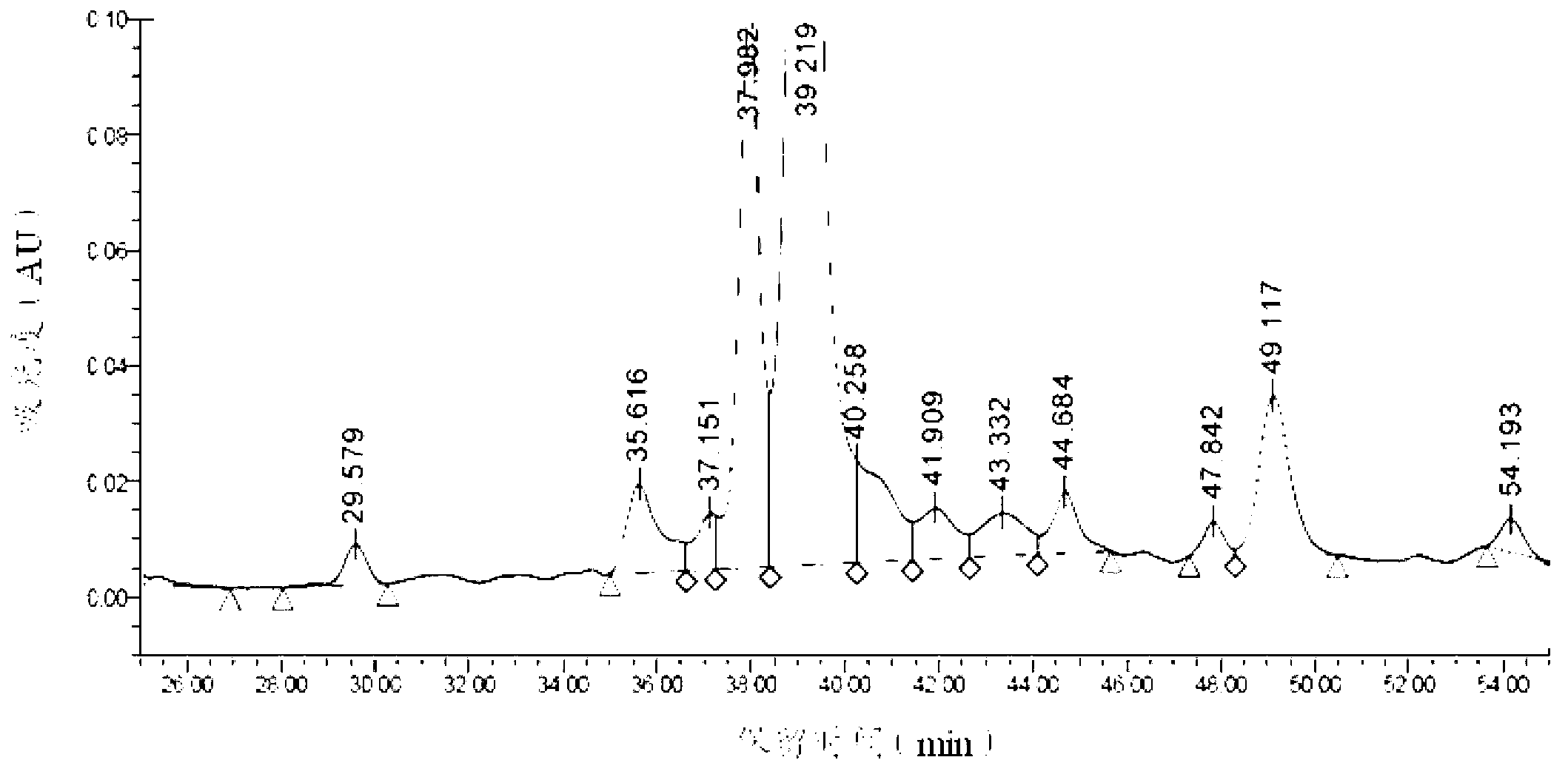
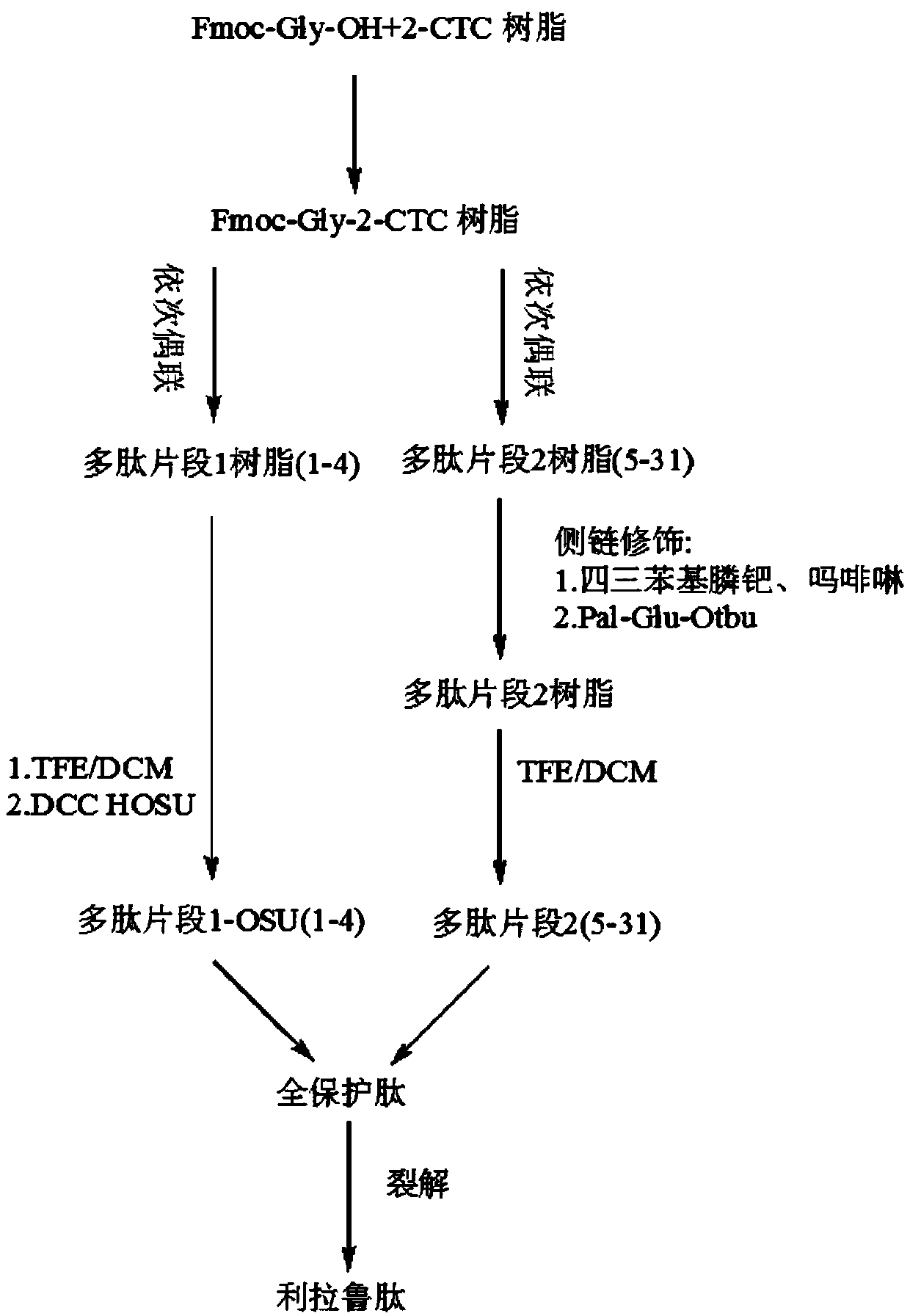
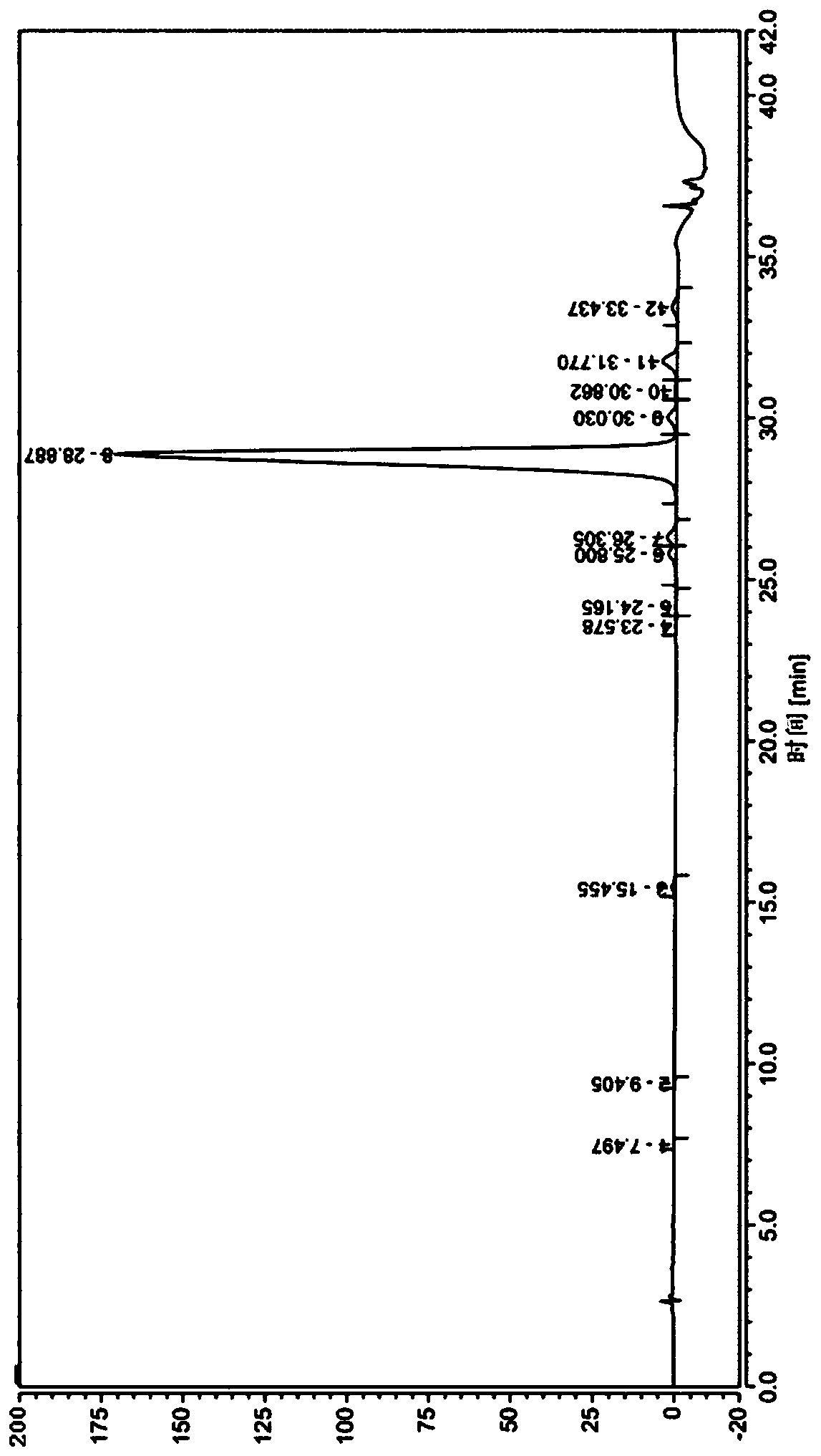
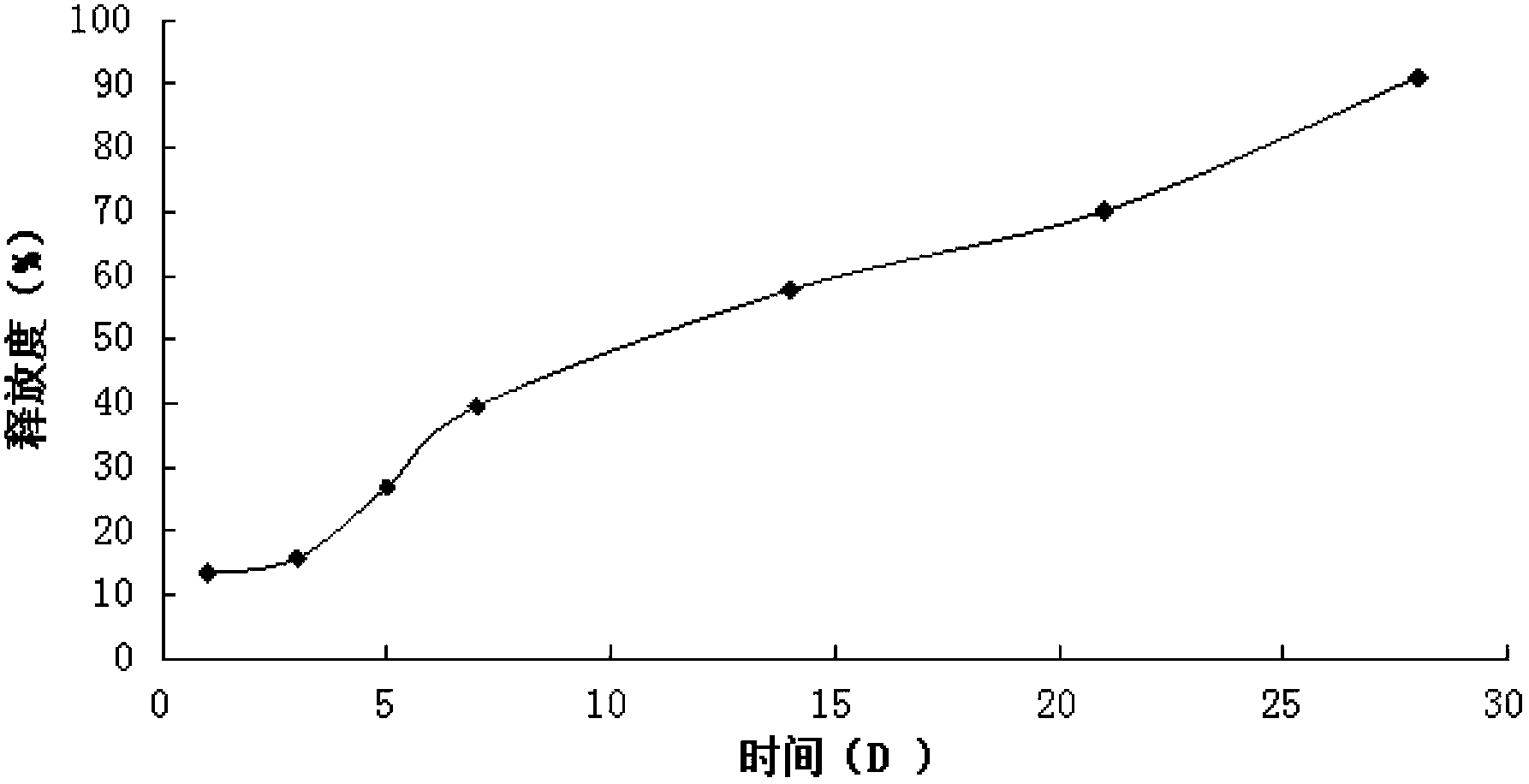
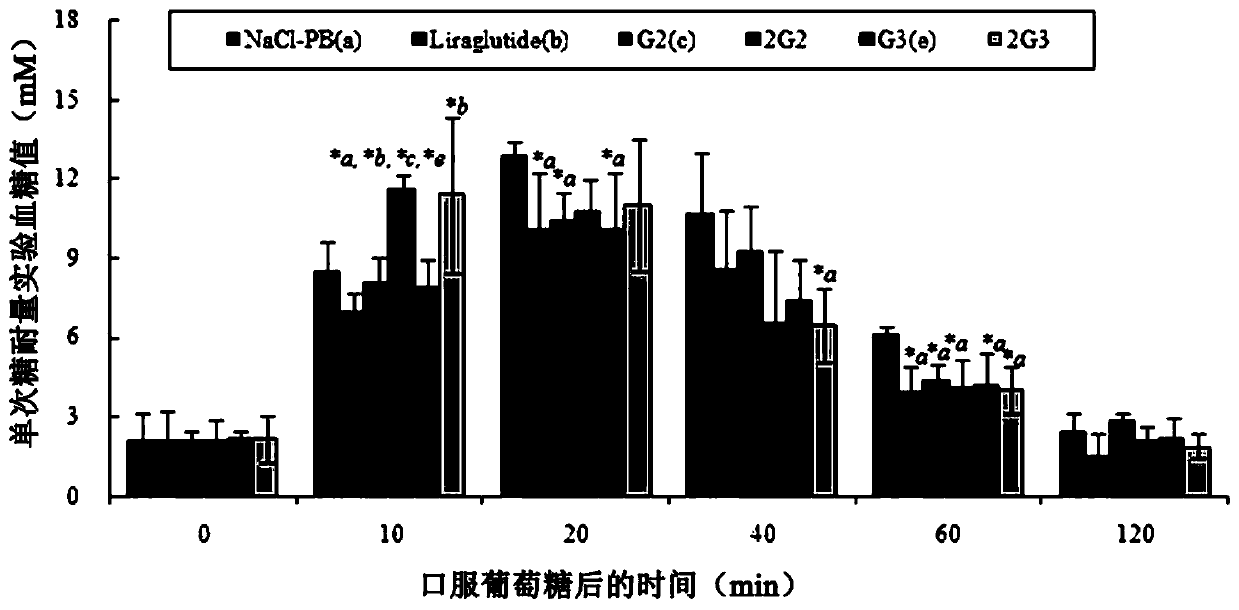
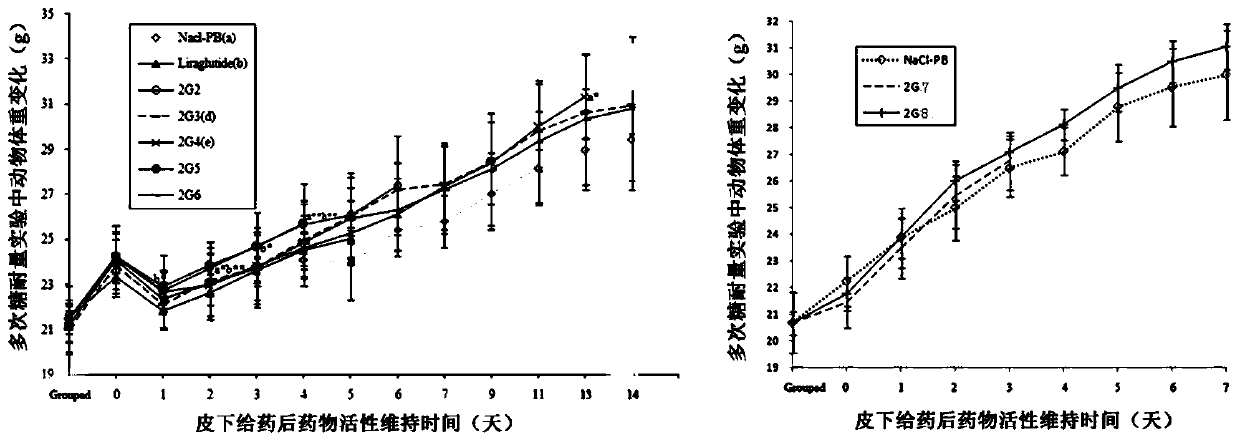
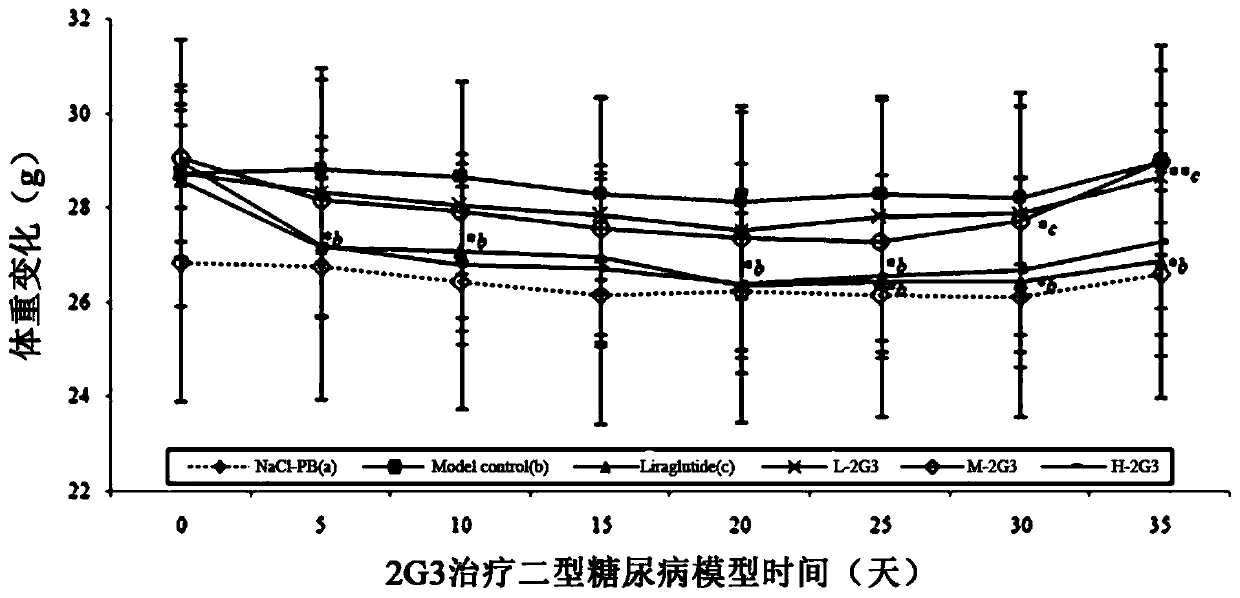
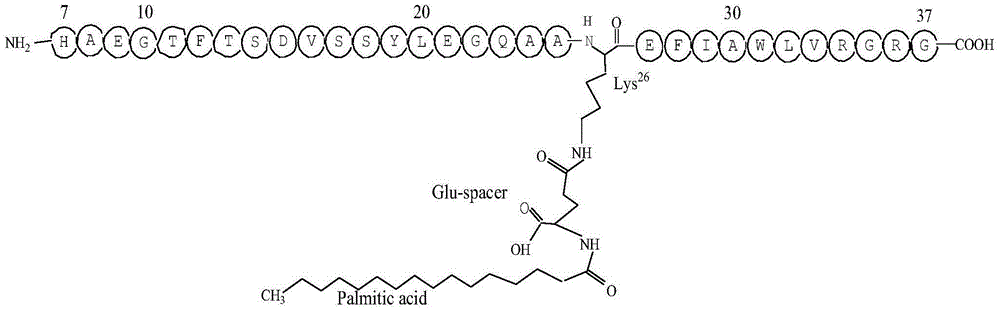
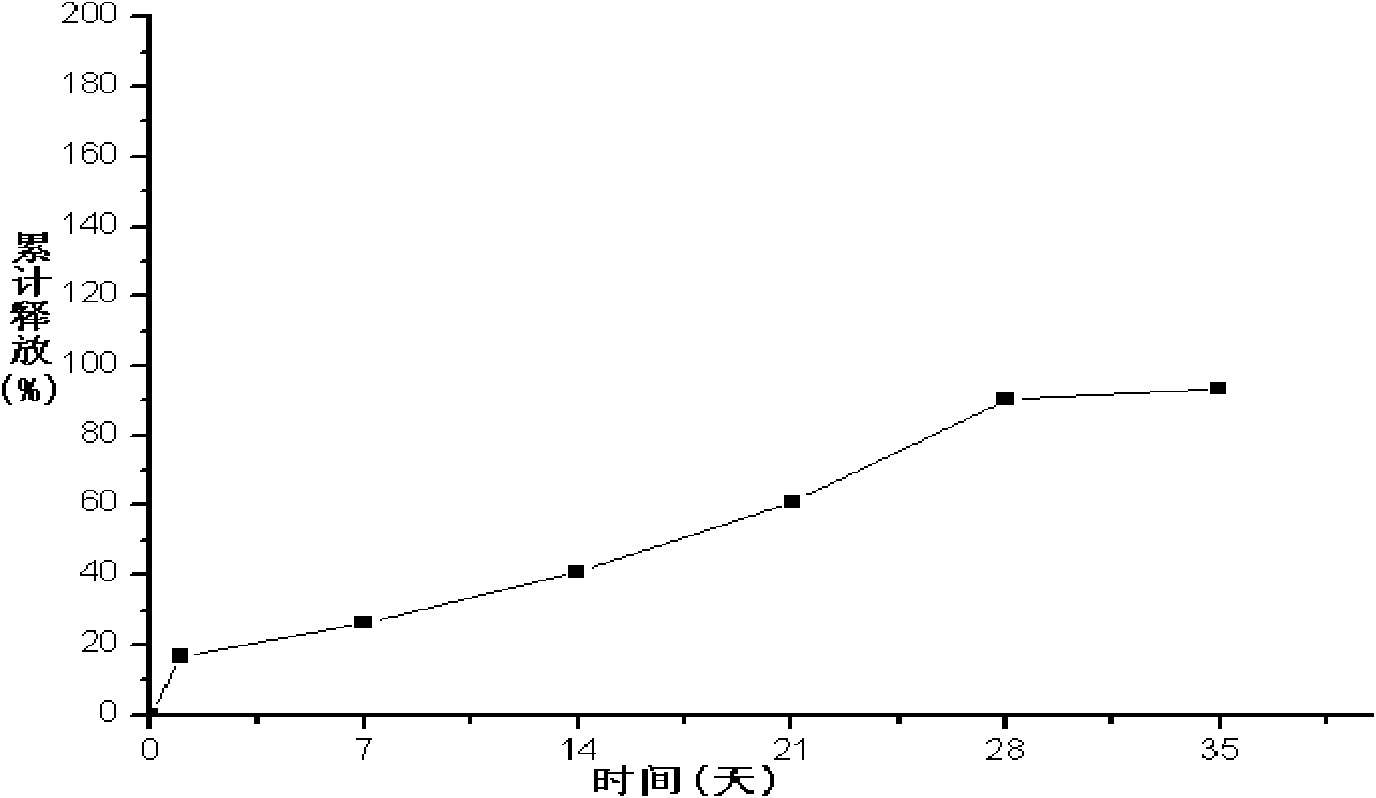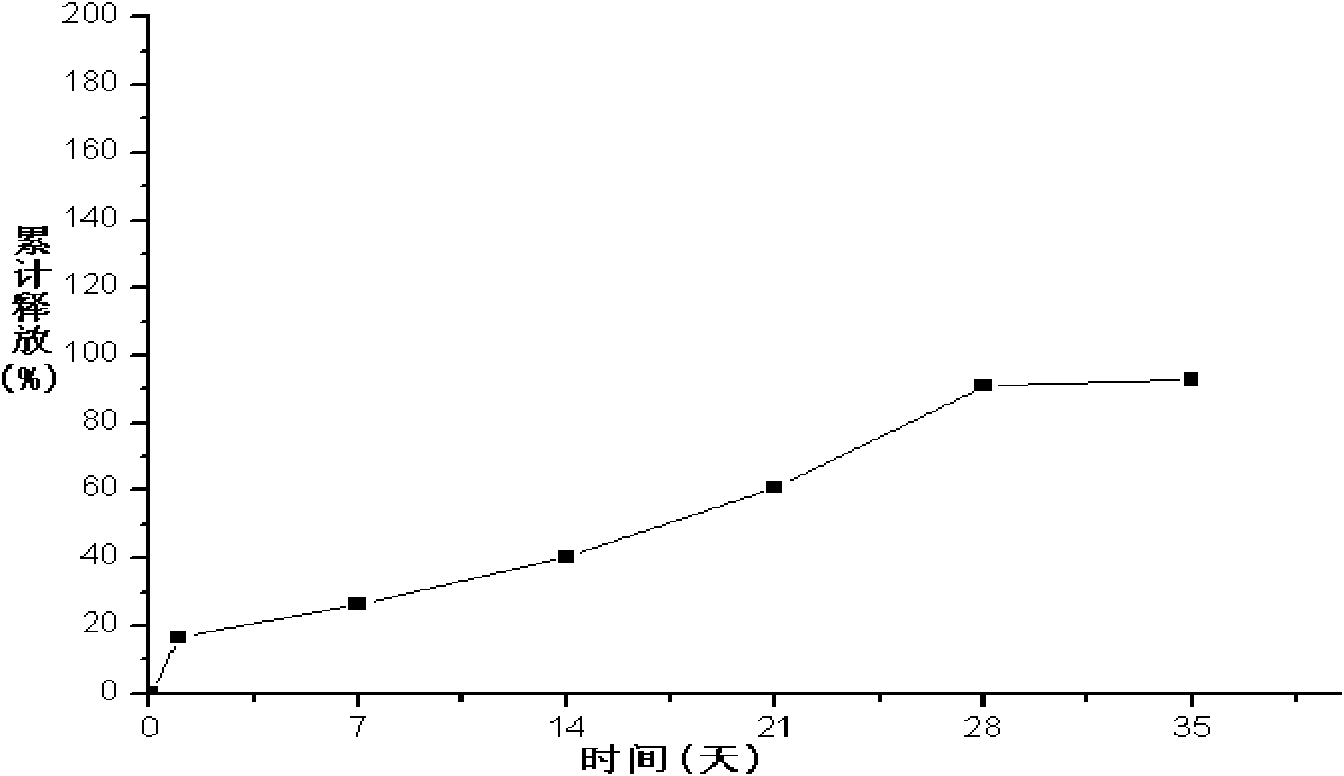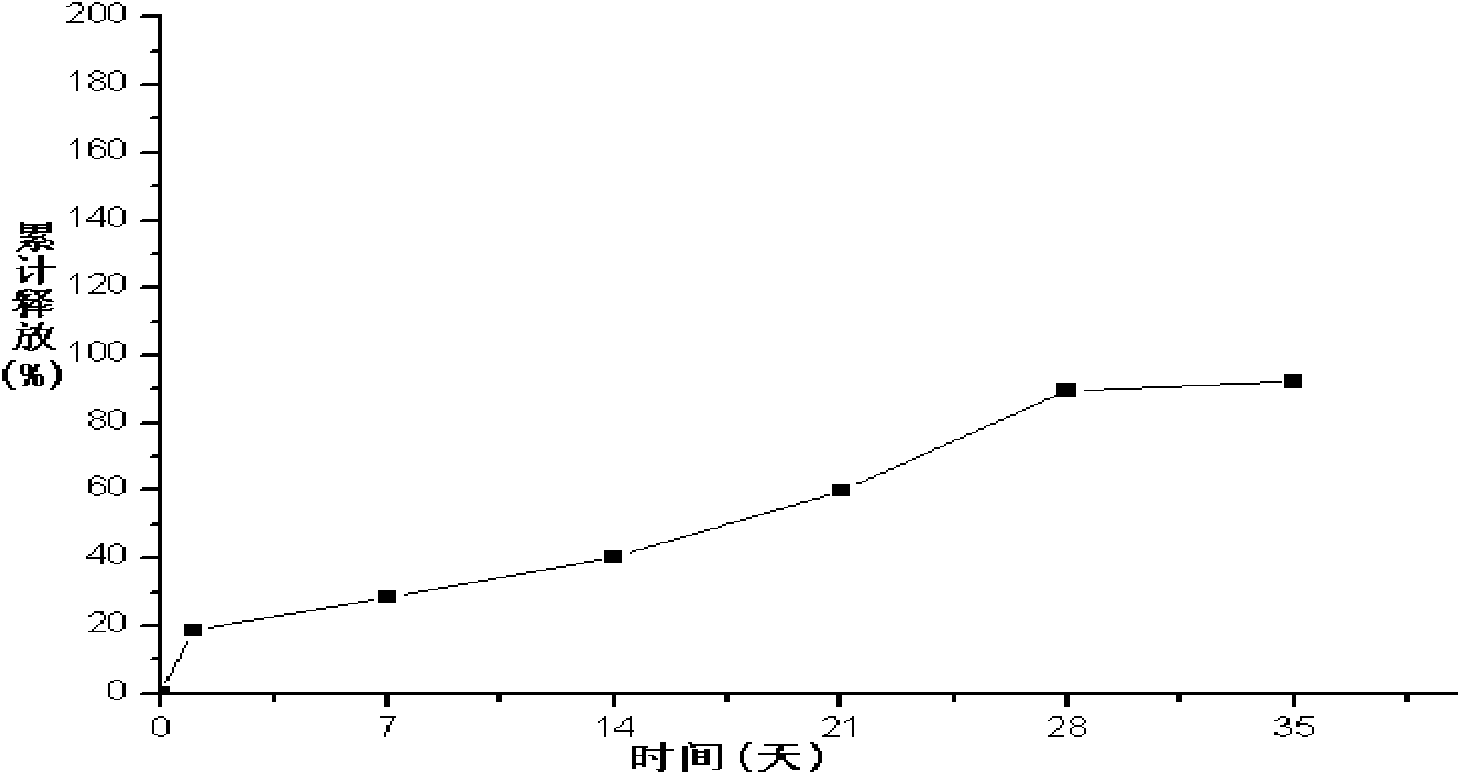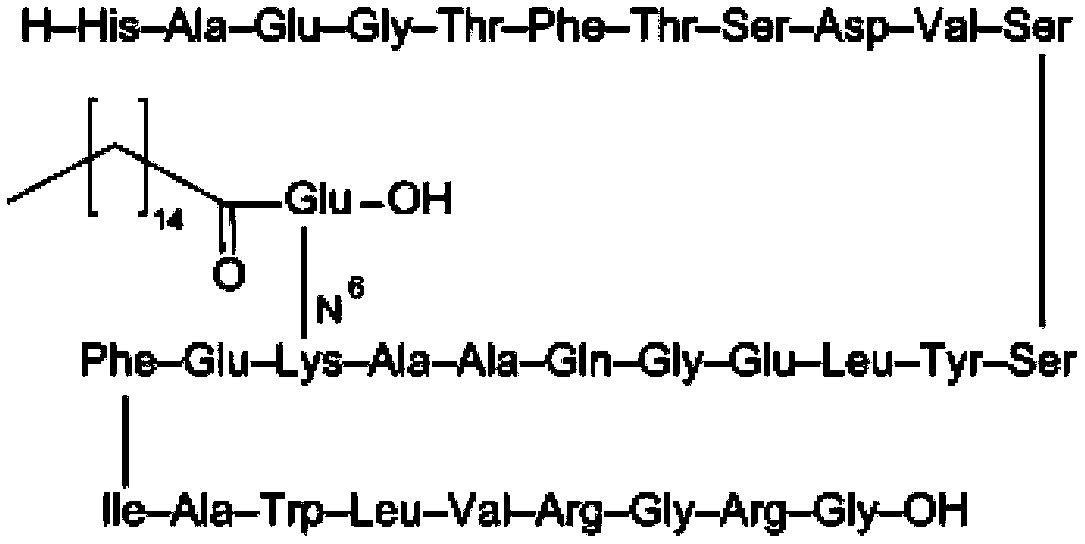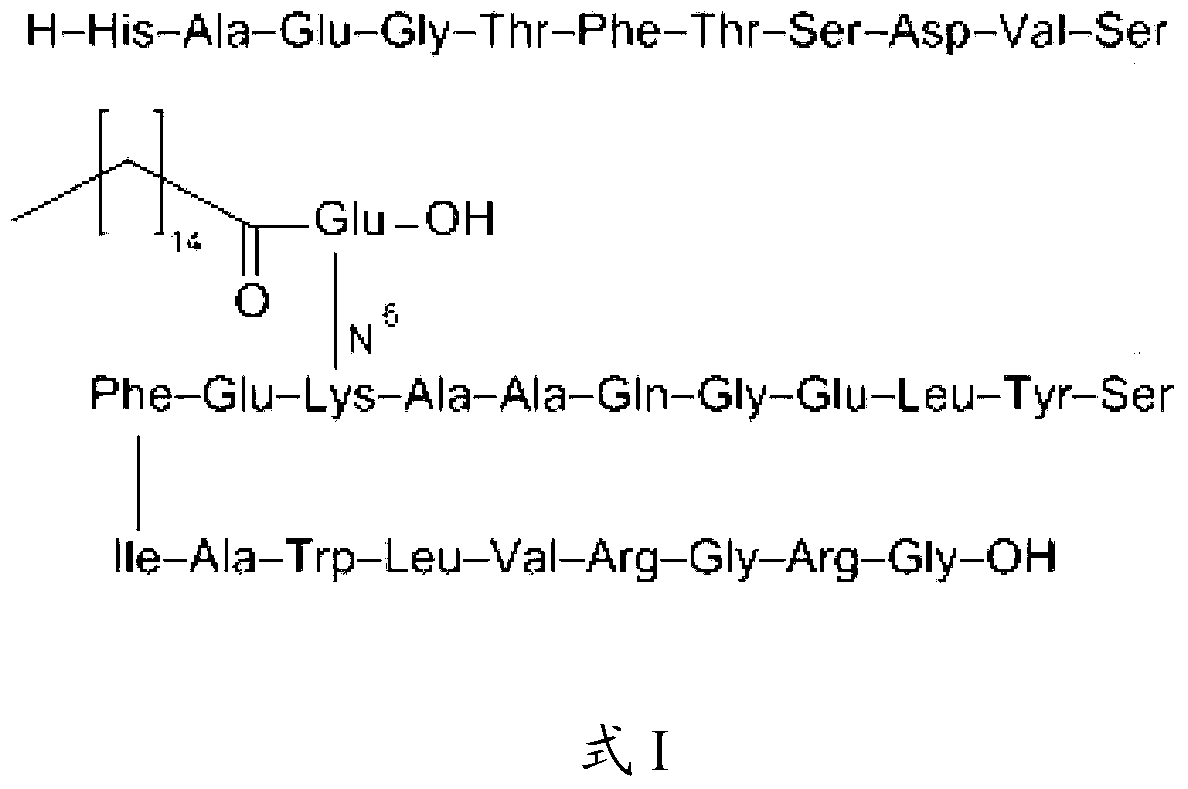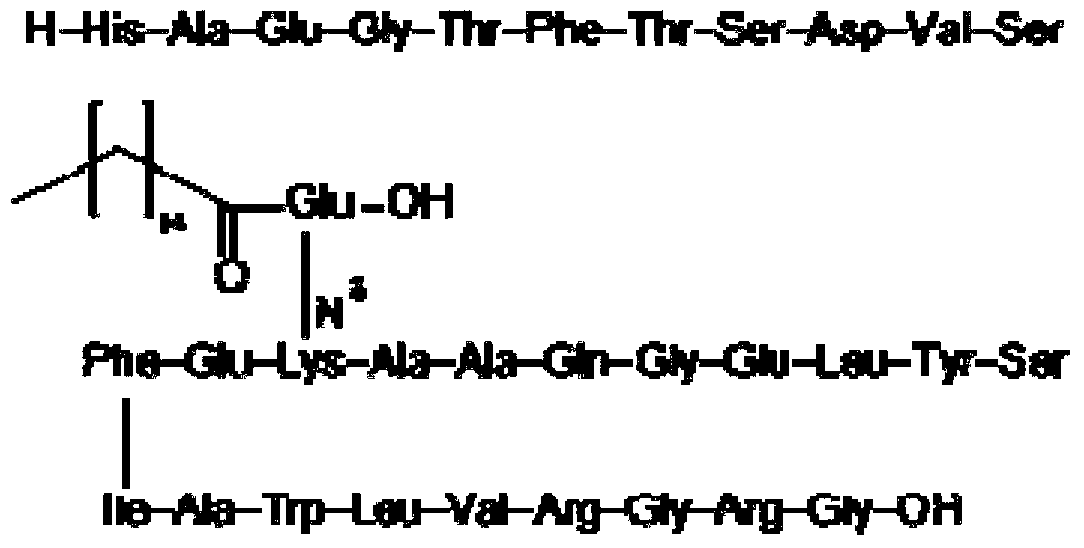Patents
Literature
147 results about "Liraglutide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Liraglutide is used either alone or with other medications, and with a proper diet and exercise program, to control high blood sugar. It is used in people with type 2 diabetes.
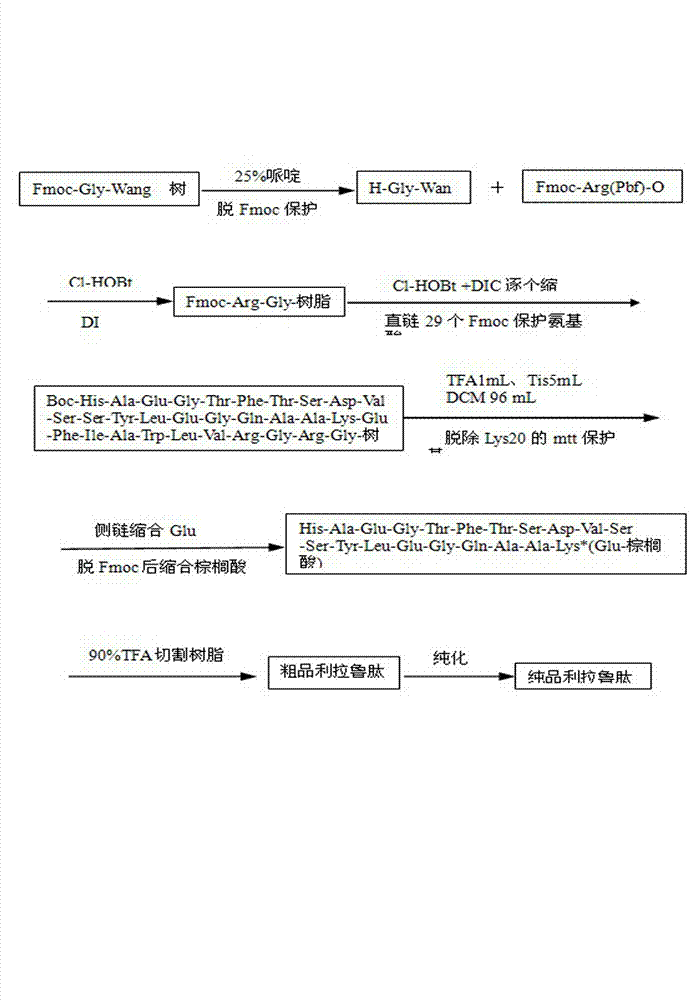
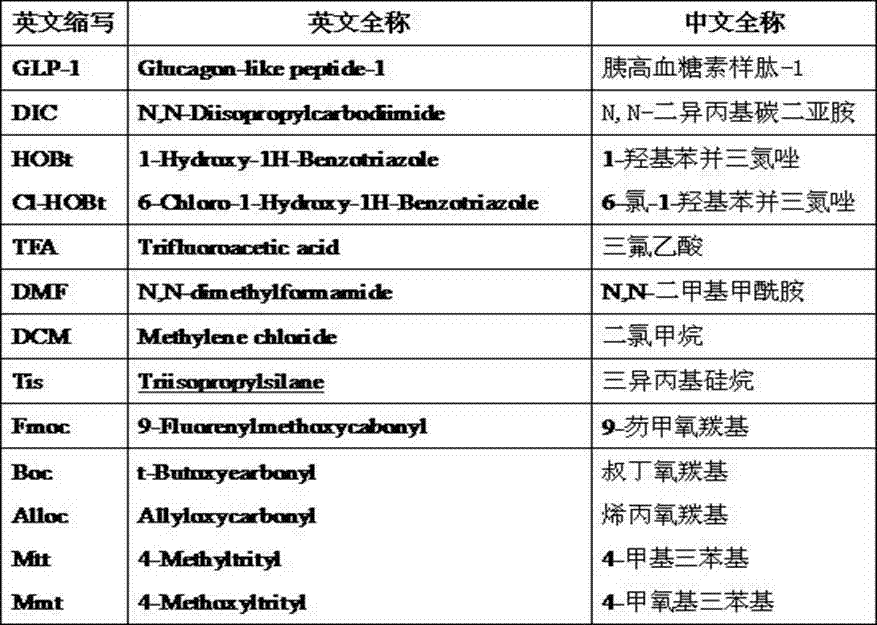
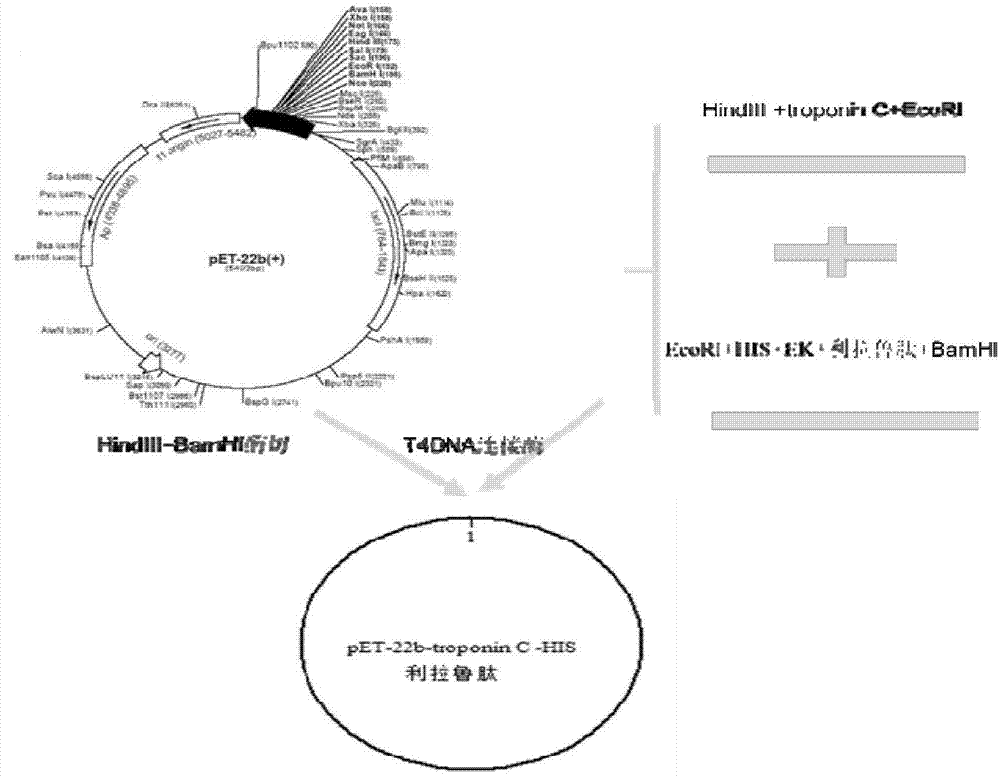
Method for synthesizing liraglutide
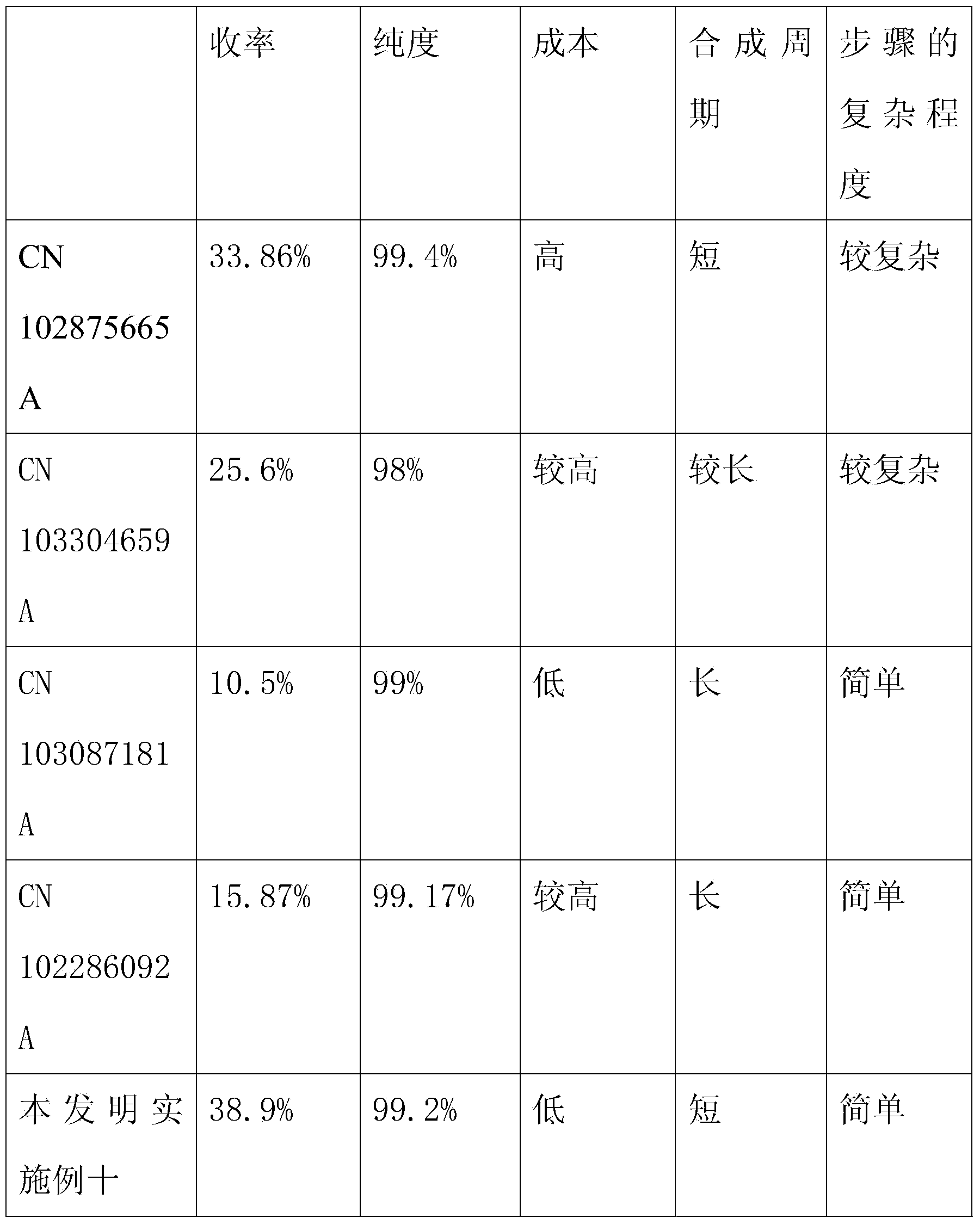
ActiveCN102875665AShort synthesis cycleImprove efficiencyPeptide preparation methodsBulk chemical productionLiraglutideAmino acid
The invention relates the field of medical synthesis and discloses a method for synthesizing liraglutide. The method includes: firstly synthesizing five polypeptide fragments of amino acid from first to fourth, amino acid from fifth to tenth, amino acid from eleventh to sixteenth, amino acid from seventeenth to twenty-fourth, and amino acid from twenty-fifth to thirty first according to amino acid sequence of the liraglutide main chain from N end to C end, and coupling the five polypeptide fragments to synthesize the liraglutide. Synthesizing of the five fragments can be performed simultaneously, synthesizing cycle is shortened greatly, total yield of the liraglutide can be increased, and accordingly the method is better than existing synthesizing methods.
Owner:HYBIO PHARMA
Solid-phase synthesis method for liraglutide
ActiveCN103864918AShort synthesis cycleImprove efficiencyPeptide preparation methodsBulk chemical productionSynthesis methodsSide chain
The invention discloses a solid-phase synthesis method for liraglutide. The solid-phase synthesis method for liraglutide comprises the following steps: 1) firstly synthesizing first to tenth amino acid segments 3, eleventh to nineteenth amino acid segments 2, and twentieth to thirty-first amino acid segments 1, wherein the twentieth lysine adopts Fmoc-Lys(Mtt)-OH, and the first histidine adopts Boc-His(Trt)-OH; 2) synthesizing pal-Glu(OH)-Otbu by adopting a liquid phase synthesis method; 3) selectively removing the Mtt protecting group on the twentieth lysine by use of 5% TFA (Trifluoroacetic Acid), connecting pal-Glu(OH)-Otbu with the side chain of the twentieth lysine to obtain a segment 4; 4) sequentially connecting the segments 2, the segments 3 and the segments 4 to obtain peptide resin completely protected by liraglutide; 5) cracking, purifying, and lyophilizing to obtain the liraglutide product.
Owner:哈尔滨吉象隆生物技术有限公司
Synthetic method of liraglutide
ActiveCN103304660AHigh selectivityHigh purityPeptide preparation methodsBulk chemical productionChemical synthesisDrugs levels
The invention discloses a full chemical synthetic method for hybridization of a solid phase and a liquid phase of liraglutide. The method comprises the following steps: chemically synthesizing a liraglutide precursor protected by N terminal and a cetyl derivative; de-protecting to remove tail end protection to obtain a target polypeptide. The liraglutide precursor semi-protected is obtained by polypeptide solid-phase synthesis, and the precursor purified to the drug level enters into the next chemical synthesis.
Owner:SHANGHAI AMBIOPHARM
Liraglutide long-acting microsphere injection and preparation method thereof
ActiveCN102085355AReduce the burden of treatmentImprove Medication AdherencePowder deliveryPeptide/protein ingredientsTreatment burdenMicrosphere
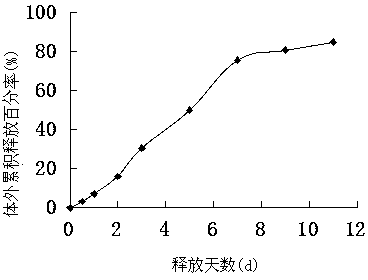
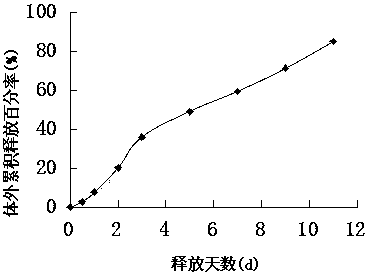
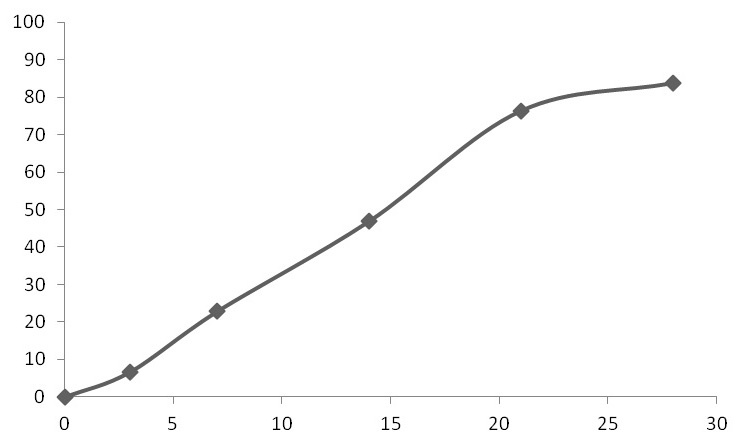
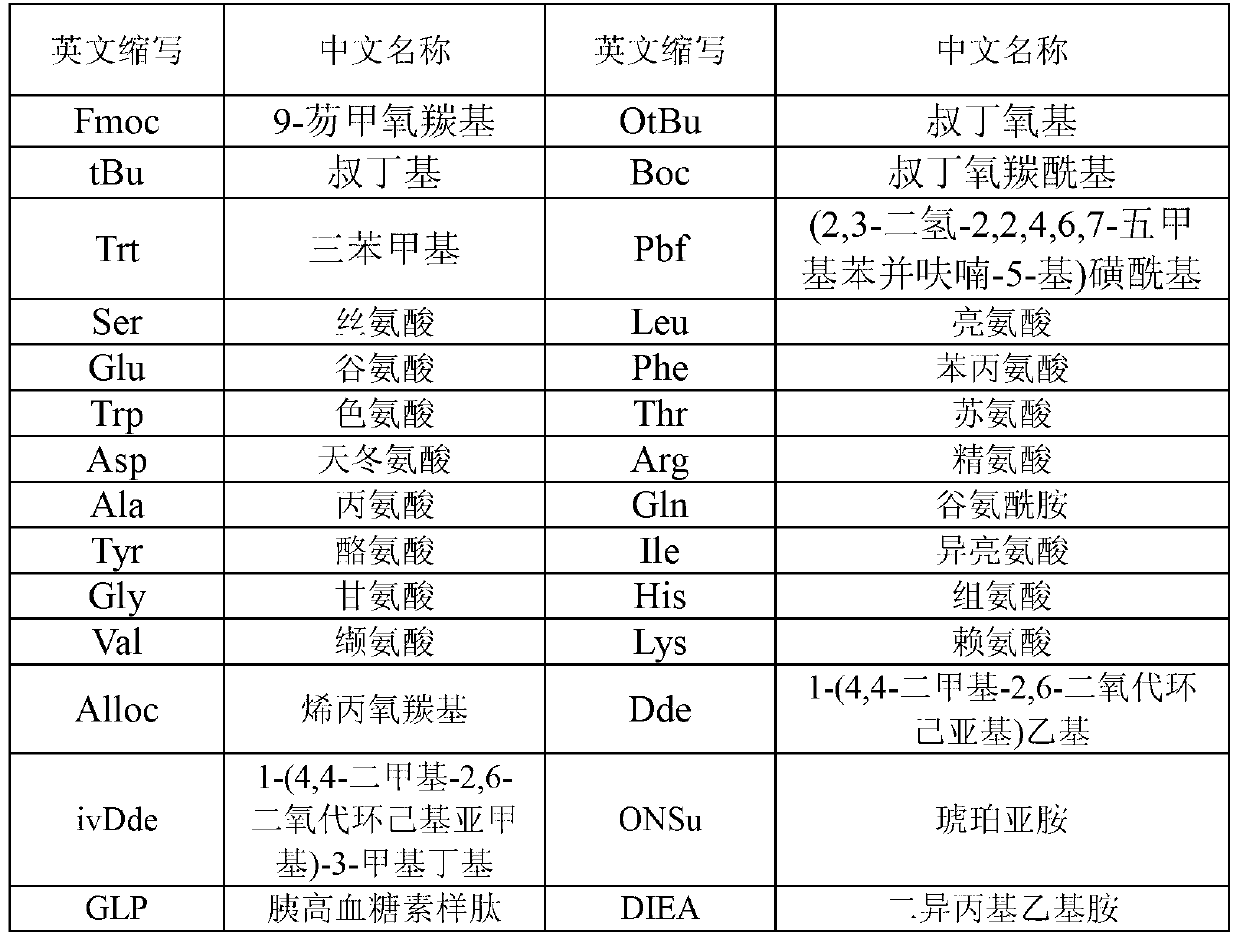
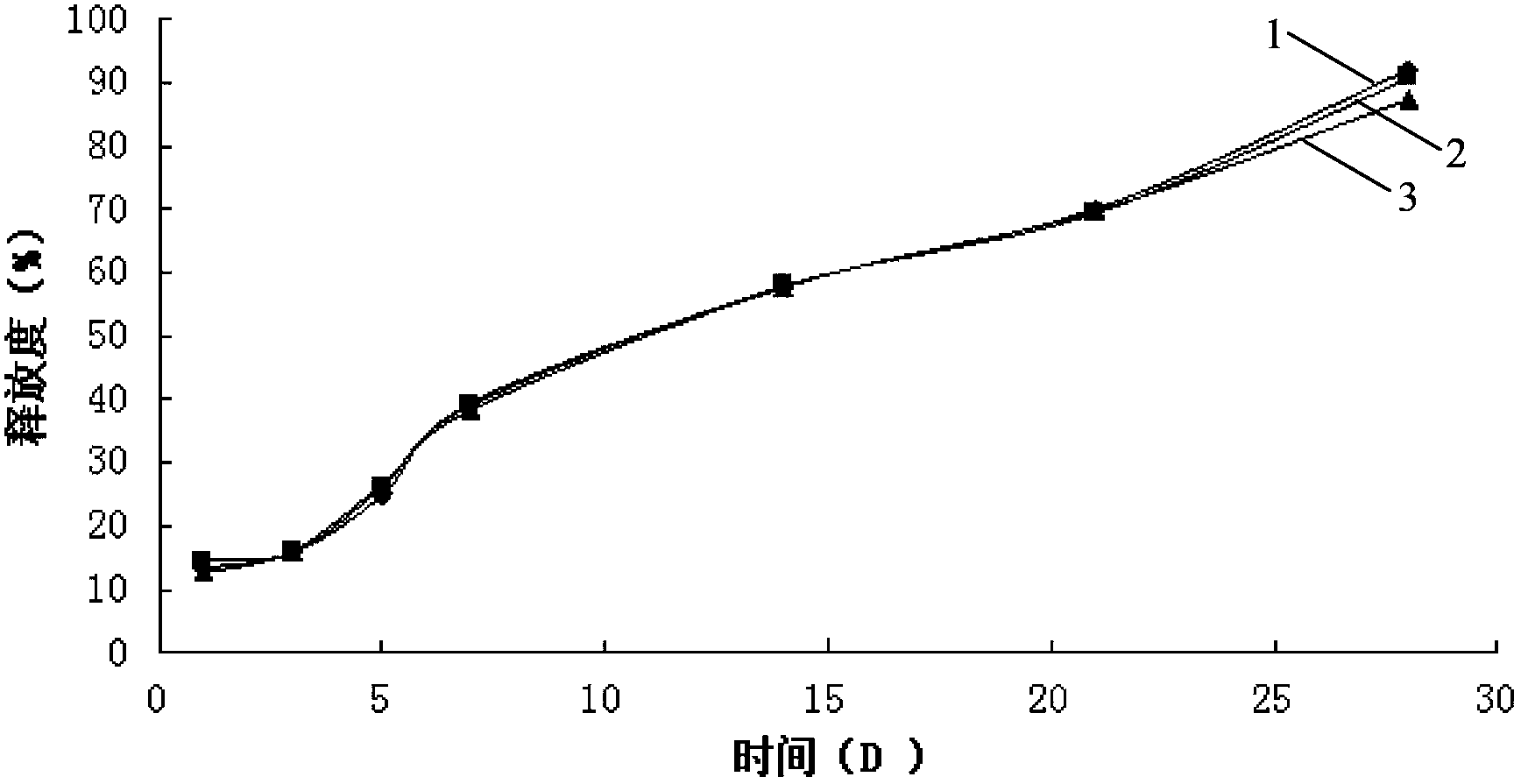
The invention provides a long-acting microsphere injection as an antidiabetic medicament, the long-acting microsphere injection comprises liraglutide, PLGA (poly(lactic-co-glycolic acid)), excipients and a surfactant, and the invention simultaneously relates to a preparation method of the injection. Liraglutide long-acting sustained-release microspheres provided by the invention are designed to perform subcutaneous injection once every 28 days, thereby greatly reducing treatment burden on a patient, improving medication compliance and reducing treatment cost; simultaneously, results of in vitro release studies, animal experiments and the like prove that the obtained sustained-release microspheres can slowly release a medicament for a long time in vitro and in vivo.
Owner:蚌埠丰原涂山制药有限公司
Complete solid-phase synthesis method for liraglutide
InactiveCN103145828AImprove reaction efficiencyHigh purityPeptide preparation methodsBulk chemical productionFreeze-dryingSide chain
The invention discloses a complete solid-phase synthesis method for liraglutide. 2-Cl-TrtResin is enabled to serve as a solid-phase carrier. DIC / HOBt is enabled to serve as a condensation agent. After processing of microwave reaction technology, reaction time is shortened, and condensation efficiency is improved. According to side chain modification, novel ivDde side chain protected lysine is adopted and side chain modification synthesis is carried out. During the process, 20% piperidine is adopted to get rid of Fmoc protection until linear chain polypeptide synthesis is finished. Then, after hydrazine hydrate is adopted to get rid of ivDde protection, a side chain modification reaction is carried out. Obtained liraglutide with complete protection on the solid phase carrier is processed by trifluoroacetic acid, and crude liraglutide is obtained. After purification and freeze-drying by a C18 column, pure liraglutide is obtained. After strong negative ion salt conversion and free-drying, acetic acid liraglutide acetate is obtained. The complete solid-phase synthesis method for the liraglutide is simple in operation, short in synthesis cycle, low in production cost, few in accessory substance, high in product yield and beneficial for mass production.
Owner:宁波瑞达医药科技有限公司
Solid-phase synthesis method of liraglutide
InactiveCN103087181ALess usableSmall quantityPeptide preparation methodsBulk chemical productionSide chainWang resin
The invention discloses a method for synthesizing liraglutide. The method comprises the following steps of: 1, selecting Fmoc-Gly-Wang resin and N terminal Fmoc protected and side chain protected amino acid as raw materials, wherein lysine on a 26th site adopts Fmoc-Lys(Mtt)-OH or Fmoc-Lys(Mmt)-OH, histidine on an N terminal adopts Boc-His(Trt)-OH or Boc-His(Boc)-OH; 2, selectively removing a Mtt or Mmt protecting group on the lysine by adopting 1 percent TFA (Trifluoroacetic Acid); 3, sequentially coupling one g-glutamic acid and palmitic acid on a side chain; and 4, cutting resin by the TFA to obtain a crude product of the liraglutide, and carrying out preparative liquid chromatography purification and lyophilization to obtain a pure product of the liraglutide. The method for synthesizing the liraglutide is simple in steps, saves time and labor, is less in difficult sequences in a coupling process, simple and easy to operate during side chain de-protection, less in byproducts and high in yield, and is suitable for industrialized production.
Owner:刘卫 +1
Method for preparing liraglutide by convergent synthesis
ActiveCN104650219AHigh purityHigh yieldPeptide preparation methodsBulk chemical productionSide chainPeptide fragment
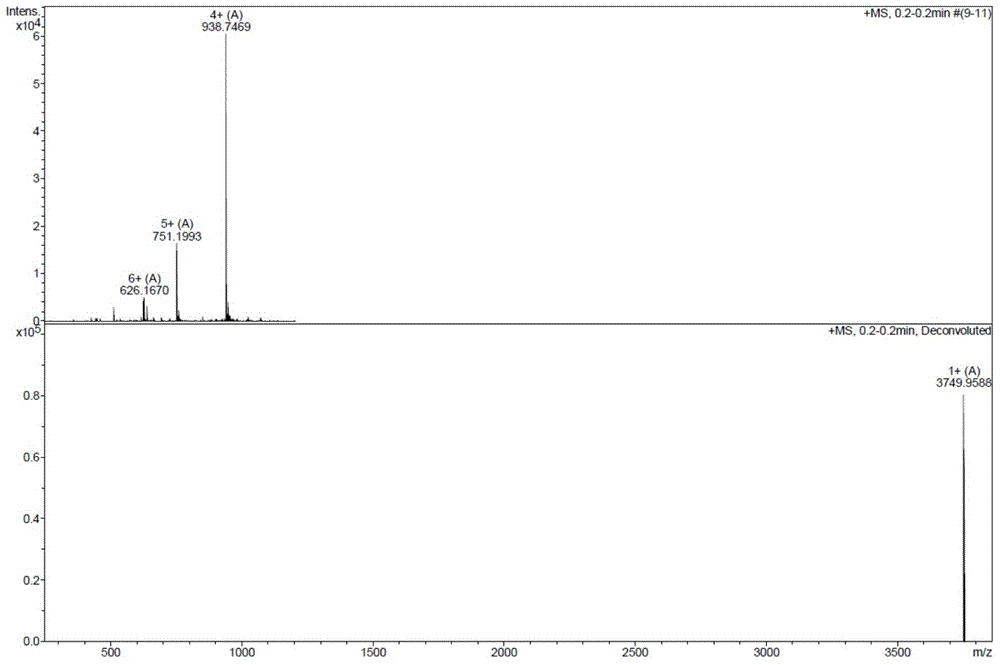
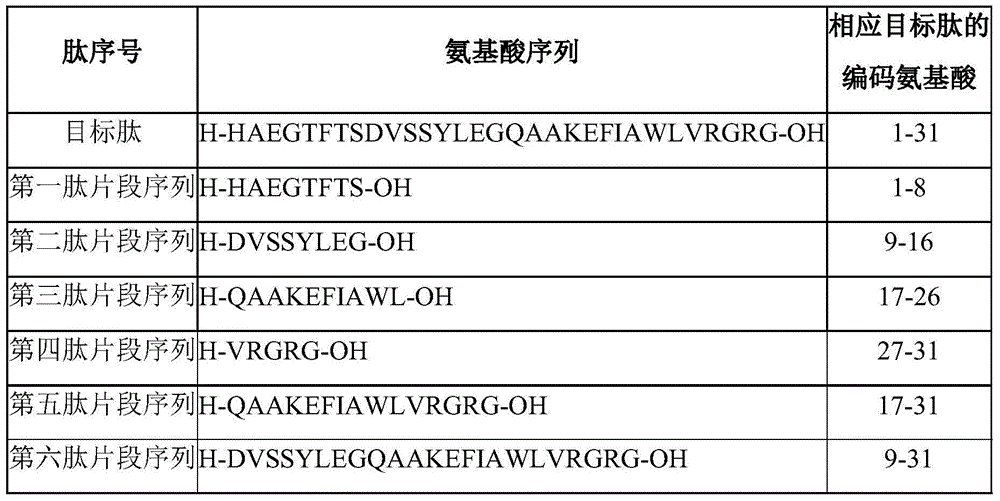
The invention discloses a method for preparing liraglutide by convergent synthesis. The method comprises the steps of performing solid phase synthesis to obtain four side chain protected peptide fragment sequences, gradually coupling the peptide fragments in a solution system to obtain an all-protected liraglutide straight chain polypeptide, removing the side chain protection of the 20th Lys, performing modification to form all-protected liraglutide, then cracking to remove protecting groups to obtain a crude liraglutide peptide, purifying and exchanging salt to obtain liraglutide; wherein in the four peptide fragment sequences, the first peptide fragment sequence is 1st to 8th amino acids in the liraglutide sequence, the second peptide fragment sequence is 9th to 16th amino acids in the liraglutide sequence, the third peptide fragment sequence is 17th to 26th amino acids in the liraglutide sequence, and the fourth peptide fragment sequence is 27th to 31st amino acids in the liraglutide sequence. By adopting the method, the yield is improved, and the synthesis cost is greatly reduced; and the method is favorable for large-scale and industrialized production.
Owner:LANZHOU UNIVERSITY
Liraglutide sustained-release microsphere preparation and preparation method thereof
InactiveCN104382860AUniform particle sizeLow burst ratePeptide/protein ingredientsMetabolism disorderSucroseMicrosphere
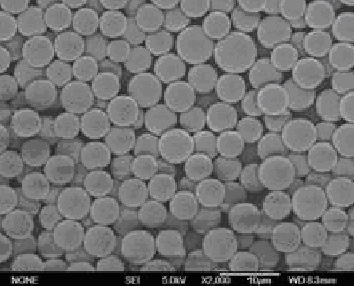
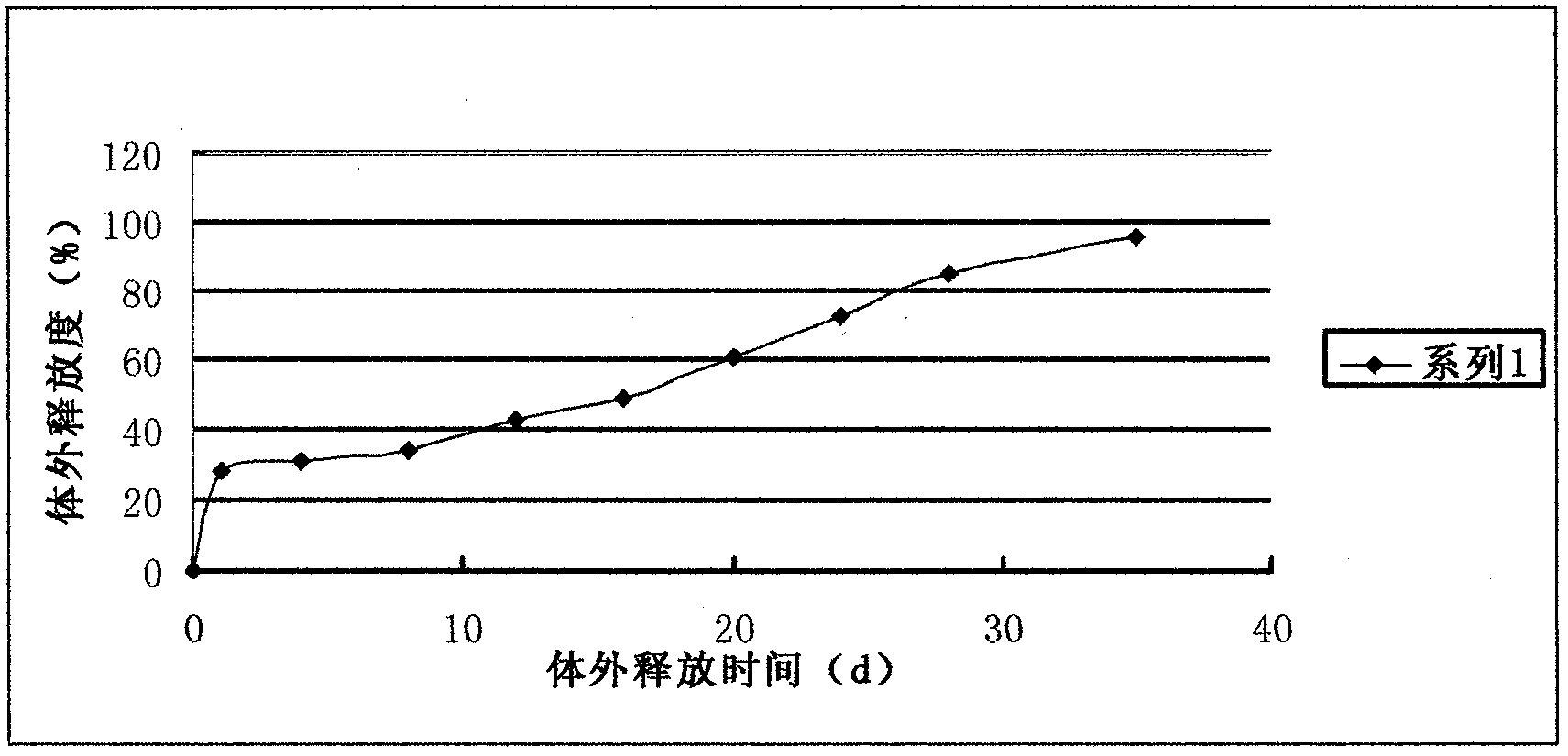
The invention relates to a liraglutide sustained-release micro sphere preparation and a preparation method thereof. The liraglutide sustained-release microsphere preparation comprises 5mg to 100mg of liraglutide, 0.5mg to 10mg of a protective agent and 50mg to 1000mg of a polylactic acid-glycolic acid copolymer, wherein the protective agent is one or a mixture of a plurality of sucrose, mycose, gelatin, mannitol, glycine, lysine and human serum albumin; the molecular weight of the polylactic acid-glycolic acid copolymer is 5000-20000 Daltons, and the ratio of polylactic acid to glycolic acid in the polylactic acid-glycolic acid copolymer is 1:3 to 3:1. According to the liraglutide sustained-release microsphere preparation disclosed by the invention, regular microspheres and medicines uniformly distributed in the microspheres can be obtained by just emulsifying and volatizing an organic solvent; processing procedures are simple; operation is simple; good repeatability is realized in preparation; the prepared liraglutide sustained-release microsphere preparations in batches have no remarkable difference; the obtained microspheres are uniform in grain size, narrow in distribution, controllable in grain size, round and orderly in surfaces and low in burst release rate.
Owner:浙江美华鼎昌医药科技有限公司 +1
Liraglutide sustained-release microsphere preparation and preparation method thereof
InactiveCN102429876AHigh drug loadingHigh encapsulation efficiencyPeptide/protein ingredientsMetabolism disorderLactideMicrosphere
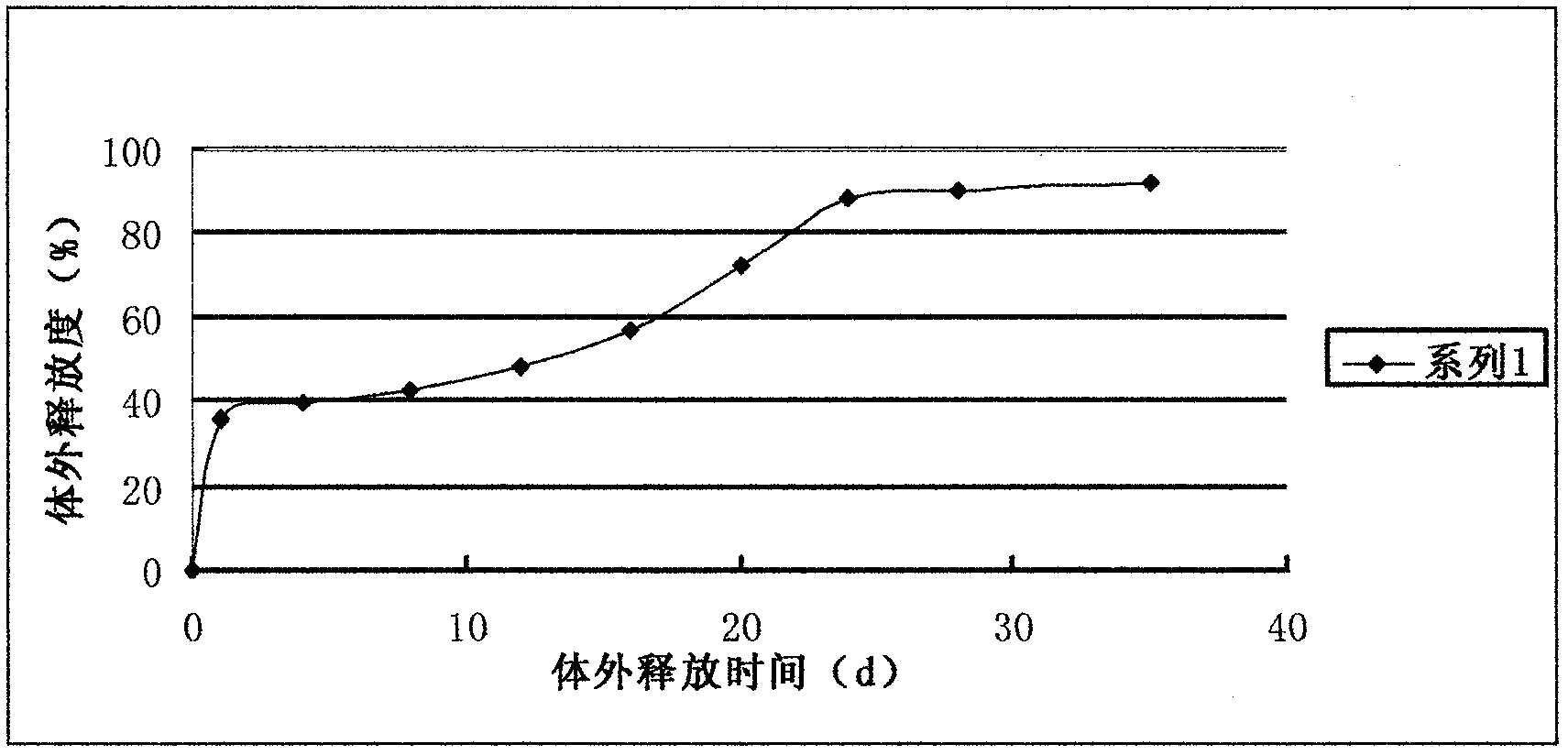
The invention relates to a formula of a liraglutide sustained-release microsphere preparation. The preparation mainly comprises 5 to 60 parts of liraglutide and 50 to 200 parts of biodegradable substrate material, wherein the biodegradable substrate material is polylactic acid (PLA), polylactic acid-glycollic acid (PLGA) block copolymer, glycolide-lactide (PLCG) copolymer, polyglycolic acid (PGA), polycaprolactone (PCL), polylactic acid-glycollic acid-polycaprolactone (PLGA-PCL) copolymer or PLCG-polycaprolactone (PLCG-PCL) or a mixture of any two or more than two of the compounds, preferably the PLGA-PCL copolymer. The sustained-release microspheres have a high encapsulating rate and a good sustained-release effect.
Owner:HYBIO PHARMA
Synthetic method of liraglutide
The invention relates to the field of polypeptide synthesis, and especially relates to a synthetic method of liraglutide. The method is as follows: peptide resin is obtained by successively coupling a third polypeptide fragment, a second polypeptide fragment and a first polypeptide fragment with a fourth polypeptide fragment-resin, and then the liraglutide is obtained by side chain modification, cracking, purification and freeze drying. By use of the synthetic method of the liraglutide, the purity and yield of crude peptide can be improved, and the method is conducive to the purification, can improve the yield of the product, shortens the synthesis time, and is suitable for industrialized production.
Owner:HYBIO PHARMA
Preparation method of liraglutide
ActiveCN106699871ALow costReduce generationPeptide preparation methodsBulk chemical productionSide chainFreeze-drying
The invention discloses a preparation method of liraglutide, and belongs to the technical field of medicines. The method comprises the following steps of (1) carrying out solid-phase synthesis to form four peptide segments of the liraglutide, wherein the peptide segment 1 is the (1-9)th amino acids in a liraglutide main chain sequence, the peptide segment 2 is the (10-14)th amino acids in the liraglutide main chain sequence, the peptide segment 3 is the (15-23)th amino acids in the liraglutide main chain sequence and the peptide segment 4 is the (24-31)th amino acids in the liraglutide main chain sequence; (2) stepwise coupling various peptide segments to obtain an all-protected liraglutide main chain peptide; (3) removing a protecting group of the 20th lysine and completing connection of side chains to obtain all-protected liraglutide peptide resin; and (4) carrying out cracking, purification and freeze-drying to obtain the liraglutide. The preparation method is high in production efficiency and stable in process, and a product is high in purity, high in yield and suitable for industrial production.
Owner:哈药集团股份有限公司 +1
Method for synthesizing liraglutide
ActiveCN104004083AReduce carrier usageAvoid couplingPeptide preparation methodsBulk chemical productionAmino acid synthesisCoupling
The invention relates to the field of medicine synthesis, and discloses a method for synthesizing liraglutide. The method includes the steps that three polypeptide fragments of the first to the fourth amino acid, the fifteenth to the sixteenth amino acid and the seventeenth to thirty-first amino acid are synthesized at first according to the amino acid sequence from N end to C end of a main chain of liraglutide, and then the three polypeptide fragments and the other amino acid are connected according to the sequence from the C end to the N end in a coupling mode to synthesize the liraglutide. According to the method, the three fragments can be synthesized simultaneously, coupling and acidolysis with much carrier resin are avoided, the use quantity of resin carriers is greatly reduced, the synthesis cycle is shortened, and the complexity level of synthetic process is simplified on the premise that high total recovery and purity are guaranteed.
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
Preparation method of liraglutide intermediate polypeptide
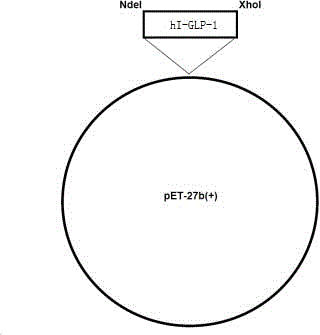
The invention belongs to the technical field of polypeptide preparation and relates to a preparation method of a liraglutide intermediate GLP-1(7-37). The preparation method utilizes a genetic recombination technology. Compared with chemical synthesis, the preparation method reduces impurities and improves purity and a yield. The preparation method comprises the following steps of introducing plasmids with hI-GLP-1(7-37) gene sequences into cells of escherichia coli by a gene engineering method to construct recombinant engineering bacteria, carrying out fermentation induction to obtain a hI-GLP-1(7-37) expression fusion protein, and carrying out renaturation, digestion conversion and separation purification to obtain GLP-1(7-37).
Owner:JIANGSU WANBANG BIOPHARMLS +1
Solid-phase preparation method of liraglutide
ActiveCN103304659AHigh purityHigh yieldPeptide preparation methodsGlucagonsCouplingCombinatorial chemistry
The invention relates to the field of polypeptide synthesis and in particular relates to a solid-phase preparation method of liraglutide. The preparation method has the beneficial effects that difficult sequences or single fragments containing the difficult sequences are connected to resins; amino acid connection is carried out by adopting a stepwise coupling method in other regions; the purity and yields of coarse peptides are obviously improved; and the cost is relatively low.
Owner:HYBIO PHARMA
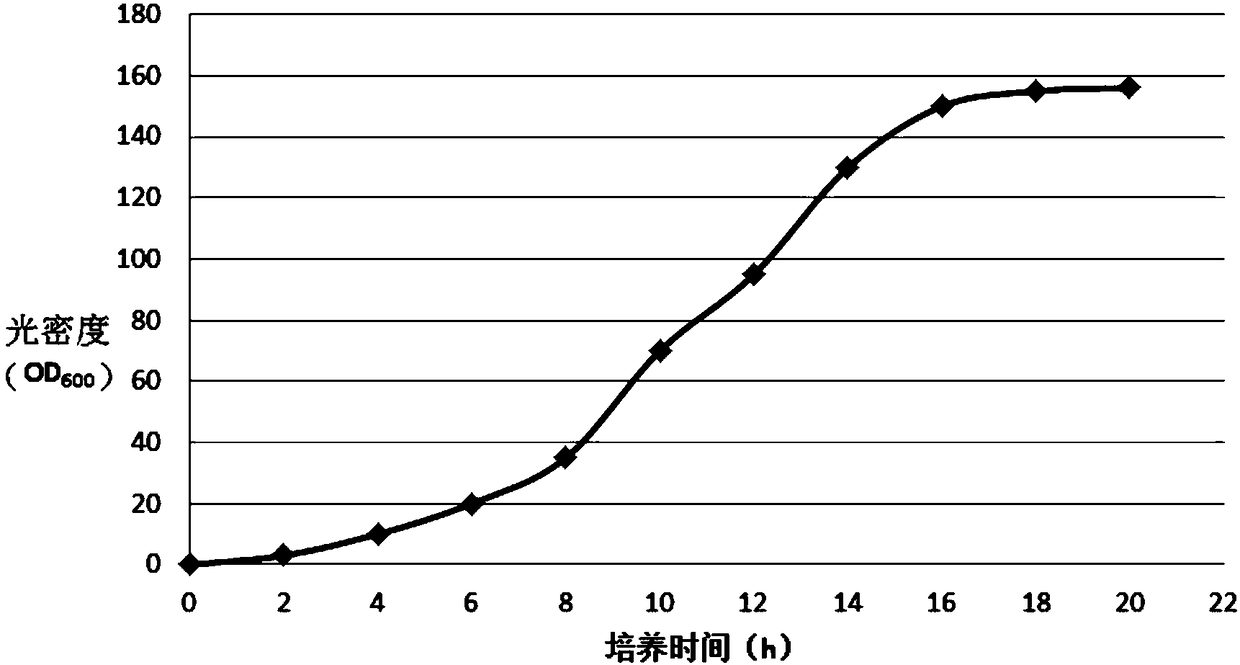
Method for efficiently expressing recombinant liraglutide
InactiveCN104745597ASimple and fast operationIncrease productionBacteriaPeptide preparation methodsEscherichia coliLiraglutide
The invention relates to the biomedical field, and in particular relates to a method for efficiently expressing recombinant liraglutide. The method for efficiently expressing recombinant liraglutide is used for performing gene cloning and transformed expression. The recombinant liraglutide is constructed, and liraglutide protein is prepared through an escherichia coli expression technology. Compared with the two methods, the method disclosed by the invention is simple and convenient to operate, high in yield and low in cost.
Owner:杭州北斗生物技术有限公司
Method for synthesizing liraglutide
ActiveCN107960079AReduce generationGood yieldPeptide-nucleic acidsPeptide preparation methodsDipeptideFreeze-drying
A synthesis method for low-racemization impurity liraglutide comprises the following steps: performing synthesis to obtain a propeptide, coupling 2 to 5 peptides comprising Thr-Phe on the propeptide by using a solid-phase synthesis method; further, performing solid-phase synthesis to obtain a liraglutide resin; the liraglutide resin is cracked after modification, or the liraglutide resin is directly cracked, purified and frozen dry, so as to obtain the liraglutide. The provided liraglutide synthesis method effectively restrains or reduces the generation of racemization impurity D-Thr5 highly similar to a product property, which facilitates the purification of the coarse liraglutide, and the high yield of the liraglutide is ensured, thereby greatly reducing production costs; during the synthesis of the liraglutide, the syntheses between dipeptide fragments, tripeptide fragments, the tetrapeptide fragments and pentapeptide fragments and the Gly-resin or the syntheses between the combination of the dipeptide fragments, the tripeptide fragments, the tetrapeptide fragments and pentapeptide fragments and the Gly resin can be carried out at the same time, and accordingly the synthesis time is shortened to some extent.
Owner:深圳市健翔生物制药有限公司
Synthetic method for liraglutide
ActiveCN105732798AReduce synthesisReduce difficultyPeptide preparation methodsGlucagonsFreeze-dryingCombinatorial chemistry
The invention relates to products in the field of medicines, and provides a synthetic method for liraglutide, which includes the following steps: 1) condensing fragments to obtain various liraglutide intermediates; 2) deprotecting and cracking full-protected liraglutide; and 3) purifying and freeze-drying the liraglutide. The method employs a 4+5+7+6+9 synthetic method, wherein synthesis on five fragments is carried out at the same time, so that synthetic time of the product is greatly reduced. Meanwhile, through step-by-step analysis of synthesis factors, comprising His-Ala-Glu-Gly, Thr-Phe-Thr-Ser-Asp, Val-Ser-Ser-Tyr-Leu-Glu-Gly, Gln-Ala-Ala-N6-[N-(1-oxohexadecyl)-Glu]Lys-Glu-Phe, and Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly-OH, synthesis difficulty of difficult peptide sequence in solid-phase synthesis is reduced, scale amplification in solid-phase synthesis is solved and synthetic efficiency is improved. Because fragment synthesis reduces purifying difficulty effectively, the production cost is greatly reduced.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Preparation method of liraglutide intermediate polypeptide
ActiveCN108191981ALow costHigh expressionPolypeptide with localisation/targeting motifBacteriaHigh concentrationEscherichia coli
The invention belongs to the technical field of preparation methods of polypeptides and particularly relates to a preparation method of liraglutide intermediate polypeptide GLP-1 (glucagon-like peptide-1) (7-37). The method comprises the following main steps: constructing recombinant liraglutide engineering bacteria, expressing liraglutide intermediate fusion protein in a form of an inclusion bodyunder Echerichia coli induction, and performing denaturation, renaturation, enzyme digestion, separation and purification to obtain the liraglutide intermediate polypeptide GLP-1 (7-37). Expression is changed into intracellular insoluble inclusion body expression by changing recombinant sequence signal peptides, and expression quantity is increased greatly; the washed inclusion body is subjectedto alkali dissolution, a large quantity of denaturant is not needed, the inclusion body with high concentration of protein concentration being 20-30 g / L is added to an inclusion body dissolution buffer, denaturation and renaturation time does not exceed 1 h, and enzyme digestion can be performed after dissolution; procedures are reduced, operation volume is reduced, reagent cost is reduced, and industrialized enlargement is facilitated; UniSP-50XS cation exchange is adopted for separation and purification, and separation degree is high. The purity of the liraglutide intermediate polypeptide prepared with the method reaches 87% or higher, and the yield is higher than 85%.
Owner:AMPHASTAR NANJING PHARMA
Preparation method for liraglutide
InactiveCN105294853AIncrease the number ofImprove polycondensationPeptide preparation methodsBulk chemical productionFluid phaseBackbone chain
The invention provides a preparation method for liraglutide. The method employs a solid-liquid phase combined manner and adopts a means of inserting Fmoc-HmbAla-OH / Fmoc-HmbGly-OH or Fmoc-DmbAla-OH / Fmoc-DmbGly-OH to overcome the problem of polycondensation. The preparation method comprises the following main steps: (1) connecting amino acids corresponding to the main chain of liraglutide by using a sequential coupling method, wherein all the amino acids except amino acids specially labeled in step (2) use amino acids with N-terminal Fmoc protection and common side chain protection as raw materials; (2) applying Fmoc-(Hmb)Gly-OH / Fmoc-(Hmb)Ala-OH or Fmoc-(Dmb)Gly-OH / Fmoc-(Dmb)Ala-OH to prevention of polymerization during connection of the main chain of liraglutide; (3) carrying out cutting, removing all the protective groups and carrying out connection of Plamitoryl-Glu(OSu)-OtBu in a liquid phase; and (4) removing a tBu protective group and carrying out purification so as to obtain liraglutide. The preparation method for liraglutide is simple to operate, effectively overcomes the problem of polycondensation in peptide synthesis and greatly improves yield; and prepared liraglutide has high purity and stable and reliable quality.
Owner:CHINESE PEPTIDE CO
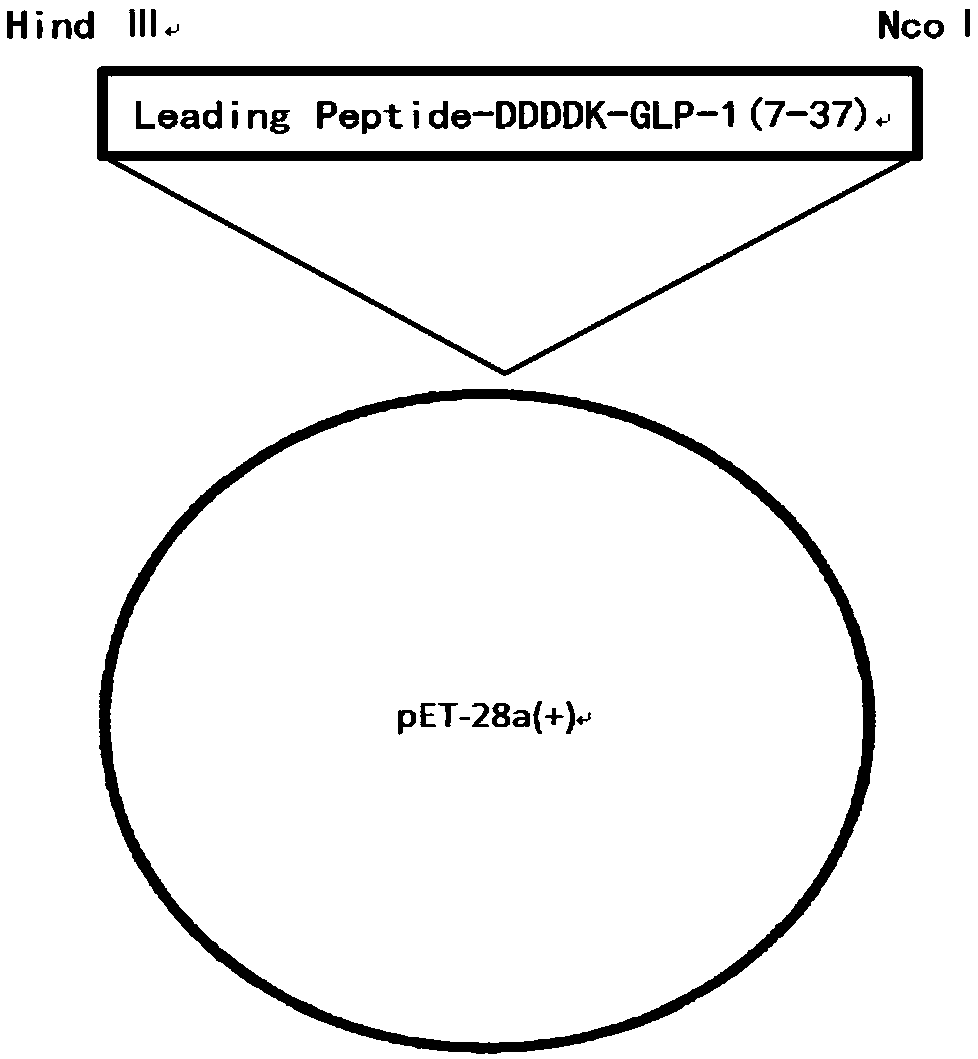
Fusion protein and method for preparing liraglutide intermediate polypeptide
ActiveCN110128552AShorten the enzymatic digestion timeLow costBacteriaAntibody mimetics/scaffoldsEnzymatic digestionEscherichia coli
The invention belongs to the technical field of polypeptide preparation, and in particular, relates to a fusion protein and a method for preparing a liraglutide intermediate polypeptide GLP-1 (7-37).The method includes the main steps: constructing recombinant liraglutide intermediate engineering bacteria, expressing a GLP-1 (7-37) fusion protein by culture and induction of escherichia coli, and carrying out denaturation, renaturation, enzyme digestion and separated purification to obtain the intermediate polypeptide GLP-1 (7-37). Through changing a sequence of leading peptide, the expressionmode becomes an intracellular insoluble inclusion body expression, and the expression quantity is remarkably increased; inclusion bodies after washing are dissolved at high pH, and a large amount of denaturants are not needed to use, the inclusion bodies after washing are added to a inclusion body dissolved buffer solution at a high protein concentration of 5-40 g / L, the renaturation time does notexceed 1 h, and enterokinase digestion is carried out immediately after dissolution; the renaturation process can be reduced, the enzymatic digestion system is reduced, the cost of chemical reagentscan be reduced, and industrial amplification is facilitated; ion exchange separation and purification can be used, and the separation degree is high. The prepared liraglutide intermediate polypeptideGLP-1 (7-37) reaches 92% or more and the yield is more than 87%.
Owner:AMPHASTAR NANJING PHARMA +1
Preparation method of liraglutide
InactiveCN103288951AHigh yieldEasy to purifyPeptide preparation methodsBulk chemical productionSide chainFreeze-drying
The invention relates to the field of polypeptide synthesis, and particularly relates to a preparation method of liraglutide. The preparation method comprises the following steps: coupling Gly and resin to obtain Gly-resin; carrying out primary successive coupling on the Gly-resin to obtain polypeptide segment-resin; and carrying out side chain modification, secondary successive coupling, cracking, purification and freeze-drying on the polypeptide segment-resin to obtain the liraglutide, wherein the sequence of the polypeptide segment in the polypeptide segment-resin is disclosed as SEQ ID NO:1, and the sequence of the liraglutide is disclosed as SEQ ID NO:2. The side chain modification process comprises the following steps: removing the protective group on the Lys side chain in the polypeptide segment-resin, and sequentially coupling Fmoc-Glu-OtBu and palmityl chloride. The liraglutide prepared by the preparation method has fewer impurities, is easy to purify and can enhance the yield of the final product.
Owner:HYBIO PHARMA
Method for synthesizing liraglutide
InactiveCN107903317AHigh purityHigh yieldPeptide preparation methodsBulk chemical productionSide chainCombinatorial chemistry
The invention discloses a method for synthesizing liraglutide. The method comprises the steps of performing solid phase synthesis to obtain the first to fourth sites of amino acid of a liraglutide sequence as a first fragment; performing solid phase synthesis to obtain the fifth to thirty-first sites of amino acid of the liraglutide sequence, removing side chain protection groups of the twentiethsite of Lys and coupling Pal-gamma-Glu to be used as a second fragment; and coupling the first fragment and the second fragment, synthesizing full protection liraglutide, cracking and settling to obtain the liraglutide. The method for synthesizing the liraglutide improves the purity of crude peptide, greatly reduces material costs and purification costs, and is suitable for industrial large-scaleproduction.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Liraglutide preparation method
InactiveCN103275209AEasy to purifyEasy to operatePeptide preparation methodsGlucagonsCoupling reactionLiraglutide
The invention belongs to the technical field of polypeptide medicament preparation methods, particularly relates to a liraglutide preparation method, and aims to solve the technical problems of complex operation process, high purification difficulty of a crude product and low product yield in the existing method. The technical scheme for solving the technical problems is as follows: the liraglutide preparation method comprises the following steps: performing coupling reaction on an N-protected GLP1(7-37) peptide segment and N-alpha-PAL-gamma-Glu(OtBu)-ONSu under the action of alkali to obtain a liraglutide-protected peptide, removing the N-protection, and performing acidolysis to obtain a liraglutide crude product; and purifying to obtain a liraglutide pure product, wherein the sequence of the used GLP1(7-37) peptide segment is as follows: R-His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly-OH; and R is Fmoc, Dde or ivDde. The new liraglutide preparation method provided by the invention has the advantages that the method is simple to operate, the crude product is easy to purify and the product is high in yield.
Owner:CHENGDU SHENGNUO BIOPHARM
GLP-I analogue liraglutide sustained-release microspheres and preparation method thereof
InactiveCN103142488AImprove adaptabilityEasy to acceptPeptide/protein ingredientsMetabolism disorderSide effectMedicine
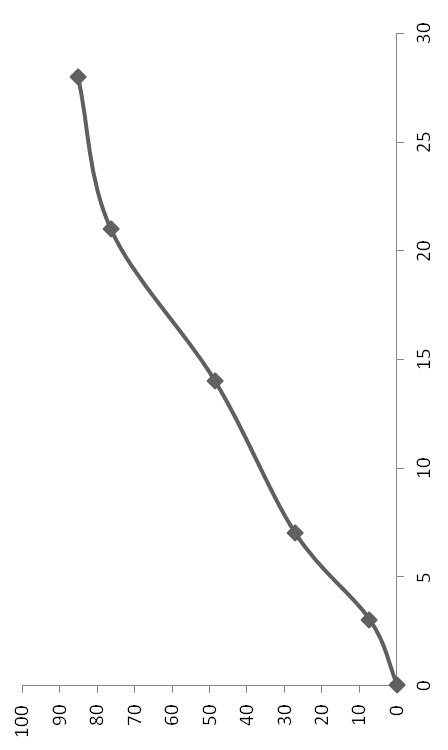
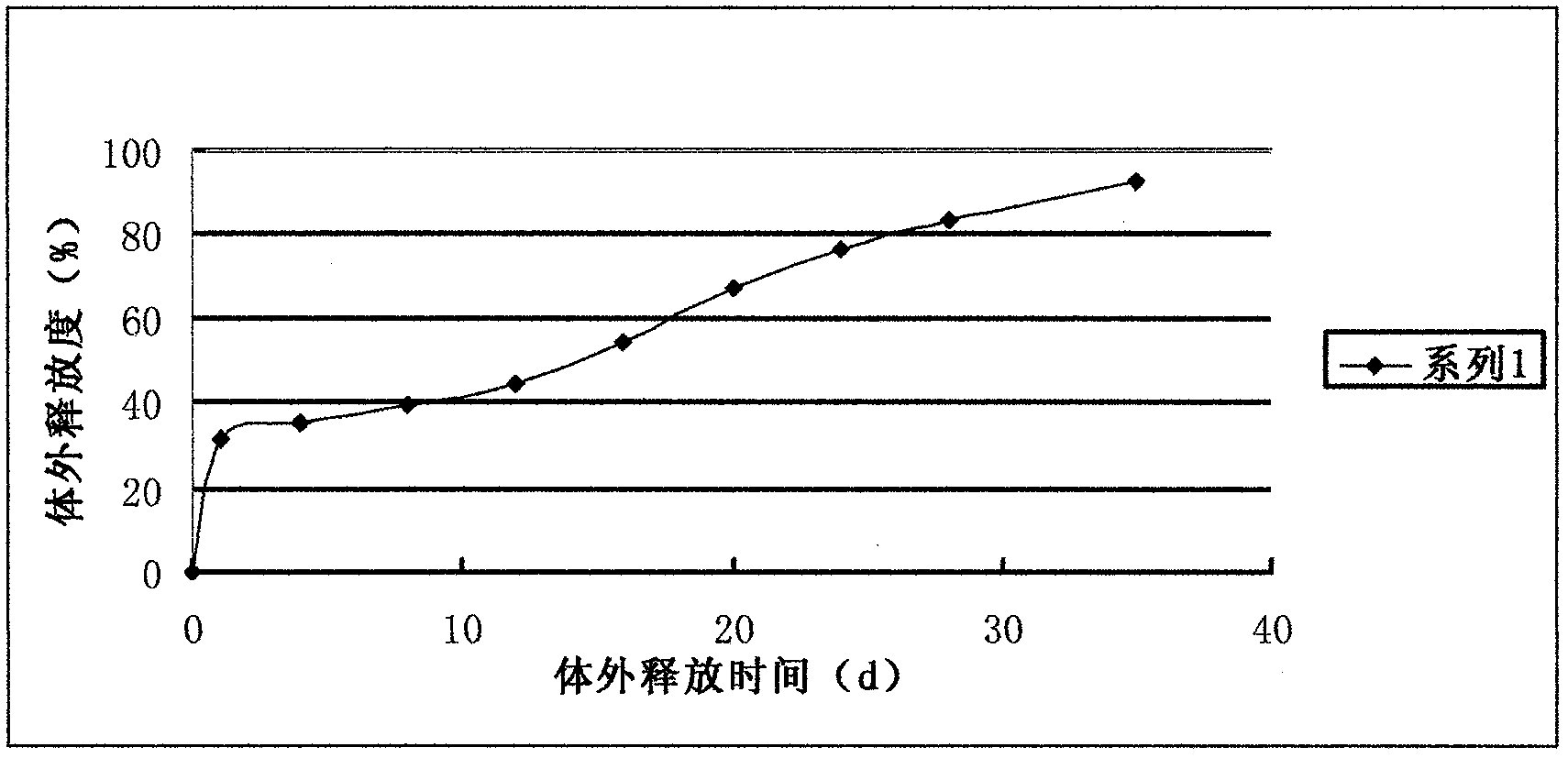
The invention discloses a GLP-I analogue liraglutide sustained-release microspheres and a preparation method thereof. The GLP-I analogue liraglutide sustained-release microspheres comprise, by weight, 0.5 to 30% (w / w) of GLP-I analogue liraglutide, 70 to 99.5% of a polymer which has the molecular weight of 5000 to 300000 dalton, can be biodegraded and has biocompatibility, and 0 to 10% of pharmaceutically acceptable auxiliary materials. The GLP-I analogue liraglutide sustained-release microspheres have the average grain size of 5 to 40 micrometers and an encapsulation ratio more than 90%. The GLP-I analogue liraglutide sustained-release microspheres have a sustained-release period of several days or several months, obviously reduce use frequency, improve GLP-I analogue liraglutide bioavailability, reduce toxic and side effects and are conducive to clinical treatment.
Owner:SHENZHEN JYMED TECH
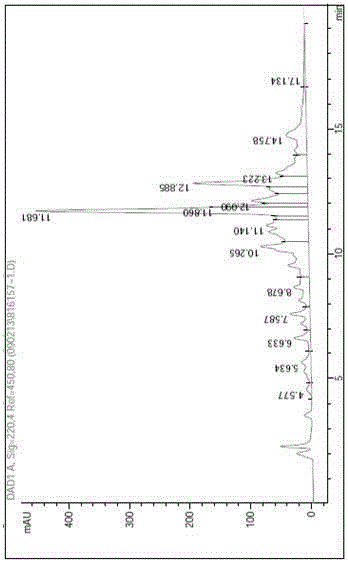
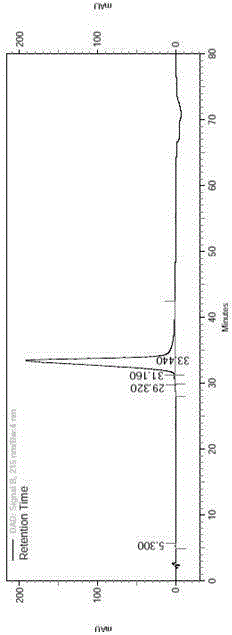
Chromatographic method for effectively improving purification yield of synthetic peptide
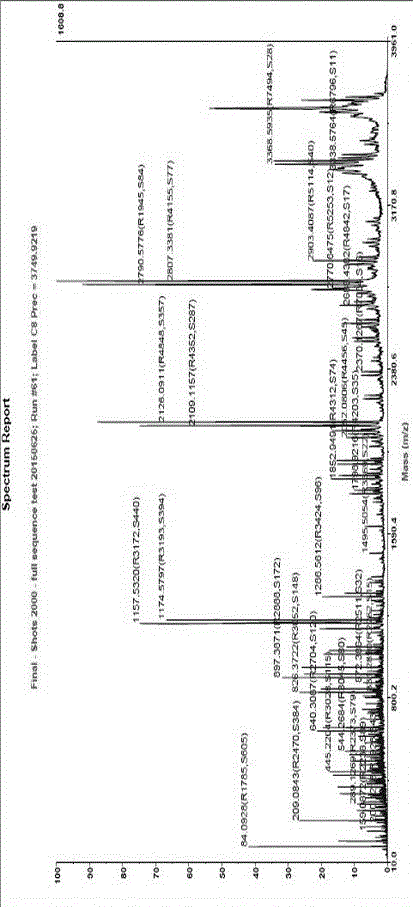
ActiveCN110540587AHigh yieldImprove the overall yield of purificationSolid sorbent liquid separationPeptide preparation methodsSynthetic Peptide PurificationMass spectrometry
The invention belongs to the field of pharmaceutical preparation, and relates to a chromatographic method for efficiently improving the purification yield of synthetic peptide, in particular to a method for improving the purification yield of liraglutide. According to the method for improving the purification yield of the liraglutide, impurities whose physicochemical properties have small differences from that of a target substance such asachiral enantiomeric impurities can be effectively removed, and the method is particularly suitable for purification of samples with complex impurity spectra; and meanwhile, the technical problems such as small loading quantity of peptide products, large individual impurities, low purity, and low efficiency can also be solved.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD +1
A liraglutide in-situ gel preparation and a preparing method thereof
InactiveCN104069485AReduce releaseRelieve painPeptide/protein ingredientsMetabolism disorderGel preparationOrganic solvent
The invention relates to the field of medicine preparations, in particular, the invention relates to a liraglutide in-situ gel preparation and a preparing method thereof. The liraglutide in-situ gel preparation comprises liraglutide, an in-situ gel material and an organic solvent. Based on g / g / mL, the mass volume ratio of the liraglutide, the in-situ gel material and the organic solvent is (0.5-1.5):(2-6):(14-30). The liraglutide in-situ gel preparation has a function of sustained release and avoids peak and valley phenomena of plasma concentrations. The medicine function time is long and the number of times of taking the medicine is reduced, thus largely alleviating pain of patients and improving compliance of patients. The liraglutide in-situ gel preparation is nonirritant to skin and connective tissues. The preparing method is simple, suitable for large-scale production, high in medicine loading capacity and low in cost.
Owner:HYBIO PHARMA
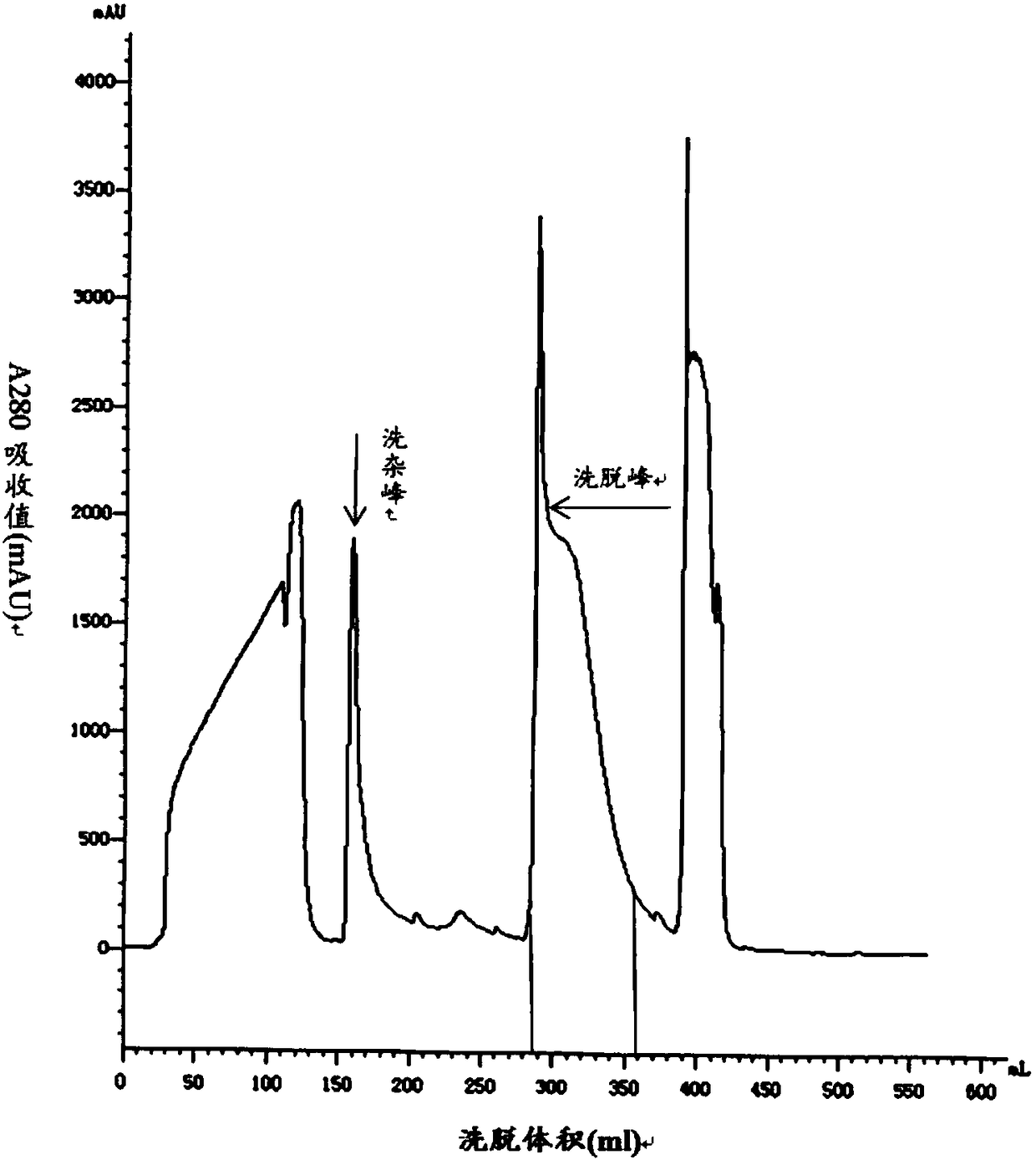
Method for purifying solid-phase synthetic crude liraglutide
InactiveUS20150051372A1High purityHigh yieldPeptide/protein ingredientsSolid sorbent liquid separationAcetonitrileAqueous solution
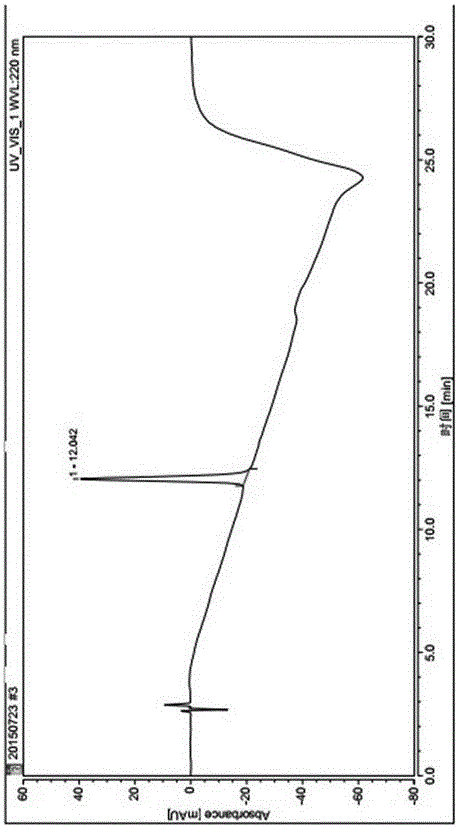
The present invention relates to the field of biomedicine, and in particular, to a method for purifying solid-phase synthetic crude liraglutide. The method comprises: dissolving solid-phase synthetic crude liraglutide in an aqueous acetonitrile solution to obtain a crude peptide solution; and obtaining liraglutide with high purity and high yield through four-step HPLC purification.
Owner:HYBIO PHARMA
Preparation method of liraglutide
ActiveCN107056927AShorten the production cycleHigh synthetic yieldPeptide preparation methodsBulk chemical productionSide chainCoupling
The invention discloses a preparation method of liraglutide. The preparation method comprises the following steps: using a deprotection reagent Fmoc-Gly-wang resin for deprotection, and removing Fmoc protective groups; coupling three protective amino acids at the 12th-14th sites by protective peptide fragments X; coupling three protective amino acids at 20-22 sites by protective peptide fragments Y; coupling protective amino acid at the 31th site by a Boc-His(trt)-OH protective form; removing alloc protection of lysine side chains for main-chain peptide resin of the liraglutide, coupling the side chains one by one and thus obtaining peptide resin of the liraglutide; cracking the peptide resin of the liraglutide in cracking liquid and thus obtaining a liraglutide crude product; purifying the liraglutide crude product and thus obtaining a liraglutide fine product. The preparation method disclosed by the invention has the advantages that segment coupling is carried out on amino sites which are easiest for folding in the process of synthesizing the main chain of the liraglutide, so that the problems of multiple times of coupling and low access rate for the amino acids due to folding in the synthesis process are avoided, the production cycle of the liraglutide is shortened, and the synthetic yield of the liraglutide is increased.
Owner:SICHUAN JISHENG BIOPHARM CO LTD
GLP-1 similar peptide modified dimer different in configuration and application of preparation method thereof in treating II-type diabetes
ActiveCN110845601AProlonged hypoglycemic effectHigh specific activityPeptide/protein ingredientsMetabolism disorderDisulfide bondingDimer
The invention provides application of novel glucagon peptide 1 fatty acid modified or unmodified dimer different in configuration in pancreas protection or hypoglycemic effect during treatment of II-type diabetes. The dimer is formed by connecting two identical GLP-1 monomers containing cysteine through disulfide bond formed by cysteine oxidation. Hypoglycemic duration of GLP-1 dimer is remarkablyprolonged without lowering activity of H-shaped GLP-1 homodimer (the disulfide bond is formed inside peptide chain), in-vivo continuous activity of GLP-1 analogue dimer can last for 19d while in-vivoactivity of liraglutide which is positive control drug is 3d, or in-vivo activity duration is remarkably prolonged when compared with long-acting GLP1 similar peptide which has already been reportedat present, so that technical progress of long-acting GLP1 drug is greatly promoted while convenience is brought to clinical application and popularization of the same. U-shaped homodimer (the disulfide bond is formed at the terminal C of the peptide chain) does not have impact on blood glucose but can obviously protect exocrine portion cells like pancreatic acini and catheters, thereby having a pancreas protecting function.
Owner:深圳纳福生物医药有限公司
Solid-liquid combined preparation method for liraglutide
ActiveCN105111303AGood water solubilityHigh yieldPeptide preparation methodsGlucagonsChemical synthesisDipeptide
The invention relates to the field of peptide synthesis, especially to a solid-liquid combined chemical preparation method for liraglutide. The method provided by the invention can simplify conventional preparation process for liraglutide and improve the quality of a final product. According to the invention, dipeptide monomer Fmoc-Lys(N-epsilon-(gamma-Glu(N-Boc)-OtBu)-OH is synthesized for the first time and is applied to preparation of liraglutide; as Fmoc-Ala-Ala-OH participates in preparation of liraglutide, generation of Ala impurity peptide in which position 24 or 25 is deleted is avoided, purification difficulty is reduced and yield is improved; and trifluoro-acetylated liraglutide (unmodified) has good water-solubility, which facilitates reversed phase chromatographic purification and preparation and protects an amino group at terminal N from side reaction in the process of palmitic acid modification, so the yield of the final product is greatly improved. Through implementation of the method, the problems that related impurity peptides in the final product exceed standard and overall yield is low in conventional chemical synthesis of liraglutide are overcome; the final product with purity of greater than 99.5% and simple impurity of less than 0.1% is obtained; and production cost is lowered.
Owner:JINAN KANGHE MEDICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com