Method for preparing iron oxide red and hydrochloric acid from hot galvanized waste acid
A combined preparation technology of iron oxide red, which is applied in the field of industrial waste resource treatment, can solve the problems of complex process flow, large iron oxide particle size, and high production cost, and achieve simple process flow, short material reaction time, and fast reaction rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
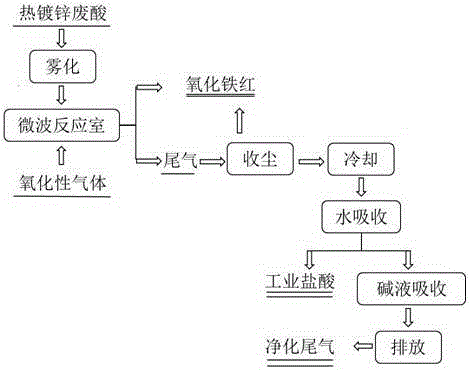
[0031] like figure 1 Shown, this hot-dip galvanizing waste acid is jointly prepared the method for iron oxide red and hydrochloric acid, and its concrete steps are as follows:
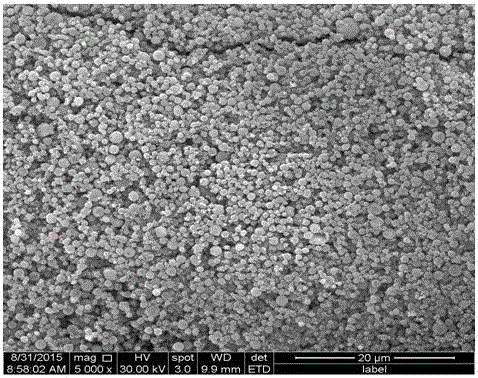
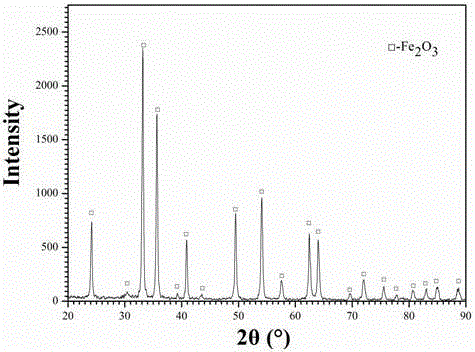
[0032] (1) First, hot-dip galvanizing waste acid (FeCl 2 40%, FeCl 3 0.1%, HCl0.2%, impurity content (calcium, magnesium, manganese salts, etc.) 0.05%, the balance is water) using an atomizer with an atomization rate of 0.25m 3 / h is sprayed into the microwave reaction chamber, and then the flow rate is 300m from the bottom of the microwave reaction chamber 3 / h of oxidizing gas (oxidizing gas is pure oxygen), pyrolysis at a temperature of 350°C for 10s to prepare iron oxide red particles and tail gas containing hydrochloric acid;
[0033] (2) The tail gas containing hydrochloric acid obtained in step (1) is dust-collected to recover iron oxide red particles, and the remaining tail gas is cooled and absorbed by water to obtain industrial hydrochloric acid, and the remaining tail gas after water abso...
Embodiment 2
[0036] like figure 1 Shown, this hot-dip galvanizing waste acid is jointly prepared the method for iron oxide red and hydrochloric acid, and its concrete steps are as follows:
[0037] (1) First, hot-dip galvanizing waste acid (FeCl 2 8%, FeCl 3 5%, HCl4%, impurity content (calcium, magnesium, manganese salts, etc.) 0.3%, the balance is water) using an atomizer with an atomization rate of 0.3m 3 / h is sprayed into the microwave reaction chamber, and then passed through the bottom of the microwave reaction chamber with a flow rate of 50m 3 / h of oxidizing gas (oxidizing gas is pure oxygen), pyrolyzed at 450°C for 8s to prepare iron oxide red particles and tail gas containing hydrochloric acid;
[0038] (2) The tail gas containing hydrochloric acid obtained in step (1) is dust-collected to recover iron oxide red particles, and the remaining tail gas is cooled and absorbed by water to obtain industrial hydrochloric acid, and the remaining tail gas after water absorption is pas...
Embodiment 3
[0041] like figure 1 Shown, this hot-dip galvanizing waste acid is jointly prepared the method for iron oxide red and hydrochloric acid, and its concrete steps are as follows:
[0042] (1) First, hot-dip galvanizing waste acid (FeCl 2 25%, FeCl 3 4%, HCl2%, impurity content (calcium, magnesium, manganese salts, etc.) 0.2%, the balance is water) using an atomizer with an atomization rate of 0.35m 3 / h sprayed into the microwave reaction chamber, and then passed through the bottom of the microwave reaction chamber with a flow rate of 100m 3 / h of oxidizing gas (oxidizing gas is pure oxygen), pyrolyzed for 6s at a temperature of 500°C to prepare iron oxide red particles and tail gas containing hydrochloric acid;
[0043](2) The tail gas containing hydrochloric acid obtained in step (1) is dust-collected to recover iron oxide red particles, and the remaining tail gas is cooled and absorbed by water to obtain industrial hydrochloric acid, and the remaining tail gas after water a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com