Heat-conduction-enhanced ionic liquid composite phase-change heat-storage material based on modified graphene, and preparation method thereof
A technology of ionic liquid and thermal conductivity enhancement, applied in the field of composite materials, can solve the problems of low latent heat of phase change, reduce production cost, and restrict the application of graphene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
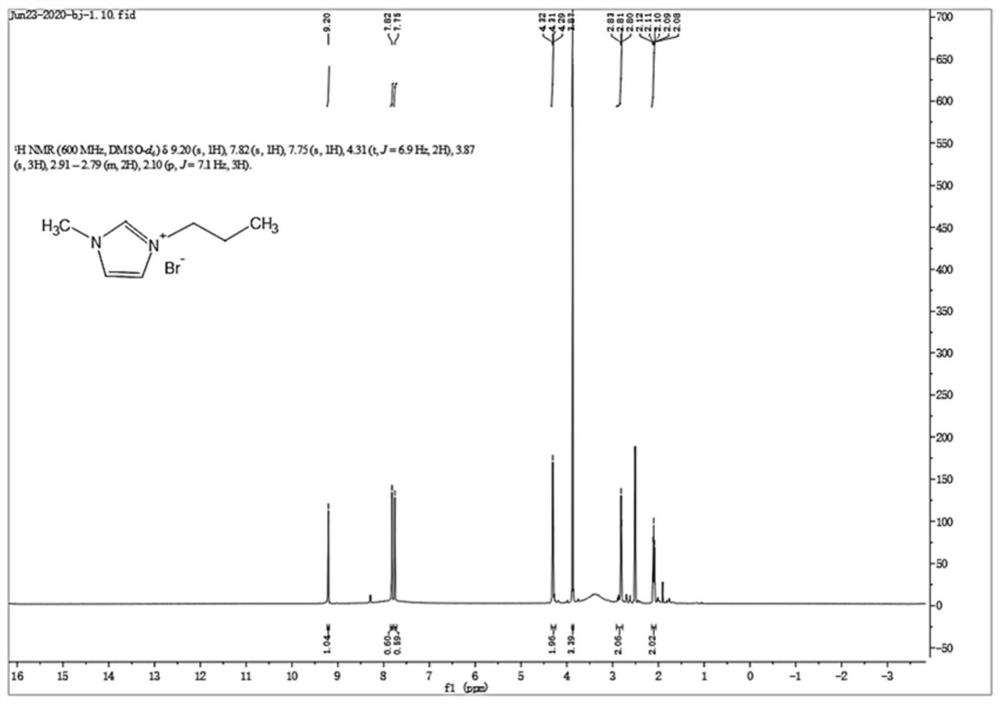
[0079]1. Take 1-methylimidazole (0.1mol) and 3-bromopropylamine hydrobromide (0.1mol) into a three-necked flask respectively, and add 50mL of ethanol. The apparatus was purged with nitrogen to remove air and moisture. Heated and refluxed in a water bath at 90°C for 24 hours, the stirring speed was 400 rpm, and the temperature of condensed water was 5°C. After the reaction, the reaction solution was rotary evaporated to obtain the crude product, which was redissolved in ethanol, and KOH (0.1 mol) was added to deprotect the amino group, stirred for 12 hours, filtered, and the filtrate was rotary evaporated to obtain an alkyl group containing terminal amino groups. Crude product of imidazole ionic liquid. The crude product was dissolved in a mixed solvent of ethanol-tetrahydrofuran (volume ratio 1:4), filtered, and the filtrate was rotary evaporated to remove the mixed solvent. The product was recrystallized in ethyl acetate to obtain a white solid, which was dried at 40°C for 2...
Embodiment 2
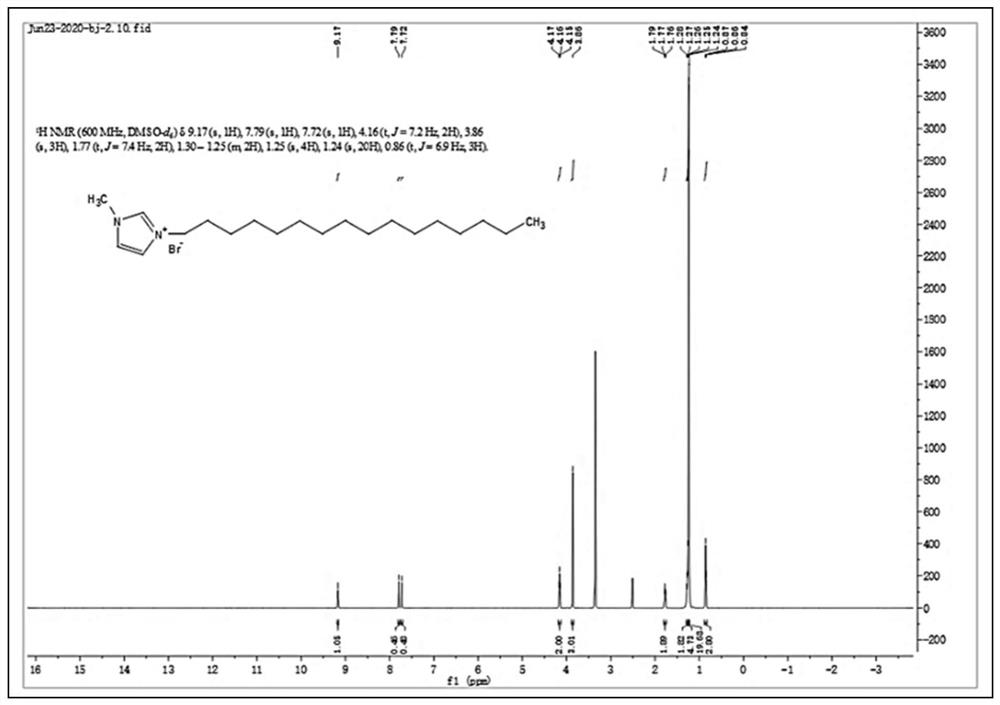
[0084] 1. Take 1-methylimidazole (0.5mol) and 3-bromopropylamine hydrobromide (0.5mol) into a three-necked flask respectively, and add 250mL of ethanol. The apparatus was purged with nitrogen to remove air and moisture. Heated and refluxed in a water bath at 90°C for 48 hours, the stirring speed was 500 rpm, and the temperature of condensed water was 5°C. After the reaction was completed, the reaction solution was rotary evaporated to obtain a crude product, which was redissolved in ethanol, and KOH (0.55mol) was added to deprotect the amino group, stirred for 12 hours, filtered, and the filtrate was rotary evaporated to obtain an alkyl group containing terminal amino groups. Crude product of imidazole ionic liquid. The crude product was dissolved in a mixed solvent of ethanol-tetrahydrofuran (3:2), filtered, and the filtrate was rotary evaporated to remove the mixed solvent. The product was recrystallized in ethyl acetate to obtain a white solid, which was dried at 40°C for ...
Embodiment 3
[0089] 1. Take 1-methylimidazole (0.5mol) and 3-bromopropylamine hydrobromide (0.55mol) into a three-necked flask respectively, and add 250mL of ethanol. The apparatus was purged with nitrogen to remove air and moisture. Heated and refluxed in a water bath at 90°C for 24 hours, the stirring speed was 400 rpm, and the temperature of condensed water was 5°C. After the reaction was completed, the reaction solution was rotary evaporated to obtain a crude product, which was redissolved in ethanol, and KOH (0.6 mol) was added to deprotect the amino group, stirred for 12 hours, filtered, and the filtrate was rotary evaporated to obtain an alkyl group containing terminal amino groups. Crude product of imidazole ionic liquid. The crude product was dissolved in a mixed solvent of ethanol-tetrahydrofuran (1:4), filtered, and the filtrate was rotary evaporated to remove the mixed solvent. The product was recrystallized in ethyl acetate to obtain a white solid, which was dried at 40°C for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Enthalpy | aaaaa | aaaaa |
| Thermal diffusivity | aaaaa | aaaaa |
| Thermal conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com