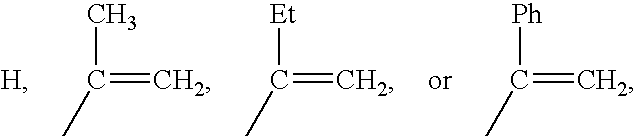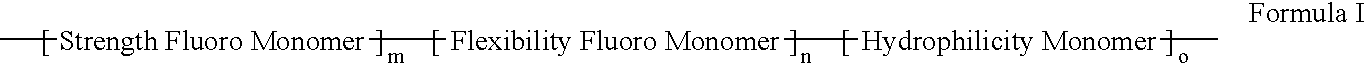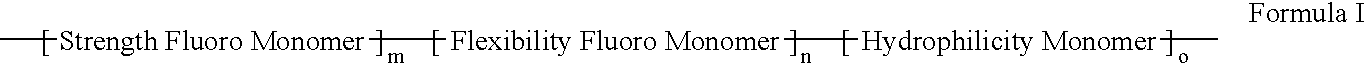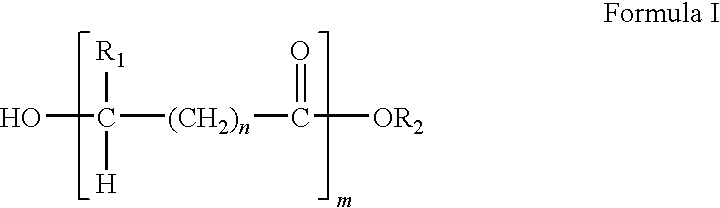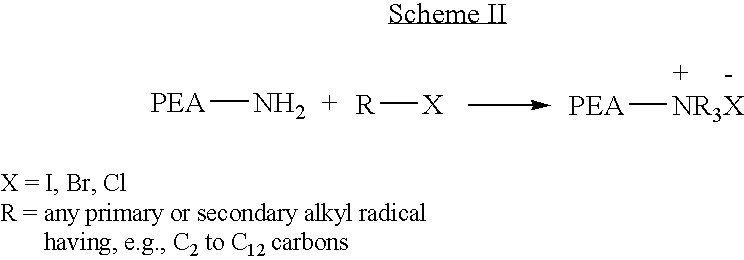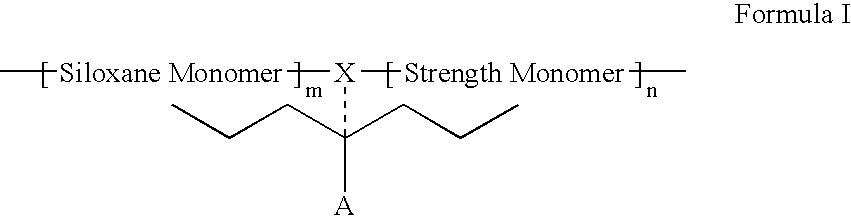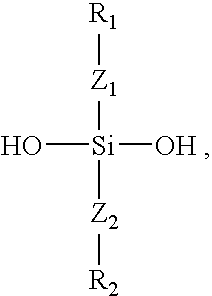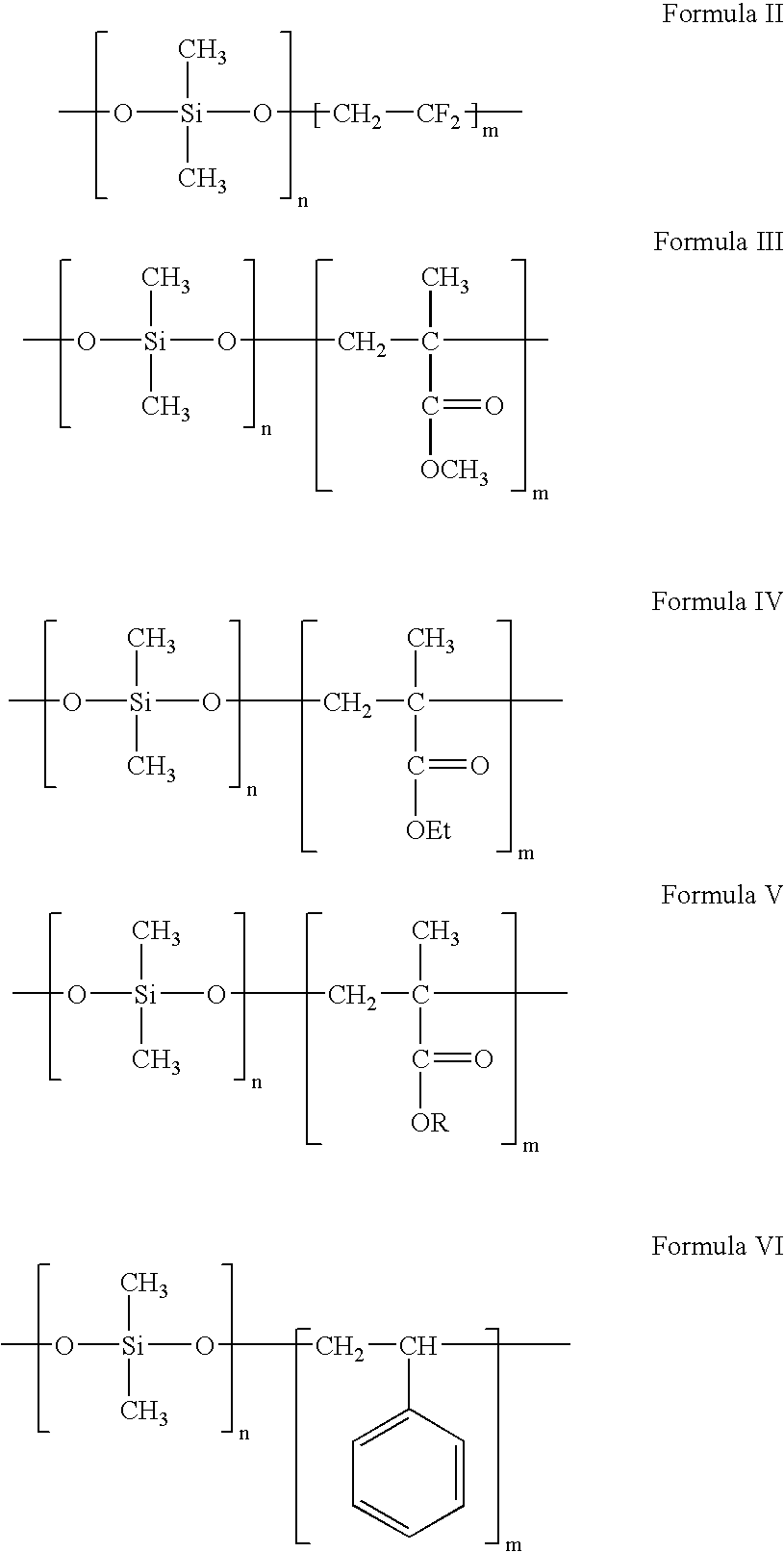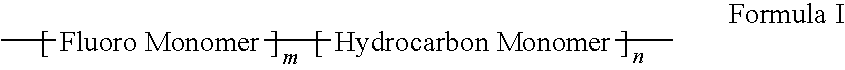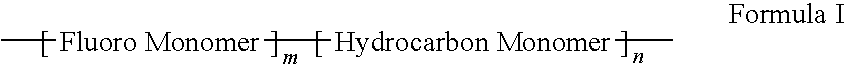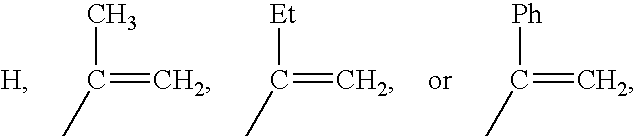Patents
Literature
56 results about "Claudication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pain or tightness in the lower leg due to inadequate blood flow.
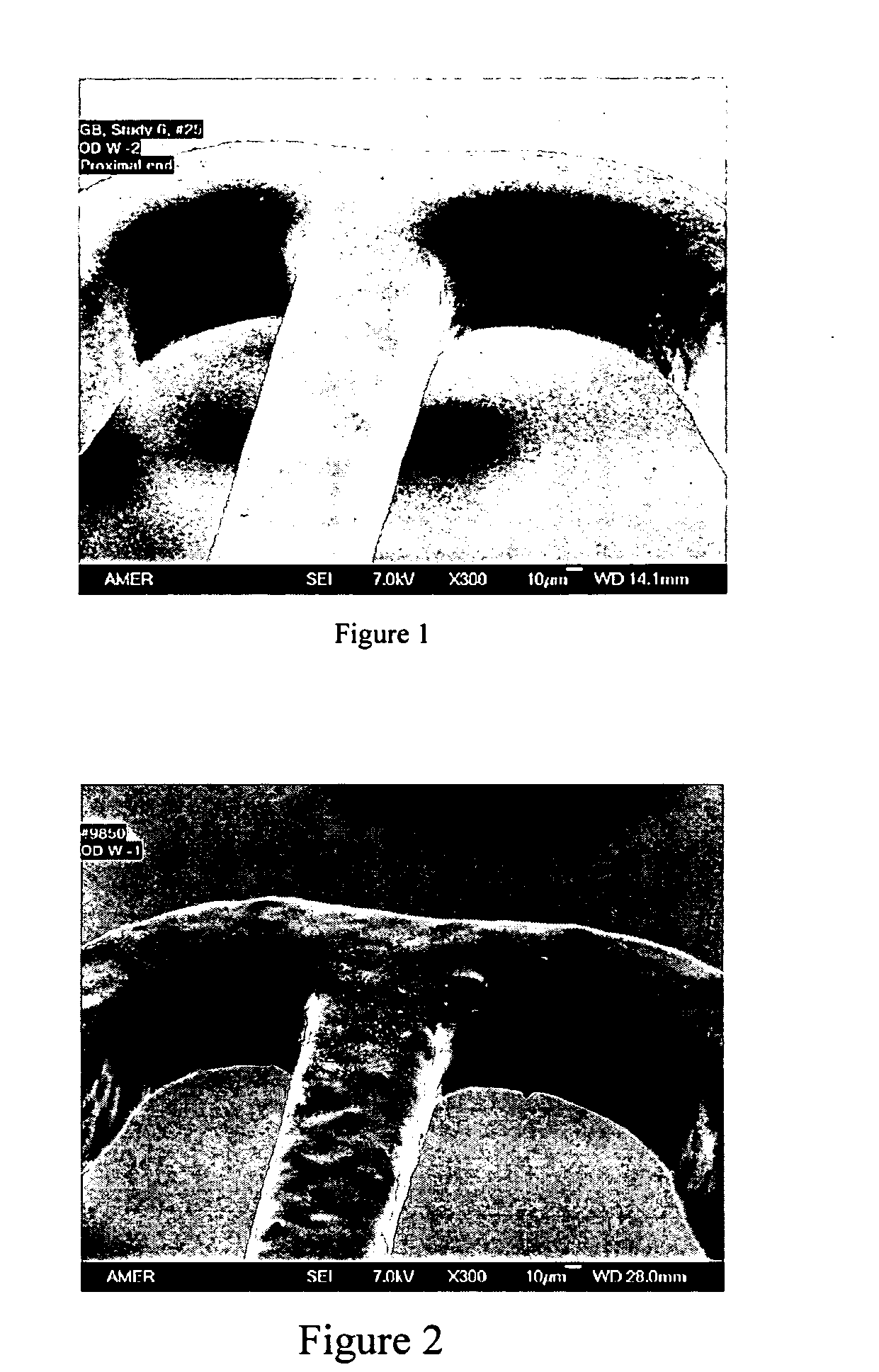
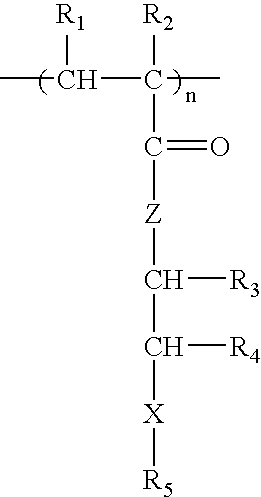
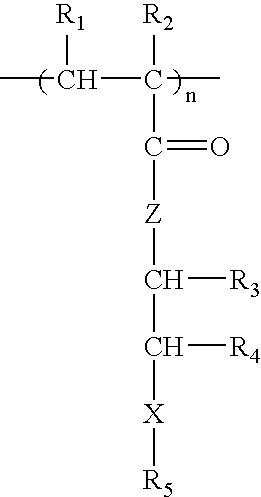
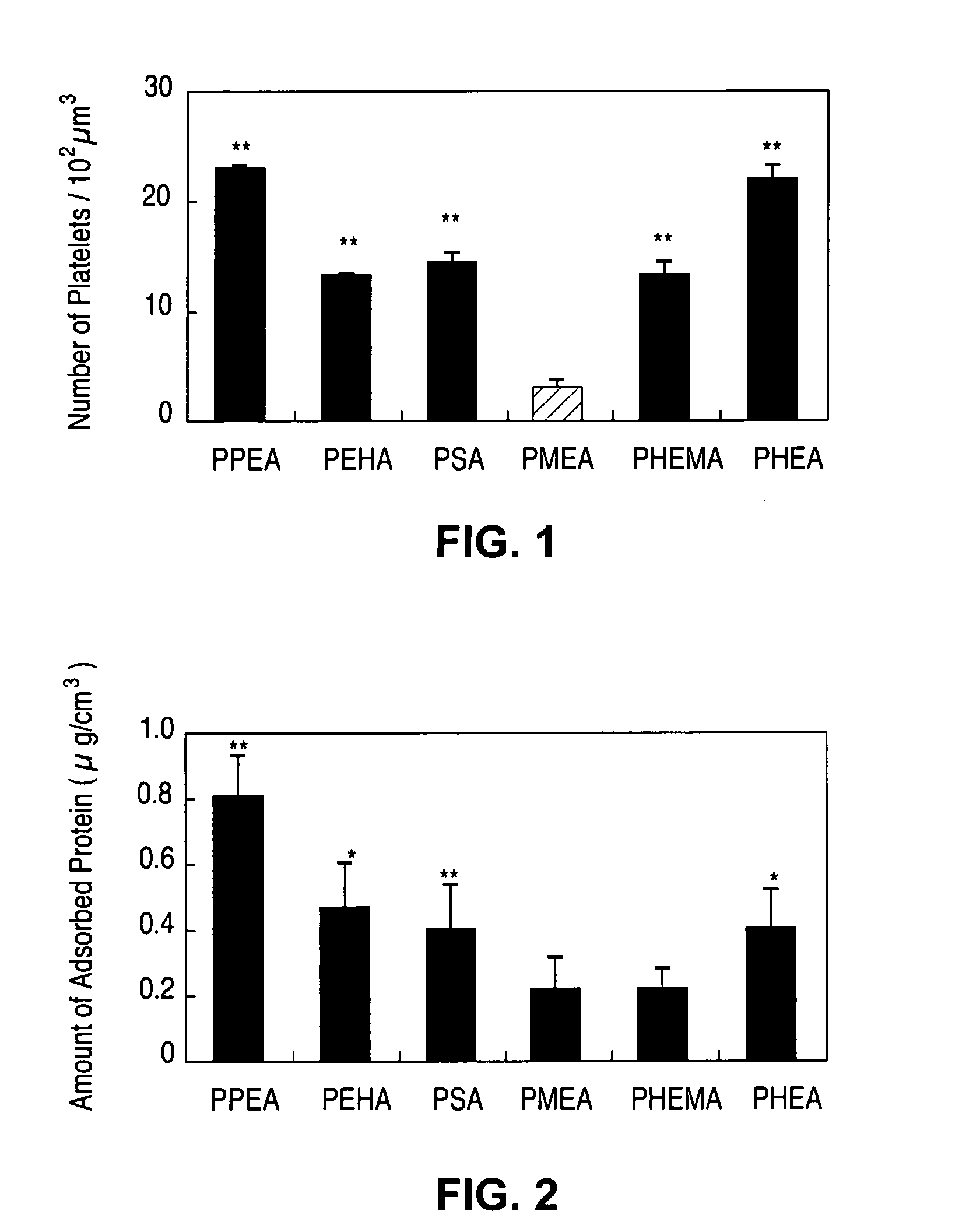
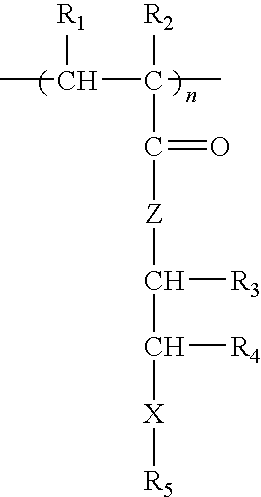
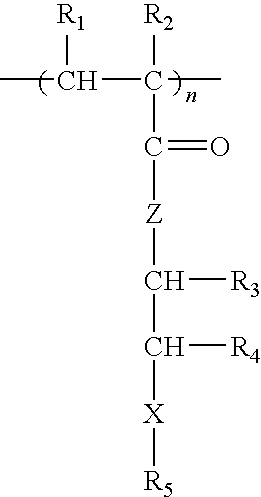
Methacrylate copolymers for medical devices
A polymer of hydrophobic monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains the polymer and another biocompatible polymer. The polymer or polymer blend and optionally a biobeneficial material and / or a bioactive agent can form a coating on an implantable device such as a drug delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
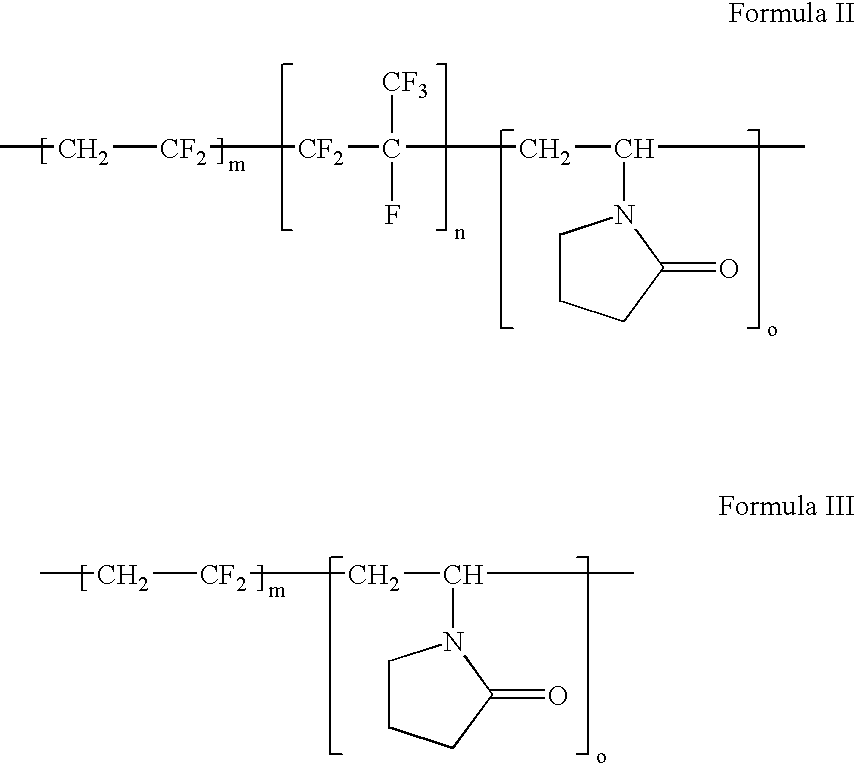
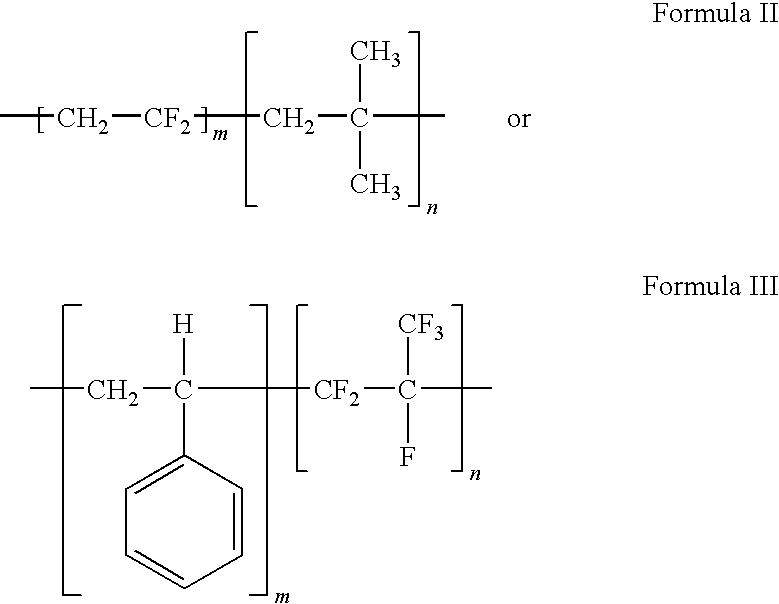
Polymers of fluorinated monomers and hydrophilic monomers
ActiveUS20060047095A1Improve propertiesProvide flexibilityFibre treatmentSurgeryDiseasePolymer science
A polymer of fluorinated monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains a polymer of fluorinated monomers and another biocompatible polymer. The polymer of fluorinated monomers or polymer blend described herein and optionally a bioactive agent can form a coating on an implantable device such as a drug-delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
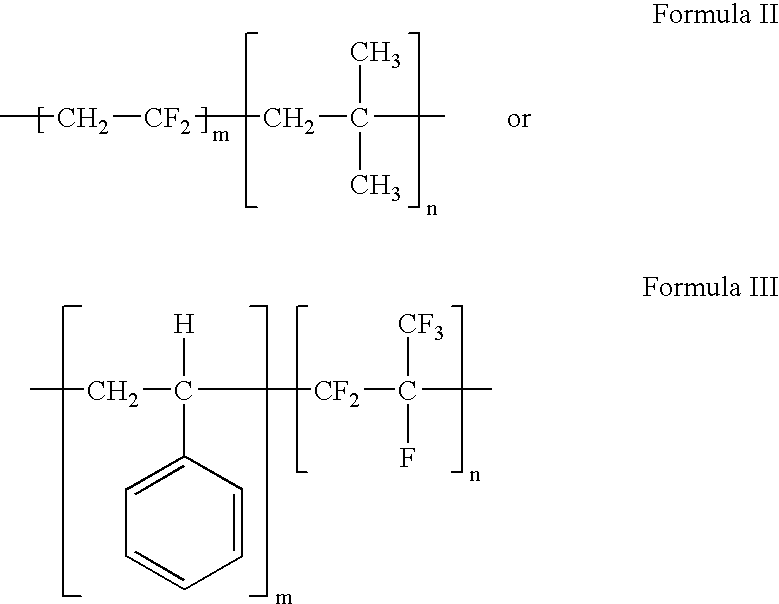
Polymers of fluorinated monomers and hydrocarbon monomers
InactiveUS20060134165A1Provide mechanical strengthGive flexibilityStentsSurgeryAbnormal tissue growthPolymer science
A polymer of fluorinated monomers and hydrocarbon monomers is provided. It is also provided a polymer blend that contains a polymer formed of fluorinated monomers and hydrocarbon monomers and another biocompatible polymer. The polymer or polymer blend described herein and optionally a bioactive agent can form an implantable device such as a stent or a coating on an implantable device such as a drug-delivery stent, which can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
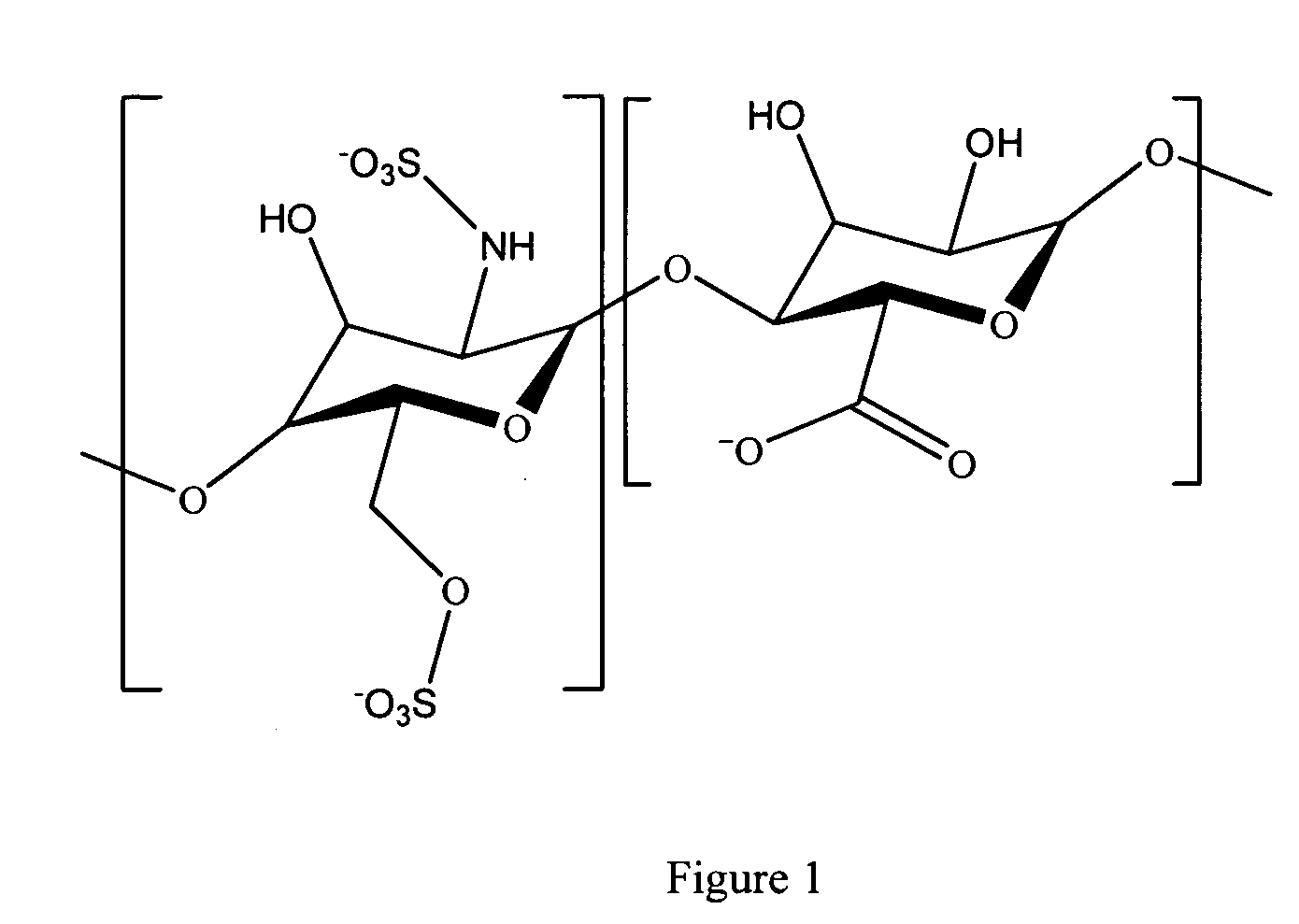
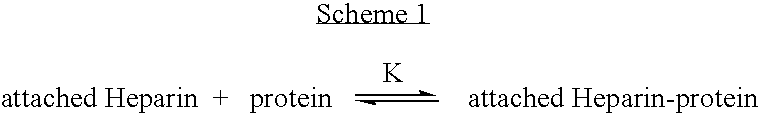
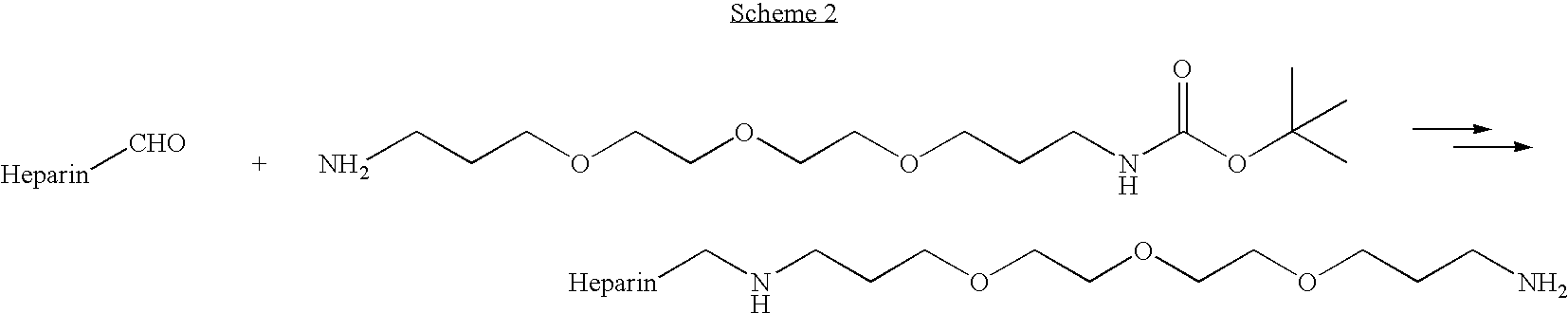
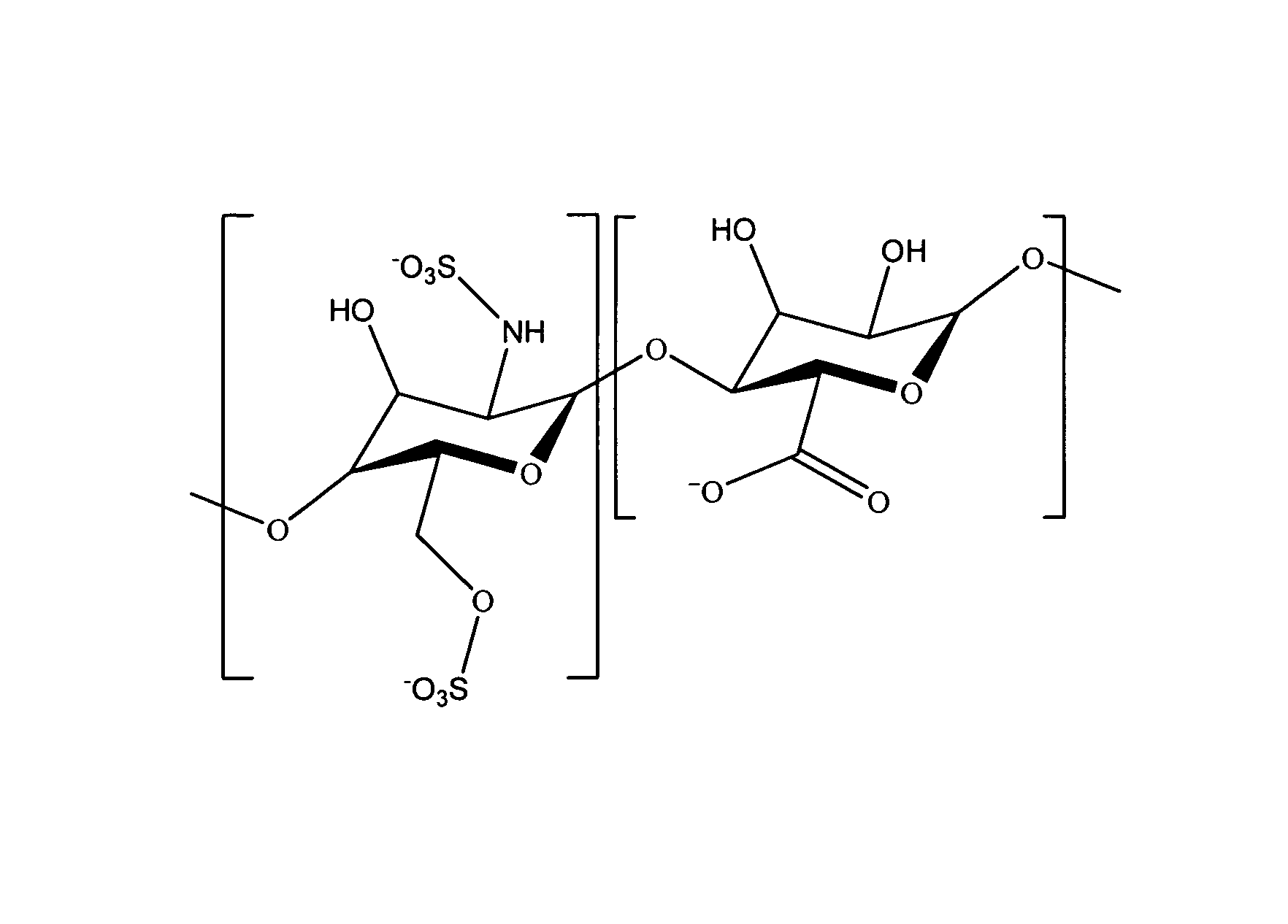
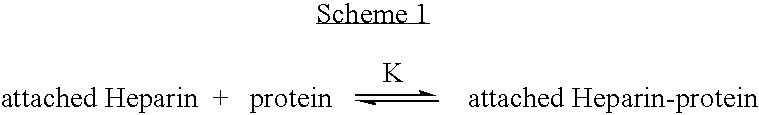
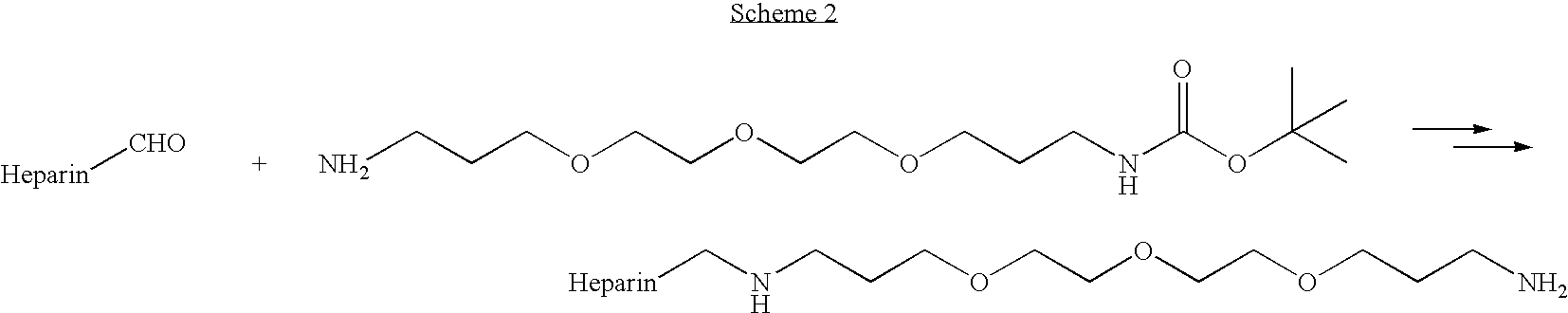
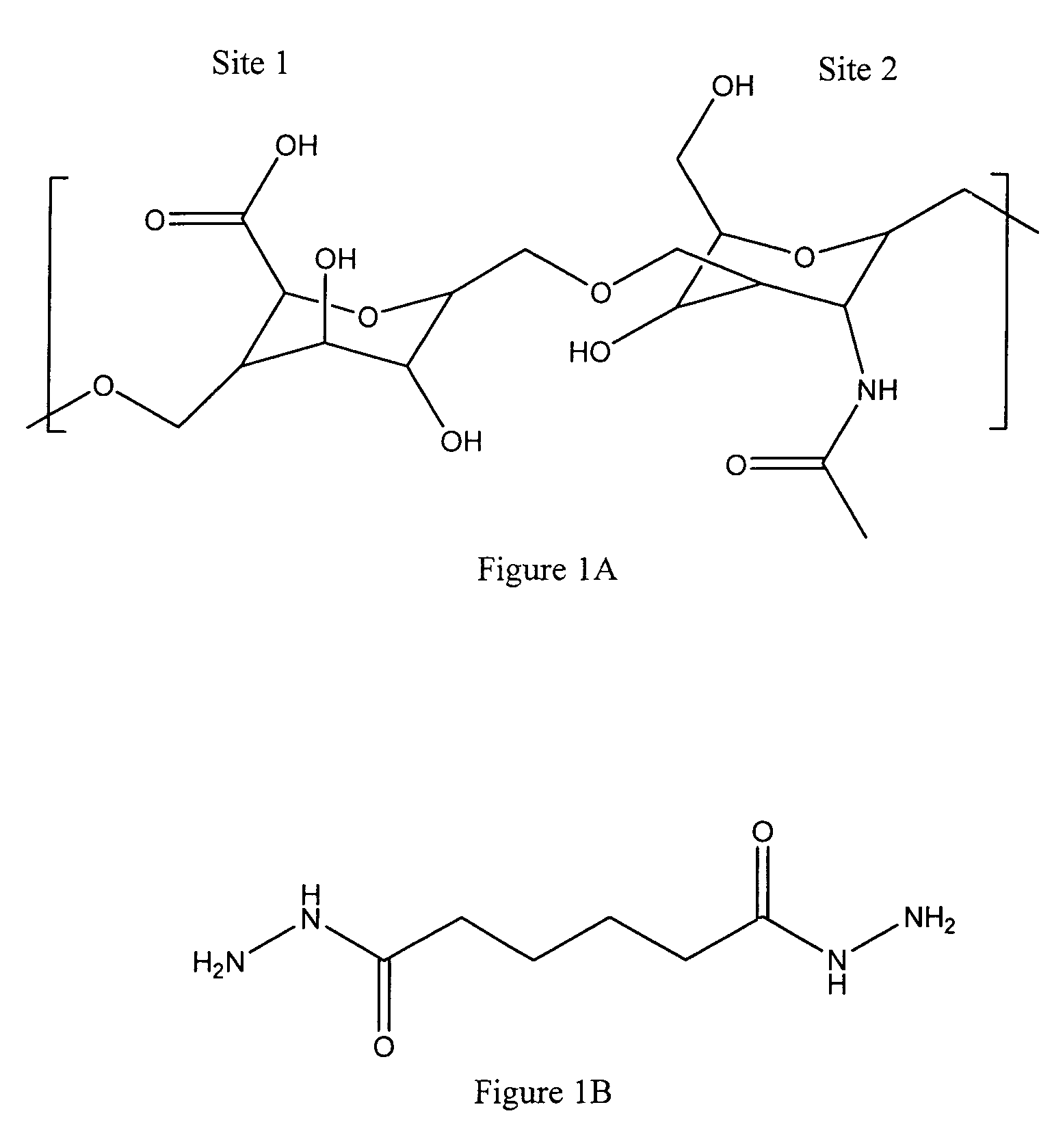
Antifouling heparin coatings
A medical device comprising a coating thereon comprising a biocompatible polymer and heparin is provided herein. Heparin is coupled with the biocompatible polymer via a spacer having a grouping that renders a binding site of the heparin molecule accessible by a binding protein. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Beneficial effects of increasing local blood flow
InactiveUS20110028548A1Increase oxygenationImprove tissue nutritionBiocidePeptide/protein ingredientsArginineNitric oxide
Owner:STRATEGIC SCI & TECH
Plasticizers for coating compositions
A biocompatible plasticizer useful for forming a coating composition with a biocompatible polymer is provided. The coating composition may also include a biobeneficial polymer and / or a bioactive agent. The coating composition can form a coating on an implantable device. The implantable device can be used to treat or prevent a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
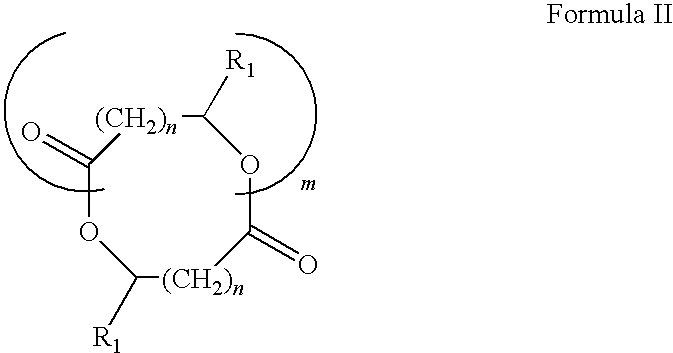
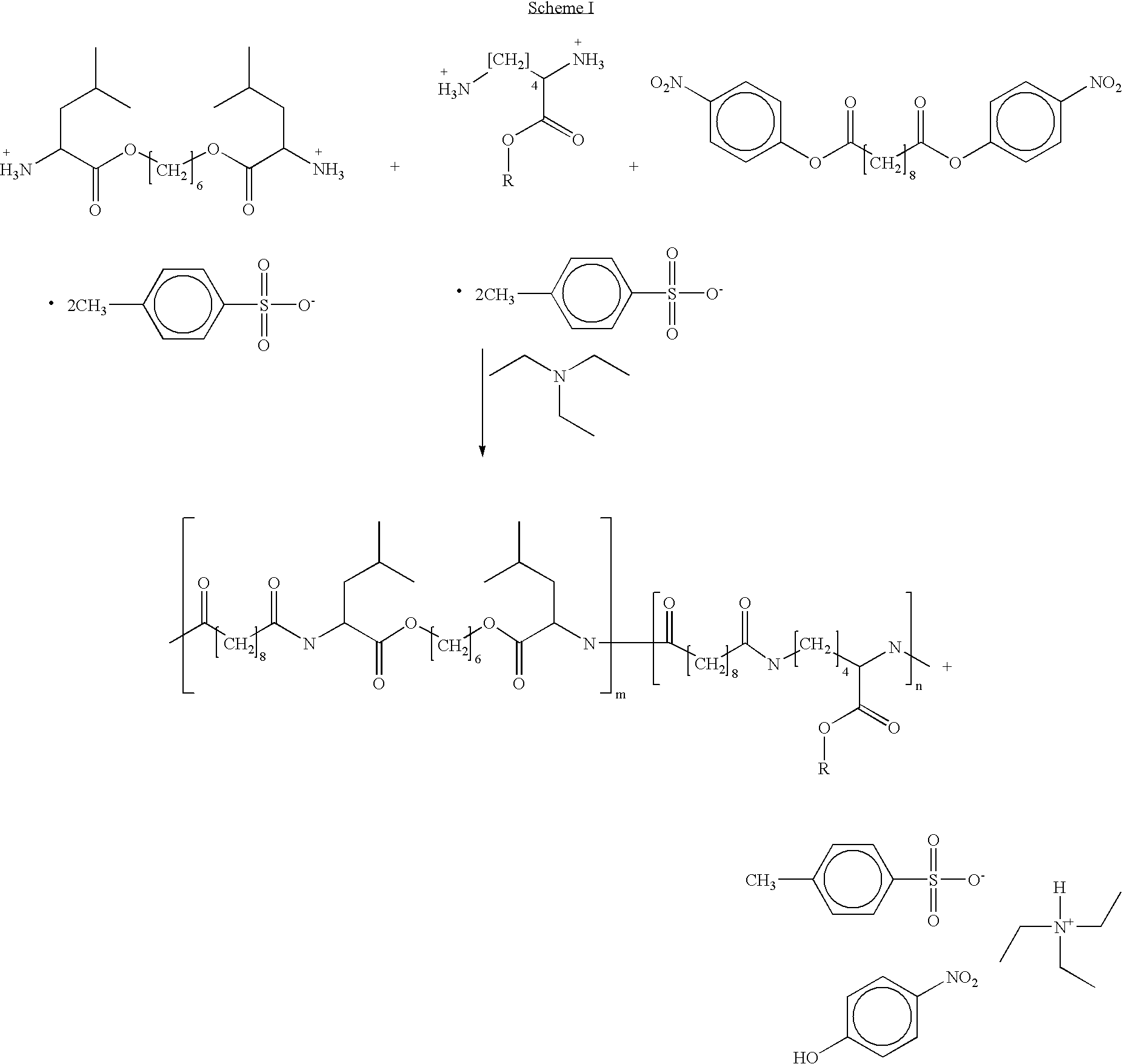
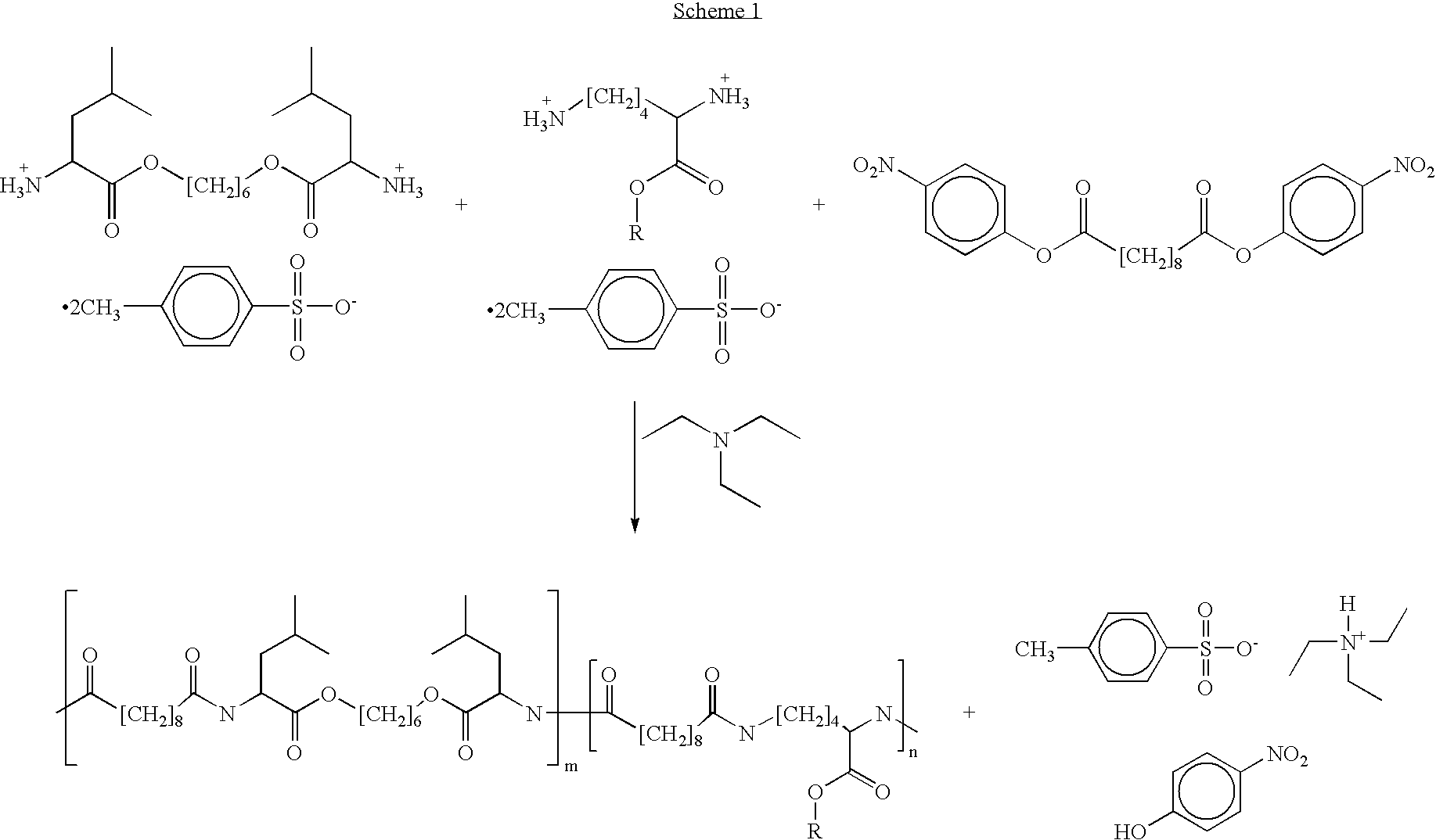
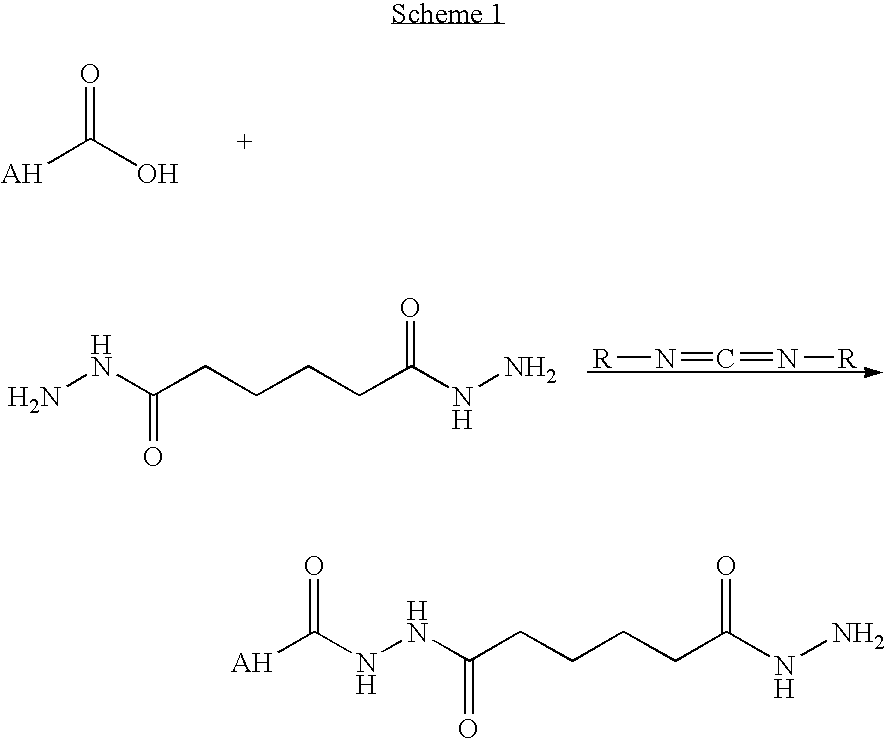
Blends of poly(ester amide) polymers
ActiveUS7166680B2Improved stability and drug release rate and mechanical characteristicReduce degradationSurgeryCatheterDiseasePEA polymer
Provided herein is a poly(ester amide) (PEA) polymer blend and a polymeric coating containing the PEA polymer blend. The PEA polymer blend has a Tg above the Tg of poly(ester amide benzyl ester) (PEA-Bz) or the Tg of poly(ester amide TEMPO). The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
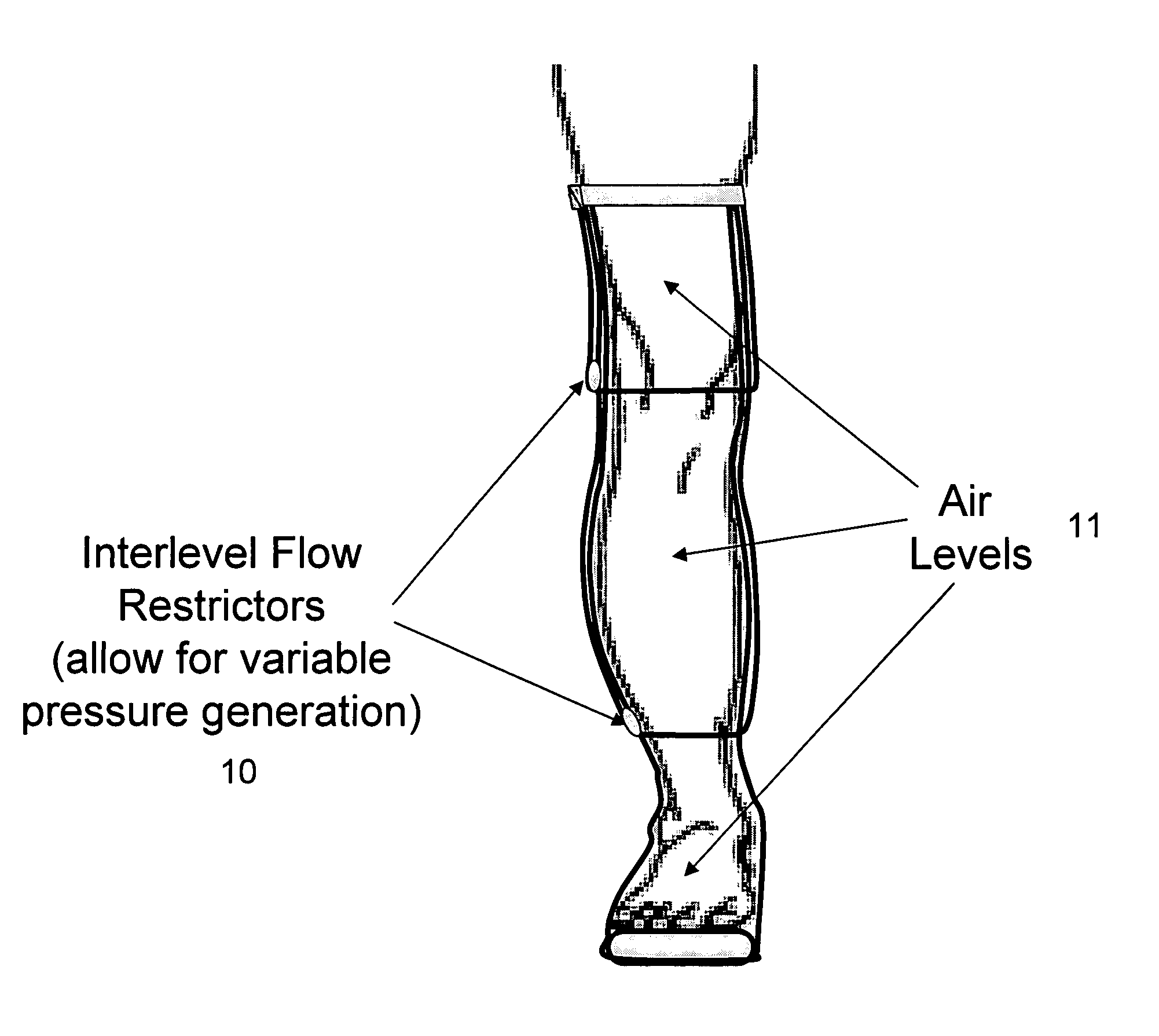
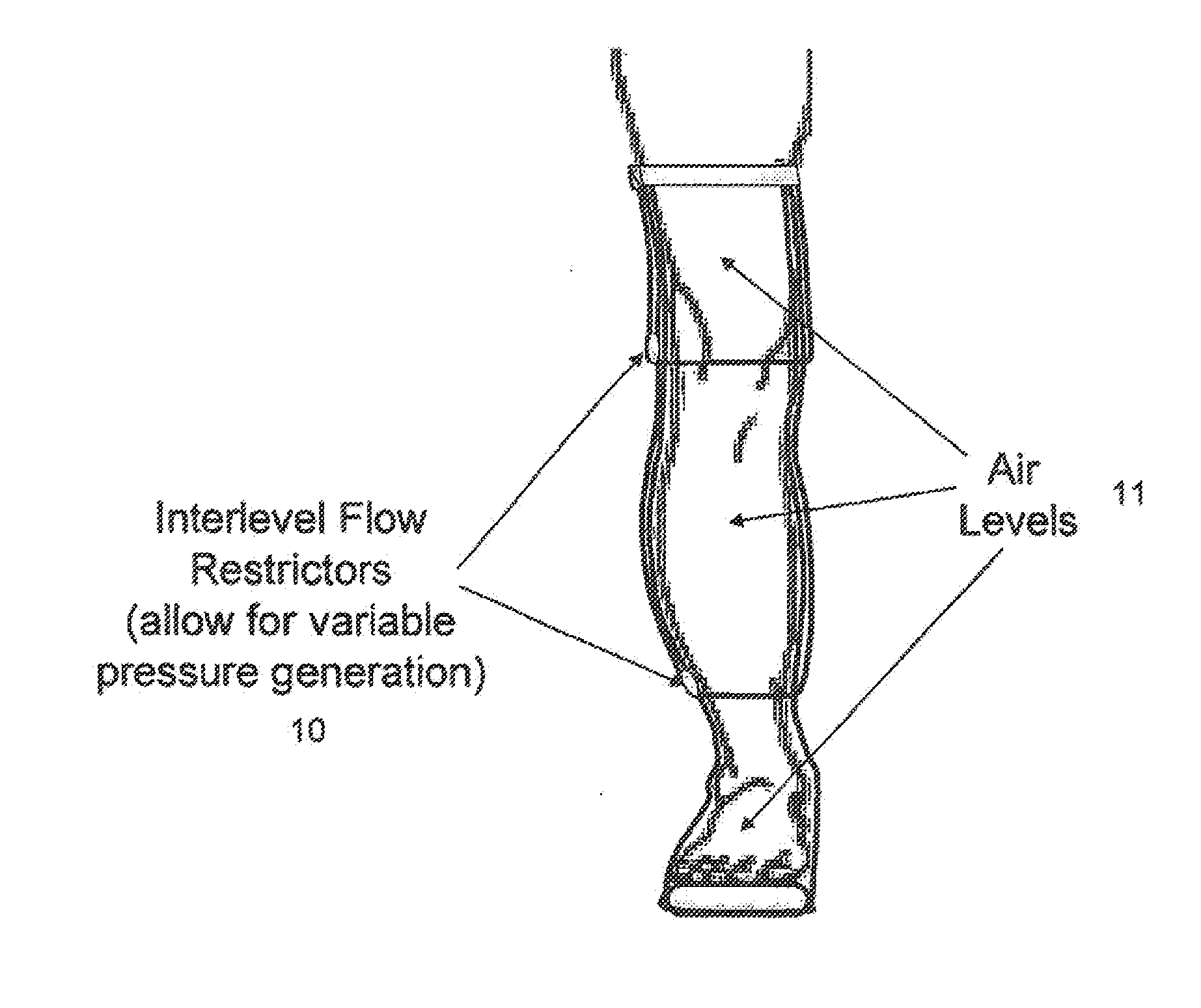
Method and apparatus for treating wound using negative pressure therapy
InactiveUS7896823B2Easy to returnReduce edemaBlood stagnation preventionPneumatic massageVacuum assistedThrombus
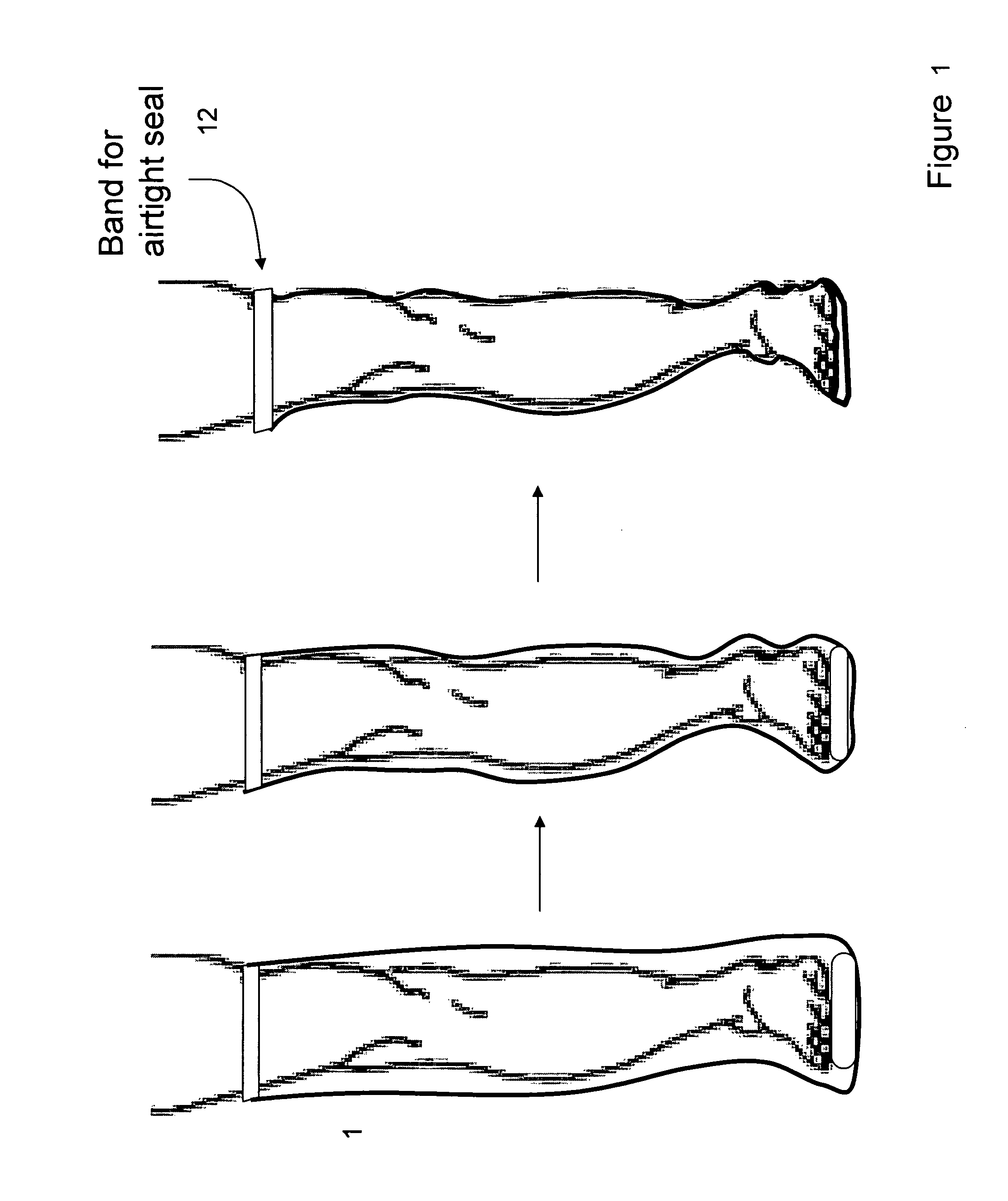
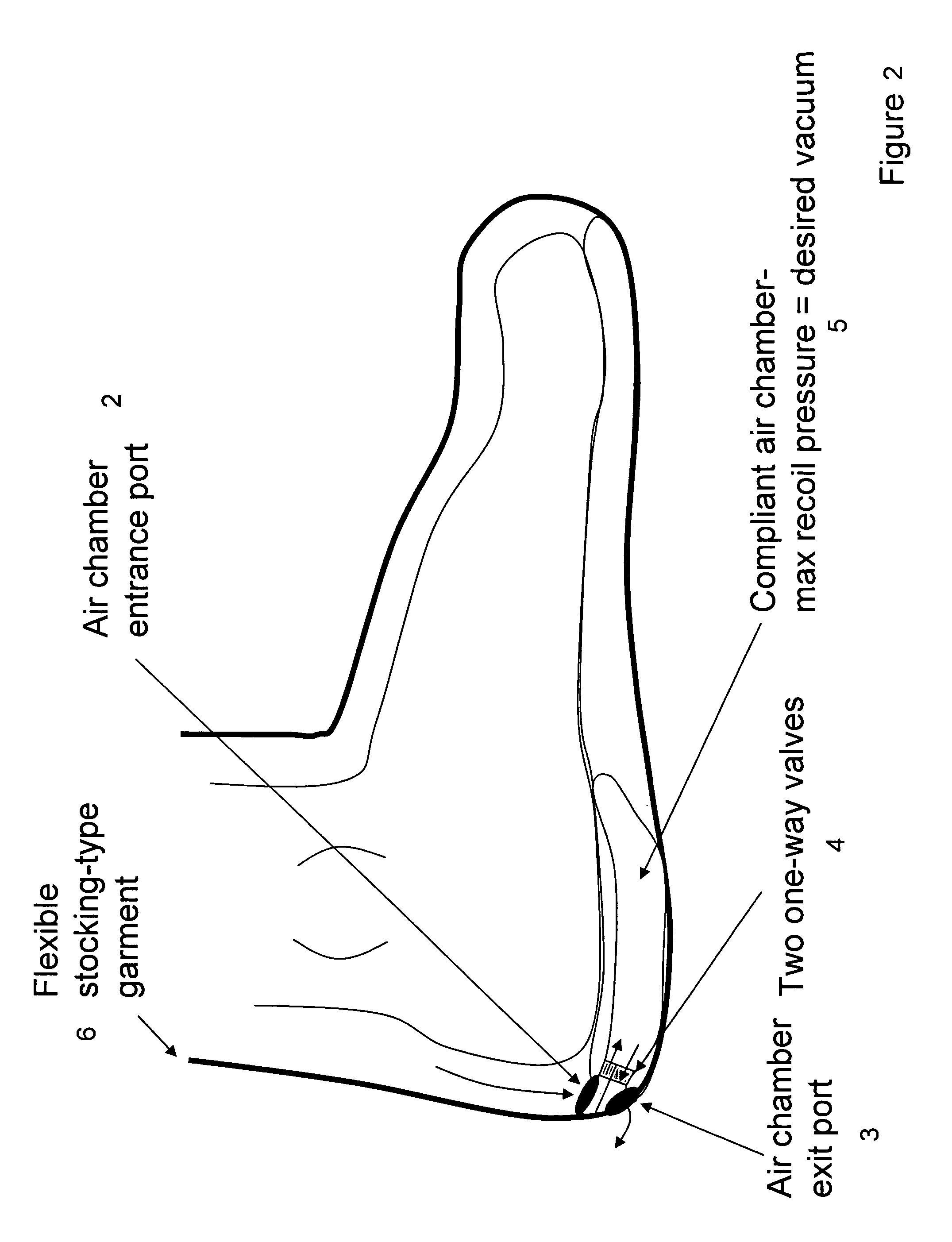
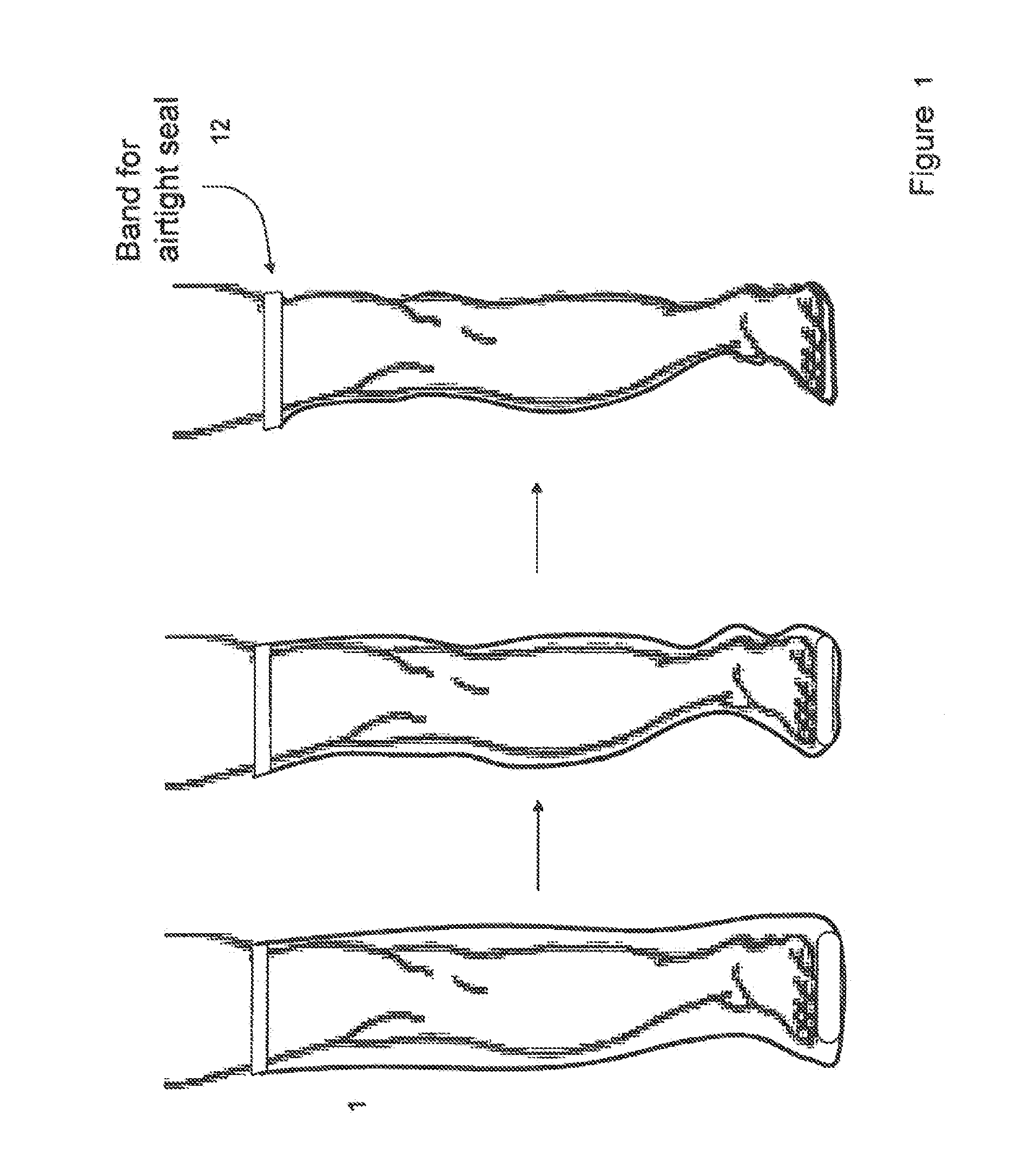
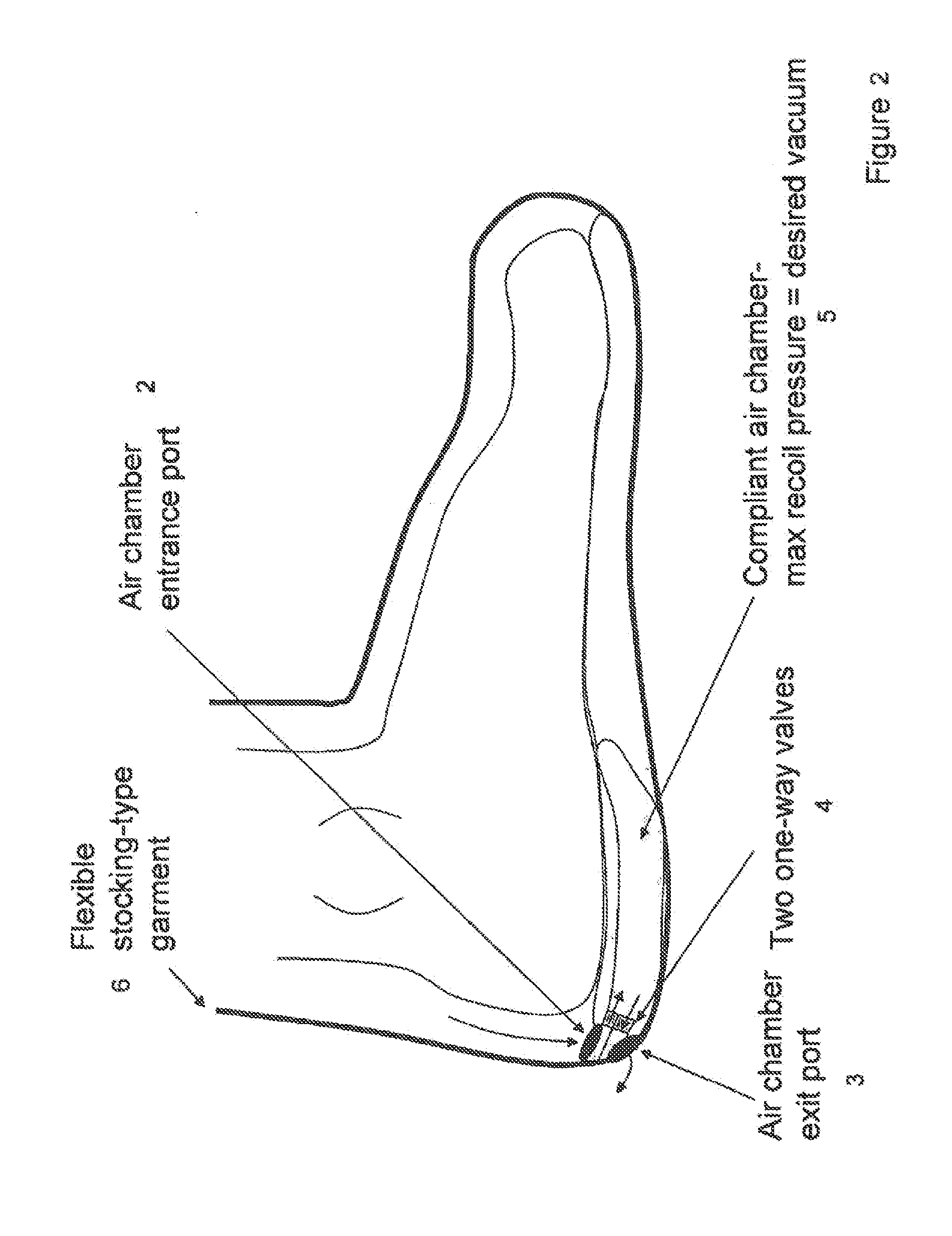
Devices that employ external compression stocking-type garments in the treatment of edema, chronic wounds, deep venous thrombosis prevention or claudication all share a number of significant limitations. These include the frequent need for custom fitting to assure an appropriate fit, vigilant maintenance to assure a continued “good fit,” limited compliance with proper use by patients and difficulty of application. There is a large body of evidence demonstrating that patients often decline to wear the compressive stockings as prescribed or in the form that would be most beneficial because they find these devices to be difficult to put on and take off.Building on the limitations of existing therapies, and distilled lessons learned from the field of prosthetics and wound healing, the present invention employs vacuum-assisted negative pressure to provide compression and help pump fluid from the tissues of affected limbs. The device is embodied in the form of a flexible stocking-like garment that will utilize a pumping mechanism to generate negative pressure around the limb and thus create vacuum compression that will mobilize fluid in a limb and increase venous return to the heart. Through the use of a circumferential wrap, the present invention provides a major advance in both the distribution of vacuum and the securing of the device over the limb.
Owner:THERANOVA LLC
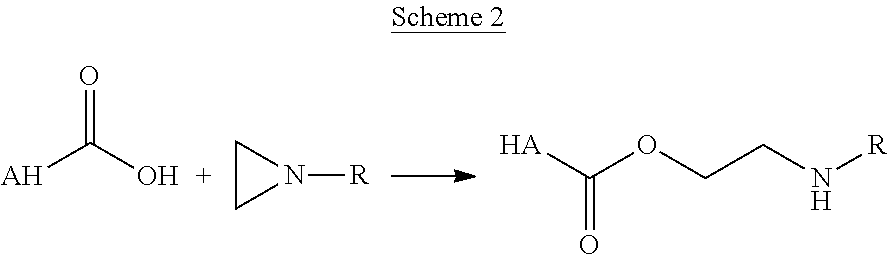
End-capped poly(ester amide) copolymers
Provided herein is an end-capped poly(ester amide) PEA) polymer and the method of making the polymer. The PEA polymer is substantially free of active amino end groups and / or activated carboxyl groups. The PEA polymer can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Blends of poly(ester amide) polymers
Provided herein is a poly(ester amide) (PEA) polymer blend and a polymeric coating containing the PEA polymer blend. The PEA polymer blend has a Tg above the Tg of poly(ester amide benzyl ester) (PEA-Bz) or the Tg of poly(ester amide TEMPO). The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Poly(ester amide) filler blends for modulation of coating properties
InactiveUS20060093842A1Improve stabilityIncrease drug release rateOrganic active ingredientsNervous disorderAbnormal tissue growthPEA polymer
Provided herein is a PEA polymer blend and coatings or implantable devices formed therefrom. The PEA polymer blend is formed of a PEA polymer and a material capable of hydrogen bonding with the PEA. The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
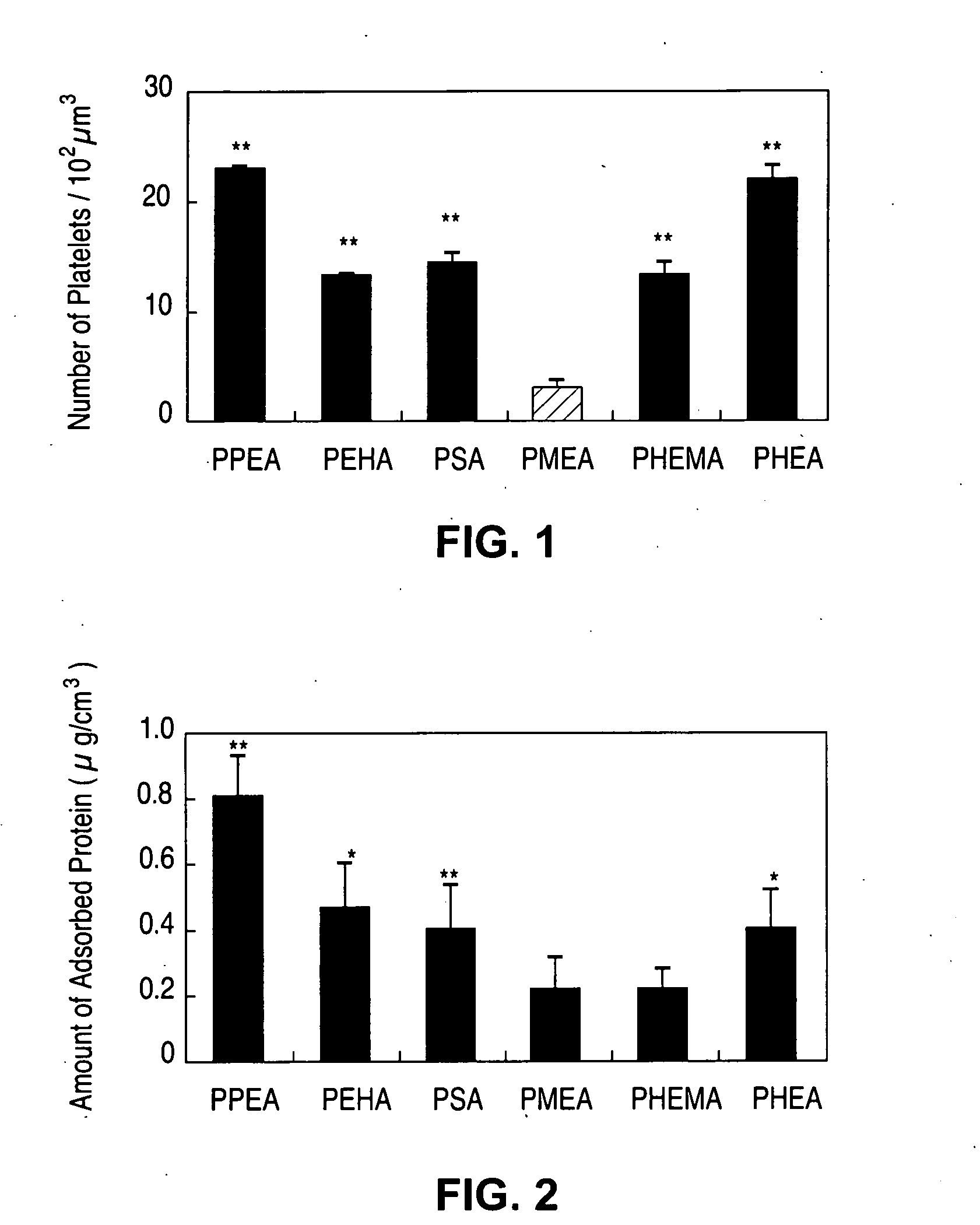
Implantable devices formed of non-fouling methacrylate or acrylate polymers
Implantable devices formed of or coated with a material that includes a polymer having a non-fouling acrylate or methacrylate polymer are provided. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
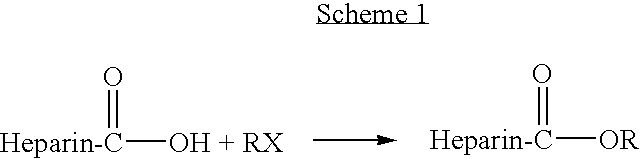
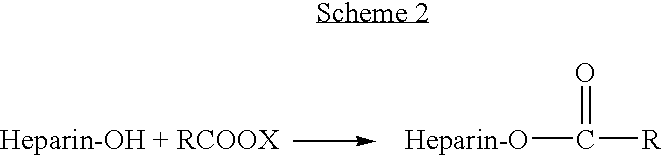
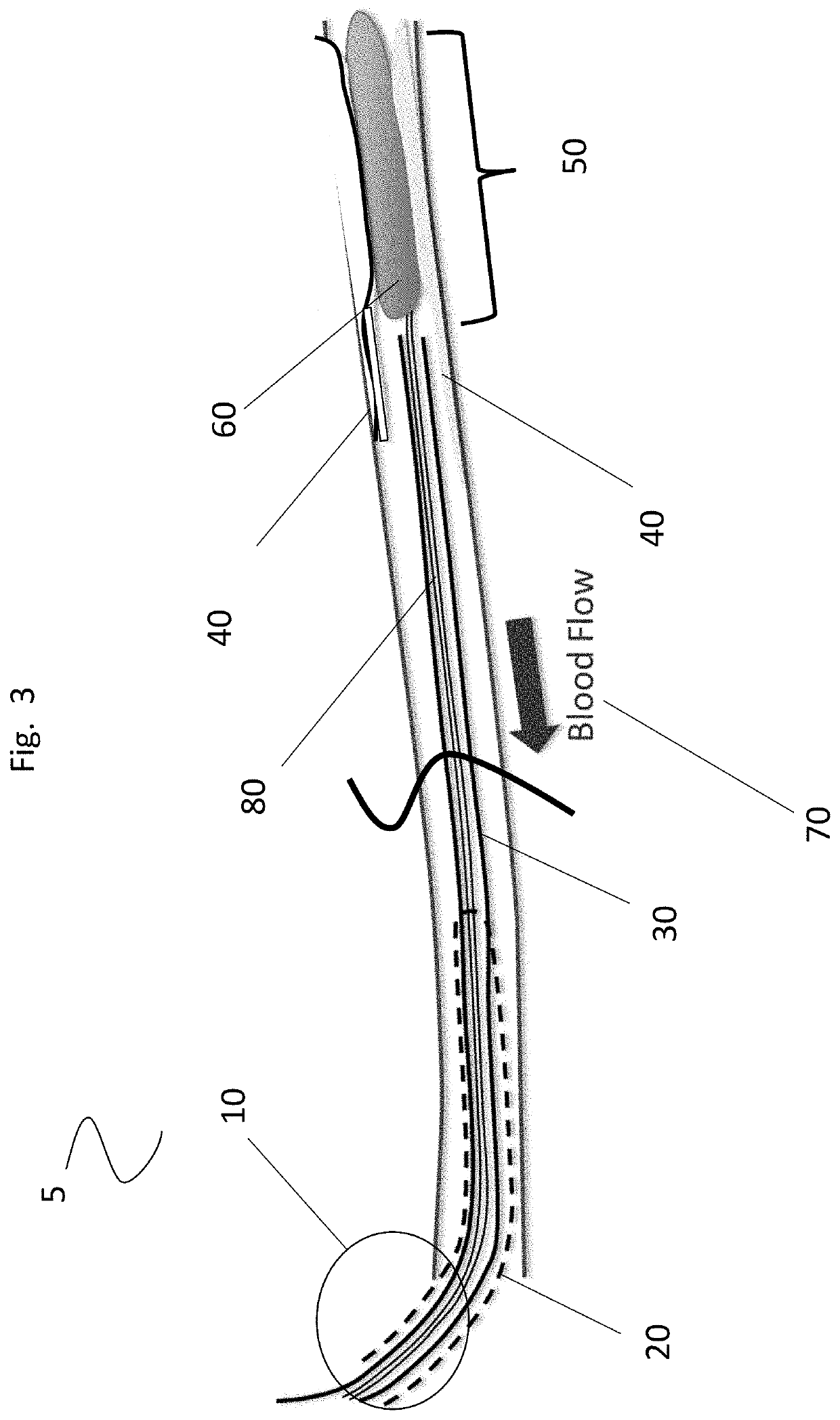
Heparin prodrugs and drug delivery stents formed therefrom
InactiveUS20060014720A1Organic active ingredientsPeptide/protein ingredientsDiseasePercent Diameter Stenosis
A prodrug comprising a heparin and a drug is provided. The prodrug can be used to form a coating on a medical device. The prodrug can also be used with a polymeric material to form a coating on a medical device. The polymeric material can be a hydrophobic polymer, a hydrophilic polymer, a non-fouling polymer, or combinations thereof. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
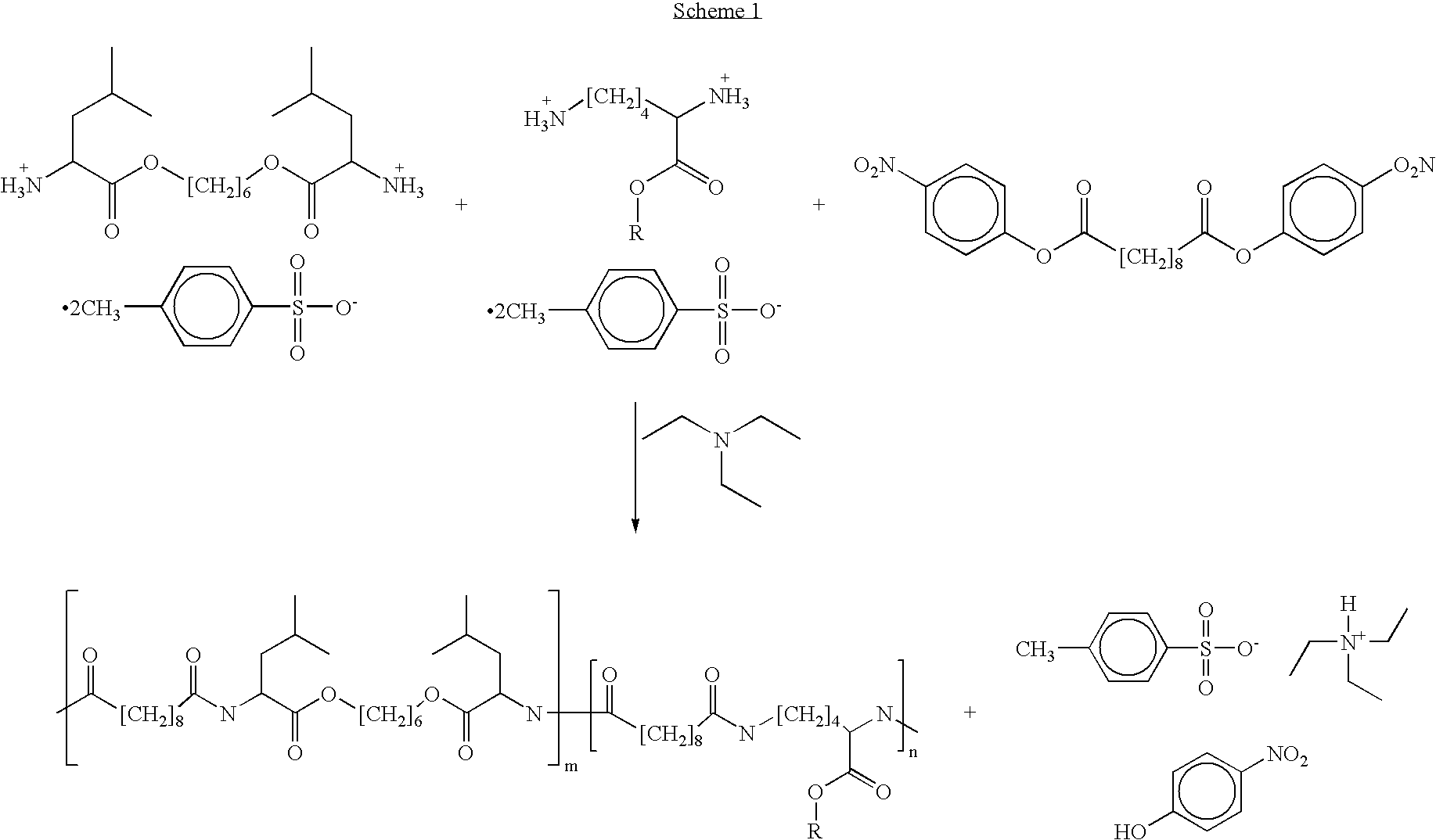
Poly(ester amide) block copolymers
Provided herein is a copolymer that includes a soft block (A) that contains poly(ester amide) (PEA) and a hard block (B). The copolymer can be any of AB, ABA or BAB type block copolymers. By varying the relative amount of the PEA block and the hard block, one can obtain a copolymer with a Tg for mechanical integrity in drug-delivery stent applications. The copolymer can be used alone or optionally in combination with a biobeneficial material and / or a biocompatible polymer to form an implantable device or a coating on an implantable device. When the copolymer is used in combination with a biobeneficial material, the copolymer and the biobeneficial material can be co-deposited or applied in sequence during the coating process. A coating formed of the copolymer may also include a bioactive agent. The implantable device can be implanted in a patient to treat, prevent, or ameliorate a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, and / or tumor obstruction.
Owner:ABBOTT CARDIOVASCULAR
Poly(ester amide) filler blends for modulation of coating properties
InactiveUS7390497B2Improved stability and drug release rate and mechanical characteristicOrganic active ingredientsNervous disorderPEA polymerDevice form
Provided herein is a PEA polymer blend and coatings or implantable devices formed therefrom. The PEA polymer blend is formed of a PEA polymer and a material capable of hydrogen bonding with the PEA. The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Poly(ester amide) block copolymers
Owner:ABBOTT CARDIOVASCULAR
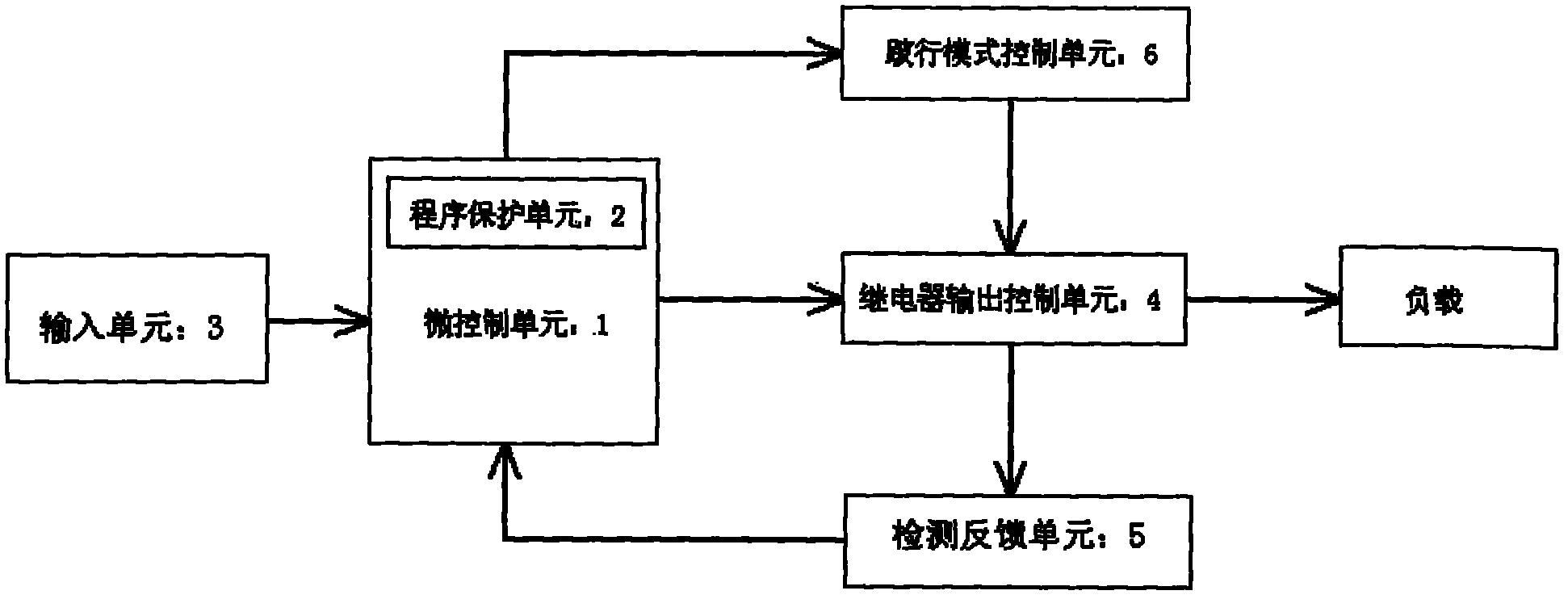
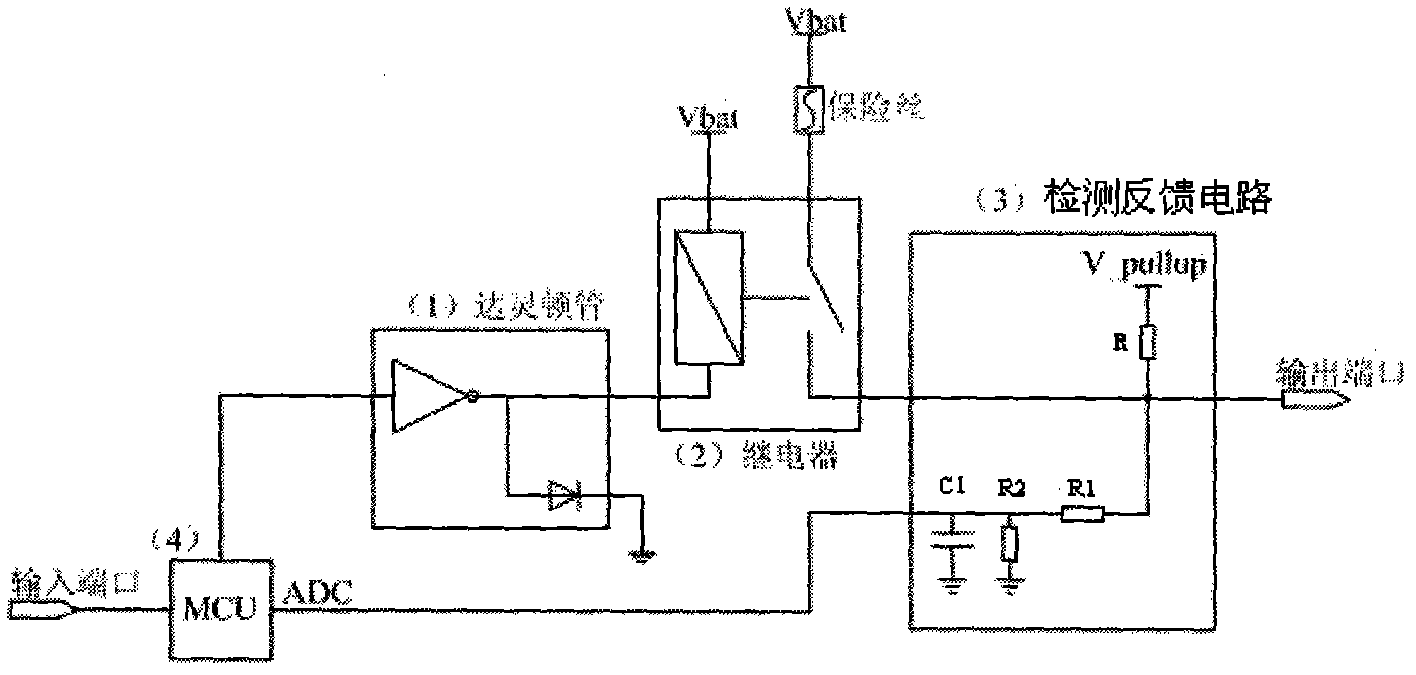
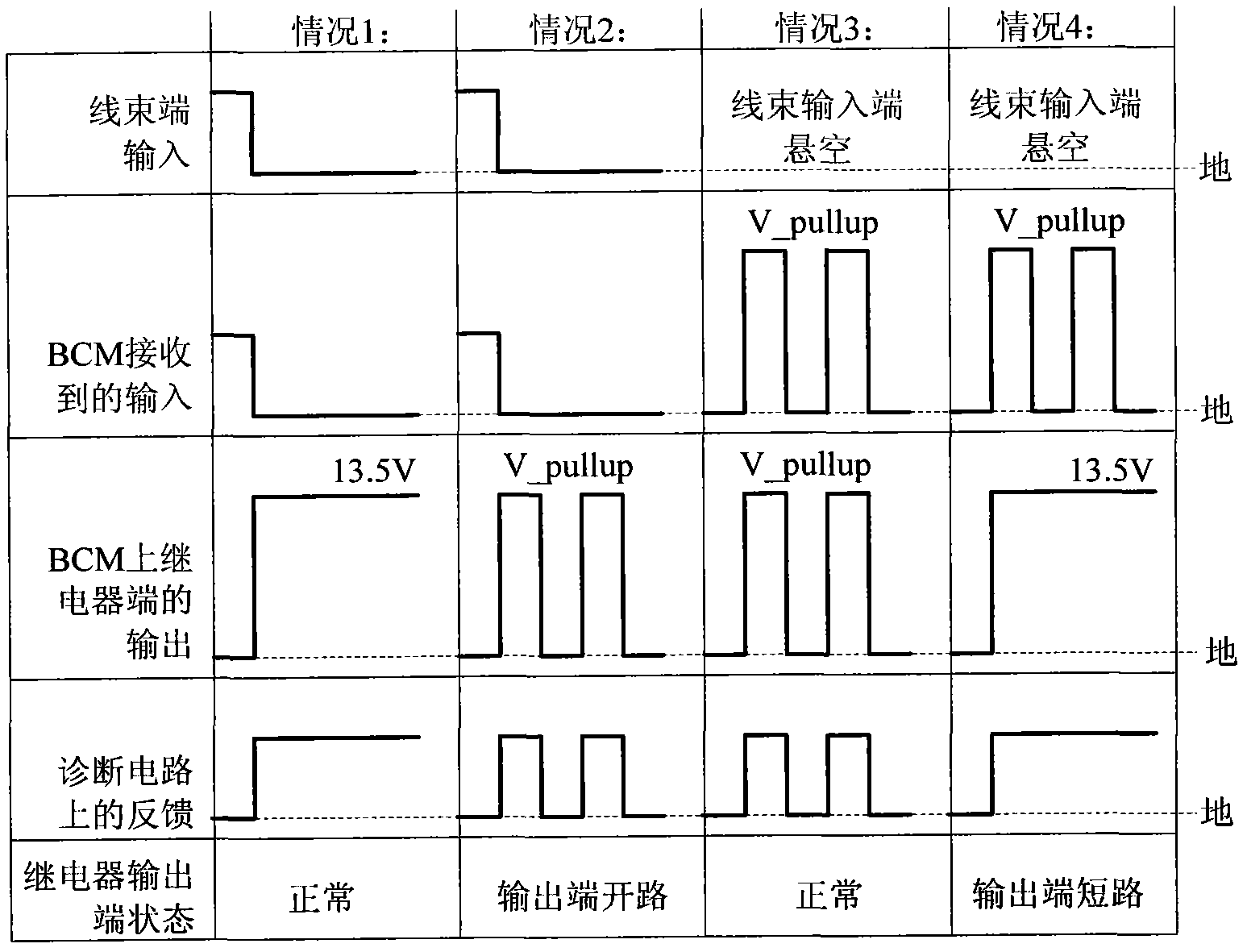
Automobile body control system
InactiveCN102063121AEasy to useImprove stabilityElectric testing/monitoringControl systemEngineering
The invention discloses an automobile body control system, comprising an input unit, a micro control unit and a claudication type control unit, wherein the input unit is used for receiving an input signal and transmitting the input signal to the micro control unit, the micro control unit is used for controlling a relay output control unit to drive a load to operate according to the input signal; and the claudication type control unit is used for controlling the relay output control unit to drive partial load to operate when the micro control unit is in failure. The automobile body control system provided by the invention is additionally provided with the claudication type control unit, and the relay output control unit is controlled to drive partial load to operate continuously when the micro control unit is in failure, thus ensuring normal use of the automobile body control system in a vehicle operating process and improving the stability of the automobile body control system.
Owner:CHERY AUTOMOBILE CO LTD
Beneficial effects of increasing local blood flow
ActiveUS20130289059A1Increase oxygenationImprove tissue nutritionBiocidePeptide/protein ingredientsArginineInjury mouth
The present invention generally relates to the improvement of tissue health by increasing local blood flow. In some aspects of the invention, increased local blood flow is effected by the transdermal delivery of the nitric oxide precursor L-arginine and / or its derivatives alone, or optionally in conjunction with an adjunct such as theophylline. The transdermal delivery is effected, in certain embodiments through the means of a hostile biophysical environment, such as that created by a high ionic strength environment. Various pathological states caused by, or occurring in conjunction with, insufficient blood flow, can be treated using the systems and methods of the invention as described herein. In other embodiments, increased blood flow using the systems and methods of the invention may result in enhanced healing, for example, through greater availability of the constituents of the blood. Examples of conditions which may benefit from increased blood flow include, but are not limited to, erectile dysfunction, hair loss, female sexual dissatisfaction, sagging facial or other body tissue, peripheral vascular disease including claudication, neuropathy, skin ulcers, bone healing, wound healing, viral and bacterial infection, and skin grafting.
Owner:STRATEGIC SCI & TECH
Heparin prodrugs and drug delivery stents formed therefrom
A prodrug comprising a heparin and a drug is provided. The prodrug can be used to form a coating on a medical device. The prodrug can also be used with a polymeric material to form a coating on a medical device. The polymeric material can be a hydrophobic polymer, a hydrophilic polymer, a non-fouling polymer, or combinations thereof. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Implantable devices formed of non-fouling methacrylate or acrylate polymers
Implantable devices formed of or coated with a material that includes a polymer having a non-fouling acrylate or methacrylate polymer are provided. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Polymers containing siloxane monomers
Owner:ABBOTT CARDIOVASCULAR
Beneficial effects of increasing local blood flow
ActiveUS9226909B2Increase ionic strengthReduce deliveryBiocidePeptide/protein ingredientsArginineInjury mouth
The present invention generally relates to the improvement of tissue health by increasing local blood flow. In some aspects of the invention, increased local blood flow is effected by the transdermal delivery of the nitric oxide precursor L-arginine and / or its derivatives alone, or optionally in conjunction with an adjunct such as theophylline. The transdermal delivery is effected, in certain embodiments through the means of a hostile biophysical environment, such as that created by a high ionic strength environment. Various pathological states caused by, or occurring in conjunction with, insufficient blood flow, can be treated using the systems and methods of the invention as described herein. In other embodiments, increased blood flow using the systems and methods of the invention may result in enhanced healing, for example, through greater availability of the constituents of the blood. Examples of conditions which may benefit from increased blood flow include, but are not limited to, erectile dysfunction, hair loss, female sexual dissatisfaction, sagging facial or other body tissue, peripheral vascular disease including claudication, neuropathy, skin ulcers, bone healing, wound healing, viral and bacterial infection, and skin grafting.
Owner:STRATEGIC SCI & TECH
Method and apparatus for negative pressure therapy
InactiveUS20110295168A1Easy to returnReduce edemaBlood stagnation preventionPneumatic massageVacuum assistedVenous thrombosis
Devices that employ external compression stocking-type garments in the treatment of edema, chronic wounds, deep venous thrombosis prevention or claudication all share a number of significant limitations. These include the frequent need for custom fitting to assure an appropriate fit, vigilant maintenance to assure a continued “good fit,” limited compliance with proper use by patients and difficulty of application. There is a large body of evidence demonstrating that patients often decline to wear the compressive stockings as prescribed or in the form that would be most beneficial because they find these devices to be difficult to put on and take off. Building on the limitations of existing therapies, and distilled lessons learned from the field of prosthetics and wound healing, the present invention employs vacuum-assisted negative pressure to provide compression and help pump fluid from the tissues of affected limbs. The device is embodied in the form of a flexible stocking-like garment that will utilize a pumping mechanism to generate negative pressure around the limb and thus create vacuum compression that will mobilize fluid in a limb and increase venous return to the heart. Through the use of a circumferential wrap, the present invention provides a major advance in both the distribution of vacuum and the securing of the device over the limb.
Owner:THERANOVA LLC
System and method for treatment of the claudification of the superficial femoral and proximal popliteal artery
PendingUS20200171282A1Restoration of proper blood flowSafe removalBalloon catheterExcision instrumentsArteria femoralisSuperior genicular arteries
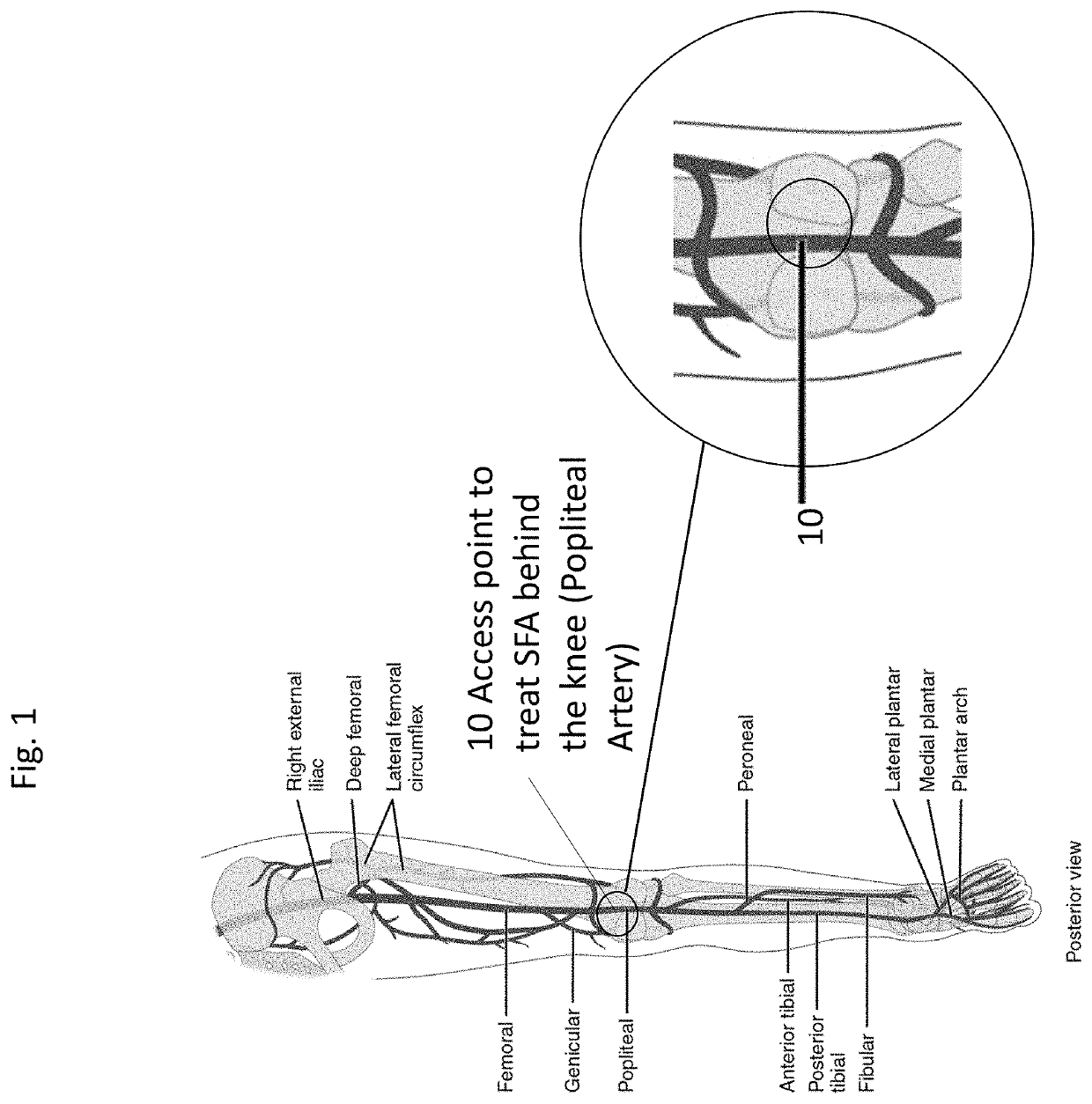
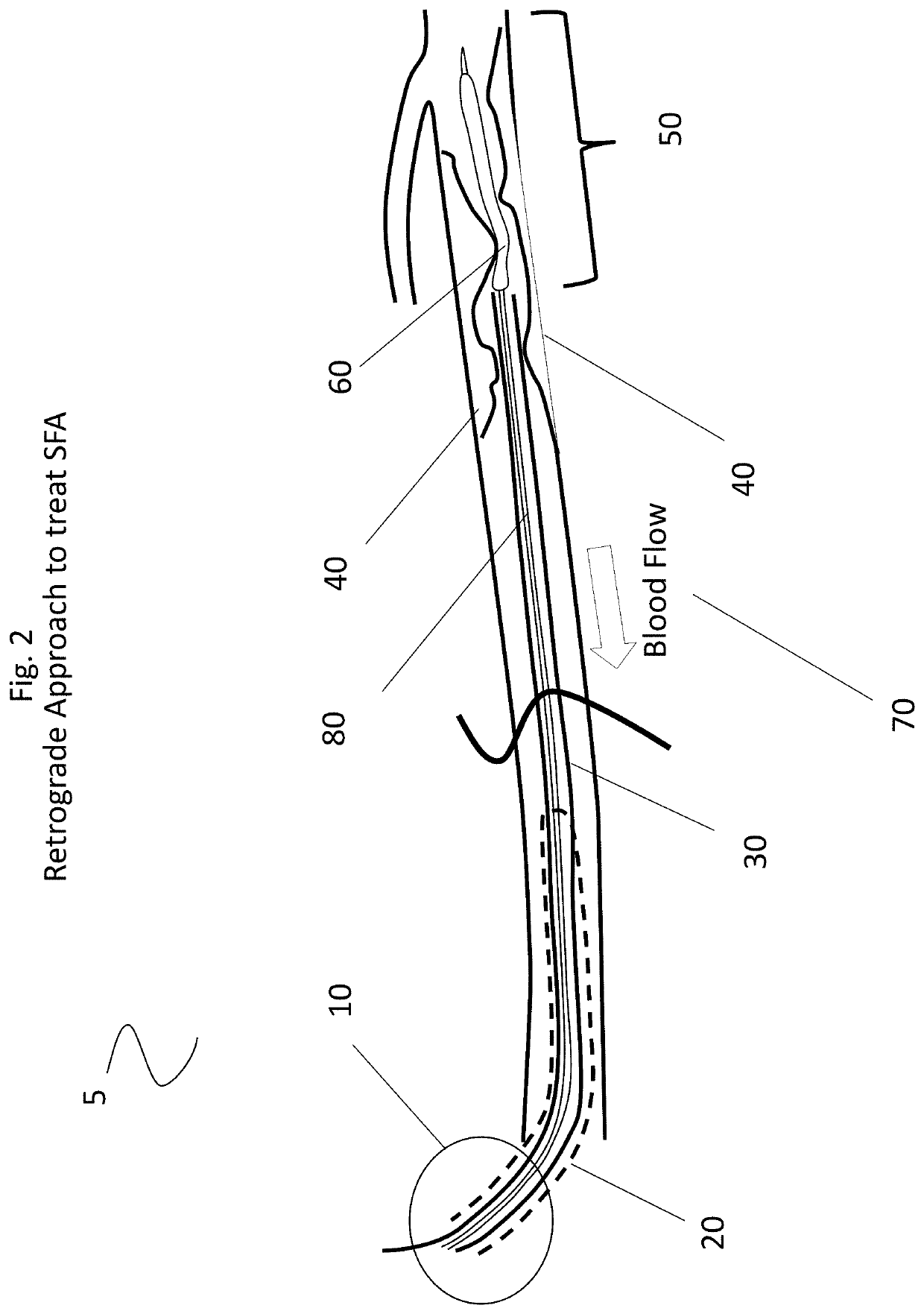
A system and accompanying methods for treating a blockage of the superior femoral artery including utilization of a puncture insertion mechanism, an introducer sheath, a guide catheter and an expandable device. Entry into the body is established through the popliteal and advancing retrograde through the popliteal artery toward the lower superior femoral artery region and in some embodiments utilizing an introducer sheath, a guide catheter and a balloon are featured.
Owner:MICRO MEDICAL SOLUTIONS INC
Antifouling heparin coatings
Owner:ABBOTT CARDIOVASCULAR
Polymers of fluorinated monomers and hydrocarbon monomers
InactiveUS7604818B2Provide mechanical strengthGive flexibilityStentsFibre treatmentPolymer scienceActive agent
A polymer of fluorinated monomers and hydrocarbon monomers is provided. It is also provided a polymer blend that contains a polymer formed of fluorinated monomers and hydrocarbon monomers and another biocompatible polymer. The polymer or polymer blend described herein and optionally a bioactive agent can form an implantable device such as a stent or a coating on an implantable device such as a drug-delivery stent, which can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Methacrylate copolymers for medical devices
A polymer of hydrophobic monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains the polymer and another biocompatible polymer. The polymer or polymer blend and optionally a biobeneficial material and / or a bioactive agent can form a coating on an implantable device such as a drug delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Traditional Chinese medicine preparation for treating rheumatism and rheumatoid and preparation method thereof
InactiveCN105288544AEnhance immune functionIncrease blood flowAntipyreticAnalgesicsRheumatismArtemisia carvifolia
The invention relates to a traditional Chinese medicine preparation for treating rheumatism and rheumatoid and a preparation method thereof. The traditional Chinese medicine preparation is prepared from the following traditional Chinese medicine raw materials of lysimachia foenum-graecum, lemongrass, scandent schefflera stem and leaf, Artemisia carvifolia, fresh ginger, beautiful millettia root, Semiliquidambar cathayensis, garden balsam stem, Caulis Spatholobi, Cudrania cochinchinensis, Parabarium micranthum, philippine flemingia root, folium artemisiae argyi, radix clematidis, lucid ganoderma, astragalus, Uncaria rhynchonphylla and Kadsura coccinea. The medicine can be used for effectively treating rheumatism and rheumatoid diseases, has an excellent curative effect on low back and leg pain, numbness of limb, intermittent claudication and bone pain caused by lumbar disc herniation.
Owner:GUANGXI CHUNLIANG BIOTECH CO LTD
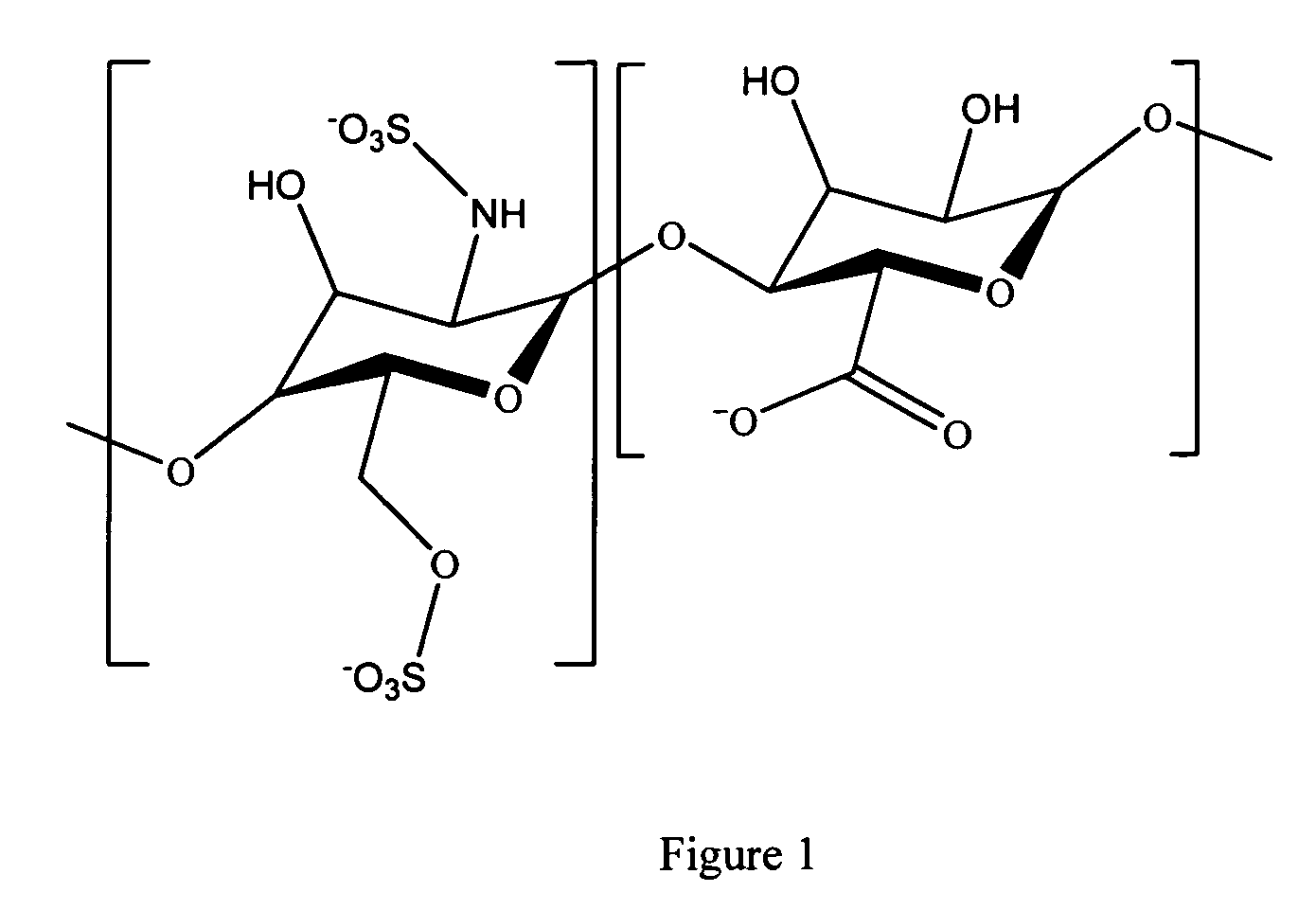
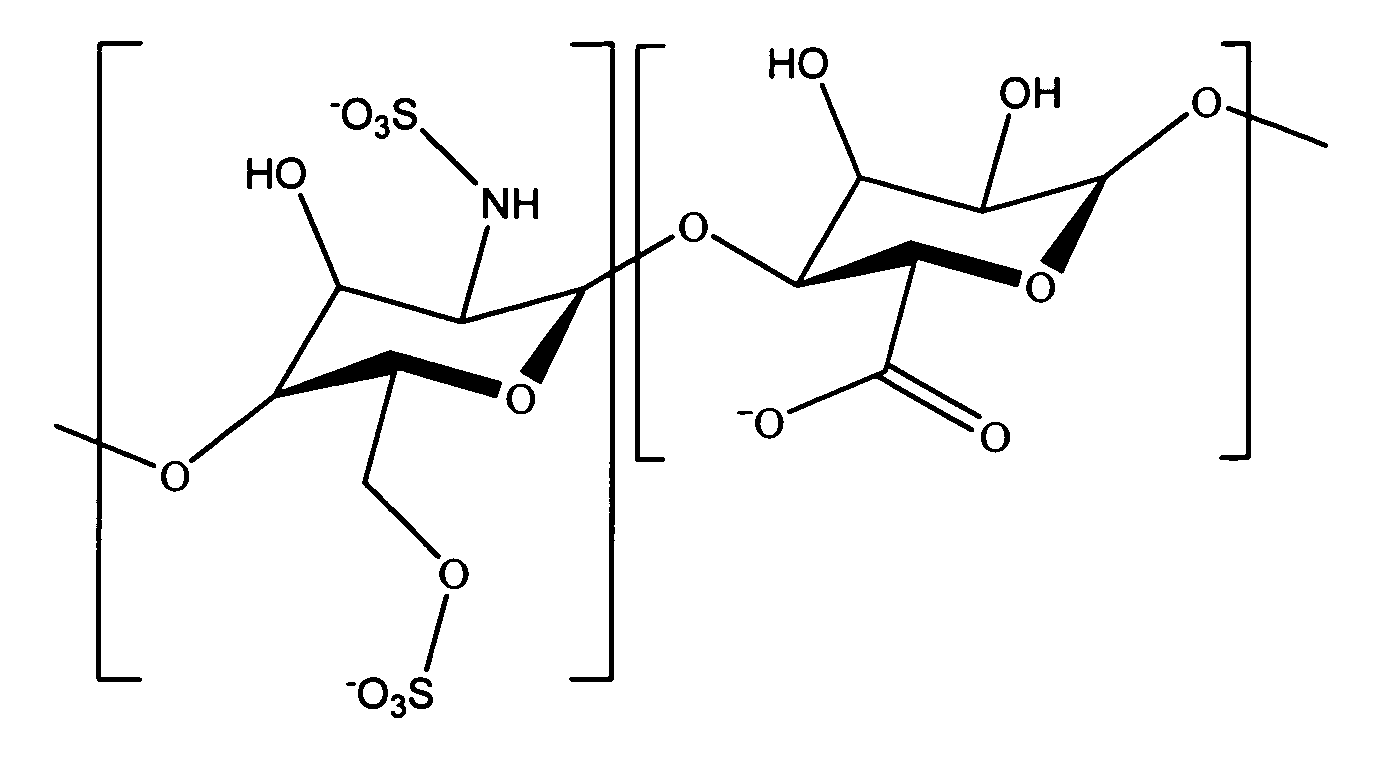
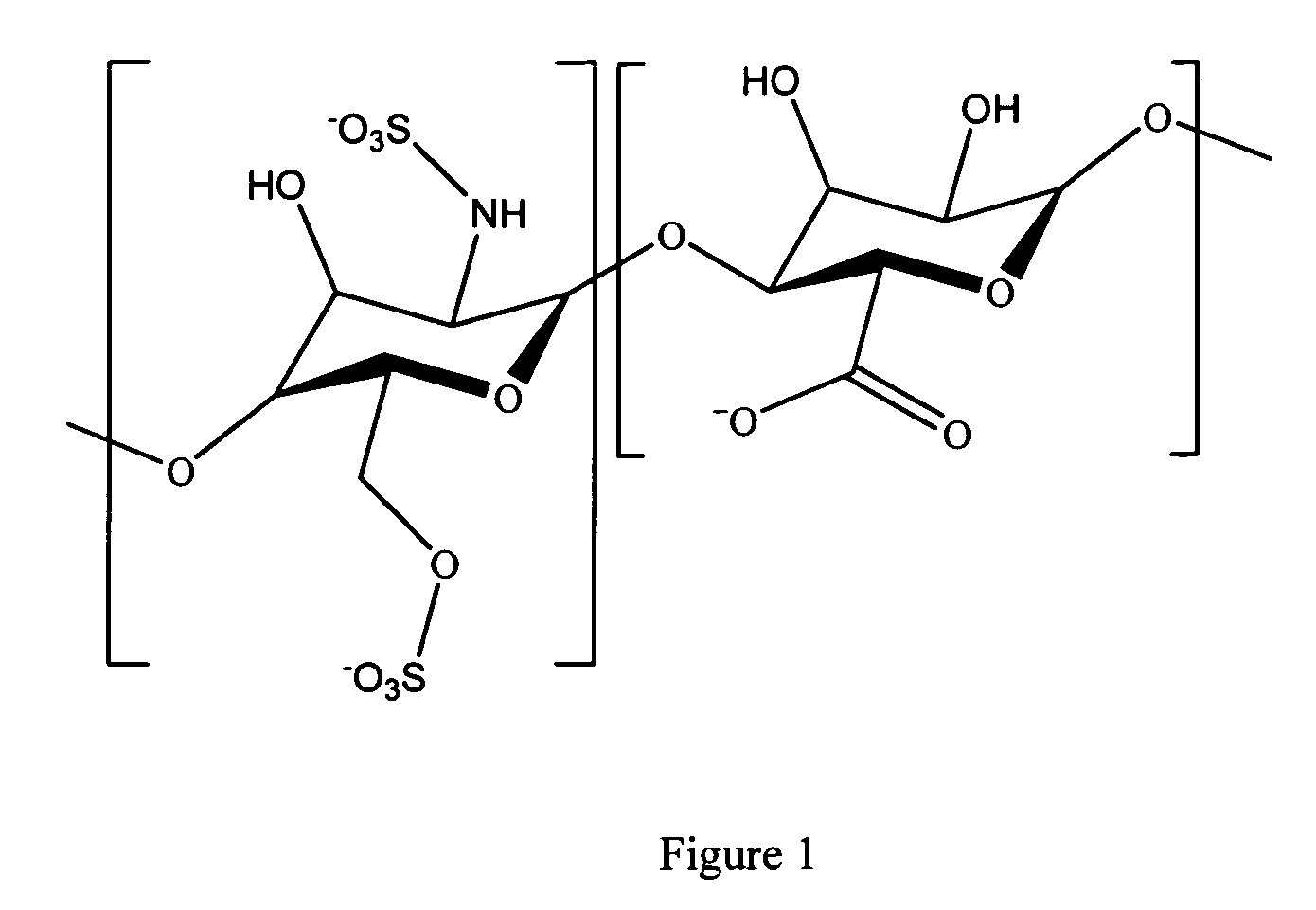
Hyaluronic acid based copolymers
Owner:ABBOTT CARDIOVASCULAR
Chinese medicine composition orally taken for curing cervical vertebra osteoproliferation
InactiveCN101181597AAnthropod material medical ingredientsSkeletal disorderOsteoproliferationHeadaches
The invention discloses a Chinese traditional medicine combination which is internally used for curing cervical vertebra hyperosteogeny, belonging to the field of traditional Chinese medicine. The invention is characterized in that the active ingredients of the traditional Chinese medicine combination are prepared by the following raw materials by weight: 15g prepared rhizome of rehmannia, 12g of dogwood, 15g of medlars, 10g of dodder, 30g of radix salviae miltiorrhizae, 21g of leatherleaf milletia, 12g of rhizoma corydalis, 21g of garden balsam stems, 12g curcuma, 10g of pangolin and 12g of earthworm. When preparing the medicine, the impurities in the raw materials are removed, and the materials are decocted with water, then the medicine is taken one dose and twice per day, morning and night. The invention can achieve the effects for dissipating blood stasis, removing obstruction in collaterals, relieving pain and eliminating swelling, fundamentally restraining hyperosteogeny, effectively promoting blood circulation, and repairing local damage of tissue, thereby achieving the aim of eliminating a series of symptoms such as dizziness, headache, myasthenia of limbs, interim claudication, arms and shoulders acroanesthesia, pain in waist, legs, feet and heel, and the symptom that the patients can not take care of themselves caused by dedifferentiation such as hyperosteogeny, etc.
Owner:胡玉翠
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com