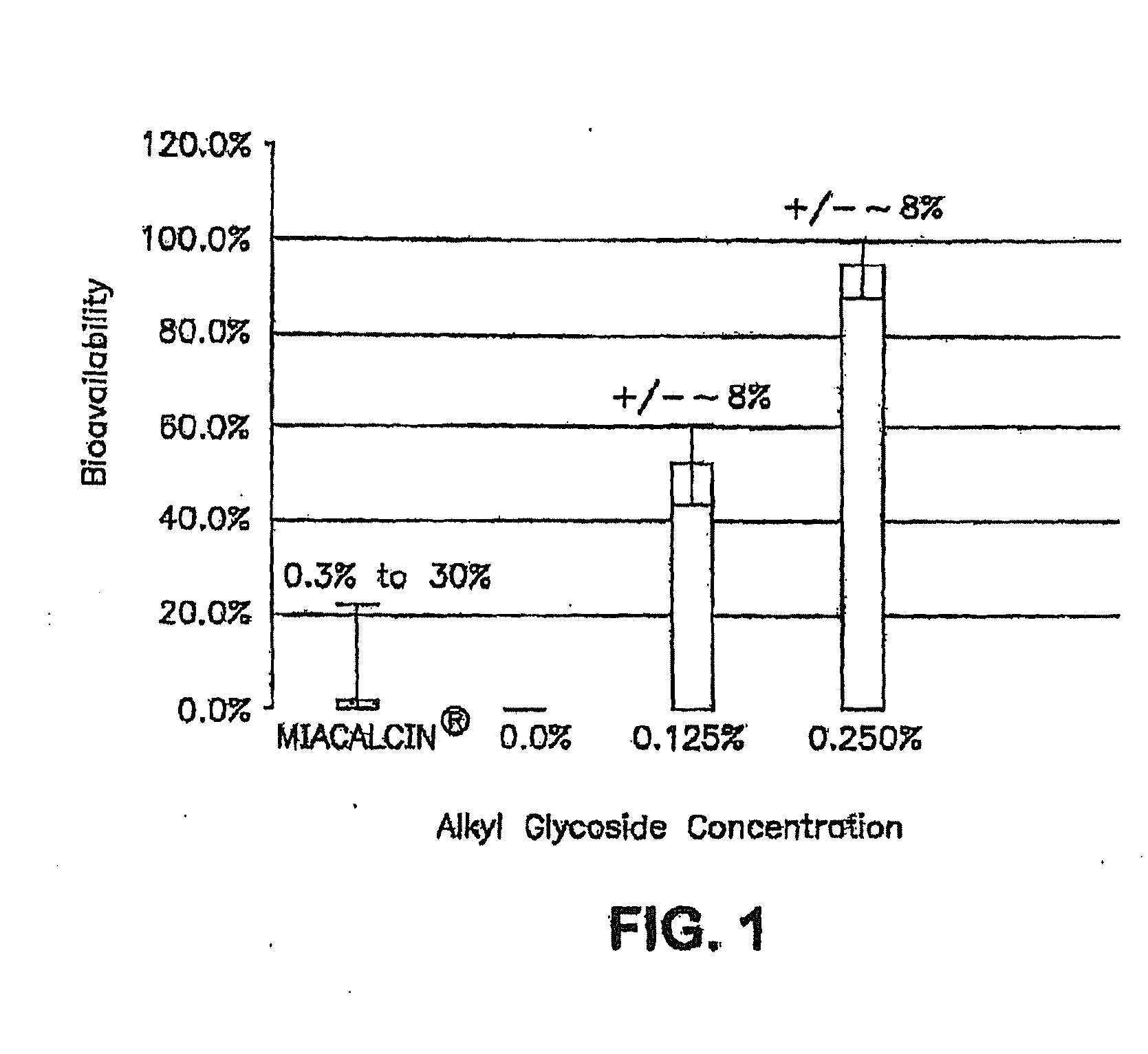
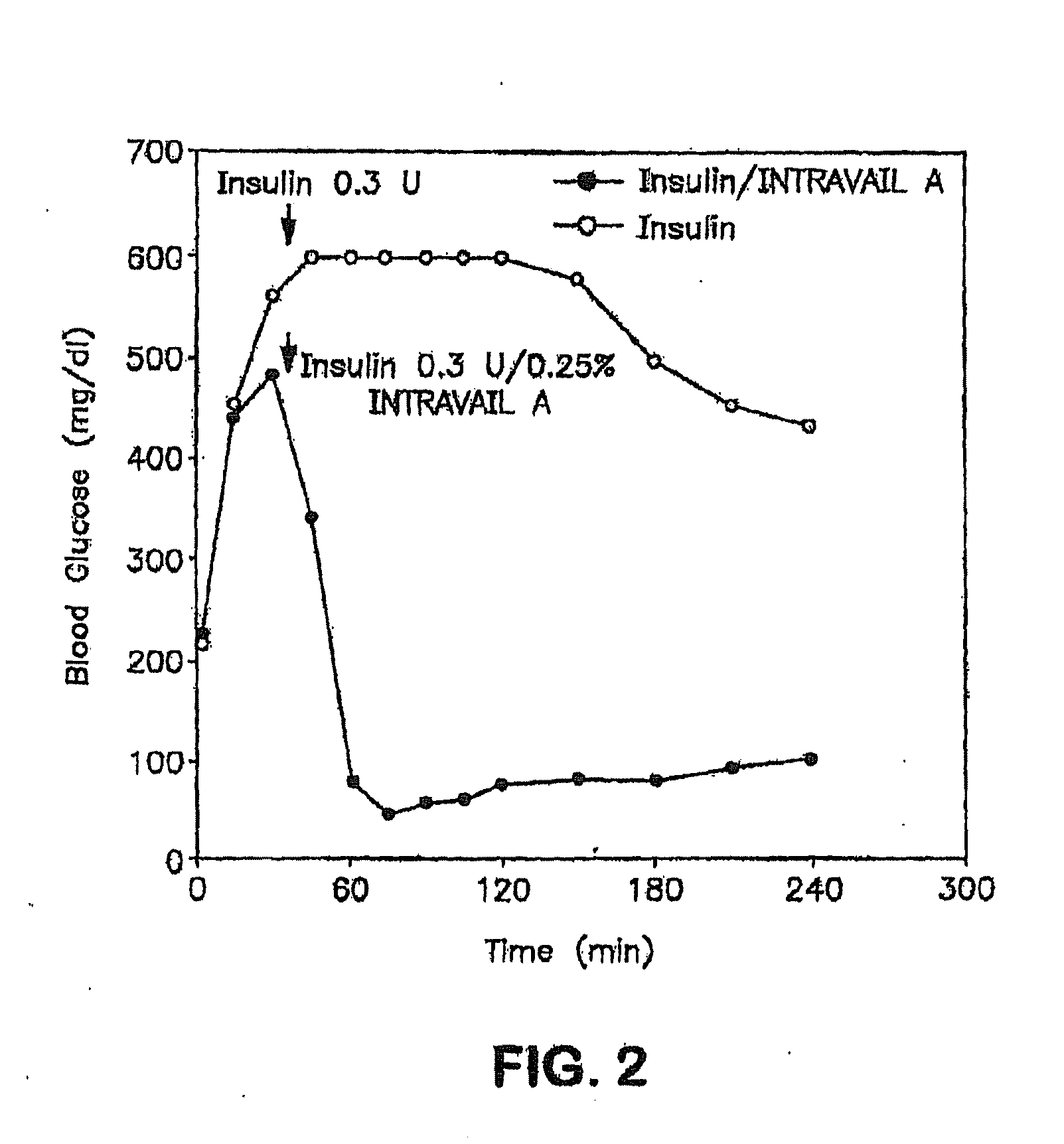
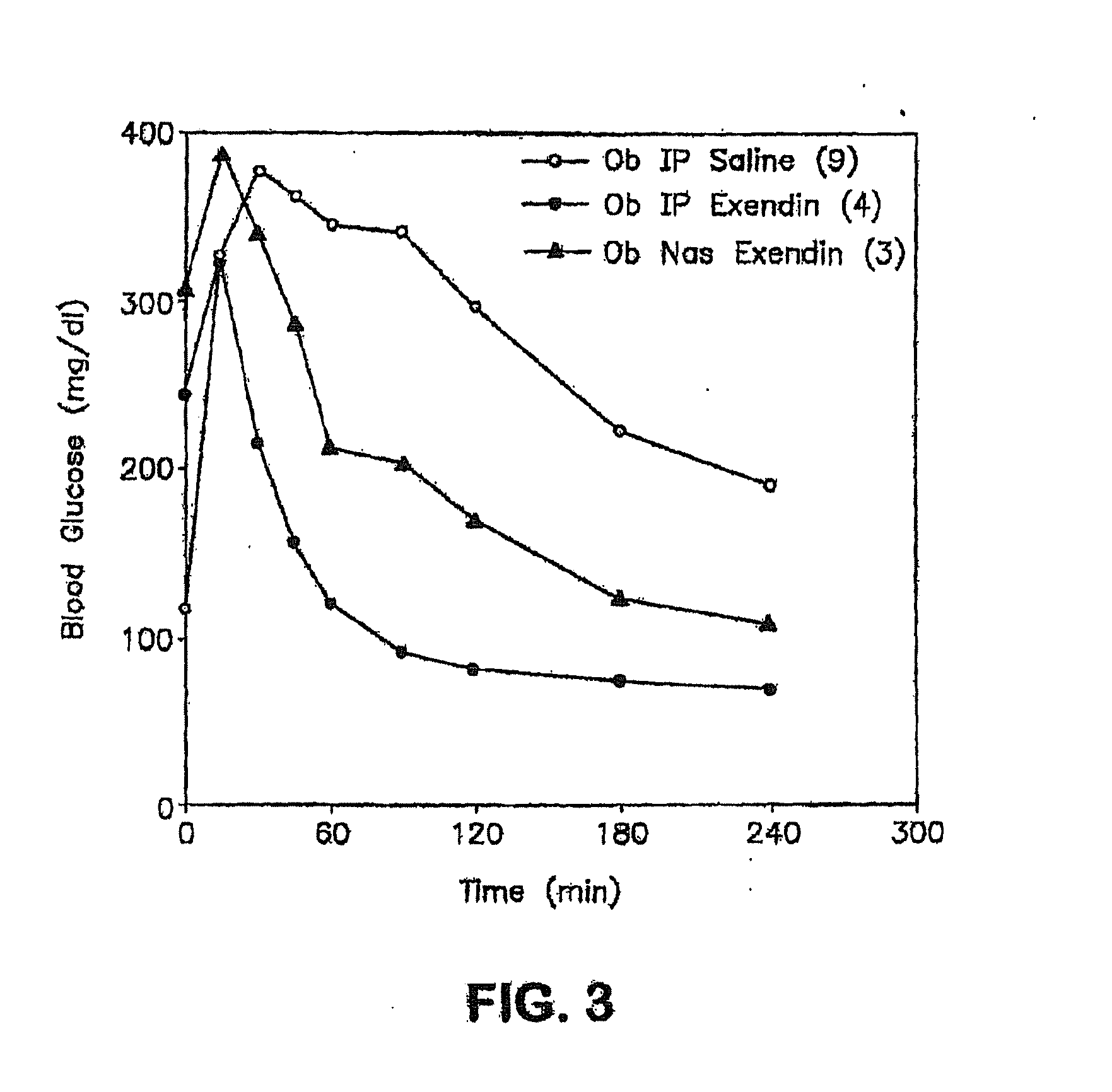
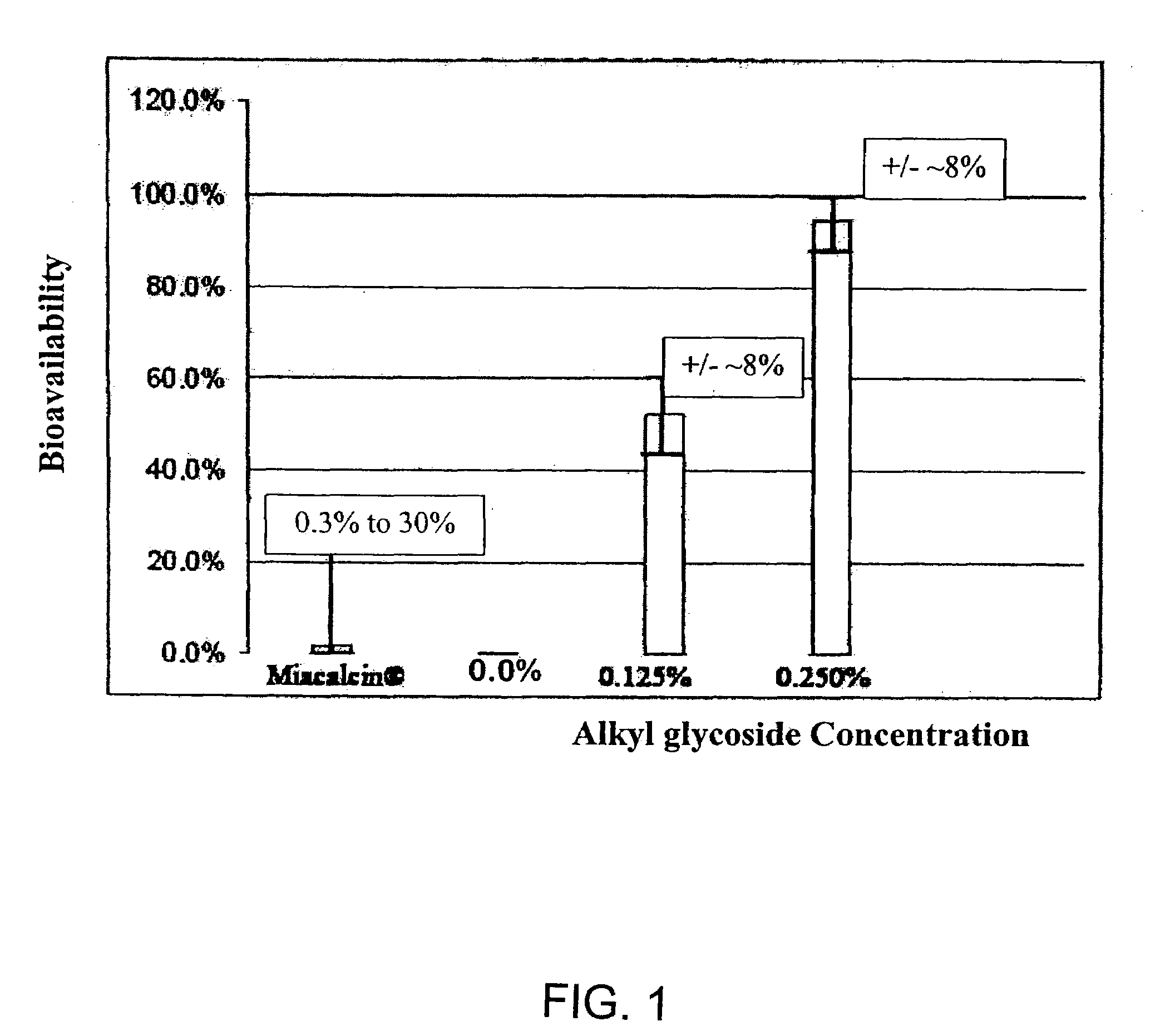
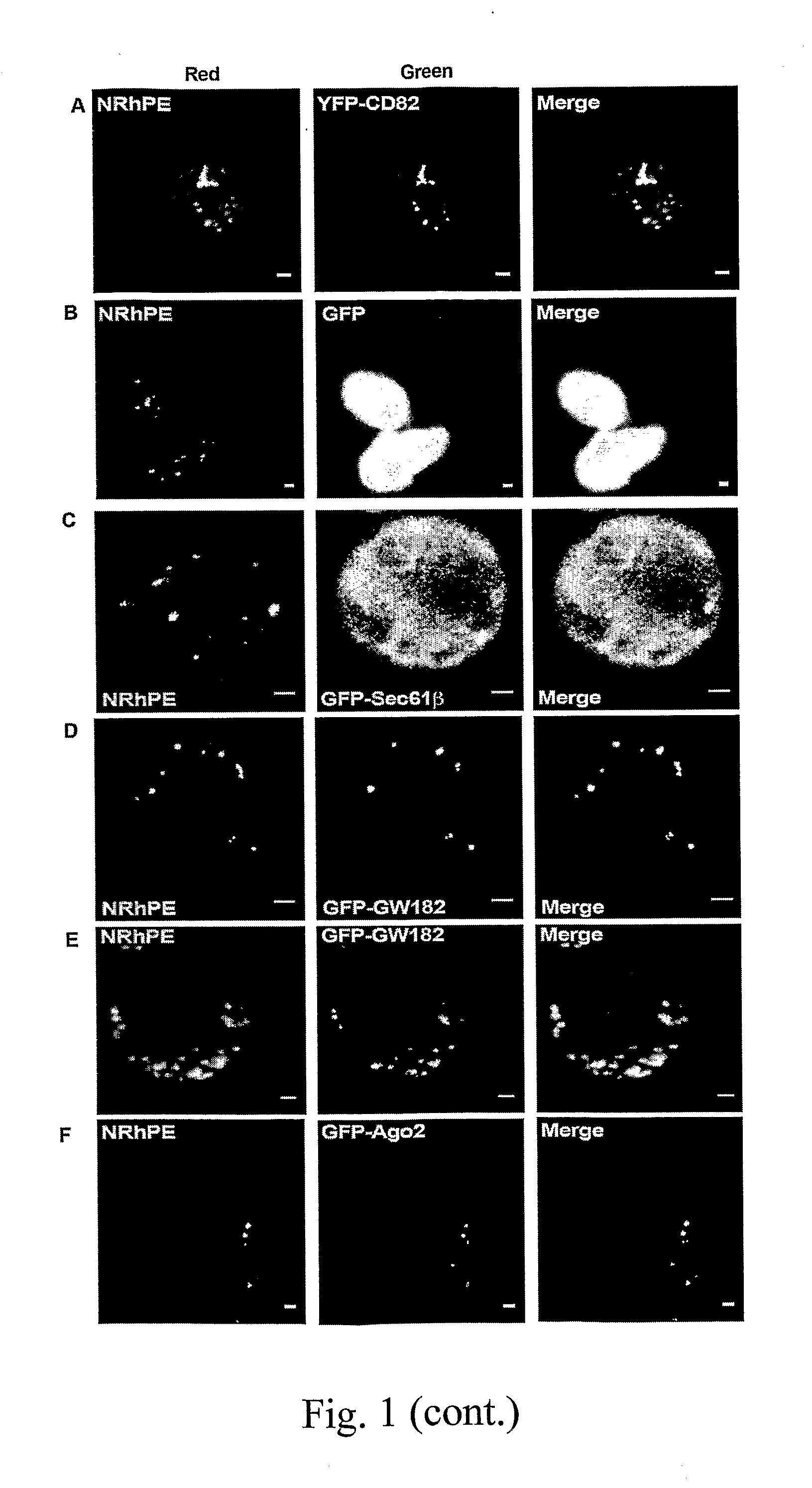
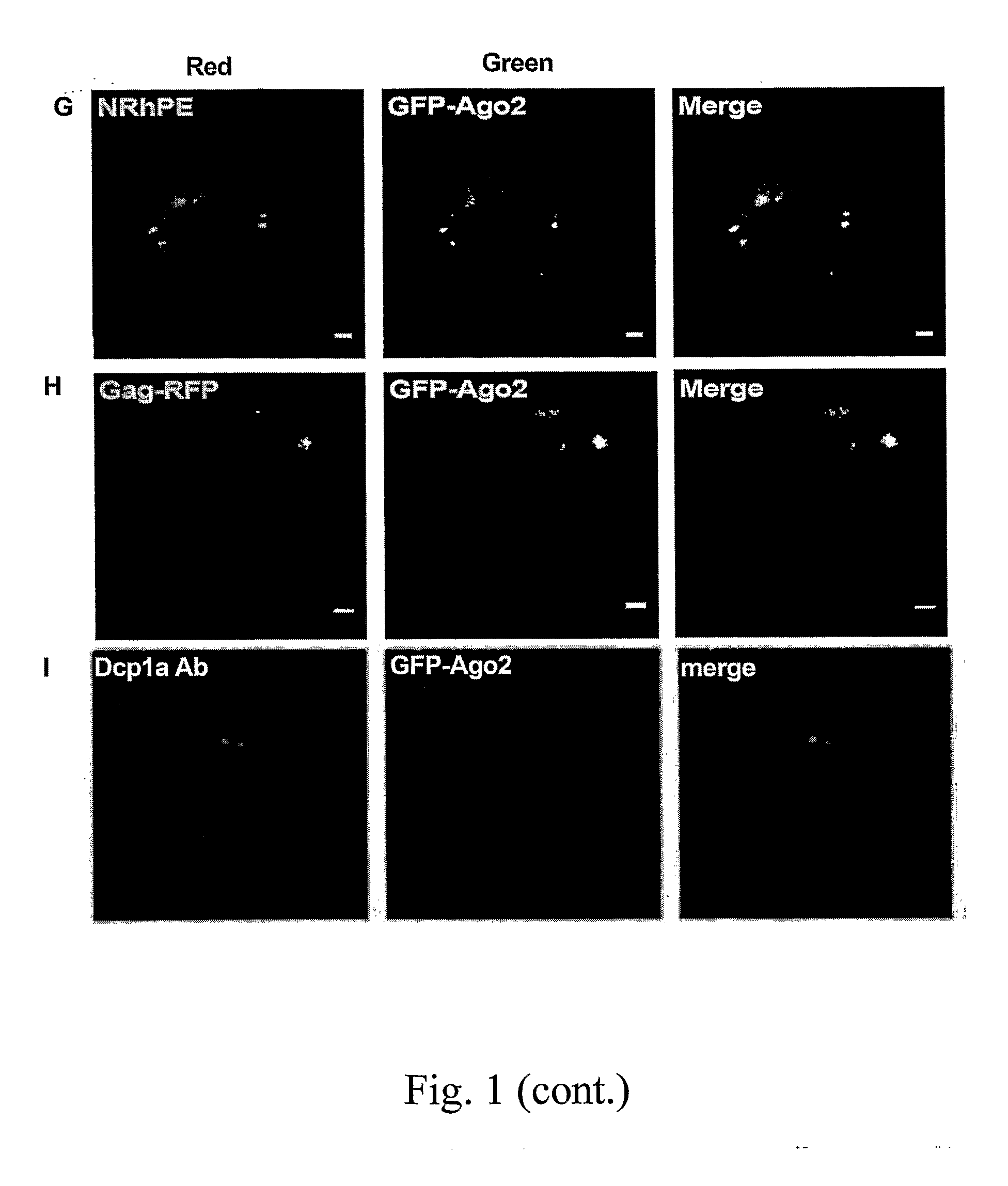
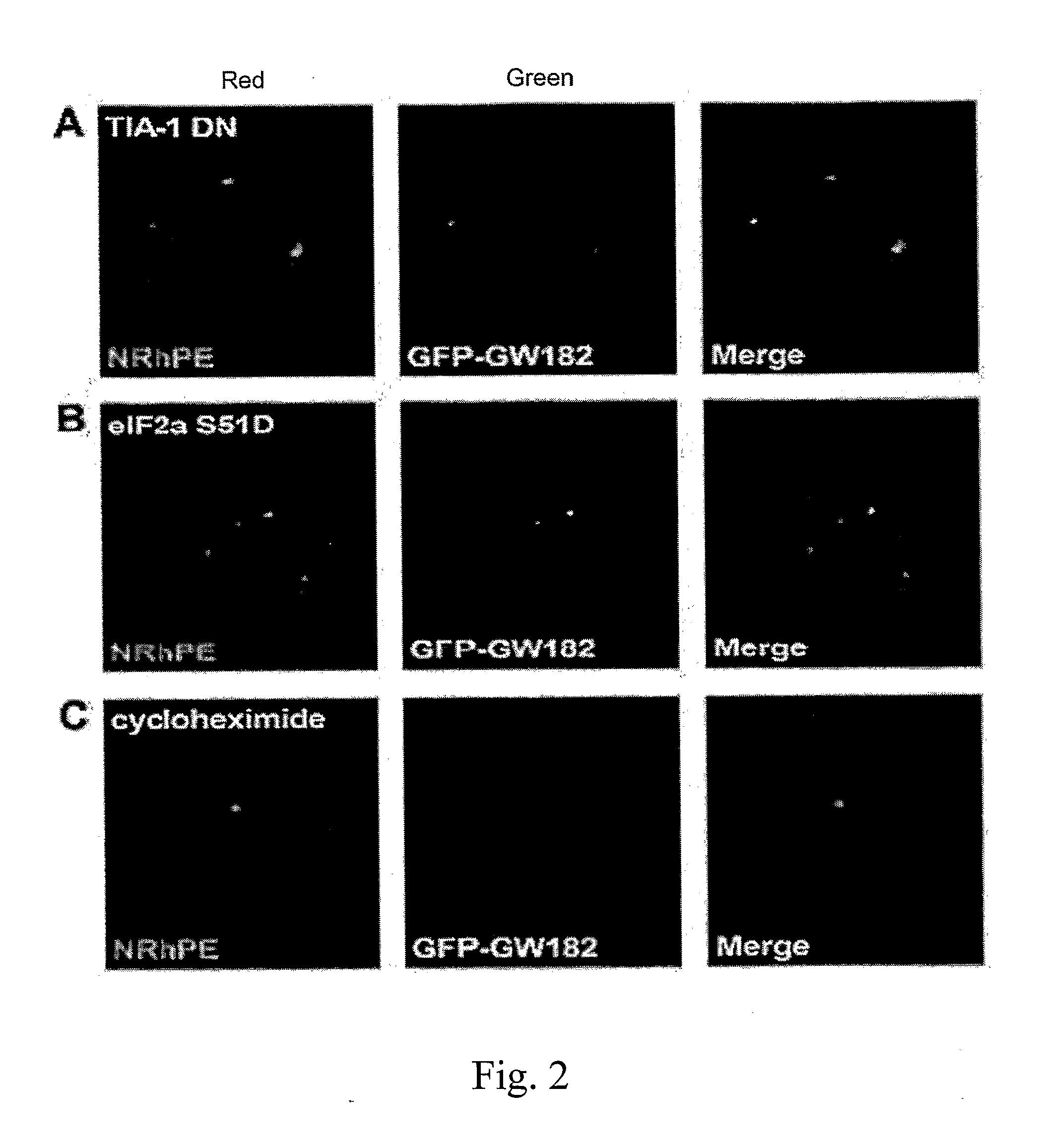
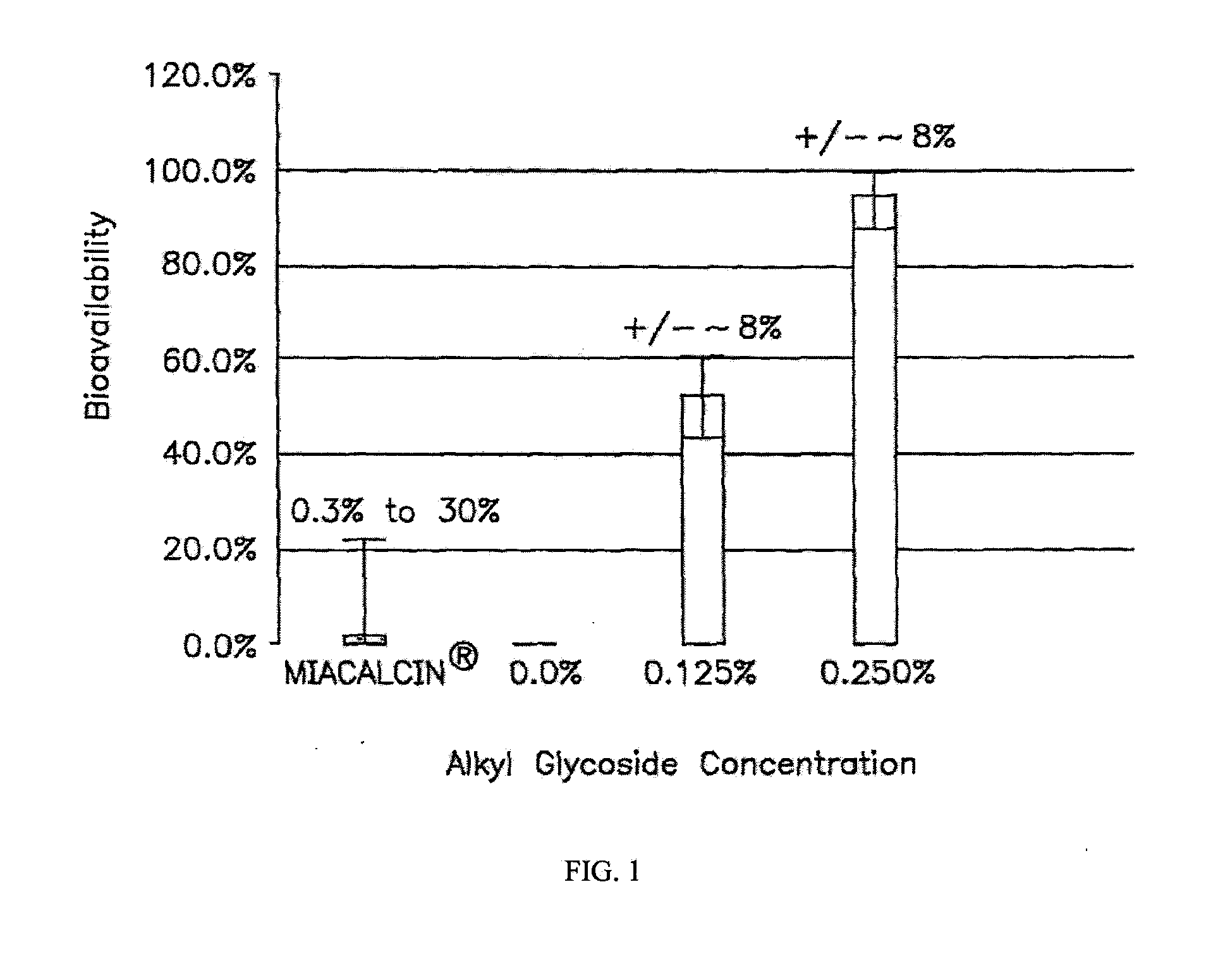
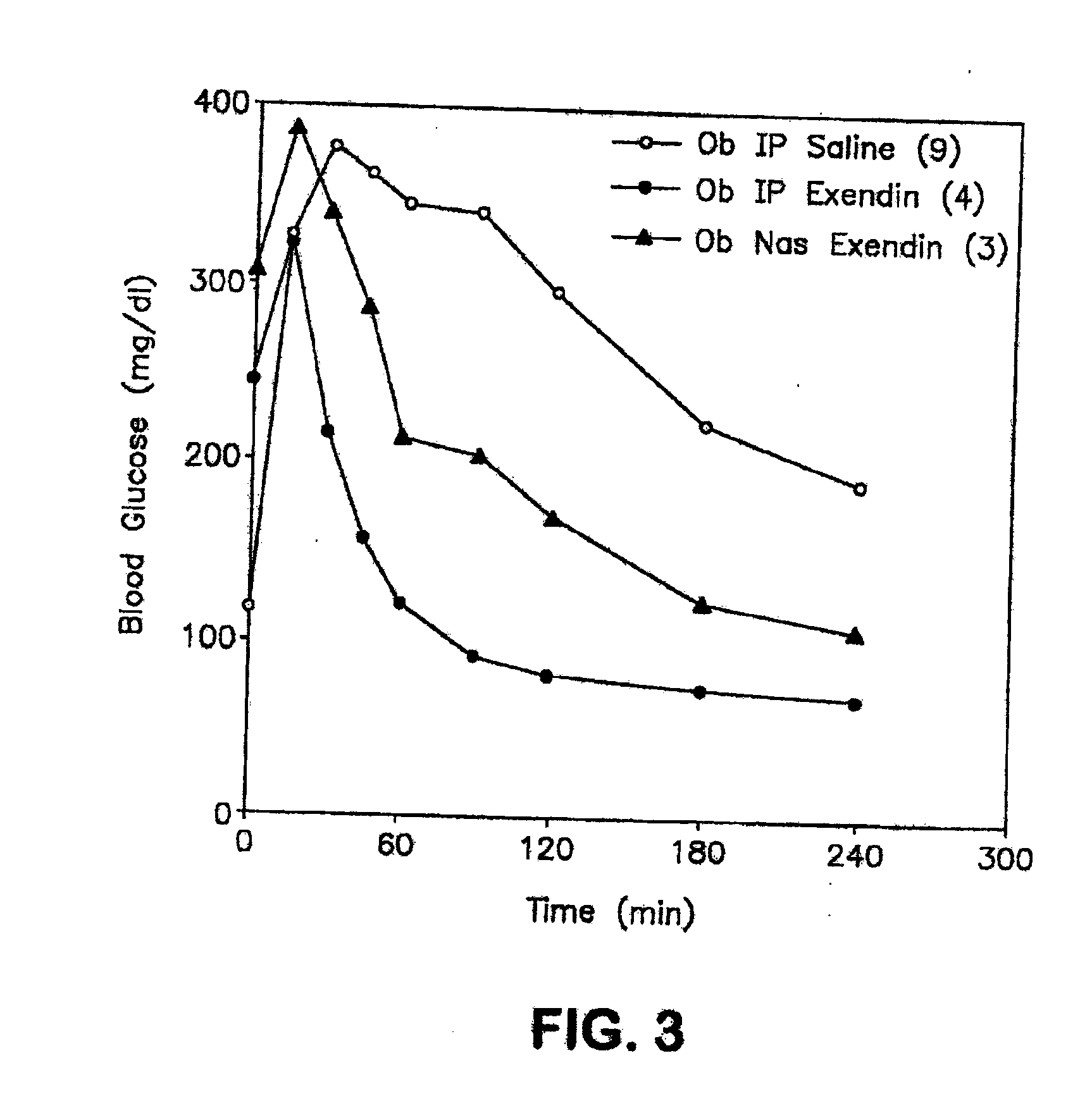
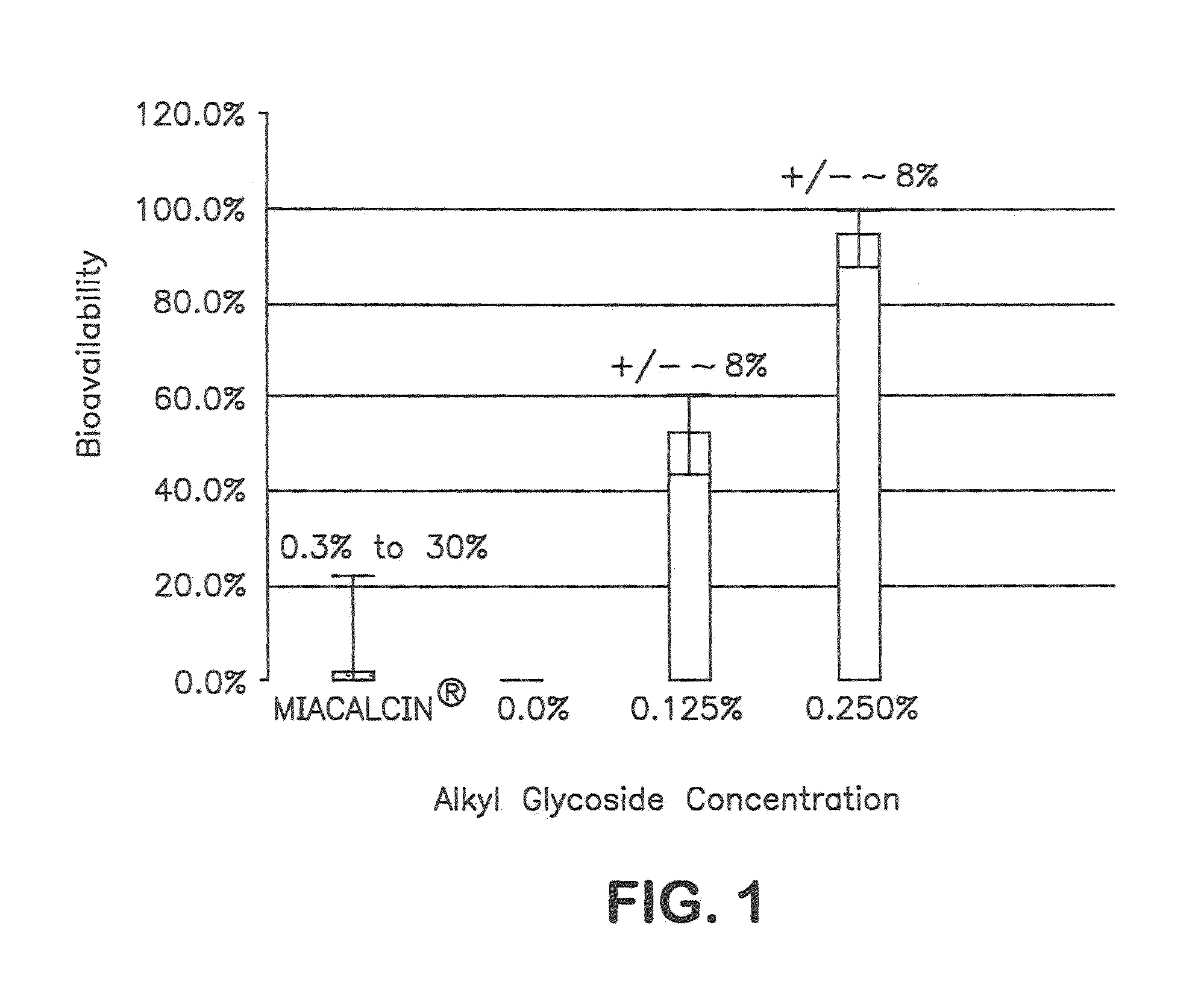
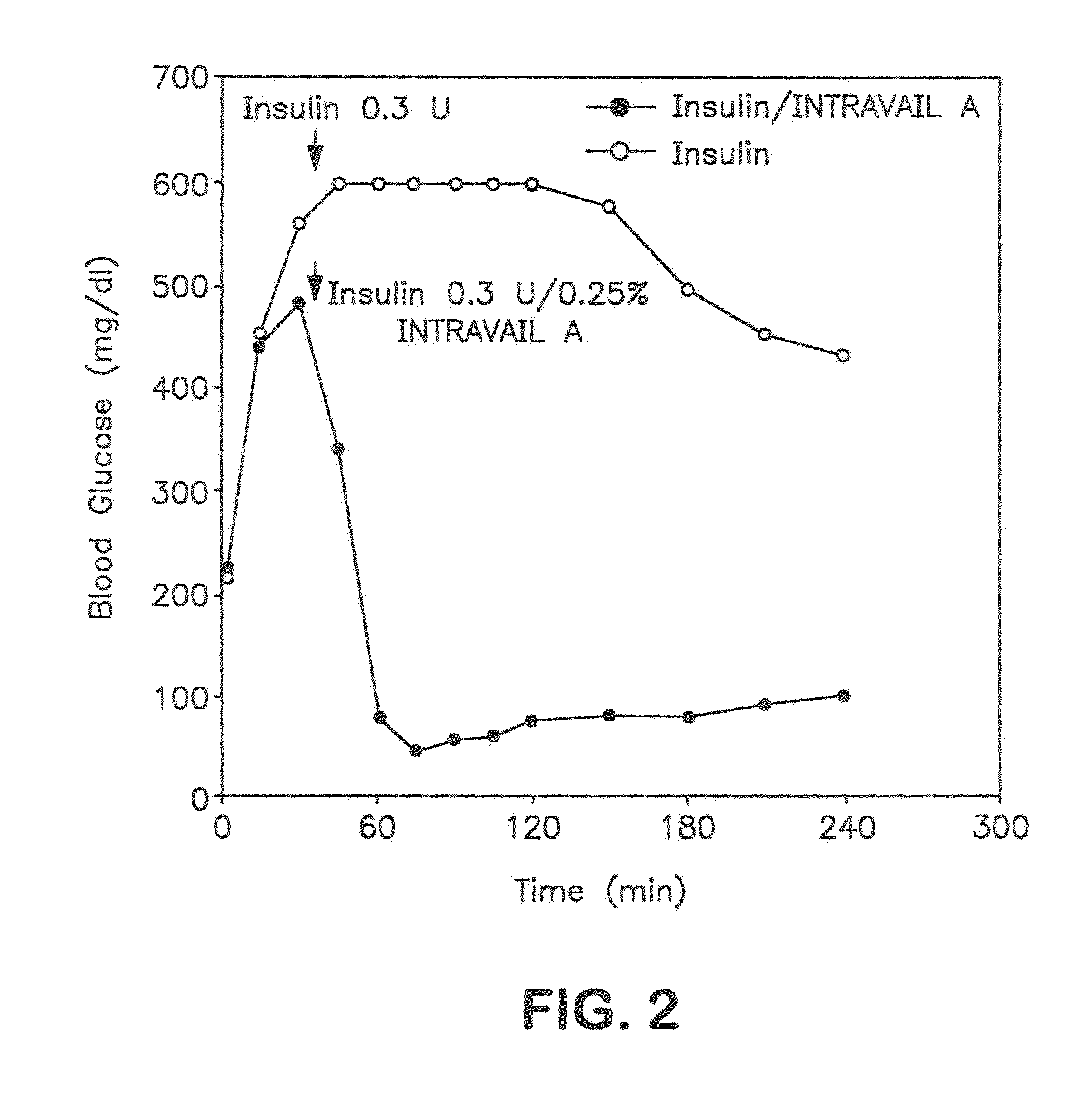
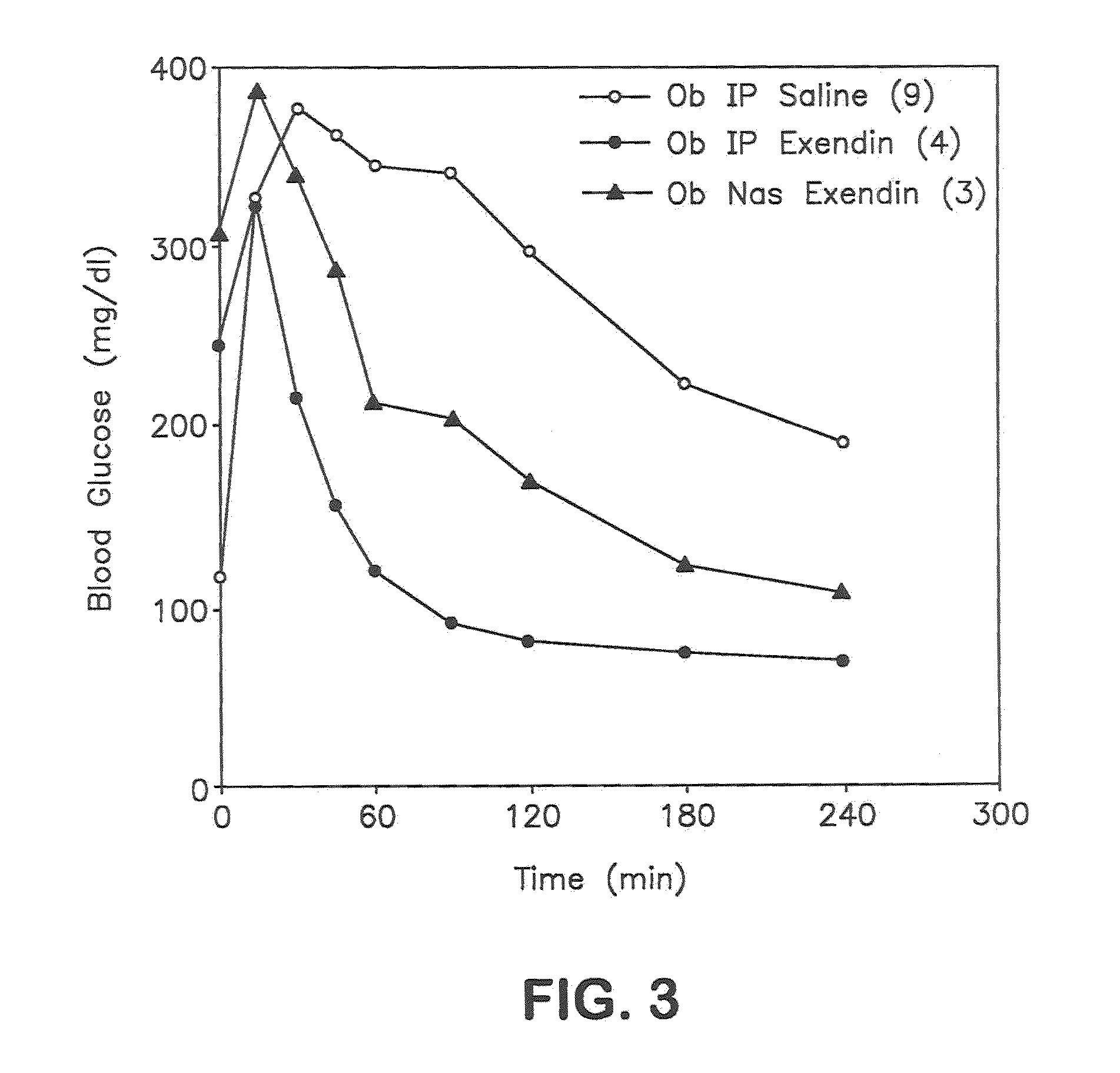
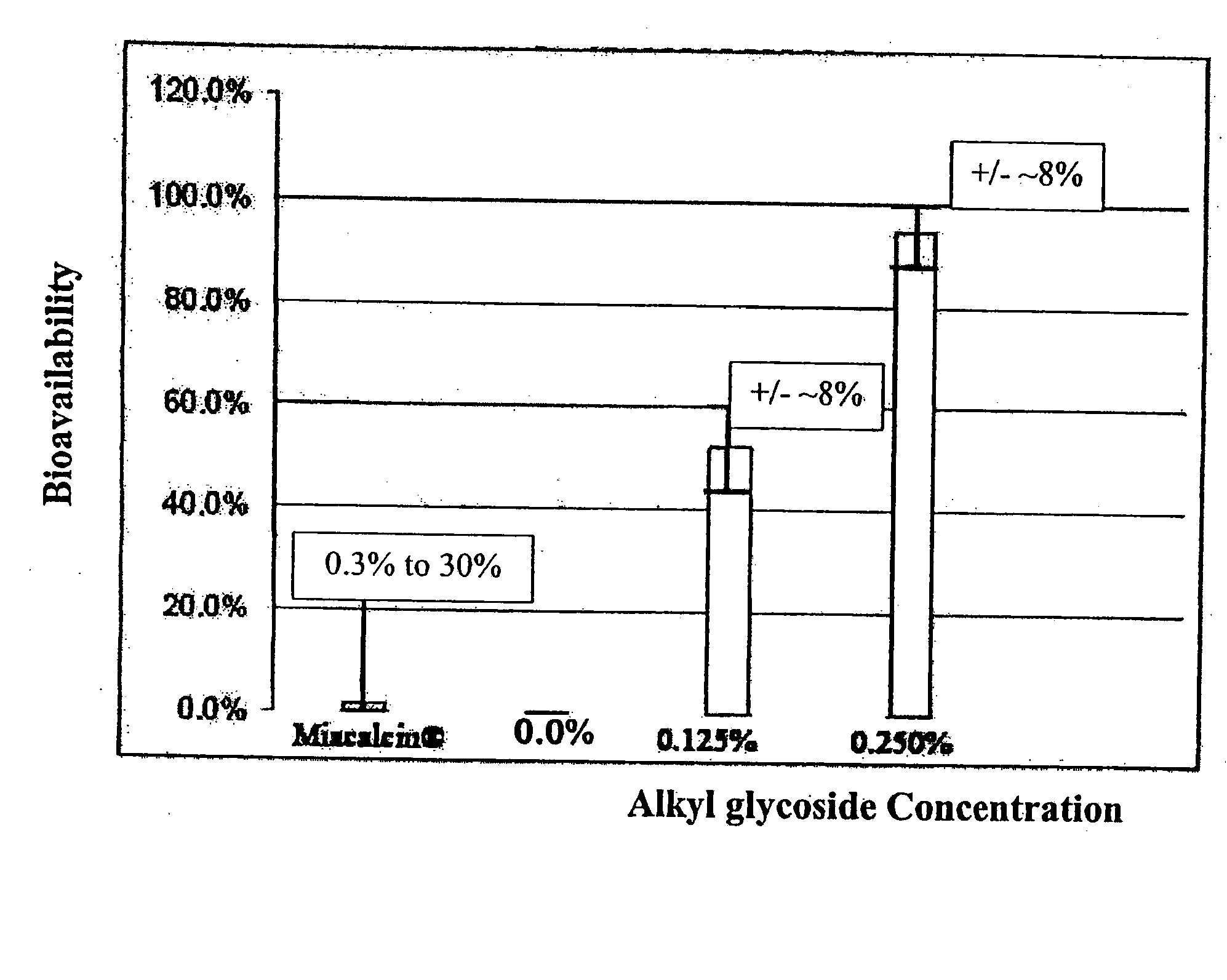
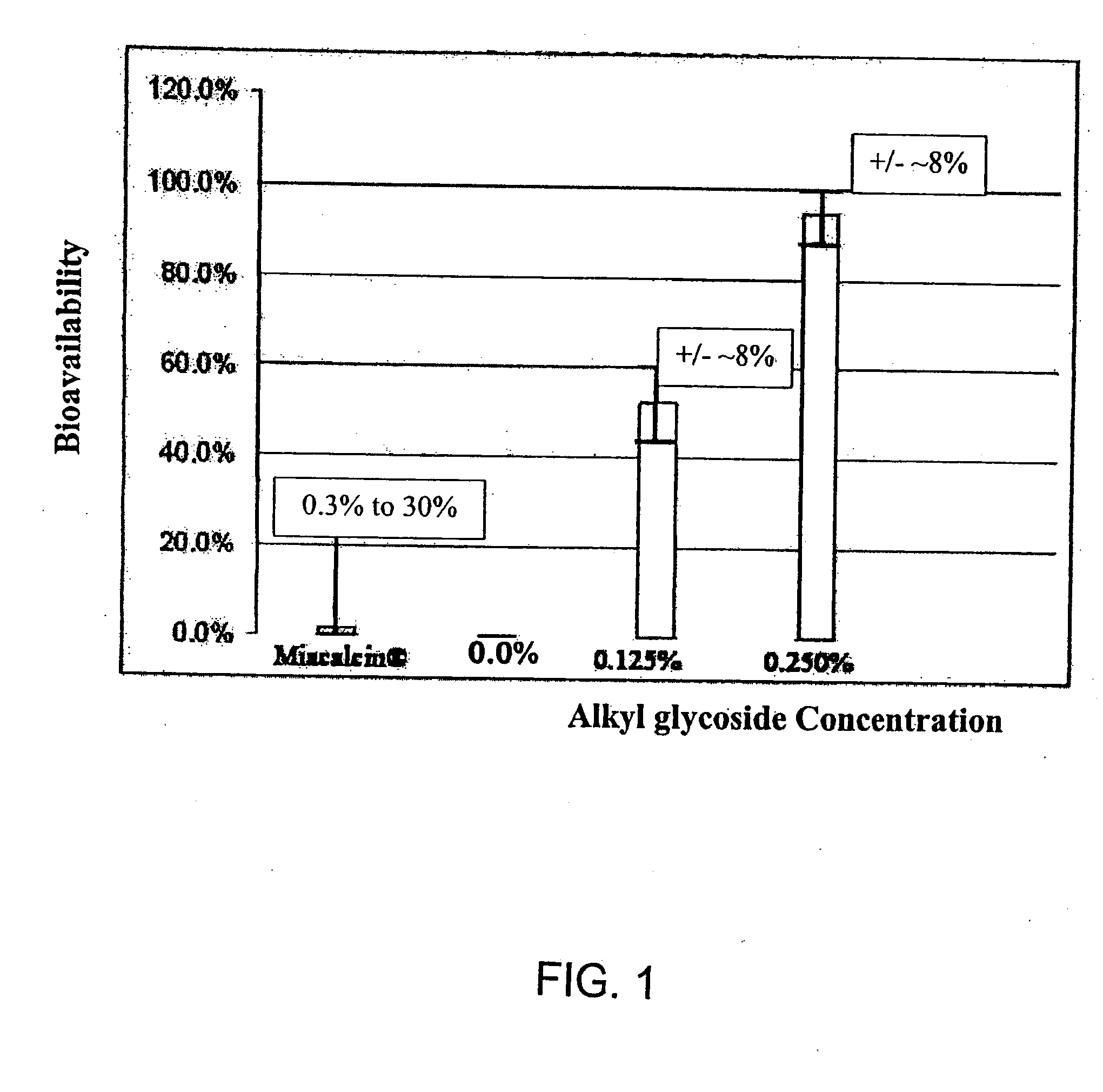
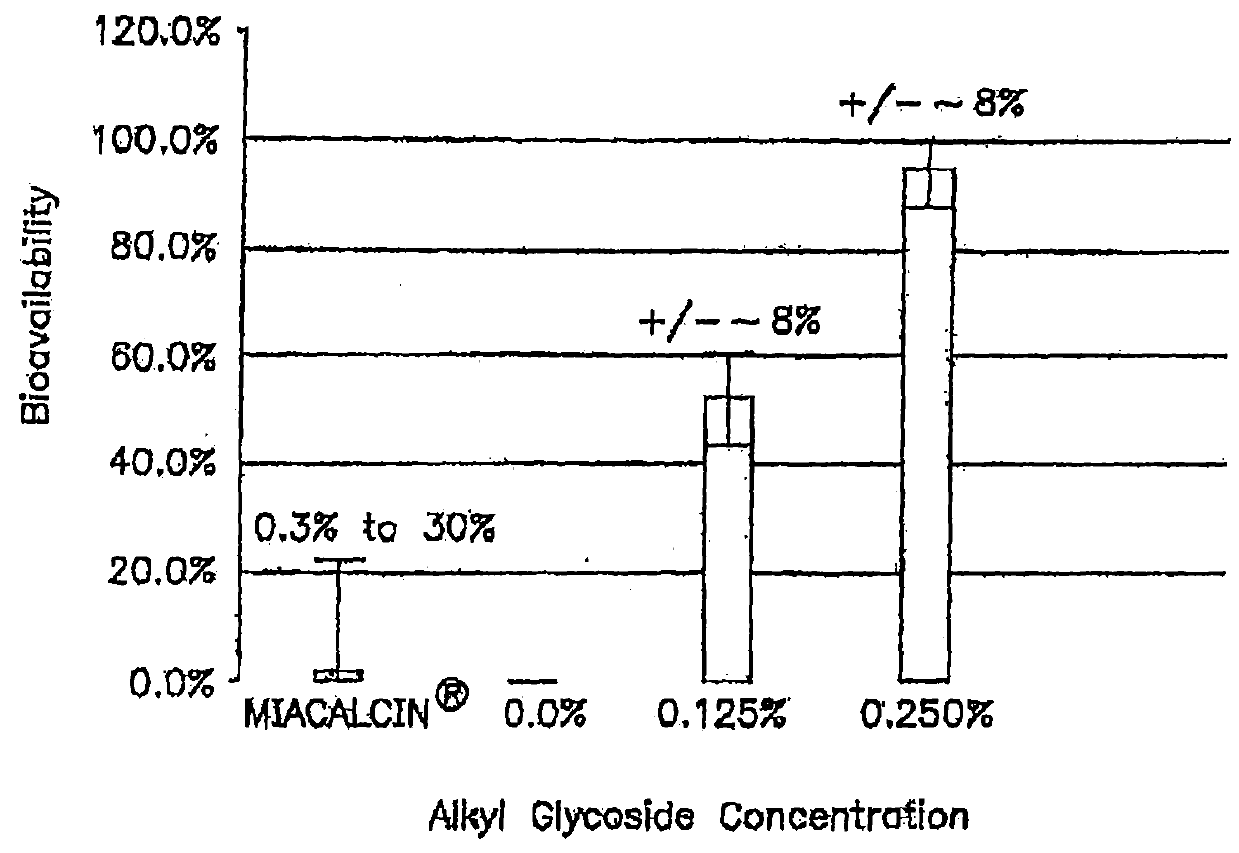
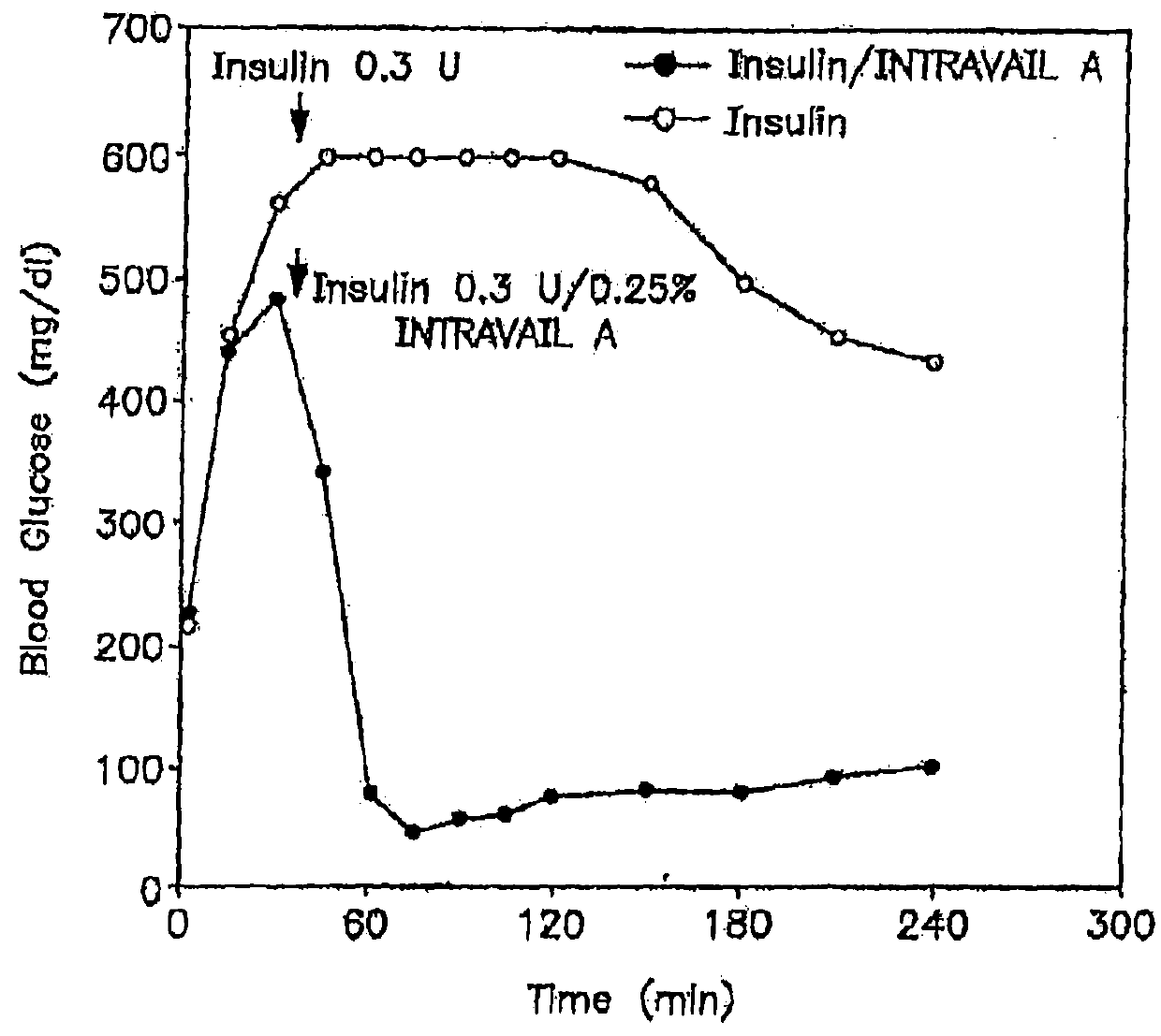
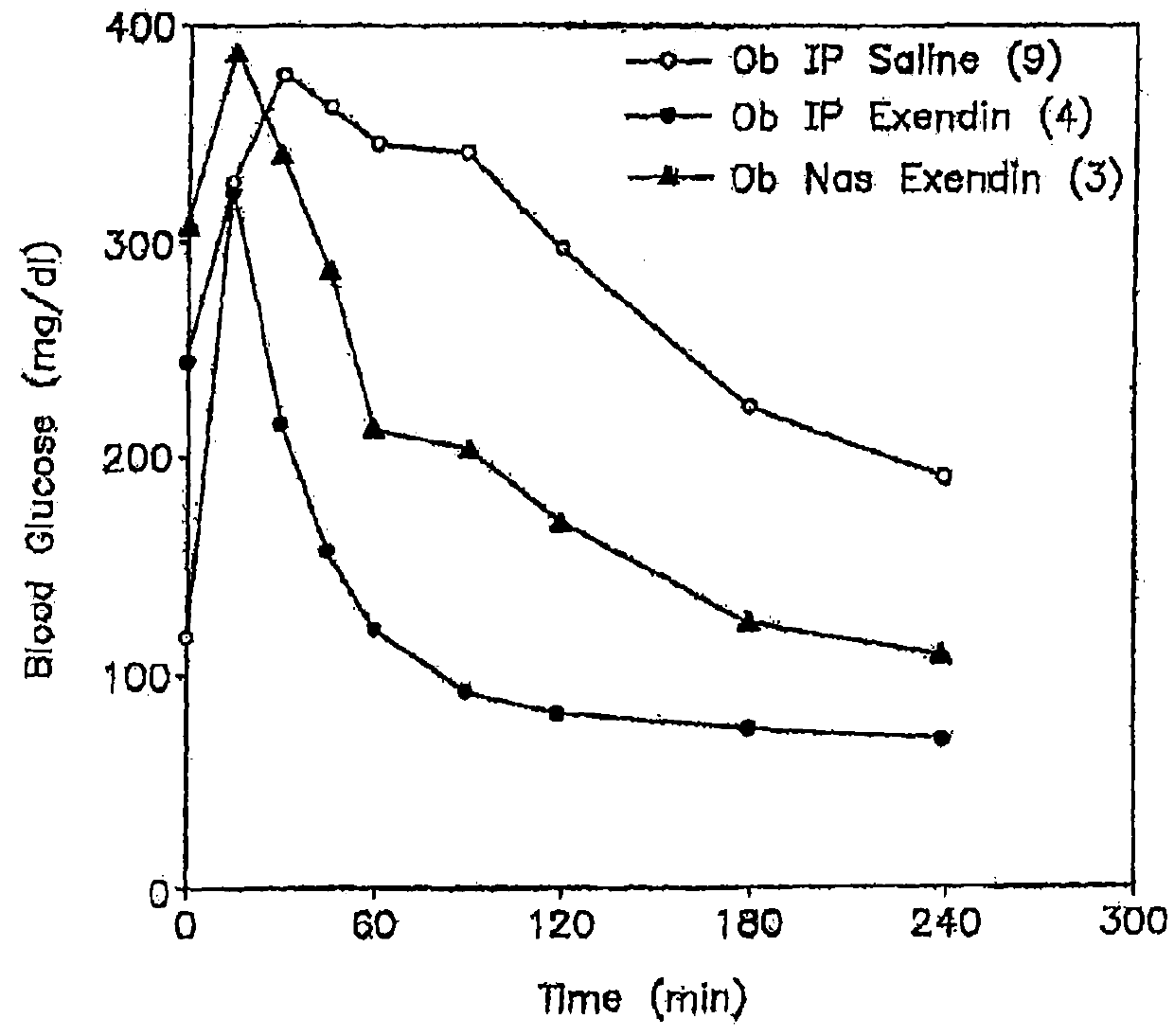
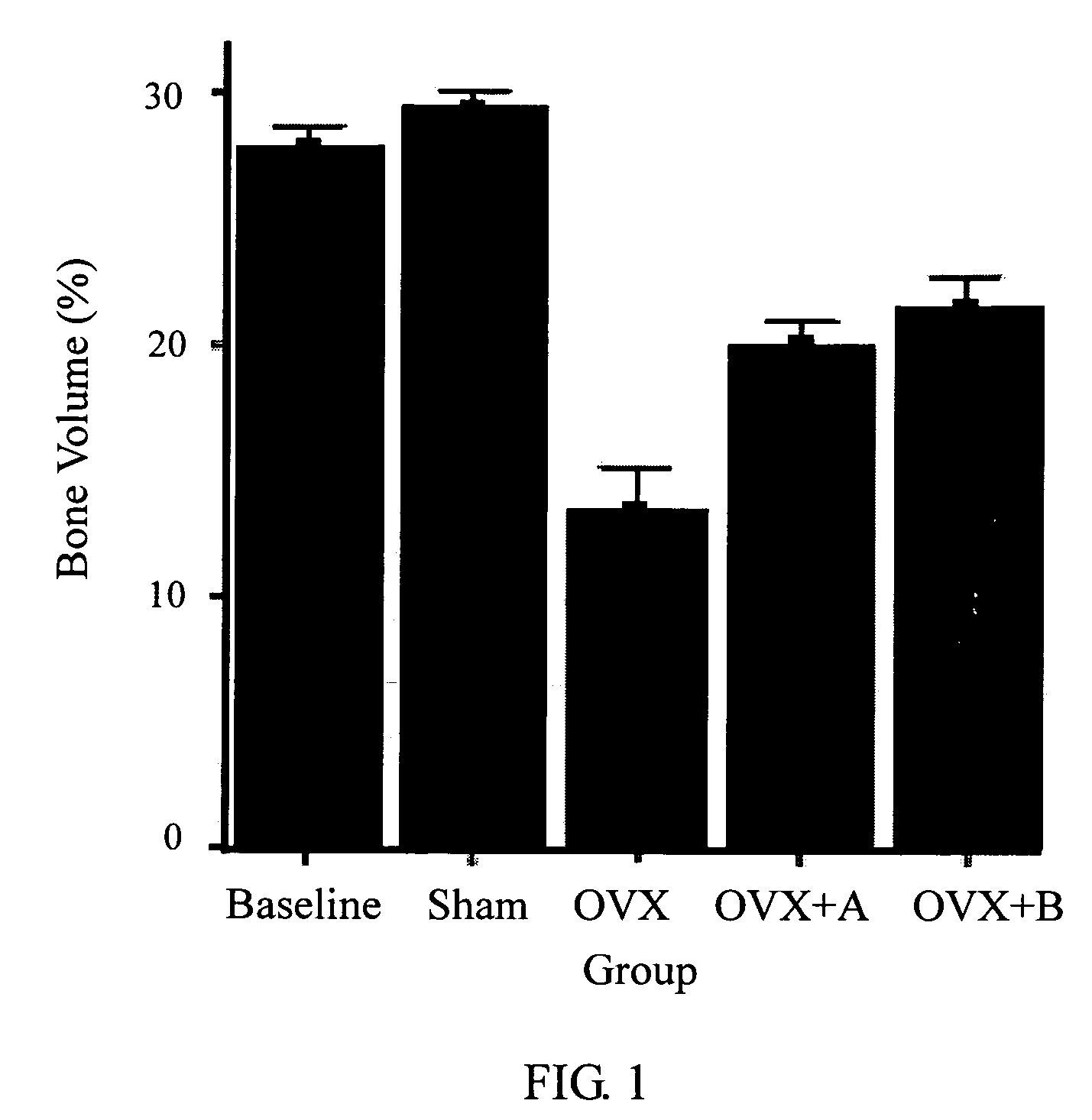
Patents
Literature
40results about How to "Toxic effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
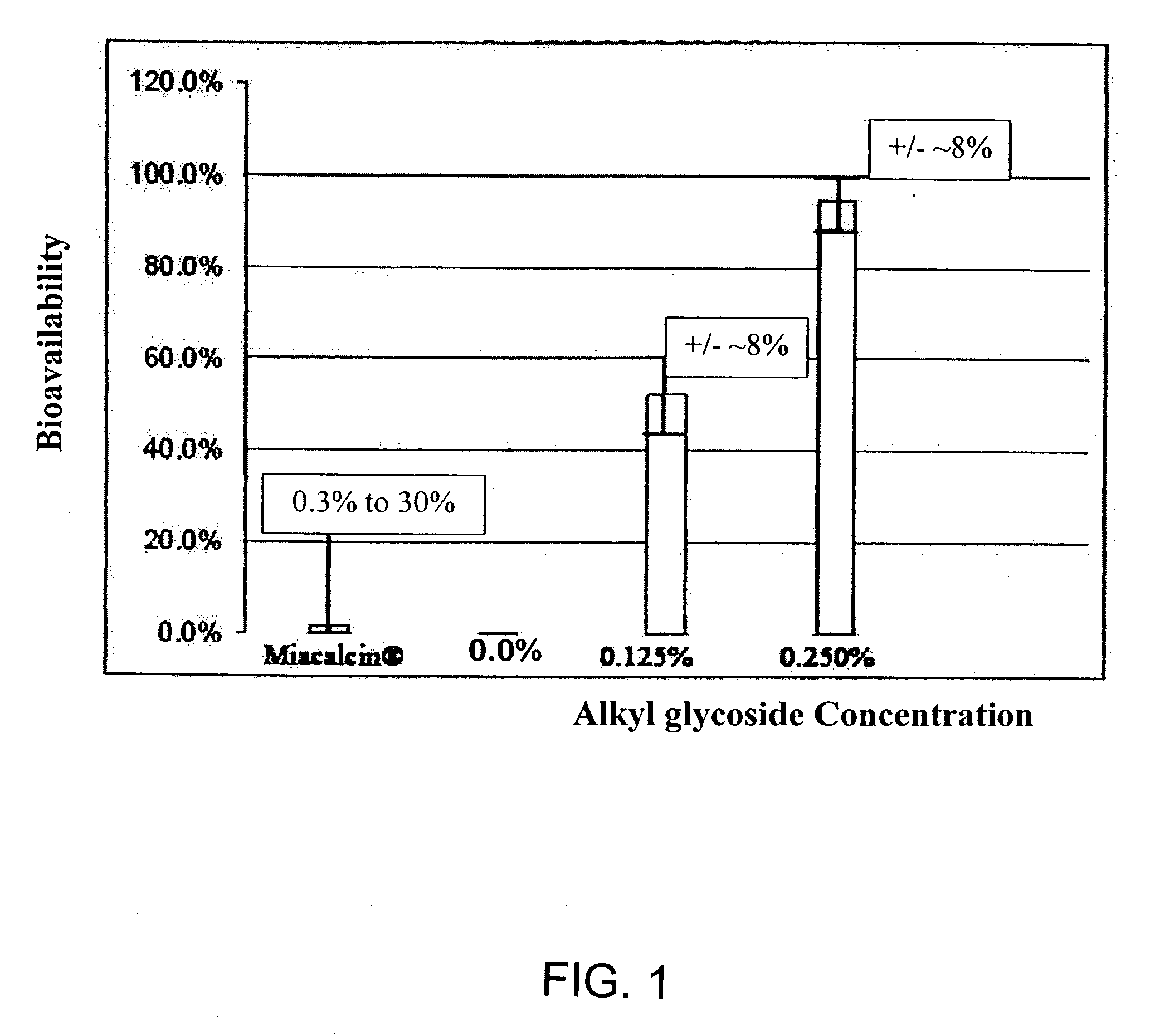
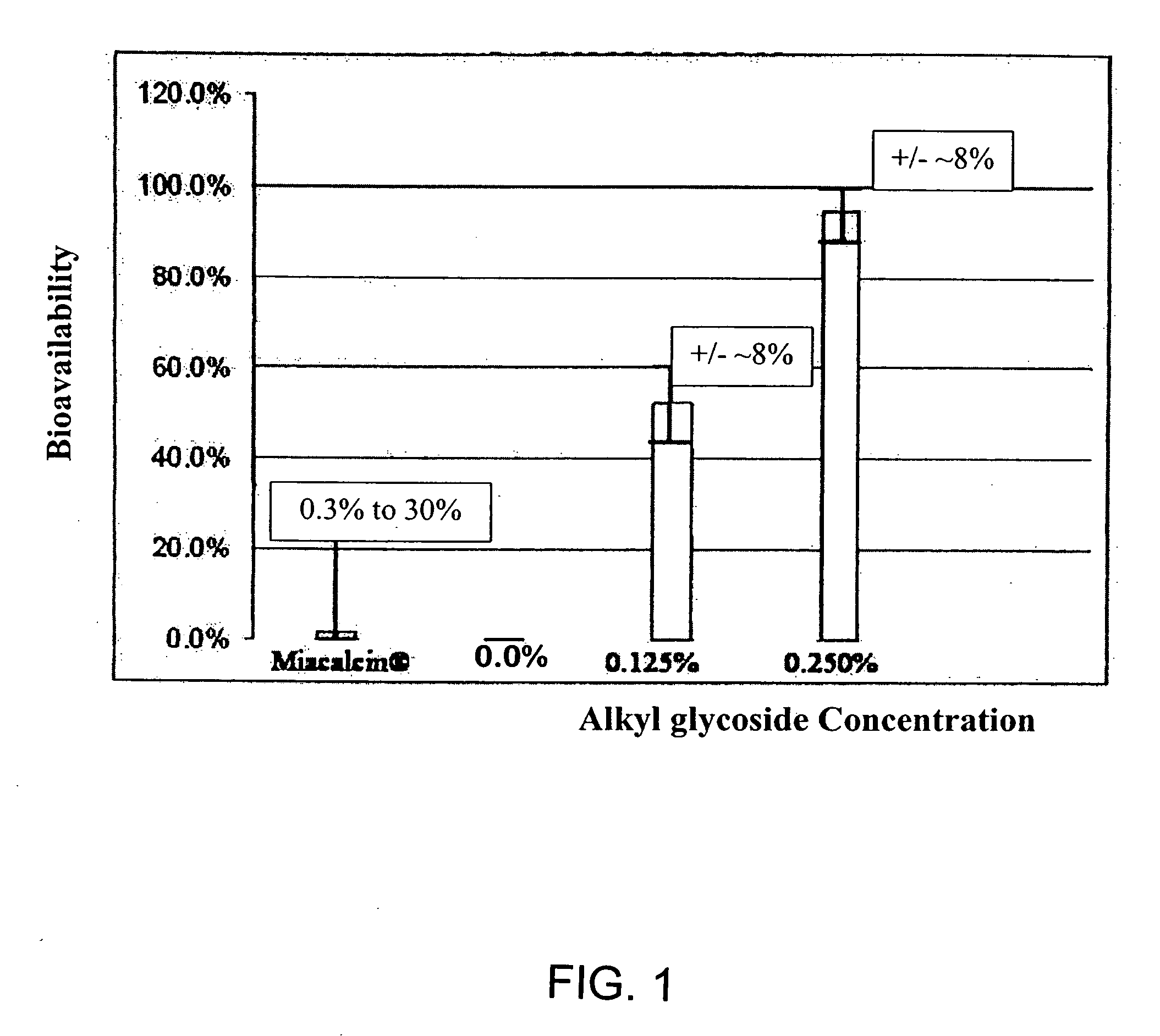
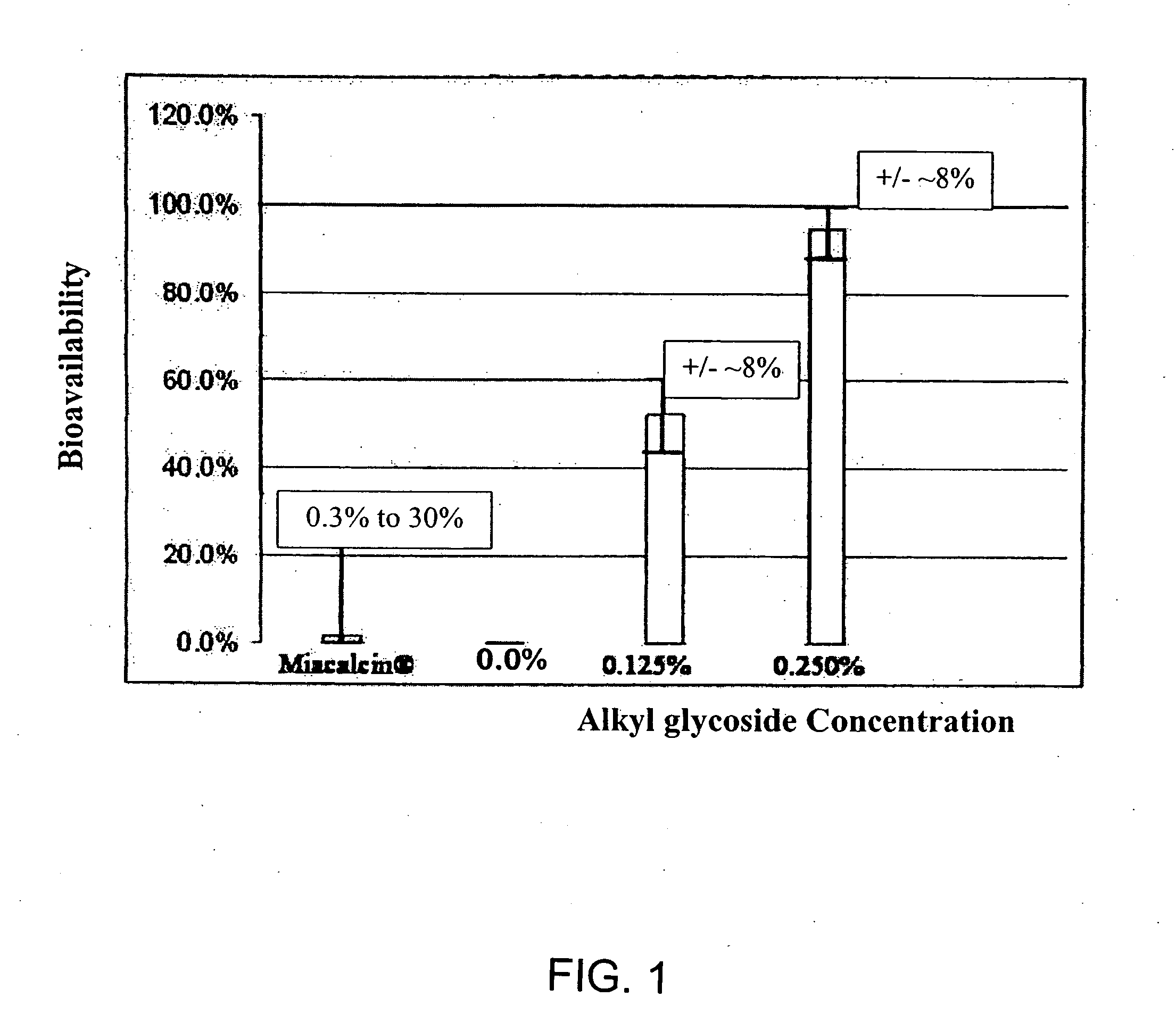
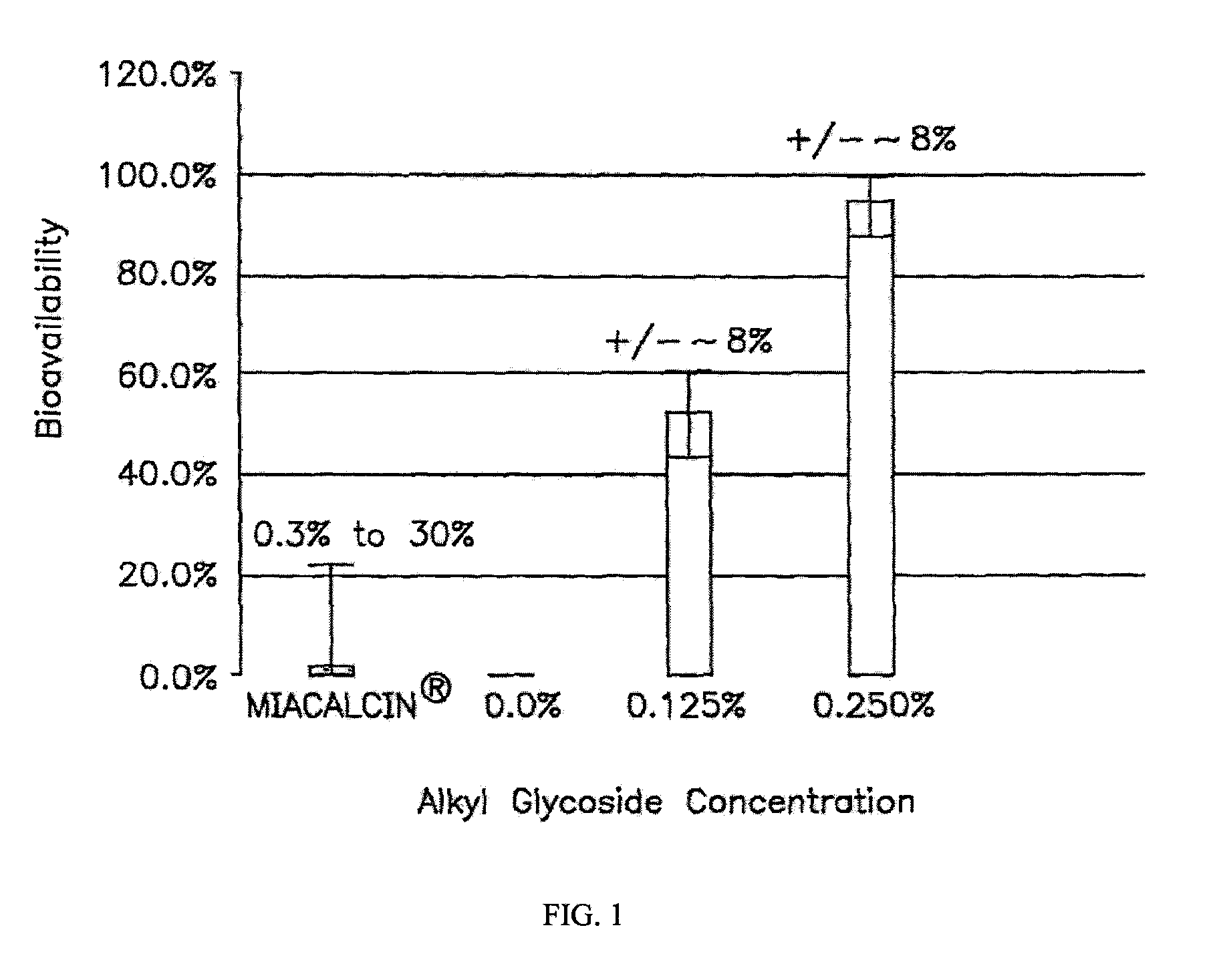
Compositions for oral drug administration
InactiveUS20140162965A1Promote absorptionImprove bioavailabilityBiocideCarbohydrate active ingredientsGlycosideOral medication
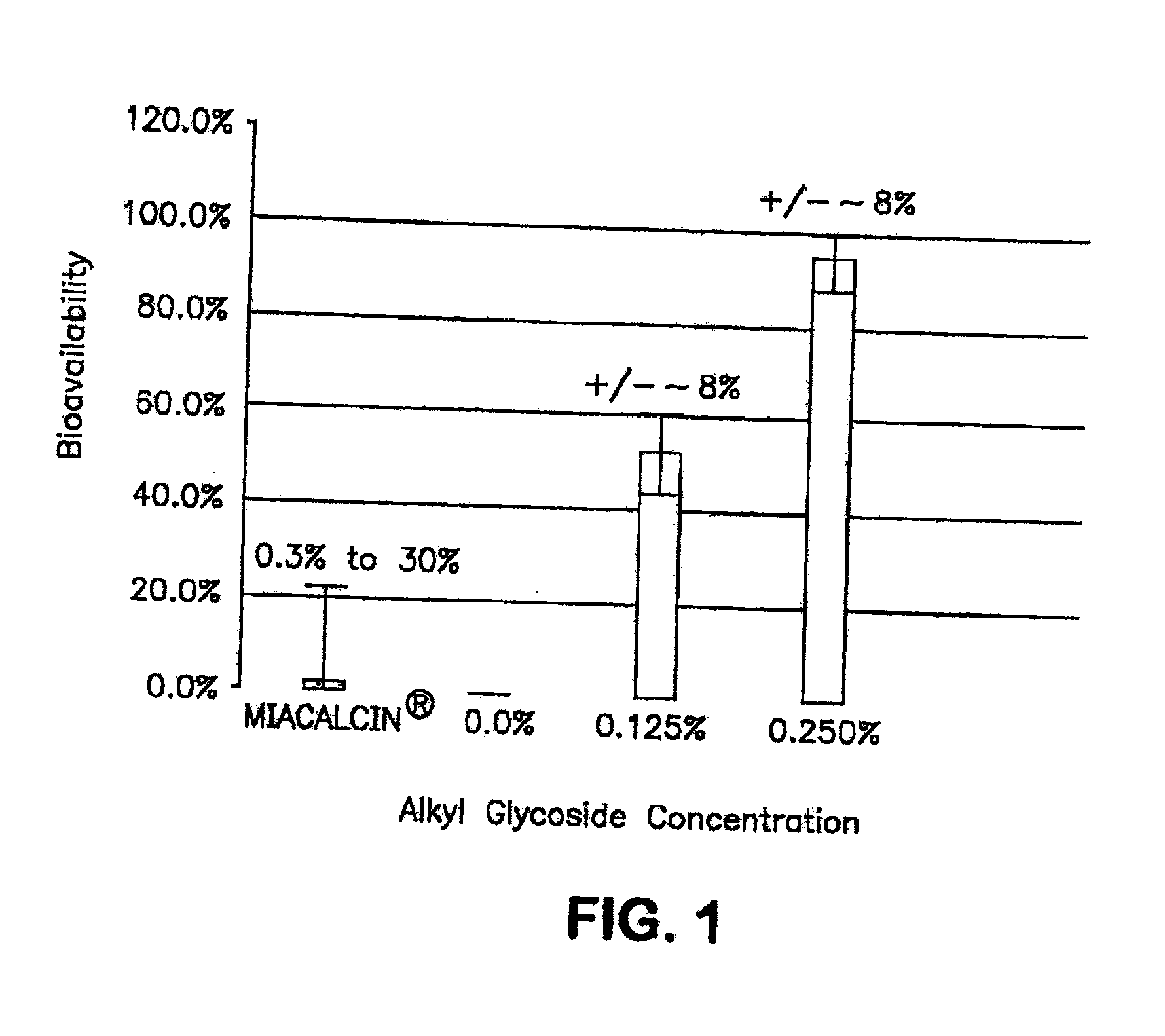
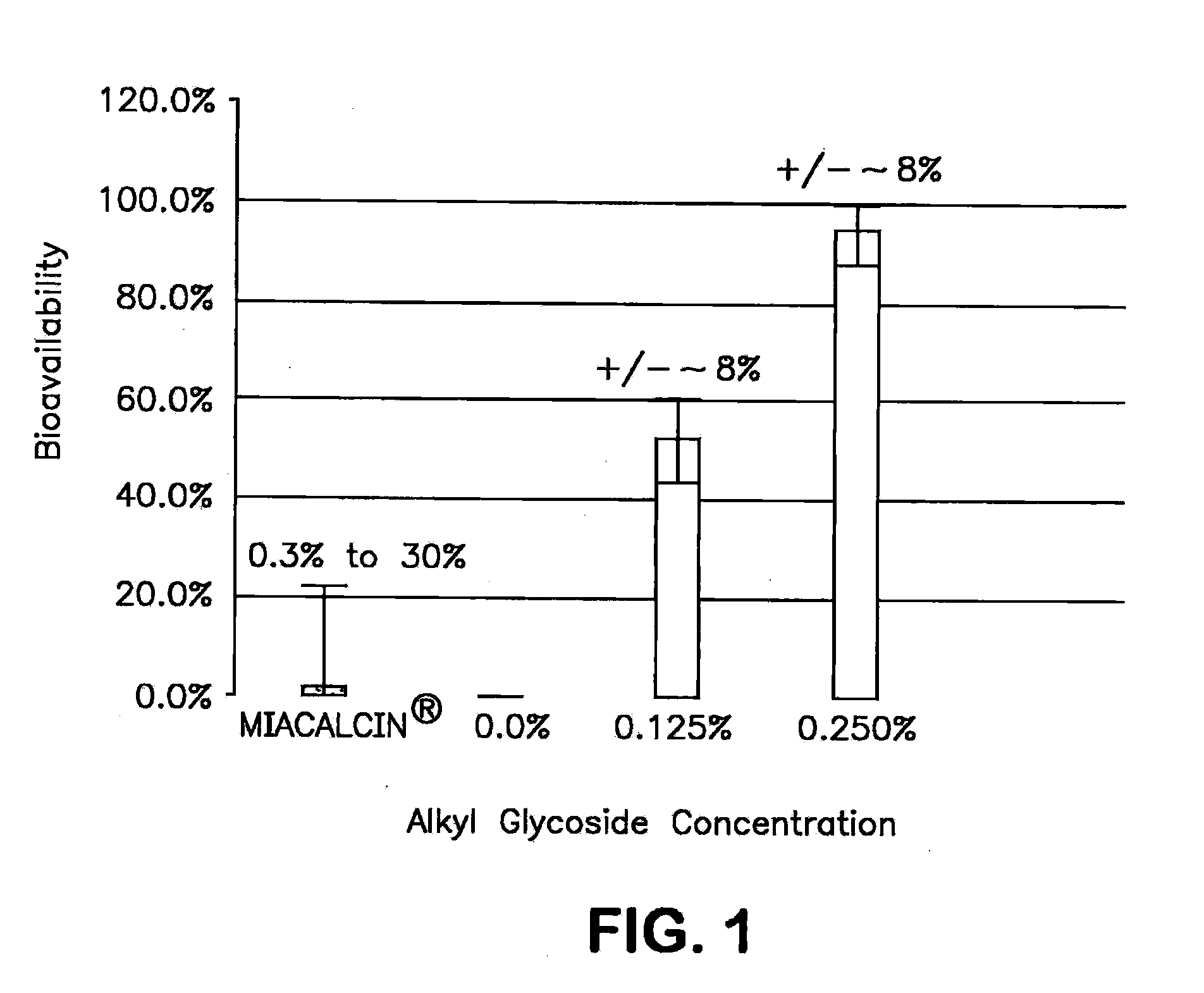
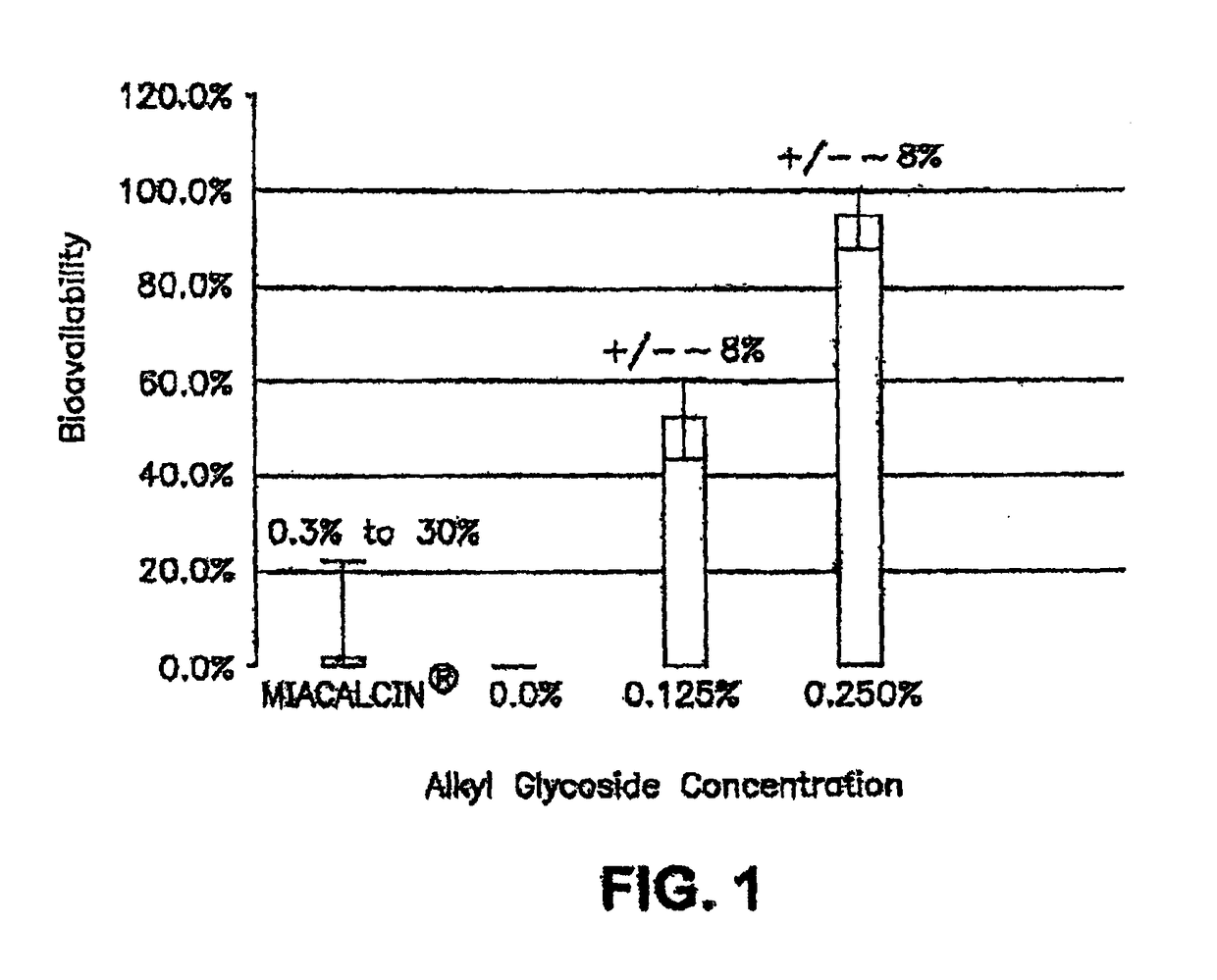
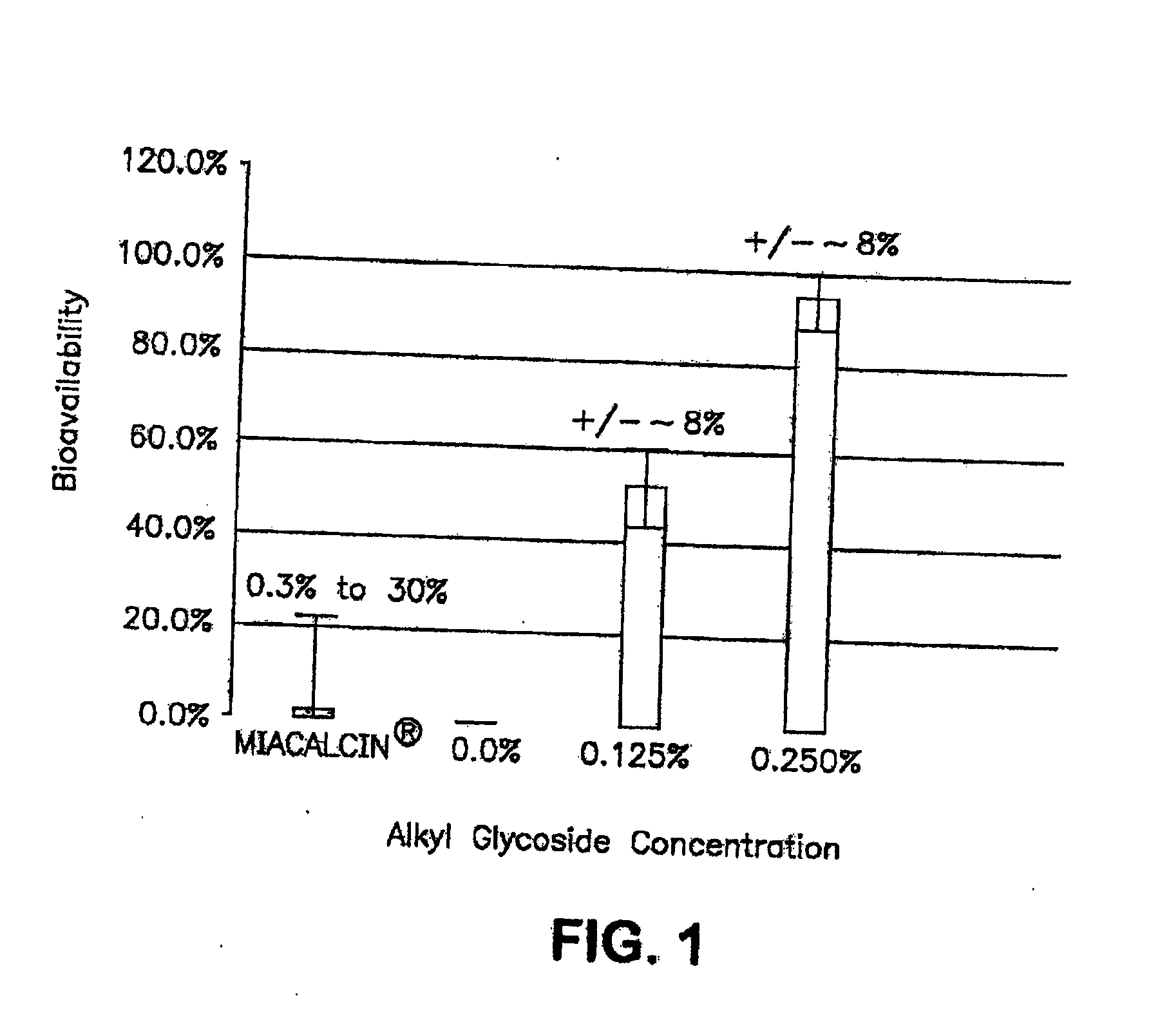
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms. In various aspects, the invention provides compositions and methods for oral delivery in the form of a tablet.
Owner:AEGIS THERAPEUTICS LLC
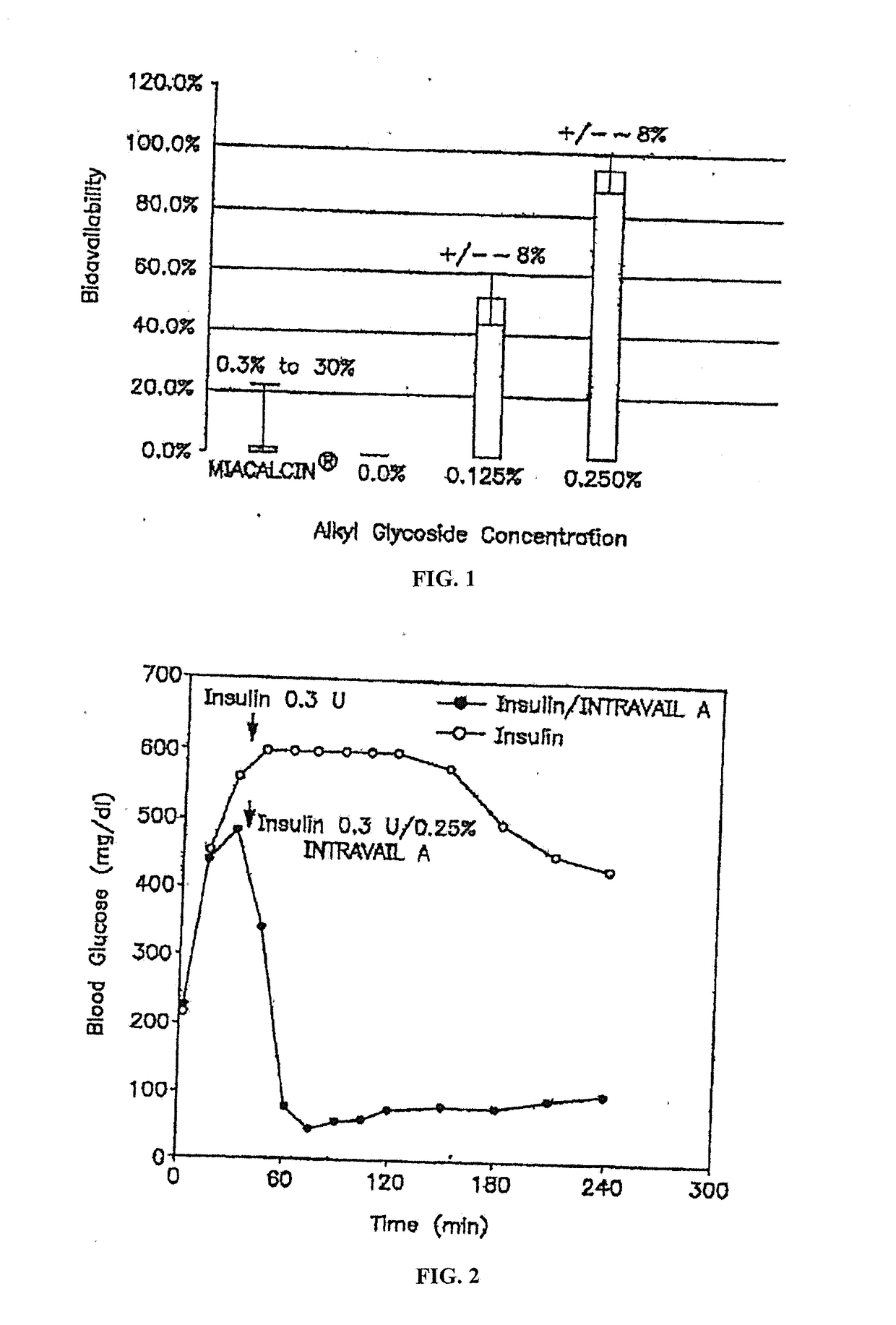
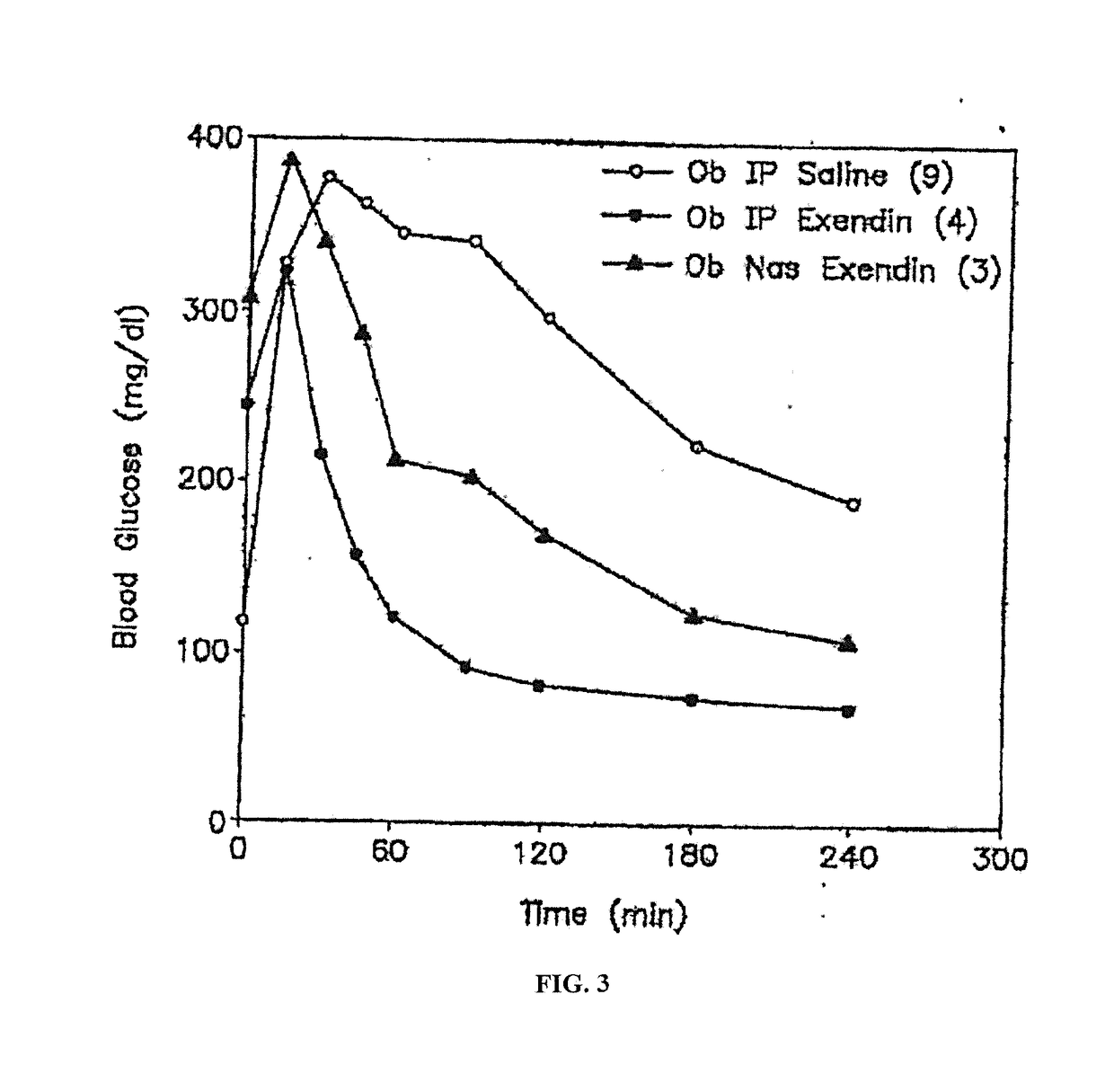
Absorption Enhancers for Drug Administration
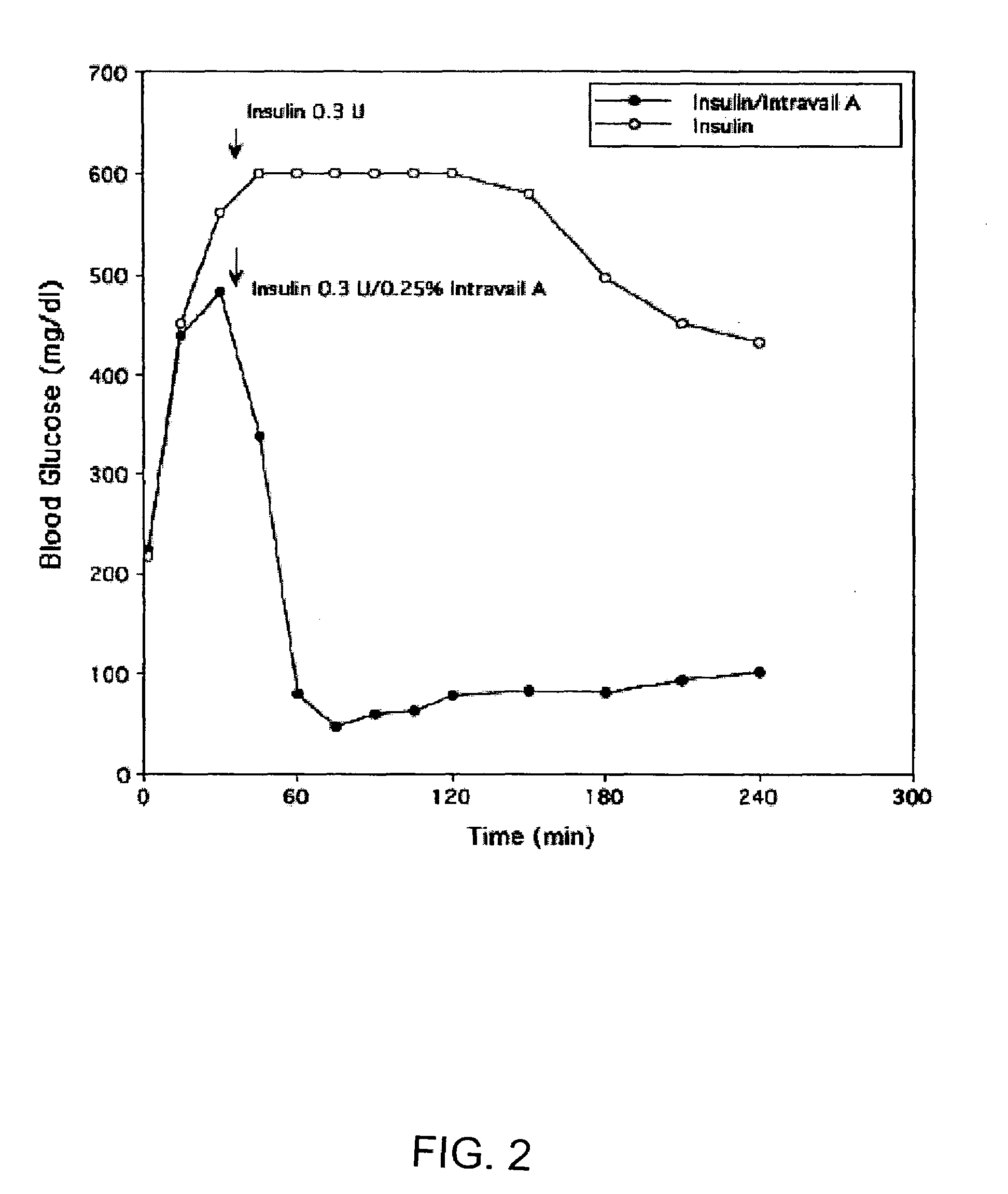
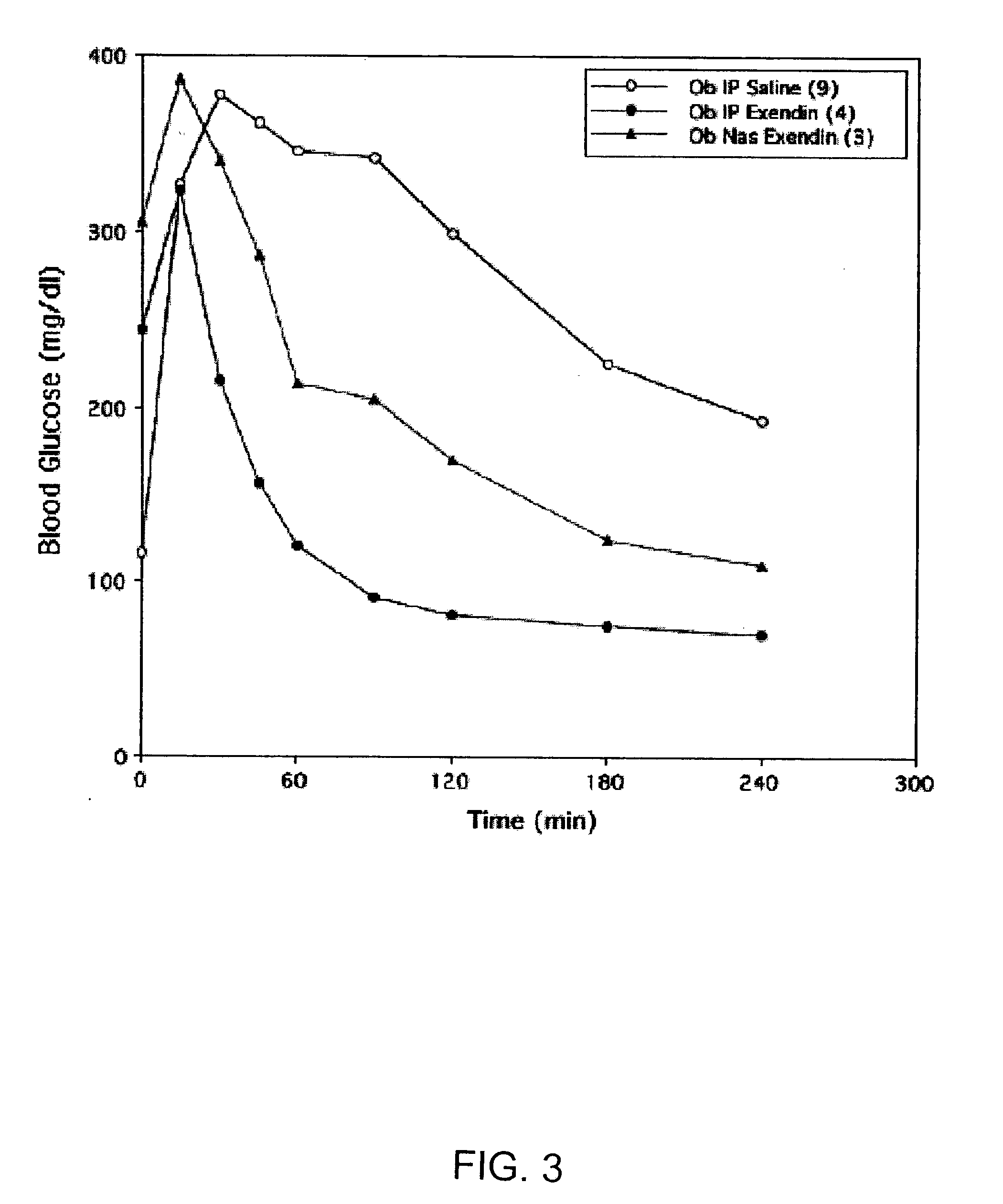
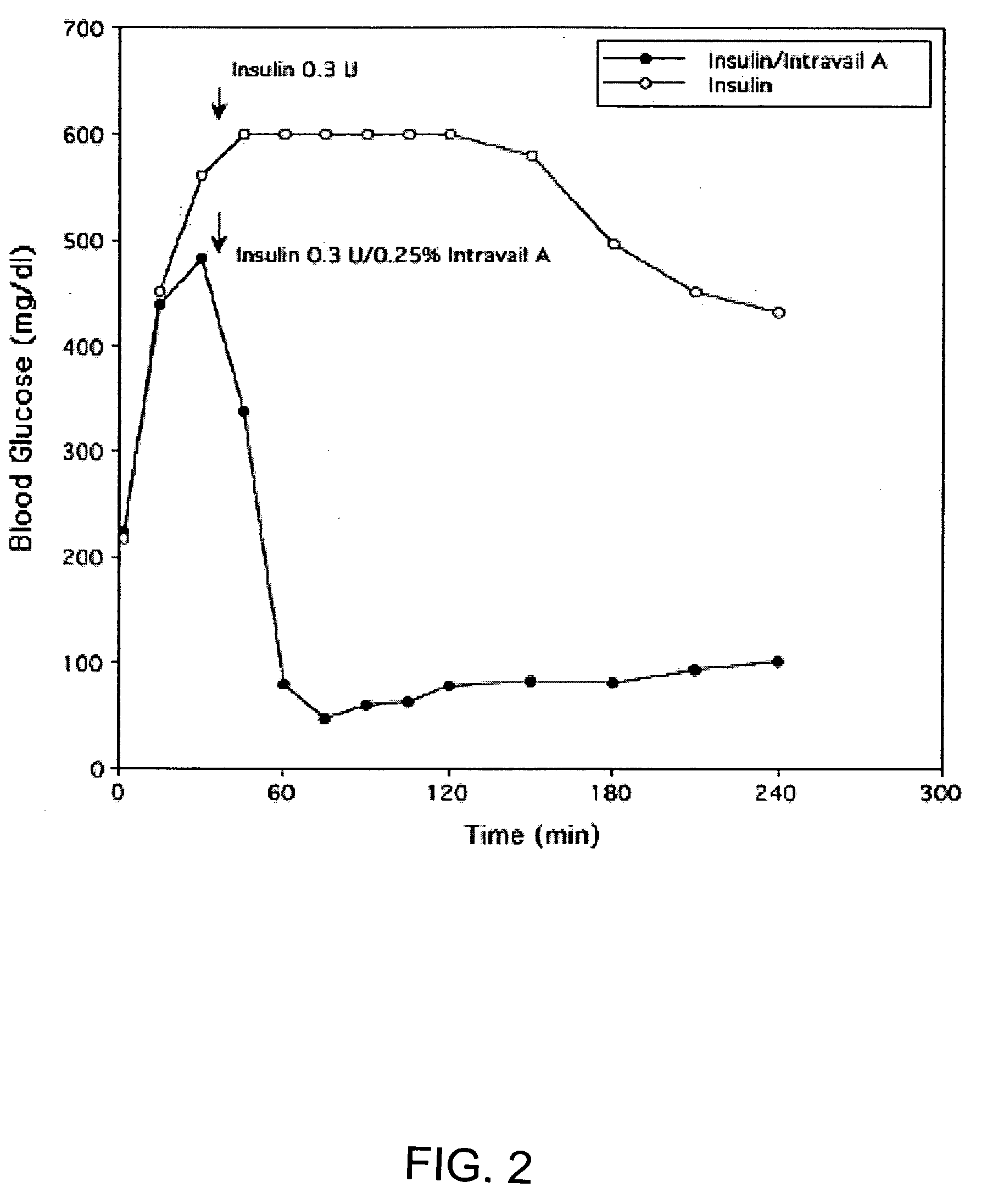
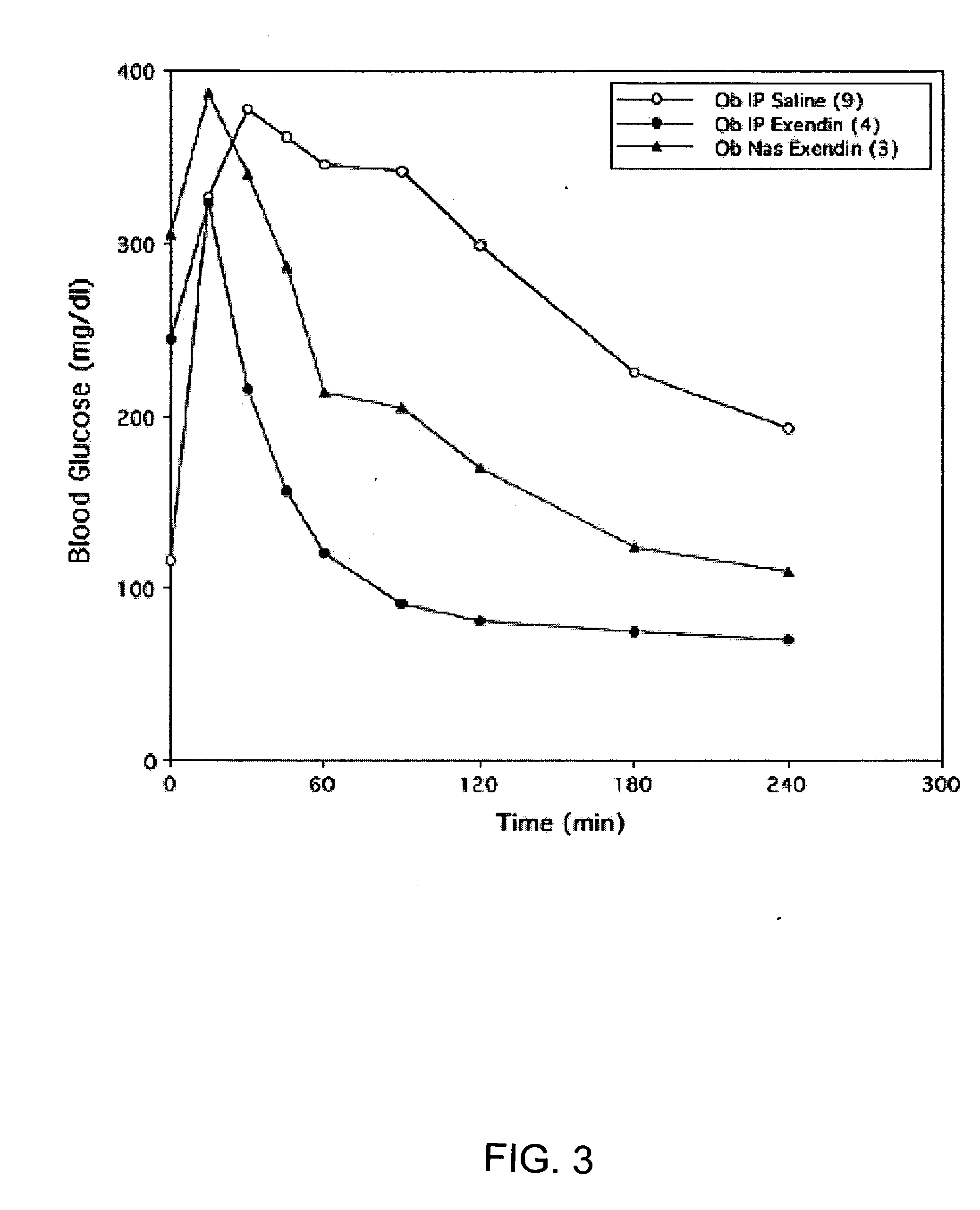
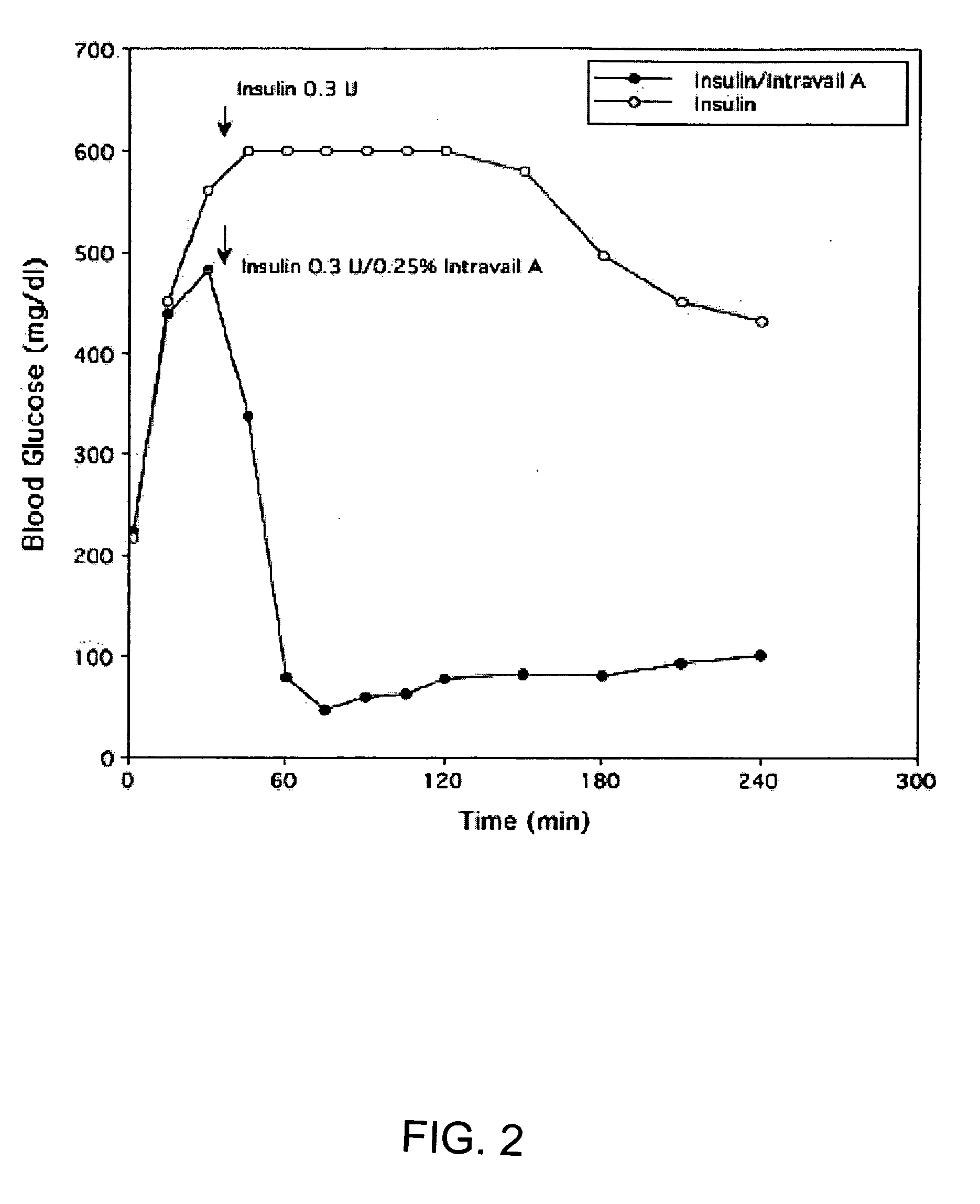
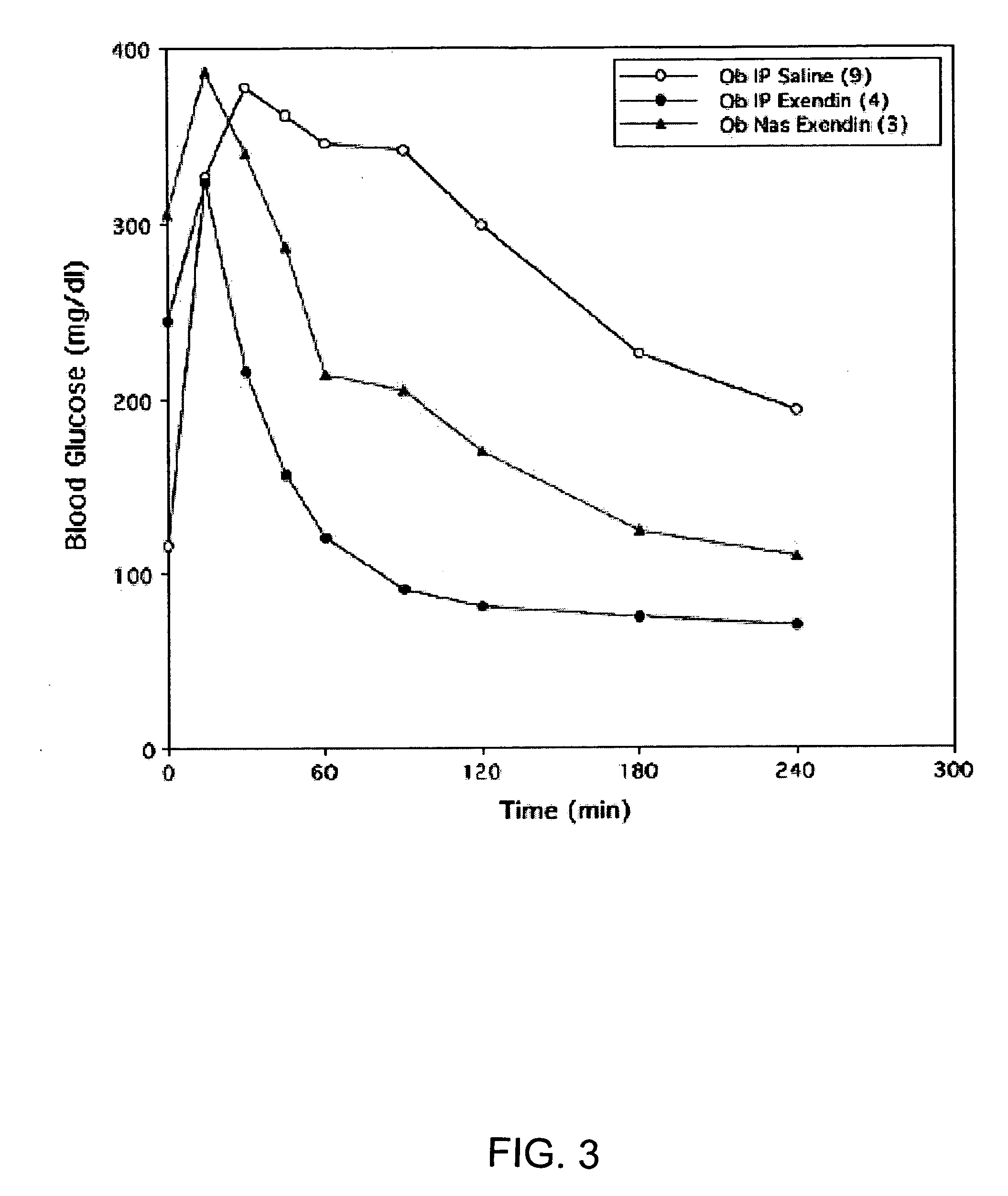
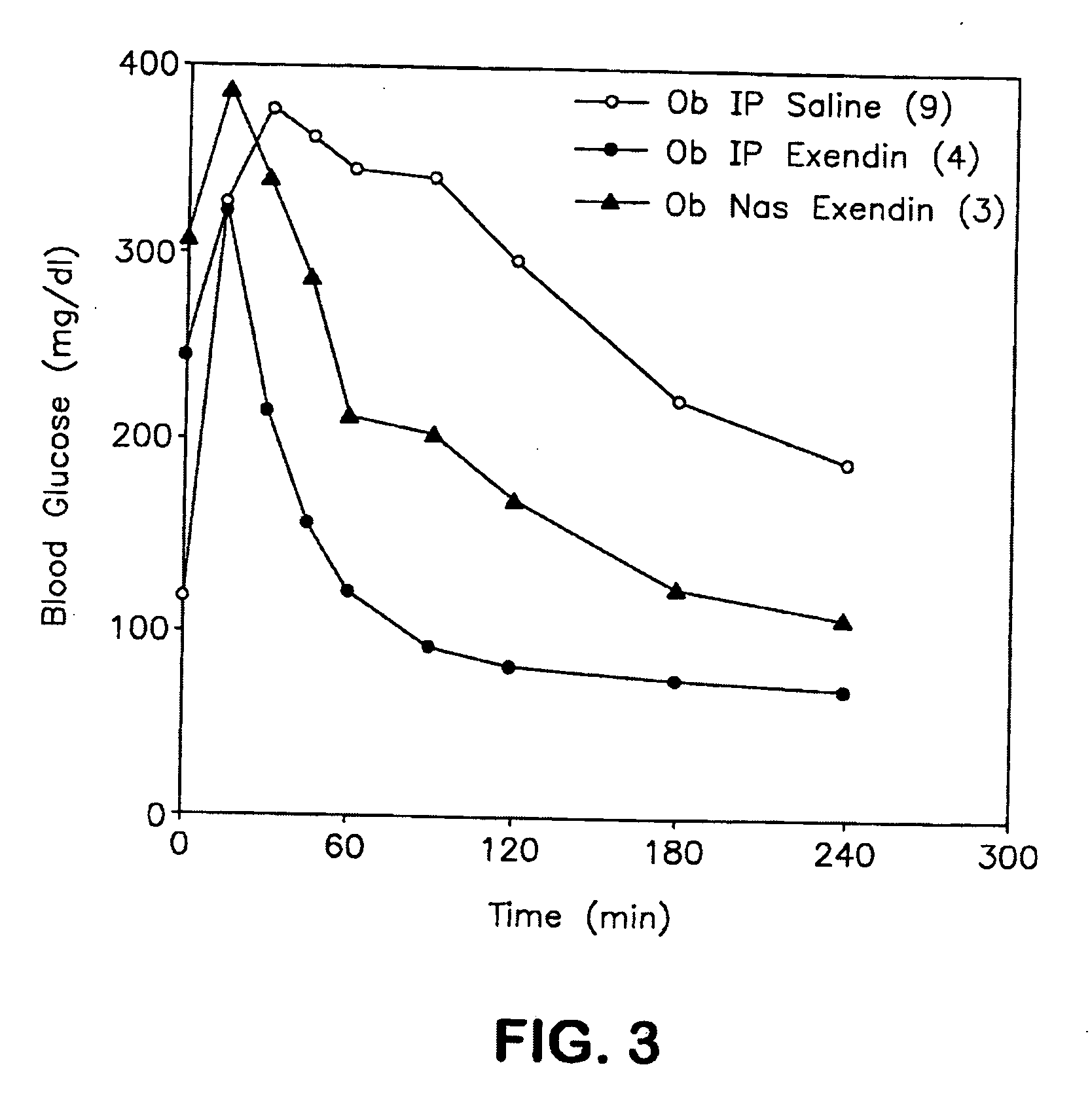
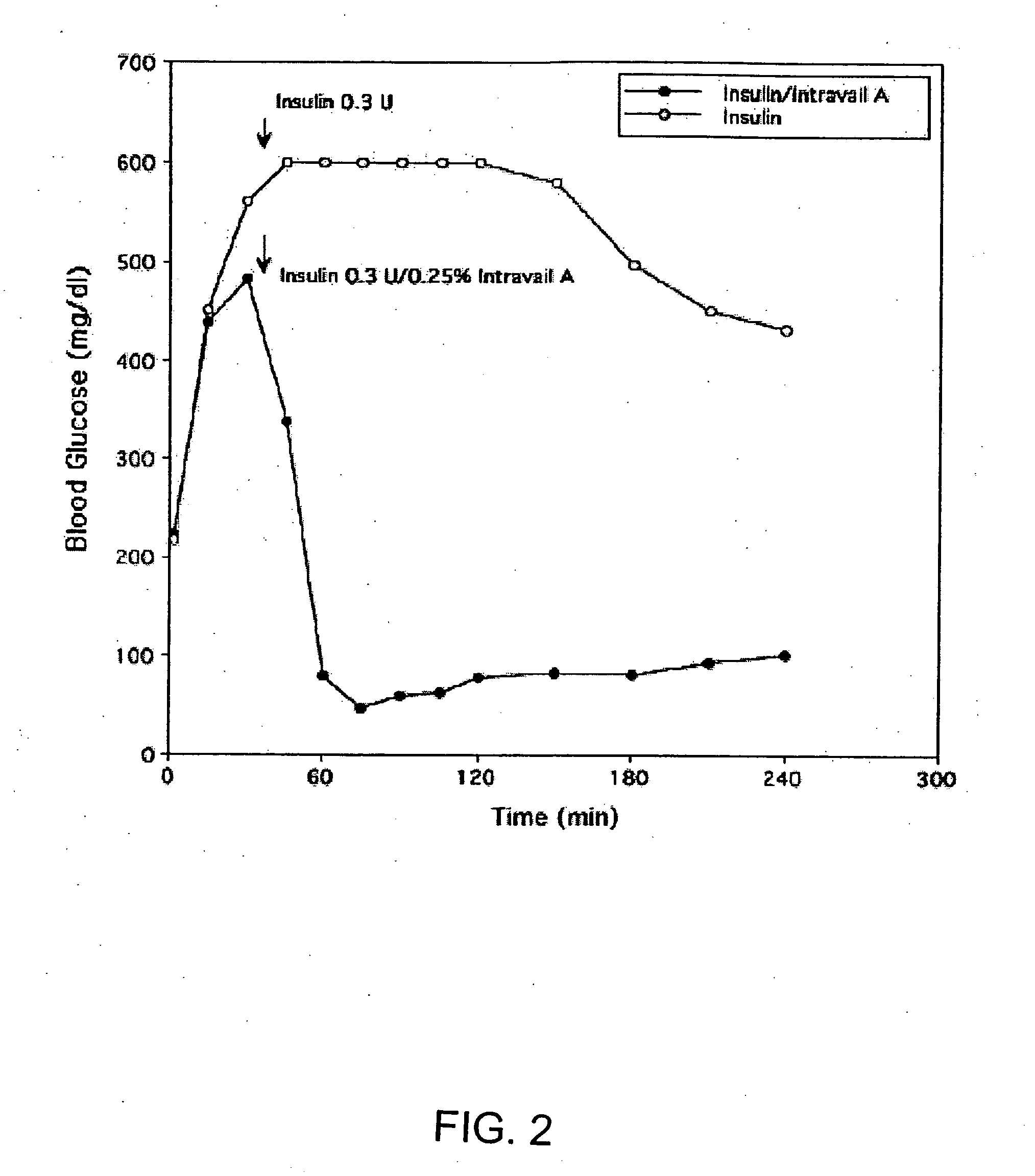
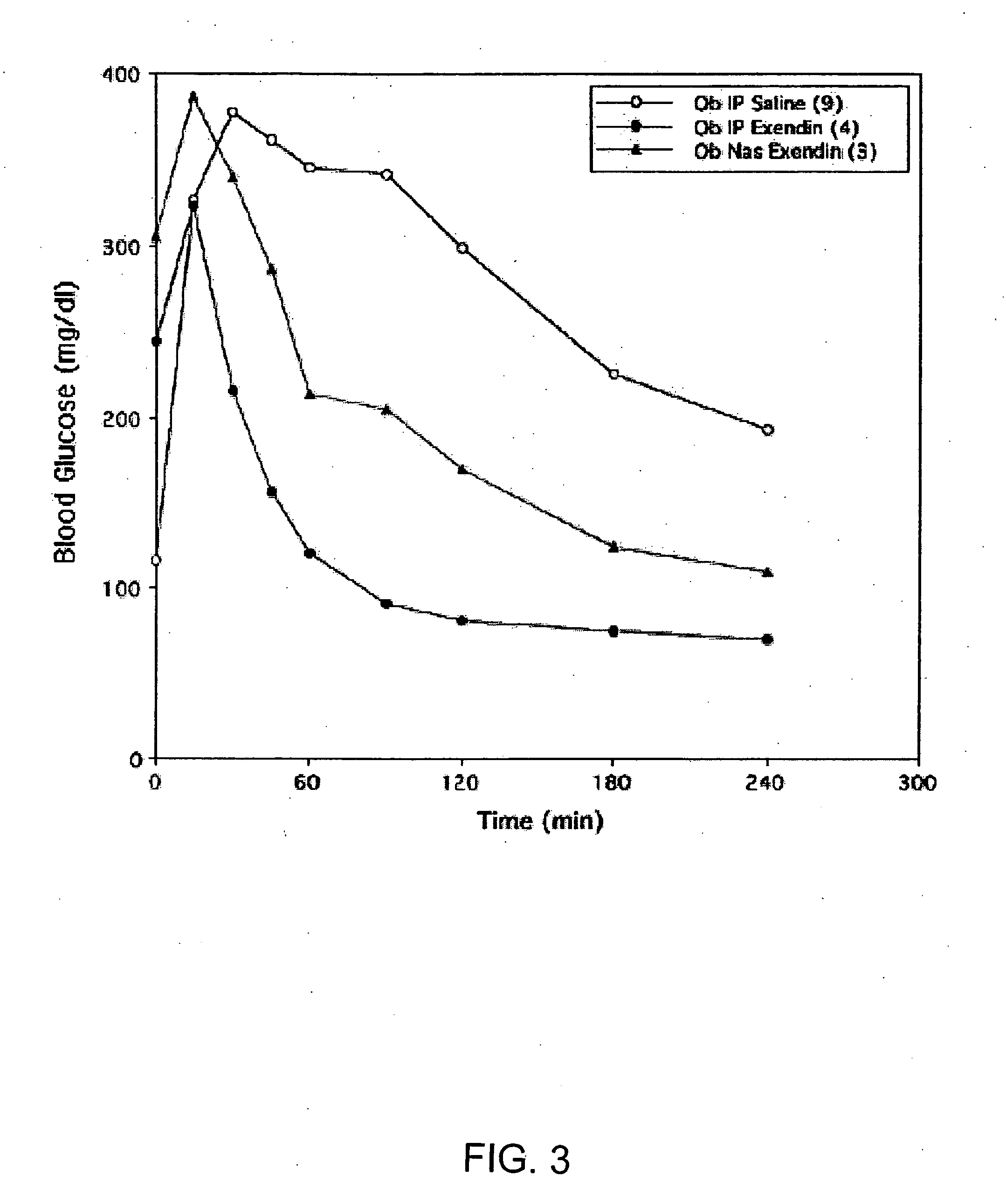
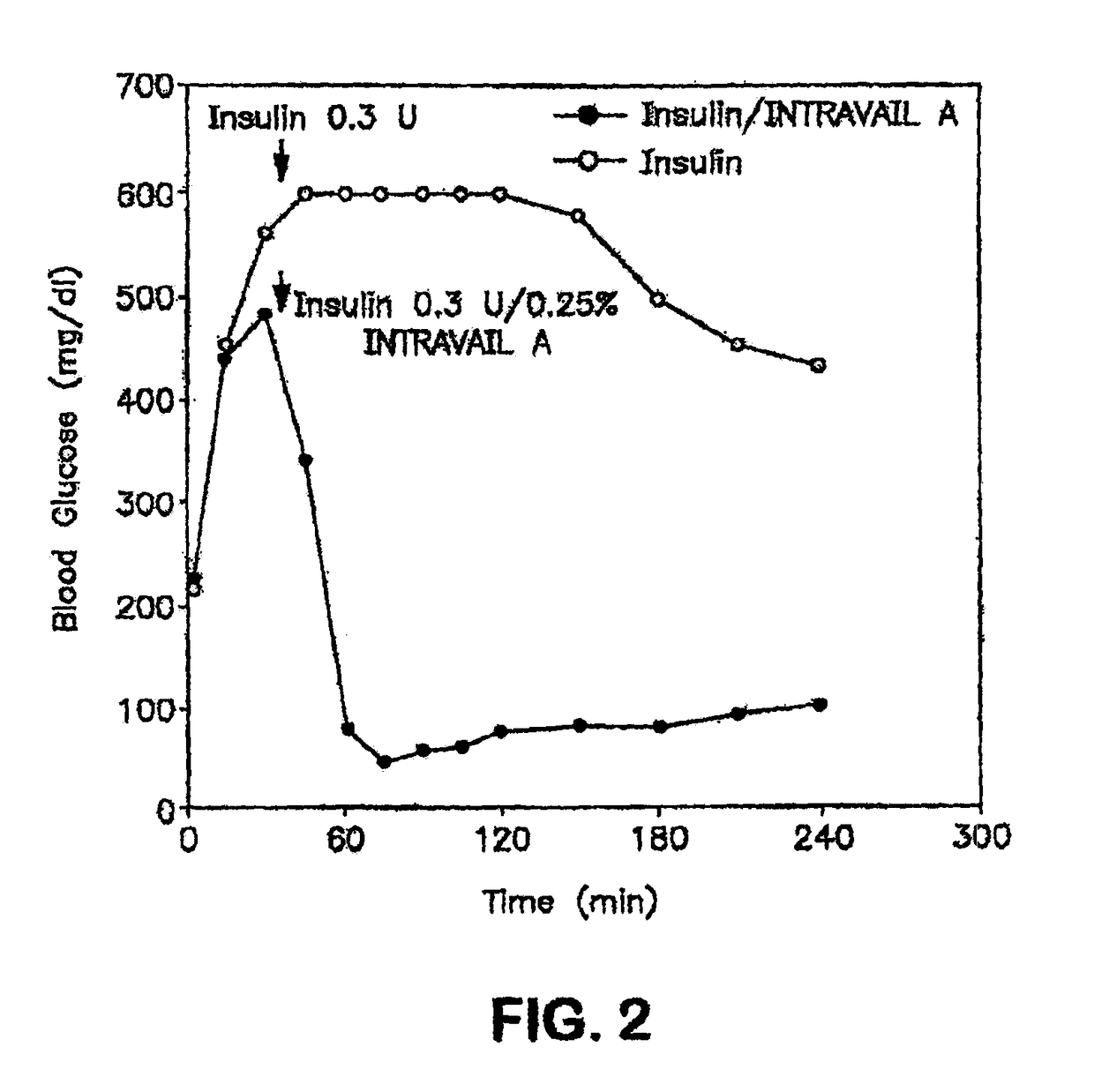
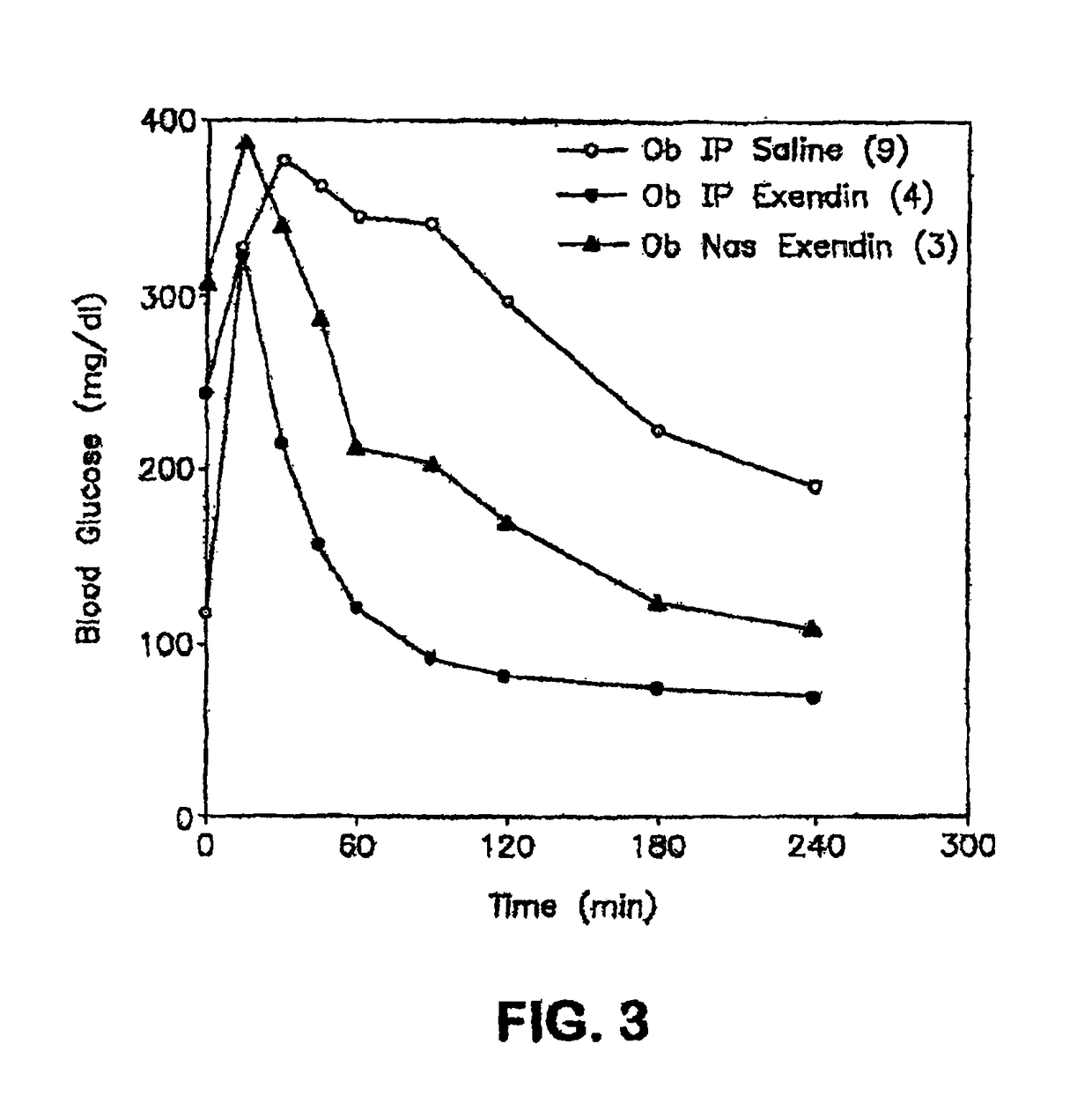
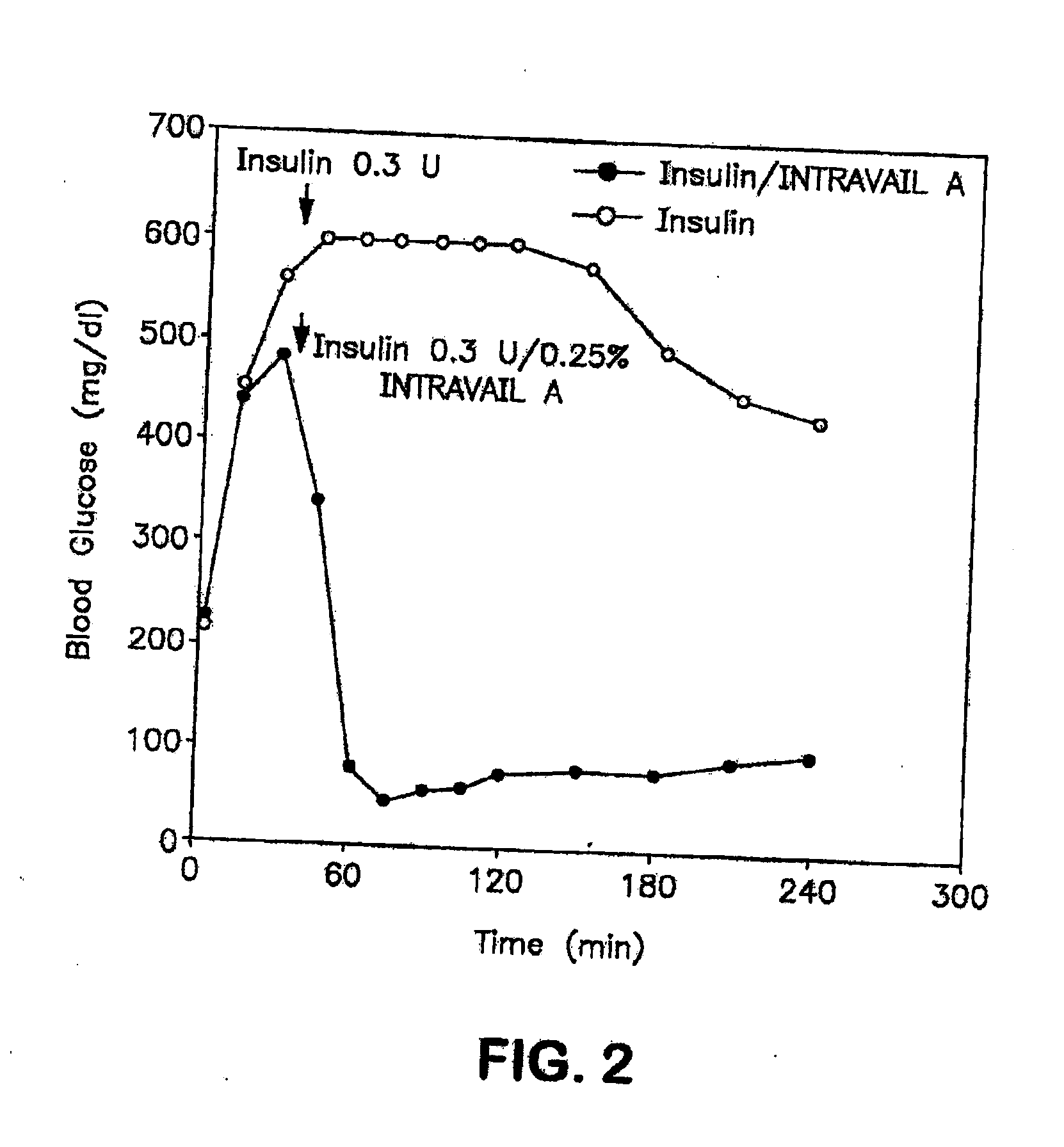
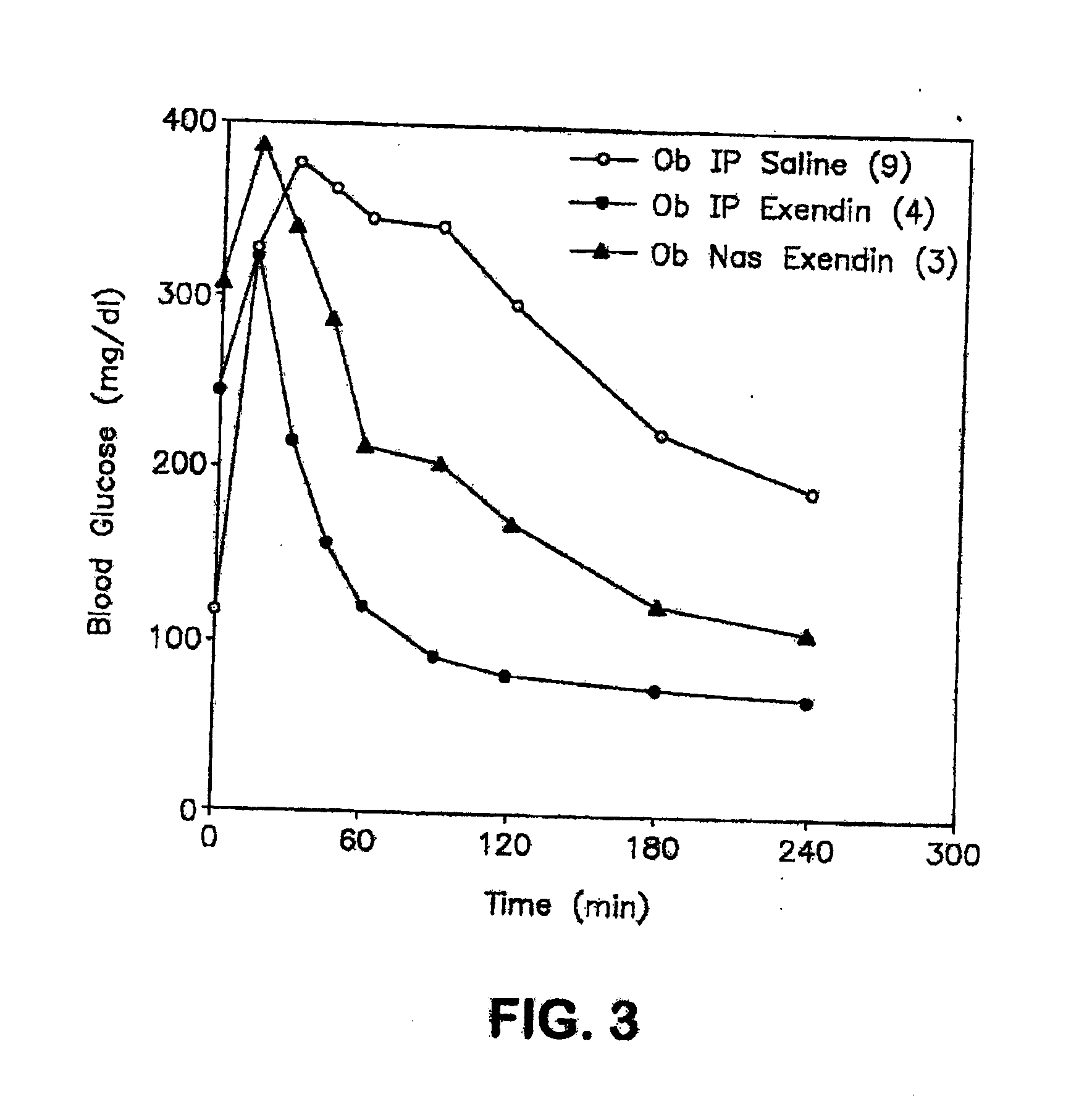
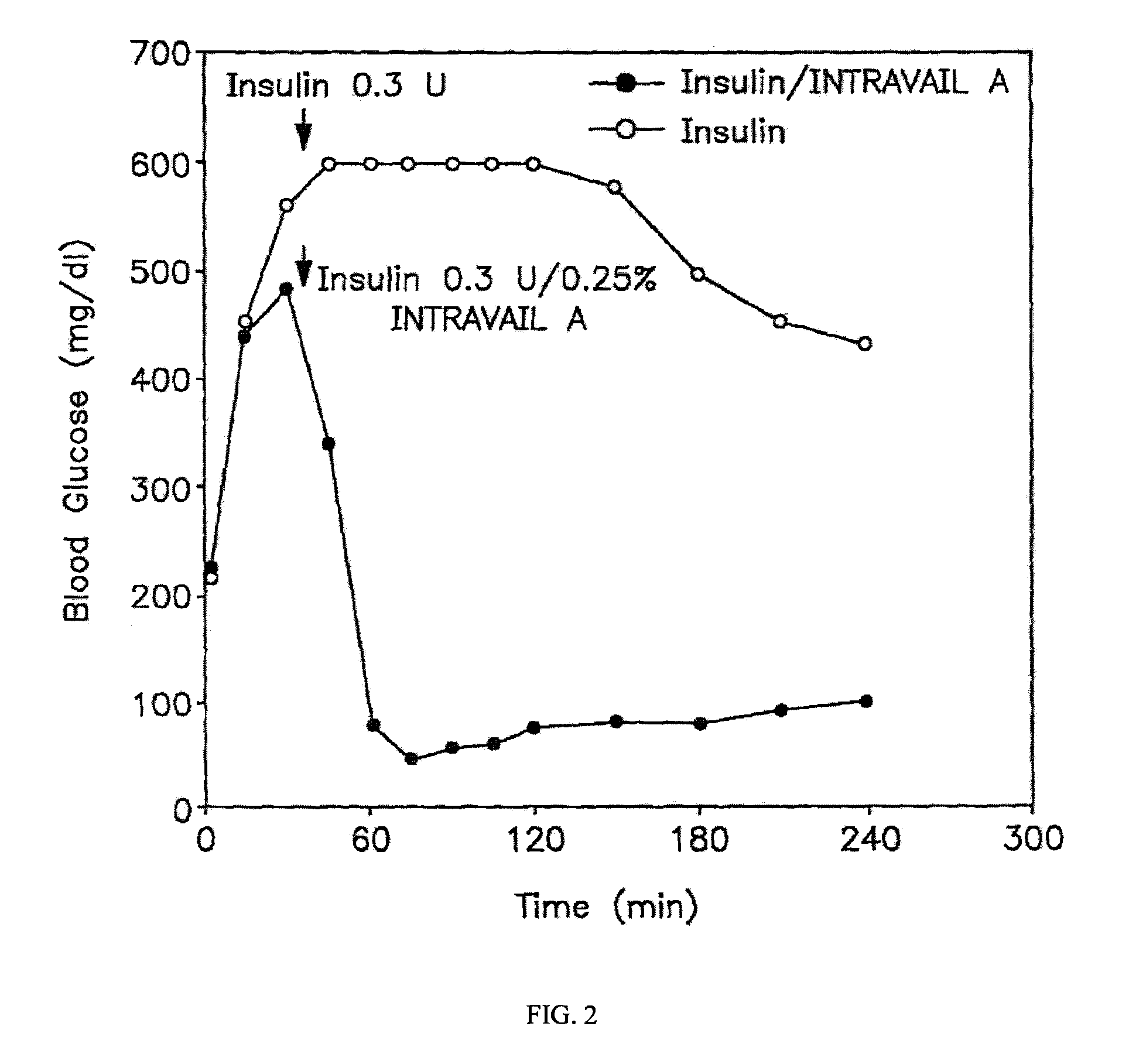
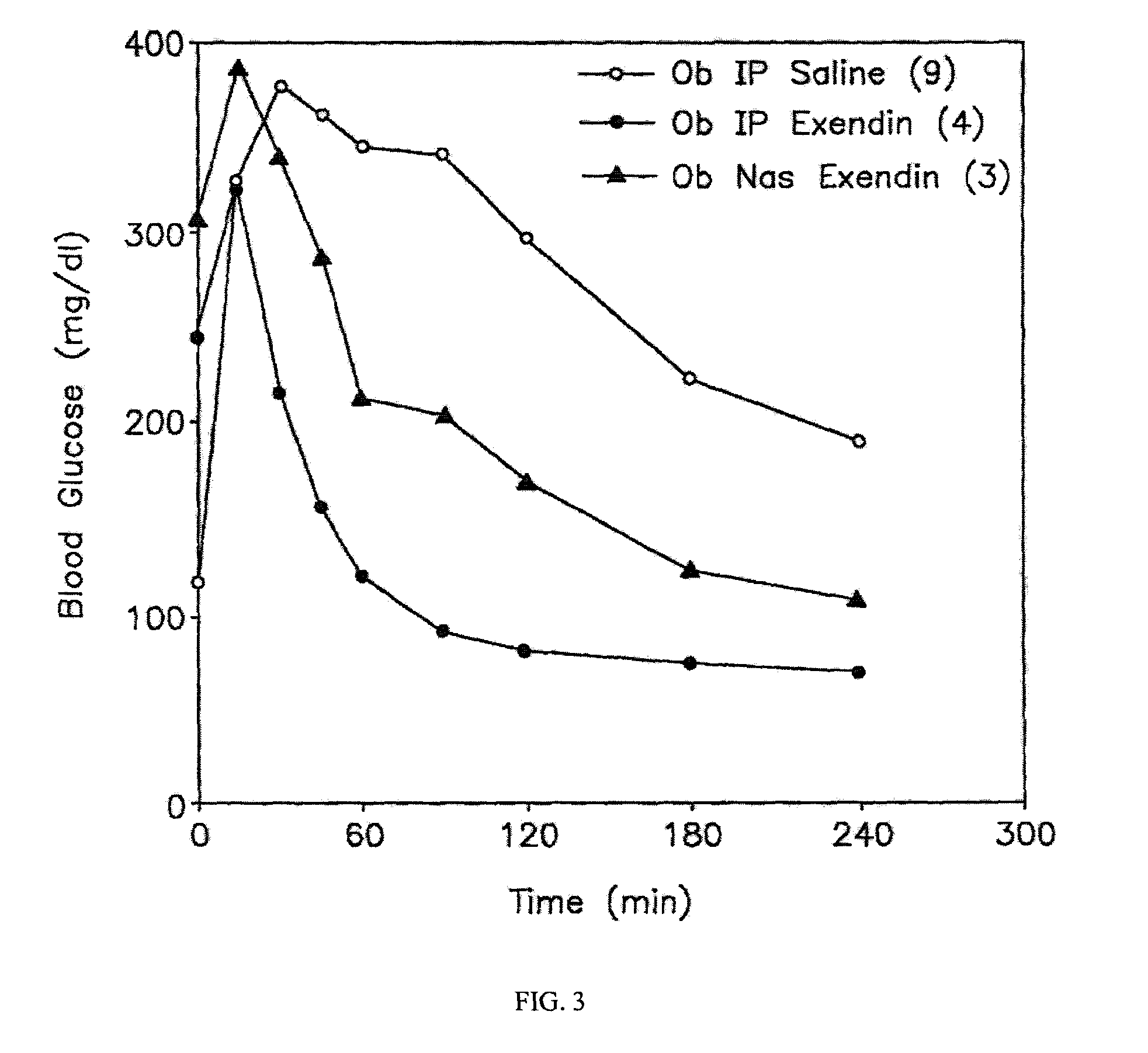
ActiveUS20080299079A1Improve absorption and bioavailabilityToxic effectsBiocideNervous disorderActive agentPancreatic hormone
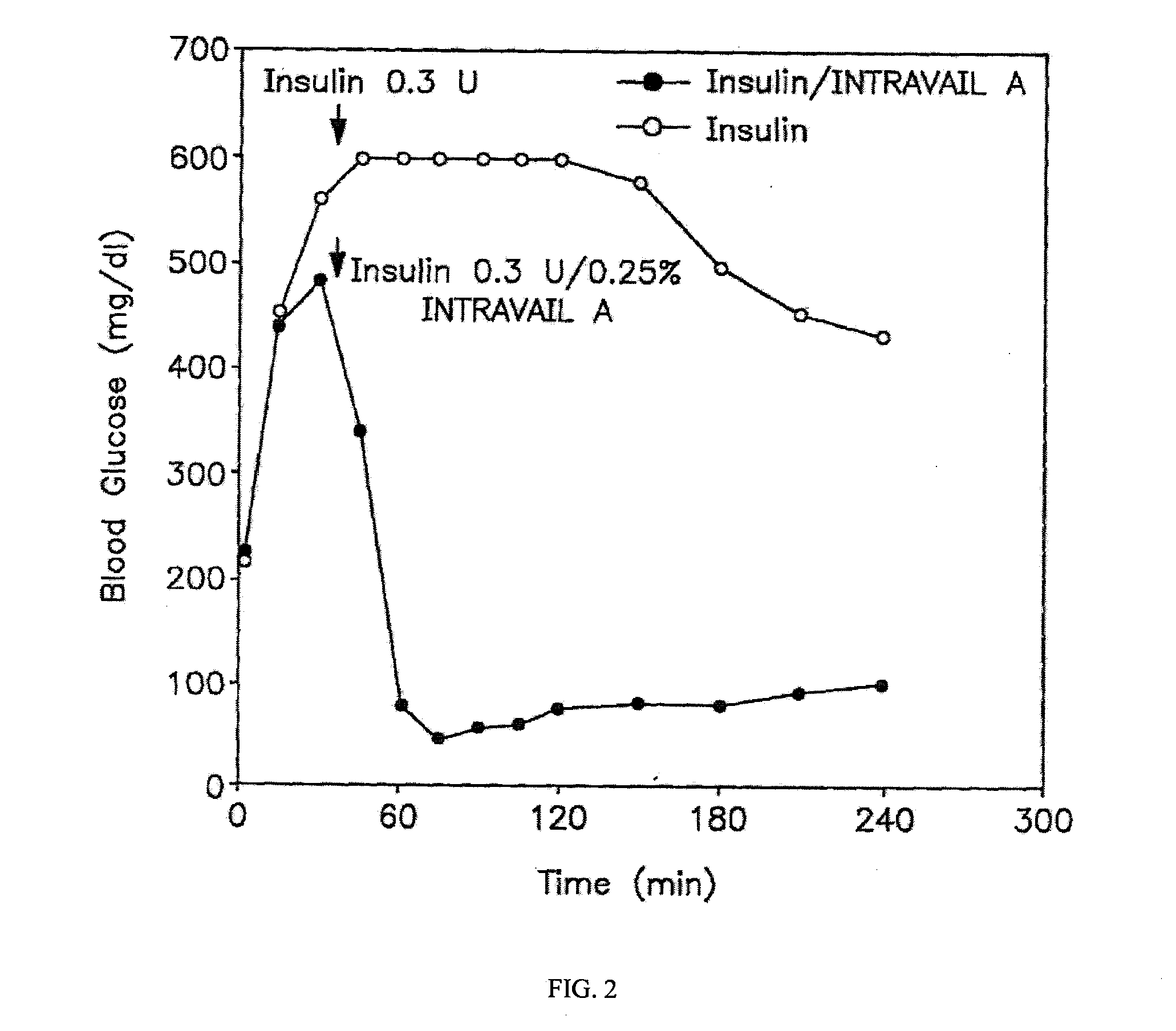
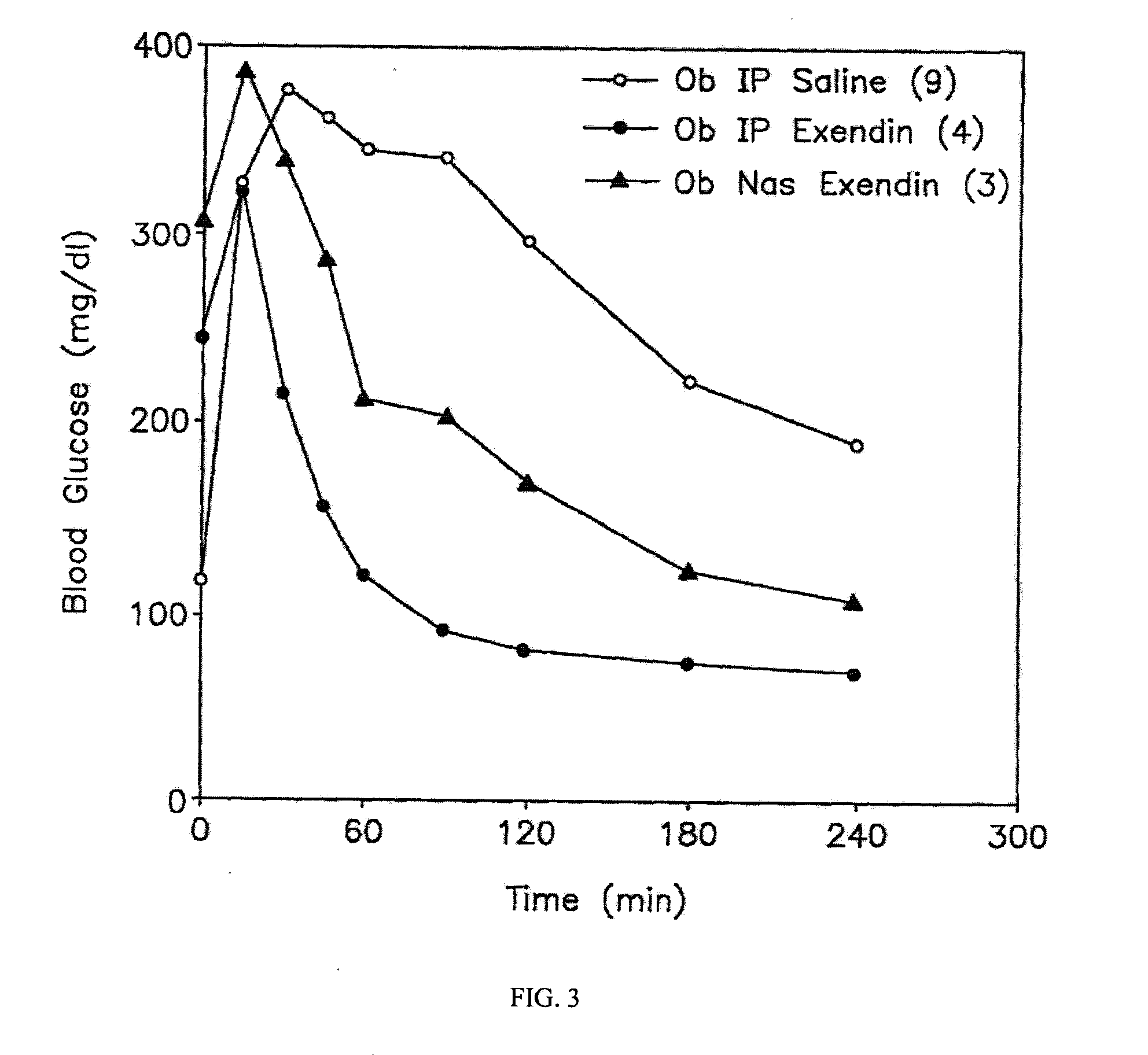
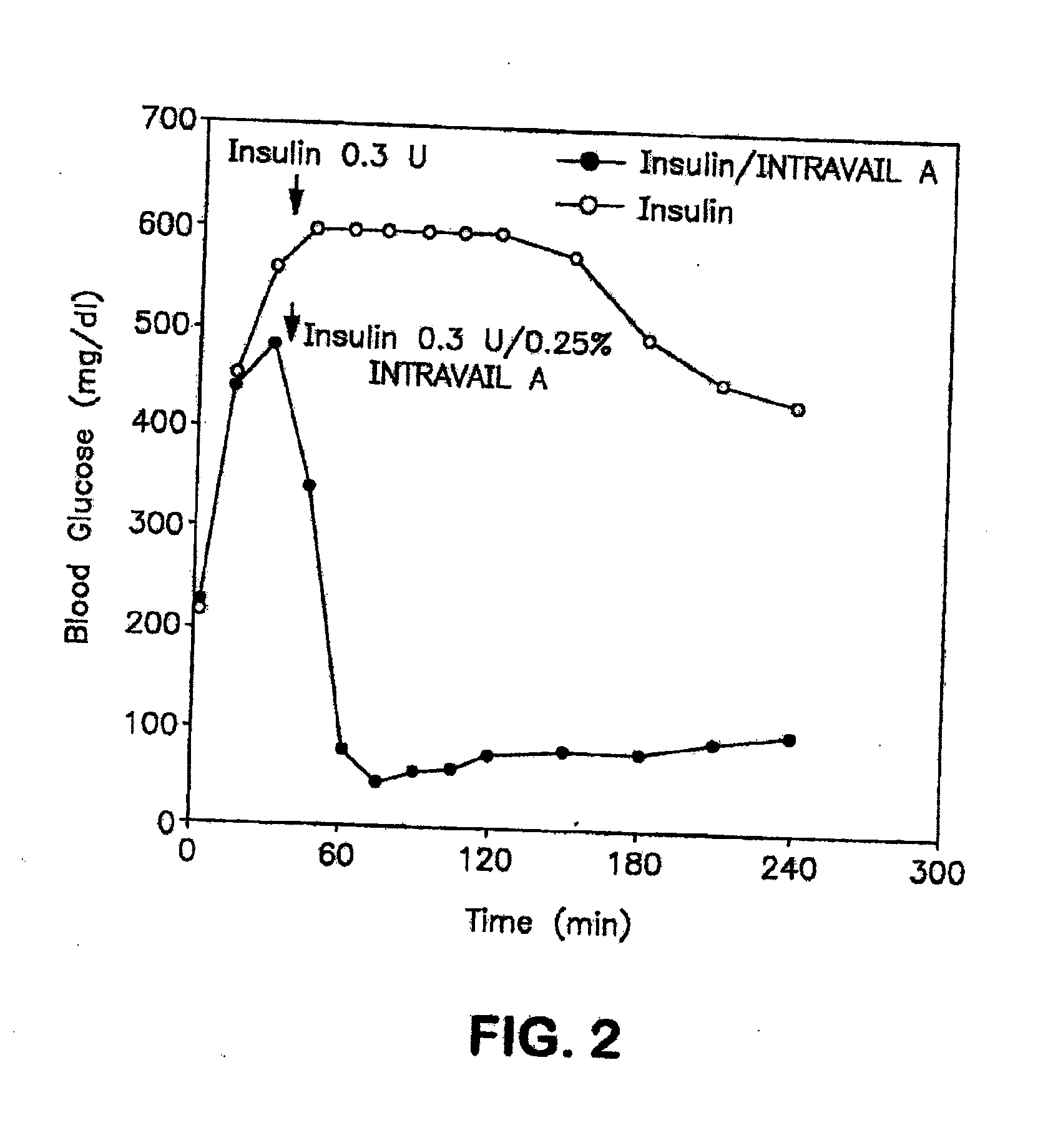
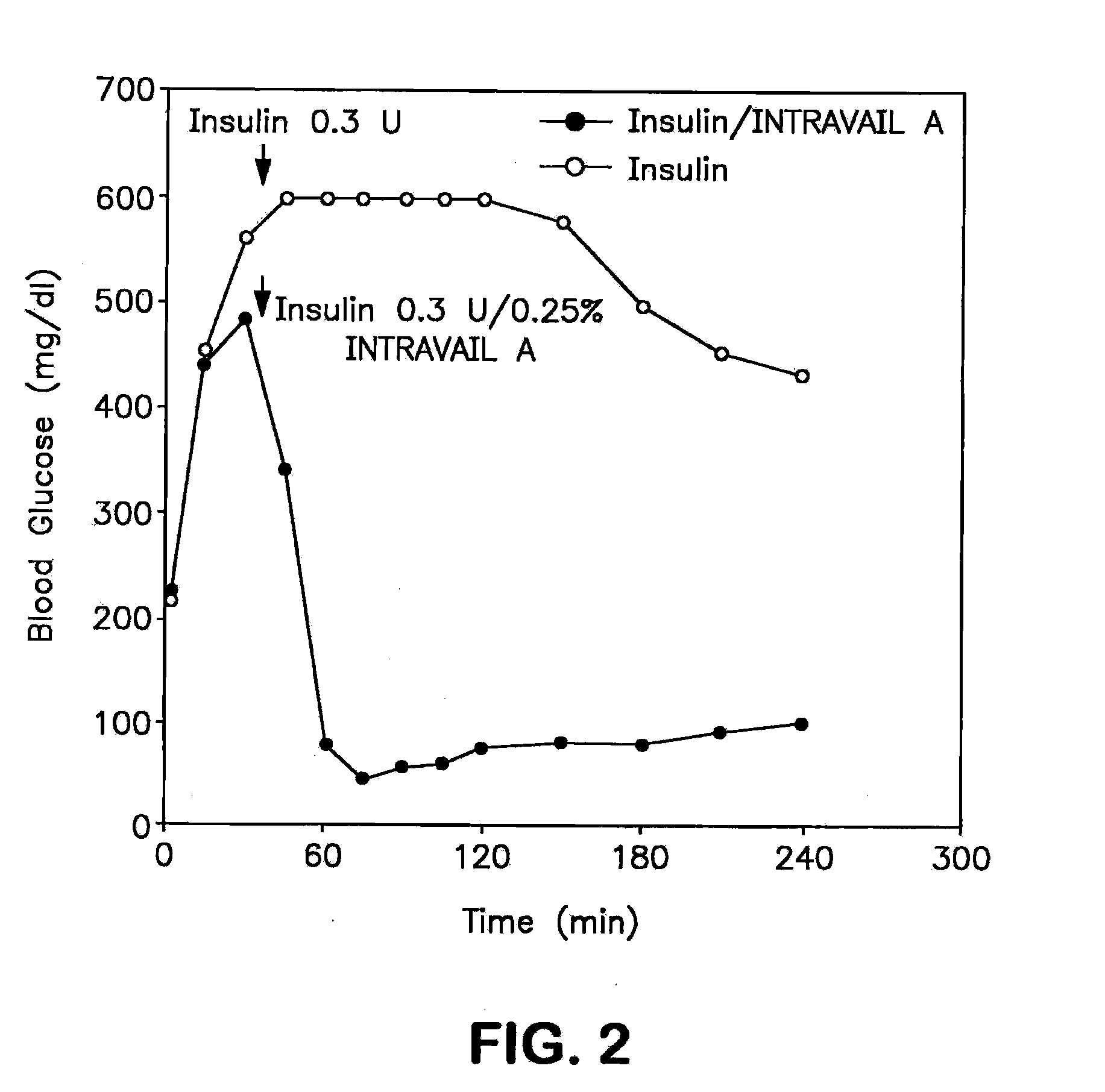
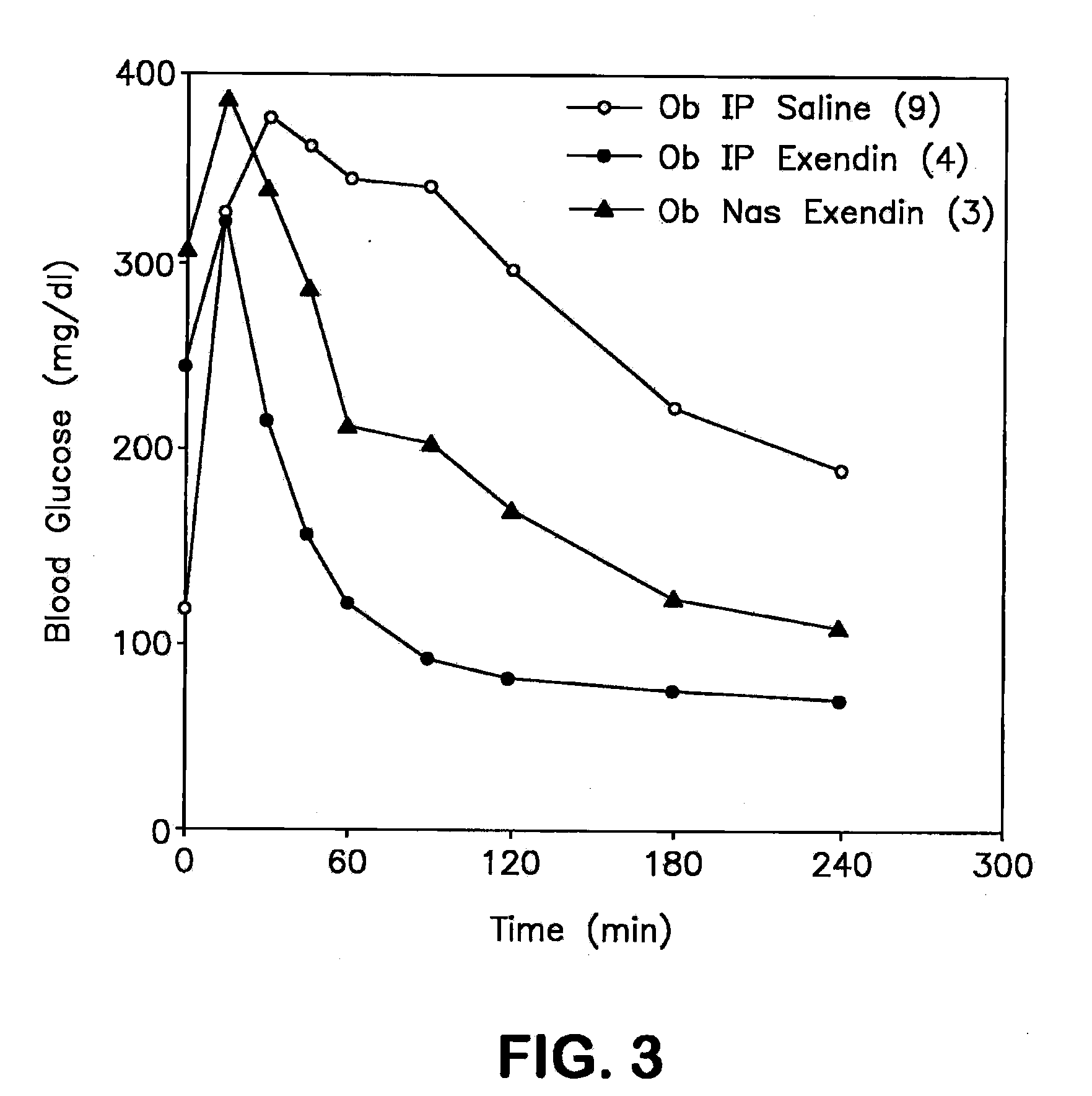
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC
Absorption enhancers for drug administration
InactiveUS20060046962A1Improve absorption and bioavailabilityHigh bioavailabilitiesBiocideNervous disorderDrugDrug administration
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:UAB RES FOUND +1
Absorption enhancers for drug administration
InactiveUS20060045869A1Avoid effectIncrease absorption and bioavailability of drugBiocideNervous disorderDrugDrug administration
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC +1
Absorption enhancers for drug administration
InactiveUS20060045868A1Improve absorption and bioavailabilityToxic effectsPowder deliveryBiocideInhalationDrug administration
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC +1
Compositions for Drug Administration
InactiveUS20090047347A1Improve absorption and bioavailabilityToxic effectsPowder deliveryBiocideDrug metabolismHepatic first pass effect
The present invention provides compositions and methods and for speeding the onset of drug action and reducing the first-pass effect drug metabolism in fast-dispersing drug formulations.
Owner:AEGIS THERAPEUTICS LLC
Compositions for Drug Administration
ActiveUS20090163447A1Promote absorptionImprove bioavailabilityBiocideNervous disorderMedicineChain length
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms.
Owner:AEGIS THERAPEUTICS LLC
Antibacterial compositions for drug administration
InactiveUS20060046969A1Avoid effectIncrease absorption and bioavailability of drugBiocidePeptide/protein ingredientsDrugDrug administration
An antibacterial composition having at least one alkyl glycoside, at least one saccharide, and at least one therapeutic agent, wherein the alkyl glycoside has an alkyl chain length from about 12 to about 14 carbon atoms and wherein the saccharide has antibacterial activity, thereby providing for a composition having antibacterial activity.
Owner:AEGIS THERAPEUTICS LLC
Use of endo-lysosomal system and secreted vesicles (exosome-like) in treatments and diagnostics based on small RNA and experimental study of small RNA
InactiveUS20110177054A1Increase reduce activityGood effectOrganic active ingredientsBiocideRegulatory rnaLipid formation
The present invention relates to a method for determining the delivery rates and / or efficiency of a siRNA, miRNA or related molecule to target organs or cells, a kit and the use of proteins or lipids involved in the formation of the endolysosomal system for modulating the activity and / or the cell-to-cell transfer of RNA, small RNA, for example miRNA, siRNA and piRNA, mRNA or non-coding RNA.It finds many applications in particular in methods for identifying the target(s) of miRNA or siRNA therapeutics, in methods for determining the efficiency of a treatment with siRNA and / or miRNA therapeutics, in methods for determining the efficiency of a treatment with siRNA and / or miRNA therapeutics, and in methods for genotyping and / or characterizing the condition of a person, a tumor or a fetus.
Owner:CENT NAT DE LA RECHERCHE SCI
Compositions for drug administration
ActiveUS20100160378A1Improve absorption and bioavailabilityToxic effectsBiocideNervous disorderChain length5-HT receptor
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject, as well as compositions and methods for providing migraine pain relief. The compositions include at least one alkyl glycoside and at least one therapeutic agent, such as a 5-HT receptor agonist, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms.
Owner:AEGIS THERAPEUTICS LLC
Compositions for drug administration
InactiveUS20110257096A1Promote absorptionImprove bioavailabilityPeptide/protein ingredientsSomatostatinsChain lengthDrug administration
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms. In various aspects, the invention provides compositions and methods for oral delivery of peptides containing non-naturally occurring structures including D-amino acids and / or chain cyclization.
Owner:AEGIS THERAPEUTICS LLC
Alkylglycoside compositions for drug administration
ActiveUS8268791B2Improve absorption and bioavailabilityToxic effectsBiocideNervous disorderGlycosideDrug administration
Owner:AEGIS THERAPEUTICS LLC
Antibacterial compositions for drug administration
ActiveUS20100068209A1Promote absorptionImprove bioavailabilityBiocidePharmaceutical delivery mechanismMicroorganismGlycoside formation
Non-toxic aqueous compositions are provided having antibacterial activity. Specifically, the invention provides aqueous alkyl glycoside or saccharide alkyl ester compositions which meet the antimicrobial effectiveness test criteria set forth in USP 31 <51> in preventing growth of specified bacteria and fungi.
Owner:AEGIS THERAPEUTICS LLC
Catheter having antimicrobial coating
InactiveUS20130245568A1Good biocompatibilityAvoid introducingPharmaceutical delivery mechanismCatheterTitanium surfaceCatheter
A catheter for human or animal vascular engagement is provided. The catheter has at least one interior lumen defined by a sidewall having an exterior circumferential. A titanium surface area is positioned along the circumferential surface entirely or at one or both ends to enhance lubricity. Additionally included may be an anti pathogenic and / or anti microbial surface area positioned adjacent to the titanium surface area of the circumferential surface.
Owner:PFM MEDICAL
Compositions for drug administration
ActiveUS20080200418A1Improve absorption and bioavailabilityToxic effectsPharmaceutical delivery mechanismCarbohydrate active ingredientsGlycosideChain length
A composition having at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms and wherein the therapeutic agent is an oligonucleotide.
Owner:AEGIS THERAPEUTICS LLC
Compositions for drug administration
ActiveUS20140107145A1Improve absorption and bioavailabilityToxic effectsBiocidePharmaceutical delivery mechanismChain lengthBioavailability
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms.
Owner:INDIVIOR UK +1
Compositions for drug administration
ActiveUS9895444B2Improve absorption and bioavailabilityToxic effectsOrganic active ingredientsPharmaceutical delivery mechanismChain lengthDrug administration
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms.
Owner:INDIVIOR UK +1
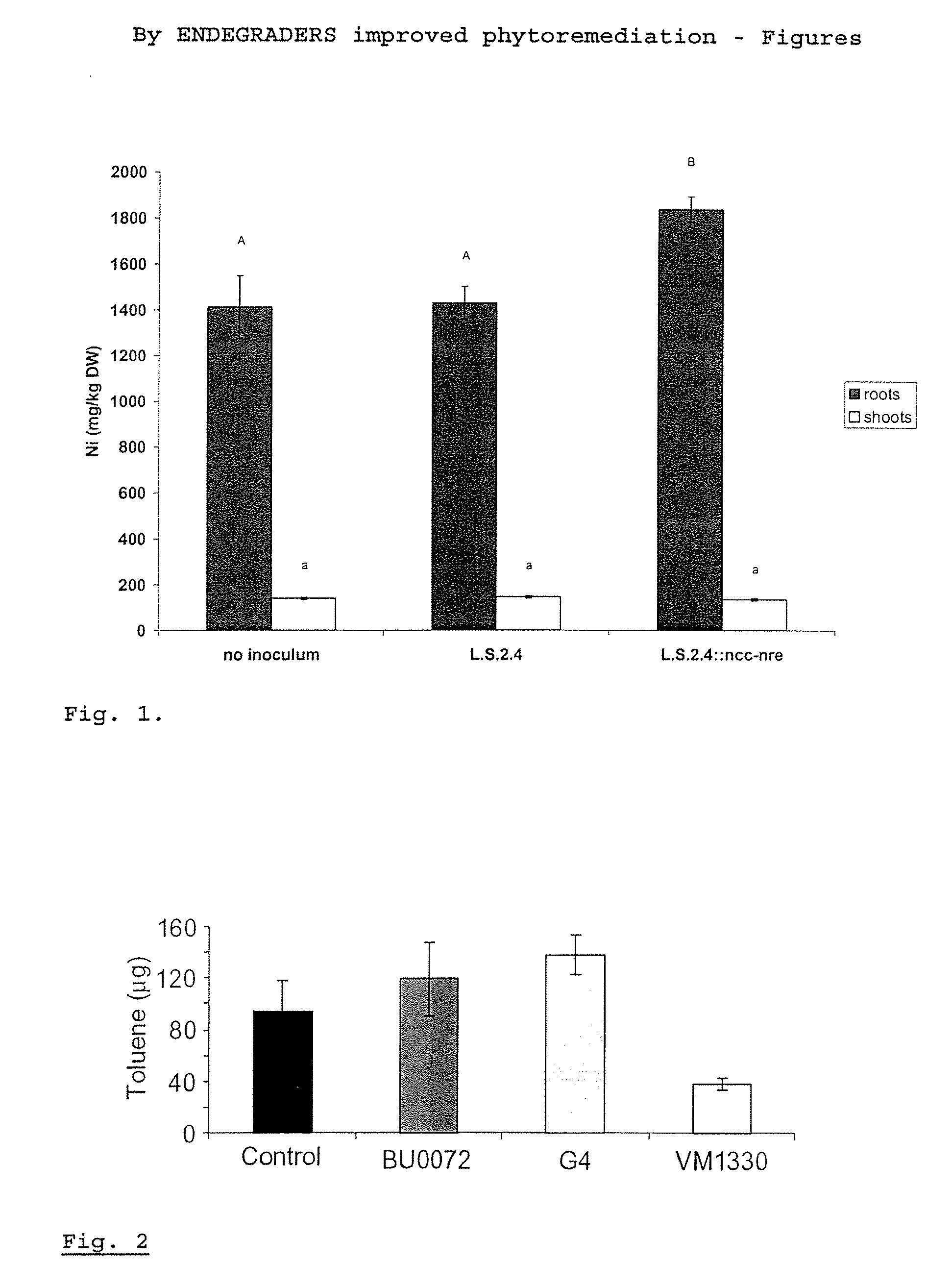
Method for Improving Phytoremediation Treatment of a Contaminated Medium
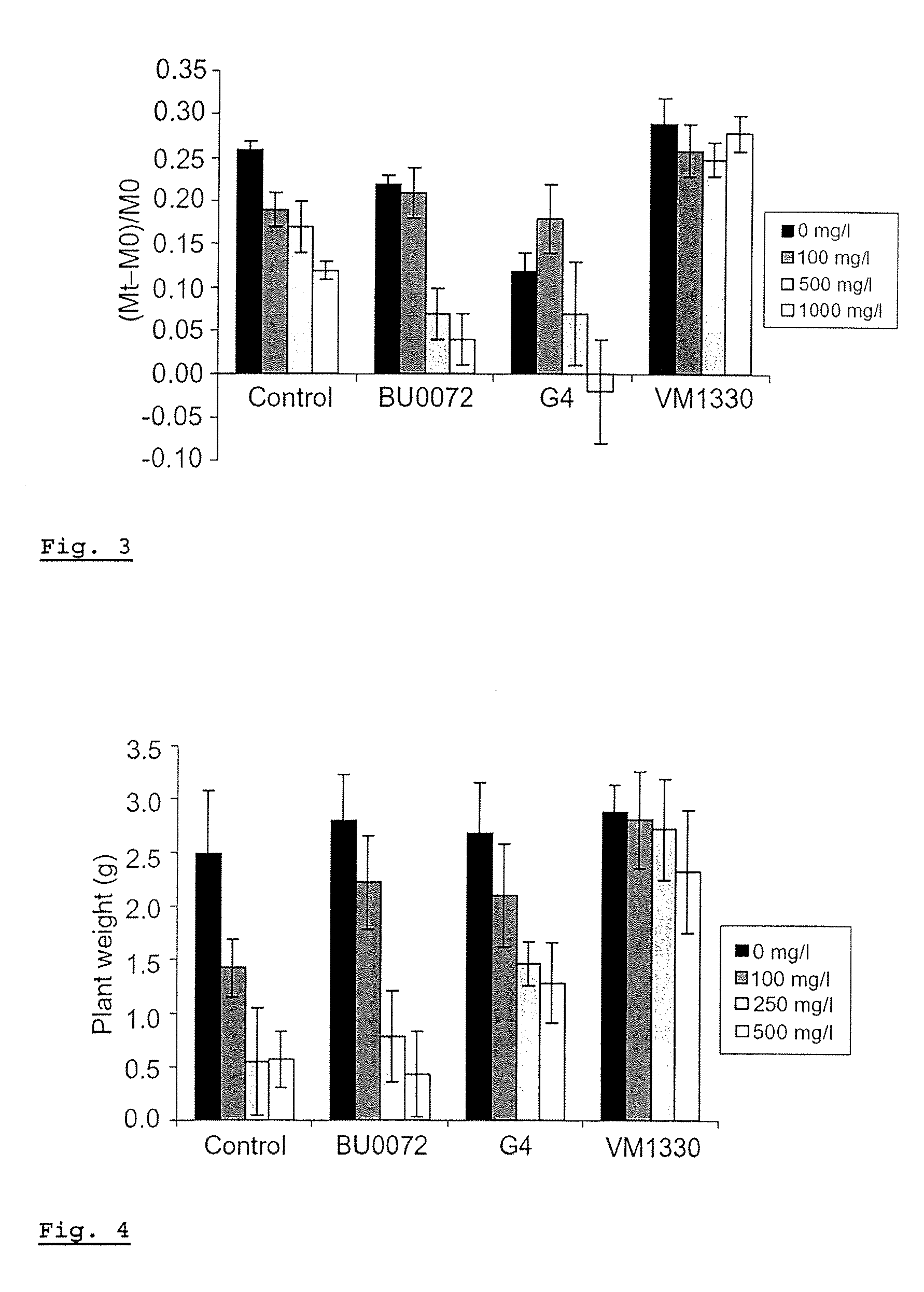
InactiveUS20070101461A1Efficient degradationReduce accumulationBryophytesSolid waste disposalPhytotoxicityCompound (substance)
The invention concerns a method for the phytoremediation treatment of a medium contaminated with at least one element selected from the group consisting of (preferably water soluble and volatile) organic pollutants, heavy metals, radionuclides or a mixture thereof, comprising the step of cultivating upon the contaminated medium a plant associated with an endophytic microorganism able to improve the phytoremediation of the plant, to reduce phytotoxicity of chemicals. The invention further relates to methods for improving phytoremediation by directly modifying members of the endogenous endophytic community of a plant, via horizontal gene transfer. Another part of the invention relates to plants associated with such endophytes and / or plants with a modified endogenous endophytic community.
Owner:VAN DER LELIE DANIEL +7
Compositions for drug administration
InactiveUS20120021980A1Improve absorption and bioavailabilityToxic effectsPeptide/protein ingredientsMetabolism disorderChain lengthDrug administration
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms. In various aspects, the invention provides compositions and methods for oral delivery of glucagon-like peptide-1 analogs, such as exenatide, albiglutide, taspoglutide, liraglutide and lixisenatide.
Owner:AEGIS THERAPEUTICS LLC
Compositions for oral drug administration
ActiveUS20180104344A1Improve absorption and bioavailabilityToxic effectsCarbohydrate active ingredientsPharmaceutical non-active ingredientsGlycosideChain length
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms. In various aspects, the invention provides compositions and methods for oral delivery in the form of a tablet.
Owner:AEGIS THERAPEUTICS LLC
Pharmaceutical composition containing bakuchiol for treating woman osteoporosis
InactiveUS7714026B2Increased riskPrevent and treat osteoporosisBiocideHydroxy compound active ingredientsOral medicationOsteopetrosis
The present invention discloses a novel use of bakuchiol or an extract containing bakuchiol in preventing or treating a woman suffering osteoporosis. An embodiment of this novel use is a pharmaceutical composition containing bakuchiol or an extract containing bakuchiol, which can be in the dosage forms of topical use, oral administration, injection or sustained release. The present invention also discloses a novel use of bakuchiol or an extract containing bakuchiol in preventing or treating a woman suffering breast cancer.
Owner:SINPHAR PHARM CO LTD
Modified Y type molecular sieve
ActiveCN103086397AMulti-surface acid contentExcellent anti-alkali nitrogen performanceFaujasite aluminosilicate zeoliteRare earthMolecular sieve
The present invention discloses a modified Y type molecular sieve, which is characterized in that the molecular sieve is modified by using rare earth, iron, copper and phosphorus, wherein rare earth content in the molecular sieve is 8-23 wt% (calculated as rare earth oxide), iron content in the molecular sieve is 0.1-3.0 wt% (calculated as Fe2O3), copper content in the molecular sieve is 0-3.0 wt% (calculated as CuO), phosphorus content in the molecular sieve is 0-2.0 wt% (calculated as P2O5), and sodium oxide content in the molecular sieve is 0.1-2.5 wt%. According to the modified Y type molecular sieve, a molecular sieve acid amount measured by <31>P MAS NMR after absorbing tri-n-butylphosphine oxide is 1.400-4.500 mmol.g<-1>, and a molecular sieve acid amount measured by <31>P MAS NMR after absorbing trimethyl phosphine oxide (TMPO) is 2.300-6.600 mmol.g<-1>. The molecular sieve has a high acid amount, especially a high outer surface acid amount, and can be adopted as an active component to effectively resist harm effects on the molecular sieve acid center by the basic nitrogen macromolecules so as to provide an excellent anti-basic nitrogen performance.
Owner:CHINA PETROLEUM & CHEM CORP +1
Absorption enhancers for drug administration
ActiveUS20120196941A1Improve absorption and bioavailabilityToxic effectsBiocideNervous disorderIrritationInhalation
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC
Compositions for drug administration
ActiveUS8642564B2Improve absorption and bioavailabilityToxic effectsBiocideGenetic material ingredientsGlycosideChain length
A composition having at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms and wherein the therapeutic agent is an oligonucleotide.
Owner:AEGIS THERAPEUTICS LLC
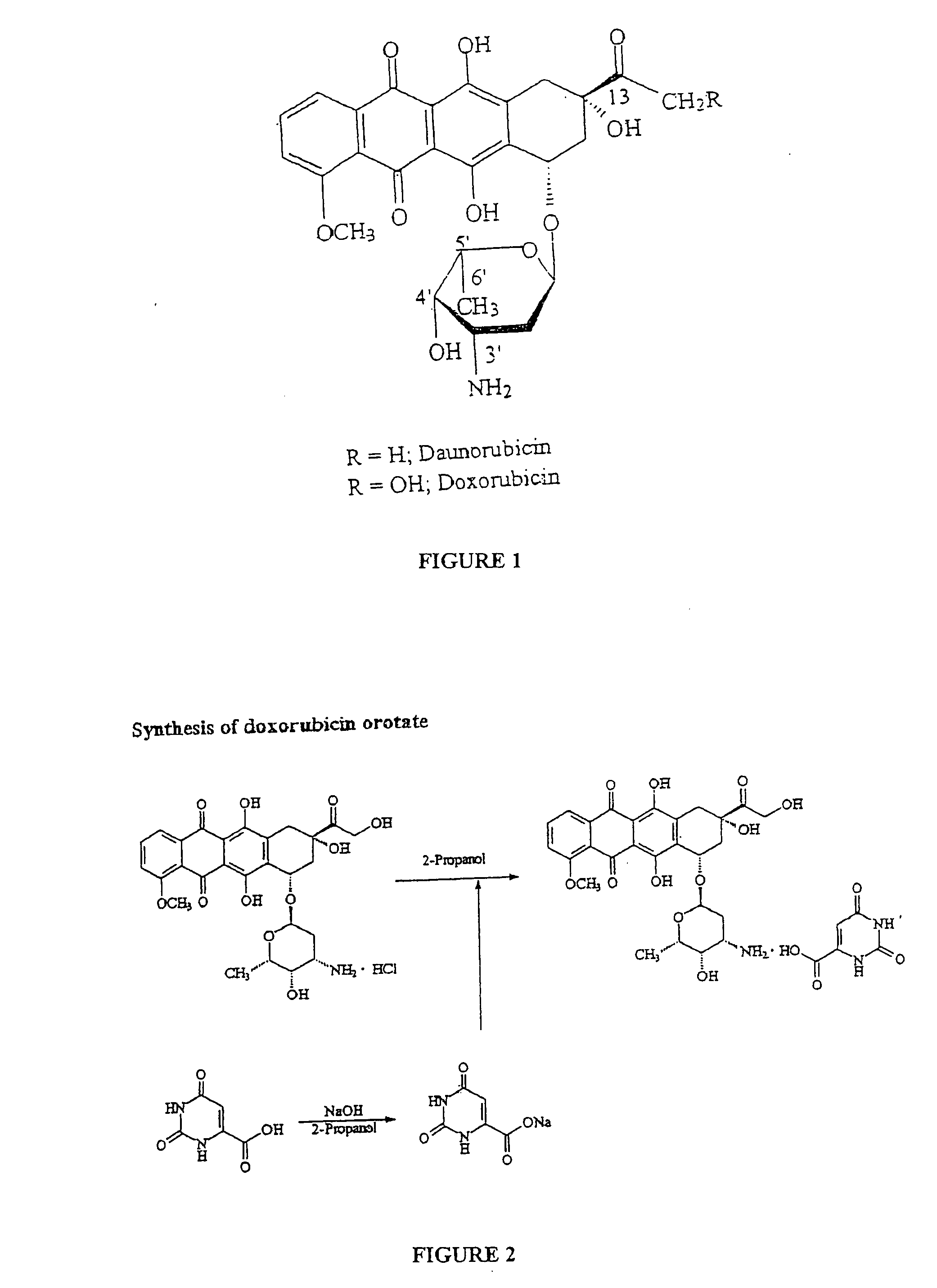
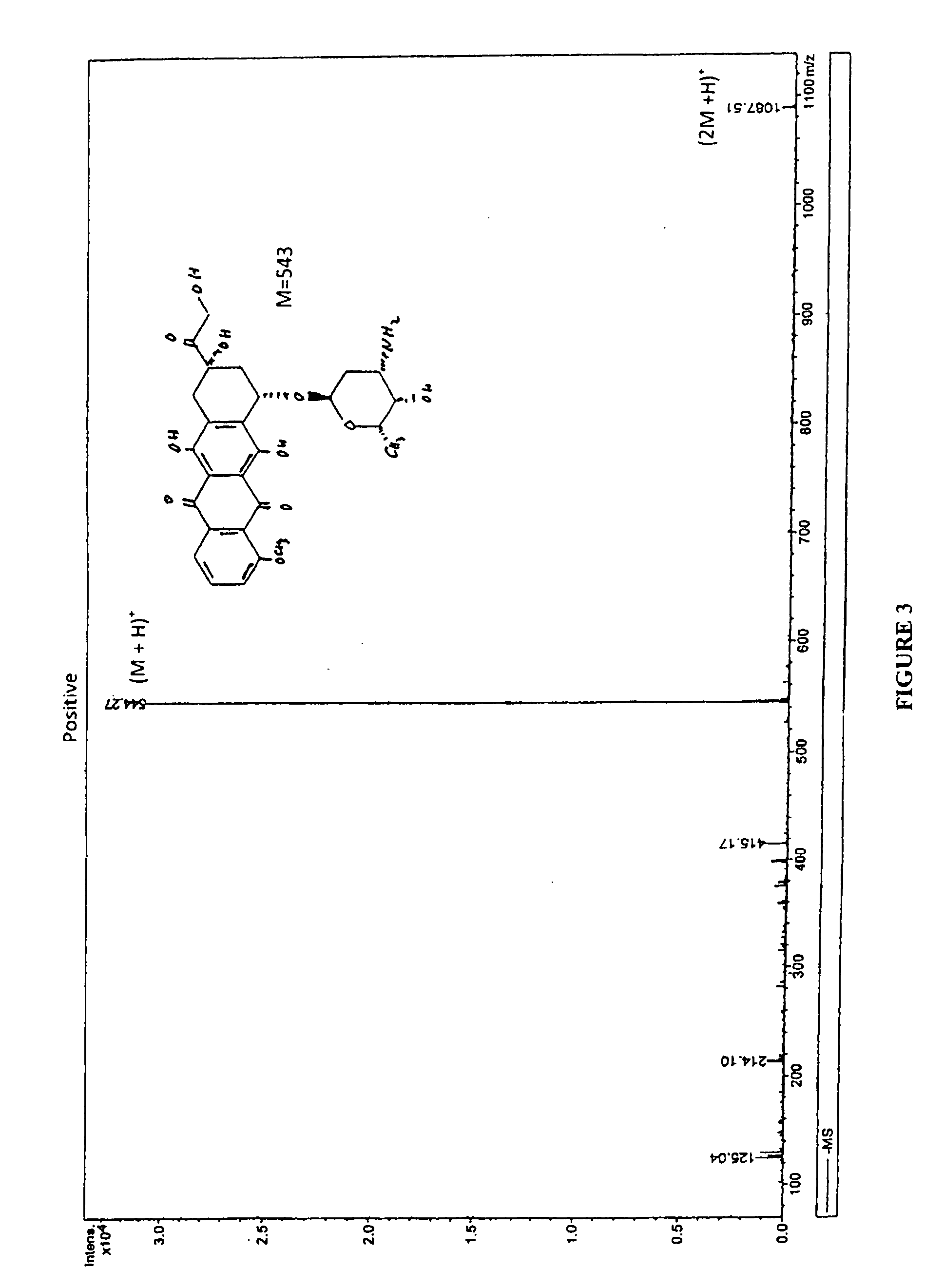
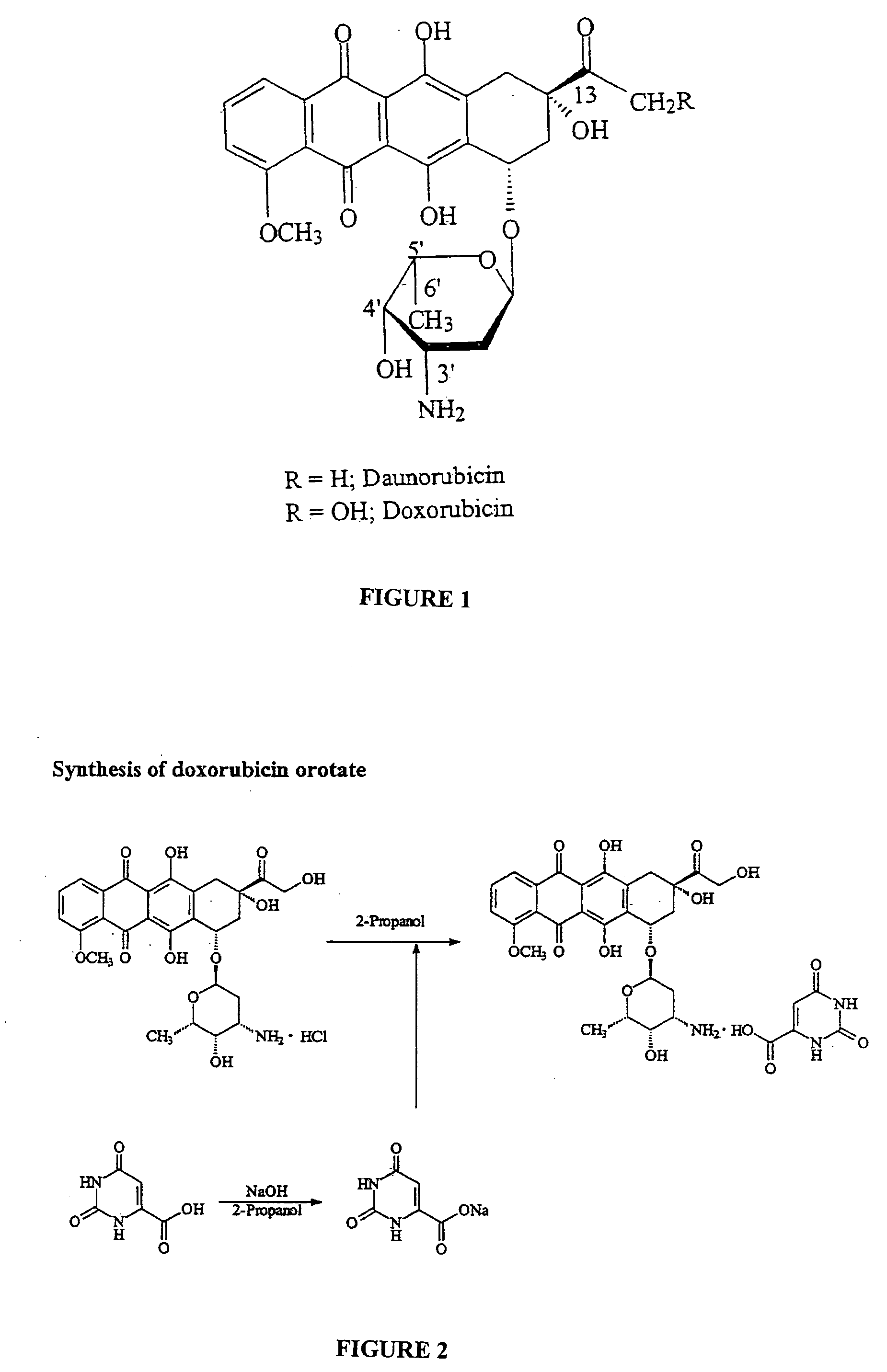
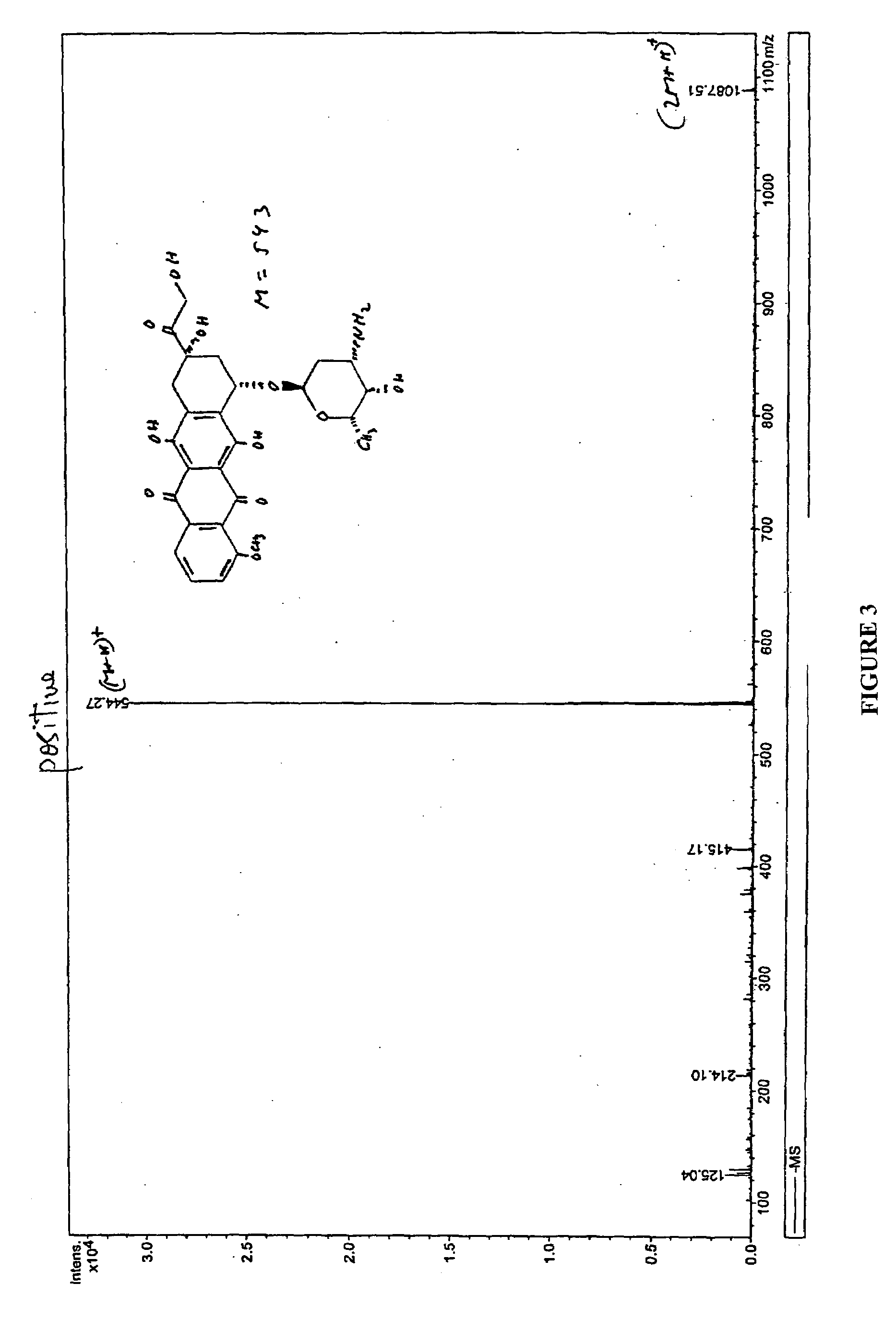
Compositions and methods of reducing tissue levels of drugs when given as orotate derivatives
ActiveUS20090131344A1Low toxicityToxic effectsSalicyclic acid active ingredientsBiocideSide effectTissue toxicity
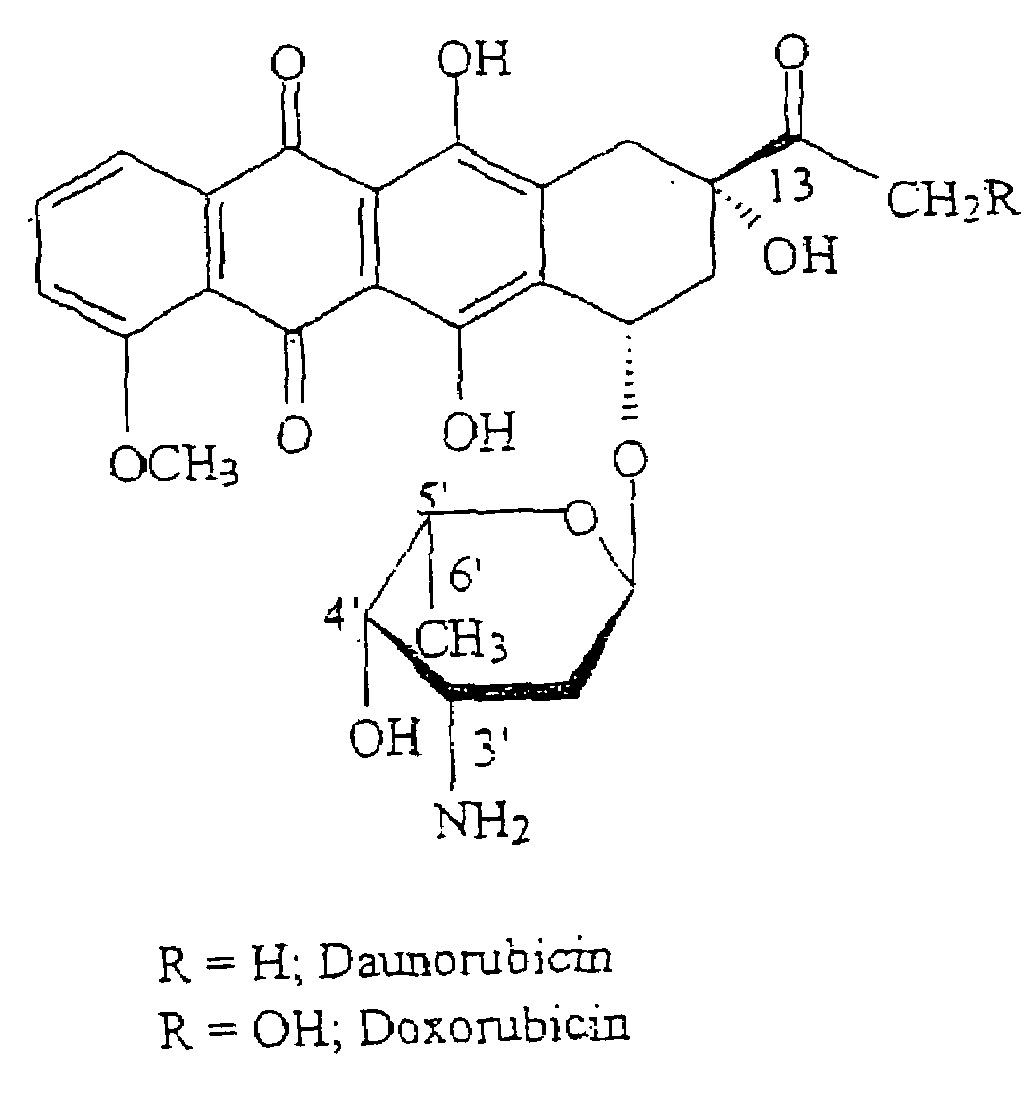
This invention is in the field of chemical restructuring of pharmaceutical agents known to cause tissue toxicity as a side effect, by producing their orotate derivatives. More particularly, it concerns orotate derivatives of the anthracyclines, doxorubicin and daunorubicin, that are found to reduce levels of the pharmaceutical agent in noncancerous tissues. These orotate derivatives are equally efficacious in inhibiting the SCCAKI-1 kidney tumor in animals and the reduction in the heart tissue of doxorubicin compared with doxorubicin HCl suggests a reduction in toxicity induced by free radical generation by the anthracyclines.
Owner:SAVVIPHARM INC
Compositions for drug administration
InactiveUS20180000942A1Promote absorptionImprove bioavailabilityOrganic active ingredientsInorganic non-active ingredientsChain lengthBioavailability
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms.
Owner:AEGIS THERAPEUTICS LLC
Pharmaceutical composition containing bakuchiol for treating woman osteoporosis
InactiveUS20050256209A1Increased riskPrevent and treat osteoporosisBiocideHydroxy compound active ingredientsOral medicationMedicine
The present invention discloses a novel use of bakuchiol or an extract containing bakuchiol in preventing or treating a woman suffering osteoporosis. An embodiment of this novel use is a pharmaceutical composition containing bakuchiol or an extract containing bakuchiol, which can be in the dosage forms of topical use, oral administration, injection or sustained release. The present invention also discloses a novel use of bakuchiol or an extract containing bakuchiol in preventing or treating a woman suffering breast cancer.
Owner:SINPHAR PHARM CO LTD
Compositions and methods of reducing tissue levels of drugs when given as orotate derivatives
ActiveUS20080025949A1Lower drug concentrationLow toxicityBiocideSalicyclic acid active ingredientsSide effectTissue toxicity
This invention is in the field of chemical restructuring of pharmaceutical agents known to cause tissue toxicity as a side effect, by producing their orotate derivatives. More particularly, it concerns orotate derivatives of the anthracyclines, doxorubicin and daunorubicin, that are found to reduce levels of the pharmaceutical agent in noncancerous tissues. There orotate derivatives are equally efficacious in inhibiting the SCCAKI-1 kidney tumor in animals and the reduction in the heart tissue of doxorubicin compared with doxorubicin HCl suggests a reduction in toxicity induced by free radical generation by the anthrracyclines.
Owner:SAVVIPHARM INC
Compositions for drug administration
ActiveUS8440631B2Improve absorption and bioavailabilityToxic effectsBiocideNervous disorder5-HT receptorChain length
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject, as well as compositions and methods for providing migraine pain relief. The compositions include at least one alkyl glycoside and at least one therapeutic agent, such as a 5-HT receptor agonist, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms.
Owner:AEGIS THERAPEUTICS LLC
Method for improving phytoremediation treatment of a contaminated medium
InactiveUS20050150003A1Efficient degradationReduce accumulationMicroorganismsContaminated soil reclamationPhytotoxicityCompound (substance)
A method for the phytoremediation treatment of a contaminated medium with at least one element selected from the group consisting of (preferably water soluble and volatile) organic pollutants, heavy metals, radionuclides or a mixture thereof, comprising the step of cultivating upon said contaminated medium a plant associated with an endophytic microorganism able to improve the phytoremediation of said plant, to reduce phytotoxicity of chemicals, and the step of recovering the elements present in said plant.
Owner:VAN DER LELIE DANIEL +7
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com