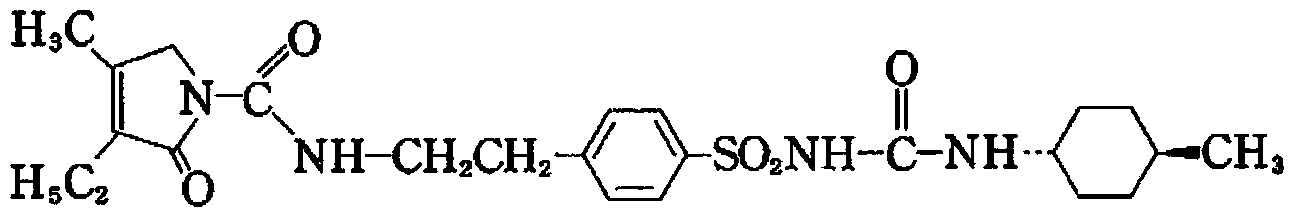
Glimepiride dropping pill
A technology of urea dripping pills and Glimepiride, applied in the field of glimepiride dropping pills, can solve the problems of poor stability of glimepiride, high temperature, high humidity instability, and good stability, and achieve good drug stability and preparation The process is simple and the effect of reducing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Preparation Process:
[0032] (1) Heat polyoxyethylene (40) stearate in a 60℃ water bath to melt;
[0033] (2) Crush the glimepiride, copovidone, and calcium ammonium alginate through a 120-mesh sieve, weigh them according to the prescription, mix them evenly, add them to the molten carrier material, stir evenly, keep warm at 60°C, and set aside;
[0034] (3) Drop the drug mixture solution prepared in (2) into dimethyl silicone oil below 10° C. to cool, filter, centrifuge to remove the oil, and package to get it.
Embodiment 2
[0036]
[0037]
[0038] Preparation Process:
[0039] (1) Heat polyoxyethylene (40) stearate in a 60℃ water bath to melt;
[0040] (2) Crush the glimepiride, copovidone, and calcium ammonium alginate through a 120-mesh sieve, weigh them according to the prescription, mix them evenly, add them to the molten carrier material, stir evenly, keep warm at 60°C, and set aside;
[0041] (3) Drop the drug mixture solution prepared in (2) into dimethyl silicone oil below 10° C. to cool, filter, centrifuge to remove the oil, and package to get it.
Embodiment 3
[0043]
[0044] Preparation Process:
[0045] (1) Heat polyoxyethylene (40) stearate in a 60℃ water bath to melt;
[0046] (2) Crush the glimepiride, copovidone, and calcium ammonium alginate through a 120-mesh sieve, weigh them according to the prescription, mix them evenly, add them to the molten carrier material, stir evenly, keep warm at 60°C, and set aside;
[0047] (3) Drop the drug mixture solution prepared in (2) into dimethyl silicone oil below 10° C. to cool, filter, centrifuge to remove the oil, and package to get it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com