Patents
Literature
1266 results about "Octadecane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
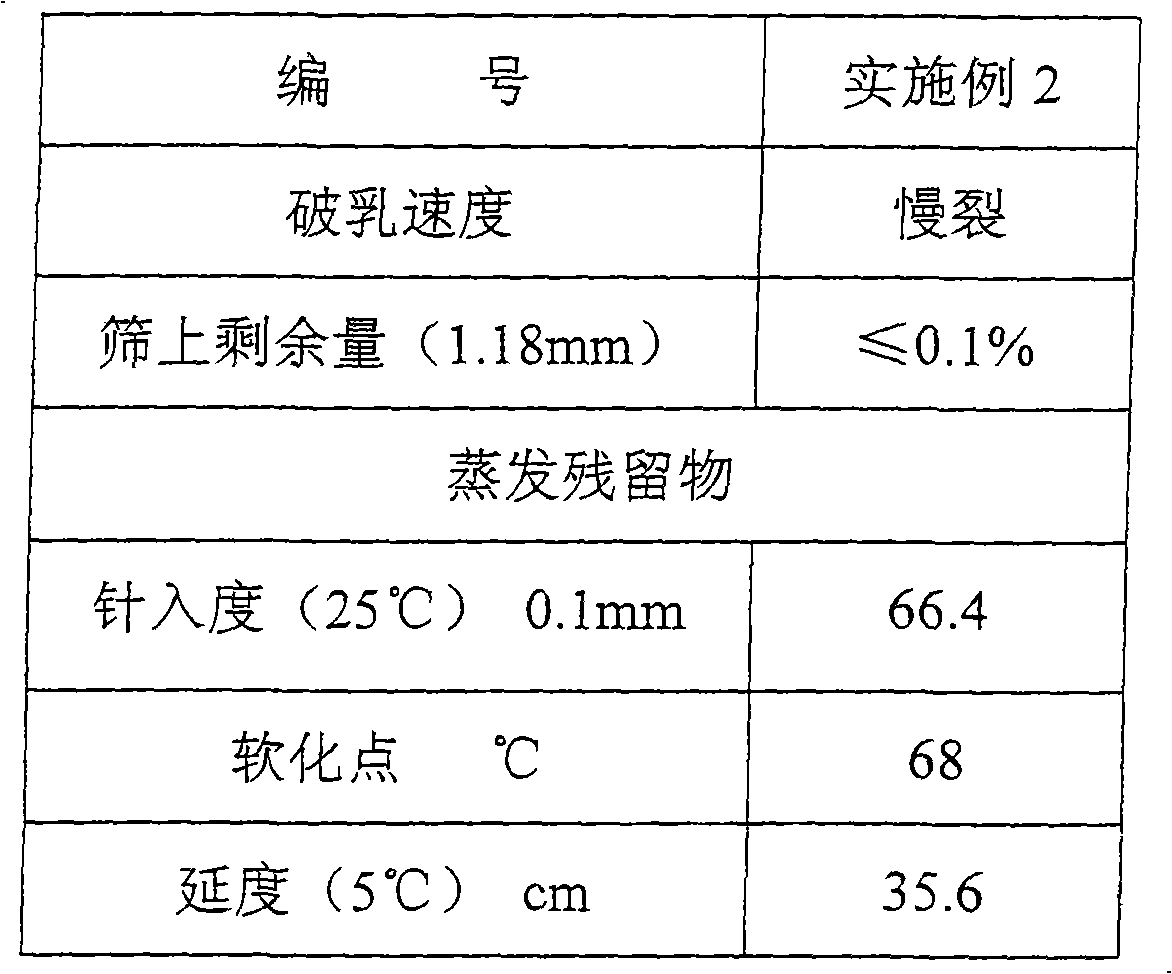
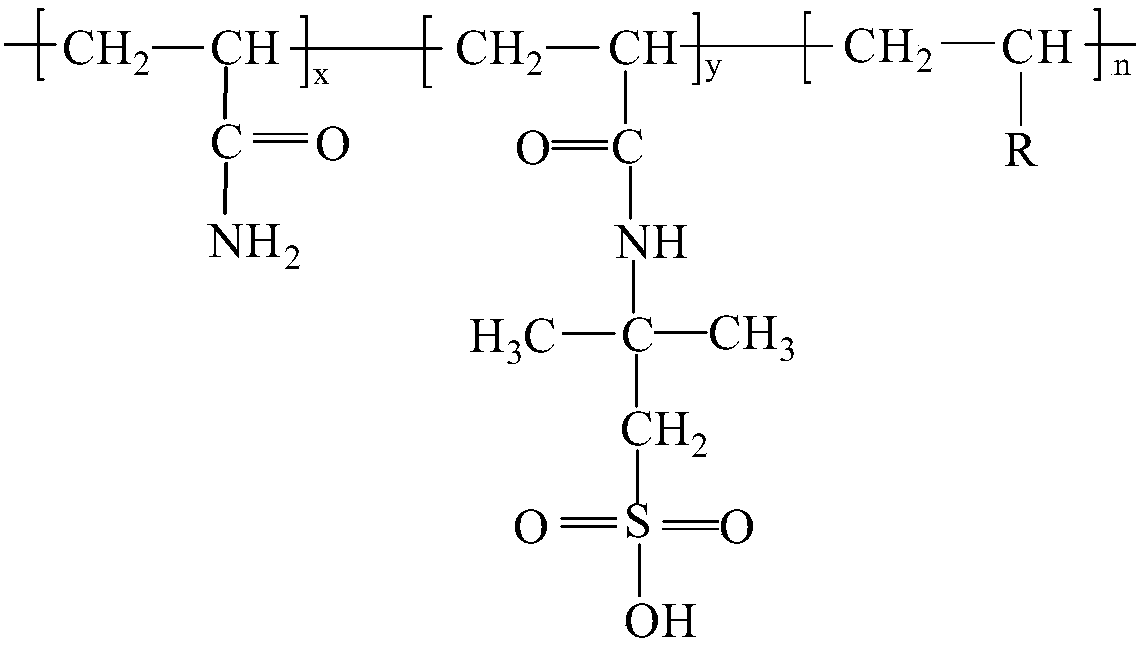
Octadecane is an alkane hydrocarbon with the chemical formula CH₃(CH₂)₁₆CH₃.
Antimicrobial isopropyl alcohol and organofunctional silane solution
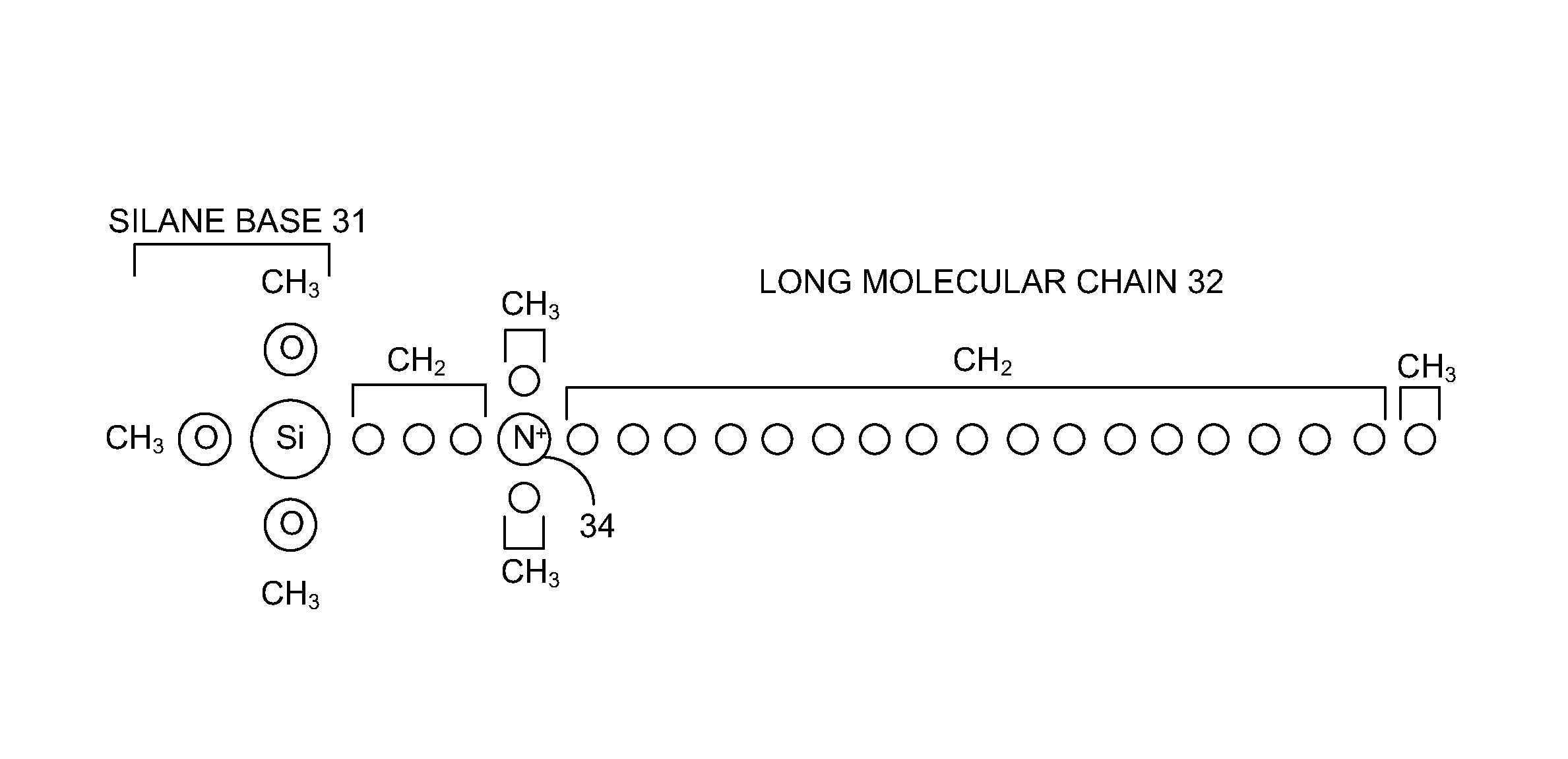
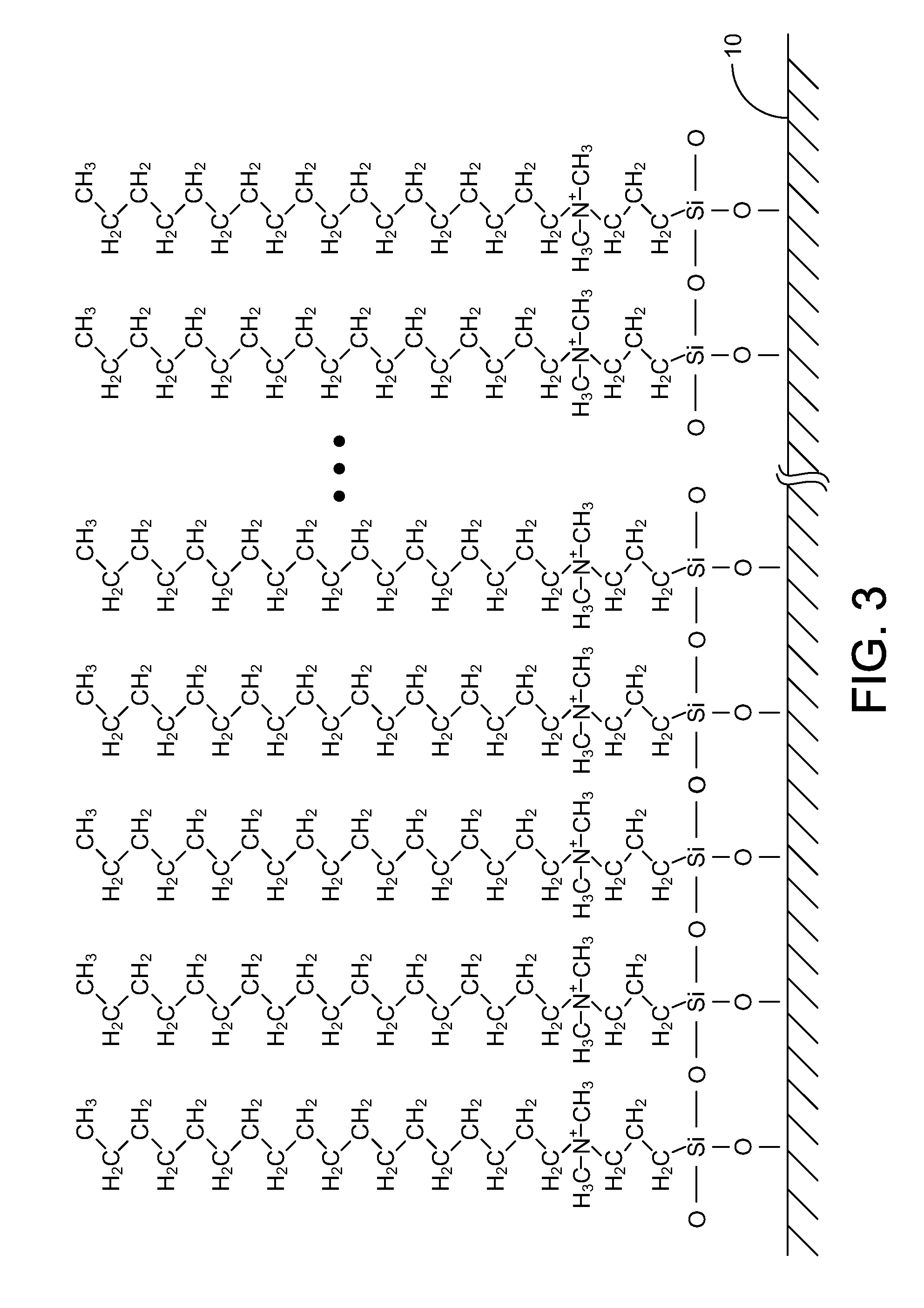
A surface of an object may be treated using an antimicrobial wipe presoaked in an antimicrobial treatment solution. Alternatively, the antimicrobial treatment solution may be sprayed directly on the surface. The antimicrobial treatment solution may be made of isopropyl alcohol and an unreacted organofunctional silane antimicrobial substance that is substantially free from arsenic, silver, tin, heavy metals and polychlorinated phenols. The antimicrobial substance may include any one of: 3 trimethoxysilylpropyloctadecyldimethyl ammonium chloride; hyaluronan and its derivatives; triclosan; and a copolymer of chloropropyltrihydroxysilane and octadecylaminodimethyltrihydroxysilylpropyl ammonium chloride.
Owner:PARASOL MEDICAL
Emulsified metal antirusting agent and preparation method thereof
ActiveCN101659802AImprove rust resistanceExcellent anti-rustAnti-corrosive paintsWater basedSarcosine
The invention relates to an emulsified metal antirusting agent and a preparation method thereof. The antirusting agent comprises the components by weight percentage of 2-2.5% of dicarboxylic acid, 6-6.5% of triethanolamine, 1-1.5% of monoethanolamine, 5-6.5% of synthesized borate, 8-10% of dodecenylbutadioic acid, 4.5-6% of octadecane oeoyl sarcosine acid, 0.1% of benzotriazole and 66.9-73.4% of water. The preparation method comprises the following steps of: firstly preparing a water-base antirusting agent; subsequently adding the octadecane oeoyl sarcosine acid, the synthesized borate and thedodecenylbutadioic acid in a reaction kettle; and mixing the solution, thus obtaining the emulsified antirust product. The method utilizes the strong adsorption function between the antirusting agentand the metal, displaces the residual oil dirt out of the surface of the metal, leads the surface of the metal to be adsorbed by a hydrophobic protective film; no oil dirt is remained on the surfaceof the metal that is processed by the antirusting agent, and the surface of the metal is not easy to be polluted by the dust; the antirusting agent can be used directly; furthermore, the components contains no nitrite, thus being safe and environment-friendly for use.
Owner:华阳新兴科技(天津)集团有限公司
Preparation method of modified superfine calcium carbonate powder and product of preparation method
InactiveCN106433220AGood dispersionModified dispersion effect is goodPigment treatment with macromolecular organic compoundsPigment treatment with organosilicon compoundsPolyesterDispersity
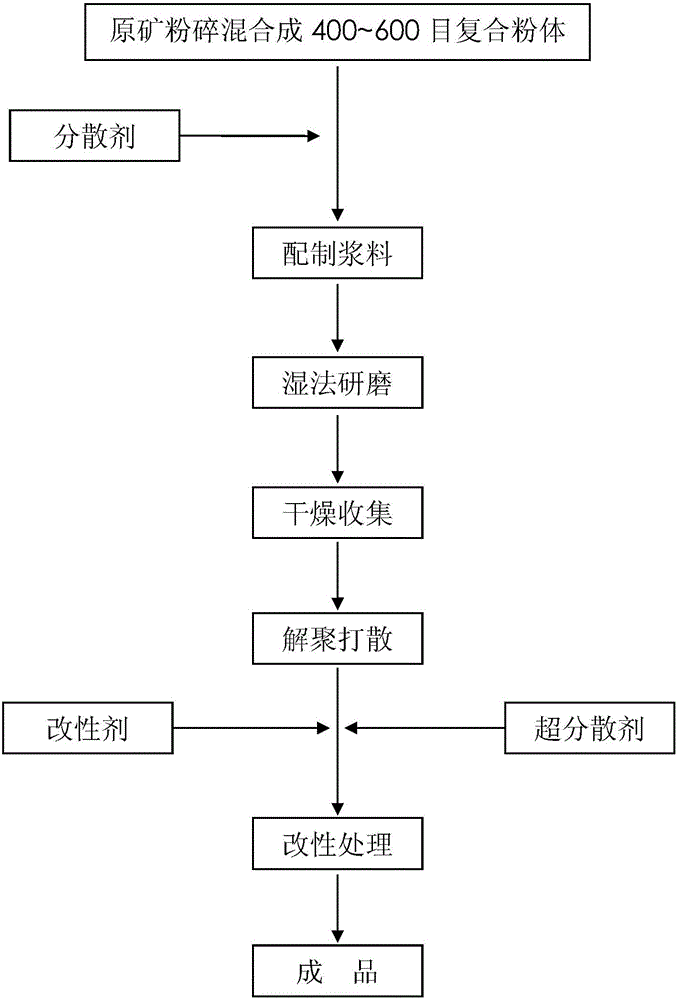
The invention provides a preparation method of modified superfine calcium carbonate powder. The preparation method comprises following steps: raw material mixing, slurry preparation, wet grinding, drying collection, depolymerization scattering and modified treatment, wherein a dispersing agent at least comprises ammonium polyacrylate salt, sodium hexametaphosphate and sodium polyacrylate salt; a modifying agent comprises one or more of octadecylamine, polymethyl methacrylate, a titanate coupling agent and a silane coupling agent; and a hyperdispersant comprises one or more of a polyester type hyperdispersant, a polyether type hyperdispersant, a polyacrylate hyperdispersant and a polyolefin hyperdispersant. By virtue of the preparation method, the dispersity of the powder can be improved, the dry-wet cover effects of building coatings can be improved, and the addition amount of titanium dioxide can be reduced.
Owner:JIANGXI GUANGYUAN CHEM
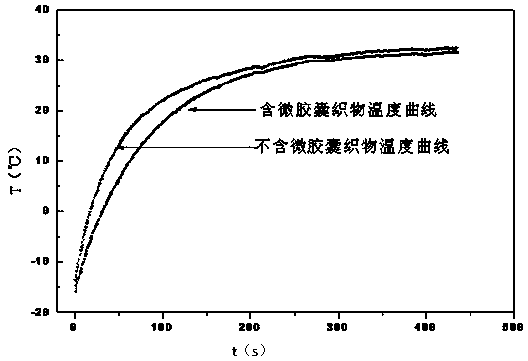
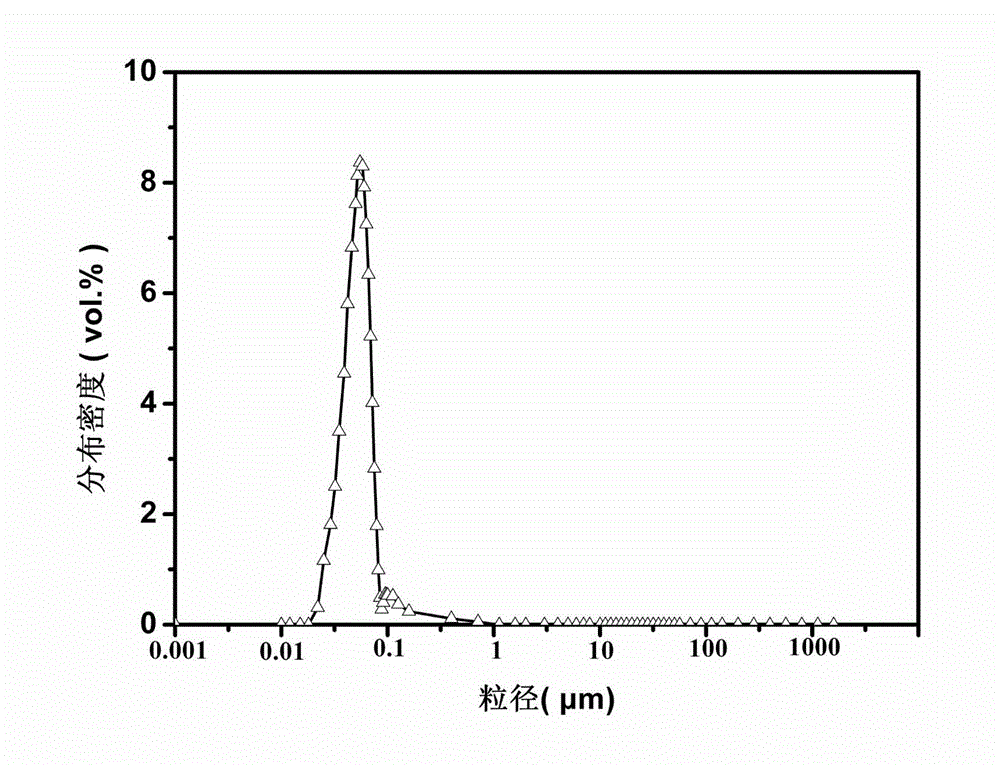
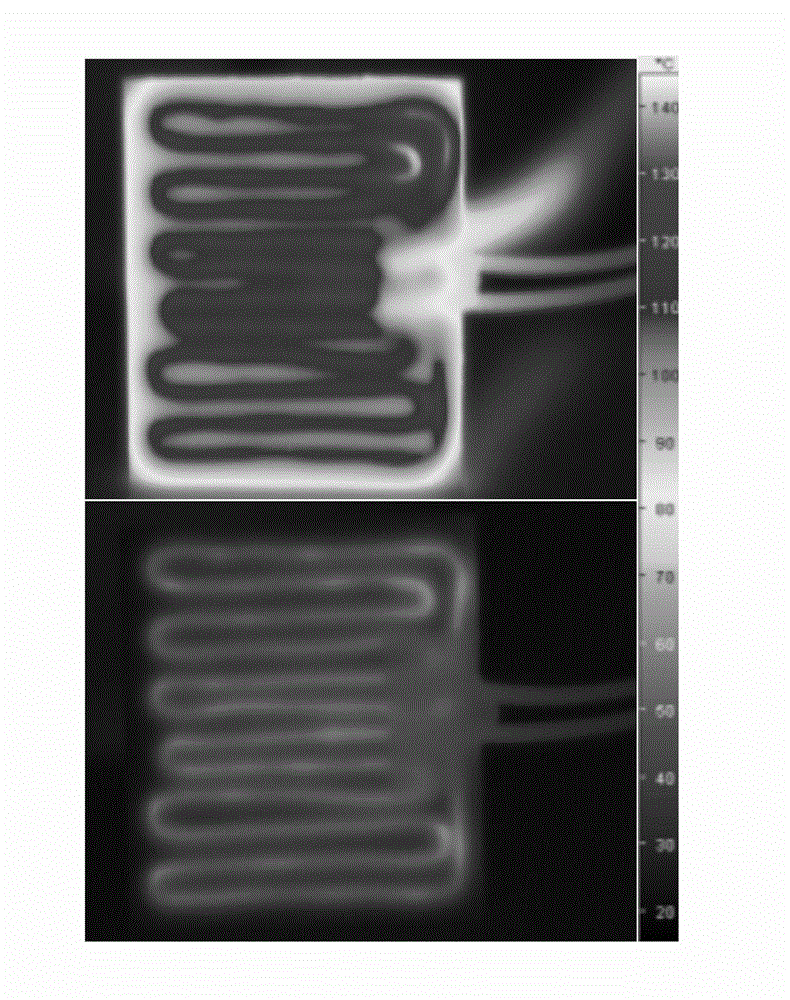
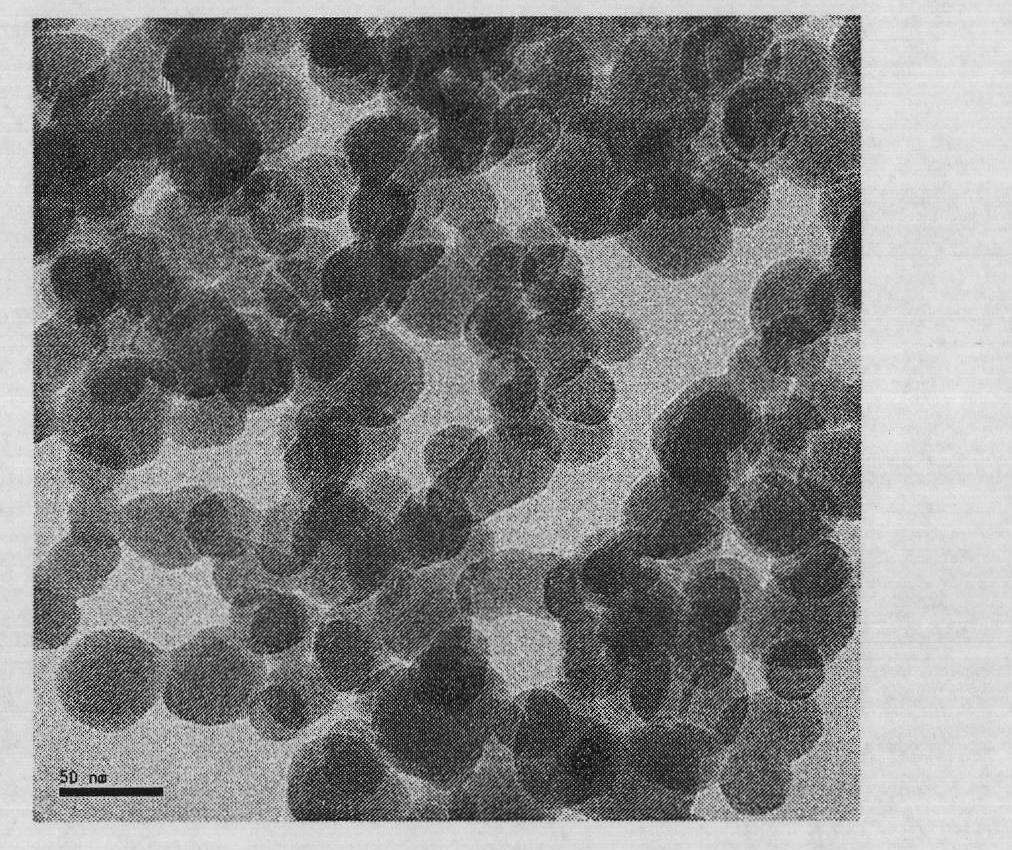
Phase change micro-capsule for clothing thermoregulation as well as preparation method and application thereof
InactiveCN103937461AImprove performanceNo corrosionHeat-exchange elementsVegetal fibresPolymer scienceN-hexadecane
The invention discloses a phase change micro-capsule for clothing thermoregulation as well as a preparation method and application thereof. The phase change micro-capsule for clothing thermoregulation comprises a core material and a wall material, wherein the core material is a mixture of n-octadecane and n-hexadecane, and the molar ratio between n-octadecane and n-hexadecane is (3 : 1)-(24 : 1); the wall material is methyl methacrylate; the phase change micro-capsule is applied to preparation of a clothing thermoregulation material. The phase change micro-capsule is stable in performance, non-toxic, non-corrosive and low in cost, can not cause super-cooling and super-heating phenomenon in use, has a melting and solidification temperature range just in the temperature range in which a human body can feel comfortable, is uniform in particle size distribution and appropriate in melting heat and solidification heat, meets the flexibility of finished fabrics in a later period, has a relatively large heat storage effect, and is suitable for performing thermoregulation on clothing.
Owner:SOUTH CHINA UNIV OF TECH
Surface treatment of nanoparticles to control interfacial properties and method of manufacture
InactiveUS20050222325A1Material nanotechnologySynthetic resin layered productsPersonal care3-mercaptopropyltrimethoxysilane
A surface treated particle comprising a plurality of inorganic, metallic, semi-metallic, and / or metallic oxide particles and a star-graft copolymer with looped and / or linear polymeric structure on a star-graft copolymer, obtainable by a heterogeneous polymerization reaction in the particle surface proximity, encapsulating at least a portion of said particles and a method for making the same. The surface treatment comprises: Si (w, x, y, z), where: w, x, y, and z are mole percent tetrafunctional, trifunctional, difunctional, and monofunctional monomeric units, respectively; w, x, y, and z are about 0-50, 0-50, 5-99, and 0-5, respectively; w is tetraethylorthosilicate; x is selected from the group consisting of γ-glycidoxypropyltrimethoxysilane, γ-methacryloxypropyltrimethoxysilane, methyltrimethoxysilane, n-propyltrimethoxysilane, isobutyltrimethoxysilane, n-hexyltrimethoxysilane, n-octyltrimethoxysilane, n-octadecyltrimethoxysilane, phenyltrimethoxysilane, 3-(trimethoxysilyl)propylsuccinic anhydride, heptadecafluorotrimethoxysilane, 3-isocyanatopropyltrimethoxysilane, 2-(diphenylphosphino)ethyltrimethoxysilane, 3-aminopropyltrimethoxysilane, 3-mercaptopropyltrimethoxysilane, n-(trimethoxysilylpropyl)EDTA, pentafluorophenylpropyltrimethoxysilane, trifluoropropyltrimethoxysilane, and the triethoxy-containing counterparts of these monomers; y is selected from the group consisting of dicyclohexyldimethoxysilane, diethyldiethoxysilane, dimethyldichlorosilane, dimethyldiethoxysilane, dimethyldimethoxysilane, diphenyldiethoxysilane, diphenyldimethoxysilane, di-n-hexyldichlorosilane, n-hexylmethyldichlorosilane, methyldodecyldiethoxysilane, n-octylmethyldimethoxysilane, and the diethoxy-containing counterparts of these monomers; and z is selected form the group consisting of n-octadecyldimethylmethoxysilane, triethylsilanol, trimethylethoxysilane, trimethylmethoxysilane, and the ethoxy-containing counterparts of these monomers. Product(s) per se, defined as surface treated ZnO and / or TiO2, and the use of the product(s) per se in personal care formulations are excluded.
Owner:NANOPHASE TECH CORP
N-octadecane phase change micro-emulsion as well as preparation method and application thereof
InactiveCN103146349ASimple ingredientsEasy to prepareHeat-exchange elementsInorganic saltsActive agent
The invention discloses an n-octadecane phase change micro-emulsion. The micro-emulsion comprises water and the following components in mass fraction: 5-40% of n-octadecane, 2-20% of surfactant, 0-20% of fatty alcohols and 0-3% of inorganic salts. The preparation method comprises the following steps of: firstly mixing n-octadecane, the surfactant, fatty alcohol and the inorganic salts according to the ratio of the mass fraction, then adding the remained water at 30-40 DEG C, stirring uniformly and standing to obtain the n-octadecane phase change micro-emulsion. The n-octadecane phase change micro-emulsion can be applied to a microelectronic system thermal management, and is used for micro-channel efficient heat dissipation cooling working medium in a thermal management system. The micro-emulsion and the application thereof have the advantages of high latent heat of phase change, wide applicable temperature range, good stability and the like.
Owner:NAT UNIV OF DEFENSE TECH
Method for quickly and efficiently testing liquid absorptivity of pole piece
InactiveCN106814004AHigh flash pointHydrophobicWeighing by absorbing componentObservational errorPole piece
The invention relates to a method for quickly and efficiently testing liquid absorptivity of a pole piece. The method comprises the following steps: cutting a pole piece sample, and weighing the pole piece sample to obtain the mass M0; putting the weighed pole piece sample into a container; pouring a soaking solution into the container, and immersing the pole piece sample; putting the container into a vacuum drying oven, and keeping for 15-20 minutes; taking out the pole piece, wiping the free soaking solution on the surface of the pole piece sample by using filter paper, and weighing the pole piece sample to obtain the mass M1; and calculating the liquid absorptivity epsilon=(M1-M0) / M0 and the electrolyte maintenance dose M=epsilon*M0*rho1 / rho2, wherein rho1 is the electrolyte concentration, and rho2 is the density of the soaking solution. The used octadecane has the advantages of high stability, high flash point and low volatility, is hydrophobic, and thus, is suitable to be used as a solvent for soaking the pole piece. The method overcomes the defects of long time consumption, big measurement errors, great environmental pollution caused by the electrolyte and potential hazards to the operating personnel when the electrolyte is used for soaking the pole piece and testing the liquid absorptivity in the past.
Owner:ZHEJIANG CHAOWEI CHUANGYUAN INDUSTRAIAL
Cation water-based nano silicon dioxide and preparation method and application thereof
InactiveCN101914313AImprove stabilityImprove uniformityCoatingsPigment treatment with organosilicon compoundsWater basedSlurry
The invention discloses cation water-based nano silicon dioxide and preparation method and application thereof. When in preparation, water, octadecyl dimethyl benzyl ammonium chloride and concentrated hydrochloric acid are added into a reactor to be stirred and dissolved; nano silicon dioxide micro powder is added into the reactor, stirring is carried out, so as to obtain low viscosity semi-transparent solution, and aminosilane coupling agent is dropwise added into the reactor while stirring; the pH value of the solution is regulated to 3-4, and the solution is continuously stirred, so as to obtain semi-transparent cation water-based nano silicon dioxide. The invention provides a cation surfactant and an aminosilane coupling agent into water phase, the surface characteristic of nano silicon dioxide and rheological property of dispersion are effectively improved, a nano silicon dioxide water-based dispersion with low viscosity, high stability and high uniformity is obtained, and further a high light waterproof coating slurry can be prepared.
Owner:NANTONG NAWEI DIGITAL MATERIAL TECH
Nanoparticles for targeted delivery of active agents to the lung
InactiveUS20110064652A1Fast and safe and easy deliveryEnhanced local lung deliveryOrganic active ingredientsBiocideActive agentDelivery system
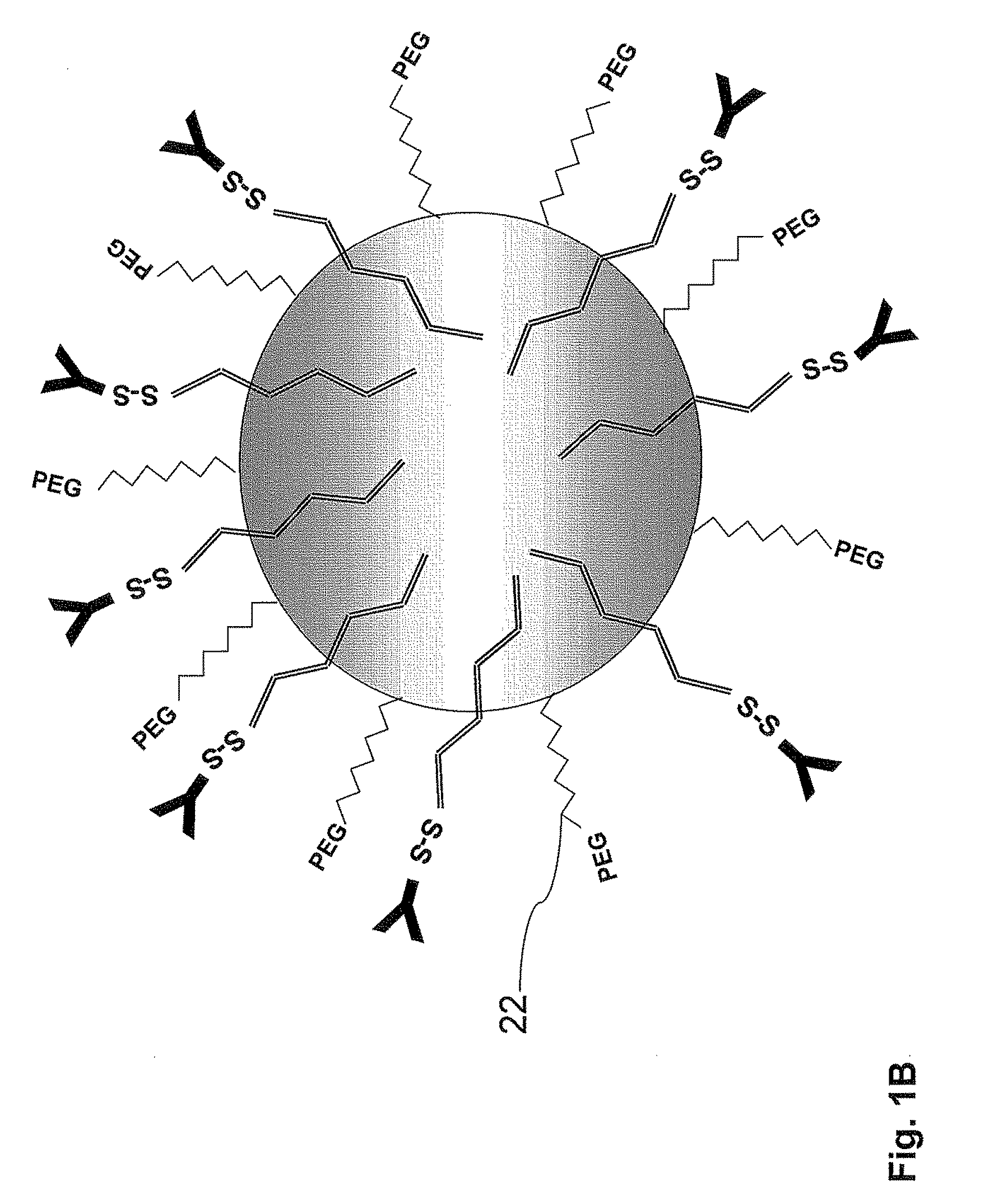
The present invention concerns a delivery system administered to the lung preferably by inhalation comprising a polymer-based nanoparticle; and a linker comprising a first portion non-covalently anchored to said nanoparticle, wherein at least part of said first portion comprises a hydrophobic / lipophilic segment embedded in said nanoparticle; and a second portion comprising a coupling group, preferably a maleimide compound, exposed at the outer surface of said nanoparticle. In accordance with one embodiment, the delivery system comprises one or more targeting agents, each covalently bound to said coupling group, preferably maleimide compound, and is administered as an aerosol in the therapy or diagnosis of lung cancer or bronchial dysplasia. In accordance with yet another embodiment, the delivery system comprises a drug and / or a radiopharmaceutical and / or a contrasting agent. A specific example for a linker in accordance with the invention is octadecyl-4-(maleimideomethyl)cyclohexane-carboxylic amide (OMCCA).
Owner:BORLAK JURGEN +3
QuEchERS method package for efficiently and homogeneously extracting residues of pesticide and veterinary drug
ActiveCN103698195AEasy to operateEasy to joinPreparing sample for investigationSilica gelAmmonium sulfate
The invention discloses a QuEchERS method package for efficiently and homogeneously extracting residues of a pesticide and a veterinary drug. The QuEchERS method package comprises an extraction pipe containing ammonium sulfate, a buffer salting-out phase-splitting salt package containing a mixture of anhydrous magnesium sulfate and sodium chloride, a needle cylinder type membrane filter containing a mixture of PSA, graphitized carbon black and a C18 reverse phase silica gel filler, and a salting package tool, wherein the PSA is an N-propylethylendiamine-modified silica gel filler and the C18 reverse phase silica gel filler is an octadecyl-modified reverse phase silica gel filler. According to the method, the technical feature of the combination of extraction, salting-out phase-splitting and dehydration purification is fully utilized, devices, materials and operating steps are reasonable to form, the structure is simple, and the operation is easy and convenient.
Owner:山东青云实验耗材有限公司
High-temperature behavior stable modified emulsified asphalt and method for preparing the same
InactiveCN101492571AStable performance at high temperatureImprove performanceBuilding insulationsSlurryPyrophosphoric acid
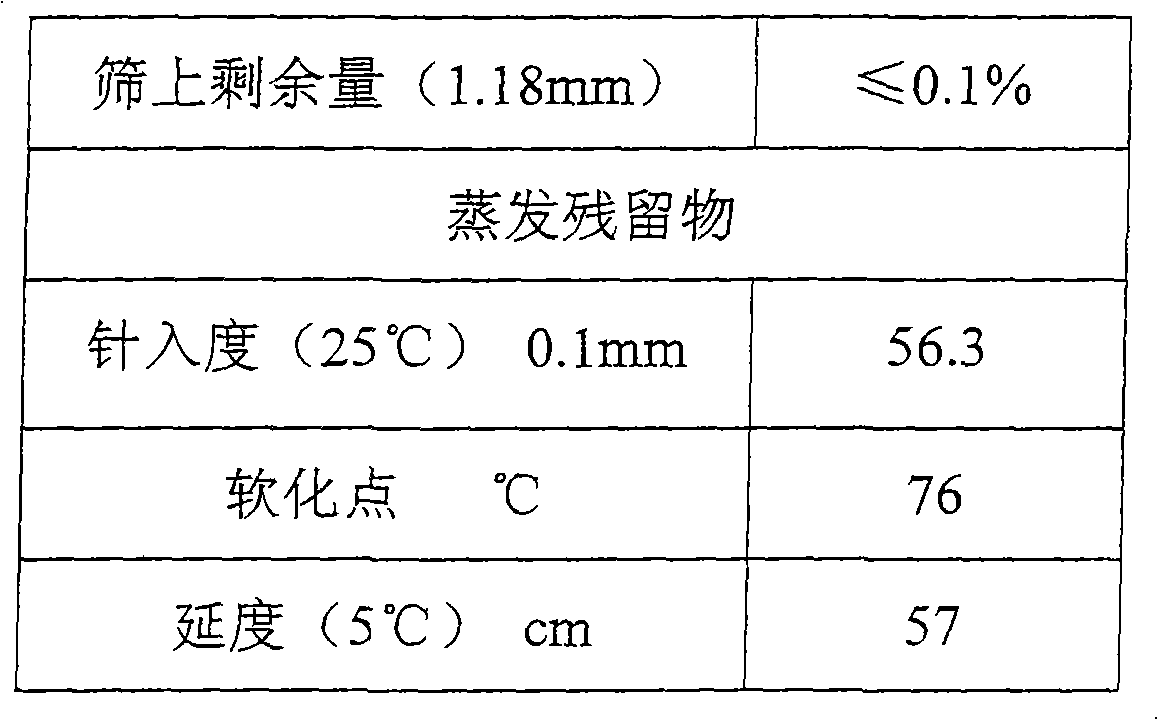
The invention relates to stable high temperature performance modified emulsified asphalt and a preparation method thereof. Based on the weight of matrix asphalt, the mixture of phosphorus-containing compound is 0.1-5%, compound emulsifying agent is 0.5-5%, modifying agent rubber latex is 1-10%. The components and weight percentage of the mixture of the phosphorous-containing compound are: 35%-40% of polyphosphoric acid, 20%-30% of pyrophosphoric acid, and 35%-40% of phosphoric acid. The components and weight percentage of the compound emulsifying agent are: 25%-30% of CAPB, 5%-10% of octadecane DTMAC and 60%-65% of water. And the modified agent rubber latex is styrene butadiene latex. Compared with the prior art, the modified emulsified asphalt prepared by the invention has the following prominent advantages that the softening point of evaporated residue of the modified emulsified asphalt is high; the modified emulsified asphalt has stable high temperature performance, simple processing technique, convenient operation, and high performance and is applicable to the micro-surface treatment of the slurry seal.
Owner:SHANGHAI SINOFRA FINE CHEM
Method for separating and detecting tenofovir alafenamide and relevant substances thereof
The invention relates to a method for separating and detecting tenofovir alafenamide and relevant substances thereof. The method can be used for detecting a tenofovir alafenamide sample by adopting an octadecyl-bonded ethylene bridge hybridization silica gel chromatographic column. According to the separating and detecting method disclosed by the invention, diastereomers and other relevant substances of the tenofovir alafenamide can be effectively separated and detected. The method has the characteristics of simplicity, convenience, accuracy and reliability, and is suitable for controlling the quality of tenofovir alafenamide products in industry.
Owner:SUNSHINE LAKE PHARM CO LTD
Phase change material of safety urea resin micro-capsule and preparation method thereof
InactiveCN103484078AIncrease profitPrevent leakageHeat-exchange elementsMicroballoon preparationHexadecanePolymer science
The invention discloses a safety urea resin micro-capsule and a preparation method of the safety urea resin micro-capsule. The safety urea resin micro-capsule is prepared by carrying out in-situ polymerization on an organic phase change material functioning as a core material and urea resin functioning as a wall material, wherein the urea resin functioning as the wall material is obtained by subjecting formaldehyde and ureophil at the molar ratio of 1:1.4-1.6 to condensation polymerization; the organic phase change material functioning as the core material is one or more of liquid paraffin, hexadecane, n-octadecane and solid paraffin, and based on the ratio of the mass of the core material, namely the organic phase change material, to the total mass of the formaldehyde and ureophil in the wall material, the ratio of the core material to the wall material is 1.2-1.5:1. On the basis of ensuring the uniform particle size and high enveloping ratio of the safety urea resin micro-capsule disclosed by the invention, the content of the formaldehyde meets E1 of the European Norm (EN) standard, namely, the content of the formaldehyde in the micro-capsule is less than 10mg / 100g.
Owner:SHANGHAI INST OF TECH
Preparation and application method of water-based paint remover
InactiveCN103013221ATake off quicklyReduce corrosionChemical paints/ink removersSodium lactateActive agent
The invention discloses a preparation and application method of a water-based paint remover. The water-based paint remover consists of sodium hydroxide, a surface active agent and a composite additive. The mass fraction of sodium hydroxide is 3%-10% that of the surface active agentis 0.5%-2%, that of the composite additive is 1%-6%, and the rest is water. The surface active agent is octadecyl dihydroxy diethyl betaine; and the composite additive is composed of three of sodium lactate, sodium malate, sodium sarcosinate, natrium aceticum and sodium gluconate. The water-based paint remover disclosed by the invention has the characteristics of being efficient, environmentally-friendly, safe, nontoxic, barely corrosive and hard to volatilize, can be used for removing the paint from common metal and plastic base materials, and is simple and easy to prepare and use.
Owner:GUANGDONG UNIV OF TECH
Perovskite quantum dot gel and preparation method thereof
InactiveCN106675550AHigh transparencyIncrease flexibilityMaterial nanotechnologyNanoopticsQuantum yieldRoom temperature
The invention relates to perovskite quantum dot gel and a preparation method thereof. The method comprises the following steps of mixing octadecene and PbX2 (0.138g of PbBr2 in green quantum dots, 0.105g of PbI2 and 0.052g of PbBr2 in red quantum dots), degassing in vacuum, heating at 120 DEG C for an hour, and putting the ODE and PbX2 mixture into a nitrogen environment; adding oleic acid and APTES, adding cesium oleate prepared in the step a at 145 DEG C, cooling to room temperature, stirring and hydrolyzing so as to form a silicon dioxide-coated perovskite quantum dot sol solution; and drying to obtain the silicon dioxide-coated perovskite quantum dot gel. The prepared perovskite quantum dot gel has good transparency and flexibility and high quantum yield, and the long-term stability can be kept in a protonic solvent.
Owner:JILIN UNIV
Fingerprint pattern quality control method for cordyceps sinensis bacterium powder raw material in herbs medicaments for strengthening the body resistance and activating blood and dissolving stasis
ActiveCN101293002AGuarantee normal implementationHigh sensitivityFungiComponent separationHplc fingerprintRetention time
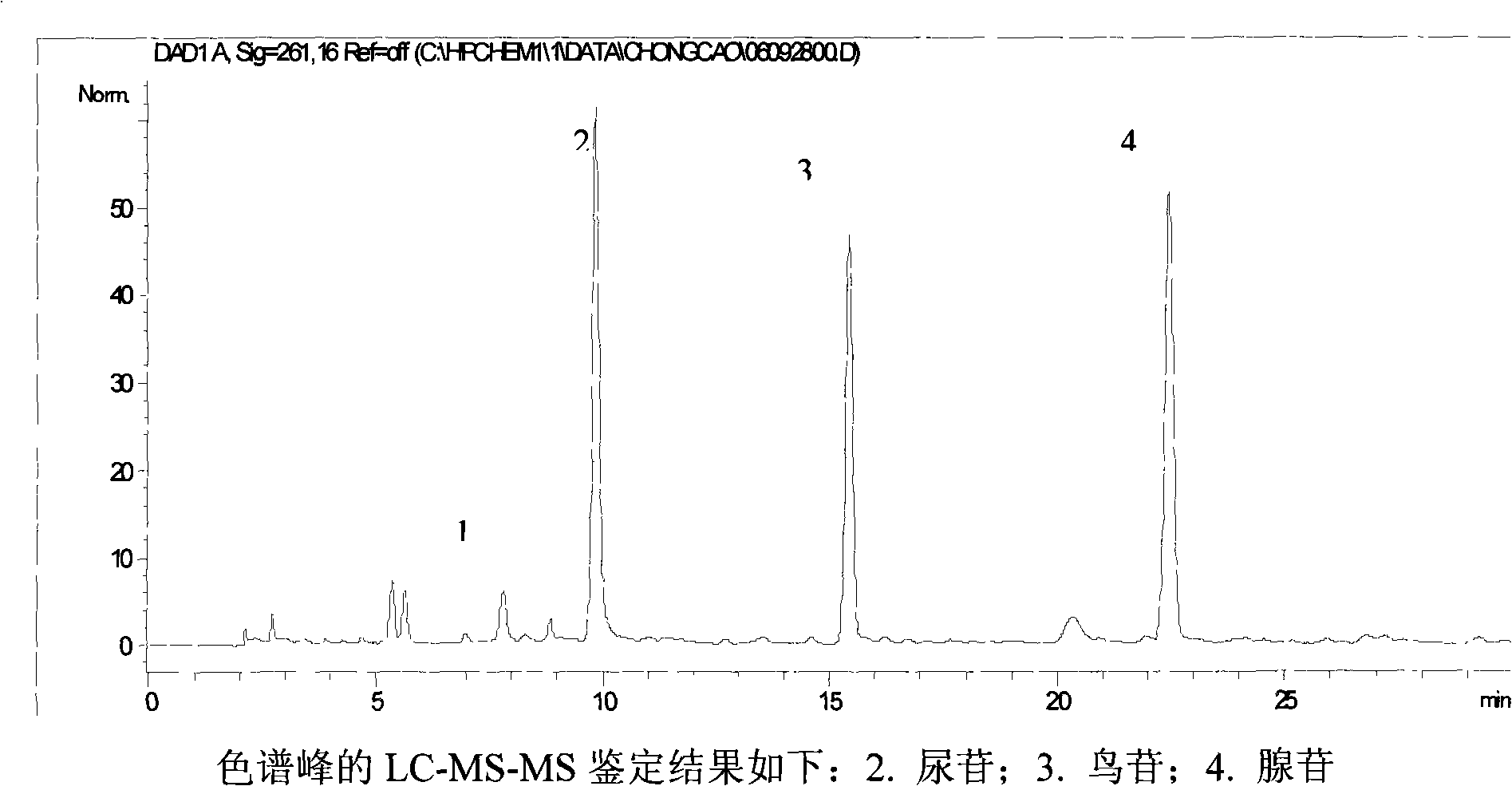
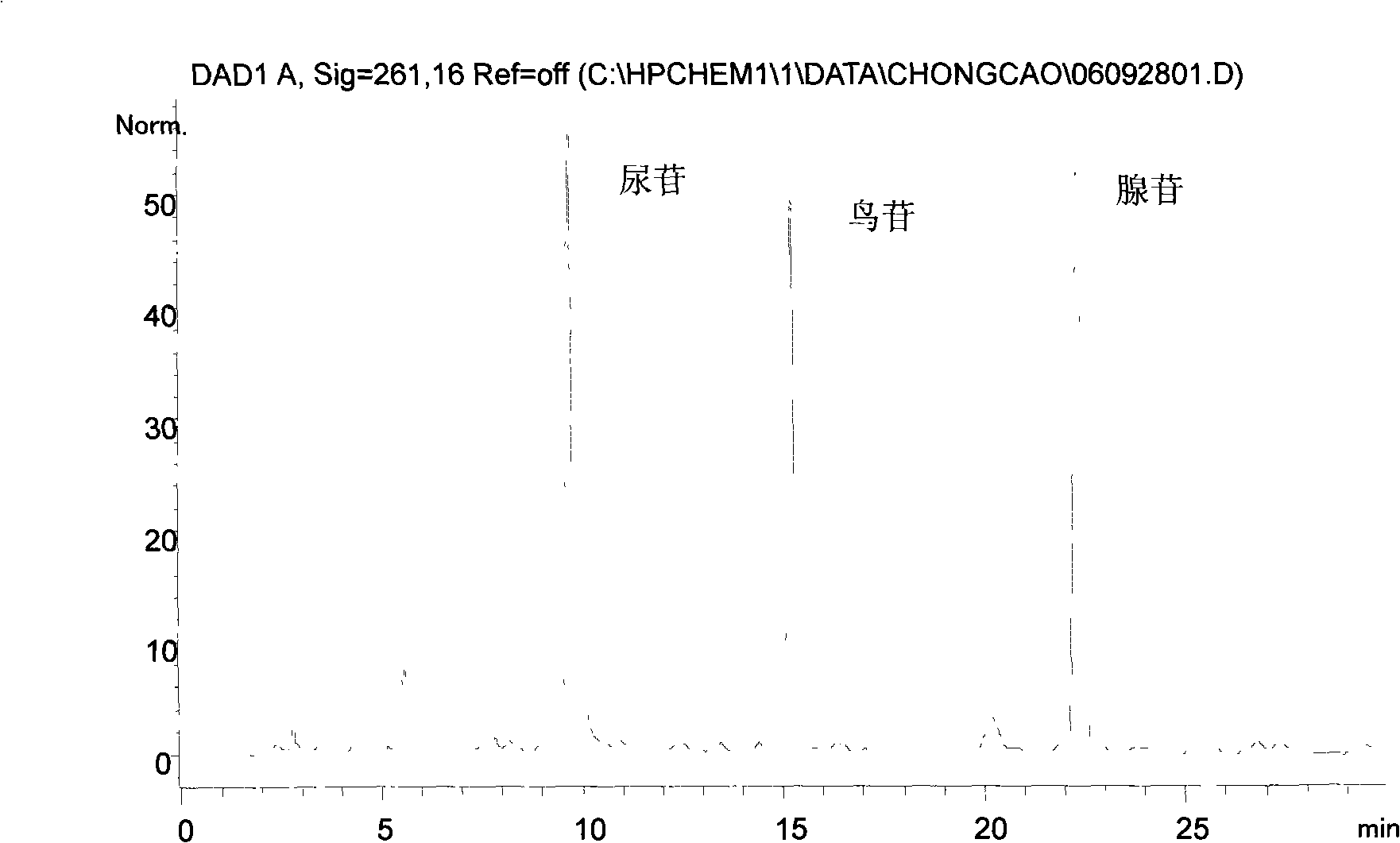
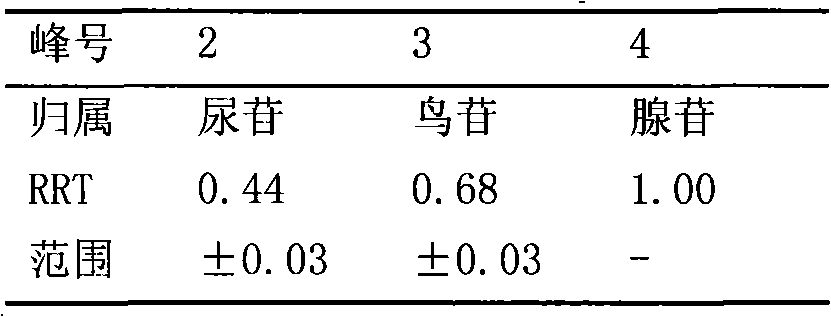
The invention relates to a control method of the fingerprint spectrum quality of cordyceps sinensis powder raw material in botanical drug for strengthening vital qi and removing blood stasis, comprising the steps that: (1) cordyceps sinensis powder is extracted: 0.100g of cordyceps sinensis powder is taken, purified water is added, the ultrasonic extraction, the filtration and the sample injection are carried out; (2) the gradient elution with mobile phase is carried out: octadecyl silane bonded silica gel is taken as a filler, water and acetonitrile are taken as mobile phase to carry out the gradient elution for 0 to 30min and 0 to 7 percent B; (3) a standard fingerprint spectrum is established: the HPLC standard fingerprint spectrum of the cordyceps sinensis powder is determined, and 3 characteristic peaks are selected; (4) the quality control of the fingerprint spectrum is carried out: the relative retention time of No.2 peak uridine, No.3 peak guanosine and No.4 peak adenosine are 0.44 plus or minus 0.03, 0.68 plus or minus 0.03 and 1.00 respectively; the HPLC fingerprint spectrum of the sample is compared with the contrast fingerprint spectrum. The similarity calculated by the 5 common peaks is not less than 0.9, (5) the preparation of the cordyceps sinensis powder raw material is carried out; the control method has good repetitivity and can fully reflect the basic characteristics of nucleoside ingredients of the cordyceps sinensis powder.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV
Method for purifying teriparatide acetate
ActiveCN102993293AOperableHigh yieldPeptide preparation methodsParathyroid hormonesGradient elutionSilica gel
The invention provides a method for purifying teriparatide acetate, comprising the following steps: dissolving crude peptide by using the aqueous solution of 10-30% of acetic acid and 5-20% of acetonitrile in volume ratio; carrying out gradient elution and purification on a crude peptide solution; adjusting the pH value of the aqueous solution of 0.1-0.4% of sulfuric acid and 0.1-0.4% of acetic acid in volume ratio by using ammonia water to be 5.0-6.0; obtaining the solution as phase A and acetonitrile as phase B; eluting when the gradient of the phase B is 20-40%; salting out; washing by using an ammonium acetate solution containing 3-10% of acetonitrile; converting acetate by using the high performance liquid chromatography of the octadecylsilane chemically bonded silica gel; and eluting an acetic acid aqueous solution acetonitrile system. The invention aims to provide a method for purifying the teriparatide acetate, which is simple in operation, high in yield and purity, and favorably realizes industrialization.
Owner:HYBIO PHARMA
Method for detecting related substances of ibuprofen and its sodium salt and preparation
The invention provides a method for detecting related substances of ibuprofen or its sodium salt and preparation. The related substances comprise impurities A, B, C, D, E and F. The method comprises (1) preparation of a test sample solution: taking an appropriate amount of ibuprofen or ibuprofen sodium raw materials, putting the materials into a certain volume of a container, dissolving the materials, diluting the solution until a desired volume marked by a scale, shaking the solution, and filtering the solution to obtain a certain concentration of the test sample solution, and (2) sample detection: pouring the test sample solution into a chromatographic instrument, and acquiring a chromatogram map of the related substances separated effectively under chromatographic conditions of use of a chromatographic column containing octadecyl silane chemically bonded silica as a filler having size of 250*4.6mm and pore sizes of 5 micrometers and having a column temperature of 20 to 40 DEG C, use of a mobile phase having a volume ratio of organic phase acetonitrile to a water phase phosphoric acid aqueous solution of 32% to 48% and phosphoric acid content of 0.01 to 0.1% in the phosphoric acid aqueous solution, a flowing rate of 1.0 to 2.3 ml / min, and a detection wavelength of 205 to 225nm. The method can realize effective separation of a variety of impurities.
Owner:CHINA PHARM UNIV
Preparation method of nanoscale organobentonite
ActiveCN104760968AGuarantee product qualityShorten the production cycleMaterial nanotechnologySilicon compoundsSodium BentoniteMass ratio
The invention discloses a preparation method of nanoscale organobentonite, which comprises the following six steps: (1) bentonite raw ore processing, (2) sodium modification, (3) centrifugation purification, (4) inorganic acid modification, (5) organic coating reaction, and (6) a post treatment stage. The preparation method has the characteristics that through substep purification, a mass ratio of a sodium agent to suspending liquid is kept at 0.3%; in an inorganic acid modification process, improvements are performed by adjustment of a PH value to subacid and a charging mode in the organic coating reaction stage; the bentonite is subjected to delaminating dispersion, centrifugation purification and superfine grading to prepare pure montmorillonite with the content greater than 95%; then, double octadecyl dimethyl ammonium chloride is used for performing the organic coating reaction; finally crushing to a nanoscale particle size is performed, that is, the average lamellar thickness is less than 25 nanometers; and the preparation method ensures the product quality, shortens the production cycle greatly, lowers the production cost, and is suitable for large-scale popularization and application.
Owner:HUANGSHAN BAIYUE ACTIVATED CLAY
Method for separating and measuring acetylcysteine enantiomers
ActiveCN101968470AStrong UV Absorbing PropertiesEasy to measureComponent separationPhosphateEnantiomer
The invention provides a method for separating and measuring acetylcysteine enantiomers. In the method, before acetylcysteine is added in a chromatographic column, a derivatization reagent is used to perform derivatization, wherein the derivatization reagent is N(alpha)-(5-fluoro-2,4-dinitrophenyl)-L-amino acid compound. The method for separation and measurement combines the high performance liquid chromatography (HPLC) or high performance liquid chromatography-mass spectrum (HPLC-MS), and the used chromatographic column uses octadecylsilane chemically bonded silica as filler. The method for separation and measurement comprises the following steps: (1) taking acetylcysteine or a preparation with acetylcysteine, dissolving in low-concentration acid solution, adjusting the pH value of the mixed solution to 6.0-8.0 with alkaline solution to obtain a sample solution for testing; (2) mixing the acetylcysteine solution with 1mol / L of carbonate solution, adding N(alpha)-(5-fluoro-2,4-dinitrophenyl)-L-amino acid compound to mix evenly; (3) reacting the solution obtained by the step (2) at 40-60 DEG C in a dark place, adding hydrochloric acid solution after the reaction; (4) adding the solution obtained by the step (3) in a drying solution with the prestored phosphorus pentoxide and potassium hydroxide, adding phosphate buffer solution-acetonitrile, dissolving residues through ultrasonic treatment, filtering to obtain filtrate which is used as a testing solution; and (5) adopting HPLC or HPLC-MS to separate and measure the testing solution prepared by the step (4).
Owner:湖北新生源生物工程有限公司
Boron nitride/graphene double-heat-conduction-base aerogel composite phase change material and preparation method thereof
ActiveCN111662688ALow densityContinuous network structureHeat-exchange elementsFreeze-dryingPyrrolidinones
The invention discloses a boron nitride / graphene double-heat-conduction-base aerogel composite phase change material. The material is formed by compounding modified boron nitride / graphene aerogel andn-octadecane by adopting a vacuum impregnation method. The double-heat-conduction aerogel is prepared by taking graphene oxide, modified boron nitride, polyvinylpyrrolidone and ethylenediamine as rawmaterials to prepare boron nitride / graphene hydrogel, freeze-drying the boron nitride / graphene hydrogel and then calcining the boron nitride / graphene hydrogel at a constant temperature. Polyvinylpyrrolidone is used as a cross-linking agent, and ethylenediamine is used as a reducing agent. A preparation method of the composite phase change material comprises the following steps: 1) preparing modified boron nitride; 2) preparing boron nitride / graphene double-heat-conduction-base aerogel; and 3) preparing the boron nitride / graphene double-heat-conduction-base aerogel composite phase change material. When the material is applied as a phase change material, the heat conductivity coefficient is 0.9-1.6 W / (m.K); wherein the phase change temperature is 19-32 DEG C, and the phase change latent heatis 200-220 J / g. The composite phase change material has the following advantages: 1, the heat conductivity coefficient is improved by 738%; 2, the leakage problem in the phase change process is effectively solved; and 3, the phase-change latent heat and the heat stability are high;
Owner:GUILIN UNIV OF ELECTRONIC TECH
Method for assaying impurities in apremilast and preparations thereof through liquid chromatography
ActiveCN105588886AAccurate measurementQuality is easy to controlComponent separationFluid phaseSilanes
The invention discloses a method for separating and assaying impurities in apremilast and preparations thereof through liquid chromatography. In the method, octadecylsilane chemically bonded silica is employed as a filler in a chromatography column; a buffer solution is employed as a mobile phase A; and a methanol-acetonitrile mixture solvent is employed as a mobile phase B, wherein a gradient elution method is employed in the mobile phases to assay the impurities in apremilast and the preparations thereof. The method can effectively separate and assay unknown impurities and known impurities from the apremilast. The method has strong specificity, is high in accuracy, is easy to use and can be used for effectively control the quality of the apremilast and preparations thereof.
Owner:CHONGQING PHARMA RES INST
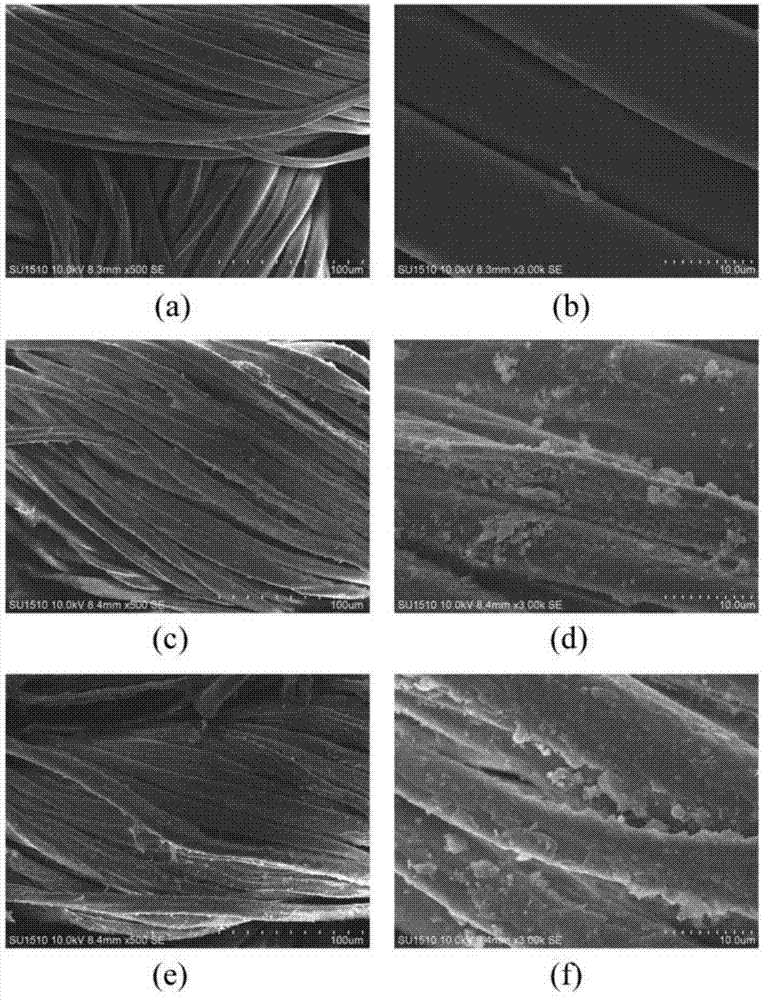
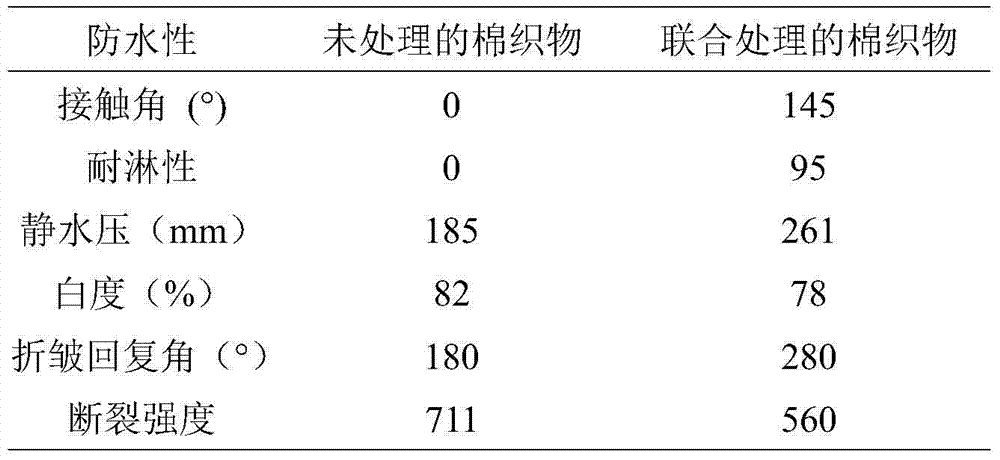
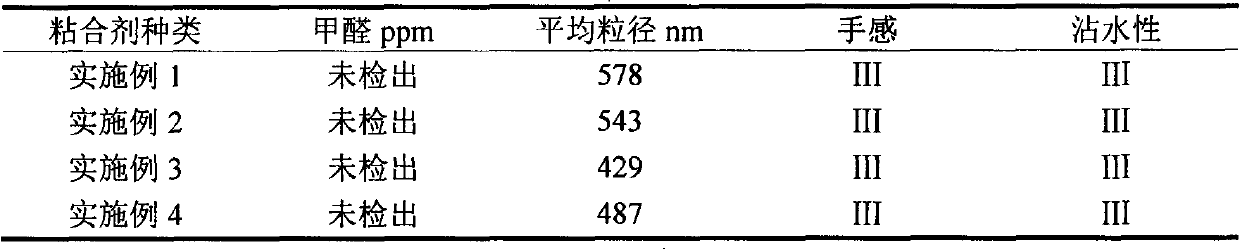
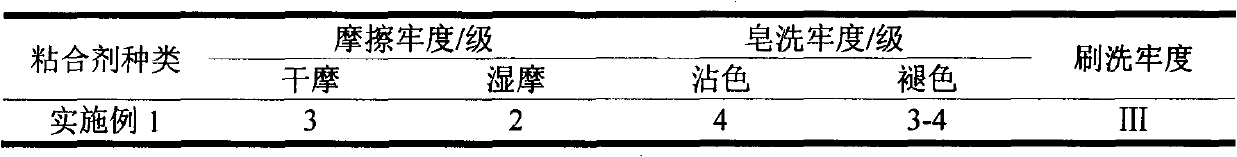
Finishing method of cotton-hydrophobic fabric based on BTCA-TEOS-OA combined treatment
ActiveCN104711852ALower surface energyAvoid environmental problemsLiquid/gas/vapor removalVegetal fibresSurface energyPre treatment
The invention discloses a finishing method of a cotton-hydrophobic fabric based on BTCA-TEOS-OA combined treatment and belongs to the technical field of functional textiles. The finishing method is characterized in that 1,2,3,4-butanetetracarboxylic acid is adopted for pretreating the fabric so as to increase the capability of reaction between the fabric and a following chemical reagent and function as a bridge; tetraethoxysilane is hydrolyzed and condensated under the acidic or alkali condition so as to form particulate matters on the surface of the fabric and play a role in roughening the fabric; due to long-chained alkane type octadecylamine, the surface energy of the fabric can be effectively reduced, and further the environmental problem caused by total-fluoride energy is avoided. The finishing method disclosed by the invention has the advantages that the traditional processes of soaking, rolling, drying and baking are adopted, and a product is good in uniformity and repeatability and low in cost. BTCA, TEOS and OA adopted in the finishing process need to be carried out under the condition of weak acid and weak alkaline, the baking temperature is low and the damage on the fabric is less. The finishing method can be used for finishing the hydrophobic function of the cotton fabrics.
Owner:高青如意纺织有限公司
Environment-friendly emulsion modified acrylate pigment dyeing adhesive and manufacturing method thereof
ActiveCN102633954AGood rubbing fastnessExcellent wash fastnessDyeing processGraft polymer adhesivesEnvironmental resistanceEmulsion
The invention relates to an environment-friendly emulsion modified acrylate pigment dyeing adhesive and a manufacturing method of the environment-friendly emulsion modified acrylate pigment dyeing adhesive. Through improvement of additive order and relavent process parameters of materials such as a combination of methyl methacrylate, methyl acrylate, ethyl acrylate, glycidyl acrylate and acrylic acid, linear block polyether octodecane amino ternary polymerization modified silicon oil, emulsifier and the like, the environment-friendly emulsion modified acrylate pigment dyeing adhesive and the manufacturing method aim at solving the long-term technical problems that fabric feels hard, crockfastness is poor, rubbing fastness is low, hardness of dyed fabric is higher, adhesive force is low and environment is polluted, so as to realize the beneficial technical effects of good crockfastness, high rubbing fastness, soft feeling on dyed fabric, good hydrophily of the dyed fabric, better adhesive force and environment-friendly production process.
Owner:JIANGSU GOLDEN AUTUMN ELASTIC FABRICS
Biogenic Turbine And Diesel Fuel
The present invention provides fully renewable turbine and diesel fuels derived completely from biomass sources. In one embodiment the fully renewable turbine fuel is comprised of mesitylene and at least one alkane. Preferably, the turbine fuel comprises from about 50 to 99 wt % mesitylene and from about 1 to 50 wt % of at least one alkane. In another embodiment the diesel fuel comprises mesitylene, octadecane, and optionally octane or nonane. Preferably, the diesel fuel comprises from about 50 to 99 wt % mesitylene, and from about 1 to 50 wt % octadecane. These biomass derived fuels may be formulated to have a wide range of cetane values and differing freezing and boiling points.
Owner:SWIFT ENTERPRISES
Heat storage phase-changing material and method for producing the same
InactiveCN101508886APrevent leakageSimple methodHeat storage plantsHeat-exchange elementsFiltrationFORMALDEHYDE SOLUTION
A heat accumulation phase-change material solves the problem that core materials are easy to leak after being absorbed by porous mass and is used for the fields such as warming, heat preservation, etc. The heat accumulation phase-change material comprises diatomite, expansive soil or expansion graphite, lauxite and core material which is paraffin, octadecane or cetane. A method for manufacturing the heat accumulation phase-change material comprises: step 1, carbamide is dissolved in water or absolute ethyl alcohol; step 2, the obtained solution is added with the core material and Tween-20 to be evenly stirred; step 3, the diatomite and the expansive soil or the expansion graphite are added into the mixed solution to be distilled and stirred until the absolute ethyl alcohol or the water is completely evaporated; step 4, when the temperature is lowered by 1-10 DEG C under the melting point of the core material, the obtained mixture is added with formaldehyde solution with the mass percentage being 37% and water having the mass being 5 times heavier than that of diatomite and expansive soil or the expansion graphite; step 5, the pH value is adjusted to be 3-4, and the reactants react for 3h and are processed by suction filtration, water rinse and desiccation; finally, the heat accumulation phase-change material is obtained.
Owner:BEIJING JIAOTONG UNIV +1
Hydrophobic associated polymer modified magnetic nano-thickener and preparation method thereof
InactiveCN109456740AEfficient viscosity increaseEfficient functionFluid removalDrilling compositionPolymer modifiedPolymer science
The invention discloses a hydrophobic associated polymer modified magnetic nano-thickener and a preparation method thereof. The hydrophobic associated polymer modified magnetic nano-thickener is a core-shell structured nanocomposite prepared by taking nanometer Fe3O4 as the core, modifying the Fe3O4 through oleic surfactants and grafting an amphiphilic high-polymer compound, wherein the amphiphilic high-polymer compound is a hydrophobic associated water-soluble ternary polymer prepared by copolymerizing a water-soluble monomer, a heat-resistant and anti-salt monomer and a hydrophobic monomer,the water-soluble monomer is acrylamide, the heat-resistant and anti-salt monomer is 2-acrylamide-2-methylpropanesulfonic acid, and the hydrophobic monomer is styrene, n-octyl acrylate or octadecyl dimethyl allyl ammonium chloride. The hydrophobic associated polymer modified magnetic nano-thickener helps solve the problems of high cost and poor thickening performance of nano-thickeners and reutilization difficulty of polymer thickeners and is an efficient thickening and recycling integrated nano-thickener obtained by modifying low-toxicity magnetic nanometer materials and amphiphilic high-polymers; the preparation method of the hydrophobic associated polymer modified magnetic nano-thickener has no need for high-temperature and high-pressure reaction conditions and helps reduce the operation difficulty and the production cost.
Owner:XI'AN PETROLEUM UNIVERSITY
Preparation method of carbamide resin phase change microcapsule
InactiveCN107903877AImprove thermal stabilityHeat-exchange elementsMicroballoon preparationOil phaseSolvent
The invention discloses a preparation method of a carbamide resin phase change microcapsule, and belongs to the field of preparation of textile materials. The method is characterized by comprising thefollowing steps: dissolving n-octadecane and TDI in a cyclohexane solvent, so as to form a uniformly mixed oil phase system; weighing an emulgator and OP-10, feeding the emulgator and OP-10 into a flask containing distilled water, and stirring, so as to form a uniformly mixed aqueous phase system; pouring the oil phase into an aqueous phase flask, and stirring with a homogenizer, so as to form uniform O / W emulsion; transferring the emulsion into a three-mouth flask, and reducing the rotate speed; uniformly mixing DETA and distilled water, dropwise adding the DETA and distilled water into theemulsion at a constant speed, and slowly performing temperature reaction after dropwise adding is accomplished, so as to obtain microcapsule suspension; performing suction filtration on an obtained product, washing the obtained product through distilled water and alcohol, performing suction filtration, and finally drying an obtained filter cake in a vacuum drying oven, so as to obtain a n-octadecane / carbamide resin phase change microcapsule. The carbamide resin / n-octadecane phase change microcapsule has the advantages that the enthalpy value of the microcapsule is 95.81 J / g, the packaging efficiency is 92.13 percent, and the thermal stability of the microcapsule taking carbamide resin as a wall material is excellent.
Owner:SHAANXI ALLIANCE LOGISTICS
Method for separating and determining rivaroxaban related substances through liquid chromatography
The invention belongs to the field of analytical chemistry, and discloses a method for separating and determining rivaroxaban related substances and the content thereof through liquid chromatography. The method is characterized in that the content of rivaroxaban and related substances thereof can be quantitatively determined through using a chromatographic column with octadecylsilane chemically bonded silica as a packing material and using a certain ratio of buffer salt solution-organic phase as a mobile phase, so the quality of rivaroxaban can be effectively controlled. The method has the advantages of strong specificity, high accuracy and simple operation.
Owner:AVENTIS PHARMA HAINAN
Acetic acid atosiban, and method for detecting content of preparation of acetic acid atosiban and relevant substances
ActiveCN101696959AStrong maneuverabilityStrong specificityComponent separationChromatographic columnAmount of substance
The invention relates to an acetic acid atosiban, and a method for detecting content of preparation of acetic acid atosiban and relevant substances. The detection method is an efficient liquid-phase chromatography method, which comprises the following chromatography conditions that a chromatographic column takes octadecylsilane chemically bonded silica (C18) as filling agent; 0.05mol / L phosphate buffer solution is taken as mobile phase A and acetonitrile as mobile phase B to carry out gradient elution; a detection wavelength ranges from 205 to 225nm; the flow speed ranges between 0.8 and 1.2ml / min and the concentration of a prepared sample ensures that each milliliter of the sample contains 0.1 to 15mg of polypeptide; and the sample size is controlled between 5 and 200mu l. The efficient liquid-phase chromatography method can accurately detect a sample and the content of impurities of the sample at the same time and realizes complete sample separation during analysis and ideal reproducibility of analysis result, thereby providing a simple and reliable method for quality control and analysis during production process.
Owner:HAINAN ZHONGHE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com