Patents
Literature
445results about "Parathyroid hormones" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dispersible macromolecule compositions and methods for their preparation and use
A process for preparing ultrafine powders of biological macromolecules comprises atomizing liquid solutions of the macromolecules, drying the droplets formed in the atomization step, and collecting the particles which result from drying. By properly controlling each of the atomization, drying, and collection steps, ultrafine dry powder compositions having characteristics particularly suitable for pulmonary delivery for therapeutic and other purposes may be prepared.
Owner:NOVARTIS FARMA
Polypeptide compositions with improved stability
InactiveUS7022674B2Good chemical stabilityLower Level RequirementsPeptide/protein ingredientsMetabolism disorderLinear correlationGlycerol
The present invention provides means to improve the chemical stability of aqueous, parenteral pharmaceutical compositions comprising a polypeptide and glycerin. Reactive aldehydes are identified in commercial glycerins, and means for reducing such are provided. Convenient means are provided to assay for reactive aldehydes in glycerin, and a strong linear correlation between the level of reactive aldehydes in glycerin and chemical stability of compositions comprising a polypeptide and glycerin is demonstrated. The invention includes aqueous compositions comprising a polypeptide and glycerin having improved chemical stability compared to compositions previously known.
Owner:ELI LILLY & CO
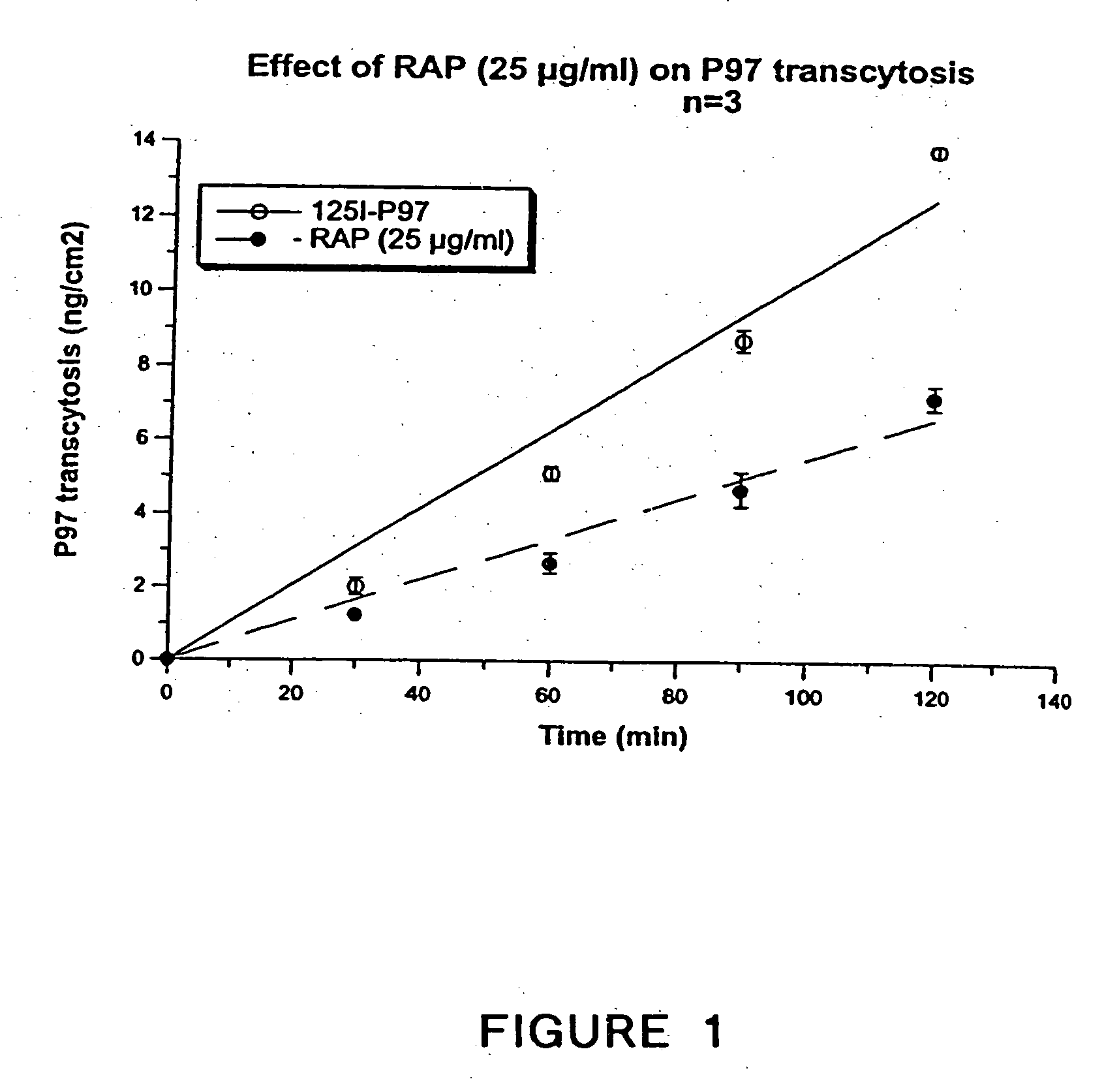
Megalin-based delivery of therapeutic compounds to the brain and other tissues
InactiveUS20050042227A1Easy to transportImprove propertiesBiocideNervous disorderBlood–brain barrierDrug delivery
Owner:HORIZON ORPHAN LLC
Method of Drug Formulation Based on Increasing the Affinity of Active Agents for Crystalline Microparticle Surfaces
ActiveUS20070059374A1Reduce solubilityPromote associationPowder deliveryOrganic active ingredientsActive agentMicroparticle
Methods are provided for promoting the adsorption of an active agent to microparticles by modifying the structural properties of the active agent in order to facilitate favorable association to the microparticle.
Owner:MANNKIND CORP
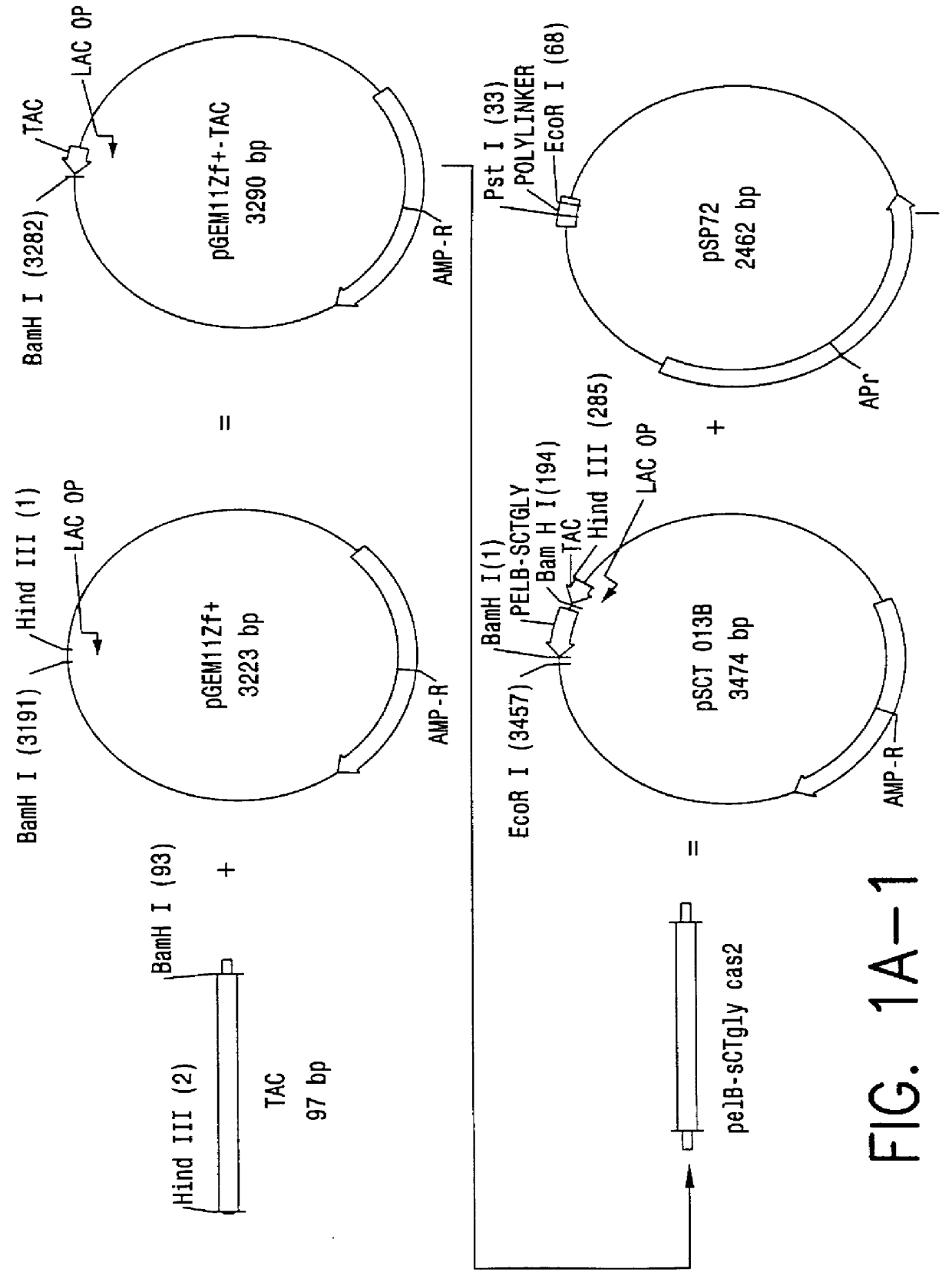
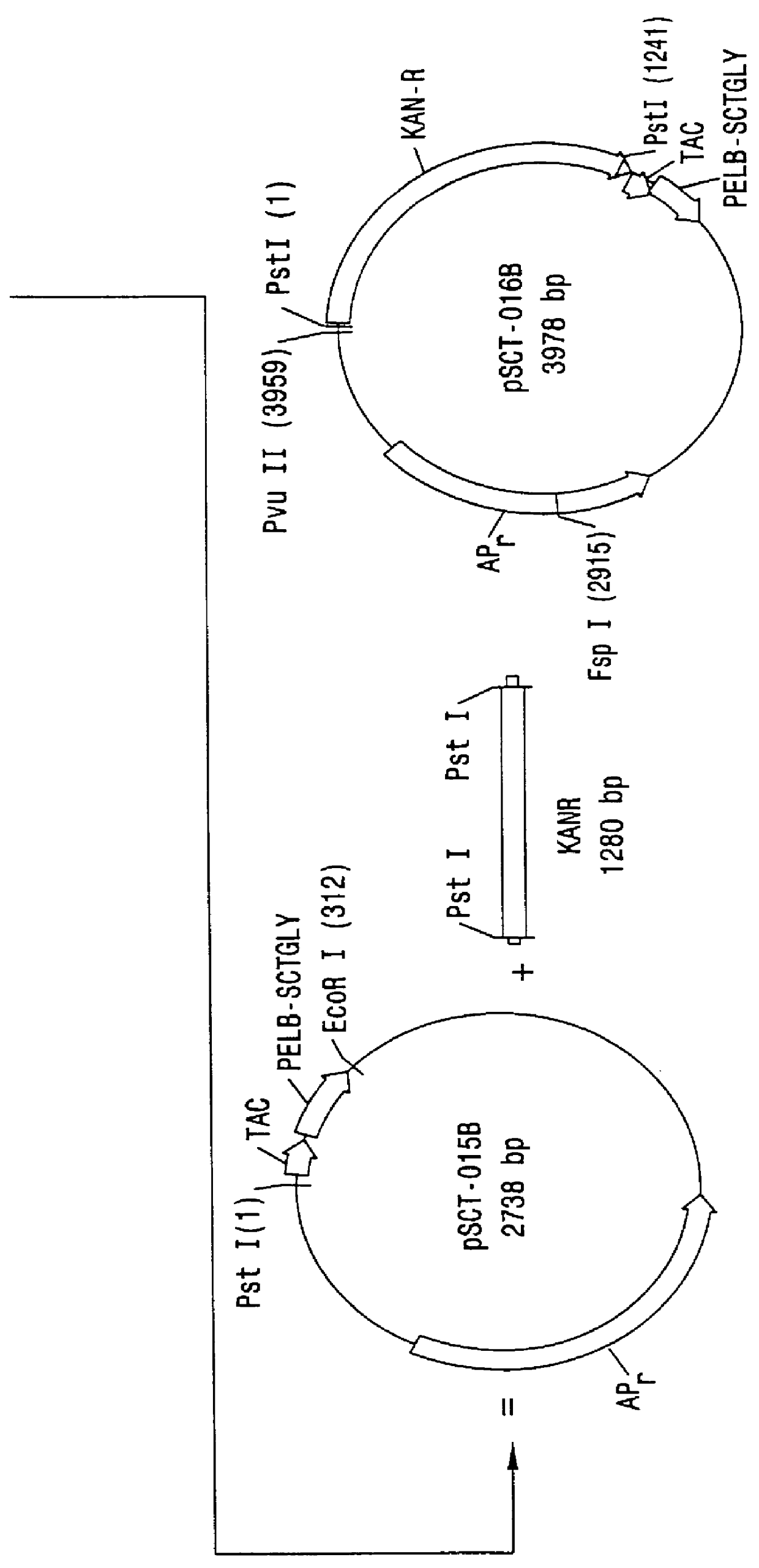
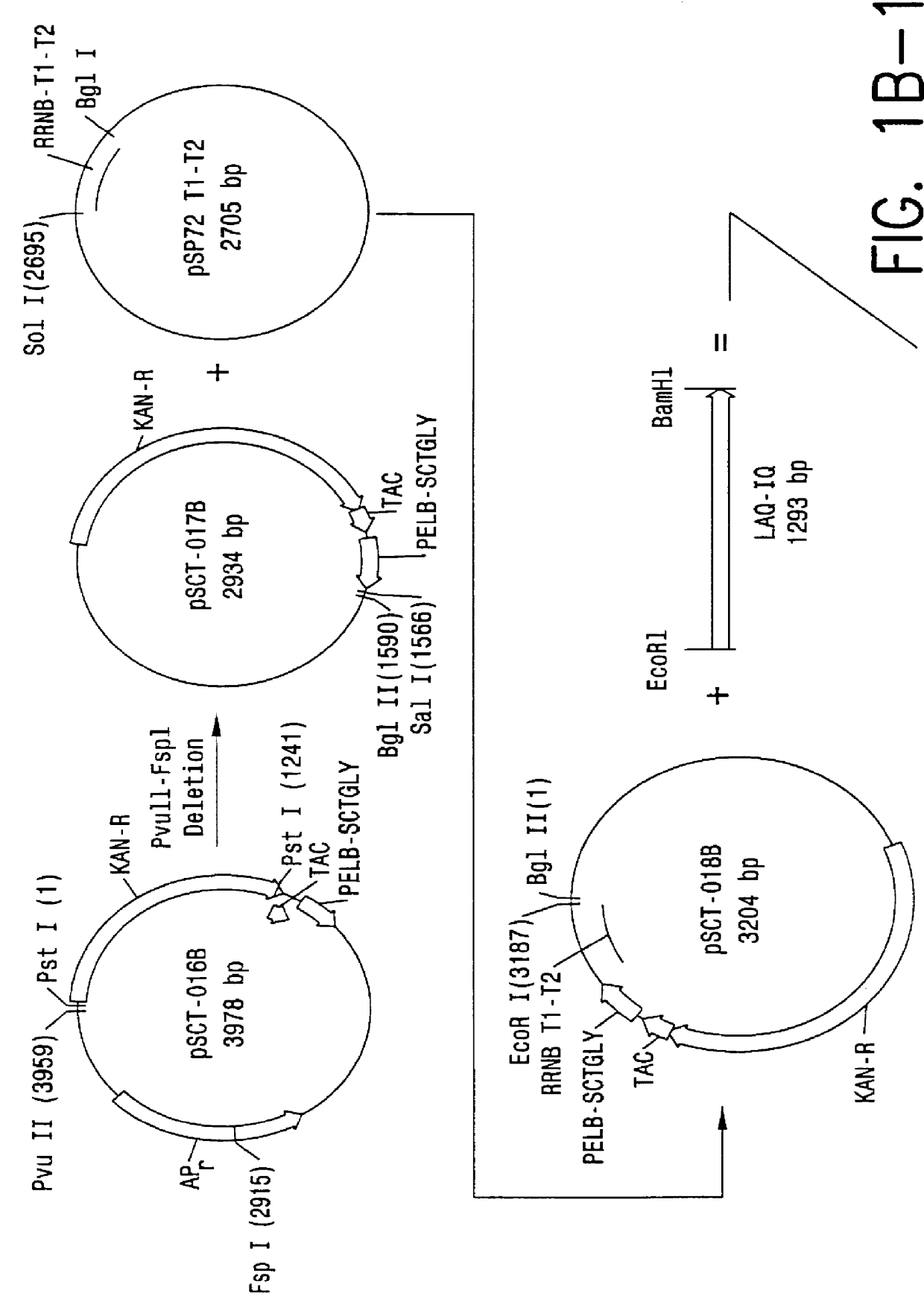
Direct expression of peptides into culture media
InactiveUS6103495AReduced viabilityImprove breathabilityBacteriaPeptide/protein ingredientsGrowth phaseBiotechnology
Expression systems are disclosed for the direct expression of peptide products into the culture media where genetically engineered host cells are grown. High yield was achieved with novel vectors, a special selection of hosts, and / or fermentation processes which include careful control of cell growth rate, and use of an inducer during growth phase. Special vectors are provided which include control regions having multiple promoters linked operably with coding regions encoding a signal peptide upstream from a coding region encoding the peptide of interest. Multiple transcription cassettes are also used to increase yield. The production of amidated peptides using the expression systems is also disclosed.
Owner:ENTERIS BIOPHARMA
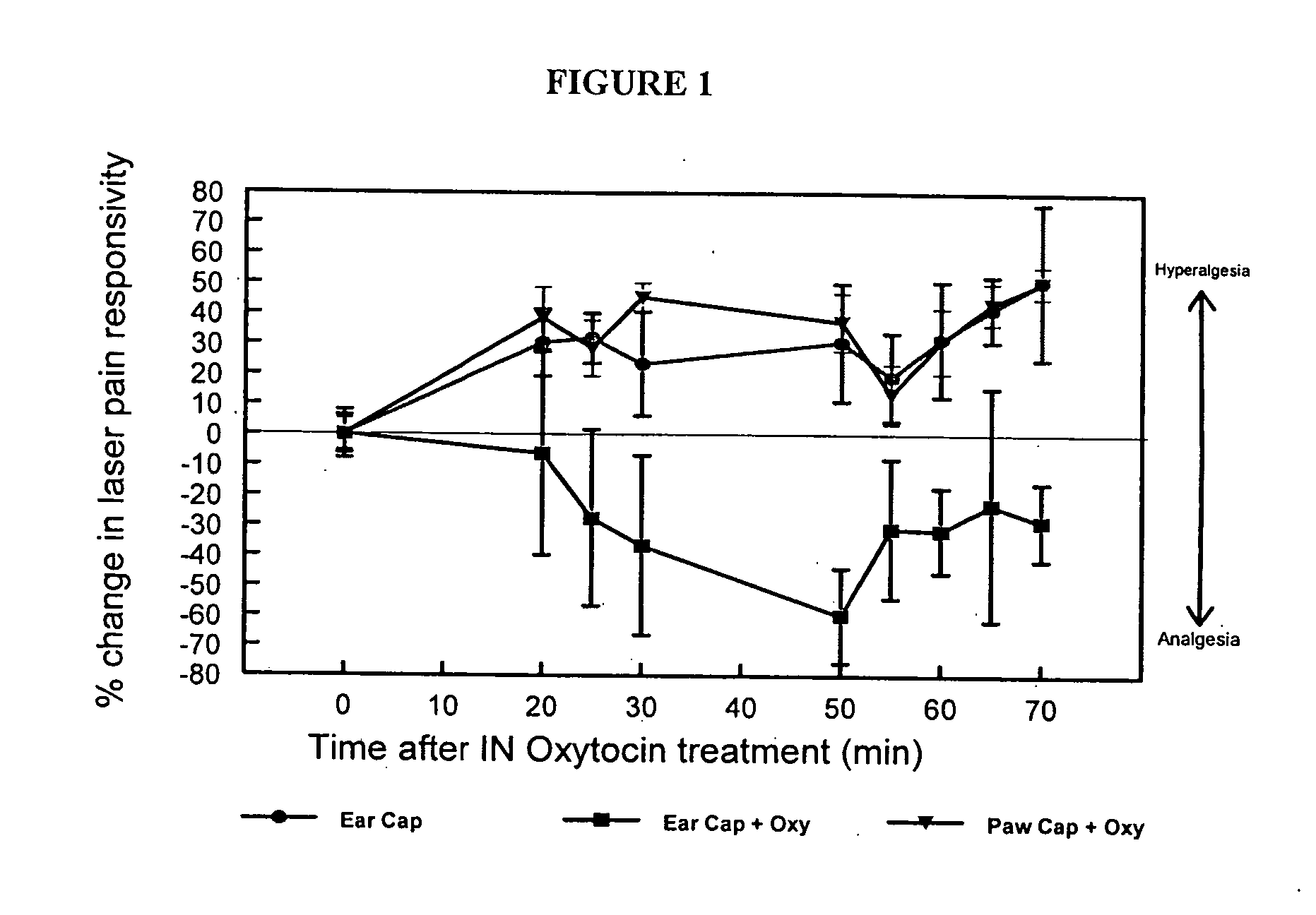
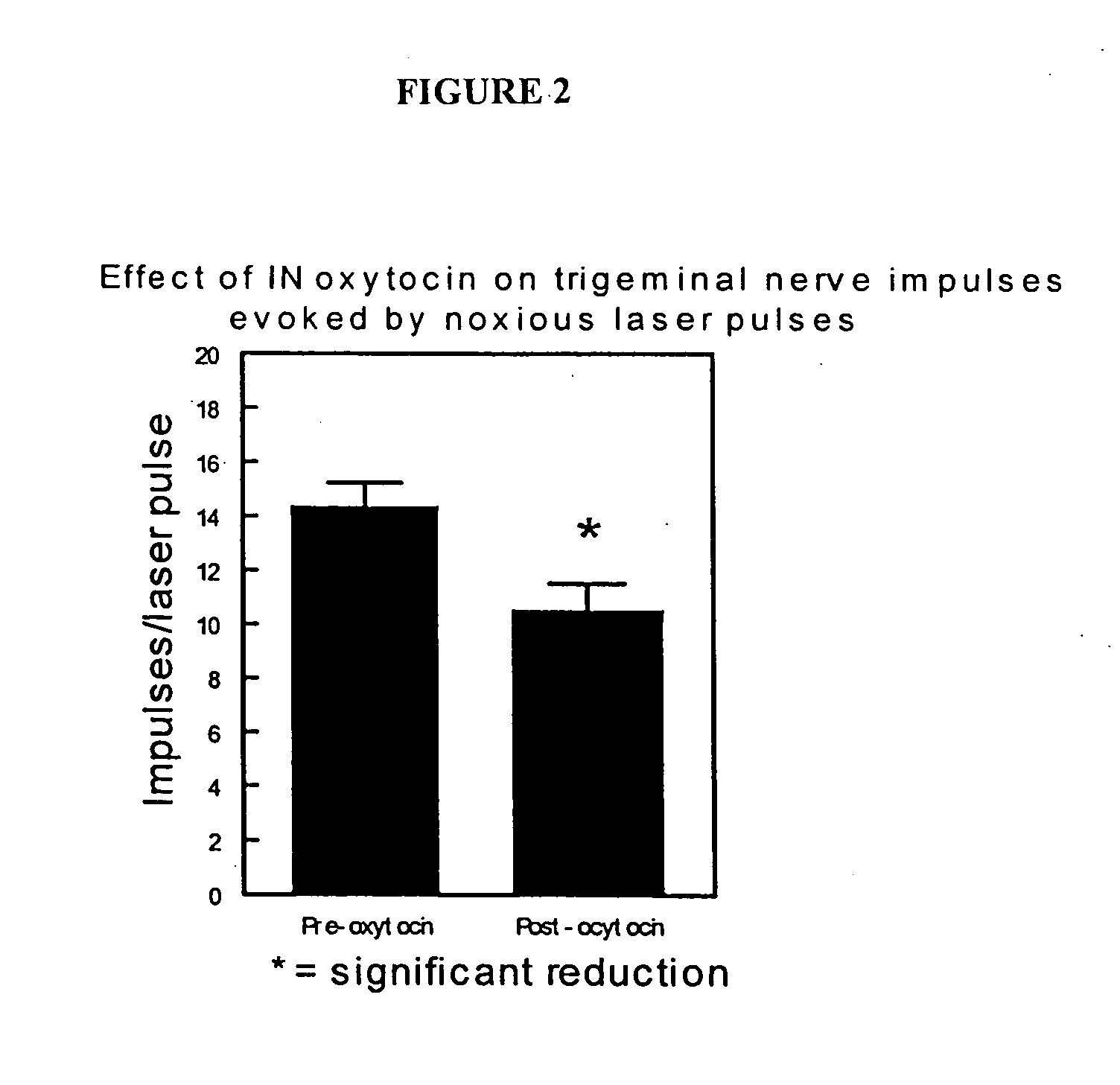
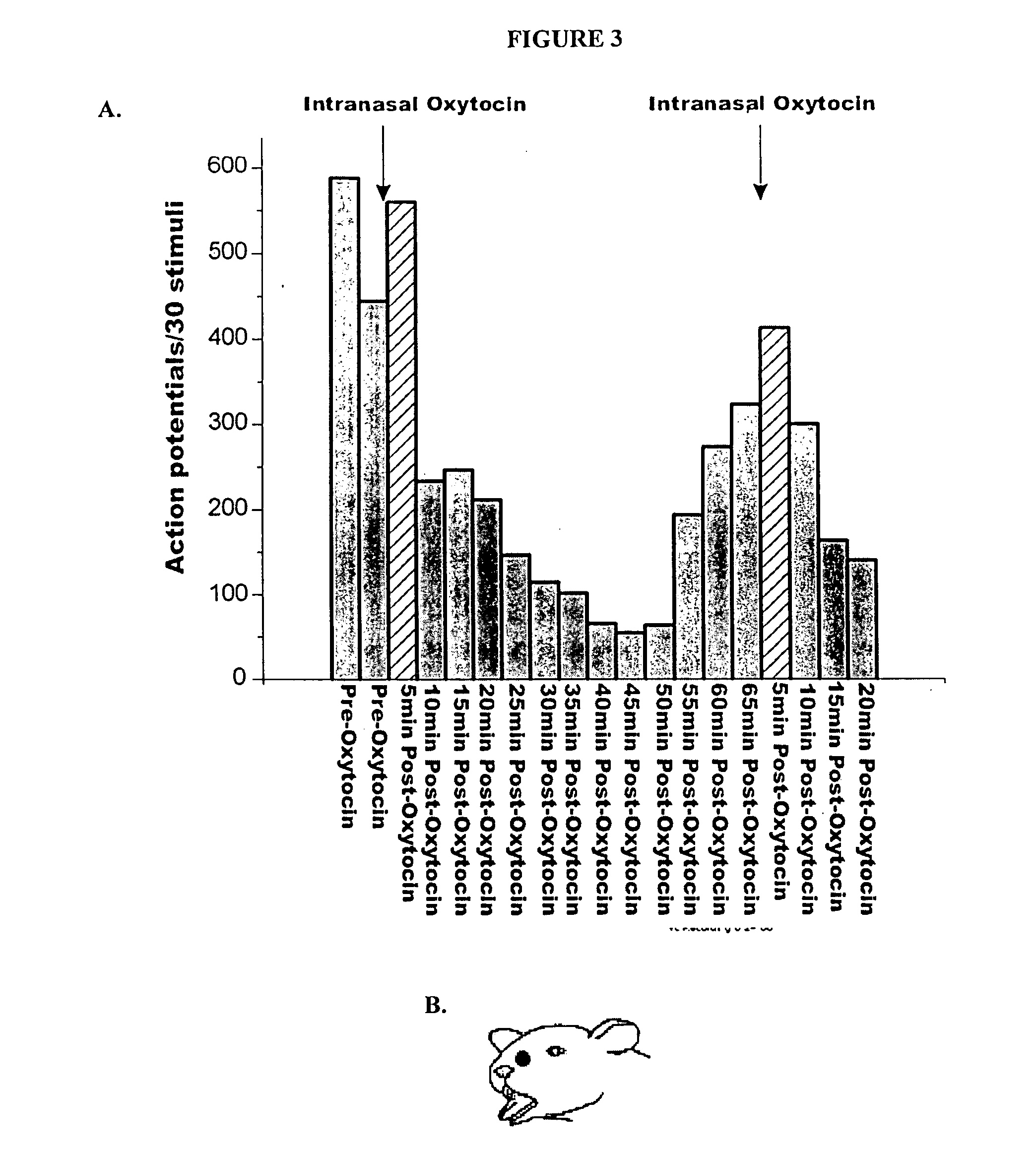
Methods for treatment of headaches by administration of oxytocin
InactiveUS20070054843A1Reduction of pain ratingReduce probabilityOrganic active ingredientsNervous disorderHeadache DisordersTrigeminal neuralgia
The present invention relates to methods for the treatment of headache and headache disorders. The methods comprise administration of an oxytocin peptide for the treatment of primary and secondary headaches or trigeminal neuralgia.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +2
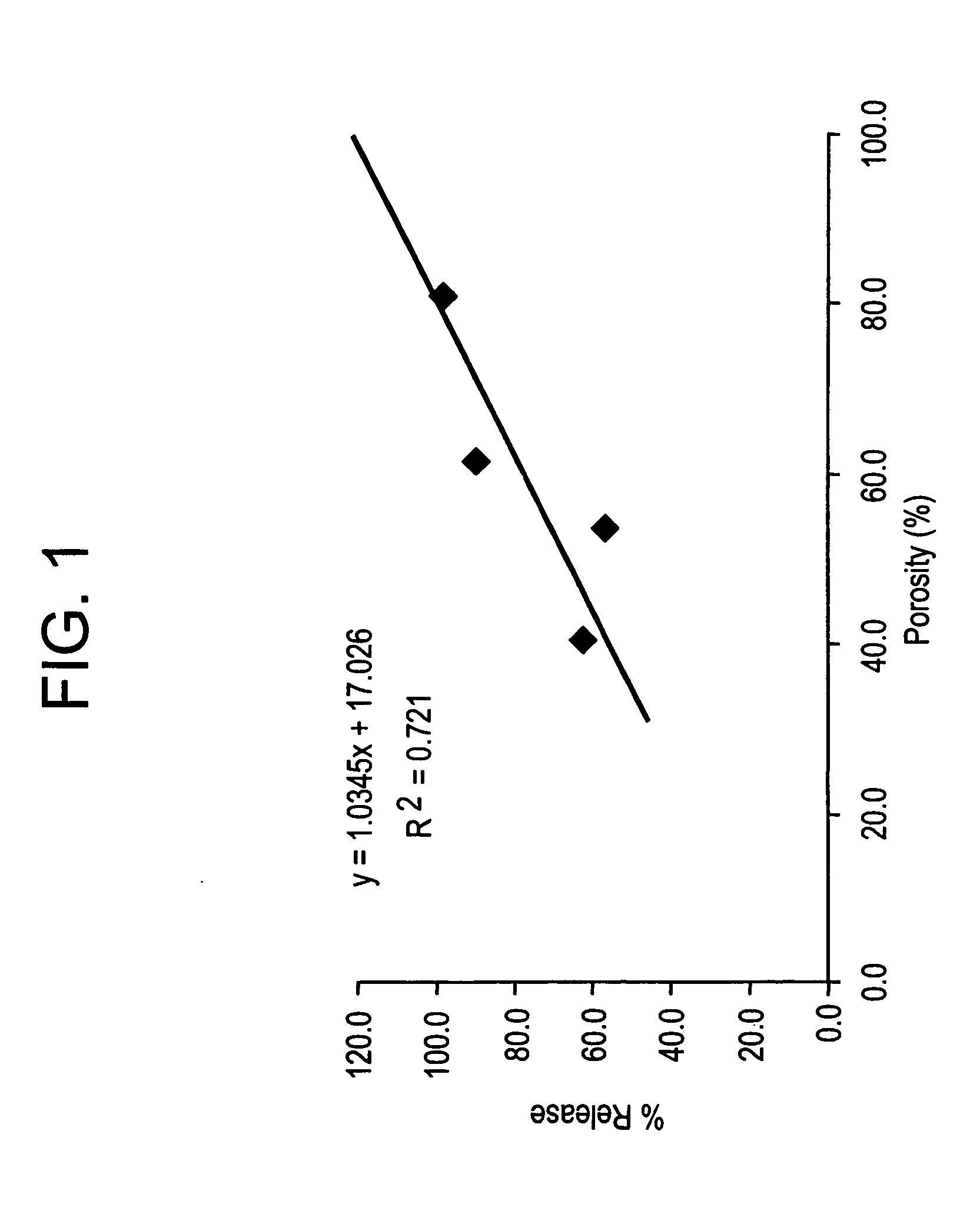
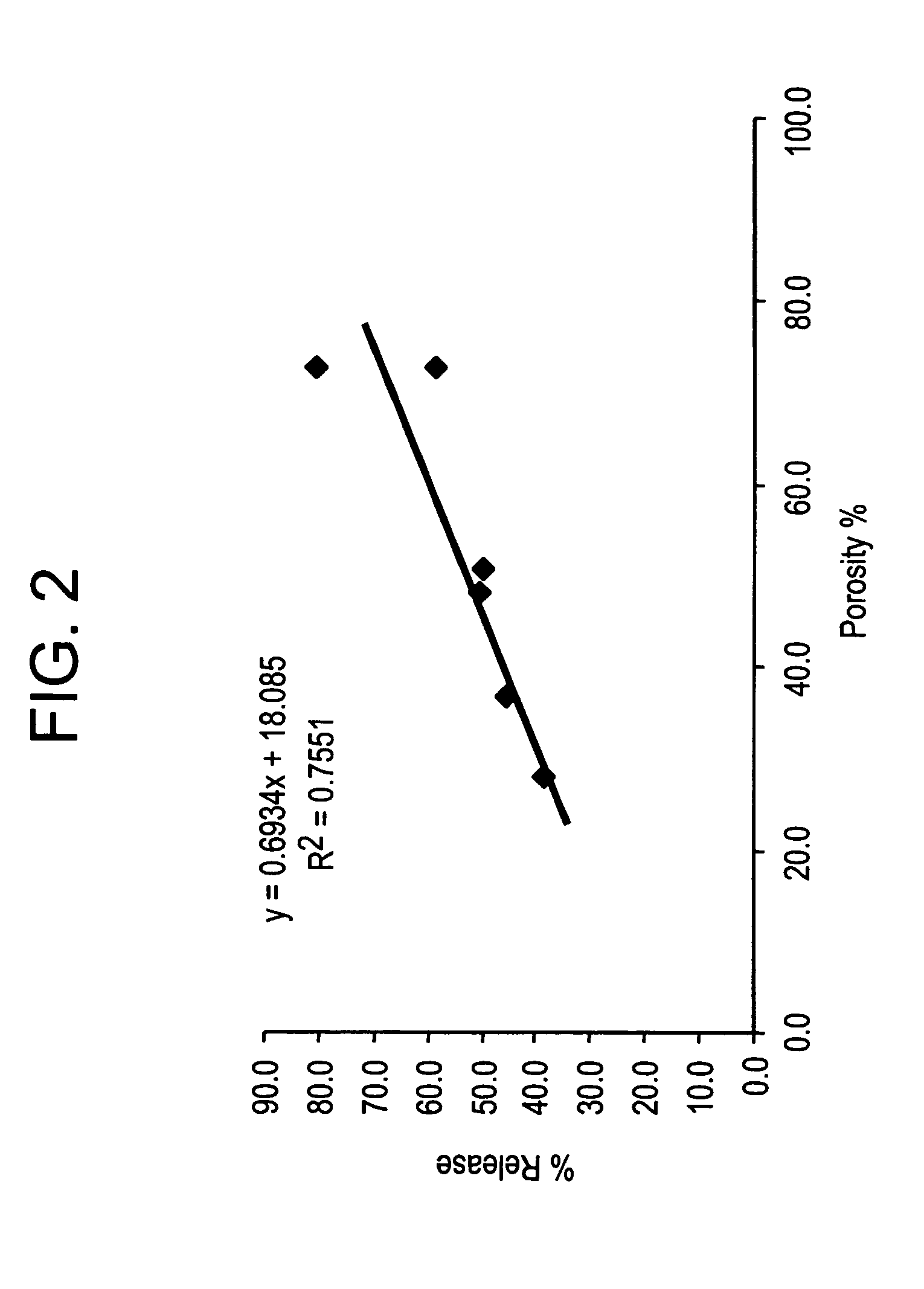
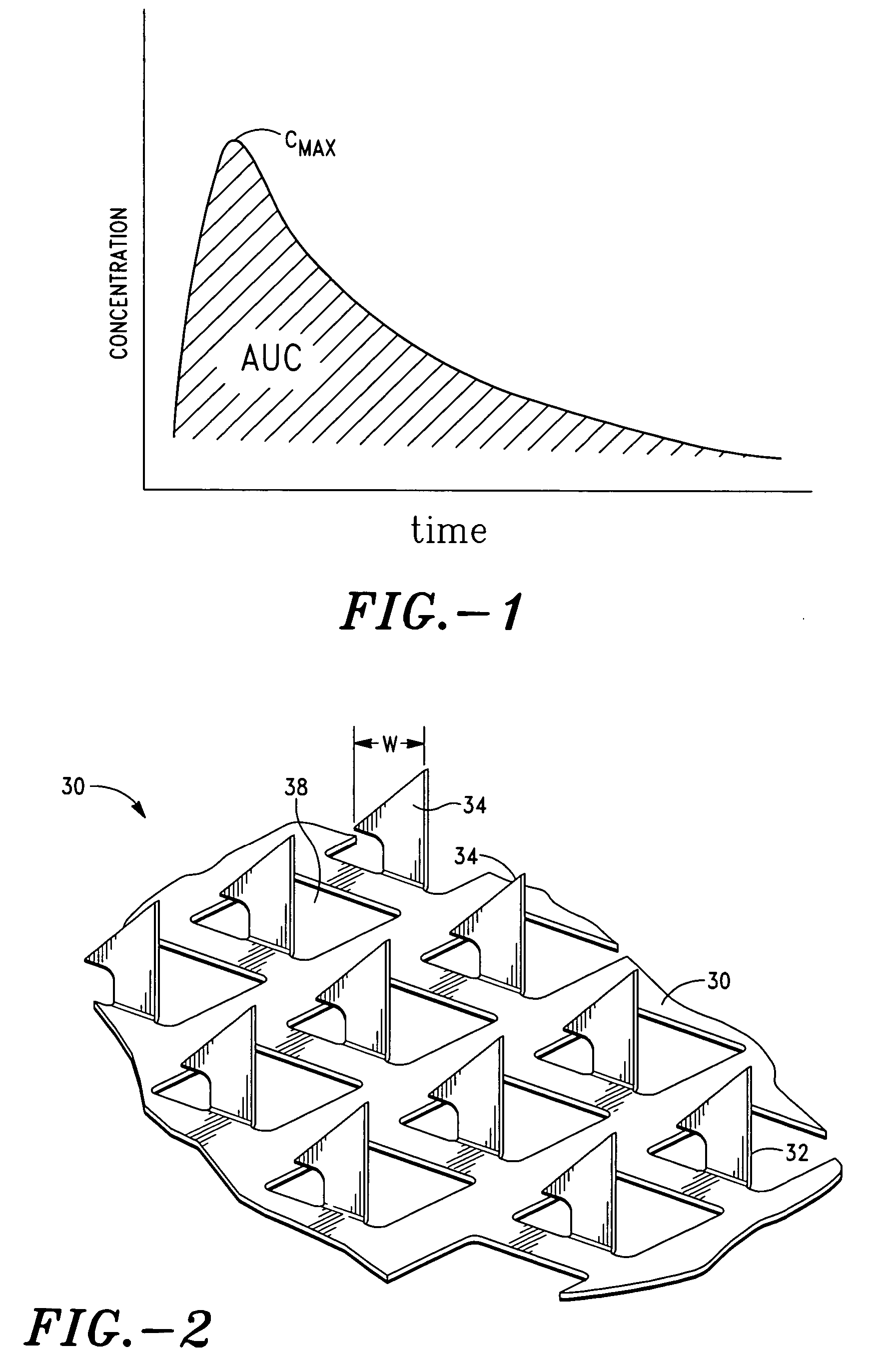
Injectable, oral, or topical sustained release pharmaceutical formulations
Pharmaceutical formulations and methods are provided for the sustained delivery of a pharmaceutical agent to a patient by injection, by oral administration or by topical administration. The injectable formulation includes porous microparticles which comprise a pharmaceutical agent and a matrix material, wherein upon injection of the formulation a therapeutically or prophylactically effective amount of the pharmaceutical agent is released from the microparticles for at least 24 hours. The oral formulation includes porous microparticles which comprise a pharmaceutical agent and a matrix material, wherein a therapeutically or prophylactically effective amount of the pharmaceutical agent is released from the microparticles for at least 2 hours following oral administration. The topical formulation includes porous microparticles which comprise a pharmaceutical agent and a matrix material, wherein a therapeutically or prophylactically effective amount of the pharmaceutical agent is released from the microparticles for at least 2 hours following topical administration.
Owner:ACUSPHERE INC
Compositions capable of facilitating penetration across a biological barrier
This invention relates to novel penetrating compositions including one or more effectors included within a water soluble composition, immersed in a hydrophobic medium. The invention also relates to methods of treating or preventing diseases by administering such penetrating compositions to affected subjects.
Owner:CHIASMA INC
Absorption enhancers for drug administration
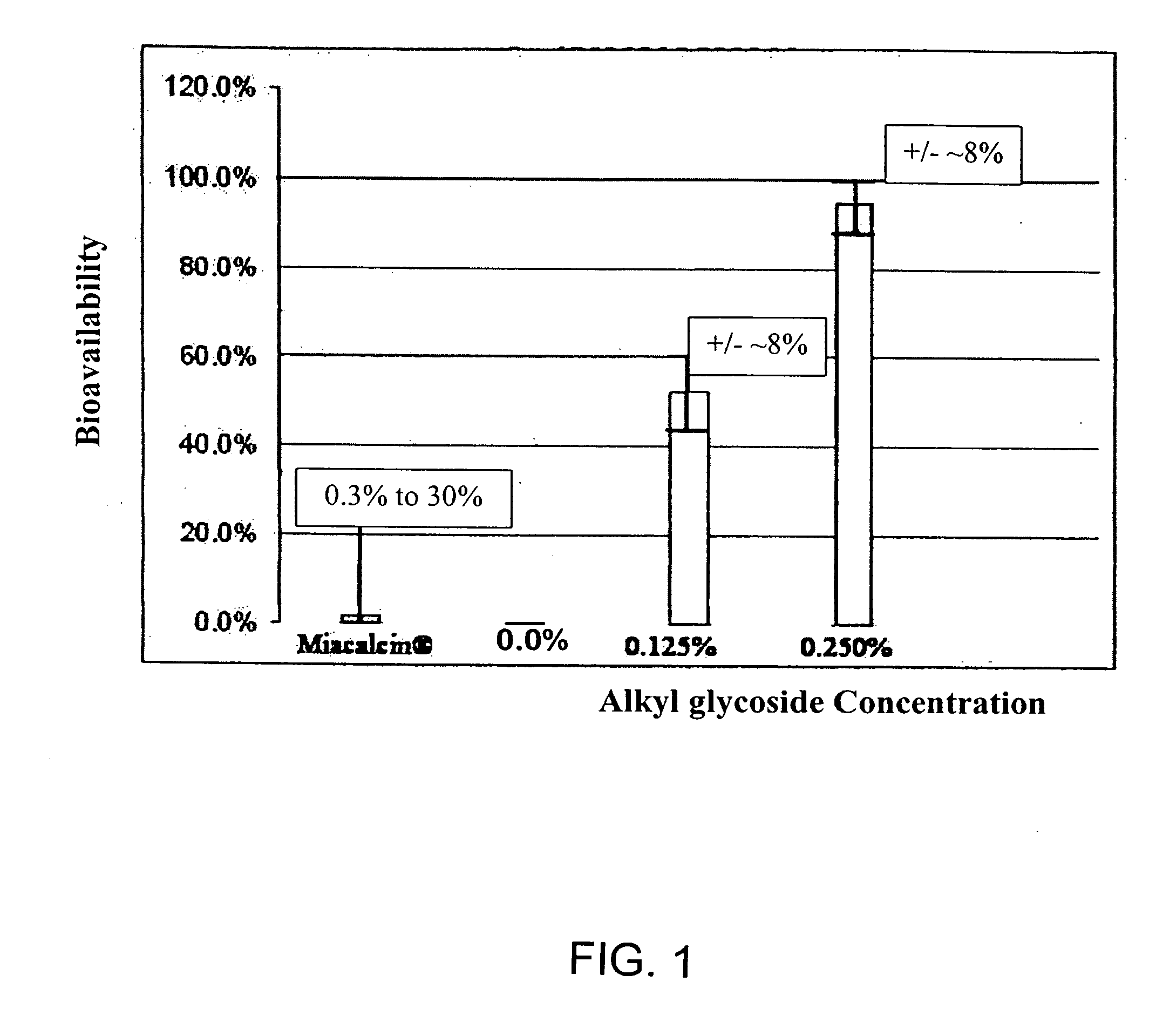
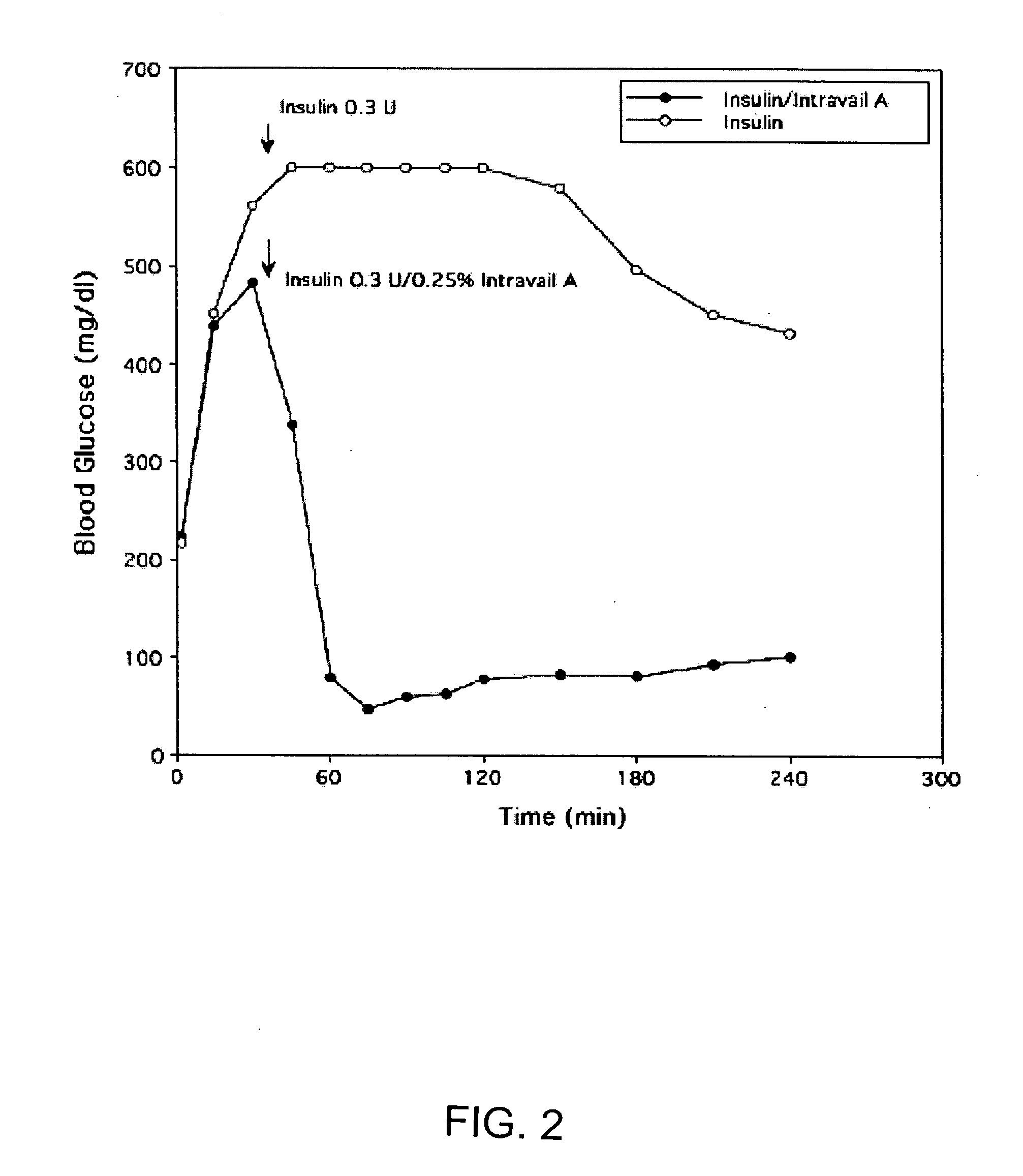
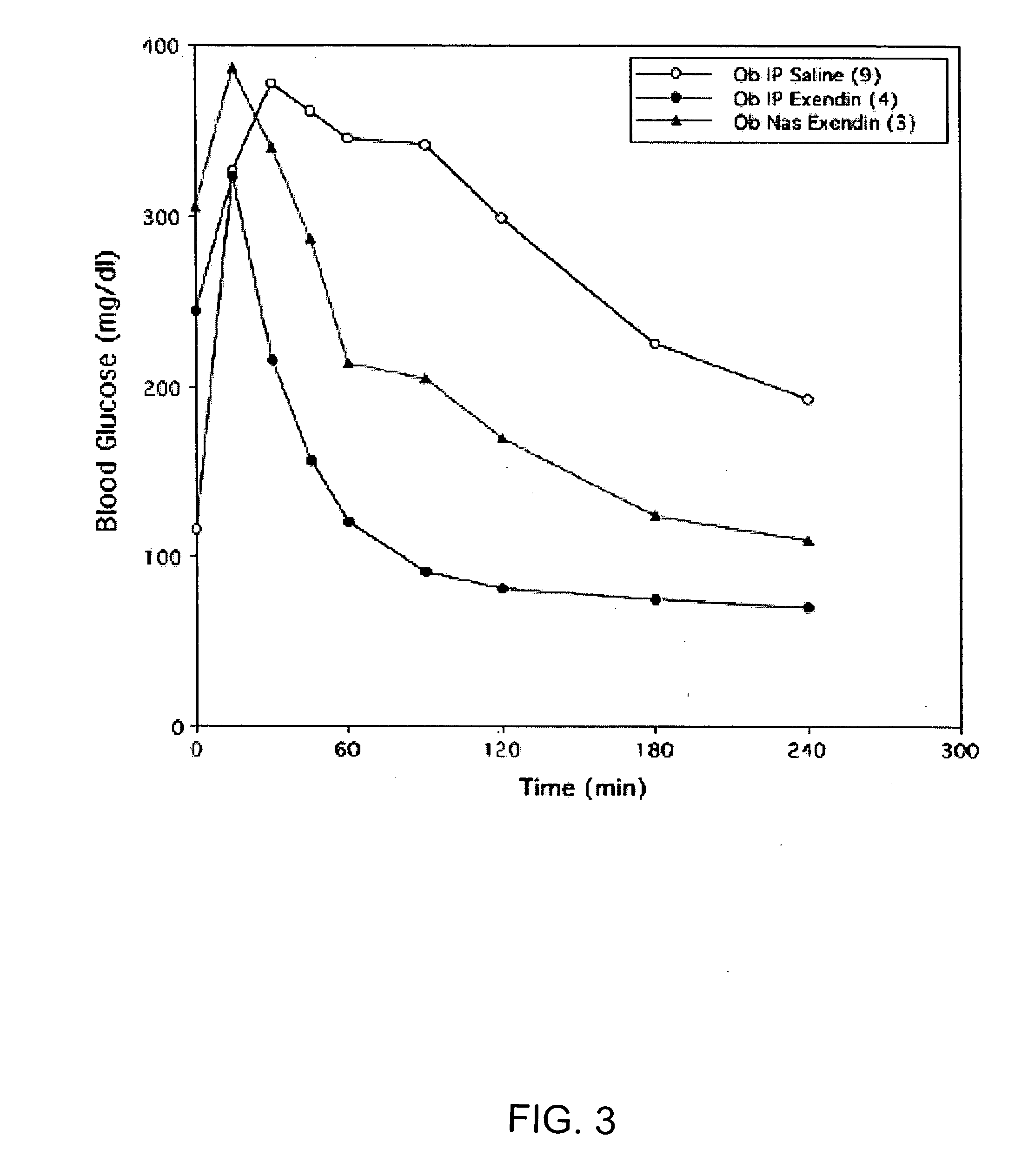
InactiveUS20060045868A1Improve absorption and bioavailabilityToxic effectsPowder deliveryBiocideInhalationDrug administration
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC +1
Parathyroid hormone (pth) containing pharmaceutical compositions for oral use
InactiveUS20070155664A1Quick releaseSufficient amountOrganic active ingredientsPeptide/protein ingredientsRegimenBlood plasma
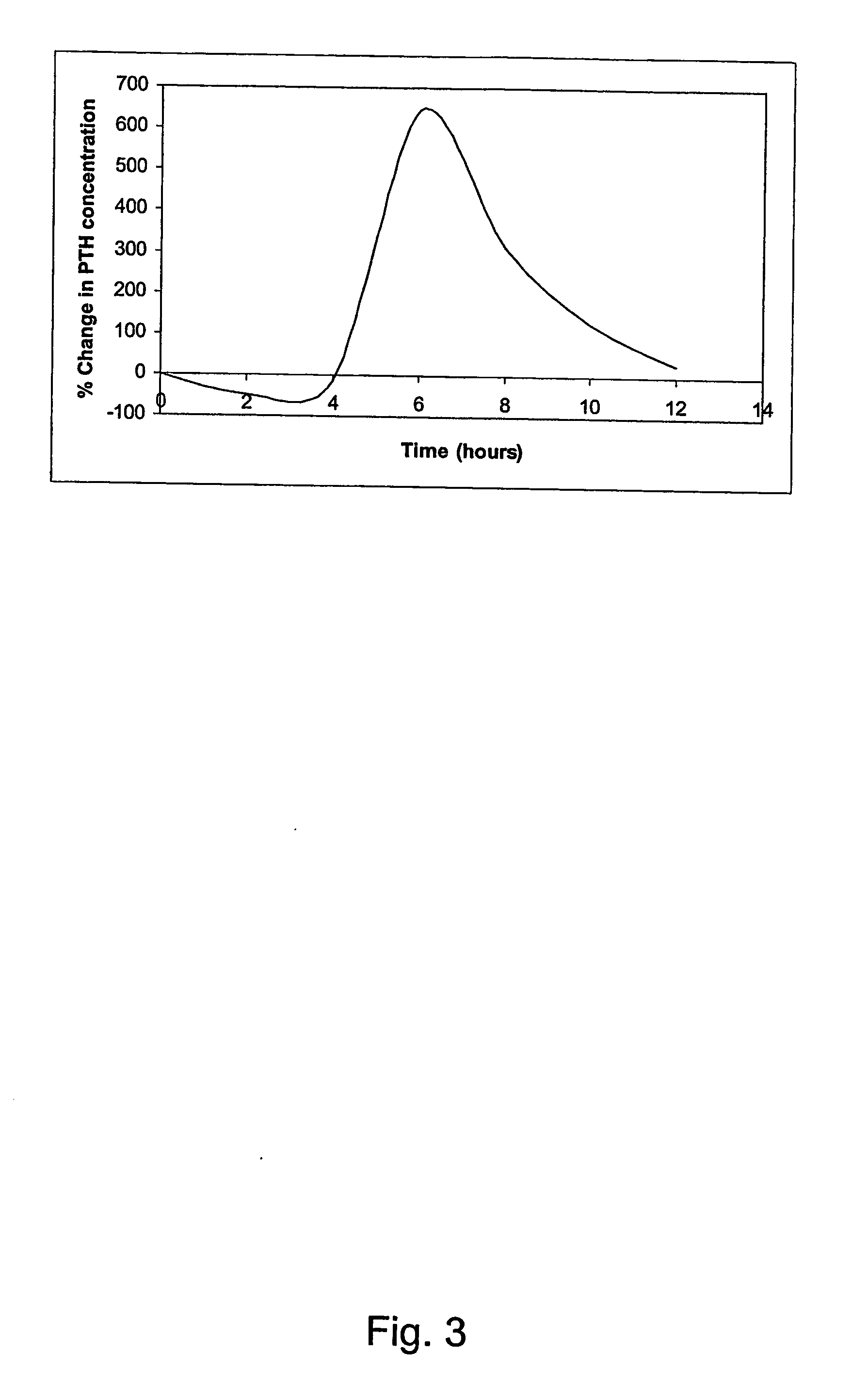
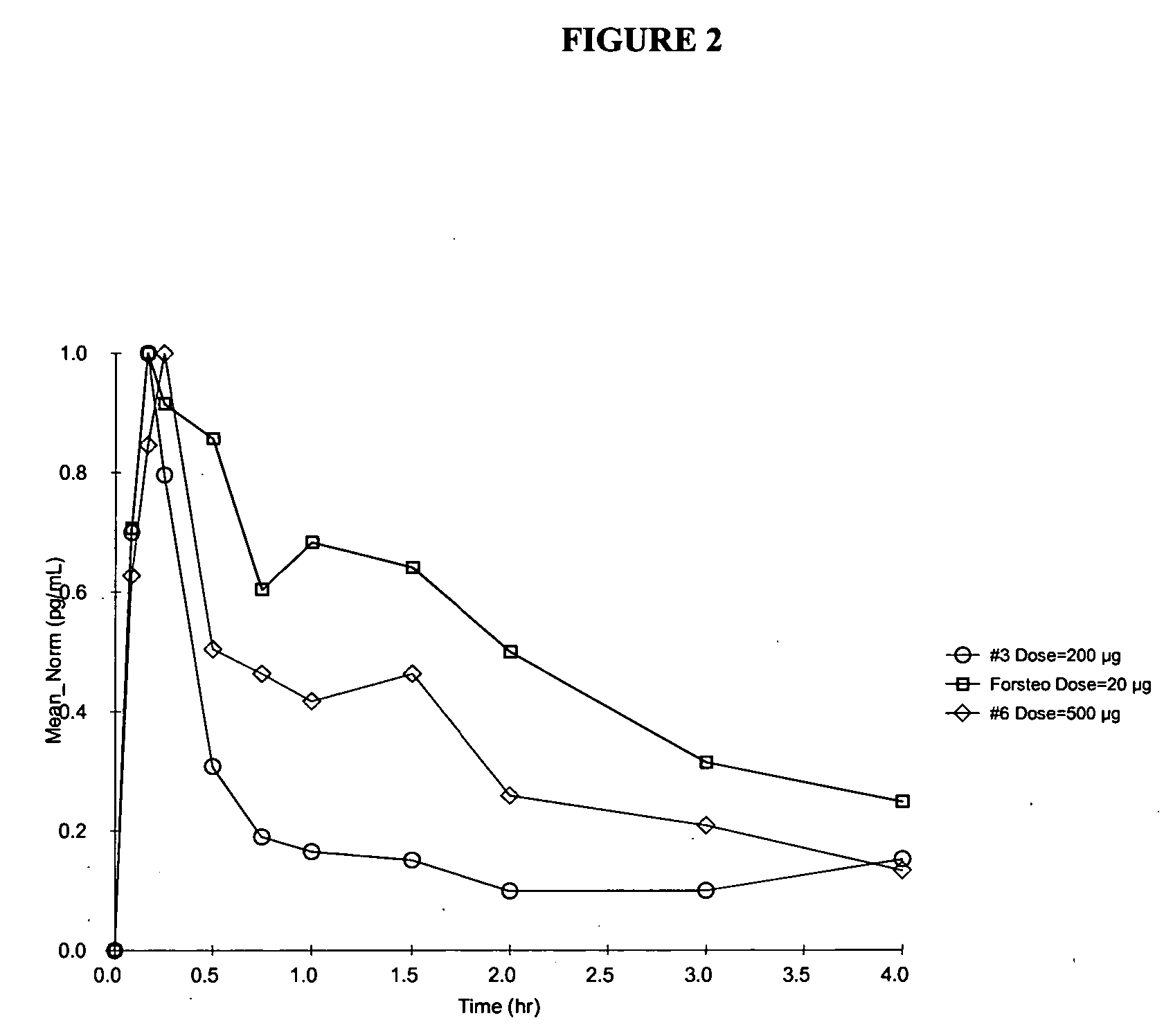
A pharmaceutical composition for oral administration comprising PTH, wherein the in vitro release of PTH-when tested in a dissolution test of pharmacopoeia standard-is delayed with at least 2 hours and once the release starts, at least 90% w / w such as, e.g., at least 95% or at least 99% of all PTH contained in the composition is released within at the most 2 hours. The composition may also comprises a calcium containing compound and / or a vitamin, D. In particular, PTH is administered in combination with a calcium-containing compound for the treatment or prevention of bone-related diseases, so that I) an effective amount of a calcium-containing compound is administered to lower the plasma level of endogenous PTH, and II) an effective amount of PTH is administered to obtain a peak concentration of Pm once the endogeneous PTH level is lowered. This present a potential therapeutic or prophylactic regimen for bone-related disorders including osteoporosis.
Owner:NYCOMED DANMARK AS
Method for delivery of monomeric or dimeric insulin complexed to diketopiperazine microparticles
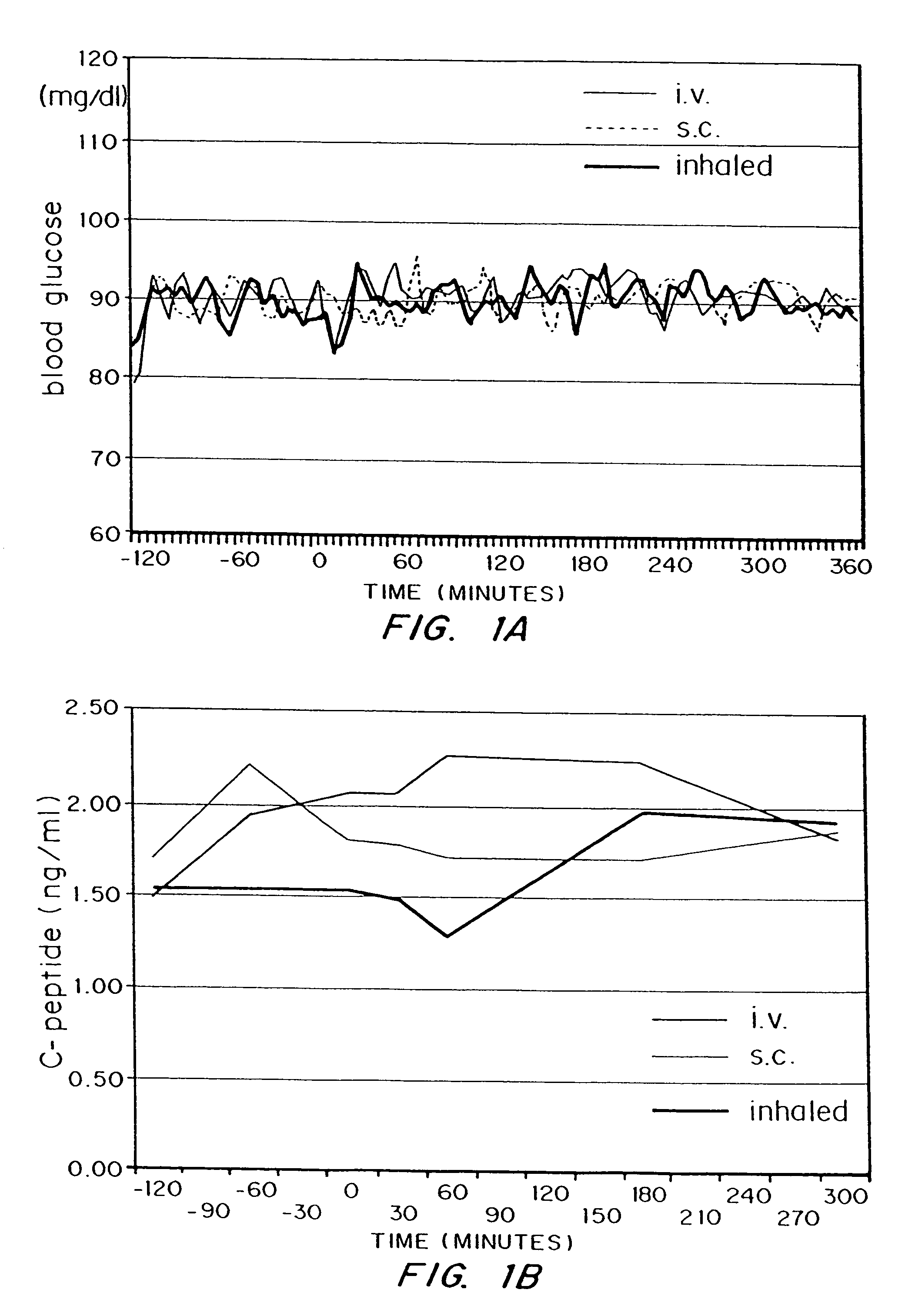
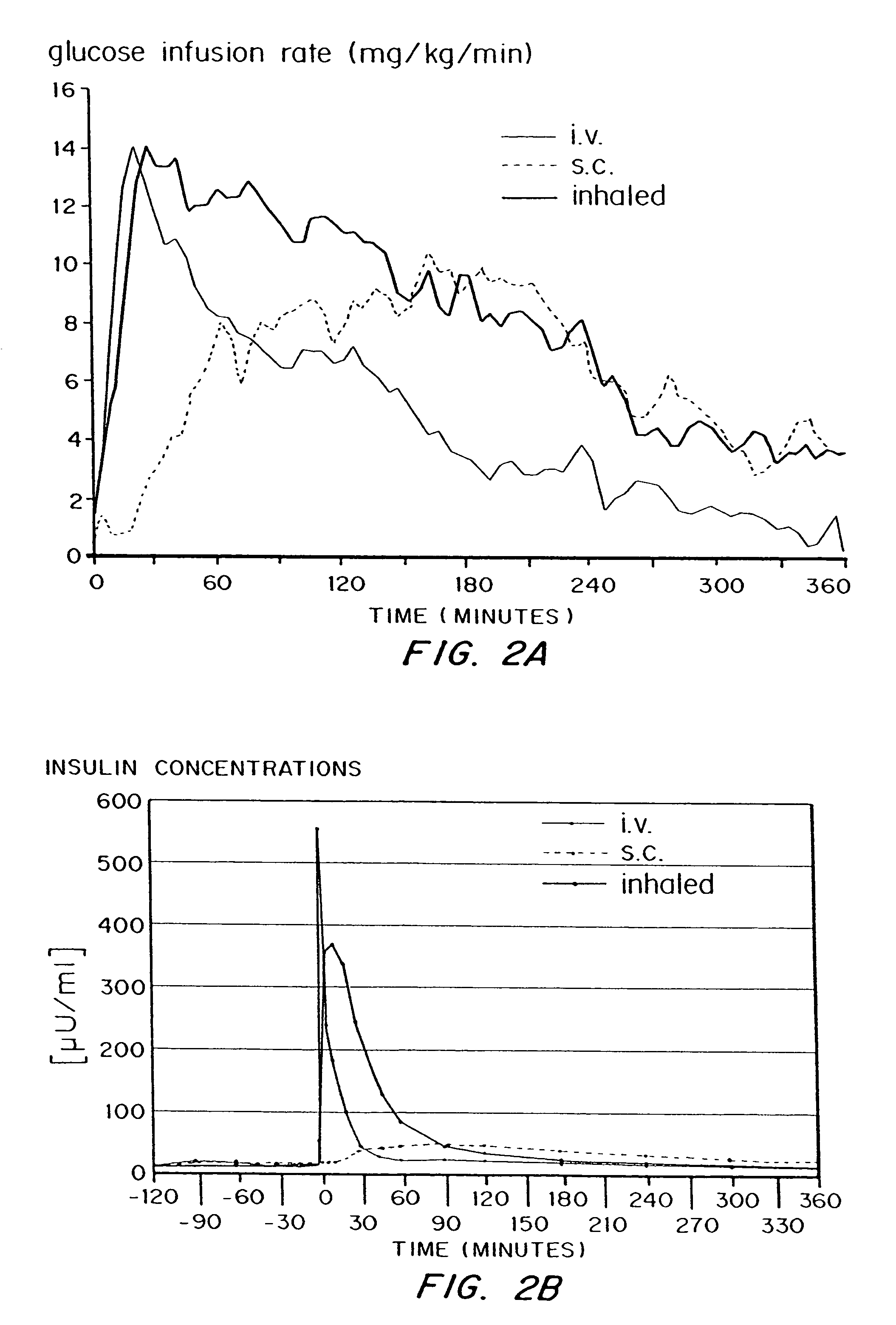
InactiveUS7648960B2Rapid increase in blood agent concentrationEasy to transportPowder deliverySpray deliveryBlood insulinBlood agent
Methods are provided for purifying peptides and proteins by incorporating the peptide or protein into a diketopiperazine or competitive complexing agent to facilitate removal of one or more impurities from the peptide or protein. Formulations and methods also are provided for the improved transport of active agents across biological membranes, resulting for example in a rapid increase in blood agent concentration. The formulations include microparticles formed of (i) the active agent, which may be charged or neutral, and (ii) a transport enhancer that masks the charge of the agent and / or that forms hydrogen bonds with the target biological membrane in order to facilitate transport. In one embodiment insulin is administered via the pulmonary delivery of microparticles comprising fumaryl diketopiperazine and insulin in its biologically active form. This method of delivering insulin results in a rapid increase in blood insulin concentration that is comparable to the increase resulting from intravenous delivery.
Owner:MANNKIND CORP
Activatable clostridial toxins
InactiveUS20090018081A1Long duration of therapyOvercome resistanceHydrolasesPeptide/protein ingredientsClostridial toxinNucleic acid
Owner:ALLERGAN INC
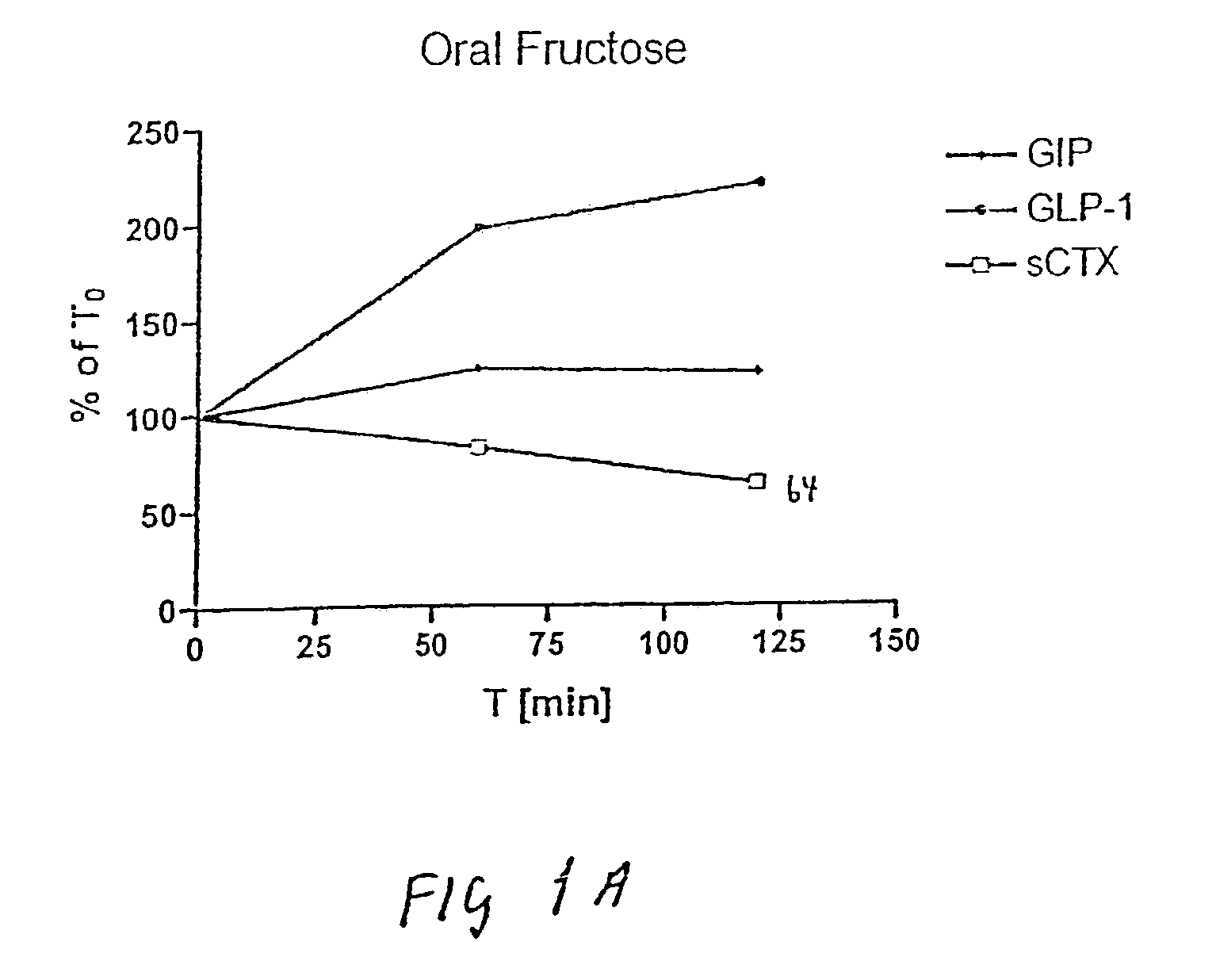
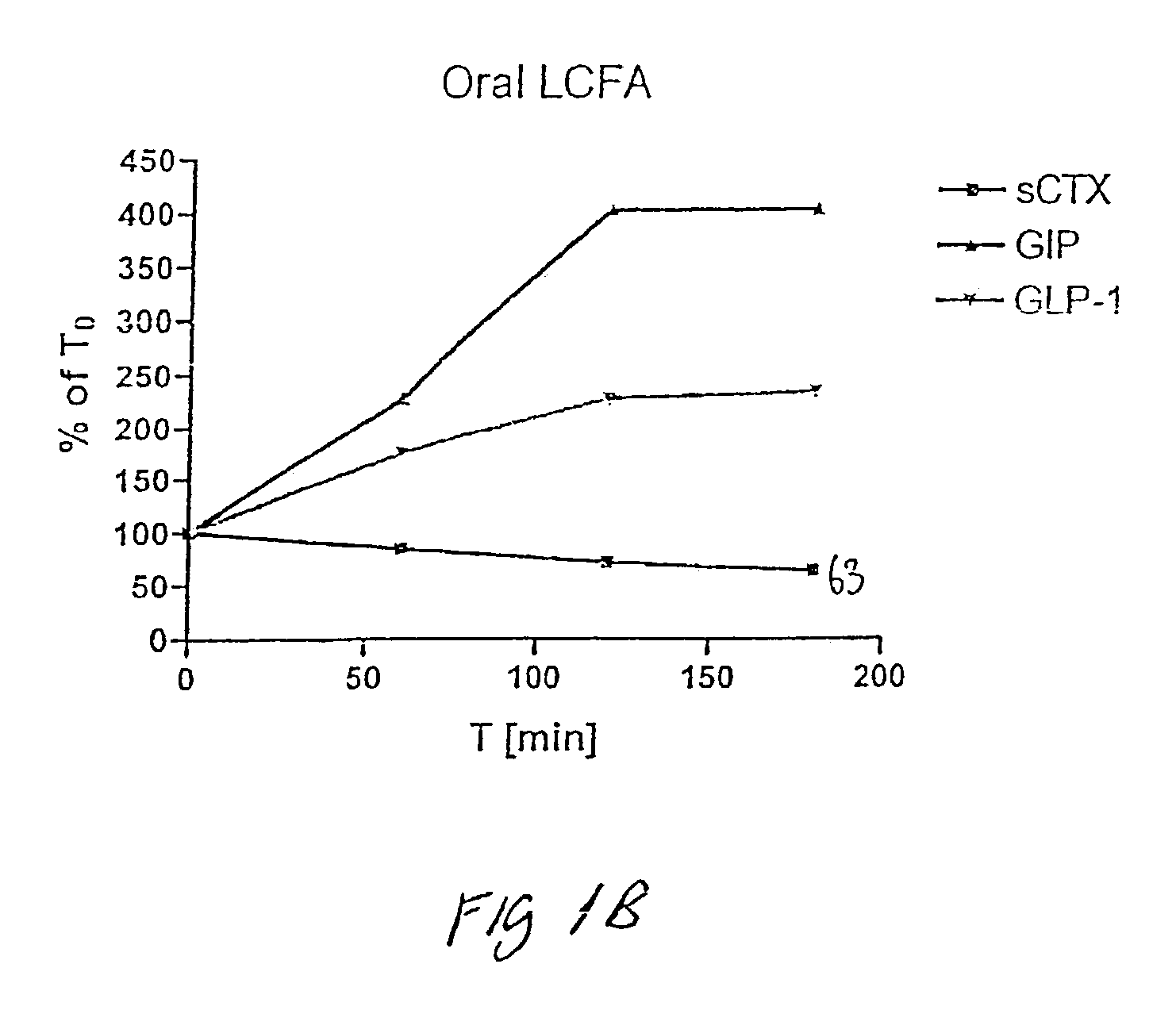
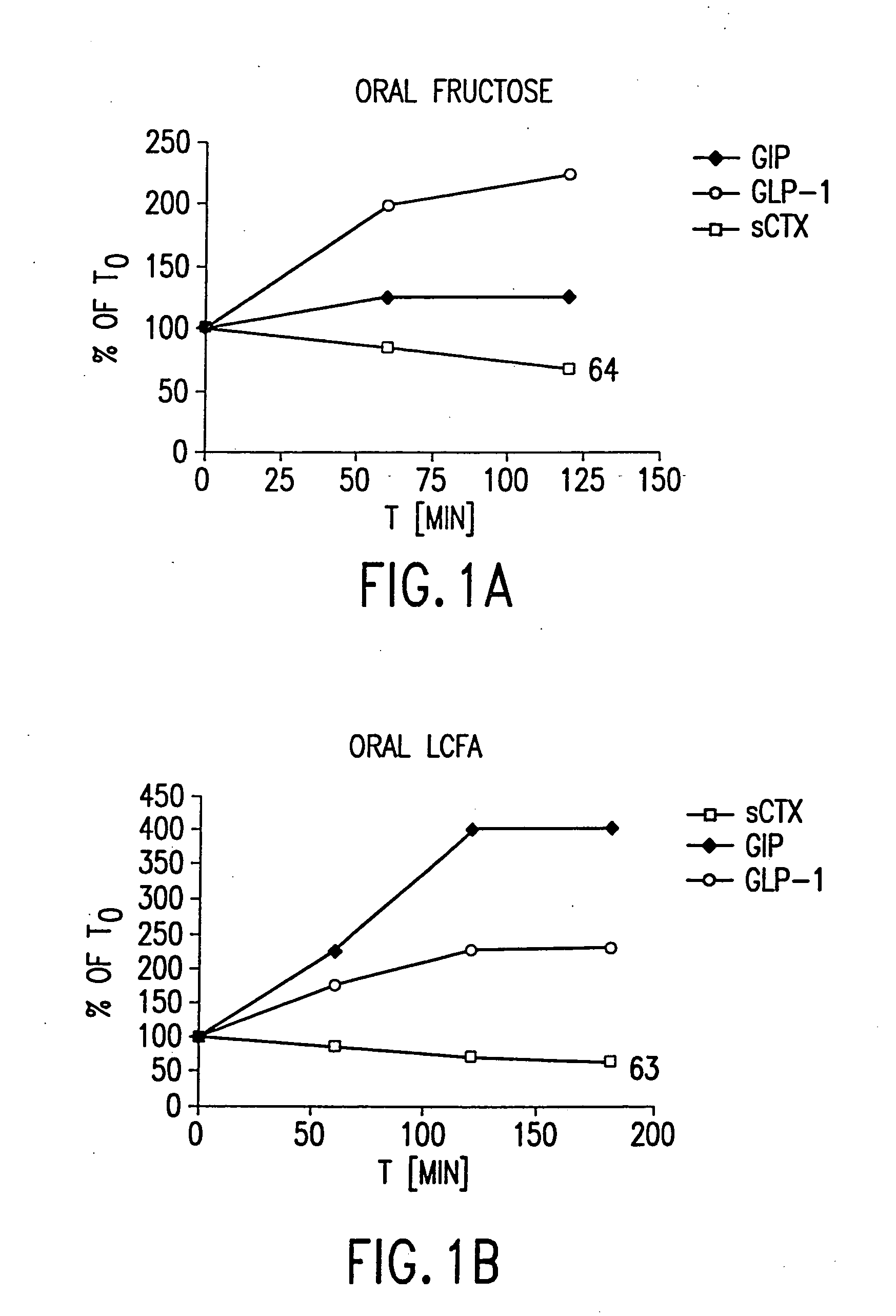
Use of GLP for the treatment, prevention, diagnosis, and prognosis of bone-related and nutrition-related disorders
The present invention relates to methods for prevention and treatment of bone-related or nutrition-related disorders using a GLP molecule or GLP activator either alone or in combination with another therapeutic. The present invention also encompasses methods of diagnosing or monitoring the progression of a disorder. The invention also encompasses methods of monitoring the effectiveness of treatment of the invention.
Owner:SANOS BIOSCI
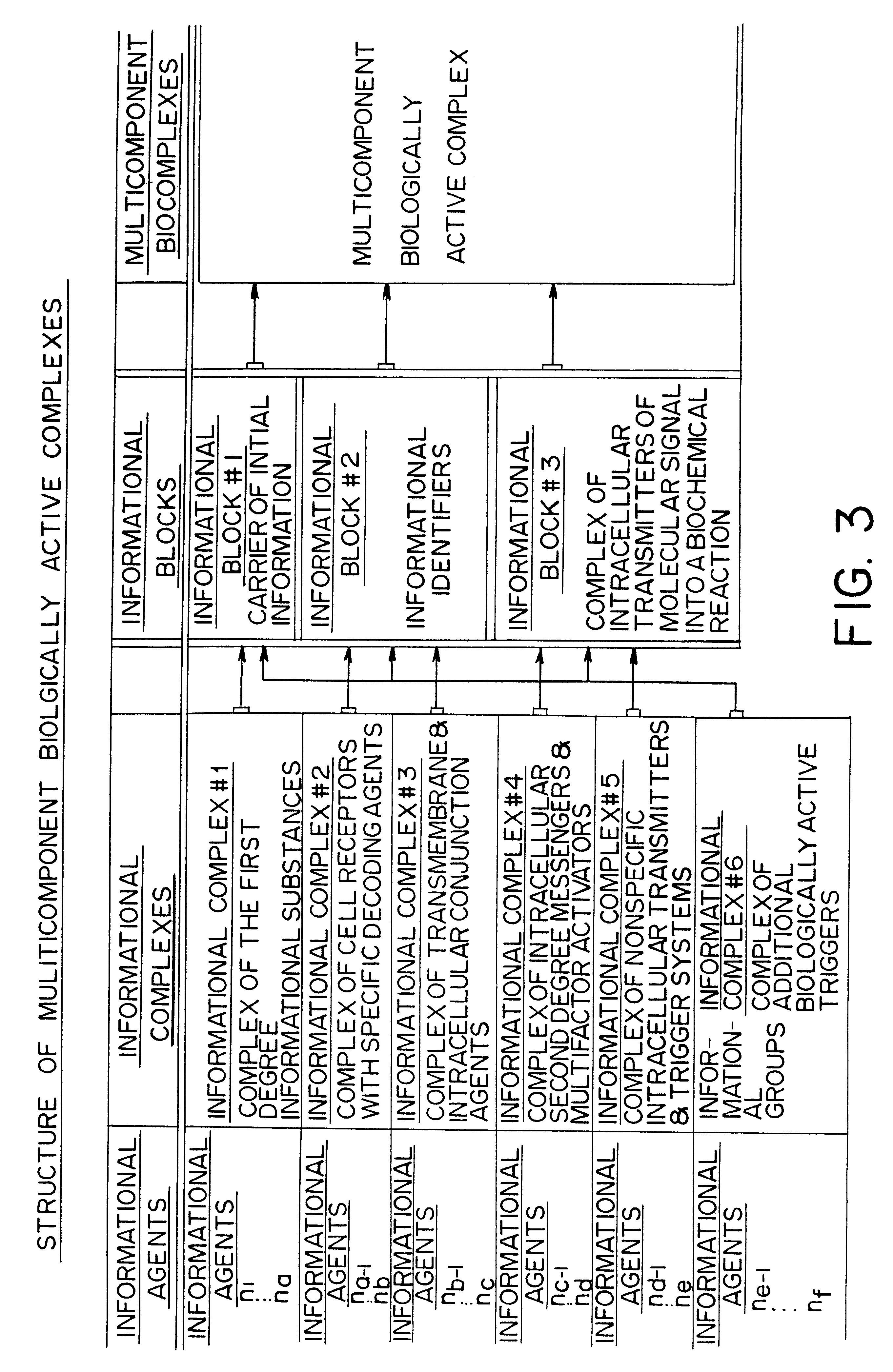
Therapeutic methods utilizing naturally derived bio-active complexes and delivery systems therefor
InactiveUS6303588B1Restore lipid membraneHigh oxygen utilizationCosmetic preparationsBiocideMedicineNormal cell
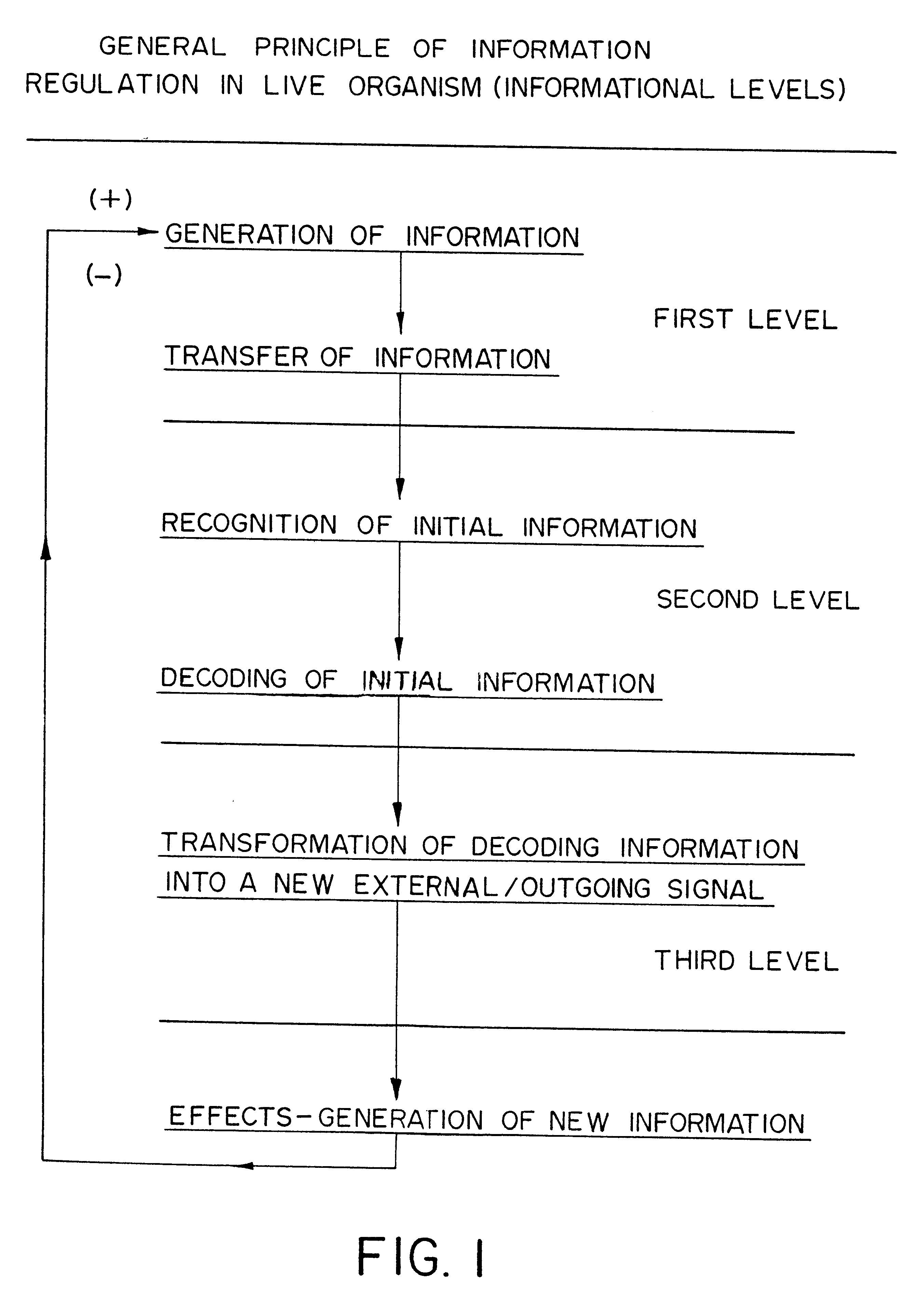
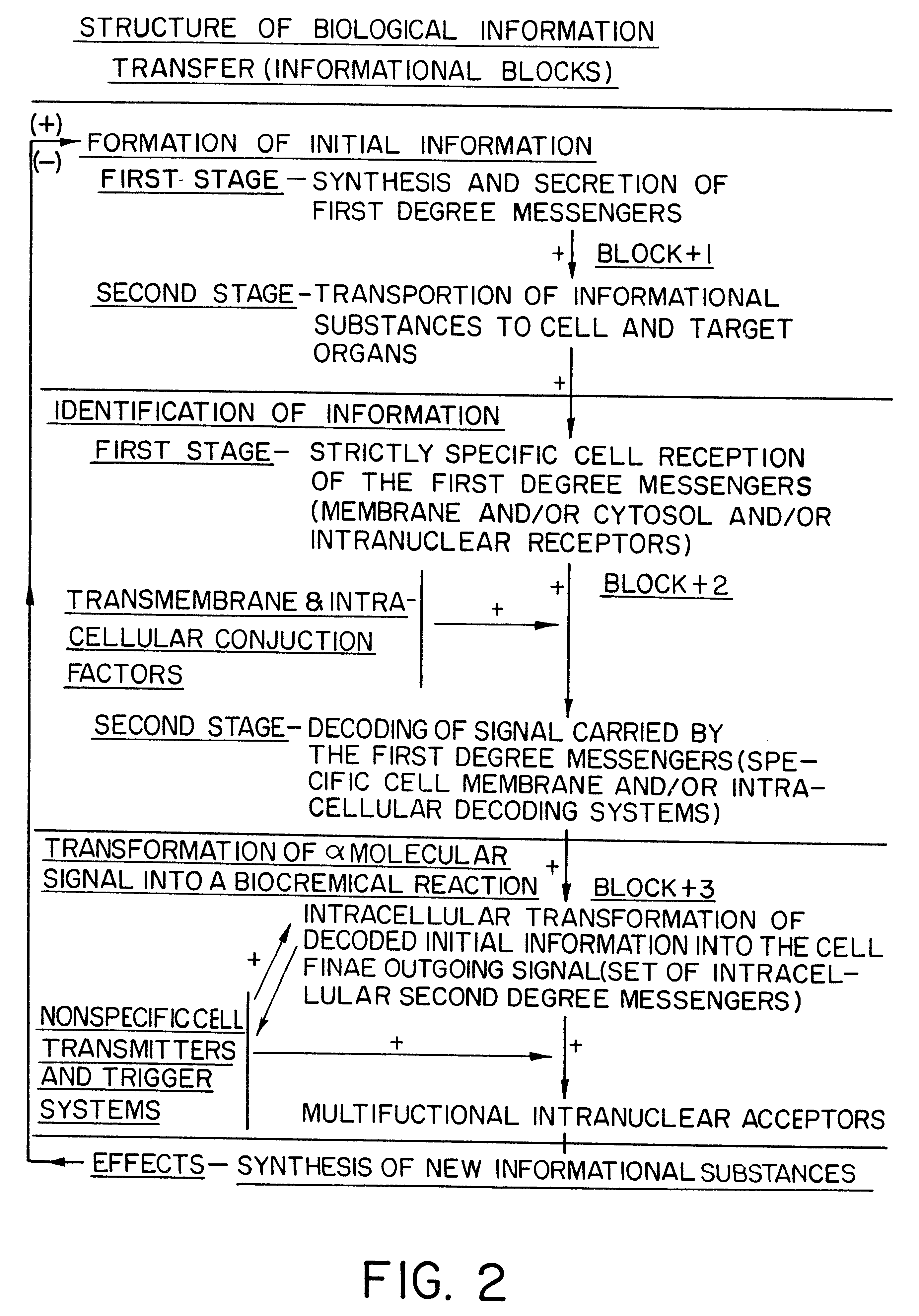
Methods are disclosed for correcting biological information transfer in a patient in need of such therapy which comprise administration to a patient of a composition comprising a therapeutically effective amount of a biocomplex comprising at least one bioactive agent from each of the three informational blocks of biological information transfer, each agent being present in an amount sufficient to correct the biological information transfer of the patient under treatment and resulting in the resumption of normal cell metabolism, said amount being less than the buffering amount of said agent; together with a carrier therefor.
Owner:DANIELOV MICHAEL M
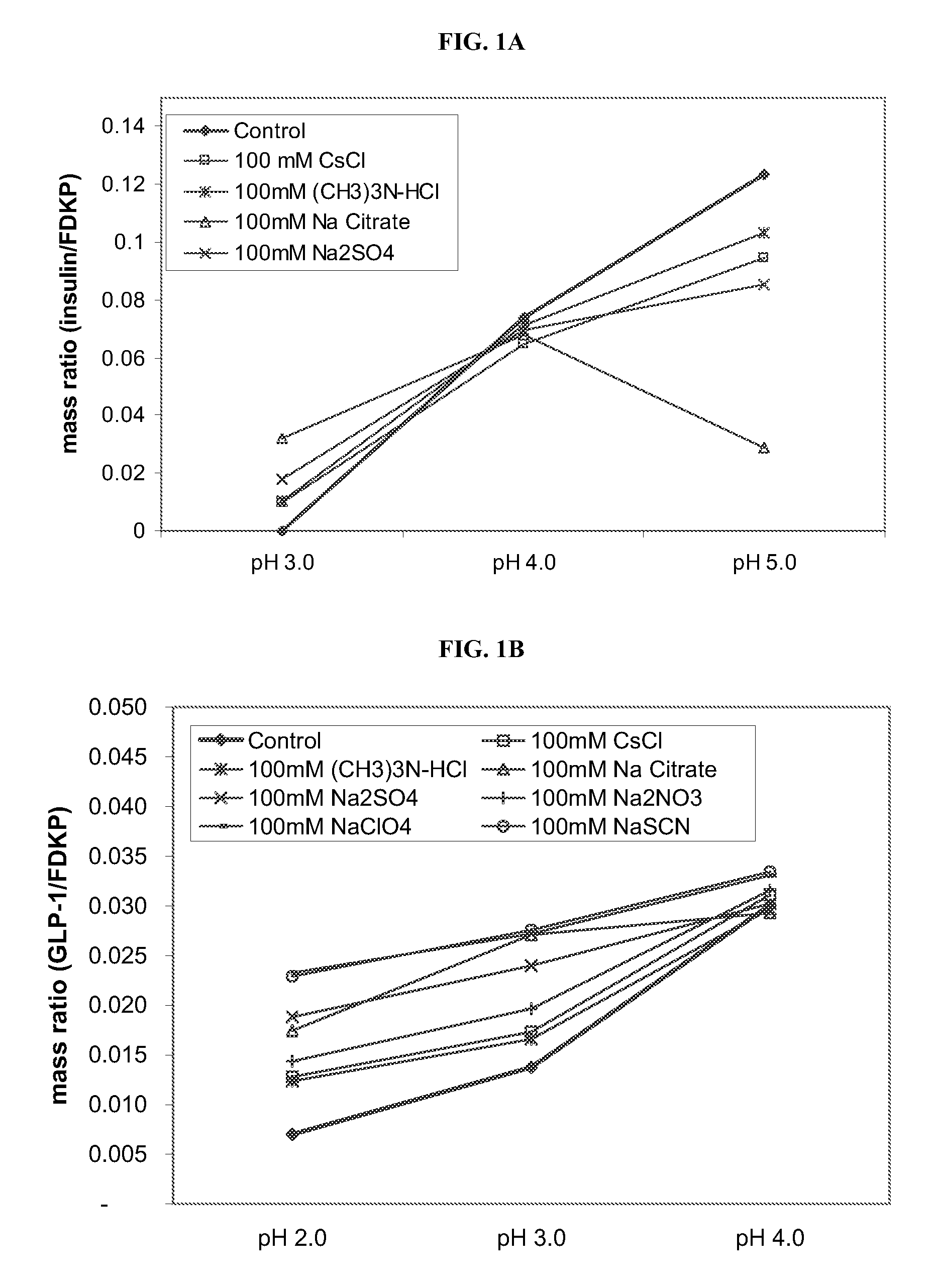
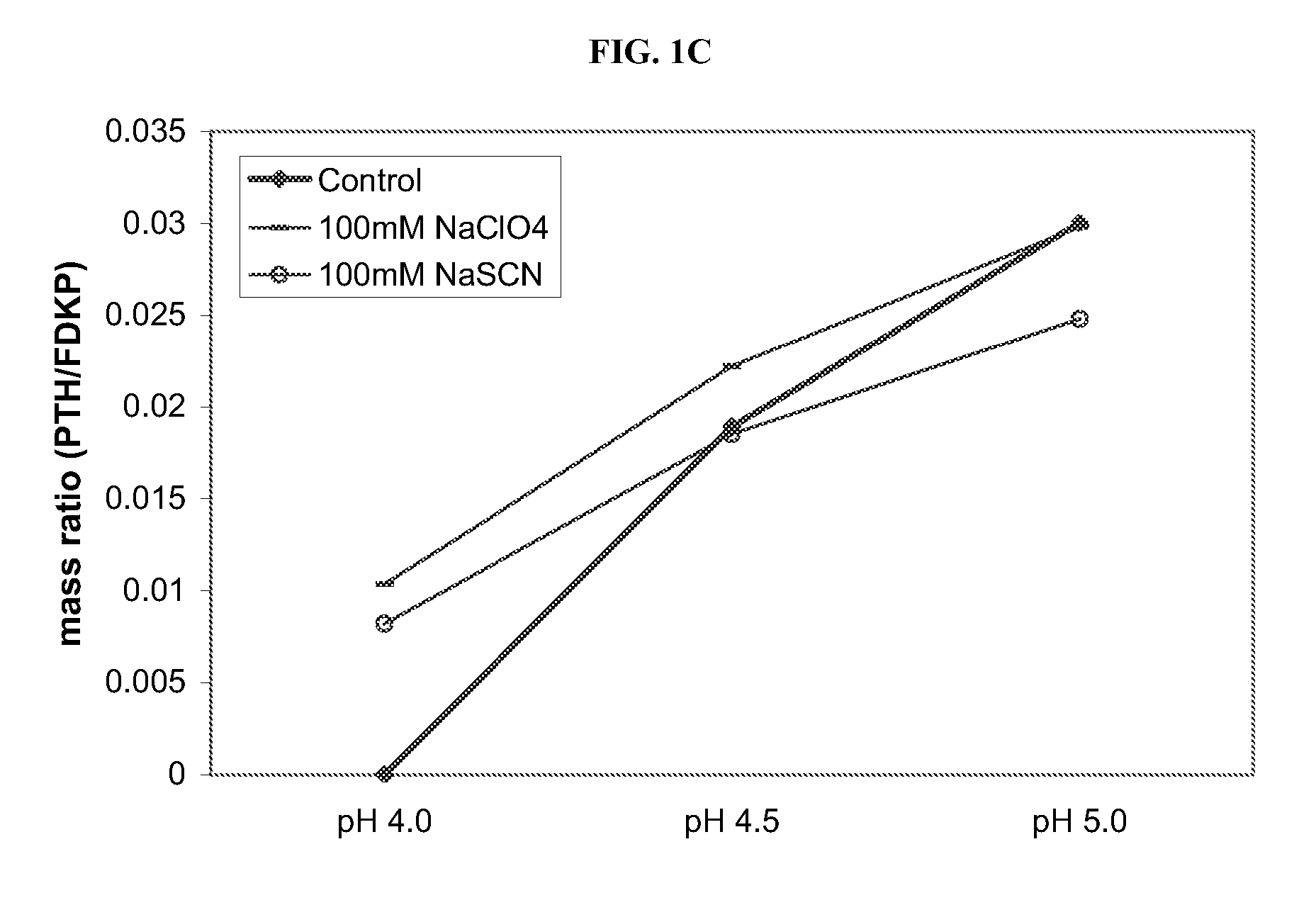
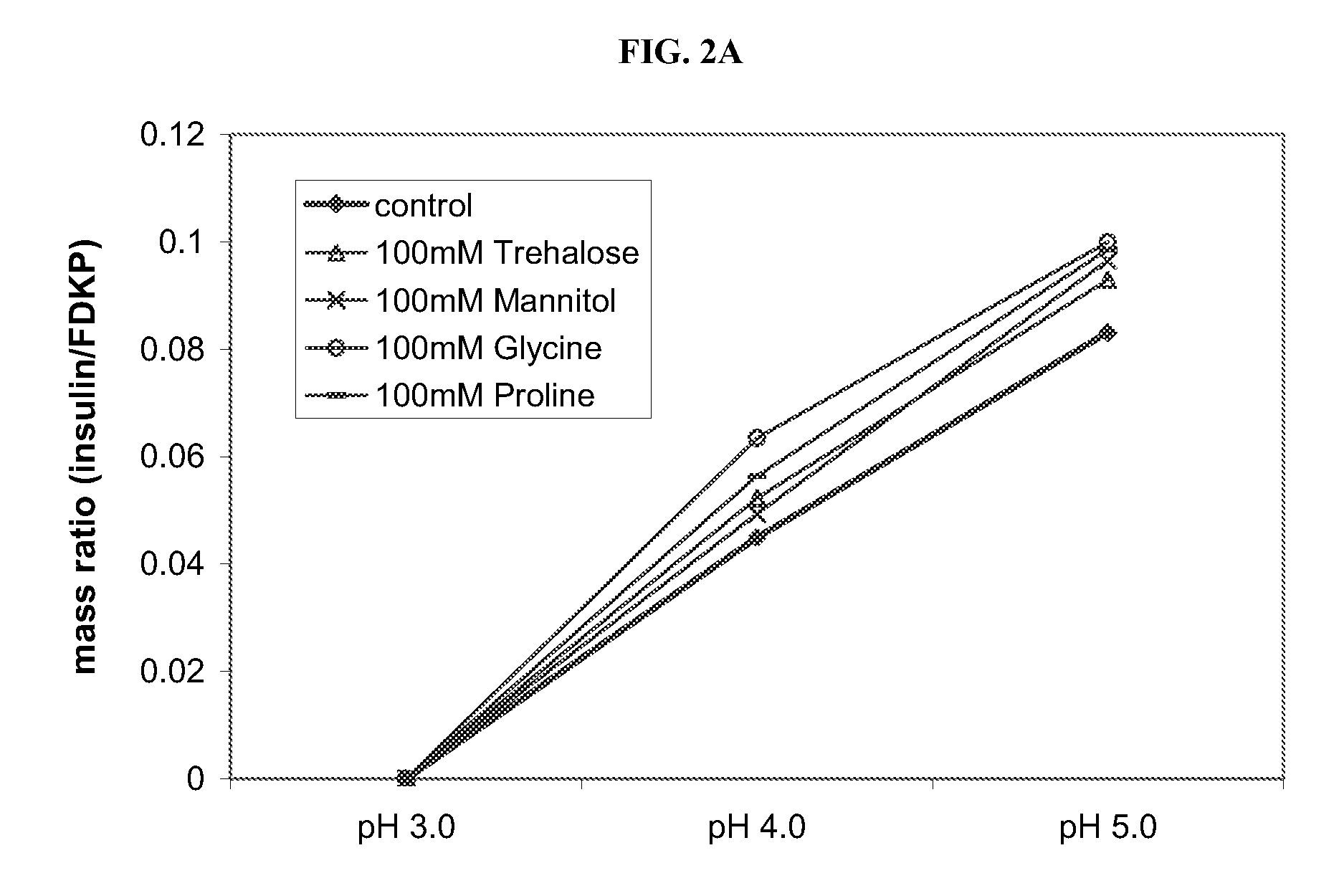
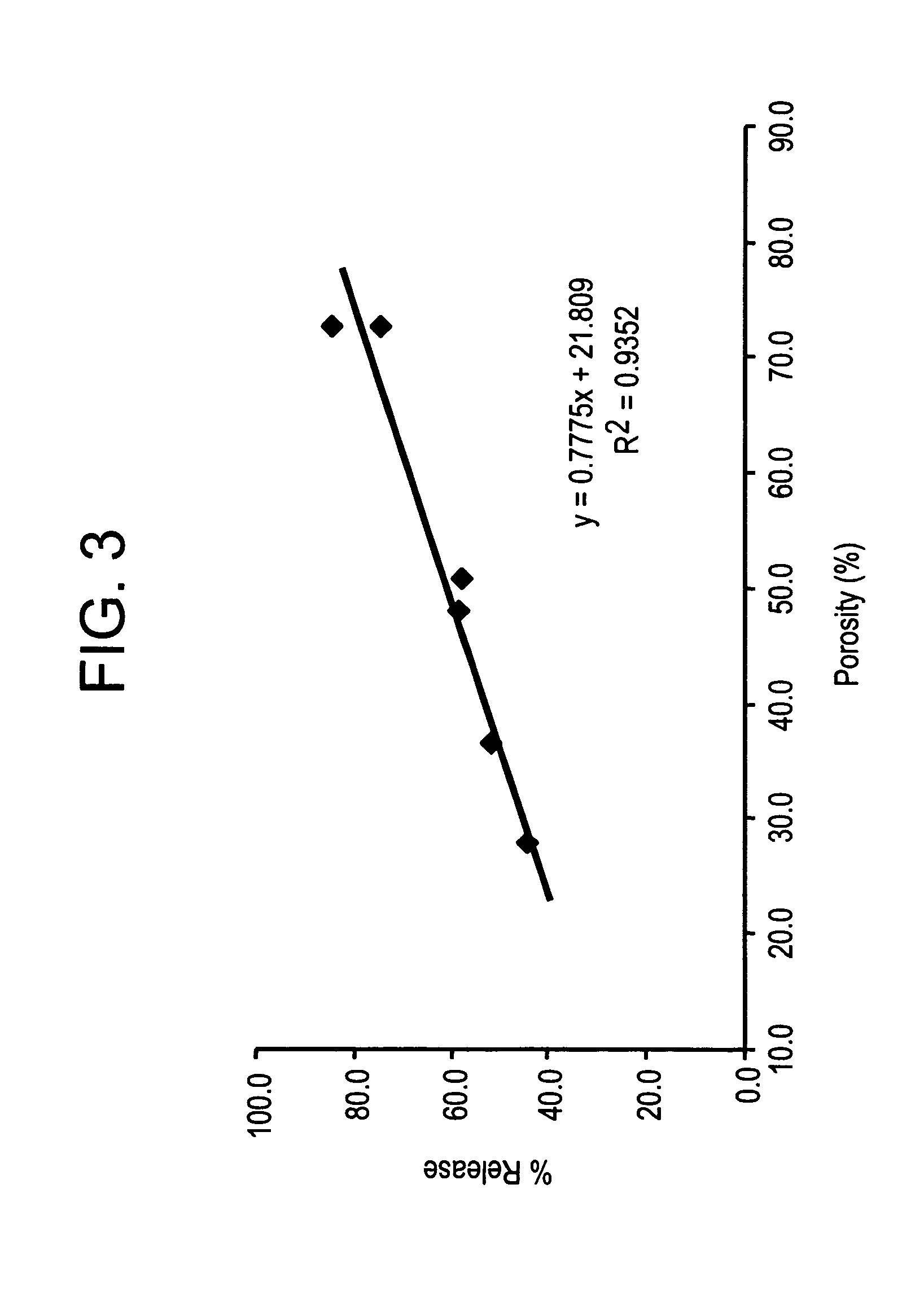
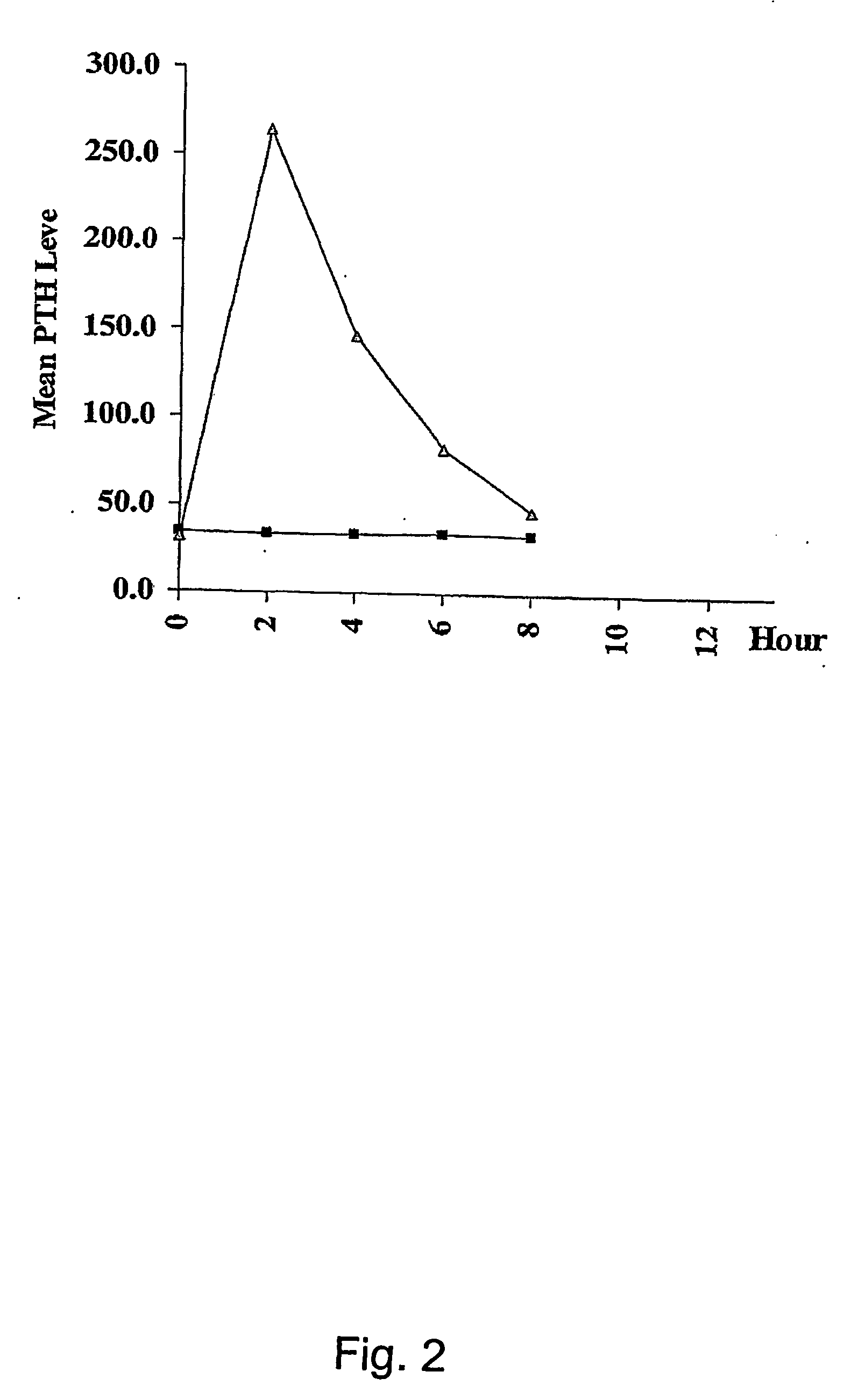
Compositions and methods for enhanced mucosal delivery of parathyroid hormone
InactiveUS20050276843A1Reduce damage-causing attritionHigh bonding strengthBiocideOrganic active ingredientsOsteoporosisPrevention breast cancer
Pharmaceutical compositions and methods are described comprising at least a parathyroid hormone peptide (PTH) preferably PTH1-34 and one or more mucosal delivery-enhancing agents for enhanced nasal mucosal delivery of PTH, for treating or preventing osteoporosis or osteopenia in a mammalian subject, preferably a human.
Owner:MARINA BIOTECH INC
Polycationic calcium modulator peptides for the treatment of hyperparathyroidism and hypercalcemic disorders
ActiveUS20090023652A1Reduce serum PTHReduce serum calciumPeptide/protein ingredientsAntipyreticHypercalcemic disorderHyperparathyroidism
The present invention provides methods and kits for treating hyperparathyroidism, bone disease and / or hypercalcemic disorders. In particular, methods for lowering serum PTH and serum calcium using polycationic calcium modulator peptides are provided. The calcium modulator peptides can be used to treat subjects having, for example: primary, secondary or tertiary hyperparathyroidism; hypercalcemia of malignancy; metastatic bone disease; or osteoporosis.
Owner:KAI PHARMA
Agents and methods for enhancing bone formation
ActiveUS20060270645A1Improve impactPromotes bone formationBiocidePeptide/protein ingredientsMedicineOxysterol
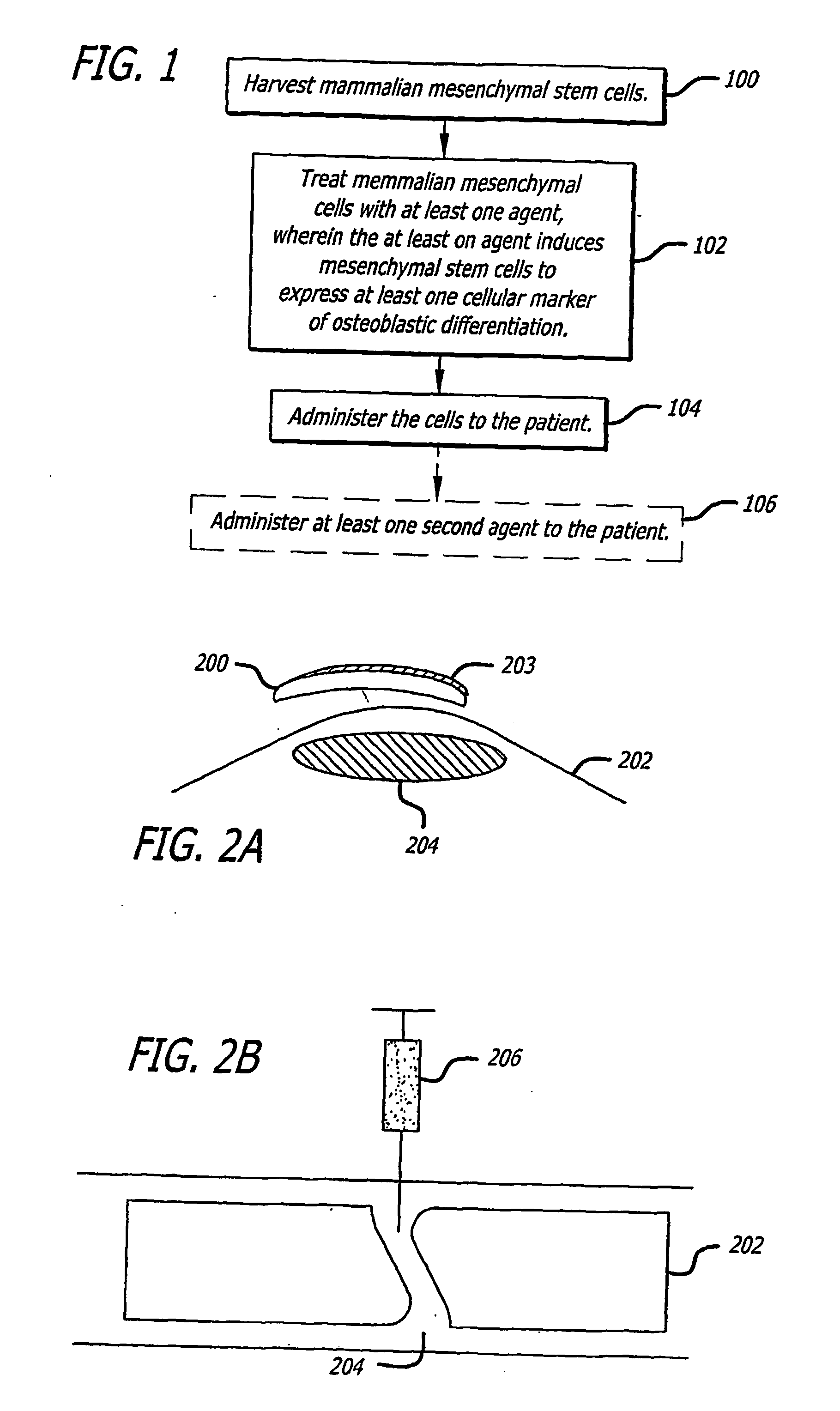
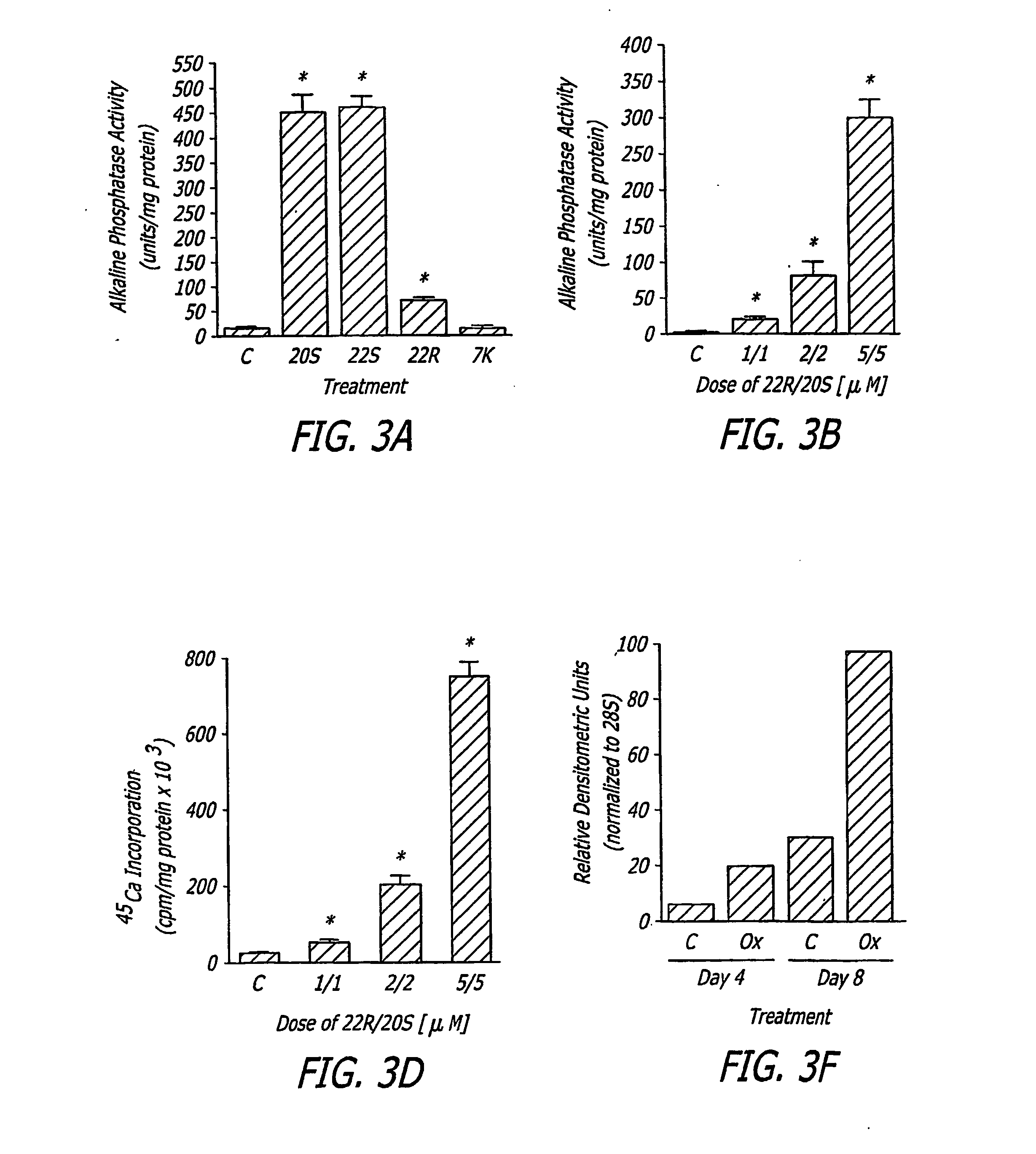
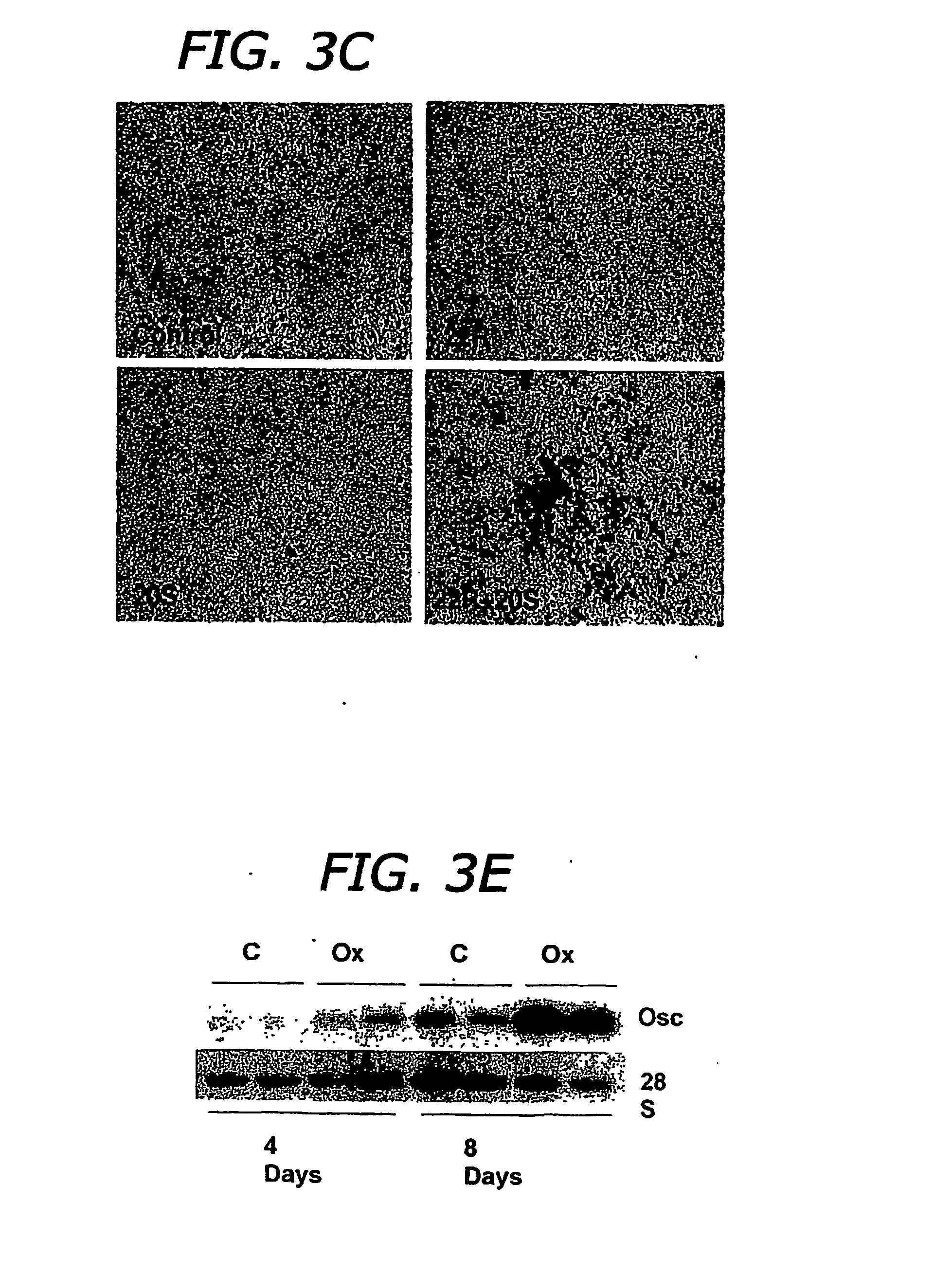
The present invention discloses agents and methods for inducing osteoblastic cellular differentiation, as well as the use of such agents and method to treat patients to maintain bone mass, enhance bone formation and / or bone repair. Exemplary agents include oxysterols, alone or in combination with particular oxysterols, or other agents known to assist in bone formation. The invention further includes medicaments including oxysterols for the treatment of bone disorders, local injections of oxysterols or cells (206) and implants (202) having agents or cells (203) to facilitate bone repair.
Owner:RGT UNIV OF CALIFORNIA
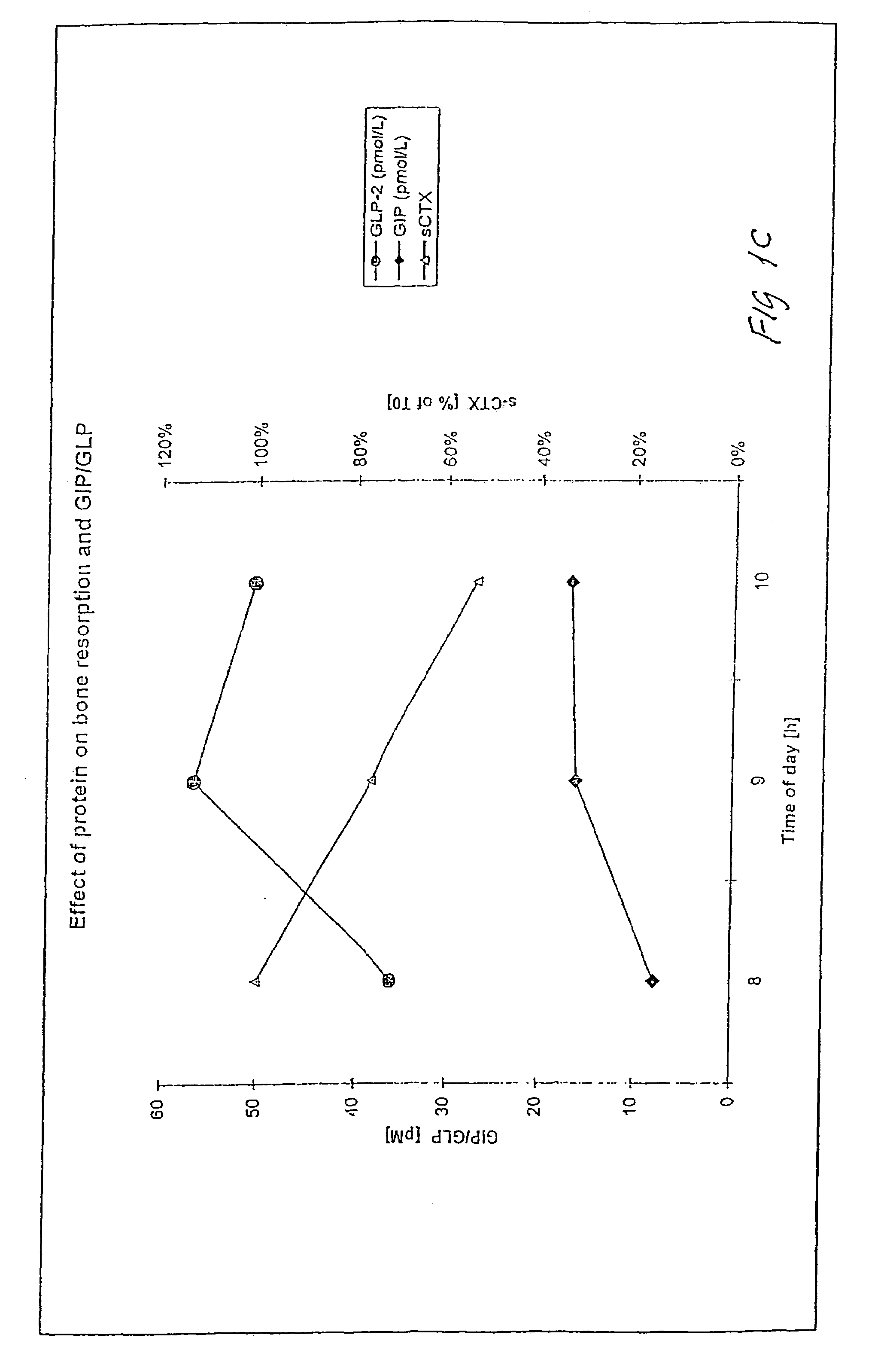
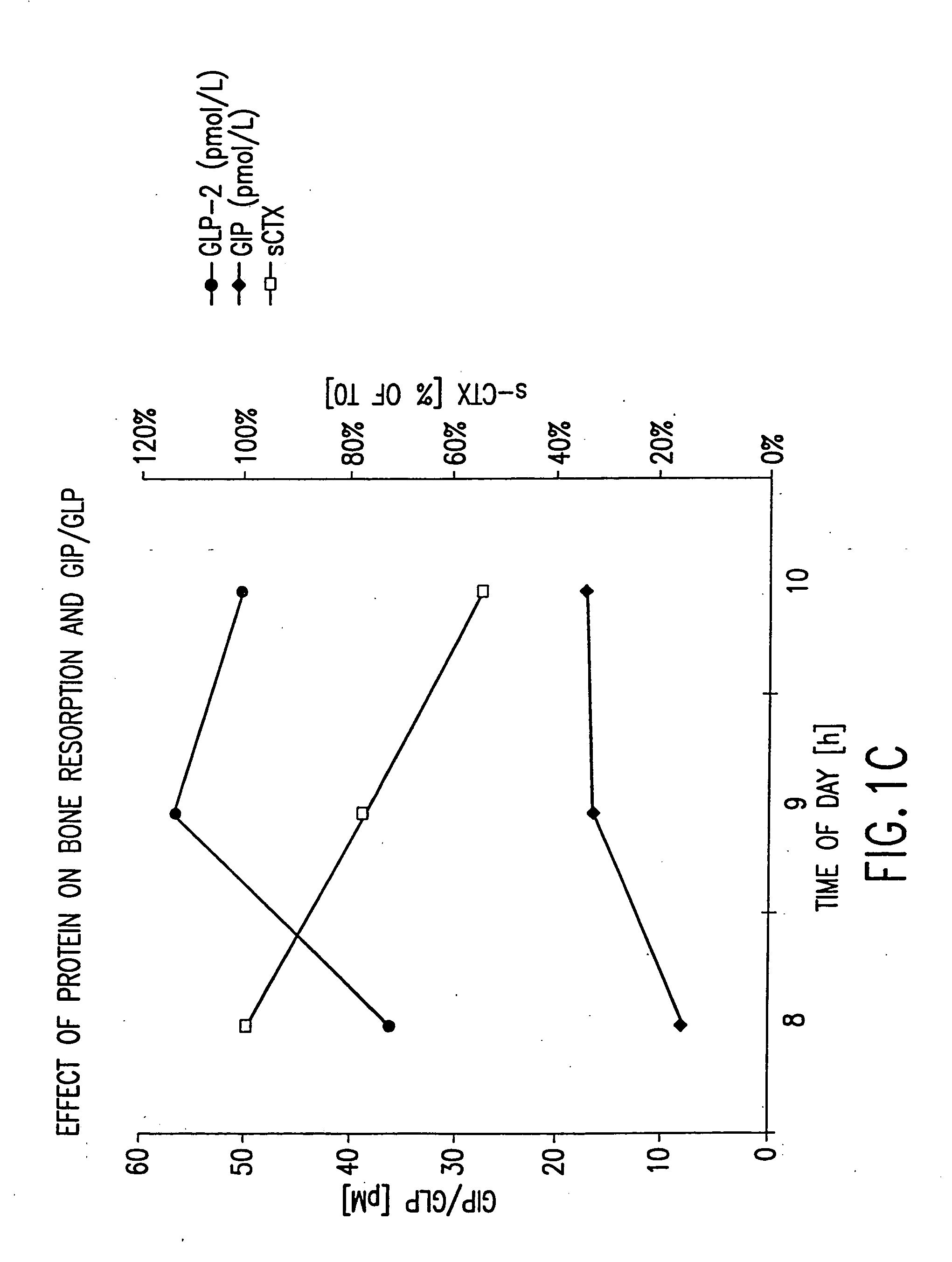
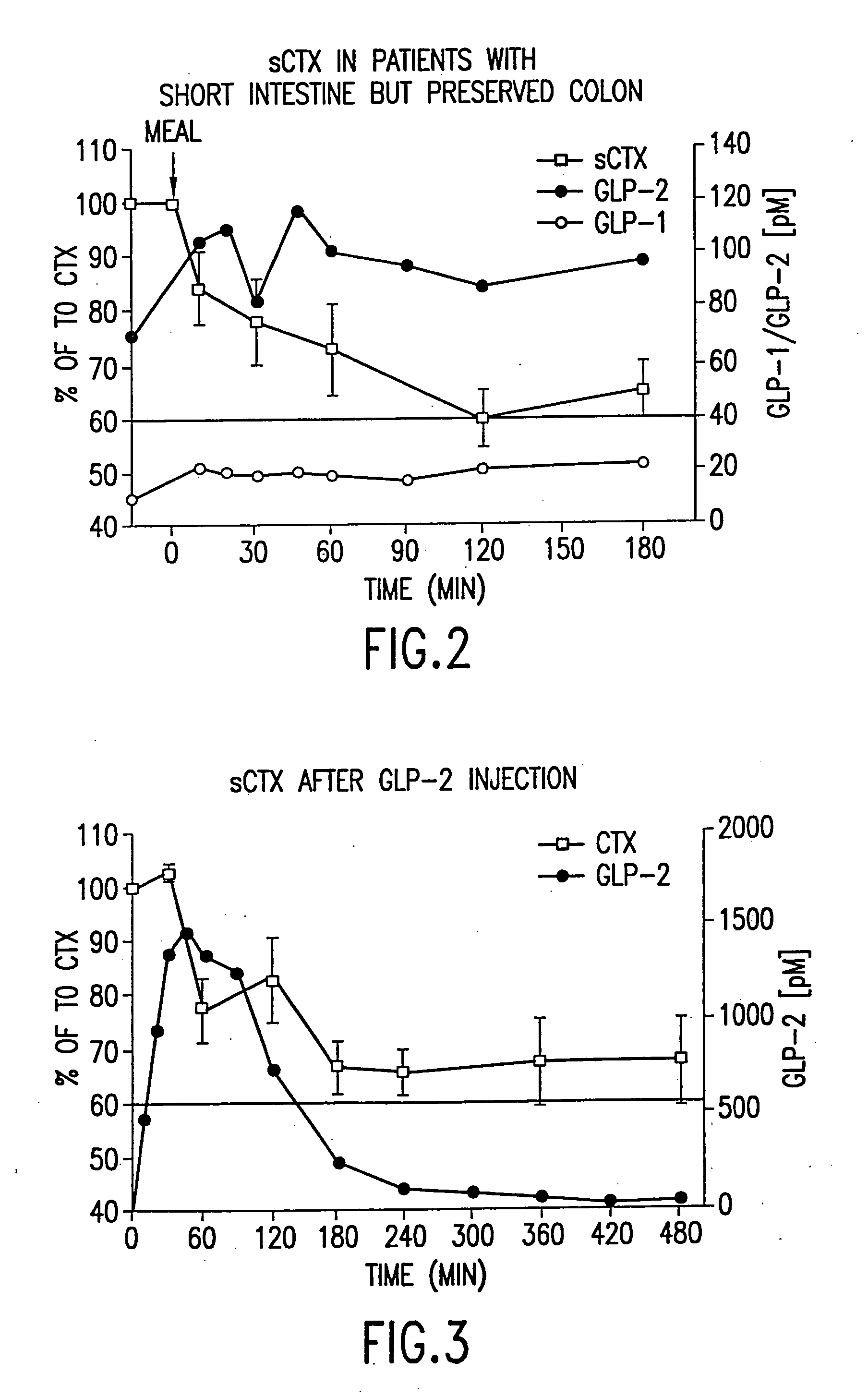
Use of GLP-2 and related compounds for the treatment, prevention, diagnosis, and prognosis of bone-related disorders and calcium homeostasis related syndromes
The present invention relates to methods for prevention and treatment of bone-related disorders and calcium homeostasis related syndromes using a GLP-2 molecule or GLP-2 activator either alone or in combination with another therapeutic. The present invention also encompasses methods of diagnosing or monitoring the progression of a disorder. The invention also encompasses methods of monitoring the effectiveness of treatment of the invention.
Owner:SANOS BIOSCI
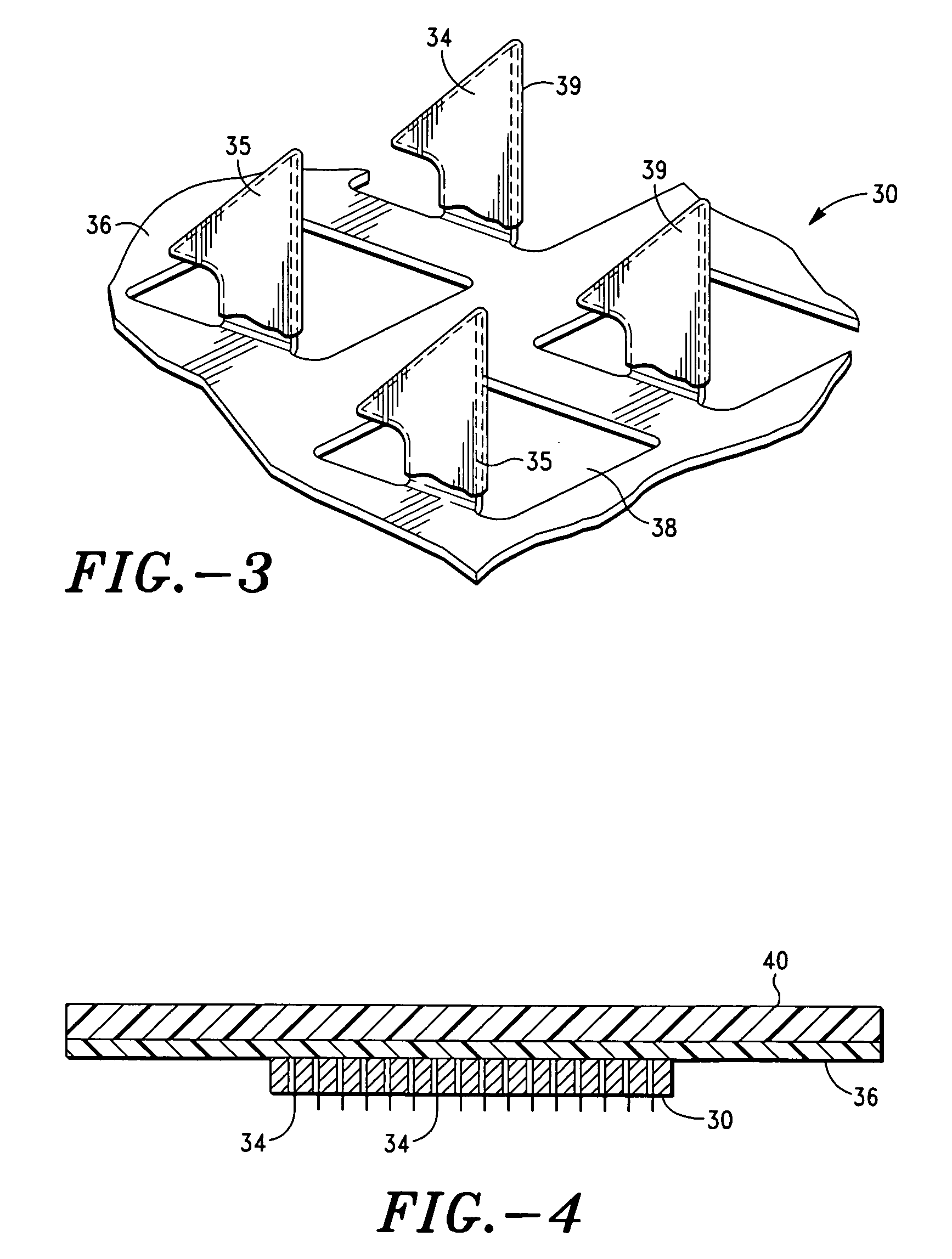
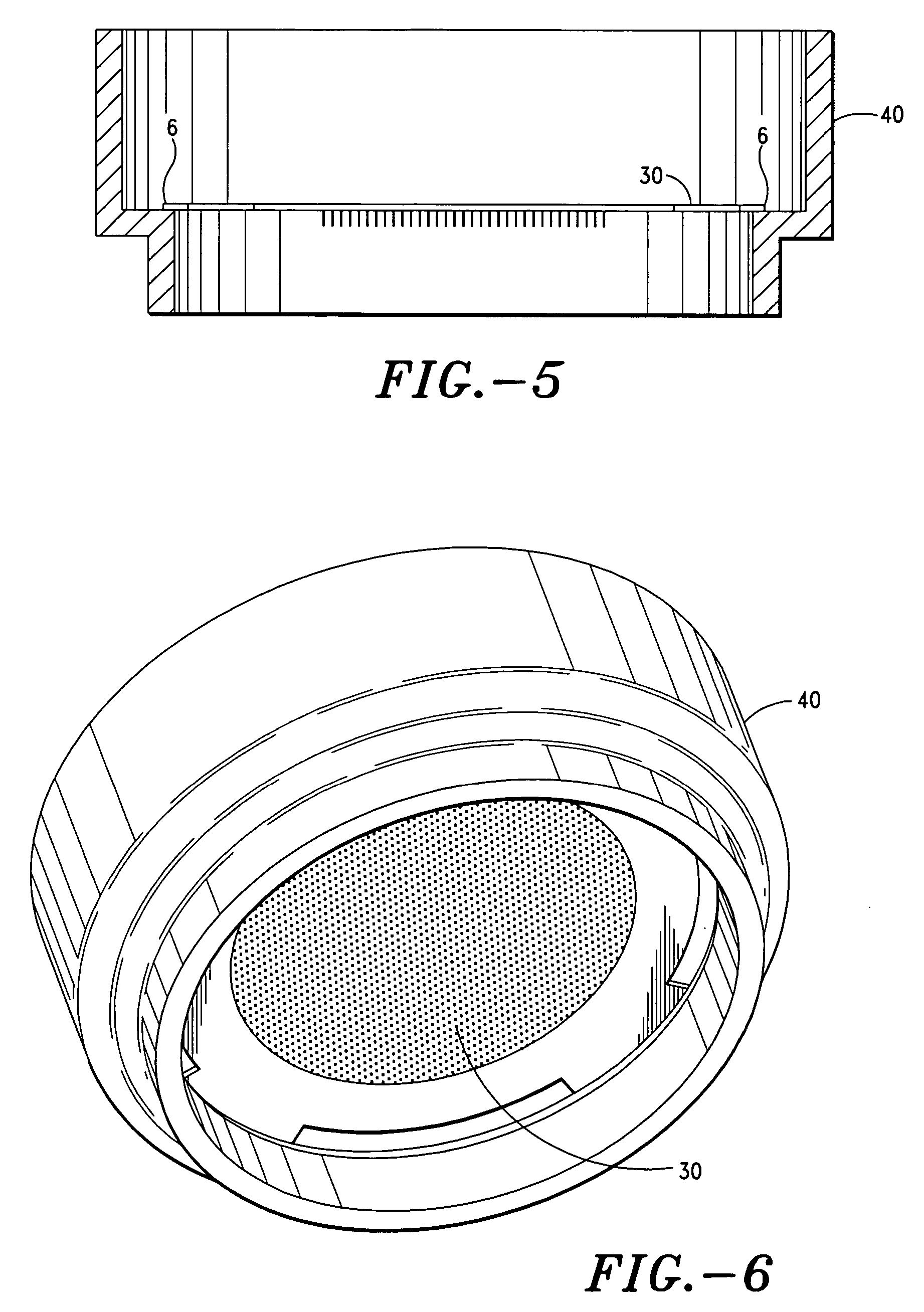
Apparatus and method for transdermal delivery of parathyroid hormone agents
ActiveUS20050256045A1Minimize and eliminate bleedingMinimize and eliminate and irritationPeptide/protein ingredientsPharmaceutical delivery mechanismActive agentBiocompatible coating
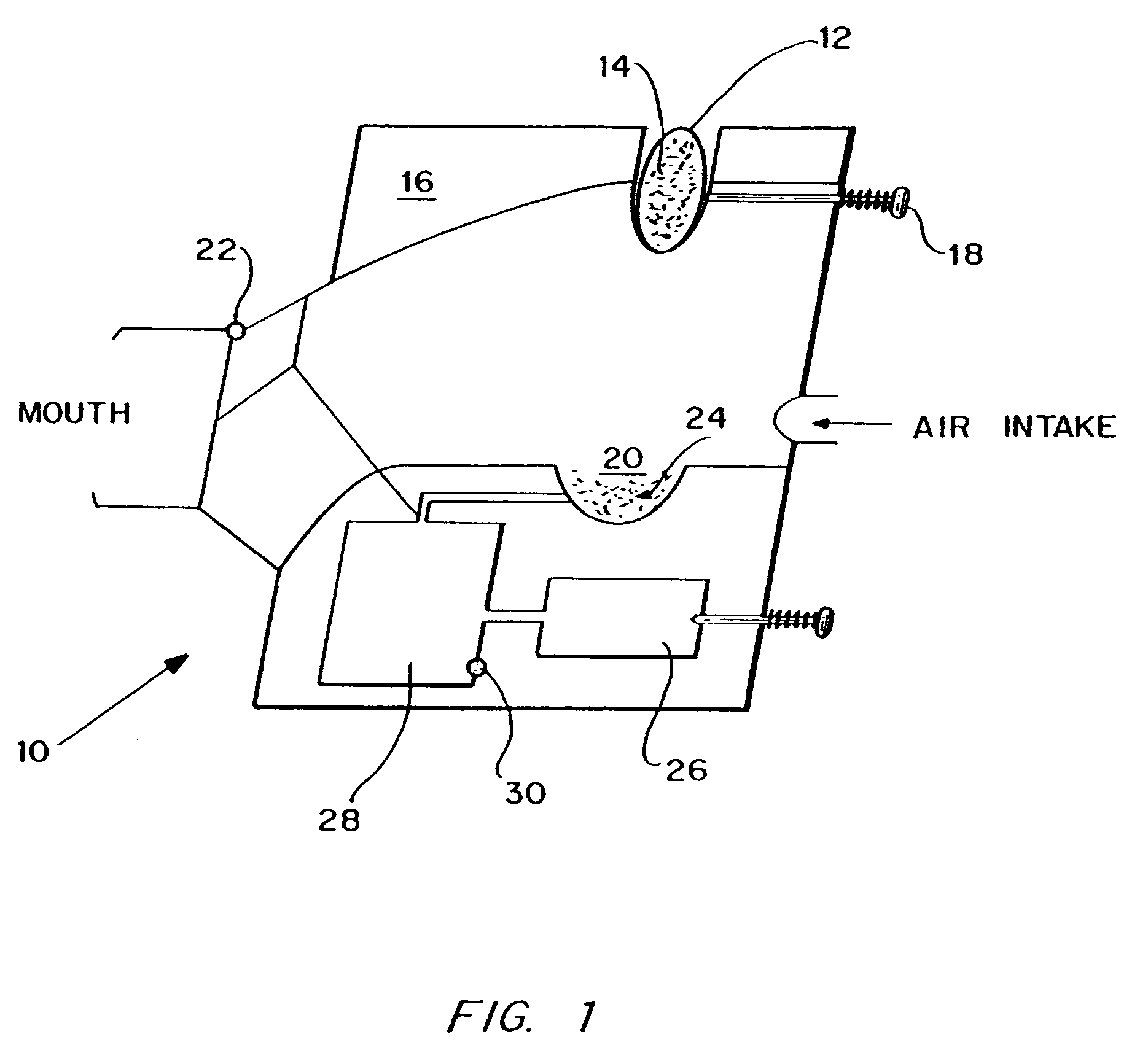
An apparatus and method for transdermally delivering a biologically active agent comprising a delivery system having a microprojection member (or system) that includes a plurality of microprojections (or array thereof) that are adapted to pierce through the stratum corneum into the underlying epidermis layer, or epidermis and dermis layers. In one embodiment, the PTH-based agent is contained in a biocompatible coating that is applied to the microprojection member.
Owner:ALZA CORP
Aerosolized active agent delivery
InactiveUS20050090798A1Improve bioavailabilityImprove the immunityFactor VIIPowder deliveryInspiratory flowActive agent
The present invention is directed to methods and devices for delivering an active agent formulation to the lung of a human patient. The active agent formulation may be in dry powder form, it may be nebulized, or it may be in admixture with a propellant. The active agent formulation is delivered to a patient at an inspiratory flow rate of less than 17 liters per minute. The bioavailability of the active agent was found to increase at these flow rates when compared to inspiratory flow rates of 17 liters per minute or more.
Owner:NOVARTIS FARMA
Fast-acting oral peptide pharmaceutical products
ActiveUS8093207B2Increase delivery speedImprove bioavailabilityTetrapeptide ingredientsSkeletal disorderActive agentDrug product
Owner:ENTERIS BIOPHARMA
Stable formulations for parenteral injection of peptide drugs
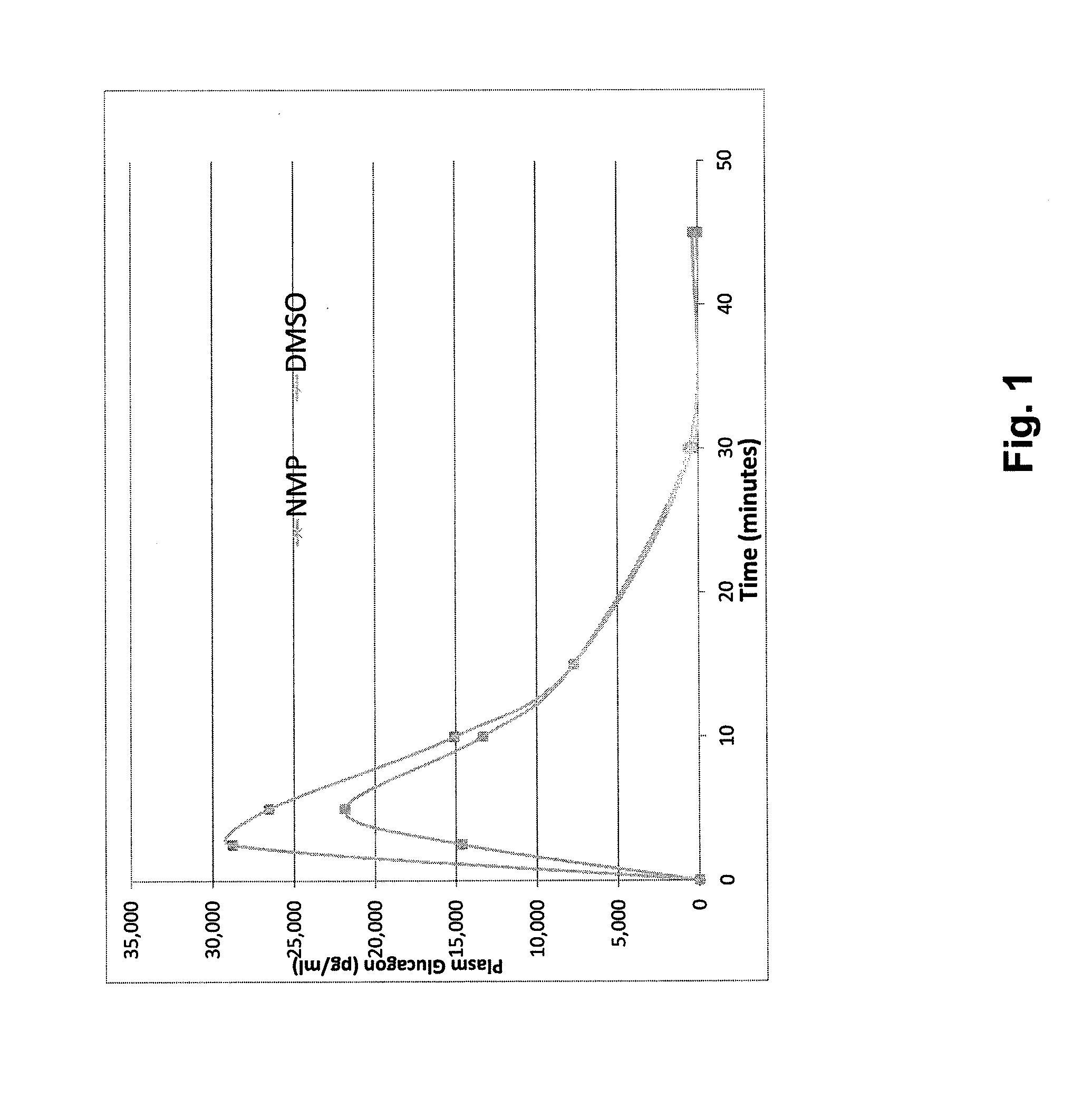
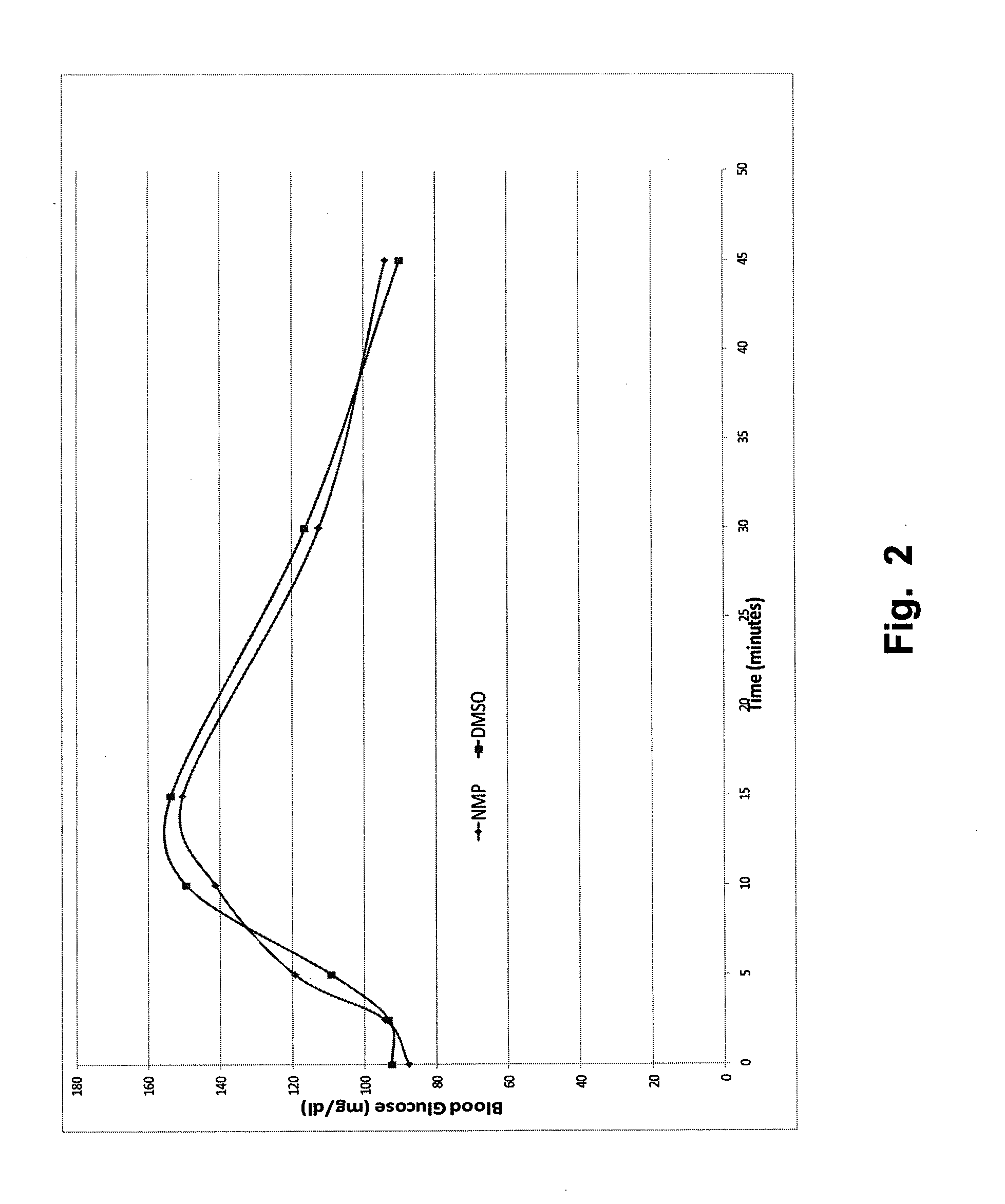
Stable formulations for parenteral injection of peptide drugs and methods of using such stable formulations are provided. In particular, the present invention provides stable formulations for parenteral injection of glucagon and methods of using such glucagon formulations to treat hypoglycemia, especially severe hypoglycemia in emergency situations.
Owner:XERIS PHARMA
Fast-acting oral peptide pharmaceutical products
ActiveUS20070134279A1Reduce likelihood of proteolytic degradationProtection attackTetrapeptide ingredientsSkeletal disorderActive agentBioavailability
Owner:ENTERIS BIOPHARMA
Stable pharmaceutical dosage forms of teriparatide
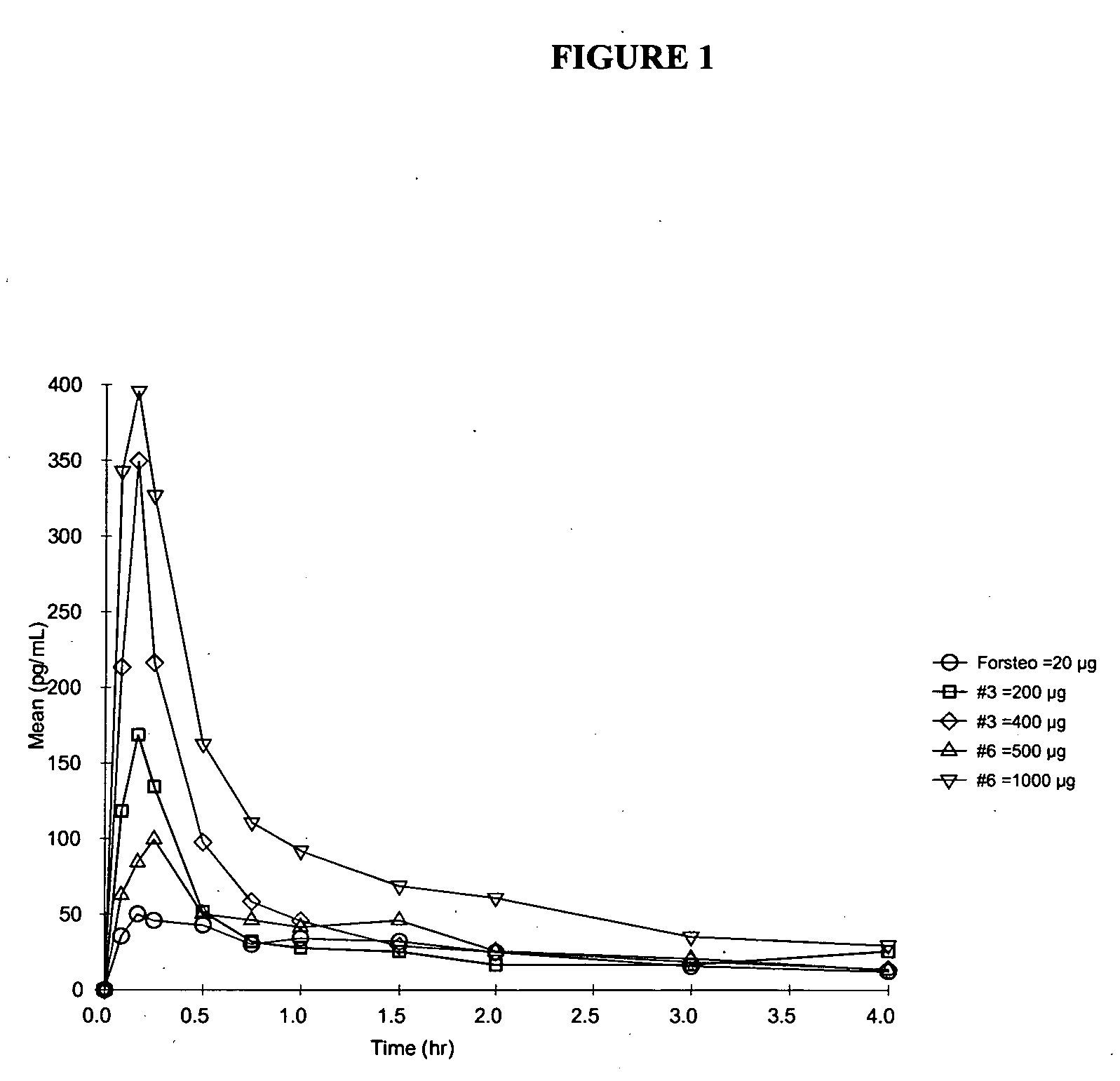
InactiveUS20060189533A1Low viscosityReduce adhesionPeptide/protein ingredientsPharmaceutical delivery mechanismTeriparatideBioavailability
A parathyroid hormone (1-34) (PTH) dosage form is described that is suitable for multi-use administration. A dosage form of parathyroid hormone (1-34) (PTH) comprising an aqueous pharmaceutical formulation for aerosolized intranasal delivery of PTH having a bioavailability of about 5% or greater, wherein the formulation comprises a therapeutically effective amount of PTH and polysorbate, and wherein least 90% of the PTH can be recovered after storage for 24 weeks at 5° C.
Owner:NASTECH PHARMA
Method for drug delivery to the pulmonary system
Drug delivery to the pulmonary system has been achieved by encapsulation of the drug to be delivered in microparticles having a size range between 0.5 and ten microns, preferably in the range of two to five microns, formed of a material releasing drug at a pH of greater than 6.4. In a preferred embodiment, the drug delivery system is based on the formation of diketopiperazine microparticles which are stable at a pH of 6.4 or less and unstable at pH of greater than 6.4, or which are stable at both acidic and basic pH, but which are unstable at pH between about 6.4 and 8. Other types of materials can also be used, including biodegradable natural and synthetic polymers, such as proteins, polymers of mixed amino acids (proteinoids), alginate, and poly(hydroxy acids). In another embodiment, the microparticles have been modified to effect targeting to specific cell types and to effect release only after reaching the targeted cells.
Owner:MANNKIND CORP
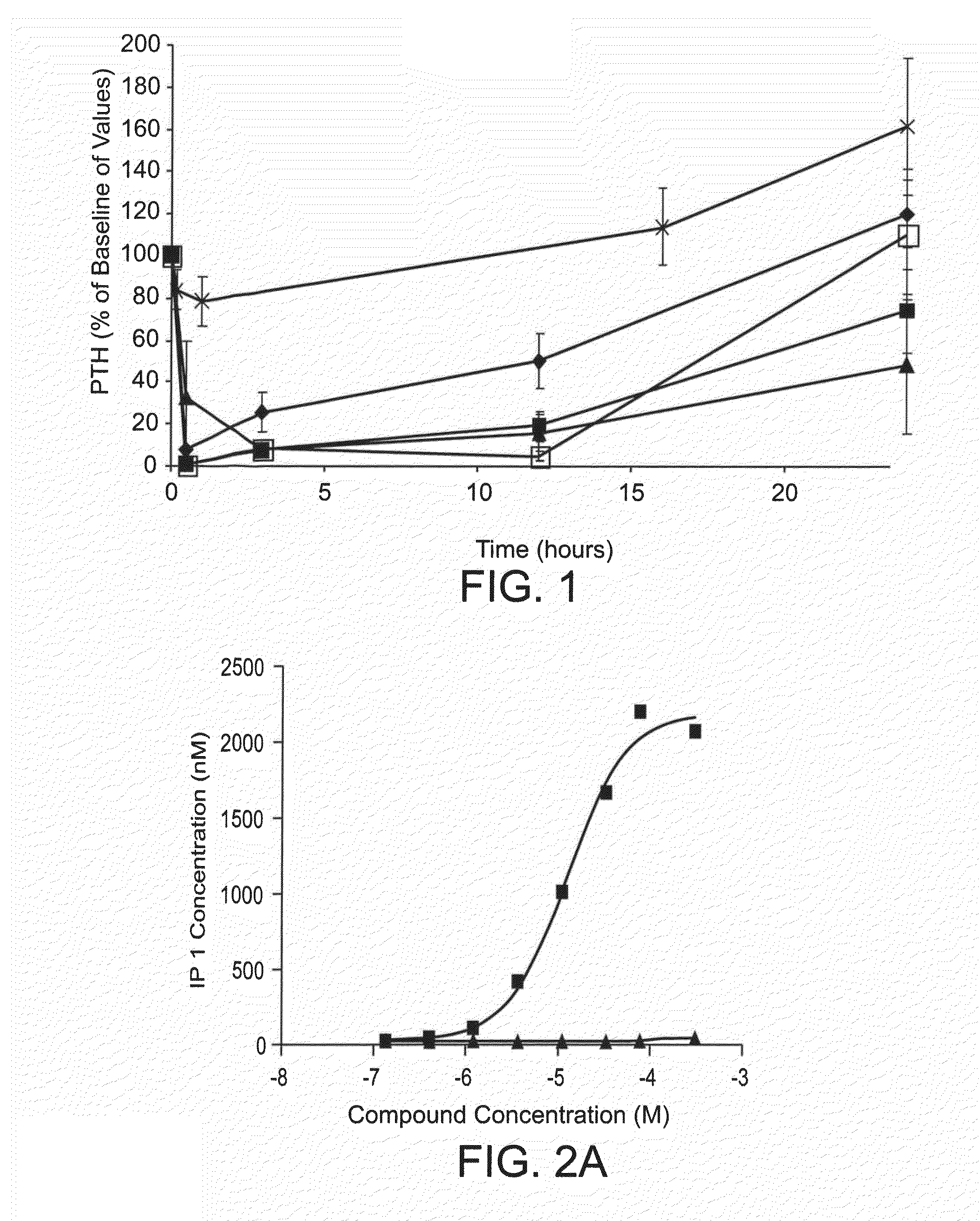
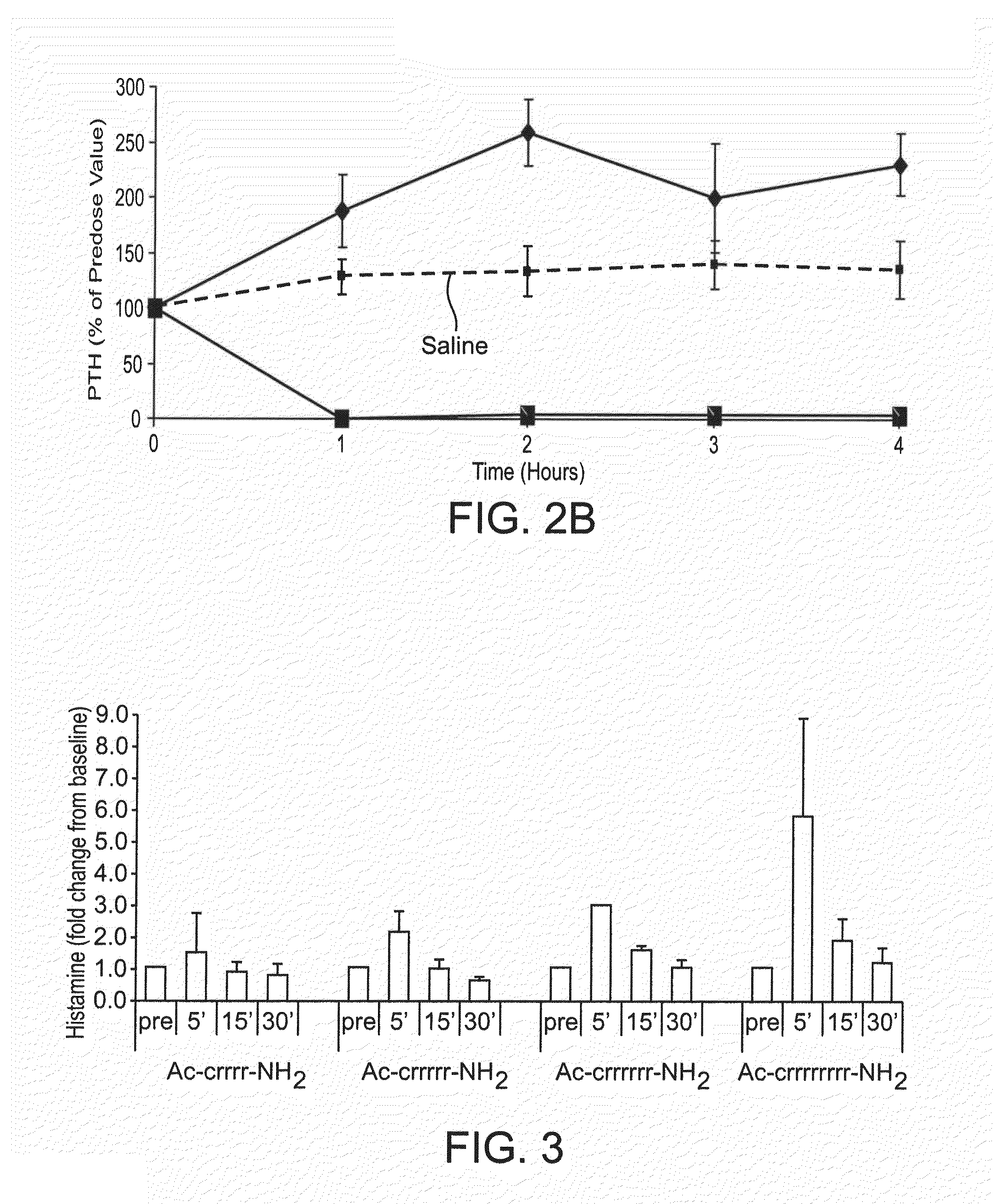
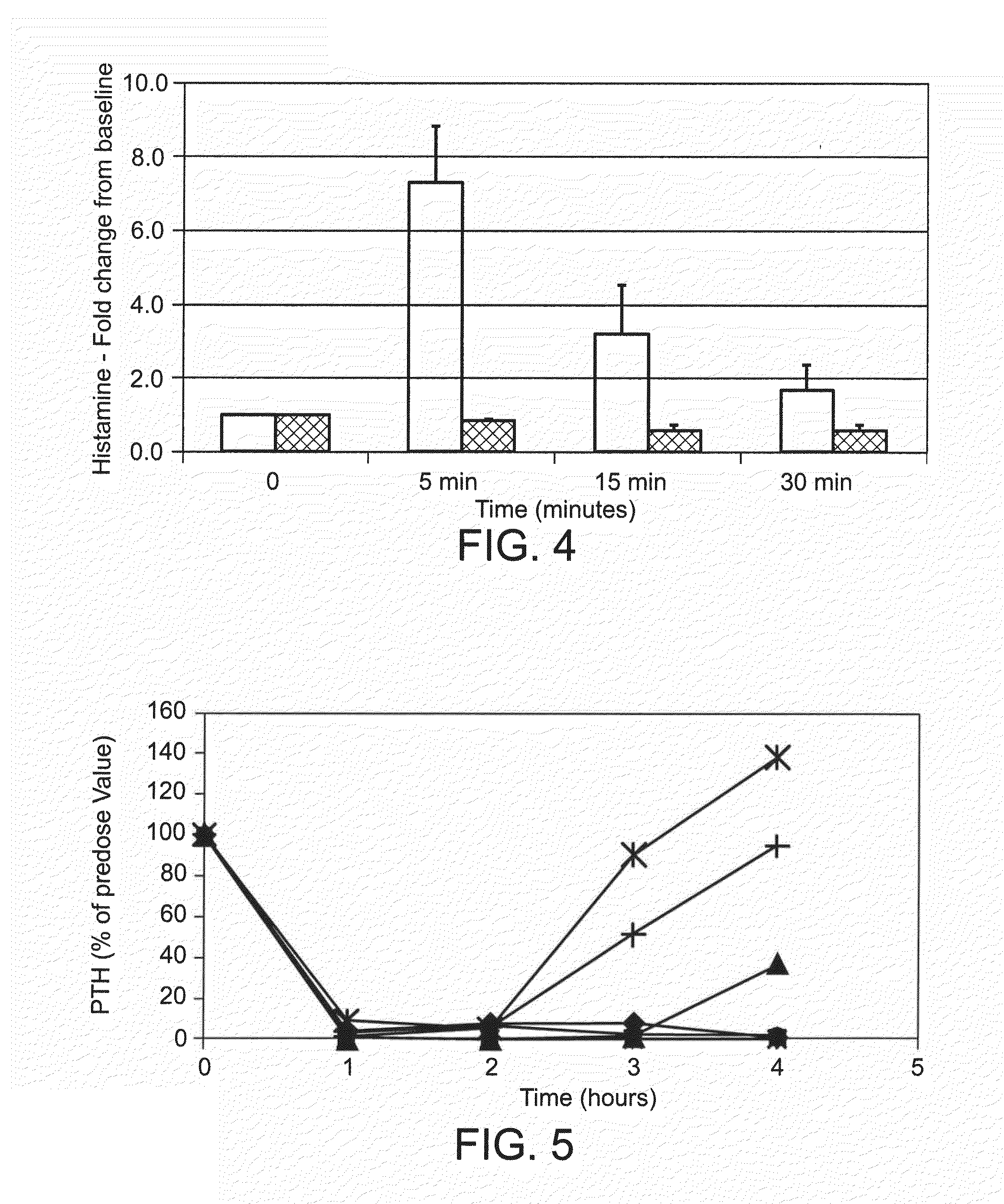
Therapeutic agents for reducing parathyroid hormone levels
ActiveUS20110028394A1Easy to transportReduce deliveryOrganic active ingredientsPeptide/protein ingredientsSerum igeThiol
Compounds having activity for lowering parathyroid hormone levels are described. In one embodiment, the compounds are comprised of a contiguous sequence of subunits, X1-X2-X3-X4-X5-X6-X7, wherein the X1 subunit comprises a thiol-containing moiety and the distribution of charge on the X2-X7 subunits provides the desired activity. Methods of using the compounds for treating hyperparathyroidism, bone disease and / or hypercalcemic disorders are also described, and in particular, methods for lowering plasma PTH and serum calcium are provided. The compounds can be used to treat subjects having, for example: primary, secondary or tertiary hyperparathyroidism; hypercalcemia of malignancy; metastatic bone disease; or osteoporosis.
Owner:KAI PHARMA
Method for promoting bone growth
InactiveUS20050037089A1Avoid defectsPromote bone growthBiocidePeptide/protein ingredientsDietary supplementBone remodeling
A method for treating a patient to promote bone growth, comprising: administering to the patient a therapeutic amount of a bone remodeling agent effective to stimulate bone growth, and a dietary supplement comprising calcium and phosphorus, in a therapeutic amount effective to prevent a deficiency in the amount of calcium or phosphorus available for bone growth.
Owner:RHONDIA INC
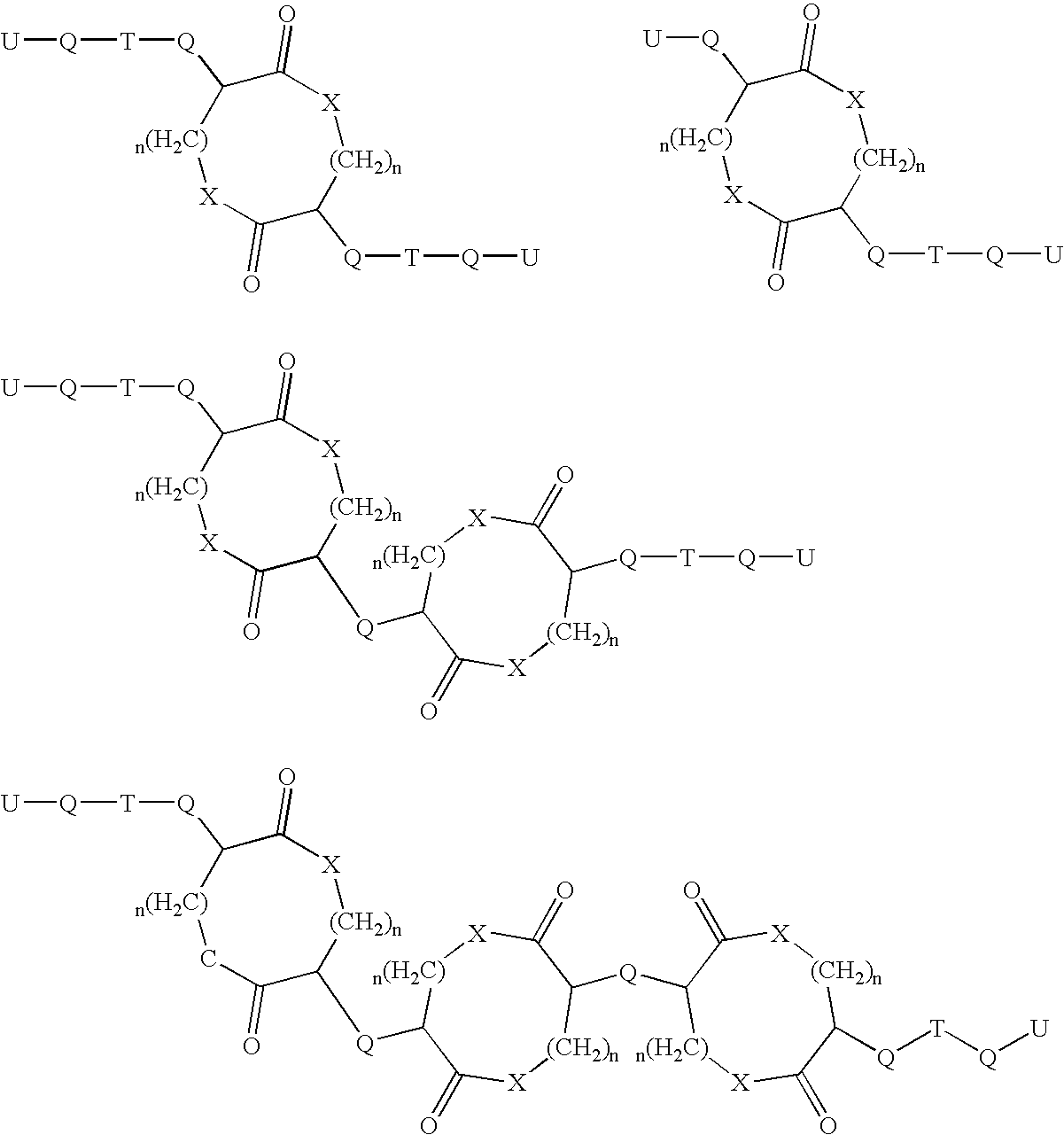
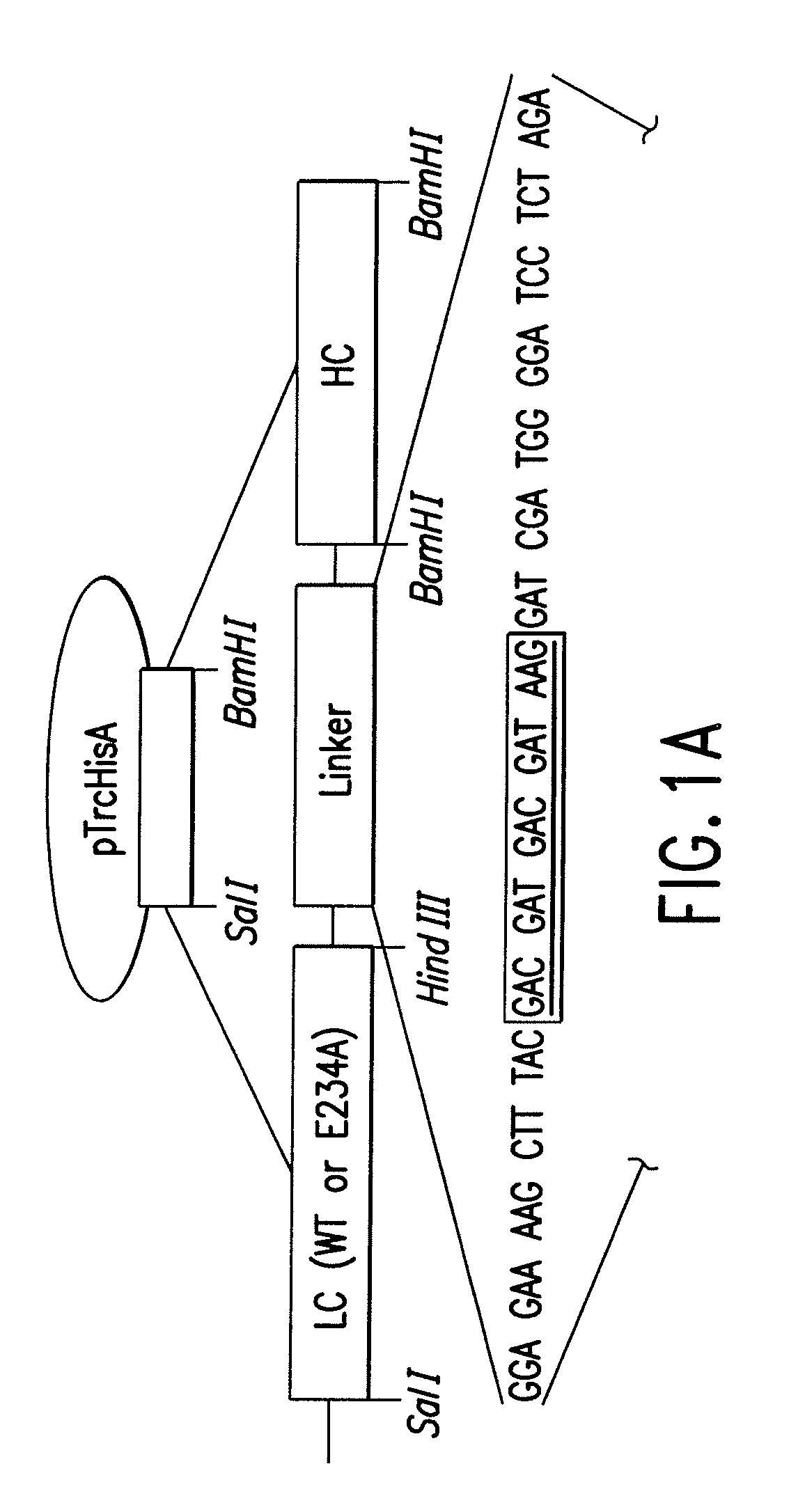
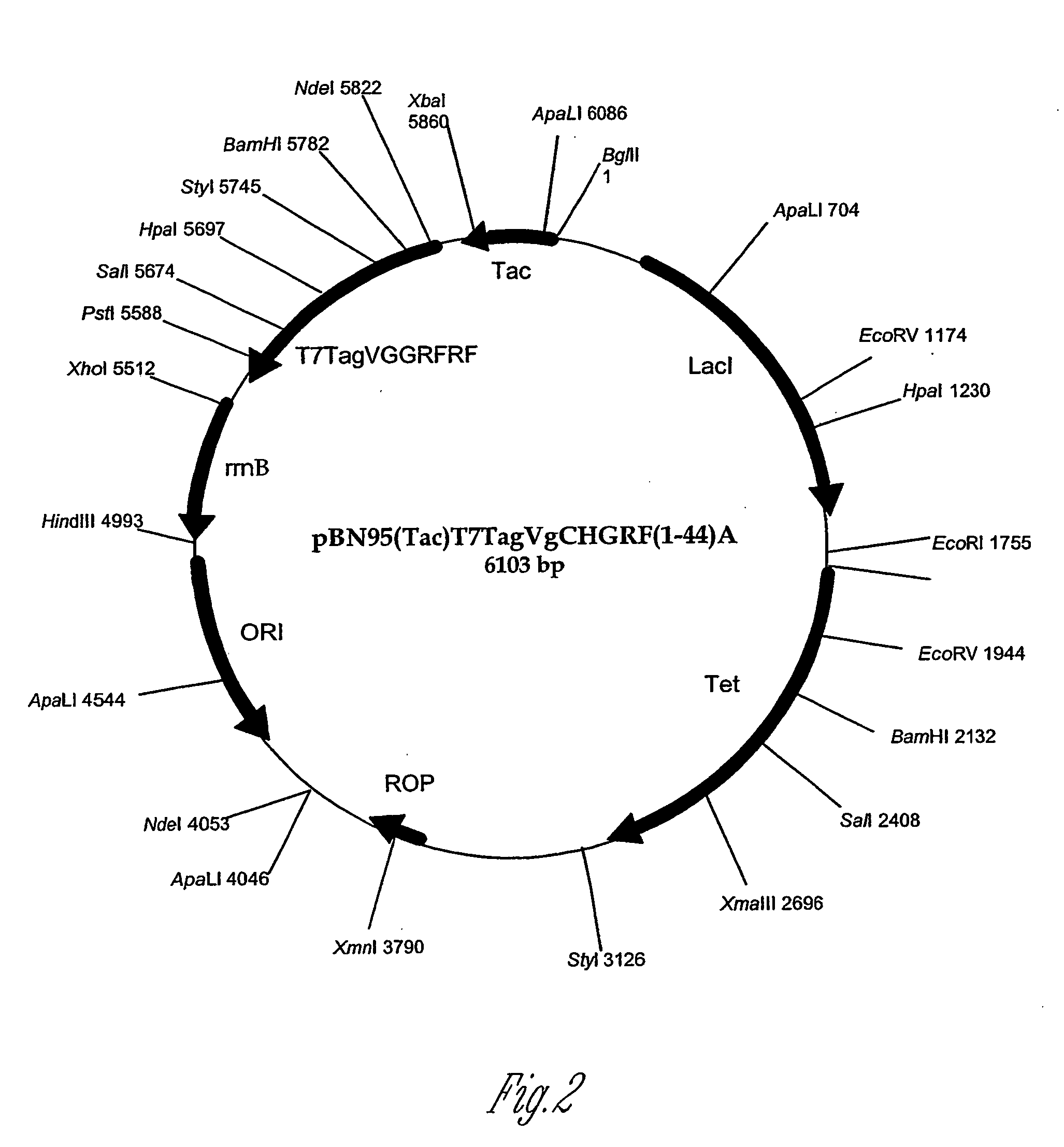
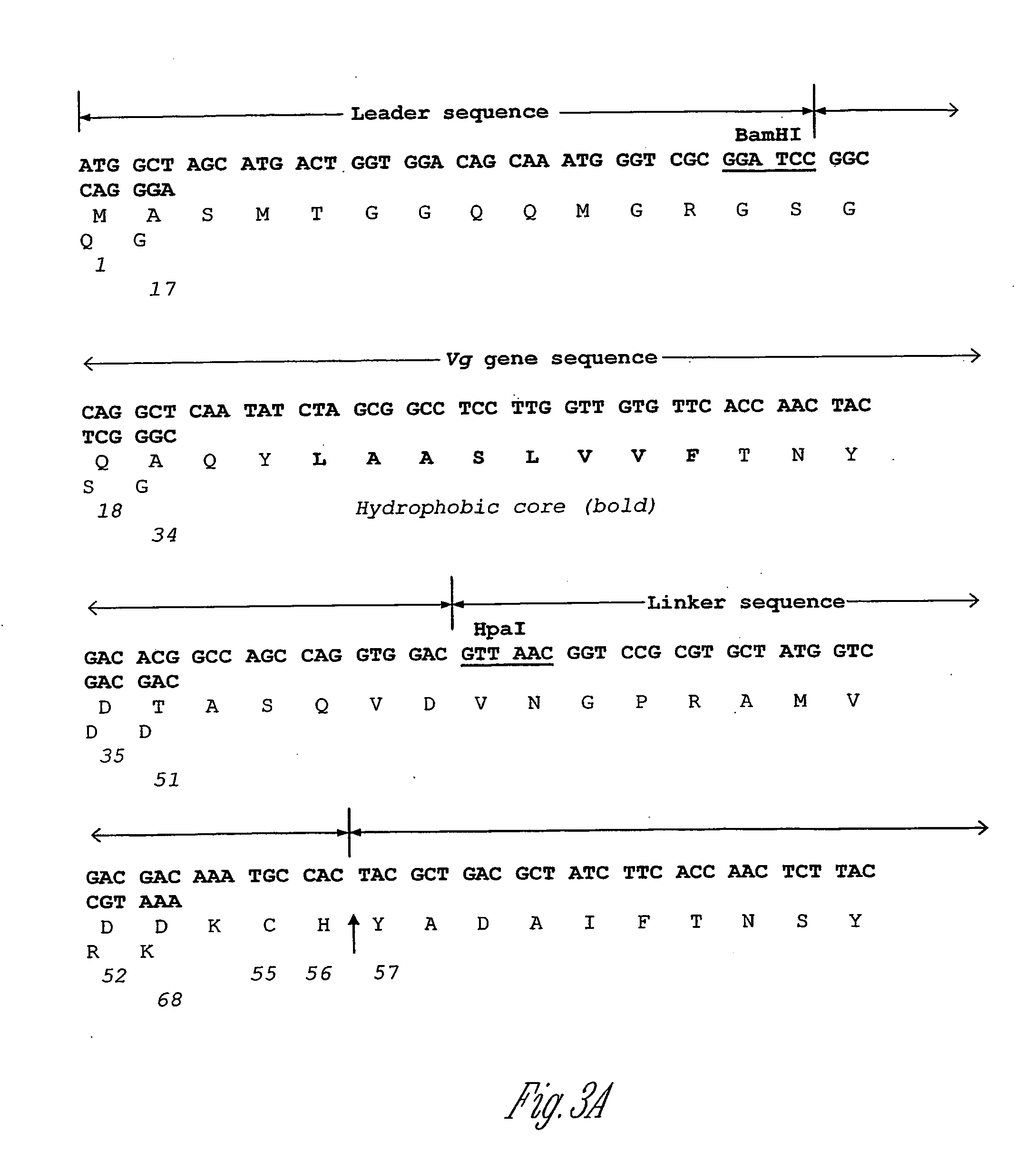
PTH functional domain conjugate peptides, derivatives thereof and novel tethered ligand-receptor molecules
InactiveUS7057012B1Improve efficacyHigh potencyPeptide/protein ingredientsImmunoglobulinsAgonist drugsPharmaceutical drug
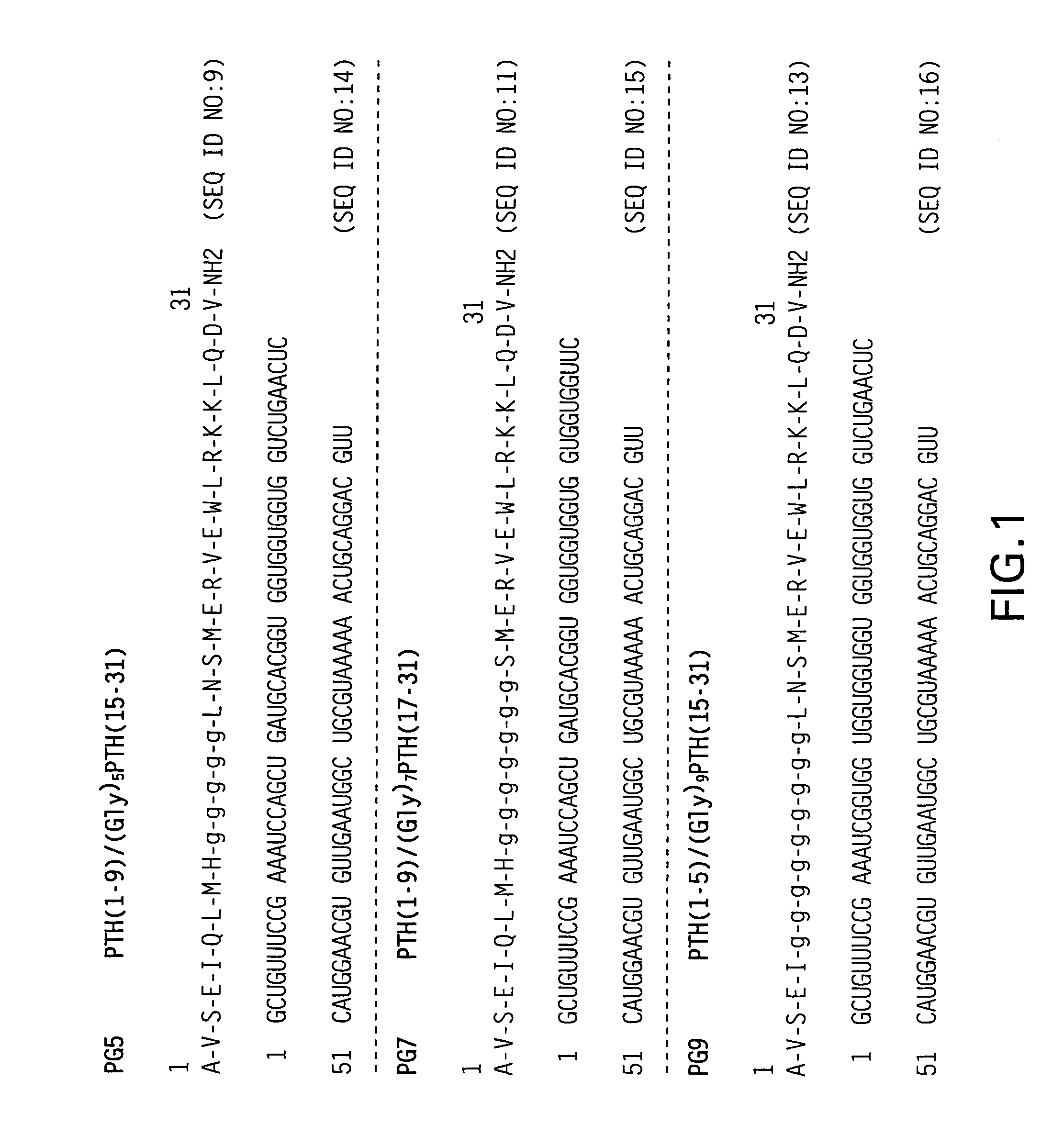
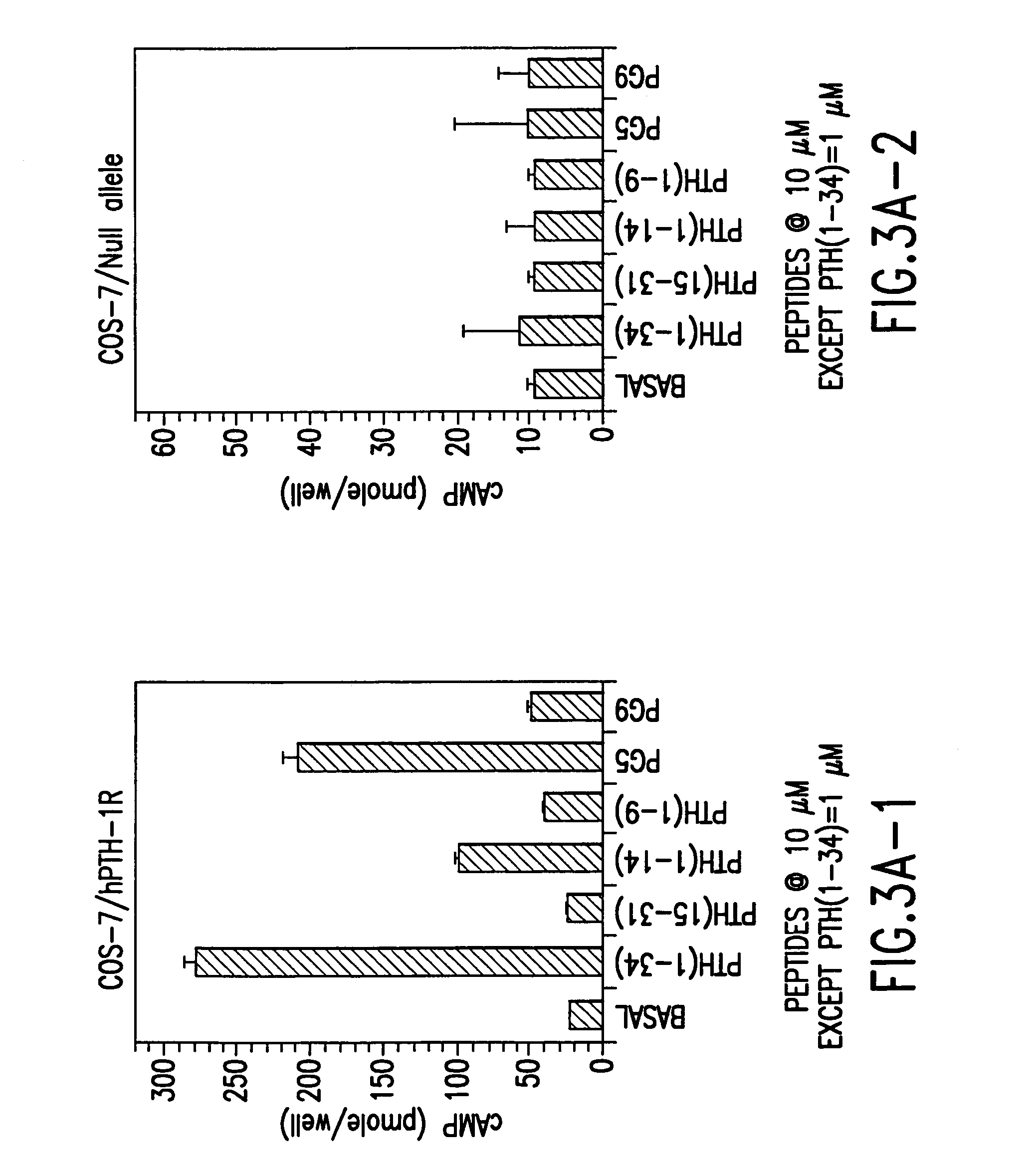
Novel parathyroid hormone (PTH) peptides and analogs thereof of the PTH(1–34) fragment are disclosed that combine the N-terminal signaling domain (residues 1–9) and the C-terminal binding domain (residues 15–31) via a linker. Nucleic acid molecules and peptides for PTH(1–9)-(Gly)5-PTH(15–31) (PG5) and (1–9)-(Gly)7-PTH(15–31) and a novel PTH receptor are disclosed. Additionally, methods of screening for PTH agonists, pharmaceutical compositions and methods of treatment are disclosed.
Owner:THE GENERAL HOSPITAL CORP
Methods and DNA constructs for high yield production of polypeptides
ActiveUS20050221444A1Improve usabilityImprove purification effectPeptide/protein ingredientsTissue cultureInclusion bodiesDNA construct
Owner:MEDTRONIC INC
Connective Tissue Stimulating Peptides
ActiveUS20050288229A1Stimulating development maintenance repairCosmetic preparationsPeptide/protein ingredientsHydroxyprolineDrug
Novel peptides are described which comprise an amino acid motif selected from the group consisting of “PG”, “GP”, “PI” and “IG”and having up to 10 amino acids upstream and / or downstream of the amino acid motif, wherein “P” in the motif is proline or hydroxyproline and the peptide stimulates the development, maintenance and repair of bone, cartilage and associated connective tissue. The invention further relates to pharmaceutical compositions of these peptides, as well as therapeutic and prophylactic uses of such peptides.
Owner:OCTANE ORTHOBIOLOGICS INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com