Method for preparing iron oxide red
A technology of iron red and iron ions, which is applied in the chemical industry, can solve the problems of slow dissolution of harmful components, occupying large land for storage, environmental pollution, etc., to solve the problem of iron slag storage, high iron removal efficiency, and environmental burden effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
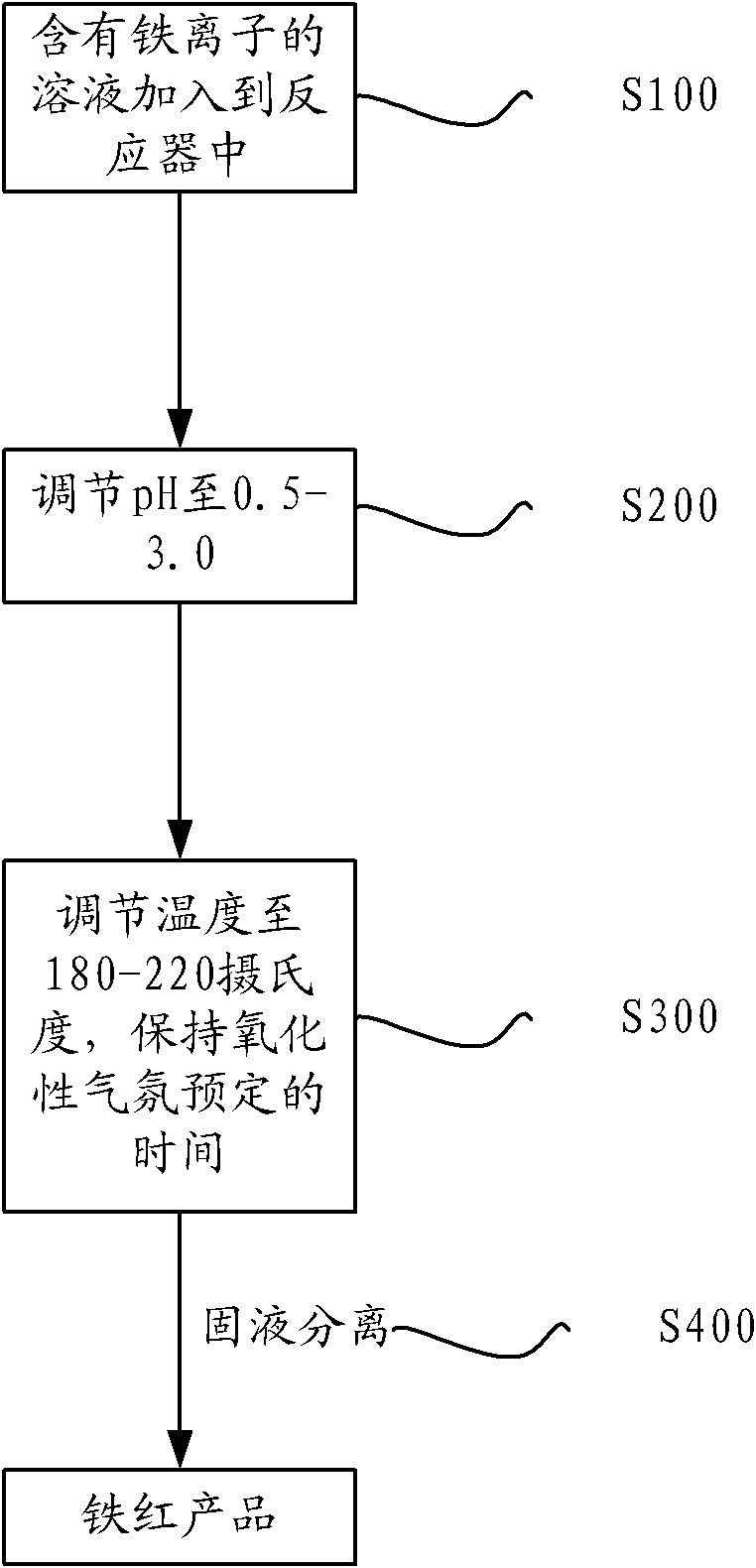
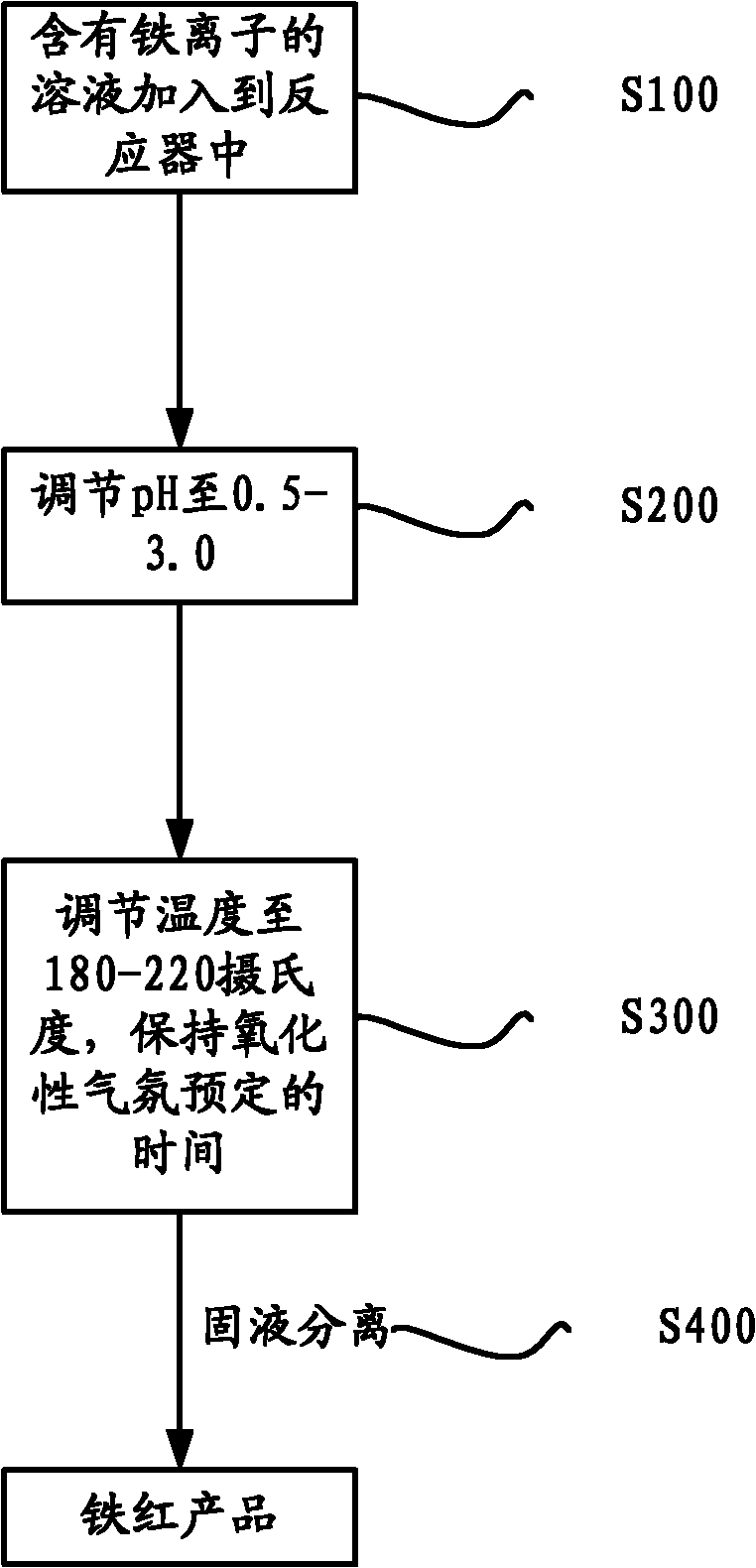
[0034] Take 1L of nickel laterite acid leaching solution, its iron content is 42g / L (98% of which is ferric iron). Adjust its pH to 2.1 with NaOH, add it to an autoclave, control the temperature at 200°C, oxygen partial pressure 0.2MPa, filter after reaction, wash with 3 times the amount of water slurry, and dry at 100°C.
[0035] After analysis, the iron precipitation rate is 96.6%, the iron oxide red content is >97%, and the Ni precipitation rate is <1.9%.
example 2
[0037] Take 1L of nickel laterite acid leaching solution, its iron content is 42g / L (98% of which is ferric iron). Adjust its pH to 2.3 with NaOH, add it to an autoclave, control the temperature at 210°C, cycle the seed crystal 4 times, oxygen partial pressure 0.2MPa, filter after reaction, wash with 3 times the amount of water slurry, and dry at 100°C.
[0038] After analysis, the iron precipitation rate is 98.0%, the iron oxide red>97%, and the Ni precipitation rate<1.2%.
example 3
[0040] Get 1L of solution before zinc ore iron deposit precipitation, its iron content 20g / L (wherein 98% is ferric iron). Adjust its pH to 2.0 with NaOH, add it to an autoclave, control the temperature at 190°C, cycle the seed crystal 4 times, oxygen partial pressure 0.2MPa, filter after reaction, wash with 3 times the amount of water slurry, and dry at 100°C.
[0041] After analysis, the iron precipitation rate is 95.4%, the iron oxide red>95%, and the Zn precipitation rate<1.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com