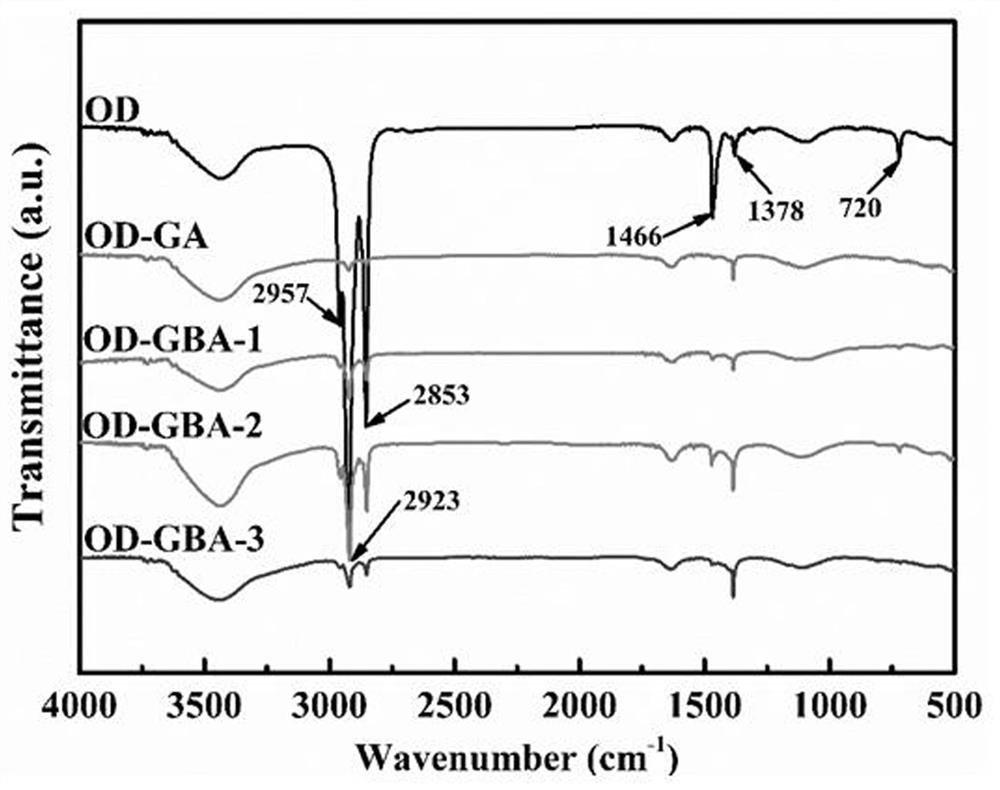
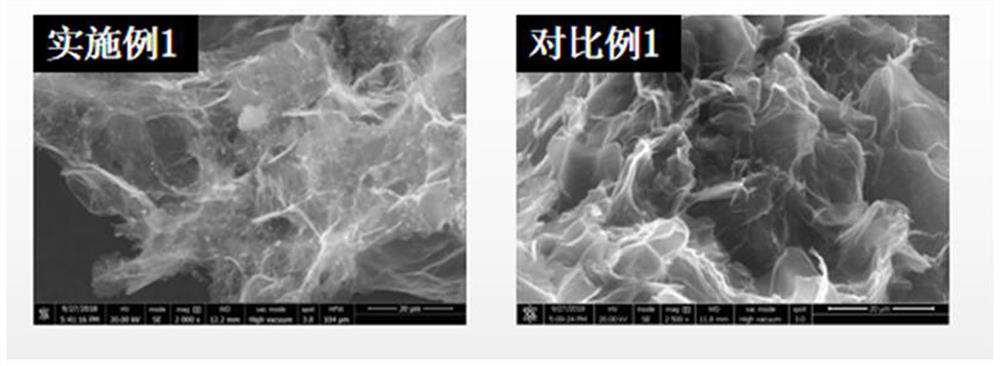
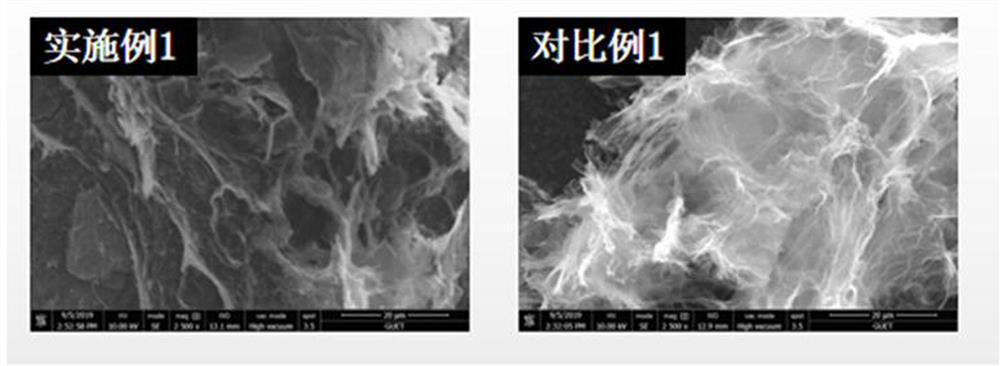
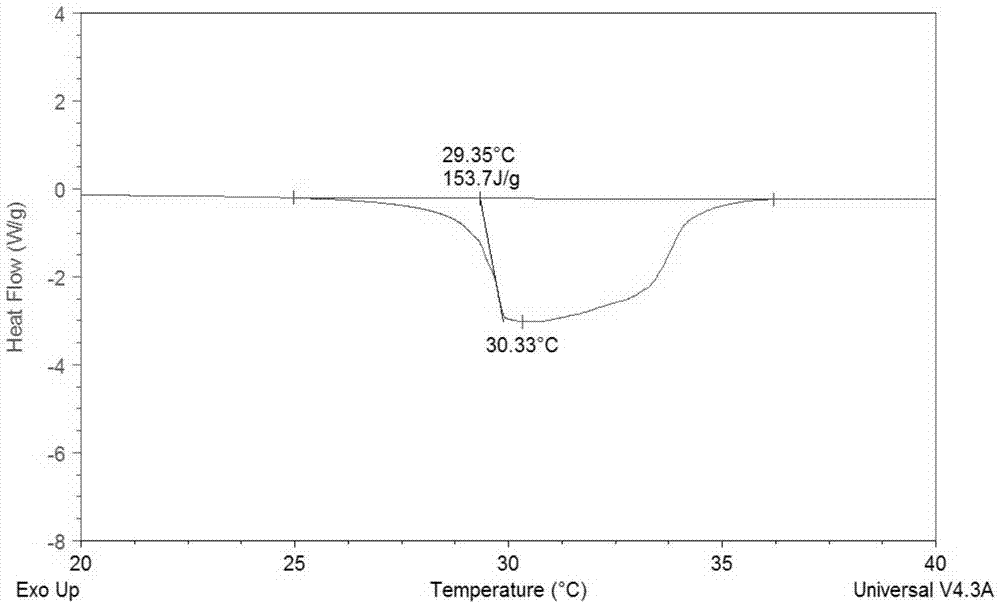
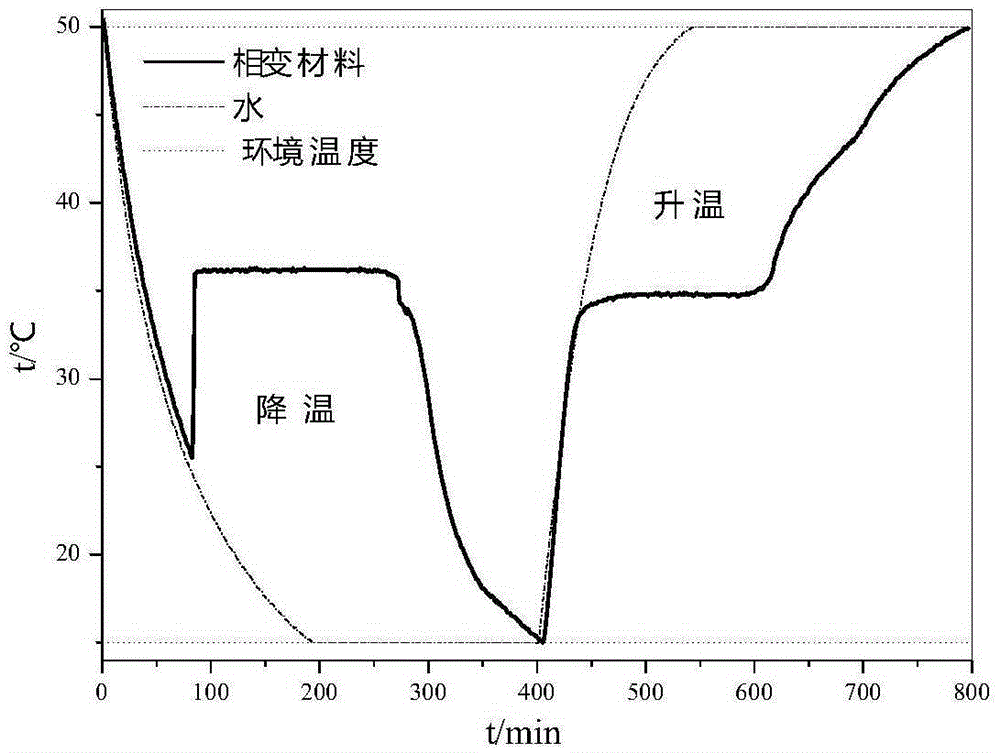
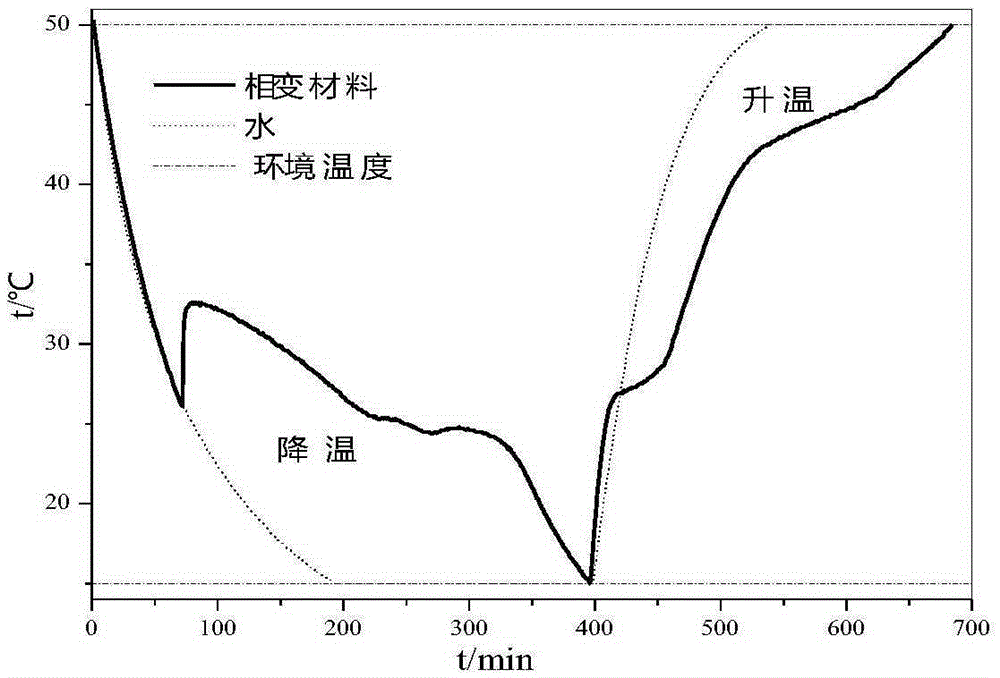
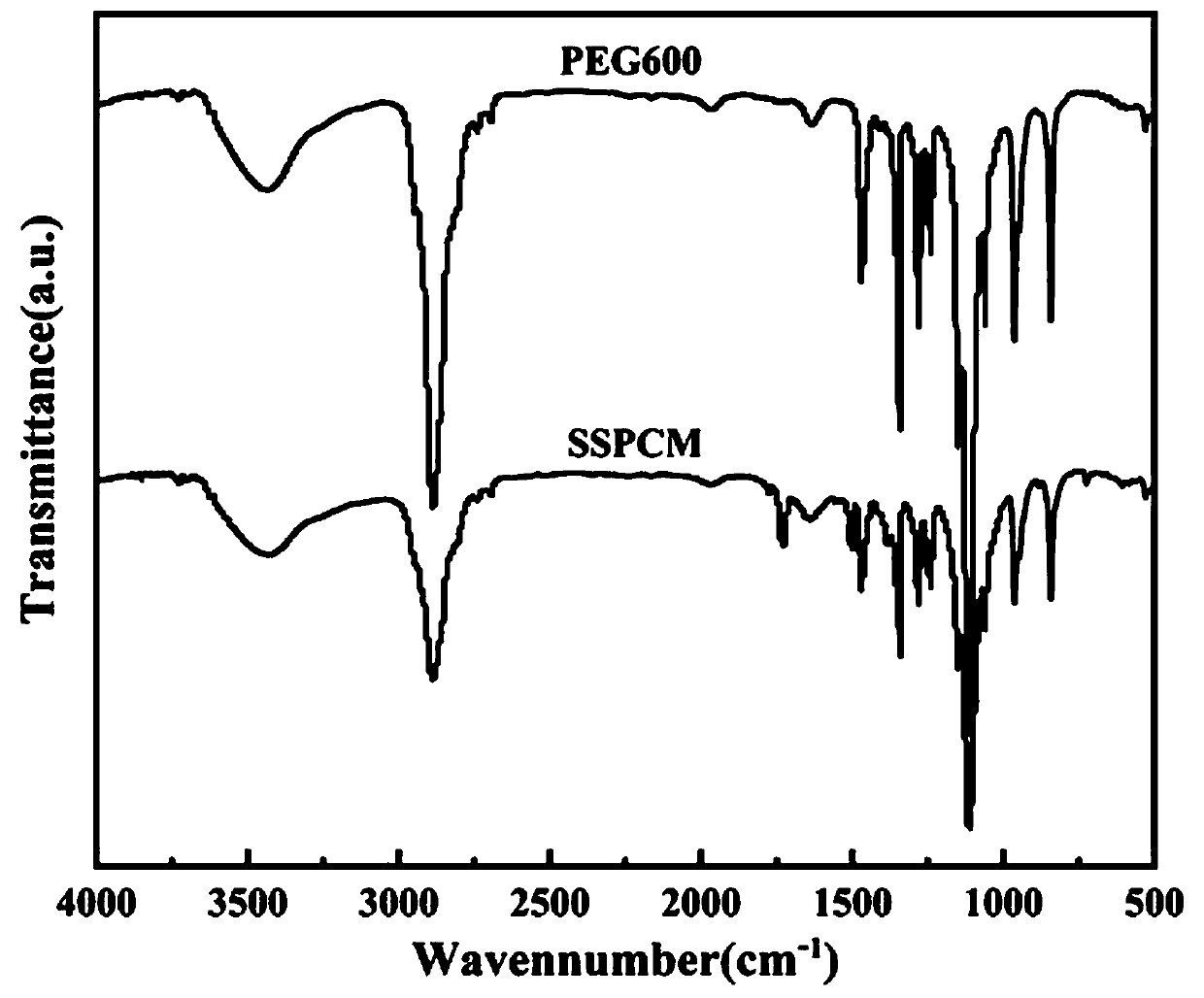
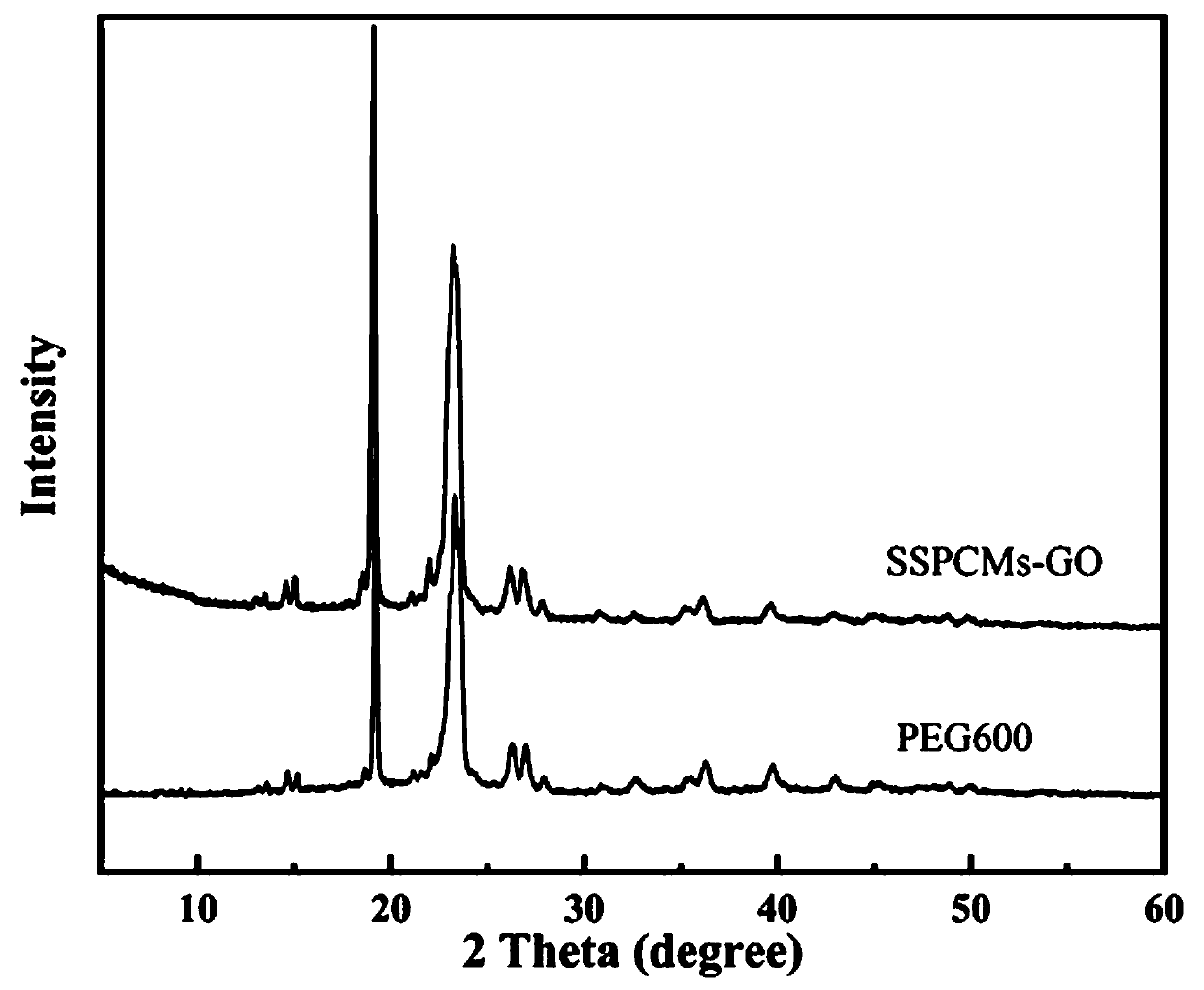
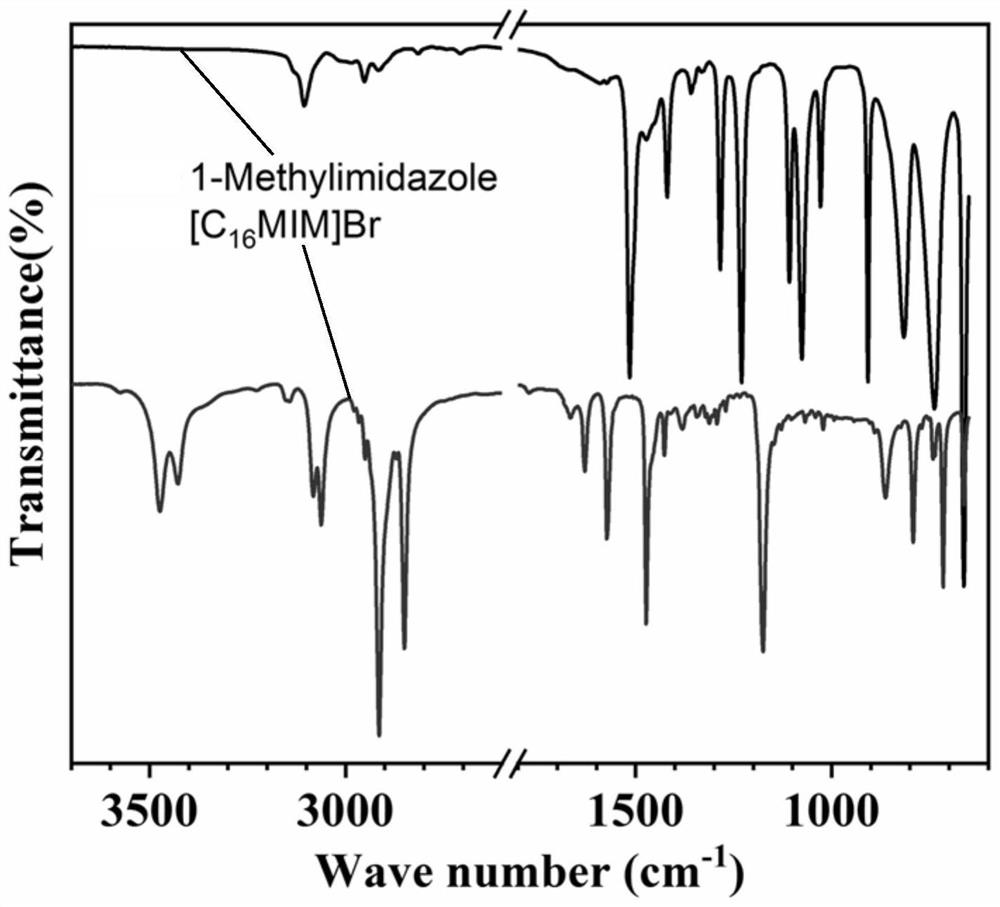
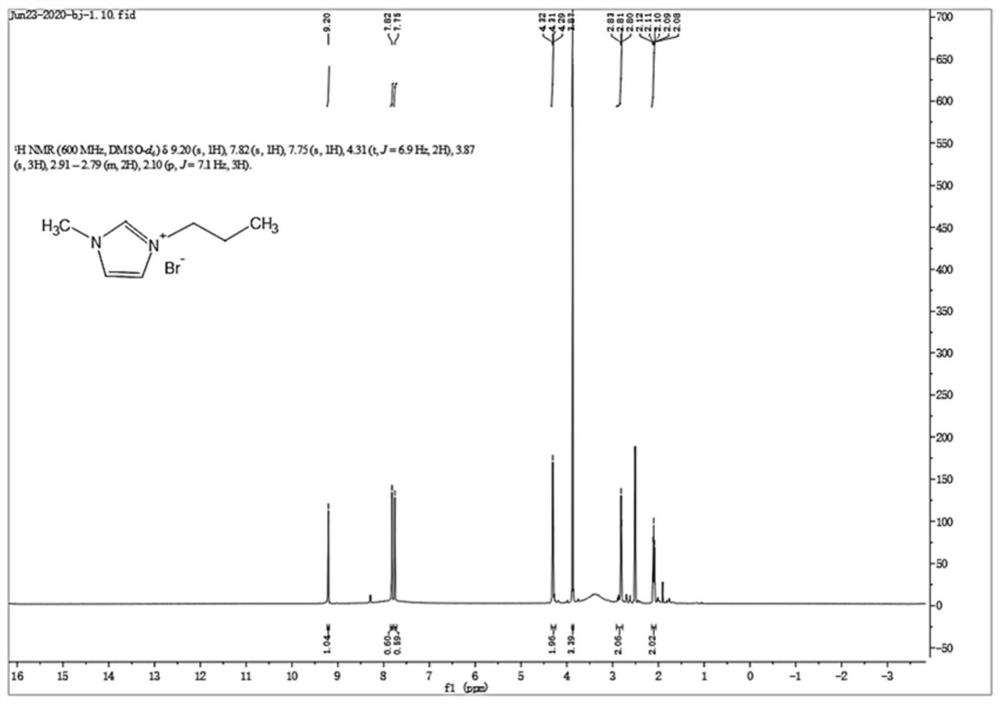
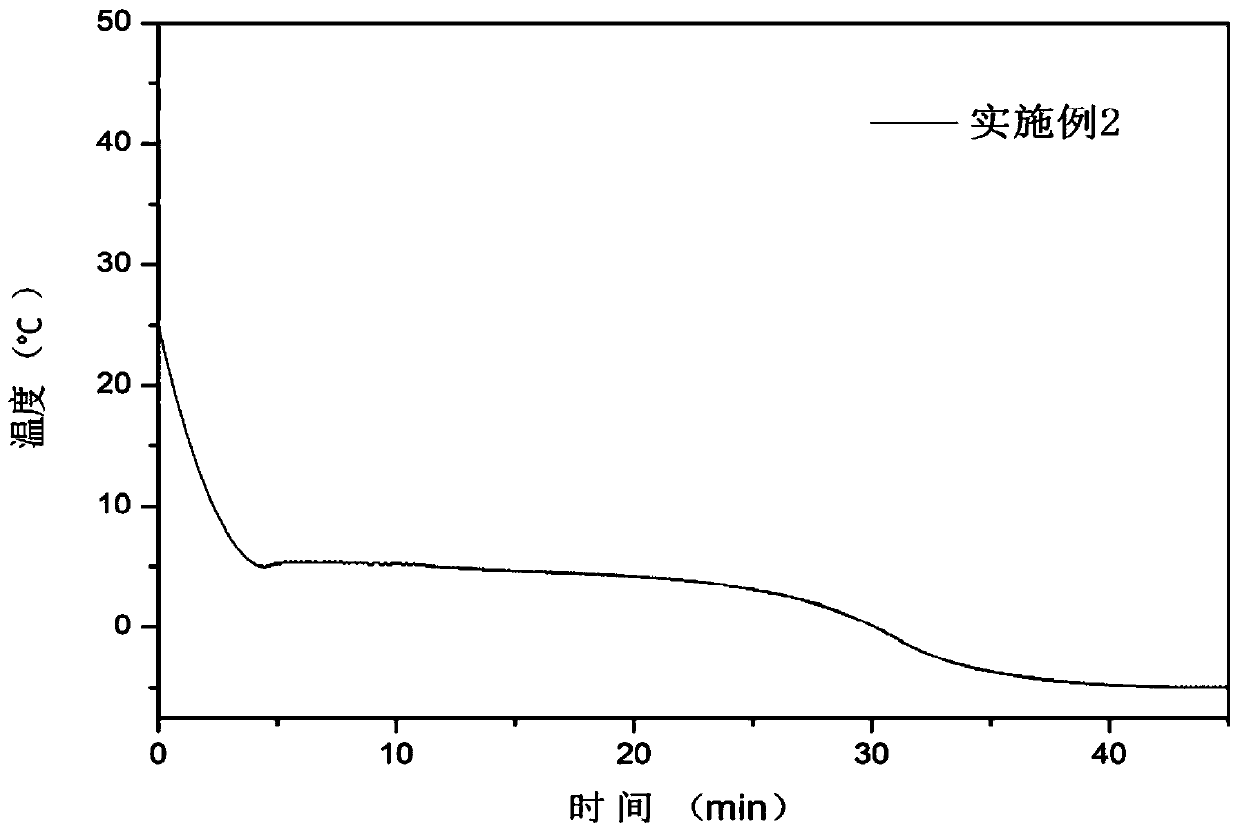
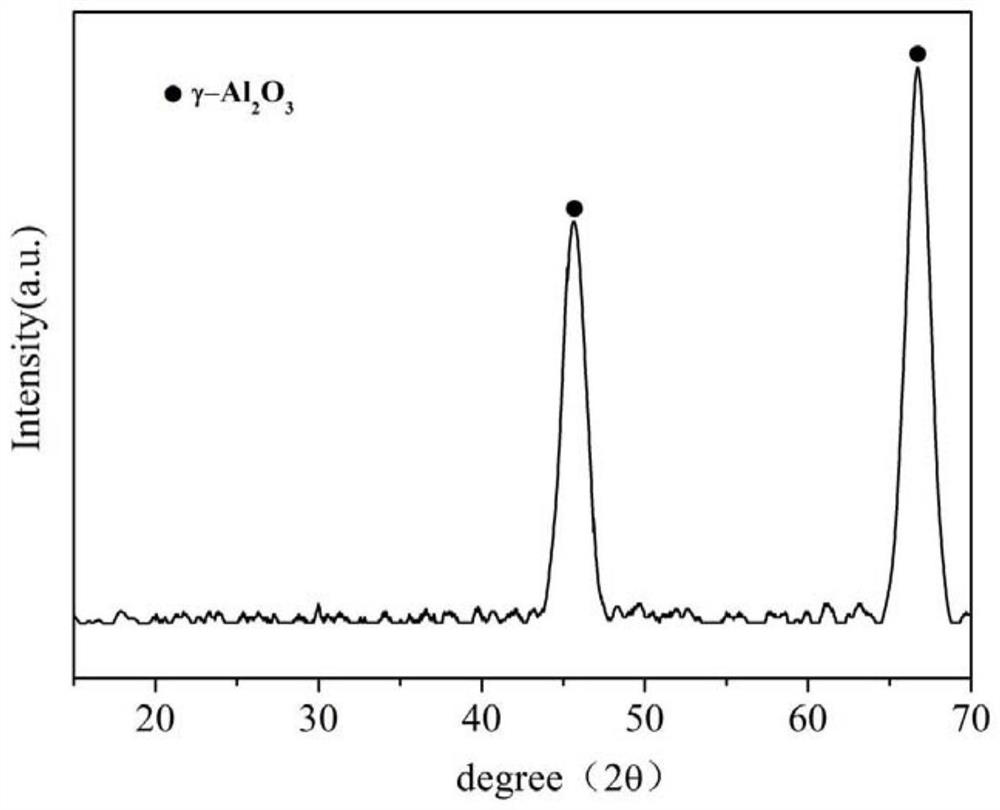
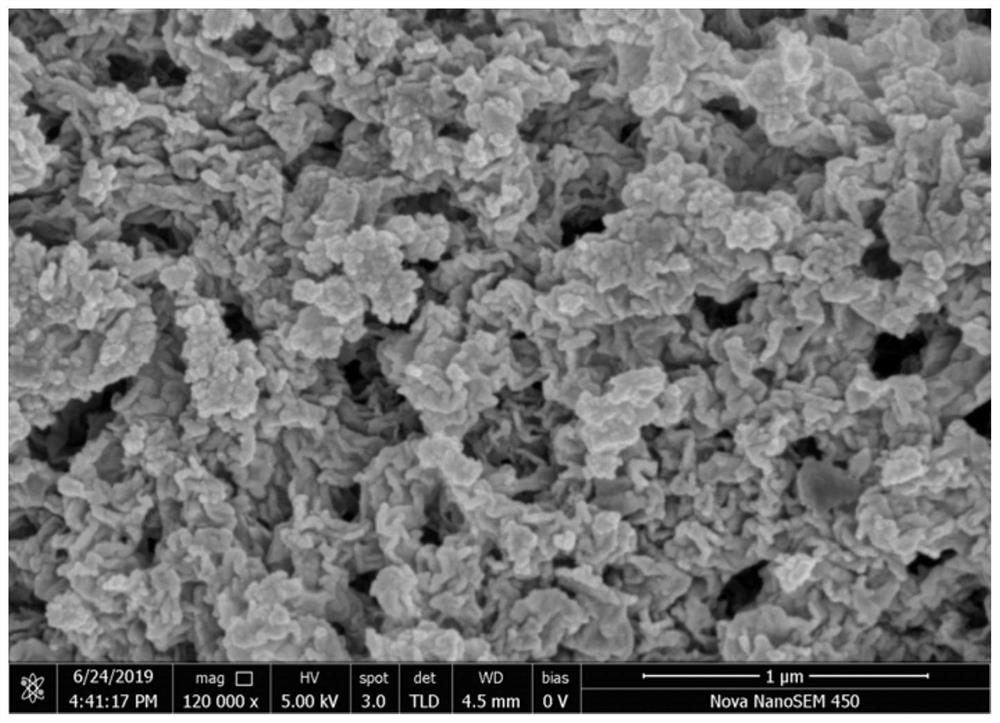
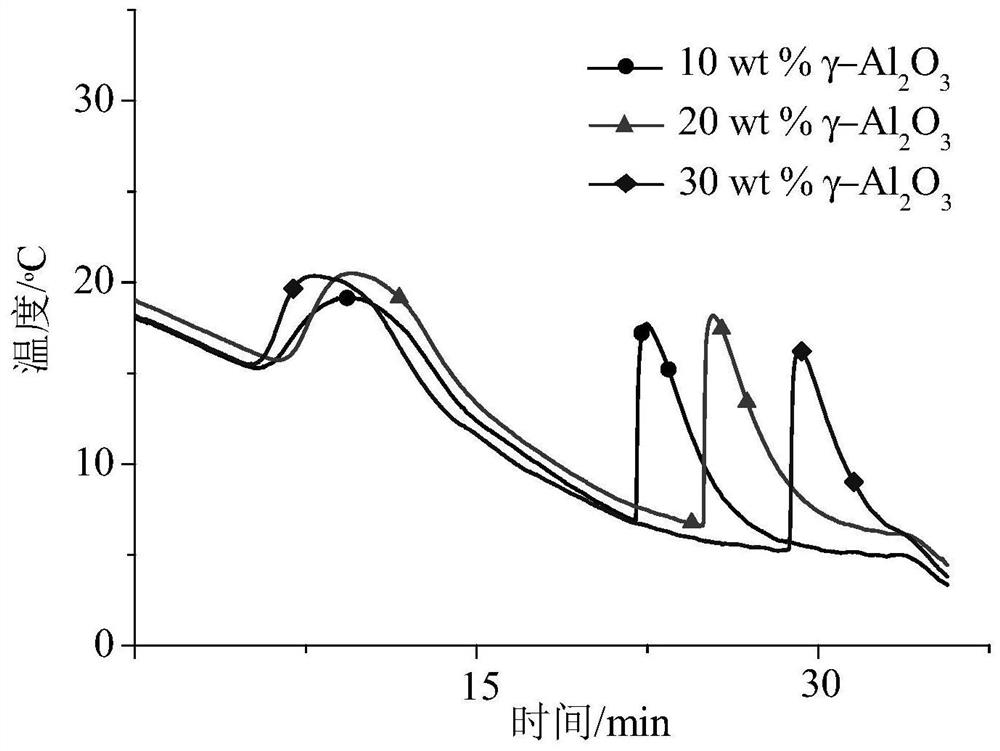
Patents
Literature
71results about How to "Phase transition temperature is suitable" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phase-transition composite material, preparation method and application thereof
ActiveCN102408877APhase transition temperature is suitableLarge latent heat of phase changeClimate change adaptationHeat proofingParaffin waxThermal insulation
The invention relates to a phase-transition composite material which comprises: A) 30-65% of phase-transition materials which are low melting point paraffin with a melting point of 25-45 DEG C and / or dodecanol; B) 25-45% of carrier materials which are high density polyethylane and / or ethane-vinylacetate copolymer; C) 5-15% of inorganic fillers which are porous substances and are selected from one or two of expanded perlite and expandable graphite; D) 1-10% of heat-conduction reinforcing agents; and E) 1-10% of fire retardants. The composite material has an appropriate phase transition temperature which matches the building ambient temperature, has great phase transition latent heat, excellent heat-preservation and heat-insulation performance, is fireproof and flame-retardant, is easy to process, and has greatly reduced phase-transition material leakage. The invention also relates to a method for preparing the phase-transition composite material, which comprises the thermal insulation mortar of the phase-transition composite material and a method of heat preservation of a wall by using the mortar.
Owner:BEIJING UNIV OF CHEM TECH +1
Preparation method of interpenetrating network formed phase-change material
InactiveCN102061148AGood shaping effectGood strengthHeat-exchange elementsConjugated synthetic polymer artificial filamentsTemperature resistanceMethylene bisacrylamide
The invention relates to a preparation method of an interpenetrating network formed phase-change material, comprising the following steps of: adding N-hydroxymethyl acrylamide as a network monomer, N, N-methylene-bisacrylamide as a cross linking agent, polyethylene glycol as a phase-change material and distilled water into a flask, ultrasonic-dispersing for 5 minutes after dissolving (the molar ratio of the cross linking agent to the network monomer is (1 / 7):(1 / 11), the mass ratio of the network monomer to the water is (1 / 6):(1 / 14), and the proportion of the polyethylene glycol accounts for 40-80 percent of the total mass); adding ammonium persulfate as an initiator and continuously ultrasonic-dispersing for 5 minutes, wherein the mass fraction of the initiator accounts for 1.5-3.5 percent of the network monomer; and reacting at a temperature of 70-80 DEG C for 3-4 hours to generate interpenetrating network hydrogel, and then drying to a constant weight to obtain the interpenetrating network formed phase-change material. The preparation method has a simple process and a low production cost and is friendly to environment. Moreover, the phase-change material has the advantages of high enthalpy (reaching over 108.41J / g), less loss, suitable phase-change temperature (32-42 DEG C) and good performance in high temperature resistance.
Owner:DALIAN POLYTECHNIC UNIVERSITY
High molecular solid/solid phase changing material with net type and comb type mixed structure and its preparing method
InactiveCN1616588APhase transition temperature is suitableSolid state goodHeat-exchange elementsPhase change enthalpyPolyethylene glycol
The present invention relates to a kind of high molecular solid / solid phase changing material with mixed net and comb structure, and features that polyglycol with two active end radical and polyglycol with one active end radical are fixed onto the high molecular skeleton material to form 3D mixed net and comb structure. The material of the present invention has relatively great phase change enthalpy up to 120 J / g, proper phase change temperature capable of being altered in 0-65 deg.c, stable solid state before and after phase change without supercooling, separating and other unstable phenomenon, high mechanical strength, high solvent resistance, good machining performance, no toxicity, no leakage, no corrosion, no pollution, long service life and other advantages. The present invention may be used widely in solar energy utilization, afterheat recovering, intelligent air conditioner and other fields.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Phase change energy storage material and preparation method thereof
InactiveCN101519581AReversible solid-solid phase change energy storage propertiesPhase transition temperature is suitableHeat-exchange elementsCellulosePolymer science
The invention discloses a phase change energy storage material and a preparation method thereof. The method comprises the following steps: 1) polyethylene glycol reacts with a crosslinking agent at the temperature of 50 DEG C to 80 DEG C for 4 hours to 8 hours and a polyethylene glycol prepolymer is obtained; and 2) the polyethylene glycol prepolymer and a cellulose ionic liquid solution react at the temperature of 60 DEG C to 80 DEG C for 4 hours to 8 hours. The mass portions of polyethylene glycol are 50 to 95 and the mass portions of the crosslinking agent are 1 to 20. The mass portions of cellulose are 2 to 40 and the concentration of the cellulose ionic liquid solution is 2 percent to 5 percent by mass percentage. The material realizes the storage and release of energy through reversible solid-solid phase conversion, the phase change temperature is at 25 DEG C to 60 DEG C, enthalpy of phase change can be up to 150 J / g, and the material is non-toxic or harmless and has high energy storage density, good thermal stabilization, no liquid leakage and phase separation, simple preparation technique and excellent application prospect in the fields of energy storage and temperature control.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Mining temperature reduction coolant and preparation method thereof
InactiveCN101824304AEnvironmentally friendlyIncrease elasticityHeat-exchange elementsFreezing Point TemperatureCross-link
The invention relates to a mining temperature reduction coolant and preparation method thereof. The invention takes macromolecular compound polyvinyl alcohol as basic raw material, cross-linking agent in certain proportion is added, wherein the cross-linking agent is 10% borax solution and the like; then freezing point temperature reduction auxiliary agent is added to prepare gel-like coolant, the prepared coolant is injected into a forming container and is frozen and formed in an icehouse the temperature of which is lower than minus 15 DEG C, and then the formed coolant is unfrozen at room temperature and is filled into a packaging bag. The mining temperature reduction coolant of the invention has the following characteristics: (1) elasticity and flexibility are good, comfortable sensation can be obtained when human body touches the coolant; (2) cold accumulation material has major latent heat of phase change and appropriate phase change temperature; (3) the coolant can be repeatedly used, can not be cataklastic, can not contract and can produce no separated water; (4) the coolant can be in close contact with human body when in use, and refrigeration effect can be fully played; (5) no toxin, odour or pollution is produced; (6) cold insulating plate and cold insulating bag of the outer packing of the coolant has the function of 'environmental protection and high efficiency cold insulation heat preservation'.
Owner:ANHUI PROVINCE COAL SCI RES INST
Fused salt phase change heat storage device applied to solar air conditioner
InactiveCN102252545AImprove heat transfer efficiencyGuarantee safety and reliabilityHeat storage plantsEnergy storageHeat conductingHeat storage material
The invention relates to a fused salt phase change heat storage device applied to a solar air conditioner. The fused salt phase change heat storage device applied to the solar air conditioner is characterized by comprising a heat storage chamber box, heat storage material accommodating pipelines, a heat storage material and heat conducting oil; a heat storage space is formed in the heat storage chamber box, the heat storage chamber box is provided with an oil inlet and an oil outlet, the oil inlet and the oil outlet are communicated with the heat storage space respectively, 2 to 200 heat storage material accommodating pipelines are arranged in the heat storage space of the heat storage chamber box, the heat storage material accommodating pipelines are fixed with the heat storage chamber box, and the heat storage material is filled in the heat storage material accommodating pipelines; and a heat transfer medium between the outer walls of the heat storage material accommodating pipelines and the inner wall of the heat storage chamber box is the heat conducting oil. The fused salt phase change heat storage device has the characteristics of simple structure, safety, reliability and high heat exchange efficiency.
Owner:WUHAN UNIV OF TECH
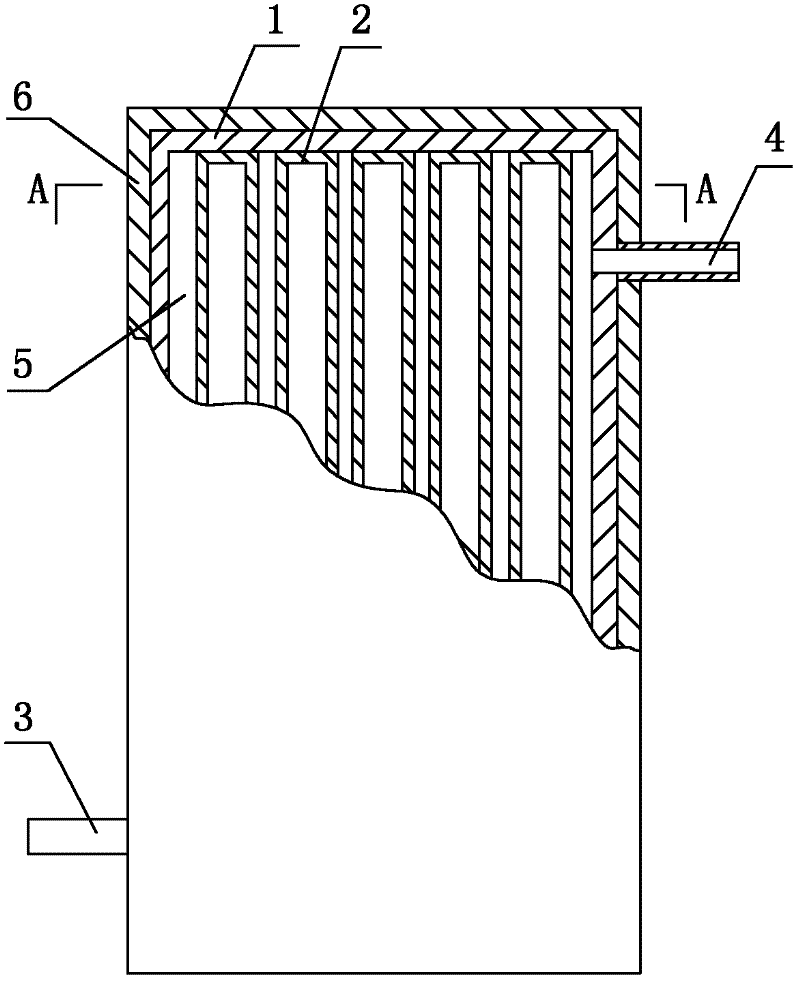
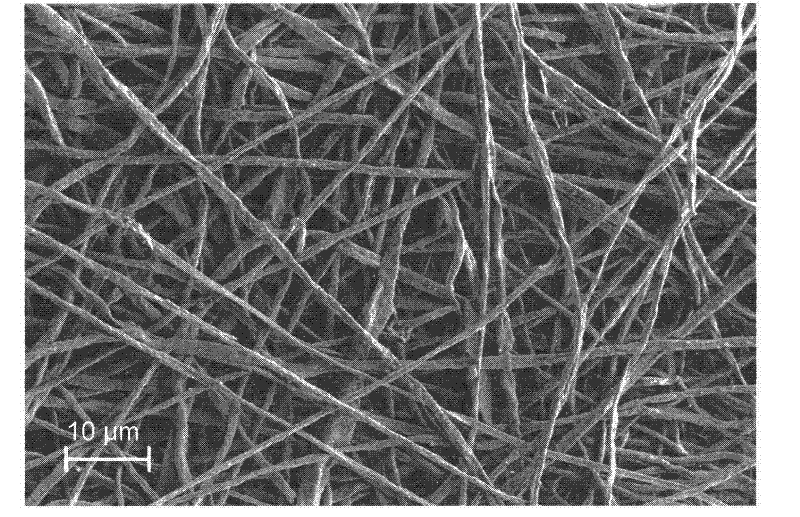
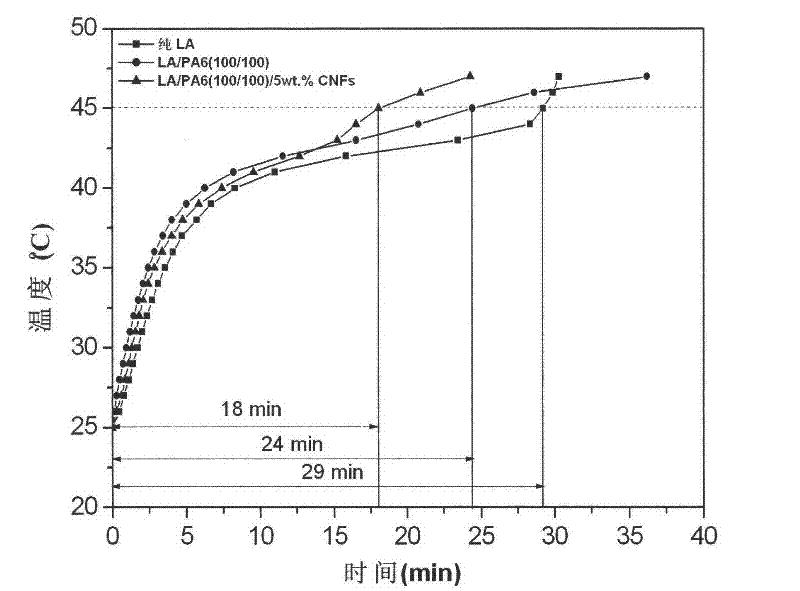
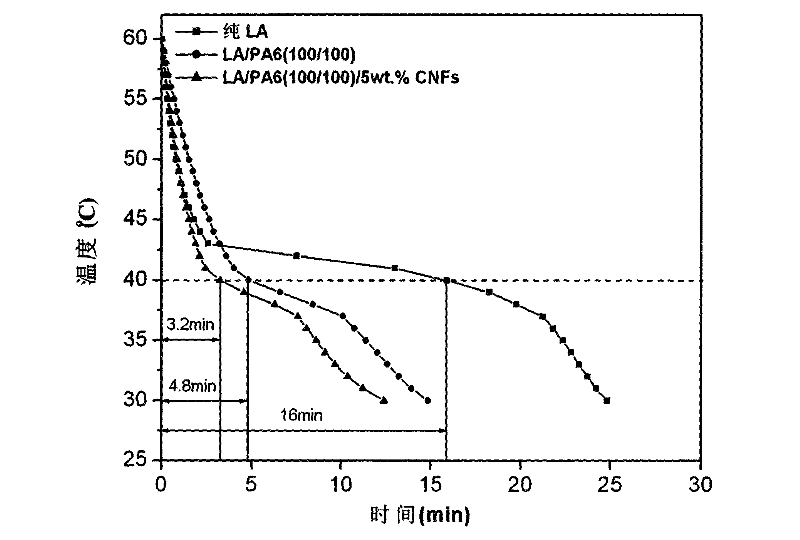
Method for enhancing thermal conductivity of phase-change energy-storage superfine composite polyamide fiber and method for producing phase-change energy-storage superfine composite polyamide fiber
InactiveCN102517793APhase transition temperature is suitableHigh latent heat of phase changeFilament/thread formingNon-woven fabricsElectrospinningCarbon nanofiber
A production method for enhancing thermal conductivity of a fatty acid / polyamide-6 nano composite phase-change material belongs to the technical fields of nanocomposites, phase change materials, electrostatic spinning and the like, and particularly relates to a method of using an electrostatic spinning device to produce a nano composite fiber membrane of fatty acid / pollyamide-6 / carbon nano fibers. The production method is simple in process and easy in control. By effectively combining the production method with the electrostatic spinning technique, the produced fiber is small in diameter and uniform in distribution, the produced nano composite phase change material is uniform, stable and less prone to leakage, and has the thermal conductivity more evident than that of the conventional phase change material. Therefore, the material is more convenient in processing and application, and more widely applicable.
Owner:JIANGNAN UNIV
Boron nitride/graphene double-heat-conduction-base aerogel composite phase change material and preparation method thereof
ActiveCN111662688ALow densityContinuous network structureHeat-exchange elementsFreeze-dryingPyrrolidinones
The invention discloses a boron nitride / graphene double-heat-conduction-base aerogel composite phase change material. The material is formed by compounding modified boron nitride / graphene aerogel andn-octadecane by adopting a vacuum impregnation method. The double-heat-conduction aerogel is prepared by taking graphene oxide, modified boron nitride, polyvinylpyrrolidone and ethylenediamine as rawmaterials to prepare boron nitride / graphene hydrogel, freeze-drying the boron nitride / graphene hydrogel and then calcining the boron nitride / graphene hydrogel at a constant temperature. Polyvinylpyrrolidone is used as a cross-linking agent, and ethylenediamine is used as a reducing agent. A preparation method of the composite phase change material comprises the following steps: 1) preparing modified boron nitride; 2) preparing boron nitride / graphene double-heat-conduction-base aerogel; and 3) preparing the boron nitride / graphene double-heat-conduction-base aerogel composite phase change material. When the material is applied as a phase change material, the heat conductivity coefficient is 0.9-1.6 W / (m.K); wherein the phase change temperature is 19-32 DEG C, and the phase change latent heatis 200-220 J / g. The composite phase change material has the following advantages: 1, the heat conductivity coefficient is improved by 738%; 2, the leakage problem in the phase change process is effectively solved; and 3, the phase-change latent heat and the heat stability are high;
Owner:GUILIN UNIV OF ELECTRONIC TECH
Low-temperature calcium chloride hexahydrate heat-storage material and preparation method
InactiveCN103923613AHigh phase change enthalpyPhase transition temperature is suitableHeat-exchange elementsCelluloseCalcium Chloride Hexahydrate
The invention discloses a low-temperature calcium chloride hexahydrate heat-storage material and a preparation method. The prepared low-temperature calcium chloride hexahydrate phase-change heat-storage material comprises a nucleating agent and a thickener according to mass percent, wherein the nucleating agent is borax, alumina or sodium metasilicate nonahydrate, the thickener is sodium carboxymethl cellulose (CMC), wherein calcium chloride hexahydrate is a phase-change base material, and the usage amount of the calcium chloride hexahydrate is 95%-98%; the preparation method comprises the step of: respectively adding 1wt% of borax and 1% of CMC or 1% alumina and 4% of CMC or 1% of sodium metasilicate nonahydrate and 2% CMC for modifying the calcium chloride hexahydrate, to obtain the low-temperature calcium chloride hexahydrate heat-storage material. The phase-change latent heat of the low-temperature calcium chloride hexahydrate heat-storage material is about 150J / g, the phase-change temperature of the low-temperature calcium chloride hexahydrate heat-storage material is at 25-30DEG C, the cooling degree is less than 2DEG C, and the heat suction and release performances of the low-temperature calcium chloride hexahydrate heat-storage material after being circulated for 3000 times are stable. The low-temperature calcium chloride hexahydrate heat-storage material has excellent application prospects in agricultural facilities and residential housing.
Owner:NORTHWEST A & F UNIV
Phase-change thermal energy-storage material applicable to heat-pump water heater and preparation method thereof
InactiveCN103351850ALarge latent heat value of phase changePhase transition temperature is suitableHeat-exchange elementsThermal energyThermal energy storage
The invention provides a phase-change thermal energy-storage material applicable to a heat-pump water heater. The phase-change thermal energy-storage material comprises, by weight, 20 to 30 of stearic acid, 30 to 40 of palmitic acid, 10 to 20 of lauric acid, 20 to 30 of paraffin, 3 to 5 of a carbon nanofiber, expandable graphite and NaCl, wherein the additive amount of the expandable graphite accounts for 12 to 20% of the weight of the paraffin, and the additive amount of NaCl accounts for 3 to 8% of the total weight of the stearic acid, the palmitic acid, the lauric acid and the paraffin. The invention further discloses a preparation method for the phase-change thermal energy-storage material. Compared with the prior art, the prepared phase-change thermal energy-storage material provided by the invention has the advantages of a great potential heat value of phase change, an appropriate phase-change temperature, great thermal energy-storage density, a small volume expansion ratio, stable performance, no toxicity, no pollution, no corrosion to metals, easily available raw materials, easy preparation, etc.
Owner:MAANSHAN NBWAVE HEAT ENERGY SCI
Phase change heat storage material and its preparing method
InactiveCN1944567AReduced stabilityAvoid interactionHeat-exchange elementsSodium acetateHeat storage material
The present invention discloses the composition and preparation process of phase change heat accumulating material. The phase change heat accumulating material consists of crystalline sodium acetate, borax as additive and polyacryamide mainly. The preparation process includes grinding and mixing the said components, heating the mixture to smelting and pouring into packing container; or includes dissolving crystalline sodium acetate, adding borax via stirring, adding polyacryamide via stirring and final pouring into packing container. The phase change heat accumulating material of the present invention has the advantages of great heat accumulating amount, low supercooling degree, proper phase change temperature, no phase separation after long term use, etc.
Owner:SHANGHAI MARITIME UNIVERSITY
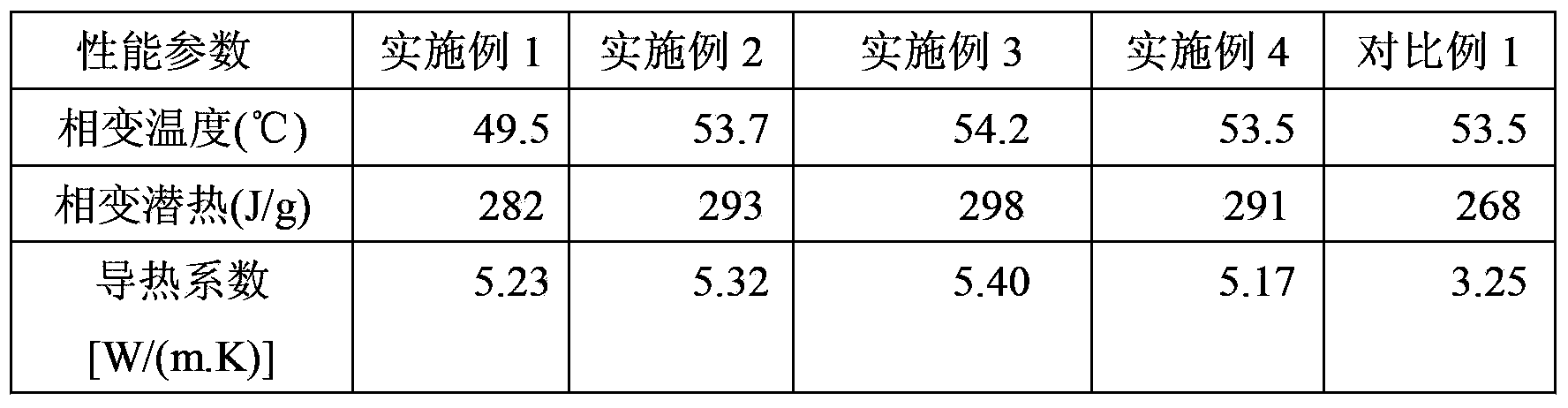
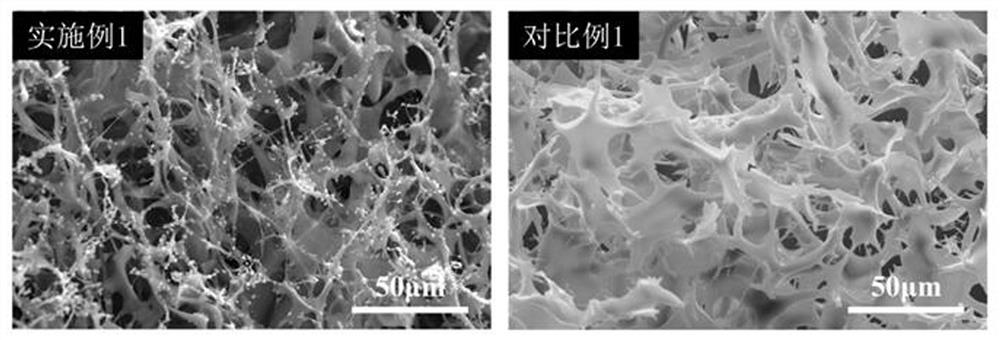
Boron nitride/pea meal double-heat-conduction-base carbon aerogel as well as preparation method and application thereof
InactiveCN113150746AIncrease added valueImprove adsorption capacityNitrogen compoundsCarbon preparation/purificationMetallurgyFreeze-drying
The invention relates to a boron nitride / pea meal double-heat-conduction-base carbon aerogel. According to the invention, boron nitride, pea meal and a cross-linking agent are used as raw materials, the boron nitride is firstly prepared into modified two-dimensional nano lamellar boron nitride, then the modified two-dimensional nano lamellar boron nitride, the pea meal and the cross-linking agent are subjected to a water bath curing reaction, freeze drying and low-temperature calcination. The preparation method comprises the following steps: 1) preparing modified two-dimensional nano lamellar boron nitride; and (2) preparing the boron nitride / pea meal double-heat-conduction-base carbon aerogel. According to the application of the product as a phase change material, the boron nitride / pea meal double-heat-conduction-base carbon aerogel composite phase change material is obtained by compounding with polyethylene glycol, the phase change temperature is 39-55 DEG C, the phase change latent heat is 168-171J / g, and the heat conductivity coefficient is 0.46-0.58 W / (m.K). The method has the following advantages: 1, the raw materials are low in cost, easy to obtain and environment-friendly; 2, the heat conductivity coefficient is improved by 187%; 2, the leakage problem is avoided; and 3, the phase change latent heat and the thermal stability are high;.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Phase change material applied to water heater and preparation method thereof
InactiveCN101649187ALarge latent heat value of phase changePhase transition temperature is suitableHeat-exchange elementsCorrosionVolume expansion
The invention discloses a phase change material applied to a water heater, which is characterized by comprising the following components in percentage by weight: 30-50 of pitch, 50-70 of paraffin, 5-8of graphite and NaCl with the adding content of 3-8 percent of pitch in weight. The invention also discloses a preparation method of the phase change material. Compared with the prior art, the invention has the advantages that the pitch and the graphite mainly play roles of conducting heat and maintaining all the components to be even; the NaCl is used for preventing crystallization, and the paraffin has the effect of storing heat; and the obtained phase change material has the advantages of large phase change potential heat value and heat storage density, proper phase change temperature, small volume expansion ratio, stable performance, no poison and pollution, no corrosion on metal objects, easy acquisition of raw materials, easy manufacture, and the like by the mutual synergetic effectof the four substances.
Owner:MAANSHAN NBWAVE HEAT ENERGY SCI
Shape-stabilized phase-change heat-storage cooling pavement material suitable for high-temperature area
The invention relates to a preparation method and application method of a shape-stabilized phase-change heat-storage cooling pavement material suitable for a high-temperature area. The method is characterized in that a shape-stabilized phase-change material is prepared from stearic acid, palmitic acid and expanded perlite at the ratio of 2:3:2.5 by adopting a vacuum impregnation method. The application method of the shape-stabilized phase-change heat-storage cooling pavement material is a design method of mixing the shape-stabilized phase-change material into an asphalt mixture and adopting asphalt mastic as the mixture. The prepared shape-stabilized phase-change heat-storage cooling pavement material suitable for the high-temperature area has good cooling and heat-insulating properties, the phase change temperature is 52.8 DEG C, the phase change enthalpy can reach 132J / g, the pavement temperature can be reduced to 4.4 DEG C and the problem of an asphalt pavement track in the high-temperature area can be effectively solved.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
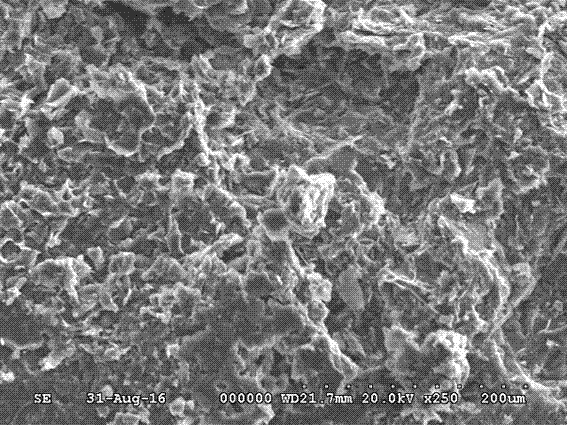
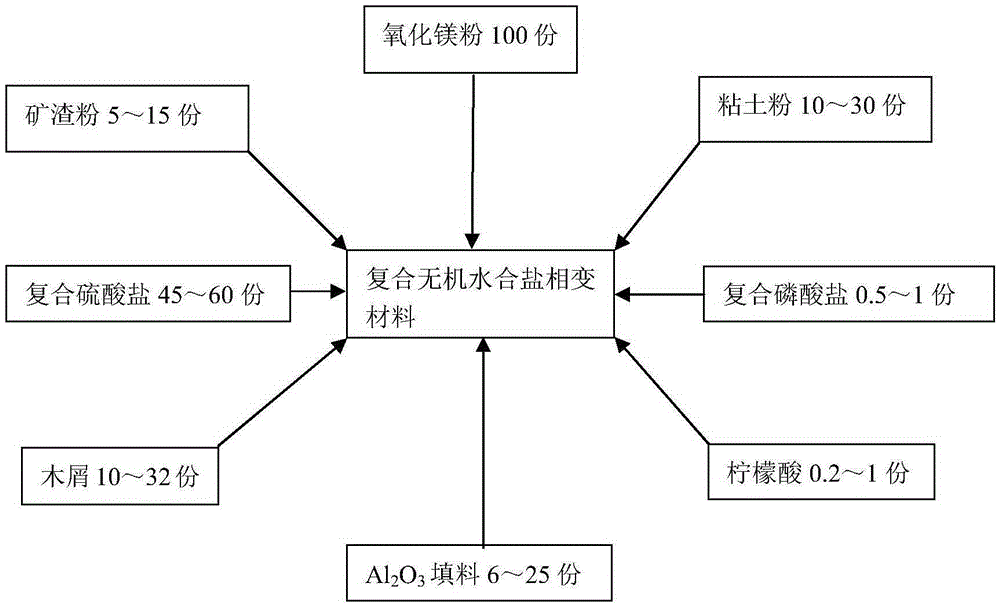
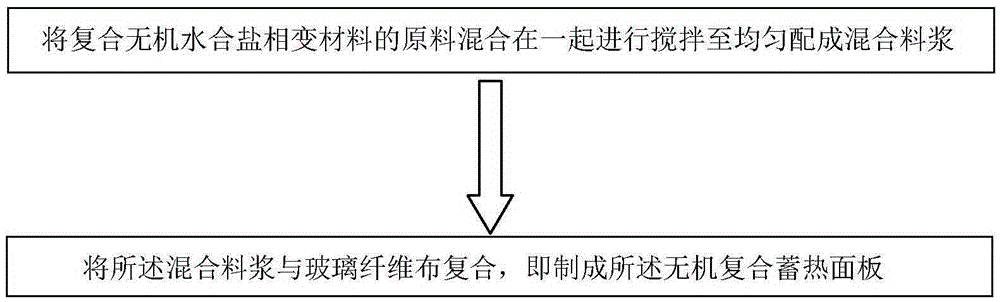
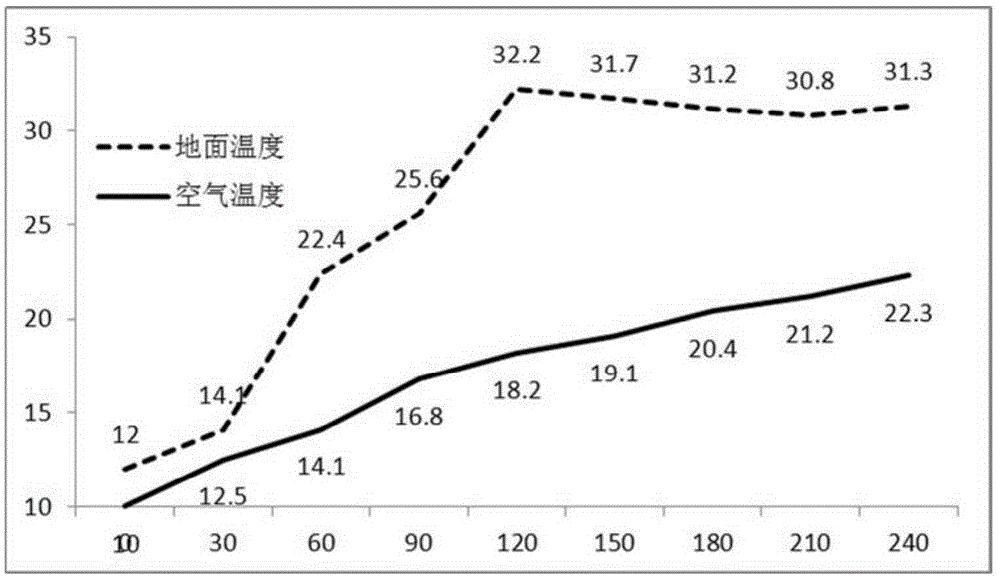
Composite inorganic hydrous salt phase-change material and preparation method of composite inorganic thermal storage panel
InactiveCN105399385APhase transition temperature is suitableHigh energy storage densityCeramicwarePhosphateHigh energy
The invention relates to the field of composite building decoration materials, in particular to a composite inorganic hydrous salt phase-change material and a preparation method of a composite inorganic thermal storage panel. The composite inorganic hydrous salt phase-change material comprises raw materials in parts by weight as follows: 100 parts of magnesia powder, 10-30 parts of clay powder, 15-25 parts of slag powder, 6-25 parts of Al2O3 filler, 45-60 parts of composite sulfate, 10-32 parts of saw dust, 0.2-1 part of citric acid, 0.5-1 part of composite phosphate and 75-85 parts of water. The raw materials of the composite inorganic hydrous salt phase-change material are mixed together and stirred until uniform stirring is realized, mixed slurry is prepared, then the mixed slurry is composited with glass fiber cloth, and the composite inorganic thermal storage panel is prepared. According to the technical scheme, the material not only has proper phase-change temperature, high energy storage density, low degree of supercooling and avoidance of layering, but also has the effects of good stability, high thermal conduction coefficient, avoidance of metal corrosion, water resistance and the like.
Owner:上海唐盾材料科技有限公司
Phase-change energy storage medium
InactiveCN106221675APhase transition temperature is suitableLow costHeat-exchange elementsStrontium chloride hexahydrateCarboxymethyl cellulose
The invention disclose a room temperature phase-change energy storage medium. The phase-change energy storage medium comprises a eutectic mixture composed of water, calcium chloride, magnesium chloride and magnesium nitrate. The preparation method of the phase-change energy storage medium comprises the following steps: mixing and heating the above components according to proportion, completely melting, uniformly stirring, and adding a certain amount of strontium chloride hexahydrate and carboxymethyl cellulose, namely, the liquid phase can be used as the phase-change energy storage medium. The phase-change energy storage medium disclosed by the invention has the features that the phase-change temperature is close to room temperature, the material is environmentally friendly and the cost is low.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Phase change energy storage medium
ActiveCN105238363AStable phase transition temperatureThe phase transition temperature point is stableHeat-exchange elementsSodium acetateRoom temperature
The invention discloses a room-temperature phase change energy storage medium which is a eutectic mixture composed of water, disodium hydrogen phosphate, sodium silicate and sodium acetate. A preparation method of the phase change energy storage medium comprises the following steps: the components are mixed according to the formula and are heated until completely molten; the mixture is well mixed, and the liquid phase can be used as the phase change energy storage medium. The phase change temperature of phase change energy storage medium is close to room temperature. The phase change energy storage medium also has the advantages of environment-friendly materials, low cost, and the like.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Stable-shape nanocomposite phase change material and preparation method thereof
InactiveCN105885796AHigh phase change enthalpyImprove thermal conductivityHeat-exchange elementsTemperature controlIn situ polymerization
The invention relates to a stable-shape nanocomposite phase change material and a preparation method thereof. The stable-shape nanocomposite phase change material mainly comprises the following components: polyethylene glycol (PEG), acrylamide (AM), clay, an initiator and a catalyst; the components are proportionally added; and a novel nanocomposite phase change material is prepared by an in-situ polymerization method, wherein the PEG is used as a phase change material, and a three-dimensional network composed of polyacrylamide (PAM) and clay serves as a support. The phase change material has relatively high latent heat of phase change, proper phase change temperature, relatively high heat conductivity coefficient and relatively good heat stability. The novel phase change material overcomes the shortcomings of frequent leakage and low heat conductivity coefficient in the phase change of traditional phase change materials; the material can keep a stable shape without encapsulation; and the preparation method is simple while the cost is low. The nanocomposite phase change material is expected to be applied to the fields such as energy-saving materials in buildings, solar energy storage and release and heat-accumulation temperature control clothing.
Owner:TIANJIN POLYTECHNIC UNIV
Anti-insect and anti-mite healthcare quilt and preparation method thereof
InactiveCN106283602APhase transition temperature is suitableImprove mechanical propertiesBiochemical fibre treatmentNon-woven fabricsHuman bodyAreca palm
The invention discloses an anti-insect and anti-mite healthcare quilt which comprises a quilt cover, a quilt interior fixing cover and a quilt interior filler, wherein the quilt interior filler is prepared from the following raw materials in parts by weight: 10-15 parts of pineapple leaf fiber, 10-15 parts of flax fiber, 3-5 parts of radix stemonae, 1-2 parts of flos chrysanthemi indici, 2-4 parts of pericarpium zanthoxyli, 2-4 parts of areca catechu, 1-2 parts of clove, 1-2 parts of myrrh, 1-2 parts of honeysuckle stem, 30-40 parts of polyethylene glycol terephthalate, 0.5-1 part of polyethylene glycol monooleate and 10-15 parts of functional aid. The healthcare quilt disclosed by the invention has the advantages of intelligent temperature control, good warmth keeping property and air permeability, resistance to heat and aging, high human comfort and good usability; meanwhile, the healthcare quilt has the healthcare functions of purifying air and promoting metabolism of human body, prevents insects and mites, realizes good cleanness, integrates environmental protection, warmth keeping and healthcare functions, and meets the market needs of higher level.
Owner:安徽军民被装保障有限公司
Heat-insulated constant-temperature plastic profile containing shape-stabilized phase change material and production process of heat insulated constant-temperature plastic profile
ActiveCN103302932APhase transition temperature is suitableLarge latent heat of phase changeSynthetic resin layered productsHeat-exchange elementsChemistryThermoplastic
The invention discloses a heat-insulated constant-temperature plastic profile containing a shape-stabilized phase change material and a production process of the heat-insulated constant-temperature plastic profile. The heat-insulated constant-temperature plastic profile comprises a thermoplastic profile, wherein the thermoplastic profile is uniformly provided with a plurality of concave holes; active carbon on which sodium sulfate is absorbed is arranged in the concave holes; a thermoplastic sheet covering layer is arranged on the thermoplastic profile; and the active carbon on which sodium sulfate is absorbed is covered in the concave holes by the thermoplastic sheet covering layer. The heat-insulated constant-temperature plastic profile has the advantages that sodium sulfate decahydrate is used as the phase change material, and sodium sulfate is proper in phase change temperature, large in latent heat of phase change and low in price; the active carbon is a porous material, and the liquid-phase added pressure of micropores can play roles in sealing and storing phase change liquid; the sizes of heterogeneous points on the walls of the micropores and the sizes of the pores are small, so that the nucleation and crystallization of the sodium sulfate decahydrate can be favorably realized, and then the degree of supercooling of the sodium sulfate decahydrate is reduced; and the active carbon is packaged by using hollow thermoplastics with the concave holes, so that the problem of phase change material leakage is solved, and the shape and size of the material can be adjusted.
Owner:JIANGSU ZHONGHENG PET ARTICLES JOINT CO LTD
Polyimide grafted polyethylene glycol composite solid-solid phase change material and preparation method thereof
The invention discloses a polyimide grafted polyethylene glycol composite solid-solid phase change material. The material mainly comprises polyethylene glycol, polyimide and graphene oxide, wherein amutual cross-linked network structure is formed through the interaction of functional groups and hydrogen bonds among polyethylene glycol, a polyimide precursor and graphene oxide molecules, and thena stable mutual cross-linked skeleton structure is formed through further thermal cross-linking of the polyimide precursor under the inert atmosphere high-temperature condition. The material disclosedby the invention is a stratified structure with cross-linked pores. The preparation method comprises the following steps of 1) preparing a mixed solution of a graphene oxide modified polyimide precursor; 2) and (3) preparing the stratified cross-linked porous structure composite solid-solid phase change material.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Flexible polymer material coolant and preparation method thereof
InactiveCN110845998ANo pollution in the processNot corrosiveHeat-exchange elementsProtective garmentPolyvinyl alcoholGlycerol
The invention discloses a flexible polymer material coolant and a preparation method thereof, and relates to the technical field of coolants, wherein the coolant is prepared from 2-4 parts of polyvinyl alcohol, 1.5-2.5 parts of propylene glycol, 1-3 parts of glycerol, 1-2 parts of borax and 60-70 parts of water. The preparation method comprises: under a heating condition, preparing a polyvinyl alcohol solution, and stirring to dissolve the polyvinyl alcohol solution; maintaining the temperature, adding propylene glycol and glycerol, and fully and uniformly stirring after each addition; preparing a borax solution, and heating to the same temperature; mixing the borax solution and the obtained solution, and uniformly stirring to obtain the coolant; and transferring into a packaging bag, cooling to a room temperature, and then freezing. According to the invention, the components and the ratio thereof of the coolant are reasonably selected, particularly part of glycerol is added as the cold accumulation aid, and the adding of the glycerol achieves the effect of the cooling aid and the effect of the softening agent, so that the prepared coolant is still soft after being frozen and accumulated, and can well fit a human body when being applied to protective clothing so as to improve wearing comfort.
Owner:HEFEI UNIV
Method for preparing composite phase change materials on basis of polished ceramic tile waste materials
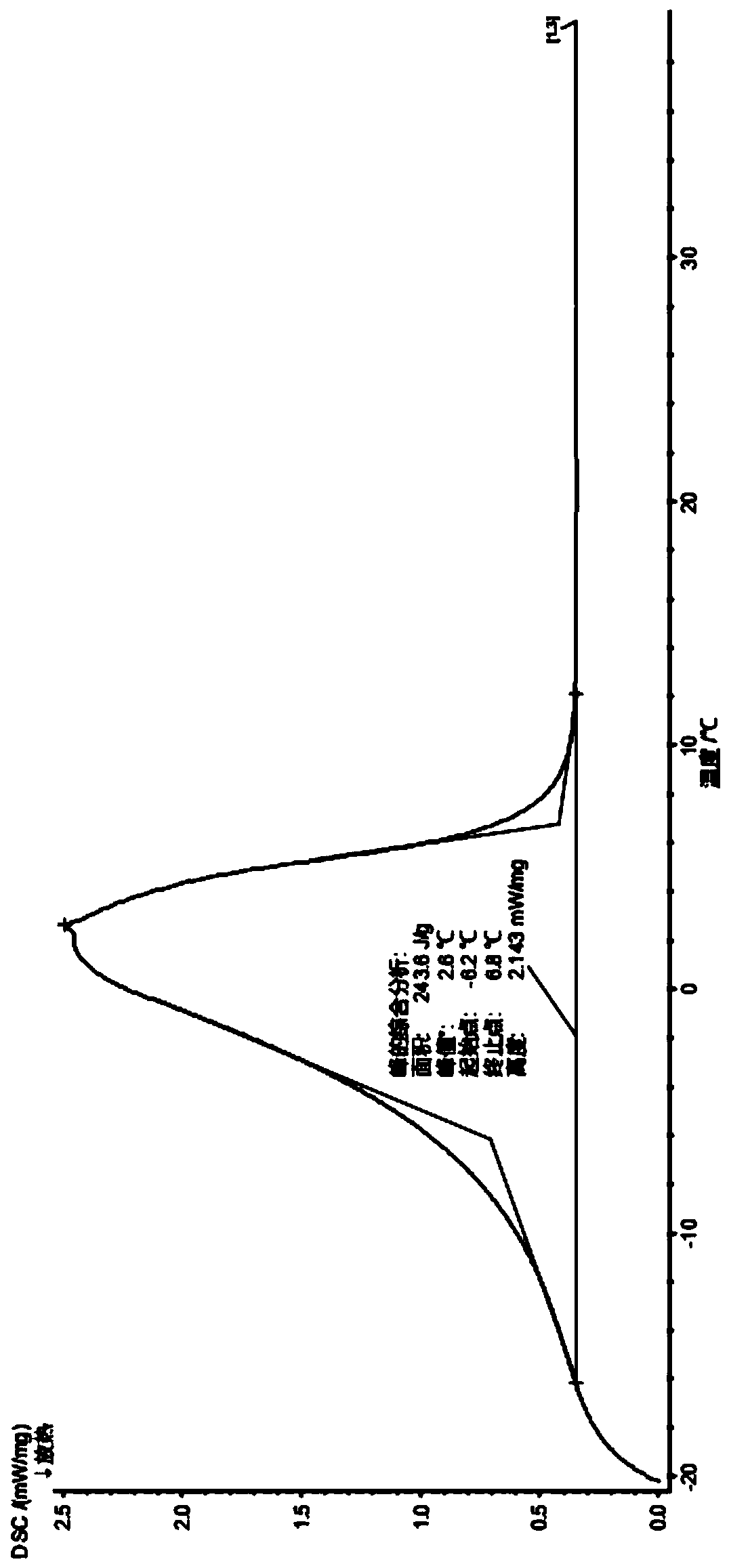
The invention discloses a method for preparing composite phase change materials on the basis of polished ceramic tile waste materials. The method includes steps of (1), preparing lauric acid / stearic acid eutectic phase change materials with the phase change temperatures of approximately 30 DEG C; (2), calcining the lauric acid / stearic acid eutectic phase change materials in muffle furnaces to obtain finished porous ceramic powder; (3), pouring the finished porous ceramic powder into hydrochloric acid solution, soaking the finished porous ceramic powder in the hydrochloric acid solution, and stirring the finished porous ceramic powder in the hydrochloric acid solution from time to time until bubble is unavailable; (4), placing activated ceramic powder in modification liquid, placing the modification liquid with the activated ceramic powder into water bath kettles, carrying out oscillation for 10 min, then carrying out suction filtration, sequentially washing the activated ceramic powderby absolute ethyl alcohol and distilled water by 4-5 times and drying the activated ceramic powder at the temperature of 105 DEG C for standby application; (5), weighing modified porous ceramic powder and the lauric acid / stearic acid eutectic phase change materials, heating and melting the modified porous ceramic powder and the lauric acid / stearic acid eutectic phase change materials, sufficiently mixing the modified porous ceramic powder and the lauric acid / stearic acid eutectic phase change materials with one another, further adding cement into the modified porous ceramic powder and the lauric acid / stearic acid eutectic phase change materials, and stirring the cement, the modified porous ceramic powder and the lauric acid / stearic acid eutectic phase change materials to obtain the composite phase change materials. The method has the advantages that processes for preparing the composite phase change materials, composite phase change materials are simple, the method is high in waste material utilization rate, the composite phase change materials have high phase change latent heat, are good in heat stability and have the appropriate phase change temperatures as compared with generalphase change materials, and accordingly the method is high in industrialization ability and has an excellent application prospect.
Owner:FOSHAN UNIVERSITY
Temperature-sensitive graphene oxide and preparation method thereof
ActiveCN106832156APhase transition temperature is suitableHigh phase change enthalpyHeat-exchange elementsPhase change enthalpyEthyl acetate
The invention discloses temperature-sensitive graphene oxide and a preparation method thereof. The preparation method comprises the following steps: 1) uniformly dispersing graphene oxide in a dispersing agent; 2) adding graphene oxide dispersion liquid and a reactive monomer into a reaction container, stirring, introducing inert gas, dropwise adding an initiator, then heating to the temperature of 60-70 DEG C, and stirring until reaction is completed, so that reaction mixed liquor is obtained, wherein the reactive monomer is at least one of n-alkyl methacrylate and n-alkyl acrylate or mixture of the two; 3) precipitating by utilizing an alcohol solvent, then dissolving precipitates into the dispersing agent, centrifuging for removing graphene oxide which is not involved in the reaction, and collecting precipitates II; and 4) dissolving the precipitates II into acetone or ethyl acetate, repeatedly centrifuging, collecting precipitates, and carrying out vacuum drying, so that the temperature-sensitive graphene oxide is obtained. The preparation method disclosed by the invention has the advantages that a phase change material is grafted to the surface of graphene oxide, the temperature-sensitive graphene oxide with stable performance is obtained, and the temperature-sensitive graphene oxide has high phase change enthalpy value and good thermal stability.
Owner:TIANJIN POLYTECHNIC UNIV
Heat-conduction-enhanced ionic liquid composite phase-change heat-storage material based on modified graphene, and preparation method thereof
PendingCN113072916AHigh purityPhase transition temperature is suitableHeat-exchange elementsPhase-change materialLatent heating
The invention discloses a heat-conduction-enhanced ionic liquid composite phase-change heat-storage material based on modified graphene and a preparation method thereof. The preparation method comprises the steps that firstly, covalent functional modification is conducted on graphene oxide through amino-terminated ionic liquid, and the modified graphene oxide reacts with hydrazine hydrate to be reduced, so the dispersity and interfacial compatibility of a graphene filler in an ionic liquid phase-change material matrix are improved, and the heat conduction characteristic of the composite material is improved; and furthermore, the modified graphene is mixed with the imidazolium ionic liquid phase-change material matrix, and conditions such as rotary evaporation and drying are controlled to obtain a mixture of the modified graphene with different loading capacities and the ionic liquid matrix phase-change material. The material provided by the invention has the characteristics of large phase change latent heat and good thermal stability; and meanwhile, the thermal conductivity is increased by 4.4%-127.9% compared with ionic liquid without modified graphene, and the thermal energy storage and conduction characteristics of the material are remarkably improved.
Owner:XI AN JIAOTONG UNIV
Phase-change energy storage ceramsite and preparation method thereof
The invention discloses phase-change energy storage ceramsite and a preparation method thereof, relating to the technical field of phase-change energy storage materials. The phase-change energy storage ceramsite contains the following raw materials in parts by weight: 50-70 parts of diatom plate polishing waste, 3-5 parts of quick lime, 20-50 parts of gel materials, 20-30 parts of solid-liquid organic phase-change energy storage materials and 15-40 parts of water. The preparation method comprises the steps of mixing the raw materials in a formula ratio, and carrying out granulation and room-temperature maintenance, so as to obtain the ceramsite. According to the phase-change energy storage ceramsite, the diatom plate polishing waste and the quick lime are taken as the raw materials, the solid-liquid organic phase-change energy storage materials are taken as an energy storage material, the raw materials and the energy storage material are matched with the gel materials so as to generatethe light and high-strength phase-change energy storage ceramsite, so that the application defect of fusion leakage of an organic phase-change material is remedied by virtue of the microstructure advantages and relatively high adsorption capacity of diatom plate polishing powder, and the high-added value functional utilization of the diatom plate polishing waste is realized. By utilizing fire-free and steam-free manners, the pretreatment of the raw materials is omitted, so that the preparation method has the advantages of simple preparation process, low cost, energy saving and environmental protection and the like.
Owner:江苏古德乐环保科技有限公司 +1
Phase-change cold storage compound and preparation method thereof
InactiveCN111320969AHigh thermal conductivityPrevent phase separationHeat-exchange elementsPhysical chemistryCarbon nanomaterials
The invention relates to a phase-change cold storage compound and a preparation method thereof. The phase-change cold storage compound comprises sodium sulfate decahydrate, a nucleating agent, a thickening agent and a temperature regulator. Wherein based on 100 parts by mass of the sodium sulfate decahydrate, the mass parts of the nucleating agent, the thickening agent and the temperature regulator are respectively 0.5-5 parts, 0.5-5 parts and 10-20 parts; wherein the phase-change cold storage compound further comprises a carbon nano material, and based on 100 parts by mass of the sodium sulfate decahydrate, the mass part of the carbon nano material is 1-5 parts. By optimizing the formula design of the phase-change cold storage composite material, the composite material has the characteristics of safety, no toxicity, low production cost, small degree of supercooling, high cold storage density and good material stability, and can be applied to use scenes needing 4-8 DEG C, such as a phase-change cold storage system of an air conditioner.
Owner:内蒙古信敏惠纳米科技有限公司
Porous alumina composite shaped phase change material and preparation method thereof
ActiveCN111849422AEasy temperature adjustmentEnhance heat storage and discharge functionClimate change adaptationHeat-exchange elementsMolten stateOxide composite
The invention discloses a porous alumina composite shaped phase change material and a preparation method thereof. The preparation method comprises the steps: putting Na2SO4.10H2O into a constant-temperature water bath kettle, and heating to a molten state; and then adding porous aluminum oxide according to the mass ratio of 10-30 wt%, uniformly stirring and mixing, and then putting the mixture into a vacuum drying oven for adsorption and fixation, so as to prepare the porous aluminum oxide composite shaped phase-change material. The porous alumina composite shaped phase-change material prepared by the method can effectively enhance the heat storage and release functions of a solar greenhouse wall body, thereby meeting the growth requirements of plants.
Owner:NORTHWEST A & F UNIV
Low temperature step phase change heat storage and cold storage device
InactiveCN103344146ALarge heat storageReduce subcoolingHeat storage plantsCarboxymethyl celluloseEngineering
The invention discloses a low temperature step phase change heat storage and cold storage device which comprises black plastic pipe bodies and a long fixing plate. The two ends of each black plastic pipe body are fixed or directly bonded to the long fixing plate respectively. A material A or a material B is arranged inside the black plastic pipe bodies, the black plastic pipe bodies which are adjacent to the black plastic pipe bodies with the material A are all provided with the material B, and the black plastic tube bodies which are adjacent to the black plastic tube bodies with the material B are all provided with the material A. The material A is composed of 80%-84% of Na2SO4 10H2O, 1%-3% of borax, 1%-2% of sodium carboxymethyl cellulose, 3%-6% of white carbon black, 2%-4% of NaCl, and 0.5%-1% of sodium hexametaphosphate. The material B is composed of 80%-94% of CaC12H2O, 1%-3% of barium hydroxide, 0.5%-2% of NaCl, 0.5%-2% of sepiolite, and 0.5%-2% of sodium silicate. The low temperature step phase change heat storage and cold storage device prolongs heat storage and heat releasing time, and achieves the effect of saving energy.
Owner:DALIAN GUOXIANG TECH DEV
High molecular solid/solid phase changing material with net type and comb type mixed structure and its preparing method
InactiveCN1263821CHigh phase change enthalpyPhase transition temperature is suitableHeat-exchange elementsPhase change enthalpyPolyethylene glycol
The present invention relates to a kind of high molecular solid / solid phase changing material with mixed net and comb structure, and features that polyglycol with two active end radical and polyglycol with one active end radical are fixed onto the high molecular skeleton material to form 3D mixed net and comb structure. The material of the present invention has relatively great phase change enthalpy up to 120 J / g, proper phase change temperature capable of being altered in 0-65 deg.c, stable solid state before and after phase change without supercooling, separating and other unstable phenomenon, high mechanical strength, high solvent resistance, good machining performance, no toxicity, no leakage, no corrosion, no pollution, long service life and other advantages. The present invention may be used widely in solar energy utilization, afterheat recovering, intelligent air conditioner and other fields.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com