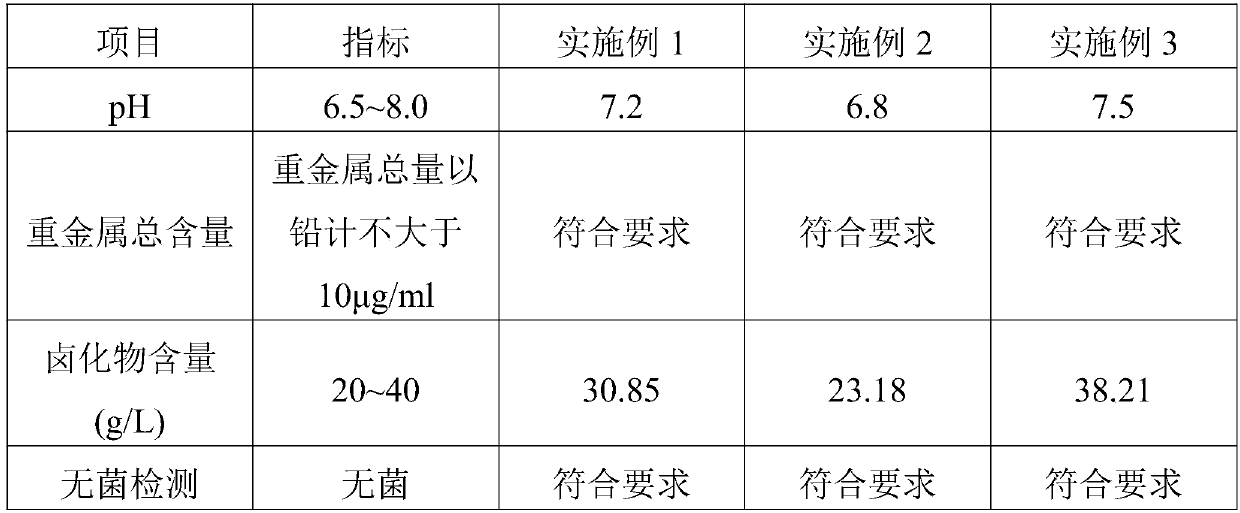
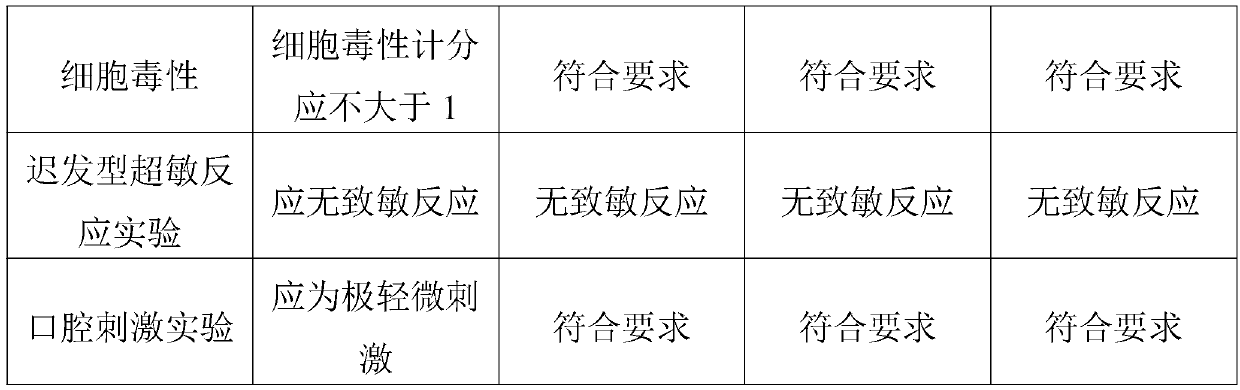
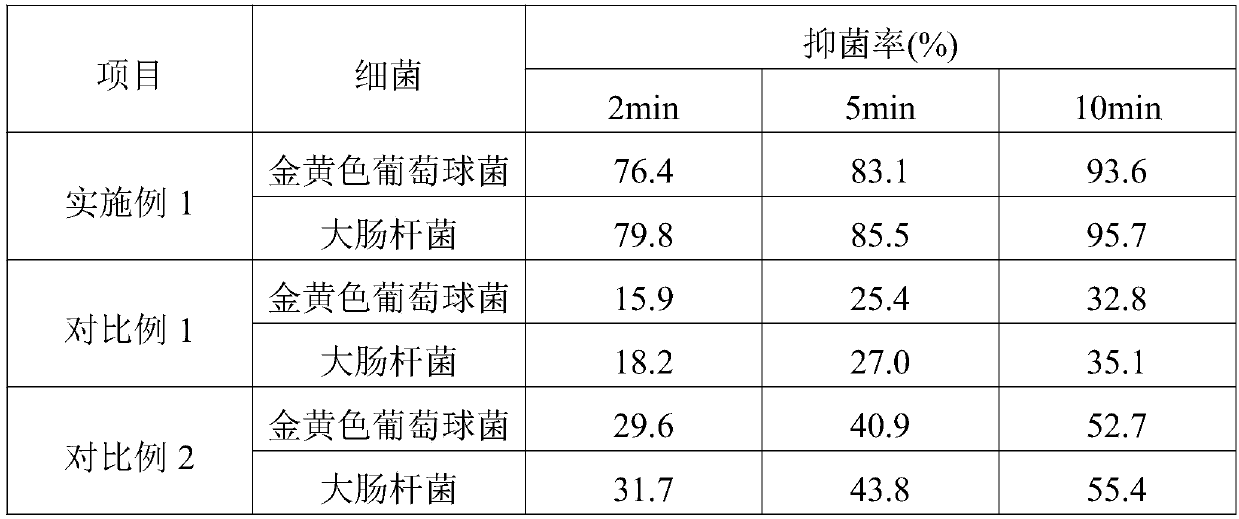
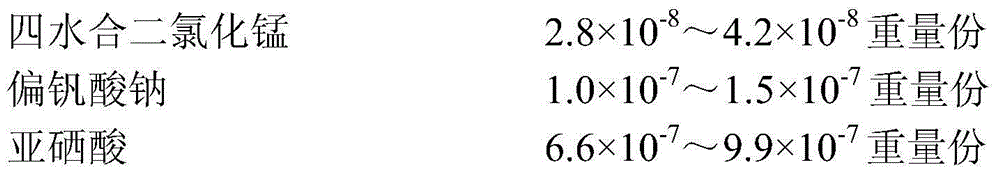Patents
Literature
43 results about "Strontium chloride hexahydrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Strontium chloride is a metallic salt that is composed of the elements strontium and chloride in the chemical formula SrCl2, and it has a variety of limited, specific uses. In the past, it was the most common ingredient in certain toothpastes where it is often in the chemical form of strontium chloride hexahydrate, or SrCl2 6H2O.
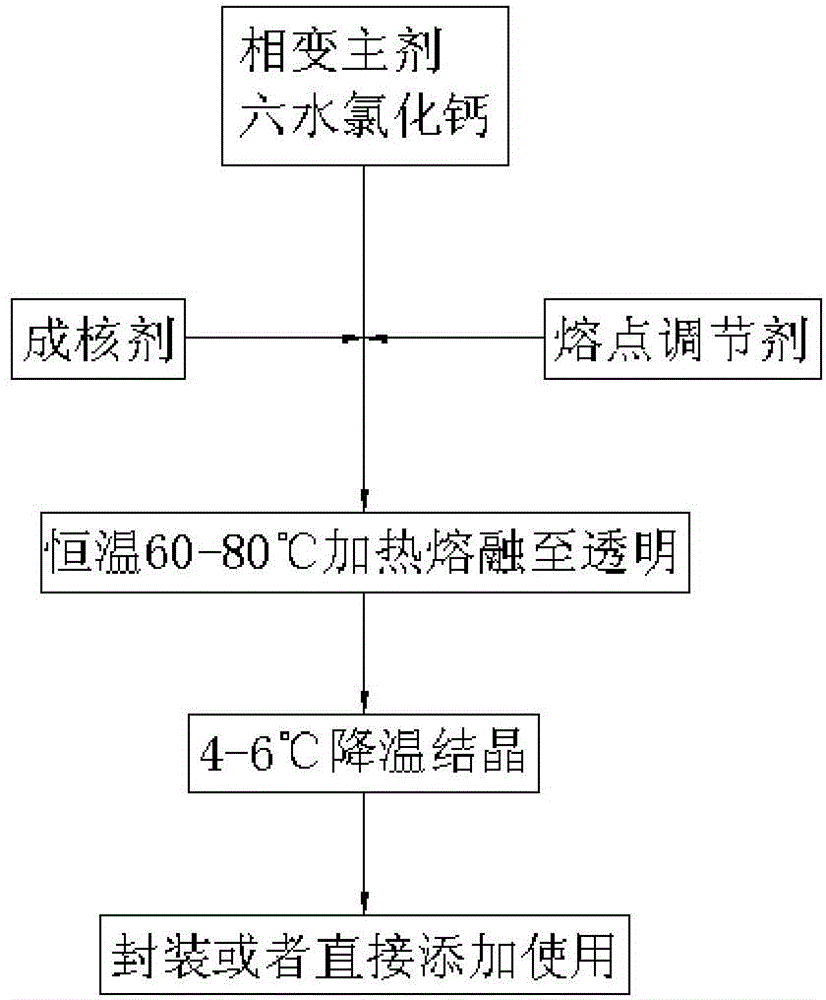
Phase change material and preparation method thereof
InactiveCN104419381ALow melting pointImprove heat storage capacityHeat-exchange elementsStrontium chloride hexahydrateSuper cooling
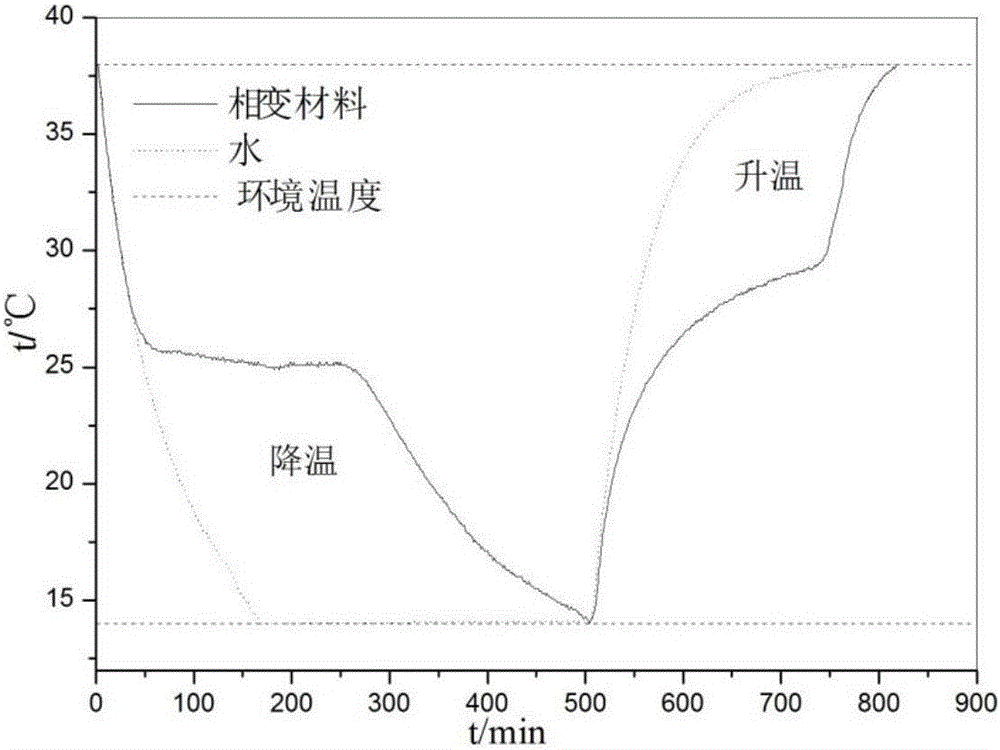
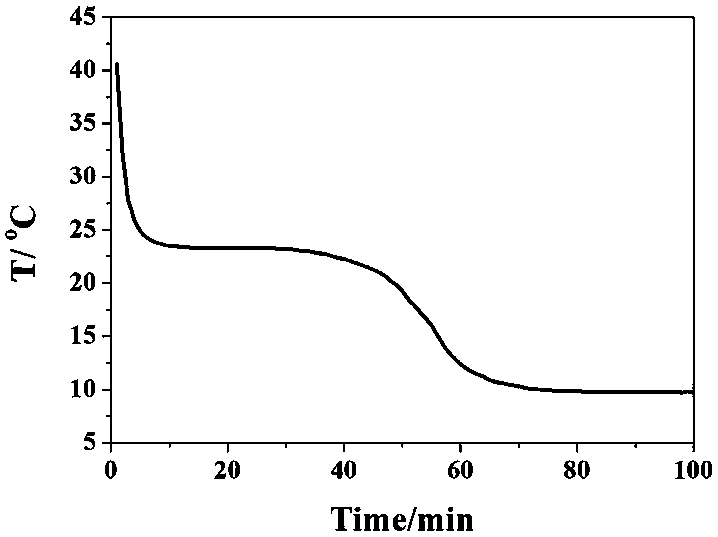
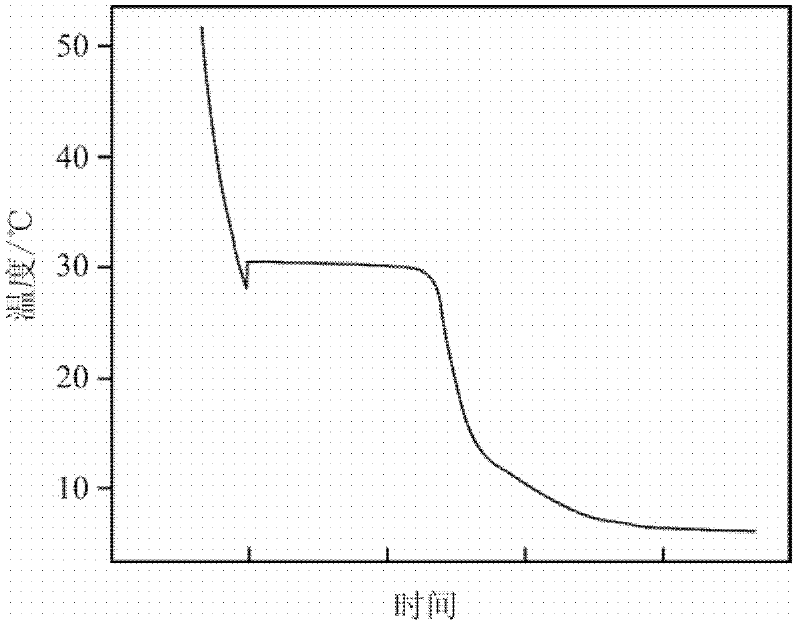
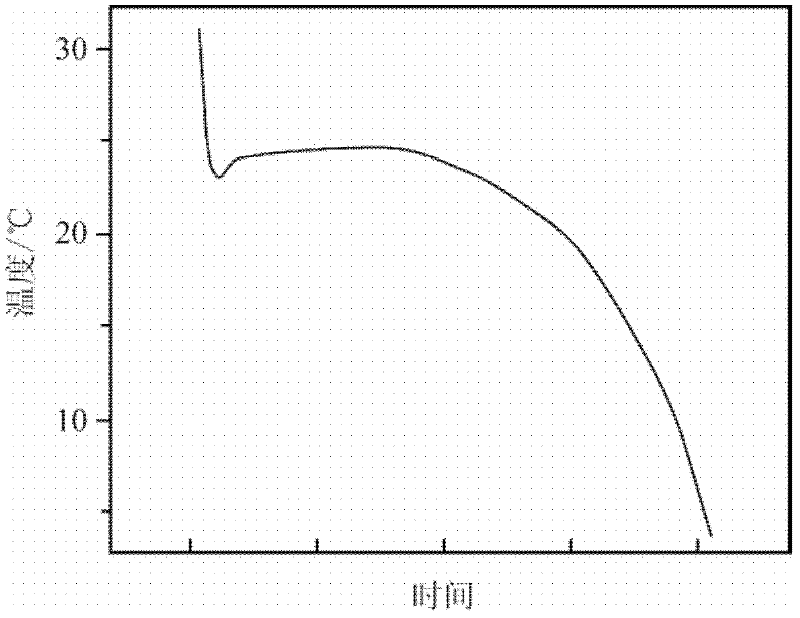
The invention discloses a phase change material and a preparation method thereof. The phase change material comprises the following preparation raw materials: calcium chloride hexahydrate, strontium chloride hexahydrate and a melting point regulator, wherein the raw materials comprise the following components in percentage by mass: 80-90% of calcium chloride hexahydrate, 5-10% of strontium chloride hexahydrate and 5-10% of the melting point regulator. The preparation method of the phase change material specifically comprises the following steps: weighing calcium chloride hexahydrate, strontium chloride hexahydrate and the melting point regulator, and mixing the raw materials according to mass parts to prepare a solution; heating the solution in a constant-temperature water bath at 60-80 DEG C, and continuously stirring until the raw materials are completely molten and the solution becomes a transparent liquid; and crystallizing the transparent liquid at a low temperature of 4-6 DEG C to obtain the phase change material. The phase change material disclosed by the invention is simple in preparation process, only needs three steps namely mixing, melting and crystallization, and is less in adopted raw material type and cheap and easily-available in raw material; and the prepared phase change material is non-toxic and non-corrosive, the phase change temperature can be adjusted between 24 DEG C and 31 DEG C, the latent heat of phase change is 120-170J / g, the super-cooling degree is less than 10 DEG C, and the phase change process is reversible.
Owner:GUANGZHOU HKUST FOK YING TUNG RES INST
Method for preparing anhydrous magnesium chloride by utilizing magnesium chloride hexahydrate
ActiveCN102491384AReduce dosageEasy to recycleMagnesium chloridesStrontium chloride hexahydrateMagnesium chloride hexahydrate
The invention relates to a method for preparing anhydrous magnesium chloride by utilizing magnesium chloride hexahydrate. The method comprises the following steps of (1) mixing and ball-milling the magnesium chloride hexahydrate and ammonium chloride to obtain ammonium camallite or mixture containing the ammonium camallite, (2) heating the mixture and performing preliminary dehydration to preparelower-water ammonium camallite or mixture containing the lower-water ammonium camallite, and (3) placing a covering on the product obtained in the step (2) and performing heating and reaction to prepare anhydrous magnesium chloride. The method for preparing the anhydrous magnesium chloride by utilizing the magnesium chloride hexahydrate can shorten production process of the anhydrous magnesium chloride, improve production efficiency and reduce production cost and input cost for environmental protection.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Efficient composite phase change material and preparation method thereof
InactiveCN108531136AGood effectAdjustable phase change temperatureHeat-exchange elementsStrontium chloride hexahydrateLithium bromide
The invention discloses an efficient composite phase change material and a preparation method thereof. The efficient composite phase change material is composed of 96.5%-75% of a thermal medium; 0.5%-3% of a nucleating agent; 3%-7% of a thickener; 1-15% of a high-efficiency conducting agent; and 0.1%-10% of a melting point regulator. The thermal medium is prepared by mixing one or more aqueous solutions of inorganic salts sodium sulfate, sodium carbonate, calcium chloride, lithium bromide and the like. The nucleating agent is any one of strontium chloride hexahydrate, potassium chloride, sodium tetraborate decahydrate, barium sulfate, or calcium fluoride. The thickener is any one or a mixture of several of the following substances in arbitrary proportion: diatomite, attapulgite, polyacrylamide and carboxymethylcellulose. The high-efficiency conducting agent is: (a) metal filler, (b) carbon filler, or (c) ceramic filler. The melting point regulator is any one of ammonium chloride, ammonium sulfate or ammonium nitrate. The efficient composite phase change material provided by the invention can rapidly play a conduction role in 60s to 180s, and the supercooling degree is 1.5DEG C.
Owner:曹斌斌
Method for recovering strontium slag
InactiveCN102328947ASolve difficult to recycleIncrease valueCalcium/strontium/barium chloridesStrontium chloride hexahydrateResource utilization
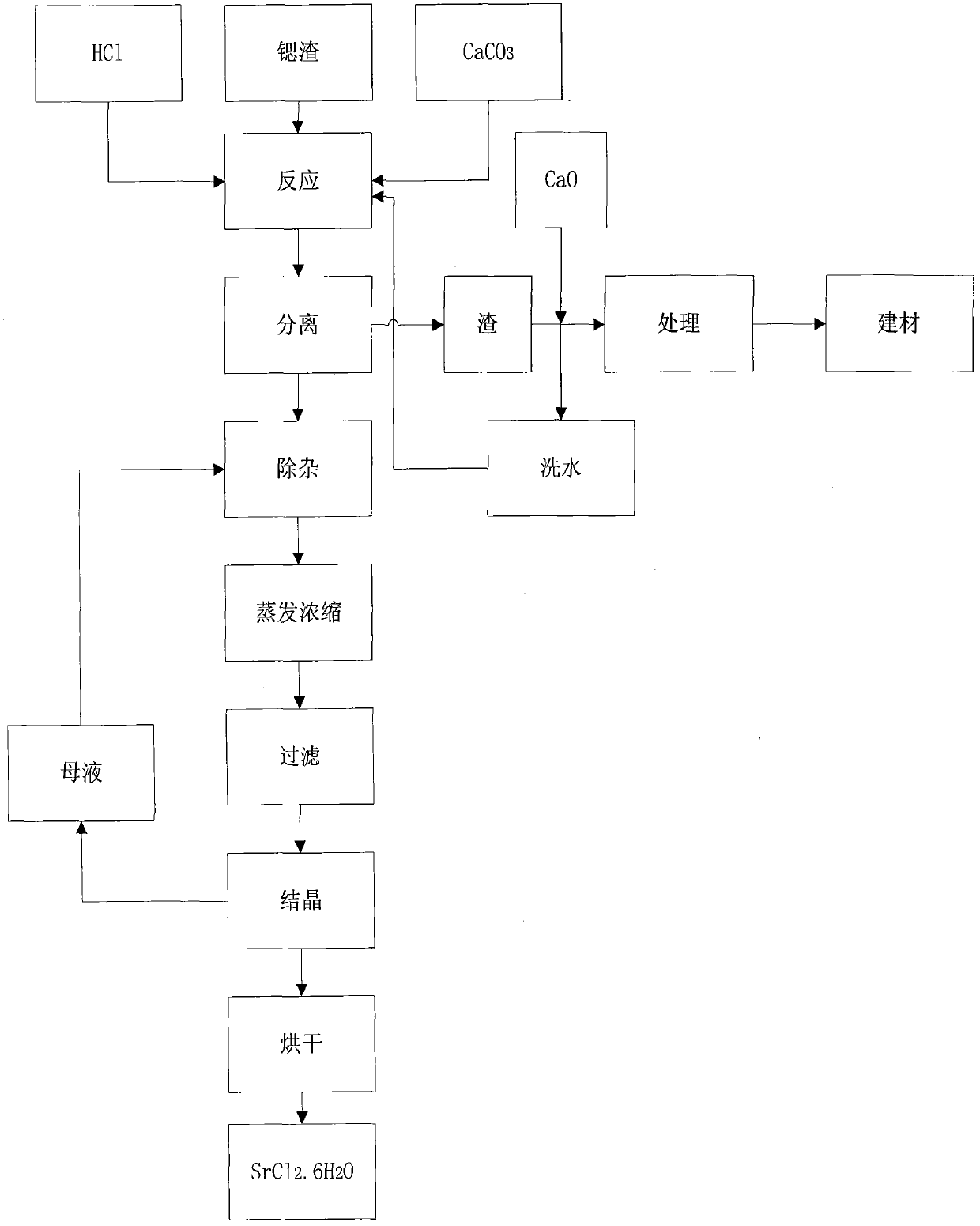
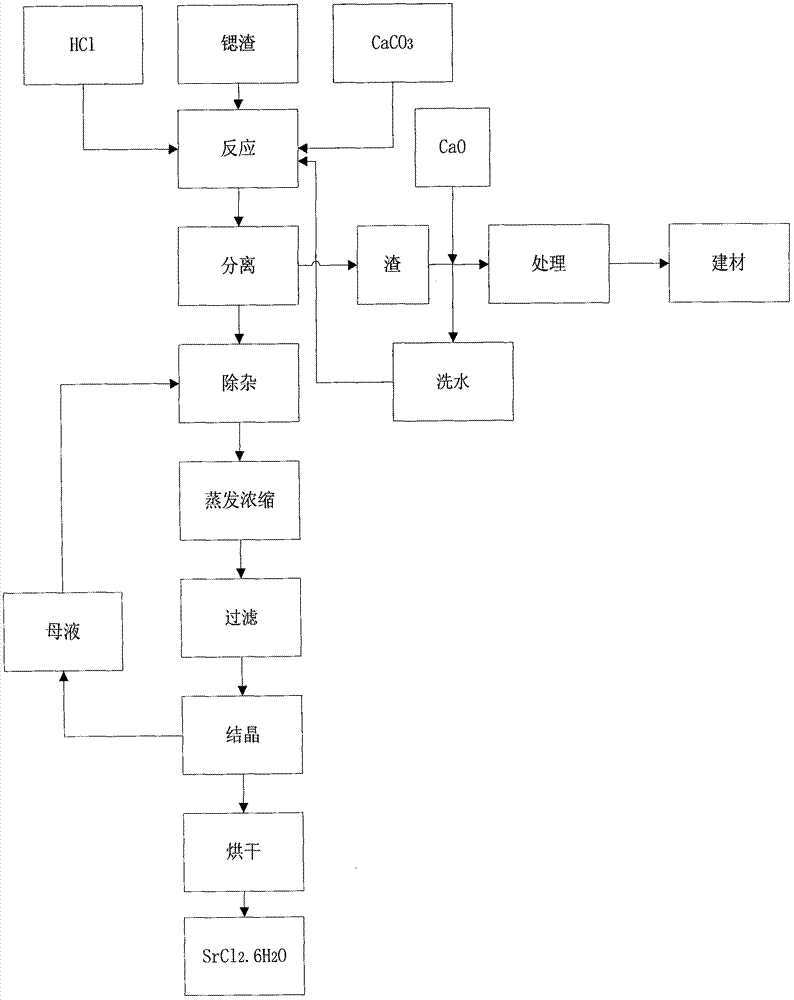
The invention relates to a method for recovering strontium slag. The method comprises the following main steps: (1) adding hydrochloric acid in the strontium slag under the condition of stirring until the pH value of a reaction system is stabilized between 1 and 2; (2) adding a calcium carbonate source substance in the reaction system obtained in the step (1) until the concentration of SO3<2-> isless than 2.0g / L; (3) regulating the pH value of the solution obtained in the step (2) to 6-7, carrying out solid-liquid separation, and washing a solid phase; and (4) decontaminating and treating mother liquor obtained in the step (3) so as to obtain a strontium chloride hexahydrate crystal product. By utilizing a double decomposition reaction of a calcium salt in a solution of strontium slag dissolved with hydrochloric acid; due to the different acid effects of sulfites of calcium and strontium under the condition of an acidic medium, the strontium salt product with relatively high value can be obtained through a replacement reaction, thereby solving the problem that strontium in strontium sulfite is difficult to recover, improving the recovery rate of the strontium salt and improving resource utilization rate.
Owner:GUIZHOU REDSTAR DEVING
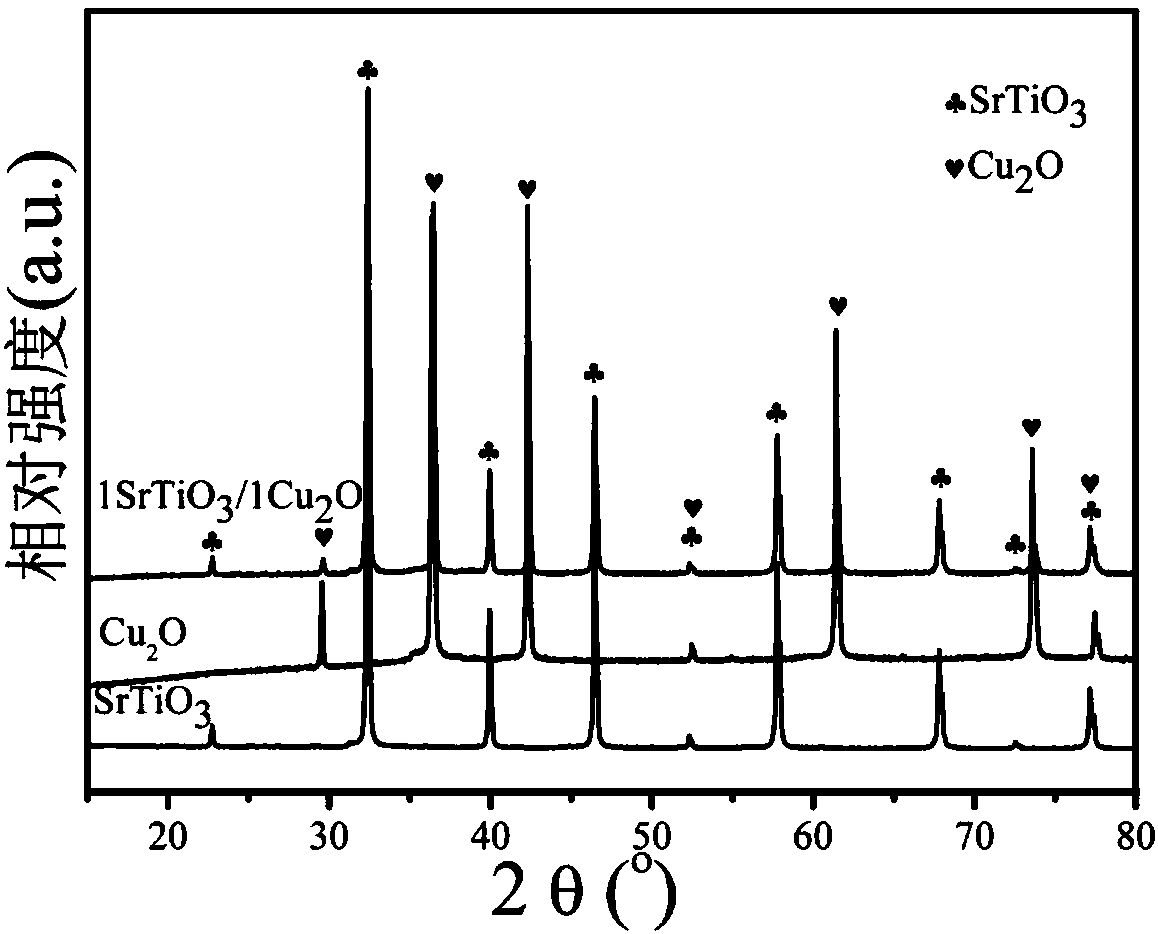
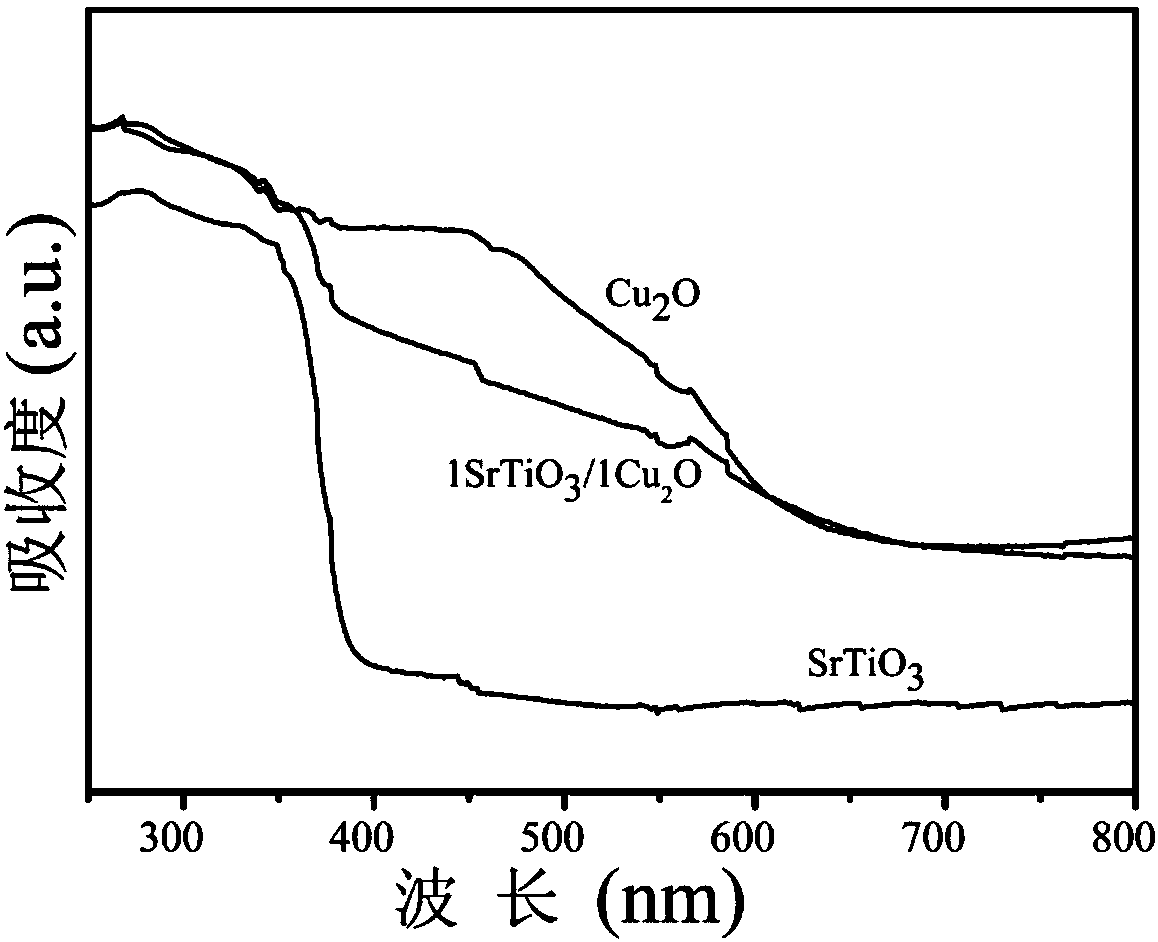
Novel preparation method and application of SrTiO3/Cu2O junction composite nanomaterial
ActiveCN107930633AUseful for pointsWater/sewage treatment by irradiationWater treatment compoundsHeterojunctionStrontium chloride hexahydrate
Owner:JIANGSU UNIV OF TECH
Biomimetic preparation method of strontium carbonate crystal
InactiveCN108675335AUse low concentrationCompact structureStrontium carbonatesNanotechnologyStrontium carbonateStrontium chloride hexahydrate
The invention discloses a biomimetic preparation method of a strontium carbonate crystal, wherein a coronary strontium carbonate nano crystal is prepared by a biomimetic method in a specific communication device by adopting strontium chloride hexahydrate and ammonium bicarbonate as reactants and sesbania gum as a biological regulation agent. According to the target product coronary strontium carbonate nano crystal, the corolla diameter is 3,000-6,000nm; the corolla is composed of nano rods with diameter being 50-80nm; the corolla structure is tight, with purity being greater than or equal to 99% and yield being 97-99%. The method disclosed by the invention has the characteristics of mild conditions, low energy consumption, large corolla diameter, tight corolla structure, high purity, highyield, low preparation cost, etc.
Owner:NANCHANG HANGKONG UNIVERSITY
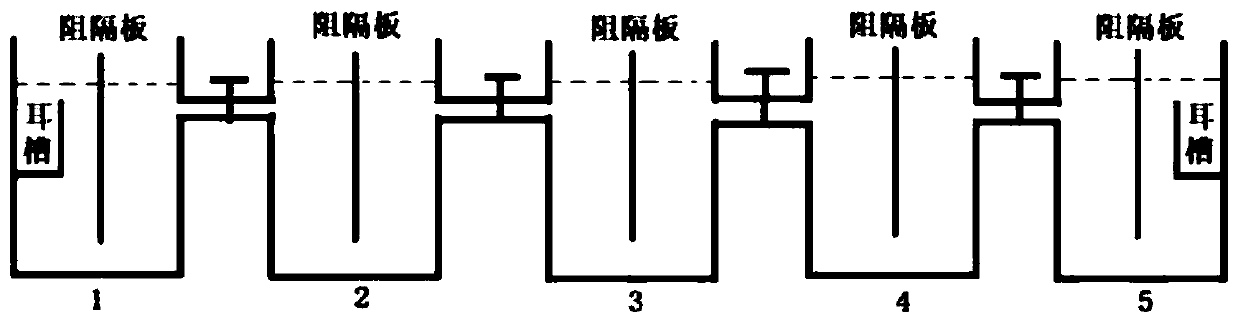
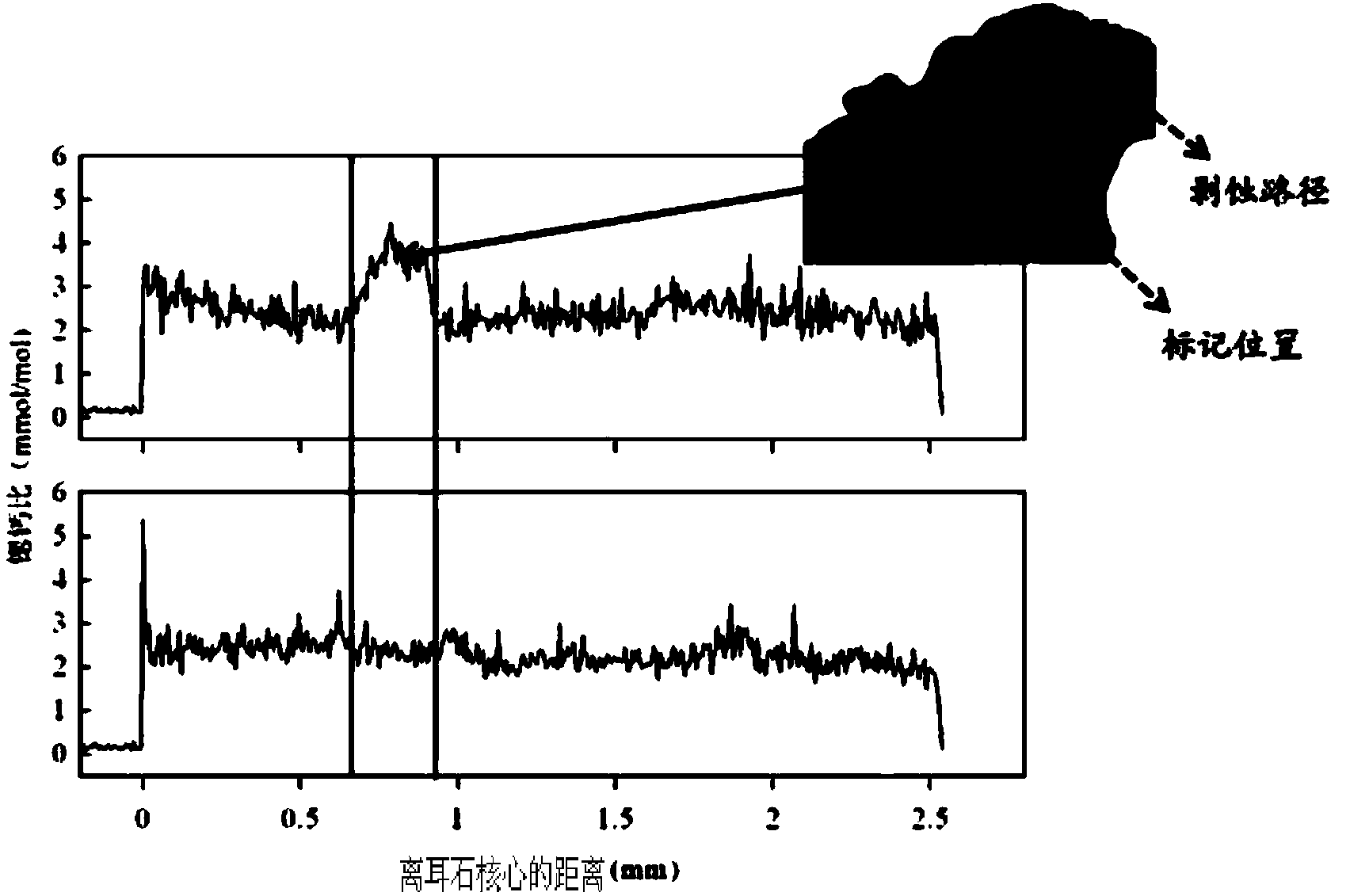
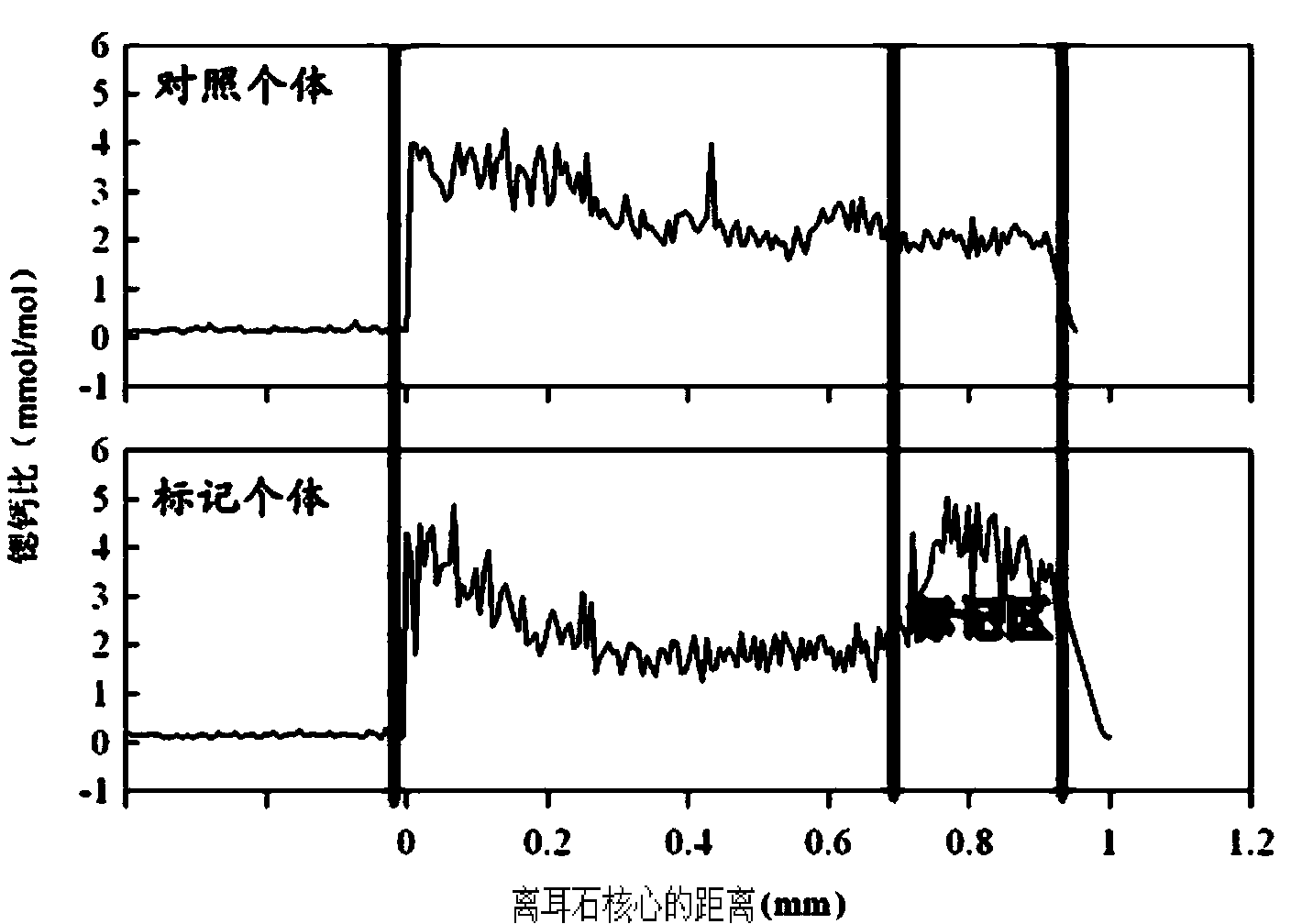
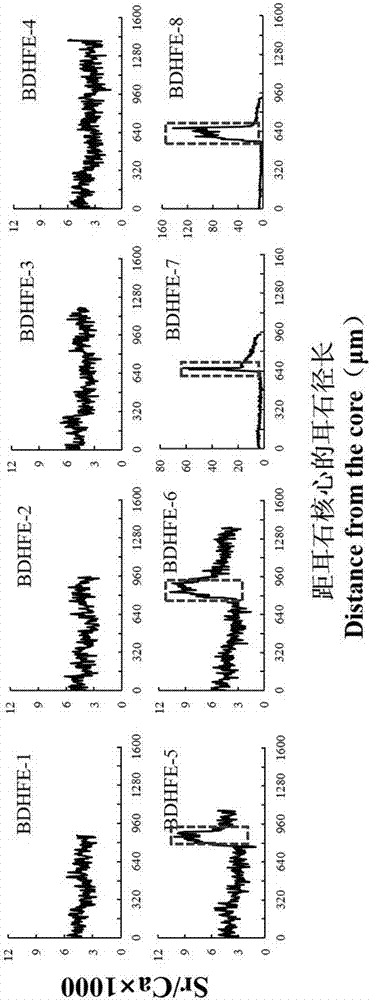
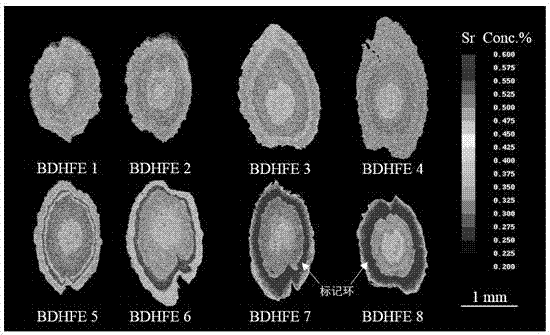
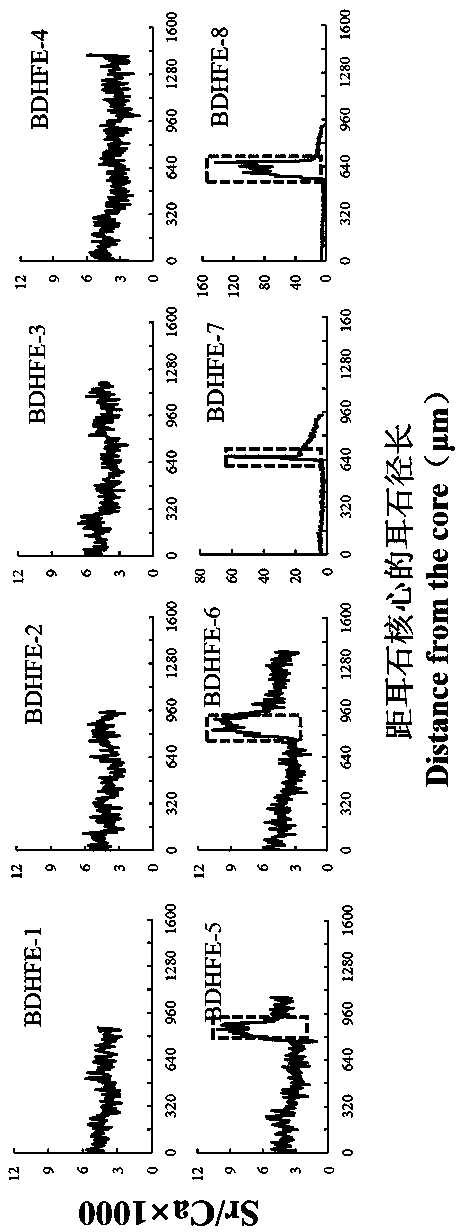
Marking method for proliferation and releasing of large yellow croakers
InactiveCN104106481ANarrow peak of Sr/Ca ratioEasy to identifyClimate change adaptationPisciculture and aquariaStrontium chloride hexahydrateStrontium chloride
The invention relates to a marking method for proliferation and releasing of large yellow croakers. The marking method includes the steps that hexahydrate strontium chloride is added in a nursery pond for releasing the large yellow croakers, the concentration of strontium ions in water of the nursery pond is increased to 0.05-0.10 g / L, strontium marking is conducted on fries with the age being 30-40 days, the marking time is 10 days, and after marking, strontium element content detection is conducted on an otolith abrasive disc. The marking method is simple in technology, has stability and convenience, can provide an effective means for evaluation of the proliferation and releasing effects of the large yellow croakers, can be used for tracking and monitoring the releasing effect of the large yellow croakers for a long-time, and provides a scientific basis for the fishery management department.
Owner:EAST CHINA SEA FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Phase-change energy storage medium
InactiveCN106221675APhase transition temperature is suitableLow costHeat-exchange elementsStrontium chloride hexahydrateCarboxymethyl cellulose
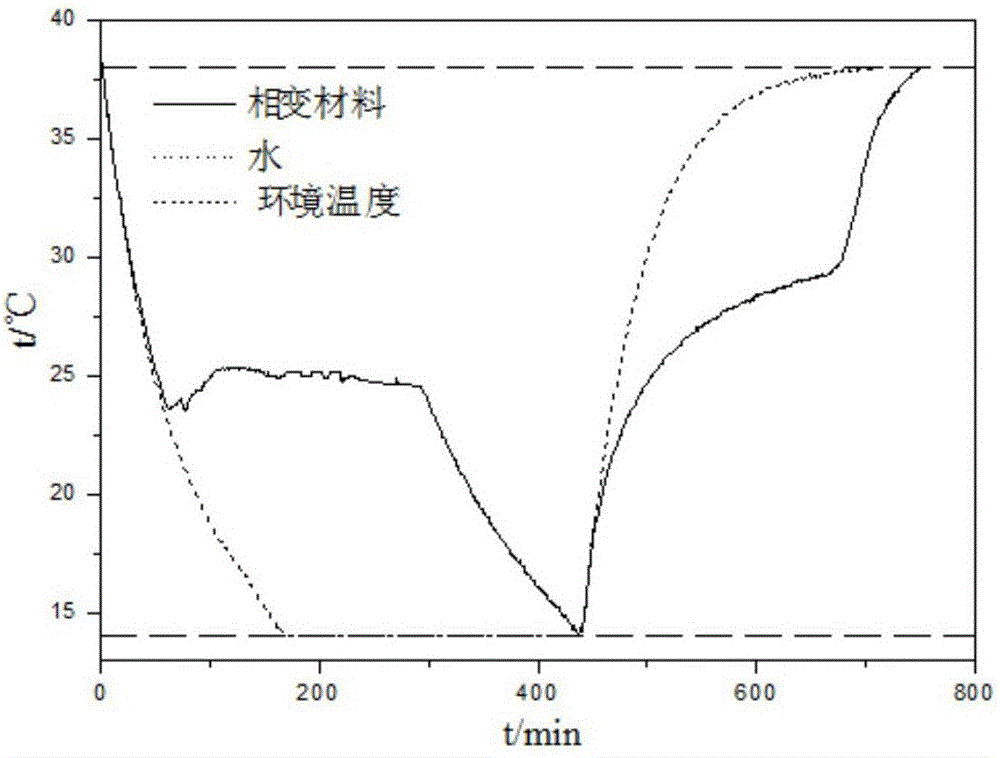
The invention disclose a room temperature phase-change energy storage medium. The phase-change energy storage medium comprises a eutectic mixture composed of water, calcium chloride, magnesium chloride and magnesium nitrate. The preparation method of the phase-change energy storage medium comprises the following steps: mixing and heating the above components according to proportion, completely melting, uniformly stirring, and adding a certain amount of strontium chloride hexahydrate and carboxymethyl cellulose, namely, the liquid phase can be used as the phase-change energy storage medium. The phase-change energy storage medium disclosed by the invention has the features that the phase-change temperature is close to room temperature, the material is environmentally friendly and the cost is low.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
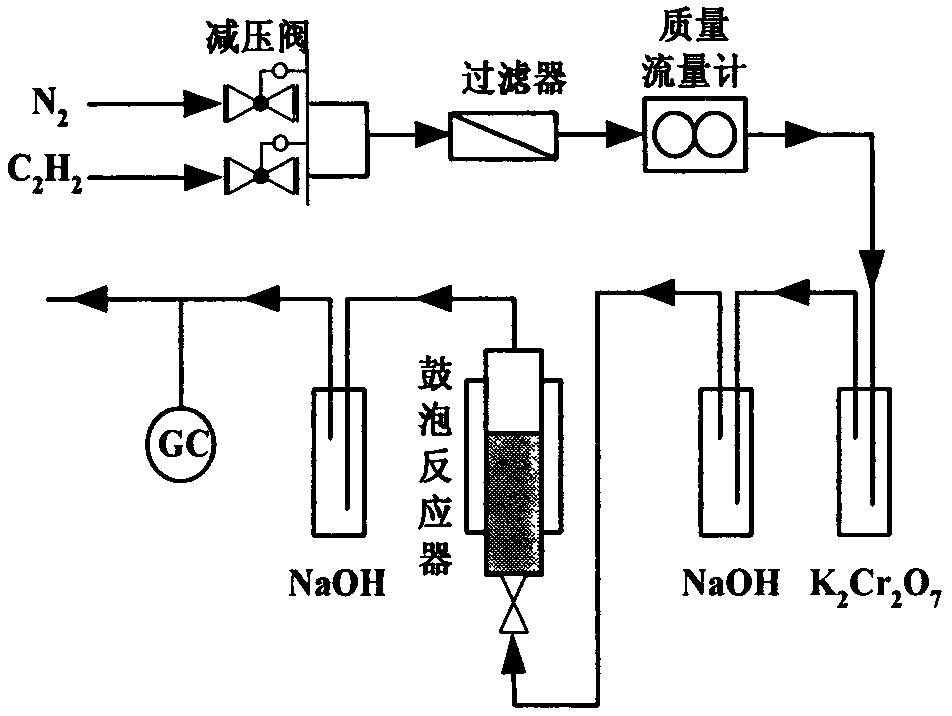
Method for preparing vinylacetylene through acetylene dimerization by utilizing modified nieuwland catalyst
InactiveCN103467236AEasy to makeLow costPhysical/chemical process catalystsHydrocarbonsStrontium chloride hexahydrateEthyl Chloride
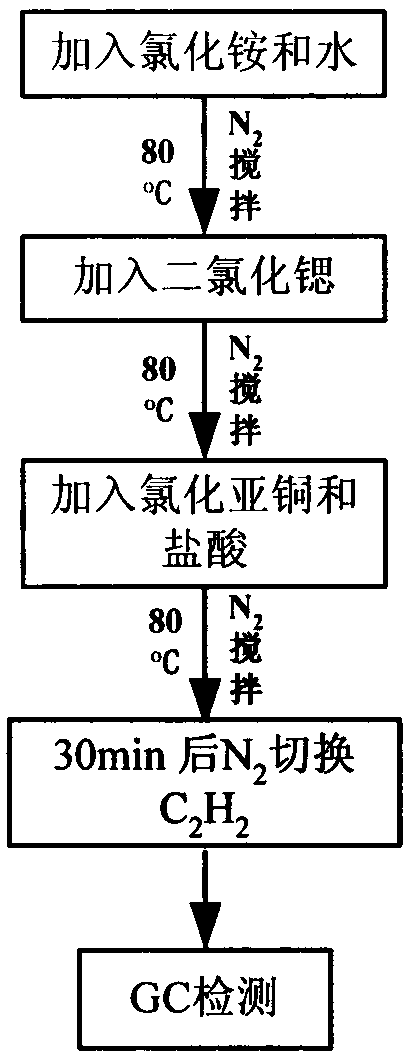
The invention relates to a method for preparing vinylacetylene through acetylene dimerization by utilizing a strontium chloride modified nieuwland catalyst and an application method. The method is characterized in that a strontium chloride hexahydrate is used as a cocatalyst, cuprous chloride is used as a main catalyst, ammonium chloride is used as a cosolvent, an acid environment is provided by hydrochloric acid, and water is used as a solvent to prepare a copper-strontium-inorganic chloride salt complex to serve as the catalyst. Under the condition, the generation of high polymers in the acetylene dimerization reaction is obviously reduced and the selectivity of vinylacetylene is significantly improved.
Owner:SHIHEZI UNIVERSITY
Production method of caking-proof strontium chloride hexahydrate
ActiveCN102303888AGuaranteed crystallizationGuaranteed complete crystallizationCalcium/strontium/barium chloridesStrontium chloride hexahydrateSlurry
The invention relates to a production method of strontium chloride hexahydrate for liquid crystal glass base plate production. The production method comprises the following steps: a, concentrating a strontium chloride solution at the temperature of less than 100 DEG C to 38-44 baume degrees; b, cooling and crystallizing the solution, wherein the specifically steps are as follows: firstly adding crystal seeds, which are strontium chloride hexahydrate crystals with a particle diameter of less than 1 millimeter, at the charging ratio above 2 kg / m<3> and controlling cooling speed; c, ageing a crystal slurry solution reaching the temperature for 1.5-3 hours under the stirring condition; and d, putting aged crystal slurry in an automatic centrifugal machine for dehydration, and drying with cold wind so as to obtain a finished product. According to the production method provided by the invention, the surface of the strontium chloride hexahydrate crystal has extremely low adhered water and low impurities; by a stacking test for 6 months, no caking phenomenon occurs; and by a stacking test for 9 months, little caking phenomenon occurs (the ratio of caking is 5-8%), and the cakes can be loosened by lightly pinching with hands.
Owner:CHONGQING YUANHE FINE CHEM
Composite phase-change material of encapsulating calcium chloride hexahydrate by calcium-based metal organic framework
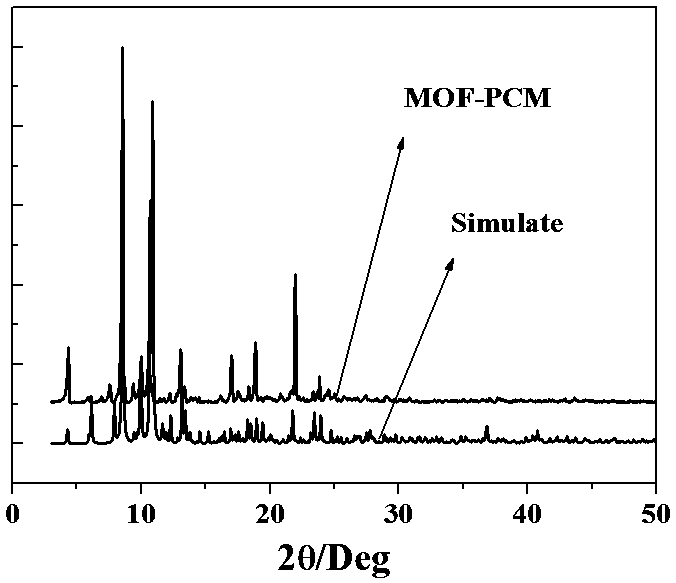
InactiveCN107794003ANo leaksSolve the coldHeat-exchange elementsHydration reactionStrontium chloride hexahydrate
The invention relates to a composite phase-change material in which calcium chloride hexahydrate is encapsulated by a calcium-based metal-organic framework and a preparation method thereof, belonging to the field of phase-change materials. The composite phase change material of the present invention is composed of 65%-90% inorganic hydrate salt phase change material-calcium chloride hexahydrate and 10%-35% calcium-based metal organic framework. The feature of the present invention is that calcium chloride hexahydrate is confined in the nano-scale pores of the metal-organic framework, and the calcium chloride that cannot be completely dissolved after melting will deposit in the pores of the metal-organic framework, which can fully contact with water to ensure the next time The solidification and crystallization process proceeds smoothly without phase separation; the crystal form difference between the calcium-based metal-organic framework and calcium chloride hexahydrate is within 15%, and the metal-organic framework can be used as a nucleation matrix for calcium chloride hexahydrate crystallization , making the supercooling of calcium chloride hexahydrate within 2oC. The composite phase change material of the present invention solves the problems of leakage, supercooling and phase separation of calcium chloride hexahydrate, and at the same time can maintain stable performance in the air, and more importantly, the raw material is cheap, the process is simple, and the reaction conditions are mild , and its industrial production has high competitiveness.
Owner:湖北赛默新能源科技有限公司
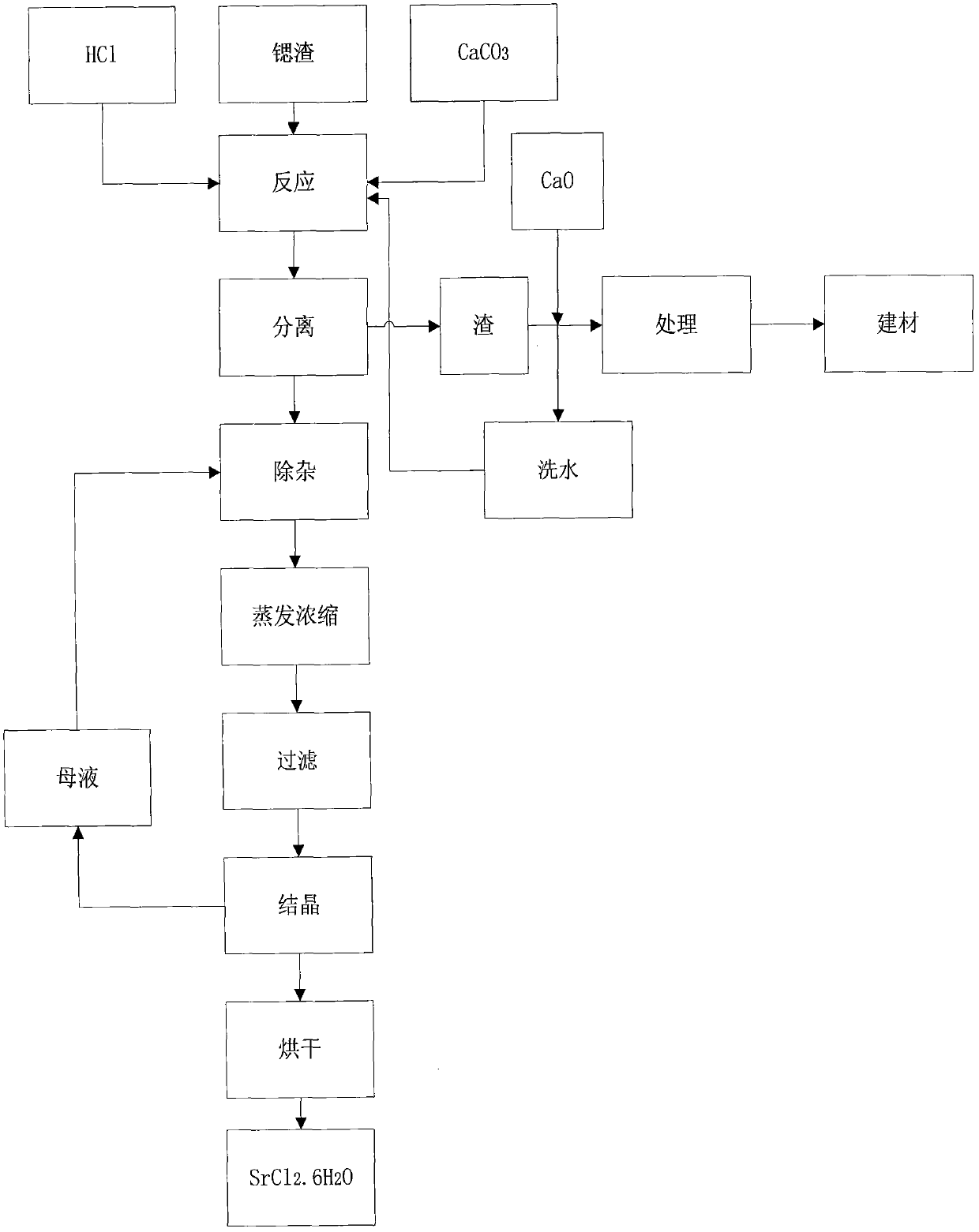
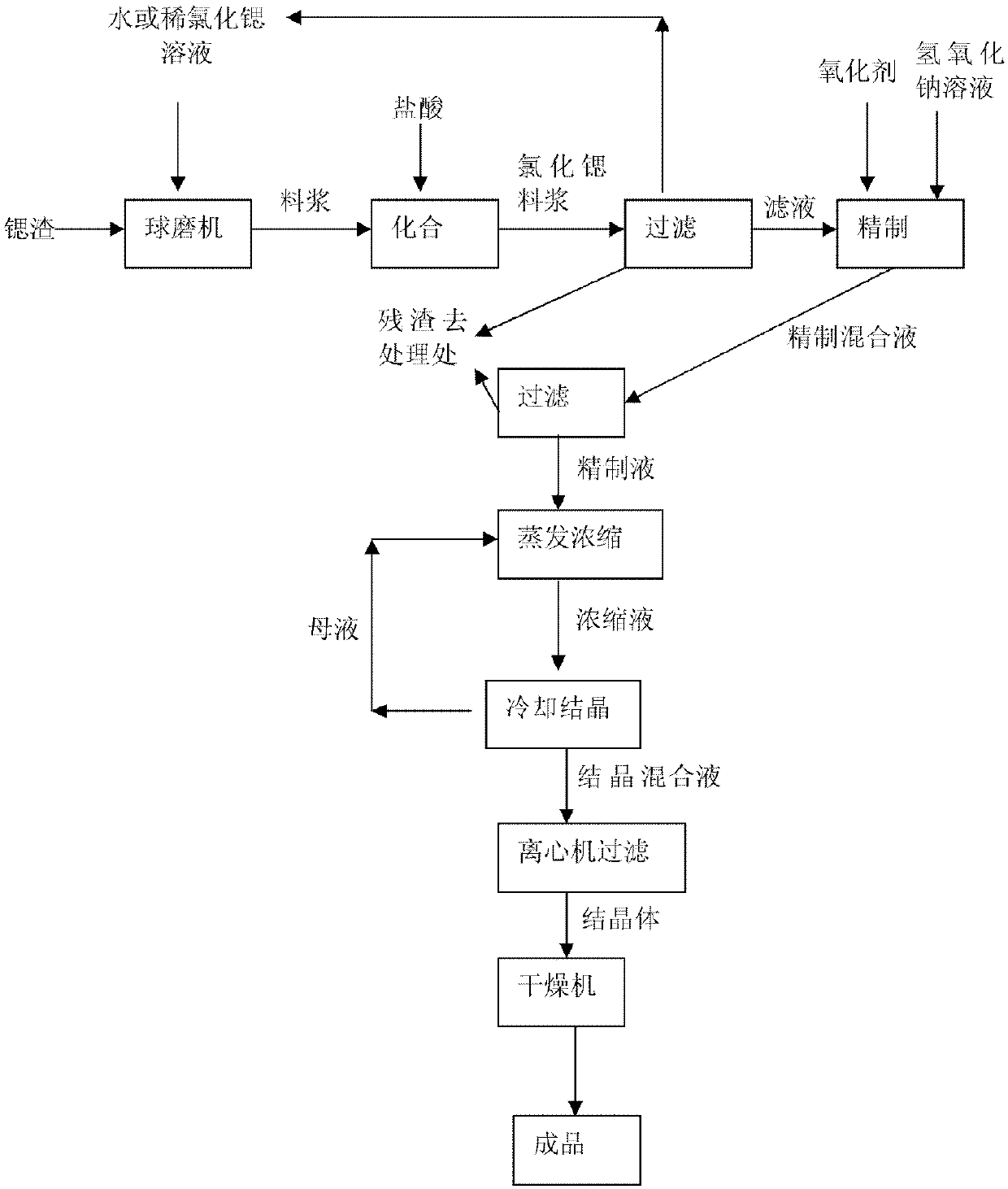
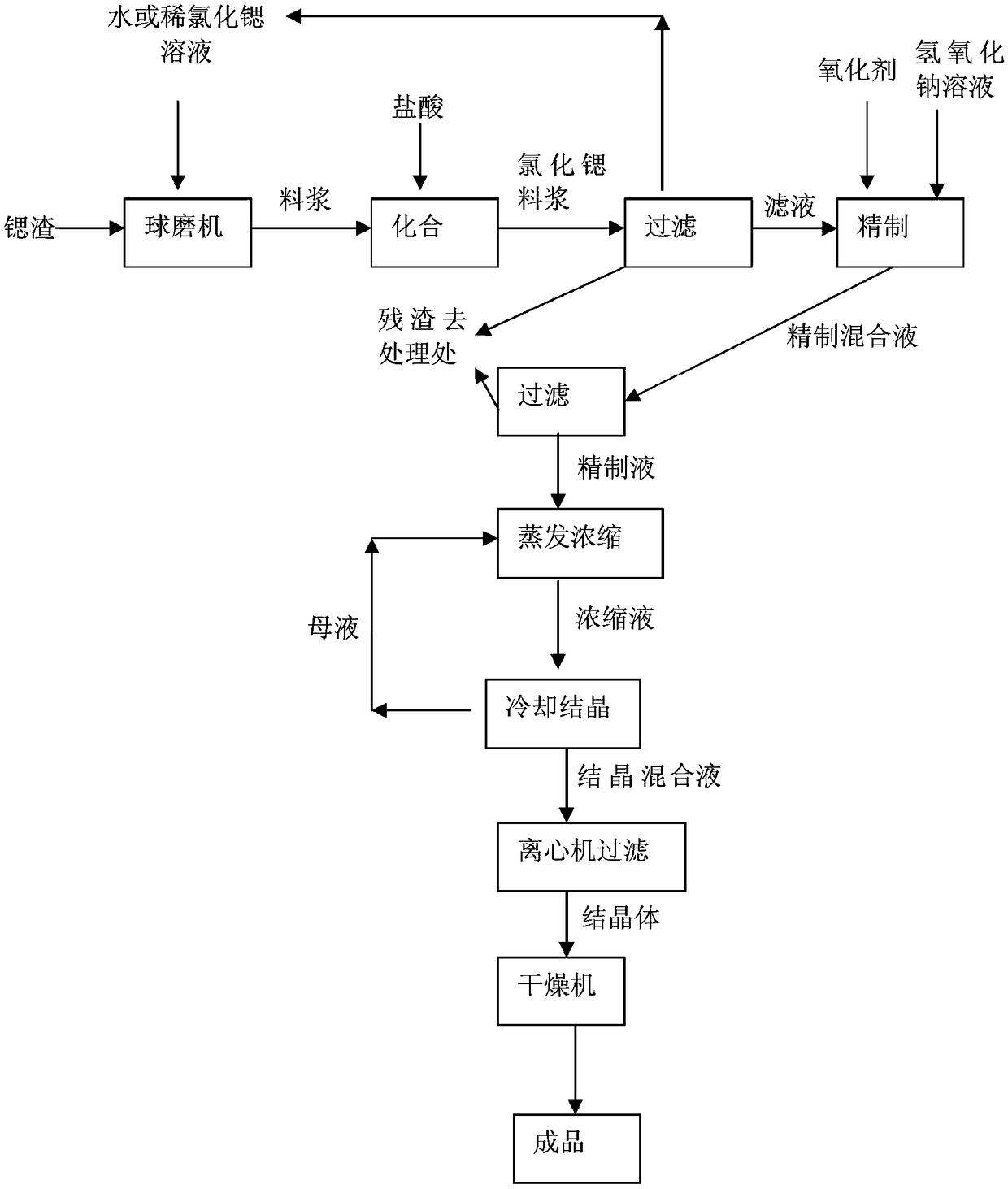
Method for producing strontium chloride by means of strontium slag, and strontium chloride prepared by using method
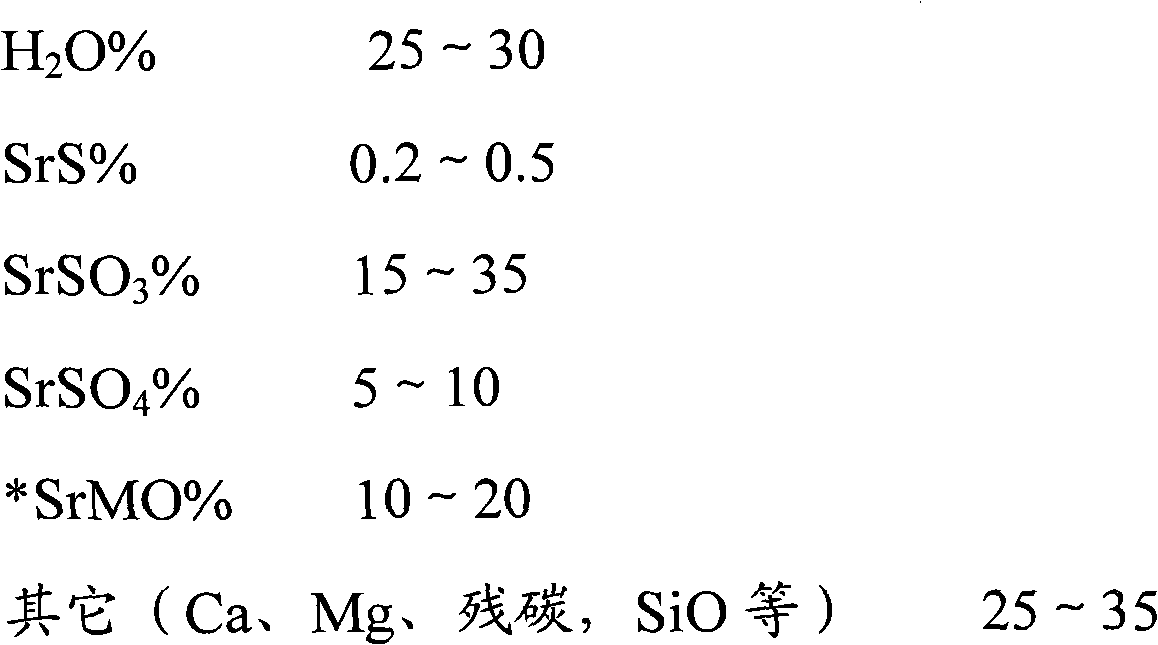
ActiveCN107673389AImprove qualityLow costCalcium/strontium/barium chloridesStrontium chloride hexahydrateSlag
The invention belongs to the field of chemical production, and discloses a method for producing strontium chloride by means of strontium slag, and the strontium chloride prepared by using the method.The invention provides the method for producing the strontium chloride by means of the strontium slag, and the strontium chloride prepared by using the method. The method for producing the strontium chloride by means of the strontium slag comprises the following steps: weighing 100-200kg of celestite and 50-250kg of feed coal, putting the weighed raw materials into a rotary kiln, carrying out reduction roasting under the high temperature condition, and grinding the strontium slag into slurry by means of a ball mill; adding hydrochloric acid into the slurry until solid particles in the slurry are no longer dissolved; filtering to remove residues, and refining filtrate; crystallizing, and filtering and washing crystals with a centrifugal machine to obtain strontium chloride hexahydrate. After the method is adopted, the quality of the strontium chloride is improved, the production efficiency is increased, and the yield is increased.
Owner:UPCHEM CHINA
Phase change energy storage material
ActiveCN102408878ASolve the problem of high supercoolingLarge latent heat of phase changeHeat-exchange elementsStrontium chloride hexahydrateCrystal structure
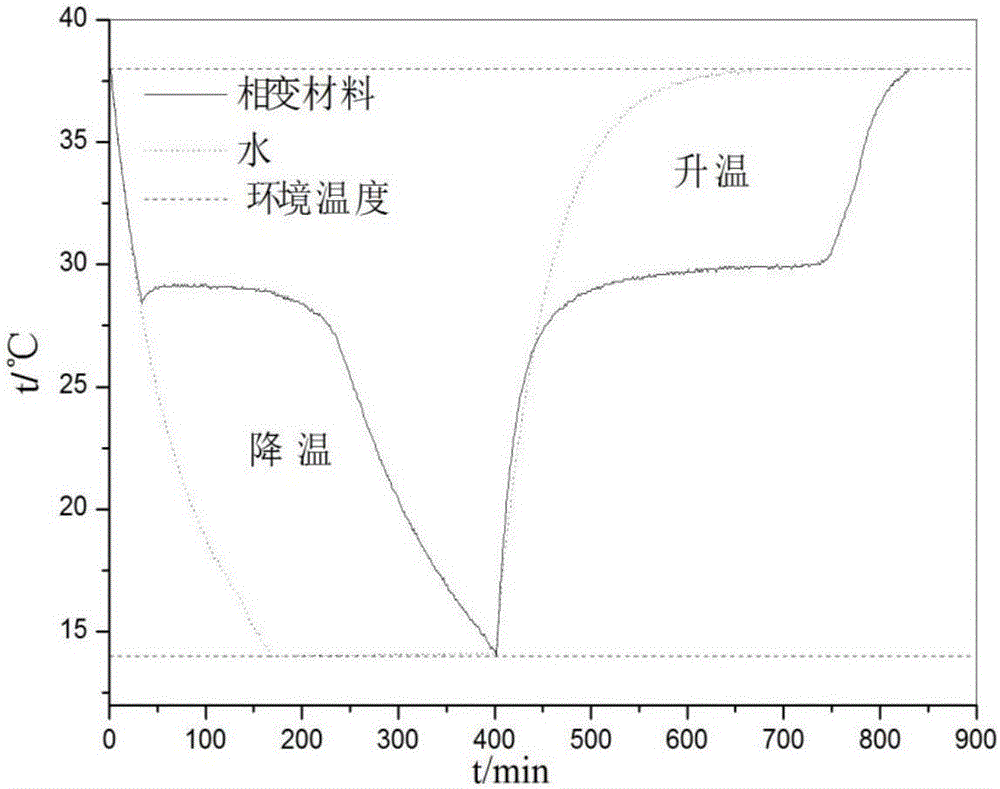
The invention discloses a phase change energy storage material composed of antarcticite, nucleating agents, thickening agents and a thermal conducting agent. The nucleating agents are barium sulfate and strontium chloride hexahydrate. Compared with prior art, the invention adopts barium sulfate and strontium chloride hexahydrate as the nucleating agents. As the nucleating agents match with antarcticite in terms of crystal structure characteristics, crystal lattice parameters, and physical properties, etc., and thickening agents and other components are added for cooperation, the problem of large supercooling degree of phase change energy storage materials in prior art is solved. Meanwhile, due to great latent heat of phase transition in antarcticite, the phase change energy storage material of the invention is also characterized by great latent heat of phase change. Experiment results show that, the phase change energy storage material prepared in the invention has a supercooling degree of only 0.4DEG C.
Owner:北京昌日新能源科技有限公司 +1
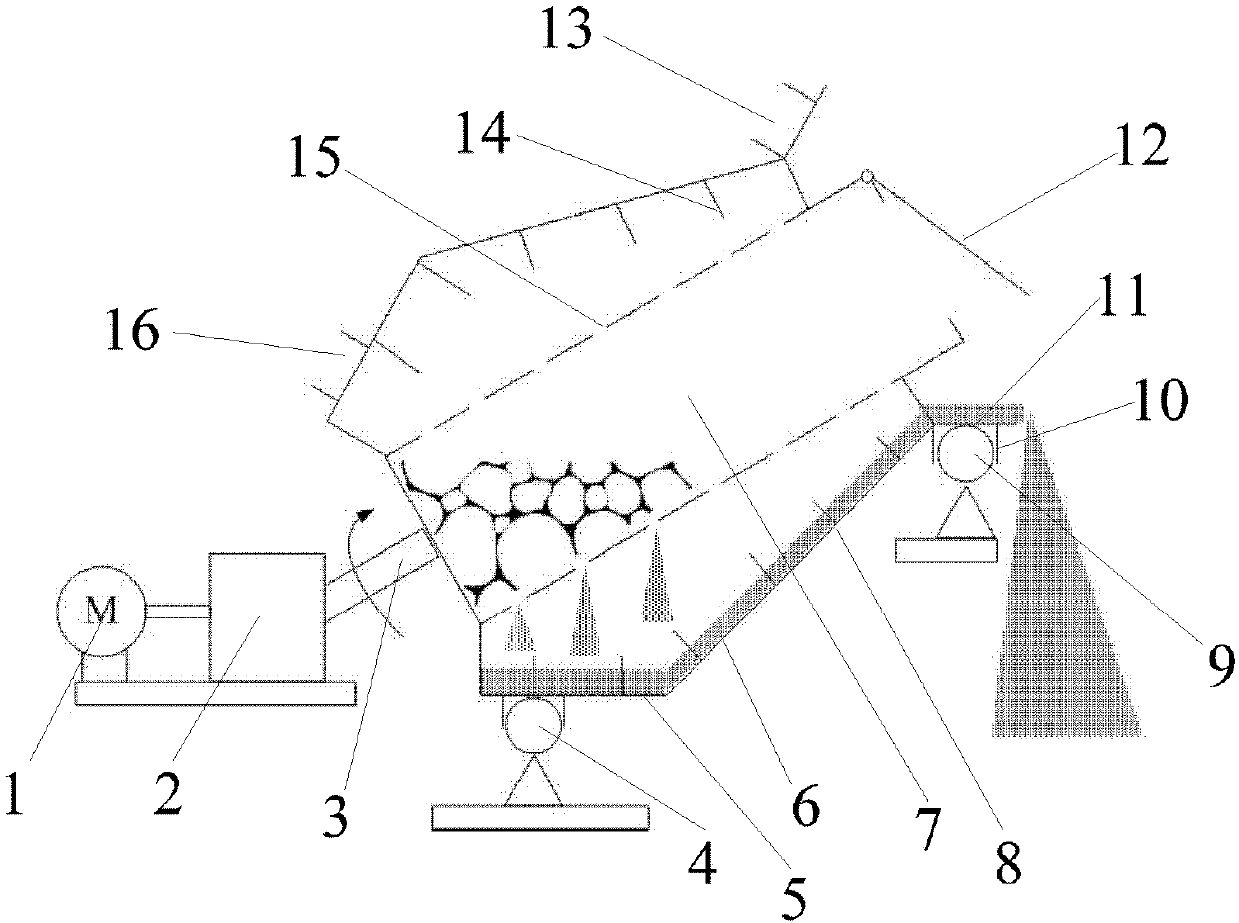
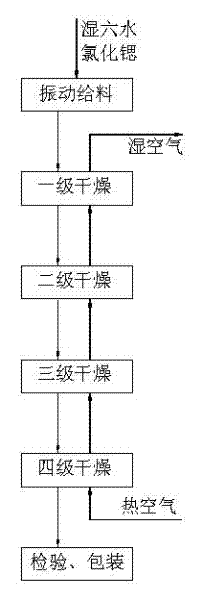
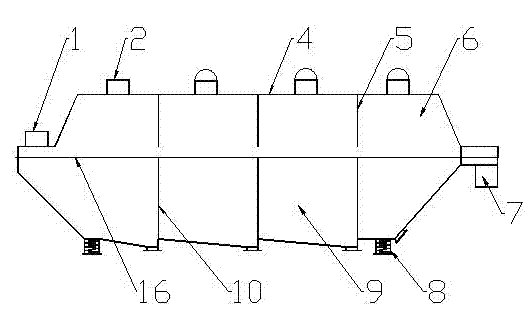
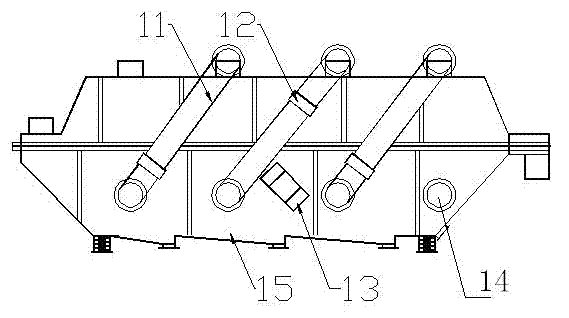
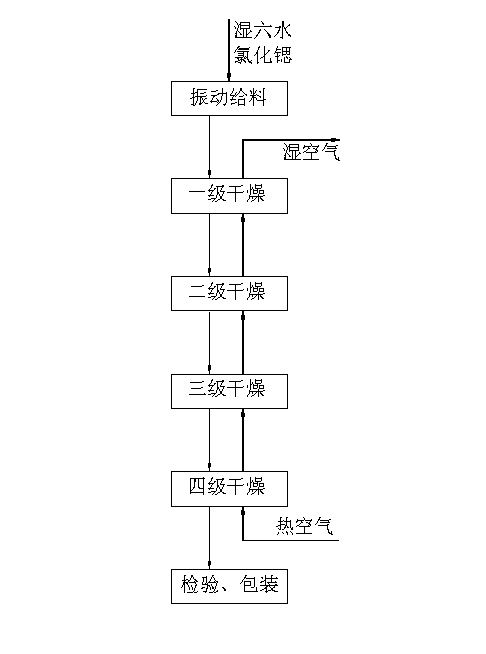
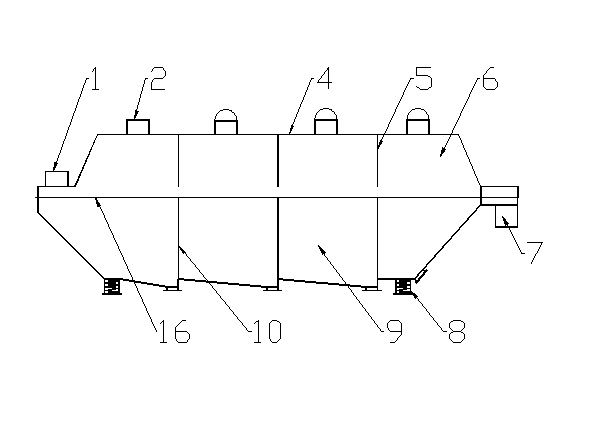
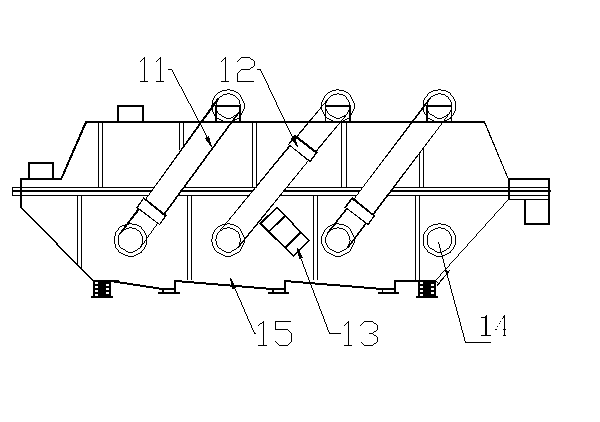
Method and device for drying strontium chloride hexahydrate
ActiveCN102226628AReduce installed capacityReduce heat consumptionDrying solid materials with heatHearth type furnacesStrontium chloride hexahydrateFluidized bed
The invention provides a method and device for drying strontium chloride hexahydrate. Strontium chloride hexahydrate sequentially passes through a plurality of fluidizing cavities of a fluidizing bed, and hot air at the temperature of 50-100 DEG C enters into the fluidizing bed from a last fluidizing cavity. The hot air reversely and sequentially enters into each fluidizing cavity to carry out counter-current drying on materials in the fluidizing cavity. By adopting the method and device provided by the invention, the drying process is easy to control. The air used for drying the materials iscontacted with the materials for multiple times, and the utilization rate of heat energy is high. The air used for drying is fully contacted with the materials, thus a product is uniformly dried, andthe phenomenon that crystal water is removed as partial strontium chloride hexahydrate is excessively dried can be prevented.
Owner:CHONGQING UNIV OF POSTS & TELECOMM
An animal cell culture medium, a preparing method thereof and applications of the culture medium
InactiveCN106032526AThe composition is simple and clearEasy to getVertebrate cellsArtificial cell constructsLithium chlorideCell culture media
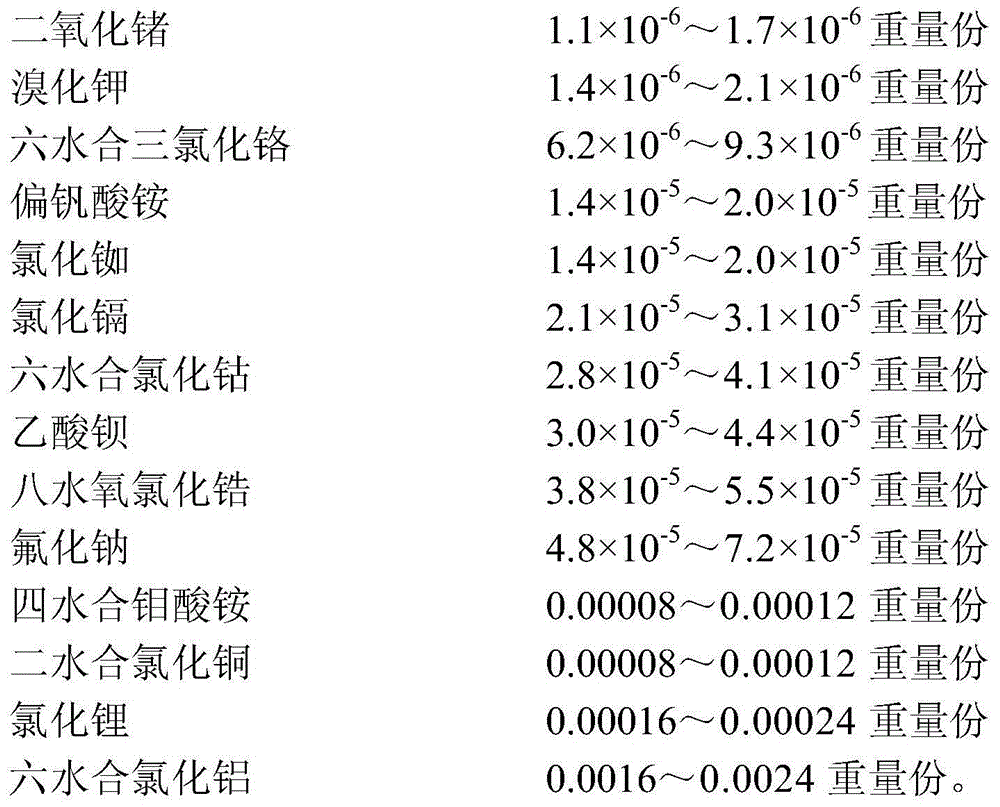
An animal cell culture medium, a preparing method thereof and applications of the culture medium are provided. The culture medium comprises 150-230 parts by weight of amino acids or salts thereof, 180-280 parts by weight of carbohydrates, 160-260 parts by weight of inorganic salts, 1-2 parts by weight of vitamins and 0.002-0.003 part by weight of trace elements. The trace elements comprise manganese chloride tetrahydrate, sodium metavanadate, selenious acid, germanium dioxide, potassium bromide, chromium chloride hexahydrate, ammonium metavanadate, rubidium chloride, cadmium chloride, cobalt chloride hexahydrate, barium acetate, zirconyl chloride octahydrate, sodium fluoride, ammonium molybdate tetrahydrate, copper chloride dihydrate, lithium chloride and aluminium chloride hexahydrate. The culture medium is simple and definite in components, convenient to prepare and use, stable in quality, and low in batch differences, and can be particularly used for culturing a plurality of animal cells. Cell growth states are good and stable.
Owner:中生天信和(无锡)生物科技有限公司
Simple method and device for preparing coral strontium carbonate crystal
InactiveCN109179473AImprove production efficiencyReduce energy consumptionStrontium carbonatesStrontium carbonateStrontium chloride hexahydrate
The invention relates to a simple method and a device for preparing a coral strontium carbonate crystal. The coral strontium carbonate crystal is prepared by using strontium chloride hexahydrate and ammonium bicarbonate as reactants and starch as a biomimetic assembly agent. The strontium chloride hexahydrate, ammonium bicarbonate and a starch solution are added into the designed reaction device,and are reacted at room temperature; a crystal generated in the device is filtered 48 h after the above reaction, is washed with distilled water three times, and then is dried at 105 DEG C for 1 h toobtain the coral strontium carbonate crystal composed of crystal rods. The diameter of every crystal rod is 100-200 nm, and the length is 0.6-2.0 [mu]m; and the purity of the product is equal to or more than 99%, and the yield is 95-97%. The method and the device have the characteristics of high preparation efficiency, environmental friendliness, low energy consumption, easiness in industrial production, and low production cost.
Owner:NANCHANG HANGKONG UNIVERSITY
Flounder-otolith-element marking method
ActiveCN107258647AImprove stabilityPermanent preservationAccessory food factorsPisciculture and aquariaHigh concentrationStrontium chloride hexahydrate
The invention provides a flounder-otolith-element marking method. The flounder-otolith-element marking method is characterized by including the following steps that (1) strontium chloride hexahydrate and fish feed are mixed to be even, a strontium element is contained in the fish feed, and marked feed is obtained; (2) to-be-marked flounders are fed with the marked feed; (3) the flounders are fed with the marked feed for 1 time to 2 times every day, and feeding time is at least 3 days; (4) the content of the strontium chloride hexahydrate at least accounts for 0.8% of the whole mass of the marked feed. The strontium element can be precipitated on a growth ring of otolith through the physiological process such as blood transmission, an element annule is formed, and the fingerprint marker effect of releasing a flounder otolith element is generated; the fingerprint marker stability of the otolith strontium element is good, is not influenced by the outside, and can be preserved permanently; the strontium chloride hexahydrate are harmless to to-be-marked fishes, and both the fatality rate of the high concentration and the fatality rate of the low concentration are quite low.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI +1
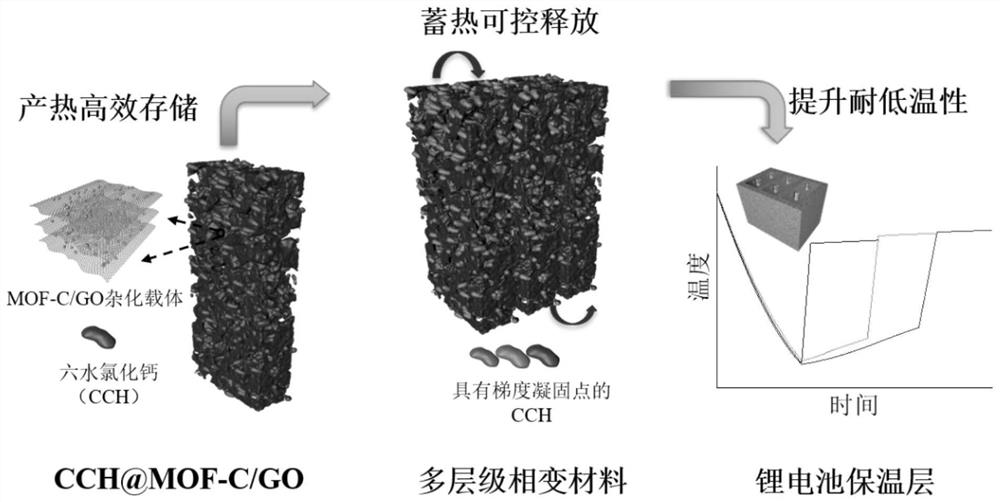
Multi-level phase change composite material and preparation method and application thereof
ActiveCN113652208AReduce heat exchangeImprove low temperature resistanceSecondary cellsHeat-exchange elementsStrontium chloride hexahydrateFreeze-drying
The invention provides a multi-level phase change composite material and a preparation method and application thereof.The preparation method adopts a step-by-step self-assembly method and comprises the steps that firstly, an organic metal frame MIL-100 (Fe) is synthesized through a hydrothermal method, high-temperature roasting treatment is conducted, and a carbide MOF-C is prepared; MOF-C and graphene oxide are mixed to form a solution, hydrothermal synthesis is performed and freeze-drying is performed to form an aerogel carrier; a phase-change material calcium chloride hexahydrate is loaded by adopting vacuum impregnation, the freezing point of each gradient phase-change layer is adjusted by changing the addition amount of a nucleating agent strontium chloride hexahydrate, and finally the phase-change layers with different freezing points are combined into the multi-level phase-change composite material through an adhesive. The multi-level composite phase change material provided by the invention can efficiently store waste heat of the battery, and can controllably release the stored heat when the environment temperature is reduced to heat the battery, so that the low-temperature resistance of the battery is improved.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Inorganic phase-change constant-temperature material and preparation method thereof
InactiveCN111117573APrevent leakageImprove performanceHeat-exchange elementsStrontium chloride hexahydrateCellulose
Owner:北京中海前沿材料技术有限公司
SrTiO3 nanometer material preparation method
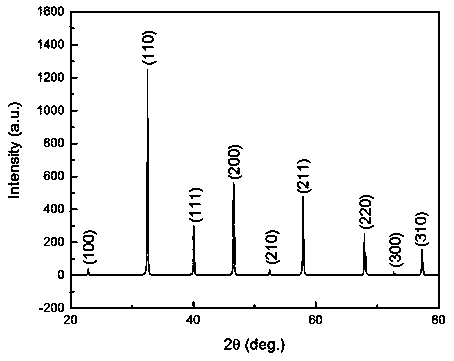
InactiveCN108706626AThe experiment process is simpleEasy to operateTitanium compoundsAlkaline earth titanatesStrontium titanateStrontium chloride hexahydrate
The invention discloses an SrTiO3 nanometer material preparation method and belongs to the field of functional materials. The SrTiO3 nanometer material preparation method comprises the following stepsof by adopting methanol as a solvent, adding chemical raw materials including citric acid, strontium chloride hexahydrate and butyl titanate in the methanol solvent, stirring and dissolving to obtaina mixed solution, and performing gel formation, drying and calcining treatment on the solution to obtain a strontium titanate material with excellent performance. For the preparation method providedby the invention, by adopting the methanol as the solvent, the method is simple in technological process, obtained strontium titanate is stable in quality, high in purity and good in uniformity, the preparation period is greatly shortened, and the production efficiency is improved at the same time.
Owner:KUNMING UNIV OF SCI & TECH
Calcium chloride hexahydrate composite phase change thermal insulation mortar
The invention discloses calcium chloride hexahydrate-containing composite phase change thermal insulation mortar, which comprises mortar, and is characterized in that the mortar also comprises 5-30 wt% of a phase change energy storage material, the phase change energy storage material comprises calcium chloride hexahydrate, strontium chloride hexahydrate and sodium carboxymethyl cellulose, the weight part ratio of calcium chloride hexahydrate to strontium chloride hexahydrate to sodium carboxymethyl cellulose is (85-99): (1-10): (1-5). The composite phase change material is added into the thermal insulation mortar, so that the thermal insulation performance of the mortar is greatly improved, a good living environment is provided for organisms in a greenhouse, meanwhile, the use of fossil fuel is reduced, energy is saved, and the construction progress of ecological rural areas is promoted.
Owner:HEBEI AGRICULTURAL UNIV.
Preparation device and method of bundle-shaped strontium carbonate crystal
InactiveCN109019654ALow priceHigh purityMaterial nanotechnologyStrontium carbonatesStrontium chloride hexahydrateStrontium carbonate
The invention provides a preparation device and method of a bundle-shaped strontium carbonate crystal. In a reaction device, strontium chloride hexahydrate and ammonium bicarbonate are used as reactants and gelatin is used as a bionic induction agent to prepare the bundle-shaped strontium carbonate crystal. After reaction is finished, a product is filtered and washed and is dried at 105 DEG C for1h to obtain the bundle-shaped strontium carbonate crystal composed of a crystal bar. The reaction device is composed of a No. 1 material groove, a sedimentation groove and a No. 2 material groove. The diameter of the crystal bar in bundle-shaped strontium carbonate is 30 to 50nm and the length of the crystal bar is 200 to 700nm; the product has the purity greater than or equal to 99 percent of the yield of 96 to 98 percent. The product provided by the invention has the advantages of high purity, high yield, small diameter, large crystal length-diameter ratio, low raw material price, simple and feasible method, low energy consumption, convenience for industrial production and the like.
Owner:NANCHANG HANGKONG UNIVERSITY
Method for producing strontium chloride from strontium slag
InactiveCN109502623ALow costSave resourcesCalcium/strontium/barium chloridesStrontium chloride hexahydrateFiltration
The invention discloses a method for producing strontium chloride from strontium slag, which comprises the following steps that S100, strontium slag is sent into a ball mill; S200, the strontium slagis ground into slurry by the ball mill; S300, hydrochloric acid is added into the slurry until solid particles in the slurry are not dissolved anymore; S400, residual is removed through filtration, and the filtrate is refined; S500, cooling crystallization is carried out; S600, the crystals are filtered and washed by a centrifuge, so that strontium chloride hexahydrate is obtained. The invention realizes the recycling of strontium salt.
Owner:赵怡欣
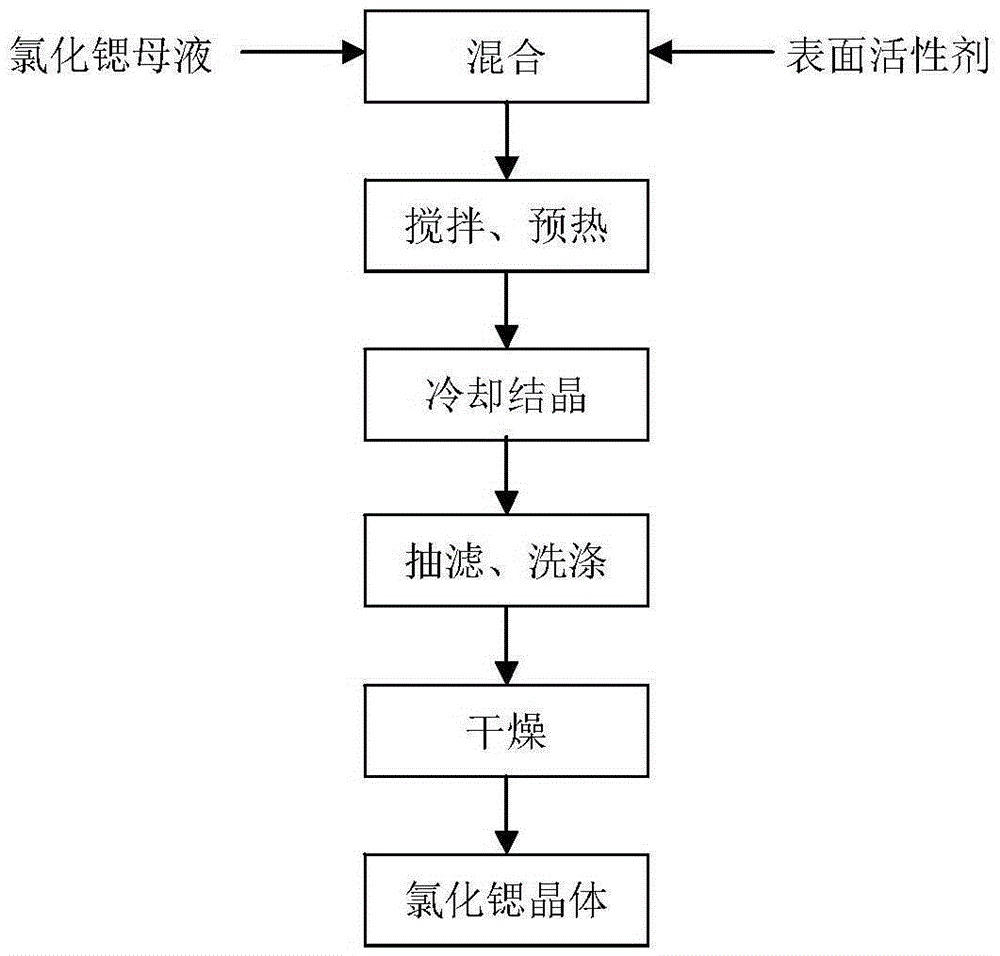
A kind of preparation method of gritty strontium chloride hexahydrate
ActiveCN104211101BSimple processLow costCalcium/strontium/barium chloridesStrontium chloride hexahydrateFiltration
The invention relates to a preparation method of a coarse sand-shaped strontium chloride hexahydrate. The preparation method comprises the following steps: adding a strontium chloride mother solution and a morphological control agent in a crystallizer according to a certain proportion, preheating and stirring to ensure that the strontium chloride mother solution and the morphological control agent are fully mixed; performing cooling crystallization at a constant cooling rate and a stirring rotating speed; performing suction-filtration; washing; and drying to prepare the strontium chloride hexahydrate product. According to the preparation method disclosed by the invention, due to the existence of a small amount of morphological control agent, the growth orientation of crystals in a crystallization process is remarkably changed, and thus the crystals of strontium chloride hexahydrate are changed into coarse sand shapes from needle shapes. After being dried, the strontium chloride hexahydrate product which is large in particle size, good in morphology and uniform in distribution can be obtained; and the strontium chloride hexahydrate product can be easily filtered, resists agglomeration and has good liquidity.
Owner:EAST CHINA UNIV OF SCI & TECH
Cleaning solution for antibacterial repair and preparation method of cleaning solution
InactiveCN109091497AGood antibacterial effectImprove nasal congestionAntibacterial agentsHydroxy compound active ingredientsStrontium chloride hexahydratePotassium
The invention belongs to the field of nasal nursing and particularly relates to a cleaning solution for antibacterial repair and a preparation method of the cleaning solution. The cleaning solution isprepared from 15%-25% of sodium chloride, 8%-12% of magnesium chloride hexahydrate, 0.5%-1% of calcium chloride, 0.1%-0.5% of potassium chloride, 0.01%-0.05% of strontium chloride hexahydrate, 0.5%-3% of anhydrous sodium sulfate, 0.05%-0.2% of sodium hydrogen carbonate, 0.002%-0.2% of xylitol, 0.1%-0.5% of alum, 0.01%-0.3% of hyaluronic acid, 0.005%-0.1% of menthol and the balance of water. The cleaning solution has remarkable bacteriostasis effect, can obviously improve symptoms of nasal obstruction, rhinocnesmus, inflammation and mycteroxerosis of rhinitis patients, and is high in safety and suitable for masses of rhinitis patients.
Owner:GUANGZHOU RAINHOME PHARM&TECH CO LTD
Method for recovering strontium slag
InactiveCN102328947BSolve difficult to recycleIncrease valueCalcium/strontium/barium chloridesStrontium chloride hexahydrateResource utilization
The invention relates to a method for recovering strontium slag. The method comprises the following main steps: (1) adding hydrochloric acid in the strontium slag under the condition of stirring until the pH value of a reaction system is stabilized between 1 and 2; (2) adding a calcium carbonate source substance in the reaction system obtained in the step (1) until the concentration of SO3<2-> isless than 2.0g / L; (3) regulating the pH value of the solution obtained in the step (2) to 6-7, carrying out solid-liquid separation, and washing a solid phase; and (4) decontaminating and treating mother liquor obtained in the step (3) so as to obtain a strontium chloride hexahydrate crystal product. By utilizing a double decomposition reaction of a calcium salt in a solution of strontium slag dissolved with hydrochloric acid; due to the different acid effects of sulfites of calcium and strontium under the condition of an acidic medium, the strontium salt product with relatively high value can be obtained through a replacement reaction, thereby solving the problem that strontium in strontium sulfite is difficult to recover, improving the recovery rate of the strontium salt and improving resource utilization rate.
Owner:GUIZHOU REDSTAR DEVING
Method for drying strontium chloride hexahydrate
ActiveCN102226628BReduce installed capacityReduce heat consumptionDrying solid materials with heatHearth type furnacesStrontium chloride hexahydrateFluidized bed
Owner:CHONGQING UNIV OF POSTS & TELECOMM
A method for labeling otolith elements of flounder fish
ActiveCN107258647BImprove stabilityPermanent preservationAccessory food factorsPisciculture and aquariaStrontium chloride hexahydrateHigh concentration
The invention provides a flounder-otolith-element marking method. The flounder-otolith-element marking method is characterized by including the following steps that (1) strontium chloride hexahydrate and fish feed are mixed to be even, a strontium element is contained in the fish feed, and marked feed is obtained; (2) to-be-marked flounders are fed with the marked feed; (3) the flounders are fed with the marked feed for 1 time to 2 times every day, and feeding time is at least 3 days; (4) the content of the strontium chloride hexahydrate at least accounts for 0.8% of the whole mass of the marked feed. The strontium element can be precipitated on a growth ring of otolith through the physiological process such as blood transmission, an element annule is formed, and the fingerprint marker effect of releasing a flounder otolith element is generated; the fingerprint marker stability of the otolith strontium element is good, is not influenced by the outside, and can be preserved permanently; the strontium chloride hexahydrate are harmless to to-be-marked fishes, and both the fatality rate of the high concentration and the fatality rate of the low concentration are quite low.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI +1
Inorganic phase-change constant-temperature material and preparation method thereof
InactiveCN111073605APrevent leakageImprove performanceHeat-exchange elementsStrontium chloride hexahydratePotassium persulfate
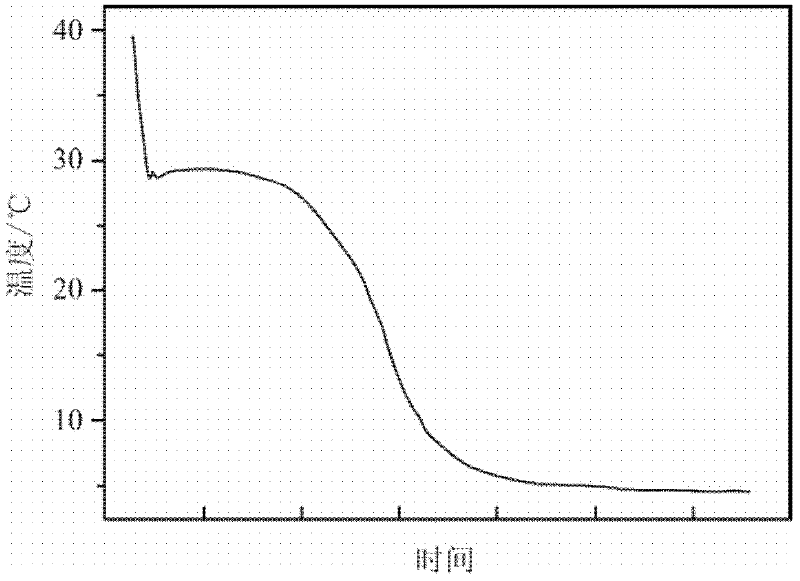
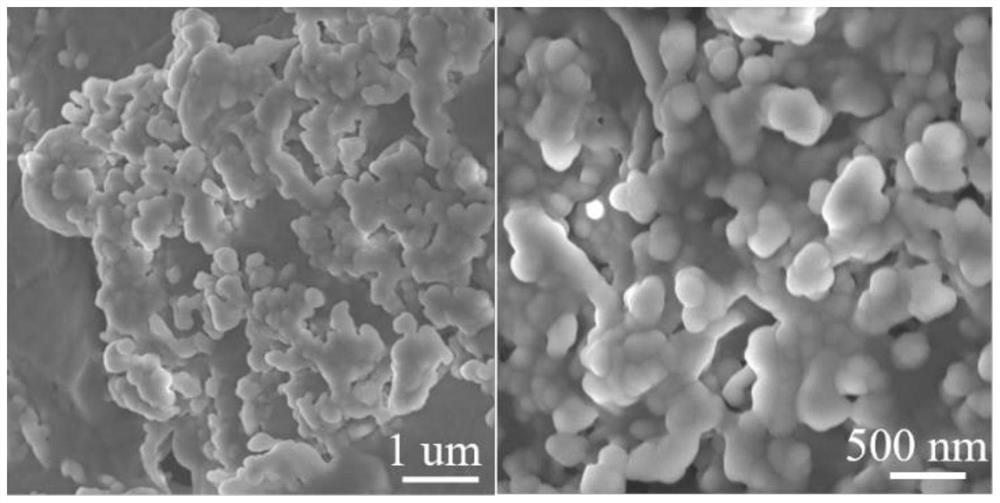
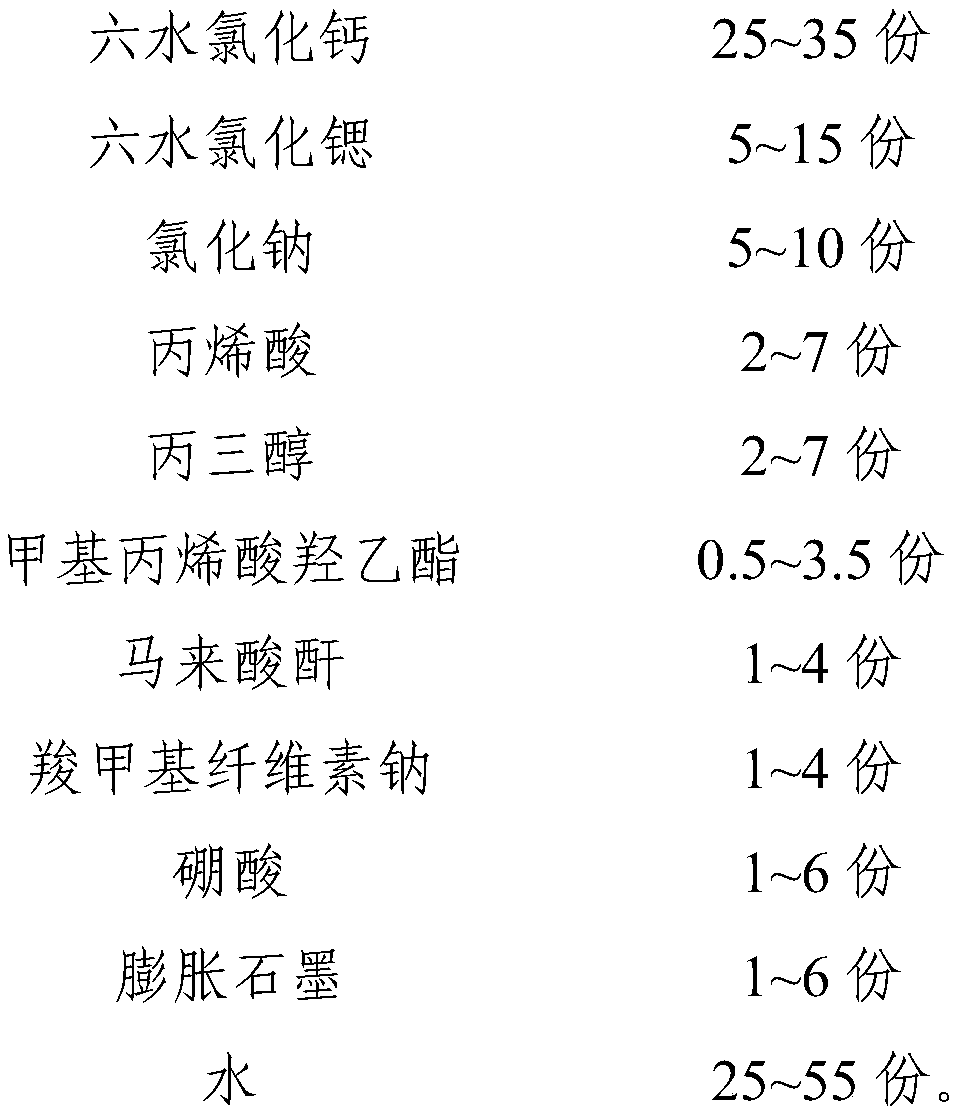
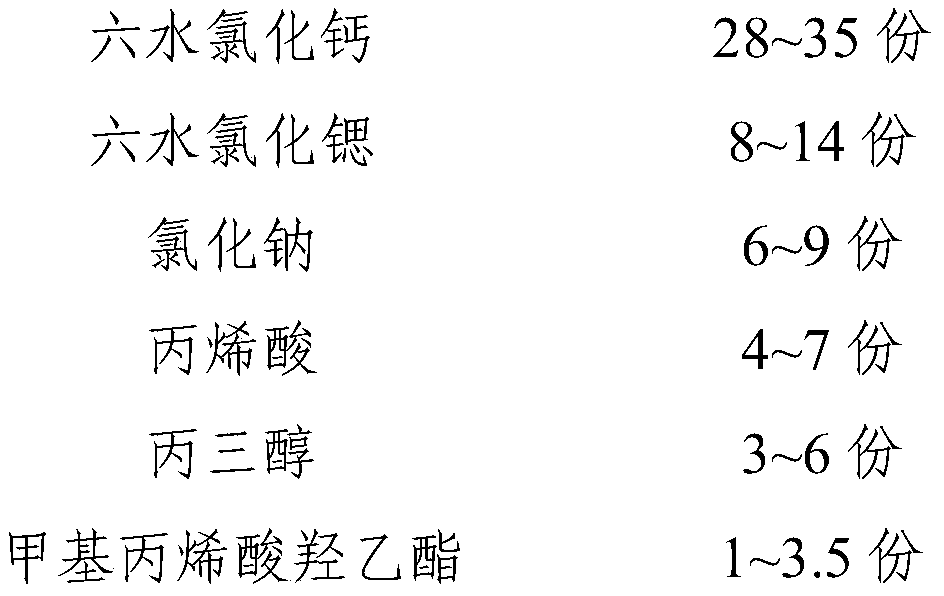
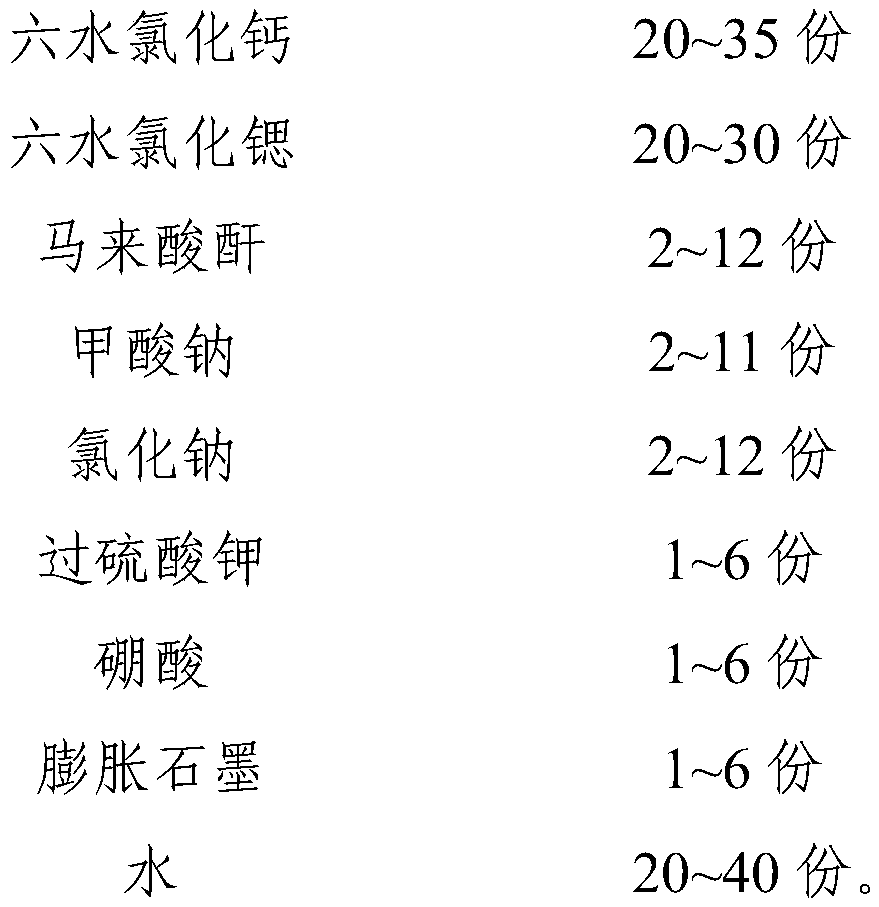
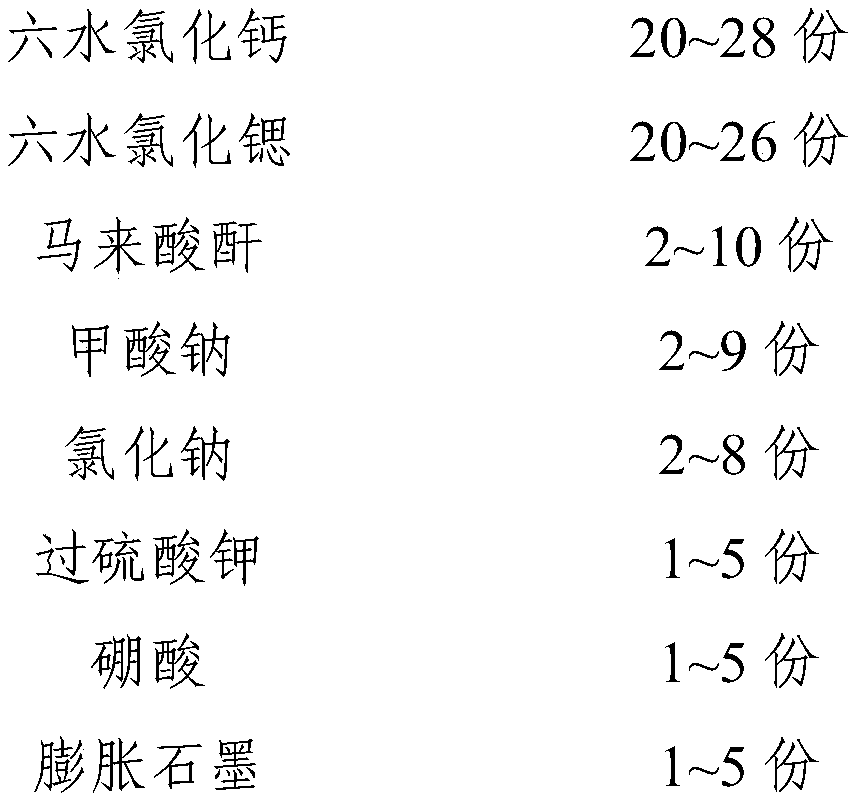
The invention relates to a phase-change material, in particular to an inorganic phase-change constant-temperature material and a preparation method thereof. The inorganic phase-change constant-temperature material comprises calcium chloride hexahydrate, strontium chloride hexahydrate, maleic anhydride, sodium formate, sodium chloride, potassium persulfate, boric acid and expanded graphite; whereinthe total amount of the boric acid and the expanded graphite accounts for 2-12% of the total amount of the inorganic phase-change constant-temperature material; wherein the mass ratio of the boric acid to the expanded graphite is (1-6): (1-6). According to the inorganic phase-change constant-temperature material, the phase-change temperature is 39 DEG C, the supercooling degree is 0.3 DEG C, thephase-change latent heat is not lower than 221 KJ / Kg, the phase-change process is reversible, and the number of times of recycling is not lower than 10000; and in addition, the situation of thermal physical property degradation does not exist in the circulation process, and leakage from a base body is not likely to happen.
Owner:北京中海前沿材料技术有限公司
Orange-red rear-earth phosphors and preparation method thereof
InactiveCN103059843AStrong excitationStrong orange-red emissionLuminescent compositionsStrontium chloride hexahydrateStrontium bromide
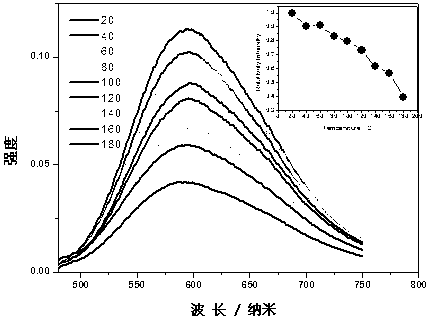
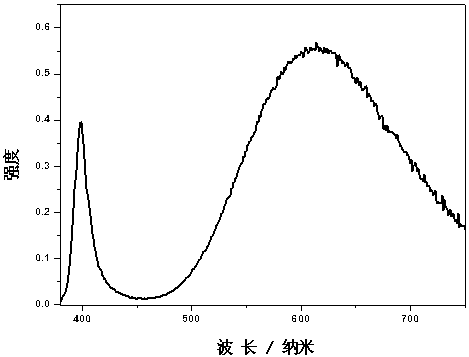
The invention relates to orange-red rear-earth phosphors and a preparation method thereof. The orange-red rear-earth phosphors are prepared from strontium carbonate, calcium chloride, strontium chloride hexahydrate, strontium fluoride, strontium bromide, aluminum hydroxide, boric acid and europium oxide serving as raw materials by adopting a high-temperature solid state method. The preparation method comprises the following steps of: uniformly mixing the raw materials according to proportions, pre-sintering for 3-6 hours at 400-500 DEG C, cooling to a room temperature, taking out the raw materials and uniformly grinding again, reducing and sintering at a CO atmosphere for 4-10 hours at 1000-1200 DEG C, and grinding fine obtained sinters to obtain final products. The orange-red rear-earth phosphors have high luminous efficiency, good stability and very high quenching temperature and are suitable for InGaN chips giving out ultraviolet lights with about 400nm wavelength.
Owner:YUNNAN MINZU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com