Method for preparing high purity iron oxide yellow and iron oxide red using titanium dioxide byproduct ferrous sulfate
A technology for iron oxide red and iron oxide yellow, applied in the field of preparation of iron oxide yellow and iron oxide red, can solve the problems of complex production process, high production cost, low product purity and the like, and achieves short technological process, low production cost, High degree of impurity removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
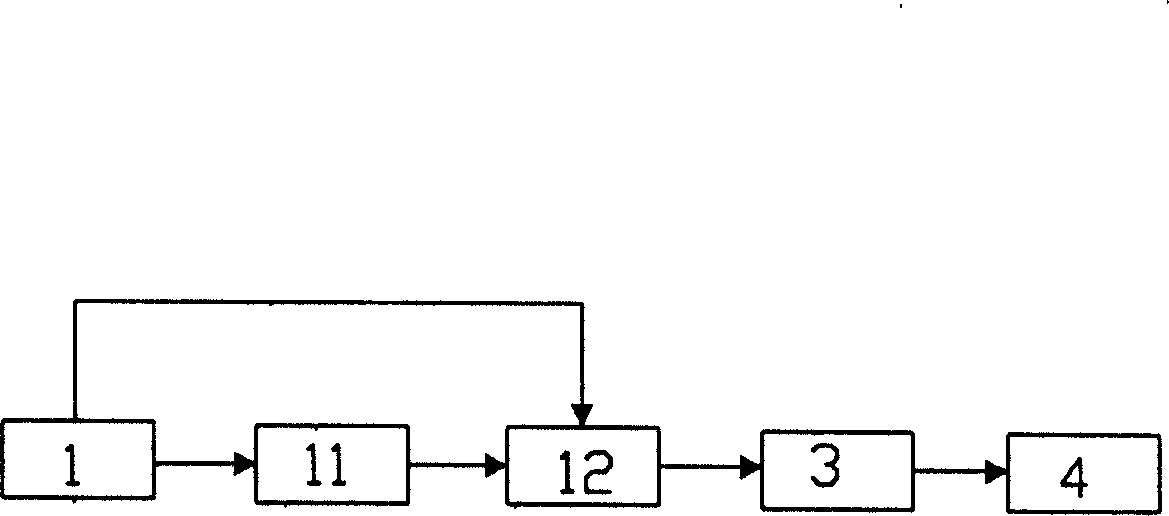
[0029] The method for preparing high-purity iron oxide yellow and iron oxide red from titanium dioxide by-product ferrous sulfate. The concentration of ferrous sulfate is 20%. Titanium dioxide is produced by sulfuric acid method. Ferrous sulfate is the main component in the by-product ferrous sulfate, followed by Also contains Mg, Al, Si, Ca, Ti, Zn, Ba, Mn and other elements. These elements have a great impact on the production of iron oxide yellow and iron oxide red, so the first step is to refine the above-mentioned ferrous sulfate solution 1:
[0030] There are two steps of impurity removal and separation in the refining 1 process. In this embodiment, the two steps of impurity removal are carried out at room temperature. The first step of impurity removal and separation is to remove titanium 11 and other impurities, and dilute it with water or adjust it with a dilute alkali solution. The pH value of above-mentioned solution is greater than 1.5, makes titanium ion Ti 3+ Co...
Embodiment 2
[0042] The concentration of ferrous sulfate in the present embodiment is 1mol L -1 After dissolving, use concentrated NaOH solution to directly adjust the pH value to 5.5, hydrolyze the titanium and ferric iron in the solution, filter, and carry out impurity removal and refining 1, and the filtrate after impurity removal and refining 1 is adjusted to pH value with 20% NaOH solution 3-7, the solution is dark green ferrous sulfate colloid, under the condition of temperature lower than 60 ℃, then oxidize 2 to the colloid to obtain iron oxide yellow; iron oxide yellow is calcined at 300 ℃ After 4, it is transformed into iron oxide red with a purity of more than 96%, which meets the national standard for producing iron oxide red by sulfuric acid method.
[0043] The present invention refines and removes impurities from the by-product ferrous sulfate solution of titanium dioxide under the condition that the temperature is lower than 60°C. By adjusting the pH value of the solution, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com