Biosynthetic cartilaginous matrix and methods for their production
a biosynthetic cartilaginous matrix and biosynthetic technology, applied in the direction of biocide, plant growth regulators, skeletal/connective tissue cells, etc., can solve the problems of expensive procedures and extensive medical procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
In Vitro Study of Chondrogenesis of hAC-Loaded MPEG-PLGA Scaffolds
These in vitro studies were done in order to evaluate the degree of chondrogenic matrix synthesis in a 3-dimensional scaffold system base on a polymer part and a cellular / hydrogel part. The results from this study will indicate whether the tested scaffold system could be a candidate in an in vivo cartilage repair study.
The scaffold system tested in this in vitro study is composed of three major parts:1. The polymer part: MPEG-PLGA (methoxypolyetheleneglycol-block-poly(lactide-co-glycolide)), Coloplast NS.2. The cellular part; human articular chondrocytes (hACs)3. The hydrogel part; Fibrin-based gel.
The MPEG-PLGA polymer is able to absorb liquid due to its hydrophillic characteristics and in this way a cellular suspension can be distributed into the scaffold structure. The cellular part used for this in vitro study is normal hACs of low passages, which is an important parameter affecting the degree of matrix synthesis ...
example 2
Degradation of Scaffolds of MPEG-PLGA 2.000-30.000 and EDC Cross-Linked Gelatine in Wound Exudate, 10% FCS in DMEM, FCS and PBS. A 14-Day Study
The present example demonstrates the degradation of MPEG-PLGA and EDC cross-linked gelatine, when incubated in wound exudates, medium, serum and PBS at 37° C. for up to 14 days.
Material and Methods
Eight mm biopsies were punched out of MPEG-PLGA and EDC cross-linked gelatine (150506E) scaffold and placed in it's own well in a 48 well plate. The scaffolds were covered by 1 ml of respectively wound exudates (debrided ischemic diabetic leg ulcer with low elastase level, collected using VAC therapy, properly corresponding to acute wound exudates), medium (10% Fetal Calf Serum (FCS) in Dulbecco's Modified Eagle's Medium (DMEM)), FCS and PBS pH 7.4. The scaffolds were tested in duplicates.
The plates were incubated for 1, 3, 8 and 14 days at 37° C. RH 50% after which the scaffolds were placed on a glass plate and photographed.
Result and Conclusion as...
example 3
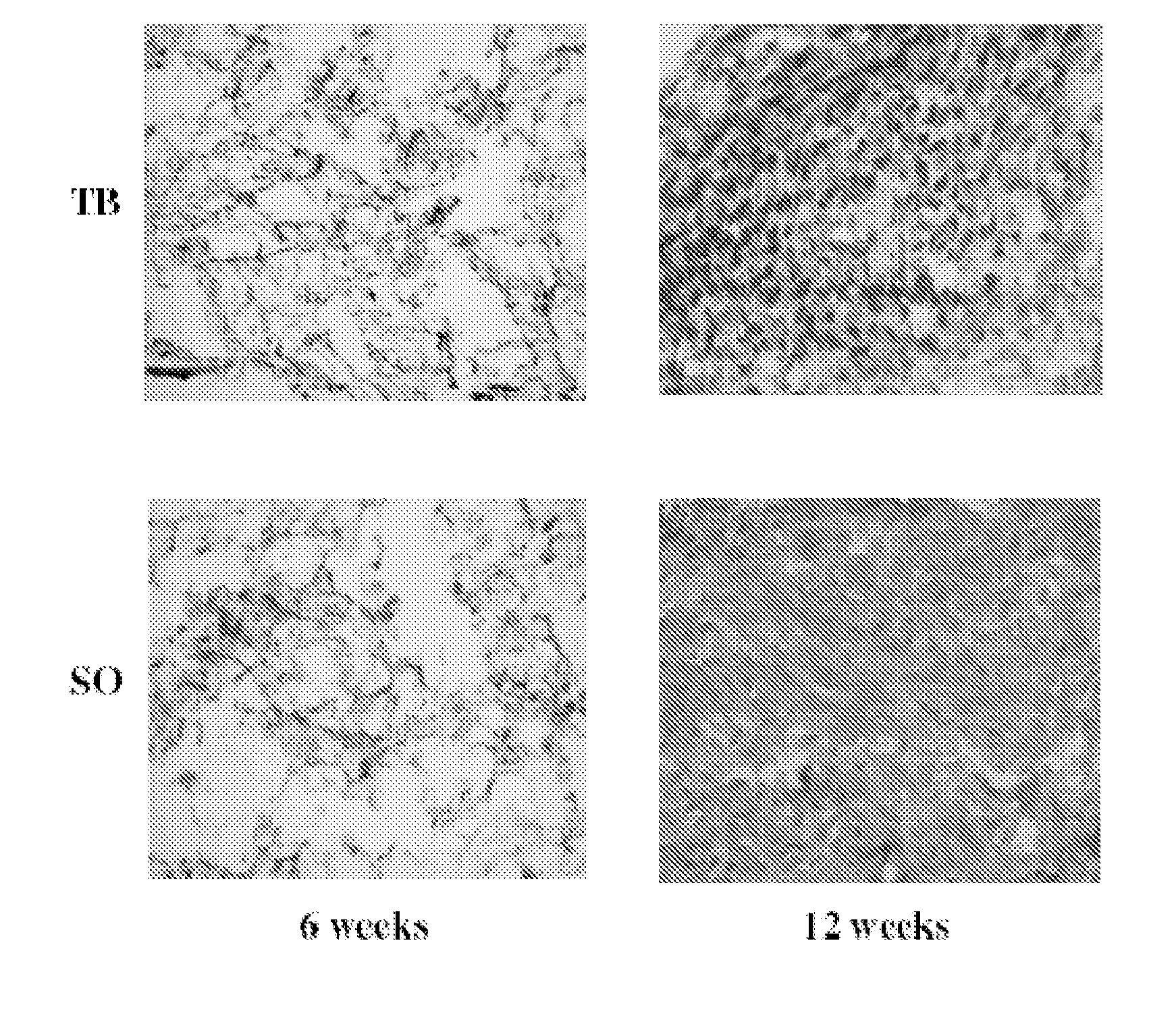
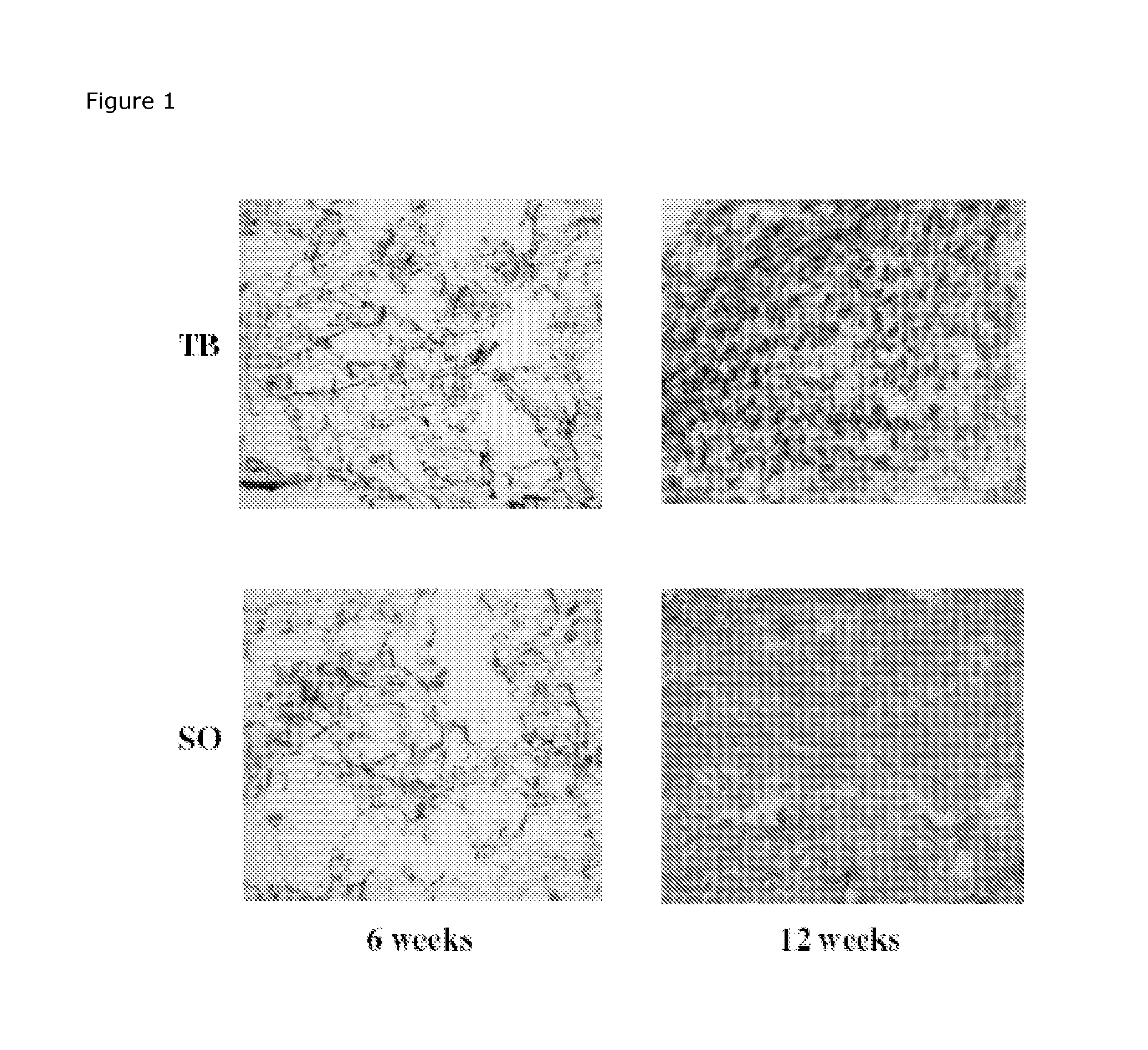
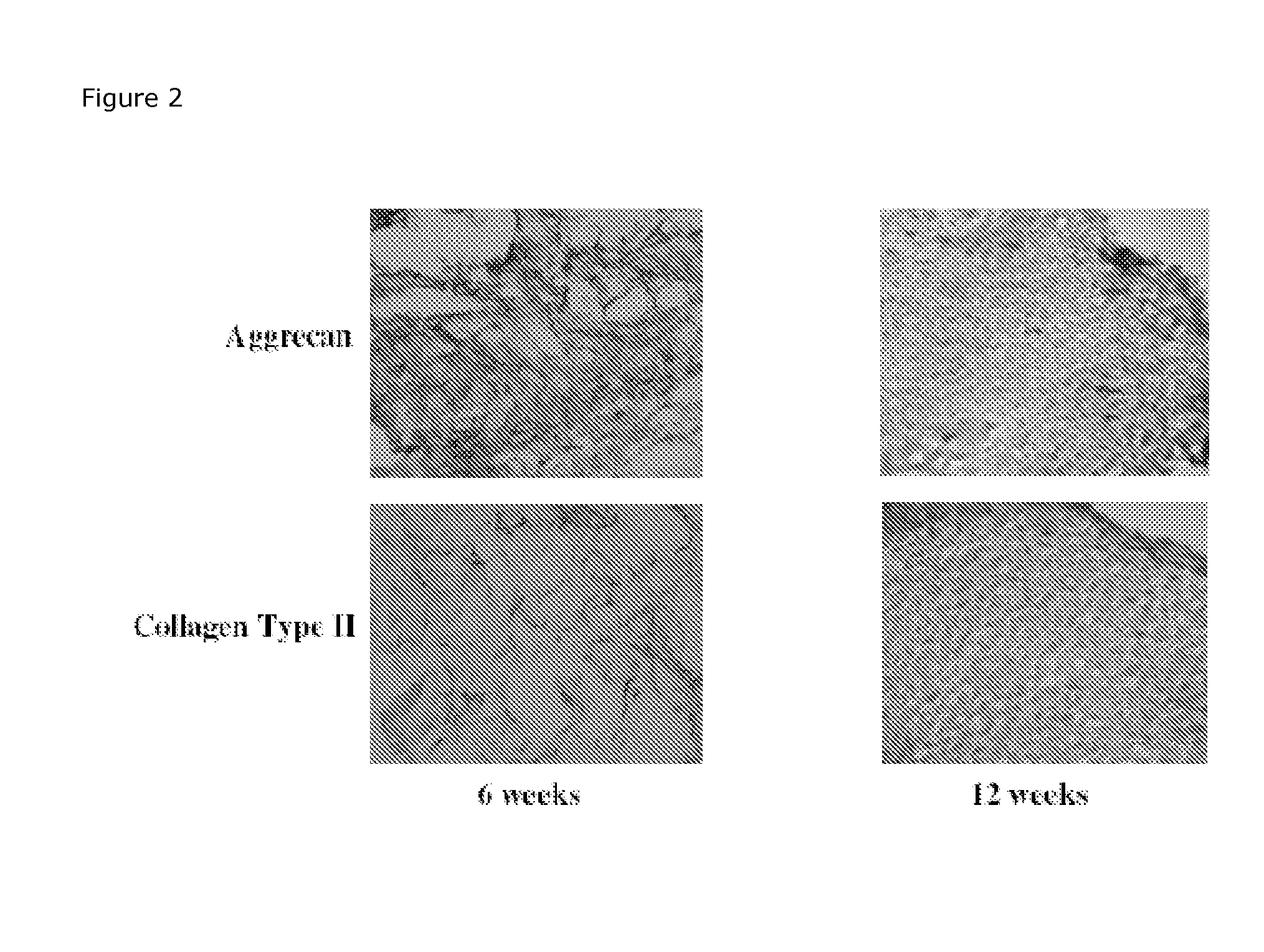
Determination of Remaining Scaffold Material in In Vitro Cultured Cartilage
Preparation of Scaffolds of Mpeg-Plga: Metoxy-Polyethylene Glycol—Poly(Lactide-Co-glycolide) (Mn 2.000-30.000, L:G 1:1) are dissolved in 1,4-dioxane to solutions containing 4%. Ten ml of the solution are poured into a 7.3×7.3 cm mould and frozen at −5° C. and lyophilised at −20° C. for 5 h and 20° C. for approx 15 h. The samples should afterwards be placed in draw (hydraulic pump) in desiccators for 24 h.
Scaffolds of MPEG-PLGA will be cultivated with chondocytes to produce in vitro cartilage as described previously. After cultivation will the scaffolds be placed in Lillys fixative for 3 days before the scaffolds are embedded in paraffin and sectioning into 8 μm slices.
An appropriate histological staining technique like Meyer's haematoxylin erosion (HE), Masson's trichrome or similar will be used to stain the new tissue but not the scaffold material. Digital images (10× and 20× magnifications) will be taken as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com