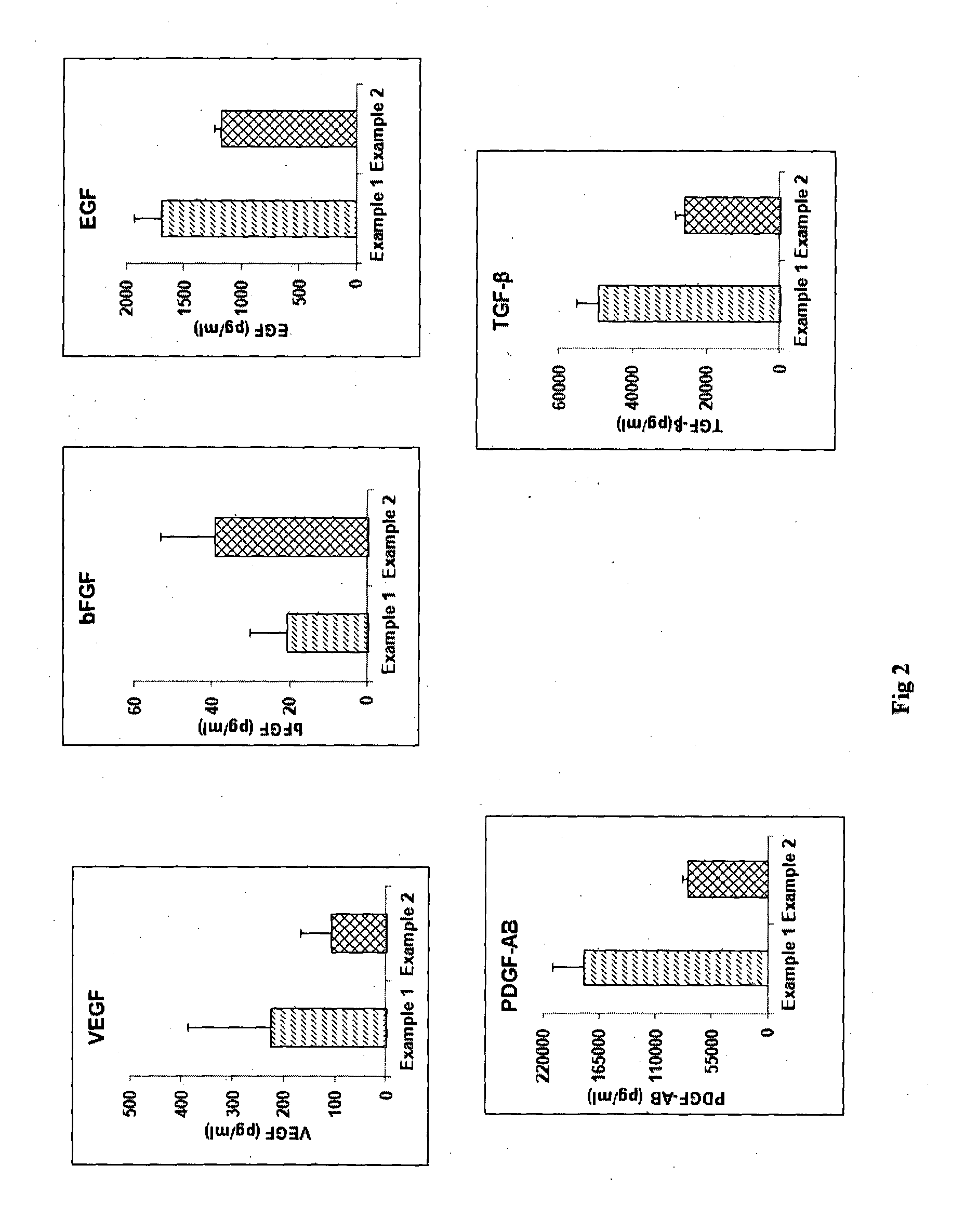
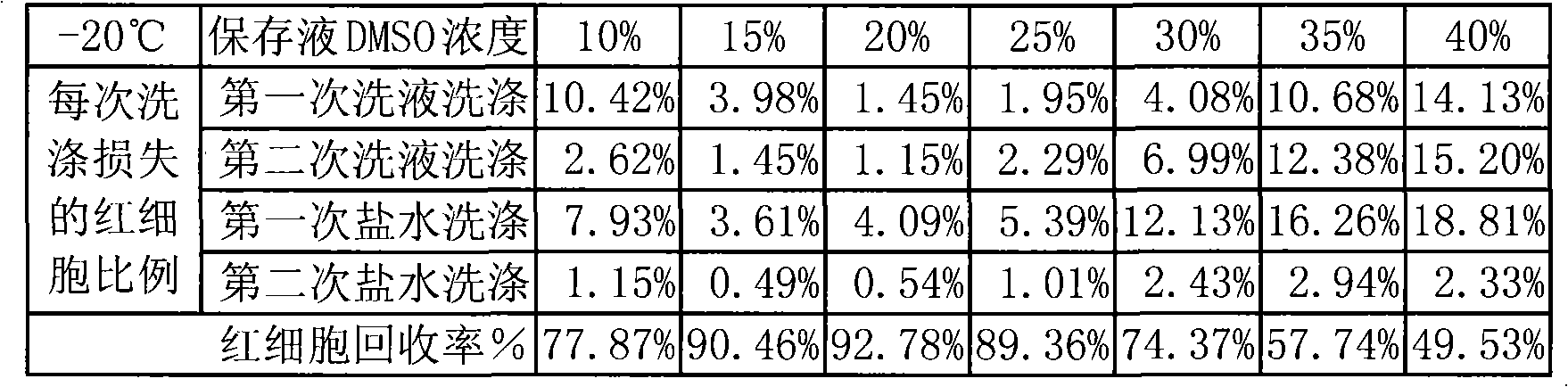
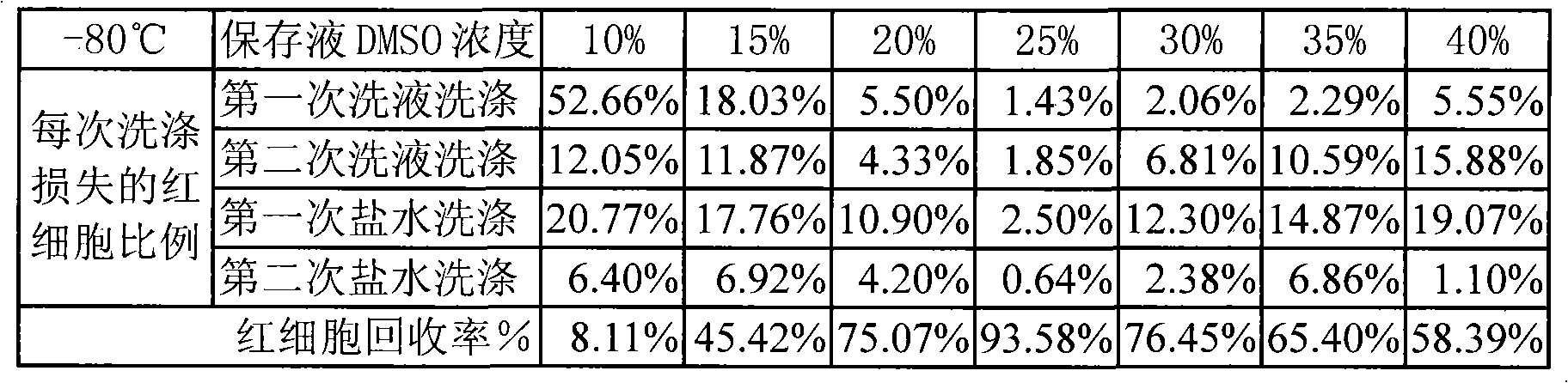
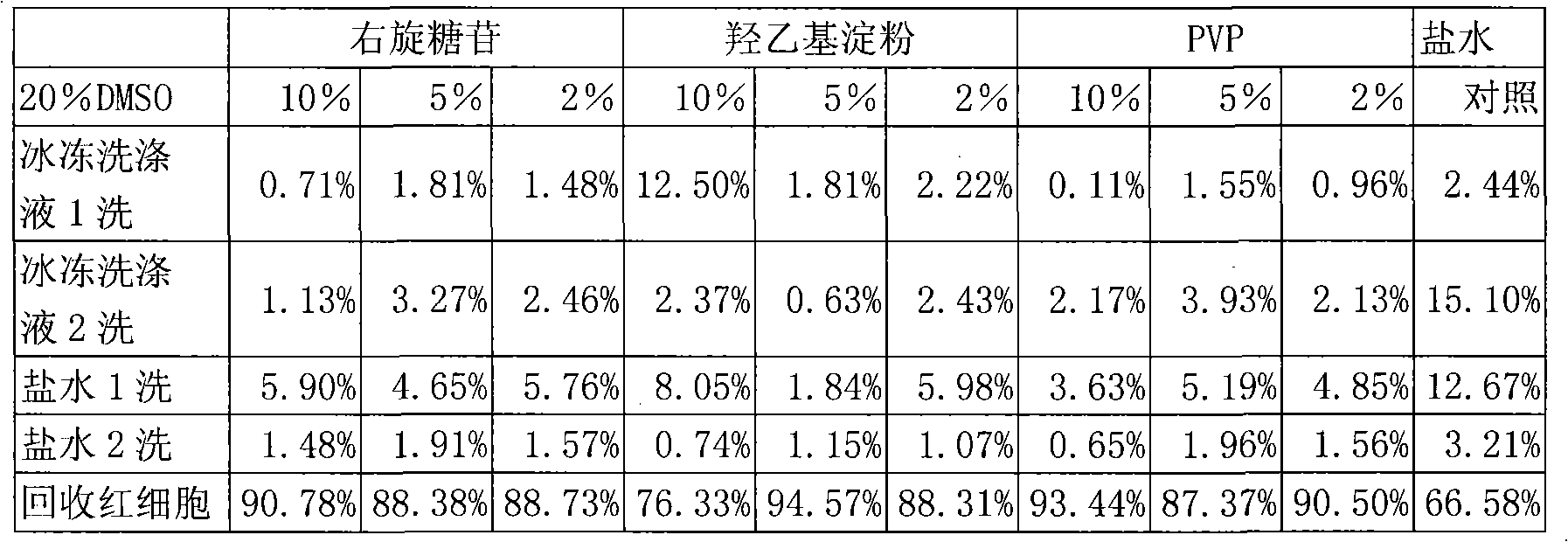
Patents
Literature
154 results about "Isotonic Solutions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Solutions having the same osmotic pressure as blood serum, or another solution with which they are compared. (From Grant & Hackh's Chemical Dictionary, 5th ed & Dorland, 28th ed)
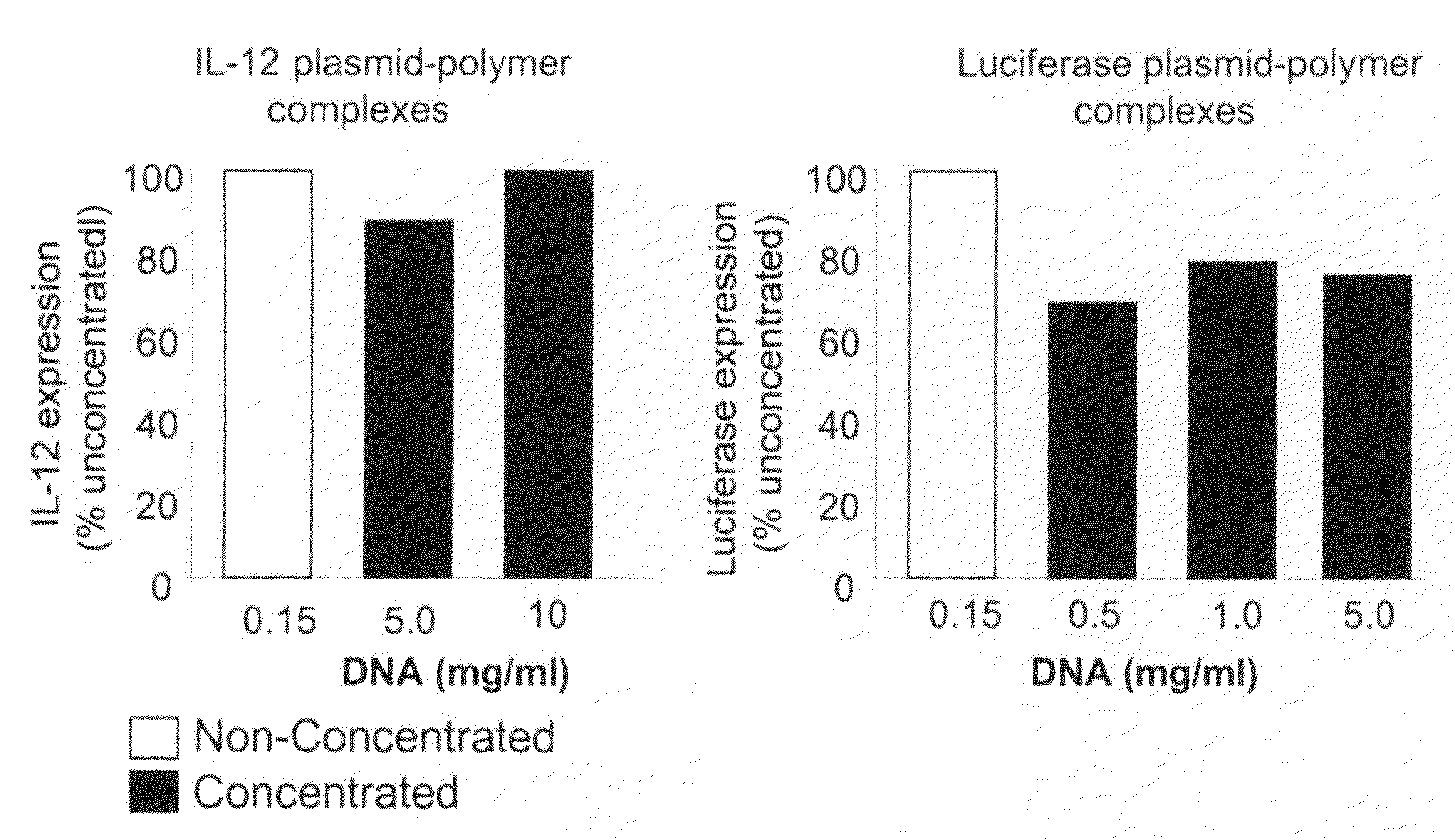
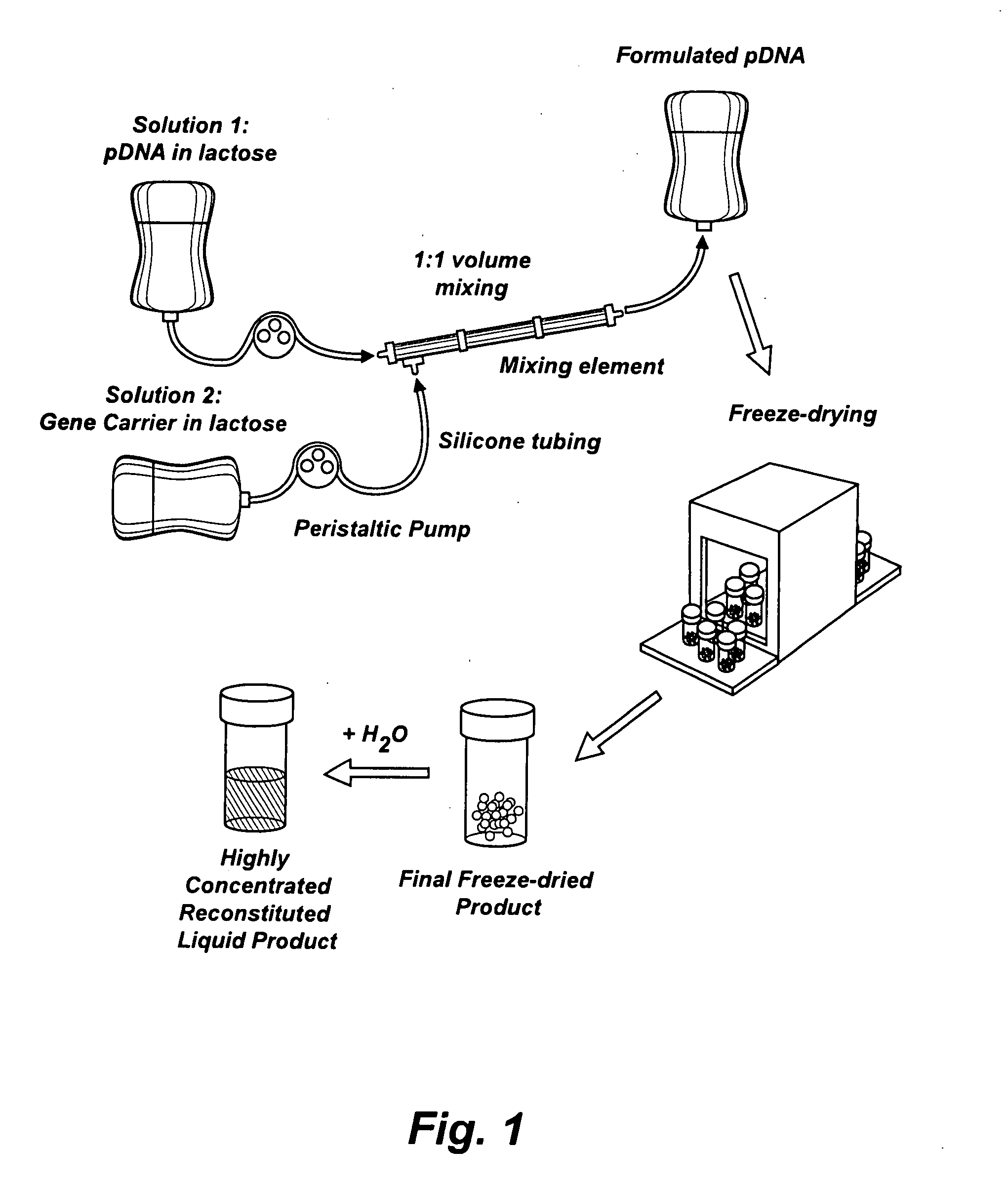
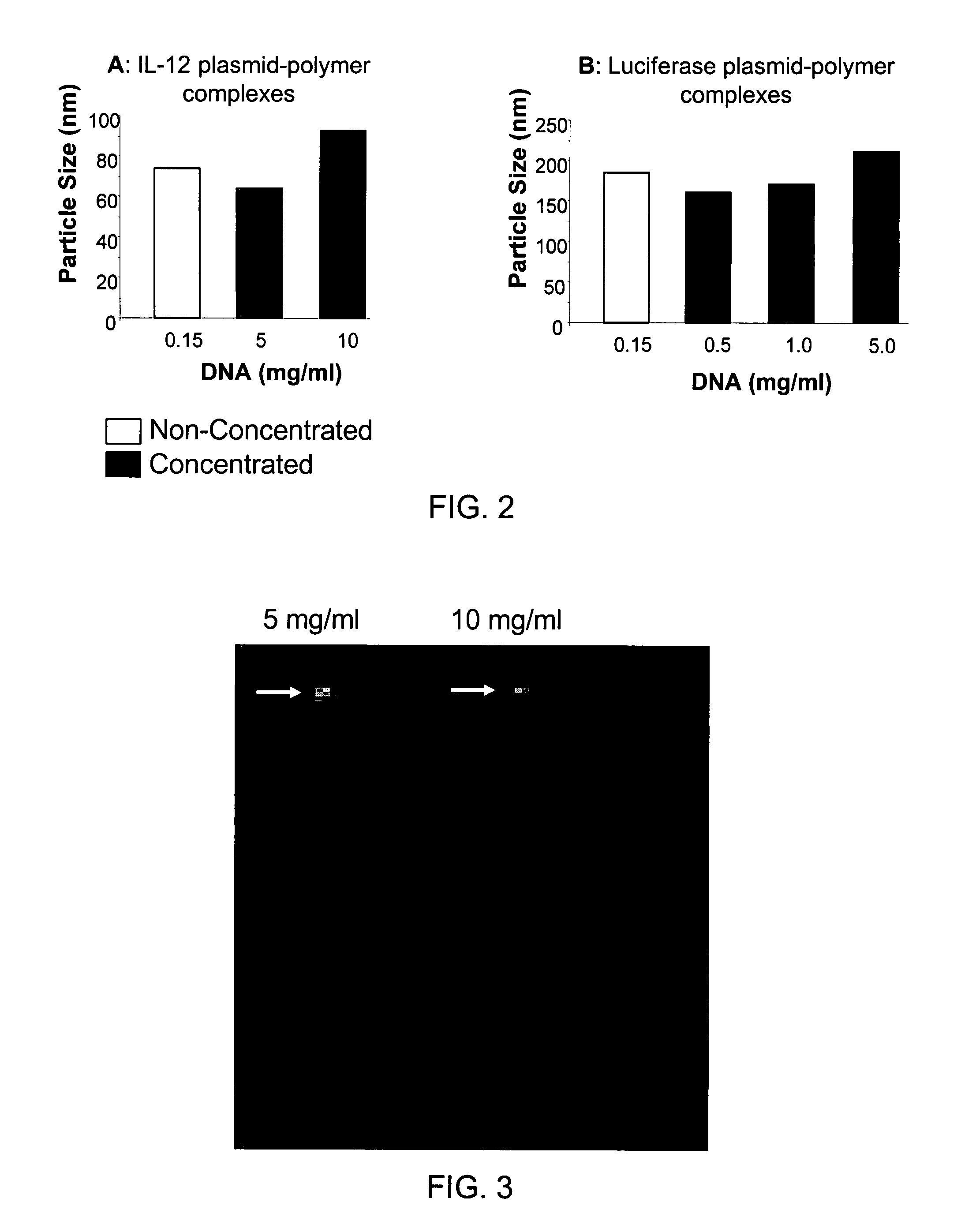
Nucleic Acid-Lipopolymer Compositions
InactiveUS20090042829A1Increase efficiency and dosing flexibilityEfficiently be lyophilizedSpecial deliveryPeptide/protein ingredientsCholesterolFiller Excipient
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:CLSN LAB
Composition, method of preparation & application of concentrated formulations of condensed nucleic acids with a cationic lipopolymer
UndeterminedUS20090042825A1Increase efficiency and dosing flexibilitySpecial deliveryPeptide/protein ingredientsFiller ExcipientCholesterol
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:EXPRESSION GENETICS INC
Cleaning and devitalization of cartilage
InactiveUS20080077251A1Improve recellularizationBone implantDead animal preservationCellular DebrisMedicine
The invention is further directed to producing a cleaned, disinfected, and devitalized cartilage graft by optionally cleaning and disinfecting the cartilage graft; treating the cartilage graft in a pretreatment solution; treating the cartilage graft in an extracting solution; washing the extracted cartilage graft with a rinsing solution; and subsequently soaking the devitalized cartilage graft in a storage solution. The devitalized cartilage graft is essentially free from metabolically viable and / or reproductively viable cells and the rinsing solution is hypotonic solution or isotonic solution. The present invention is further directed to a cleaned, disinfected, and devitalized cartilage graft and a process for cleaning, disinfecting, and devitalizing cartilage grafts. The invention also relates to a process for repairing a cartilage defect and implantation of a cartilage graft into a human or animal by crafting the cartilage matrix into individual grafts, disinfecting and cleaning the cartilage graft, applying a pretreatment solution to the cartilage graft, removing cellular debris using an extracting solution to produce a devitalized cartilage graft, implanting the cartilage graft into the cartilage defect with or without an insertion device, and sealing the implanted cartilage graft with recipient tissue. The devitalized cartilage graft is optionally recellularized in vitro, in vivo, or in situ with viable cells to render the tissue vital before or after the implantation. The devitalized cartilage graft is also optionally stored between the removing cellular debris and the recellularizing steps.
Owner:LIFENET HEALTH
Nucleic Acid-Lipopolymer Compositions
ActiveUS20130065942A1Increase efficiency and dosing flexibilityEfficiently be lyophilizedSpecial deliveryPeptide/protein ingredientsFiller ExcipientCholesterol
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:CLSN LAB
Salt solution for colon cleansing
The field of colonic diagnostic and surgical procedures is hampered by the lack of optimal means available to cleanse the colon. A compromise between convenient, distasteful, solid or low volume, hyperosmotic solutions which cause considerable fluid and electrolyte imbalances in patients and large volume, difficult to consume, iso-osmotic solutions has had to be made heretofore. This invention describes a low volume, hyper-osmotic solution consisting of sulfate salts with and with out polyethylene glycol. Unlike prior art, this composition is useful for the cleansing of the bowel and, in lower volumes, as a laxative, without producing clinically significant changes in bodily function.
Owner:BRAINTREE LAB
Cross-linked hyaluronic acid cell-scaffold material and preparation method and application
ActiveCN104225677APromote regenerationProvide temporary protectionOrganic active ingredientsSurgical drugsCross-linkFreeze-drying
The invention relates to a cross-linked hyaluronic acid cell-scaffold material and its preparation method and application. The cross-linked hyaluronic acid cell-scaffold material is obtained by crosslinking of high-molecular hyaluronate and low-molecular hyaluronate. The proportion of hyaluronate disaccharide molecules which take part in the crosslinking is 0.5%-20%, and expansion rate of the hyaluronate disaccharide molecules in an isotonic solution is 80%-110%. The preparation method of the cell-scaffold material comprises two freeze drying steps. Hyaluronate mixed with a cross-linking agent is formed firstly; after a heating reaction, water is added for swelling to form gel; and freeze drying is then carried out to obtain the porous scaffold material. The scaffold has abundant pores, has a certain mechanical strength and pore size and has good hydroscopicity and biocompatibility. The scaffold can be used as a tissue engineering cell-scaffold in promoting cartilage injury repairing and also can be used for preparing a haemostasis anti-adhesion material.
Owner:INST OF BIOPHARM OF SHANDONG PROVINCE
II-type collagen joint cartilage fluid and preparation method thereof
ActiveCN101810855APromotes self-healingImprove lubrication functionPeptide/protein ingredientsSkeletal disorderHazardous substanceHybrid protein
The invention provides II-type collagen joint cartilage fluid and a preparation method thereof. Bacteria, viruses and other hazardous substances of the joint cartilage are removed through degreasing-> serum removing-> surfactant treatment-> oxidant treatment-> disinfection and other links. Hybrid protein and other denatured protein of the cartilage are removed through acid hydrolysis, salt precipitation, dialysis and other processes, and high-purity and high-activity II-type collagen solution is prepared. Non-toxin and non-heat raw materials and auxiliary materials and isotonic solution are mixed in an appropriate ratio, and filled in a sterile non-heat disposable injector, to prepare II-type collagen joint cartilage fluid for disposable injection. Other components of the II-type collagen joint cartilage fluid comprise the auxiliary materials and the isotonic solution. The II-type collagen joint cartilage fluid can play the roles of lubrication, pain relieving and joint activity degree improving to the injured joints, fundamentally realizes the internal repair to the cartilage tissue, and is applicable to a patient with various joint cartilage injuries and all types of arthritis.
Owner:GUANGZHOU TRAUER BIOTECH
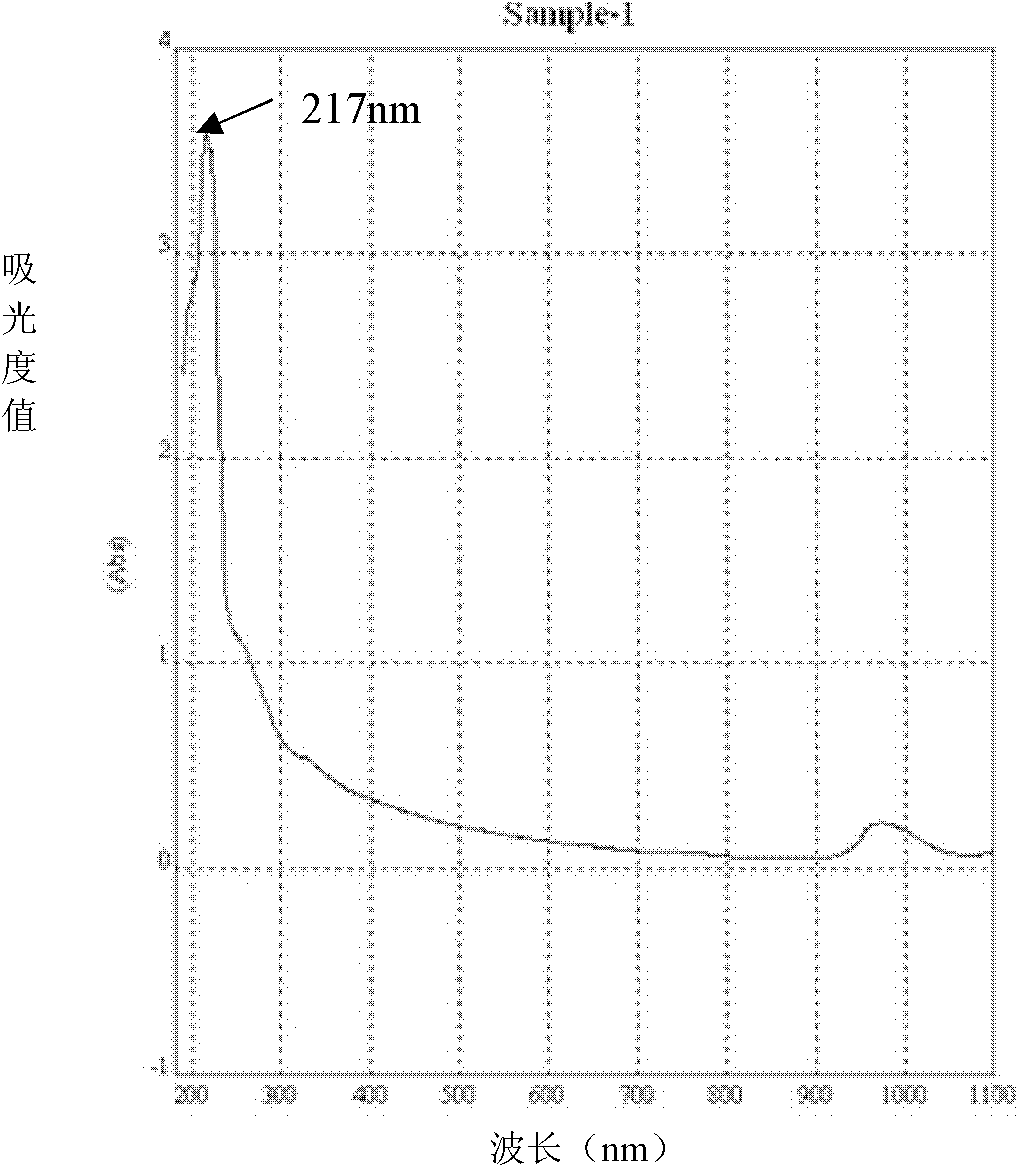
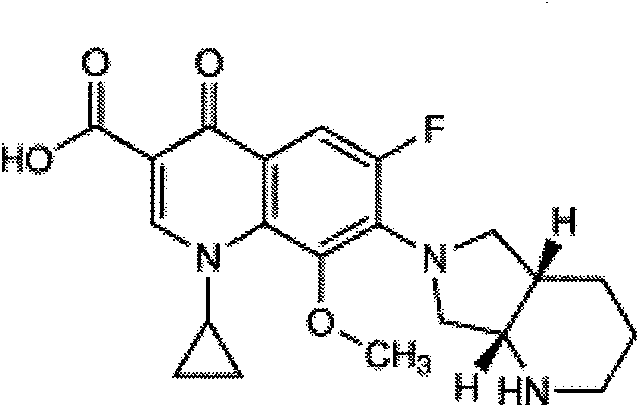
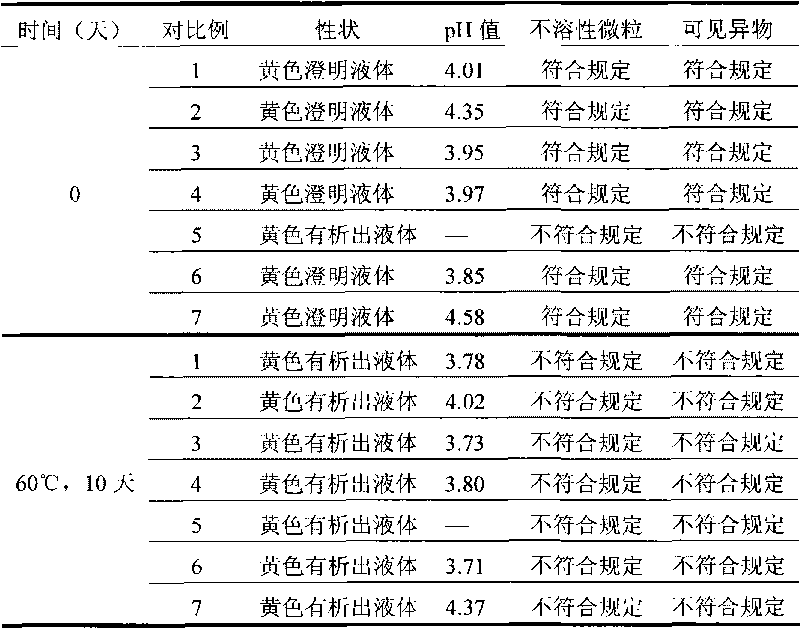
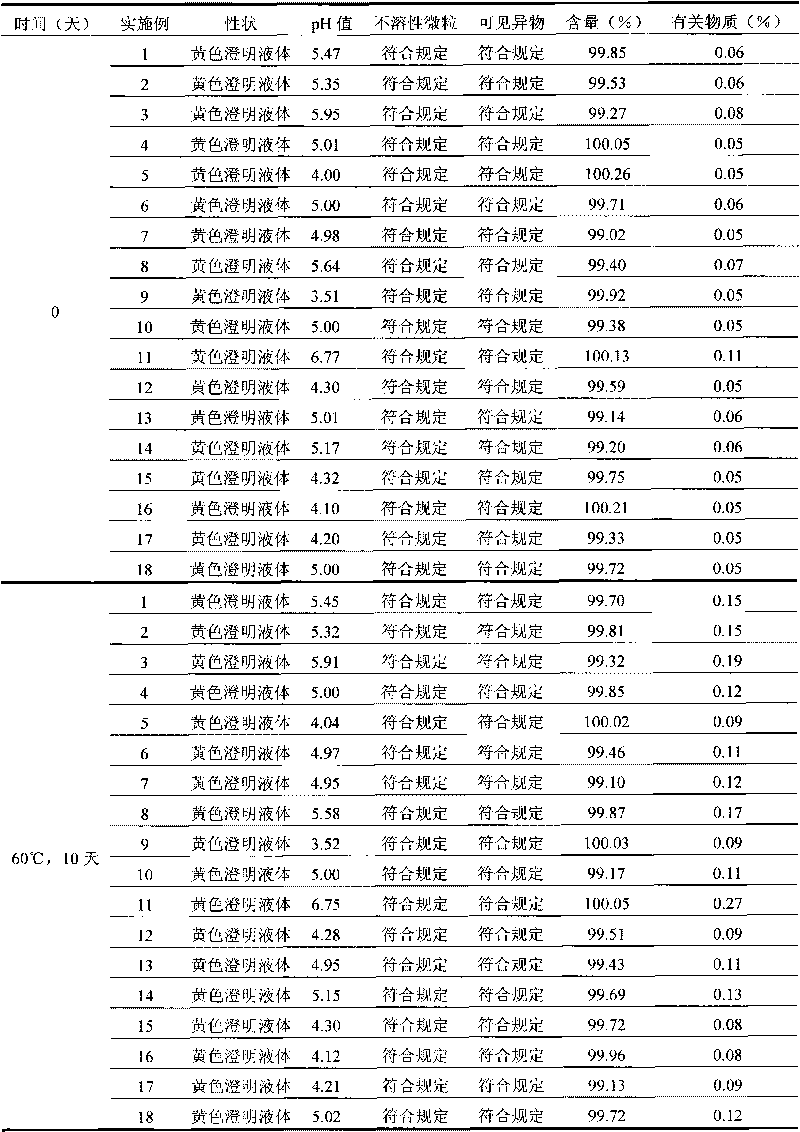
Moxifloxacin aqueous solution type injection
InactiveCN101732246AGood water solubilityImprove stabilityAntibacterial agentsOrganic active ingredientsO-Phosphoric AcidMedicine
The invention discloses a moxifloxacin aqueous solution type injection which contains moxifloxacin or pharmaceutically acceptable salt, weak acid sodium salt or phosphoric acid sodium salt and water for injection, wherein the content of the moxifloxacin is 0.8-4 percent (g / ml), and the molar concentration of the weak acid sodium salt or the phosphoric acid sodium salt is 0.0002-1mol / L. The moxifloxacin aqueous solution type injection product has strong dissolubility, human body acceptable pH value, easier control of product quality, stability in a storage period, good compatibility with clinical common isotonic solution, low production cost, small volume and convenient transportation and storage.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Natural-tissue-derived decellularized and decalcified bone material and preparation method thereof
InactiveCN105435307AImprove biological activityImprove manufacturing speedProsthesisCell-Extracellular MatrixBiocompatibility Testing
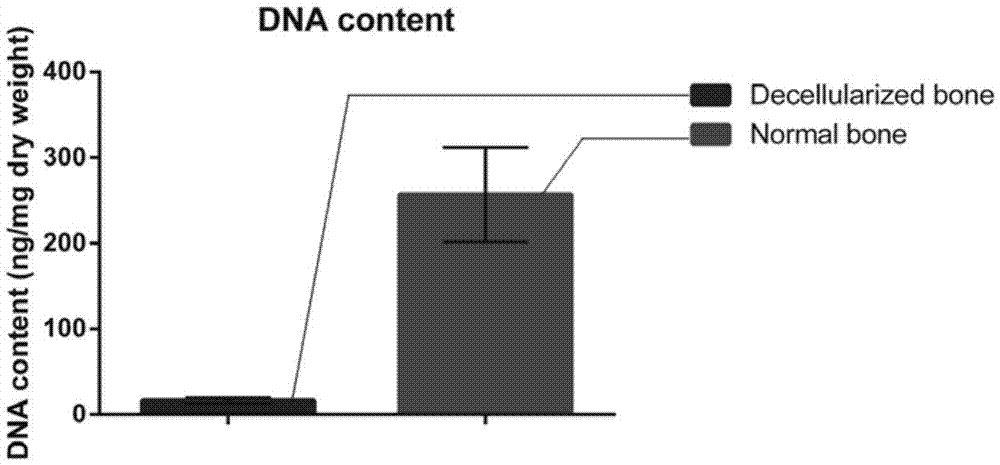
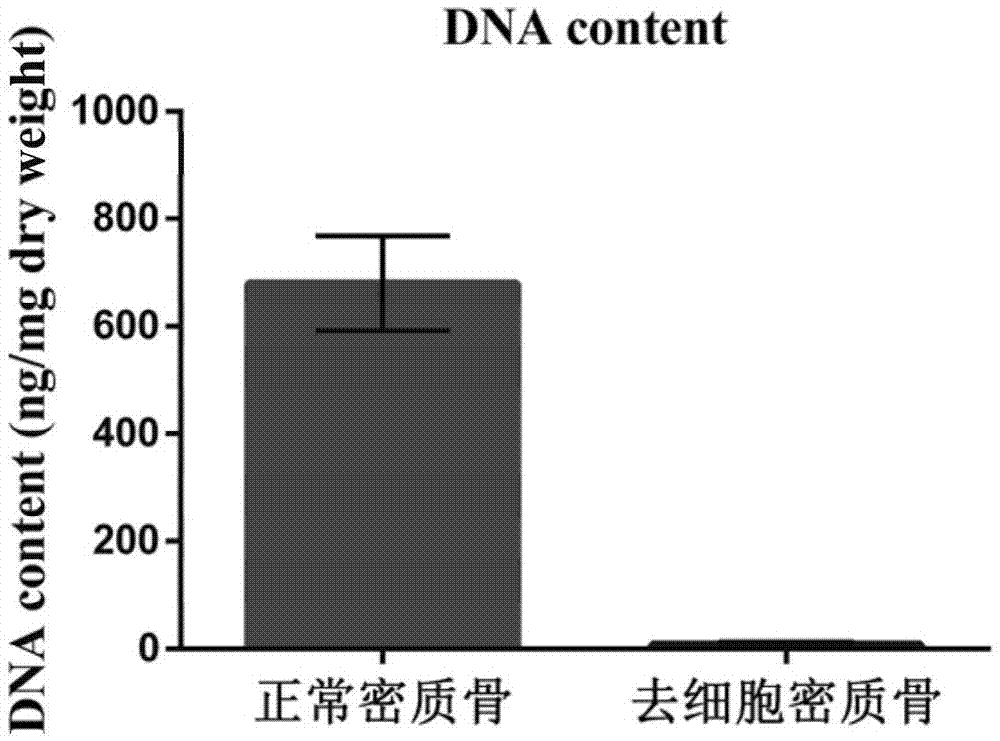
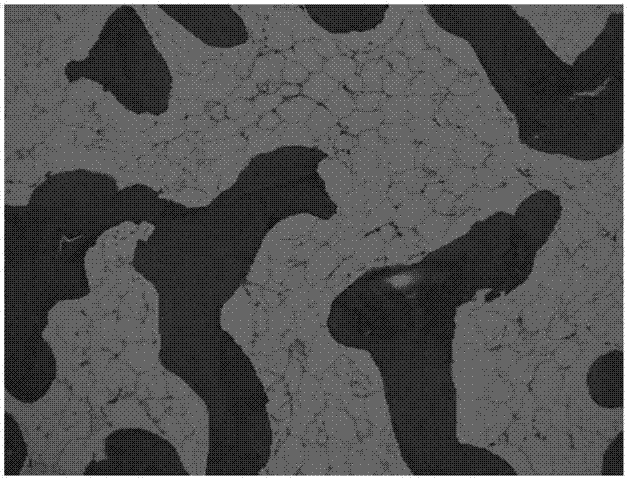
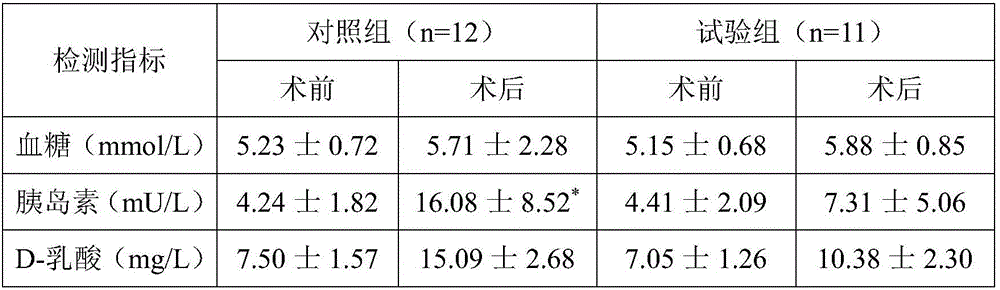
The invention discloses a preparation method of a natural-tissue-derived decellularized and decalcified bone material. According to the method, any bone tissue of a mammal is treated with a protease inhibitor-containing normal saline buffer, an organic solvent, Tirton X-containing PBS (phosphate buffer saline), SDS (sodium dodecyl sulfonate)-containing PBS, pancreatin-containing PBS, deoxyribonuclease-containing PBS, an EDTA (ethylene diamine tetraacetic acid) isotonic solution and ultrasonic waves, and the decellularized and decalcified bone material is obtained. Cancellous bones and cortical bones can be decellularized completely and simultaneously, the conditions are mild, damage to an ECM (extracellular matrix) is avoided, rapidness and stability are realized, and the obtained decellularized and decalcified bone material has the advantages of good biocompatibility, high plasticity, high biomechanical strength and the like and can be used for clinically repairing bone regeneration and repair disorder such as bone defects, nonunion and the like caused by various causes.
Owner:GUANGXI MEDICAL UNIVERSITY
Liquid eye drop composition
A composition that is used as an eye treatment contains reduced glutathione, vitamin A and vitamin E, as well as one or more of zinc sulfate, boric acid and potassium as buffering agents. The composition also may contain a lubricant and a preservative. The composition is a sterile isotonic solution. The composition is used in a method of treating eyes for the alleviation of irritations and / or dryness, as well as for the prevention and treatment of cataracts.
Owner:BRASWELL A GLENN +2
Trans-epithelial osmotic collyrium for the treatment of keratoconus
Trans-epithelial osmotic collyrium for the treatment of keratoconus by corneal cross-linking with UV rays, of the type consisting of a riboflavin-based solution and an absorption enhancement adjuvant consisting of benzalkonium chloryde (BAC). The solution has a hypoosmolarity degree ranging between −70% and −30% over an isotonic solution and contains a BAC concentration accordingly ranging between 0.005% and 0.035%.
Owner:AVEDRO
Special nutritional supplement agent for preoperative patients
InactiveCN105795481AReduce resistancePromote absorptionOrganic active ingredientsMetabolism disorderIsotonic SolutionsInsulin resistance
The invention discloses a special nutritional supplement agent for preoperative patients. The nutritional supplement agent contains maltodextrin, levulose, glutamine, sodium ions and potassium ions. When the preoperative nutritional agent is taken, the nutritional agent can be compounded into an isotonic solution of which the osmotic pressure level is similar to that of plasma, so that the possibility of diarrhoea caused by that a great deal of liquid enters intestinal tracts through tissues due to osmotic pressure differences can be obviated; the feeling that an operation patient feels thirsty and tired can be obviously improved, and the symptoms of nausea, vomit, diarrhoea, abdominal distension and the like cannot be caused; complete emptying can be realized within 79-107 minutes, and experiments confirm that taking the nutritional agent for 2 hours before the operation is safe; in addition, the nutritional agent also has good effects of improving insulin resistance and maintaining intestinal mucosal barriers.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
Kit for obtaining multiple-organ and tissue extracellular matrix and using method of kit
The invention discloses a kit for obtaining a multiple-organ and tissue extracellular matrix and a using method of the kit. The kit for obtaining the multiple-organ and tissue extracellular matrix comprises a nonionic detergent I, an ionic detergent I, an ionic detergent II, a hypertonic solution, a hypotonic solution, an isotonic solution, a nucleic acid removal agent and a tissue bulking agent. According to the kit and a method for using the kit provided by the invention, acellularized scaffolds of multiple organs and tissues can be obtained in a short time, and the structure and most of structure of the extracellular matrix are maintained, so that product materials are provided for research and application of regenerative medicines and tissue engineering.
Owner:ZHEJIANG DISAI BIOTECHNOLOGY CO LTD
Optimised formulation of tobramycin for aerosolization
InactiveUS20050163722A1Improve complianceMaximum efficacyAntibacterial agentsBiocideTobramycinCystic fibrosis lungs
The invention provides a tobramycin formulation for delivery by aerosolization in the form of additive-free, isotonic solution whose pH has been optimised to ensure adequate shelf-life at room temperature. Said formulation can be advantageously used for the treatment and prophylaxis of acute and chronic endobronchial infections, in particular those caused by the bacterium Pseudomonas aeruginosa associated to lung diseases such as cystic fibrosis.
Owner:CHIESI FARM SPA
Use of bacterial phage associated lysing enzymes for treating streptococcal infections of the upper respiratory tract-
A composition for treatment of bacterial infections of the eye is disclosed which comprises a lytic enzyme composition specific for the infecting bacteria, and a carrier for delivering said lytic enzyme.. The carrier for delivering at least one lytic enzyme to the eye may be but is not limited to the use of an isotonic solution..
Owner:THE ROCKEFELLER UNIV
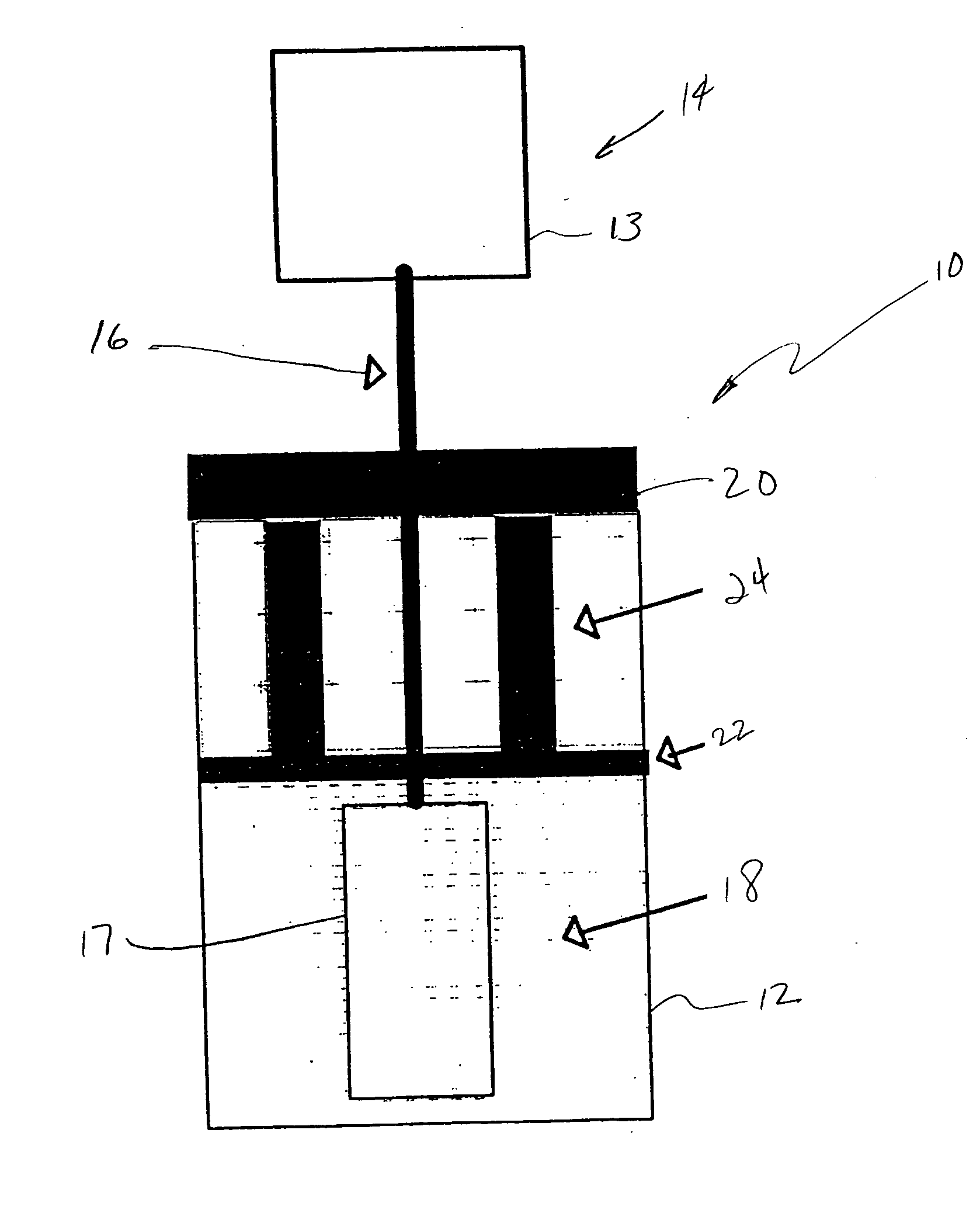
Lysis/resealing process and device for incorporating an active ingredient in erythrocytes
ActiveCN101031339AOrganic active ingredientsPeptide/protein ingredientsBiochemical engineeringBULK ACTIVE INGREDIENT
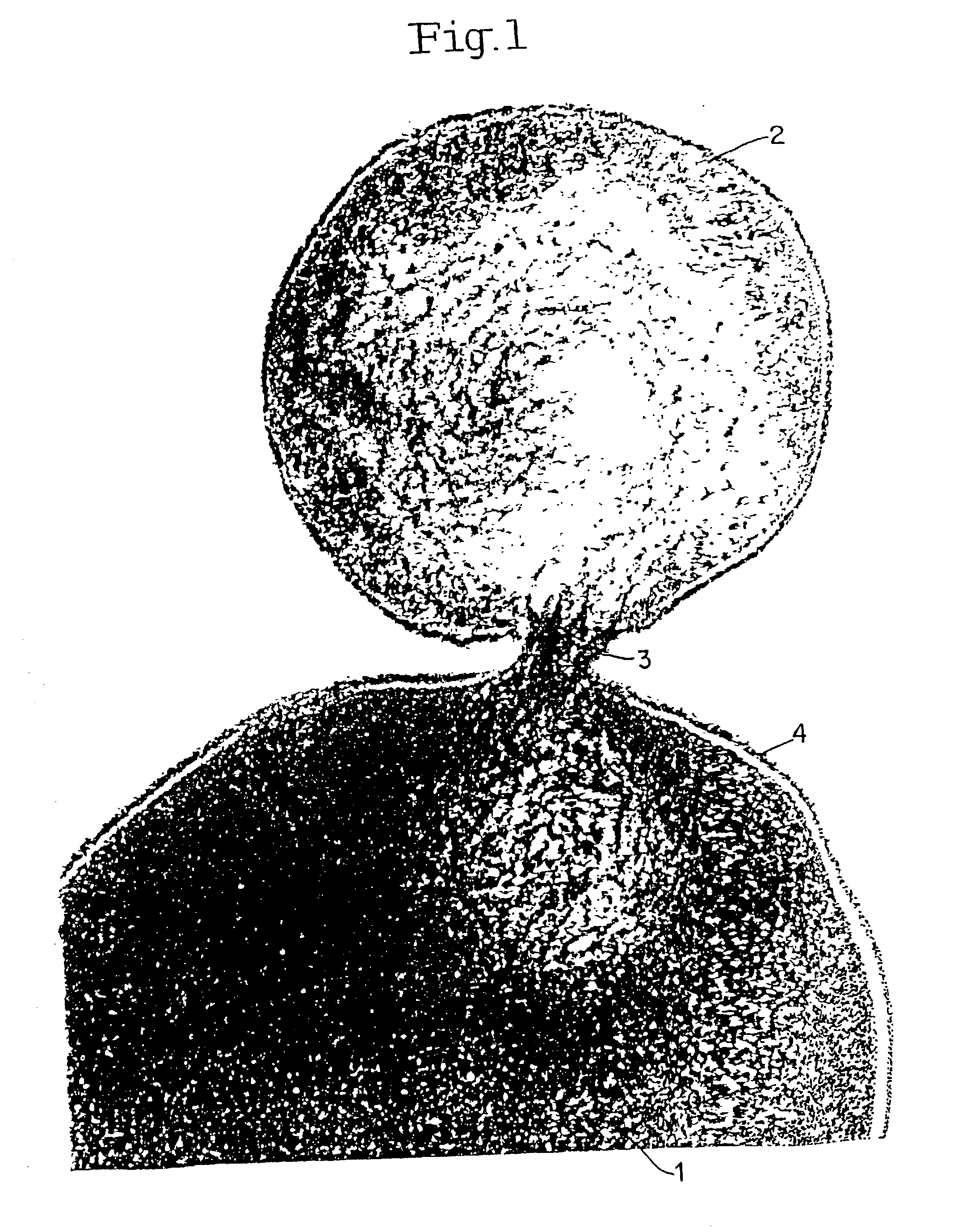
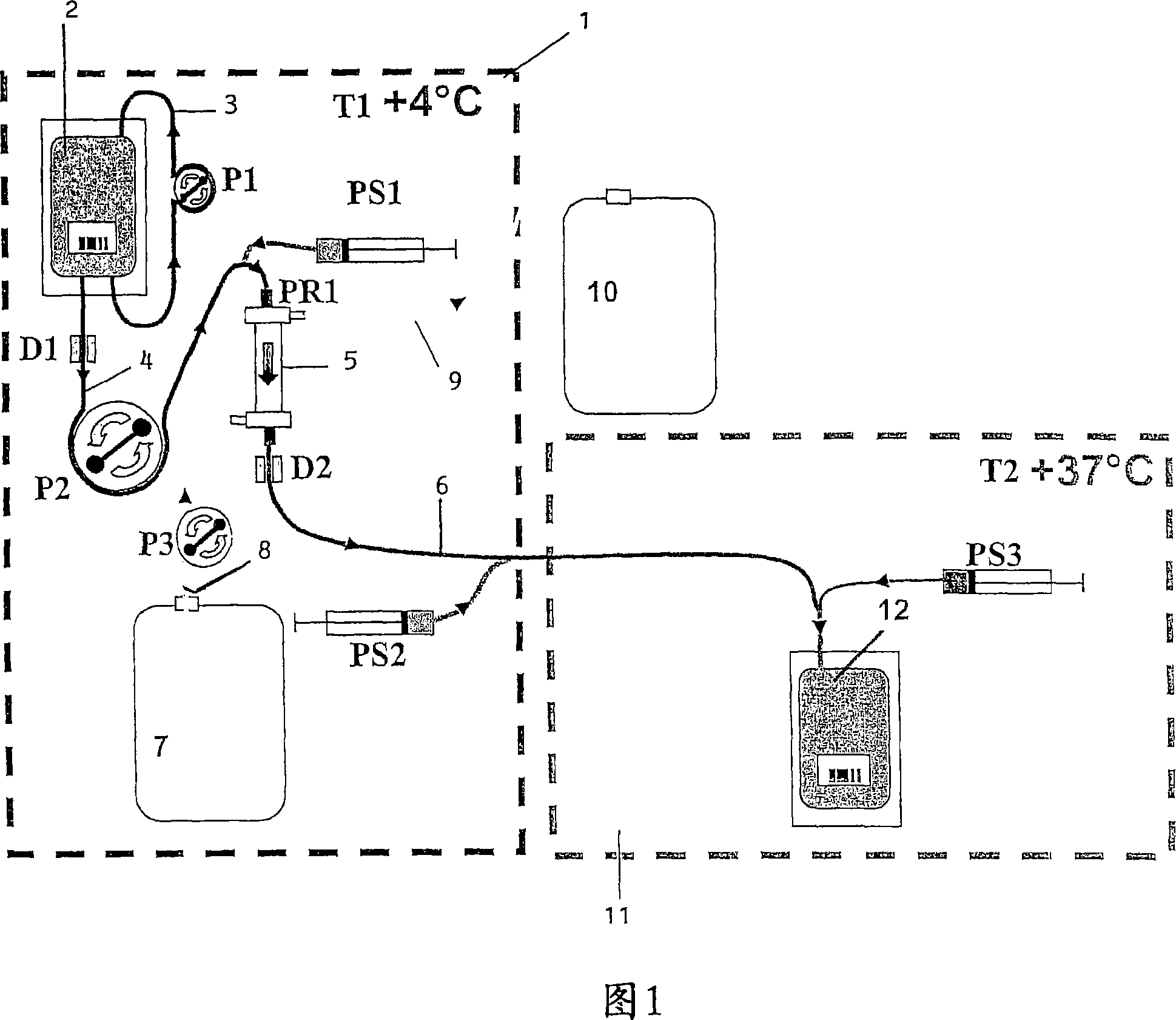
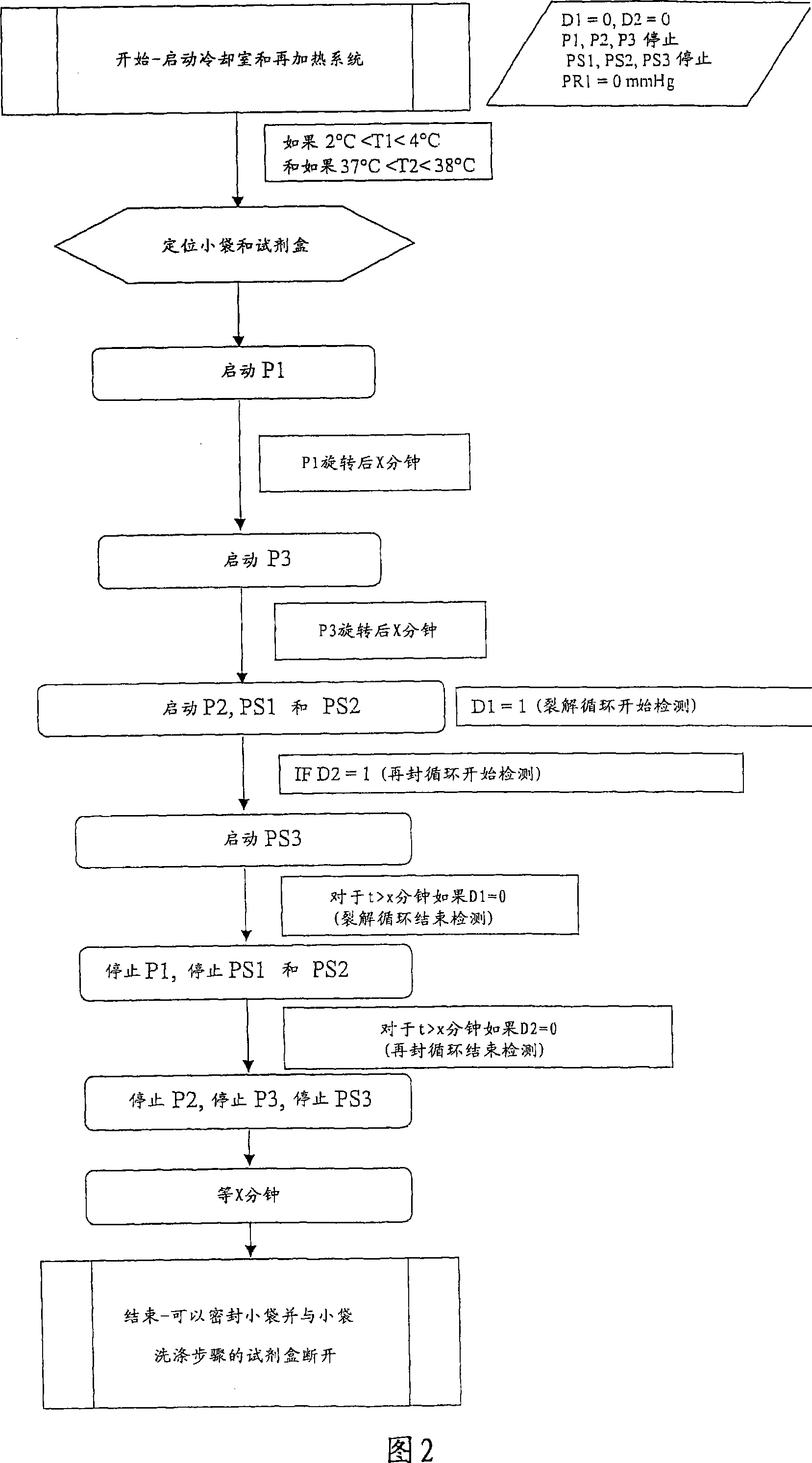
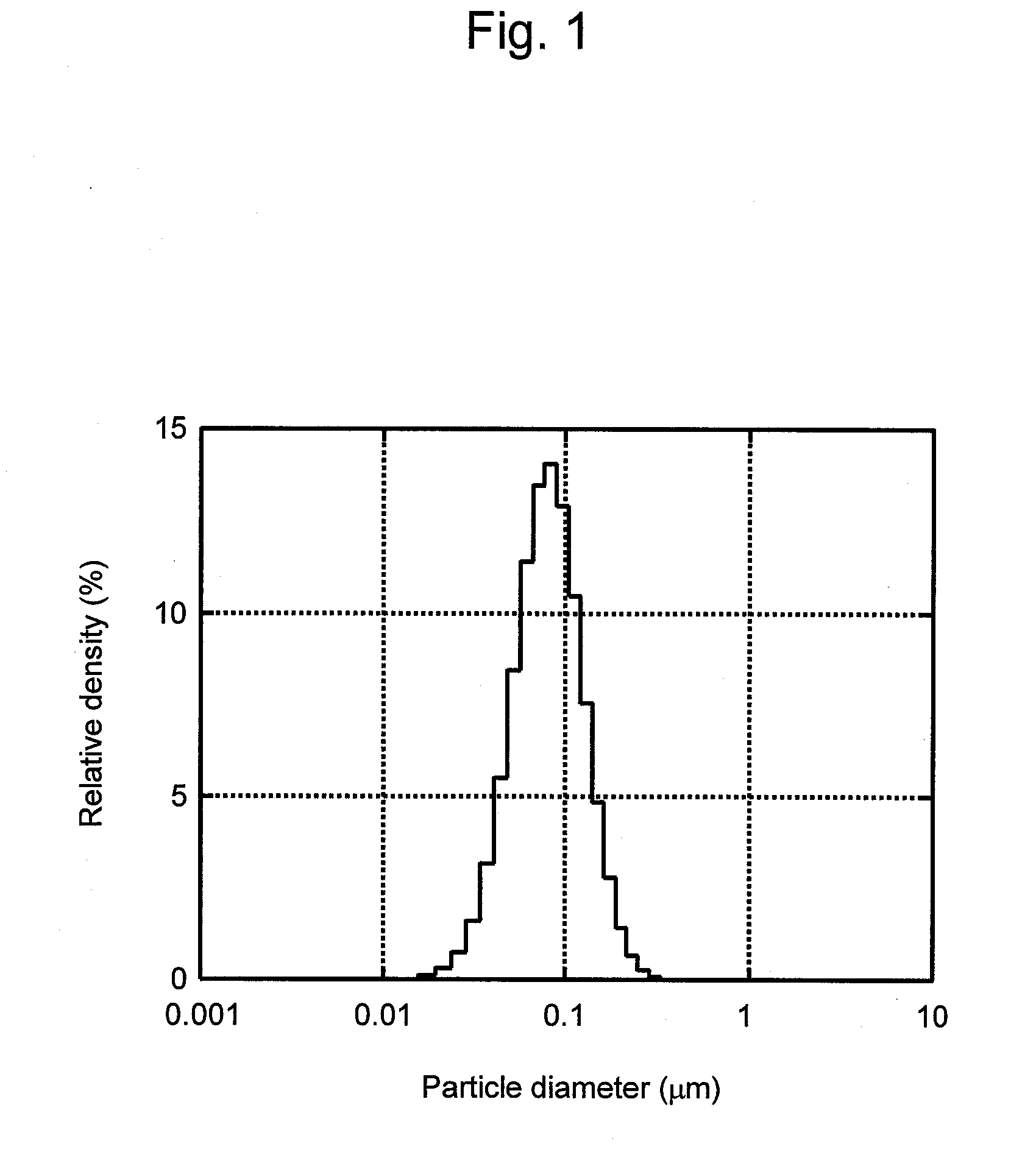
Lysis / resealing process for incorporating an active ingredient, the process comprising the following steps: (1) - placing a globular concentrate in suspension in an isotonic solution having a haematocrit level which is equal to or greater than 65 %, with refrigeration at from + 1 to + 8 DEG C, (2) - measuring the osmotic fragility based on a sample of erythrocytes from that same globular concentrate, preferably on a sample of the suspension obtained in step (1), (3) - lysis and internalisation procedure of the active ingredient, inside the same chamber, at a temperature which is constantly maintained at from + 1 to + 8 DEG C, comprising allowing the erythrocyte suspension having a haematocrit level which is equal to or greater than 65 % and a hypotonic lysis solution which is refrigerated at from + 1 to + 8 DEG C, to circulate in a dialysis cartridge; the lysis parameters being adjusted in accordance with the osmotic fragility previously measured; and (4) - resealing procedure carried out in a second chamber at a temperature of from + 30 to + 40 DEG C by means of a hypertonic solution. Figure 1.
Owner:ERYTECH PHARMA
Rupid red blood cell freezing and defreezing method, and freeze protective liquid thereof
InactiveCN1935989AImprove effectivenessImprove securityDead animal preservationTissue cultureSaline waterBiocompatibility Testing
The invention relates to experiment using reagent erythrocyte long term storage technique. The erythrocyte icing protecting solution / thawing solution is formed by isotonic solution and macromolecular substance with biocompatibility. The osmotic pressure of the former is 250-400mOsm / L; the molecular weight of the latter is 20000-400000; and its density is 15-40%. The invention utilizes isotonic protecting solution, adds into macromolecular substance colloidal medium to form erythrocyte icing protecting solution to make it not occur hyper-osmotic state inside and outside. And in thawing process, it uses the solution likes with the icing protecting solution to thaw the erythrocyte, washes by physiological saline to remove the macromolecular substance to gain the erythrocyte brine suspended solution. Using in the correlation testing, the whole icing and thawing procedure is about 15min; and the yield of the erythrocyte can reach over 80%.
Owner:SHANGHAI BLOOD CENT
Method of preparing a growth factor concentrate derived from human platelets
The invention relates to a method of preparing an intra-dermally, intra-articularly, sub-dermally or topically administrable growth factor concentrate derived from human platelets. The method comprises the steps of suspending human platelets in multiple electrolyte isotonic solution; snap-freezing the suspension; thawing the frozen suspension; and sterile-filtering the suspension. In particular, in this method, a fixed number of platelets is suspended in a fixed volume of multiple electrolyte isotonic solution to obtain the required concentration of growth factors in the growth factor concentrate, snap-freezing of the suspension is carried out at a temperature of −120° C. to −200° C., thawing of the frozen suspension is carried out at 25° C. to 37° C., and cellular debris are separated from the thawed suspension and the resultant suspension of growth factors is diluted with an isotonic medium before sterile-filtering.
Owner:KASIAK RES PVT
Rapid freezing and thawing process for erythrocyte in refrigerator and freezing protection liquid and scrubbing liquid used by the process
InactiveCN101333514AImprove final yieldReduced operating requirementsPharmaceutical containersMedical packagingSaline waterHemolysis
The invention relates to a technique for preserving erythrocytes in low temperature refrigerator for a long time. Wherein, erythrocyte frozen protective solution is of high osmotic pressure, and contains macromolecular substances with biocompatibility and DMSO (dimethyl sulfoxide). Washing solution is composed of basically isotonic solution and macromolecular substances with biocompatibility. Frozen stock solution can make the erythrocytes go into the hypertonic condition without severe hemolysis. By using macromolecule colloidal medium, prepared erythrocyte washing solution can make the erythrocytes break away from the hypertonic condition so that the erythrocytes are not in severe hemolytic condition. Then the macromolecular substances are washed away by normal saline, and erythrocyte saline suspension is obtained. When the technique is applied in relevant experiments, the total frozen preservation process lasts about one minute, while the unfreezing and washing processes last about fifteen minutes. Thus, the achievement rate of the erythrocytes is high.
Owner:SHANGHAI BLOOD CENT
Circulating tumor cell dyeing kit and use thereof
ActiveCN102980793AAvoid lossSimple and fast operationPreparing sample for investigationMicrometerPhosphate
The invention provides a circulating tumor cell dyeing kit and a use thereof. The circulating tumor cell dyeing kit is suitable for the field of biotechnology and the medical science. The circulating tumor cell dyeing kit comprises a filter, a basophilic dyeing liquid, an eosinophilic dyeing liquid, an isotonic solution and a stationary liquid, wherein the basophilic dyeing liquid, the eosinophilic dyeing liquid, the isotonic solution and the stationary liquid are sub-packaged. The filter comprises a filter membrane having aperture sizes of 8 micrometers. The basophilic dyeing liquid is an eosin Y methanol solution. The eosinophilic dyeing liquid is a phosphate buffer solution containing methlene blue and an azure I. The stationary liquid is a phosphate buffer solution containing paraformaldehyde and bovine serum albumin. The circulating tumor cell dyeing kit has good dying effects, a low cost and short operation time, can be prepared simply, is convenient for operation and greatly improves work efficiency.
Owner:WUHAN YZY MEDICAL SCI & TECH
Gas bubble-generating agent
InactiveUS20080019923A1Ultrasonic/sonic/infrasonic diagnosticsInfrasonic diagnosticsChemical LinkageBoiling point
This invention provides a gas bubble-generating agent that can be used as a contrast medium or a blocking agent in vivo. Such gas bubble-generating agent is produced by a method for producing a gas bubble-generating agent comprising the following steps of: (a) preparing a mixed solution of an amphiphilic substance, an amphiphilic substance comprising a water-soluble polymer chemically bound thereto, a hardly water-soluble substance having a boiling point of lower than 60° C. at atmospheric pressure, and a physiologically acceptable isotonic solution; (b) pressurizing the mixed solution; and (c) centrifuging the mixed solution after the step of pressurization, wherein a molar concentration of the amphiphilic substance in the mixed solution prepared in step (a) is 10 times or more higher than that of the amphiphilic substance comprising a water-soluble polymer chemically bound thereto.
Owner:HITACHI LTD
Collagen eye drops and preparation method thereof
ActiveCN102210856ATo promote metabolismImprove the living environmentSenses disorderPeptide/protein ingredientsVasoconstrictor AgentsCell migration
Owner:XIAN GIANT BIOGENE TECH CO LTD
Method for determining red blood cell penetration fragility
InactiveCN1588062AEasy to operateShorten the timeMaterial analysis by observing effect on chemical indicatorTransmissivity measurementsTurbidityFragility
The invention relates to a determining method for the erythrocyte osmotic brittleness. It adopts turbidimetric analytic method as below: mixing the blood sample with the hypotonic solution with specific osmotic pressure, taking the undissolved erythrocyte as the main particle forming the turbidity of the mixed solution., selecting a undissolved erythrocyte which has higher absorbency to it, taking the specific wave length that the soluble coloured substance such as the free hemoglobin which is dissolved from erythrocyte have a weaker absorbency to it as the determining wave length, making turbidimetric analysis on the spectrophotometer: calculating the value of the haemolytic rate which is in direct ratio with the erythrocyte osmotic brittleness by comparing the different absorbency of erythrocyte in different duration of time and on the same determining condition within the hypotonic solution with the said specific osmotic pressure, or by comparing the different absorbency of erythrocyte between in the isotonic solution and in the above said hypotonic solution with specific osmotic pressure. The invention has the advantages of high accuracy, good repeatability of measurement, simple procedure, high velocity of determination, capability of realizing the dynamic determination and the full automatic analysis easily.
Owner:潘干华
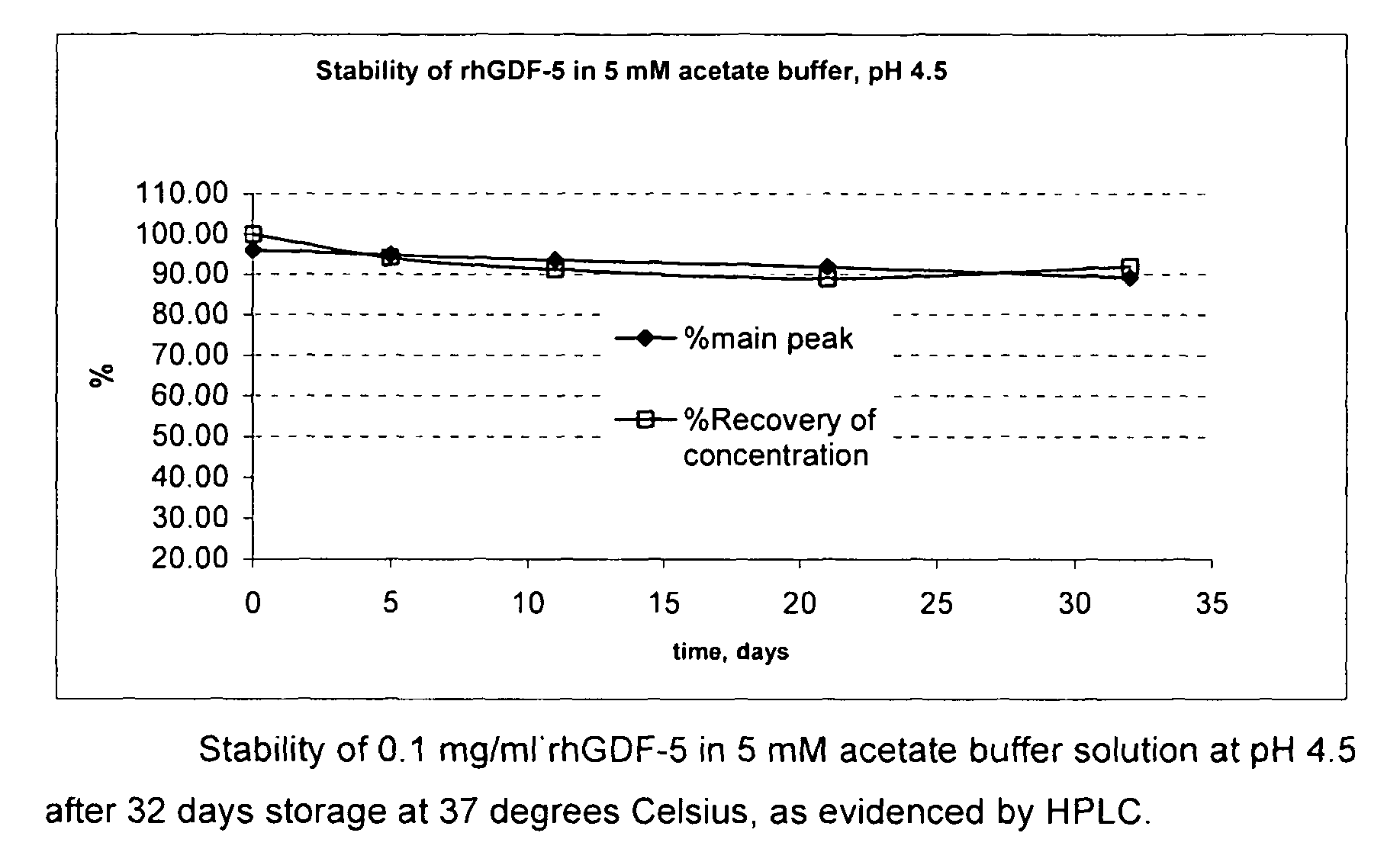
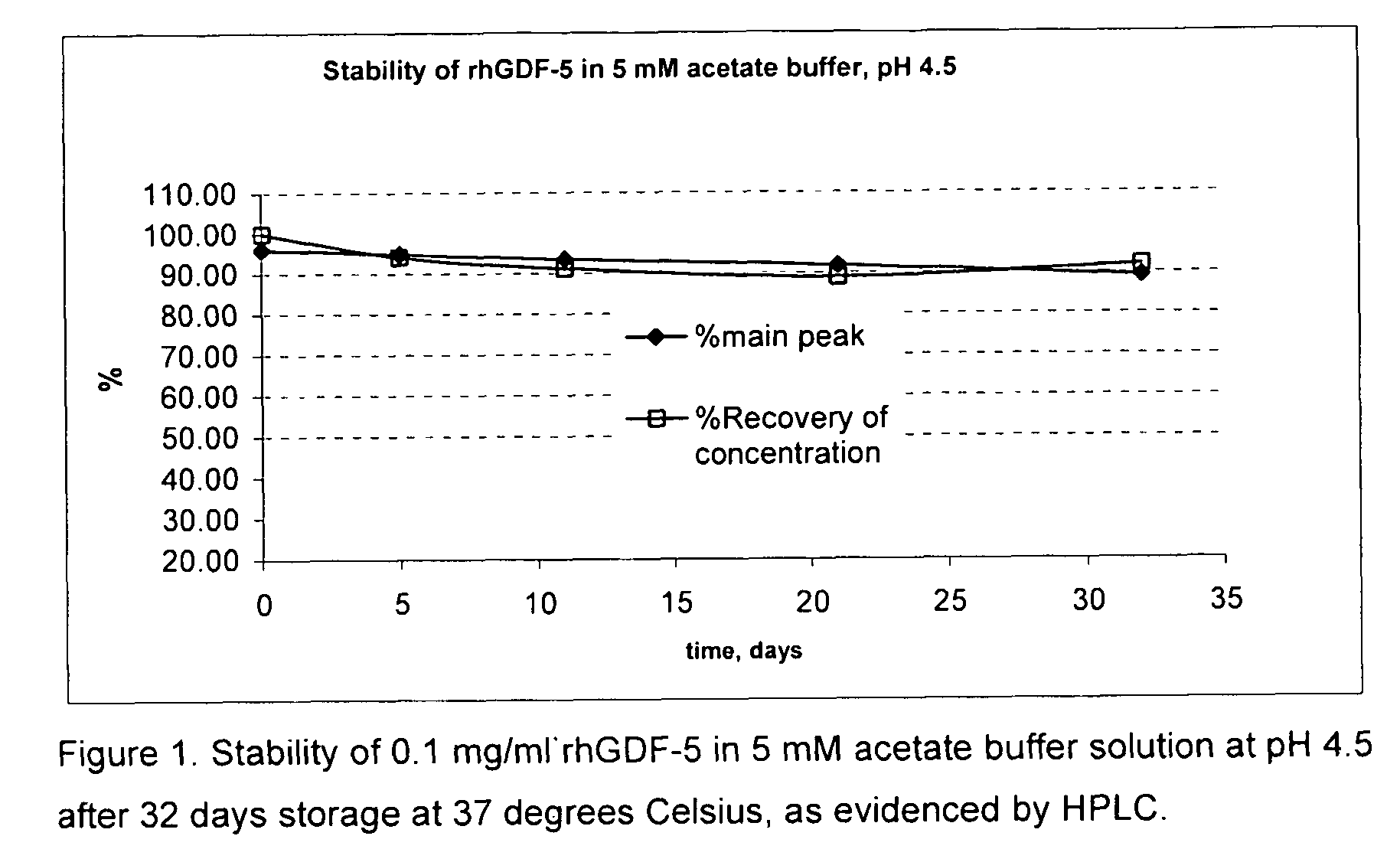
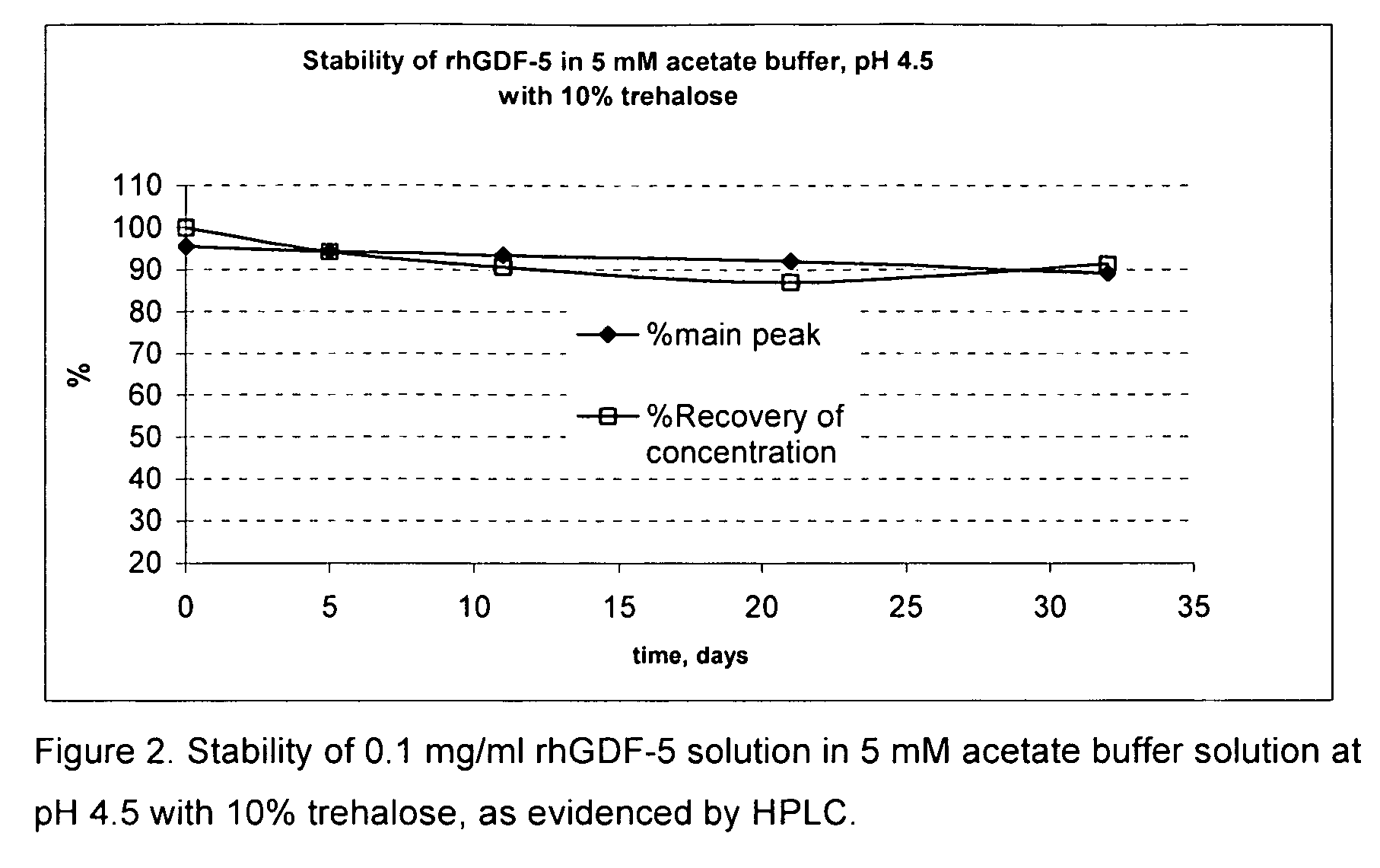
Liquid buffered GDF-5 formulations
InactiveUS7947649B2Improve propertiesStability of GDF-Peptide/protein ingredientsPharmaceutical delivery mechanismAcetic acidIsotonic Solutions
Improved formulations and methods are provided for stabilizing a solution of bone morphogenetic protein. The compositions comprise an acetate buffered solution of GDF-5 and other excipients wherein the solution has a pH of from about 4.2 to about 5.3, thereby providing for a biologically isotonic solution having improved stability of the GDF-5 protein during storage, handling, and use.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
Blood cell separation method for portunus trituberculatus
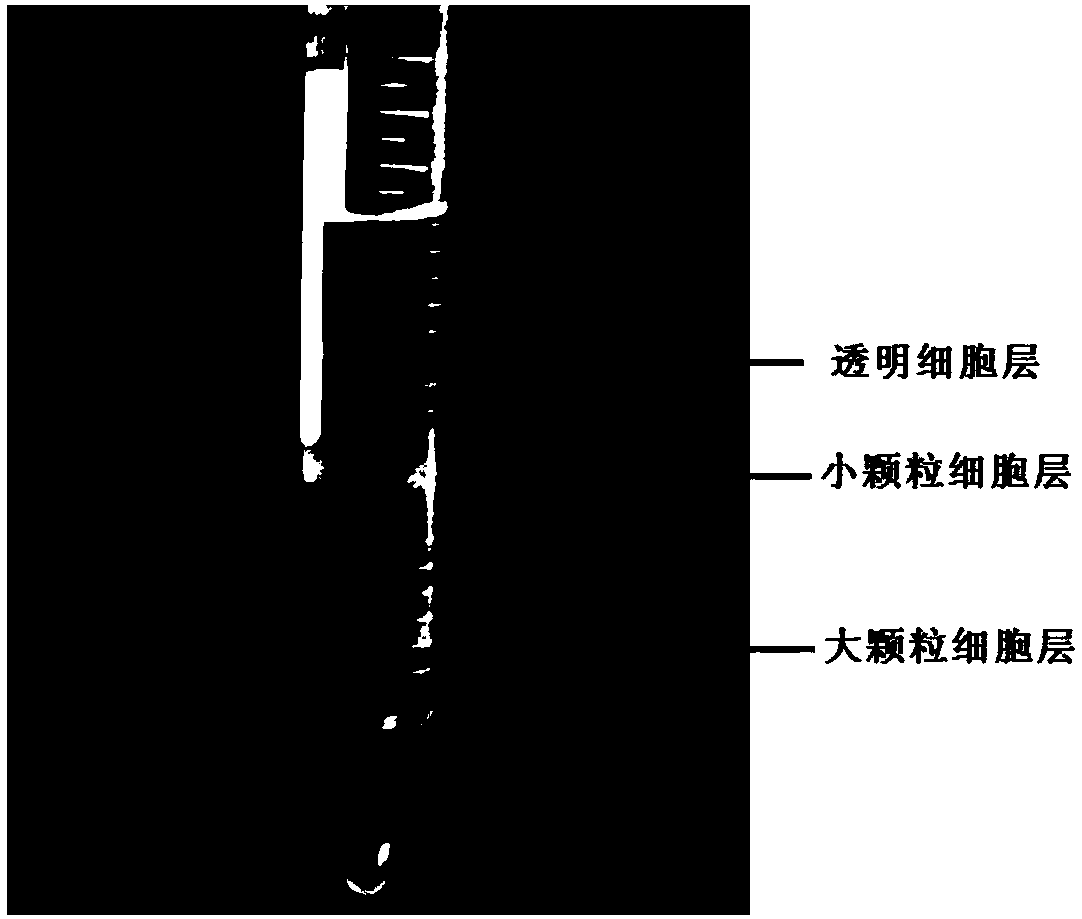
ActiveCN108118024AHigh activityEasy to operateCell dissociation methodsInvertebrate cellsGranular cellRed blood cell
The invention discloses a blood cell separation method for portunus trituberculatus. The method comprises the following steps: diluting iodixanol with an isotonic solution obtained by regulating an osmotic pressure of a PBS buffer solution by utilizing a sodium chloride solution, and performing gradient preparation to obtain three iodixanol solutions of different concentrations; forming gradient solutions of different densities by adopting a cushion technology, and standing to form a continuous density gradient solution to serve as a cell separation solution; collecting blood cells of portunustrituberculatus, and taking mixed cell suspension obtained by diluting the isotonic solution; slowly dropping the mixed cell suspension into the cell separation solution, centrifuging, and dividing the blood cells of portunus trituberculatus into three layers, wherein the uppermost layer refers to clear cells, the intermediate layer refers to small granular cells, and the lowest layer refers to large granular cells. The method has the advantages that only one-step density gradient centrifugation is adopted, the operation is simple, and experiments discover that the separated blood cells haveexcellent viabilities and can be used for cell culture.
Owner:NINGBO UNIV
Manufacturing method of medical sterilized isotonic solution having low-concentratedly controlled free chlorine including hypochlorous acid therein
InactiveCN101631898ALow free chlorine concentrationGreat sterilization effectElectrolysis componentsPharmaceutical product form changeSaline waterElectrolysis
The present invention relates to a manufacturing method of medical sterilized normal saline, more specifically, to such a method for manufacturing sterilized normal saline for medical purpose with effective sterilizing efficacy comprising: a step of disposing at least one electrode set immersed in saline solution of pH 4.0 to pH 7.5 including a pair of electrodes with flat surface separated from each other by an interval between lmm and 3mm, the flat surfaces of the electrodes facing each other; and a step of supplying 30mA to 200mA direct current to the electrodes by applying 2.4V to 3.3V DC power to the electrodes; wherein free chlorine is reliably and stably generated as having concentration range between 0.17ppm and 6ppm from electrolysis between electrodes.
Owner:韩国突起株式会社
Gas bubble-generating agent
InactiveUS20100158816A1Ultrasonic/sonic/infrasonic diagnosticsDiagnostic recording/measuringChemical LinkageIn vivo
This invention provides a gas bubble-generating agent that can be used as a contrast medium or a blocking agent in vivo. Such gas bubble-generating agent is produced by a method for producing a gas bubble-generating agent comprising the following steps of: (a) preparing a mixed solution of an amphiphilic substance, an amphiphilic substance comprising a water-soluble polymer chemically bound thereto, a hardly water-soluble substance having a boiling point of lower than 60° C. at atmospheric pressure, and a physiologically acceptable isotonic solution; (b) pressurizing the mixed solution; and (c) centrifuging the mixed solution after the step of pressurization, wherein a molar concentration of the amphiphilic substance in the mixed solution prepared in step (a) is 10 times or more higher than that of the amphiphilic substance comprising a water-soluble polymer chemically bound thereto.
Owner:HITACHI LTD
Methods of preventing surgical site infections
InactiveUS20160120936A1Increased mortalityAntibacterial agentsBiocideSurgical site infectionSurgical incision
Methods for preventing a tissue infection associated with the site of a tissue disruption, such as a surgical incision. The methods include contacting tissue at the site with a composition comprising ε-polylysine in a physiologically-acceptable carrier, such as an isotonic solution, powder or hemostatic material containing ε-polylysine. Also provided are kits for preparing antibacterial ε-polylysine compositions.
Owner:BIOMET MFG CORP
Method of preparing an immunologically inert graft material from body tissue and material made with the method
InactiveUS20050238688A1Mammal material medical ingredientsLavatory sanitoryCell-Extracellular MatrixMedicine
Owner:BRENNEN MEDICAL
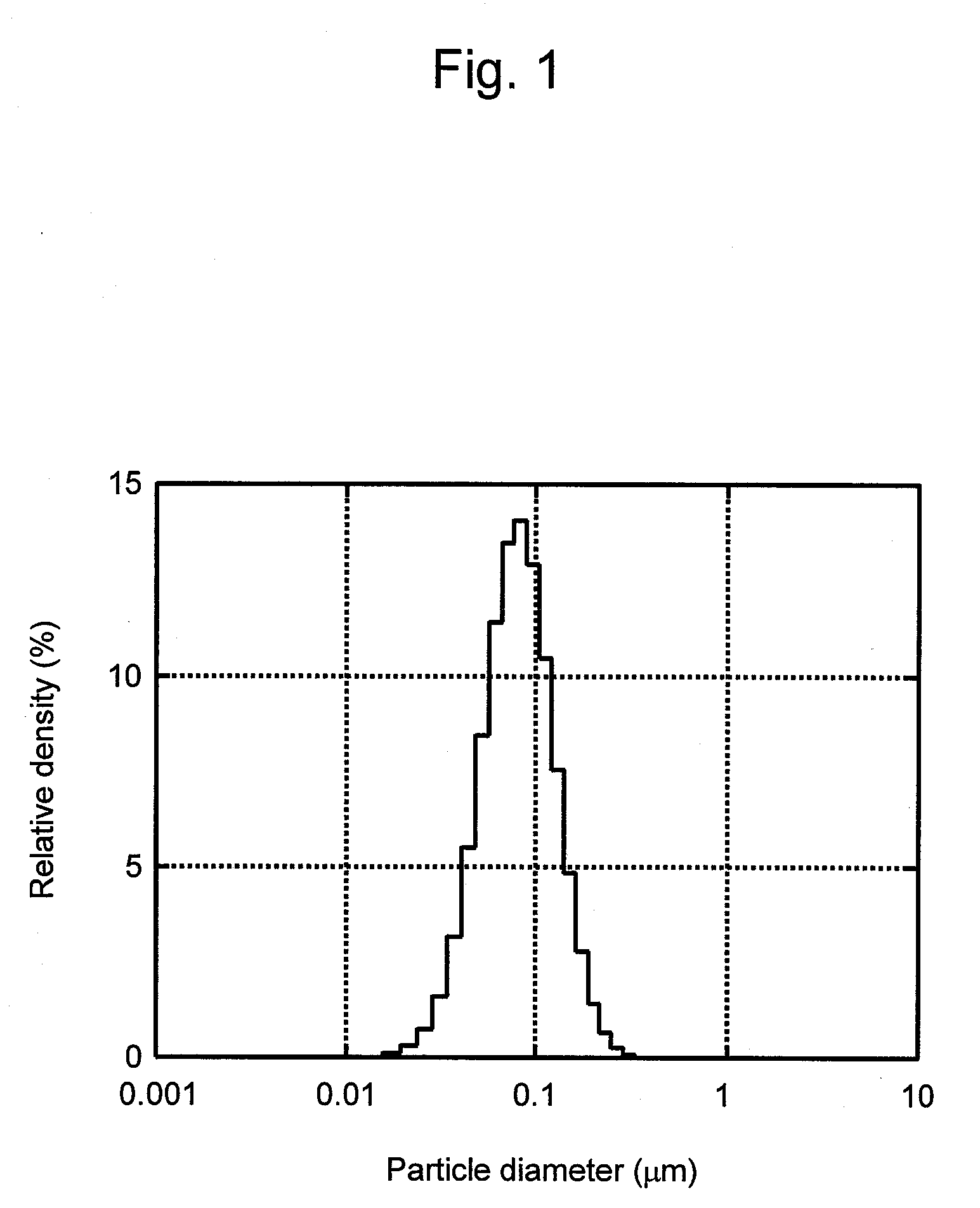
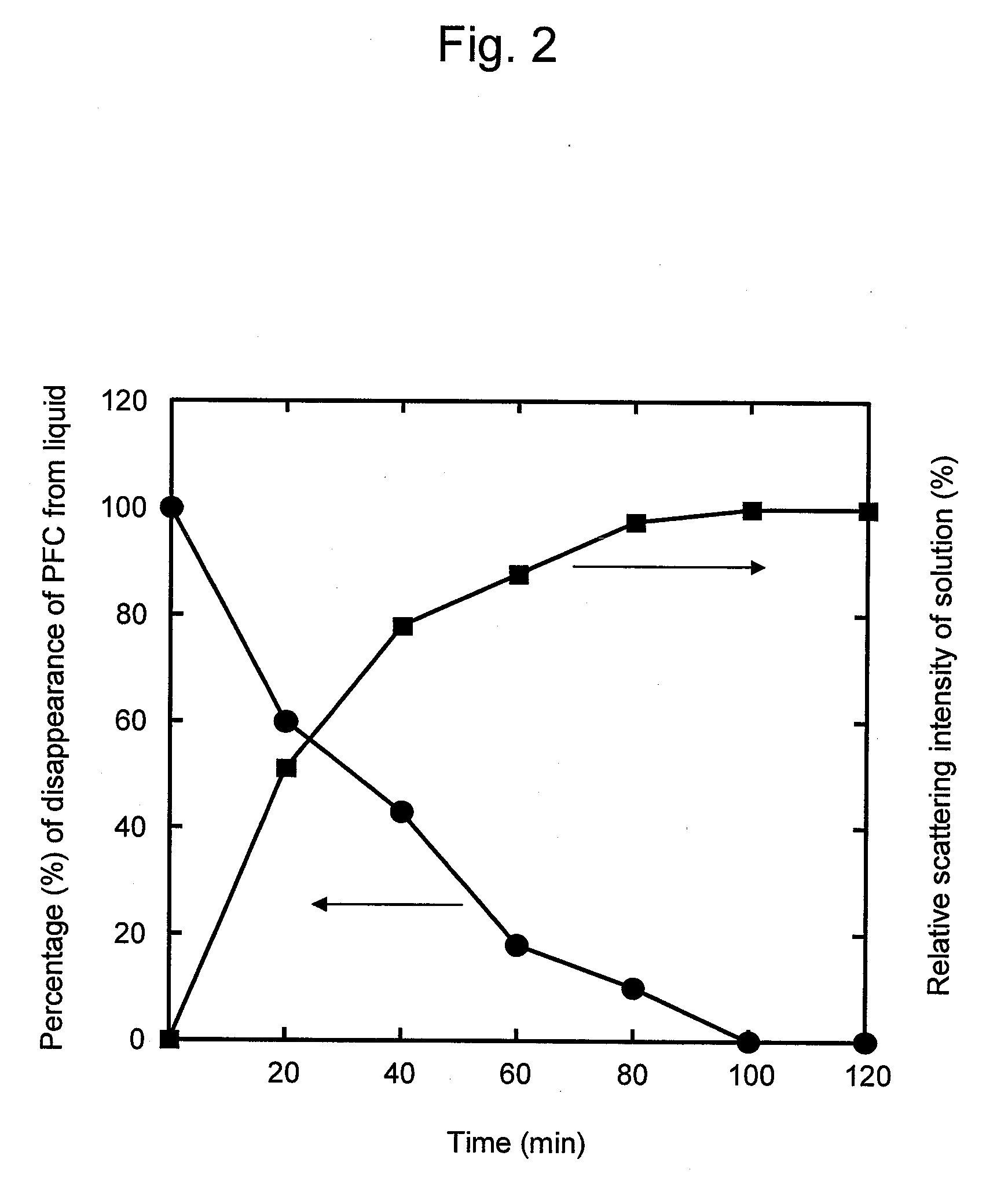
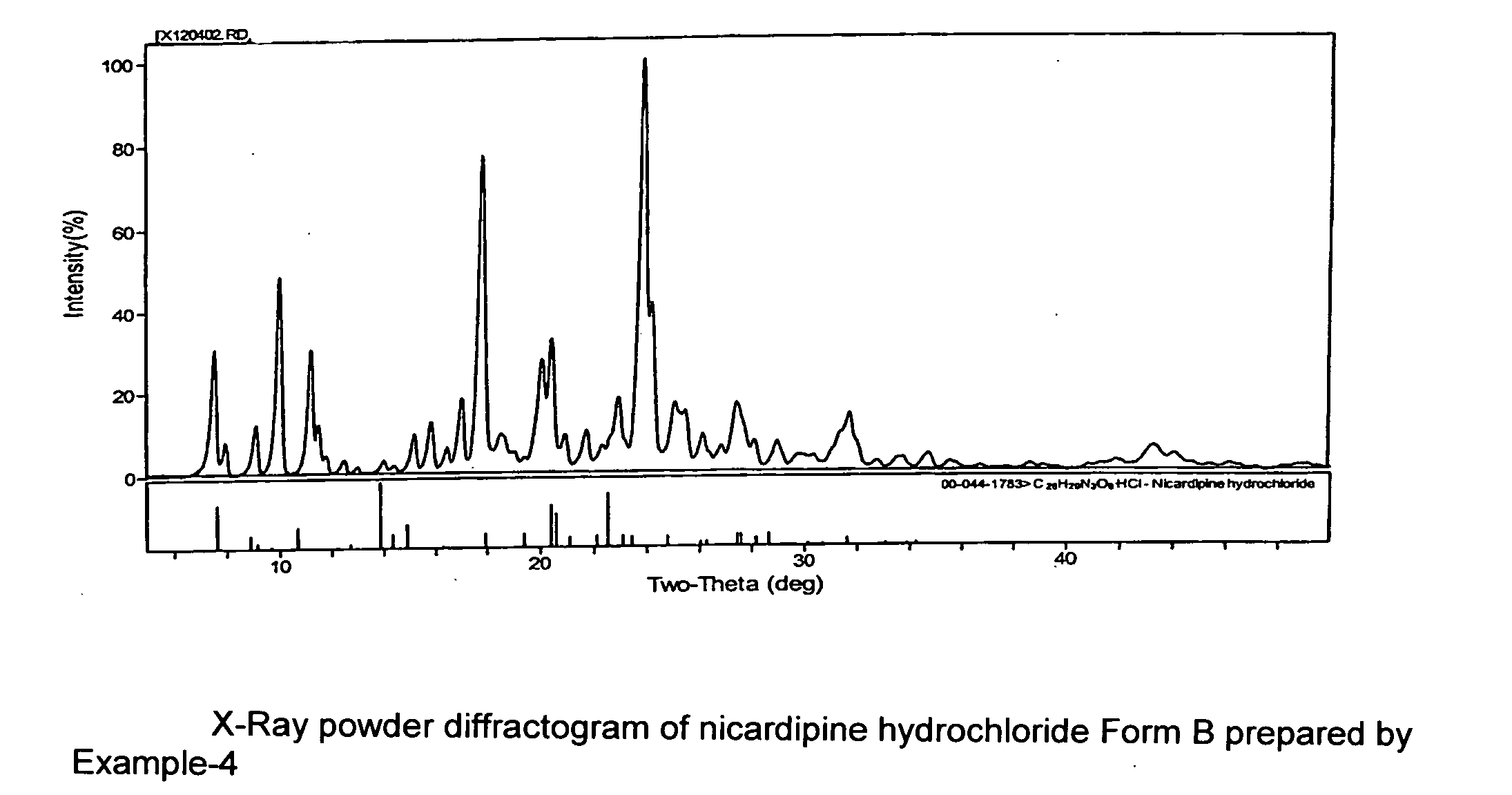
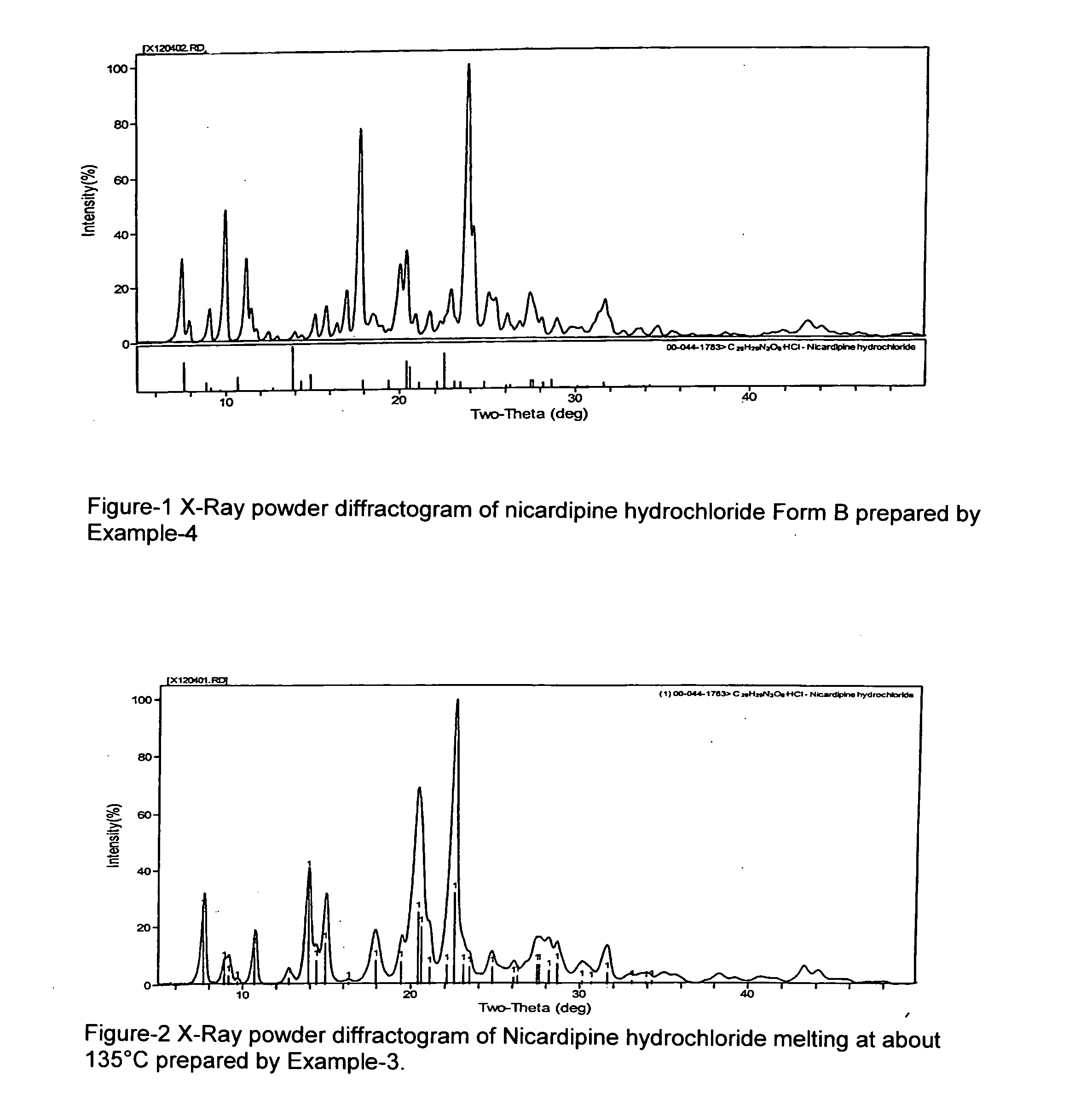
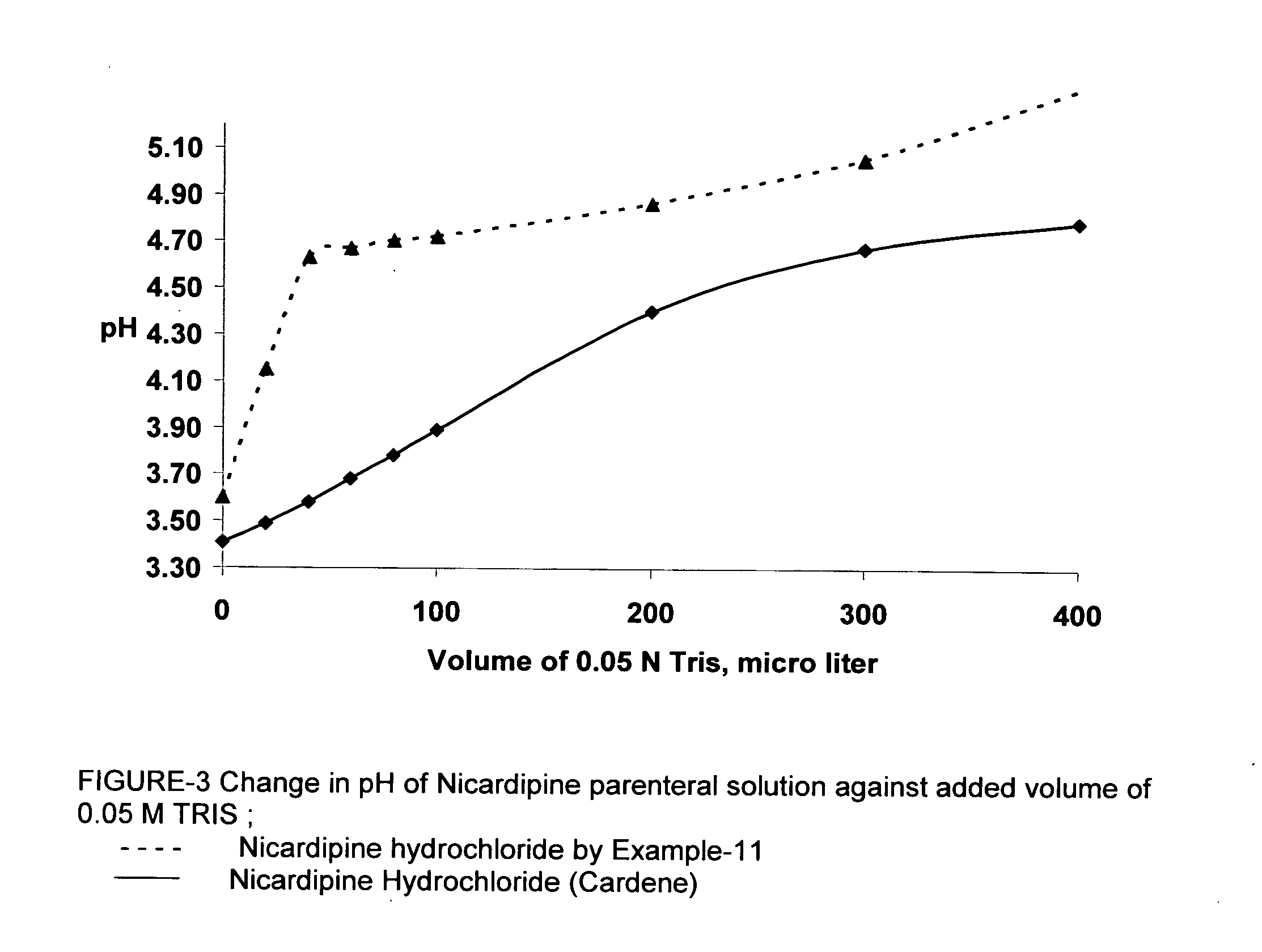
Processes of manufacturing substituted-1,4-dihydropyridines, improved aqueous solutions thereof, and processes of manufacturing the solutions
A process of preparing a stable parenteral solution of a 1,4-dihydropyridine salt, such as nicardipine hydrochloride, in an acidic aqueous medium. The presence of L-arginine in the solution enhances the solubility of the salt, which is poorly soluble in water.An aqueous, injectable isotonic solution at pH about 3.5-3.6 consists essentially of nicardipine hydrochloride, L-arginine, and a sugar alcohol.An improved single pot manufacturing process for obtaining unsymmetrical 1,4-dihydropyridines by using more than one mole equivalent of aldehyde with respect to the other reactants (amino crotonate and acetoacetate ester). The reaction can be conducted in a solvent present at 20 times the amount of any one component.A process for changing one polymorph of nicardipine hydrochloride (Form A) into another (Form B), and a separate process for the reverse (Form B into Form A).
Owner:NAVINTA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com