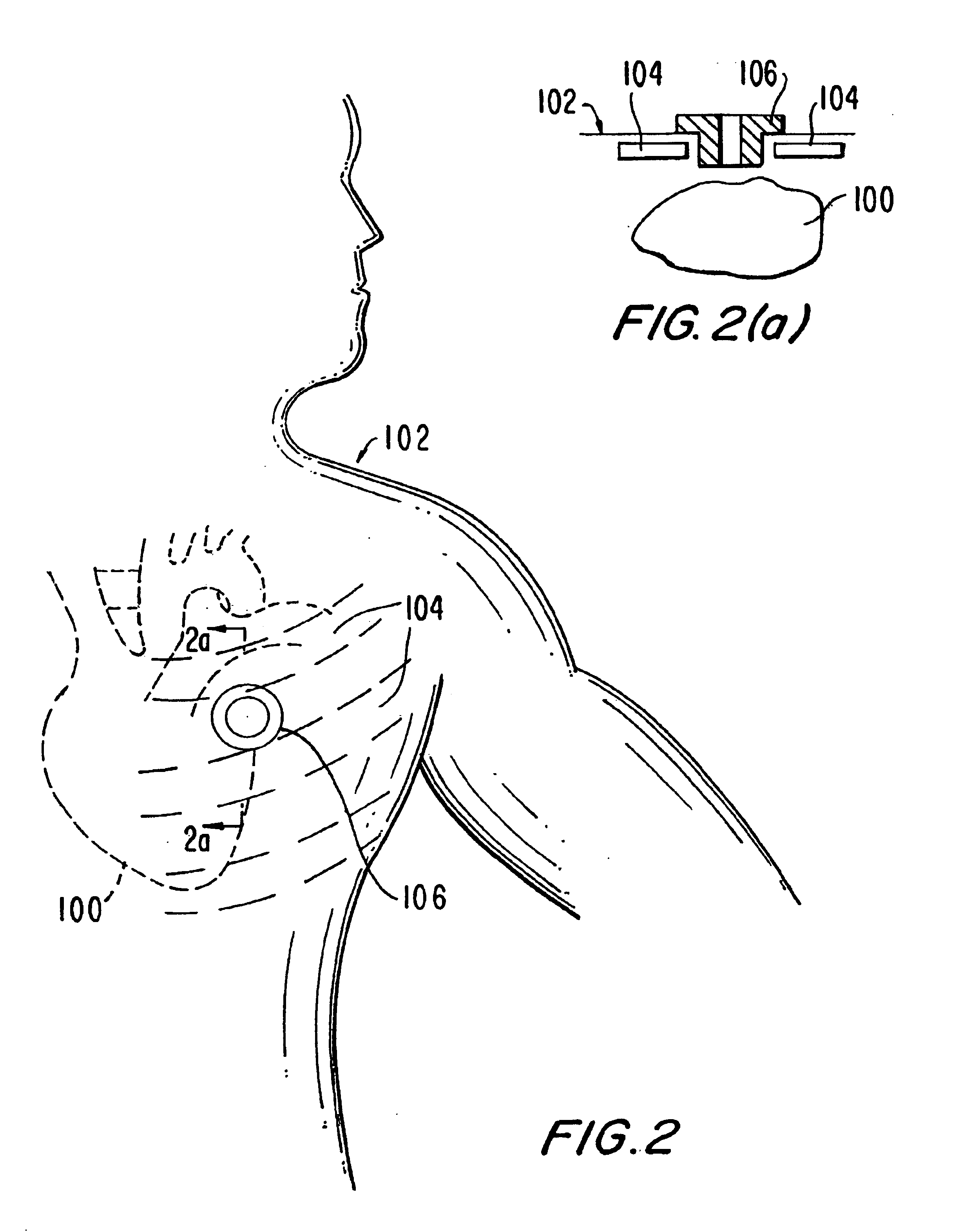
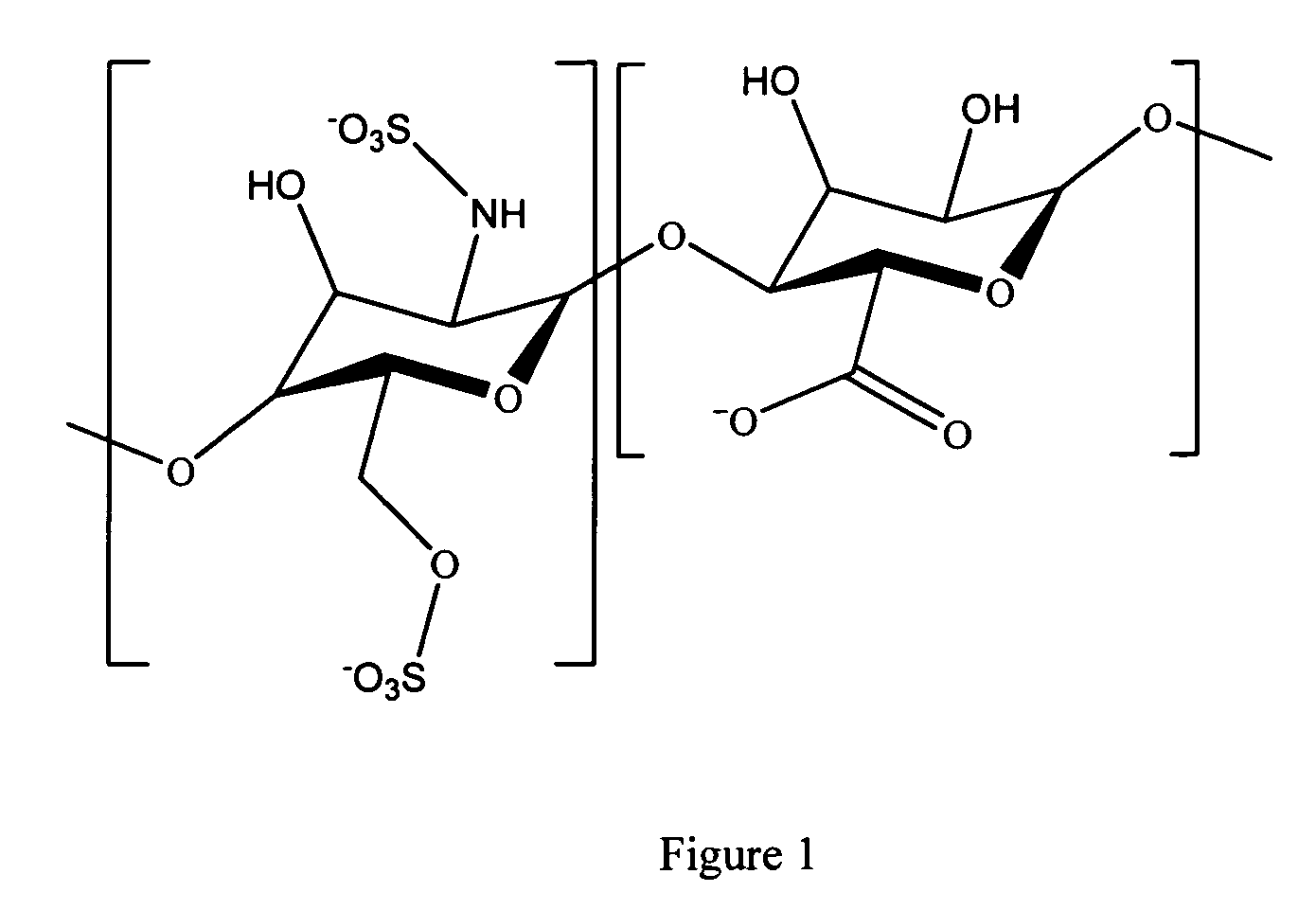
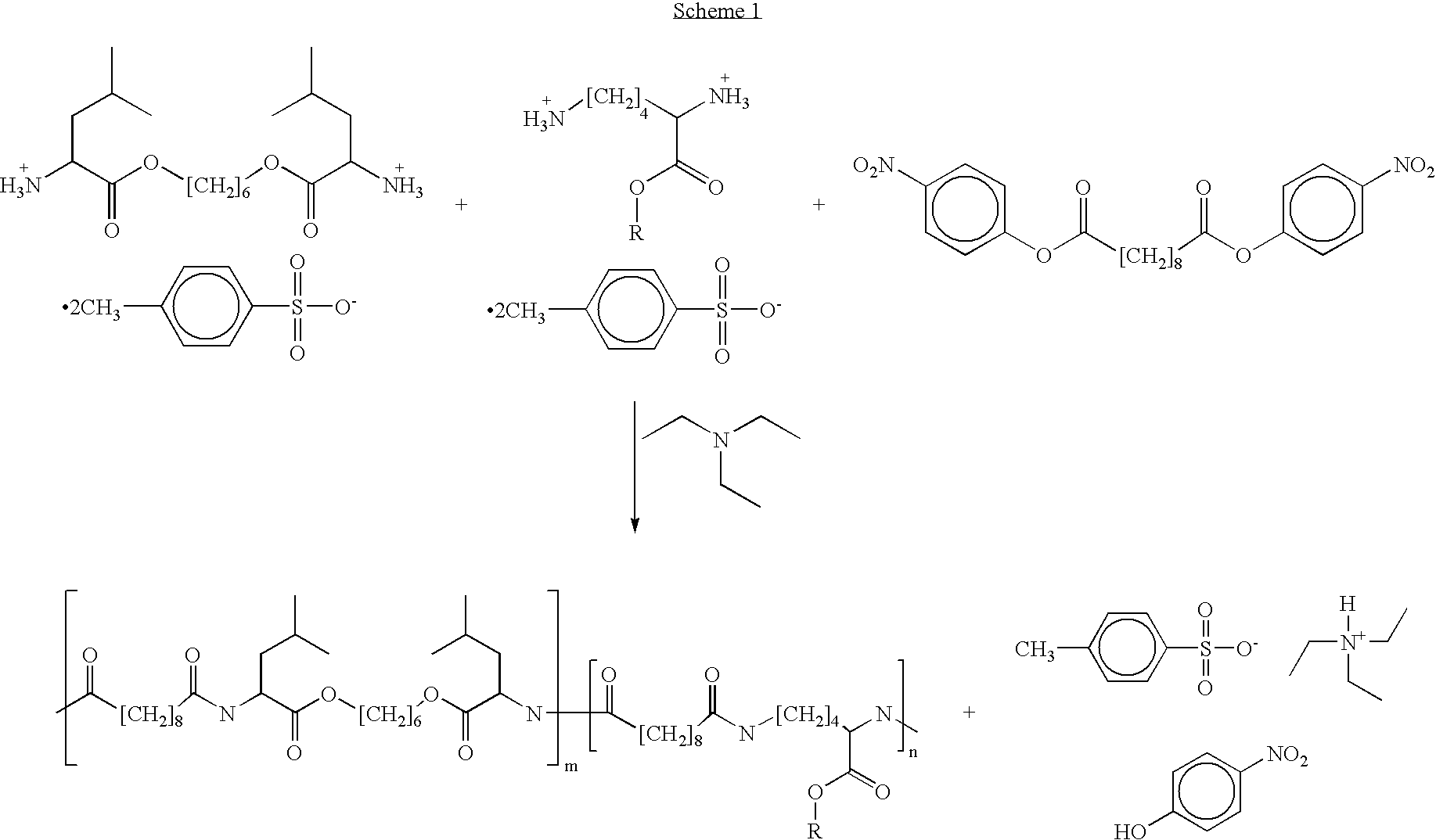
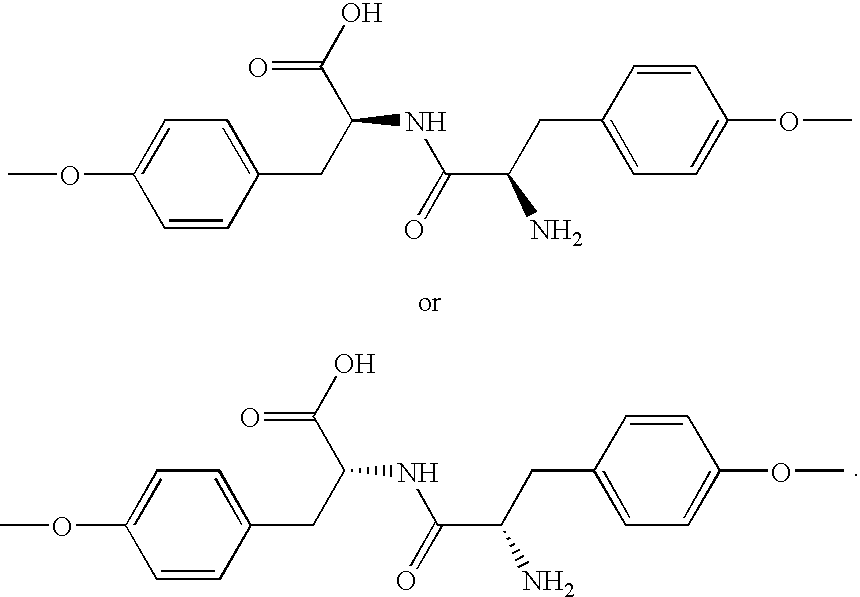
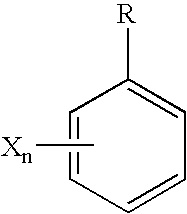
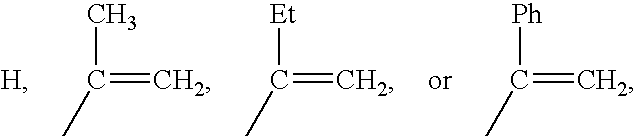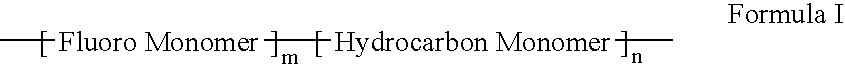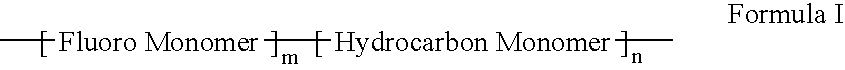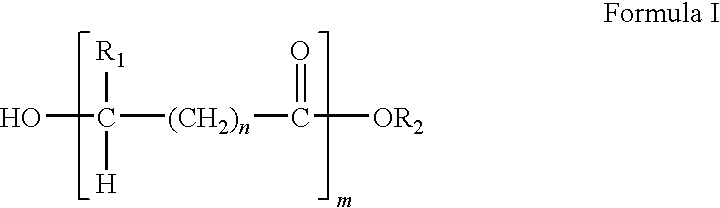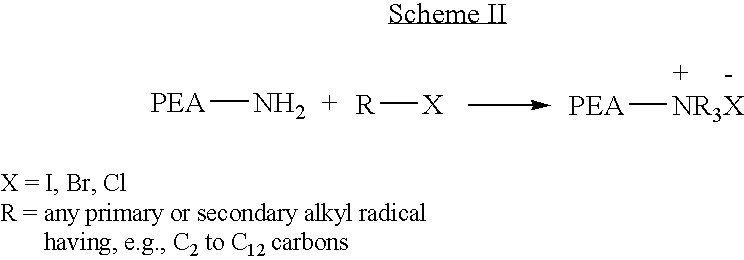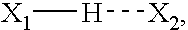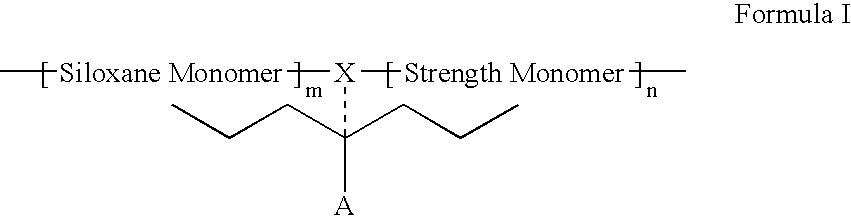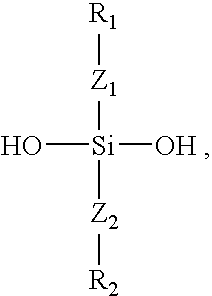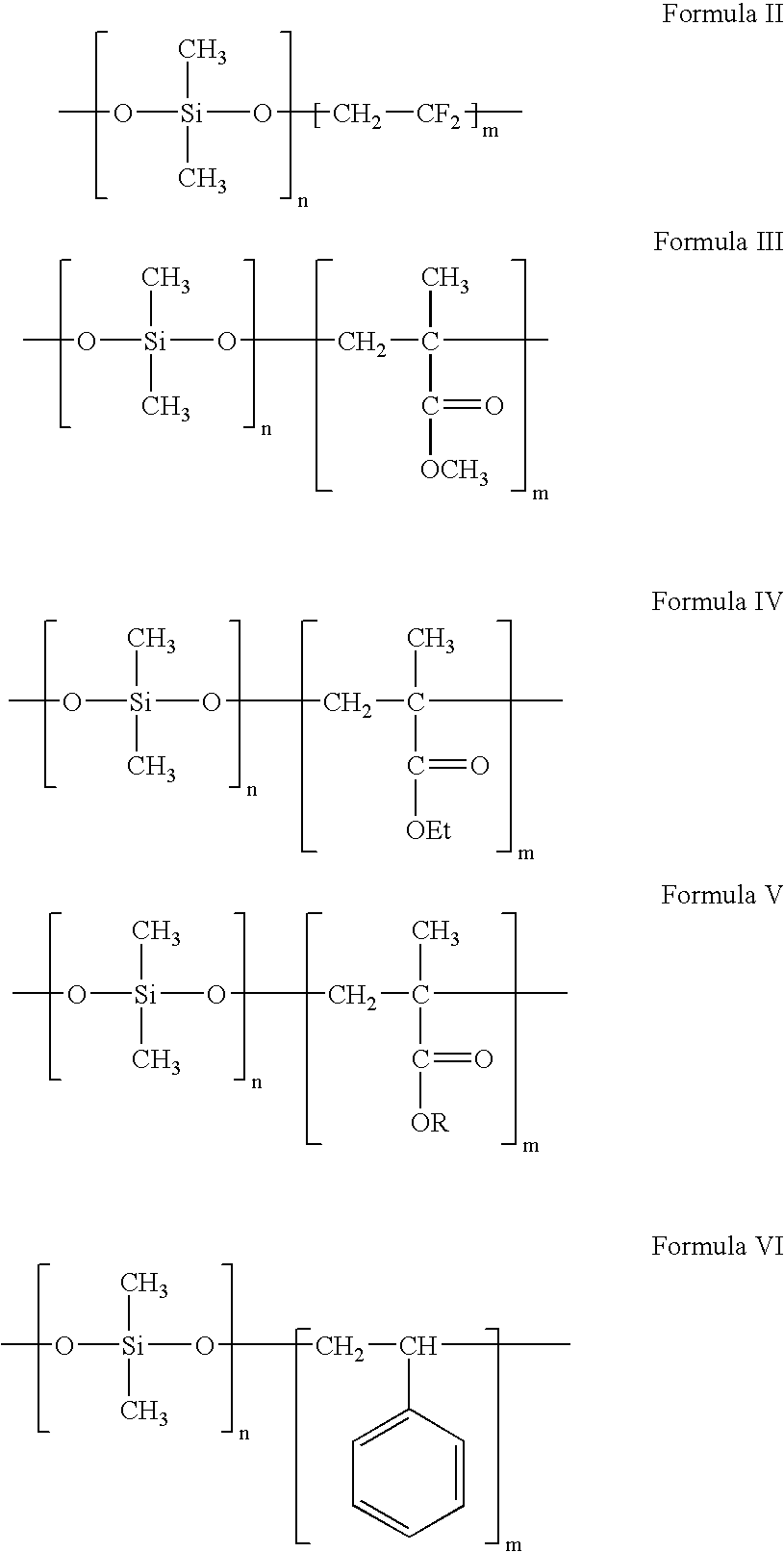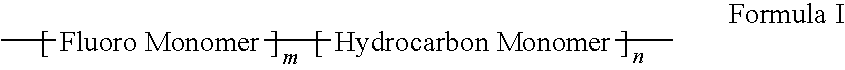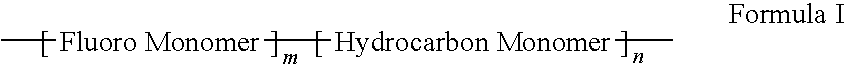Patents
Literature
46 results about "Artificial graft" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
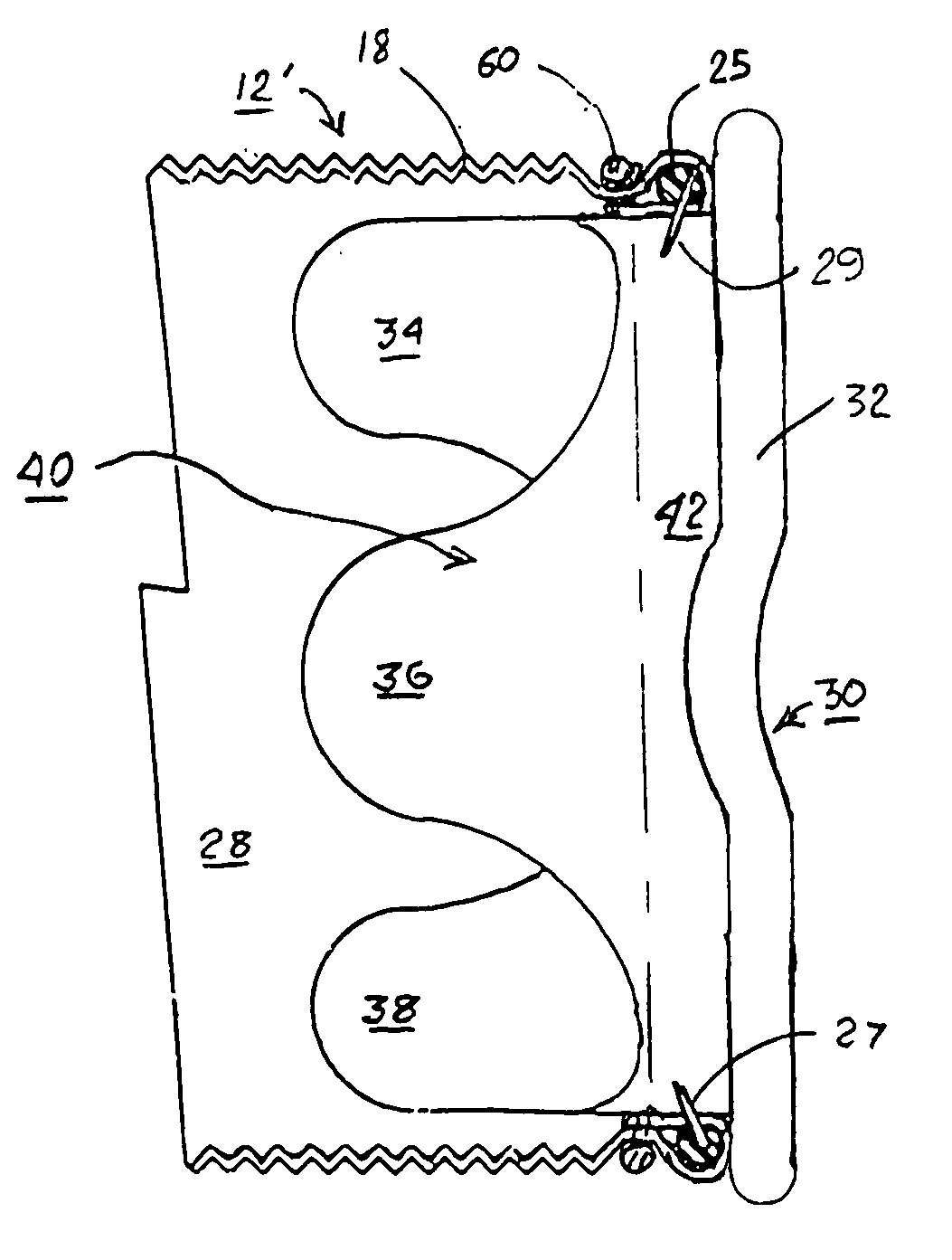
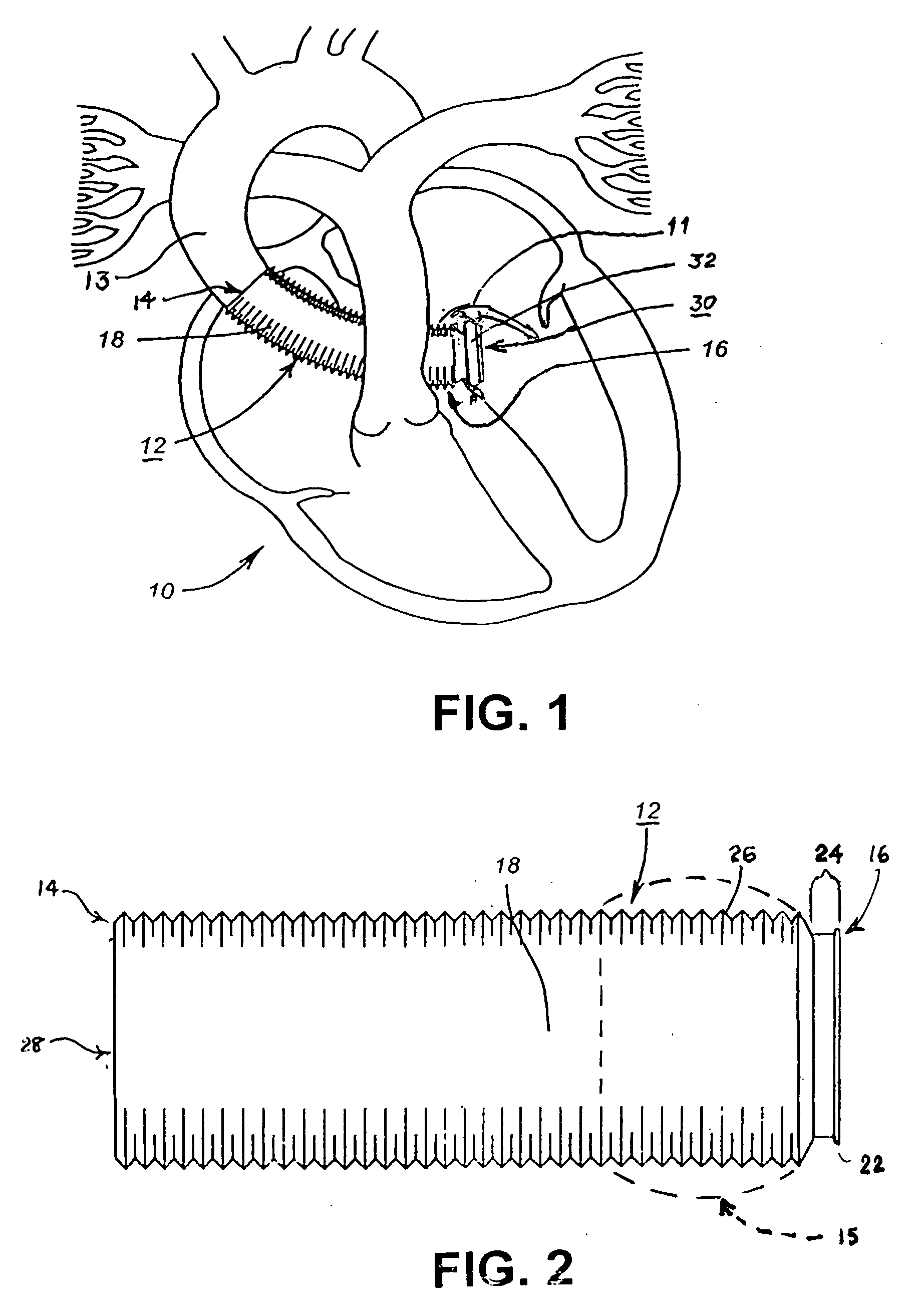
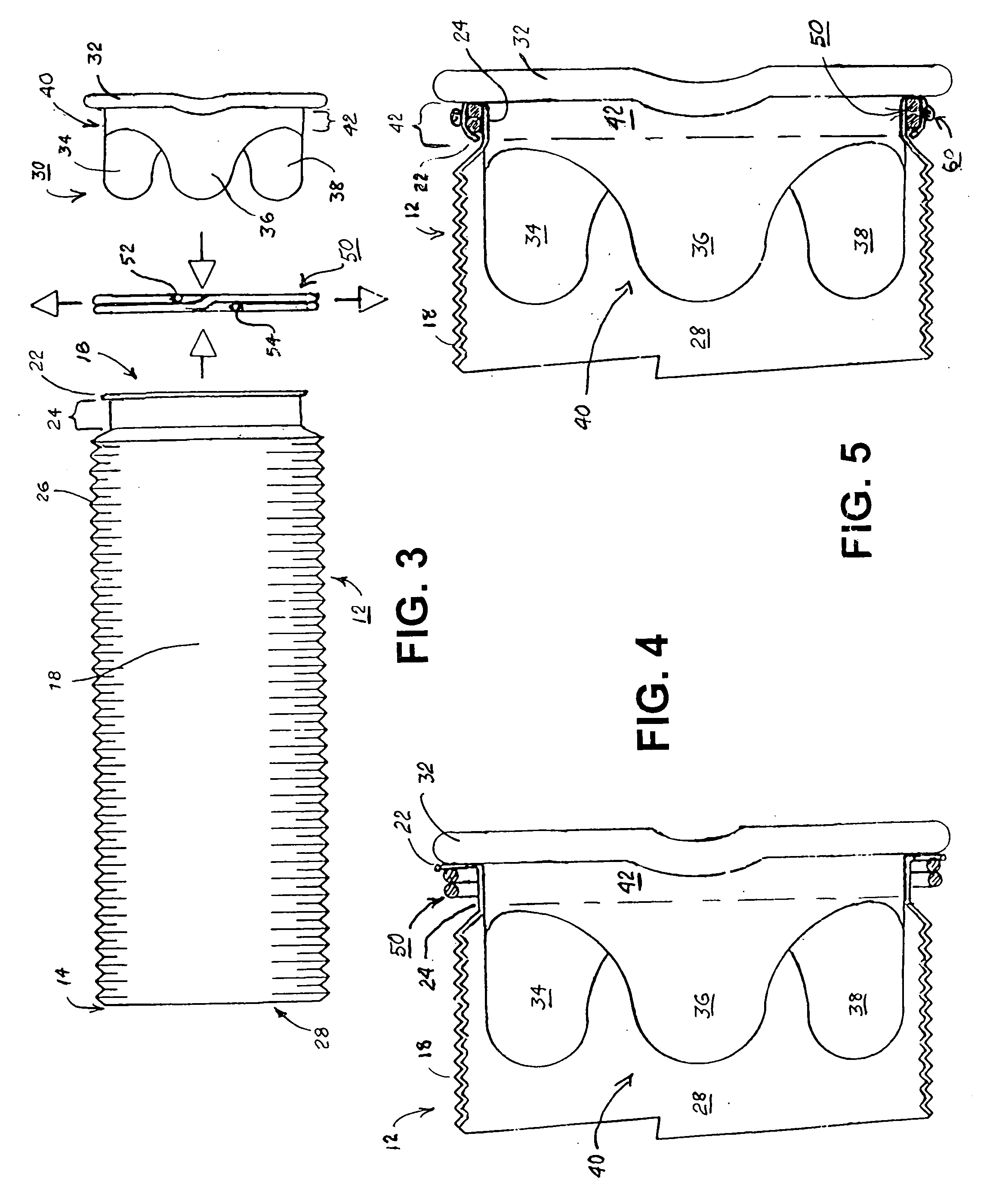
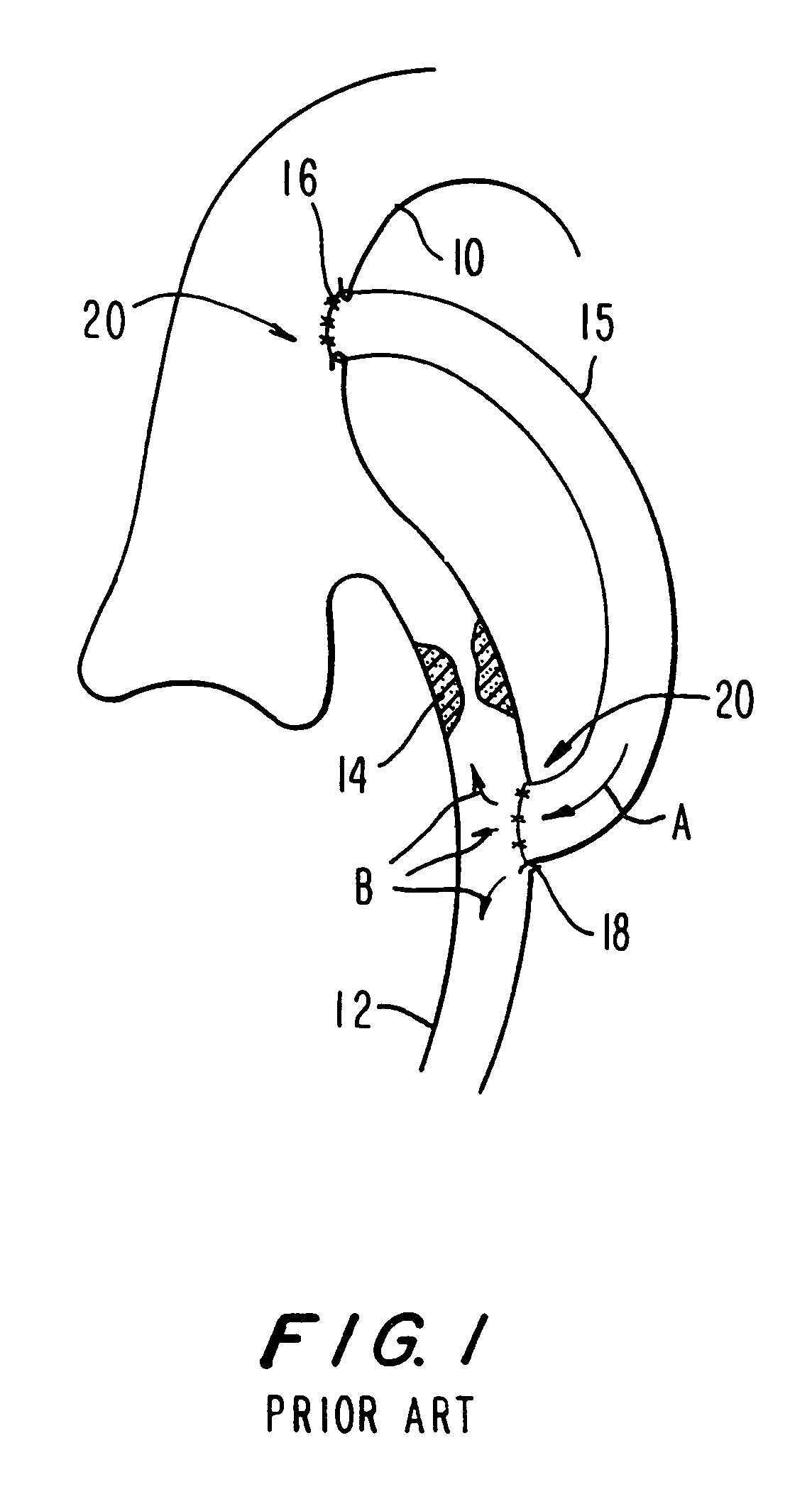
Methods and apparatus for coupling an allograft tissue valve and graft
InactiveUS20060085060A1Prevent leakageMinimal degradationHeart valvesBlood vesselsAscending aortaProsthesis
Improvements to prosthetic heart valves and grafts for human implantation, particularly to methods and apparatus for coupling a prosthetic heart valve with an artificial graft during a surgical procedure to replace a defective heart valve and blood vessel section, e.g., the aortic valve and a section of the ascending aorta, are disclosed. An annular exterior surface of the prosthetic heart valve is fitted within a vascular graft lumen to dispose the vascular graft proximal end overlying the annular exterior surface, and the proximal end of an elongated vascular graft is compressed against the valve annular exterior surface in a manner that inhibits blood leakage between the vascular graft and the prosthetic heart valve.
Owner:MEDTRONIC INC
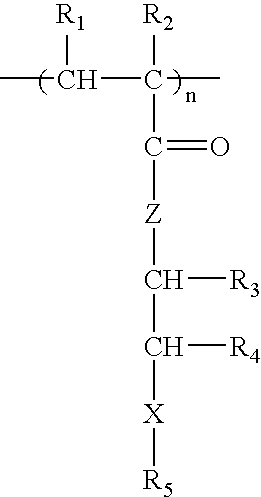
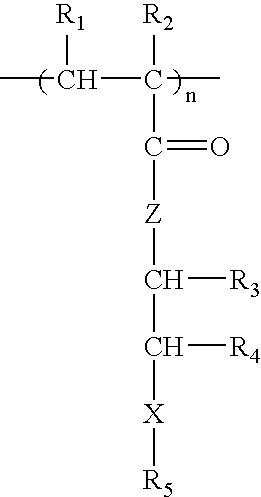
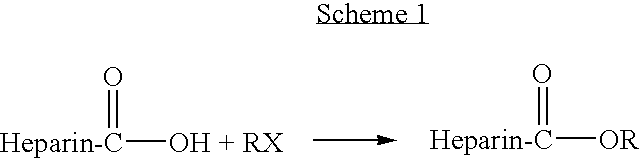
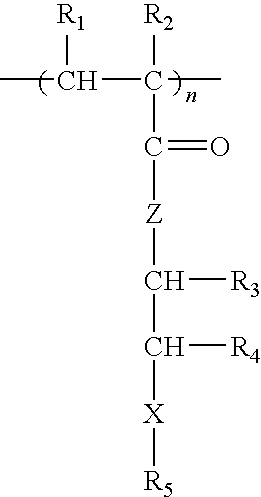
Methacrylate copolymers for medical devices
A polymer of hydrophobic monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains the polymer and another biocompatible polymer. The polymer or polymer blend and optionally a biobeneficial material and / or a bioactive agent can form a coating on an implantable device such as a drug delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
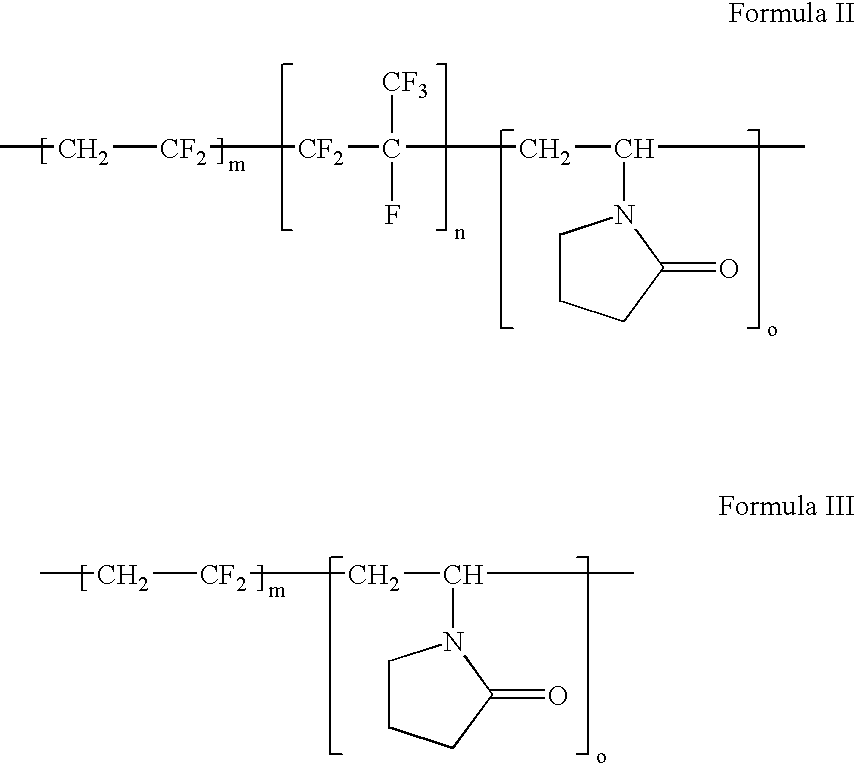
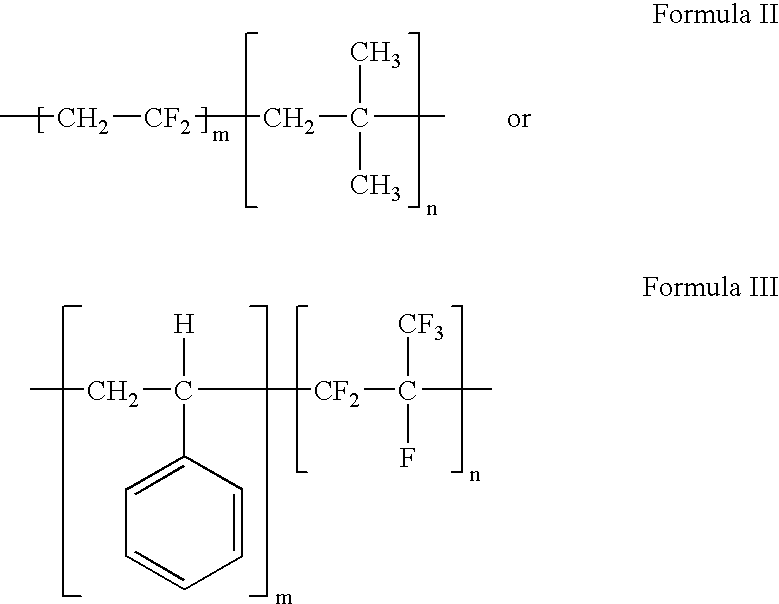
Polymers of fluorinated monomers and hydrophilic monomers
ActiveUS20060047095A1Improve propertiesProvide flexibilityFibre treatmentSurgeryDiseasePolymer science
A polymer of fluorinated monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains a polymer of fluorinated monomers and another biocompatible polymer. The polymer of fluorinated monomers or polymer blend described herein and optionally a bioactive agent can form a coating on an implantable device such as a drug-delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
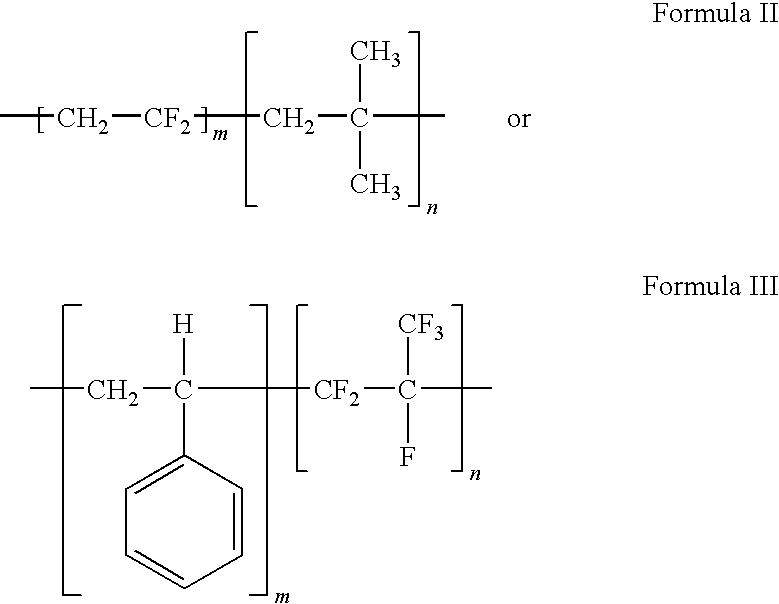
Polymers of fluorinated monomers and hydrocarbon monomers
InactiveUS20060134165A1Provide mechanical strengthGive flexibilityStentsSurgeryAbnormal tissue growthPolymer science
A polymer of fluorinated monomers and hydrocarbon monomers is provided. It is also provided a polymer blend that contains a polymer formed of fluorinated monomers and hydrocarbon monomers and another biocompatible polymer. The polymer or polymer blend described herein and optionally a bioactive agent can form an implantable device such as a stent or a coating on an implantable device such as a drug-delivery stent, which can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
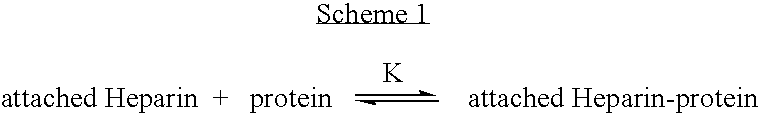
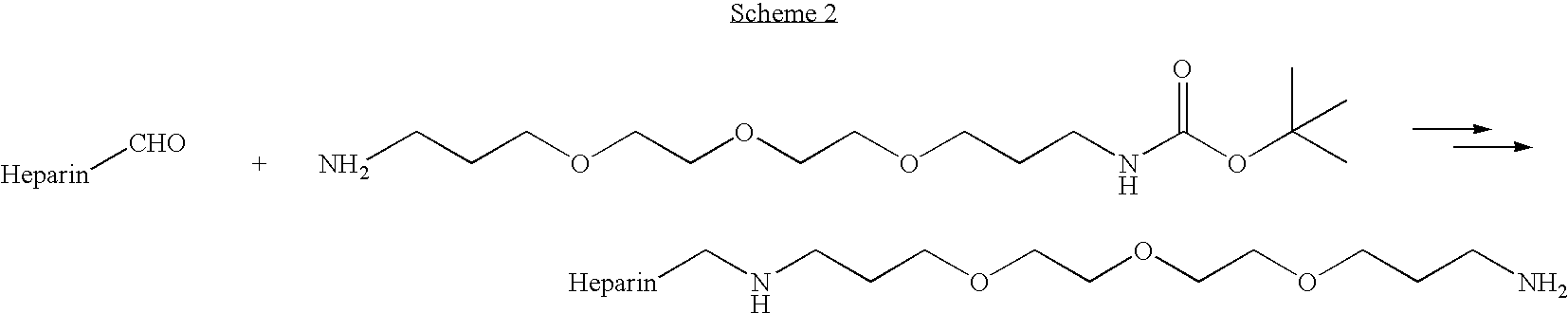
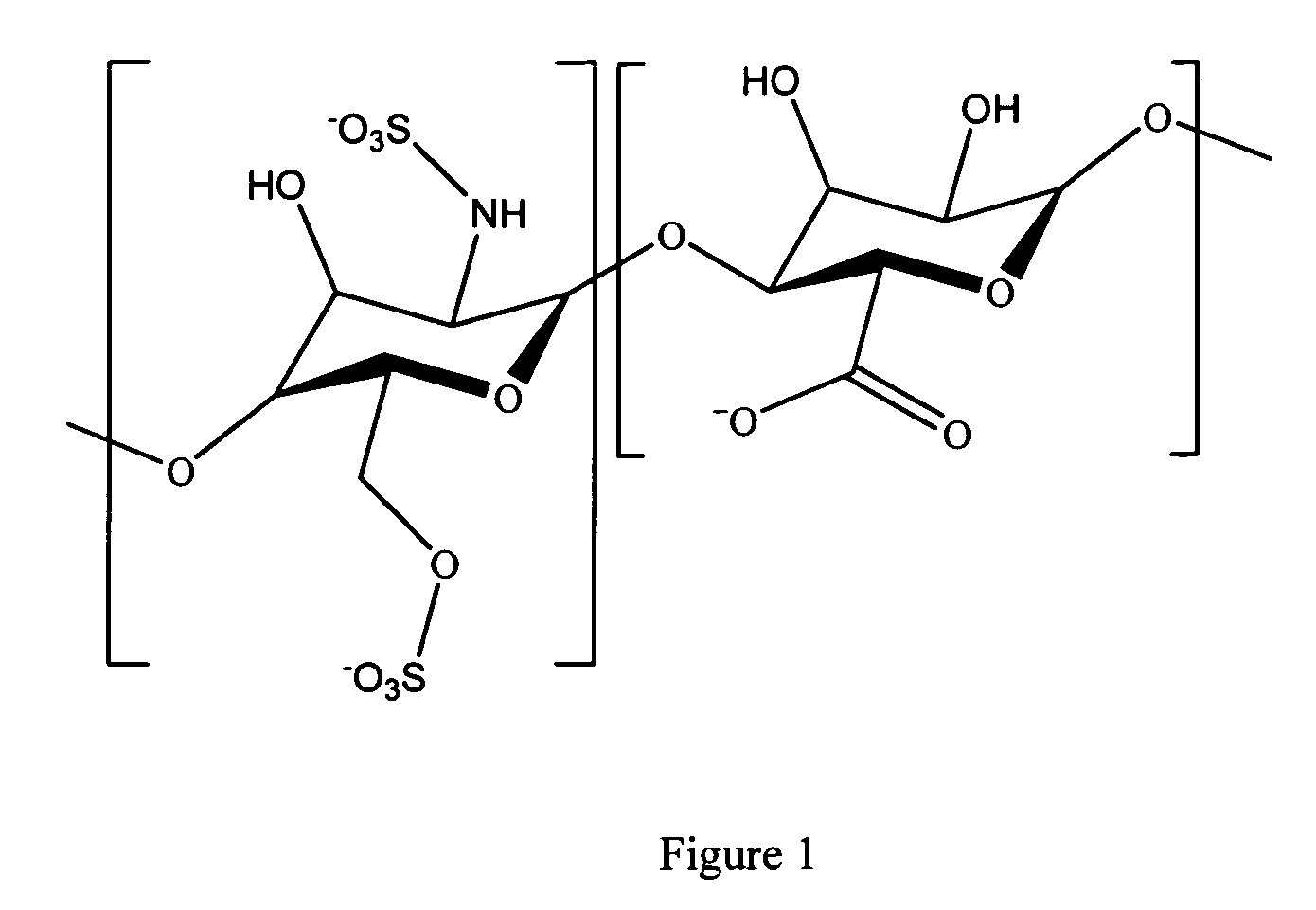
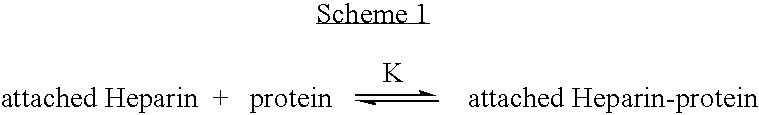
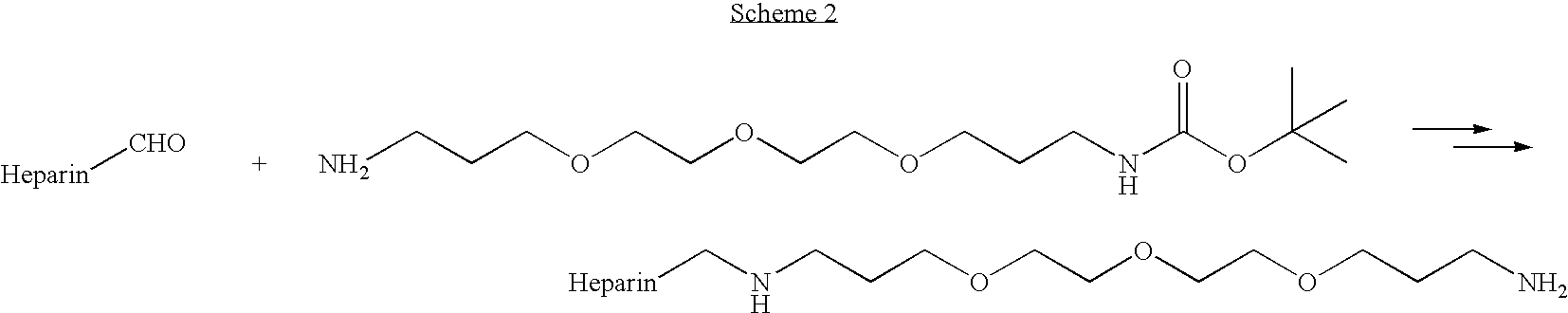
Antifouling heparin coatings
A medical device comprising a coating thereon comprising a biocompatible polymer and heparin is provided herein. Heparin is coupled with the biocompatible polymer via a spacer having a grouping that renders a binding site of the heparin molecule accessible by a binding protein. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Plasticizers for coating compositions
A biocompatible plasticizer useful for forming a coating composition with a biocompatible polymer is provided. The coating composition may also include a biobeneficial polymer and / or a bioactive agent. The coating composition can form a coating on an implantable device. The implantable device can be used to treat or prevent a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
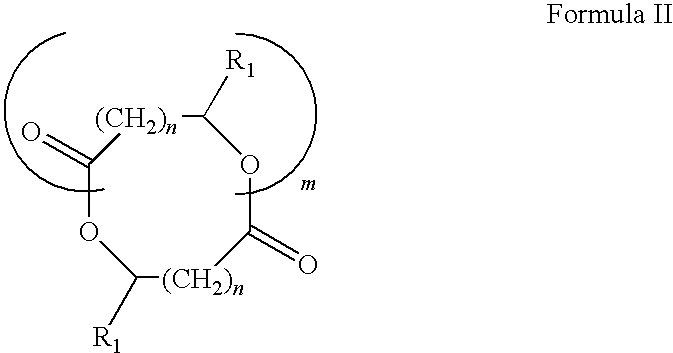
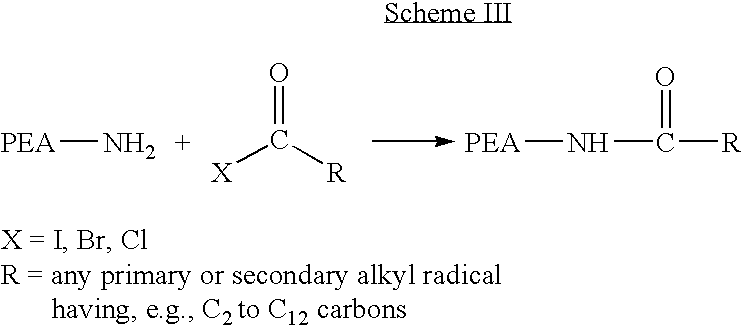
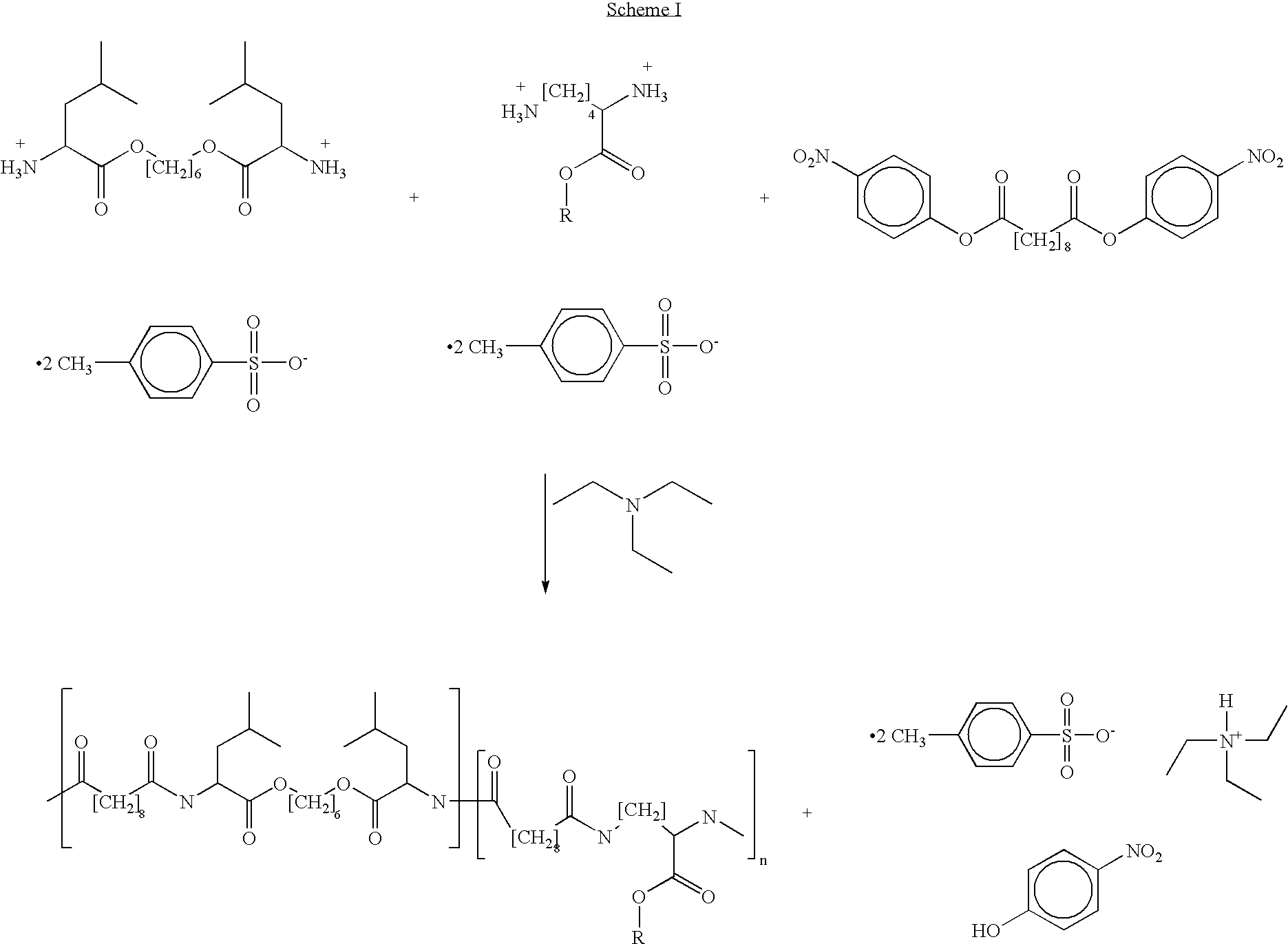
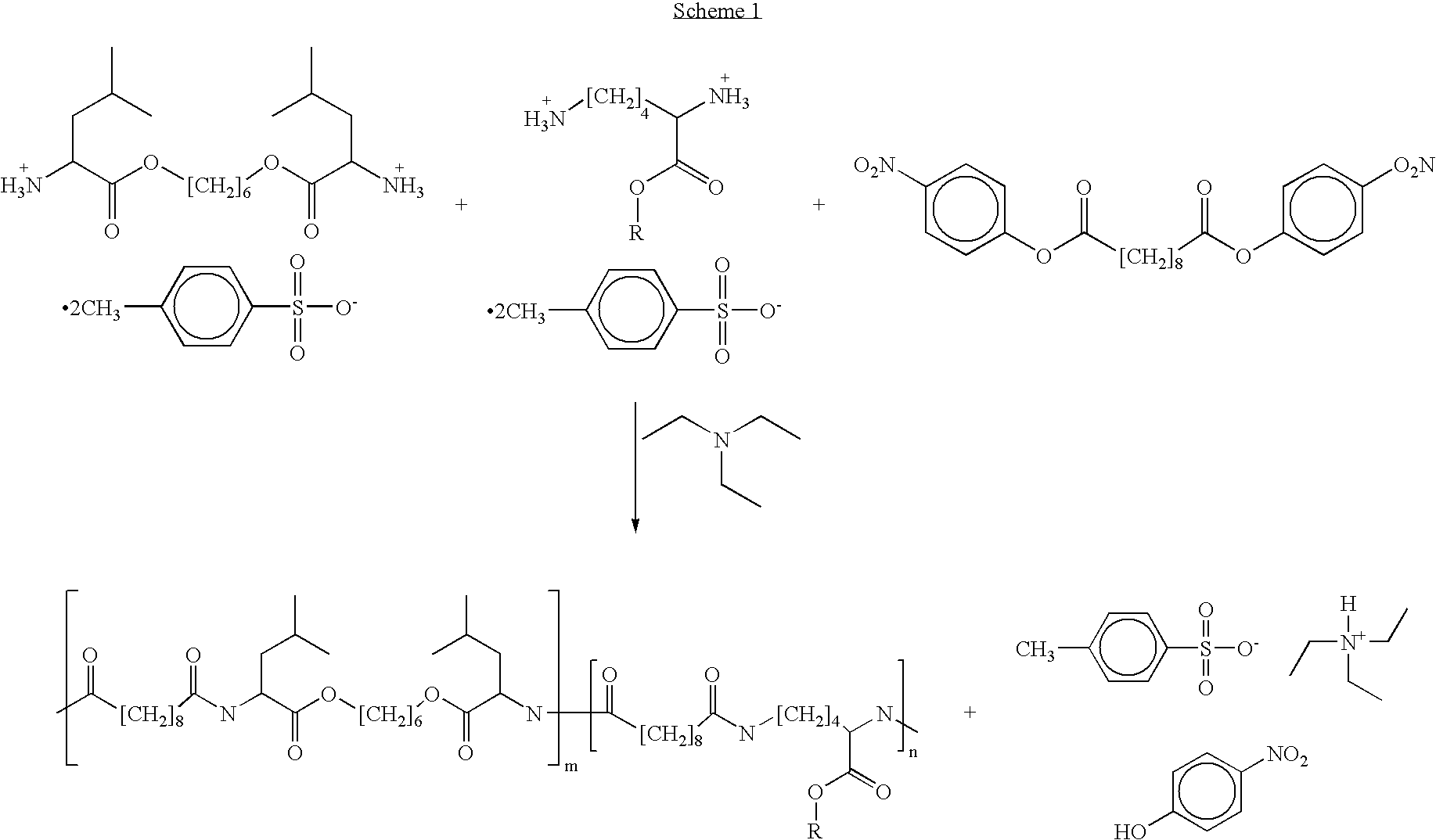
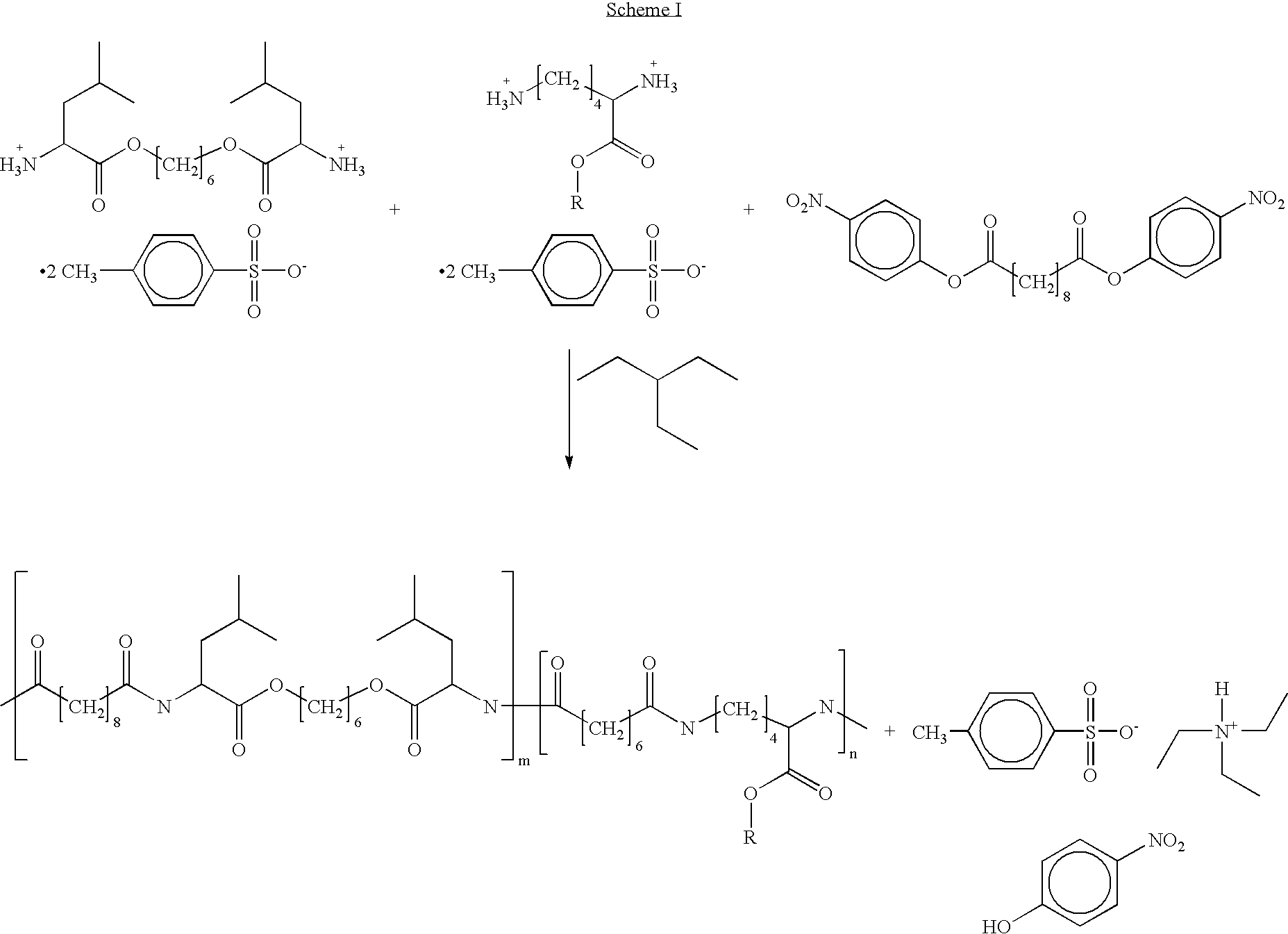
Blends of poly(ester amide) polymers
ActiveUS7166680B2Improved stability and drug release rate and mechanical characteristicReduce degradationSurgeryCatheterDiseasePEA polymer
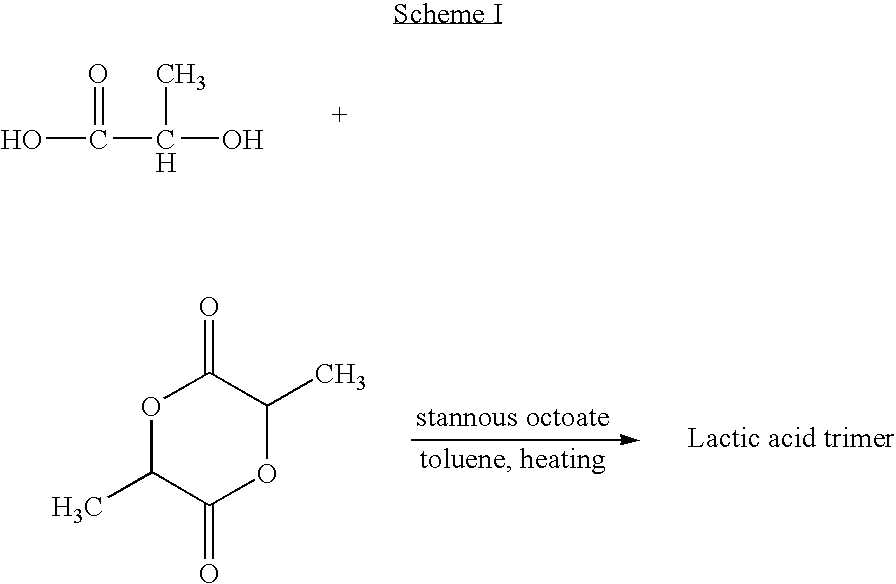
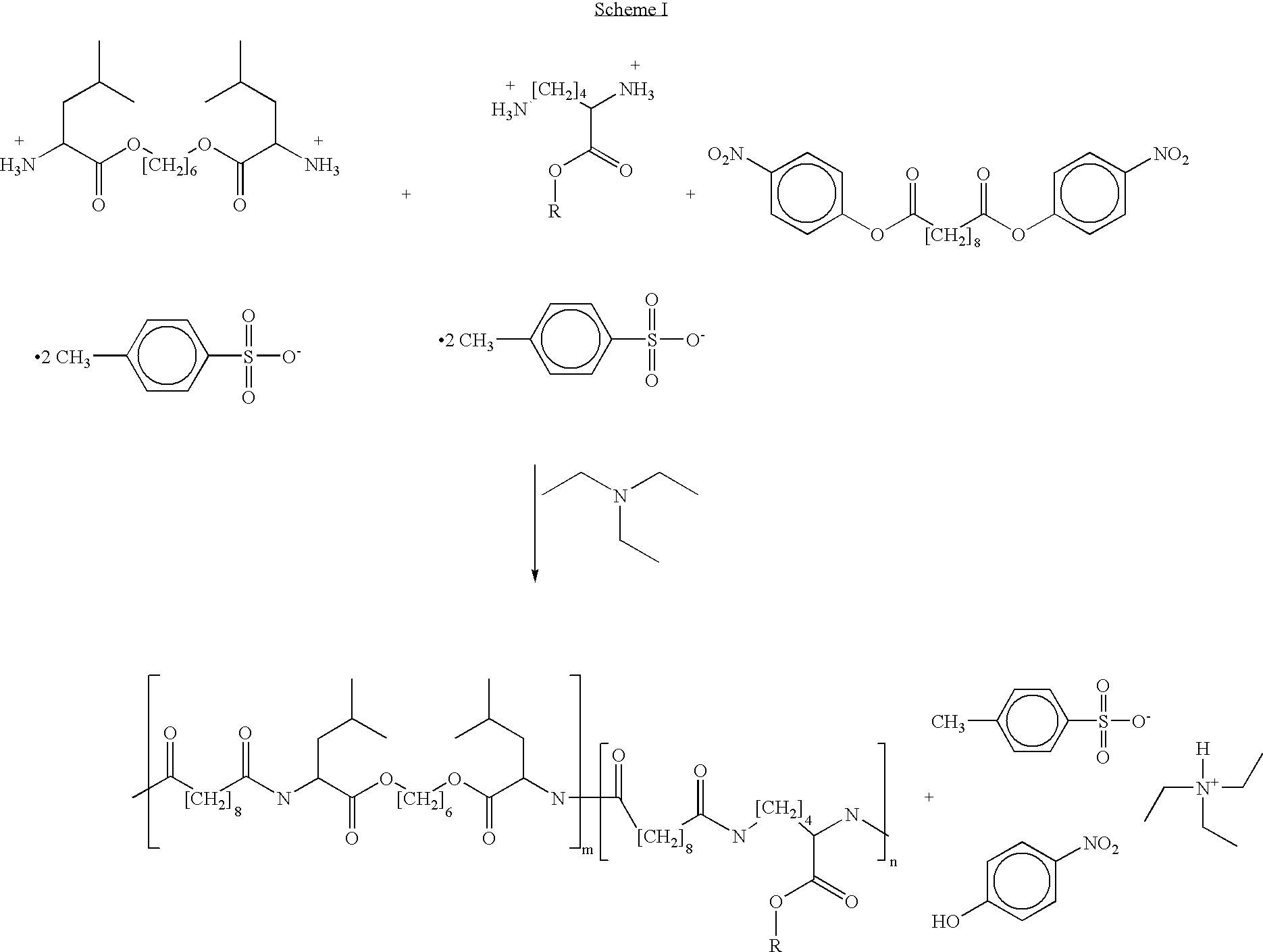
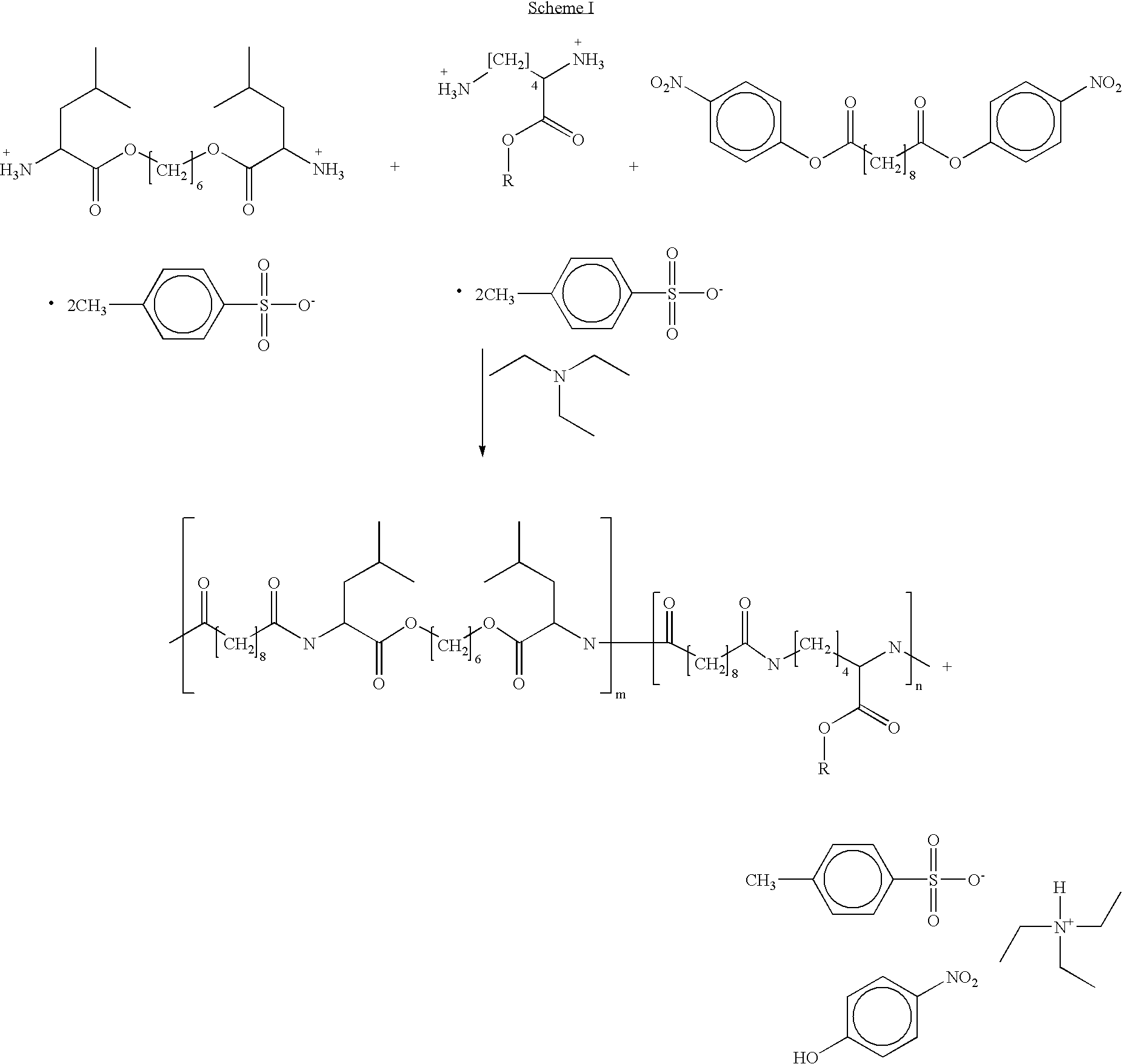
Provided herein is a poly(ester amide) (PEA) polymer blend and a polymeric coating containing the PEA polymer blend. The PEA polymer blend has a Tg above the Tg of poly(ester amide benzyl ester) (PEA-Bz) or the Tg of poly(ester amide TEMPO). The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
End-capped poly(ester amide) copolymers
Provided herein is an end-capped poly(ester amide) PEA) polymer and the method of making the polymer. The PEA polymer is substantially free of active amino end groups and / or activated carboxyl groups. The PEA polymer can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Blends of poly(ester amide) polymers
Provided herein is a poly(ester amide) (PEA) polymer blend and a polymeric coating containing the PEA polymer blend. The PEA polymer blend has a Tg above the Tg of poly(ester amide benzyl ester) (PEA-Bz) or the Tg of poly(ester amide TEMPO). The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
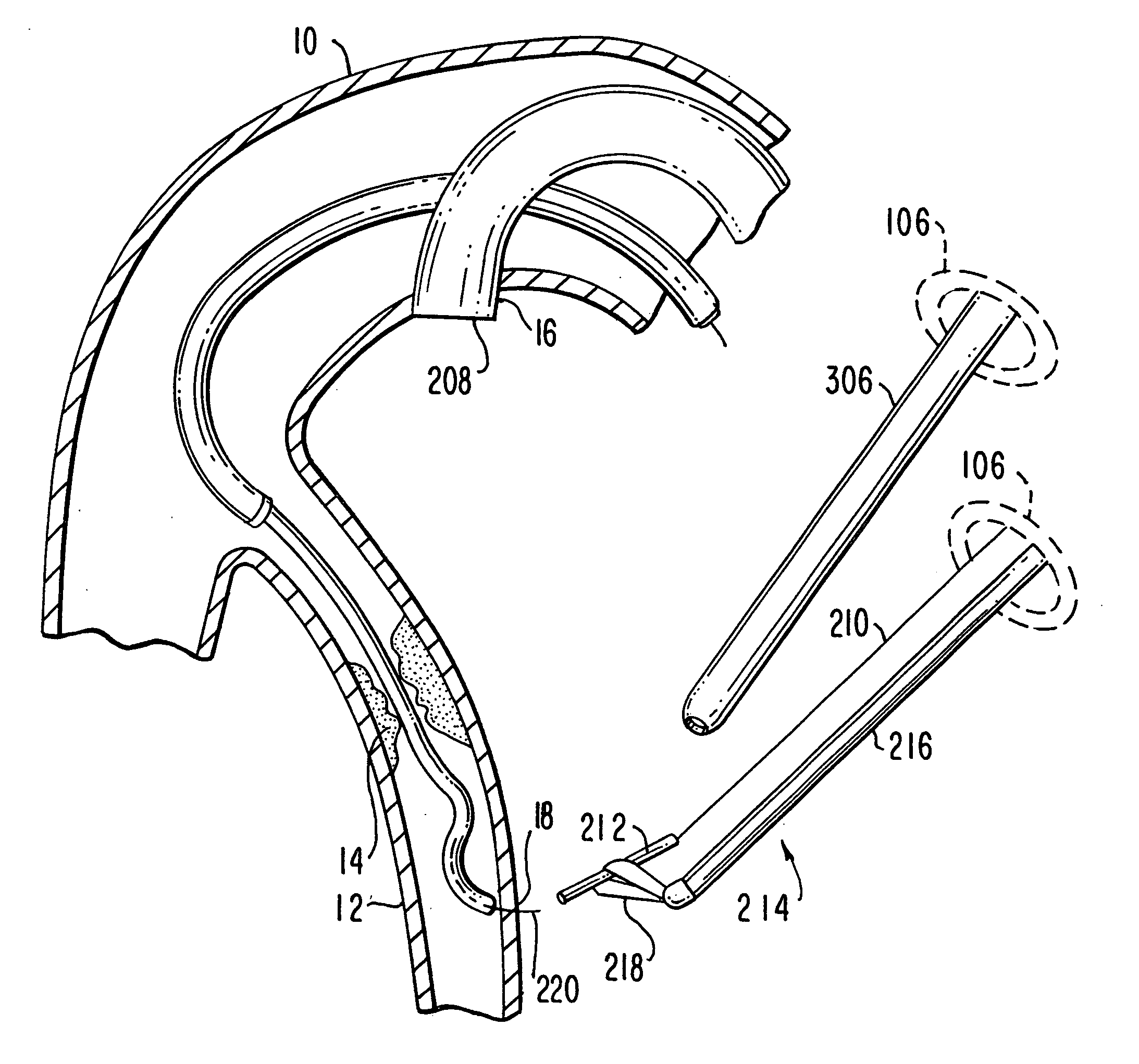
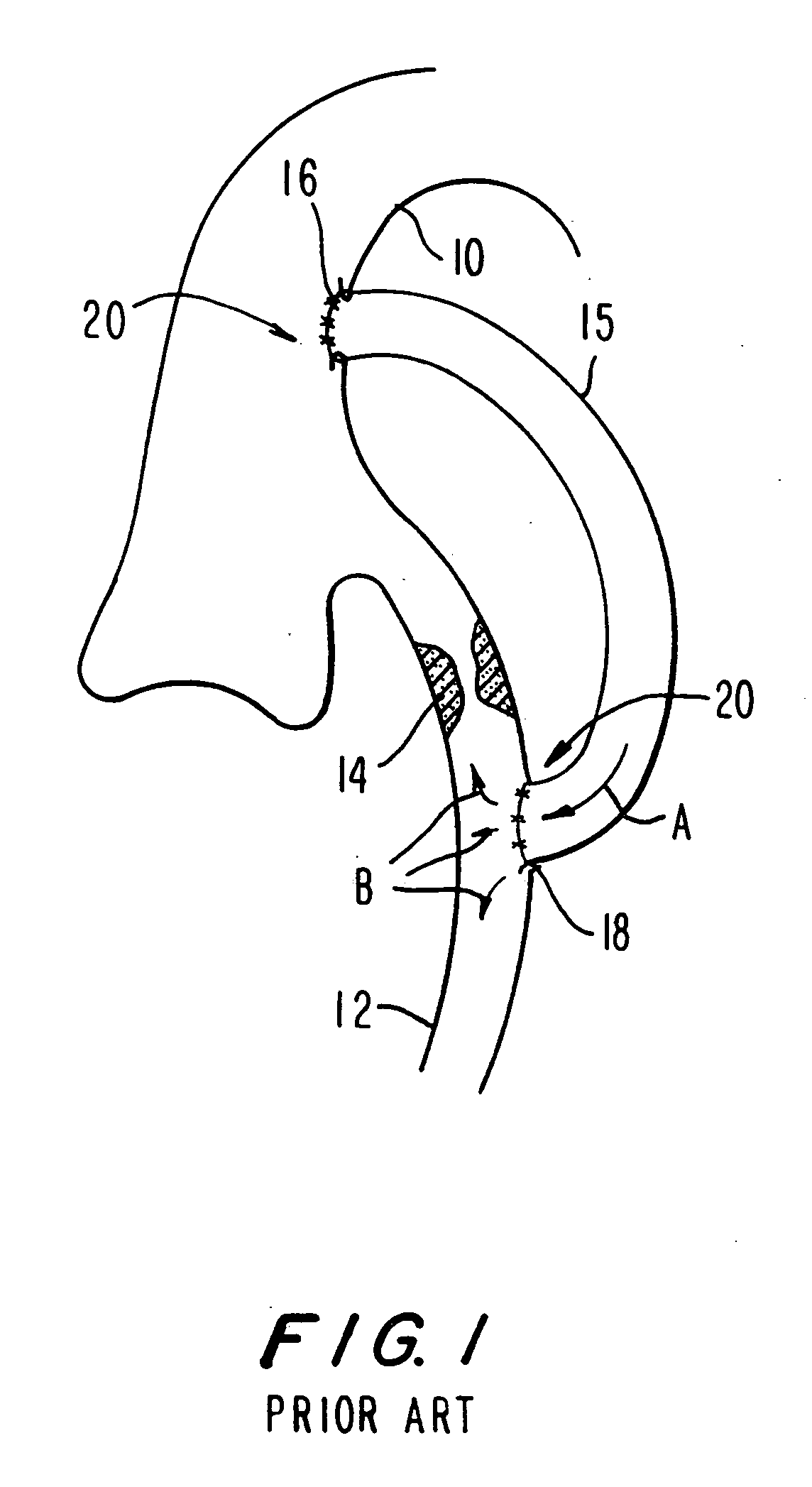
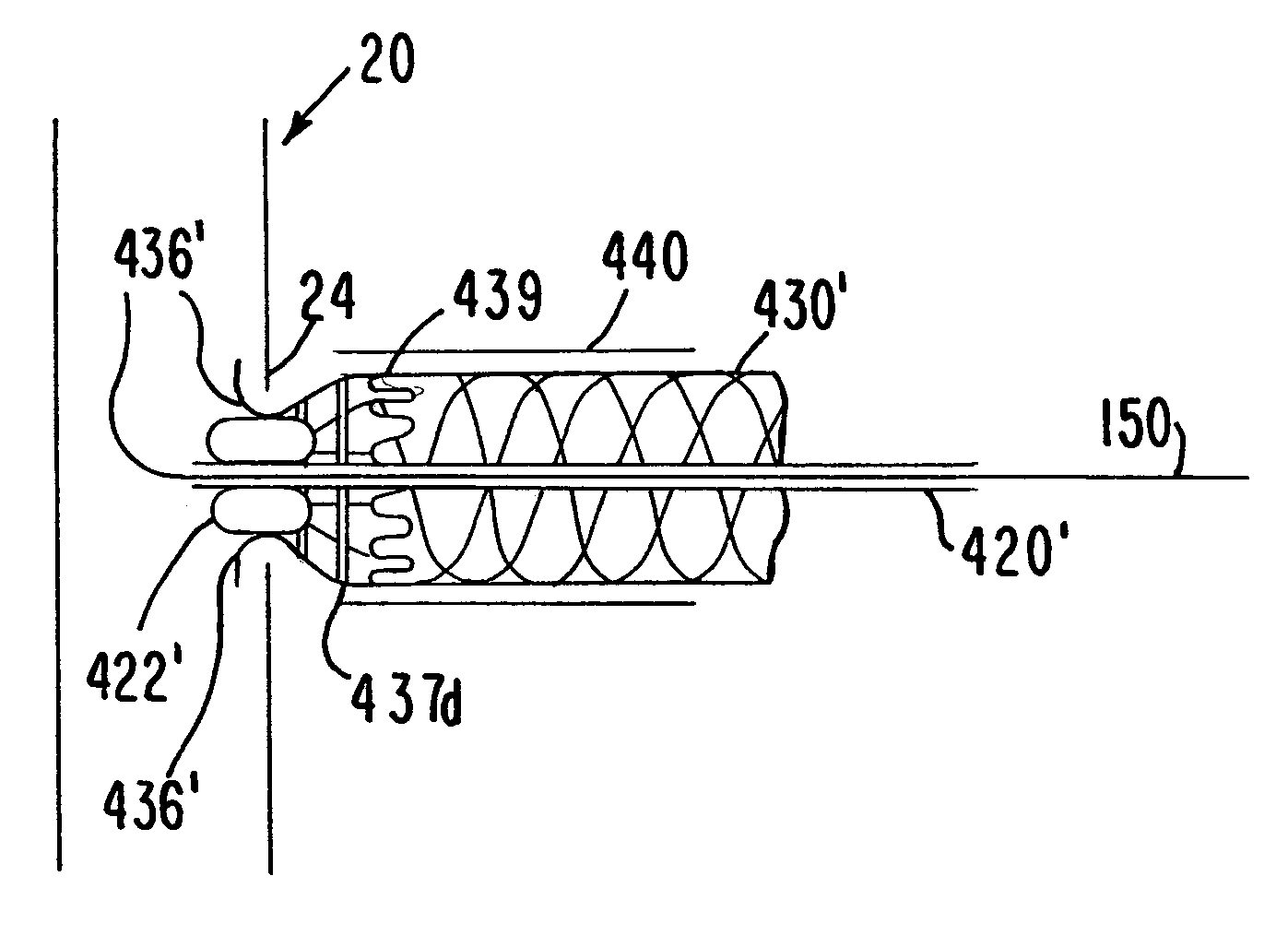
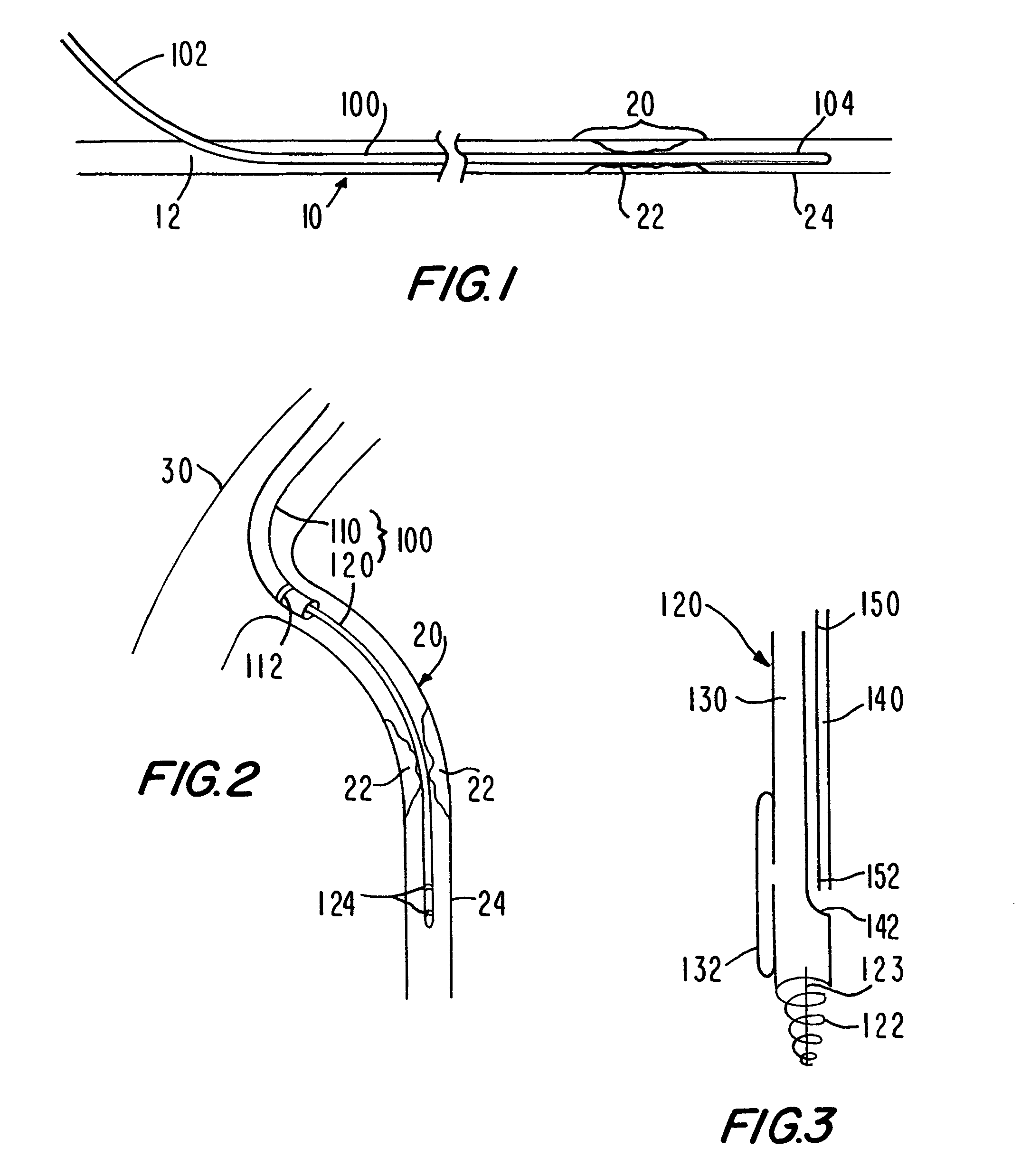
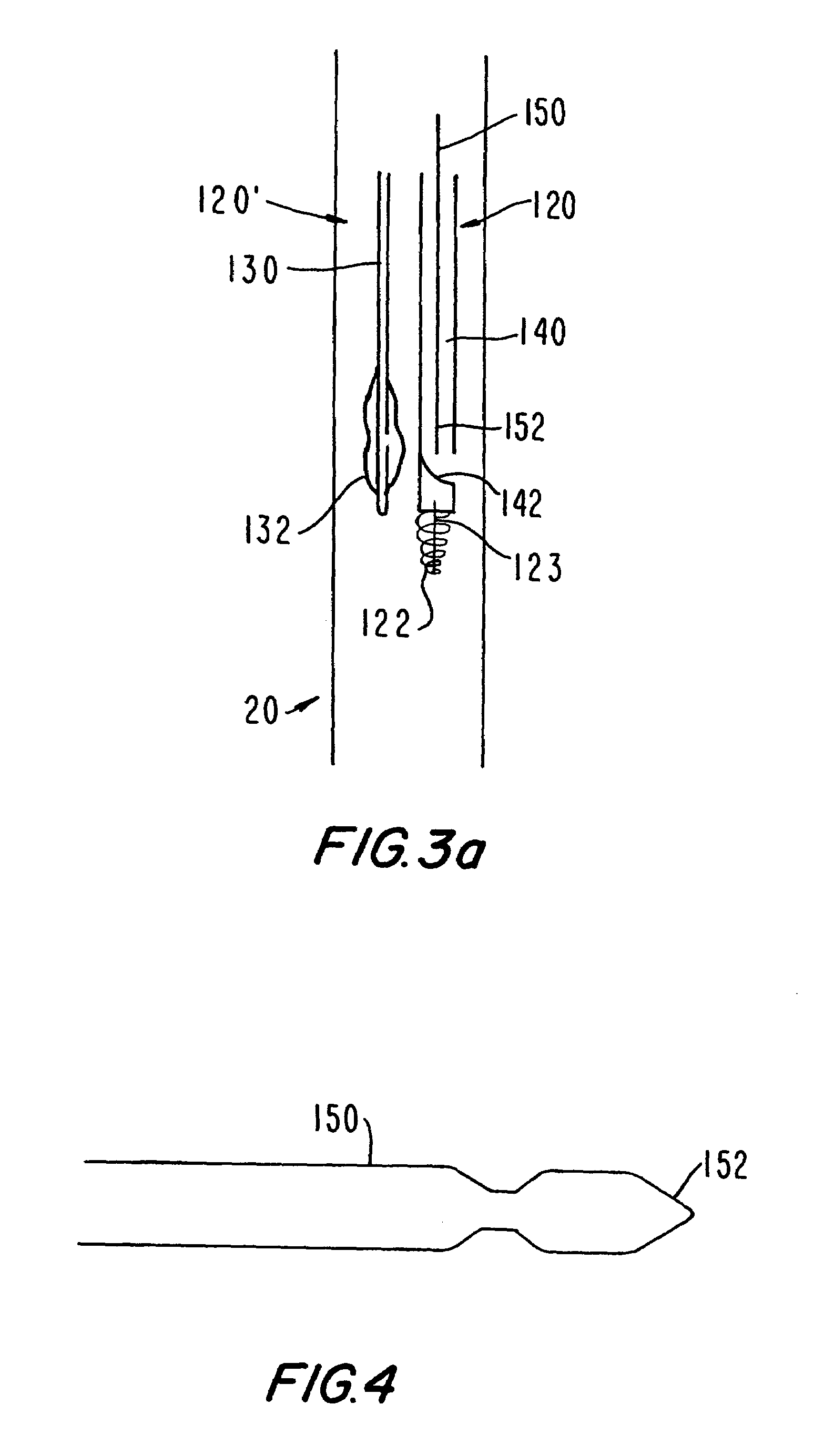
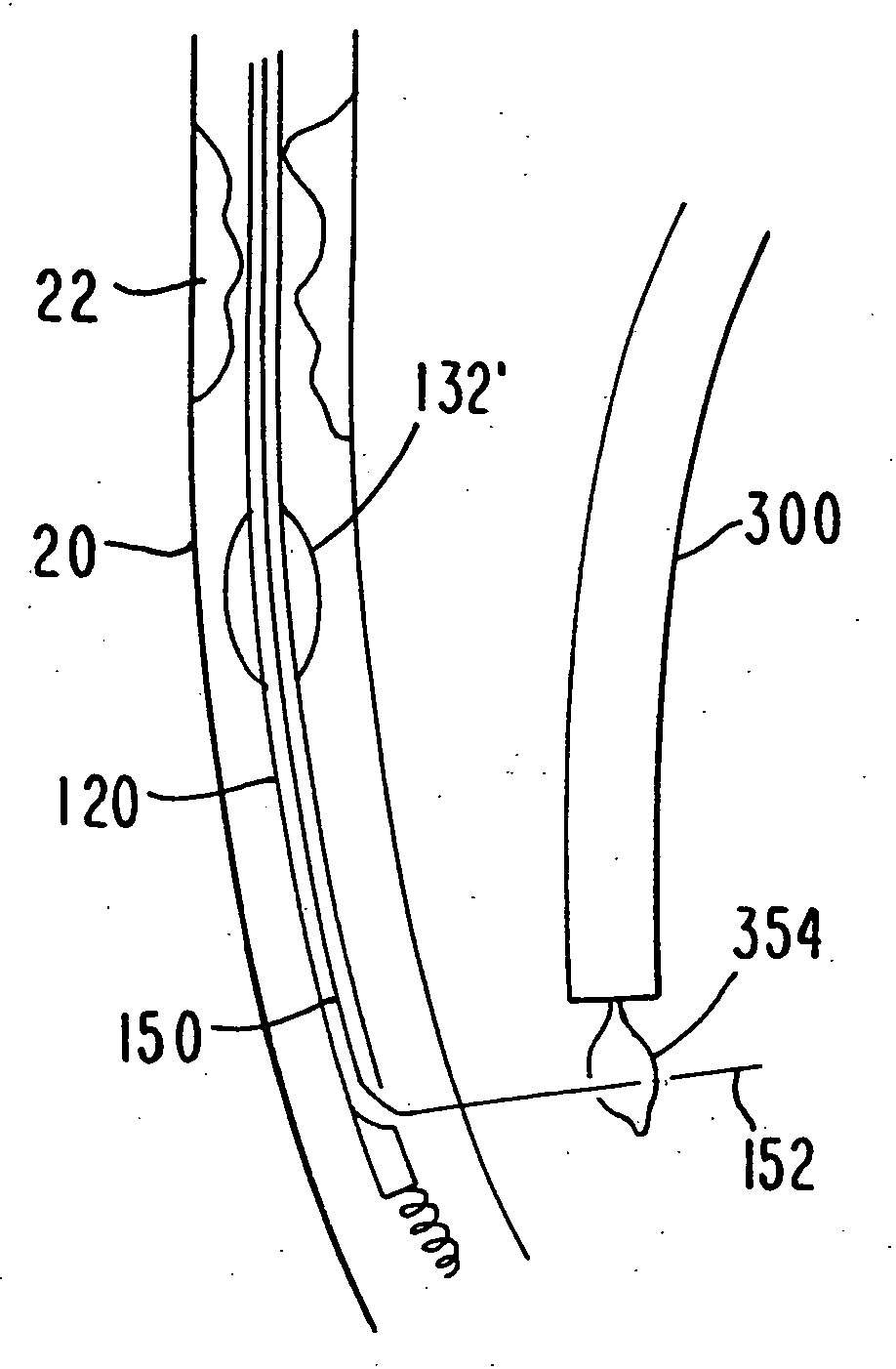
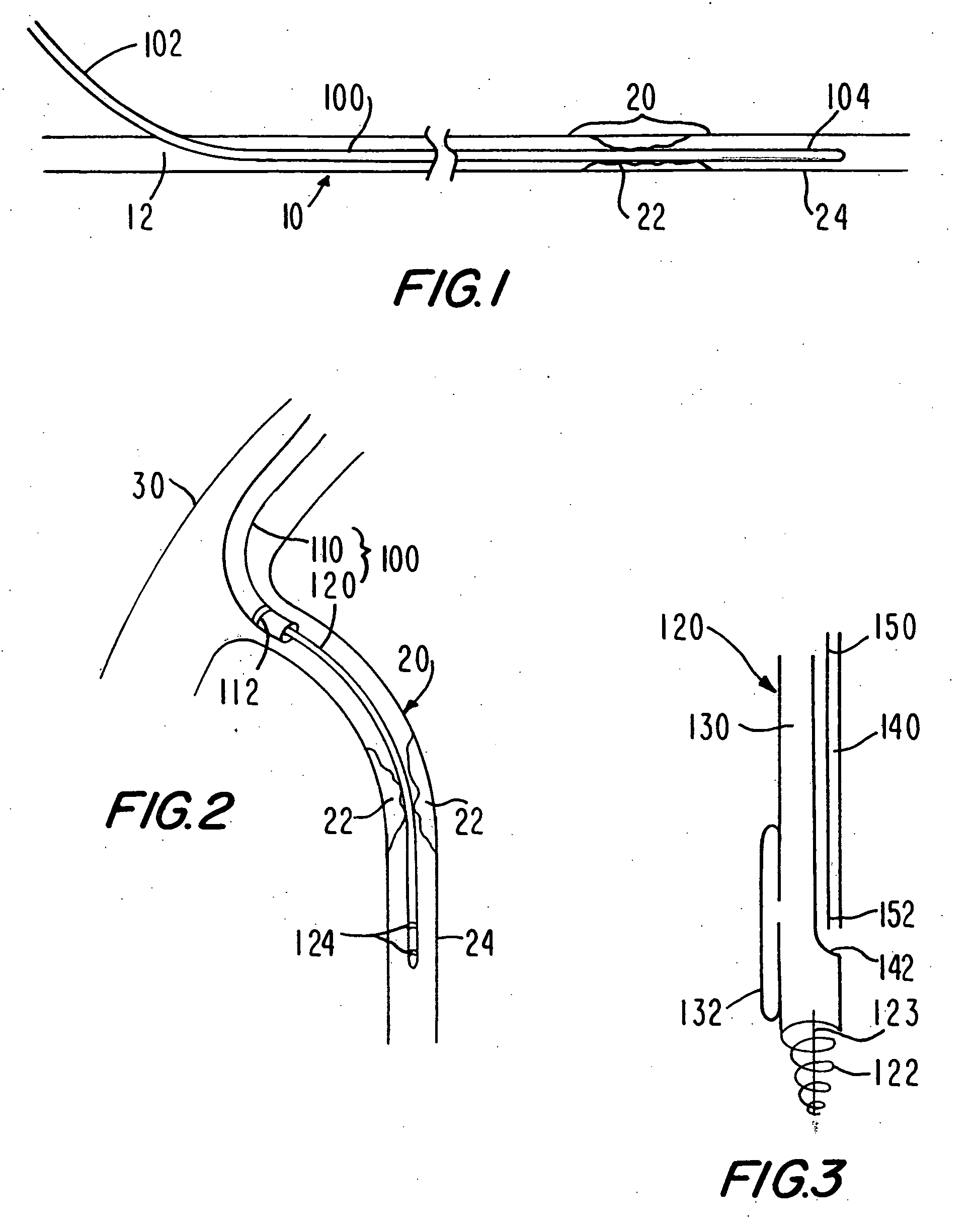
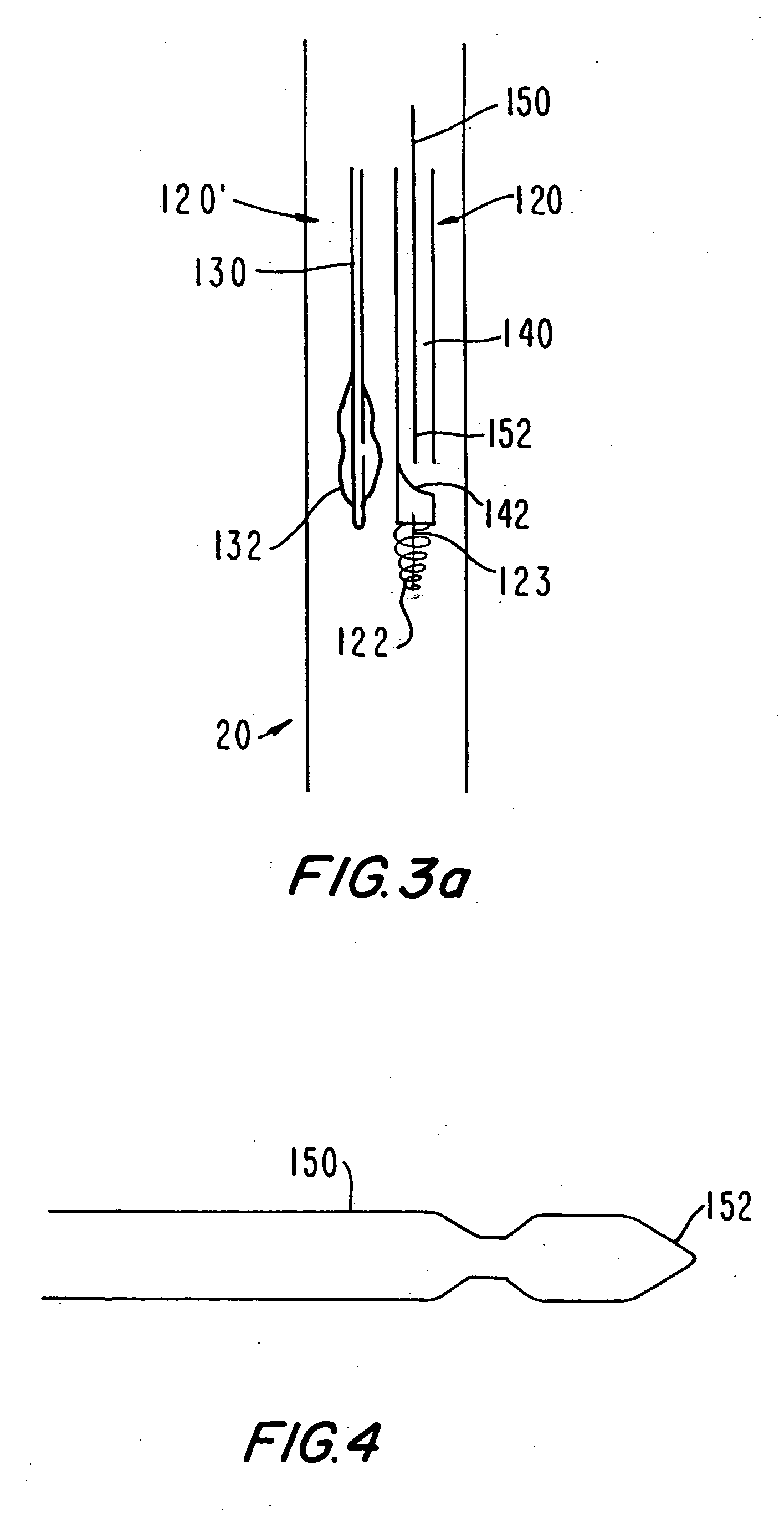
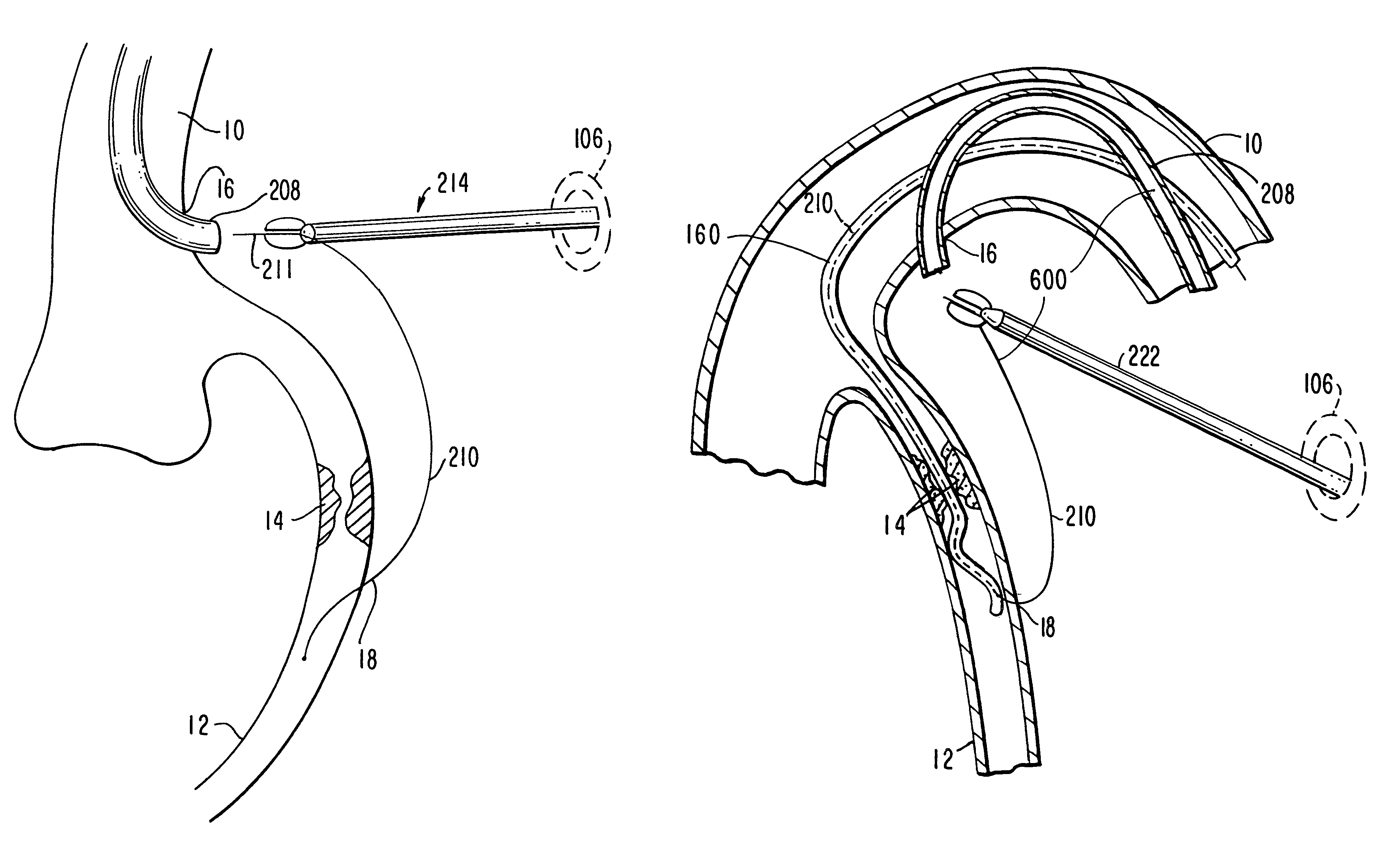
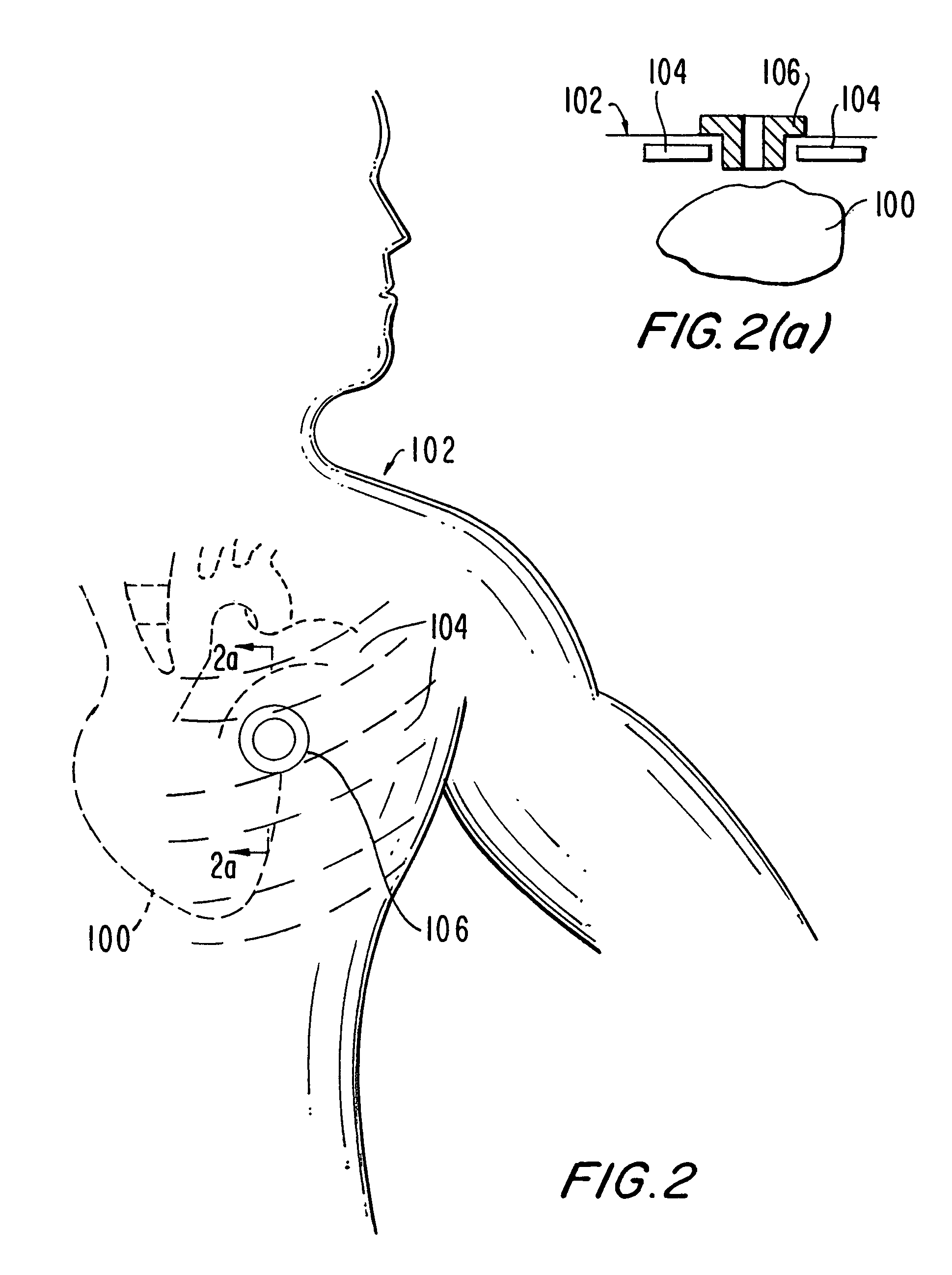
Medical grafting methods and apparatus
Methods and apparatus for delivering and installing a new length of tubing between two sections of a patient's existing body organ tubing and at least partly outside of that existing structure. For example, the new length of tubing may be for the purpose of providing the patient with a coronary bypass. The new tubing may be an artificial graft, a natural graft (harvested elsewhere from the patient), or both. The new tubing is installed at the operative site primarily by providing at least one graft location with instrumentation inserted through the patient's existing tubular body organ structure. Assistance in installing the new tubing may be provided by minimally invasive surgical access openings in the patient's chest. The tubing may be delivered through the patient's existing tubular body structure or, alternatively, through the surgical access openings.
Owner:ST JUDE MEDICAL ATG
Poly(ester amide) filler blends for modulation of coating properties
InactiveUS20060093842A1Improve stabilityIncrease drug release rateOrganic active ingredientsNervous disorderAbnormal tissue growthPEA polymer
Provided herein is a PEA polymer blend and coatings or implantable devices formed therefrom. The PEA polymer blend is formed of a PEA polymer and a material capable of hydrogen bonding with the PEA. The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Implantable devices formed of non-fouling methacrylate or acrylate polymers
Implantable devices formed of or coated with a material that includes a polymer having a non-fouling acrylate or methacrylate polymer are provided. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Medical grafting methods and apparatus
Methods and apparatus for delivering and installing a new length of tubing between two sections of a patient's existing body organ tubing and at least partly outside of that existing structure. For example, the new length of tubing may be for the purpose of providing the patient with a coronary bypass. The new tubing may be an artificial graft, a natural graft (harvested elsewhere from the patient), or both. The new tubing is installed at the operative site primarily by providing at least one graft location with instrumentation inserted through the patient's existing tubular body organ structure. Assistance in installing the new tubing may be provided by minimally invasive surgical access openings in the patient's chest. The tubing may be delivered through the patient's existing tubular body structure or, alternatively, through the surgical access openings.
Owner:ST JUDE MEDICAL ATG
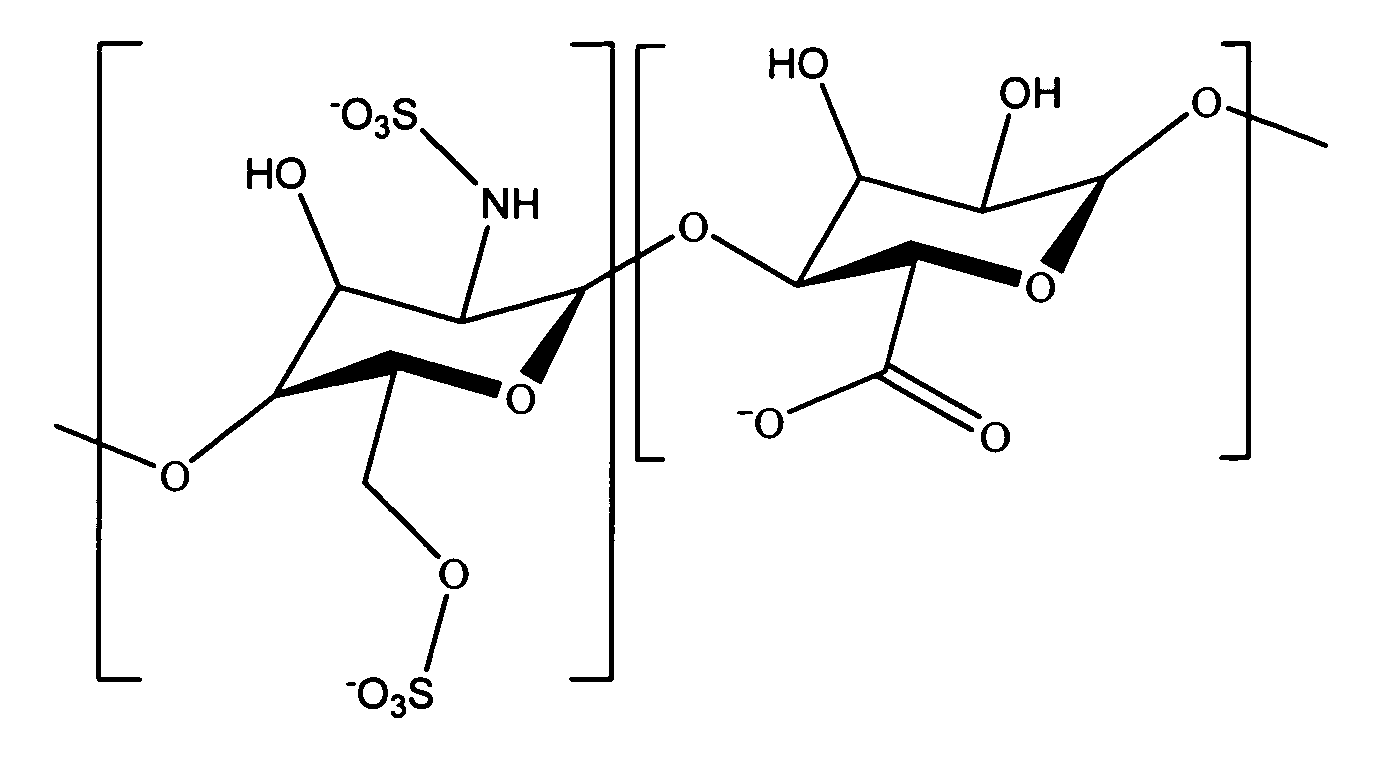
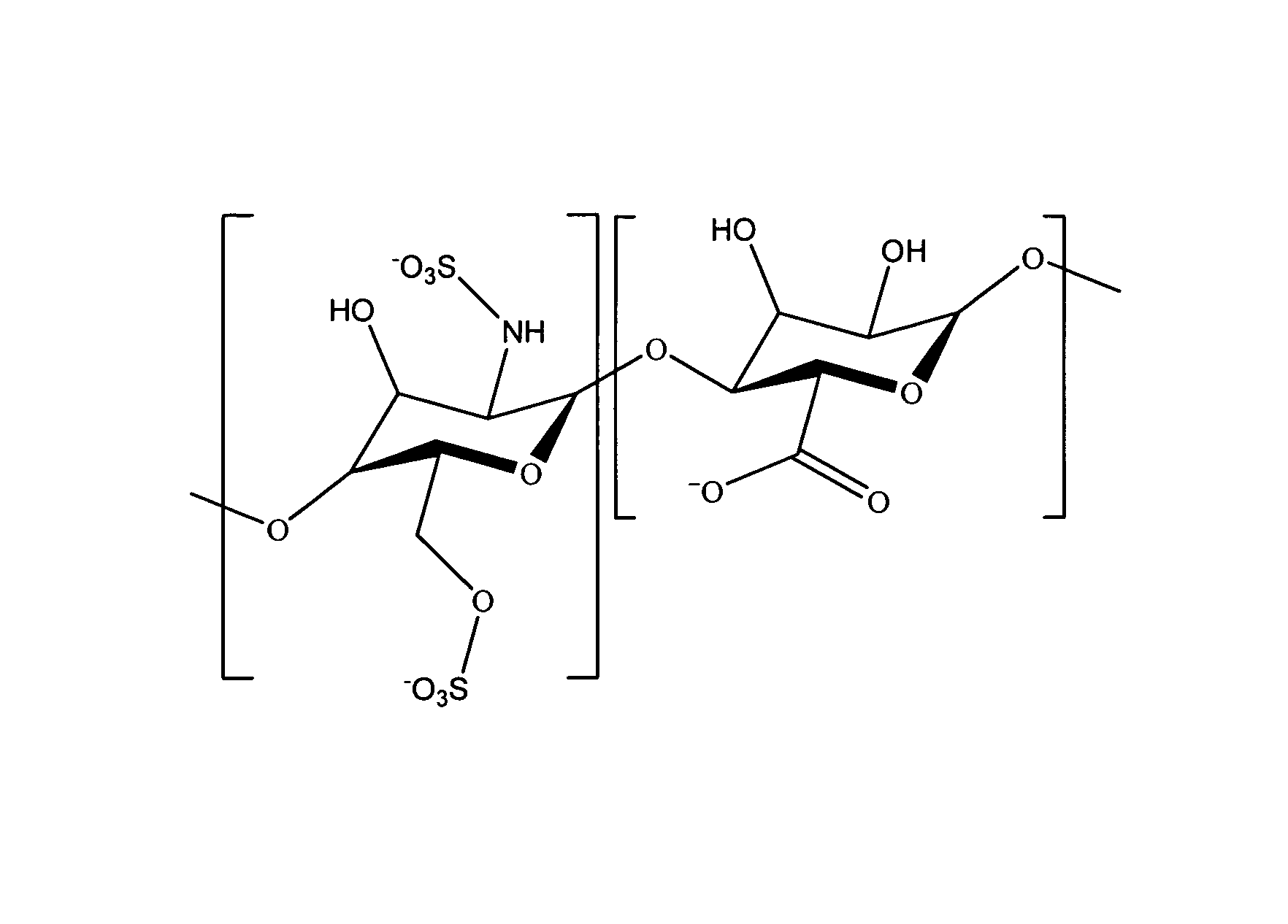
Heparin prodrugs and drug delivery stents formed therefrom
InactiveUS20060014720A1Organic active ingredientsPeptide/protein ingredientsDiseasePercent Diameter Stenosis
A prodrug comprising a heparin and a drug is provided. The prodrug can be used to form a coating on a medical device. The prodrug can also be used with a polymeric material to form a coating on a medical device. The polymeric material can be a hydrophobic polymer, a hydrophilic polymer, a non-fouling polymer, or combinations thereof. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Medical grafting methods and apparatus
InactiveUS7578829B2Enhance their bio-utilityElasticityStentsBalloon catheterBody organsSurgical site
Methods and apparatus for delivering and installing a new length of tubing between two sections of a patient's existing body organ tubing and at least partly outside of that existing structure. For example, the new length of tubing may be for the purpose of providing the patient with a coronary bypass. The new tubing may be an artificial graft, a natural graft (harvested elsewhere from the patient), or both. The new tubing is delivered to and installed at the operative site primarily by working through the patient's existing tubular body organ structure. This avoids the need for any significant surgery on the patient. The artificial grafts may have shapes other than tubular. Certain procedural and apparatus aspects of the invention have uses other than in connection with grafting in general or tubular grafting in particular.
Owner:ST JUDE MEDICAL ATG
Poly(ester amide) block copolymers
Provided herein is a copolymer that includes a soft block (A) that contains poly(ester amide) (PEA) and a hard block (B). The copolymer can be any of AB, ABA or BAB type block copolymers. By varying the relative amount of the PEA block and the hard block, one can obtain a copolymer with a Tg for mechanical integrity in drug-delivery stent applications. The copolymer can be used alone or optionally in combination with a biobeneficial material and / or a biocompatible polymer to form an implantable device or a coating on an implantable device. When the copolymer is used in combination with a biobeneficial material, the copolymer and the biobeneficial material can be co-deposited or applied in sequence during the coating process. A coating formed of the copolymer may also include a bioactive agent. The implantable device can be implanted in a patient to treat, prevent, or ameliorate a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, and / or tumor obstruction.
Owner:ABBOTT CARDIOVASCULAR
Poly(ester amide) filler blends for modulation of coating properties
InactiveUS7390497B2Improved stability and drug release rate and mechanical characteristicOrganic active ingredientsNervous disorderPEA polymerDevice form
Provided herein is a PEA polymer blend and coatings or implantable devices formed therefrom. The PEA polymer blend is formed of a PEA polymer and a material capable of hydrogen bonding with the PEA. The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Poly(ester amide) block copolymers
Owner:ABBOTT CARDIOVASCULAR
Medical grafting methods and apparatus
InactiveUS20060161195A1Enhance their bio-utilityElasticityStentsBalloon catheterBody organsCoronary arteries
Methods and apparatus for delivering and installing a new length of tubing between two sections of a patient's existing body organ tubing and at least partly outside of that existing structure. For example, the new length of tubing may be for the purpose of providing the patient with a coronary bypass. The new tubing may be an artificial graft, a natural graft (harvested elsewhere from the patient), or both. The new tubing is delivered to and installed at the operative site primarily by working through the patient's existing tubular body organ structure. This avoids the need for any significant surgery on the patient. The artificial grafts may have shapes other than tubular. Certain procedural and apparatus aspects of the invention have uses other than in connection with grafting in general or tubular grafting in particular.
Owner:DONGBU HITEK CO LTD
Heparin prodrugs and drug delivery stents formed therefrom
A prodrug comprising a heparin and a drug is provided. The prodrug can be used to form a coating on a medical device. The prodrug can also be used with a polymeric material to form a coating on a medical device. The polymeric material can be a hydrophobic polymer, a hydrophilic polymer, a non-fouling polymer, or combinations thereof. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Implantable devices formed of non-fouling methacrylate or acrylate polymers
Implantable devices formed of or coated with a material that includes a polymer having a non-fouling acrylate or methacrylate polymer are provided. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
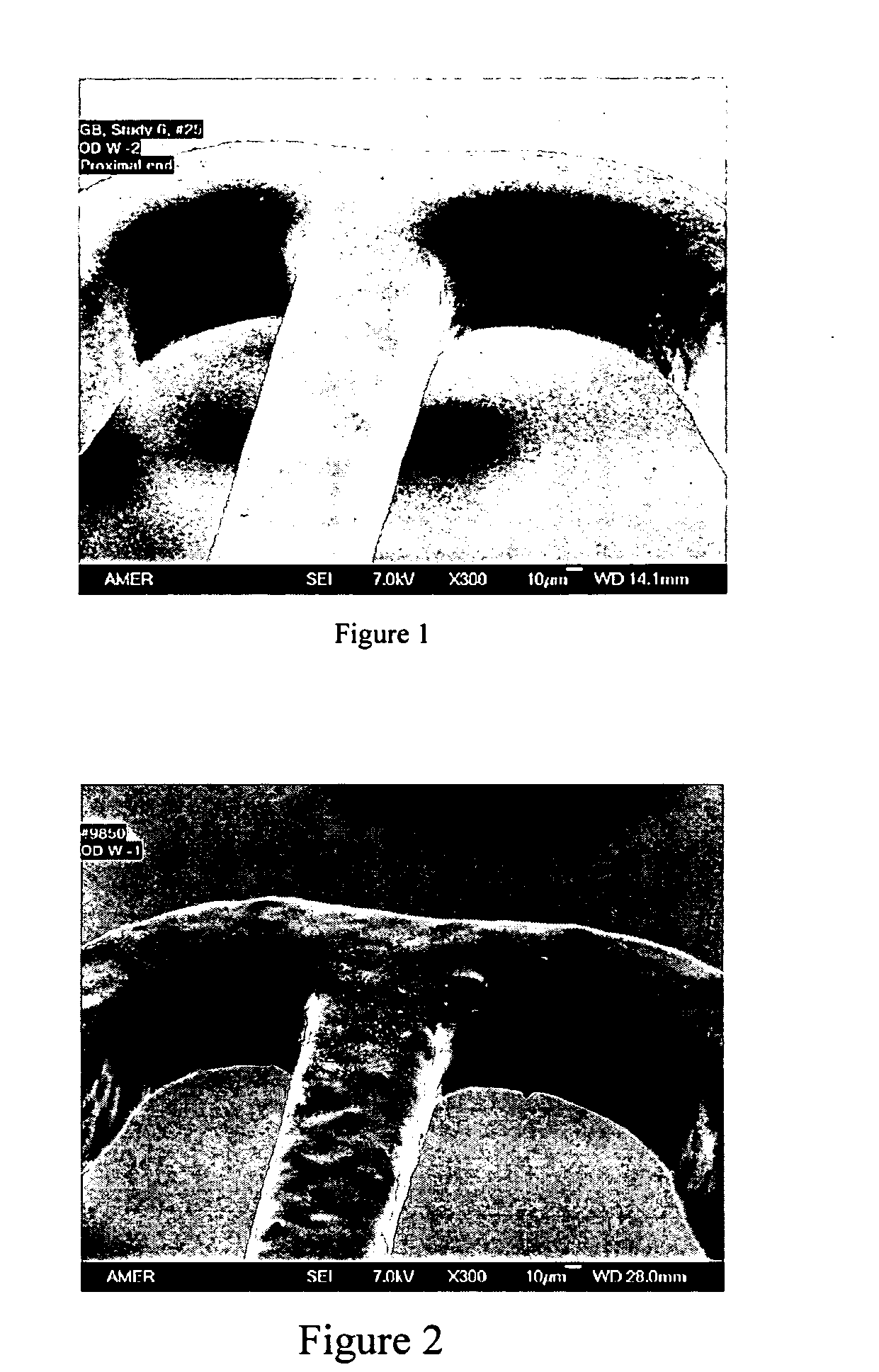
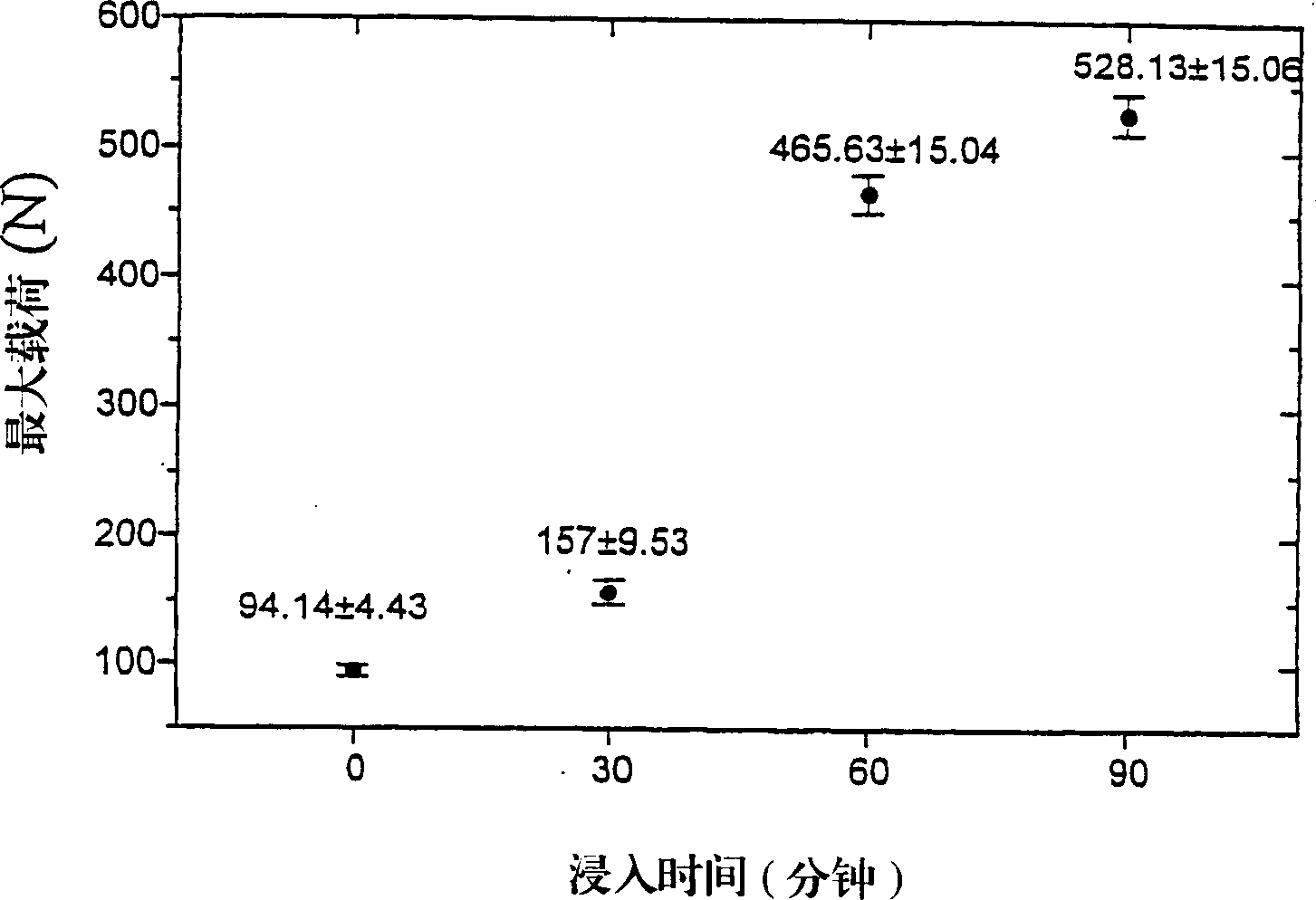
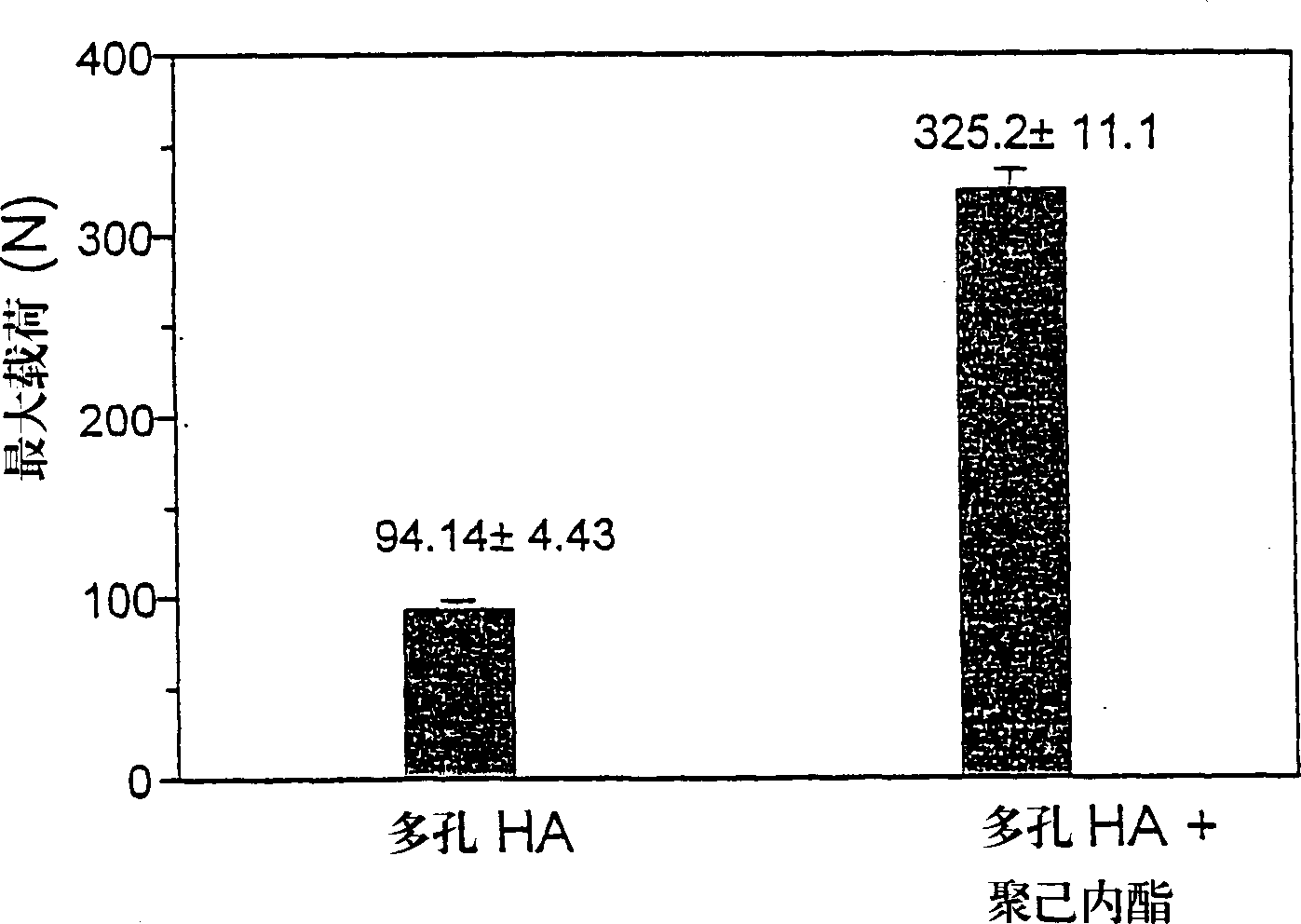
Porous synthetic bone graft and method of manufacture thereof
A process for preparing artificial bone is described which comprises: (i) preparing a mixture of a finely divided bio-compatible ceramic powder, an organic binder and a pore-forming agent in an inert liquid to form a body and causing at least some of the pore-forming agent to align along a common axis; (ii) optionally shaping the resulting body; (iii) allowing the pore-forming agent to form a porous structure in the body; (iv) heating the shaped body to a temperature sufficient to fix the porous structure and; (v) further heating the body to eliminate residues of organic binder and pore-forming agent and to fuse it.
Owner:ORTHOGEM LTD
Polymers containing siloxane monomers
Owner:ABBOTT CARDIOVASCULAR
Medical grafting methods and apparatus
Methods and apparatus for delivering and installing a new length of tubing between two sections of a patient's existing body organ tubing and at least partly outside of that existing structure. For example, the new length of tubing may be for the purpose of providing the patient with a coronary bypass. The new tubing may be an artificial graft, a natural graft (harvested elsewhere from the patient), or both. The new tubing is installed at the operative site primarily by providing at least one graft location with instrumentation inserted through the patient's existing tubular body organ structure. Assistance in installing the new tubing may be provided by minimally invasive surgical access openings in the patient's chest. The tubing may be delivered through the patient's existing tubular body structure or, alternatively, through the surgical access openings.
Owner:ST JUDE MEDICAL ATG
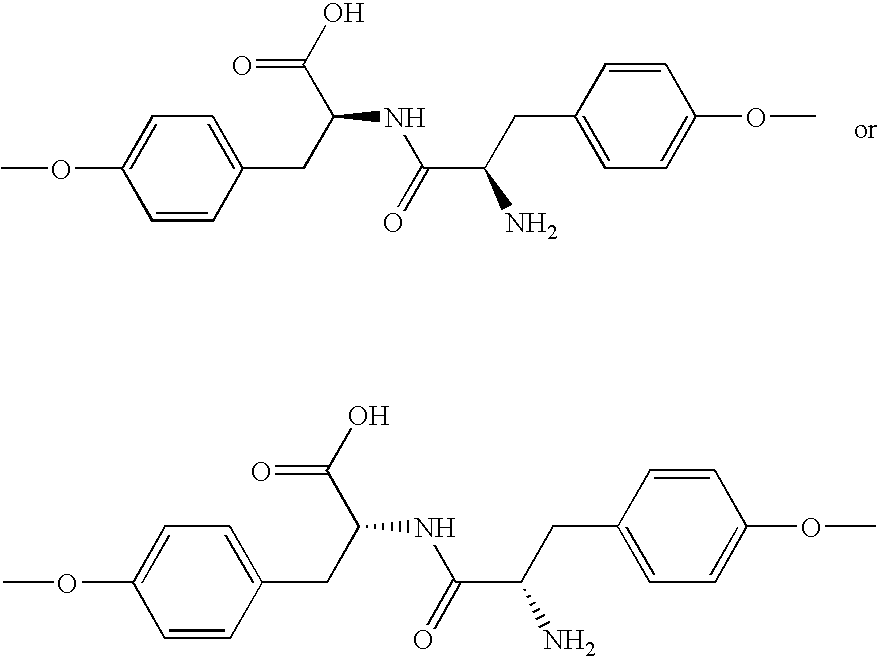
Poly(ester amide) block copolymers
Provided herein is a copolymer that includes a soft block (A) and a hard block (B) comprising a tyrosine di-peptide. The copolymer can be any of AB, ABA or BAB type block copolymers. The soft block can include a PEA polymer. A coating formed of the copolymer may also include a bioactive agent. The implantable device can be implanted in a patient to treat, prevent, or ameliorate a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, and / or tumor obstruction.
Owner:ABBOTT CARDIOVASCULAR
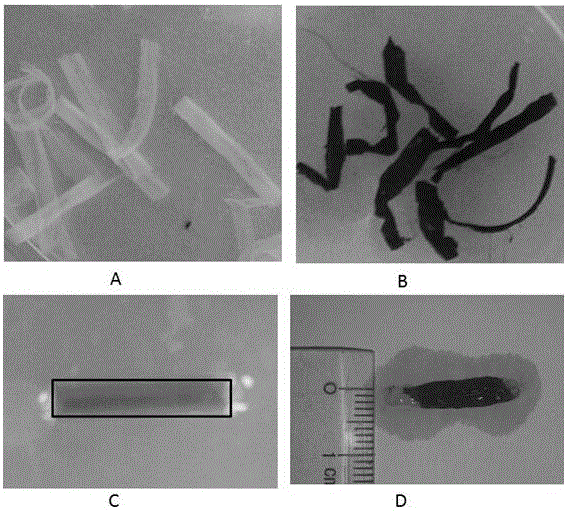
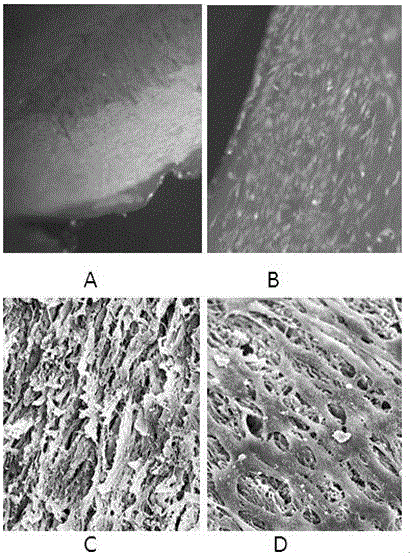
Artificial nerve graft constructed based on chip-type acellularized scaffold and preparation method of artificial nerve graft
ActiveCN106730034AAvoid the disadvantages that if the strength is too small, it will easily remain, and if the strength is too high, it will be crushedImproved cell loading capacityTissue regenerationProsthesisAdhesiveSpinal cord
The invention relates to an artificial nerve graft constructed based on a chip-type acellularized scaffold and a preparation method of the artificial nerve graft, belonging to the field of tissue engineering. According to the artificial nerve graft and the preparation method, heterogeneous animal medulla spinalis, skeletal muscle or tendon acellularized tissue slices are adopted as a bracket, genipin is adopted for crosslinking cytokines, autologous ecto mesenchymal stem cells are planted on the bracket, and fibrous protein is taken as an adhesive for constructing the artificial nerve graft, so that the repairing of the nervous tissue is promoted. The artificial nerve graft and the preparation method have the advantages that the preparation cost is low, the period is short, the preparation raw materials are easily available, and the preparation is simple and is easy to operate. The constructed artificial nerve graft has low immunogenicity, contains a large number of autologous stem cells and cell factors, and is beneficial for adjusting the nerve injury microenvironment, and promoting the nerve regeneration and myelinogenesis. The artificial nerve graft can be used for preparing peripheral nerve and medulla spinalis defect, and has the broad application prospect.
Owner:JIANGSU UNIV
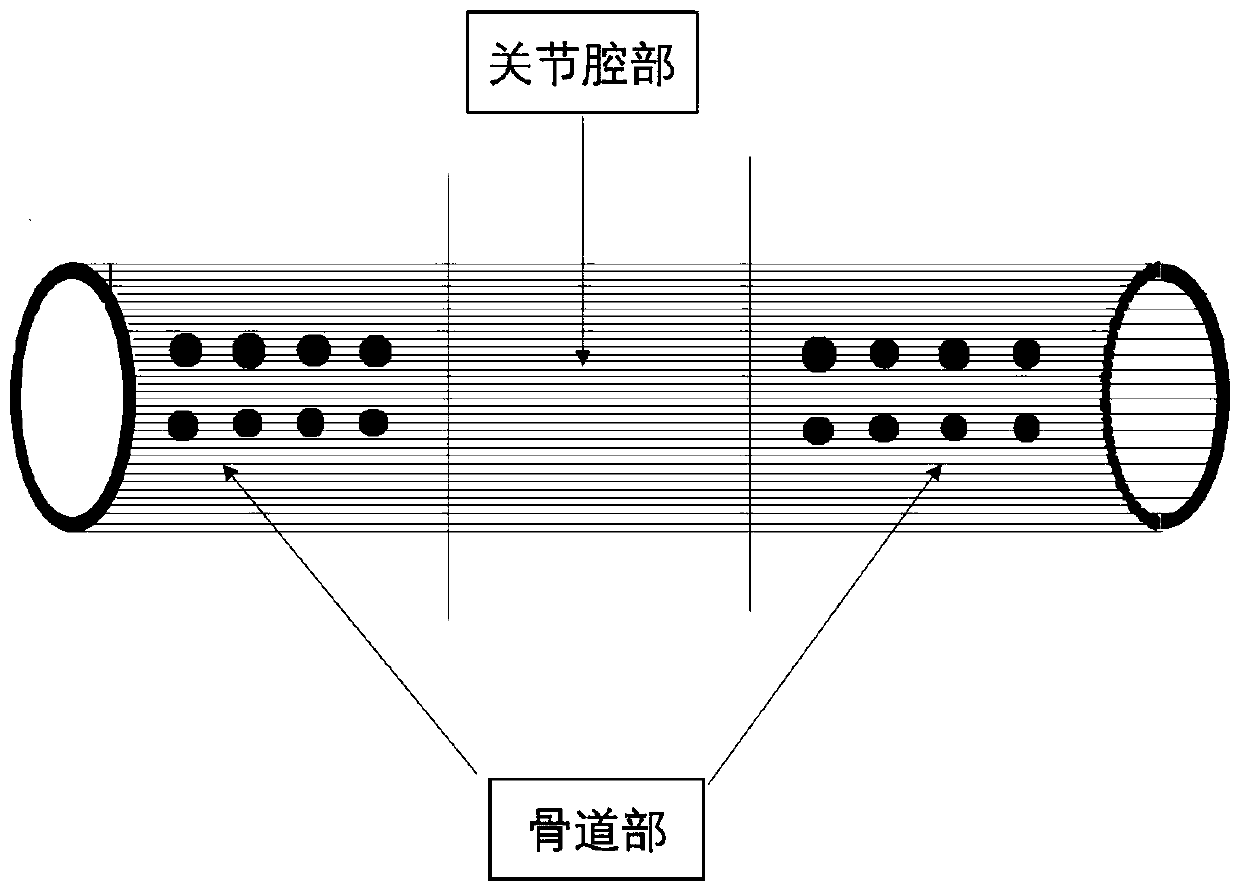
Segmented and gradient functional tendon sleeve implanting device and preparation method thereof
PendingCN110693629AIncreased adhesion proliferationPromotes regenerative remodelingPharmaceutical delivery mechanismLigamentsBone tunnelJoint cavity
The invention discloses a segmented and gradient functional tendon sleeve implanting device and a preparation method thereof. The preparation method includes the steps: (1) firstly, a three-dimensional bracket structure containing a bone tunnel part and a joint cavity part and having a porous hollow cylindrical structure shape is prepared through an electrostatic spinning or textile weaving method; (2) surface modification is conducted on wefts of the joint cavity part through a physical or chemical method, and surface modification comprises protein and small molecular compound modification; (3) gradient modification is conducted on the bone tunnel part through a physical or chemical method, and gradient modification comprises bone material, protein and small molecular compound modification; and (4) a needle threading marker hole is reserved in the outer surface of the bone tunnel part, a traction line is arranged, and thus the segmented and gradient functional tendon sleeve implantingdevice is obtained. The segmented and gradient functional tendon sleeve implanting device can achieve a promoting enhancement effect on clinical ACL repair through autograft tendon and allogenic tendon grafts and artificial grafts and ligament regeneration and tendon-bone healing of other ligament and tendon injuries, and provide mechanical protection without forming of stress shielding.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Antifouling heparin coatings
Owner:ABBOTT CARDIOVASCULAR
Polymers of fluorinated monomers and hydrocarbon monomers
InactiveUS7604818B2Provide mechanical strengthGive flexibilityStentsFibre treatmentPolymer scienceActive agent
A polymer of fluorinated monomers and hydrocarbon monomers is provided. It is also provided a polymer blend that contains a polymer formed of fluorinated monomers and hydrocarbon monomers and another biocompatible polymer. The polymer or polymer blend described herein and optionally a bioactive agent can form an implantable device such as a stent or a coating on an implantable device such as a drug-delivery stent, which can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Porous scaffold for tissue engineering and preparation method thereof
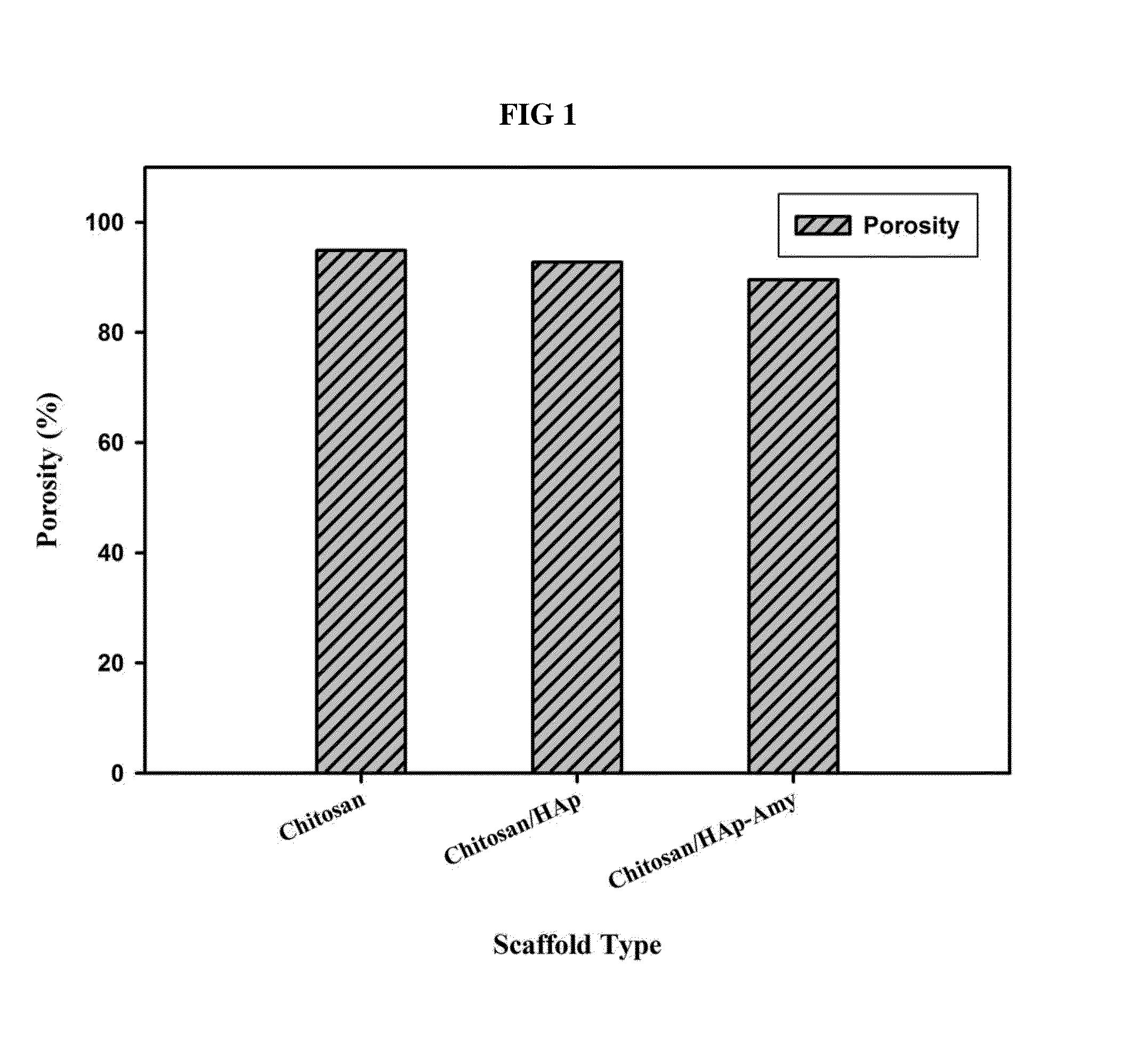
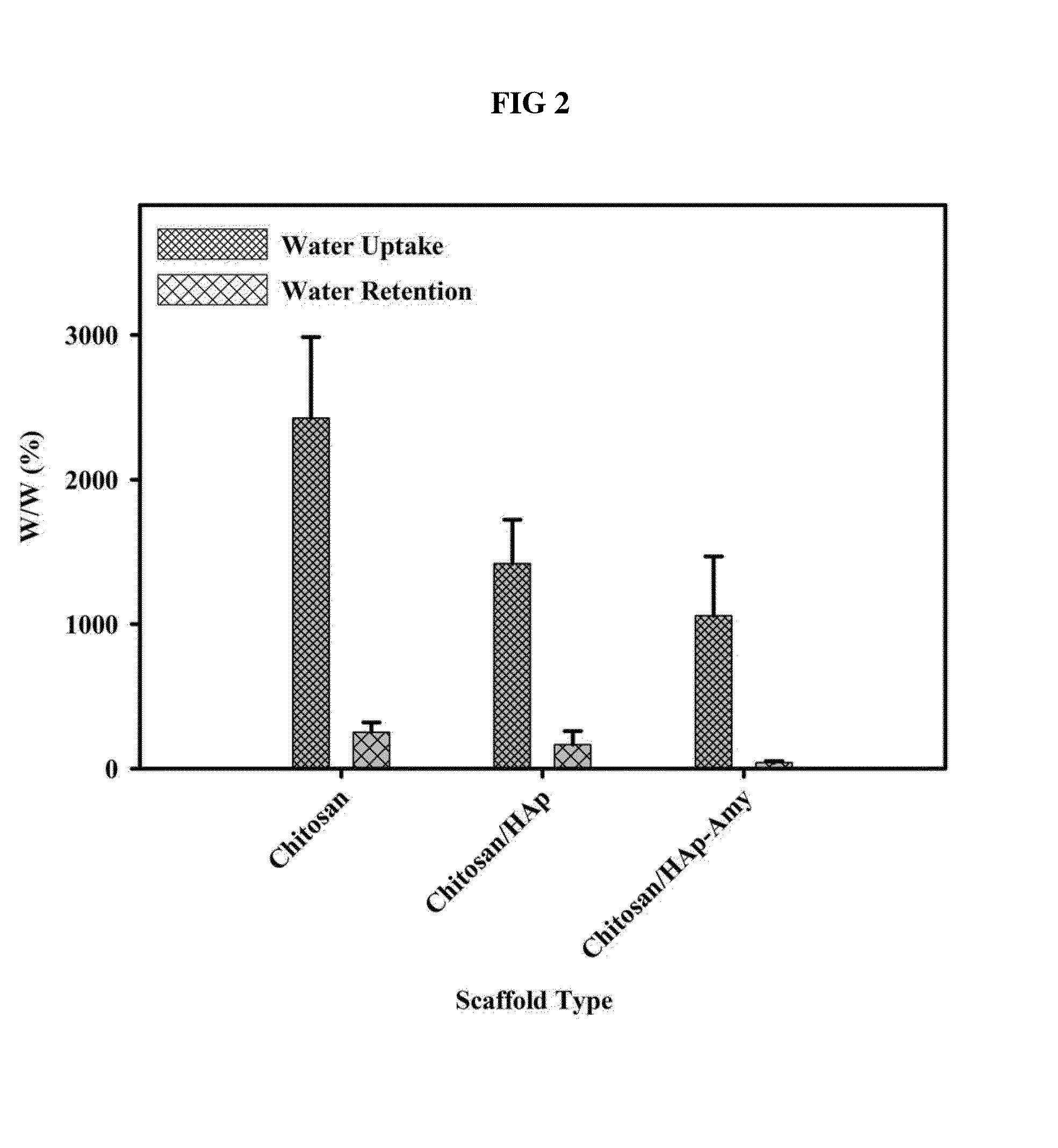
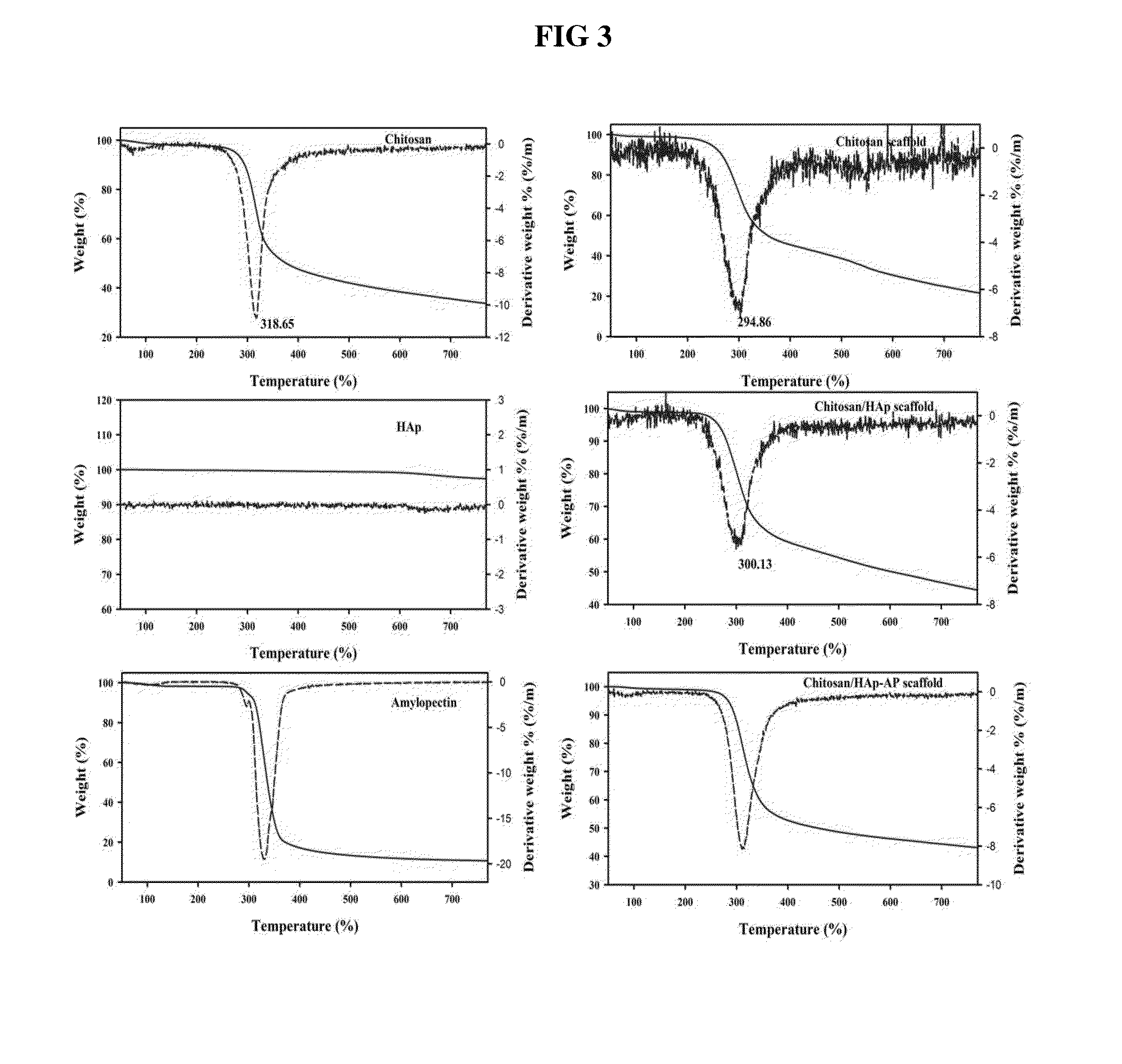
InactiveUS20140314824A1Efficient cell proliferationGuaranteed normal transmissionBiocideInorganic phosphorous active ingredientsHuman bodyBiocompatibility Testing
A porous artificial transplant material to replace autogenous bone with excellent biocompatibility, cytocompatibility and biodegradability is provided. More specifically, a porous scaffold for tissue engineering including chitosan / hydroxyapatite-amylopectin (Chitosan / HAp-AP) and a preparation method thereof are provided. The porous scaffold for tissue engineering has cross linkage among chitosan, hydroxyapatite and amylopectin, which provides advantageous effect including superior cell proliferation and transmission, and excellent thermal stability and mechanical strength. Further, considering excellent biocompatibility and biodegradability which do not harm human body, the porous scaffold can be widely used as an artificial transplant material to replace autogenous bone in biomedical field.
Owner:PUKYONG NAT UNIV IND ACADEMIC COOPERATION FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com