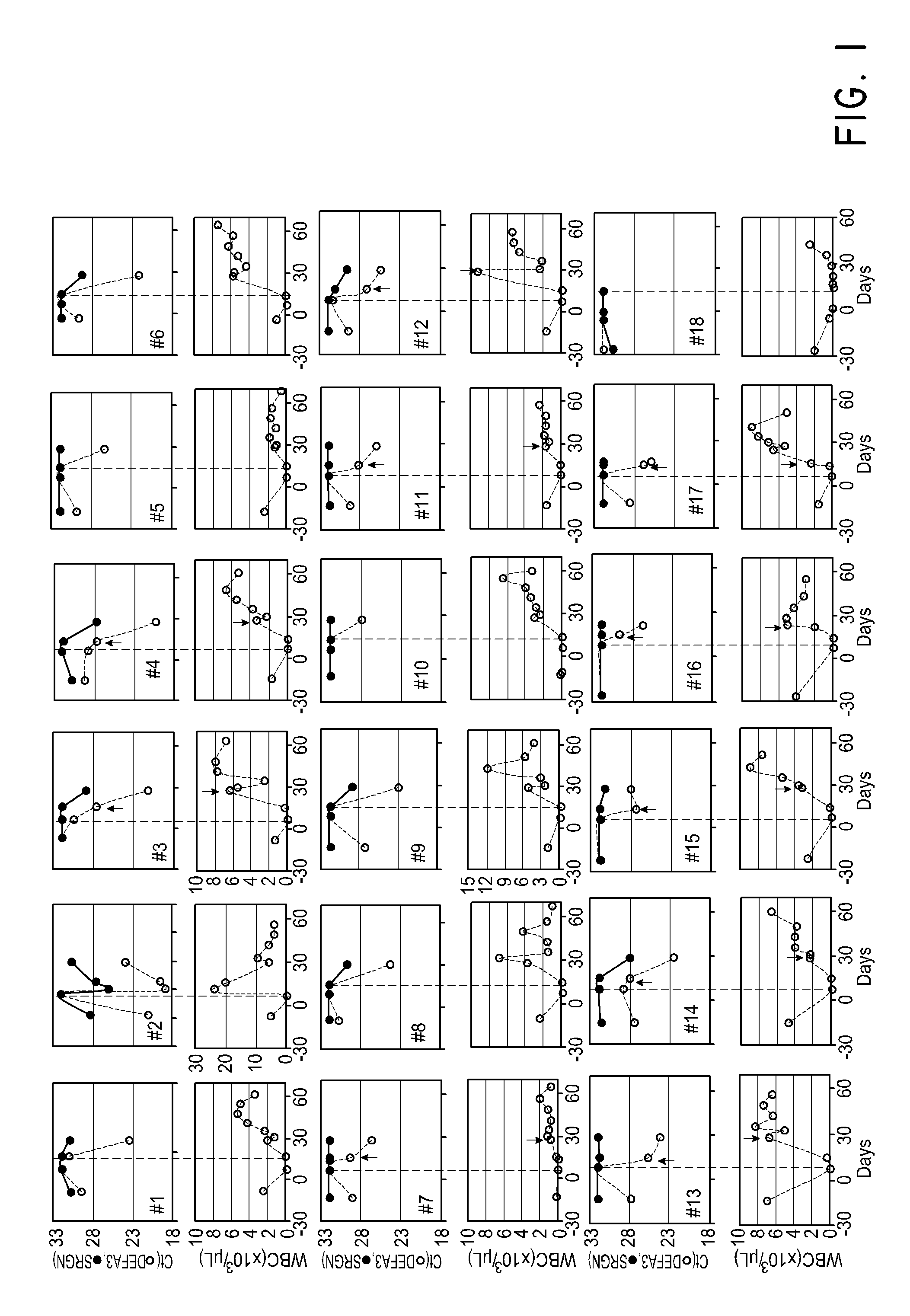
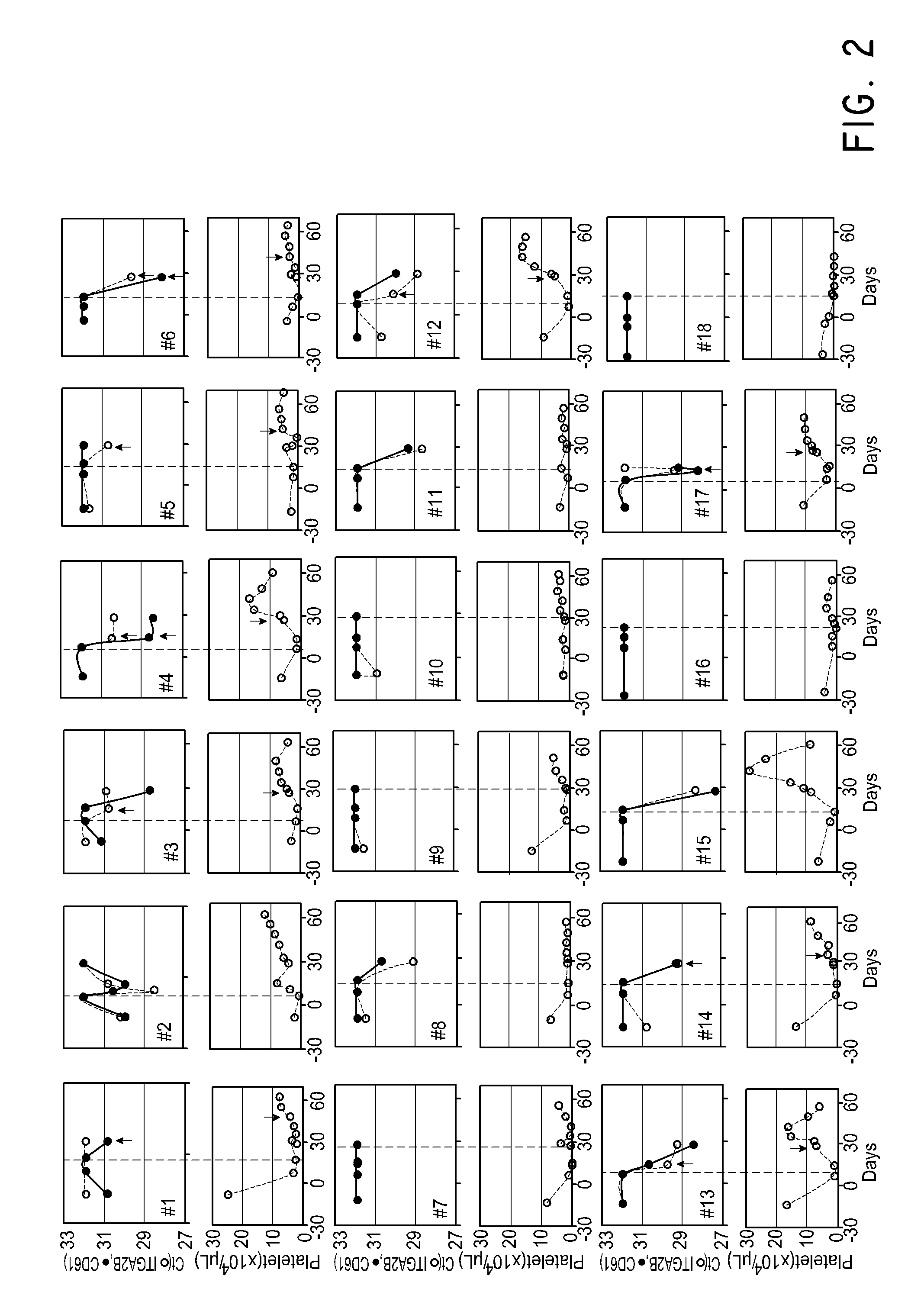
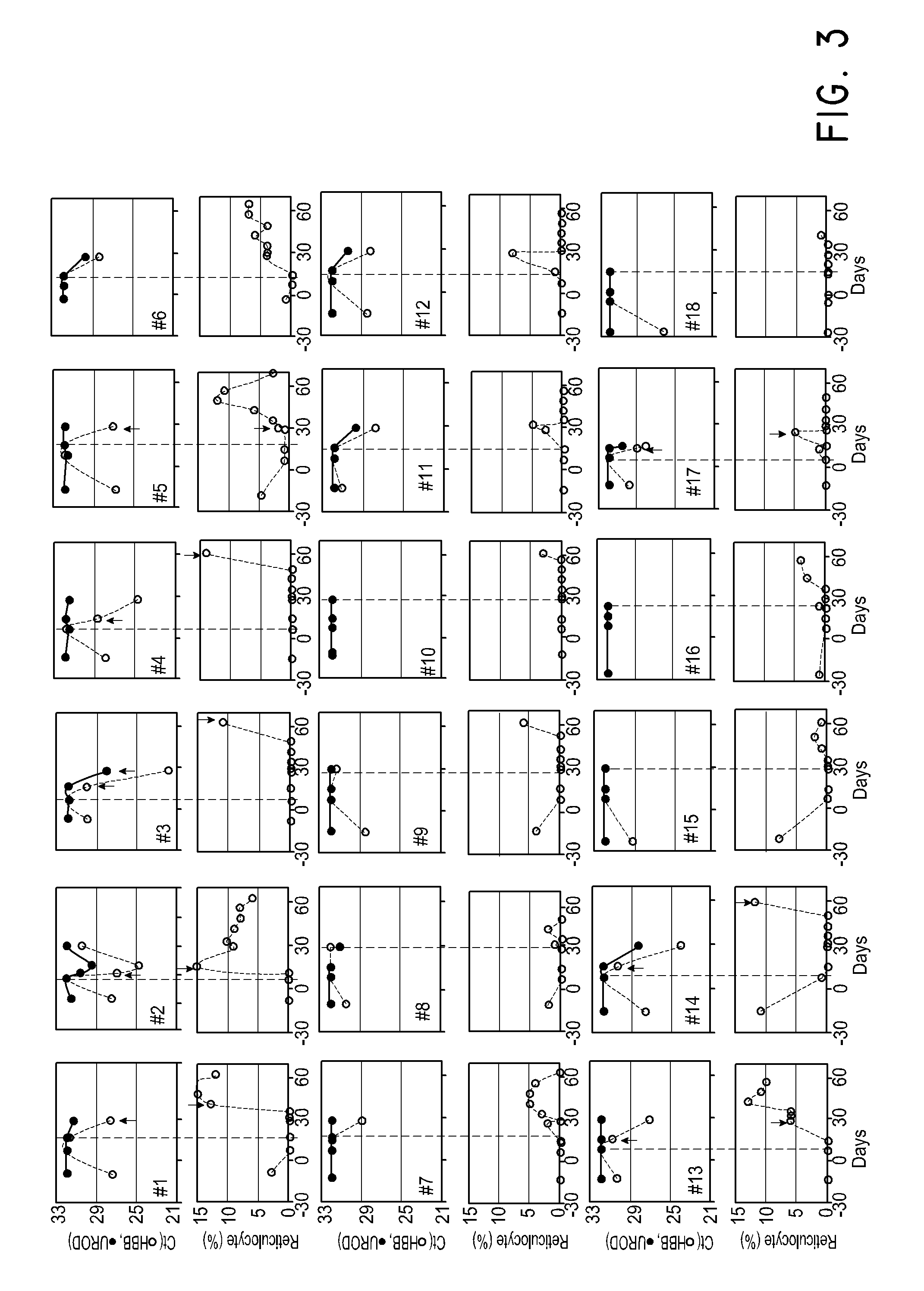
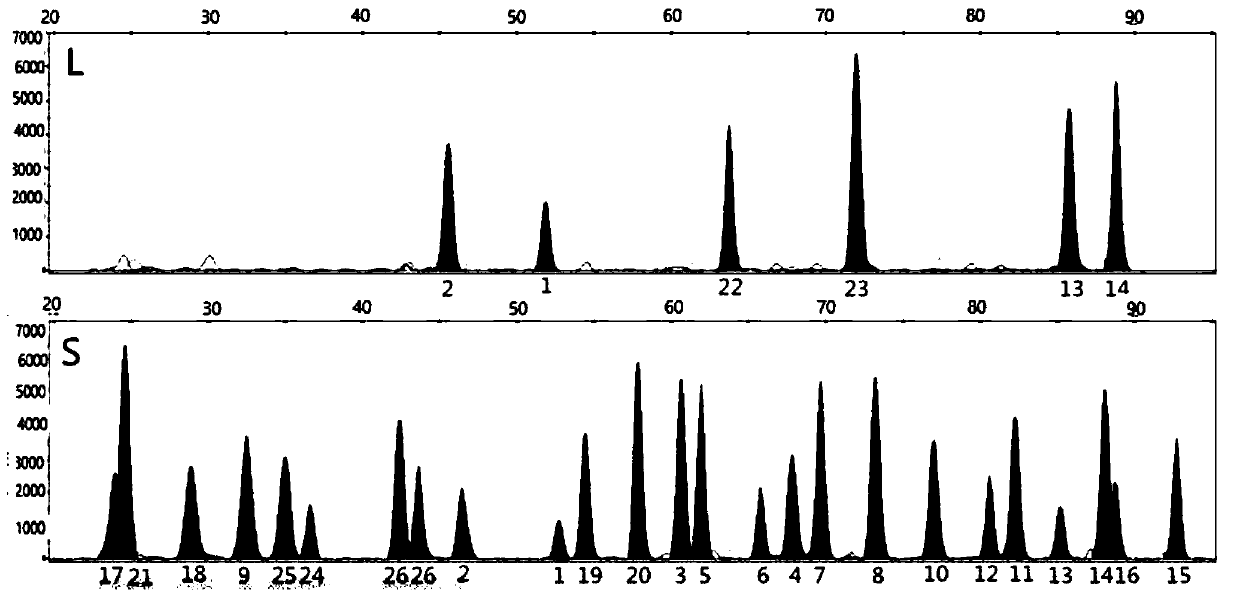
Patents
Literature
40 results about "Bone marrow transplantations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods for ex-vivo expanding stem/progenitor cells
InactiveUS20060205071A1Increase productionCulture processArtificial cell constructsProgenitorBone marrow transplantations
Methods of ex-vivo expansion of fetal and / or adult progenitor, and umbilical cord blood, bone marrow or peripheral blood derived stem cells in bioreactors for bone marrow transplantation, transfusion medicine, regenerative medicine and gene therapy.
Owner:GAMIDA CELL
Human mesenchymal progenitor cell
InactiveUS20050059147A1Microbiological testing/measurementArtificial cell constructsProgenitorBone marrow transplantations
The present invention provides isolated pluri-differentiated human mesenchymal progenitor cells (MPCs), which simultaneously express a plurality of genes that are markers for multiple cell lineages, wherein the multiple cell lineages comprise at least four different mesenchymal cell lineages (e.g., adipocyte, osteoblast, fibroblast, and muscle cell) and wherein each of the markers is specific for a single cell lineage. The present invention also method for isolating and purifying human mesenchymal progenitor cells from Dexter-type cultures for characterization of and uses, particularly therapeutic uses for such cells. Specifically, isolated MPCs can be used for diagnostic purposes, to enhance the engraftment of hematopoietic progenitor cells, enhance bone marrow transplantation, or aid in the treatment or prevention of graft versus host disease.
Owner:UNIV OF SOUTH FLORIDA
Methods for mobilizing hematopoietic facilitating cells and hematopoietic stem cells into the peripheral blood
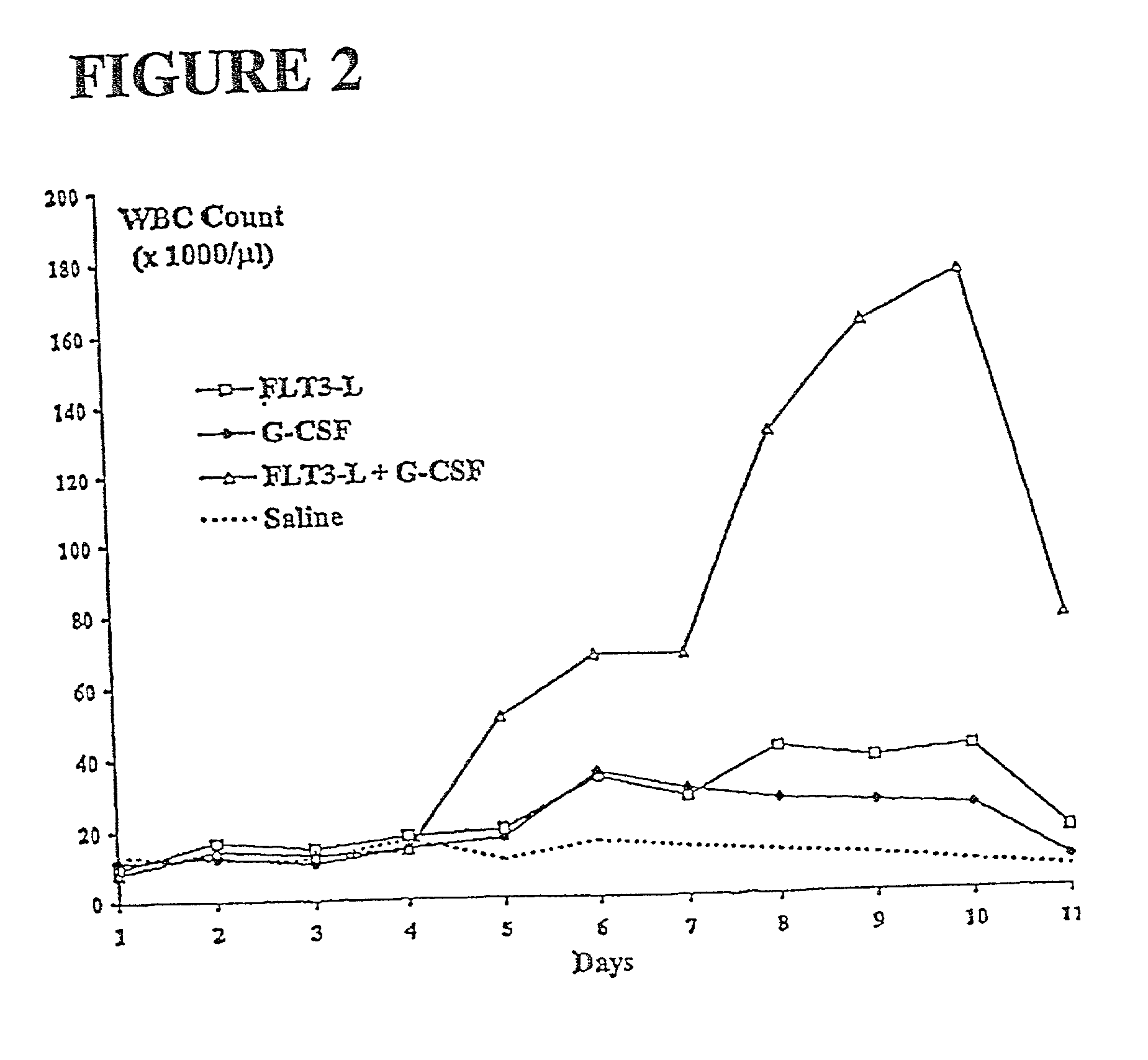
The present invention relates to methods for mobilizing hematopoietic facilitating cells (FC) and hematopoietic stem cells (HSC) into a subject's peripheral blood (PB). In particular, the invention relates to the activation of both FLT3 and granulocyte-colony stimulating factor (G-CSF) receptor to increase the numbers of FC and HSC in the PB of a donor. The donor's blood contains both mobilized FC and HSC, and can be processed and used to repopulate the destroyed lymphohematopoietic system of a recipient. Therefore, PB containing FC and HSC mobilized by the method of the invention is useful as a source of donor cells in bone marrow transplantation for the treatment of a variety of disorders, including cancer, anemia, autoimmunity and immunodeficiency. Alternatively, the donor's hematopoietic tissue, such as bone marrow, can be treated ex vivo to enrich selectively for FC and HSC populations by activating appropriate cell surface receptors.
Owner:ILDSTAD SUZANNE T +1
Mobilization of hematopoietic cells
ActiveUS20050142103A1Effective level of cellEffective levelingAntibacterial agentsOrganic active ingredientsDiseaseHematopoietic cell
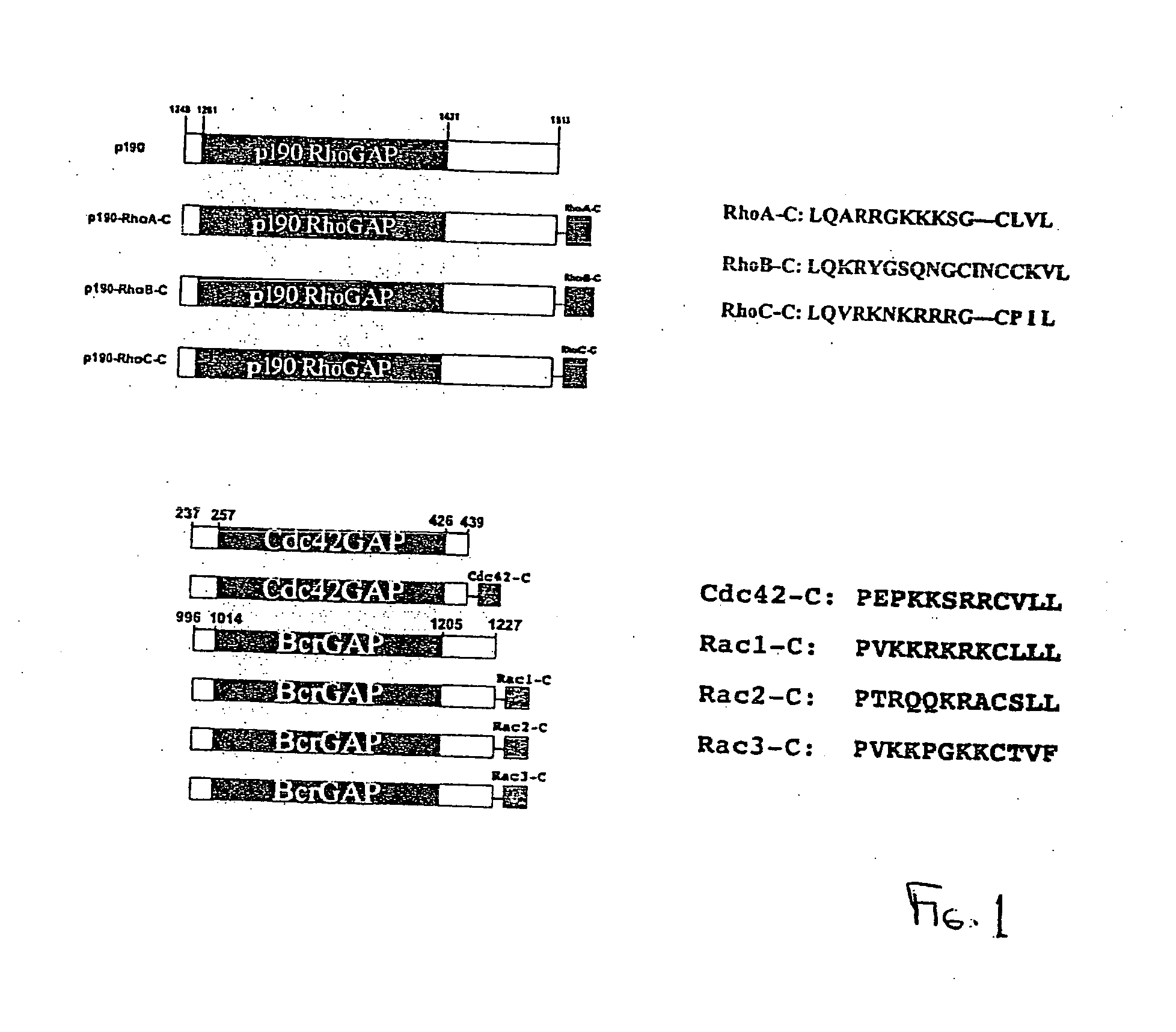
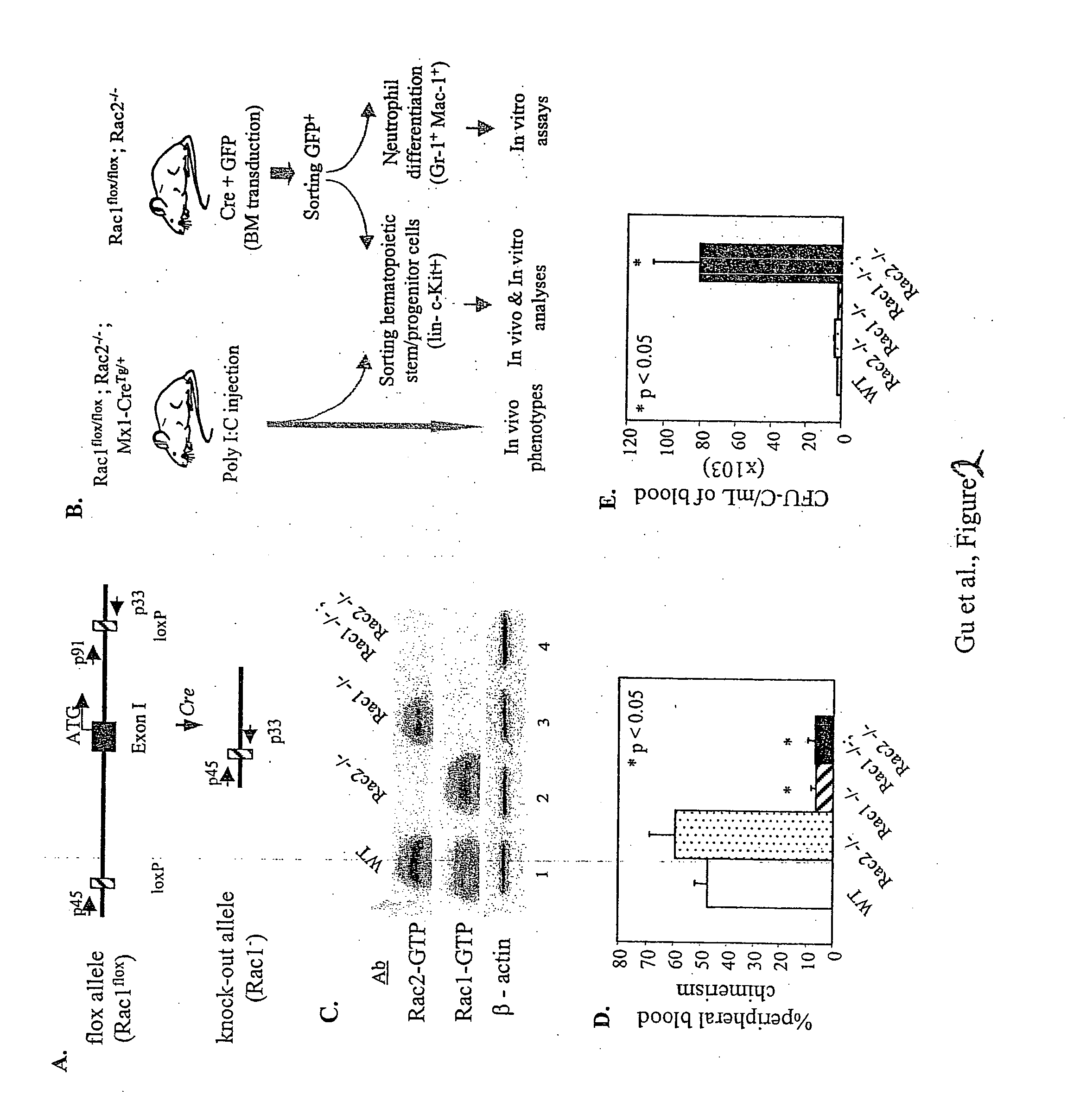
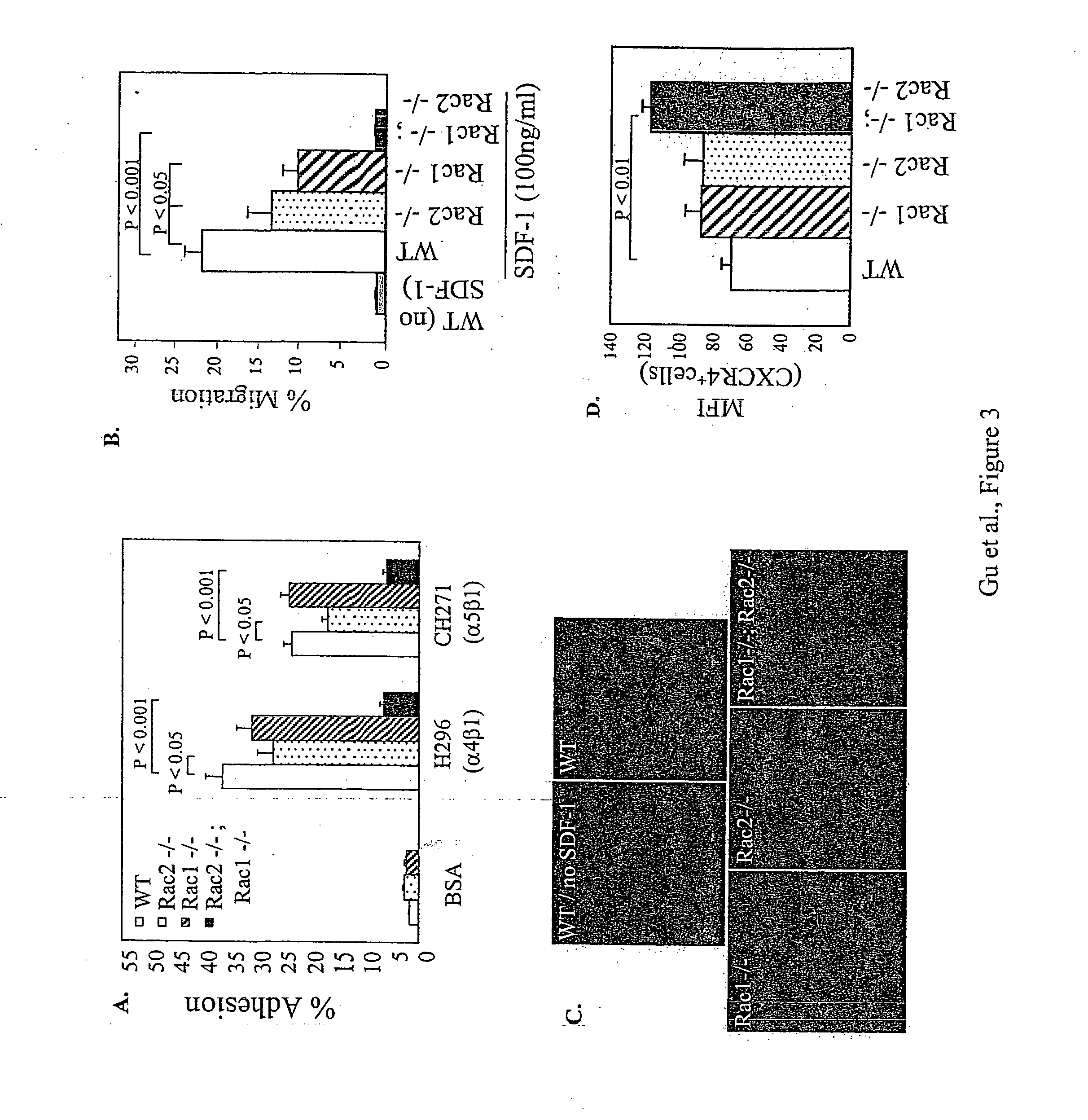
Improved methods and pharmaceutical compositions are provided herein for mobilizing hematopoietic progenitor cells from bone marrow into peripheral blood, comprising the administration of an effective amount of an inhibitor of GTPases, such as Rac1 and Rac2 alone or in combination. Specifically, methods are disclosed for mobilizing hematopoietic stem cells into a subject's peripheral blood. In particular, embodiments of the method involve inhibition of both Rac1 and Rac2 GTPases to increase the numbers of hematopoietic stem cells into a subject's peripheral blood of a subject. The subject's blood can be processed and used to repopulate the destroyed lymphohematopoietic system of a recipient and may in the future be utilized to repair a variety of non-hematopoietic tissues. Therefore, hematopoietic stem cells mobilized into a subject's peripheral blood by the method of the invention is useful as a source of donor cells in bone marrow transplantation for the treatment of a variety of disorders, including cancer, anemia, autoimmunity and immunodeficiency. They can also be used for increasing white blood cell survival and for chemotherapy.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
ASSESSMENT OF BONE MARROW RECOVERY BY MEASURING PLASMA EXOSOME mRNAS
InactiveUS20130172208A1Microbiological testing/measurementLibrary screeningBone marrow transplantationsExosome
The present disclosure relates to the characterization of bone marrow conditions through the quantification of bone marrow-associated markers. In several embodiments, the bone marrow-associated markers are expressed in exosomes, which can be obtained from biological fluid samples, such as plasma or whole blood. In several embodiments, quantification of such markers allows for the assessment of bone marrow recovery following bone marrow transplantation.
Owner:HITACHI CHEM CO LTD +1
Method for analyzing mixed sample DNA
PendingCN110157786ASuitable for forensic testingProtection securityMicrobiological testing/measurementOrgan transplantationBone marrow transplantations
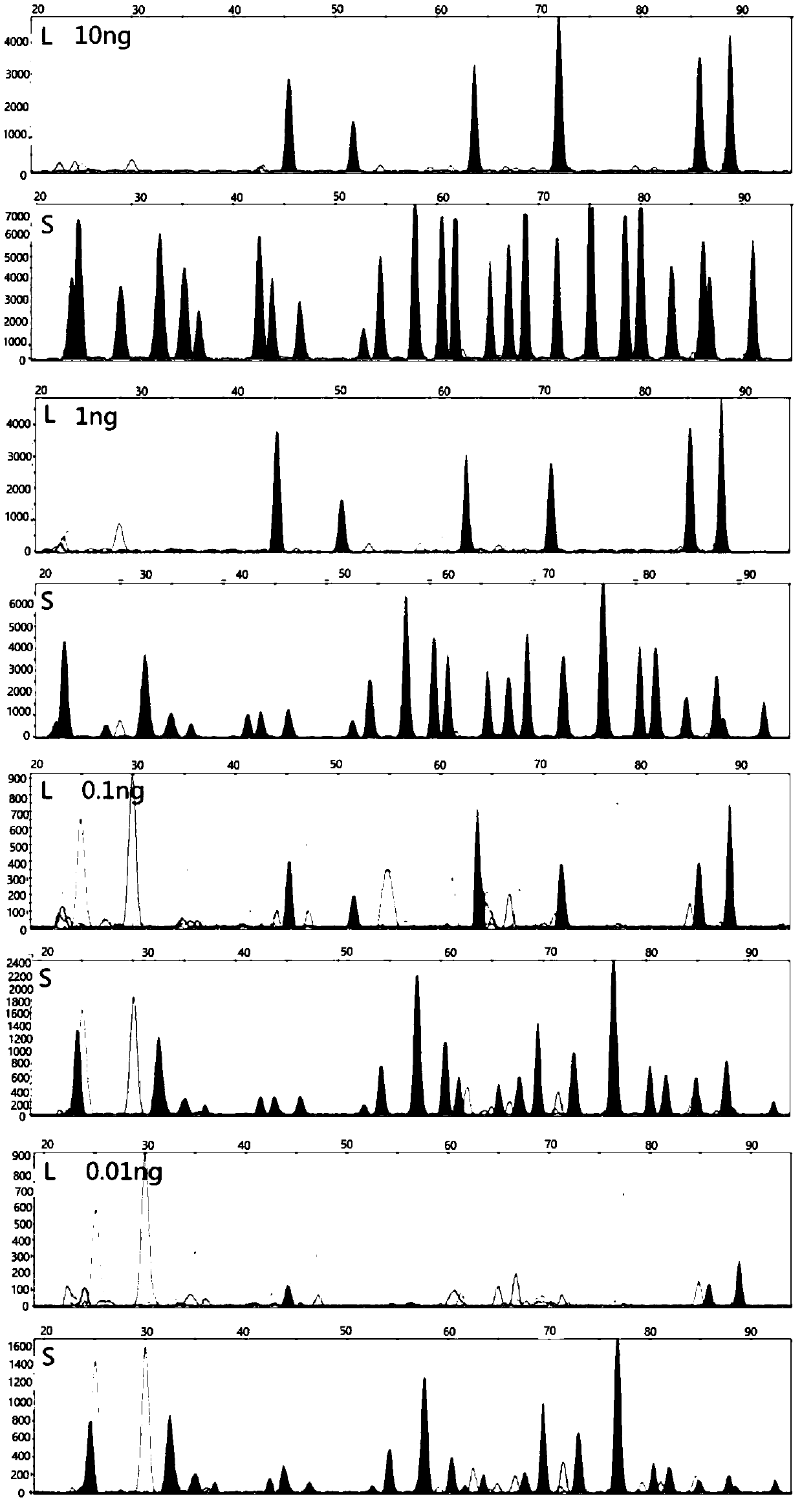
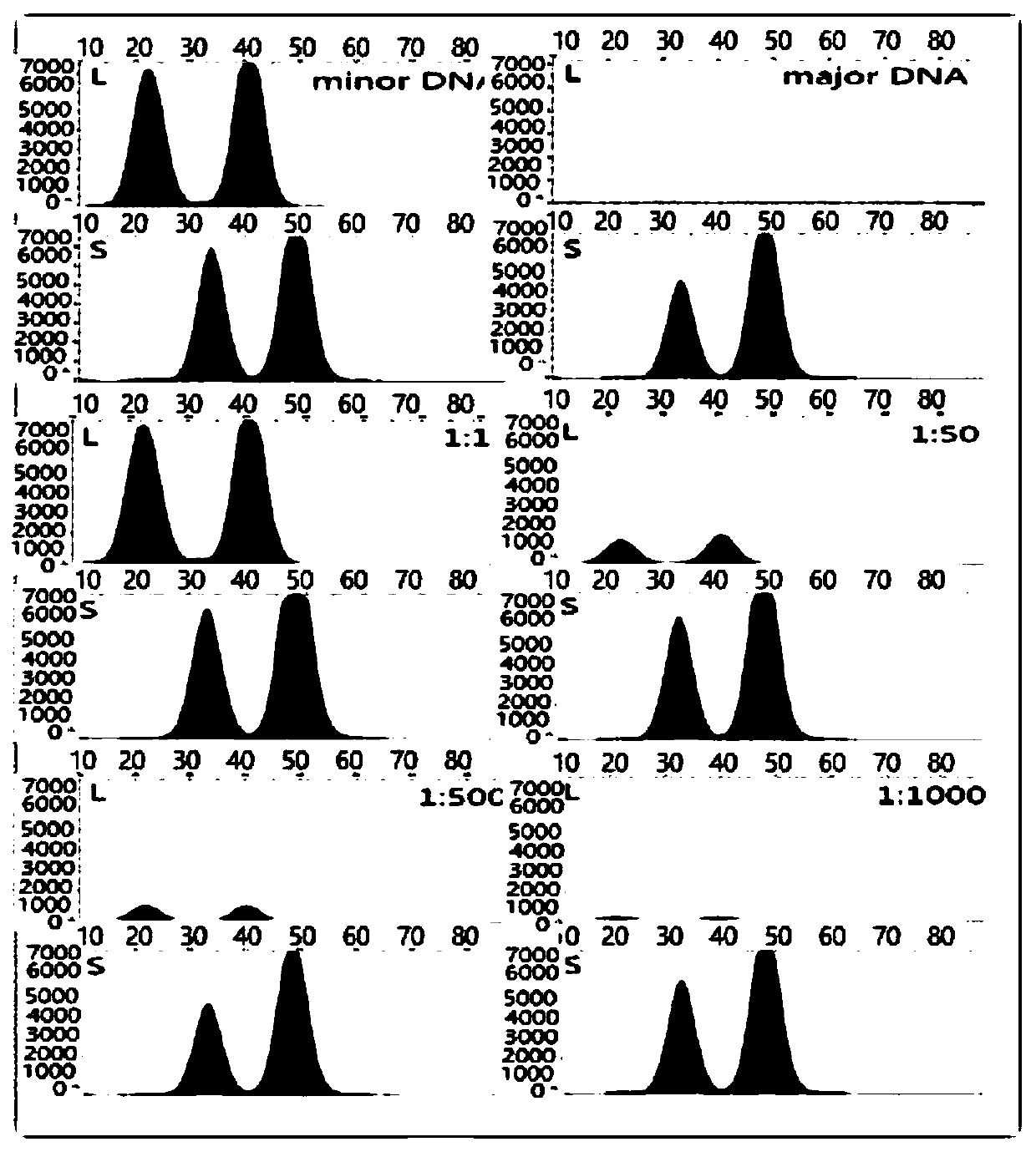
The invention discloses a method for analyzing mixed sample DNA. The mixed sample DNA is analyzed with a method combining INDEL with Microhaplotypes; in other words, father source or mother source DNAof an individual is sorted through differential amplification of the INDEL, and then individual distinguishing is conducted through the Microhaplotypes on DNA molecules; the method is named a DIP-Microhaplotypes method. The method has the advantages that analysis of a mixed DNA sample of two samples and detection of mixed stain sensitivity mainly depend on the INDEL, following detection of SNP isrelated to a detection means, and mixed stains can be successfully analyzed and used for excluding or confirming the individual; an INDEL-SNP primer has species specificity and is suitable for forensic medicine detection; the method is possibly used for monitoring transplant ingredients in peripheral blood after a bone marrow transplantation operation or other organ transplantation operations; the method is possibly used for all cases where individuals need to be distinguished when the mixed sample is involved.
Owner:SHANXI MEDICAL UNIV
Human mesenchymal progenitor cell
InactiveUS20050142119A1BiocideMicrobiological testing/measurementProgenitorBone marrow transplantations
There is provided an isolated pluri-differentiated human mesenchymal progenitor cells (MPCs), a method for isolating and purifying human mesenchymal progenitor cells from Dexter-type cultures, and characterization of and uses, particularly therapeutic uses for such cells. Specifically, there is provided isolated MPCs which can be used for diagnostic purposes, to enhance the engraftment of hematopoietic progenitor cells, enhance bone marrow transplantation, or aid in the treatment or prevention of graft versus host disease.
Owner:SOUTH FLORIDA UNIVESITY OF
Blockade of T cell migration into epithelial GVHD target tissues as an approach to achieving anti-tumor effects against lymphohematopoietic malignancies without GVHD
InactiveUS7498023B2BiocideMammal material medical ingredientsHematopoietic cellHaematopoietic cell transplantation
Antagonists of T cell migration are used to reduce GVHD in recipients of hematopoietic cell grafts. The administration of antagonists of T cell migration can be used in combination with conventional methods of bone marrow transplantation and in combination with the administration of donor leukocytes.
Owner:THE GENERAL HOSPITAL CORP
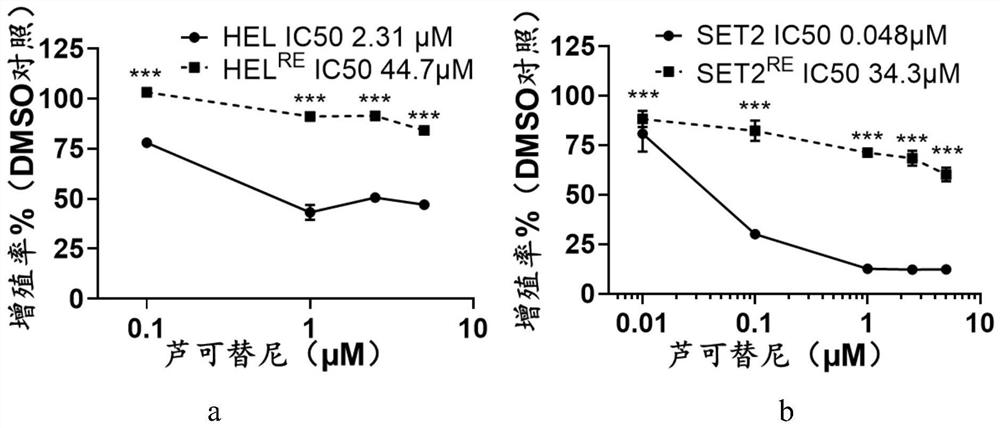
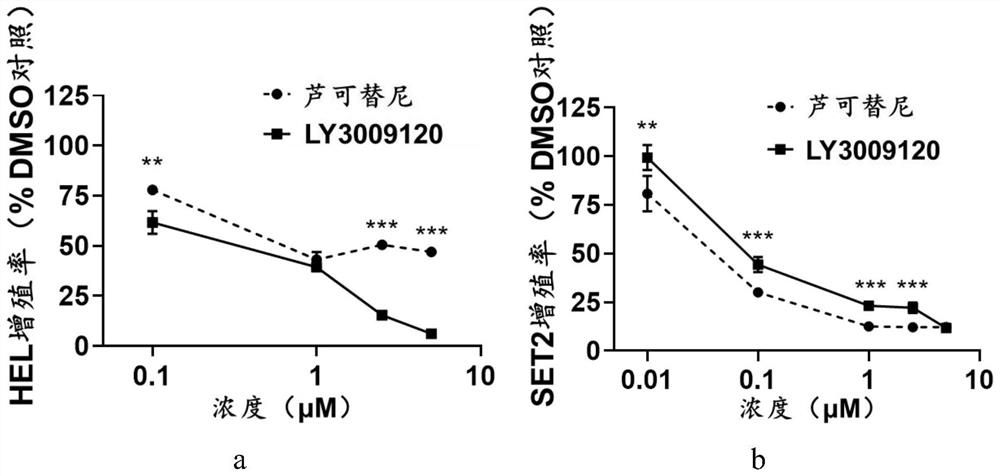
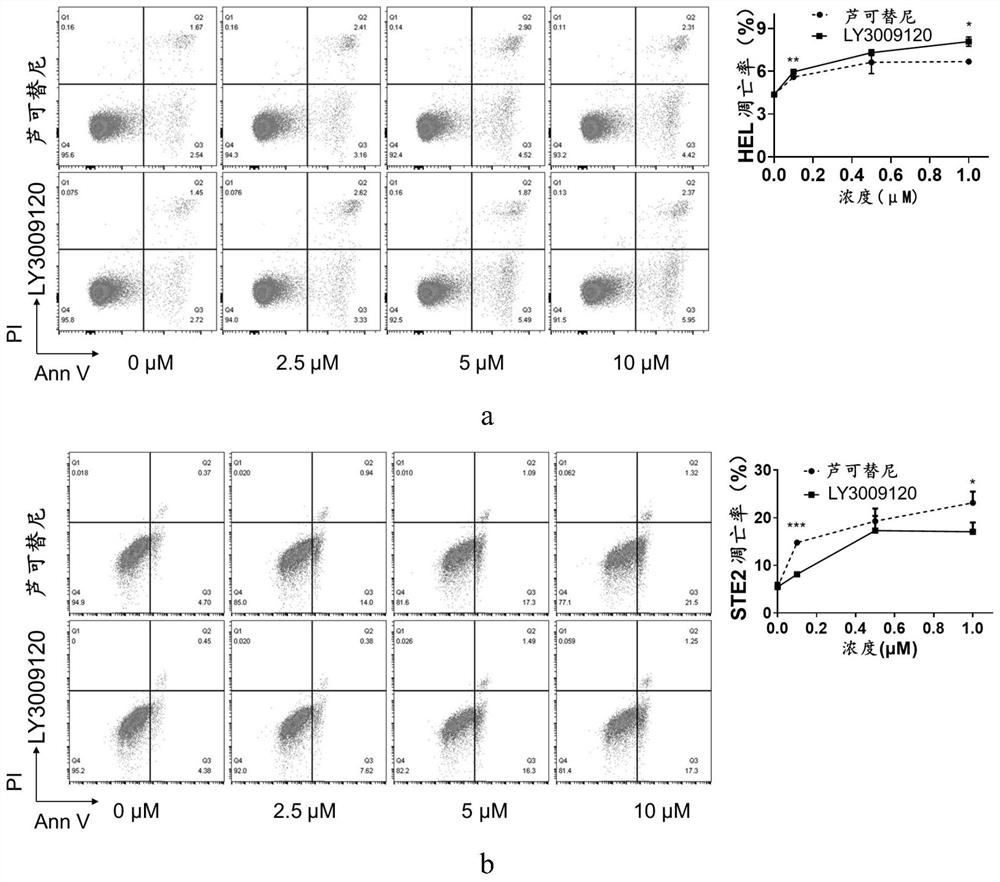
Application of LY3009120 in preparation of medicine for treating myeloproliferative tumors
ActiveCN113082035ALow costOrganic active ingredientsAntineoplastic agentsBone marrow fibrosisChemical synthesis
The invention relates to the technical field of medicines, in particular to application of LY3009120 in preparation of a medicine for treating myeloproliferative tumors. The myeloproliferative tumors comprise polycythemia vera, primary thrombocythemia and myelofibrosis, and particularly, the myeloproliferative tumors resistant to rucotinib. When the LY3009120 is used for treating the myeloproliferative tumors, a new treatment way is provided for the majority of patients with the myeloproliferative tumors, and more choices are provided for clinicians and the patients. For a myeloproliferative tumor patient with drug resistance to rucotinib, LY3009120 can provide continuous oral drug treatment for the patient, and bone marrow transplantation is avoided. LY3009120 can be chemically synthesized, and the cost is lower than that of a biological agent. And through a first-stage clinical experiment, the side reaction is less and lighter, and the tolerance of a clinical patient is good.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
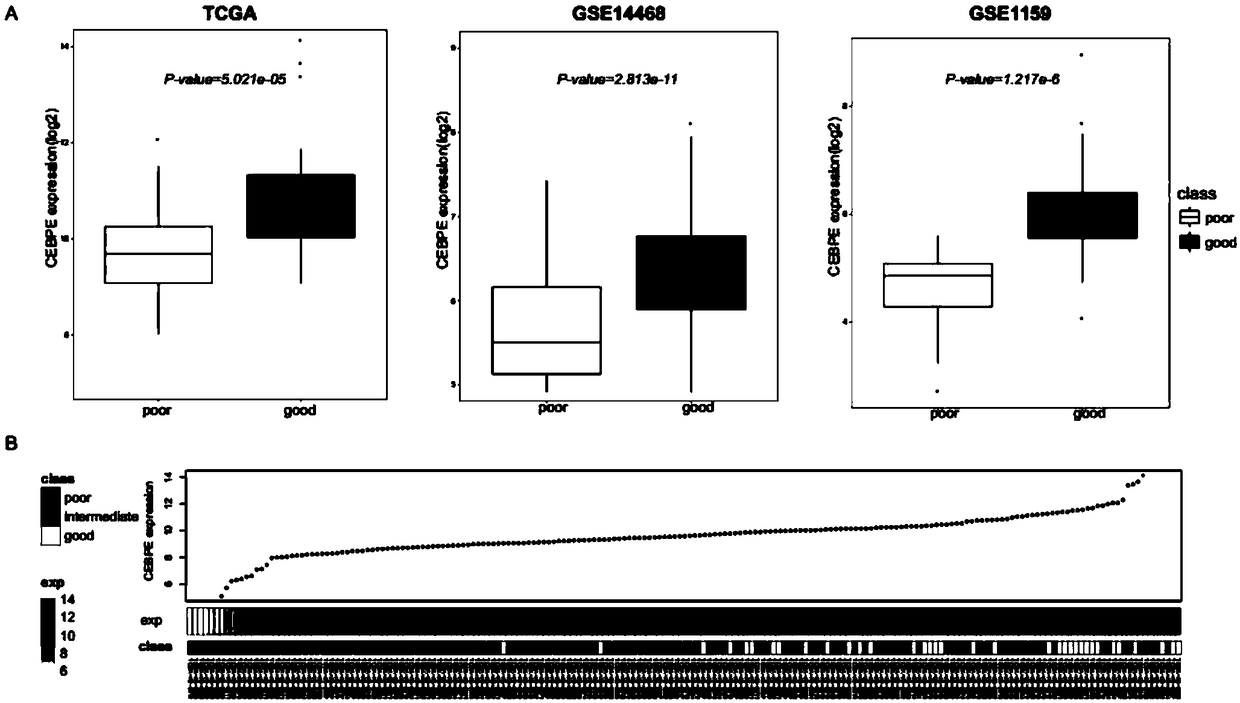
Genetic marker for risk assessment on AML (acute myelogenous leukemia) and application of genetic marker
InactiveCN109402252AMicrobiological testing/measurementBiological material analysisLower riskBone marrow transplantations
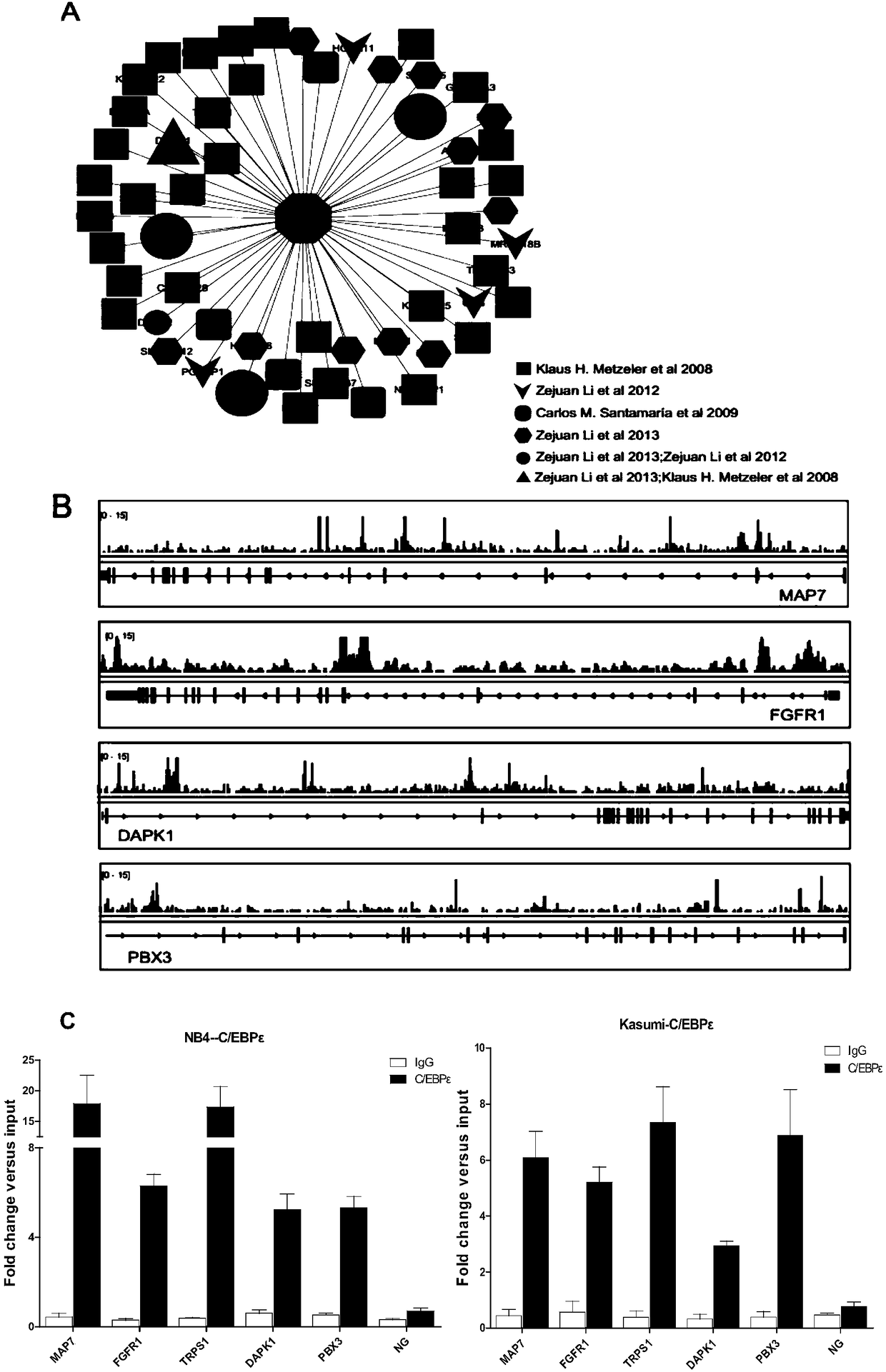
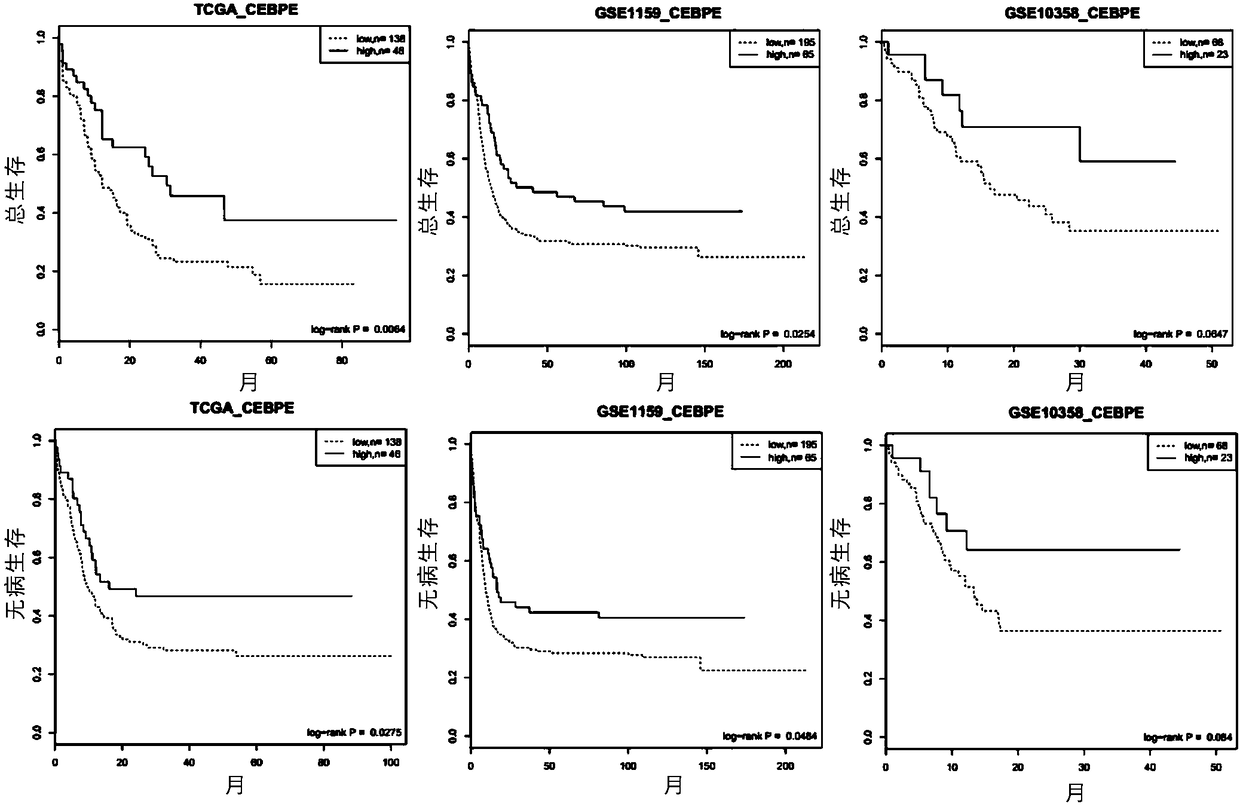
The invention relates to a genetic marker for risk assessment on AML (acute myelogenous leukemia) and application of the genetic marker. The inventor generally analyzes a key gene causing occurrence of AML and development of the disease and identifying CEBPe as a key gene causing survival and recurrence of AML by integrating clinical experimental data and public data resources and utilizing various bioinformatics analysis methods, so that a diagnosis method and a diagnosis kit taking the CEBPe as a target spot are provided. The bioinformatics analysis thinking and methods and the related diagnosis kit are especially suitable for analyzing and predicting the survival time and the recurrence sitation of AML clinical patients. Additionally, the gene diagnosis kit taking the CEBPe as the target spot can also be used for subdividing non-aged AML patients and AML patients having middle and low risks, such as an oncogenic non-mutation sample and the like, and guiding whether allogeneic bone marrow transplantation is conducted.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Treatment of aspergillus infections with alpha thymosin peptides
A method for treating a human infected with Aspergillus by using thymosin alpha 1 as an immuno-stimulator in activating dendritic cells. The method is particularly useful in preventing an infection by Aspergillus in an immuno-compromised host being treated with a bone marrow transplantation.
Owner:SCICLONE PHARM INT LTD
Human stem cell growth factor as well as production method and application of polyethylene glycol (PEG) modified human stem cell growth factor
InactiveCN102559725AInduced expression condition optimizationHigh expressionCosmetic preparationsPeptide/protein ingredientsHalf-lifeCuticle
The invention discloses a human stem cell growth factor as well as a production method and application of a polyethylene glycol (PEG) modified human stem cell growth factor. The production method comprises the following steps of: fusing an h-SCF (Stem Cell Factor)-alpha sequence and an SUMO (Small Ubiquitin-Related Modifier) sequence and constructing to obtain an SUMO-rhSCF-alpha fused gene expression vector; transferring the SUMO-rhSCF-alpha fused gene expression vector into a host bacteria to obtain engineering bacteria; culturing the engineering bacteria and inducing to express an SUMO-rhSCF-alpha fused protein; and cutting off an SUMO part to obtain an rhSCF-alpha protein. By using the production method, the high soluble expression and large-scale purification of the rhSCF-alpha protein in cell plasmas are realized, and the activity of the obtained rhSCF-alpha protein is high. The invention also discloses the production method of the PEG modified human stem cell growth factor; the half-life period of the PEG modified human stem cell growth factor obtained by using the method is remarkably prolonged, and the activity of the PEG modified human stem cell growth factor in promoting the proliferation of red blood cells is also remarkably improved; and the PEG modified human stem cell growth factor can be applied to the preparation of medicines for hypohemia therapy, reconstruction and recovery of a hematopoietic function after chemoradiotherapy and a bone marrow transplantation operation, stem cell ex-vivo expansion and gene therapy and cosmetics for promoting the metabolism of epidermal cells, repairing aged and damaged skin cells, delaying the aging of skin and the like.
Owner:GUANGZHOU JINAN BIOMEDICINE RES & DEV CENT
Human-derived CD-19 chimeric antigen receptor T lymphocyte vector and application thereof
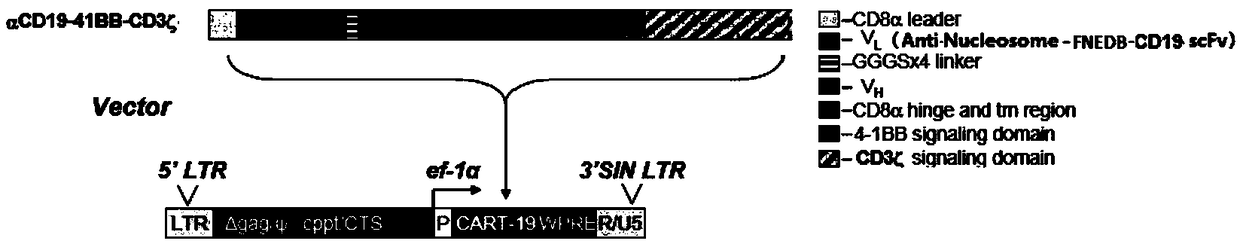
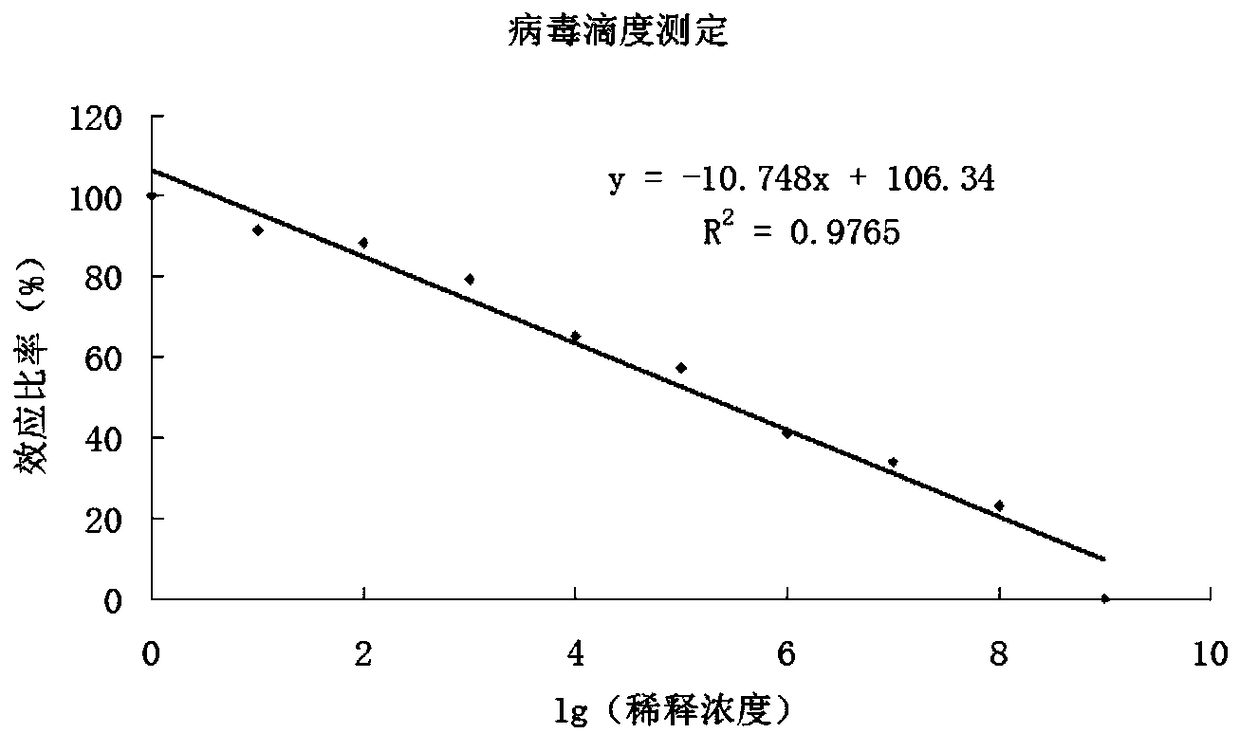
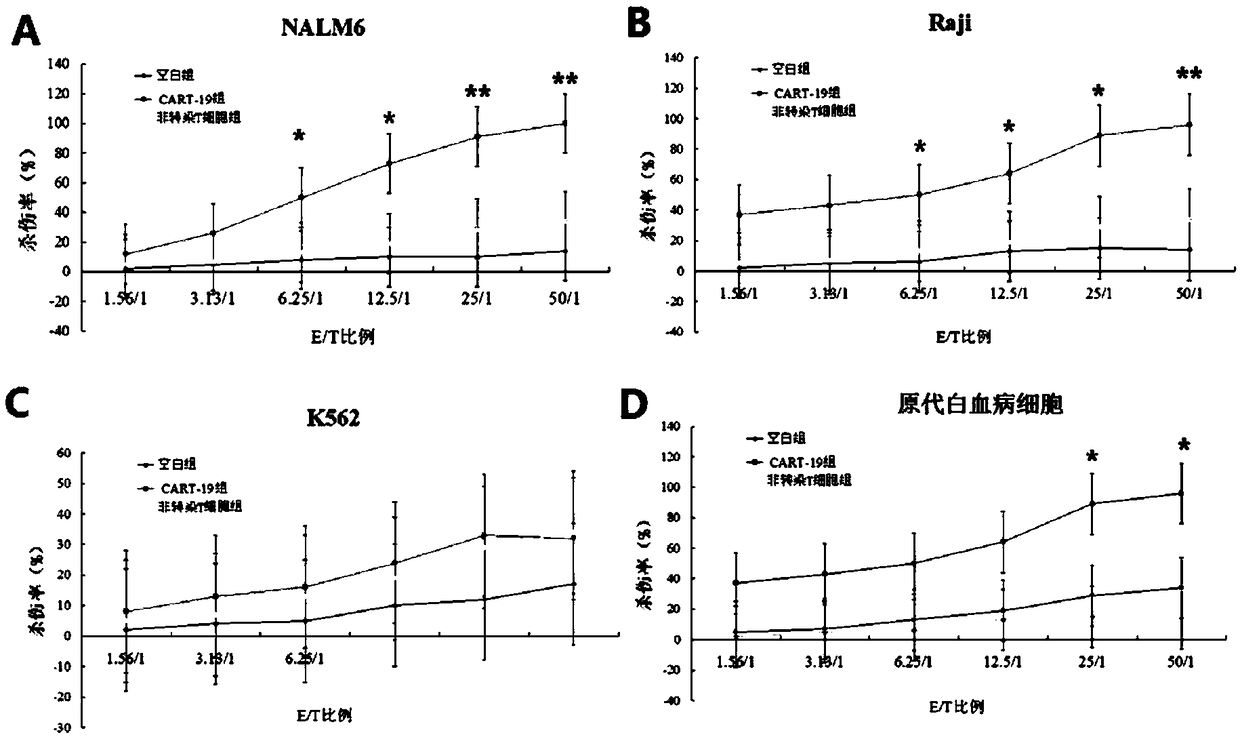
InactiveCN108823247AIncreased suicide geneImprove conversion efficiencyGenetically modified cellsMammal material medical ingredientsMalignant lymphomaT-Cell Specificity
The invention discloses a human-derived CD-19 chimeric antigen receptor T lymphocyte vector which is characterized by comprising a plasmid vector for coding single-chain variable region genes of CD19monoclonal antibodies, nucleosomal histone monoclonal antibodies and FNEDB monoclonal antibodies. The vector is a lentiviral vector. The invention further discloses application of the human-derived CD-19 chimeric antigen receptor T lymphocyte vector in preparation of CATR cells. The vector is used for transduction of T lymphocytes, natural killer cells and mononuclear cells, and is applied to treating tumors, preferably treating reoccurrence in malignant lymphoma drugs as well as neoplastic hematologic disorders and lymphomas which are refractory and unsuccessful in bone marrow transplantationmatching. The human-derived CD-19 chimeric antigen receptor T lymphocyte vector disclosed by the invention has the beneficial effects that the vector constructed in the invention can achieve the effects of enhancing the targeted CD19 antigen transgenic T lymphocyte specificity, overcoming the tumor microenvironment inhibited immune cellular function and controlling cytokine storm in clinic treatment.
Owner:山东省医学科学院附属医院 +1
Movable bone marrow transplantation bin
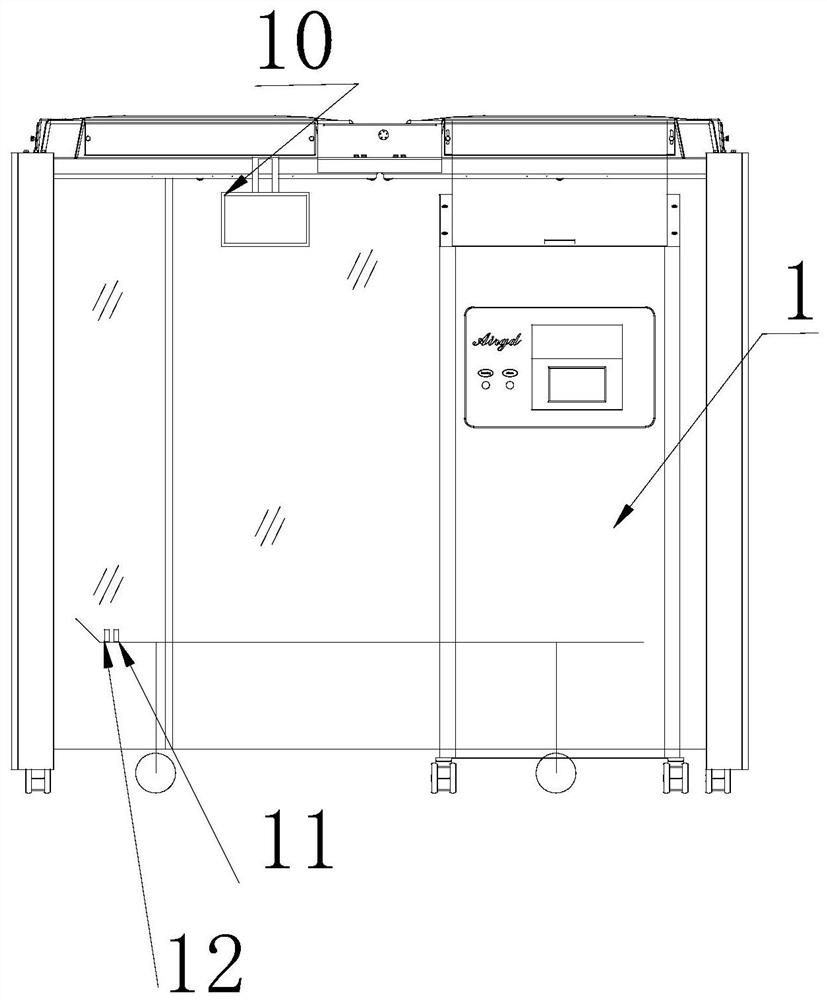
InactiveCN111910959AQuick combination, disassembly and retractionEasy to moveBuilding constructionsDeodrantsBone marrow transplantationsBiomedical engineering
The invention relates to a movable bone marrow transplantation bin. According to the technical scheme, the movable bone marrow transplantation bin comprises a plasma air disinfection machine and a laminar flow clean cover assembled with the plasma air disinfection machine. The modular design is adopted, rapid and convenient combination, disassembly and storage can be achieved, imported medical universal trundles with locking are all installed at the bottom of the transplantation bin, and overall movement is convenient.
Owner:WEIHAI AIRGD MEDICAL EQUIP CO LTD
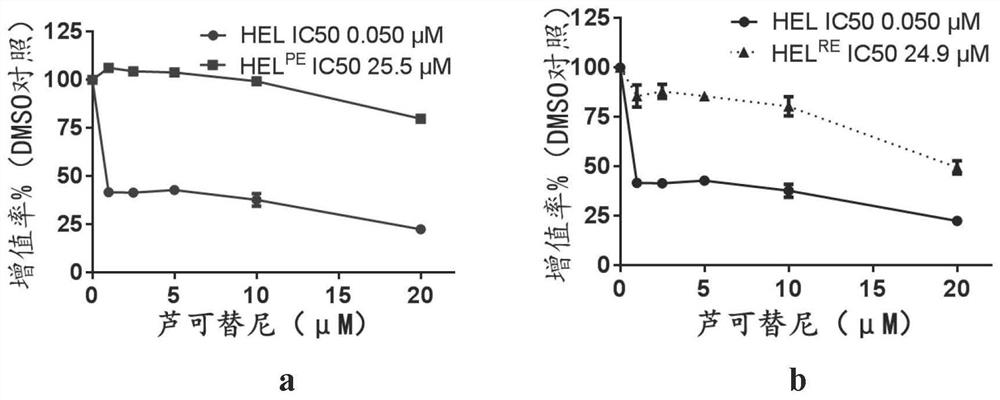
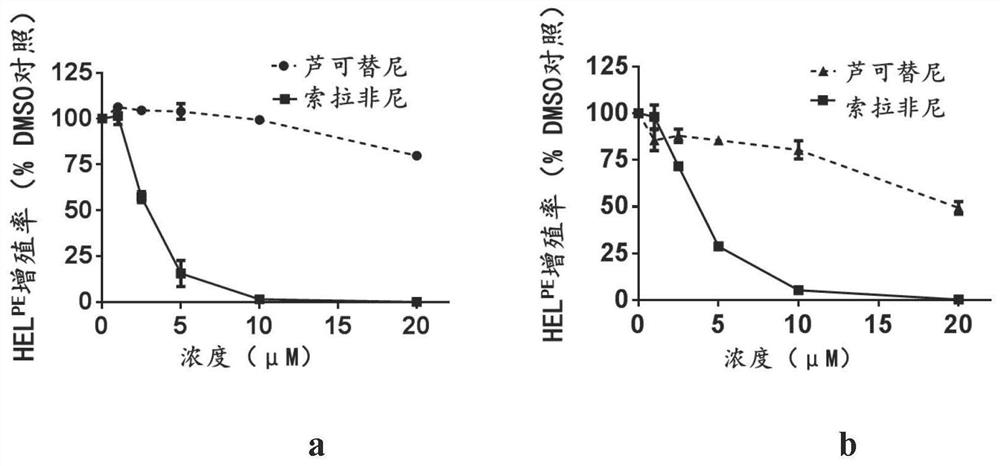
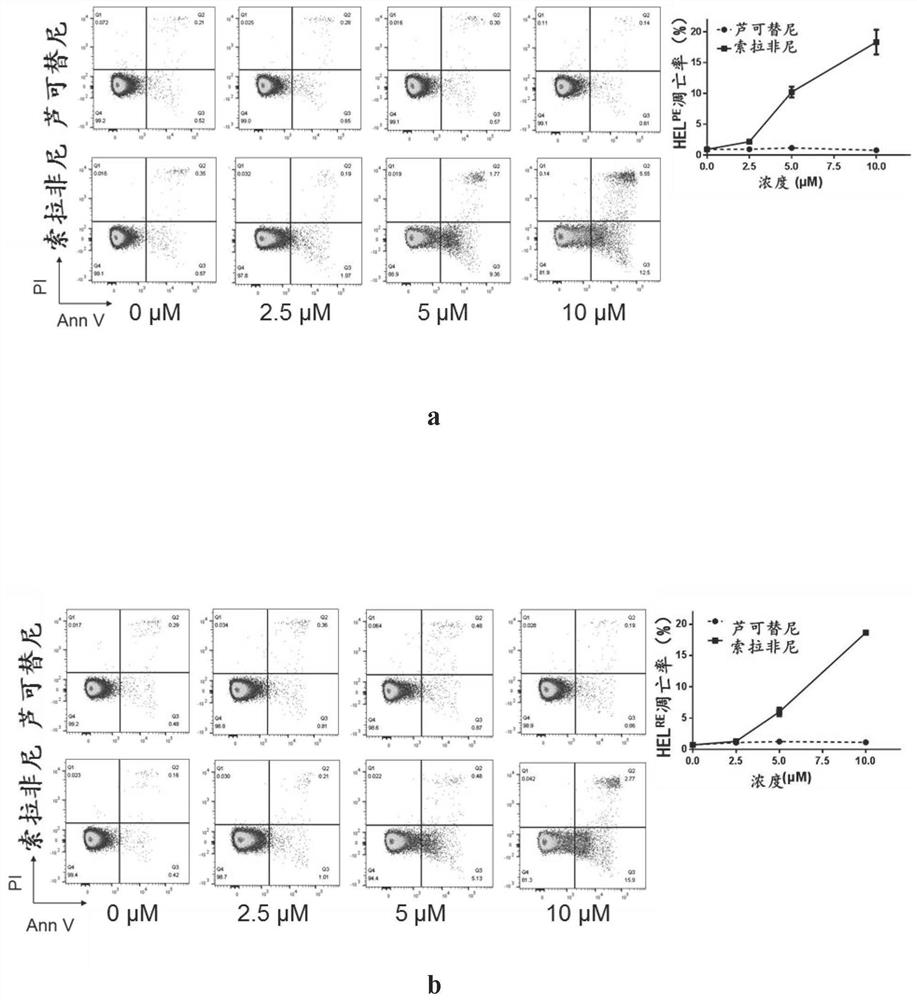
New application of sorafenib, regorafenib and analogues or derivatives thereof
ActiveCN111870600AExempt from transplantSmall burdenAntineoplastic agentsHeterocyclic compound active ingredientsChemical synthesisRed blood cell
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Human Mesenchymal Progenitor Cell
The present invention provides isolated pluri-differentiated human mesenchymal progenitor cells (MPCs), which simultaneously express a plurality of genes that are markers for multiple cell lineages, wherein the multiple cell lineages comprise at least four different mesenchymal cell lineages (e.g., adipocyte, osteoblast, fibroblast, and muscle cell) and wherein each of the markers is specific for a single cell lineage. The present invention also method for isolating and purifying human mesenchymal progenitor cells from Dexter-type cultures for characterization of and uses, particularly therapeutic uses for such cells. Specifically, isolated MPCs can be used for diagnostic purposes, to enhance the engraftment of hematopoietic progenitor cells, enhance bone marrow transplantation, or aid in the treatment or prevention of graft versus host disease.
Owner:UNIV OF SOUTH FLORIDA
Determining the replicative history of lymphocytes
ActiveUS20070003951A1Reduce riskEasy and accurate quantificationSugar derivativesMicrobiological testing/measurementNucleotideControl cell
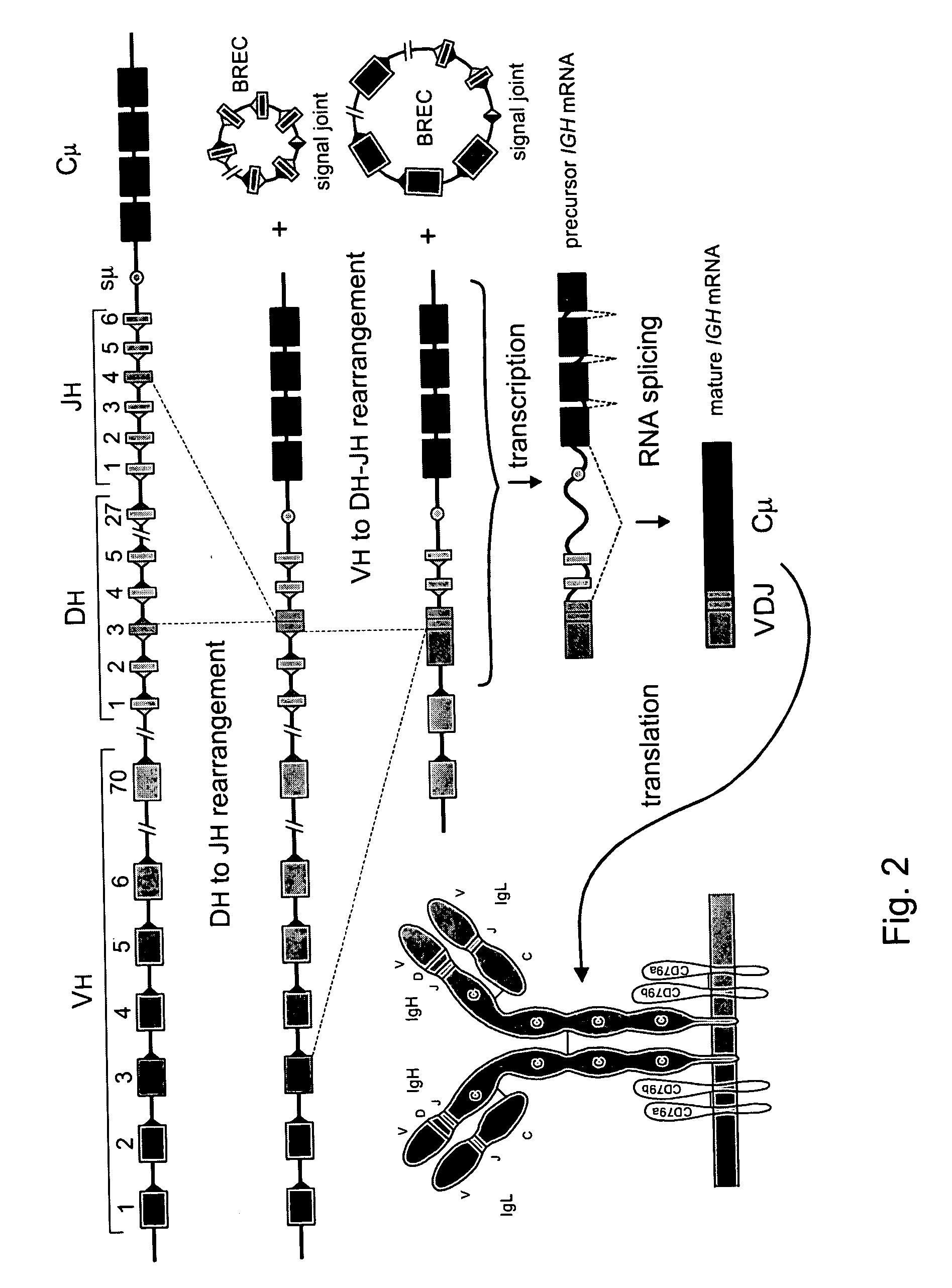
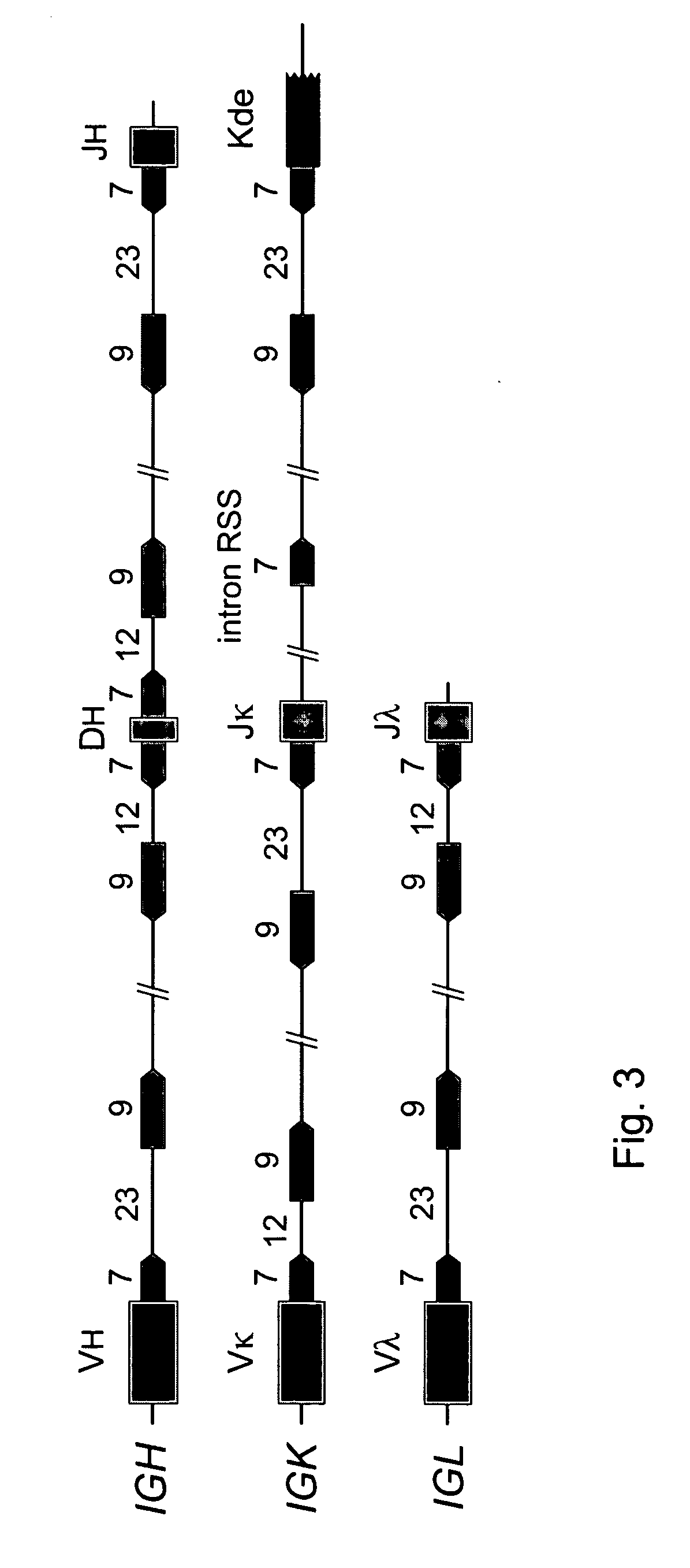
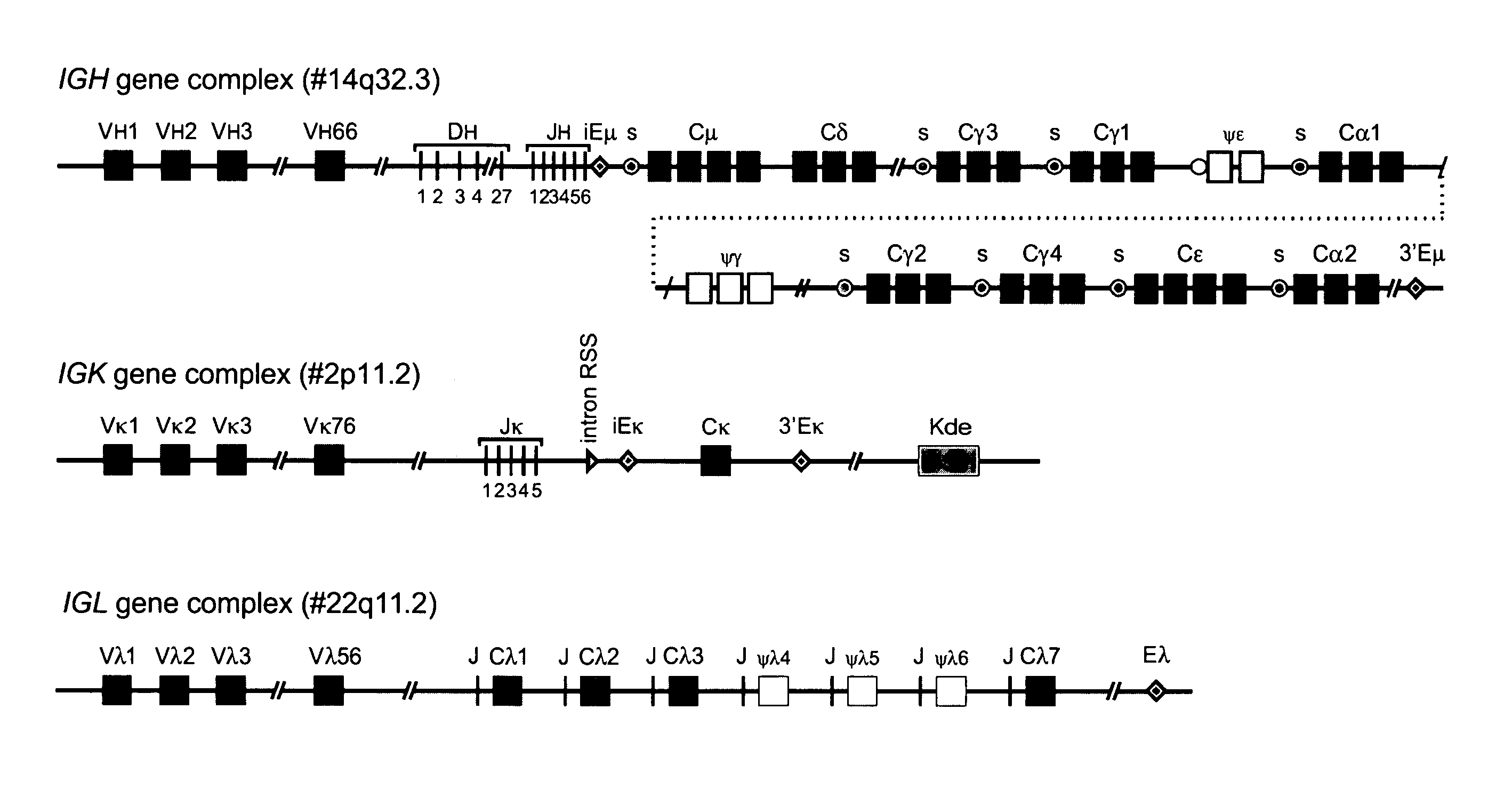
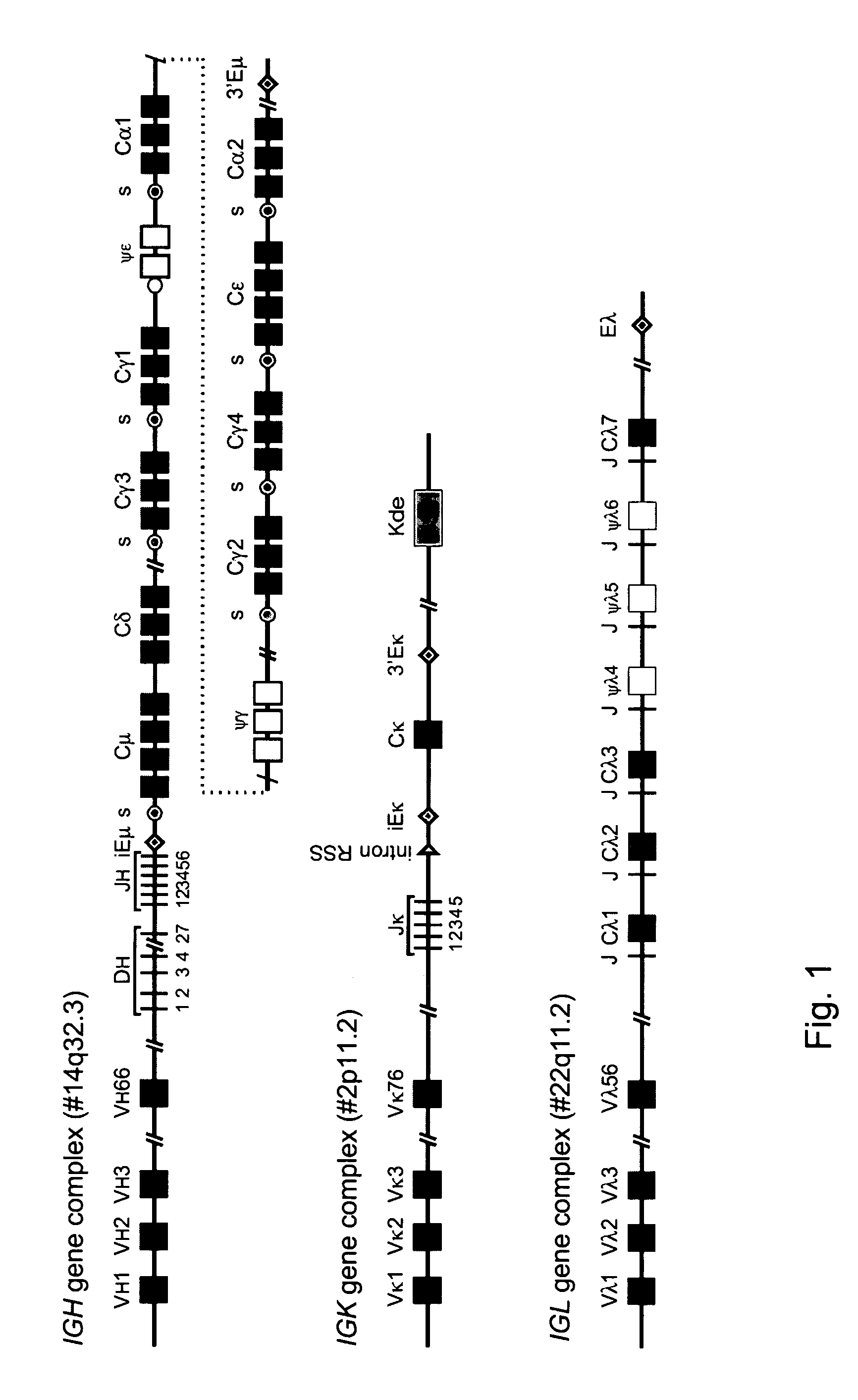
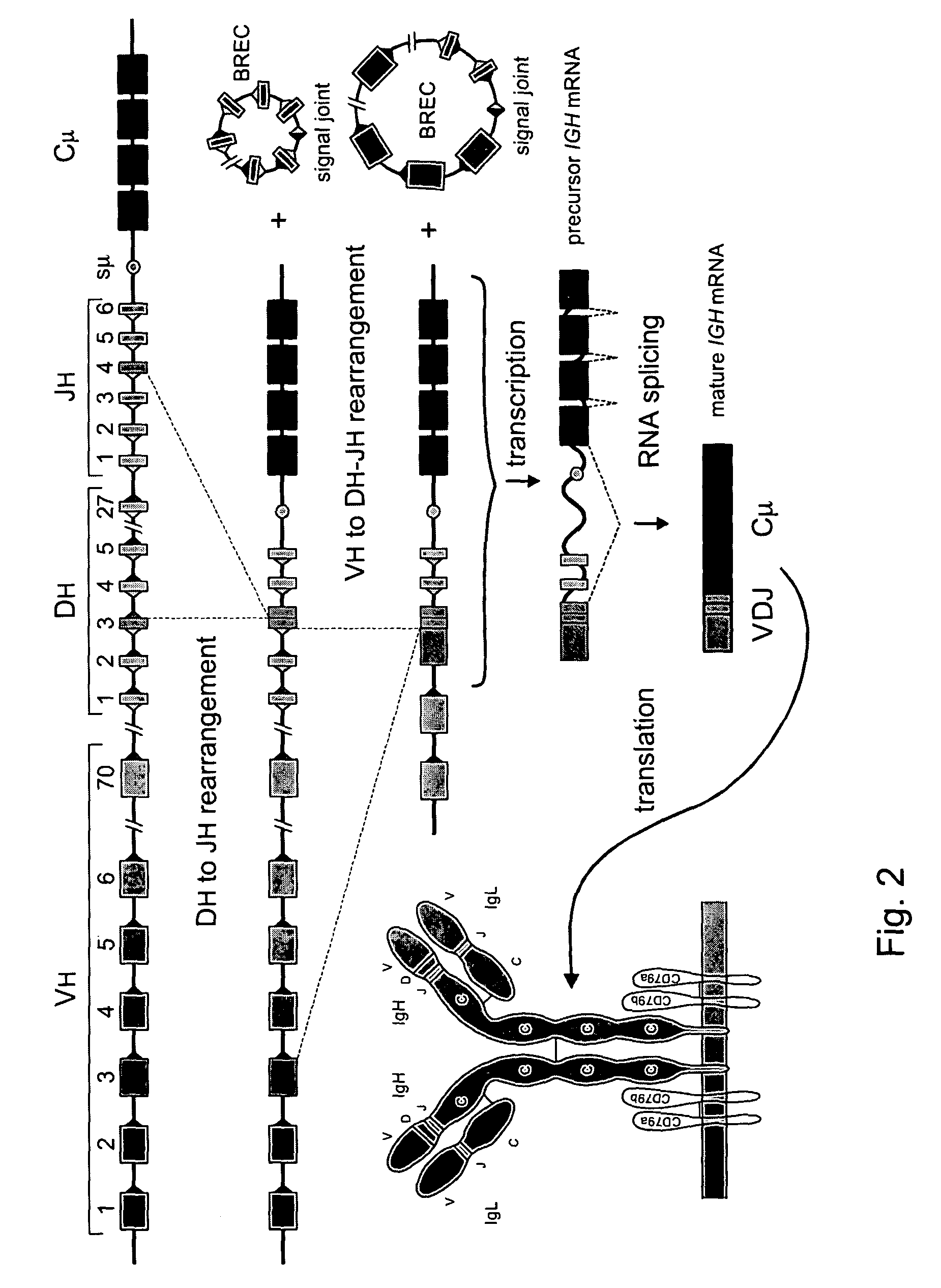
The invention relates to the field of immunology and immunodiagnostics. Provided is a method for determining the replicative history of a lymphocyte, preferably a B cell, the method comprising detecting a signal joint nucleotide sequence on an extrachromosomal circular excision product in the lymphocyte, wherein the excision product is deleted from a chromosome to give a chromosomal-coding joint nucleotide sequence, wherein the coding joint is retained in the chromosome, and detecting the coding joint nucleotide sequence in the lymphocyte. Also provided are primers, probes and a control cell for use in a method of the invention. A method provided herein is among others advantageously used to assess recovery of the precursor B-cell compartment, for example, in a patient following bone marrow transplantation
Owner:ERASMUS UNIVERSITY ROTTERDAM
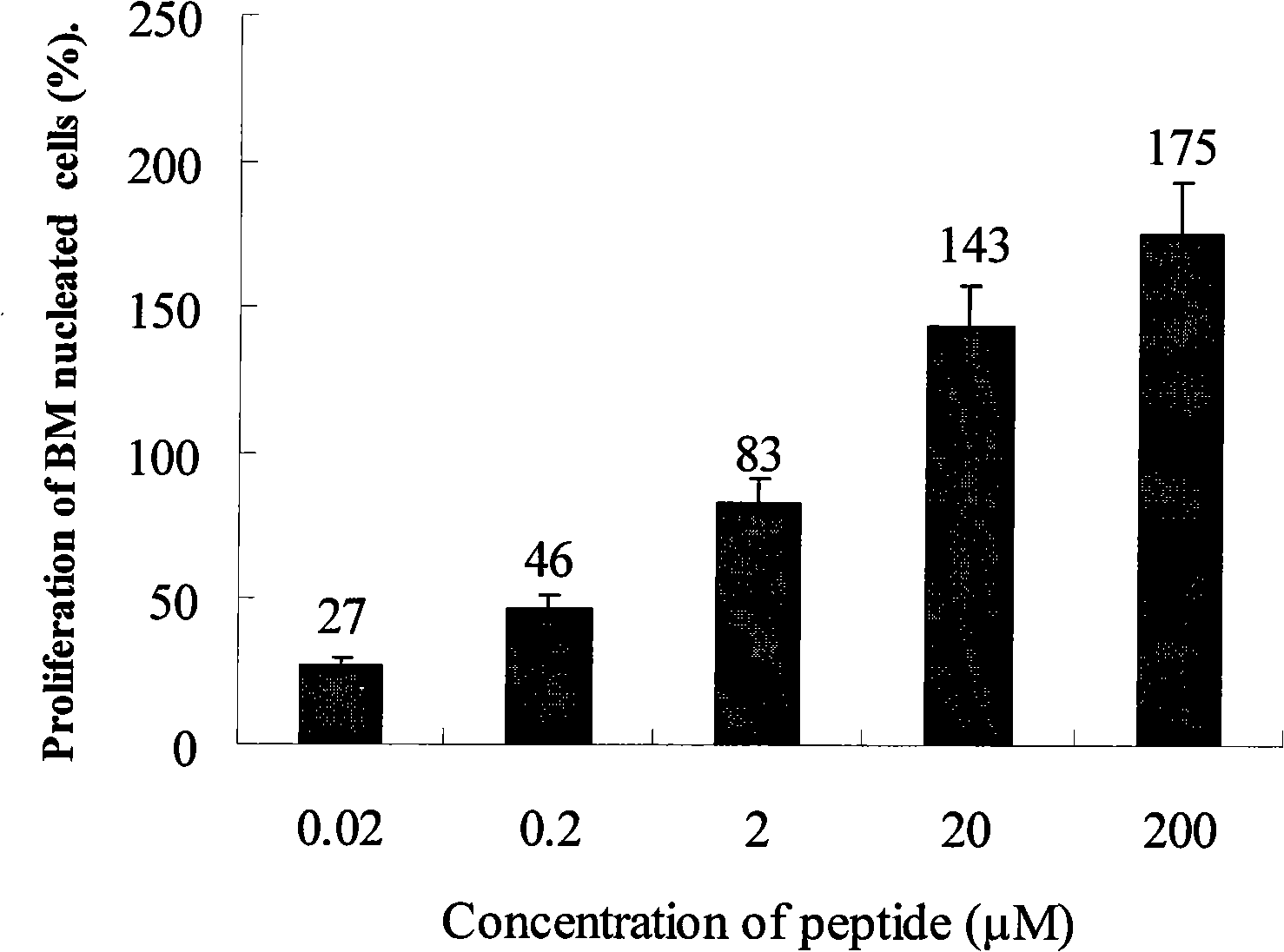
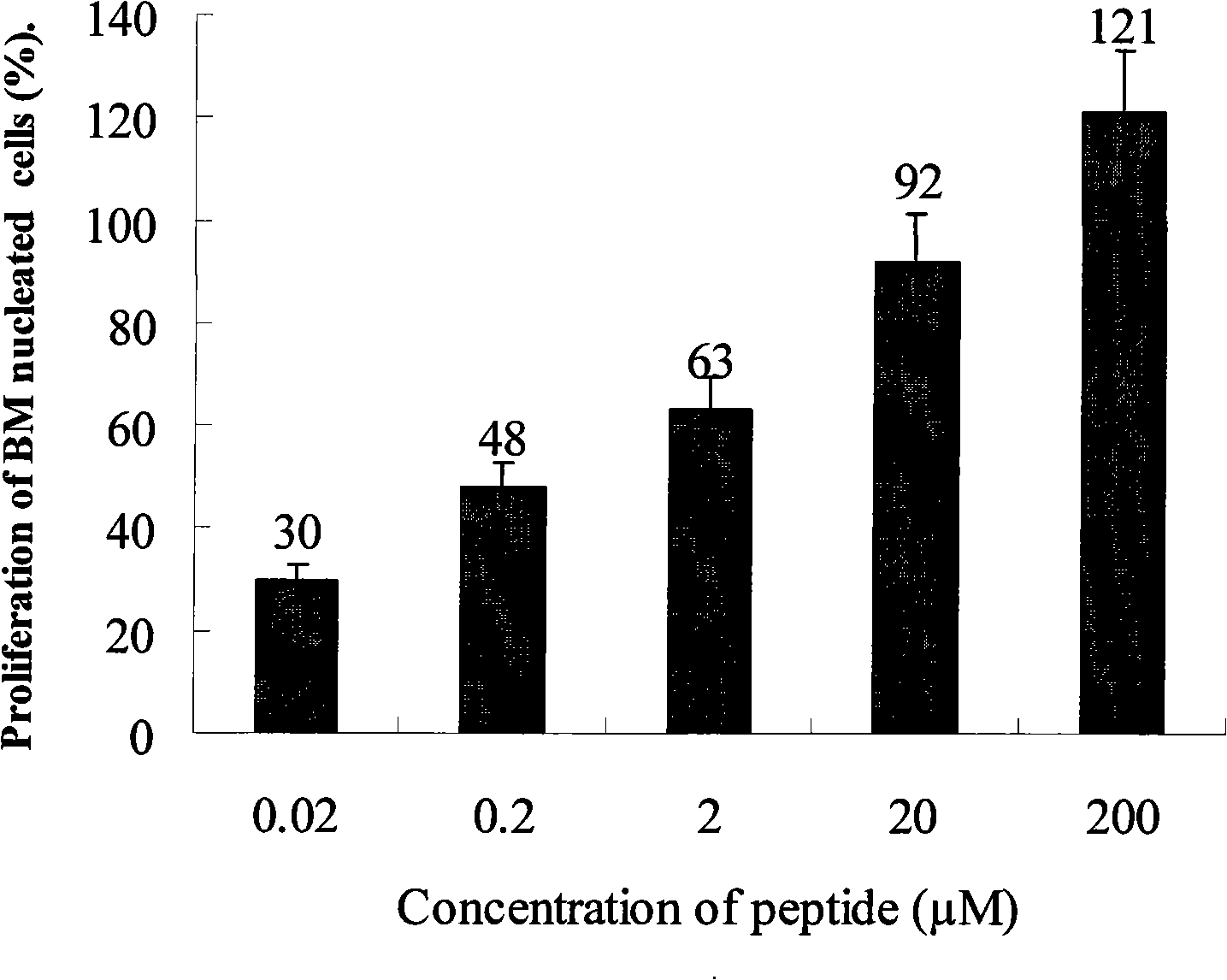
Peptide medicaments for accelerating medulla hematopoiesis cell proliferation
InactiveCN101270151APromote proliferationNormal structureOrganic active ingredientsSugar derivativesHematopoietic cellMiddle medulla
The present invention provides a peptide drug that can promote the proliferation of marrow hematopoietic cells. The main amino acid sequence of the short peptide fragments, analogs, derivants and variants of the short peptide is larger than or equal to 70 percent, equal to and larger than or equal to 90 percent similar to the main amino acid sequence of the short peptide. The short peptide, nucleotides, short peptide fragments, short peptide analogs, short peptide derivants and short peptide variants are used for the preparation of drugs for treating manifold marrow hematopoietic cell defection diseases, congenital anaemia, aplastic anemia and various hemorrhagic anemia diseases and can be applied to the bone marrow transplantation of leukemia patients, etc., that is, the peptide drug of the present invention can treat pancytopenia caused by hemopoietic stem cell defects.
Owner:FUZHOU UNIV
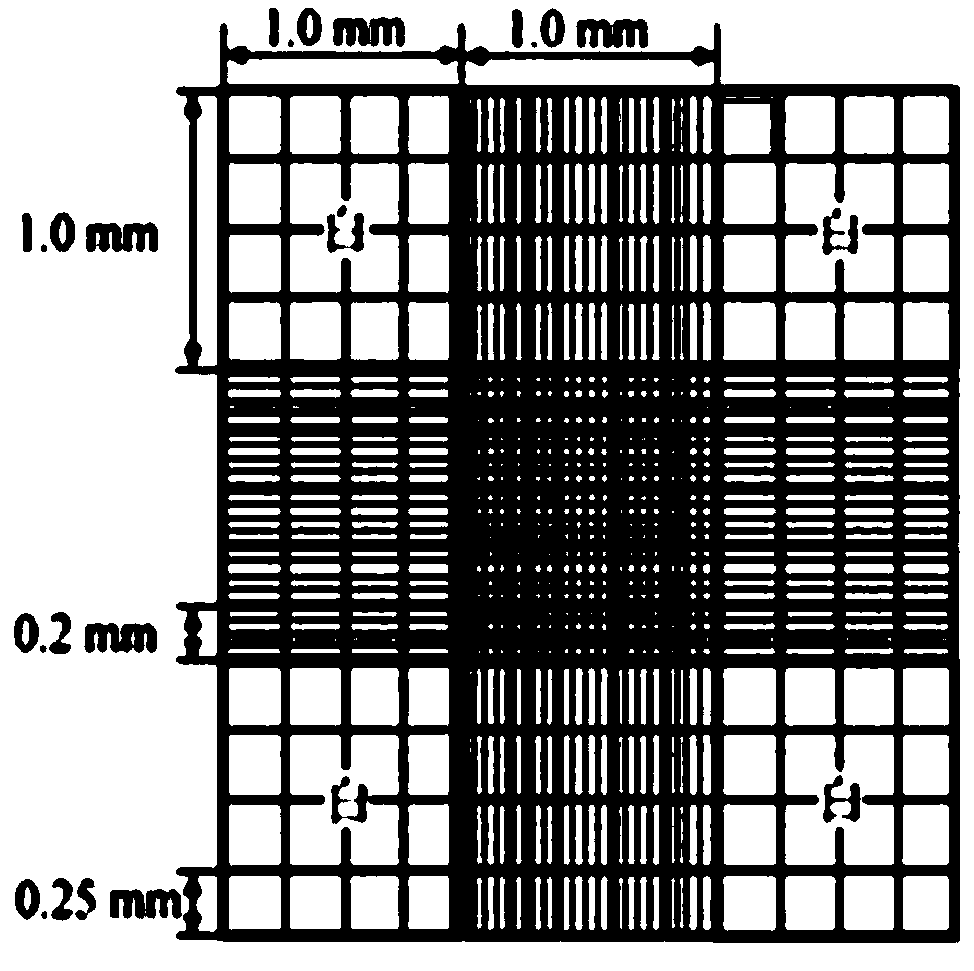
Method for counting reticulocyte by using improved Neubauer counting plate
InactiveCN108827860AMeet the requirementsLow priceIndividual particle analysisCounting reticulocytesCurative effect
The invention relates to the technical field of reticulocyte counting, in particular to a method for counting reticulocyte by using an improved Neubauer counting plate. According to the method, the normalized operation for directly counting the reticulocyte by using the improved Neubauer counting plate is established, the result of the method is consistent with a method approved by the authority,and a satisfactory effect is achieved; the trouble of preparing a blood smear by using a Miller ocular is reduced, and the error caused by nonuniform distribution of the reticulocyte is avoided; the operation procedures are simplified, and the method has the advantages of being simple, direct, low in price, fast, practical, accurate, reliable, capable of realizing normalized and standardized operation and capable of being widely popularized in clinic; and the method has great significance in anemia diagnosis, differential diagnosis, curative effect observation, prognosis monitoring and the judgment of the success of bone marrow transplantation. The method comprises the following steps of (1) preparing a cell suspension; (2) selecting the reticulocyte counting plate; and (3) carrying out counting.
Owner:CANGZHOU MEDICAL COLLEGE
Peptide medicament series for accelerating medulla hematopoiesis cell proliferation
InactiveCN101270152APromote cell proliferationNormal cell structureOrganic active ingredientsSugar derivativesBone Marrow Blood-Forming CellPeptide analog
The present invention provides peptide drug series that can promote the proliferation of marrow hematopoietic cells. The main amino acid sequence of the short peptide fragments, analogs, derivants and variants of the short peptide of the peptide drug series is larger than or equal to 70 percent equal to and larger than or equal to 90 percent similar to the main amino acid sequence of the short peptide. The short peptide, nucleotides, short peptide fragments, short peptide analogs, short peptide derivants and short peptide variants are used for the preparation of drugs for treating manifold marrow hematopoietic cell defection diseases, congenital anaemia, aplastic anemia and various hemorrhagic anemia diseases and can be applied to the bone marrow transplantation of leukemia patients, etc., that is, the peptide drug of the present invention can treat pancytopenia caused by hemopoietic stem cell defects.
Owner:FUZHOU UNIVERSITY
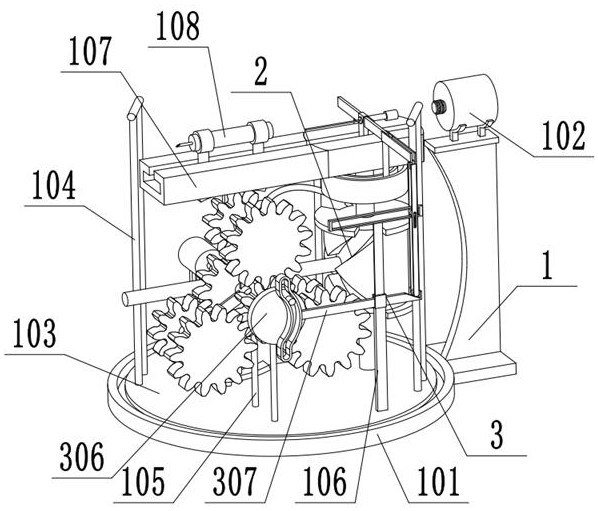
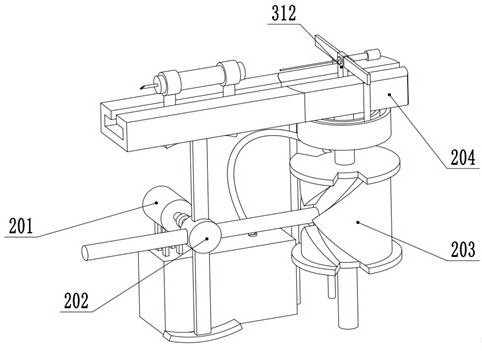
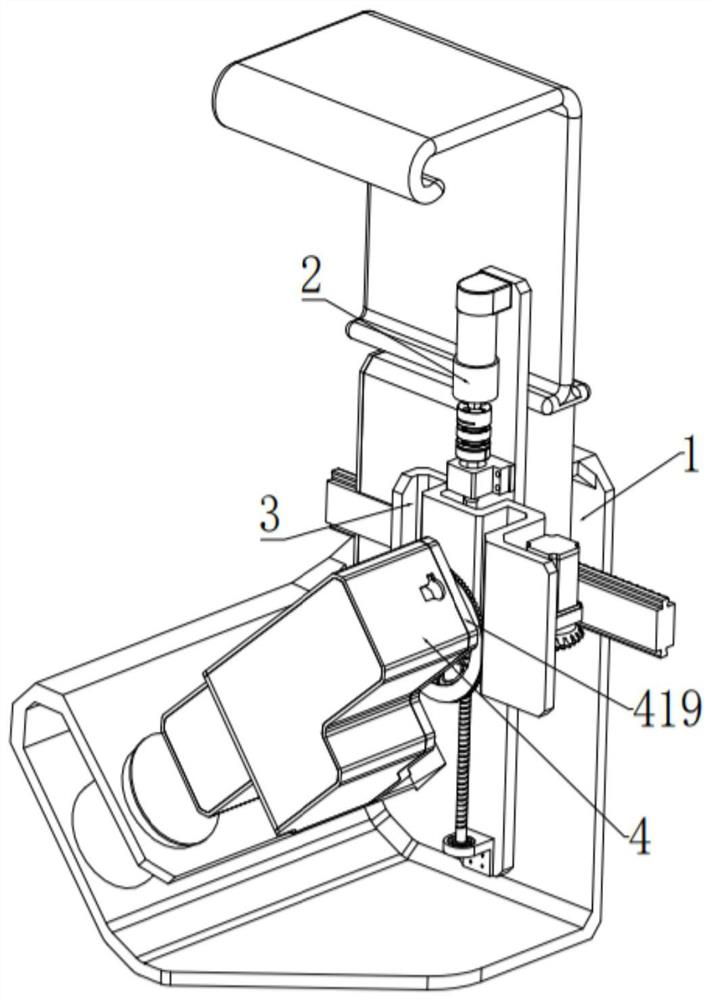
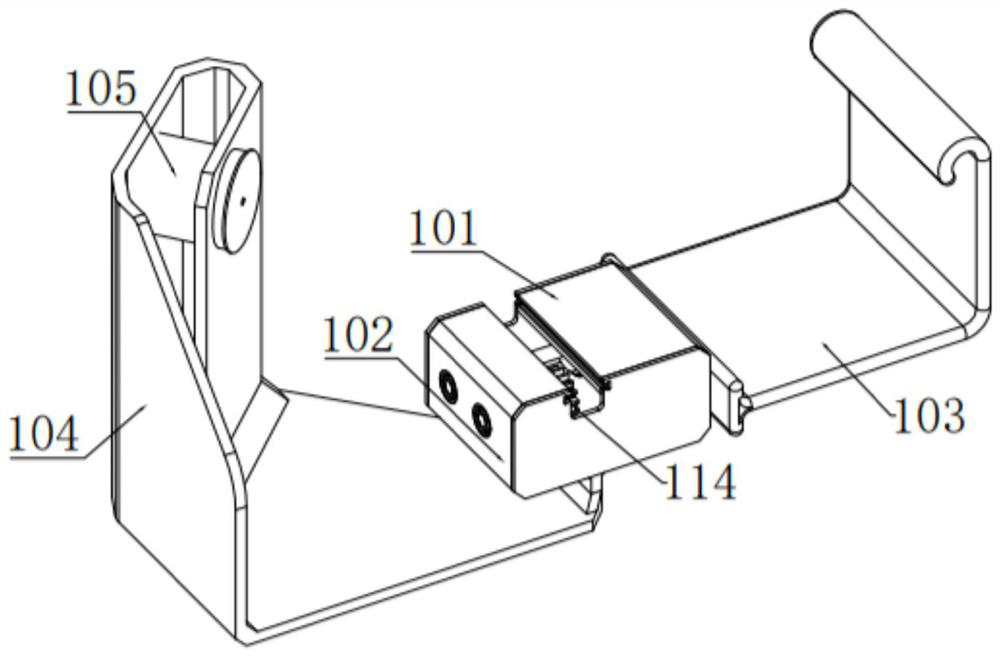
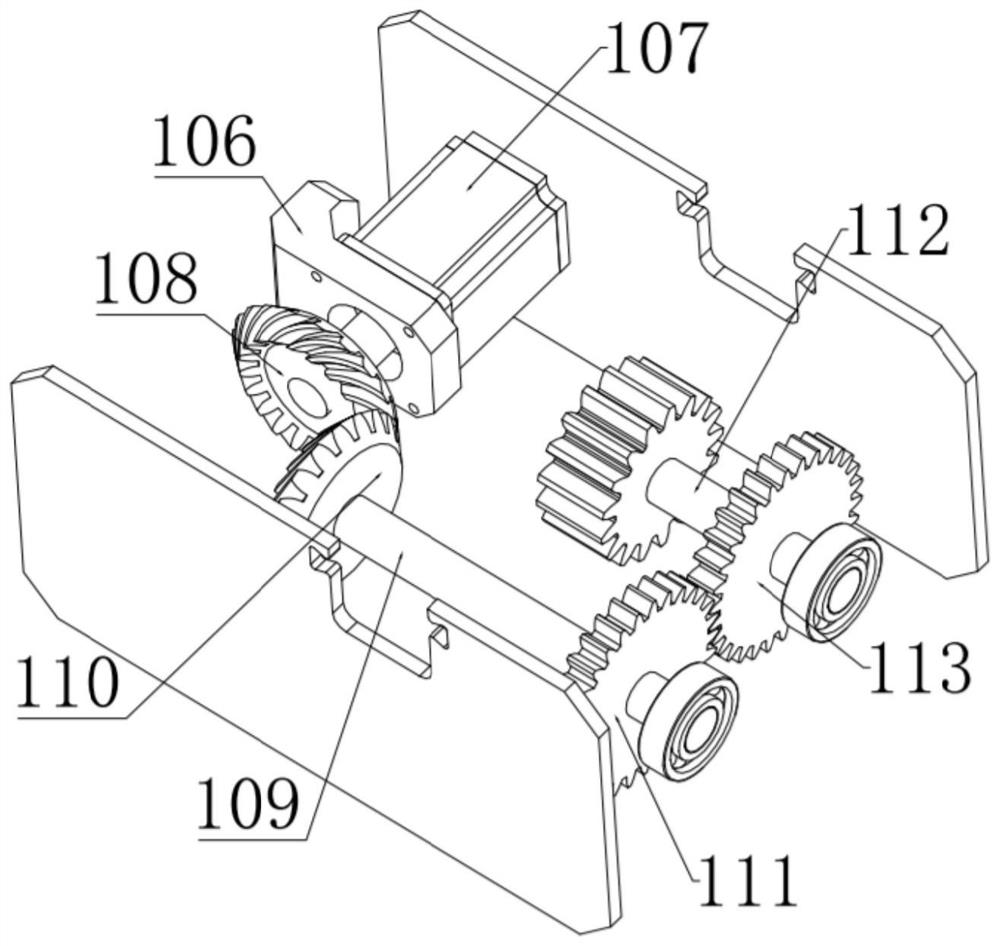
Bone marrow transplantation device for treating cancer
InactiveCN112754615AEasy to operateHigh degree of automationSurgical needlesMedical devicesBone marrow transplantationsSurgery
The invention discloses a bone marrow transplantation device for treating cancer. The bone marrow transplantation device comprises a turntable base, a rotating structure and a push plate structure; the turntable base is used for changing the direction of the upper part of the device; the rotating structure is used for controlling a needle core and the like to rotate; the push plate structure is used for pushing the needle core to horizontally slide; and the operation of semi-automatic bone marrow transplantation is realized by the arrangement of the above parts.
Owner:大泗医疗生物科技有限公司
Stem cell preparation for preventing acute graft versus host disease
InactiveCN101269237AGood treatment effectReduce incidenceProsthesisHuman bodyBone marrow transplantations
The invention belongs to the stem cells and immunology field, and relates to a stem cell preparation for preventing acute graft-versus-host disease. The stem cell preparation for preventing acute graft-versus-host disease of the invention is a stem cell preparation jointly grafted from mesenchymal stem cells and hematopoietic stem cells. The stem cell preparation is infused into the receptors in the human body through vein, the infusion dosage of the mesenchymal stem cells is adjusted according to the receptors, and the infusion dosage of the hematopoietic stem cells refers to the conventional bone marrow transplantation program. The stem cell preparation of the invention is applicable to the patient the bone marrow of which is transplanted, can reduce the outbreak rate of the acute graft-versus-host disease (GVHD), can lower the severity degree obviously, and can provide a new way for comprehensively solving the problems raised after hematopoietic stem cells transplantation.
Owner:SUN YAT SEN UNIV
Strong medicine composition for treating chronic granulocytic leukemia and preparation thereof
InactiveCN108524577APromote hematopoietic functionPromote value-added effectAntineoplastic agentsPlant ingredientsSide effectChronic myeloid leukaemia
The invention discloses a strong medicine composition for treating chronic granulocytic leukemia and preparation thereof. The strong medicine composition and the preparation thereof are simple in formula, safe and mild; by combining cusiae and folium isatidis, the functions of clearing away heat and toxic materials, cooling and activating blood, removing stasis, dispersing blood stasis, strengthening the body resistance to eliminate pathogenic factors, protecting visceral organs, regulating qi and blood and enhancing immunity are achieved, the effect of promoting recovery of the normal marrowhematopoietic function is achieved, the effective component indirubin extracted from cusiae and folium isatidis has the obvious killing effect on leukemia cells, and the effect of promoting T lymphocyte proliferation is achieved; in addition, the strong medicine composition is scientific and reasonable in formula, the side effects are small, the preparation method is simple, the preparation cost is low, the composition becomes effective rapidly, the chronic granulocytic leukemia does not relapse almost, no bone marrow transplantation needs to be performed during chronic granulocytic leukemia treatment, little pain is caused to a patient, the patient accepts the composition easily, the body and economic burdens can be greatly relieved, and the great popularization value is achieved.
Owner:广西忠宁制药有限公司
New Short Nucleotide Tandem Repeat Sequence Site and Its Application
ActiveCN104099325BHigh heterozygosityHigh resolutionMicrobiological testing/measurementDNA preparationWhite blood cellAmniotic fluid
The invention discloses a new short nucleotide tandem repeat site and application thereof. A short tandem repeat locus G11S0001 has a sequence shown as SEQ ID NO.1, and can be used for preparation of (a) reagents or kits for genetic relationship analysis; (b) reagents or kits for individual identification; (c) reagents or kits for paternity testing or blood relationship analysis; (d) reagents or kits for detecting whether maternal blood contamination exists in extracted amniotic fluid; and / or (e) kits for detecting whether leucocytes in a bone marrow transplantation receptor are substituted by donor cells. The short tandem repeat locus G11S0001 has high discrimination, and can effectively analyze the genetic relationship.
Owner:GENESKY DIAGNOSTICS SUZHOU
New short nucleotide tandem repeat site and application thereof
ActiveCN104099325AHigh heterozygosityHigh resolutionMicrobiological testing/measurementDNA preparationWhite blood cellNucleotide
The invention discloses a new short nucleotide tandem repeat site and application thereof. A short tandem repeat locus G11S0001 has a sequence shown as SEQ ID NO.1, and can be used for preparation of (a) reagents or kits for genetic relationship analysis; (b) reagents or kits for individual identification; (c) reagents or kits for paternity testing or blood relationship analysis; (d) reagents or kits for detecting whether maternal blood contamination exists in extracted amniotic fluid; and / or (e) kits for detecting whether leucocytes in a bone marrow transplantation receptor are substituted by donor cells. The short tandem repeat locus G11S0001 has high discrimination, and can effectively analyze the genetic relationship.
Owner:GENESKY DIAGNOSTICS SUZHOU
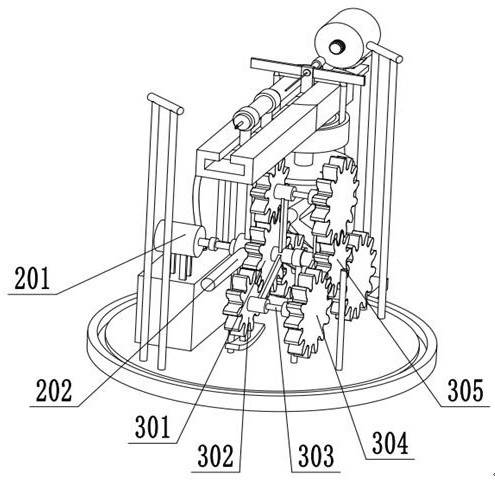
Bone marrow transplantation device for treating cancer
InactiveCN112716573AAutomatic placementImprove efficiencySurgical needlesSuction devicesBone marrow transplantationsBiomedical engineering
The invention discloses a bone marrow transplantation device for treating cancer, which comprises a translation device, a lifting device, a rotating device and a transplantation device which are arranged on a rack, a mechanical group in the translation device operates to drive the lifting device to translate, and mechanical groups in the lifting device and the rotating device operate to drive the transplantation device to rotate; after a transplanting needle tube on the transplanting device is parallel to the center of the bone marrow test tube, the mechanical groups in the translation device and the lifting device operate to drive the transplanting needle tube to be inserted into the bone marrow test tube to extract bone marrow, and after bone marrow extraction, the mechanical groups in the translation device and the lifting device operate to drive the transplanting needle tube to be away from the test tube and close to a transplanted patient; the mechanical group in the rotating device rotates by a transplanting angle, and the mechanical groups in the translation device and the lifting device operate to perform bone marrow transplantation on the affected group.
Owner:张素华
Determining the replicative history of lymphocytes
ActiveUS9487830B2Reduce riskEasy and accurate quantificationSugar derivativesMicrobiological testing/measurementImmunodiagnosticsNucleotide
Owner:ERASMUS UNIVERSITY ROTTERDAM
New short nucleotide tandem repeat site and application thereof
ActiveCN104099326AHigh heterozygosityHigh resolutionMicrobiological testing/measurementDNA preparationWhite blood cellNucleotide
The invention discloses a new short nucleotide tandem repeat site and application thereof. A short tandem repeat locus G7S0005 has a sequence shown as SEQ ID NO.1, and can be used for preparation of (a) reagents or kits for genetic relationship analysis; (b) reagents or kits for individual identification; (c) reagents or kits for paternity testing or blood relationship analysis; (d) reagents or kits for detecting whether maternal blood contamination exists in extracted amniotic fluid; and / or (e) kits for detecting whether leucocytes in a bone marrow transplantation receptor are substituted by donor cells. The short tandem repeat locus G7S0005 has high discrimination, and can effectively analyze the genetic relationship.
Owner:GENESKY DIAGNOSTICS SUZHOU
Fenugreek protein, its separation and extraction method and its application in the preparation of bone marrow hsc clearance auxiliary agent
ActiveCN113214351BSimple ingredientsQuality controllableDough treatmentPeptide/protein ingredientsAdjuvantCentrifugation
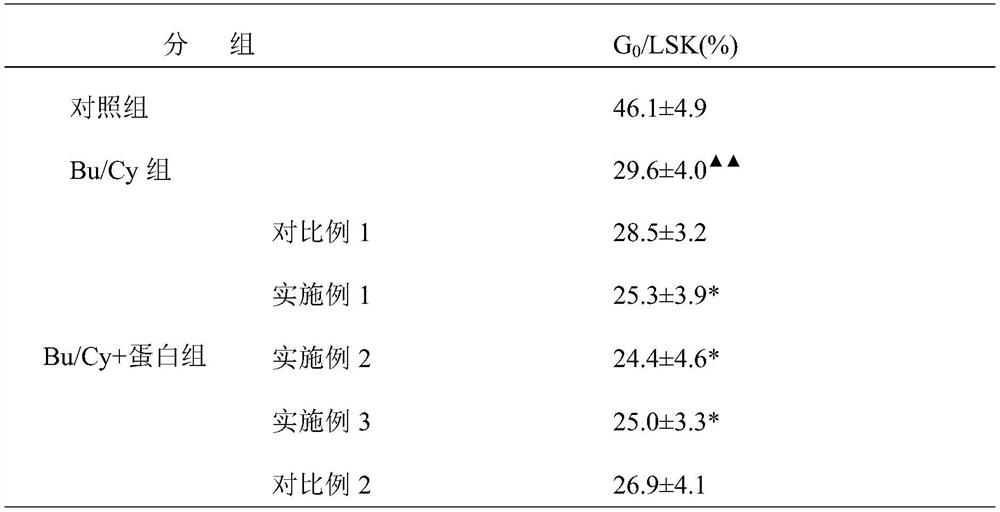
The invention discloses a fenugreek protein, a method for separating and extracting the fenugreek protein, and an application in preparing a bone marrow HSC clearing auxiliary agent. The method for separating and extracting fenugreek protein comprises the following steps: slowly adding ammonium sulfate to the fenugreek extract to a saturation of 40%, centrifuging, and collecting the supernatant; continuing to add ammonium sulfate to a saturation of 70%, Centrifuge to collect the precipitate; dialyze and freeze-dry the precipitate to obtain fenugreek protein. Fenugreek protein of the present invention can significantly reduce G in the LSK cell population 0 The ratio of cells, which have the function of enhancing bone marrow HSC depletion in bone marrow transplantation preconditioning of thalassemia iron overloaded mice, can be used as an adjuvant for bone marrow HSC depletion in thalassemia bone marrow transplantation preconditioning.
Owner:SOUTH CHINA NORMAL UNIVERSITY
New short nucleotide tandem repeat site and application thereof
ActiveCN104099327AHigh heterozygosityHigh resolutionMicrobiological testing/measurementDNA preparationWhite blood cellNucleotide
The invention discloses a new short nucleotide tandem repeat site and application thereof. A short tandem repeat locus G5S0001 has a sequence shown as SEQ ID NO.1, and can be used for preparation of (a) reagents or kits for genetic relationship analysis; (b) reagents or kits for individual identification; (c) reagents or kits for paternity testing or blood relationship analysis; (d) reagents or kits for detecting whether maternal blood contamination exists in extracted amniotic fluid; and / or (e) kits for detecting whether leucocytes in a bone marrow transplantation receptor are substituted by donor cells. The short tandem repeat locus G5S0001 has high discrimination, and can effectively analyze the genetic relationship.
Owner:GENESKY DIAGNOSTICS SUZHOU
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com