Patents
Literature
34 results about "Bone marrow transplantation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
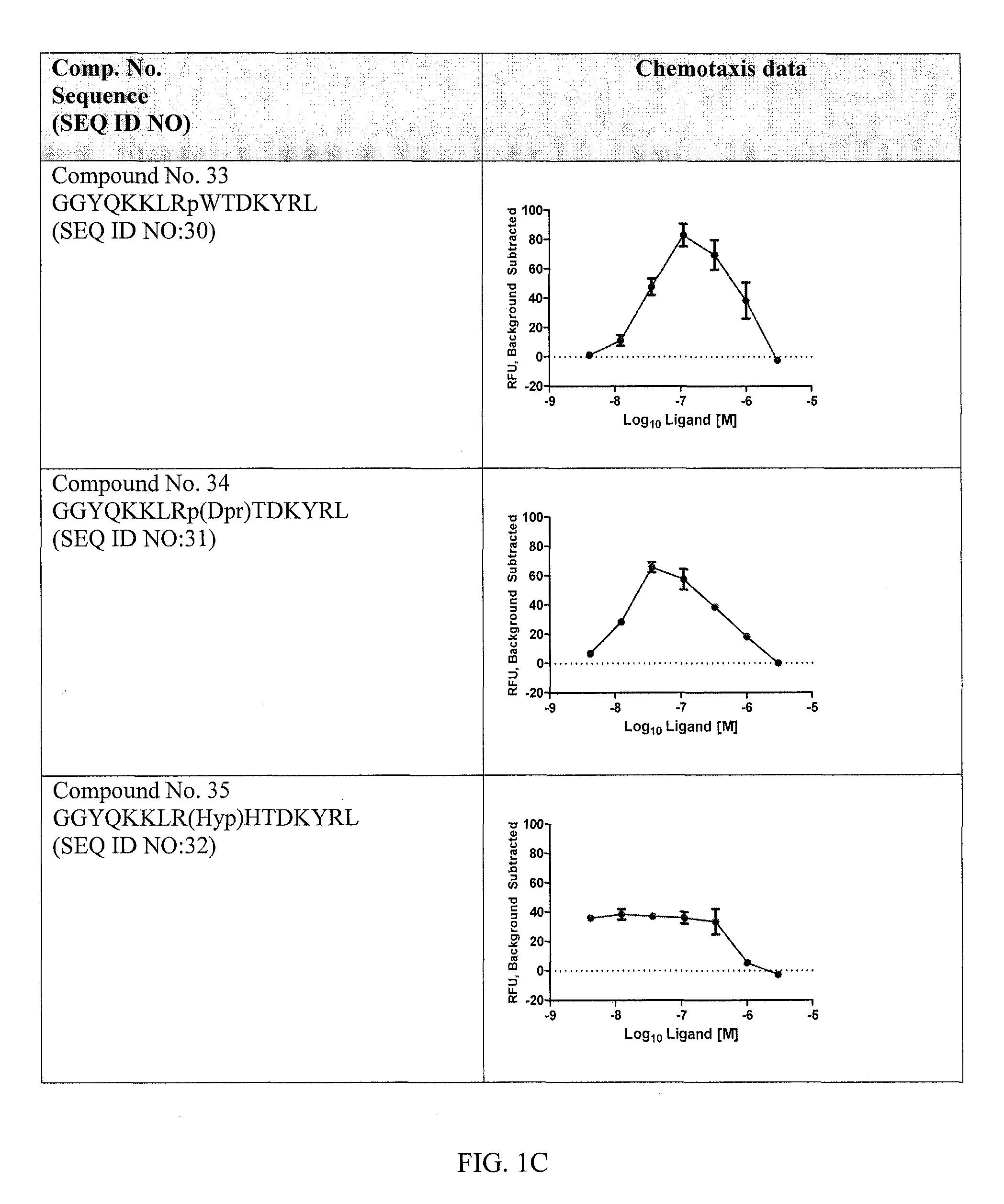
Method of preventing or treating organ, hematopoietic stem cell or bone marrow transplant rejection
InactiveUS20080182845A1Inhibitory activityOrganic chemistryImmunological disordersBone marrow transplantationSurgery
Methods of preventing or treating organ, hematopoietic stem cell or bone marrow transplant rejection are disclosed.
Owner:ABBOTT LAB INC
Use of compounds having ccr antagonism
InactiveUS20050245537A1Effective preventionEffective treatmentBiocideSenses disorderAutoimmune conditionAutoimmune disease
It is intended to provide preventives / remedies for graft-versus-host disease and / or rejection in organ or bone marrow transplantation, rheumatoid arthritis, autoimmune diseases, allergic diseases, ischemic cerebral cell injury, myocardial infarction, chronic nephritis and arteriosclerosis. The above object can be achieved by preventives / remedies for graft-versus-host disease and / or rejection in organ or bone marrow transplantation, rheumatoid arthritis, autoimmune diseases, allergic diseases, ischemic cerebral cell injury, myocardial infarction, chronic nephritis and arteriosclerosis characterized by containing a specific compound having a CCR (CC chemokine receptor) antagonism.
Owner:TAKEDA PHARMA CO LTD +1
Test strip for specifically measuring IgM, IgG and IgA blood group antibodies
The invention belongs to the field of biological medicines and discloses an immunochromatography test paper technology, which is used for specifically measuring the negative, positive and titer of IgM, IgG and IgA blood group antibodies in blood or body fluid or tissue fluid or secretion of people. The technology comprises the following steps: (1) preparing a sample filter membrane and a sample pad; (2) preparing a tracer and a tracing pad; (3) preparing a fiber membrane detection area and a quality control area; (4) labeling a test strip; (5) assembling the test strip; (6) injecting a sample and detecting; and (7) reading a result. The test strip can be used for measuring IgM anti-A and anti-B antibody titer, monitoring implantation of bone marrow transplantation and measuring the titer of immune IgG anti-A and anti-B antibodies of individuals, particularly prenatal pregnant women and newborns, the reverse typing of blood groups can be accurately judged by specifically measuring the IgM anti-A and anti-B antibodies, and the reverse typing of the blood group of an individual can be judged through saliva without blood sampling by specifically measuring IgA anti-A and anti-B antibodies in secretion.
Owner:邵超鹏
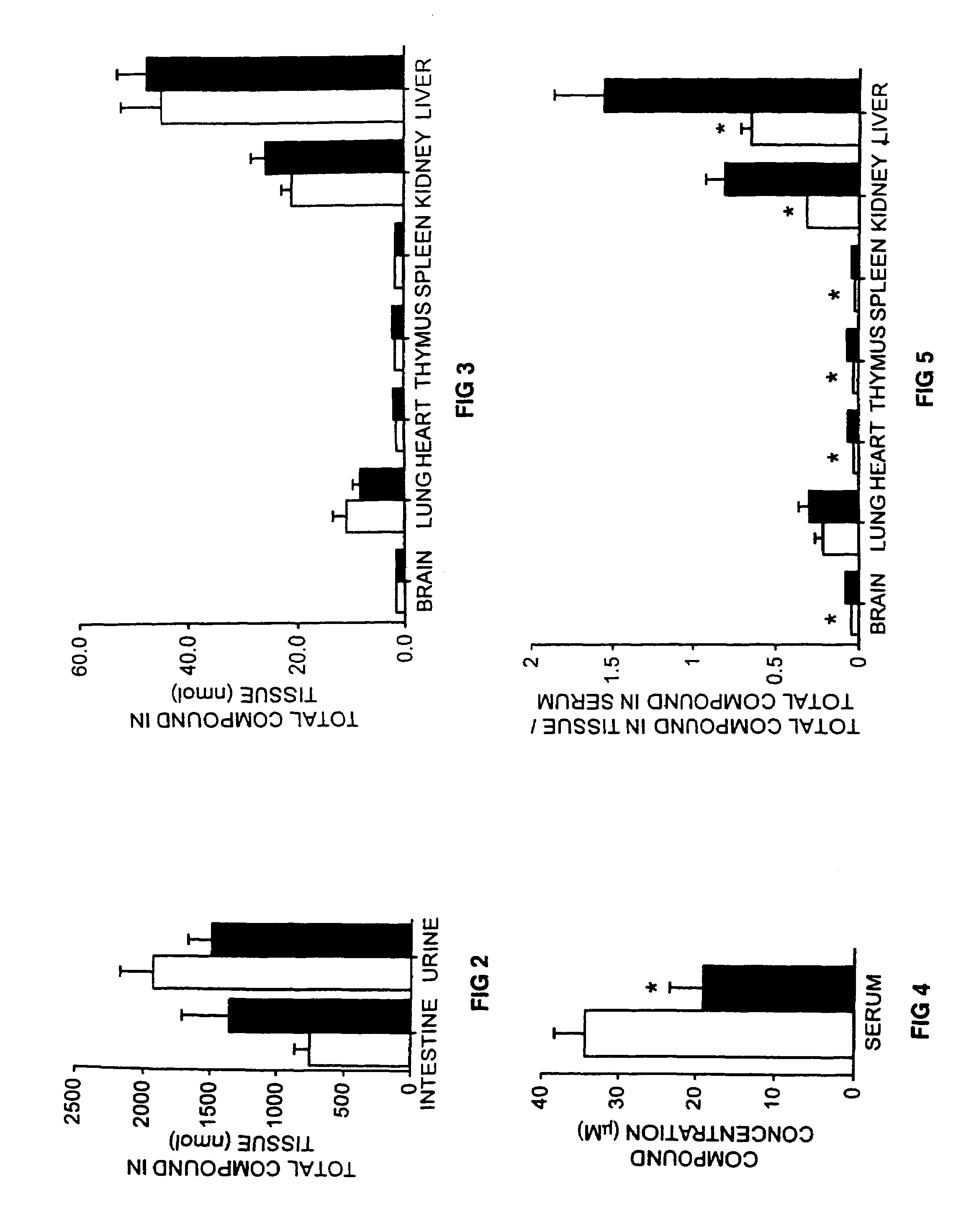
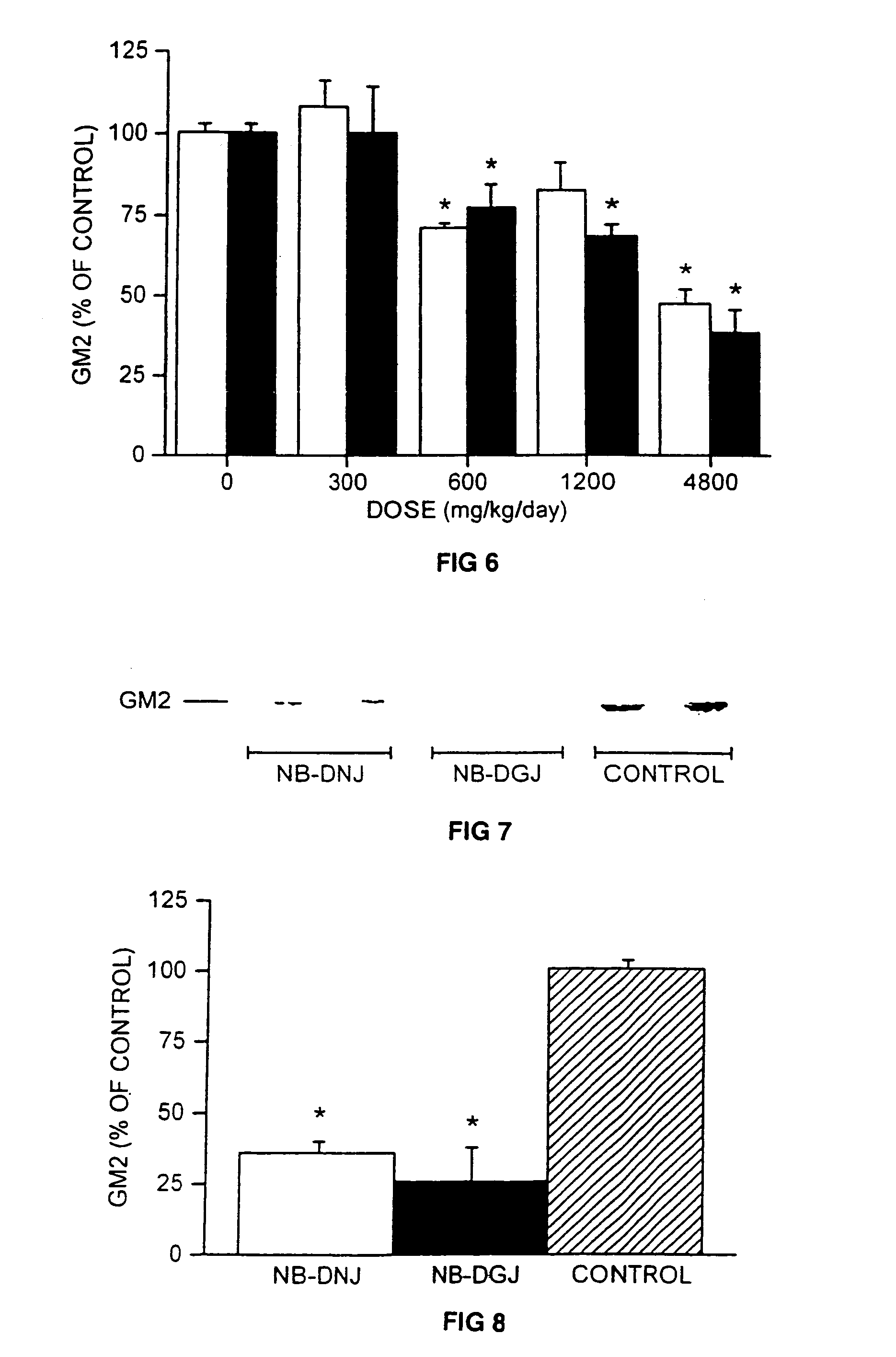
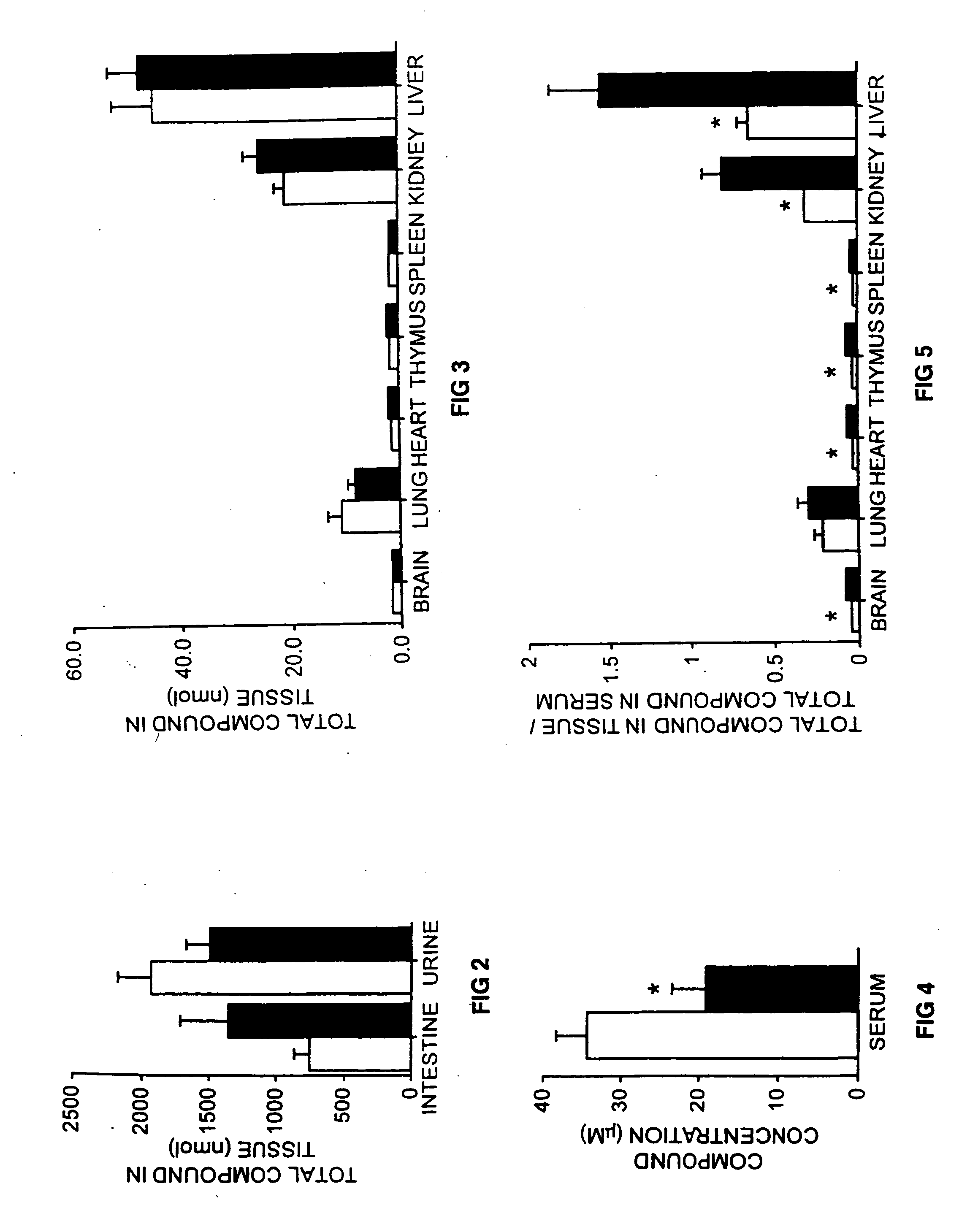
Therapeutic compositions and methods of treating glycolipid storage related disorders
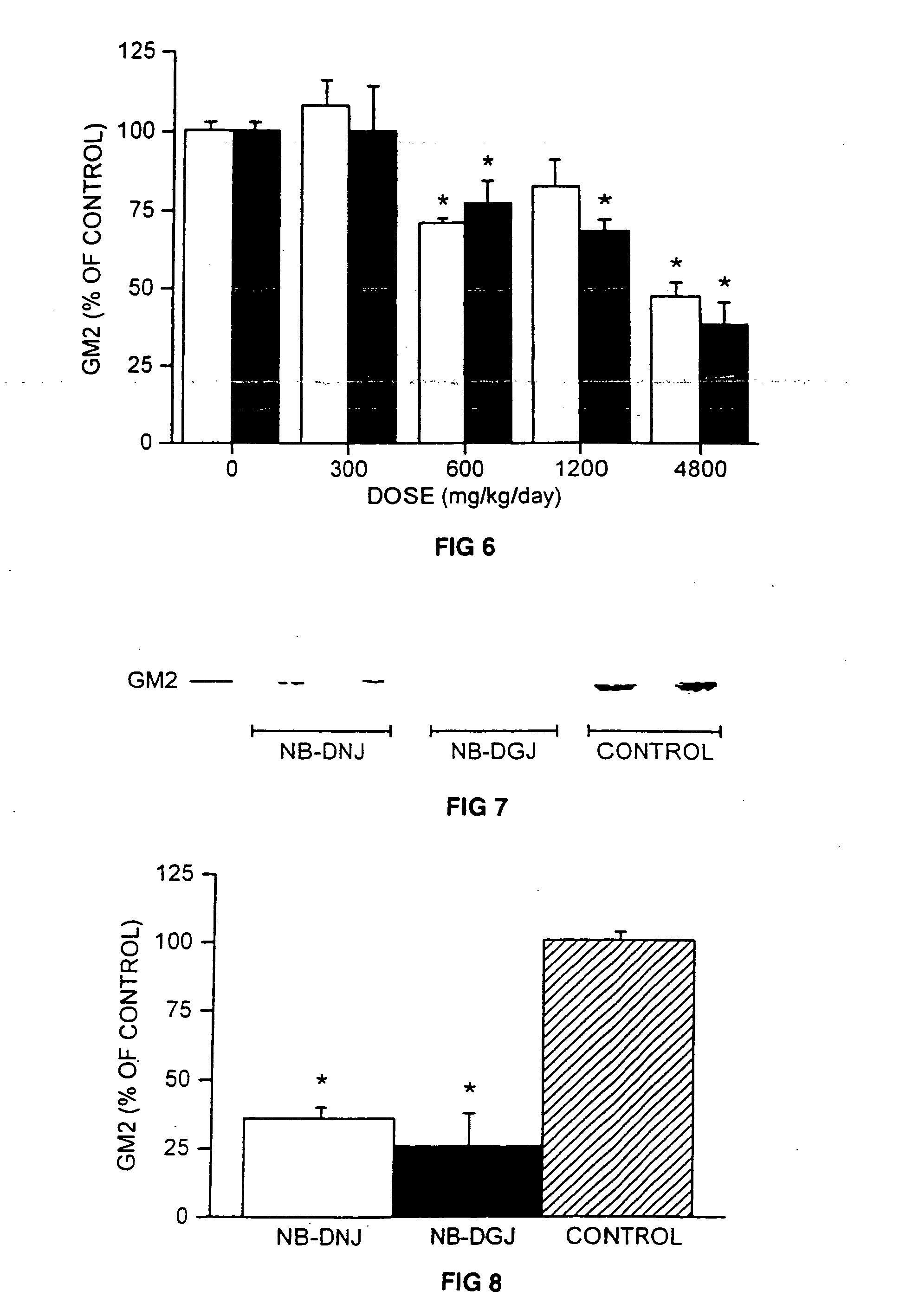
InactiveUS7348000B2Increase enzyme activityEfficacyBiocideNervous disorderN-butyldeoxygalactonojirimycinGm1 ganglioside
Owner:ACTELION PHARM LTD
Therapeutic compositions and methods of treating glycolipid storage related disorders
InactiveUS20050075305A1Increase enzyme activityEfficacyBiocideNervous disorderGlycolipid synthesisN-butyldeoxygalactonojirimycin
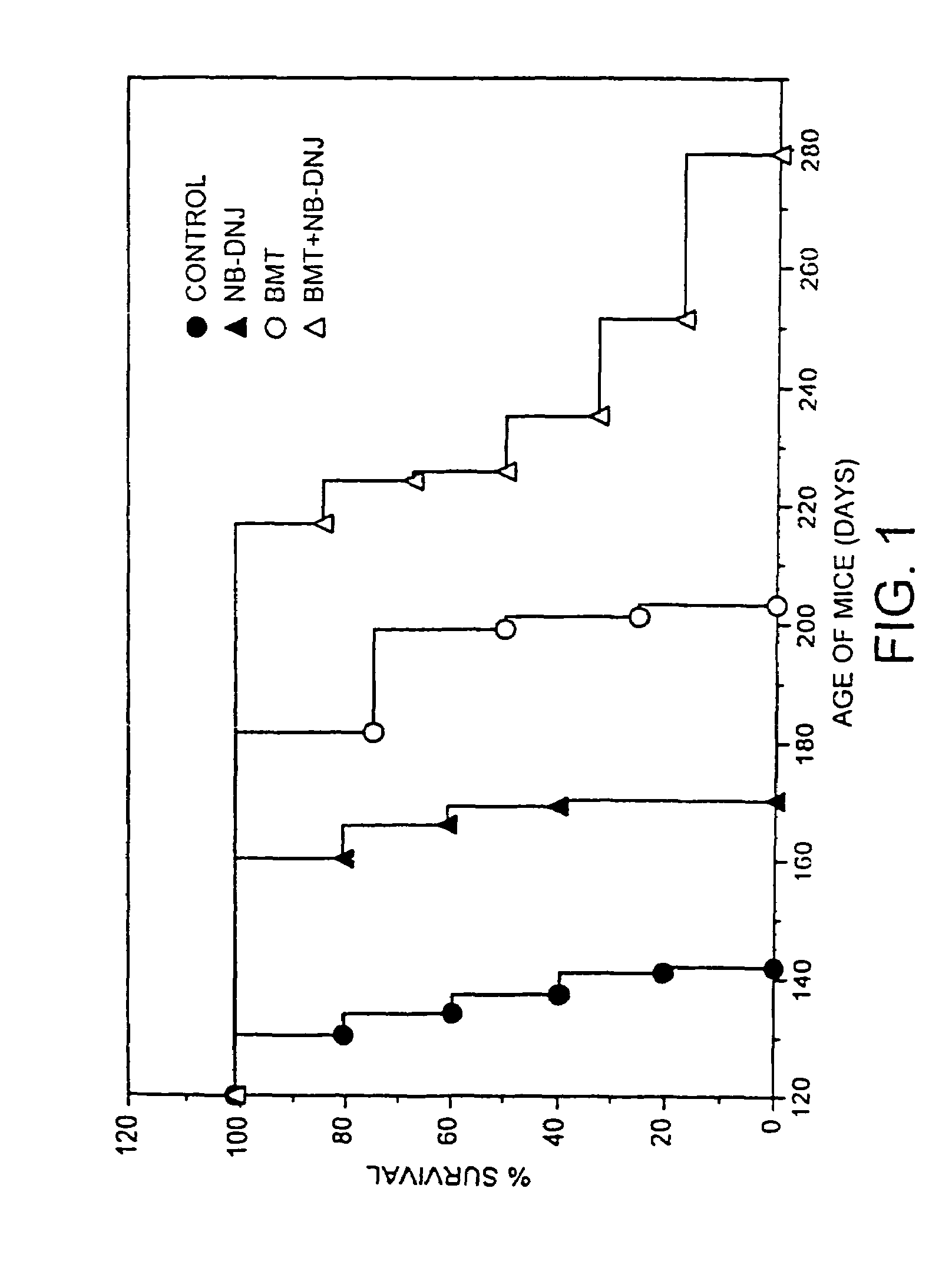
A method for treating a glycolipid storage-related disorder, comprising administering a therapeutically effective amount of an inhibitor of glycolipid synthesis in combination with an agent capable of increasing the rate of glycolipid degradation or in combination with bone marrow transplantation. Inhibitors of glycolipid synthesis include N-butyldeoxynojirimycin (NB-DNJ), N-butyldeoxygalactonojirimycin (NB-DGJ) or N-nonyldeoxynojirimycin (NN-DNJ). Glycolipid storage-related disorders include Gaucher disease, Sandhoff's disease, Fabry's disease, Tay-Sach's disease, Niemann-Pick C storage disease, GM1 gangliosidosis, genetic disorders in which neuronal glycolipid accumulation contributes to disease pathology.
Owner:ACTELION PHARM LTD
Recombinant human platelet auxin/dry cell factor fusion protein and preparation thereof
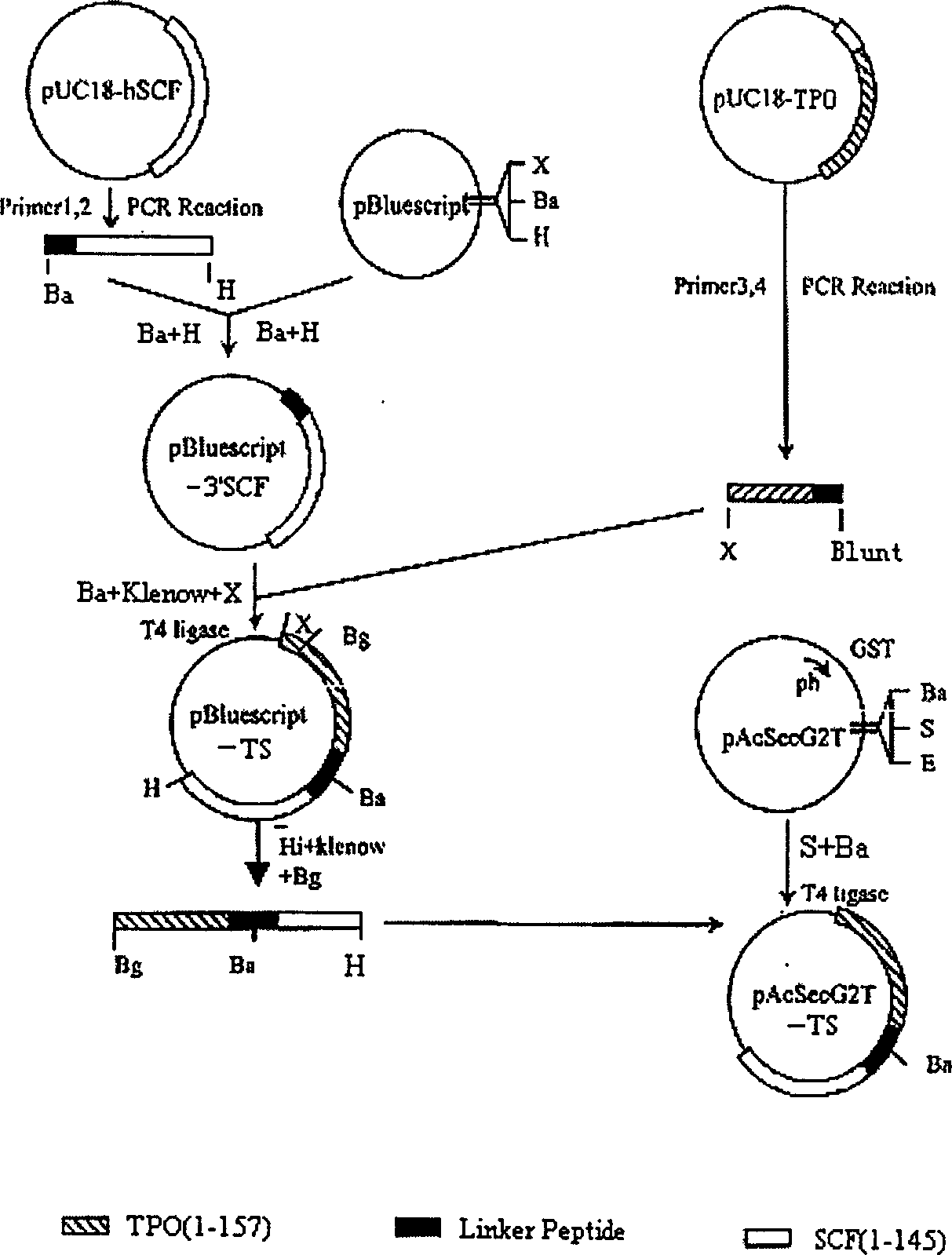
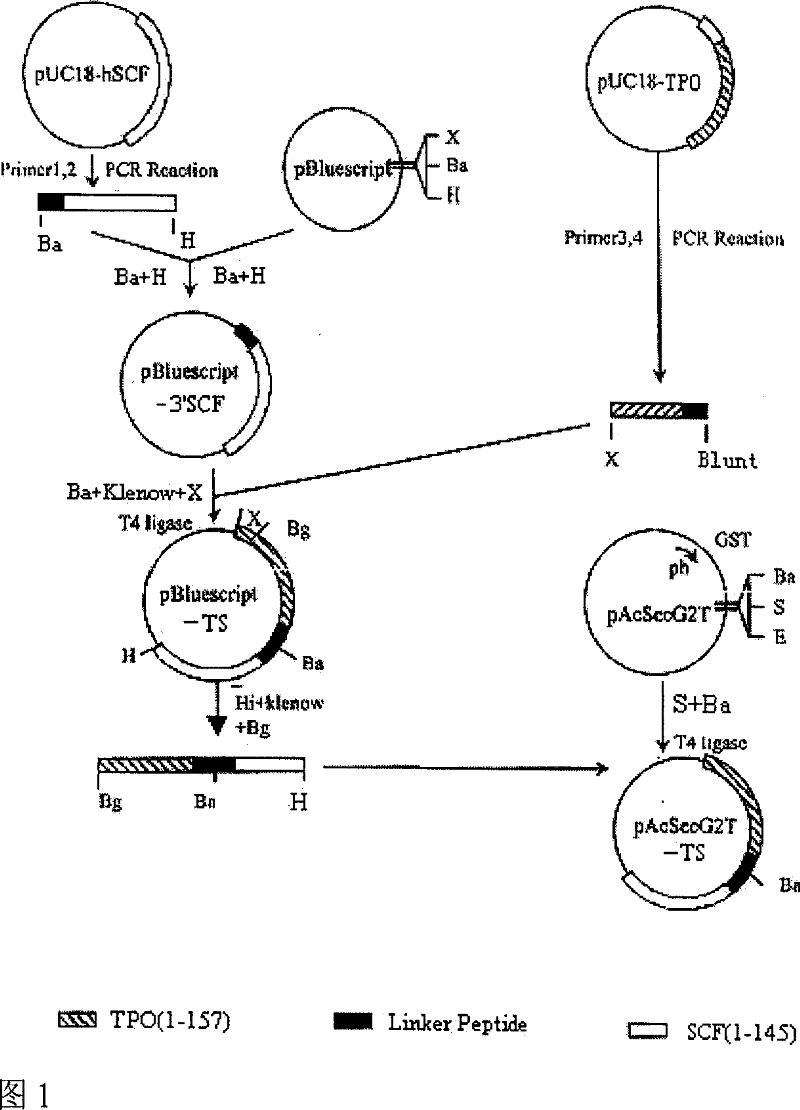
The invention is the gene engineering preparation polypeptide remedy technology field of biology technology. The aid is to build a double function fusion albumen gene of rhTPO / SCF, express and prepare with more biology activityú¼less side-effect rhTPO / SCF in insect cell. The gene of rhTPO / SCF includes 1-157 amino acids coding sequence with TPO, 16 amino acids continual peptide coding sequence, 1-145 amino acids coding sequence of SCF and codoní»s DNA fragments. By taking the rhTPO / SCF into TF1 cells experiment, which preparation from the infect recombination virus growing in adherence S19 cell, it mensurates that the specific activity is 2.0 í10 to the power 5 units / mg; by Mo7e cells experiment, the specific activity is 8.3í10 to the power 5 units / mg. It can be used to cure low blood platelet cause by radiotherapy, chemotherapy, bone marrow transplantationíú
Owner:NANJING UNIV
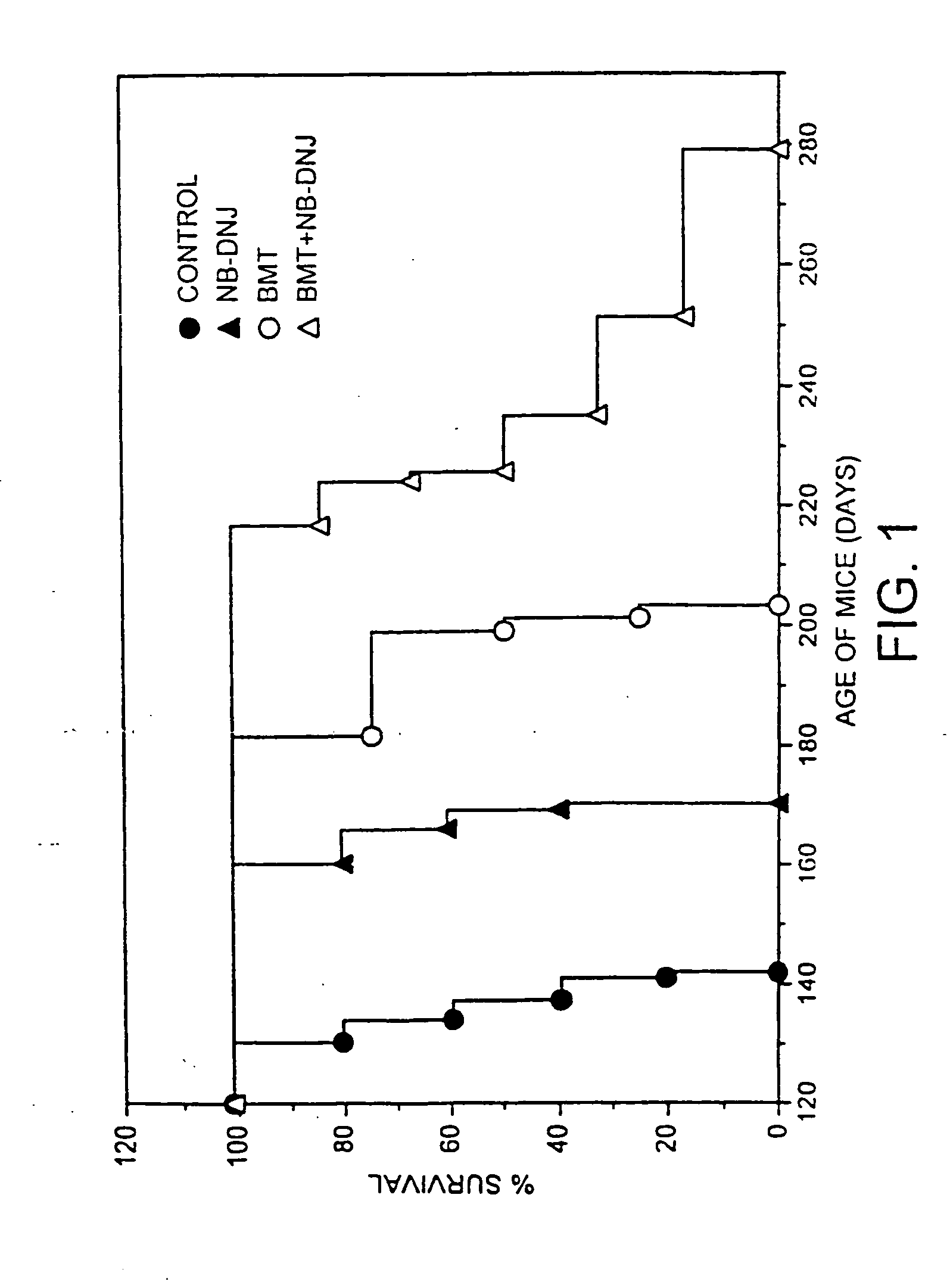
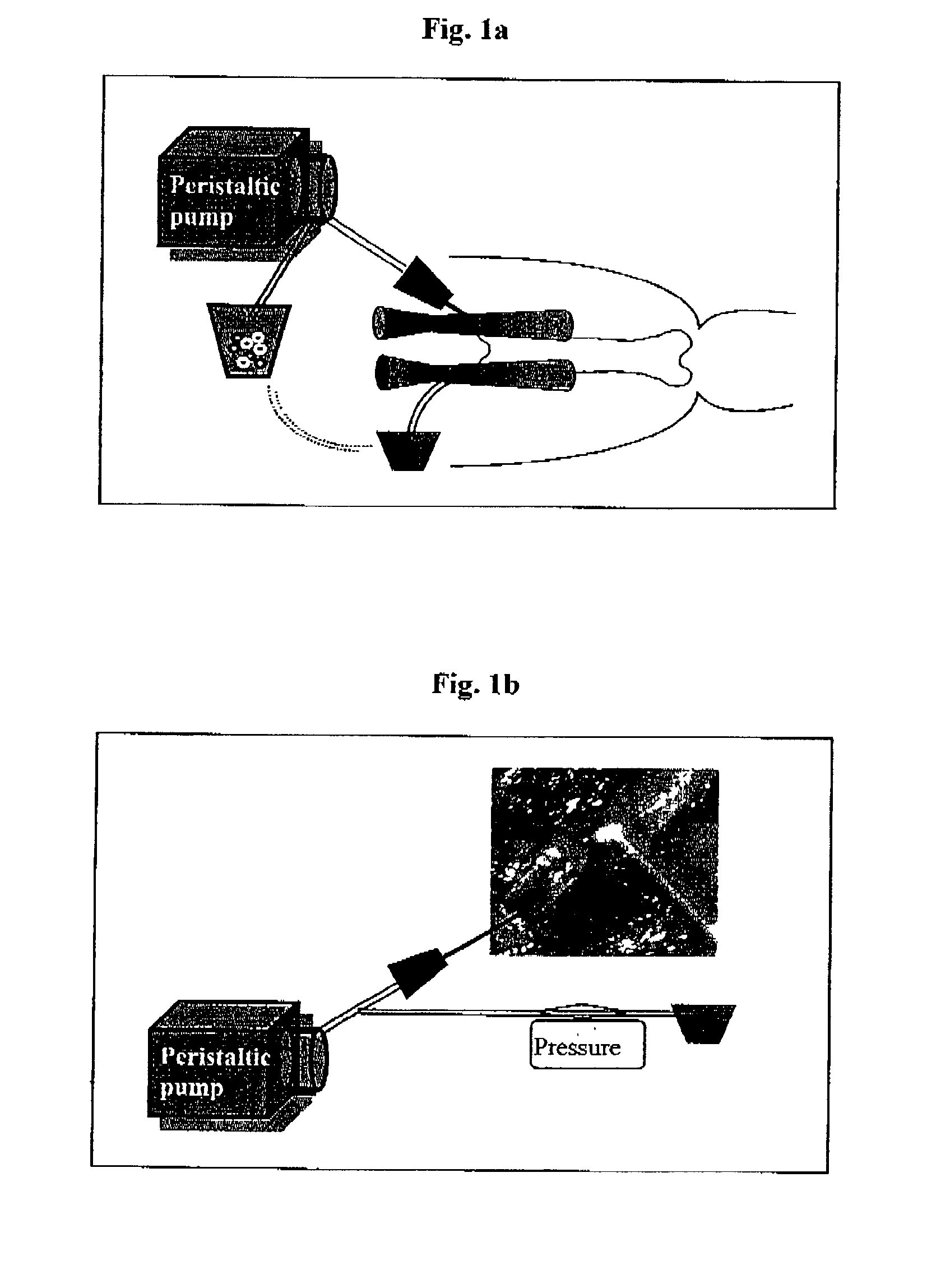
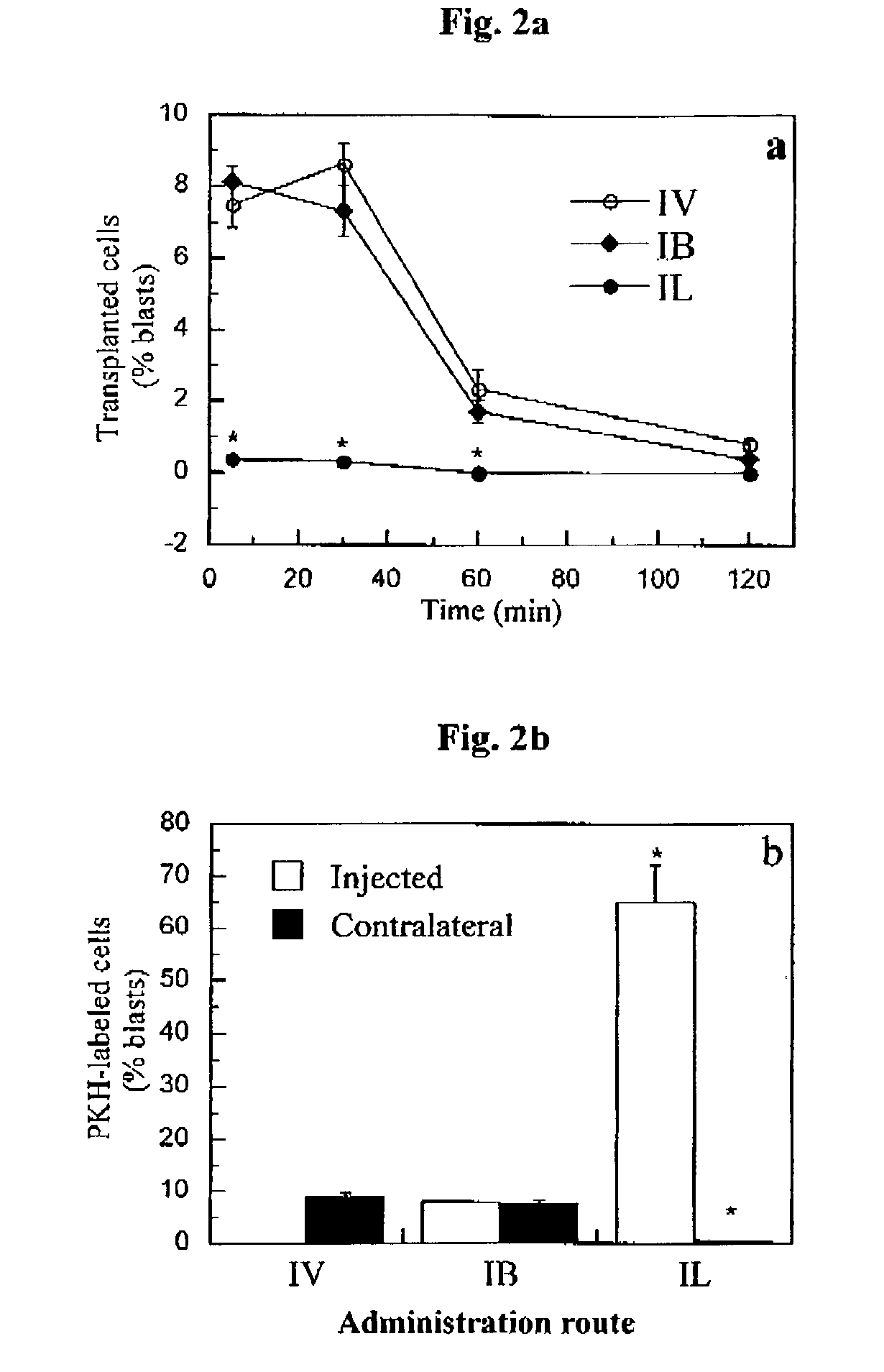
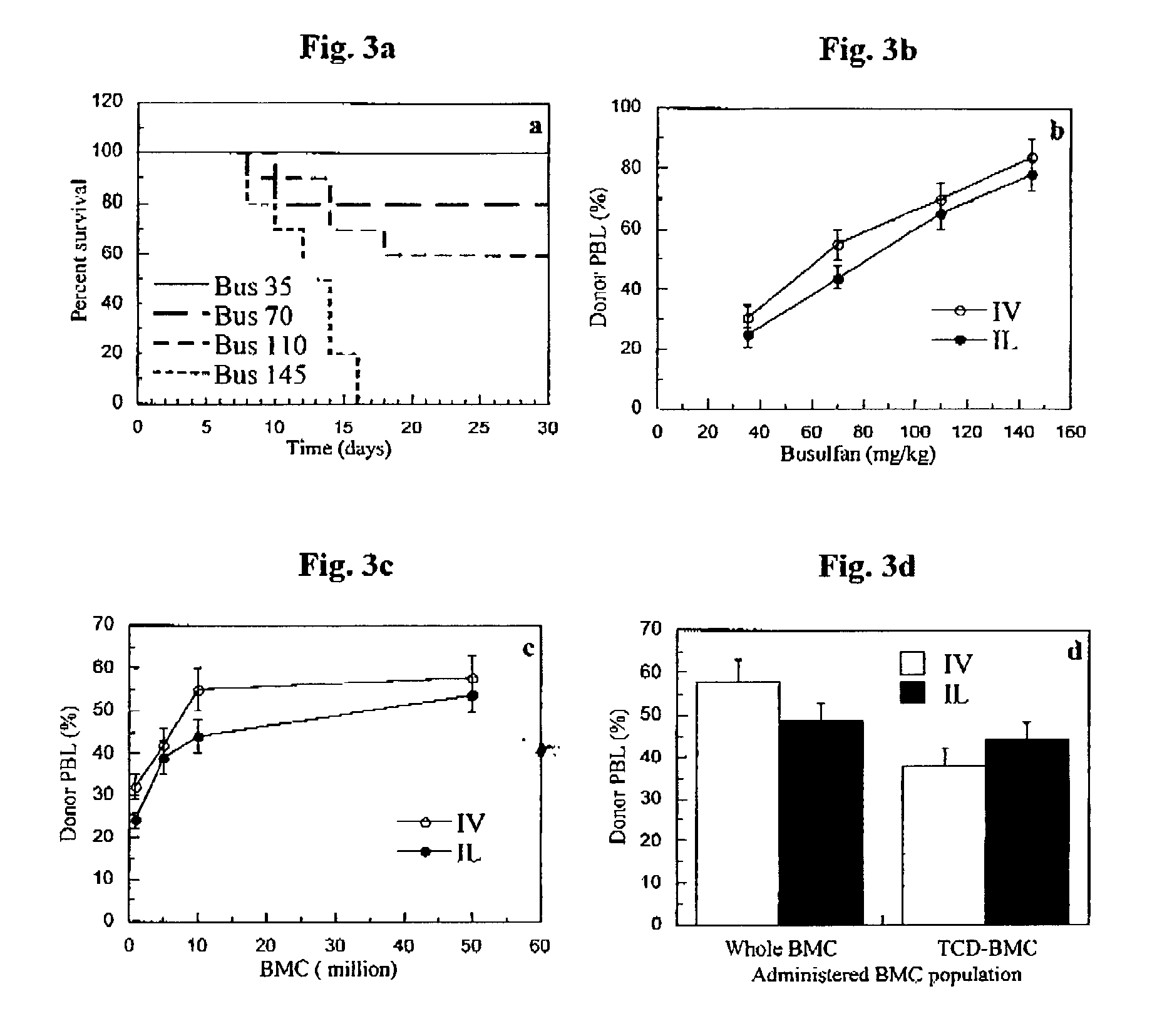
Method of inducing immune tolerance via blood/lymph flow-restricted bone marrow transplantation
ActiveUS7138144B2Reduced doses of bone marrow cellsPreventing graft-versus-host diseaseBiocideGenetic material ingredientsImmune toleranceLymph flow
Owner:CELLECT BIOTECH
Human stem cell growth factor as well as production method and application of polyethylene glycol (PEG) modified human stem cell growth factor
InactiveCN102559725AInduced expression condition optimizationHigh expressionCosmetic preparationsPeptide/protein ingredientsHalf-lifeCuticle
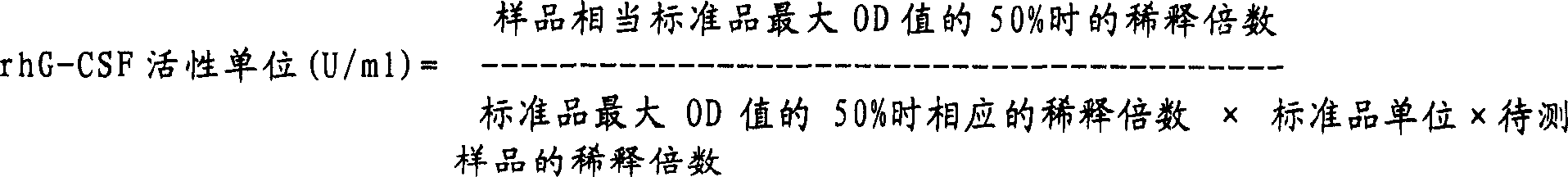
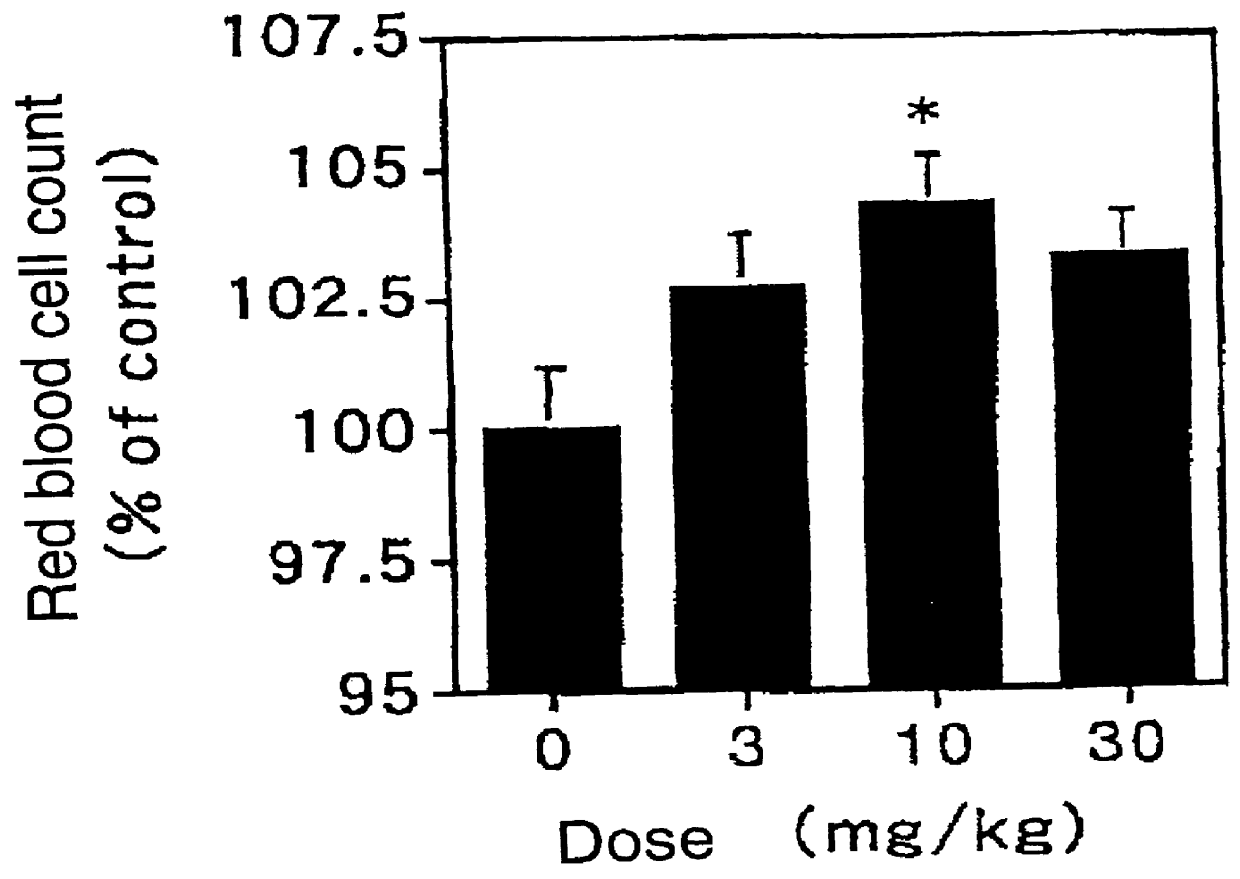
The invention discloses a human stem cell growth factor as well as a production method and application of a polyethylene glycol (PEG) modified human stem cell growth factor. The production method comprises the following steps of: fusing an h-SCF (Stem Cell Factor)-alpha sequence and an SUMO (Small Ubiquitin-Related Modifier) sequence and constructing to obtain an SUMO-rhSCF-alpha fused gene expression vector; transferring the SUMO-rhSCF-alpha fused gene expression vector into a host bacteria to obtain engineering bacteria; culturing the engineering bacteria and inducing to express an SUMO-rhSCF-alpha fused protein; and cutting off an SUMO part to obtain an rhSCF-alpha protein. By using the production method, the high soluble expression and large-scale purification of the rhSCF-alpha protein in cell plasmas are realized, and the activity of the obtained rhSCF-alpha protein is high. The invention also discloses the production method of the PEG modified human stem cell growth factor; the half-life period of the PEG modified human stem cell growth factor obtained by using the method is remarkably prolonged, and the activity of the PEG modified human stem cell growth factor in promoting the proliferation of red blood cells is also remarkably improved; and the PEG modified human stem cell growth factor can be applied to the preparation of medicines for hypohemia therapy, reconstruction and recovery of a hematopoietic function after chemoradiotherapy and a bone marrow transplantation operation, stem cell ex-vivo expansion and gene therapy and cosmetics for promoting the metabolism of epidermal cells, repairing aged and damaged skin cells, delaying the aging of skin and the like.
Owner:GUANGZHOU JINAN BIOMEDICINE RES & DEV CENT
Radation therapy methods
InactiveUS20080039363A1Reduce tissue damageImprove efficiencyPeptide/protein ingredientsEnergy modified materialsMegakaryocyte productionAngiotensinogen mrna
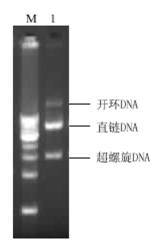
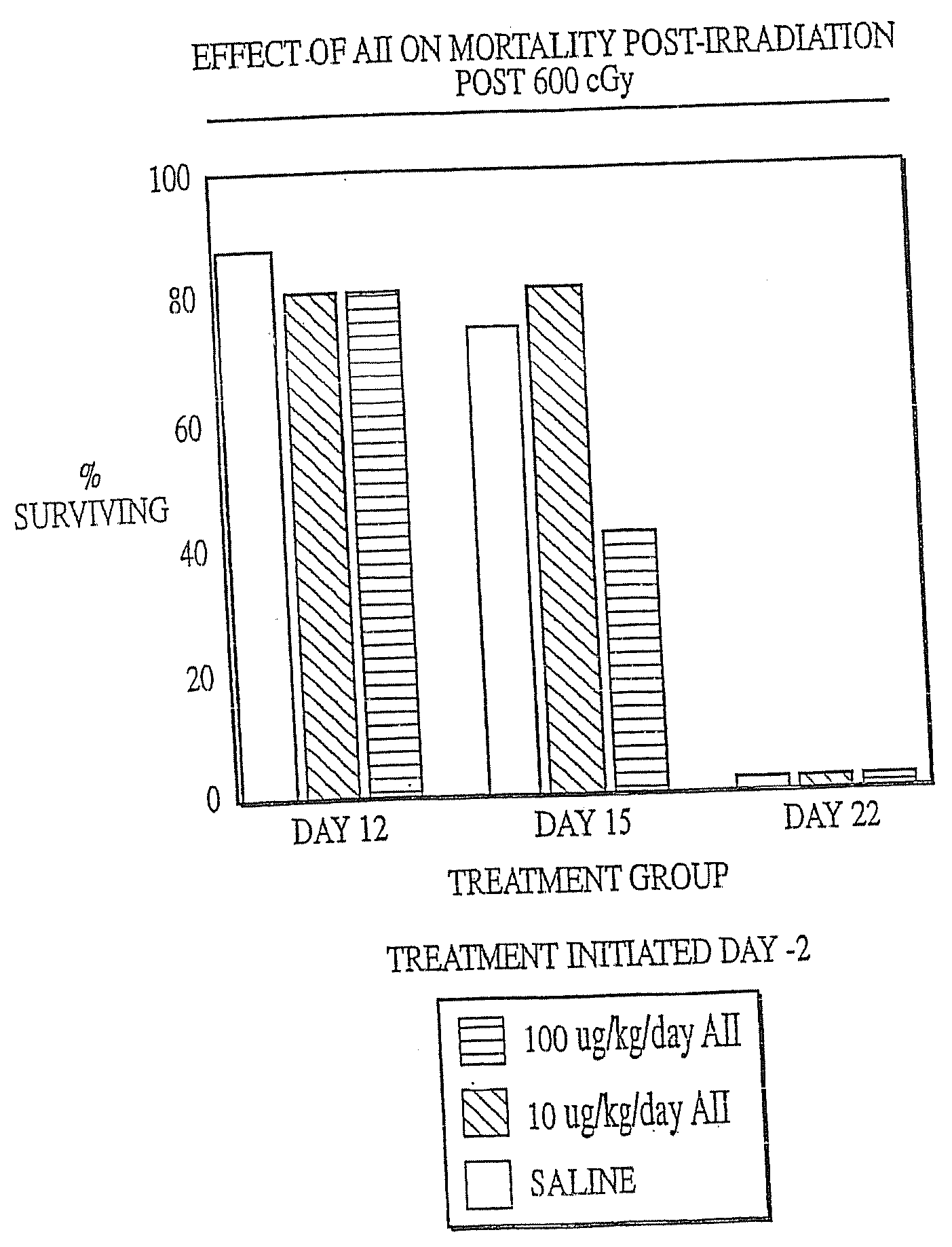
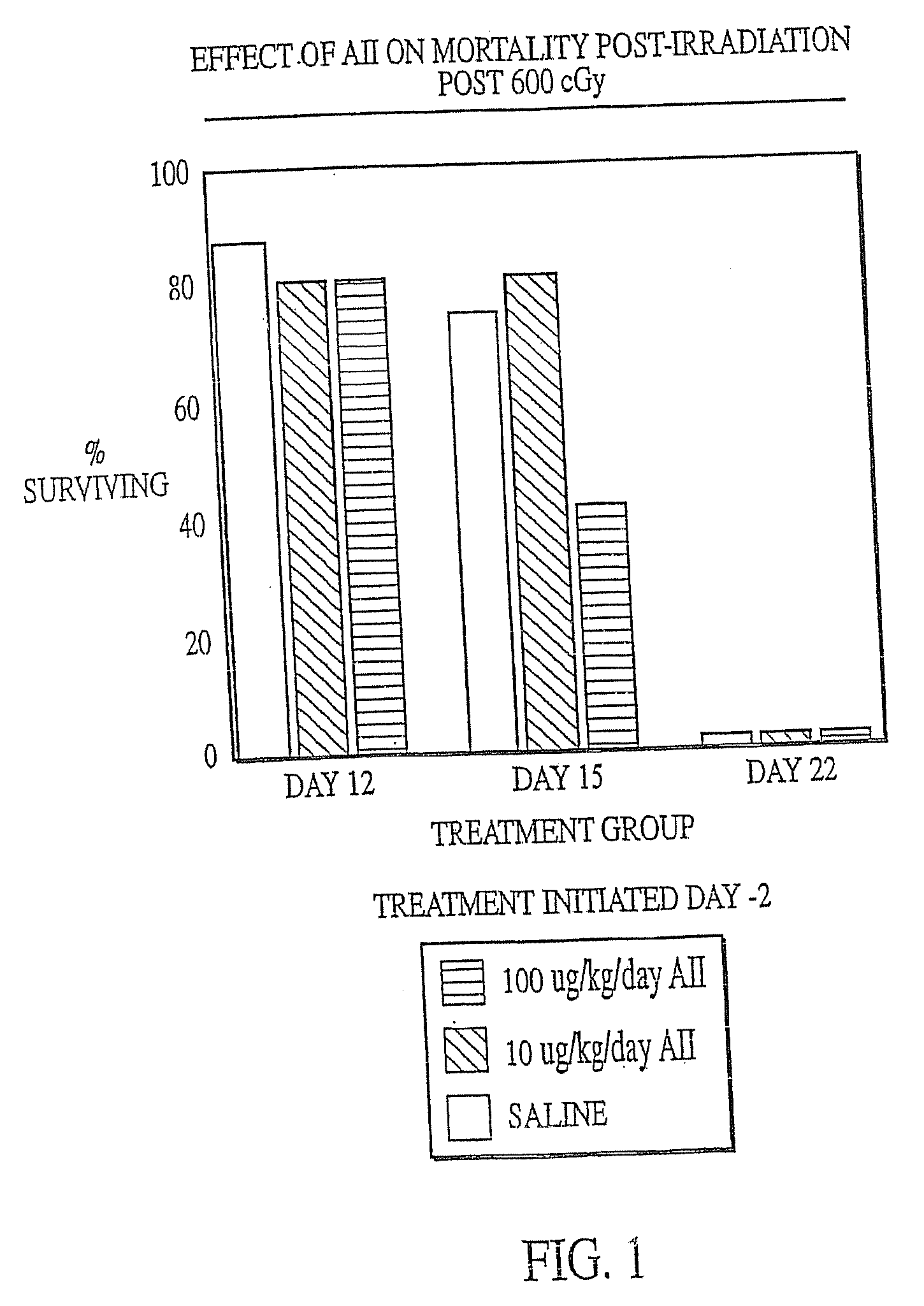
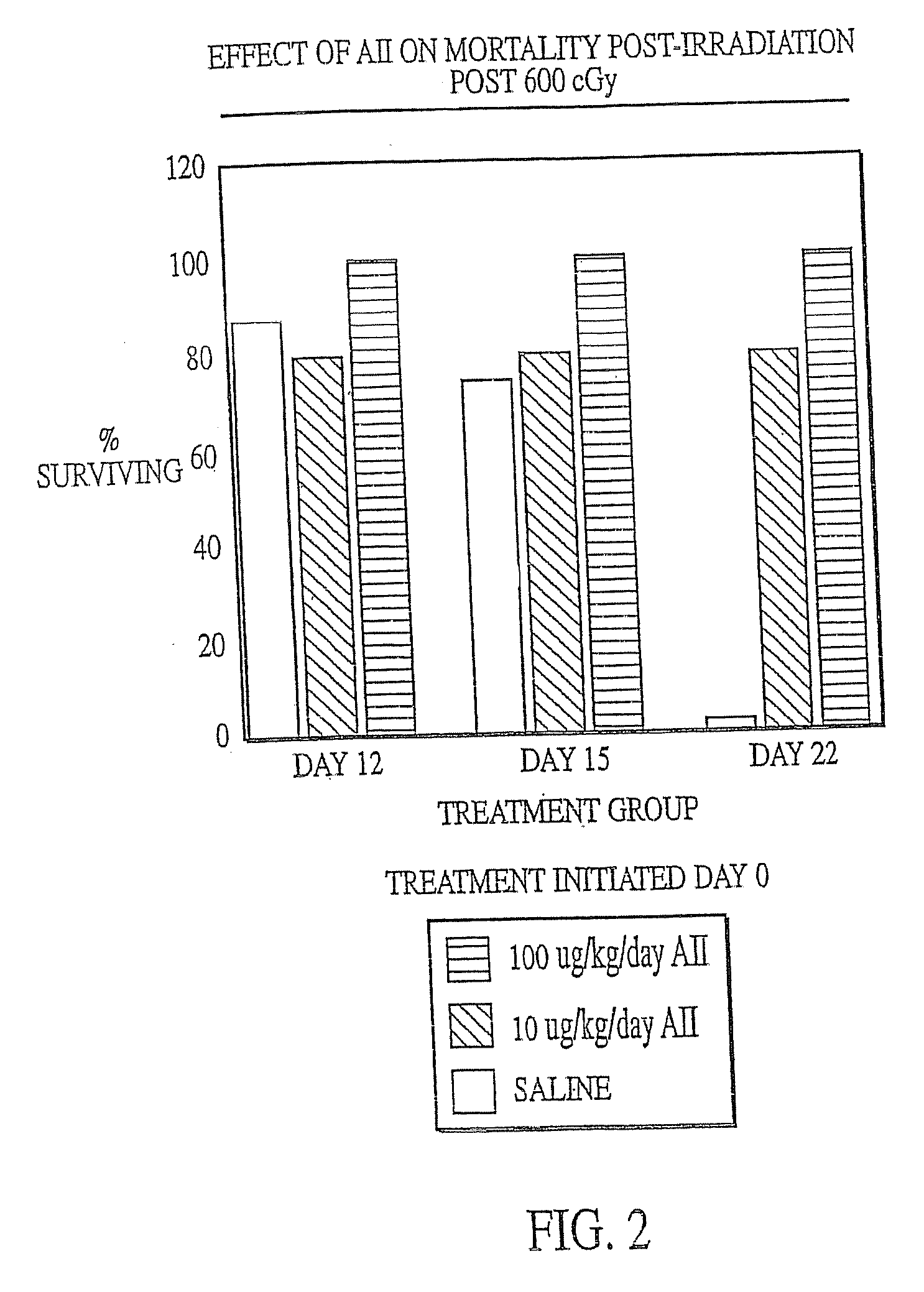
The present invention provides methods and kits for mitigating radiation induced tissue damage, improving the effectiveness of radiation therapy, to support bone marrow transplantation, and promoting megakaryocyte production and mobilization and platelet production, each method comprising the administration of an effective amount of angiotensinogen, angiotensin I (AI), AI analogues, AI fragments and analogues thereof, angiotensin II (AII), AII analogues, AII fragments or analogues thereof or AII AT2 type 2 receptor agonists.
Owner:UNIV OF SOUTHERN CALIFORNIA
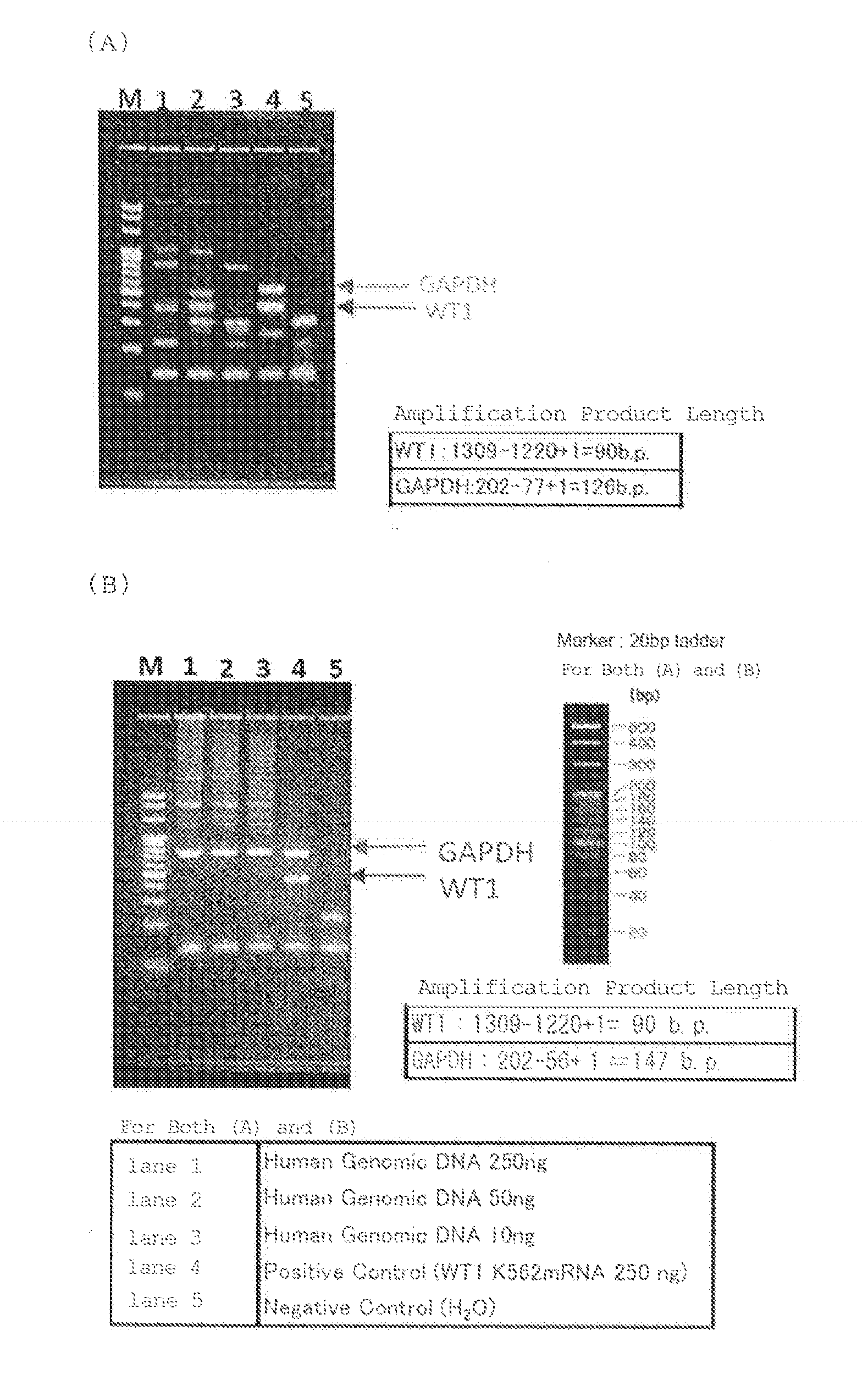
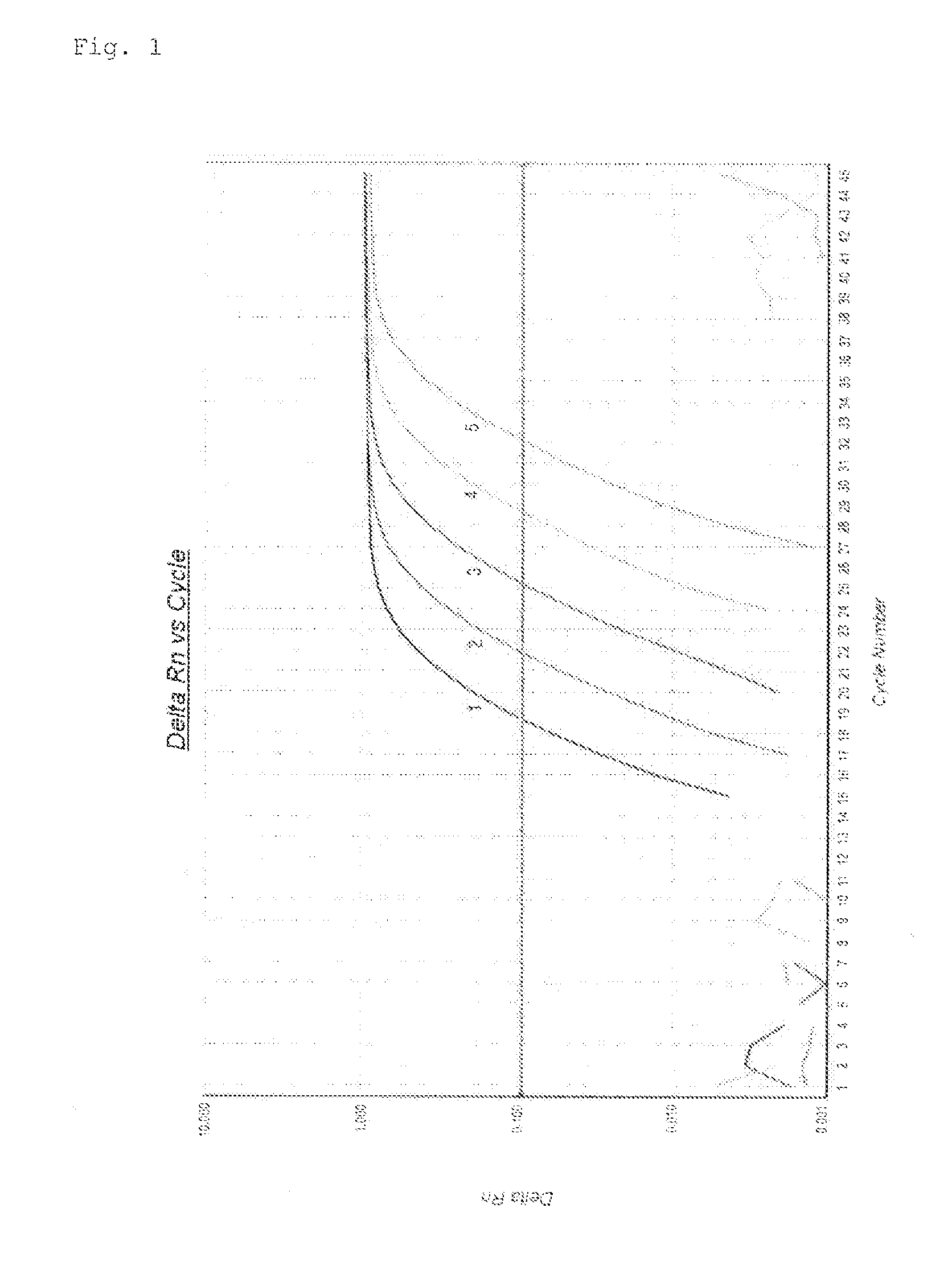
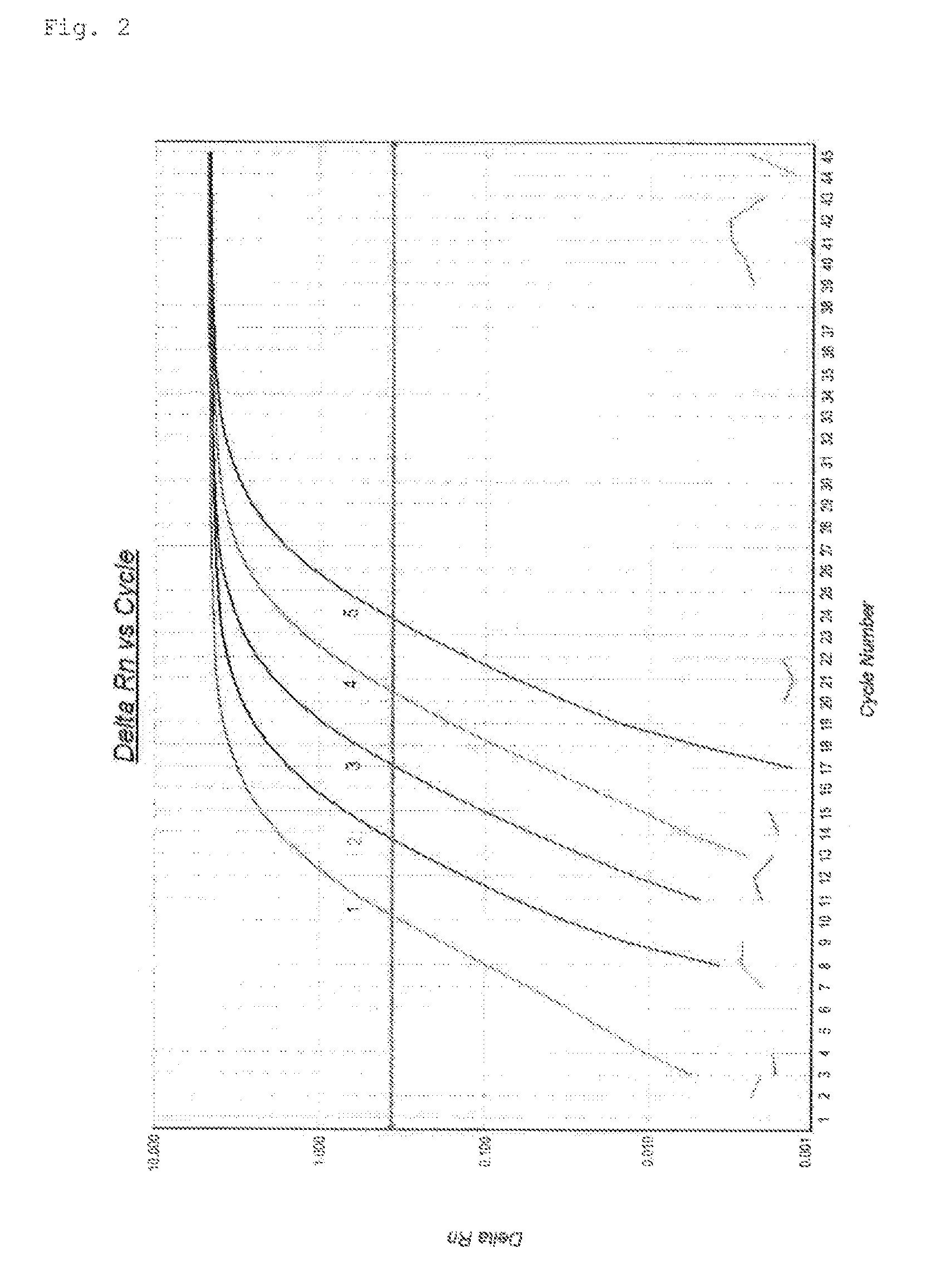
Quantification method for expression level of WT1 mRNA
ActiveCN104937112AEasy to measureSimple and fast operationMicrobiological testing/measurementRecombinant DNA-technologyHousekeeping geneQuantification methods
Provided is a method for quantifying, with ease, in a short time period, and with high sensitivity, human WT1 mRNA expression level which can be used to diagnose cancers such as leukaemia and solid carcinoma, and which can be used to determine bone-marrow transplantation times. This quantification method for human WT1 mRNA expression level uses one-step RT-PCR to quantify human WT1 mRNA expression level, and is characterized in that a reverse transcription reaction and an elongation reaction of human WT1 mRNA and a housekeeping gene (mRNA) are simultaneously and continuously progressed in the same vessel.
Owner:OTSUKA PHARM CO LTD
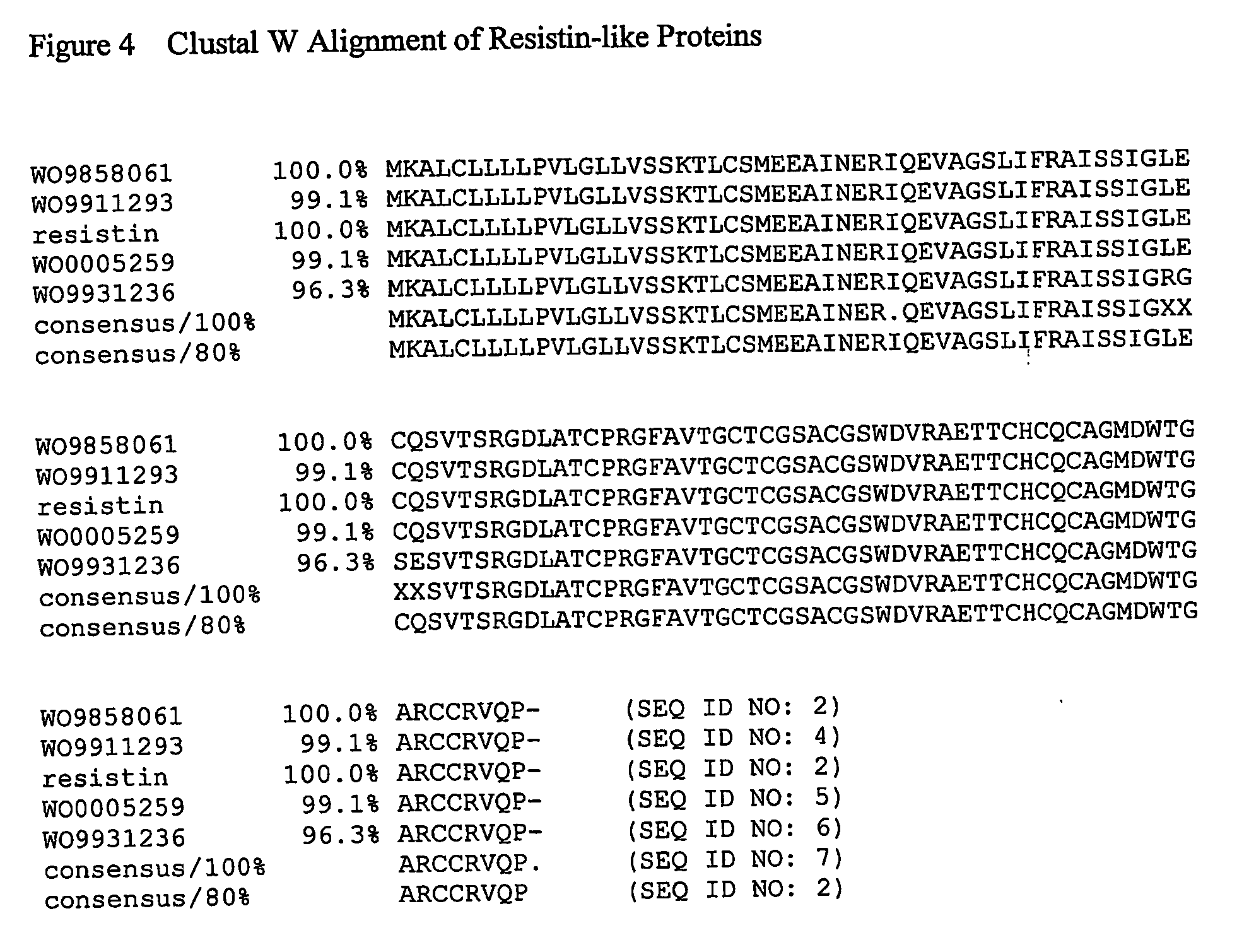
Use of resistin to treat hematopoietic disorders
InactiveUS20050233954A1Increase the number ofPeptide/protein ingredientsAntineoplastic agentsDiseaseResistin
The present invention relates to a method of using a mammalian gene sequence and polypeptides encoded thereby to treat mammalian hematopoietic disorders. More specifically the present invention relates to methods of using compositions comprising at least one resistin agonist, resistin polynucleotide and / or resistin polypeptide for the prevention and / or treatment of mammalian hematopoietic disorders, including, but not limited to, anemia, leukemia, and hematopoietic conditions caused by bone marrow transplantation or chemo- / radiation therapy.
Owner:ELI LILLY & CO
Recombinant human platelet auxin/dry cell factor fusion protein and preparation thereof
The invention is the gene engineering preparation polypeptide remedy technology field of biology technology. The aid is to build a double function fusion albumen gene of rhTPO / SCF, express and prepare with more biology activity, less side-effect rhTPO / SCF in insect cell. The gene of rhTPO / SCF includes 1-157 amino acids coding sequence with TPO, 16 amino acids continual peptide coding sequence, 1-145 amino acids coding sequence of SCF and codon's DNA fragments. By taking the rhTPO / SCF into TF1 cells experiment, which preparation from the infect recombination virus growing in adherence S19 cell, it mensurates that the specific activity is 2.0 *10 to the power 5 units / mg; by Mo7e cells experiment, the specific activity is 8.3*10 to the power 5 units / mg. It can be used to cure low blood platelet cause by radiotherapy, chemotherapy, bone marrow transplantation.
Owner:NANJING UNIV
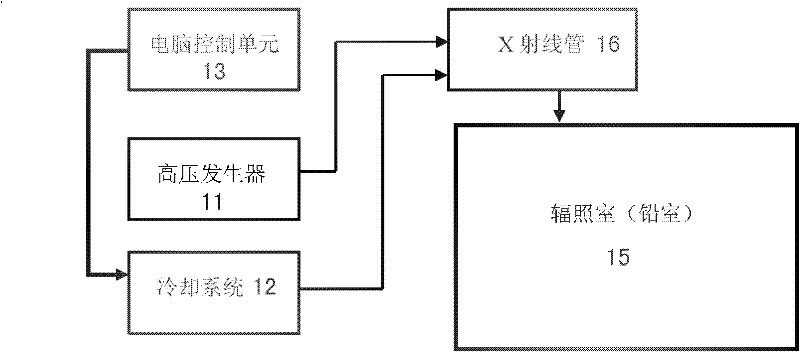
Biological irradiation device of high energy X-rays
InactiveCN102543244AReduce manufacturing costImprove the protective effectX-ray/gamma-ray/particle-irradiation therapyIrradiation devicesX-rayEngineering
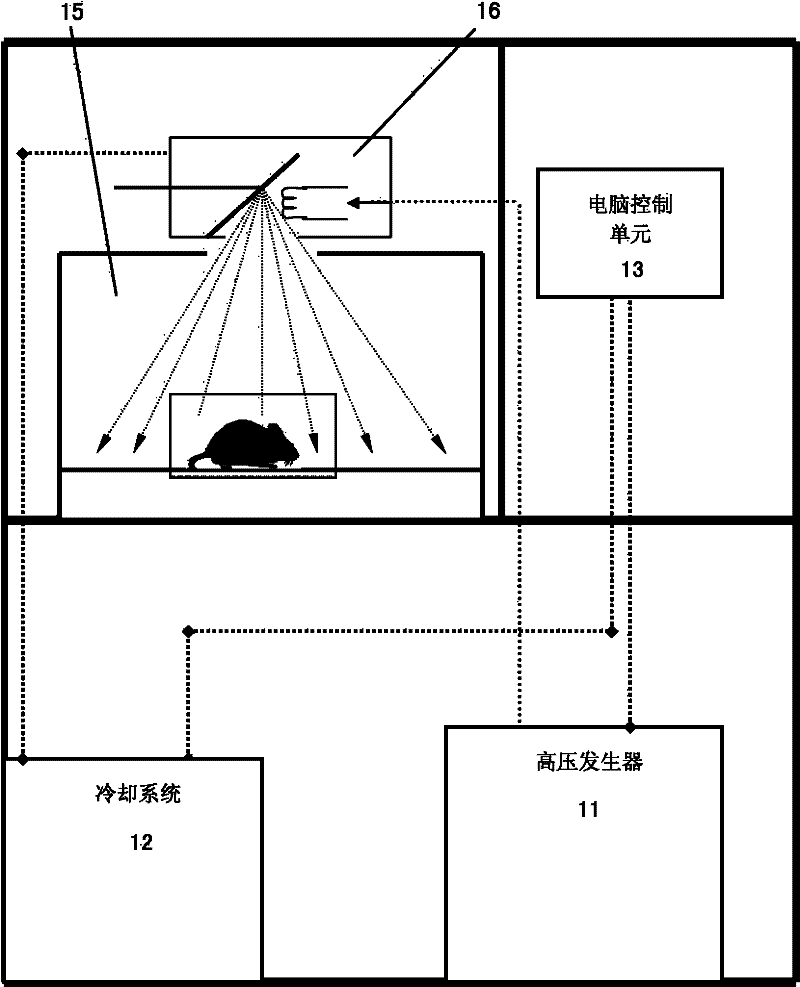
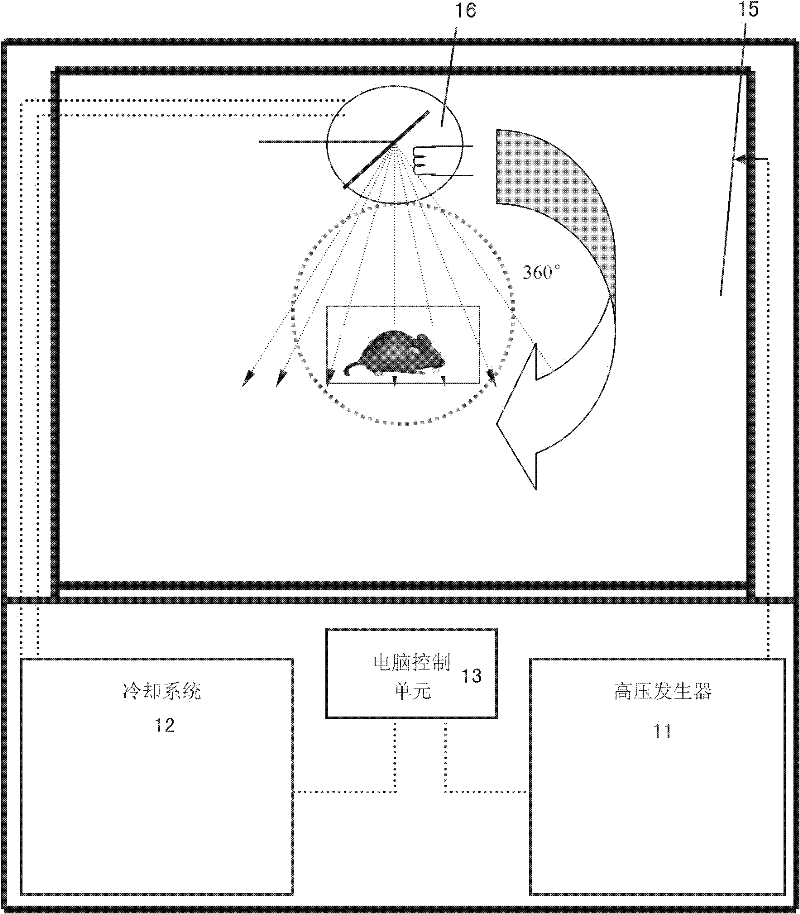
The invention relates to a biological irradiation device of high energy X-rays, which comprises a high pressure generator, a cooling system, a computer control unit, an input end connecting and controlling the computer control unit and the cooling system, at least one X-ray pipe and a radiation chamber. Each X-ray pipe comprises a pipe head, a control end of the pipe head is connected with the high pressure generator and the output end of the cooling system, the pipe head is used for emitting the X-rays, the radiation chamber is a closed space and is used for shielding the X-rays, and the pipe head is arranged in the radiation chamber. The device can achieve energy X-ray irradiation on cells of specific animal organism or microorganism, plants and animals in the radiation chamber. The biological irradiation device is applicable to various scientific research fields such as bone marrow transplantation, human genomics, immunology, oncology phymatology, radioecology, cells, microorganism inactivation and sterilization of the animals infected with infectious microorganisms.
Owner:王全兴
Transduced peptide-humanized granular leukocyte colony stimulating factor fusion protein and its medicinal composition
InactiveCN101074266AEasy to purifyDoes not affect the structurePeptide/protein ingredientsAntiinfectivesAbnormal tissue growthColony-stimulating factor
A transduction peptide-humanized granulocyte colony stimulating factor fusion protein, nucleic acid molecule of nucleic acid sequence for encoding fusion protein, expression carrier containing the nucleic acid molecule and its usage of fusion protein in preparation of medicine are disclosed. It can be used for neutrophil reduction after tumor radiant chemo-radiotherapy, hematopoietic restoration after bone marrow transplantation, peripheral blood stem cells mobilization, anti-infective therapy, aregenerative anemia, systemic lupus erythematosus and immune response adjustment.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Traditional Chinese medicine composition for treating leukemia
InactiveCN105796989APhysical damageRelieve painUnknown materialsAntineoplastic agentsMedicineCurative effect
The invention relates to a traditional Chinese medicine composition for treating leukemia.The traditional Chinese medicine composition comprises 120-360 parts of hairyvein agrimonia herb (processed), 60-180 parts of lalang grass rhizome (processed), 60-180 parts of common cephalanoplos herb (processed), 60-180 parts of rehmannia root (processed), 60-180 parts of dandelion (processed), 30-90 parts of buffalo horn (processed), 15-45 parts of forsythia (processed), 15-45 parts of tenacious condorvine stem (processed), 15-45 parts of white paeony root (processed), 15-45 parts of cochinchnese asparagus root (processed), 15-45 parts of stir-baked fructus gardeniae (processed), 15-45 parts of fresh node of lotus rhizome (processed), 10-30 parts of lotus leaf (processed), 10-30 parts of bark of tree peony root (processed), 10-30 parts of dried tangerine or orange peel (processed) and 60-180 parts of pitted date (processed).The traditional Chinese medicine composition for treating leukemia has the advantages of low cost, short treatment period and effective in treatment without toxic or side effects.In addition, by means of the traditional Chinese medicine composition, radiotherapy, chemotherapy, operations and bone marrow transplantation are not required.
Owner:艾小军
Treatment of Aspergillus infections with alpha thymosin peptides
A method for treating a human infected with Aspergillus by using thymosin alpha 1 as an immuno-stimulator in activating dendritic cells. The method is particularly useful in preventing an infection by Aspergillus in an immuno-compromised host being treated with a bone marrow transplantation.
Owner:SCICLONE PHARM INT LTD
Human stem cell growth factor injection and preparation method thereof
ActiveCN104922698ALittle side effectsImprove bioavailabilityPeptide/protein ingredientsGenetic material ingredientsCuticleBone marrow transplantation
The invention relates to a human stem cell growth factor injection and a preparation method thereof. The injection can realize a slow-release technical effect, can reduce the medicine taking times of a patient, can improve the compliance, can be used for preparation of drugs for anemia treating, reconstruction and recovery of the hematopoietic function after chemoradiotherapy and bone marrow transplantation, drugs for stem cells in vitro amplification and gene therapy drugs, and can be applied to the field of cosmetics for promoting skin cell metabolism, repairing aging and damaged skin cells, delaying skin aging and the like.
Owner:GUANGZHOU JINAN BIOMEDICINE RES & DEV CENT
Ketone derivatives and their medical applications
This invention relates to ketones represented by the following formula and to drugs in which such a ketone or pharmacologically acceptable salt thereof is an effective component. The ketones of the present invention encourage the production of platelets, red blood cells, white blood cells and the like, and can be used to prevent or treat cytopaenia brought about by cancer chemotherapy, radiotherapy, bone marrow transplantation and drug therapy, or by immunological abnormality or anaemia, and the like.
Owner:TORAY IND INC
QUANTIFICATION METHOD FOR EXPRESSION LEVEL OF WT1 mRNA
InactiveUS20160333415A1Easy to operateLess effortMicrobiological testing/measurementHousekeeping geneQuantification methods
A method for quantifying the expression level of human WT1 mRNA conveniently, in a short period of time, and with high sensitivity is provided. The method can be used for diagnosing cancer, such as leukemia and solid cancer, or for determining when to perform bone marrow transplantation. The method is for quantifying the expression level of human WT1 mRNA by one-step RT-PCR and comprises simultaneously subjecting the human WT1 mRNA and a housekeeping gene (mRNA) to reverse transcription and extension reactions carried out sequentially in the same vessel.
Owner:OTSUKA PHARM CO LTD
Novel application of acetylcysteine
InactiveCN105412058AQuick Pain ReliefQuickly relieve painOrganic active ingredientsAerosol deliveryWound healingOral ulcers
The invention provides novel application of the acetylcysteine; the acetylcysteine is suitable for treating oral ulcer; and an acetylcysteine gel, which is prepared according to the application, simultaneously has medicinal functions of rapidly relieving pain and preventing ulcer, and specifically inhibiting further spread of ulcer in patients receiving radiotherapy, chemotherapy and bone marrow transplantation and promoting wound healing.
Owner:TIANJIN KUNJIAN BIOLOGICAL PHARMA
Quantification method for expression level of WT1 mRNA
InactiveUS10280467B2Easy to operateLess effortMicrobiological testing/measurementFermentationHousekeeping geneSolid cancer
A method for quantifying the expression level of human WT1 mRNA conveniently, in a short period of time, and with high sensitivity is provided. The method can be used for diagnosing cancer, such as leukemia and solid cancer, or for determining when to perform bone marrow transplantation. The method is for quantifying the expression level of human WT1 mRNA by one-step RT-PCR and comprises simultaneously subjecting the human WT1 mRNA and a housekeeping gene (mRNA) to reverse transcription and extension reactions carried out sequentially in the same vessel.
Owner:OTSUKA PHARM CO LTD
New Short Nucleotide Tandem Repeat Sequence Site and Its Application
ActiveCN104099326BHigh heterozygosityHigh resolutionMicrobiological testing/measurementDNA preparationDNA paternity testingNucleotide
The present invention discloses a new short nucleotide tandem repeat locus and its application, the short tandem repeat locus G7S0005, the sequence is shown in SEQ ID NO.: 1, used to prepare (a) for genetic relationship Reagents or kits for analysis; (b) reagents or kits for individual identification; (c) reagents or kits for paternity testing or blood relationship analysis; (d) for detecting whether there is maternal blood in the extracted amniotic fluid Contaminated reagents or kits; and / or (e) kits for detecting whether leukocytes in recipients have been replaced by donor cells after bone marrow transplantation. The short tandem repeat locus G7S0005 has a high degree of discrimination and can effectively analyze genetic relationships.
Owner:GENESKY DIAGNOSTICS SUZHOU
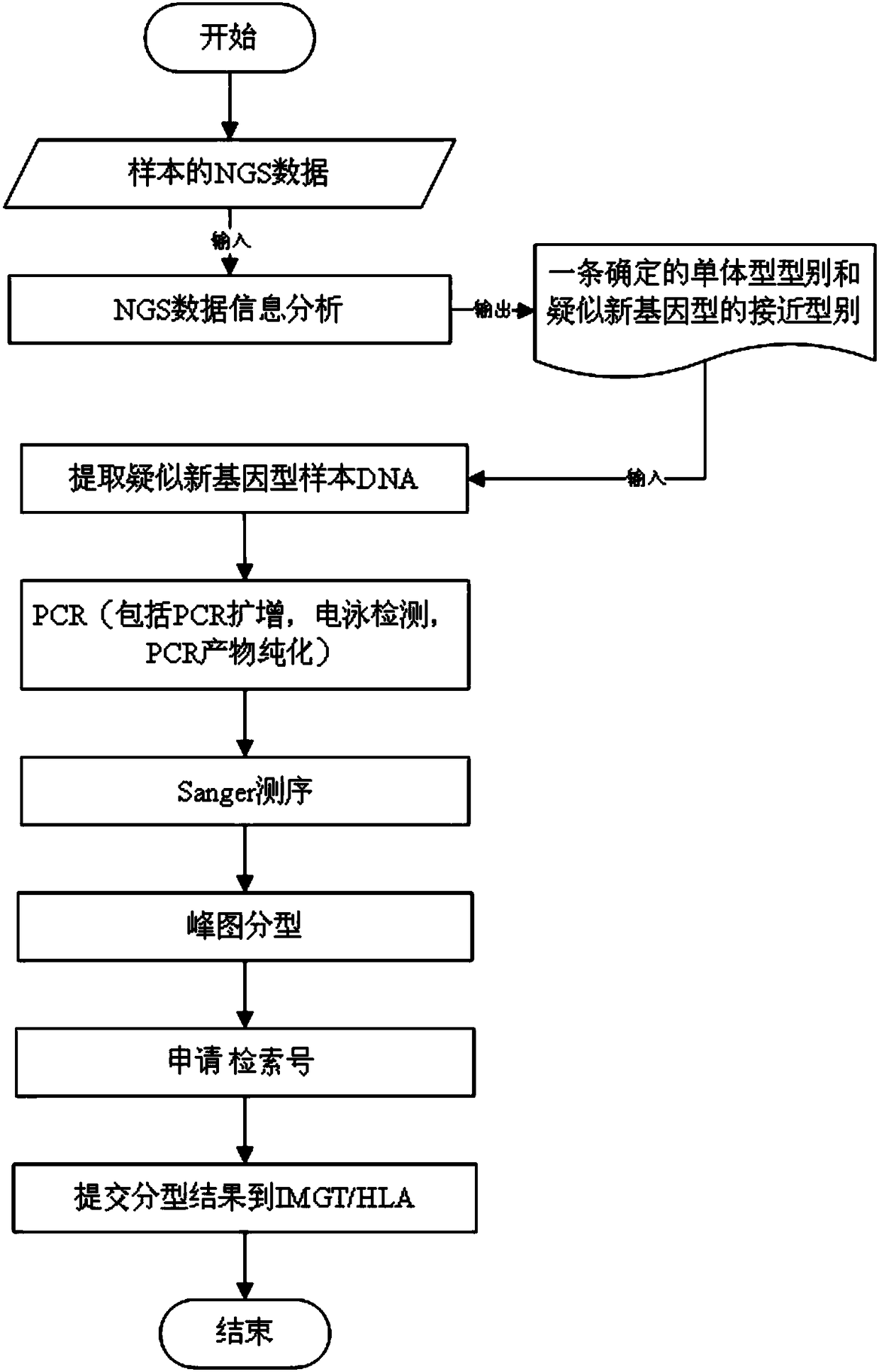
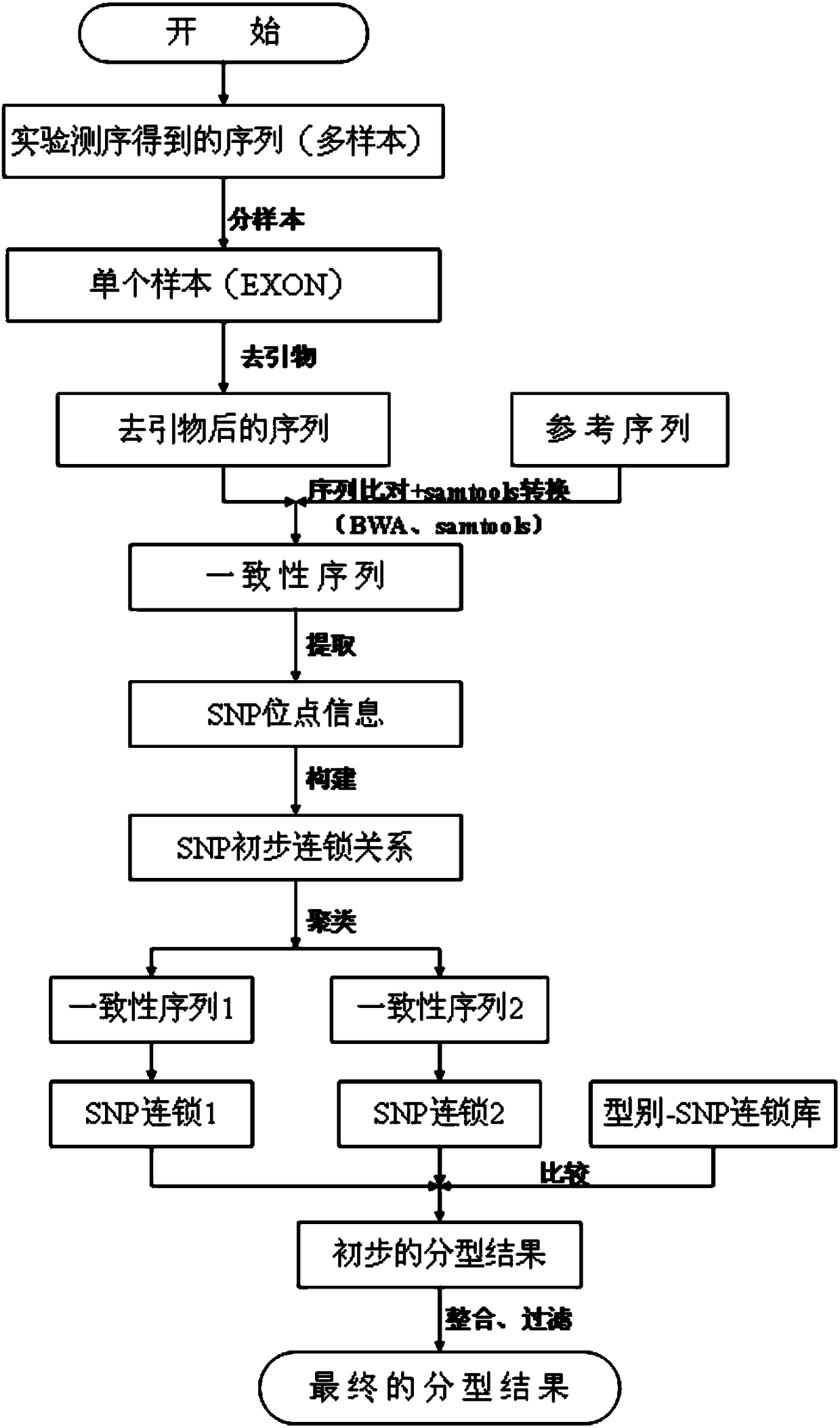
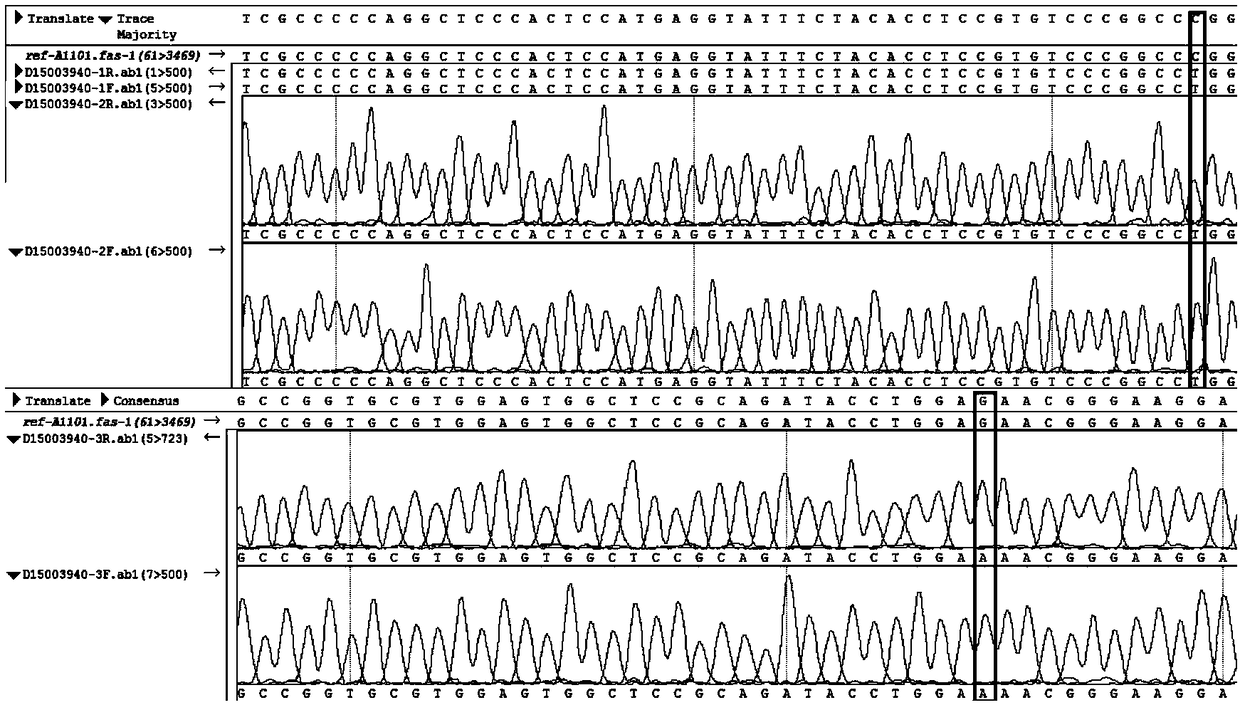
Genotype sequences for HLA typing
ActiveCN108624671AImprove treatment efficiencyImprove typing accuracyMicrobiological testing/measurementDNA/RNA fragmentationDiseaseNucleotide
The invention provides a set of genotype sequences for HLA typing. The genotype sequences comprise a nucleotide sequence as shown in SEQ ID No. 1, and optionally comprise at least one of the nucleotide sequences as shown in SEQ ID No. 2-10. The genotype sequences for HLA typing of the invention enrich a HLA database, and can effectively improve typing accuracy, thereby improving the treatment efficiency of related diseases, such as thalassemia, acute leukemia and bone marrow transplantation.
Owner:BGI GENOMICS CO LTD
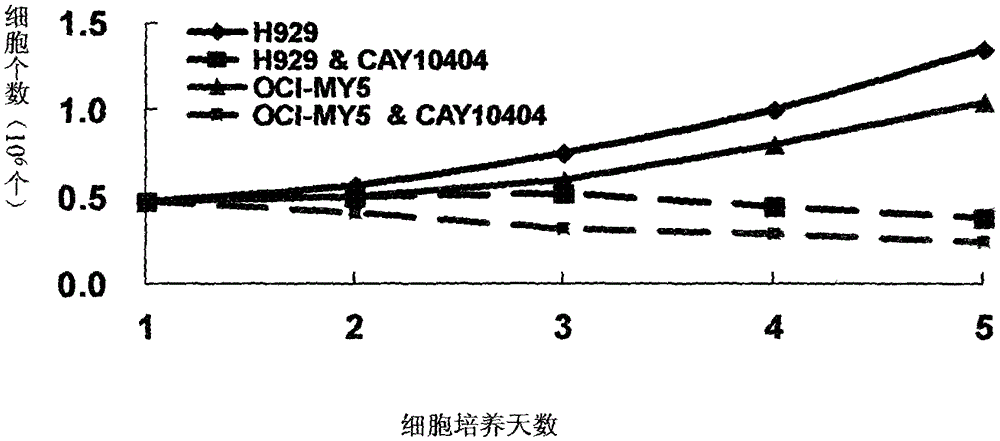
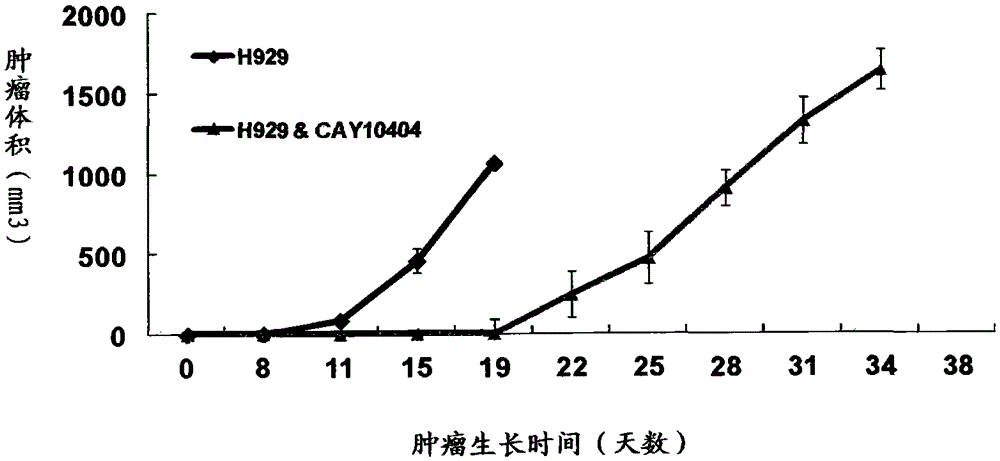
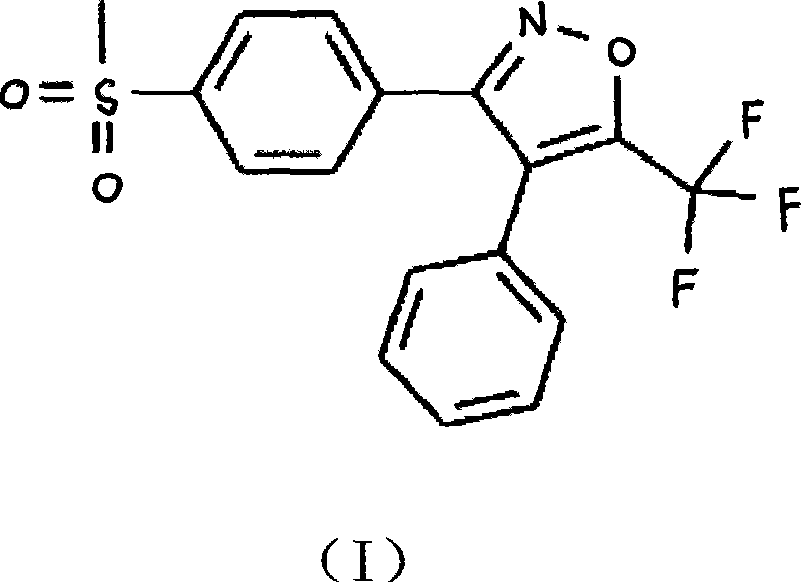
Application of cay10404 in the preparation of multiple myeloma drugs
The invention discloses a medicine for treating multiple myeloma, and specifically relates to application of CAY10404 to prepare a pharmaceutical composition for treating multiple myeloma. CAY10404 is mainly used in tumor cell Wnt signal pathway and used to inhibit accumulation of beta-catenin protein in the pathway. The beneficial effects comprise that CAY10404 is strong in targeting ability and clear in action mechanism, and is mainly used in tumor cell Wnt signal pathway and used to inhibit accumulation of beta-catenin protein in the pathway so as to realize the purpose of inhibiting tumor growth. Compared with other medicines for treating multiple myeloma, such as doxorubicin, the medicine provided by the invention has relatively substantial action effect. Clinic tests show that the medicine has treatment effect on variety of multiple myeloma patients, and is relatively good in treatment effect on especially a patient subjected to bone marrow transplantation. Treatment for 30 examples of clinic test patients shows that the total effective rate is 96.7%. Additionally, the medicine has synergistic effect when used by combining bortezomib and doxorubicin and the like.
Owner:CHINA PHARM UNIV +5
CXCR4 receptor compounds
The invention relates generally to compounds which are allosteric modulators {e.g., positive and negative allosteric modulators, and allosteric agonists) of the G protein coupled receptor for stromal derived factor 1 (SDF-I), also known as the CXCR4 receptor. The CXCR4 receptor compounds are derived from the intracellular loops and domains of the CXCR4 receptor. The invention also relates to the use of these CXCR4 receptor compounds and pharmaceutical compositions comprising the CXCR4 receptor compounds in the treatment of diseases and conditions associated with CXCR4 modulation such as bone marrow transplantation, chemosensitization, cancer, metastatic disease (e.g., cancer), auto-immune disease (e.g., rheumatoid arthritis), fibrosis disease (e.g., pulmonary), AIDS infection, cardiovascular disease, uveitis, inflammatory diseases, celiac disease HIV infection and stem cell-based regenerative medicine.
Owner:ACER THERAPEUTICS INC
New Short Nucleotide Tandem Repeat Sequence Site and Its Application
ActiveCN104046617BHigh heterozygosityHigh resolutionMicrobiological testing/measurementDNA preparationDNA paternity testingWhite blood cell
The present invention discloses a new short nucleotide tandem repeat locus and its application, the short tandem repeat locus G4S0001, the sequence is shown in SEQ ID NO.1, used to prepare (a) for genetic relationship analysis (b) Reagents or kits for individual identification; (c) Reagents or kits for paternity testing or blood relationship analysis; (d) Used to detect whether there is maternal blood contamination in the extracted amniotic fluid and / or (e) a kit for detecting whether leukocytes in a recipient are replaced by donor cells after bone marrow transplantation. The short tandem repeat locus G4S0001 has a high degree of discrimination and can effectively analyze genetic relationships.
Owner:GENESKY DIAGNOSTICS SUZHOU
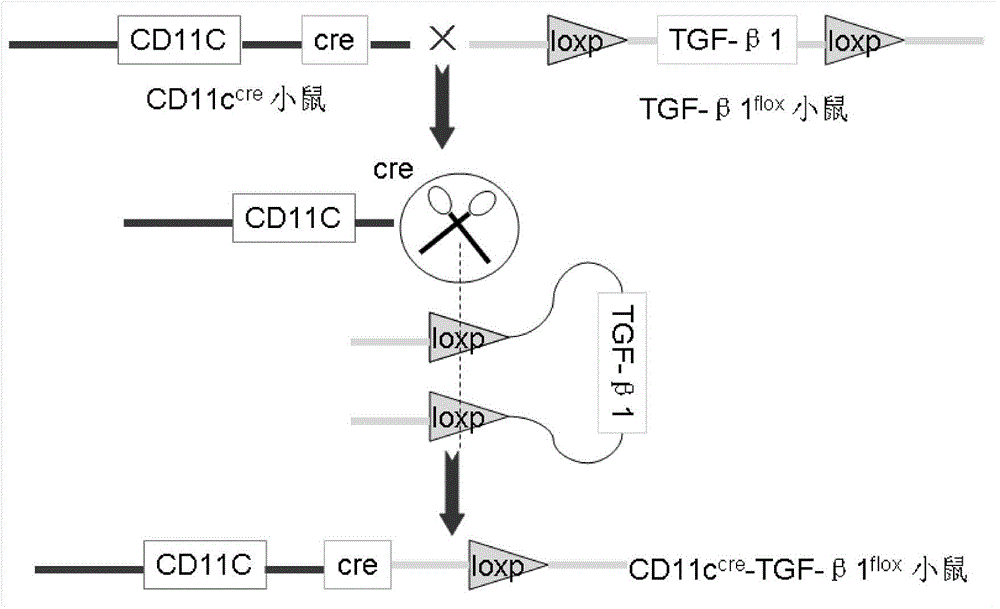
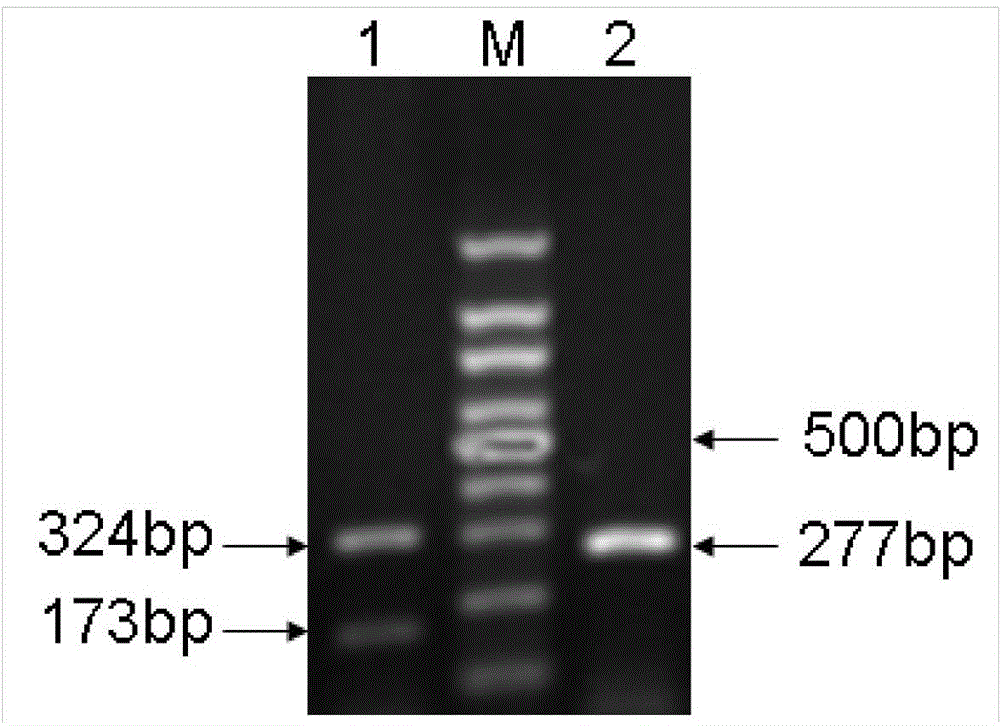
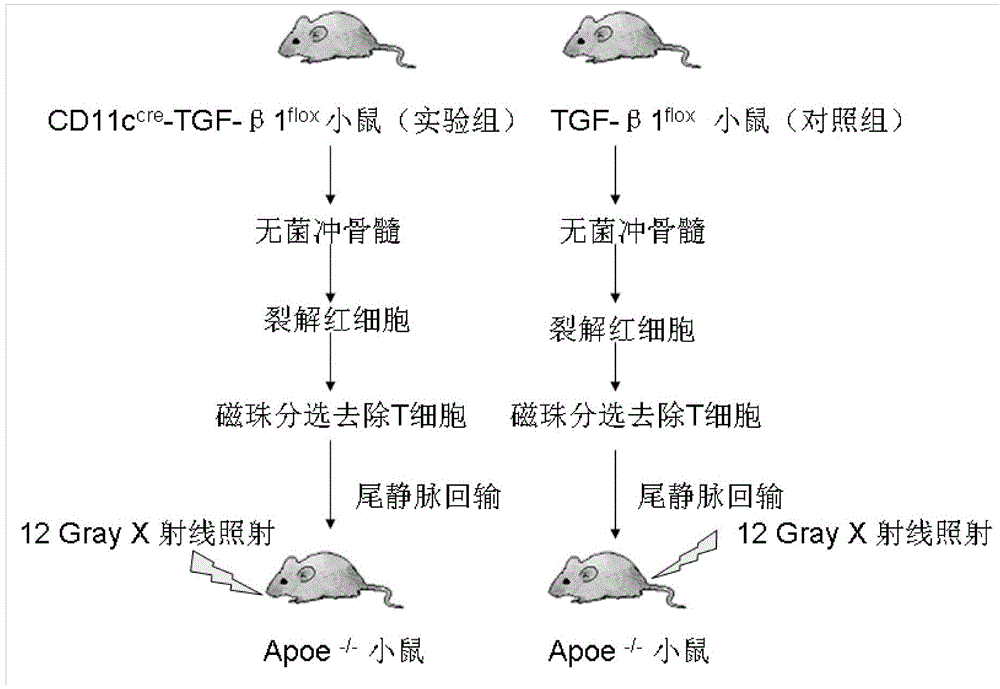
Application of dendritic cell expressed TGF-beta 1 (transforming growth factor-beta1) in preparing anti-atherosclerosis medicaments
InactiveCN104147593APeptide/protein ingredientsCardiovascular disorderBone marrow transplantationTgf beta1
The invention discloses application of dendritic cell expressed TGF-beta 1 (transforming growth factor-beta1) in preparing anti-atherosclerosis medicaments. According to the application, a mouse dendritic cell (DC) unexpressed TGF-beta1 is successfully hybridized by means of a cre-lox technology, and the bone marrow of the mouse is embedded in a susceptible mouse Apoe- / - with atherosclerosis by means of bone marrow transplantation, and the mouse is induced to generate atherosclerosis by means of high fat diet. Results discover that the DC unexpressed TGF-beta1 causes increase of total cholesterol of plasma and remarkable increase of the plaque area of atherosclerosis, and indicate that the DC expressed TGF-beta1 is capable of resisting atherosclerosis by reducing the cholesterol level of plasma; the DC expressed TGF-beta1 can be used for preparing anti-atherosclerosis medicaments based on the anti-atherosclerosis effect of the DC expressed TGF-beta1, and a novel direction is provided to development of anti-atherosclerosis medicaments.
Owner:TAISHAN MEDICAL UNIV
New Short Nucleotide Tandem Repeat Sequence Site and Its Application
ActiveCN103966207BHigh heterozygosityHigh resolutionMicrobiological testing/measurementDNA preparationDNA paternity testingNucleotide
The present invention discloses a new short nucleotide tandem repeat locus and its application, the short tandem repeat locus G2S0002, the sequence is shown in SEQ ID NO.: 1, used to prepare (a) for genetic relationship Reagents or kits for analysis; (b) reagents or kits for individual identification; (c) reagents or kits for paternity testing or blood relationship analysis; (d) for detecting whether there is maternal blood in the extracted amniotic fluid Contaminated reagents or kits; and / or (e) kits for detecting whether leukocytes in recipients have been replaced by donor cells after bone marrow transplantation. The short tandem repeat locus G2S0002 has a high degree of discrimination and can effectively analyze genetic relationships.
Owner:GENESKY DIAGNOSTICS SUZHOU
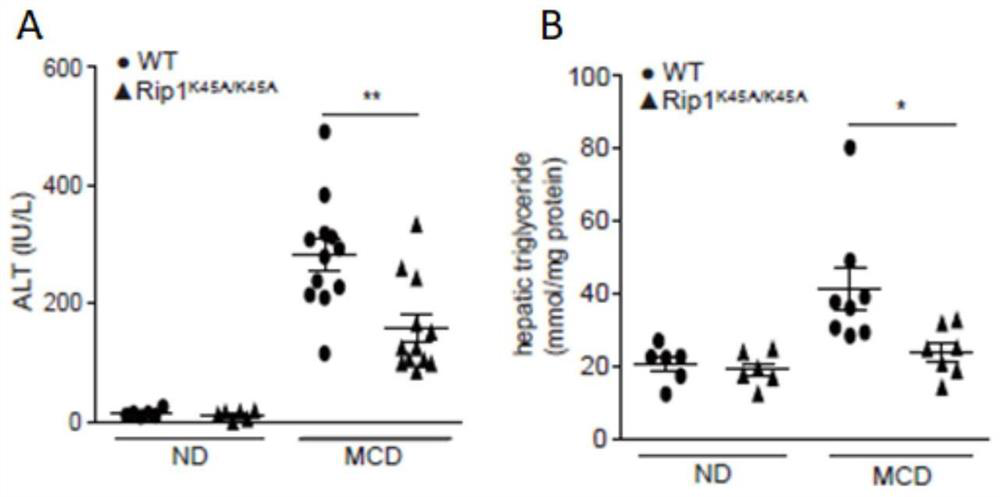
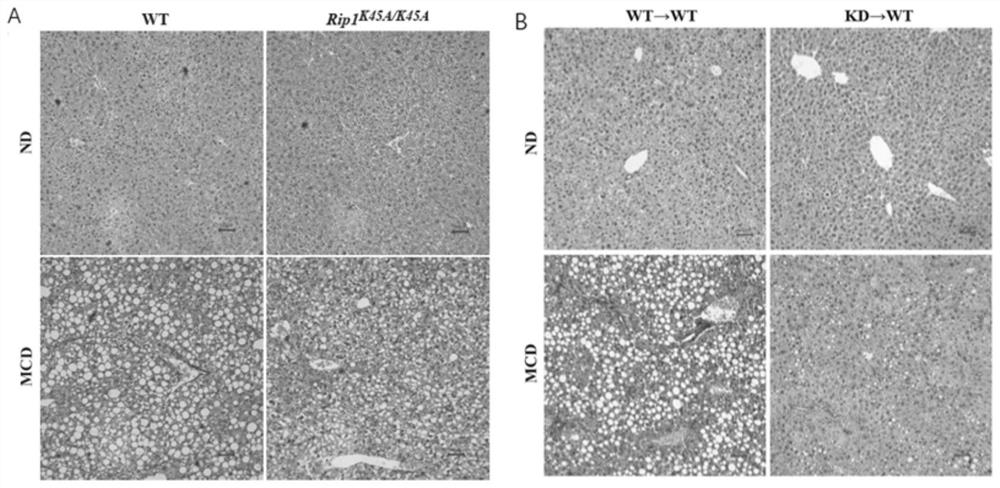
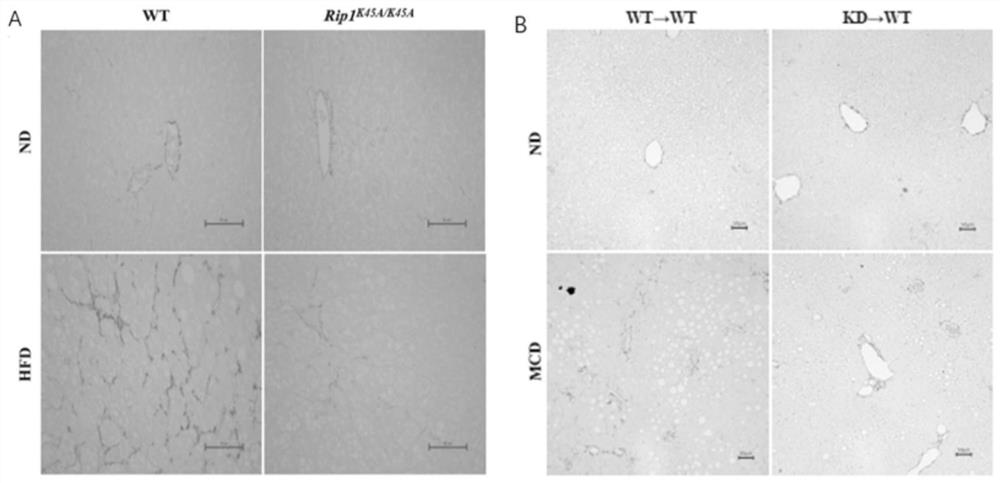
Application of macrophage-targeting RIPK1 and RIPK1 inhibitor in screening and preparing liver injury diagnosis and treatment medicine
PendingCN113069546AReduce contentReduce savingsCompounds screening/testingOrganic active ingredientsStainingTG - Triglyceride
The invention discloses application of macrophage-targeting RIPK1 and an RIPK1 inhibitor in screening and preparing a liver injury diagnosis and treatment medicine RIPK1 inactivated mouse bone marrow is transplanted into a normal C57 mouse to construct a non-alcoholic fatty liver, cirrhosis and hepatic fibrosis model, a result shows that the liver function of the mouse with transplanted RIPK1 inactivated bone marrow is obviously superior to that of a mouse with transplanted C57 mouse bone marrow, the contents of glutamic-pyruvic transaminase and triglyceride in serum are obviously reduced, a lipid component pathological staining result shows that the mouse with transplanted RIPK1 inactivated bone marrow can obviously relieve fatty liver lesion and significantly reduce lipid accumulation, and it is found that RIPK1 inactivation can significantly reduce inflammation and death of palmitic acid-induced bone marrow macrophages. A macrophage-targeting RIPK1 active site can be used as a medicine target for screening and treating liver injury diseases such as fatty liver, cirrhosis and hepatic fibrosis, and the RIPK1 inhibitor can be used for preparing the medicine for treating the liver injury diseases.
Owner:NANJING UNIV OF SCI & TECH
Enteral nutritional preparation containing marine bioactivity polysaccharide as well as preparation method and application thereof
ActiveCN101703248BEnhanced inhibitory effectIncrease lethalityOrganic active ingredientsMetabolism disorderSide effectBone marrow transplantation
An enteral nutrition comprises (mass%) marine bioactive polysaccharide 0.5-0.8%, enzymolysis article of shell fish meat 25-35%, fruit and vegetable powder 10-20%, and sodium-iron-EDTA 0.05-0.1%. The marine bioactive polysaccharide can be Pinctada martensii glycosaminoglycan, sea cucumber chondroitin sulfate or fishbone chondroitin sulfate. The enteral nutrition can further comprise soy protein isolate 35-45%, kelp powder 1-5% and refined konjak powder 3-5%. The enteral nutrition has high heat, high protein, high iron, low fat, multiple vitamins and easy absorbability. It is used as food for people needing much iron, especially for leukemia patient, bone marrow transplantation patient. It is also used with chemotherapy medicine to increase the curative effect of the medicine, improve the functions of gastrointestinal tract, enhance the immunity, and reduce side effects such as anemia and accompanying infection. Its preparation method includes that the raw materials are crushed with 60 meshes sieve and sterilized for 2 minutes with 800 kW power microwave after mixing and drying.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com