Process for synthesizing glimepiride raw material medicine
A synthesis process and technology of APIs, which are applied in the directions of drug combinations, pharmaceutical formulations, organic active ingredients, etc., can solve the problems of incomplete reaction, complicated products, complicated methods, etc., so as to reduce the generation of isomer impurities and improve the yield. efficiency and production efficiency, and the effect of simple purification operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
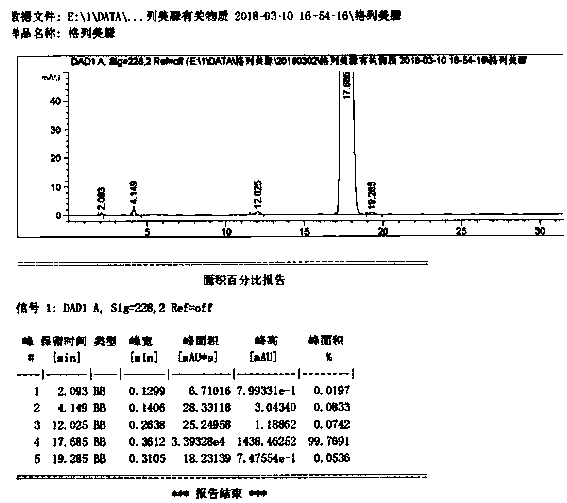
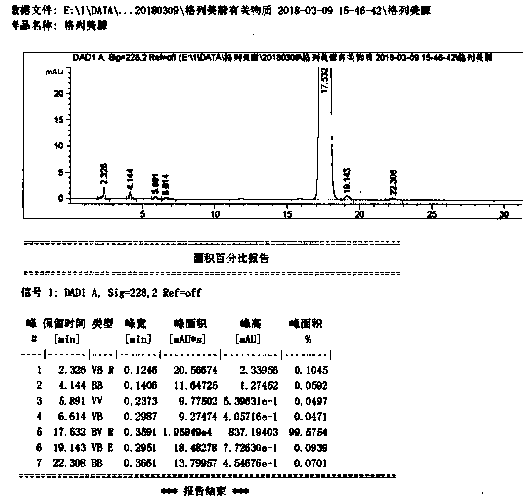
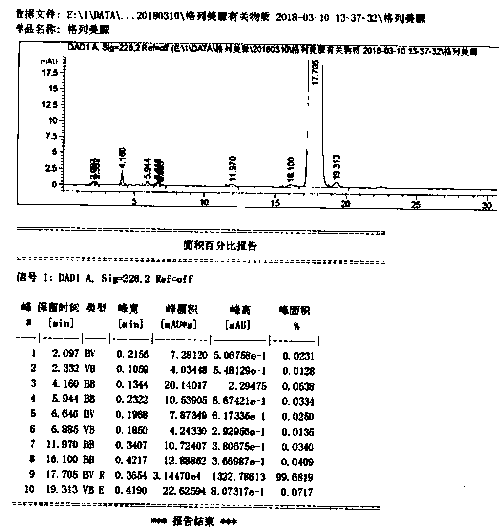
Image
Examples
Embodiment Construction
[0096] The technical solutions of the present invention will be described in detail below in conjunction with specific embodiments.
[0097] Step 1: Addition I
[0098] Add 12kg of toluene, 5.0kg of compound A, and 7.06kg of compound B into a 50L glass reactor; start stirring and heating, and heat up to 120-130°C for reflux reaction for 2 hours; take samples and send them to HPLC for central control; after the reaction, cool down to 20- Filter at 25°C; vacuum-dry the filter cake to obtain 9.25 kg of solid intermediate 1, with an HPLC purity of >99% and a molar yield of 85%. The filtrate was directly applied to the next batch of addition I reaction, and the above operation was repeated to obtain 10.33 kg of intermediate 1 with a HPLC purity of >99% and a molar yield of 97%.
[0099] The second step: sulfonation
[0100] Add 53.10kg of dichloromethane and 10.23kg of intermediate 1 into a 100L glass reactor, start stirring, cool down to -5-0°C, slowly add 17.58Kg of chlorosulfo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com