Patents
Literature
903 results about "Trial drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phase 0 trials are optional first-in-human trials. Single subtherapeutic doses of the study drug or treatment are given to a small number of subjects (10 to 15) to gather preliminary data on the agent's pharmacodynamics (what the drug does to the body) and pharmacokinetics (what the body does to the drugs).
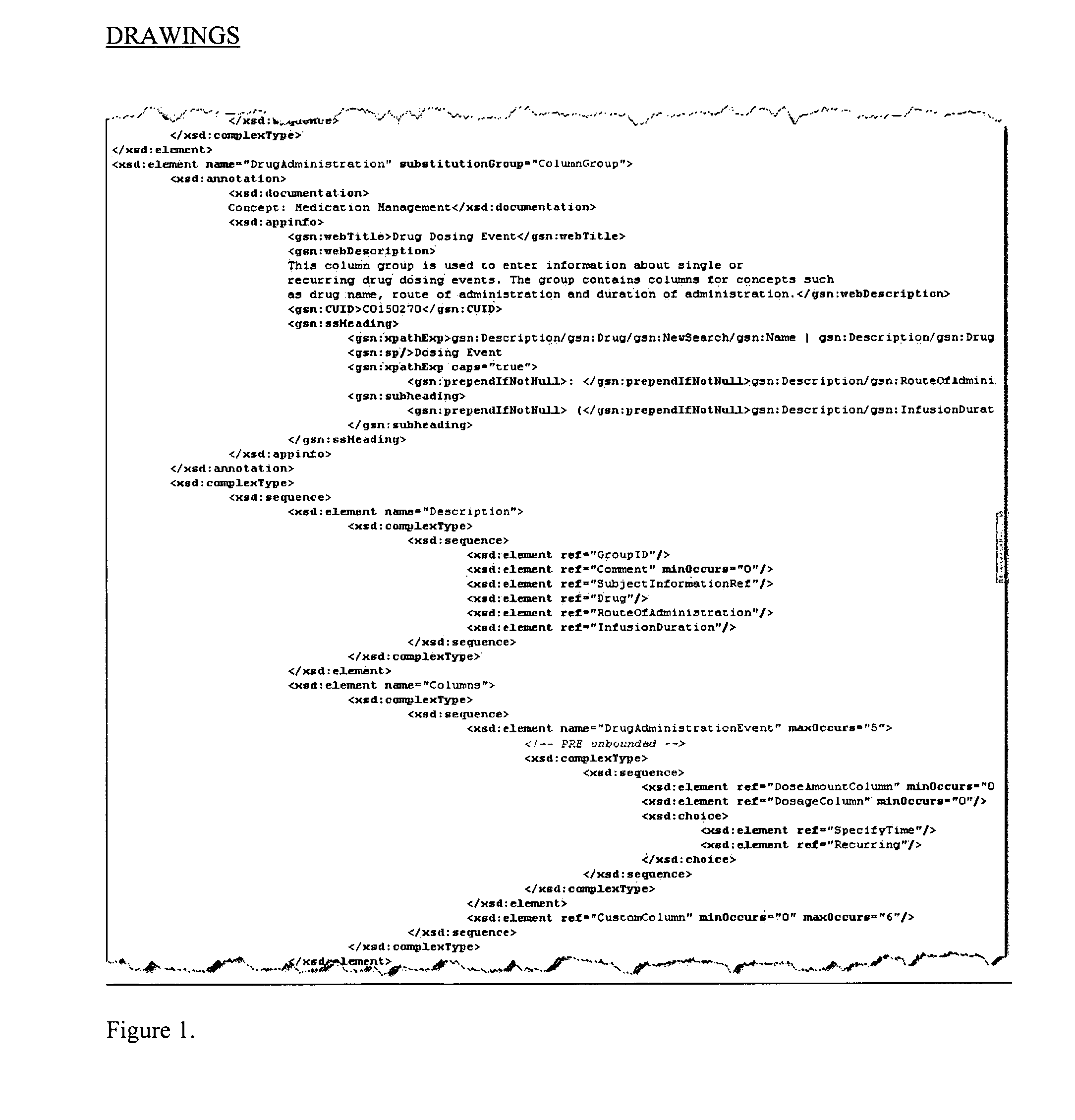
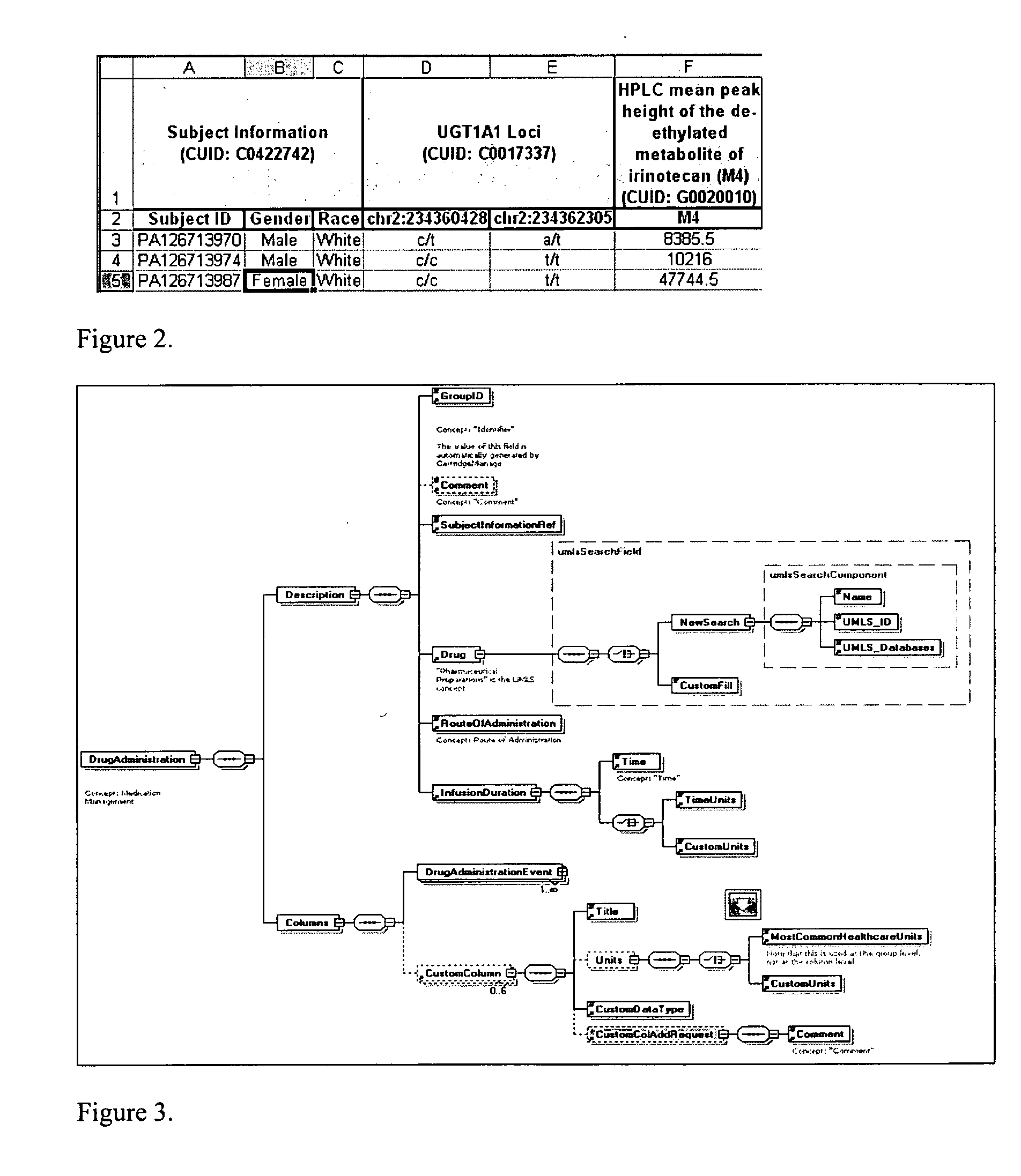
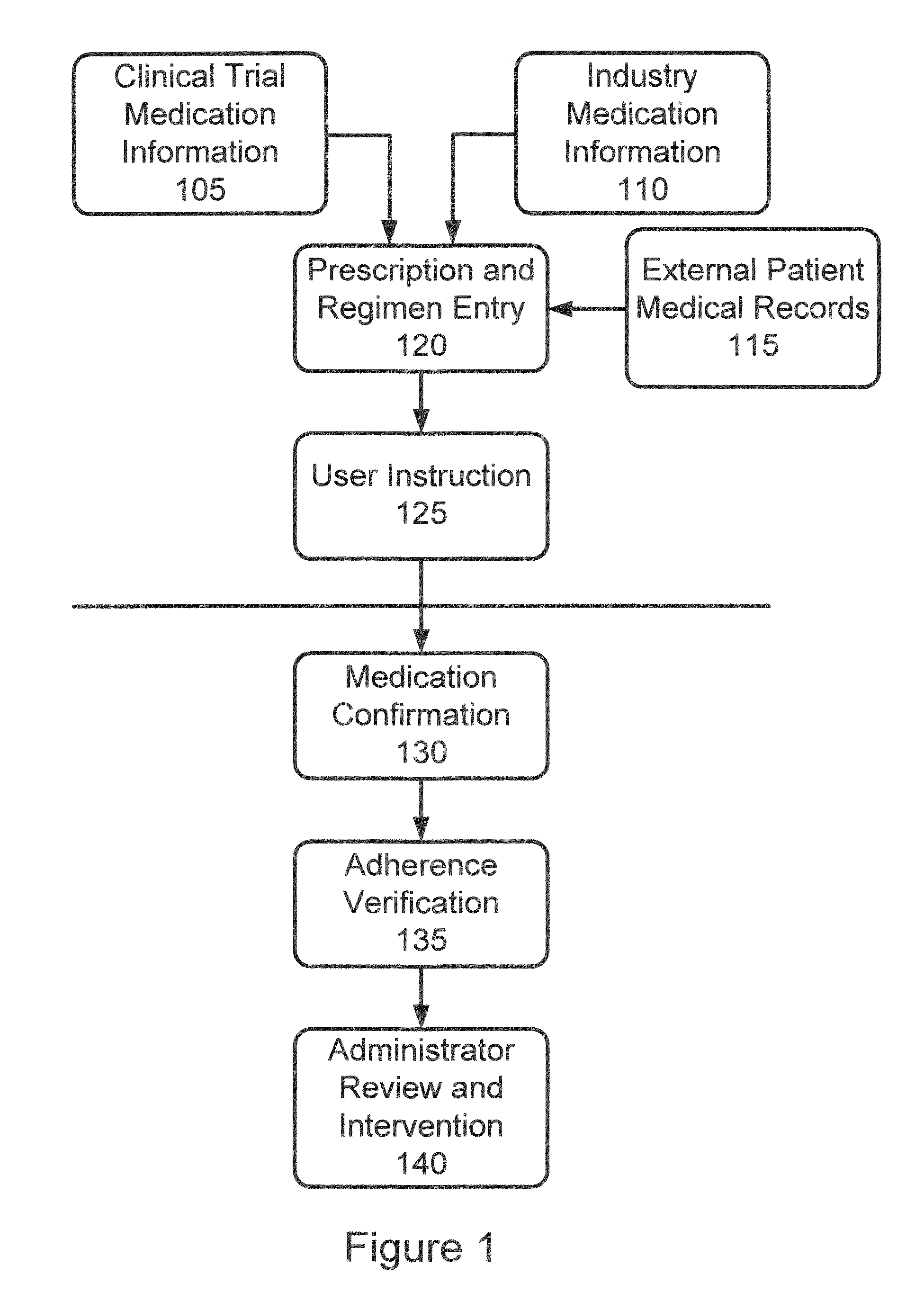
System and method for integrating and validating genotypic, phenotypic and medical information into a database according to a standardized ontology
InactiveUS20070178501A1Safest and most effective treatmentGood decisionData processing applicationsMicrobiological testing/measurementData validationMedical record
The system described herein enables clinicians and researchers to use aggregated genetic and phenotypic data from clinical trials and medical records to make the safest, most effective treatment decisions for each patient. This involves (i) the creation of a standardized ontology for genetic, phenotypic, clinical, pharmacokinetic, pharmacodynamic and other data sets, (ii) the creation of a translation engine to integrate heterogeneous data sets into a database using the standardized ontology, and (iii) the development of statistical methods to perform data validation and outcome prediction with the integrated data. The system is designed to interface with patient electronic medical records (EMRs) in hospitals and laboratories to extract a particular patient's relevant data. The system may also be used in the context of generating phenotypic predictions and enhanced medical laboratory reports for treating clinicians. The system may also be used in the context of leveraging the huge amount of data created in medical and pharmaceutical clinical trials. The ontology and validation rules are designed to be flexible so as to accommodate a disparate set of clients. The system is also designed to be flexible so that it can change to accommodate scientific progress and remain optimally configured.
Owner:NATERA
Computational method and system to perform empirical induction
InactiveUS6317700B1Reduce the amount of solutionPharmaceutical delivery mechanismDigital computer detailsIntervention measuresThe Internet
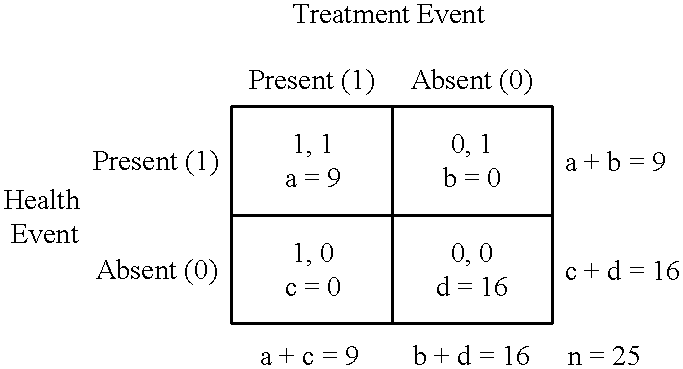
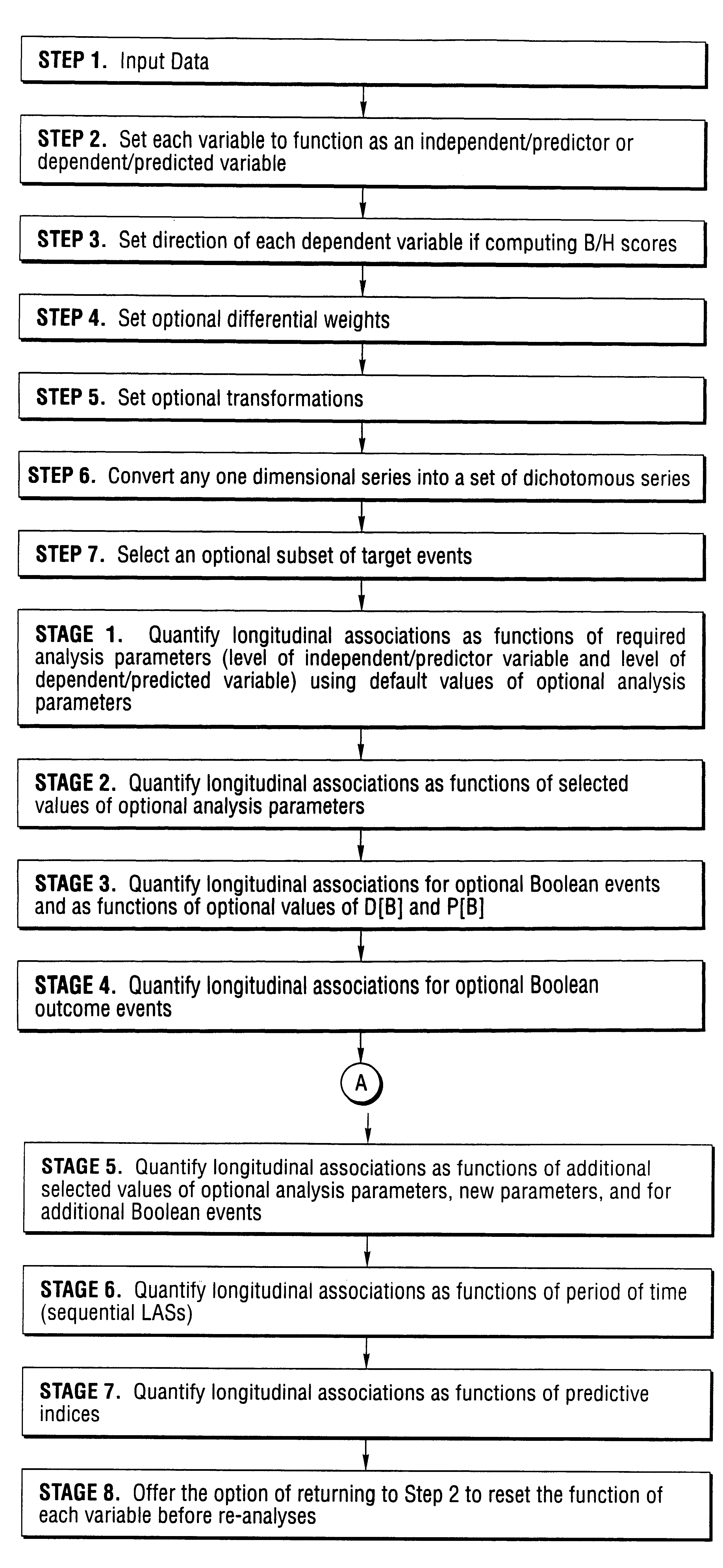
The present invention is an improved computational method and system of empirical induction that can be used to arrive at generalized conclusions and make predictions involving longitudinal associations between and among variables and events. Empirical induction is used to gain scientific knowledge, to develop and evaluate treatments and other interventions, and to help make predictions and decisions. The invention, which is distinct from and often complementary to the statistical method, is applied to repeated measures and multiple time-series data and can be used to quantify, discover, analyze, and describe longitudinal associations for individual real and conceptual entities. Major improvements include provisions to define Boolean independent events and Boolean dependent events and to apply analysis parameters such as episode length and episode criterion for both independent and dependent variables, persistence after independent events, and delay and persistence after Boolean independent events. These improvements are in addition to levels of independent and dependent variables, delay after independent events, and provision to quantify benefit and harm across two or more dependent variables. Additional improvements include provisions to quantify longitudinal associations as functions of period or time and to compute values of predictive indices when there are two or more independent variables. Major applications and uses of the invention include data mining, the conduct of clinical trials of treatments for the management or control of chronic disorders, health-effect monitoring, the quantification and analysis internal control in adaptive systems, analyses of serial functional images, analyses of behavior and behavior modification, and use to create computerized devices and systems whose behavior can be modified by experience. The present invention is best implemented on the Internet.
Owner:BAGNE MILLER ENTERPRISES INC
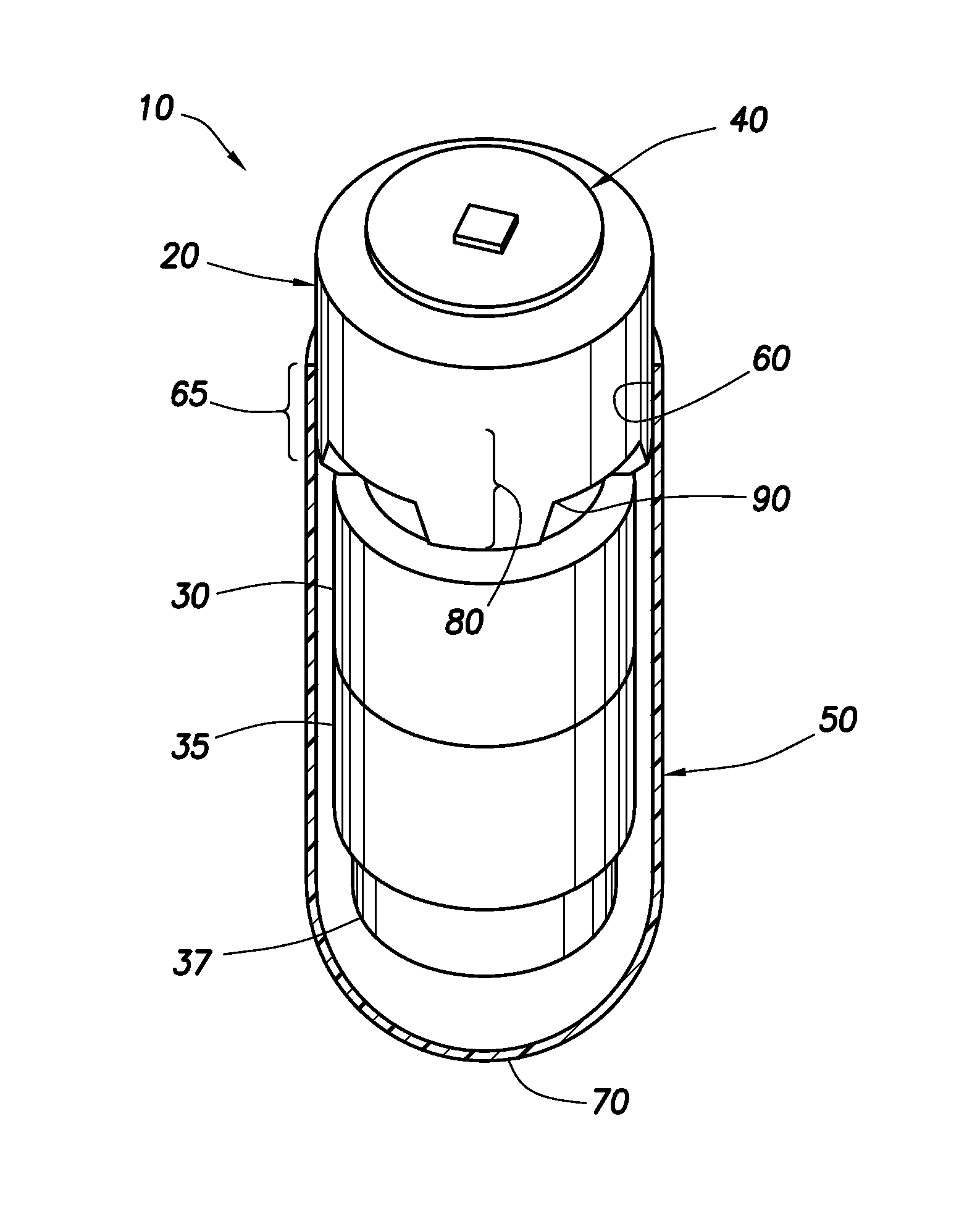
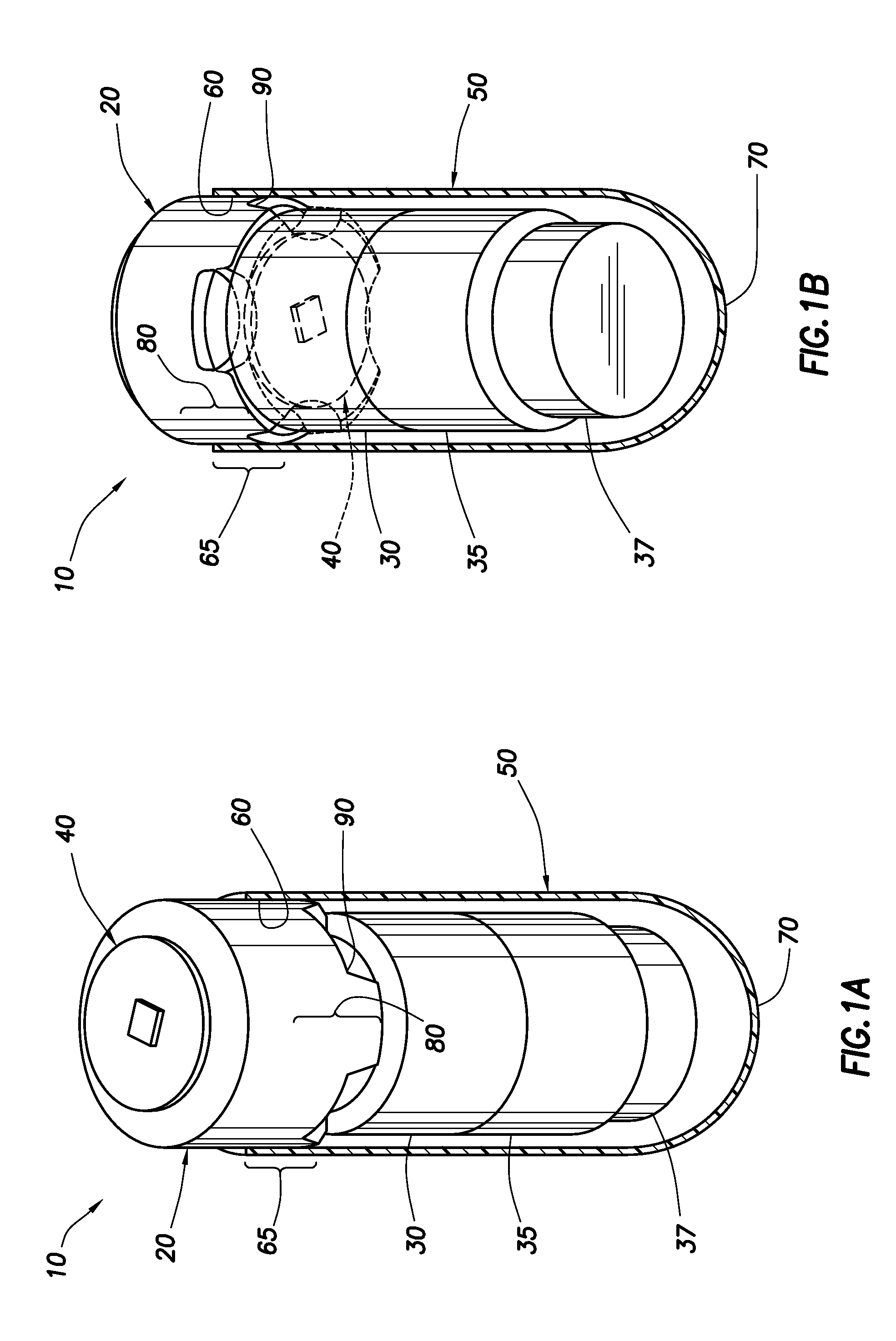
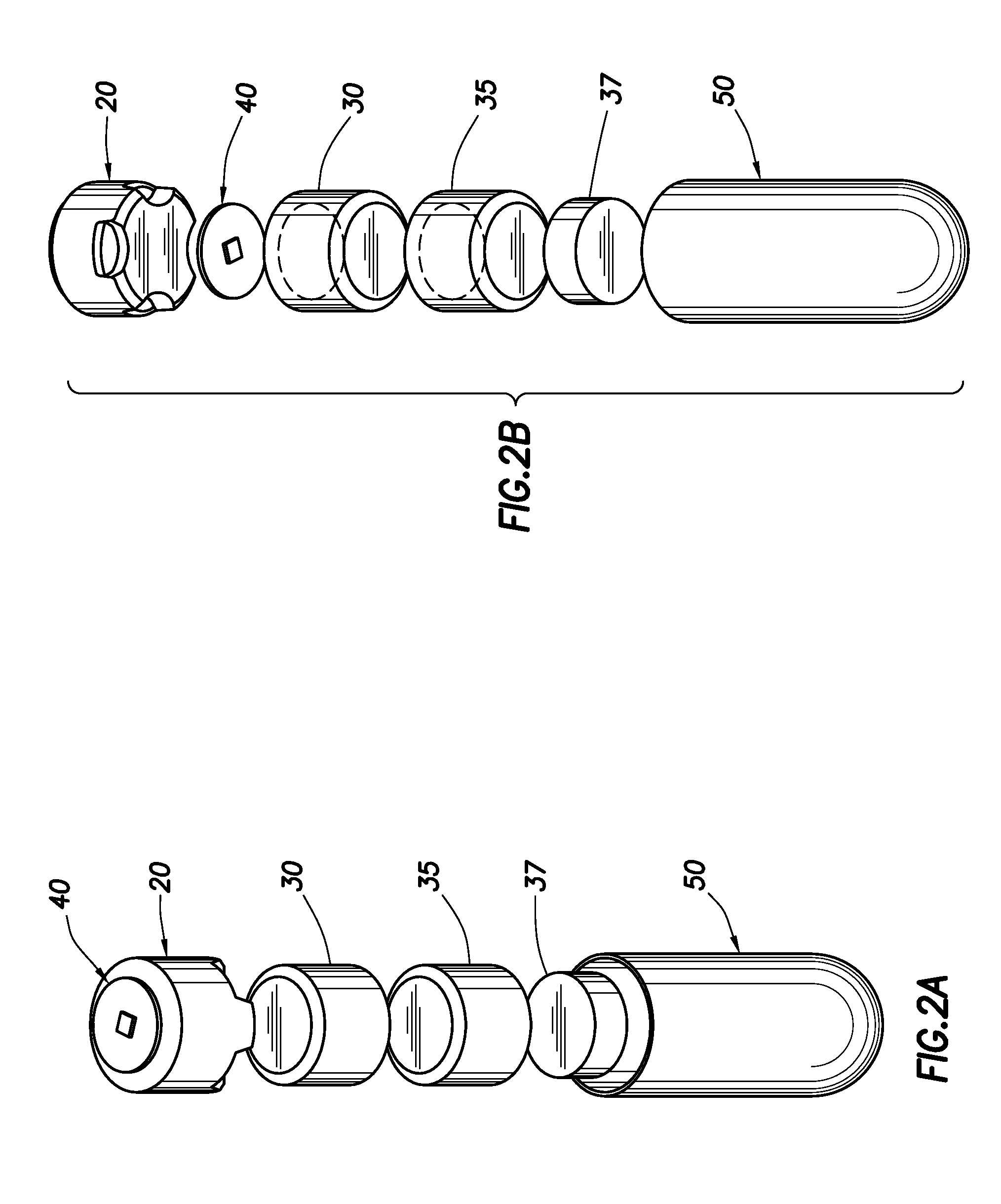
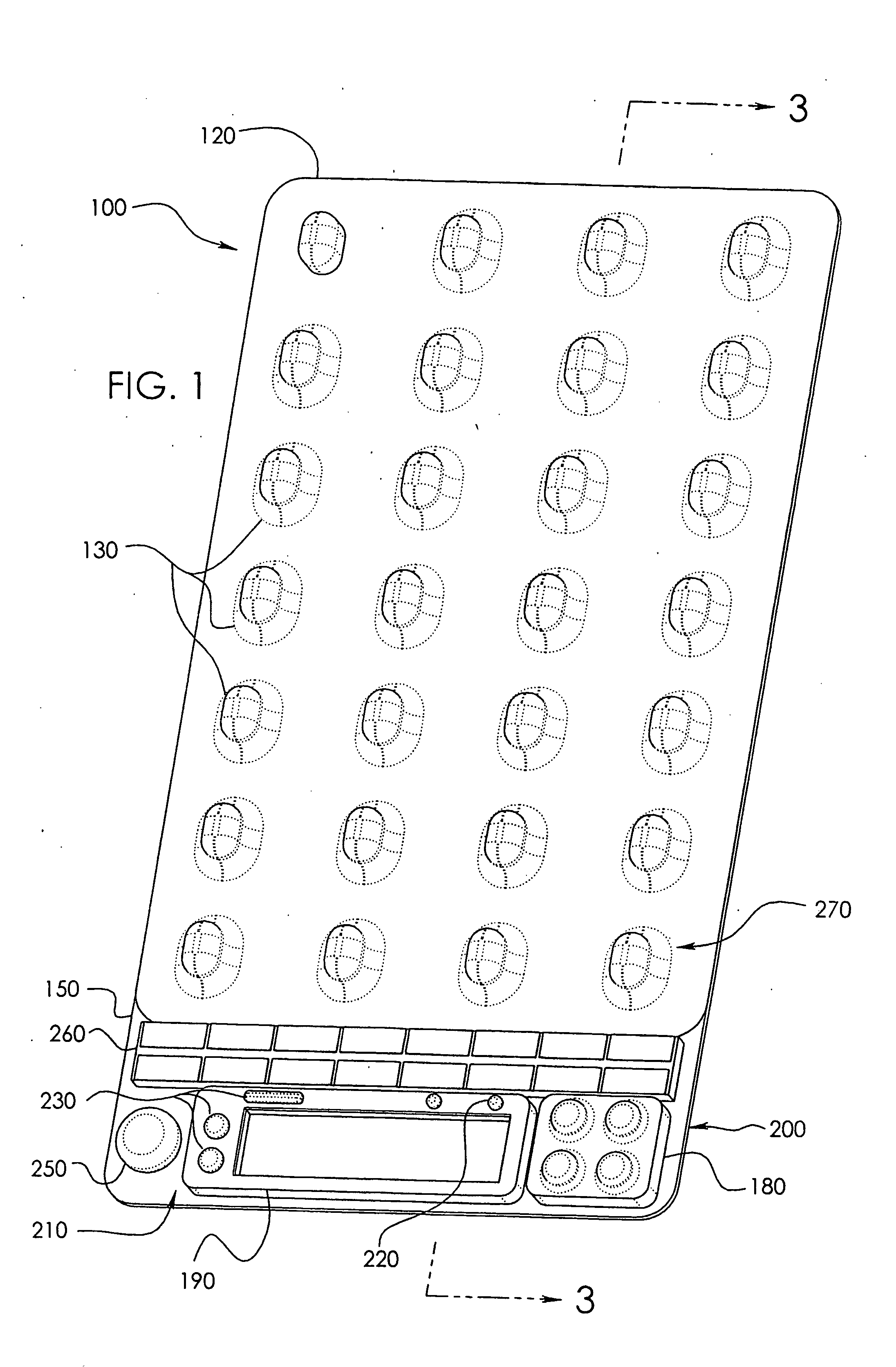
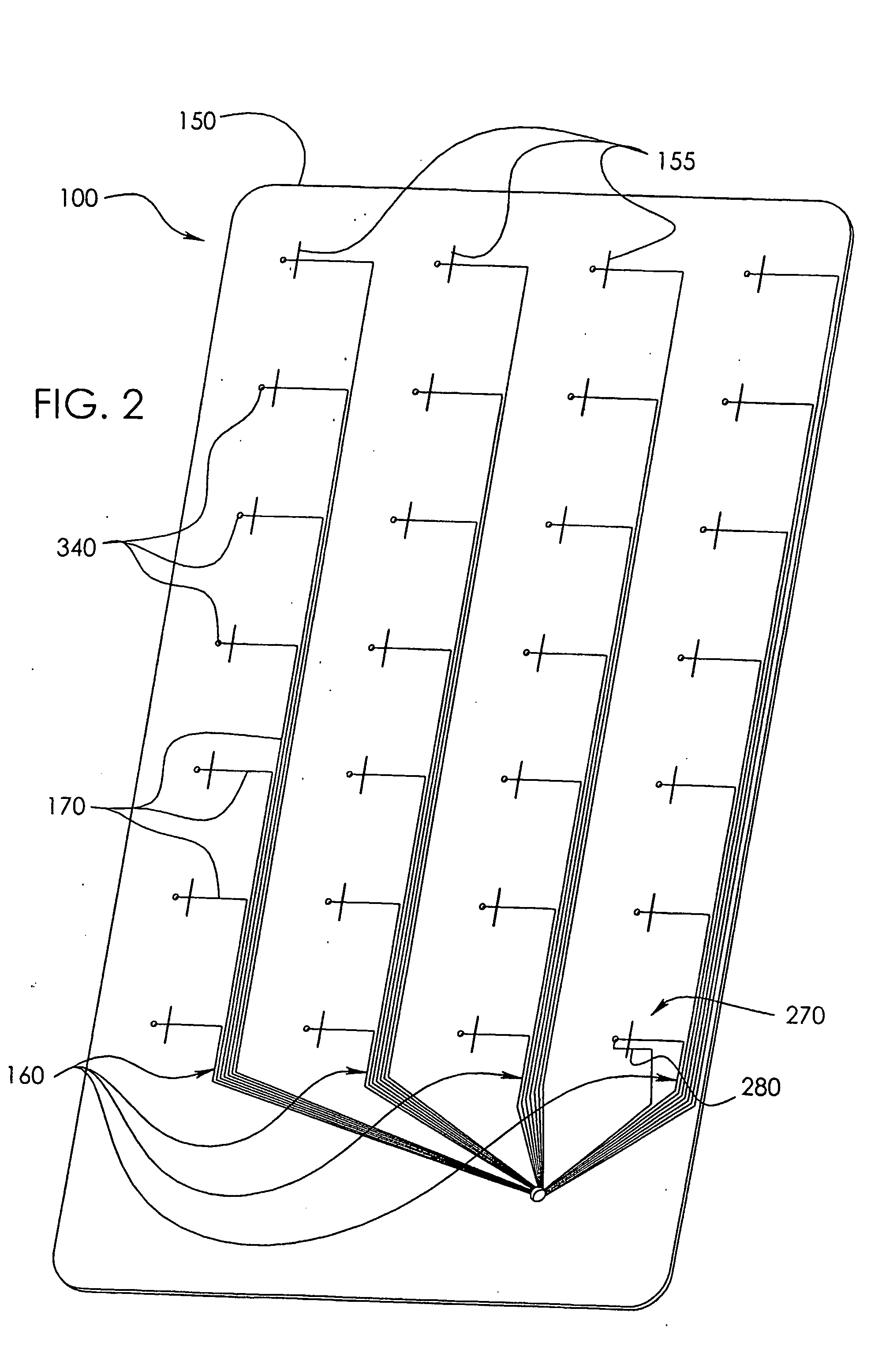
Needle Array Assembly and Method for Delivering Therapeutic Agents
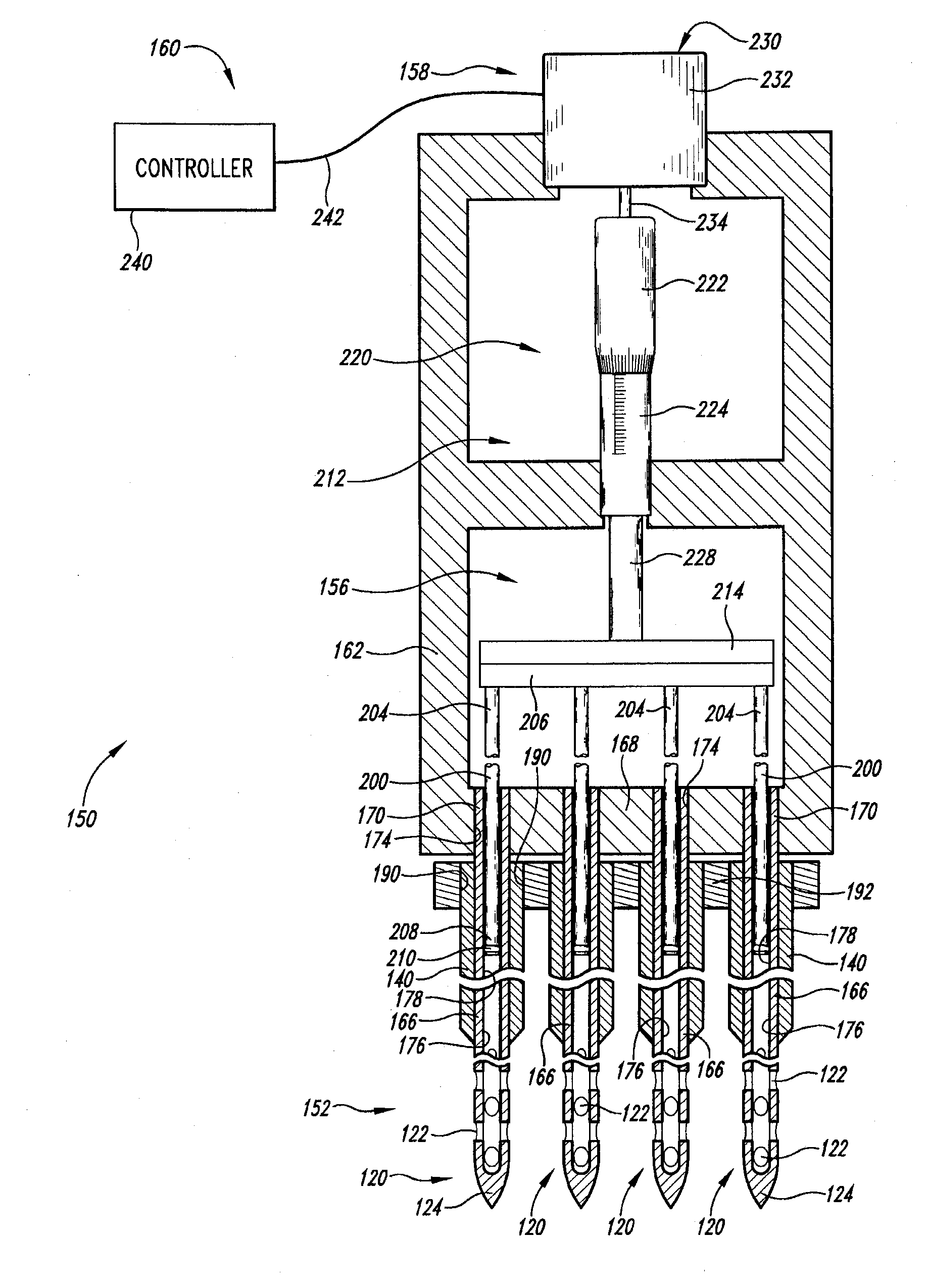
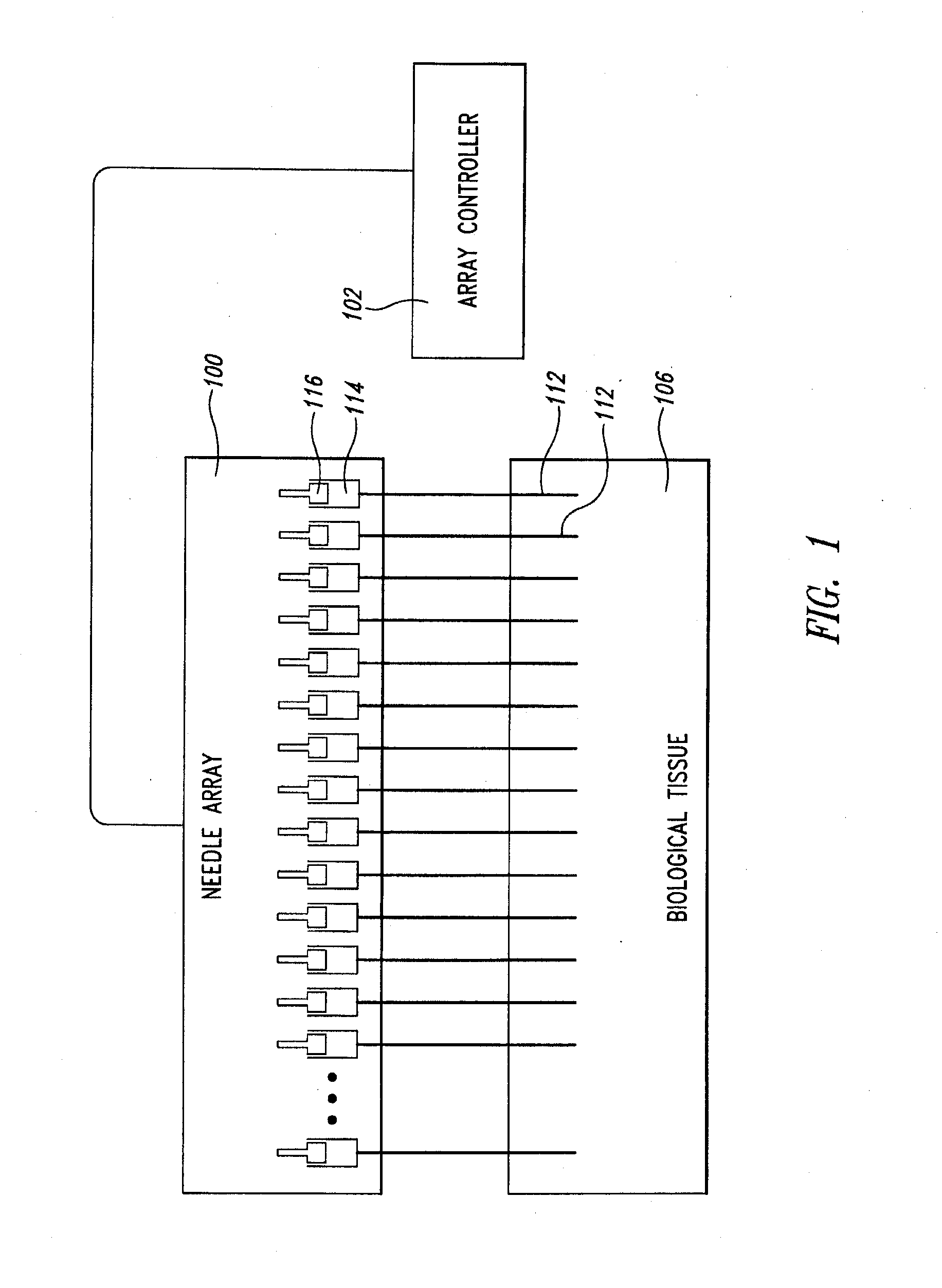
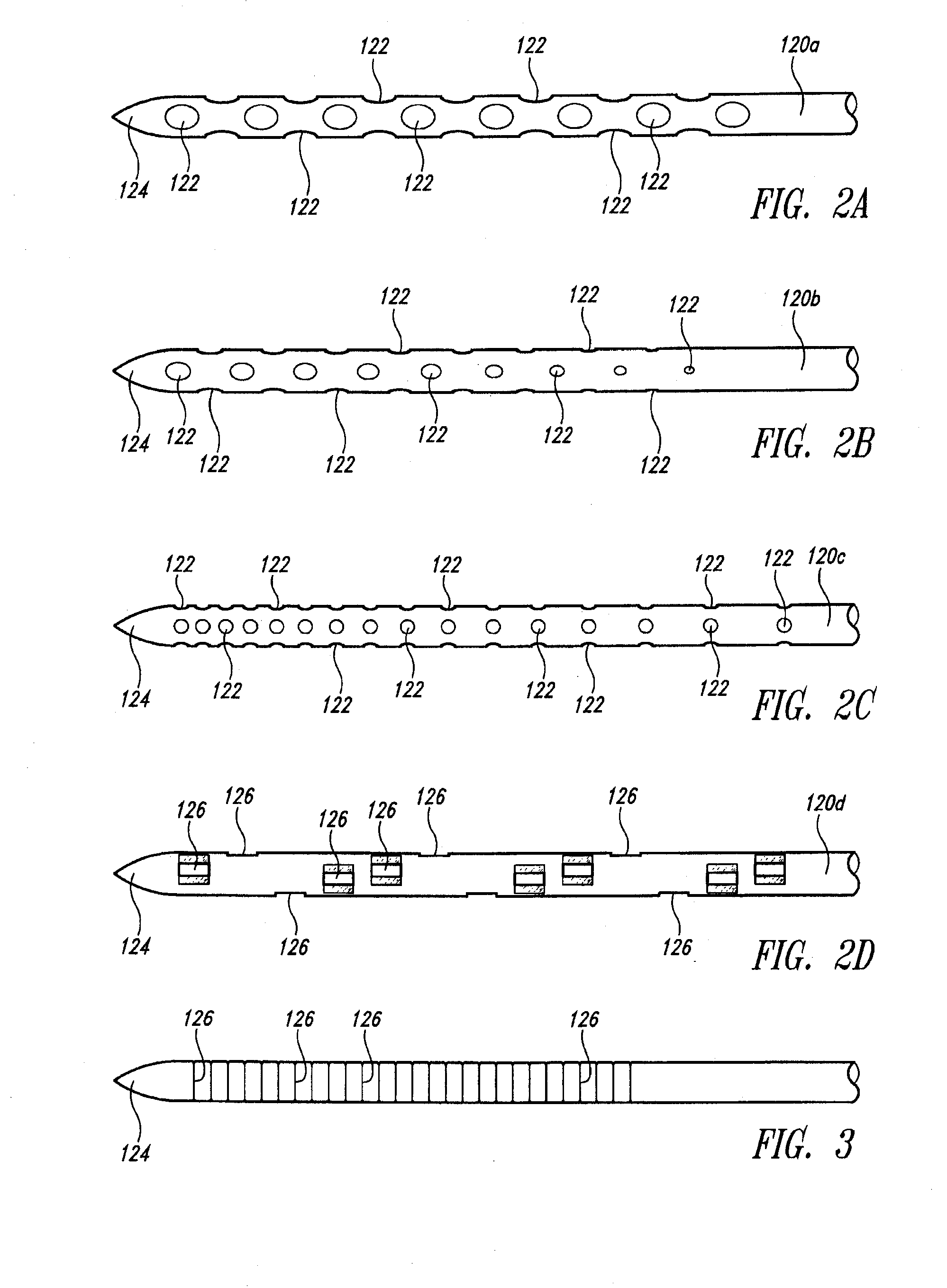
A fluid delivery device includes an array of needles, each in fluid communication with a respective reservoir. Respective actuators are coupled so as to be operable to drive fluid from the reservoirs via needle ports. Each needle can have a plurality of ports, and the ports can be arranged to deliver a substantially equal amount of fluid at any given location along its length. A driver is coupled to the actuators to selectively control the rate, volume, and direction of flow of fluid through the needles. The device can simultaneously deliver a plurality of fluid agents along respective axes in solid tissue in vivo. If thereafter resected, the tissue can be sectioned for evaluation of an effect of each agent on the tissue, and based on the evaluation, candidate agents selected or deselected for clinical trials or therapy, and subjects selected or deselected for clinical trials or therapeutic treatment.
Owner:FRED HUTCHINSON CANCER RES CENT
High throughput correlation of polymorphic forms with multiple phenotypes within clinical populations
A computer-assisted method of looking for pharmacologic targets, in which large numbers of persons are enrolled in drugh clinical trials, they are medically examined and documented, tissue samples are taken, the tissue samples are genotyped, and an examination is made of the genotypes to try to ascertain associations between the genotypes and the documented disease phenotypes of the patients.
Owner:SMITHKLINE BECKMAN CORP
Pharmaceutical dosages delivery system
Owner:PROTEUS DIGITAL HEALTH INC
Methods of identifying compounds that modulate body weight using the OB receptor
The present invention relates to the discovery, identification and characterization of nucleotides that encode Ob receptor (ObR), a receptor protein that participates in mammalian body weight regulation. The invention encompasses obR nucleotides, host cell expression systems, ObR proteins, fusion proteins, polypeptides and peptides, antibodies to the receptor, transgenic animals that express an obR transgene, or recombinant knock-out animals that do not express the ObR, antagonists and agonists of the receptor, and other compounds that modulate obR gene expression or ObR activity that can be used for diagnosis, drug screening, clinical trial monitoring, and / or the treatment of body weight disorders, including but not limited to obesity, cachexia and anorexia.
Owner:MILLENNIUM PHARMA INC
Methods for monitoring patients with severe sepsis and septic shock and for selecting treatments for these patients
InactiveUS20080311554A1Effective treatmentMicrobiological testing/measurementBiological testingClinical efficacyPhysiologic Test
Methods of identifying, monitoring and matching patients with appropriate treatments who are at risk for developing a systemic inflammatory condition using a systemic mediator-associated physiologic test profile are provided. The methods of the present invention increase the likelihood of demonstrating clinical efficacy in clinical trial datasets.
Owner:SLOTMAN GUS J
Medicament for relieving pain of human body and preparation method thereof
ActiveCN101632737APromoting blood circulation and removing blood stasisGood anti-inflammatory and detumescenceAntipyreticAnalgesicsRheumatismHyperostosis
The invention discloses a pharmaceutical composition with effects of promoting blood circulation by removing blood stasis, anti-inflammatory and detumescence, and relieving pain and a preparation method thereof. The pharmaceutical composition is prepared from the following raw materials: 20-100 parts by mass of rhubarb, 20-110 parts by mass of gardenia and 10-100 parts by mass of baikal skullcap root. The preparation method of the pharmaceutical composition comprises the following steps: extracting the raw materials with water or ethanol for preparing the pharmaceutical composition, and then filtering and collecting filtrate to obtain the pharmaceutical composition. Clinical trials show that the pharmaceutical composition has favorable curative effect of relieving pains in neck and shoulders, waist and legs, joints and muscles caused by rheumatism, hyperosteogeny, traumatic injury, and the like.
Owner:吉林一正药业集团有限公司
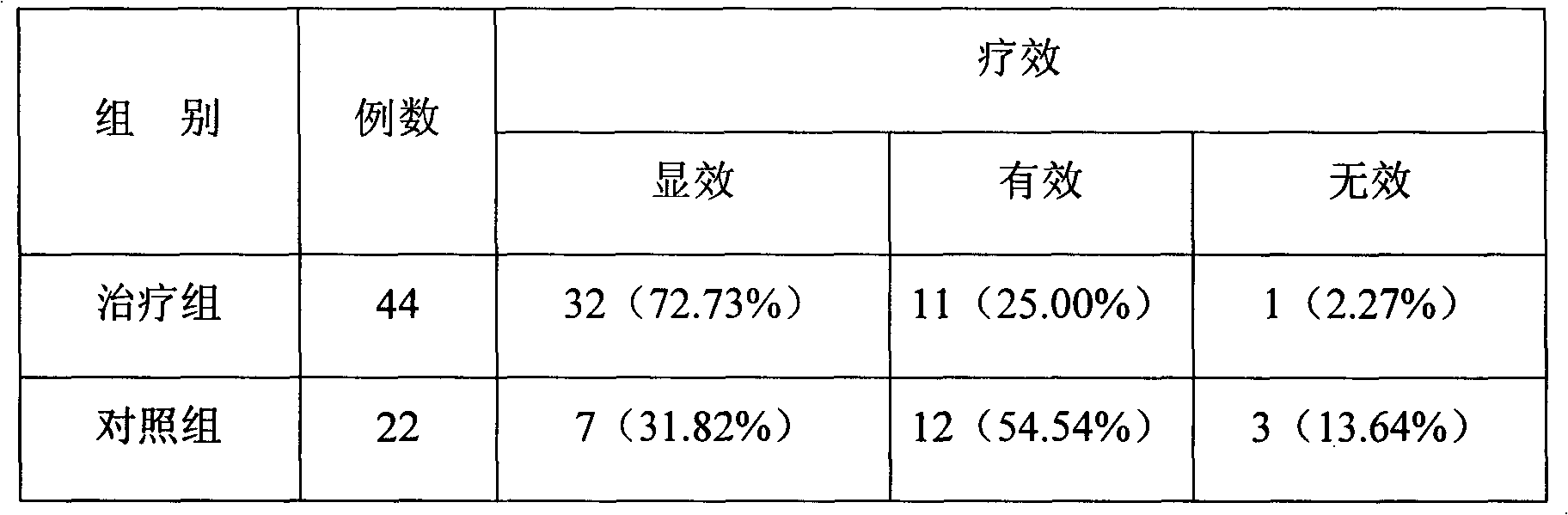
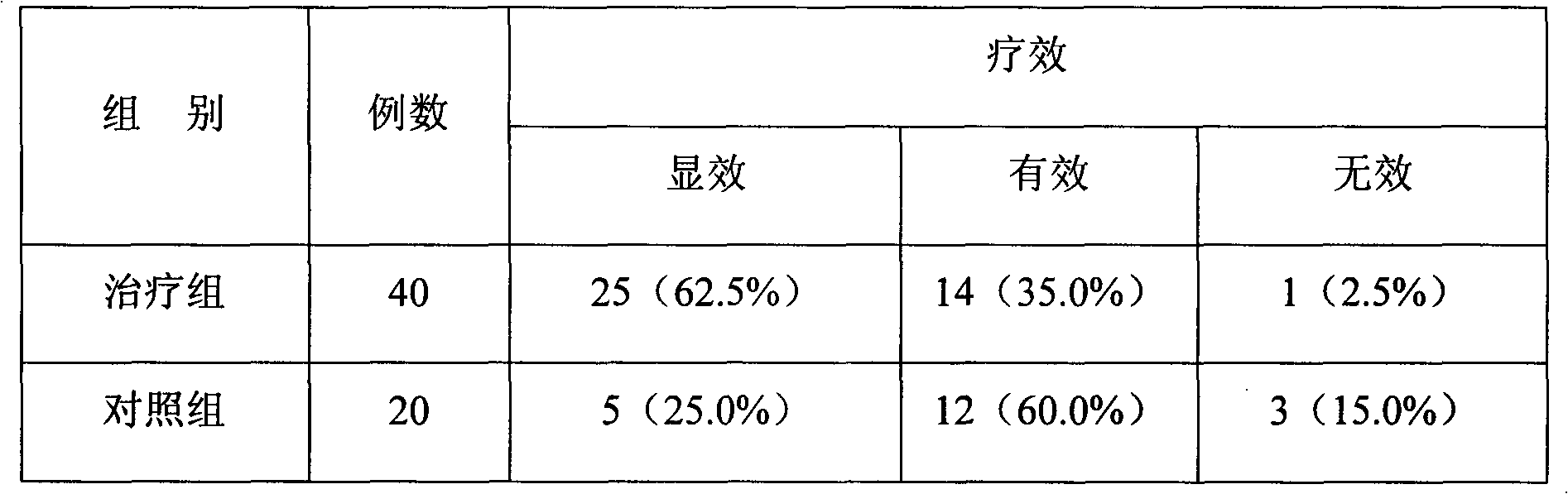
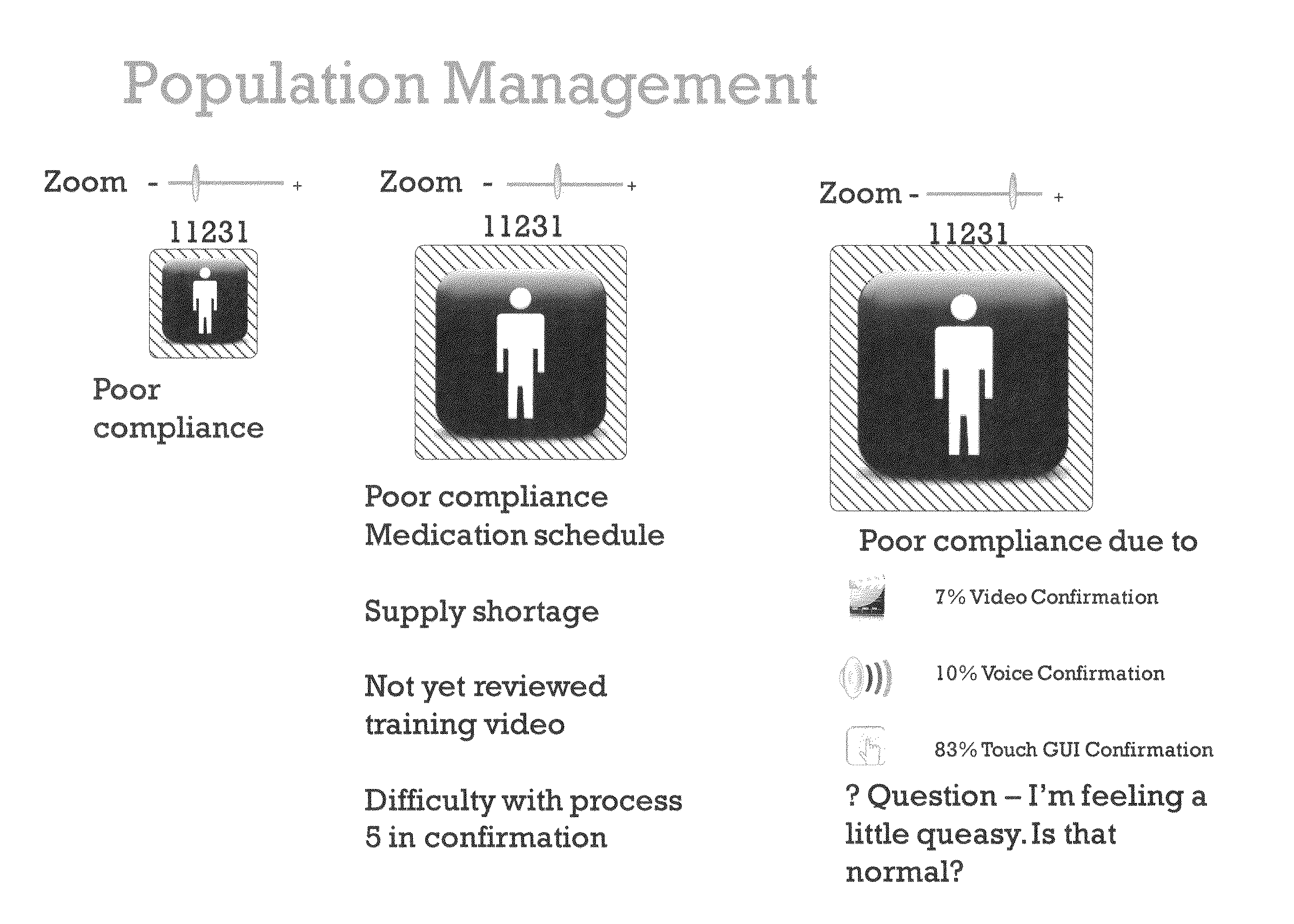
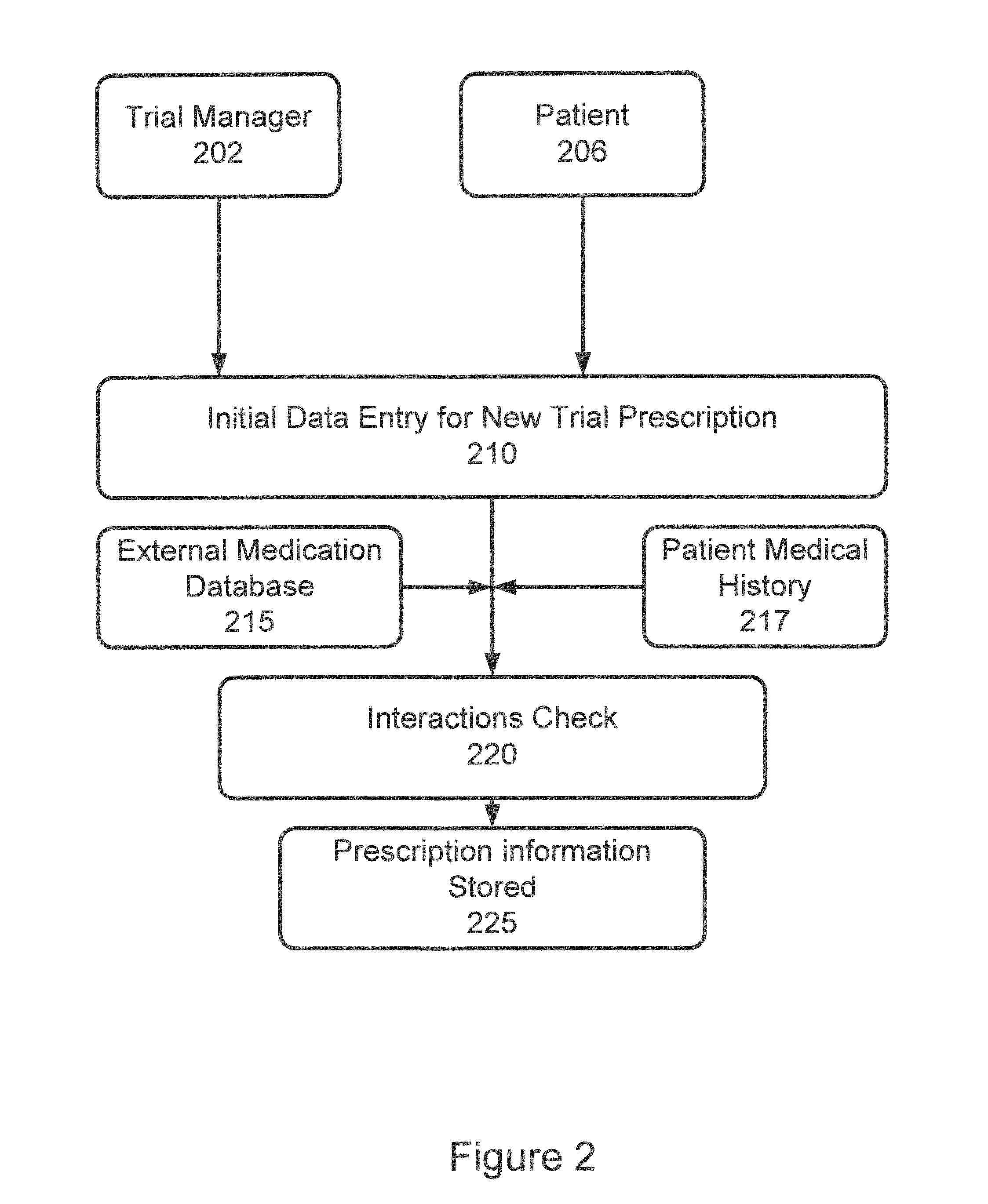
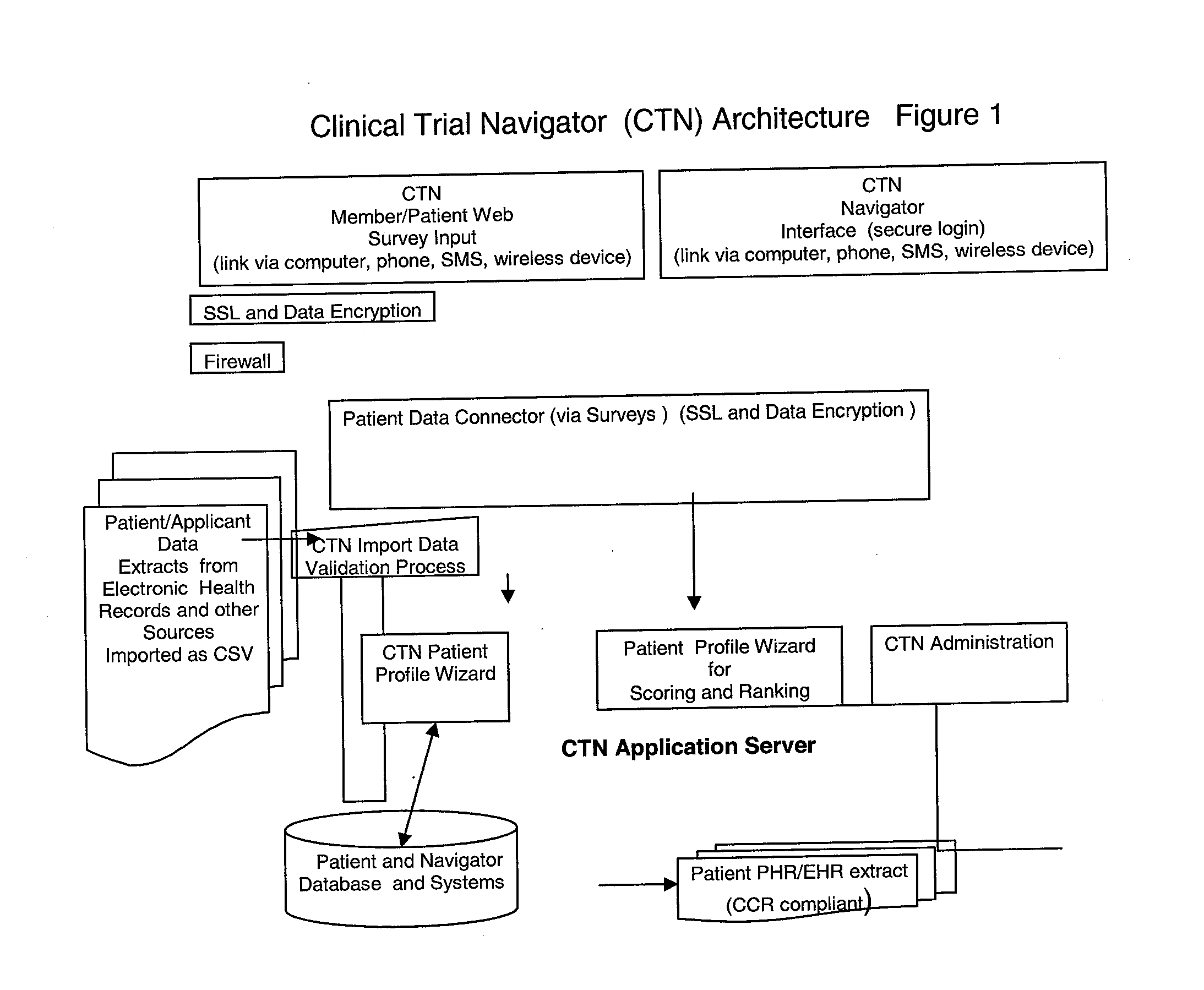
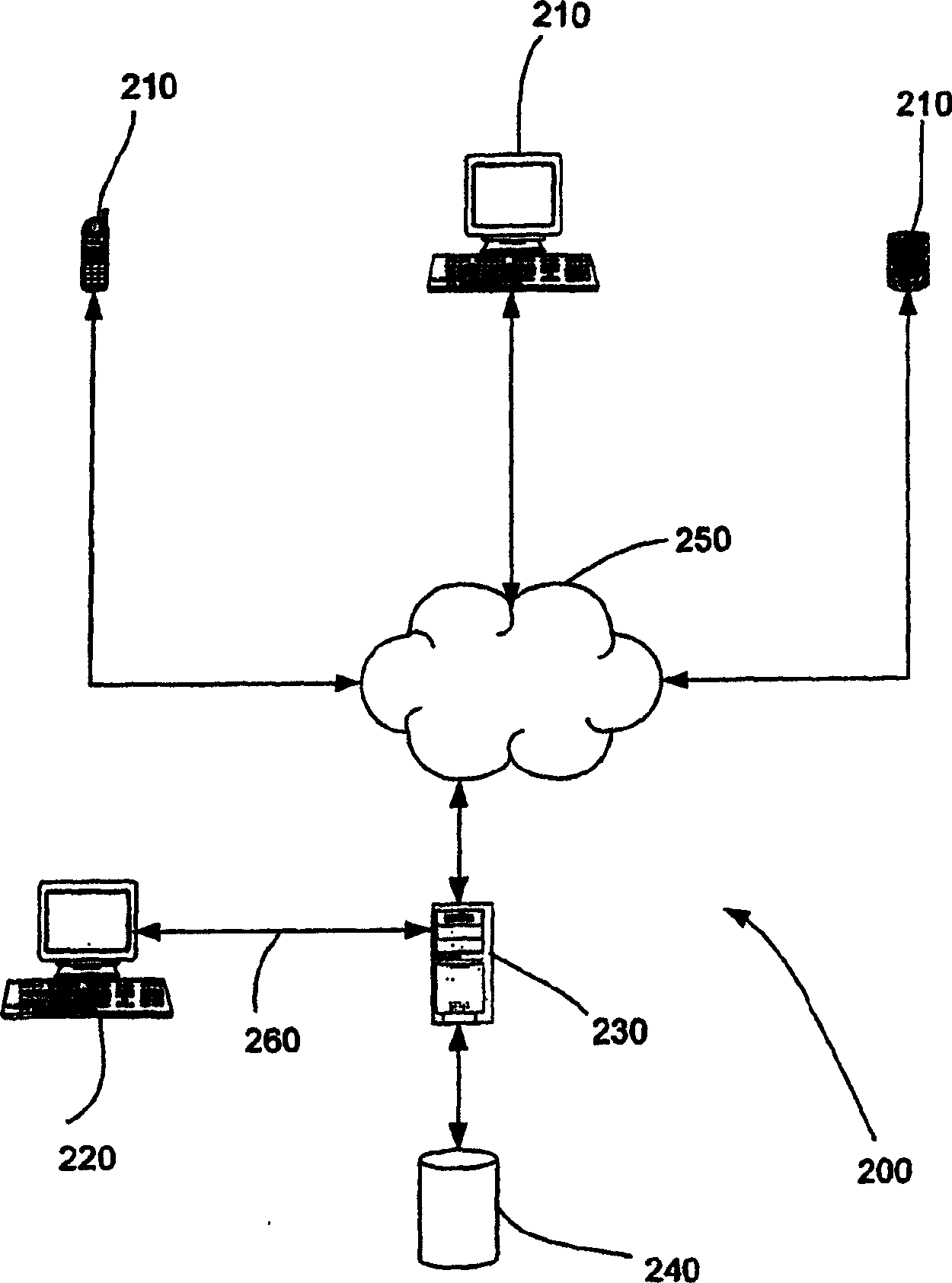
Method and Apparatus for Management of Clinical Trials
ActiveUS20110153361A1Effective corporate compliance programMinimize forgetfulnessDrug and medicationsNutrition controlGraphicsTrial drug
A system and method of a clinical trial is provided. The system comprises a summary page providing an overview of each clinical trial participant in a graphical format, each clinical trial participant being represented by a unique clinical trial participant identifier and one or more clinical trial participant identifier modifiers applicable to modify one or more of the clinical trial participant identifiers, each modifier indicative of a different status of the particular participant identifier to which it is applied. A zoom selector is provided for zooming in on a subset of the clinical trial participant identifiers included with the summary page. Upon selection of a particular level of zoom, a corresponding amount of detailed information related to the subset of the clinical trial identifiers is provided; the detailed information including at least an indication of the level of compliance of a clinical trial participant to a prescribed clinical trial protocol represented by a corresponding clinical trial participant identifier, and further information related to any particular clinical trial participant identifier modifiers applied to any one of the clinical trial participant identifiers included within the subset.
Owner:AI CURE TECH
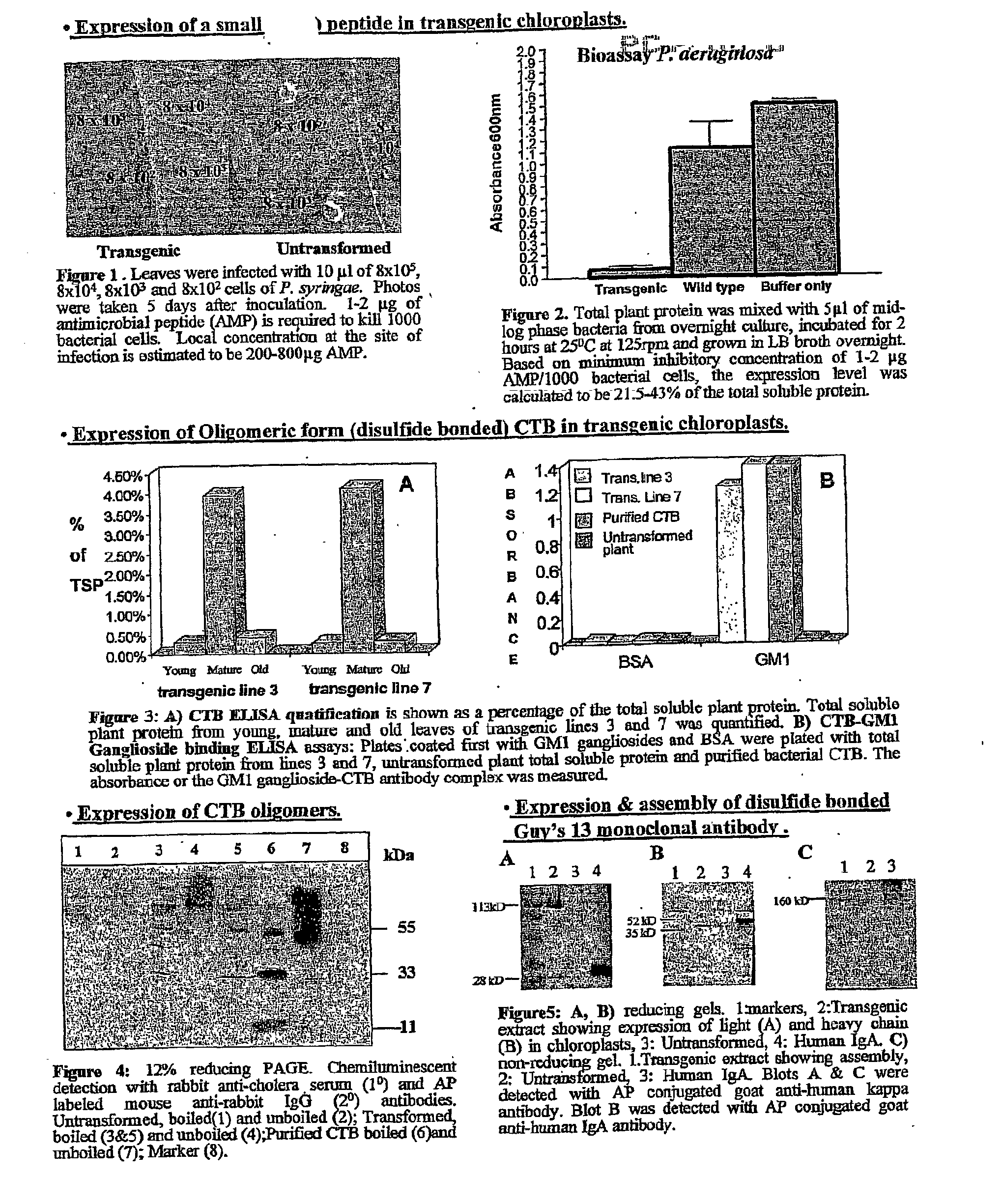
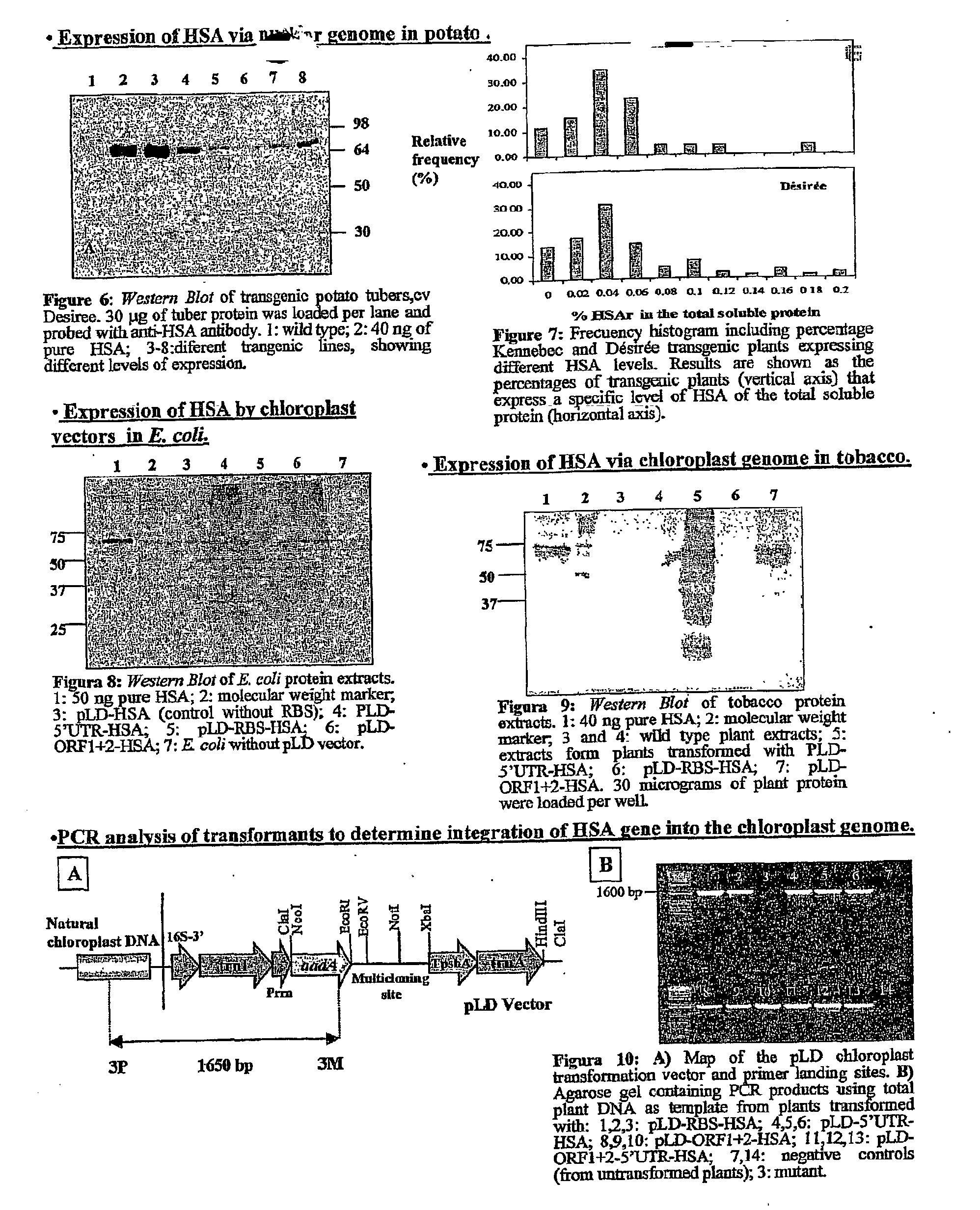
Pharmaceutical proteins, human therapeutics, human serum albumin, insulin, native cholera toxic b submitted on transgenic plastids
InactiveUS20030204864A1Eliminate needLarge biomassBiocidePeptide/protein ingredientsEscherichia coliInsulin-like growth factor
Transgenic chloroplast technology could provide a viable solution to the production of Insulin-like Growth Factor I (IGF-I), Human Serum Albumin (HSA), or interferons (IFN) because of hyper-expression capabilities, ability to fold and process eukaryotic proteins with disulfide bridges (thereby eliminating the need for expensive post-purification processing). Tobacco is an ideal choice because of its large biomass, ease of scale-up (million seeds per plant), genetic manipulation and impending need to explore alternate uses for this hazardous crop. Therefore, all three human proteins will be expressed as follows: a) Develop recombinant DNA vectors for enhanced expression via tobacco chloroplast genomes b) generate transgenic plants c) characterize transgenic expression of proteins or fusion proteins using molecular and biochemical methods d) large scale purification of therapeutic proteins from transgenic tobacco and comparison of current purification / processing methods in E. coli or yeast e) Characterization and comparison of therapeutic proteins (yield, purity, functionality) produced in yeast or E. coli with transgenic tobacco f) animal testing and pre-clinical trials for effectiveness of the therapeutic proteins. Mass production of affordable vaccines can be achieved by genetically engineering plants to produce recombinant proteins that are candidate vaccine antigens. The B subunits of Enteroxigenic E. coli (LTB) and cholera toxin of Vibrio cholerae (CTB) are examples of such antigens. When the native LTB gene was expressed via the tobacco nuclear genome, LTB accumulated at levels less than 0.01% of the total soluble leaf protein. Production of effective levels of LTB in plants, required extensive codon modification. Amplification of an unmodified CTB coding sequence in chloroplasts, up to 10,000 copies per cell, resulted in the accumulation of up to 4.1% of total soluble tobacco leaf protein as oligomers (about 410 fold higher expression levels than that of the unmodified LTB gene). PCR and Southern blot analyses confirmed stable integration of the CTB gene into the chloroplast genome. Western blot analysis showed that chloroplast synthesized CTB assembled into oligomers and was antigenically identical to purified native CTB. Also, GM1,-ganglioside binding assays confirmed that chloroplast synthesized CTB binds to the intestinal membrane receptor of cholera toxin, indicating correct folding and disulfide bond formation within the chloroplast. In contrast to stunted nuclear transgenic plants, chloroplast transgenic plants were morphologically indistinguishable from untransformed plants, when CTB was constitutively expressed. The introduced gene was stably inherited in the subsequent generation as confirmed by PCR and Southern blot analyses. Incrased production of an efficient transmucosal carrier molecule and delivery system, like CTB, in transgenic chloroplasts makes plant based oral vaccines and fusion proteins with CTB needing oral administration a much more practical approach.
Owner:AUBURN UNIV +1
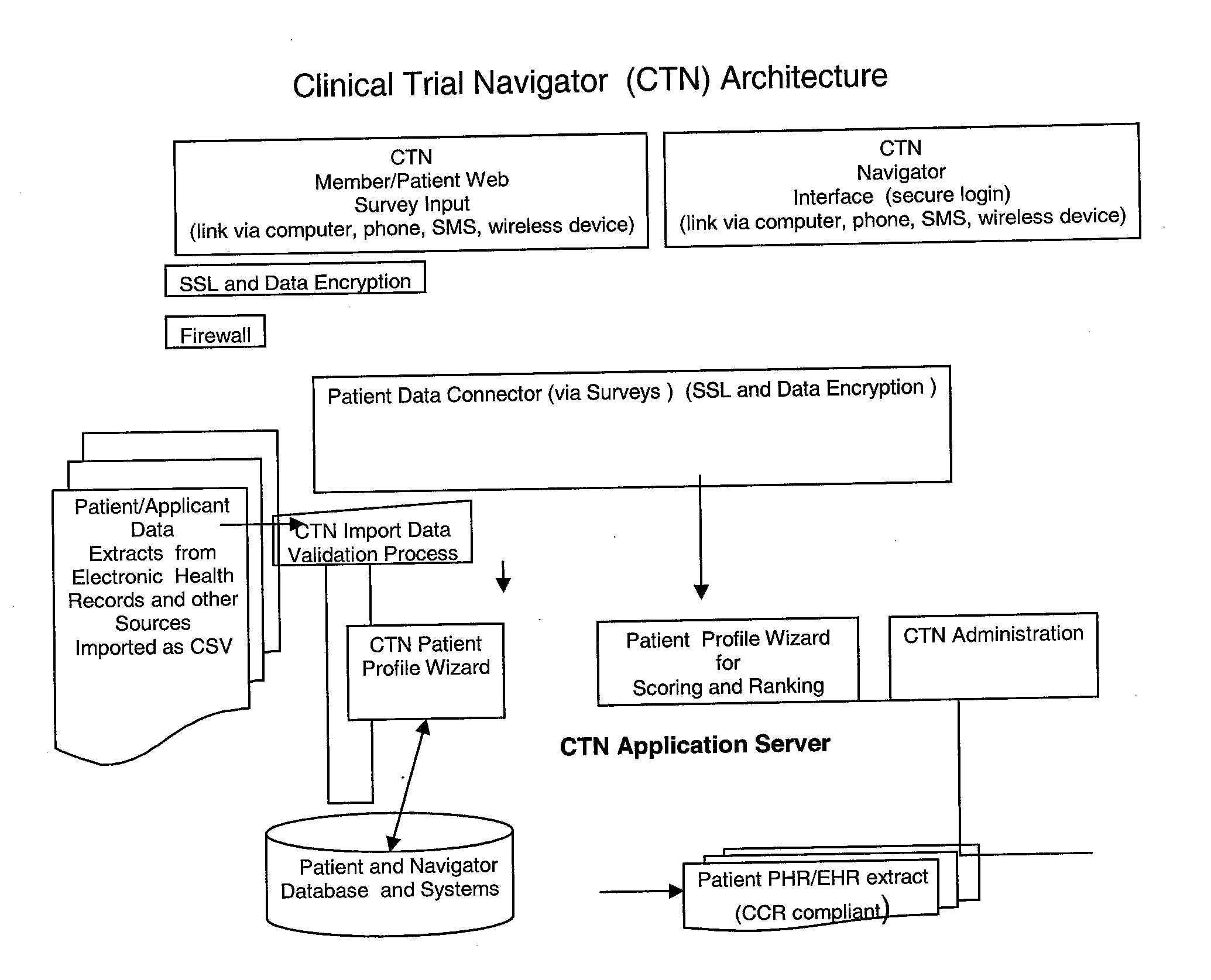
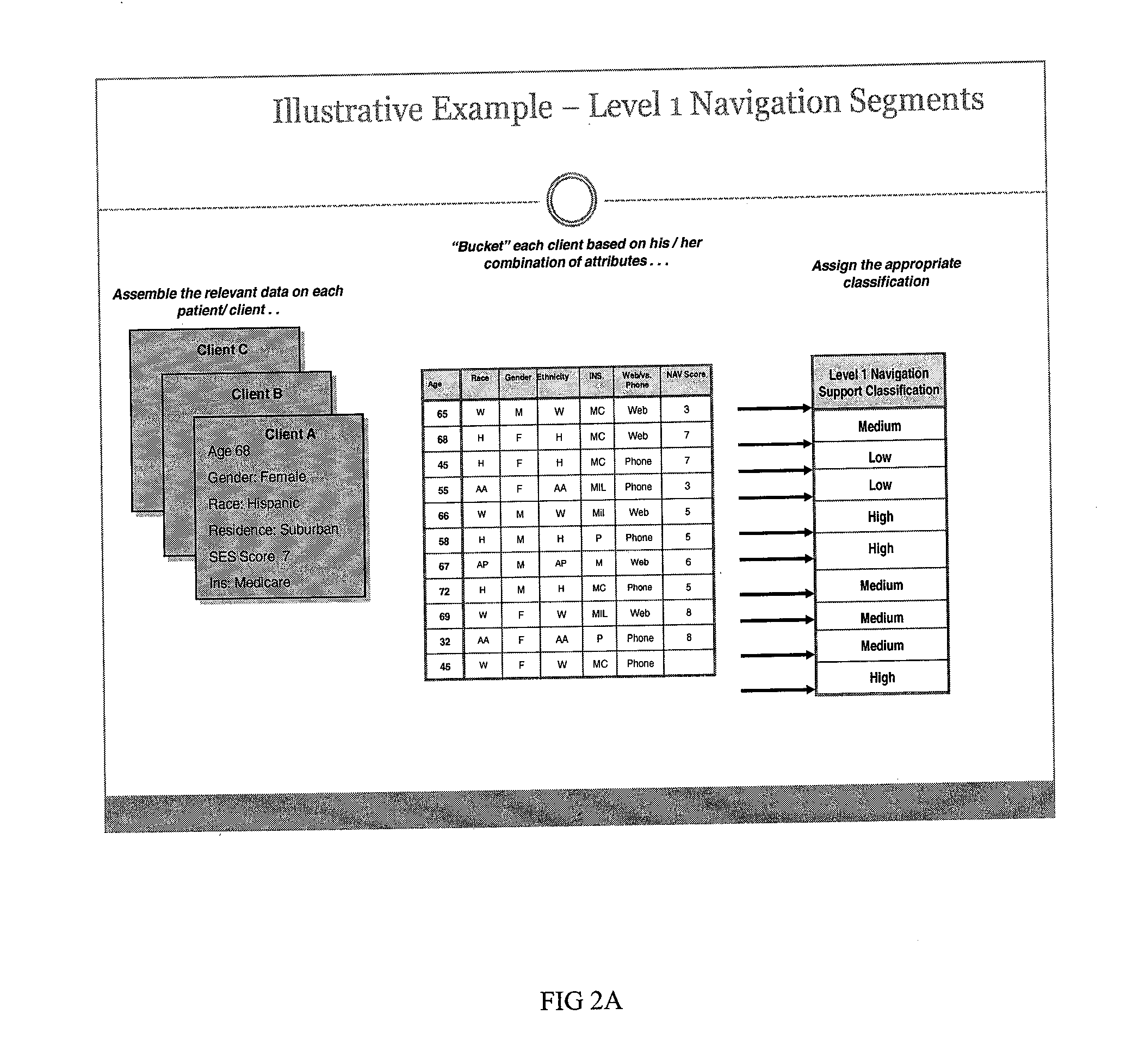
Clinical Trial Navigation Facilitator
InactiveUS20100332258A1Accelerate clinical trialIncreased recruitmentMarketingSpecial data processing applicationsTrial drugRanking
A method of improving clinical trial recruitment and retention includes creating patient trial scores based on ranking of patient traits or characteristics, creating patient clusters based on statistical similarity and allocating clinical trial navigation resources to patients groups based on need as indicated by cluster rankings.
Owner:TEXAS HEALTHCARE & BIOSCI INST
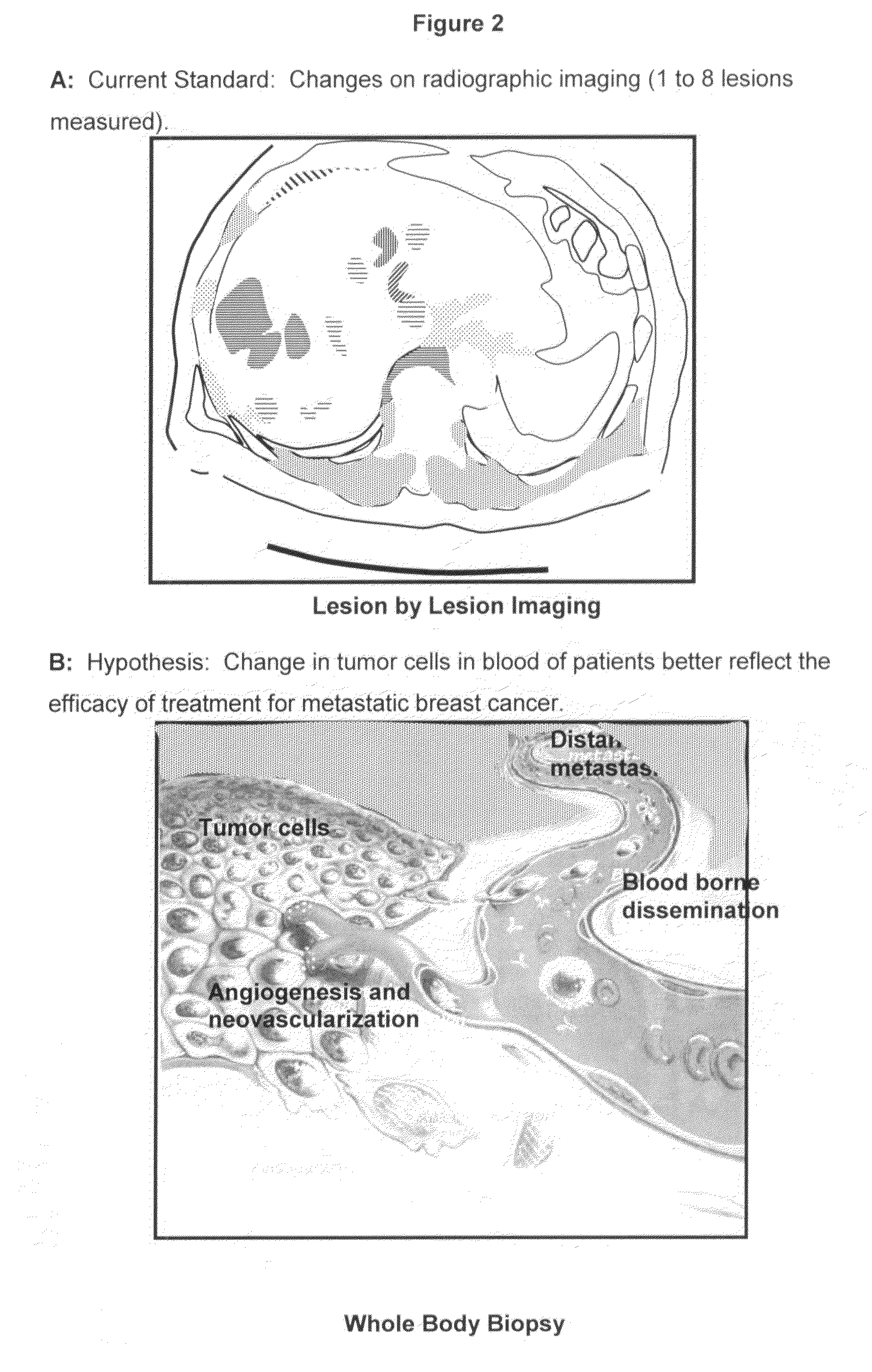
Method for predicting progression free and overall survival at each follow-up time point during therapy of metastatic breast cancer patients using circulating tumor cells
InactiveUS20090061456A1Less side effectsImprove the quality of lifeDisease diagnosisBiological testingOncologyDisease progression
A cancer test having prognostic utility in predicting time to disease progression, overall survival, and response to therapy in patients with MBC based upon the presence and number of CTC's. The Cell Spotter® System is used to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. The mean time to survival of patients depended upon a threshold number of 5 CTC's per 7.5 ml of blood. Detection of CTC's in metastatic cancer represents a novel prognostic factor in patients with metastatic cancers, suggests a biological role for the presence of tumor cells in the blood, and indicates that the detection of CTC's could be considered an appropriate surrogate marker for prospective therapeutic clinical trials.
Owner:VERIDEX LCC
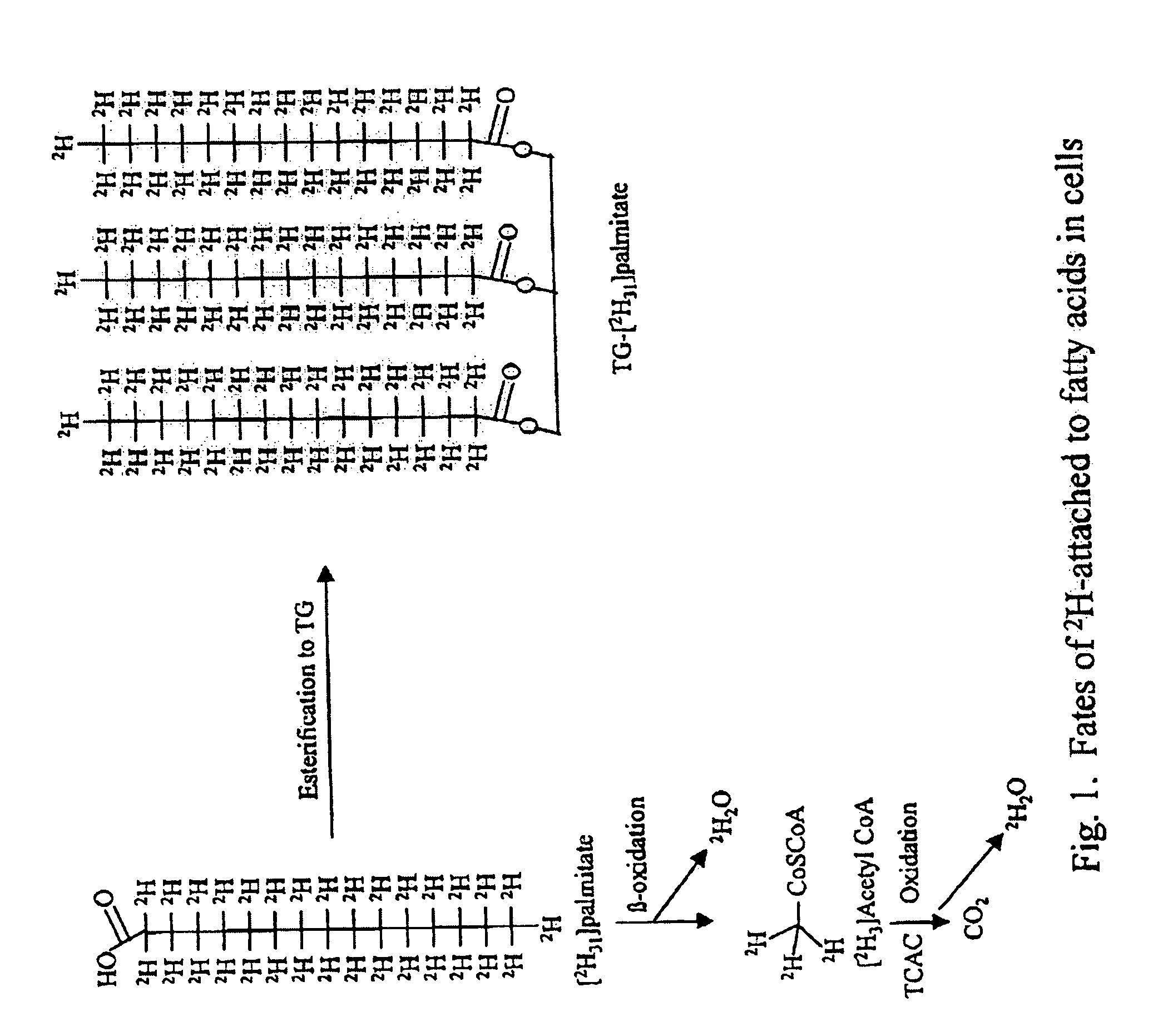
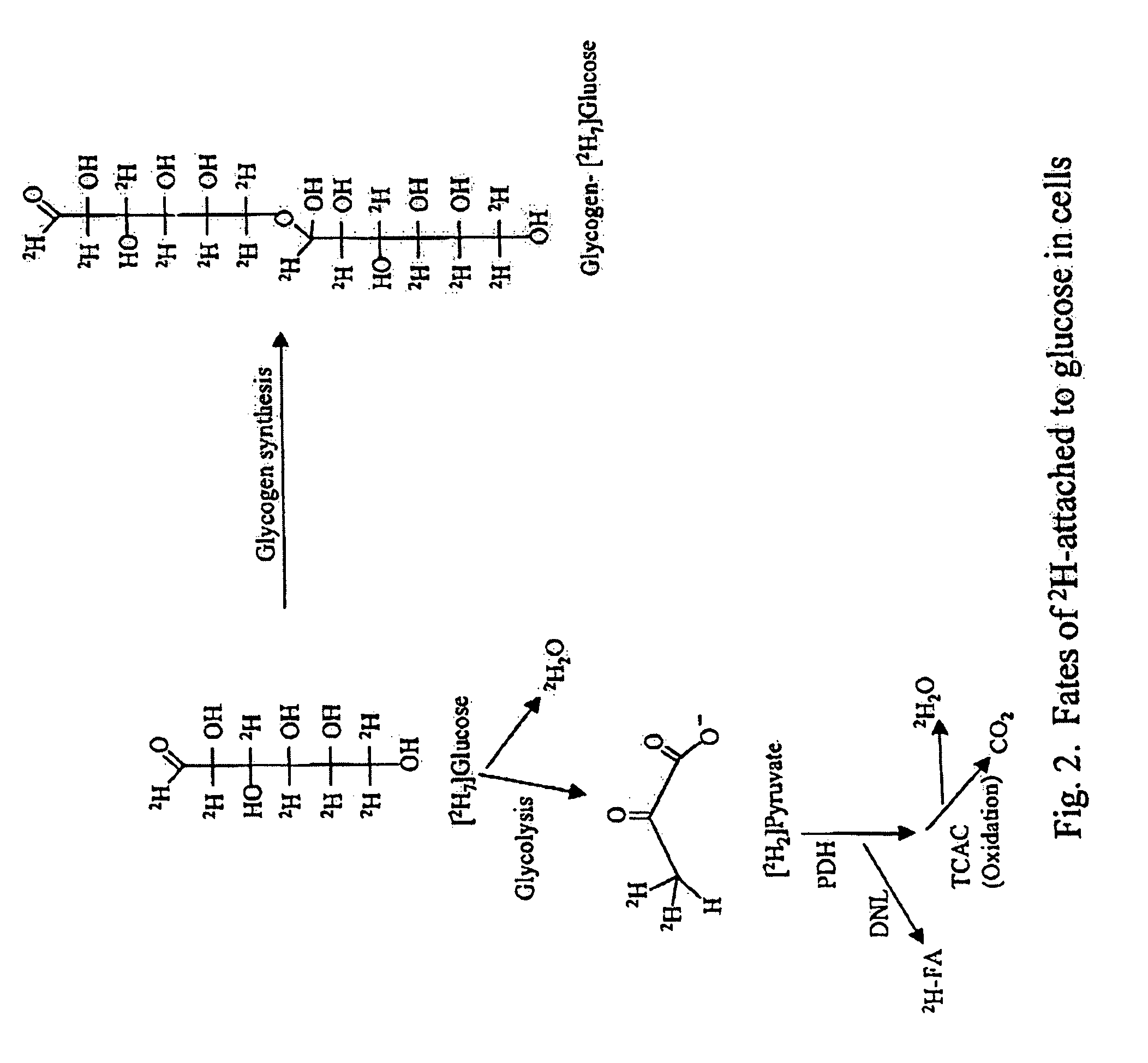
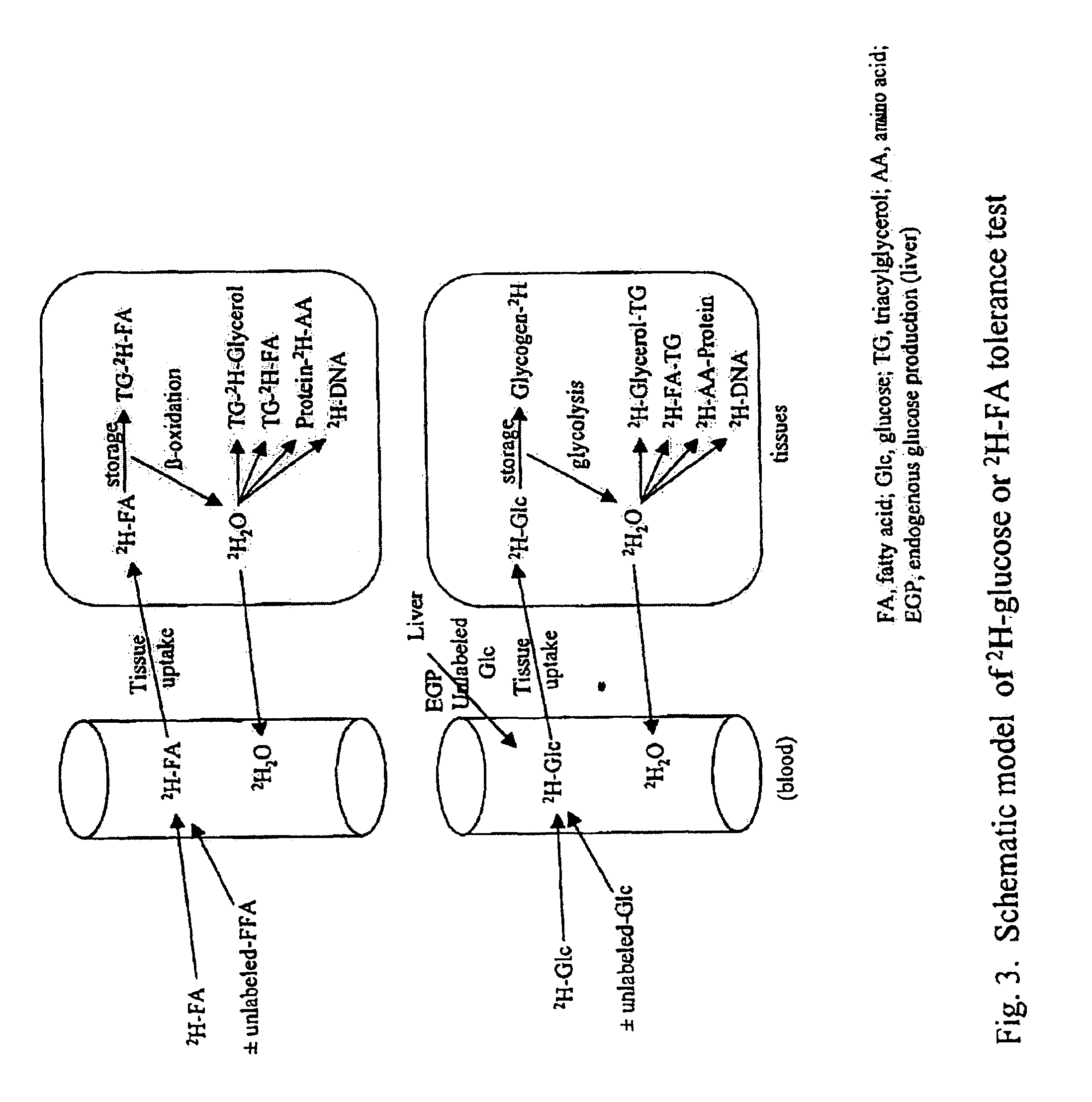
Methods for determining the metabolism of sugars and fats in an individual
ActiveUS7504233B2Compounds screening/testingIn-vivo radioactive preparationsTrial drugPreclinical testing
Provided herein are methods for determining the metabolism of one or more sugars and / or fatty acids, and applications thereof. Such applications include determining the rate of glycogen synthesis and glycolysis, which are believed to be early markers for predicting elevated risk of diabetes and cardiovascular disease. Other applications include methods for screening drugs that effect sugar and / or fatty acid metabolism. The methods are useful for at least partially characterizing drugs for desirable or undesirable (toxic) characteristics. Drugs that are at least partially characterized using the methods of the invention can then be further developed in pre-clinical testing and clinical trials. Such drugs may be found to be useful in treating obesity, diabetes, cardiovascular disease, and other disorders of metabolism.
Owner:RGT UNIV OF CALIFORNIA
Micelle encapsulation of therapeutic agents
ActiveUS20110076308A1Effective dissolutionToxic reductionBiocideDispersion deliveryLarge doseActive agent
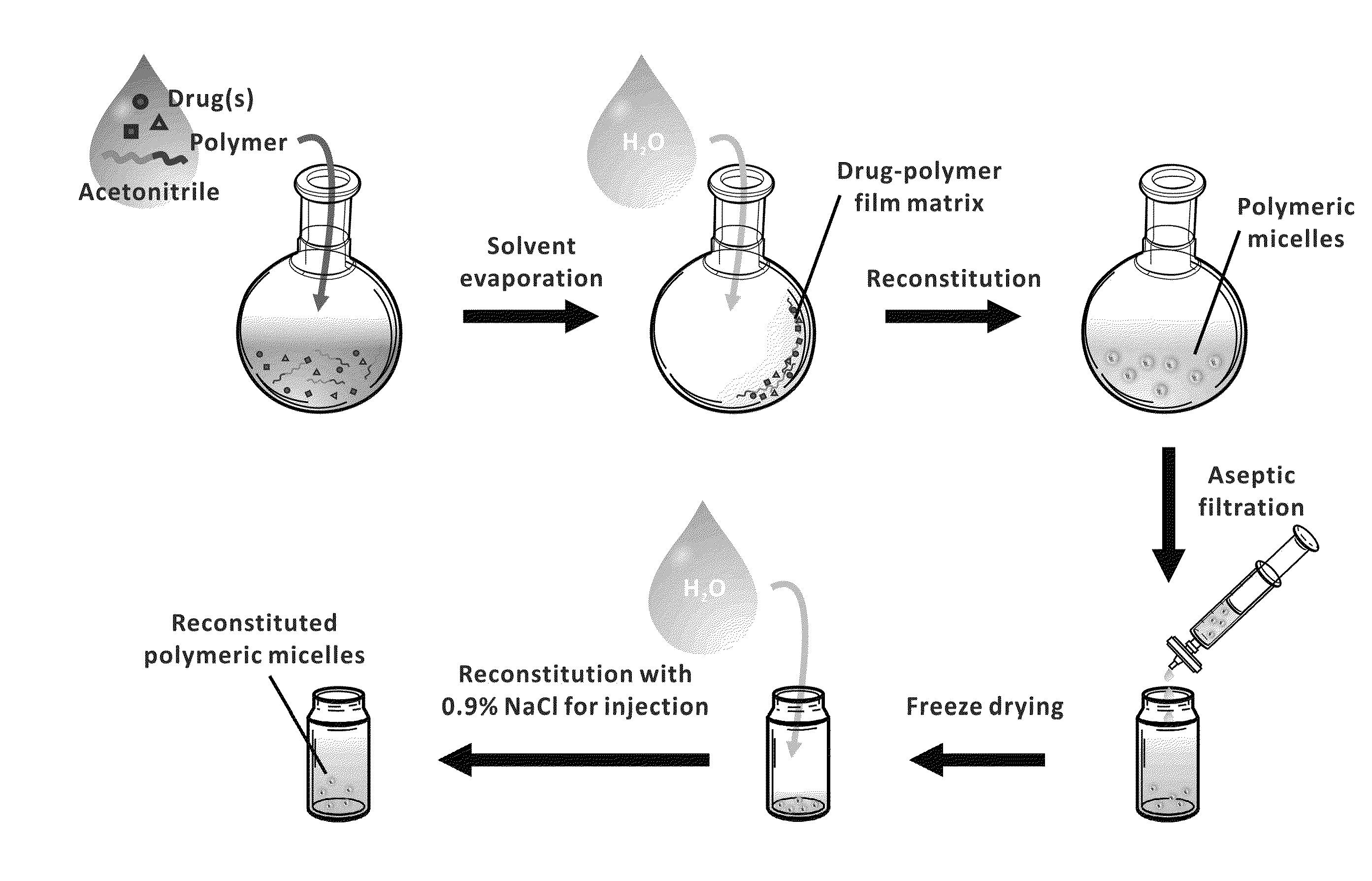
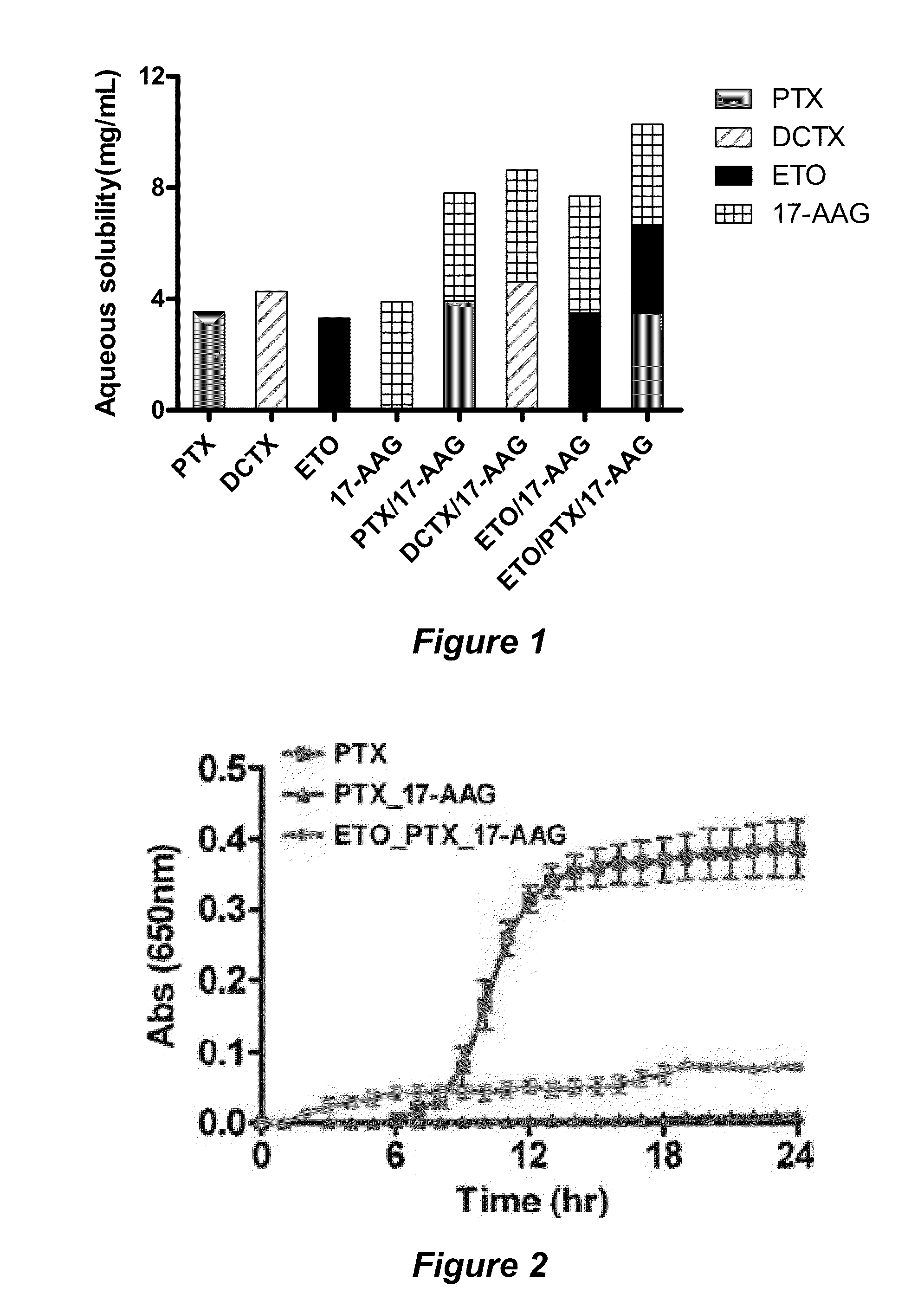
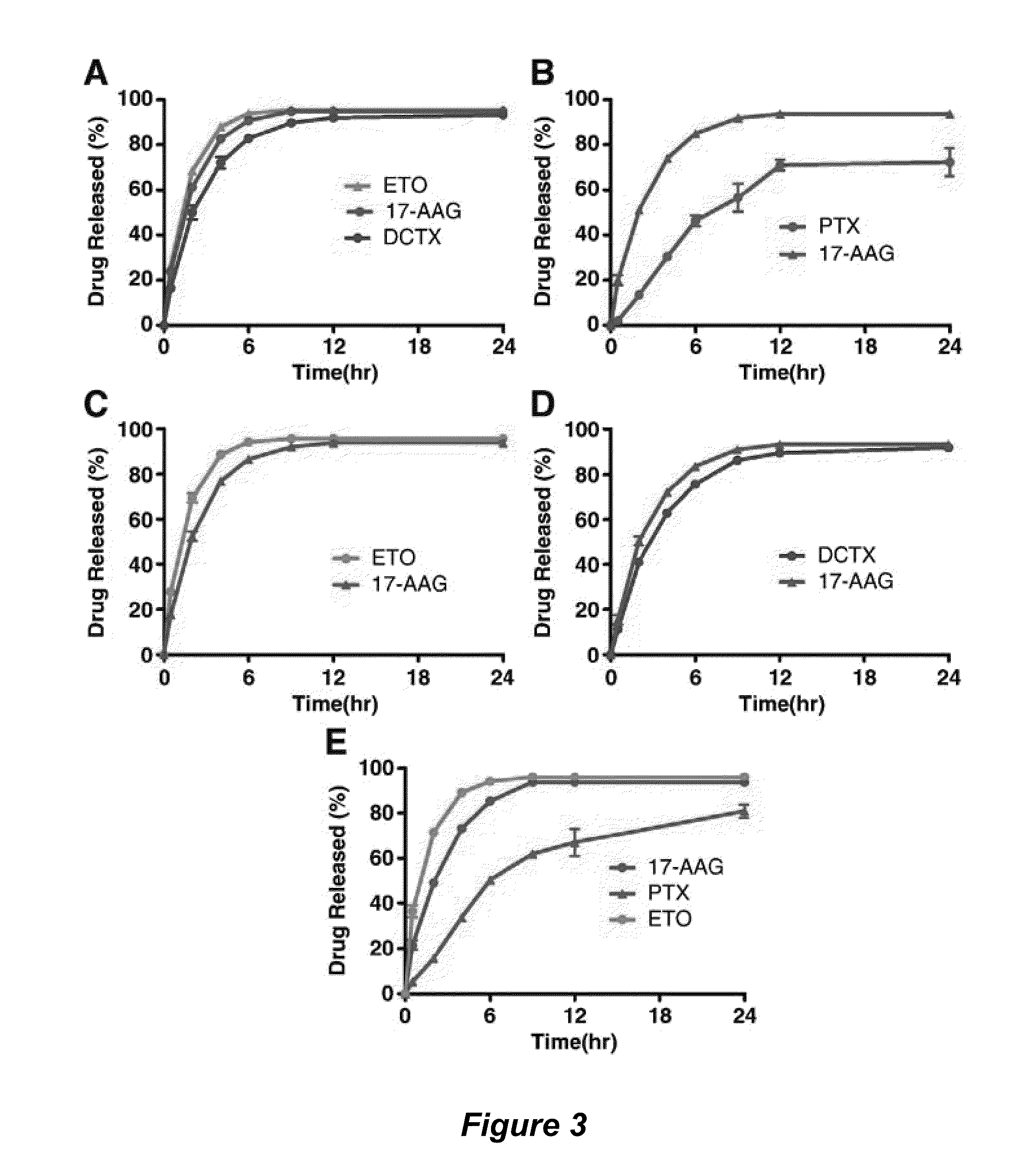
The invention provides active agents, such as paclitaxel, rapamycin, or 17-AAG, encapsulated by safe poly(ethylene glycol)-block-poly(lactic acid) (“PEG-b-PLA”) micelles. The compositions provide effective solubilization of drug combinations, such as paclitaxel, rapamycin, and 17-AAG, as well as others described herein. A significant advantage of PEG-b-PLA as a carrier is that it is less toxic than Cremophor® EL or DMSO, which are used in currently known compositions. Additionally, PEG-b-PLA micelles are easier to handle than DMSO and they do not possess a foul odor, which is a problem with formulations currently in clinical trials. Accordingly, the invention provides stable and biocompatible drug formulations that improve bioavailabilty without causing toxicity. It was also found that larger doses of individual drugs in micelle formulations can be administered compared to non-micelle formulations.
Owner:WISCONSIN ALUMNI RES FOUND
Unit dose compliance monitoring and reporting device and system
InactiveUS20090065522A1Reduce pressureEffective mergerDrug and medicationsCoin-freed apparatus detailsData displayCompliance Monitoring
A unit dose medication compliance monitoring and reporting apparatus and system (100) that includes a dispenser shell (120) formed with dose compartments (130). A retainer sheet (150) affixed to the shell (120) seals each compartment (130) and partially bursts upon dispensing. A sensor network and monitoring and reporting circuitry (160, 170) records dispensing times and determines an average time interval, which can be reported with other data on an integral data display (190). The system (100) can thereby monitor and report patient compliance with prescription regimens. Additional data can be recorded and displayed for augmented patient compliance assistance and analysis, which data can include customized informational messages, telephone and other patient support contact information, unit doses dispensed and remaining, reminder alarms, identification data, prescription regimens, among other data. In myriad variations and alternative preferred configurations, the devices and systems have demonstrated efficacy in minimalist to complex monitoring and reporting applications ranging from routine prescription monitoring to detailed clinical trial assessments.
Owner:BENOUALI NADIR
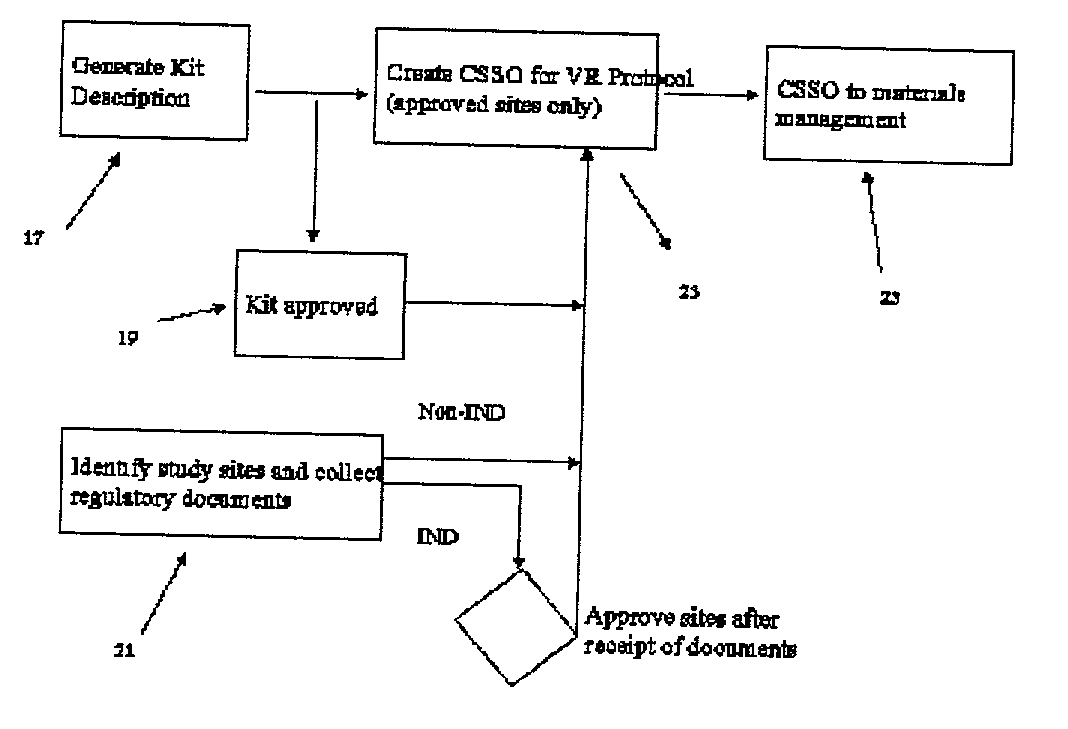
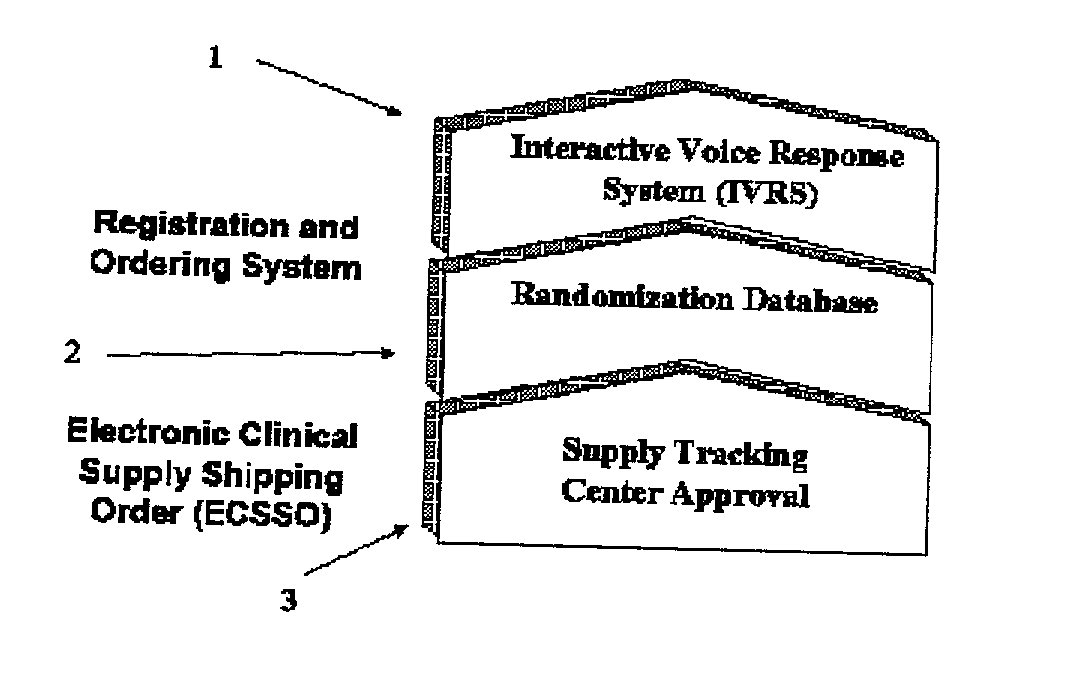
Registration and ordering system
InactiveUS20020029155A1Small sample sizeSave significant re-programming effortDrug and medicationsAnimal feeding devicesTrial drugBlinded study
This invention relates to a process and system for managing blinded studies such as medical clinical trials. It is made up of three components a supply tracing approval module, a module for randomizing supplies and an interactive voice or online response system for requesting materials.
Owner:SMITHKLINE BECKMAN CORP
Functional probiotic solid beverage capable of maintaining intestinal health and improving immunity and preparation method thereof
InactiveCN109329419AKeep healthyImprove immunityMilk preparationIsomaltooligosaccharideLactobacillus fermentum
The present invention belongs to the technical field of functional foods and relates to a functional probiotic solid beverage capable of maintaining intestinal health and improving immunity and a preparation method thereof. The functional probiotic solid beverage is composed of the following components in parts by weight: 20-30 parts of sorbitol, 10-30 parts of skim milk powder, 10-20 parts of stachyose, 1-20 parts of resistant dextrin, 1-15 parts of whole milk powder, 1-15 parts of isomaltitol, 1-10 parts of isomaltooligosaccharide, 1-10 parts of oligofructose, 0.01-1 part of bifidobacteriumlongum powder, 0.01-1 part of bifidobacterium lactis powder, 0.01-1 part of lactobacillus acidophilus powder, 0.01-1 part of lactobacillus rhamnosus powder MP-108, 0.01-1 part of lactobacillus paracasei powder and 0.01-1 part of lactobacillus fermentum powder. The present invention also provides the preparation method of the functional probiotic solid beverage. The functional probiotic solid beverage is prepared by using a modern technology, convenient to carry and high in safety. Clinical trials show that compared with the probiotic group and the prebiotic group, the functional probiotic solid beverage has remarkable effects on maintaining the intestinal health and enhancing the immunity.
Owner:吉林标普生物科技有限公司
Methods of Determining Patient Response By Measurement of HER-2 Expression
Methods are provided for determining or otherwise assessing the response of a patient to treatment, in particular, to cancer treatment. The methods include the analysis of samples for the presence or the absence of HER2 markers alone or in conjunction with other biomarkers, such as HER3 markers. In certain examples, the probable time to progression can be determined by first determining HER2 positive patients and then further stratifying by using the presence or the absence of a second biomarker (e.g, HER3 markers). In addition, the data can be used to track a patient's response to a treatment regimen, assessing the expected success of treating a patient using a particular regiment, determining the effects of a treatment regiment or for categorizing a patient in order to create a homogenous group for a clinical trial.
Owner:LAB OF AMERICA HLDG
Traditional Chinese medicinal external-use liniment for muscle and bone ache
ActiveCN102488869ASimple preparation processLow costAnthropod material medical ingredientsAntipyreticGleditsia triacanthosRhizome
The invention relates to a traditional Chinese medicinal external-use liniment for muscle and bone ache. The liniment is prepared by steps of crushing raw materials into coarse powders and immersing the coarse powders by medicinal alcohol, wherein the raw materials contain Jasminum lanceolarium, Chinese honey locust thorn, Chinese prickly ash, rhizoma atractylodis, wild aconite root, Aconitum carmichaeli, pawpaw, cudweed herb, achyranthes root, Sargentodoxa cuneata, bark of ash, Ligusticum wallichii, notopterygium root, dahurian angelica root, clematis root, levisticum, speranskia herb, corydalis tuber, piper wallichii, ramulus loranthi, Cortex Periplocae, turmeric, sparganium, Curcuma zedoaria, Dalbergia Odorifera, pseudo-ginseng, Dipsacus asperoides, safflower, cassia twig, ramulus mori, Chinese cassia tree, radix paeoniae rubrathe, banksia rose, Peach Seed, spatholobus stem, ragwort, Dictamnus dasycarpus Turcz, Japanese eupatorium, Chinese angelica, ground beetle, cibotium rhizome,Asarum Herb, Pine Nodular Branch, common cnidium fruit, Caulis Erycibes, senecio, dandelion, morinda officinalis and Smilax glabra. The liniment has efficacy of invigorate blood circulation, freeing the channels, dispelling wind, dehumidifying, activating blood stasis, detoxicating, reducing swelling and resolving mass, is used for spinal column, shoulder and muscle and bone joint ache, is suitable for a wide range of symptoms, has good curative effect, and is convenient to preserve, carry and use. It is proved through clinical trials that the cure rate reaches more than 80% and the effectiverate reaches 100% after the liniment is used.
Owner:湖南苑医堂中医药开发有限公司
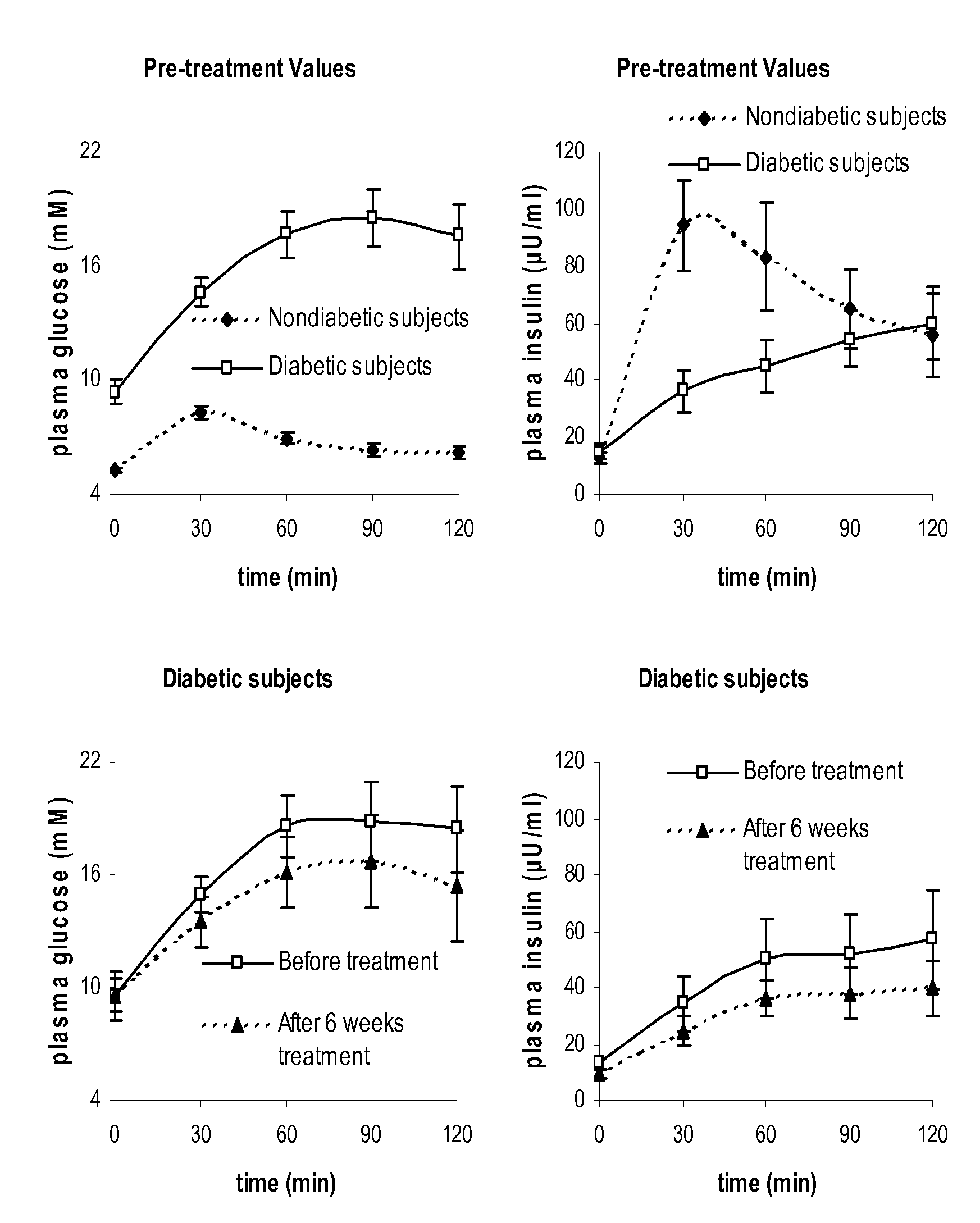
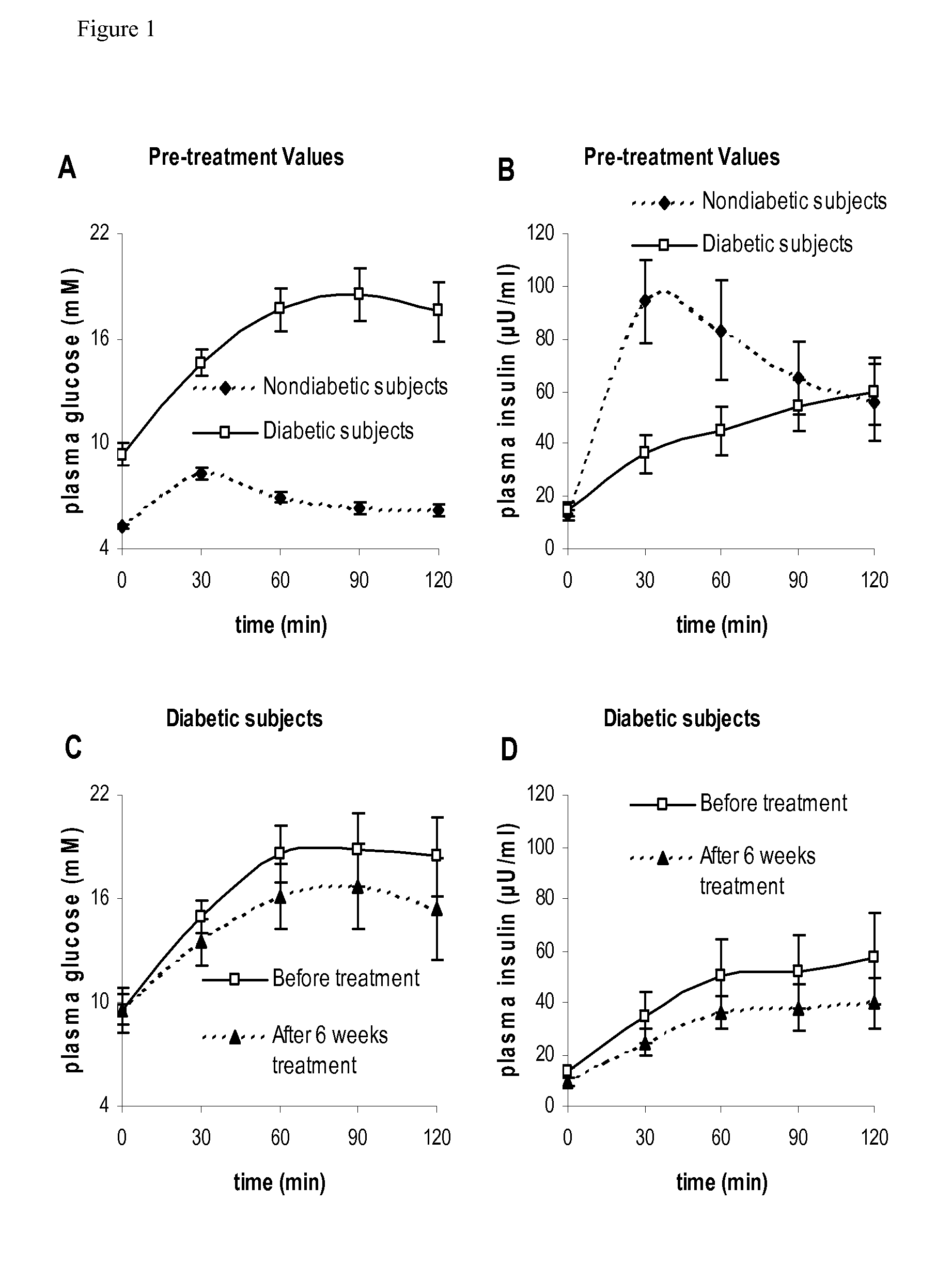
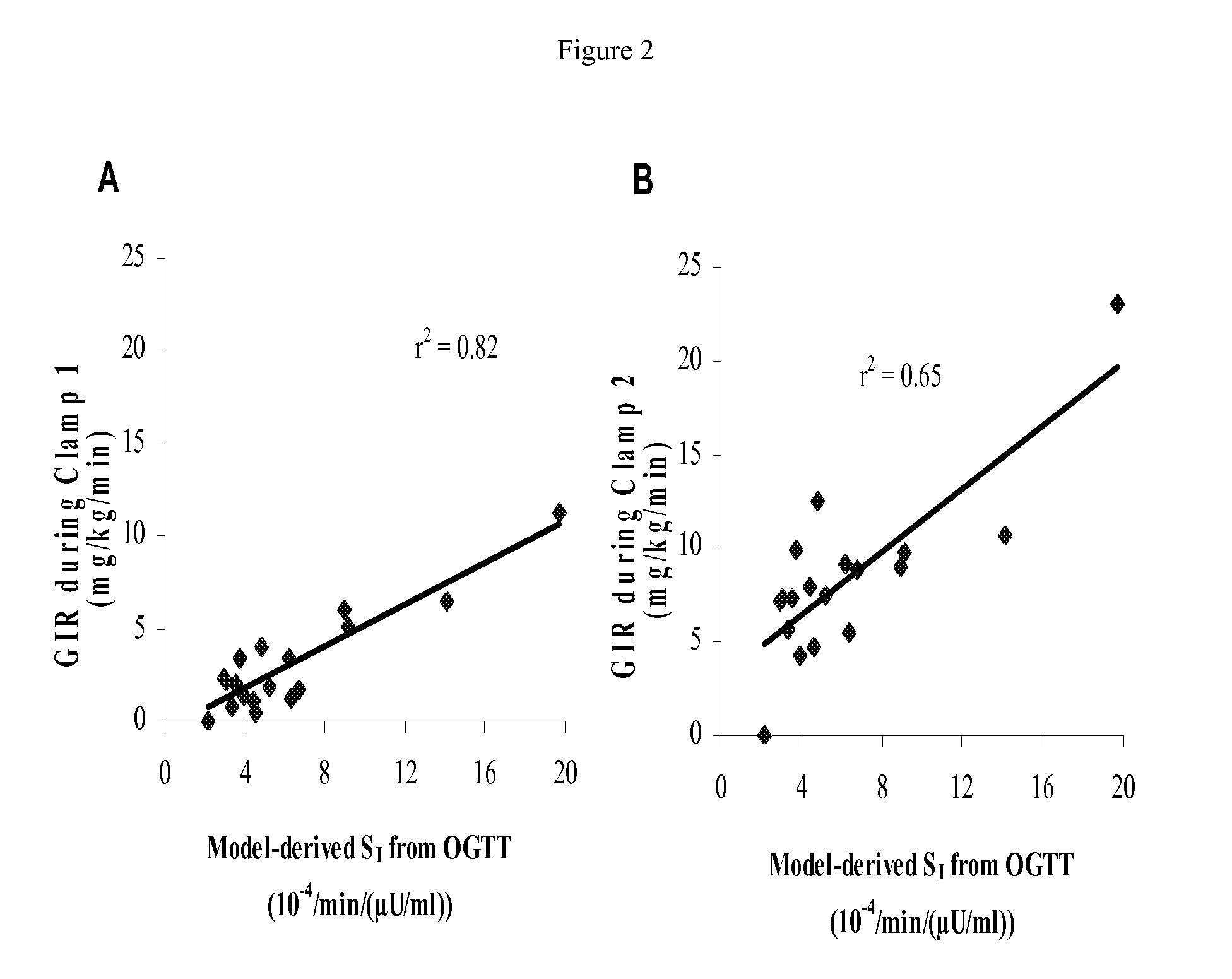
Method for Determining Insulin Sensitivity and Glucose Absorption
InactiveUS20080262745A1Large-scale clinical trialsDifference in insulin sensitivityHealth-index calculationMicrobiological testing/measurementPresent methodPatient type
The present invention encompasses a model-based method for determining insulin sensitivity and glucose absorption from oral glucose tolerance tests or mixed meals. The present invention has several advantages over current methods. The technique requires about four to six blood samples taken over about two to three hours following glucose ingestion and is therefore applicable to large-scale clinical trials. The analysis involves a reduced version of the classical minimal model, a method for describing glucose absorption using only two parameters, and an integral approach enabling the parameters to be obtained using simple algebra. The present method robustly identifies differences in insulin sensitivity in different patient types as well as improvements in insulin sensitivity arising from pharmaceutic therapy. In addition, insulin sensitivity measurements obtained with the present method are highly correlated with results from hyperinsulinemic clamps (r2>0.8). This method is therefore a practical and robust method for determining insulin sensitivity under physiologic conditions.
Owner:VERIDEX LCC
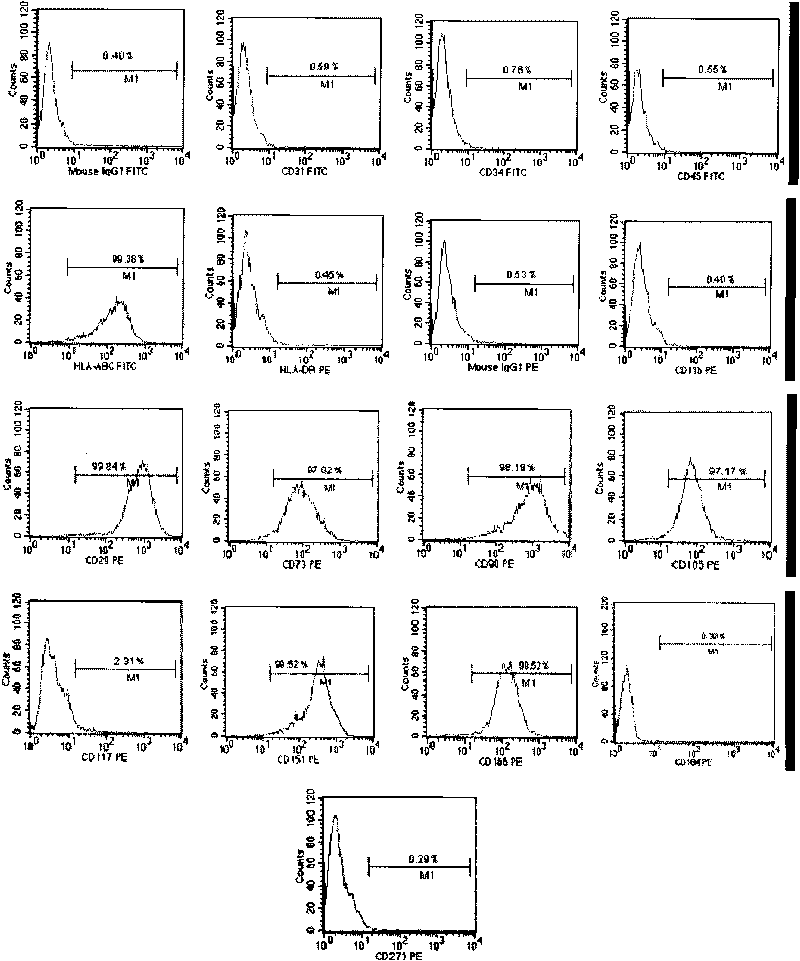
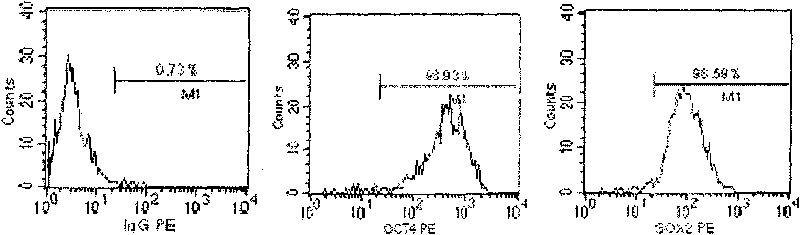
Sub totipotential stem cell and preparation method and application thereof
The invention discloses a method for preparing a population of?human pluripotent stem cells and the application thereof. The preparation of stem cells is characterized by comprising the following steps: CD151<+>, CD31<->, Sox<2+> pluripotent stem cells are separated and collected from human umbilical cord and or placenta tissues; the cells adhere to grow in a culture vessel under a predetermined condition and expand through passage 20 or above to be still stable in gene expression. The population of cells of this invention do not form teratoma after injection into animals. The human pluripotent stem cells highly express CD151, OCT4 and Sox-2 as specific markers of embryonic stem cells, as well as specific markers of epidermic cells, endothelial cells, thrombocytes, dendritic cells, while lack expression of CD31, CD34, CD45 and HLA-II. The pluripotent stem cells are also characterized as being able to adhere to tissue culture plastic and having the potential to differentiate into three germ layers: endoderm, mesoderm and ectoderm. These pluripotent stem cells are able to be used as carrier cells of gene therapy and for the treatment of diseases caused by cell damage or cell aging. The present invention provides a method of isolating, purifying and culturally expanding of a population of human pluripotent stem cell for preparing the high purity injection preparation. The preparation of stem cells has a good therapeutic effect on the treatment of diseases caused by cell damage or cell aging in animal and human clinical trials. The preparation also has no toxic side effect and no immune rejection.
Owner:BEIJING HEALTH & BIOTECH (H&B) CO LTD
Pure traditional Chinese medicine preparation for treating hyperlipemia
InactiveCN101455791AWith promoting blood circulation and removing stasisReduce cholesterolPowder deliveryMetabolism disorderSalvia miltiorrhizaSide effect
The invention provides a pure traditional Chinese medicine preparation for treating hyperlipemia, and is prepared from the following traditional Chinese medicine raw materials: cassia seed, Alisma orientale, radix polygoni multiflori, cattail pollen, evening primrose, hawthorn, folium ginkgo, rhubarb, Viscum album, Salvia miltiorrhiza, apocynum, lotus leaf, polyporus lucidus, rhizoma gastrodiae, plantain, dried orange peel, medlar, achyranthes, radix bupleuri, medicated leaven, safflower, giant knotweed, and malt. The preparation is prepared by using traditional Chinese herbal medicines as raw materials through scientific prescription composing and repeated verification of clinical trial, and accords with the prior theory of the traditional Chinese medicine. After the medicines are combined, medicine effect has synergic action, and the pure traditional Chinese medicine preparation has functions of promoting blood circulation by removing blood stasis, reducing cholesterol, regulating and lowering blood fat, and preventing atherosclerosis. The preparation has the outstanding advantages of no toxic side effect, high radical cure efficiency, low treatment cost, and short treatment course, so the preparation has good social benefit after wide popularization.
Owner:朱新华
Medicament for treating burn by 'dry, moistening, dressing' three in one bandaging method
InactiveCN101439104ANot easy to seepPromote oxidationHeavy metal active ingredientsAnthropod material medical ingredientsForsythiaCoptis teeta
The invention relates to a medicine for treating burning by three-in-one combination bandaging strapping of 'dryness', 'moist', and 'dressing'. The medicine has the main characteristics that the medicine comprises medicinal powder, dressing and plaster, and is prepared by mainly using purple gromwell root, coptis teeta, bismuth subnitrate tablets, chloramphenicol tablet, rheum officinale, the dried rhizome of rehmannia, forsythia, the root of red-rooted salvia, safflower, angelica, borneol and the like according to according to a certain proportioning. Compared with the traditional treatment method, the medicine can moisten a burn sore surface when the burn sore surface is dry and can dry the burn sore surface when the burn sore surface is moist, can soften, oxidize in proper sequence, can clear away sores, naturally promotes skin so that low degree burn can fast heal and high degree burn does not need skin-grafting. The medicine proved by many cases of clinical trial has precise healing efficacy and high cure rate.
Owner:黄群才
Fungal fibrinolytic enzyme and cultivating method thereof
ActiveCN101503682AThrombolytic effect is goodNo obvious acute toxicityMicroorganism based processesEnzymesBiotechnologyCordyceps
The invention discloses a fungal fibrinolytic enzyme and a preparation method thereof. The fungal fibrinolytic enzyme is prepared by taking a Cordyceps militaris strain as a strain and performing liquid fermentation culture, separation and purification. The relative molecular weight of the fibrinolytic enzyme is about 21,000. The Cordyceps militaris fibrinolytic enzyme has good thrombolytic performance, is free from obvious acute toxicity, has prospects for clinical trial, development and application, and adds a new member to a rare fibrinolytic enzyme family. Particularly, a preferable fibrinolytic-enzyme Cordyceps militaris strain C.LSG-1 obtained through severe screening is rough in growth conditions, short in enzyme production cycle and capable of harvesting a large amount of Cordyceps militaris mycelium during enzyme production, and is the strain excellent in production performance. The Cordyceps militaris fibrinolytic enzyme is an extracellular enzyme which is particularly beneficial to subsequent separation and purification during preparation. The fungal fibrinolytic enzyme takes corn protein with extensive sources as a main culture medium, thereby having low cost for raw materials and providing a novel way for developing and utilizing the prior resources.
Owner:QIQIHAR UNIVERSITY
Kahalalide compounds for use in cancer therapy
InactiveUS20050054555A1Avoid toxicityClinical improvementBiocideOrganic active ingredientsTrial drugCancer therapy
Owner:PHARMA MAR U
Traditional Chinese medicine for treating scalp seborrheic dermatitis
InactiveCN104027468ADandruff subsidedRegression of lesionsDermatological disorderPlant ingredientsMedicinal herbsTrial drug
The invention relates to a traditional Chinese medicine for treating scalp seborrheic dermatitis. The traditional Chinese medicine comprises, by weight ratio, the following raw medical materials of 12 parts of screen grass, 9 parts of eggplant roots, 15 parts of millennium mugwort, 9 parts of cladonia alpestris, 6 parts of pellionia repens, 6 parts of flos sophorae and 12 parts of radix rehmanniae. The clinical trials prove that the traditional Chinese medicine can treat scalp seborrheic dermatitis safely and effectively.
Owner:俞天阳
Interactive technique for optimizing drug development from the pre-clinical phases through phase-IV
InactiveUS20040107084A1Chemical property predictionMedical simulationPatient populationPhases of clinical research
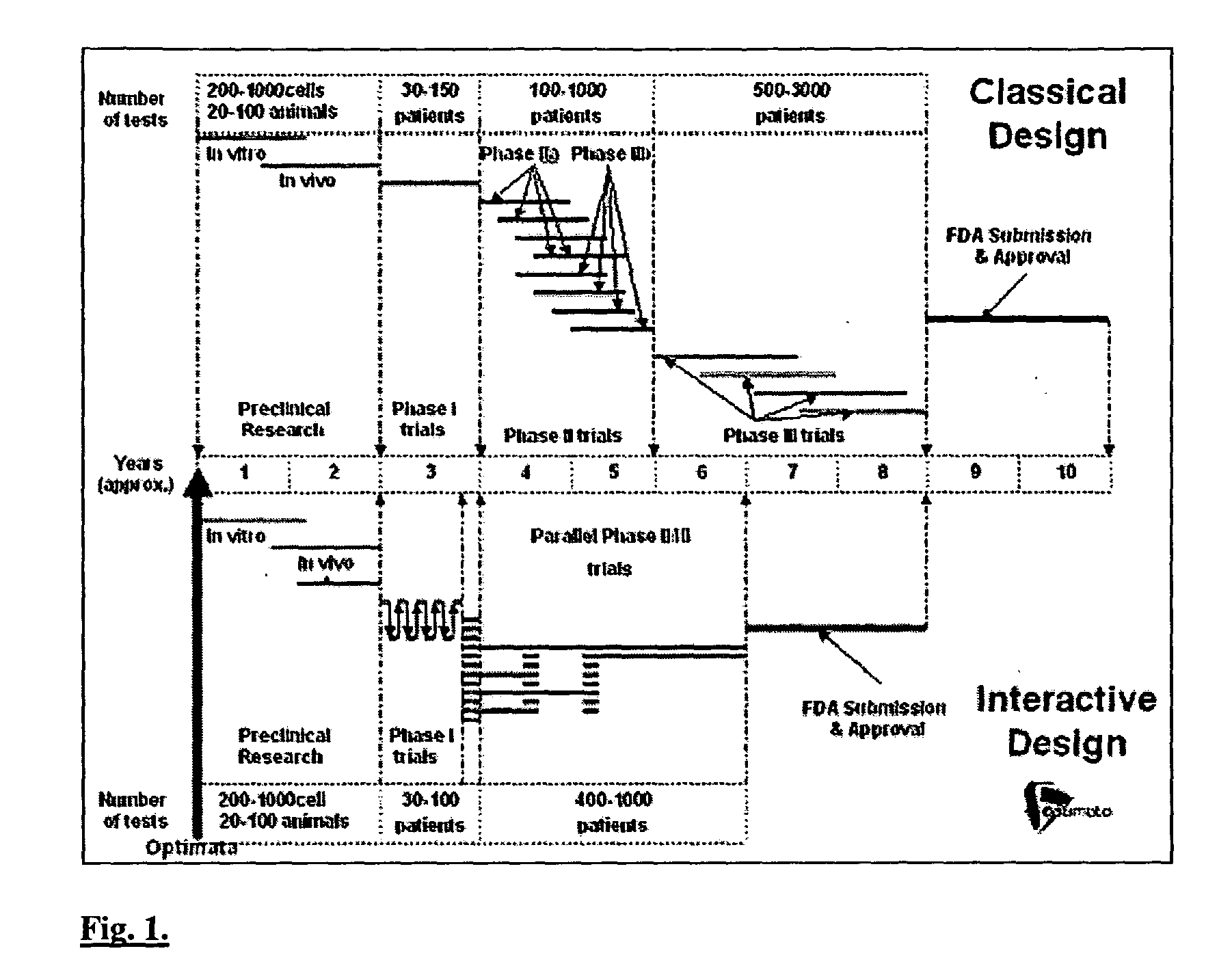
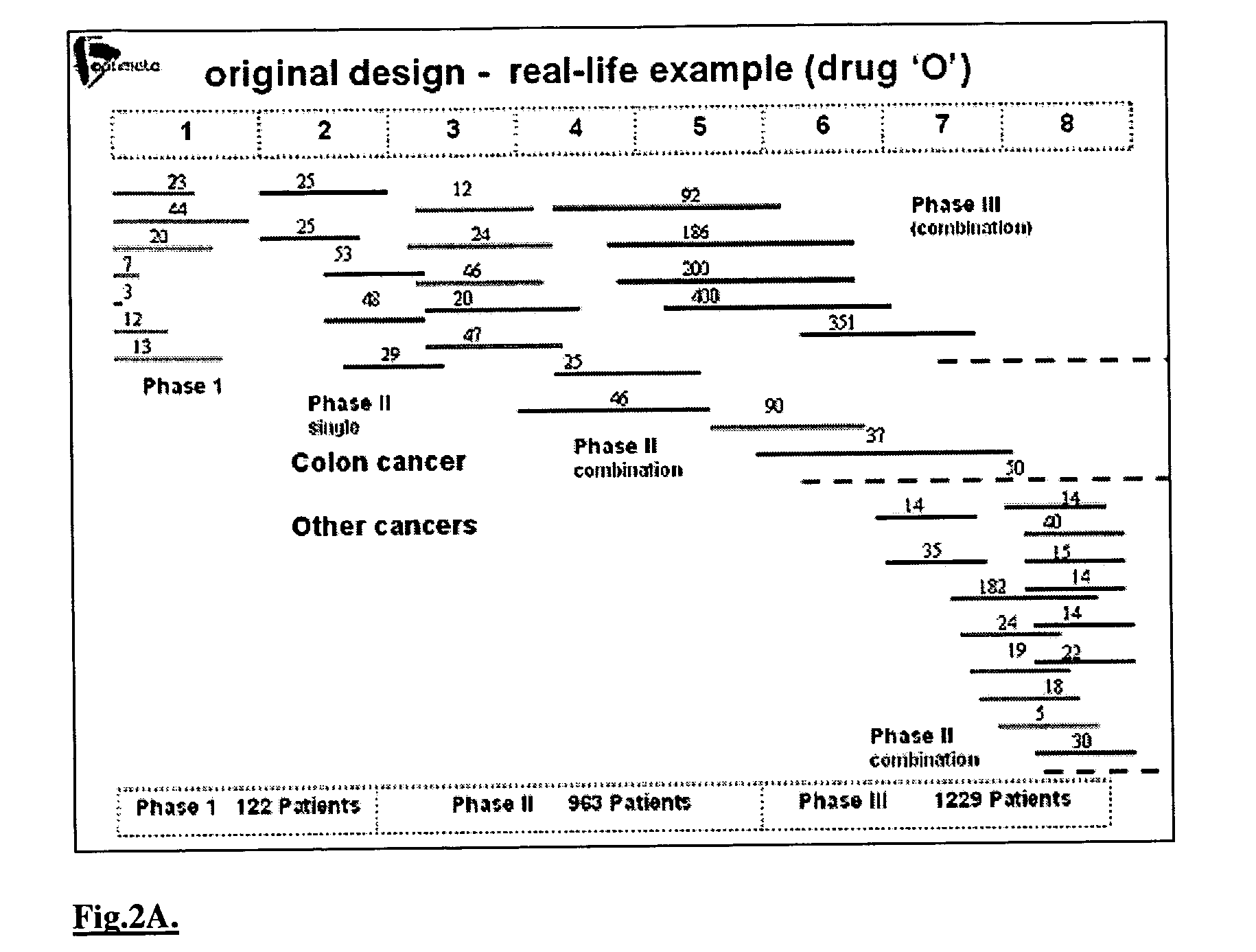
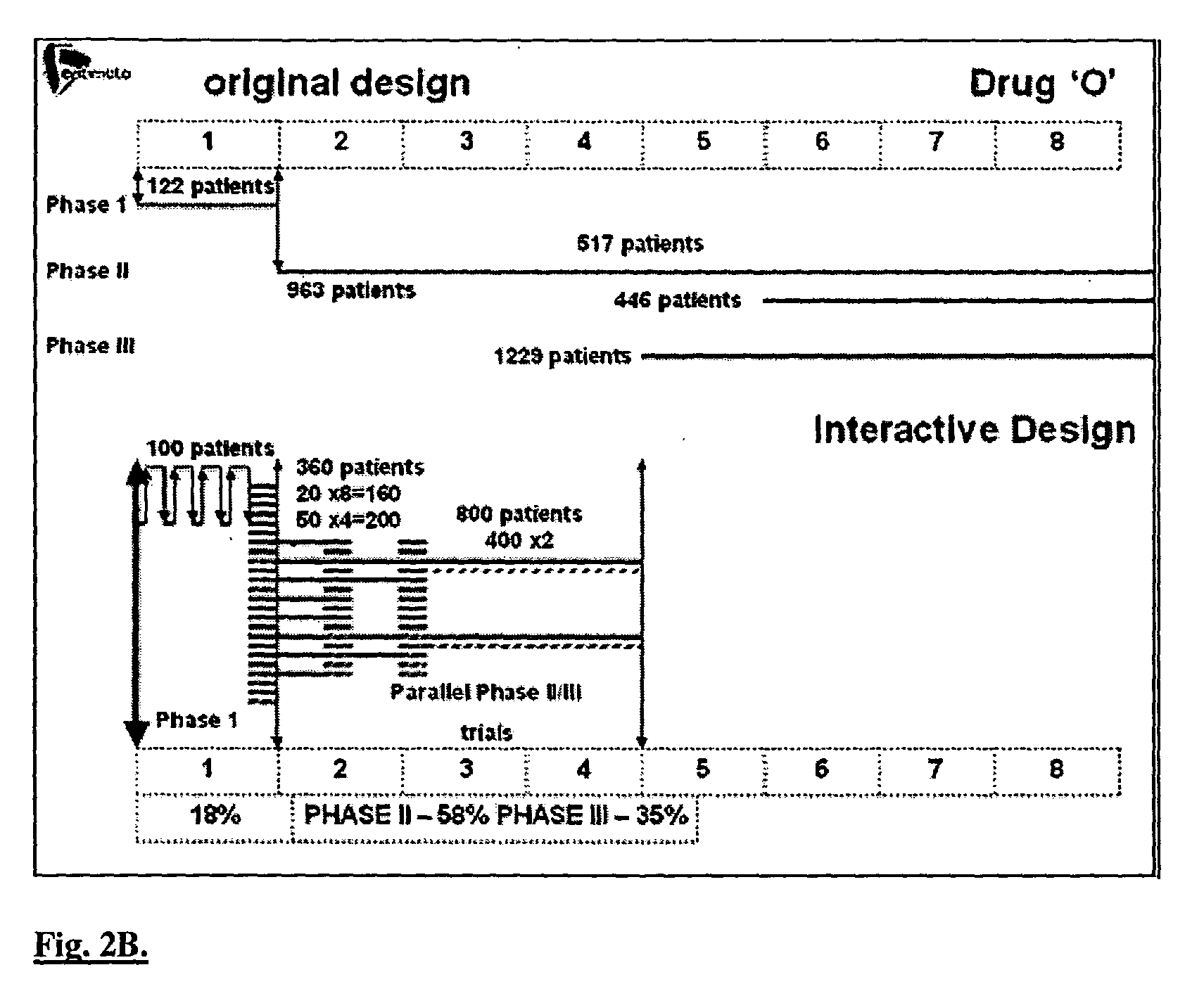
A method of performing interactive clinical trials for testing a new drug comprising performing a pre-clinical phase in which a computer model for pharmacokinetics and pharmacodynamics of the drug is created and adjusted based on in vitro studies and in vivo studies in animals. A phase I clinical research is performed in which a clinical trial on at least a single dose is performed in parallel with performing computer simulation studies using the computer model. The computer model is adjusted based on comparison of the results of the clinical research and the computer simulation. A maximal tolerated dose, minimum effective dose, and a recommended dose is determined based on the phase I clinical research in conjunction with the computer simulations. The drug is checked for cumulative effects and providing this information to the computer model. Multiple simulations are performed using the computer model with different doses and dosing intervals. An optimal protocol is determined for the most responsive patient populations and indications for a phase II clinical trial. Phase II clinical trial is performed where a number of small scale clinical trials are performed in parallel based on results of the above. The interim results are analyzed to choose the most promising regimens for continued clinical trials. Phase III clinical research is performed for chosen indications by chosen protocols. Phase IV studies are performed for post-marketing subpopulation analysis and long term product safety assessment.
Owner:OPTIMATA
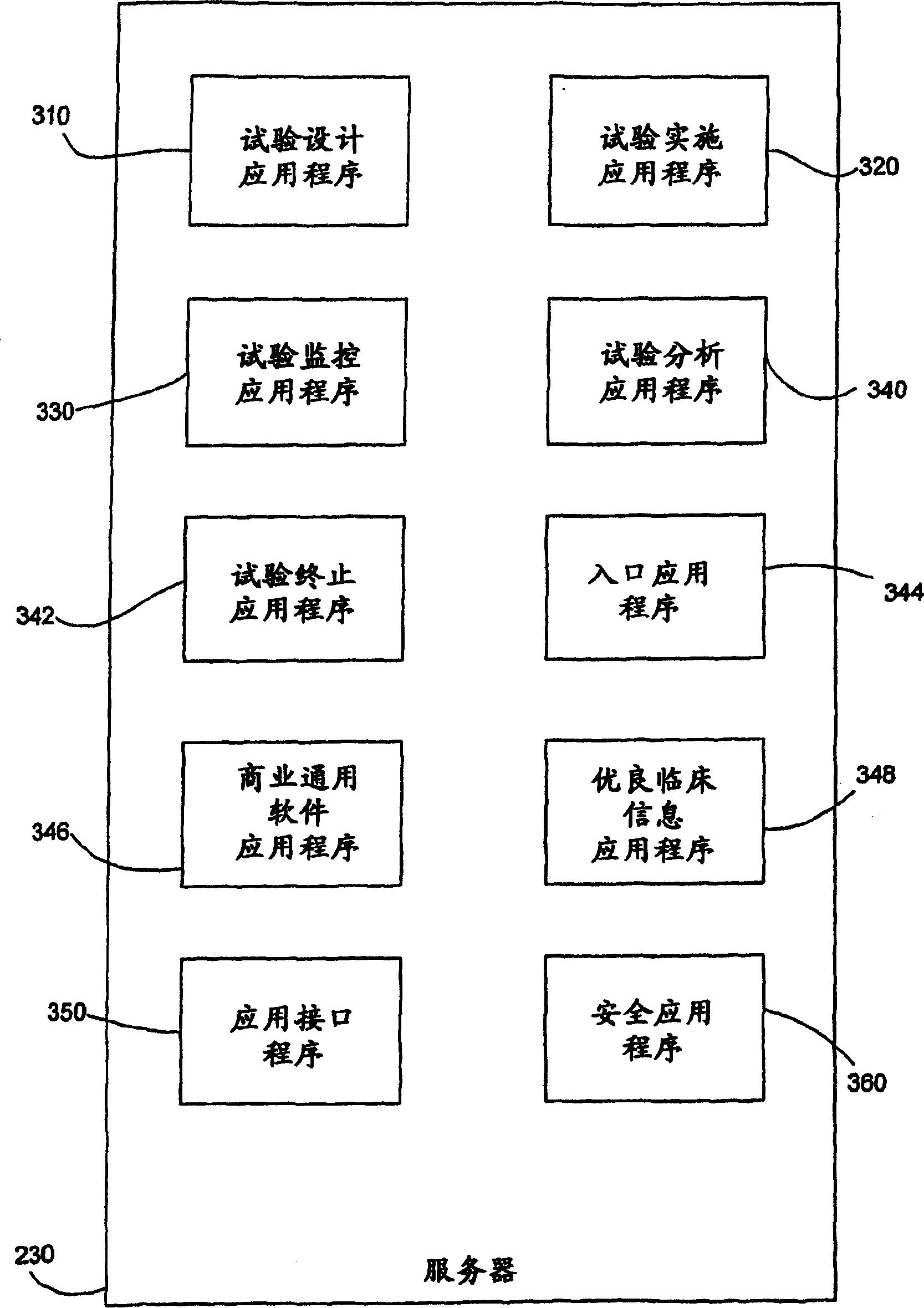
Systems and methods for clinical trials information management
Embodiments of the invention relate to systems and methods for managing clinical trials. A system for managing clinical trials includes a web client, client, server, and patient record database. Depending on the user's role in the clinical trial process, the server provides many different applications that the user can run. These applications are divided into core components and non-core components. Information from the patient records database is reported based on the role of the user accessing the information in the clinical trial process. Users with different roles are called stakeholders. Stakeholders include funders, administrators, researchers, sites, patients, and supervisors. Disclosed herein are methods for reporting clinical trial information, monitoring clinical trial events, scheduling and tracking appointments, providing superior clinical information, stopping a trial, and presenting information to interested parties.
Owner:CAPITAL SURINI GROUP INT
Traditional Chinese medicine formula capable of clearing phlegm and relieving cough
InactiveCN101167944ADefinite curative effectGood curative effectRespiratory disorderAluminium/calcium/magnesium active ingredientsSide effectPinellia
The invention relates to a Chinese traditional medicament for eliminating phlegm and relieving a cough in the Chinese traditional medicament field. The technical proposal of the invention is that the proportion by weight of the materials is that peucedanum root 10-15, almond 9, mulberry leaves 10-15, common anemarrhena 10-15, liriope 8-10, scutellaria 8-10, roast ephedrae 8-10, asarum 8-10, blackberry lily 8-10, plaster stone 20-25, schisandra 8-10, baked licorice 8-10, prepared pinellia 8-10, Japanese honeysuckle 10-15, tussilago farfara 10-15, loquat leaves 10-15, balloonflower 8-10, and liquorice 4-6. The effects of the invention is that the Chinese herbal composition of the invention takes ventilating the lung and resolving phlegm, relieving cough and asthma, and modulating and combining as the target; the invention completely employs the Chinese herbal configuration; the curative effect is accurate and stable; the period of the treatment is short; the curing cost is low; and the toxic and side effects are small. The clinical trial proves that the cure rate is 87% and the effective rate is 92% when the invention has been used for 5 to 7 days.
Owner:张津
Detection and characterization of microorganisms
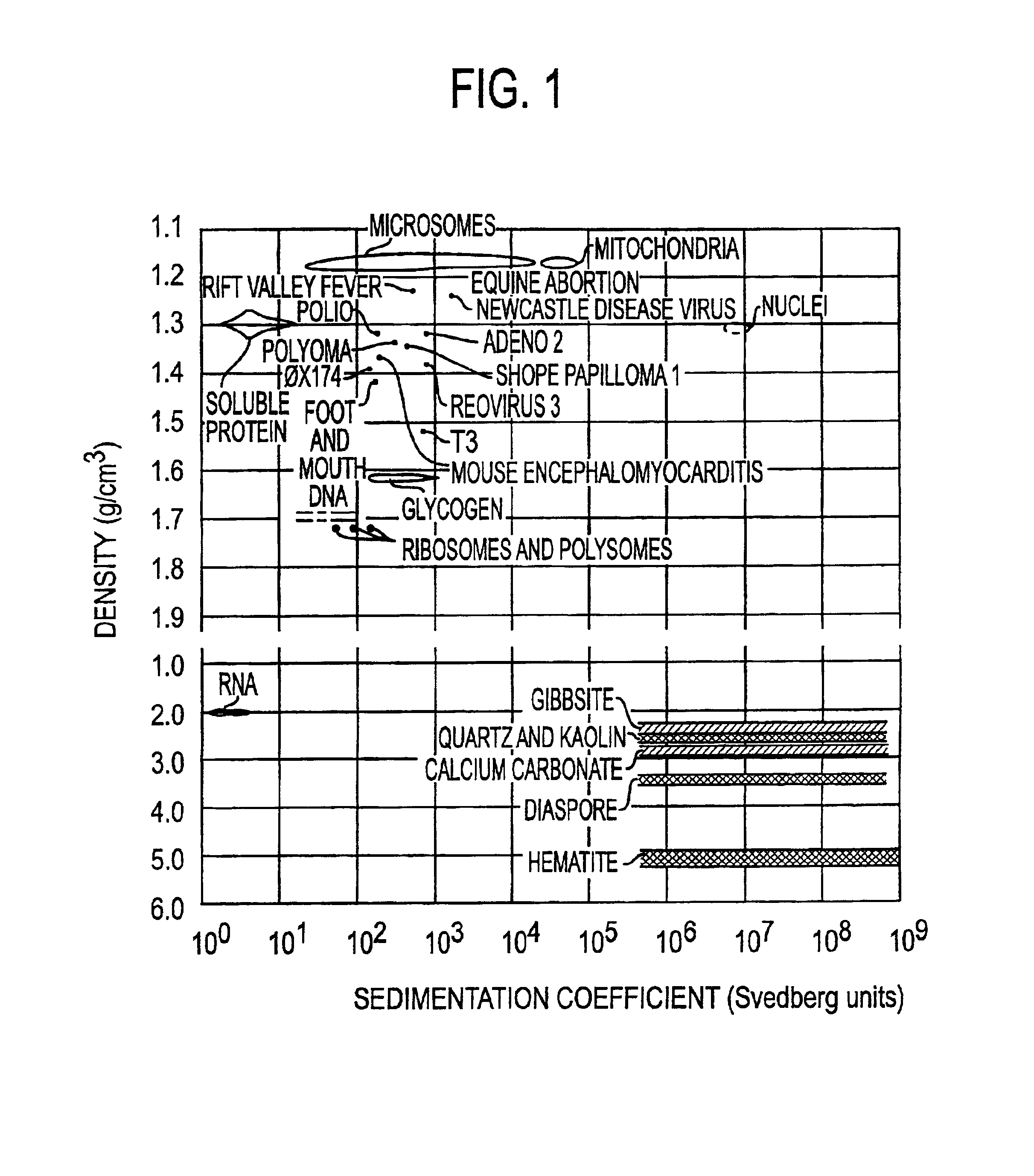
InactiveUS6911312B2Quick distinctionRapid discovery of new infectious agentsBioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceRapid identification
A method for separating microorganisms, especially infectious agents, from a mixture by two dimensional centrifugation on the basis of sedimentation rate and isopycnic banding density, for sedimenting such microorganisms through zones of immobilized reagents to which they are resistant, for detecting banded particles by light scatter or fluorescence using nucleic acid specific dyes, and for recovering the banded particles in very small volumes for characterization by mass spectrometry of viral protein subunits and intact viral particles, and by fluorescence flow cytometric determination of both nucleic acid mass and the masses of fragments produced by restriction enzymes. The method is based on the discovery that individual microorganisms, such as bacterial and viral species, are each physically relatively homogeneous, and are distinguishable in their biophysical properties from other biological particles, and from non-biological particles found in nature. The method is useful for distinguishing infections, for identifying known microorganisms, and for discovering and characterizing new microorganisms. The method provides very rapid identification of microorganisms, and hence allows a rational choice of therapy for identified infectious agents. A particularly useful application is in clinical trials of new antibiotics and antivirals, where it is essential to identify at the outset individuals infected with the targeted infectious agent.
Owner:LARGE SCALE PROTEOMICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com