Patents
Literature
133results about How to "Increase infectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
AAV vectors produced in insect cells
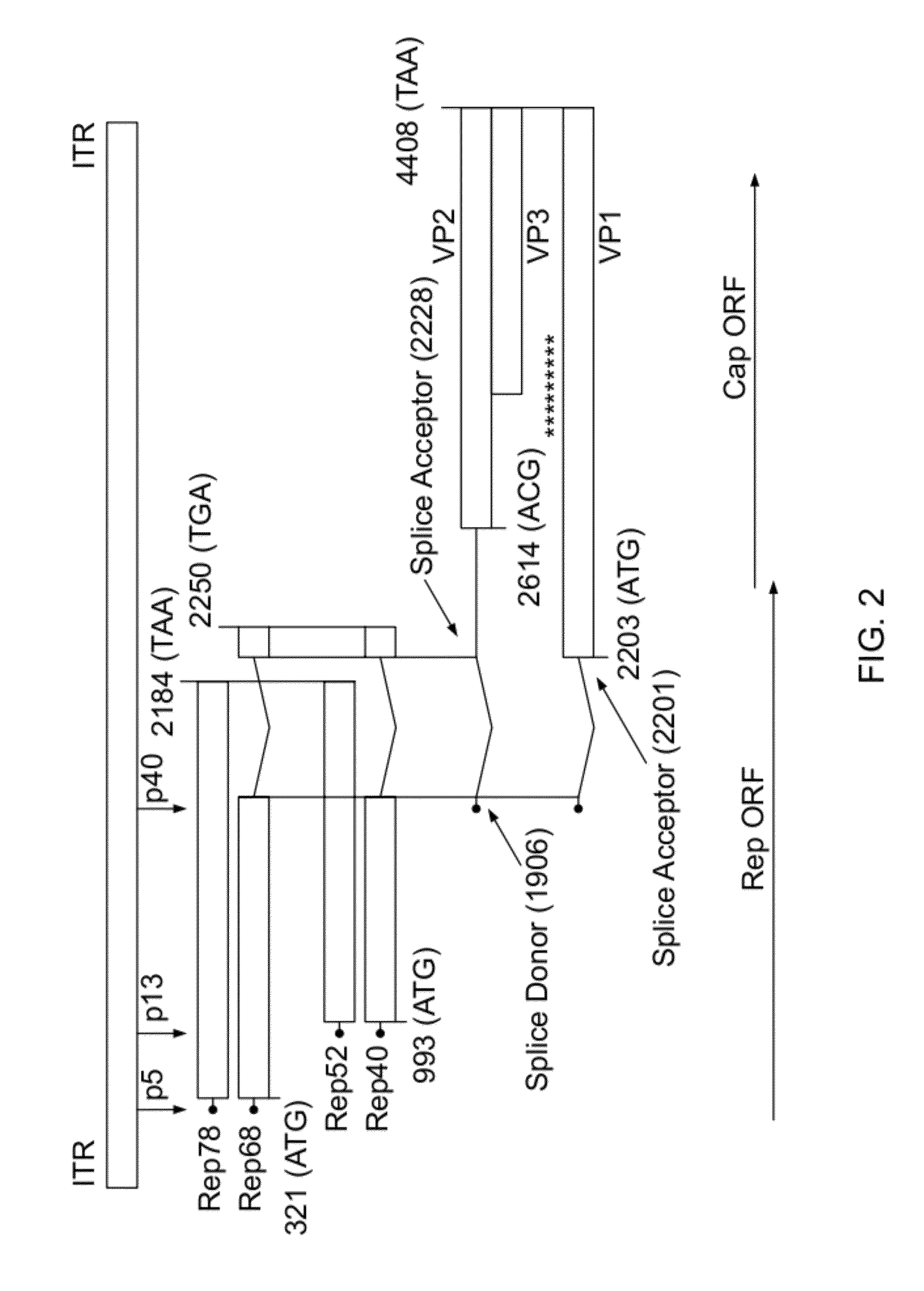
ActiveUS8163543B2Improve stabilityReduced expression levelAnimal cellsGenetic material ingredientsNucleotideViral vector
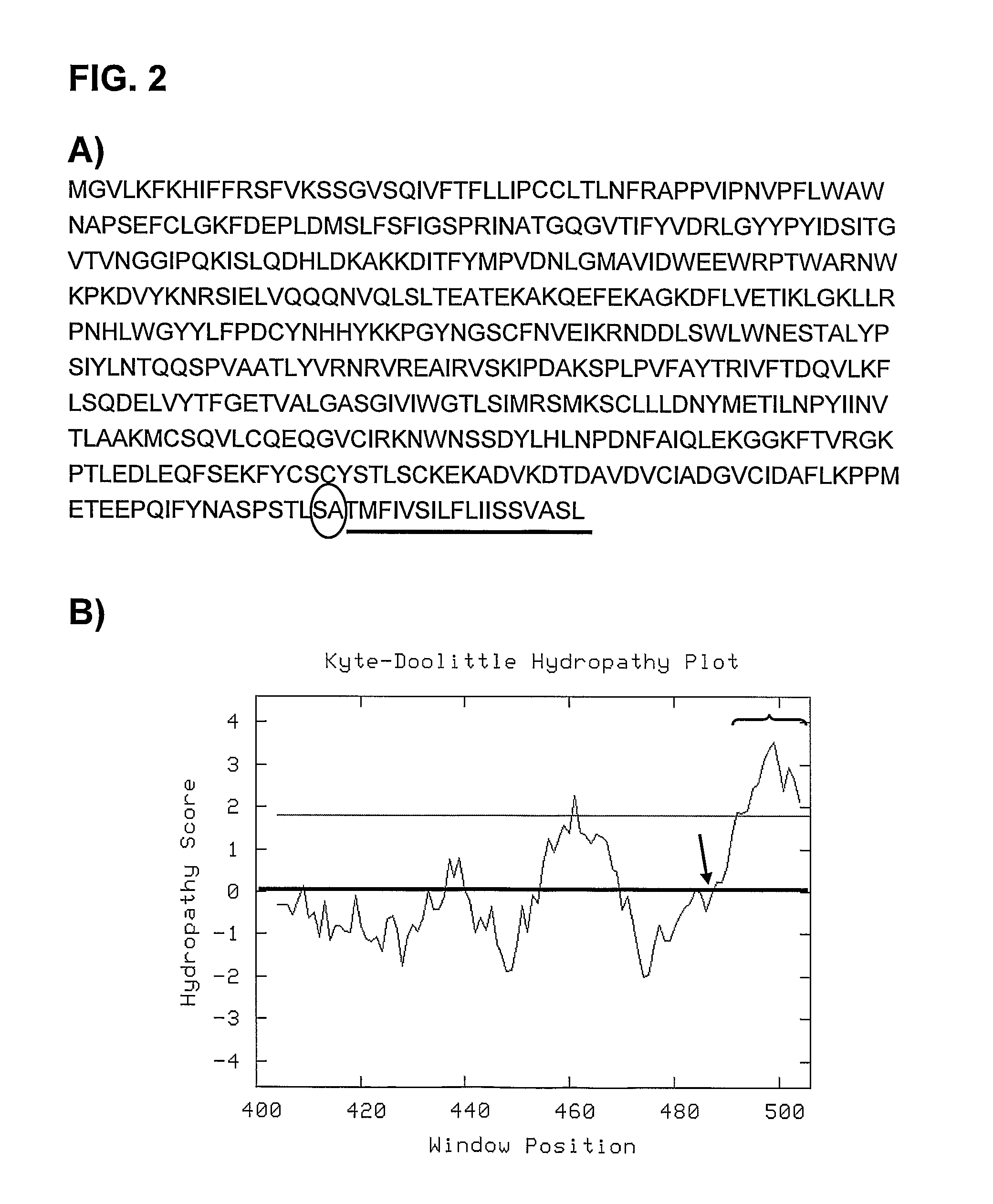
The present invention relates to the production of adeno-associated viral vectors in insect cells. The insect cells therefore comprise a first nucleotide sequence encoding the adeno-associated virus (AAV) capsid proteins, whereby the initiation codon for translation of the AAV VP1 capsid protein is a non-ATG, suboptimal initiation codon. The insect cell further comprises a second nucleotide sequence comprising at least one AAV inverted terminal repeat (ITR) nucleotide sequence; a third nucleotide sequence comprising a Rep52 or a Rep40 coding sequence operably linked to expression control sequences for expression in an insect cell; and, a fourth nucleotide sequence comprising a Rep78 or a Rep68 coding sequence operably linked to expression control sequences for expression in an insect cell. The invention further relates to adeno-associated viral vectors with an altered ratio of the viral capsid proteins that provides improved infectivity of the viral particles.
Owner:UNIQURE IP BV
Compositions and methods to prevent AAV vector aggregation
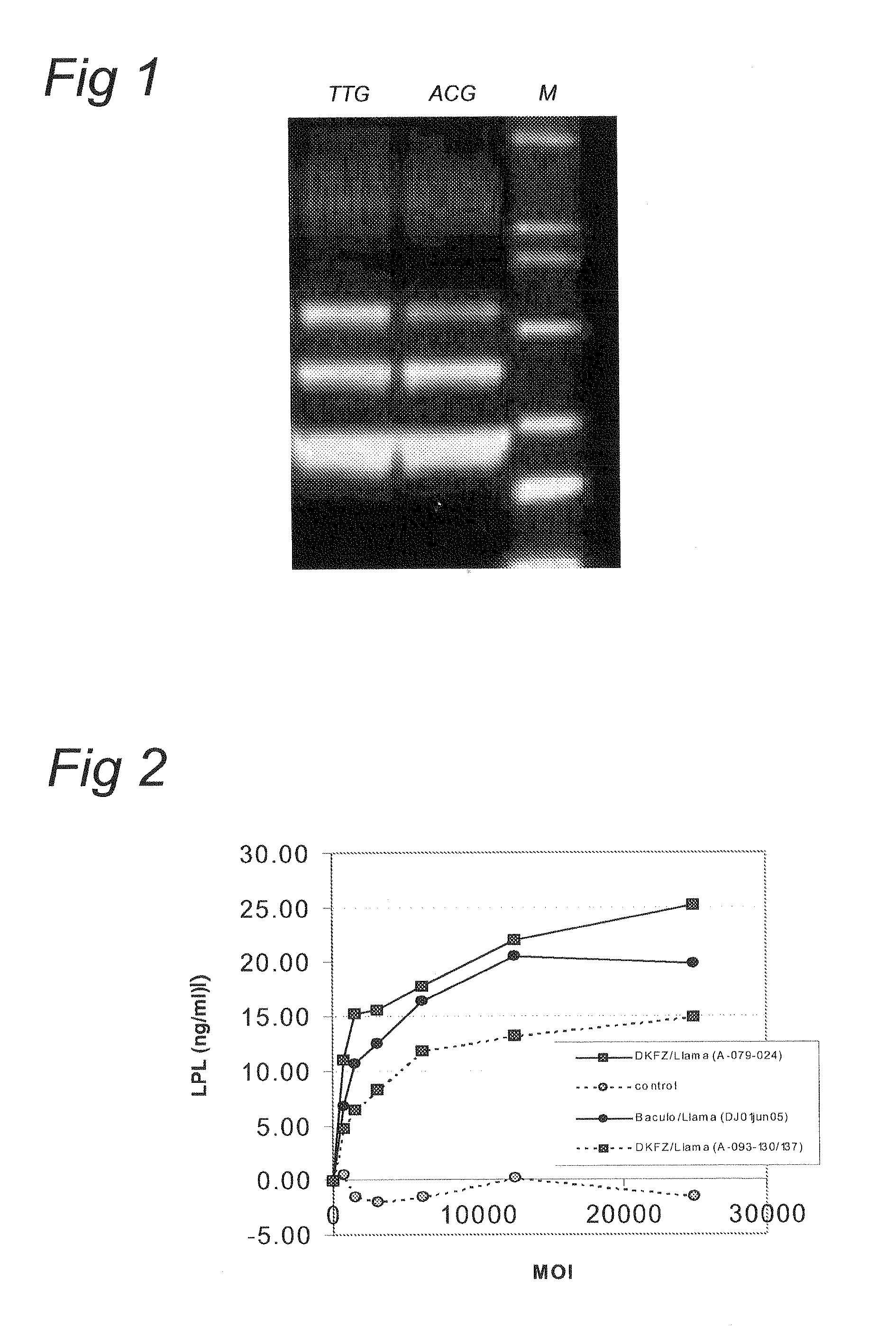
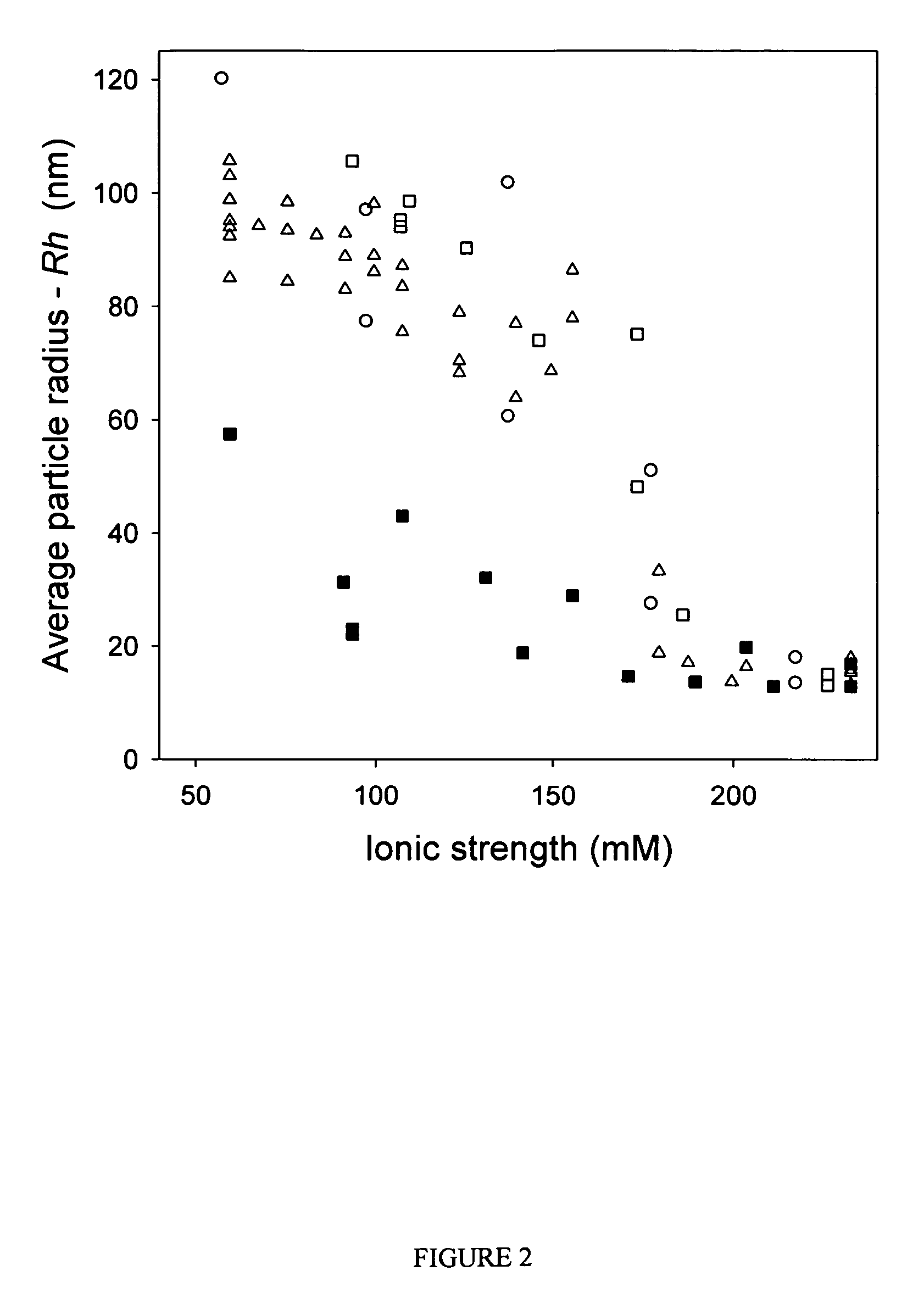
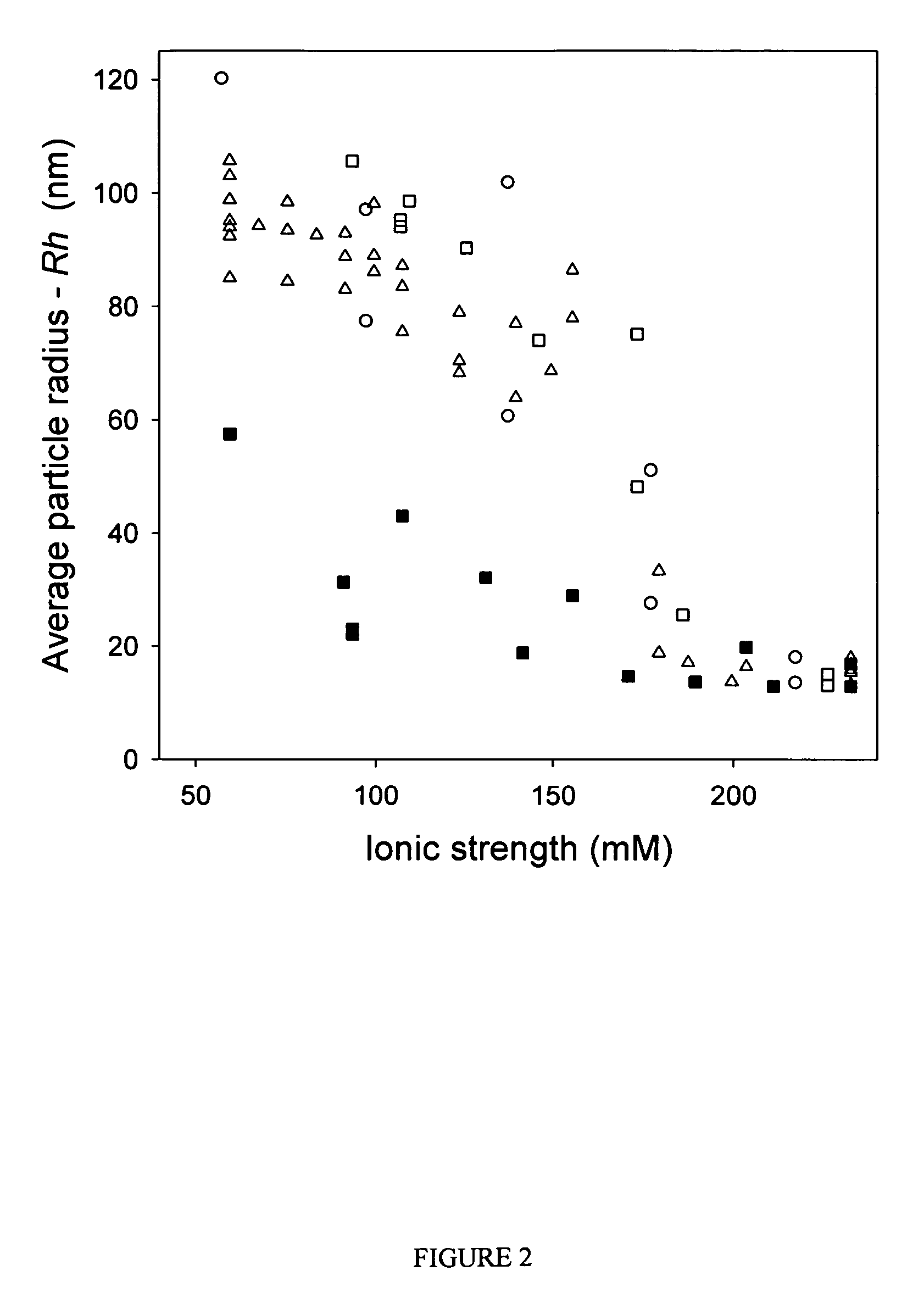
ActiveUS7704721B2Increase ionic strengthImprove efficiencyRecovery/purificationSsDNA virusesFreeze thawingIonic strength
Compositions and methods are provided for preparation of concentrated stock solutions of AAV virions without aggregation. Formulations for AAV preparation and storage are high ionic strength solutions (e.g. μ˜500 mM) that are nonetheless isotonic with the intended target tissue. This combination of high ionic strength and modest osmolarity is achieved using salts of high valency, such as sodium citrate. AAV stock solutions up to 6.4×1013 vg / mL are possible using the formulations of the invention, with no aggregation being observed even after ten freeze-thaw cycles. The surfactant Pluronic® F68 may be added at 0.001% to prevent losses of virions to surfaces during handling. Virion preparations can also be treated with nucleases to eliminate small nucleic acid strands on virions surfaces that exacerbate aggregation.
Owner:GENZYME CORP
Core structure of gp41 from the HIV envelope glycoprotein
InactiveUS6506554B1Increase infectivityHigh activityPeptide/protein ingredientsMicrobiological testing/measurementHiv envelopeDesigning drug
Described are the crystal structure of the alpha-helical domain of the gp41 component of HIV-1 envelope glycoprotein which represents the core of fusion-active gp41, methods of identifying and designing drugs which inhibit gp41 function and drugs which do so.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Purging of cells using viruses
InactiveUS20020037543A1Preventing graft-versus-host diseasesHigh selectivityNervous disorderPeptide/protein ingredientsAutoimmune conditionGraft versus host disease induction
The subject invention relates to viruses that are able to purge (reduce or eliminate) undesirable cells in a mixture of cells. Undesirable cells can include neoplastic cells, cells mediating graft-versus host diseases, and autoimmune cells. The subject invention also relates to the purging of undesirable cells from bone marrow or peripheral blood cell harvests in the treatment of mammals including cancer patients, transplant recipients, and patients with autoimmune disease.
Owner:PRO VIRUS
Formulation of adenovirus for gene therapy
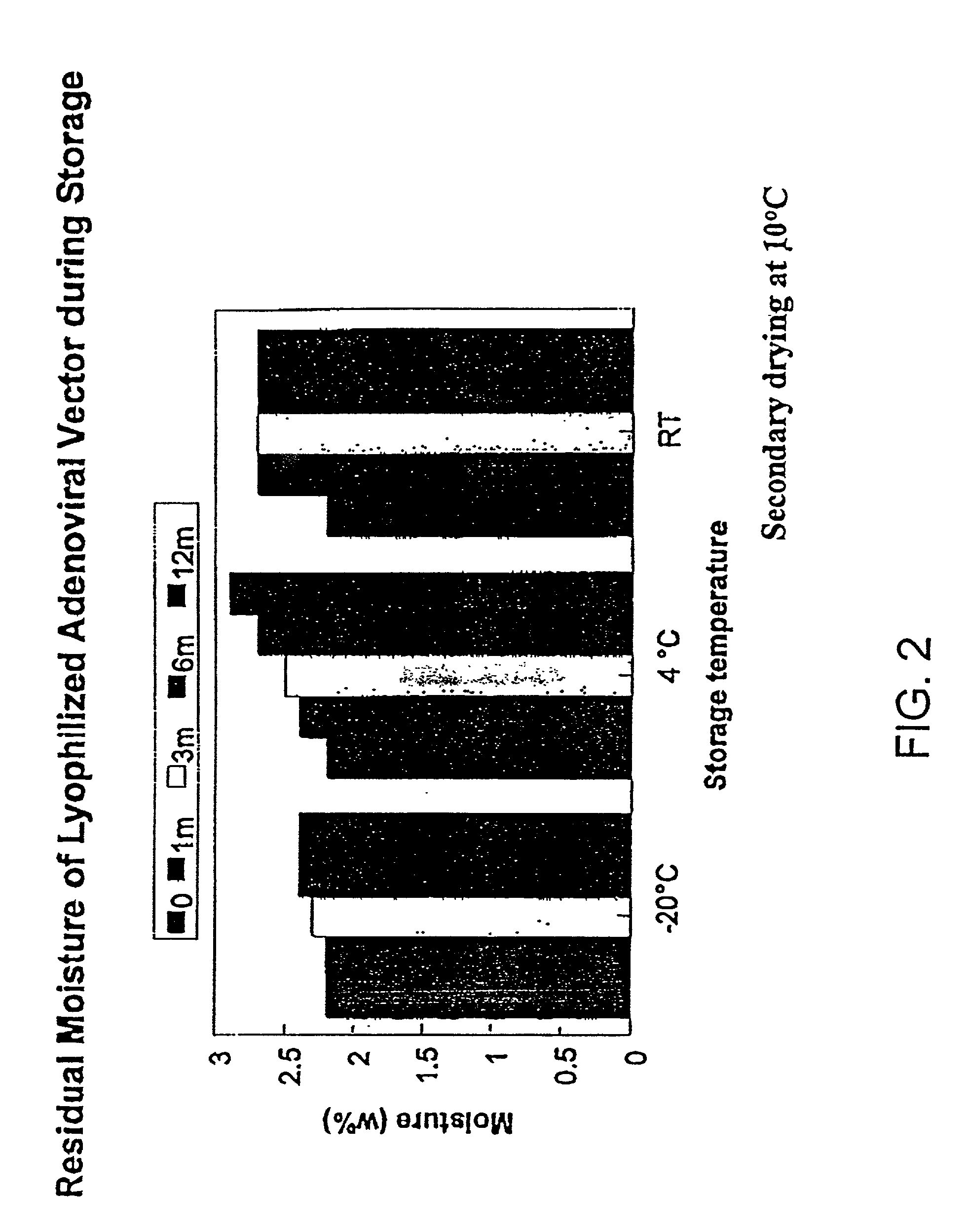
The present invention addresses the need to improve the long-term storage stability (i.e. infectivity) of vector formulations. In particular, it has been demonstrated that for adenovirus, the use of bulking agents, cryoprotectants and lyoprotectants imparts desired properties that allow both lyophilized and liquid adenovirus formulations to be stored at 4° C. for up to 6 months and retain an infectivity between 60–100% of the starting infectivity.
Owner:JANSSEN VACCINES & PREVENTION BV
Targeting viruses using a modified sindbis glycoprotein
InactiveUS20060127981A1High expressionIncrease infectivitySsRNA viruses negative-senseSsRNA viruses positive-senseInfected cellDisease
The present invention relates to viruses that are engineered to contain a surface ligand molecule which targets the virus to a cell of interest. In particular non-limiting embodiments, the cell of interest is desirably ablated and may be a cancer cell, an infected cell, a cell exhibiting a non-malignant proliferative disorder, or a cell of the immune system. Alternatively, the cell of interest is a target for gene therapy.
Owner:UNIVERSITY OF PITTSBURGH
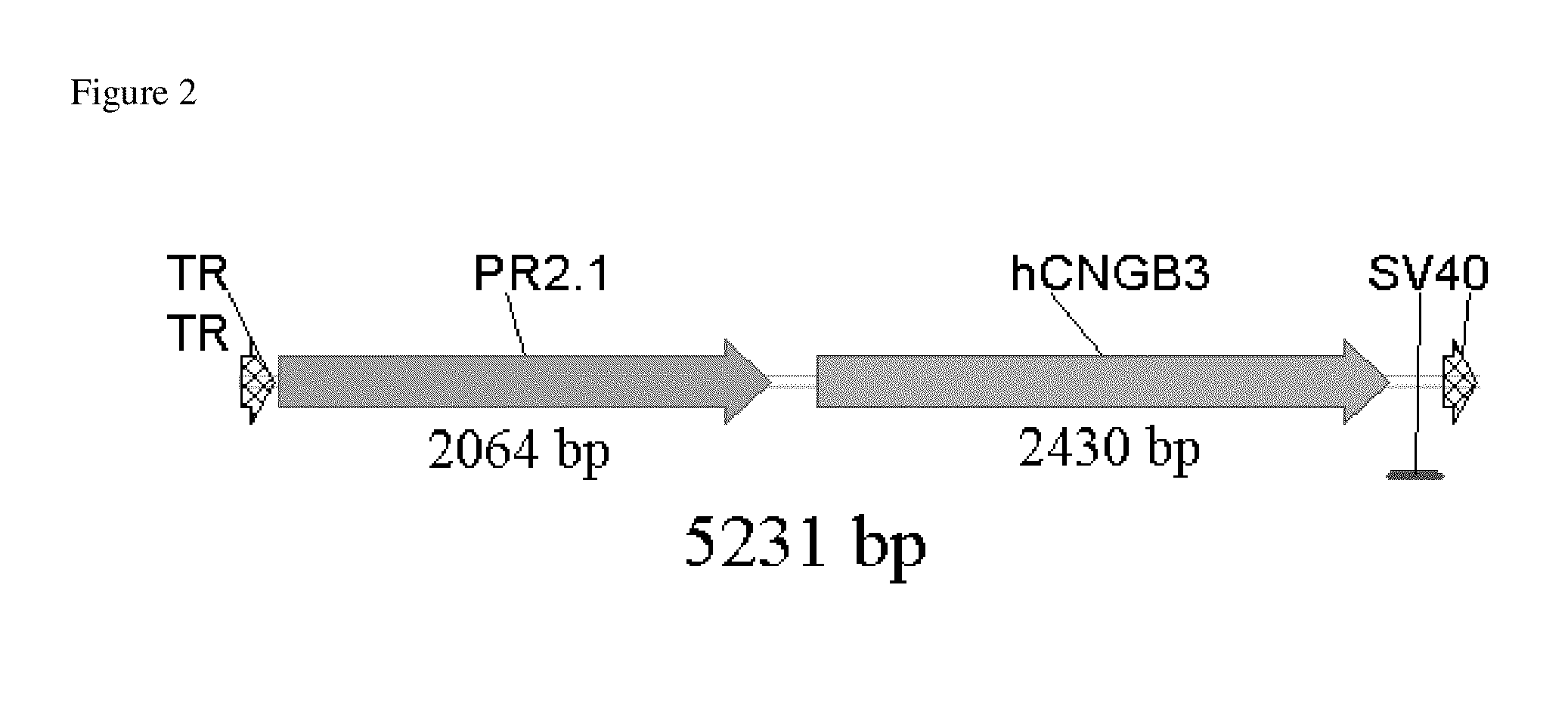
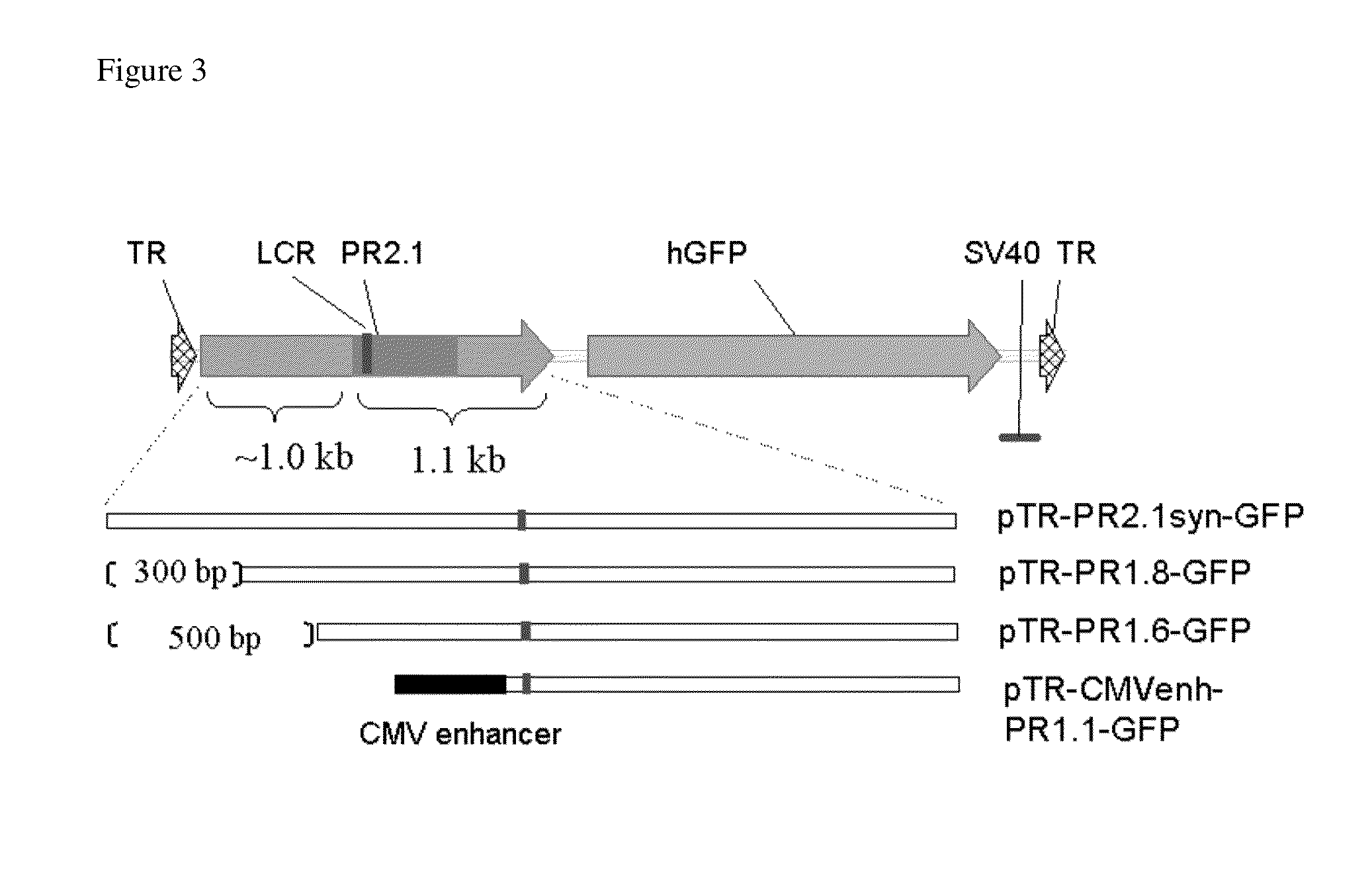
Promoters, expression cassettes, vectors, kits, and methods for the treatment of achromatopsia and other diseases
Owner:APPL GENETIC TECH CORP
Simian adenovirus nucleic acid- and amino acid-sequences, vectors containing same, and uses thereof
ActiveUS20120027788A1Low cross-reactivityReduce adverse effectsFungiVirusesDiseaseSimian Adenoviruses
The present invention relates to novel adenovirus strains with an improved sero-prevalence. In one aspect, the present invention relates to isolated polypeptides of adenoviral capsid proteins such as hexon, penton and fiber protein and fragments thereof and polynucleotides encoding the same. Also provided is a vector comprising the isolated polynucleotide according to the invention and adenoviruses comprising the isolated polynucleotides or polypeptides according to the invention and a pharmaceutical composition comprising said vector, adenovirus, polypeptide and / or polynucleotide. The invention also relates to the use of the isolated polynucleotides, the isolated polypeptides, the vector, the adenoviruses and / or the pharmaceutical composition for the therapy or prophylaxis of a disease.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Targeting viruses using a modified sindbis glycoprotein
InactiveUS7429481B2High expressionIncrease infectivitySsRNA viruses negative-senseSsRNA viruses positive-senseNon malignantInfected cell
Owner:UNIVERSITY OF PITTSBURGH
Porcine reproductive and respiratory syndrome vaccine, based on isolate JA-142
InactiveUS6641819B2Improve efficiencyShorten the timeSsRNA viruses positive-senseViral antigen ingredientsPhysiologyVirus strain
Substantially avirulent forms of atypical porcine reproductive and respiratory syndrome (PRRS) virus and corresponding vaccines are provided which result from cell culture passaging of virulent forms of PRRS. The resultant avirulent atypical PRRS virus is useful as a vaccine in that PRRS specific antibody response is elicited by inoculation of host animals, thereby conferring effective immunity against both previously known strains of PRRS virus and newly isolated atypical PRRS virus strains. The preferred passaging technique ensures that the virus remains in a logarithmic growth phase substantially throughout the process, which minimizes the time required to achieve attenuation. The present invention also provides diagnostic testing methods which can differentiate between animals infected with field strains and attenuated strains of PRRSV.
Owner:US SEC AGRI +1
Methods and compositions for gene delivery to on bipolar cells
InactiveUS20170007720A1Overcome limitationsImprove efficiencyVectorsPeptide/protein ingredientsGene deliveryRetinitis pigmentosa
Disclosed are capsid-modified rAAV expression vectors, as well as infectious virions, compositions, and pharmaceutical formulations that include them. Also disclosed are methods of preparing and using novel capsid-protein-mutated rAAV vector constructs in a variety of diagnostic and therapeutic applications including, inter alia, as delivery agents for diagnosis, treatment, or amelioration of one or more diseases, disorders, or dysfunctions of the mammalian eye. Also disclosed are methods for intravitreal delivery of therapeutic gene constructs to retinal neuron cells, and specifically to ON bipolar cells, of the mammalian eye, as well as use of the disclosed compositions in the manufacture of medicaments for a variety of in vitro and / or in vivo applications including the treatment of retinitis pigmentosa, melanoma-associated retinopathy, and congenital stationary night blindness.
Owner:THE UNIV OF BRITISH COLUMBIA +1
Compositions and methods to prevent AAV vector aggregation
ActiveUS20060035364A1High infectivity titerHigh transduction efficiencyMicrobiological testing/measurementRecovery/purificationChemistryIonic strength
Compositions and methods are provided for preparation of concentrated stock solutions of AAV virions without aggregation. Formulations for AAV preparation and storage are high ionic strength solutions (e.g. μ˜500 mM) that are nonetheless isotonic with the intended target tissue. This combination of high ionic strength and modest osmolarity is achieved using salts of high valency, such as sodium citrate. AAV stock solutions up to 6.4×1013 vg / mL are possible using the formulations of the invention, with no aggregation being observed even after ten freeze-thaw cycles. The surfactant Pluronic® F68 may be added at 0.001% to prevent losses of virions to surfaces during handling. Virion preparations can also be treated with nucleases to eliminate small nucleic acid strands on virions surfaces that exacerbate aggregation.
Owner:GENZYME CORP
Cell lines for production of replication-defective adenovirus
ActiveUS20060270041A1Promote infectionIncrease productionSugar derivativesGenetically modified cellsPolymerase LDefective virus
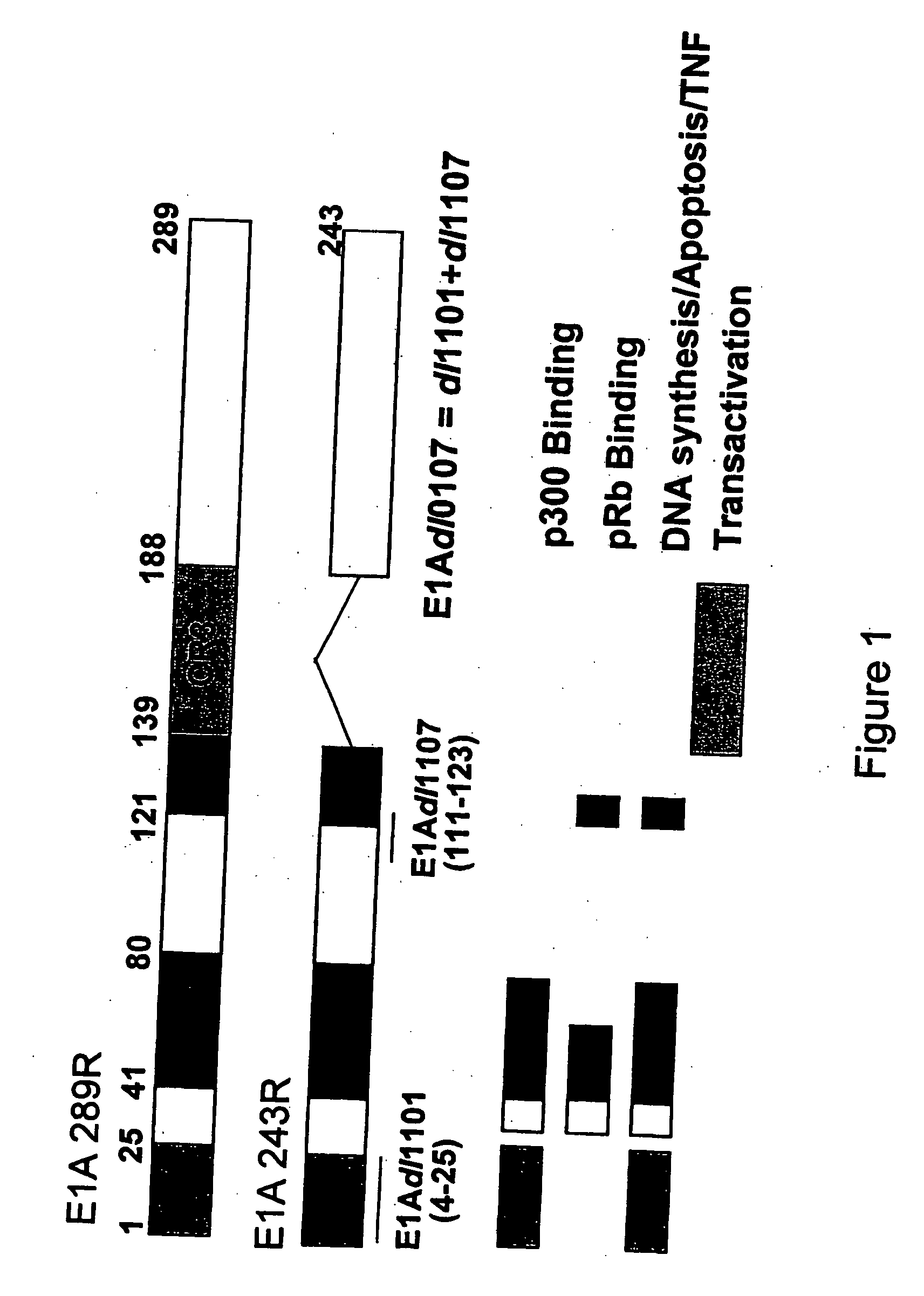
The present invention provides cell lines for the production of E1-deleted adenovirus (rAd) vectors that complement E1A and E1B functions. The present invention also provides cell lines for the production of E1- and E2-deleted adenovirus vectors that complement E1A, E1B and E2B polymerase functions. The invention provides particular cell lines that complement E1A function by insertion of an E1A sequence containing mutations in the 243R and 289R proteins and an E1B sequence comprising the E1B-55K gene. Production yields in the resulting producer cell lines, designated SL0003 and SL0006, were similar to those obtained from 293 cells without generation of detectable recombinant replication competent adenovirus (“RCA”).
Owner:CANJI
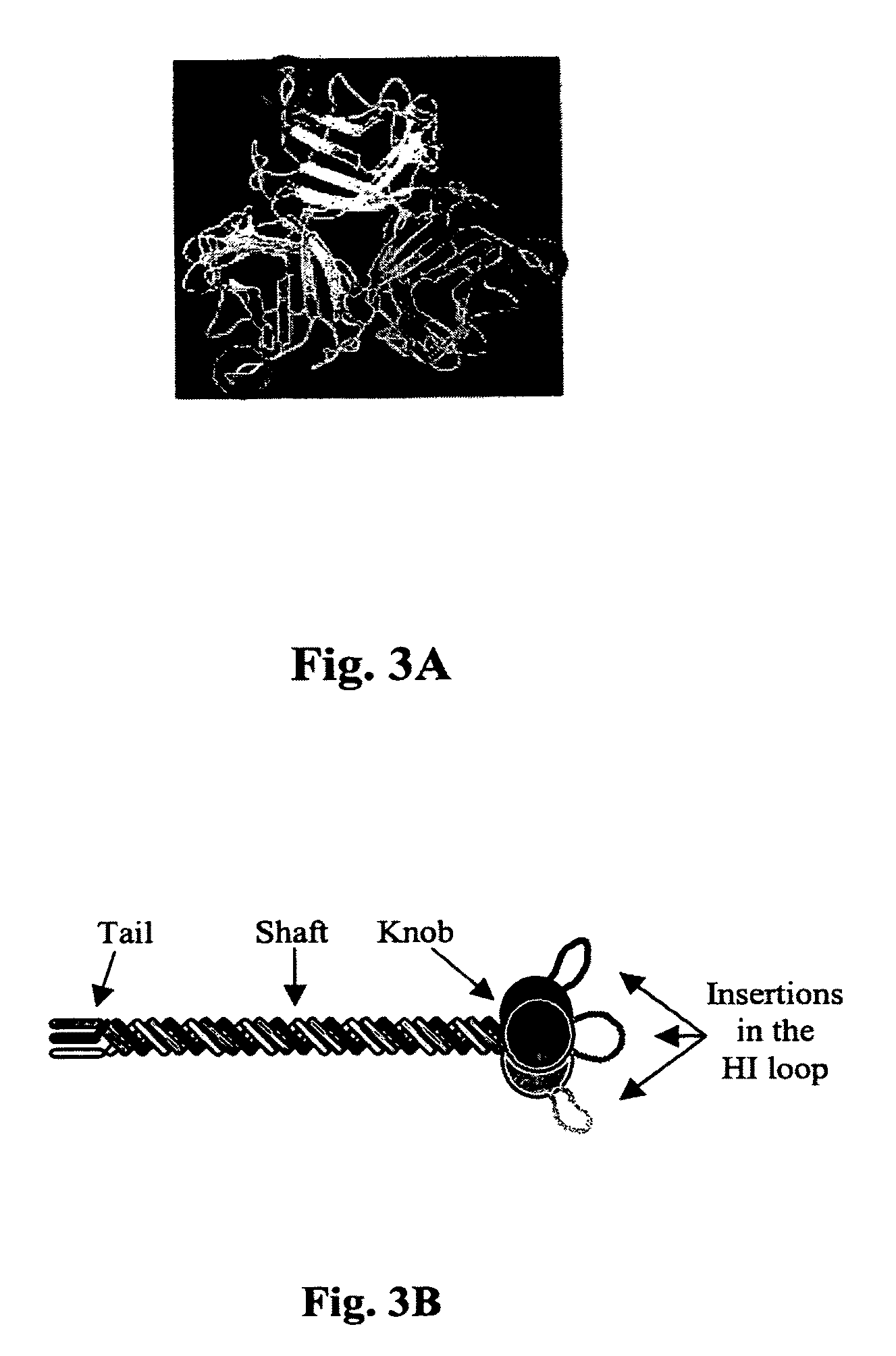
Viral Vectors with Improved Properties
InactiveUS20120220492A1Increase infectivityGenetic therapy composition manufacturePeptidesMutated proteinADAMTS Proteins
Methods to improve the tropism or other features of a virus are disclosed. Such methods can be used to prepare, e.g., DNA or plasmid libraries of variants of a gene encoding a viral capsid or envelope protein having a randomly inserted restriction site, libraries of viral clones with such variant genes with a randomly inserted restriction site or polypeptide sequence targeting a receptor expressed by a specific type of mammalian cells. Described are also methods to prepare mosaic viruses, i.e., viral particles wherein copies of one or more capsid or envelope proteins originate from different sources. These methods can be used to prepare mosaic viruses of a specific mixture of wild-type and mutant proteins, or of different types of mutant proteins.
Owner:MT SINAI SCHOOL OF MEDICINE
Infectivity-enhanced conditionally-replicative adenovirus and uses thereof
InactiveUS8168168B2Increase infectivityStrong specificityBiocideAntibody mimetics/scaffoldsFiberCoxsackie-Adenovirus Receptor
A modified adenovirus capable of overcoming the problem of low level of coxsackie-adenovirus receptor (CAR) expression on tumor cells and methods of using such adenovirus are provided. The fiber protein of the adenovirus is modified by insertion or replacement so as to target the adenovirus to tumor cells, and the replication of the modified adenovirus is limited to tumor cells due to specific promoter control or mutations in E1a or E1b genes.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
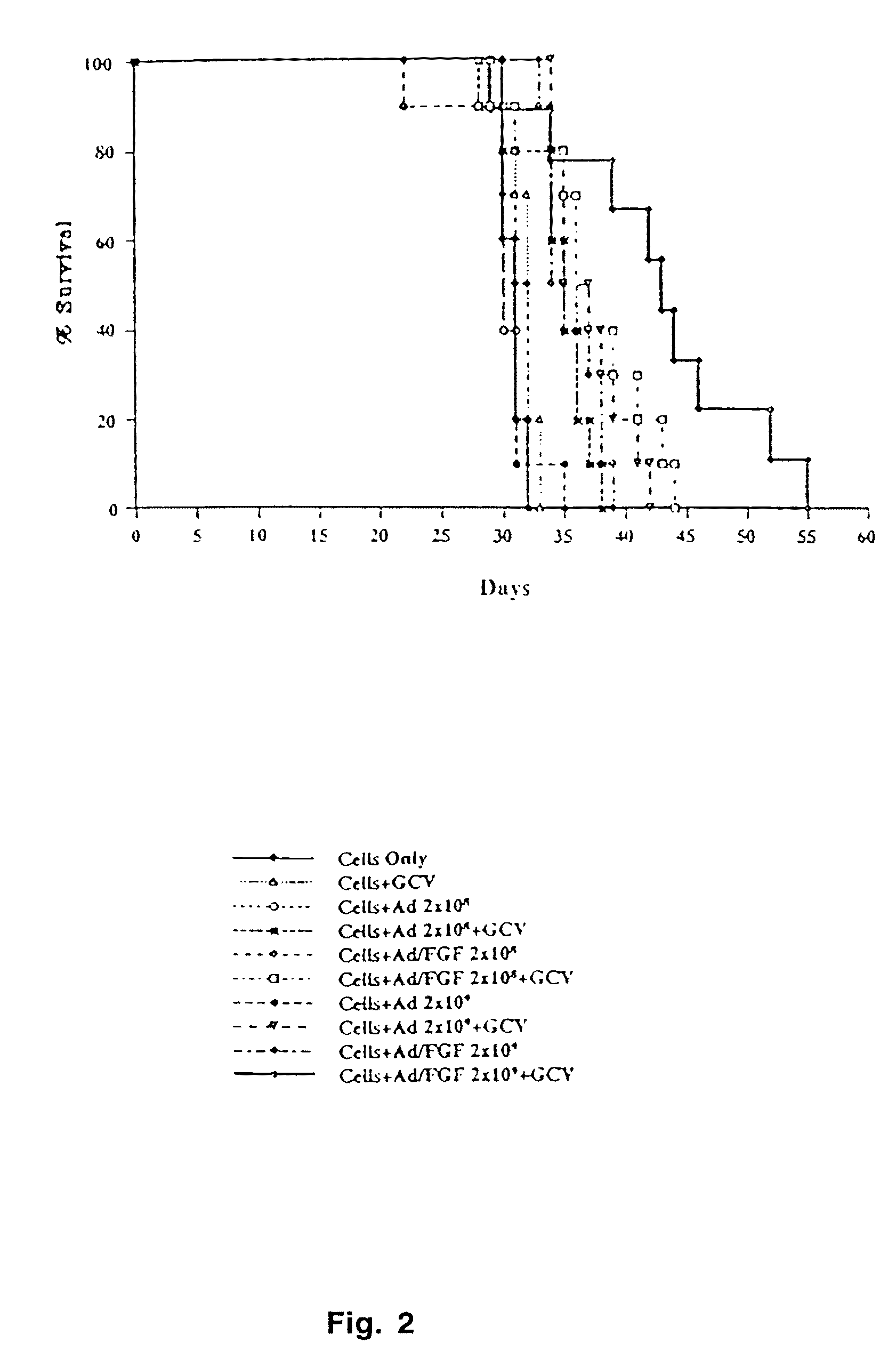
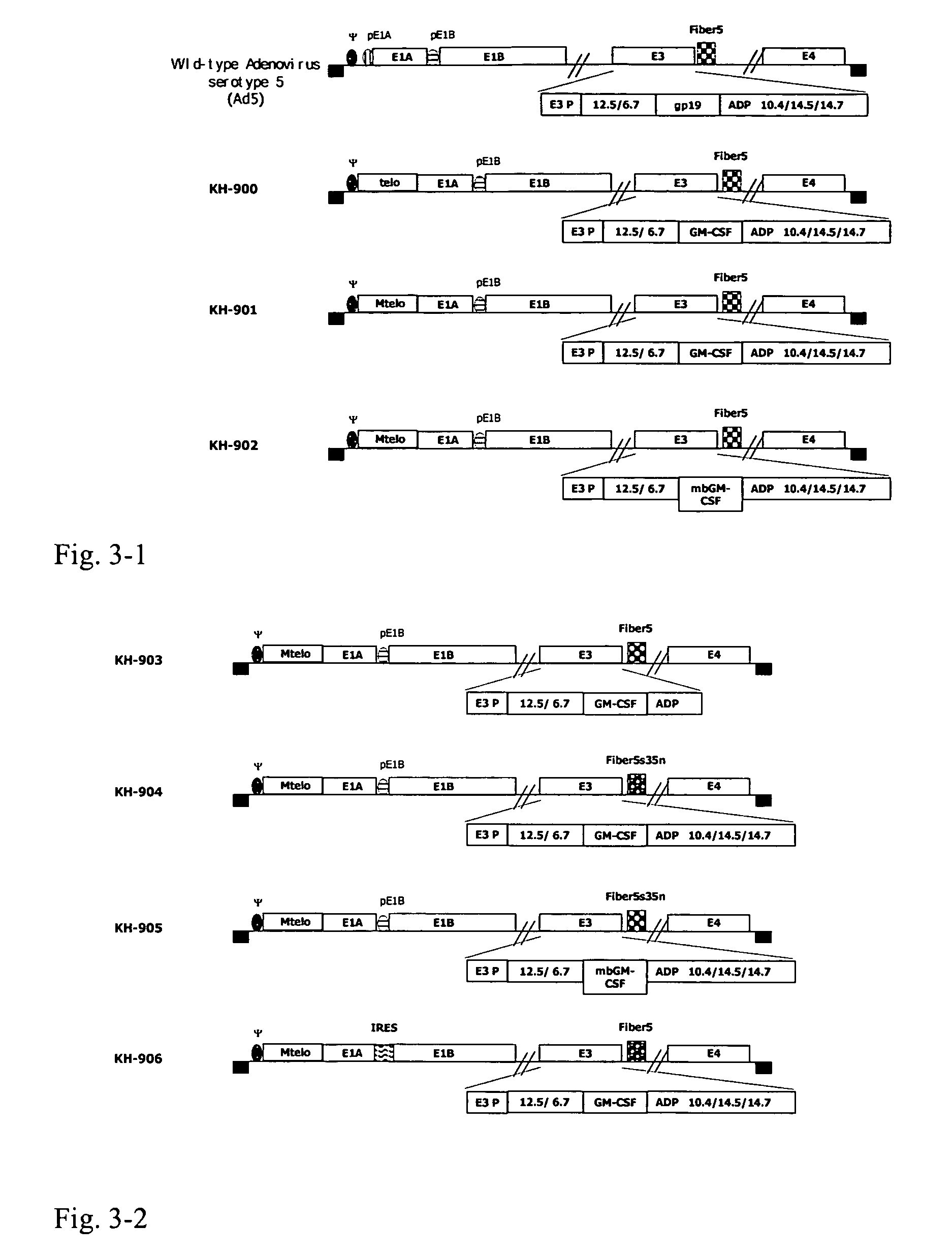
Construction of oncolytic adenovirus recombinant specifically expressing immune modulatory factor GM-CSF in tumor cells and uses thereof
ActiveUS7951585B2Strong specificityIncrease infectivityBiocidePeptide/protein ingredientsOncolytic adenovirusWilms' tumor
The present invention relates to gene therapy for tumors, specifically, it relates to the construction of oncolytic adenovirus recombinant, which specifically expresses immune modulatory factor GM-CSF in tumor cells and uses thereof.
Owner:CHENGDU KANGHONG BIOTECH
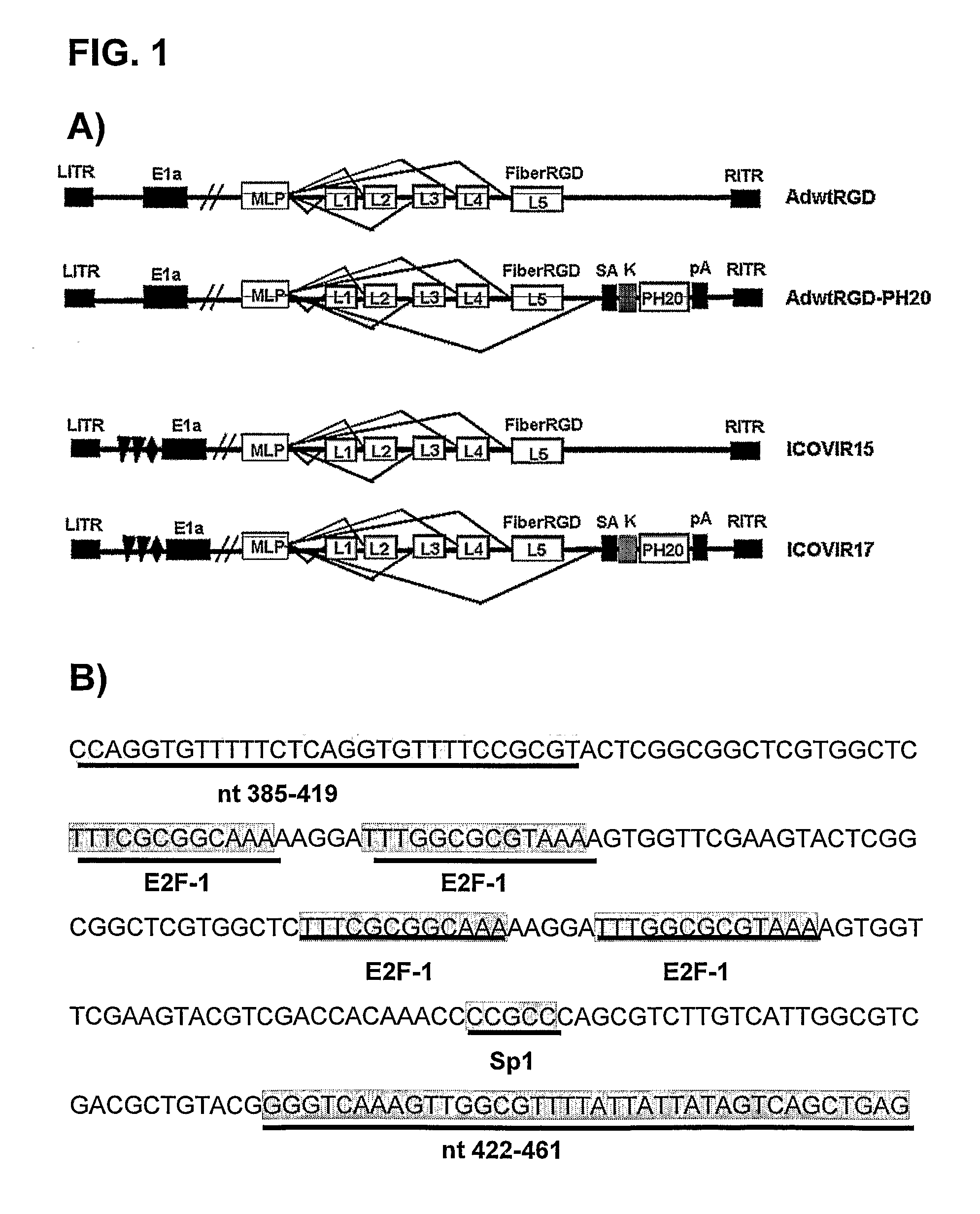
Oncolytic adenoviruses for treating cancer
ActiveUS20120148535A1Increase of oncolytic effectIncrease antitumour efficayBiocidePeptide/protein ingredientsEnzymeTumour volume
The invention is related to an oncolytic adenovirus that comprises a sequence encoding a hyaluronidase enzyme inserted in its genome. This adenovirus spreads more efficiently in the tumour mass and therefore the oncolytic effect is increased. Injecting the oncolytic adenovirus of the invention endovenously, tumour volume regressions are obtained. Therefore the oncolytic adenovirus of the present invention is useful for the treatment of a cancer or a pre-malignant state of cancer.
Owner:FUNDACIO INST DINVESTIGACIO BIOMEDICA DE BELLVITGE IDIBELL +1
Baculoviruses, insecticidal compositions, and methods for control of invertebrates
InactiveUS6521454B1Increase infectivityIncrease flexibilityBiocideSugar derivativesInvertebrateComputational biology
Owner:US SEC AGRI
Lecanicillium lecanii and application of Lecanicillium lecanii to control of greenhouse pests
The invention belongs to the technical field of biological control of pests and relates to a Lecanicillium lecanii and application of the Lecanicillium lecanii to control of greenhouse pests. The strain is Lecanicillium lecanii, is preserved in CGMCC (China General Microbiological Culture Collection Center) with the preservation number CGMCC No.8453, can be used for controlling greenhouse pests like whiteflies, aphids and thrips, and can be used in pollution-free vegetable and green food vegetable production greenhouses.
Owner:谢明
Viral vectors with improved properties
InactiveUS20060286545A1Efficient retargetingIncrease infectivityVectorsGenetic therapy composition manufactureMutated proteinRestriction site
Methods to improve the tropism or other features of a virus is disclosed. Such methods can be used to prepare, e.g., DNA or plasmid libraries of variants of a gene encoding a viral capsid or envelope restriction site, libraries of viral clones with such variant genes with a randon-dy inserted restriction site or polypeptide sequence targeting a receptor expressed by a specific type of mammalian cells. Described are also methods to prepare mosaic viruses, i.e., viral particles wherein copies of one or more capsid or envelope proteins originate from different sources. These methods can be used to prepare mosaic viruses of a specific mixture of wild-type and mutant proteins, or of different types of mutant proteins.
Owner:MT SINAI SCHOOL OF MEDICINE
Method of sanitizing a biological tissue
ActiveUS20050202137A1Preserve the flavorPreserve colorMilk preservationFruit and vegetables preservationBiotechnologyMicroorganism
A method for treating biological tissue, particularly meats for human consumption, so as to sanitize the tissue, is described. The method inactivates microorganisms and pathogenic prions (proteins) in the tissue.
Owner:URTHTECH
Culture media for edible mycorrhizal fungi and symbiotic seedlings and synchronous culture method by utilizing culture media
ActiveCN107711290AGood growth activityImprove survival rateMagnesium fertilisersAlkali orthophosphate fertiliserHyphomycetesBiotechnology
The invention discloses culture media for edible mycorrhizal fungi and symbiotic seedlingsand and a synchronous culture method by utilizing the culture media. The culture media for the edible mycorrhizal fungi and the symbiotic seedlings comprise an edible mycorrhizal fungus strain mycelium culture medium and an edible mycorrhizal fungus symbiosis seedling synthesis culture medium, and the ediblemycorrhizal fungus strain mycelium culture medium includes a solid culture medium used for expanding reproduction of the mycelium and a liquid culture medium used for culturing mycelium which is usedfor inoculation of the mycorrhizal symbiotic seedlings. The synchronous culture method for implementing edible mycorrhizal fungus symbiotic seedling synthesis by utilizing the above culture media comprises the following steps: (1) a cultivation method for the edible mycorrhizal fungus strain mycelium, (2) a cultivation method for the edible mycorrhizal fungus symbiotic seedlings. According to theinvention, cultivation of the edible mycorrhizal fungus mycelium is divided into two stages including solid culture and liquid culture, liquid fungus strains are adopted for inoculation, and meanwhilethe edible mycorrhizal fungus mycelium and symbiotic plants in the mycorrhizal seedling synthesis culture medium grow together, so that the infection ability of the mycelium is increased by 30% or more, the success rate of the inoculation is increased by 20%-30%, and the survival rates of symbiotic plant seedlings and the mycorrhizal fungus mycelium are both increased by 20% or more.
Owner:INST OF EDIBLE FUNGI SHANXI ACAD OF AGRI SCI
Compositions and methods for diagnosing and/or treating influenza infection
ActiveUS20110033490A1Enhanced human bindingIncrease infectivitySsRNA viruses negative-sensePeptide/protein ingredientsGlycanReagent
Owner:MASSACHUSETTS INST OF TECH
Cell culture system of a hepatitis c genotype 3a and 2a chimera
InactiveUS20100093841A1Efficient and sustainable growthDifferential efficiencyOrganic active ingredientsSsRNA viruses positive-senseGenomic sequencingNS5A
The present inventors have developed a culture system for genotype 3a, which has a high prevalence worldwide. Since intergenotypic recombinant genomes exploiting the replication characteristics of JFH1 will be a valuable tool for the genotype specific study of the replaced genes and related therapeutics, the present inventors constructed a genotype 3a / 2a (S52 / JFH1) recombinant containing the structural genes (Core, E1, E2), p7 and NS2 of strain S52 and characterized it in Huh7.5 cells. S52 / JFH1 and J6 / JFH viruses passaged in cell culture had comparable growth kinetics and yielded similar peak HCV RNA titers and infectivity titers. Direct genome sequencing of cell culture derived S52 / JFH1 viruses identified putative adaptive mutations in Core, E2, p7, NS3 and NS5A; clonal analysis revealed, that all genomes analyzed exhibited different combinations of these mutations. Finally, viruses resulting from transfection with RNA transcripts of five S52 / JFH1 recombinant containing these combinations of putative adaptive mutations performed as efficiently as J6 / JFH viruses in Huh7.5 15 cells and were all genetically stable after viral passage. In conclusion, the present inventors have developed a robust and genetically stable cell culture system for HCV genotype 3a.
Owner:HVIDOVRE HOSPITAL
Formulation of adenovirus for gene therapy
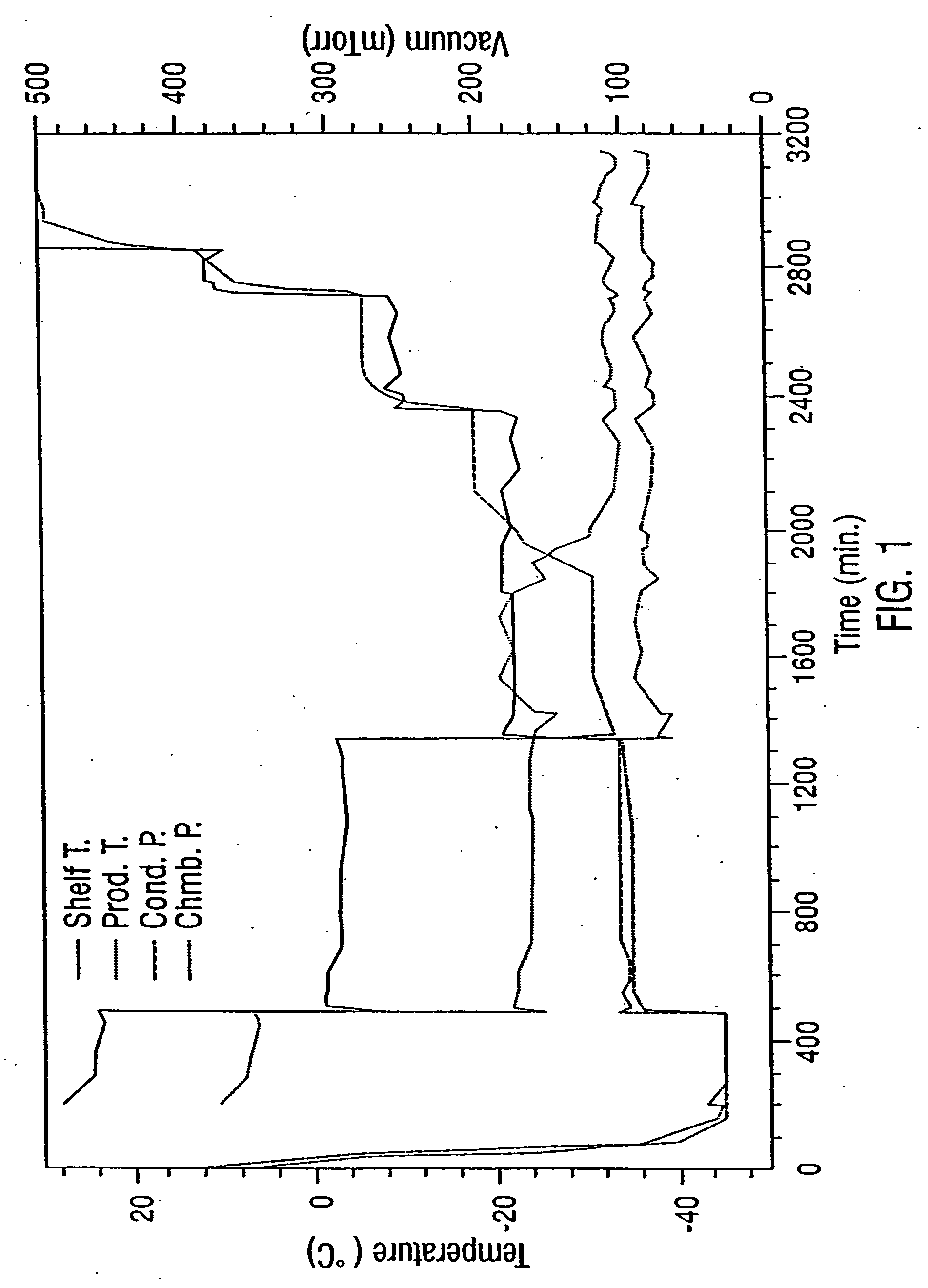
InactiveUS20050175592A1Increase infectivityLow residual moistureBiocidePowder deliveryBiologyInfectivity
The present invention addresses the need to improve the long-term storage stability (i.e. infectivity) of vector formulations. In particular, it has been demonstrated that for adenovirus, the use of bulking agents, cryoprotectants and lyoprotectants imparts desired properties that allow both lyophilized and liquid adenovirus formulations to be stored at 4° C. for up to 6 months and retain an infectivity between 60-100% of the starting infectivity.
Owner:JANSSEN VACCINES & PREVENTION BV
Purging of cells using viruses
InactiveUS20040109878A1Preventing graft-versus-host diseasesHigh selectivityBiocideNervous disorderAutoimmune conditionGraft versus host disease induction
The subject invention relates to viruses that are able to purge (reduce or eliminate) undesirable cells in a mixture of cells. Undesirable cells can include neoplastic cells, cells mediating graft-versus host diseases, and autoimmune cells. The subject invention also relates to the purging of undesirable cells from bone marrow or peripheral blood cell harvests in the treatment of mammals including cancer patients, transplant recipients, and patients with autoimmune disease.
Owner:UNIVERSITY OF OTTAWA +1
Phage lyase composite powder and preparation method and application thereof
PendingCN110592056AMaintain micro-ecological balanceIncrease daily weight gainAccessory food factorsLyasesPhage lysisLyase
The invention relates to phage lyase composite powder. The composite powder composition comprises the following components in percentage by mass: 0.15%-0.2% of phage lyase TSPpgh, 0.15%-0.2% of phagelyase MMPpgh, 0.3%-0.5% of trehalose, 0.1%-0.2% of citric acid, 0.2%-0.3% of mannitol, 0.2%-0.3% of vitamin C and 98.3%-98.9% of diatomite. The phage lyase composite powder is subjected to in-vitro antibacterial activity detection by adopting a two layer plating method. After the composite powder is stored for 12 months at normal temperature, the enzyme activity is kept at 95% or above (the enzymeactivity loss is within 5%). The composite lyase preparation can be used for replacing antibiotics to inhibit proliferation of harmful pathogenic bacteria such as escherichia coli, salmonella and staphylococcus aureus in animal intestines, and can maintain intestinal microecological balance.
Owner:KUNMING UNIV OF SCI & TECH
Simian adenovirus nucleic acid- and amino acid-sequences, vectors containing same, and uses thereof
ActiveUS9718863B2Low cross-reactivityReduce adverse effectsVirusesBacteriaSimian AdenovirusesDisease
The present invention relates to novel adenovirus strains with an improved seroprevalence. In one aspect, the present invention relates to isolated polypeptides of adenoviral capsid proteins such as hexon, penton and fiber protein and fragments thereof and polynucleotides encoding the same. Also provided is a vector comprising the isolated polynucleotide according to the invention and adenoviruses comprising the isolated polynucleotides or polypeptides according to the invention and a pharmaceutical composition comprising said vector, adenovirus, polypeptide and / or polynucleotide. The invention also relates to the use of the isolated polynucleotides, the isolated polypeptides, the vector, the adenoviruses and / or the pharmaceutical composition for the therapy or prophylaxis of a disease.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Modification of polypeptides
ActiveUS9932367B2Increase infectivityExtended stayPeptide preparation methodsBiological testingPeptide displayIon-exchange resin
Owner:BICYCLE THERAPEUTICS
Infectivity-enhanced conditionally-replicative adenovirus and uses thereof
InactiveUS20120207711A1Increase infectivityStrong specificityBiocideAntibody mimetics/scaffoldsCoxsackie-Adenovirus ReceptorInfectivity
A modified adenovirus capable of overcoming the problem of low level of coxsackie-adenovirus receptor (CAR) expression on tumor cells and methods of using such adenovirus are provided. The fiber protein of the adenovirus is modified by insertion or replacement so as to target the adenovirus to tumor cells, and the replication of the modified adenovirus is limited to tumor cells due to specific promoter control or mutations in E1a or E1b genes.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com