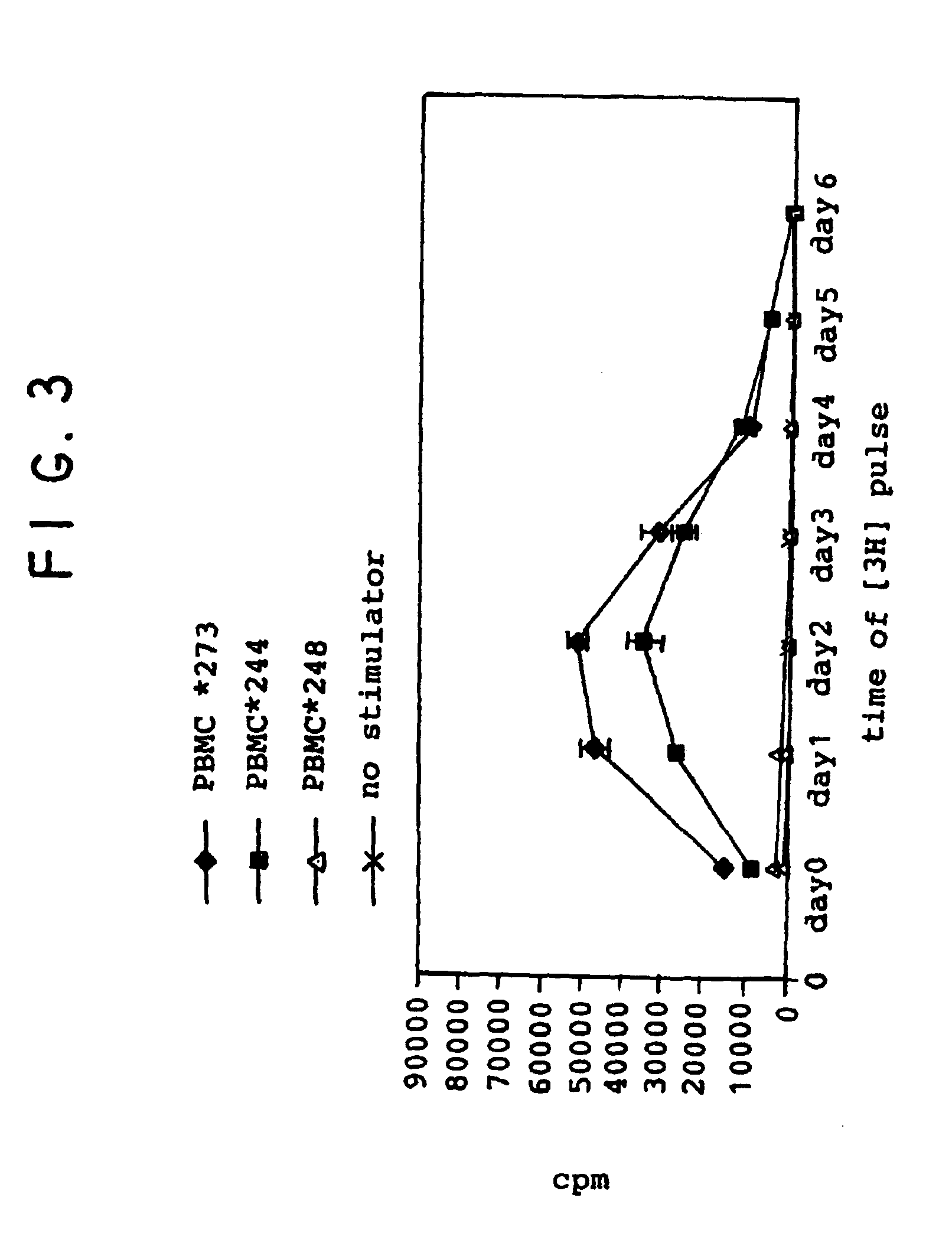
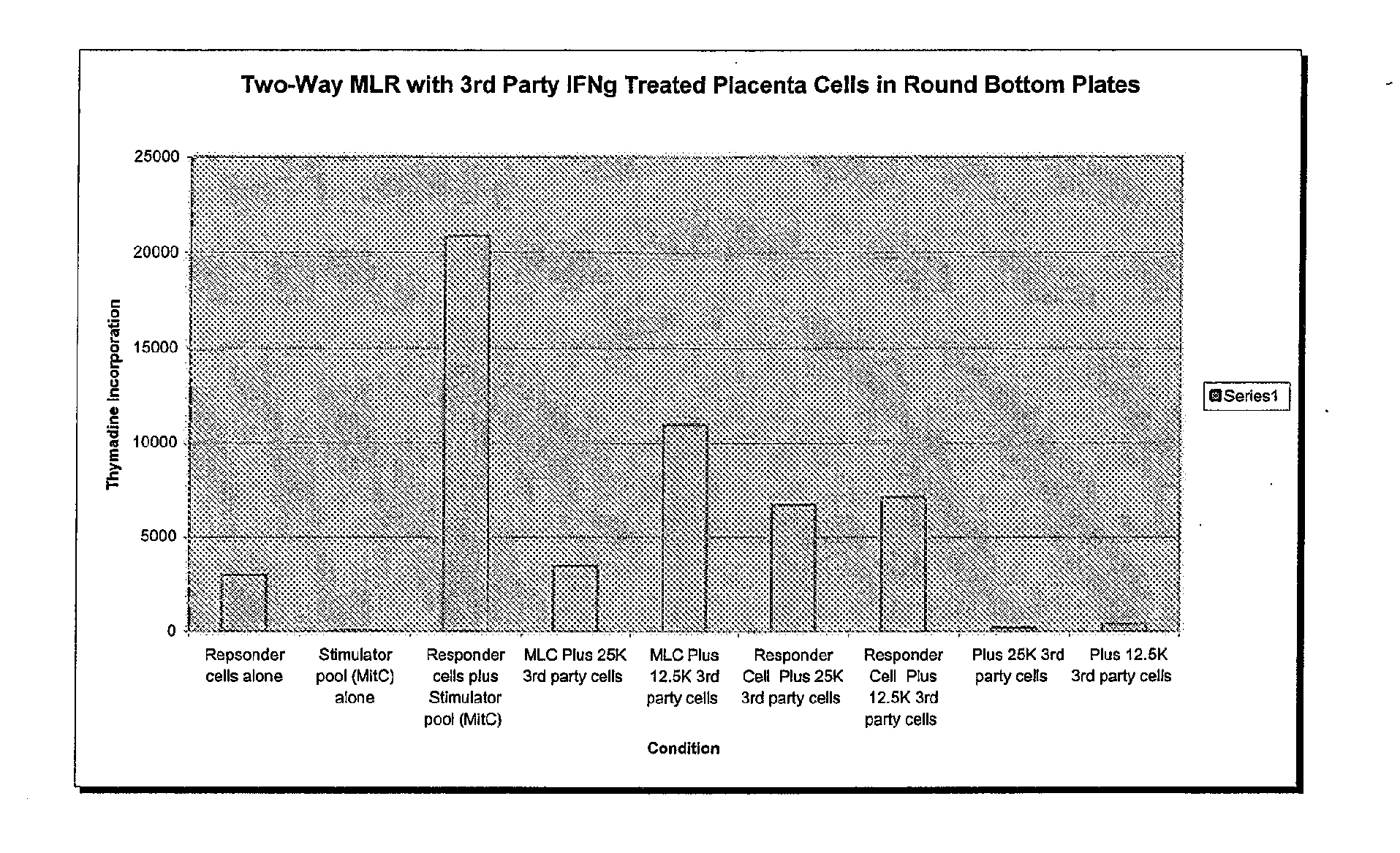
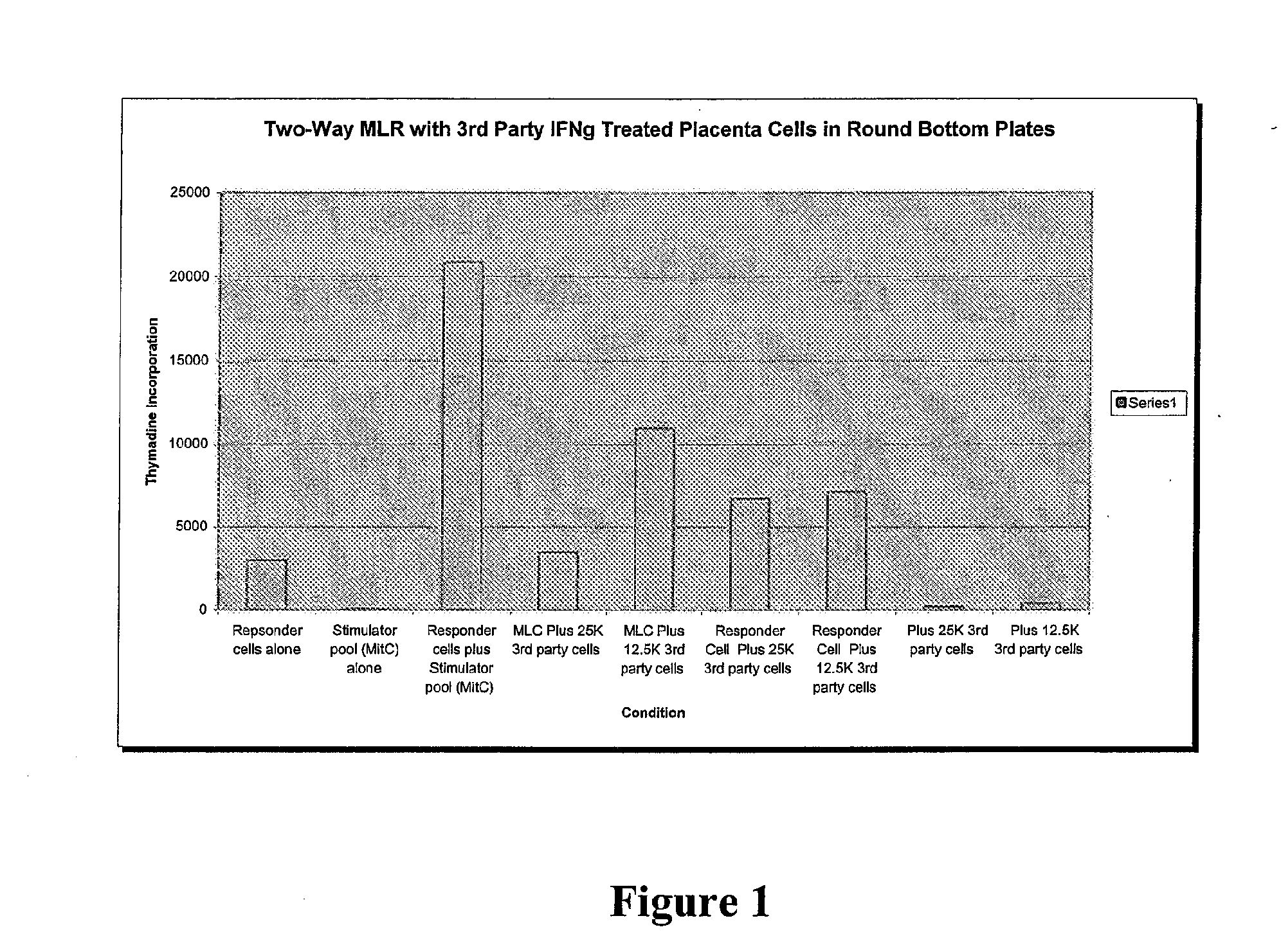
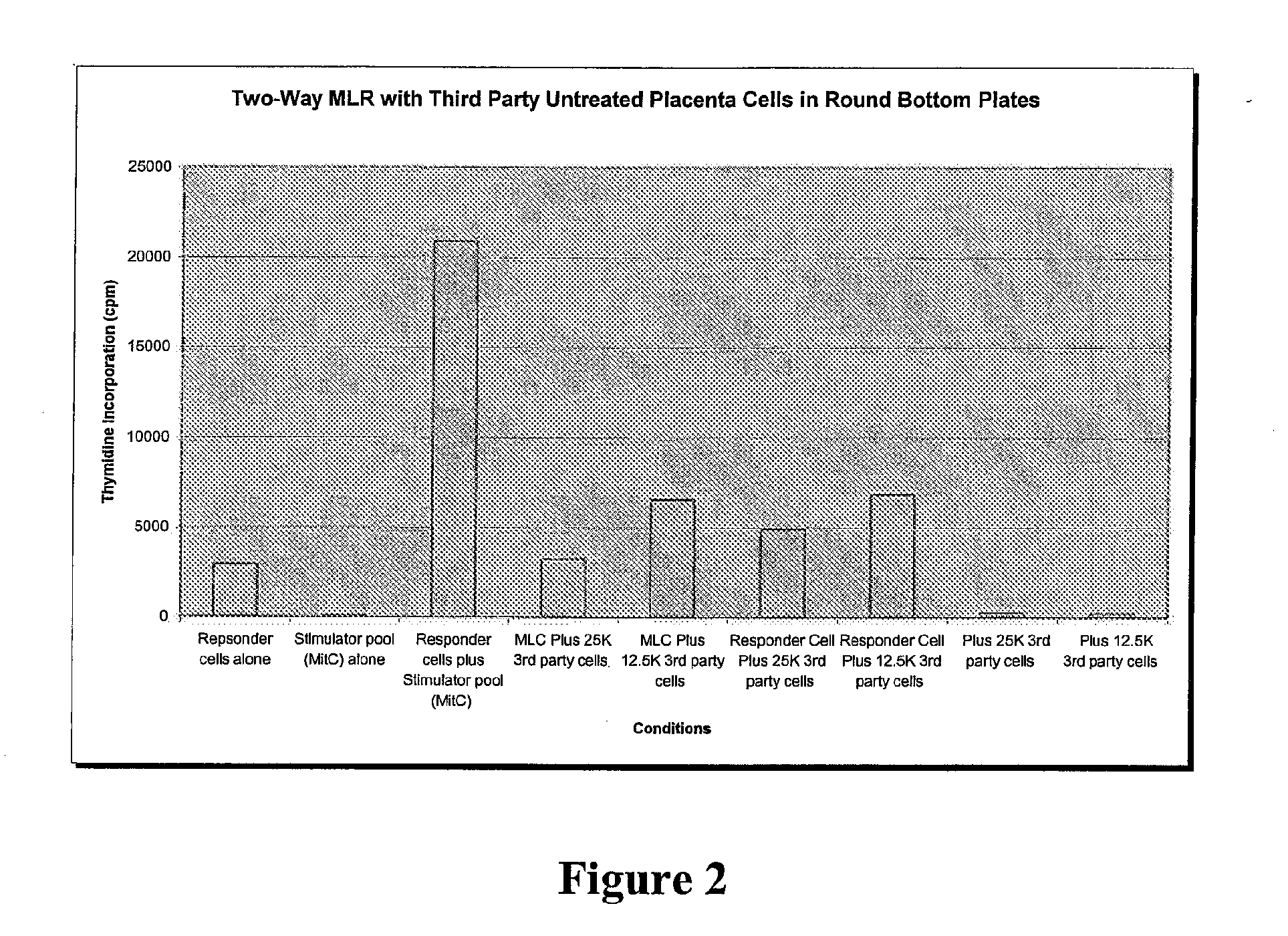
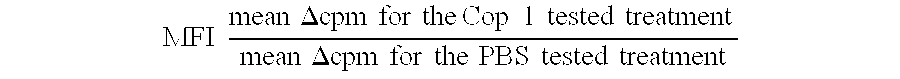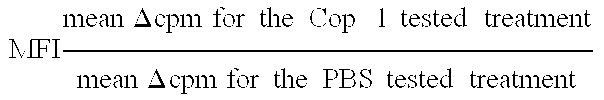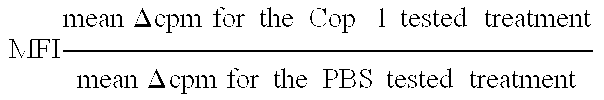Patents
Literature
203 results about "Graft versus host disease induction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
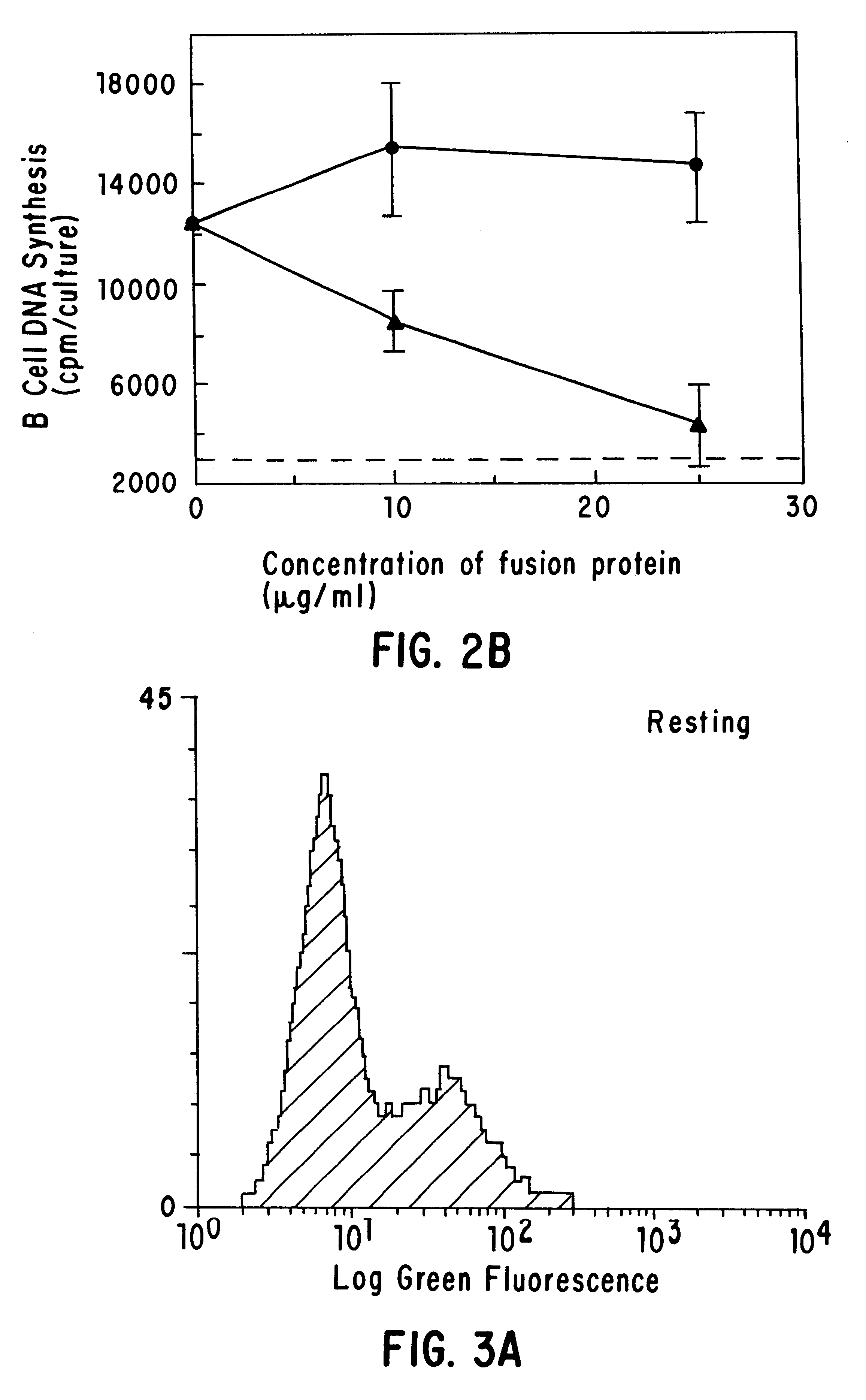
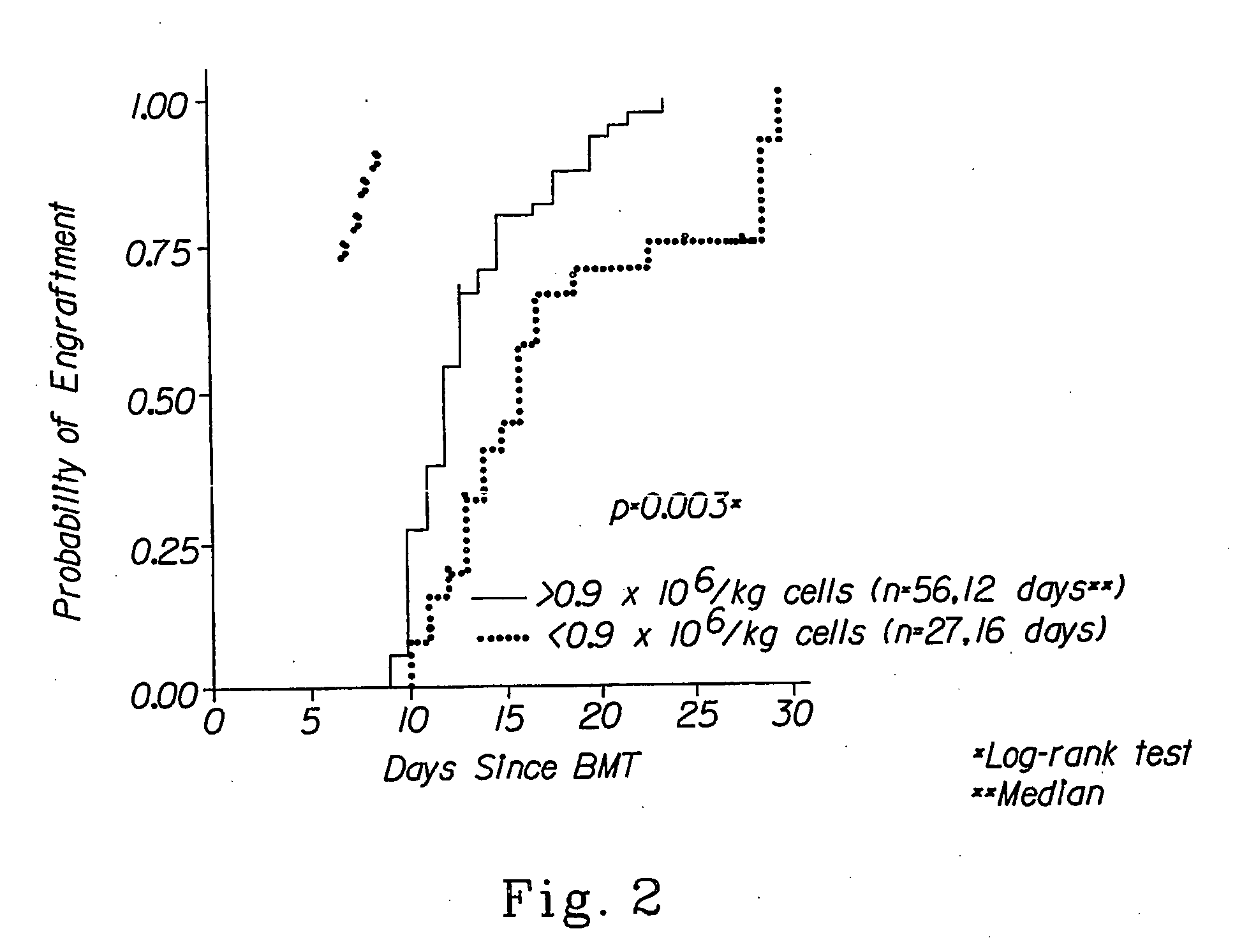
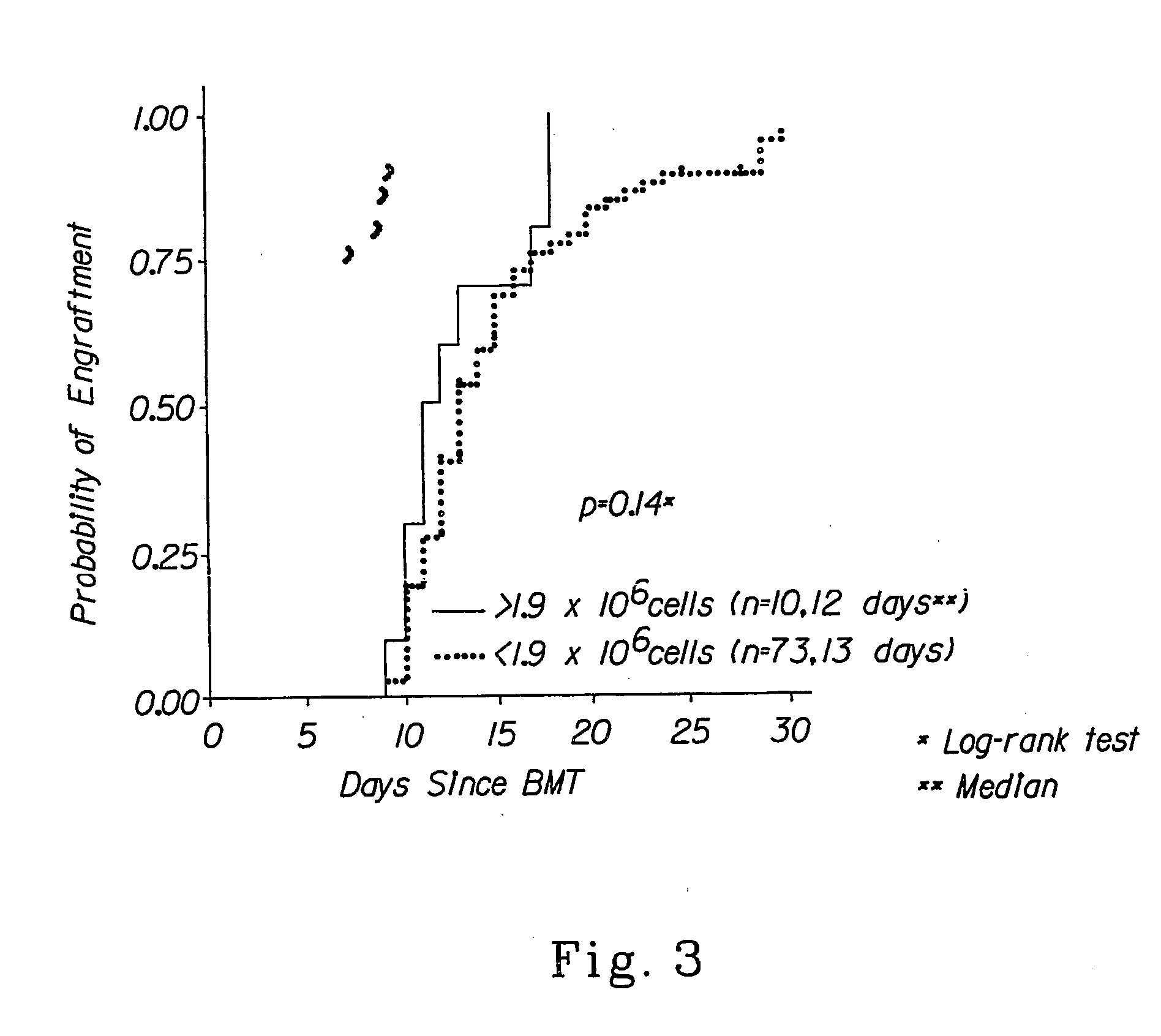
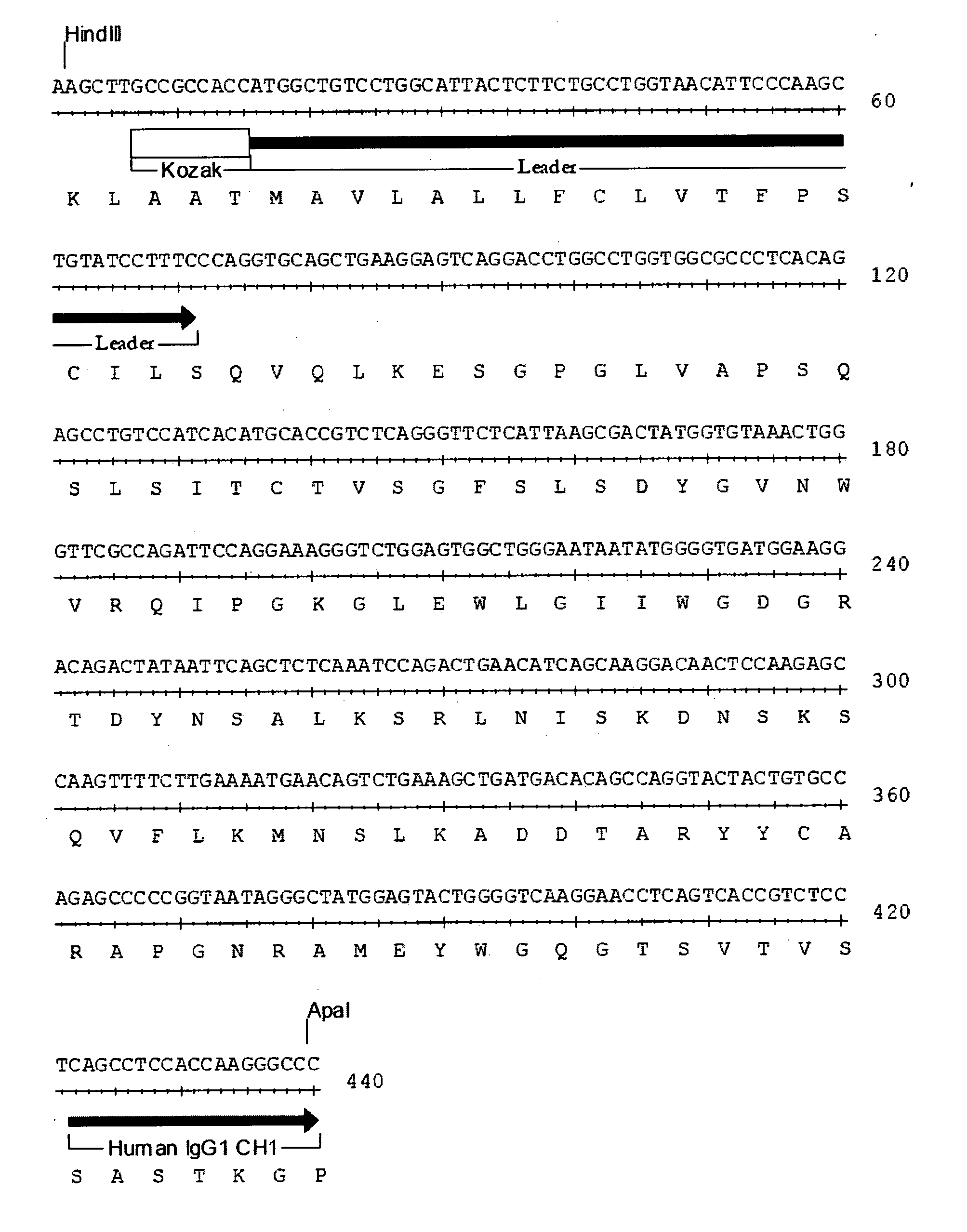
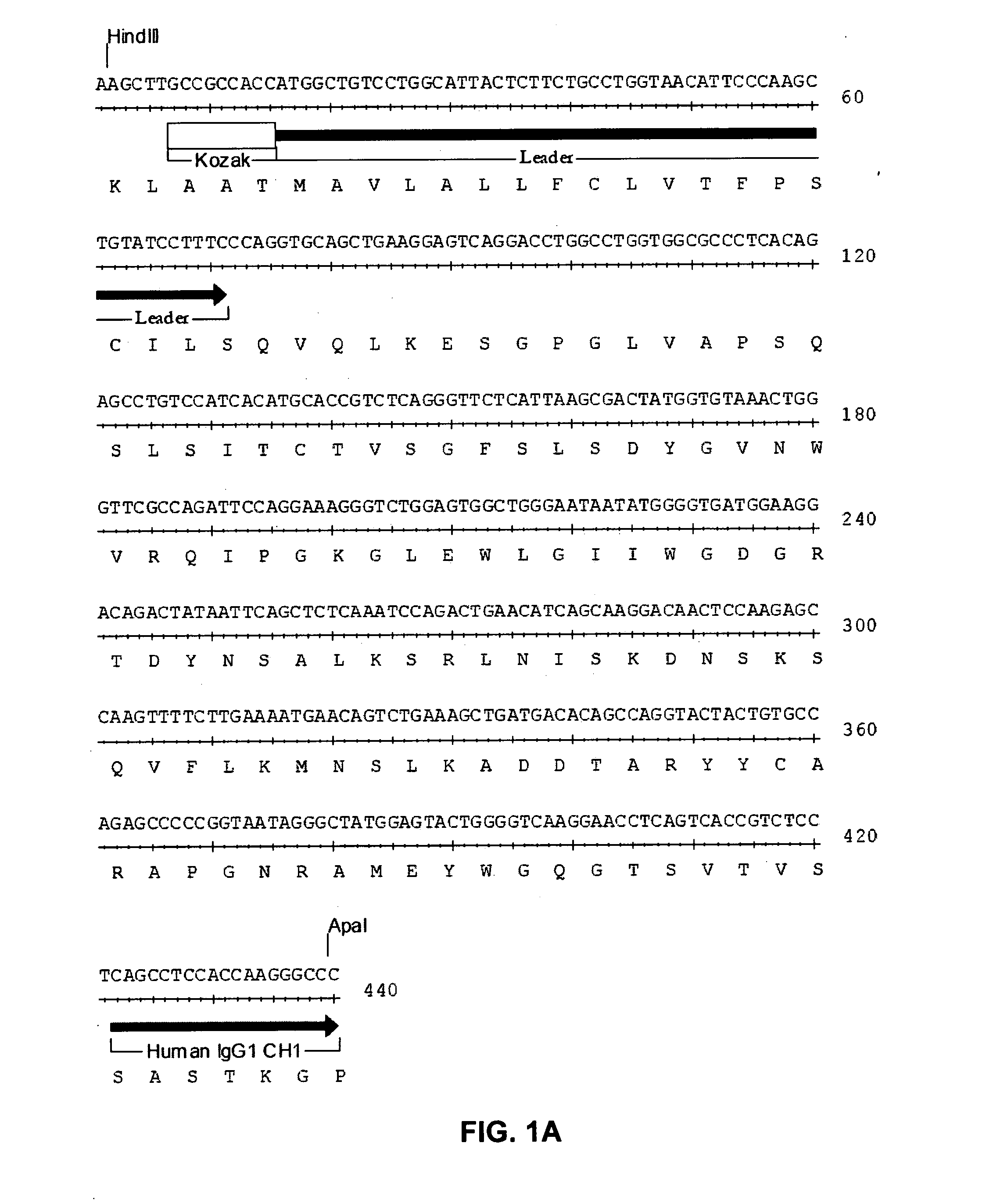
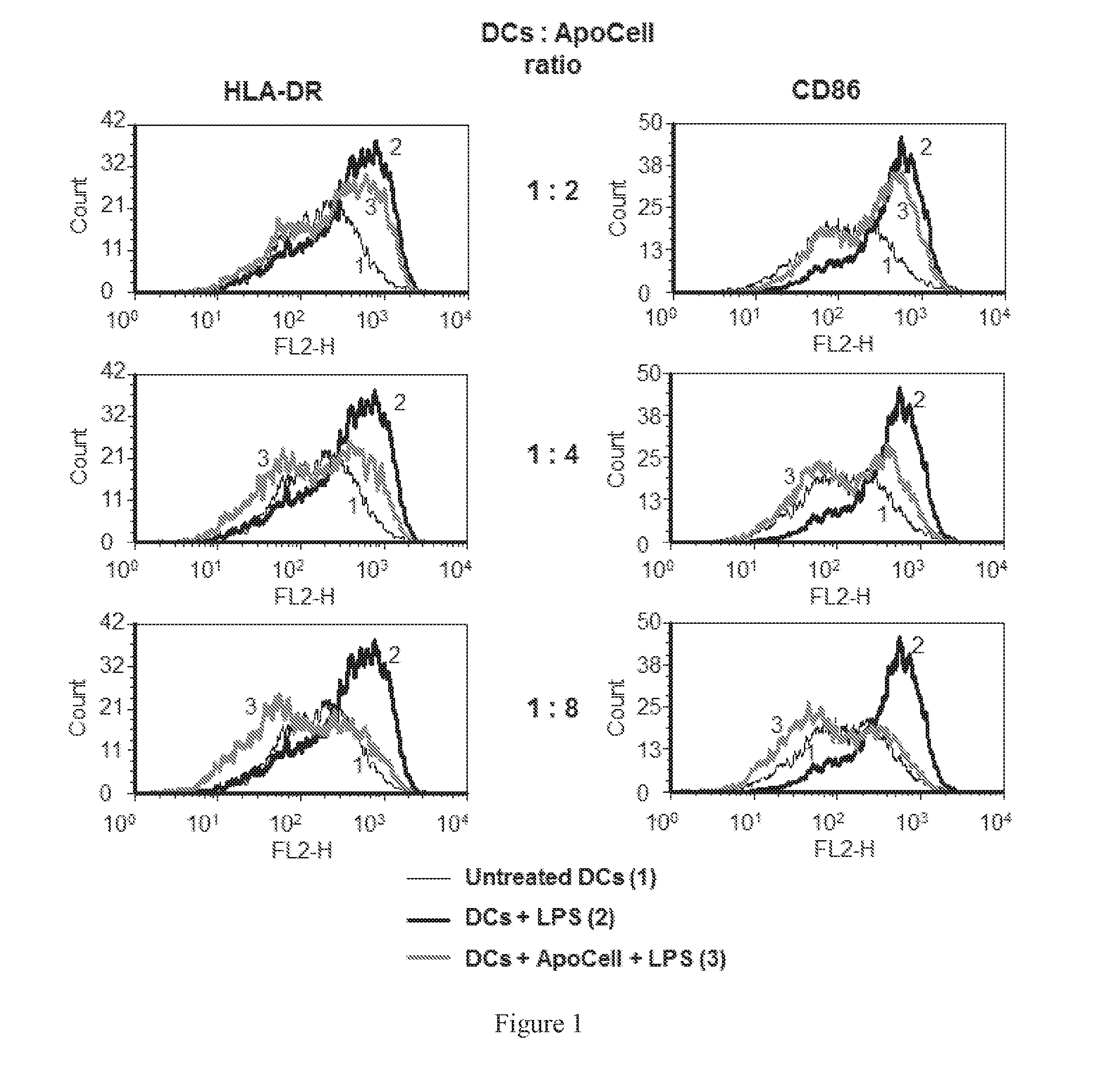
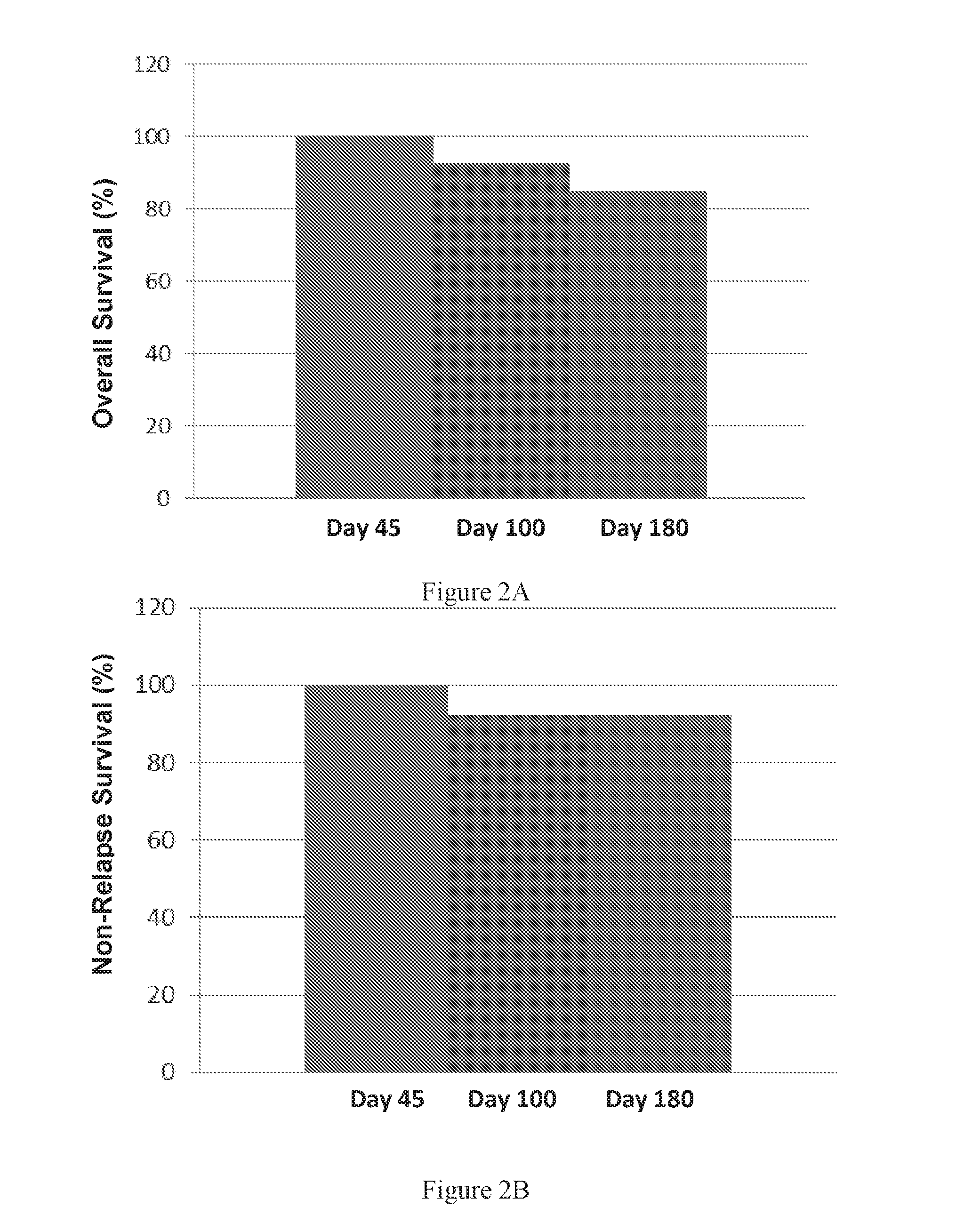
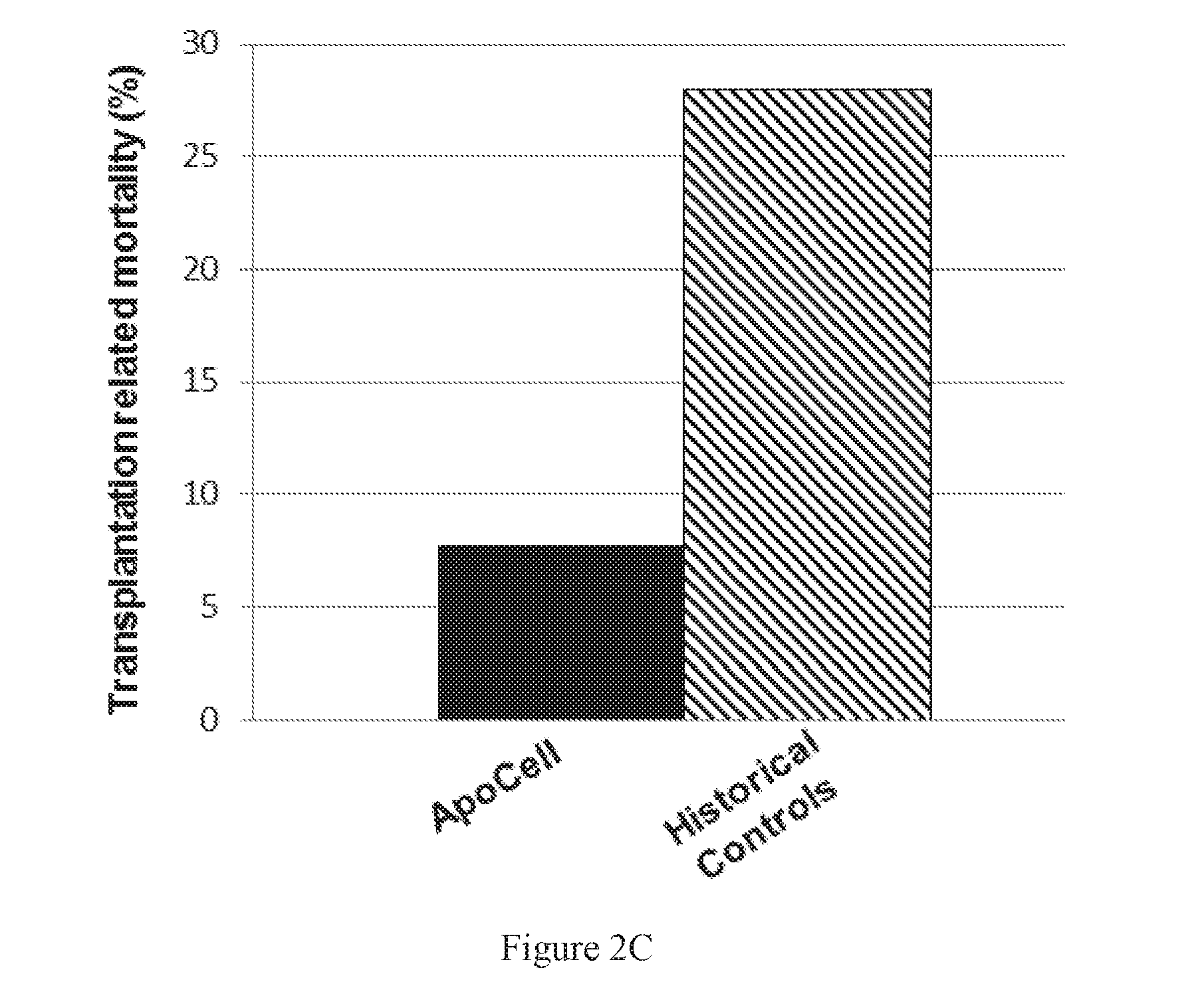
Autologous graft-versus-host disease induction in advanced breast cancer: role of peripheral blood progenitor cells ... The purpose of the present study was to investigate the impact of the use of peripheral blood progenitor cells (PBPCs) on the induction of autologous graft-versus-host disease (GVHD) in patients with advanced breast cancer. 14 ...
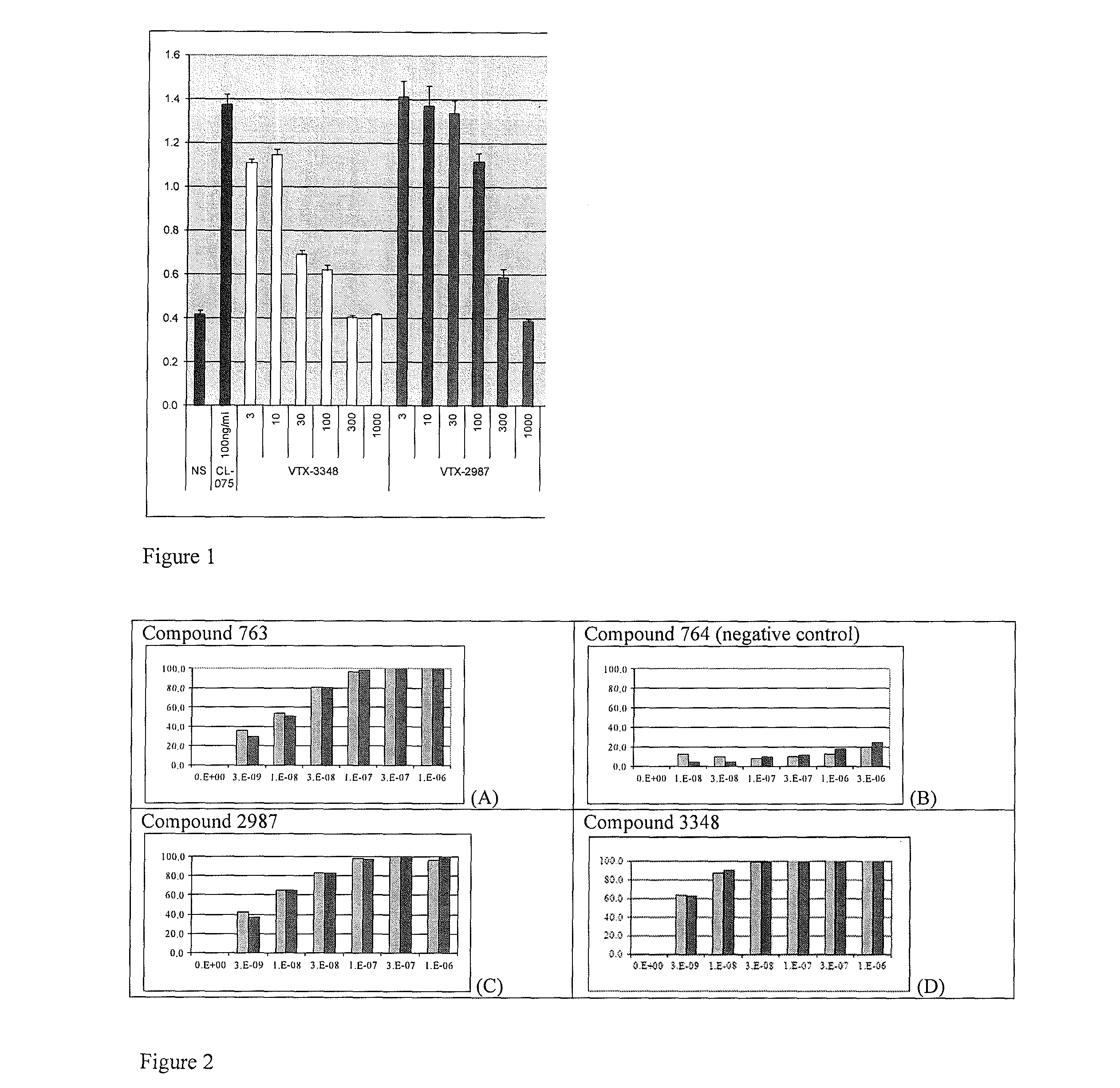
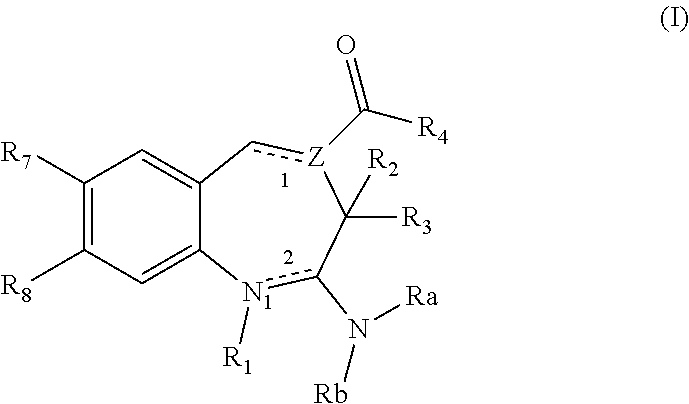
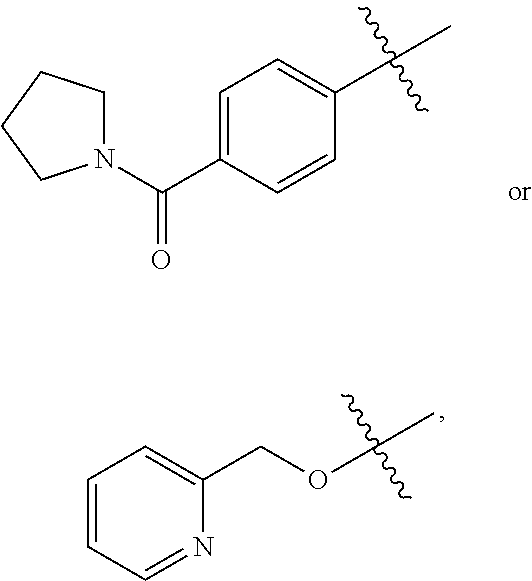
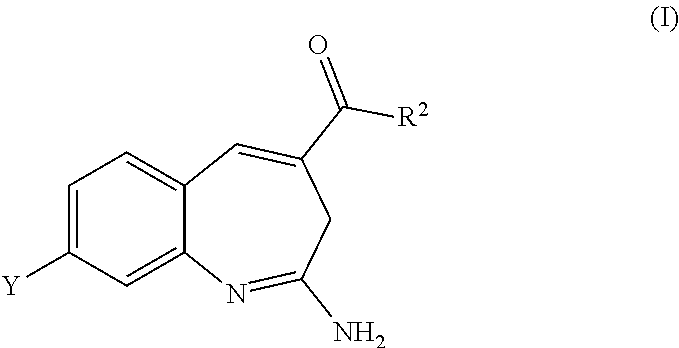
Substituted Benzoazepines As Toll-Like Receptor Modulators
Provided are compositions and methods useful for modulation of signaling through the Toll-like receptors TLR7 and / or TLR8. The compositions and methods have use in treating or preventing disease, including cancer, autoimmune disease, fibrotic disease, cardiovascular disease, infectious disease, inflammatory disorder, graft rejection, or graft-versus-host disease.
Owner:ARRAY BIOPHARMA +1
Substituted benzoazepines as toll-like receptor modulators
ActiveUS20110118235A1Efficient modulationAntibacterial agentsBiocideTLR8Graft versus host disease induction
Provided are compositions and methods useful for modulation of signaling through the Toll-like receptors TLR7 and / or TLR8. The compositions and methods have use in treating or preventing disease, including cancer, autoimmune disease, infectious disease, inflammatory disorder, graft rejection, and graft-verses-host disease.
Owner:ARRAY BIOPHARMA +1
Mesenchymal stem cells for prevention and treatment of immune responses in transplantation
InactiveUS6875430B2Ameliorate a response by the immune systemPrevent and reduce T cell responseBiocideMammal material medical ingredientsGraft versus host disease inductionGraft-versus-host disease
A method of reducing an immune response to a transplant in a recipient by treating said recipient with an amount of mesenchymal stem cells effective to reduce or inhibit host rejection of the transplant. The mesenchymal stem cells can be administered before, at the same time as, or after the transplant. Also disclosed is a method of inducing a reduced immune response against a host by foreign tissue, i.e., graft versus host disease, by treatment with mesenchymal stem cells.
Owner:MESOBLAST INT
Compositions and methods for inhibiting adverse immune response in histocompatibility-mismatched transplantation
ActiveUS20070264269A1Effective for adverse immune responseAntipyreticAnalgesicsGraft versus host disease inductionCell based
Cell-based compositions and methods of their use to inhibit an adverse immune response such as graft versus host disease or rejection of transplanted tissue in a transplant recipient that is histocompatibility mismatched to the transplant donor are disclosed. The compositions and methods utilize postpartum-derived cells, such as cells derived from the placenta or umbilicus.
Owner:DEPUY SYNTHES PROD INC
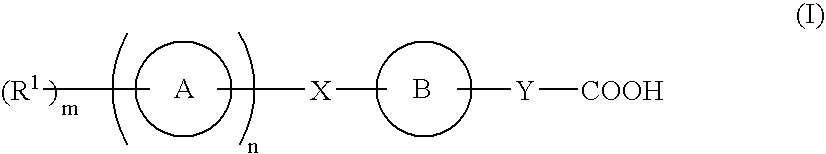
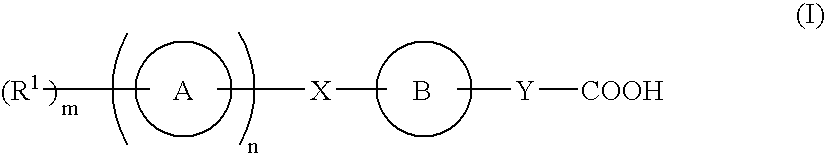
Compound capable of binding s1p receptor and pharmaceutical use thereof
InactiveUS20070167425A1Enhance pharmacological effectsLittle side effectsBiocideOrganic chemistryAutoimmune conditionHydrogen atom
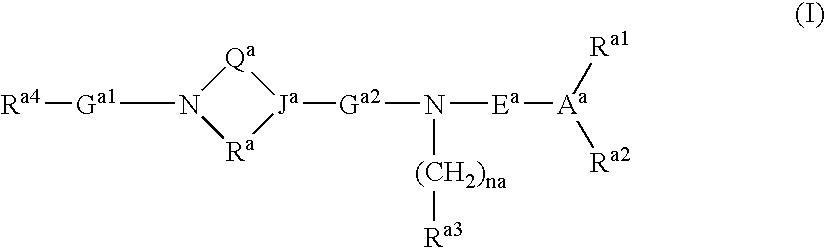
A compound having an ability to bind to an S1P receptor (particularly EDG-6, preferably EDG-1 and EDG-6), for example, the compound represented by formula (I) of the present invention, a salt thereof, a solvate thereof or a prodrug thereof is useful for prevention and / or treatment of rejection of transplantation, graft-versus-host disease, autoimmune disease, allergic disease and the like. wherein ring A is a cyclic group; ring B is a cyclic group which may have substituent(s); X is a spacer having 1 to 8 atoms in its main chain, etc.; Y is a spacer having 1 to 10 atoms in its main chain, etc.; n is 0 or 1, wherein when n is 0, m is 1 and R1 is a hydrogen atom or a substituent, and wherein when n is 1, m is 0 or an integer of 1 to 7 and R1 is a substituent, and wherein m is 2 or more, R1s are the same or different.
Owner:ONO PHARMA CO LTD
Gene expression analysis of pluri-differentiated mesenchymal progenitor cells and methods for diagnosing a leukemic disease state
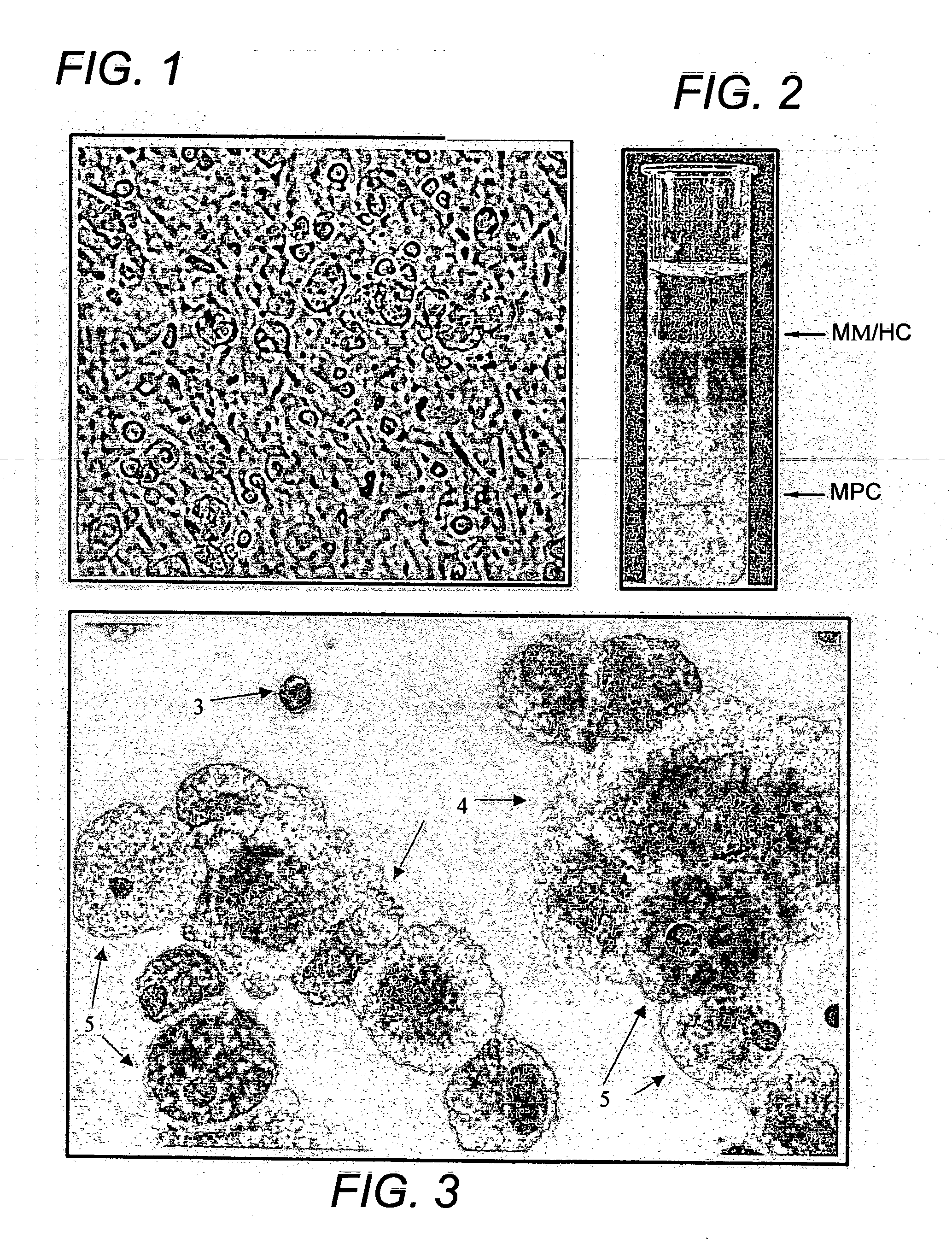
Pluri-differentiated human mesenchymal progenitor cells (MPCs) are isolated. A method isolates and purifies human mesenchymal progenitor cells from Dexter-type cultures for characterization of and uses, particularly therapeutic uses for such cells. Specifically, isolated MPCs can be used for diagnostic purposes, to enhance the engraftment of hematopoietic progenitor cells, enhance bone marrow transplantation, or aid in the treatment or prevention of graft versus host disease.
Owner:SOUTH FLORIDA UNIVESITY OF
Methods and compositions for immunotherapy and detection of inflammatory and immune-dysregulatory disease, infectious disease, pathologic angiogenesis and cancer
InactiveUS20060140936A1Antibacterial agentsOrganic active ingredientsDendritic cellAutoimmune condition
Methods and compositions for immunotherapy of inflammatory and immune-dysregulatory diseases, using multispecific antagonists that target at least two different markers are disclosed. The different targets include (i) proinflammatory effectors of the innate immune system, (ii) coagulation factors, and (iii) targets specifically associated with an inflammatory or immune-dysregulatory disorder, with a pathologic angiogenesis or cancer, or with an infectious disease, wherein the targets included in group (iii) are neither a proinflammatory effector of the immune system nor a coagulation factor. When the multispecific antagonist reacts specifically with a target associated with an inflammatory or immune-dysregulatory disorder, with a pathologic angiogenesis or cancer, or with an infectious disease, it also binds specifically with at least one proinflammatory effector of the immune system or at least one coagulation factor. Thus, the multispecific antagonist contains at least one binding specificity related to the diseased cell or condition being treated and at least one specificity to a component of the immune system, such as a receptor or antigen of B cells, T cells, neutrophils, monocytes and macrophages, and dendritic cells, a modulator of coagulation, or a proinflammatory cytokine. The multispecific antagonists are used in the treatment of various diseases that are generated or exacerbated by, or otherwise involve, proinflammatory effectors of the innate immune system or coagulation factors. Such diseases more particularly include acute and chronic inflammatory disorders, autoimmune diseases, giant cell arteritis, septicemia and septic shock, coagulopathies (including diffuse intravascular coagulation), neuropathies, graft versus host disease, infectious diseases, acute respiratory distress syndrome, granulomatous diseases, transplant rejection, asthma, cachexia, myocardial ischemia, and atherosclerosis. Other diseases also responsive to these therapies include cancers and conditions with pathological angiogenesis.
Owner:IMMUNOMEDICS INC
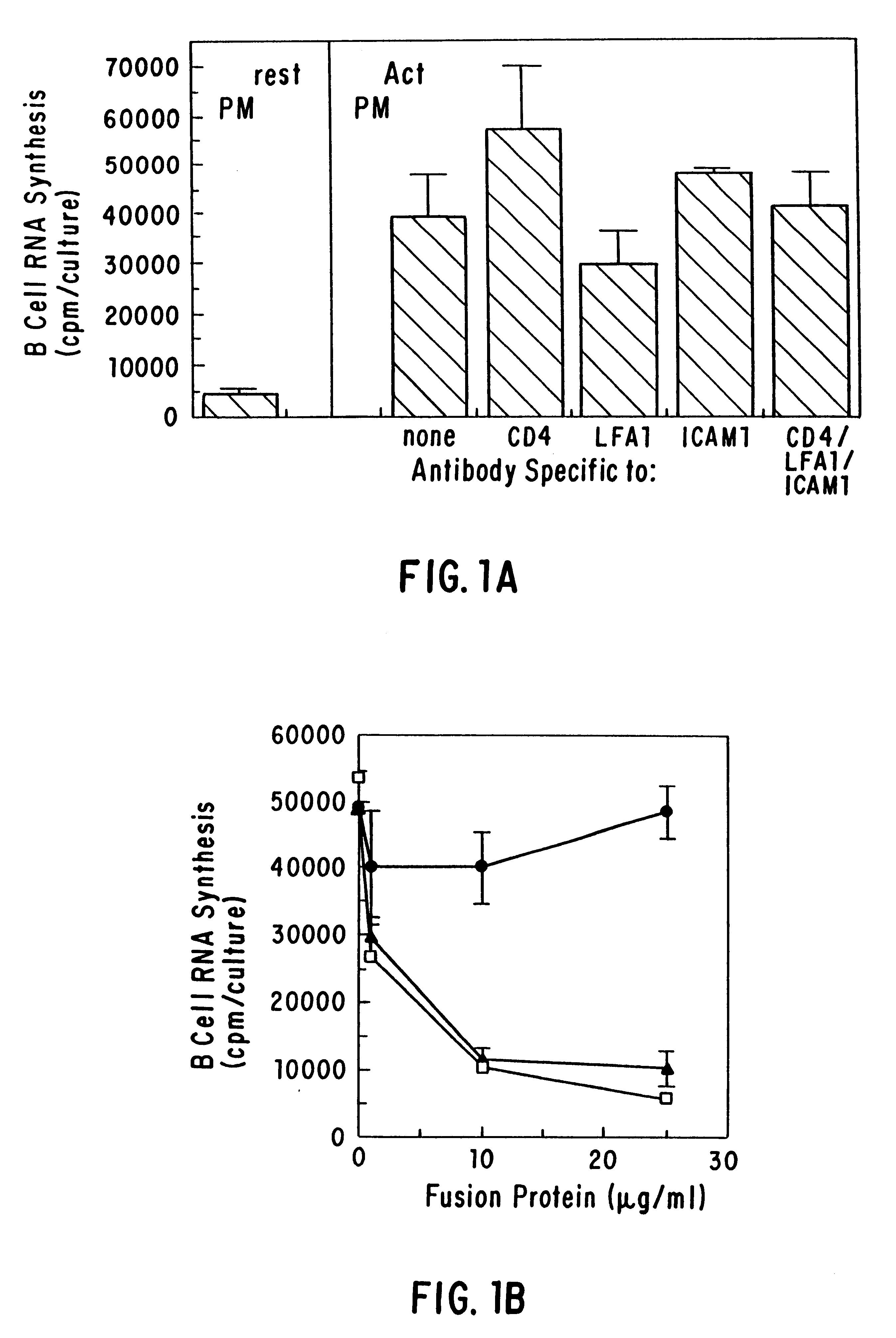
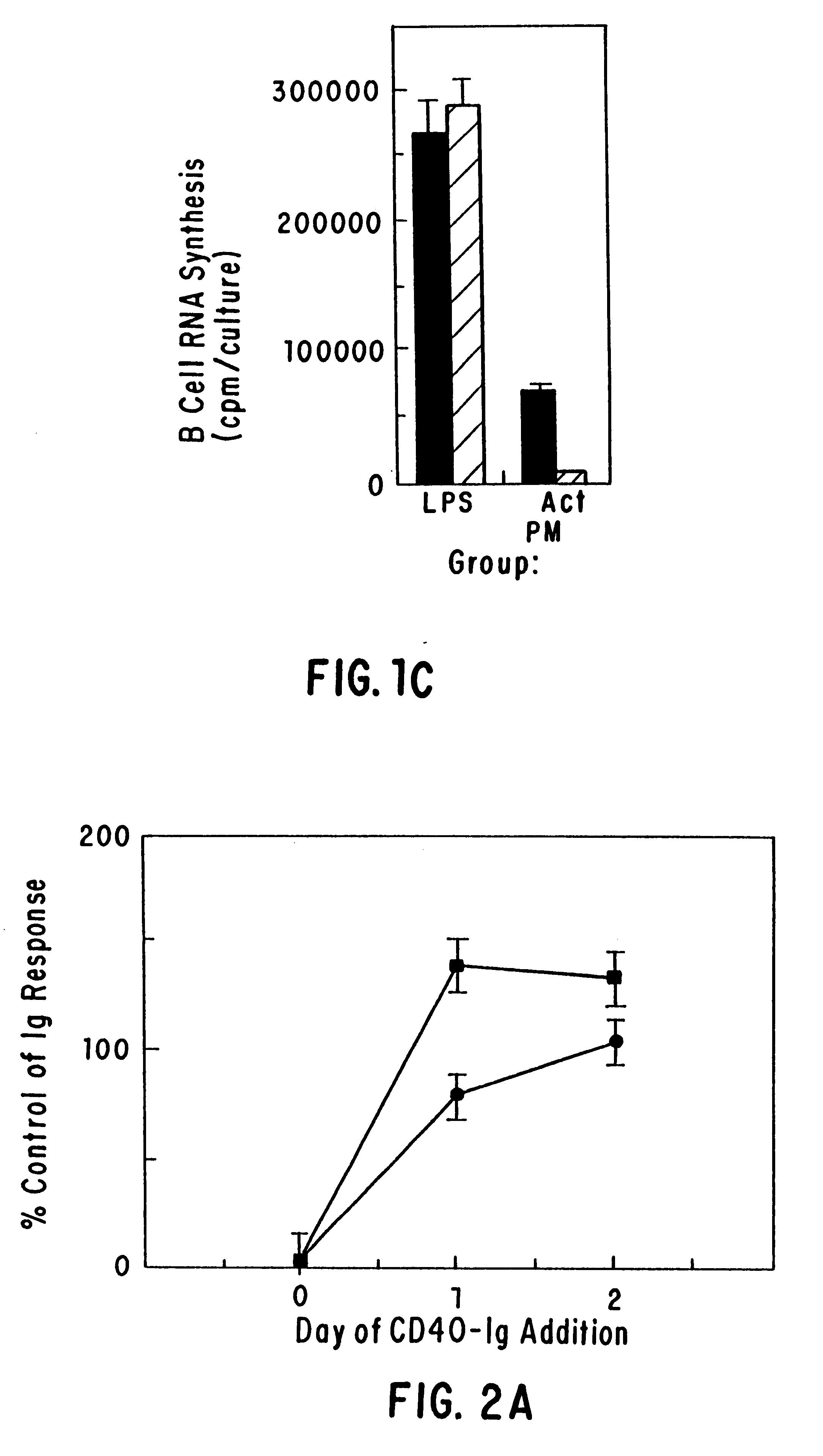
Inhibiting B cell activation with soluble CD40 or fusion proteins thereof
The present invention relates to a counter-receptor, termed CD40CR, for the CD40 B-cell antigen, and to soluble ligands for this receptor, including fusion molecules comprising at least a portion of CD40 protein. It is based, at least in part, on the discovery that a soluble CD40 / immunoglobulin fusion protein or antibody specific for gp39 on T cells was able to inhibit helper T-cell mediated B-cell activation by binding to a novel 39 kD protein receptor on helper T-cell membranes. The present invention provides for a substantially purified CD40CR receptor; for soluble ligands of CD40CR, including antibodies as well as fusion molecules comprising at least a portion of CD40 protein; and for methods of controlling B-cell activation which may be especially useful in the treatment of allergy or autoimmune disease, including graft-versus-host disease and rheumatoid arthritis.
Owner:BRISTOL MYERS SQUIBB CO +1
Apoptosis inducing molecule II and methods of use
The present invention relates to a novel member of the TNF-Ligand superfamily. More specifically, isolated nucleic acid molecules are provided encoding a human Apoptosis Inducing Molecule II (AIM II). AIM II polypeptides are also provided, as are vectors, host cells and recombinant methods for producing the same. The invention further relates to screening methods for identifying agonists and antagonists of AIM II activity. Also provided are therapeutic methods for treating lymphadenopathy, aberrant bone development, autoimmune and other immune system diseases, graft versus host disease, rheumatoid arthritis, osteoarthritis and to inhibit neoplasia, such as tumor cell growth.
Owner:HUMAN GENOME SCI INC
Method for generating t-cells compatible for allogenic transplantation
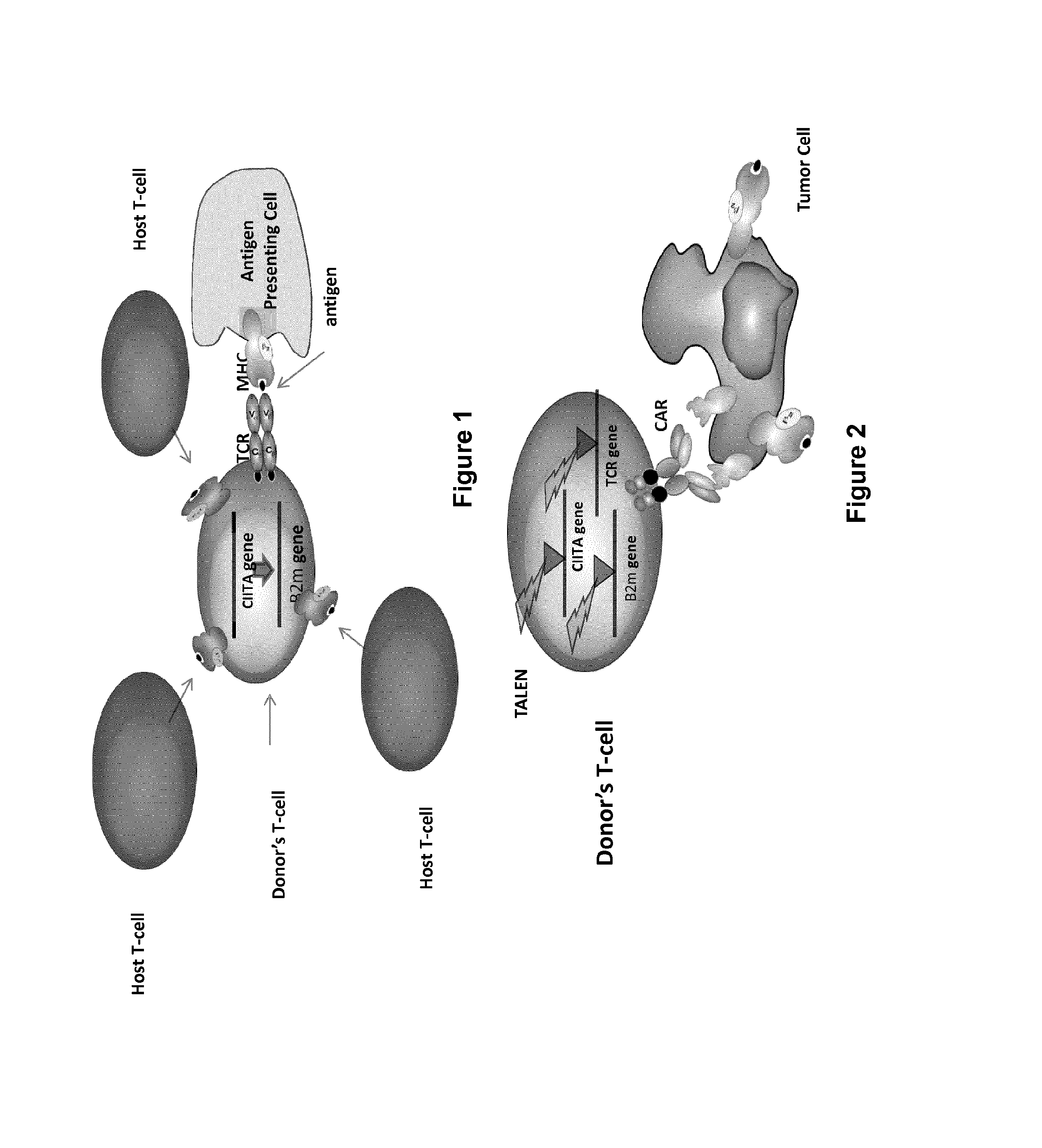
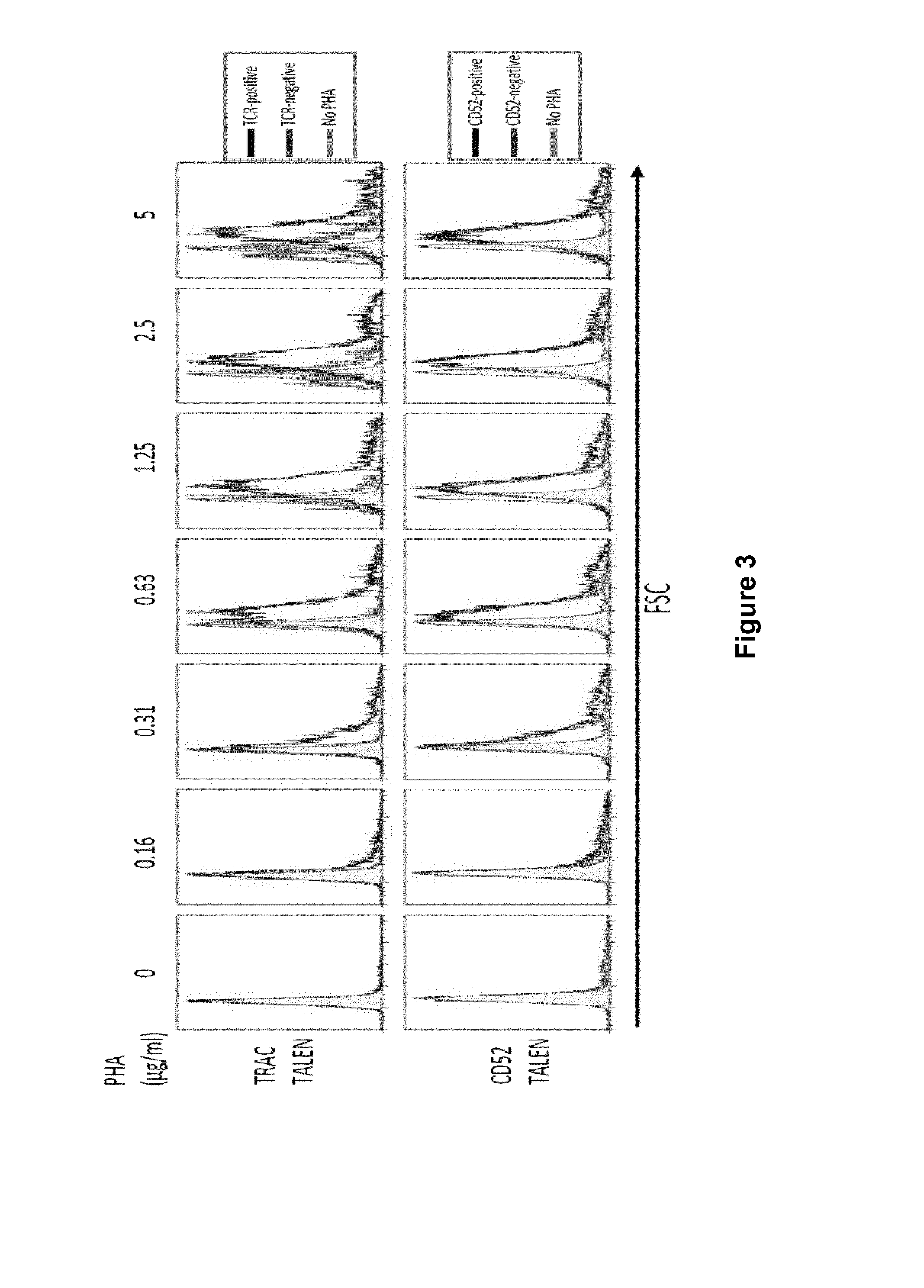
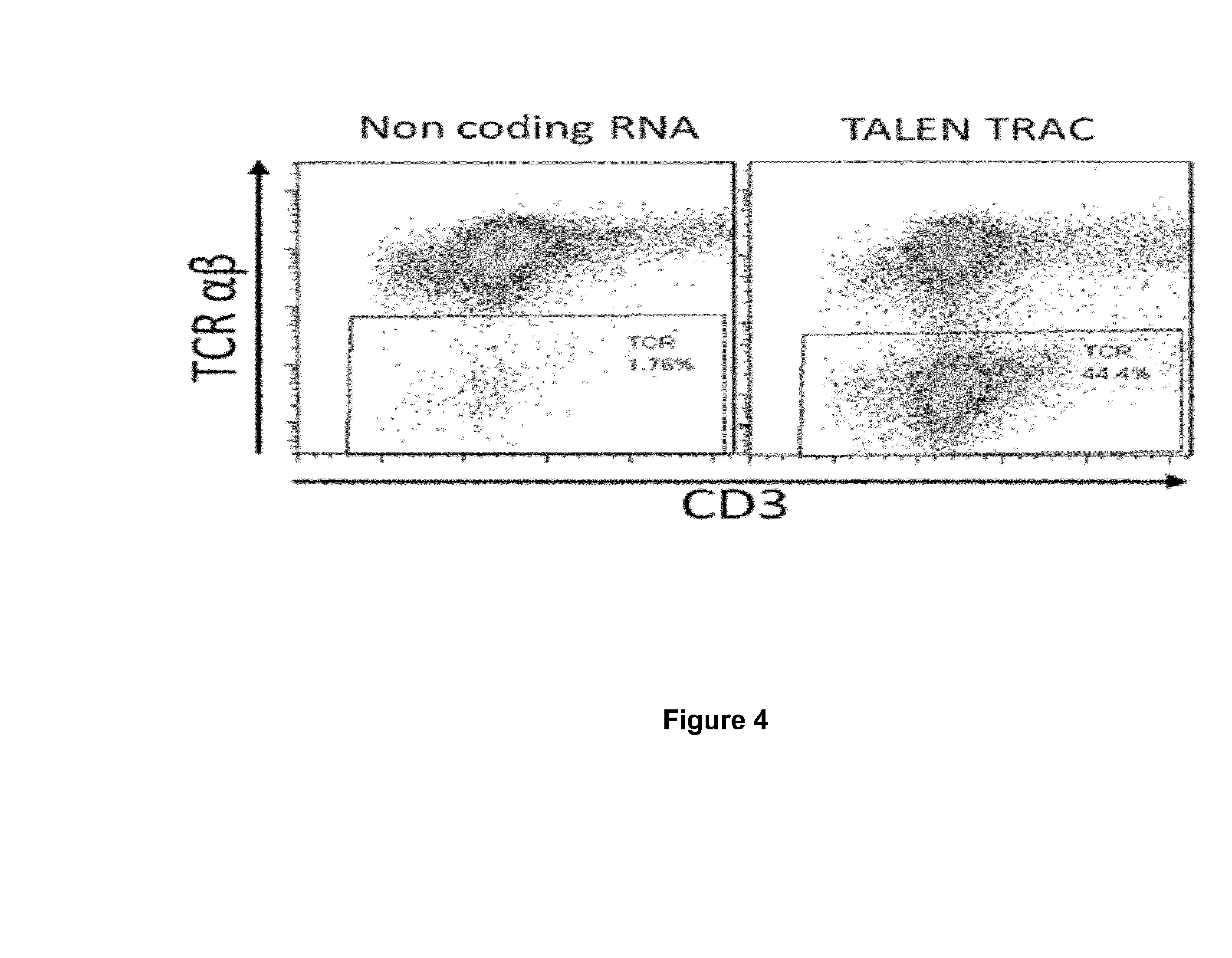
InactiveUS20170016025A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyCIITAAuto immune disease
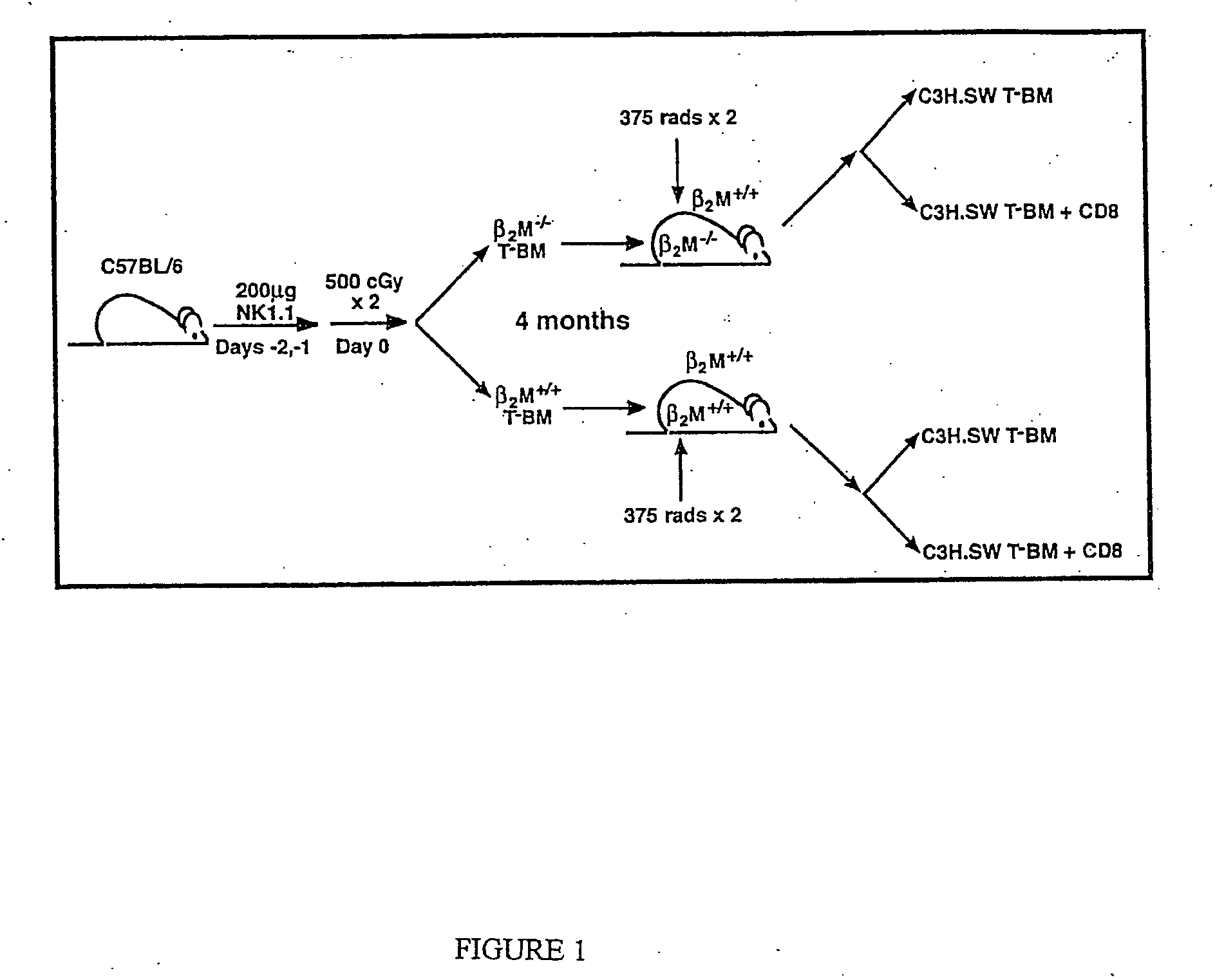
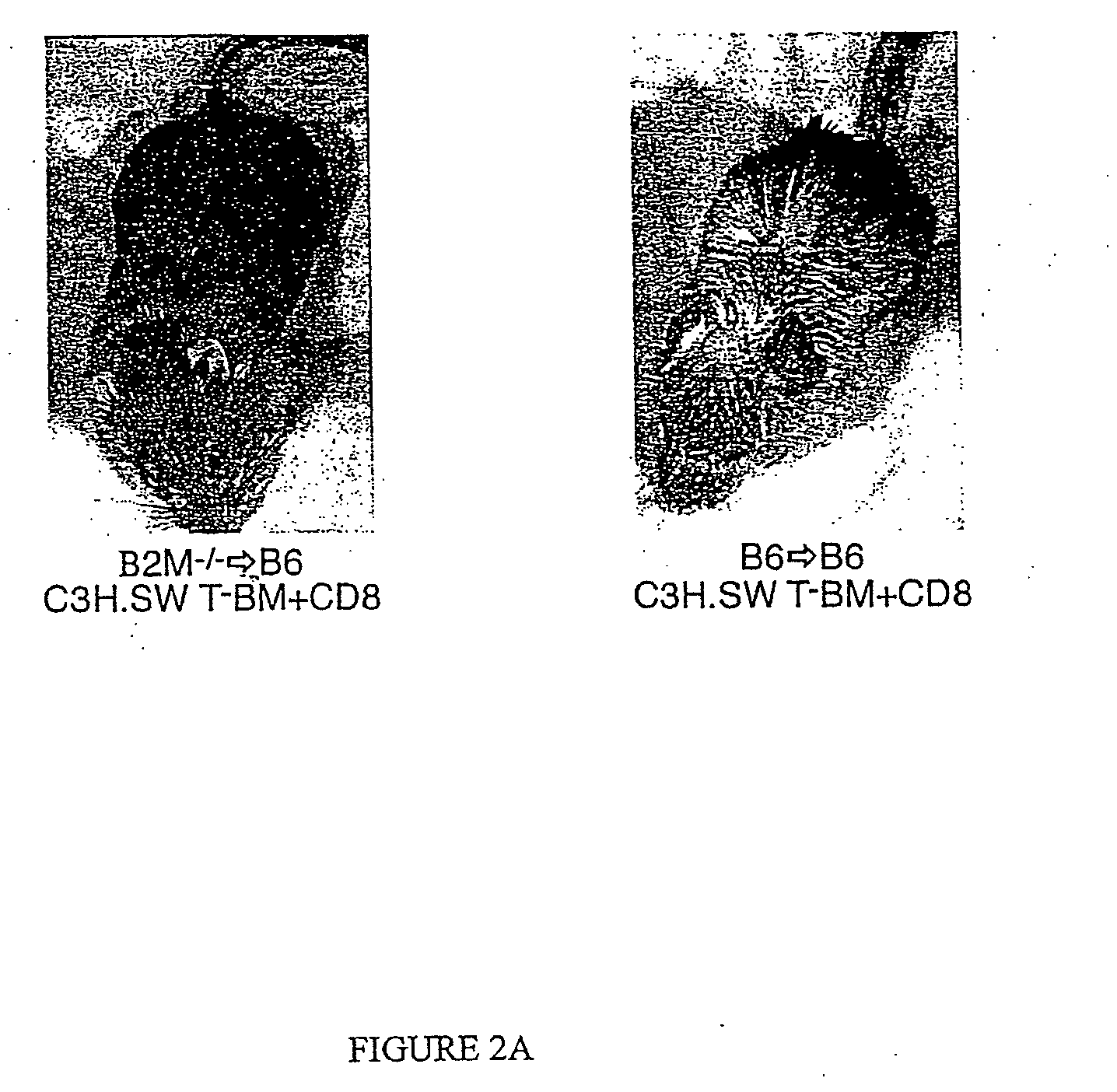
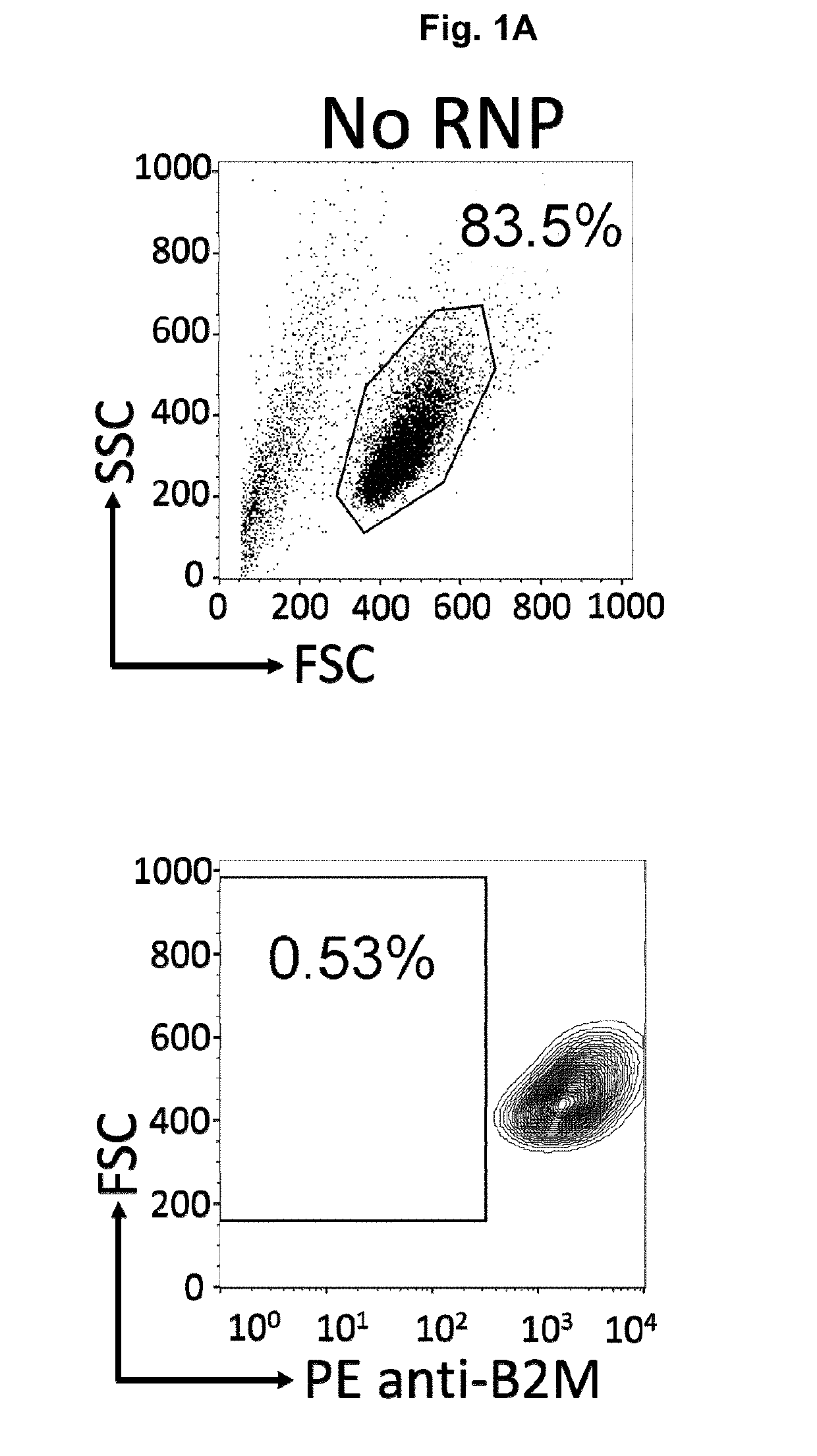
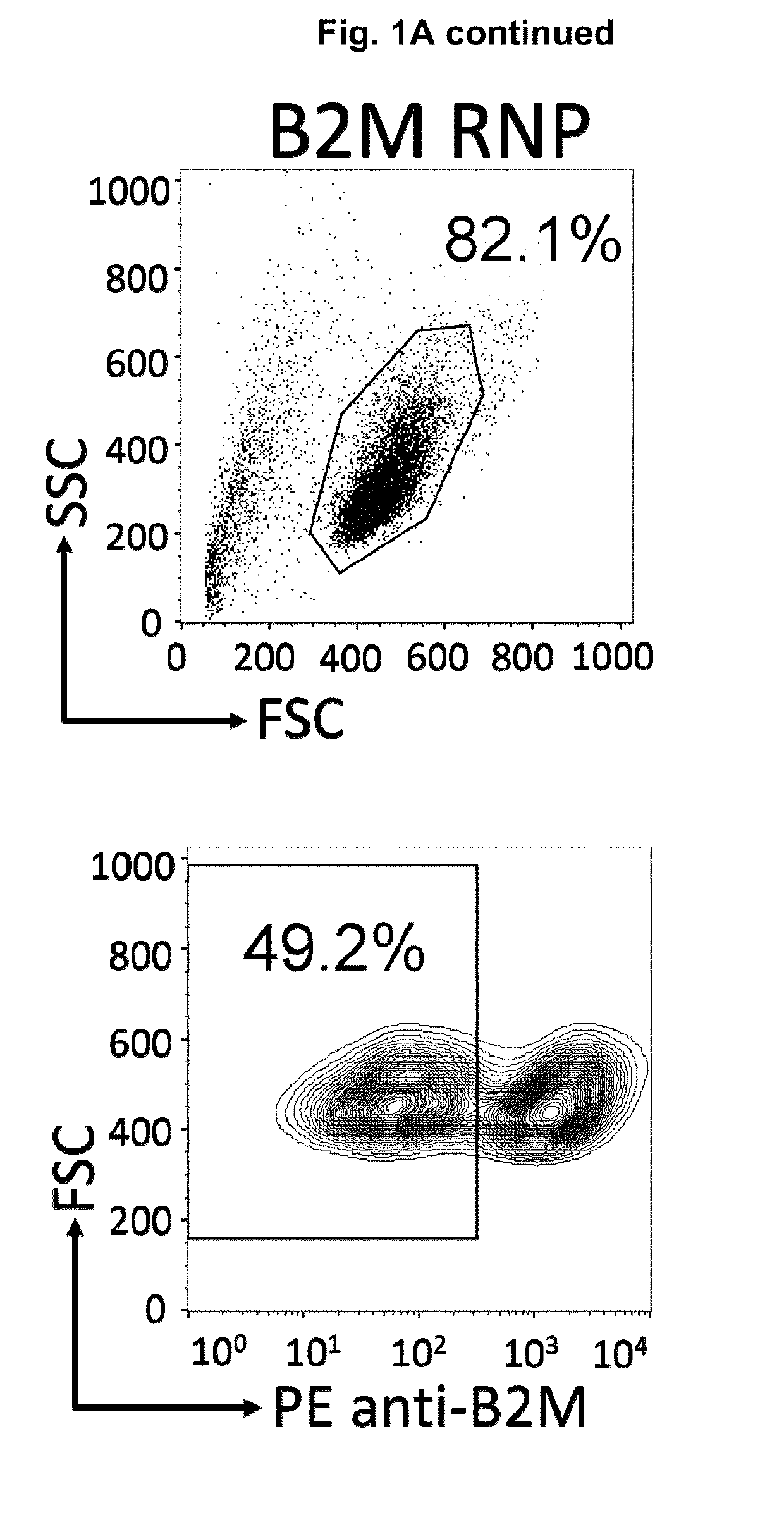
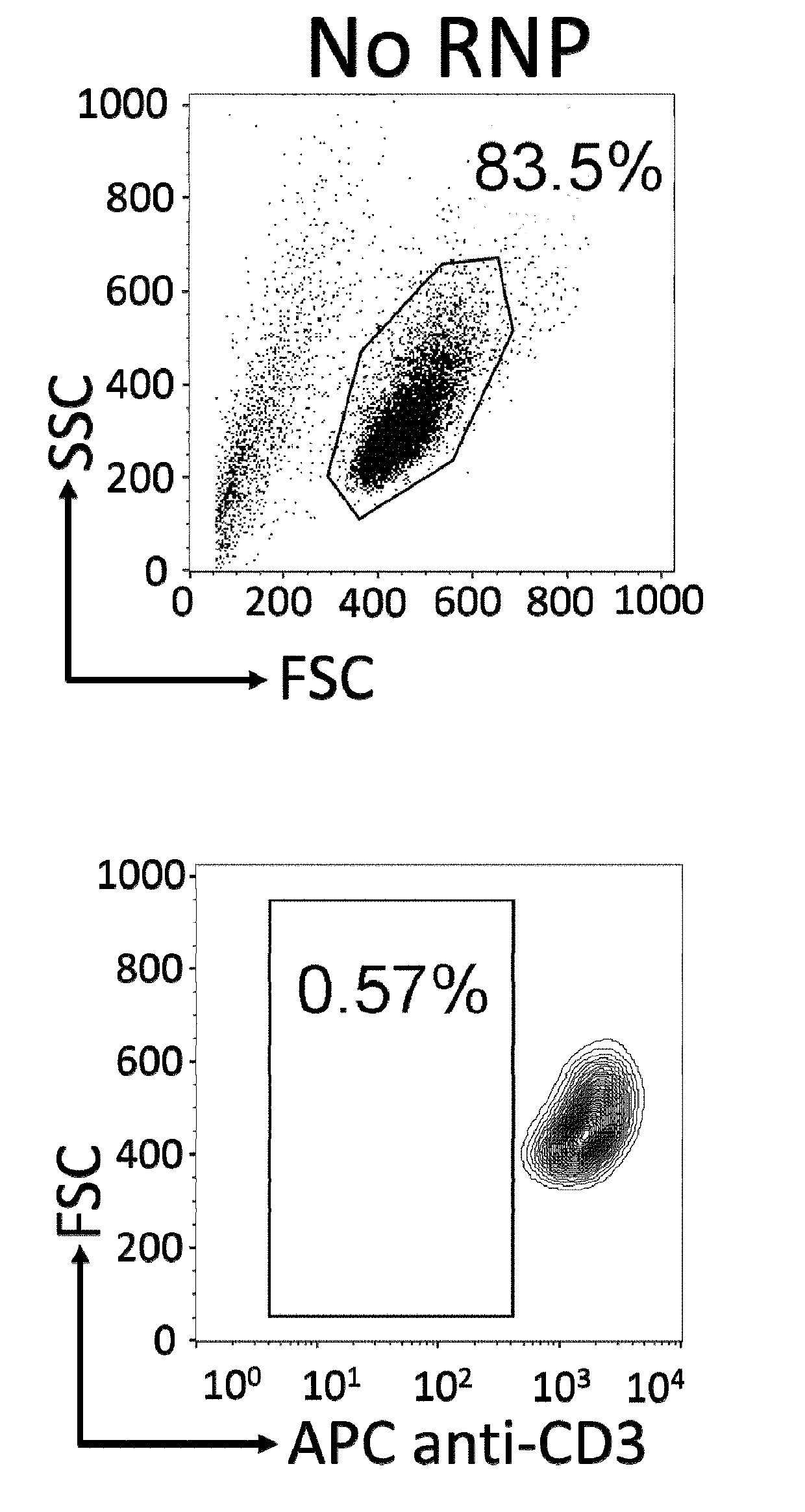
The present invention pertains to engineered T-cells, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered T-cells of the invention are characterized in that the expression of beta 2-microglobulin (B2M) and / or class II major histocompatibility complex transactivator (CIITA) is inhibited, e.g., by using rare-cutting endonucleases able to selectively inactivating by DNA cleavage the gene encoding B2M and / or CIITA, or by using nucleic acid molecules which inhibit the expression of B2M and / or CIITA. In order to further render the T-cell non-alloreactive, at least one gene encoding a component of the T-cell receptor is inactivated, e.g., by using a rare-cutting endonucleases able to selectively inactivating by DNA cleavage the gene encoding said TCR component. In addition, expression of immunosuppressive polypeptide can be performed on those modified T-cells in order to prolong the survival of these modified T cells in host organism. Such modified T-cell is particularly suitable for allogeneic transplantations, especially because it reduces both the risk of rejection by the host's immune system and the risk of developing graft versus host disease. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer, infections and auto-immune diseases.
Owner:CELLECTIS SA
Cellular compositions which facilitate engraftment of hematopoietic stem cells while minimizing the risk of gvhd
The present invention provides for cellular compositions which facilitate engraftment of hematopoietic stem cells from a syngeneic, allogeneic or xenogeneic donor. The cellular compositions of the invention facilitate engraftment while minimizing the risk of graft versus host disease in the graft recipient. According to a preferred embodiment of the invention, a cell composition is provided, which cell composition comprises hematopoietic stem cells, such as CD34+ cells and / or facilitating cells, in combination with αβ TCR+ T cells. The invention also relates to methods of using the cellular compositions of the invention to induce donor specific tolerance in a recipient, thus allowing the transplantation of donor organs, cells and tissues. Also disclosed are methods of treating leukemia and cancer as well as infectious diseases caused by viruses.
Owner:JEWISH HOSPITAL HEALTHCARE SERVICES
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20070258981A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAntigenAutoimmune condition
Owner:MEDIMMUNE LLC +1
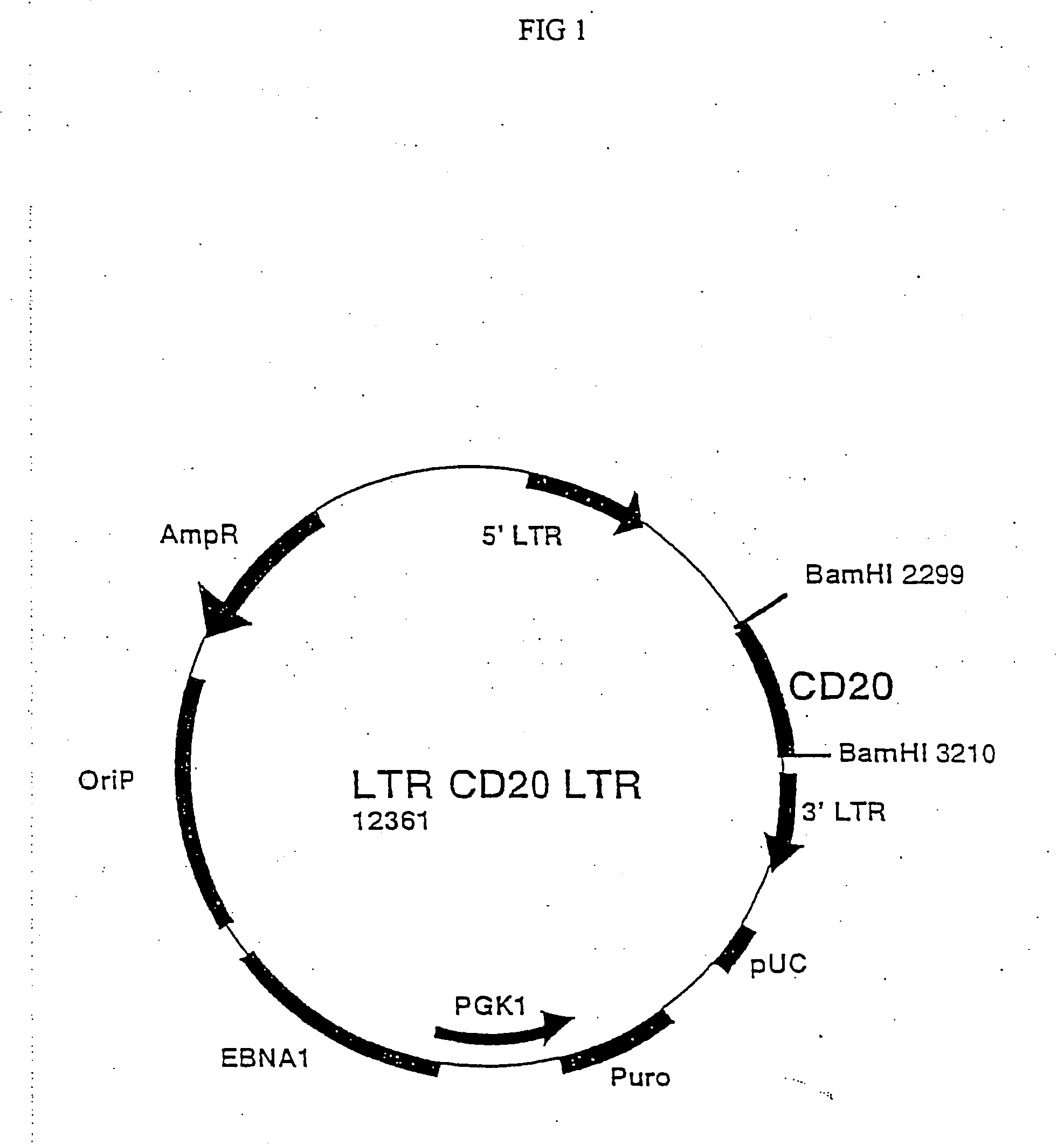
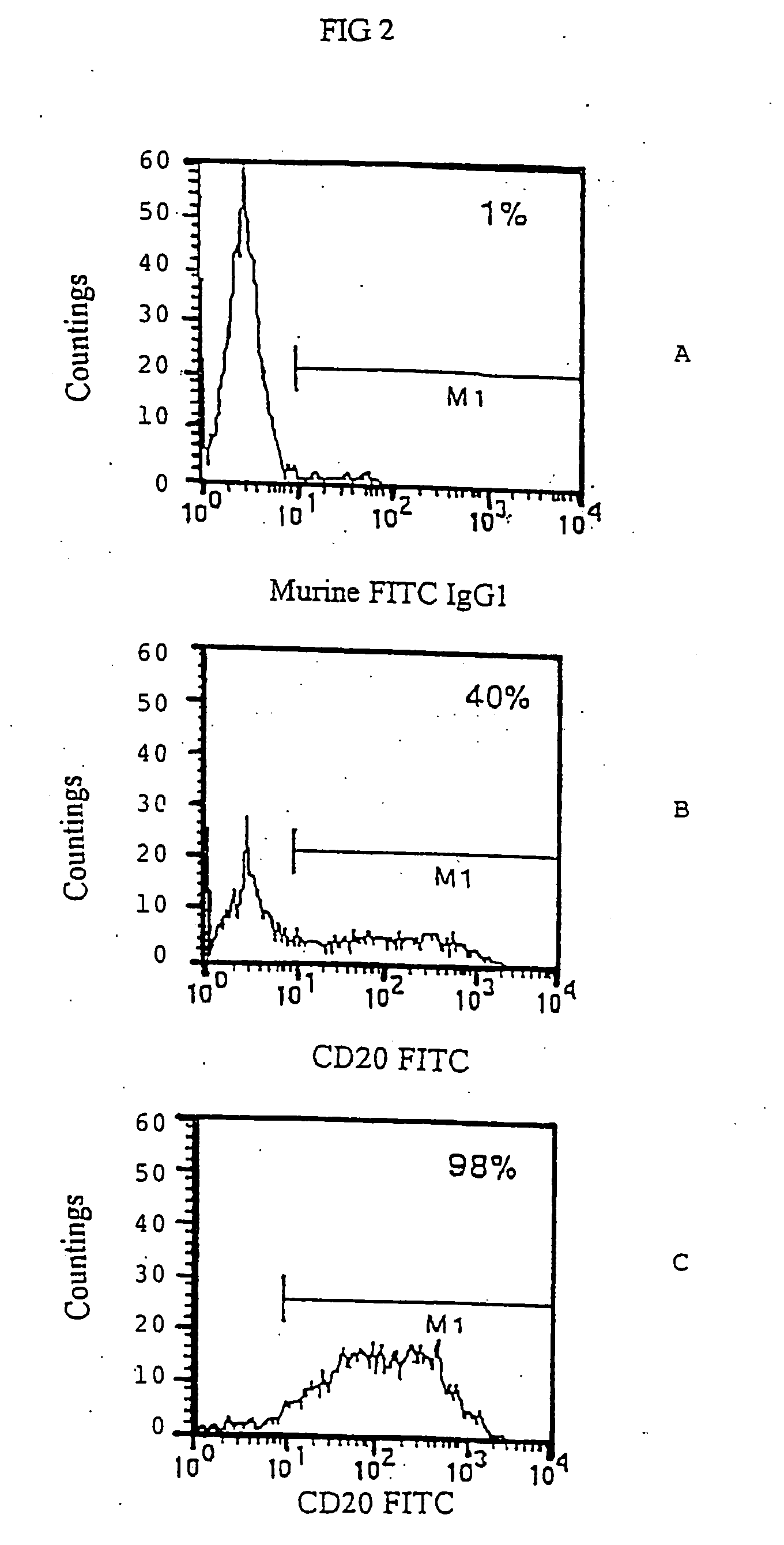
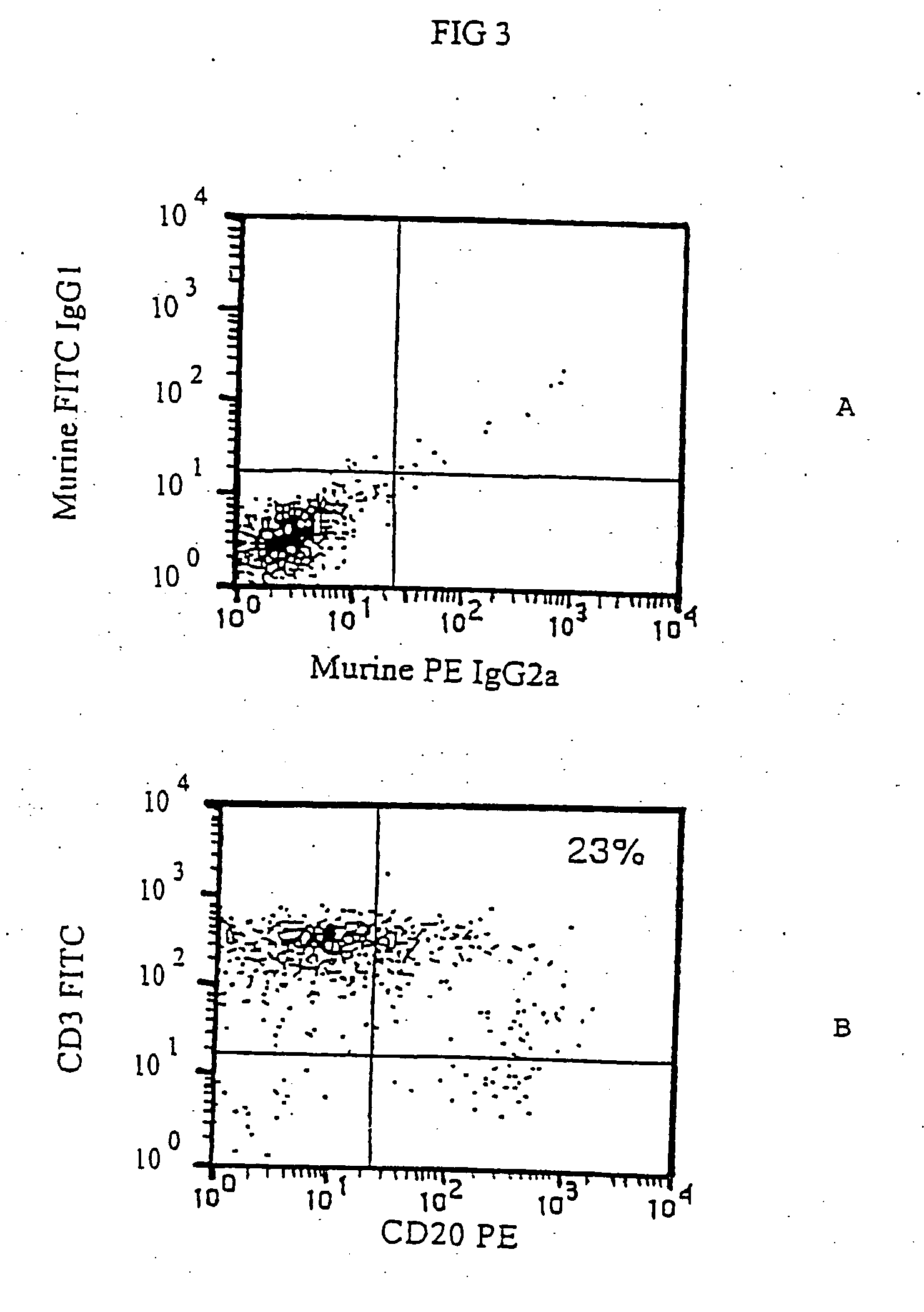
Use of antibodies against CD20 for the treatment of the graft versus host disease
It is described the use of antibodies against exogenous surface antigens not present on normal human T lymphocytes for the preparation of compositions for the treatment of the graft versus host disease in patients who have received T lymphocytes transduced with such exogenous surface antigens.
Owner:CONSIGLIO NAT DELLE RICERCHE
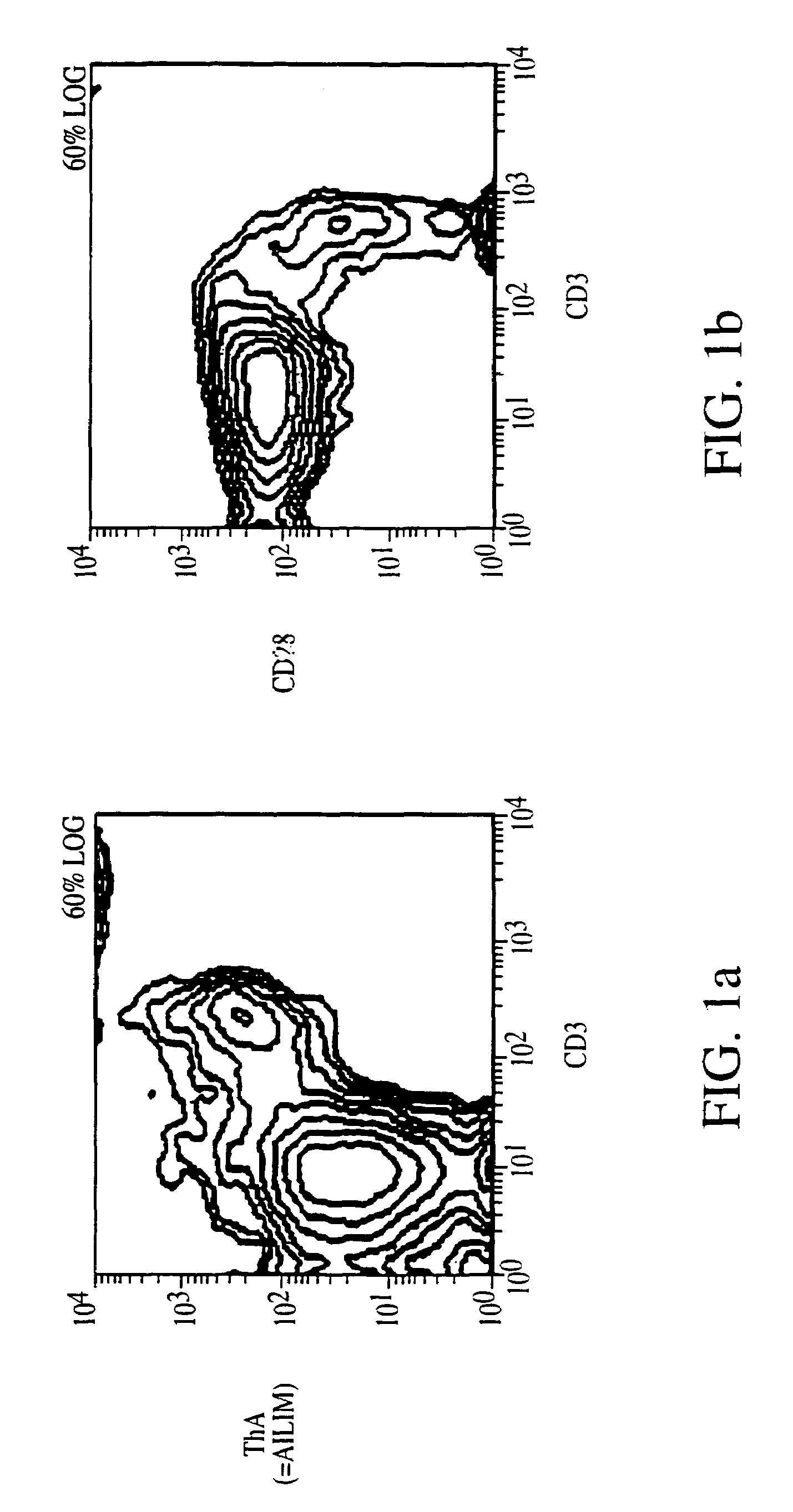
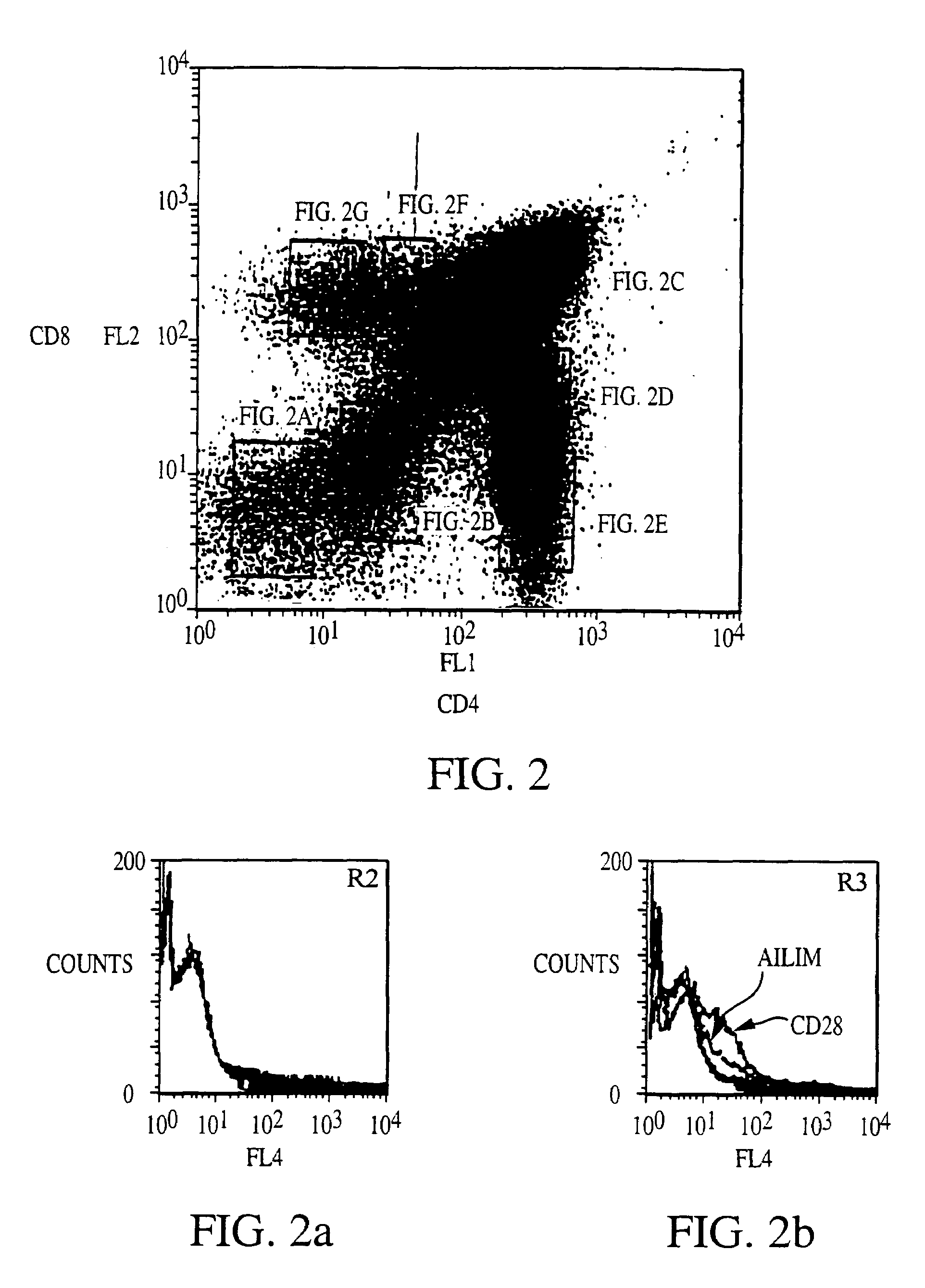
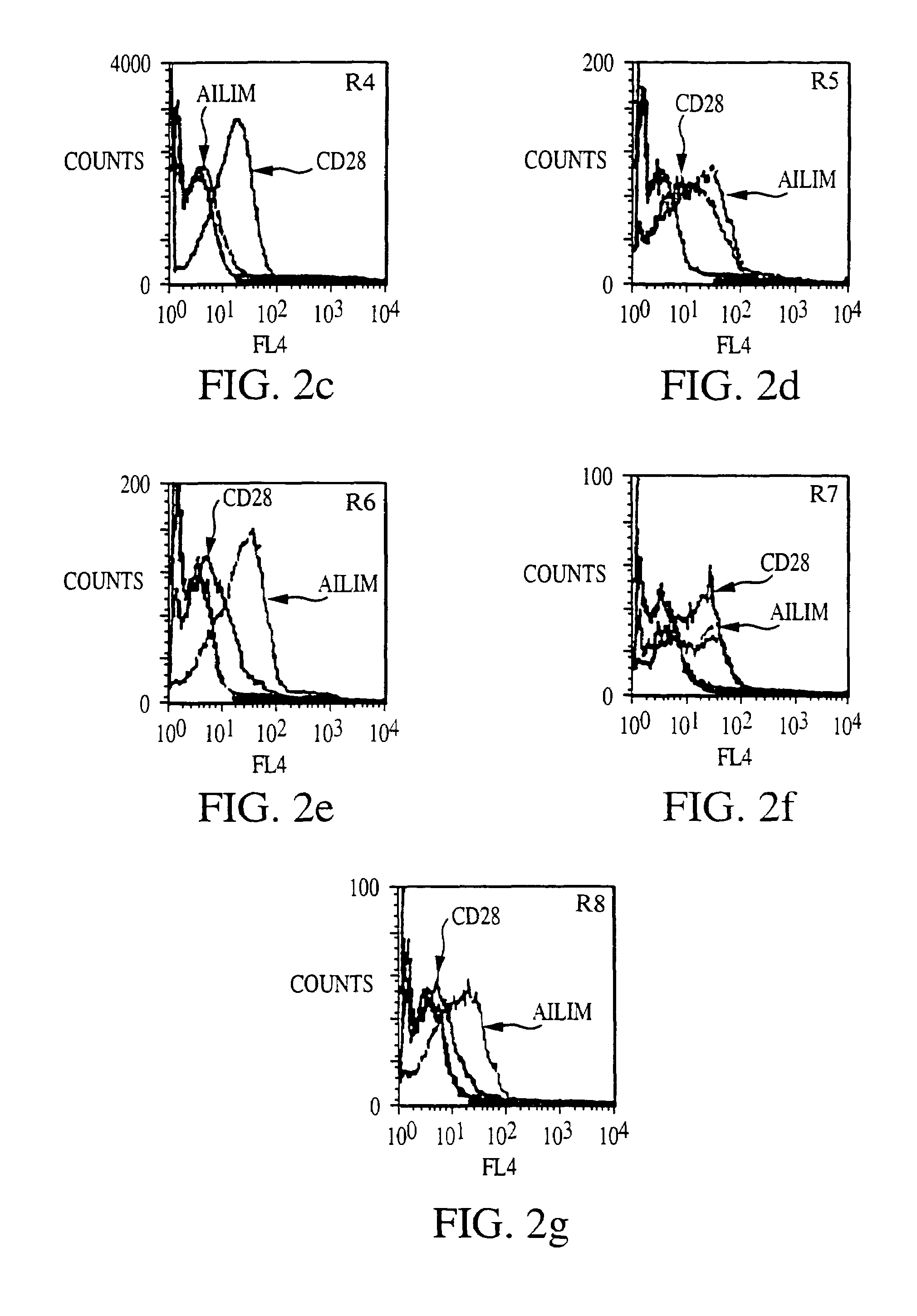
Methods of preventing or treating graft versus host reaction by administering an antibody or portion thereof that binds to AILIM
InactiveUS7465445B2Modulate productionPrevent diseaseOrganic active ingredientsBiocideAntigenTherapeutic effect
An antibody against AILIM (alternatively called JTT-1 antigen, JTT-2 antigen, ICOS and 8F4) was found to have a significant therapeutic effect on arthrosis, for example, rheumatoid arthritis and osteoarthritis, graft versus host disease, graft immune rejection, inflammation (hepatitis and inflammatory bowel diseases), diseased condition accompanied by the excessive production of an antibody against a foreign antigen triggered by immunological sensitization by the antigen.
Owner:JAPAN TOBACCO INC
Purging of cells using viruses
InactiveUS20020037543A1Preventing graft-versus-host diseasesHigh selectivityNervous disorderPeptide/protein ingredientsAutoimmune conditionGraft versus host disease induction
The subject invention relates to viruses that are able to purge (reduce or eliminate) undesirable cells in a mixture of cells. Undesirable cells can include neoplastic cells, cells mediating graft-versus host diseases, and autoimmune cells. The subject invention also relates to the purging of undesirable cells from bone marrow or peripheral blood cell harvests in the treatment of mammals including cancer patients, transplant recipients, and patients with autoimmune disease.
Owner:PRO VIRUS
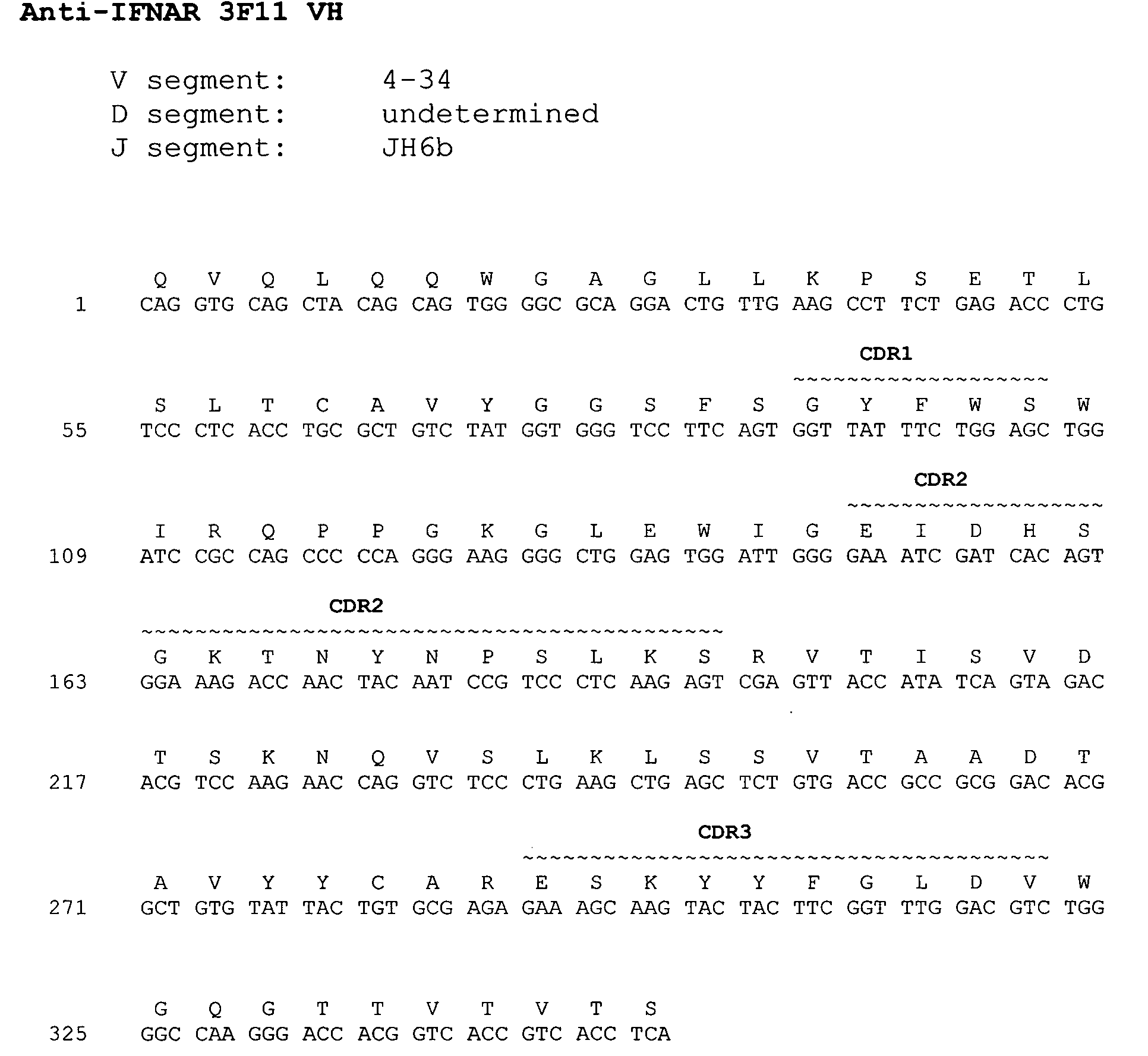
Interferon alpha receptor 1 antibodies and their uses
ActiveUS20060029601A1Inhibit biological activityNervous disorderAntipyreticTransplant rejectionGraft versus host disease induction
The present invention provides isolated human monoclonal antibodies that bind to IFNAR-1 and that are capable of inhibiting the biological activity of Type I interferons. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for inhibiting Type I interferon-mediated disorders using the antibodies of the invention, including methods for treating autoimmune disorders, transplant rejection or Graft Versus Host Disease using the antibodies of the invention.
Owner:ER SQUIBB & SONS INC
Prevention, decrease, and/or treatment of immunoreactivity by depleting and/or inactivating antigen presenting cells in the host
InactiveUS20060222633A1Efficiently presentedPreventing graft versus host diseaseBiocideBacterial antigen ingredientsAntigenGraft versus host disease induction
The invention includes compositions and methods for depleting and / or inactivating antigen presenting cells, or for otherwise impairing the biological function of antigen presenting cells, which compositions are useful for treatment of graft versus host disease and other immune diseases.
Owner:YALE UNIV
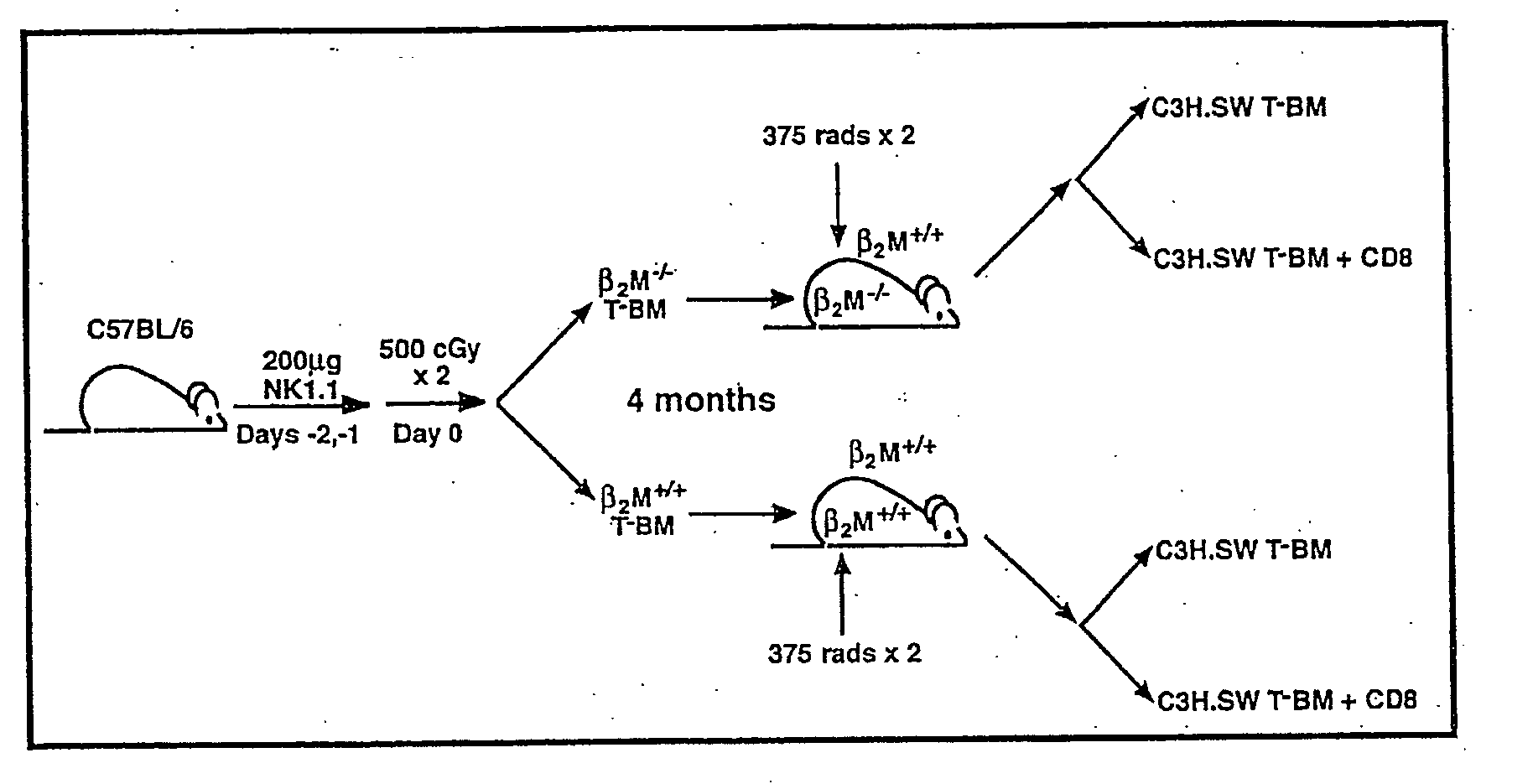
Methods for enhancing engraftment of purified hematopoietic stem cells in allogenic recipients
InactiveUS20070098693A1Facilitate HSC engraftmentEnhance engraftmentBiocidePeptide/protein ingredientsDendritic cellGraft versus host disease induction
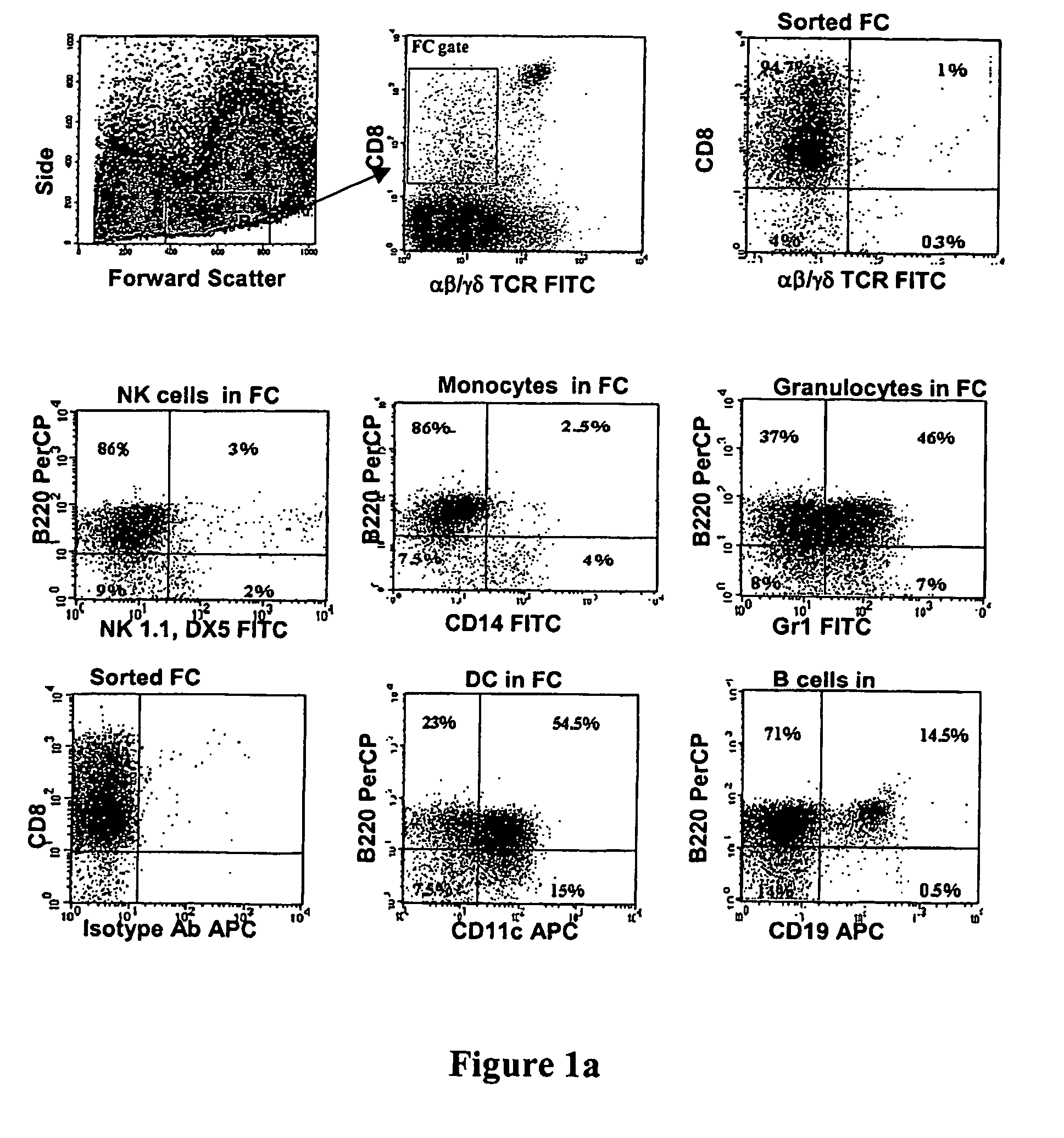
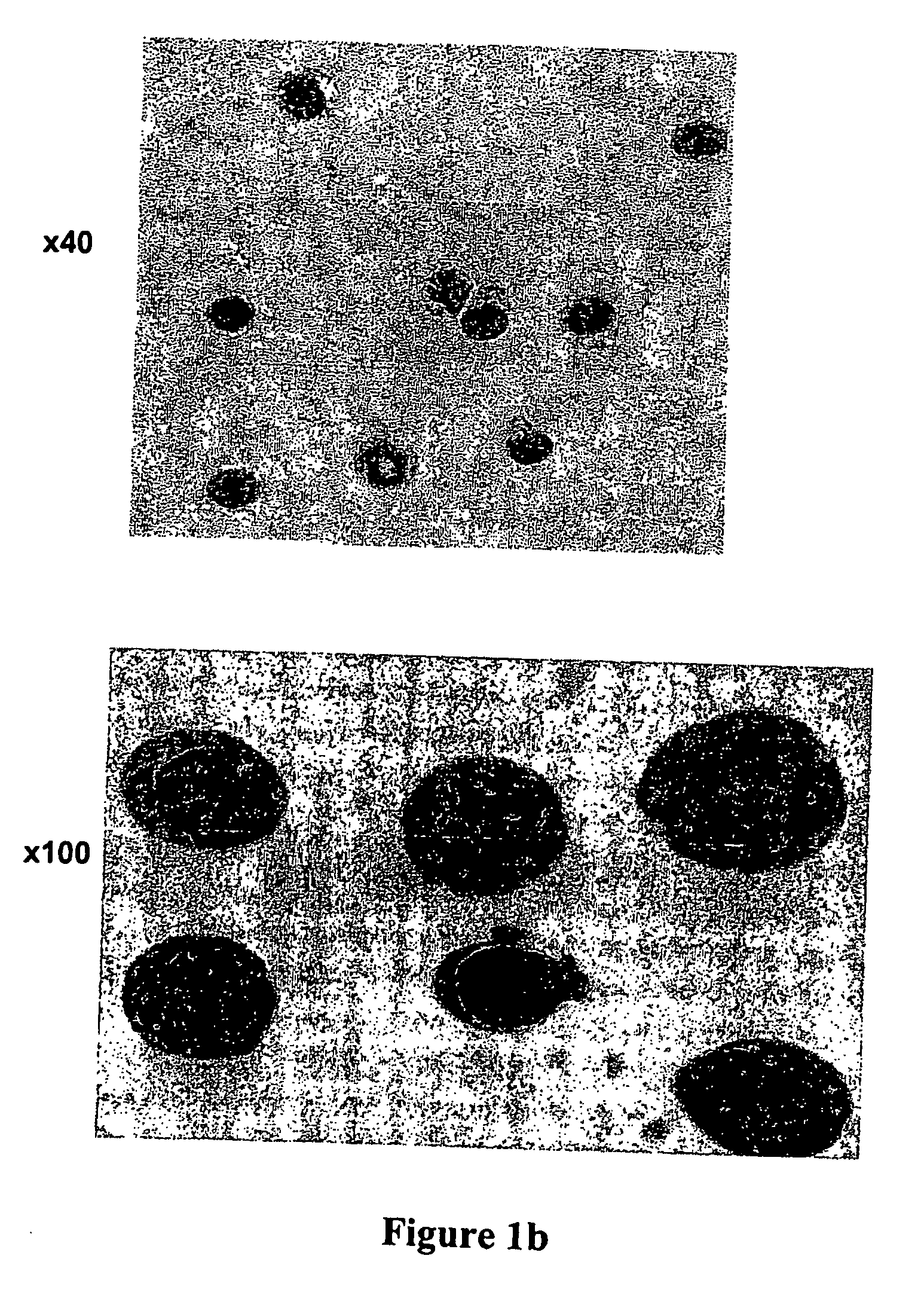
CD8+ / TCR− bone marrow cells facilitate engraftment of hemapoietic stem cells (HSQ in allogeneic recipients without causing graft versus host disease. The present invention identifies the main subpopulation (55-65%) of CD8+ / TCR− facilitating cells (FQ as plasmacytoid precursor dendritic cells (p-preDC). The present invention notably demonstrates that FC and p-preDC share many phenotypic, morphological, functional features, including IFN-α production, activation and survival after stimulation, and expansion and maturation after FIO-Ligand (FL) treatment. FL mobilized FC, the majority of which express a pre-DC phenotype, facilitate HSC engraftment. Although p-preDC significantly enhance HSC engraftment, they do so with less efficiency than FC. The present invention for the first time defines a direct functional role for p-preDC in HSC engraftment and will have a significant impact on strategies to design effective facilitating cell-based therapies for transplantation.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Human mesenchymal progenitor cell
InactiveUS20050059147A1Microbiological testing/measurementArtificial cell constructsProgenitorBone marrow transplantations
The present invention provides isolated pluri-differentiated human mesenchymal progenitor cells (MPCs), which simultaneously express a plurality of genes that are markers for multiple cell lineages, wherein the multiple cell lineages comprise at least four different mesenchymal cell lineages (e.g., adipocyte, osteoblast, fibroblast, and muscle cell) and wherein each of the markers is specific for a single cell lineage. The present invention also method for isolating and purifying human mesenchymal progenitor cells from Dexter-type cultures for characterization of and uses, particularly therapeutic uses for such cells. Specifically, isolated MPCs can be used for diagnostic purposes, to enhance the engraftment of hematopoietic progenitor cells, enhance bone marrow transplantation, or aid in the treatment or prevention of graft versus host disease.
Owner:UNIV OF SOUTH FLORIDA
Use of compounds having ccr antagonism
InactiveUS20050245537A1Effective preventionEffective treatmentBiocideSenses disorderAutoimmune conditionAutoimmune disease
It is intended to provide preventives / remedies for graft-versus-host disease and / or rejection in organ or bone marrow transplantation, rheumatoid arthritis, autoimmune diseases, allergic diseases, ischemic cerebral cell injury, myocardial infarction, chronic nephritis and arteriosclerosis. The above object can be achieved by preventives / remedies for graft-versus-host disease and / or rejection in organ or bone marrow transplantation, rheumatoid arthritis, autoimmune diseases, allergic diseases, ischemic cerebral cell injury, myocardial infarction, chronic nephritis and arteriosclerosis characterized by containing a specific compound having a CCR (CC chemokine receptor) antagonism.
Owner:TAKEDA PHARMA CO LTD +1
Methods for generating antigen-specific effector T cells
InactiveCN101415827AImmunoglobulin superfamilyMicroinjection basedAutoimmune conditionAutoimmune disease
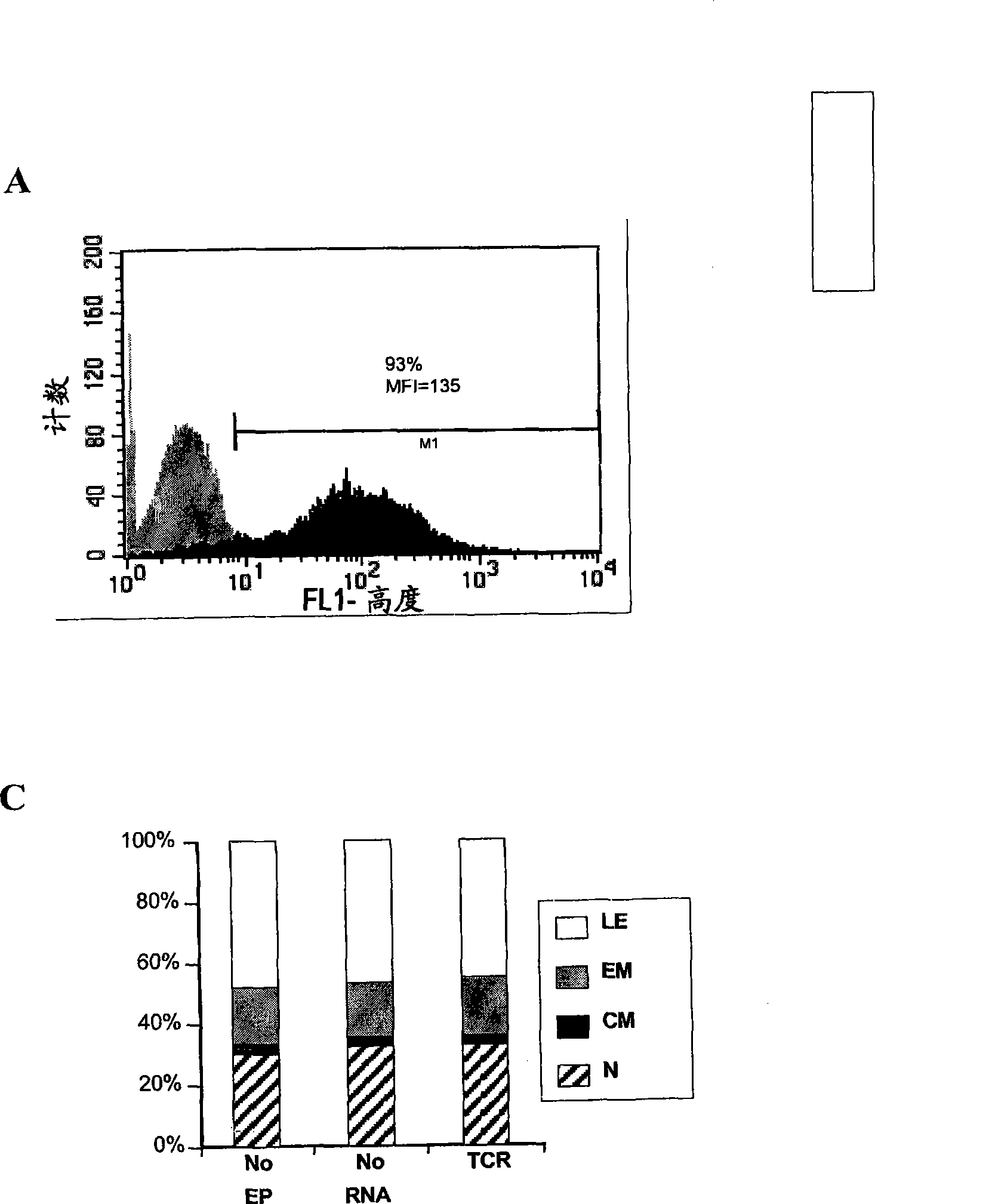
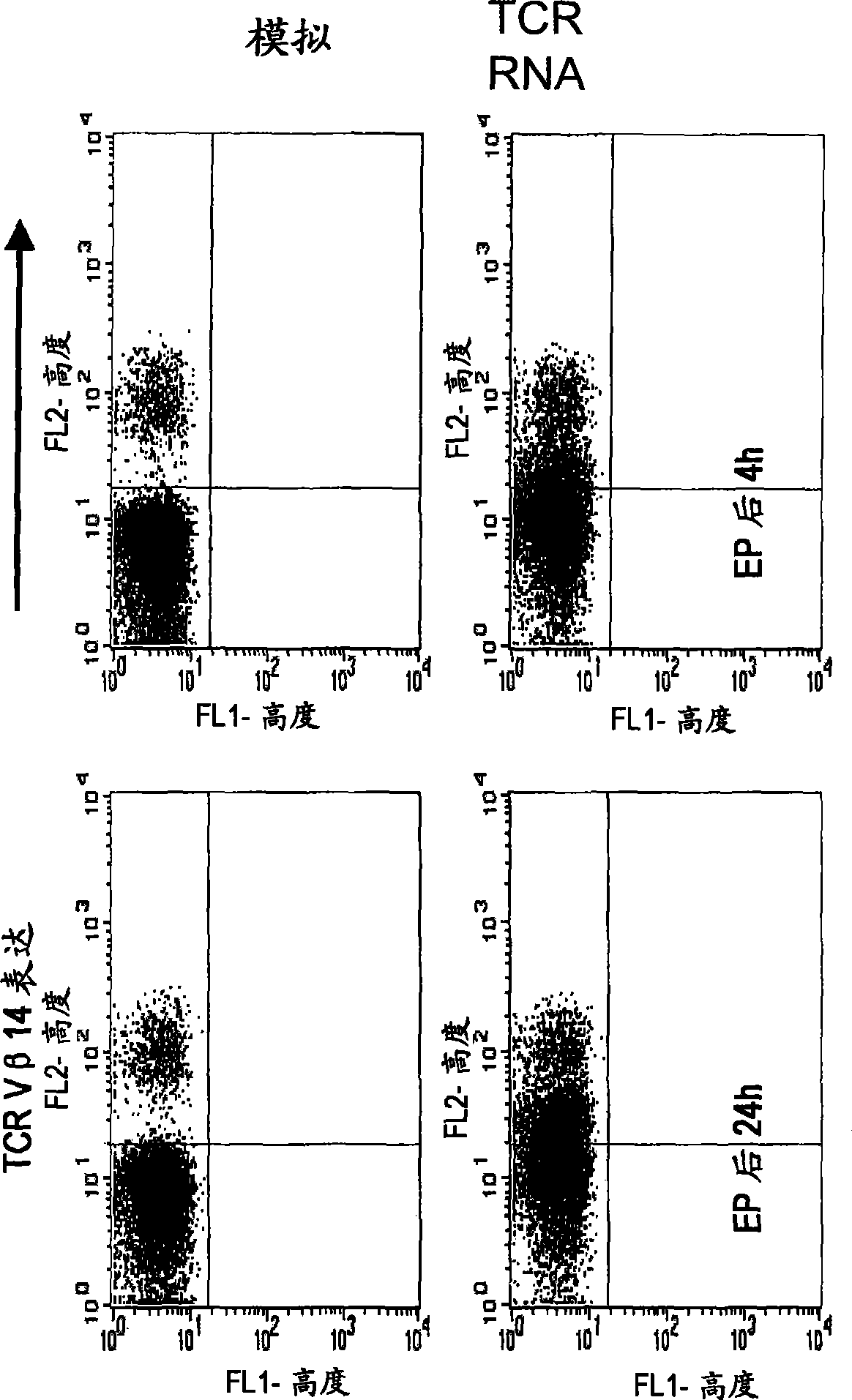
The invention relates to T cells transiently transfected with RNA, especially RNA encoding a T cell receptor and / or FoxP3, and methods of transfecting T cells with RNA by electroporation. Compositions of the invention include an effector T cell transiently transfected with RNA encoding a T cell receptor (TCR) specific for an antigen, wherein the T cell demonstrates effector function specific for cells presenting the antigen in complex with an MHC molecule. Treg cells comprising an exogenous RNA encoding FoxP3 are also provided. The transfected T cells are useful for immunotherapy, particularly in the treatment of tumors, pathogen infection, autoimmune disease, transplant rejection and graft versus host disease.
Owner:ARGOS THERAPEUTICS INC
Methods for the treatment and prevention of inflammatory diseases
The invention includes processes mainly for the treatment of a inflammatory diseases, such as inflammatory bowel disease, arthritis, atherosclerosis, asthma, allergy, inflammatory kidney disease, circulatory shock, multiple sclerosis, chronic obstructive pulmonary disease, skin inflammation, periodontal disease, psoriasis and T cell-mediated diseases of immunity, including allergic encephalomyelitis, allergic neuritis, transplant allograft rejection, graft versus host disease, myocarditis, thyroiditis, nephritis, systemic lupus erthematosus, and insulin-dependent diabetes mellitus. The processes involve treating a patient with a pharmaceutical composition containing an active ingredient that inhibits the activity of sphingosine kinase.
Owner:APOGEE BIOTECHNOLOGY CORP
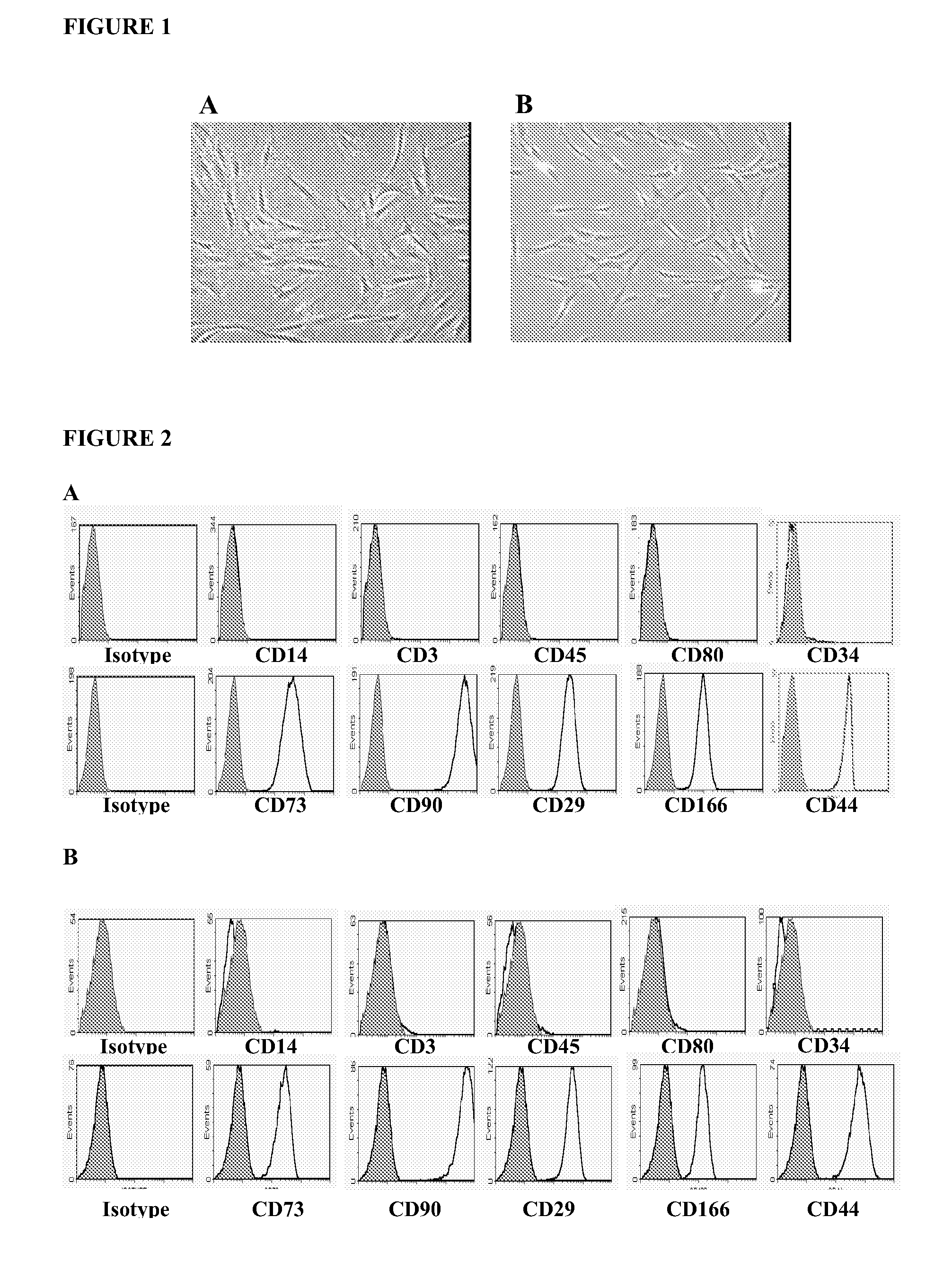
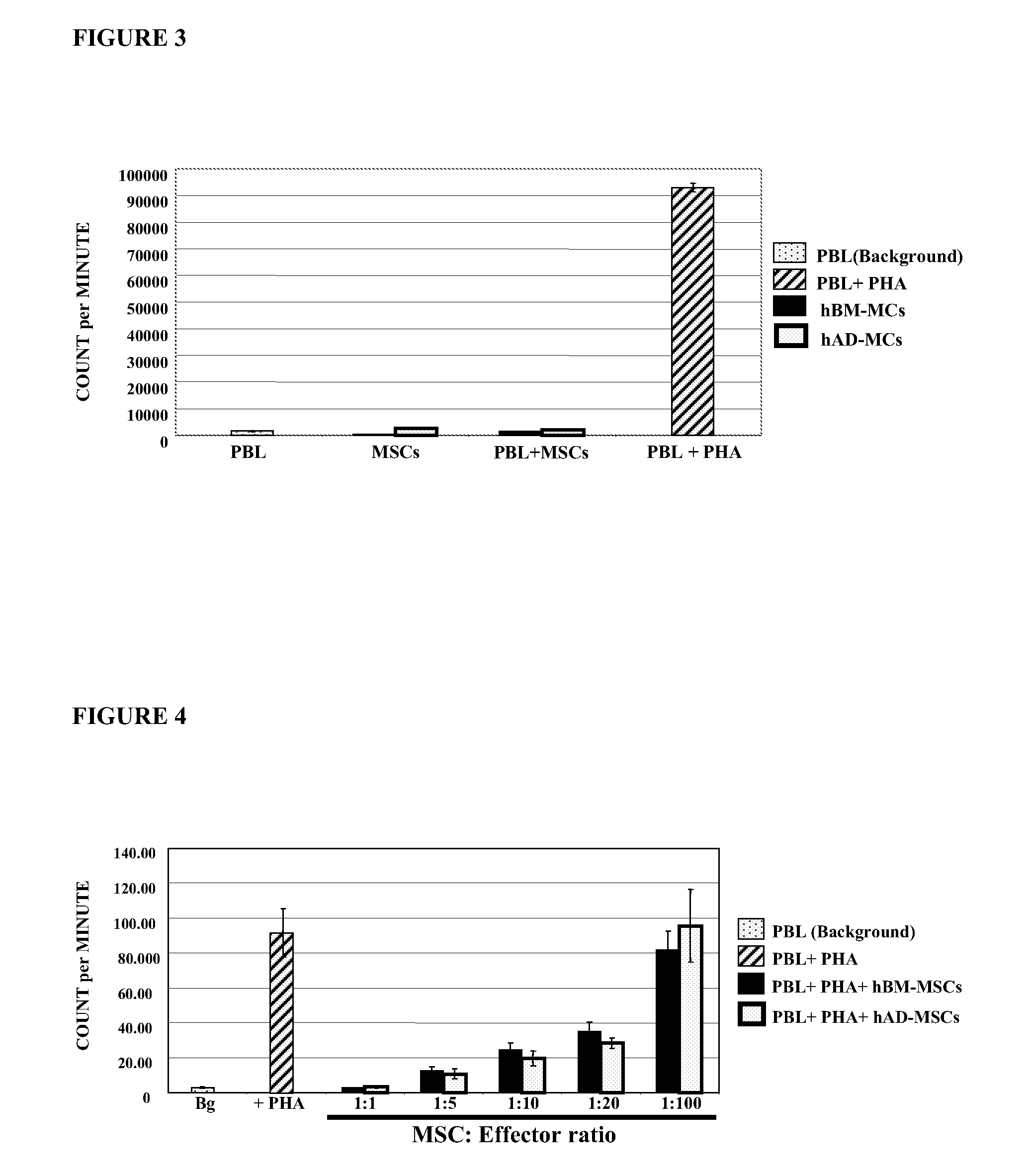
Use of adipose tissue derived mesenchymal stem cells for the treatment of graft versus host disease
ActiveUS20090148419A1Improve severityReduce mortalityBiocideMammal material medical ingredientsAllo hsctGraft versus host disease induction
The present invention relates to the use of a particular type of adipose tissue derived mesenchymal stem cells (AD-MSCs), which exert immunosuppressive properties, in the manufacture of a pharmaceutical composition for the prevention and treatment of the graft-versus-host disease (GVHD) produced after allogeneic hematopoietic stem cell transplantation.
Owner:CENT DE INVESTIGACIONES ENERGETICAS MEDIO AMBIENTALLES Y TECNOLOGICAS (C I E M A T) +2
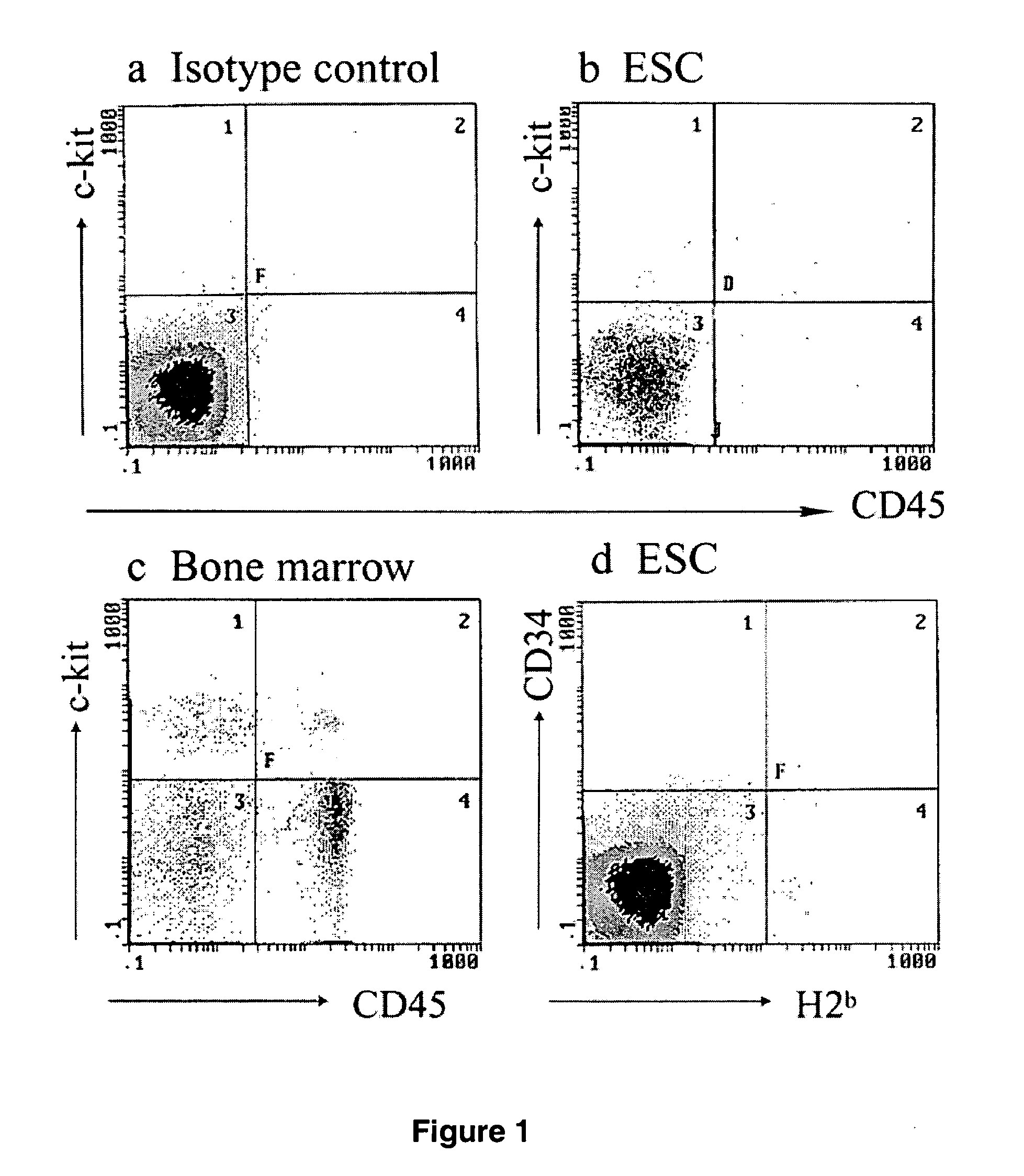
Methods and compositions for obtaining hematopoietic stem cells derived from embryonic stem cells and uses thereof
InactiveUS20050221482A1Good conditionMammal material medical ingredientsArtificial cell constructsAutoimmune conditionAutoimmune disease
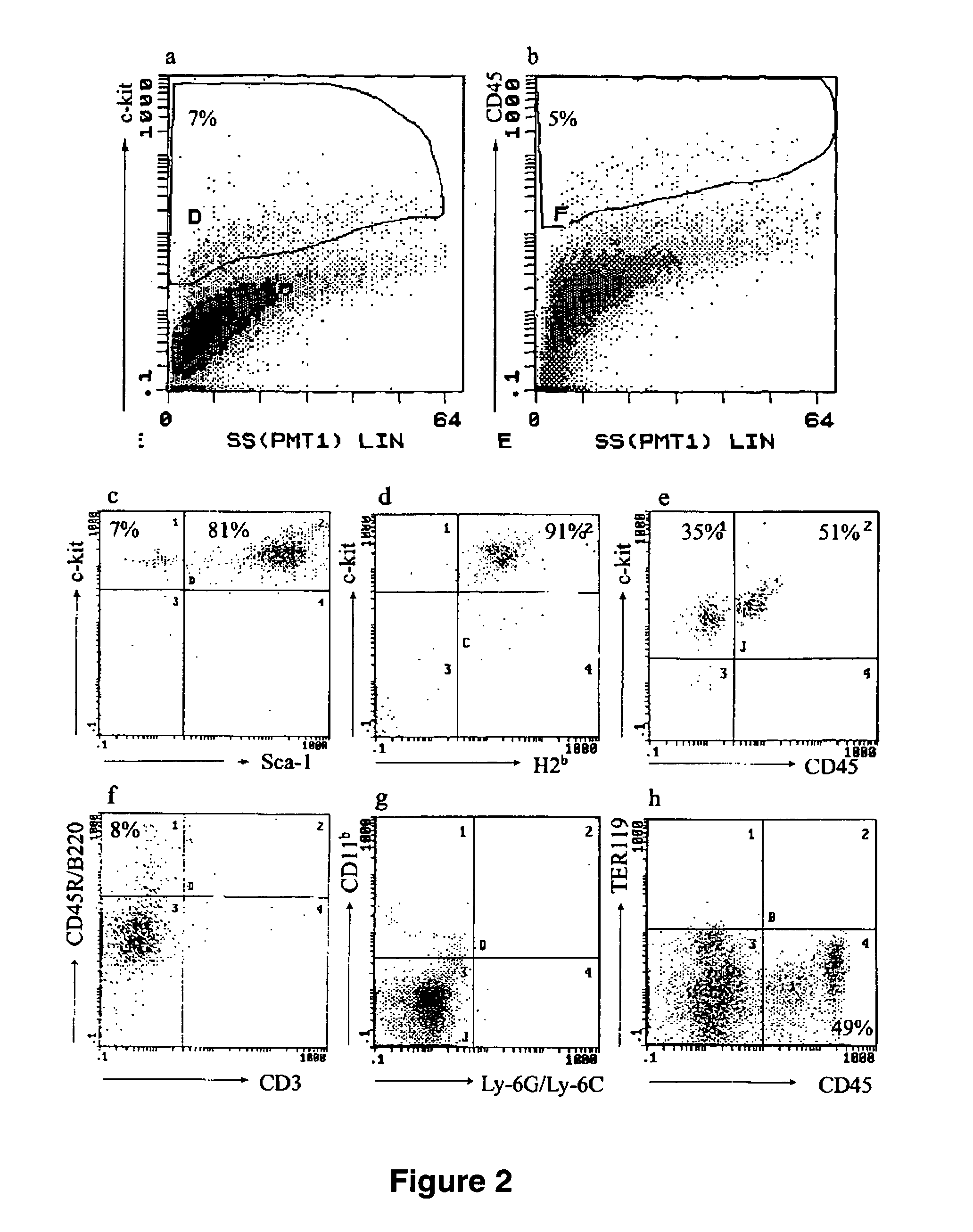
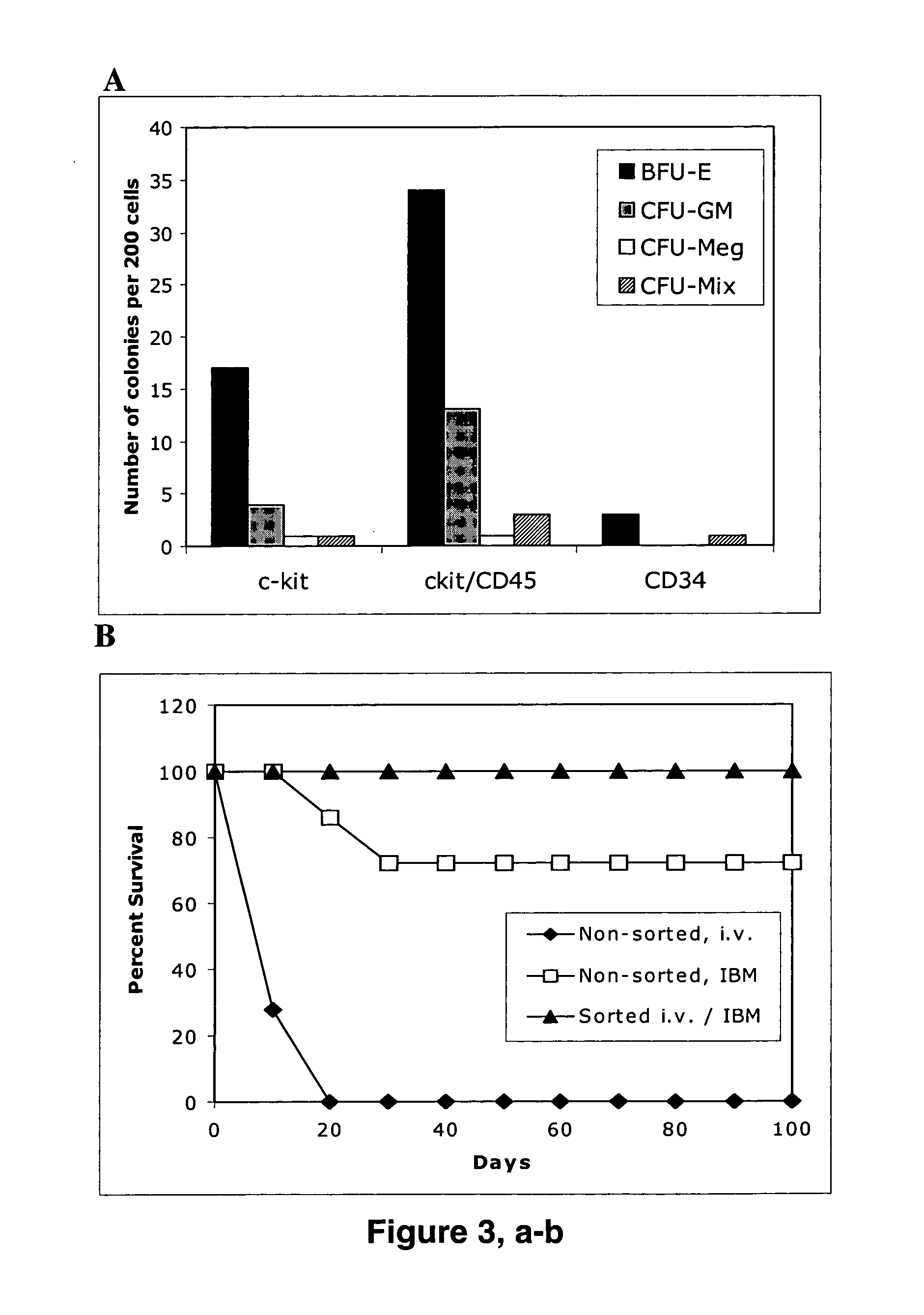
The present invention provides an isolated population of adult hematopoietic stem cells (HSC) derived from embryonic stem cells (ESC) induced to differentiate in vitro by culturing ESCs in a medium with stem cell factor, interleukin (IL)-3, and IL-6. HSC of immunophenotype c-kit+ or c-kit+ / CD45+ from this population are isolated and injected either intra bone marrow or intravenously into myeloablated recipient individuals. This method allows for the establishment of banks of allogeneic ESC-derived adult stem cells for treatments of autoimmune diseases, immune deficiencies and induction of immunotolerance during organ transplantation. These allogeneic ESC-derived adult hematopoietic stem cells (HSC) may be used in reconstituting bone marrow, without the development of teratomas or graft versus host disease, despite crossing histocompatibility barriers. Additionally, allogeneic ESC-derived HSC can be used to prevent the development of autoimmune diseases or organ rejection during transplantation.
Owner:NEWLINK GENETICS +1
Allogeneic cellular immunotherapy for invasive pulmonary aspergillosis (IPA)
ActiveUS20060115487A1Snake antigen ingredientsVertebrate antigen ingredientsAllogeneic cellGraft versus host disease induction
A method for stimulating the immune system in immunocompromised patients in order to treat opportunistic infection. The method involves the infusion of intentionally mismatched allogeneic cells. In order to prevent graft vs. host disease complications, the allogeneic cells can be irradiated prior to infusion.
Owner:MIRROR BIOLOGICS INC +1
Pharmaceutical compositions comprising synthetic peptide copolymers and methods for preventing and treating GVHD and HVGD
Compositions and methods for treating and preventing host-versus-graft disease and graft-versus-host disease comprising as active ingredient random copolymers of amino acids comprising one amino acid from at least three of the following groups: (a) lysine and arginine; (b) glutamic acid and aspartic acid; (c) alanine and glycine; and (d) tyrosine and tryptophan; with the proviso that the random copolymer is not Copolymer 1 or D-Copolymer 1 when the disease being treated is graft-versus-host disease.
Owner:YEDA RES & DEV CO LTD
Immortalized car-t cells genetically modified to elminate t-cell receptor and beta 2-microglobulin expression
InactiveUS20190175651A1None is suitable for purposeTumor rejection antigen precursorsGenetic material ingredientsT cellAuto immune disease
The present invention pertains to engineered immortalized T-cell lines, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered immortalized T-cell lines of the invention are characterized in that the expression of endogenous T-cell receptors (TCRs) and beta 2-microglobulin (B2M) is inhibited, e.g., by using an endonuclease able to selectively inactivate the TCR and B2M genes in order to render the immortalized T-cells non-alloreactive. In addition, expression of immunosuppressive polypeptide can be performed on those engineered immortalized T-cells in order to prolong the survival of these T-cells in host organisms. Such engineered immortalized T-cells are particularly suitable for allogeneic transplantations, especially because it reduces both the risk of rejection by the host's immune system and the risk of developing graft versus host disease. The invention opens the way to standard and affordable adoptive immunotherapy strategies using immortalized T-cells for treating cancer, infections and auto-immune diseases.
Owner:JANSSEN BIOTECH INC
Therapeutic apoptotic cell preparations, method for producing same and uses thereof
ActiveUS20150275175A1Reduce morbidityReduced incidence of hepatotoxicityBiocideOrganic active ingredientsInflammatory Bowel DiseasesGraft versus host disease induction
The present application provides pharmaceutical compositions comprising a population of mononuclear-enriched cells in an early-apoptotic state, methods for the production of said compositions and uses thereof in the treatment of diseases characterized by pathological immune responses. The pharmaceutical compositions may be used in treatment of conditions such as, but not limited to, graft versus host disease (GVHD) and autoimmune diseases including but not limited to inflammatory bowel disease, gout and arthritis.
Owner:ENLIVEX THERAPEUTICS R&D LTD
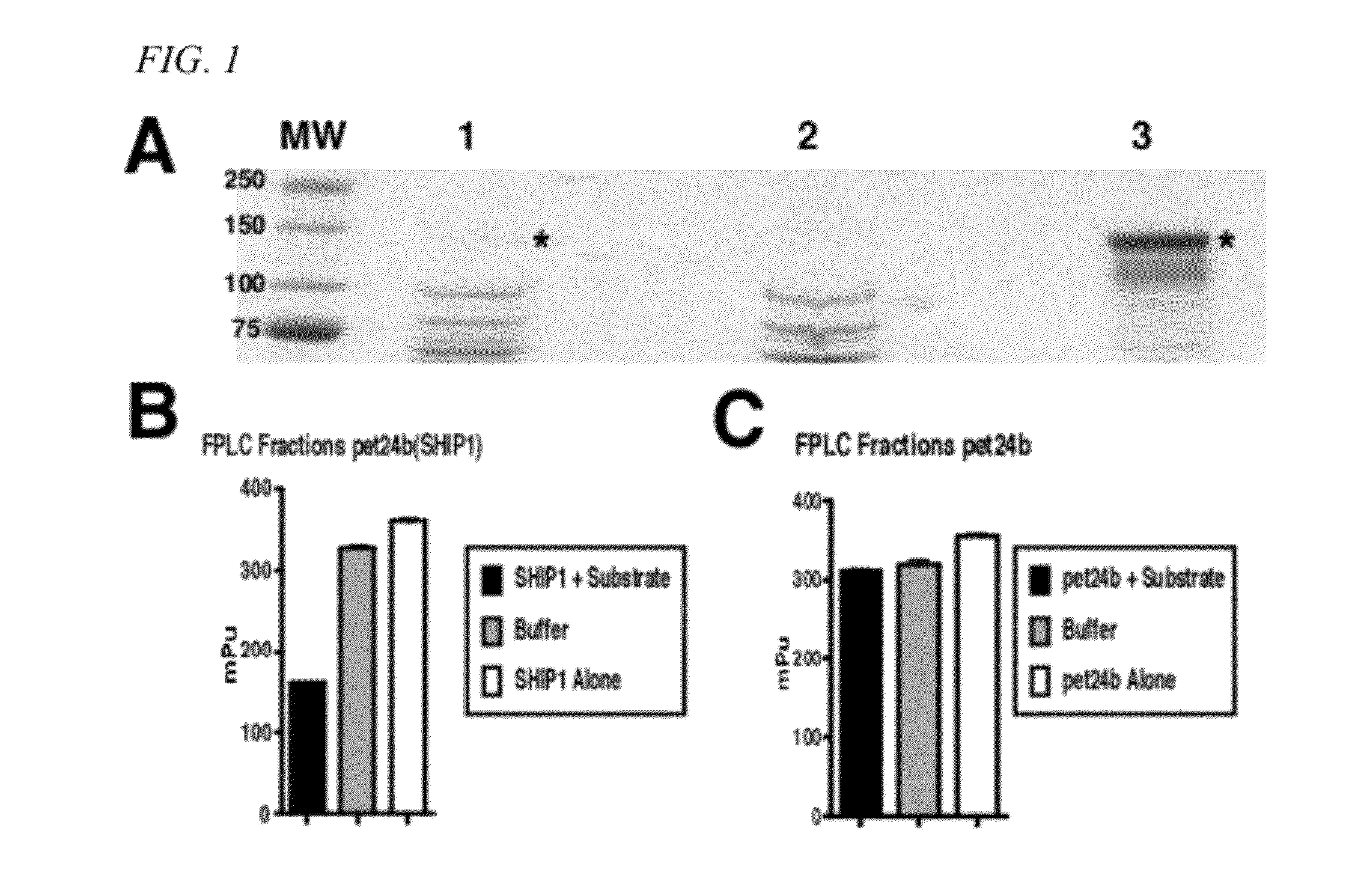
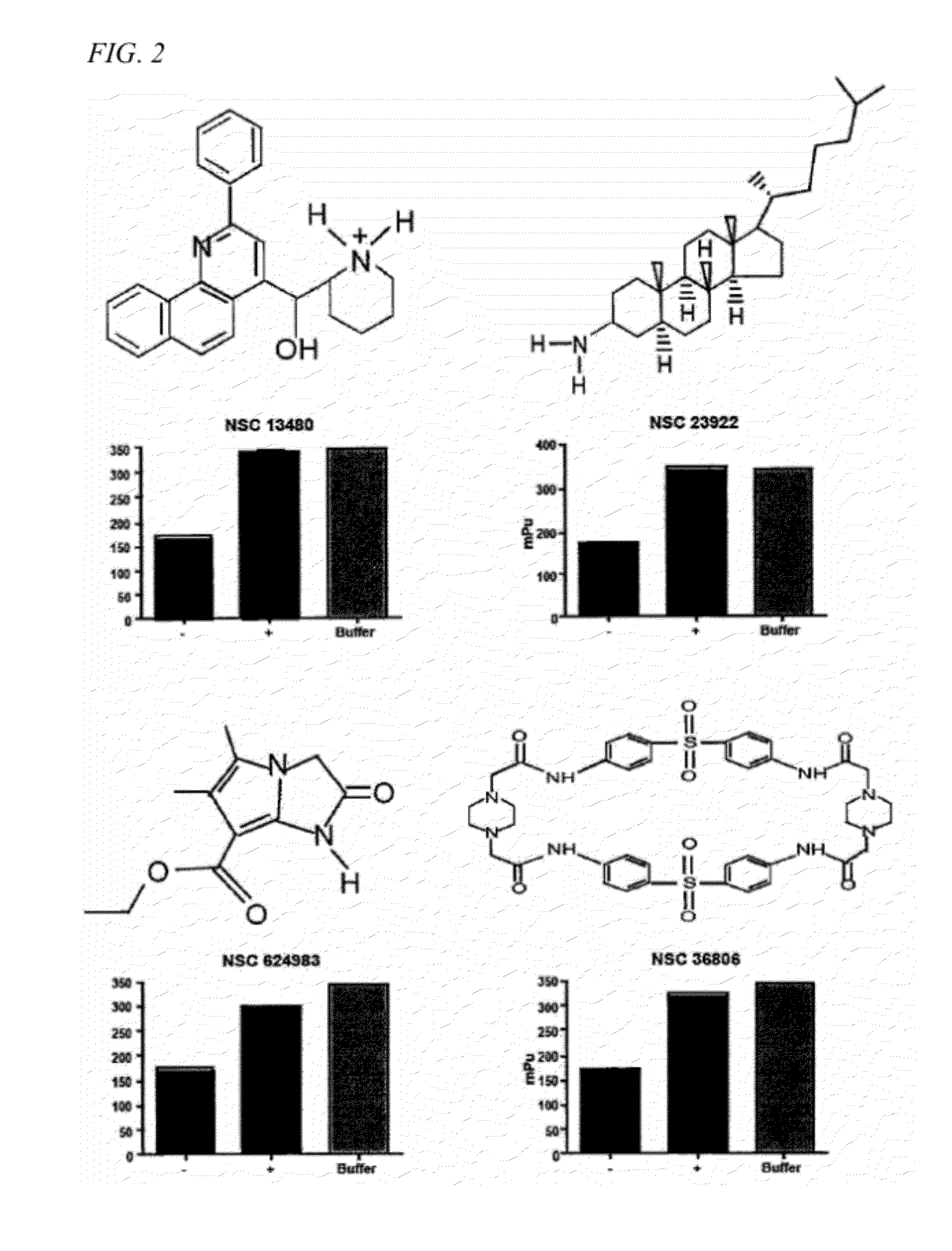
Method of modulating ship activity
InactiveUS20120178725A1Easy to optimizeMobilizeBiocideMetabolism disorderGraft versus host disease inductionAllogeneic transplantation
A method of treating or preventing an immune disorder, such as graft versus host disease, in a subject. The method includes the administering a SHIP1 inhibitor, such as 3α-aminocholestane, to a subject in need of treatment. Thus, SHIP1 inhibitors taught herein represent a novel class of small molecules that have the potential to enhance allogeneic transplantation, boost innate immunity and improve the treatment of hematologic malignancies.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC
Tolerogenic vaccine and method
InactiveUS20060093580A1Increased proliferationBiocidePeptide/protein ingredientsRegulatory T cellAutoimmune condition
Methods and compositions are provided for treating autoimmune diseases such as diabetes, rheumatoid arthritis, inflammatory bowel disease, and other conditions involving undesired immune responses such as allergies, including food allergies, and graft-versus-host disease. In one embodiment disclosed, regulatory / suppressor T cells are selected or expanded in culture using a phospholipase D (PLD) inhibitor to prevent growth of effector T cells and a growth factor to stimulate the regulatory cells. Antigen-specific regulatory / regulatory T cells can be produced by this method. The regulatory T cells can then be administered to a patient in need of suppressive immunotherapy. In another embodiment, PLD inhibitor, growth factor, and an antigen for which antigen-specific suppressive immunotherapy is desired are administered to a patient via injection, oral or topical administration, or other means known to the art.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com