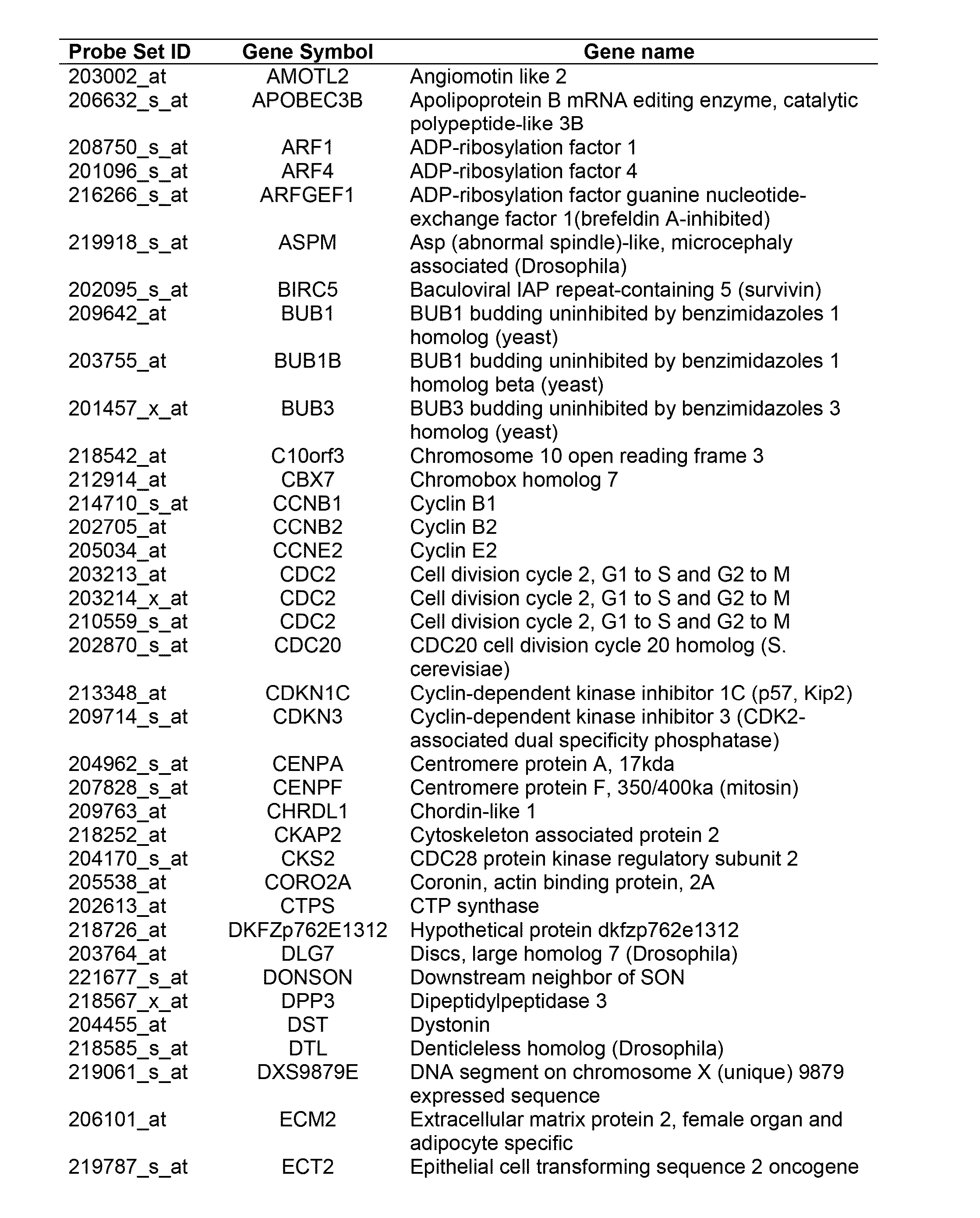
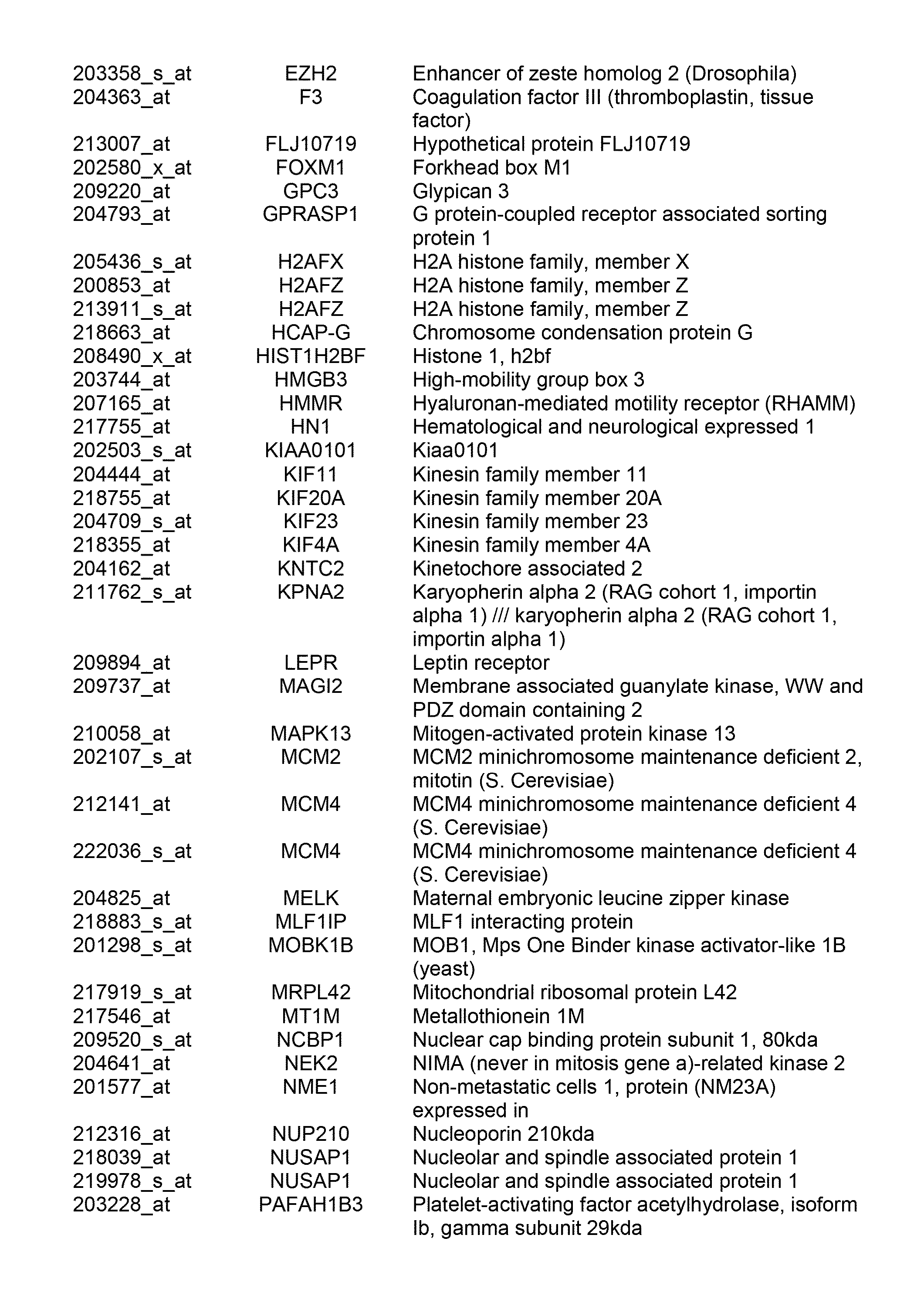
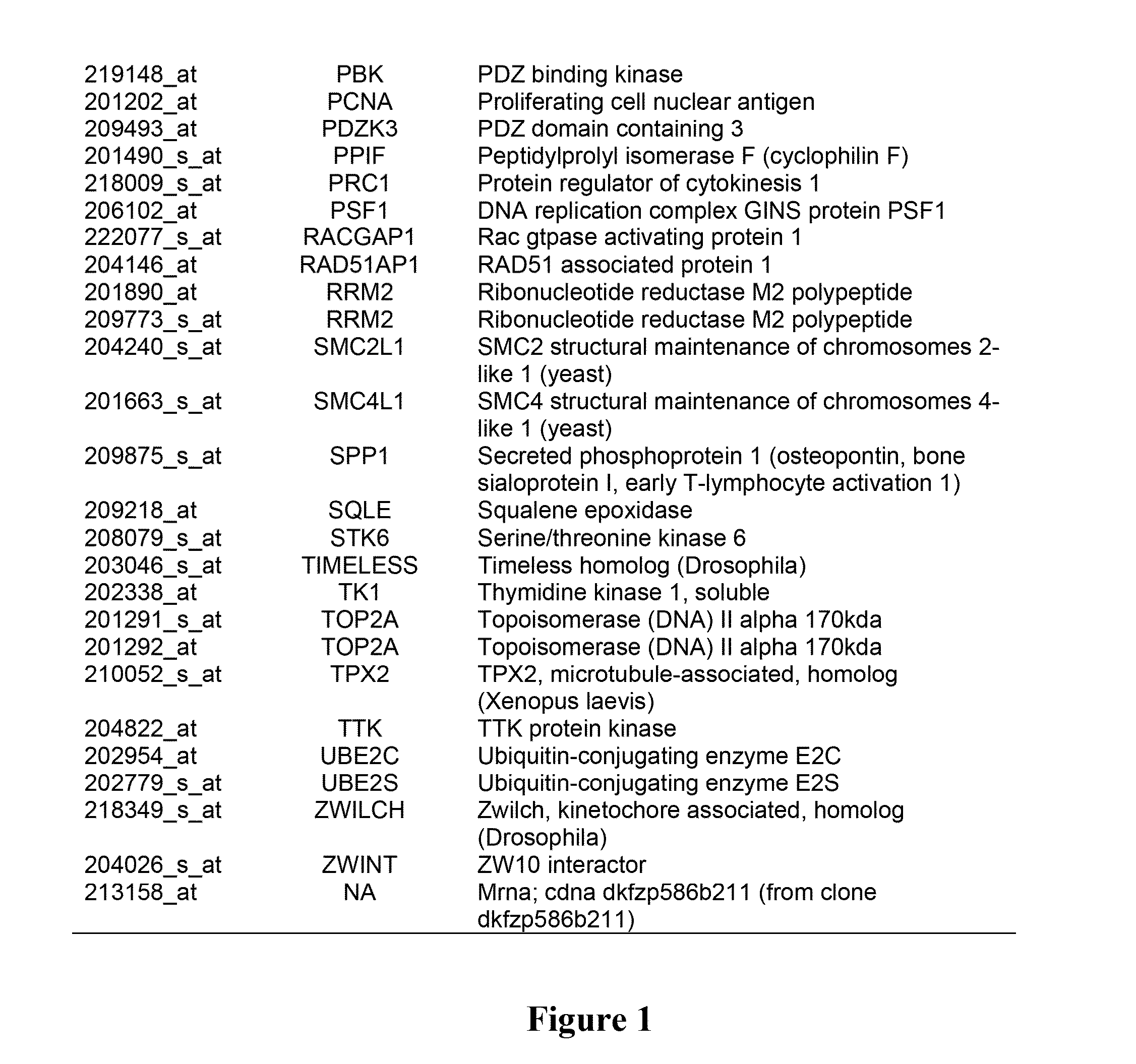
Patents
Literature
51 results about "Prognostic factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Prognostic factor. Any feature of a disease or of a patient's presentation that suggests that he or she will be affected for better or worse by an illness. See also: factor.
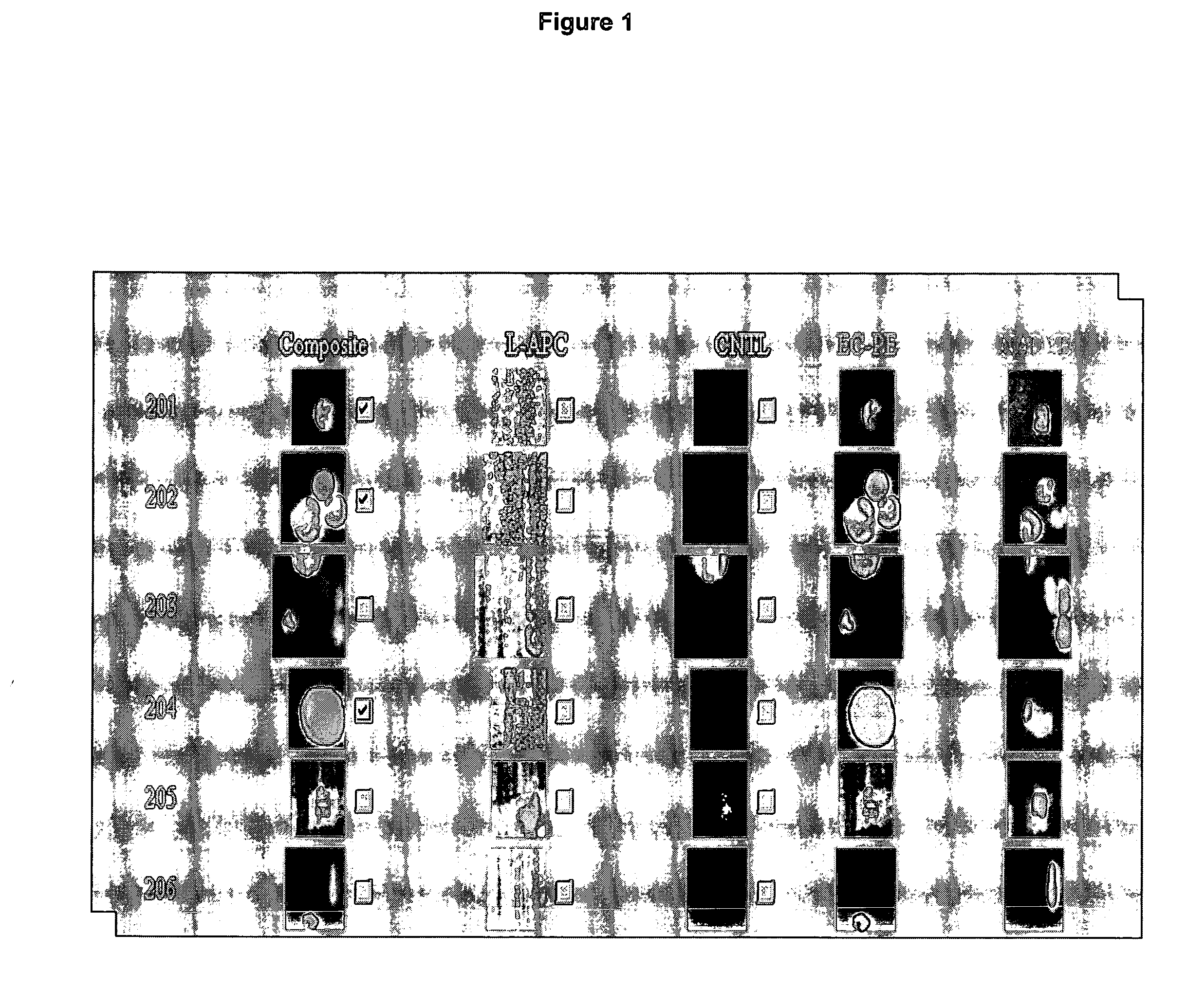
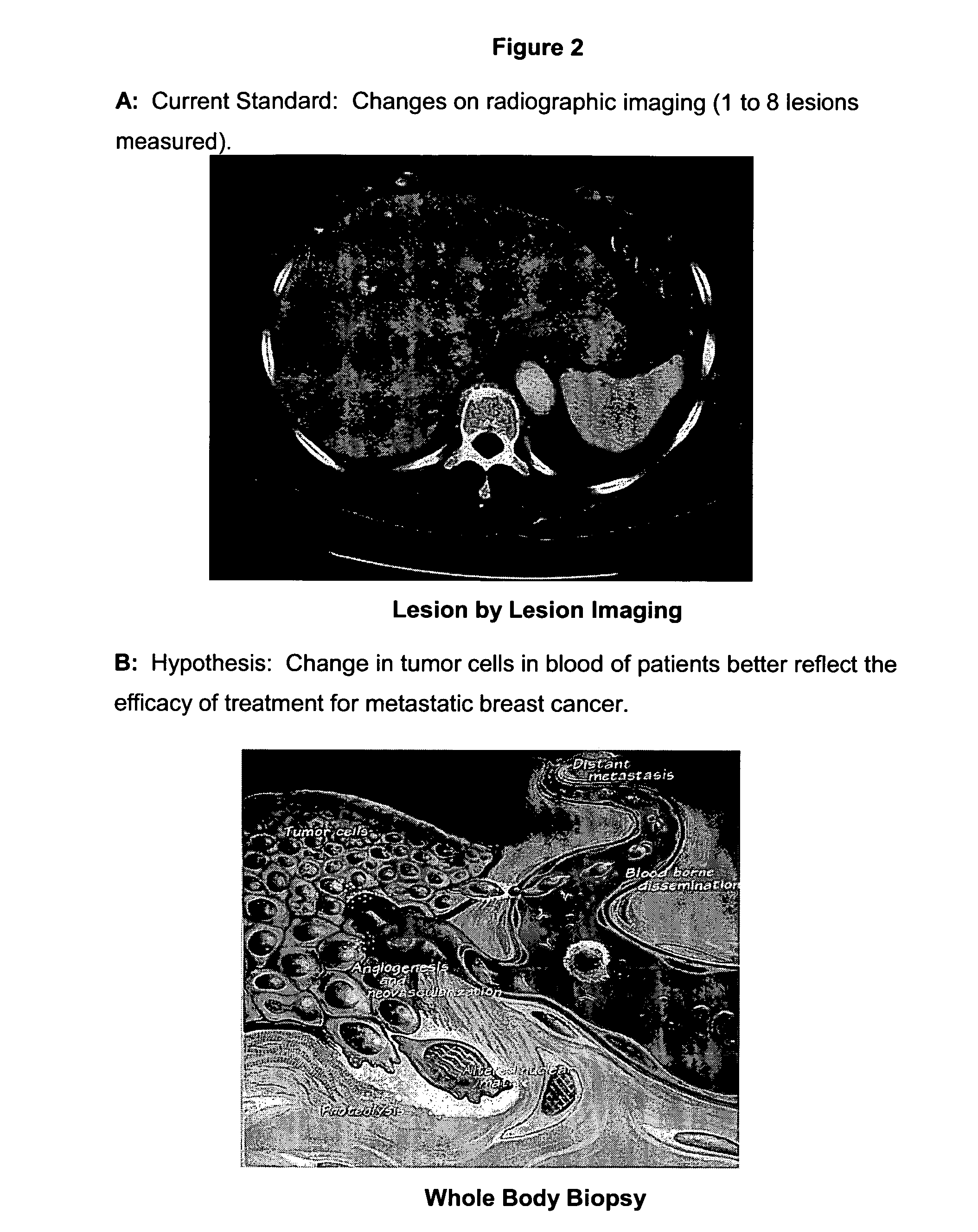
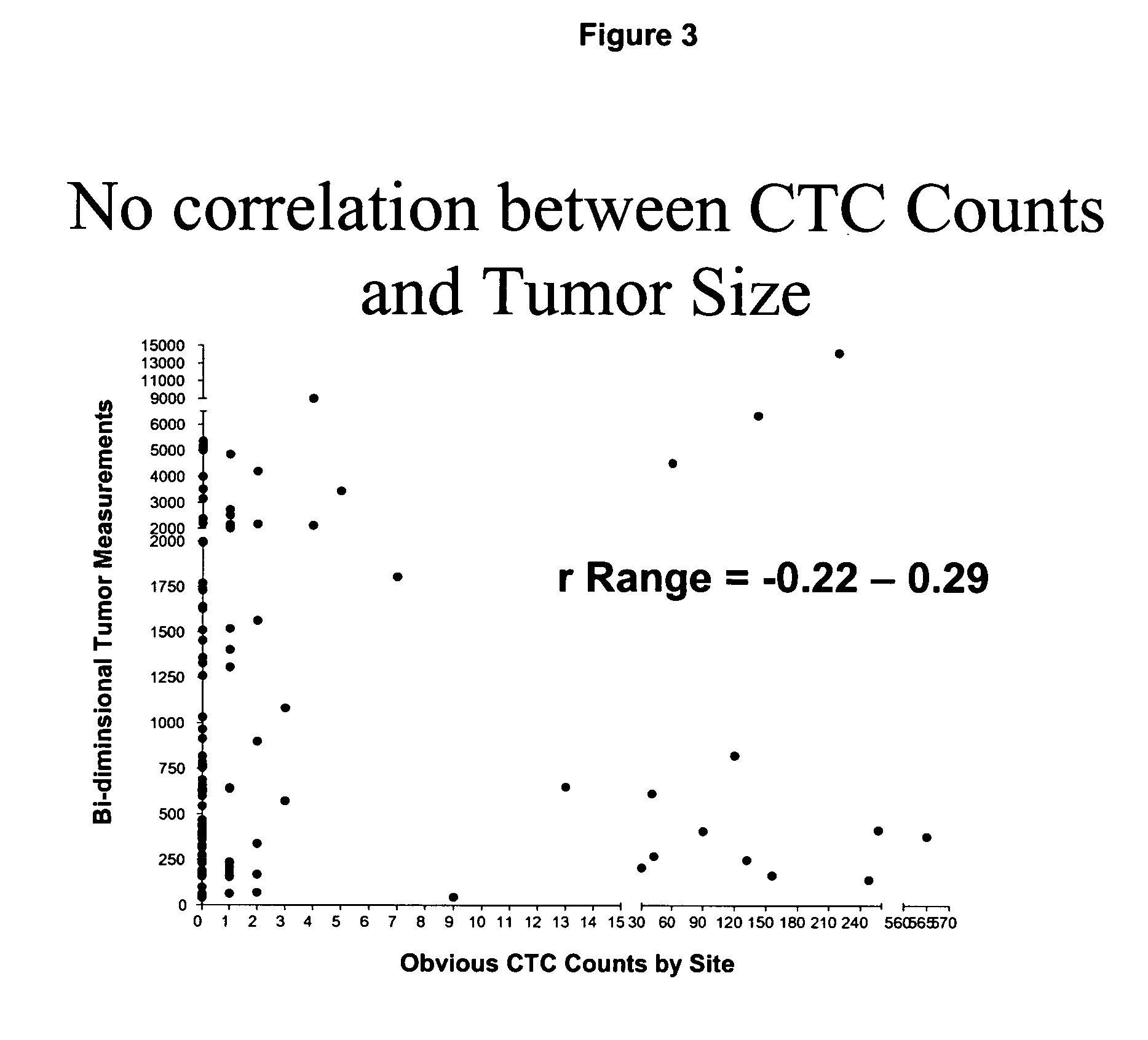
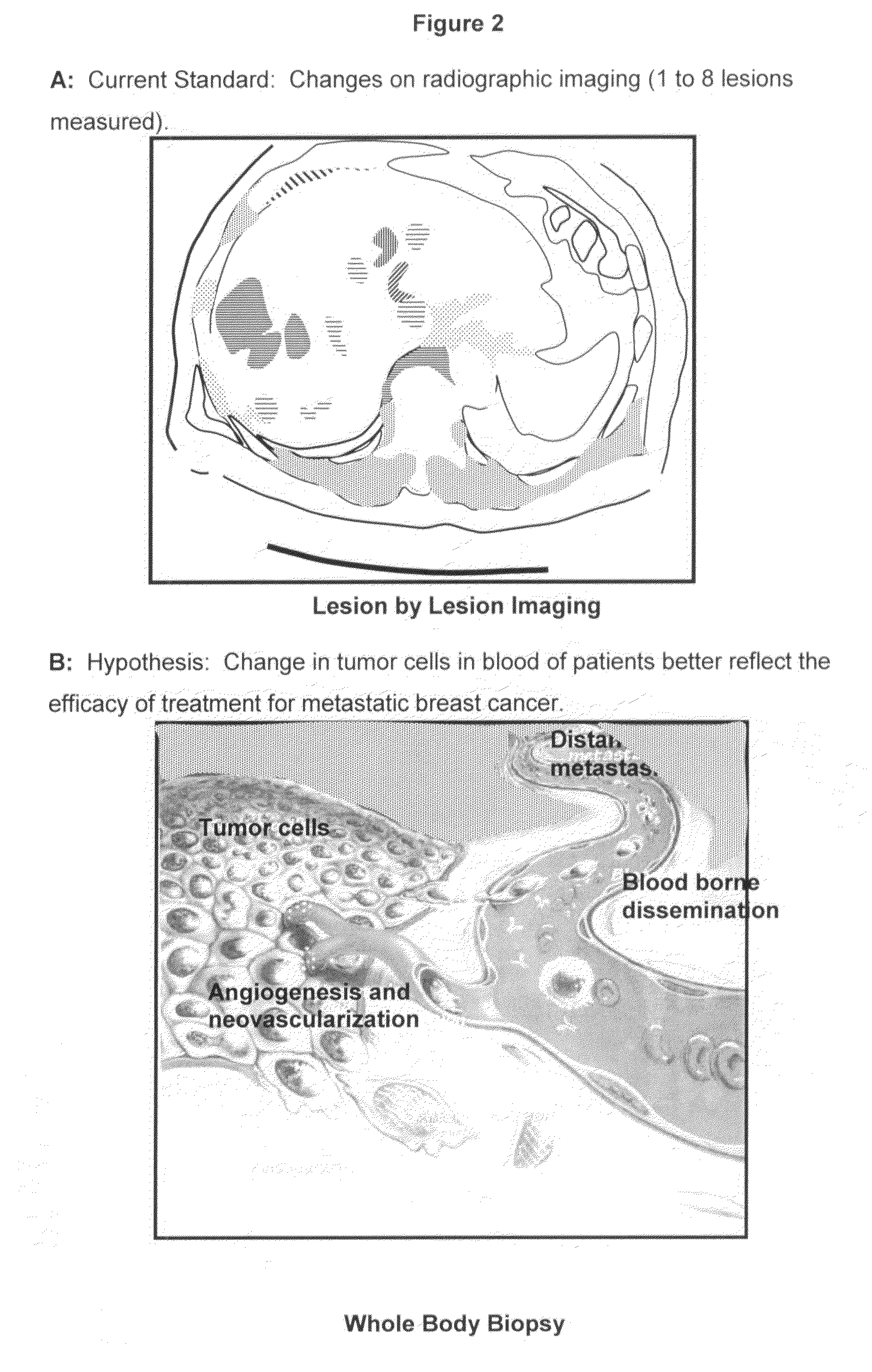
Circulating tumor cells (CTC's): early assessment of time to progression, survival and response to therapy in metastatic cancer patients
InactiveUS20070037173A1Less side effectsImprove the quality of lifeMicrobiological testing/measurementBiological testingClinical trialOncology
A cancer test having prognostic utility in predicting time to disease progression, overall survival, and response to therapy in patients with MBC based upon the presence and number of CTC's. The Cell Spotter® System is used to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. The mean time to survival of patients depended upon a threshold number of 5 CTC's per 7.5 ml of blood. Detection of CTC's in metastatic cancer represents a novel prognostic factor in patients with metastatic cancers, suggests a biological role for the presence of tumor cells in the blood, and indicates that the detection of CTC's could be considered an appropriate surrogate marker for prospective therapeutic clinical trials.
Owner:JANSSEN DIAGNOSTICS LLC
Molecular lymphatic mapping of sentinel lymph nodes
ActiveUS20050142556A1Luminescence/biological staining preparationSugar derivativesAbnormal tissue growthRadioactive tracer
The present invention describes a method for identification and labeling of sentinel lymph nodes (SLNs) and the presence or absence of lymph node metastases as an important diagnostic and prognostic factor in early stage cancers of all types. The method, know as Molecular Lymphatic Mapping, uses traditional dye / radioactive tracer based techniques in conjunction with a nucleic acid marker to identify and label the SLN, not only for current diagnostic methods, but for archival purposes. In addition, MLM can be used to deliver a therapeutic gene or genes to the SLN to activate tumor immunity to tumor cells, and / or to inhibit tumor metastases. The methods may be combined with therapeutic intervention including chemotherapy and radiotherapy.
Owner:JOHN WAYNE CANCER INST
Method for predicting progression free and overall survival at each follow-up time point during therapy of metastatic breast cancer patients using circulating tumor cells
InactiveUS20090061456A1Less side effectsImprove the quality of lifeDisease diagnosisBiological testingOncologyDisease progression
A cancer test having prognostic utility in predicting time to disease progression, overall survival, and response to therapy in patients with MBC based upon the presence and number of CTC's. The Cell Spotter® System is used to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. The mean time to survival of patients depended upon a threshold number of 5 CTC's per 7.5 ml of blood. Detection of CTC's in metastatic cancer represents a novel prognostic factor in patients with metastatic cancers, suggests a biological role for the presence of tumor cells in the blood, and indicates that the detection of CTC's could be considered an appropriate surrogate marker for prospective therapeutic clinical trials.
Owner:VERIDEX LCC
MTA1 is a predictive and prognostic factor in human breast cancer
The present invention is directed to providing a prognosis of disease—free survival for a cancer patient. The invention further relates to methods of prognosticating recurrence or metastasis by determining a level of an MTA1 polypeptide in the patient. Yet further, the methods allow for prognosticating and / or predicting micrometastasis in a patient and offer the improvement in the efficacy of clinical treatment of the patient.
Owner:BAYLOR COLLEGE OF MEDICINE
Breast cancer recurrent risk assessment 21 gene detection primer and application thereof
InactiveCN107058523ASimple and fast operationStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationWilms' tumorAdjuvant chemotherapy
The invention relates to a breast cancer recurrent risk assessment 21 gene detection primer and application thereof. The breast cancer recurrent risk assessment 21 gene detection primer comprises a nucleotide sequence as shown in an SEQ ID NO.: 1 to 42, and belongs to the technical field of molecular detection. The detection primer provided by the invention utilizes the advantage of fluorescent quantitation, and adopts fluorescent quantitation polymerase chain reaction to carry out amplification detection on 21 genes for breast cancer recurrent risk assessment, so that whether clinical measurements such as adjuvant chemotherapy are needed or not is considered. A kit provides a PCR (Polymerase Chain Reaction) amplification primer sequence of the 21 genes for breast cancer recurrent risk assessment, and the primer is applicable to detecting ER positive patients, HER2 negative patients, axillary node negative patients, moderately and poorly differentiated patients with the tumor size being 0.6 to 1.0cm and adverse prognostic factors, or patients with the tumor size is larger than 1cm, has the advantages of high sensitivity and specificity, stability, promptness, convenience in operation and the like, can well meet the clinical application of breast cancer recurrent risk assessment, and can be beneficial for reducing invalid medication administration, improving medication administration accuracy, and reducing financial burdens of the patients.
Owner:温州迪安医学检验所有限公司 +1
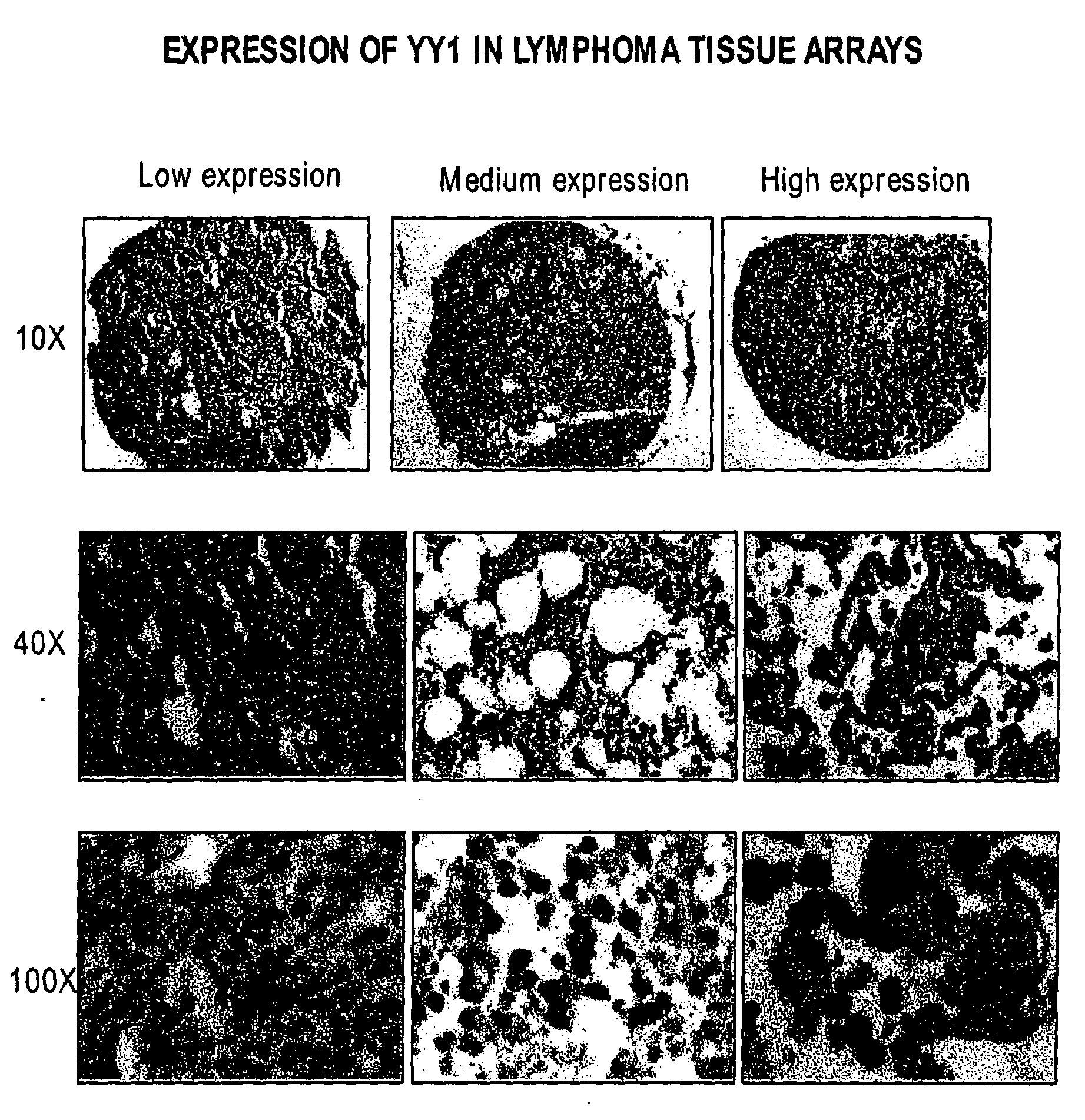
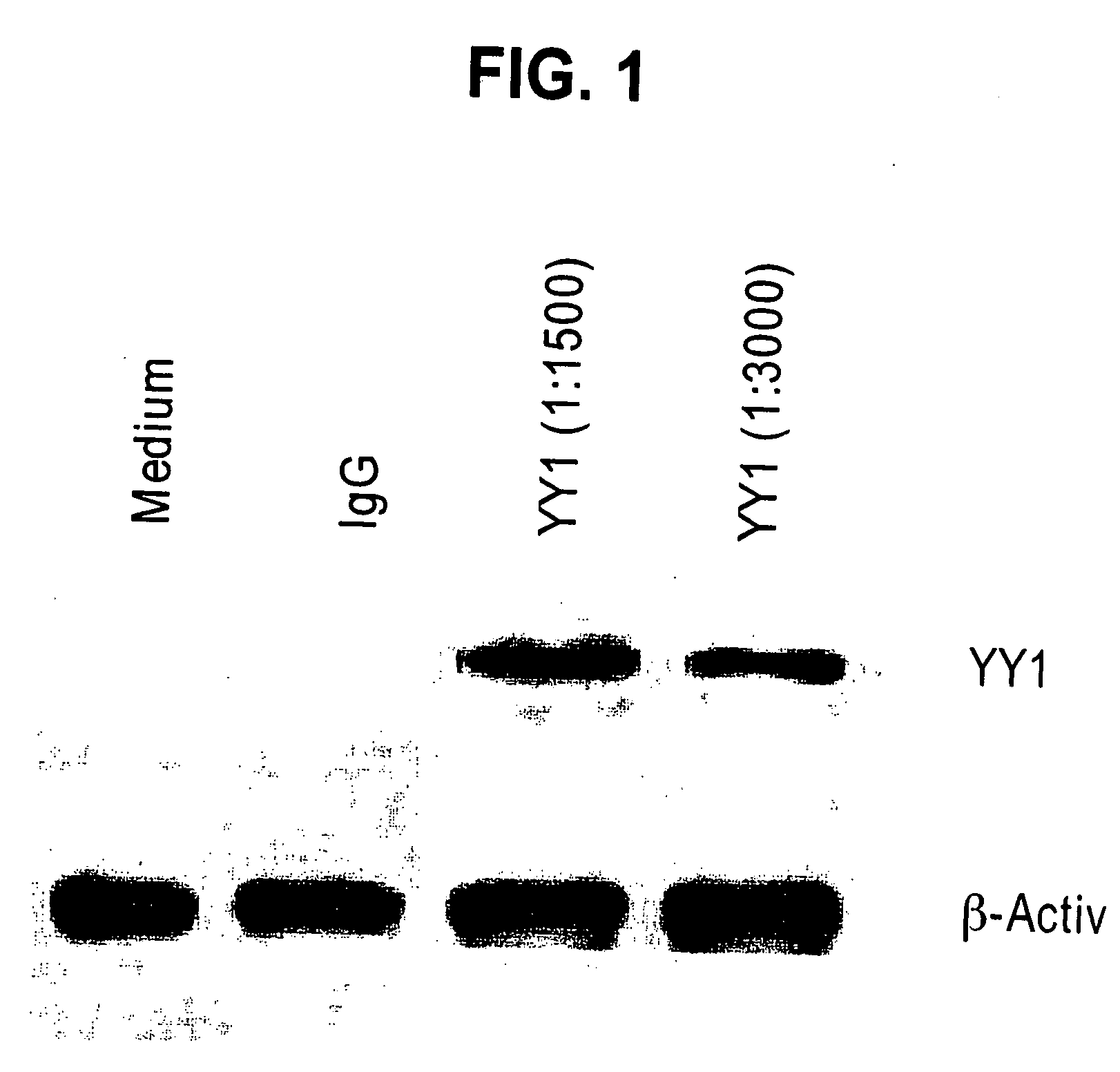
Therapeutic and Prognostic Factor Yy1 in Human Cancer
The present invention provides for the first time YY1, a transcription factor gene over-expressed and / or functionally overactive in human cancer. The present invention provides methods of diagnosing and providing a prognosis for cancer such as prostate cancer, as well as methods of drug discovery. YY1 is also a therapeutic target for treatment of cancer resistant to conventional and experimental cancer therapeutics. Inhibition of YY1 expression and / or activity sensitizes resistant tumor cells to cytotoxic treatments, including chemotherapy, radiation therapy, hormonal therapy, and immunotherapy.
Owner:RGT UNIV OF CALIFORNIA
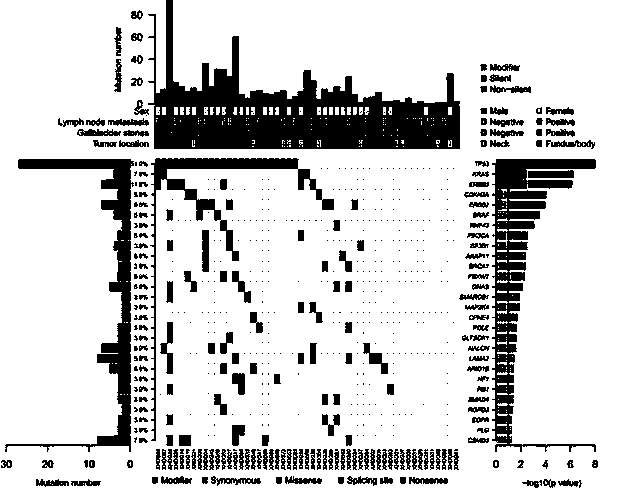
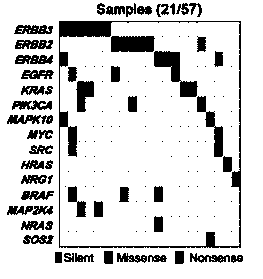
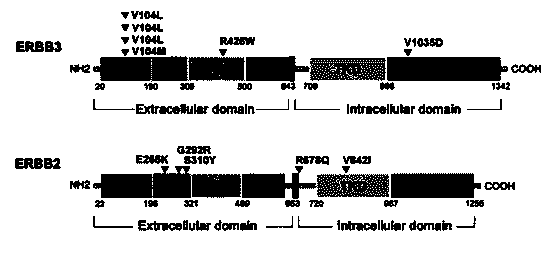
ERBB signal pathway mutation targeted sequencing method for prognosis evaluation of gallbladder carcinoma
InactiveCN104032001AHelp to establishAids in diagnosisMicrobiological testing/measurementGenes mutationSignaling Pathway Gene
The invention relates to an ERBB signal pathway mutation targeted sequencing method for the prognosis evaluation of gallbladder carcinoma and provides an application of an ERBB signal pathway mutation targeted sequencing reagent / kit for gallbladder carcinoma patients. In the invention, the whole-genome exon technology is adopted for performing mutation target sequencing of the ERBB signal pathway of a gallbladder carcinoma patient; and in combination with the pathological clinical data of the gallbladder carcinoma patient, the Kaplan-Meier survival curve analysis indicates that the lifetime of the patient after operation is closely related to the gene mutation of the ERBB signal pathway, and the multiple-factor analysis of Cox risk regression model indicates that the gene mutation of the ERBB signal pathway can serve as an independent prognosis factor of gallbladder carcinoma. The prompt of screening the ERBB signal pathway related gene mutation of the gallbladder carcinoma cancer is favorable to the gene-level diagnosis and parting of gallbladder carcinoma, the establishment of therapeutic target, the prognosis analysis and the like.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
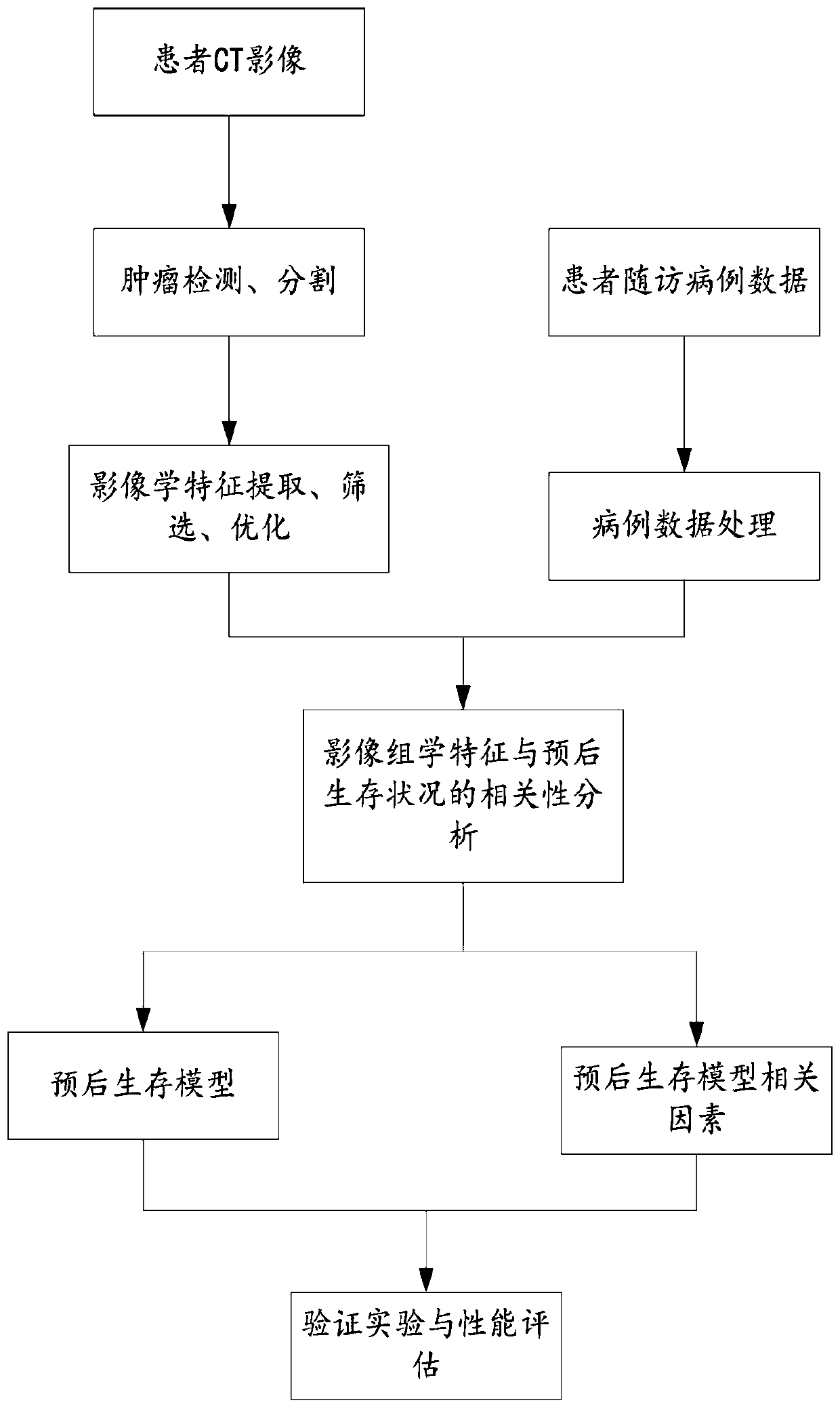
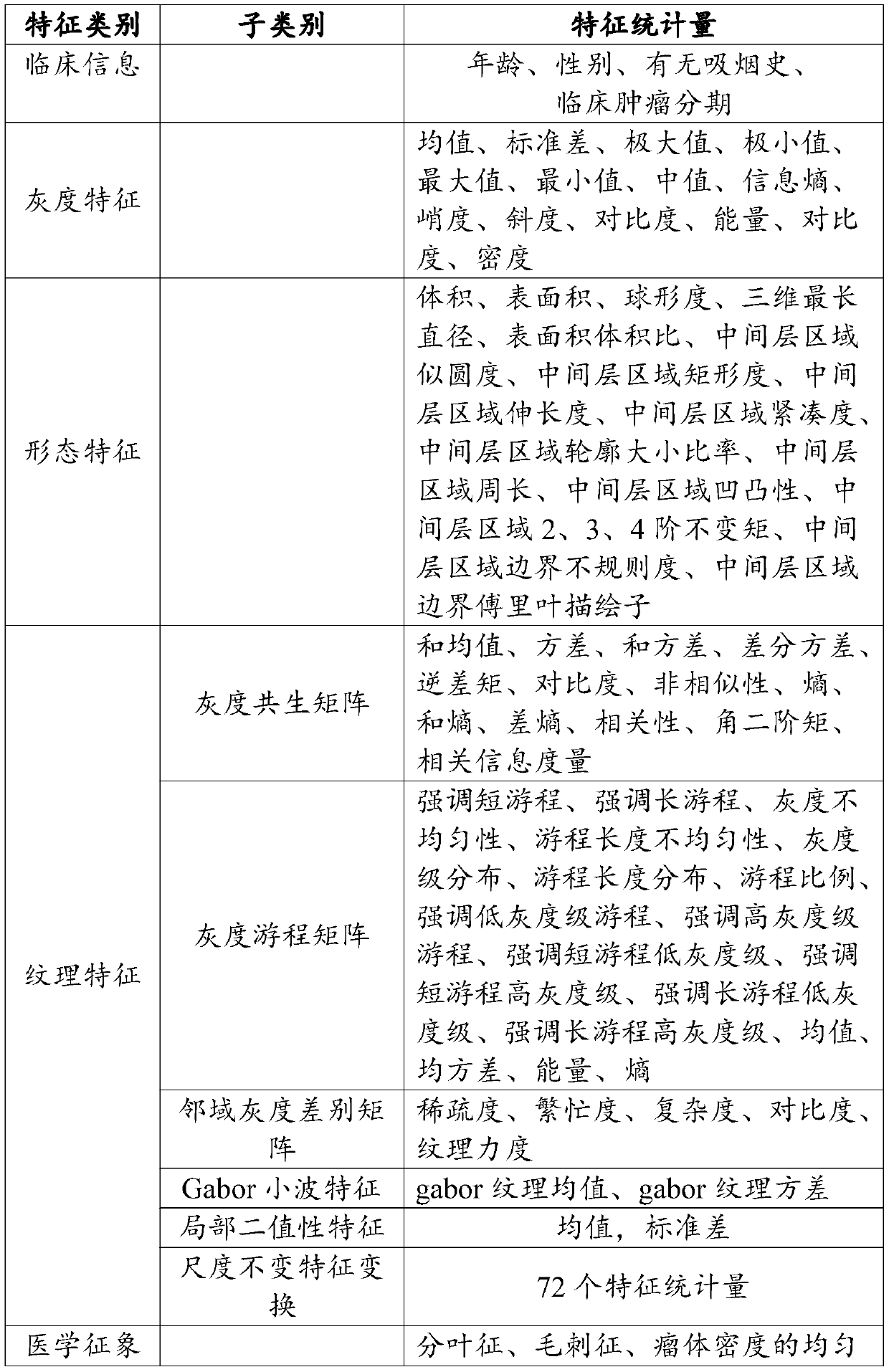
Analytical method for prognosis survival case of non-small cell lung cancer
PendingCN109887600AGuaranteed accuracyMedical automated diagnosisData informationCorrelation analysis
The invention provides an analytical method for prognosis survival case of non-small cell lung cancer. The analytical method for prognosis survival case of non-small cell lung cancer designs an experiment to perform prognosis survival analysis and research on a non-small cell lung cancer patient, constructs a prognostic analysis model for the non-small cell lung cancer based on CT image omics characteristics, according to a traditional imaging omics research framework, performing tumor segmentation, characteristic extraction, characteristic screening, and modeling of a correlation analysis andprognosis survival analysis model for image omics characteristics and the prognosis survival case on the data of the non-small cell lung cancer patient to obtain an image omics prognostic factor andprognosis survival analysis model correlated with prognosis survival significance of the patient with the non-small cell lung cancer, so as to provide the doctor with data information including the survival time of the patient and a series of late lesion development situations, and at the same time, to evaluate the performance of the prognosis survival model to ensure the accuracy of the prognosissurvival model.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
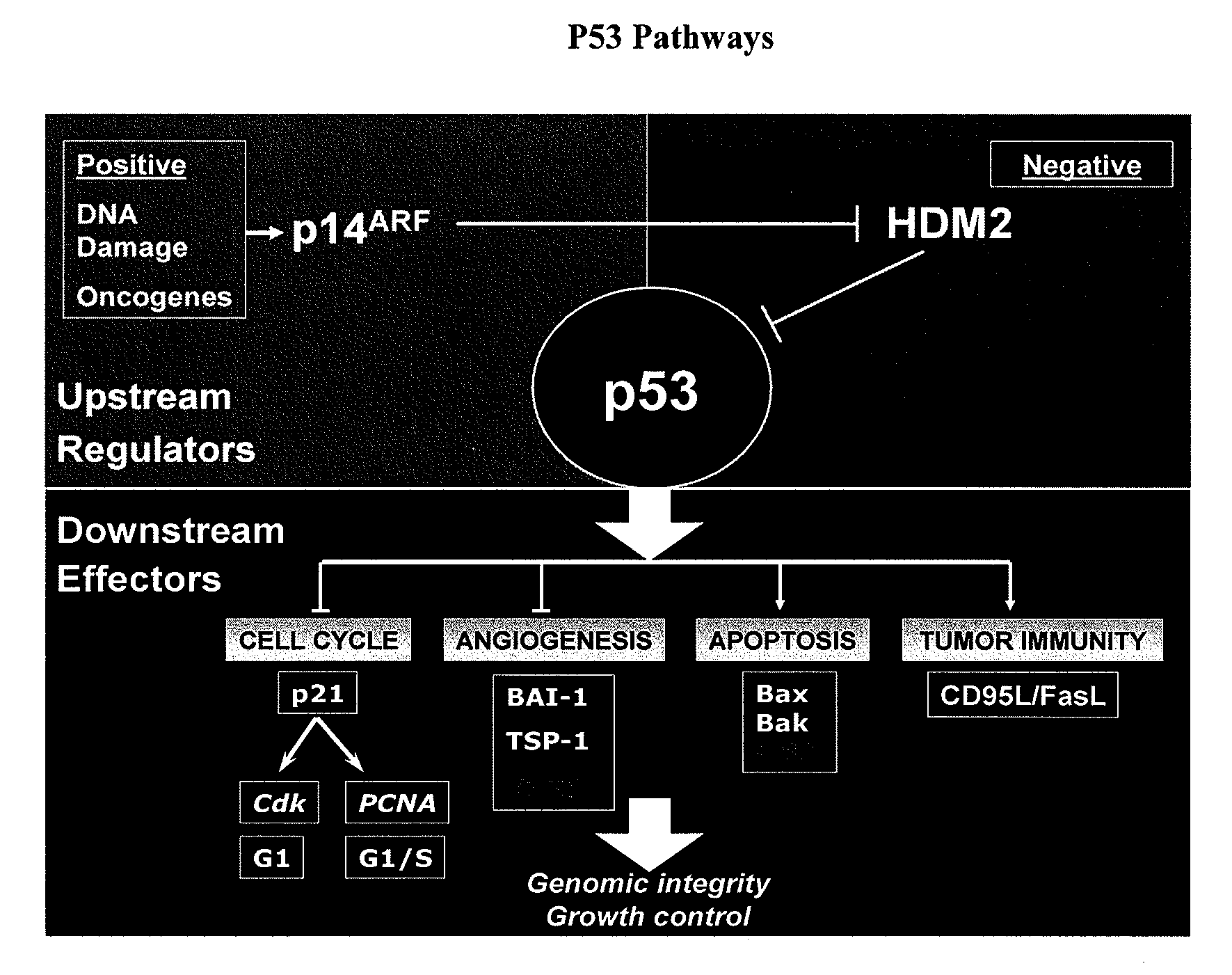
Prognostic factors for Anti-hyperproliferative disease gene therapy
InactiveUS20070231304A1Reduce the burden onShrink tumor sizeBiocideOrganic active ingredientsCancer genesFhit gene
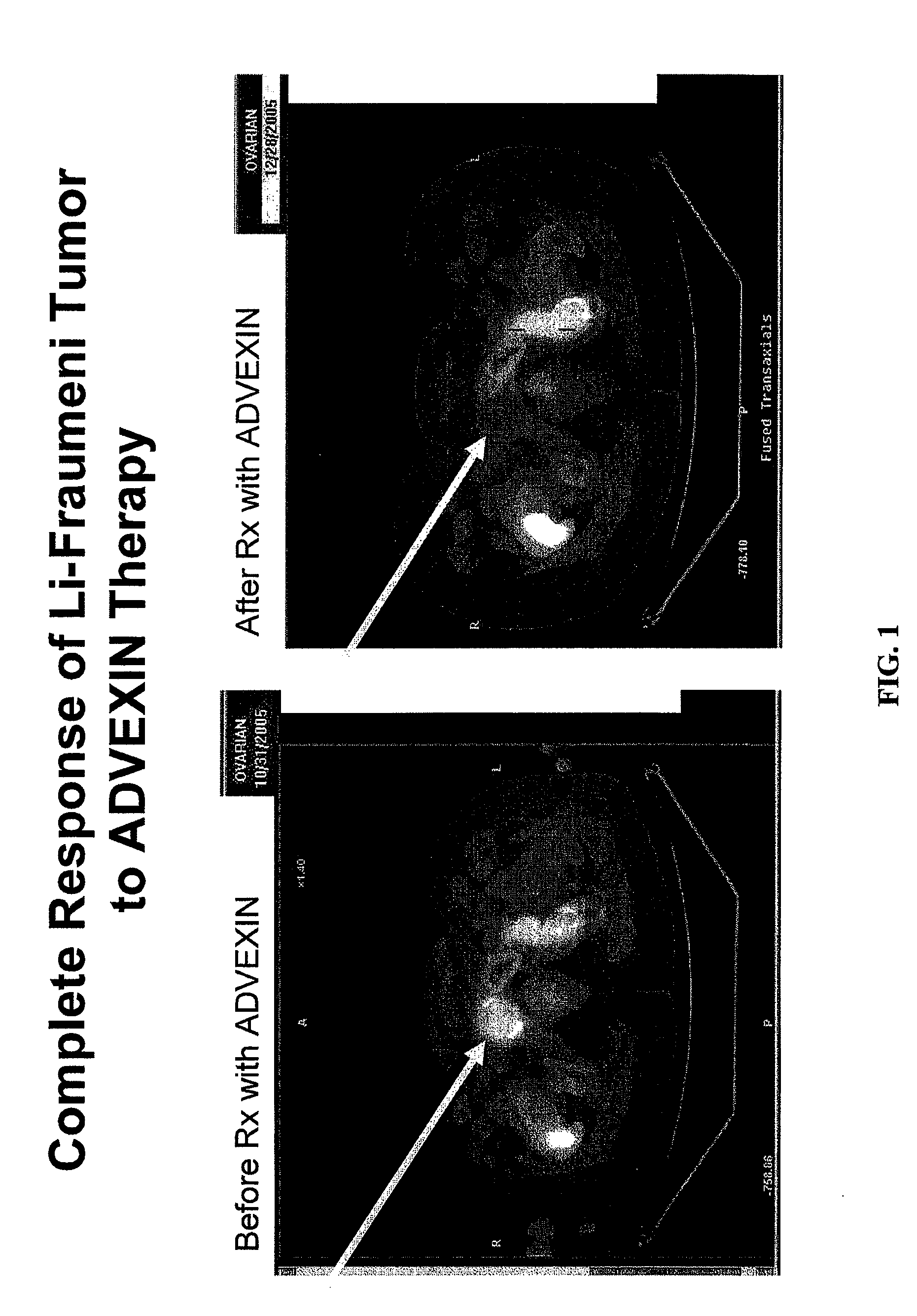
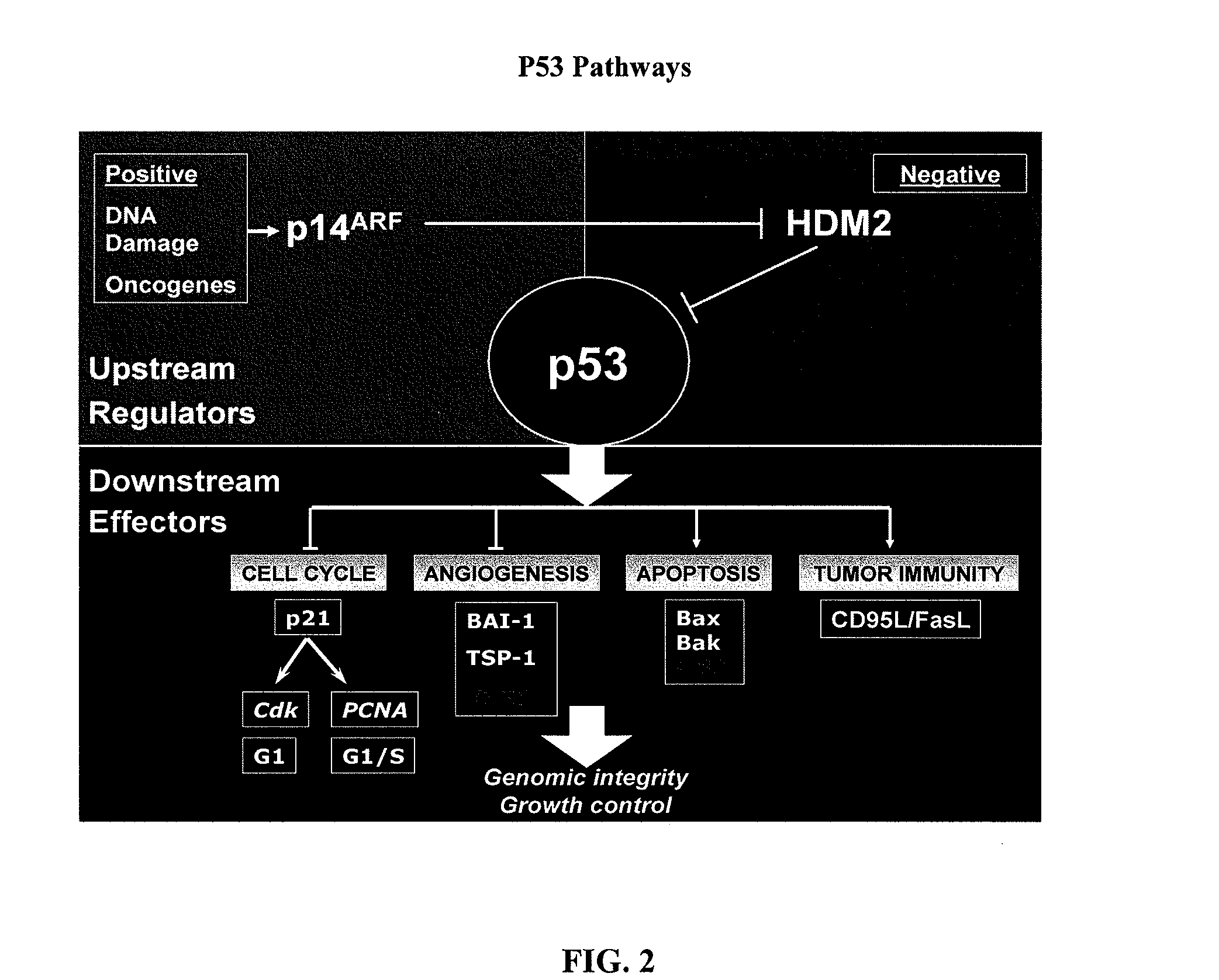
The present invention relates to the identification of various prognostic factors that predict response in patients with hyperproliferative disease such as cancer to gene therapy, and their use in methods of treating such patients with an anti-hyperproliferative disease gene therapy. Also described are methods of treatment for Li Fraumeni syndrome, and for assessing anti-cancer gene therapy using PET scans.
Owner:INTROGEN THERAPEUTICS INC
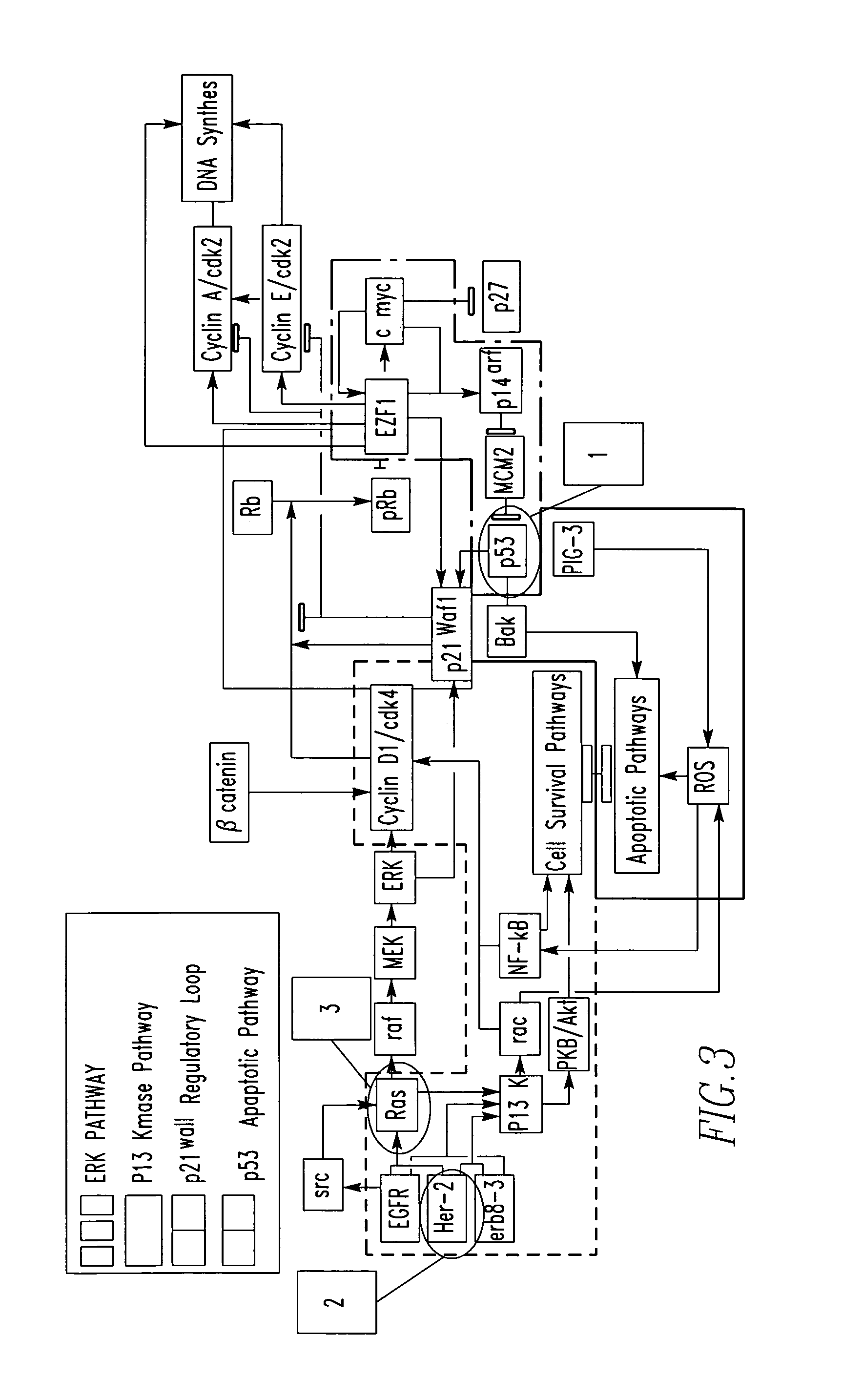
Laser microdissection and microarray analysis of breast tumors reveal estrogen receptor related genes and pathways
InactiveCN101965190APeptide/protein ingredientsMicrobiological testing/measurementPathway analysisLaser micro dissection
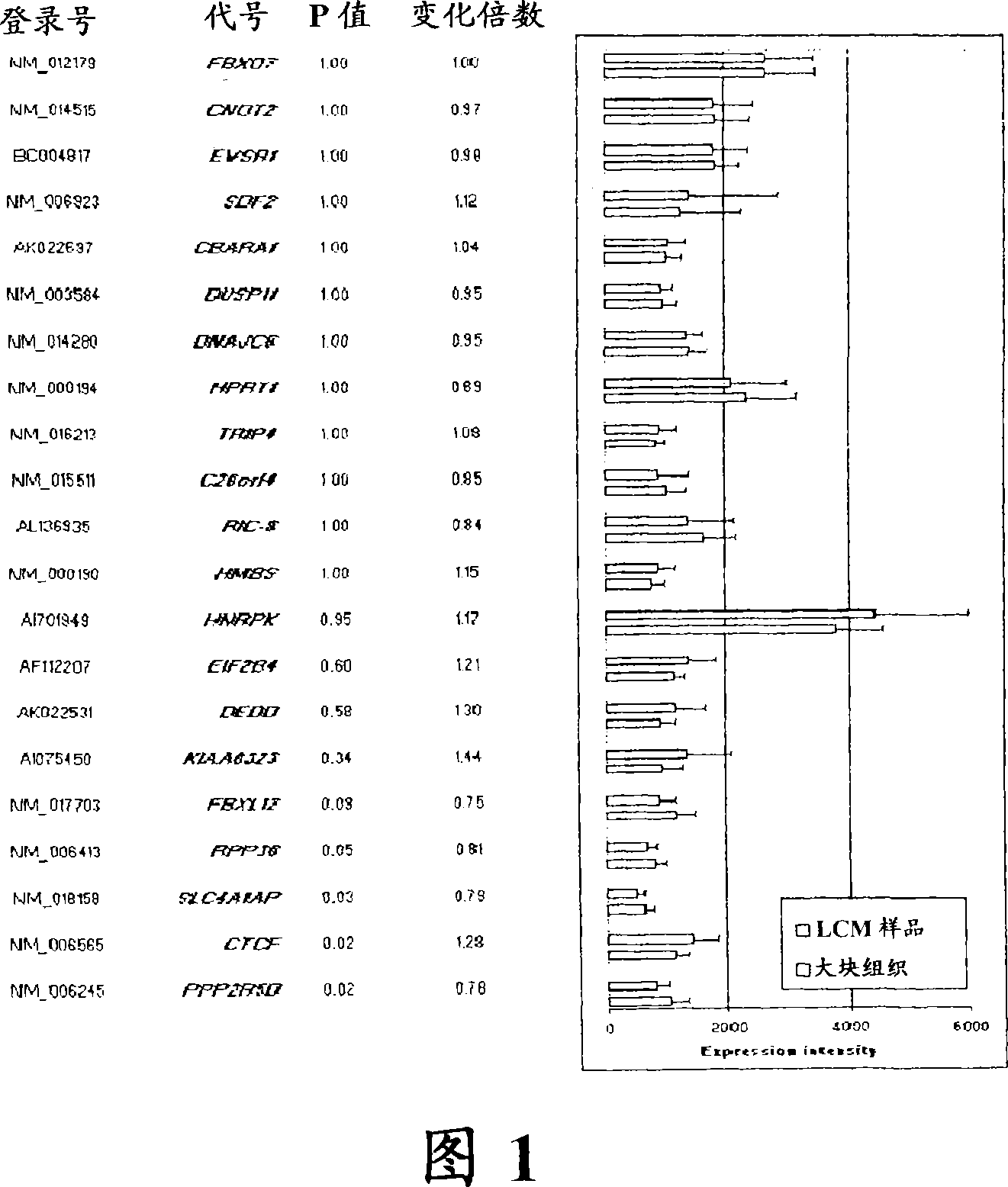
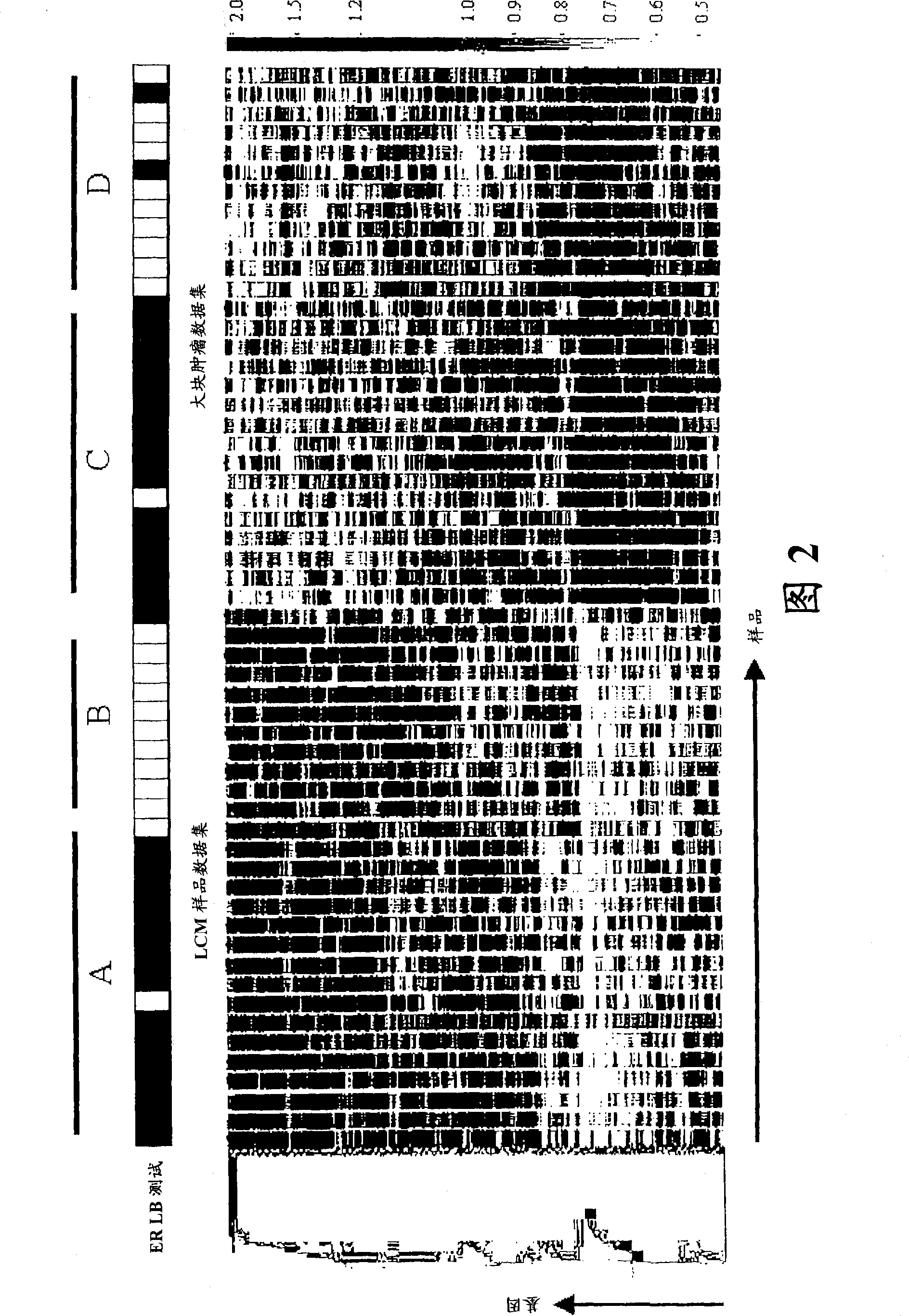
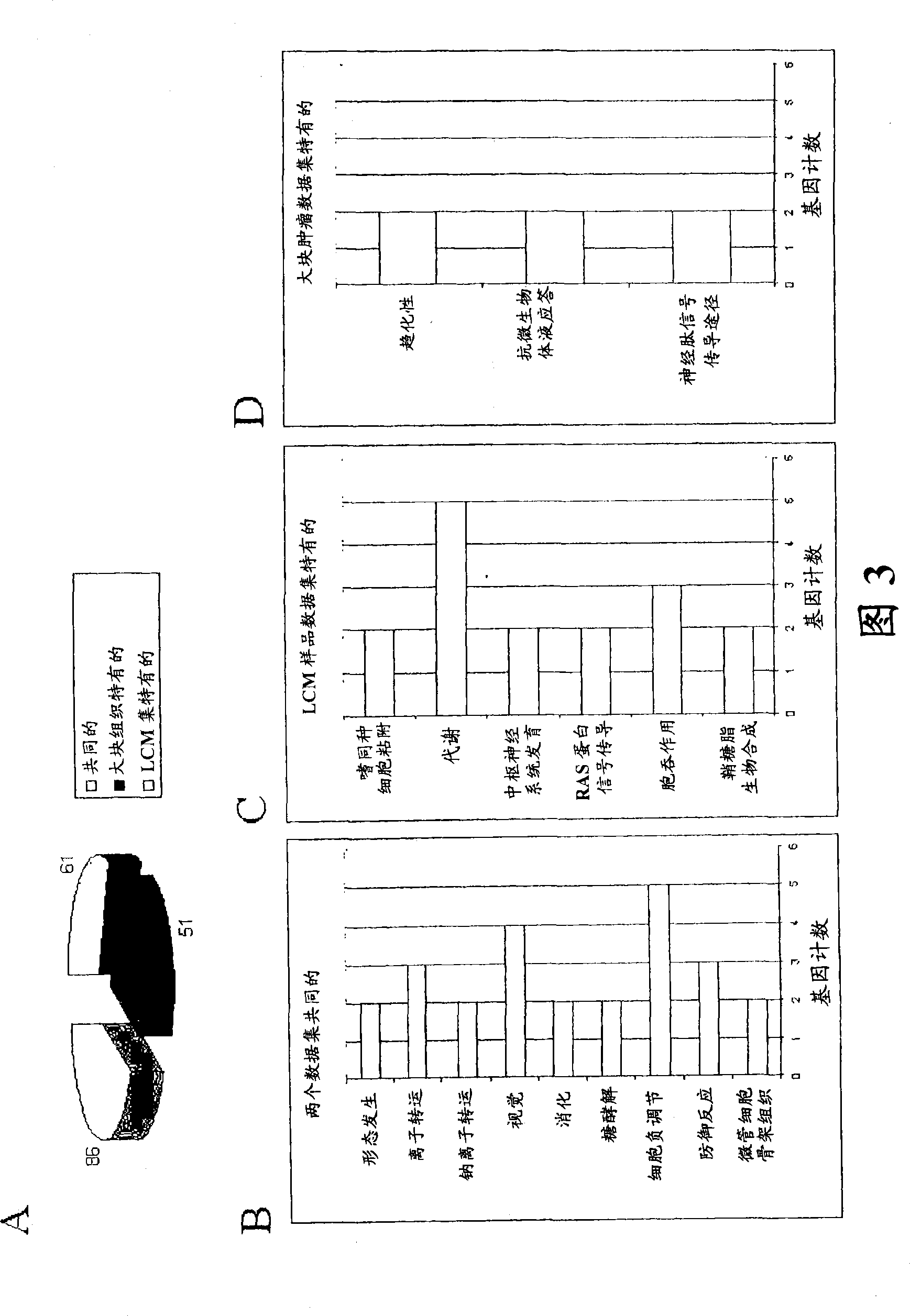
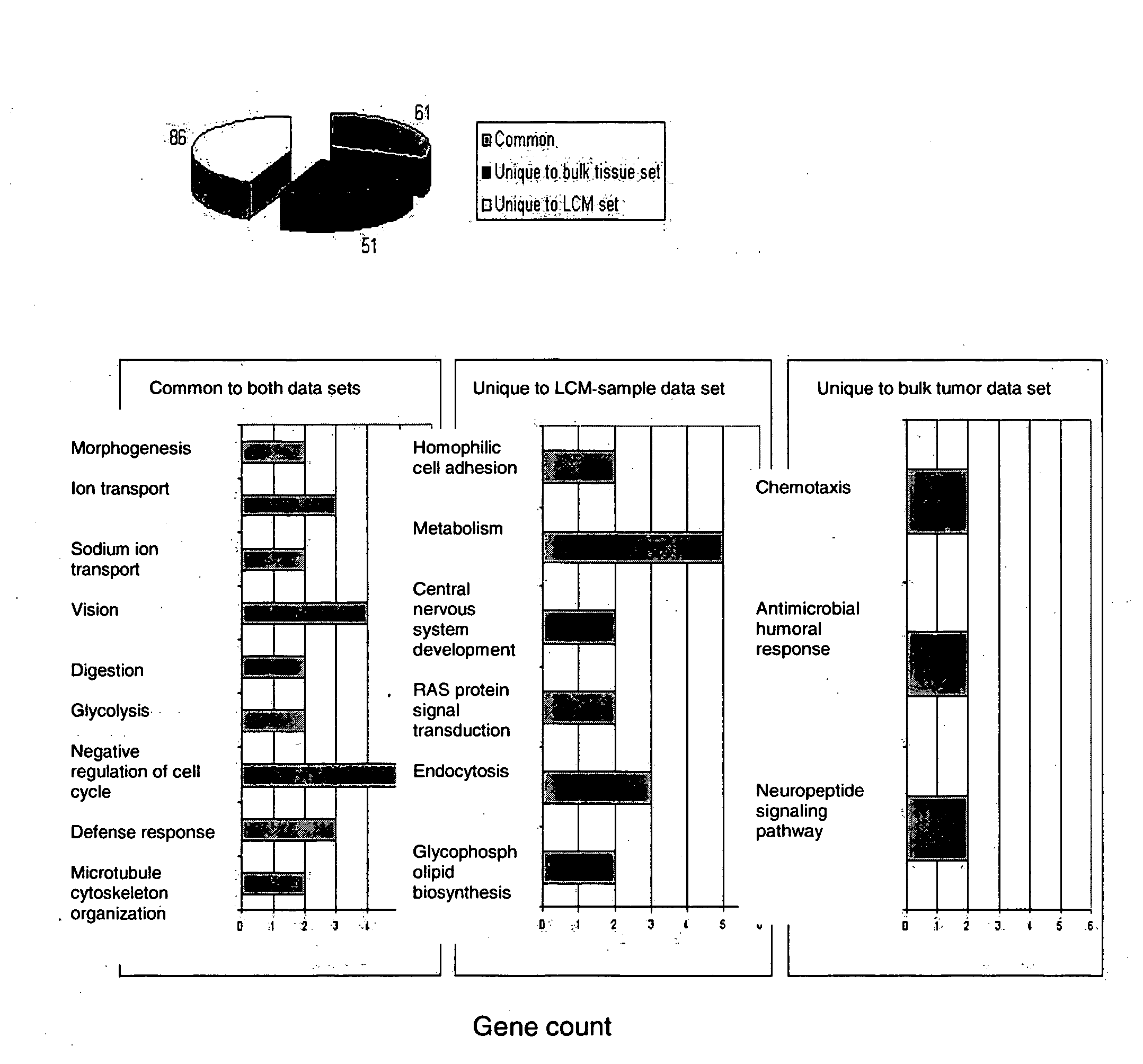
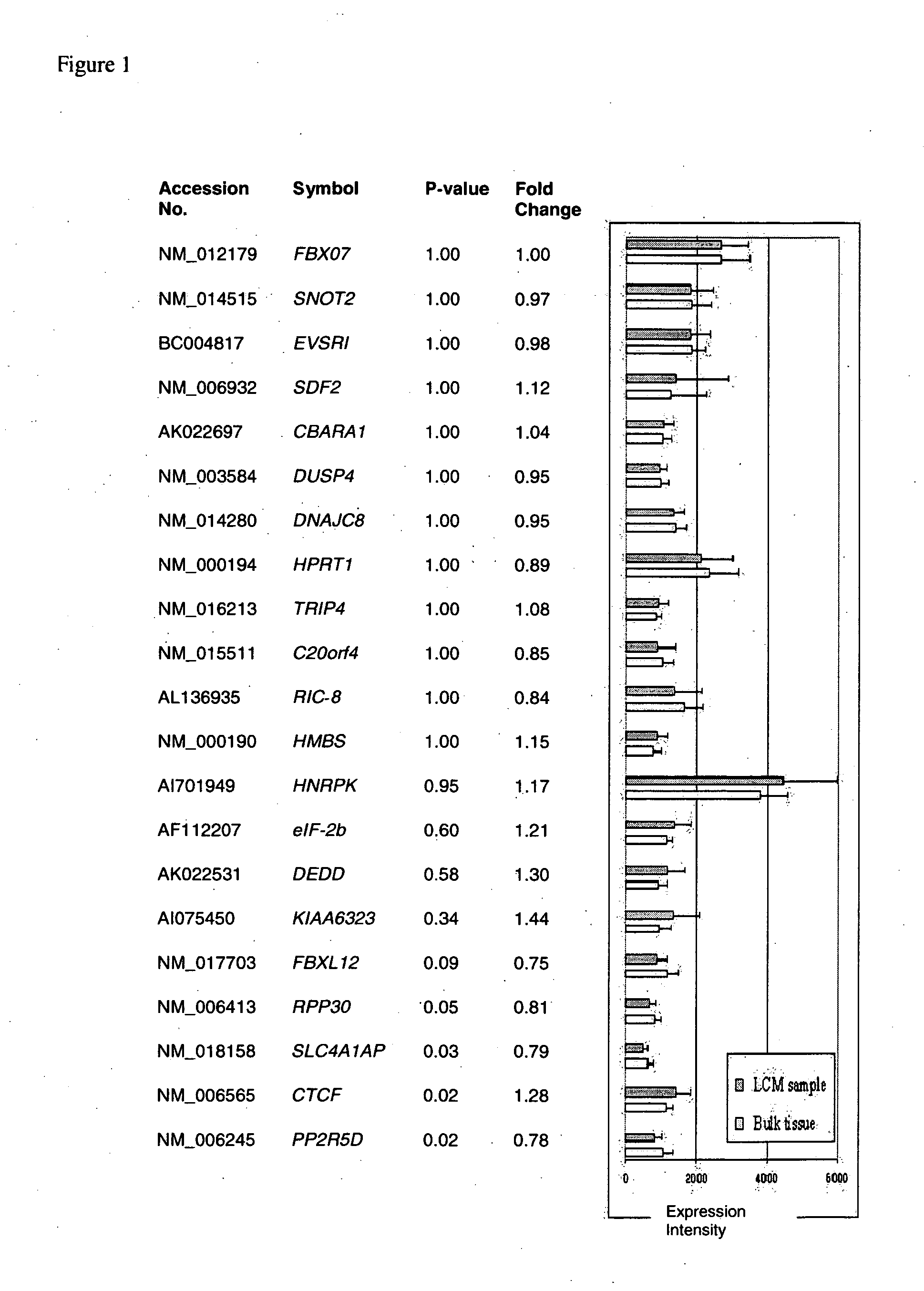
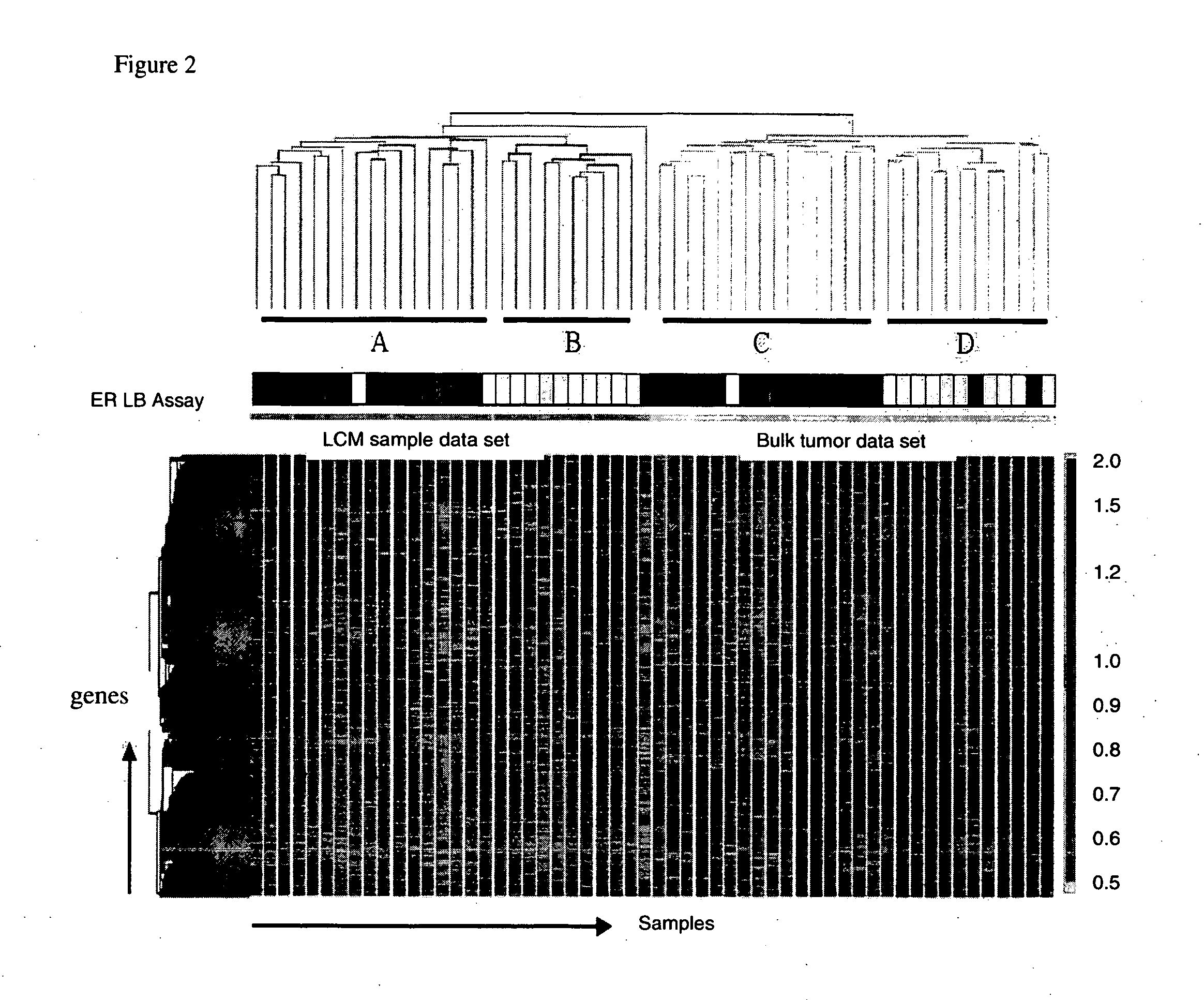
About 70% to 80% of breast cancers express estrogen receptor-a (ERa), and estrogens play important roles in the development and growth of hormone-dependent tumors. Together with lymph node metastasis, tumor size and histological grade, ER status is considered one of the prognostic factors in breast cancer, and an indicator for hormonal treatment. 147 genes and 112 genes with significant P- value and having significant differential expression between ER+ and ER- tumors were identified from the LCM data set and bulk tissue data set, respectively. 61 genes were found to be common in both data sets, while 85 genes were unique to the LCM data set and 51 genes were present only in the bulk tumor data set. Pathway analysis with the 85 genes using Gene Ontology suggested that genes involved in endocytosis, ceramide generation, Ras / ERK / Ark cascade, and JAT- STAT pathway may play roles related to ER. The gene profiling with LCM-captured tumpr cells provides a unique approach to characterize and study epithelial tumor cells and to gain an insight into signaling pathways associated with ER.
Owner:VERIDEX LCC
ELISA (enzyme linked immunosorbent assay) kit for detecting endothelial cell specific molecule-1 (ESM-1) of tumour marker
InactiveCN102175850AImprove early diagnosis ratePredictable outcomeBiological testingGastric carcinomaHorseradish peroxidase
The invention provides an ELISA (enzyme linked immunosorbent assay) kit for detecting endothelial cell specific molecule-1 (ESM-1) of a tumour marker, wherein the kit comprises (1) a coated antibody, wherein the coated antibody is a mouse anti-human ESM-1 antibody; (2) a detection antibody, wherein the detection antibody is a biotinylated goat anti-human Endocan antibody; (3) an enzyme linked antibody, wherein the enzyme liked antibody is a rabbit anti-goat IgG (immunoglobulin G) marked by a horseradish peroxidase; (4) a human Endocan standard substance; and (5) a colour development substrate of the enzyme linked antibody. By the kit provided by the invention, the early diagnosis rate of gastric cancer can be improved under a relatively small wound; the biomarker is an independent prognostic factor and is relevant to vessel invasion, distant metastasis and general classification of gastric cancer, so that prognosis can be predicted, states of the illness can be monitored, and treatments can be guided.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL
Krüppel-like transcriptional factor KLF4/GKLF and uses thereof
The present invention proivdes methods of identifying new carcinoma oncogenes or analyzing functions of known carcinoma oncogenes by transformation of RK3E cells. Also provided are methods of using nuclear localization of Krüppel-like factor 4 (KLF4, or termed Gut-Enriched Krüppel-Like Factor, GKLF) as a marker and prognostic factor for aggressive early-stage breast carcinoma.
Owner:UAB RES FOUND
Methods for detecting antibodies
Methods for detection of any antibody utilizing a standardized approach applicable to any antibody which provides highly specific assays specific for individual or multiple antibodies. The methods enable improved pharmacokinetic analysis during development and clinical use of antibody-based therapies as well as determination of diagnostic and / or prognostic factors.
Owner:RGT UNIV OF CALIFORNIA
Gene biomarkers for prediction of susceptibility of ovarian neoplasms and/or prognosis or malignancy of ovarian cancers
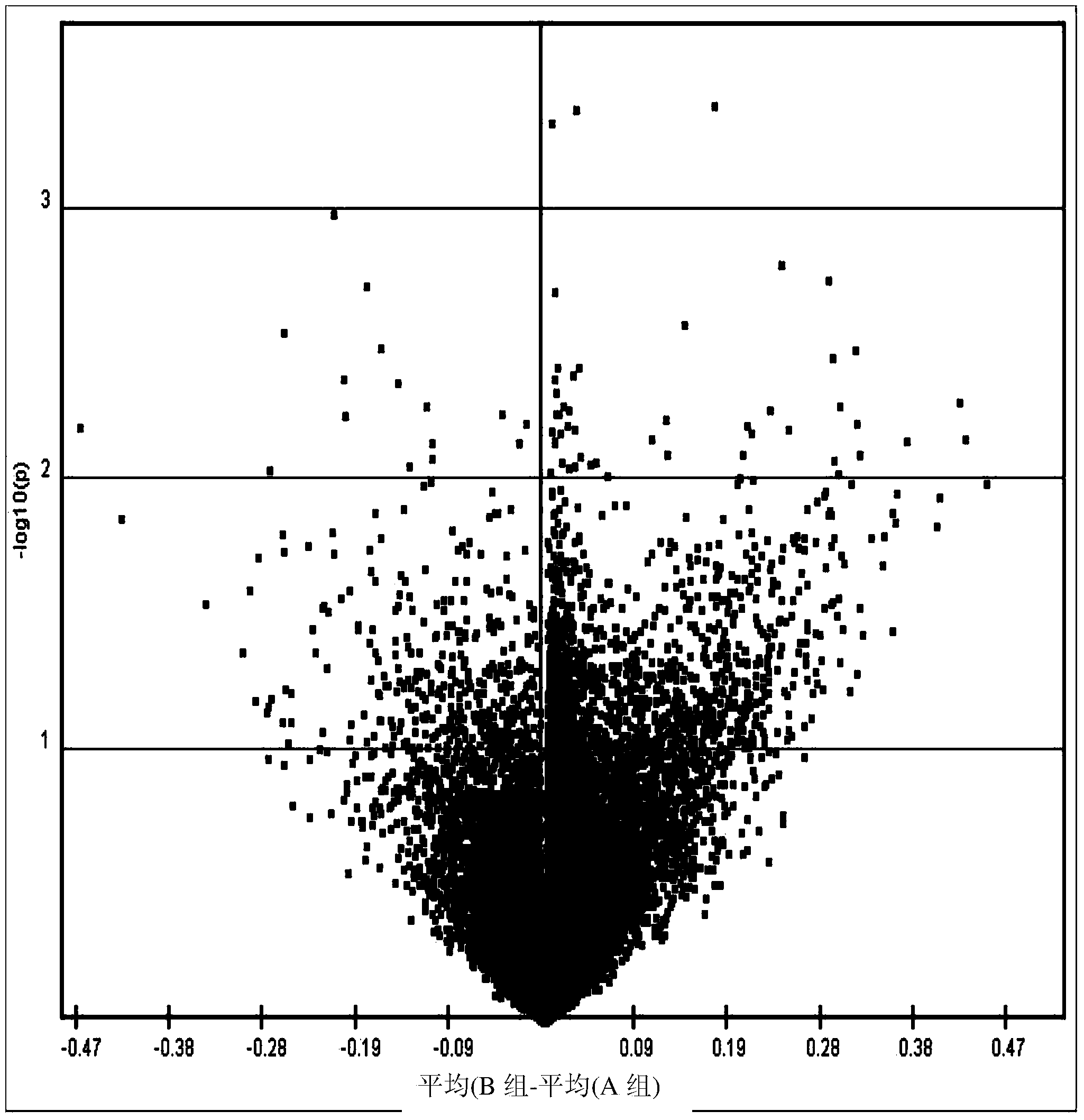
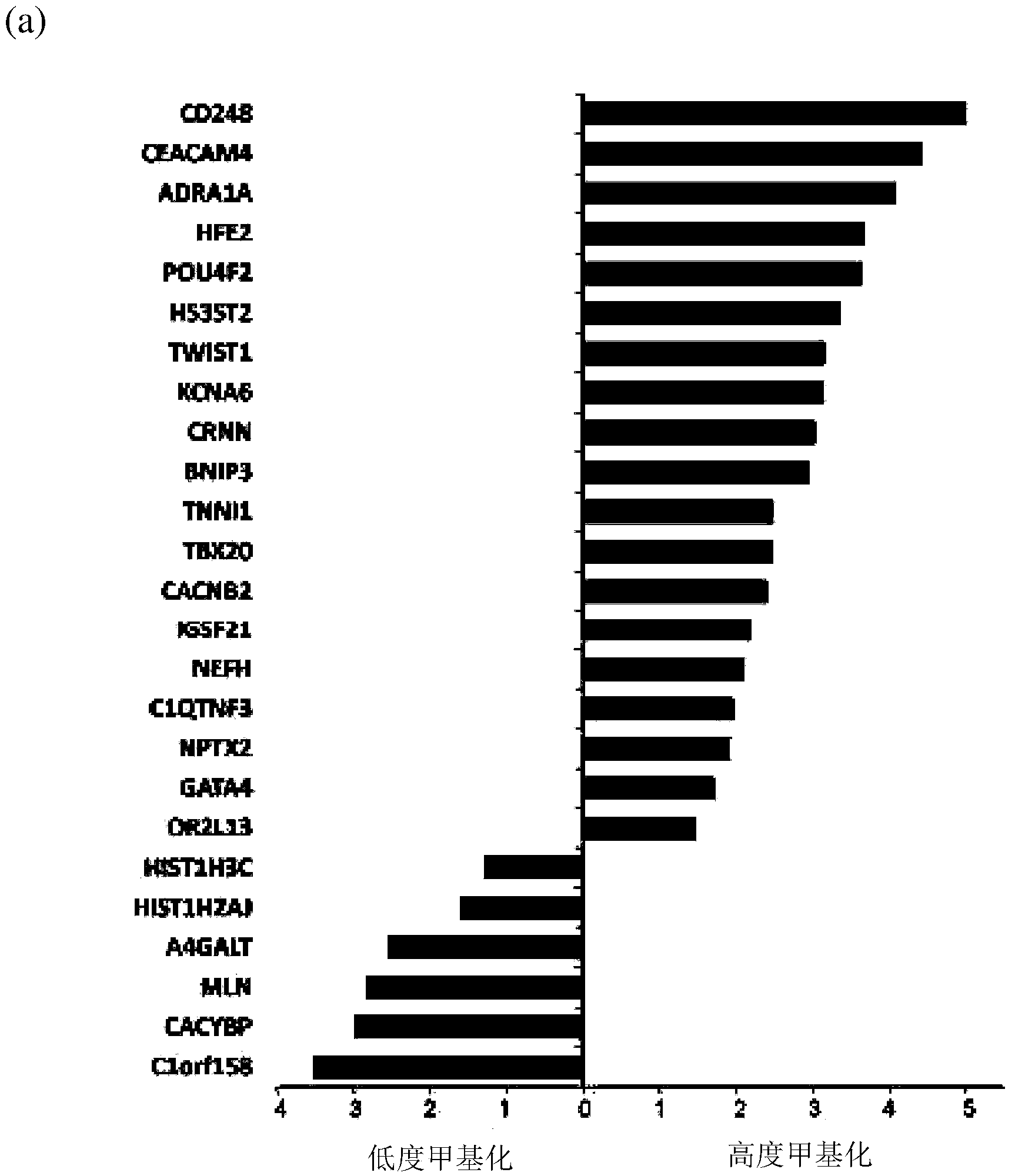
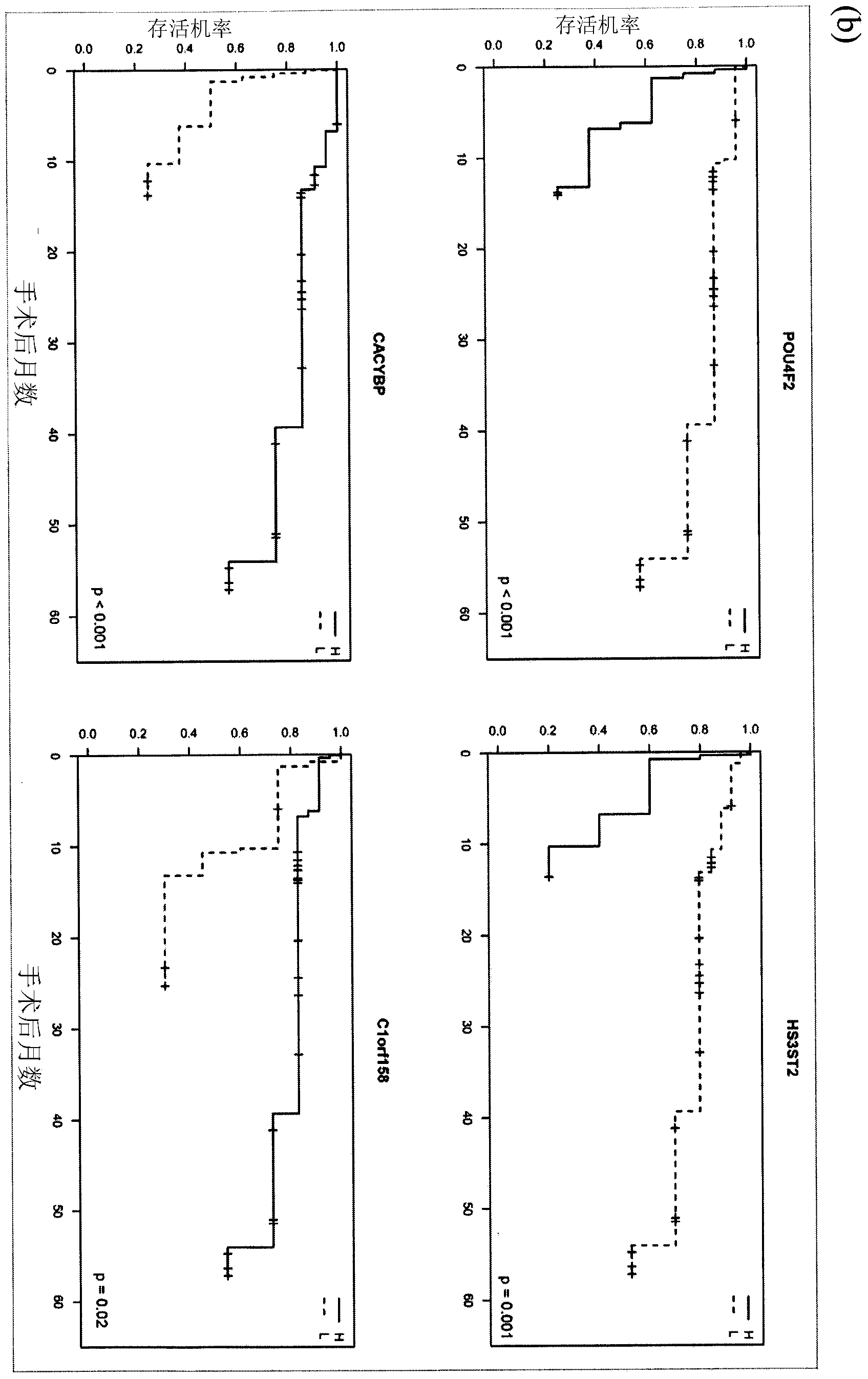
The disclosure provides methylome analysis using DNA methylation biomarkers for predicting ovarian cancer prognosis and detecting malignant ovarian cancer. Being independent prognostic factors for patients with current treatment protocols, these DNA methylations are also important biomarkers for treating patients with individualized medicine in future including using chemotherapy, especially the demethylation agents or other epigenetic drugs.
Owner:DCB USA +1
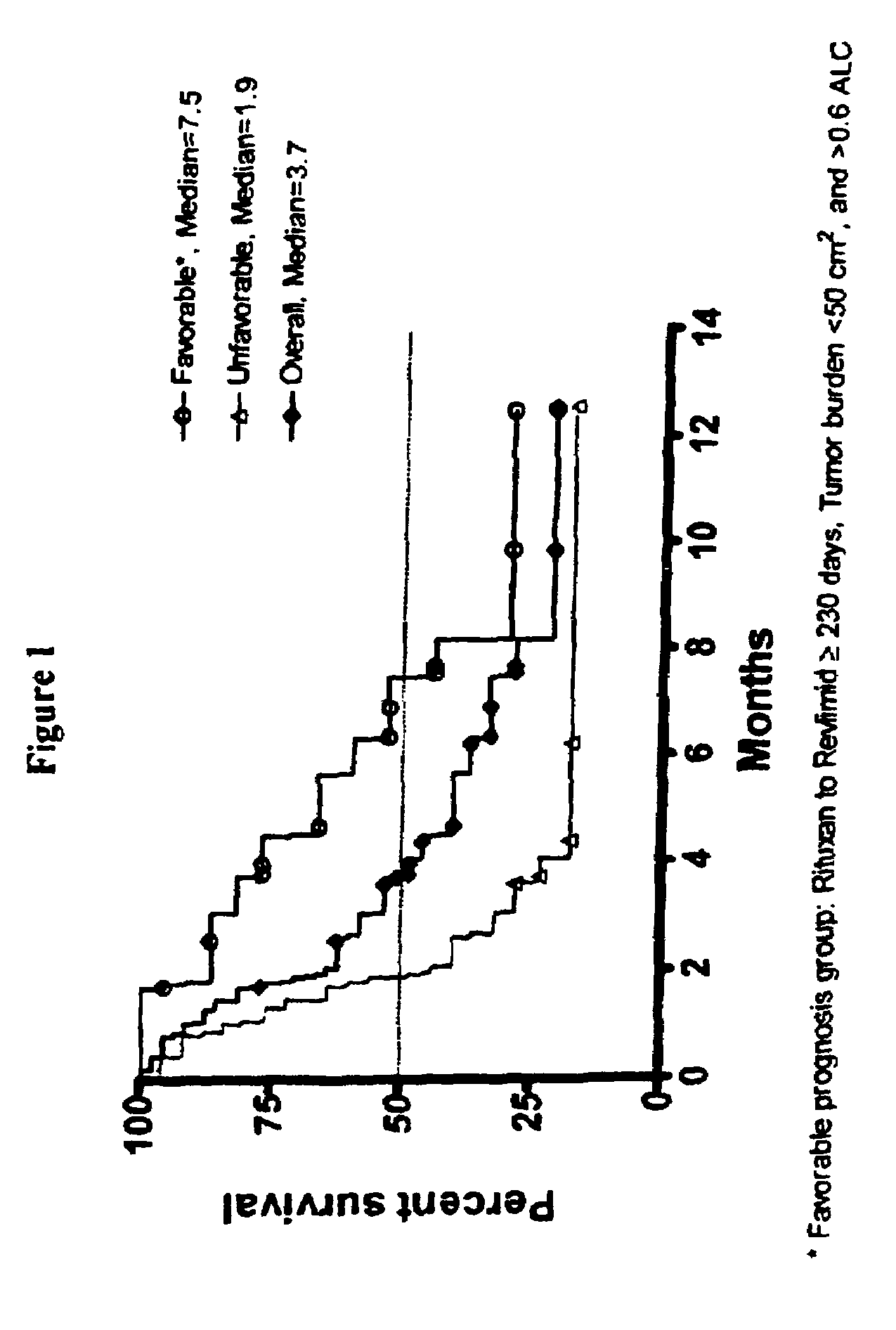
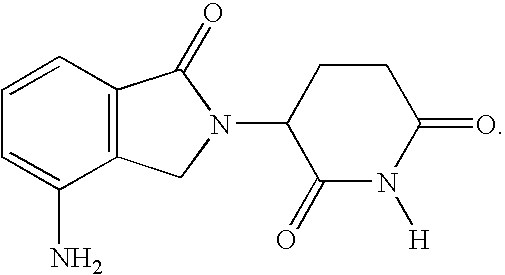
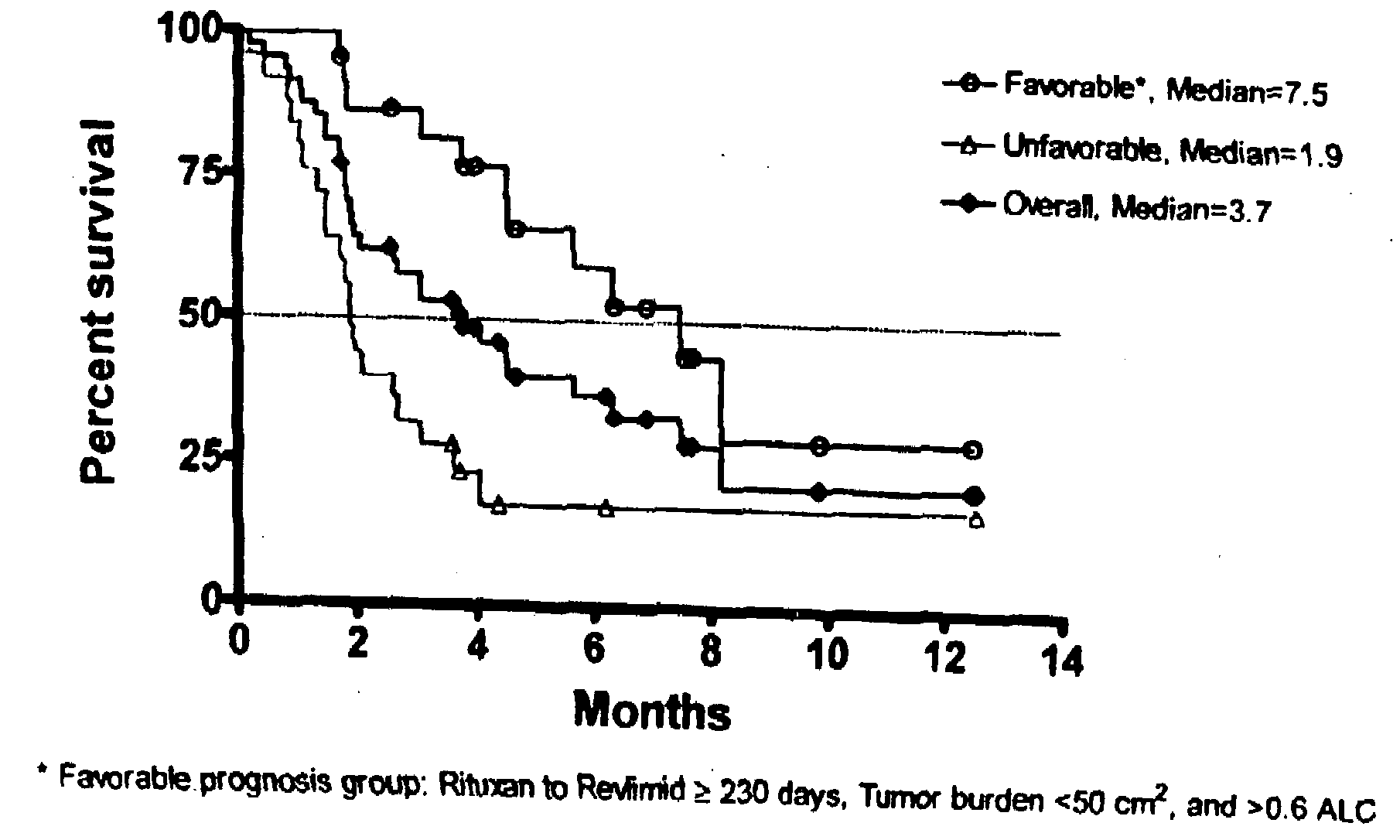
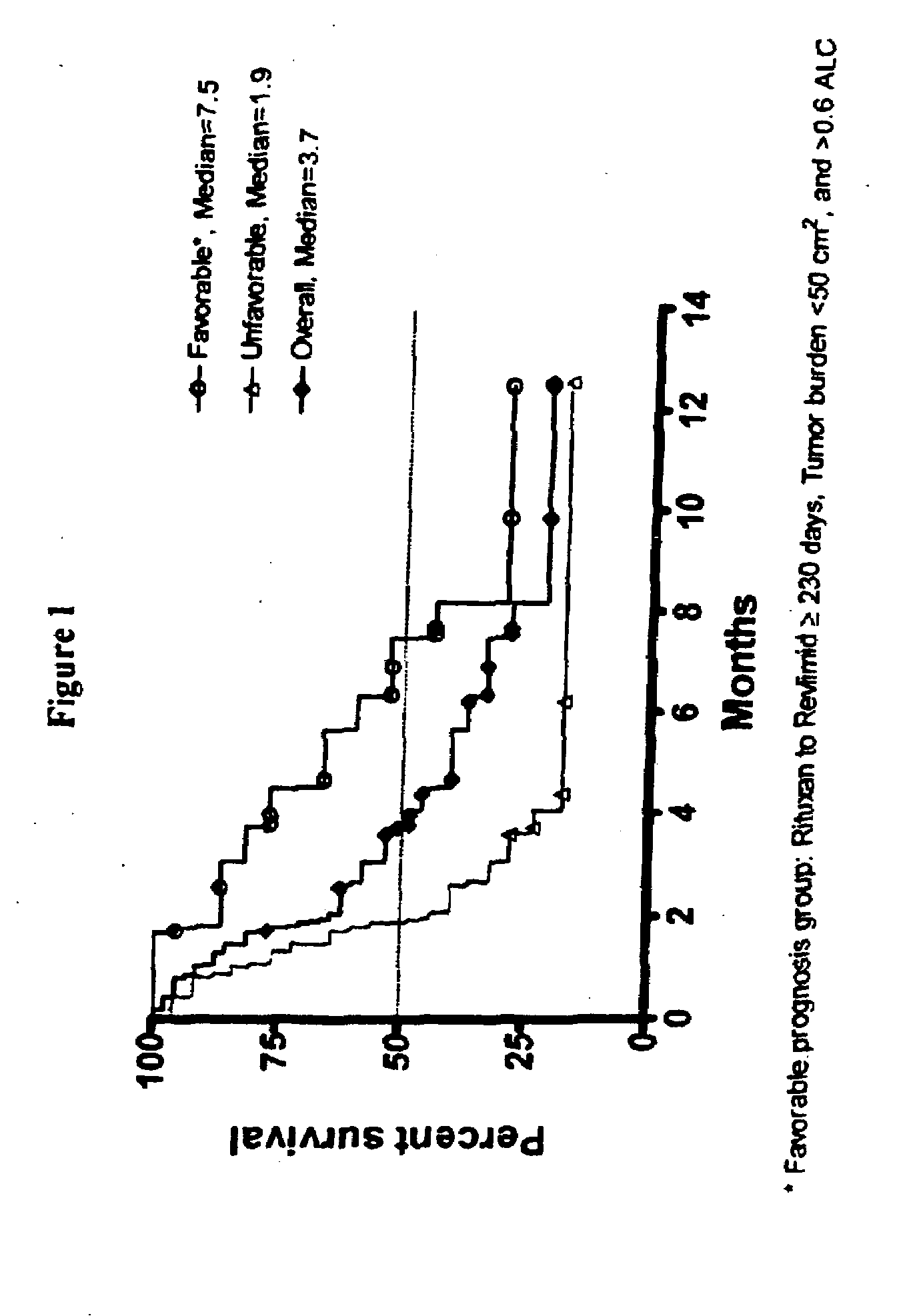
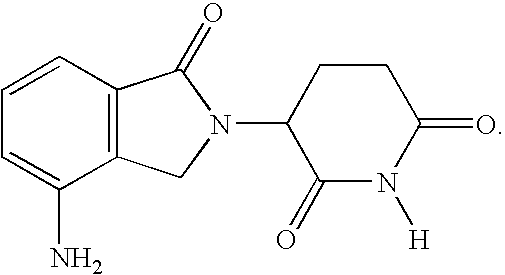
Methods for treating lymphomas in certain patient populations and screening patients for said therapy
Methods for predicting a response of a patient having a lymphoma to a therapy regimen of 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione using prognostic factors of a patient's disease burden, absolute lymphocyte count or time since last rituximab therapy are disclosed. Specific methods of treating a lymphoma encompass the administration of 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione to a patient who has one or more of the favorable profiles, alone or in combination with immunosuppressive agents such as rituximab.
Owner:CELGENE CORP
Methods for treating lymphomas in certain patient populations and screening patients for said therapy
InactiveUS20090081203A1High response rateBiocideAntibody ingredientsAbsolute lymphocyte countRegimen
Methods for predicting a response of a patient having a lymphoma to a therapy regimen of 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione using prognostic factors of a patient's disease burden, absolute lymphocyte count or time since last rituximab therapy are disclosed. Specific methods of treating a lymphoma encompass the administration of 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione to a patient who has one or more of the favorable profiles, alone or in combination with immunosuppressive agents such as rituximab.
Owner:CELGENE CORP
Method of diagnosing early stage non-small cell lung cancer
InactiveUS20130252831A1Easy to predictMicrobiological testing/measurementLibrary screeningPrincipal component analysisSmoking status
A “malignancy-risk” (MR) gene signature score was developed with abundant proliferative genes using principal component analysis. This MR gene signature was shown to be a predictive and prognostic factor of overall survival in early-stage NSCLC. The malignancy-risk signature showed a significant association with OS, with poor survival seen in patients having a higher MR score and better survival seen in patients having a low MR score. As a prognostic factor, the MR gene signature showed a positive correlation with TNM stage, histologic grade, and smoking status. Combination of the MR signature with each clinical parameter often showed the best survival in the low MR group with good clinical outcome. The MR gene profile, tested with a PCA scoring method, discriminated overall survival in lung cancer patients was a predictor independent of pathological staging and other clinical parameters.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC
Method for prognostic factor analysis regarding cancers
InactiveUS7412333B2Analogue computers for chemical processesBiological testingAbnormal tissue growthWilms' tumor
Owner:INTELLIGENT ONCOTHERAPEUTICS
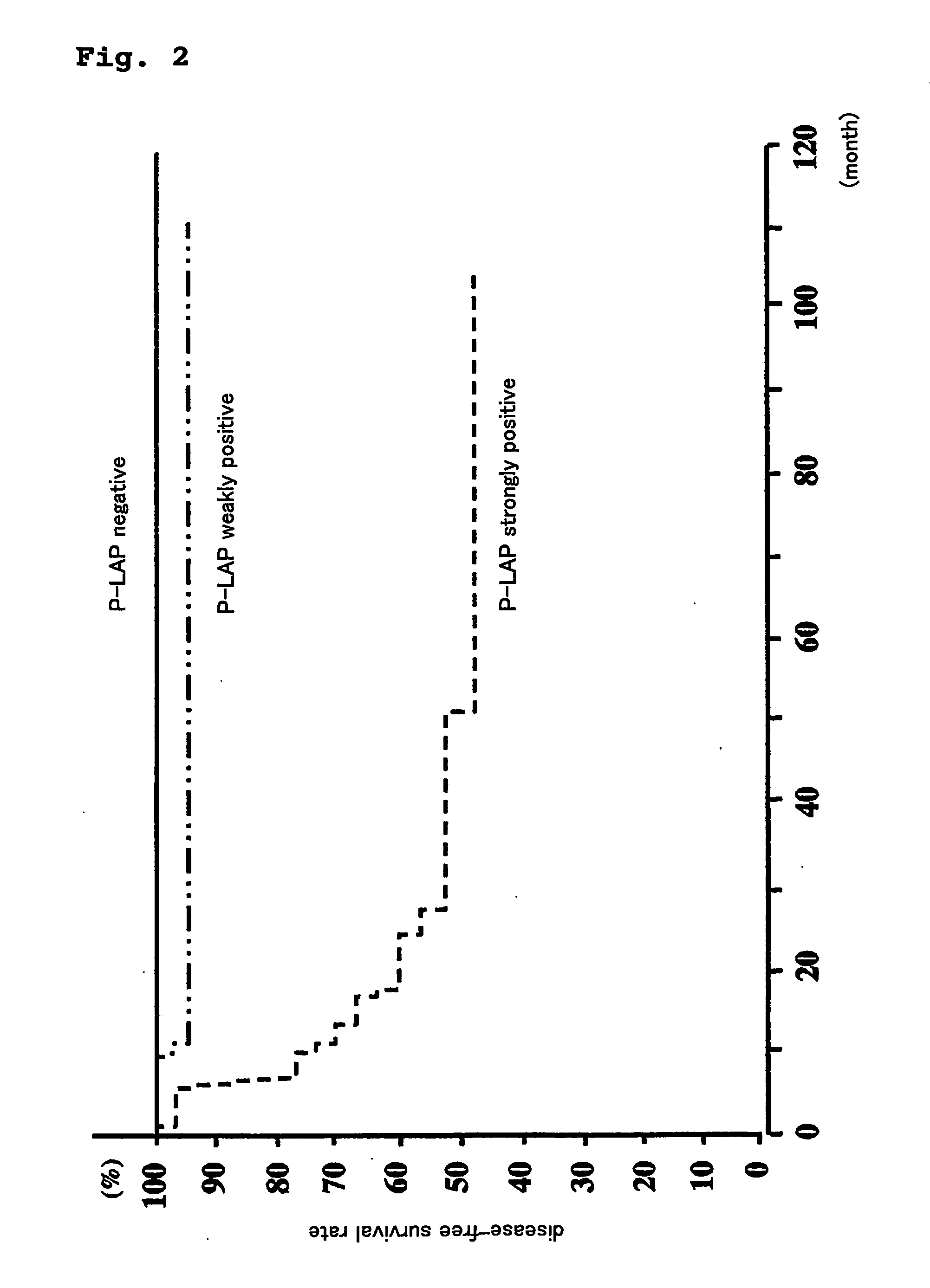
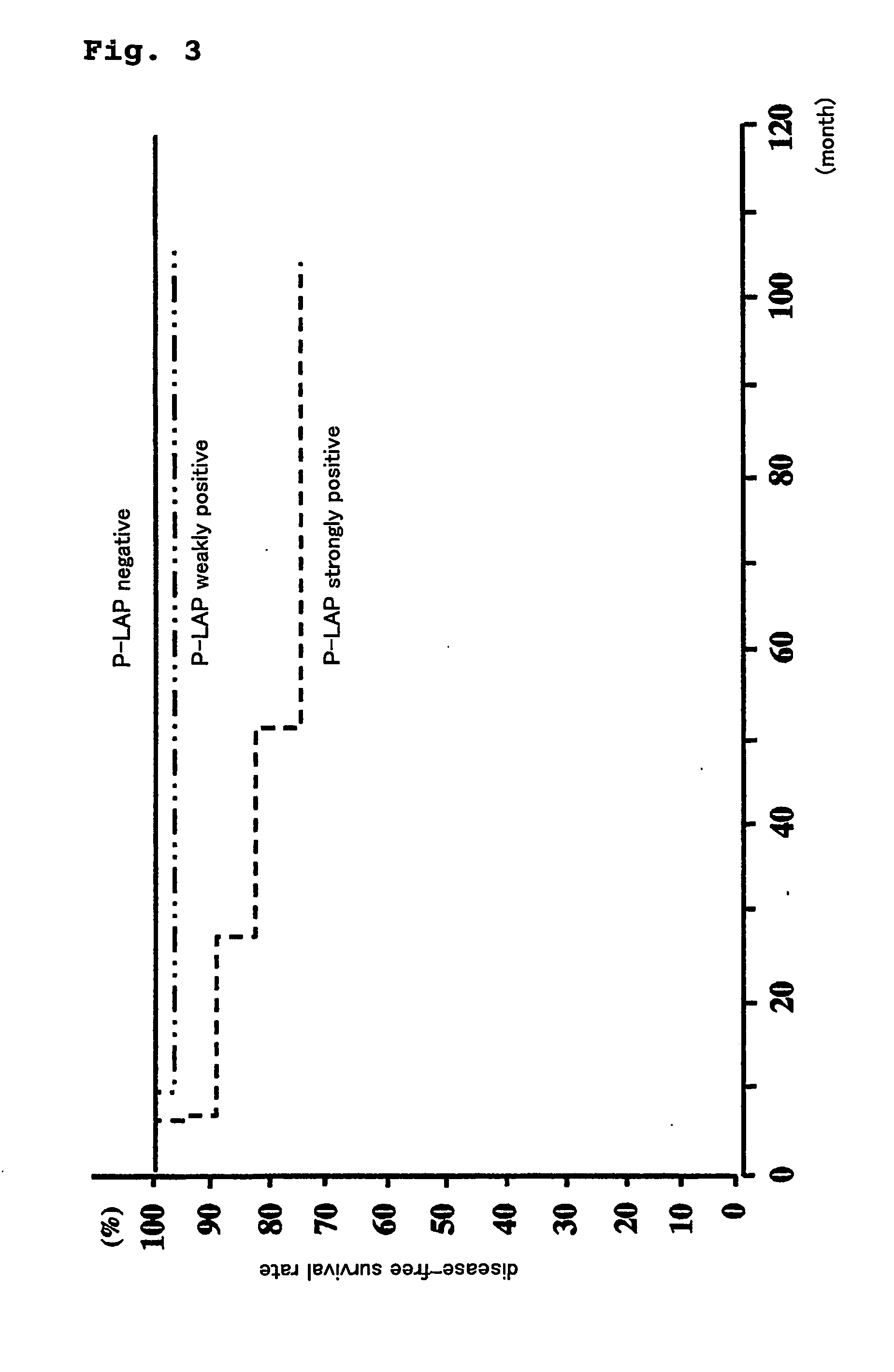
Method for prognostic evaluation of carcinoma using anti-p-lap antibody
ActiveUS20070020705A1Accurate prognostic evaluationAppropriate treatmentBiological material analysisAntibody ingredientsSurgical operationAntigen-antibody reactions
The present invention provides a reagent for prognostic evaluation of carcinoma patients, comprising an anti-P-LAP antibody as an active ingredient. Also, the present invention provides a method for determination of P-LAP which is a prognostic factor in carcinoma patients comprising (a) a step of contacting carcinoma tissues obtained from carcinoma patients by surgical operation with an anti-P-LAP antibody and (b) a step of measuring the intensity of the specific antigen-antibody reaction between P-LAP present in the carcinoma tissues and anti-P-LAP antibody.
Owner:SUNTORY HLDG LTD
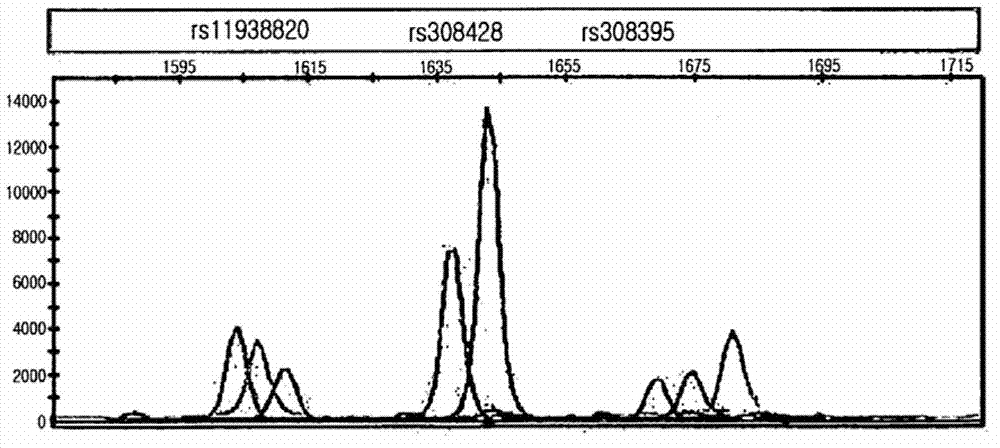
Single nucleotide polymorphism for prognosis of hepatocellular carcinoma
ActiveCN102906277AImprove poor prognosisSugar derivativesMicrobiological testing/measurementHepatocellular carcinomaSurgery
The present invention relates to a single nucleotide polymorphism (SNP) for the prognosis of hepatocellular carcinoma, wherein the SNP shows a significant correlation with an over-expression of MTA1 which is a useful prognostic factor for prediction of recurrence or poor survival after hepatocellular carcinoma surgery. Therefore, the SNP can be used in developing micro-arrays or diagnosis kits for the prognosis of hepatocellular carcinoma, and in screening drugs for improving prognosis of hepatocellular carcinoma to thereby improve poor prognosis of hepatocellular carcinoma after surgery.
Owner:郑永化
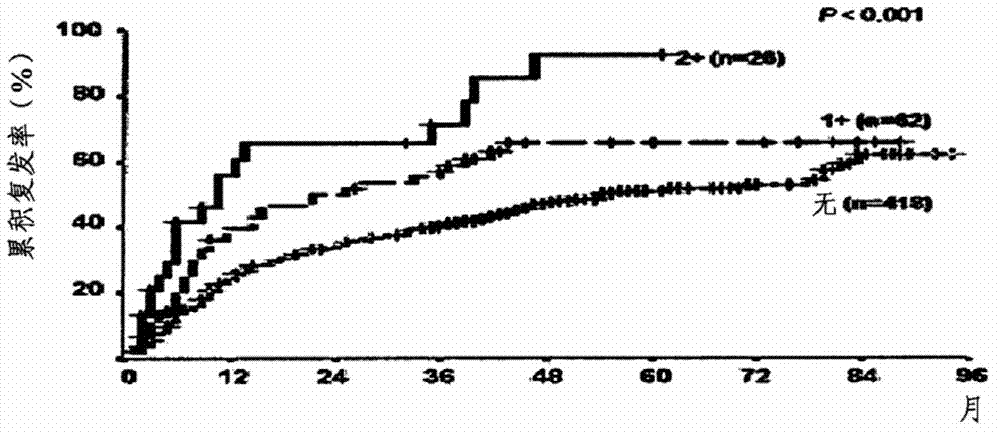
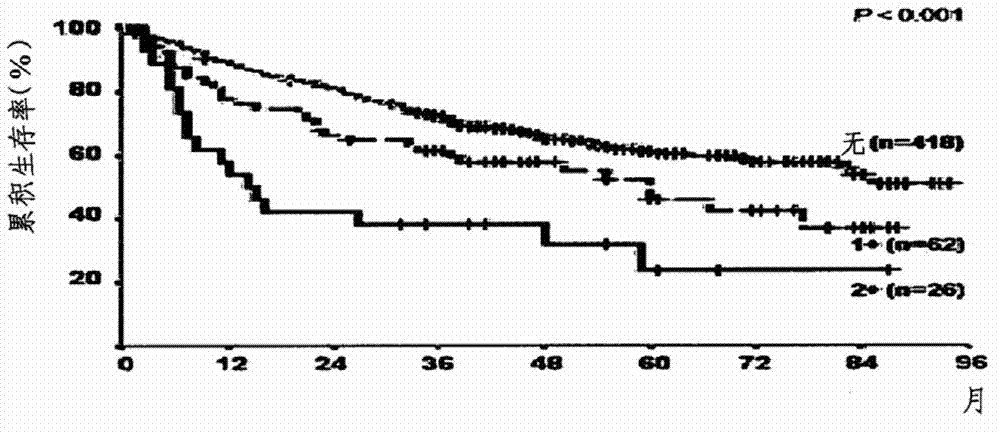
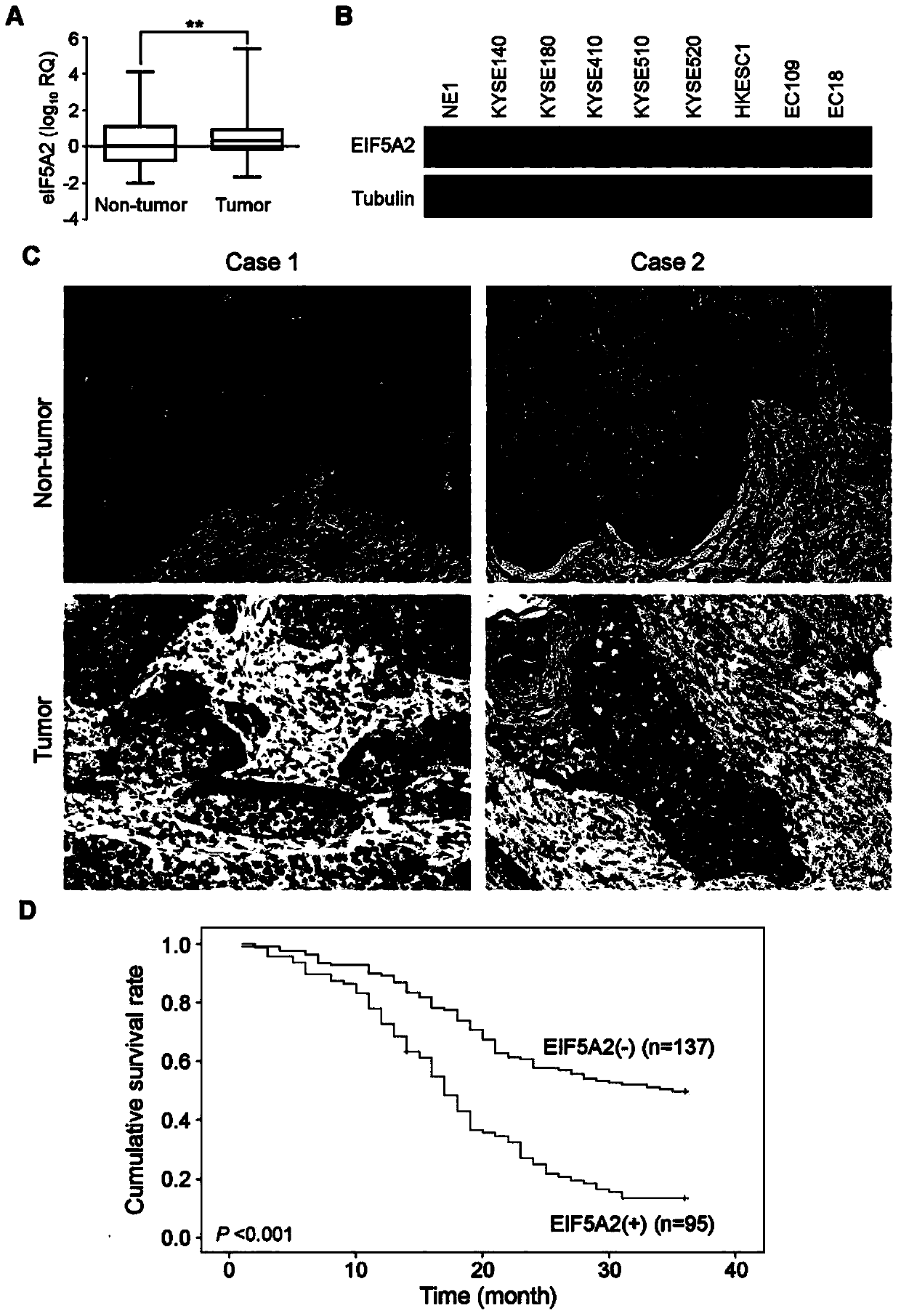
Application of EIF5A2 to preparation of esophageal squamous cell carcinoma prognosis reagent
ActiveCN103468799APromote formationPromote invasionMicrobiological testing/measurementMaterial analysisOxygen deprivationNode metastasis
The invention discloses application of EIF5A2 to preparation of an esophageal squamous cell carcinoma prognosis reagent. Real-time quantitative PCR (Polymerase Chain Reaction) detection data shows that expression of the EIF5A2 in esophageal squamous cell carcinoma tissues is obviously higher than that in corresponding non-tumor tissues. An immunohistochemical data analysis result shows that expression of the EIF5A2 is positively correlated to lymphatic metastasis, the tumor invasion depth and neoplasm staging. Kaplan-Meier analysis shows that a patient overexpressed by the EIF5A2 has poor overall survival (p is less than 0.001). Multivariate analysis also proves that the EIF5A2 is an independent prognostic factor. The EIF5A2 possibly takes important effects in invasion and metastasis of esophageal squamous cell carcinoma. Further experimental data proves that overexpression of the EIF5A2 can be caused by gene amplification and oxygen deprivation; the EIF5A2 can be combined into a promoter region of HIF1a to up-regulate expression of the HIF1a; and the EIF5A2 can induce epithelial-mesenchymal transition conversion and promote angiopoiesis of the esophageal squamous cell carcinoma so as to promote invasion and metastasis of the esophageal squamous cell carcinoma. Knockout of the EIF5A2 can obviously inhibit tumor metastasis and the silent EIF5A2 is expected to be a potential target of esophageal squamous cell carcinoma treatment.
Owner:SUN YAT SEN UNIV CANCER CENT
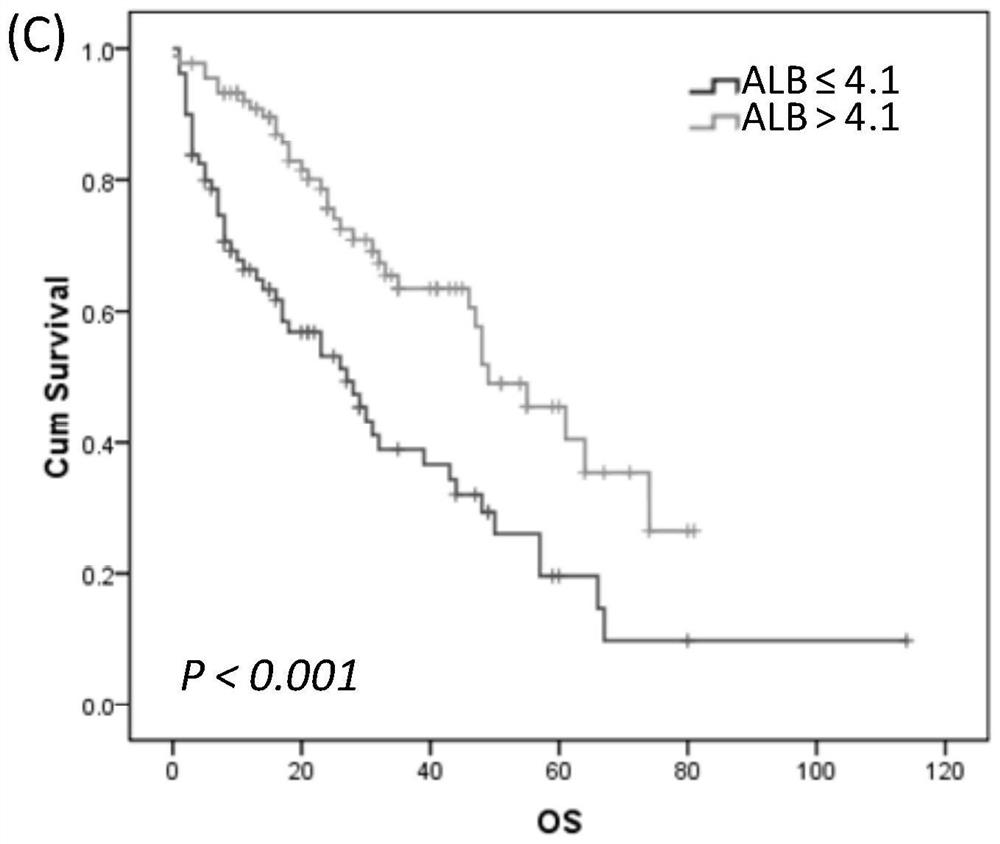
Establishment method and application of primary central nervous system lymphoma prognosis model based on albumin and ECOG-PS
PendingCN113470753AEase of evaluationAccurate assessmentHealth-index calculationBiostatisticsSerum albuminRisk groups
The invention discloses a primary central nervous system lymphoma prognosis model based on albumin and ECOG-PS. The model comprises related prognosis factors, risk groups corresponding to evaluation scores of different states of the prognosis factors and prognosis states corresponding to the risk groups, wherein the prognosis factors are composed of serum albumin content and ECOG-PS. According to the invention, the serum albumin content and ECOG-PS are integrated as prognosis evaluation indexes of primary central nervous system lymphoma for the first time, and a novel prognosis evaluation model for patients with primary central nervous system lymphoma is established.
Owner:CANCER CENT OF GUANGZHOU MEDICAL UNIV
Single nucleotide polymorphism for predicting prognosis of hepatocellular carcinoma
InactiveUS20130017975A1Improved prognosisUseful prognosisSugar derivativesNucleotide librariesNucleotideHepatocellular carcinoma
Single nucleotide polymorphisms (SNP) for predicting prognosis of hepatocellular carcinoma after curative surgical resection are provided. The SNPs have a significant correlation with an over-expression of MTA1 which is useful prognostic factor for prediction of prognosis or poor survival after curative surgical resection of hepatocellular carcinoma. Therefore, the SNPs can be used in developing micro-arrays or test kits for prediction of the prognosis of hepatocellular carcinoma, and in screening drugs to improve poor prognosis of hepatocellular carcinoma after curative surgical resection.
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION
Laser microdissection and microarray analysis of breast tumors reveal estrogen receptor related genes and pathways
InactiveUS20080305959A1Sugar derivativesMicrobiological testing/measurementPathway analysisEstrogen receptor
About 70% to 80% of breast cancers express estrogen receptor-α (ERα), and estrogens play important roles in the development and growth of hormone-dependent tumors. Together with lymph node metastasis, tumor size and histological grade, ER status is considered one of the prognostic factors in breast cancer, and an indicator for hormonal treatment. 147 genes and 112 genes with significant P-value and having significant differential expression between ER+ and ER− tumors were identified from the LCM data set and bulk tissue data set, respectively. 61 genes were found to be common in both data sets, while 85 genes were unique to the LCM data set and 51 genes were present only in the bulk tumor data set. Pathway analysis with the 85 genes using Gene Ontology suggested that genes involved in endocytosis, ceramide generation, Ras / ERK / Ark cascade, and JAT-STAT pathway may play roles related to ER. The gene profiling with LCM-captured tumor cells provides a unique approach to characterize and study epithelial tumor cells and to gain an insight into signaling pathways associated with ER.
Owner:VERIDEX LCC
Method and apparatus for prognostic factor analysis regarding cancers
InactiveUS20020198664A1Analogue computers for chemical processesBiological testingWilms' tumorCancer research
A method for treating a patient with cancer. The method includes the steps of obtaining cells from a cancerous tumor in the patient. Then there is the step of performing multiple correlated measurements on each cell to obtain cell data for each cell regarding each cell's place in a genetic evolutionary sequence that had occurred in the tumor. Next there is the step of determining from the cell data a likelihood of recurrence of the cancer by minimizing false negatives. A method for treating a patient with cancer. The method includes the steps of obtaining cells from a cancerous tumor in the patient. Then there is the step of obtaining cell data for each cell regarding each cell's place in a genetic evolutionary sequence that had occurred in the tumor. Next there is the step of recognizing distinctive false negative patterns from the cell data. Then there is the step of correlating the false negative patterns to aberrations in mitogenic signaling to determine a likelihood of recurrence of the cancer in the patient. An apparatus for treating a patient with cancer. The apparatus includes a mechanism for obtaining cell data from cells of a cancerous tumor in the patient to obtain cell data for each cell regarding each cell's place in a genetic evolutionary sequence that has occurred in the tumor. The apparatus includes a mechanism for determining from the cell data a likelihood of recurrence of the cancer by minimizing false negatives.
Owner:INTELLIGENT ONCOTHERAPEUTICS
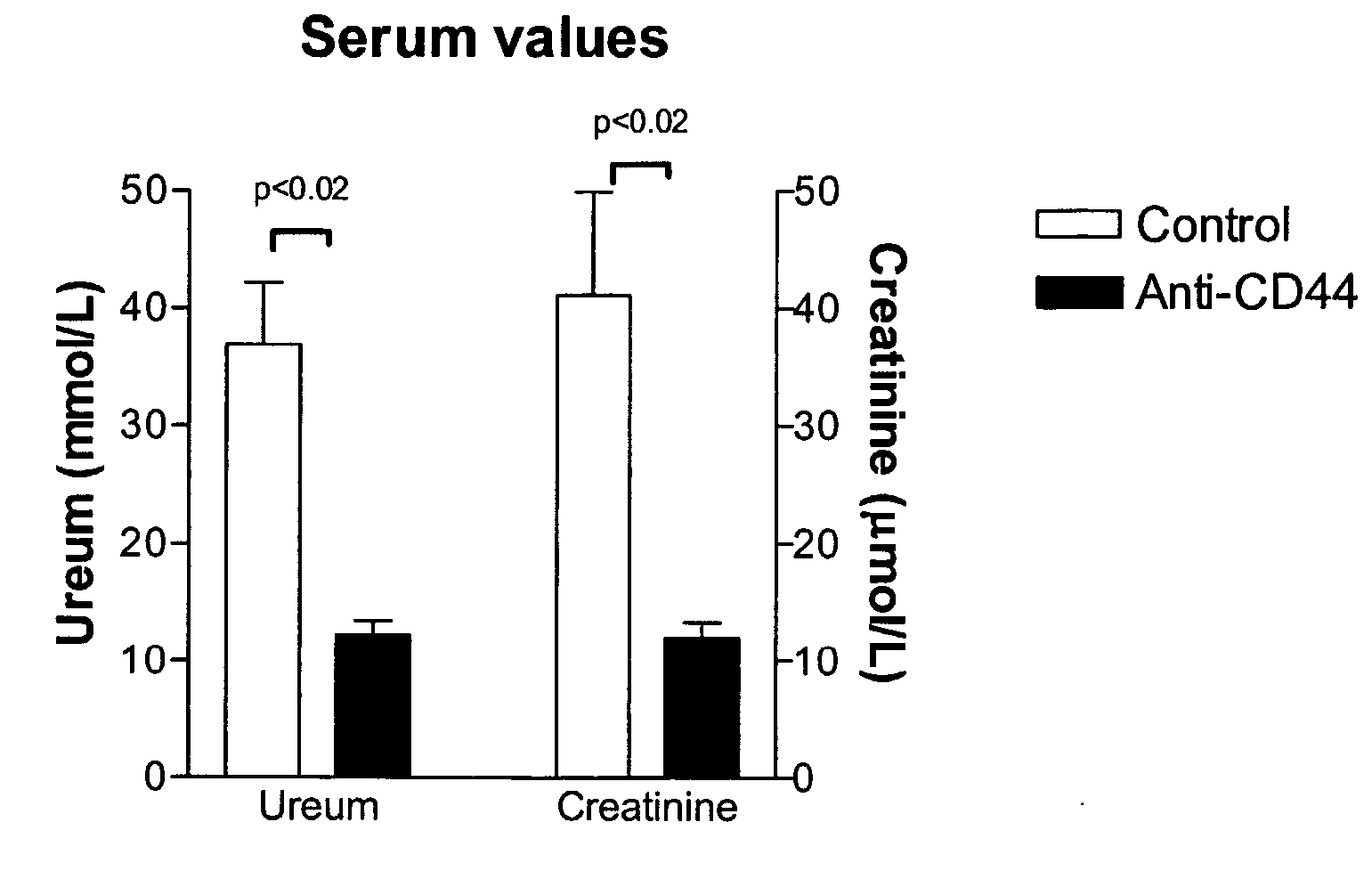
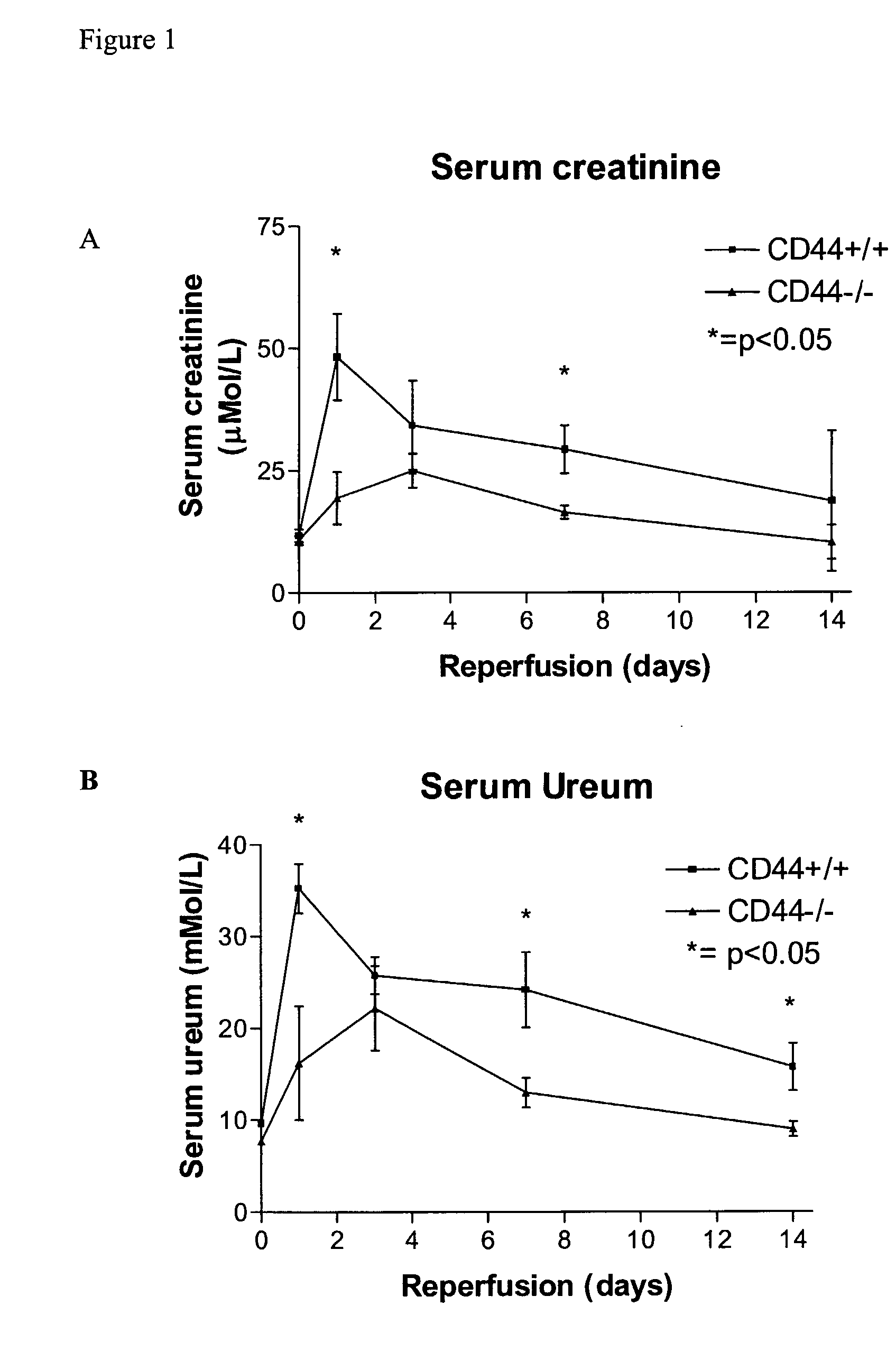
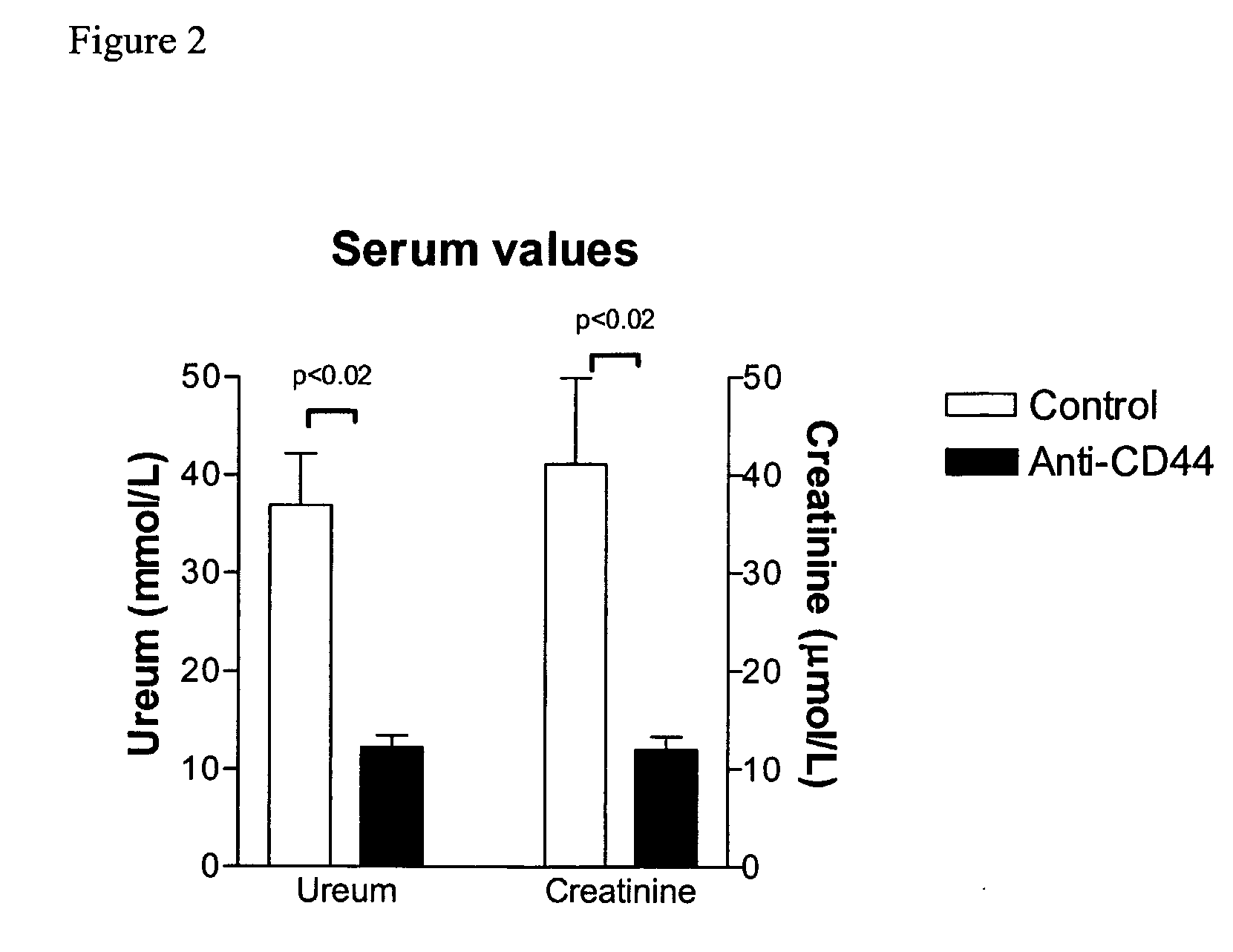
Cd44-Targeting for Reducing/Preventing Ischemia-Reperfusion-Injury
InactiveUS20070280930A1Easy to storePrevent and reduce injuryOrganic active ingredientsBiocideReperfusion injuryOrgan transplantation
The present invention relates to CD44 binding molecules, preferably anti-CD44 antibodies, and their use in methods for the prevention or reduction of ischemia-reperfusion injury in e.g. solid organ transplantation or in patients in shock. The invention further relates to methods wherein levels of soluble CD44 are determined in e.g. serum or urine as a prognostic factor for the risk of organ rejection.
Owner:ROUSCHOP KASPER MATHIAS ANTOON +1
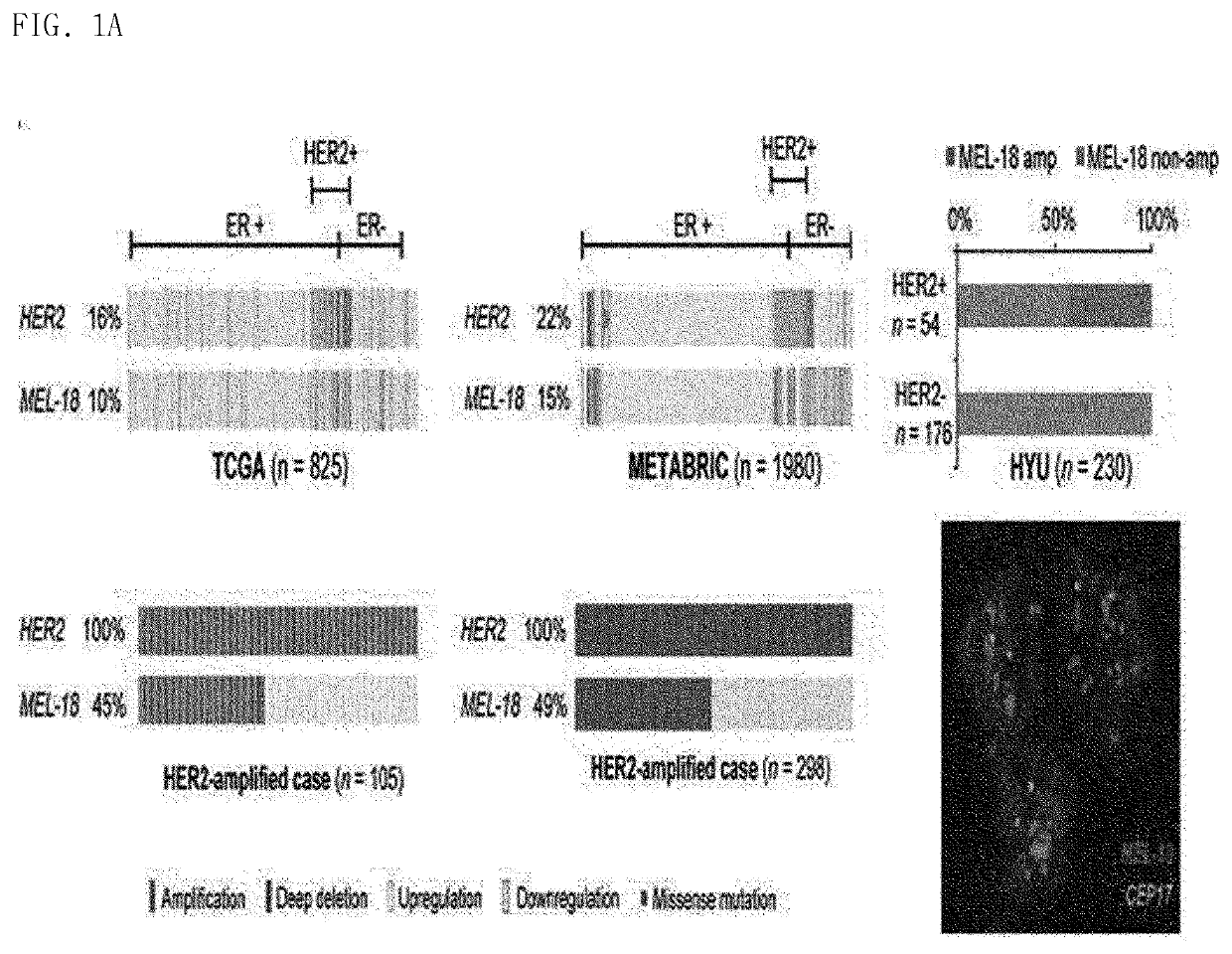
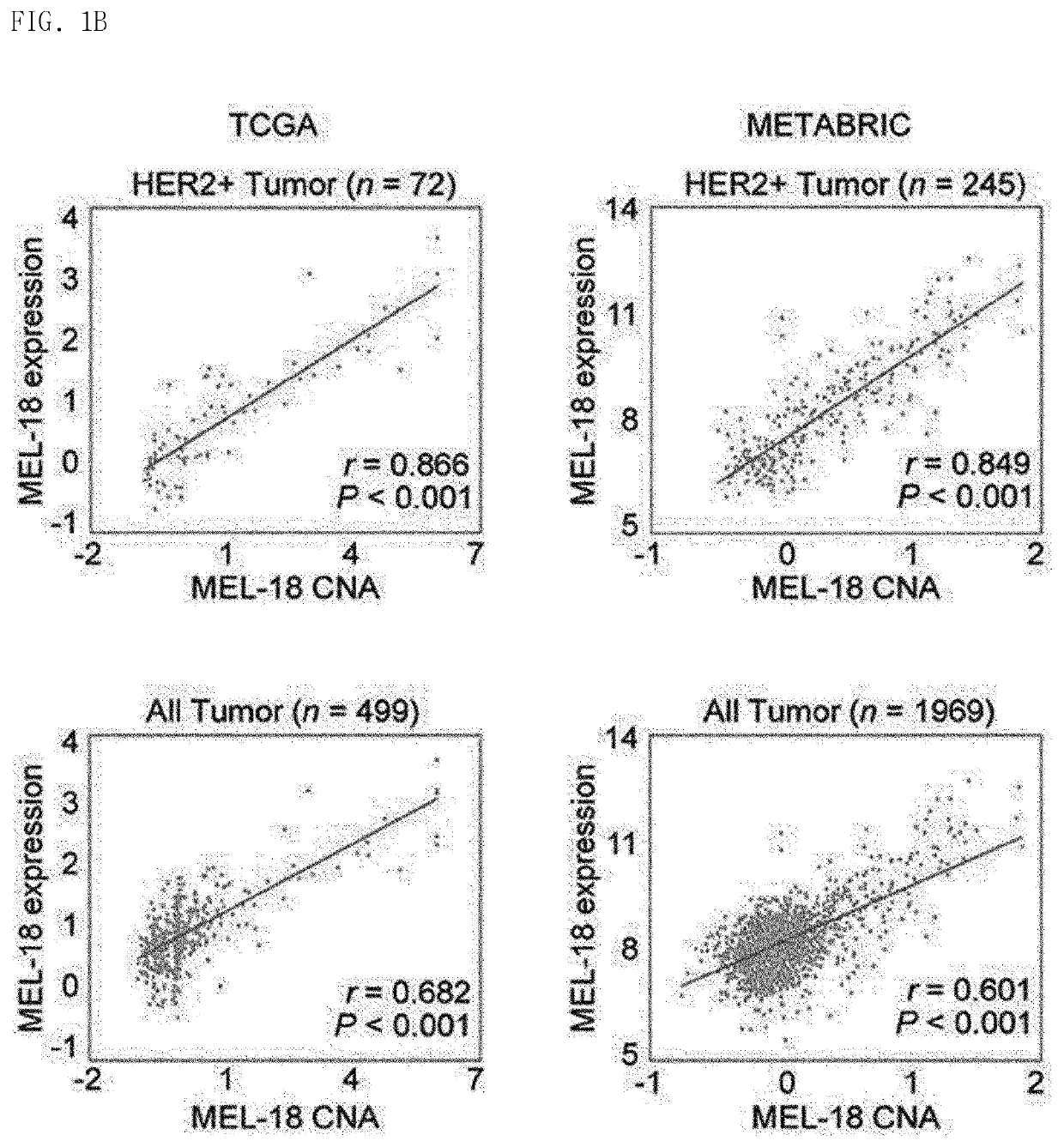
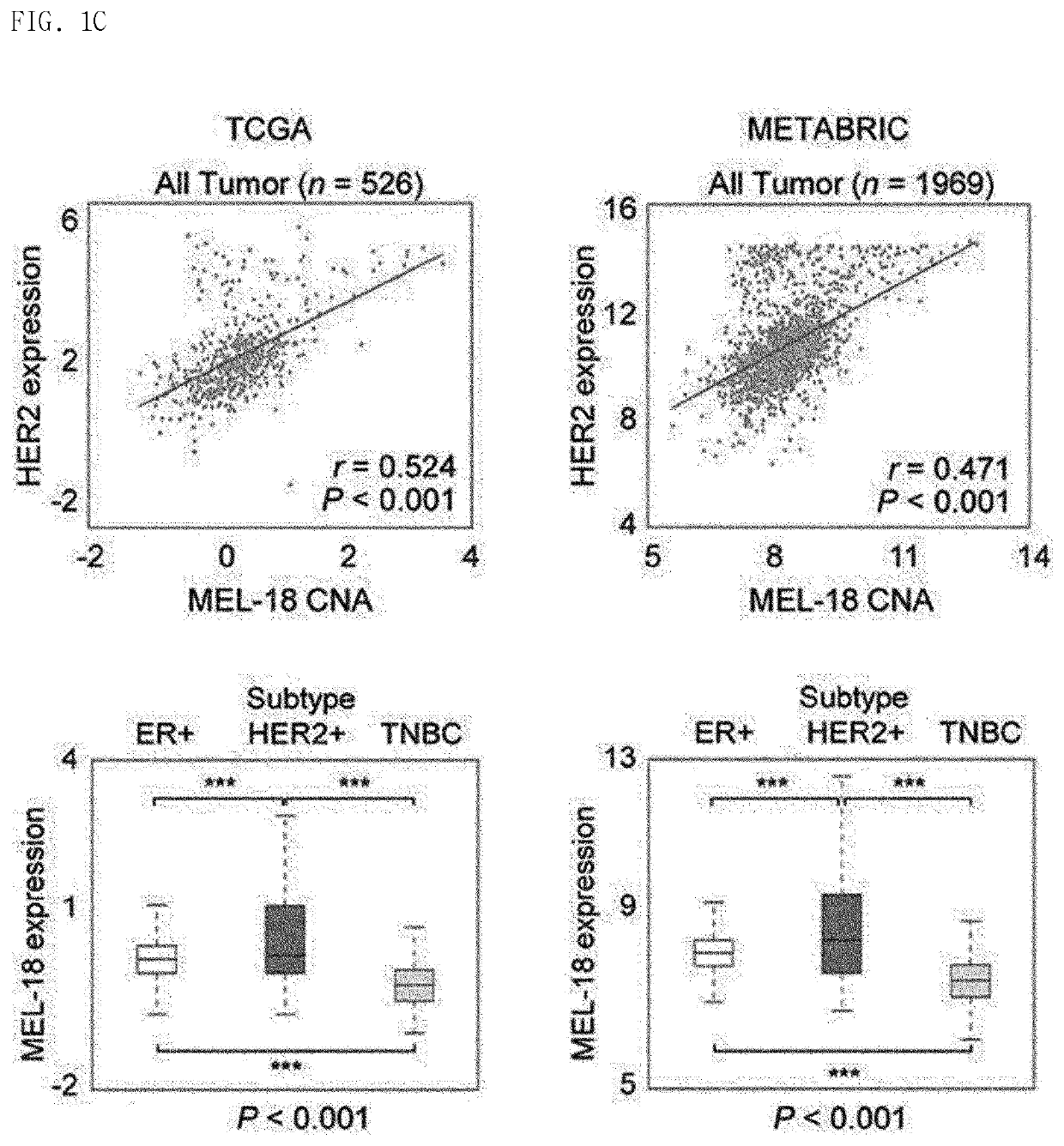
Biomarker for her2-positive cancer and Anti-her2 therapy and applications thereof
ActiveUS20210010084A1Good curative effectEffective treatmentMicrobiological testing/measurementImmunoglobulinsTherapy resistantEfficacy
The present invention provides MEL-18, which is a biomarker for human epidermal growth factor receptor2 (HER2)-positive cancer and anti-HER2 therapy, and a use thereof. According to the present invention, MEL-18 is a prognostic factor or predictor for a response of subjects to an anti-HER2-targeted drug in HER2-POSITIVE cancer and may be used in companion diagnostics for HER2-targeted drugs in subjects with HER2-positive cancer. Therefore, HER2-positive cancer may be more effectively treated by overcoming resistance to HER2 therapeutic agents and enhancing therapeutic efficacy by determining whether ADAM10 / 17 inhibitors are administered or co-administered with HER2-targeted drugs.
Owner:IUCF HYU (IND UNIV COOP FOUNDATION HANYANG UNIV)
Companion diagnostic biomarker for Anti-her2 therapy and use thereof
PendingUS20200248271A1Good effectOvercome resistanceCompound screeningApoptosis detectionTherapy resistantTherapeutic effect
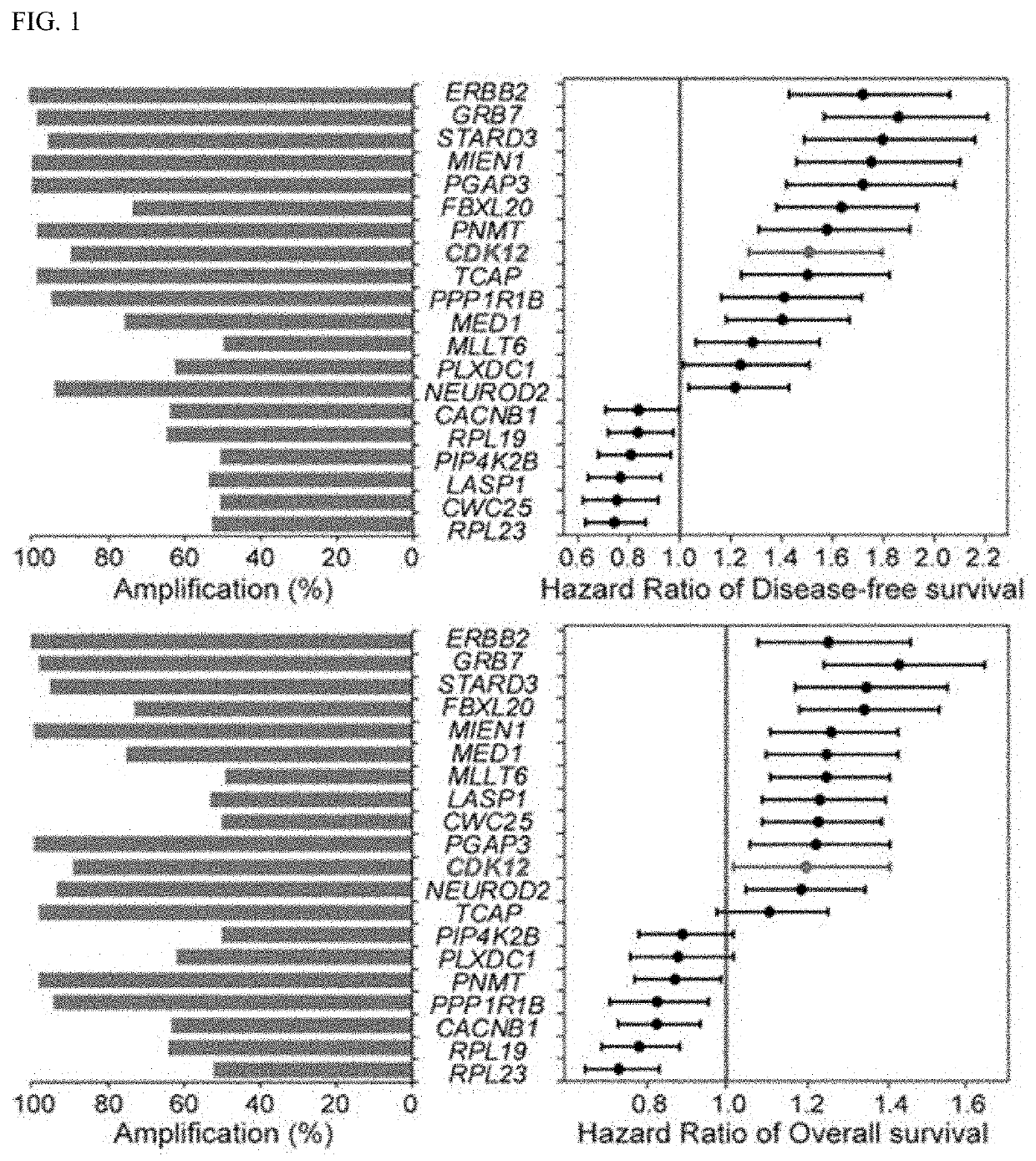
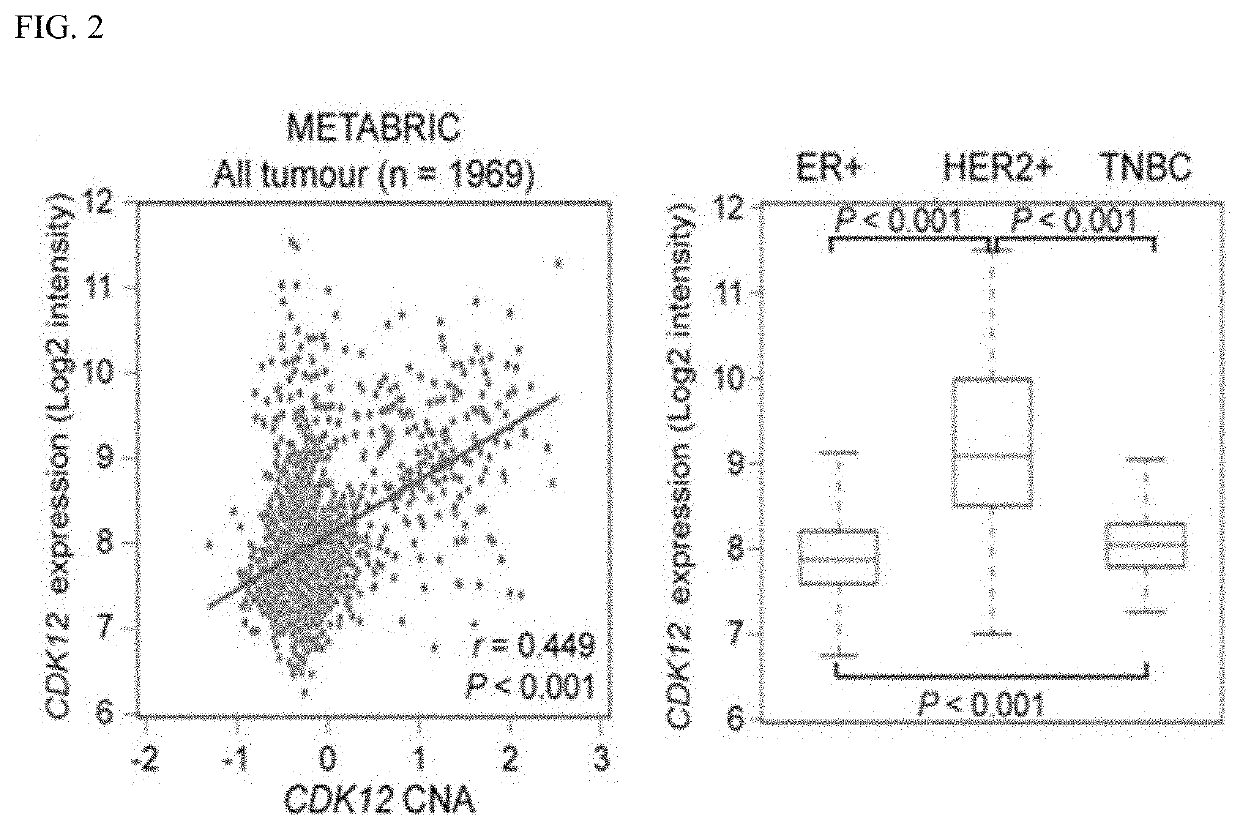
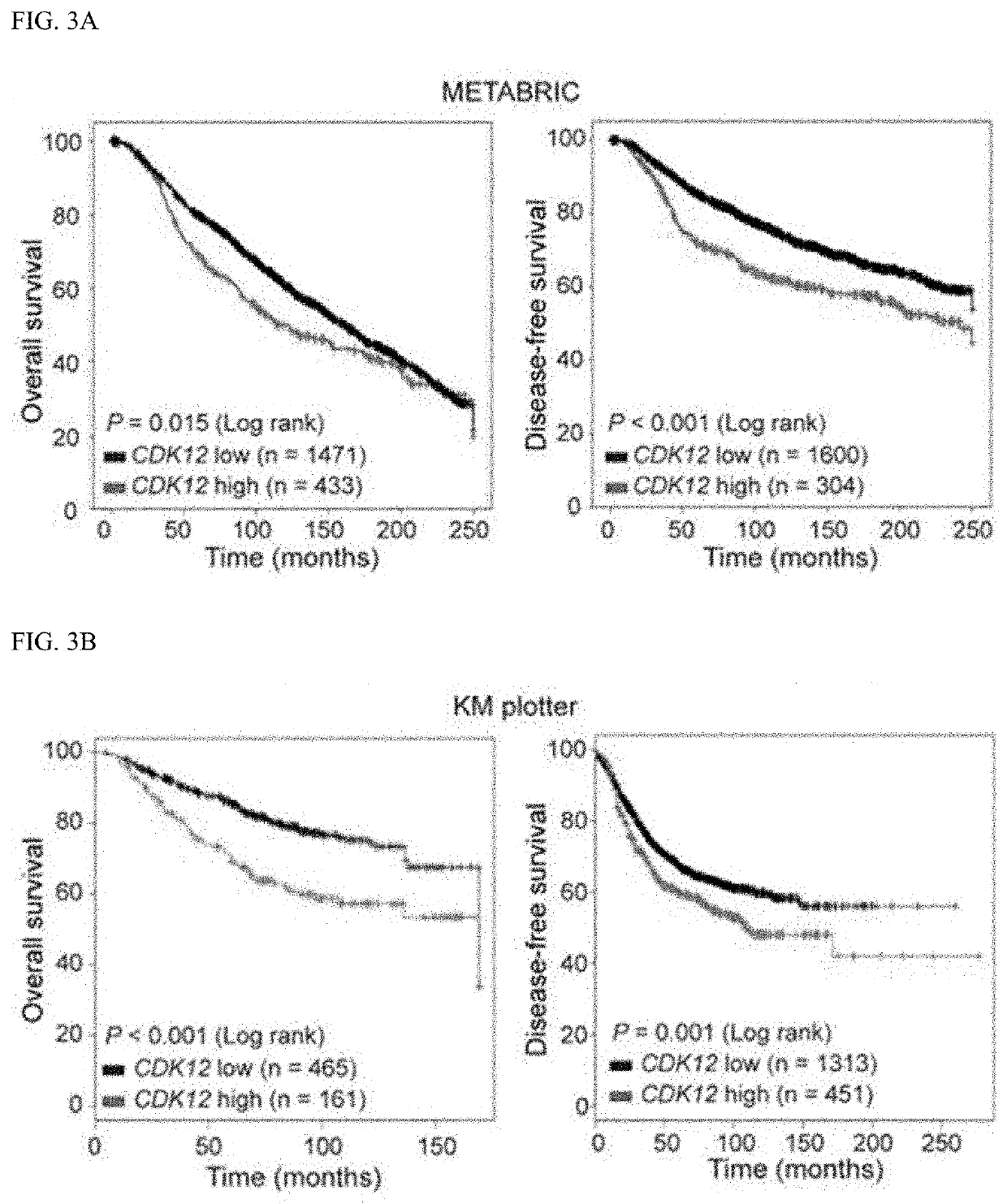
A function of cyclin-dependent kinase 12 (CDK12) as a biomarker for a human epidermal growth factor receptor 2 (HER2)-positive cancer and anti-HER2 therapy, and a use of the CDK12 are provided. The CDK12 may be used for companion diagnostics of a HER2-targeted therapeutic agent for a subject having HER2+ cancer as a prognostic factor and a predictive factor for response of the subject to the anti-HER2-targeted therapeutic agent in HER2+ cancer, or used to check the probability of expressing resistance to the HER2-targeted therapeutic agent. When the CDK12 is amplified or expressed at a level higher than a reference value, the CDK12 may be suppressed to overcome the resistance to the HER2 therapeutic agent and improve a therapeutic effect, thereby improving the therapeutic efficiency of HER2-positive cancer.
Owner:IUCF HYU (IND UNIV COOP FOUND HANYANG UNIV)
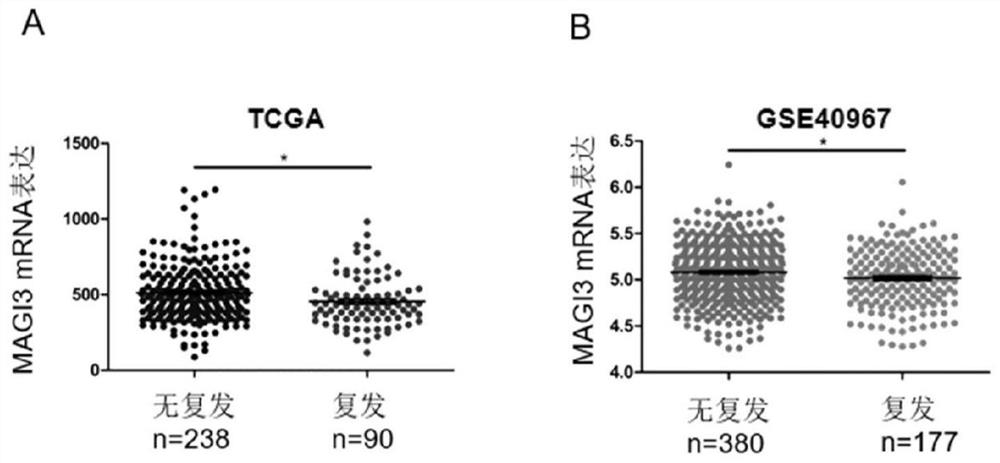
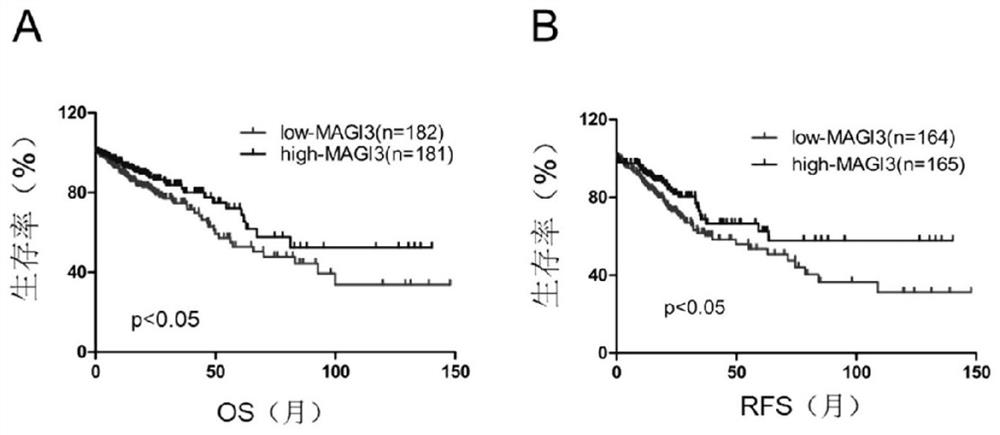
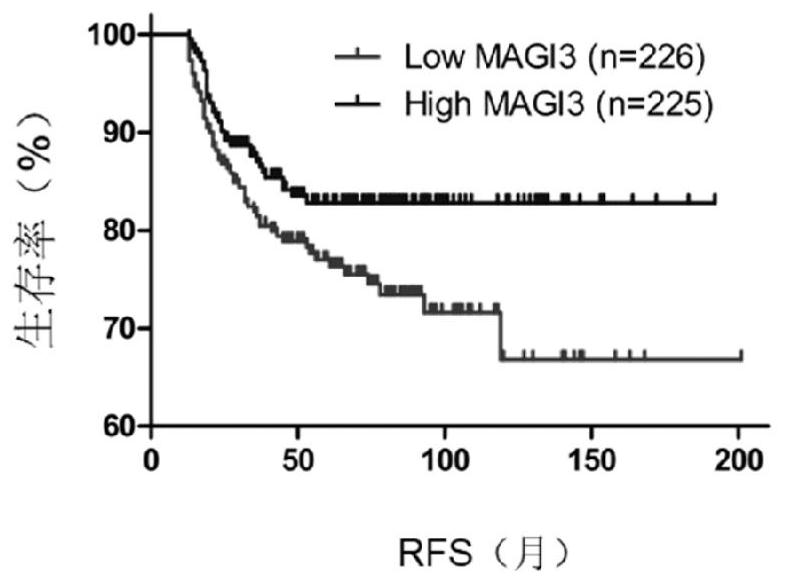
Application of MAGI3 in prediction of prognosis or chemosensitivity of colorectal cancer patient
ActiveCN112522405AMicrobiological testing/measurementBiological material analysisChemotherapy regimenPharmaceutical drug
The invention relates to application of MAGI3 in prediction of prognosis or chemosensitivity of a colorectal cancer patient. Researches prove that the MAGI3 in patients with postoperative recurrence and patients with short lifetime shows low expression, so that the MAGI3 can be used as a useful prognostic factor for predicting whether the postoperative recurrence occurs or not or predicting the lifetime of patients with colorectal cancer . In addition, the invention proves that the effective rate of colorectal cancer patients with high MAGI3 expression level on a chemotherapy scheme based on 5-Fu is higher than that of patients with low expression MAGI3, so that the MAGI3 can be used as an index for predicting the chemotherapy effectiveness of the colorectal cancer patients. Accordingly, aproduct for predicting prognosis of colorectal cancer patients and a drug for enhancing chemosensitivity of the colorectal cancer patients can be developed, so that prognosis of the patients is improved.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Gene biomarkers for prediction of susceptibility of ovarian neoplasms and/or prognosis or malignancy of ovarian cancers
InactiveUS20150072947A1Predict riskPredict susceptibilityBiocidePeptide/protein ingredientsDNA methylationNeoplasm
The present invention uses methylomic analysis and discovers DNA methylation biomarkers for prediction of ovarian cancer prognosis and detection of malignant ovarian cancer. In addition to being independent prognostic factors for patients with current treatment protocols, these DNA methylations are important biomarkers for individualized medicine for future chemotherapy (especially the demethylation agents or other epigenetic drugs).
Owner:NAT DEFENSE MEDICAL CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com