Long-acting sustained-release cell scaffold, and preparation method and application thereof
A cell scaffold and slow-release technology, which is applied in the fields of pharmaceutical formulation, medical science, and capsule delivery, can solve the problems of short drug release time, secondary injury, and easy recurrence, and achieve good mechanical tolerance, metabolic activity, and strong Effects of Mechanical Tolerance and Prolonged Drug Release Period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
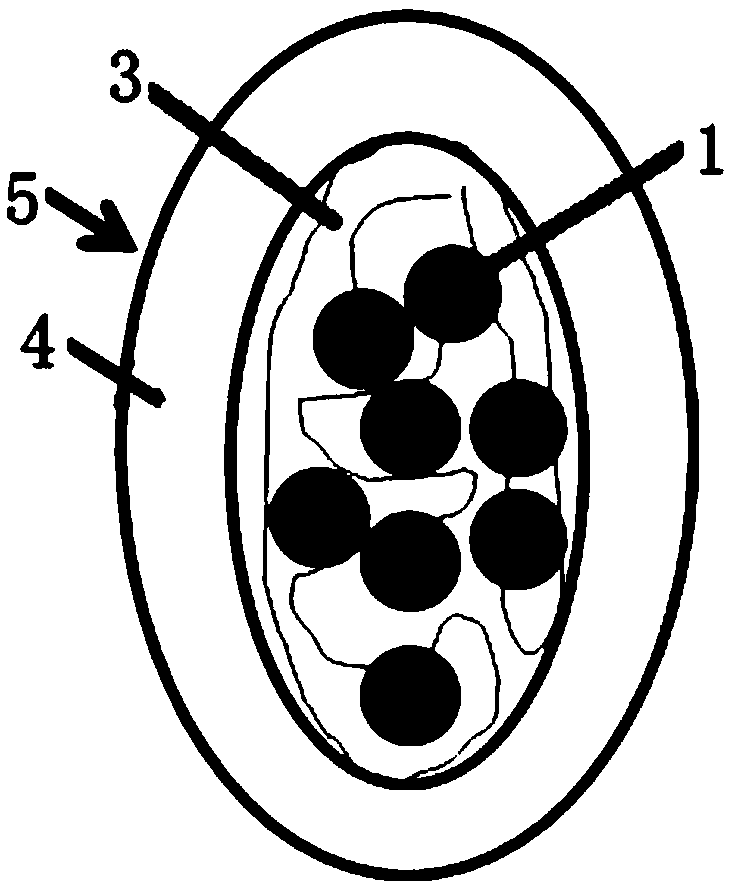
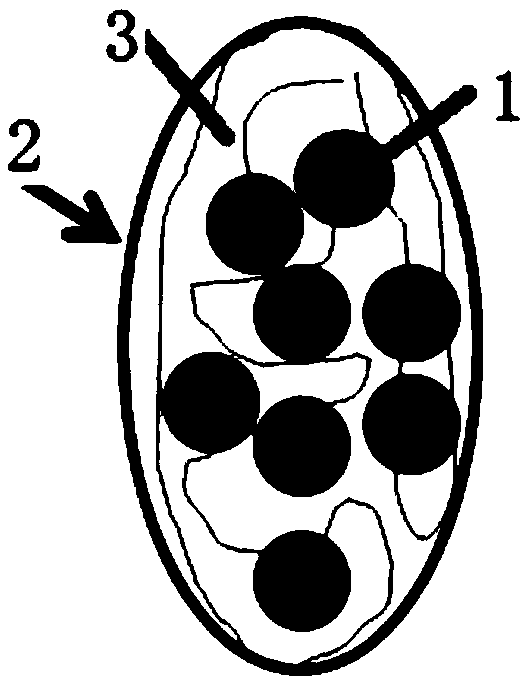
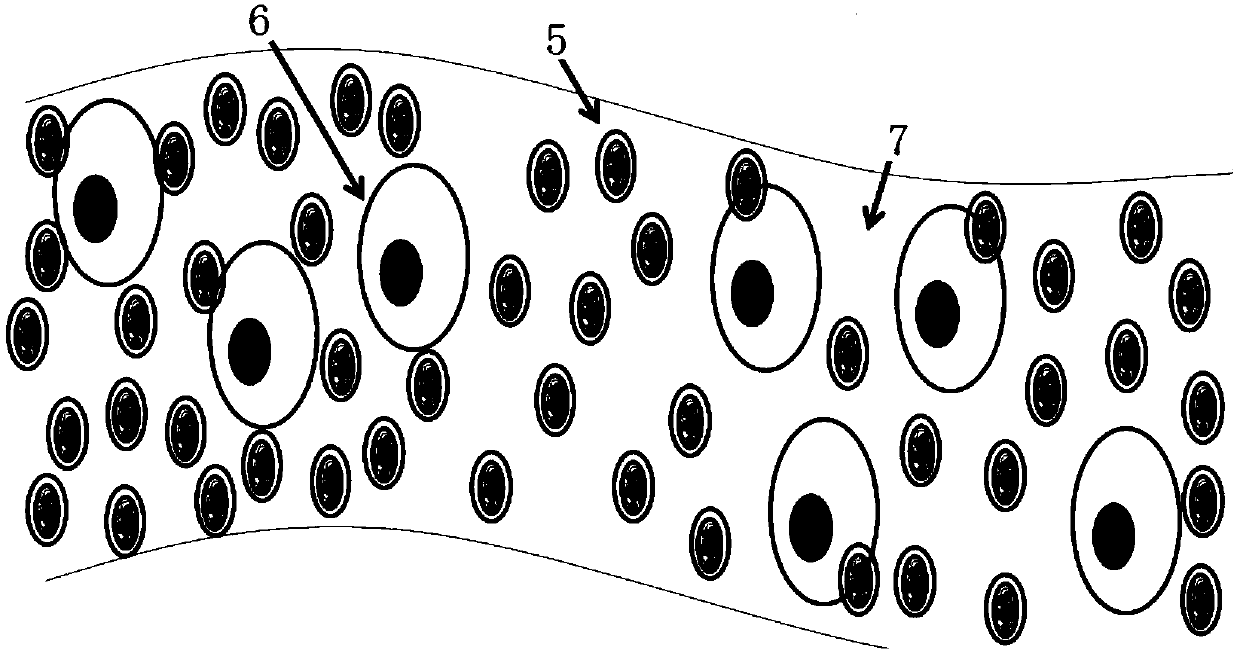
[0044] combine Figure 1 to Figure 6 As shown, a long-acting slow-release cell scaffold provided in this embodiment includes drug-cytokine protein 1, silk fibroin 3 and composite protein 4, and the drug-cytokine protein 1 is mixed with silk fibroin 3 The cytokine sustained-release microspheres 2 formed by coating, the complex protein 4 is coated on the outer layer of the cytokine sustained-release microspheres 2, and the silk fibroin 3 is a silk fibroin regulated by calcium salt self-assembly , the complex protein 4 includes gelatin that can escape the immune attack of the body and can be degraded and metabolized by cells; the drug-cytokine protein 1 includes but not limited to basic fibroblast bFGF with a concentration of 0-2 mg / ml, and a concentration of 0 Transforming growth factor beta 1 at 2 mg / ml, transforming growth factor beta 2 at a concentration of 0-2 mg / ml, transforming growth factor beta 3 at a concentration of 0-2 mg / ml and BMP-2 at a concentration of 0-2 mg / ml; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com