Acellular cartilage matrix and preparation method thereof
A kind of acellular cartilage and matrix technology, applied in medical science, tissue regeneration, prosthesis and other directions, can solve the problems of high immunogenicity and toxicity, poor mechanical properties of acellular cartilage matrix, etc., to reduce the dosage and improve the decellularization effect. , improve cytotoxicity or enhance immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
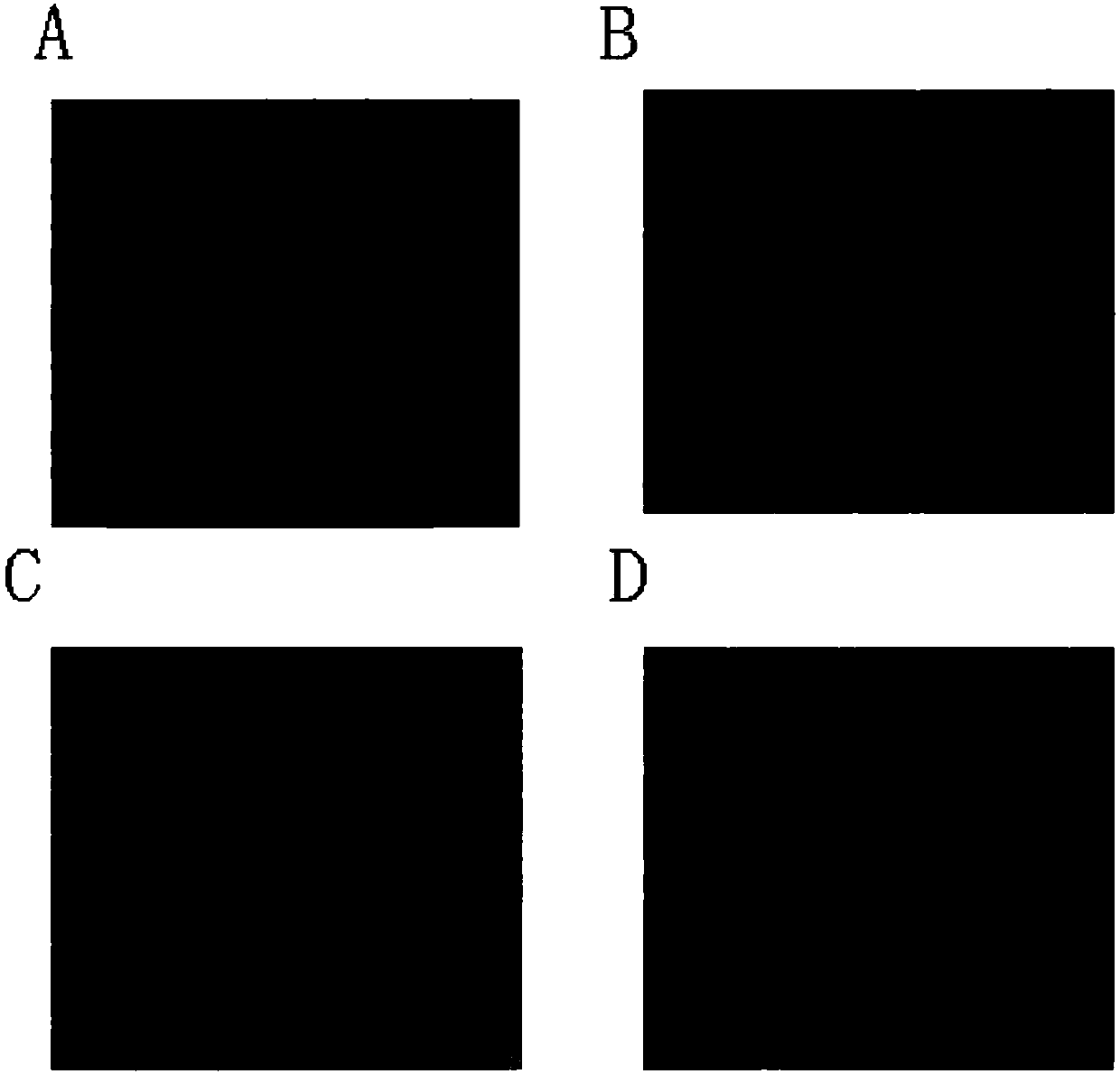
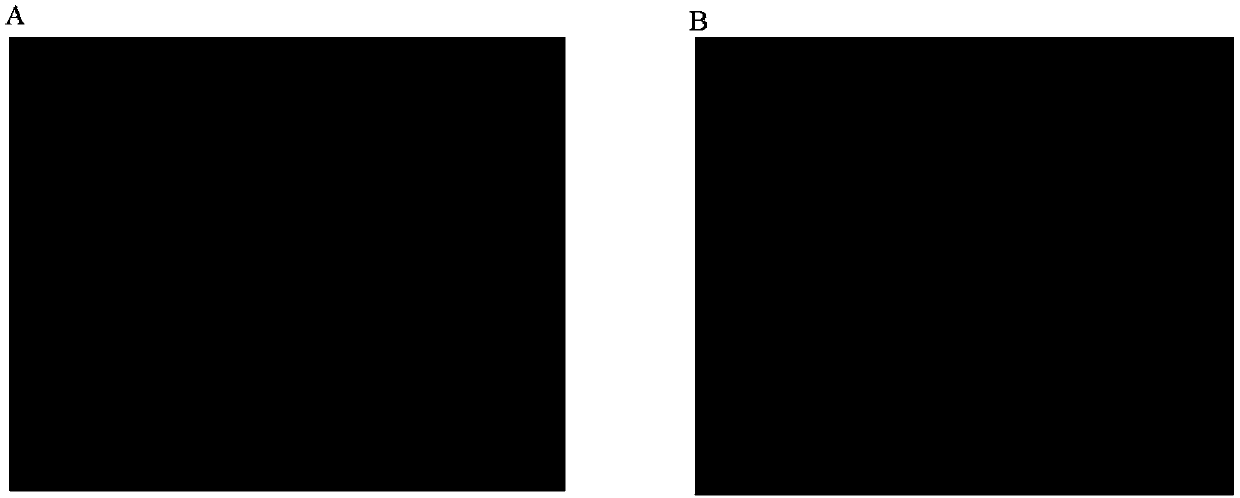
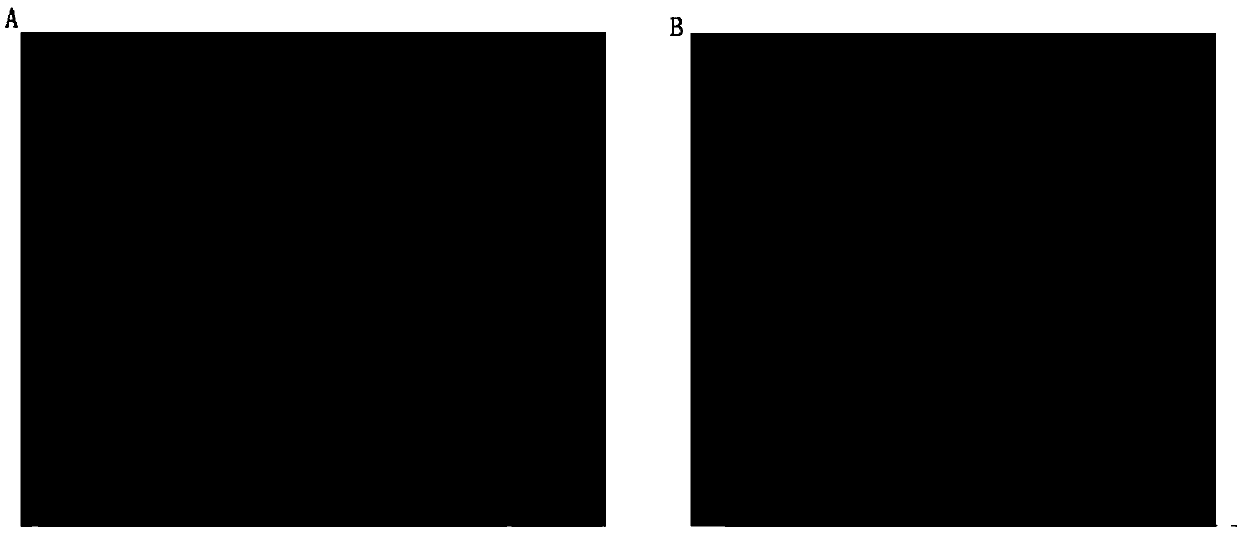
Examples
Embodiment 1
[0045] The preparation method of the decellularized cartilage matrix in this embodiment comprises the following steps:
[0046] (1) Pre-cleaning treatment: Take 800g of fresh cartilage tissue and add it to normal saline, soak it, then take out the cartilage tissue and add it to ultrapure water, ultrasonically clean it with an ultrasonic cleaner, and then rinse it continuously with ultrapure water.
[0047] (2) Repeated freezing and thawing treatment: the cartilage tissue was first frozen at -80°C for 3 hours, then thawed at room temperature for 4 hours, and repeated freezing and thawing 4 times.
[0048] (3) Hypotonic treatment: add cartilage tissue to 10L Tris-Hcl solution that has been sterilized, and incubate at room temperature for 20 hours. Wherein, the pH of the Tris-Hcl solution is 8, and the concentration is 10 mM.
[0049] (4) Trypsin treatment: put the cartilage tissue into a trypsin solution, and heat in a water bath at 37° C. for 8 hours. Wherein, the trypsin sol...
Embodiment 2
[0059] The preparation method of the decellularized cartilage matrix in this embodiment comprises the following steps:
[0060] (1) Pre-cleaning treatment: Take 1 kg of fresh cartilage tissue and add it to normal saline, soak it, then take out the cartilage tissue and add it to ultrapure water, ultrasonically clean it with an ultrasonic cleaner, and then rinse it continuously with ultrapure water.
[0061] (2) Repeated freezing and thawing treatment: the cartilage tissue was first frozen at -80°C for 2 hours, then thawed at room temperature for 6 hours, and repeated freezing and thawing 6 times.
[0062] (3) Hypotonic treatment: add cartilage tissue into sterilized Tris-Hcl solution, and incubate at room temperature for 15 hours. Wherein, the pH of the Tris-Hcl solution is 8, the volume is 10 L, and the concentration is 10 mM.
[0063] (4) Trypsin treatment: the cartilage tissue was washed with ultrapure water, put into trypsin solution, and heated in a water bath at 37° C. f...
Embodiment 3
[0073] The preparation method of the decellularized cartilage matrix in this embodiment comprises the following steps:
[0074] (1) Pre-cleaning treatment: Take 500g of fresh cartilage tissue and add it to normal saline, soak it, then take out the cartilage tissue and add it to ultrapure water, ultrasonically clean it with an ultrasonic cleaner, and then rinse it continuously with ultrapure water.
[0075] (2) Repeated freezing and thawing treatment: the cartilage tissue was first frozen at -70°C for 4 hours, then thawed at 30°C for 1 hour, and repeated freezing and thawing twice.
[0076] (3) Hypotonic treatment: the cartilage tissue was added to a sterilized 8 mM Tris-Hcl solution, and incubated at room temperature for 30 h. Wherein, the pH of the Tris-Hcl solution is 8, and the volume is 10 L.
[0077] (4) Trypsin treatment: the cartilage tissue was washed with ultrapure water, put into trypsin solution, and heated in a water bath at 37° C. for 24 hours. Wherein, the tryp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com