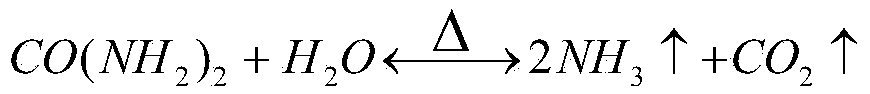Patents
Literature
120 results about "Iron oxide cycle" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
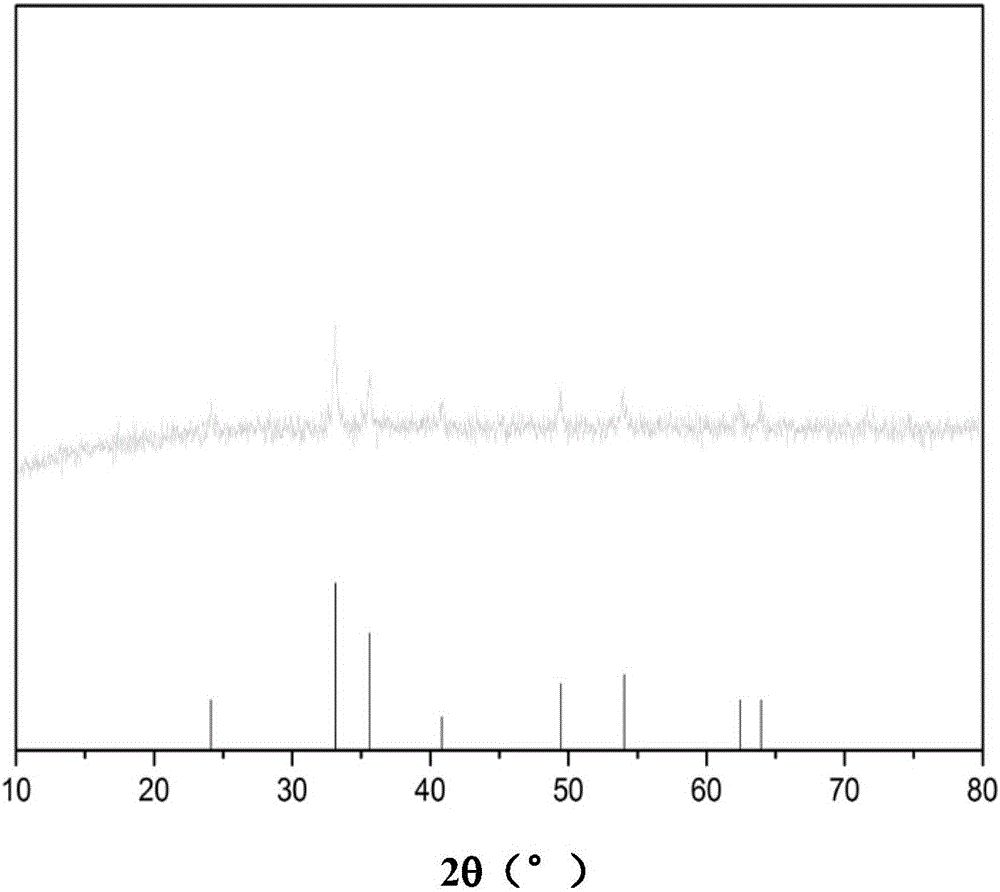
The iron oxide cycle (Fe₃O₄/FeO) is the original two-step thermochemical cycle proposed for use for hydrogen production. It is based on the reduction and subsequent oxidation of iron ions, particularly the reduction and oxidation from Fe³⁺ to Fe²⁺. The ferrites, or iron oxide, begins in the form of a spinel and depending on the reaction conditions, dopant metals and support material forms either Wüstites or different spinels.
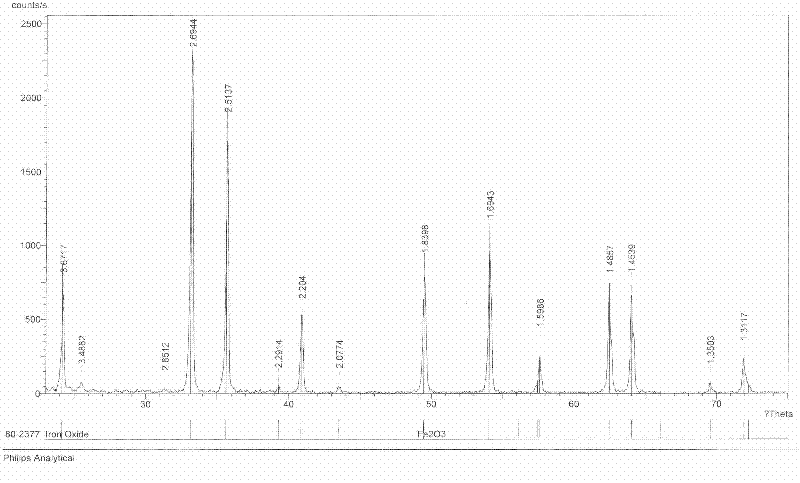
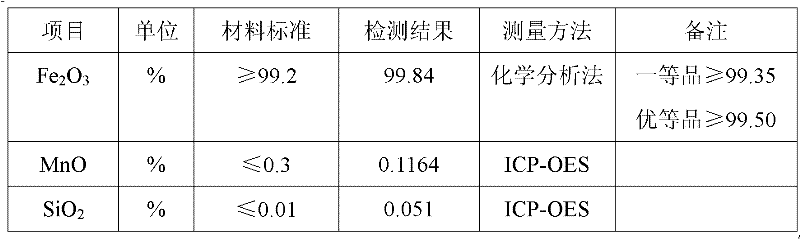
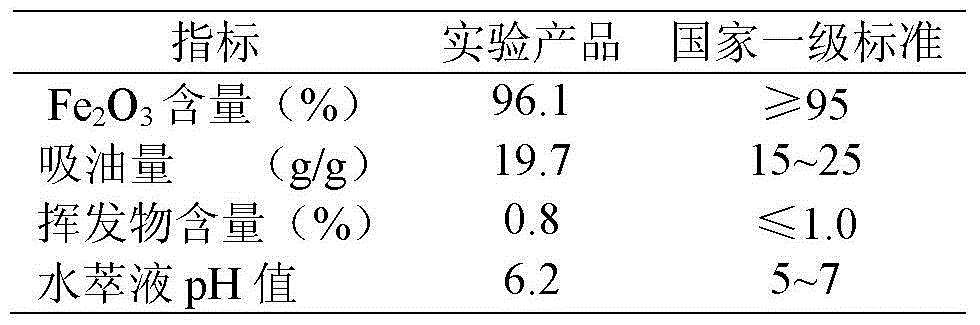
Method for producing iron oxide red by using ferrous sulfate as titanium dioxide byproduct
The invention discloses a method for producing oxide red by using ferrous sulfate as a titanium dioxide byproduct. The method comprises the following process steps of: firstly, dissolving the ferrous sulfate as the titanium dioxide byproduct with water of which the temperature is 50-80DEG C at normal pressure to form an aqueous solution; then adding ammonia water into the aqueous solution; adjusting the pH value of the aqueous solution to 2.2-4.0; adding soluble fluoride into the aqueous solution; reacting at the temperature of 50-80DEG C and normal pressure for 20-60 minutes by stirring; and after the reaction time reaches, separating a precipitation to obtain a purified liquid, wherein the addition mount of the soluble fluoride is 0.3-10 percent of the mass of the ferrous sulfate as the titanium dioxide byproduct; secondly, condensing and crystallizing the purified liquid obtained in the first step to obtain a pure ferrous sulfate crystal; and thirdly, dehydrating the pure ferrous sulfate crystal obtained in the second step at the temperature of 200-350DEG C and normal pressure for 20-60 minutes and calcining the reacted product at the temperature of 800-900DEG C and normal pressure for 1-4h to obtain the iron oxide red.
Owner:SICHUAN UNIV
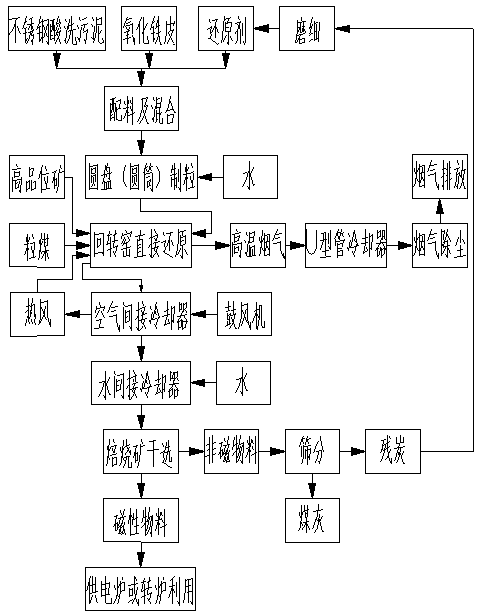
Stainless steel acid pickling sludge treatment process
The invention relates to stainless steel acid pickling sludge treatment process. The stainless steel acid pickling sludge treatment process includes the steps that (1) after stainless steel acid pickling sludge, iron oxide scale and reduction coal are prepared and mixed according to the mass ratio of 50:50:(10-20), granular materials with water content of 10-12% and particle size of 3-5mm are produced by a granulating machine; (2) after the granular materials are dried and pre-heated in a rotary kiln, the granular materials are sent to a high temperature reducing zone to be reduced under the temperature of 1150-1250 DEG C for 90-120min, and high-volatility pea coal and high grade pea ore are sprayed into the rotary kiln to be subjected to combined hydrocarbon reduction and carbon cycle oxygenation reduction; and (3) after taken out of the kiln, the high temperature roasting materials are cooled and magnetically separated, granular metallization products used by a power supply furnace or a converter can be obtained. According to the stainless steel acid pickling sludge treatment process, while the stainless acid pickling sludge and the iron oxide scale of steel enterprises are treated, iron, nickel and chrome resources of materials can be effectively used, and high quality metallization materials with the nickel and the chrome are produced.
Owner:JIUQUAN IRON & STEEL GRP
Method for producing iron oxide yellow and iron oxide red by using waste hydrochloric acid solution
The invention provides a method for producing iron oxide yellow and iron oxide red by using a waste hydrochloric acid solution. The method comprises the following steps: 1, purifying the waste hydrochloric acid solution: taking a steel waste acid solution, adjusting the pH value, adding a certain amount of waste sheet iron, carrying out controlled temperature hydrolysis for 18-24h, adding a flocculating agent, standing for above 24h, and taking the obtained supernatant; 2, preparing crystal seeds: adding the solution obtained in step 1, liquid alkali and water into a crystal seed tank, controlling the temperature and the pH value, and carrying out a preliminary oxidation reaction on ferrous chloride to generate a small amount of iron oxide nuclei; 3, carrying out an oxidation reaction: pumping the nuclei into an oxidation tank, adding sheet iron and water, and introducing compressed air to fully oxidize in order to generate iron oxide crystals on the nuclei; 4, processing byproducts; and 5, carrying out purifying post-treatment. A raw material used in the invention is the steel cleaning hydrochloric acid waste liquid, and is processed to generate useful products comprising iron oxide red and iron oxide yellow, so the waste is changed to valuables. Pigments produced through the method have the advantages of good staining degree, pure and right color, and good client feedback.
Owner:天津市大港华明化工厂
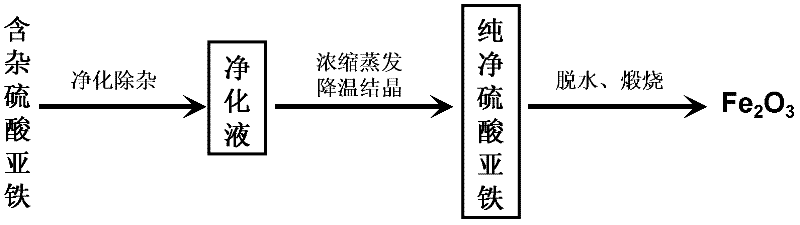
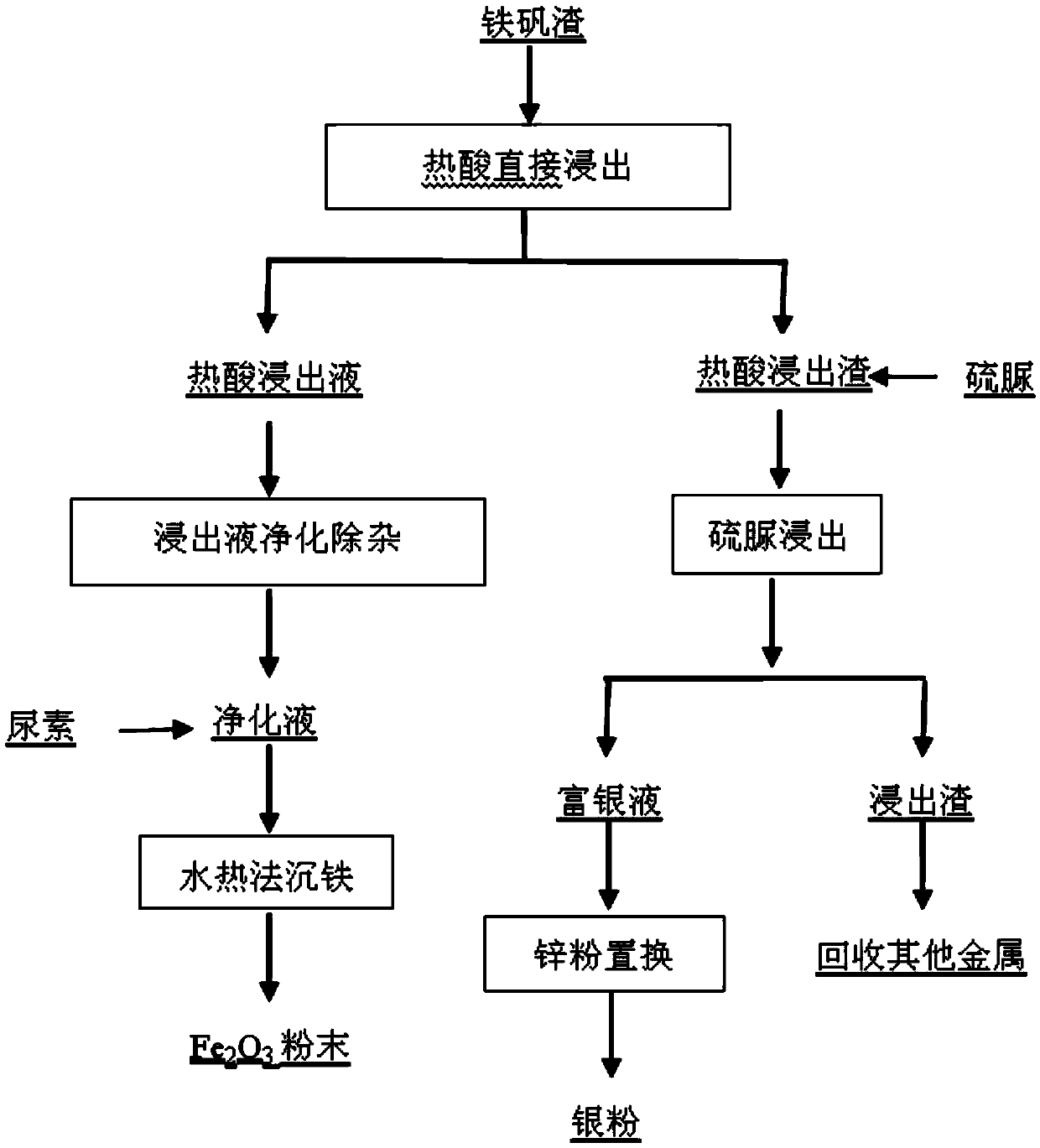
Method for extracting and separating iron from industrial waste iron sludge and preparing ferrous sulfate and iron oxide red
InactiveCN102674480AReduce consumptionNo emissionsFerric oxidesIron sulfatesSludgeAmmonium Hydrogen Carbonate
The invention discloses a method for extracting and separating iron from industrial waste iron sludge and preparing ferrous sulfate and iron oxide red and relates to a technological method for chemical extraction and separation. The method is an organic solvent effect crystallization process for extracting and separating iron from industrial waste iron sludge and preparing ferrous sulfate and iron oxide red, consists of four technical processes and realizes the chemical goal of extracting and separating iron and preparing ferrous sulfate and iron oxide red. The four technical processes are respectively as follows: 1) a diluted sulfuric acid leaching process of industrial waste iron sludge; 2) an iron crystallization and separation process under the effect of monobasic alcohol serving as organic solvent; 3) an iron oxide red preparation process through reaction between ferrous sulfate solution and ammonium hydrogen carbonate and oxidizing roasting of sediment; and 4) an ammonium sulfate preparation process through evaporative crystallization of sedimentation solution. The method is suitable for extracting and separating iron element from all kinds of iron-containing minerals and industrial waste residues, valuable components are comprehensively utilized and the environmental pollution and the resource wastage are reduced.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method for preparing iron oxide particles having controllable morphology and size
InactiveCN107628648AEffective regulationStrong controllabilityFerroso-ferric oxidesNanotechnologyOctahedronMagnetite Nanoparticles
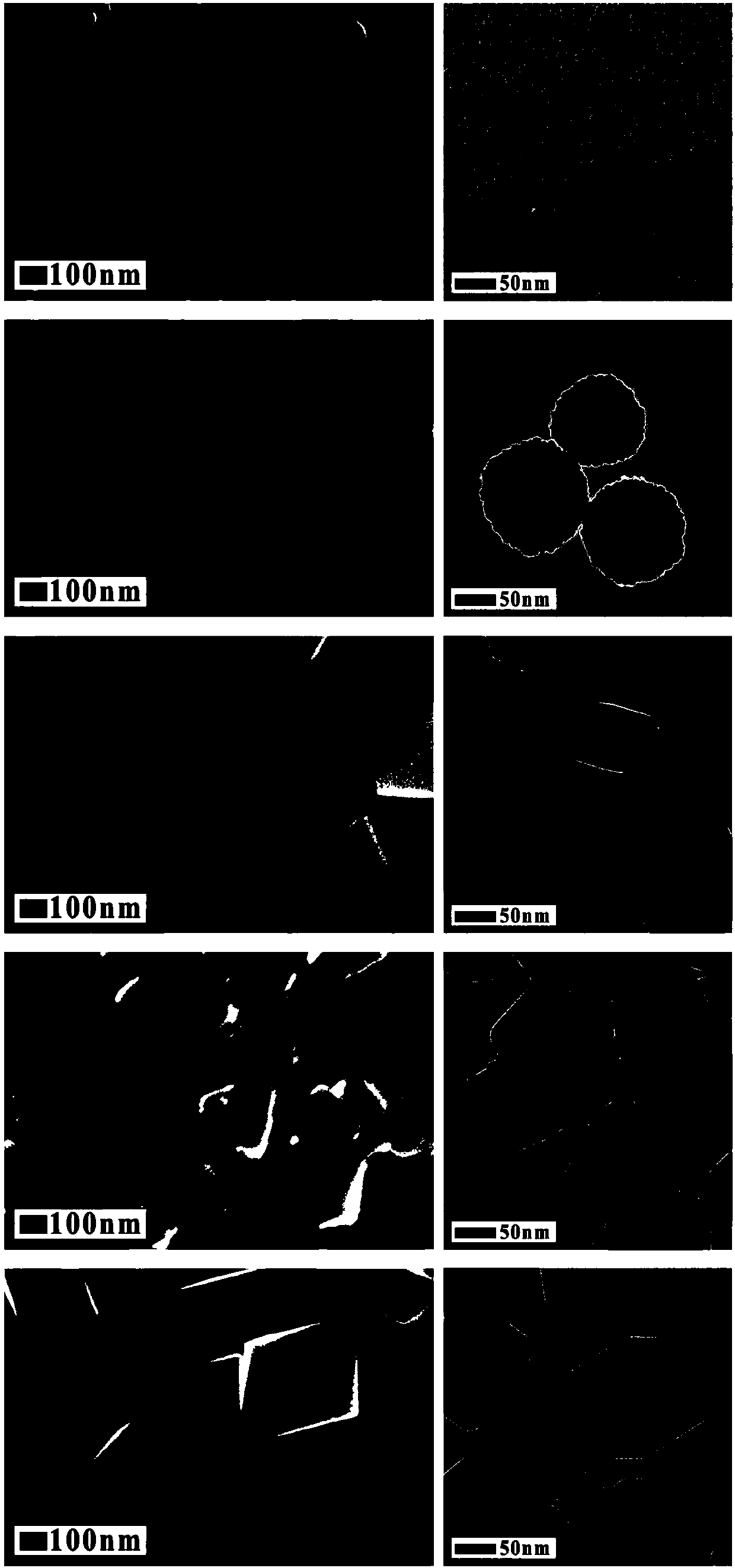
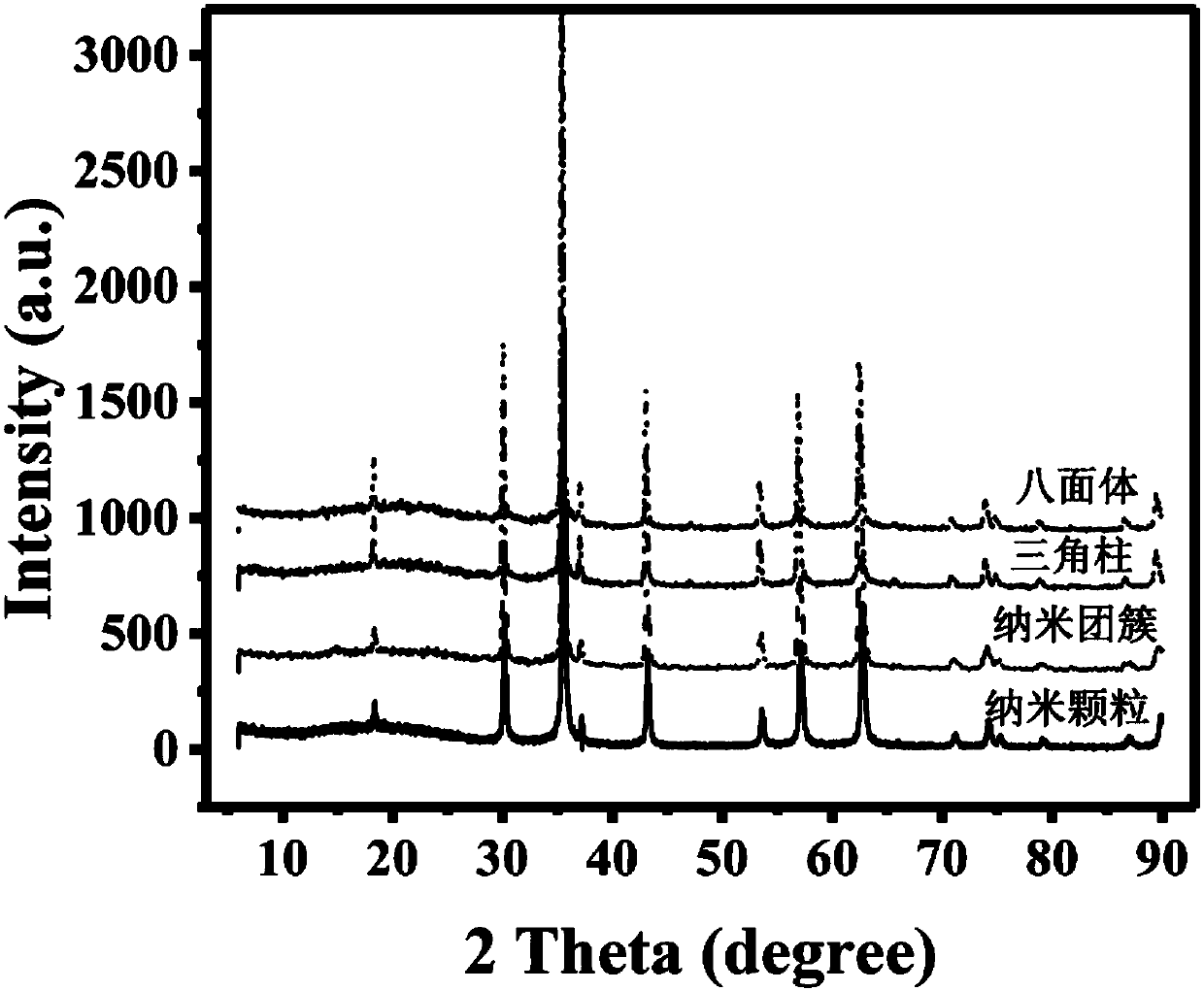
The invention relates to a method for preparing iron oxide particles having controllable morphology and size. The method comprises the following steps: 1, adding a surfactant into an organic solvent,fully stirring the surfactant and the organic solvent to obtain a uniformly dispersed suspension, adding an inorganic alkali and a soluble iron salt into the suspension, and fully stirring the inorganic alkali, the soluble iron salt and the suspension until the inorganic alkali and the soluble iron salt are completely dissolved; 2, pouring a solution prepared in step 1 into a closed heating container, and carrying out a thermal reaction; and 3, separating a precipitate in the obtained reaction solution through a centrifuging or magnet process, repeatedly washing the precipitate with ethanol and deionized water, and carrying out vacuum drying to obtain a black solid which is the final product. The accurate and highly-efficient regulation of the size and the morphology of nano-particles is realized through adjusting the proportion of the reaction solvent and the addition amount of the inorganic alkali under same reaction conditions, the size of the obtained particles is 20-400 nm, and solid particles, nano-clusters, triangular prisms, regular octahedrons and other various morphologies can be obtained. The preparation method has the advantages of simplicity, and green and environmentally-friendly raw materials and technology, the product has the advantages of high crystallinity, stable structure, uniform size and gram level reaching yield, and the preparation method is of great guidance significance to preparing magnetic nano-particles.
Owner:TSINGHUA UNIV
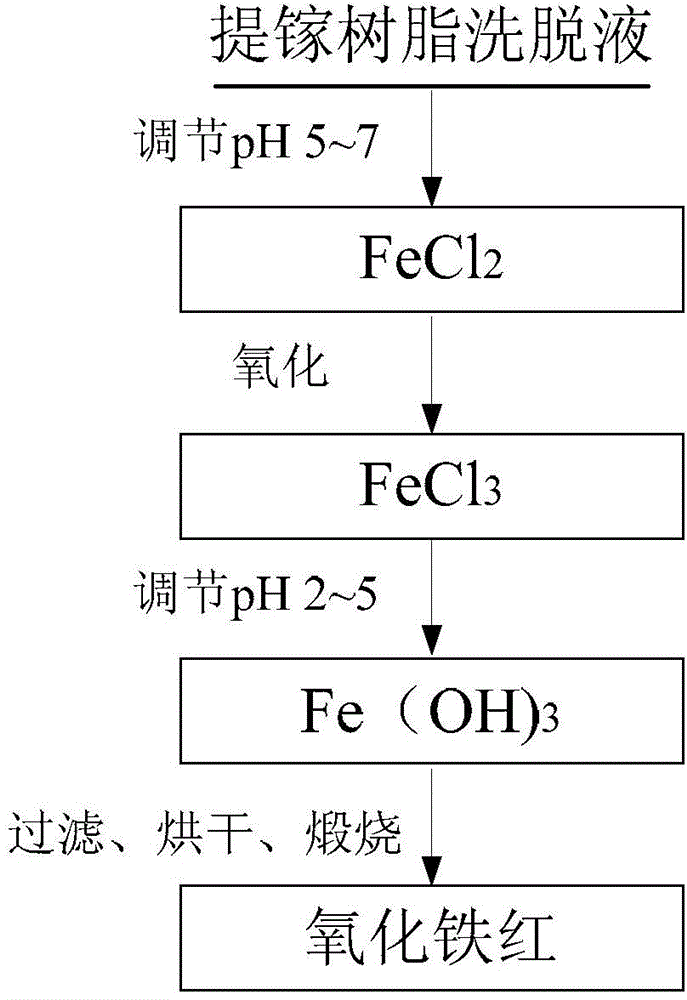
Iron oxide red and preparation method thereof
The invention discloses iron oxide red and a preparation method thereof. The preparation method comprises the steps of using a gallium extraction resin eluant as a raw material, treating the gallium extraction resin eluant and obtaining the iron oxide red. The gallium extraction resin eluant produced in an aluminum oxide production process by a pulverized fuel ash acid method is subjected to the treatment such as purification, refining, precipitation and burning, and a high index iron oxide red product meeting a national primary standard and having iron oxide content up to 99.3wt% is obtained. Therefore, the method shortens a technological flow, saves energy sources, produces a better economic benefit, opens up a new efficient treatment approach for composing the complicated gallium extraction resin eluant, has an important significance in boosting gallium extraction, realizes waste utilization and also provides a new preparation approach for producing the iron oxide red at the same time.
Owner:CHINA SHENHUA ENERGY CO LTD +1
Process method for extracting and separating iron from industrial waste iron mud and preparing iron oxide red
InactiveCN102703689AReduce consumptionNo emissionsFerric oxidesProcess efficiency improvementSlagEvaporation
The invention discloses a process method for extracting and separating iron from industrial waste iron mud and preparing iron oxide red and relates to a chemical engineering separation method. The process method disclosed by the invention is of a new process for extracting and separating the iron from the industrial waste iron mud and preparing the iron oxide red; the process method comprises four technical operations, and the chemical engineering purpose of not only extracting and separating the iron, but also preparing a high-added value product, namely the iron oxide red; and the four technical operations are respectively as follows: (1) a dilute sulfuric acid-dilute hydrochloric acid mixed acid leaching process of the industrial waste iron mud and an acid leaching solution reduction process; (2) the process for preparing the iron oxide red by enabling a ferrous sulfate solution to react with a carbonate and performing oxidizing roasting on a precipitate intermediate; (3) the process for preparing ammonia sulfate by crystallizing a precipitation solution under the action of an organic solvent; and (4) the process for recovering the organic solvent by evaporation and preparing ammonium chloride by evaporation. The process method disclosed by the invention is suitable for extracting and separating iron element from various iron-containing minerals and industrial waste slag, and by using the process method, valuable components can be comprehensively utilized, and environmental pollution and the waste of resources can be reduced.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
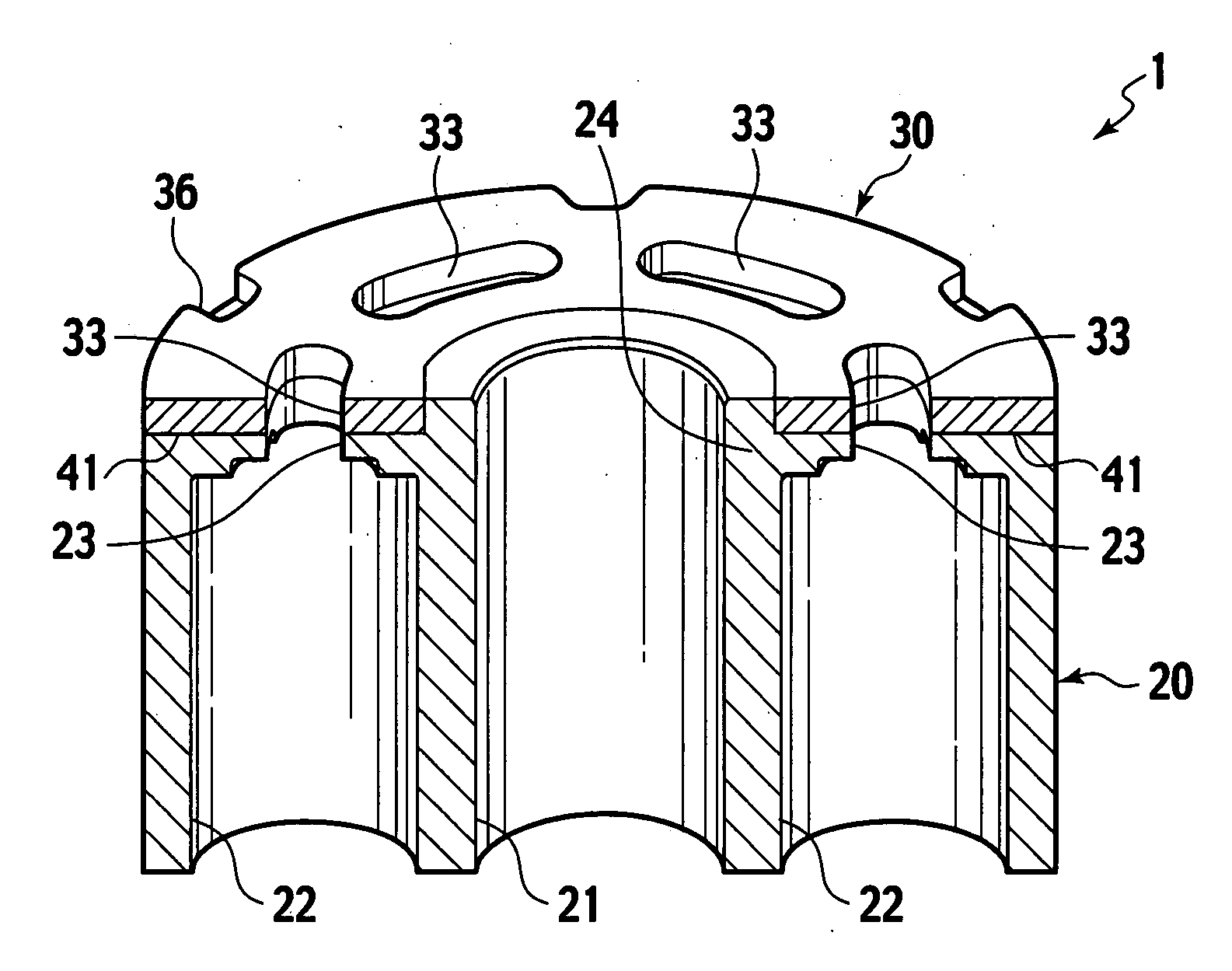
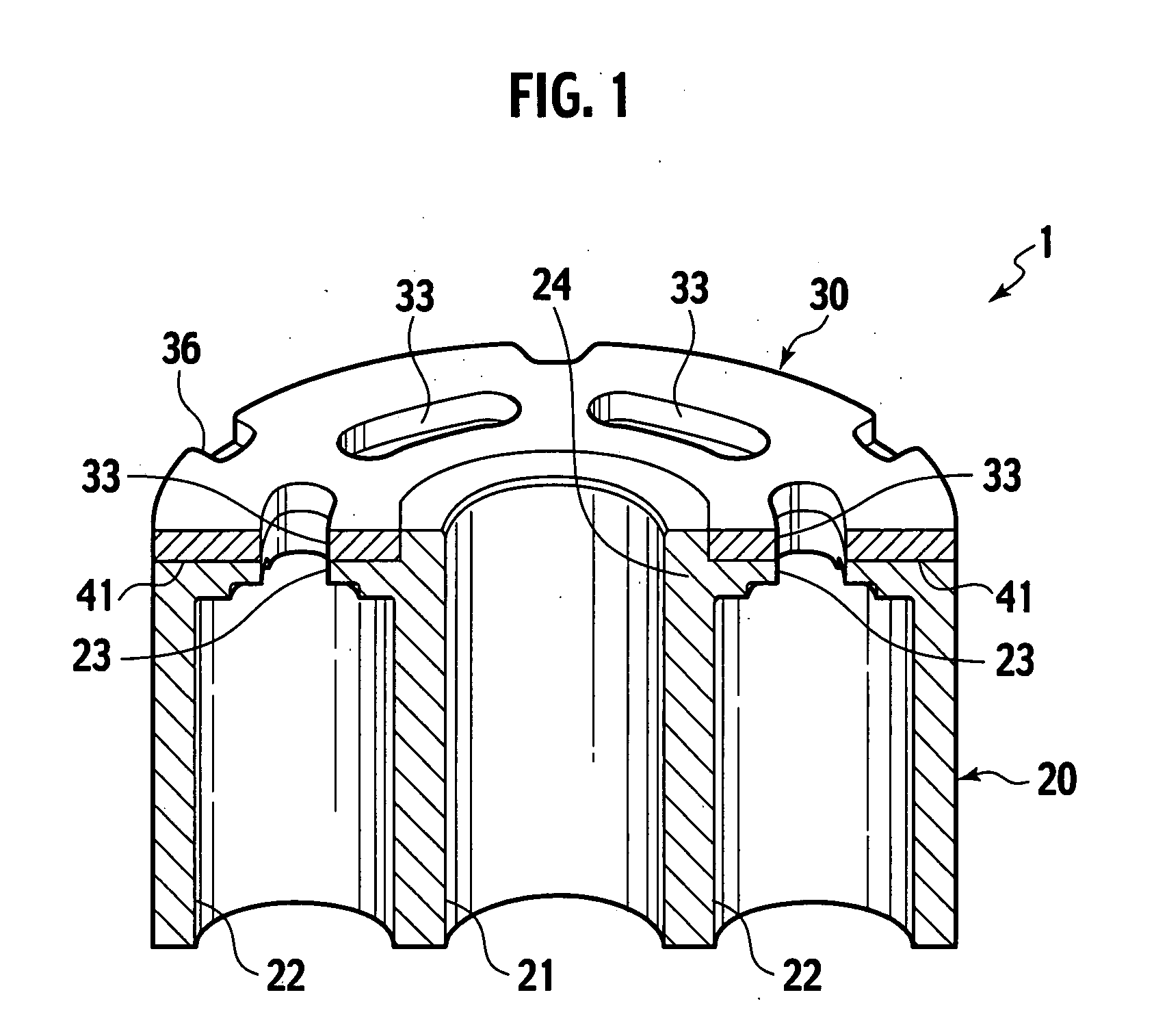
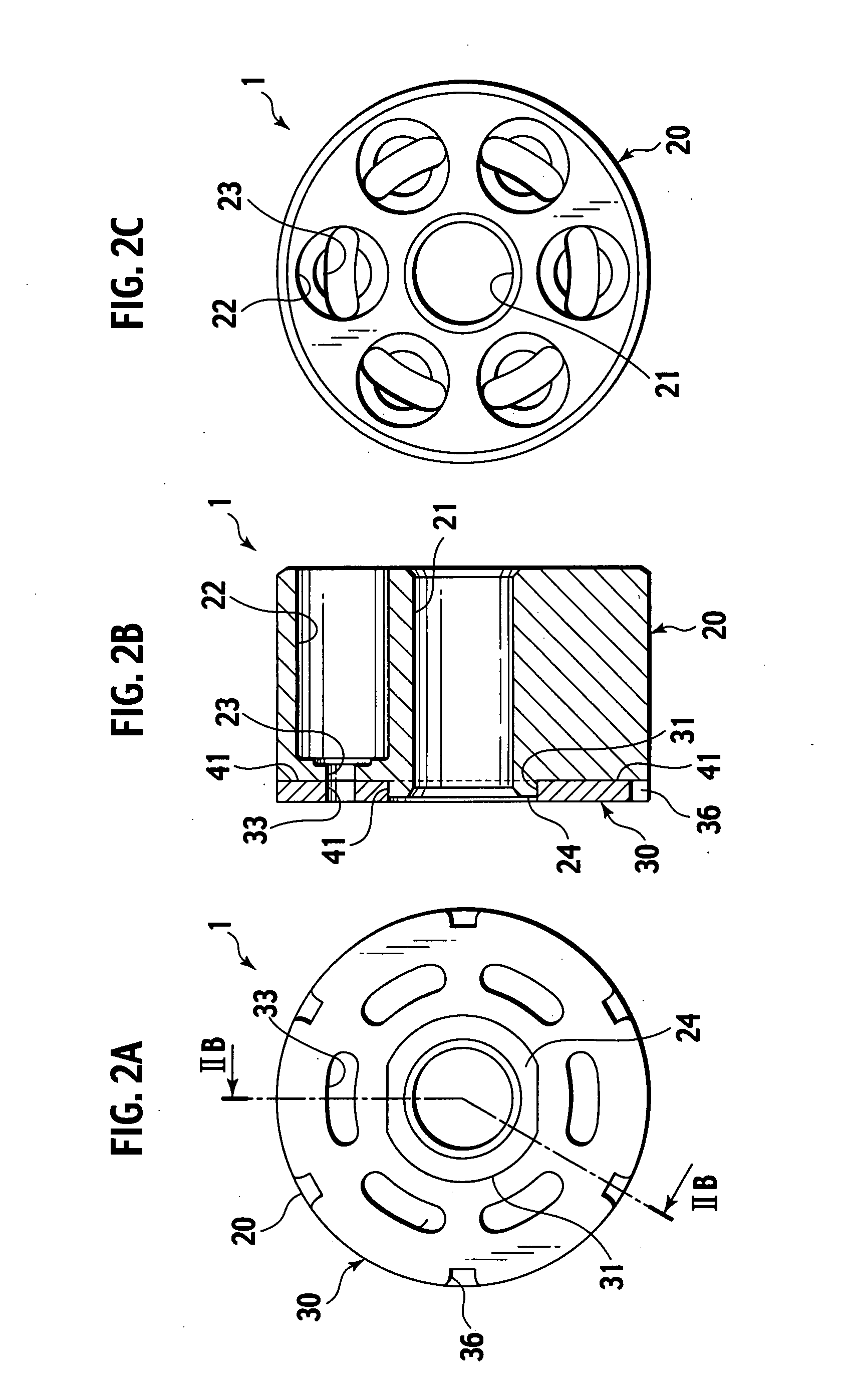
Sintered composite machine part and manufacturing method thereof
Disclosed are a sintered composite machine part as a cylinder block for piston pumps or piston motors, and a manufacturing method thereof. The machine part has an air-tight main body of sintered porous iron alloy and having an iron oxide layer on the surface; and a sliding part of sintered porous copper alloy being bonded direct to the main body. The sliding part is to be slid in tight contact with a fluid supply / return device. The manufacturing is made by preparing a main body of sintered porous iron alloy and a green compact for sliding part from a raw material metal powder having a composition corresponding to the copper alloy; sintering the green compact for sliding part in contact with the main body to bond the sliding part to the main body by diffused junction; and subjecting the main body to steam treatment to provide an iron oxide layer.
Owner:HITACHI POWDERED METALS COMPANY
Method of producing iron oxide red using ferrous sulphate
ActiveCN1903735ANo pollution in the processShort reaction timePigmenting treatmentFerric oxidesSulfateFerrous sulphate
The present invention provides a method for producing iron oxide red by using ferrous sulfate. Said method includes the following steps: (1), placing ferrous sulfate and ammonia water into a reactor; (2), introducing oxygen gas to make oxidation reaction; (3), discharging material, filtering, washing, drying and pulverizing so as to obtain the iron oxide red.
Owner:PANZHIHUA LIYU MINING
Method for preparing iron oxide red by utilizing iron-containing wastes and iron oxide red pigment
The invention relates to the technical field of chemical industry and provides a method for preparing iron oxide red by utilizing iron-containing wastes. The method comprises the following steps: converting an iron element in the iron-containing wastes into ferric hydroxide, ferrous hydroxide or a mixture of ferric hydroxide and ferrous hydroxide; then carrying out constant-temperature drying for3 to 5h at 120 to 180 DEG C in the presence of oxygen gas, so as to obtain dry powder; storing the dry powder for 40 to 180min under a sealed condition at 400 to 800 DEG C, so as to obtain gamma-Fe2O3. The method provided by the invention can be used for recycling the iron-containing wastes and resources are avoided; the yield of iron oxide red is high and the economic benefits are relatively high. The invention further provides an iron oxide red pigment; the iron oxide red pigment is obtained through gamma-Fe2O3 in a standardized manner; iron oxide red pigment has relatively excellent economic benefits.
Owner:CHANGZHOU SANLE ANTICORROSIVE MATERIAL CO LTD
Method for preparing iron oxide red
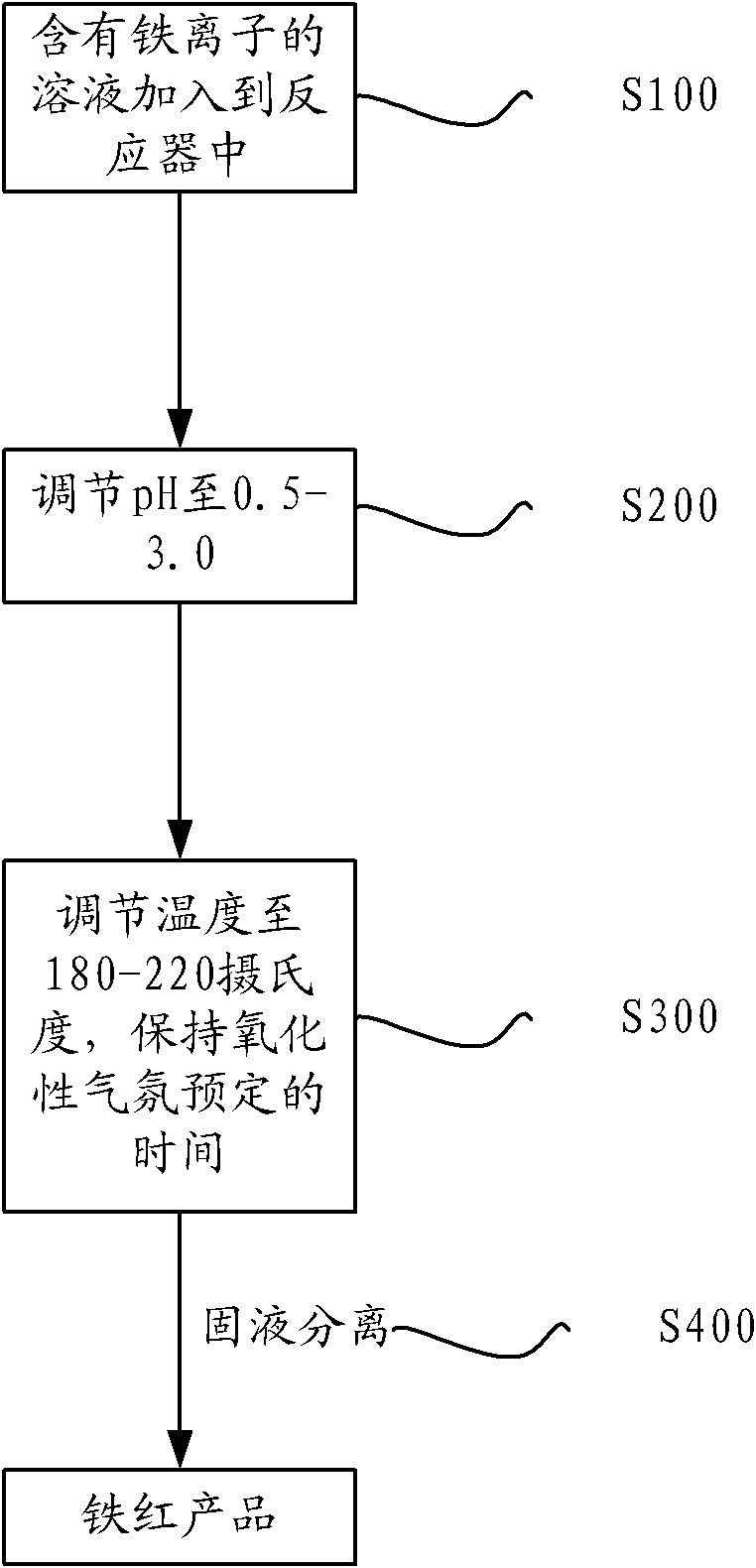
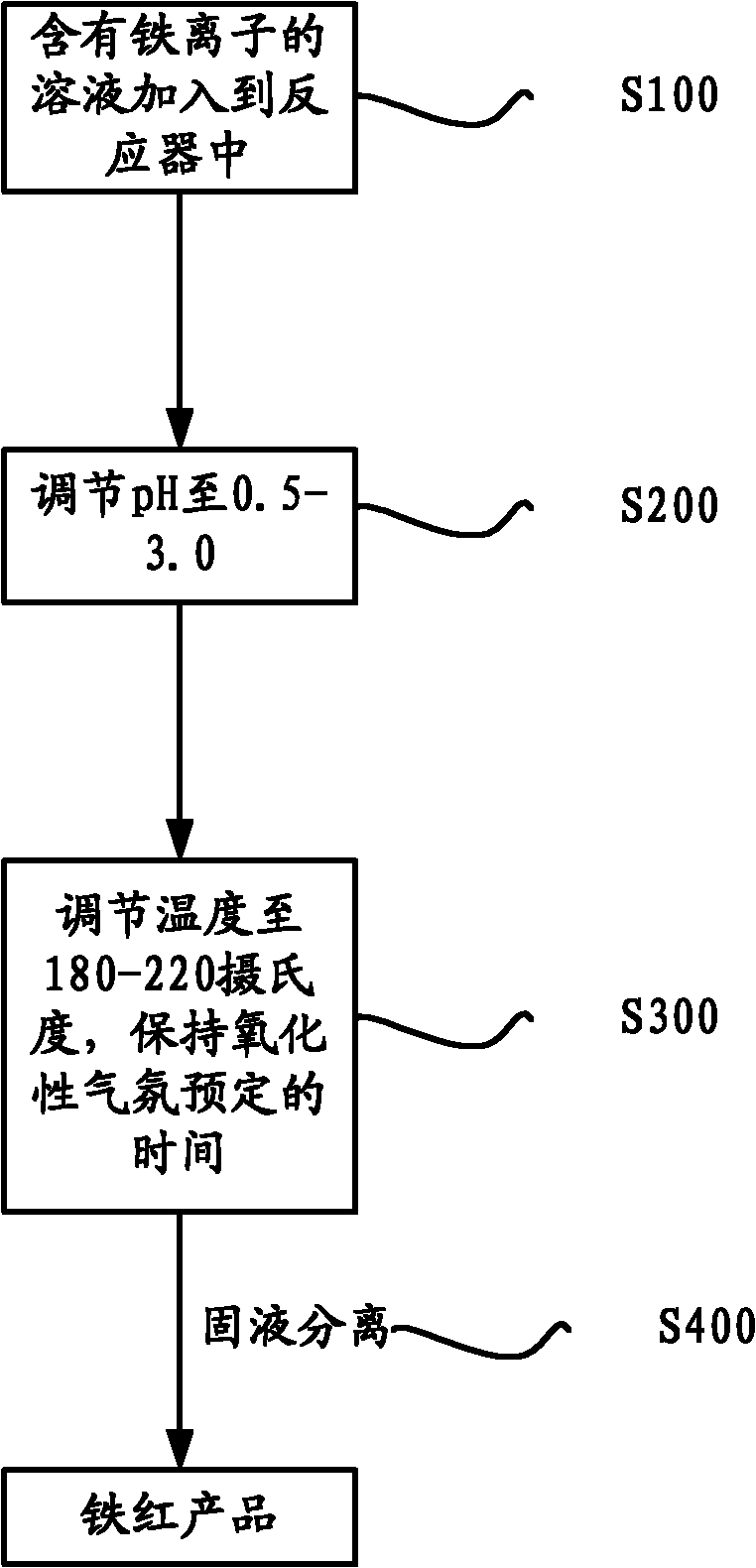
ActiveCN102153148AEfficient preparationSolve storage problemsFerric oxidesFerric ionIron oxide cycle
The invention provides a method for preparing iron oxide red by utilizing a solution containing iron ions, wherein the iron ions are at least one kind of ferrous ions and ferric ions. The method comprises the following steps of: (1) adding the solution containing iron ions into a reactor; (2) adjusting the pH value of the solution to be 0.5-3.0; (3) adjusting the temperature in the reactor to be 180-220 DEG C, maintaining the temperature for a predetermined time at oxidizing atmosphere to obtain a solid-liquid mixture containing ferric oxides; and (4) carrying out solid liquid separation on the solid-liquid mixture to obtain an iron oxide red product. By utilizing the method, iron in the solution can be effectively recycled and the iron oxide red can be prepared.
Owner:CHINA ENFI ENGINEERING CORPORATION
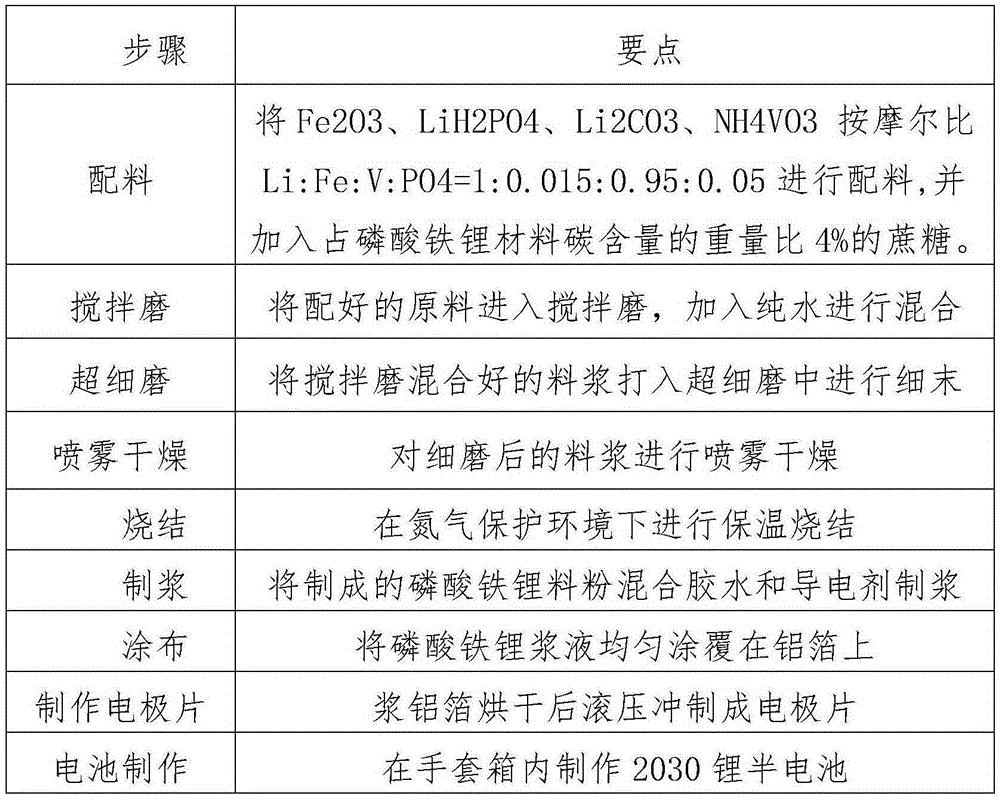
Method for preparing lithium iron phosphate positive electrode material by taking pickling iron oxide red as raw material
ActiveCN110021747AReduce manufacturing costGood effectCell electrodesSecondary cellsLithium iron phosphateMixed materials
The invention provides a method for preparing a lithium iron phosphate positive electrode material by taking pickling iron oxide red as a raw material. The method comprises the steps of (1) mixing a lithium source, an iron source, a phosphate source and a carbon source to obtain a mixed material, wherein the iron source comprises the pickling iron oxide red; and (2) sintering the mixed material the step (1) under a protection gas to obtain a carbon-coated lithium iron phosphate positive electrode material. The product pickling iron oxide red is recycled by utilizing a pickling waste liquid andis used as the iron source, and the carbon-coated LiFePO4 positive electrode material is prepared by a carbon thermal reduction method; and meanwhile, the electrochemical performance of the lithium iron phosphate positive electrode material is improved by metal and non-metal ion doping on a product, the production cost of the carbon-coated LiFePO4 positive electrode material is reduced, and larger utilization space can be brought for industrial production. Moreover, the method is simple in preparation process and high in controllability and is suitable for industrial production.
Owner:东北大学秦皇岛分校
Method for preparing iron oxide red from pyrite roasting residues by using hydrothermal method
The invention discloses a method for preparing iron oxide red from pyrite roasting residues by using a hydrothermal method. The method comprises the following steps: adding a sulfuric acid solution into a reaction device, slowly adding the pyrite roasting residues, reacting at the reaction temperature of 110-120 DEG C for 3-5 hours, and filtering the ore pulp, thereby obtaining an acid leaching solution; diluting, regulating the ferrous concentration by using hydrogen peroxide, so that the molar ratio of Fe<2+> to Fe<3+> in the solution is 0.1-0.15, adding stronger ammonia water to regulate the pH value to be 8-10, and fully stirring, thereby obtaining Fe(OH)3 and Fe(OH)2 colloid of the precursor; transferring the precursor into a high pressure kettle, carrying out a hydrothermal reaction, and washing, filtering, drying and grinding the reaction products, thereby obtaining the iron oxide red product. According to the method disclosed by the invention, the iron oxide red product of which various quality indexes meet the first grade requirements in the national standard can be obtained, the particles refer to pseudo cubes of uniform size, and industrial high-quality ammonium sulfate can be obtained from the filtrate of the hydrothermal reaction products.
Owner:CHANGSHU ROCKWOOD PIGMENT
Stable nitrogen-doped carbon nanotube and iron oxide composite anode material and preparation method thereof
InactiveCN105375009AImproved magnification performanceImprove efficiencyCell electrodesSecondary cellsIron(II) oxideNew energy
The invention relates to the field of new energy materials and electrochemistry, in particular to a stable nitrogen-doped carbon nanotube and iron oxide composite anode material and a preparation method thereof. With a nitrogen-doped carbon nanotube as a main body, the stable nitrogen-doped carbon nanotube and iron oxide composite anode material is characterized in that iron oxide particles are loaded on the outer surface of the nitrogen-doped carbon nanotube, wherein the iron oxide is a main component; and the load amount of the iron oxide accounts for 10%-90% of total weight of the iron oxide particles and the nitrogen-doped carbon nanotube. The stable nitrogen-doped carbon nanotube and iron oxide composite anode material is simple in preparation technology, low in production cost, friendly to environment, high in safety and good in experimental repeatability.
Owner:SHANDONG YUHUANG NEW ENERGY TECH +1
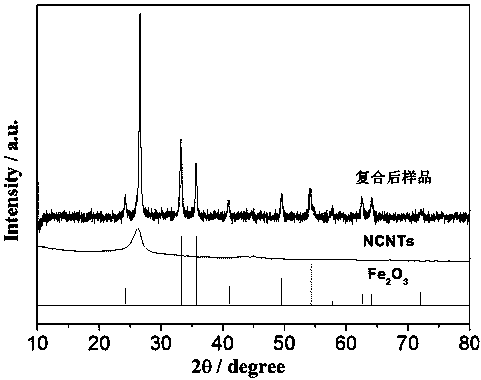
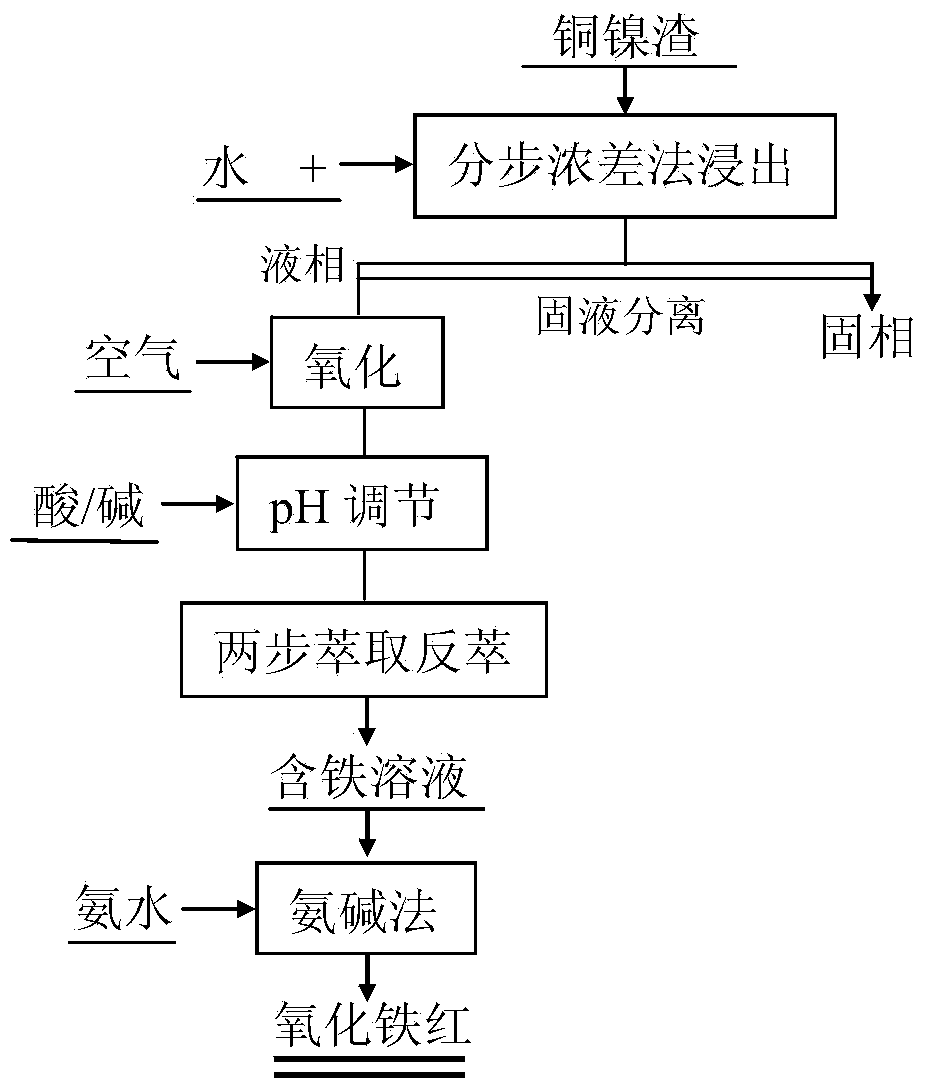
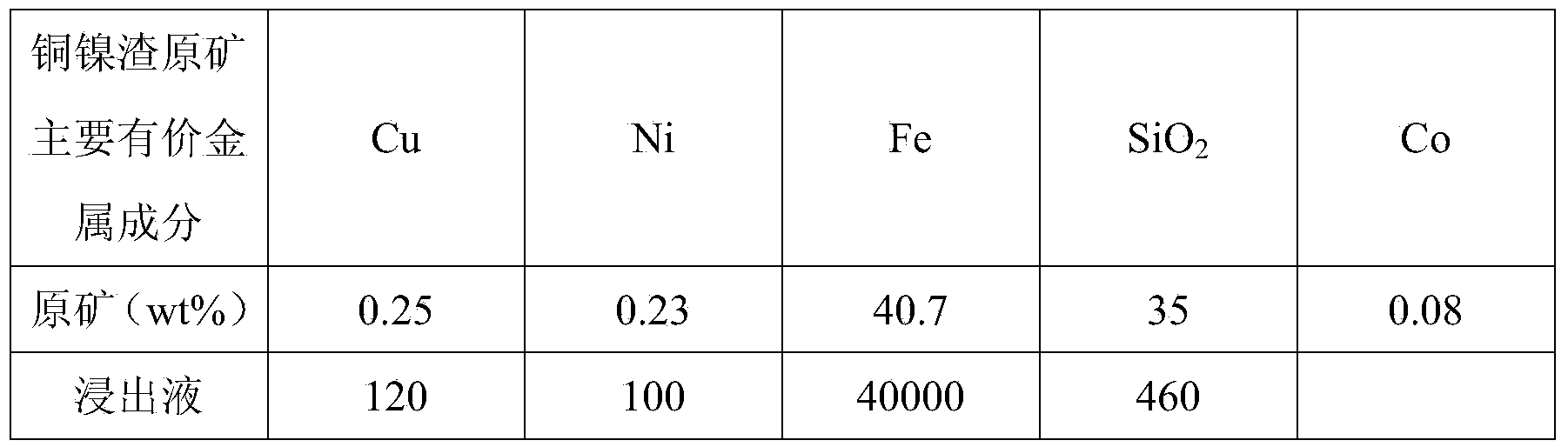
Method for preparing iron oxide red by using copper nickel slag
InactiveCN103834814AHigh purityDissolution inhibitionFerric oxidesProcess efficiency improvementFerric hydroxideSlag
The invention discloses a method for preparing iron oxide red by using copper nickel slag. The method comprises the steps: sequentially performing step concentration difference leaching by using copper nickel slag as a raw material, oxidizing a leaching solution, adjusting a pH value, extracting and reversely extracting and purifying, obtaining a ferric hydroxide precipitation by using an ammonia-alkali process, and calcining at a low temperature to prepare the iron oxide red. The method for preparing the iron oxide red with high purity by using the copper nickel slag has the characteristics of low cost, small energy consumption, environmental friendliness, and high recovering rate of iron in the copper nickel slag, and meets the industrialized production requirement.
Owner:斯莱登(北京)化工科技有限公司
Iron oxide red for lithium iron phosphate and preparation method and application thereof
The invention belongs to the technical field of new energy powder, and in particular, relates to a preparation method of iron oxide red for lithium iron phosphate. The preparation method comprises the steps: dispersing electronic-grade industrial ultra-pure water and steel factory iron oxide red; sieving the iron oxide red suspension with a sieve, and sieving to remove large-particle matters; grinding the sieved iron oxide red suspension with a horizontal sand mill; carrying out magnetic filtration; adding an iron-based compound; treating the mixed solution with a material water separation device to obtain an iron oxide red slurry; carrying out spray drying; mixing and dispersing with electronic-grade industrial ultra-pure water, treating with the material water separation device, carrying out spray drying, and thus obtaining the iron oxide red for lithium iron phosphate. Compared with a process for preparing iron oxide red for lithium iron phosphate through a precipitation method, the preparation method has the advantages of low environmental effect, high material obtaining rate, good dispersion, narrower particle size distribution, and higher specific surface area; and compared with a conventional Ruthner method for iron oxide red for lithium iron phosphate, the method has the advantage that the performance has relatively large improvement.
Owner:SHANGHAI BAOSTEEL MAGNETICS
Mildew-proof wood plastic material and production method thereof
The intention discloses a mildew-proof wood plastic material which contains 50-60 parts of recycled high-density polyethylene (HDPE) plastics, 130-140 parts of wood powder, 20-30 parts of light calcium carbonate, 6-8 parts of coupling reagents, 1-3 parts of zinc stearate, 1-3 parts of ethylene bis stearamide (EBS), 1-3 parts of paraffins, 0.4-0.6 part of ultraviolet (UV) absorber UV-531, 0.4-0.6 part of antioxidant 1010, 3-4 parts of iron oxide red 110B, 0.7-0.9 part of iron oxide brown 66 and 0.6-0.8 part of carbon black 3113 according to weight. Moisture content of the wood powder is no more than 3%, and fineness of the wood powder is 60-80 meshes. A production method of the mildew-proof wood plastic material includes the following steps: (1) premix preparing; (2) semi-finished product pelleting material preparing and (3) extrusion molding. According to the mildew-proof wood plastic material and the production method thereof, the obtained mildew-proof wood plastic material cannot mildew when meeting water or being used in severe environments such as the humid environment for a long time.
Owner:桐乡市大毅塑木有限公司
Process for synthesizing iron oxide brown
The invention discloses a process for synthesizing iron oxide brown, comprising the following steps of: feeding a ferrous sulfate solution into a reaction kettle; slowly adding caustic soda liquid in three times; then heating to a temperature in the reaction kettle of 75-78 DEG C and incubating for 50-70 min; adding water to dilute mixed slurry until solid content of the mixed slurry is 8-10%; slowly adding sodium nitrite into the reaction kettle and introducing oxygen simultaneously; then turning on spraying equipment to sufficiently mix the slurry, oxygen gas and NO which is generated in a reaction process; continuously sampling and comparing a sample with a standard sample in the reaction process; stopping the reaction when the colored light of a product is similar to the yellowish-brown or reddish brown colored light of standard sample; and carrying out plate pressure filtration, washing, drying and crashing the taken-out slurry so as to obtain the product. The iron oxide brown produced by the invention includes brownish red iron oxide brown and brownish yellow iron oxide brown. Compared with the traditional method for preparing the iron oxide brown by physically mixing iron oxide red, iron oxide black and iron oxide yellow, the iron oxide brown produced by the invention is stable in quality and pure in colored light, the synthesis method is simple and the operation is convenient.
Owner:ANHUI MINGZHU PIGMENT TECH
Contrast agent for photoacoustic imaging and photoacoustic imaging method utilizing same
ActiveCN102573925AStrong photoacoustic signalHigh antigen binding performanceEnergy modified materialsSolution deliveryInfraredIron oxide nanoparticles
Owner:CANON KK
Preparation method for iron oxide red
The invention discloses a preparation method for iron oxide red. The preparation method comprises the following steps: extracting ferrous ions in waste hydrochloric acid pickling liquid to prepare ferrous sulfate; adding ammonia water into the ferrous sulfate for oxidation and neutralization reactions so as to obtain iron oxide red sediment; and carrying out separating and drying. The method has the following advantage: iron oxide red is prepared by using the waste hydrochloric acid pickling liquid, so the utilization rate of the waste hydrochloric acid pickling liquid is improved.
Owner:江苏江盛南节能科技有限公司
Novel magnesium oxysulfate sanding floor for light-weight steel building and preparation method thereof
InactiveCN105948690AUniform thicknessImprove machinabilitySynthetic resin layered productsGlass/slag layered productsGlass fiberFoaming agent
The invention relates to a building material and in particular relates to a sanding floor for a light-weight steel building and a preparation method thereof. The sanding floor comprises a bottom slurry layer, one layer of non-woven fabric, three layers of glass fiber fabrics, a medium material layer, three layers of glass fiber fabrics and a sanding layer in sequence from bottom to top and is shown in the accompany drawing in the description in detail. The bottom slurry layer is prepared from 100 parts of light burned magnesium oxide, 40 to 60 parts of magnesium sulfate heptahydrate, 50 to 70 parts of water, 20 to 60 parts of talcum powder, 6 to 15 parts of latex powder, 1 to 5 parts of modifying agent and 3 to 10 parts of any one of iron oxide red, iron oxide yellow, iron oxide green, iron oxide blue and carbon black. The medium material layer is composed of 100 parts of the light burned magnesium oxide, 40 to 80 parts of the magnesium sulfate heptahydrate, 60 to 100 parts of the water, 20 to 60 parts of sawdust, 4 to 40 parts of other fillers, 1 to 5 parts of modifying agent and 1 to 10 parts of any one of the iron oxide red, the iron oxide yellow, the iron oxide green, the iron oxide blue and the carbon black. The sanding layer is composed of 100 parts of the light burned magnesium oxide, 40 to 80 parts of the magnesium sulfate heptahydrate, 60 to 100 parts of the water, 20 to 60 parts of wood meal, 0.5 to 2 parts of de-foaming agent, 1 to 5 parts of modifying agent and 1 to 10 parts of any one of the iron oxide red, the iron oxide yellow, the iron oxide green, the iron oxide blue and the carbon black.
Owner:布鲁科技(常州)股份有限公司
Method for preparing iron oxide red and hydrochloric acid from hot galvanized waste acid
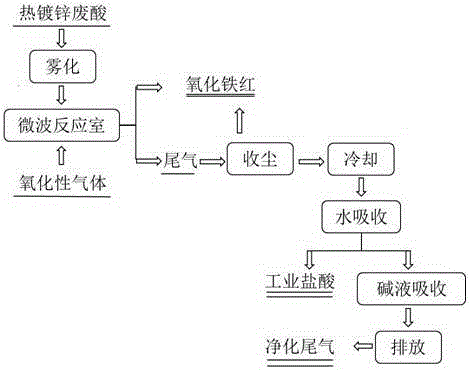
InactiveCN105271429AFast heatingShort reaction timeChlorine/hydrogen-chlorideFerric oxidesMicrowaveToxic industrial waste
The invention relates to a method for preparing iron oxide red and hydrochloric acid from hot galvanized waste acid and belongs to the technical field of resourceful treatment of industrial waste. Firstly, the hot galvanized waste acid is sprayed into a microwave reaction chamber through an atomizer at the atomization rate of 0.1-1.0 m<3> / h, then an oxidizing gas is introduced into the microwave reaction chamber from the bottom of the microwave reaction chamber at the flow rate of 50-300 m<3> / h and is pyrolyzed for 1-10 s at the temperature of 350 DEG C-850 DEG C, and iron oxide red particles and tail gas containing hydrochloric acid are prepared; the tail gas containing hydrochloric acid is subjected to dust collection, the iron oxide red particles are recovered, remaining tail gas is cooled and absorbed with water, industrial hydrochloric acid is obtained, and tail gas remaining after water absorption is absorbed by an alkaline solution and is exhausted after reaching an industrial waste gas exhaust standard. The method aims to solve the problem of difficulty in treatment of hot galvanized waste acid, and iron oxide red with high purity and small particle size is prepared.
Owner:KUNMING UNIV OF SCI & TECH
Method for preparing high-purity iron powder through iron oxide red
InactiveCN107745132AReduced iron powder with low silicon contentReduce energy consumptionApparent densityIron powder
The invention discloses a method for preparing high-purity iron powder through iron oxide red and belongs to the technical field of powder metallurgy production. The problems that as for reduced ironpowder production in an existing powder metallurgy technology, the technological process is long, operation is difficult in the high-temperature environment, the cost is high, and energy consumption is high are solved. The method comprises the steps of raw material preparation, oxidizing roasting, material cooling, material reduction, smashing and grading. When the iron oxide red is used for preparing the high-purity iron powder through the method, after the iron oxide red obtained by pickling of a carbon steel thin plate is subjected to oxidizing roasting pretreatment, the iron oxide red withthe iron grade being 60%-70% is reduced, reduced iron powder with the iron grade being 98% or above can be obtained, and the yield of iron powder reduction operation reaches 65% or above; according to the reduced iron powder, the silicon content is less than 0.15%, the carbon content is less than 0.02%, the apparent density is 2-3 g / cm<3>, and the flowability is 25-34 s / 50g. According to the method, the conditions are simple, energy consumption is low, the physical-chemical performance and metallurgical performance of the iron powder are good, and the reduction technological process is short.
Owner:JIUQUAN IRON & STEEL GRP +1
Method for degradation of high salinity organic wastewater and synchronous preparation of iron oxide
InactiveCN107381863AExtraordinary recovery abilityWater treatment parameter controlWater treatment compoundsWastewaterSalinity
Relating to the technical field of wastewater treatment, the invention in particular discloses a method for degradation of high salinity organic wastewater and synchronous preparation of iron oxide. The method at least includes the steps of: firstly adjusting the pH value of the high salinity organic wastewater to 4.0-6.5, adding an iron oxide catalyst in advance, then adding an oxidizing agent and Fe<2+> for reaction, and controlling the pH value at 4.0-6.5 in the reaction process; and conducting filtration at the end of reaction, adding water into a filter cake for dispersion, then adding Fe<2+>, and carrying out heating reaction to generate iron oxide red or iron oxide yellow. According to the invention, the catalyst iron oxide is added in advance to induce reaction, then Fe<2+> and the oxidizing agent are added simultaneously for in-situ generation of a lot of nano-iron oxide catalyst, the .OH generation efficiency and organic matter degradation speed are improved, the TOC removal rate is 80% or above, the degradation effect is excellent, and at the end of degradation, a pigment grade iron oxide product can be obtained, thus achieving waste utilization.
Owner:HEBEI NORMAL UNIV
Method for preparing iron oxide red from jarosite slag
ActiveCN105366728AImprove leaching rateHigh purityFerric oxidesProcess efficiency improvementVulcanizationSlag
The invention discloses a method for preparing iron oxide red from jarosite slag. The method includes the steps of leaching of jarosite slag through hot acid, impurity removing of leaching liquid and direct preparing of iron oxide red through a hydrothermal method. In the process of leaching of jarosite slag through hot acid, leaching is conducted for 1-4 hours at the temperature of 60-100 DEG C, and hot acid leaching liquid is obtained; in the process of impurity removing of leaching liquid, copper is removed through iron powder replacement, lead, cadmium and arsenic are removed through vulcanization, H<2>O<2> is oxidized, and purified liquid is obtained; in the process of direct preparing of iron oxide red through the hydrothermal method, an additive is added to the purified liquid, a reaction is conducted for 0.5-3 hours at the temperature of 120-150 DEG C, and a product of the iron oxide red is obtained. In the iron oxide red preparation process, the iron precipitation rate is higher than 93%, and the purity of the iron oxide red is higher than 97%. The method is simple in technological process, and efficient recovery of iron from the jarosite slag is achieved to the maximum degree.
Owner:CENT SOUTH UNIV
Method for producing iron oxide red by using concussive sludge
ActiveCN103449532ACause harmOvercome operational complexitySludge treatmentFerric oxidesSludgeFree cooling
The invention discloses a method for producing iron oxide red by using concussive sludge. The method comprises the following steps of: preparing a blank piece into concussive sludge through a concussion process, heating the concussive sludge to remove moisture out of the concussive sludge; electrically heating the treated concussive sludge into an un-sealed container to remove organic substances out of the concussive sludge; and heating the treated concussive sludge in the un-sealed container, and then naturally cooling to obtain an iron oxide red product with purity of over 90%. Compared with many methods for producing the iron oxide red, having the problems of complicated operation and high raw material cost, the method provided by the invention has the beneficial effect that waste substances including raw edges, burrs and the like in blank piece processing are effectively utilized, the steps of the method are simple, and the iron oxide red can be obtained through effective treatment.
Owner:郑培学
Method for preparing iron oxide red from coal ash
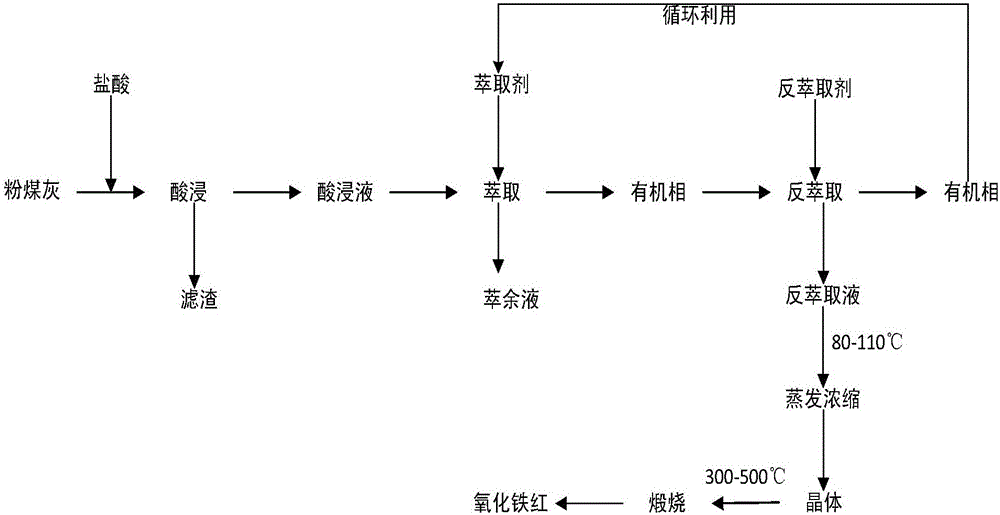
ActiveCN105819517AIncrease profitImprove qualityIron compounds preparationFerric oxidesEvaporationDigestion
The invention belongs to the field of environmental chemistry and discloses a method for preparing iron oxide red from coal ash .The method comprises the steps of 1, acid leaching, wherein digestion is conducted on coal ash with acid solution to obtain acid leaching solution; 2, extraction, wherein the acid leaching solution is extracted with an extracted organic phase to obtain extract liquor; 3, back extraction, wherein back extraction is conducted on the extract liquor with a back extractant to obtain back extraction solution; 4, evaporation and concentration, wherein the back extraction solution is evaporated, concentrated and crystallized; 5, calcination, wherein crystals are calcined to obtain iron oxide red .According to the method, iron oxide red is prepared with coal ash as the raw material, recycling application of coal ash waste is achieved, the utilization rate of valuable metal in coal ash is increased, and the environment problem is solved; an adopted extraction agent can be recycled, so that industrial production cost is reduced; the quality of prepared iron oxide red is high, and purity is 95% or more and meets the GB1863-2008 product technology requirement.
Owner:JINAN UNIVERSITY
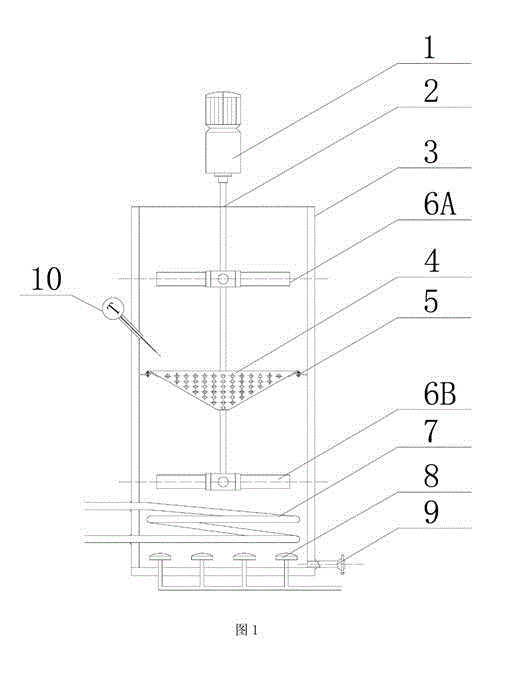
Reactor for preparing iron oxide red
The present invention relates to a reactor for preparing iron oxide red. The reactor comprises a reaction tank, a variable-frequency motor, a main shaft, stirring paddles, a heating coil pipe, aeration disks and a conical air distribution plate, wherein the top portion of the reaction tank is provided with the variable-frequency motor and the main shaft, the main shaft is provided with two sets of the stirring paddles, the conical air distribution plate is arranged between the two sets of the stirring paddles and on the inner wall of the reaction tank, and the bottom portion of the reaction tank is provided with the heating coil pipe and the aeration disks. According to the present invention, the frequency converter is utilized to control the variable-frequency motor so as to achieve different stirring rates and control the mixing uniformity of the liquid in the reaction tank; and the air required by the reaction is introduced into the aeration disks on the bottom portion, and rises to the conical air distribution plate so as to be secondarily and uniformly dispersed.
Owner:ZHENJIANG JINSHENGYUAN INFORMATION TECH
Vehicular disc brake rotor and manufacturing method of vehicular disc brake rotor
ActiveUS9541144B2Improve wear resistance and corrosion resistanceFriction characteristicBraking discsSolid state diffusion coatingFrictional coefficientNitrogen
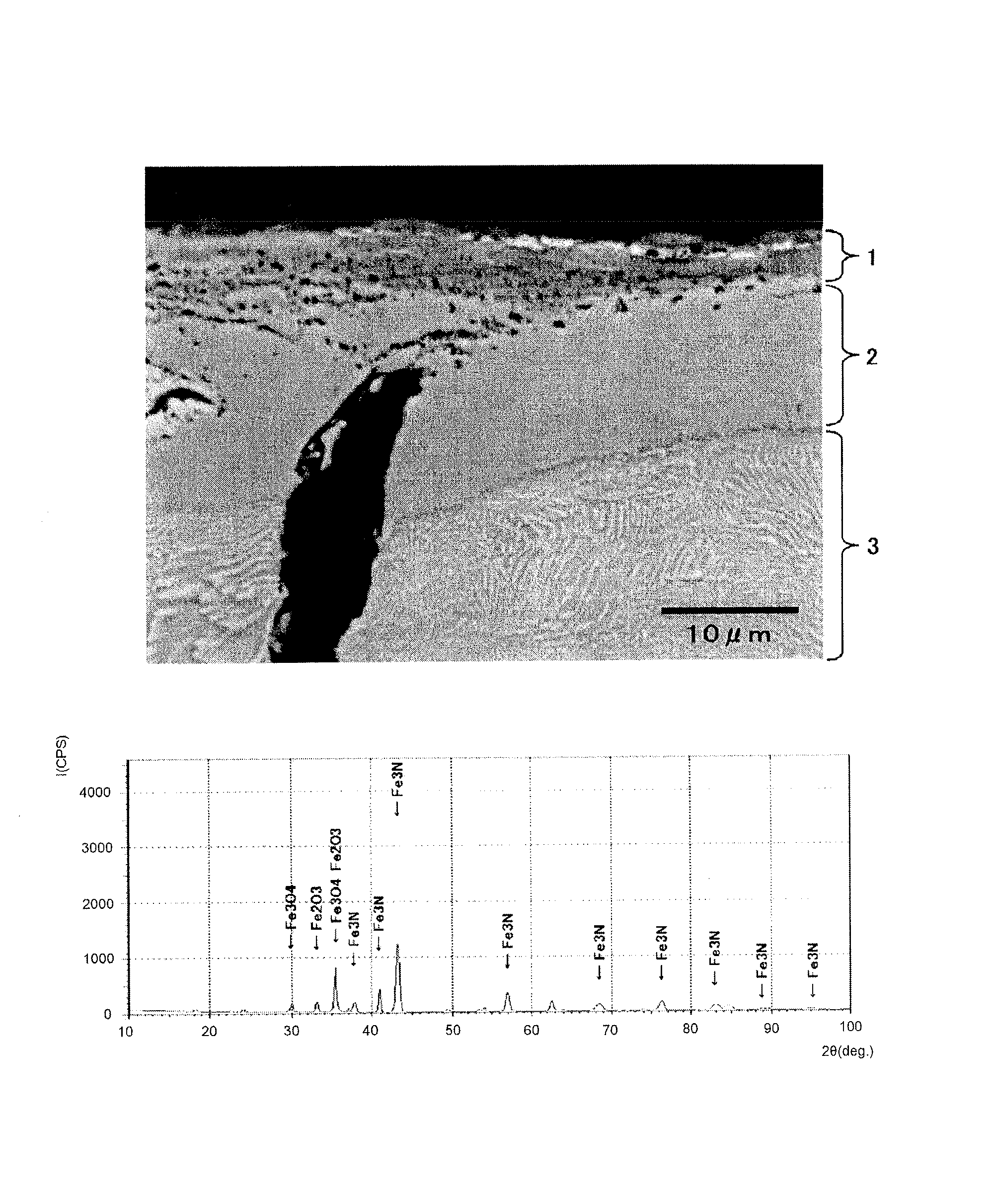
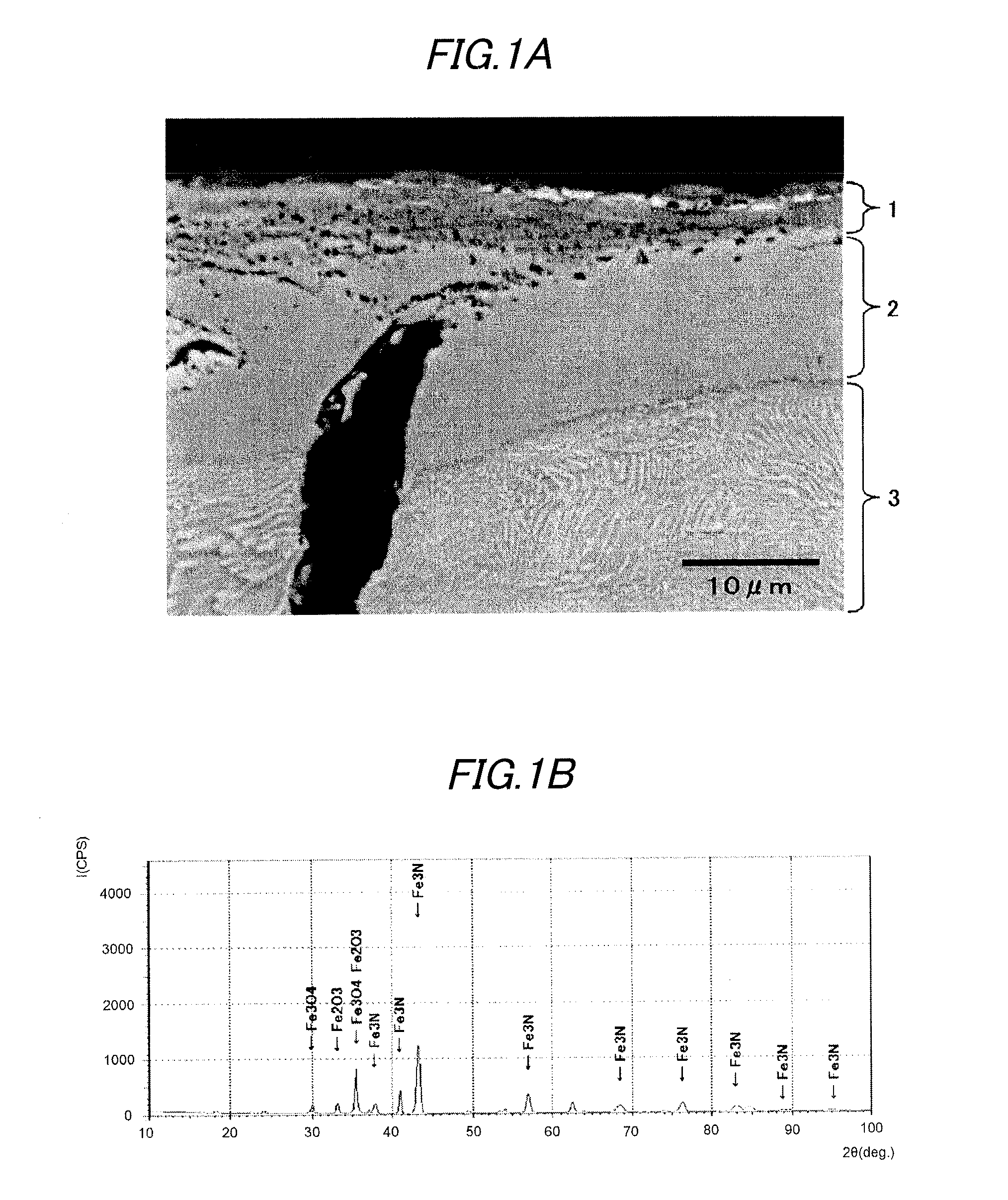
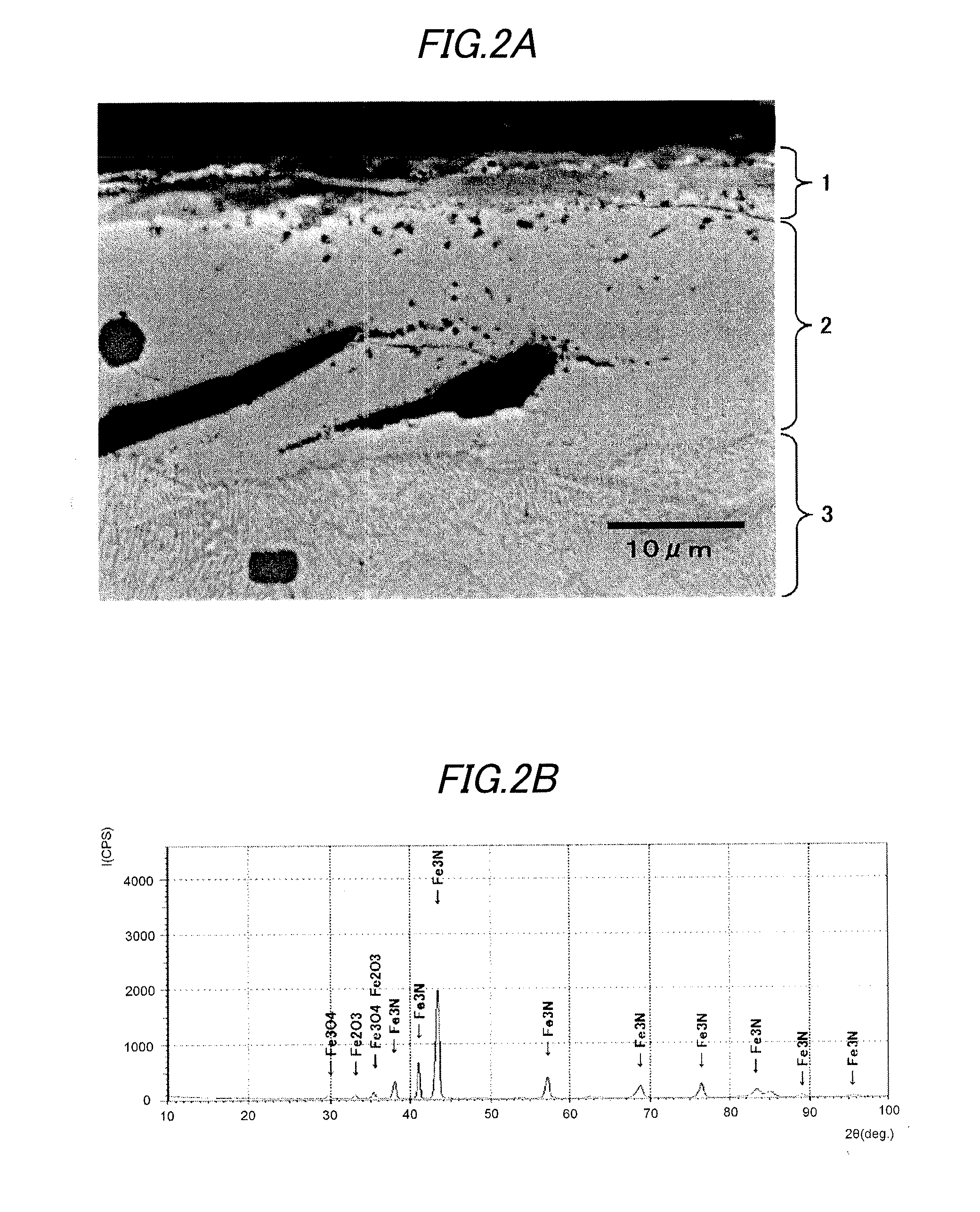
A vehicular disc brake rotor is provided with a cast-iron base, a nitrogen diffusion layer formed on the cast-iron base, a nitrogen compound layer formed on the nitrogen diffusion layer, and an iron oxide layer including Fe3O4 and formed on the nitrogen compound layer. In a burnish and a first re-burnish of a “Passenger car-Braking device-Dynamometer test procedures” (which is based on JASO C 406:2000), a change ratio of a frictional coefficient between the burnish and the first re-burnish is 10% or less.
Owner:AKEBONO BRAKE IND CO LTD
Method for preparing permanent magnet strontium ferrite by utilizing iron oxide red
InactiveCN105439208ASmall granularityUniform compositionIron compoundsStrontium carbonateGranularity
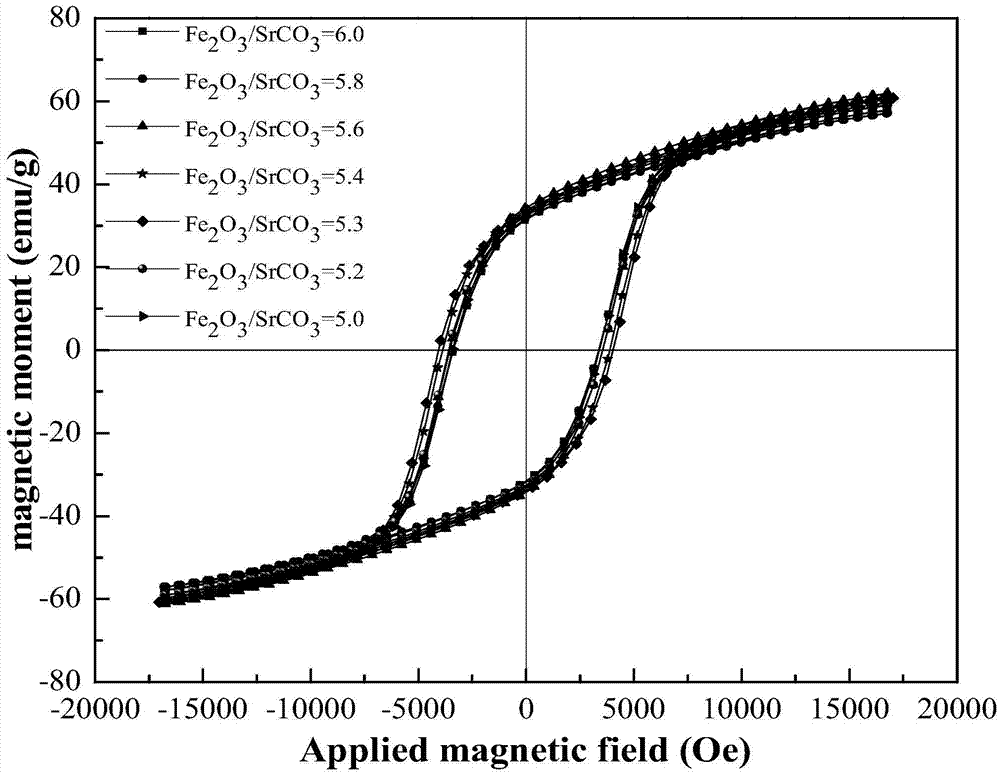
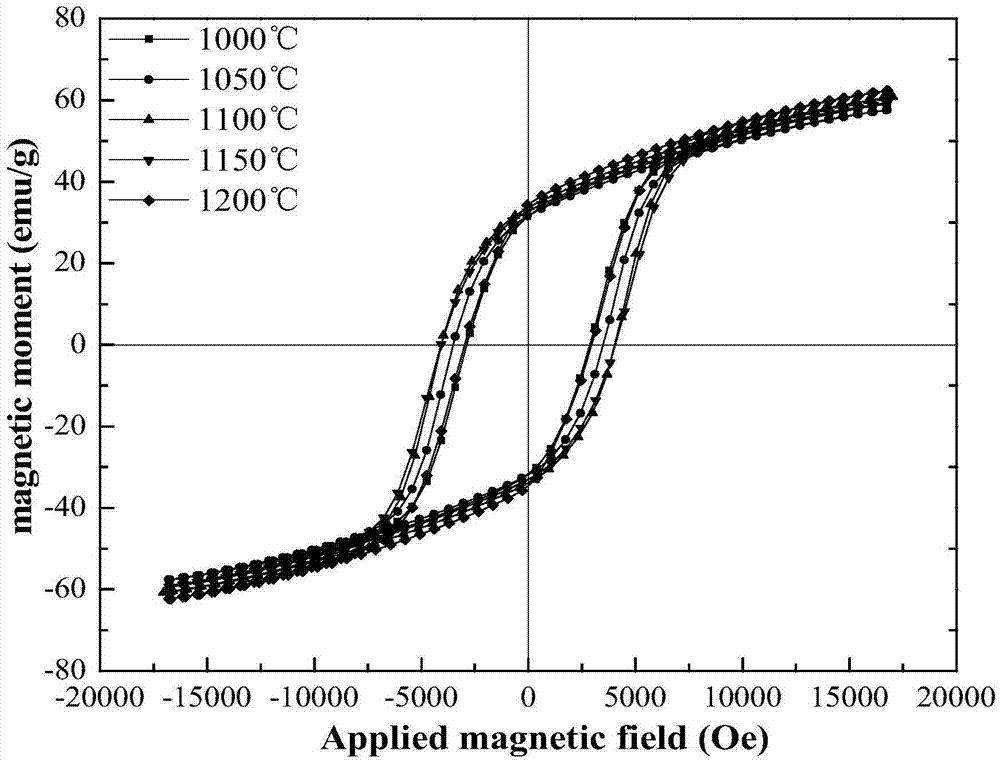
The invention relates to a method for preparing a permanent magnet strontium ferrite by utilizing iron oxide red and belongs to the fields of comprehensive utilization of resources and material synthesis. The method for preparing the permanent magnet strontium ferrite by utilizing the iron oxide red comprises the following steps: (1) mixing the iron oxide red obtained by rolling steel scale and strontium carbonate, and then carrying out ball milling; (2) mixing the material obtained through the ball milling with fused salt, and then calcining; and (3) cleaning the materials obtained after reaction, and drying, so that the permanent magnet strontium ferrite product is obtained. The method for preparing the permanent magnet strontium ferrite by utilizing the iron oxide red has the advantages that the synthetic permanent magnet strontium ferrite is small in granularity and uniform in composition, large-scale production can be realized, and the fused salt can be recycled; value-added exploration of a secondary resource, namely the iron oxide red obtained by rolling the steel scale, is realized, and the energy is saved; the prepared permanent magnet strontium ferrite is uniform in granularity distribution, and the grain size is 0.5-3mu m; and the coercive force value is 2687-4043Oe, the residual magnetism is 32.92-35.37emu / g, the saturated magnetic intensity is 59.57-62.29emu / g, and the maximum magnetic energy product is 0.42-0.61MG.Oe.
Owner:NORTHEASTERN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com