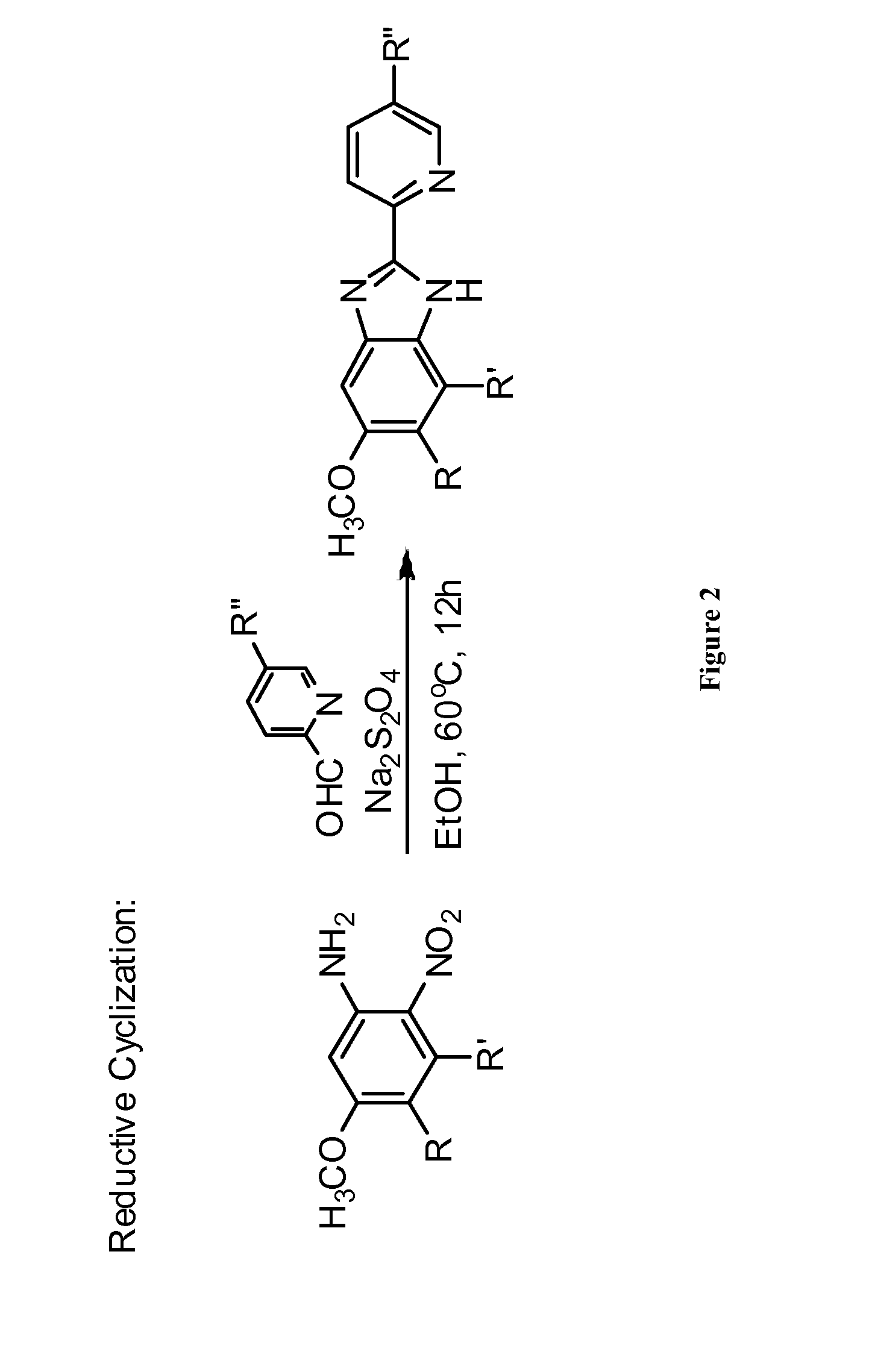
Patents
Literature
32 results about "Pharmacokinetic analysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
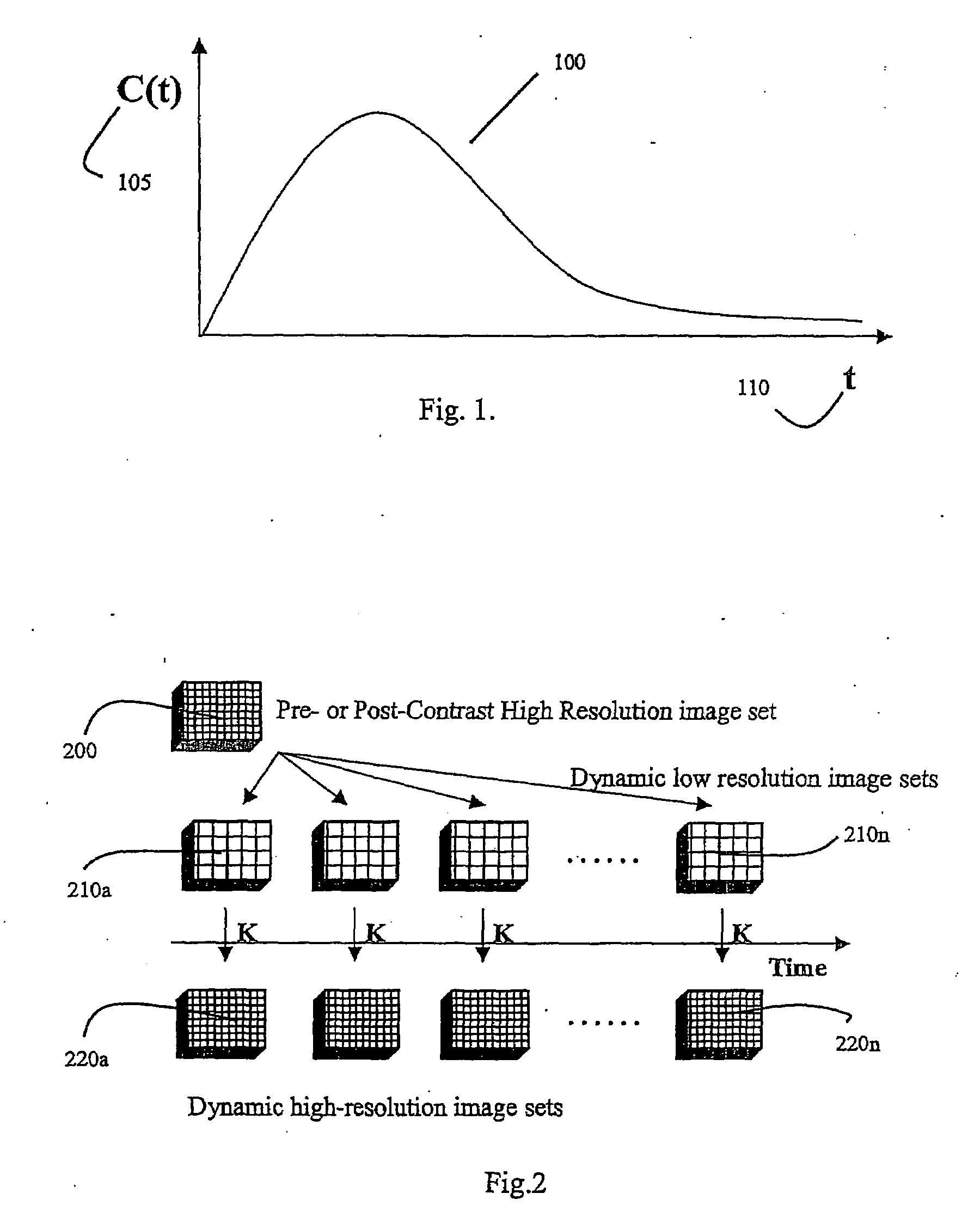
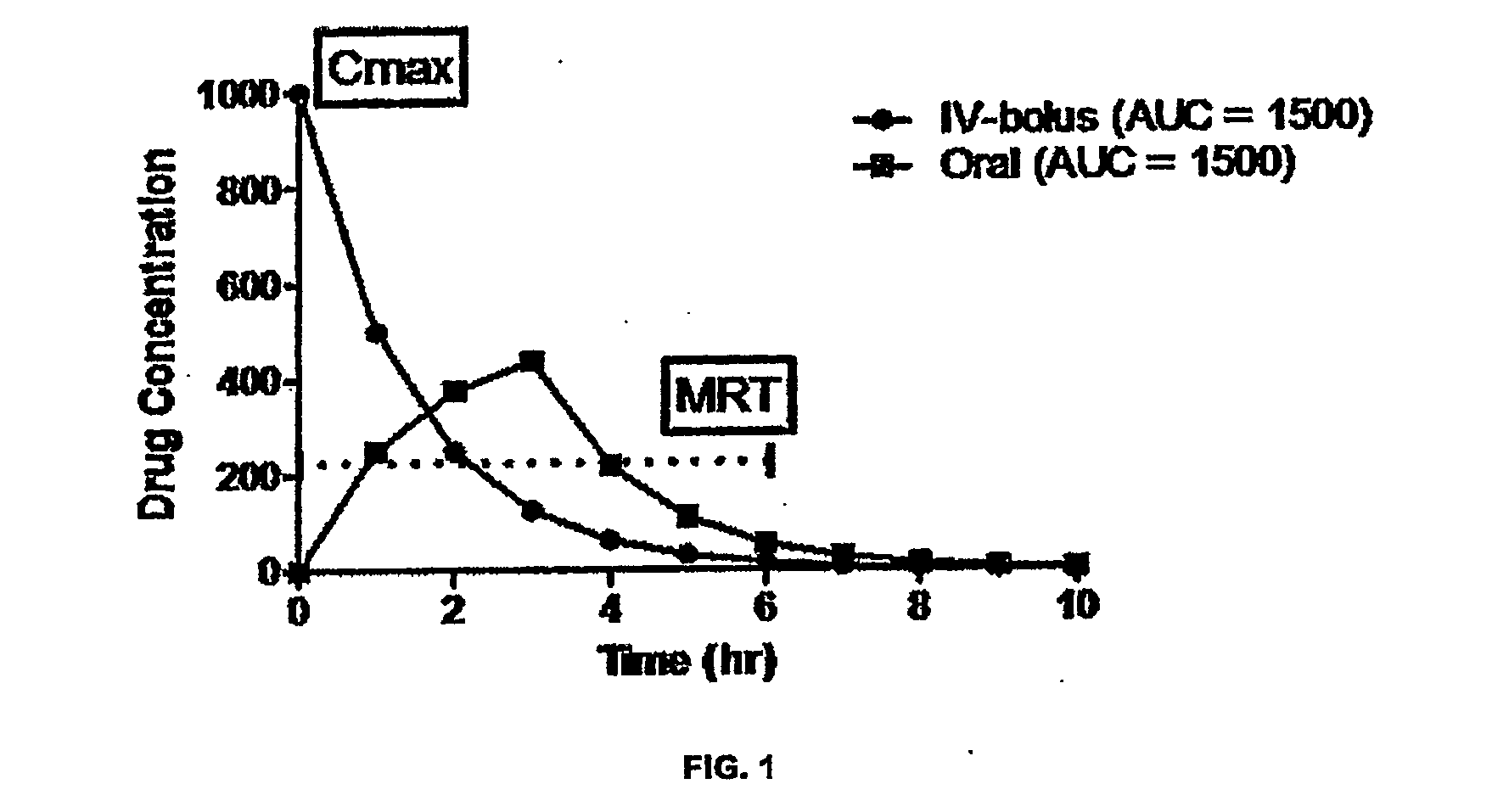
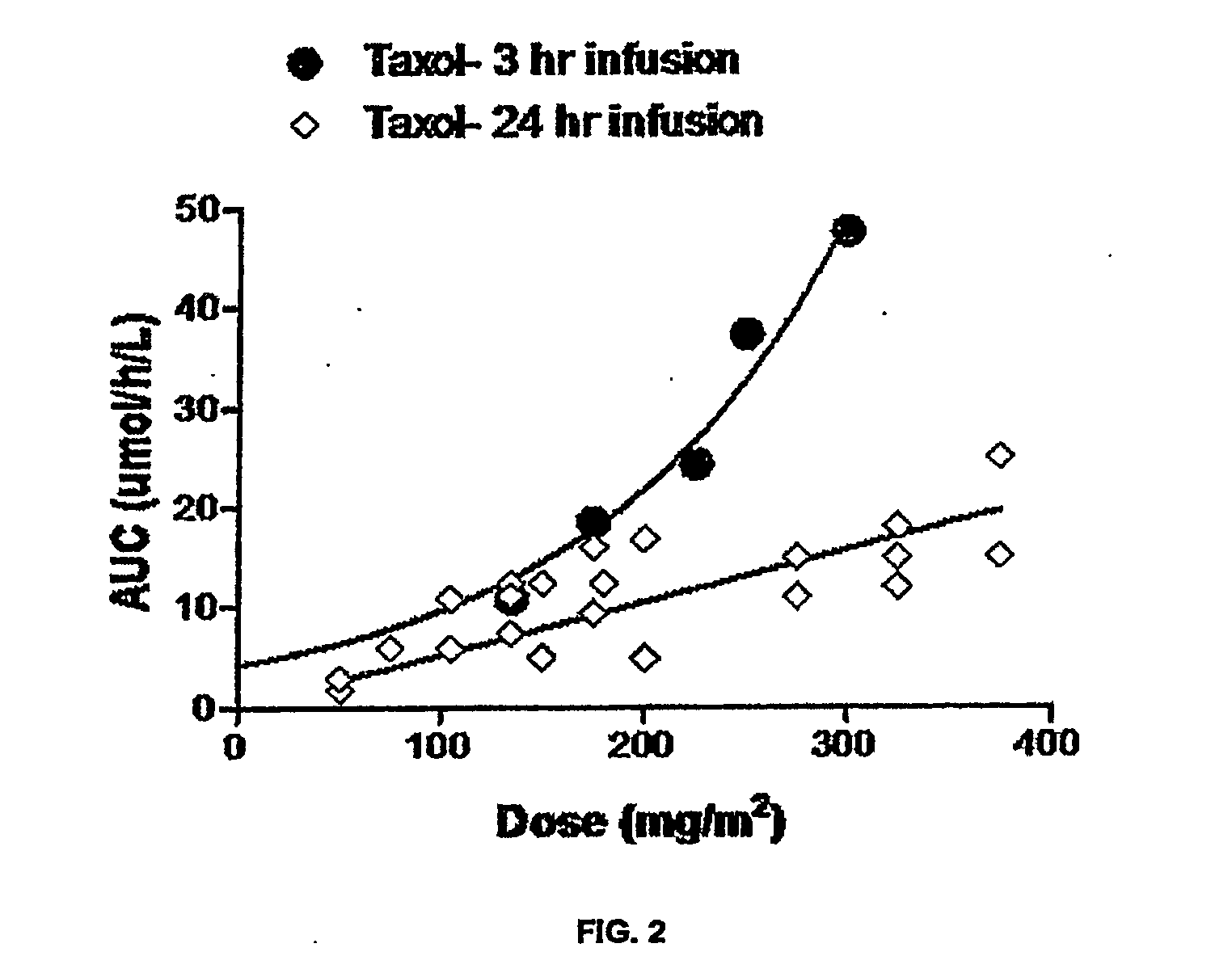
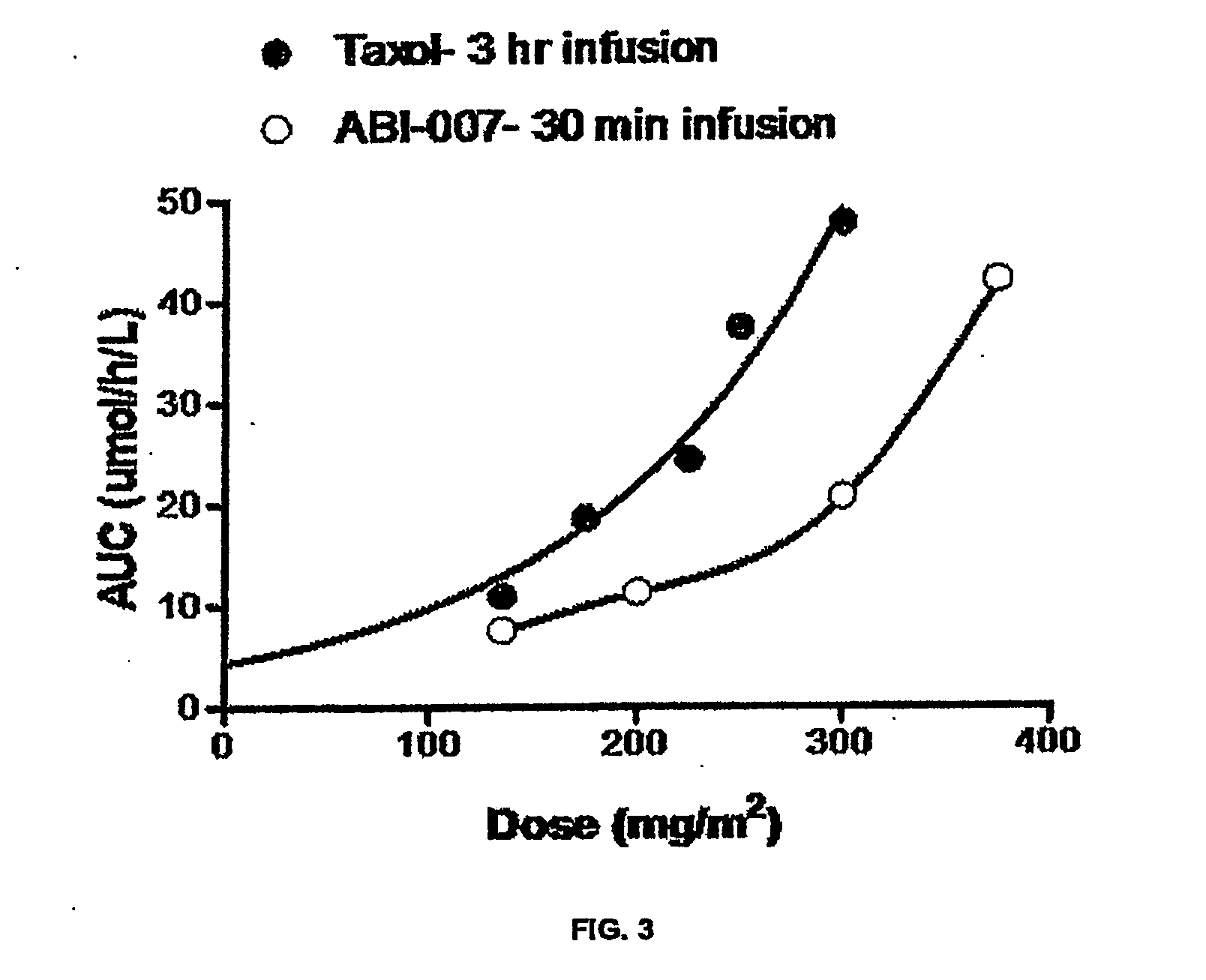
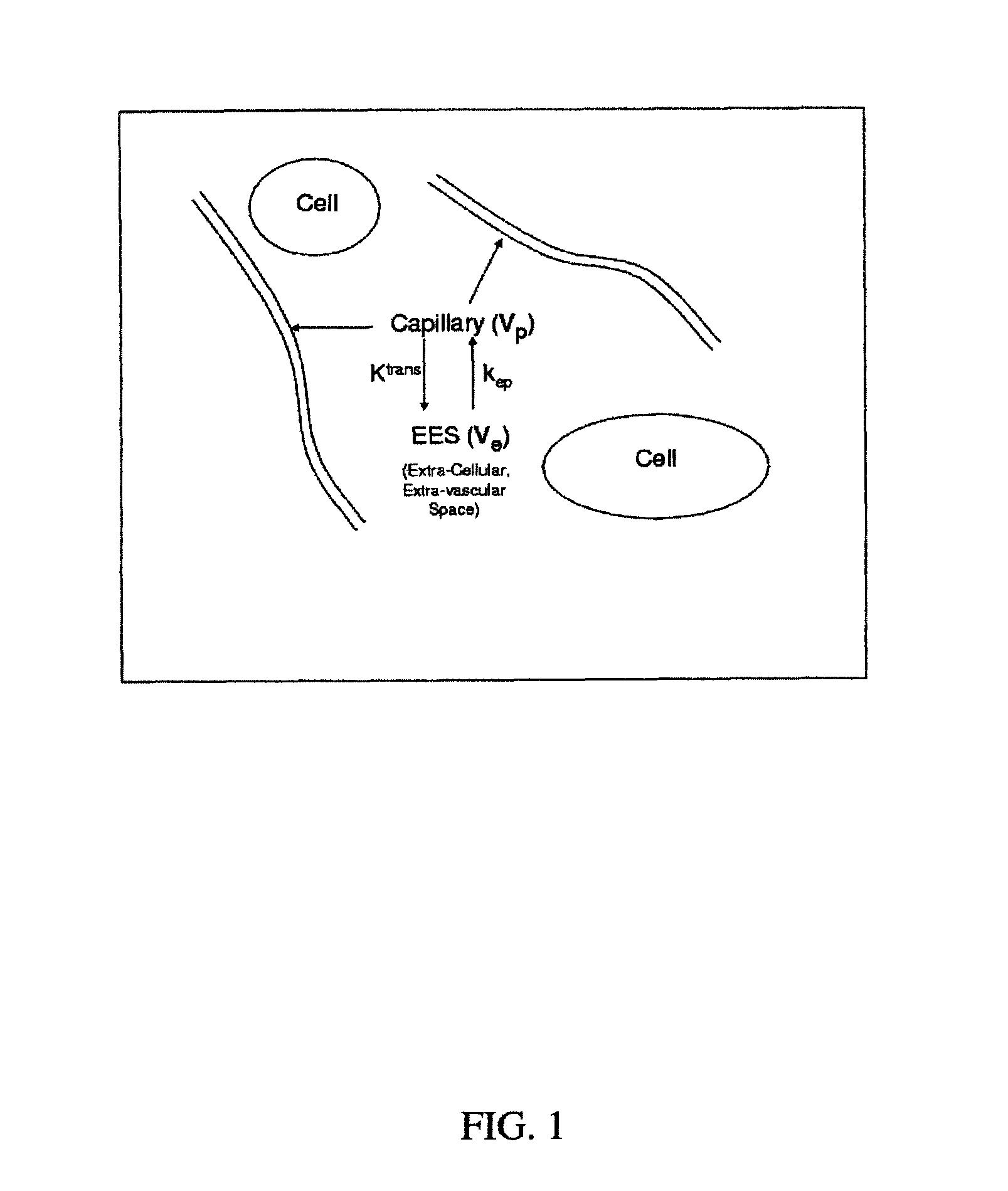
Analysis of pharmacokinetic (PK) data is concerned with defining the relationship between the dosing regimen and the body's exposure to drug as indicated by the concentration time curve to determine a dose.
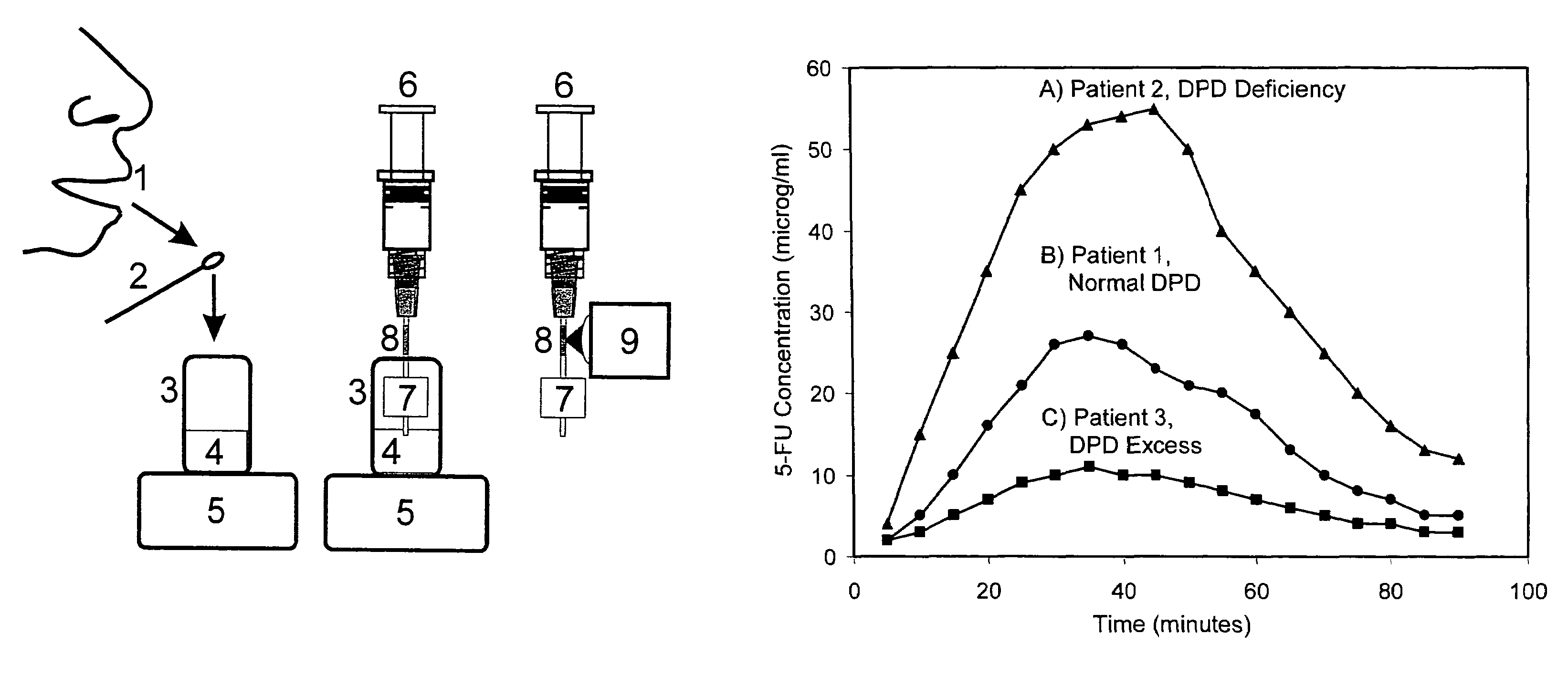
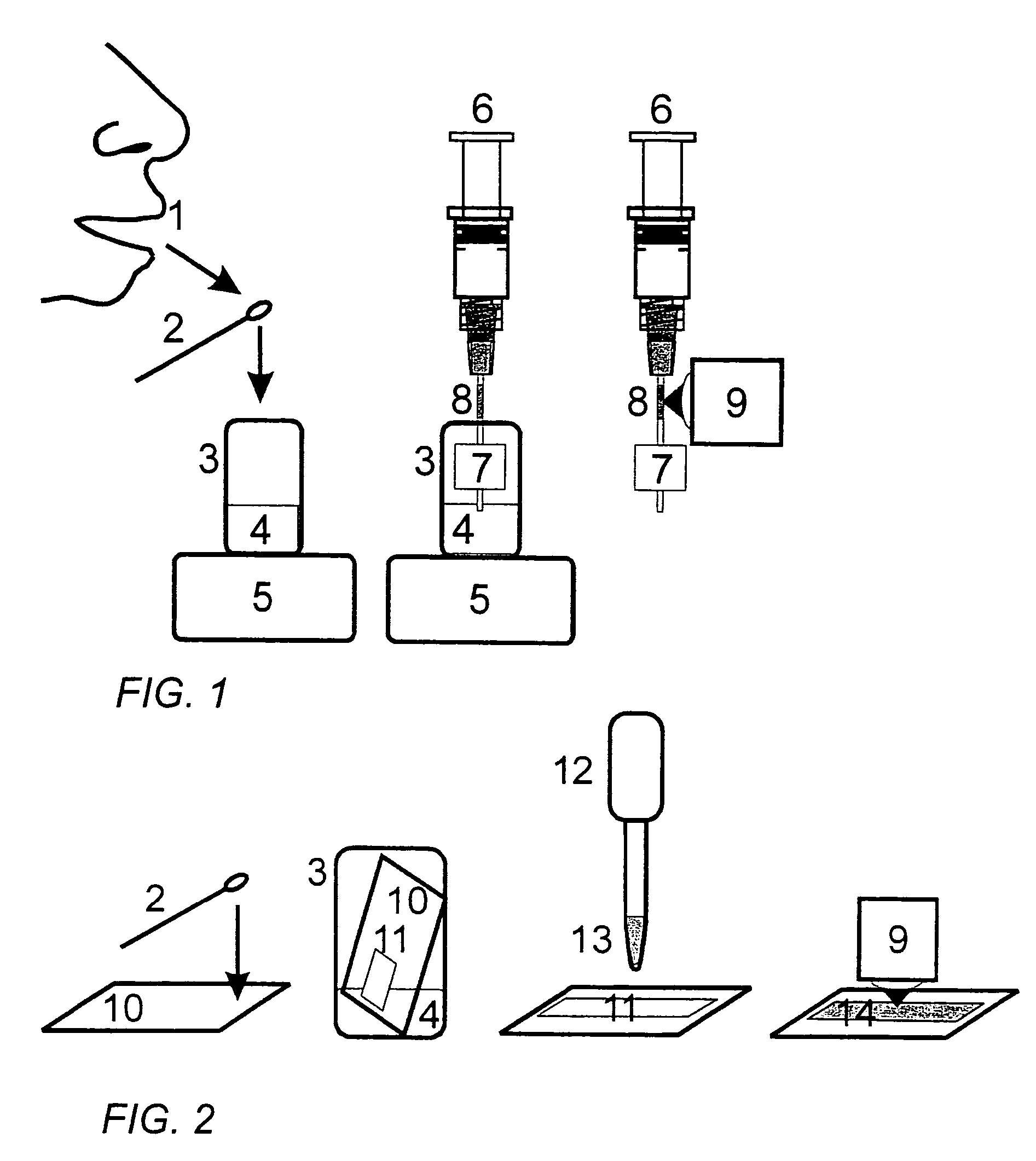
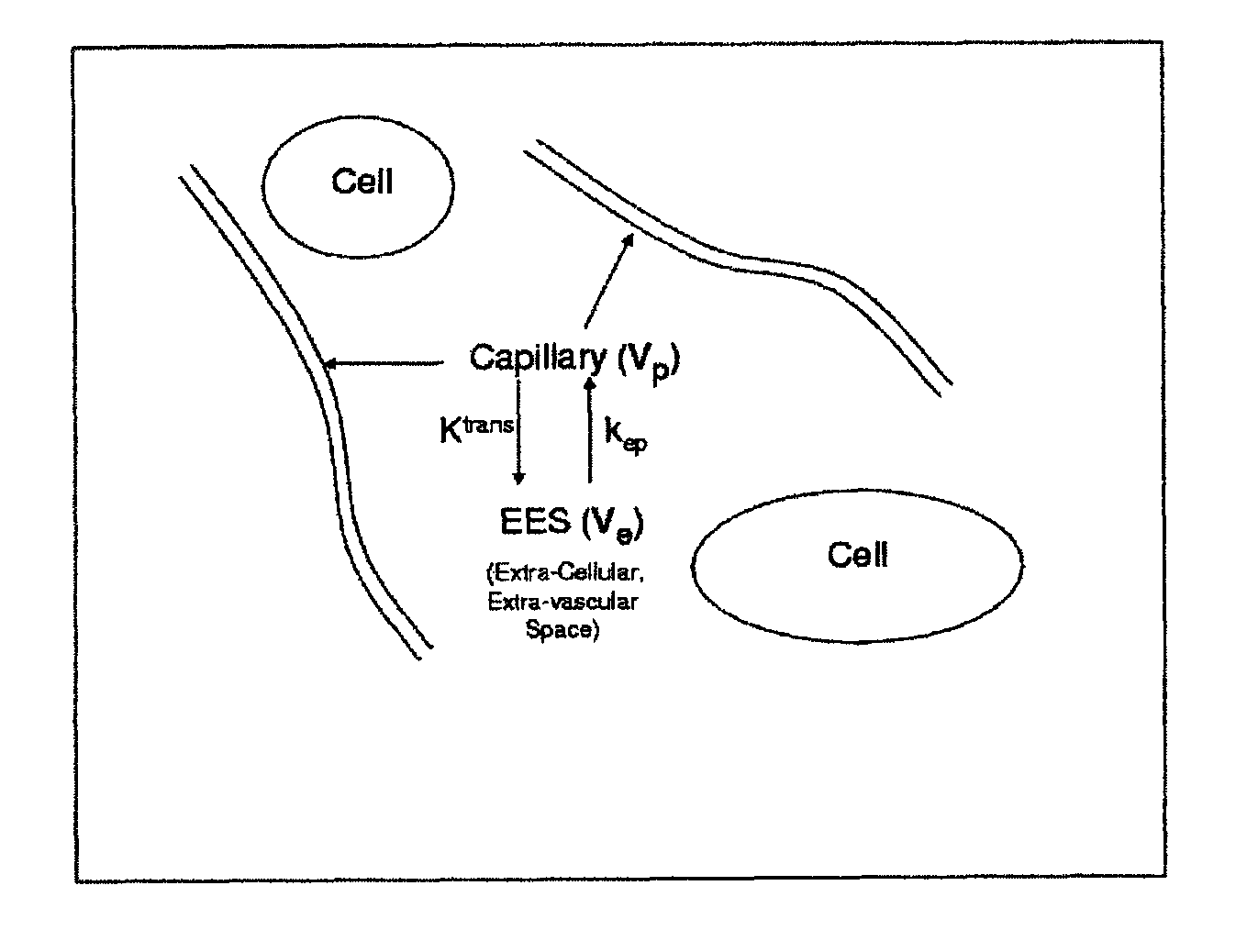
Method for tracking of contrast enhancement pattern for pharmacokinetic and parametric analysis in fast-enhancing tissues using high-resolution MRI
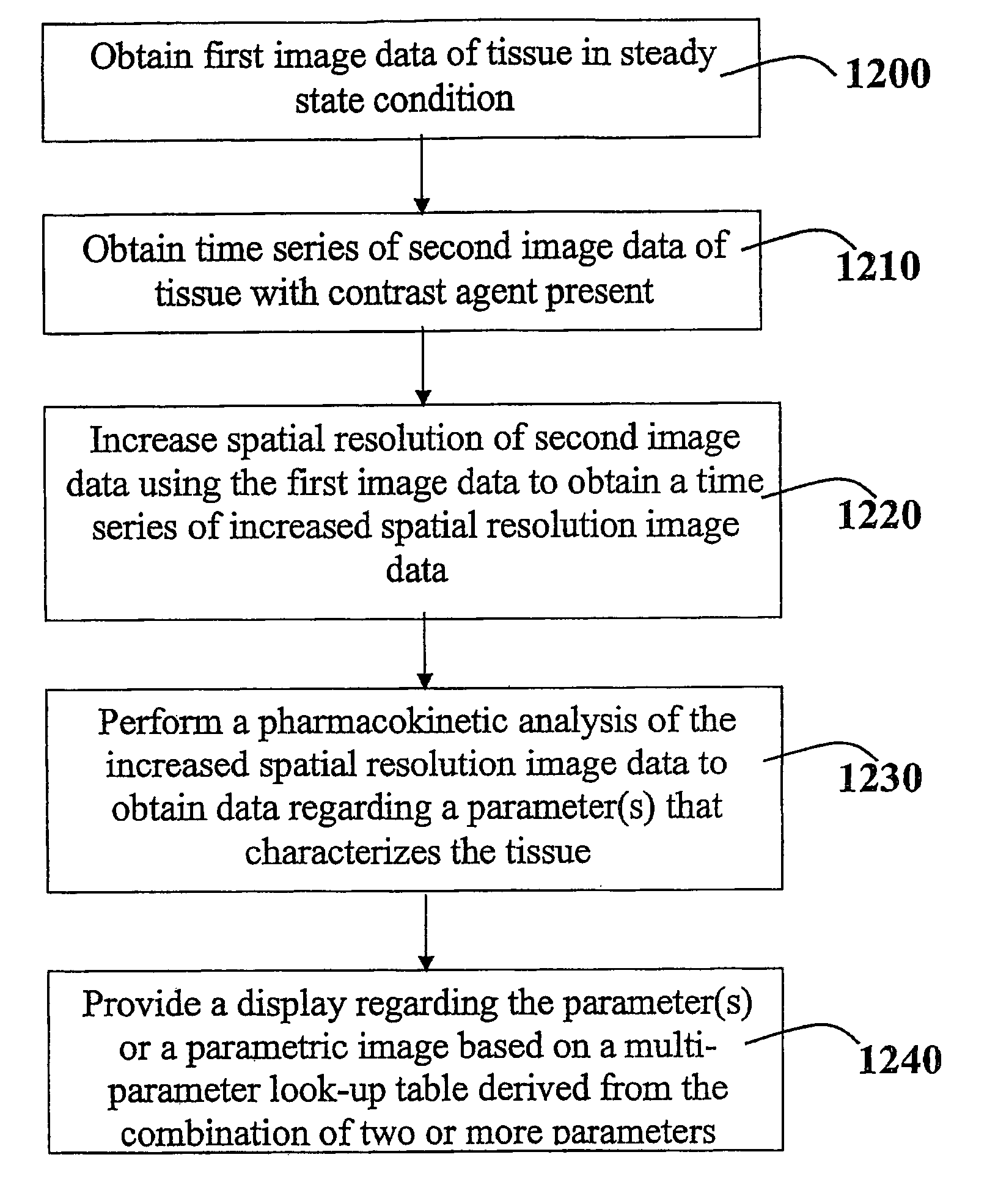
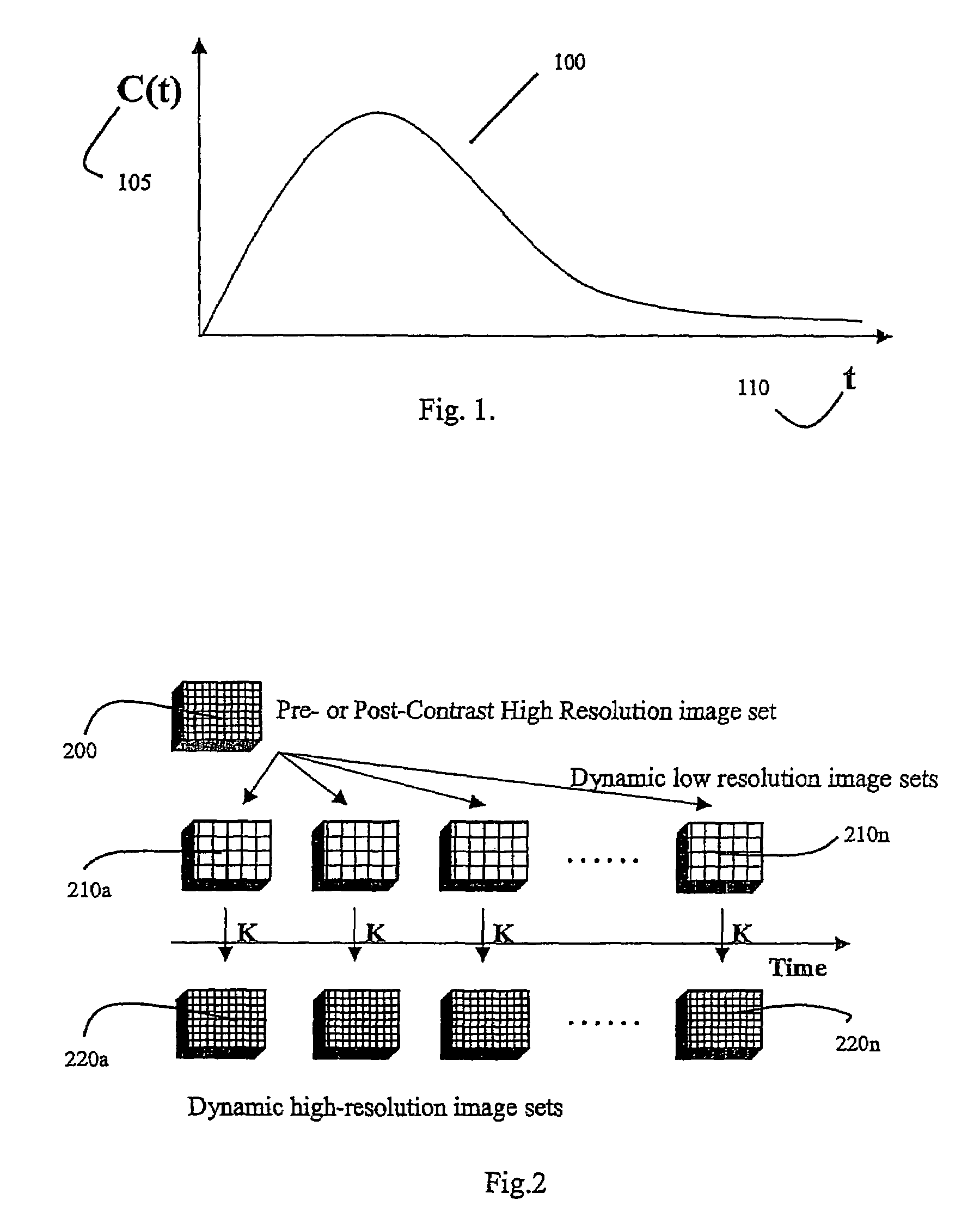
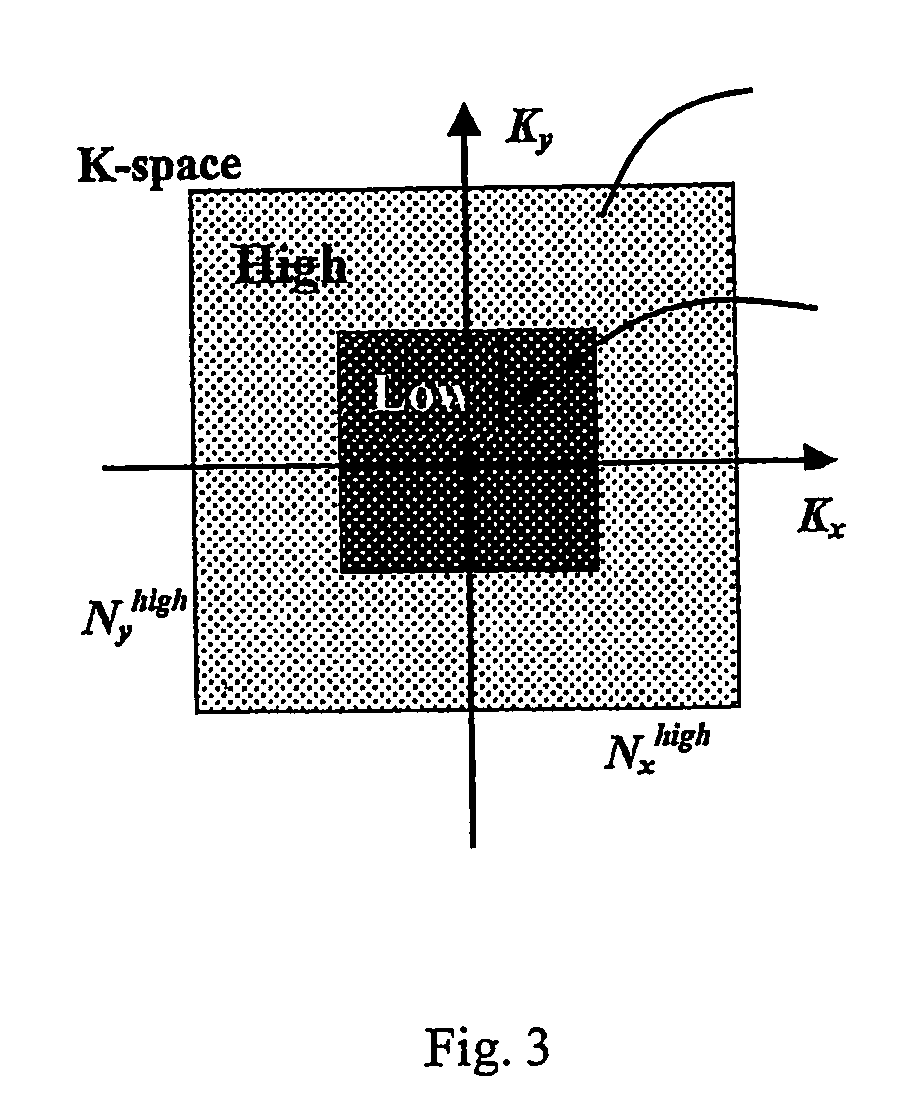
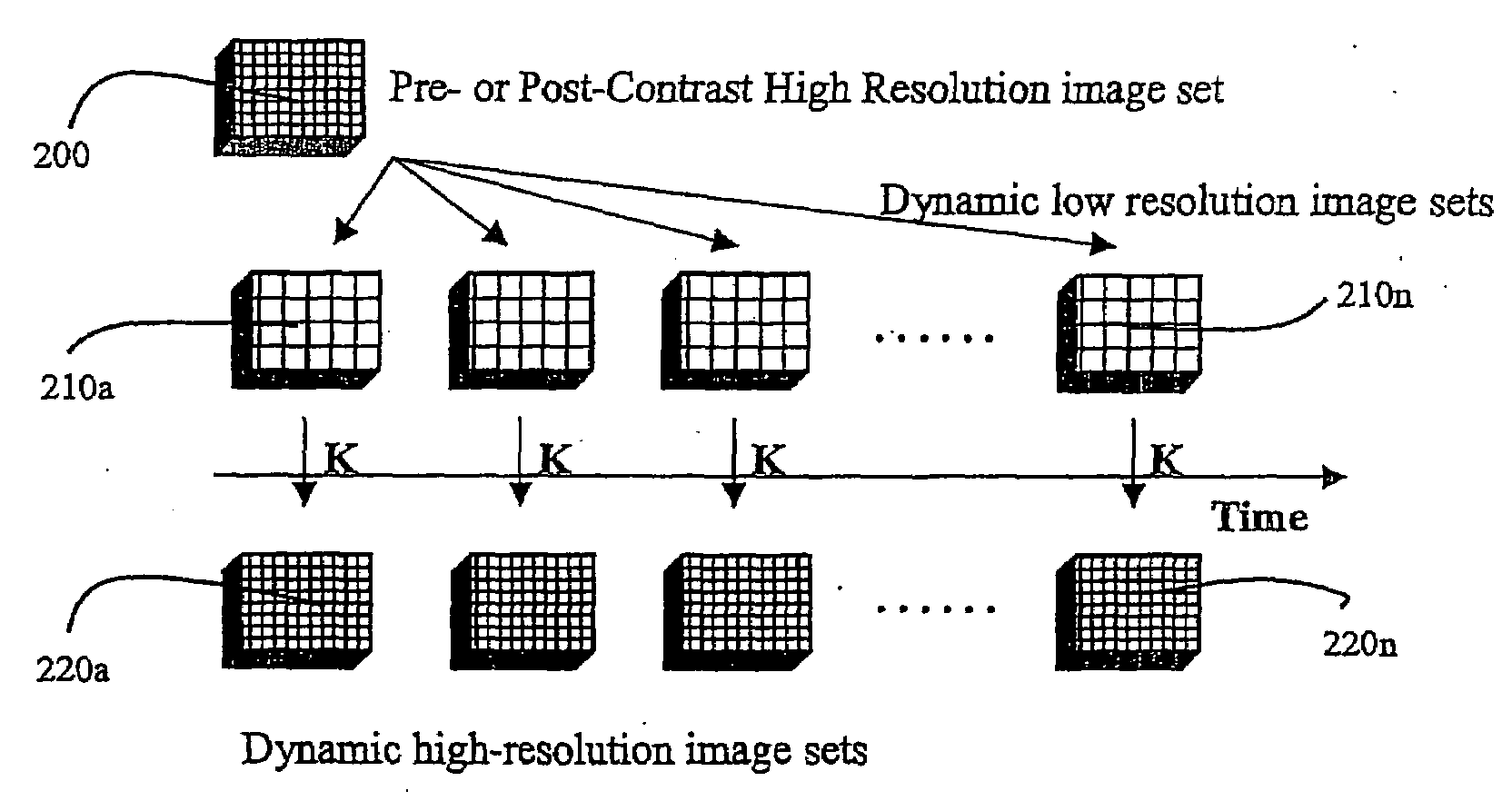
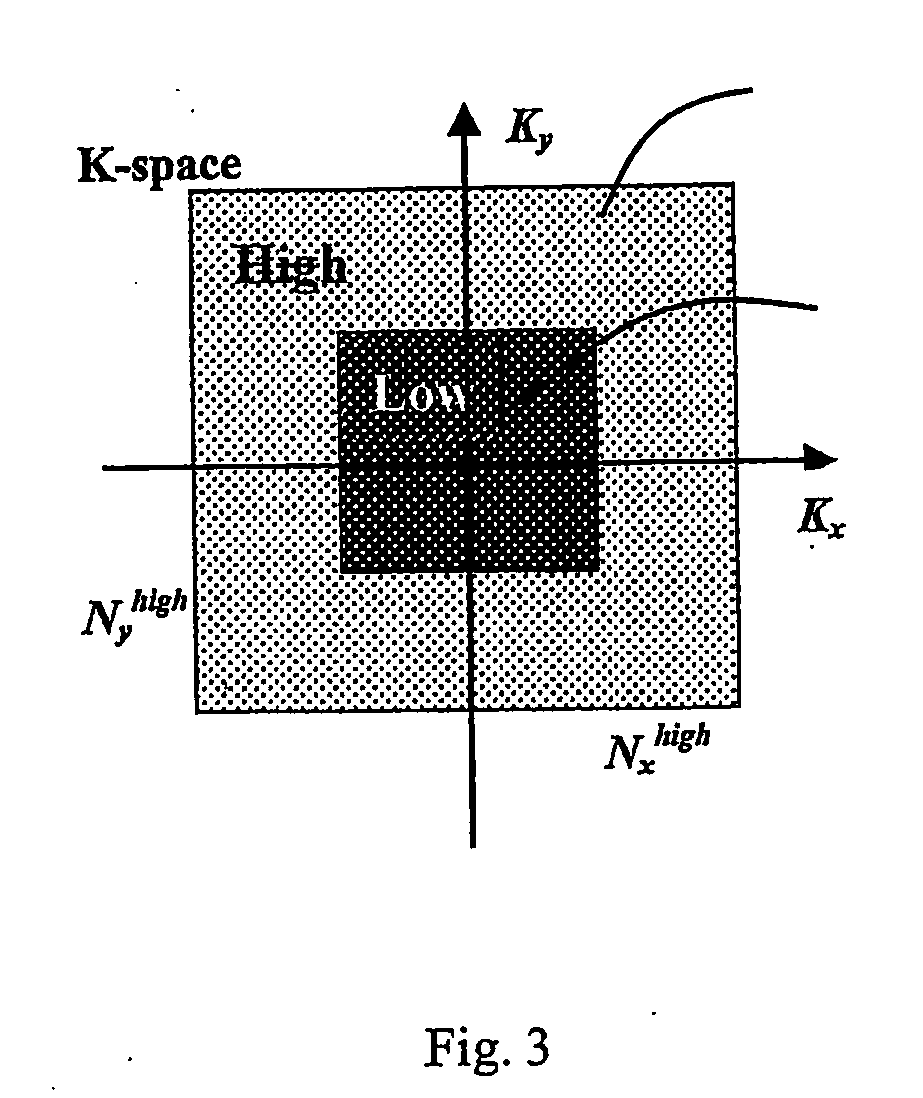
A method for performing a high-resolution pharmacokinetic analysis for calculation of tissue parameters for a fast-enhancing tissue enables medical personnel to accurately determine pharmacokinetic parameters in fast-enhancing tissues. The method includes obtaining mask image data of the tissue when it is in a steady state condition, obtaining a time series of image data of the tissue when the contrast agent is flowing in the tissue, and increasing a spatial resolution of the time series of image data using the mask image data to obtain a time series of increased spatial resolution image data. The method further includes performing a pharmacokinetic analysis to obtain data including at least one parameter that characterizes the tissue, providing a multi-parameter look-up table derived from a combination of two or more parameters, and providing a display including one parameter or a parametric image, where the parametric image is derived from the look-up table.
Owner:KONINKLJIJKE PHILIPS NV
Method for tracking of contrast enhacement pattern for pharmacokinetic and parametric analysis in fast-enhancing tissues using high -resolution mri
A method for performing a high-resolution pharmacokinetic analysis for calculation of tissue parameters for a fast-enhancing tissue enables medical personnel to accurately determine pharmacokinetic parameters in fast-enhancing tissues. The method includes obtaining mask image data of the tissue when it is in a steady state condition, obtaining a time series of image data of the tissue when the contrast agent is flowing in the tissue, and increasing a spatial resolution of the time series of image data using the mask image data to obtain a time series of increased spatial resolution image data. The method further includes performing a pharmacokinetic analysis to obtain data including at least one parameter that characterizes the tissue, providing a multi-parameter look-up table derived from a combination of two or more parameters, and providing a display including one parameter or a parametric image, where the parametric image is derived from the look-up table.
Owner:KONINKLJIJKE PHILIPS NV
Methods for detecting antibodies
Methods for detection of any antibody utilizing a standardized approach applicable to any antibody which provides highly specific assays specific for individual or multiple antibodies. The methods enable improved pharmacokinetic analysis during development and clinical use of antibody-based therapies as well as determination of diagnostic and / or prognostic factors.
Owner:RGT UNIV OF CALIFORNIA
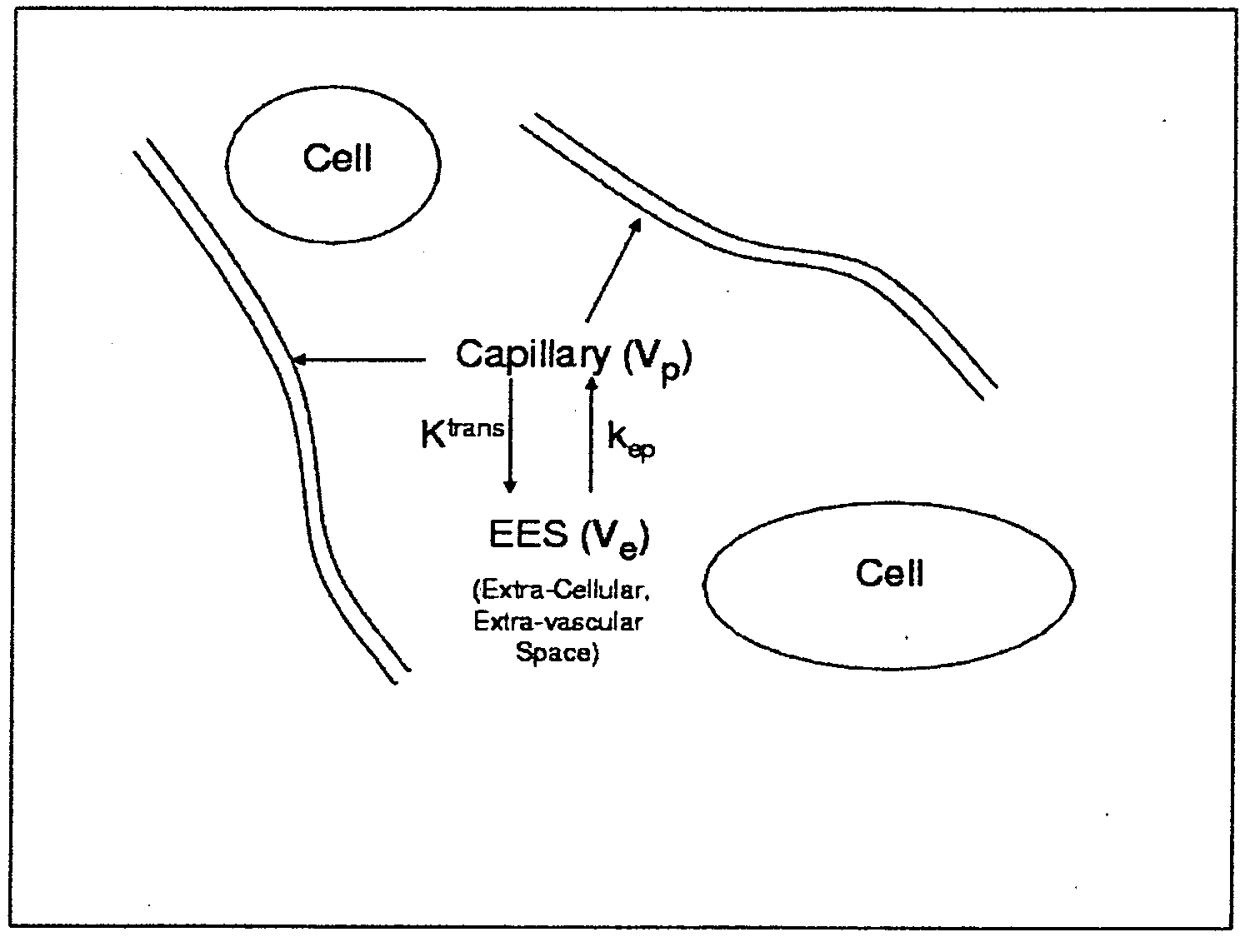
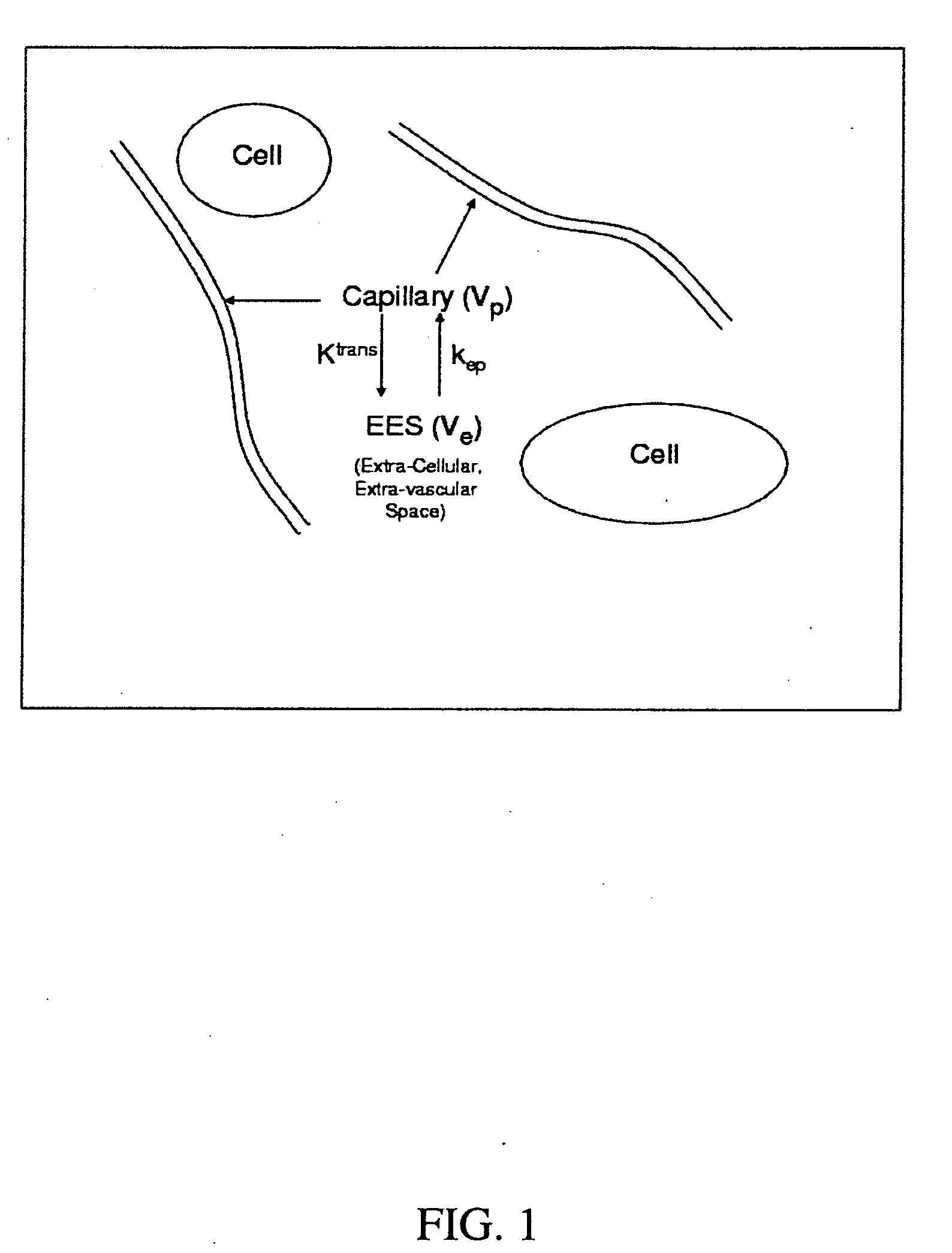
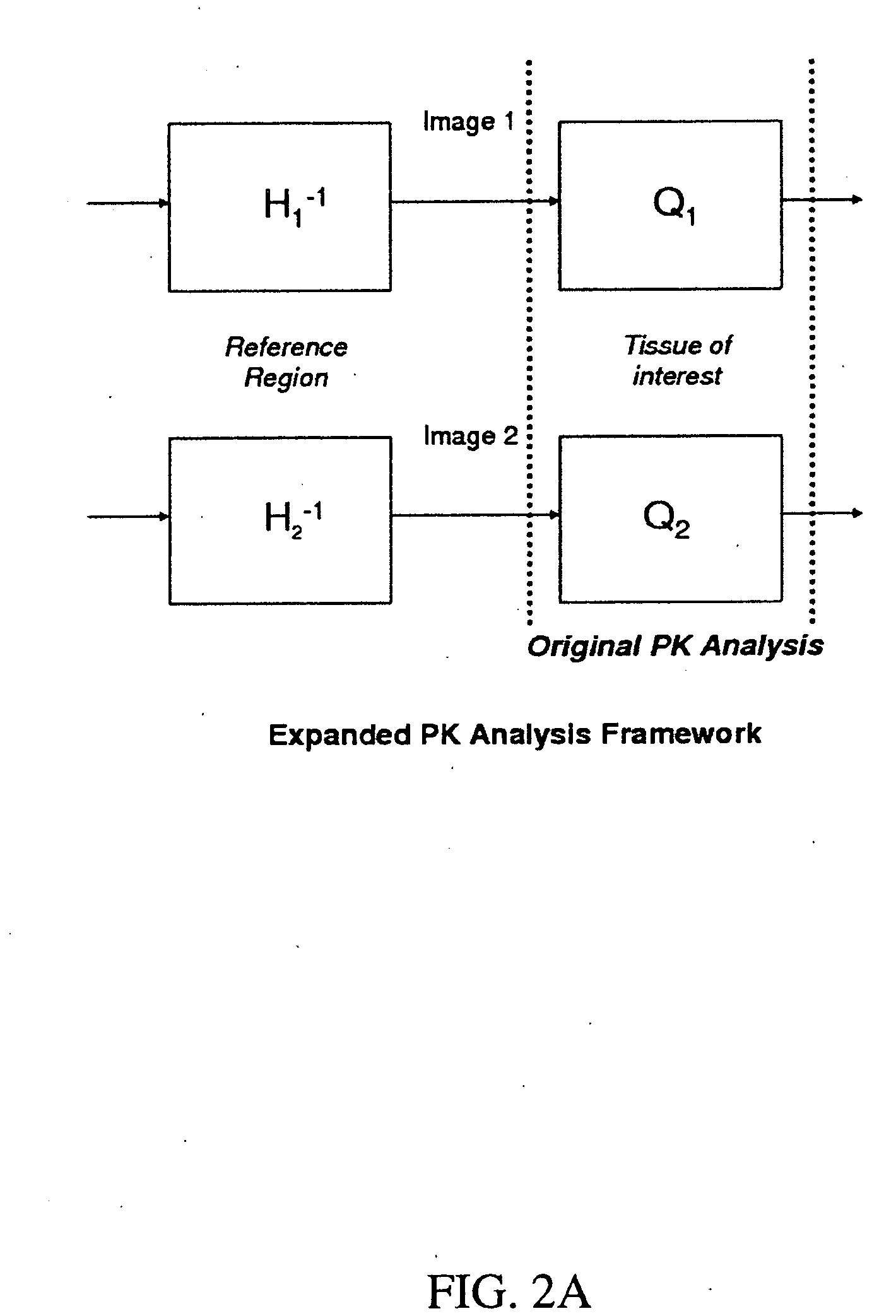
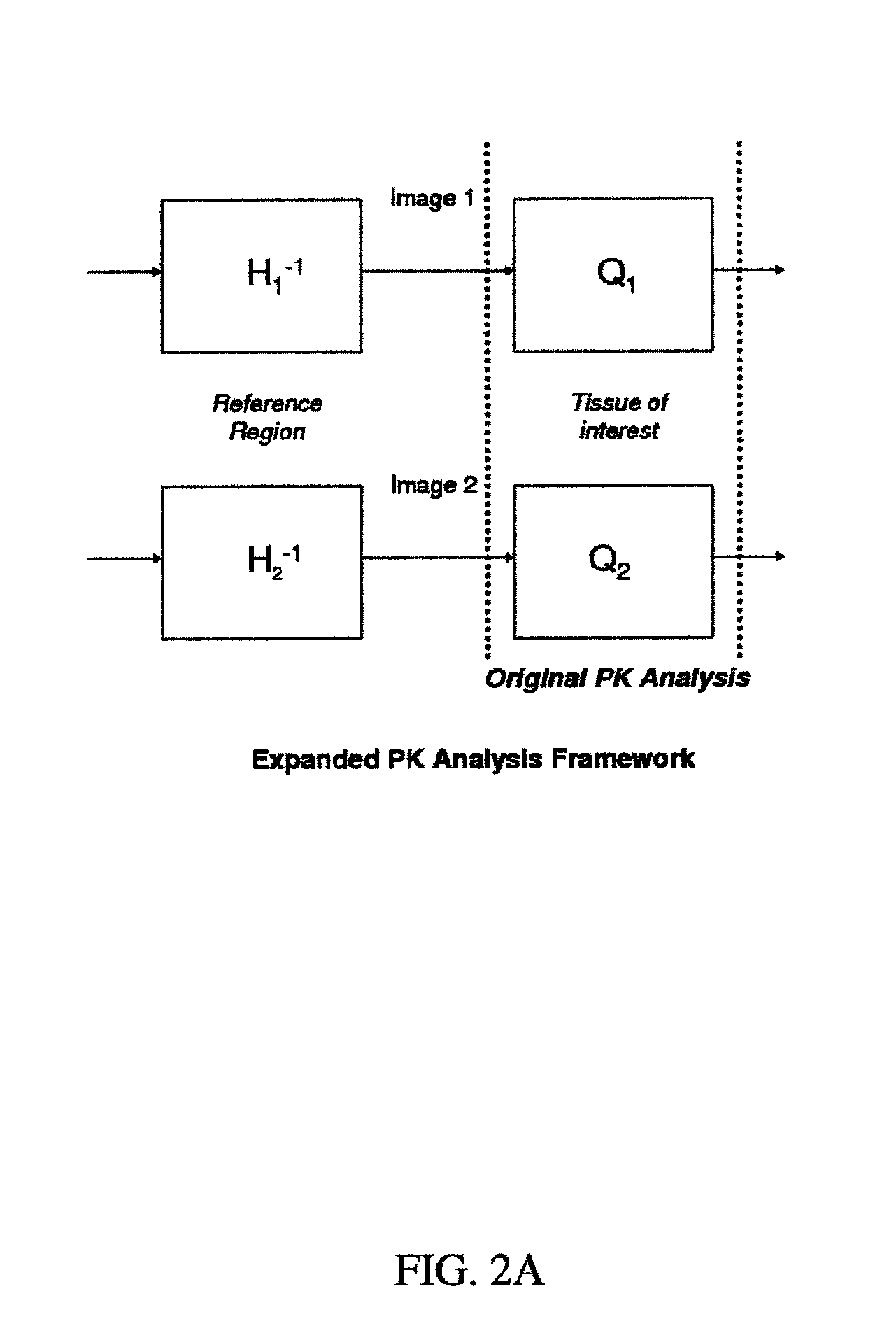
Expanded Pharmacokinetic Model for Population Studies in Breast Magnetic Resonance Imaging (MRI)
A method for pharmacokinetic analysis, including: receiving time-series medical image data of a patient introduced with a contrast agent; identifying a reference region in the medical image data; identifying a plurality of points of interest in the medical image data; measuring an intensity of voxels in the reference region; and for each point of interest, measure an intensity of voxels therein, use the measured reference region and point of interest intensities to obtain an expression relating the point of interest's voxel concentration to that of the reference region, wherein the expression is a five-parameter nonlinear model with no reference to an arterial input function; and obtain values for each of the five-parameters by solving the expression and use the obtained values to determine whether the point of interest is malignant.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
Analytical method for pharmacokinetics of multicomponent traditional Chinese medicine
InactiveCN102175783AMeet the speed of analysisMeet analysis requirementsComponent separationChemical solutionIn vivo
The invention provides an analytical method for pharmacokinetics of a multicomponent traditional Chinese medicine, and belongs to the technical field of medicine component analysis. Quantitative reactive ions of various components in bulk pharmaceutical chemicals are built in a multiple reaction monitor scanning manner of liquid chromatogram-tandem mass spectrometry and taking the bulk pharmaceutical chemicals as 'standard substances', and the concentrations of various traditional Chinese medicine components in a biological sample are relatively quantified. The method comprises the following specific steps: preparing a bulk pharmaceutical chemical solution; building a liquid chromatogram-mass spectrometry analysis condition; testing in vivo; measuring the biological sample; and processing the data. The method provided by the invention ensures that the quantitative analysis on multicomponent traditional Chinese medicine in the biological sample is realized without the standard substance. By utilizing the method, the pharmacokinetic analysis is performed on the known or unknown components in a traditional Chinese preparation. The result shows that the precision and accuracy obtained by the method conform to the requirements of relevant specifications of SFDA (the State Food and Drug Administration).
Owner:JILIN UNIV
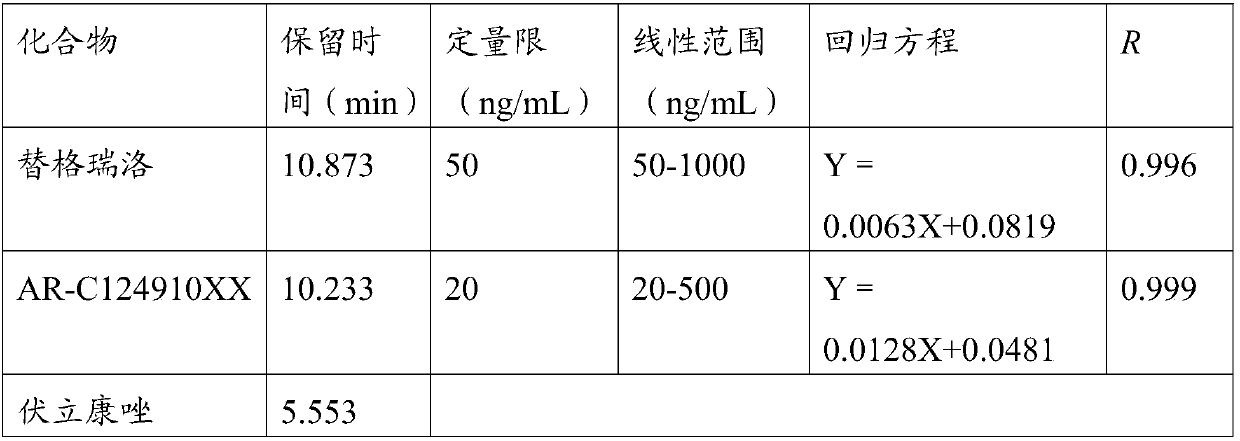
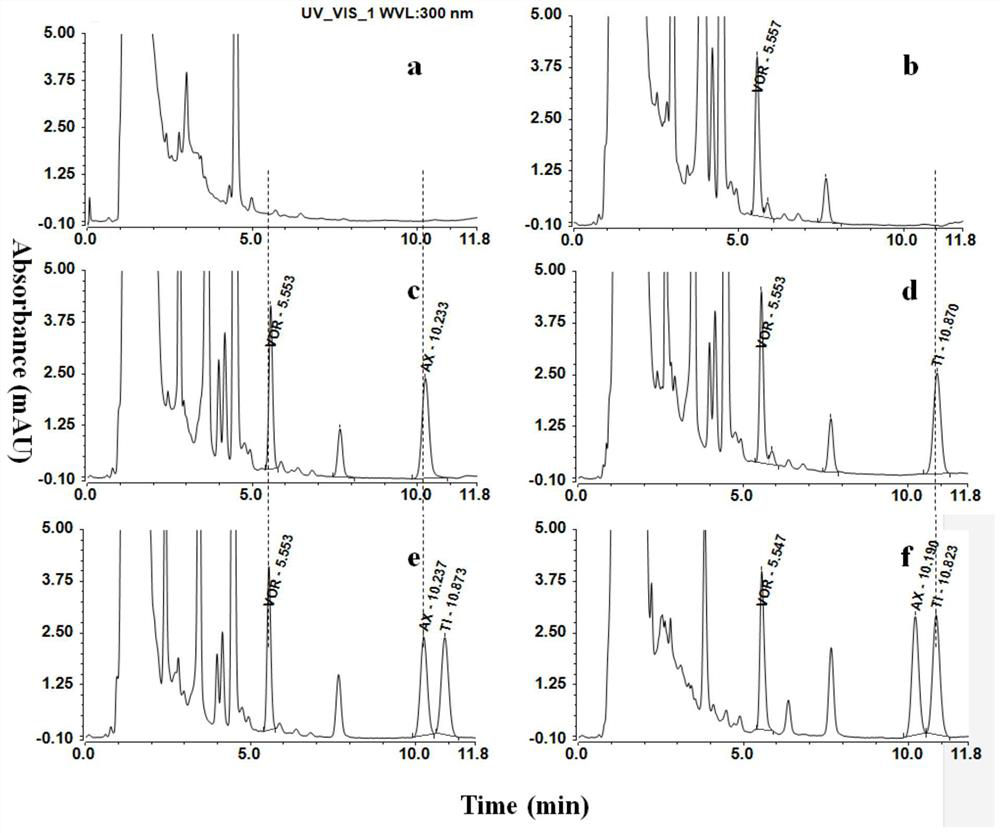
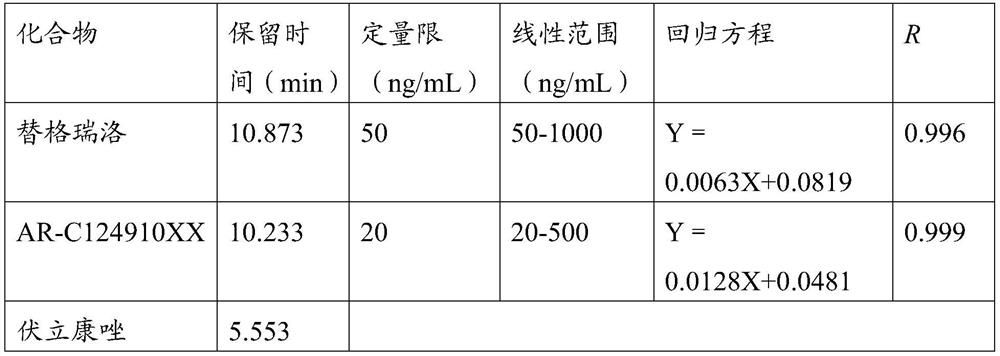
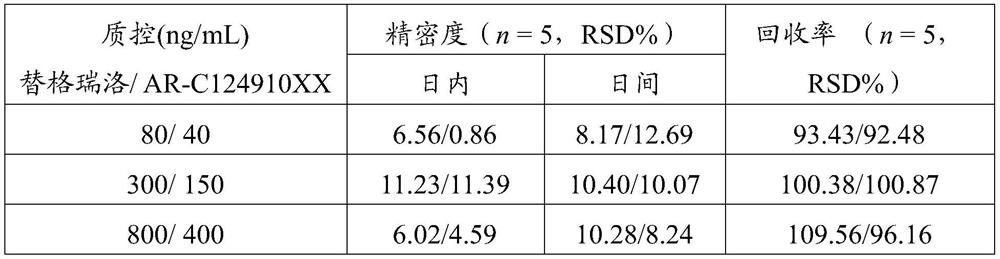
Detection method of ticagrelor in human plasma and active metabolite concentration thereof
The invention discloses a detection method of ticagrelor in human plasma and active metabolite concentration thereof. The method comprises the following steps: (1) preparing a standard working solution; (2) treating a sample; (3) manufacturing a standard curve; (4) quantitatively analyzing: treating a test sample according to the method of step (2), and computing according to a standard curve equation obtained in the step (3) to obtain the ticagrelor in the human plasma and the concentration of the active metabolite concentration thereof. The ticagrelor in the human plasma and the concentration of the active metabolite concentration thereof are determined by applying the high-performance liquid chromatography (HPLC), the ticagrelor in the human plasma and the concentration of the active metabolite concentration thereof can be accurately quantified; through the reasonable selection of a chromatographic column, a pre-treatment method, mobile phase and a mass spectrum condition and the optimization of the flow velocity, the mobile phase proportion, the chromatographic condition and like experimental conditions, the series of methodology verifies that the whole quantitative detection method is practicable, simple and convenient for operation, good in repeatability, good in accuracy, and strong in operability, and can be directly applied to the plasma sample detection analysis of the clinical patient and the related pharmacokinetics analysis.
Owner:HARBIN MEDICAL UNIVERSITY
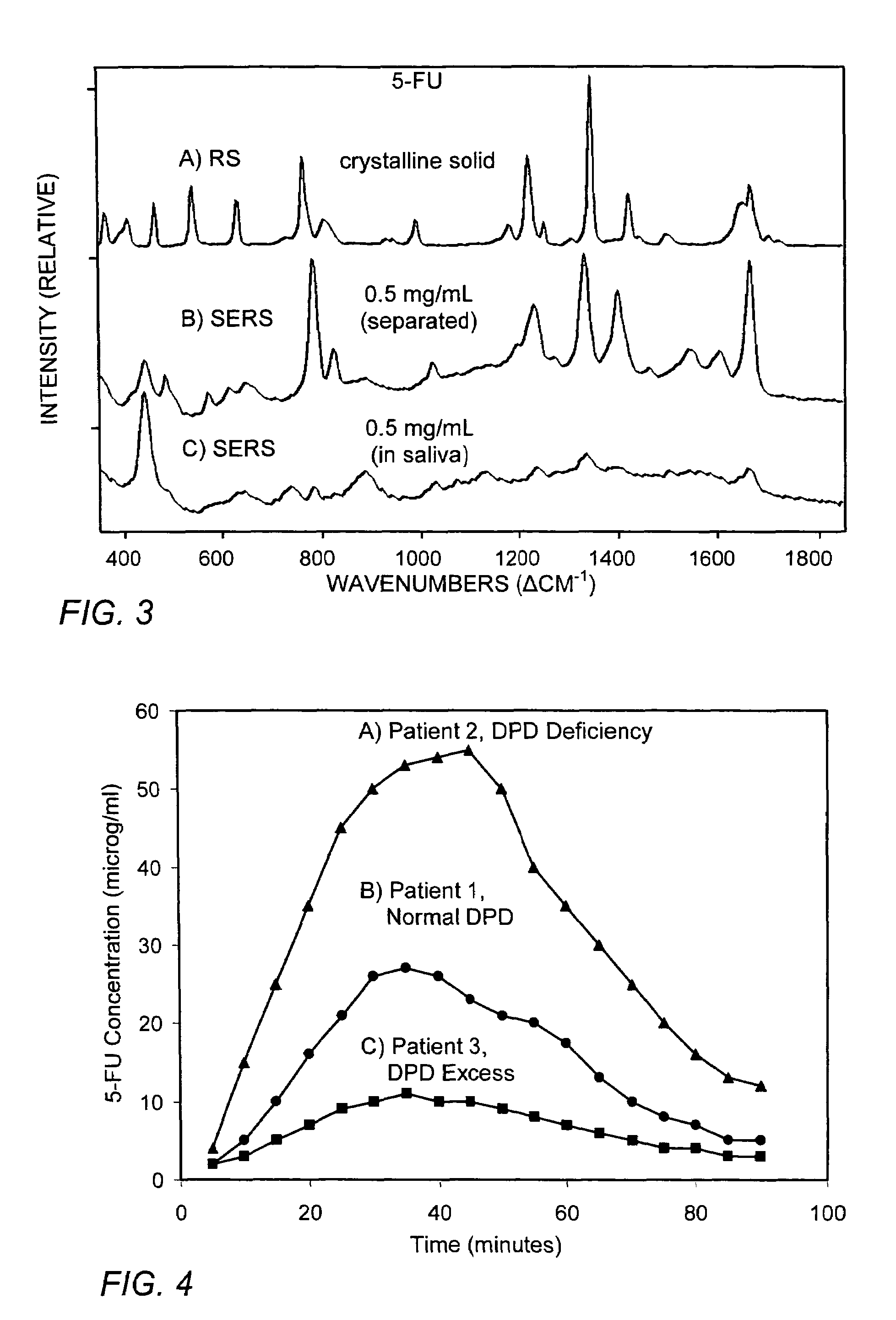
SERS method for rapid pharmacokinetic analysis of drugs in saliva
ActiveUS7393692B2Quantity maximizationAnalysis using chemical indicatorsComponent separationMetaboliteMedicine
The method and apparatus rapidly separate drugs and their metabolites from saliva and, in a continuous sequence of steps, rapidly detect, identify and quantify them through surface-enhanced Raman spectroscopy.
Owner:REAL TIME ANALYZERS
Methods and compositions for therapeutic drug monitoring and dosing by point of care pharmacokinetic profiling
InactiveUS20140349862A1Good curative effectReduce dosageOrganic active ingredientsLibrary screeningPoint of careSelf sampling
Disclosed are methods and kits for pharmacokinetic profiling employing point-of-care or point of service self-sampling and allowing for dosage adjustments based on the pharmacokinetic profiles.
Owner:AUTOTELIC
Small RNA detection method and use thereof
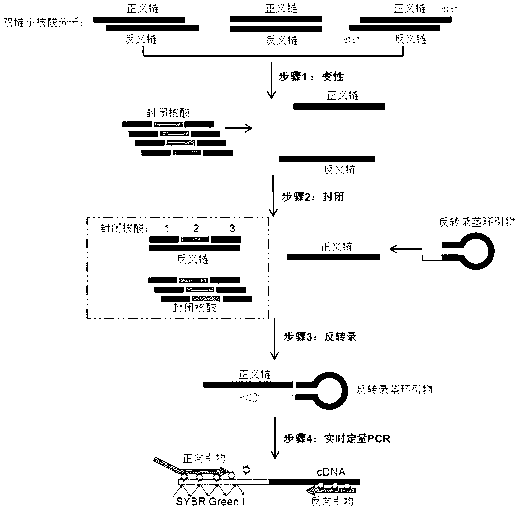
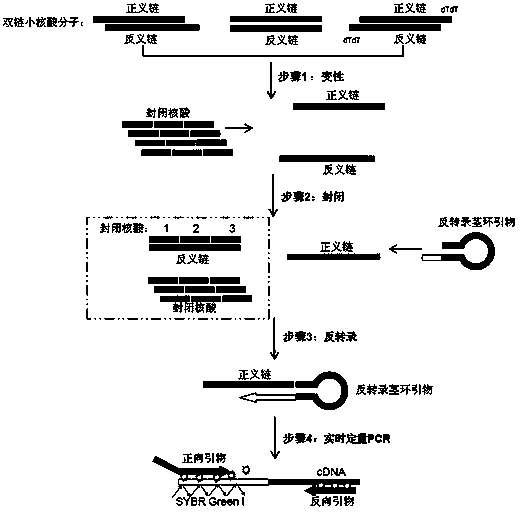
The invention relates to a small RNA detection method and a use thereof and belongs to the field of double-stranded small RNA detection. The small RNA detection method comprises the following steps of 1, designing multiple continuous or discontinuous closed RNAs which are complementary with one chain of a small RNA, a reverse transcription stem-loop primer which is complementary with an end 3' of the other chain of the small RNA, and a forward primer and a reverse primer for quantitative PCR, and 2, detecting the small RNA by denaturation, enclosing, inverse transcription and quantitative PCR. The small RNA detection method has simple processes, a low detection cost, a detection limit reaching a femtogram grade, high detection efficiency, strong singularity and high sensitivity. The small RNA detection method can be widely used for quantitative analysis, qualitative analysis and pharmacokinetic analysis of a small RNA drug, for amplification, specific identification and differentiation of a small RNA molecule, and especially for molecular diagnosis, small RNA drug metabolism and small RNA drug functional study.
Owner:BIOMICS BIOTECH
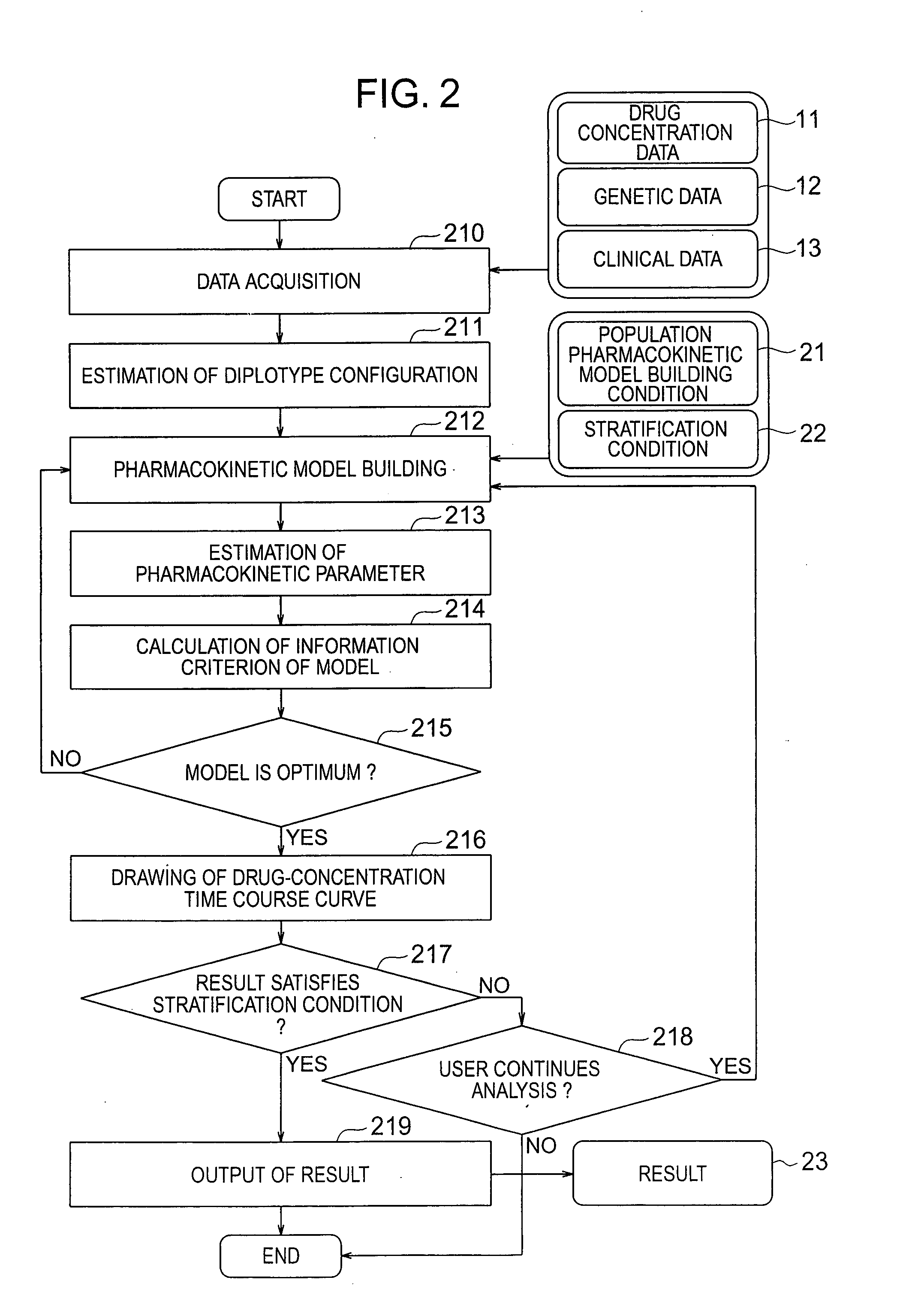
Pharmacokinetic analysis system and method thereof
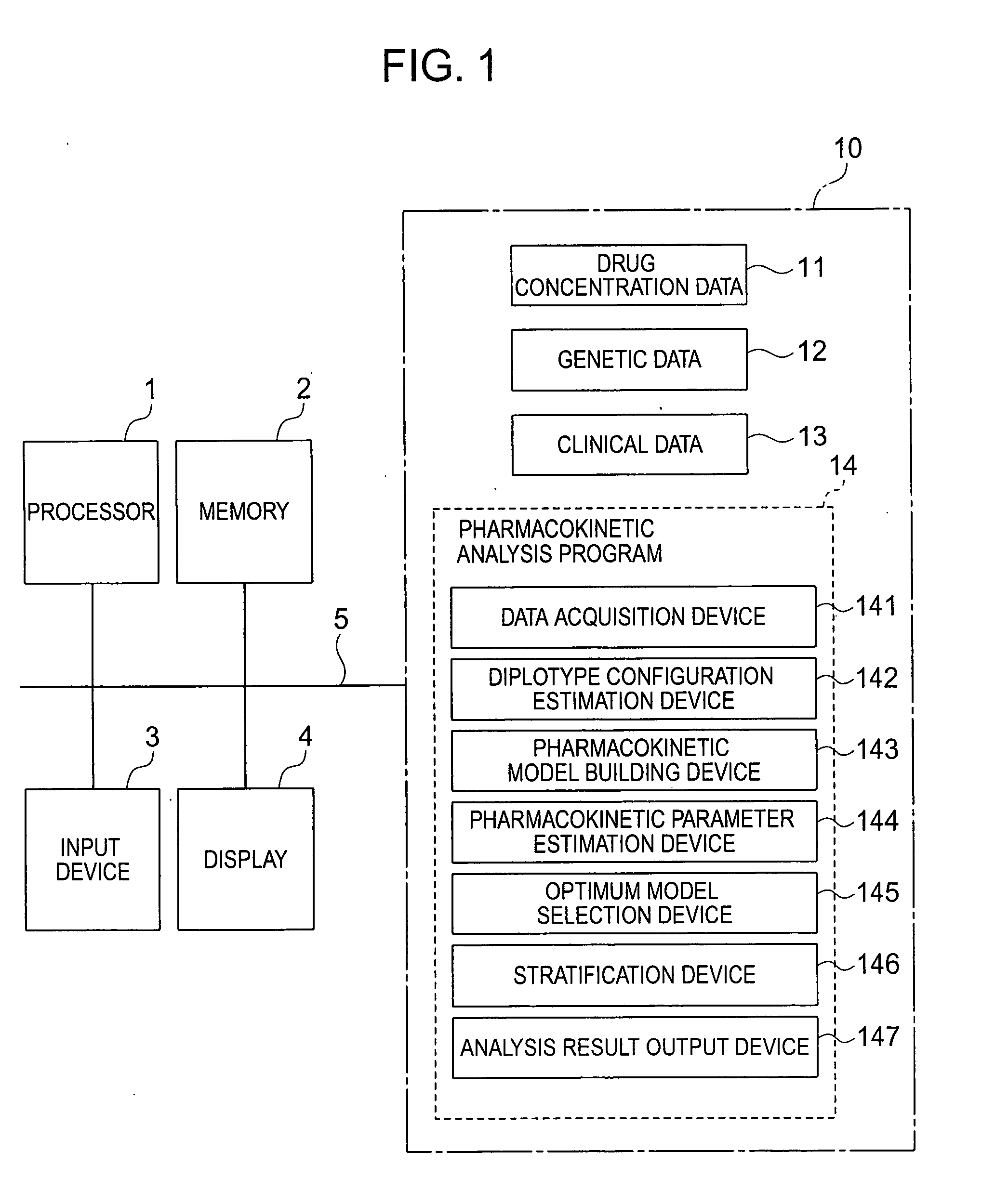
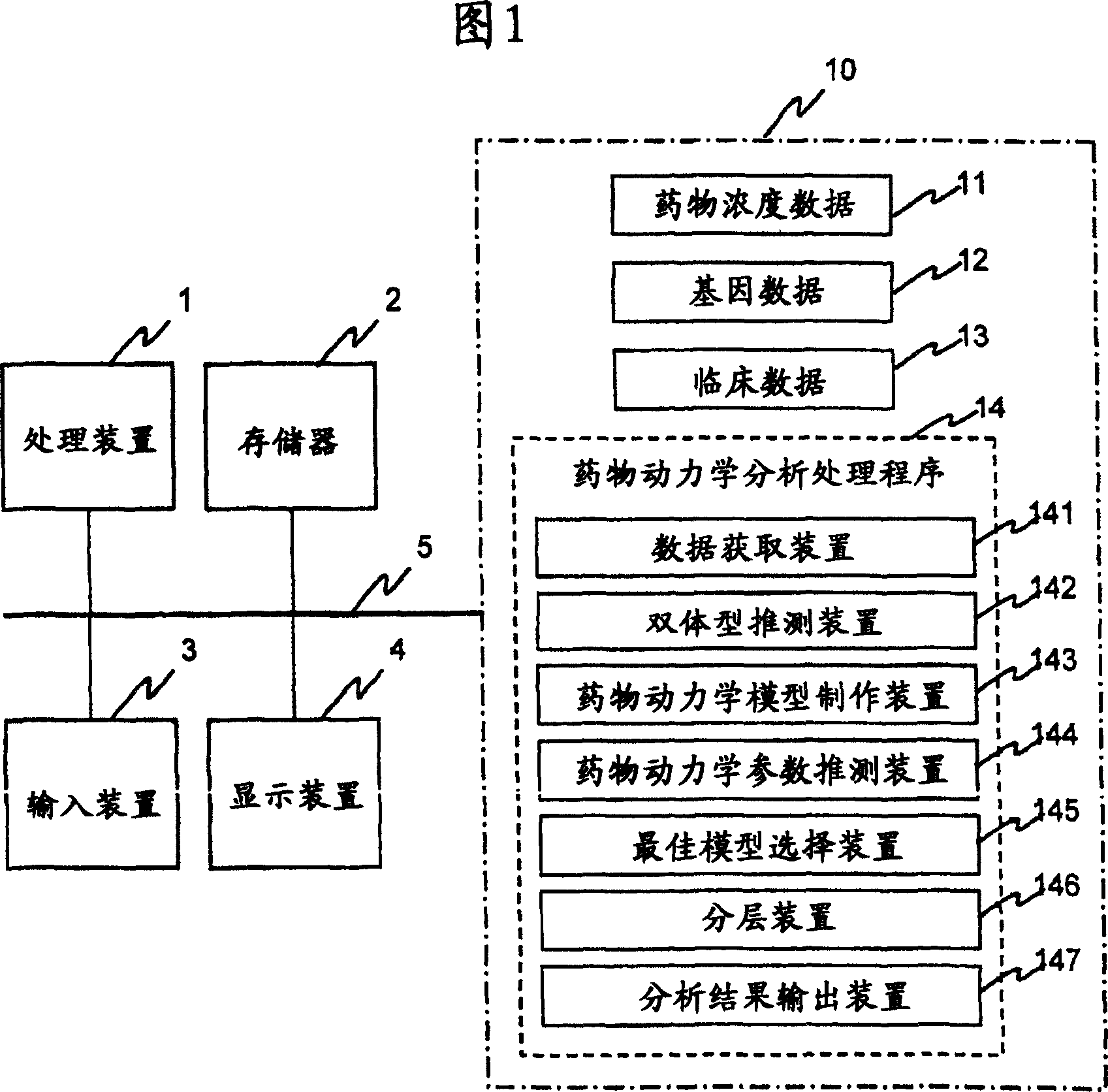
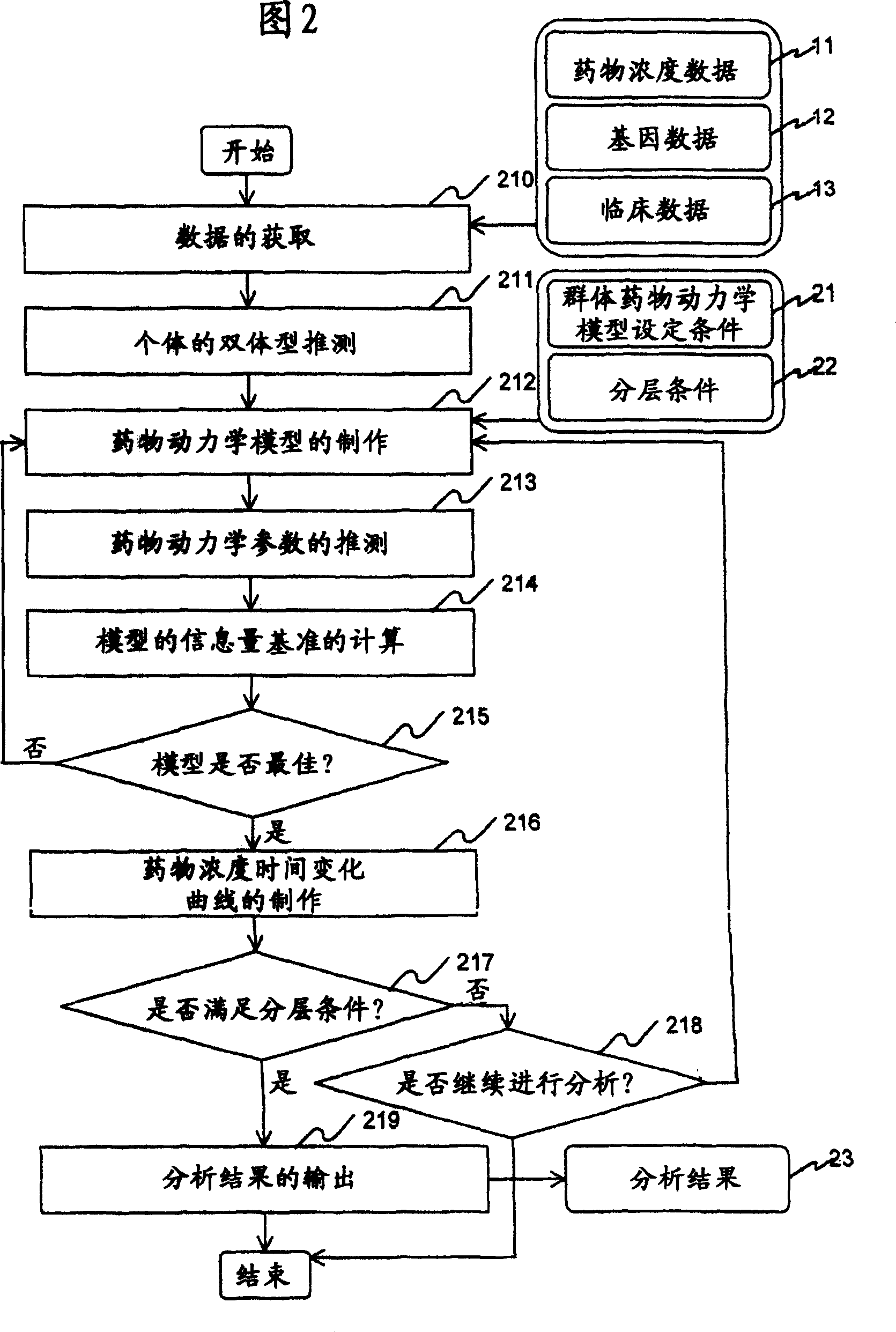
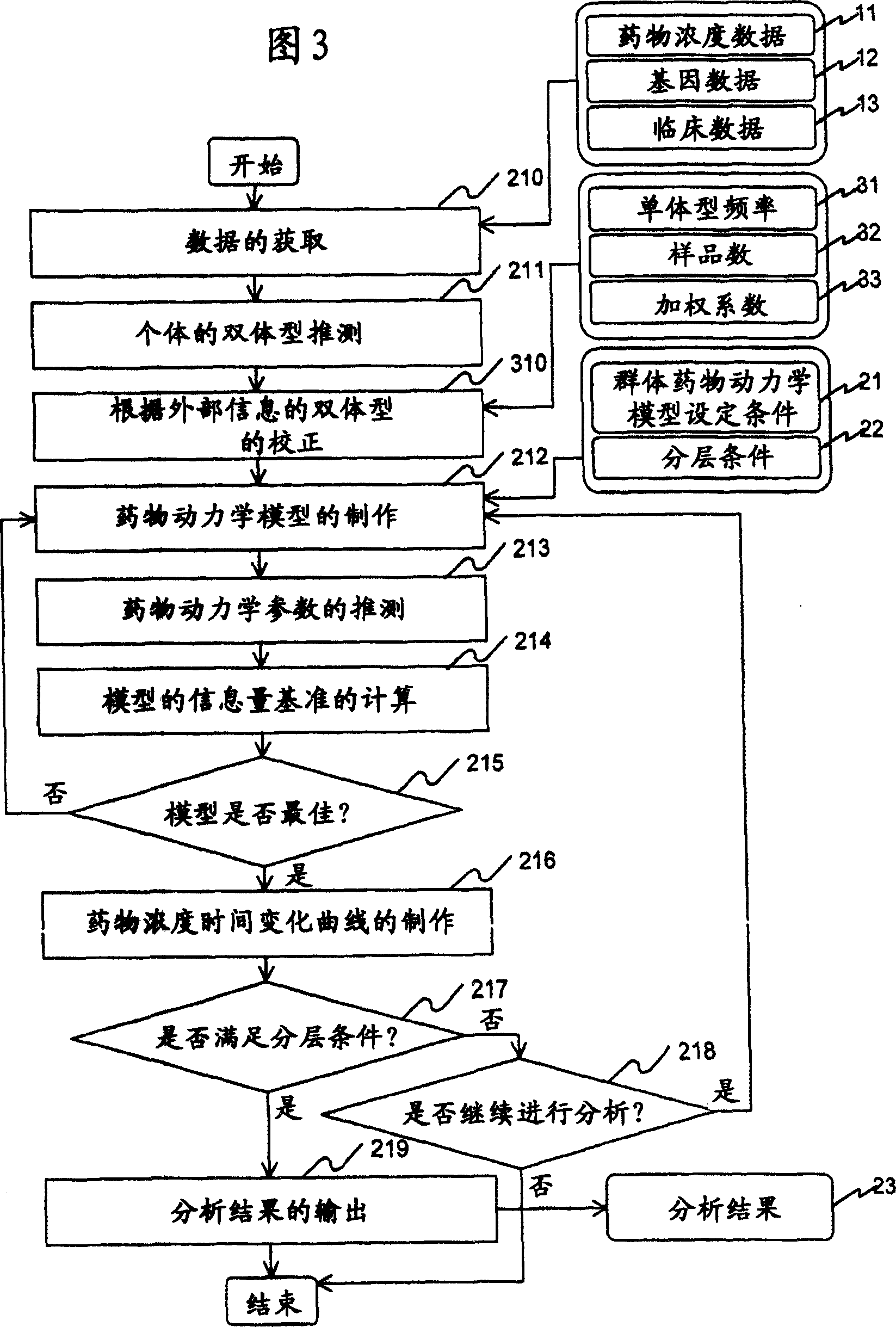
InactiveUS20070239416A1Improve accuracyImprove estimation accuracyChemical property predictionData processing applicationsAlgorithmPharmacokinetic modeling
There are provided a system and a method therefor for analyzing pharmacokinetics in such a manner that the influence of genetic polymorphism of an individual is taken into consideration, and a system and a method therefor for allowing implementation of high-accuracy haplotype frequency estimation and diplotype configuration estimation even in a case where the number of individuals is small from which data is obtainable when making pharmacokinetic analysis. A diplotype configuration estimation step estimates diplotype configurations of individuals. Next, a pharmacokinetic model building step expresses pharmacokinetic parameters as functions of the diplotype configurations of the individuals, thereby configuring pharmacokinetic models. Moreover, a pharmacokinetic parameter estimation step estimates the pharmacokinetic parameters. Here, a diplotype configuration correction step corrects the estimation result of the diplotype configurations of the individuals, thereby implementing an enhancement in the estimation accuracy.
Owner:HITACHI LTD
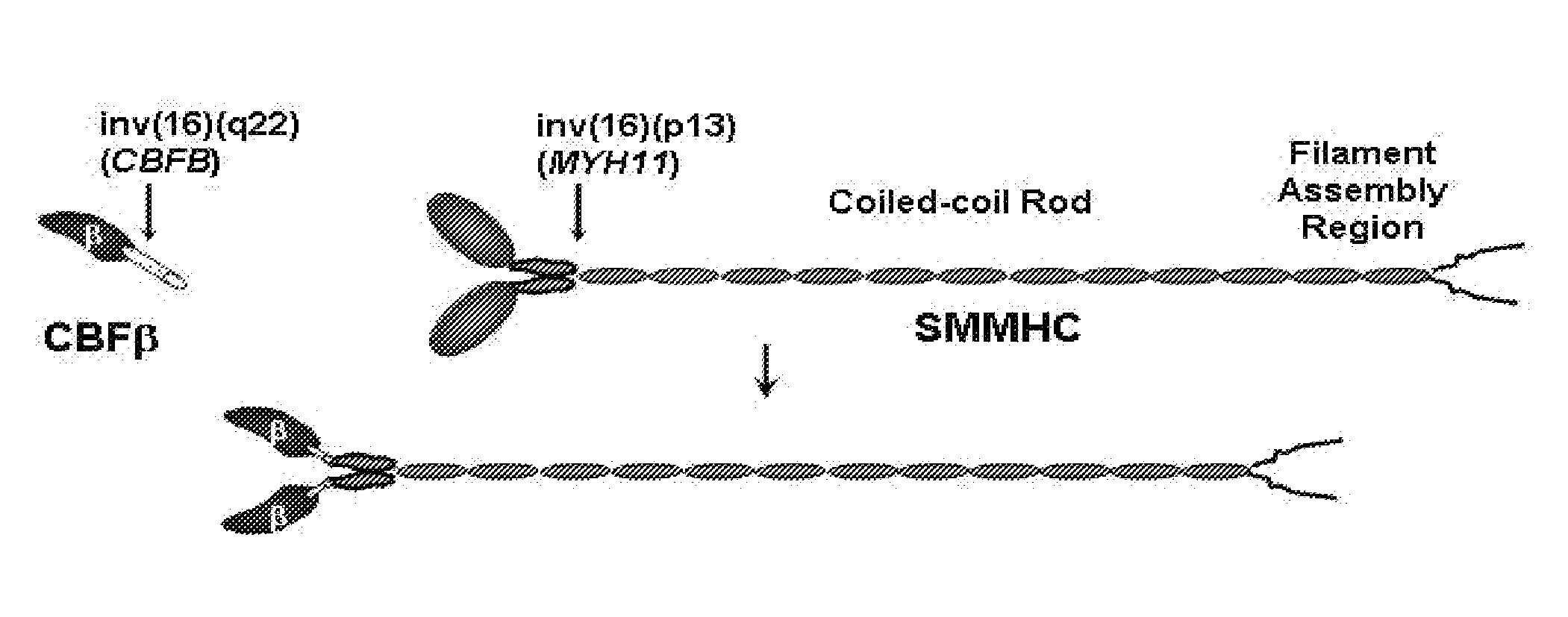
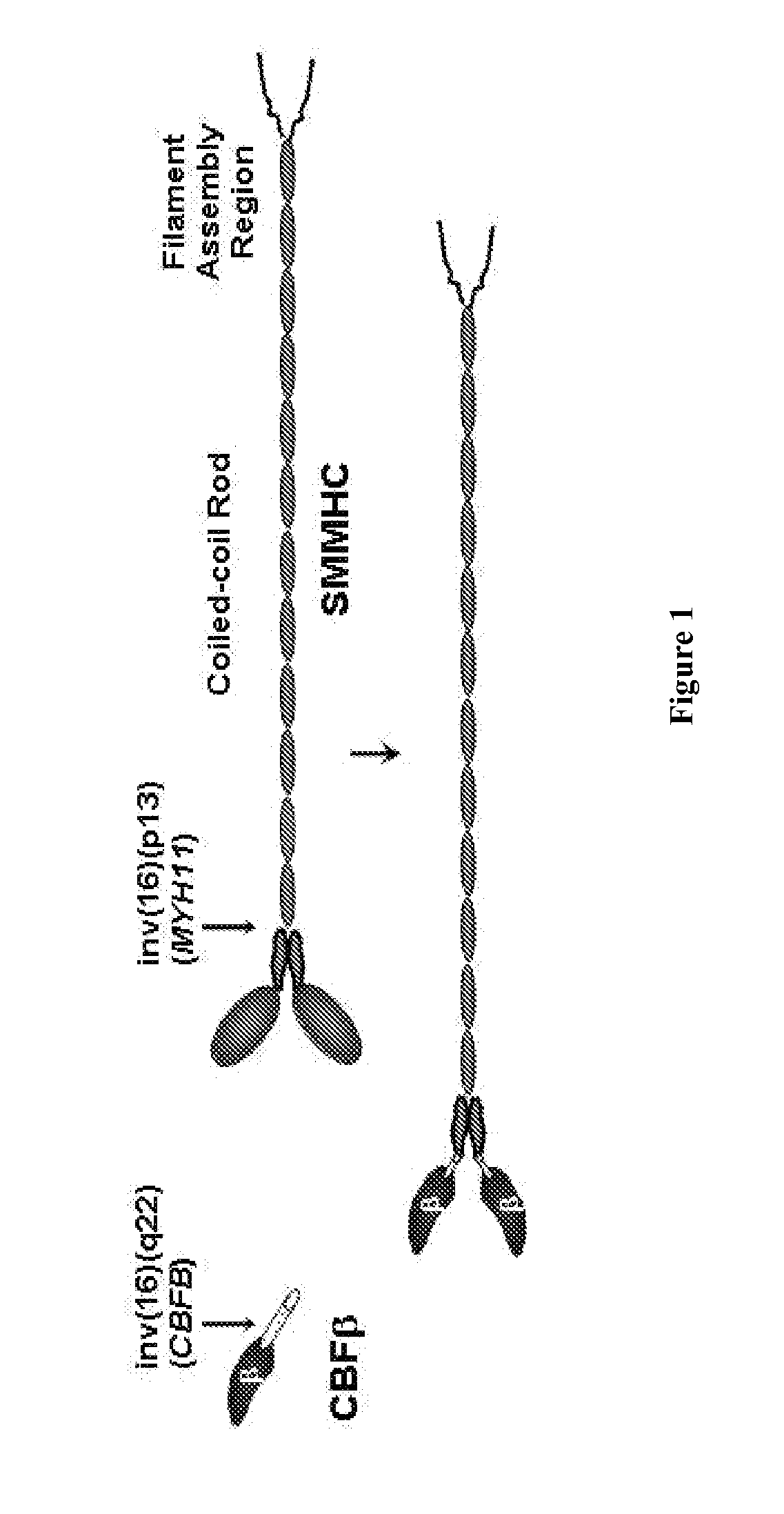
Inhibitors of inv(16) leukemia
This invention describes the development of targeted small molecule inhibitors of the inv(16) fusion, the causative agent in ˜12% of acute myeloid leukemia (AML). The inv(16) fusion results in expression of the CBFβ-SMMHC fusion protein in the blood cells of afflicted patients. The present invention provides compounds which inhibit the function of both CBFβ and the CBFβ-SMMHC fusion. These compounds block the growth of an inv(16) leukemia cell line as well as increase its apoptosis, while showing minimal effects against non inv(16) cell lines. As a mechanism to develop inhibitors with selectivity for the CBFβ-SMMHC fusion protein, the present invention further provides dimeric derivatives of these compounds which show both increased potency as well selectivity for CBFβ-SMMHC. These compounds show potent inhibition of an inv(16) leukemia cell line with minimal effects on non inv(16) cell lines. Analysis of the pharmacokinetics of the developed compounds has made it possible to improve the lifetime of the compound in the plasma of mice to a level commensurate with long-term treatment.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
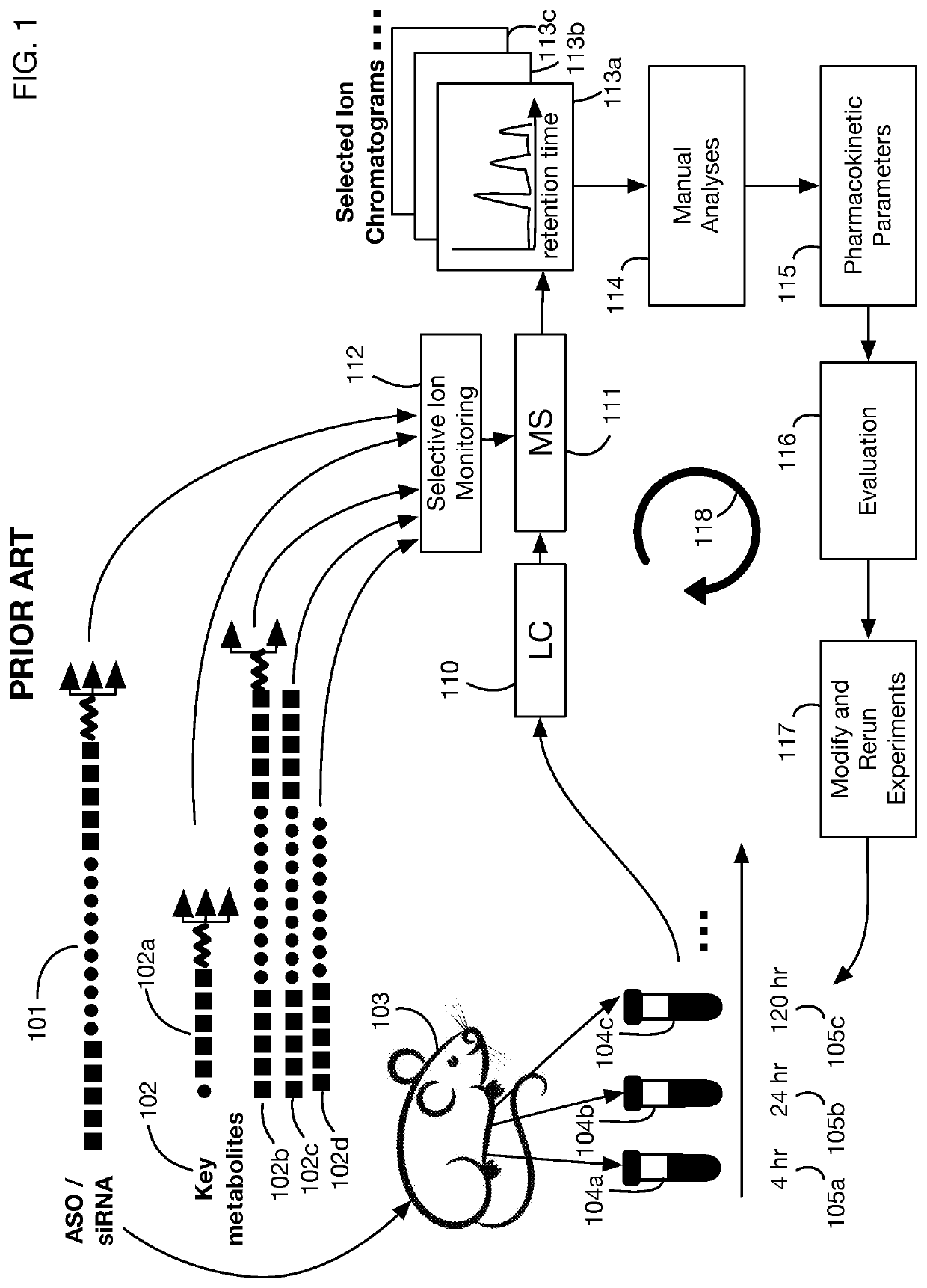
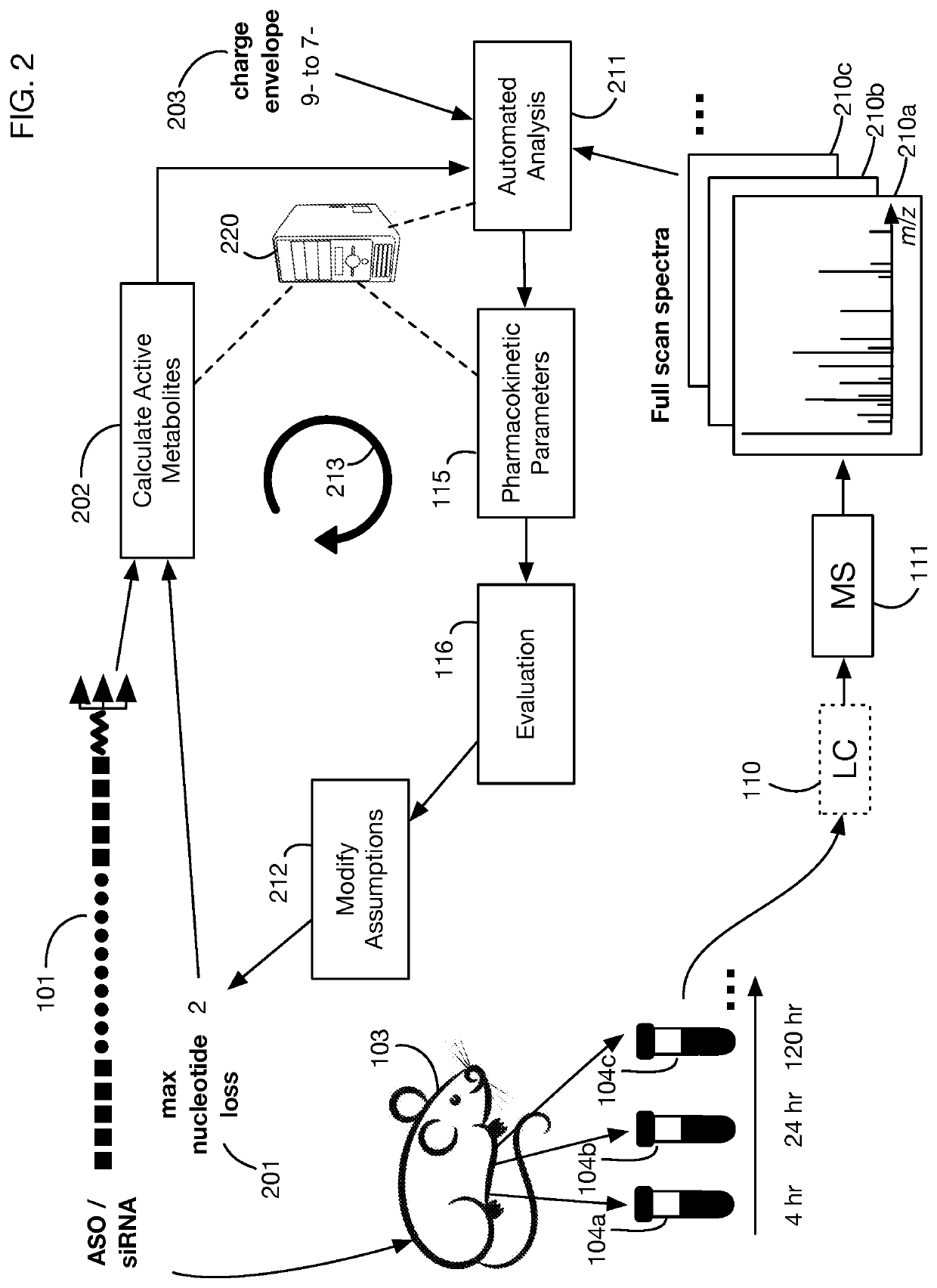
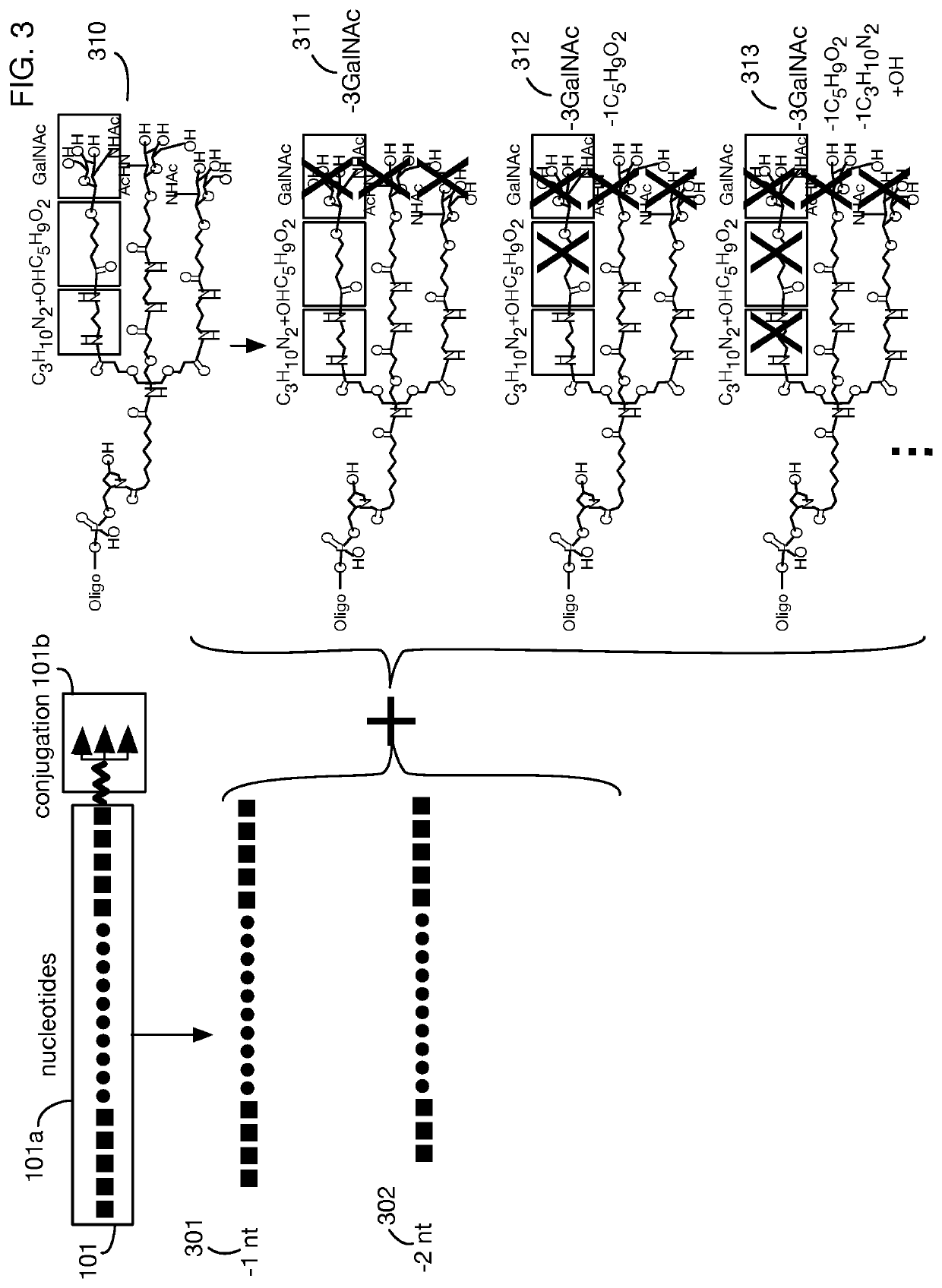
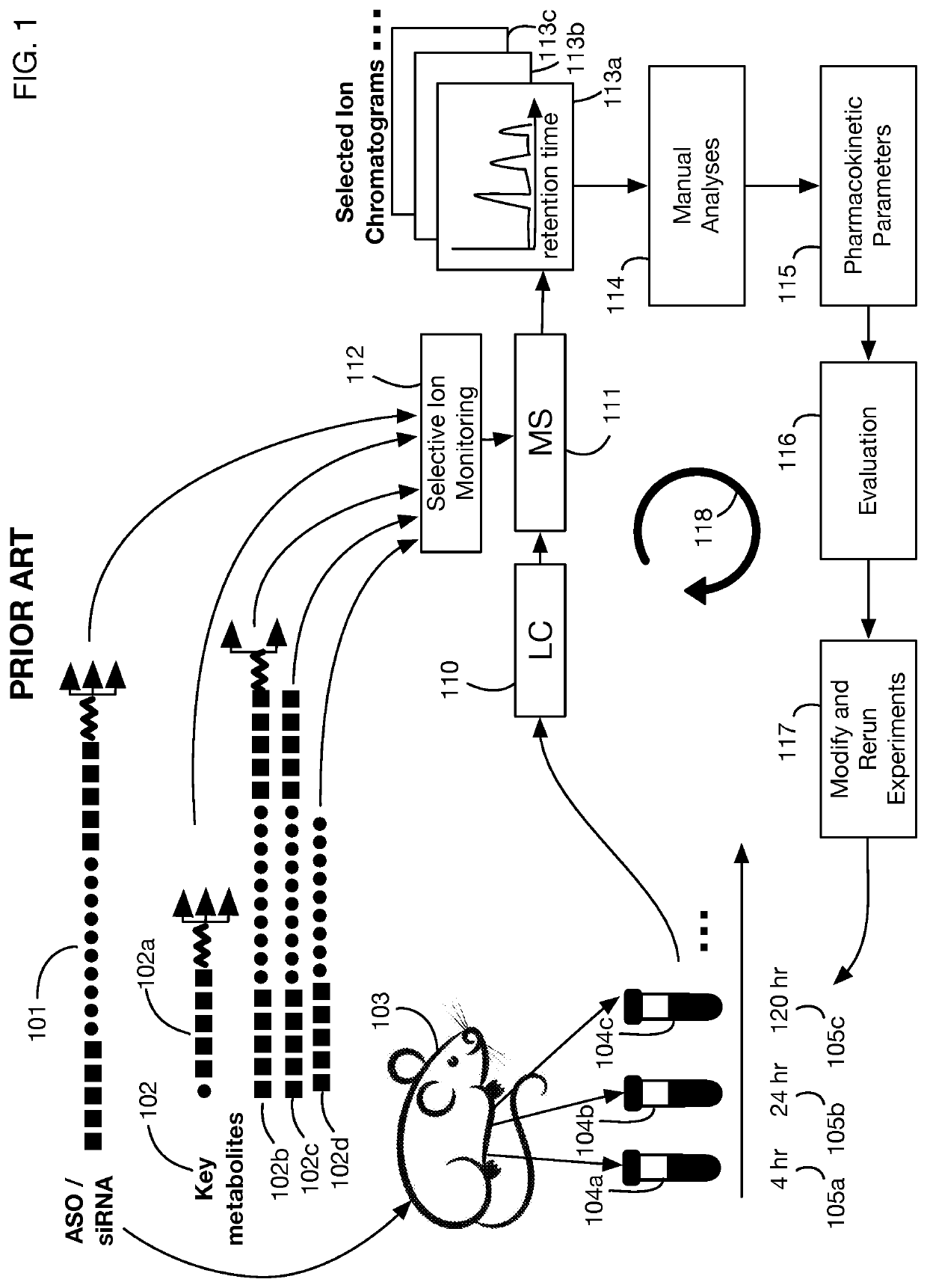
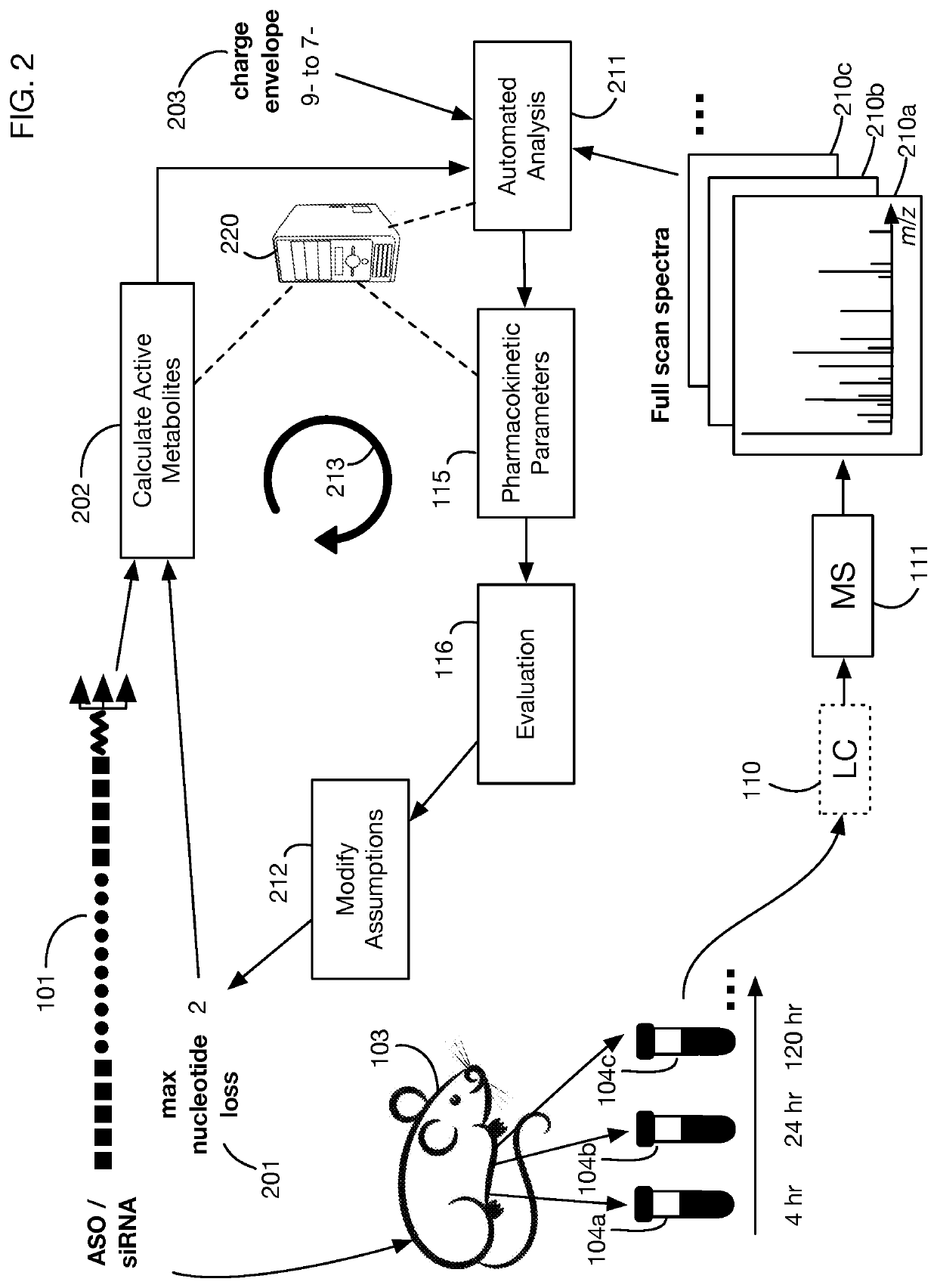
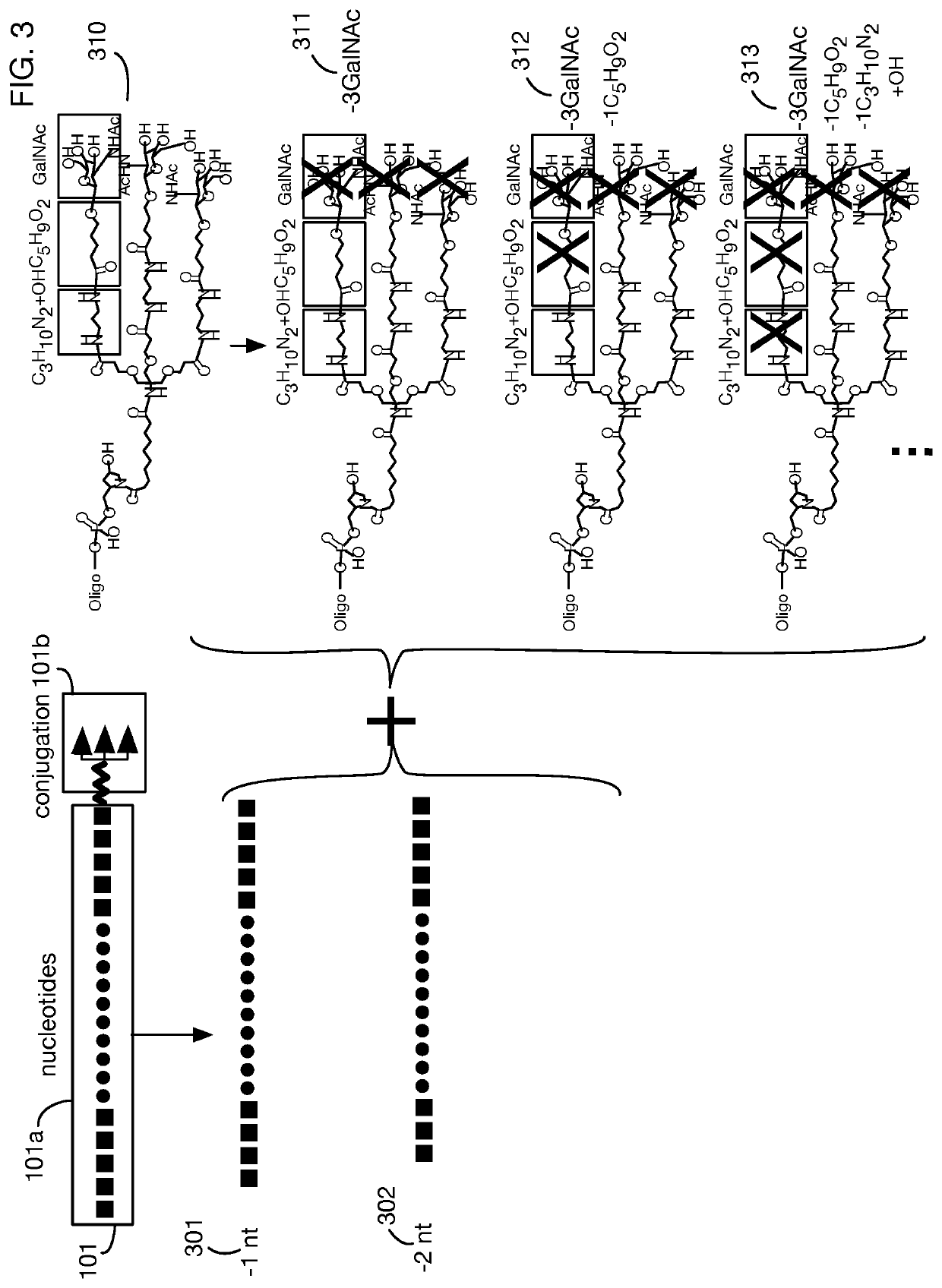
System that generates pharmacokinetic analyses of oligonucleotide total effects from full-scan mass spectra
System that automates analysis of mass spectrometry data for oligonucleotides to generate pharmacokinetic parameters and models. A user inputs an oligonucleotide sequence and a maximum number of nucleotides that may be lost during metabolism while retaining therapeutic effectiveness. The system calculates the possible active metabolites and develops a mass spectrum filter for the mass-to-charge ratio of ions for these metabolites. Full-scan spectra are analyzed to calculate the total concentration of these active molecules present in a time series of samples. Pharmacokinetic models and parameters are calculated from the time series of total concentration. Because full-scan spectra are captured, assumptions may be modified and analyses may be quickly rerun without collecting additional data. Overall pharmacokinetic analysis is therefore much more streamlined and efficient, reducing cost, delay, and the need for a mass spectrometrist who is highly skilled in spectral analysis.
Owner:BIOTUNE COMPUTATIONS LLC
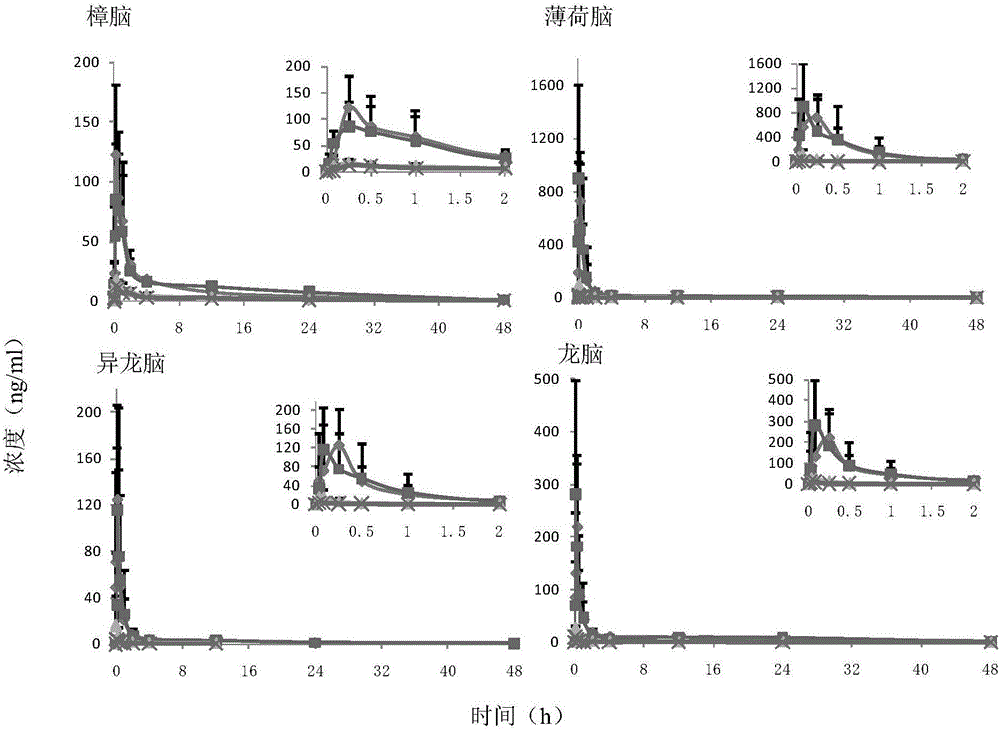
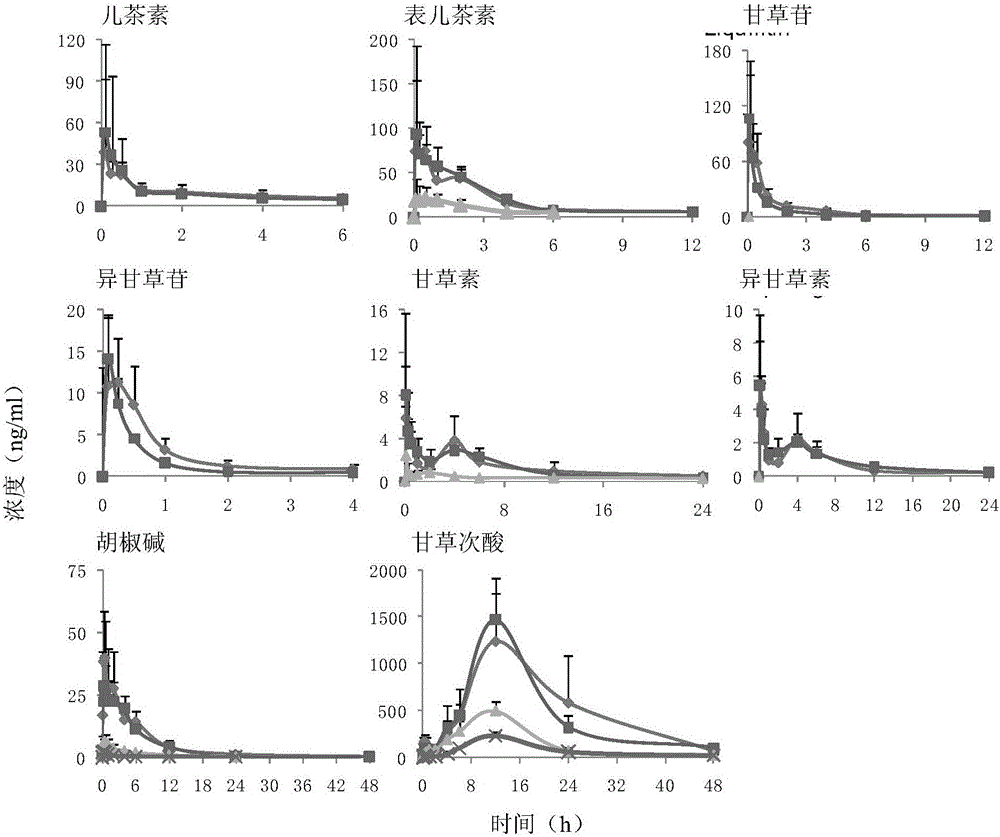
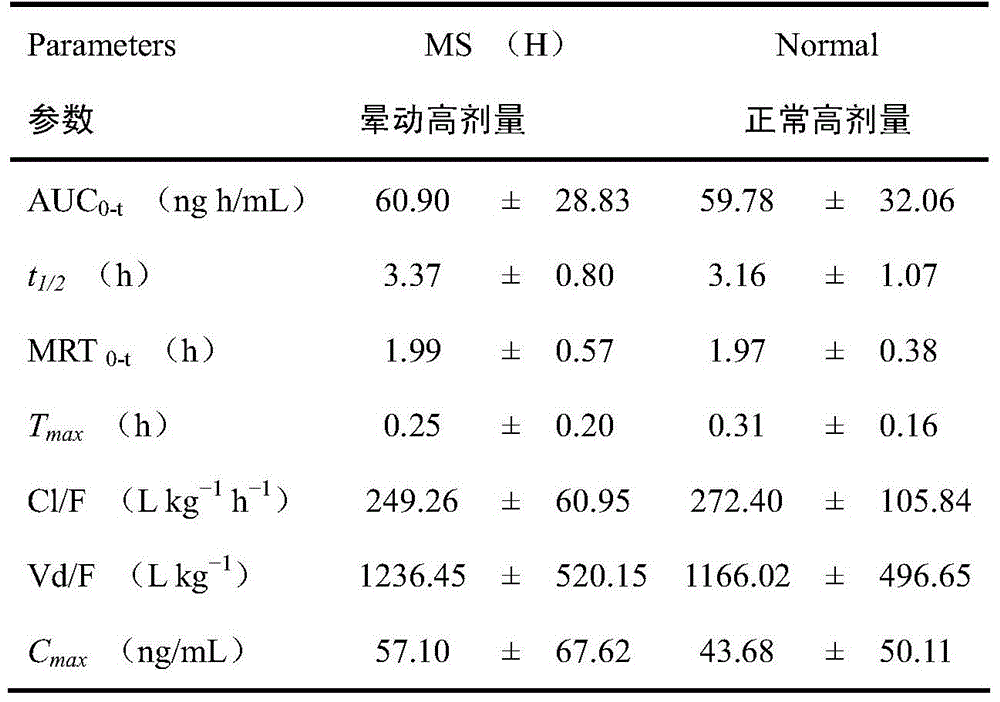
Method for structuring motion sickness model of dragon tiger panacea
InactiveCN105727315ACompounds screening/testingHydroxy compound active ingredientsMotion sicknessAdditive ingredient
The method for constructing the motion sickness model of Long Hu Ren Dan comprises the following steps: (1) animal preparation: clean rats; (2) establishment of motion sickness model; (3) administration and sample collection; (4) Sample determination and (5) pharmacokinetic analysis and statistical analysis, calculate the plasma drug concentration of the active ingredient in the corresponding batch through the accompanying standard curve of each determination batch, and obtain the concentration-time data. In this study, the 8 non-volatile components LC-MS / MS and 4 volatile components HS-SPDE-GC-MS / MS biological sample analysis methods in Longhu Rendan were used to study the normal and Pharmacokinetic study rules in rats with motion sickness. The results of the present invention can provide help for understanding the anti-motion sickness medicinal substance of Longhuren Dan.
Owner:SHANGHAI ZHONGHUA PHARMA
Expanded pharmacokinetic model for population studies in breast magnetic resonance imaging (MRI)
A method for pharmacokinetic analysis, including: receiving time-series medical image data of a patient introduced with a contrast agent; identifying a reference region in the medical image data; identifying a plurality of points of interest in the medical image data; measuring an intensity of voxels in the reference region; and for each point of interest, measure an intensity of voxels therein, use the measured reference region and point of interest intensities to obtain an expression relating the point of interest's voxel concentration to that of the reference region, wherein the expression is a five-parameter nonlinear model with no reference to an arterial input function; and obtain values for each of the five-parameters by solving the expression and use the obtained values to determine whether the point of interest is malignant.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
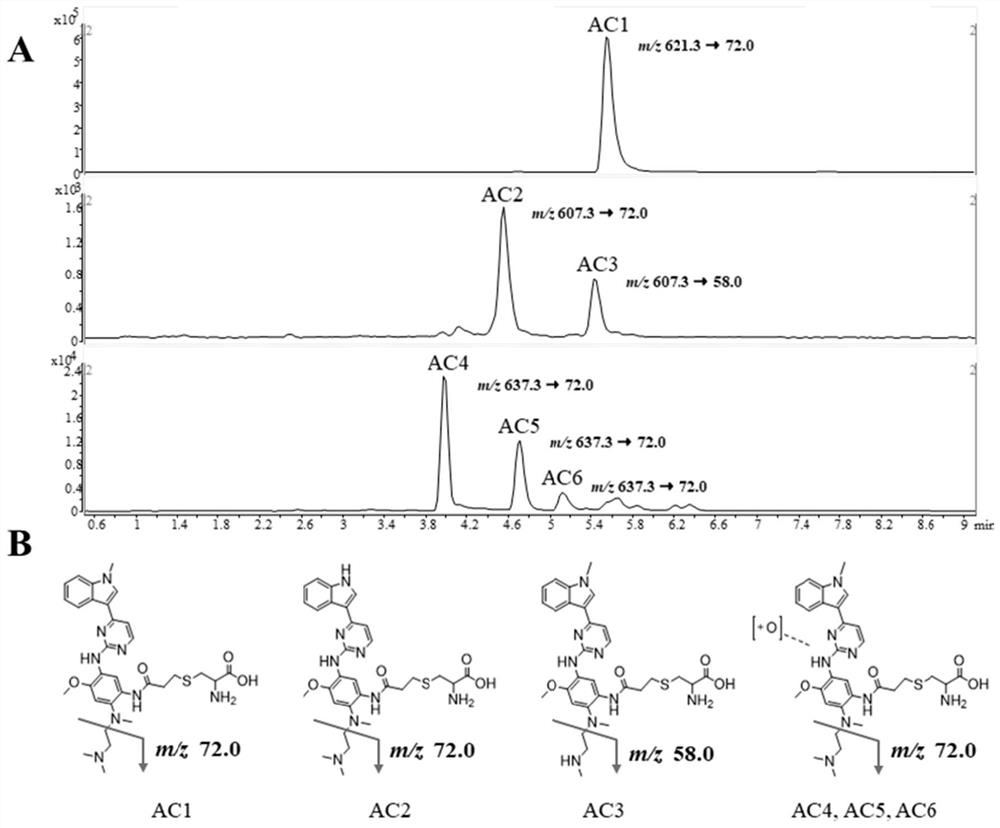
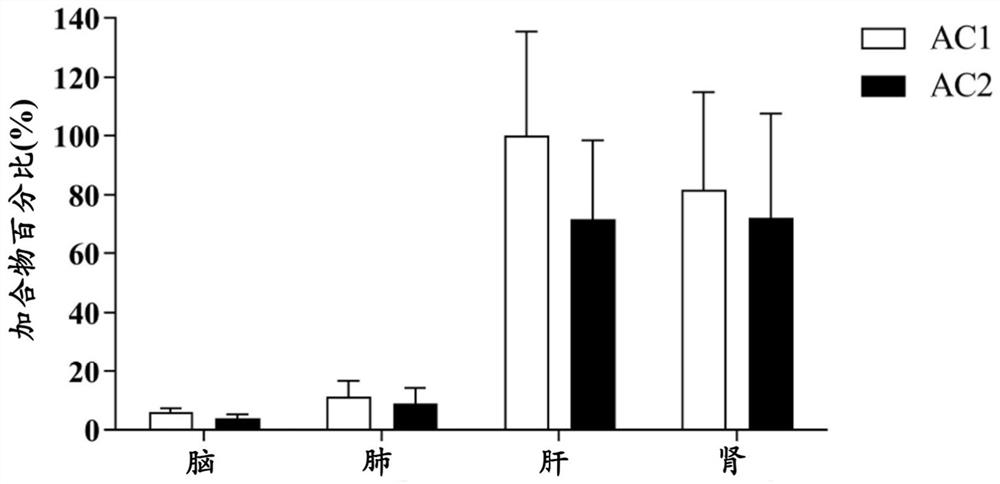
Pharmacokinetic analysis method for covalent drug and metabolite thereof
The invention discloses a method for quantitatively analyzing the modification level of a covalent drug and a metabolite of the covalent drug on protein from the amino acid level, and is applied to the research of pharmacokinetics. The method comprises the following steps: (1) adding a covalent drug and a capture reagent into an in-vitro incubation system for incubation, taking incubation liquid for analysis, identifying an adduct formed by the covalent drug and a metabolite thereof and the capture reagent, and determining a metabolite structure with covalent modification capacity and modified target amino acid; (2) preparing a standard substance of the adduct in the step (1), detecting chromatographic and mass spectrometric data of the standard substance through UHPLC-QQQ-MS, and establishing a quantitative analysis method; and (3) carrying out enzymolysis on a biological drug delivery sample of the covalent drug, detecting the obtained enzymolysis product by adopting UHPLC-QQQ-MS, and determining the content of the adduct in the biological drug delivery sample according to the detection result in combination with the chromatographic and mass spectrometric data in the step (2).
Owner:MACAU UNIV OF SCI & TECH
A preparation transdermal pharmacokinetic analysis method and device
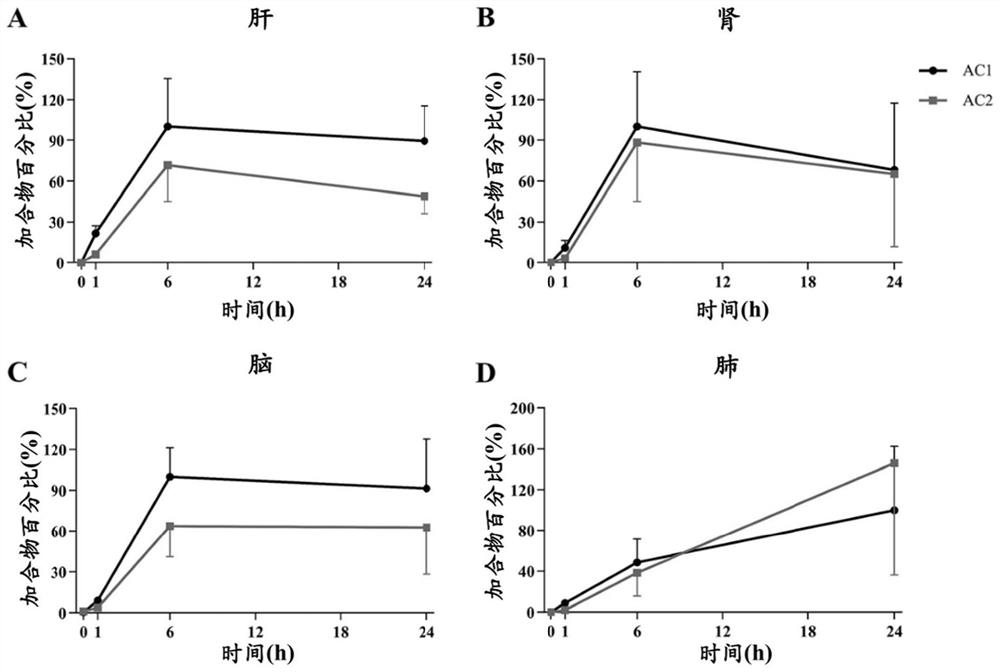
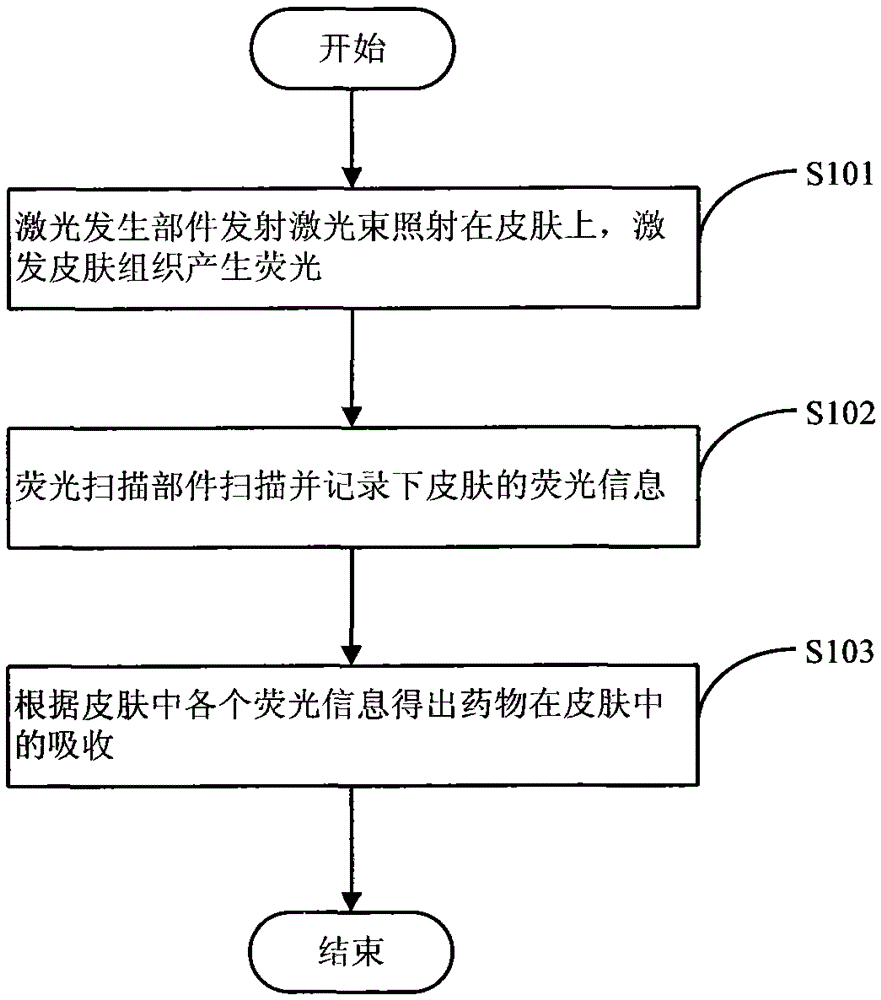
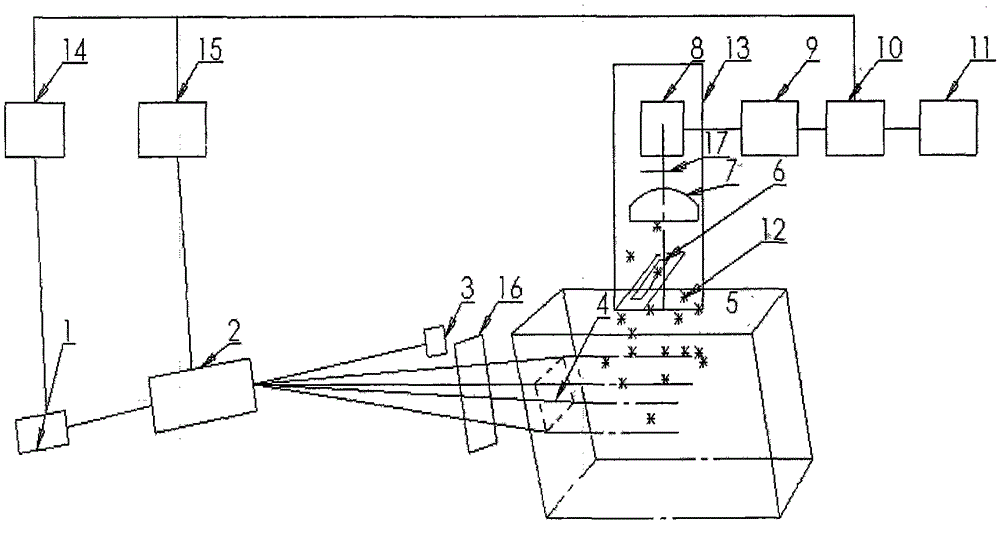
ActiveCN103356171BTruly reflect the actual situation of dynamicsTrue reflection of absorption characteristicsDiagnostic recording/measuringSensorsDrug administrationMedical treatment
The invention belongs to the technical field of medical devices, and discloses a novel preparation transdermal pharmaceutical analytical method and a device. The device comprises a laser generation component, an audio-optical deflection scanning component, a signal detection component of a controllable spatial region, a signal processor, a display, a simulated three-dimensional imaging module and a controlling and actuating mechanism. According to the novel preparation transdermal pharmaceutical analytical technical method and the device, a transdermal pharmaceutical effect is obtained by detecting action mechanisms of laser and photodynamic laser-induced fluorescence of each layer of skin; a novel optical technique is applied in absorption detection of transdermal drug delivery; absorption of a drug in the skin is reflected by detecting the three-dimensional fluorescence in the skin; an evaluation mechanism of transdermal pharmacokinetics is reflected; a transdermal metabolic rule of a preparation is analyzed; and the method and the device have very important significance in studying the action mechanism of the drug and formulating a drug administration scheme.
Owner:泰州准唯光电科技有限公司 +1
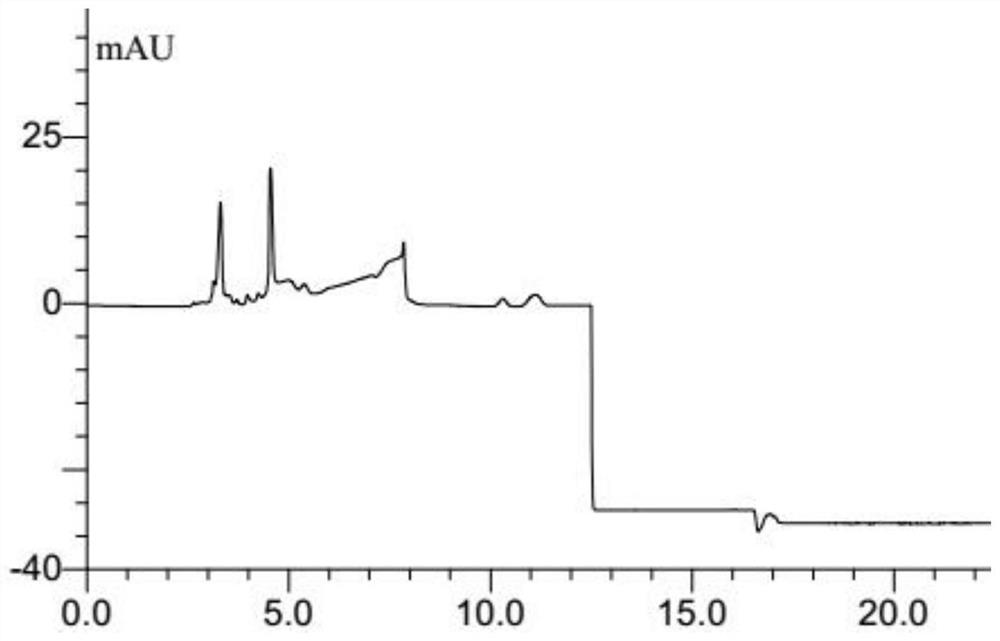
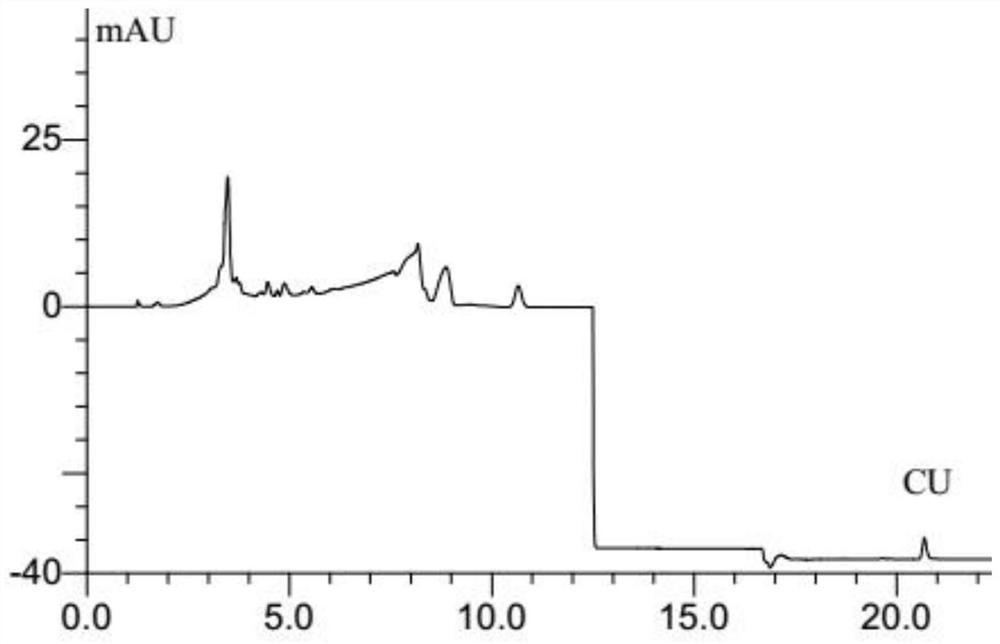
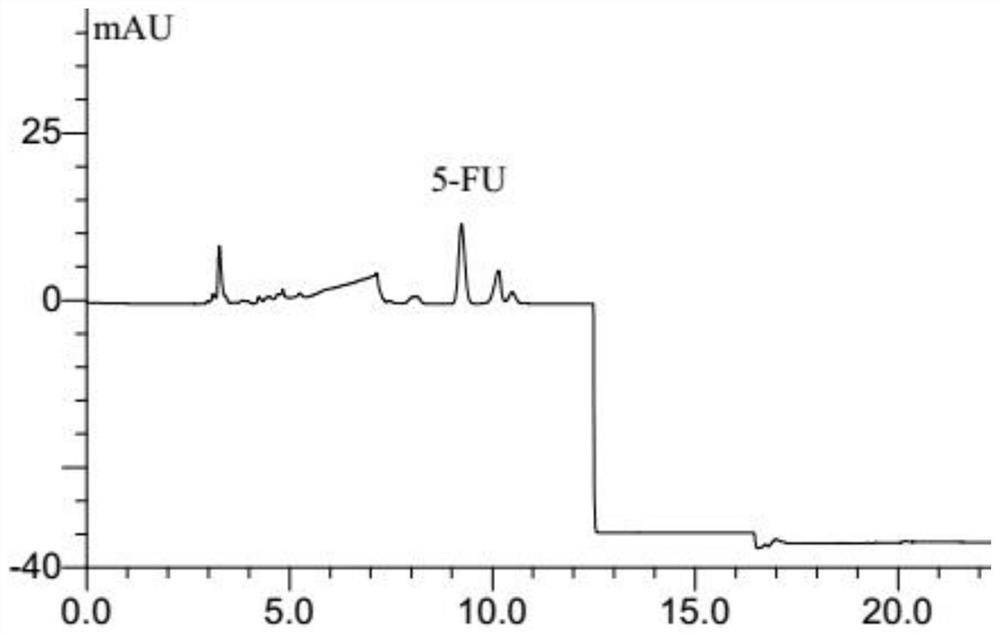
Simultaneous determination of curcumin and 5-fluorouracil in plasma by hplc analysis method
ActiveCN108956816BOvercoming large differences in polarityOvercome the defect of low recovery rateComponent separationPharmaceutical drugBlood plasma
The invention belongs to the technical field of pharmacokinetic analysis, and relates to an HPLC analysis method for simultaneously measuring the concentrations of curcumin and 5-fluorouracil in animal plasma. The initial steps include the preparation of standard solution, sample pretreatment, sample separation, detection of sample peak area and preparation of standard curve. The method overcomes the difficulty that curcumin and 5-fluorouracil are greatly different in polarity and is not easily eluted and separated in the same chromatographic system, and can simultaneously determine the concentrations of curcumin and 5-fluorouracil in plasma.
Owner:SOUTHWEST MEDICAL UNIVERISTY
Detection antibody of anti-VEGFR-2 monoclonal antibody, application and detection method
ActiveCN112094351ASensitive detectionAccurate detectionNucleic acid vectorImmunoglobulinsAntiendomysial antibodiesBiology
The invention provides a detection antibody of an anti-VEGFR-2 monoclonal antibody, application and a detection method. The detection antibody comprises a heavy chain variable region and a light chainvariable region, and the amino acid sequence of the heavy chain variable region is selected from SEQ ID NO: 5, SEQ ID NO: 6, SEQ ID NO: 7 or SEQ ID NO: 8; and the amino acid sequence of the light chain variable region is selected from SEQ ID NO: 9, SEQ ID NO: 10, SEQ ID NO: 11 or SEQ ID NO: 12. According to the detection antibody, the detection antibody aiming at the anti-VEGFR-2 monoclonal antibody is obtained by screening, the antibody can be used for rapidly, sensitively and accurately detecting the drug concentration of the anti-VEGFR-2 monoclonal antibody in body fluid, and a reliable research method is provided for assisting the clinical diagnosis of the anti-VEGFR-2 monoclonal antibody and the pharmacological and pharmacokinetic analysis of a clinical test.
Owner:BEIJING DONGFANG BIOTECH +1
A fluorescent negative staining method for detecting living cells
ActiveCN104345052BDoes not affect activityEasy to judge the phagocytosis ratePreparing sample for investigationFluorescence/phosphorescencePresent methodStaining
The invention discloses a method for detecting living cells by fluorescent negative staining. Fluorescent staining reagents stain whole cells, and when cells phagocytize opaque nano-particles, the nano-particles are enriched in the phagocytic body. Cut off the detection light so that it appears as a fluorescent negative staining area on the fluorescence image. According to the size of the negative staining area, the phagocytosis rate of the cells to the nanoparticles can be easily judged without affecting the activity of the cells. The CFSE fluorescence negative staining method of the cell labeling detection method of the present invention can be used for the detection of labeling rate and labeling amount after nano-iron-labeled cells, and for evaluating the pharmacodynamics and pharmacokinetics analysis of nano-iron-type new drugs, and the method Cells do not need to be fixed, CFSE staining is simple and convenient, and maintains the viability of cells.
Owner:江苏禄亿生生物科技有限公司
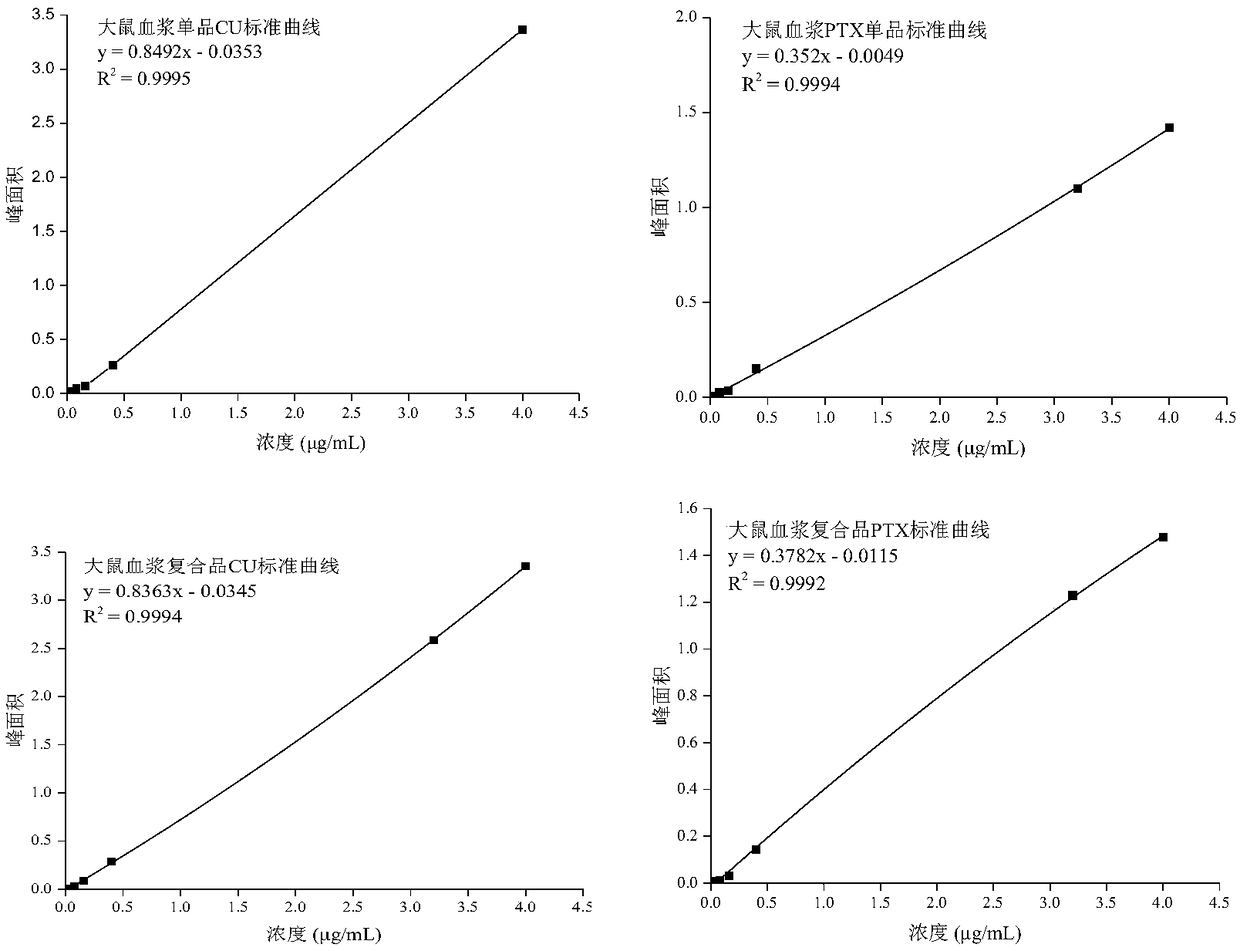
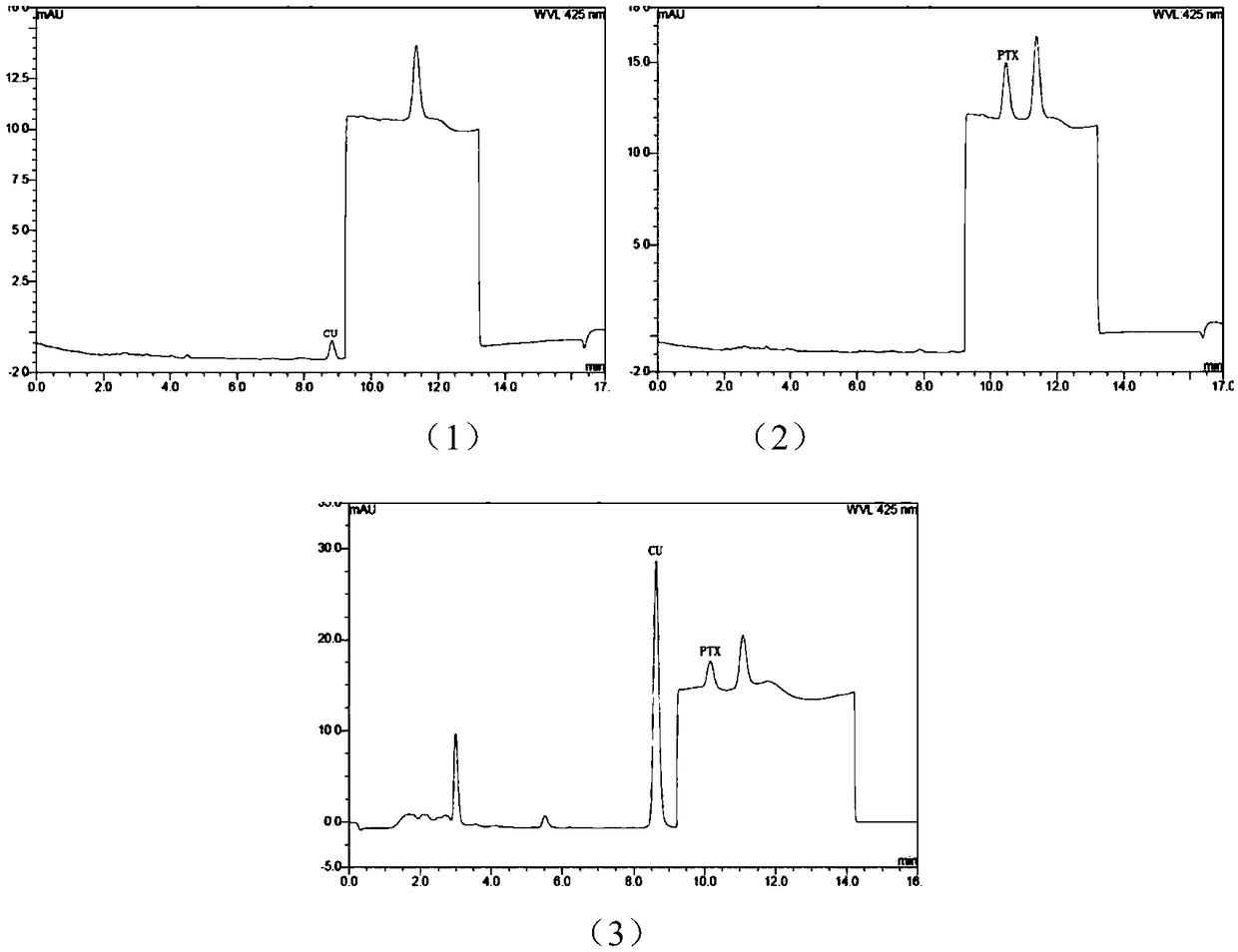
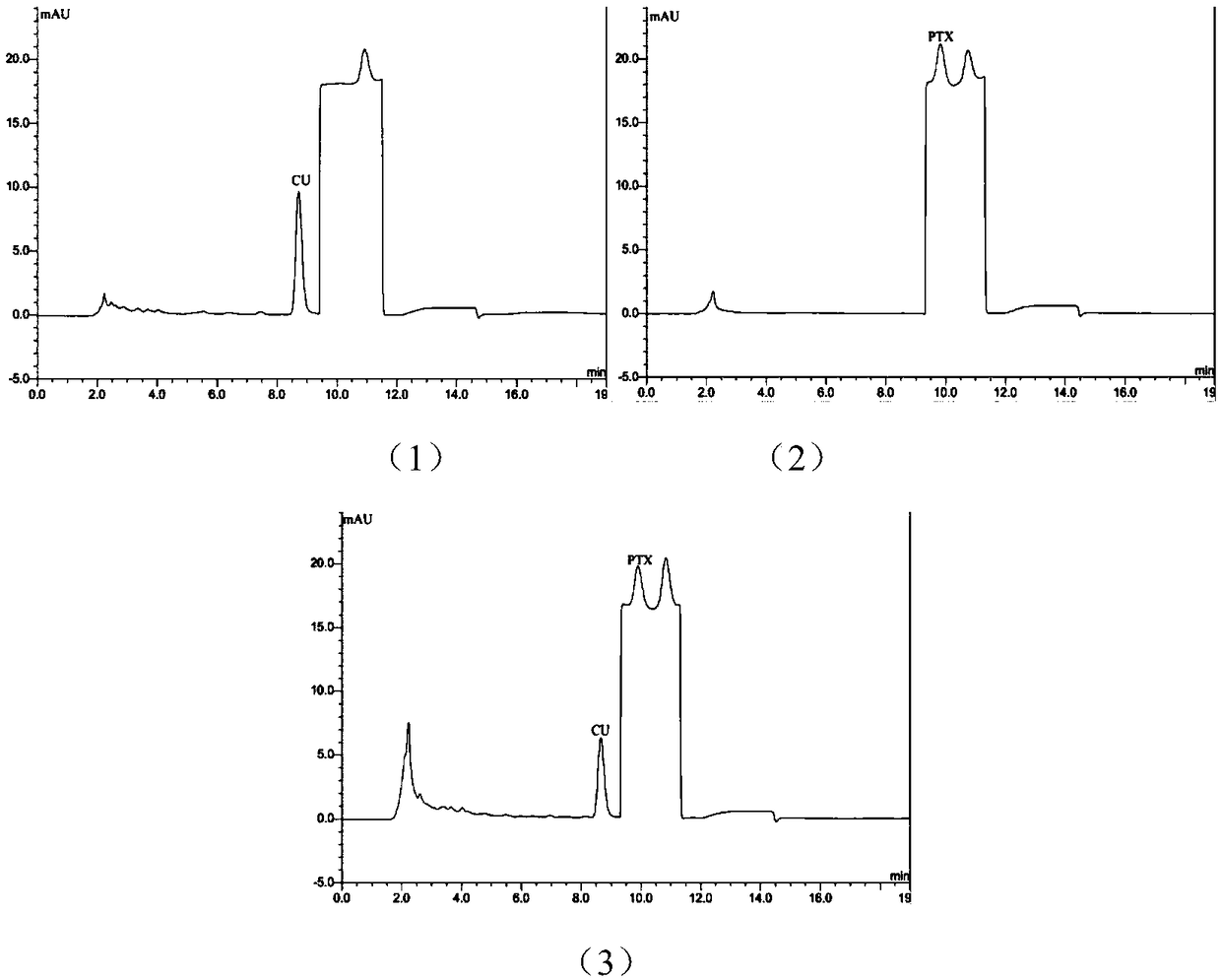
HPLC (High Performance Liquid Chromatography) analysis method for determining content of curcumin and paclitaxel in biological sample
ActiveCN109100435ARealize simultaneous measurementImprove the efficiency of pharmacokinetic studiesComponent separationHplc methodGradient elution
The invention relates to the field of pharmacokinetic analysis, in particular to an HPLC (High Performance Liquid Chromatography) method for determining the content of curcumin and paclitaxel in a biological sample. The method comprises a standard curve establishment step and a sample detection step in sequence. The HPLC analysis method is characterized in that a Luna@5[mu]m C18 (4.6mmx250mm) chromatographic analysis column and a gradient elution variable-wavelength method are adopted. Through adoption of the method, simultaneous determination of the content of curcumin and paclitaxel in the biological sample is realized by only HPLC; the efficiency of pharmacokinetic study of curcumin and paclitaxel combination is improved rapidly; the development process of curcumin and paclitaxel combination can be promoted rapidly; and the time, manpower and economic cost is greatly lowered for research and development enterprises and scientific research institutions.
Owner:SOUTHWEST MEDICAL UNIVERISTY
A detection antibody, application and detection method of anti-vegfr-2 monoclonal antibody
ActiveCN112094351BSensitive detectionAccurate detectionNucleic acid vectorImmunoglobulinsAntiendomysial antibodiesAmino acid
Owner:BEIJING DONGFANG BIOTECH +1
A method for detecting the concentration of ticagrelor and its active metabolites in human plasma
ActiveCN109633063BStrong specificityImprove accuracyComponent separationPretreatment methodTicagrelor
The invention discloses a method for detecting the concentration of ticagrelor and its active metabolites in human plasma, which comprises the following steps: (1) preparation of a standard working solution; (2) sample processing; (3) preparation of a standard curve; 4) Quantitative analysis: take the test sample and process it according to the method of step (2), and calculate according to the standard curve equation obtained in step (3), to obtain the concentration of ticagrelor and its active metabolite in the sample to be tested. The present invention uses high-performance liquid chromatography (HPLC) to measure the concentration of ticagrelor and its active metabolites in human plasma, and accurately quantifies the concentration of ticagrelor and its active metabolites in human plasma. Reasonable selection of methods, mobile phases, mass spectrometry conditions, etc., optimization of experimental conditions such as flow rate, mobile phase ratio, and chromatographic conditions, etc. After a series of methodological verifications, the entire quantitative detection method is practical, easy to operate, high in repeatability, and good in accuracy , strong operability, and can be directly applied to the detection and analysis of plasma samples of clinical patients and related pharmacokinetic analysis.
Owner:HARBIN MEDICAL UNIVERSITY
Method for Screening Active Components, Synergistic and Antagonistic Components in Compound Panax notoginseng Using Rat Blood Stasis Model
ActiveCN104826135BActivity evaluation results are objective and effectiveIn-vivo testing preparationsTime lagModern medicine
The invention belongs to the technical field of medicine, and relates to a method for evaluating the pharmacological activity of traditional Chinese medicine in vivo. The specific technical scheme is: after administering the traditional Chinese medicine to experimental animals, evaluate the changing trend of pharmacological indicators, and collect and administer the medicine at the best time for the action of the traditional Chinese medicine. The biological fluids of animals are reinjected into the experimental animals after appropriate treatment, and then the change trend of the pharmacological indicators is evaluated and compared with the data obtained after direct administration of traditional Chinese medicine to evaluate the time-lag effect of traditional Chinese medicine on the pharmacological indicators. The invention combines the whole pharmacokinetics of the traditional Chinese medicine based on the modern analysis technology to evaluate the effective components and the synergistic or antagonistic components in the effective substances of the traditional Chinese medicine.
Owner:SHENYANG PHARMA UNIVERSITY
System that generates pharmacokinetic analyses of oligonucleotide total effects from full-scan mass spectra
ActiveUS20200402617A1More simplifiedMicrobiological testing/measurementBiostatisticsNucleotideTherapeutic effect
System that automates analysis of mass spectrometry data for oligonucleotides to generate pharmacokinetic parameters and models. A user inputs an oligonucleotide sequence and a maximum number of nucleotides that may be lost during metabolism while retaining therapeutic effectiveness. The system calculates the possible active metabolites and develops a mass spectrum filter for the mass-to-charge ratio of ions for these metabolites. Full-scan spectra are analyzed to calculate the total concentration of these active molecules present in a time series of samples. Pharmacokinetic models and parameters are calculated from the time series of total concentration. Because full-scan spectra are captured, assumptions may be modified and analyses may be quickly rerun without collecting additional data. Overall pharmacokinetic analysis is therefore much more streamlined and efficient, reducing cost, delay, and the need for a mass spectrometrist who is highly skilled in spectral analysis.
Owner:BIOTUNE COMPUTATIONS LLC
Small RNA detection method and use thereof
The present invention relates to a nucleic acid detection method and its application, especially the detection of double-stranded small nucleic acids, characterized in that the method is specifically designed to design several continuous or discontinuous closed nucleic acids complementary to one strand of the small nucleic acid , a reverse transcription stem-loop primer complementary to the 3' end of the other strand of the small nucleic acid, and a forward primer and a reverse primer for quantitative PCR, through denaturation, blocking, reverse transcription and quantitative PCR methods to detect small nucleic acid. The invention has the advantages of simple operation, low detection cost, detection limit up to femtogram level, high detection efficiency, strong specificity, high sensitivity and the like. The present invention can be applied to the quantitative analysis, qualitative analysis, pharmacokinetic analysis of small nucleic acid drugs and the amplification, specific identification and identification of small nucleic acid molecules, especially in molecular diagnosis, small nucleic acid drug metabolism and small nucleic acid drug function research. Wide range of uses.
Owner:BIOMICS BIOTECH
Pharmacokinetic analysis method for monitoring therapeutic drugs
PendingCN114155978AGood curative effectReduce the risk of adverse reactionsDrug referencesComputational theoretical chemistryMetaboliteMedication monitoring
A pharmacokinetic analysis method for monitoring therapeutic drugs comprises the following steps: (1) establishing a plasma concentration determination method: establishing the plasma concentration determination method capable of simultaneously monitoring the therapeutic drugs and main metabolites thereof in a plasma sample of a patient by adopting UPLC-MS / MS, and performing methodological verification on the established plasma concentration determination method; (2) establishing a pharmacokinetic model; and (3) monitoring treatment drugs. The pharmacokinetic analysis method for monitoring the therapeutic drug is reasonable in design, provides a basis for individualization of clinical medication of the therapeutic drug by taking the therapeutic drug as a monitoring target, taking blood concentration monitoring as a core and combining pharmacokinetic research data, and provides a basis for clinical medication individualization of the therapeutic drug by monitoring the therapeutic drug for a patient. The curative effect of the treatment medicine can be obviously improved, the occurrence risk of adverse reaction of the treatment medicine is reduced, and the application prospect is wide.
Owner:SUZHOU LEO BIOTECH
Magnetic resonance contrast agent based on hyaluronic acid as well as preparation method and application of magnetic resonance contrast agent
ActiveCN114805632AAchieve early diagnosisAchieve accuracyIn-vivo testing preparationsLiver fibrosisIn vivo
The invention provides a magnetic resonance contrast agent based on hyaluronic acid as well as a preparation method and application thereof, and belongs to the technical field of medical imaging. The magnetic resonance contrast agent is a hyaluronic acid-based polymer, and the structure of the polymer is shown as a formula (I). The relaxation rate of the liver fibrosis specific MRI imaging contrast agent HTCDGd is three times that of clinical small molecule DTPA-Gd. Pharmacokinetic analysis, MRI and fluorescence imaging confirm that HTCDGd circulates in vivo for a long time and accumulates effectively in the liver. For the diagnosis of hepatic fibrosis, HTCDGd shows excellent fibrosis tissue targeting ability, through MRI enhancement, not only are differences of different degrees of hepatic fibrosis tissue enhancement degrees and longer imaging window observation periods provided, but also morphological characteristics of different degrees of hepatic fibrosis can be directly displayed, and early diagnosis and accurate staging of hepatic fibrosis are realized. The results show that as a non-invasive detection method, HTCDGd has huge potential in the aspect of detecting and accurately monitoring hepatic fibrosis change.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
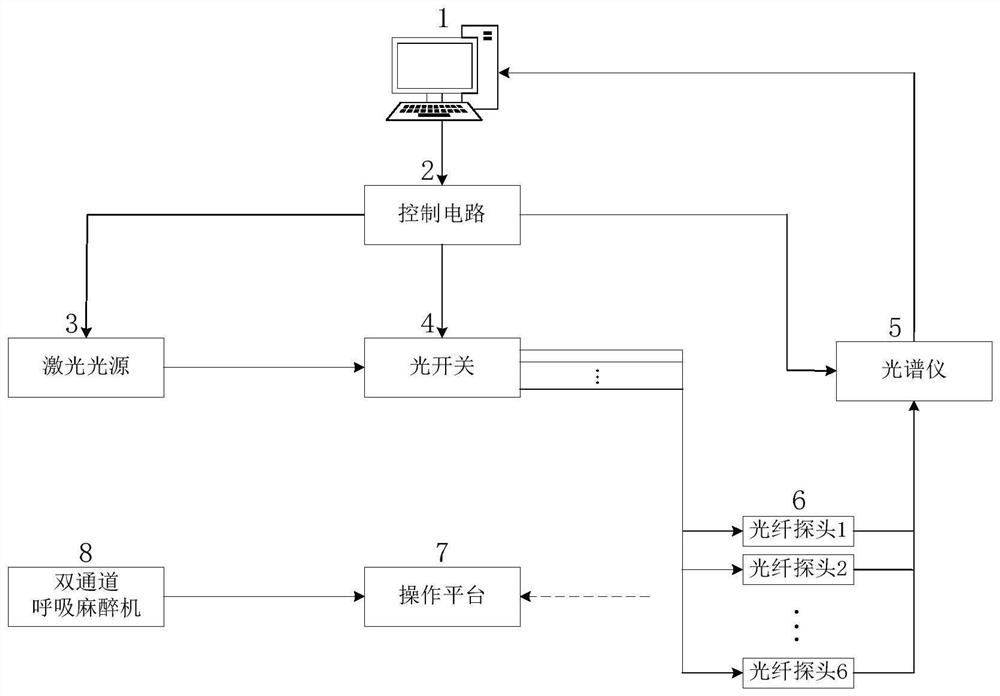
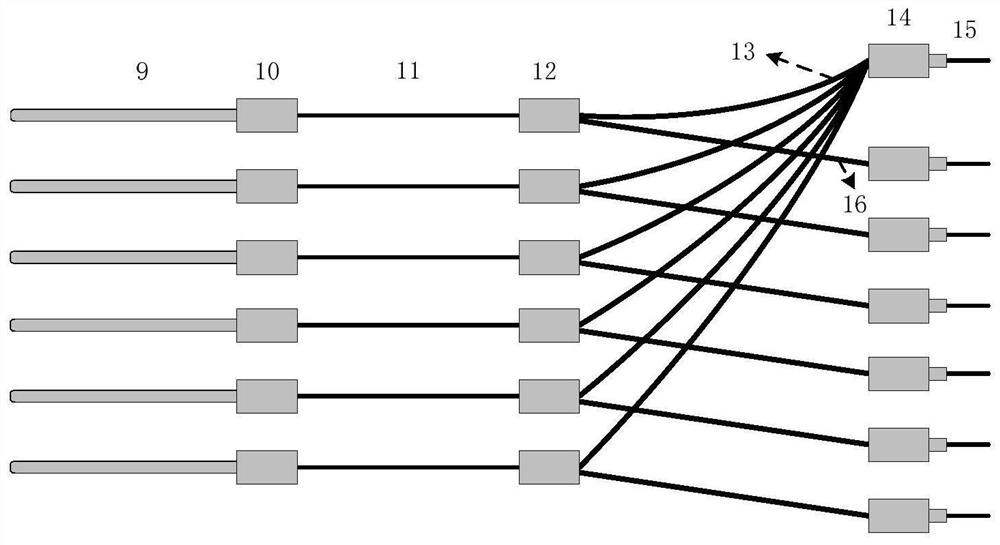
Multi-channel in-vivo pharmacokinetic analysis system based on fluorescence monitoring
PendingCN113633261ARealize monitoringAccurate biological dataDiagnostics using spectroscopyDiagnostics using fluorescence emissionTime-division multiplexingFluorescence spectrometer
The invention discloses a multi-channel in-vivo pharmacokinetic analysis system based on fluorescence monitoring. The multi-channel in-vivo pharmacokinetic analysis system comprises a light source, a multi-path optical switch, a fluorescence spectrometer, a Y-shaped optical fiber probe group, a control circuit, an operation platform, a dual-channel anesthesia machine and an upper computer. According to the invention, multi-channel real-time in-vivo monitoring can be realized, and more accurate biological data relative to in-vitro data can be obtained; optical fiber arrangement is designed according to a slit of the spectrometer, time division multiplexing is performed on the spectrometer by utilizing switching of an optical switch, and simultaneous monitoring of parts such as different tissues, organs and blood vessels of a small animal by six channels is realized; and different physiological pharmacokinetic models are established for analysis according to in-vivo data of a plurality of tissues on an upper computer, so that more accurate data is provided for metabolism of new drugs, and clinical transformation and application of the new drugs are promoted.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Rapid pretreatment method for simultaneously detecting glimepiride and metformin in blood plasma
ActiveCN111693636AGood magnetic responseSimplified processing stepsComponent separationOther chemical processesGlimepiridumBlood plasma
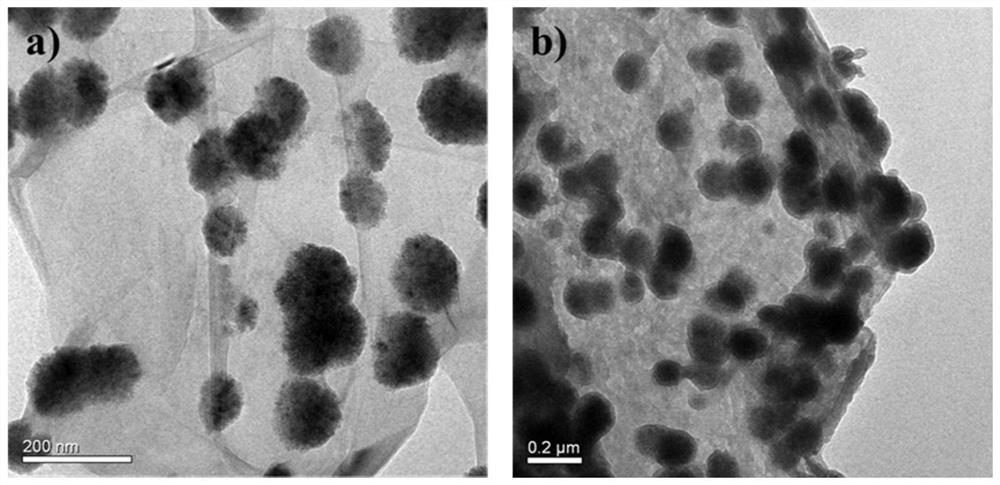
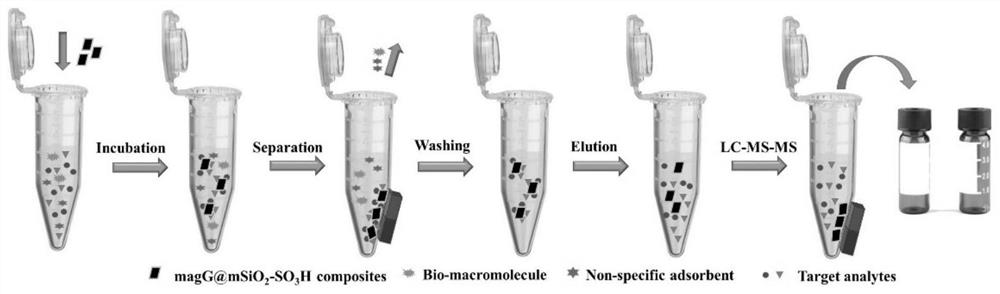
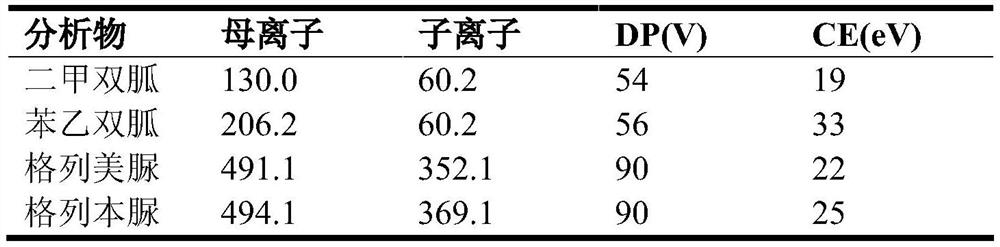
The invention belongs to the technical field of analysis, and relates to a rapid pretreatment method for detecting glimepiride and metformin in blood plasma, in particular to a rapid pretreatment method for enriching and detecting glimepiride and metformin in blood plasma based on a magnetic mesoporous graphene composite material. According to the method, the functionalized magnetic mesoporous graphene material is synthesized, and is used for the pretreatment process of the plasma sample and the method comprises the steps of extraction, cleaning, elution and detection, the sample treatment steps are obviously simplified, and the sample treatment time is shortened; the method can effectively eliminate interference caused by a matrix effect, is wide in linear range, good in recovery rate andhigh in sensitivity, can be used for simultaneously detecting the concentrations of glimepiride and metformin in blood plasma, and is further used for in-vivo pharmacokinetic analysis of glimepirideand metformin.
Owner:FUDAN UNIV
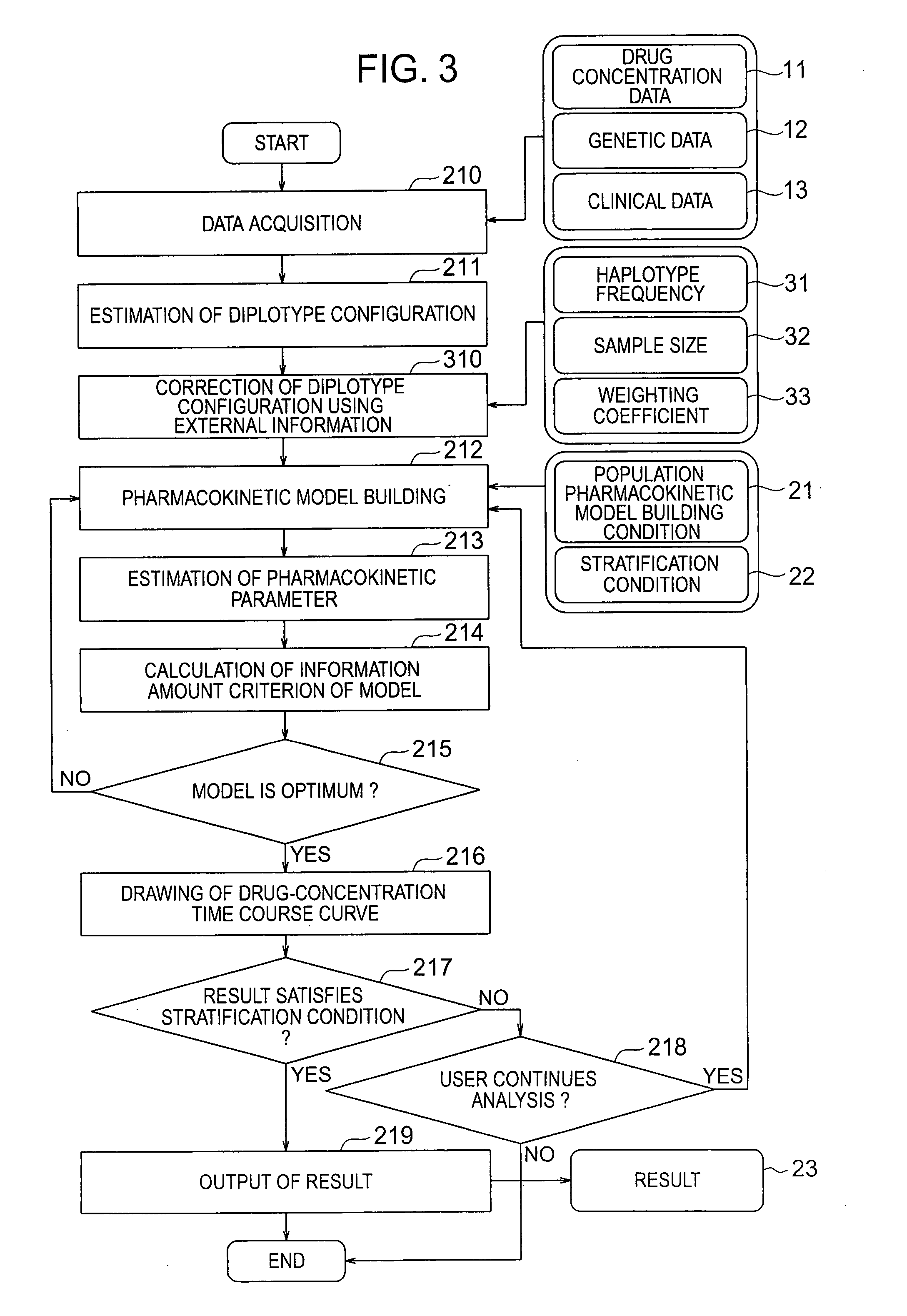
Pharmacokinetic analyzing system and method
InactiveCN101049260AImprove guessing accuracyChemical property predictionData processing applicationsHaplotypeComputer science
Systems and methods for analyzing pharmacokinetics in such a manner that the influence of genetic polymorphisms of an individual are taken into consideration, and for allowing implementation of high-accuracy haplotype frequency estimation and diplotype configuration estimation even in cases where the number of individuals is small from which data is obtainable when making pharmacokinetic analyses.
Owner:HITACHI LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com