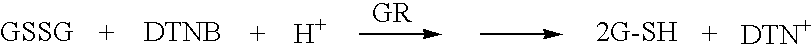Patents
Literature
496 results about "Surgical therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
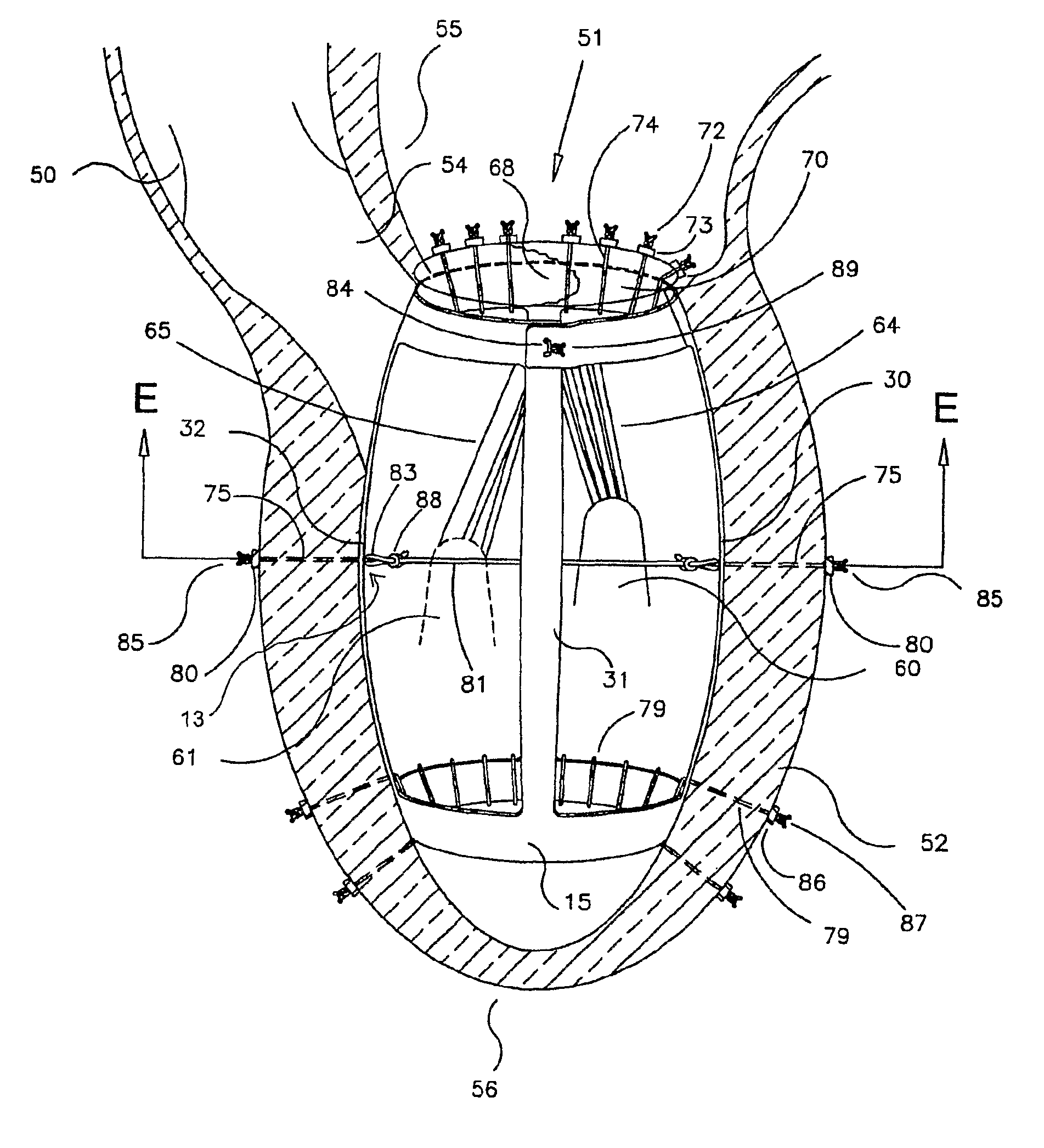
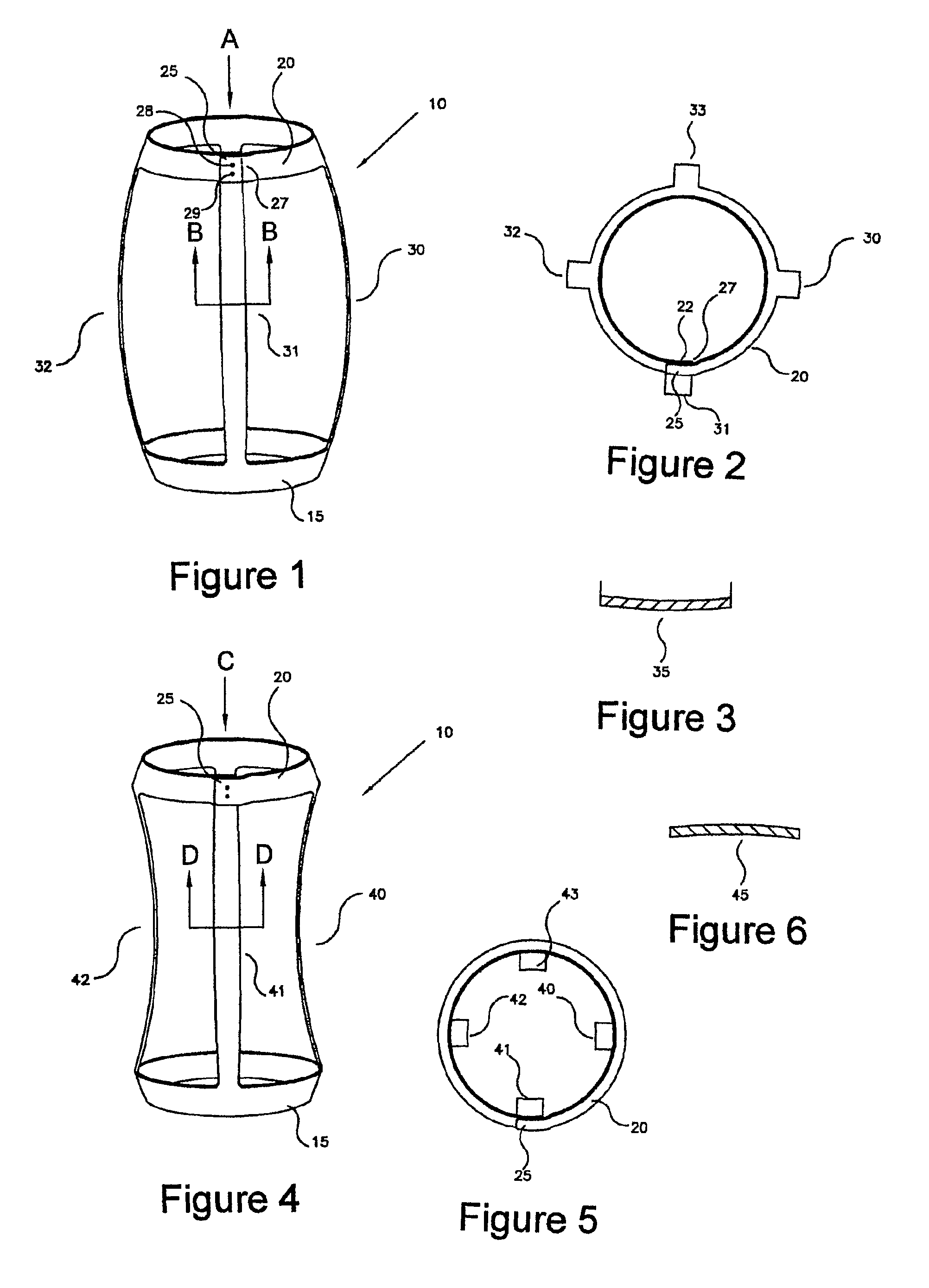
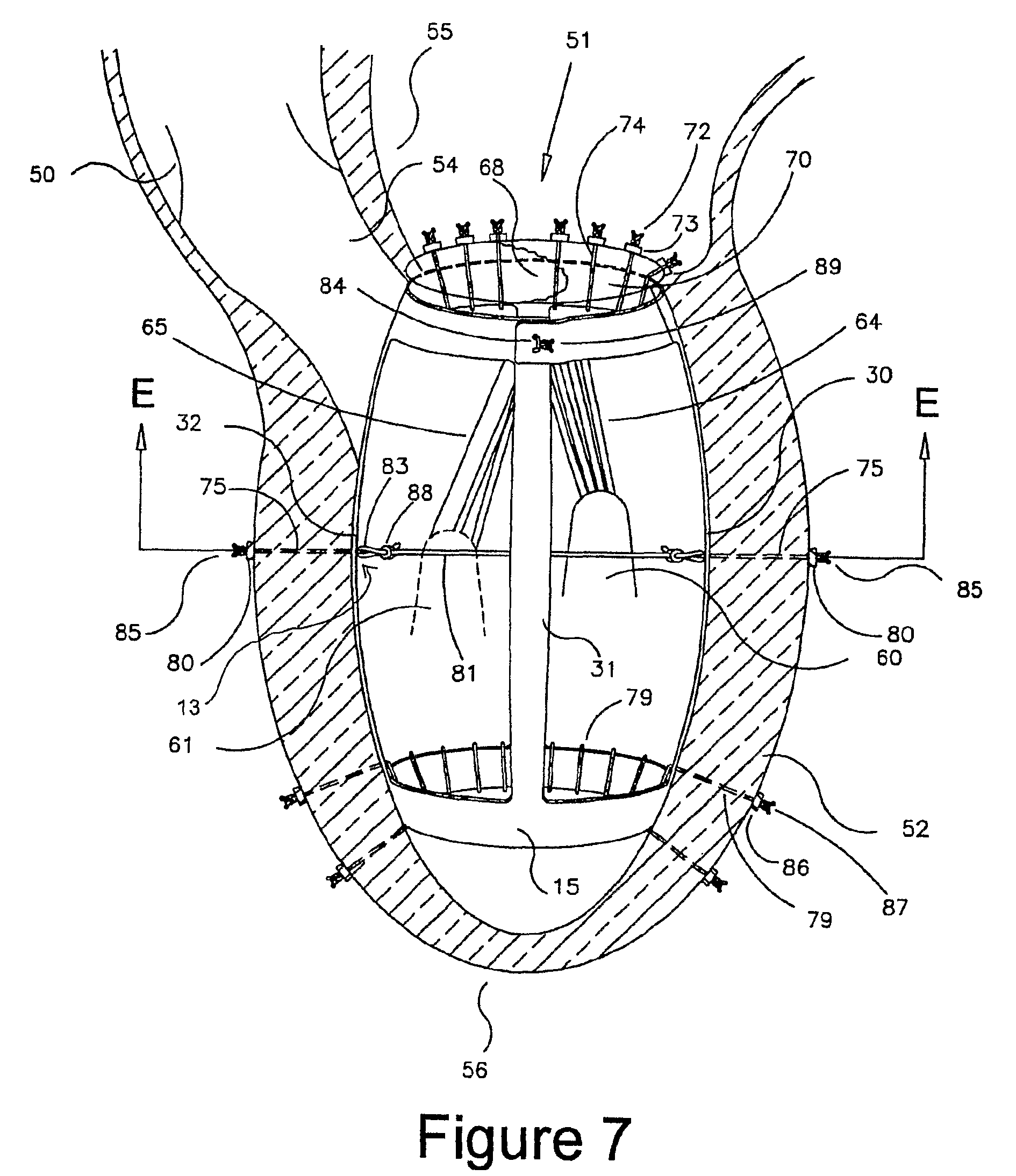
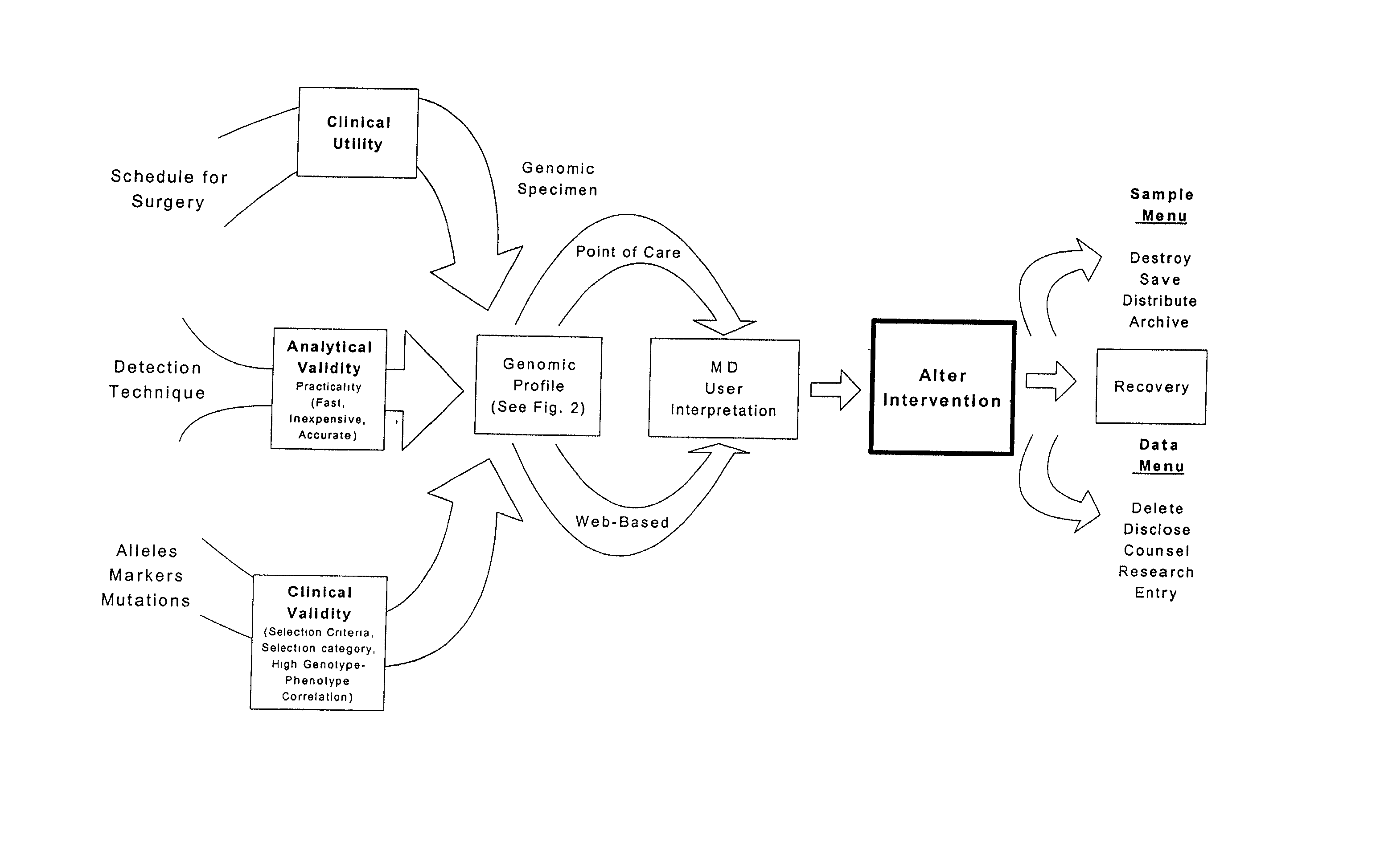
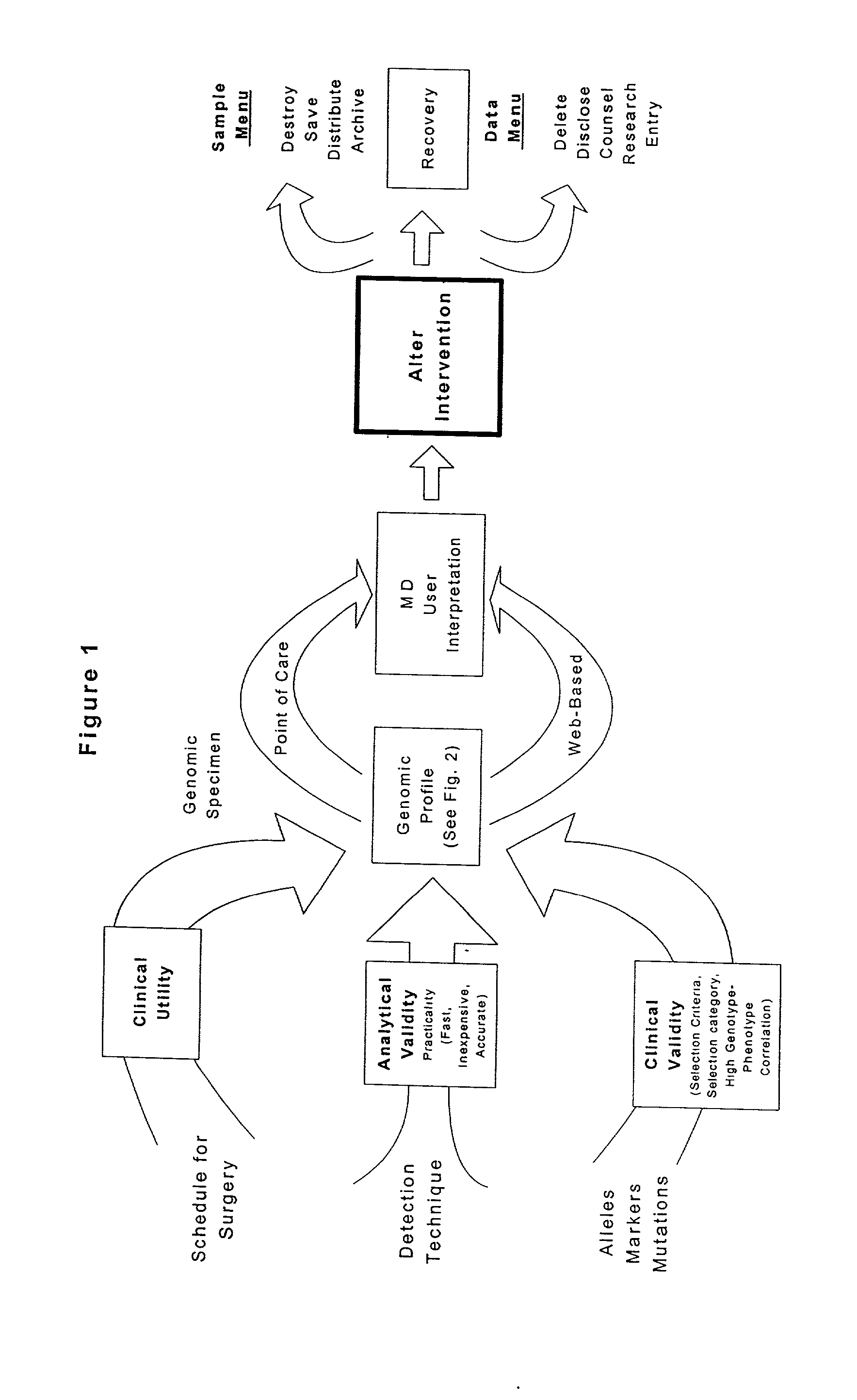
Patient-specific hemodynamics of the cardio vascular system
ActiveUS20100241404A1Minimize model instabilityMore benefitMedical simulationAnalogue computers for chemical processesInstabilityRetrograde Flow
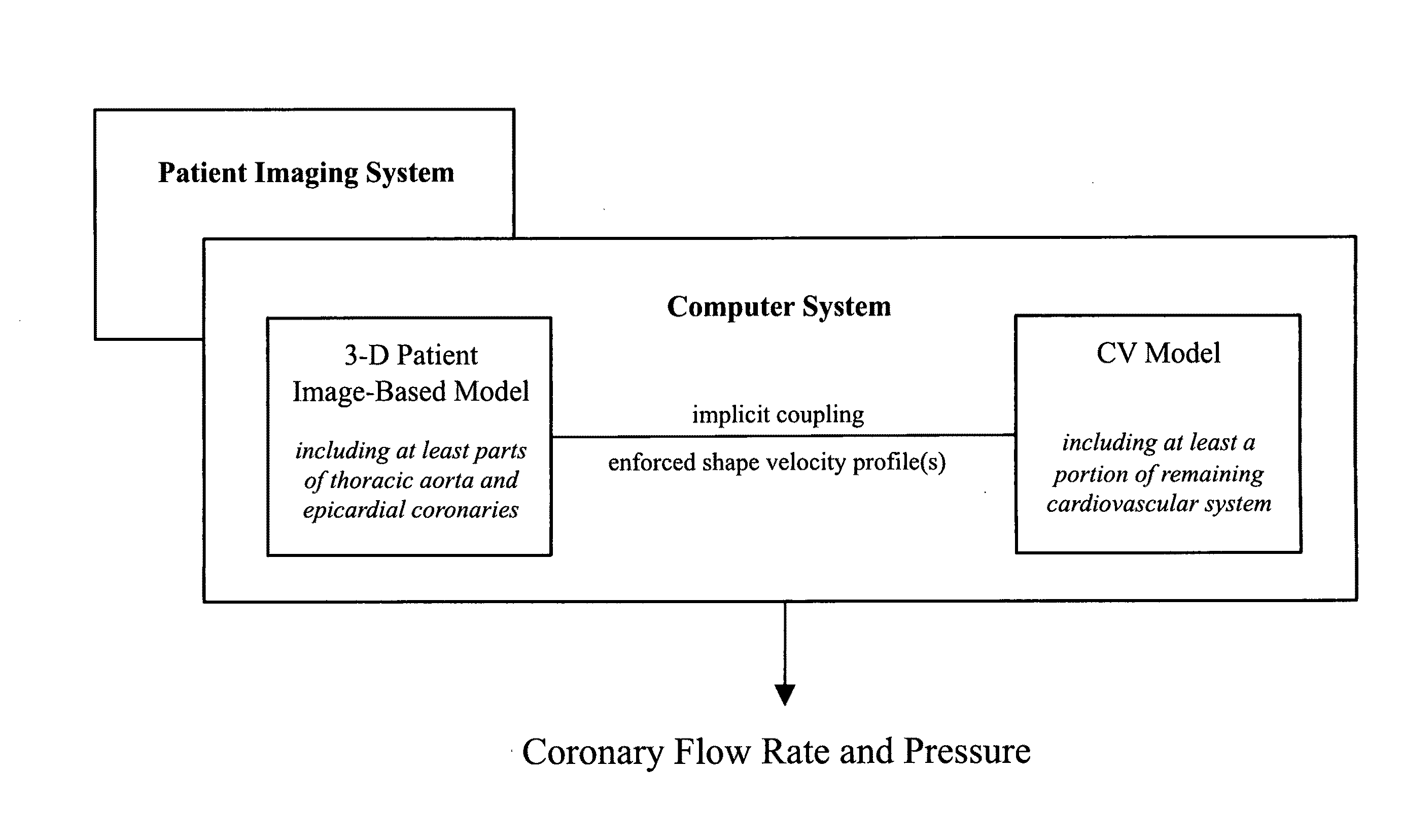
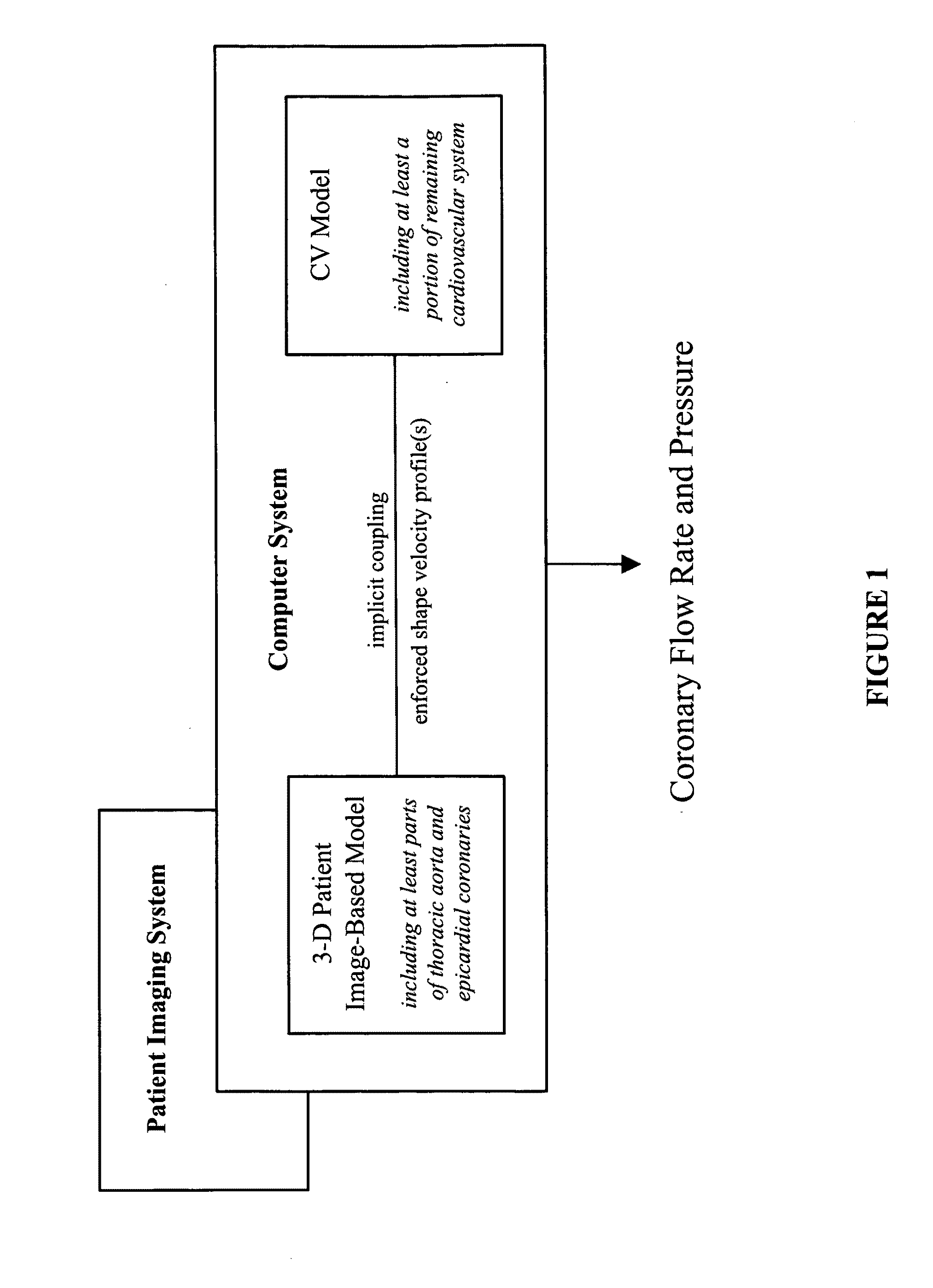
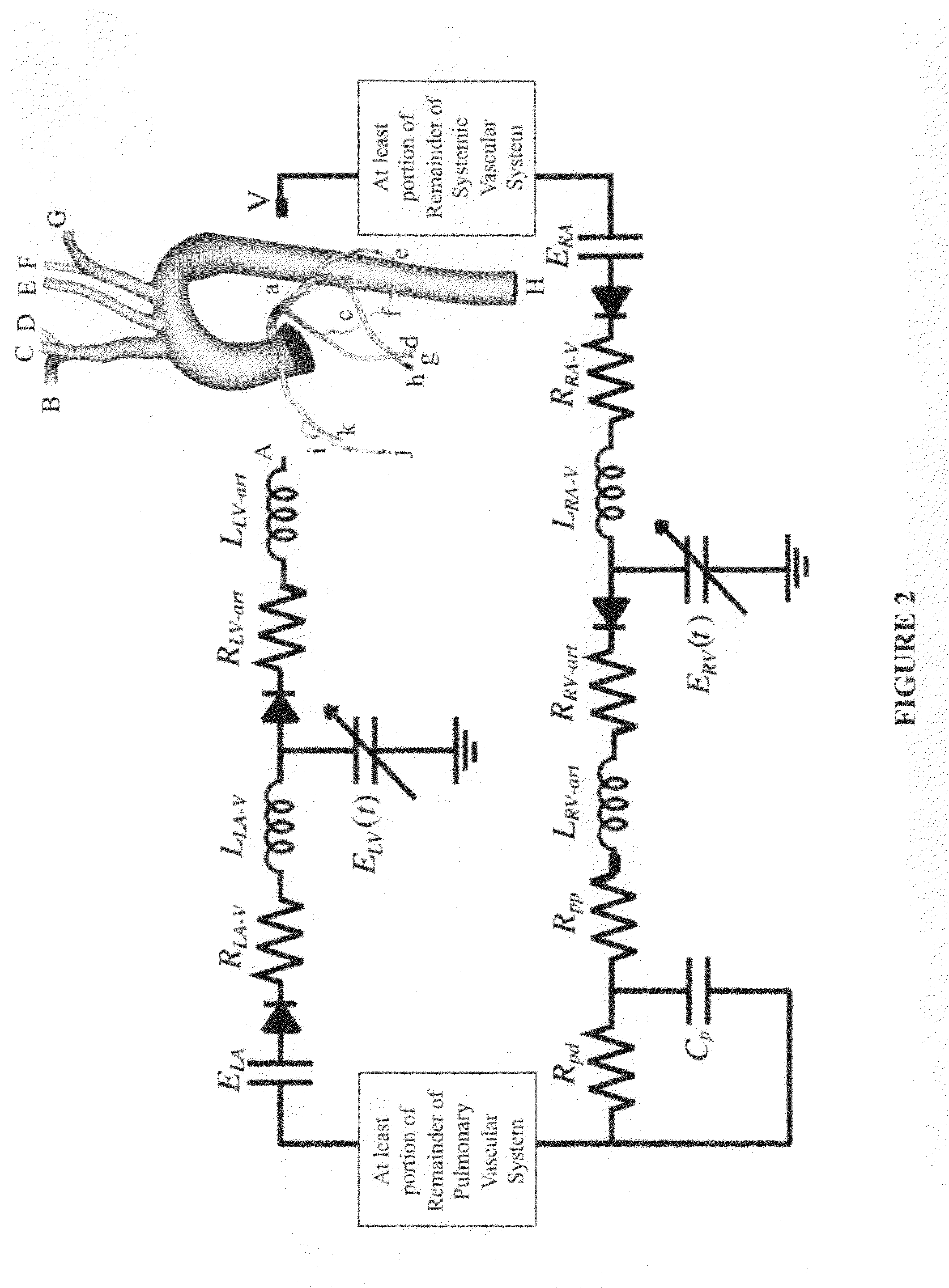
A noninvasive patient-specific method is provided to aid in the analysis, diagnosis, prediction or treatment of hemodynamics of the cardiovascular system of a patient. Coronary blood flow and pressure can be predicted using a 3-D patient image-based model that is implicitly coupled with a model of at least a portion of the remaining cardiovascular system. The 3-D patient image-based model includes at least a portion of the thoracic aorta and epicardial coronaries of the patient. The shape of one or more velocity profiles at the interface of the models is enforced to control complex flow features of recirculating or retrograde flow thereby minimizing model instabilities and resulting in patient-specific predictions of coronary flow rate and pressure. The invention allows for patient-specific predictions of the effect of different or varying physiological states and hemodynamic benefits of coronary medical interventions, percutaneous coronary interventions and surgical therapies.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
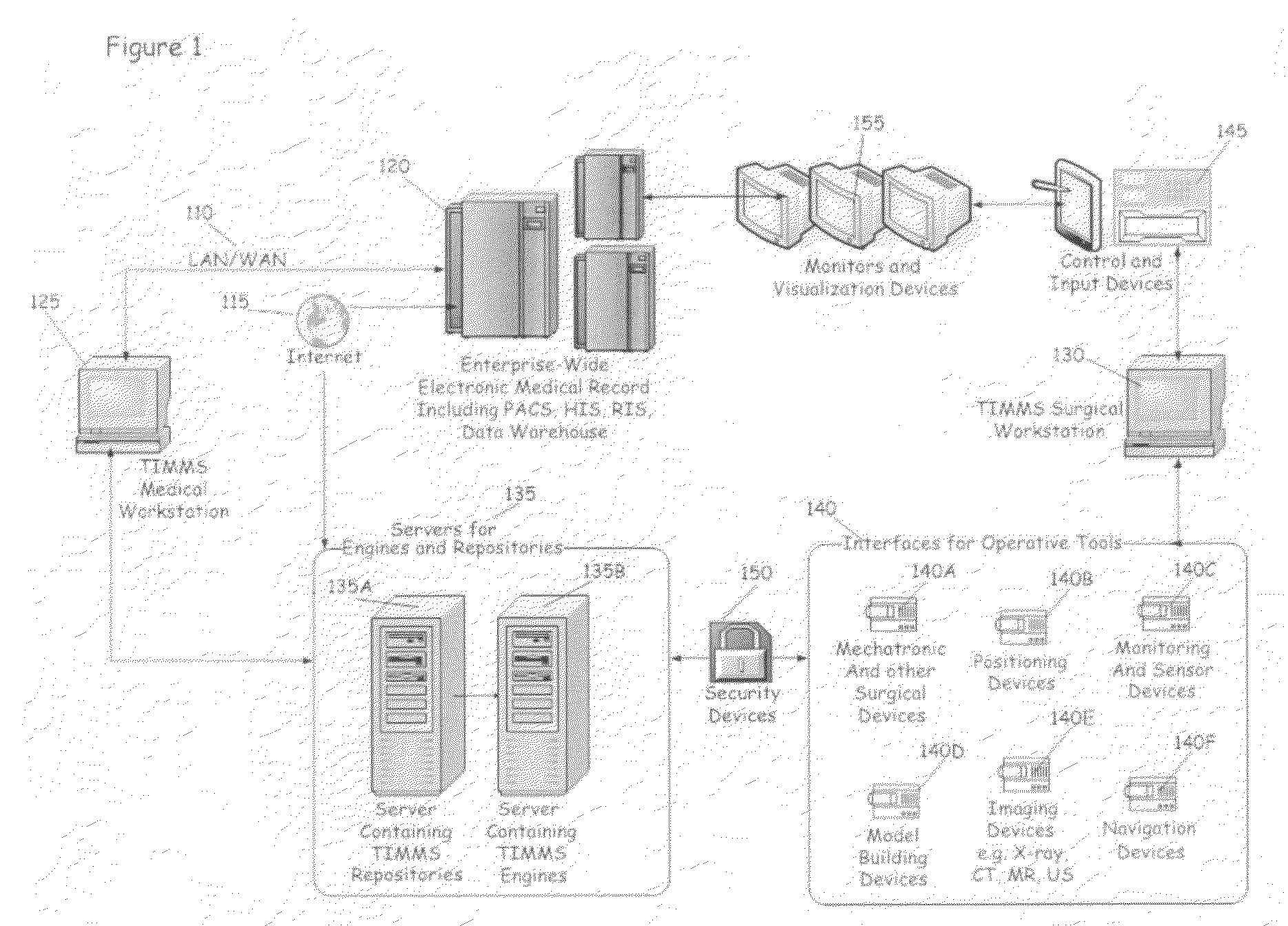
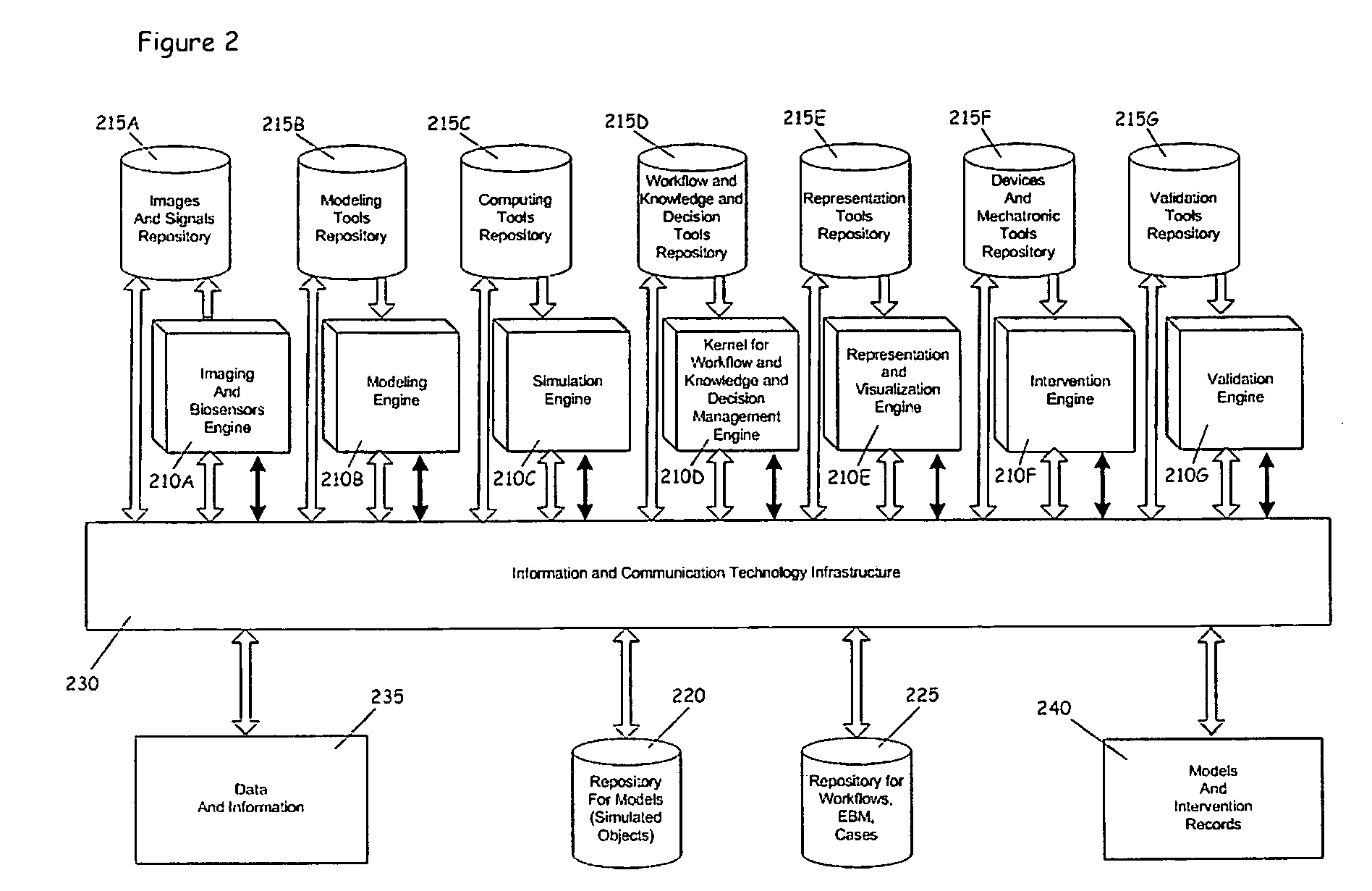
Process for comprehensive surgical assist system by means of a therapy imaging and model management system (TIMMS)
InactiveUS20090326336A1Increase successMechanical/radiation/invasive therapiesDiagnostic recording/measuringModel managementMedical record
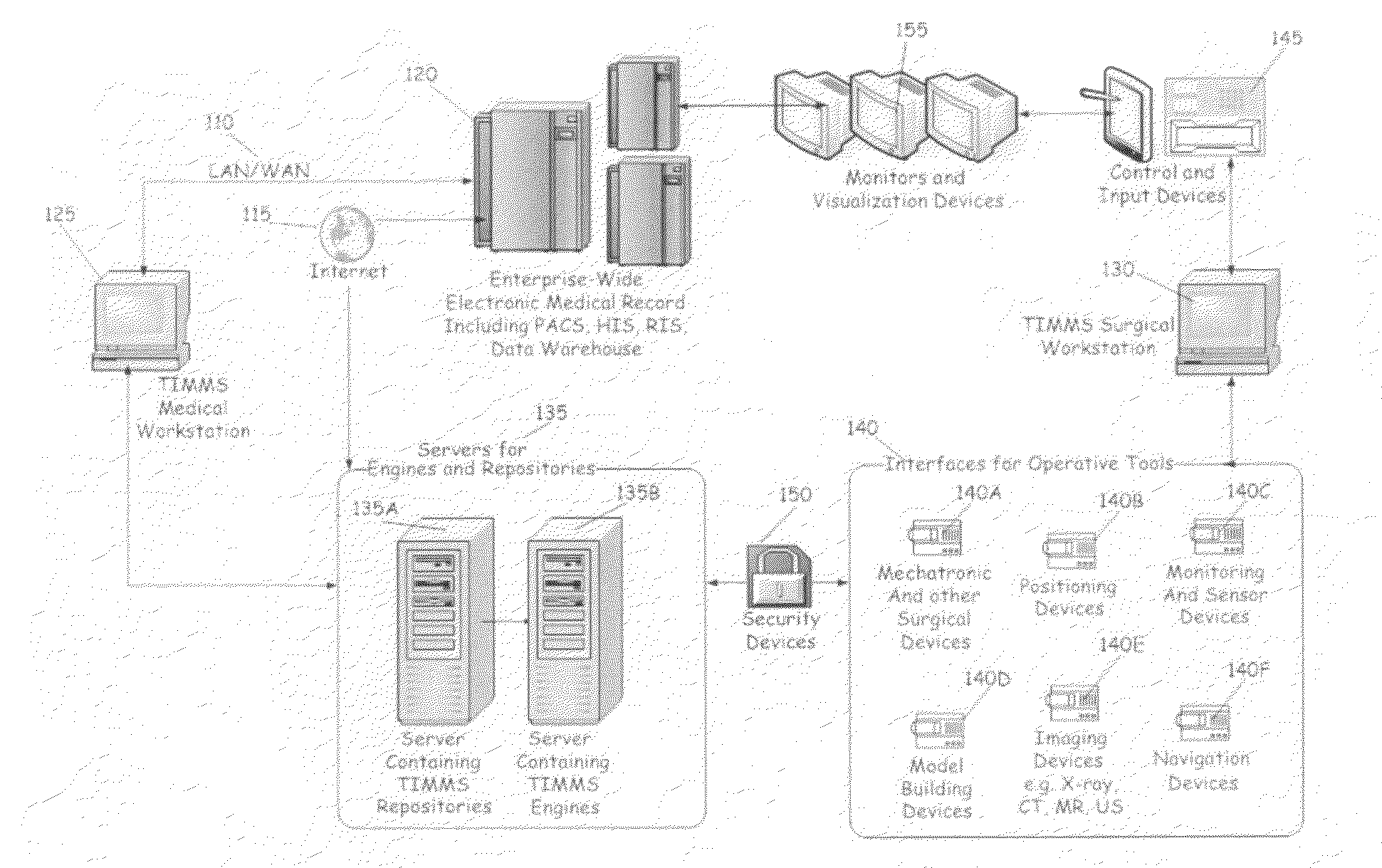
This invention provides a process and system for a comprehensive surgical assist system, called a Therapy Imaging and Model Management System (TIMMS), which combines and integrates all of the necessary information and communication technology; workflow analysis, data processing and data synthesis; interactive interfaces between surgeon and mechatronic devices; and, cognitive agents; to provide comprehensive assistance and guidance throughout complex medical and surgical therapies, such as image guided surgery. The components of this invention, which are modular, scalable and may be distributed in location, act synergistically to provide functionality and utility that exceeds the sum of its individual parts.A method of performing surgery on a patient comprising the step of comparing a chosen patient's data to statistical data in a repository of patient data to develop a patient specific model, wherein the data comprises information from two or more sub databases selected from the group consisting of workflow data, electronic medical records, diagnostic data, biological data, measurement data, anatomical data, physiological data, genetic data, molecular data, imaging data, chemical data, clinical laboratory data, simulated data, coordinate data and surgical result and wherein the patient specific model aids in the preoperative, operative or post operative phase of surgery performed in real time on the patient.
Owner:LEMKE HEINZ ULRICH +1
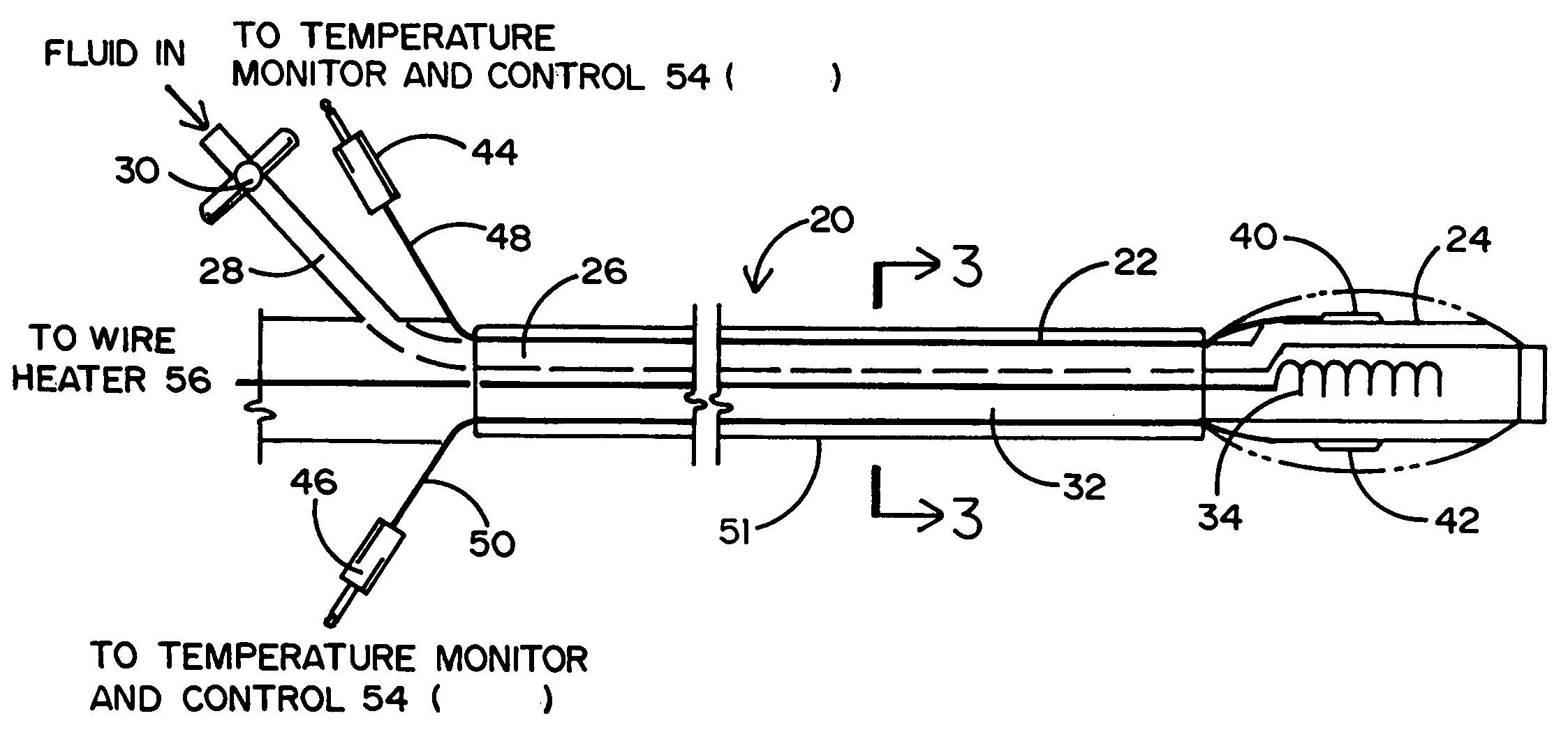
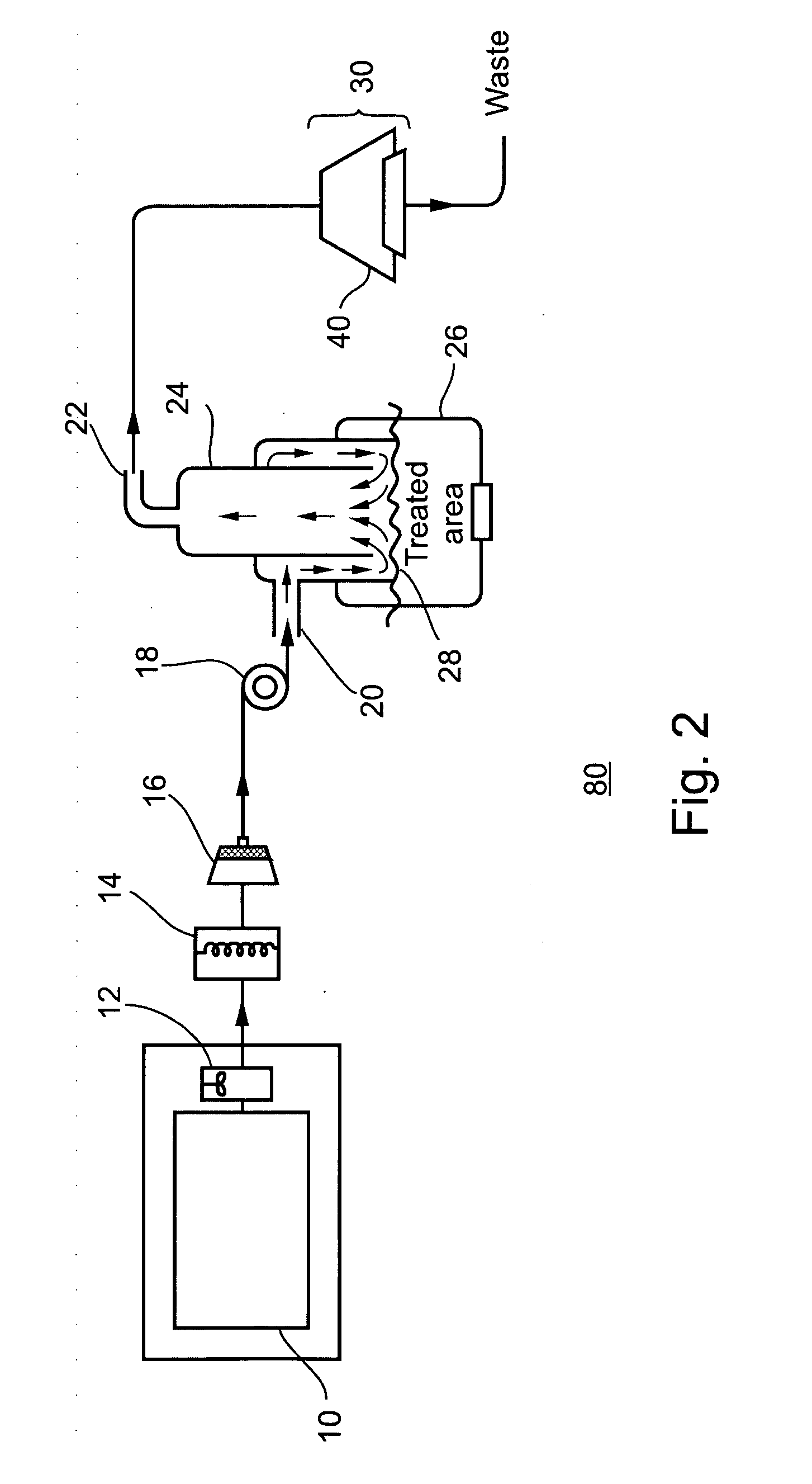
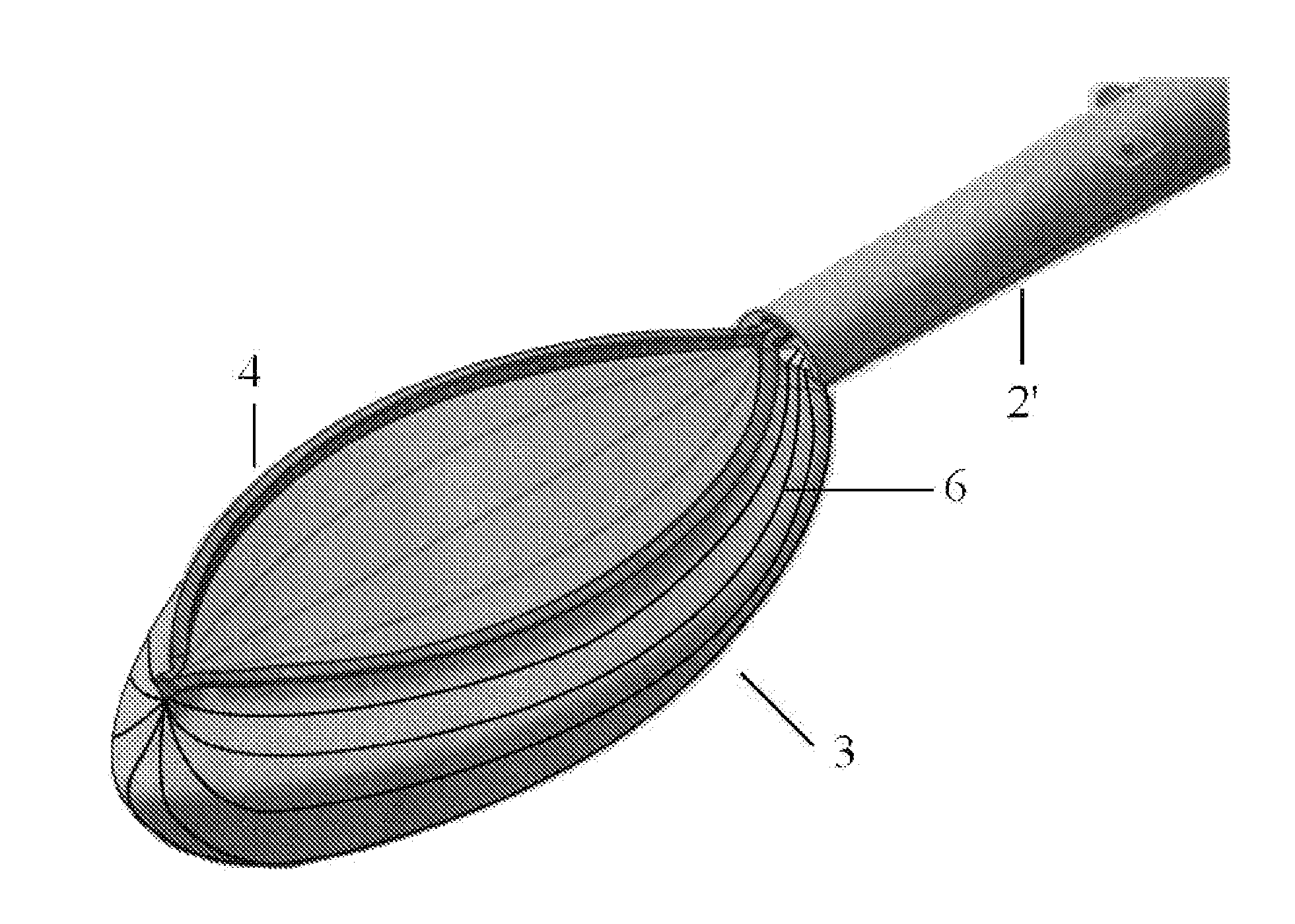
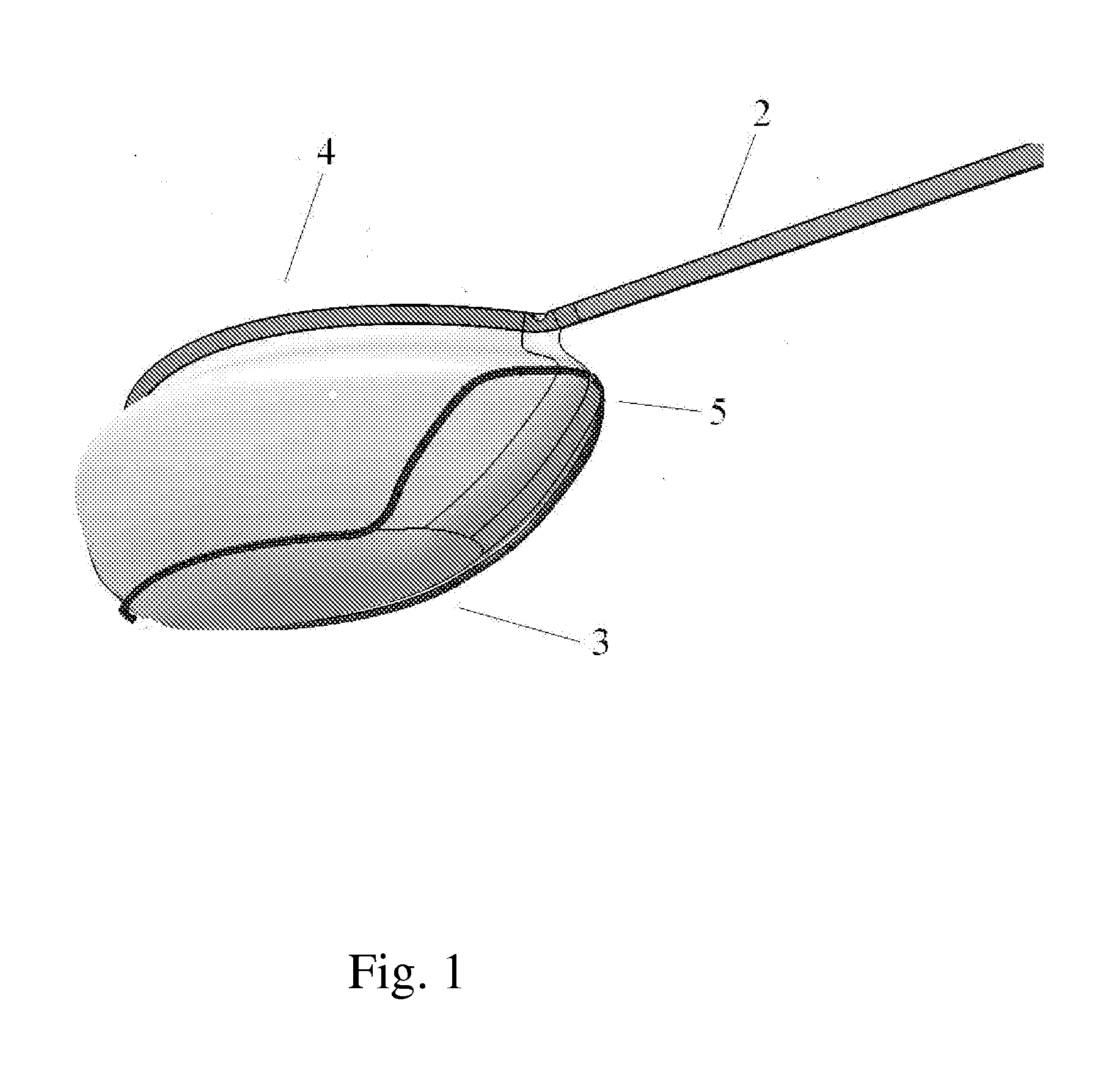
Tissue protective system and method for thermoablative therapies
InactiveUS20060118127A1Possible removalDiagnosticsSurgical instruments for heatingCancer cellNeurovascular bundle
A tissue protective system and method having particular application in thermoablative surgical therapies where heat or cold is used to create a kill zone for treating cancer cells as well as malignant or benign tumors in a targeted internal tissue area (e.g., the prostate) of a patient while sparing an adjacent benign internal tissue area (e.g., a neurovascular bundle). One of a hollow sheath or a balloon that is carried by a balloon catheter is located within an access opening that is made by a needle trocar inserted between the targeted tissue area in need of treatment and the benign tissue area to be protected in order to hold the protected tissue area off the targeted tissue area and away from the lethal temperature of the kill zone. The balloon of the balloon catheter is inflated in the access opening via a balloon channel which runs longitudinally through the catheter. At least one temperature sensor is mounted on the balloon and responsive to the temperature near the benign tissue area to be protected. Heat or cold is provided to the balloon from a heating wire or a circulating fluid, depending upon the temperature that is sensed by the temperature sensor.
Owner:CHINN DOUGLAS O
Shape-sensing expandable member
ActiveUS20090005674A1Easy procedureSufficient informationUltrasonic/sonic/infrasonic diagnosticsCatheterThree-dimensional spaceTransducer
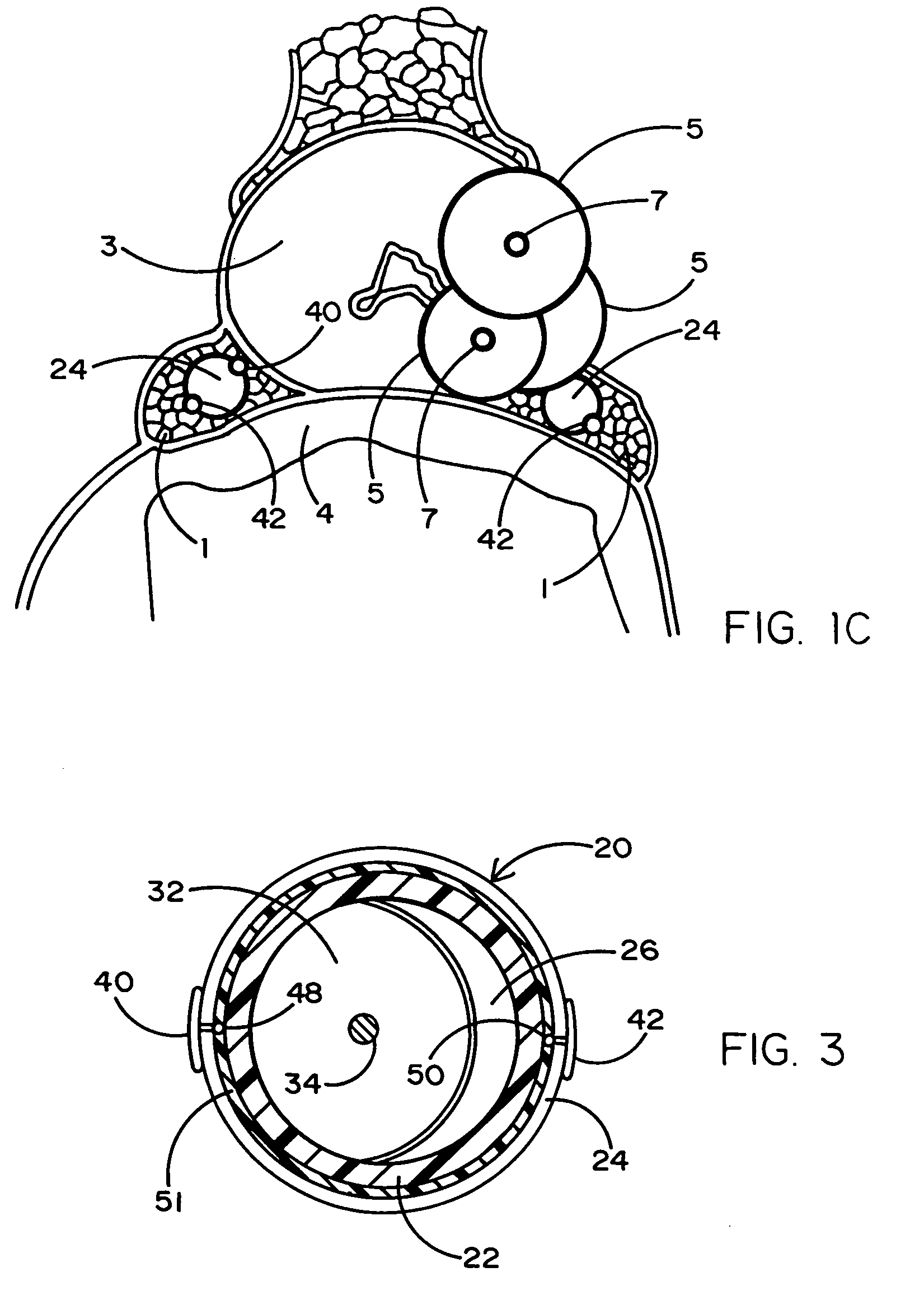
An expandable member for characterizing a three-dimensional space within a mammalian body is described. The expandable member is delivered to a target region in a deflated state where it is expanded by inflation of the member. Sensory transducers that contact the member relay sensory information generated when the member is in an expanded state to a microprocessor located outside the body. Using the sensory information, a data-driven picture that characterizes the three-dimensional space within the body is created with a microprocessor. The apparatus is useful in preparation for minimally invasive surgical therapy.
Owner:NIDUS MEDICAL
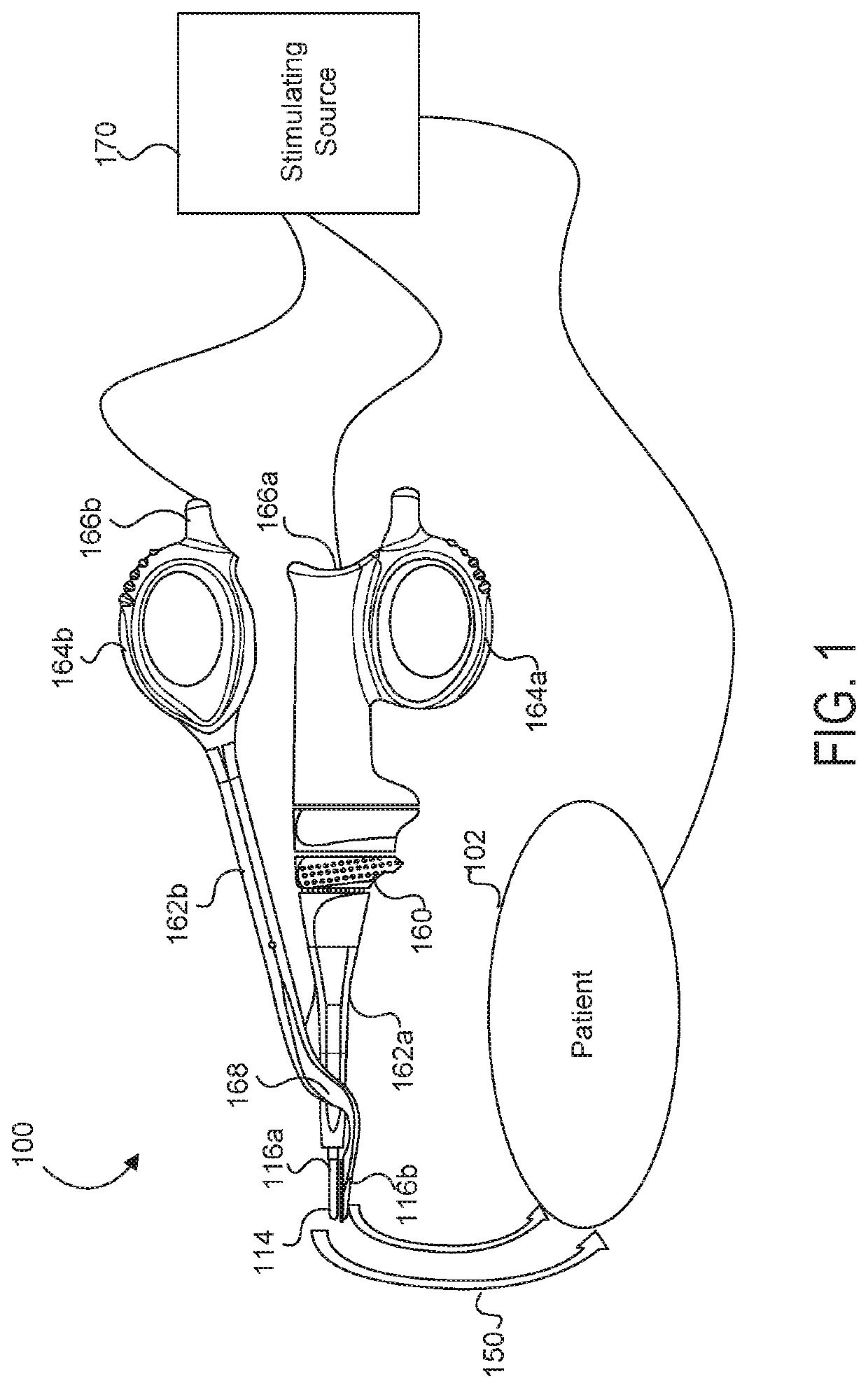
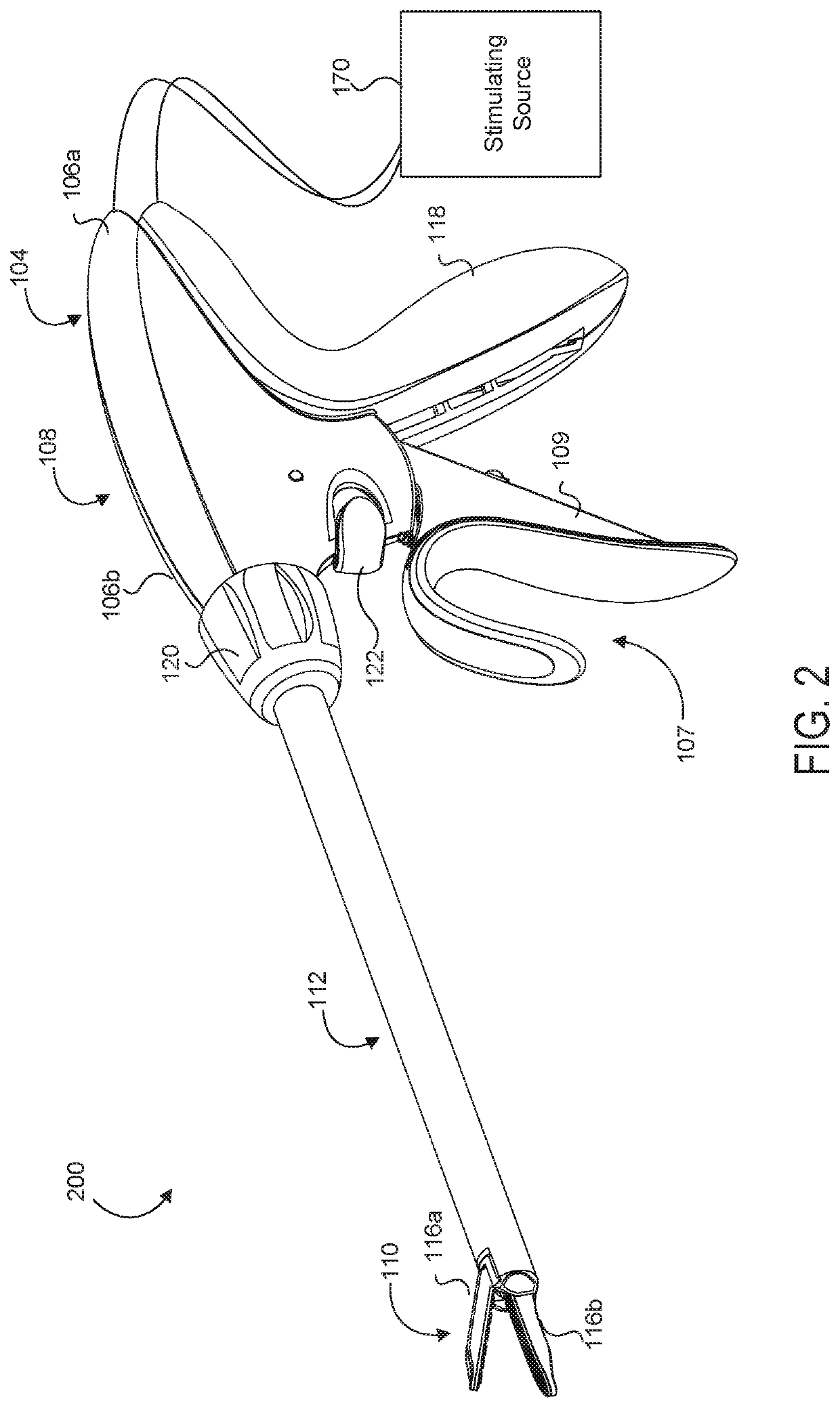
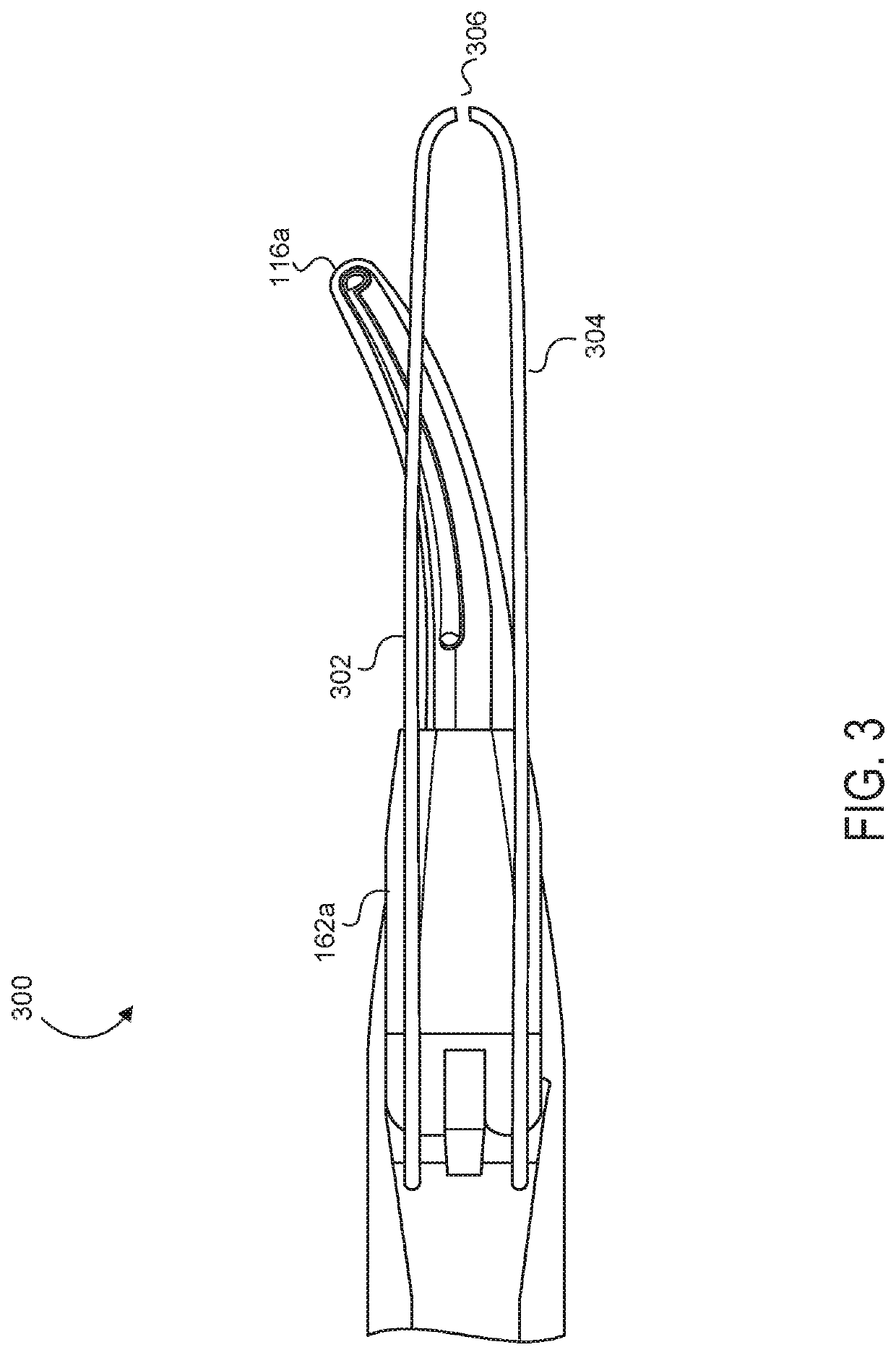
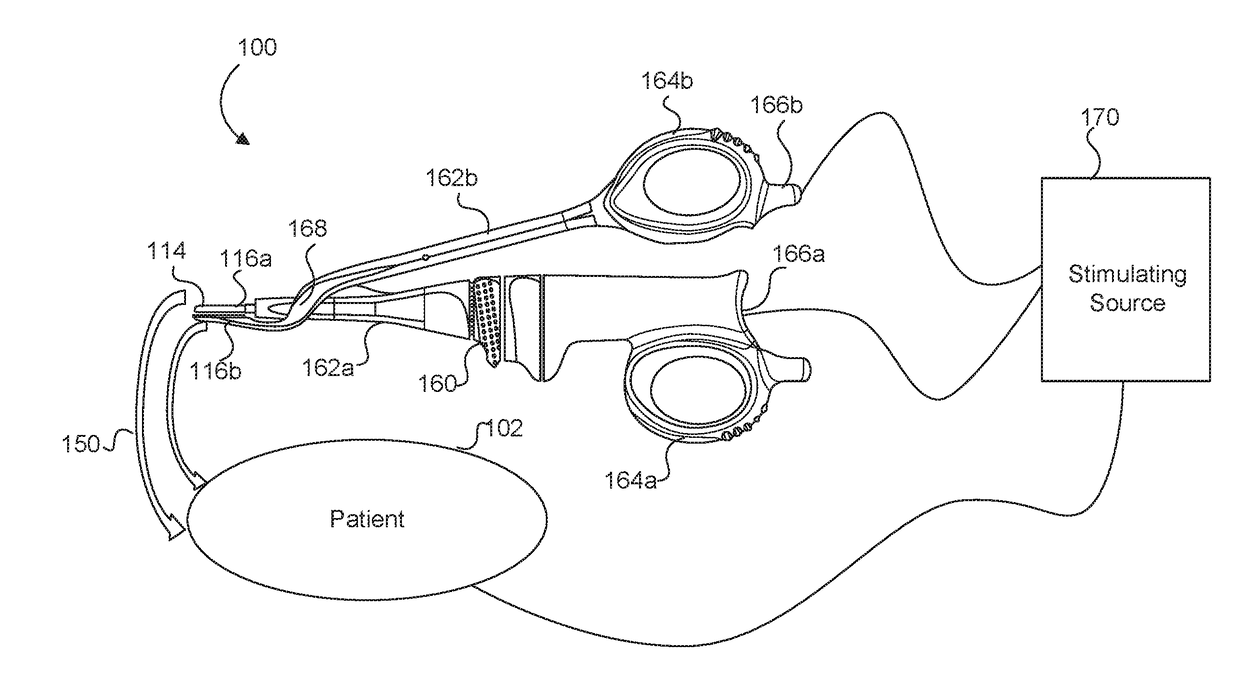
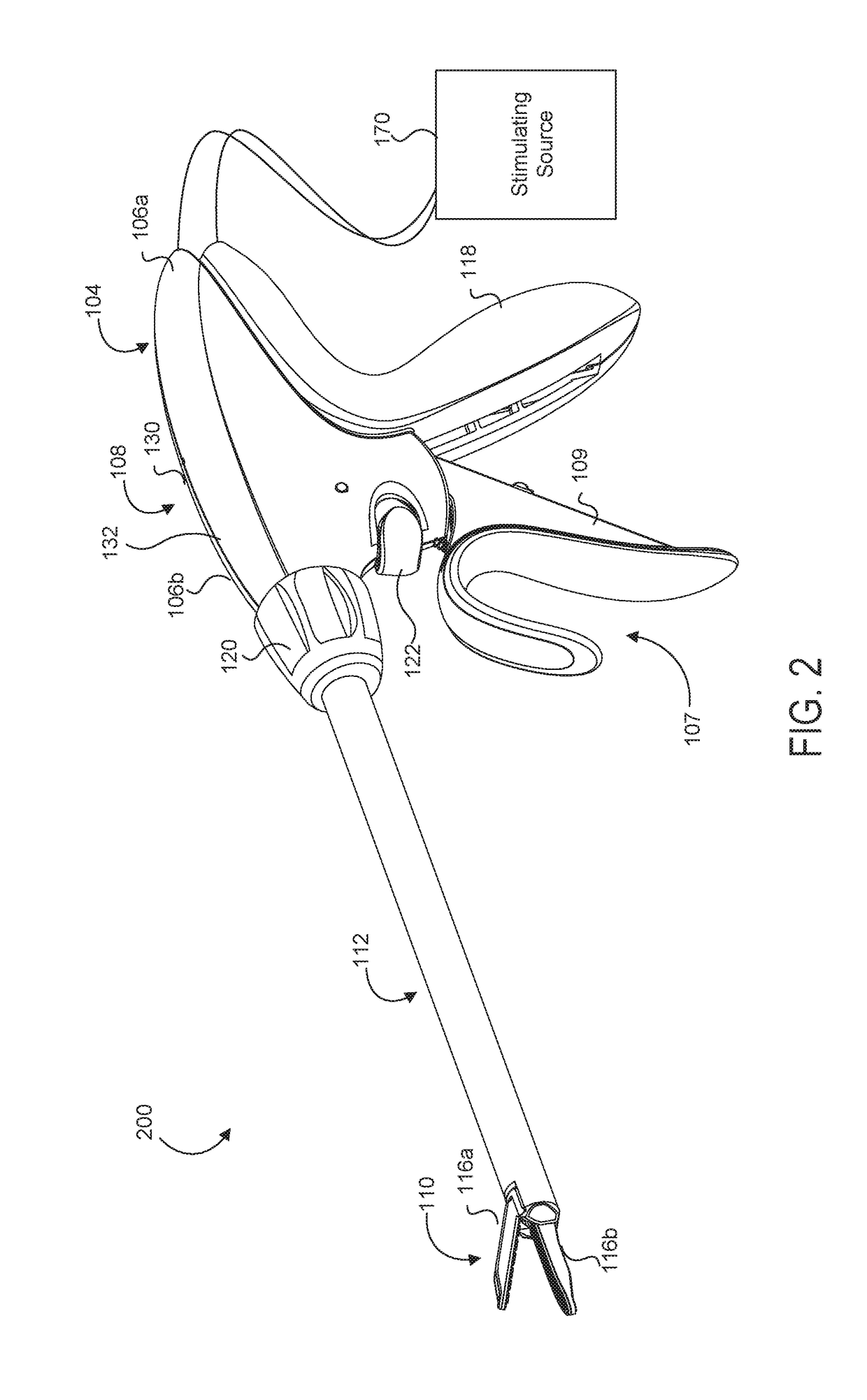
Medical device with a bilateral jaw configuration for nerve stimulation
ActiveUS20200078085A1Spinal electrodesImplantable neurostimulatorsSurgical operationNerve stimulation
Aspects of the present disclosure are presented for a single surgical instrument configured to grasp, seal, and / or cut tissue through application of therapeutic energy, and also detect nerves through application of non-therapeutic electrical energy. A medical device may include two jaws at an end effector, used to apply therapeutic energy and to perform surgical procedures. The therapeutic energy may be in the form of ultrasonic vibrations or higher voltage electrosurgical energy. One of the jaws may be configured to cut tissue through application of the blade. In addition, one or both of the two jaws may be configured to apply nontherapeutic energy for nerve stimulation probing. The application of therapeutic energy may be disabled while the nontherapeutic nerve stimulation energy is applied, and vice versa. The nontherapeutic nerve stimulation energy may be applied to the use of one or more probes positioned near one or both of the jaws.
Owner:CILAG GMBH INT
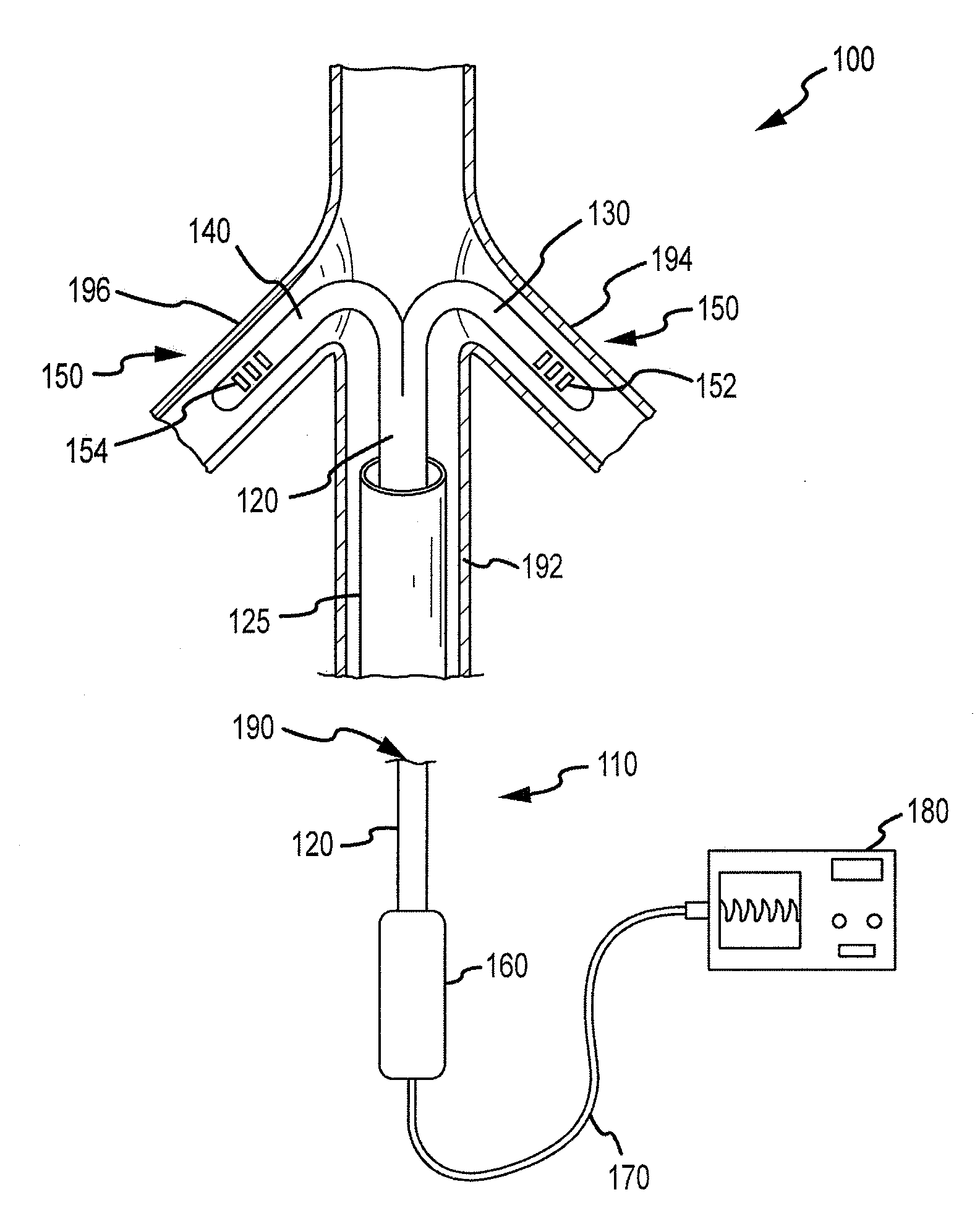
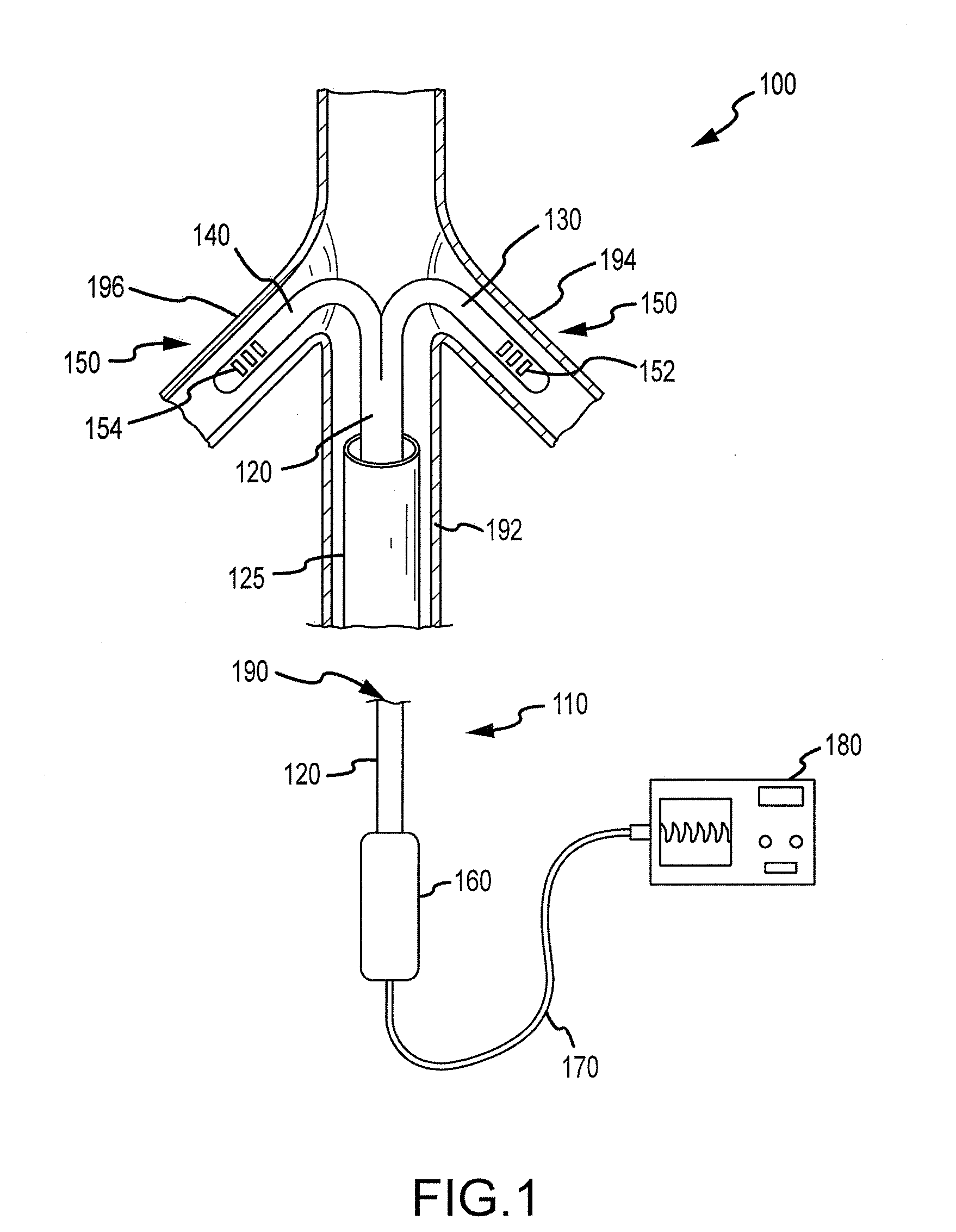
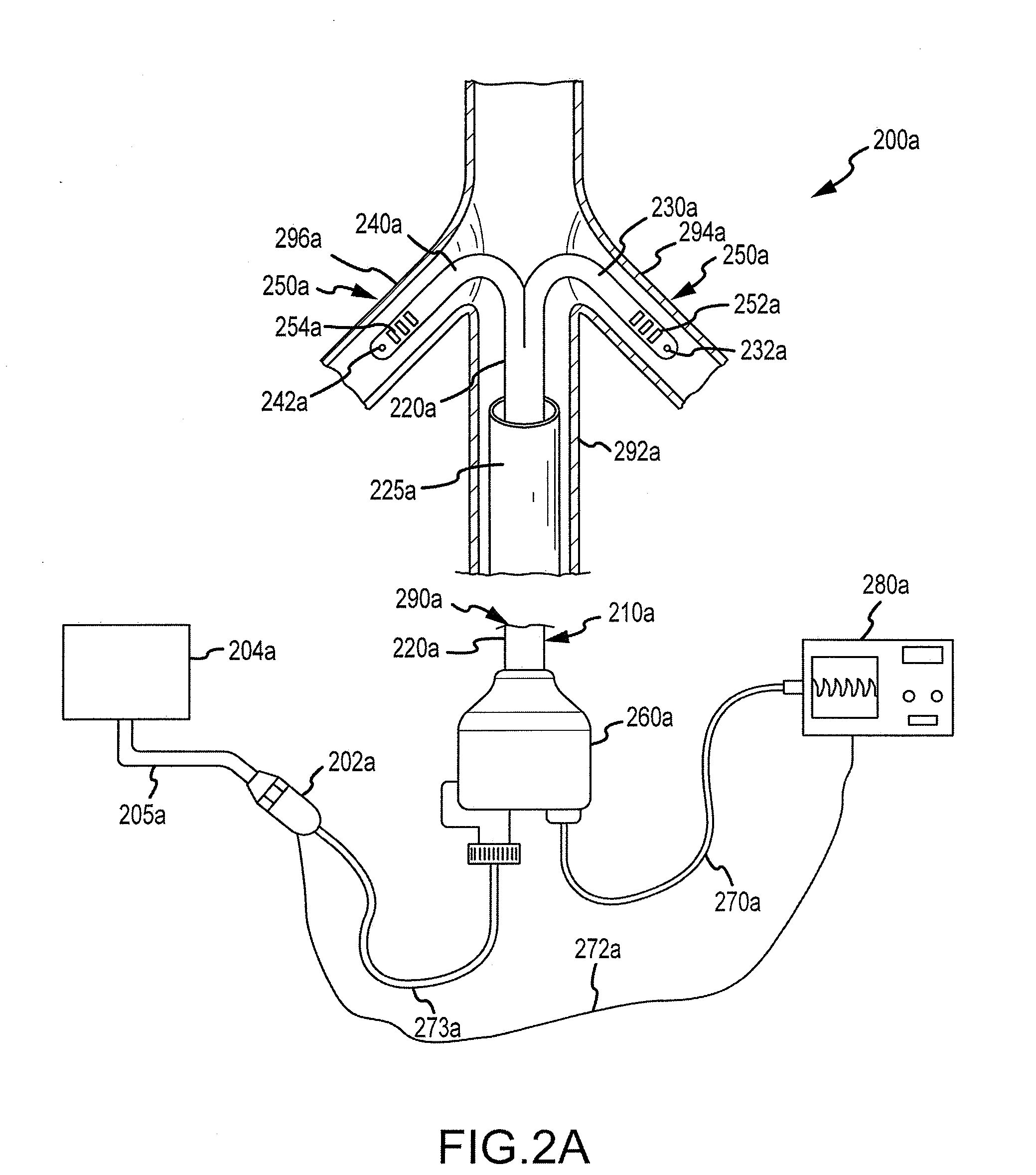
Renal assessment systems and methods
Techniques for assessing a physiological profile of a patient include advancing a catheter shaft of a bifurcated renal catheter system into an aorta of the patient, deploying branches of the bifurcated renal catheter system into the renal arteries of the patient, detecting a renal arterial physiological parameter with a sensing mechanism, and assessing the physiological profile of the patient based on the physiological parameter. Related techniques include modifying or initiating pharmacological or surgical treatments for the patient based on the assessment.
Owner:FLOWMEDICA
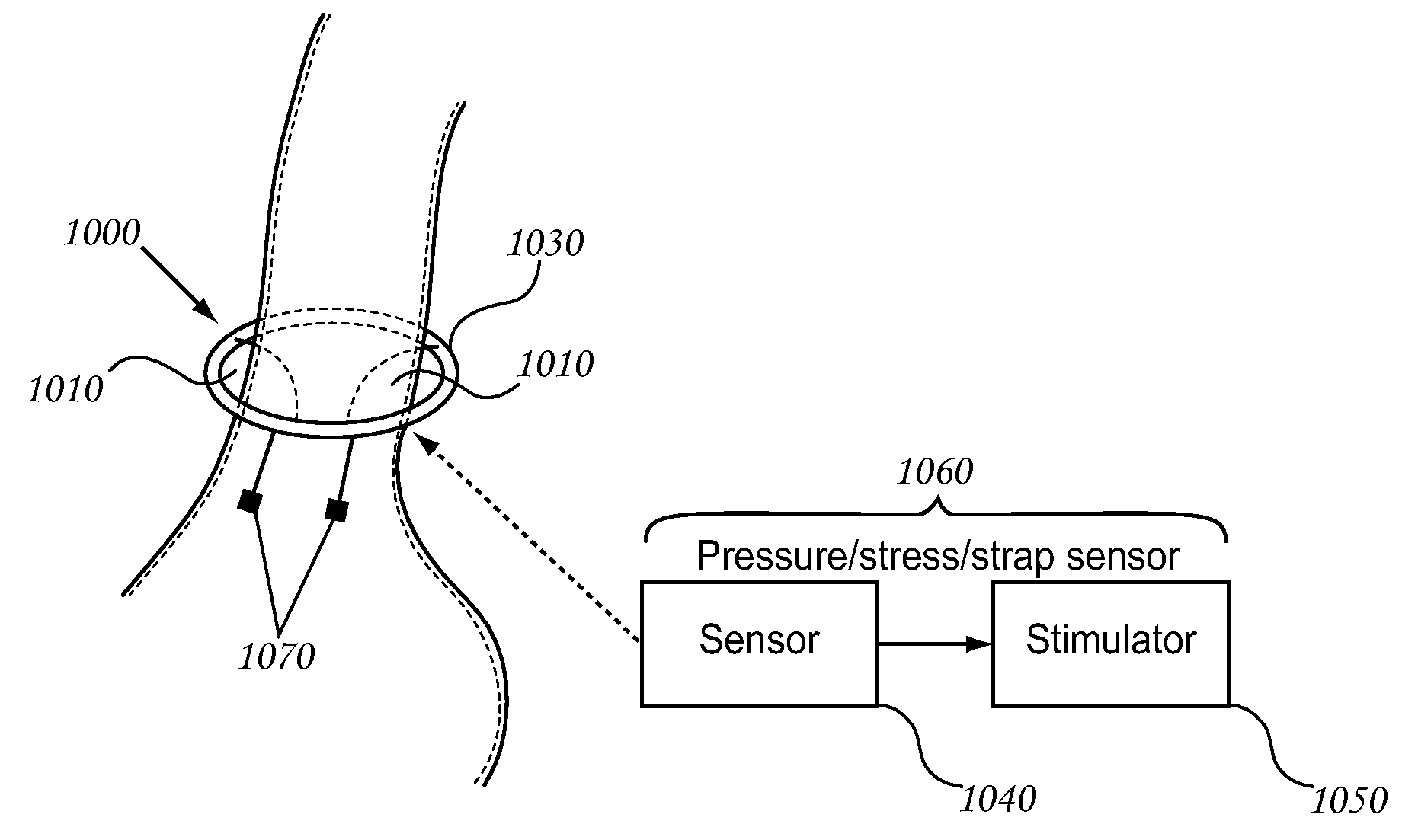
Management Systems For The Surgically Treated Obese Patient
InactiveUS20060189889A1Increase heightLonger-term implantationSuture equipmentsElectrotherapyWireless transmissionPatient management
In one embodiment, a pressure sensing system is described which transmits data to a patient management system external to a patient. The pressure sensing system can rigidly couple to an implantable port or flexibly couple to an implantable port. In some embodiments, the pressure sensing system communicates with a hydraulic actuating system. In some embodiments, the pressure sensing system is implantable and comprises a circuit capable of wireless transmission through the skin of a patient to an external receiver which is part of a patient management system. A patient management system is described which receives up to date as well as historical data from the pressure sensing system and manages the these data in the context of a patient database.
Owner:GERTNER MICHAEL
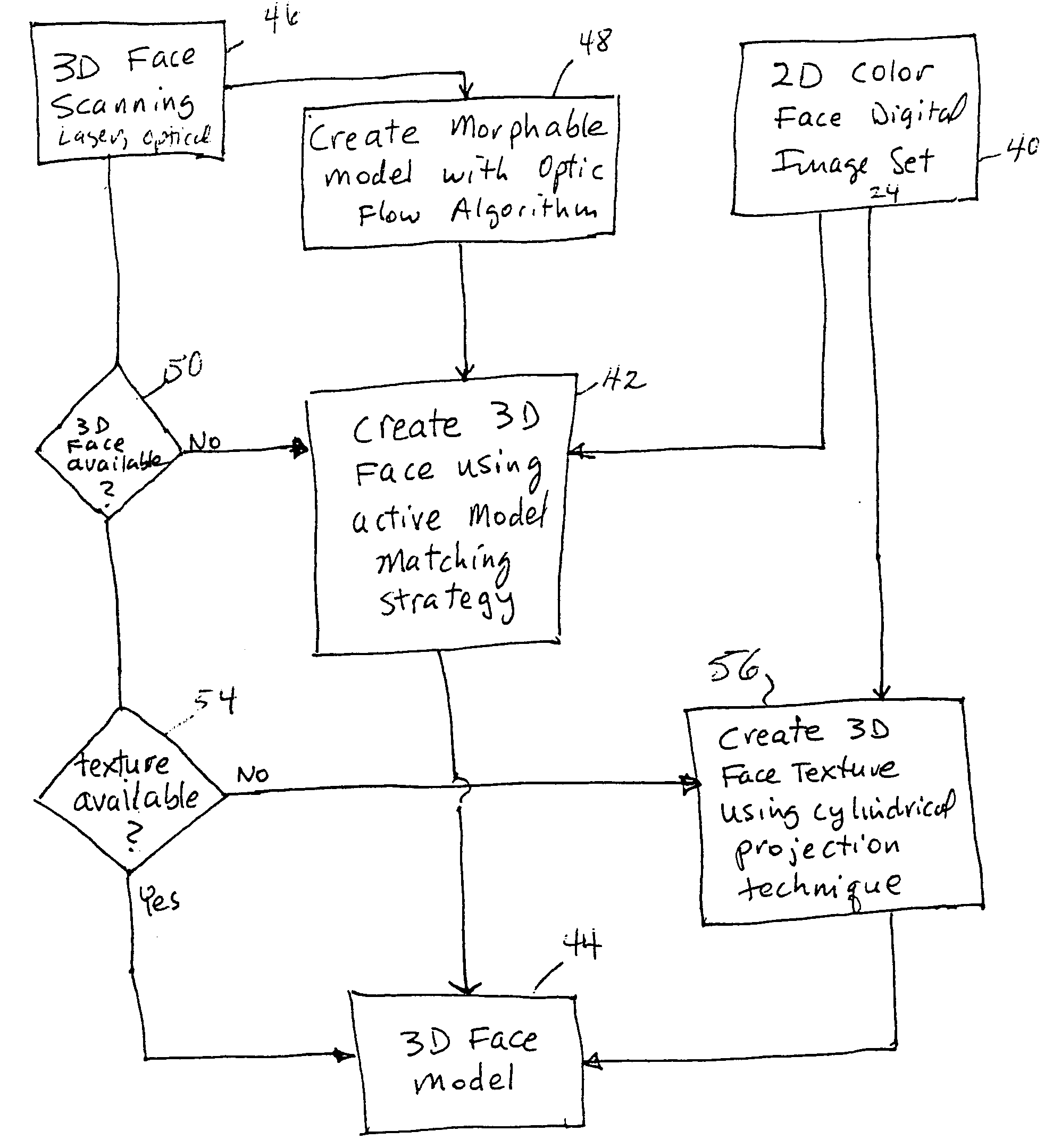
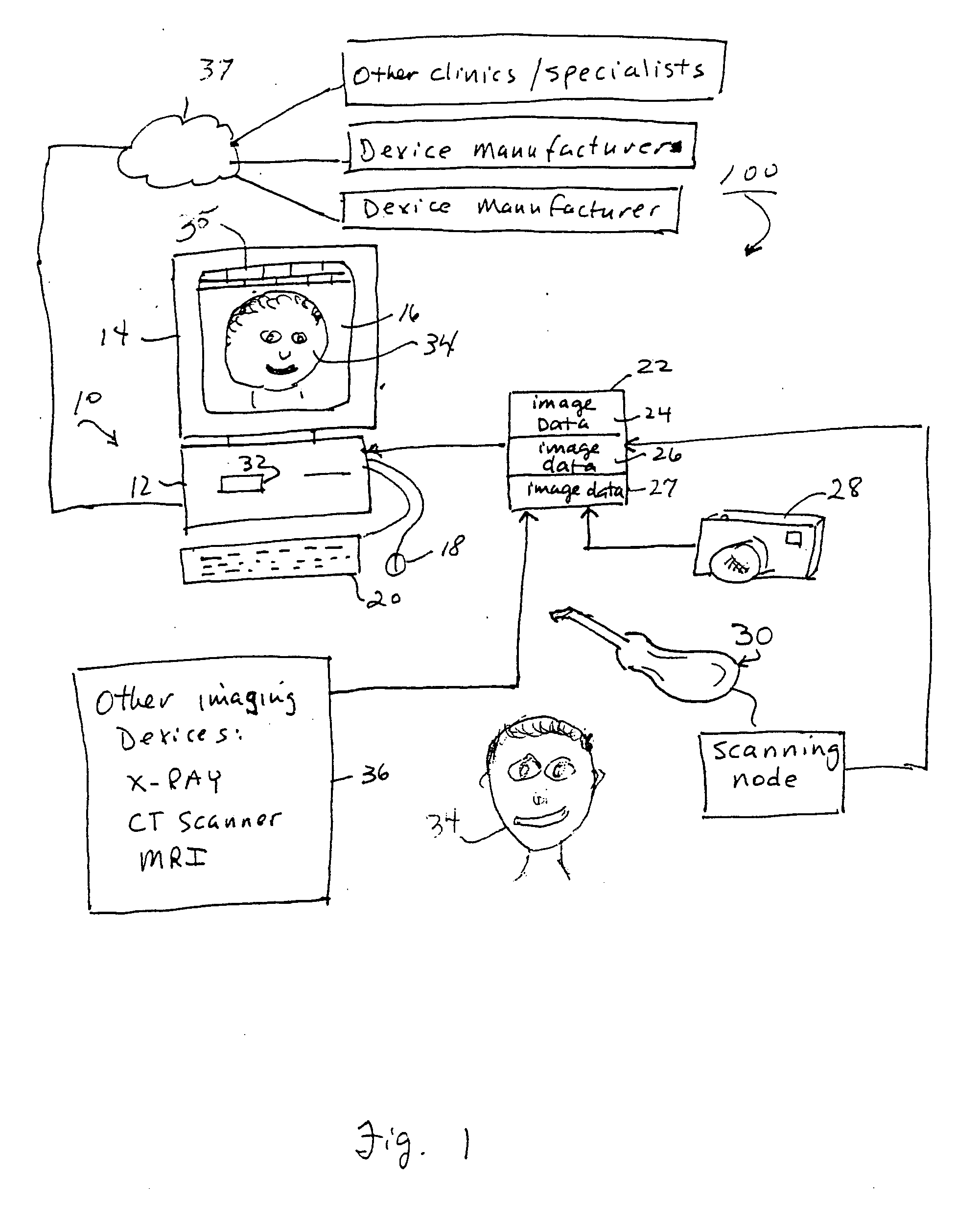
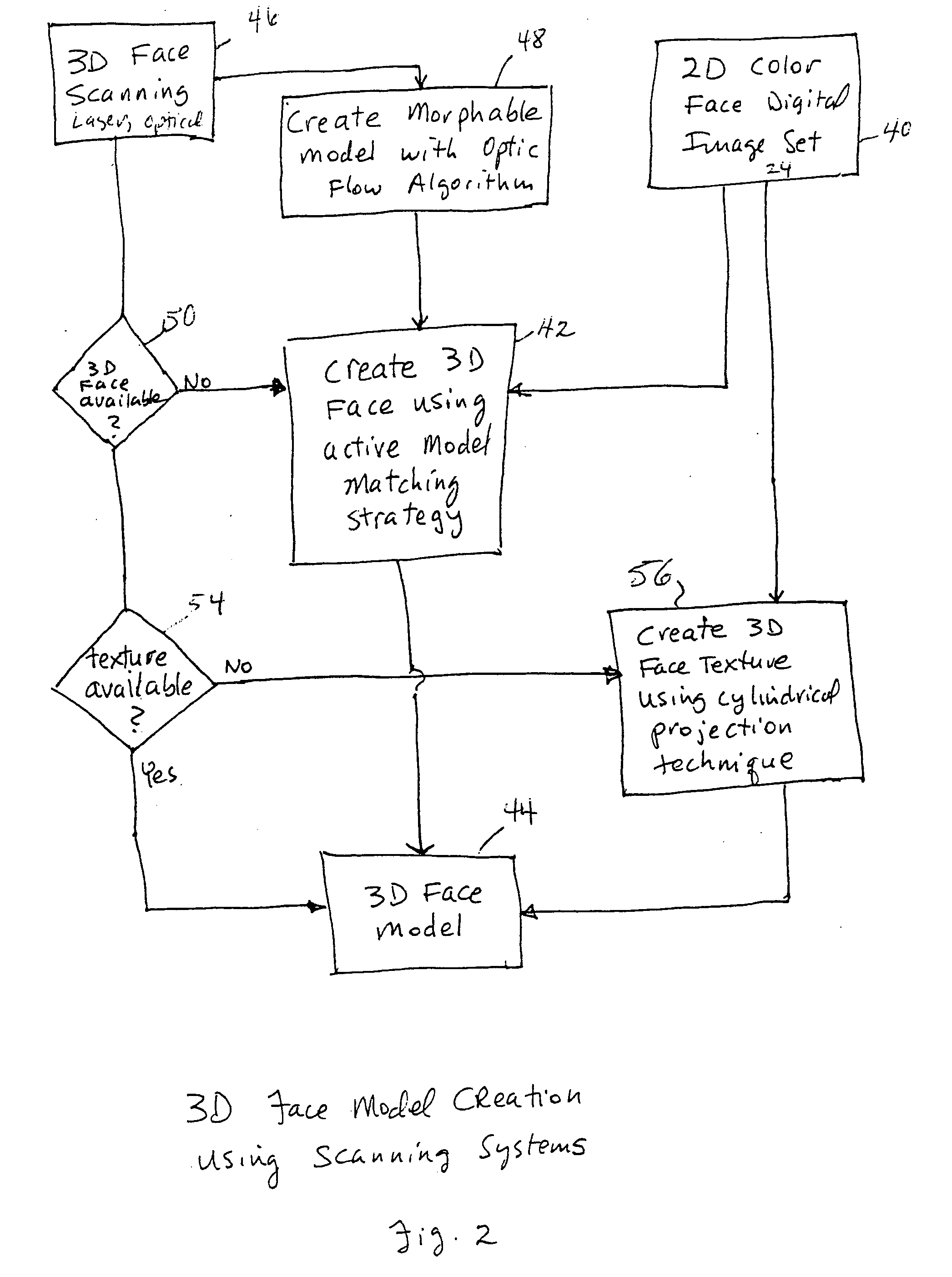
Unified workstation for virtual craniofacial diagnosis, treatment planning and therapeutics
An integrated system is described in which digital image data of a patient, obtained from a variety of image sources, including CT scanner, X-Ray, 2D or 3D scanners and color photographs, are combined into a common coordinate system to create a virtual three-dimensional patient model. Software tools are provided for manipulating the virtual patient model to simulation changes in position or orientation of craniofacial structures (e.g., jaw or teeth) and simulate their affect on the appearance of the patient. The simulation (which may be pure simulations or may be so-called “morphing” type simulations) enables a comprehensive approach to planning treatment for the patient. In one embodiment, the treatment may encompass orthodontic treatment. Similarly, surgical treatment plans can be created. Data is extracted from the virtual patient model or simulations thereof for purposes of manufacture of customized therapeutic devices for any component of the craniofacial structures, e.g., orthodontic appliances.
Owner:ORAMETRIX
Method and apparatus for the surgical treatment of congestive heart failure
ActiveUS7758491B2Effective of treatingReduce manufacturing costSuture equipmentsAnnuloplasty ringsSurgical treatmentSystole
An apparatus implantable in a heart ventricle includes a frame configured to engage an inner circumferential periphery of the ventricle and to expand and contract between an expanded state corresponding to a desired end diastolic diameter of the ventricle and a contracted state corresponding to a desired end systolic diameter of the ventricle. A bistable structure is operatively associated with the frame. The bistable structure mechanically assists movement of the ventricle toward both an end systolic diameter during systole and an end diastolic diameter during diastole. The bistable structure may be integrally formed of the frame. A method of implanting the apparatus in a heart ventricle includes surgically accessing a ventricle, inserting the apparatus in the ventricle and attaching the device to a portion of myocardium defining an inner circumferential periphery of the ventricle.
Owner:GENESEE BIOMEDICAL
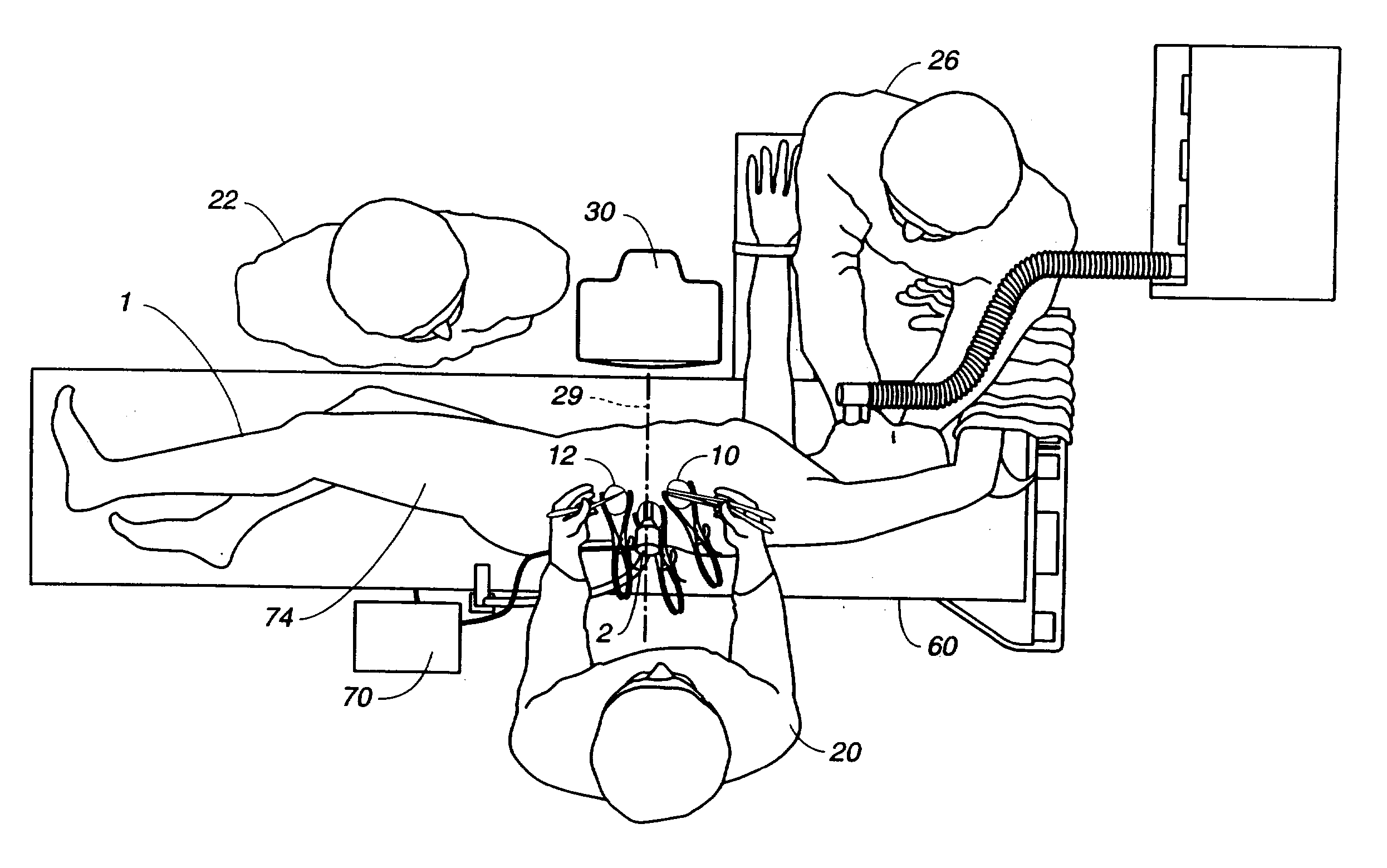
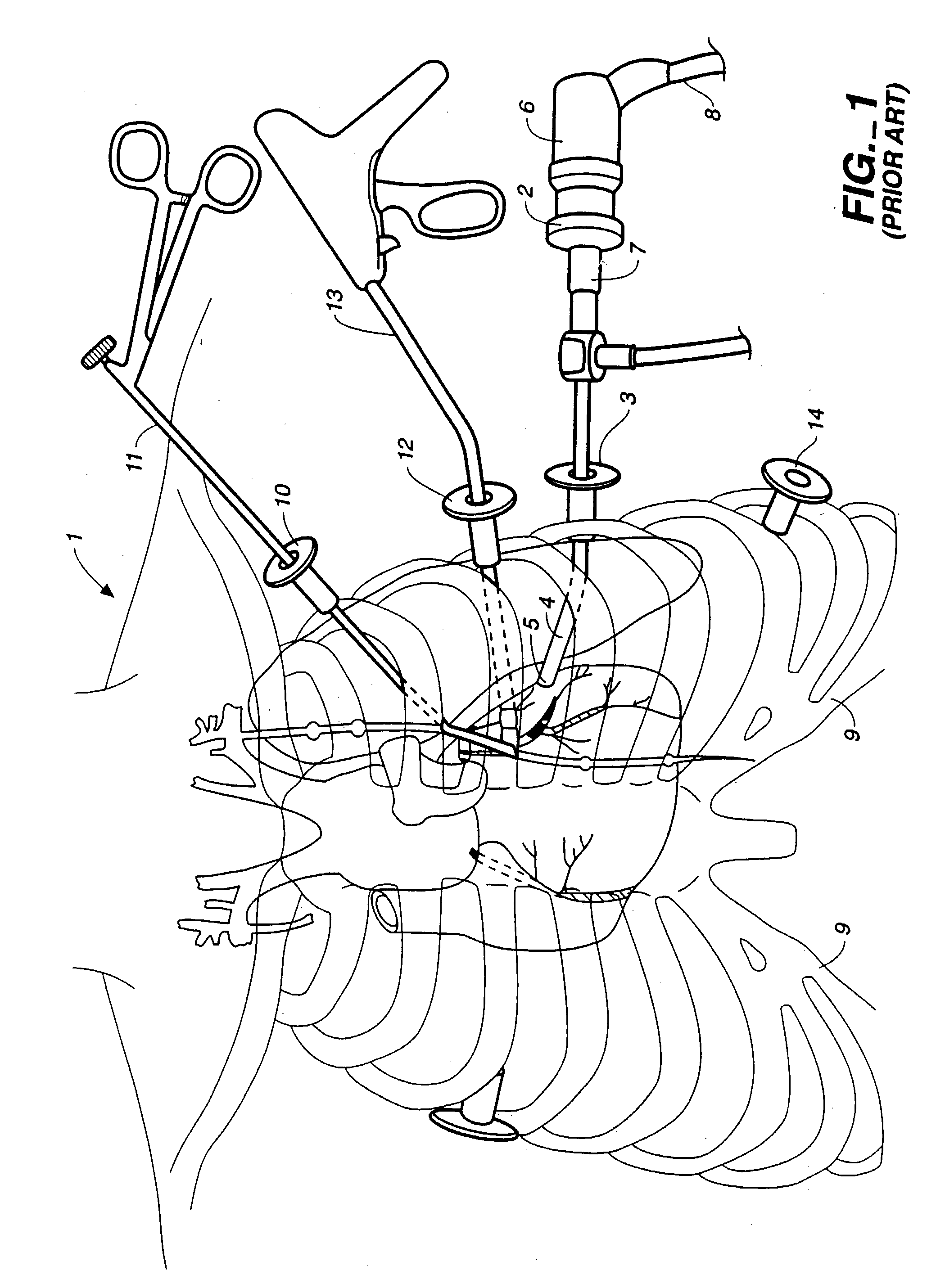
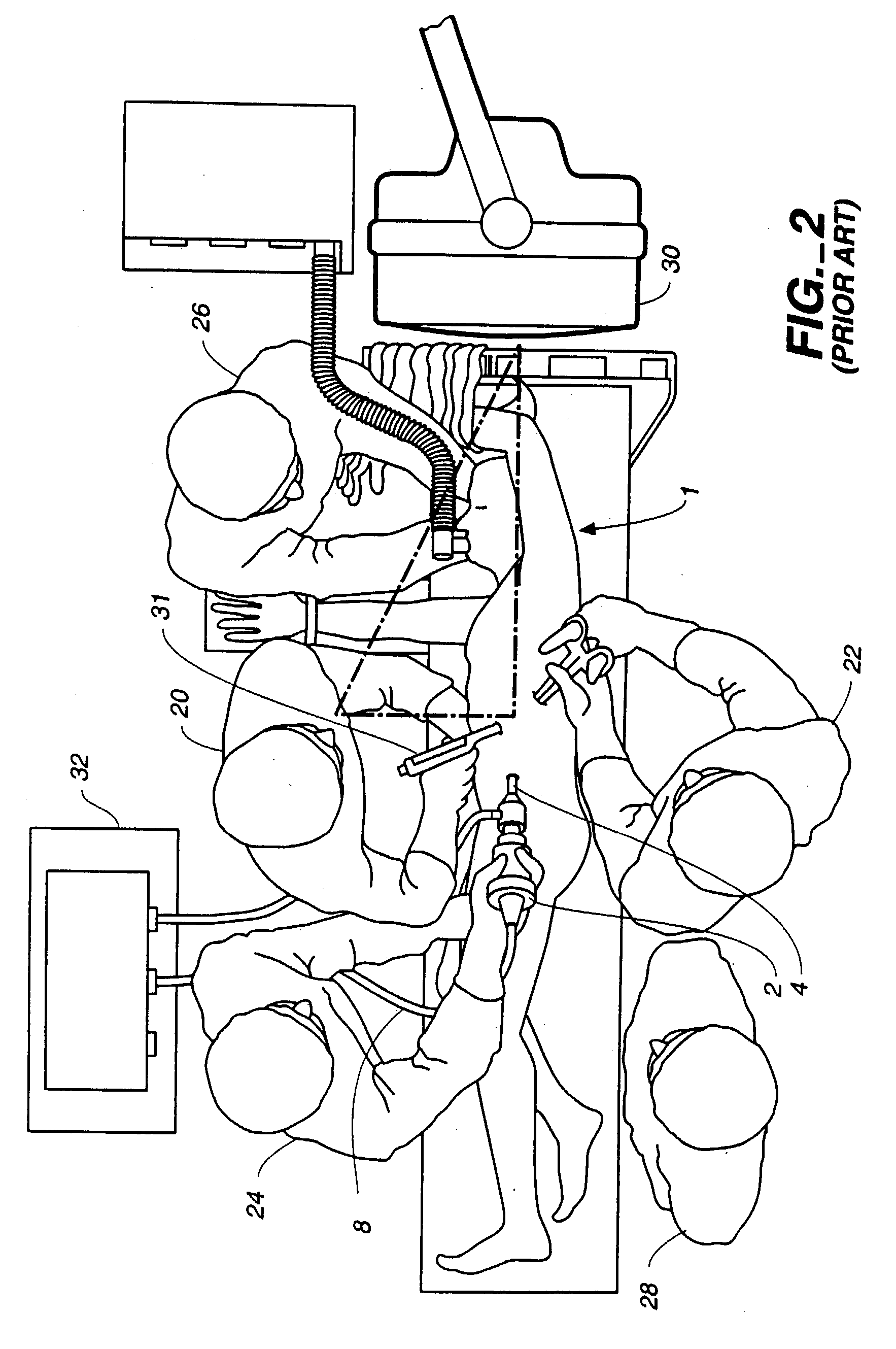
Visualization during closed-chest surgery
InactiveUS20030153810A1Easy alignmentMinimize scopeSuture equipmentsTelevision system detailsOperating theatresEngineering
An improvement in a method for closed-chest, video-assisted diagnostic or surgical treatment of a patient is provided. The improvement comprises draping a video monitor with a transparent sterile surgical drape and positioning the draped monitor within the surgical field so that a surgeon can perform an internal surgical or diagnostic procedure and view it on the monitor to provide improved visual alignment for the surgeon. A flexible, sterile drape for covering the video monitor is disclosed. An apparatus for assisting the surgeon in performing closed-chest, video-assisted surgical or diagnostic treatment of a patient is disclosed which comprises a movable cabinet having an adjustable arm to which is affixed a video monitor that can be extended into the surgical field of an operating room to improve the visualization of the surgery performed by the surgeon. An improved operating table for assisting a surgeon in performing closed-chest, video-assisted surgical or diagnostic treatment of a patient is also disclosed along with endoscopic visualization apparatus and an improved design of a view scope that allows the doctor to get closer to a patient during the operation.
Owner:ATRICURE
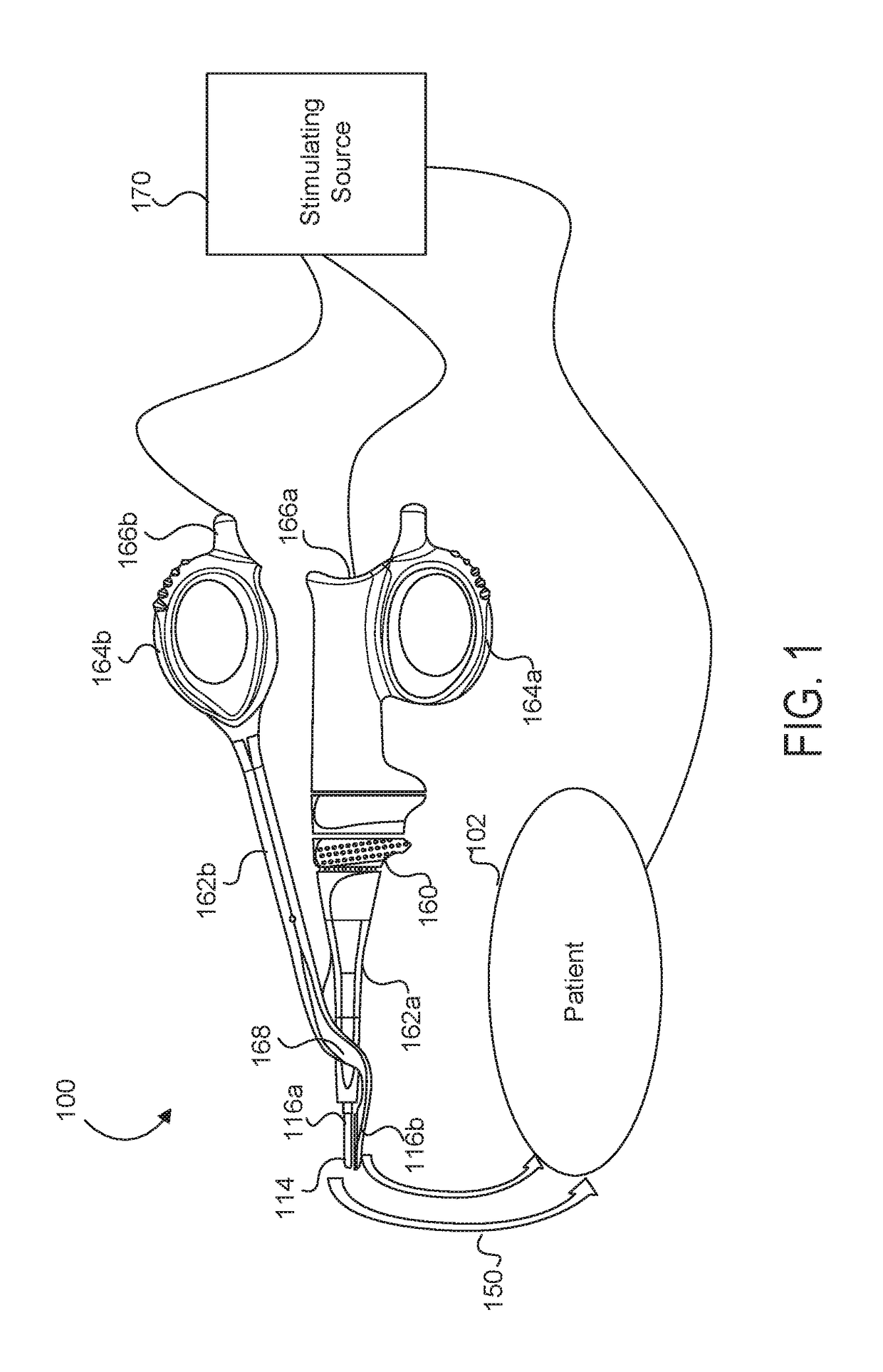
Medical device with a bilateral jaw configuration for nerve stimulation
ActiveUS20170319265A1Increase energy levelDecreased energy levelSpinal electrodesImplantable neurostimulatorsMedicineSacral nerve stimulation
Aspects of the present disclosure are presented for a single surgical instrument configured to grasp, seal, and / or cut tissue through application of therapeutic energy, and also detect nerves through application of non-therapeutic electrical energy. A medical device may include two jaws at an end effector, used to apply therapeutic energy and to perform surgical procedures. The therapeutic energy may be in the form of ultrasonic vibrations or higher voltage electrosurgical energy. One of the jaws may be configured to cut tissue through application of the blade. In addition, one or both of the two jaws may be configured to apply nontherapeutic energy for nerve stimulation probing. The application of therapeutic energy may be disabled while the nontherapeutic nerve stimulation energy is applied, and vice versa. The nontherapeutic nerve stimulation energy may be applied to the use of one or more probes positioned near one or both of the jaws.
Owner:CILAG GMBH INT
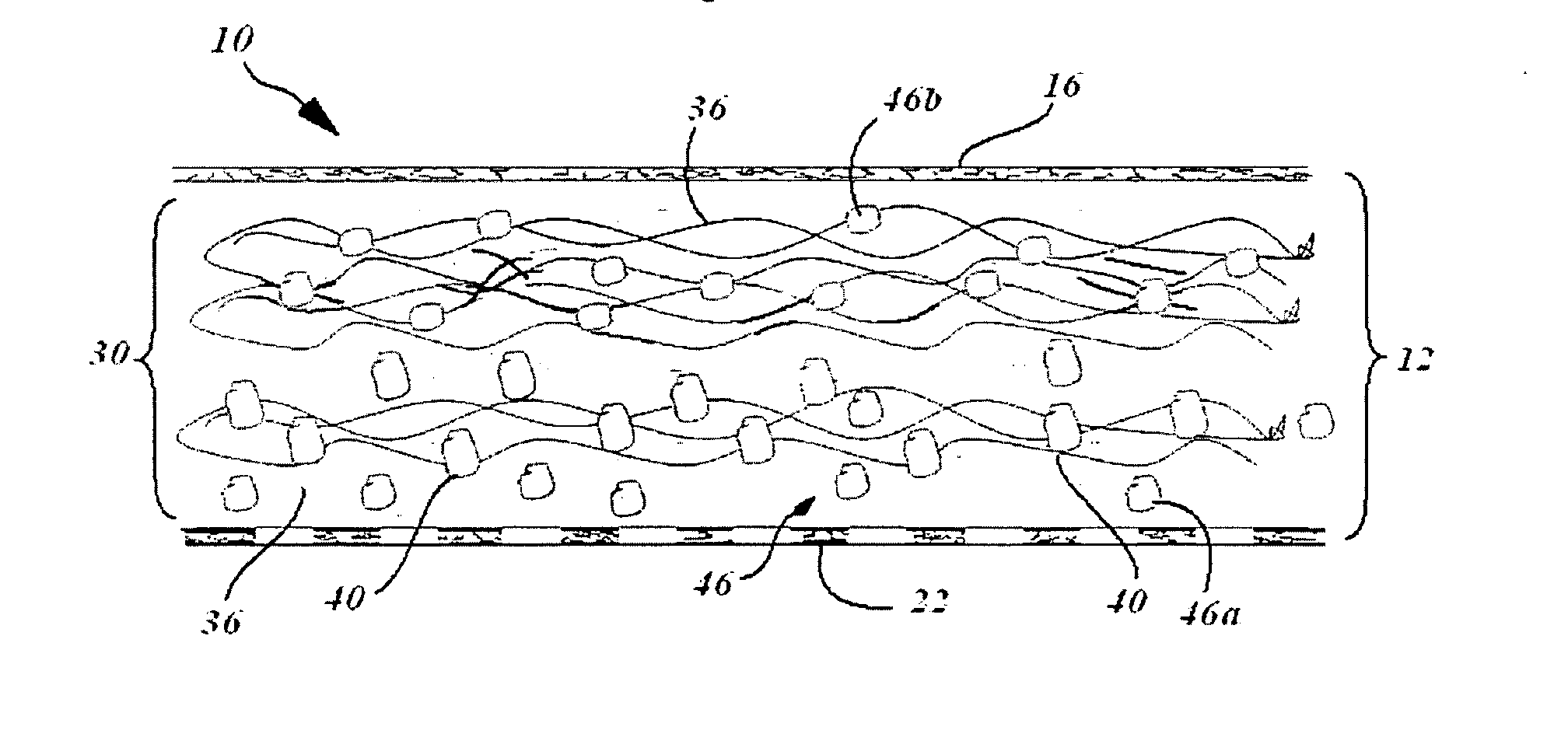
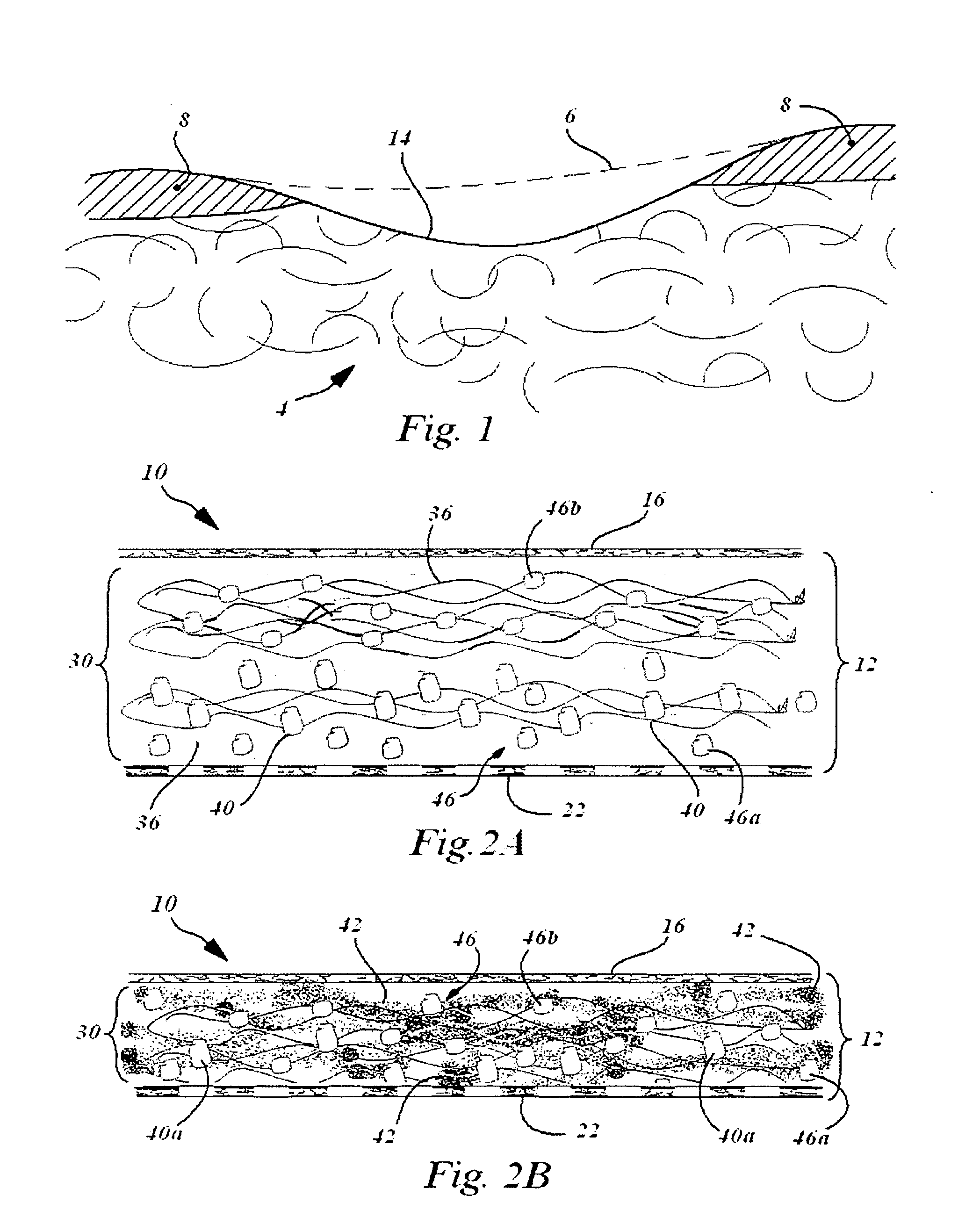
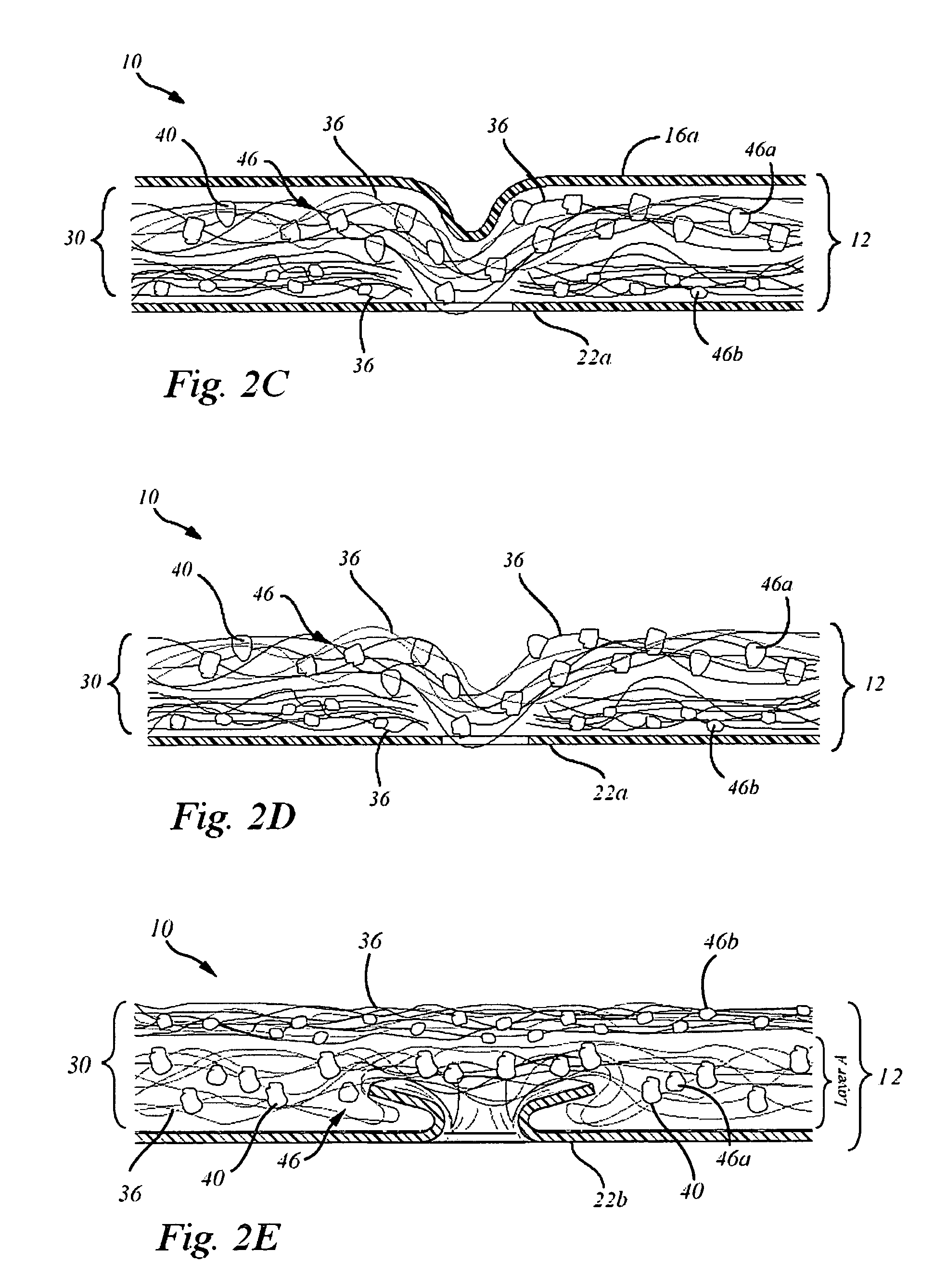
In situ system for intra-articular chondral and osseus tissue repair
Disclosed is a device and method which provide a surgical therapy for in situ treatment and repair of intra-articular cartilage lesions and / or defects. The device is an implantable laminate cartilage repair patch which is bio-compatible and physiologically absorbable. The cartilage repair patch has a first outer cell occlusive layer; a second outer, cell porous layer adapted to be disposed proximate a subchondral bone wound site; and a cartilagenic matrix disposed between the first and second layers. The cartilagenic matrix is a sink for diffusion of autologous stem cells and includes chemical components promoting generation of hyaline-like cartilage in the presence of the autologous stem cells. The method of the present invention provides the autologous compositions, which when used in combination with the repair patch provides a therapeutic system to regenerate replacement hyaline-like intraarticular cartilage.
Owner:LABE MEDIDOM
Antientity tumour medicinal composition containing topoenzyme inhibitor
A composite medicine for treating tumor by locally putting it in the tumor is composed of the active anticancer components (topoenzyme inhibitor and its symergist chosen from taxol-type anticancer medicnie, antineoplastic antibiotic and antimetabolitic medicine) and the medicinal auxiliary (biocompatible and biodegradable high-molecular polymer).
Owner:DASEN BIOLOGICAL PHARMA CO LTD
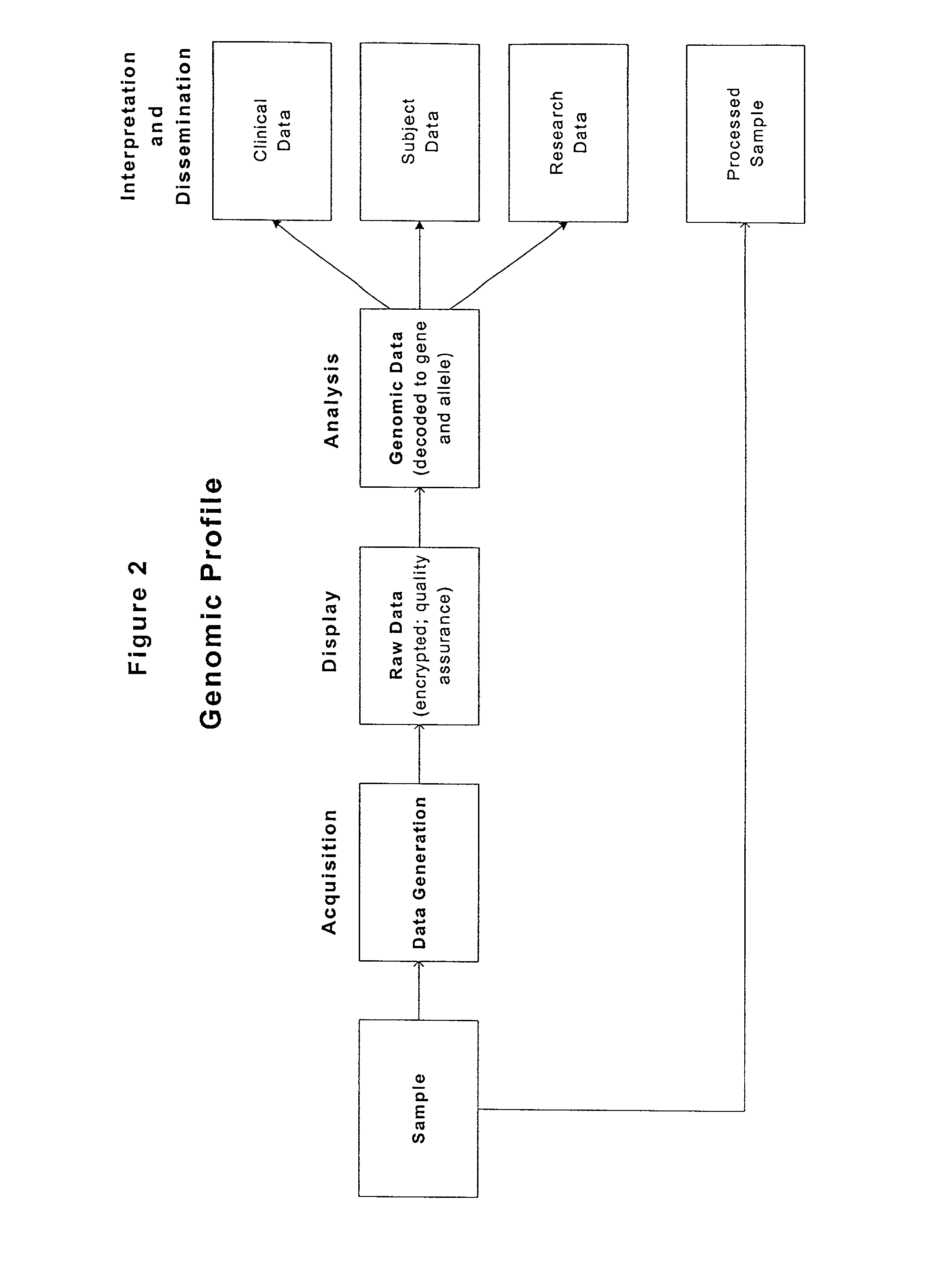
Methods and compositions for perioperative genomic profiling
InactiveUS20020110823A1Decrease timeTime- and cost-effectiveMicrobiological testing/measurementRecombinant DNA-technologyDrugPerioperative
The present invention relates to methods for perioperative genomic screening of subjects, in particular to perioperative screening for markers indicative of responses to anesthesia and other perioperative or operative treatments and procedures. The present invention also provides compositions for use in screening methods. The methods and compositions of the present invention find use in tailoring a subject's medical or surgical treatment to reflect genetic information that predicts a subject's response to medications or techniques used in the procedure.
Owner:HOGAN KIRK
Crosslinked elastin and process for producing the same
InactiveUS7125960B2Prevent disengagementPowder deliveryHydrolasesAdhesion processRegeneration tissue
A crosslinked elastin, a water-soluble crosslinking agent to be used for crosslinking, molded elastin articles, medical instruments and regeneration tissues using the crosslinked elastin, and a surgical therapy method and regeneration treatment wherein the medical instruments are employed. There is provided a biocompatible functional material having elasticity suitable for transplantation into the body without causing detachment of cell adhesion proteins.
Owner:JNC CORP
Controlled enzymatic removal and retrieval of cells
Method and device, including a uniquely operative applicator, and pharmaceutical compositions, for the controlled, non-surgical removal and retrieval of cells from a variety of skin lesions and tissue surfaces are disclosed. A synergistic effect of proteolytic digestion of the intracellular matrix and "stripping" flow is achieved by treating a defined area with a controlled, continuous stream of proteolytic enzyme solution, causing gentle but effective tissue erosion. Isolated cells from the skin lesion and / or tissue surface may be collected from the protease solution stream for histological analysis and / or cell culture, affording a method of "enzymatic biopsy". The protease solution may be supplemented with anesthetics, coagulants, anticoagulants and antibiotics to decrease the discomfort, erythema, bleeding, risk of infection and scarring traditionally associated with surgical treatment of skin lesions. Delivery of precise levels of catalytic activity is ensured by controlled activation of stable, inactivated enzyme stock solutions and powders shortly prior to application.
Owner:RAMOT AT TEL AVIV UNIV LTD
Anticarcinogenic internal implant agent
InactiveCN1679950APharmaceutical delivery mechanismAmide active ingredientsTreatment effectWhole body
An anticancer in vivo implant applied locally is prepared from the anticancer nitrosourea-type medicine, the anticancer synergist chosen from nitrogen mustard compound, mitotic inhibitor and antimetabolic anticancer medicine, and the biocompatible and biodegradable high-molecular polymer as medicinal additive.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
Adjuvant immune therapy in the treatment of solid tumors through modulation of signaling pathways following engagement of humoral and cell mediated responses
InactiveUS20020187130A1Evaluating efficacyAccurate assessmentBiocideSaccharide peptide ingredientsAdjuvantWhite blood cell
Owner:KINDNESS GEORGE +2
Device for Removing Tissue
A device for removing tissue by means of laparoscopy or thoracoscopy with an operating part and an optionally partially open hollow tube with a guide for operating a removing part. The removing part has the form of a zeppelin with one or more ribs. The removing part is provided on the upper side with longitudinal connecting elements.Further described is an application of such a device in a surgical treatment wherein, by use of the operating part, the removing part with the form of a zeppelin is opened out, the removed tissue is collected in the expanded bag of the removing part, and the removing part with tissue is pulled optionally at least partially into the open part of the hollow tube. The connecting elements of the removing part can then be brought together and snapped or zipped closed.
Owner:JANSEN ANTON
Combination of slow released anticancer medication
InactiveCN1660437AAddress sensitivityOvercoming toxicityAntineoplastic agentsPharmaceutical active ingredientsWhole bodyTherapeutic effect
A slowly-releasing anticancer composite medicine is composed of the hormone-kind anticancer medicine chosen from steroid-type hormone and hormone antagon for regulating the cell reproduction of hormone dependent tumor and the medicinal additive chosen from biocompatible and biodegradable high-molecular polymer for slowly releasing said anticanser medicine toward tumor.
Owner:SHANDONG LANJIN PHARMA +1
Anticancer sustained release agent containing epothilone
InactiveCN1969816AEasy injectionIncrease drug concentrationOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolDepressant
Disclosed is an anti-cancer drugs slow release agent containing Epothilone which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer active constituents include Epothilone, Epothilone derivatives, Epothilone B, Epothilone D and combination of anti-cancer drugs selected from phosphoinositide-3-kinase inhibitor, of pyrimidine analogues and / or DNA restoring enzyme inhibitor, the slow release auxiliary materials include polylactic acid and its copolymer, polyethylene glycol, PLA-COOH copolymer, di-aliphatic acid and sebacylic acid copolymer, poly(erucic aciddipolymer-sebacylic acid), poly(fumaric acid-sebacylic acid), Polifeprosan, polylactic acid and other biocompatible high polymers, the viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose. The anticancer active constituents and the slow release microspheres can also be prepared into slow release implanting agent for intra-tumor or around-tumor injection or placement for the effective suppression of tumor growth and for the appreciable enhancement for curative effects of non-operative treatments such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Anticancer sustained release injection containing epothilone derivatives
InactiveCN1969818AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismAdjuvantMicrosphere
Disclosed is an anti-cancer slow release injection containing Epothilone derivatives which comprises slow release microspheres and dissolvent, the slow release micro-balloons include anticancer drugs selected from Paclitaxel, alkyl agent and / or plant alkaloids, Epothilone derivatives and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The Epothilone derivatives are selected from Epothilone B, Epothilone D, Isoepothilone D, BMS-247550, azaepothilone B, furaepothilone D or BMS-310705. The slow release auxiliary material is selected from poly-D, L-lactic acid and its glycolic copolymer, polyethylene glycol and polylactic acid copolymer, PLA-COOH copolymer, aliphatic acid and sebacylic acid copolymer, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose, The slow release microspheres can also be prepared into slow release implanting agent, for injection or placement in or around tumor with the period of local drug release can be about 40-50 days, as a result, the local medicinal concentration can be increased selectively, and the treatment effect of the non-operative treatment methods such as chemotherapy can be improved substancially.
Owner:JINAN SHUAIHUA PHARMA TECH
Robotized installation for the positioning and movement of a component or instrument and treatment device that comprises such an installation
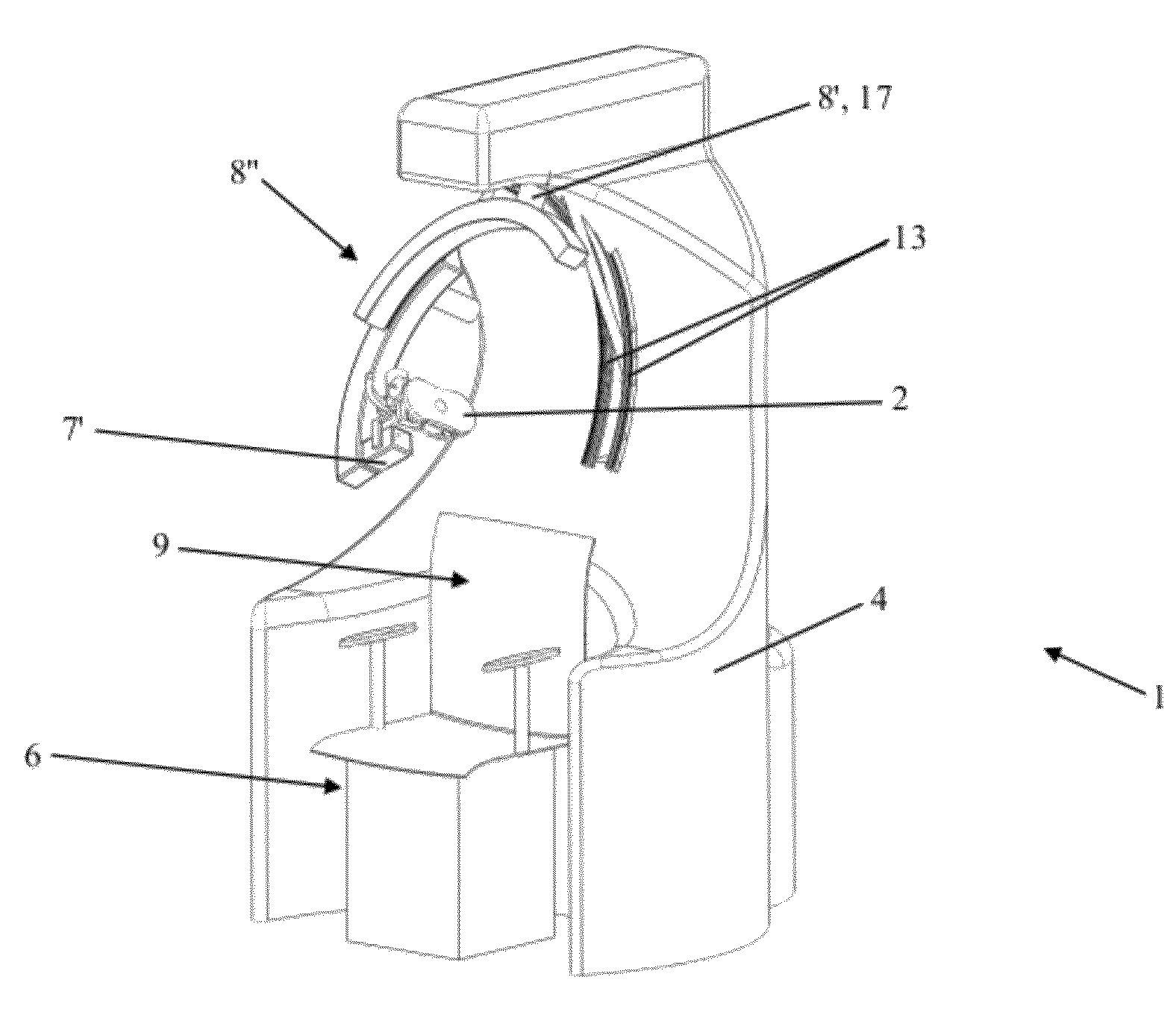
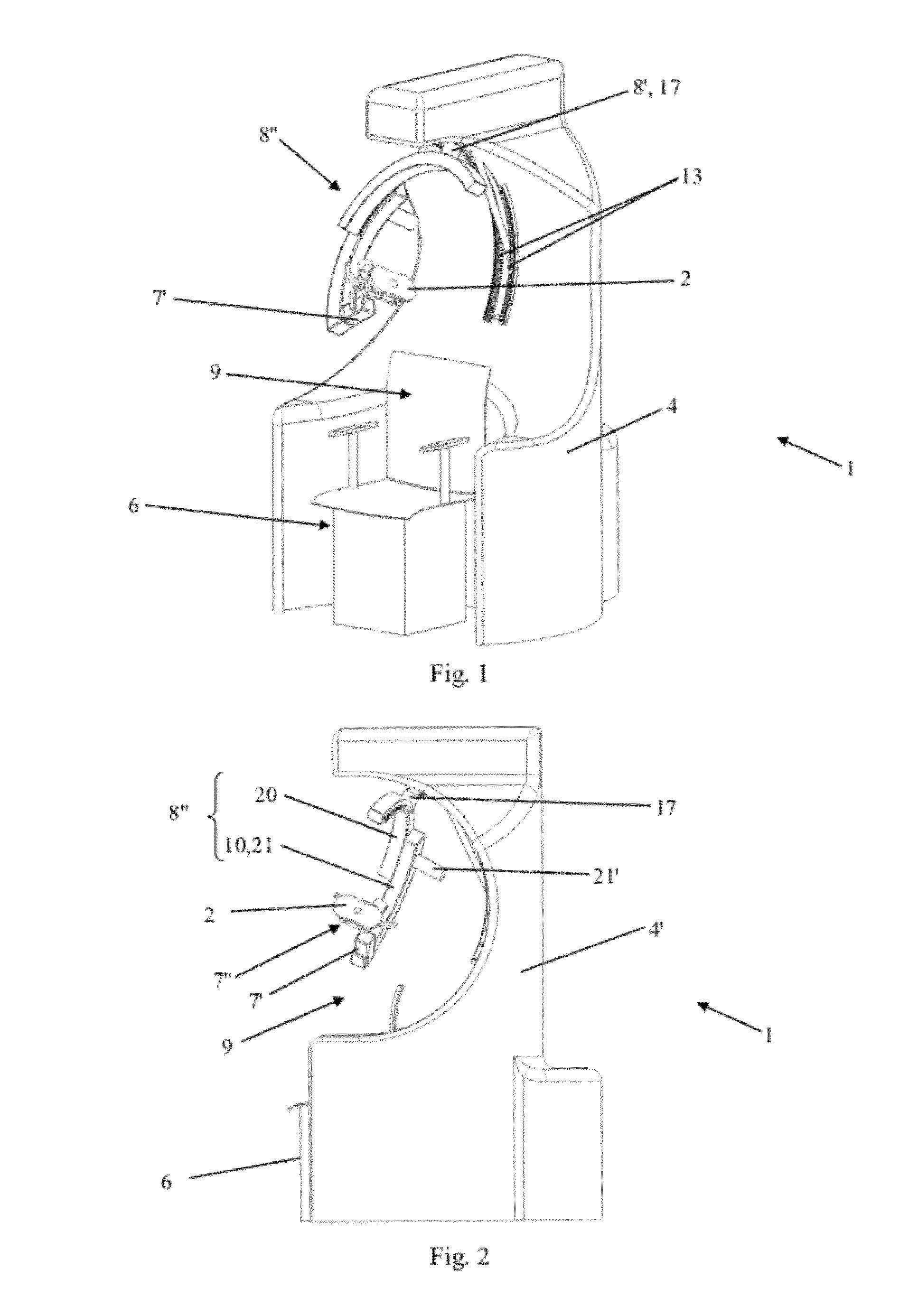
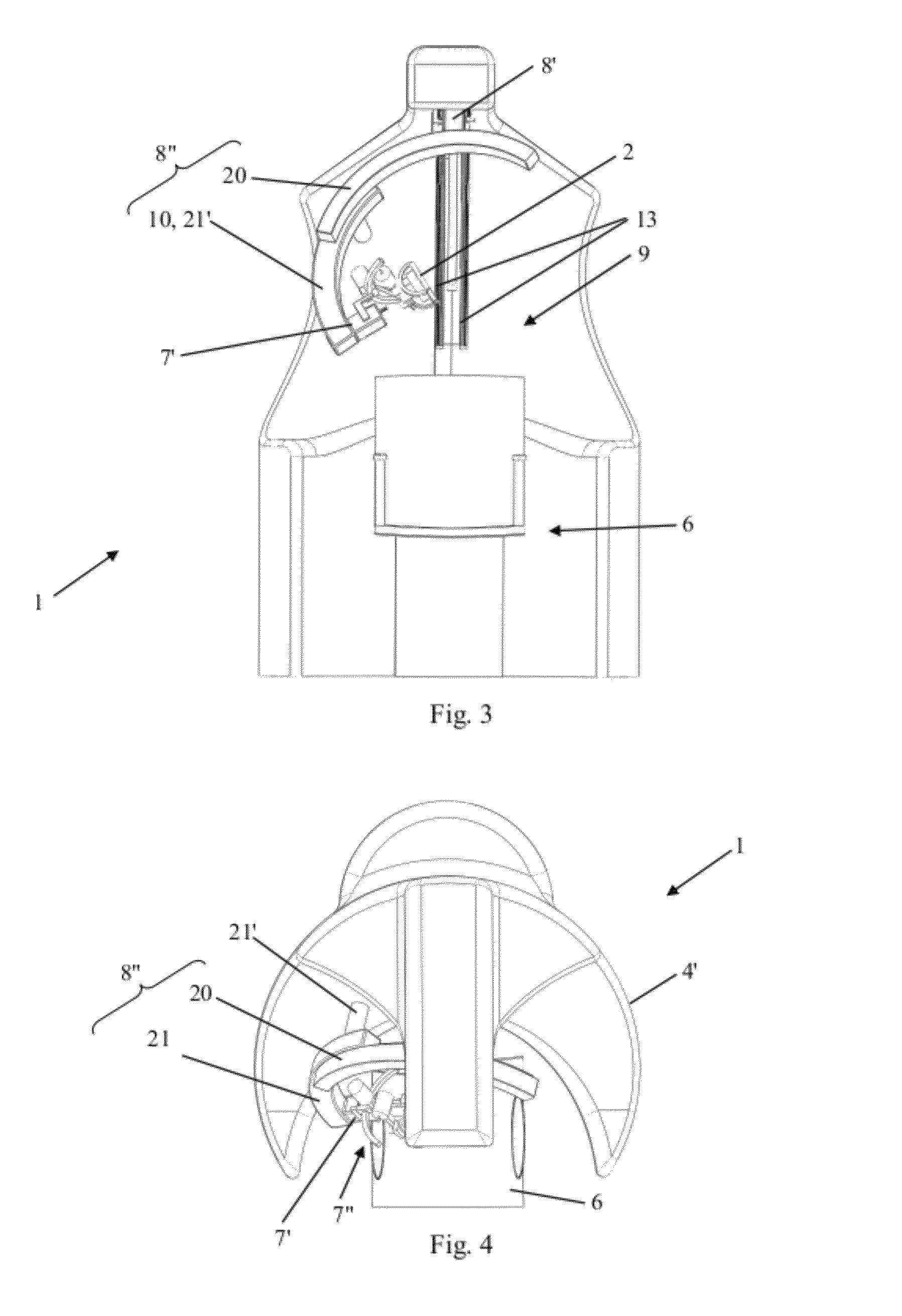
ActiveUS8303478B2Easy to controlElectrotherapyMagnetotherapy using coils/electromagnetsSurgical treatmentThree degrees of freedom
Robotized installation for the guided and controlled positioning and movement of a component or an instrument for diagnostic or surgical treatment at or around the head of a patient, whereby the installation includes a robotic device that forms a serial kinematic chain and carries component or instrument at its free and position-controlled end. The robotic device has three kinematic sub-assemblies that are mutually combined in series and include, a first sub-assembly in the form of a rotary-articulation mechanism corresponding to a serial-type, spherical kinematic arrangement with three degrees of freedom, a second sub-assembly in the form of a mechanism with linear translation along an axis, and a third sub-assembly in the form of a second rotary-articulation mechanism, integral with the moving part of the second sub-assembly and also corresponding to a serial-type spherical kinematic arrangement with three degrees of freedom.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Pharmaceutical composition for solid tumour
InactiveCN1634017AImprove anti-cancer effectIncrease and prolong concentrationAntineoplastic agentsHeavy metal compound active ingredientsTreatment effectAdjuvant
Disclosed is a pharmaceutical composition for solid tumour which comprises anticancer available composition and medicinal adjuvant, the anticancer available composition is platinum compounds, and the medicinal adjuvant mainly employs biological compactable, degradable and absorbable macromolecular polymer. The composition can lower down the whole body toxicity reaction of the medicament when locally dispensing on the tumor, selectively increase the tumor local medicinal concentration, and improve the treatment effect of the non-operative treatment methods such as chemotherapy, medicament and radiation.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
Focused ultrosound therapy combined array element phased array and multifocal shear wave imaging system
InactiveCN101690677AOvercome the disadvantage of limited focus scanning areaIncrease in sizeSurgical instruments for heatingArray elementPhased array transducer
The invention relates to a focused ultrosound therapy combined array element phased array and multifocal shear wave imaging system for focused ultrosound non-invasive surgical therapy, belonging to the technical field of biomedical instrument, in particular to a combined array element phased array transducer used for multifocal focused ultrosound therapy and radial force excitation imaging and also relates to a multifocal plane shear wave imaging system by using the combined array element phased array transducer. In the transducer, the combined array element is formed by compactly placing 2*2 or 3*3 basic matrix array elements in rows according to spherical rectangle mode, all basic matrix array elements in each combined array element are in parallel electrically and share one power driven channel; a basic matrix array element distance is left between the centers of two adjacent combined array elements along at least one direction, and each combined array element is matched with the phase and amplitude of one power driven channel so as to significantly expand the focal scanning area.
Owner:XI AN JIAOTONG UNIV
Implantable distractor whose length can be modified without reoperation and which has a j-shape
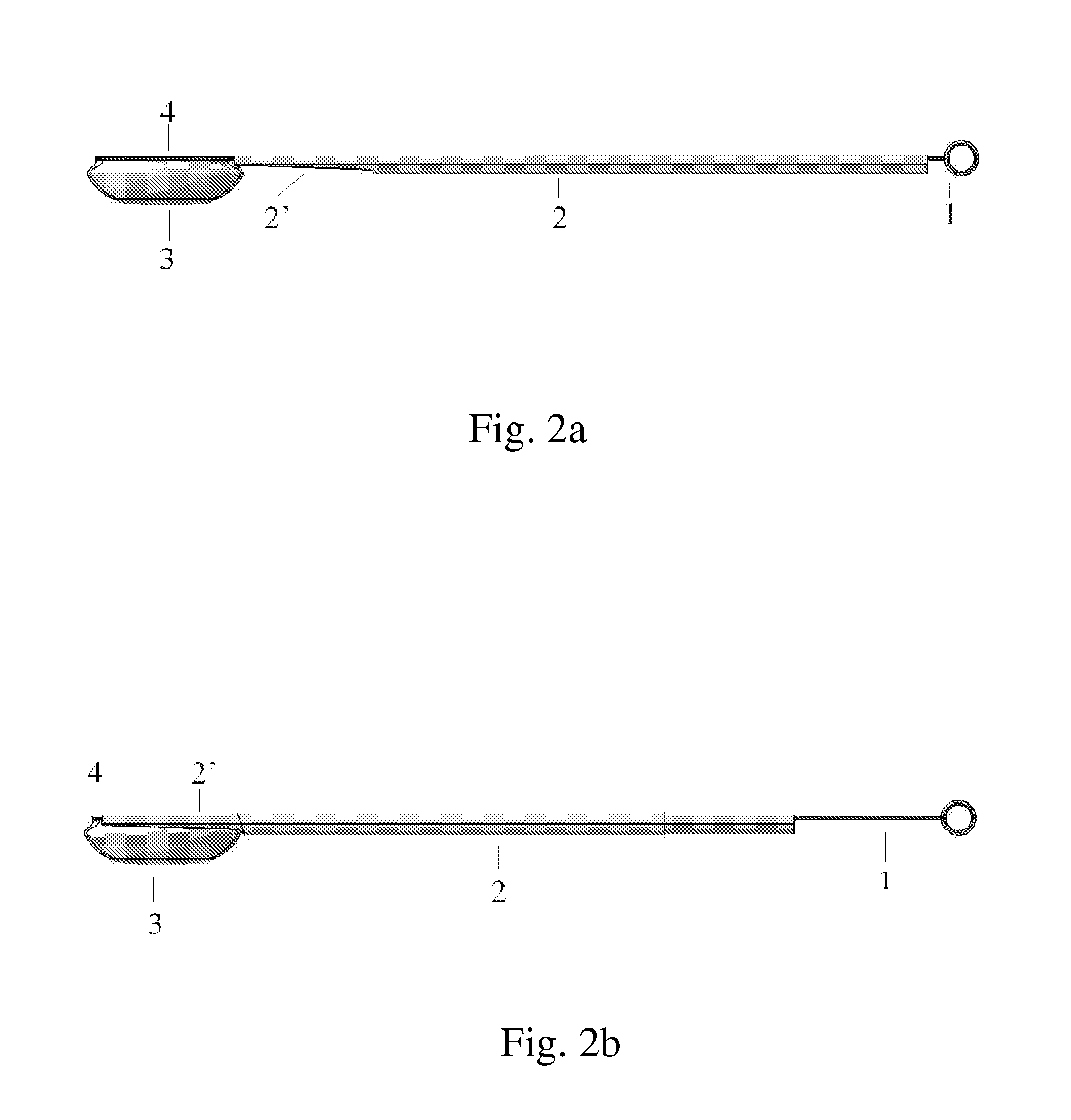
InactiveUS20100280519A1Easy to implantGood muscular coverageInternal osteosythesisProsthesisBiological bodyDistraction
This distraction device comprises a rod (1), a second rod (2) and means to control the translation of one rod relative to the other (3), so as to cause the desired distraction or compression. Each rod (1) and (2) has a first end, respectively (11) and (21), which receive means to link to a part of the organism such as hooks or screws for vertebra, ribs or the pelvis, and a second end, respectively (12) and (22), linked to the means to control the translation of one rod relative to the other (3) such that the direction of the first end (11) toward the second end (12) of the first rod (1) is roughly the same as the direction of the first end (21) toward the second end (22) of the second rod (2). The distraction device thus roughly forms a J, the bulging and rigid part of which is located outside the area concerned by the surgical treatment, facilitating its implantation. It is useful for straightening of the spine and bone lengthening, deformity correction or transport, depending on the means used to link to the organism.
Owner:SOUBEIRAN ARNAUD
Medicine for treating child hernia
InactiveCN102335270AFree from painEliminate inconvenienceDigestive systemSexual disorderSurgical operationTangerine Seed
The invention belongs to the technical field of traditional Chinese medicine, and particularly relates to a medicine for treating child hernia. The medicine consists of the following components in part by weight: 3 to 8 parts of ligusticum wallichii, 4 to 8 parts of fructus evodiae, 3 to 8 parts of bitter orange, 4 to 8 parts of cimicifuga foetida, 4 to 8 parts of fennel, 4 to 8 parts of cinnamon, 7 to 12 parts of tangerine seed, 7 to 12 parts of lychee seed, 7 to 12 common fenugreek seed, 7 to 12 parts of combined spicebush root, and 7 to 12 parts of costustoot. The child hernia can be treated by a Chinese medicinal external compression method, and pain and inconvenience caused by surgical operation can be eliminated, and the medicine has a good curative effect; meanwhile, the medicine has a good curative effect on recurrent hernia caused after the hernia is treated by operation.
Owner:尹立焱
Method for reducing biofilm formation
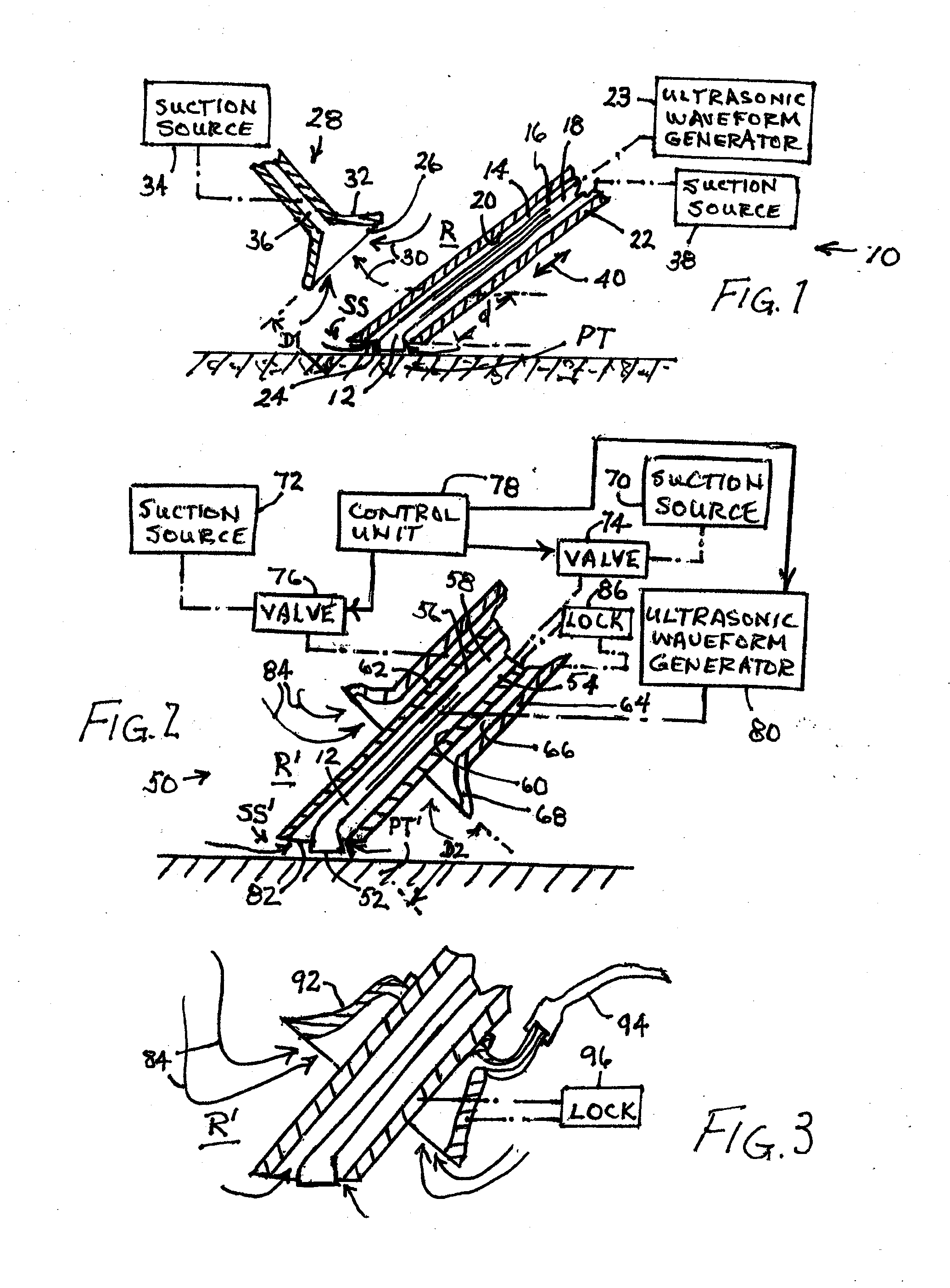
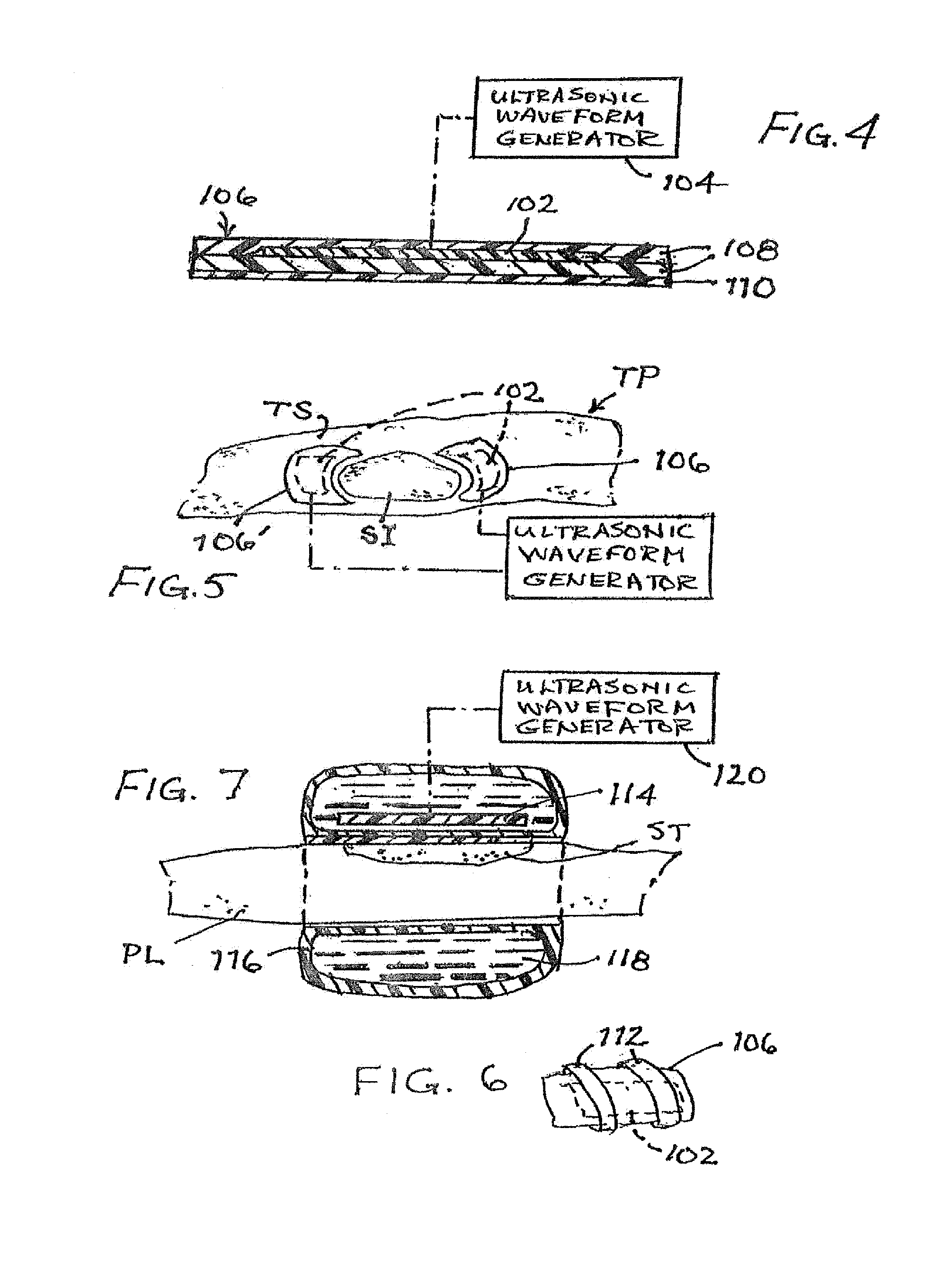
ActiveUS20160128708A1Reduce the possibilityEasy to placeSurgeryMedical devicesBiofilmSurgical treatment
A two phase method for reducing the formation of biofilm includes an evacuation of ambient air from a region about the surgical or treatment site, to extract airborne or aerosolized bacteria ejected from the site by the treatment. The extracted bacteria are prevented from settling back onto the cleansed tissue surface, thus at least reducing colonial bacteriological growth and concomitantly exuded biofilm material. A second phase involves the attachment of one or more ultrasonic transducers to the patient over or near a surgical treatment site after the surgery is terminated. Each applied ultrasonic transducer is used to vibrate the patient's tissues at the treatment site to disrupt biofilm formation.
Owner:MISONIX INC
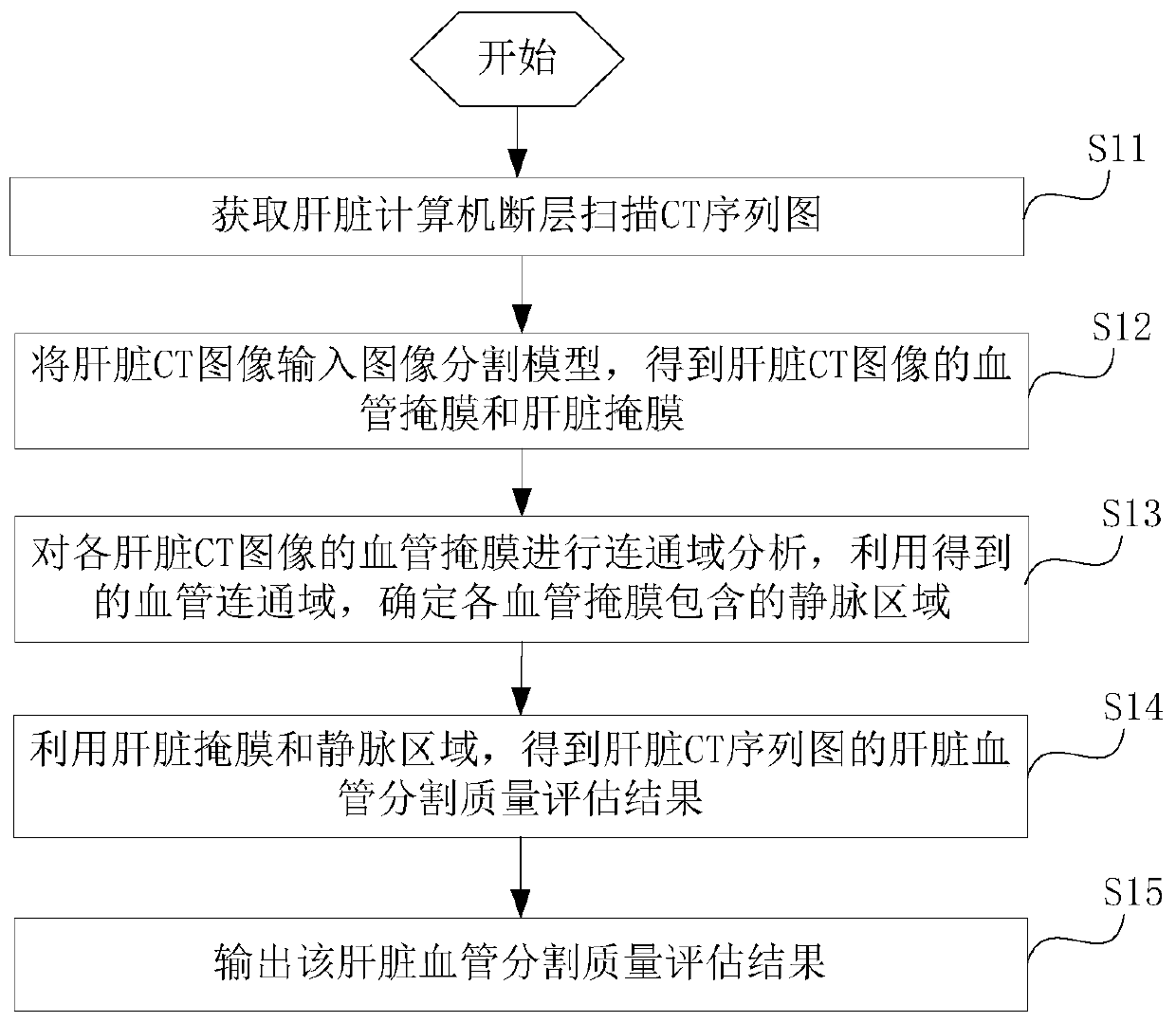
Liver image segmentation quality evaluation method and device and computer equipment
PendingCN111145206AAvoid diagnosis and treatmentImprove accessibilityImage enhancementImage analysisLiver ctLiver disease
The invention provides a liver image segmentation quality evaluation method and device and computer equipment. Inputting the acquired liver CT sequence diagram into an image segmentation model; obtaining a blood vessel mask and a liver mask contained in each liver CT image; carrying out connected domain analysis on each blood vessel mask; utilizing the obtained blood vessel connected domain; veinregions contained in the blood vessel masks are determined; and obtaining and outputting a liver blood vessel segmentation quality evaluation result of the liver CT sequence diagram acquired this timeby utilizing the determined liver mask and vein region. The medical personnel are informed of the liver blood vessel segmentation quality of the liver CT sequence diagram; therefore, it is avoided that under the condition that the liver blood vessel segmentation quality of the liver CT sequence diagram is poor, medical staff continue to refer to the liver CT sequence diagram to diagnose and treata patient, and the auxiliary effect of the liver auxiliary diagnosis system on liver disease diagnosis and surgical treatment is greatly improved.
Owner:LENOVO (BEIJING) CO LTD
Anticancer combination of medication
InactiveCN1660436AAddress sensitivityOvercoming toxicityAntineoplastic agentsPharmaceutical active ingredientsWhole bodyTherapeutic effect
An anticancer composition medicine is composed of the antimitosis medicine for suppressing the reproduction of tumor cells and the medicinal additive chosen from the biocompatible and biodegradable high-molecular polymer for slowly releasing said antimitosis medicine toward the tumor.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com