Liquid formulations of compounds active at sulfonylurea receptors
a technology of sulfonylurea and liquid formulation, which is applied in the direction of drug composition, biocide, metabolic disorder, etc., can solve the problems of difficult maintenance of constant drug concentrations in tissues and fluids, poor water soluble drug content, and inability to swallow pills, etc., to achieve rapid and readily controlled increase in circulating drug concentration, rapid onset of treatment, and rapid adjustment and maintenance of circulating drug concentrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
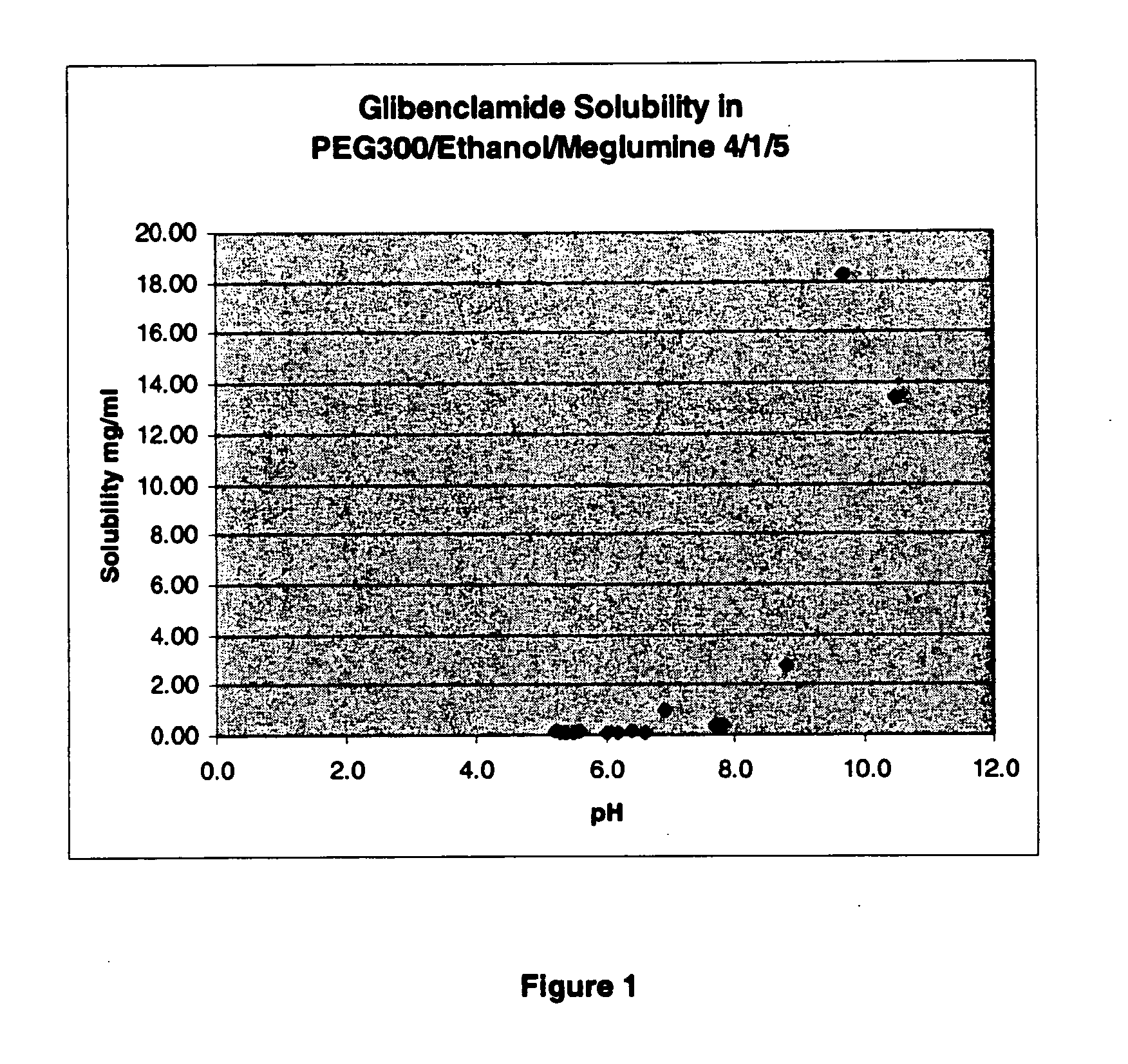
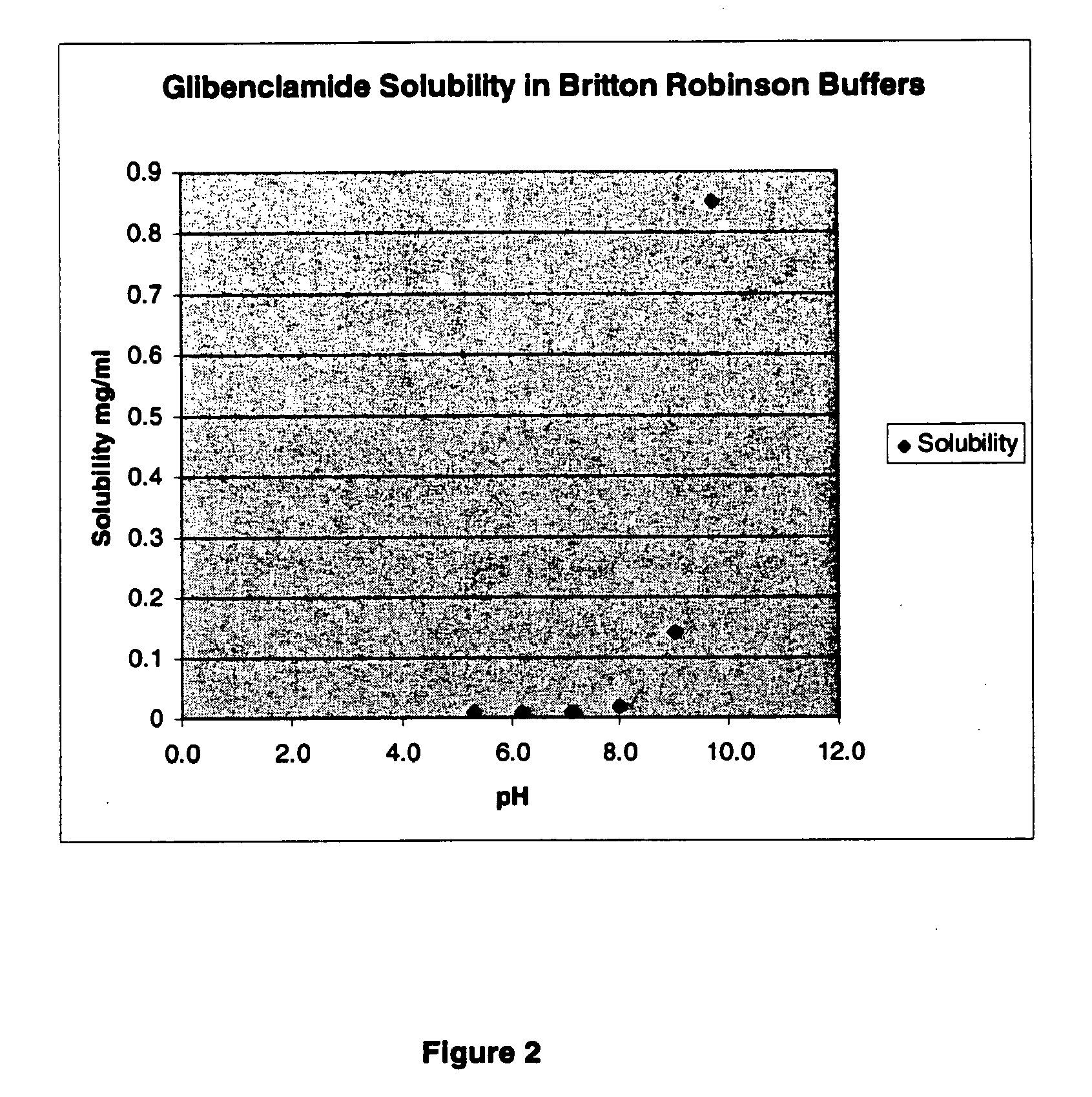
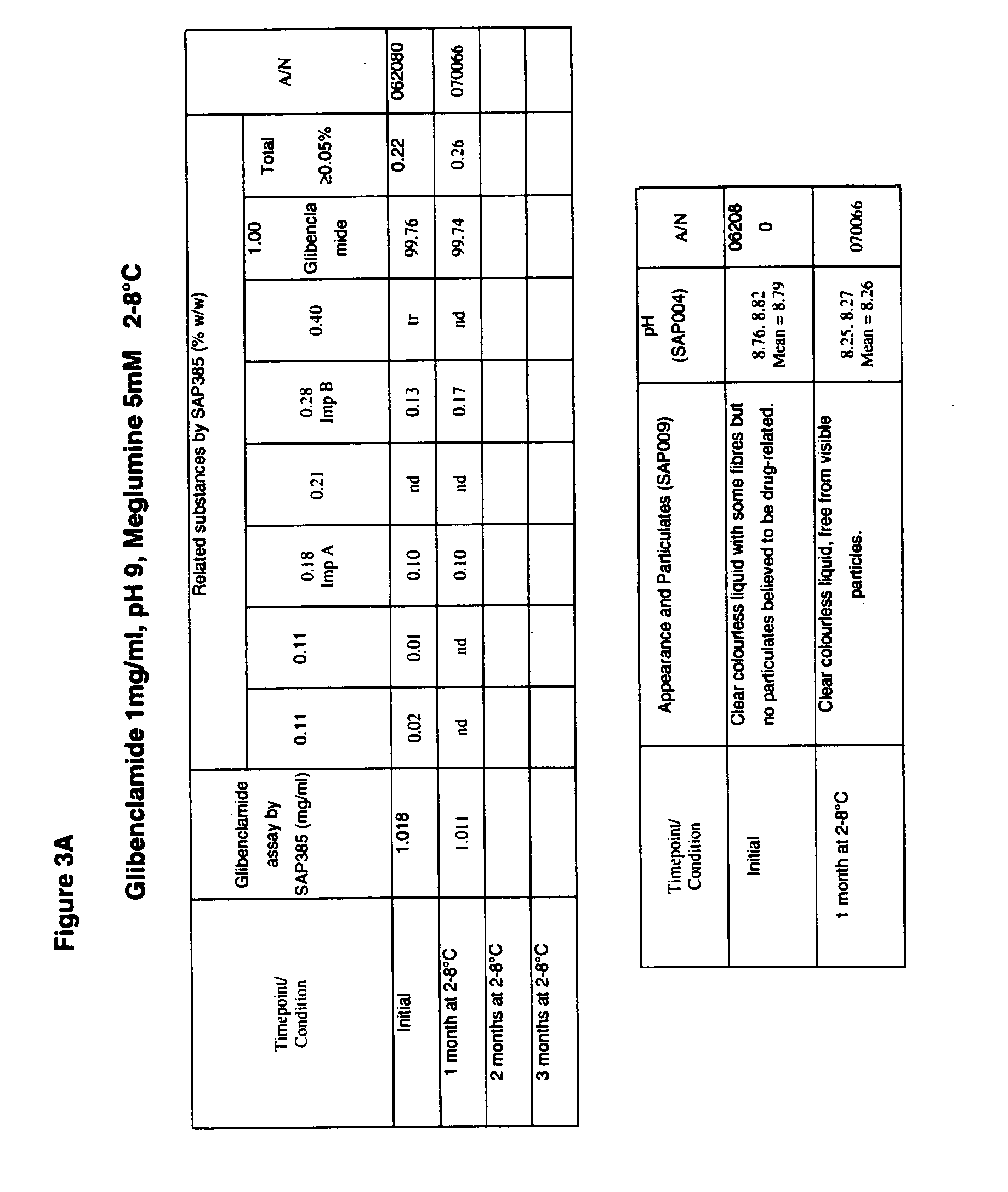
[0074]A liquid formulation having 40% PEG 300, 10% Ethanol and 50% water, at a pH of about pH 9 (8.5-9.5) included 1 mg glibenclamide per ml. This formulation may also include a buffer, such as meglumine or diethanolamine. This formulation may also include a surfactant; for example, this solution may include Tween 80 (e.g., less than about 1 ug / ml or less than about 1 ug / mg of glibenclamide). This formulation can be stored in a refrigerator (e.g., at about 2-8° C.) or can be stored at room temperature. This formulation is suitable for dilution; for example, it may be diluted 1:1, or 1:2 for use in bolus injections. This formulation may also be diluted by larger amounts of diluent, to 1:50 for example, for administration of glibenclamide as an infusion over an extended period of time. Dilution with a buffered solution allows the reduction of the solution pH to physiological levels without causing precipitation of glibenclamide. Glibenclamide remained in solution during and following ...
example 2
[0075]A further liquid formulation containing glibenclamide or tolbutamide includes 50% PEG 300, 10% Ethanol and 40% Water, and has a small quantity of Tween 80 1 ug / mg glibenclamide), at pH 8.4. This solution may be diluted by about 1:2 or 1:3. This formulation may also include a buffer, such as meglumine or diethanolamine.
example 3
[0076]A further liquid formulation containing glibenclamide or tolbutamide includes 40% PEG 300, 15% Tween 80, 10% Ethanol and 35% water, and 1 mg / ml glibenclamide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com