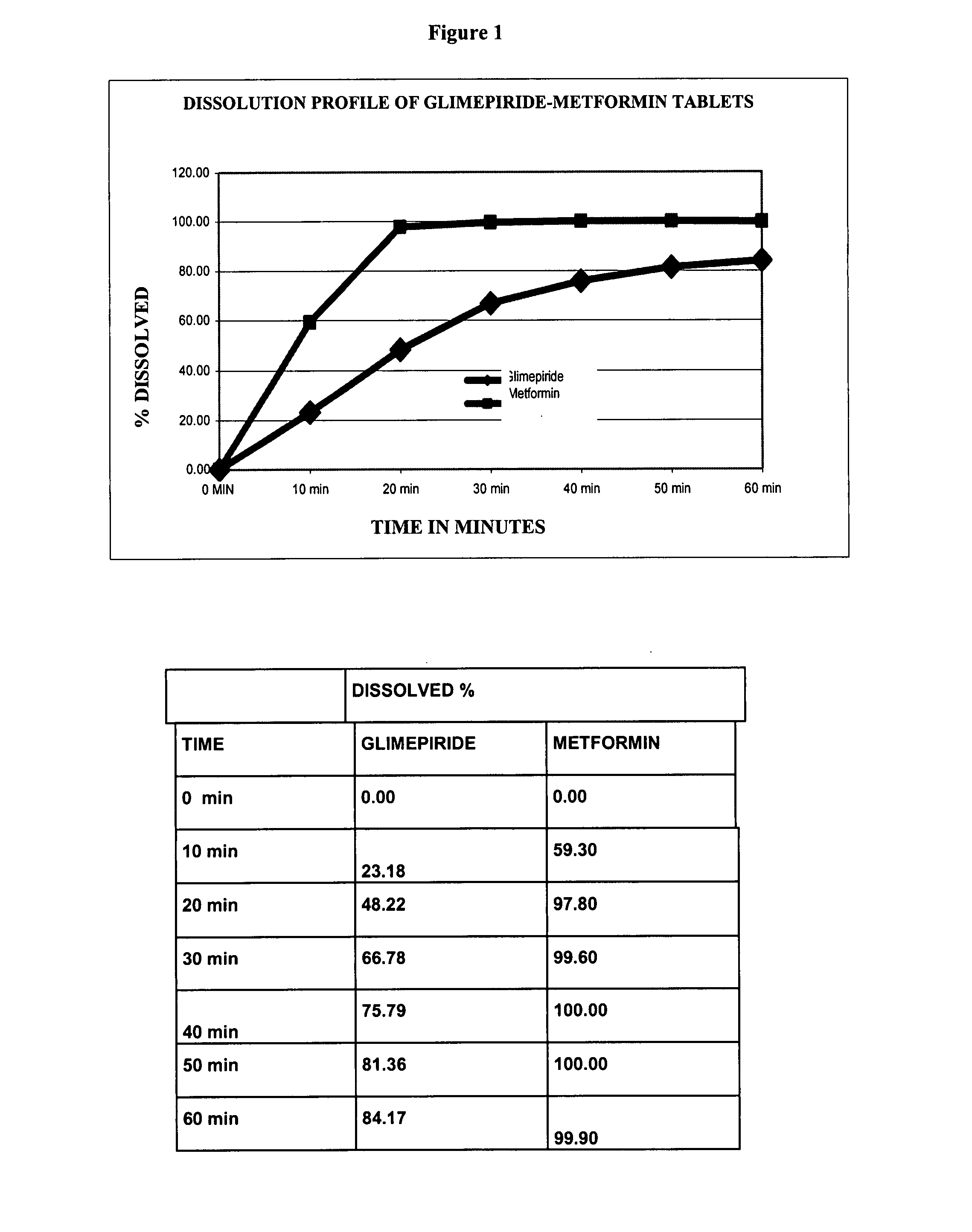
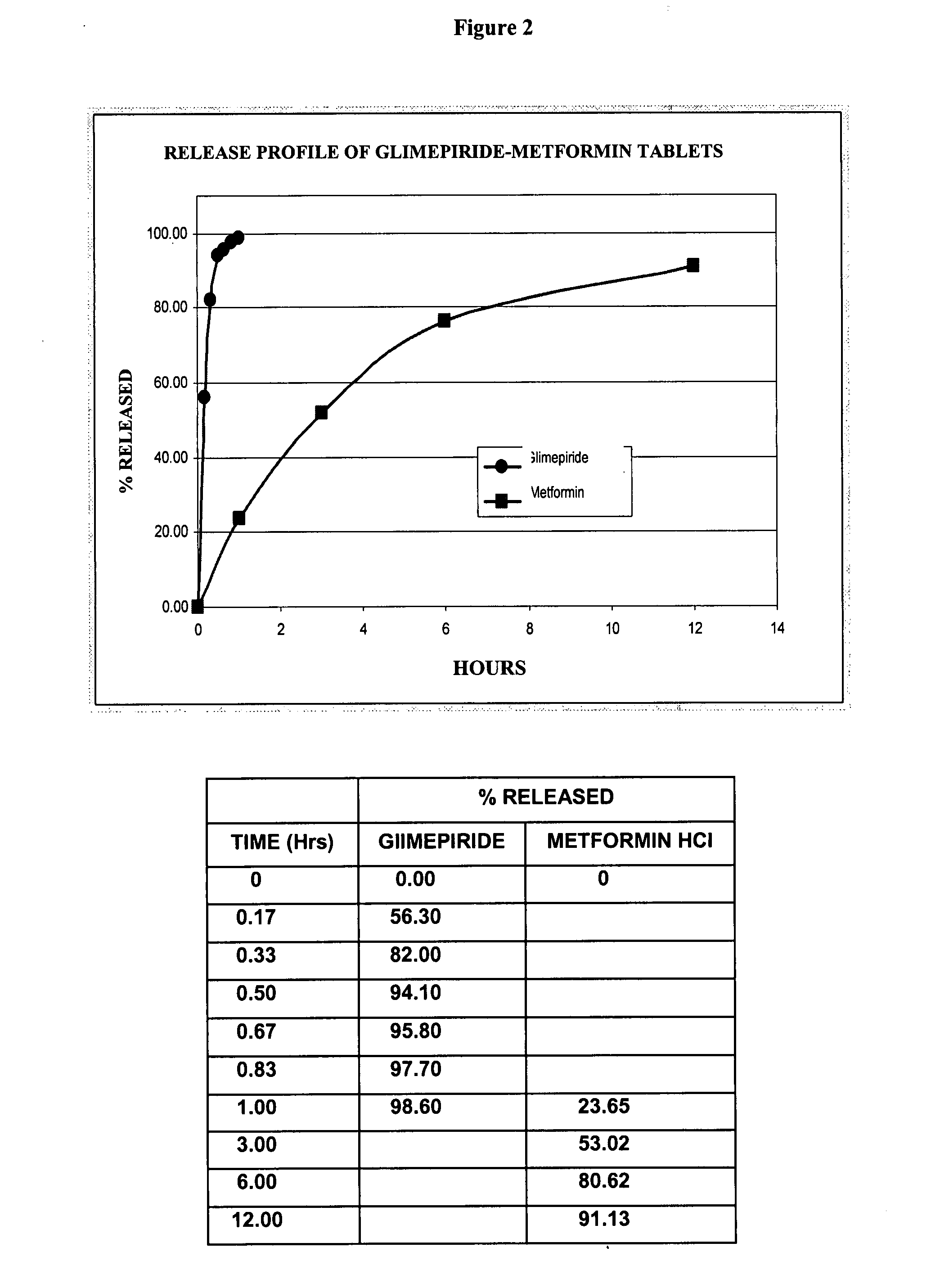
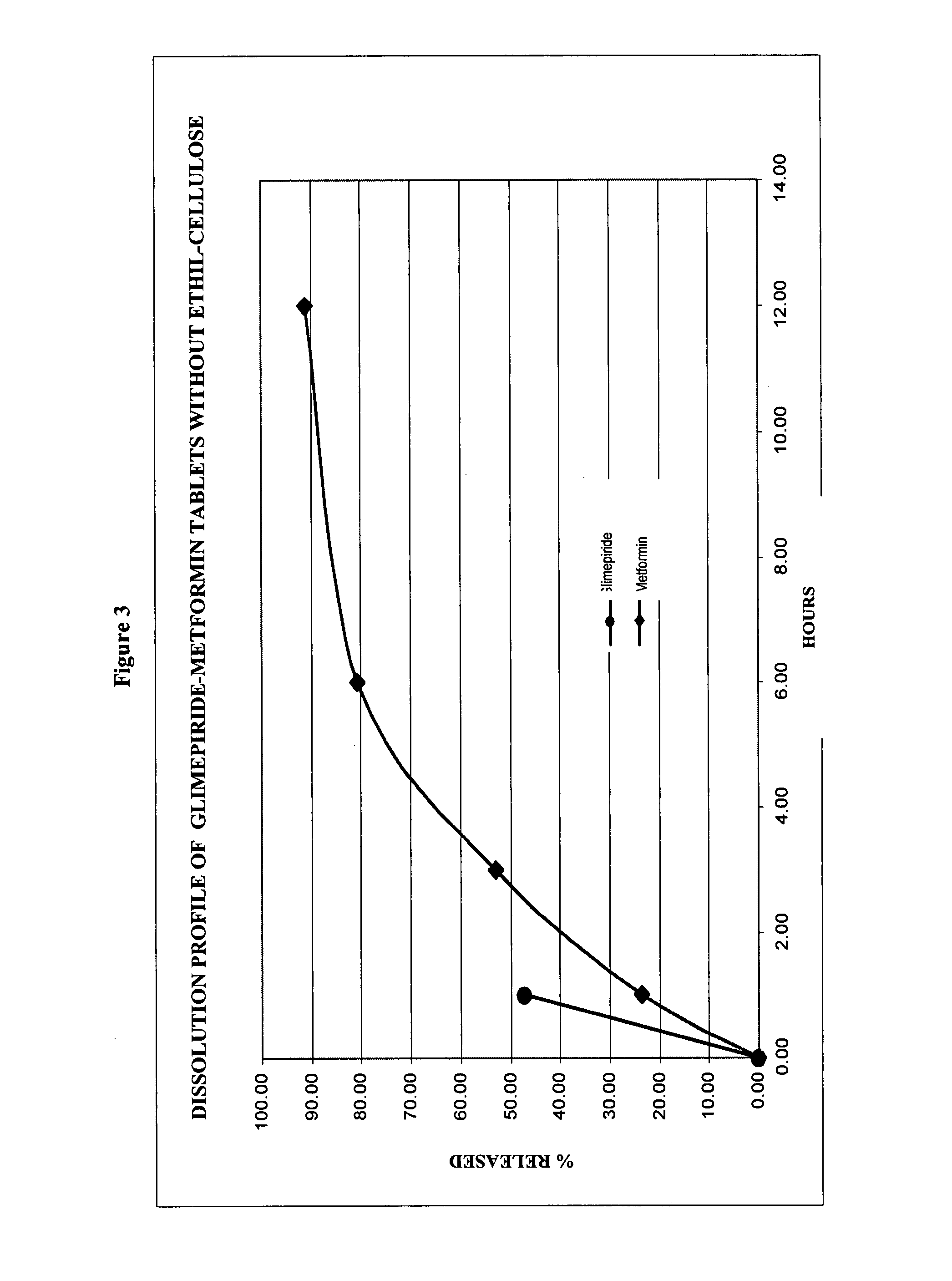
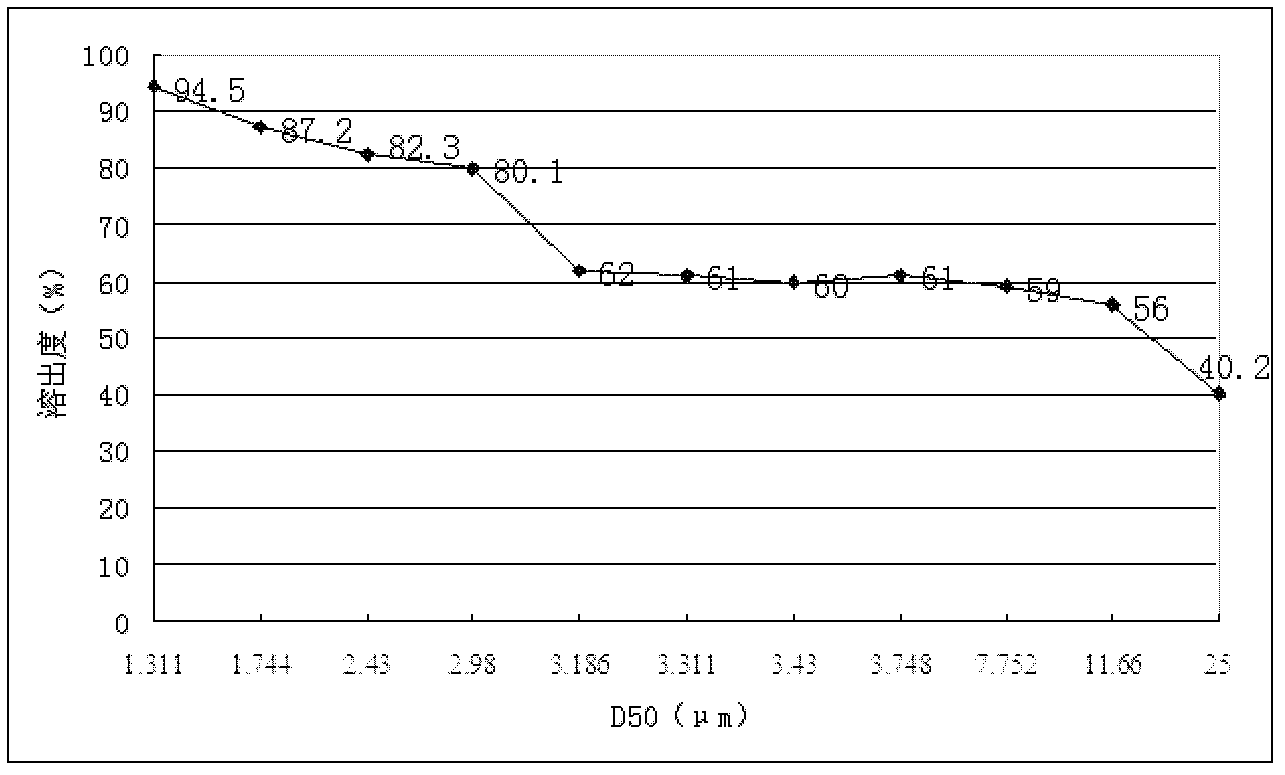
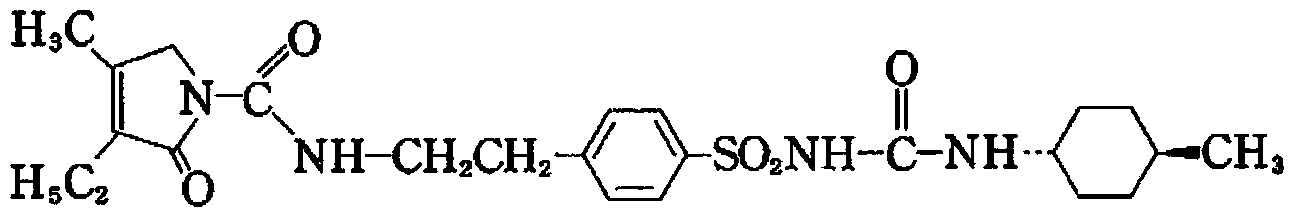
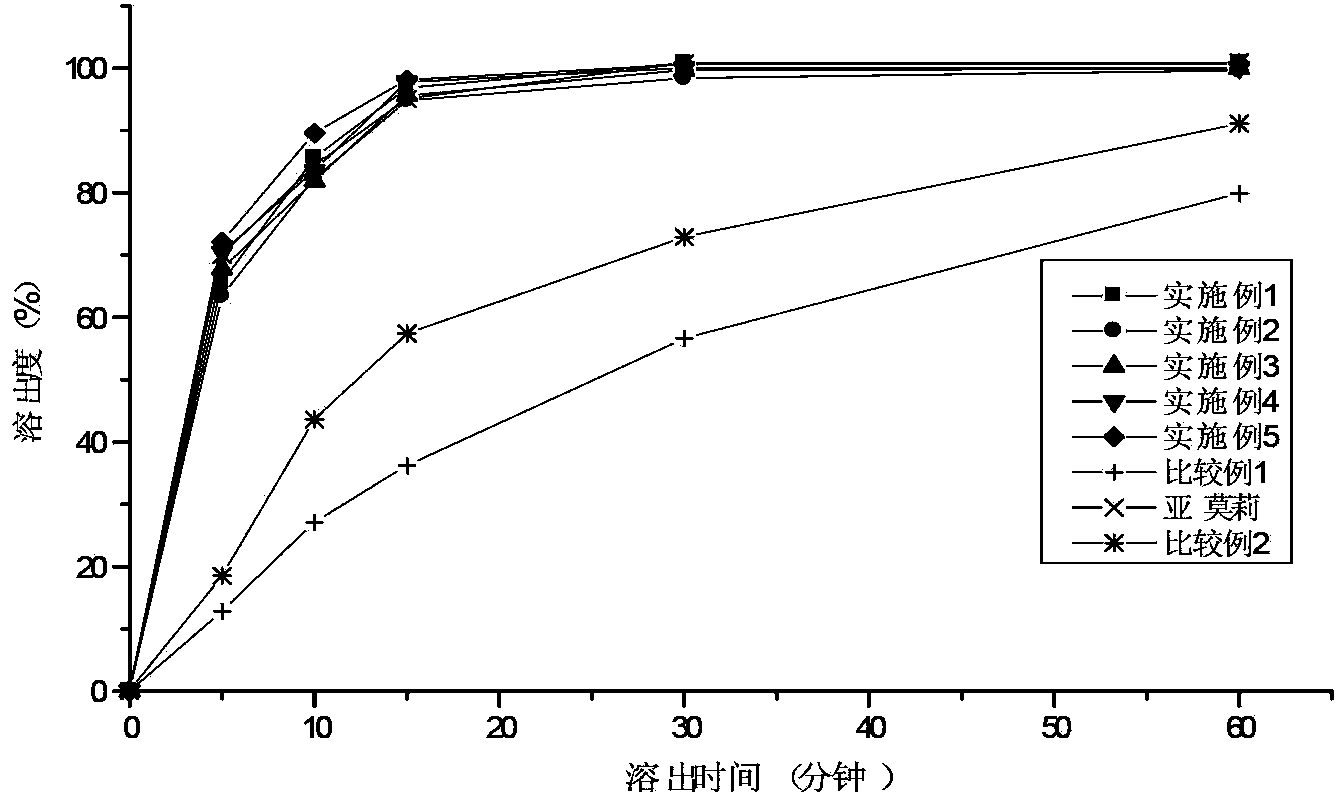
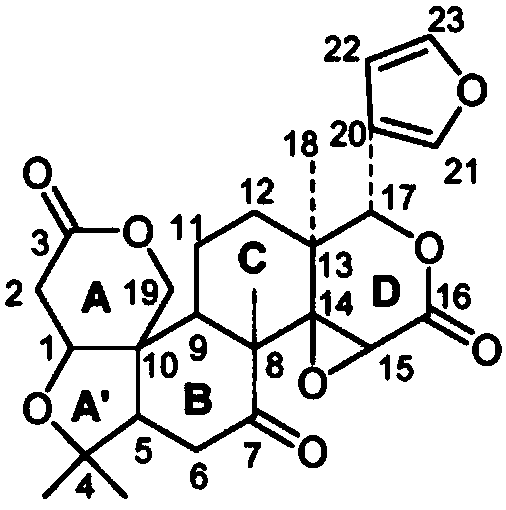
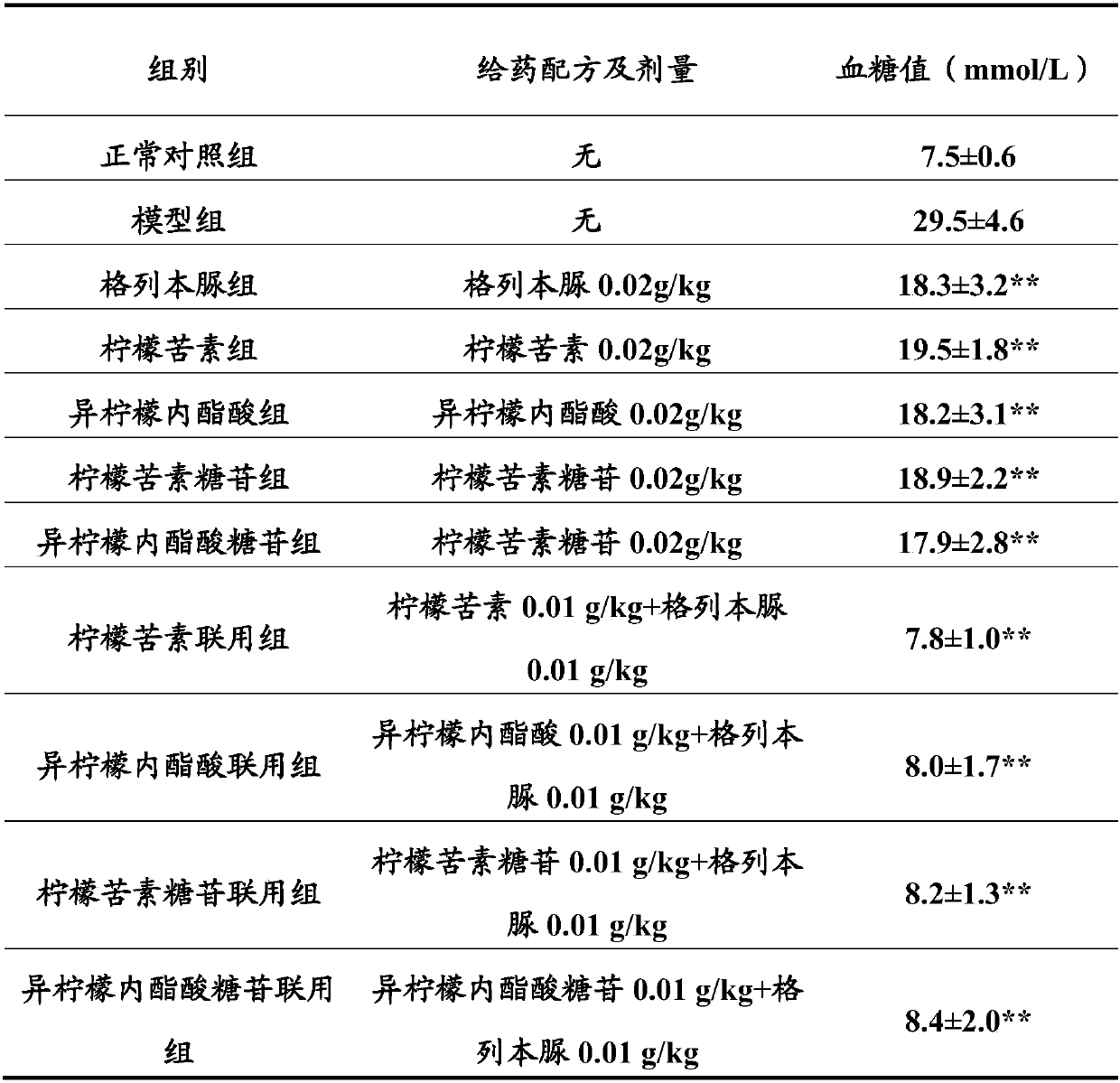
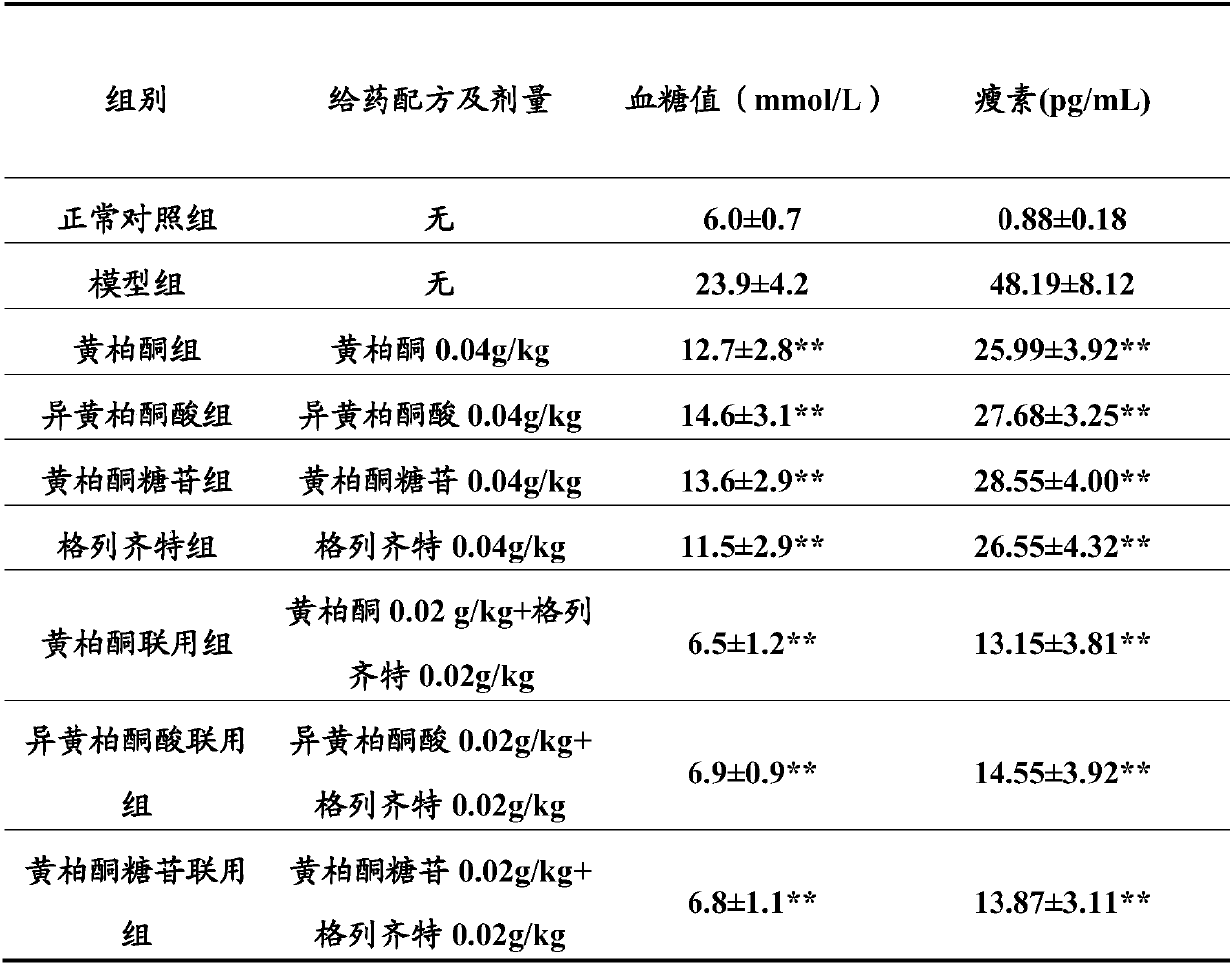
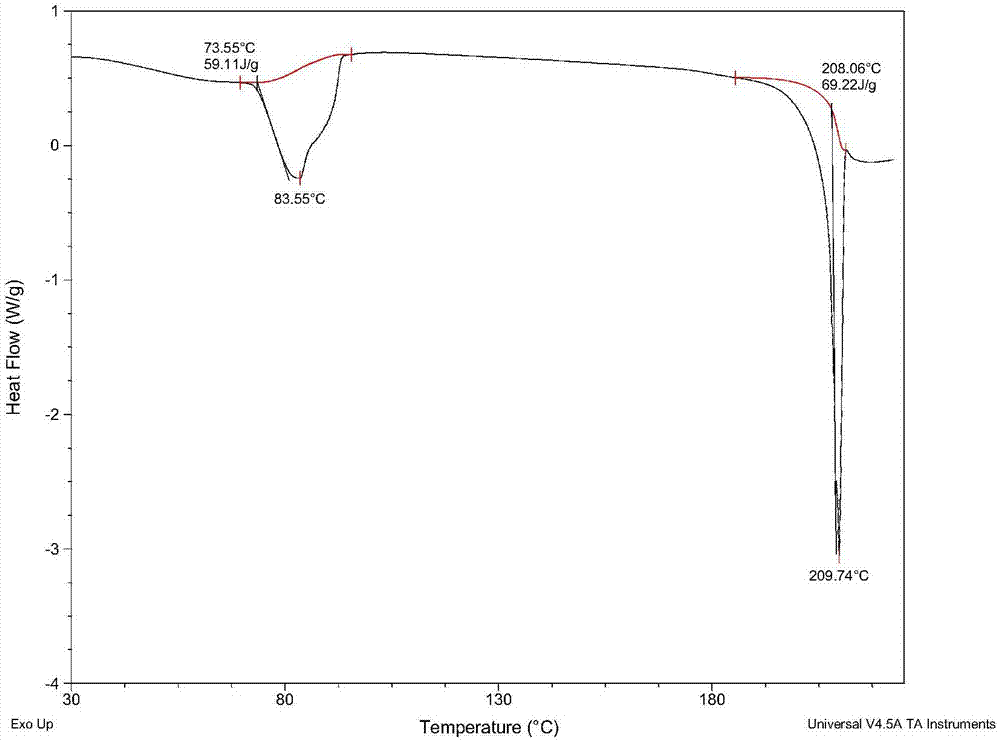
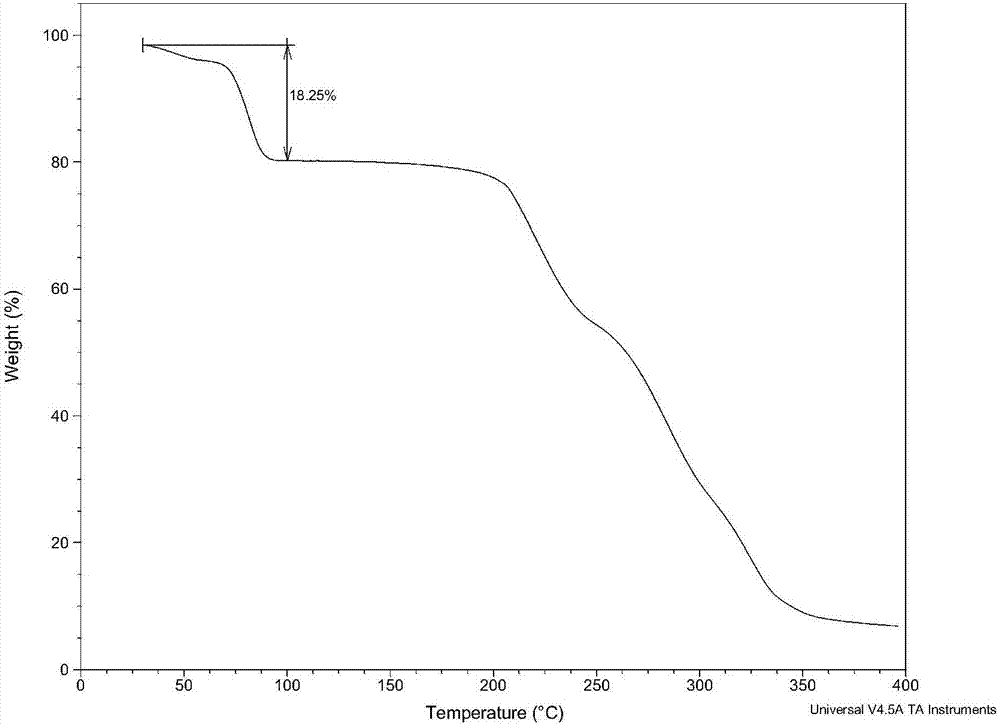
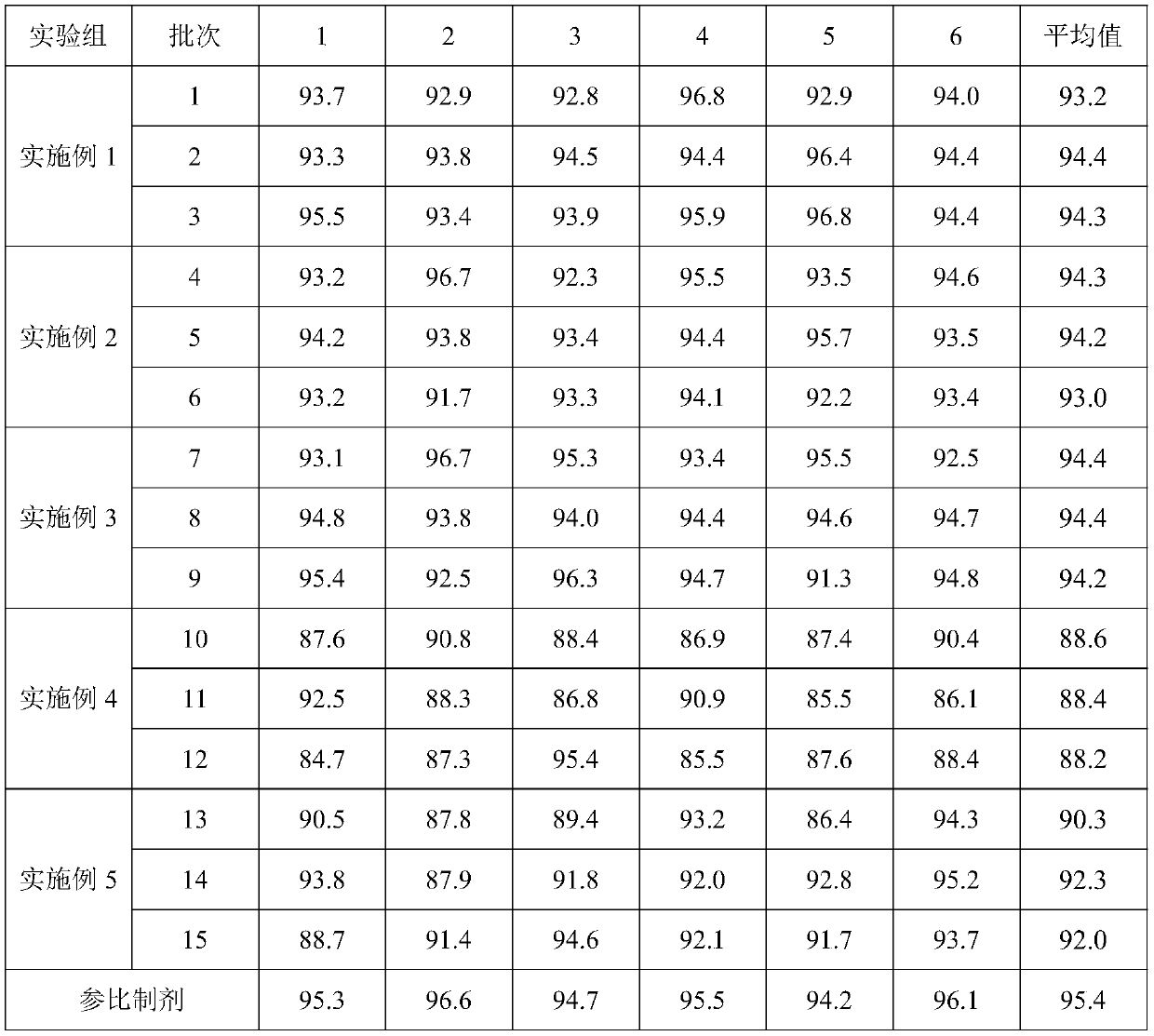
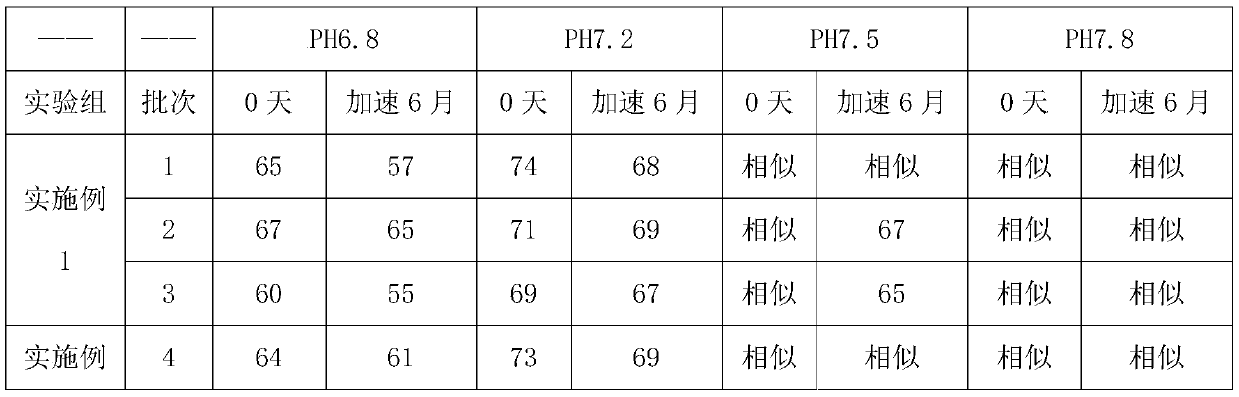
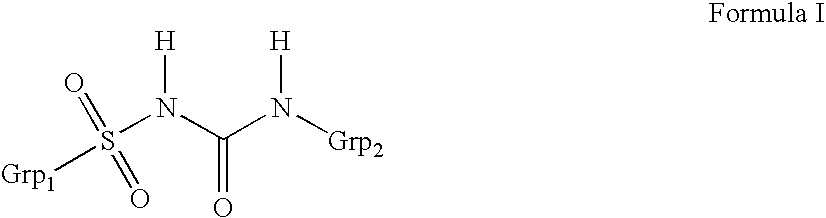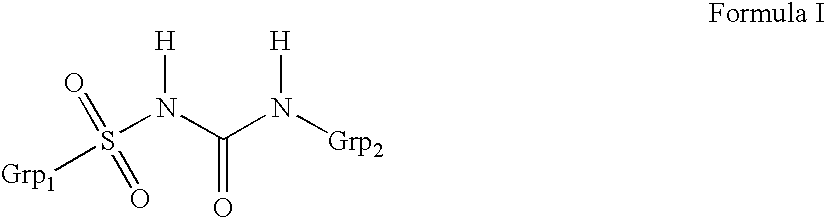Patents
Literature
82 results about "Glimepiride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
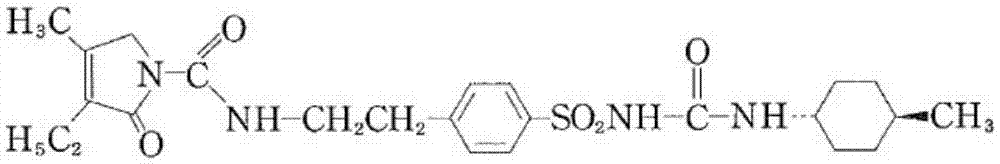
Glimepiride is used with a proper diet and exercise program to control high blood sugar in people with type 2 diabetes. It may also be used with other diabetes medications.
Stable pharmaceutical composition of immediate-release glimepiride and extended-release metformin
InactiveUS20070264331A1Avoid mixingBiocideMetabolism disorderImmediate releaseMetformin Hydrochloride
This invention is directed to a pharmaceutical composition in the form of a tablet with improved stability, as well as the process for obtaining said composition. The tablet of the present invention comprises two active ingredients comprising two oral hypoglycemic agents: one phase with a sulphonylurea, such as immediate release Glimepiride, and a second phase with a biguanide, such as extended-release Metformin hydrochloride (Metformin HCl). The biphasic tablet, which can include over 500 mg of Metformin HCl (i.e. up to 1,000 or 1,500 mg, depending on the daily requirements of each patient), is to be orally administered once or twice a day. The combination of these hypoglycemic agents has a synergic effect and therefore a greater effectiveness in controlling the blood glucose level in patients with diabetes mellitus, type 2.
Owner:LAB SILANES
Glimepiride dispersible tablet and preparation method thereof
InactiveCN102379855AImprove stabilityMetabolism disorderSulfonylurea active ingredientsSide effectCurative effect
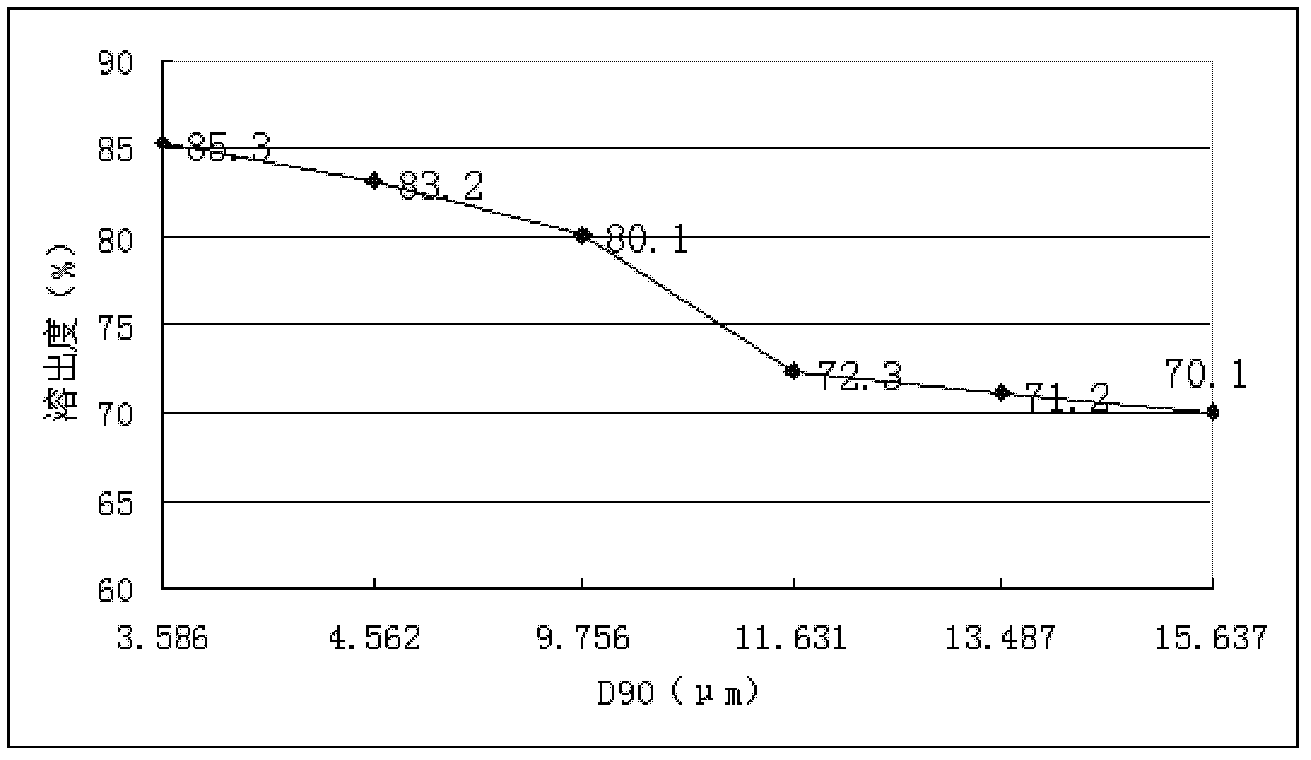
The invention discloses a glimepiride dispersible tablet and a preparation method thereof. The dispersible table is prepared by micronizing the medicament glimepiride to control the grain diameter to be lower than 10 mu m, and adding auxiliary materials. According to the prepared dispersible tablet, the stability and absorption rate of the medicament are remarkably improved, and the bioavailability and curative effect are increased, so that the dose is reduced, toxic and side effects caused by the medicament are lightened, and the problems of low dissolution rate and the like of the conventional glimepiride tablets are solved. The method is suitable for the glimepiride dispersible tablet, which is mainly used for treating II-type diabetes mellitus which cannot be controlled in diet control and exercise.
Owner:石药集团中诺药业(石家庄)有限公司
Glimepiride nano-particle capsule and preparation method thereof
InactiveCN102600106AOvercome mutual gravityImprove stabilityMetabolism disorderSulfonylurea active ingredientsSolubilityFreeze-drying
The invention relates to a glimepiride nano-particle capsule and a preparation method thereof. The problems of poor water-solubility, short half-life period, instability, low bioavailability and poor targeted therapeutic effect of the glimepiride can be effectively solved. The technical scheme for solving the problems is that the glimepiride nano-particle capsule comprises the following components in parts by weight of 1 part of glimepiride, 1-30 parts of surfactant and 3-60 parts of freeze-drying protective agent. The preparation method comprises the steps of: dissolving the glimepiride or a mixture of the glimepiride and the surfactant into an organic solvent to prepare an initial suspension, and carrying out a high-pressure homogenization method or emulsification dispersion method to obtain a suspension with an average particle size of 300+ / -60nm; and freeze-drying and screening the nano suspension, and then filling into a capsule. Through adding the proper surfactant, the mutual attraction among anno particles is overcome, a solid preparation has excellent long-term stability, long-term storage and transportation are facilitated, half-life period of medicines is prolonged, absorption degree is increased, bioavailability is improved, and a targeted therapeutic effect is good.
Owner:ZHENGZHOU UNIV
Compound tablet of pioglitazone hydrochloride, glimepiride and preparation method thereof
InactiveCN101804056ASolve the problem of exceeding the standardReduce moisture contentOrganic active ingredientsMetabolism disorderGlimepirideDissolution
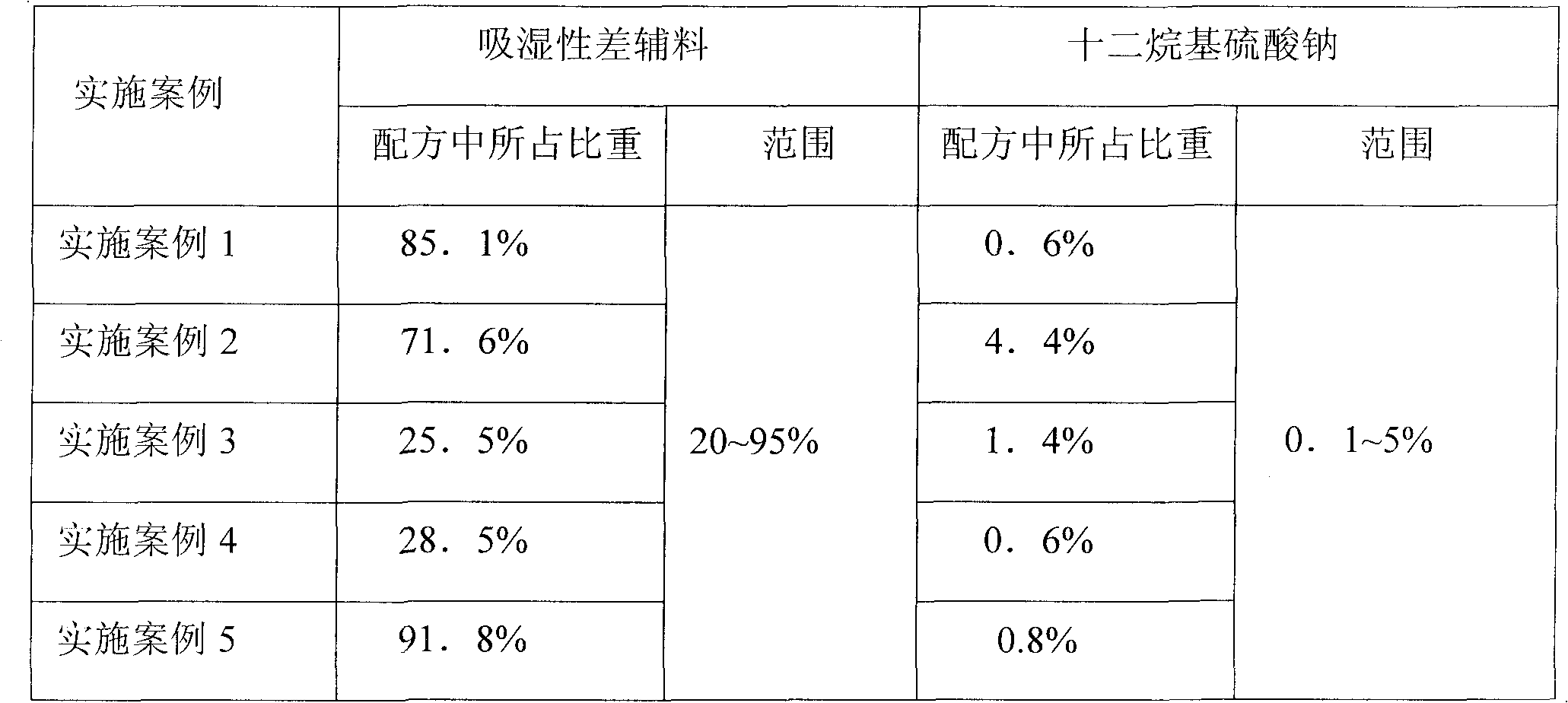
The invention relates to a compound tablet of pioglitazone hydrochloride, glimepiride and a preparation method thereof. The compound tablet contains sodium dodecyl sulfate and auxiliary with poor hygroscopicity, wherein the weight ratio of glimepiride to pioglitazone hydrochloride is 1-4:33; the content of sodium dodecyl sulfate by weight percent is 0.1-5%, and the content of the auxiliary with poor hygroscopicity by weight percent is 20-95%. The preparation method comprises the steps: the raw material of glimepiride, pioglitazone hydrochloride and the auxiliary with poor hygroscopicit are mixed into smashed mixing powder, the mixing powder, the raw material of pioglitazone hydrochloride, filler, disintegrant and lubricator are evenly mixed, and the mixture is subject to tabletting. The compound tablet solves the problems of imperfect dissolution of glimepiride, inferior stability and large industrialization difficulty of glimepiride. The preparation method according to the invention has the advantages of simple process, easy operation and better suitability for industrial production.
Owner:SHANDONG XINHUA PHARMA CO LTD
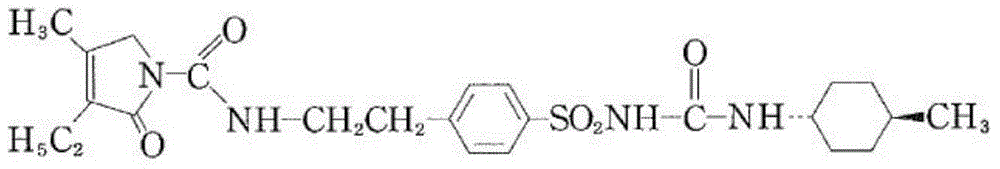
Triphosgene method for synthesizing benzene sulphanilamide, intermediate of glimepiride, drug for Type ii Diabetes Mellitus
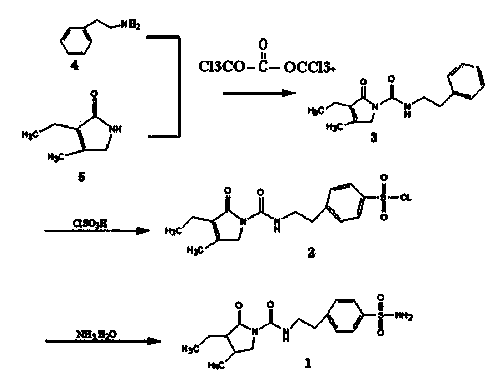
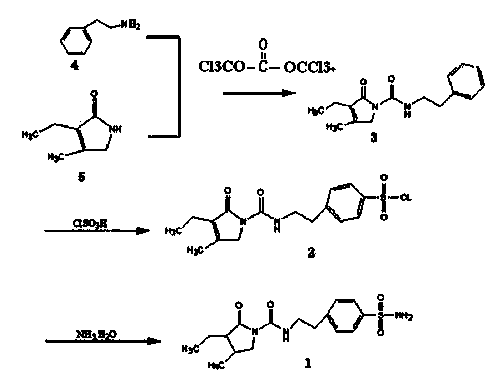
The invention discloses a triphosgene method for synthesizing benzene sulphanilamide, the intermediate of glimepiride, a drug for Type ii Diabetes Mellitus. Triphosgene is slowly dropped into a solution which contains phenethylamine and 3-ethyl-4-methyl pyrroline ketone, at a controlled temperature, so that N-[2-(3-ethyl-4-methyl-2-oxidation-3-pyrroline-1-formylamino)ethyl]-benzene (3) is obtained; the product reacts with chlorosulfonic acid to generate sulfonated product; the sulfonated product reacts with ammonia water to generate crude product benzenesulfonamide; the crude product is refined through solvent treatment to generate fine product; the reaction formula is show in the specification. The triphosgene method is simple in technology, simple in operation and high in yield; compared with the conventional method of phosgene, the solid phosgene (triphosgene) method is safer and is convenient to operate.
Owner:姜树林
Preparation of glimepiride raw material
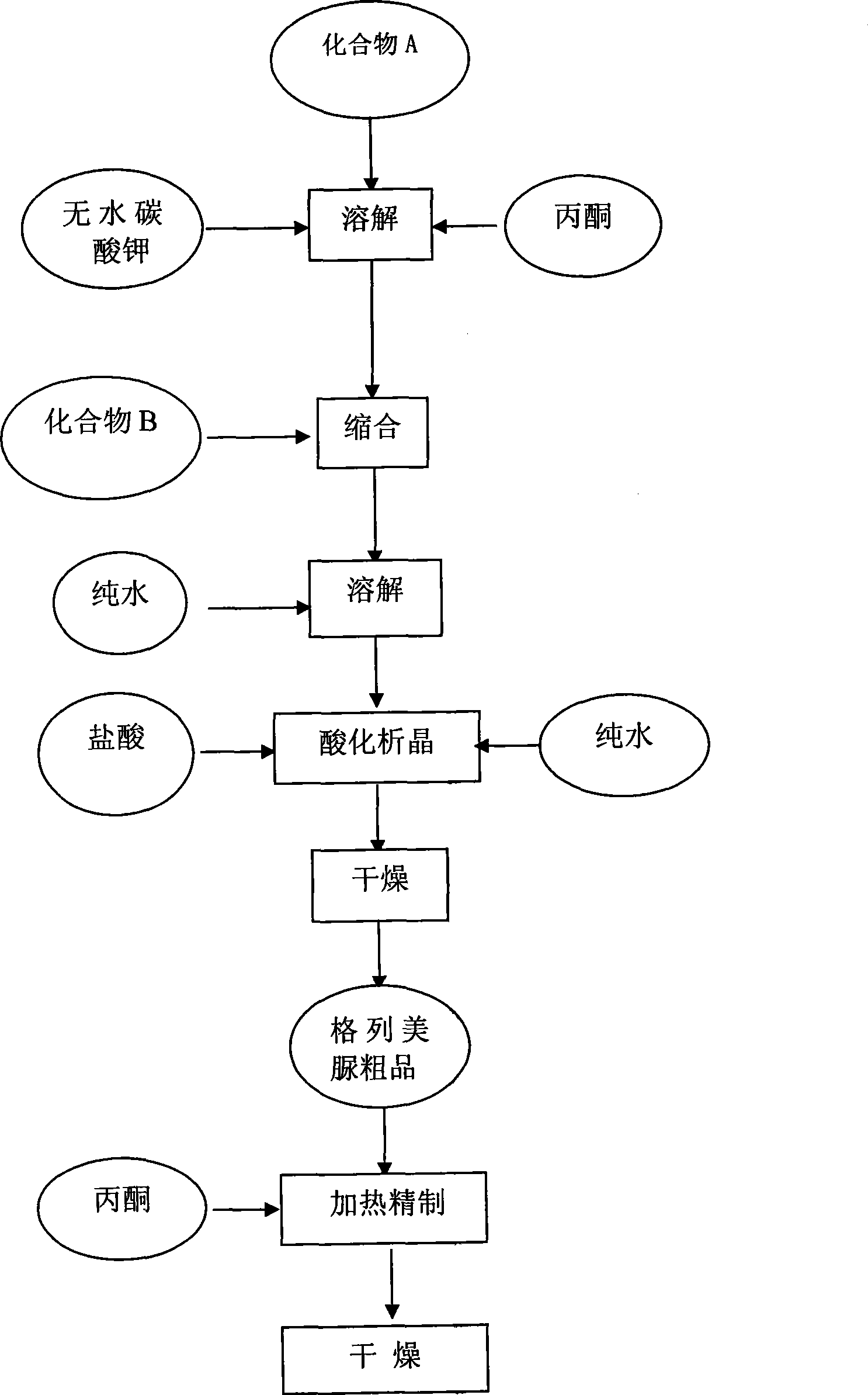
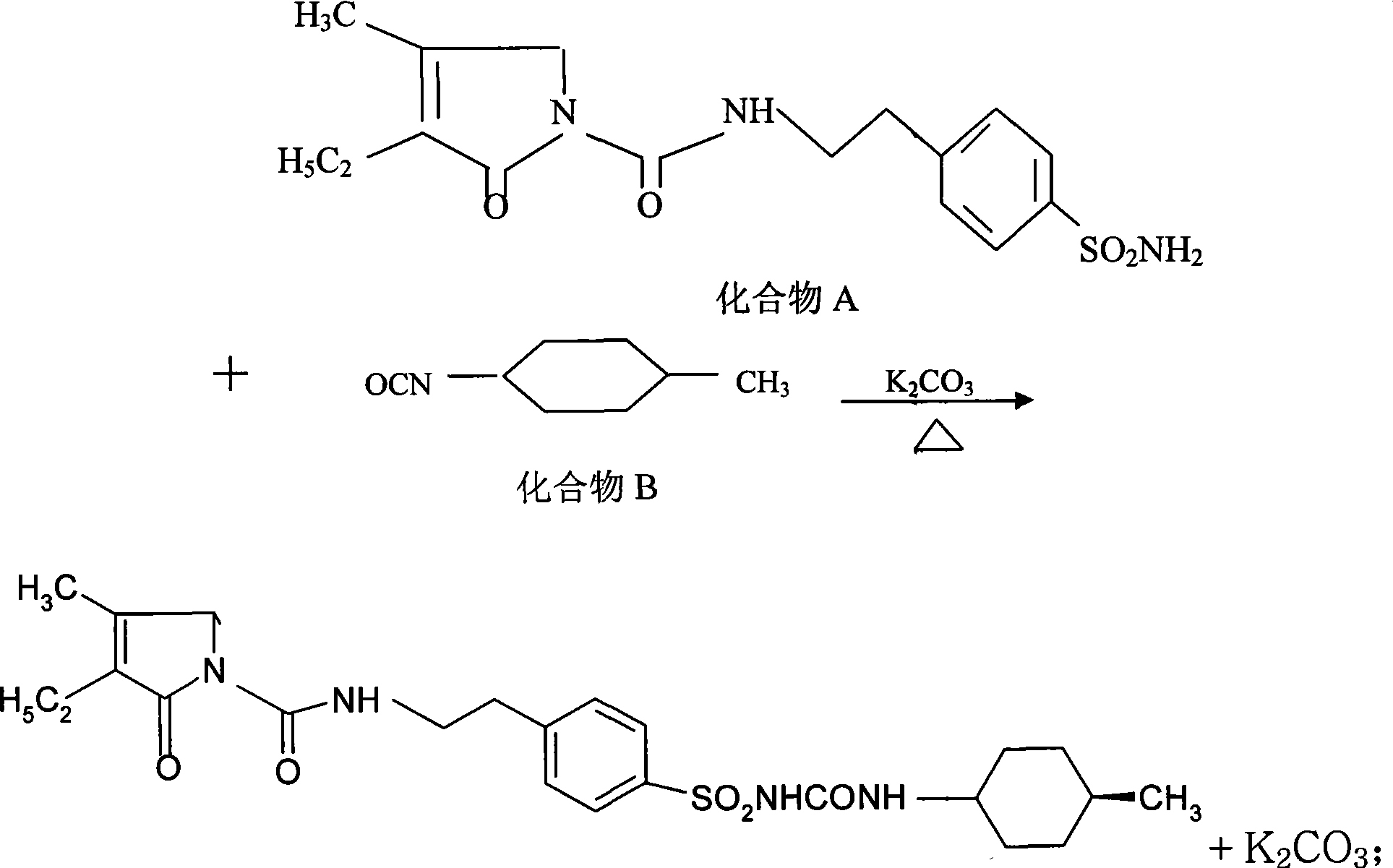
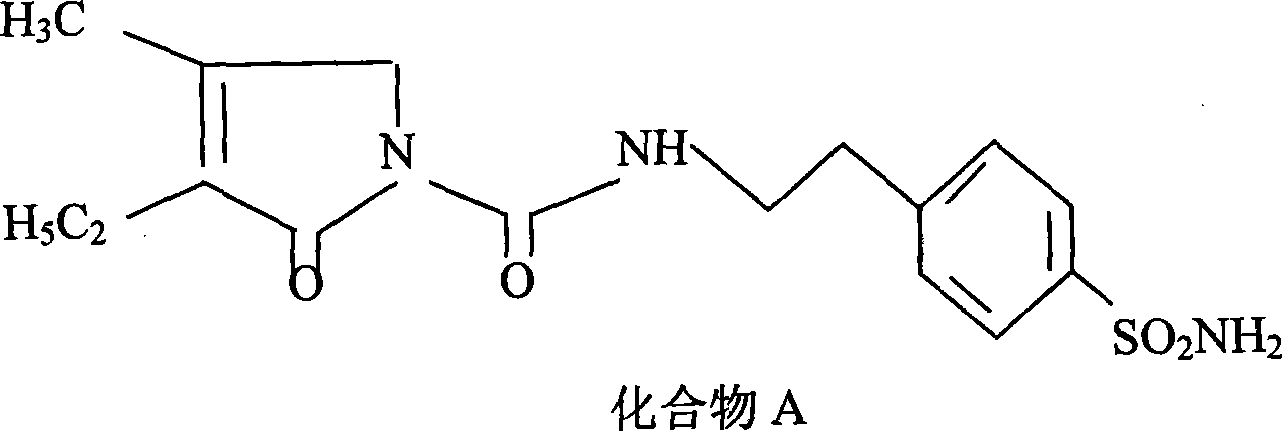
ActiveCN101486674AReduce usageResidue reductionOrganic chemistryMetabolism disorderCyclohexylisocyanateMass ratio
The invention discloses a preparation method of Glimepiride raw material, which comprises the following steps in sequence: in the presence of K2CO3, a condensation reaction is carried out between a compound A: 4-[2-(3-ethyl-4-methyl-2-keto-3-pyrroline-1-formamido)-ethyl]-benzene sulfonamide and a compound B: trans-4-methyl-cyclohexyl isocyanate of the same molar weight in an organic solvent. The mass ratio of the compound A and K2CO3 is 1:0.8-0.9; the mixture synthetic fluid of a crude Glimepiride product and K2CO3 is filtered in a suction way, and then an extract is added into pure water and dissolved to form a solution. The solution is filtered for removing impurities to obtain the aqueous solution of Glimepiride; a hydrochloric acid solution is added into the aqueous solution of the Glimepiride for acidification and then a crude product is separated; acetone is added into the crude Glimepiride product and then refined, filtered in a suction manner, dried in a vacuum environment to obtain a pure Glimepiride product. The method has the advantages of simplifying the synthetic route, selecting mature intermediates as raw materials, adding K2CO3 for promoting the condensation reaction, avoiding the use of chloroform, reducing the residual organic solvent, and being applicable to industrialized production.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Preparation method of pharmaceutical composition for treating type II diabetes
ActiveCN101984974AStable drug releaseSmall fluctuations in blood concentrationMetabolism disorderSulfonylurea active ingredientsSide effectPatient compliance
The invention relates to a preparation method of a pharmaceutical composition for treating type II diabetes, belonging to the medical preparations containing organic ingredients, particularly relating to a preparation method of a pharmaceutical composition of metformin hydrochloride and glimepiride. In the preparation method, the metformin hydrochloride and the glimepiride are taken as active ingredients of the pharmaceutical composition, the metformin hydrochloride is firstly prepared into pill cores by adopting an osmotic pump technology and then the metformin hydrochloride pill cores are coated with the glimepiride by using a coating technology. The preparation method comprises the following steps: 1), preparing the metformin hydrochloride and pharmaceutically acceptable auxiliary materials into pills which are coated with semipermeable membrane layers, punching by laser and producing the pill cores; and 2), preparing the glimepiride and the pharmaceutically acceptable auxiliary materials into a coating liquid dissolved in stomach and carrying out coating on the pill cores in step 1. The invention provides the preparation method of the pharmaceutical composition for treating the type II diabetes, wherein the pharmaceutical composition steady and slowly releases drugs, has reduced administration times, good patients compliance, small side effect and small tablet volume, is less influenced by pH values of different segments of a gastrointestinal tract, and is convenient for administration for the patients.
Owner:SHANDONG XINHUA PHARMA CO LTD
Solid self-microemulsion based on spherical crystallization technique and preparation method thereof
InactiveCN103315960AReduce liver and kidney toxicityAvoid influencePowder deliveryEmulsion deliveryNeogambogic acidCaprylic acid
The invention relates to the medical technology field, and particularly relates to a solid self-microemulsion based on a spherical crystallization technique and a preparation method thereof. The solid self-microemulsion is characterized in that: with the use of the spherical crystallization technique, the solid self-microemulsifying micoparticles are prepared from poorly water soluble drugs in a liquid phase by one step. The solid self-microemulsion with the poorly water soluble drugs comprises the components, by weight: 0.1 to 1.5 g of the poorly water soluble drugs, 4.0 g of a polyoxyethylene hydrogenated castor oil, 2.0 g of capric caprylic triglyceride, 2.0 g of tpropylene glycol, 1.0 ml of ethanol, 4.0 ml of dichloromethane, 0.5 to 1.1 g of ethylcellulose (or Eudragit RS100, RL100), 0.05 g of PEG4000, and 0.5 g of colloidal silicon dioxide. The poorly water soluble drugs include cyclosporine A, fenofibrate, glimepiride, cilnidipine, isradipine, simvastatin, baicalein, neogambogic acid, puerarin, cyclovirobuxine D, silymarin and the like.
Owner:胡容峰
Preparation method of glimepiride tablets
InactiveCN105769787ALarge particle sizeIncrease dosageMetabolism disorderSulfonylurea active ingredientsGlimepirideDrug powder
The invention discloses a preparation method of glimepiride tablets.The preparation method comprises the steps that a glimepiride raw material drug is smashed, an auxiliary material is added, a prescription process of the glimepiride tablets is optimized, the glimepiride raw material drug is micronized, the particle size of the glimepiride raw material drug powder is controlled to be smaller than 10 microns, the weight percentage of microcrystalline cellulose is controlled to be below 1%, meanwhile the weight percentage of the water-soluble auxiliary material lactose is kept above 60%, the usage amount of the water-soluble auxiliary material lactose is increased while a soft material is prepared by adopting a hydrophilic adhesive povidone aqueous solution or a povidone ethanol aqueous solution, the proportion of the microcrystalline cellulose is minimized, and meanwhile wet granulation is performed by adopting a povidone containing solution.By the adoption of the preparation method, the dissolution rate of the drug in a dissolution medium is improved, the particle size smaller than 10 microns of the raw materials can meet the requirements, energy consumption is reduced, the dissolution rate of a main drug is accelerated, the time is saved, and the drug dissolution and bioavailability are improved.
Owner:CHONGQING CONQUER PHARML
Qualitative analysis detection method for low polarity sugar-reducing chemical medicament in traditional Chinese medicine
InactiveCN101285803AImprove identification sensitivityHigh sensitivityComponent separationTesting medicinal preparationsRetention timeUltraviolet
The invention discloses a qualitative analysis detection method for illegally mixed high-polar chemical anti-diabetic components in anti-diabetic traditional Chinese medicine products. The method comprises the following steps that: 1) high efficient liquid phase chromatography conditions: an ammonium acetate-triethylamine-acetonitrile moving phase system and a C18 chromatographic column with certain specification are used, the wavelength is detected by ultraviolet, and the flow rate is 1.0ml / min; 2) analysis result: glibenclamide, glipizide, gliclazide, glimepiride, gliquidone, repaglinide, nateglinide, rosiglitazone and pioglitazone hydrochloride can realize the complete separation; 3) result judgment: when retention time of a chromatographic peak in an anti-diabetic traditional Chinese medicine product is consistent with that of anti-diabetic medicine in the step 2) and the apparent absorption is shown out, which indicate that the anti-diabetic medicine is contained in the sample to be tested. The method has the advantages of quickness, simplicity, convenience, high sensitivity, strong specialization, broad coverage and so on.
Owner:北京市东城区药品检验所
Controlled-release preparation containing metformin hydrochloride and glimepiride and preparation method of controlled-release preparation
ActiveCN105878256AImprove release uniformityImproved controlled releaseMetabolism disorderSulfonylurea active ingredientsMedicineMetformin Hydrochloride
The invention relates to the field of pharmaceutical preparations, and in particular discloses a compound controlled-release preparation of metformin hydrochloride and glimepiride and a preparation method of the controlled-release preparation. The controlled-release preparation disclosed by the invention consists of an internal controlled-release tablet which contains the metformin hydrochloride and an external drug-loaded coating which contains the glimepiride, wherein the internal controlled-release tablet which contains the metformin hydrochloride comprises a tablet core, an insoluble semipermeable coating film and drug-release small pores. The controlled-release preparation disclosed by the invention can significantly improve the linearity and the uniformity of drug release, the process (the preparation method) is simple and easy to implement, and the tablet is better in compressibility; and the glimepiride, without micronizing treatment or other special treatment, is excellent in dissolving effect.
Owner:HEFEI LIFEON PHARMA
Glimepiride dropping pill
ActiveCN104173308APromote dissolutionLess irritatingMetabolism disorderSulfonylurea active ingredientsSide effectCalcium alginate
The invention provides a glimepiride dropping pill which contains copovidone, calcium alginate ammonium and polyoxyethylene (40) stearate. The glimepiride dropping pill provided by the invention has the advantages of high dissolution rate, good stability, simple preparation process and small toxic and side effect.
Owner:SHANDONG NEWTIME PHARMA
Glymiurea dispersion tablet and its preparation method
InactiveCN1555786APromote dissolution and absorptionGood dispersionMetabolism disorderPill deliveryAdhesiveGlimepiride
A dispersing glimepiride tablet for treating type-B diabetes is prepared from glimepiride, disintegrant, diluent, lubricant, flowing aid and adhesive through proportionally mixing, granulating, baking and tabletting.
Owner:肖广常 +1
Solid compound preparation containing metformin hydrochloride and glimepiride, preparation method and application thereof
ActiveCN103505466AHigh dissolution rateImprove stabilityMetabolism disorderSulfonylurea active ingredientsWater insolubleFiller Excipient
The invention provides a solid compound preparation containing metformin hydrochloride and glimepiride. The solid compound preparation is prepared from metformin hydrochloride granules and glimepiride granules. The metformin hydrochloride granules contain metformin hydrochloride and an adhesive, and the glimepiride granules contain glimepiride, a water soluble filler, a disintegrating agent and an adhesive. The solid compound preparation does not contain water insoluble filler. The invention also provides a preparation method and application of the solid compound preparation. Compared with common preparations and preparations adopting a water insoluble filler, the solid compound preparation provided by the invention can significantly improve the dissolution rate of glimepiride, and can effectively reduce interaction of the two main drugs, thereby reducing the increase of related impurities in a placement process. The product has stable quality and good uniformity, so that the effectiveness and safety of drug use by patients can be improved.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Process for synthesizing glimepiride raw material medicine
InactiveCN108383768AReduce lossesReduce generationOrganic active ingredientsOrganic chemistryKetoneGlimepiride
The invention discloses a process for synthesizing a glimepiride raw material medicine. A compound A, namely 3-ethyl-4-methyl-3-pyrroline-2-ketone and a compound B, namely 2-phenethyl isocyanate are taken as start raw materials. The process is characterized in that when an intermediate 1 is synthesized, filtrate is applied mechanically, so that the loss of the intermediate 1 can be reduced, the yield can be increased, and the production efficiency can be improved; when an intermediate 2 is synthesized, hydrochloric ether is adopted as a solvent, so that isomer impurities can be greatly reduced, the content of the isomer impurities can be reduced to 0.5% or less from 8%, and later purification procedures can be simple to operate; when a glimepiride metallic salt is synthesized, acetonitrileis adopted as a solvent, sufficient reactions can be achieved, the reaction time can be greatly shortened, the residue of an intermediate 3 is reduced to 0.2% or less from 5-10%, in addition, a highsolvent recycling rate can be achieved. The process disclosed by the invention is simple and safe, low in production cost, high in yield, stable in intermediate and finished product quality and applicable to industrial large-scale production and hypoglycemic drug, namely glimepiride, synthesis processes with relatively great social, economic and environmental-friendly benefits.
Owner:江西博雅欣和制药有限公司
Sustained-release preparation containing metformin hydrochloride and glimepiride and preparation process thereof
The invention relates to a method for preparing slow-release agent which contains diguanil and gelimourea, wherein said slow-release agent is formed by slow-releaser and quick-releaser; the diguanil is slow-release part which will release 15-40% in first hour, and release 50-70% in fourth hour, and more than 75% at eighth hour; the gelimourea is quick-release component which will release more than 75% after 30min. The invention can avoid side effect and reduce feeding time. And gelimourea can quickly release to improve treatment and reduce blood sugar. The invention also provides relative external release property.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Liquid formulations of compounds active at sulfonylurea receptors
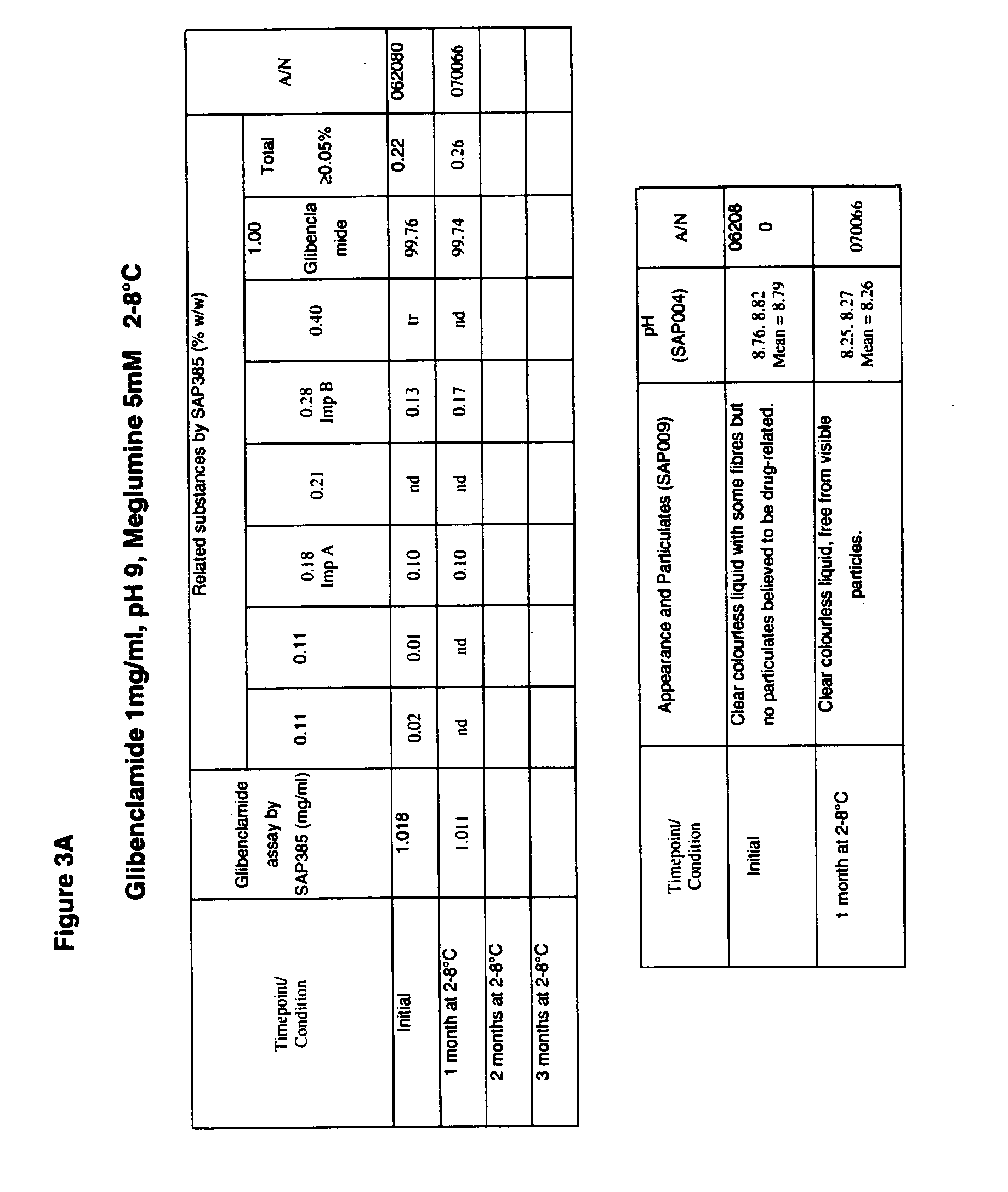
ActiveUS20170216321A1Rapid and readily controlled increase in circulating drug concentrationsQuick cureBiocideMetabolism disorderMeglitinideWater based
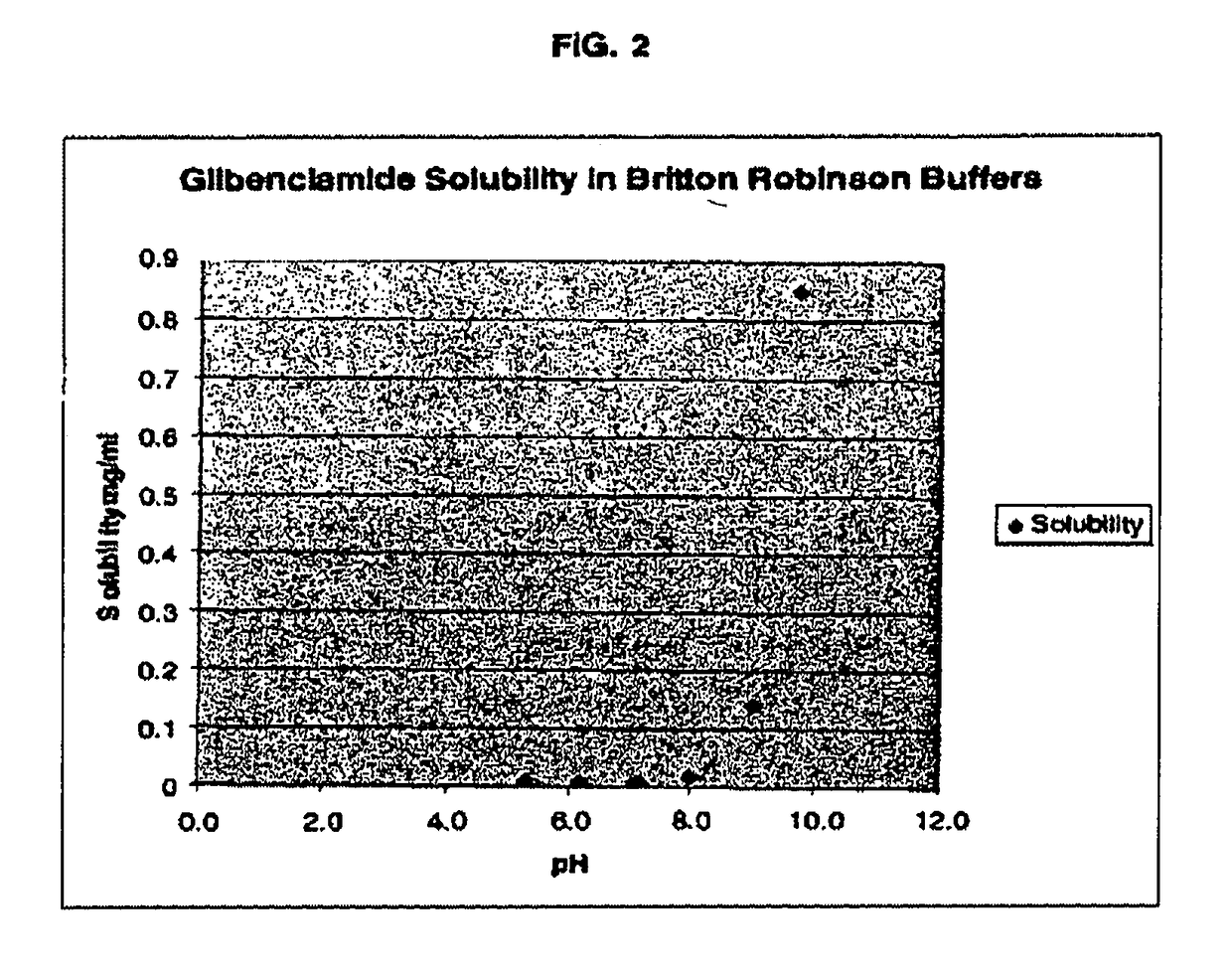
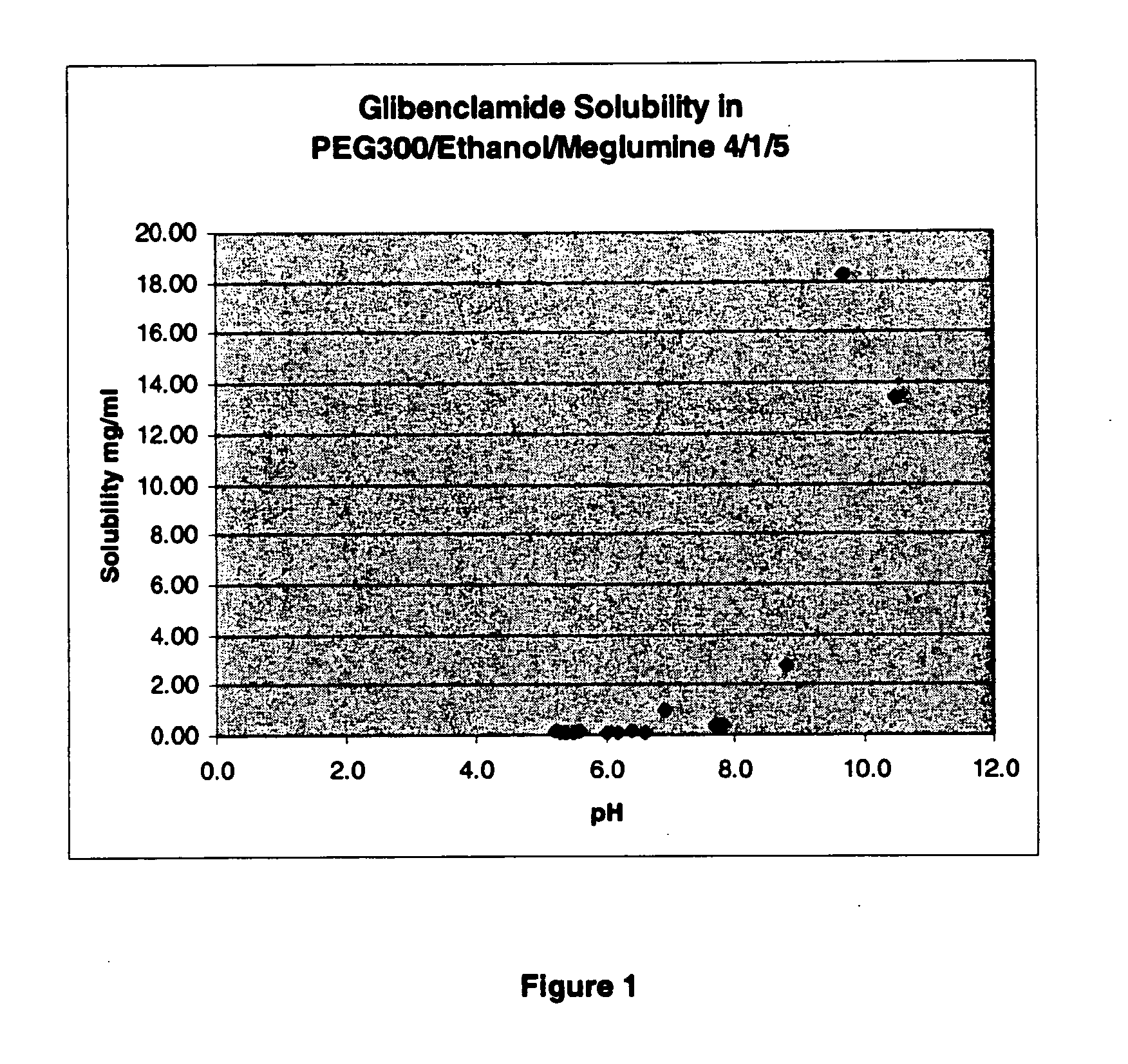
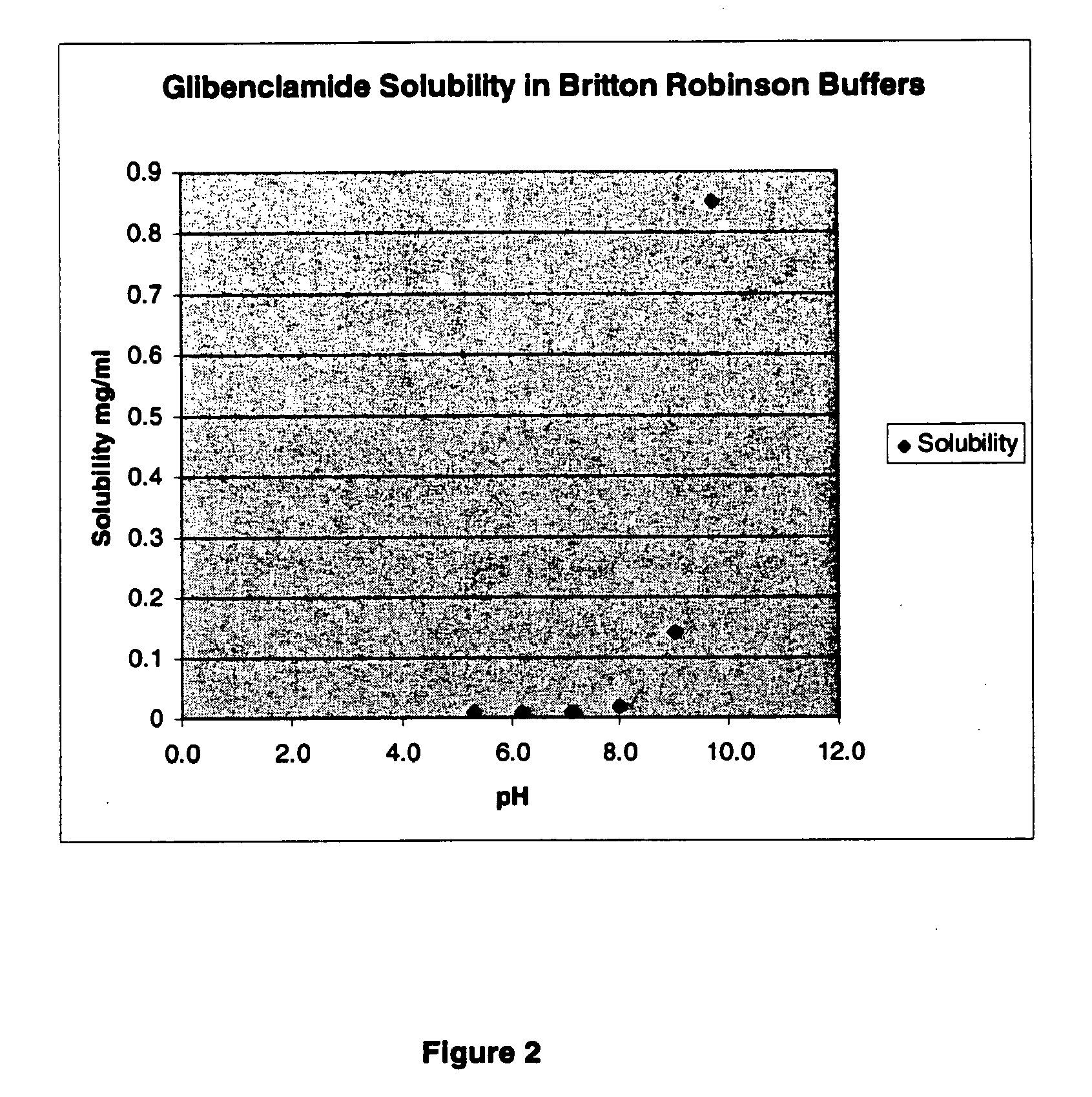
The invention provides liquid formulations of compounds that act at sulfonylurea receptors that are suitable for intravenous and intra-arterial infusion. Compounds active at a sulfonylurea receptor include glibenclamide, tolbutamide, repaglinide, nateglinide, meglitinide, midaglizole, LY397364, LY389382, glyclazide, and glimepiride. Liquid formulations may be concentrated solutions suitable for storage; may be diluted (e.g., dilution of 1:1 or 1:1.2) suitable for bolus injections, and may be further diluted (e.g., dilution of 1:10 or 1:20 or more) for intravenous and intra-arterial infusion over an extended period of time. For example, a liquid formulation may include at least about 0.05 mg / ml glibenclamide in a water-based solution including 40% polyethylene glycol 300, 10% Ethanol, 50% water, at about pH 9. The solution may include a buffer, and is suitable for storage in refrigerator or at room temperature. This solution may be diluted 1:1, or more (e.g., 1:20) without precipitation of the glibenclamide.
Owner:BIOGEN CHESAPEAKE LLC
Method for synthesizing 4-(2-aminoethyl)benzsulfamide
InactiveCN106336366AOvercome the problems of expensive, difficult operation of reaction conditions, etc.Improve responseOrganic compound preparationCarboxylic acid amides preparationGlimepirideHydrolysis
Owner:姜近仁
Liquid formulations of compounds active at sulfonylurea receptors
InactiveUS20110034560A1Rapid and readily controlled increaseRapid adjustment and ready maintenance of circulating drug concentrationBiocideMetabolism disorderWater basedMeglitinide
The invention provides liquid formulations of compounds that act at sulfonylurea receptors that are suitable for intra-venous and intra-arterial infusion. Compounds active at a sulfonylurea receptor include glibenclamide, tolbutamide, repaglinide, nateglinide, meglitinide, midaglizole, LY397364, LY389382, glyclazide, and glimepiride. Liquid formulations may be concentrated solutions suitable for storage; may be diluted (e.g., dilution of 1:1 or 1:1.2) suitable for bolus injections, and may be further diluted (e.g., dilution of 1:10 or 1:20 or more) for intravenous and intra-arterial infusion over an extended period of time. For example, a liquid formulation may include at least about 0.05 mg / ml glibenclamide in a water-based solution including 40% polyethylene glycol 300, 10% Ethanol, 50% water, at about pH 9. The solution may include a buffer, and is suitable for storage in refrigerator or at room temperature. This solution may be diluted 1:1, or more (e.g., 1:20) without precipitation of the glibenclamide.
Owner:BIOGEN CHESAPEAKE LLC
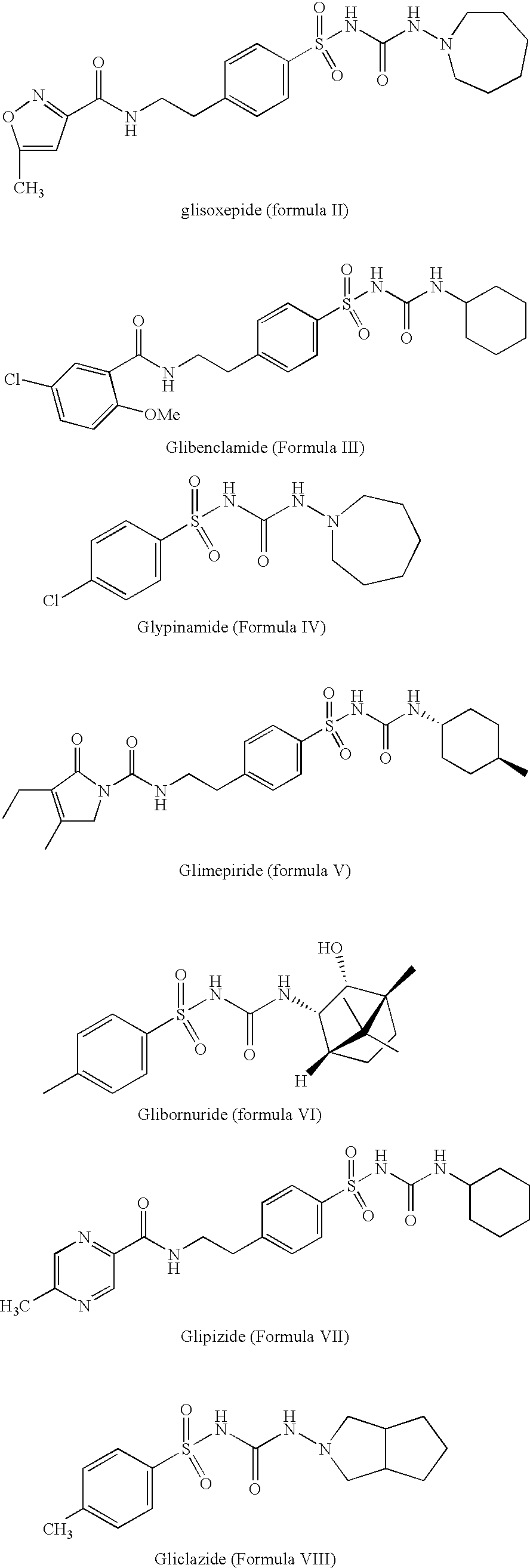
Method for manufacture of compounds related to the class of substituted sulfonyl urea anti-diabetics
InactiveUS20070255056A1Quick responseEfficient purificationOrganic compound preparationSulfonic acid amide preparationGlibornurideDiabrezide
The present invention relates to a process for preparation of sulfonyl urea compounds in high conversion rates and purity. More specifically, this invention relates to a process for manufacture of sulfonyl urea class of anti-diabetic pharmaceutical drugs in higher purity and yield. The process may effectively and economically be used to produce anti-diabetic drugs, such as glimepiride, glipizide, gliclazide, glibenclamide, glibornuride, and glisoxepide.
Owner:IPCA LAB LTD
Preparation method of pharmaceutical composition for treating type II diabetes
ActiveCN101984974BImprove complianceReduce volumeMetabolism disorderSulfonylurea active ingredientsSide effectPatient compliance
The invention relates to a preparation method of a pharmaceutical composition for treating type II diabetes, belonging to the medical preparations containing organic ingredients, particularly relating to a preparation method of a pharmaceutical composition of metformin hydrochloride and glimepiride. In the preparation method, the metformin hydrochloride and the glimepiride are taken as active ingredients of the pharmaceutical composition, the metformin hydrochloride is firstly prepared into pill cores by adopting an osmotic pump technology and then the metformin hydrochloride pill cores are coated with the glimepiride by using a coating technology. The preparation method comprises the following steps: 1), preparing the metformin hydrochloride and pharmaceutically acceptable auxiliary materials into pills which are coated with semipermeable membrane layers, punching by laser and producing the pill cores; and 2), preparing the glimepiride and the pharmaceutically acceptable auxiliary materials into a coating liquid dissolved in stomach and carrying out coating on the pill cores in step 1. The invention provides the preparation method of the pharmaceutical composition for treating the type II diabetes, wherein the pharmaceutical composition steady and slowly releases drugs, has reduced administration times, good patients compliance, small side effect and small tablet volume, is less influenced by pH values of different segments of a gastrointestinal tract, and is convenient for administration for the patients.
Owner:SHANDONG XINHUA PHARMA CO LTD
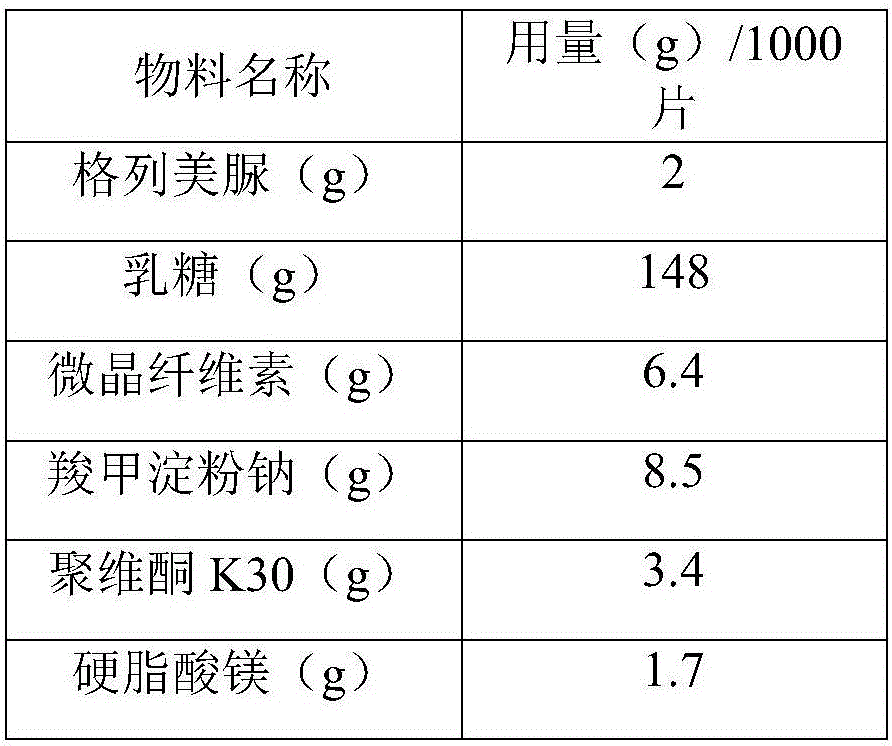
Glimepiride tablet and preparation method thereof
InactiveCN106361712ASmall particle sizeImprove liquidityMetabolism disorderSulfonylurea active ingredientsMedicineCurative effect
The invention relates to a glimepiride tablet and a preparation method thereof, belonging to the technical field of medicine. The glimepiride tablet is prepared from the following components in parts by weight: 1-2 parts of glimepiride, 74-128 parts of lactose, 3.2-6.4 parts of microcrystalline cellulose, 4.25-8.5 parts of sodium starch glycolate, 1.7-3.4 parts of povidone K30 and 0.85-1.7 parts of magnesium stearate. The glimepiride tablet provided by the invention enhances the bioavailability, thereby enhancing the curative effect. The glimepiride tablet has the advantages of higher stability, better leaching effect and higher unit-dosage use efficiency, provides a good option for medicine selection and clinical application in hospitals, and has very high economic and social meanings.
Owner:SHIJIAZHUANG HUAXIN PHARMA
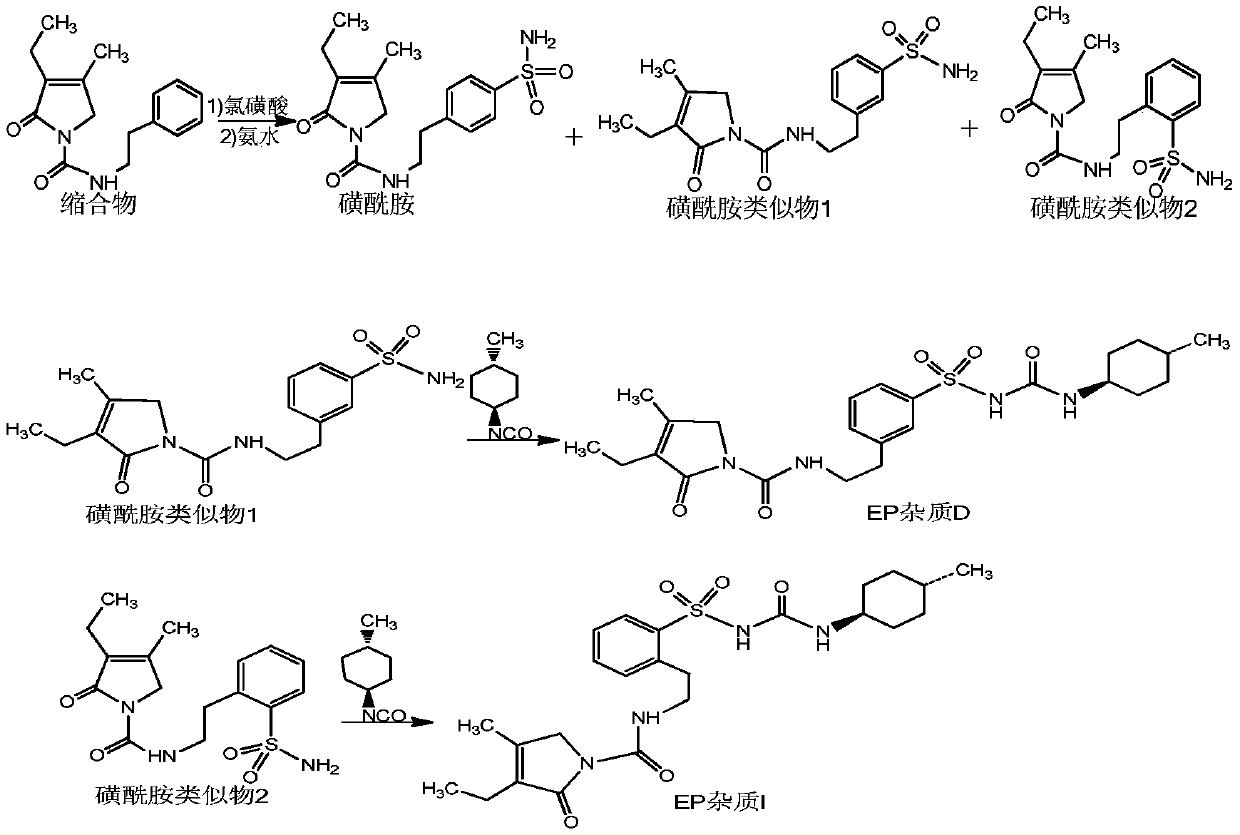
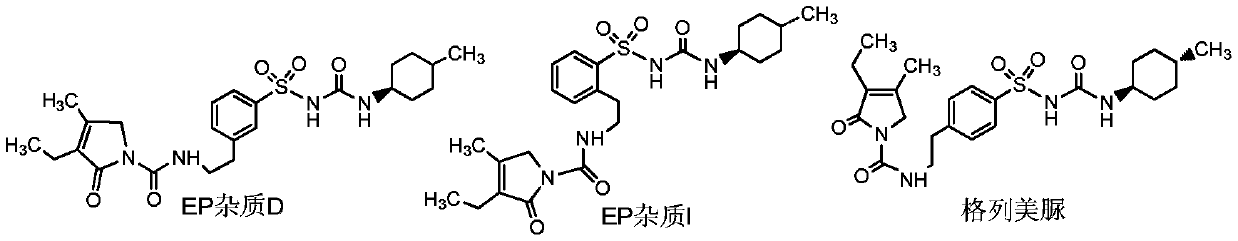
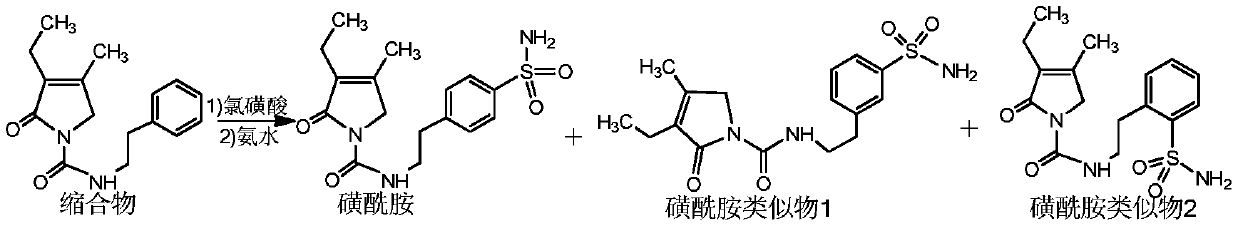
Preparation method of glimepiride EP impurities D and I
The invention provides a preparation method of glimepiride EP impurities D and I, comprising allowing 3-ethyl-4-methyl-2-oxo-3-pyrrolin-N-(2-phenethyl)formamide to reaction with chlorosulfonic acid, allowing reaction with ammonia water, carrying out liquid preparative purification to obtain sulfamide analogues 1 and 2, and subjecting the sulfamide analogues to reaction separately with trans-4-methylcyclohexyl isocyanate to obtain the glimepiride EP impurities D and I. By preparing high-purity controls of glimepiride EP impurities D and I, it is possible to affiliate main impurities in crude glimepiride, control the purity of the crude glimepiride and optimize a production technique of the crude glimepiride; more importantly, the contents of EP impurities D and I in finished glimepiride arecontrolled in advance by controlling the purity of the crude glimepiride instead of by refining at the cost of yield loss; therefore, glimepiride quality is controllable, the yield is high, and the cost is low.
Owner:SHANDONG XINHUA PHARMA CO LTD
Glimepiride tablet composition preparation and preparation method thereof
InactiveCN109481408AGuaranteed external releaseOvercome the risk of increased degradation impuritiesMetabolism disorderSulfonylurea active ingredientsCrospovidonesMedicine
The invention provides a glimepiride tablet composition preparation and a preparation method thereof. The glimepiride tablet is prepared from glimepiride, lactose, microcrystalline cellulose, copovidone, crospovidone and magnesium stearate. According to the glimepiride composition preparation, the dissolving and releasing behaviors of glimepiride tables can be effectively ensured in vitro by sufficiently utilizing the hydrogen bond effect among the crospovidone, povidone and glimepiride, the defects of low yield, large energy consumption, high cost and the like caused by micronizing glimepiride in the prior art can be overcome. The preparation process is simple, has good reproducibility and good stability, is suitable for industrial production, and has relatively high economic and social magnificence.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD +1
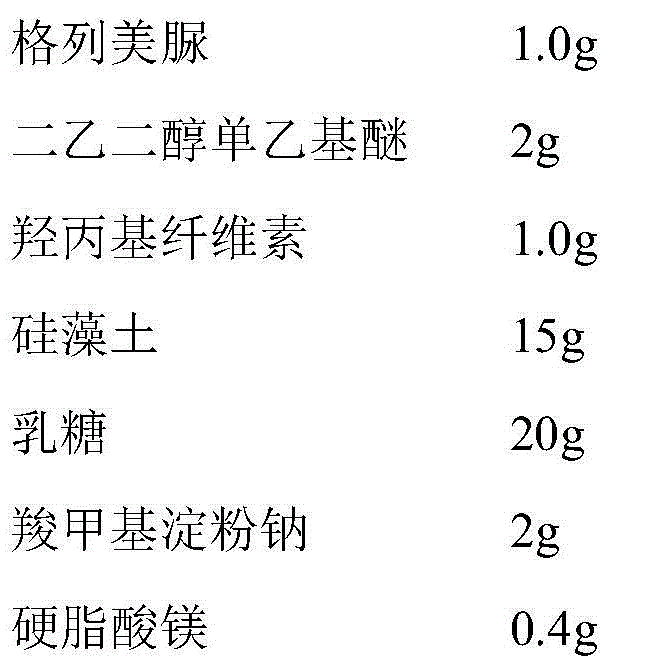
Glimepiride tablet
ActiveCN104546774ADissolution rate is fastSimple processMetabolism disorderSulfonylurea active ingredientsDiethylene glycol monoethyl etherGlimepiride
The invention belongs to the technical field of medicine, and particularly relates to a glimepiride tablet. The glimepiride tablet contains glimepiride, hydroxypropyl cellulose and vapor-phase silicon dioxide. The preparation method comprises the following steps: dissolving the glimepiride and hydroxypropyl cellulose in diethylene glycol monoethyl ether, adding the vapor-phase silicon dioxide for adsorption, uniformly mixing pharmaceutically acceptable auxiliary materials, and directly tabletting. Compared with the prior art, the glimepiride tablet has the advantages of high drug dissolution rate and simple technique, does not need to add any surfactant, and does not need micronization treatment.
Owner:SHANDONG NEWTIME PHARMA
Combination product comprising limonoids and sulfonylurea
The invention relates to a combination product comprising limonoids (pharmacologically acceptable derivatives, esters, steric isomers, salt or prodrugs) and sulfonylurea (such as glibenclamide, gliclazide, glipizide, gliquidone and glimepiride). The invention further relates to an application of the combination product to treatment and / or prevention of diabetes-related diseases and the like.
Owner:NATURAL MEDICINE INST OF ZHEJIANG YANGSHENGTANG
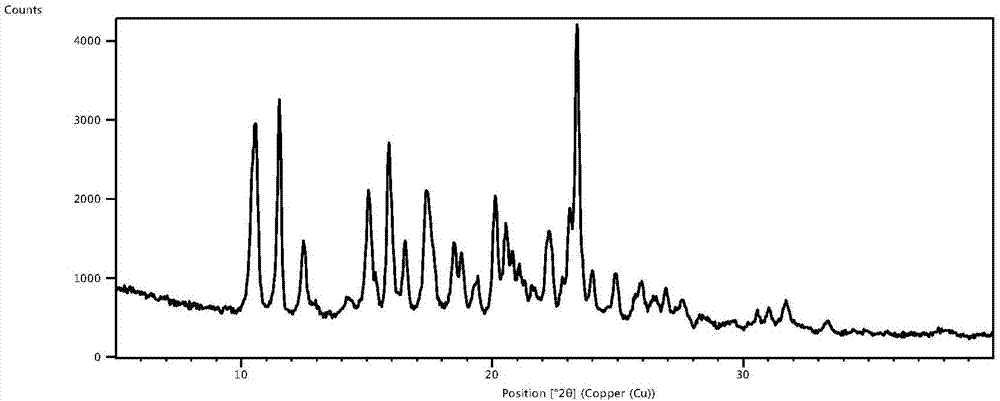
Glimepiride delta crystal form and preparation method thereof
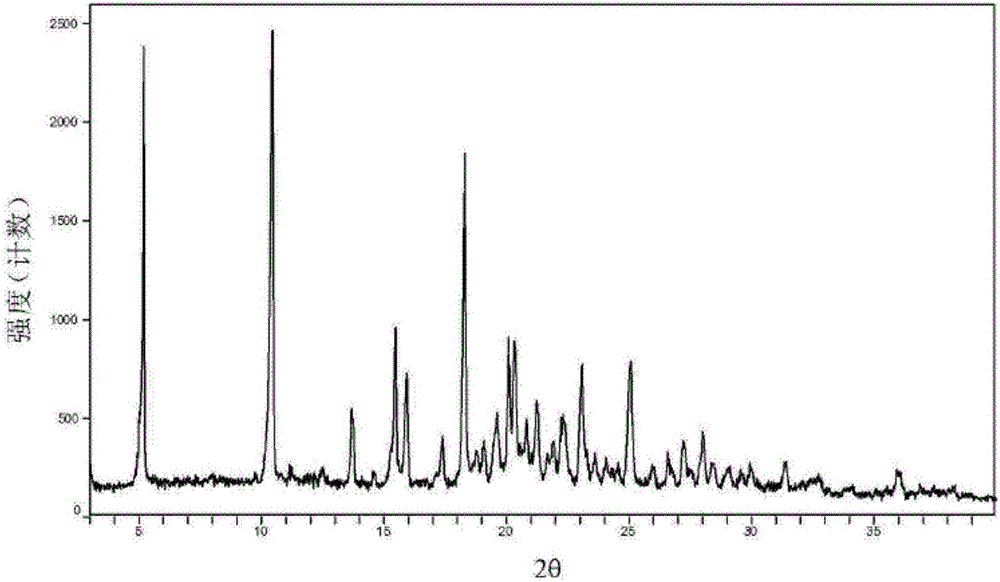
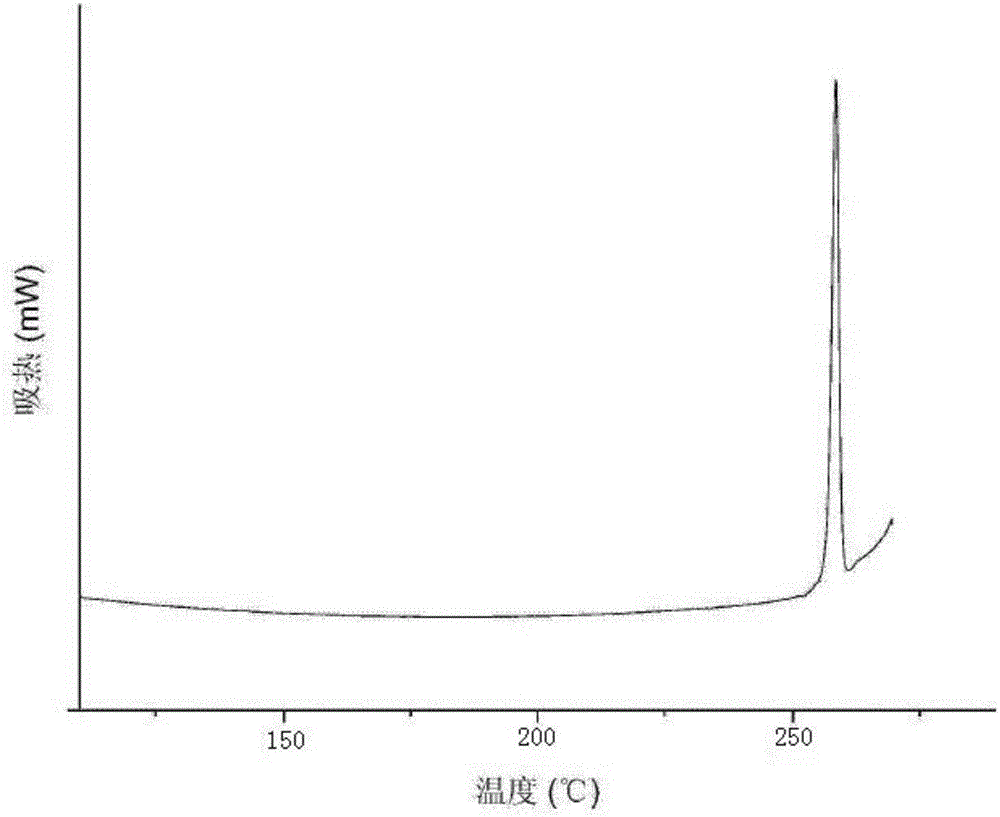
The invention relates to a glimepiride delta crystal form. A preparation method of the glimepiride delta crystal form comprises the following steps of dissolving a glimepiride crystal form I in N-methyl pyrrolidone at the temperature of 100 to 120 DEG C; crystallizing at the temperature of 80 DEG C below zero to 0 DEG C, wherein in a crystallizing process, a glimepiride delta crystal form seed crystal can be added and the crystallizing time is 24 to 72 hours; and unfreezing at the normal temperature, and filtering to obtain a solid, i.e. the glimepiride delta crystal form. The glimepiride delta crystal form disclosed by the invention is different from the glimepiride crystal form in the existing literature in powder x-ray diffraction and differential scanning spectra, so that the solid form is completely different from the existing crystal form of the glimepiride.
Owner:SHENZHEN NYCRIST TECH CO LTD
Preparation method of glimepiride tablets
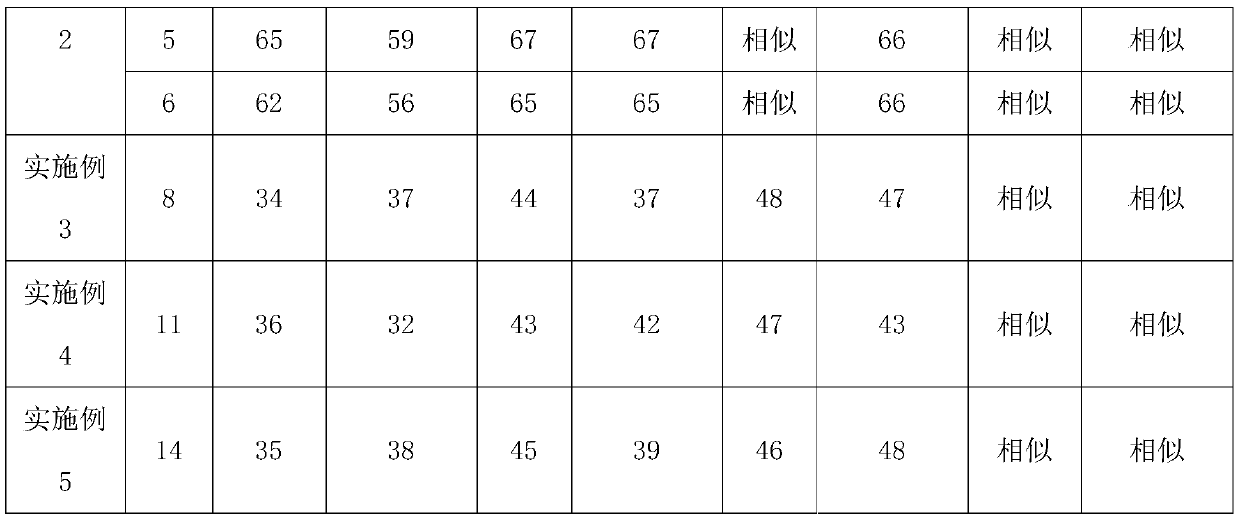
InactiveCN111135150APrescription process optimizationHigh similarityMetabolism disorderSulfonylurea active ingredientsAqueous ethanolMagnesium stearate
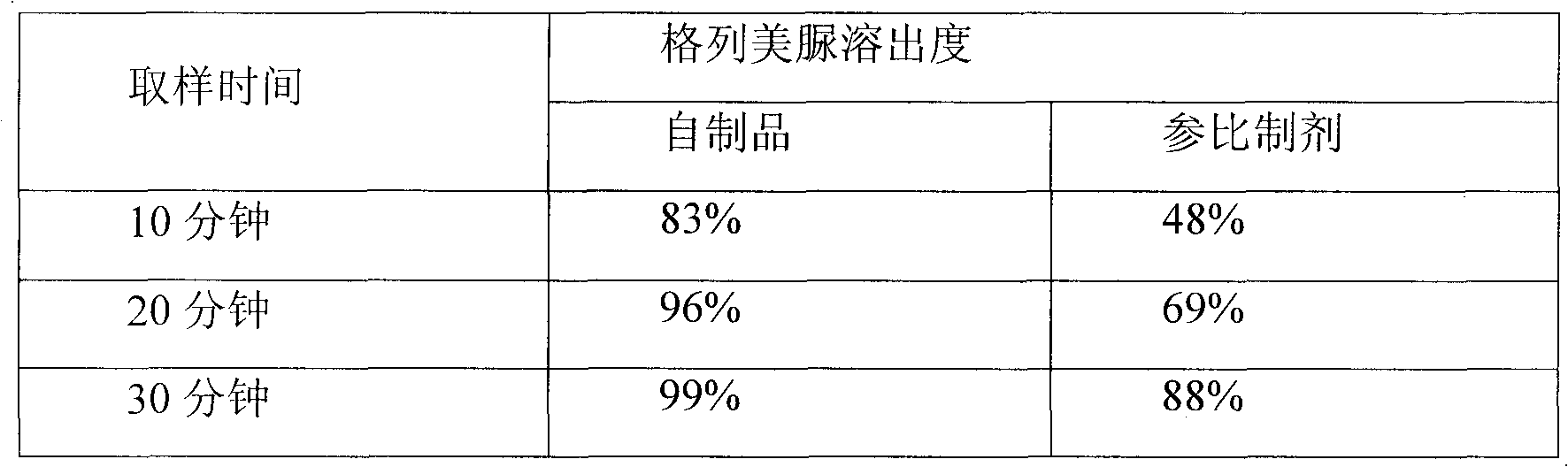
The invention relates to a preparation method of glimepiride tablets. The preparation method of the glimepiride tablets comprises the following steps: taking micronized glimepiride as a raw material;mixing the micronized glimepiride with auxiliary materials with relatively good solubilization effects, namely lactose and povidone, for a long period of time; and then, adding a certain amount of water or an aqueous ethanol solution as a moistening agent. According to the preparation method of the glimepiride tablets, the time of shearing and mixing is increased, so that the solubilization effects are promoted; and magnesium stearate, which has relatively little influence on dissolution, is added for performing mixed tablet compression, so that the final dissolution rate of the main drug is ensured with similarity of several dissolution curves between the self-made product and the reference preparation improved.
Owner:CHONGQING CONQUER PHARML
Glime piride soft capsule and its preparing method
The present invention relates to a glimepiride soft capsule and its preparation method. The content of the described soft capsule is solution type or suspension type made up by using glimepiride and one and / or several kinds of solubilization agent, cosolvent, suspension adjuvant, solubility promoter and surfactant, mixing and dissolving them.
Owner:宛六一 +1
Glimepiride tablet
ActiveCN106860408AEvenly dispersedPromote dissolutionMetabolism disorderSulfonylurea active ingredientsAlcoholCyclodextrin
The invention relates to a glimepiride tablet. The glimepiride tablet consists of glimepiride, hydroxypropyl-[beta]-cyclodextrin, deoxycholic acid and a pharmaceutically acceptable carrier. The glimepiride tablet is prepared by virtue of a method comprising the following steps: dissolving the glimepiride, the hydroxypropyl-[beta]-cyclodextrin and the deoxycholic acid to absolute ethyl alcohol; then, granulating an obtained solution with the addition of the pharmaceutically acceptable carrier; drying obtained granules; and adding a lubricating agent, and conducting mixing and tableting. In comparison with the prior art, the glimepiride tablet provided by the invention is simple in preparation process, uniform in drug dispersion, smooth in production process and rapid in drug dissolution.
Owner:SHANDONG NEWTIME PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com