Solid compound preparation containing metformin hydrochloride and glimepiride, preparation method and application thereof
A technology for metformin hydrochloride and compound preparations, which is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, and pill delivery, etc. Can't reach etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
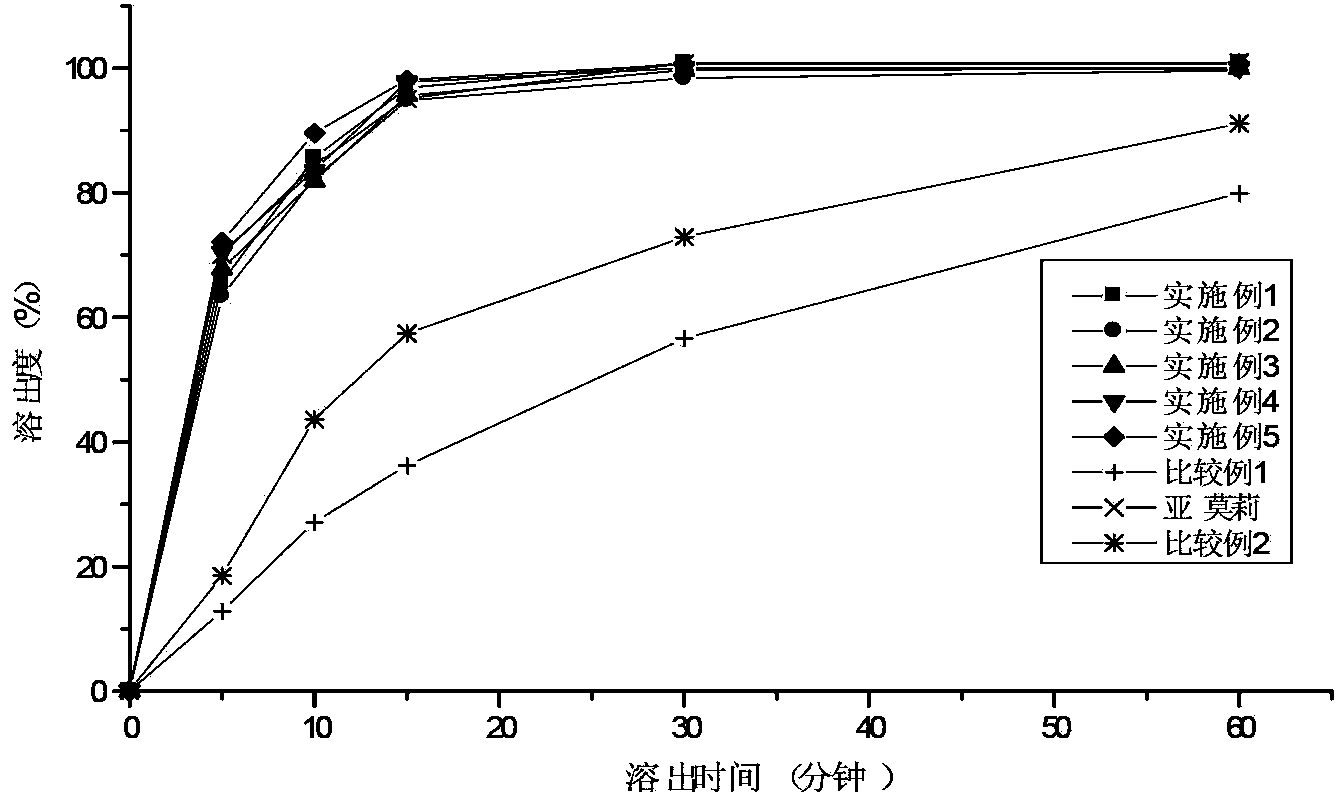
Embodiment 1
[0091] Take 1000.5g of metformin hydrochloride (particle size: 200 μ m), 20.2g of lactose, and 30.1g of mannitol, and use a high-speed stirring granulator to pre-mix for 3 minutes to make them uniform, and then add 91.4 g of povidone k30 aqueous solution (15%, w / w) , making soft materials, granulating with a swing granulator, and drying in a fluidized bed to obtain metformin hydrochloride granules with a moisture content of 0.75% by weight.
[0092] Take 40.0 g of glimepiride (particle size 2.5 μm), 200.8 g of lactose, 100 g of sucrose, and 18.0 g of croscarmellose sodium, and use a high-speed stirring granulator to pre-mix for 3 minutes to make them uniform, and then add povidone k25 aqueous solution (10%, w / w) 74.2 g of soft material, granulated by a swing granulator, and dried in a fluidized bed to obtain glimepiride granules.
[0093] According to the calculation results of the particle content, take 530.5 g of the above-mentioned metformin hydrochloride granules and 18.5 ...
Embodiment 2
[0095] Take 1000.5g of metformin hydrochloride (particle size 1000μm), 30.2g of maltodextrin, 58g of crospovidone, add 89.4g of povidone k30 aqueous solution (15%, w / w), and use a high-speed stirring granulator to pre-mix evenly , make soft material, shake granulator granulation, fluidized-bed drying obtains metformin hydrochloride granule, granule moisture 0.3% by weight, add croscarmellose sodium 1.8g, magnesium stearate 5.2g, micropowder silica gel 5.1g, Set the multi-directional mixer to mix for 5 minutes to make it uniform, and obtain the mixture containing metformin hydrochloride granules.
[0096] Take glimepiride 40.2g (particle size 20μm), lactose 1200.6g, glucose binder 1200g, xylitol 600.3g, crospovidone 50.1g, mix well, add povidone k25 aqueous solution (20%, w / w) 247.3g of soft material, granulated by a swing granulator, and dried in a fluidized bed to obtain glimepiride granules, adding 40.2g of sodium carboxymethyl starch, 5.3g of calcium stearate, 2.5g of talc...
Embodiment 3
[0099] Take 1000.3g of metformin hydrochloride (particle size 150μm), 401g of mannitol, 55.2g of dextrose, 45g of dextran, add 180.5g of povidone k25 aqueous solution (15%, w / w), and use a high-speed stirring granulator Mix uniformly in advance, make soft material, shake granulator granulation, fluidized bed drying obtains metformin hydrochloride granule, granule moisture 1.5% by weight, add sodium carboxymethyl starch 18.1g, zinc stearate 4.3g, talcum powder 2.2g, 2.0 g of micropowder silica gel was placed in a multidirectional mixer and mixed for 5 minutes to make it uniform to obtain a mixture containing metformin hydrochloride granules.
[0100] Take 40.2 g of glimepiride (particle size 1.0 μm), 300.3 g of lactose, 300 g of dextrose binder, and 30.1 g of croscarmellose sodium, mix well, add povidone k30 aqueous solution (10%, w / w ) 153.2g of soft material, granulated by a swing granulator, dried in a fluidized bed to obtain glimepiride granules, added 20.5g of croscarmello...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com