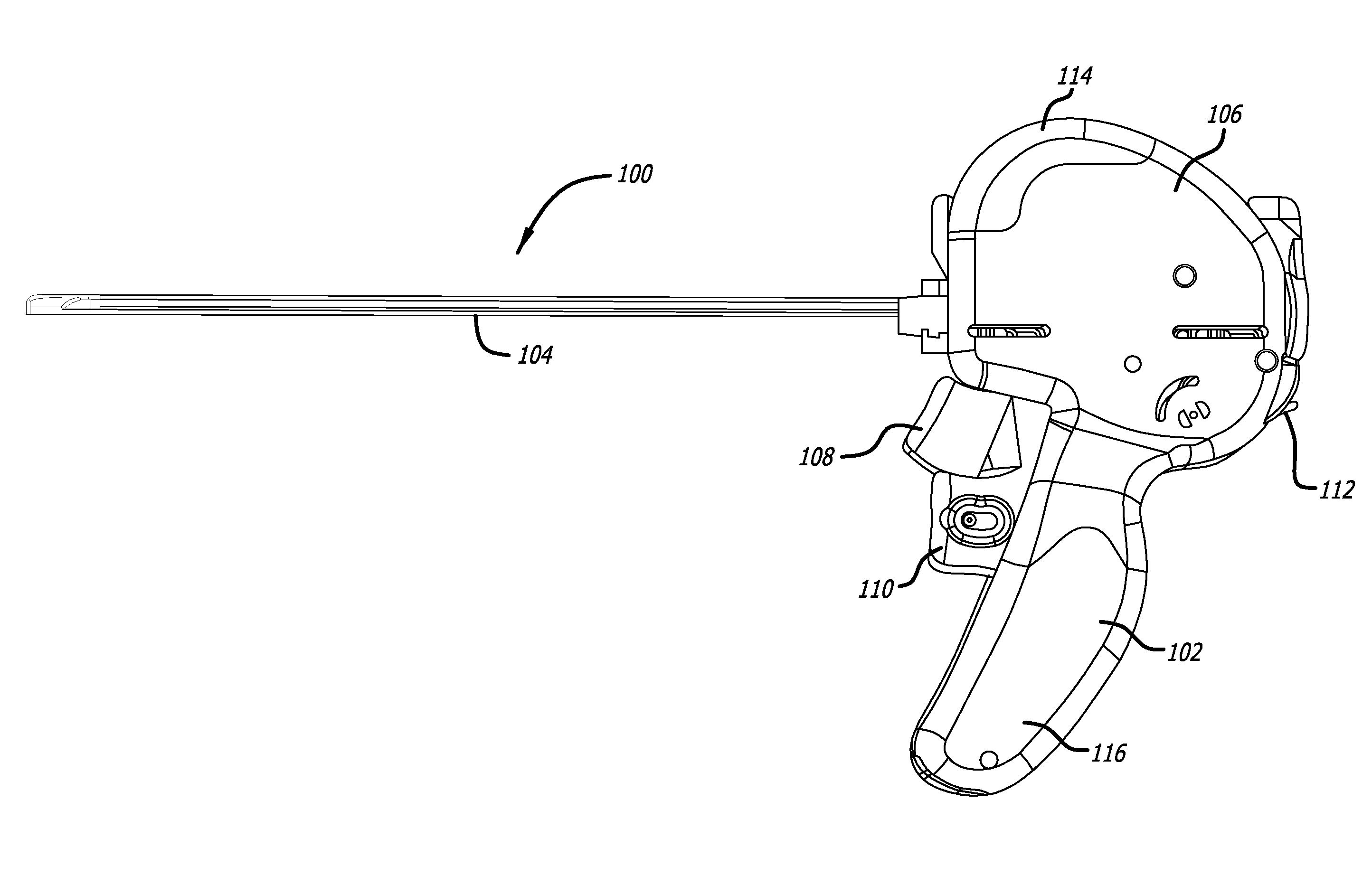
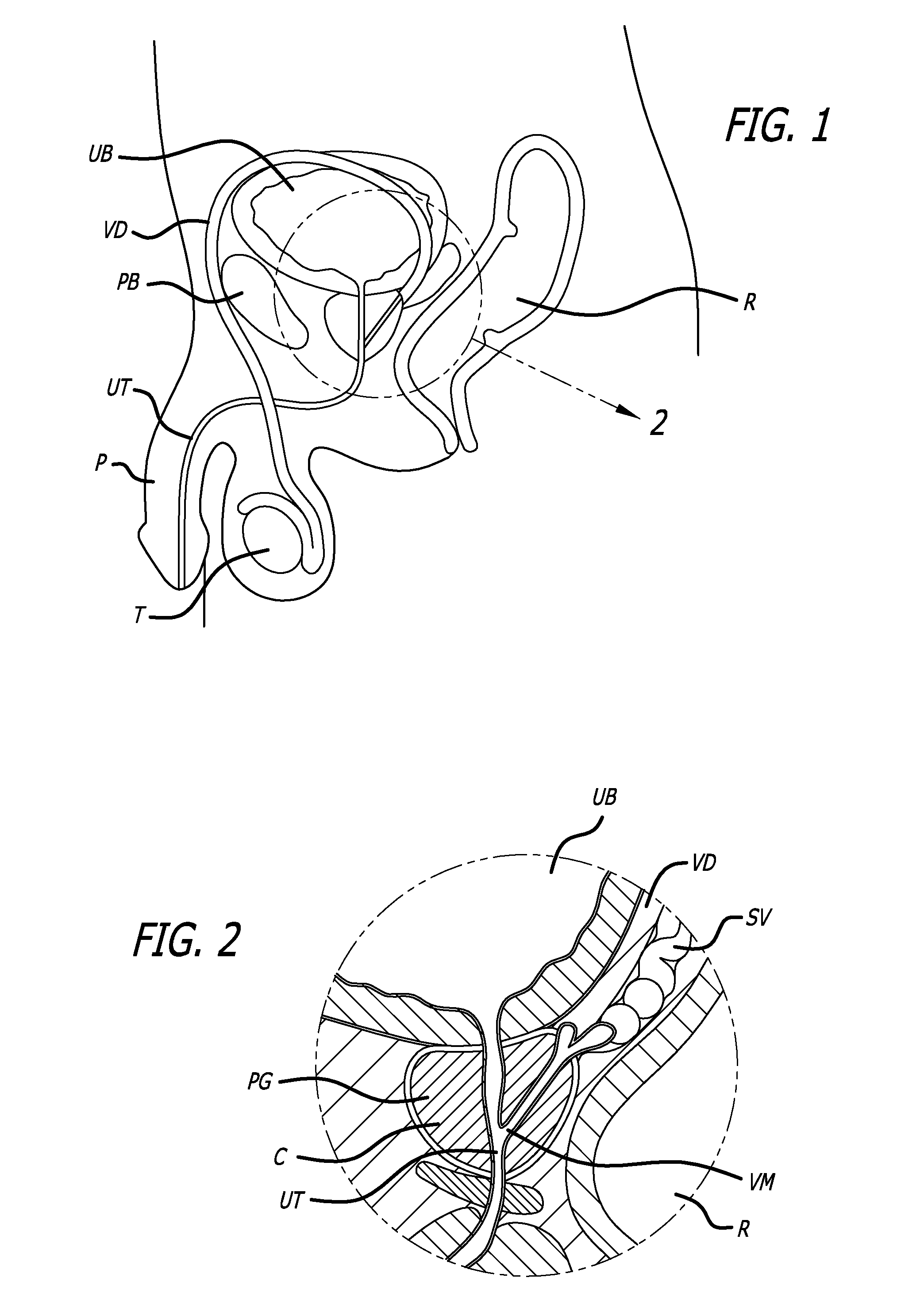
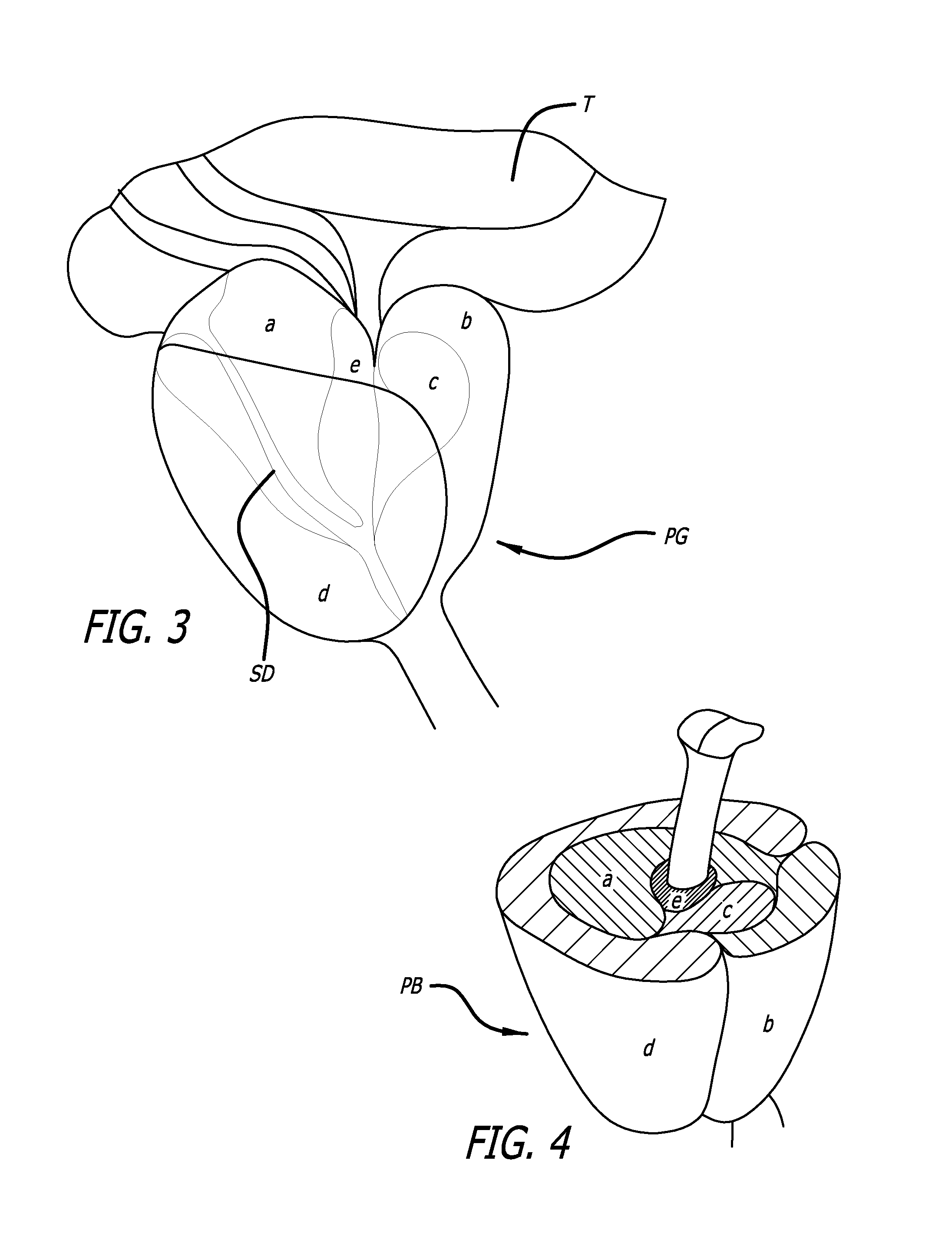
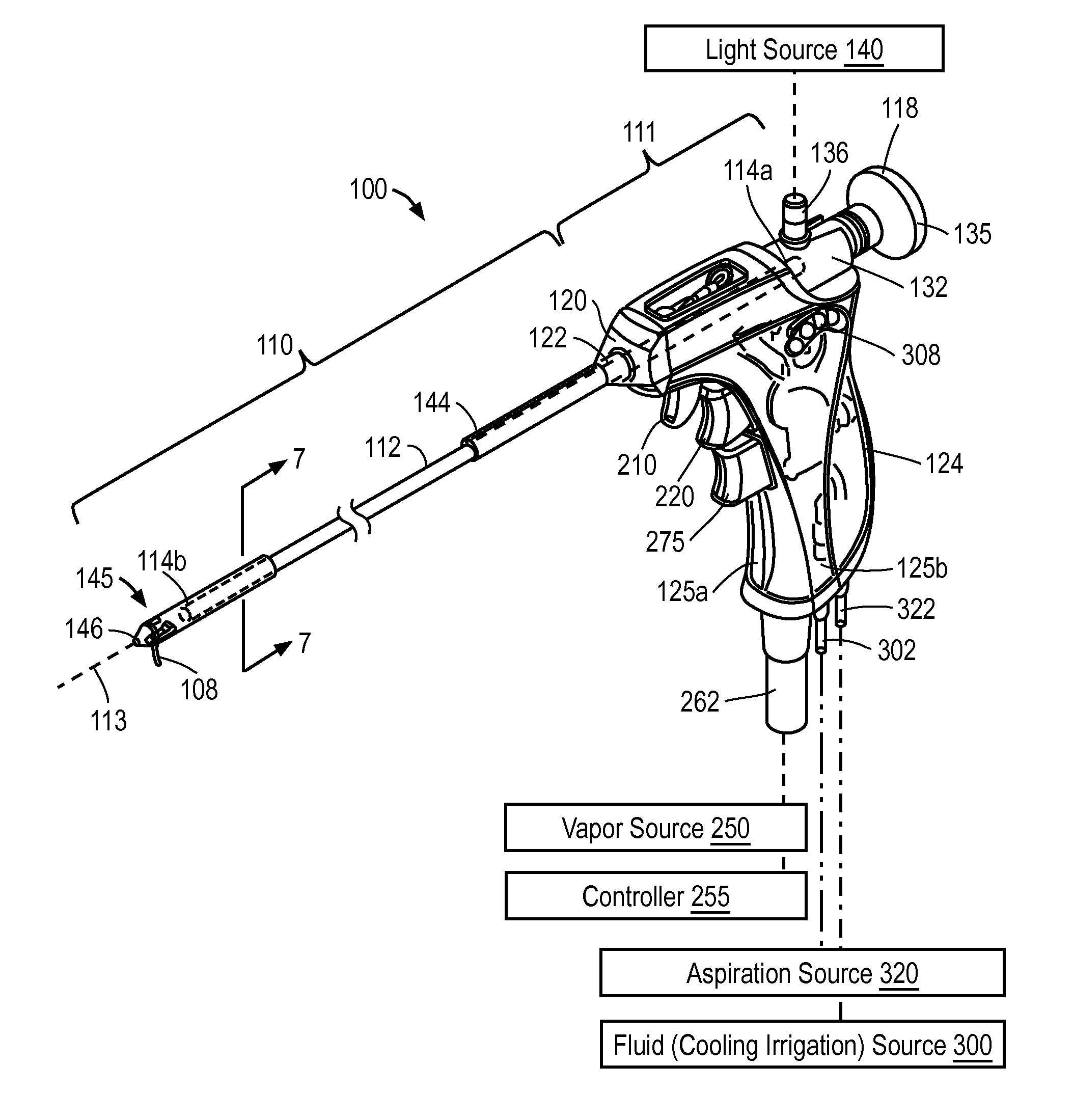
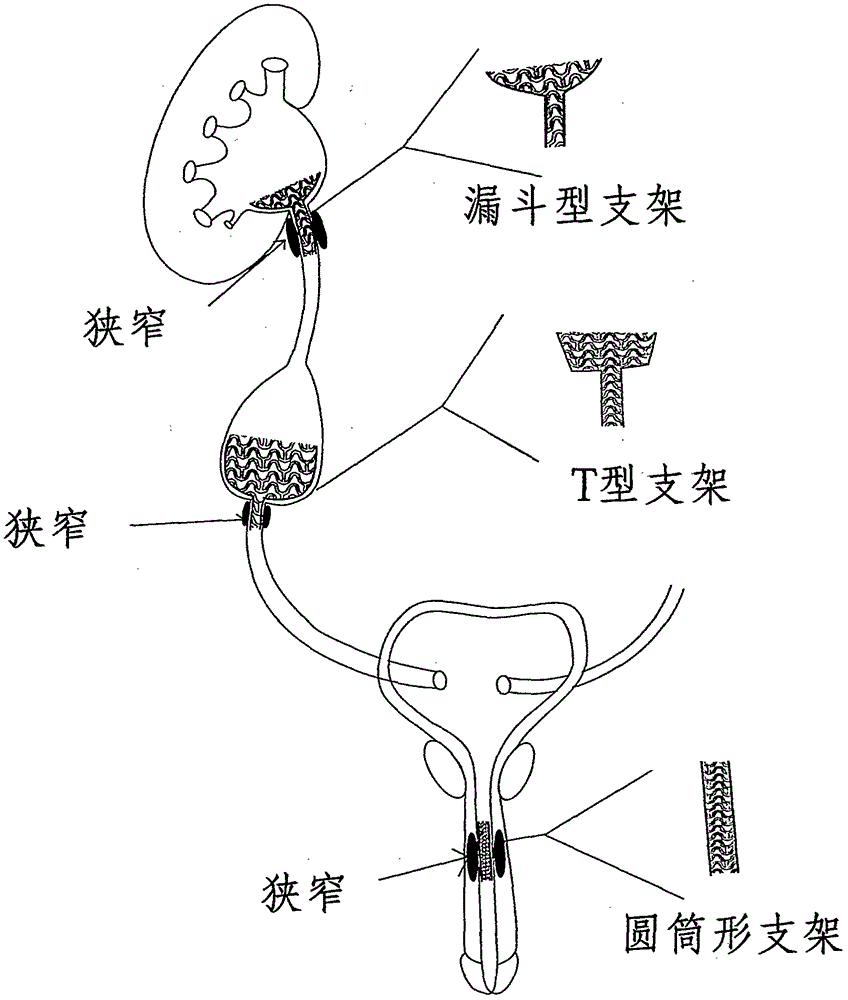
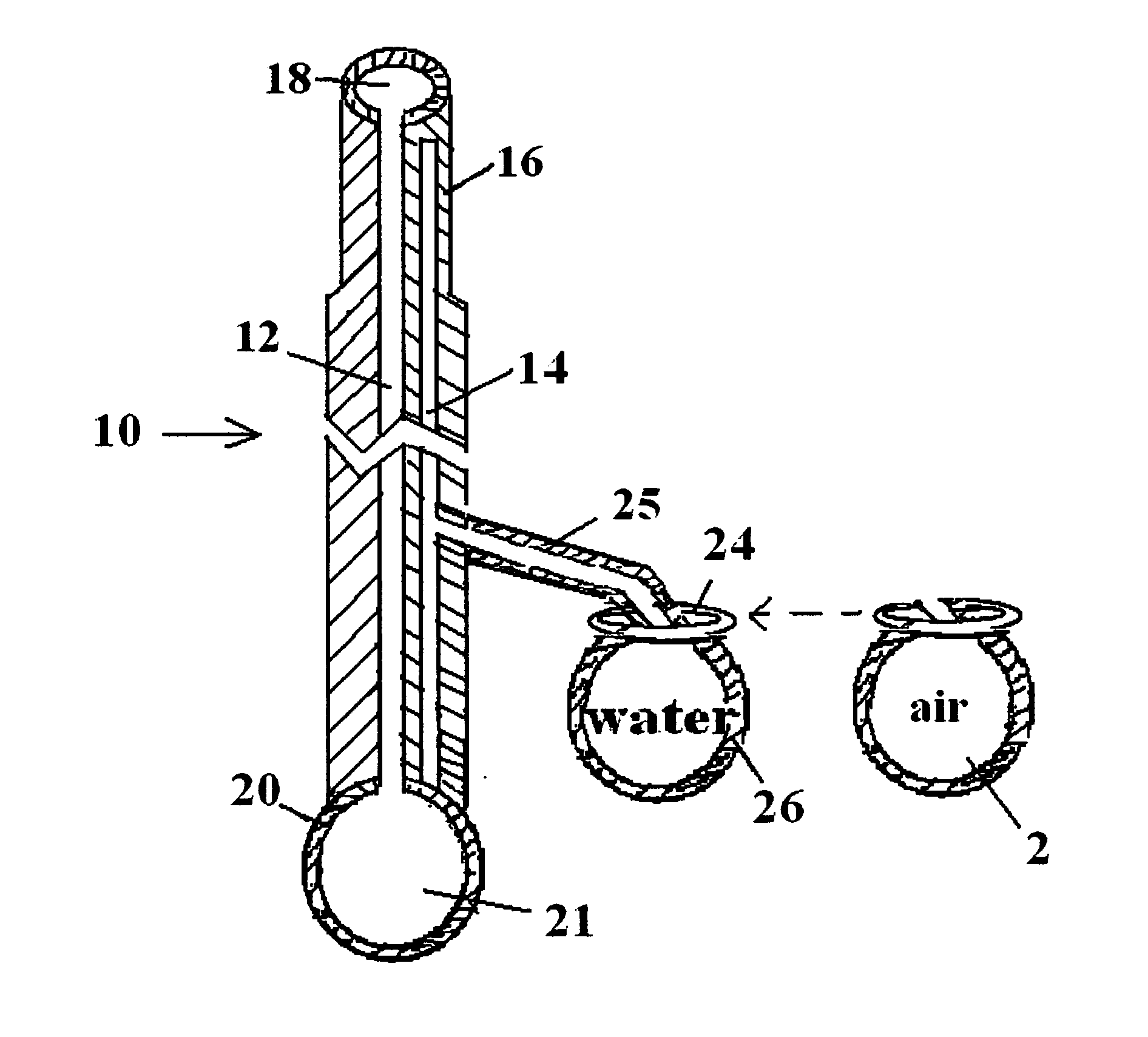
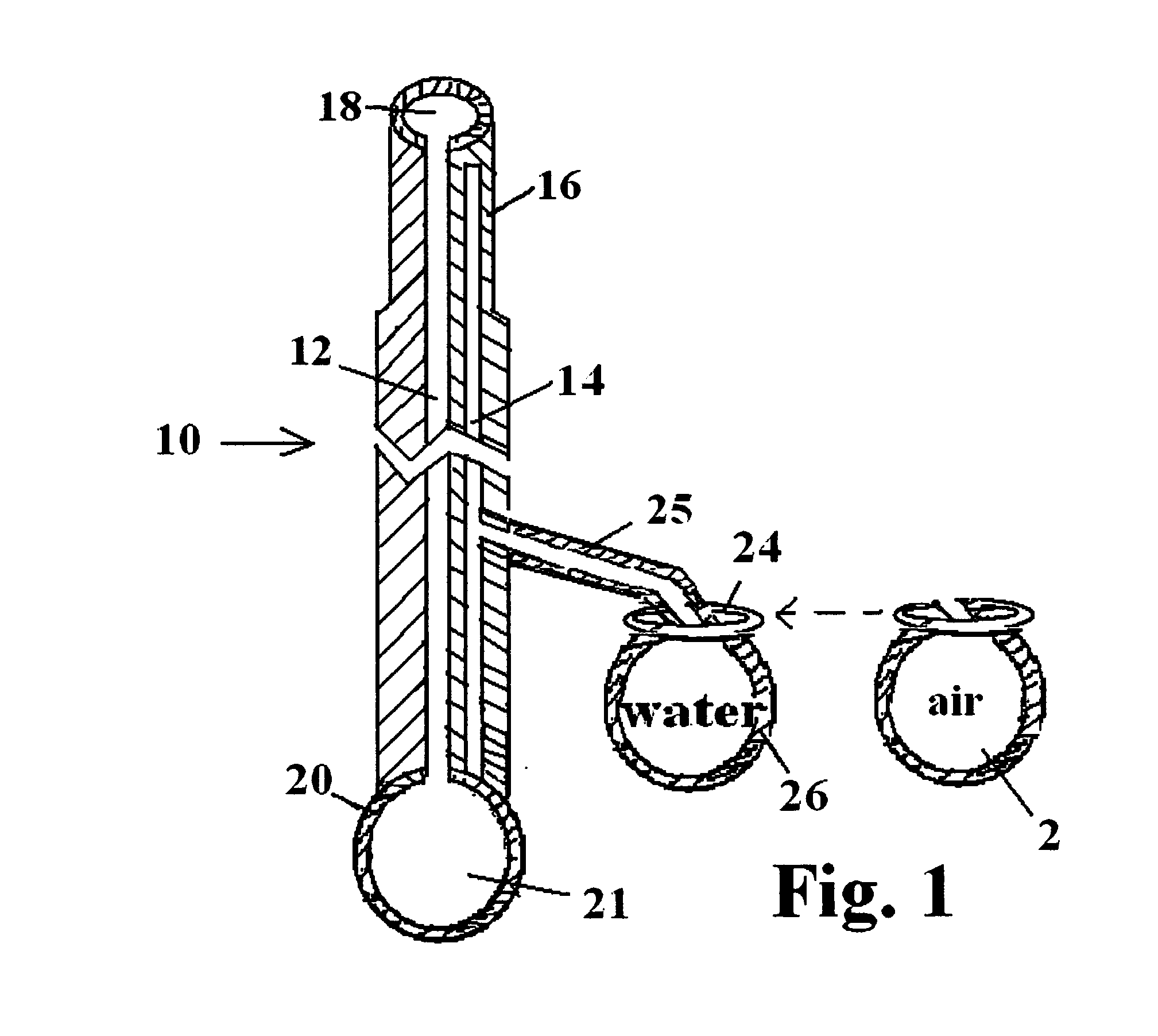
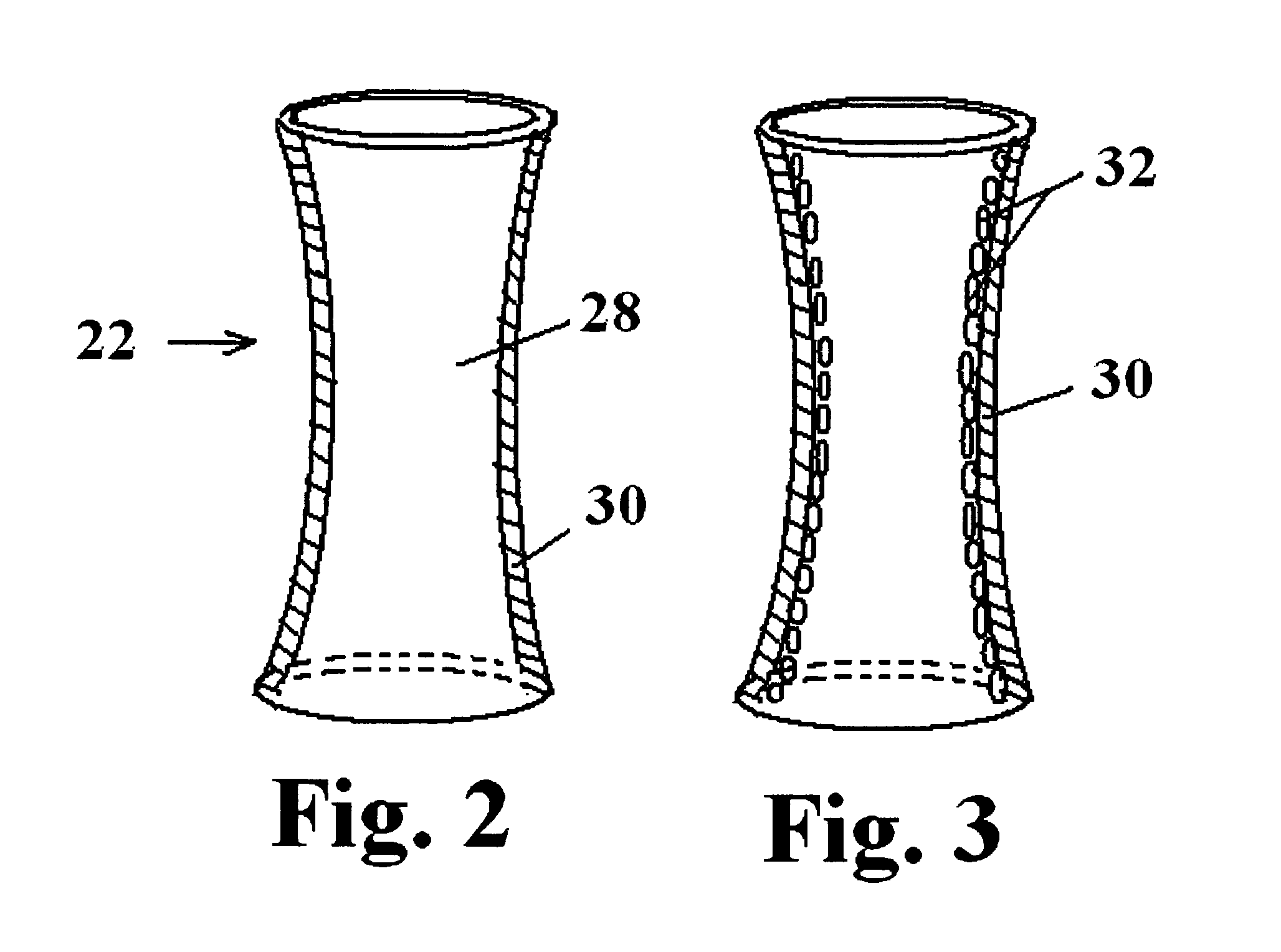
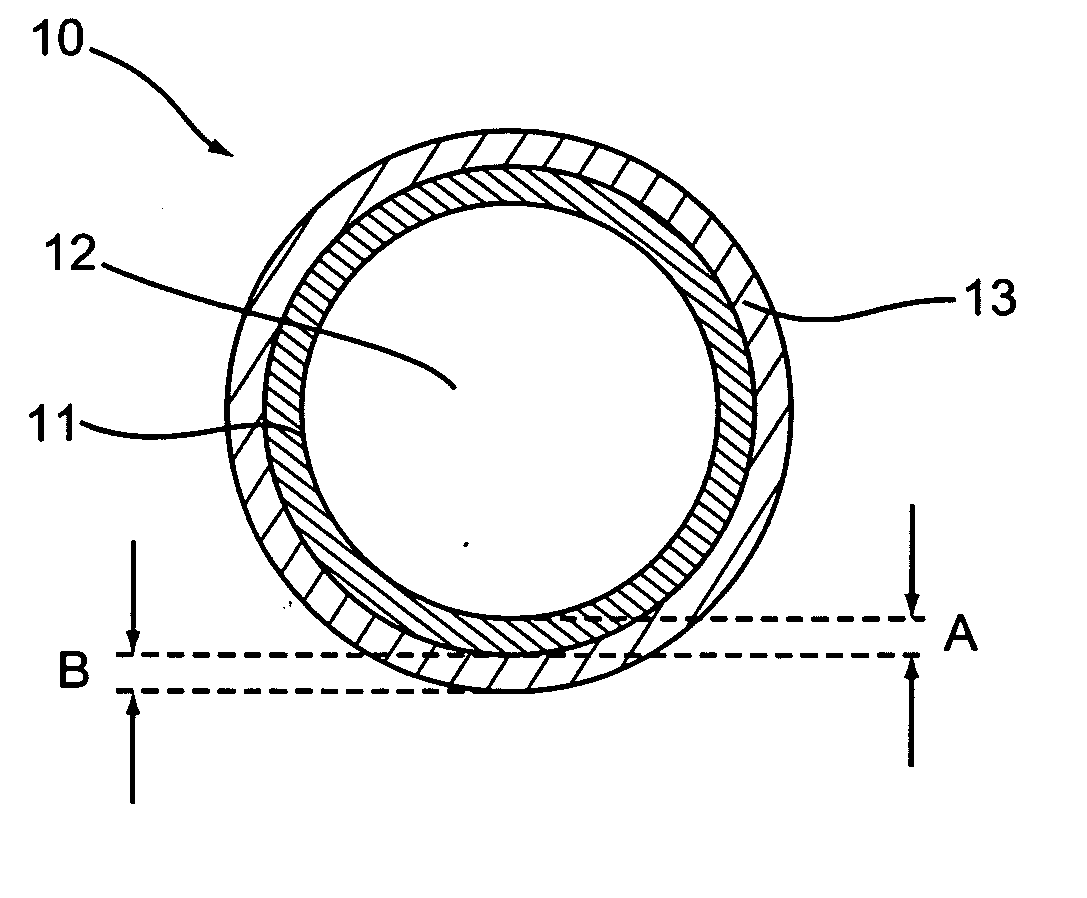
Patents
Literature
176results about "Urethrae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
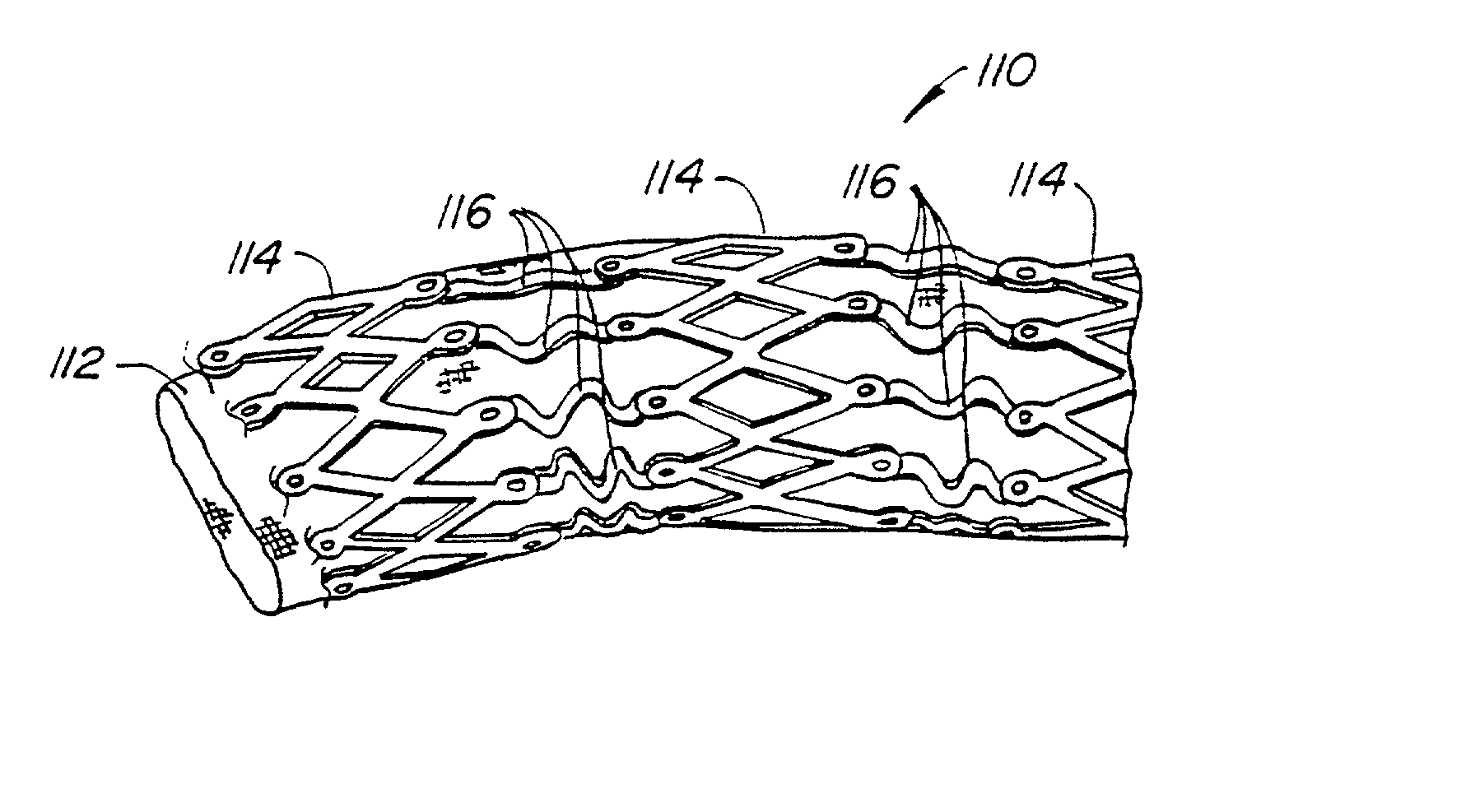
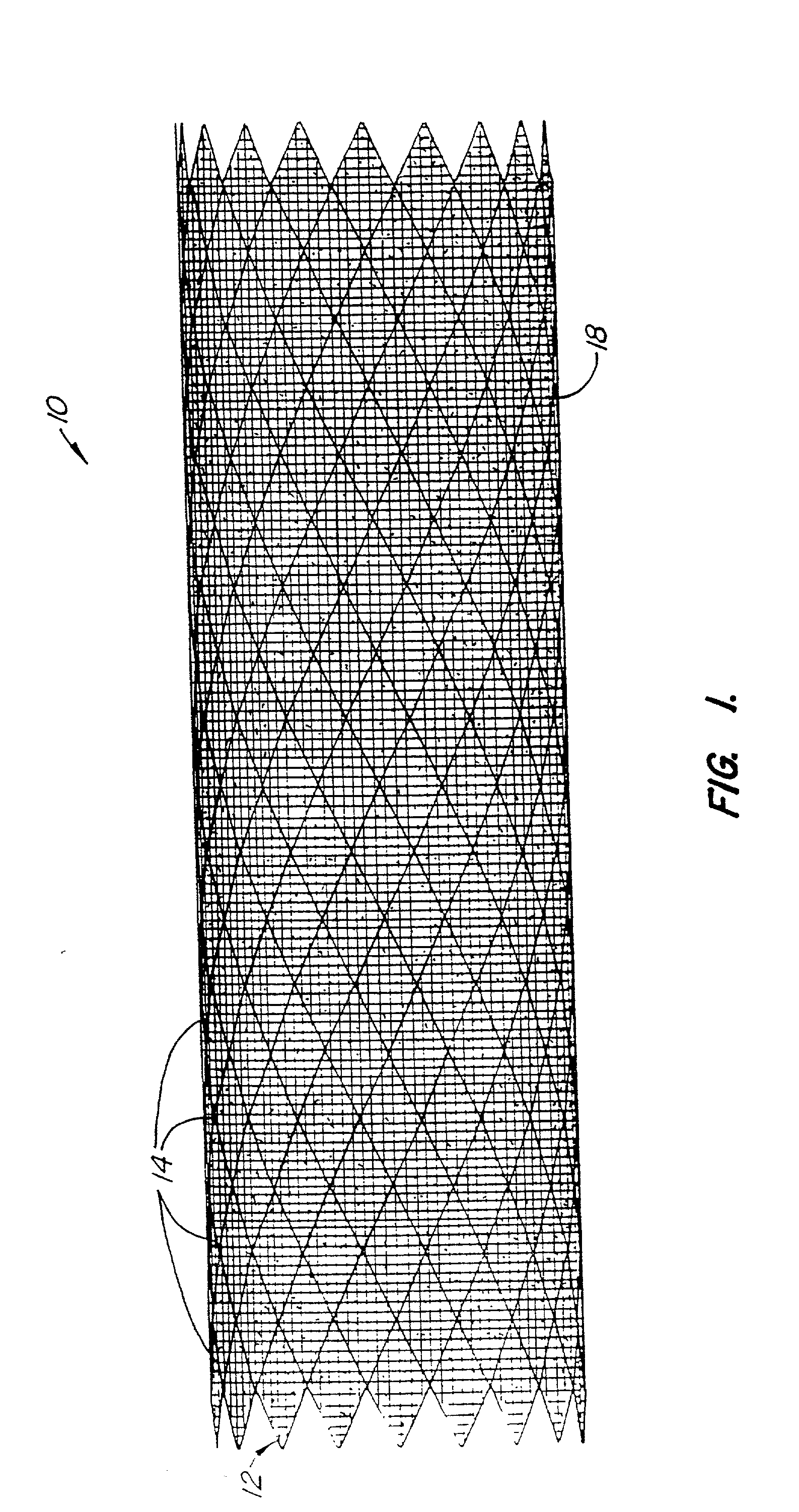
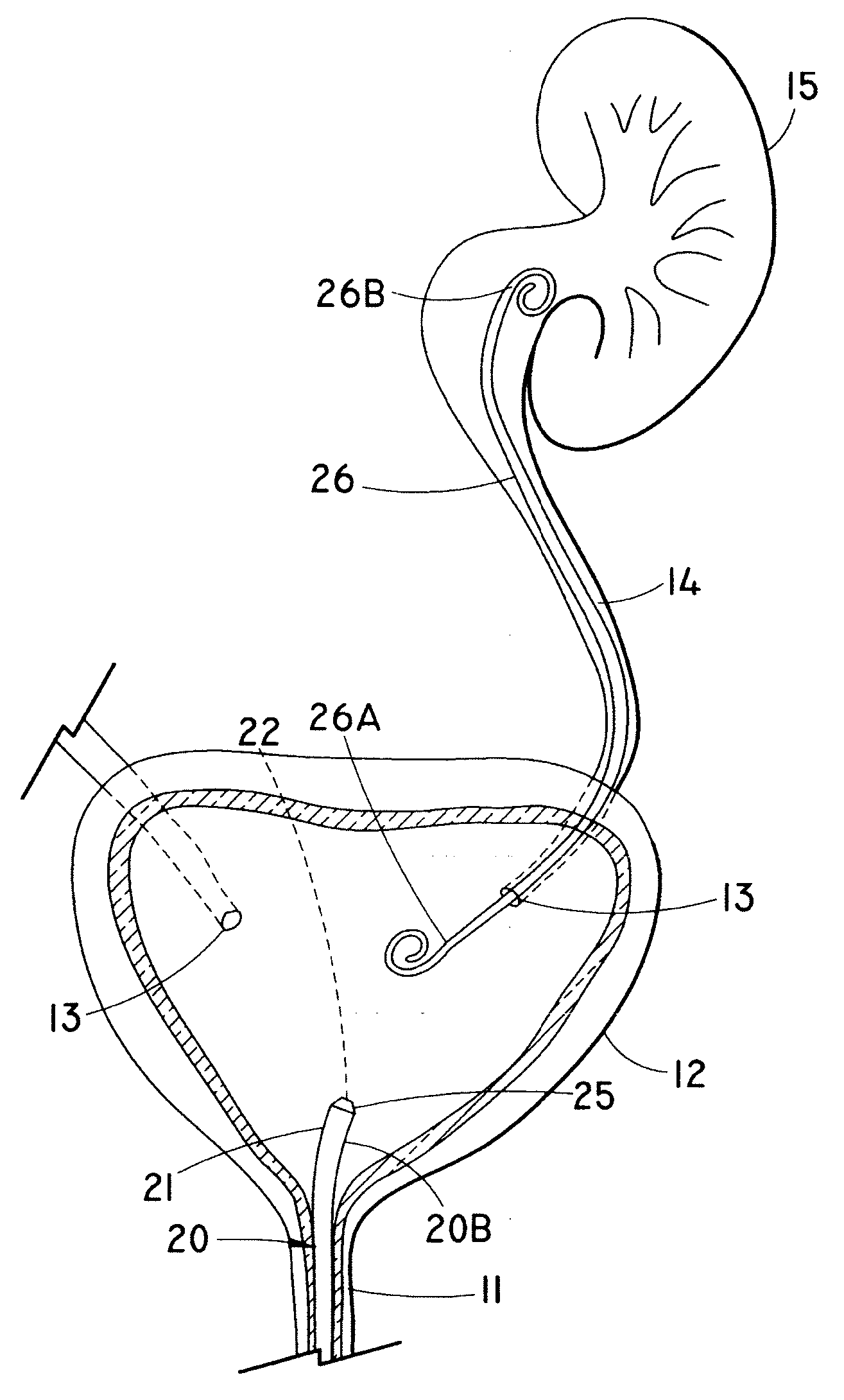
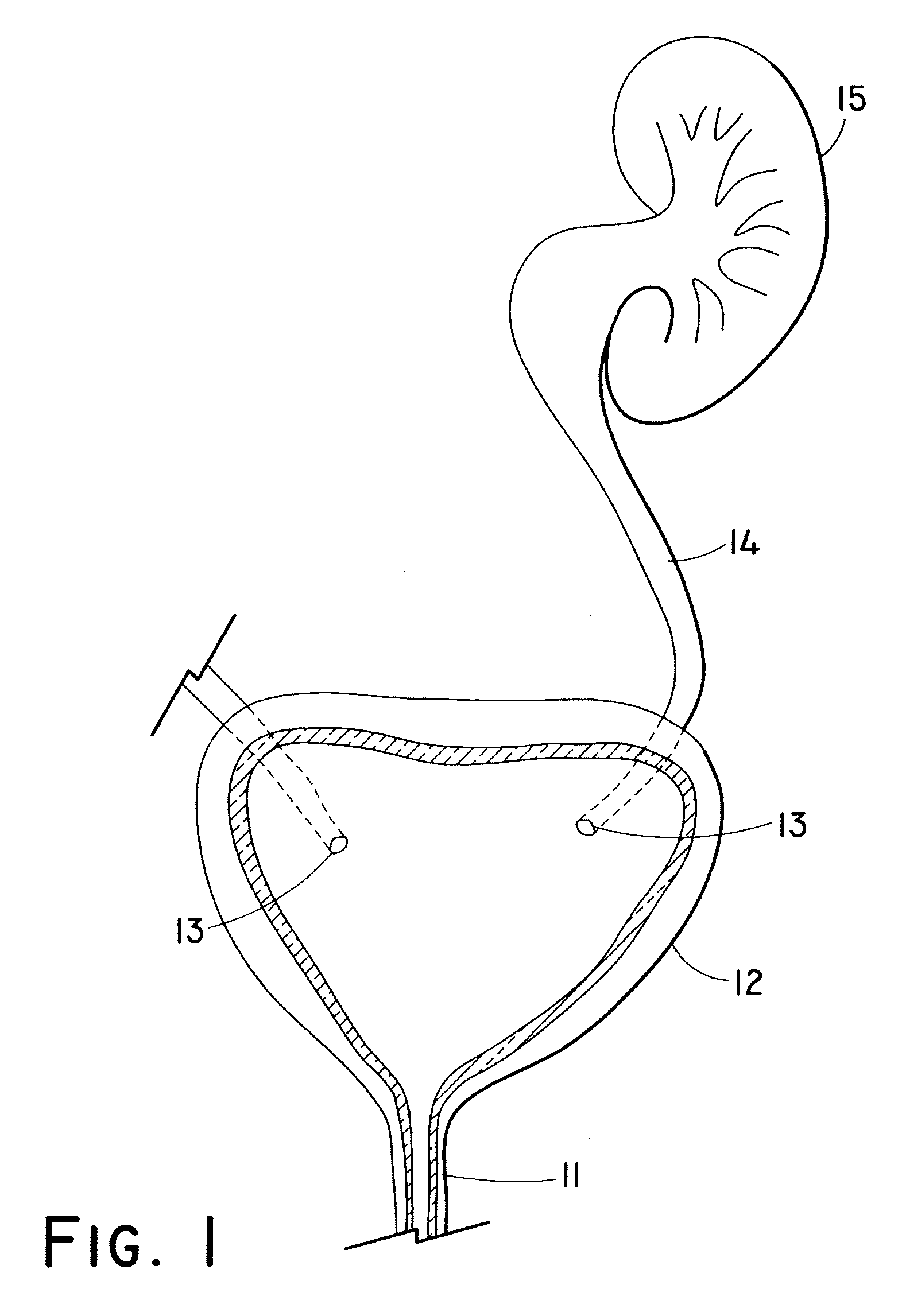
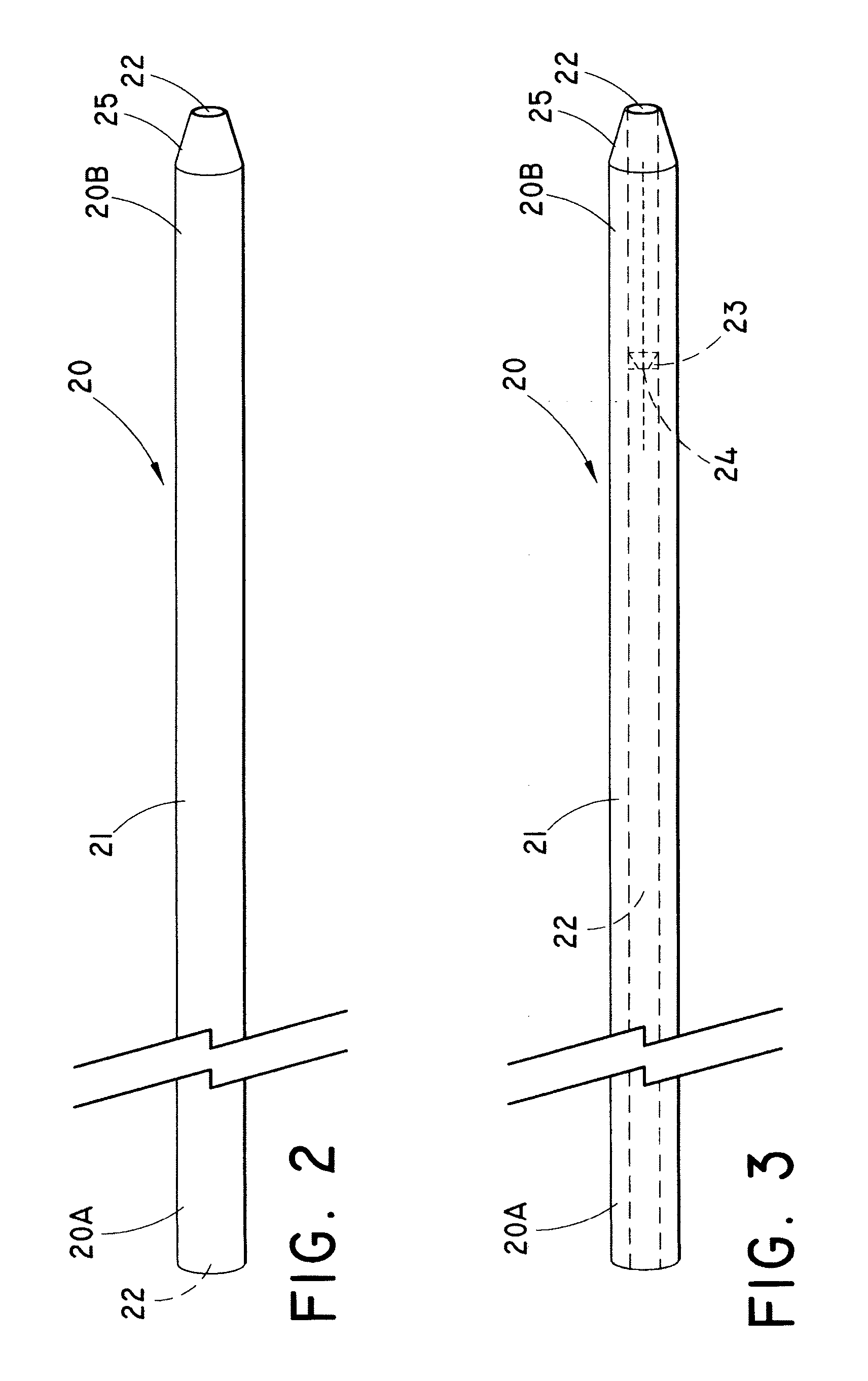
Endoluminal prostheses and therapies for highly variable body lumens
InactiveUS20020120327A1Maximize column strengthAvoid distortionStentsBile ductsY connectorProsthesis
The present invention provides a branching endoluminal prosthesis for use in branching body lumen systems which includes a trunk lumen and first and second branch lumens. The prostheses comprises a radially expandable tubular trunk portion having a prosthetic trunk lumen, and radially expandable tubular first and second branch portions with first and second prosthetic branch lumens, respectively. A radially expandable tubular Y-connector portion provides fluid communication between the prosthetic trunk lumen and the first and second prosthetic branch lumens. Although it is often considered desirable to maximize the column strength of endoluminal prostheses, and although the trunk portion will generally have a larger cross-section than much of the remainder of a branching endoluminal prostheses, the expanded trunk portion is more axially flexible than the expanded Y-connector portion, as insufficient flexibility along the trunk portion may result in leakage between the prosthesis and the trunk lumen of the body lumen system. In contrast, the Y-connector portion benefits form a less axially flexible structure to avoid distortion of the flow balance between the luminal branches.
Owner:COX BRIAN +5
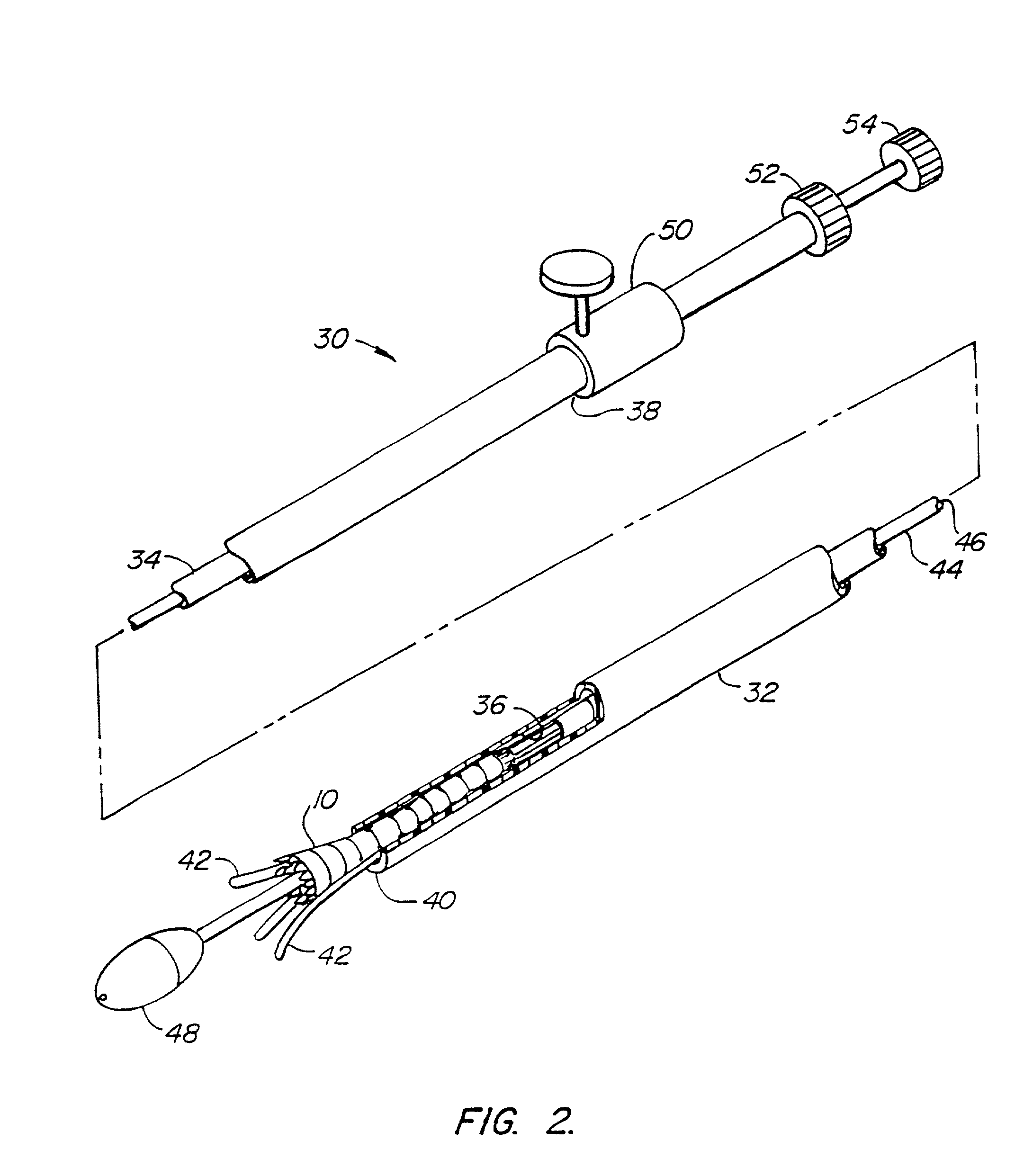
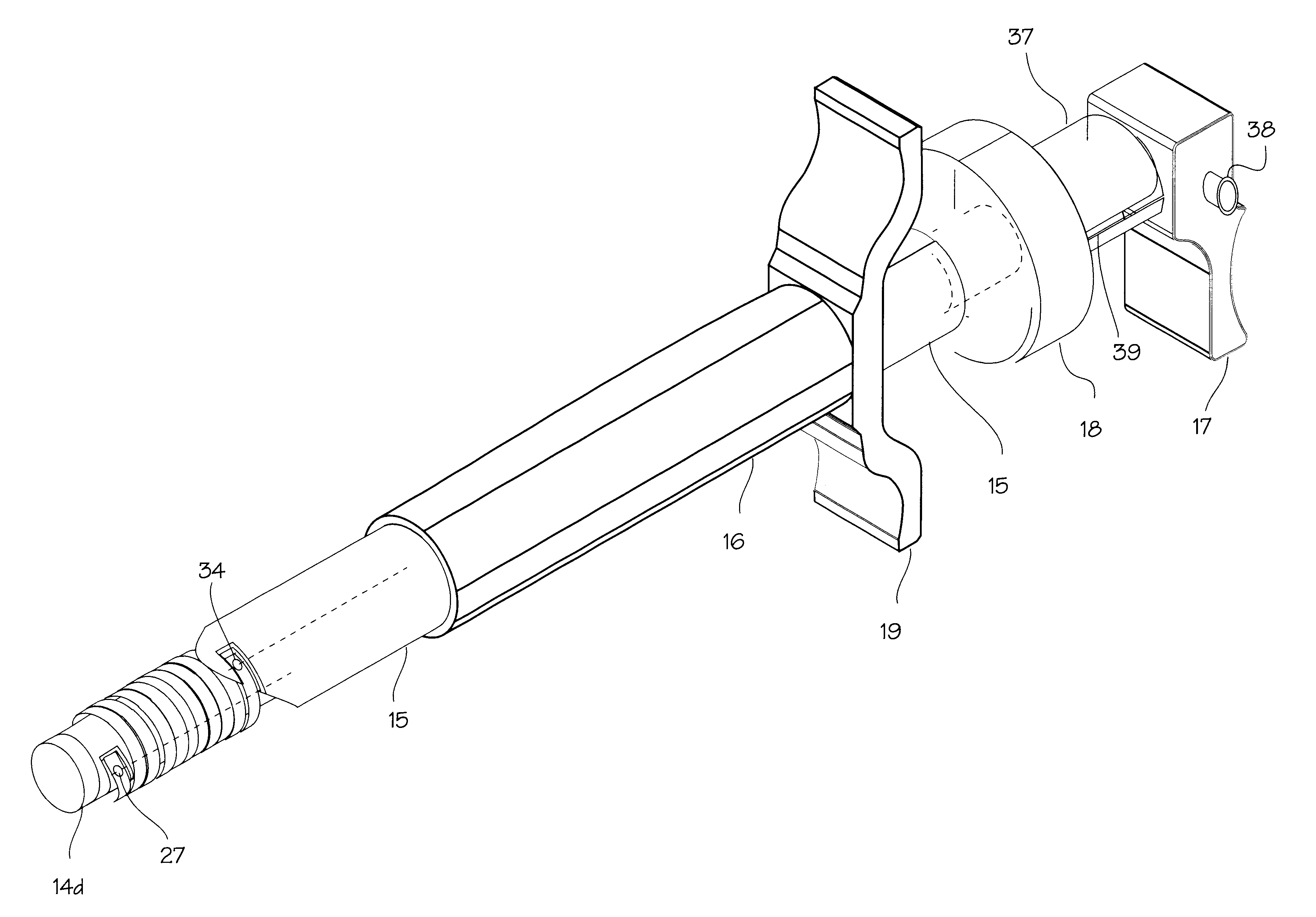
Stent delivery system
A stent delivery catheter. The catheter includes an inner catheter tube and an outer catheter tube which are rotatable relative to each other. The inner catheter and outer catheter include recesses to receive the ends of the stent, permitting pull wires to engage the stent ends without need to for the pull wires to extend radially beyond the bounds of the catheter or requiring the stent ends to protrude it into the lumen of the inner or outer catheter.
Owner:ENDOCARE
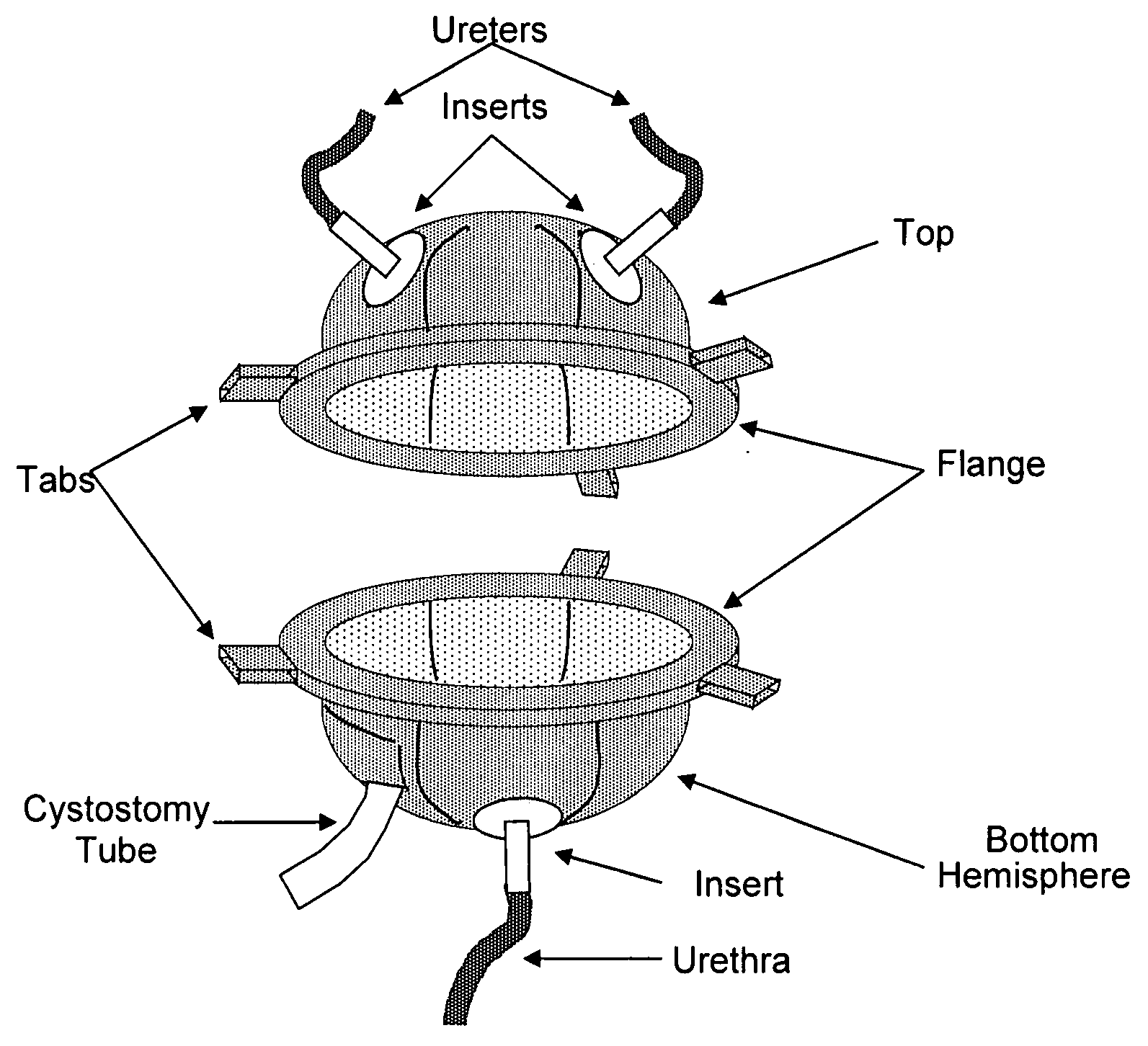
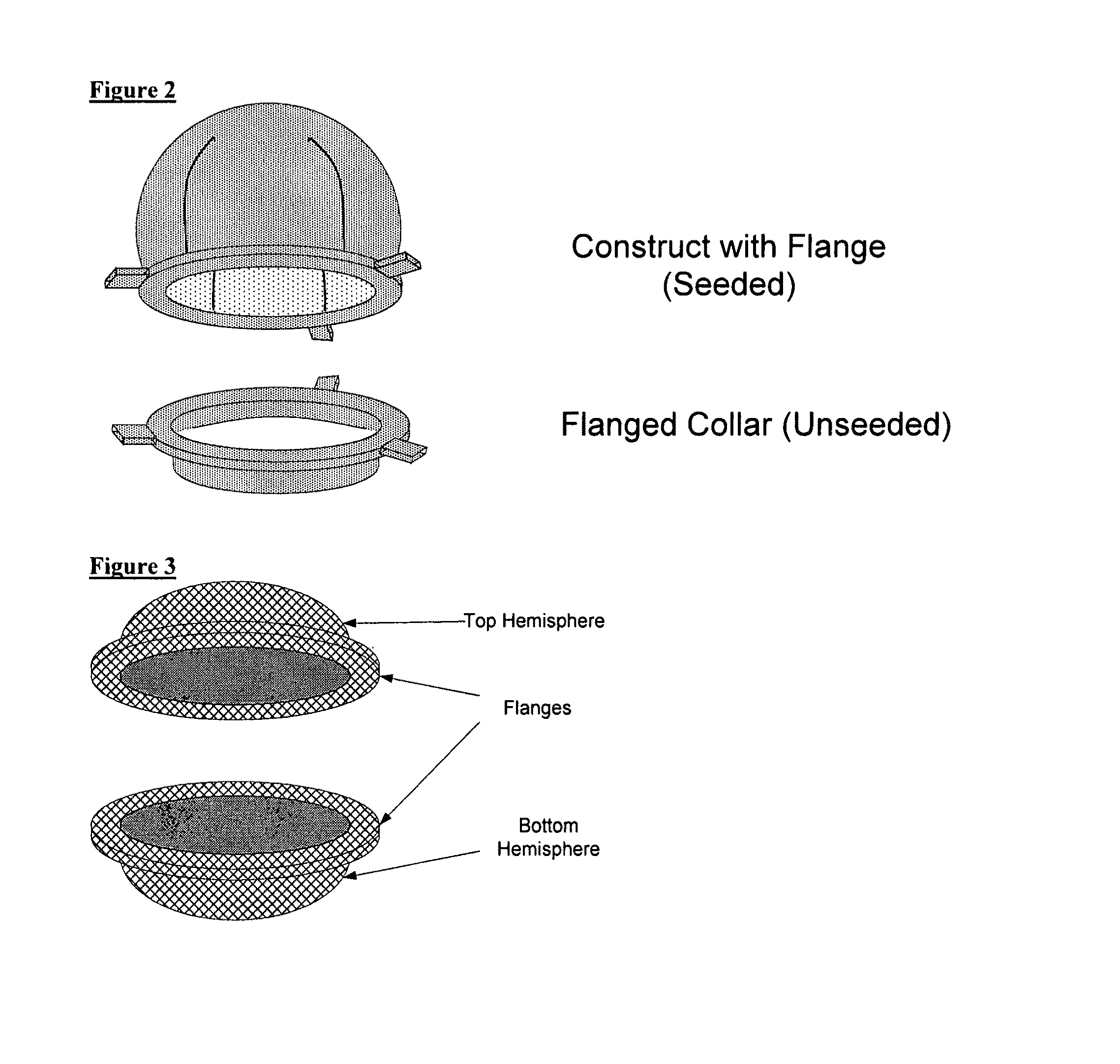
Scaffolds for organ reconstruction and augmentation
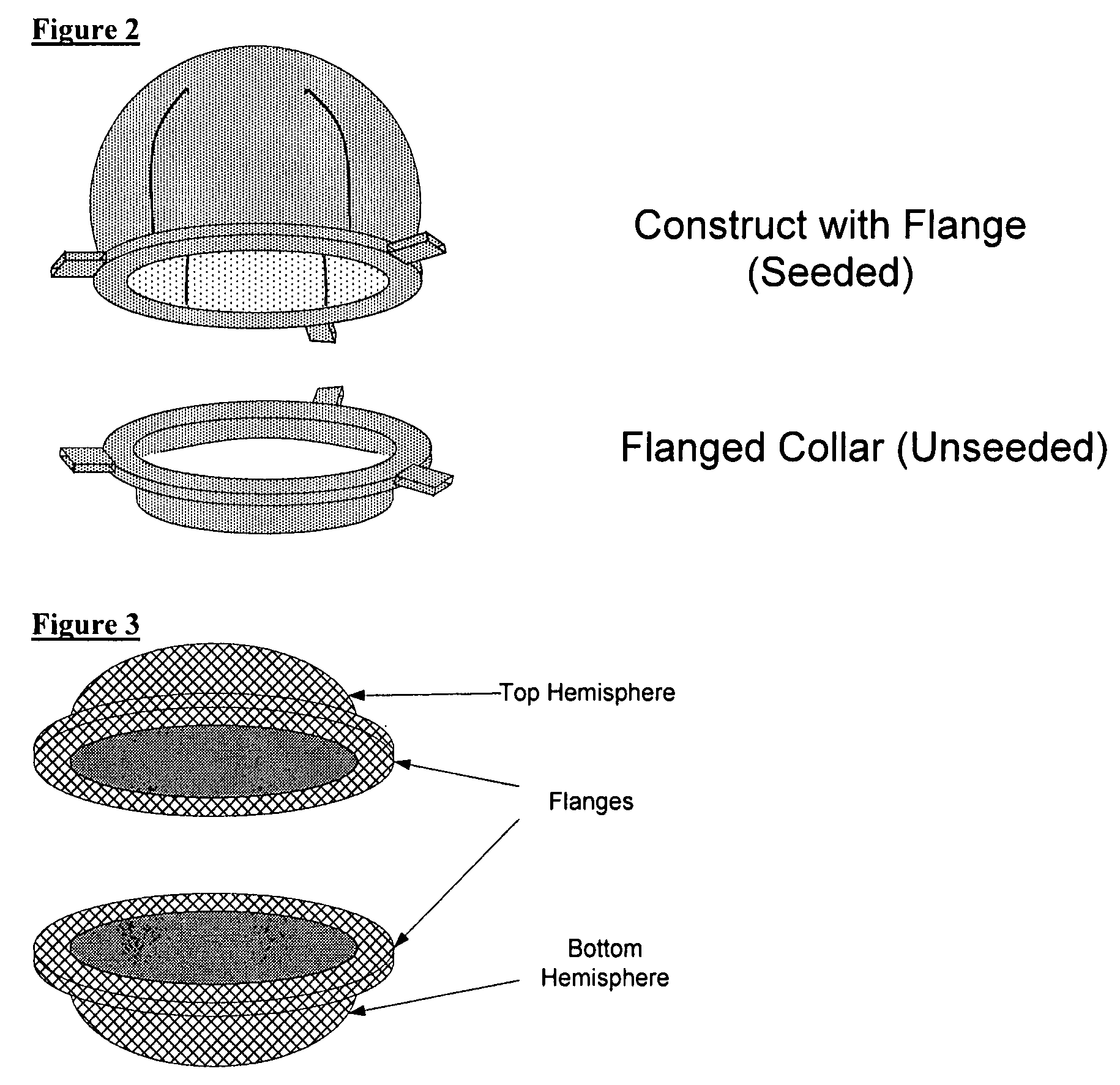
Biocompatible synthetic or natural scaffolds are provided for the reconstruction, repair, augmentation or replacement of organs or tissue structures in a patient in need of such treatment. The scaffolds are shaped to conform to at least a part of the organ or tissue structure and may be seeded with one or more cell populations. Inserts, receptacles and ports are also provided for the attachment of tubular vessels to the neo-organ scaffolds. The seeded scaffolds are implanted into the patient at the site in need of treatment to form an organized organ or tissue structure. The scaffolds may be used to form organs or tissues, such as bladders, urethras, valves, and blood vessels.
Owner:ORGAGEN INC
Self-cleansing bladder drainage device
An urethral drain having deep external drainage channels, a low-profiled bladder retention segment, and a reversibly detachable collection segment, facilitates the draining of urine and fluids from the bladder. The low-profiled retention means minimizes bladder irritations and the deep external channels reduce the occurrence of infections. Incorporation of a reduced diameter smooth segment on the catheter, proximate the location of the external urethral sphincter allows the patient to void normally and at will. Modifying the size of this smooth segment aids the function of a defective sphincter in controlling urine leakage. The drain can be worn concealed within the urethra. Flushing action from normal voiding washes out particulate matters in the urethra and the concealed drain further minimizes contamination. Together, these features improve quality of life for patients needing catheterization.
Owner:CONSERT INC
Continuous Indentation Lateral Lobe Apparatus and Method
ActiveUS20110160747A1Easy to implantPrevent disengagementSuture equipmentsSurgical needlesDiseaseDisease injury
A system and associated method for manipulating tissues and anatomical or other structures in medical applications for the purpose of treating diseases or disorders or other purposes. In one aspect, the system includes a delivery device configured to deploy and implant anchor devices for creating continuous defects or indentations in lobes of a prostate.
Owner:TELEFLEX LIFE SCI LTD
Systems and Methods for Prostate Treatment
An energy delivery probe is provided that may include any of a number of features. One feature of the energy delivery probe is that it can apply energy to tissue, such as a prostrate, to shrink, damage, denaturate the prostate. In some embodiments, the energy can be applied with a vapor media. Another feature of the energy delivery probe is that it can deploy a stent to apply tissue-compressive forces to the prostate tissue after energy delivery. Methods associated with use of the energy delivery probe are also covered.
Owner:NXTHERA INC
Device for maintaining a passage for urine through the prostate
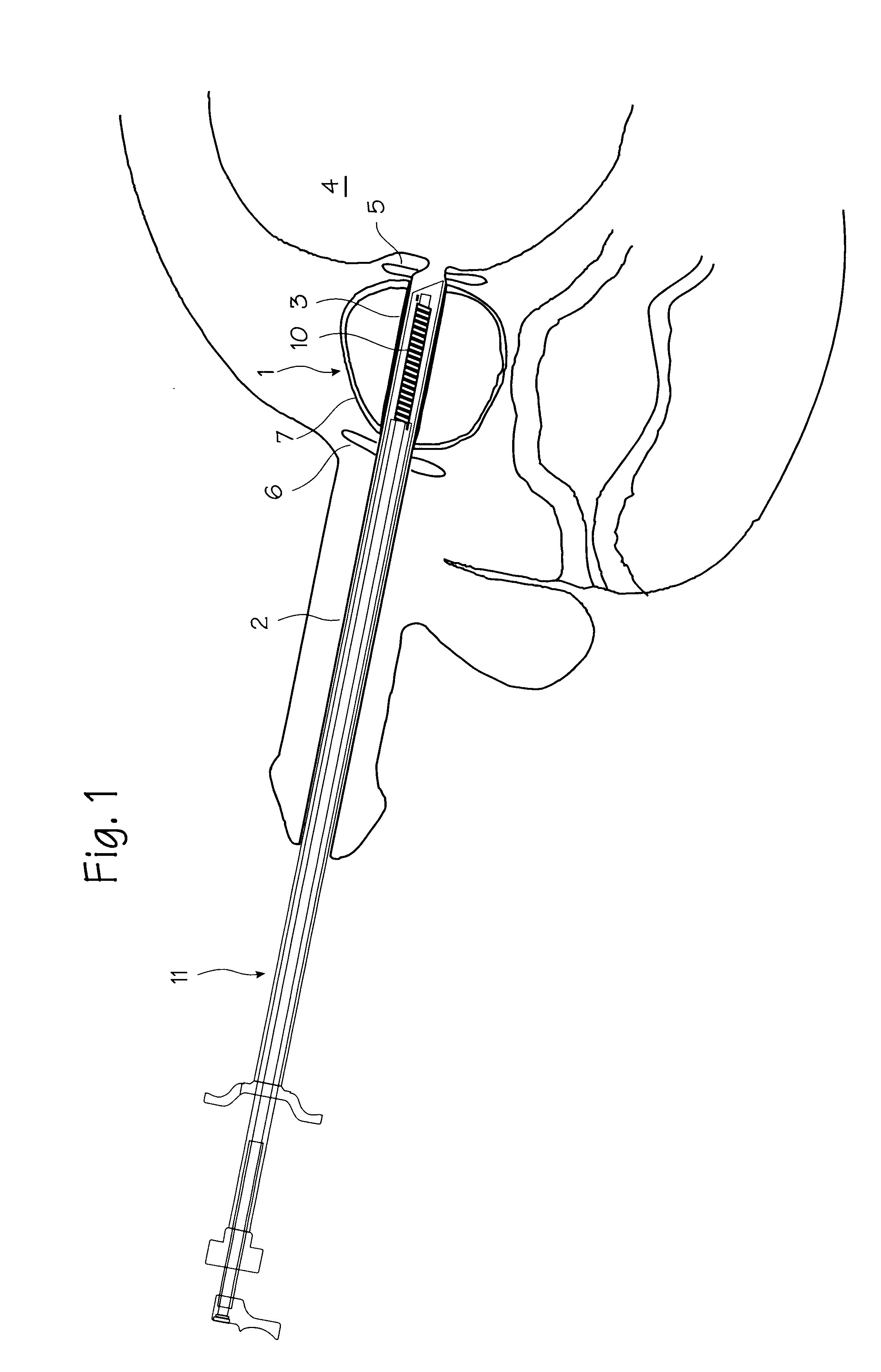
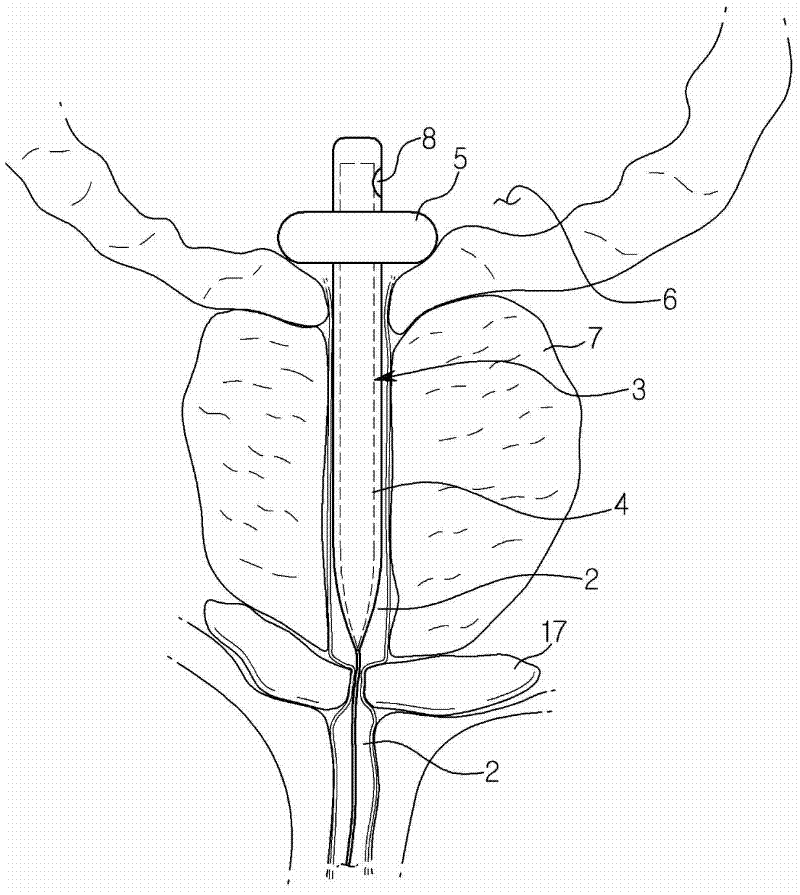
PCT No. PCT / SE96 / 00607 Sec. 371 Date Nov. 12, 1997 Sec. 102(e) Date Nov. 12, 1997 PCT Filed May 9, 1996 PCT Pub. No. WO96 / 35395 PCT Pub. Date Nov. 14, 1996A device for maintaining a passage through the prostate gland (13) after treatment of a catheter (10) inserted through the urethra into the prostate gland, the catheter (10) being provided with means (11) for heating the prostate gland. A sleeve (12) is received over the catheter (10) so as to follow the catheter (10) during insertion into a desired position within the prostate gland, and the sleeve (12) is formed to remain in the desired position when the catheter is removed from the prostate gland, thereby maintaining a passage having a predetermined lower inner diameter through the prostate gland when tissue in the prostate gland swells.
Owner:PROSTALUND OPERATIONS
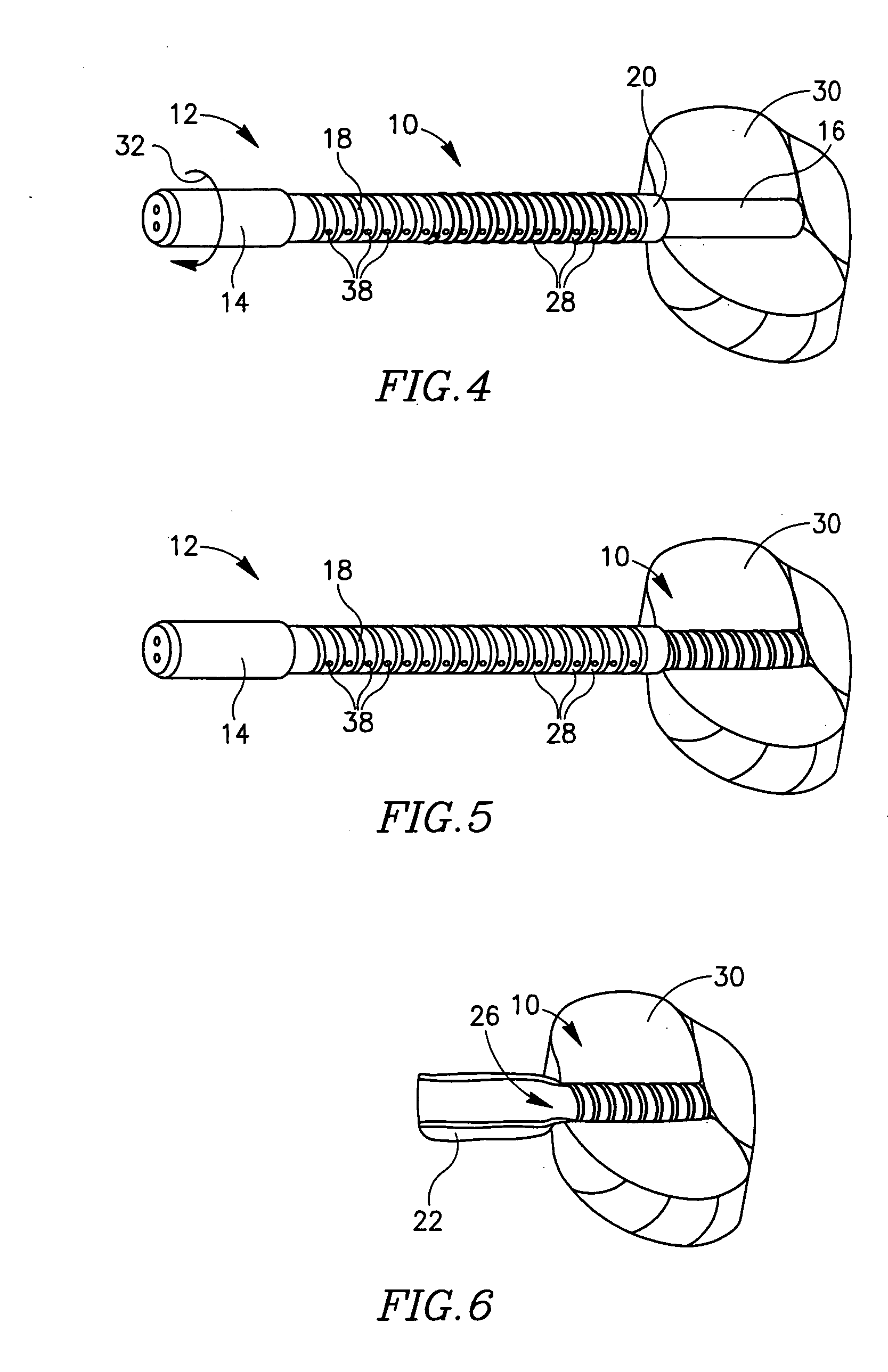
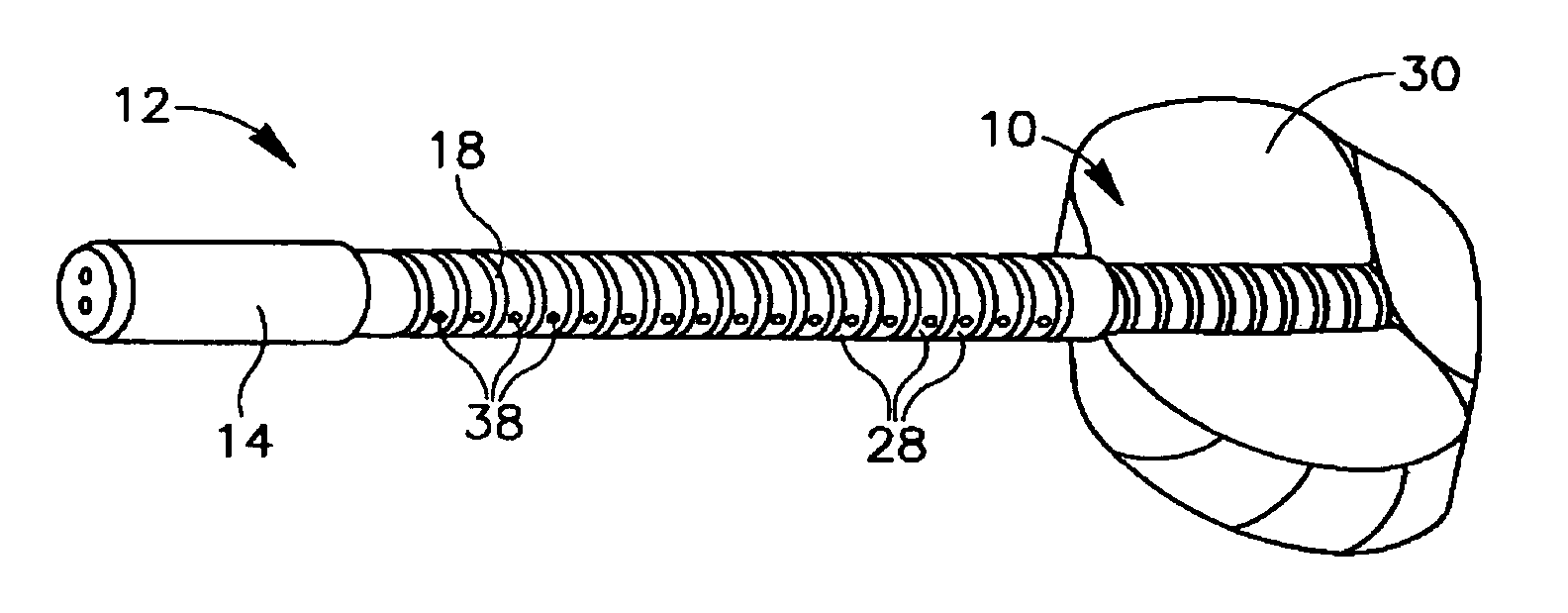
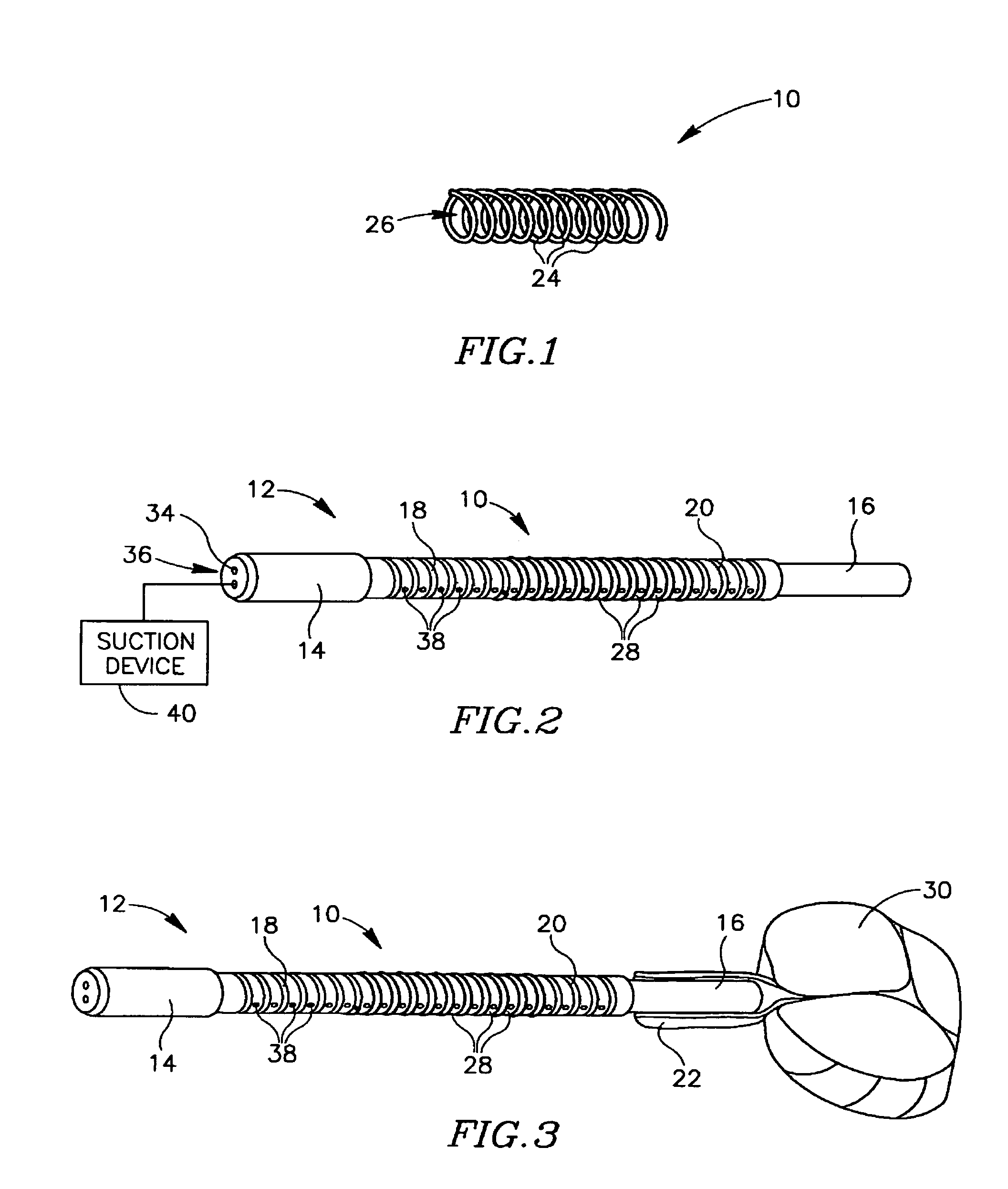
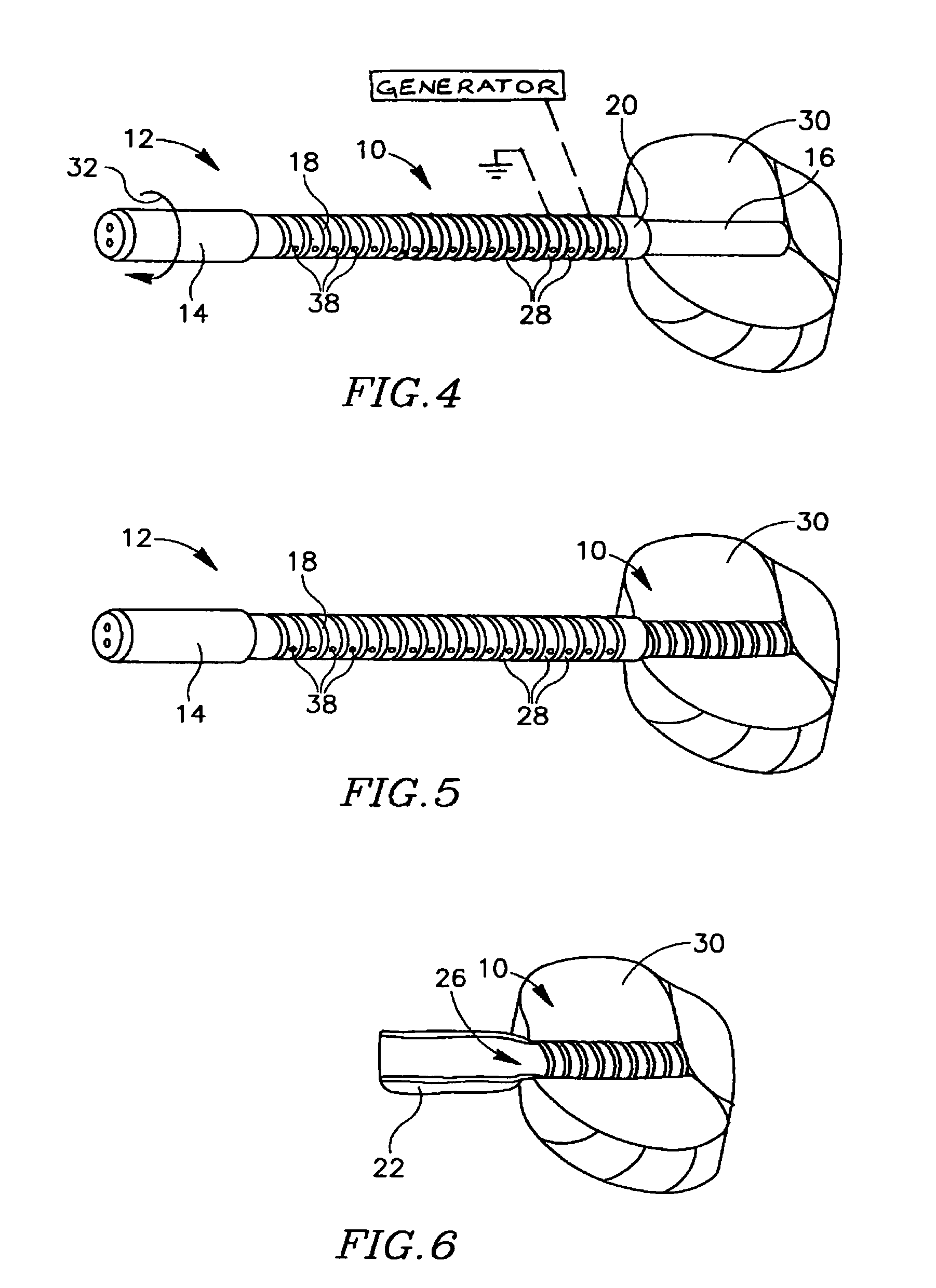
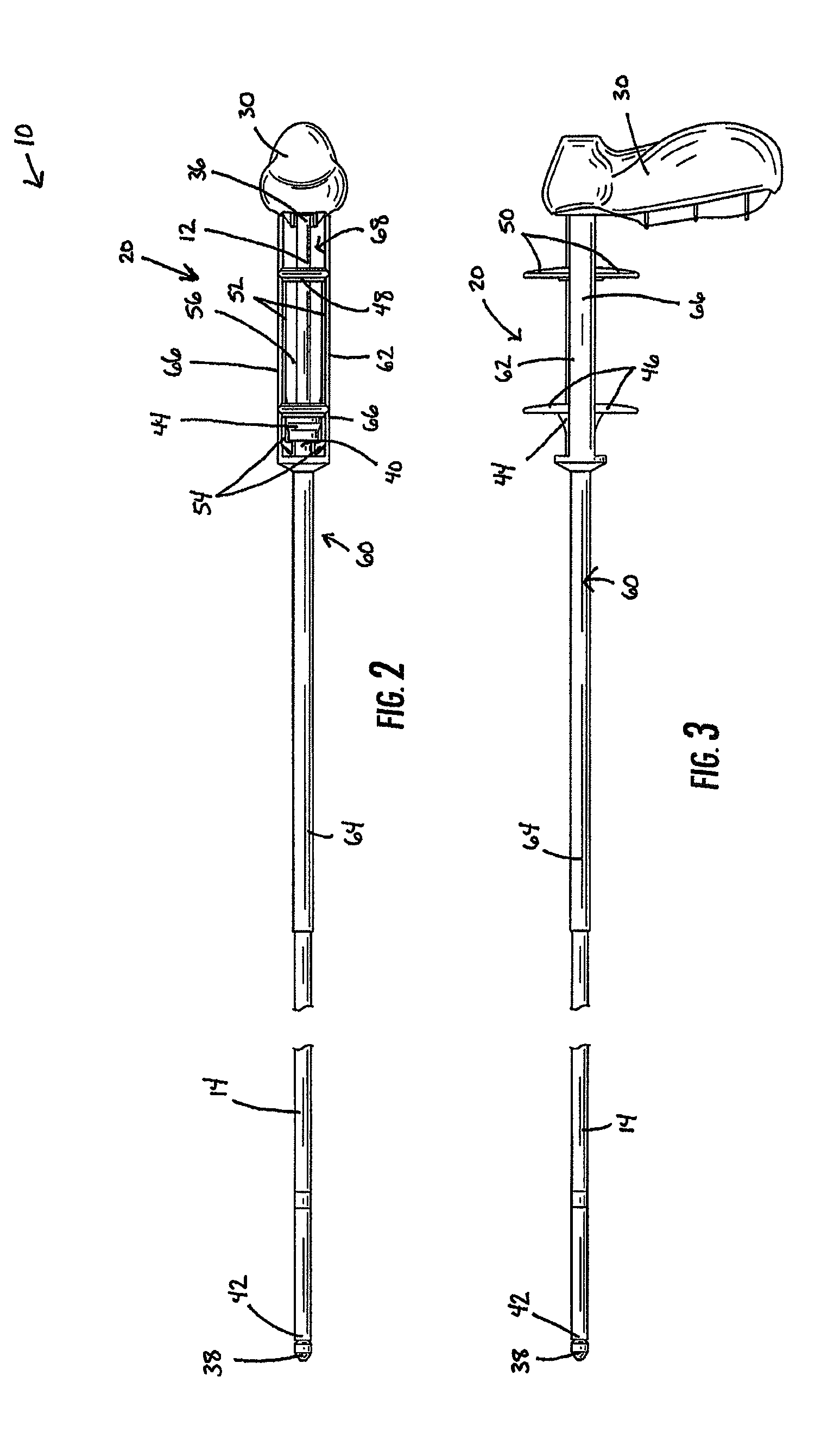
Devices for urethral treatment
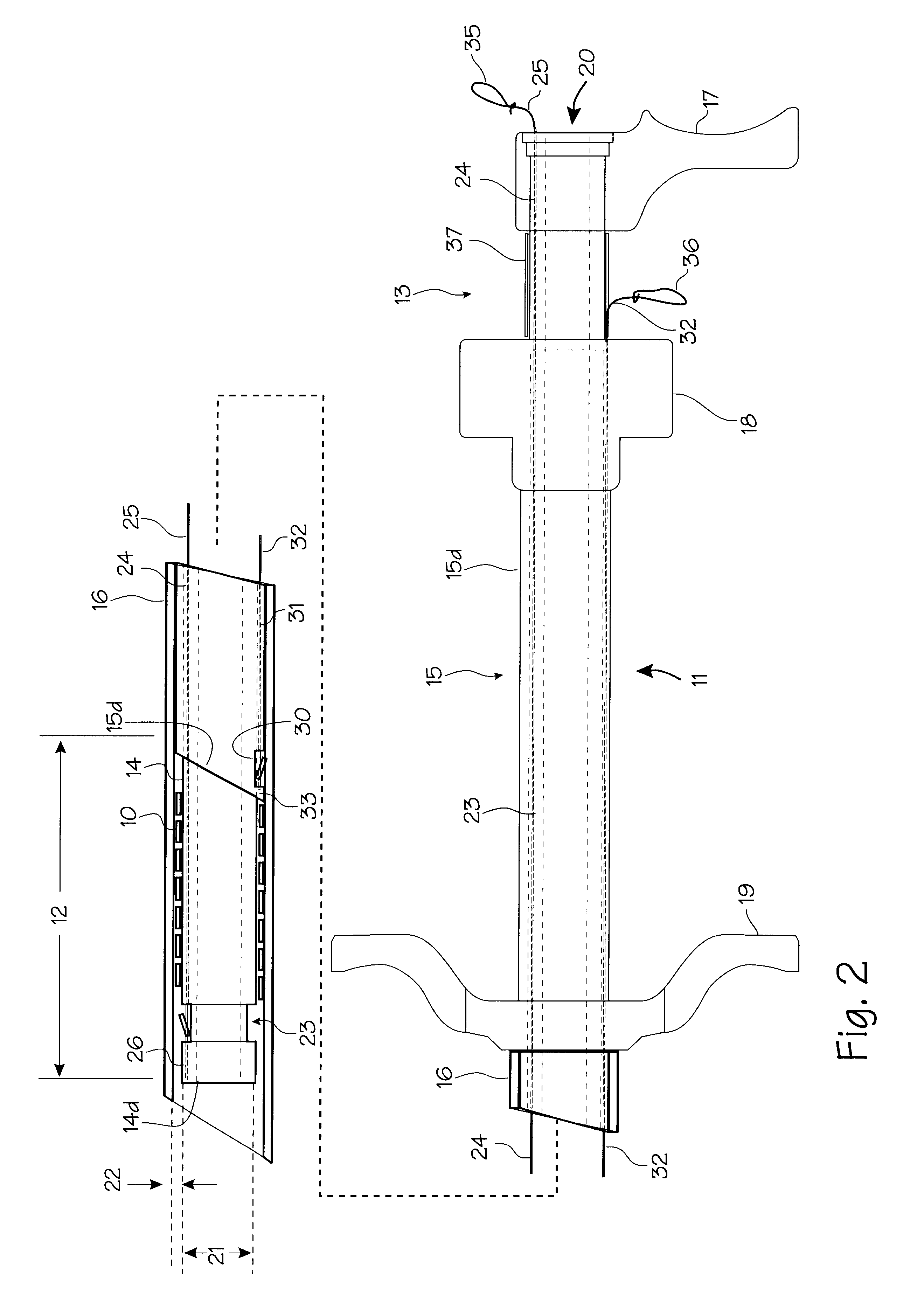
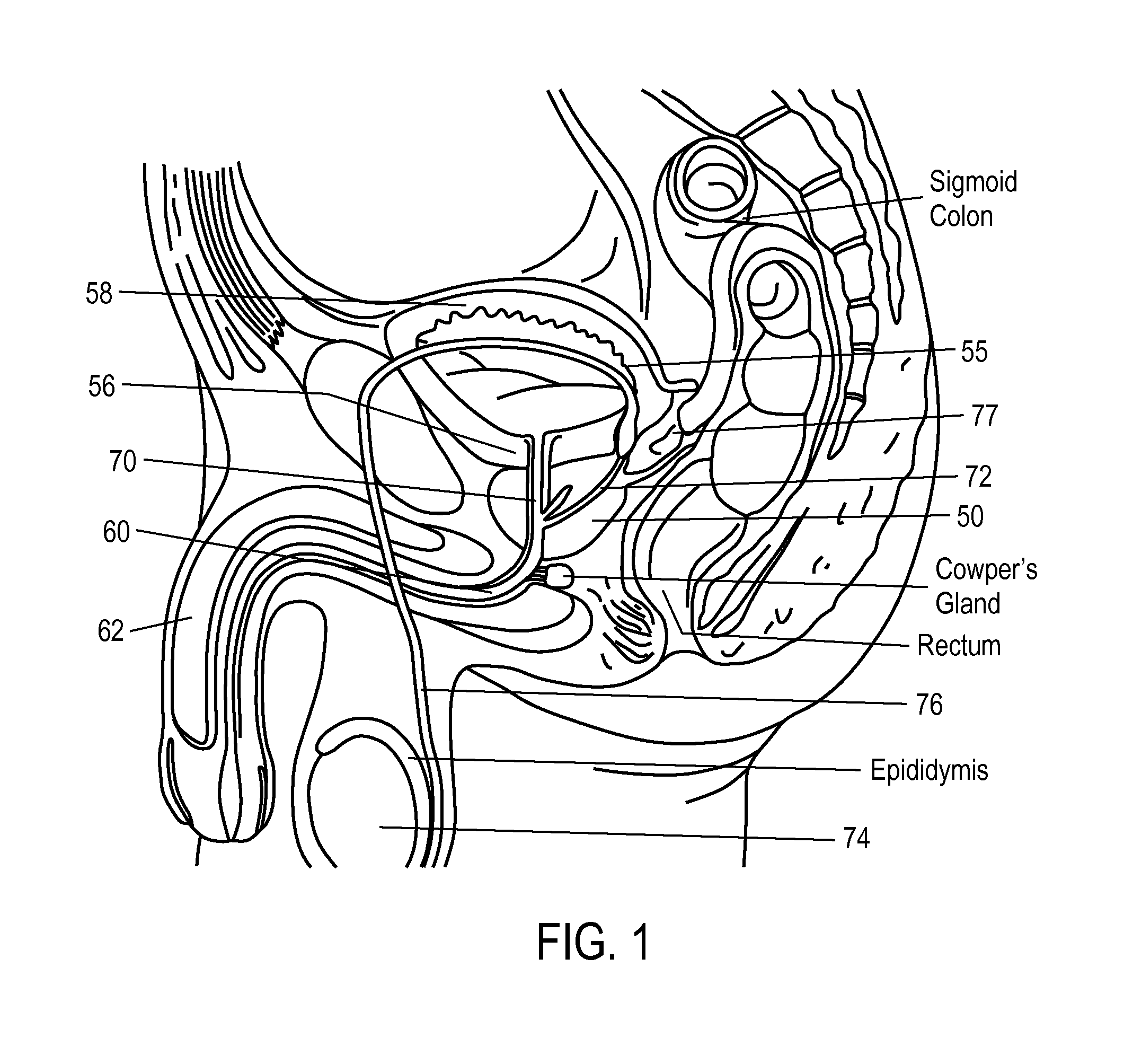
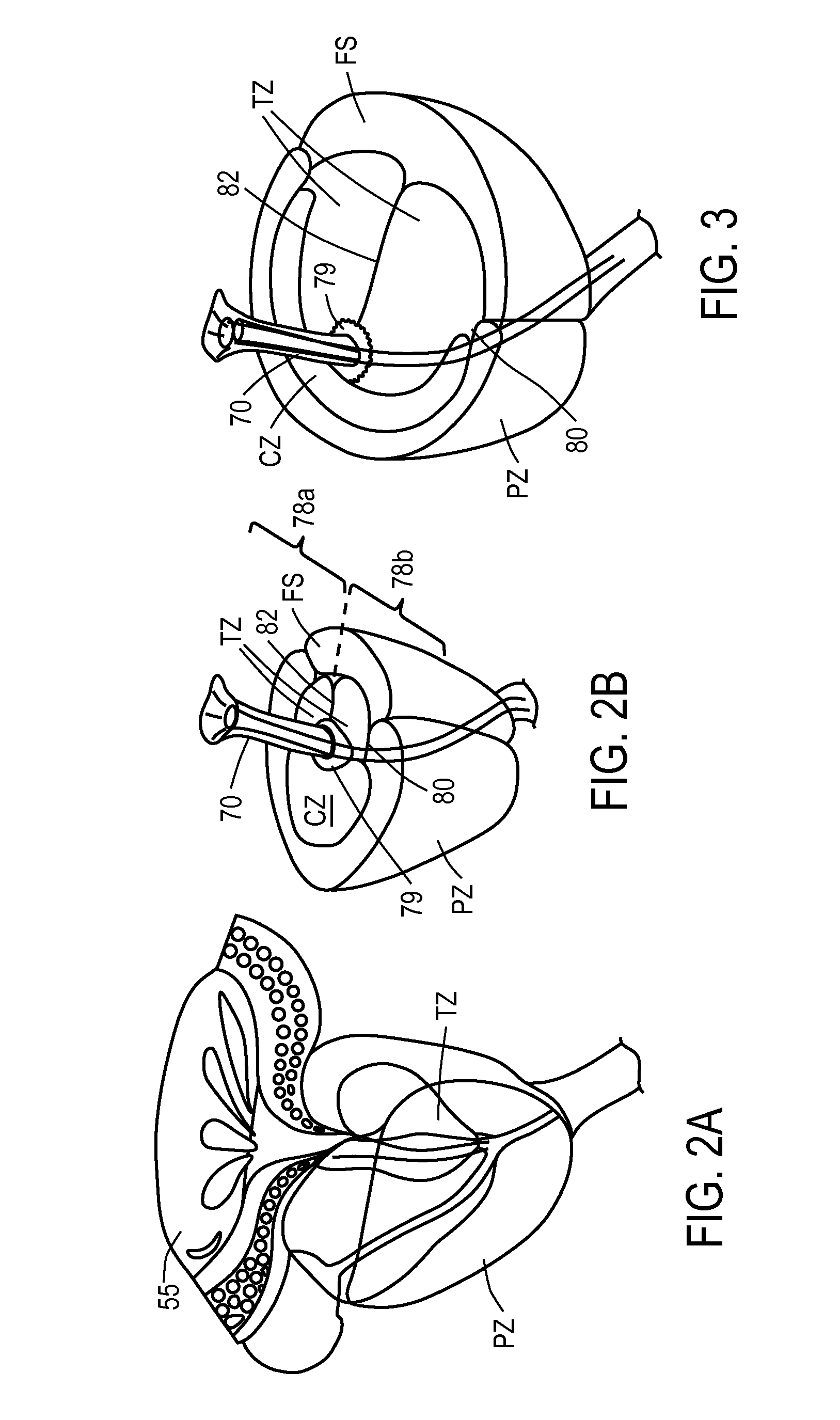
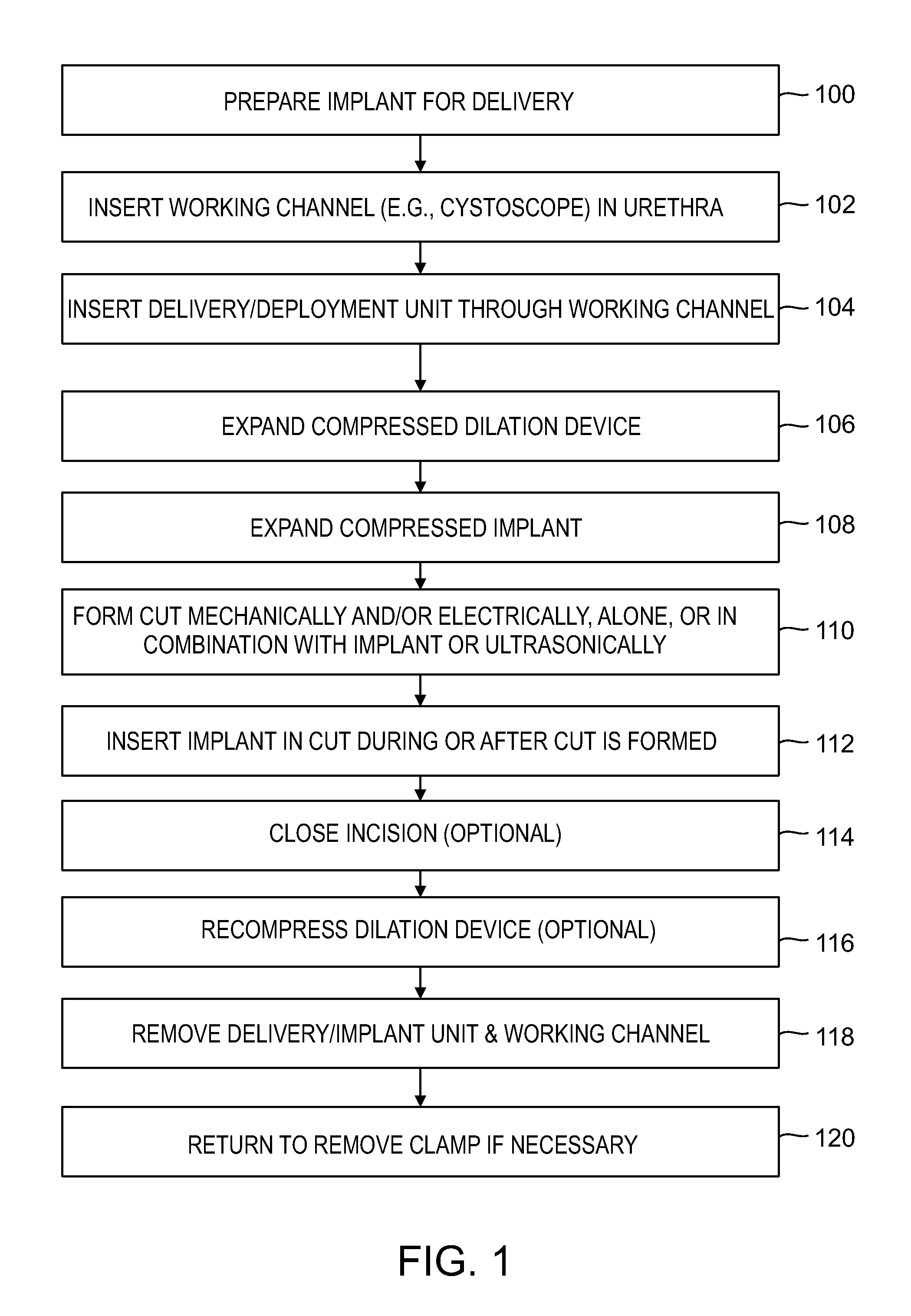
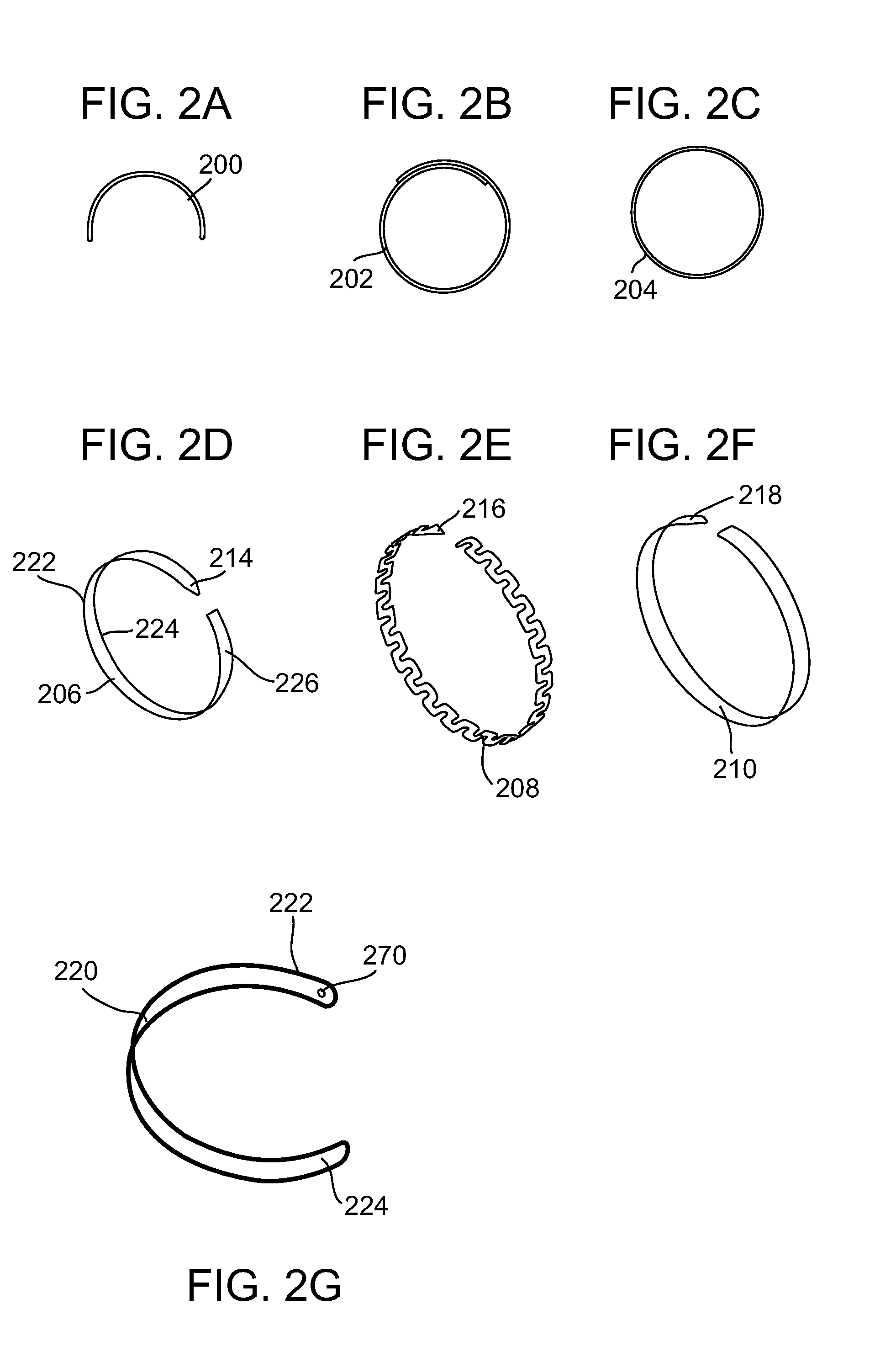
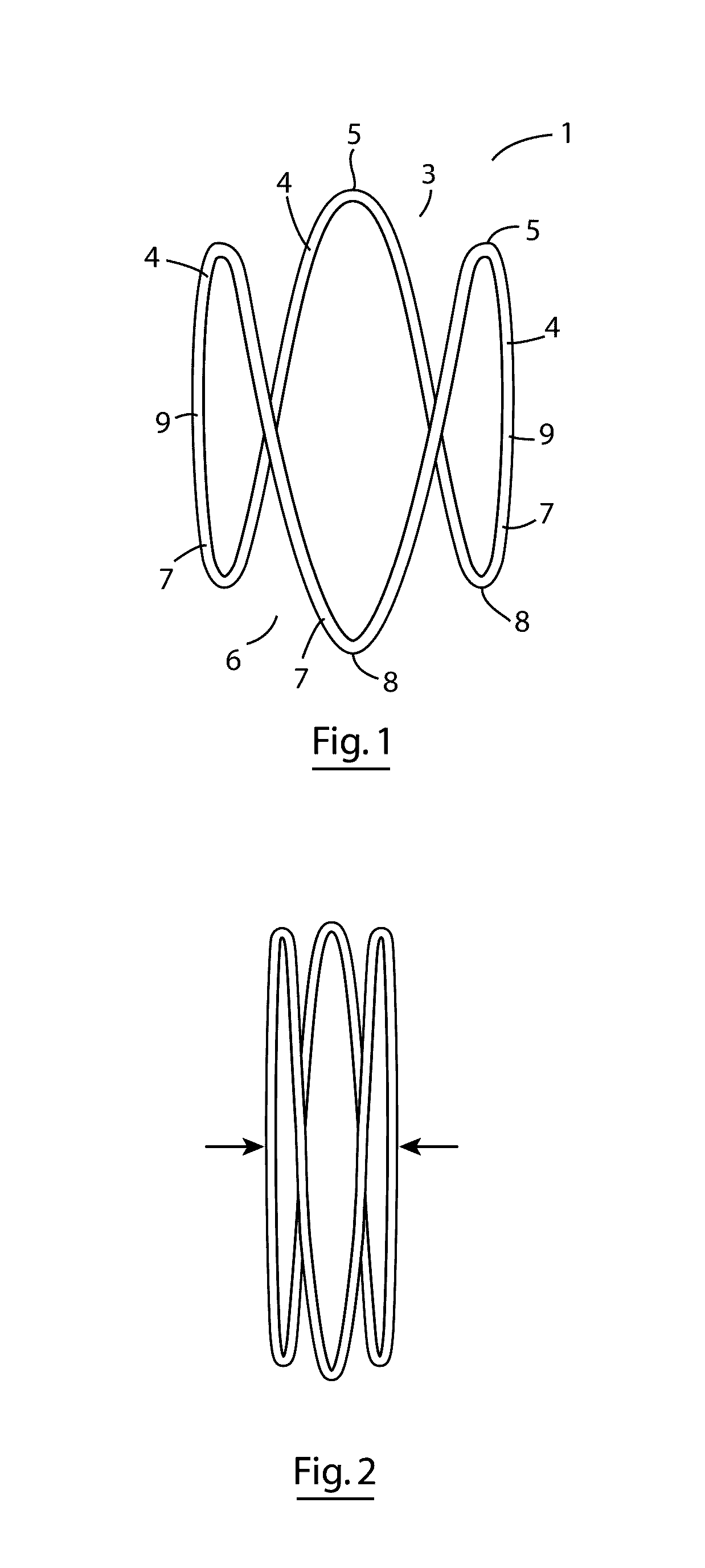
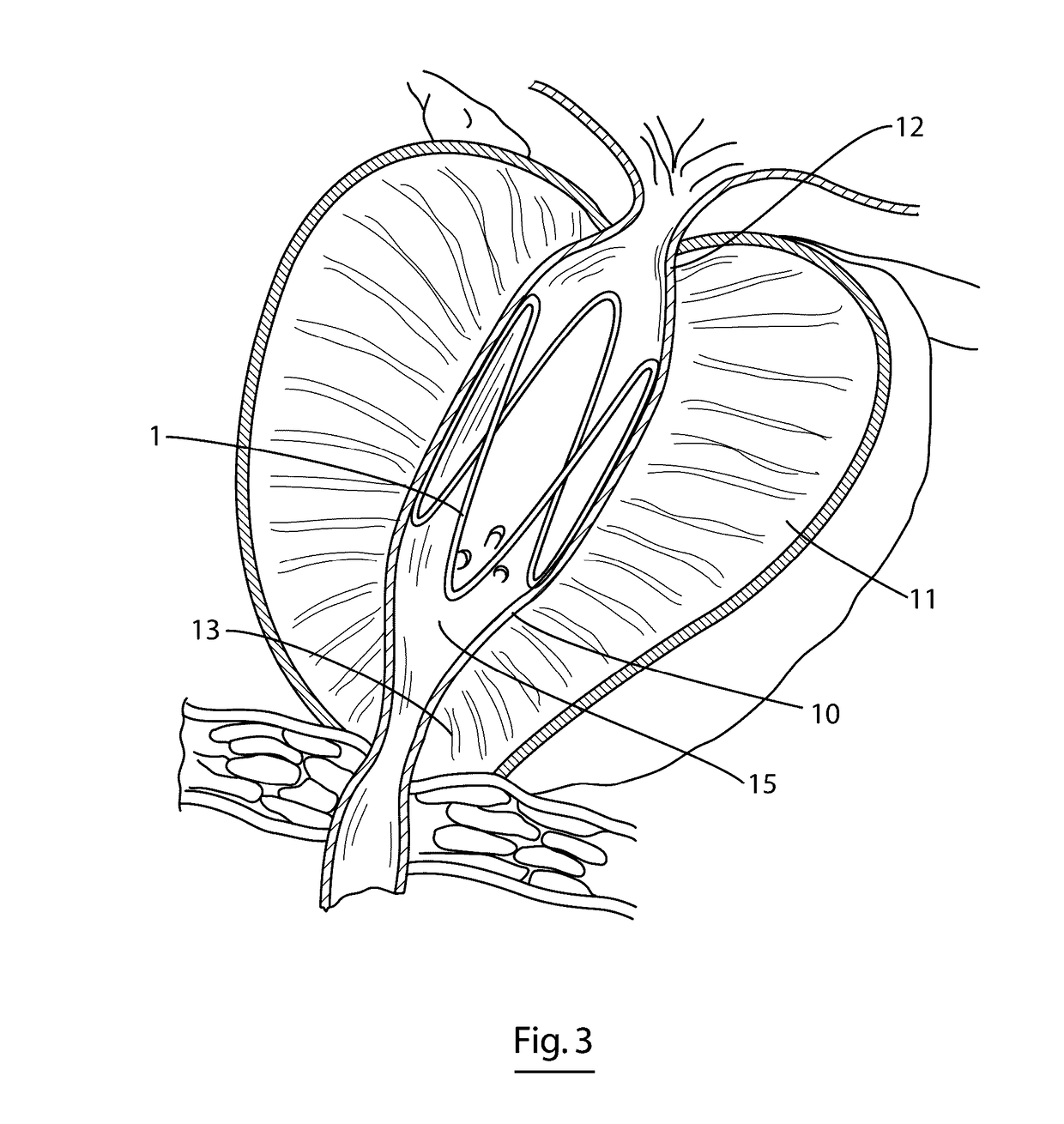
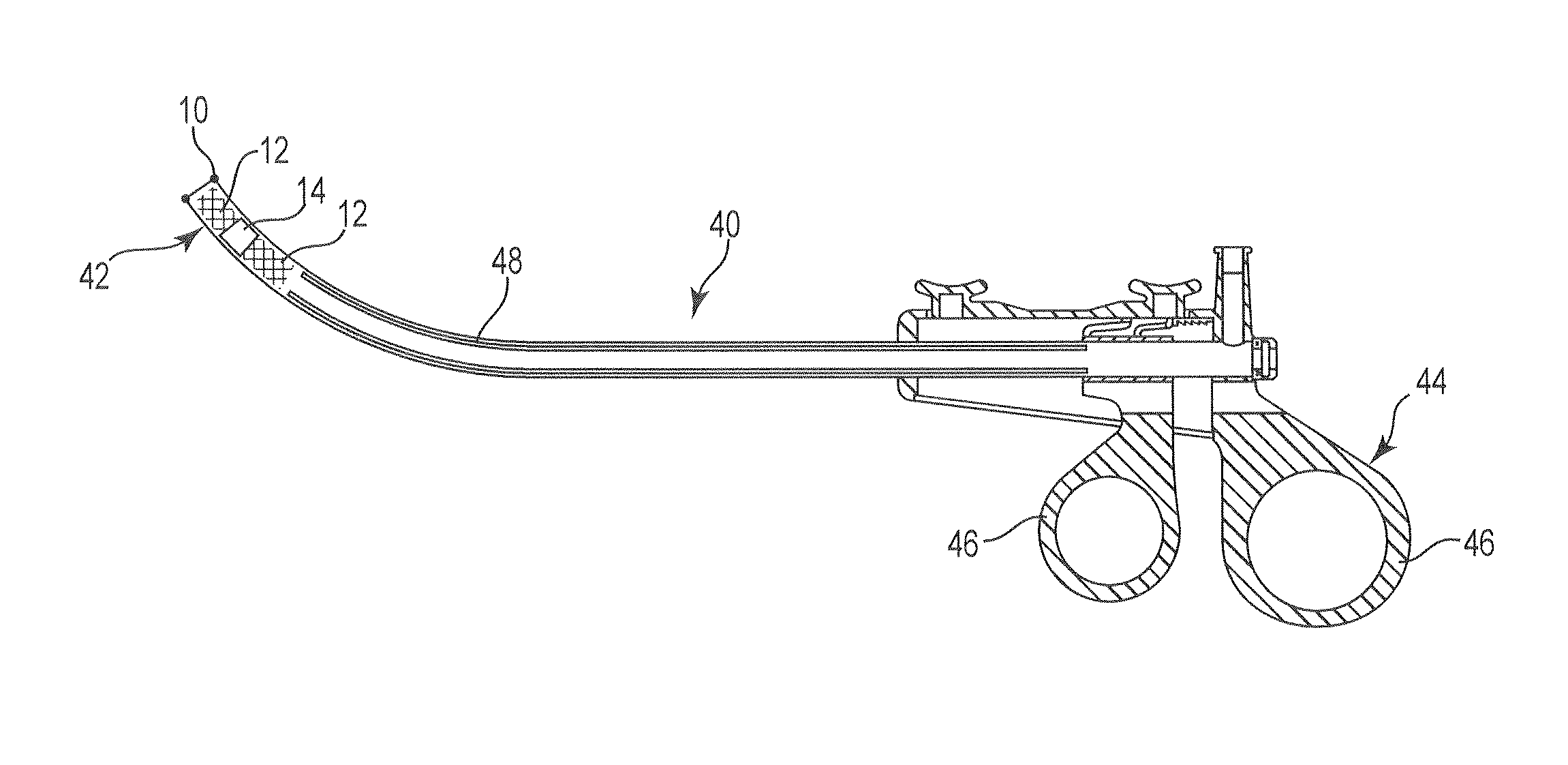
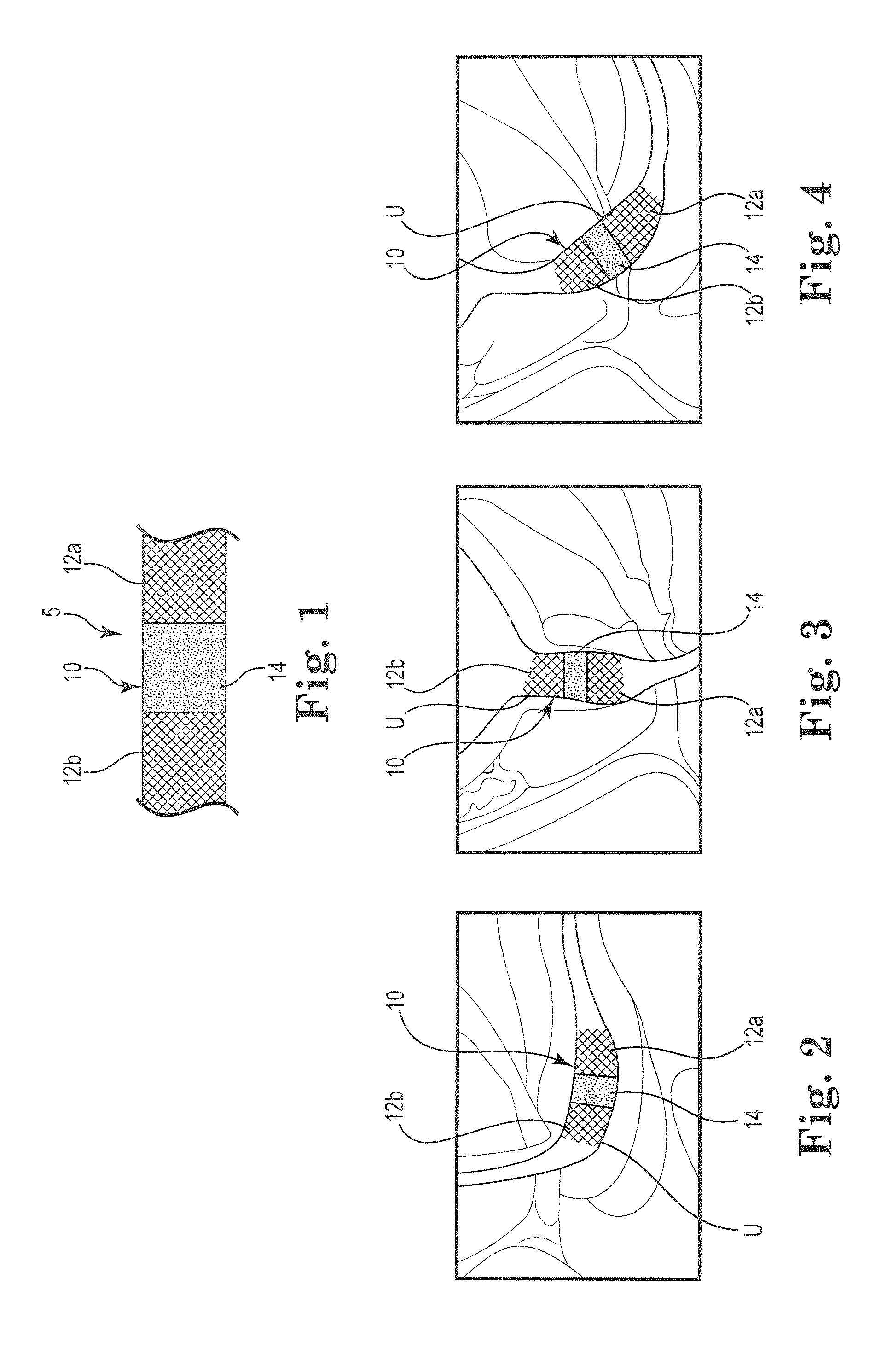
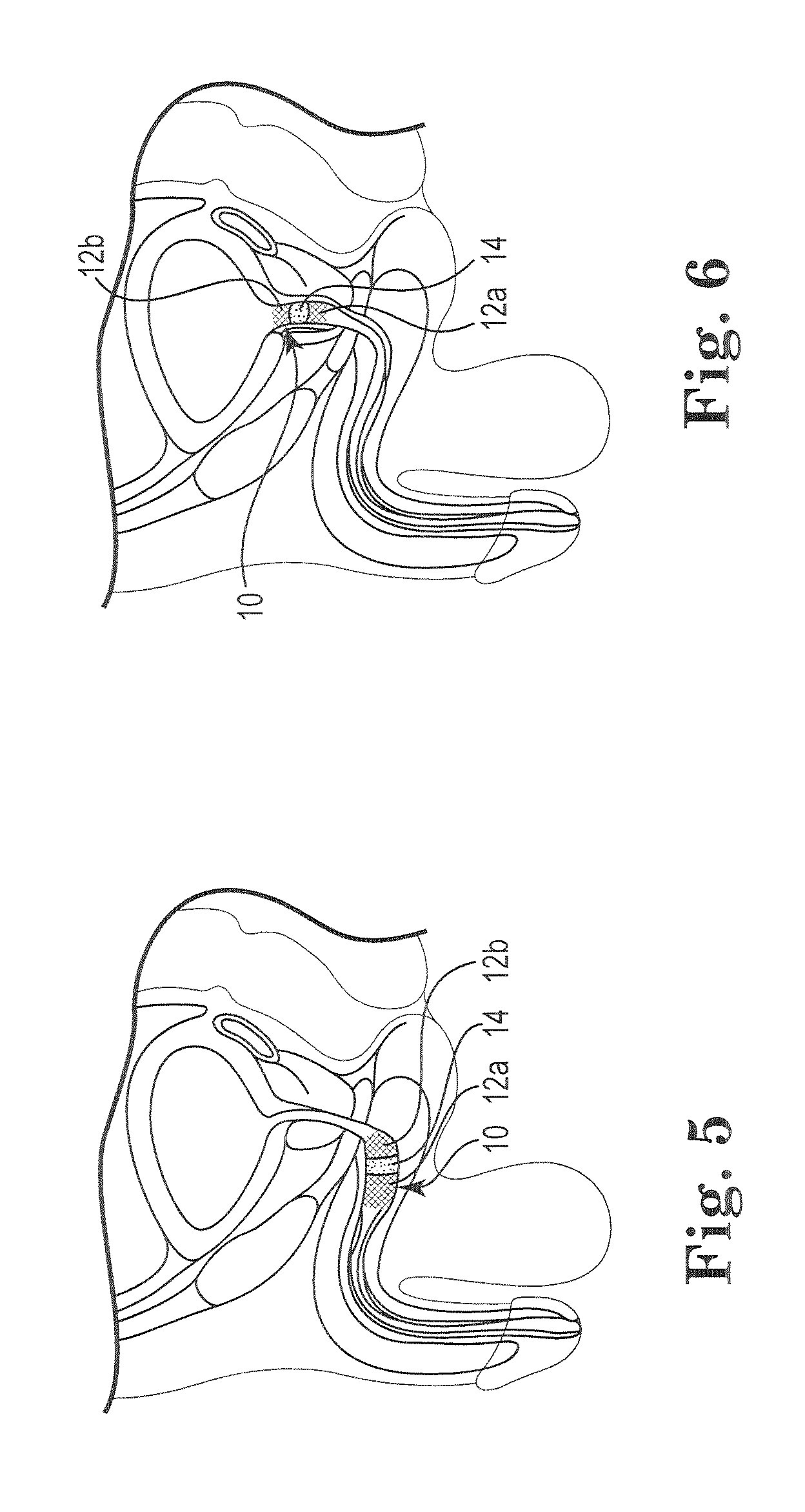
A dilation device (206) for an intra-body lumen, for example, a urethra partially occluded by an enlarged prostate in the form of a resilient curved body configured to be implanted in a cut around the lumen, and a method for dilating such a lumen by implanting a dilation device in a cut around the outside of the lumen. Also, a deployment system (300) including a delivery device for the implant, and a device for dilating the lumen before and while the cut is being made, and a method of using the implant and the deployment system. The implant may be formed of a strip or a coiled wire. The cut may be performed in part by the implant. The cut may be performed by a sharp edge, and / or by application of electrical current to the area being cut, or by a piezoelectric transducer.
Owner:PROARC MEDICAL
Indwelling body lumen expander
Indwelling body lumen expanders which allow for the maintenance and patency of a body lumen, such as the prostatic urethra to relieve urethral obstruction, are described. Because the one or more expanders are configured to be relatively larger in diameter than the diameter of the body lumen, the expanders may become invaginated into the lumen wall thus enabling the prostheses to avoid fluid exposure which in turn prevent the prostheses from becoming encrusted or calcified.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Intraurethral and extraurethral apparatus
InactiveUS20100130815A1Facilitates the proximal corkscrewing of the implantFacilitates the distal corkscrewing of the implantStentsSurgical needlesUrethraPost operative
A method is provided, including distally advancing an implant through a urethra of a patient until the implant emerges in a bladder of the patient, and facilitating expanding of a pre-operative perimeter of a portion of the urethra to a post-operative perimeter of the portion of the urethra that is larger than the pre-operative perimeter by proximally retracting the implant and implanting the implant in prostate tissue surrounding the urethra. Other embodiments are also described.
Owner:PROSTAPLANT
Implantable biocompatible expander suitable for treatment of constrictions of body lumen
An implantable biocompatible expander suitable for implantation into a urinary duct, comprises an elongated sinusoidal ring comprising at least two proximal prongs and at least two distal prongs, wherein the expander is resiliently deformable from a relaxed radially expanded orientation to a radially contracted orientation suitable for transluminal delivery through the urinary duct. The expander is configured to exert an outward radial force against a wall of the urinary duct when in-situ within the urinary duct. In particular, the expander is suitable for treatment of benign prostatic hyperplasia and configured for implantation into the prostatic urethra between, and substantially spanning the prostatic urethra between, the bladder neck and external sphincter.
Owner:TRINITY COLLEGE DUBLIN
Implant and delivery tool therefor
An implant system including a transurethral prostatic implant positioned in a prostate and including a lumen with an inner perimeter that surrounds an outer perimeter of a urethra at the prostate. The implant system may include a delivery tool including a shaft having a distal portion and an implant-holding portion proximal to the distal portion, the distal portion being sized for entry into a urethra, and the implant-holding portion being thicker than the distal portion, and an implant positioned on the implant-holding portion.
Owner:GROSS YOSEF
Implant and delivery tool therefor
An implant system including a transurethral prostatic implant positioned in a prostate and including a lumen with an inner perimeter that surrounds an outer perimeter of a urethra at the prostate. The implant system may include a delivery tool including a shaft having a distal portion and an implant-holding portion proximal to the distal portion, the distal portion being sized for entry into a urethra, and the implant-holding portion being thicker than the distal portion, and an implant positioned on the implant-holding portion.
Owner:GROSS YOSEF
Scaffolds for organ reconstruction and augmentation
Biocompatible synthetic or natural scaffolds are provided for the reconstruction, repair, augmentation or replacement of organs or tissue structures in a patient in need of such treatment. The scaffolds are shaped to conform to at least a part of the organ or tissue structure and may be seeded with one or more cell populations. Inserts, receptacles and ports are also provided for the attachment of tubular vessels to the neo-organ scaffolds. The seeded scaffolds are implanted into the patient at the site in need of treatment to form an organized organ or tissue structure. The scaffolds may be used to form organs or tissues, such as bladders, urethras, valves, and blood vessels.
Owner:ORGAGEN INC
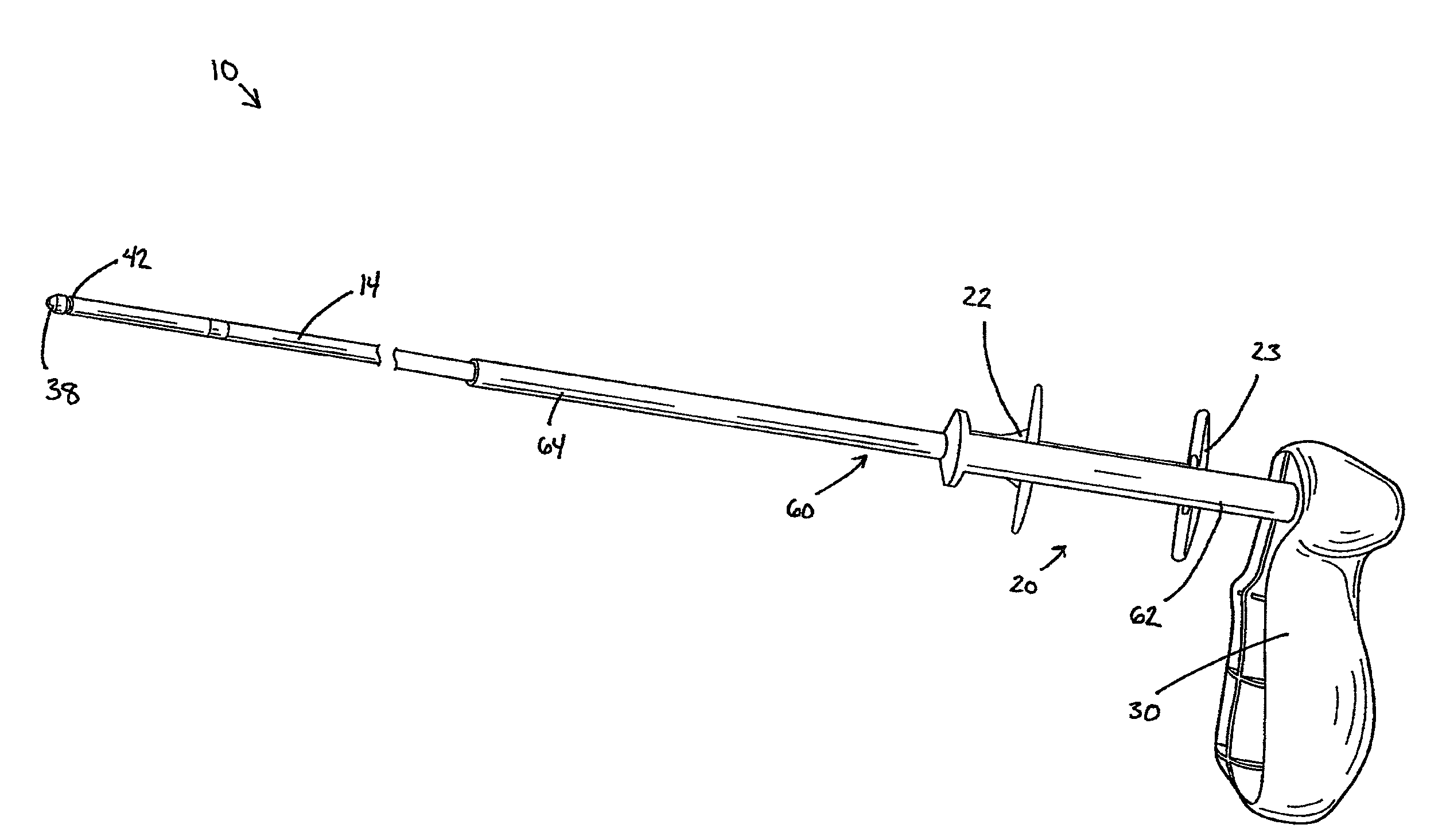
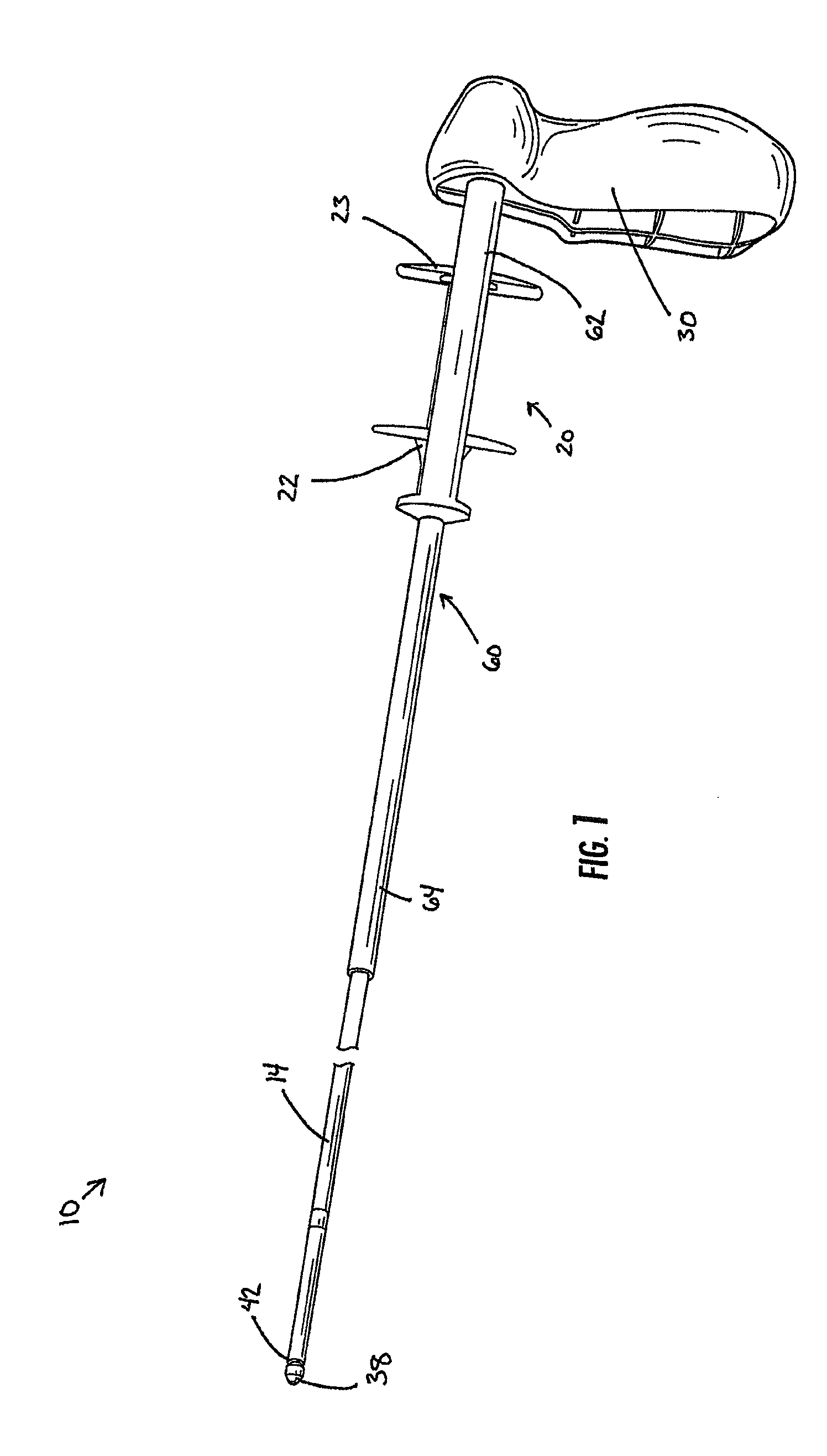
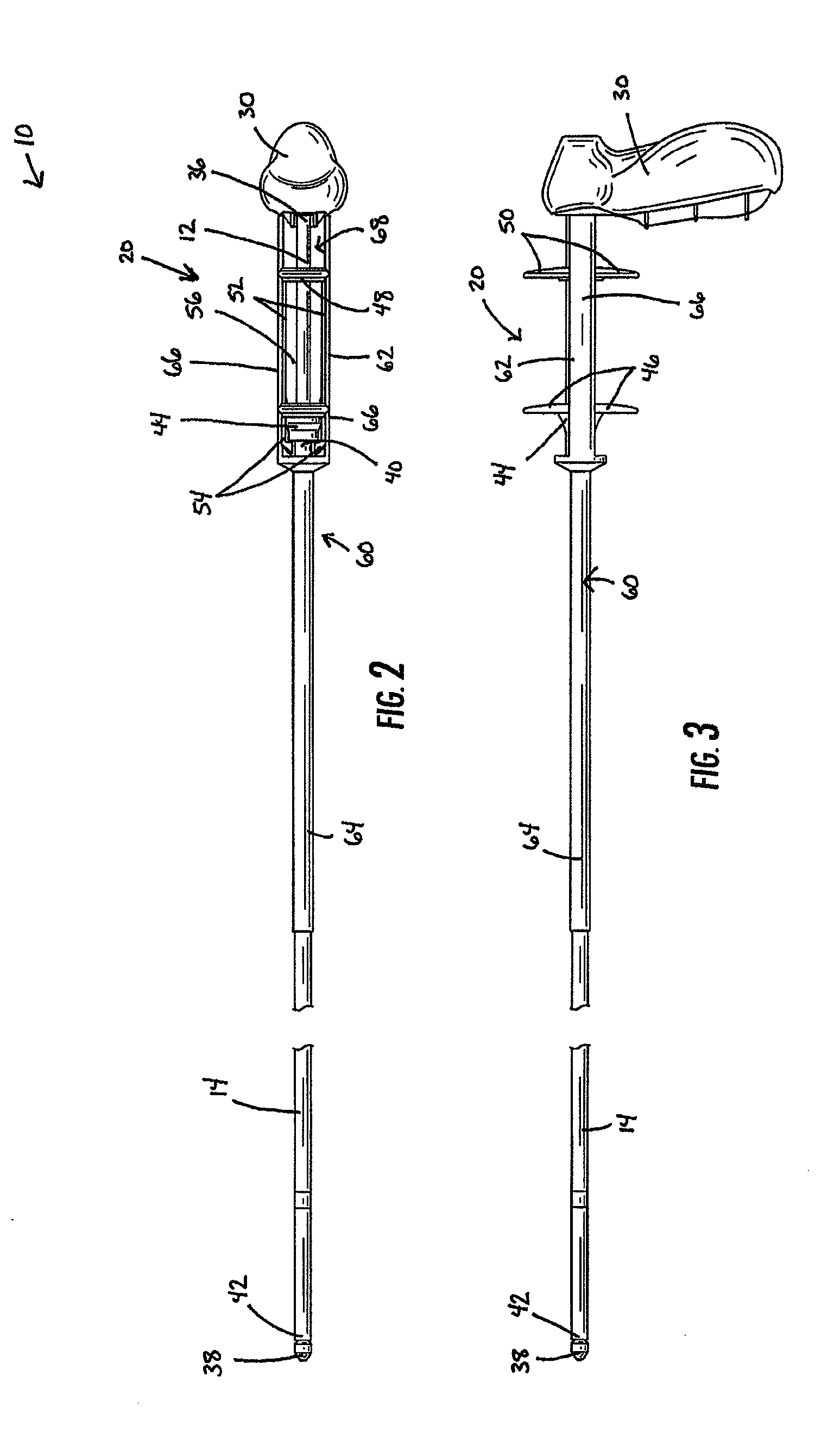
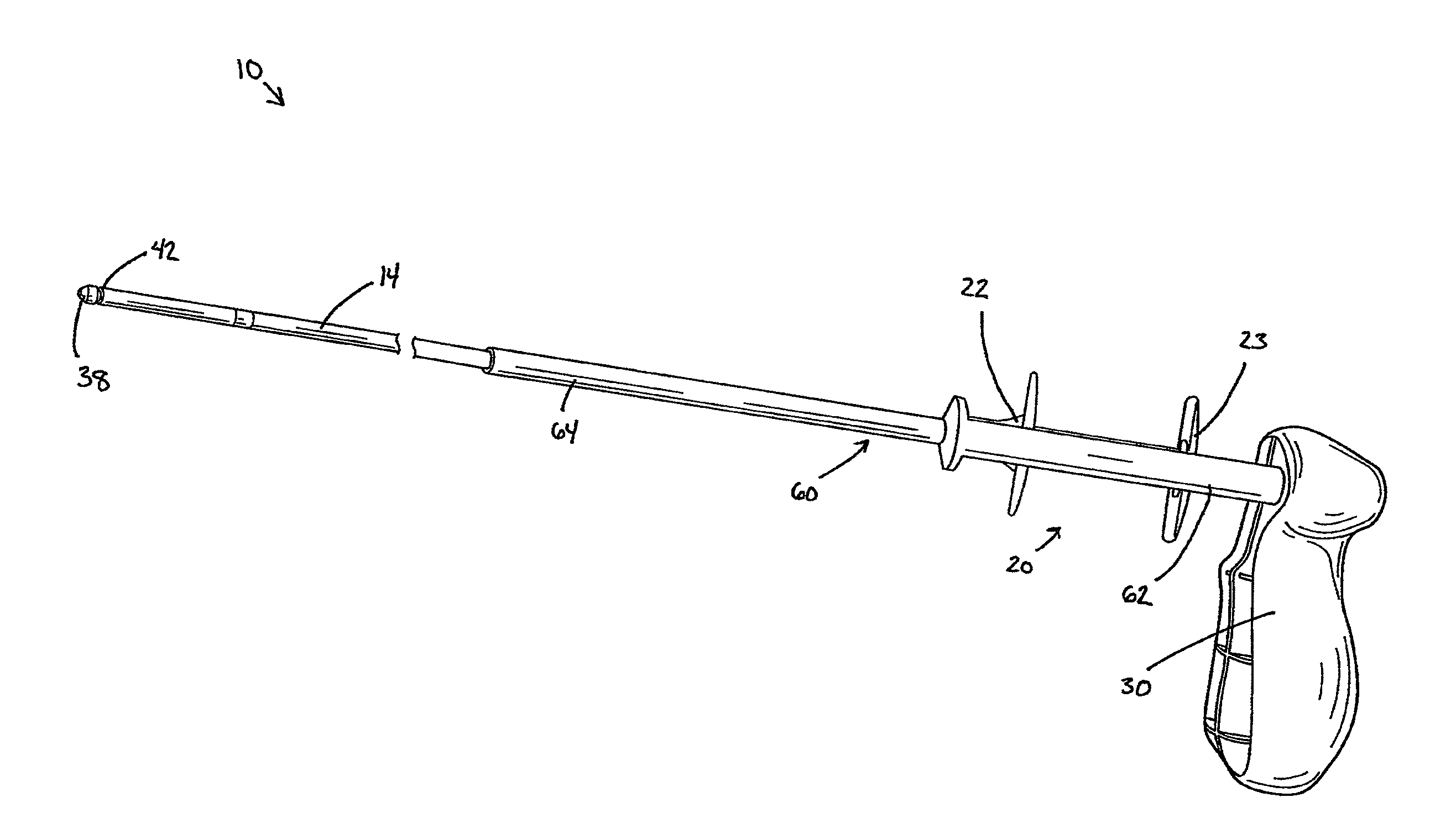
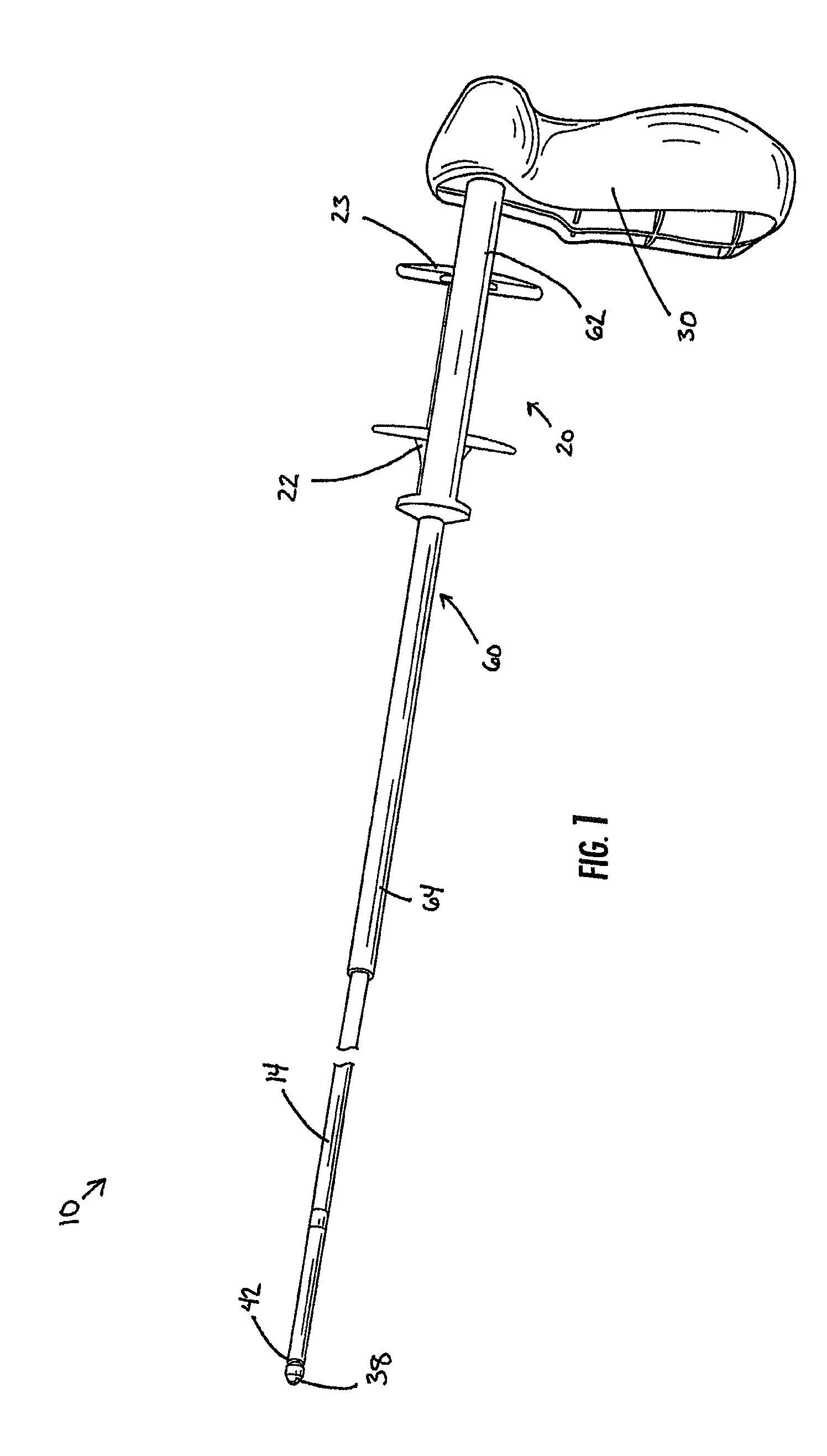
Delivery device with a protective member
A delivery device for positioning and deploying an implantable device within a lumen is provided. The delivery device includes inner and outer tubular members slidable to each other between at least a first hold position and a second release position, a handle and a deployment mechanism adapted for an operator to use a first hand for operating the delivery device and moving the tubular members between the first and second positions. The delivery device further includes a protective member adapted for insulating at least a portion of the outer tubular member from the tendency of the operator to use his or her second hand to grab the outer tubular member during operations or from other external forces.
Owner:MERIT MEDICAL SYST INC
A connectable catheter
There are provided a connectable catheter system, device and methods of use thereof. The connectable catheter system, comprising: an intermediary catheter comprising an external section and a tip section, the tip section is configured to be inserted into a body of a subject; and a reconnectable indwelling stent comprising a connecting section and a target section, the target section being configured to be located within a body of the subject, wherein the connecting section of the reconnectable indwelling stent is configured to reversibly connect, within the subject body, to the tip section of the intermediary catheter to form a continuous conduit between the intermediary catheter and the reconnectable indwelling stent.
Owner:UROGEN PHARMA LTD
Pre-Positioned Anastomosis Device and Related Methods of Use
Embodiments of a medical device and related methods of use are provided in the disclosure. The medical device includes an elongate member having a first end defining a first opening, a second end defining a second opening, and a tapered lumen extending between the first and second openings. The diameter of the first end may be smaller than that of the second end. The medical device also may include a securing mechanism protruding from one of the first or second ends to penetrate tissue.
Owner:BOSTON SCI SCIMED INC
Precision stent positioner
A positioner is provided that is able to precisely position a stent, such as a ureteral stent, by using an anatomical landmark, such as a ureteral orifice. The positioner is placed over a wire guide and advanced until the proximal portion of the stent abuts a stent-stop. The positioner and stent are together pushed until the positioner reaches the ureteral orifice. The stent can be deployed and the positioner can be removed leaving the stent correctly positioned within the kidney and bladder.
Owner:VANCE PROD INC D B A COOK UROLOGICAL INC
Degradable expandable local urine tract intracavity support system
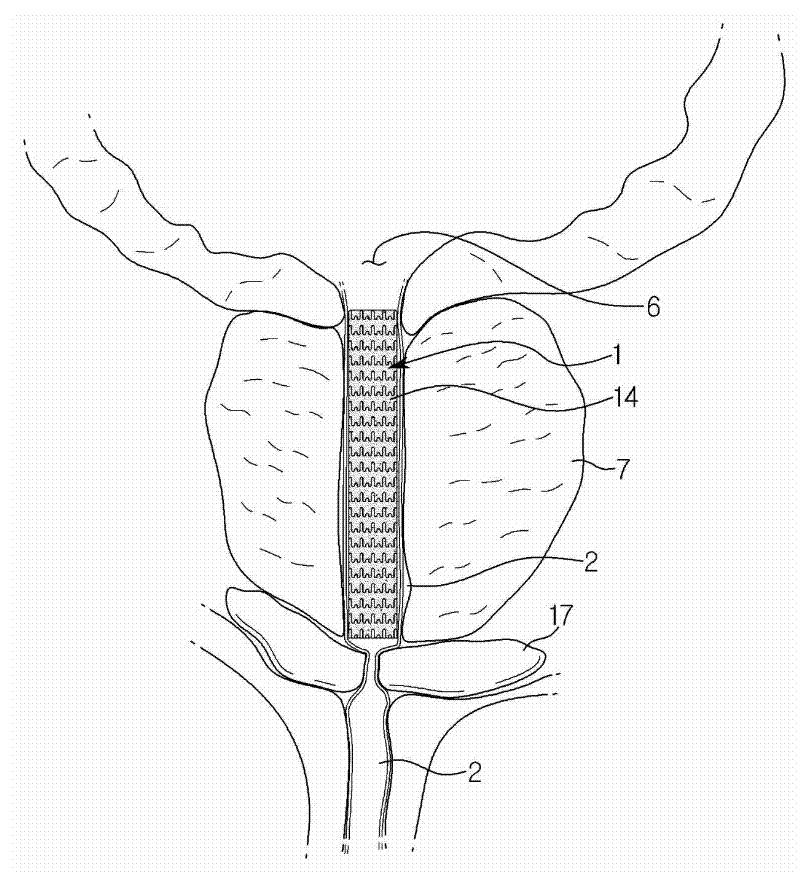
InactiveCN106473847AAct as temporary support for expansionAvoid complicationsStentsSurgeryStricture obstructionEndoluminal stent
The invention provides a degradable expandable local urine tract intracavity support system to effectively support a local lesion urine tract after urine tract stricture obstruction surgery and to prevent stricture obstruction replase due to postoperation scar hyperplasia and contracture. The support system is made of degradable material and can be degraded after scar stability and before adhesive lithogenesis formation and can be discharged with urine outside the body; and the support system comprises but is not confined to a naked rack, a drug eluting stent or a drug coating layer rack, a film covering rack, etc. The support is compressed into an exotheca with small caliber and then released after reaching a target urine tract position, then expanded and enlarged to support the lesion urine tract; the internal diameter and length of the support can be adjusted according to clinic demands; and compared with traditional double J type ureter racks and catheters, interference and damage to normal urine tract except for the stricture part can be avoided, and limitation of rack lumen inner diameter by the urine tract physiological stricture part can be avoided.
Owner:曹庆杰 +1
Delivery system and urethra stent for enlarged prostates and method
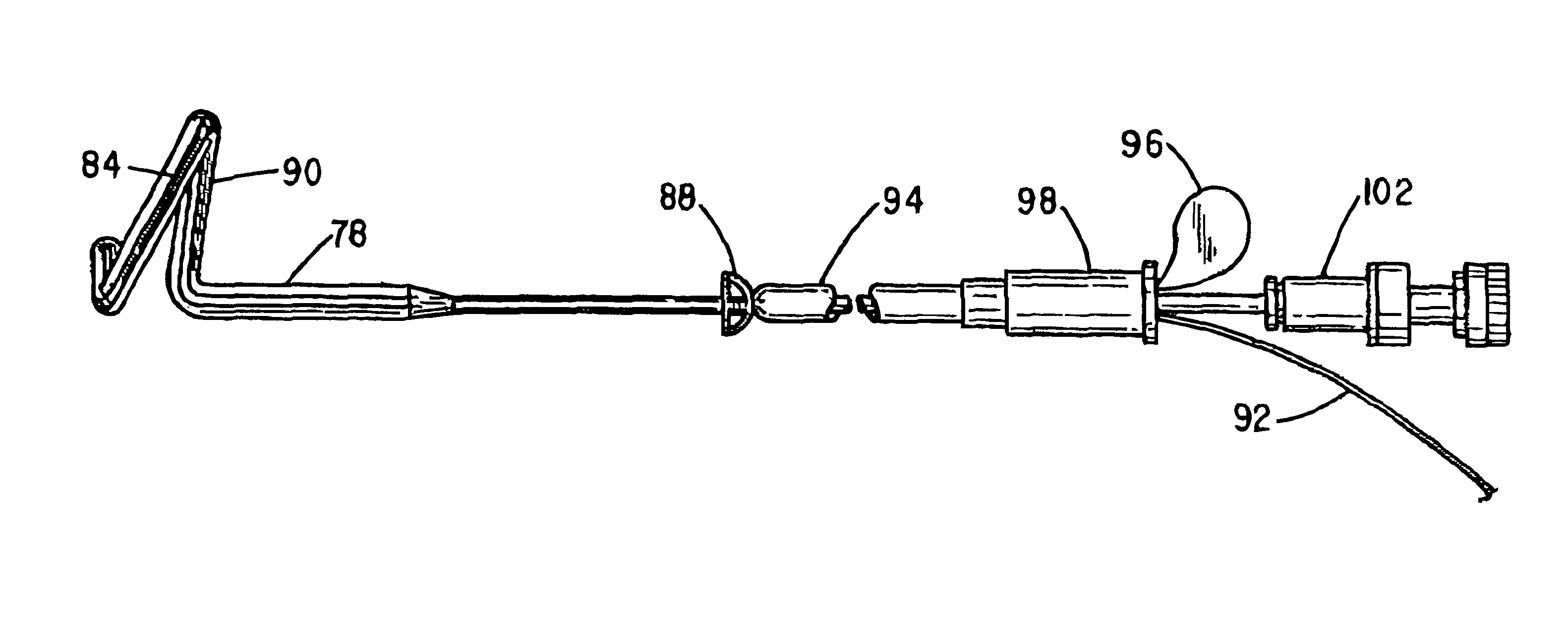
A device for placing, positioning and / or removing a stent within the urethra of a patient obstructed by an enlarged prostate positioned by use of ultrasound comprising a flexible stent inserter having a shaft of a length and size to be inserted within the urethra and extend to the bladder, with a balloon positioning tip distally mounted proximate an expandable securing segment onto which a stent is mounted; the balloon selectively inflated with air or liquid if a contrasting media is required for ultrasound location or with a liquid to unblock obstructed urethra segments, and the securing segment selectively inflated with a liquid to secure during positioning and deflated to release the stent in position to inflate the expandable securing segment to hold the stent during positioning and insertion, and in a second mode deflates the securing segment and positioning tip to release the stent within the urethra to allow the flexible stent inserter to be withdrawn and allow urine to pass through the stent.
Owner:SCHANZ V
Tissue-Engineered Constructs
InactiveUS20130013083A1Improve degradation rateImprove wettabilityUrethraeCell culture supports/coatingCell-Extracellular MatrixVein graft
Constructs including a tubular biodegradable polyglycolic acid scaffold may be coated with extracellular matrix proteins and are substantially acellular. The constructs can be utilized as an arteriovenous graft, a coronary graft, an arterial graft, a venous graft, a duct graft, a skin graft, or a urinary graft or conduit.
Owner:HUMACYTE INC
Implantable medical device with pharmacologically active ingredient
An implantable medical device comprising an inner region and an outer region positioned over at least a portion of the inner region and in contact with a surface of the inner region. The durometer of the inner region is greater than the durometer of the outer region and a pharmacologically active ingredient is present in at least a portion of the inner region or the outer region.
Owner:VANCE PROD INC D B A COOK UROLOGICAL INC
Medical delivery device with a protective member
A delivery device for positioning and deploying an implantable device within a lumen is provided. The delivery device includes inner and outer tubular members slidable to each other between at least a first hold position and a second release position, a handle and a deployment mechanism adapted for an operator to use a first hand for operating the delivery device and moving the tubular members between the first and second positions. The delivery device further includes a protective member adapted for insulating at least a portion of the outer tubular member from the tendency of the operator to use his or her second hand to grab the outer tubular member during operations or from other external forces.
Owner:MERIT MEDICAL SYST INC
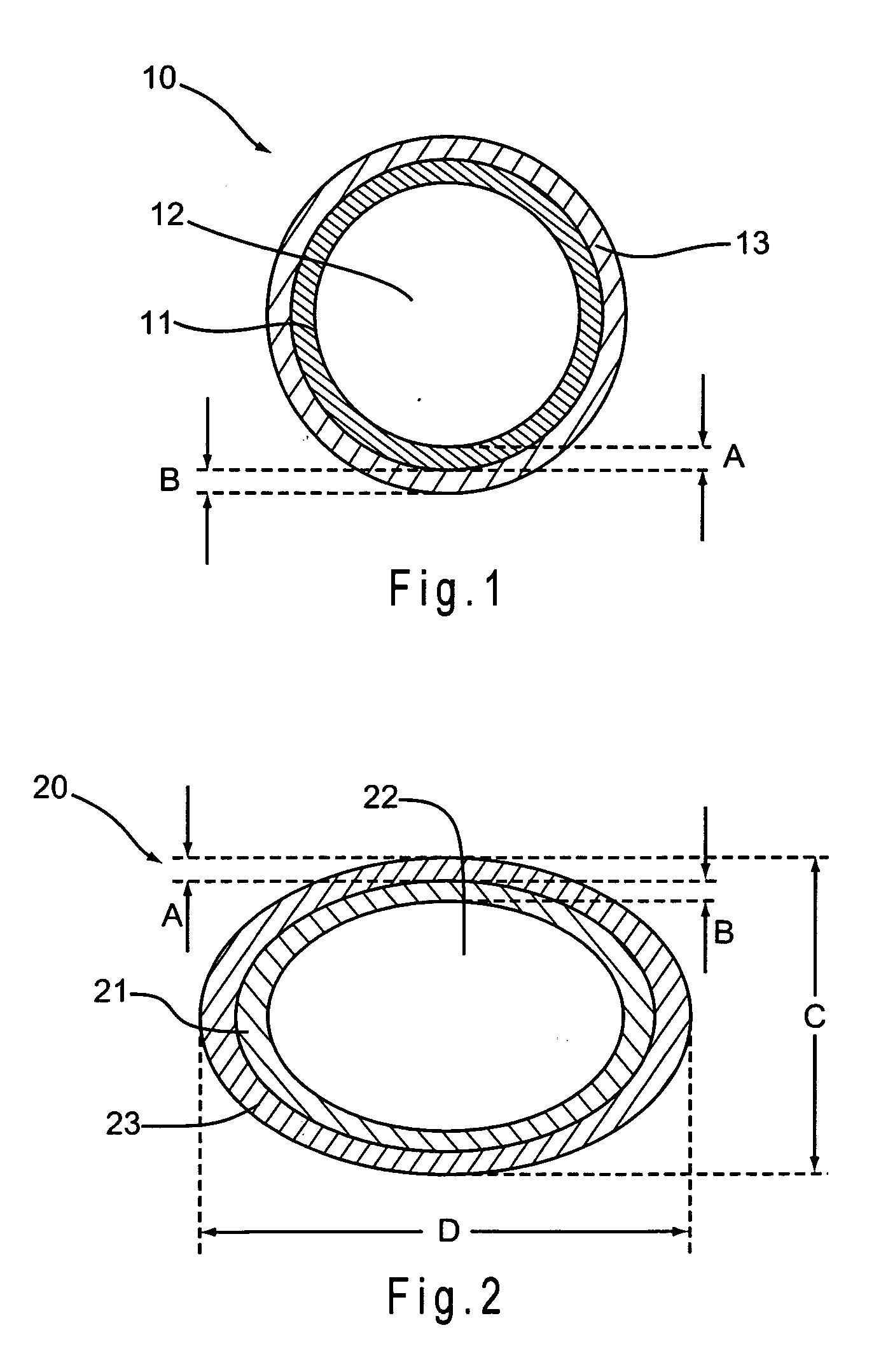
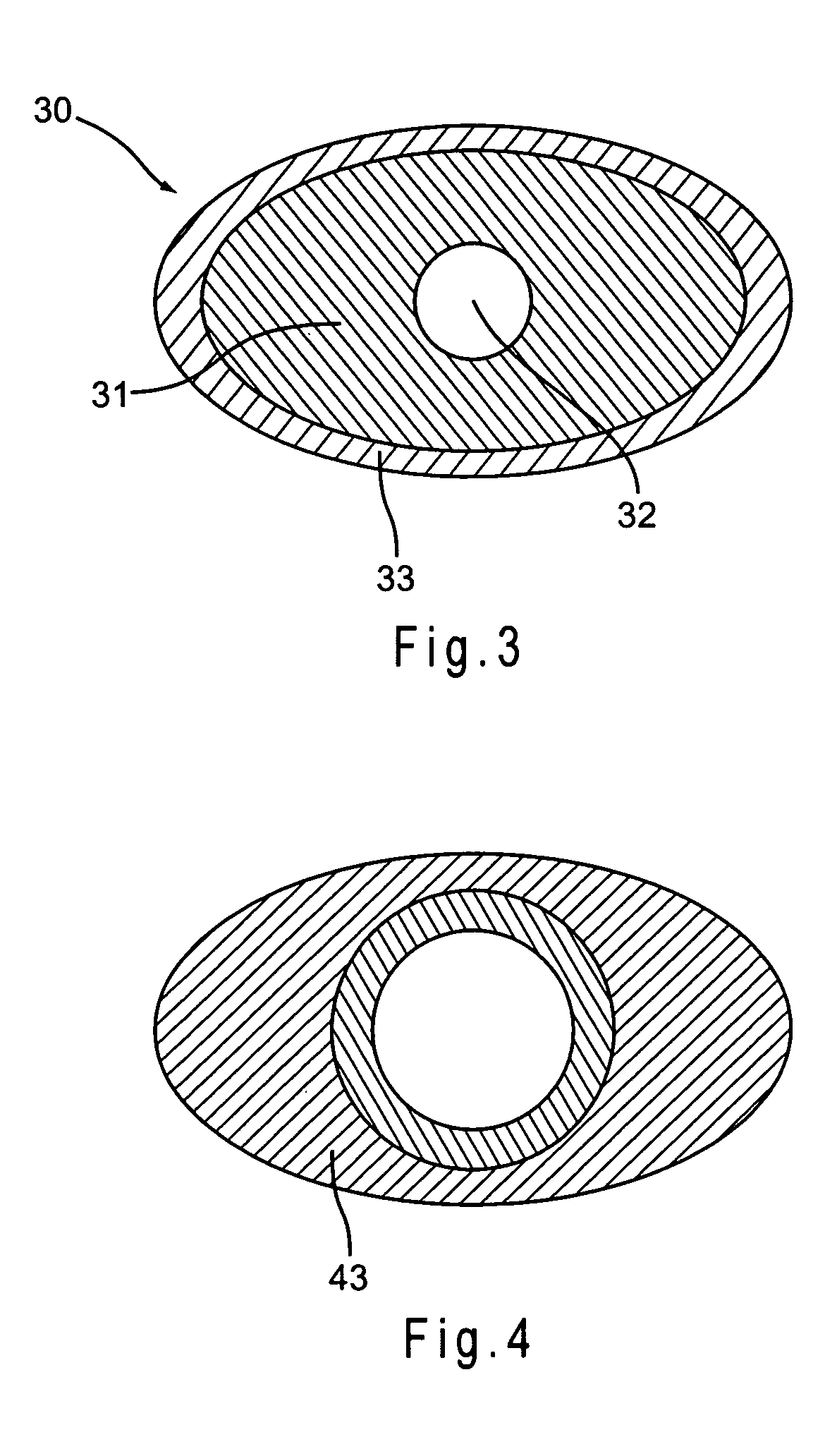
Urethral patency implant
InactiveUS20160242894A1Reduce Shrinkage ProblemsReduce and eliminate re-growthStentsUrethraeUrineUrethra
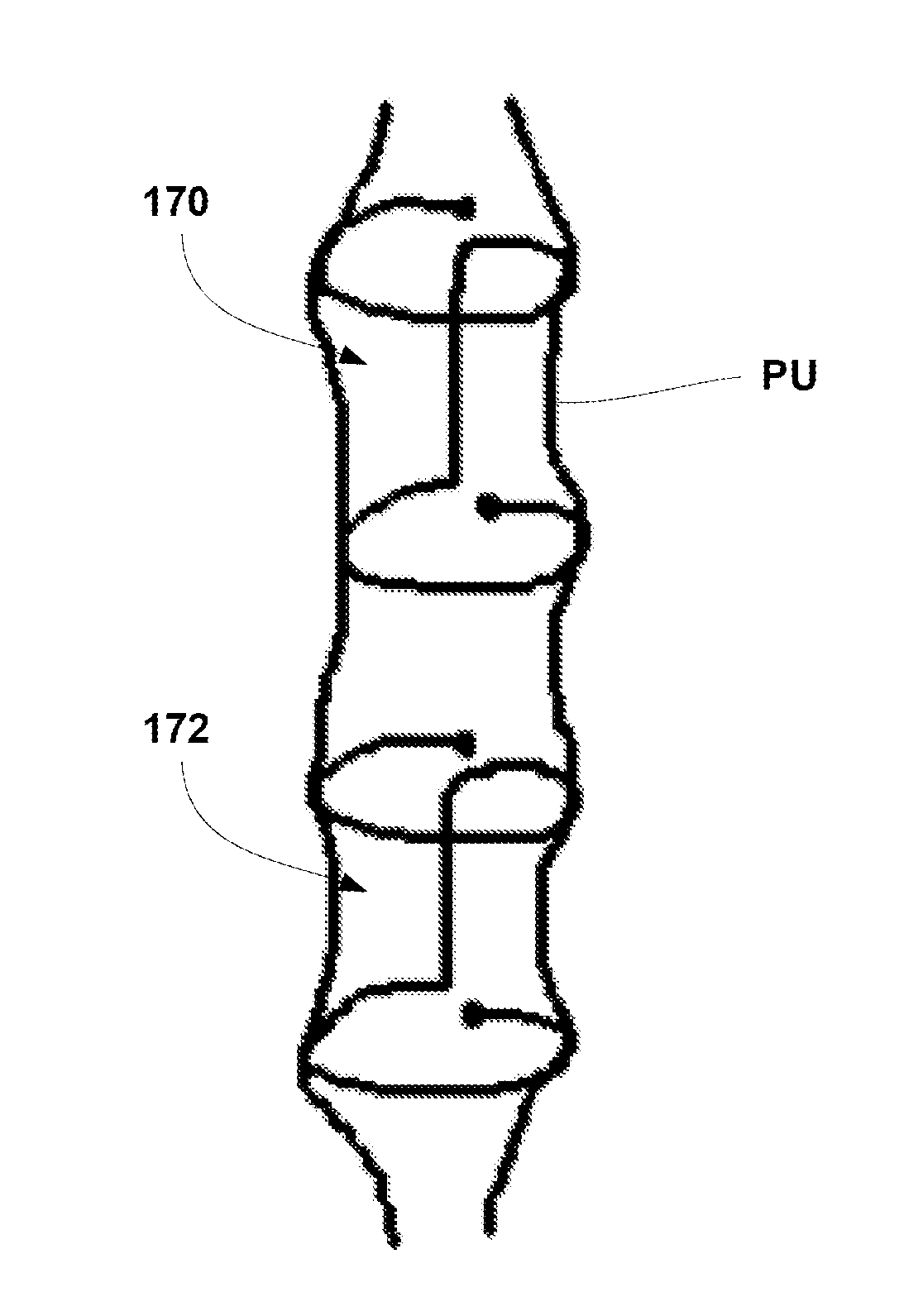
A urethral scaffold is provided for expanding a portion of the urethra that extends through the prostate. The scaffold is expandable and has a plurality of undulating circumferential members disposed between a proximal end and a distal end of the scaffold. A central section of the scaffold has a width that exceeds the width at the distal end of the scaffold. The scaffold is made of a material that reacts with urine or with tissue surrounding the urethra to reduce the volume of the scaffold such that the scaffold can self-explant without an interventional procedure.
Owner:SCAFFOLD MEDICAL AG
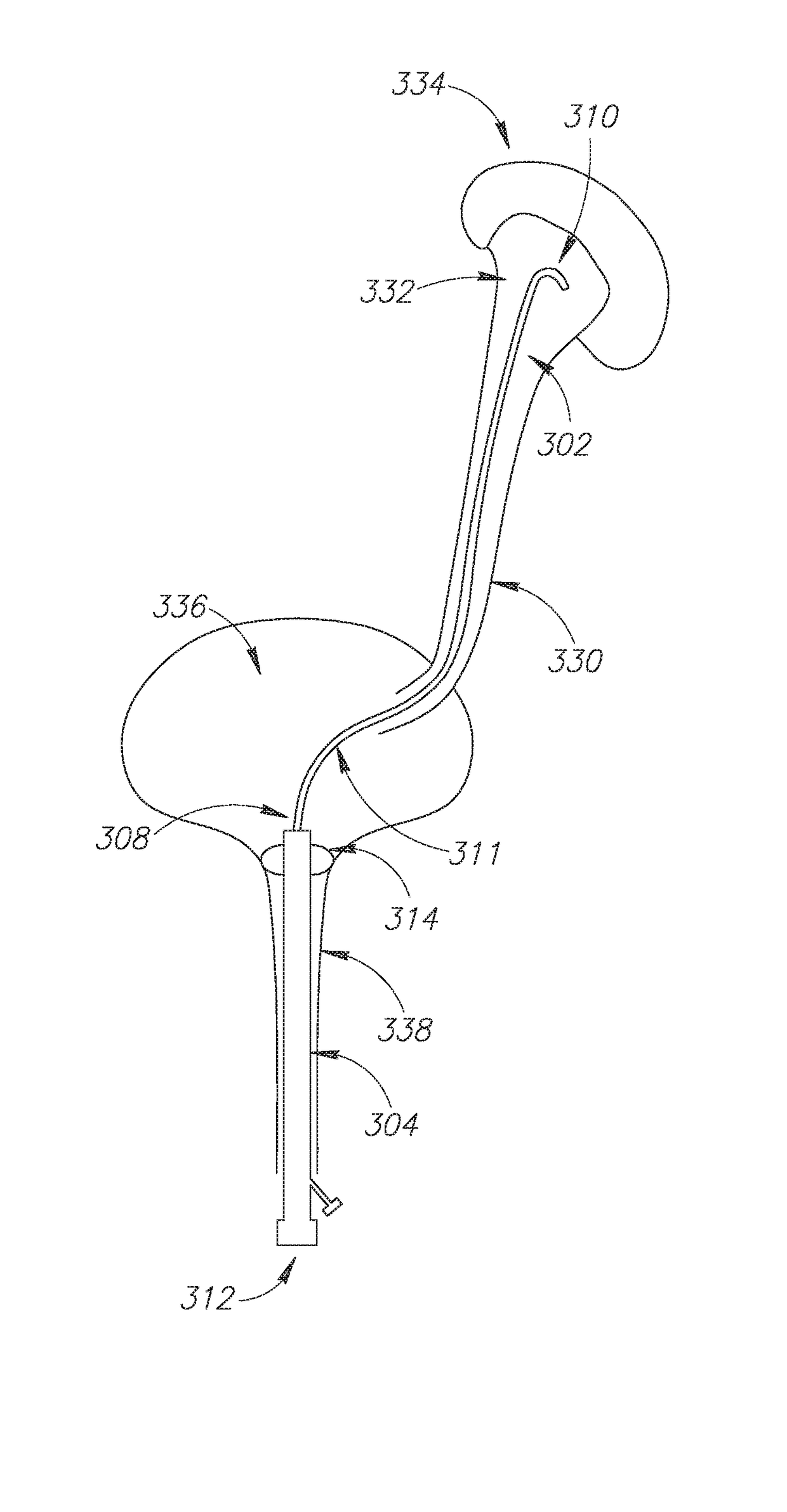
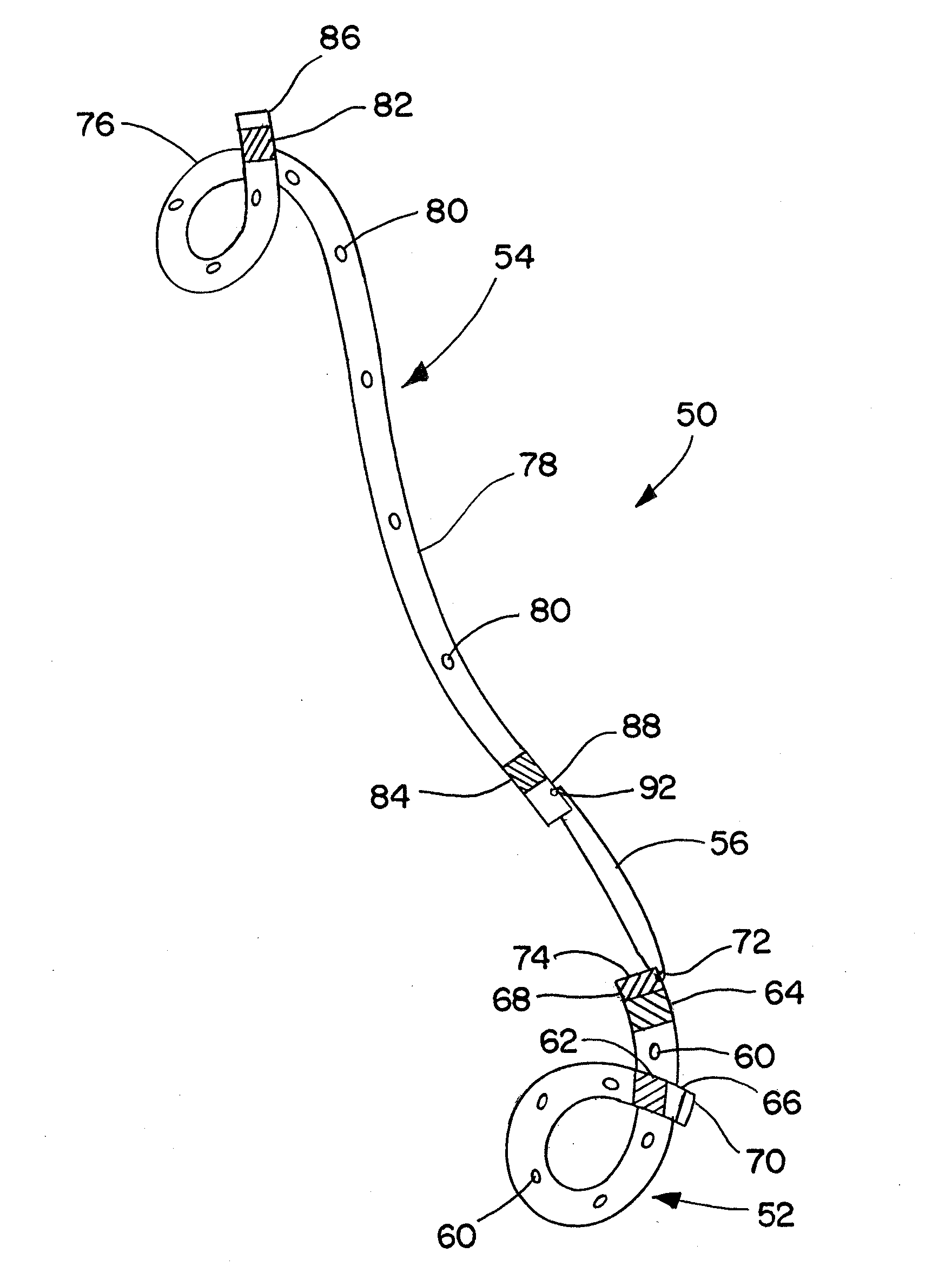
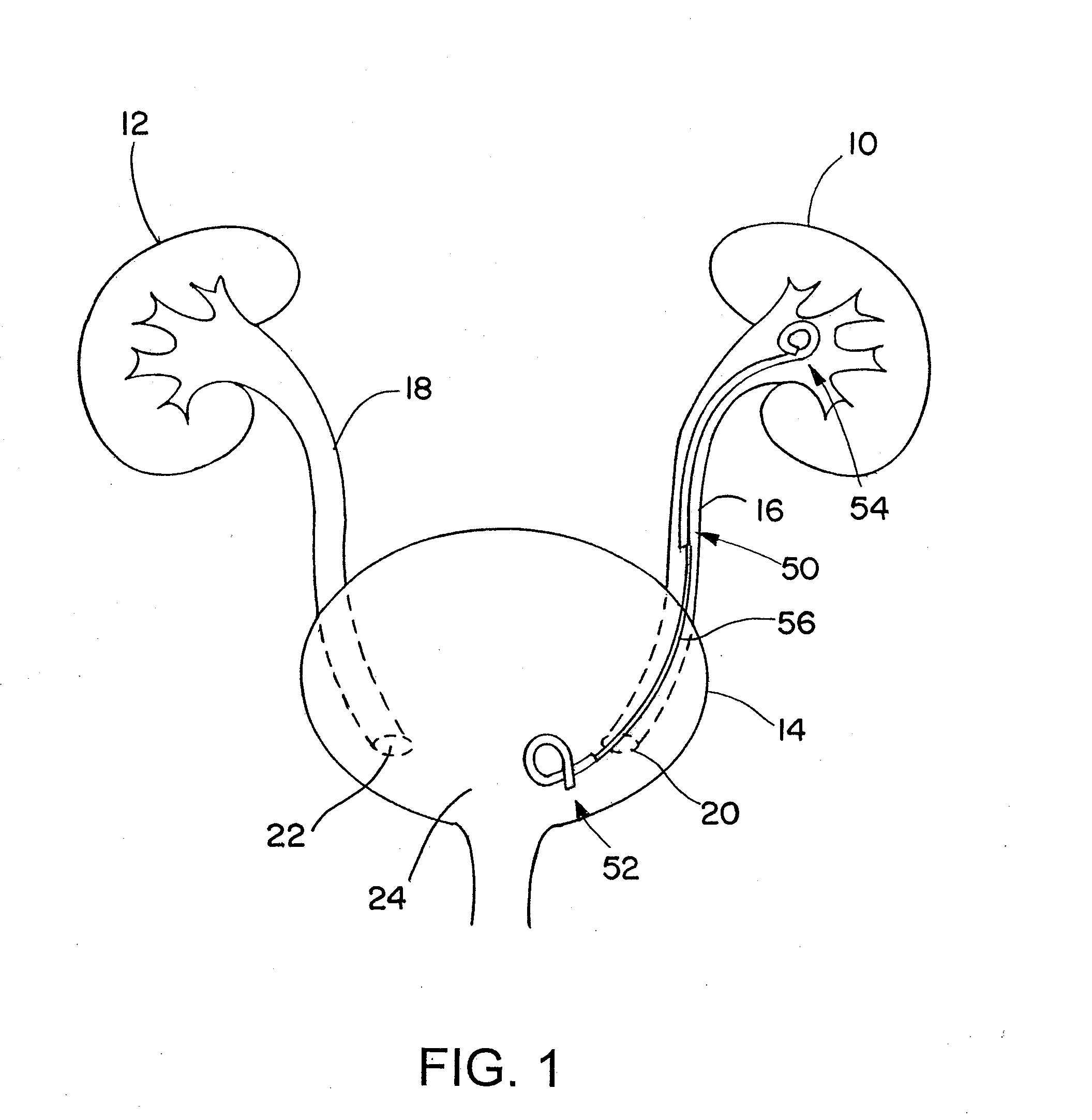
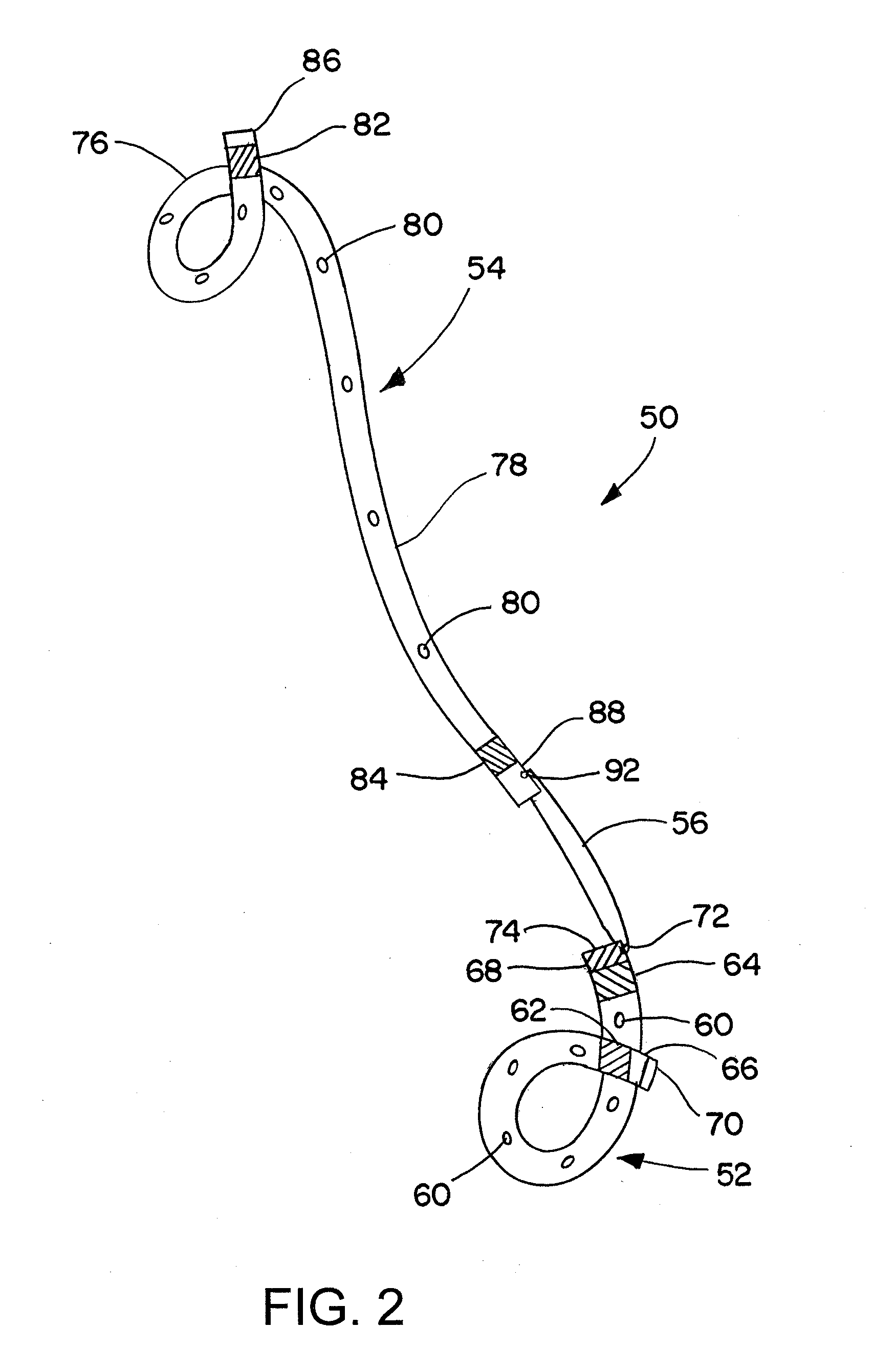
Ureteral stent for placement in a kidney and bladder
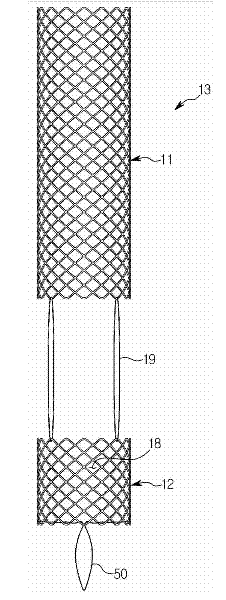
Provided is a ureteral stent (=50) including a bladder portion (=52) positioned in a bladder of a patient, a kidney portion (=54) positioned in a kidney and ureteral passageway of the patient, and one or more tethers (=56) coupling the bladder portion to the kidney portion. The ureteral stent allows urine to pass around a blockage, and allows a ureter orifice connecting the ureteral passageway to the bladder to move between a compressed state and an uncompressed state to prevent or minimize urinary reflux, flank pain, blood in the urine, etc., while allowing the bladder portion to move freely in the bladder to prevent the bladder portion from irritating the trigone muscle.
Owner:UNIV HOSPITALS HEALTH SYST
Urethral stent for the prostate
A urethral stent for the prostate including a first stent section placed into an upper portion of the urethra passing through the prostate, a second stent section placed into a lower portion of the urethra below a urinary sphincter muscle, the first and second stent sections being formed of a shape-memory alloy wire, and at least one connection filament is provided at a middle portion of the urethra passing through the urinary sphincter muscle so as to connect the first and second stent sections, thereby preventing the stent from moving to the upper or lower portions of the urethra.
Owner:TAEWOONG MEDICAL CO LTD +1
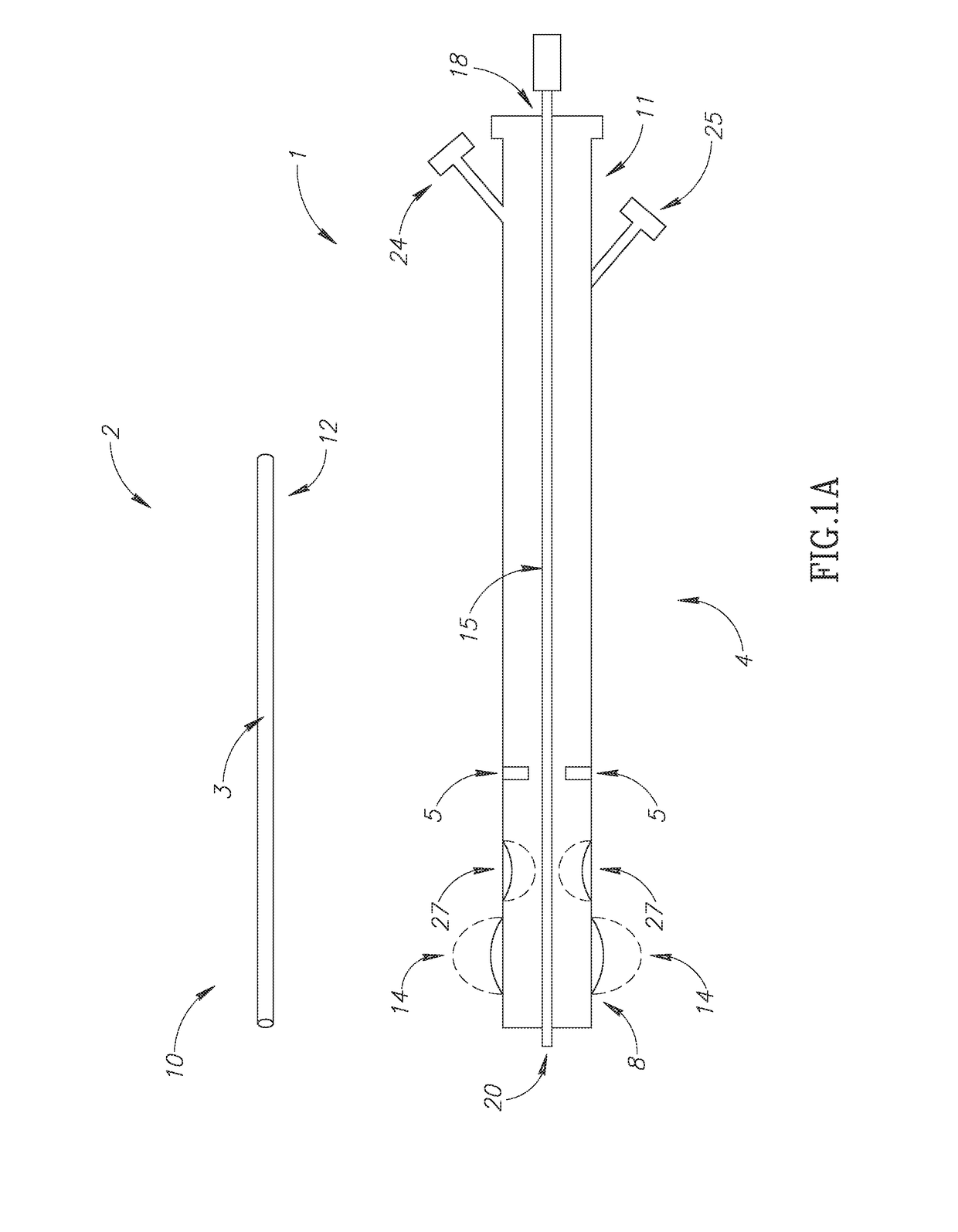
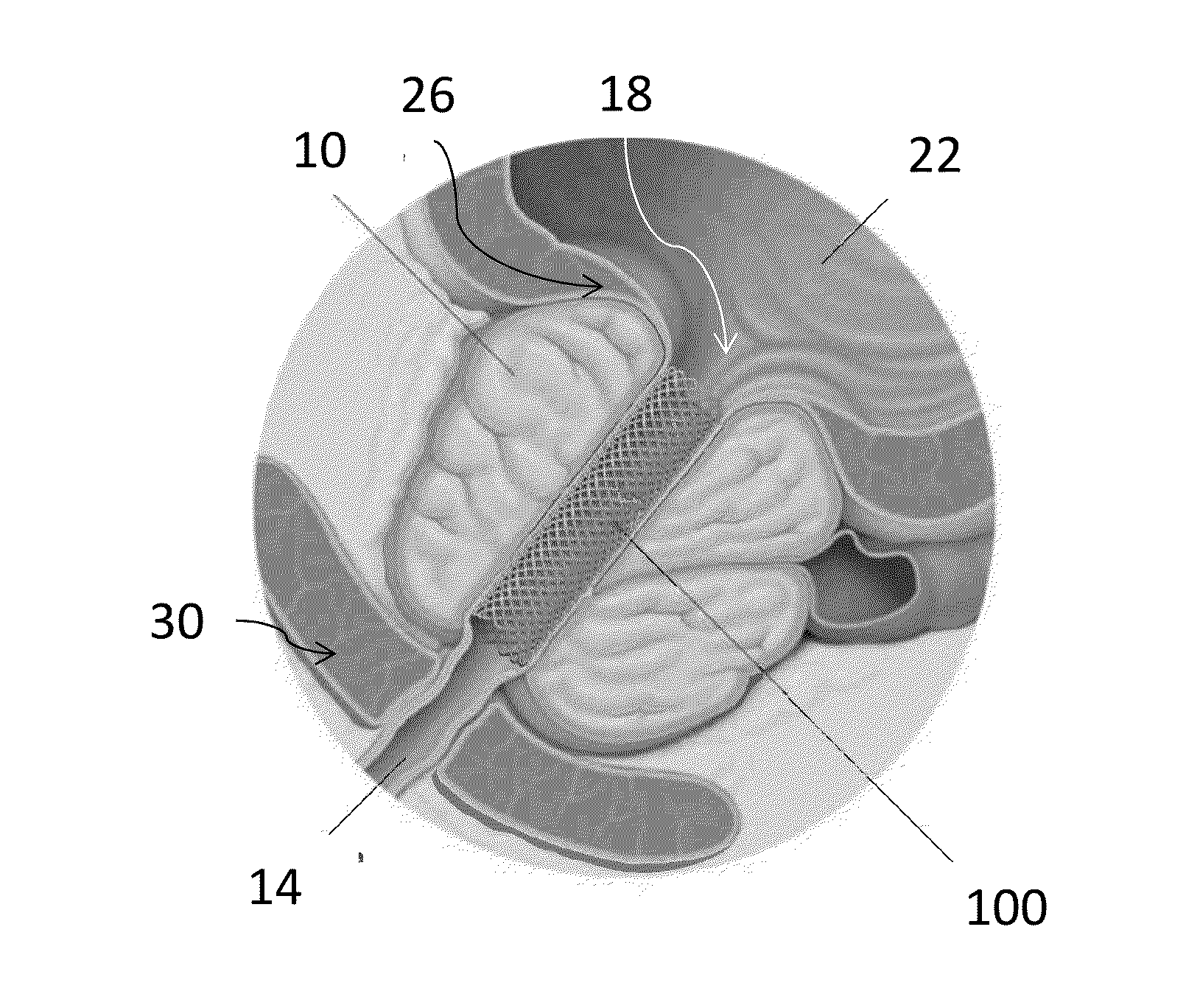
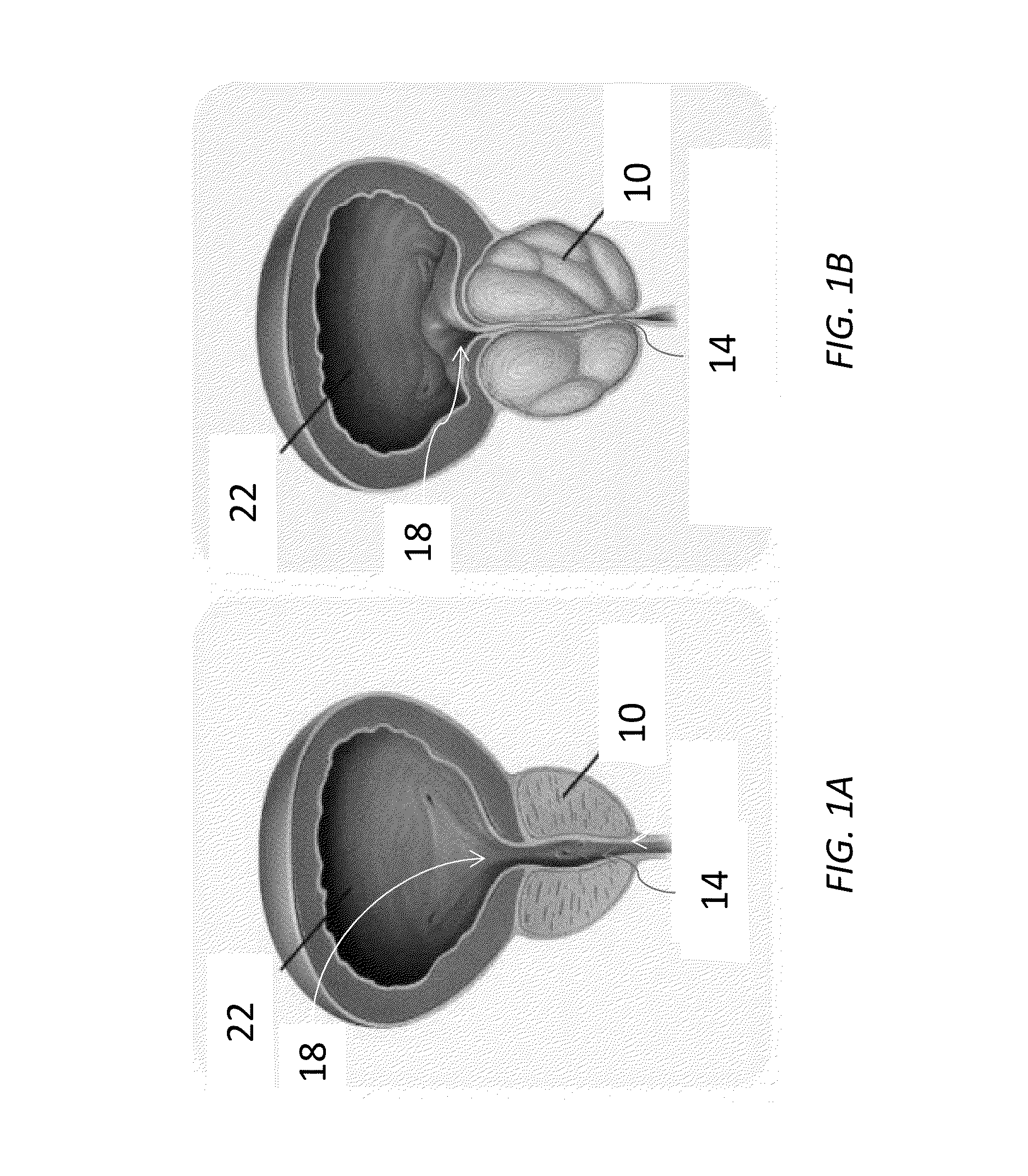
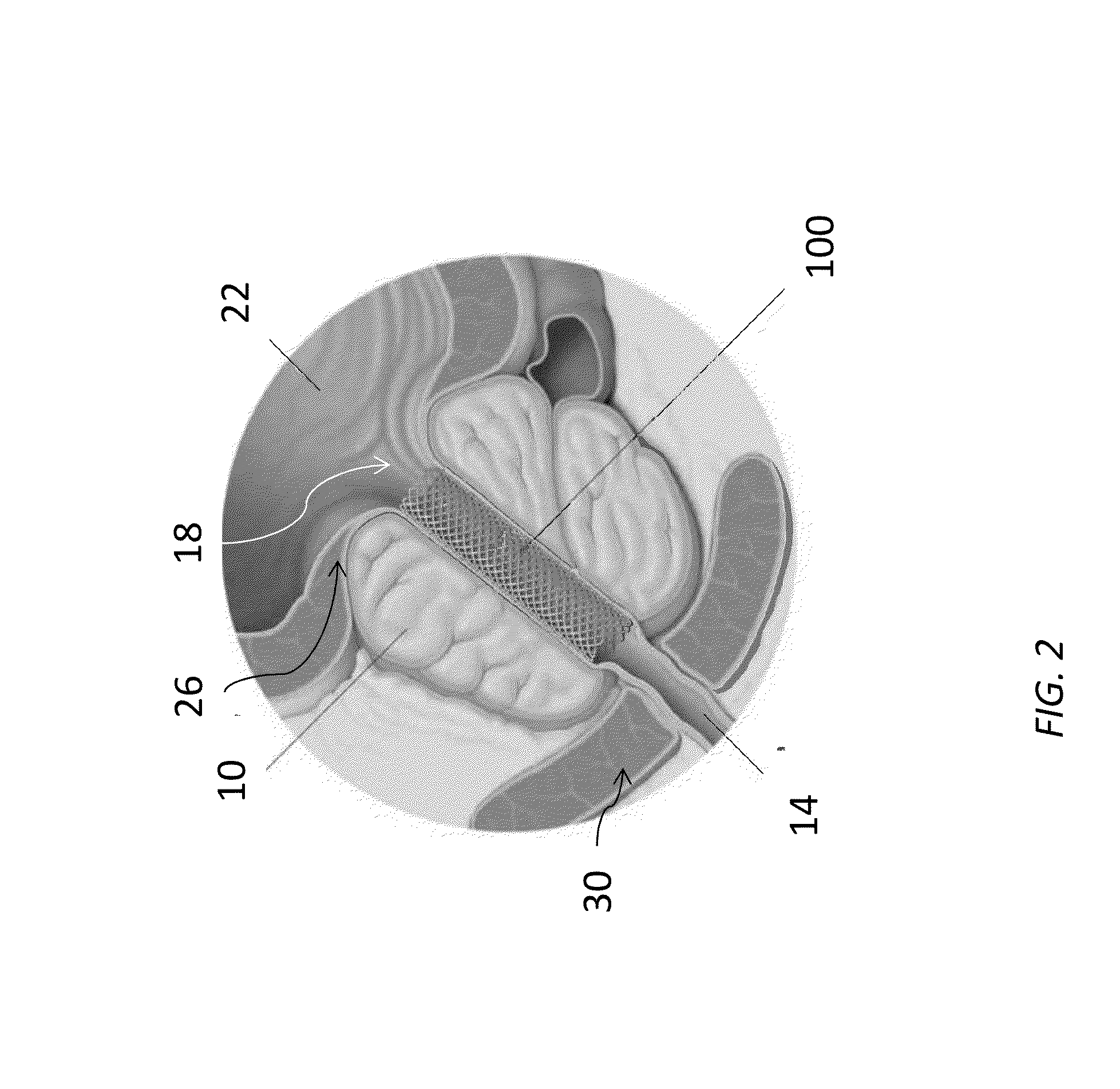
Device for positioning a stent
The invention concerns a device (23) for positioning a stent in a natural canal (22) after the passage through an obstacle at the level of a target organ (19). The device (23) comprises a stent (6), a pusher tube (16) and a guide (1). The said guide (1) is conceived to push the stent (6) through the lumen of the natural canal (22), and comprises at least one flexible part having a predetermined longitudinal stiffness and a lateral flexibility consisting in a reversible deformation from a straight position to a bent position so as to follow the curve of the natural canal (22) under the application of minimal external forces exerted by an operator. The invention is particularly adapted to the technical field of urology.
Owner:马里昂·德沃纳
Stent including anchoring members
A method for treating an intestine with an expandable scaffolding expanded within the intestine. After placing the expandable scaffolding at a target location, such as across a fistula, the first and second end portions of the expandable scaffolding are radially expanded such that the first and second end portions contact an inner surface of the intestine on opposing sides of the fistula, anchoring the first and second end portions to the intestine. Radially expanding the first and second end portions foreshortens the medial portion along the longitudinal axis such that the first and second end portions are drawn closer together along the longitudinal axis as the medial portion foreshortens to close the fistula.
Owner:BOSTON SCI SCIMED INC
Urethra pressure control valve to control incontinence
InactiveUS20090018385A1Easy to installAnti-incontinence devicesMedical devicesAbutmentControl valves
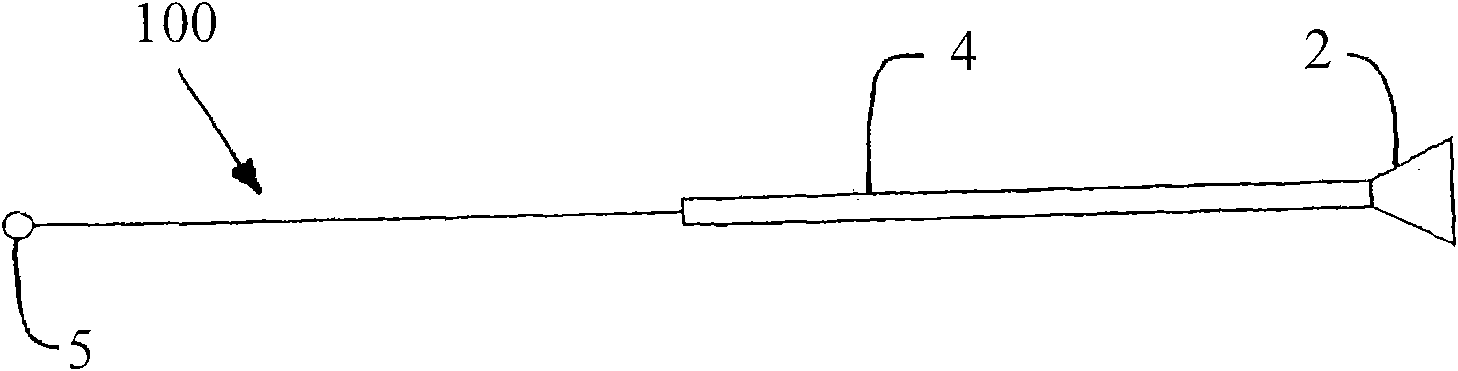
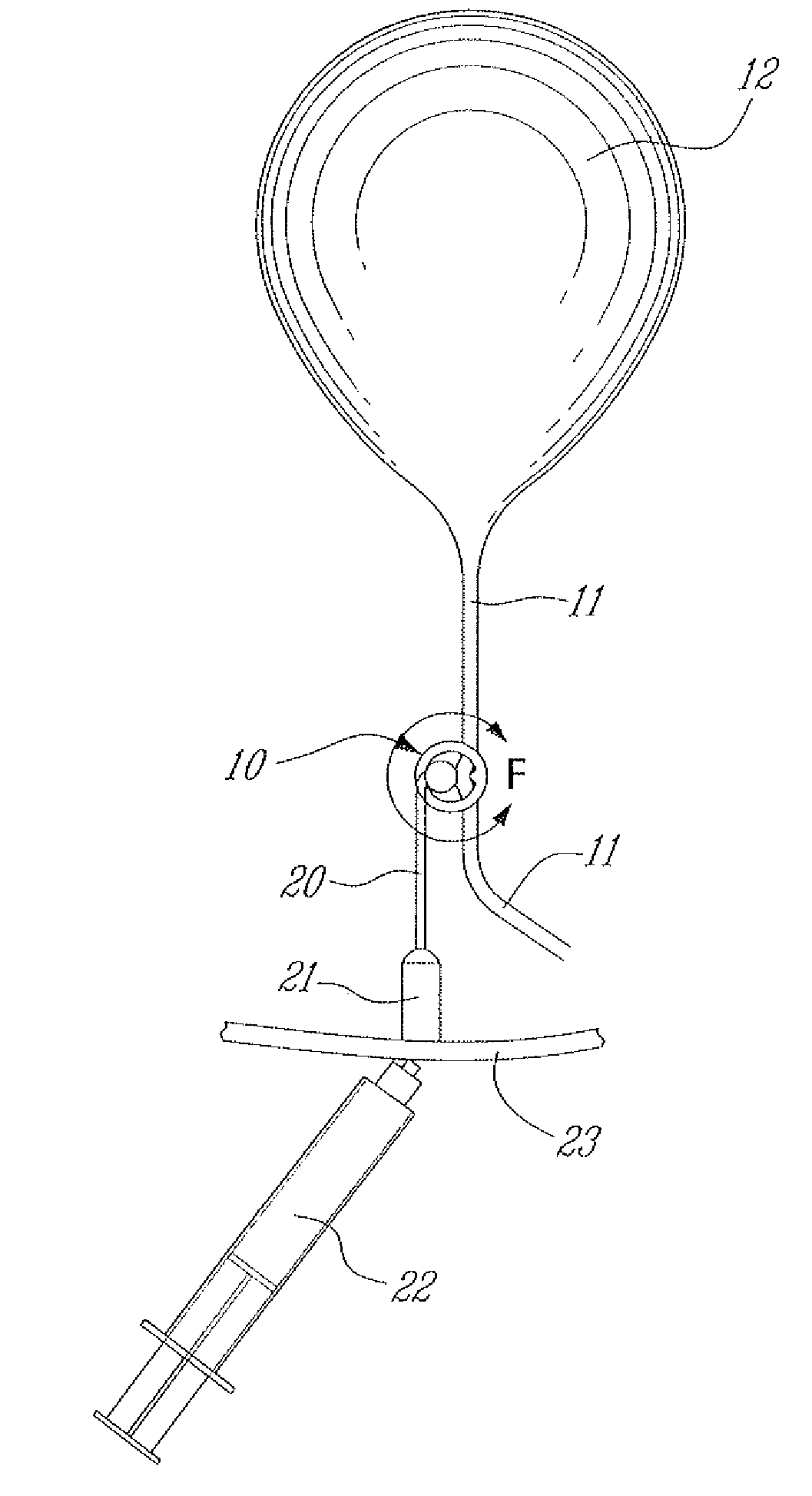
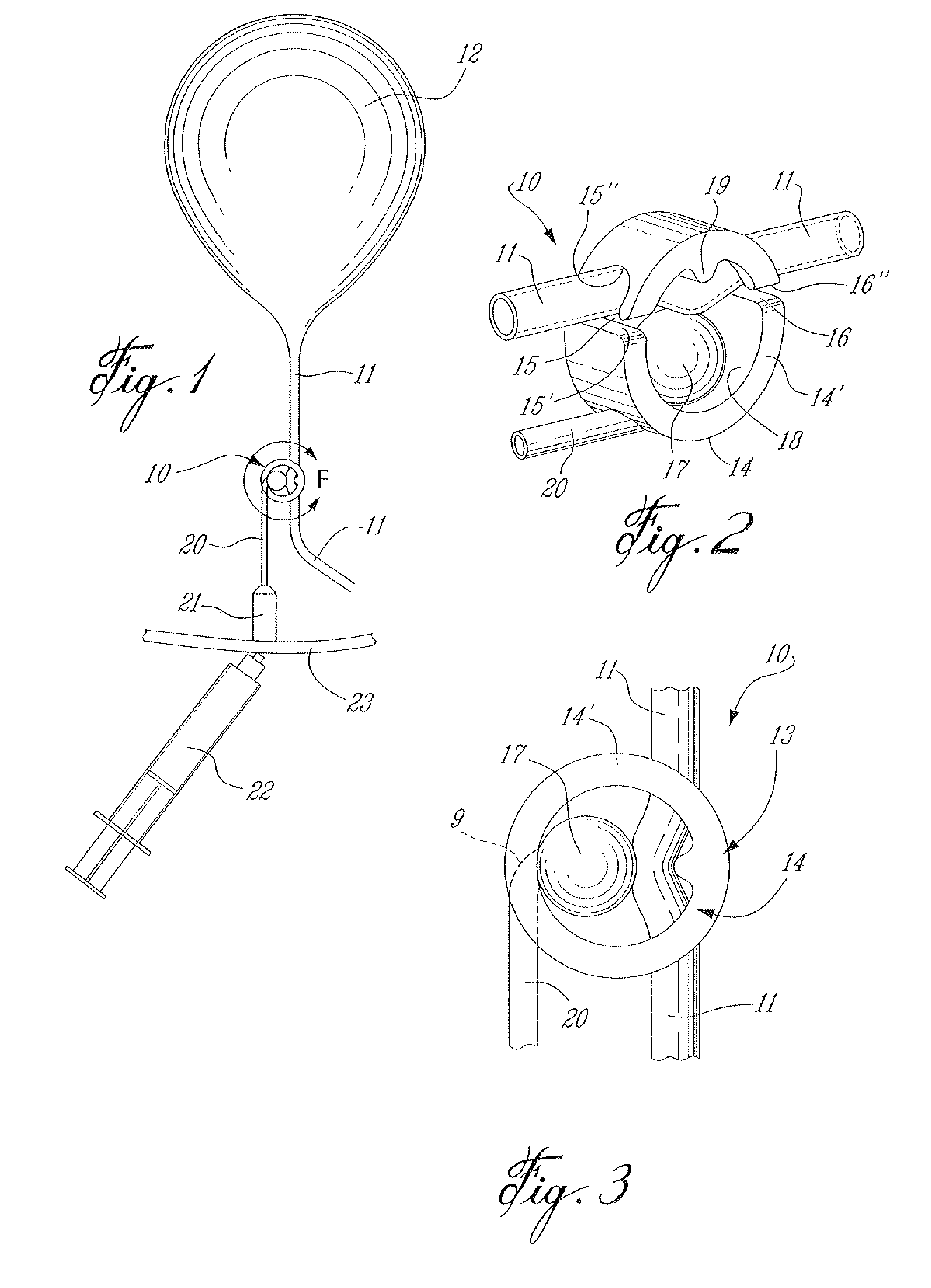
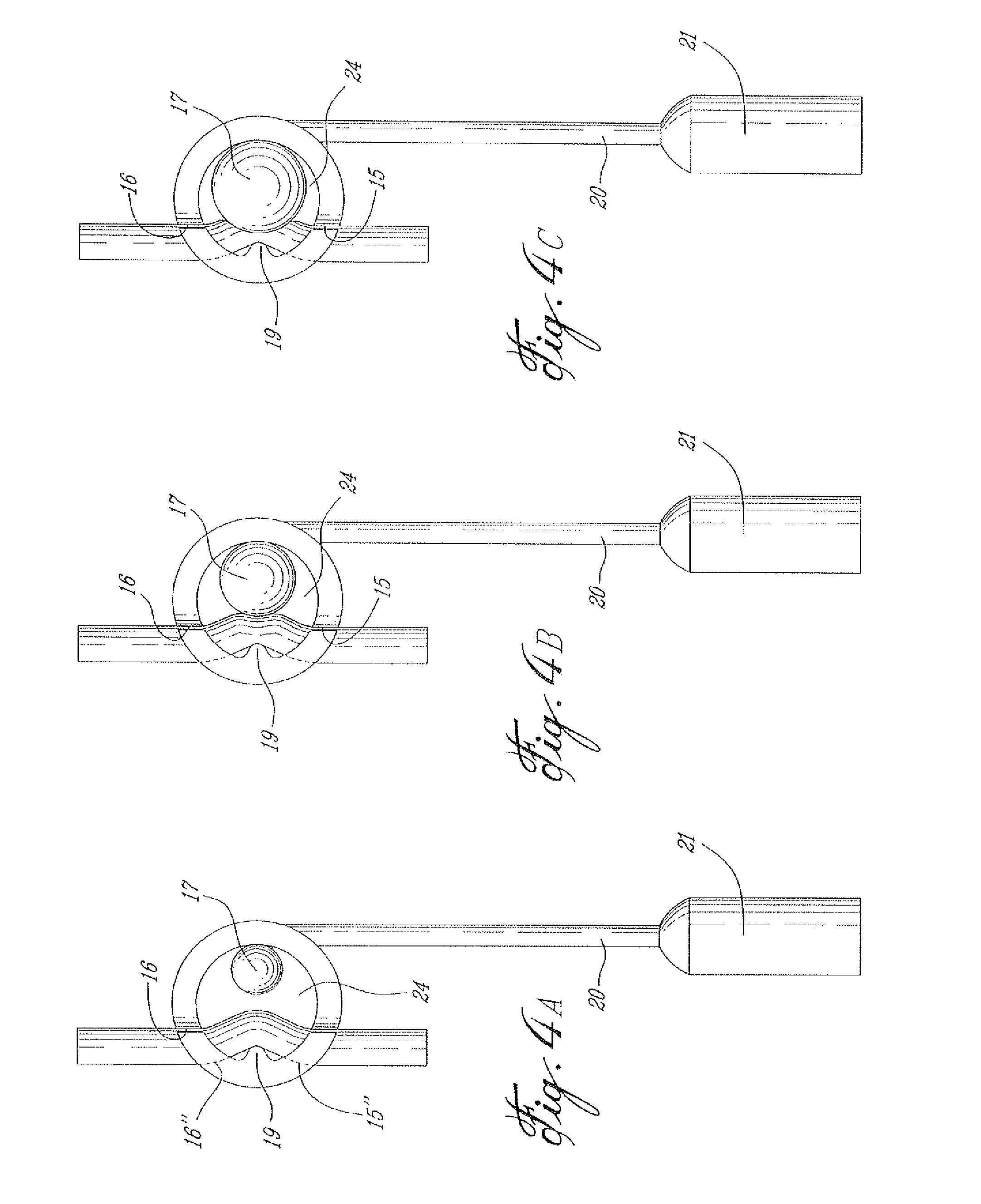
A surgically implantable urethra pressure control adjustable valve for male and female patients is described. The valve comprises a clamp positionable about a urethra in a patient's body. The clamp has a circumferential wall having a first and a second opening. The openings are oppositely spaced apart at predetermined locations to provide for the passage of the urethra through the circumferential wall. A pressure abutment element is provided inside the circumferential wall and disposed between an inner wall surface of the circumferential wall and the urethra and actuable to apply a contained control pressure against the urethra to close the urethra by pinching same against a diametrically opposed immovable abutment element formed in the circumferential wall and projecting inwardly therein. The contained controlled pressure is adjustable and selected so that liquid pressure from a bladder associated with the urethra and under the influence of muscle control will cause the urethra to open against the contained controlled pressure to discharge liquid from the bladder and to automatically close once the pressure from the bladder is discontinued by muscle control.
Owner:TRUBIANO RONALD +1
Urethral Stent System and Method
A urethral stent device comprising a mechanical valve system is provided. The urethral stent device can be delivered up through the urethra via a flexible delivery tool. The valve mechanism is adapted to open when exposed to a certain amount of intraluminal pressure and can remain open until a desired cessation of fluid flow is achieved.
Owner:BOSTON SCI SCIMED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com