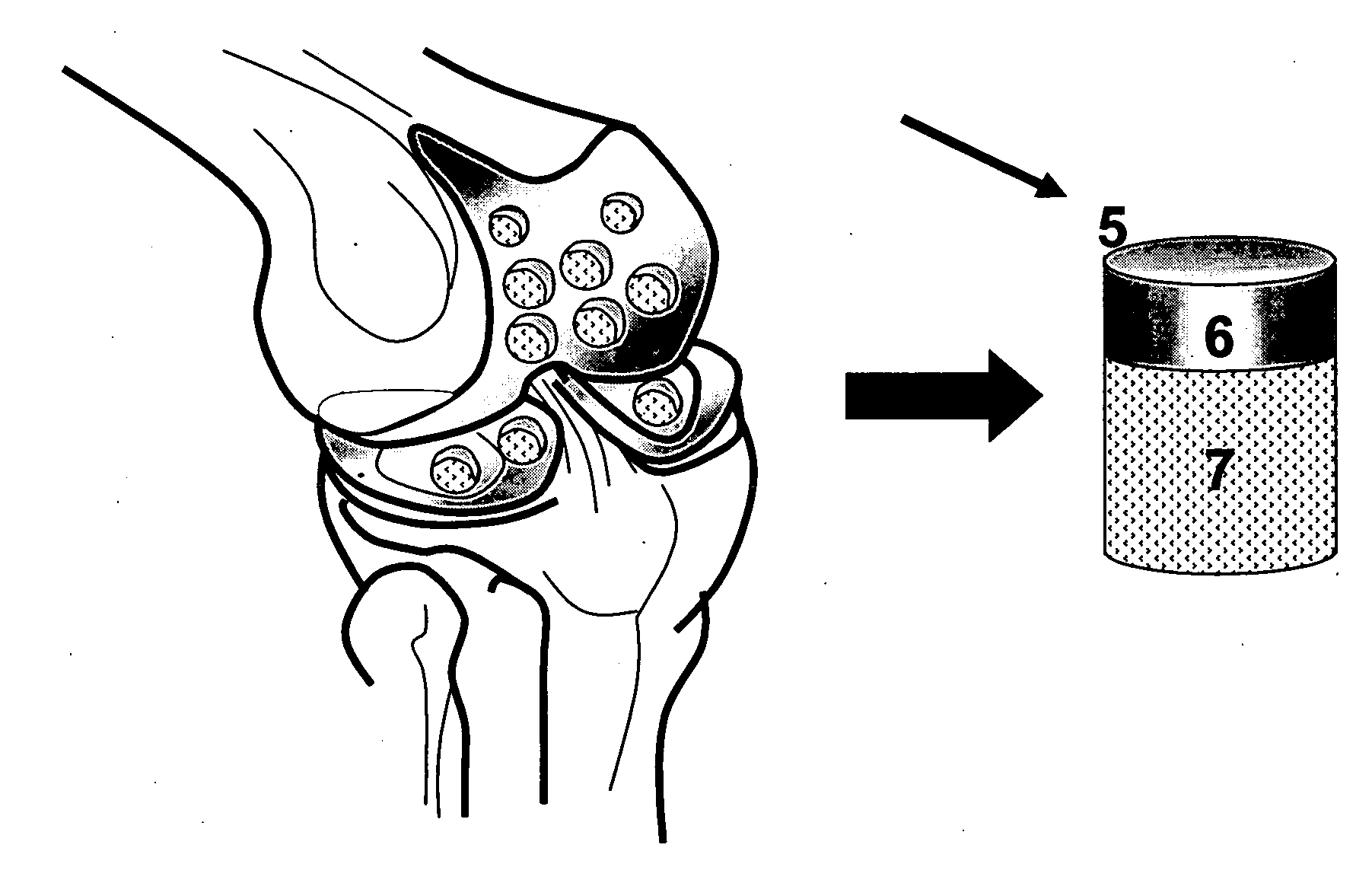
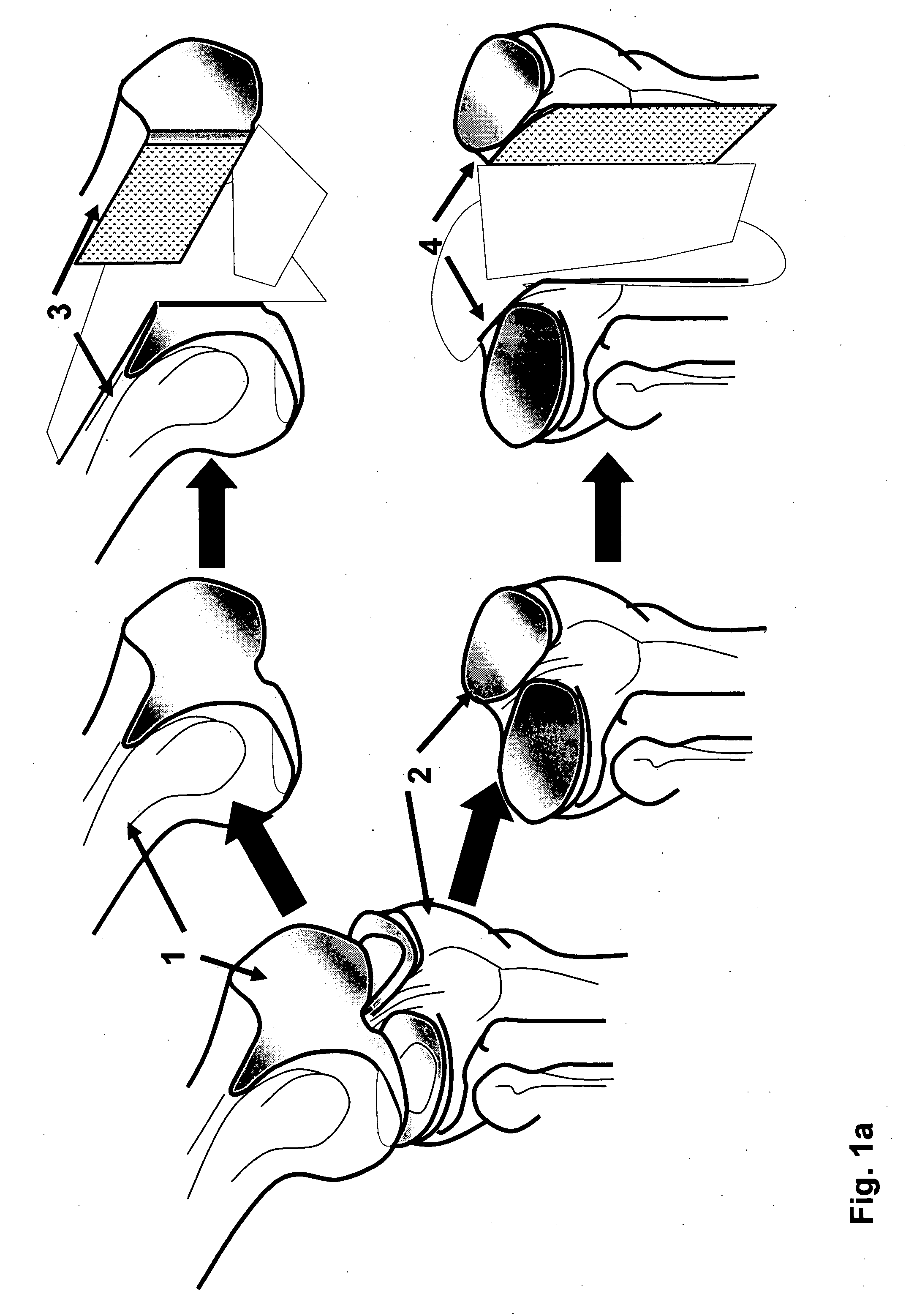
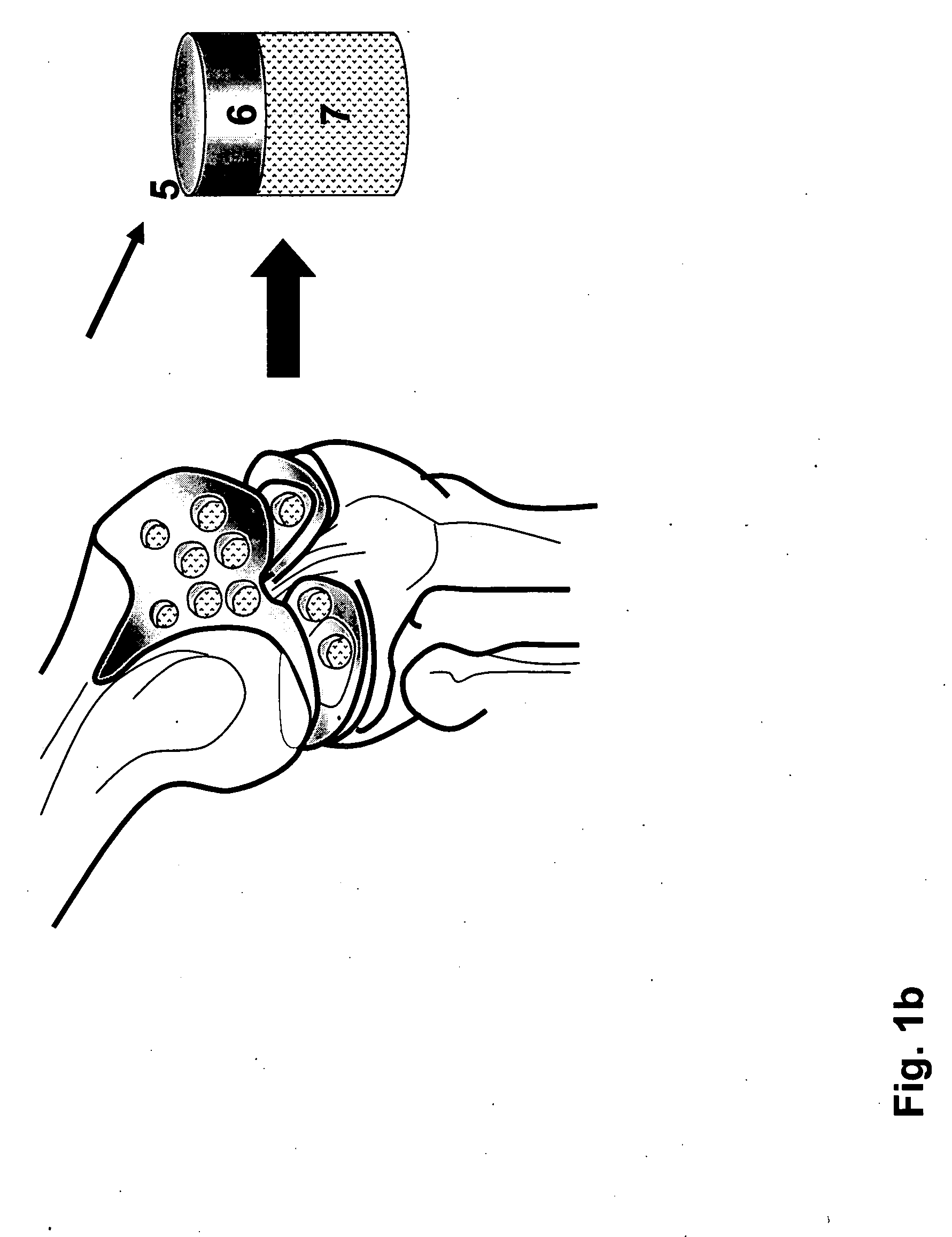
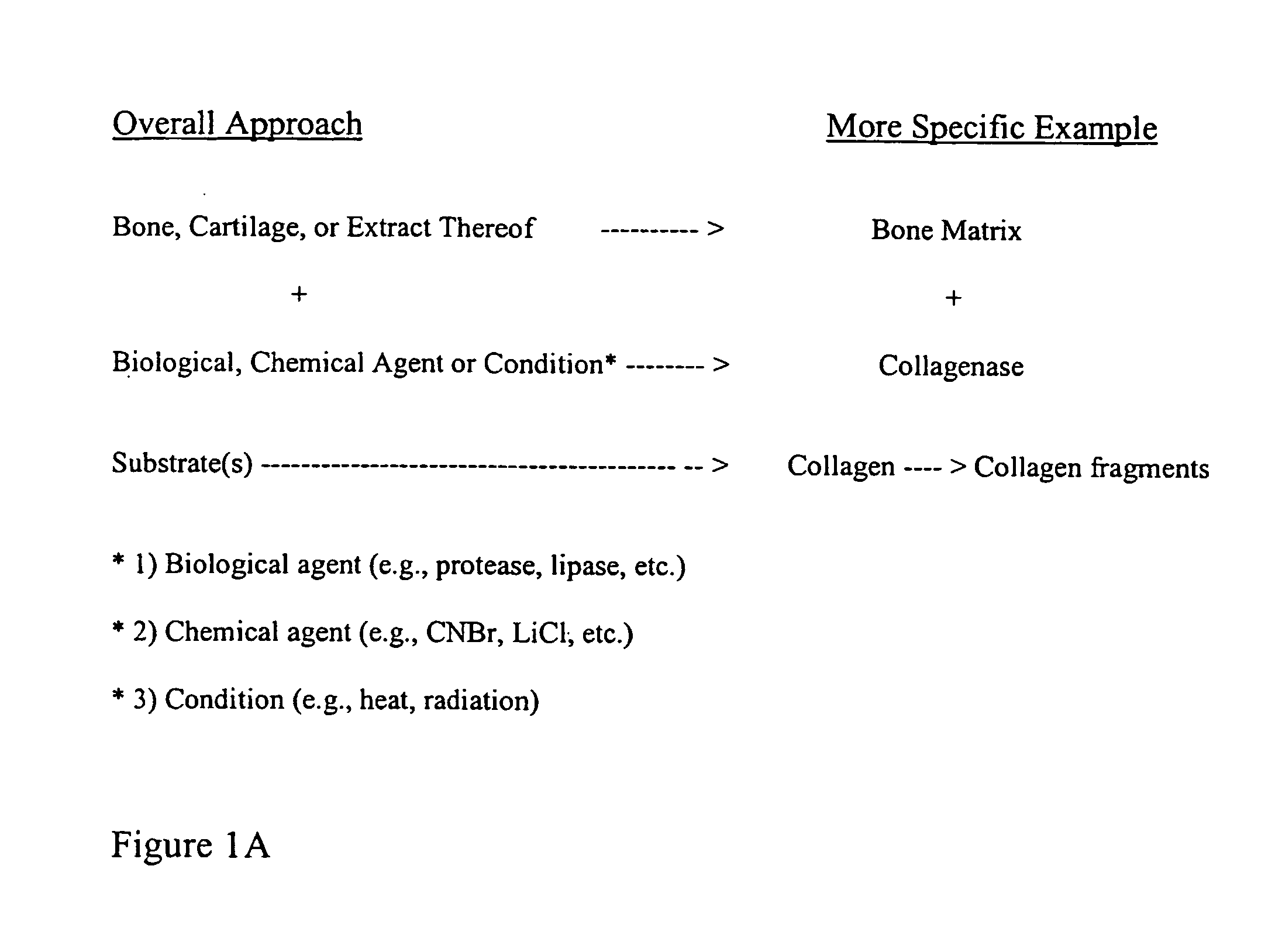
Patents
Literature
107 results about "Cartilage Matrix" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
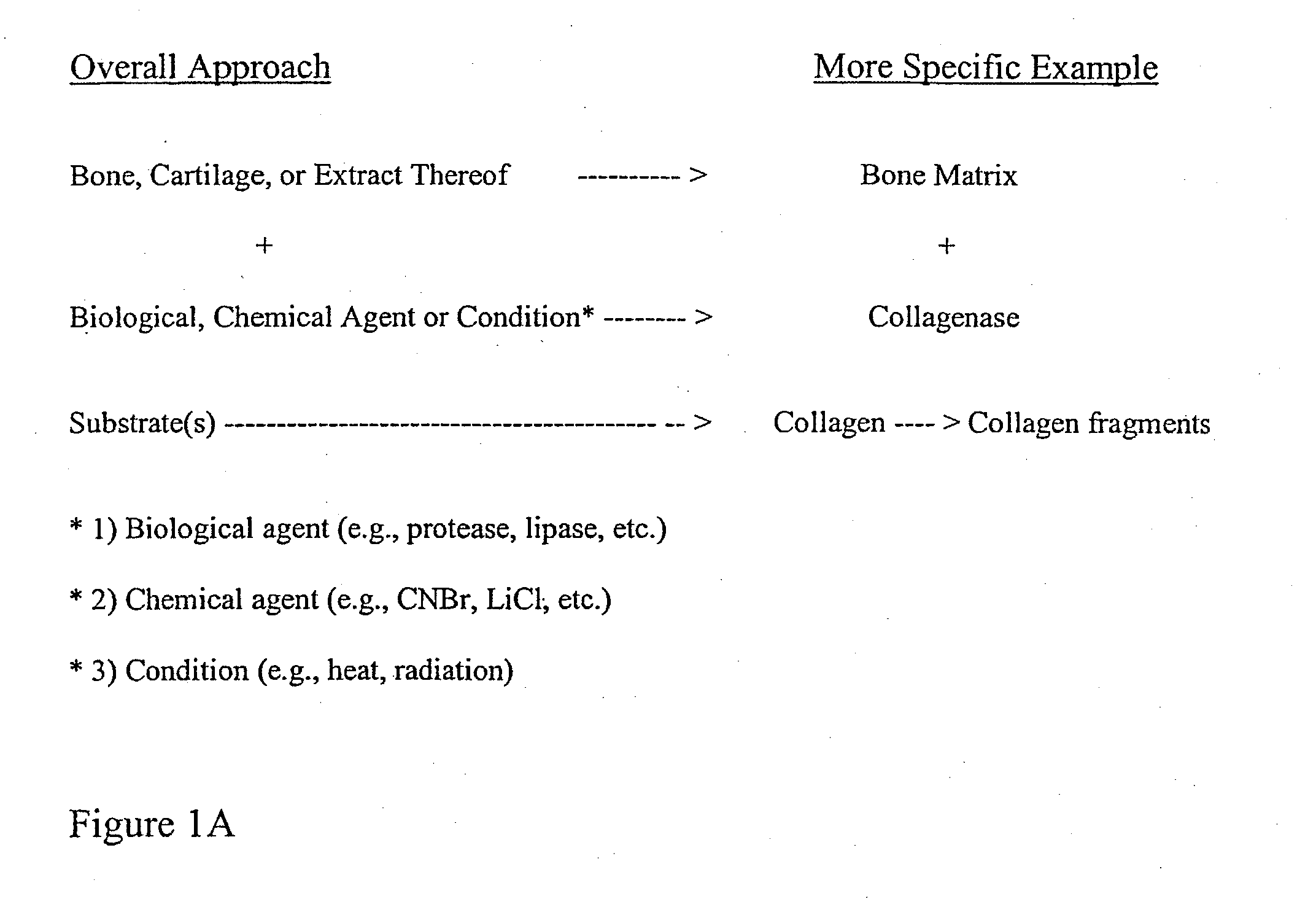
Bone matrix the intercellular substance of bone, consisting of collagenous fibers, ground substance, and inorganic salts. cartilage matrix the intercellular substance of cartilage consisting of cells and extracellular fibers embedded in an amorphous ground substance.
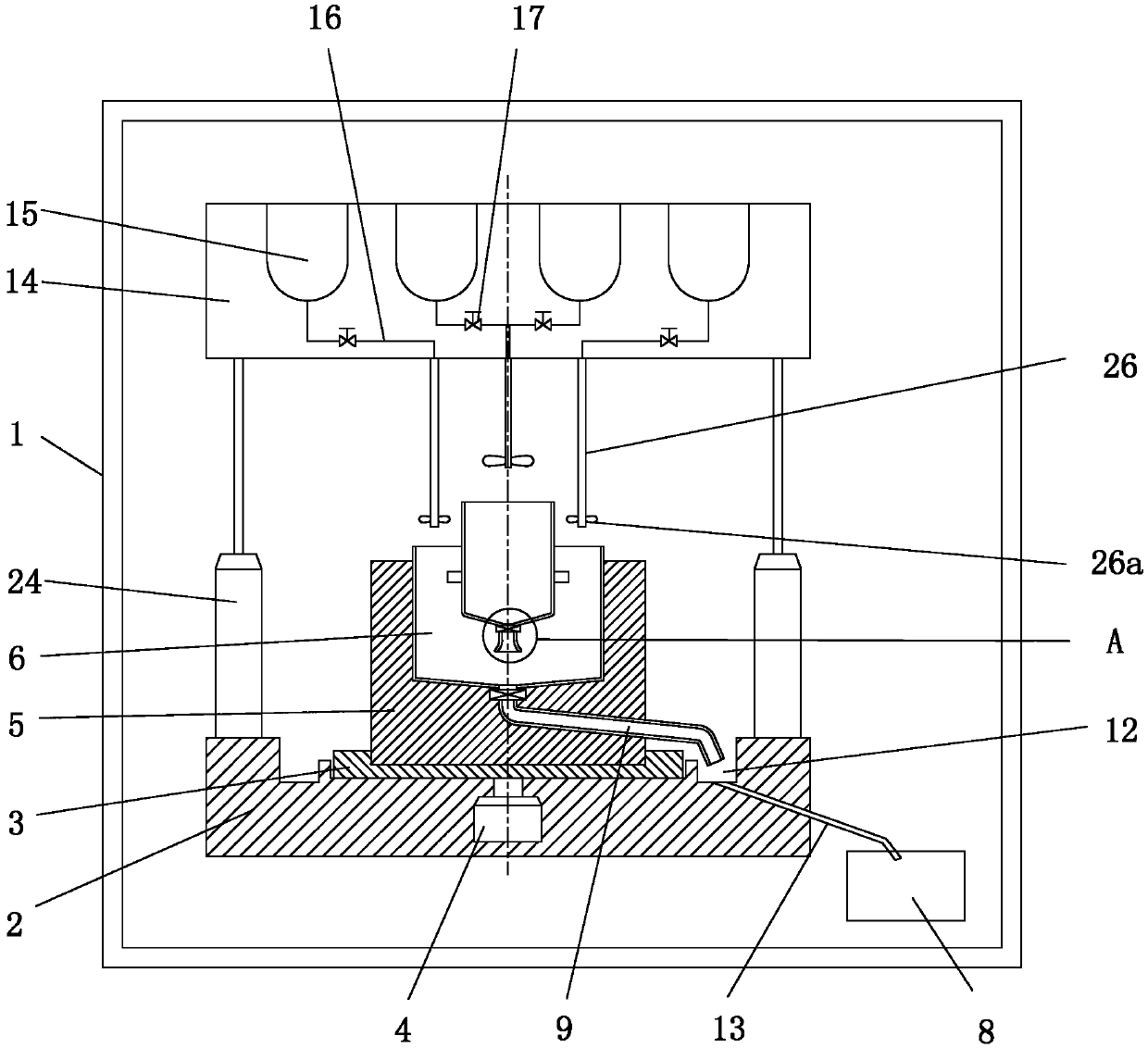
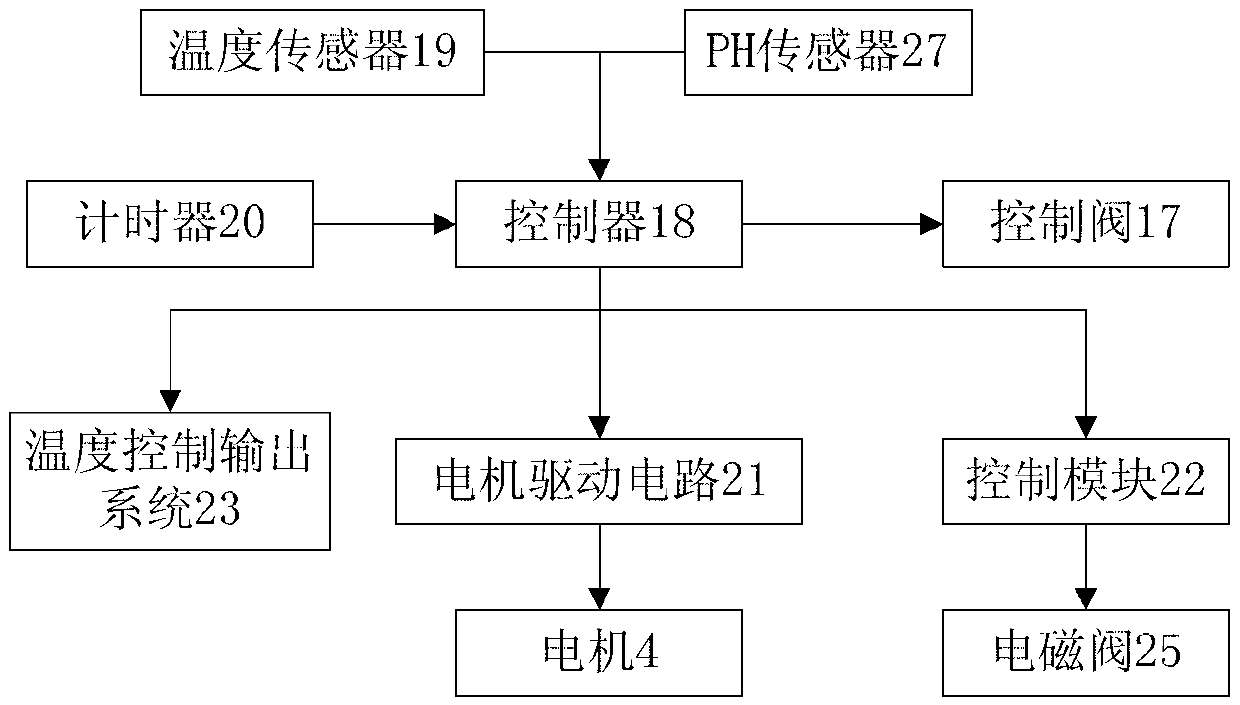
Cleaning and devitalization of cartilage
InactiveUS20080077251A1Improve recellularizationBone implantDead animal preservationCellular DebrisMedicine
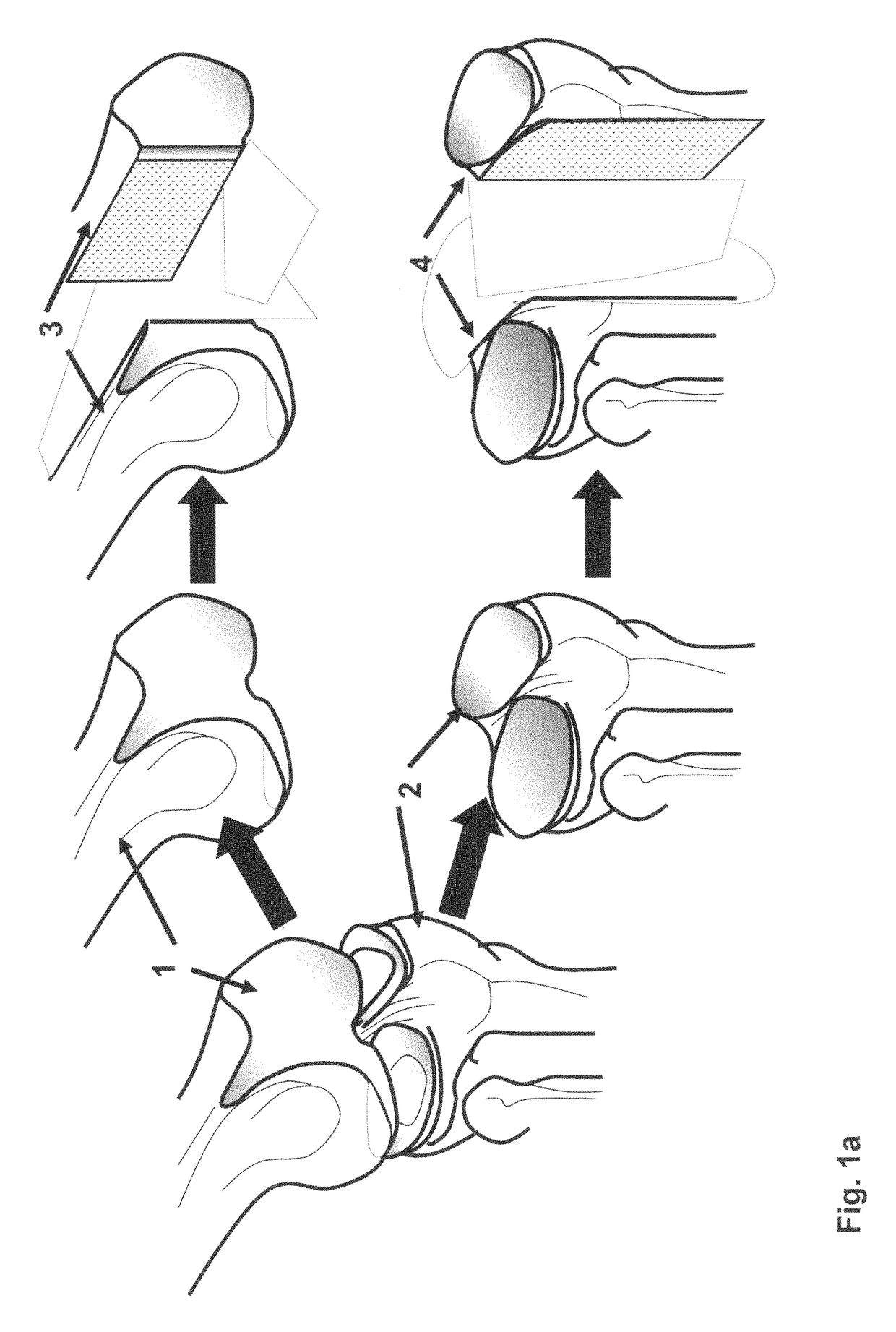
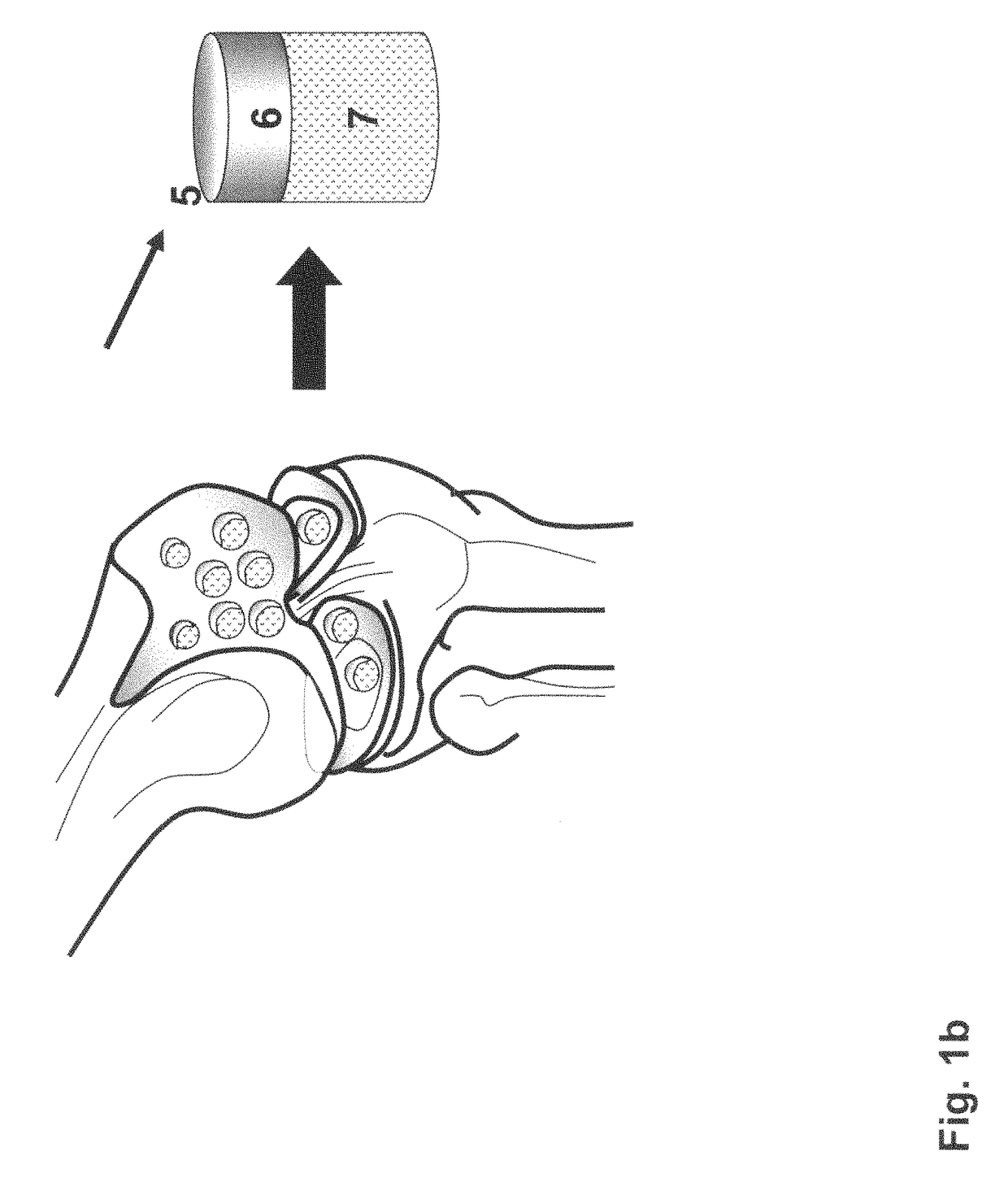
The invention is further directed to producing a cleaned, disinfected, and devitalized cartilage graft by optionally cleaning and disinfecting the cartilage graft; treating the cartilage graft in a pretreatment solution; treating the cartilage graft in an extracting solution; washing the extracted cartilage graft with a rinsing solution; and subsequently soaking the devitalized cartilage graft in a storage solution. The devitalized cartilage graft is essentially free from metabolically viable and / or reproductively viable cells and the rinsing solution is hypotonic solution or isotonic solution. The present invention is further directed to a cleaned, disinfected, and devitalized cartilage graft and a process for cleaning, disinfecting, and devitalizing cartilage grafts. The invention also relates to a process for repairing a cartilage defect and implantation of a cartilage graft into a human or animal by crafting the cartilage matrix into individual grafts, disinfecting and cleaning the cartilage graft, applying a pretreatment solution to the cartilage graft, removing cellular debris using an extracting solution to produce a devitalized cartilage graft, implanting the cartilage graft into the cartilage defect with or without an insertion device, and sealing the implanted cartilage graft with recipient tissue. The devitalized cartilage graft is optionally recellularized in vitro, in vivo, or in situ with viable cells to render the tissue vital before or after the implantation. The devitalized cartilage graft is also optionally stored between the removing cellular debris and the recellularizing steps.
Owner:LIFENET HEALTH
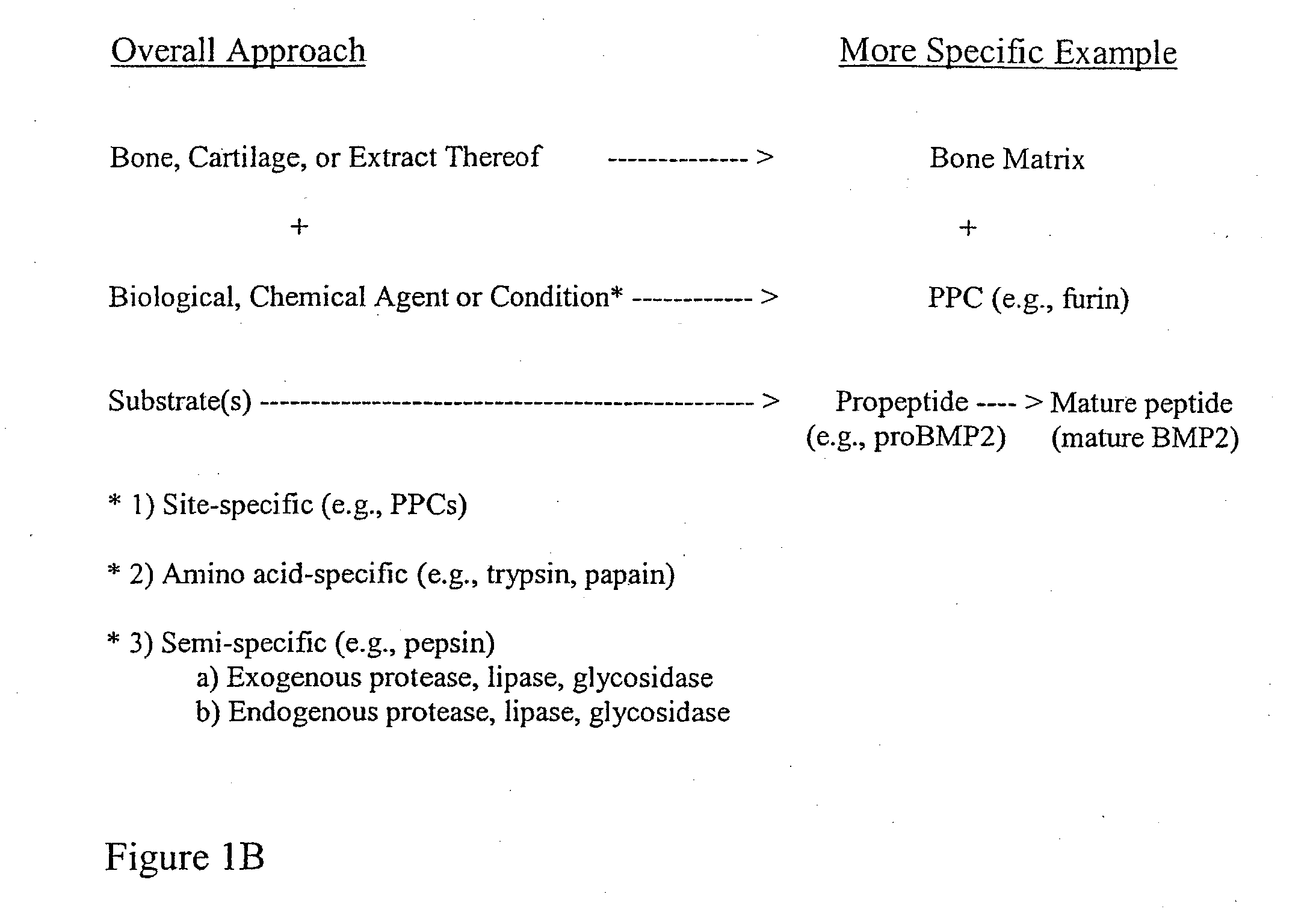
Bone matrix compositions and methods
ActiveUS20070154563A1Good osteoinductivityHigh activityHydrolysed protein ingredientsBone implantOsteoblastLine of therapy
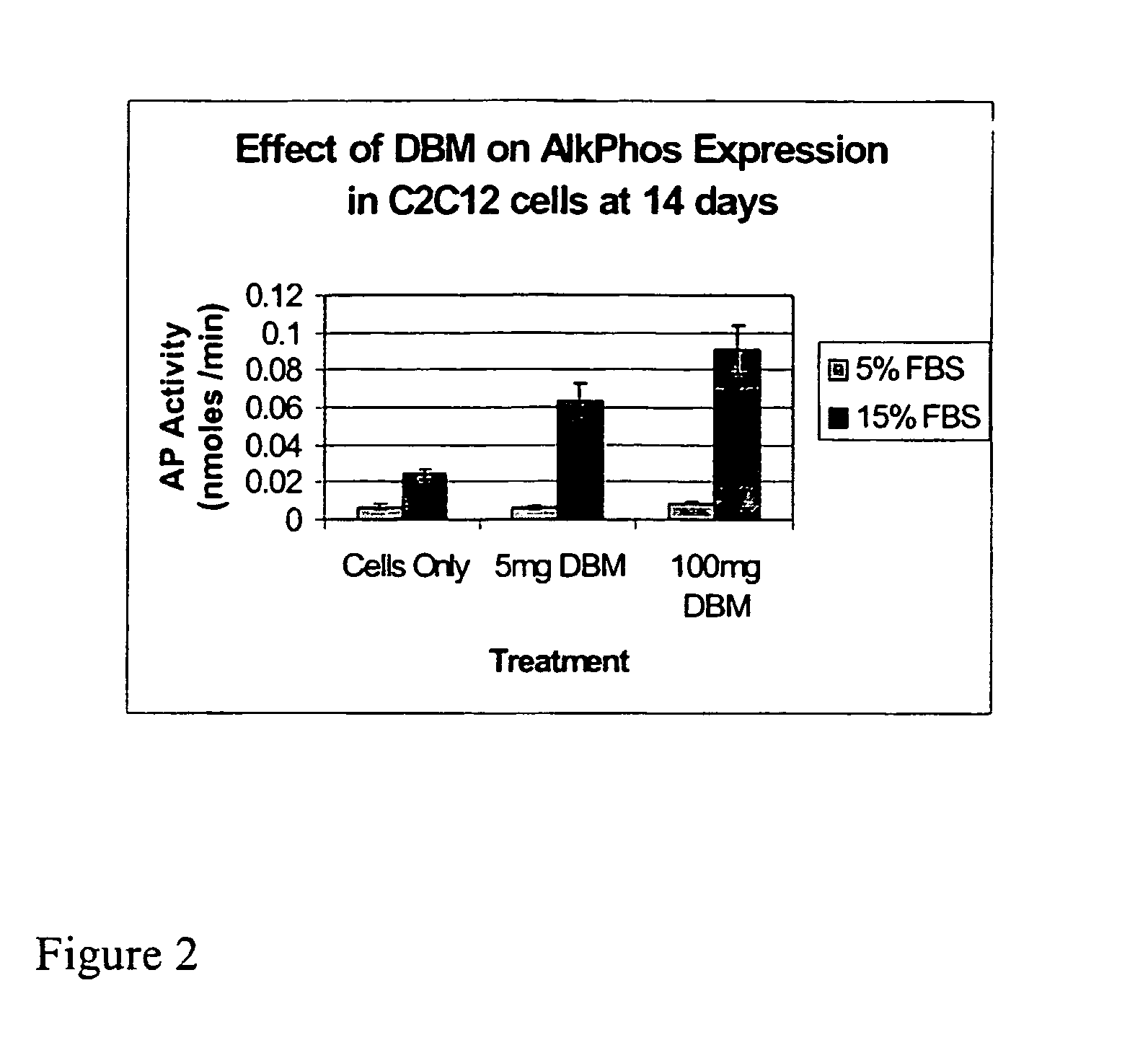
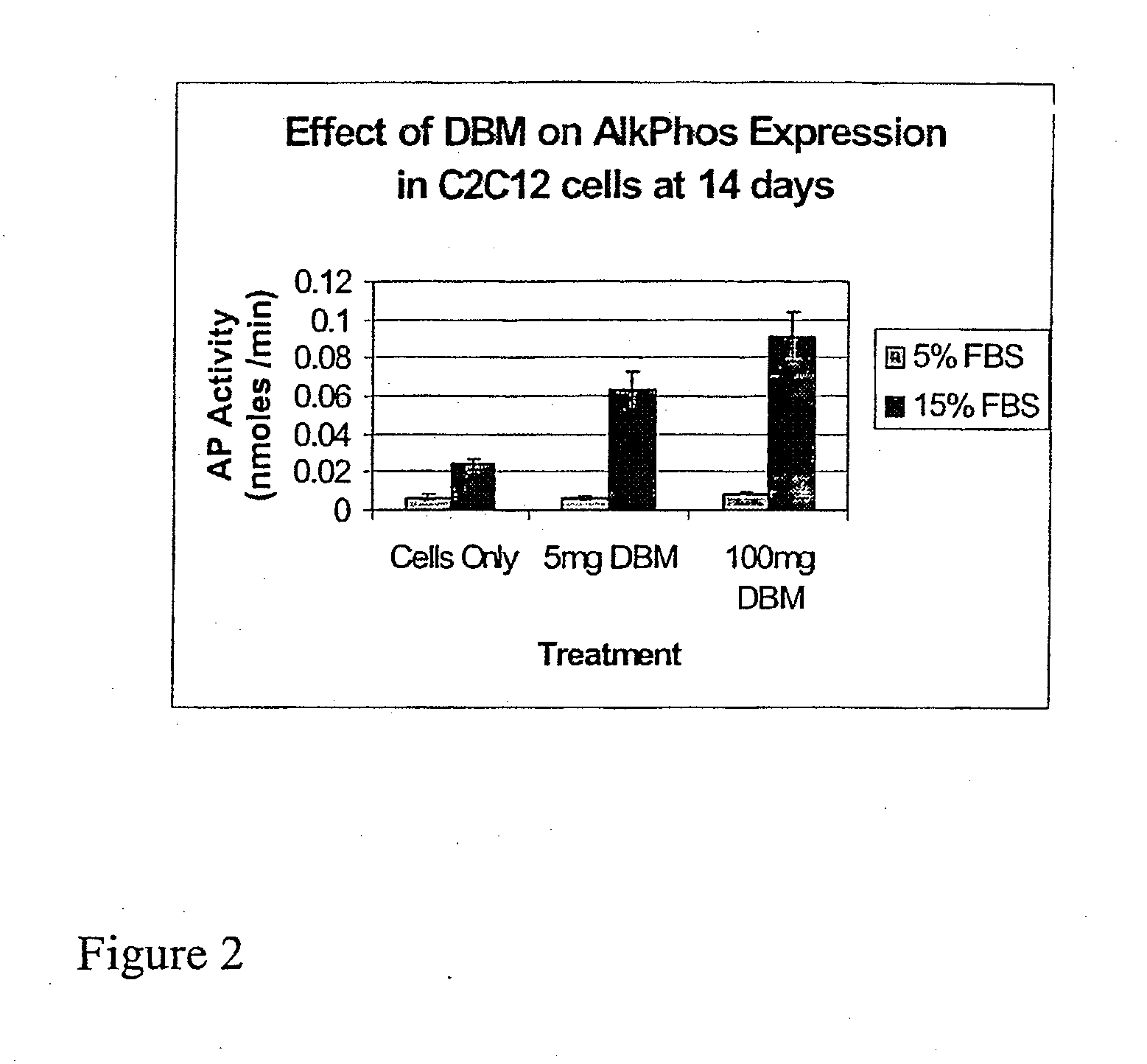
The present invention provides methods of improving the osteogenic and / or chondrogenic activity of a bone matrix, e.g., a dermineralized bone matrix (DBM), by exposing the bone matrix to one or more treatments or conditions. In preferred embodiments the bone matrix is derived from human bone. The treatment or condition may alter the structure of the bone matrix and / or cleave one or more specific proteins. Cleavage may generate peptides or protein fragments that have osteoinductive, osteogenic, or chondrogenic activity. Preferred treatments include collagenase and various other proteases. The invention further provides improved bone and cartilage matrix compositions that have been prepared according to the inventive methods and methods of treatment using the compositions. The invention further provides methods of preparing, testing, and using the improved bone matrix compositions. Ona assay comprises exposing relatively undifferentiated mesenchymal cells to a bone matrix composition and measuring expression of a marker characteristic of osteoblast or chondrocyte lineage(s). Increased expression of the marker relative to the level of the marker in cells that have been exposed to a control matrix (e.g., an inactivated or untreated matrix) indicates that the treatment or condition increased the osteogenic and / or chondrogenic activity of the bone matrix. Suitable cells include C2C12 cells. A suitable marker is alkaline phosphatase. The inventive methods increase the osteogenic and / or chondrogenic activity of human DBM when tested using this assay system.
Owner:WARSAW ORTHOPEDIC INC
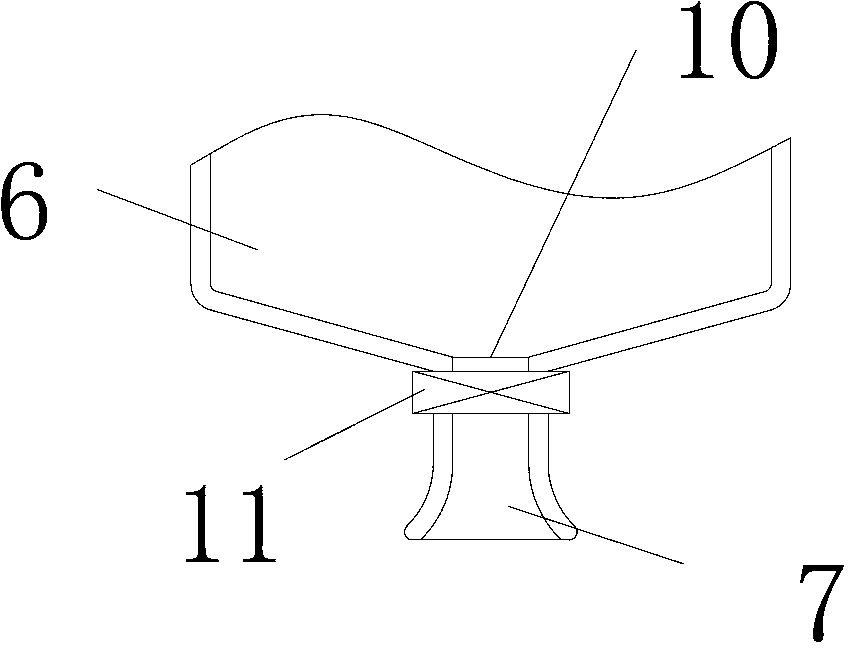
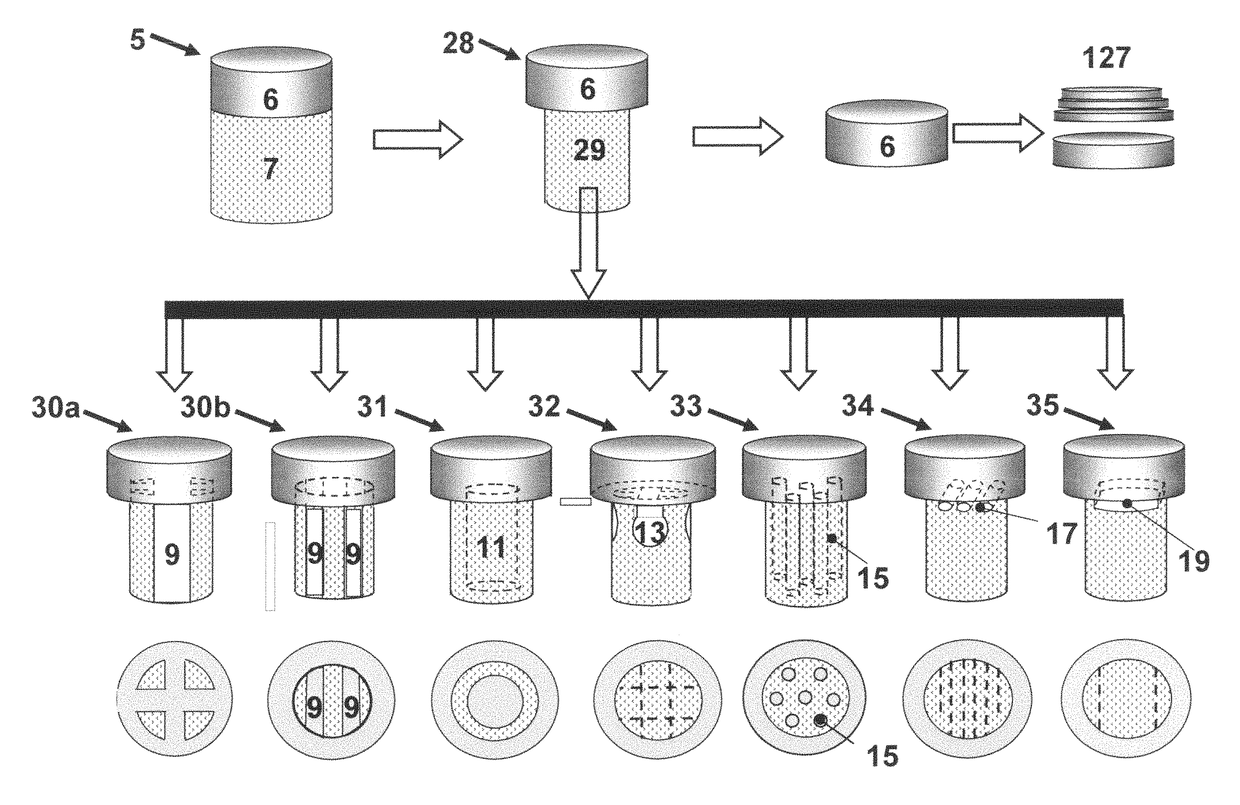
Crafting of cartilage
The invention is directed to producing a shaped cartilage matrix isolated from a human or animal where the cartilage has been crafted to facilitate disinfection, cleaning, devitalization, recellularization, and / or integration after implantation. The invention relates to a process for repairing a cartilage defect and implantation of a cartilage graft into a human or animal by crafting the cartilage matrix into individual grafts, disinfecting and cleaning the cartilage graft, applying a pretreatment solution to the cartilage graft, removing cellular debris using an extracting solution to produce a devitalized cartilage graft, implanting the cartilage graft into the cartilage defect with or without an insertion device, and sealing the implanted cartilage graft with recipient tissue. The devitalized cartilage graft is optionally recellularized in vitro, in vivo, or in situ with viable cells to render the tissue vital before or after the implantation. The devitalized cartilage graft is also optionally stored between the removing cellular debris and the recellularizing steps.
Owner:LIFENET HEALTH
Method for improving cartilage repair and/or preventing cartilage degeneration in a joint
InactiveUS20100215731A1Convenient treatmentRelieve painPowder deliveryBiocideRepair tissueActive agent
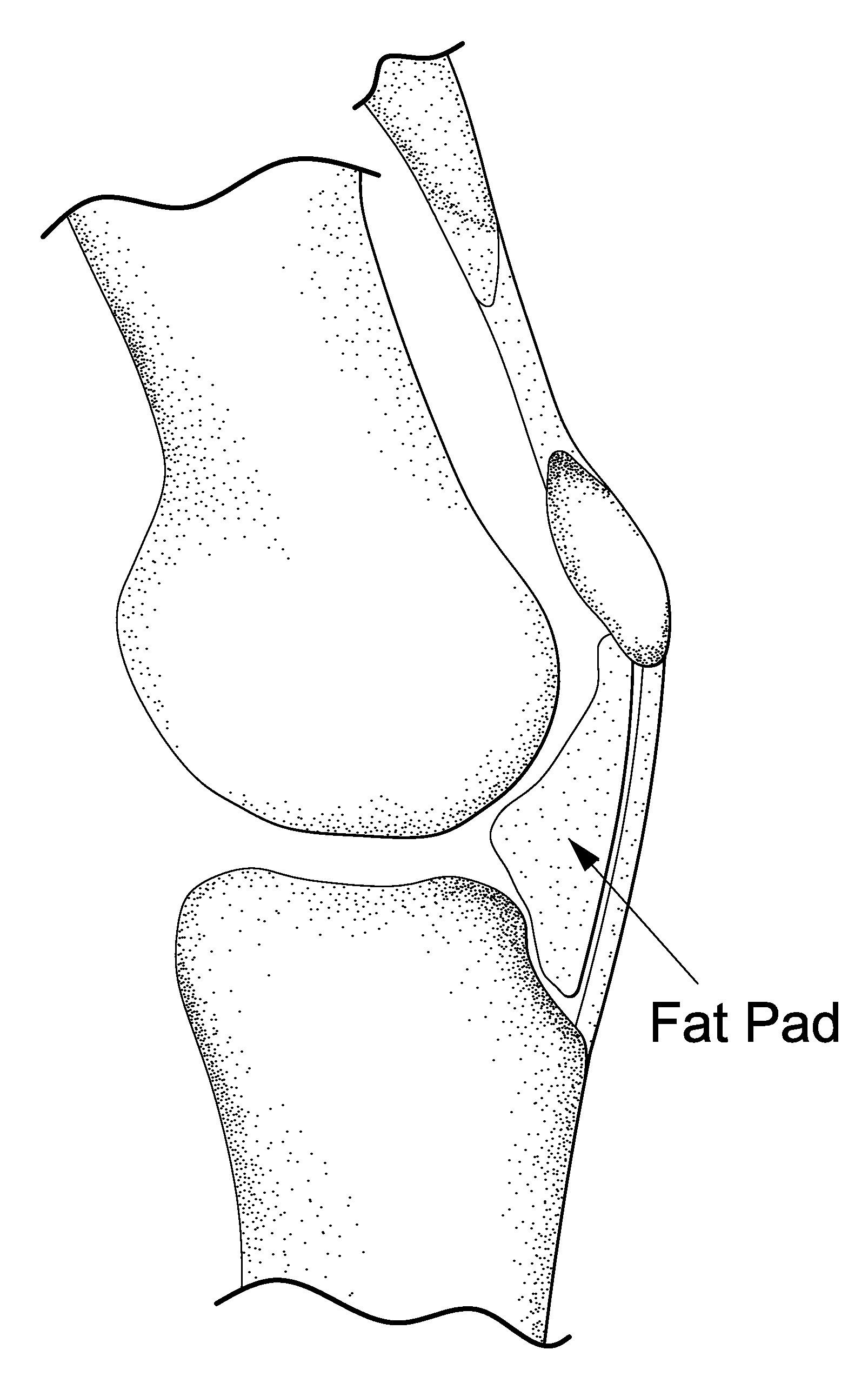
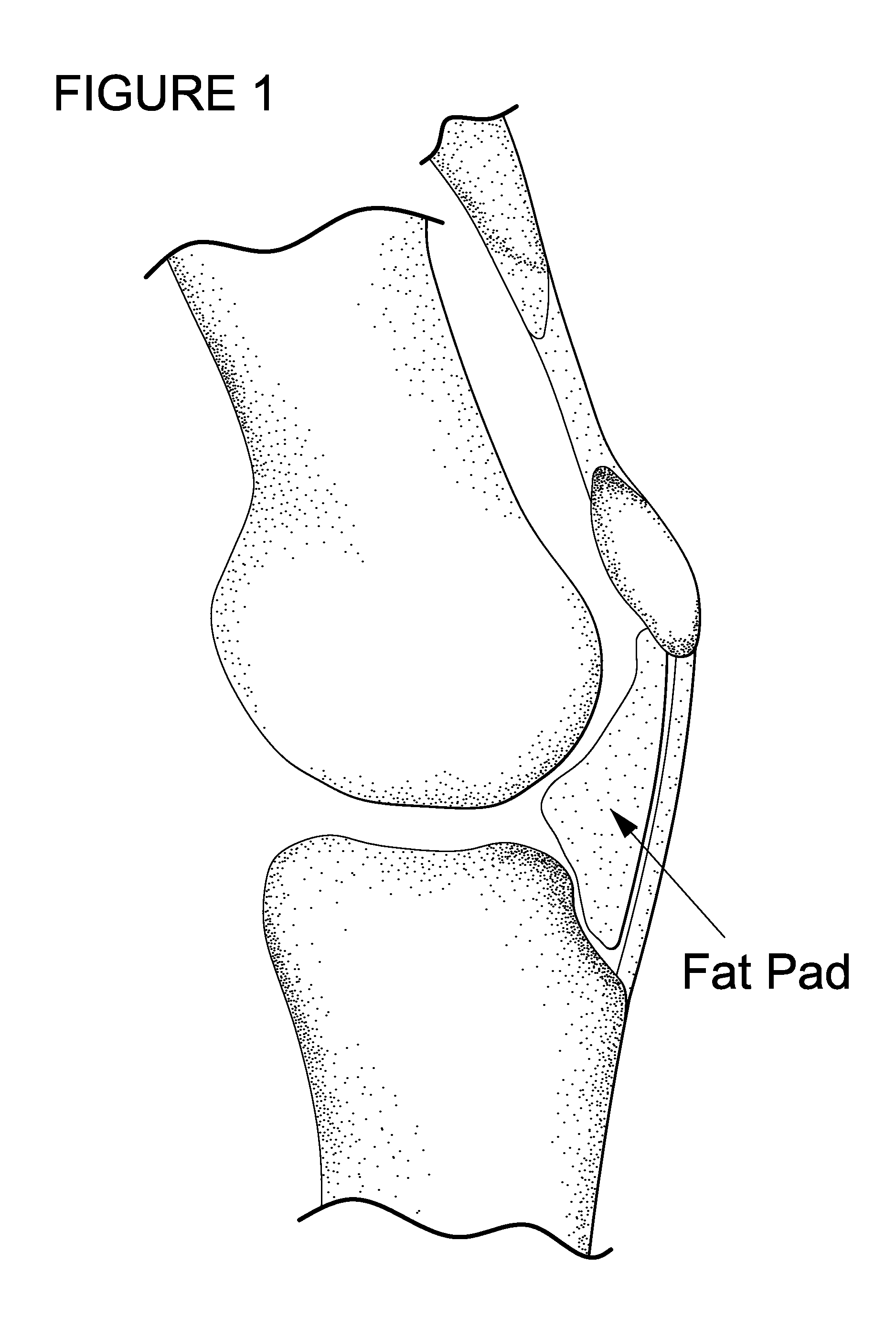
The invention is in the field of methods for medical treatment. It provides an improved method for repairing damaged cartilage and / or preventing cartilage degeneration in tissue, in particular in a joint by administering a pharmaceutically active agent directly into the fat pad of a joint. The pharmaceutically active agent is preferably selected from the group consisting of agents that stimulate chondrogenic differentiation and / or cartilage matrix synthesis; agents that inhibit osteogenesis and / or hypertrophy, anti-inflammatory agents, agents that inhibit apoptosis of chondrocytes, agents that inhibit senescence of chondrocytes and agents that enhance lubrication of a joint.
Owner:ACADEMIC HOSPITAL MAASTRICHT +1
Preparation method of co-crosslinked double-network hydrogel support for promoting cartilage injury repair
ActiveCN107281550AImprove adhesionThe effect of promoting defect healingTissue regenerationProsthesisCartilage injuryDouble network
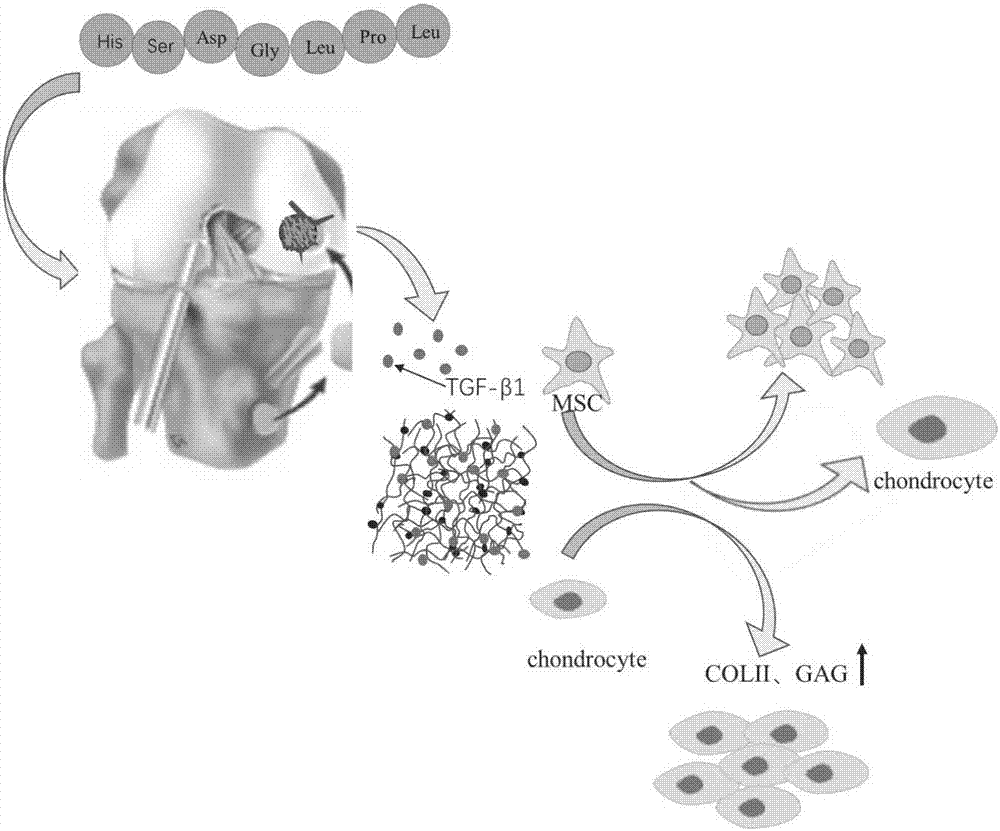
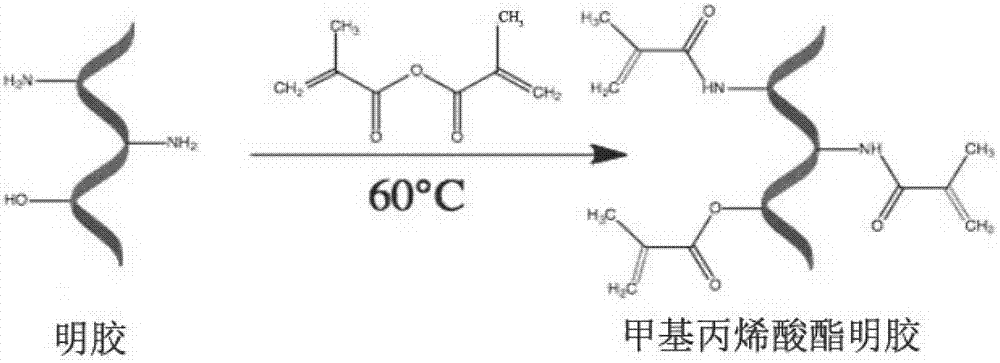
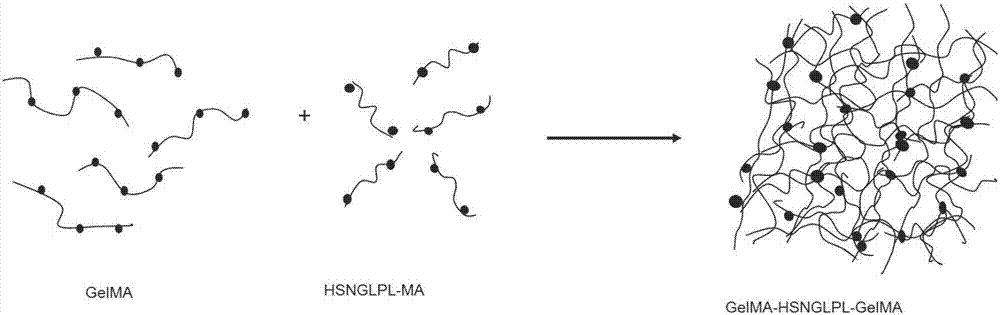
The invention belongs to the field of biomedical materials and particularly relates to a preparation method of a co-crosslinked double-network hydrogel support for promoting cartilage repair and growth. The method comprises the following steps of (1) preparation of GelMA; (2) preparation of a photo-crosslinked polypeptide; and (3) preparation of a hydrogel support, namely preparing the hydrogel support from the GelMA and the photo-crosslinked HSNGLPL under the action of a photoinitiator. The prepared hydrogel support has an obvious effect of promoting new cartilage generation and cartilage defect healing, and is capable of regulating cartilage matrix secretion of normal chondrocytes around the injured tissue, mesenchymal stem cell migration to the injured part and cartilage differentiation; by virtue of a porous structure of the hydrogel support, the HSNGLPL can be crosslinked to the surface of a porous support, endogenous TFG-beta is effectively adsorbed, the concentration of local TFG-beta beta1 is improved and the effects of the biological support in the orthopedics fields, such as a cartilage defect and a cartilage injury are achieved.
Owner:SUZHOU UNIV
In situ system for intra-articular chondral and osseus tissue repair
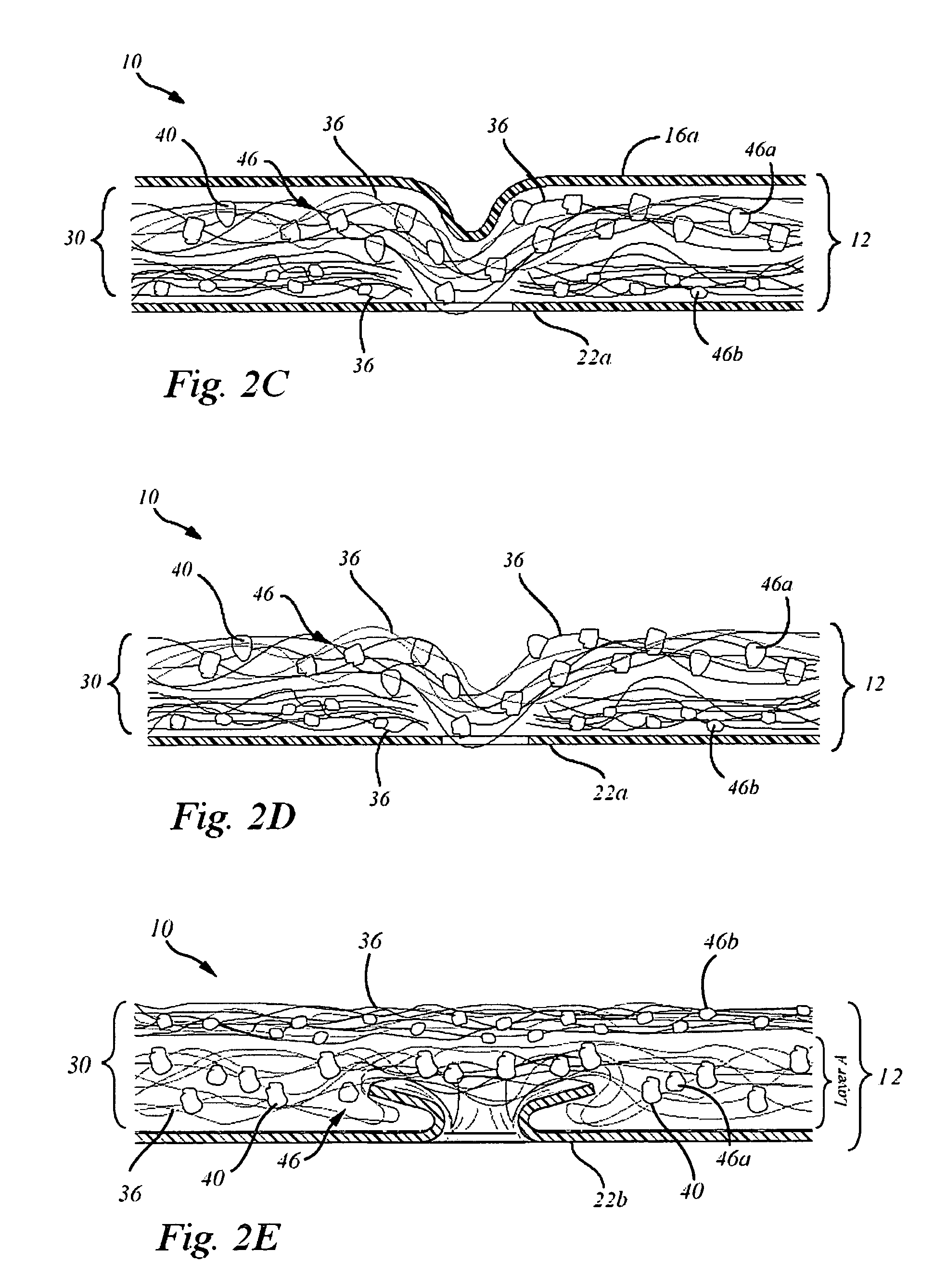
Disclosed is a device and method which provide a surgical therapy for in situ treatment and repair of intra-articular cartilage lesions and / or defects. The device is an implantable laminate cartilage repair patch which is bio-compatible and physiologically absorbable. The cartilage repair patch has a first outer cell occlusive layer; a second outer, cell porous layer adapted to be disposed proximate a subchondral bone wound site; and a cartilagenic matrix disposed between the first and second layers. The cartilagenic matrix is a sink for diffusion of autologous stem cells and includes chemical components promoting generation of hyaline-like cartilage in the presence of the autologous stem cells. The method of the present invention provides the autologous compositions, which when used in combination with the repair patch provides a therapeutic system to regenerate replacement hyaline-like intraarticular cartilage.
Owner:LABE MEDIDOM
Methods for improving mobility and controlling cartilage matrix degradation of weight-bearing articular joints
ActiveUS8060210B1Improve mobilityImproving mobility and qualityElectrotherapyRange of motionMuscle group
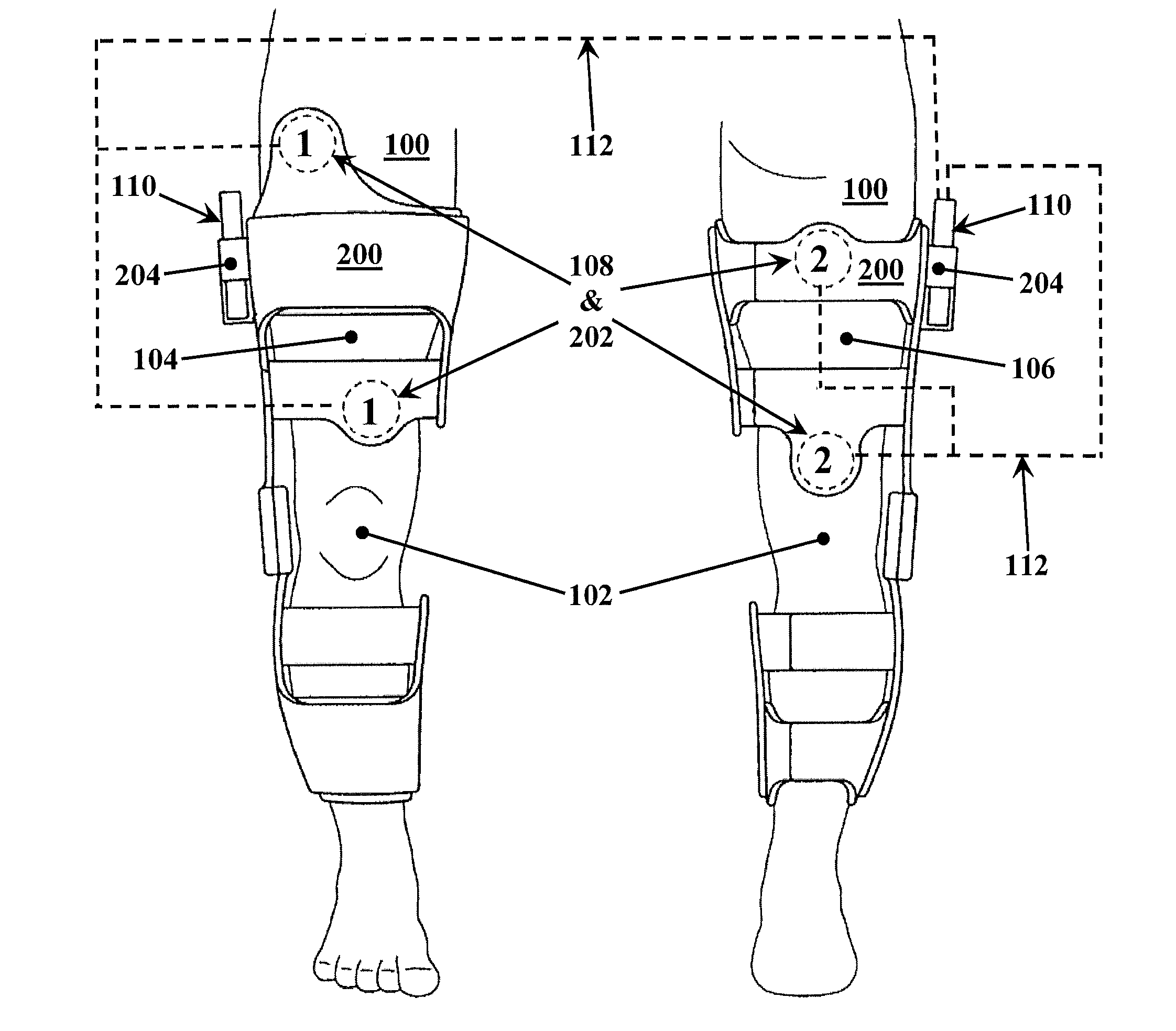
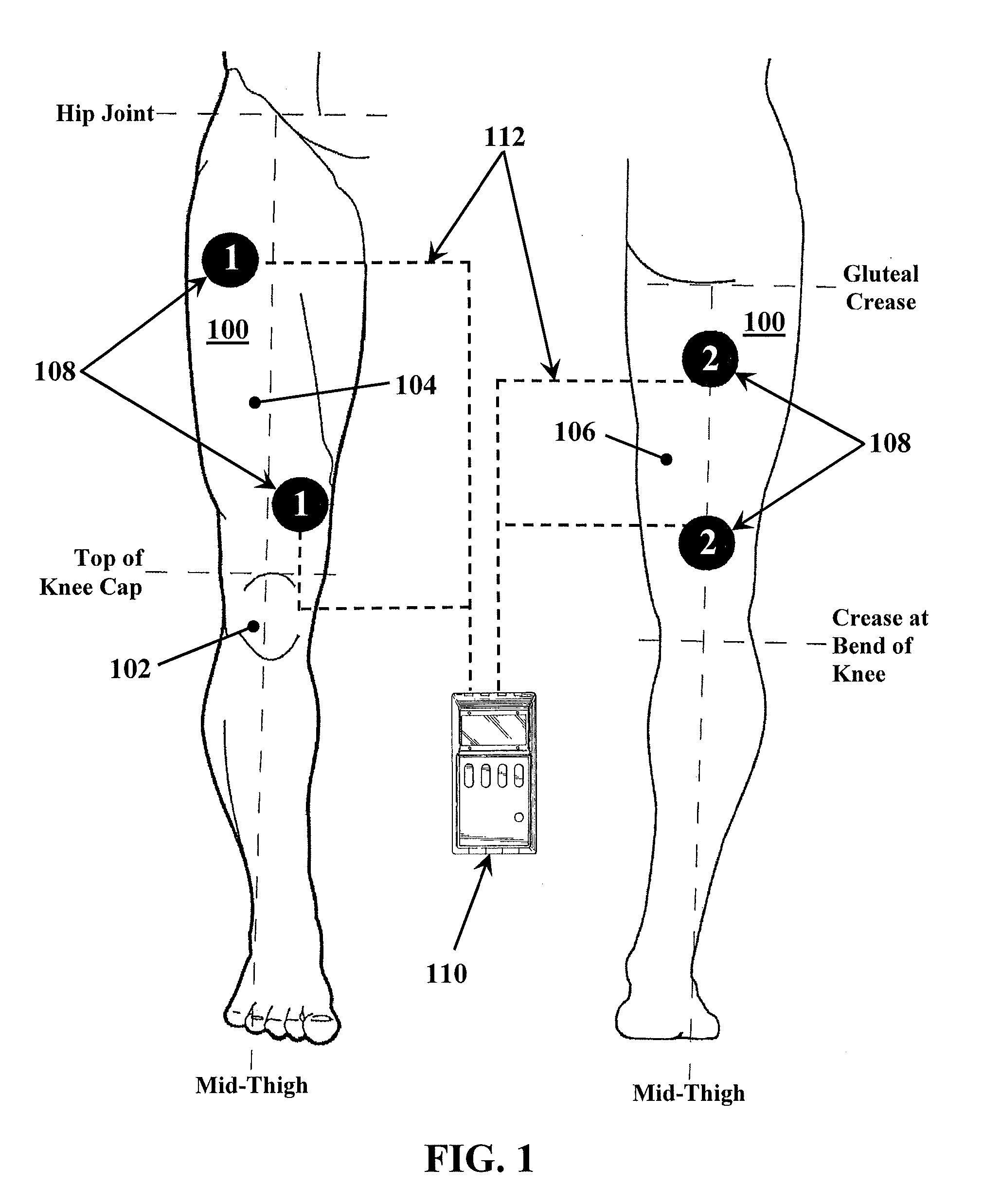
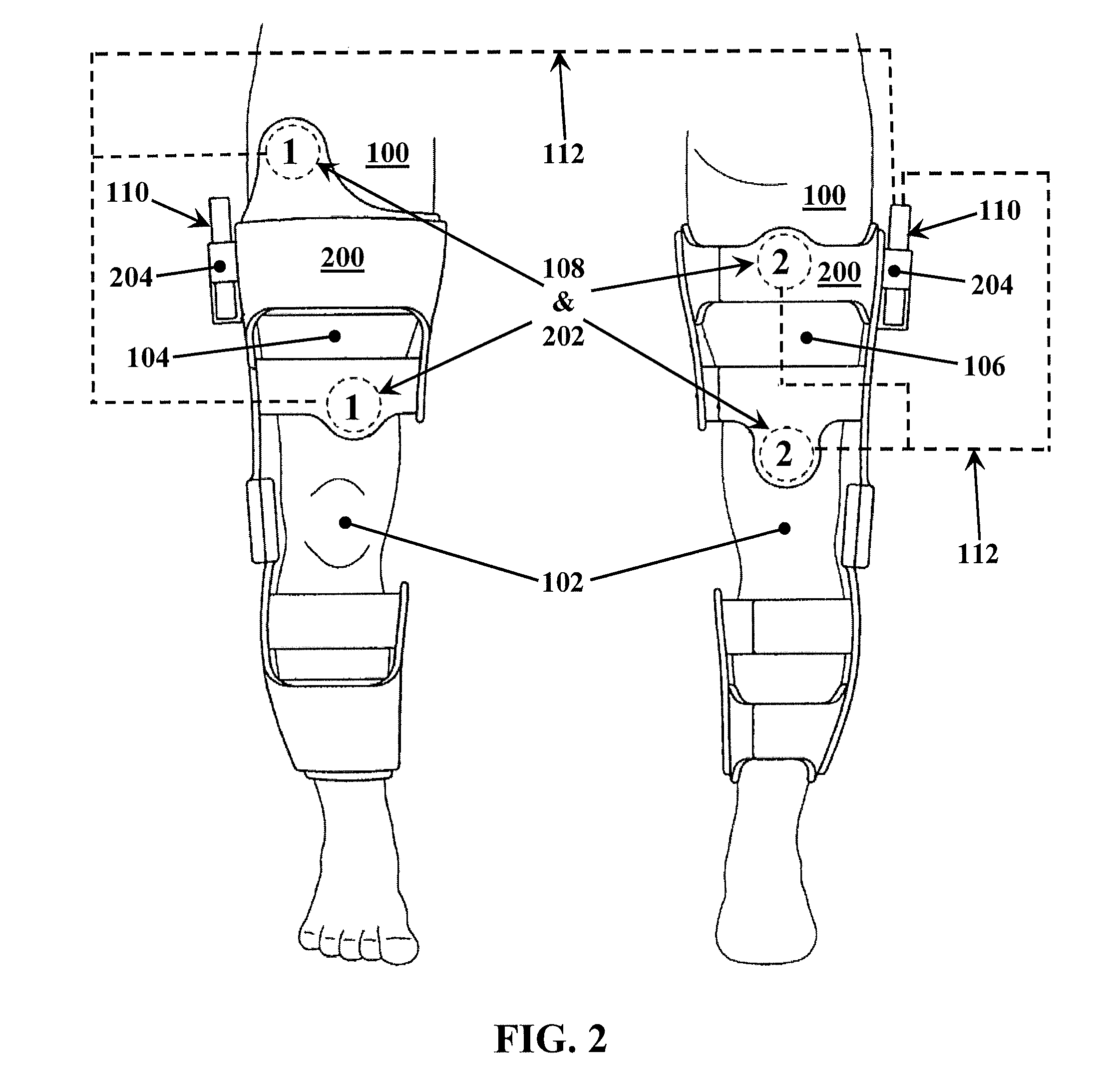
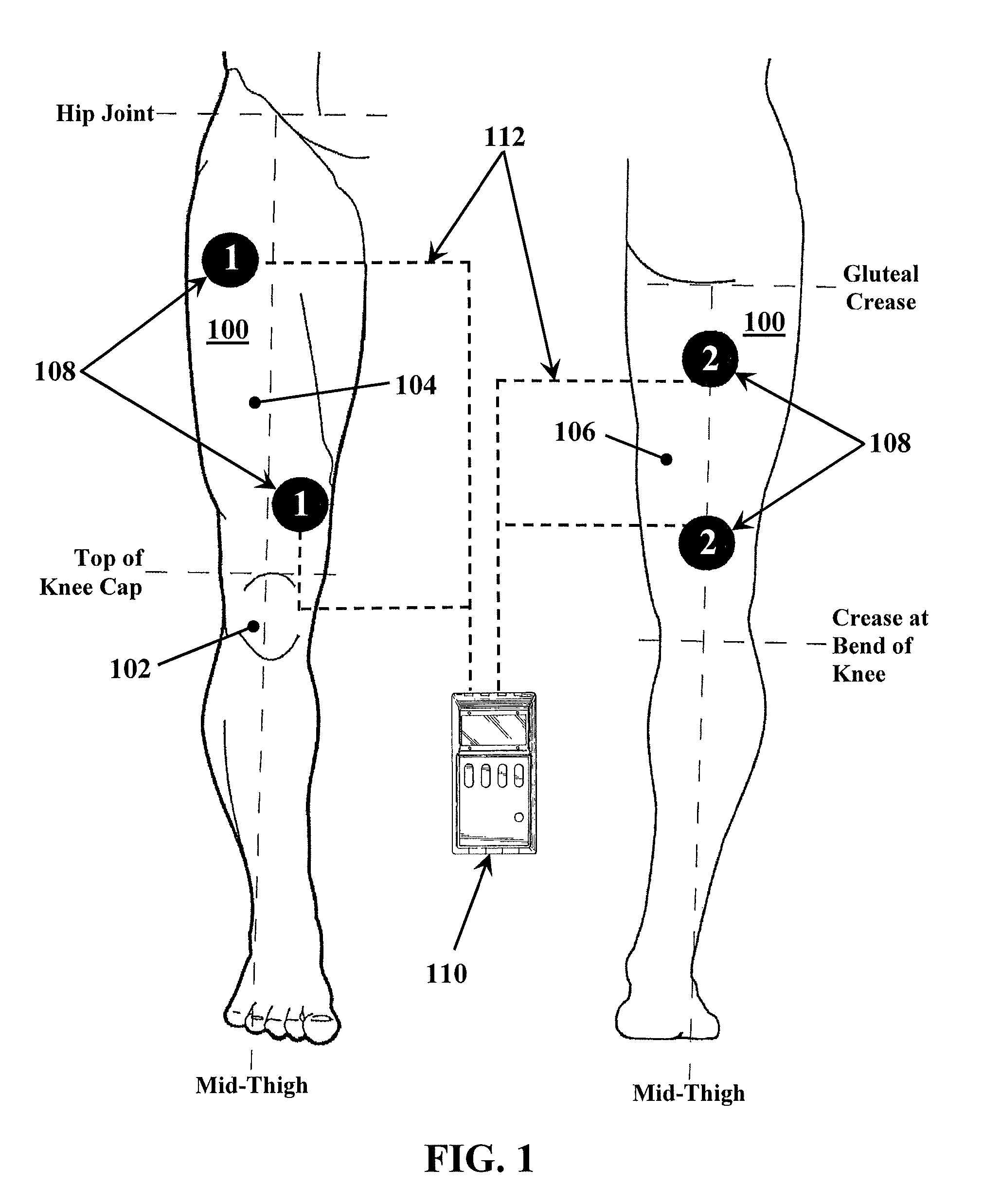
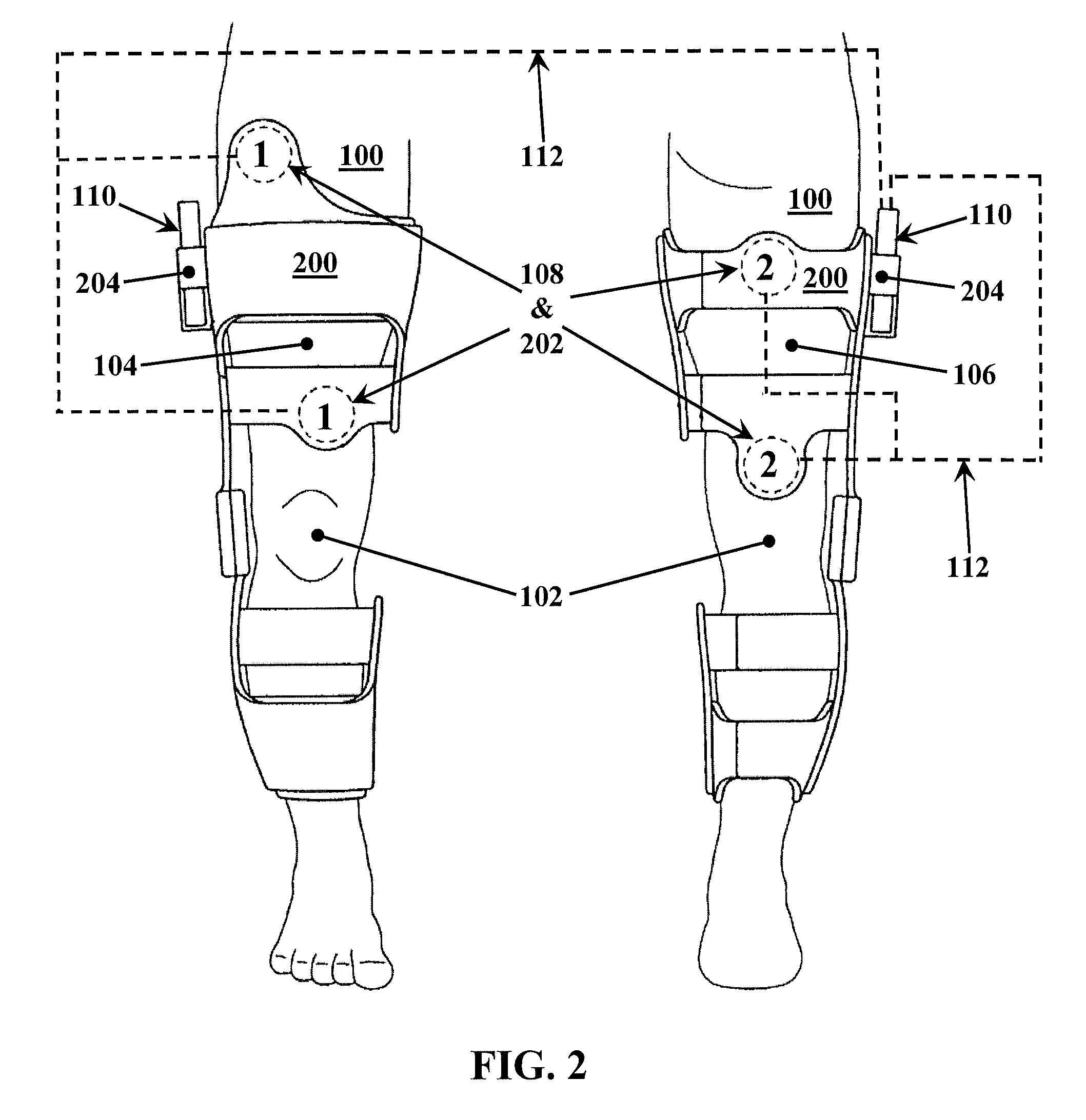
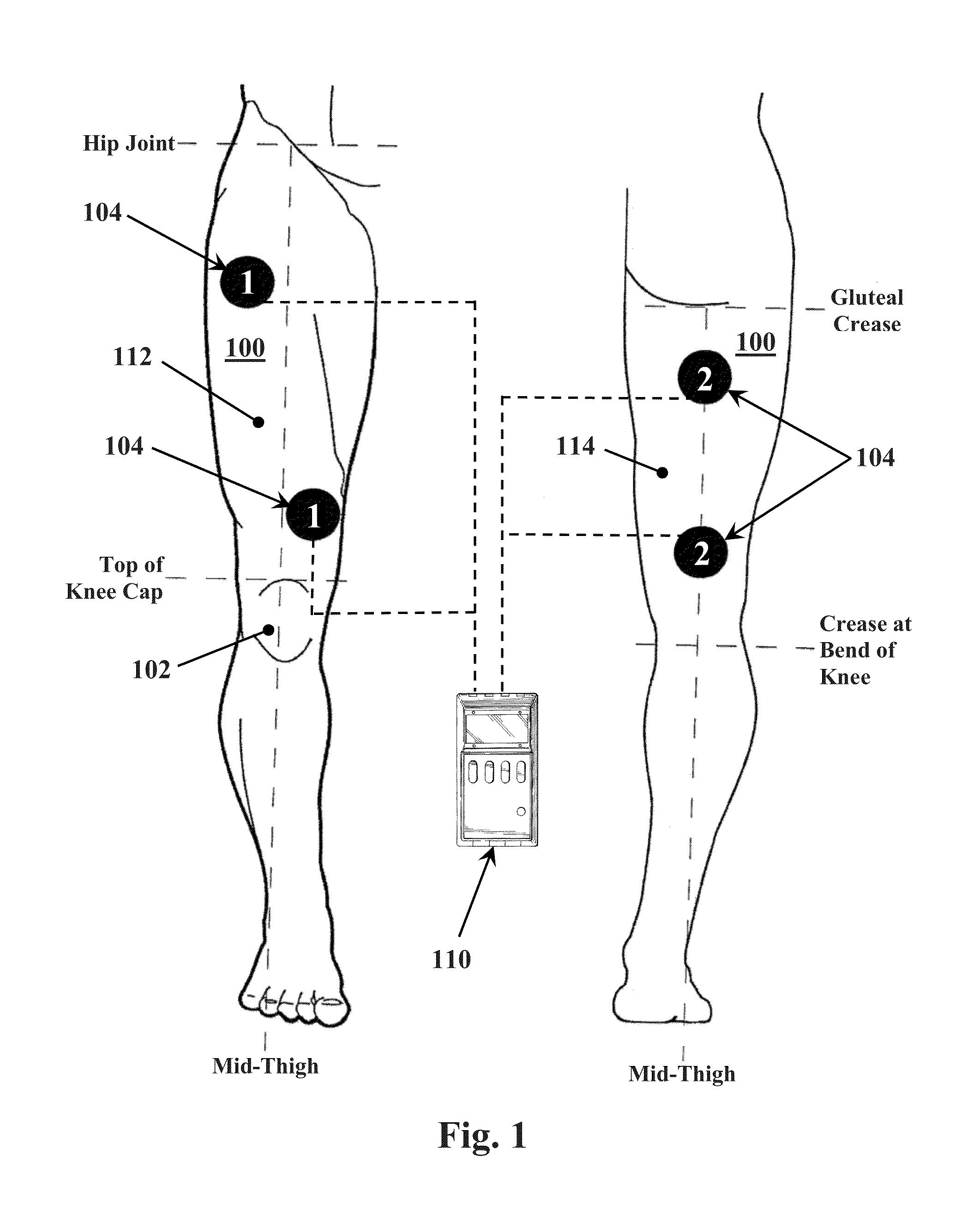
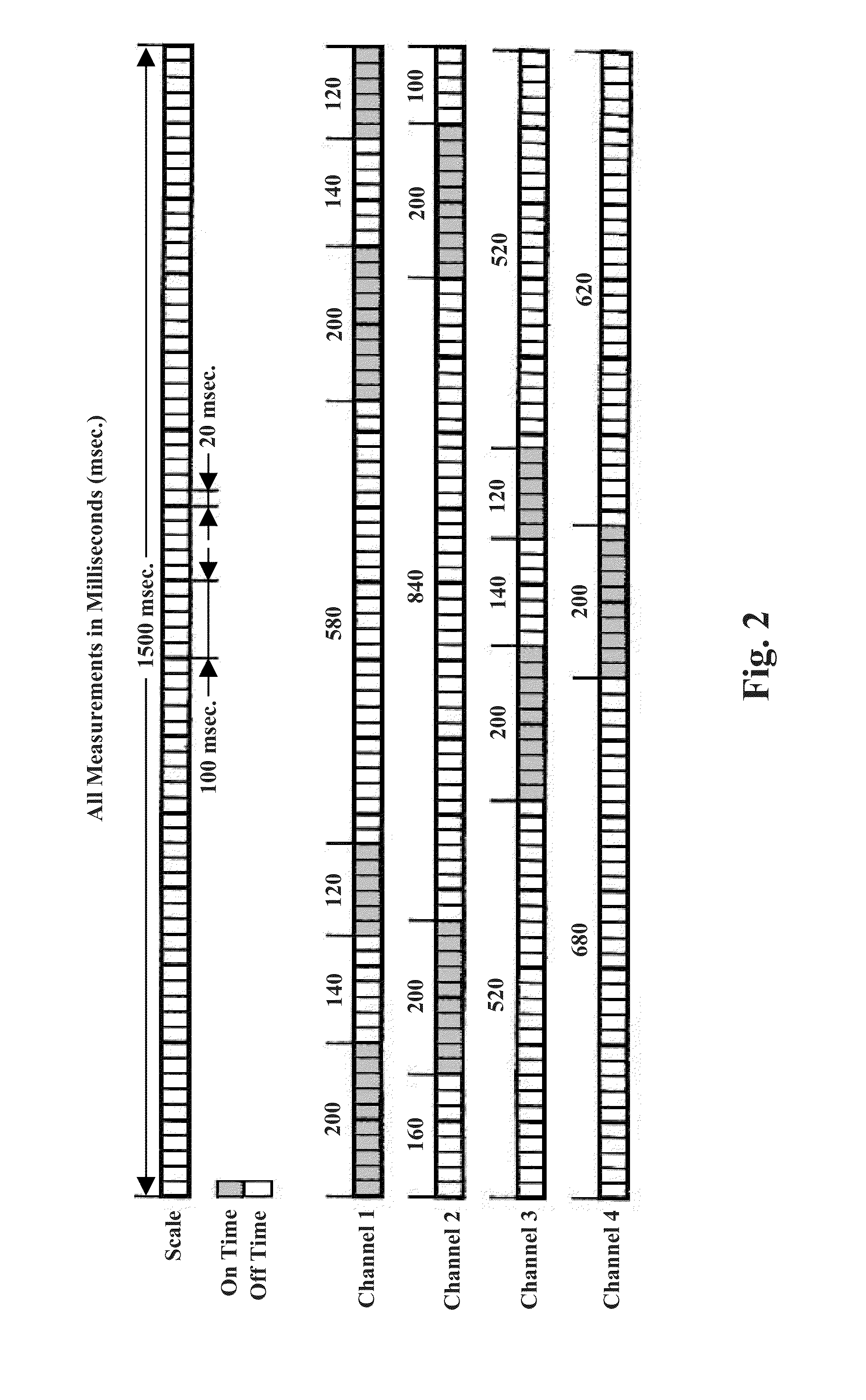
A method for improving mobility and / or the quality of synovial fluid of an affected articular joint, wherein the joint is associated with at least a first muscle group and at least a second muscle group each having an antagonistic relationship for effecting mobility of the joint through a range of motion when recruited by natural neural impulses. The method includes positioning at least two first electrodes proximate to the at least first muscle group, positioning at least two second electrodes proximate to the at least second muscle group, and applying motor-level electrical stimulation to the at least first and second muscle groups via the at least two first and second electrodes in a multiphasic pattern corresponding to a sequence of electromyographic outputs.
Owner:MEAGAN MEDICAL
Apparatus and method for stabilizing, improving mobility, and controlling cartilage matrix degradation of weight-bearing articular joints
An apparatus and method for improving mobility and / or the quality of synovial fluid of an affected articular joint are disclosed, wherein the joint is associated with at least a first muscle group and at least a second muscle group each having an antagonistic relationship for effecting mobility of the joint through a range of motion when recruited by natural neural impulses. The apparatus and method include an electro-medical device configured to apply motor-level electrical stimulation in a multiphasic pattern via at least a first channel and at least a second channel, the multiphasic pattern being programmed into the electro-medical device and corresponding to the sequence of an electromyographic output for the joint; at least two first electrodes connected to the at least first channel of said electro-medical device, the at least two first electrodes being positioned proximate to the at least first muscle group; at least two second electrodes connected to the at least second channel of said electro-medical device, the at least two second electrodes being positioned proximate to the at least second muscle group; and an applicator configured to be worn on the articular segment such that the at least two first electrodes and the at least two second electrodes are disposed between the applicator and the articular segment, the applicator being further configured to reduce compressive forces on at least one compartment of the affected joint.
Owner:MEAGAN MEDICAL
Novel chondrocyte epimatrix membrane and preparation method thereof
InactiveCN102188748ANo biological immunogenicitySuitable for growthProsthesisAdditive ingredientBlood vessel
The invention discloses a novel chondrocyte epimatrix membrane and a preparation method thereof. The membrane contains cell epimatrix ingredients which have bioactivity and cell activity, wherein the cell epimatrix comprises the following main ingredients in percentage by weight: 80 to 95 percent of bionic type II collagen in an acellular cartilage matrix and 10 to 20 percent of hyaluronic acid, 10 to 20 percent of aminopolysaccharide and the like which serve as a chondrocyte epimatrix; the chondrocyte epimatrix biological membrane prepared from the acellular cartilage matrix has the bionic chondrocyte epimatrix ingredients, is excellent in cell consistency, does not arouse immunological rejection of host cartilage tissue, can be used for repairing the damage of hyaline cartilage tissue of joints and reconstructing the hyaline cartilage tissue, and also can be used for a seed cell inoculating vector and a growth factor sustained-release vector; and the chondrocyte epimatrix contains blood vessel inhibiting factor ingredients, so the membrane also can be used for preventing the conglutination of tissue such as muscle tendons.
Owner:成都军区昆明总医院
Injectable extracellular cartilage matrix particle and application of particle to implants
InactiveCN106492285APromote growthPromote proliferationPharmaceutical delivery mechanismProsthesisCell-Extracellular MatrixTissue repair
The invention discloses an injectable extracellular cartilage matrix particle and an application of the particle to implants. Cartilage is rinsed, sterilized, washed again, repeatedly frozen and thawed and cut into sheets, the sheets are soaked, decellularized, frozen, dried and grinded at low temperature to obtain the particle, and the particle can be further matched with implant auxiliary materials to serve as injectable extracellular cartilage implants. The matrix particle has good biocompatibility, structural components of the matrix particle are extracellular matrixes of cells, growth, proliferation and differentiation of the cells are facilitated, and the matrix particle has physiological effect on shape, phenotype and movement of the cells. The implants of the particle can serve as minimally invasive injection plastic implant materials, and the natural three-dimensional structure and natural active components of the cartilage are reserved, so that the implants have filling and tissue repairing functions.
Owner:广州昕生医学材料有限公司
Biological rack material for articular cartilage and its prepn process
The present invention relates to one kind of biological rack material for articular cartilage and its preparation process. The porous rack material for articular cartilage is prepared through mixing natural cartilage matrix component with the cell, antigen and type I and type X collagen eliminated and type II gollagen, and serial physical and chemical treatments with sodium chloride crystal grain as pore forming agent. The rack material has the components the same as those of natural cartilage, high biomechanical strength, high porosity, proper degradation speed, use convenience and no influence on the body's and local immunity. The present invention may be used in the clinical repair of articular cartilage defect and in vivo and in vitro construction of tissue engineering cartilage.
Owner:孔清泉
Bone matrix compositions and methods
ActiveUS20110195052A1Improve biological activityFacilitated releaseBiocideHydrolysed protein ingredientsOsteoblastSpecific protein
The present invention provides methods of improving the osteogenic and / or chondrogenic activity of a bone matrix, e.g., a dermineralized bone matrix (DBM), by exposing the bone matrix to one or more treatments or conditions. In preferred embodiments the bone matrix is derived from human bone. The treatment or condition may alter the structure of the bone matrix and / or cleave one or more specific proteins. Cleavage may generate peptides or protein fragments that have osteoinductive, osteogenic, or chondrogenic activity. Preferred treatments include collagenase and various other proteases. The invention further provides improved bone and cartilage matrix compositions that have been prepared according to the inventive methods and methods of treatment using the compositions. The invention further provides methods of preparing, testing, and using the improved bone matrix compositions. On a assay comprises exposing relatively undifferentiated mesenchymal cells to a bone matrix composition and measuring expression of a marker characteristic of osteoblast or chondrocyte lineage(s). Increased expression of the marker relative to the level of the marker in cells that have been exposed to a control matrix (e.g., an inactivated or untreated matrix) indicates that the treatment or condition increased the osteogenic and / or chondrogenic activity of the bone matrix. Suitable cells include C2C12 cells. A suitable marker is alkaline phosphatase. The inventive methods increase the osteogenic and / or chondrogenic activity of human DBM when tested using this assay system.
Owner:WARSAW ORTHOPEDIC INC
Apparatus and method for stabilizing, improving mobility, and controlling cartilage matrix degradation of weight-bearing articular joints
An apparatus and method for improving mobility and / or the quality of synovial fluid of an affected articular joint are disclosed, wherein the joint is associated with at least a first muscle group and at least a second muscle group each having an antagonistic relationship for effecting mobility of the joint through a range of motion when recruited by natural neural impulses. The apparatus and method include an electro-medical device configured to apply motor-level electrical stimulation in a multiphasic pattern via at least a first channel and at least a second channel, the multiphasic pattern being programmed into the electro-medical device and corresponding to the sequence of an electromyographic output for the joint; at least two first electrodes connected to the at least first channel of said electro-medical device, the at least two first electrodes being positioned proximate to the at least first muscle group; at least two second electrodes connected to the at least second channel of said electro-medical device, the at least two second electrodes being positioned proximate to the at least second muscle group; and an applicator configured to be worn on the articular segment such that the at least two first electrodes and the at least two second electrodes are disposed between the applicator and the articular segment, the applicator being further configured to reduce compressive forces on at least one compartment of the affected joint.
Owner:MEAGAN MEDICAL
Method for inhibiting articular cartilage matrix calcification
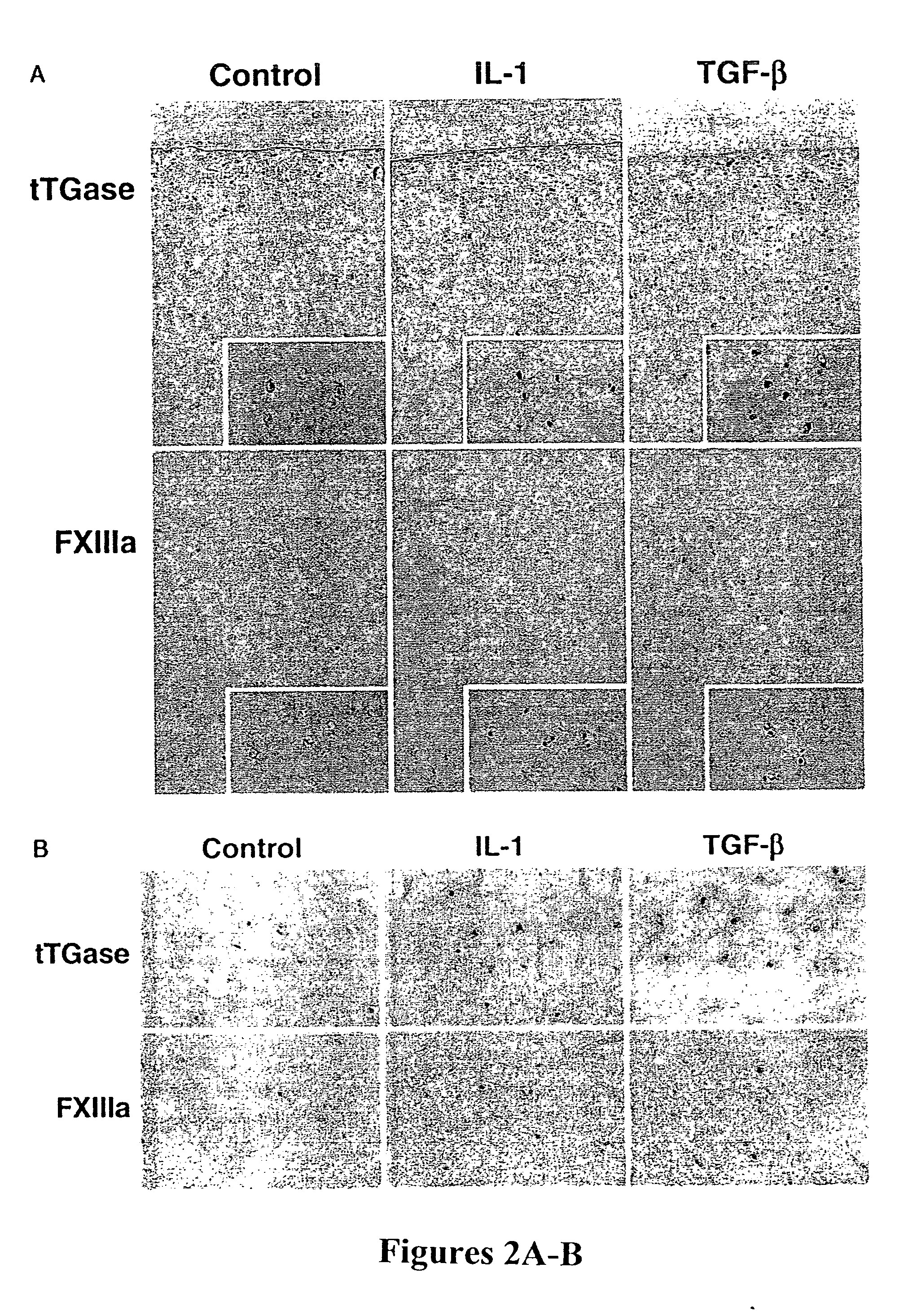
InactiveUS20040109845A1Suppresses IL-1-induced nitric oxide productionInhibits both IL-1 and TNF.alphaGenetic material ingredientsGene therapyMedicineCalcification
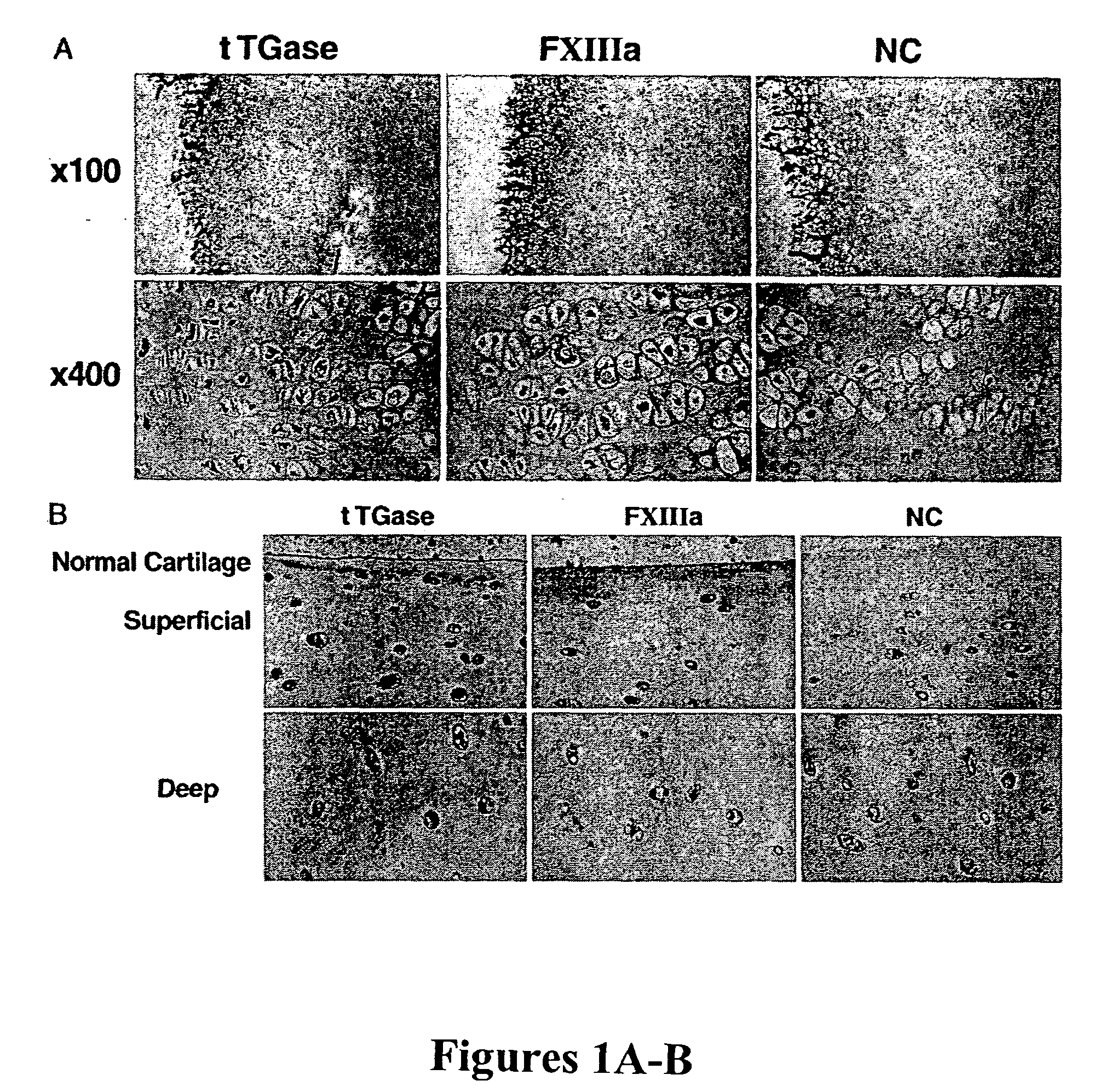
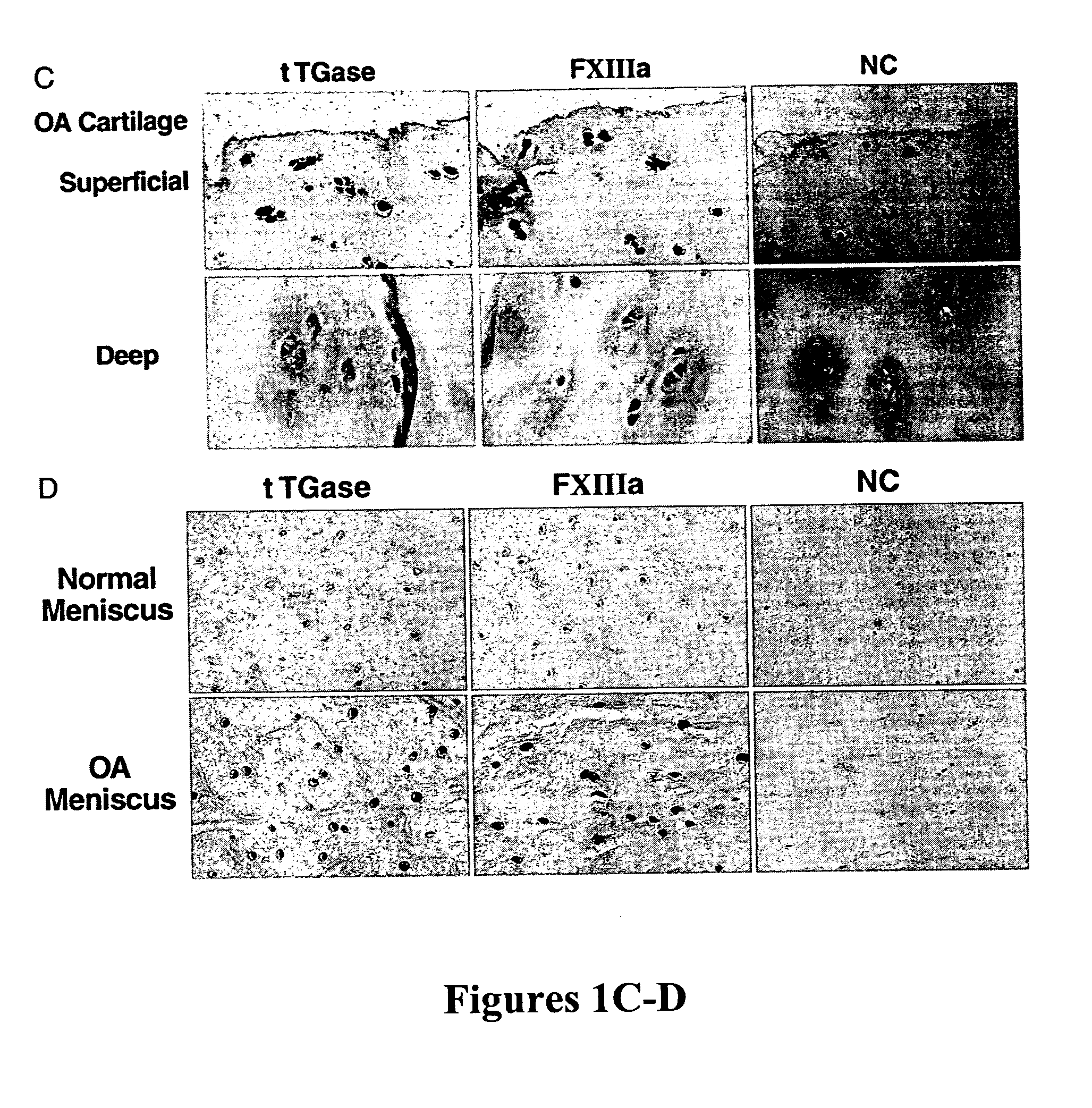
Methods of inhibiting calcification in meniscal and articular cartilage of the joints are disclosed. The methods include blocking the activation and activity of transglutaminases tTGase and Factor XIIIa. Furthermore, disclosed are methods for identifying agents that affect TGase activity and / or matrix calcification.
Owner:RGT UNIV OF CALIFORNIA
Therapeutic composition, and use of a cell-free substance
InactiveUS20100104641A1Efficient preparationHighly efficient mannerBiocidePowder deliveryCell freeMedicine
The invention relates to a therapeutic composition, a method for producing a therapeutic composition, and the use of a cell-free substance, especially a cell-free bone or cartilage matrix. The disclosed therapeutic composition comprises at least a cell-free substance obtained from stimulated stem cells and / or precursor cells. Immunogenic reactions during in vivo therapeutic use are prevented by the fact that the therapeutic composition is free from cells and contains no typically antigenic cell components. The disclosed composition can therefore be universally used for the therapeutic purposes regardless of the origin of the stem cells and / or precursor cells and utilize the natural regenerative potency thereof in a highly efficient manner for replacing tissue, e.g. for a suitable bone and / or cartilage structure.
Owner:STIFTUNG CAESAR +1
Cartilage deficiency prosthesis, preparation method thereof and integrated cartilage-bone repair material
InactiveCN101954121AGood biocompatibilityStable mechanical strengthProsthesisMass ratioArticular cartilage injuries
The invention discloses cartilage deficiency prosthesis, a preparation method thereof and an integrated cartilage-bone repair material. The prosthesis is composed of collagen and proteoglycan based on the mass ratio of 1 / 3-3, wherein, the collagen is I-type collagen or II-type collagen, and the proteoglycan comprises glycosaminoglycan, chitosan or chondroitin sulfate; and natural cartilage matrixes are longitudinally arranged in the prosthesis. The integrated cartilage-bone repair material is made by compounding the prosthesis with a bone matrix material together. The cartilage defect prosthesis of an articular cartilage matrix structure of the invention can meet the repair need of articular cartilage deficiency, effectively improve repair rate of articular cartilage injury and lower disability incidence of a patient.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Fish collagen composition as well as preparation method and application thereof
InactiveCN109156829APromote hyperplasiaPromote formationFood ingredient functionsProtein food ingredientsSucroseVitamin C
The invention discloses a fish collagen composition as well as a preparation method and application thereof. The fish collagen composition comprises 45-65 parts of fish collagen peptide, 6-15 parts ofmaltodextrin, 10-15 parts of resistant dextrin, 5-10 parts of calcium aspartate, 1-5 parts of lemon powder, 0.1-1 part of acerola cherry powder, 5-15 parts of xylitol, 0.5-2 parts of xanthan gum, 1-2parts of anhydrous citric acid, 1-2 parts of lemon essence, and 0.01-0.2 part of sucralose. The fish collagen peptide is used as a main raw material, and the calcium aspartate is used as an auxiliarymaterial for complementing collagen and calcium, so that metabolism of skeleton collagen is promoted, chondrocyte is stimulated and actuated to strengthen synthesis of a cartilage matrix, bone jointrubbing is reduced, damaged articular cartilage is repaired, bone density is increased, and osteoporosis is prevented. The lemon powder and the acerola cherry powder contain massive rich vitamin C, sothat proliferation, formation and oxidation resistance of the collagen can be promoted; the lemon powder and the acerola cherry powder generate synergy effect with the fish collagen peptide and the calcium aspartate, so that the efficacy of products for beautifying the features is improved, and the effects of smoothing skin, enabling the skin to be elastic, preventing wrinkles and delaying senescence can be achieved.
Owner:广州市顺芝堂生物科技有限公司
Methods for improving mobility and controlling cartilage matrix degradation of weight-bearing articular joints
A method for improving mobility and / or the quality of synovial fluid of an affected articular joint, wherein the joint is associated with at least a first muscle group and at least a second muscle group each having an antagonistic relationship for effecting mobility of the joint through a range of motion when recruited by natural neural impulses. The method includes positioning at least two first electrodes proximate to the at least first muscle group, positioning at least two second electrodes proximate to the at least second muscle group, and applying motor-level electrical stimulation to the at least first and second muscle groups via the at least two first and second electrodes in a multiphasic pattern corresponding to a sequence of electromyographic outputs.
Owner:MEAGAN MEDICAL
Hedgehog signaling promotes the formation of three dimensional cartilage matrices
InactiveUS7160725B2Reduce the impactPrice can be significantBiocidePeptide/protein ingredientsDiseaseMedicine
Owner:CURIS INC
Acellular cartilage matrix and preparation method thereof
PendingCN109621010AStrong cytotoxicityImmunogenicityTissue regenerationProsthesisMedicineVaccine Immunogenicity
The invention relates to the technical field of articular cartilages, in particular toarticular cartilage matrix and a preparation method thereof. Cartilage tissues are sequentially subjected to hypotonic treatment, trypsin treatment, detergent treatment and nuclease treatment to obtain the cartilage matrix. The prepared acellular cartilage matrix can maintain the three-dimensional structure of chondrocyte matrix itself to the maximum extent, have good mechanical properties, do not require an external crosslinking agent for physical or chemical crosslinking, have low immunogenicity and low toxicity, have the simple preparation method and low cost, and be suitable for industrial production.
Owner:浙江华臻医疗器械有限公司
Method for preparing decellularization cartilage matrix material
PendingCN108653814AIntegrity guaranteedQuality assuranceTissue regenerationProsthesisSaline waterPorosity
The invention discloses a 'method for preparing a decellularization cartilage matrix material', and belongs to the technical field of biological material preparation. The method comprises the following steps: (1) carrying out immobilization treatment on human or animal cartilage tissue in an immobilization agent; (2) washing the cartilage tissue after the immobilization treatment by using an appreciate solution, wherein the washing time can be more times; (3) carrying out decellularization treatment on the washed cartilage tissue in an alkali solution; (4) carrying out soaking washing treatment on the cartilage tissue after the decellularization treatment, wherein the immobilization agent is glutaraldehyde, formaldehyde or peracetic acid, the appropriate solution may be distilled water, and the alkali solution is selected from 4-15% NaOH or KOH solutions, and a washing liquid used in soaking washing treatment is normal saline. The decellularization cartilage matrix material prepared byusing the method has a porosity of 70-90% and a pore size of 12-67mu m.
Owner:BEIJING QINGYUAN WEIYE BIO TISSUE ENG
Tissue engineering cartilage framework material, as well as preparation method and device thereof
ActiveCN102580156AMaintain a high modulus of elasticityPromote regenerationBone implantCartilage cellsFiber
The invention discloses a tissue engineering cartilage framework material. The cartilage framework material provided by the invention sequentially comprises a surface layer, a middle layer and a calcification layer from the top down; the surface layer is composed of freeze-dry cartilage matrix and II-type collagen, and matrix fiber is in parallel distribution in the horizontal direction; the middle layer is composed of freeze-dry cartilage matrix, GAG, X-type collagen, II-type collagen and TGF-Beta, and matrix fiber is arranged longitudinally; and the calcification layer is composed of freeze-dry cartilage matrix, GAG, X-type collagen and sintered bovine bone power, and fiber is distributed in a three dimensional staggered manner. The prepared cartilage framework material has a cartilage repairing effect, is favorable to the integration of the framework material and subchondral bone after the framework material is planted, and promotes the regeneration of the subchondral bone; after cartilage cell compound, the framework material can be used for full-layer cartilage defect repairing, the regenerated cartilage tissue has the space structure and protein components, which are the same with those of natural cartilage; and the framework material is suitable for repairing cartilages in load bearing joints such as knee joints and the like, cartilage with higher regenerated compressive strength can be regenerated, and the abrasion performance and anti-pressure capability of the surface of the cartilage framework are enhanced.
Owner:西安博鸿生物技术有限公司
Biosynthetic cartilaginous matrix and methods for their production
An isolated, acellular biosynthetic cartilaginous matrix substantially devoid of synthetic biodegradable scaffold structure is provided. Through a method with the steps of a) contacting in vitro a population of chondrogenic cells with a synthetic biodegradable scaffold; b) culturing in vitro for a period of time said chondrogenic cells within said synthetic biodegradable scaffold so that the chondrogenic cells produce a biosynthetic cartilaginous matrix; c) substantially removing any antigen derived from said chondrogenic cells a matrix suitable of implantation into a living individual mammal, such as a human being is obtained.
Owner:COLOPLAST AS +1
Preparation method and application of silk fibroin and chitin blended nanofiber embedded hydrogel cartilage biomimetic scaffold
ActiveCN109999227AIncrease elasticityNon-cytotoxicElectro-spinningTissue regenerationCross-linkFiber
The invention discloses a preparation method and application of a silk fibroin and chitin blended nanofiber embedded hydrogel cartilage biomimetic scaffold. The method utilizes mulberry silk to prepare regenerated the silk fibroin, purifies industrial grade chitin, mixes a silk fibroin solution and a chitin solution, prepares a blended nanofiber membrane by an electrospinning technique, cross-links with ethanol to form a short fiber through a blended nanofiber, mixes the short fiber and the silk fibroin solution, conducts cross-linking by a crosslinking agent, and conducts freeze-forming to obtain a spongy cartilage bionic scaffold. In particular, the insertion of the nano short fiber increases the compressive strength and biocompatibility of the scaffold material. The method constructs the biomimetic scaffold with good biocompatibility, low immune rejection and similar composition with natural cartilage matrix, has wide material source, low cost and simple preparation process, can beused for constructing tissue engineering cartilage and repairing cartilage regression, and has good clinical application prospects.
Owner:WUHAN UNIV
Preparation method of mixed hydrogel biological material and application thereof
PendingCN110302425AThe experimental method is simpleEasy to operateTissue regenerationProsthesisCartilage MatrixNon toxicity
The invention provides a preparation method of a mixed hydrogel biological material and an application thereof. The material is prepared by mixing an acellular cartilage matrix, a Gelatin Methacryloyl(GelMA) and Hyaluronic Acid Methacryloyl (HAMA) in a certain ratio. The preparation method of all materials of the invention has the characteristics of safety, non toxicity, easy operation and low immunogenicity, can be applied to repair of clinical tympanic membrane perforation, replaces traditional treatment methods, and has wide application prospects.
Owner:THE FIRST AFFILIATED HOSPITAL OF WENZHOU MEDICAL UNIV +1
Special extraction device for main ingredient collagen II of natural cartilage matrix
ActiveCN103342748ASave human effortReduce manufacturing costConnective tissue peptidesPeptide preparation methodsControl systemAdditive ingredient
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Crafting of cartilage
The invention is directed to producing a shaped cartilage matrix isolated from a human or animal where the cartilage has been crafted to facilitate disinfection, cleaning, devitalization, recellularization, and / or integration after implantation. The invention relates to a process for repairing a cartilage defect and implantation of a cartilage graft into a human or animal by crafting the cartilage matrix into individual grafts, disinfecting and cleaning the cartilage graft, applying a pretreatment solution to the cartilage graft, removing cellular debris using an extracting solution to produce a devitalized cartilage graft, implanting the cartilage graft into the cartilage defect with or without an insertion device, and sealing the implanted cartilage graft with recipient tissue. The devitalized cartilage graft is optionally recellularized in vitro, in vivo, or in situ with viable cells to render the tissue vital before or after the implantation. The devitalized cartilage graft is also optionally stored between the removing cellular debris and the recellularizing steps.
Owner:LIFENET HEALTH
High-activity primary cartilage cell preparing method
ActiveCN104911145APromote digestionAvoid damageSkeletal/connective tissue cellsCartilage cellsMedicine
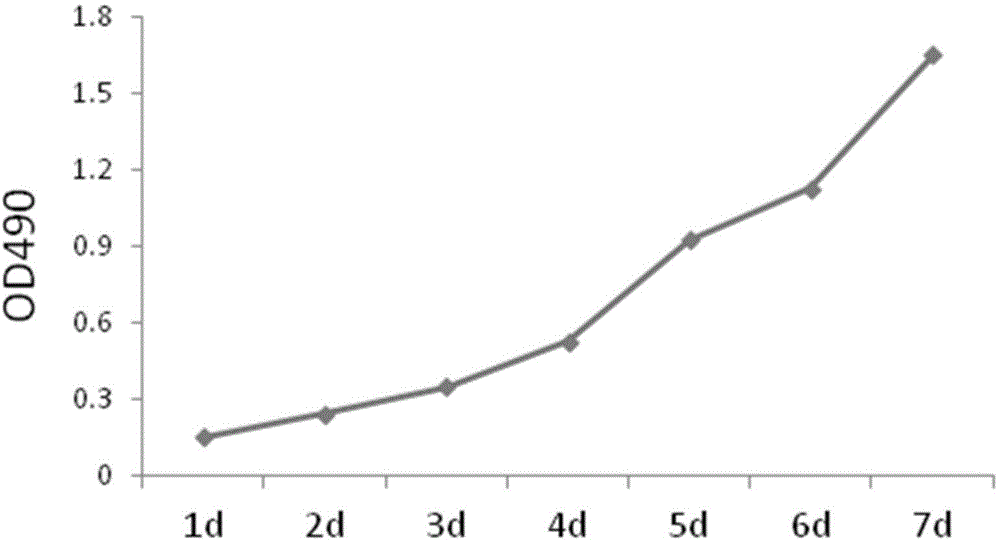
The invention discloses a high-activity primary cartilage cell preparing method. The high-activity primary cartilage cell preparing method includes the following steps that 1, cartilage tissues of joints in limbs of an immature rat are extracted; 2, a blade special for operations is adopted to cut up the cartilage tissues; 3, the cut-up cartilage tissues are placed in a constant temperature shaking table to be shaken and placed in a horizontal centrifuge for centrifugation by 1500-2000 rpm, cell sediments are left, the cartilage tissues continue to be placed in the shaking table to be digested, are separated from cartilage matrixes after being repeatedly blown and beaten by a gun head of 1 ml, and are placed in the horizontal centrifuge for centrifugation by 1500-2000 rpm, and cartilage cell sediments are left; and 4, the cartilage cell sediments are planted in a culture dish and cultured in a DMEM high-glucose culture medium containing 10% of FBS to obtain high-activity primary cartilage cells. Compared with a traditional cartilage cell extracting method, the high-activity primary cartilage cell preparing method shortens the time by a half and improves the yield of cartilage cells by at least ten times; experiments verify that the obtained cartilage cells have a good rate of increase and good cellular morphology.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
Biomaterials for regenerative medicine
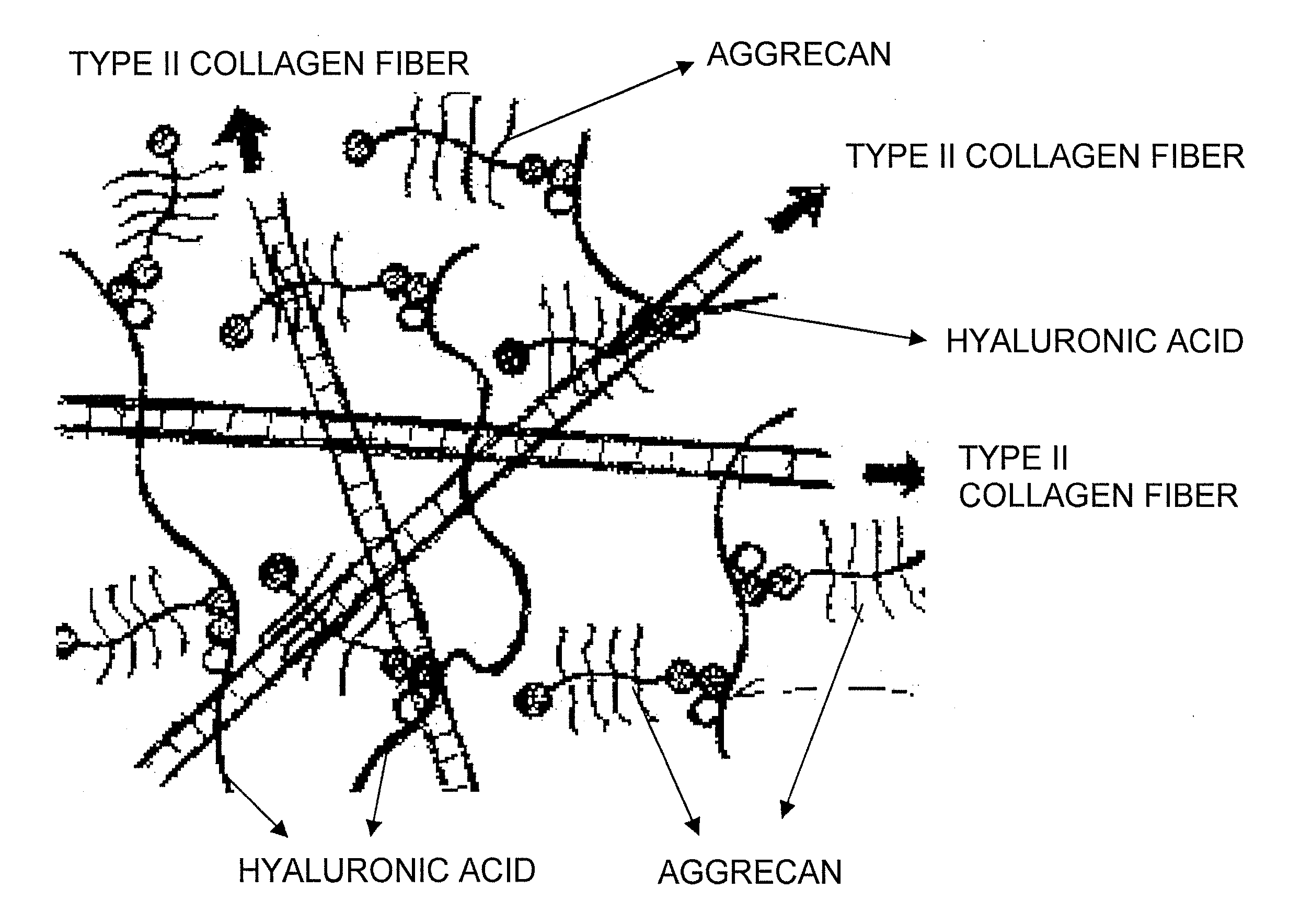
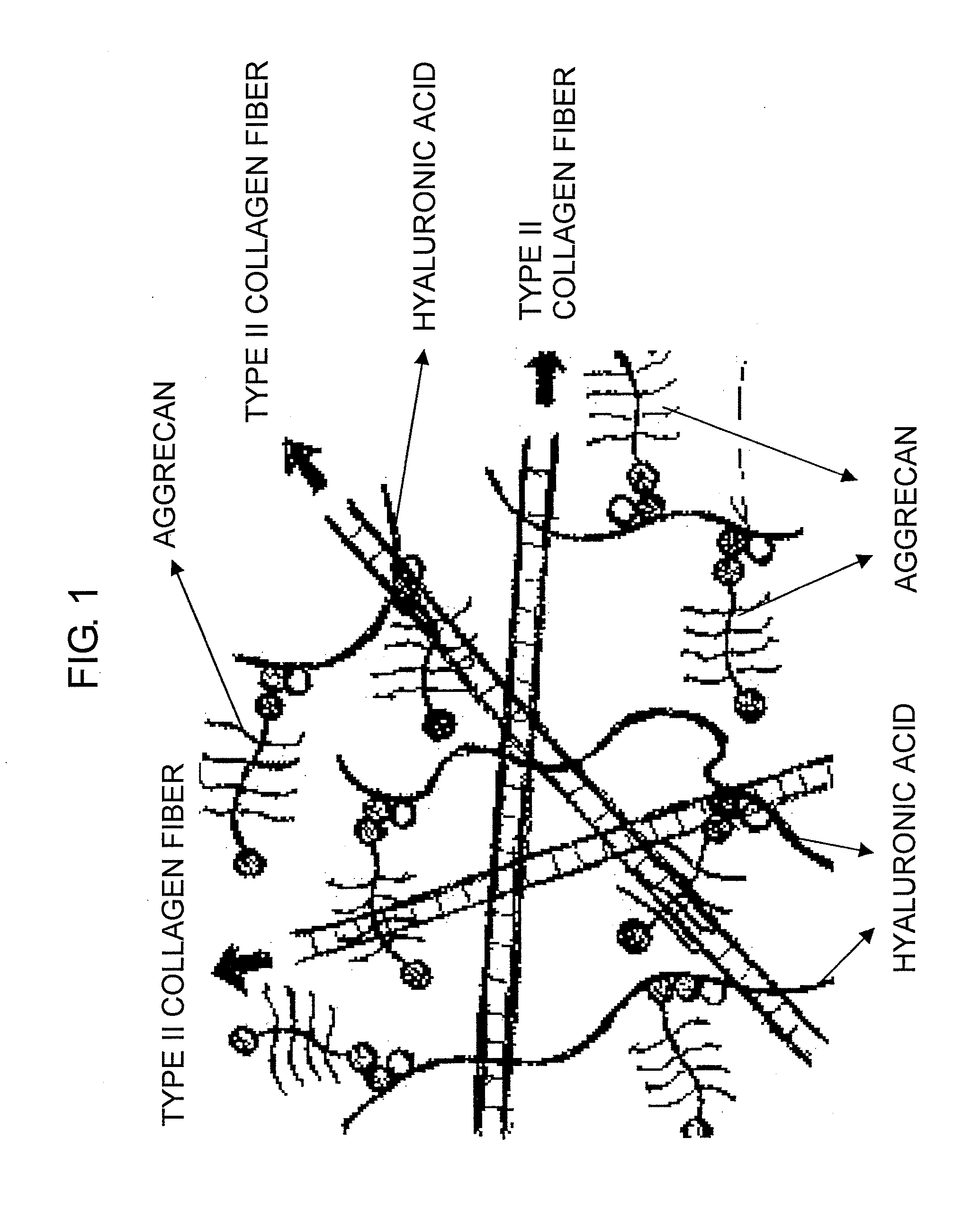
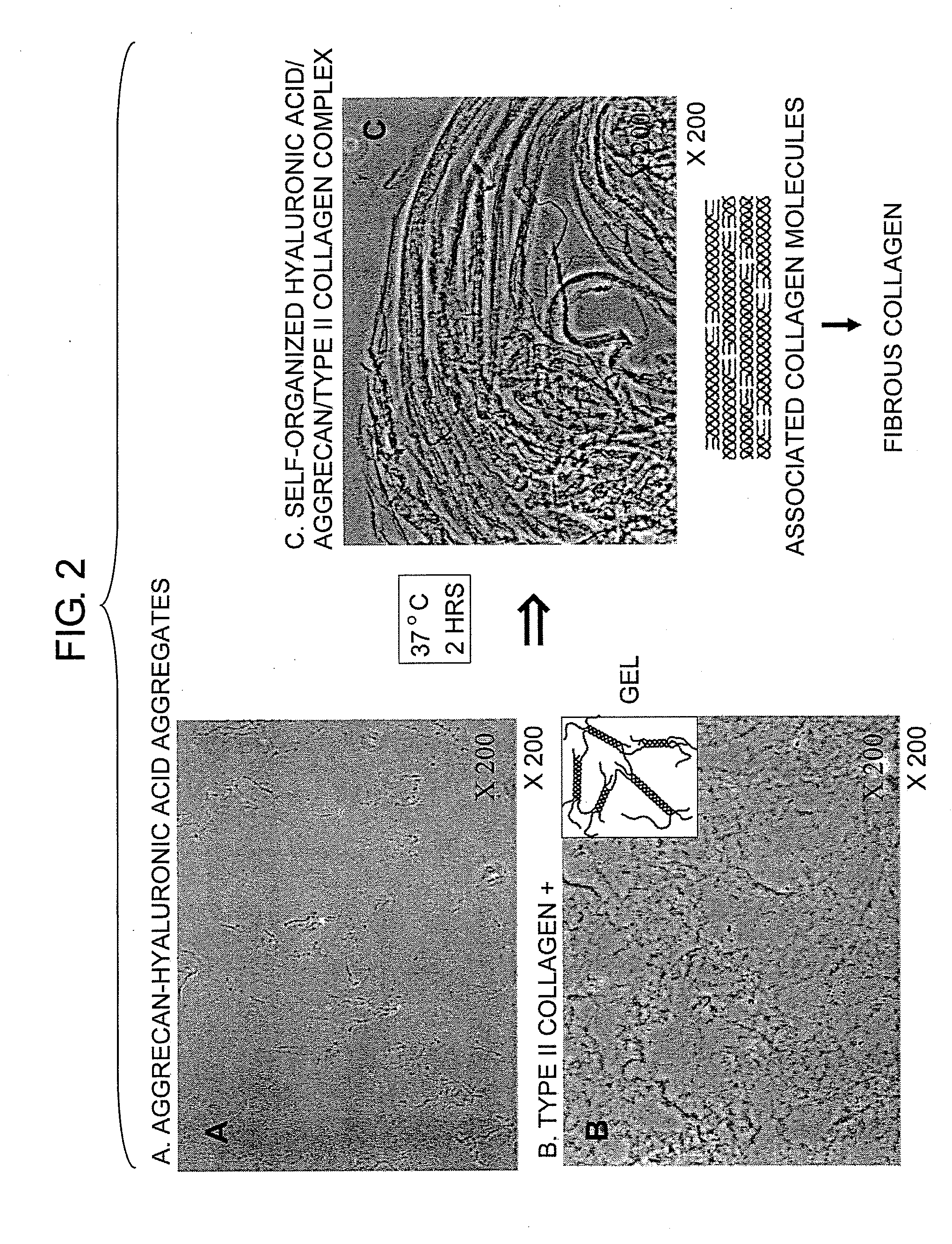
It was examined whether a cartilage-like tissue is formed under various reaction conditions using cartilage matrix components: glycosaminoglycan, proteoglycan, and collagen. The present inventors have discovered that proteoglycan bound to glycosaminoglycan through self-organization form an aggregate when the glycosaminoglycan was reacted with proteoglycan under specific concentrations and pH, and that a mesh structure composed of collagen fibers was constructed through self-organization using the aggregates as a skeleton when the aggregates were reacted with collagen molecules.
Owner:ST MARIANNA UNIV SCHOOL OF MEDICINE
Long-acting sustained-release cell scaffold, and preparation method and application thereof
ActiveCN109602952ALong release periodIncreased mechanical resistanceTissue regenerationMicrocapsulesMicrosphereCytokine
The invention discloses a long-acting sustained-release cell scaffold, and a preparation method and application thereof. The long-acting sustained-release cell scaffold comprises a drug-cytokine protein, a silk fibroin and a complex protein. The drug-cytokine protein is coated by the silk fibroin to form cytokine sustained-release microspheres; the complex protein coats the outer layers of the cytokine sustained-release microspheres; the silk fibroin is a self-assembled silk fibroin by calcium salt regulation; the complex protein comprises gelatin capable of escaping body immune attack and degradable by cells. According to the preparation method, first the cytokine sustained release microspheres are formed through silk fibroin coating, and then the cytokine sustained release microspheres are coated with the complex protein degradable by cells, so that 3-5 month long-term sustained release of the drugs can be realized; a hydrogel hybrid formed by the complex protein is more mechanicallytolerant than simple collagen and chondroitin sulfate; and the scaffold has good homology, and a long drug sustained release period, so that the cartilage tissue has better mechanical tolerance and metabolic activity during conversion of the scaffold into a cartilage matrix.
Owner:上海北陆医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com