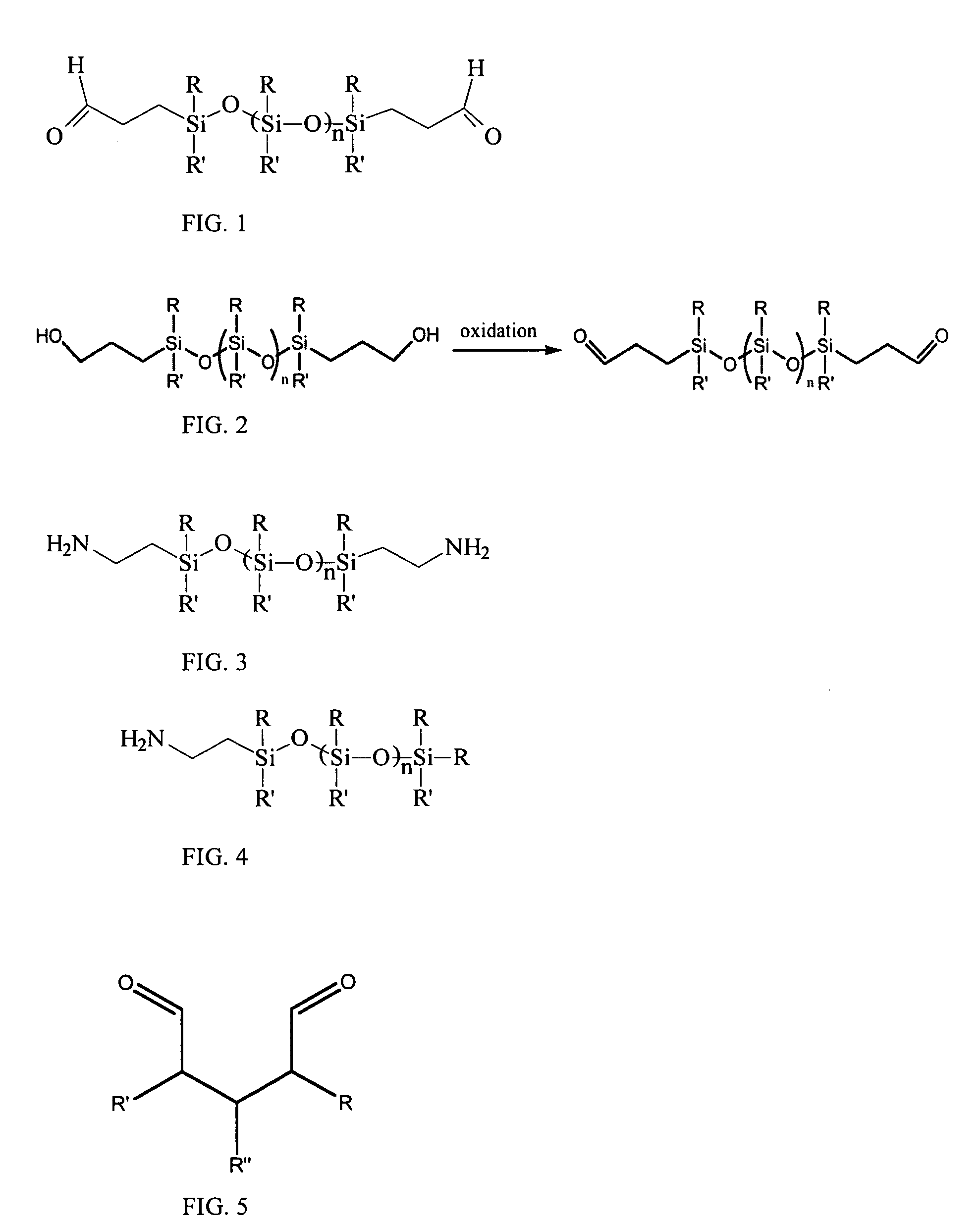
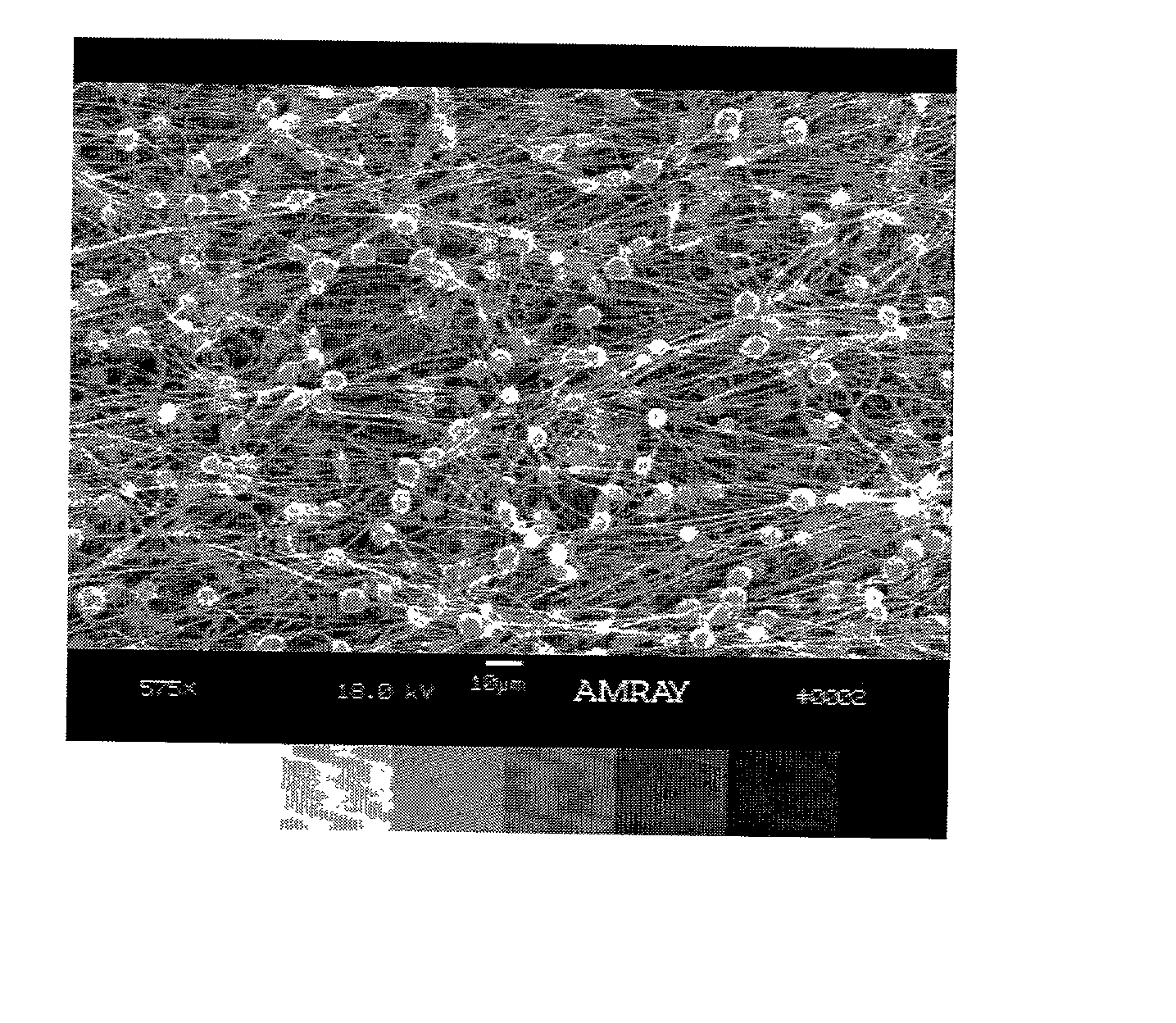
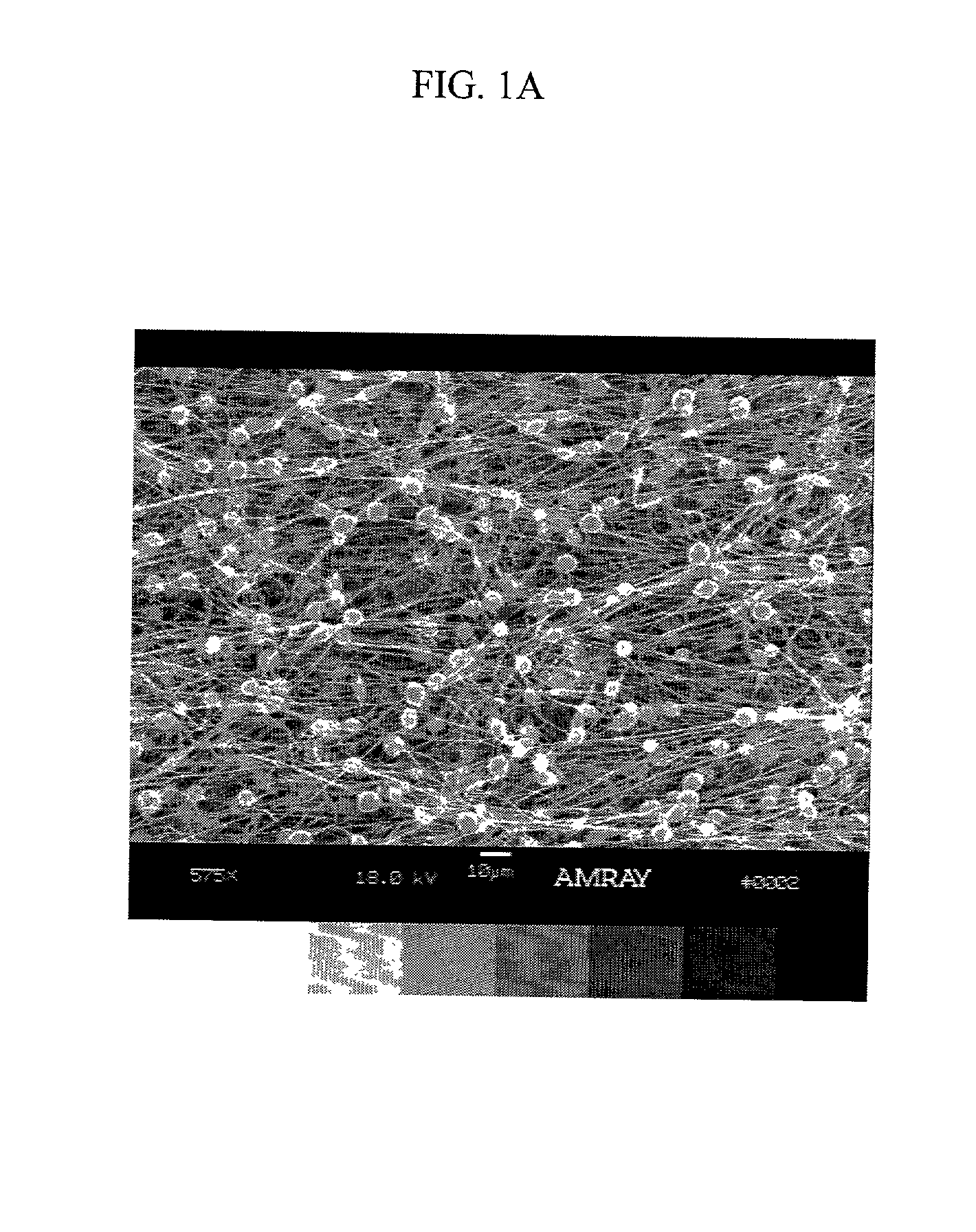
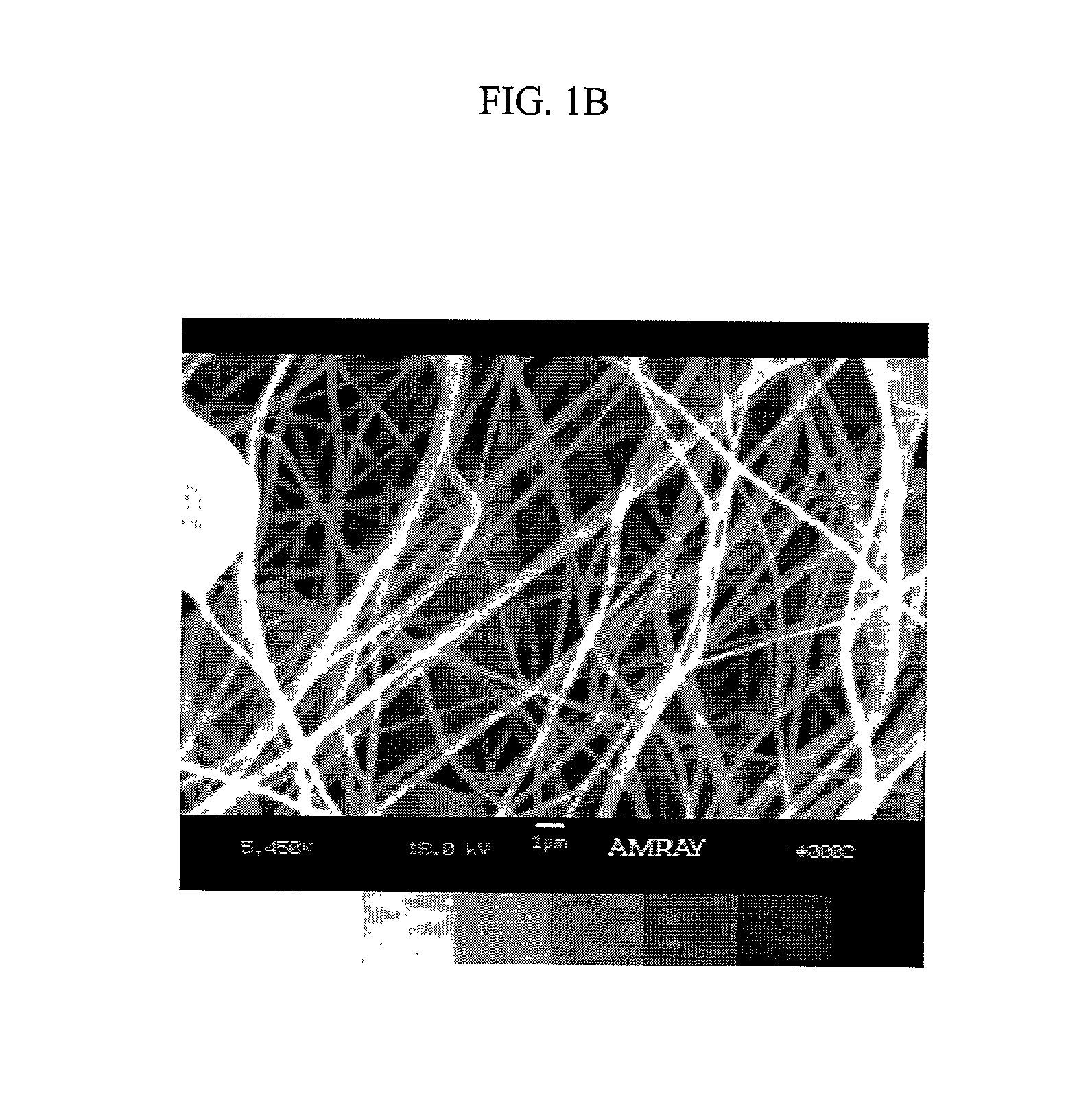
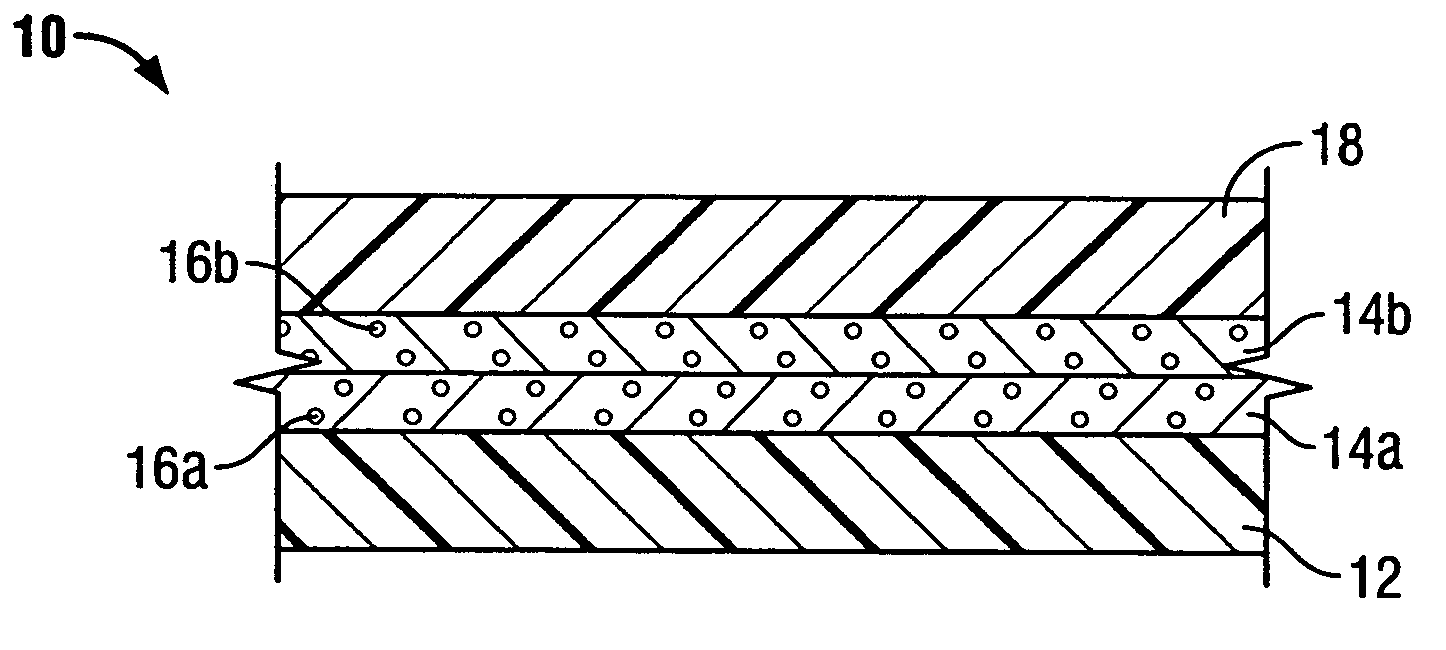
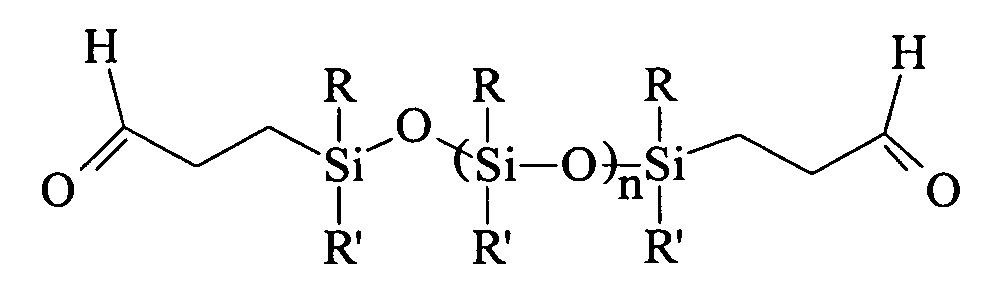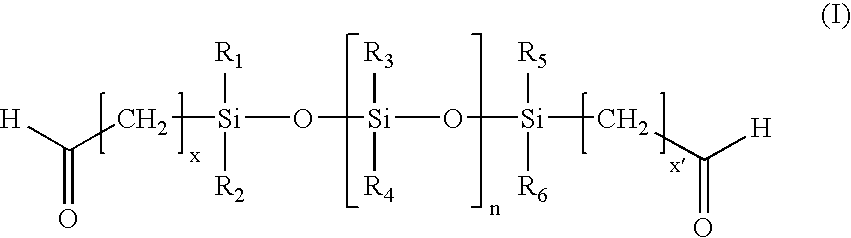Patents
Literature
469 results about "Vascular graft" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
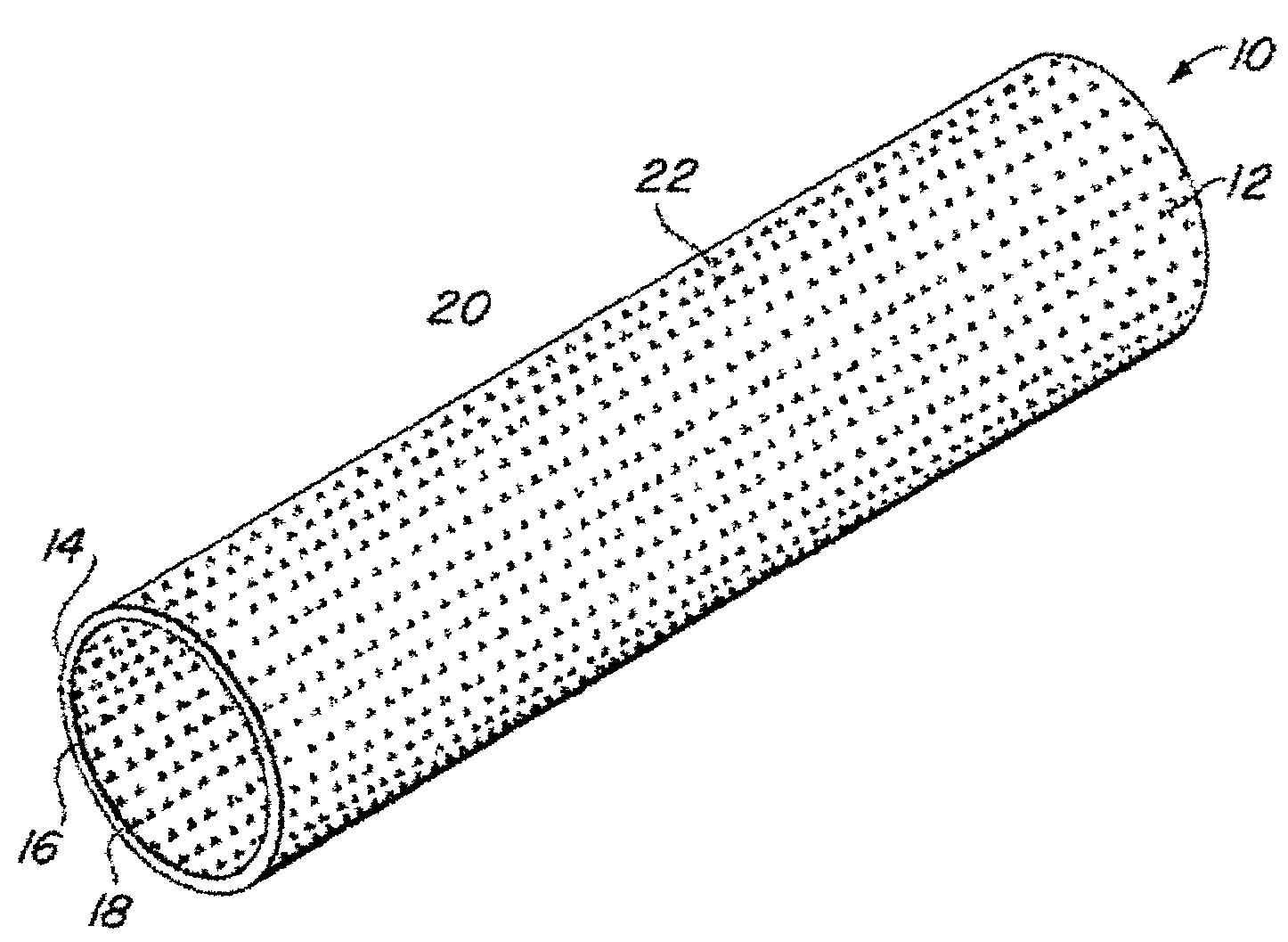
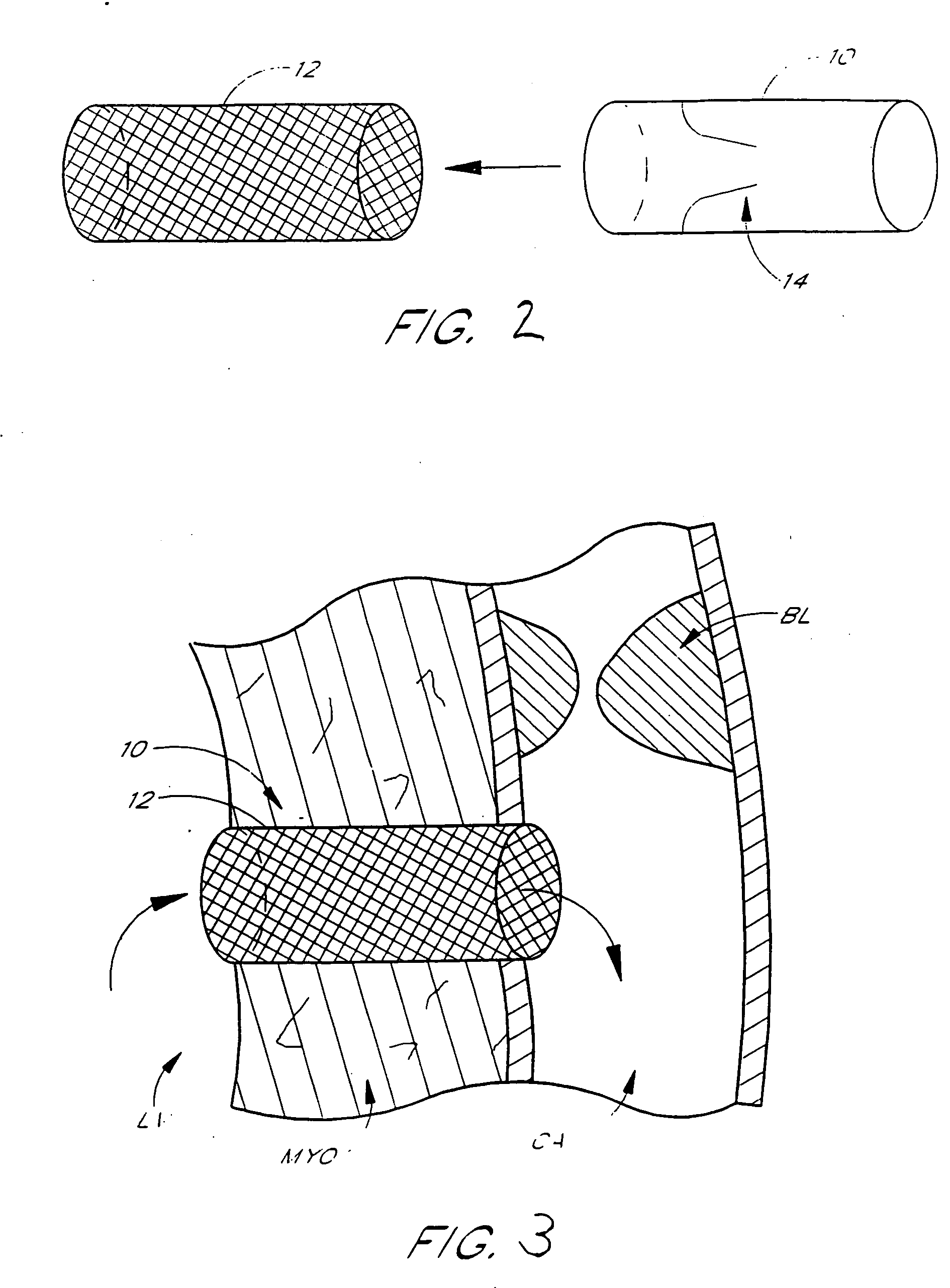
A vascular graft is a tube-shaped synthetic material surgically implanted in the veins and arteries. The diameter and length of the graft will vary depending on the size of the artery or vein being replaced. These grafts are used for peripheral vascular bypass operations and to create vein access in hemodialysis patients.
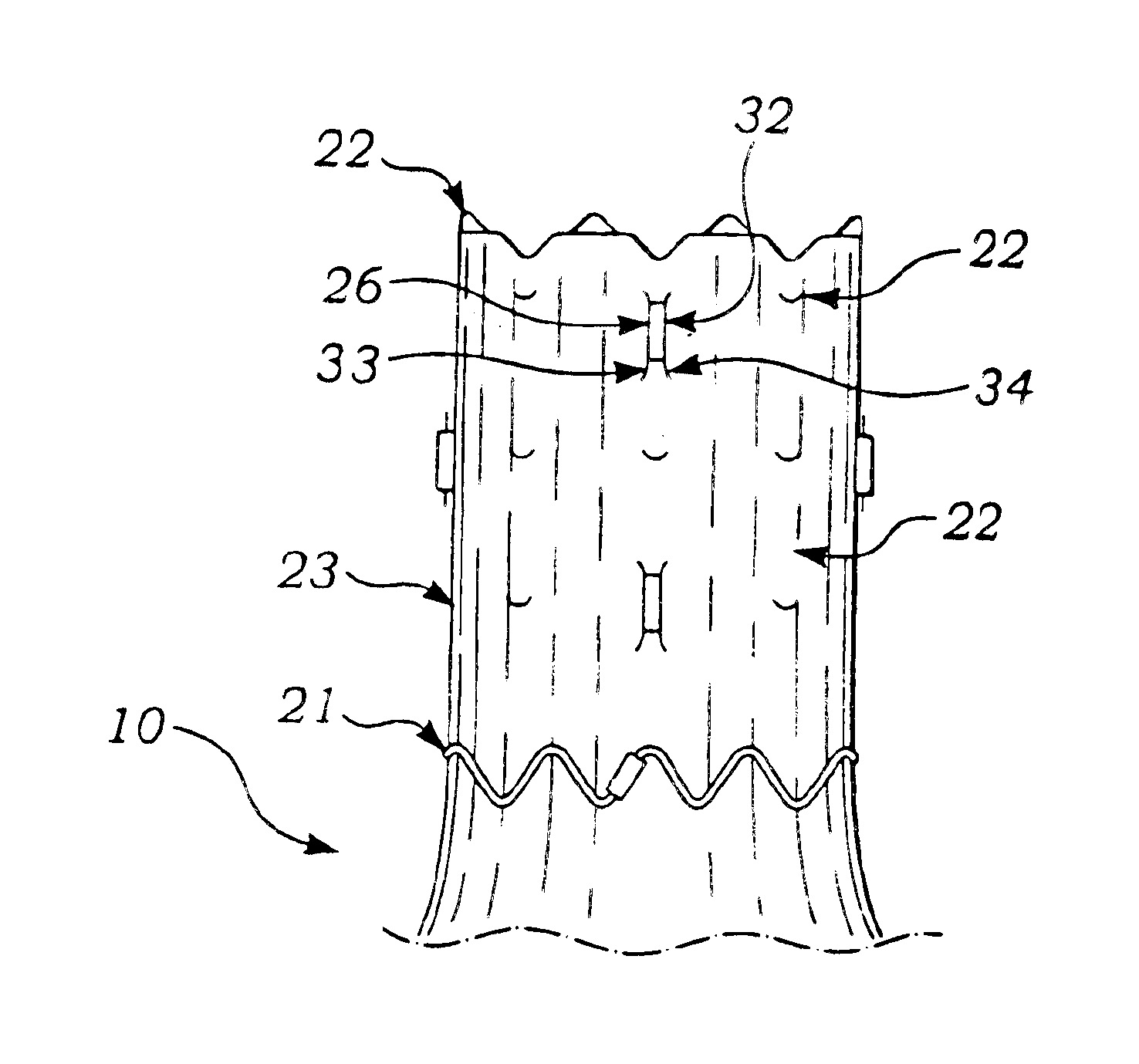
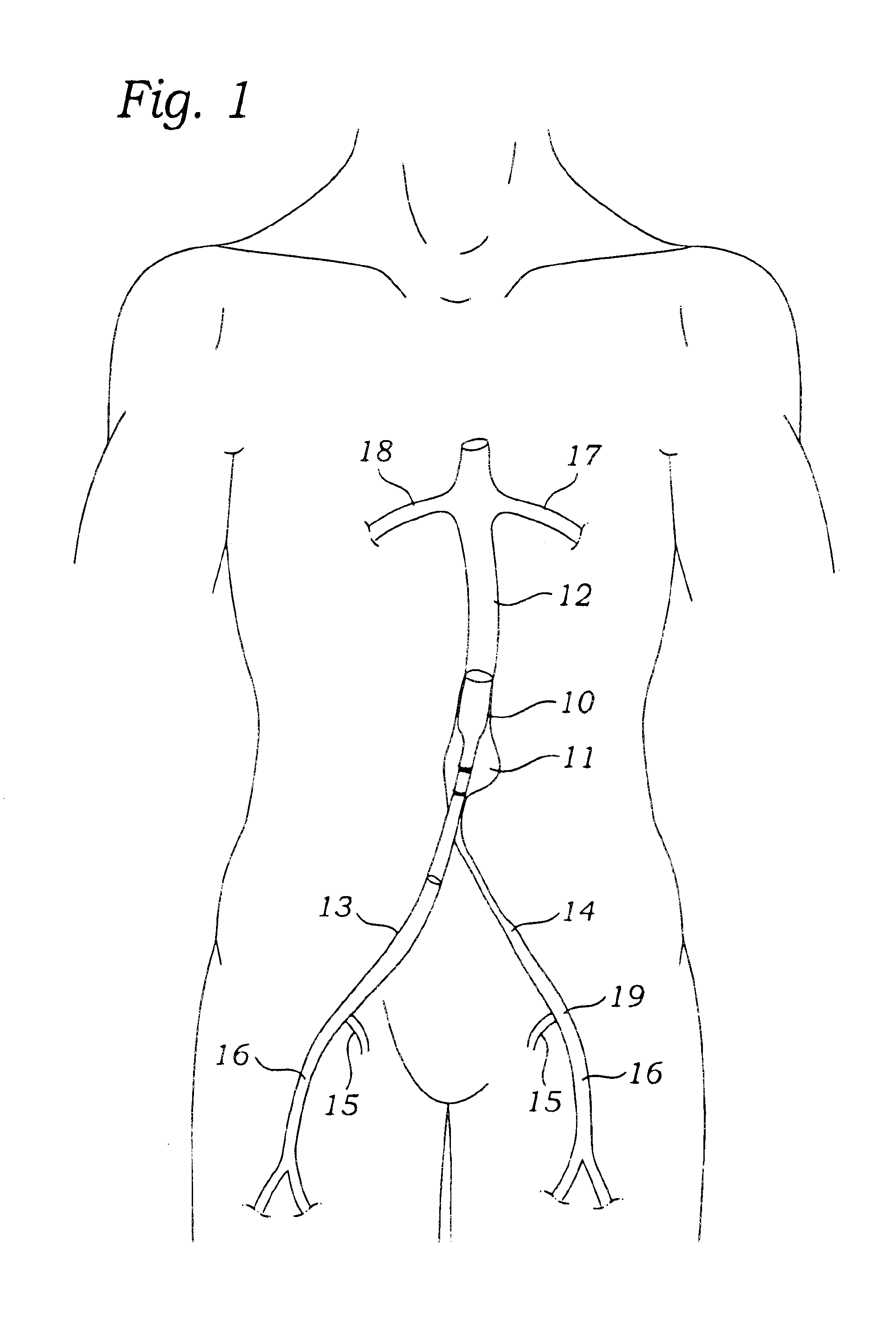
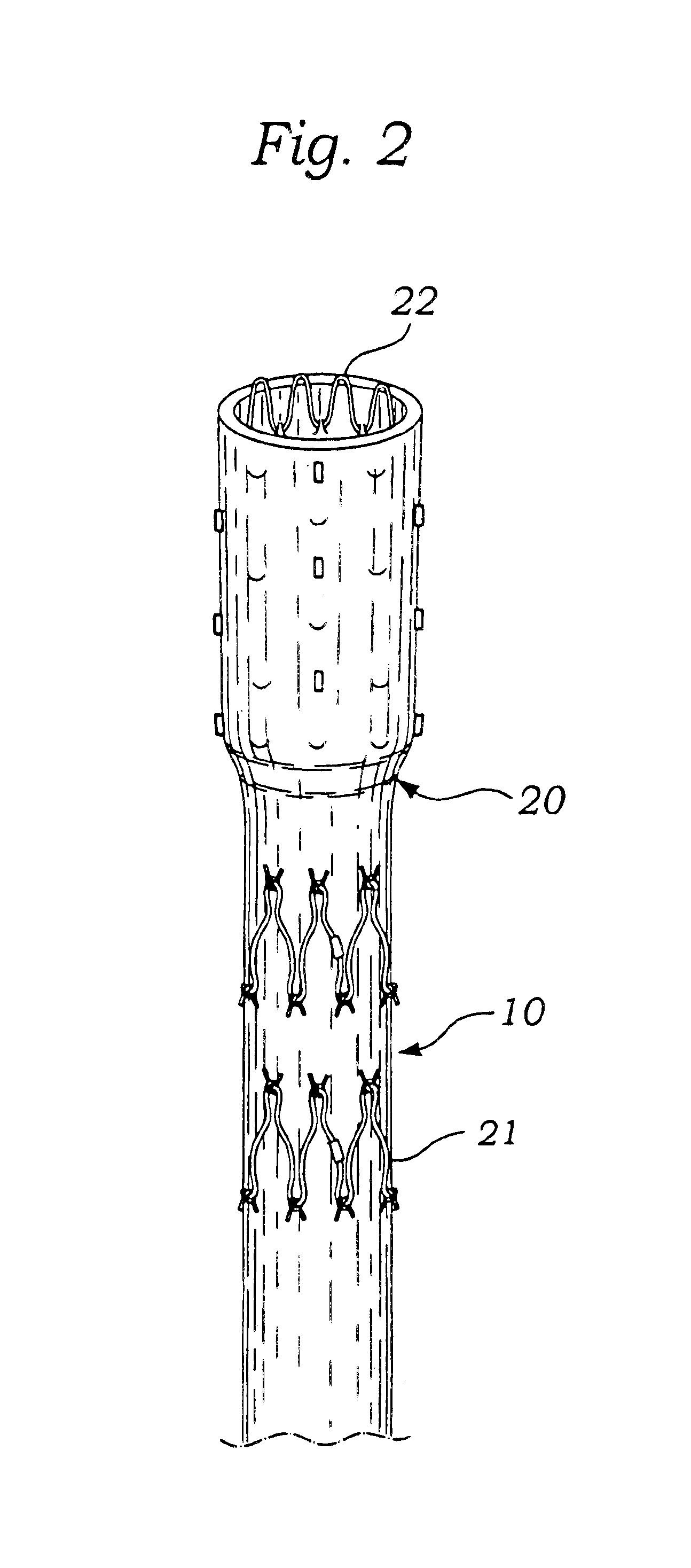
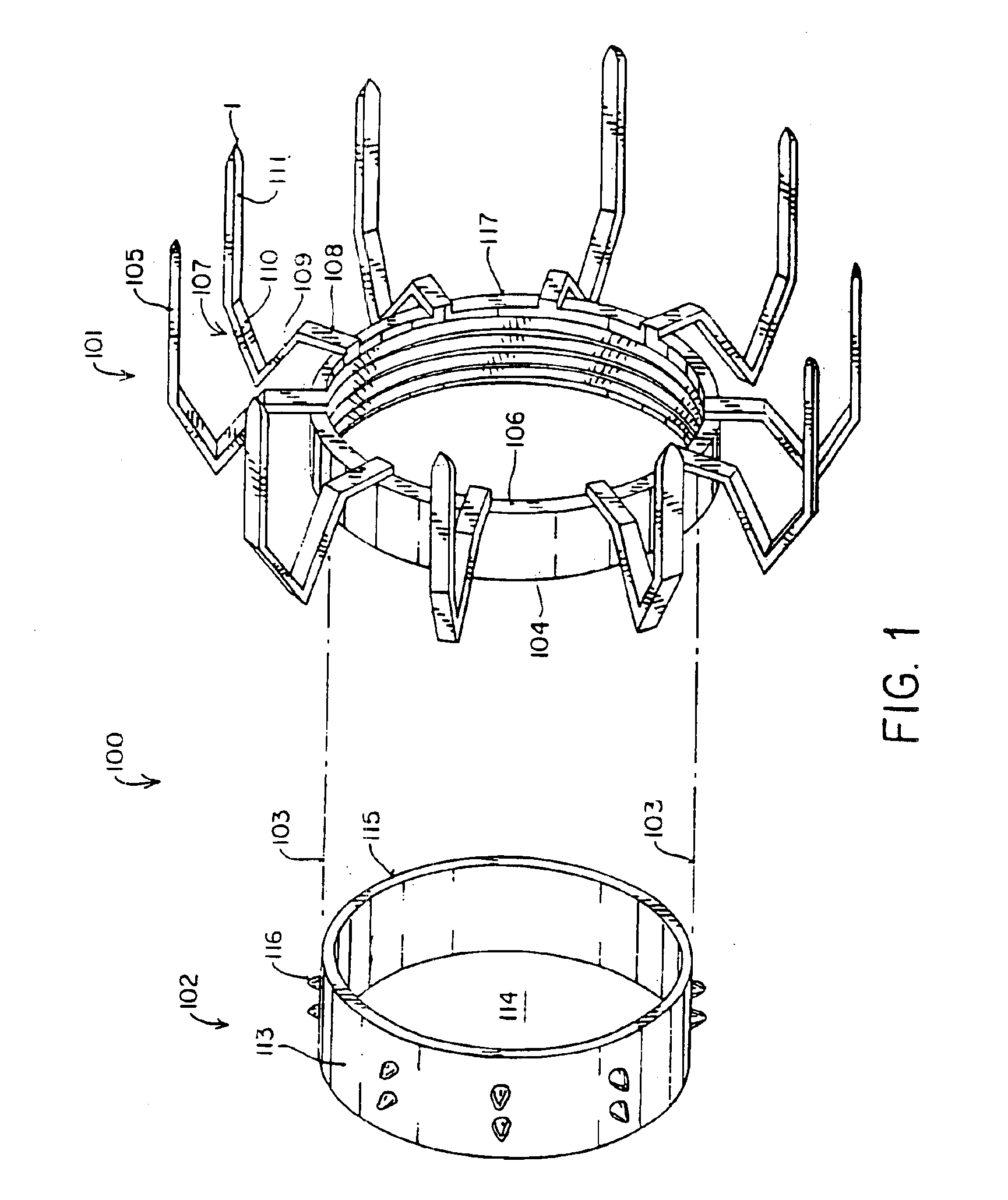
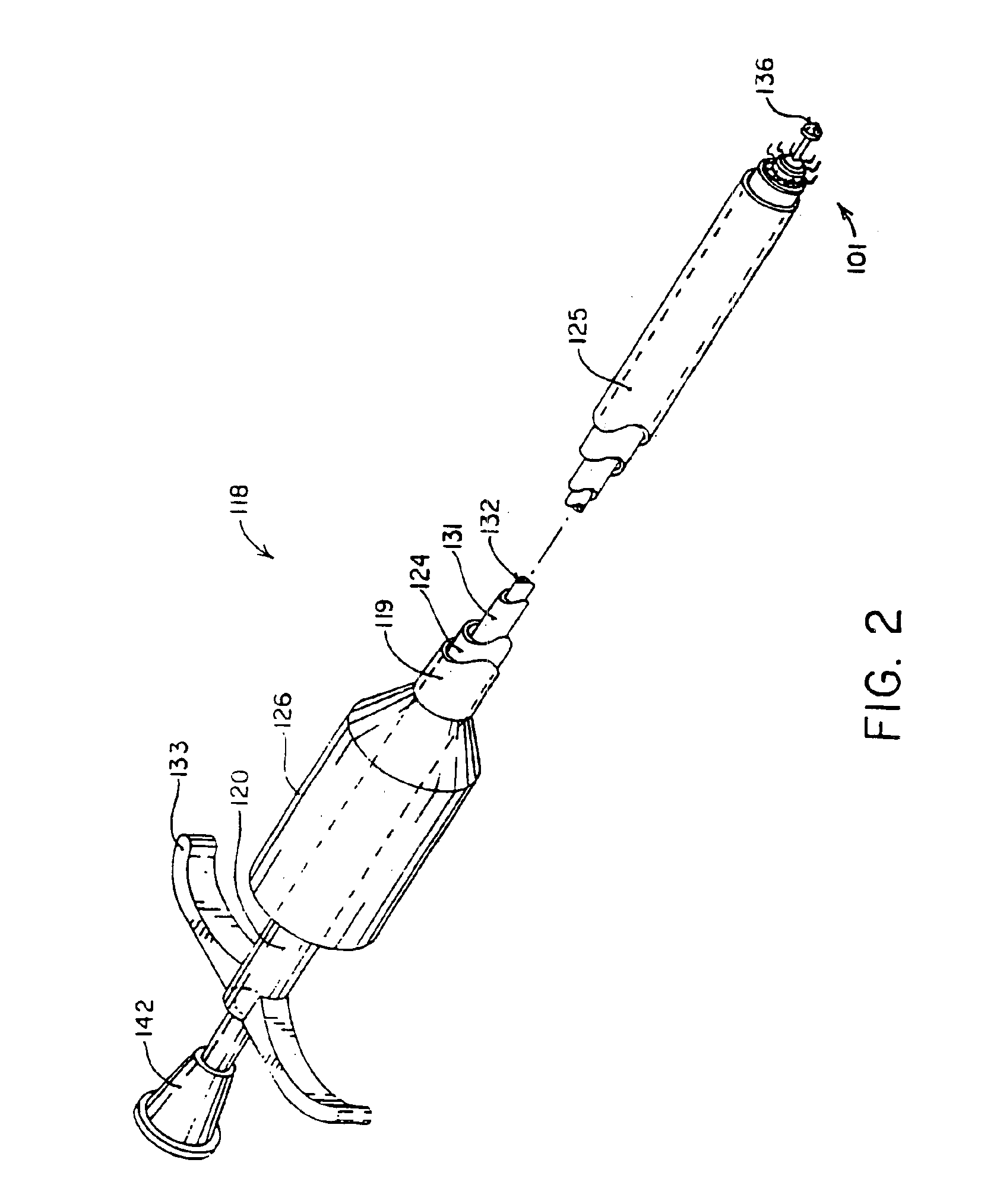
Apparatus and method for fixation of vascular grafts
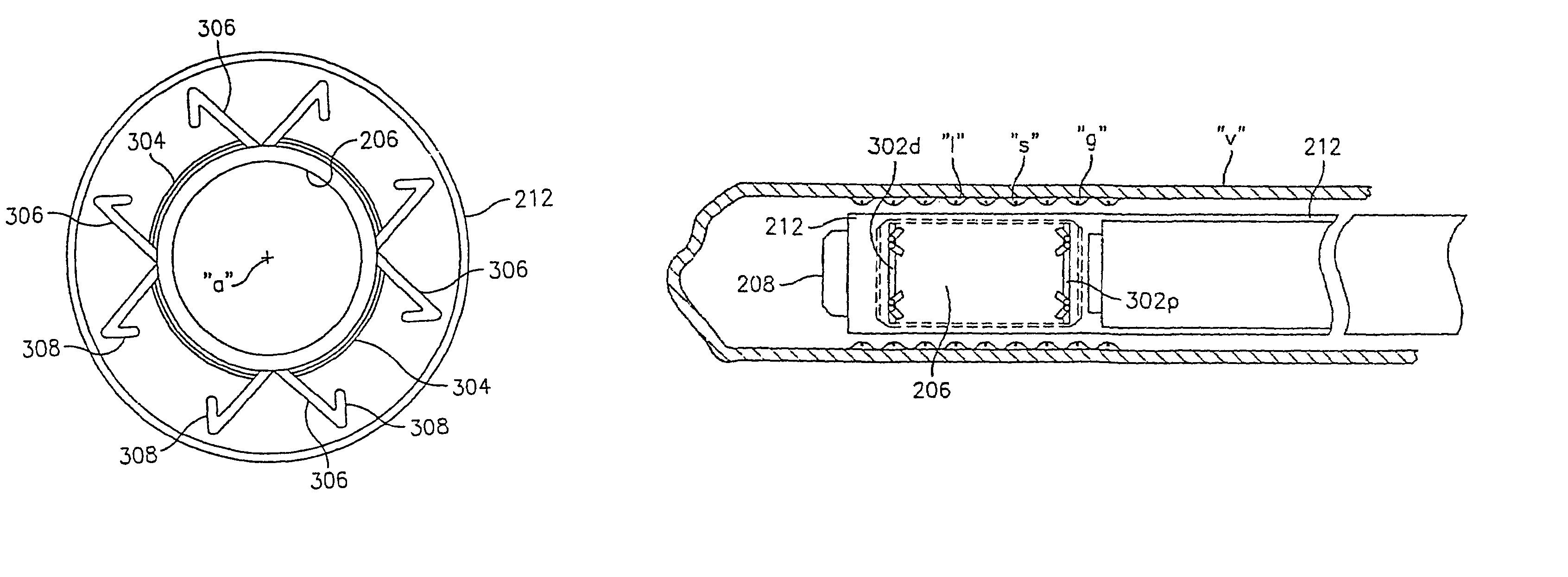
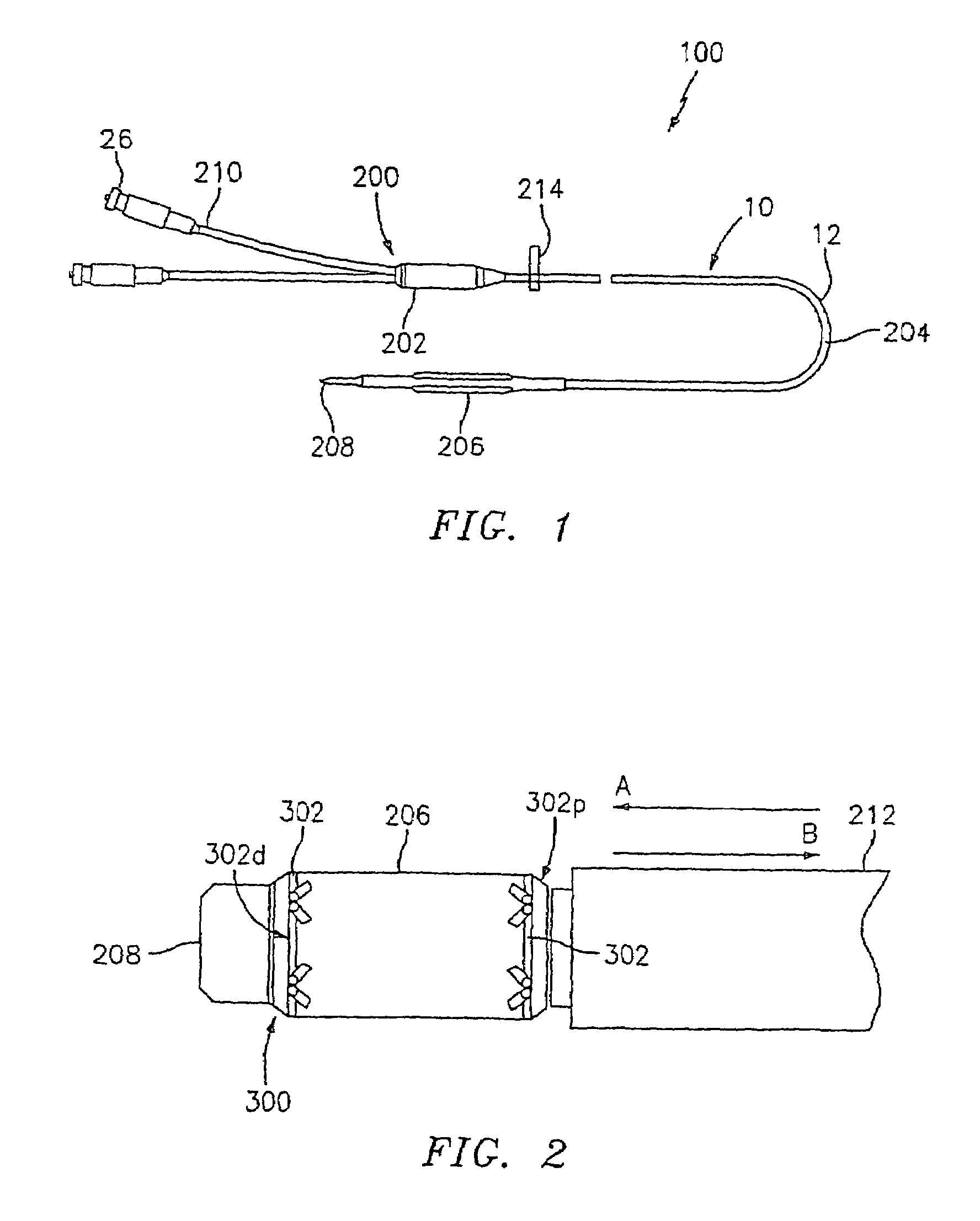
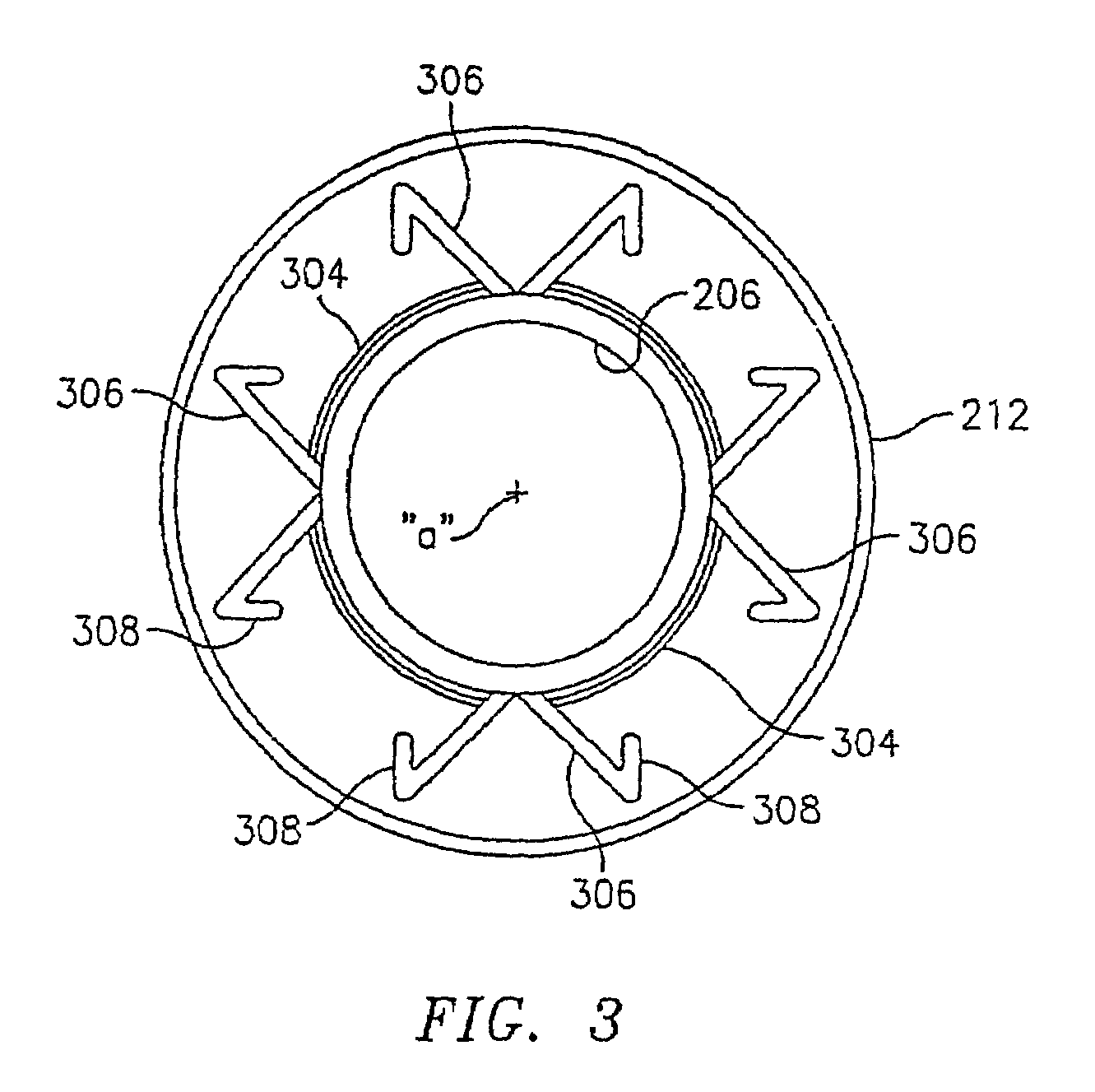
InactiveUS7351258B2Reduce leakageOvercome disadvantagesStentsBlood vesselsThree vesselsVascular graft
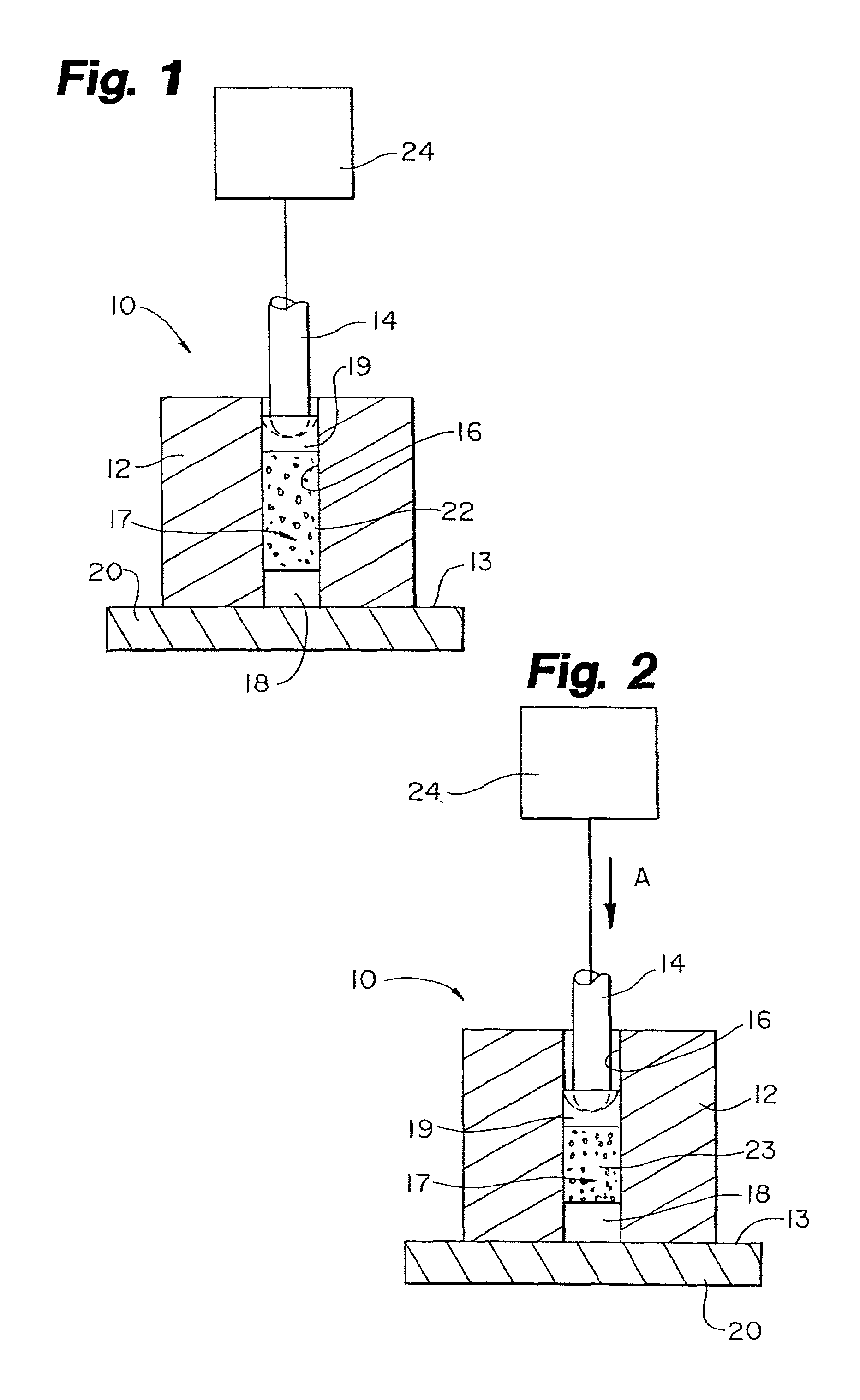
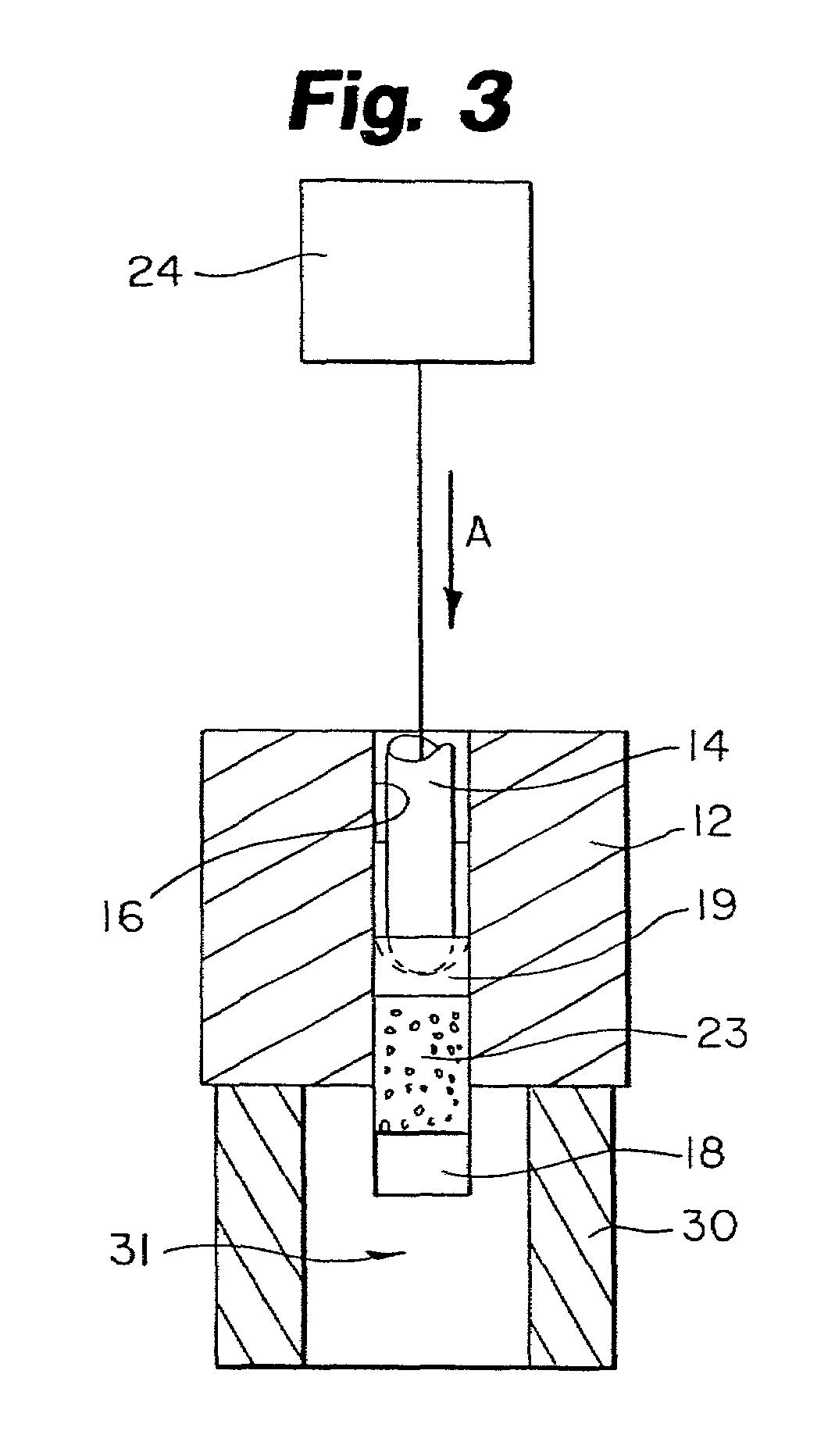
An apparatus for facilitating securement of a vascular graft within a blood vessel, includes a shaft dimensioned for passage within a blood vessel and having an expansion member movable between a contracted condition and an expanded condition and a fastener array comprising at least one fastener disposed about a peripheral portion of the expansion member. The one fastener is deployable into a wall of the blood vessel upon movement of the expansion member to the expanded condition thereof, to thereby engage the vascular graft to secure the vascular graft to a wall of the blood vessel. The fastener array preferably includes a plurality of fasteners. The fasteners may be operatively connected to each other and releasably secured to the peripheral portion of the expansion member.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
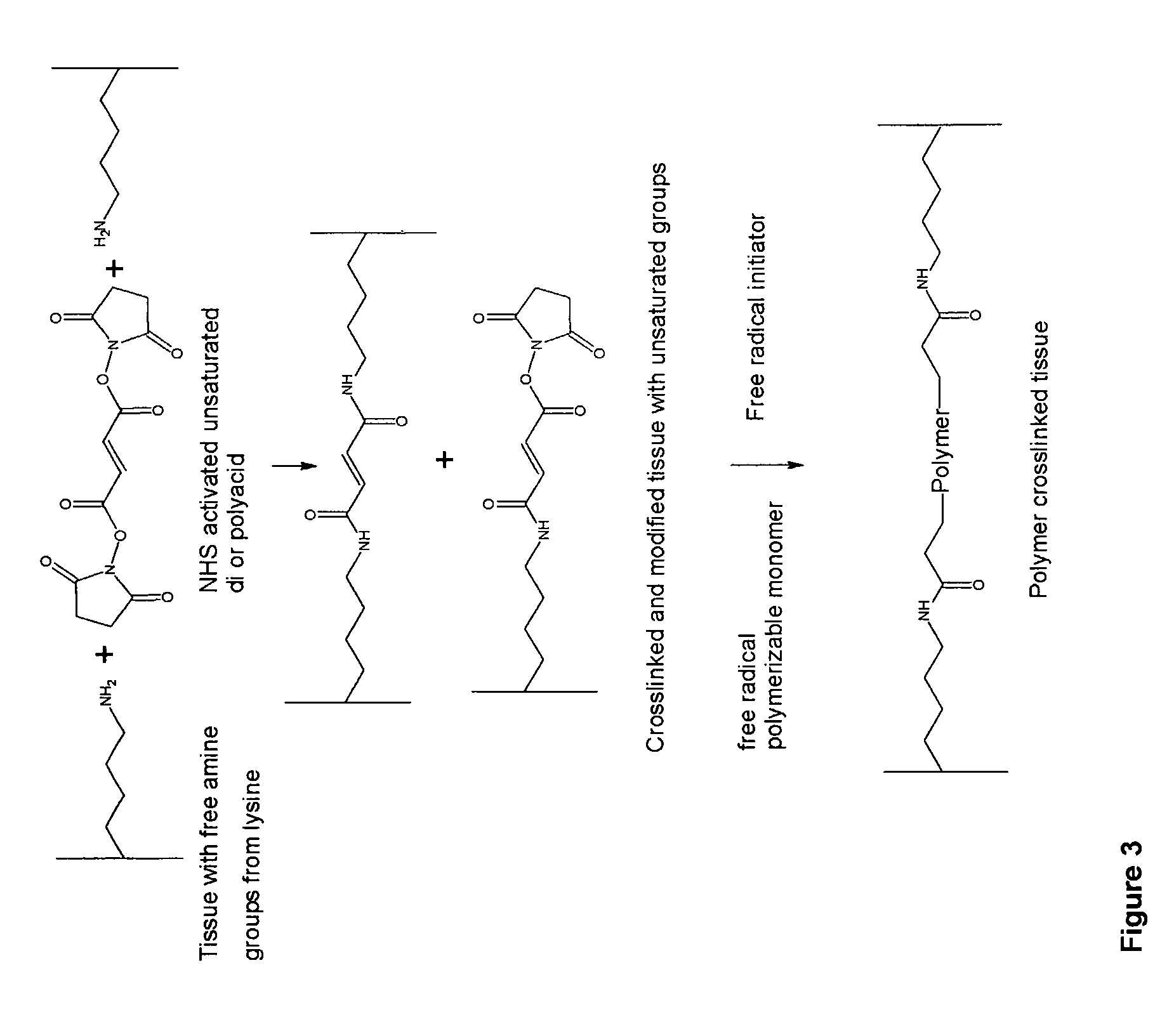
Biocompatible crosslinked polymers
InactiveUS7009034B2Improve performanceImprove visibilityUltrasonic/sonic/infrasonic diagnosticsPowder deliveryWound dressingPost operative
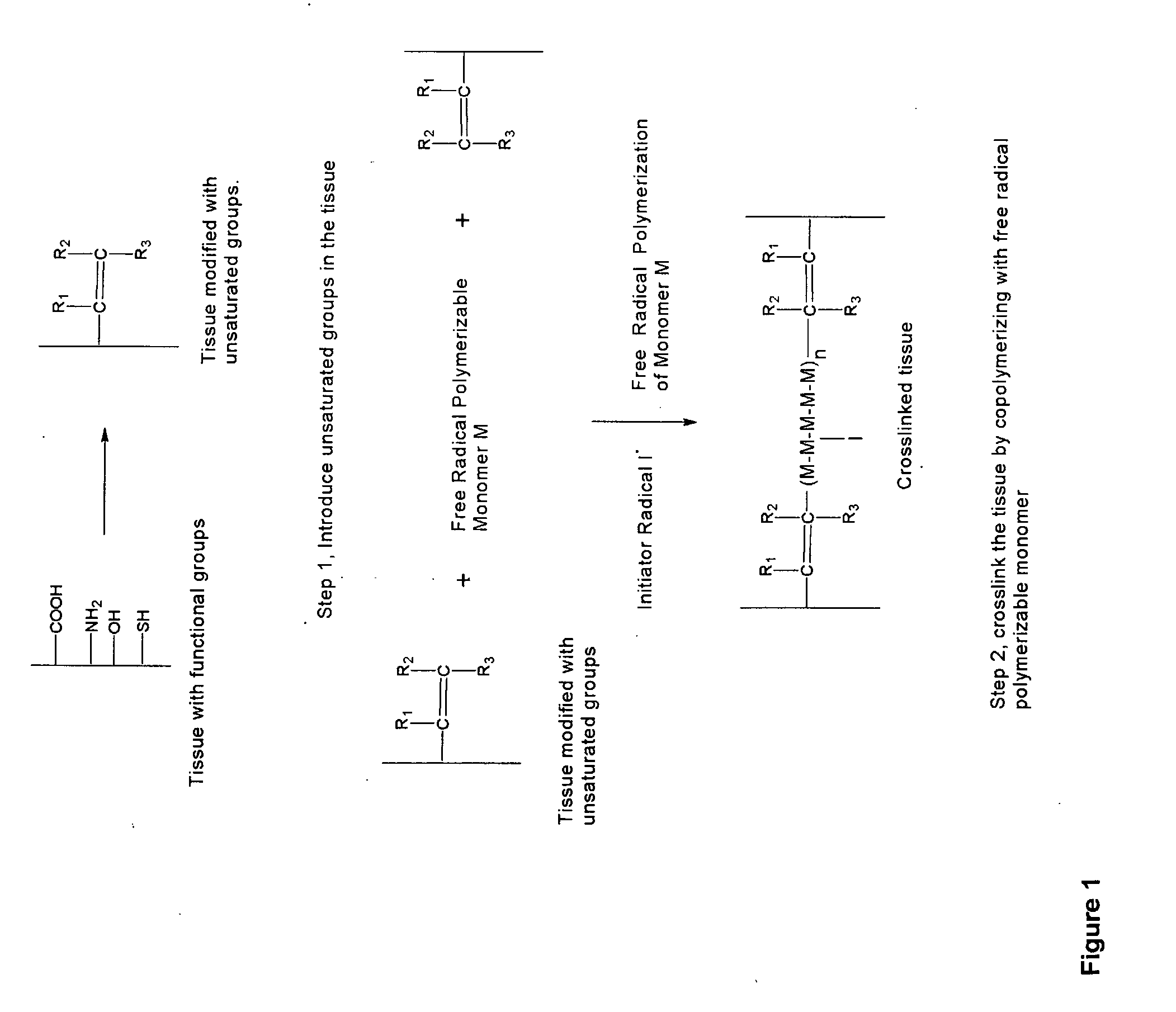
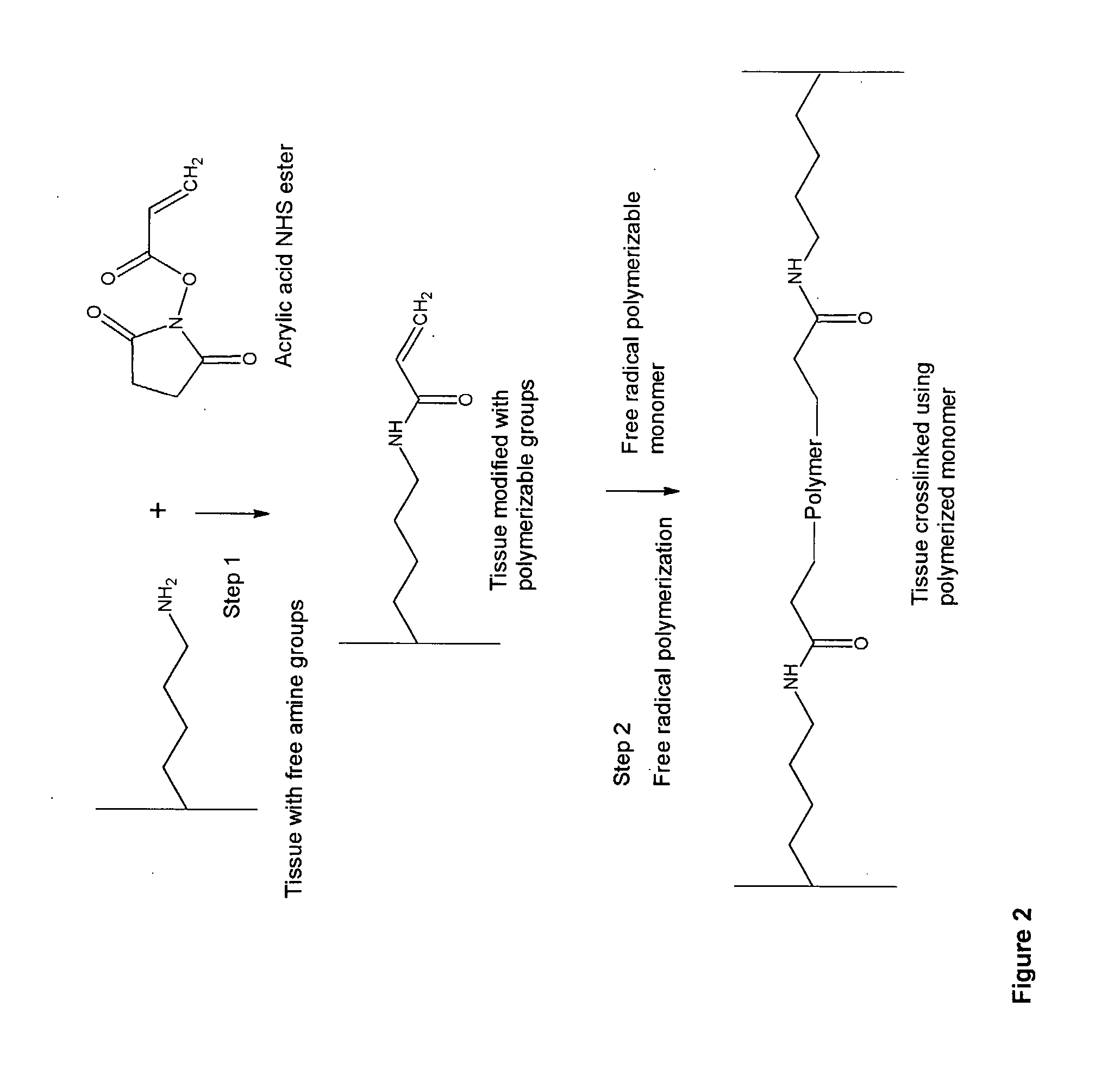
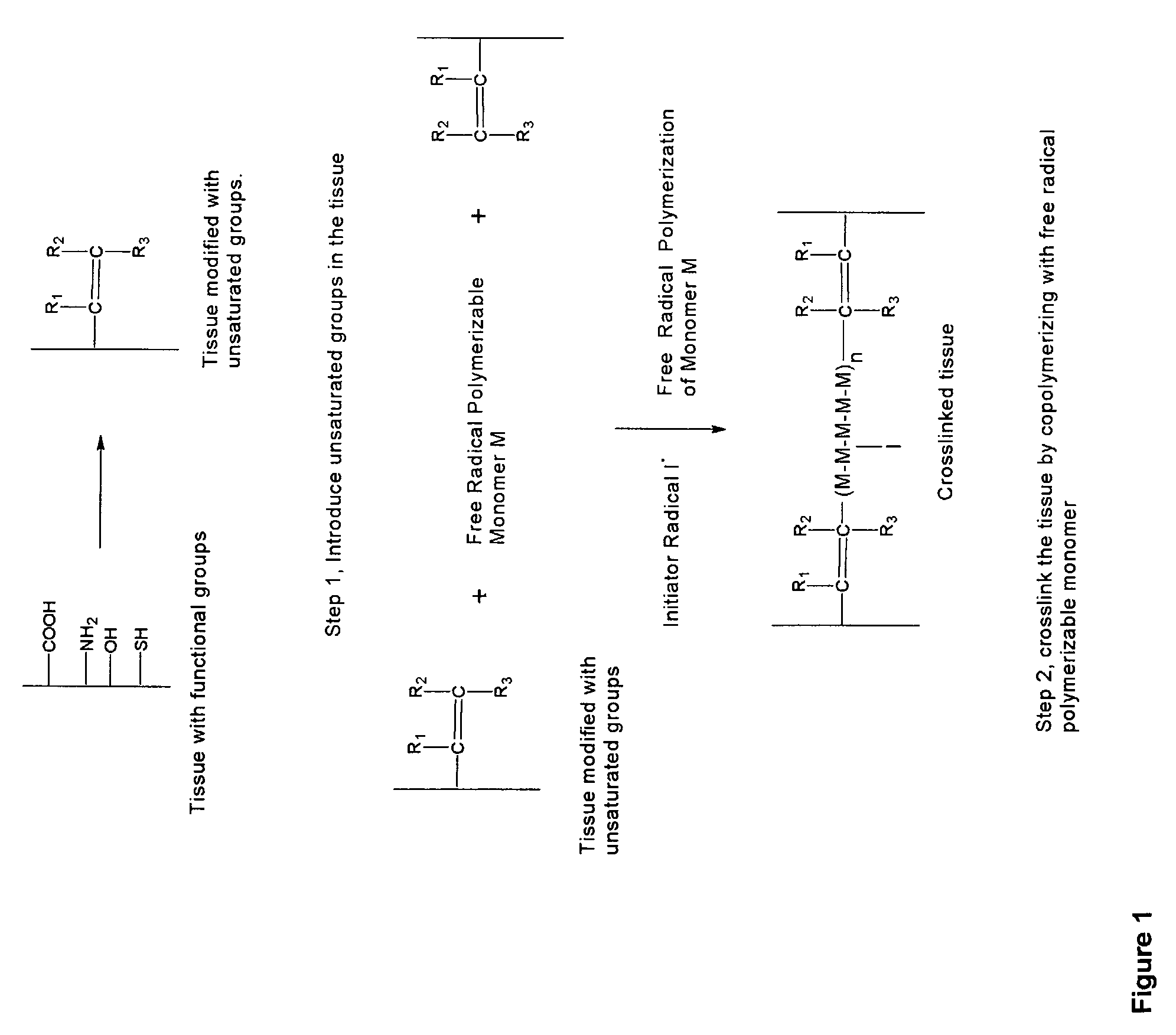
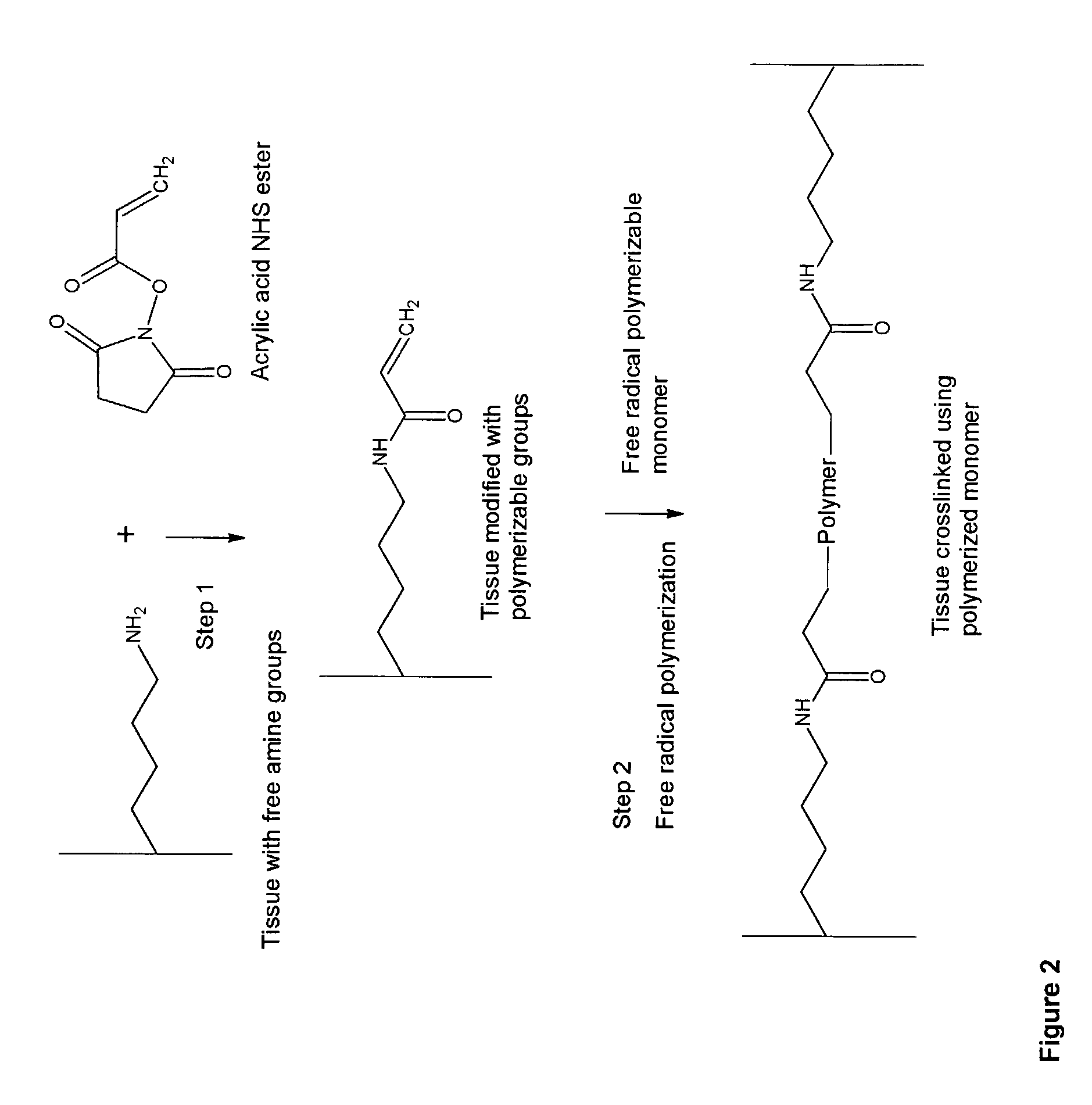
Biocompatible crosslinked polymers, and methods for their preparation and use, are disclosed in which the biocompatible crosslinked polymers are formed from water soluble precursors having electrophilic and nucleophilic functional groups capable of reacting and crosslinking in situ. Methods for making the resulting biocompatible crosslinked polymers biodegradable or not are provided, as are methods for controlling the rate of degradation. The crosslinking reactions may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked polymers and their precursors include controlled delivery of drugs, prevention of post-operative adhesions, coating of medical devices such as vascular grafts, wound dressings and surgical sealants. Visualization agents may be included with the crosslinked polymers.
Owner:INCEPT LLC
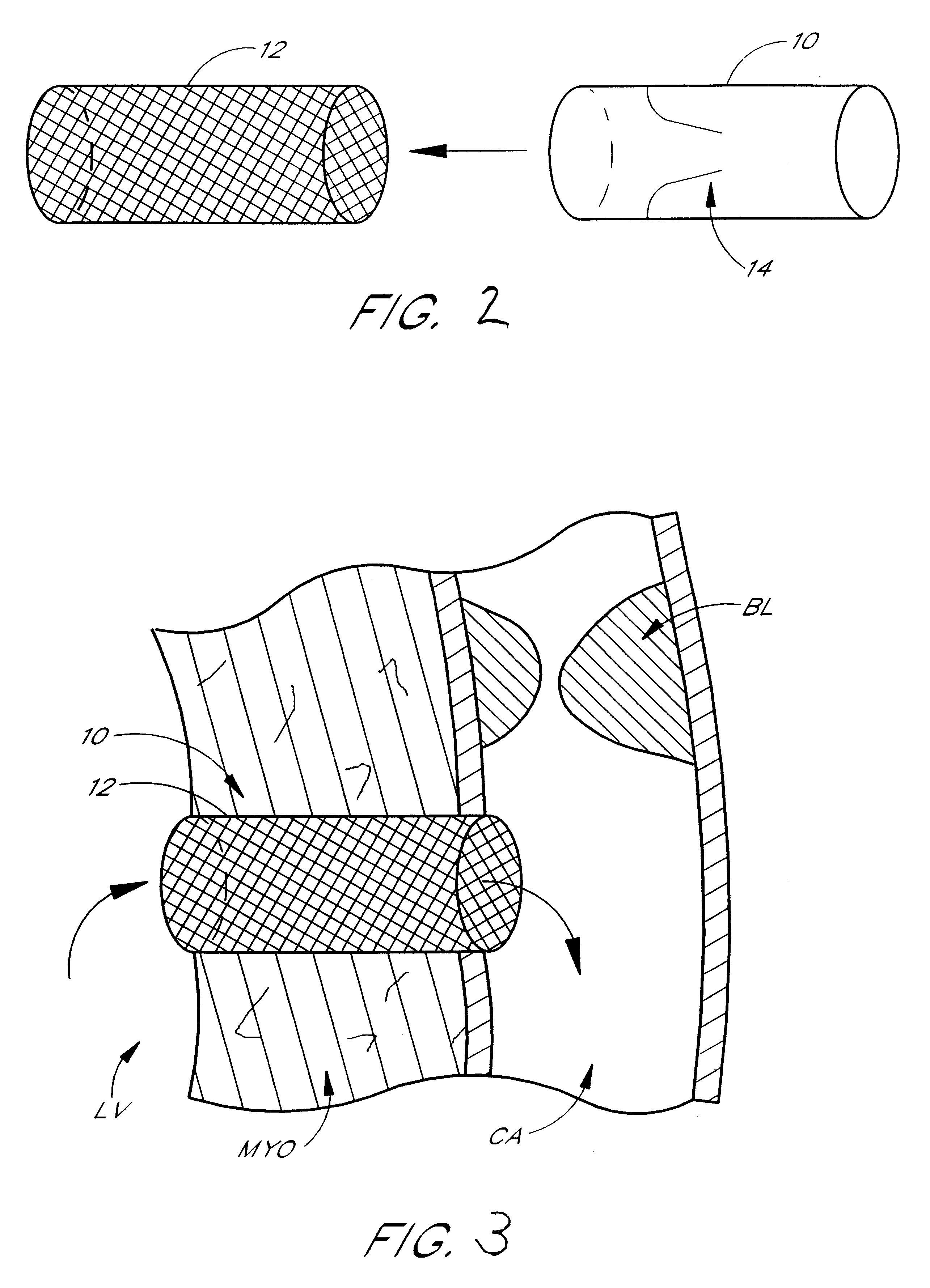
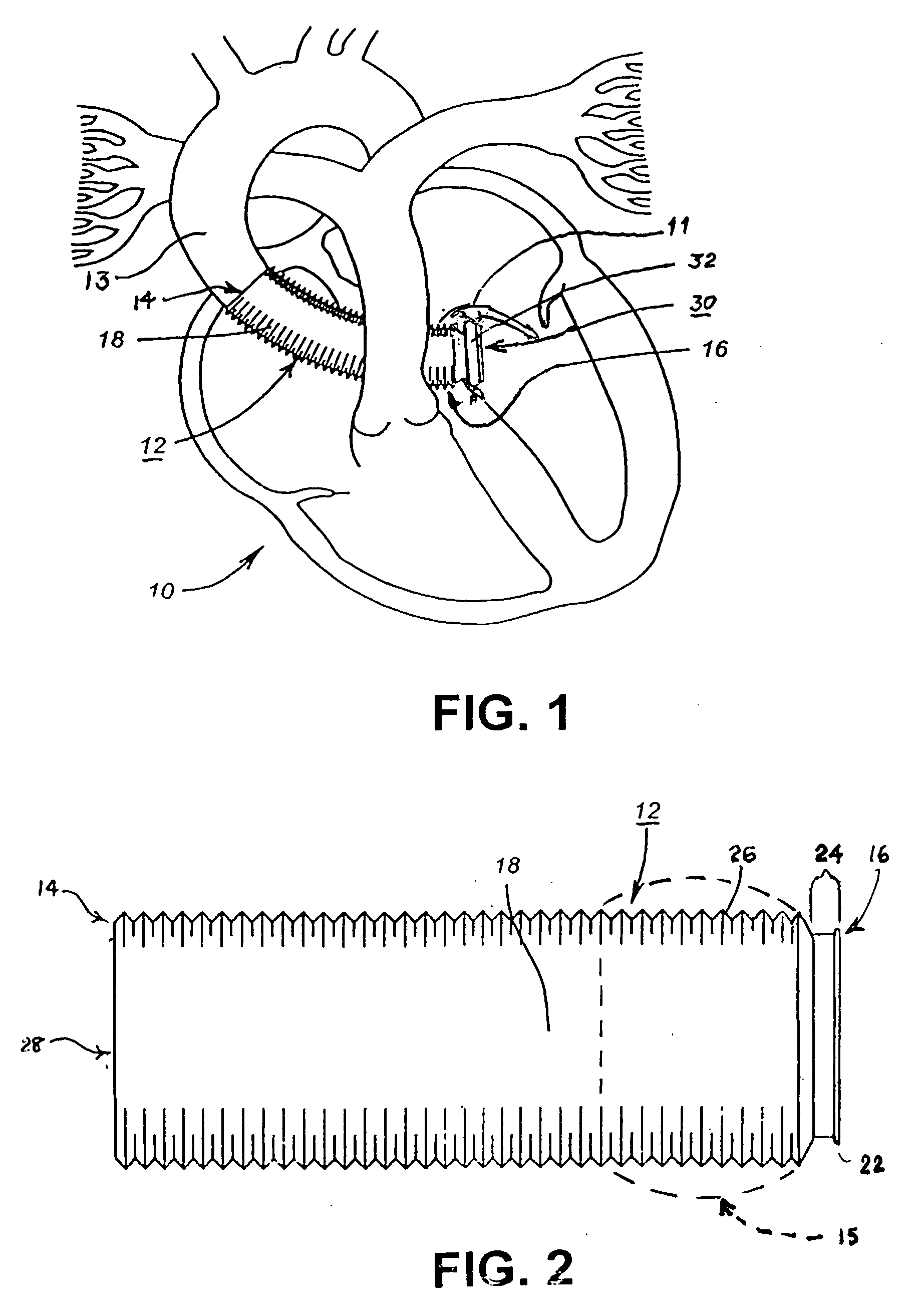
Conduit with valved blood vessel graft
Disclosed is a conduit that provides a bypass around an occlusion or stenosis in a coronary artery. The conduit is a tube adapted to be positioned in the heart wall to provide a passage for blood to flow between a heart chamber and a coronary artery, at a site distal to the occlusion or stenosis. The conduit has a section of blood vessel attached to its interior lumen which preferably includes at least one naturally occurring one-way valve positioned therein. The valve prevents the backflow of blood from the coronary artery into the heart chamber.
Owner:HORIZON TECH FUNDING CO LLC +1
Methods and apparatus for coupling an allograft tissue valve and graft
InactiveUS20060085060A1Prevent leakageMinimal degradationHeart valvesBlood vesselsAscending aortaProsthesis
Improvements to prosthetic heart valves and grafts for human implantation, particularly to methods and apparatus for coupling a prosthetic heart valve with an artificial graft during a surgical procedure to replace a defective heart valve and blood vessel section, e.g., the aortic valve and a section of the ascending aorta, are disclosed. An annular exterior surface of the prosthetic heart valve is fitted within a vascular graft lumen to dispose the vascular graft proximal end overlying the annular exterior surface, and the proximal end of an elongated vascular graft is compressed against the valve annular exterior surface in a manner that inhibits blood leakage between the vascular graft and the prosthetic heart valve.
Owner:MEDTRONIC INC
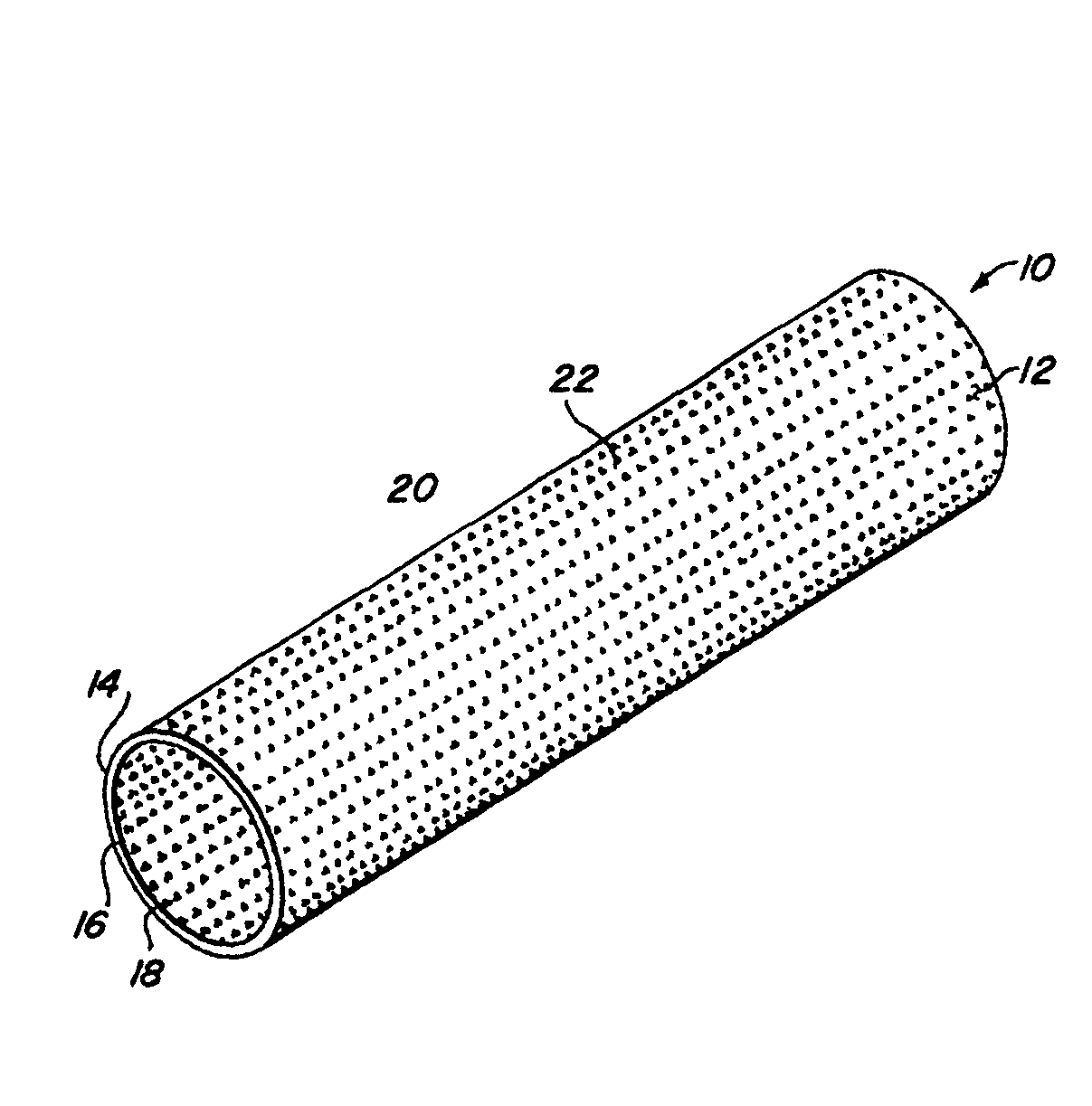
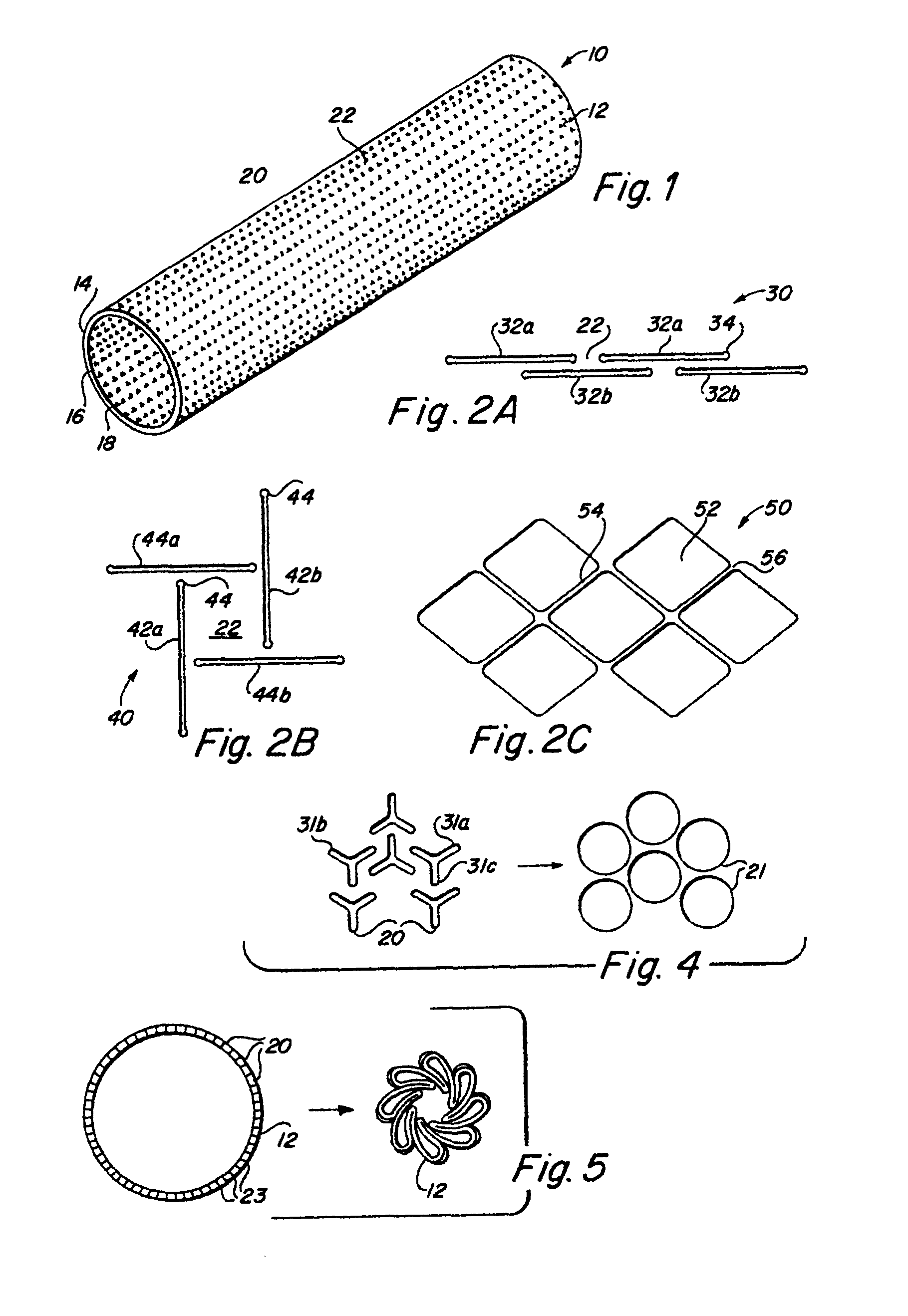
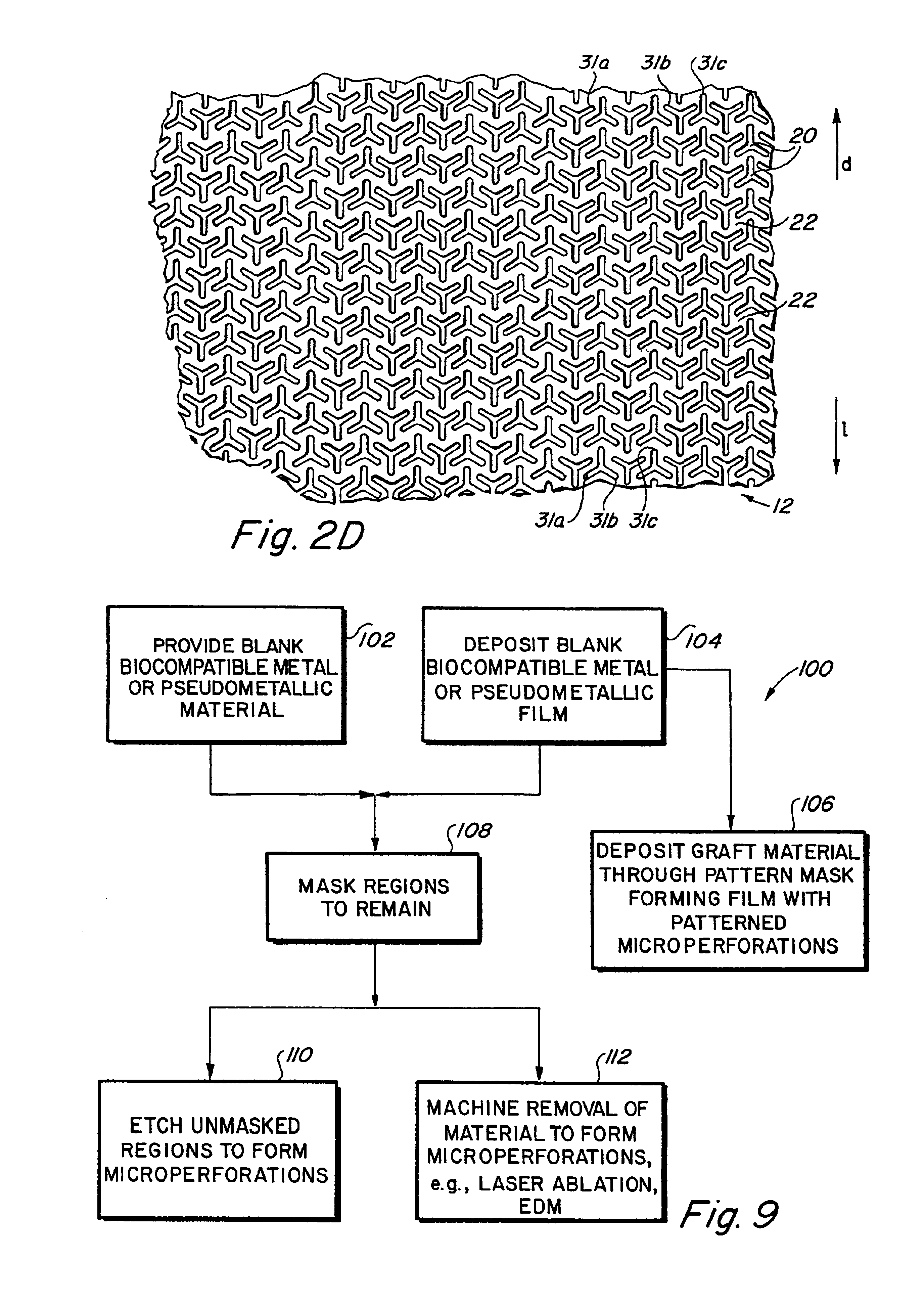
Complaint implantable medical devices and methods of making same
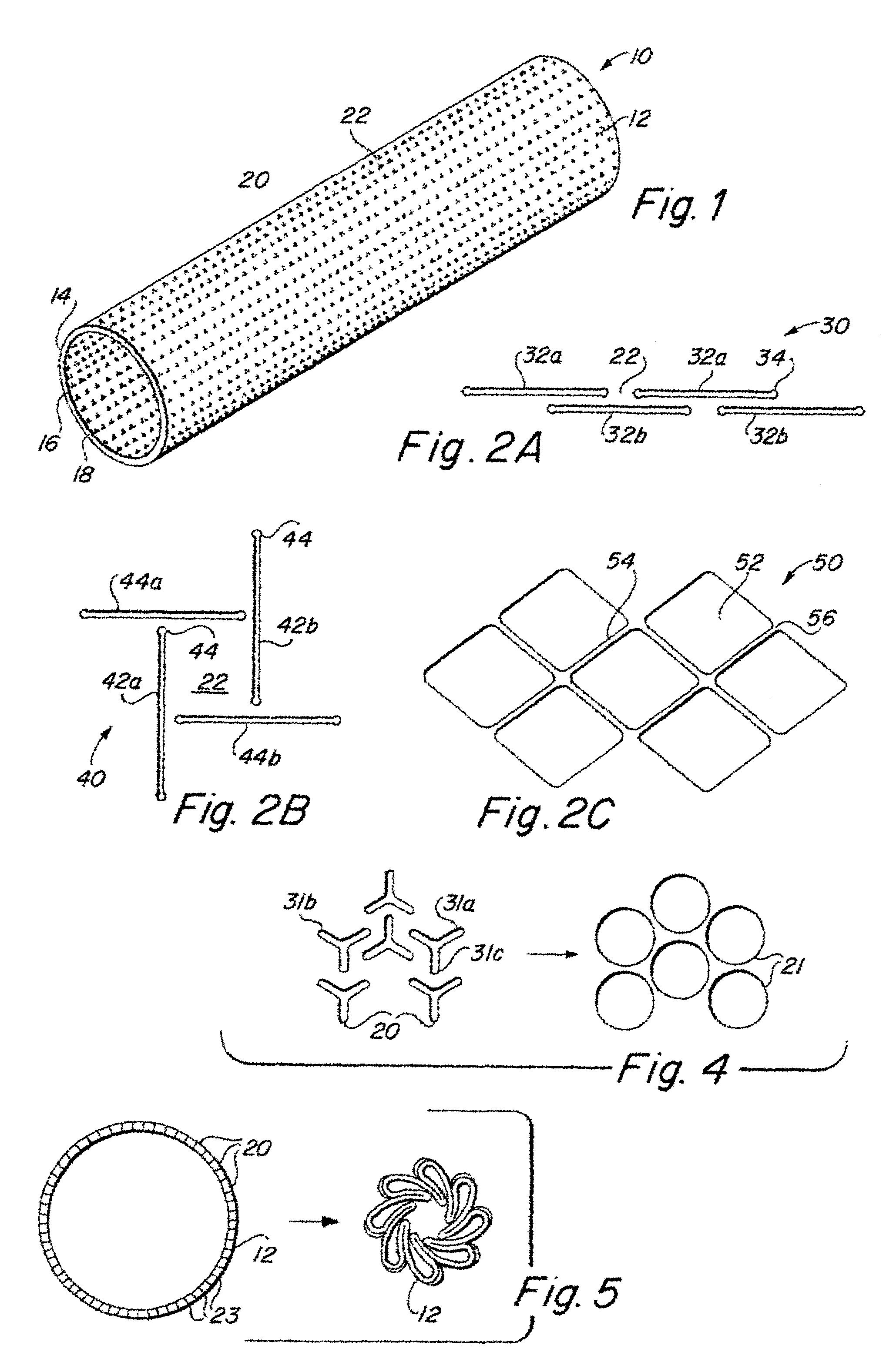
InactiveUS6936066B2Give flexibilityFacilitating transmural endothelializationStentsHeart valvesSurgical GraftMetallic materials
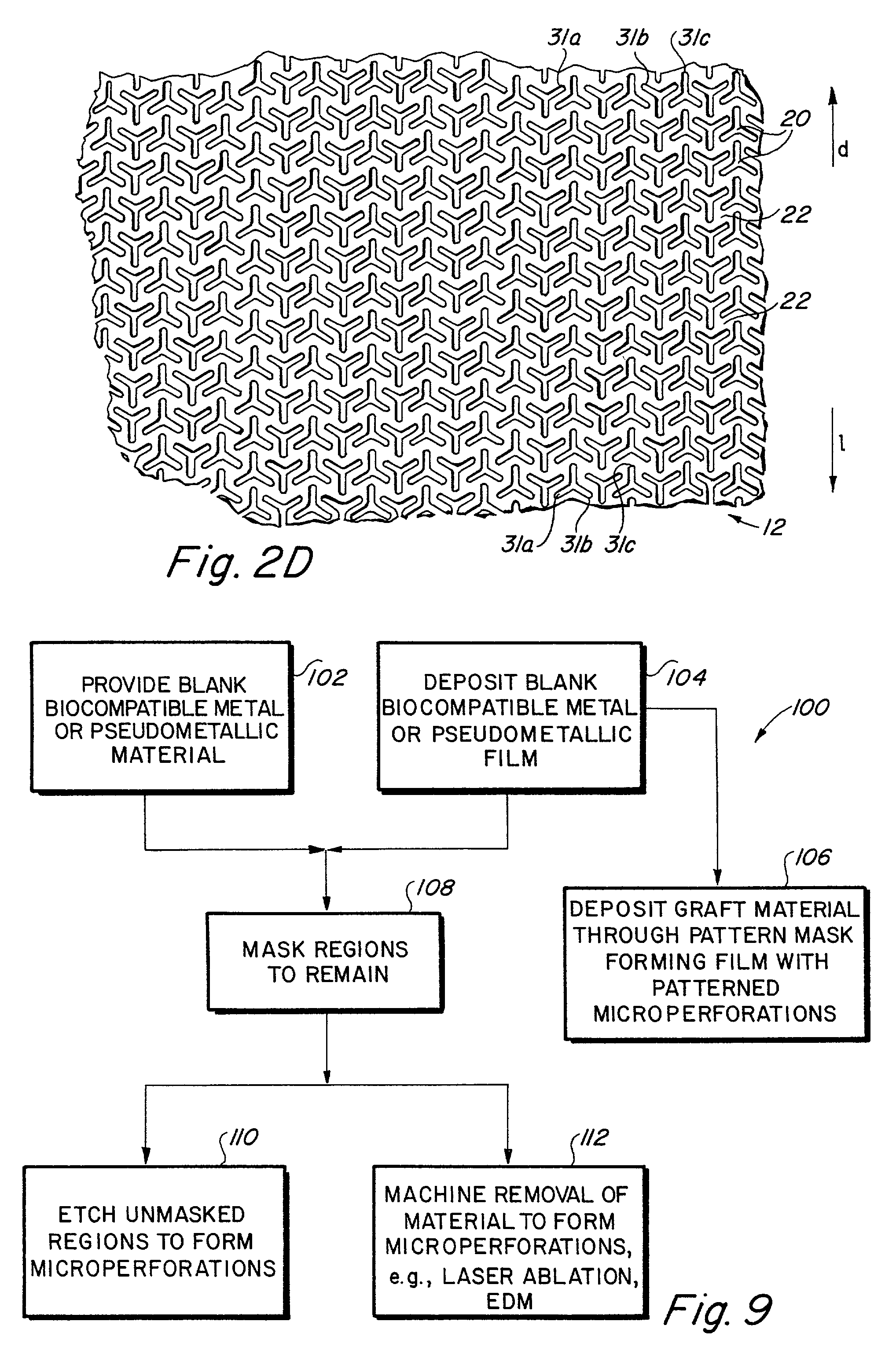
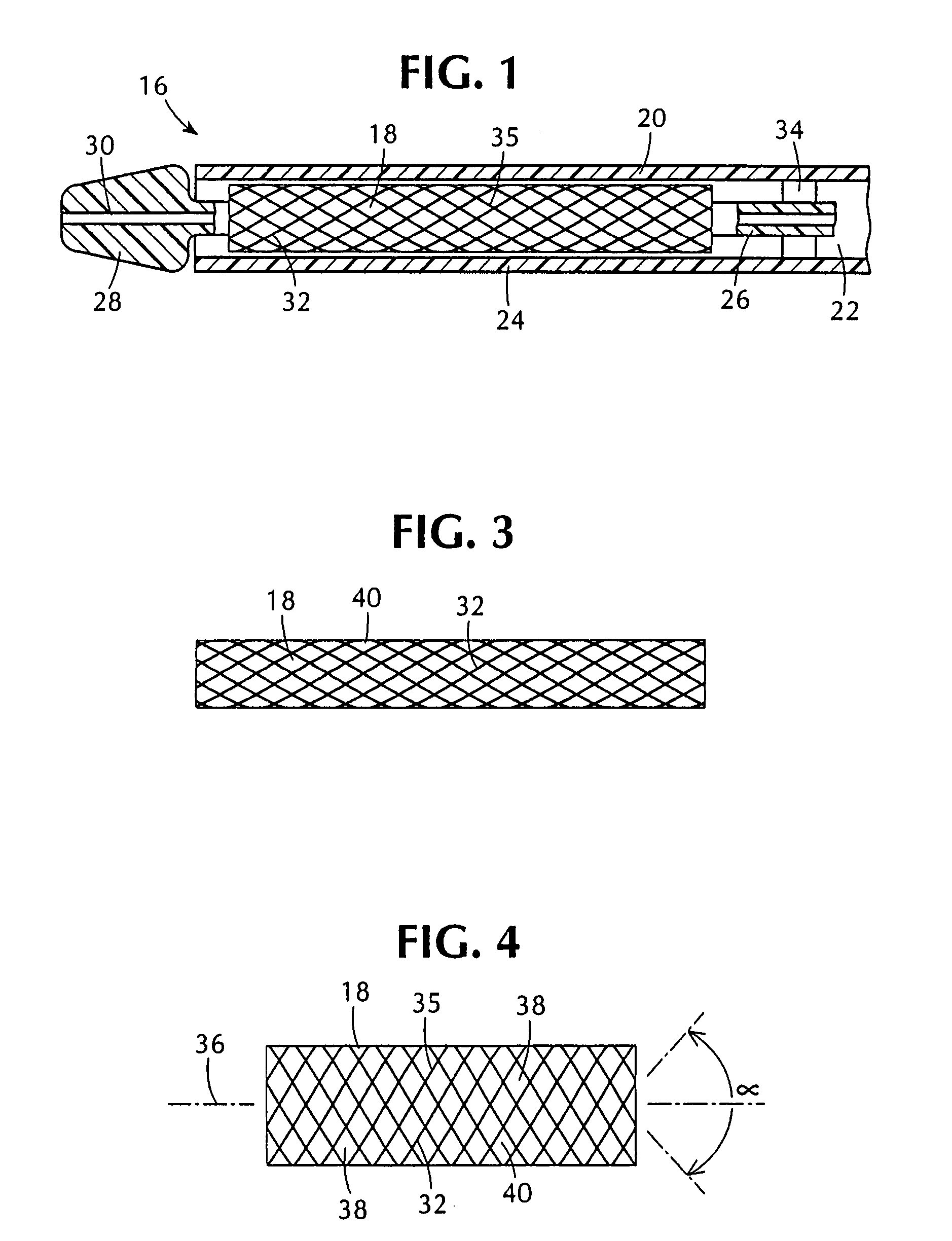
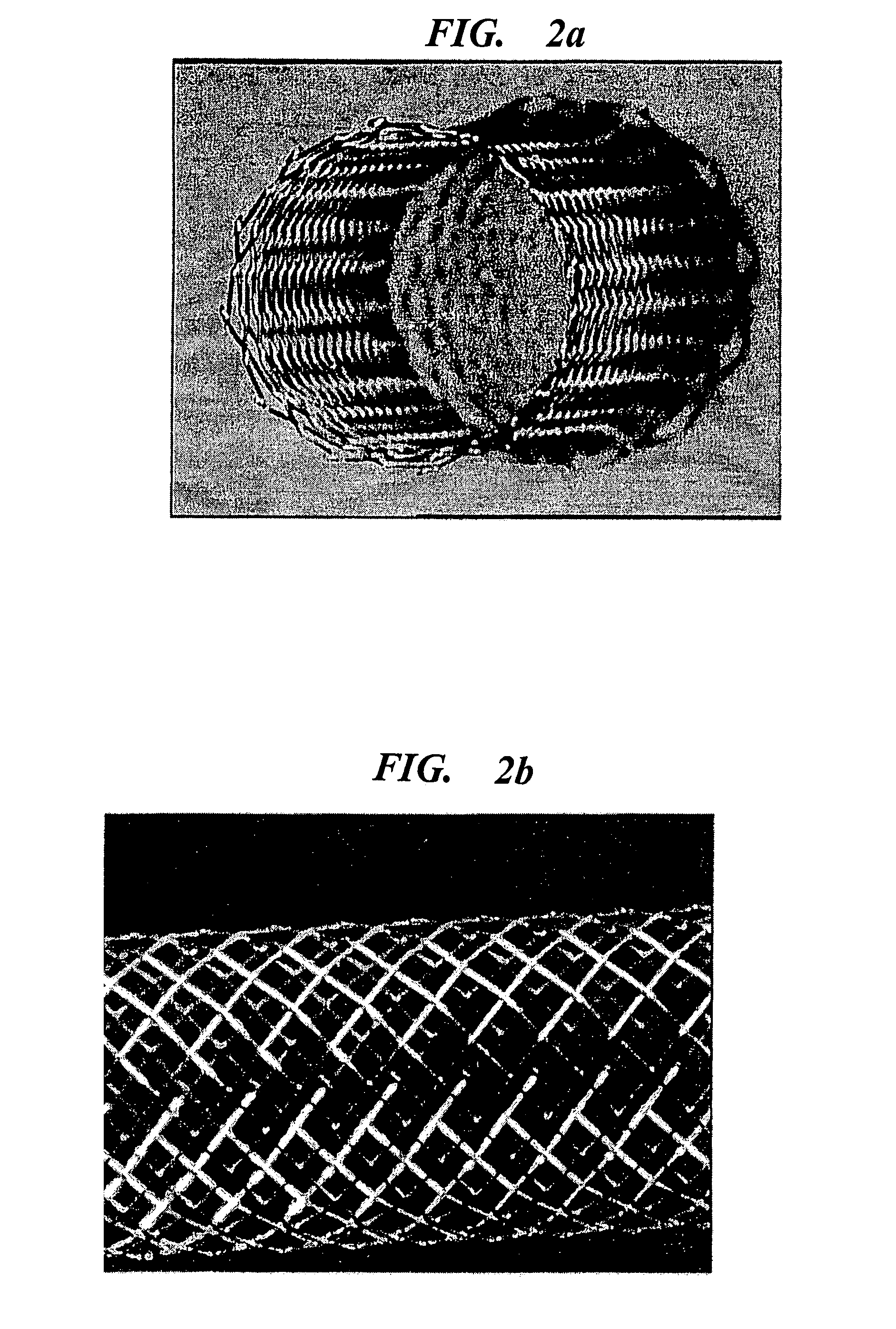
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Apparatus and methods for preventing or treating failure of hemodialysis vascular access and other vascular grafts
InactiveUS6726923B2Reduce calcificationInhibiting smooth muscle cell proliferationBiocideOrganic chemistryVascular graftBlood vessel
Owner:VASCULAR THERAPIES INC
Implantable Tissue Compositions and Method
InactiveUS20070254005A1Promotes localized deliveryImprove overall lifespanNervous disorderAntipyreticWater insolubleFixation method
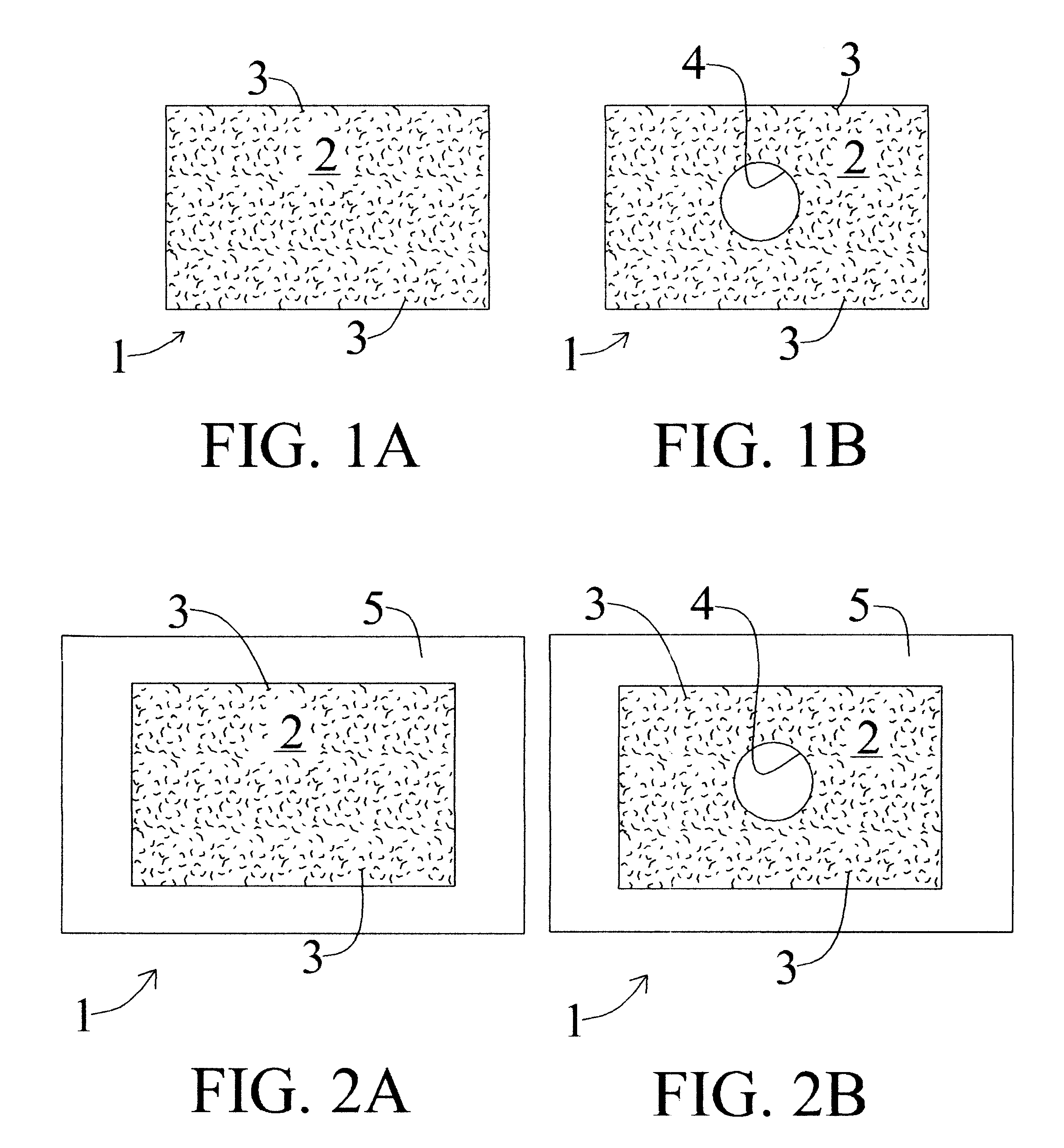
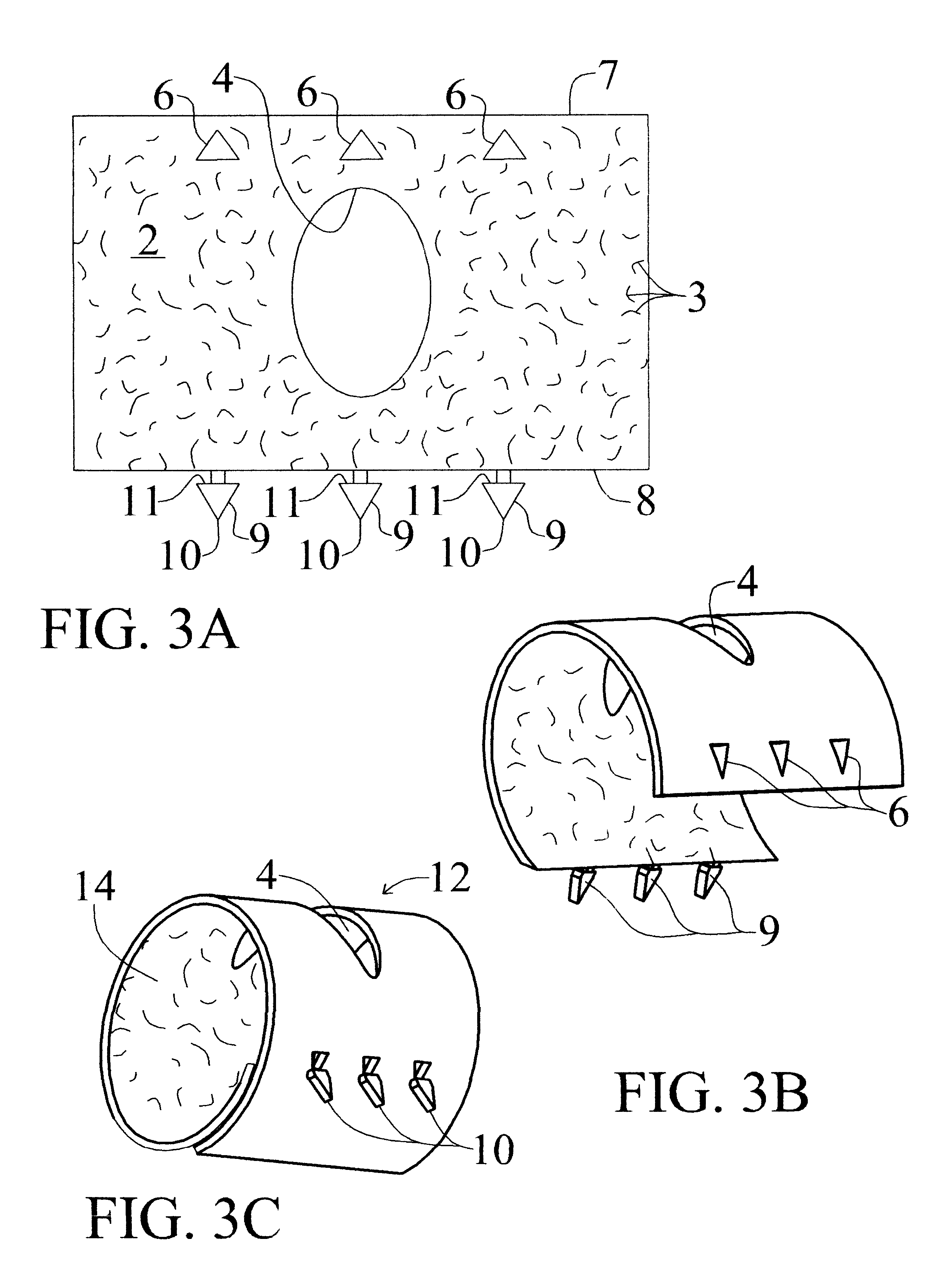
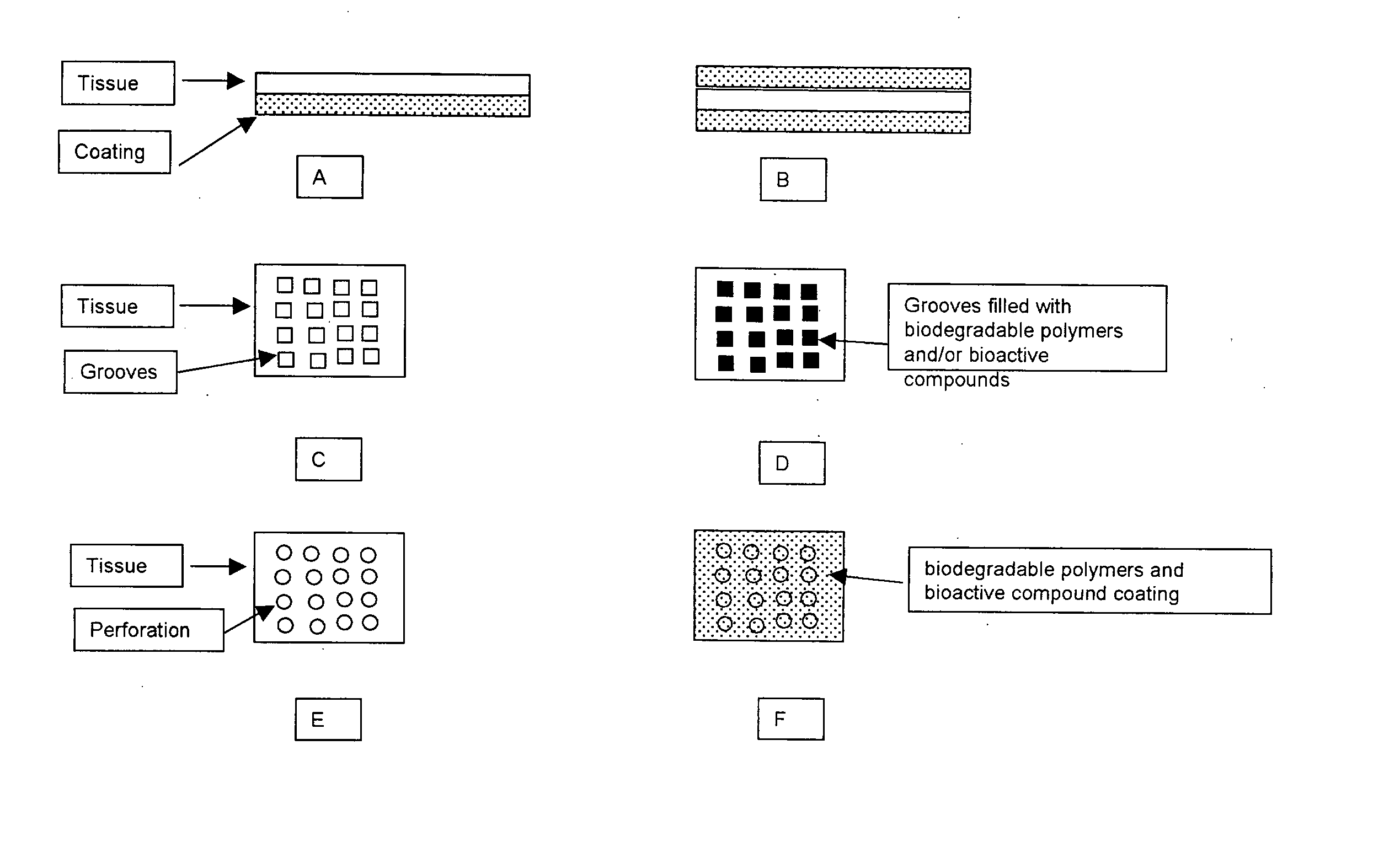
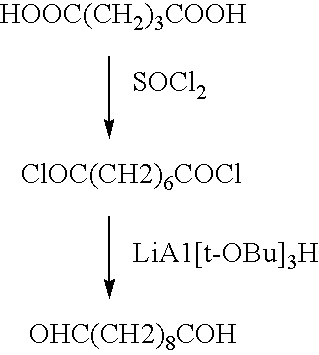
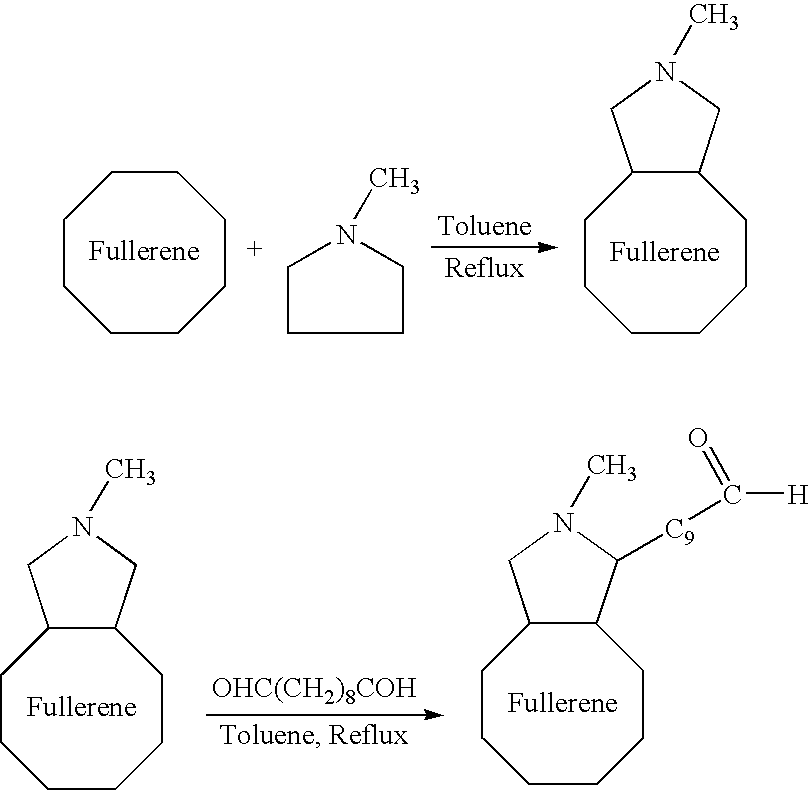
Novel implantable tissue fixation methods and compositions are disclosed. Methods and compositions of tissue, fixed using polymeric and / or variable length crosslinks, and di- or polymercapto compounds are described. Also described are the methods and compositions wherein the tissue is fixed using biodegradable crosslinkers. Methods and compositions for making radioopaque tissue are also described. Methods and compositions to obtain a degradable implantable tissue-synthetic biodegradable polymer composite are also described. Compositions and methods of incorporating substantially water-insoluble bioactive compounds in the implantable tissue are also disclosed. The use of membrane-like implantable tissue to make an implantable drug delivery patch are also disclosed. Also described are the compositions and methods to obtain a coated implantable tissue. Medical applications implantable tissue such as heart valve bioprosthesis, vascular grafts, meniscus implant, drug delivery patch are also disclosed.
Owner:PATHAK HLDG
Protein matrix materials, devices and methods of making and using thereof
InactiveUS7662409B2Enhances strength and durabilityFacilitated releaseBiocidePowder deliveryActive agentProtein materials
The present invention relates to protein matrix materials and devices and the methods of making and using protein matrix materials and devices. More specifically the present invention relates to protein matrix materials and devices that may be utilized for various medical applications including, but not limited to, drug delivery devices for the controlled release of pharmacologically active agents, encapsulated or coated stent devices, vessels, tubular grafts, vascular grafts, wound healing devices including protein matrix suture material and meshes, skin / bone / tissue grafts, biocompatible electricity conducting matrices, clear protein matrices, protein matrix adhesion prevention barriers, cell scaffolding and other biocompatible protein matrix devices. Furthermore, the present invention relates to protein matrix materials and devices made by forming a film comprising one or more biodegradable protein materials, one or more biocompatible solvents and optionally one or more pharmacologically active agents. The film is then partially dried, rolled or otherwise shaped, and then compressed to form the desired protein matrix device.
Owner:PETVIVO HLDG INC
Self-supporting metallic implantable grafts, compliant implantable medical devices and methods of making same
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Coated vascular grafts and methods of use
InactiveUS6939377B2Provide strengthProvide compliancePharmaceutical containersPretreated surfacesPorous coatingMedicine
A vascular graft, such as an AAA stent graft, includes a core zone of PET fabric with a non-porous coating comprising a polyurethane, such as Thoralon®, disposed on at least one surface. The coating provides a barrier to prevent short and long term leakage of fluids through the pores of the PET fabric core zone. A method for sealing the pores of a porous PET graft includes the step of coating at least one surface of said graft with at least one polyurethane, or mixtures and combinations thereof. Preferably, the coating is Thoralon®. A method for making a vascular prosthesis includes the steps of providing a core zone of PET fabric, and coating at least one surface of the core zone with at least one polyurethane, or mixtures and combinations thereof, such as Thoralon®. Finally, a method of repairing or treating a defective vessel includes the step of reinforcing or replacing the defective vessel with a graft according to the invention.
Owner:TC1 LLC
Non-leaching non-fouling antimicrobial coatings
InactiveUS20090155335A1Solve the lack of flexibilityPromote adequate mobilityAntibacterial agentsPeptide/protein ingredientsDendrimerFiber
Compositions containing one or more types of membrane-targeting antimicrobial agents immobilized on a substrate with activity in relevant biological environments, and methods of making and using thereof, are described herein. The antimicrobial agents retain their activity in the presence of blood proteins and / or in vivo due to improved molecular structures which allow for cooperative action of immobilized agents and hydrophilic chemistries which resist non-specific protein adsorption. Suitable molecular structures include branched structures, such as dendrimers and randomly branched polymers. The molecule structures may also include hydrophilic tethers which provide both flexibility and resistance to non-specific protein adsorption. The membrane targeting antimicrobial agent coatings can be applied to a variety of different types of substrates including medical implants such as vascular grafts, orthopedic devices, dialysis access grafts, and catheters; surgical tools, surgical garments; and bandages. The substrates can be composed of metallic materials, ceramics, polymers, fibers, inert materials such as silicon, and combinations thereof. The compositions described herein are substantially non-leaching, resistant to non-specific protein adsorption, and non-hemolytic.
Owner:ARROW INT INC
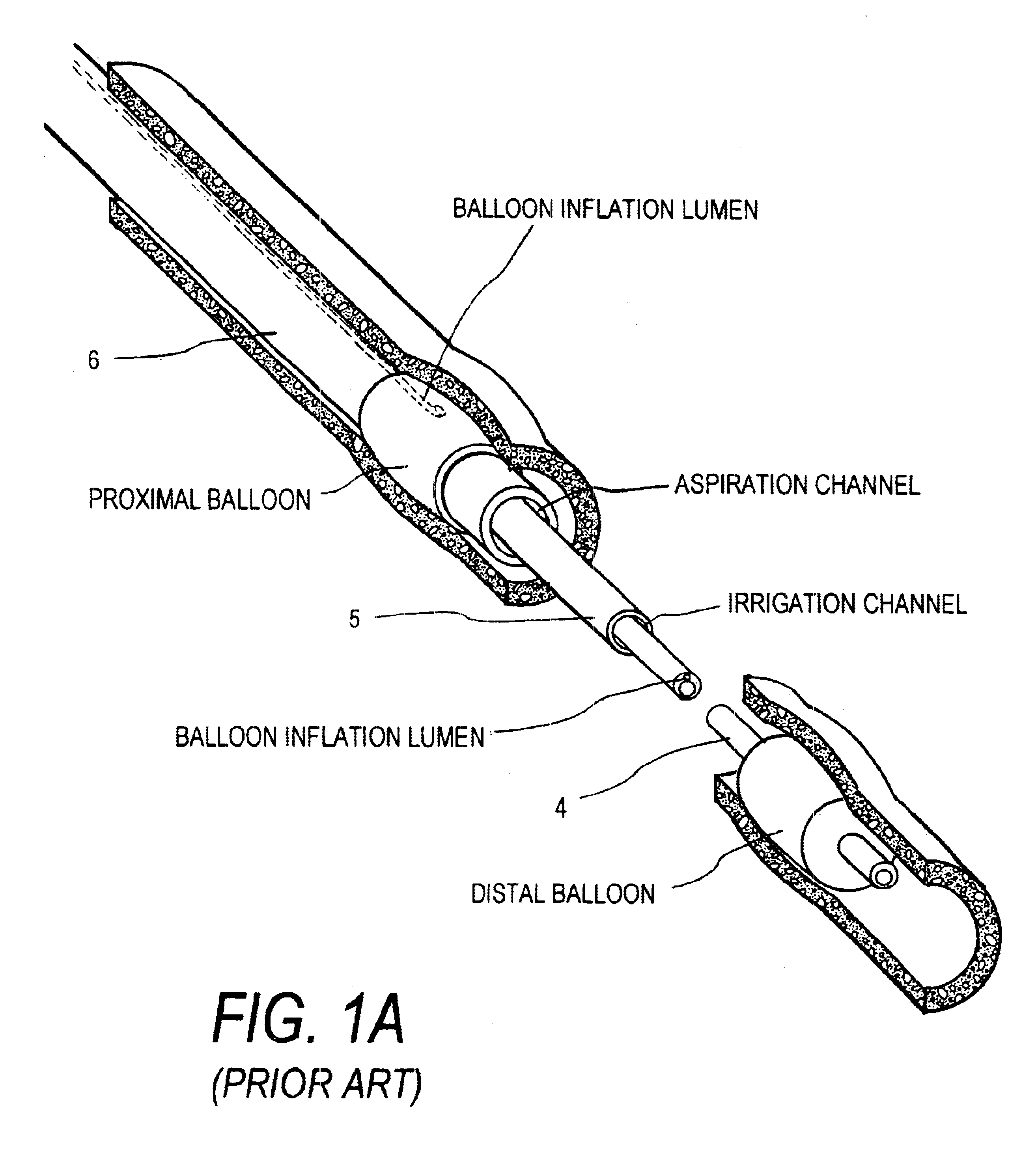
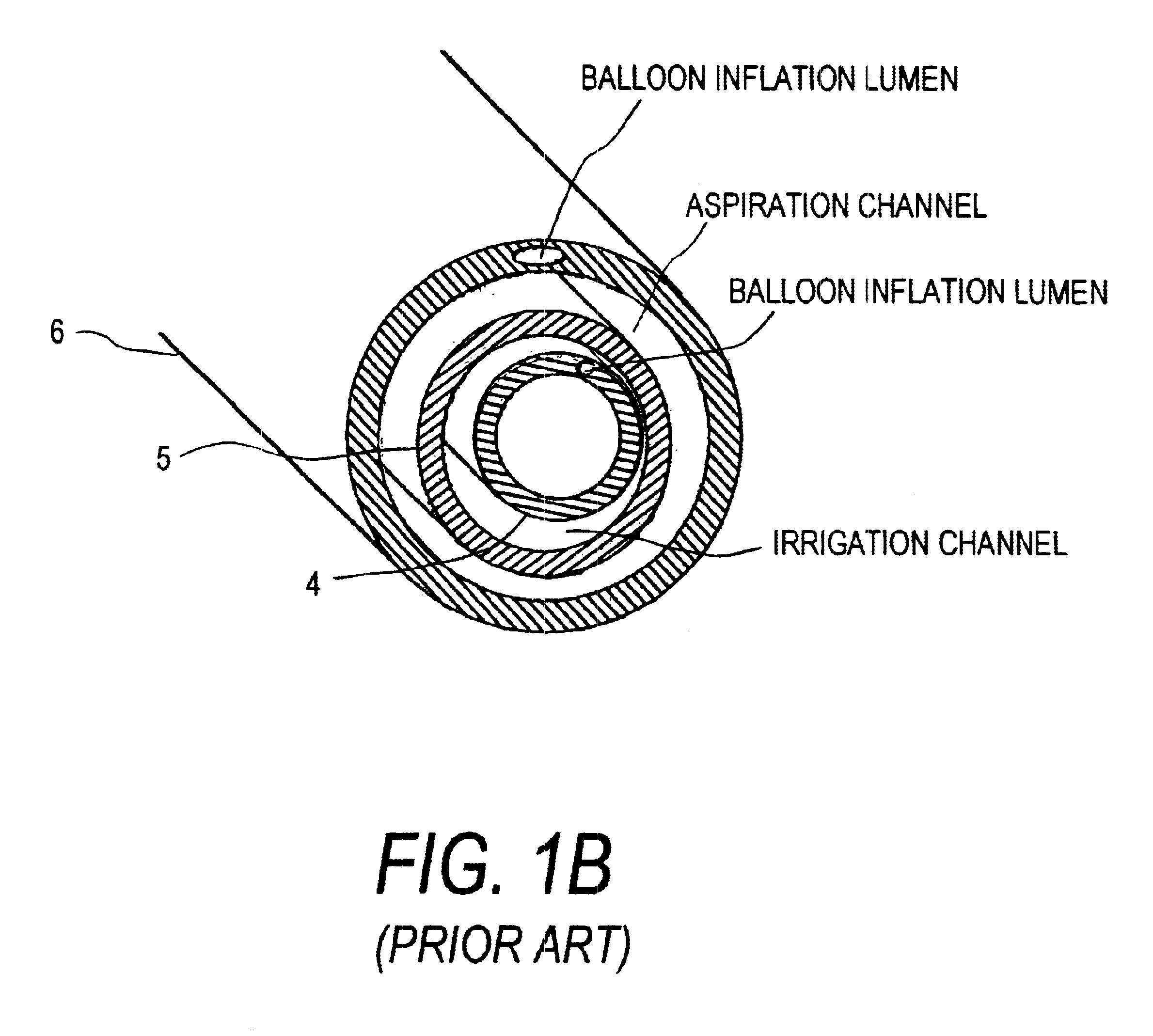
Rotational thrombectomy device
Owner:ARGON MEDICAL DEVICES
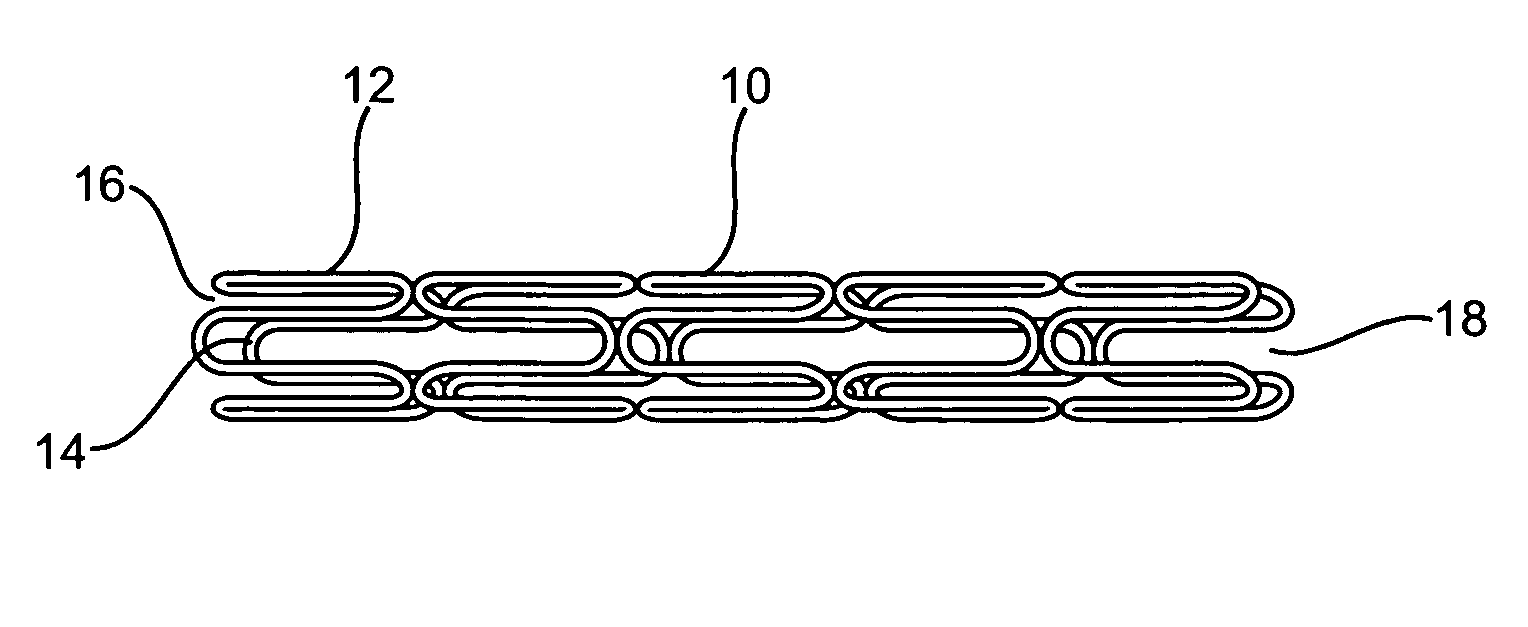
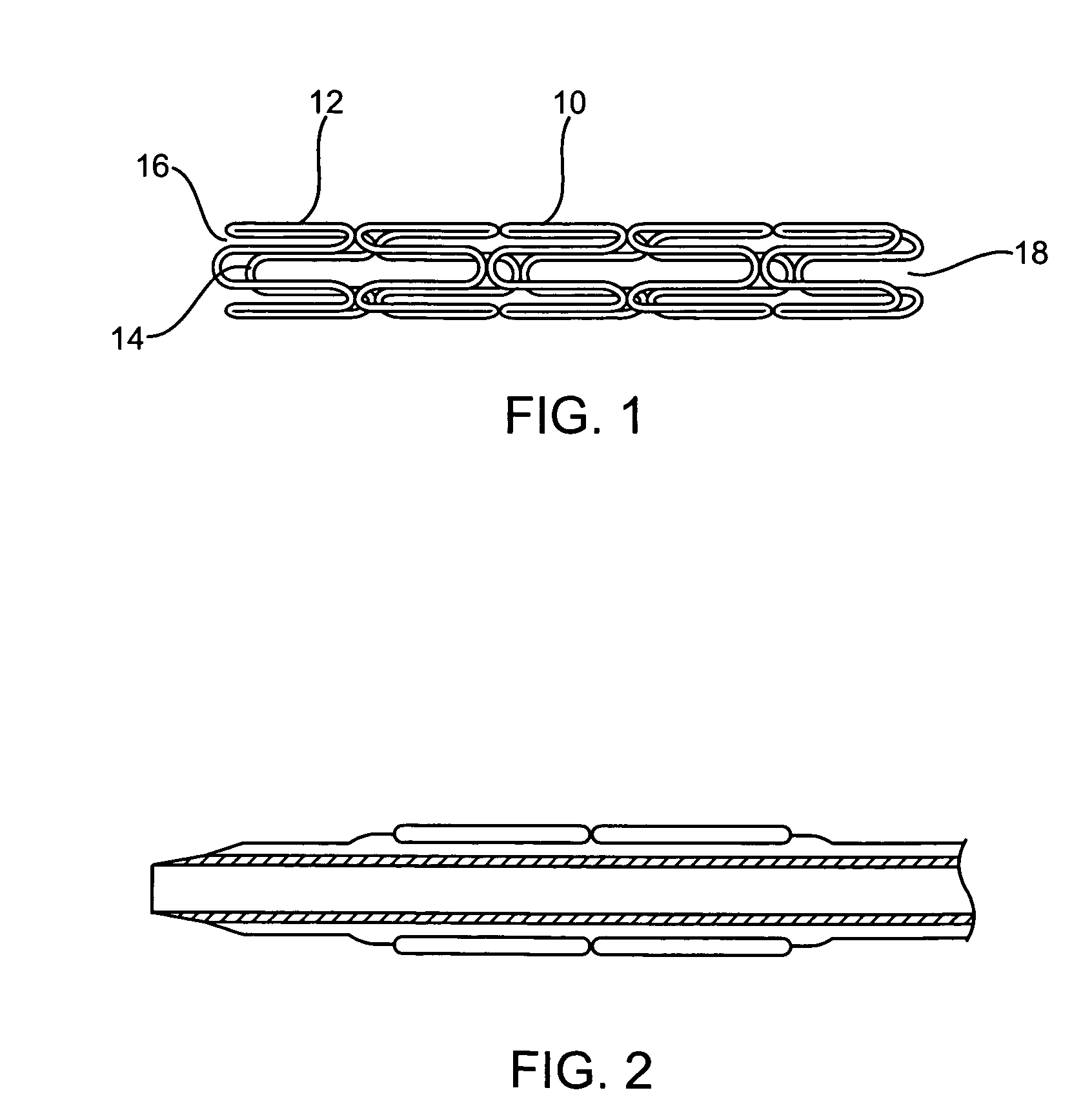
Braided composite prosthesis
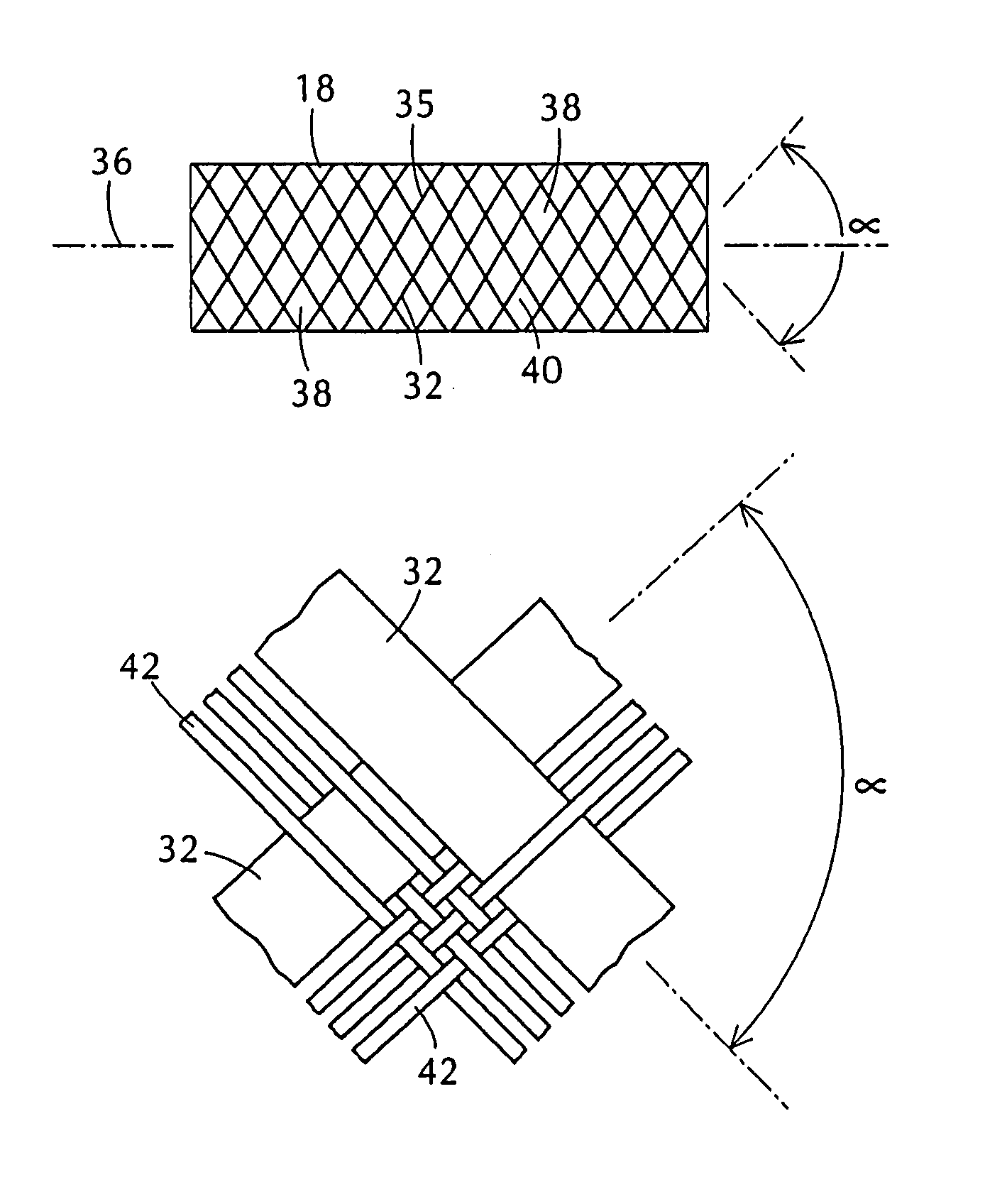
A prosthesis for transluminal implantation consists of a flexible tubular interbraided structure of metal or polymeric monofilaments, and polymeric multifilament yarns. The prosthesis can be elastically deformed to reduce its diameter through axial elongation. The monofilaments and multifilament yarns are arranged in axially spaced apart helices, concentric on a common central axis of the prosthesis. The monofilaments are selectively shaped before their interbraiding with the multifilament yarns, either by an age-hardening or other heat-setting stage, or a cold-working stage that controllably plastically deforms the strands. The shaped structural strands cooperate to impart to the prosthesis its nominal shape and resilience. The textile strands cooperate to provide a sheeting that occupies interstices between adjacent structural strands, to reduce permeability and thereby enhance the utility of the prosthesis as a vascular graft. An alternative embodiment prosthesis includes elastically and plastically deformable structural strands, selectively plastically deformed by cold-working then interbraided to form the prosthesis.
Owner:LIFESHIELD SCI
Thrombectomy device with multi-layered rotational wire
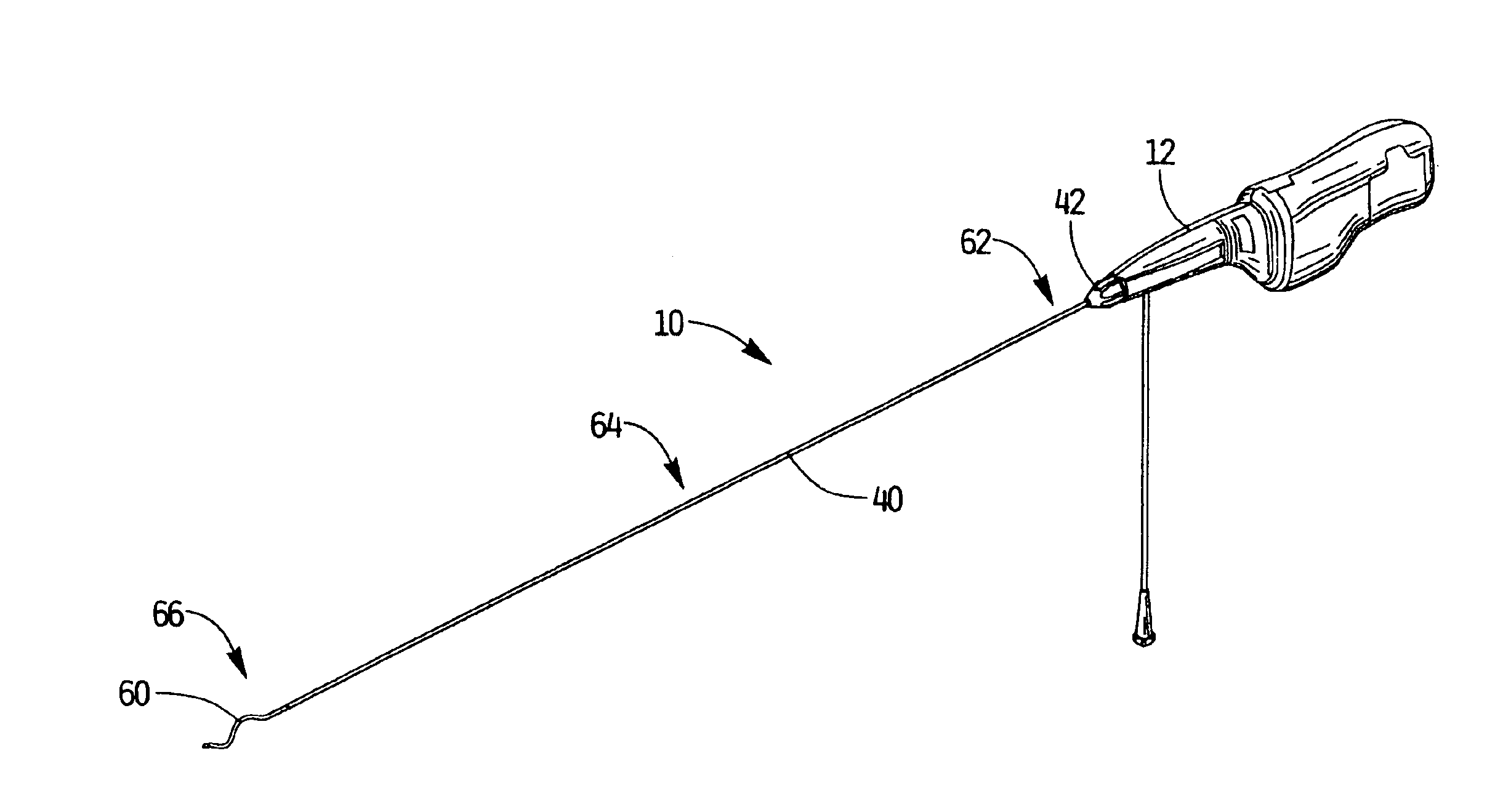
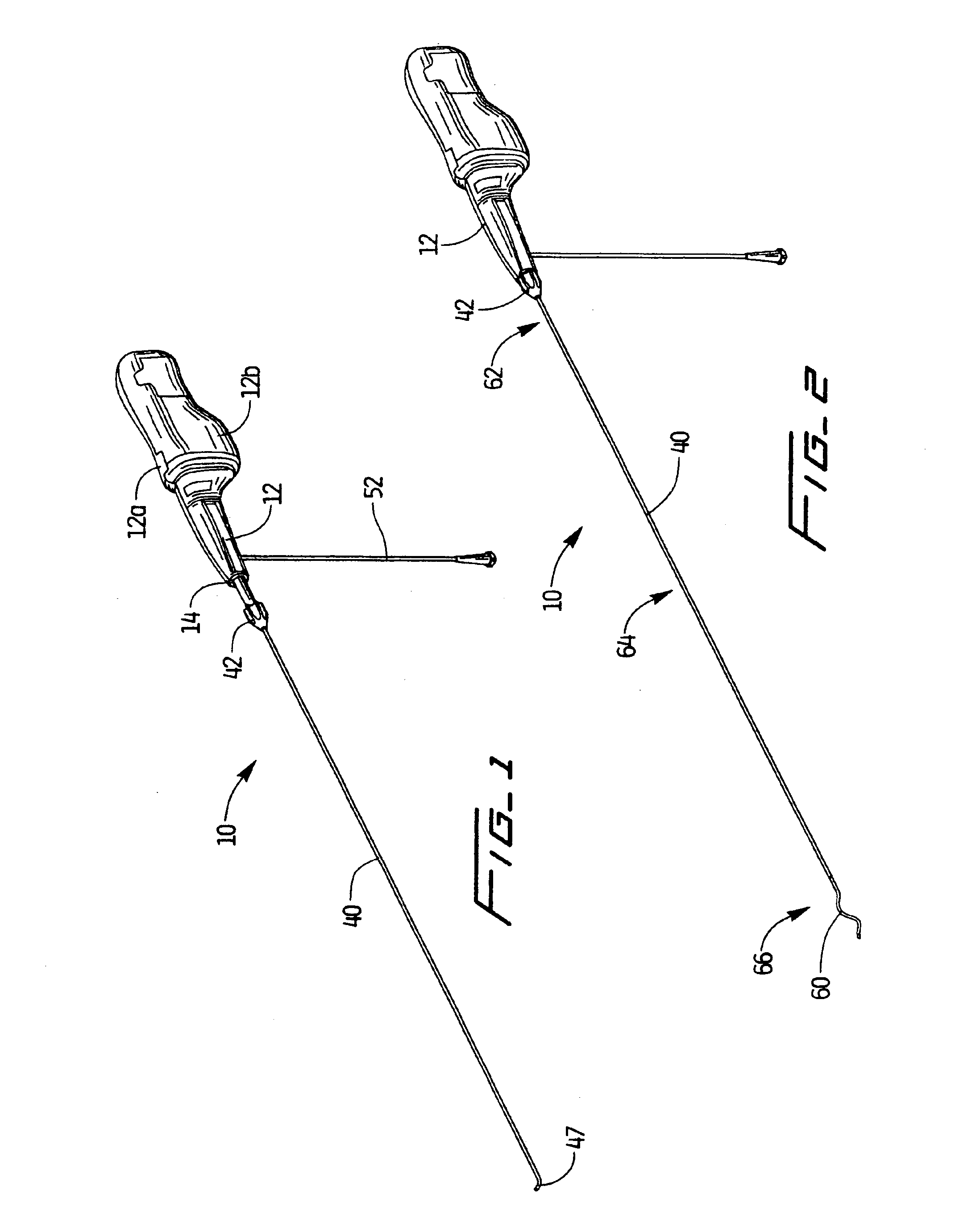
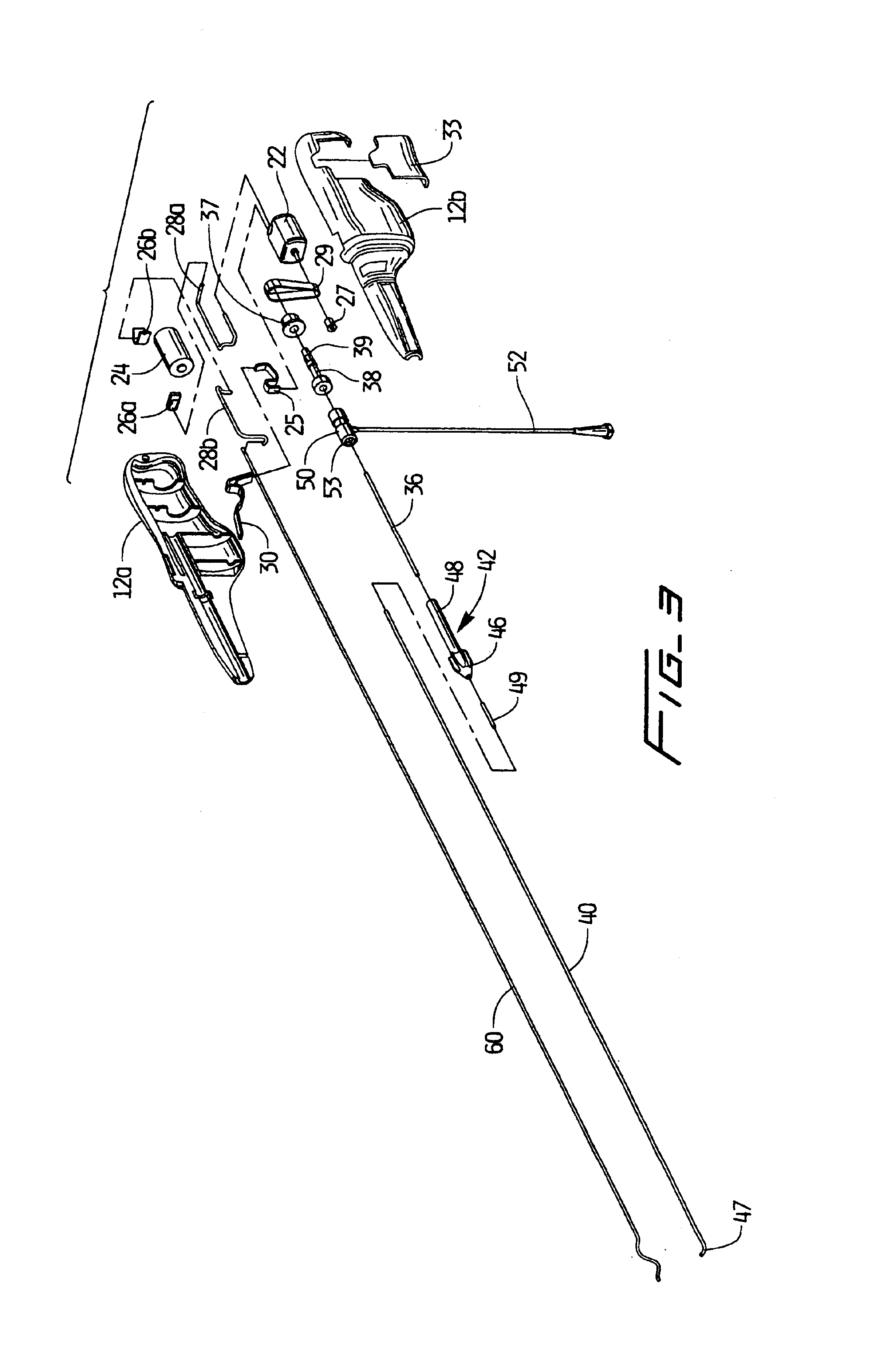
An improvement to a thrombectomy apparatus for breaking up thrombus or other obstructive material in a lumen of a vascular graft or vessel having a wire and flexible sheath relatively movable, wherein the wire is sinuous in configuration and assumes its sinuous configuration when in the deployed configuration and has a straighter configuration in the first configuration. The wire is operatively connected to a motor for rotation of the wire to enable peaks of the sinuous wire to contact a wall of the lumen to break up the thrombus or other obstructive material. The improvement to the thrombectomy apparatus comprises the wire being formed of an inner core formed by a plurality of twisted wires and an outer wire wound directly around the inner core, wherein a distal portion of the outer wire extends distal of the inner core and progressively tapers towards a distal end to form a tapered region.
Owner:ARGON MEDICAL DEVICES
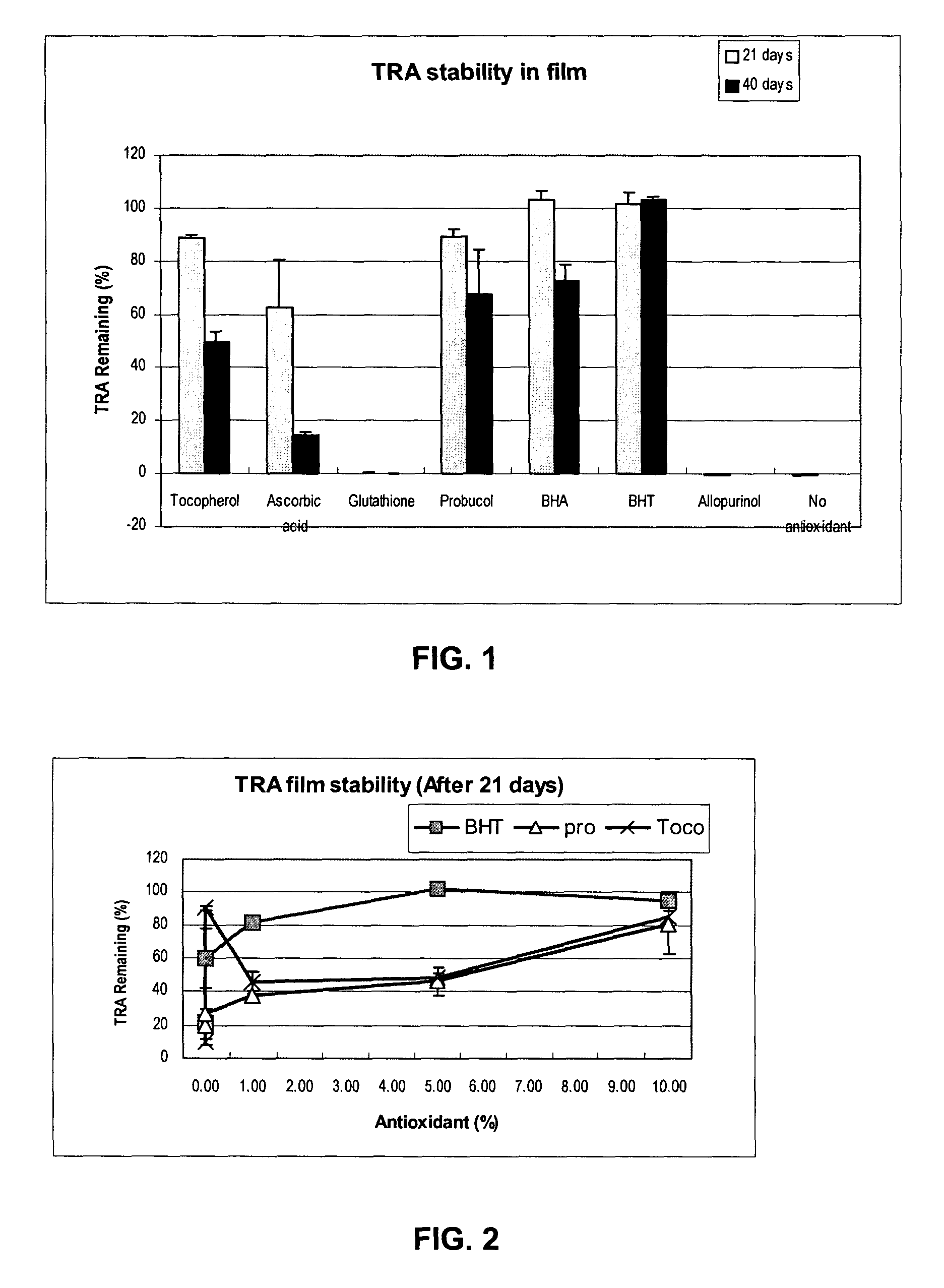
Medical devices containing antioxidant and therapeutic agent
An medical device which comprises (a) a medical device substrate and (b) a therapeutic-agent-containing region over the substrate that comprises a therapeutic agent and an antioxidant. Exemplary medical devices are implantable or insertable medical devices, such as catheters, guide wires, balloons, filters, stents, stent grafts, vascular grafts, vascular patches and shunts. Also described are methods of making devices such as those above, which methods comprise: (a) providing a solution comprising (i) solvent, (ii) the therapeutic agent, and (iii) the antioxidant; (b) providing the medical device substrate; (c) contacting the solution with the medical device substrate; and (d) removing the solvent from the solution to form the therapeutic-agent-containing region.
Owner:BOSTON SCI SCIMED INC
Implantable heart assist system and method of applying same
InactiveUS6889082B2RevitalizeReduce energy inputElectrotherapyElectrocardiographyMinimally invasive proceduresCatheter
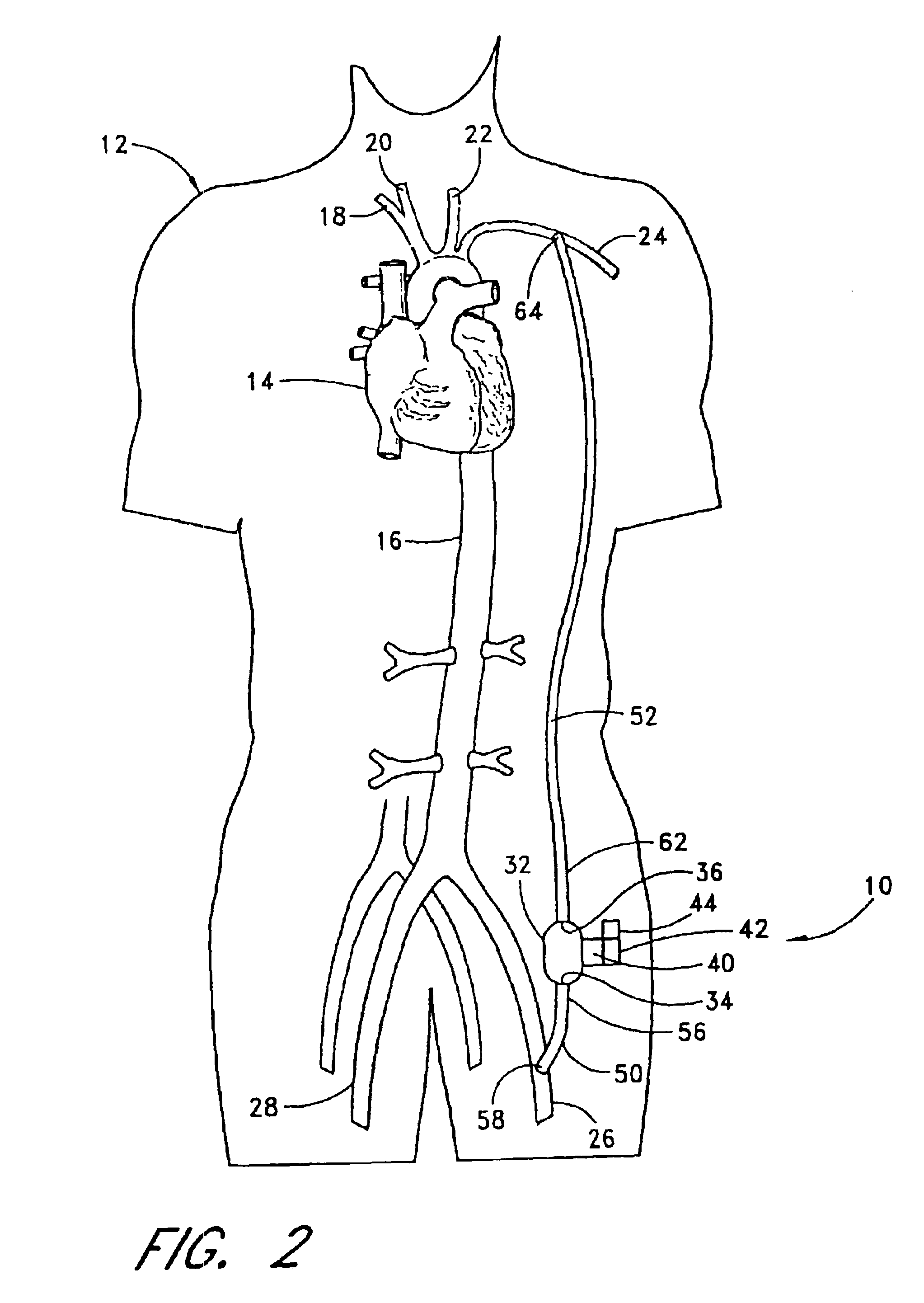
An extracardiac pumping for supplementing the circulation of blood, including the cardiac output, in a patient without any component thereof being connected to the patient's heart, and methods of using same. One embodiment provides a vascular graft that has a first end that is sized and configured to couple to a non-primary blood vessel and a second end that is fluidly coupled to a pump to conduct blood between the pump and the non-primary blood vessel. An outflow conduit is also provided that has a first end sized and configured to be positioned within the same or different blood vessel, whether primary or non-primary, through the vascular graft. The outflow conduit is fluidly coupled to the pump to conduct blood between the pump and the patient. The vascular graft may be connected to the blood vessel subcutaneously to permit application of the extracardiac pumping system in a minimally-invasive procedure.
Owner:TC1 LLC +1
Anti-calcification treatments for heart valves and vascular grafts
InactiveUS20060047343A1Reduces calcification potentialReduce calcificationSuture equipmentsHeart valvesCalcificationProsthesis
The present invention provides processes for fixation of biological tissue and / or post-fixation treatment of such tissue that result in modified tissues with reduced susceptibility to in vitro calcification when used in prosthetic devices. The invention also relates to calcification resistant biological tissue and to methods of using such tissue.
Owner:OVIATT HENRY W +1
Aorto uni-iliac graft
A generally tubular intraluminal vascular graft having a linear shape that may be radially expanded from a compressed state to an expanded state within a body vessel is disclosed. The graft includes a plurality of stent portions which take the form of undulating filaments extending circumferentially along the tubular body and forming a generally ring-shaped configuration. The graft may further be equipped with a plurality of specifically-configured engagement members disposed on the outer surface of the graft which are configured to frictionally engage an inner wall of a vessel so as to inhibit longitudinal movement of the tubular body without piercing the vessel wall.
Owner:EDWARDS LIFESCIENCES CORP
Devices and methods for performing avascular anastomosis
InactiveUS6899718B2Efficient and reliable performanceEfficient executionStaplesNailsVascular anastomosisBlood vessel
Owner:HEARTPORT
Elastomerically impregnated ePTFE to enhance stretch and recovery properties for vascular grafts and coverings
This invention relates to an elastomerically recoverable PTFE material that includes a longitudinally compressed fibrils of ePTFE material penetrated by elastomeric material within the pores defining the elastomeric matrix. The elastomeric matrix and the compressed fibrils cooperatively expand and recover without plastic deformation of the ePTFE material. This invention was used for various prosthesis, such as a vascular prosthesis like a patch, a graft and an implantable tubular stents. Furthermore, this invention discloses a method of producing the elastomerically recoverable PTFE material which include the steps of: providing the specified ePTFE, defined by the nodes and fibrils, to meet the desired end use; longitudinally compressing the fibrils of the ePTFE, the pore size sufficiently enough to permit penetration of the elastomeric material; applying the elastomeric material within the pores to provide a structurally integral elastomerically recoverable PTFE material defining an elastomeric matrix. The elastomeric material is applied to the ePTFE by dip, brush or spray coating techniques. The compression step and the application steps are interchangeable to produce the desired properties for the end use material. Finally, the elastomeric material is dried within the pores of the longitudinally compressed ePTFE to solidify the elastomeric matrix.
Owner:LIFESHIELD SCI
Compliant blood vessel graft
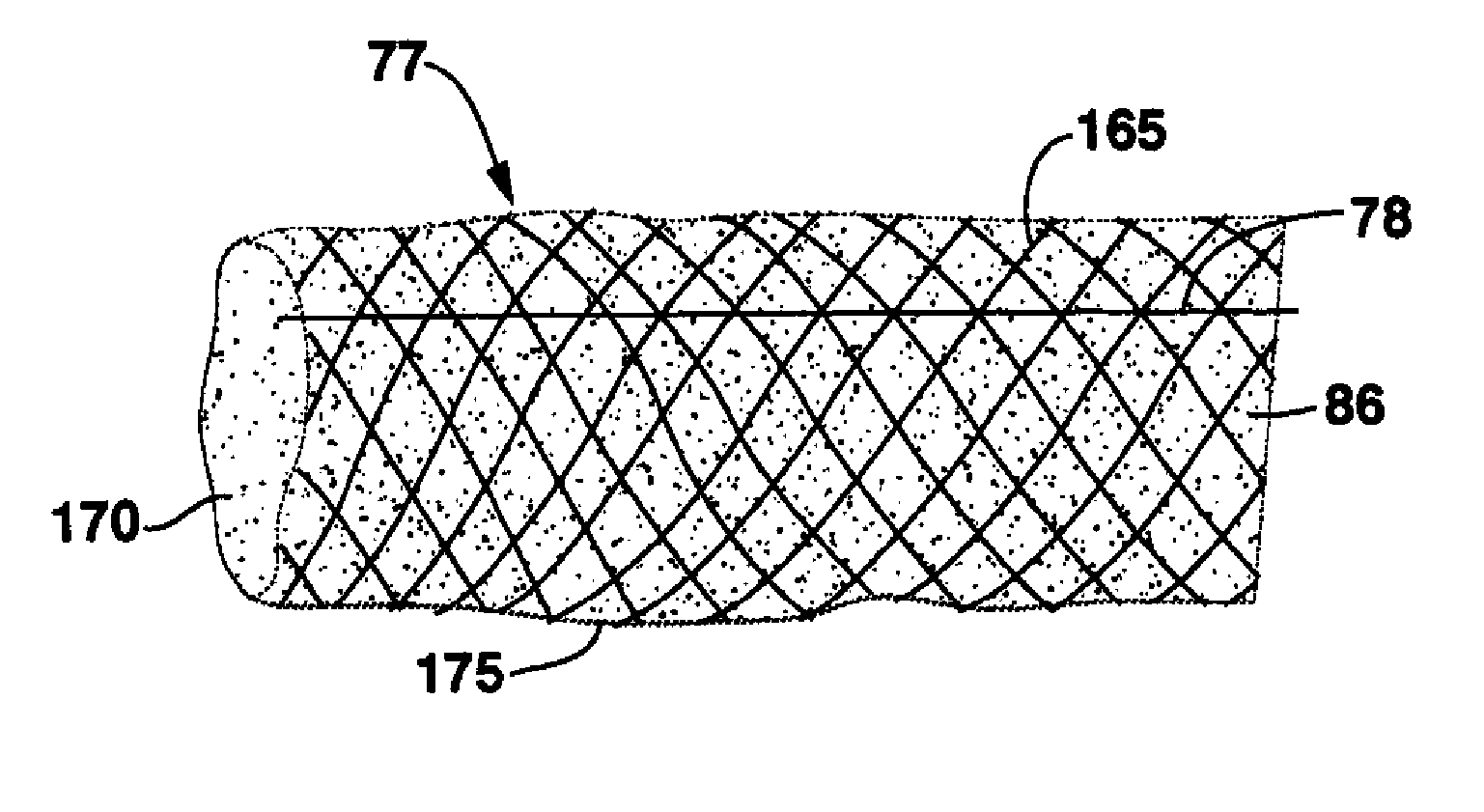
ActiveUS20070293932A1Smoothening of irregularitiesReduce the differenceStentsSurgeryVenous SegmentVenous graft
Stents and methods of using stents are provided. Stents of the invention provide external support structure for a blood vessel segment disposed within, wherein the stents are capable of resilient radial expansion in a manner mimicking the compliance properties of an artery. The stent may be formed of a knitted or braided mesh formed so as to provide the needed compliance properties. A venous graft with the stent and a vein segment disposed within is provided, wherein graft is capable of mimicking the compliance properties of an artery. Methods of selecting stents for downsizing and methods of using the stents of the invention in downsizing and smoothening are provided. Methods of replacing a section of an artery with a venous graft including a stent of the invention are provided. Methods of reducing intimal hyperplasia in implanted vein segment in a venous graft using stents of the invention are provided.
Owner:VASCULAR GRAFT SOLUTIONS
Implantable medical devices with antimicrobial and biodegradable matrices
InactiveUS20050283224A1Inhibition formationPromote tissue growthStentsBlood vesselsVenous graftActive agent
A composite vascular graft is provided, which incorporates bioactive agents that can be controllably delivered to the implantation site to deliver therapeutic materials and / or to reduce infection of the implant. The vascular graft of the present invention includes a luminal layer of ePTFE; and a biodegradable polymer layer including a bioactive agent, such as an antimicrobial agent. The biodegradable polymer layer is posited on the external surface of the luminal ePTFE layer. The graft also includes a fabric layer, which is posited on the external surface of the biodegradable layer. The graft is particularly useful as an arterial-venous graft for hemodialysis procedures.
Owner:MAQUET CARDIOVASCULAR LLC +1
Medical implants with a combination of compounds
InactiveUS20100074934A1Minimize formationImprove biological effectOrganic active ingredientsBiocideDipyridamoleFibrosis
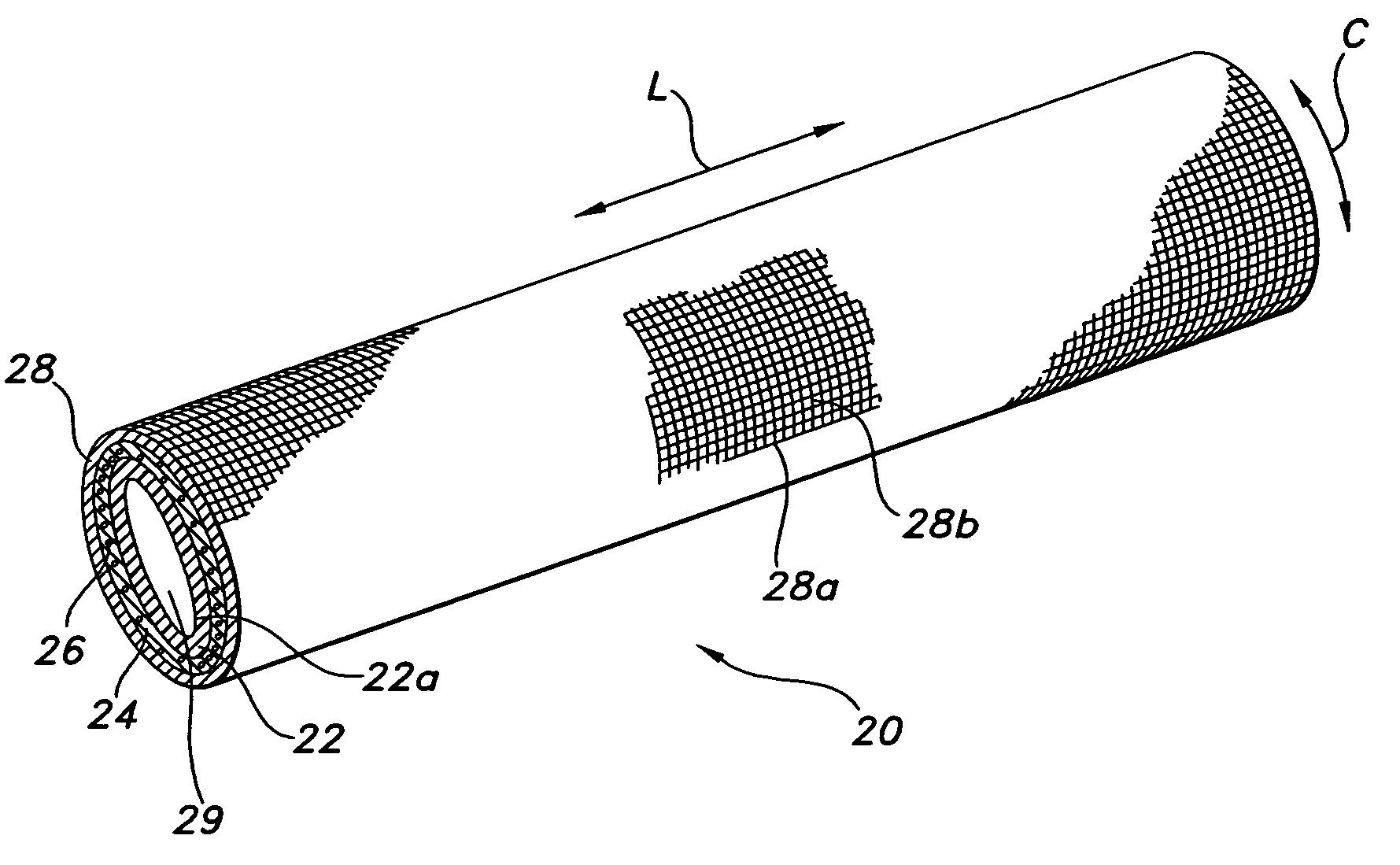
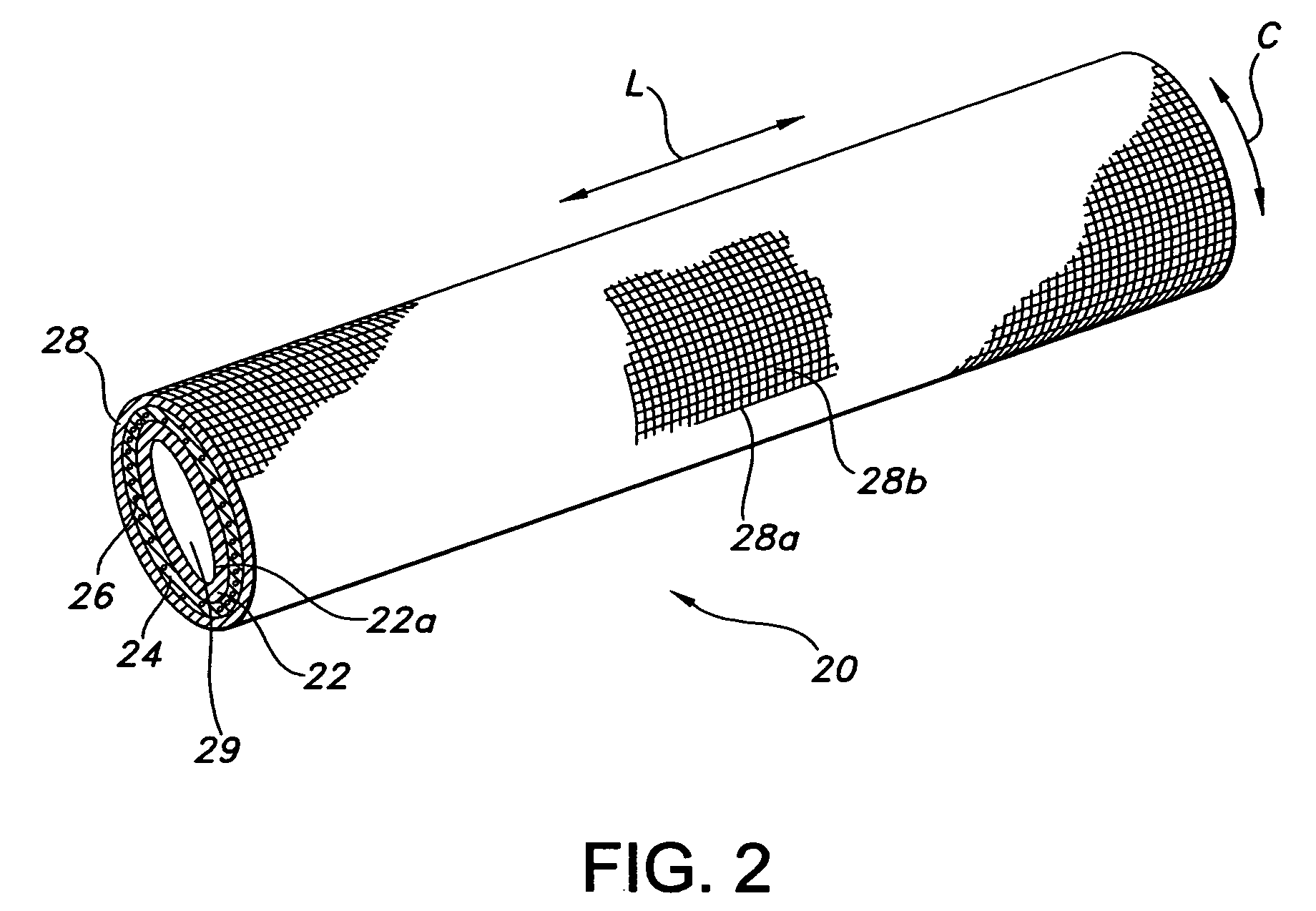
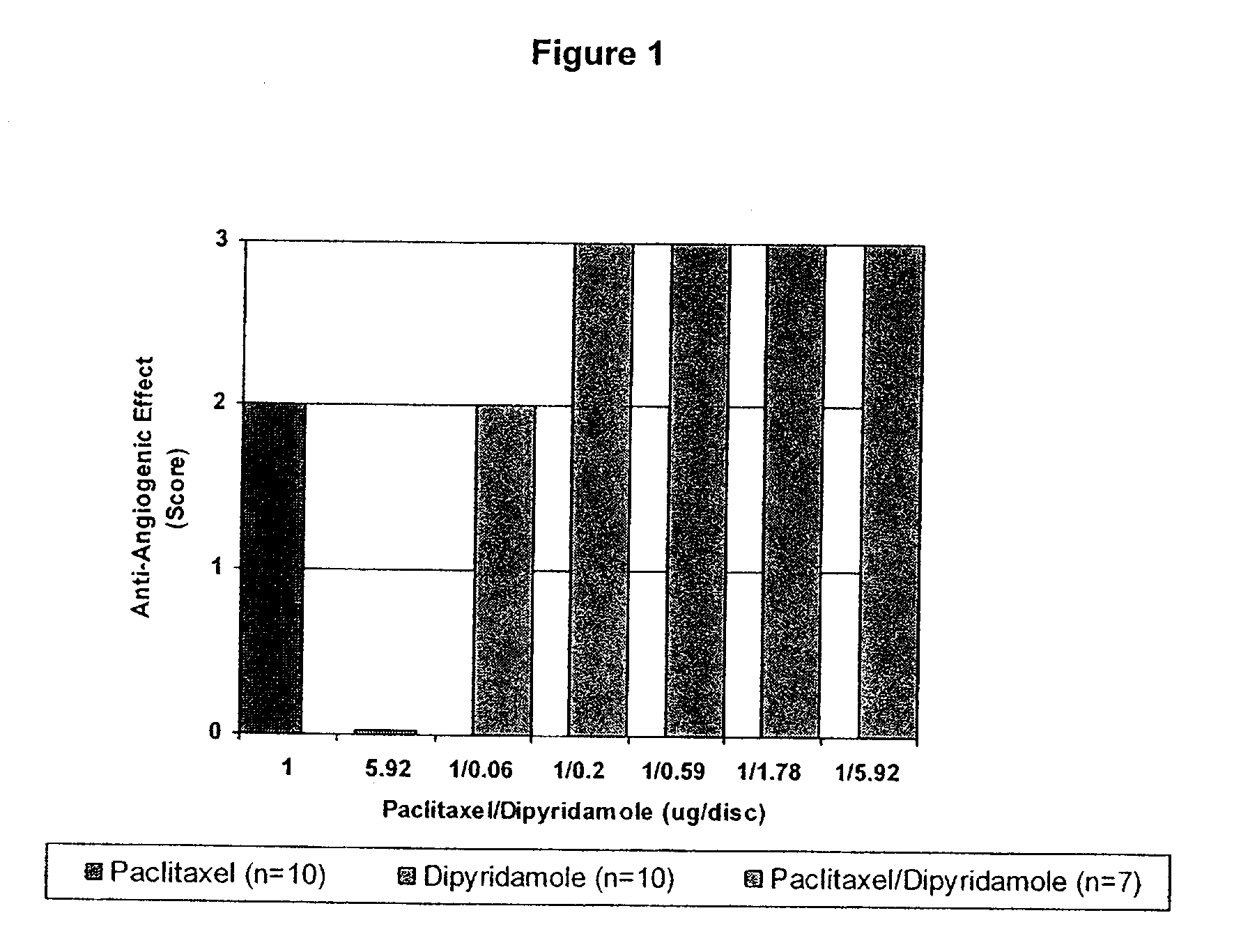
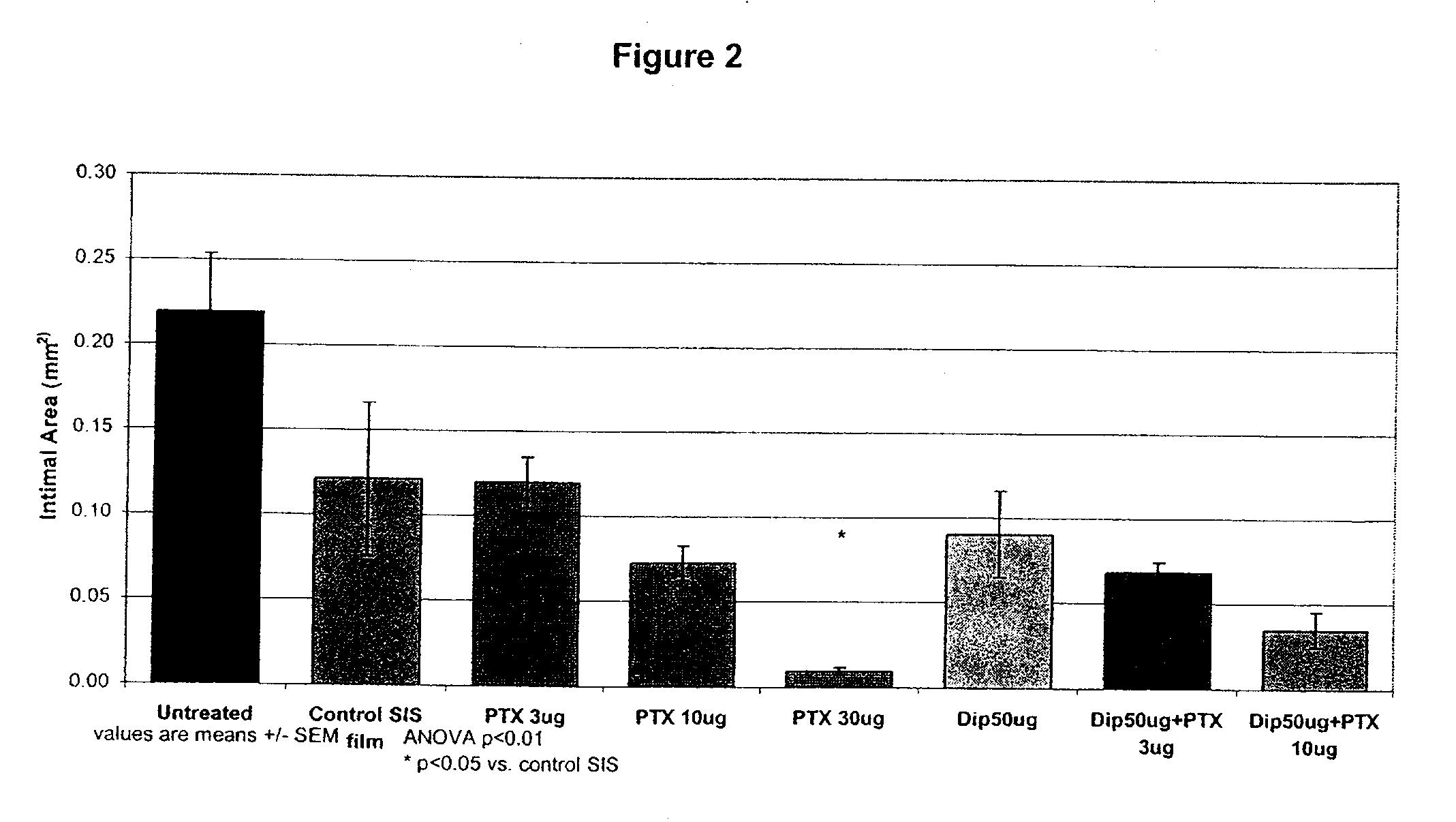
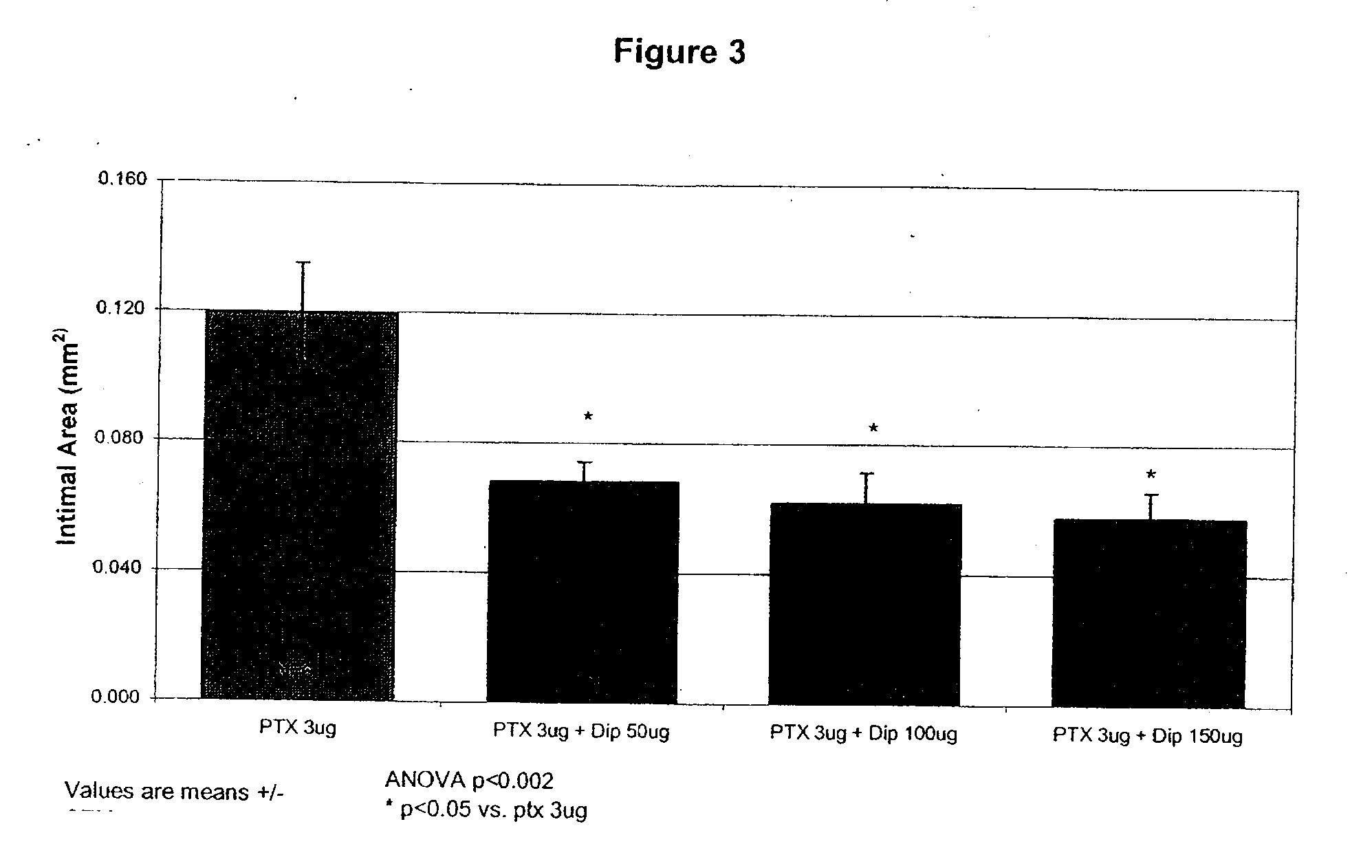
Implants are associated with a combination of paclitaxel or derivatives and dipyridamole or derivatives in order to inhibit fibrosis that may otherwise occur when the implant is placed within an animal. Exemplary implants include intravascular implants (e.g., coronary and peripheral vascular stents, catheters, balloons), non-vascular stents, pumps and sensors, vascular grafts, perivascular devices, implants for hemodialysis access, vena cava filters, implants for providing an anastomotic connection, electrical devices, intraocular implants, and soft tissue implants and fillers.
Owner:ANGIOTECH PHARMA INC
Implantable tissue compositions and method
InactiveUS7919112B2Improve overall lifespanPromotes localized deliveryNervous disorderAntipyreticWater insolubleFixation method
Novel implantable tissue fixation methods and compositions are disclosed. Methods and compositions of tissue, fixed using polymeric and / or variable length crosslinks, and di- or polymercapto compounds are described. Also described are the methods and compositions wherein the tissue is fixed using biodegradable crosslinkers. Methods and compositions for making radio-opaque tissue are also described. Methods and compositions to obtain a degradable implantable tissue-synthetic biodegradable polymer composite are also described. Compositions and methods of incorporating substantially water-insoluble bioactive compounds in the implantable tissue are also disclosed. The use of membrane-like implantable tissue to make an implantable drug delivery patch are also disclosed. Also described are the compositions and methods to obtain a coated implantable tissue. Medical applications implantable tissue such as heart valve bioprosthesis, vascular grafts, meniscus implant, drug delivery patch are also disclosed.
Owner:PATHAK HLDG
Medical devices to treat or inhibit restenosis
Implantable medical devices having anti-restenotic coatings are disclosed. Specifically, implantable medical devices having coatings of certain antiproliferative agents, particularly a certain endothelin receptor inhibitor, are disclosed. The anti-restenotic endothelin receptor inhibitor is atrasentan, and pharmaceutically acceptable derivatives thereof. The anti-restenotic medical devices include stents, catheters, micro-particles, probes and vascular grafts. Intravascular stents are preferred medical devices. The medical devices can be coated using any method known in the art including compounding the endothelin receptor inhibitor with a biocompatible polymer prior to applying the coating. Moreover, medical devices composed entirely of biocompatible polymer-endothelin receptor inhibitor blends are disclosed. Additionally, medical devices having a coating comprising at least one endothelin receptor inhibitor in combination with at least one additional therapeutic agent are also disclosed. Furthermore, related methods of using and making the anti-restenotic implantable devices are also disclosed.
Owner:MEDTRONIC VASCULAR INC
Vascular graft having a chemicaly bonded electrospun fibrous layer and method for making same
InactiveUS20030100944A1Improve surface morphologyGood tissue responseNon-woven fabricsCoatingsChemical LinkageElectrospinning
A vascular graft comprising a traditional graft material and an electrospun fibrous layer. The solvent used to reduce the material for the electrospun layer is also capable of reducing the graft material to a liquid solution. The electrospun layer is chemically bonded to the graft material, without adhesives, by either spraying the graft with the solvent prior to electrospinning or by assuring that a sufficient amount of residual solvent reaches the graft while electrospinning.
Owner:DATASCOPE INVESTMENT
Composite vascular graft including bioactive agent coating and biodegradable sheath
A composite vascular graft incorporates bioactive agents to deliver therapeutic materials and / or inhibit or reduce bacterial growth during and following the introduction of the graft to the implantation site in a vascular system. A composite vascular graft includes a porous tubular graft member. One or more biodegradable, bioactive agent coating layers are disposed over the graft member, the coating layer including at least one bioactive agent. A biodegradable sheath is disposed over the coating layer. The sheath has a rigidity greater than the flexible tubular graft member and is biodegradable to expose the coating layer so as to re-establish the flexibility of the tubular graft member.
Owner:LIFESHIELD SCI
Left ventricular conduit with blood vessel graft
Disclosed is a conduit that provides a bypass around an occlusion or stenosis in a coronary artery. The conduit is a tube adapted to be positioned in the heart wall to provide a passage for blood to flow between a heart chamber and a coronary artery, at a site distal to the occlusion or stenosis. The conduit has a section of blood vessel attached to its interior lumen which preferably includes at least one naturally occurring one-way valve positioned therein. The valve prevents the backflow of blood from the coronary artery into the heart chamber.
Owner:JENAVALVE TECH INC
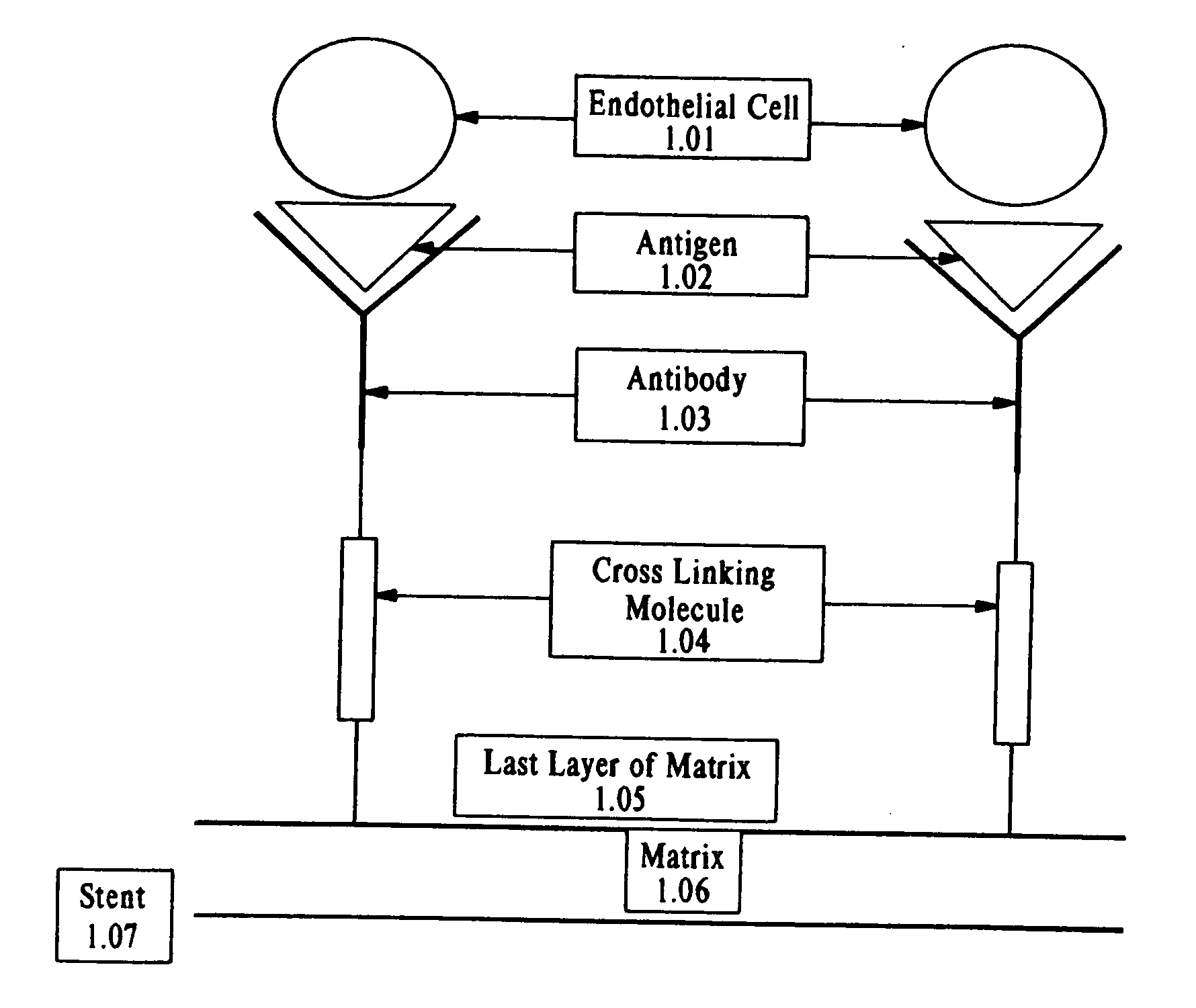
Medical device with coating that promotes endothelial cell adherence and differentiation
Compositions and methods are provided for producing a medical device such as a stent, a stent graft, a synthetic vascular graft, heart valves, coated with a biocompatible matrix which incorporates antibodies, antibody fragments, or small molecules, which recognize, bind to and / or interact with a progenitor cell surface antigen to immobilize the cells at the surface of the device. The coating on the device can also contain a compound or growth factor for promoting the progenitor endothelial cell to accelerate adherence, growth and differentiation of the bound cells into mature and functional endothelial cells on the surface of the device to prevent intimal hyperplasia. Methods for preparing such medical devices, compositions, and methods for treating a mammal with vascular disease such as restenosis, artherosclerosis or other types of vessel obstructions are disclosed.
Owner:ORBUSNEICH MEDICAL PTE LTD
Electrical discharge catheter system for extracting emboli in endovascular interventions
InactiveUS6679879B2Surgical instrument detailsFluid jet surgical cuttersMicrometerEndovascular interventions
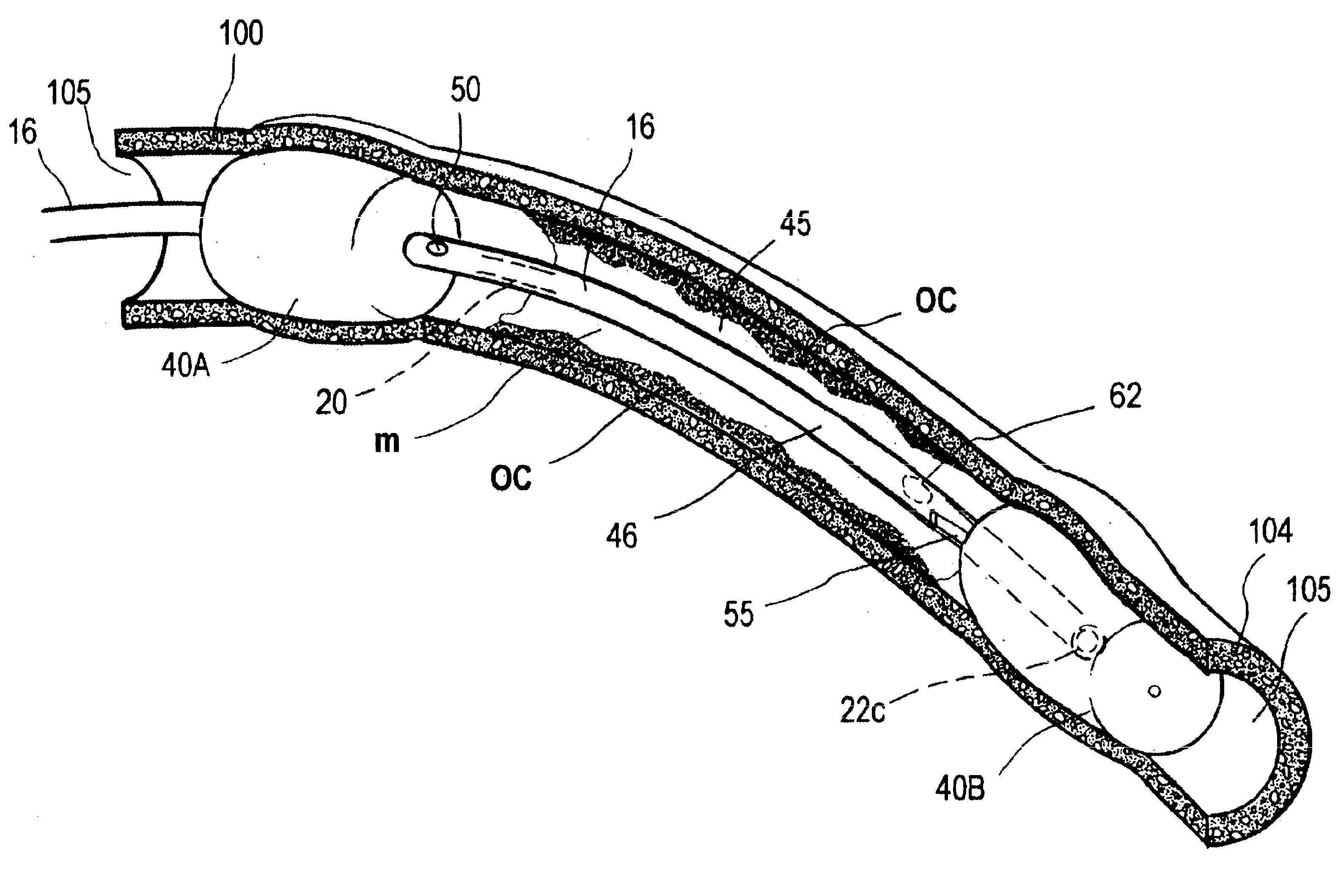
A catheter system for removing occlusive materials from a targeted endoluminal site, such as a vascular graft. The catheter system utilizes an electrical source and a controller to deliver sequences of very small electrical discharges between paired electrodes in a fluid-jet arrangement (i) to cause high fluid flow velocities in the catheter's fluid extraction pathway based on Bernoulli's Law of Pressure Differential, (ii) to create a selected level of turbulent fluid flows within the targeted site to remove occlusive material from the vessel walls and to thereafter suction fluids and entrained embolic particles into the extraction pathway, and (iii) to emulsify any embolic particles having a cross-sectional dimension larger than a couple of hundred micrometers to allows passage of the embolic particles through the elongate catheter to the catheter handle.
Owner:SHADDUCK JOHN H
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com