Preparation method of glimepiride EP impurities D and I
A technology for glimepiride and impurities, which is applied in the field of medicine and can solve the problems that the preparation method of glimepiride has not been reported in the literature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
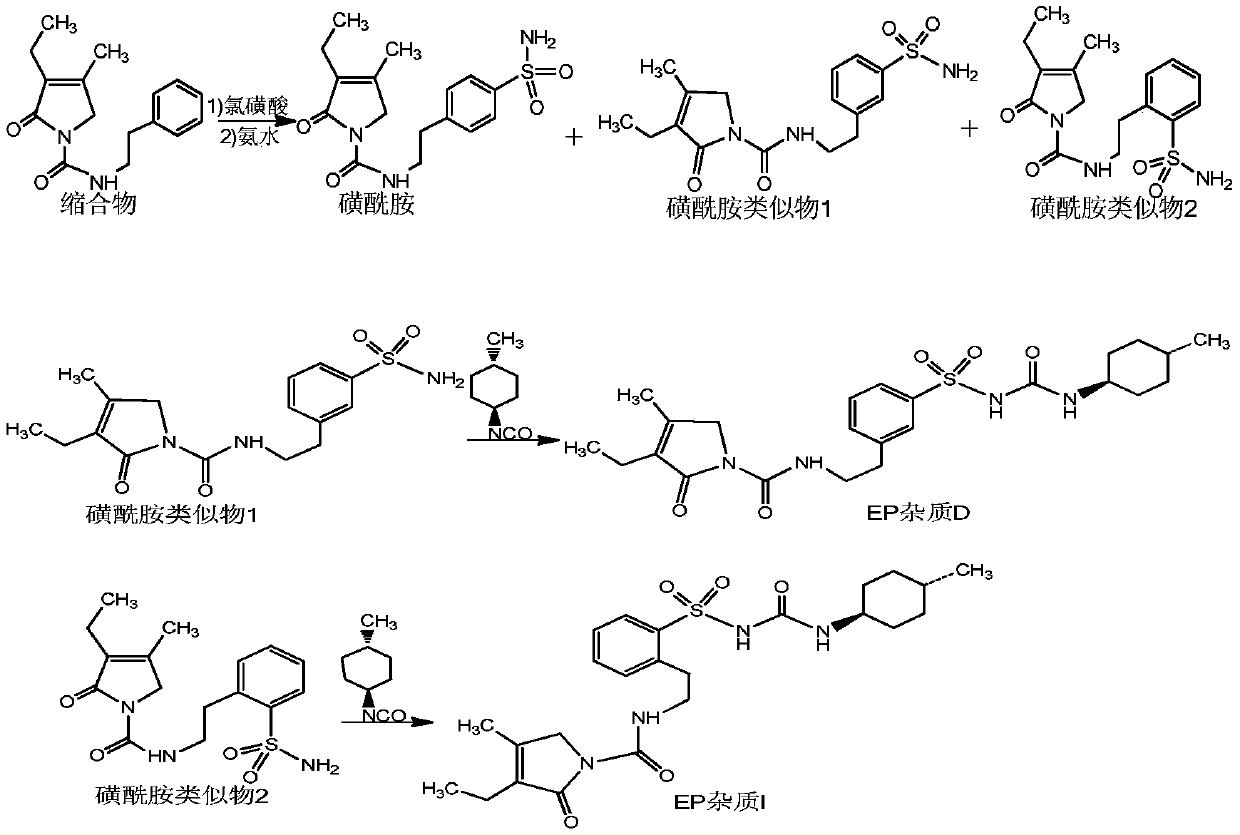
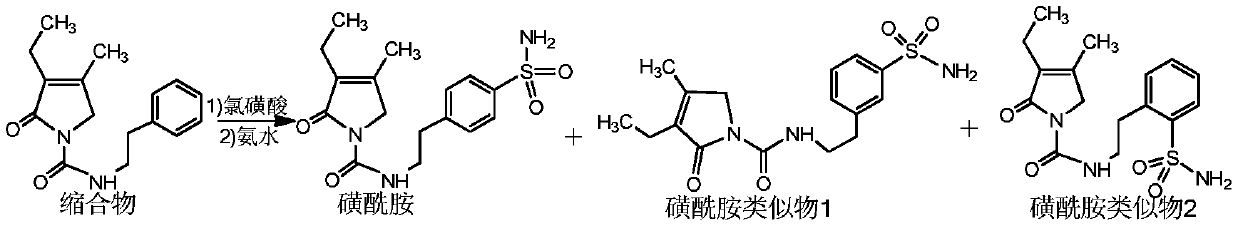
[0023] Example 1 Preparation of sulfonamide analogue 1 and sulfonamide analogue 2
[0024] Add 180g of chlorosulfonic acid to a 250ml reaction flask, lower the temperature to below 20°C, slowly add 30g of the condensate under the conditions of 10-20°C, then heat up to 40°C, and react at 40±2°C for 7 hours. After the reaction, the reaction solution was slowly poured into 1000 ml of ice water. Then filter and wash with water to obtain a white solid.
[0025] Add the white solid to a 100ml reaction flask, add 800ml of concentrated ammonia, and react at 20-25°C for 4 hours, then increase the temperature to 40±2°C and continue the reaction for 4 hours. After the reaction, the temperature was lowered to 25° C., filtered and washed with water to obtain a white solid.
[0026] Finally, the white solid was applied to high performance liquid chromatography with chromatographic conditions: Column: C18, 300*50.0mm, particle size 10μm, pore size 120A, wavelength: 225nm, mobile phase A: methanol...
Embodiment 2
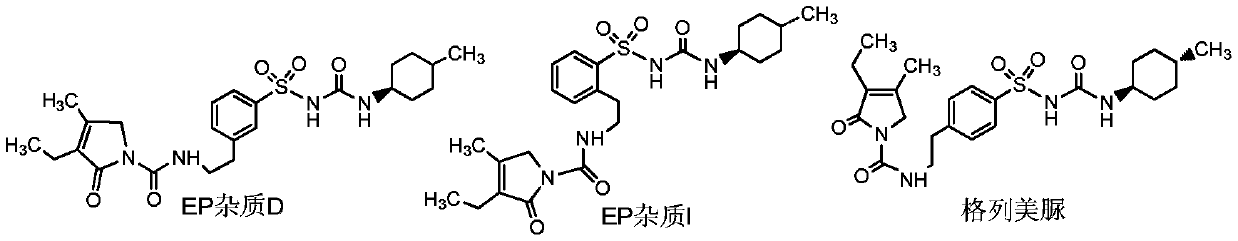
[0027] Example 2. Preparation of EP Impurity D
[0028] Add 1.0g compound 1, 0.51g anhydrous potassium carbonate, 16ml acetone into a 50ml reaction flask, raise the temperature to 50℃, and react at this temperature. After 0.5 hours, add 0.40g trans-4-methylcyclohexyl isocyanate dropwise With 4ml of acetone mixed solution, continue to react for 5 hours. After the reaction is completed, the temperature is lowered to room temperature, filtered and washed to obtain 1.10 g of EP impurity D.
Embodiment 3
[0029] Example 3 Preparation of EP Impurity D
[0030] Add 1.0g compound 1, 0.51g anhydrous potassium carbonate, 16ml acetone into a 50ml reaction flask, raise the temperature to 55℃, and react at this temperature. After 0.5 hours, add 0.60g trans-4-methylcyclohexyl isocyanate dropwise With 4ml of acetone mixed solution, continue to react for 8 hours. After the reaction is completed, it is cooled to room temperature, filtered and washed to obtain 1.35 g of EP impurity D.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com