Method for preparing iron oxide particles having controllable morphology and size
A technology of iron oxide particles and size, applied in the field of preparation of iron oxide particles, can solve the problems of inability to realize the regulation of particle size and morphology, inability to regulate particle size and morphology, and no very successful examples, and achieve good results. The effect of biocompatibility, low production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Add 40mL of ethylene glycol into a 200mL beaker, start stirring, add 0.5g of dodecylbenzenesulfonic acid and 1.0g of polyvinyl alcohol (M.W.18000), and stir for 12h;
[0031] (2) Add 2.5g ferric chloride hexahydrate FeCl 3 ·6H 2 O and 0.35g sodium hydroxide, stirred for 4h;
[0032] (3) Transfer the above 40mL solution into a hydrothermal reaction kettle, and react at 190°C for 36h;
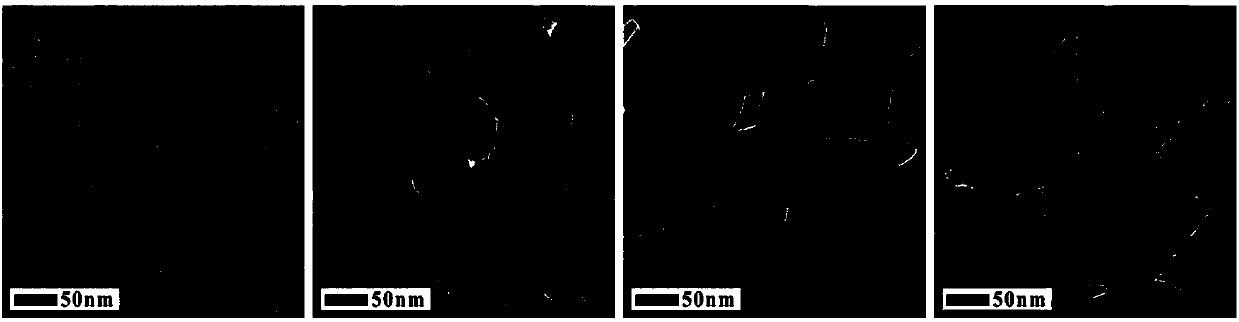
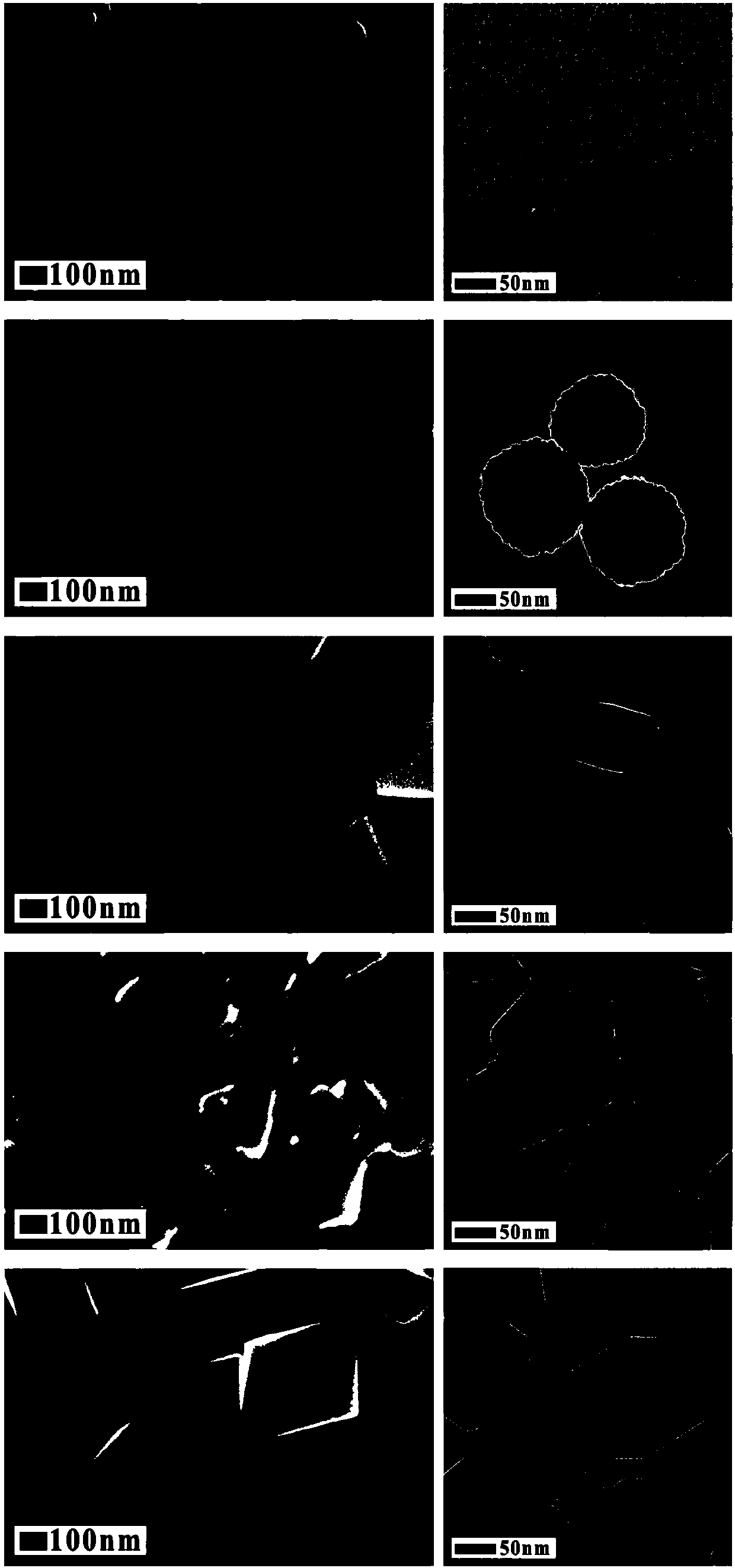
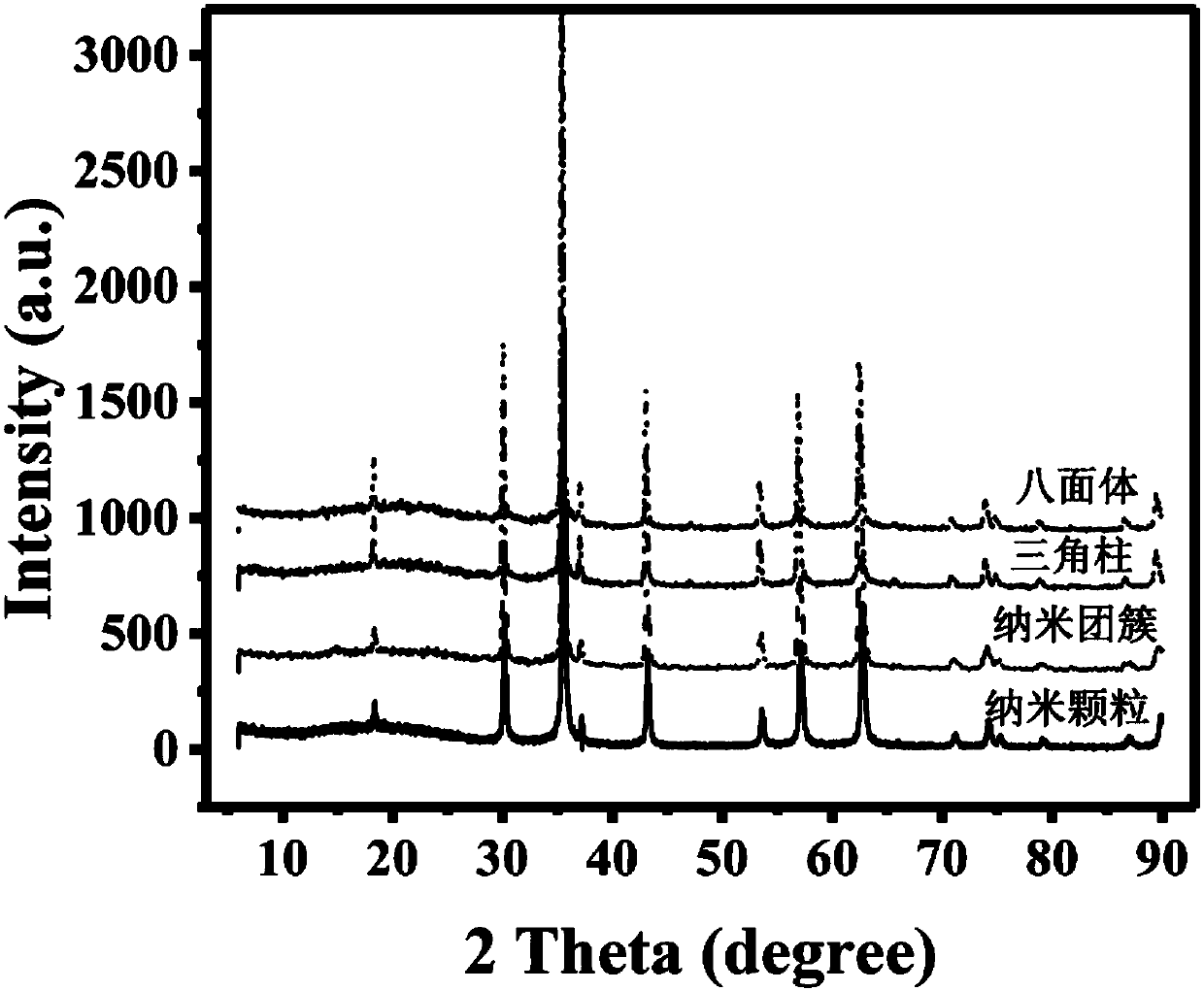
[0033] (4) After the reaction, the precipitate was separated by centrifugation, washed three times with ethanol and deionized water, and vacuum-dried at 50° C. to obtain magnetic ferric oxide nanoparticles. It can be seen from TEM characterization that the particle diameter is at ~ 20nm, the particle size distribution is uniform, and the monodispersity is good; the particle morphology is nanoparticle; XRD characterization shows that the particle is ferric oxide with high crystallinity; the product yield is ~ 87%.
Embodiment 2
[0035] Add 40mL of ethylene glycol into a 200mL beaker, start stirring, add 0.5g of dodecylbenzenesulfonic acid and 1.0g of polyvinyl alcohol (M.W.18000), and stir for 12h;
[0036] (2) Add 2.5g ferric chloride hexahydrate FeCl 3 ·6H 2 O and 1.35g sodium hydroxide, stirred for 4h;
[0037] (3) Transfer the above 40mL solution into a hydrothermal reaction kettle, and react at 190°C for 36h;
[0038] (4) After the reaction, the precipitate was separated by centrifugation, washed three times with ethanol and deionized water, and vacuum-dried at 50° C. to obtain magnetic ferric oxide nanoparticles. It can be seen from the TEM characterization that the particle diameter is at ~ 200nm, the particle size distribution is uniform, and the monodispersity is good; the particle morphology is a nano-cluster; the XRD characterization shows that the particle is ferric oxide with high crystallinity; the product yield is ~85%.
Embodiment 3
[0040] (1) Add 40mL of ethylene glycol and 20mL of diethylene glycol into a 200mL beaker, start stirring, add 0.5g of dodecylbenzenesulfonic acid and 1.0g of polyvinyl alcohol (M.W.18000), and stir for 12h;
[0041] (2) Add 2.5g ferric chloride hexahydrate FeCl 3 ·6H 2 O and 2.42g sodium hydroxide, stirred for 4h;
[0042] (3) Transfer the above 40mL solution into a hydrothermal reaction kettle, and react at 200°C for 36h;
[0043] (4) After the reaction, the precipitate was separated by centrifugation, washed three times with ethanol and deionized water, and vacuum-dried at 50° C. to obtain magnetic ferric oxide nanoparticles. The particle size distribution is uniform, the monodispersity is good, and the particle shape is regular octahedron; the product yield is ~88%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com