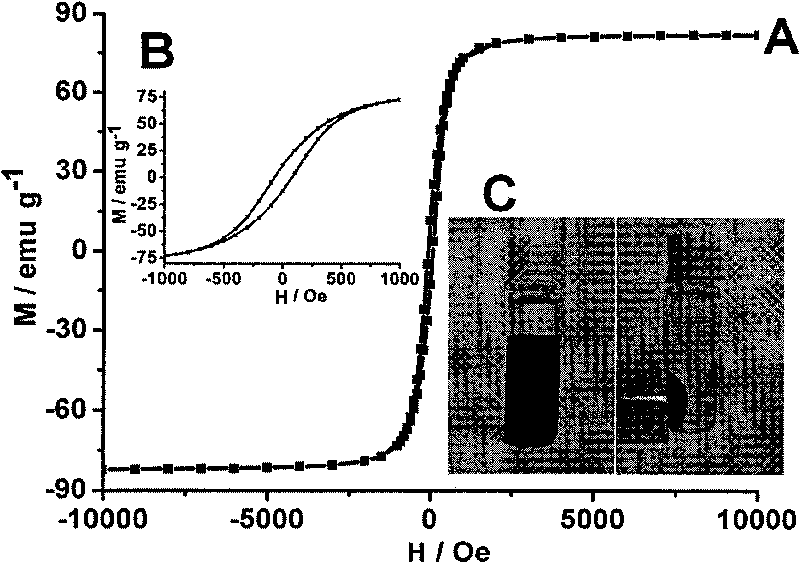
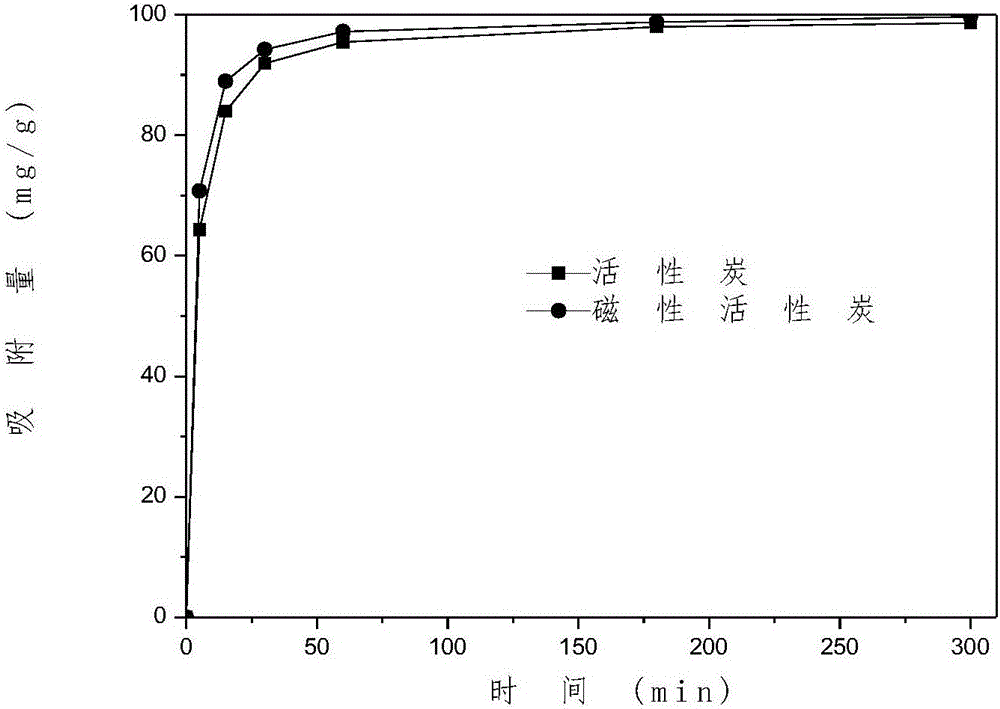
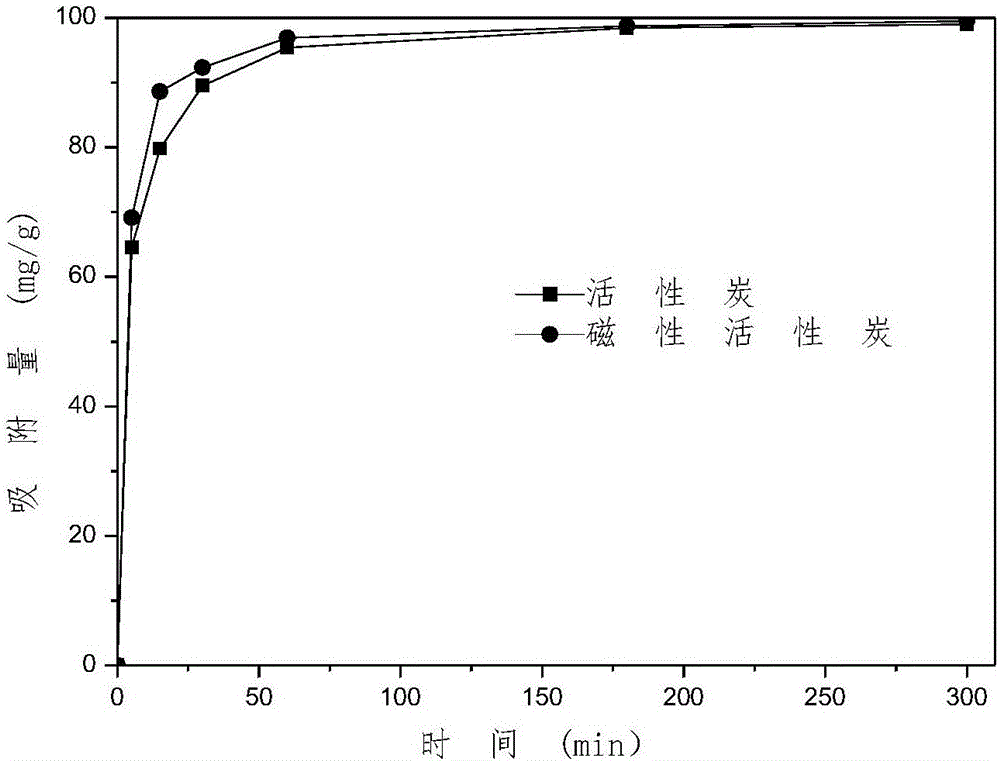
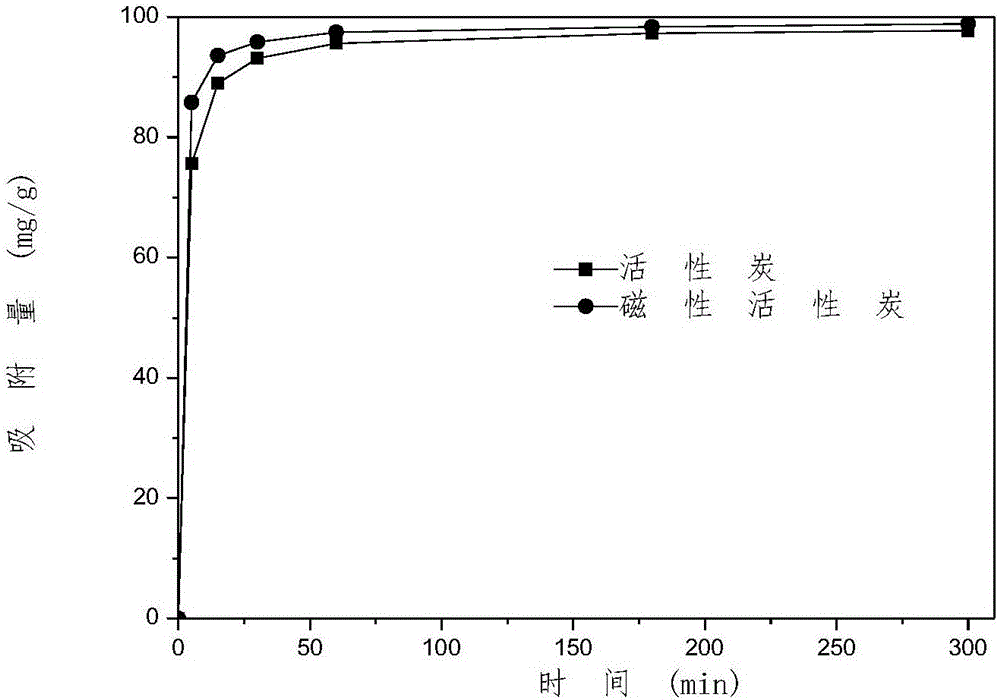
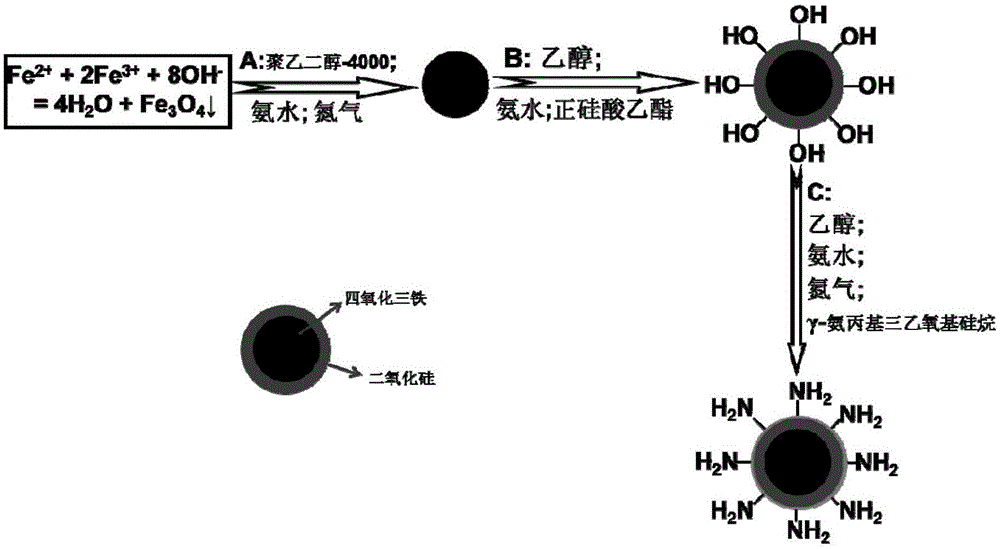
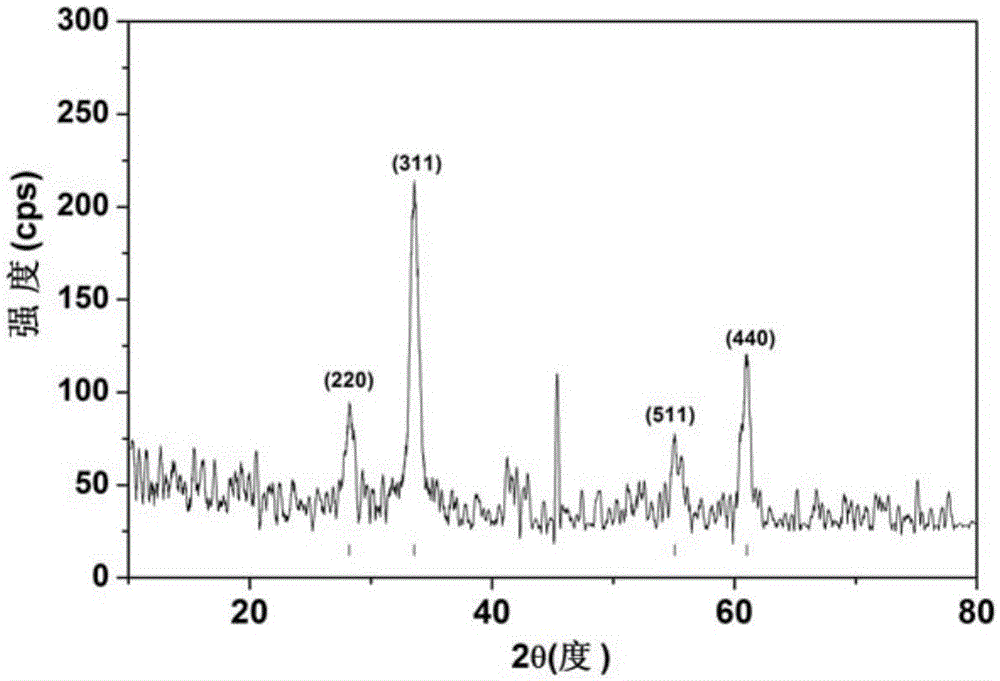
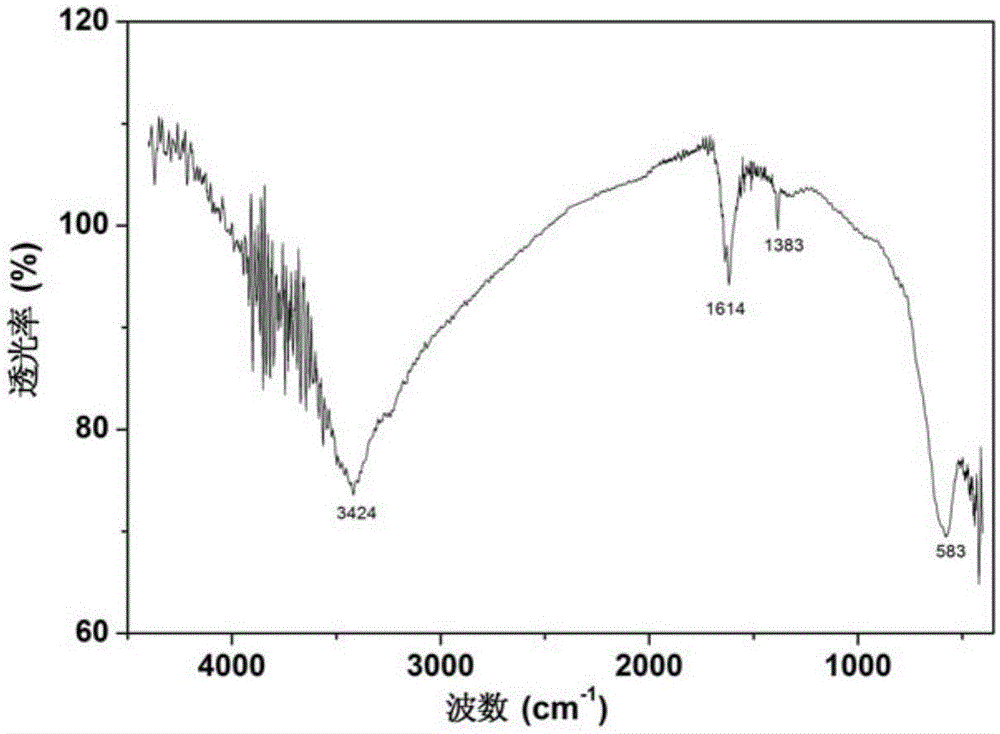
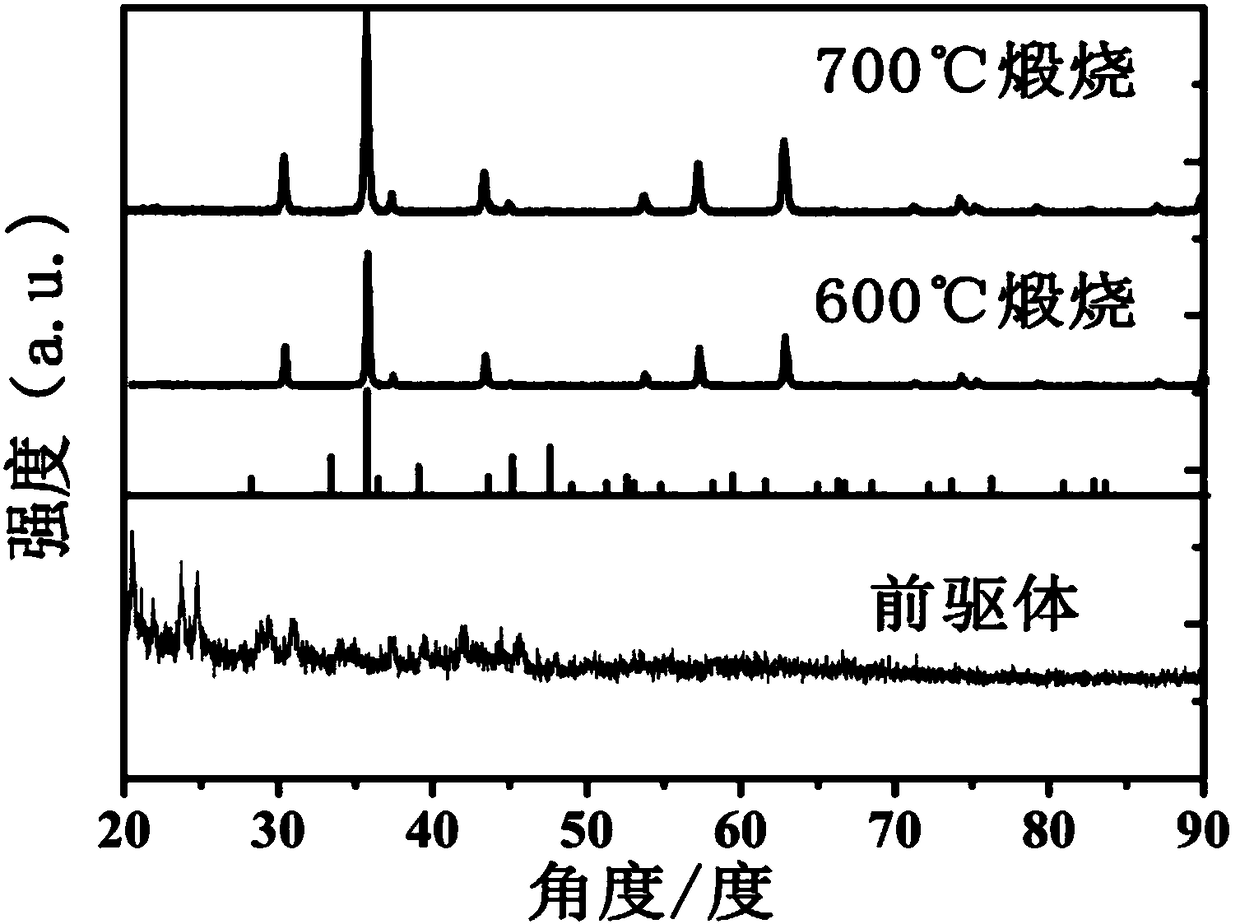
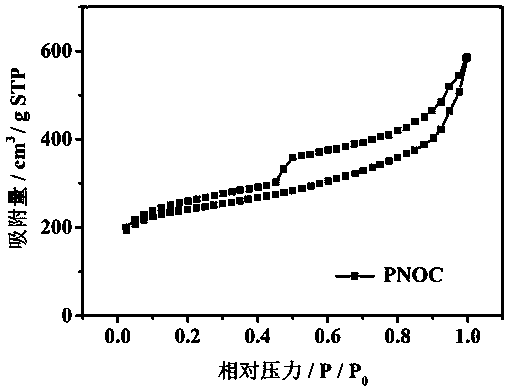
Patents
Literature
364 results about "Ferric chloride hexahydrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ferric chloride, hexahydrate 40600016 | 10025-77-1 Ferric chloride, hexahydrate, also known as Iron Chloride, is a chemical compound with a color that depends on the type of light used to view it.
Solder stripping agent and preparation method thereof
InactiveCN101962776AReduce pollutionSolution to short lifeConductive material chemical/electrolytical removalNitric oxideTin
The invention discloses a solder stripping agent, which is prepared from the following components: 500 to 650g / L of 70 percent nitric acid, 50 to 70g / L of hydrochloric acid, 4.3 to 8.6g / L of ferric nitrate or 2.9 to 5.8g / L of ferric trichloride or 4.8 to 9.7g / L of ferric chloride hexahydrate, 0.5 to 25g / L of organic acid complexing agent, 1 to 50g / L of water-soluble organic amine, 1 to 10g / L of organic corrosion inhibitor for copper, 1 to 2g / L of stabilizing agent, 1 to 5g / L of surfactant, and 0.1 to 1g / L of small molecular alcohol. The solder stripping agent can quickly strip solder or solder alloy layers thoroughly, basically cannot generate or generates little salt mist containing nitric oxides in the stripping process, and has the advantages of small silt amount, long service life of stripping solution, no strong corrosion on copper at a bottom layer, bright substrate and the like.
Owner:济南德锡科技有限公司
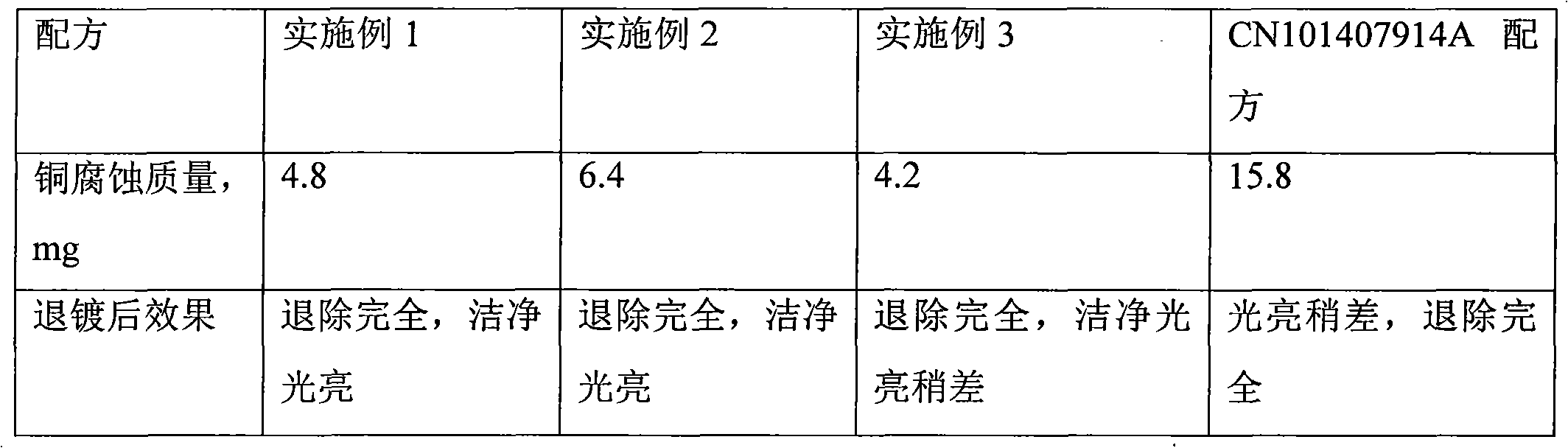
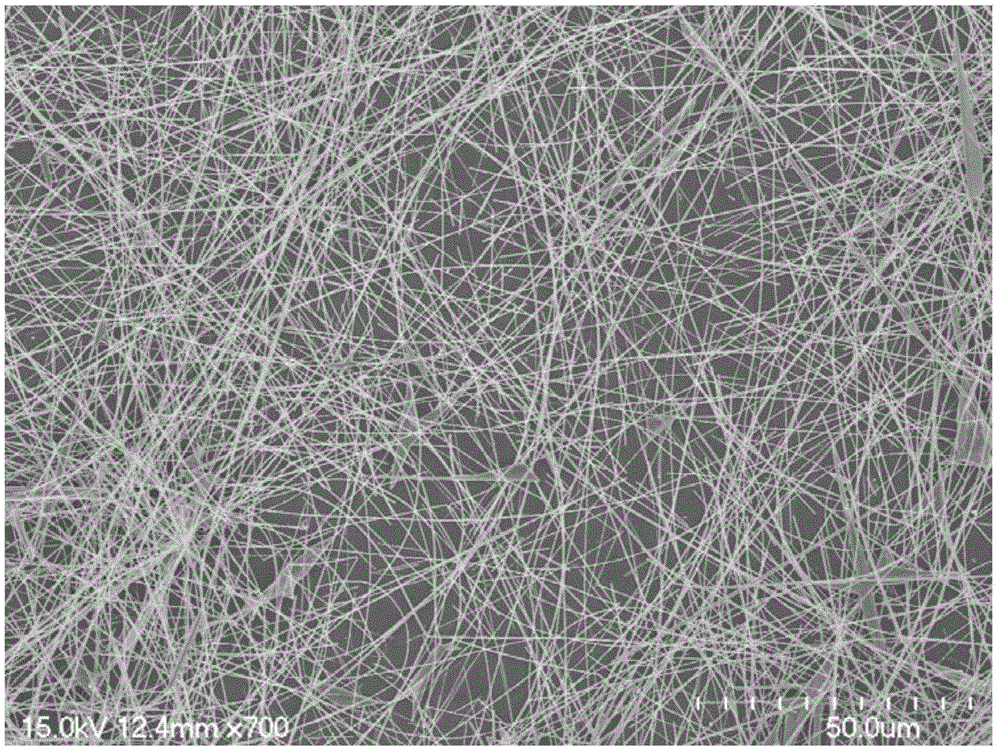
Silver nanowire preparation method
The invention relates to a silver nanowire preparation method which comprises the following steps: (a), taking polyvinylpyrrolidone, dissolving polyvinylpyrrolidone in ethylene glycol; (b), taking silver nitrate, and dissolving silver nitrate in ethylene glycol; (c), taking ferric chloride anhydrous and dissolving ferric chloride anhydrous in ethylene glycol; (d), mixing the silver nitrate-ethylene glycol solution with the polyvinylpyrrolidone-ethylene glycol solution to obtain a first mixed solution, wherein the mass ratio of polyvinylpyrrolidone to silver nitrate is 1-9 to 1; (e), respectively adding a ferric trichloride-ethylene glycol solution and an ionic liquid-ethylene glycol solution in the first mixed solution, and reacting for 2-24 hours at a temperature lower than the boiling temperature of ethylene glycol after stirring and mixing to obtain a shiny white mixed solution; (f), flushing silver nanowires obtained after solid-liquid separation with acetone, and dispersing the silver nanowires in water or absolute ethyl alcohol to obtain a nanowire dispersion liquid, wherein the step (a), the step (b) and the step (c) are not ordered. The average ratio of length and diameter of the silver nanowire is greater than 1,000.
Owner:SUZHOU UNIV
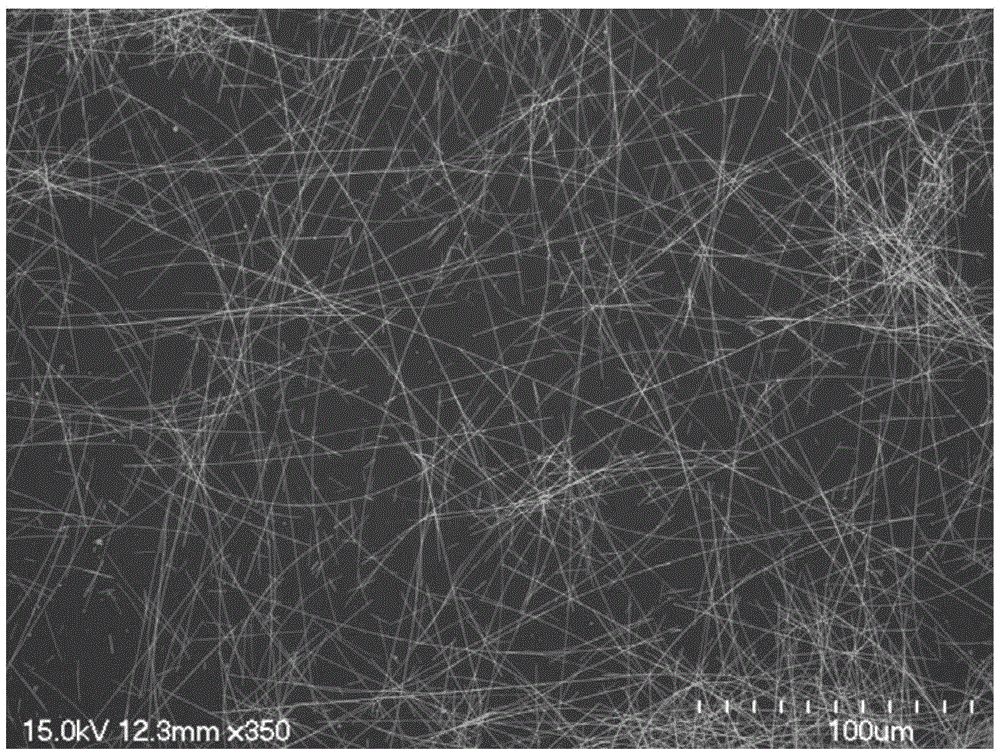
Fe2O3 micro-nano porous sphere, preparation method thereof and uses thereof
InactiveCN102616861ALarge specific surface areaImprove thermal stabilityWater/sewage treatment by irradiationOther chemical processesMicro nanoOrganic dye
The invention discloses a Fe2O3 micro-nano porous sphere, a preparation method thereof and uses thereof. The porous sphere is formed by Fe2O3 nanoparticles having a nanometer mesoporous alpha-Fe2O3 phase structure therebetween, wherein the diameter of the sphere is 500-5000nm, the specific surface area of the sphere is 15-25m<2> / g, particle sizes of the particles are 20-60nm, and pore diameters of mesopores are 2-50nm. The method comprises the following steps: mixing ferric chloride hexahydrate, ascorbic acid, urea and water, and uniformly stirring them to obtain a mixed liquid; reacting the mixed liquid under conditions that the temperature is 140-180DEG C and the pressure is a self-generated pressure for at least 4h to obtain an intermediate product; separating, washing and drying the intermediate product to obtain porous iron carbonate; and annealing the porous iron carbonate at 450-550DEG C for at least 4h, and naturally cooling the porous iron carbonate to room temperature to prepare the Fe2O3 micro-nano porous sphere. The Fe2O3 micro-nano porous sphere can be placed in water polluted by organic dyes to photocatalytically degrade under visible light, or in water polluted by potassium dichromate to adsorb.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Metal-organic frame-derived ferroferric oxide@carbon/reduced graphene oxide nano composite wave-absorbing material and preparation method thereof
ActiveCN110012656ASimple and fast operationNothing producedOther chemical processesMagnetic/electric field screeningEnvironmental resistanceN dimethylformamide
The invention discloses a ferroferric oxide@carbon / reduced graphene oxide nano composite wave-absorbing material and a preparation method thereof. By using graphene oxide as a template, ferric chloride hexahydrate as a metal salt, terephthalic acid as an organic ligand, and N,N-dimethylformamide as a solvent, the ferroferric oxide@carbon / reduced graphene oxide nano composite material is prepared by a solvothermal-high temperature pyrolysis two-step method. The preparation method is environmentally friendly, generates no toxic by-products, and has a simple preparation process. The prepared nanocomposite material has a good microwave absorption capacity, a wide absorption frequency band, a low thickness, light weight and a low filling ratio, can absorb electromagnetic waves in different wavelength bands by adjusting the addition amount of graphene oxide in a precursor and the thickness of a coating layer, and has an important application value in the field of electromagnetic absorptionand electromagnetic shielding.
Owner:ANHUI UNIV OF SCI & TECH
Comprehensive recycling process for valuable metal roasting-cyaniding gold-containing tailing
InactiveCN102168176AShort processWill not cause secondary pollutionProcess efficiency improvementIron halidesImpurityPollution
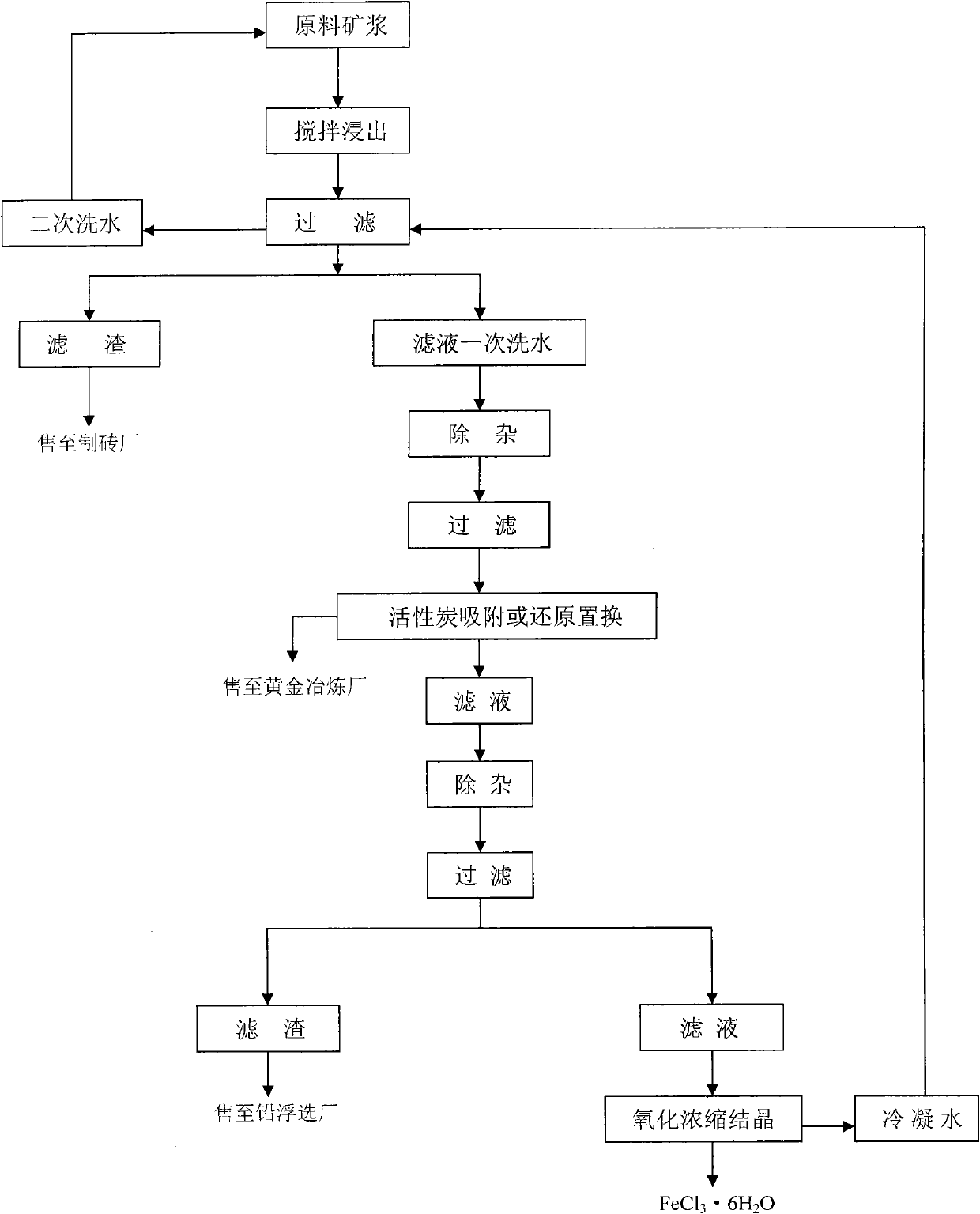
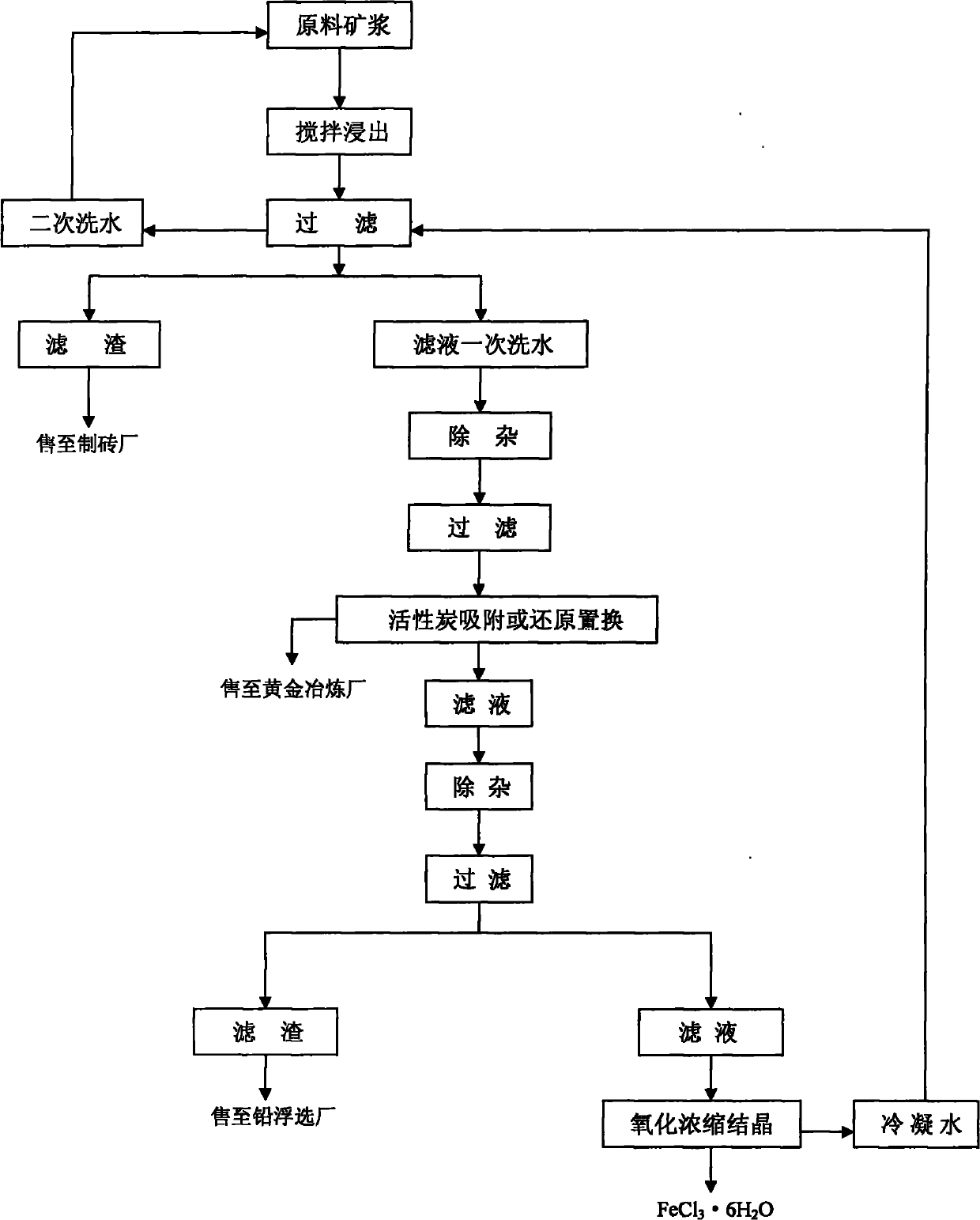
The invention discloses a comprehensive recycling process for valuable metal roasting-cyaniding gold-containing tailing, belonging to comprehensive recycling and utilization of industrial solid waste. The process comprises the following steps: selecting hydrochloric acid, chlorine and sodium hypochlorite as a leaching agent to leach gold, silver, iron, lead and arsenic to the maximum extent; adsorbing gold and silver with activated carbon, removing and recycling arsenic and lead through a series of solid-liquid separation and conversion, evaporating and crystallizing a ferric chloride solution subjected to impurity removal, thus finally producing a ferric chloride hexahydrate product, and realizing the comprehensive recycling process for valuable metal in the tailing. For the process, the available raw material sources are wide, the processing cost is low, the step-by-step impurity removal is thorough, all the impurities are recycled, no secondary pollution is generated, and the comprehensive recycling process can be used for fully realizing clean production, is environment-friendly and convenient for industrial production and has good promotion and application values.
Owner:田文学 +2
Preparation method of graphene supported ferriferrous oxide nanocomposite
InactiveCN102660220ASimple structureGood crystallizationMaterial nanotechnologyOther chemical processesCarbon nanotubeNanocomposite
The invention discloses a preparation method of a graphene supported ferriferrous oxide nanocomposite, comprising the following steps: preparing a precursor by deposition precipitation method, depositing ferric nitrate ninehydrate or ferric chloride hexahydrate on graphene in the form of iron hydroxide by using sodium hydroxide or ammonia to obtain black powder, washing and drying the obtained black powder, grinding the dried black powder, then calcining under the protection of argon, and then cooling, and reducing under the protection of a mixed gas of argon and hydrogen or argon and ammonia gas to obtain the graphene supported ferriferrous oxide nanocomposite. The preparation method is simple and stable. In the obtained composite, ferriferrous oxide is uniformly distributed on graphene and has good interface combination of the substrate graphene, thus the absorbing properties and the like of the composite as a functional material can be improved. The method also can be used for preparing carbon material supported ferriferrous oxide nanocomposites with different substrates by using carbon nanotube, graphite oxide and other carbon materials as the substrate.
Owner:TIANJIN UNIV
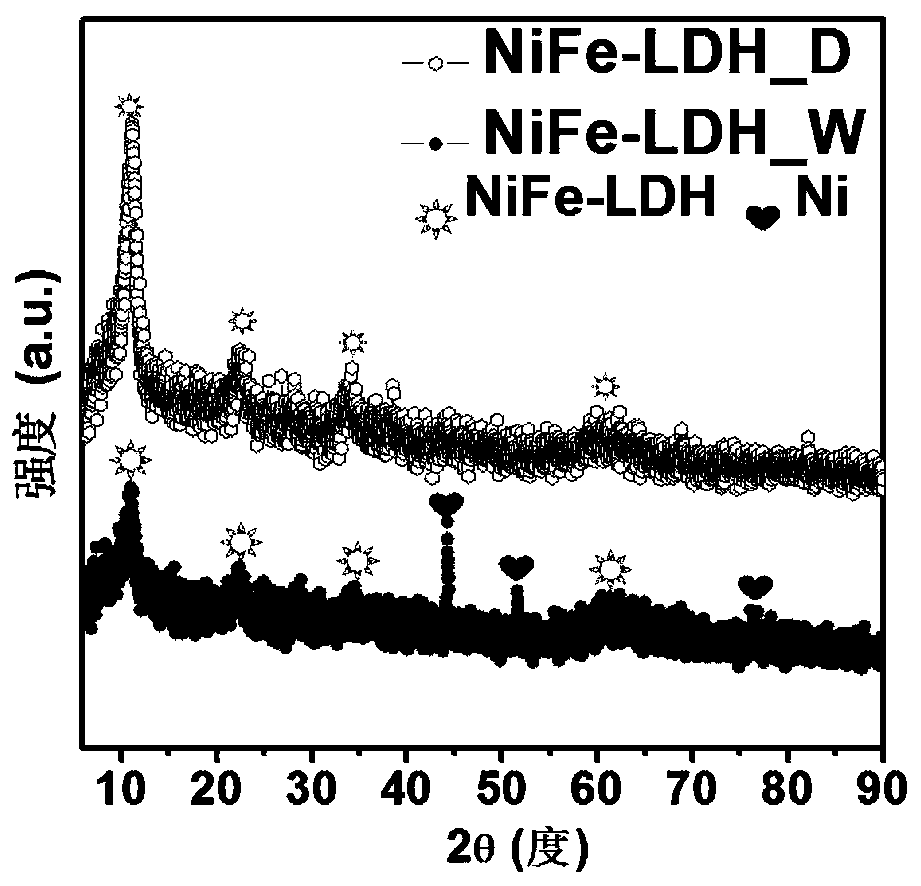
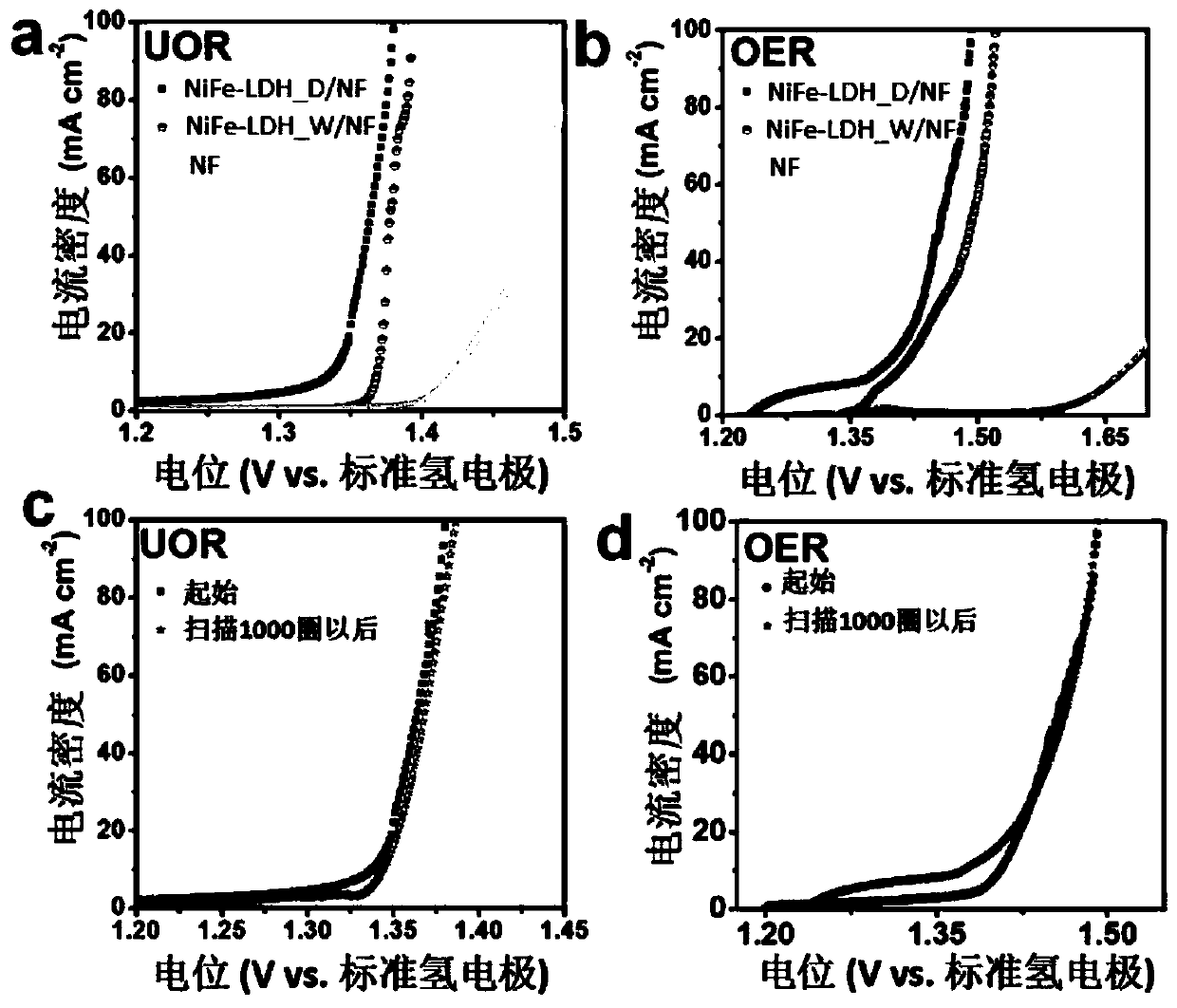
Nickel-iron double hydroxide/foamed nickel catalyst based on ferric chloride/urea eutectic solvent, and preparation method and application thereof
ActiveCN110201670ASimple processLow costCatalyst activation/preparationElectrode shape/formsElectrolysisSolvent
The invention provides a nickel-iron double hydroxide / foamed nickel catalyst based on a ferric chloride / urea eutectic solvent, and a preparation method and an application thereof. The nickel-iron double hydroxide / foamed nickel catalyst is prepared from the eutectic solvent which is prepared from ferric chloride hexahydrate and urea. The preparation method has the advantages of cheap and easily available raw materials, low cost, extremely simple operating process, easily realized reaction conditions, no high temperature, low energy consumption, short preparation period, and suitableness for industrial production. The obtained catalyst is nickel-iron double hydroxide loaded on foamed nickel, and the catalytic active component is nickel-iron double hydroxide (NiFe-LDH), and has a hierarchicalstructure that is a nanoflower structure composed of nanosheets. The catalyst has a good electrocatalytic electrolysis effect on water and urea.
Owner:SHANDONG UNIV
Method for preparing ferroferric oxide/graphene magnetic nano composite material by solvothermal one-step method
InactiveCN104261487AAvoid reunionEvenly dispersedMaterial nanotechnologyInorganic material magnetismSodium bicarbonateActive agent
The invention relates to a method for preparing a ferroferric oxide / graphene magnetic nano composite material by a solvothermal one-step method. The method comprises the following steps: by using ferric chloride hexahydrate and graphite oxide as raw materials, sodium bicarbonate as an alkali source, ethylene glycol and water as the solvent and polyvinylpyrrolidone as a surface active agent, dissolving graphite oxide, ferric chloride hexahydrate and polyvinylpyrrolidone into a mixed solvent of ethylene glycol and water, and carrying out ultrasonic treatment to obtain mixed solution A; dissolving sodium bicarbonate into ethylene glycol and carrying out ultrasonic treatment to prepare mixed solution B; after mixing the mixed solution A and B, reacting at 150-200 DEG C and at the pressure of less than 2.5 MPa for 4-12h, and then carrying out aftertreatment to obtain the ferroferric oxide / graphene magnetic nano composite material, wherein the ferroferric oxide particles are uniform, the distribution is narrow and the grain size is 10-30nm. The method is simple and convenient in process, environment-friendly and suitable for batch production.
Owner:NANCHANG HANGKONG UNIVERSITY
Method for preparing hollow spherical ferroferric oxide nano material
InactiveCN101698516AImprove stabilityHas a hollow structureNanostructure manufactureFerroso-ferric oxidesDiethylene glycolRoom temperature
The invention discloses a method for preparing a hollow spherical ferroferric oxide nano material, which comprises the following steps: dissolving ferric chloride hexahydrate in glycol or diethylene glycol and stirring the solution uniformly, wherein the dosage of the glycol or diethylene glycol is 1 to 4 milliliters of glycol or diethylene glycol per millimol of ferric chloride hexahydrate; dissolving anhydrous sodium acetate or sodium succinate or a mixture of anhydrous sodium acetate and sodium succinate in any ratio in solution prepared by the step 1 and stirring the mixed solution with ultrasonic waves to obtain uniform yellow brown sticky solution, wherein the mass ratio of the anhydrous sodium acetate or sodium succinate to the ferric chloride hexahydrate is 1:1 to 4:1; and transferring solution obtained by the step 2 to a liner of a water hydrothermal kettle, closing the kettle for reaction at 180 to 220 DEG C for 6 to 16 hours, naturally cooling the resulting product to room temperature, taking a sample out of the kettle and washing the sample with water and ethanol sequentially to obtain the hollow spherical ferroferric oxide nano material.
Owner:NANJING UNIV
Method for preparing peanut shell activated carbon-based magnetic Cr(VI) adsorbent
InactiveCN106378092AReduce manufacturing costLarge adsorption capacityOther chemical processesSpecific water treatment objectivesActivated carbonSorbent
The invention discloses a method for preparing a peanut shell activated carbon-based magnetic Cr(VI) adsorbent. The method specifically comprises the following steps: washing peanut shells, drying and grinding to obtain the needed particle size; then, calcining the peanut shell powder under liquid N2 protection, thereby obtaining an activated carbon precursor; dipping in KOH in a static state, drying, calcining in an N2 atmosphere, washing the calcined product, performing vacuum drying, thereby obtaining activated carbon; dispersing the activated carbon in ethylene glycol, adding ferric chloride hexahydrate and anhydrous sodium acetate, mixing and stirring, performing hydro-thermal treatment, washing the product, and performing vacuum drying, thereby obtaining the peanut shell activated carbon-based magnetic Cr(VI) adsorbent. The method disclosed by the invention has the advantages that the magnetic activated carbon which is easily separated after Cr(VI) adsorption is prepared by taking cheap and readily available peanut shells as raw materials, the magnetic activated carbon has the advantages of high specific surface area, good adsorptive property, excellent recycling performance and the like, the defect that magnetic particles easily drop is overcome, and the removal rate of over 90.6% also can be reached after recycling for four times.
Owner:WUHAN UNIV OF TECH
Alpha-Fe2O3 nanometer sphere preparation method
ActiveCN103073065AReduce usageShort reaction timeMaterial nanotechnologyFerric oxidesEnvironmental resistanceMicrowave method
The present invention belongs to the technical field of chemical engineering, and specifically relates to an alpha-Fe2O3 nanometer sphere preparation method. The method specifically comprises: adopting ferric chloride hexahydrate (FeCl3.6H2O) as an iron source, adopting urea (CO(NH2)2) as a hydroxide radical ion initiator, dissolving the iron source and the initiator in a mixing solvent comprising glycerin and water by using ultrasound, placing the mixed solution in a sealed condition to carry out a microwave treatment for 20-50 min at a temperature of 120-160 DEG C, naturally cooling the product from the microwave treatment, then carrying out centrifugation, carrying out alternate washing on the resulting precipitate by using ethanol and water, and drying to obtain the alpha-Fe2O3 nanometer sphere having an average diameter of 300-500 nm, wherein the whole structure of the nanometer sphere comprises rod-shaped nanometer sub-units with a size of about 20-50 nm. According to the present invention, cheap and non-toxic glycerin / water is adopted as a solvent so as to avoid use of toxic and organic reagents, and the microwave method provides characteristics of short reaction time, energy resource saving and environmental protection compared with the traditional heating method.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Method and composition for attracting arthropods by volatilizing an acid
The invention is a method for attracting mosquitoes by producing a volatilized acid by combining an acid precursor with water to produce the humidified vapor acid attractant, preferably hydrogen chloride. The humidified volatilized acid can be further combined with carbon dioxide, or carbon dioxide can be simultaneously produced to attract mosquitoes. The acid precursor can be a hydrate of ferric chloride, such as ferric chloride hexahydrate. The acid precursor can be impregnated in a carrier, and can be combined with water through exposure to water vapor in the atmosphere, the intentional addition of water, or water produced by a chemical reaction.
Owner:ICA TRINOVA
Preparation method of graphene magnetic aerogel with high adsorption performance
InactiveCN111013540AImprove adsorption capacityIncrease surface areaIon-exchange process apparatusOther chemical processesSodium acetateFreeze-drying
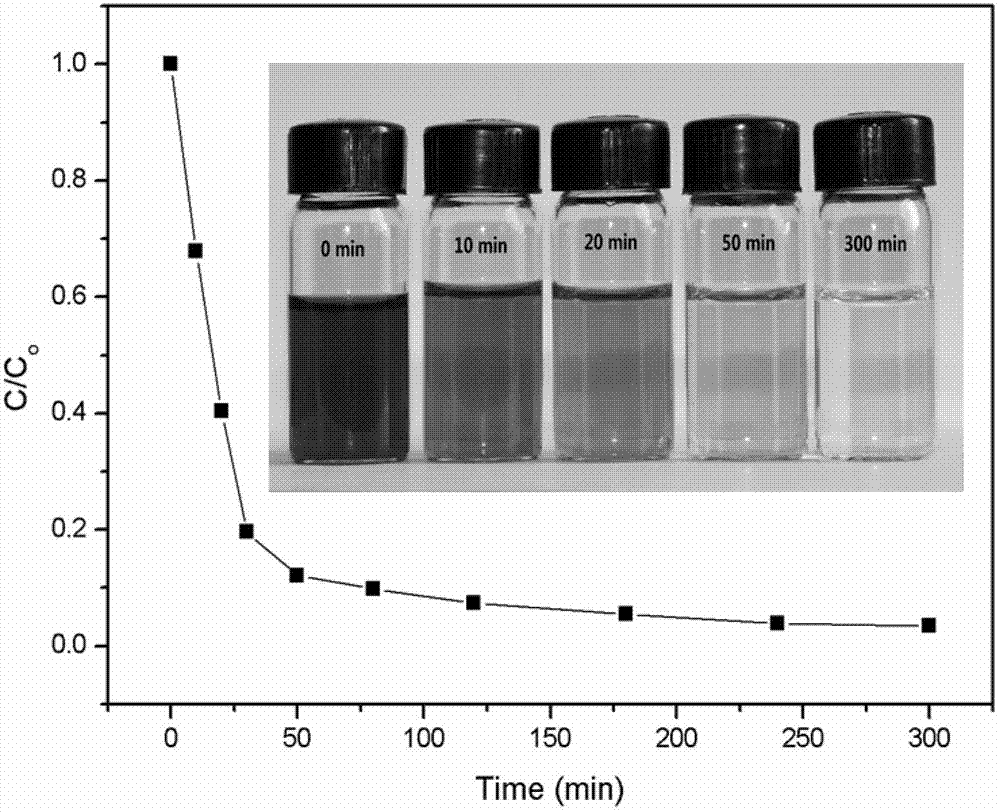
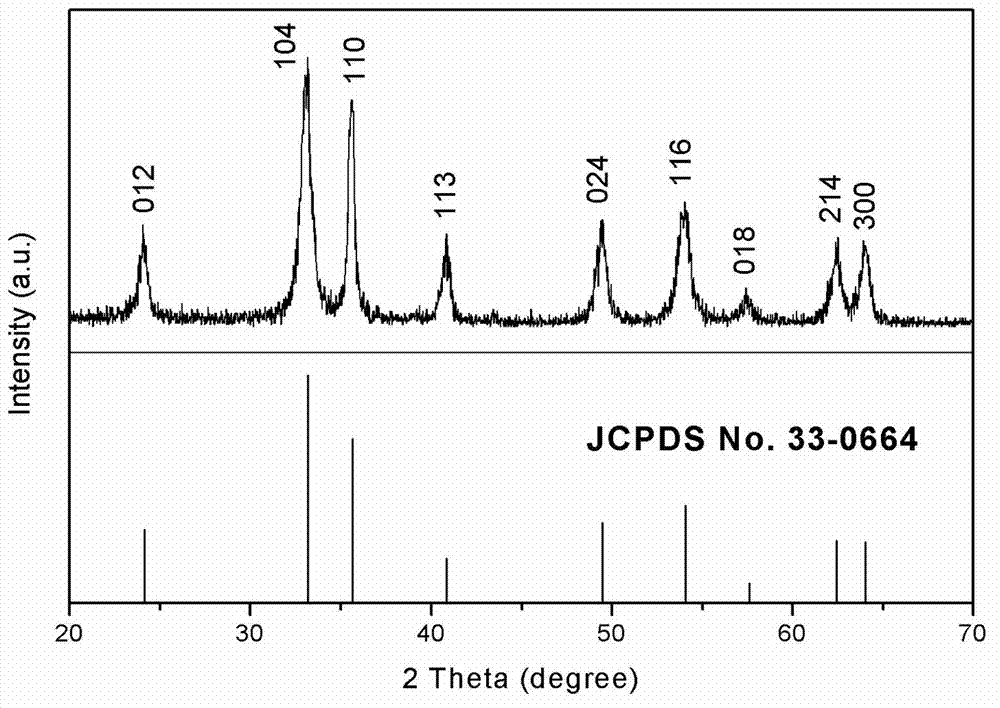
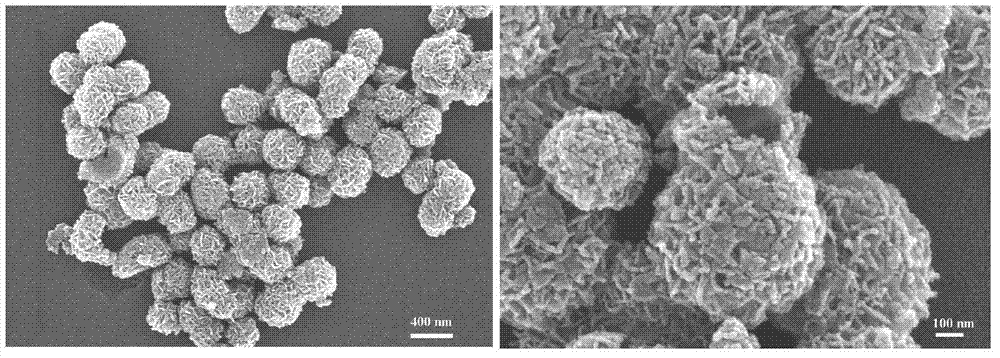
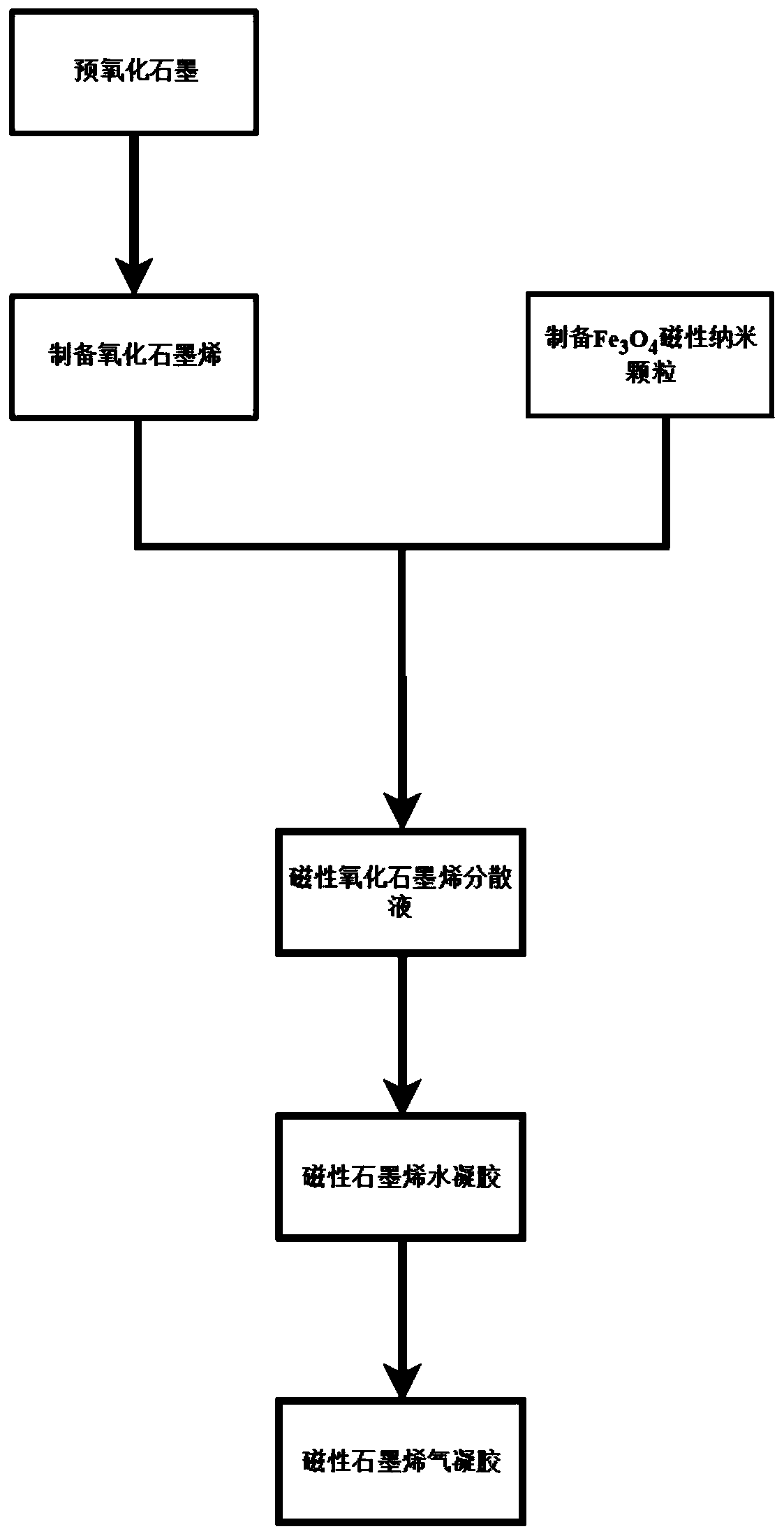
The invention relates to a preparation method of graphene magnetic aerogel with high adsorption performance. The preparation method comprises the following steps: preparing Fe3O4 magnetic nanoparticles from anhydrous sodium acetate, polyvinylpyrrolidone, ferric chloride hexahydrate and ethylene glycol; pre-oxidizing crystalline flake graphite by using a pre-oxidation method, and then preparing a graphene oxide solution by using an improved Hummers method; compounding Fe3O4 and graphene through a solvothermal method, and preparing a graphene magnetic hydrogel through reduction; and finally, preparing a graphene magnetic aerogel material through a freeze drying method. Adsorption experiments show that the prepared graphene magnetic aerogel has the advantages of high adsorption capacity, highsurface area and the like, and is greatly improved in adsorption performance on pollutants such as dyes, organic solvents, heavy metal particles and the like, so the graphene magnetic aerogel has good application prospects in the fields of sewage treatment and the like.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Preparation method for magnetic macromolecule nanoball
ActiveCN105140018AContinuous preparationFast preparationInductances/transformers/magnets manufactureMagnetic materialsPolyethylene glycolSuperparamagnetism
The invention provides a preparation method for a magnetic macromolecule nanoball. The preparation method comprises the following steps of: adding ferric chloride hexahydrate and iron dichloride tetrahydrate into water to prepare a mixed solution, dropwise adding ammonia water and a polyethylene glycol-4,000 aqueous solution for reaction, and carrying out ultrasonic dispersion and magnetic separation after reaction to obtain a Fe3O4 nanopraticle magnetic fluid; mixing the Fe3O4 nanopraticle magnetic fluid with absolute ethyl alcohol, the water and the ammonia water, dropwise adding tetraethyl orthosilicate after ultrasonic dispersion, carrying out magnetic separation after room-temperature reaction, collecting sediment and immersing the sediment in a hydrochloric acid solution, and carrying out magnetic separation after immersion to obtain a Fe3O4 composite nanopraticle magnetic fluid; and adding the Fe3O4 composite nanopraticle magnetic fluid into the absolute ethyl alcohol, adding the ammonia water after magnetic separation, dropwise adding 3-aminopropyltriethoxysilane for reaction, and carrying out magnetic separation after reaction to obtain the magnetic macromolecule nanoball. The method can be continuously and rapidly carried out, the time cost is low, the operation stability is high, the prepared magnetic macromolecule nanoball has excellent superparamagnetism, and magnetism is easy to separate and recycle.
Owner:ZHEJIANG UNIV
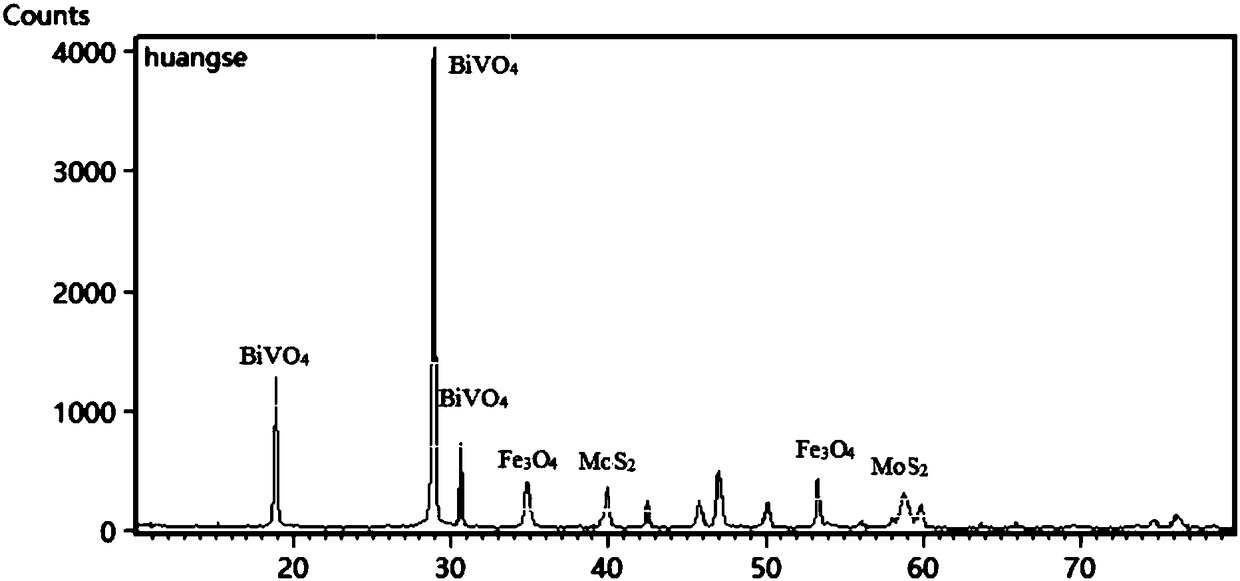
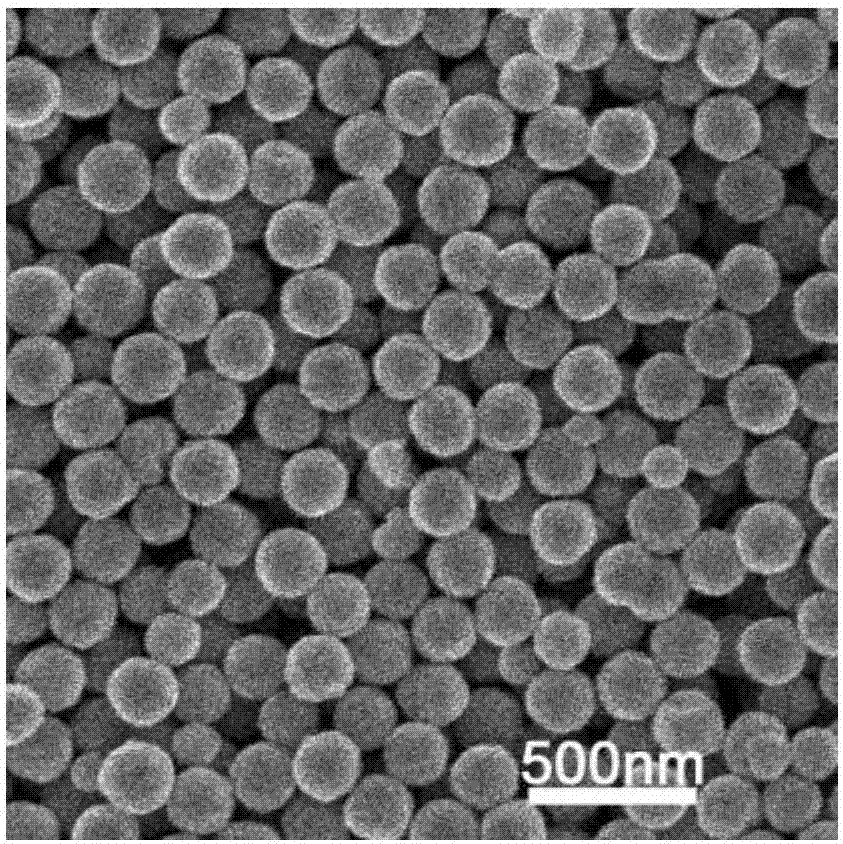
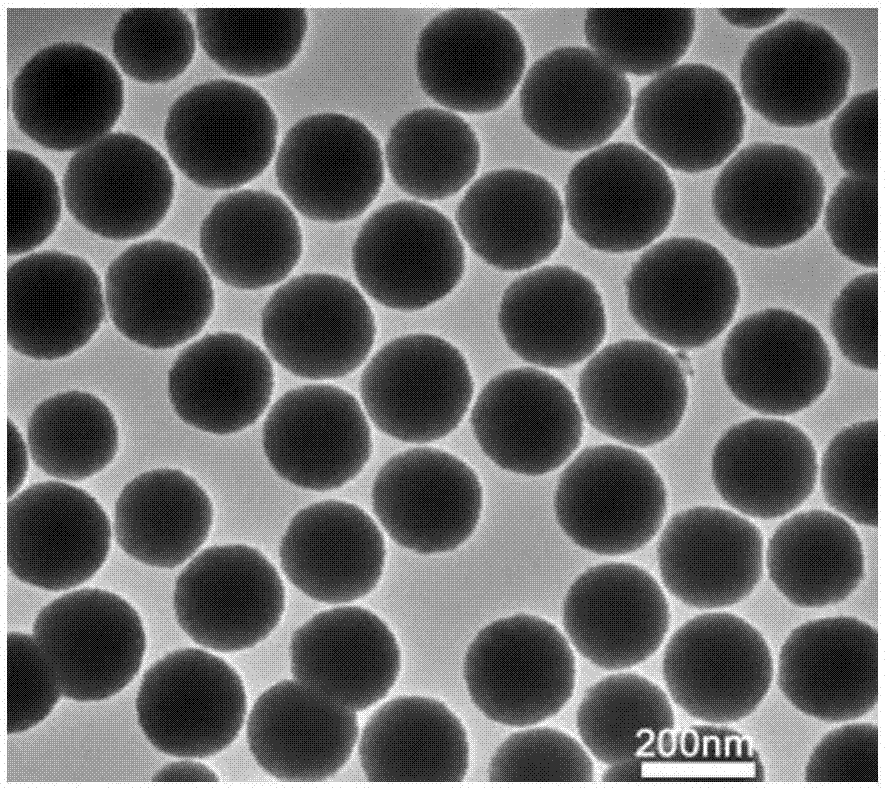
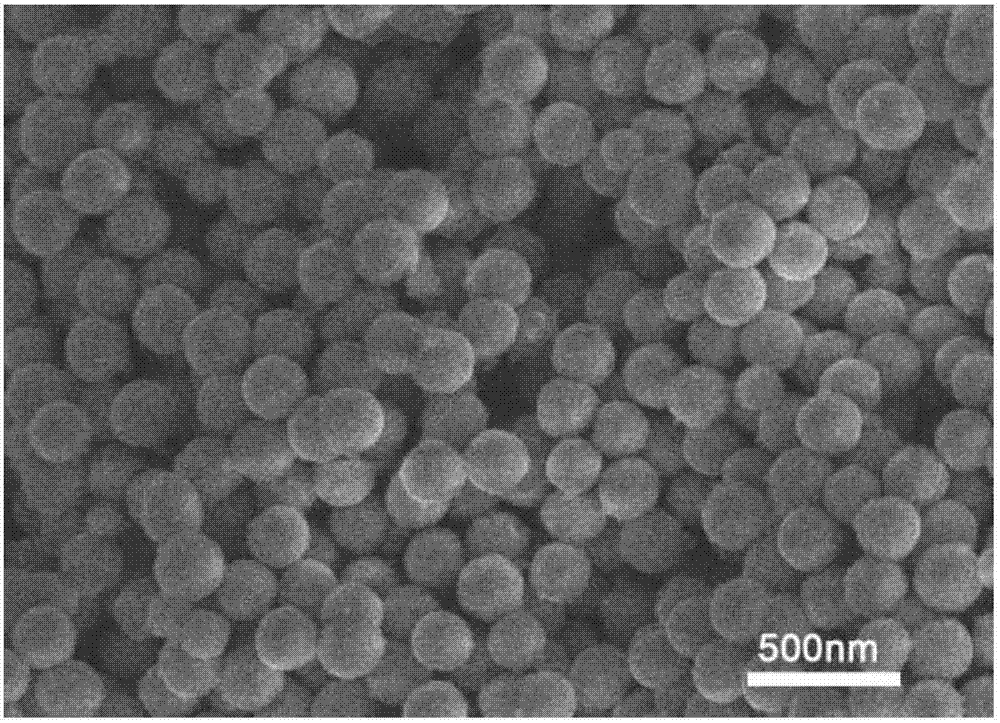
Fe3O4/MoS2/BiVO4 material preparing method, product and application of product
InactiveCN108355679AHigh reuse rateEasy to recycleWater/sewage treatment by irradiationWater treatment compoundsSodium acetateThiourea
The invention discloses a Fe3O4 / MoS2 / BiVO4 material preparing method, a product and an application of the product. The method comprises the steps: firstly, dissolving ferric chloride hexahydrate, sodium acetate and polyethylene glycol in ethylene glycol, carrying out a reaction at the temperature of 180-200 DEG C for 8-9 h, and thus obtaining ferric oxide; then, mixing sodium molybdate dehydrate,sodium molybdate tetrahydrate and thiourea evenly, adding ferroferric oxide, and carrying out a reaction for 22-24 h at the temperature of 180-200 DEG C, to obtain a Fe3O4 / MoS2 material; finally, dissolving bismuth nitrate pentahydrate and ammonium metavanadate in deionized water, adding the Fe3O4 / MoS2 material, mixing evenly, carrying out a reaction for 15-17 h at the temperature of 160-170 DEG C, and thus obtaining a Fe3O4 / MoS2 / BiVO4 composite material. When the composite material is used as a photocatalyst for treating tetracycline hydrochloride wastewater, the degradation rate can reach 90% or more, and the photocatalytic efficiency can be improved. At the same time, because of the presence of Fe3O4, the photocatalyst can be recovered more conveniently and simply for repeated experiments, the recovery cost of the photocatalyst is reduced, and the repeated utilization rate of the photocatalyst is improved.
Owner:CHANGAN UNIV
Preparation method of visible-light-driven photocatalyst Fe3O4@PDA@Ag composite microsphere
ActiveCN107442178AHigh reuse rateAvoid secondary pollutionWater/sewage treatment by irradiationWater treatment compoundsSodium acetateWater baths
The invention belongs to the technical field of photocatalysts and specifically relates to a preparation method of a visible-light-driven photocatalyst Fe3O4@PDA@Ag composite microsphere. The preparation method comprises step 1 of respectively dissolving ferric chloride hexahydrate and sodium acetate into equivalent ethylene glycol, then evenly mixing and stirring two solutions, adding a 10 to 30wt% polyacrylic acid solution to continue stirring for 6 to 12h, putting into a reaction kettle, heating to 180 to 210 DEG C and keeping reaction for 4 to 12h to obtain a black product of ferroferric oxide; step 2 of dispersing the ferroferric oxide into an ethanol solution of polyvinyl pyrrolidone, then adding a water solution of dopamine hydrochloride, performing water bath and fully and ultrasonically dispersing to obtain a mixed solution b; step 3 of dropwise adding ammonium hydroxide into the mixed solution b, continuing ultrasonic reaction for 2 to 5 hours to obtain Fe3O4@PDA composite microspheres; step 4 of adding the prepared Fe3O4@PDA composite microspheres into ammonium hydroxide and silver nitrate to be prepared into a tollens' reagent, putting in a table concentrator to be vibrated for 8 to 16hours, performing magnetic separation and performing aftertreatment to obtain the Fe3O4@PDA@Ag composite microspheres. The Fe3O4@PDA@Ag composite microspheres have an obvious effect on degrading methyl orange and can be used repeatedly.
Owner:ZHEJIANG SCI-TECH UNIV
Nanometer iron-lithium oxide composite negative electrode material and preparation method thereof
ActiveCN103560237ARaw materials are easy to getSimple preparation processMaterial nanotechnologyCell electrodesLithium oxideLithium-ion battery
The invention belongs to the technical field of preparation of electrode materials for lithium ion batteries, and particularly relates to a nanometer iron-lithium oxide composite negative electrode material and a preparation method thereof. The material is a mixture or one of Li0.5F2.5O4 and LiFeO2, is a nanometer material and is uniform in size. The particle diameter of the prepared iron-lithium oxide material is about 250-300 nanometer. The preparation method of the material comprises the following steps: firstly preparing a ferric oxide (Fe2O3) nanorod through the hydrothermal reaction of ferric chloride hexahydrate (FeC13.6H2O) and ammonium dihydrogen phosphate (NH4H2PO4), then mixing the prepared ferric oxide (Fe2O3) nanorod with lithium hydroxide monohydrate (LiOH.H2O), and carrying out high-temperature calcination so as to prepare the nanometer iron-lithium oxide composite negative electrode material. The nanometer iron-lithium oxide composite negative electrode material has regular morphology, high purity and good electrochemical performance and is relatively uniform in size; the preparation method of the nanometer iron-lithium oxide composite negative electrode material is simple in process and is easy for large-scale industrial production.
Owner:ADVANCED TECHNOLOGY & MATERIALS CO LTD
Protein-based carbon/magnetic Fe Co nanoparticle composite wave-absorbing agent and preparation method and application thereof
ActiveCN110079271AImprove absorbing performanceAbsorbing frequency bandwidthMaterial nanotechnologyOther chemical processesMagnetite NanoparticlesLarge applications
The invention provides a protein-based carbon / magnetic Fe Co nanoparticle composite wave-absorbing agent and a preparation method and application thereof, and belongs to the technical field of wave-absorbing materials. The preparation method of the protein-based carbon / magnetic Fe Co nanoparticle composite wave-absorbing agent comprises the following steps: 1) mixing water, ferric chloride hexahydrate, ferrous chloride tetrahydrate and cobalt chloride hexahydrate, and reacting for 1-2 h at the temperature of 70-100 DEG C to prepare magnetic nanoparticles; 2) mixing the magnetic nanoparticles with egg white liquid, and curing to obtain a protein-magnetic nanoparticle compound; 3) carbonizing the protein-magnetic nanoparticle compound obtained in the step 2) to obtain the protein-based carbon / magnetic Fe Co nanoparticle composite wave-absorbing agent. The material prepared by using the composite wave-absorbing agent has strong wave-absorbing performance, meanwhile has the characteristicsof wide wave-absorbing frequency band, small matching thickness of an absorber and the like, and has a large application prospect in the field of wave-absorbing materials.
Owner:JILIN UNIV
Aerogel hybrid microsphere grafted silicon composite coating and preparation method thereof
The invention discloses an aerogel hybrid microsphere grafted silicon composite coating which is prepared from the following raw materials in parts by weight: 3-5 parts of superfine silicon dioxide aerogel, 7-9 parts of ferric chloride hexahydrate, 10-12 parts of ferrous chloride tetrahydrate, 2-3 parts of citric acid, 1-2 parts of methylacryloyloxypropyl trimethoxy silane, 100-130 parts of tetraethoxysilane, 5-7 parts of hexafluorobutyl acrylate, 0.1-0.4 part of azodiisobutyronitrile, 0.1-0.2 part of sp800, 1-2 parts of chlorthal-dimethyl, 2-4 parts of hydrolyzed polymaleic anhydride, 0.6-1 part of polyacrylamide, 1-2 parts of casein, 0.3-1 part of zinc cyanurate, 0.1-0.2 part of ethyl naphthol, 3-5 parts of calcium phosphate and appropriate absolute ethyl alcohol and dimethyl formamide. In the invention, the added silicon dioxide aerogel can effectively improve the packing dispersity in resin, and the stability and impact strength of the finished product of coating are enhanced.
Owner:TONGLING JINGWEI FLUID SCI & TECH
Preparation method of magnetic nitrogen-doped carbon materials
ActiveCN103295756ANo pollution in the processGuaranteed MagneticInductances/transformers/magnets manufactureHydration reactionPtru catalyst
The invention provides a preparation method of magnetic nitrogen-doped carbon materials. The method includes: using concentrated hydrochloric acid to adjust pH of mixed solvent of anhydrous ethanol and deionized water to 1-3, sequentially and fully dissolving or dispersing oxidation carbonization agent (ferric chloride hexahydrate), carbon source (saccharides), nitrogen source (glycine or melamine), mixing well, evaporating solvent, carbonizing at 60-80 DEG C for 40-50 hours, grinding carbonized products, and carbonizing the ground carbonized products at 180-200 DEG C for 4-6 hours to obtain the magnetic nitrogen-doped carbon materials which are promising in application prospect in fields such as fuel cell catalysts and super capacitors. The preparation method is mild in reaction condition, low in cost, simple to operate, and easy to control nitrogen contents in the nitrogen-doped carbon materials.
Owner:合肥碳艺科技有限公司
Graphene-loaded nanometer Fe<3>O<4>/ZnO composite and preparation method thereof
ActiveCN105384146AImprove conductivityEvenly distributedZinc oxides/hydroxidesMagnetic/electric field screeningSolubilityElectromagnetic shielding
The invention relates to a graphene-loaded nanometer Fe<3>O<4> / ZnO composite with an electromagnetic shielding function and a preparation method thereof. Te preparation method includes the steps that firstly, ferric chloride hexahydrate and Zn powder are mixed to be uniform after no sediment exists, an alkaline solution and a graphene oxide solution are added, and after the mixture is stirred to be uniform, suspension liquid is obtained; then, the suspension liquid is placed in a reaction kettle to be subjected to constant temperature heat treatment, centrifugal separation is conducted, and obtained paste-shaped solid is placed in a tube-shaped furnace to be subjected to thermal recovery under the mixed atmosphere of H<2> / Ar after being refrigerated and dried; finally, the graphene-loaded nanometer Fe<3>O<4> / ZnO composite is obtained. The graphene-loaded nanometer Fe<3>O<4> / ZnO composite prepared through the method is high in degree of dispersion and good in water solubility, the magnetic property and the electromagnetic shielding property are remarkably enhanced, the operation process is simple and efficient, and industrialized production and application is achieved easily.
Owner:TANGSHAN JIANHUA TECH DEV
Polypyrrole coated hard carbon cathode material and preparation method thereof
The invention discloses a polypyrrole coated hard carbon cathode material and a preparation method thereof. The polypyrrole coated hard carbon cathode material adopts a hard carbon sphere as the substrate, which is coated with a polypyrrole layer. The preparation process comprises: taking glucose as the carbon source, preparing a hard carbon sphere material by a hydrothermal calcination method, taking ferric chloride hexahydrate as the oxidant, and preparing the polypyrrole coated hard carbon cathode material by an in-situ chemical oxidation method. The process involved in the invention is simple, has good repeatability, and has no need of adding additives. The prepared composite material has good electrical conductivity, thus being in favor of bringing the electrochemical performance of the lithium ion battery cathode material into play.
Owner:TIANJIN UNIV
Method for preparing super-hydrophobic copper coating on surface of aluminum alloy
InactiveCN107267967ASimple processEase of industrial productionLiquid/solution decomposition chemical coatingChemical reactionAlloy substrate
The invention discloses a method for preparing a super-hydrophobic copper coating on the surface of aluminum alloy. The method comprises the steps that firstly, a processed aluminum alloy sample is soaked into a mixed solution of a certain concentration for reaction, the mixed solution is composed of cupric sulfate pentahydrate and ferric chloride hexahydrate, and a copper coating with a plume-like microstructure is generated on the surface of the sample; secondly, the sample is put into a vacuum tube type furnace and subjected to heat treatment under vacuum for certain time, and the interface bonding strength of the surface copper coating and the aluminum alloy substrate is improved; and finally, the copper coating is subjected to low energy modification in a stearic acid absolute ethyl alcohol solution of a certain concentration, and the surface copper coating with a super-hydrophobic function is obtained. Through measuring, the static contact angle of deionized water and the surface of the super-hydrophobic copper coating is larger than 150 degrees, and the rolling angle is smaller than 10 degrees. The method for preparing the super-hydrophobic copper coating on the surface of the aluminum alloy does not need complex and expensive equipment, and is low in cost, simple in process, good in repeatability and suitable for industrialized production.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Method for preparing anhydrous magnesium chloride by utilizing magnesium chloride hexahydrate
ActiveCN102491384AReduce dosageEasy to recycleMagnesium chloridesStrontium chloride hexahydrateMagnesium chloride hexahydrate
The invention relates to a method for preparing anhydrous magnesium chloride by utilizing magnesium chloride hexahydrate. The method comprises the following steps of (1) mixing and ball-milling the magnesium chloride hexahydrate and ammonium chloride to obtain ammonium camallite or mixture containing the ammonium camallite, (2) heating the mixture and performing preliminary dehydration to preparelower-water ammonium camallite or mixture containing the lower-water ammonium camallite, and (3) placing a covering on the product obtained in the step (2) and performing heating and reaction to prepare anhydrous magnesium chloride. The method for preparing the anhydrous magnesium chloride by utilizing the magnesium chloride hexahydrate can shorten production process of the anhydrous magnesium chloride, improve production efficiency and reduce production cost and input cost for environmental protection.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Magnetic nanocomposite, and preparation and application thereof
InactiveCN110385116ALarge specific surface areaImprove adsorption capacityOther chemical processesPreparing sample for investigationPolypyrroleSorbent
The invention relates to a magnetic nanocomposite, and preparation and application thereof, belonging to the technical field of detection for heavy metals in food samples. The invention also providesa sensitive and efficient method for detecting heavy metal ions in food samples. A preparation method for the magnetic nanocomposite comprises the following steps: (1) preparing magnetic graphene oxide from graphene oxide, ferric chloride hexahydrate, and ferrous chloride tetrahydrate in an alkaline solution; (2) preparing silica-modified graphene oxide from magnetic graphene oxide and ethyl silicate in anhydrous ethanol; and (3) ) preparing a solid-phase extraction adsorbent, i.e., polypyrrole polyaniline modified-silica coated magnetic graphene oxide from pyrrole, aniline, FeCl3 solid and silica-modified graphene oxide. The prepared magnetic nanocomposite has good adsorption performance and can be used as a magnetic solid-phase extraction adsorbent to extract heavy metals such as Cr(VI)and Pb(II) in food.
Owner:沈阳信达泰康医药科技有限公司
Solvothermal method for preparing nano ferroferric oxide powder
InactiveCN103708564AComplete crystal formNarrow size distributionMaterial nanotechnologyFerroso-ferric oxidesSodium bicarbonateBrick
The invention relates to a solvothermal method for preparing nano ferroferric oxide powder. The method comprises steps of weighing a proper amount of ferric chloride hexahydrate and sodium bicarbonate, respectively dissolving into ethylene glycol so as to prepare a mixed solution A and a mixed solution B; adding a proper amount of polyvinylpyrrolidone into the solutions to be taken as surfactant; adding the mixed solution B into the mixed solution A so as to prepare brick-red mixed solution; then sealing in a high pressure reactor with teflon as the lining, putting into an air dry oven and heating for a while, after cooling down the reaction liquid, separating, washing, vacuum drying so as to obtain black powder. The solvothermal method has the advantages of having simple processes, being convenient to operate, being easily available in raw materials, being economical and efficient and environmentally friendly, and being suitable for volume production.
Owner:NANCHANG HANGKONG UNIVERSITY
Porous ferroferric oxide/ carbon nanometer rod-shaped electromagnetic wave absorbing material and preparing method and application thereof
ActiveCN108154984AHigh saturation susceptibilityImprove coercive forceMaterial nanotechnologyMagnetic/electric field screeningAbsorbent materialOxidation resistant
The invention relates to a porous ferroferric oxide / carbon nanometer rod-shaped electromagnetic wave absorbing material and a preparing method and application thereof. The porous ferroferric oxide / carbon nanometer rod-shaped electromagnetic wave absorbing material is multi-phase nanometer composite powder composed of carbon and ferroferric oxide and a nanometer rod of a porous structure with thelength being 1.0-1.2 micrometers. The preparing method comprises the steps of dissolving raw materials like ferric trichloride hexahydrate and fumaric acid into deionized water, conducting a reactionto obtain a precursor, conducting calcination under nitrogen atmosphere and directly synthesizing the porous ferroferric oxide / carbon nanometer rod composite material. The porous ferroferric oxide / carbon nanometer rod composite material is high in stability and uniformity, has the advantages of being high in electromagnetic wave absorbing performance, wide in absorbing and covering frequency range, high in corrosion resistance and oxidation resistance and low in cost and is used for manufacturing an electromagnetism absorber.
Owner:SHANDONG UNIV
Preparation method and application of octahedral nitrogen doped carbon framework material
The invention provides an octahedral nitrogen doped carbon framework material for oxygen reduction reaction of fuel cell cathodes. Firstly, terephthalic acid and ferric trichloride hexahydrate performhydrothermal reaction in DMF to obtain a precusor substance; then, in a polar solvent, the precusor substance and a nitrogen-rich substance perform reaction under the ultrasonic condition for 2-6 hours, standing, suction filtration and drying are performed, then the mixture is put in a tube furnace, nitrogen is introduced for protection, thermal treatment is performed at the temperature of 400-1200 DEG C for 1-6 hours, and a product is treated with an acid solution and is washed and dried to obtain the octahedral nitrogen doped carbon framework material. The octahedral nitrogen doped carbon framework material serves as a catalyst for oxygen reduction reaction of fuel cell cathodes. Compared with a traditional precious metal catalyst, the octahedral nitrogen doped carbon framework materialhas the advantages of being low in cost, high in activity, strong in poison tolerance capability, good in stability and the like and is an ideal catalytic material for oxygen reduction reaction of fuel cell cathodes.
Owner:NORTHWEST NORMAL UNIVERSITY
Preparation method of Fe2O3/CeO2/NiO core-shell structural microspheres
The invention discloses a preparation method of Fe2O3 / CeO2 / Ni(OH)2 core-shell structural microspheres. The preparation method comprises the following steps of: dissolving ferric chloride hexahydrate, ferric nitrate and polyvinylpyrrolidone (PVP) into deionized water; reacting at a hydrothermal condition to obtain egg-shaped alpha-Fe2O3 microspheres; dispersing the alpha-Fe2O3 microspheres into water again; adding ceric ammonium nitrate, urea and PVP to dissolve; reacting at a certain temperature to coat a CeO2 shell layer; centrifugally washing the obtained product, namely, Fe2O3 / CeO2; dispersing into water again; adding nickel sulfate, urea and PVP to dissolve; reacting at a certain temperature to coat an Ni(OH2) shell layer; centrifugally washing the obtained product, namely, Fe2O3 / CeO2 / Ni(OH)2 after the reaction is done; drying; and transferring into a muff furnace to roast to obtain Fe2O3 / CeO2 / NiO core-shell structural microspheres. The Fe2O3 / CeO2 / Ni(OH)2 microspheres and the Fe2O3 / CeO2 / NiO microspheres, prepared by the preparation method, are of a novel three-layer core-shell structure; the core layers are of a multi-level structure formed by nanometer assembly; and the microspheres can be applied to the development of novel Ni-Ce-Fe based functional materials.
Owner:徐州康翔精密制造有限公司
Method for preparing iron phosphide and carbon composite structure by utilizing carbothermic reaction
InactiveCN104084224AWell mixedMix well and evenlyPhysical/chemical process catalystsCarbon compositesPhosphate
The invention relates to a method for preparing an iron phosphide and carbon composite structure by utilizing carbothermic reaction, and particularly relates to a simple and easy method for preparing the iron phosphide and carbon composite structure. The method is suitable for preparing a composite structure of iron phosphide, other metal phosphides and carbon in large scale. The method comprises the following steps: soaking melamine by a mixed solution of ferric chloride hexahydrate and ammonium dihydrogen phosphate by adopting a soaking method in which the ferric chloride hexahydrate, the ammonium dihydrogen phosphate and the melamine are used as raw materials, then carrying out high-temperature pyrolysis on melamine under inert gas by adopting a high-temperature pyrolysis method, reducing metal phosphates into metal phosphides, thereby obtaining the iron phosphide and carbon composite structure. According to the method, the uniform refining of the iron phosphide and carbon composite structure can be achieved by changing the ratio of the ferric chloride hexahydrate to the melamine and conditions of the pyrolysis reduction, such as warming velocity, holding temperature, temperature holding time, so that the iron phosphide and carbon composite structure which is uniform in granule and excellent in catalytic performance can be obtained.
Owner:JIANGSU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com